Abstract
Background
Opioid dependence (OD) is an increasing clinical and public health problem worldwide. International guidelines recommend opioid substitution treatment (OST), such as methadone and buprenorphine, as first‐line medication treatment for OD. A negative aspect of OST is that the medication used can be diverted both through sale on the black market, and the unsanctioned use of medications. Daily supervised administration of medications used in OST has the advantage of reducing the risk of diversion, and may promote therapeutic engagement, potentially enhancing the psychosocial aspect of OST, but costs more and is more restrictive on the client than dispensing for off‐site consumption.
Objectives
The objective of this systematic review is to compare the effectiveness of OST with supervised dosing relative to dispensing of medication for off‐site consumption.
Search methods
We searched in Cochrane Drugs and Alcohol Group Specialised Register and Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, Web of Science from inception up to April 2016. Ongoing and unpublished studies were searched via ClinicalTrials.gov (www.clinicaltrials.gov) and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/).
All searches included non‐English language literature. We handsearched references on topic‐related systematic reviews.
Selection criteria
Randomised controlled trials (RCTs), controlled clinical trials (CCTs), and prospective controlled cohort studies, involving people who are receiving OST (methadone, buprenorphine) and comparing supervised dosing with dispensing of medication to be consumed away from the dispensing point, usually without supervision.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane.
Main results
Six studies (four RCTs and two prospective observational cohort studies), involving 7999 participants comparing supervised OST treatment with unsupervised treatment, met the inclusion criteria. The risk of bias was generally moderate across trials, but the results reported on outcomes that we planned to consider were limited. Overall, we judged the quality of the evidence from very low to low for all the outcomes.
We found no difference in retention at any duration with supervised compared to unsupervised dosing (RR 0.99, 95% CI 0.88 to 1.12, 716 participants, four trials, low‐quality evidence) or in retention in the shortest follow‐up period, three months (RR 0.94; 95% CI 0.84 to 1.05; 472 participants, three trials, low‐quality evidence). Additional data at 12 months from one observational study found no difference in retention between groups (RR 0.94, 95% CI 0.77 to 1.14; n = 300).There was no difference in abstinence at the end of treatment (self‐reported drug use) (67% versus 60%, P = 0.33, 293 participants, one trial, very low‐quality evidence); and in diversion of medication (5% versus 2%, 293 participants, one trial, very low‐quality evidence).
Regarding our secondary outcomes, we did not found a difference in the incidence of adverse effects in the supervised compared to unsupervised control group (RR 0.63; 96% CI 0.10 to 3.86; 363 participants, two trials, very low‐quality evidence). Data on severity of dependence were very limited (244 participants, one trial) and showed no difference between the two approaches. Data on deaths were reported in two studies. One trial reported two deaths in the supervised group (low‐quality evidence), while in the cohort study all‐cause mortality was found lower in regular supervision group (crude mortality rate 0.60 versus 0.81 per 100 person‐years), although after adjustment insufficient evidence existed to suggest that regular supervision was protective (mortality rate ratio = 1.23, 95% CI = 0.67 to 2.27).
No studies reported pain symptoms, drug craving, aberrant opioid‐related behaviours, days of unsanctioned opioid use and overdose.
Authors' conclusions
Take‐home medication strategies are attractive to treatment services due to lower costs, and place less restrictions on clients, but it is unknown whether they may be associated with increased risk of diversion and unsanctioned use of medication. There is uncertainty about the effects of supervised dosing compared with unsupervised medication due to the low and very low quality of the evidence for the primary outcomes of interest for this review. Data on defined secondary outcomes were similarly limited. More research comparing supervised and take‐home medication strategies is needed to support decisions on the relative effectiveness of these strategies. The trials should be designed and conducted with high quality and over a longer follow‐up period to support comparison of strategies at different stages of treatment. In particular, there is a need for studies assessing in more detail the risk of diversion and safety outcomes of using supervised OST to manage opioid dependence.
Plain language summary
Supervised‐dosing strategies versus take‐home opioid substitution treatment for people dependent on opioid drugs
Review question
We reviewed the evidence about the effectiveness of supervised dosing strategies in opioid substitution treatment for people dependent on opioid drugs.
Background
Opioid dependence (OD) is a global clinical and public health problem that is associated with significant burden of disease and drug‐related deaths. OD represents a complex health condition that usually requires long‐term treatment. International guidelines recommend opioid substitution treatment (OST), such as methadone and buprenorphine, as a first‐line treatment for OD. OST is a form of health care for people who are dependent on heroin, or who have become dependent after taking prescribed opioids for pain, and involves substitution of the drug that is being used inappropriately with a long‐acting opioid. OST gives people who are opioid dependent the opportunity to stabilise their lives, and to address the social and psychological dimensions that tend to accompany opioid dependence. A negative aspect of OST is that the medications used can be diverted, by being sold on the black market or used inappropriately. One strategy for minimising diversion is for OST medications to be administered under supervision (supervised dosing). With supervised dosing, access to unsupervised or take‐away doses of medication is then a privilege which can be used as a motivational and reward incentive. Supervised dosing is also associated with more frequent contact between the client and service provider offering more opportunities for therapeutic engagement. However, providing supervised dosing is more expensive for service providers, and more restrictive for clients who have to attend for dosing every day. The purpose of this review was to assess the effectiveness of supervised dosing, compared to dispensing of take‐home medication, in terms of reduction in heroin and other unsanctioned opioid use, retention in treatment, diversion of medication and adverse effects.
Search date
The evidence is current to April 2016.
Study characteristics
We identified six studies involving 7999 people receiving treatment with methadone (7786 people) or buprenorphine–naloxone (213 people ) for opioid dependence. Four of the studies were randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups), while the other two studies followed groups of people over time. Four of the studies were funded by the National Institutes for Health Research and by the Health Research Board, with one study not reporting the funding source. One study was also funded by the drug company of buprenorphine–naloxone.
Key results
At three or more months follow‐up, this review found no evidence on benefit of the supervised dosing with respect to keep people in treatment, or reduce opioid use, mortality reduction and adverse drug events. One study found that supervised dosing led to a reduction of diversion. None of the studies assessed the effect of supervised dosing on pain symptoms, drug craving, days of unsanctioned opioid use, overdose and hospitalisation.
We are unable to make any conclusion about the effectiveness of supervised dosing compared to dispensing of medication as take‐home doses, in the context of OST. Further research is required to determine the effectiveness of supervised or take‐home dosing in OST.
Quality of the evidence
Overall, the studies were moderately well‐conducted, but there was a small number of studies reporting outcomes of interest, therefore insufficient to evaluate the efficacy of intervention such as diversion, opoid use reduction, retention in treatment and frequency of unsanctioned opioid use, Furthermore, low rates of occurrence of some events between studies resulted in the overall quality of the evidence being assessed as low and very low. This indicates that further evidence would be likely to change the estimates of relative effect made in this review.
Summary of findings
Summary of findings for the main comparison. Supervised dosing with a long‐acting medication compared to unsupervised consumption for opioid dependence.
| Supervised versus unsupervised dosing | ||||||
| Patient or population: patients with opioid dependence Settings: outpatients Intervention: supervised dosing with a long‐acting medication Comparison: unsupervised consumption | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Unsupervised | Supervised | |||||
| Retention ‐ retention at the end of treatment (number of participants) Follow‐up: 3‐12 months | Study population | RR 0.99 (0.88 to 1.12) | 716 (4 studies) | ⊕⊕⊝⊝ low1 | ||
| 634 per 1000 | 628 per 1000 (558 to 710) | |||||
| Moderate | ||||||
| 653 per 1000 | 646 per 1000 (575 to 731) | |||||
| Retention ‐ retention at 3 months (number of participants) Follow‐up: mean 3 months | Study population | RR 0.94 (0.84 to 1.05) | 472 (3 studies) | ⊕⊕⊝⊝ low2 | ||
| 707 per 1000 | 664 per 1000 (594 to 742) | |||||
| Moderate | ||||||
| 737 per 1000 | 693 per 1000 (619 to 774) | |||||
| Adverse serious events (any) (number of participants) Follow‐up: mean 7.5 months | Study population | RR 0.63 (0.10 to 3.86) | 363 (2 studies) | ⊕⊝⊝⊝ very low3,4 | ||
| 77 per 1000 | 49 per 1000 (8 to 298) | |||||
| Moderate | ||||||
| 80 per 1000 | 50 per 1000 (8 to 309) | |||||
| Abstinence from unsanctioned opioid use (number of participants with urine drug screen or self‐report use) Follow‐up: mean 3 months | Study population | Not estimable | 293 (1 study) | ⊕⊝⊝⊝ very low4,5 | No difference between supervised and no supervised therapy in self‐reported heroin use at 3 months (67% vs 60%, P = 0.33) | |
| See comment | See comment | |||||
| Moderate | ||||||
| Diversion (number of participants) Follow‐up: mean 3 months | Study population | Not estimable | 293 (1 study) | ⊕⊝⊝⊝ very low4,5 | 5% respondents in the supervised group and 2% in the unsupervised group reported that "they had let another person have their drug", but it is not clear if the data referred to diversion. | |
| See comment | See comment | |||||
| Moderate | ||||||
| Frequency of unsanctioned opioid use at the end of the intervention (number of days) | See comment | See comment | Not estimable | 0 (0 study) |
See comment | No studies assessed this outcome |
|
Mortality ‐
(number of participants) Follow‐up: mean 12 months |
See comment | See comment | Not estimable | 230 (1 study) | ⊕⊕⊝⊝ low4 | It was reported one death from gastric haemorrhage at day 304 post‐enrolment, and one death from pneumonia at day 334 post‐enrolment. In one cohort study (6983 participants), the mortality rate ratio was unrelated to regular supervised methadone consumption. All‐cause mortality was lower in those with regular supervision (crude mortality rate 0.60 versus 0.81 per 100 person‐years), although after adjustment insufficient evidence existed to suggest that regular supervision was protective (mortality rate ratio = 1.23, 95% CI = 0.67–2.27). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded of two levels because all studies were at high risk for performance bias, one at high risk of detection bias and one at unclear risk for selection bias 2 Downgraded of two levels because all studies were at high risk for performance bias, one at high risk of detection bias and one at unclear risk for selection bias 3 Downgraded of two levels because two studies at high risk for performance and detection bias 4 Downgraded of two levels because OIS not met 5 Downgraded of two levels because the study was at high risk for performance and detection bias
Background
Description of the condition
Past‐year global use of opioids, including heroin and prescription painkillers, is estimated at between 28.6 million and 38 million (UNODC 2016). In 2014, 4.3 million people aged 12 or older reported current non‐medical use of prescription pain relievers and, in the same year, about 435,000 people aged 12 or older were current heroin users (SAMSHA 2015 b). The need to improve access to pain relief has influenced this rise: more than 100 million people suffer from chronic pain in the USA alone (IOM 2011; Johannes 2010), and in this context prescription opioids provide a therapeutic value for the management of pain relief and palliative care (Franklin 2009).
But in recent times prescription opioids have been increasingly used to treat chronic non‐cancer pain (Kissin 2013). The number of prescription opioids, such as hydrocodone and oxycodone products that are mostly prescribed for the treatment of moderate‐to‐severe pain have escalated from around 76 million in 1991 to nearly 207 million in 2013, with the USA their biggest consumer globally (UNODC 2014). A similar increase has been noted in Canada, where opioid consumption doubled between 2001 and 2009 (Fischer 2012). This trend has affected European countries to a lesser extent, although reports from the UK, Germany and Norway also identified an increase in the use of opioid analgesics (Schubert 2013; Zin 2014). Prescription opioid use has also risen in Australia, with a reported 150% increase in oxycodone prescriptions between 2002 and 2008 (Roxburgh 2011). Also, it is recognised that in numerous street‐drug populations in both the USA and Canada, prescription opioids have replaced heroin as the main opioid of choice (Fischer 2012; Sigmon 2006).
The health consequences associated with the rapid rise in prescription opioids worldwide have been an increase in misuse and addiction (Maxwell 2011), in inpatient hospitalisations (Ling 2011), emergency department visits (Cai 2010; SAMHSA 2015 a), and overdose. In the USA, rates of opioid overdose deaths increased significantly from 7.9 per 100.000 in 2013 to 9.0 per 100.000 in 2014, with an increase of 14%; over half of those deaths were from prescription opioids (Rudd 2016).
Description of the intervention
Opioid dependence (OD) represents a complex health condition that usually requires long‐term treatment if sustained recovery is to be achieved. The management of OD is similar to other chronic, relapsing medical conditions such as asthma, hypertension and diabetes (McLellan 2000; WHO 2004). International guidelines recommend opioid substitution treatment (OST), such as methadone and buprenorphine, as a first‐line approach in the pharmacologic treatment for OD (NICE 2007; WHO 2009), whether OD arises from the misuse of heroin or prescribed opioid medications. OST involves the administration of a long‐acting opioid (methadone or buprenorphine) in conjunction with psychosocial support. Long‐term substitution treatment has better outcomes than medically‐supervised detoxification, and should be considered within the context of a harm‐reduction approach. There is strong evidence that OST is more effective than detoxification or drug‐free counselling in terms of retention in treatment, reduction in unsanctioned opioid use, reduction in injecting use, and mortality (Dolan 2015). OST gives people who are opioid dependent the opportunity to stabilise their lives, to address the social and psychological dimensions that tend to accompany OD. It is not just about prescription of medication, but also the psychosocial support and interventions that are an integral part of OST. The physical adaptation to opioid drugs can be addressed later, when they feel more confident in their ability to become and remain abstinent (Dolan 2015; Gowing 2014). A negative aspect of OST is that the medications used can be diverted. Diversion in this context encompasses both the sale of medications on the black market, and the unsanctioned use of medications, for example by accumulating doses and then using larger amounts than prescribed, or use by injection (Larance 2011). Access to unsupervised or take‐away doses of medication is then a privilege that can be used to support contingency management approaches to treatment (Bell 2014; WHO 2009).
How the intervention might work
In the last two decades, OST diversion has been documented in countries all over the world and several studies have shown that a majority of methadone‐related deaths can be directly attributed to methadone diversion, often in patients not enrolled in any methadone maintenance treatment (MMT) programme (Cicero 2005; Seymour 2003).
To minimise diversion and misuse, it has been proposed that methadone be given under strict medical supervision (Fountain 2000; Varenbut 2007). Many countries have introduced treatment systems that require supervised dosing of OST (i.e. direct observation of methadone or buprenorphine consumption by the pharmacist or by the clinician), particularly for high‐risk patients, and supervised dosing is recommended in many clinical guidelines (WHO 2009). Supervised OST, often referred to as ‘supervised consumption’, is standard practice in many drug‐treatment centres, but it is expensive. Supervision ensures that patients take their medication as prescribed and prevents illicit drug diversion promoting stability and improving retention (WHO 2009). Both methadone and buprenorphine are sufficiently long acting to be taken once daily under supervision, if necessary. Regular contact through supervision may enhance treatment, but, on the other hand, prolonging supervision, may imply a lack of trust and may disadvantage patients as the need to attend daily for supervised dosing may interfere with employment opportunities and normal life activities. Hence, reducing the need for daily attendance for supervised dosing may improve patients' quality of life and facilitate rehabilitation and processes of reintegration into this community (Ritter 2005). In this context, qualitative studies of OST patients indicated that a flexible approach to supervision of dosing was appreciated (Dale‐Perera 2015; Notley 2013).
Why it is important to do this review
The potential benefits to patients from less restrictive dosing strategies need to be balanced with the potential risks to the community from possible diversion of OST medications.
Objectives
The objective of this systematic review is to compare the effectiveness of opioid substitution treatment (OST) with supervised dosing relative to dispensing of medication for off‐site consumption.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), and controlled clinical trials (CCTs) and prospective controlled cohort studies. The rationale for including study types other than RCTs was that it can be difficult to implement RCTs assessing supervised supply of OST medications. We did not expect to find many RCTs that answered our question.
Types of participants
People "diagnosed as opioid dependent and receiving opioid substitution treatment with either buprenorphine or methadone". Patients with additional diagnosis such as alcoholism or in methadone‐maintenance schemes were also eligible.
Types of interventions
Experimental: supervised opioid substitution treatment (methadone, buprenorphine).
Comparator: dispensing of medication to be consumed away from the dispensing point, usually without supervision.
Types of outcome measures
Primary outcomes
Retention in treatment defined as the number of participants who complete the study protocol (at any duration).
Abstinence from unsanctioned opioid use (measured by urine drug screen or self‐report use).
Diversion, defined as inappropriate use of medication by those for whom it has been prescribed, for example the selling or sharing of medication by patients to the illicit drug market.
Frequency of unsanctioned opioid use at the end of the intervention period.
Secondary outcomes
Adverse effects of medication (number of participants experiencing any adverse event).
Mortality or serious adverse event.
Severity of dependence as measured by validated scales e.g. Addiction Severity Index (ASI), Clinical Global Impression ‐ Severity scale (CGI‐S), Clinical Global Impression ‐ Observer scale (CGI‐O).
Overdoses.
Craving as measured by validated scales e.g. Brief Substance Craving Scale (BSCS), Visual Analogue Scale (VAS).
Pain, assessed by validated scales such as the Brief Pain Inventory (Cleeland 2009) and the McGill Pain Questionnaire (Melzack 1975) for prescribed opioid‐dependent people.
Aberrant opioid‐related behaviours (e.g. seeing multiple doctors for extra opioid medication, lost medication, unauthorised dose escalations), for prescribed opioid‐dependent people.
Search methods for identification of studies
Electronic searches
We searched the following bibliographic databases:
the Cochrane Drugs and Alcohol Group's Specialised Register of Trials (on 15 April 2016);
the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2016, Issue 10, (April 2016));
Embase (Elsevier, Embase.com) (1974 to 15 April 2016);
MEDLINE (EBSCO host) (1966 to 15 April 2016);
Web of Science (1990 to 15 April 2016).
The search strategy used for each database combined appropriate controlled vocabulary terms (as applicable) and free‐text terms relating to opioid use and supervised therapy. Details of the search strategies for all databases are shown in Appendix 1, Appendix 2, Appendix 3, and Appendix 4. We did not apply any filter on study design or language.
We conducted additional searches for ongoing clinical trials on the following sites:
www.clinicaltrials.gov (last searched 30 April 2016);
www.who.int/ictrp/en/, International Clinical Trials Registry Platform (last searched 30 April 2016).
We also searched relevant websites which are likely to contain evaluations of treatments for opioid dependence, such as:
Substance Abuse and Mental Health Services Administration‐SAMHSA ( www.samhsa.gov/);
Drug and Alcohol Findings (http://findings.org.uk/);
National Institute of Drug Abuse (https://www.drugabuse.gov/).
Searching other resources
We searched the bibliographies of included studies to find additional studies and used the Web of Science (ISI Web of Knowledge, April 2016) to identify additional studies that had cited included studies.
We contacted study investigators of relevant studies to request any details of any other known study.
Data collection and analysis
Selection of studies
Two review authors (RS, SV) independently screened and evaluated all titles and abstracts (where available) identified by the literature search, in accordance with the described inclusion criteria (see Criteria for considering studies for this review). We retrieved full‐text copies of papers judged to be potentially relevant by at least one author. The same review authors independently reviewed the full‐text articles for inclusion. Differences were resolved through discussion until consensus was reached. Multiple publications were collated and assessed as one study.
Data extraction and management
For trials that fulfilled inclusion criteria, two review authors (RS, SV) extracted data independently using a data extraction form. We considered information about study design, settings and number of study centres and location, characteristics of participants and interventions (type of drugs, dosage and frequency of supervised administration, additional co‐interventions), outcomes and times reported, length of follow‐up, funding and conflict of interest. We planned to extract both dichotomous and continuous outcome data, and details of the analysis (intention‐to‐treat or per‐protocol analysis). Any discrepancies between the data were resolved through discussion with the third review author. We transferred data into Review Manager software version 5.3. (RevMan 2014), and checked for accuracy.
Assessment of risk of bias in included studies
Two review authors (RS, SV) independently assessed the risk of bias of the included studies. We used the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess the risk of bias for RCTs based on seven standard criteria: adequate sequence generation; concealment of allocation; blinding of participants and providers, blinding of outcome assessor; adequately addressed incomplete outcome data; freedom from selective reporting; freedom from other risk of bias. Blinding of participants and providers and blinding of outcome assessor were assessed separately for objective outcomes (e.g. retention, use of substance of abuse measured by urine analysis) and subjective outcomes (e.g. patient self‐reported use of substance, adverse effects, diversion). We considered incomplete outcome data (avoidance of attrition bias) for all outcomes except for retention in treatment, which is the primary outcome measure in trials on the field of addiction.
For observational studies, we included in the 'Risk of bias' table some additional criteria suggested by the Effective Practice and Organization of Care (EPOC 2016) "Risk of bias" tool for non‐randomised studies. Specifically for cohort studies, we considered baseline outcome measurements, baseline characteristics, and protection against contamination. For a detailed description of the criteria used to assess risk of bias, see Appendix 5.
The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry, in terms of low, high or unclear risk. We resolved any disagreements by discussion.
Grading of evidence
We assessed the overall quality of the evidence for the primary and secondary outcomes using the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) system. The GRADE Working Group developed a system for grading the quality of evidence that takes into account issues not only related to internal validity but also to external validity, such as directness of results (GRADE 2004; Guyatt 2008; Guyatt 2011; Schünemann 2006). We prepared a 'Summary of findings' table to report the main findings of the review in a transparent and simple tabular format. In particular, the table provides key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data for retention at the end of treatment (including all follow‐up periods), retention at three months of follow‐up,and any adverse serious events.
The GRADE system uses the following criteria for assigning grades of evidence.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
Grading is decreased for the following reasons.
Serious (‐1) or very serious (‐2) limitation to study quality.
Important inconsistency (‐1).
Some (‐1) or major (‐2) uncertainty about directness.
Imprecise or sparse data (‐1).
High probability of reporting bias (‐1).
Grading is increased for the following reasons.
Strong evidence of association ‐ significant risk ratio of > 2 (0.5) based on consistent evidence from two or more observational studies, with no plausible confounders (+1).
Very strong evidence of association ‐ significant risk ratio of > 2 (< 0.5) based on direct evidence with no major threats to validity (+2).
Evidence of a dose‐response gradient (+1).
All plausible confounders would have reduced the effect (+1).
Measures of treatment effect
For dichotomous data, we presented results as risk ratios (RRs) with 95% confidence intervals (95% CIs). For continuous outcomes data, we planned to calculate the mean differences (MDs) with 95% CIs if studies used the same measurement scale. If studies used different scales we planned to calculate the standardised mean difference (SMD).
We performed meta‐analyses according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Unit of analysis issues
The unit of analysis was the participants in the included studies. In the case of studies with multiple treatment arms, we planned to combine all relevant experimental intervention groups of the study into a single group, and to combine all relevant control intervention groups into a single control group (Higgins 2011). This approach was used to prevent inappropriate double‐counting of individuals.
Assessment of heterogeneity
We considered the clinical and methodological characteristics of the included studies to evaluate a clinically meaningful summary of data. We tested statistical heterogeneity in each meta‐analysis using the I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 50%, or a P value was lower than < 0.10 for the Chi² test for heterogeneity. Following the guidance in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), we distinguished the following values to denote not important, moderate, substantial, and considerable heterogeneity, respectively: 0% to 40%, 30% to 60%, 50% to 90%, and 75% to 100%. Had we found considerable levels of heterogeneity (i.e., ≥ 75%), we planned to explore possible reasons by visually inspecting the forest plot to identify studies that might be contributing to heterogeneity.
Assessment of reporting biases
We did not assess reporting biases using funnel plots for asymmetry as an indication of publication bias because the analyses that we were able to undertake included less than 10 studies.
Data synthesis
Where data were available we carried out statistical analysis using the Review Manager software (RevMan 5.3). We combined outcome data from the individual trials through meta‐analysis where possible (comparability of intervention and outcomes between trials). We planned to pool data using a random‐effects model for all analyses because a certain degree of heterogeneity of interventions and outcomes (different duration and different follow‐up measures) was expected. Specifically, we used the Mantel‐Haenszel method for dichotomous data and planned to use the same method for continuous data.
The results are presented as the average treatment effect with its 95% CI, and the estimates of Chi² and I² are reported.
Where it was not possible to pool data or only one study was included in the comparison, we reported the outcomes in the text in a narrative way and discussed the results.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses for studies where the majority of participants were using street heroin compared to prescription opioids, but this was not possible because of the insufficient data available.
Sensitivity analysis
We planned to perform sensitivity analysis for primary outcomes by excluding trials with high risk for allocation concealment and sequence generation. This was unnecessary as none of the included studies were assessed as being at high risk for selection bias.
Results
Description of studies
Results of the search
Through bibliographic searches we identified 2171 records. After removing duplicates we screened 1651 reports and we excluded 1631 based on the title and abstract. We retrieved 20 articles for more detailed evaluation, 14 of which we excluded after reading the full text. The remaining six studies met all the inclusion criteria and were included in the review (see Figure 1). No ongoing studies were identified.
1.
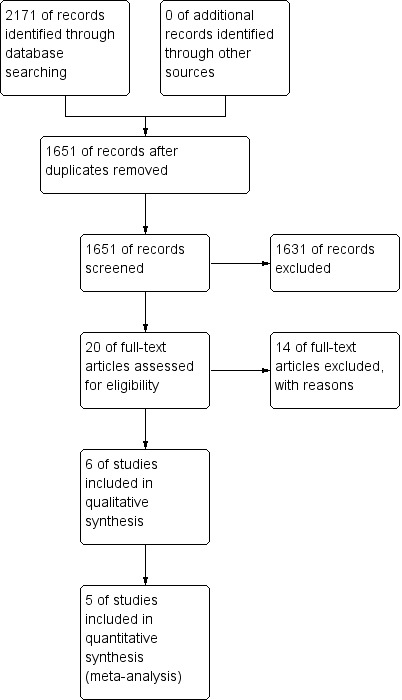
Study flow diagram.
Included studies
Details of the individual studies are provided in the Characteristics of included studies tables. We included six studies of opioid‐dependent people receiving opioid substitution treatment (OST) with a total of 7999 participants, 7587 receiving methadone (Cousins 2016; Gerra 2011; Holland 2012; Schwartz 2012), and 119 receiving buprenorphine–naloxone (Bell 2007). Holland 2014 includes 293 participants in buprenorphine (Subutex®/Suboxone®) or methadone maintenance.
Of the six included studies, four were randomised controlled trials (RCTs) (Bell 2007; Holland 2012; Holland 2014; Schwartz 2012), and two had a prospective observational cohort study design (Cousins 2016; Gerra 2011). The RCTs employed a simple randomisation procedure based on individual allocation to the intervention and control groups. In the observational studies, opioid users were recruited through interviews of all eligible patients referred to long‐term methadone treatment centres (Gerra 2011), or from a national methadone treatment register (Cousins 2016). The sample size ranged from 60 (Holland 2012, pilot study) to 6983 (Cousins 2016) participants. All studies were conducted in high‐income countries: two in the UK (Holland 2012; Holland 2014), one each from Ireland (Cousins 2016), Australia (Bell 2007), Italy (Gerra 2011), and the USA (Schwartz 2012). Two studies were single‐centre (Holland 2012; Schwartz 2012); the remaining were multicentre trials (with three centres: Gerra 2011; four centres: Bell 2007; 12 centres: Holland 2014).
Overview of study participants
Overall, the participants had a diagnosis of opioid dependence with a mean age of 35 years. The proportion of males was high, ranging from 68% (Cousins 2016) to 83% (Gerra 2011). Only one study, reported data on race, where the predominant (77%) of participants were African‐American (Schwartz 2012).
Participants were defined differently across the studies: in Bell 2007, opioid‐dependent participants, meeting the criteria for entry to maintenance treatment, were initiated on buprenorphine, usually on the day of presentation (day one). In Gerra 2011 and Cousins 2016, community centres recruited all eligible patients referred for long‐term methadone treatment. In the study conducted by Holland 2012, participants were eligible if they had confirmed opioid dependence and had been managed with supervised methadone for three months (with a two‐week ‘window’ before or after the three‐month point). In Holland 2014, eligible clients with confirmed symptoms and toxicological evidence of opioid dependency commenced maintenance therapy with methadone/buprenorphine, and in Schwartz 2012, individuals being placed on a waiting‐list for publicly‐subsidised treatment availability at one of two methadone treatment programmes (MTPs) were recruited. None of the studies included participants with prescription opioids dependence.
Participants with psychiatric co‐morbidities were included only in one study (Gerra 2011).
Treatment regimens or Interventions
All studies reported the setting of interventions. Five studies delivered the interventions in an outpatient setting (Bell 2007; Cousins 2016; Gerra 2011; Holland 2014; Schwartz 2012), and one trial was in a community pharmacy (Holland 2012). The mean duration of the treatments in five studies was 5.5 months (range three to 12 months); Cousins 2016 had a follow‐up of 72 months.
Of the included studies, four studies allocated participants to one of two groups, the intervention (supervision consumption of opioid maintenance treatment) and a control group (no supervision) (Bell 2007; Cousins 2016; Holland 2014; Schwartz 2012). Two studies allocated participants to one of three groups, two of which were control groups comprising slight variations of the same programme in terms of intensity or modality (Gerra 2011; Holland 2012).
Administration of methadone under supervision condition was studied in four studies (Cousins 2016; Gerra 2011; Holland 2012, Schwartz 2012). Gerra 2011 tested a supervised daily consumption, contingent take‐home incentives and a non‐contingent take‐home in three groups of heroin‐addicted patients attending different methadone maintenance treatment (MMT) programmes.
Holland 2012 considered supervised treatment with different intensity: a ‘twice weekly supervision’ scheme, where the pharmacist dispensed their medication daily, but supervision was on only two days per week; a "daily supervised consumption", where the pharmacist dispensed the medication to be swallowed under their supervision; "no supervision" with medication collected daily.
Schwartz 2012 assigned participants to methadone combined with psychosocial intervention of different intensities (emergency or routine counselling). Opioid substitution therapy (OST) with methadone or buprenorphine (Subutex®/Suboxone®) were used in Holland 2014.
Bell 2007 randomly assigned all participants to medication with regular attendance at drug‐treatment centres (observed dosing), or picking up medication once per week, for administration at home (unobserved dosing).
Excluded studies
We excluded 14 studies (see table of Characteristics of excluded studies.The main reasons for exclusion were the type of interventions (Amass 2001; Auriacombe 2004; Carrieri 2014; Groshkova 2013; Haasen 2007; Hutchinson 2000; Kakko 2003; Krook 2002; Lintzeris 2013; Strang 2010; Suzuki 2014; van den Brink 2003). One study was excluded because it was a protocol without any results (Lintzeris 2006). In addition, the study Bell 2008 was excluded as it is a an open‐label extension study focused on only a selected sample of patients recruited from Bell 2007.
Risk of bias in included studies
The risk of bias was generally moderate across studies with some domains of the 'Risk of bias' assessment tool being satisfied for the majority of studies; (Figure 2). We have summarised the results of the assessments for each study in Figure 3, and in the following sections.
2.
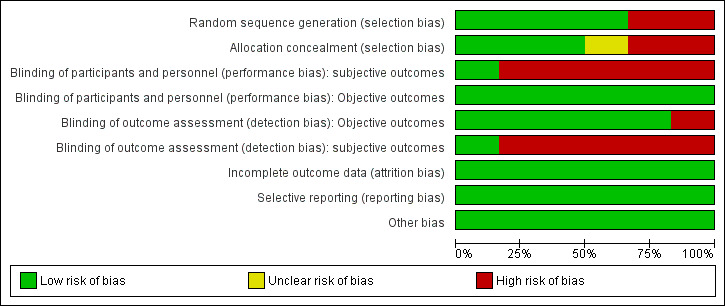
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
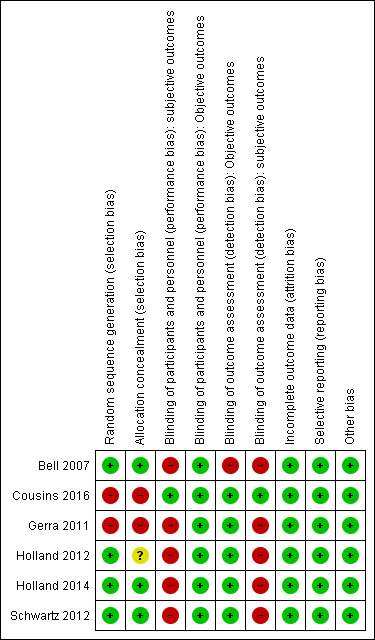
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Random sequence generation
Four studies (Bell 2007; Holland 2012; Holland 2014; Schwartz 2012) were judged at low risk of selection bias because they used a computer‐generated randomisation sequence to allocate adults to the experimental and control groups. Two studies were non‐randomised and assessed as having a high risk for selection bias (Cousins 2016; Gerra 2011).
Allocation concealment
Three studies (Bell 2007; Holland 2014; Schwartz 2012) were judged at low risk of selection bias because researchers used an adequate method for allocation concealment. We judged Holland 2012 to have an unclear risk because investigators did not clearly report the method used for allocation concealment. Two studies were judged at high risk of bias (Cousins 2016; Gerra 2011) because the study design was non‐randomised.
Blinding
Performance bias
Objective outcomes
The nature of the intervention made blinding of participants receiving the intervention and providers delivering the intervention impossible. For objective outcomes, we classified all studies as having a low risk of bias because we judged that they were not likely to be influenced by lack of blinding.
Subjective outcomes
Participants and personnel were not blinded in five of the studies for these kind of interventions (Bell 2007; Gerra 2011; Holland 2012; Holland 2014; Schwartz 2012), so we judged them to be at high risk of bias. One study (Cousins 2016) was judged at low risk of bias because it did not consider any subjective outcomes.
Detection bias
Objective outcomes
We rated five studies as having a low risk of bias for blinding of outcome assessment (Cousins 2016; Gerra 2011; Holland 2012; Holland 2014; Schwartz 2012) and one at high risk of bias (Bell 2007).
Subjective outcomes
Five studies were judged at high risk of bias because the authors declared unblinded assessment at each follow up (Bell 2007; Gerra 2011; Holland 2012; Holland 2014; Schwartz 2012). Cousins 2016 did not consider subjective outcomes.
Incomplete outcome data
All four RCTs performed an intention‐to treat analysis. When studies reported missing outcome data, they were balanced in numbers across intervention and control groups, with similar reasons. We classified all studies as low risk of bias. In the Gerra 2011 study, five participants withdrew from the study and therefore it was assessed as being at low risk of bias.
Selective reporting
Published trial registration information was available for only one study (Holland 2014). However, for all studies, we found all the outcomes listed in the 'Methods' section reported in the 'Results' section; so we judged all five studies as having low risk of bias (Bell 2007; Cousins 2016; Gerra 2011; Holland 2012; Schwartz 2012).
Other potential sources of bias
We did not find other obvious sources of bias. We classified four studies as having a low risk of other bias (Bell 2007, Holland 2012; Holland 2014; Schwartz 2012). All included trials reported sufficient data for baseline characteristics in the intervention and control group to be judged comparable. In Holland 2014, the groups differed with respect to gender, criminal convictions, and alcohol use; we judged the study as having a low risk of bias because the authors fully adjusted all analyses for each potential confounders.
The two observational studies included in the review, due to the study design, had a potential source of bias related to selection of participants and contamination of interventions. To address these bias, different measures were taken to reduce differences among the three recruiting centres and a standardised patient‐recruitment protocol was used in Gerra 2011. Moreover, the analysis were adjusted for possible confounders (gender and age) (Cousins 2016; Gerra 2011) and hence both were scored as having a low risk of potential bias.
Effects of interventions
See: Table 1
Comparison: Supervised dosing vs unsupervised
SeeTable 1
Primary outcomes
Retention
There was no significant difference in retention rate at any duration of treatment (three to 12 months) in the pooled data of four studies where participants received supervised treatment and the control group received unsupervised therapies (risk ratio (RR) 0.99, 95% confidence interval (CI) 0.88 to 1.12; four studies, n = 716), seeAnalysis 1.1.
1.1. Analysis.
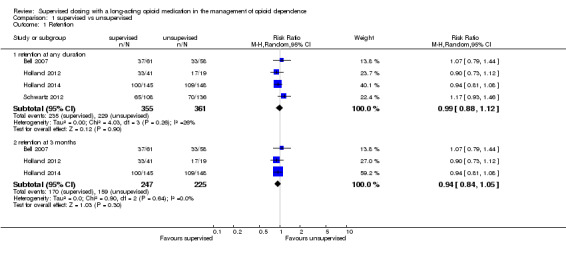
Comparison 1 supervised vs unsupervised, Outcome 1 Retention.
There was no important statistical heterogeneity in the meta‐analysis:Chi² = 4.03, df = 3 (P = 0.26); I² = 26%.The included period of duration of intervention with the most consistently available data across studies ranged between three and 12 months.
Because of differences in duration of treatment, we conducted a separate analysis of the studies that reported data on retention assessed at three months (end of treatment). The pooled estimates across these studies yielded no difference between supervised and unsupervised therapy (RR 0.94, 95% CI 0.84 to 1.05; three studies, n = 472) seeAnalysis 1.2., with no heterogeneity (I2 = 0%).
1.2. Analysis.
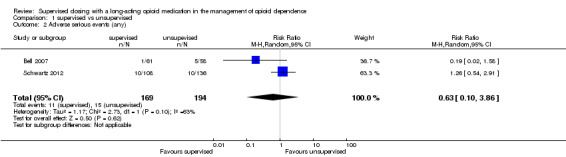
Comparison 1 supervised vs unsupervised, Outcome 2 Adverse serious events (any).
Data from observational study not included in meta analysis (Gerra 2011) reported no significant difference in retention between groups (RR 0.94, 95% CI 0.77 to 1.14; n = 300).
Abstinence end of treatment (point abstinence)
One study (Holland 2014) found no difference between supervised and no supervised therapy in self‐reported heroin use at three months (67% versus 60%, P = 0.33; n = 293).
Abstinence end of treatment (continuous abstinence)
No studies assessed this outcome.
Diversion
One study (Holland 2014) found that a total of 5% respondents in the supervised group and 2% in the unsupervised group reported that "they had let another person have their drug", but it is not clear if the data referred to diversion.
Days of unsanctioned opioid use (end of treatment)
No studies assessed this outcome.
Secondary outcomes
Serious adverse events
Two trials (Bell 2007;Schwartz 2012) reported the number of serious adverse events requiring hospitalisation such as accident, cardiovascular, respiratory, gastrointestinal diseases, urinary tract infection. Pooling these data no significant difference was found (RR 0.63, 95% CI 0.10 to 3.86, two studies, n = 363);seeAnalysis 1.2. with a high heterogeneity (Chi² =63%).
Overdoses
No studies assessed this outcome.
Mortality
Schwartz 2012 reported in the supervised group one death from gastric haemorrhage at day 304 post‐enrolment, and one death from pneumonia at day 334 post‐enrolment.
One cohort study (Cousins 2016) with data from 6983 participants and six years of follow‐up reported data on drug‐related mortality and all‐cause mortality. During follow‐up, 31 participants in the supervised group and 47 in the control group died for drug‐related effects but this was unrelated to regular supervised methadone consumption (mortality rate ratio = 0.76, 95 CI% = 0.32 to 1.80). All‐cause mortality was lower in those with regular supervision (crude mortality rate 0.60 versus 0.81 per 100 person‐years), although after adjustment insufficient evidence existed to suggest that regular supervision was protective (mortality rate ratio = 1.23, 95% CI = 0.67 to 2.27).
Severity of dependence (Addiction Severity Index (ASI), Clinical Global Impression ‐ Severity scale (CGI) etc)
One study (Schwartz 2012) with 244 participants found no significant difference for the ASI composite scores (P > 0.05).
Craving (BSCS score, VAS etc)
No studies assessed this outcome.
Pain
No studies assessed this outcome.
Aberrant opioid related behaviours
No studies assessed this outcome.
Discussion
Summary of main results
This review has examined the published evidence comparing supervised versus unsupervised consumption of opiate substitution treatment. We identified six studies, four were randomised controlled trials (RCTs) and two were cohort studies, with a total of 7999 participants. Methadone or buprenorphine under medical supervision were used. The risk of bias was generally moderate across trials. Evidence on all the primary and secondary outcomes was considered limited: only one study considered the point abstinence at end of treatment, one study investigated the diversion and the severity of dependence. Moreover, effects on drug‐related mortality were inconclusive (only one study reported this outcome). None of the studies assessed continuous abstinence at the end of treatment, days of unsanctioned opioid use, overdose, craving, pain and aberrant opioid‐related behaviours. This scarcity of data meant that we could perform a limited number of meta‐analyses. Across all of the included studies, all reported retention outcome but we did not find a difference between supervised treatment and unsupervised treatment in increased retention rate,low‐quality of evidence. Stratifying for the duration of treatment, no significant results were found at three months, low‐quality of evidence. For the outcome adverse events, meta‐analysis of two studies found no increase or decrease for any serious adverse events in those exposed to supervised therapy, very low‐quality of evidence.
Overall completeness and applicability of evidence
The strength of the evidence available is limited by the small number of studies, some of them were quite small, and by the variability of outcome measures utilised, which limits the meta‐analysis and the conclusions. Most of the included studies did not assess some of secondary outcomes prespecified in the review.
For example, based on the available data, it is not possible to draw any conclusions regarding the risk of diversion of opioid substitution treatment (OST) for unauthorised use in the treatment and in control group, while maintaining access to OST.
An important weakness of the review is the applicability of findings. First at all, most included studies were conducted with predominantly in men, stable opioid‐dependent participants receiving OST in specialised opioid‐dependence treatment programmes, and it is unclear whether findings are generalisable to other populations, for example those dependent upon prescription opioids, and women. Secondly, five of the six studies were conducted in countries where opioid substitution is widely available. Stronger evidence would be gained from replicating trials on people dependent on opioids living in other settings.
Apart from one study (Schwartz 2012), we did not identify any studies combining OST and psychosocial measures such as supportive counselling, psychotherapy, assistance with social needs such as housing, employment, education, welfare and legal problems. Authors of one systematic review reported that individual or group counselling or psychosocial support may improve abstinence rates in comparison to pharmacotherapy alone (Amato 2011).
Quality of the evidence
The major limitation of the review was the small number of studies included, two of which used an observational study design and therefore at high risk of bias. Four of the six included studies were RCTs with low risk of bias, although blinding of participants and clinicians involved in the supervised consumption of opiate substitution treatment was impossible. Thus, we cannot rule out performance bias. Our 'Risk of bias' estimates show that a main limitation is due to the high risk of detection bias (subjective outcomes) in some studies. Using GRADE, the quality of evidence was downgraded to low and very low for all outcomes (see Table 1) so that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
We found high heterogeneity when we combined data for serious adverse events outcomes (seeAnalysis 1.2), reducing the confidence that can be placed in these results. No heterogeneity resulted for the other analyses.
Potential biases in the review process
We minimised potential publication biases in the review process by searching different databases such as the Cochrane Drug and Alcohol Group's Specialised Register, MEDLINE and Embase with no limits on language or year of publication, but we only identified a small number of studies. We planned to assess the publication bias using the funnel plots but, in line with recommendations, we did not perform statistical tests of asymmetry due to the small number of trials. Alternatively, we investigated publication bias by searching for non‐published data via the website of international organisations and registers of clinical trials.
Agreements and disagreements with other studies or reviews
In this review we summarised for the first time the evidence from all randomised controlled trials that have experimented a supervised strategy in the intervention for patients with opioid dependence. To our best knowledge, no systematic review on the efficacy of supervised consumption of opiate substitution has been published.
Clinical guidelines published in 2007 and 2009 (NICE 2007; WHO 2009) recommend a period of supervised consumption of opioid medicines in the early phase of treatment, although the quality of evidence was considered low on the base of the available observational studies.
Authors' conclusions
Implications for practice.
Evidence on the use of supervised dosing in the context of opioid substitution treatment (OST) for the management of opioid dependence was limited to six studies, some of them we considered at high risk of bias. At present, there is uncertainty about the effects of supervised dosing compared unsupervised medication due to the low and very low quality of the evidence. For opioid‐dependent people receiving OST, the decision to use supervised OST versus unsupervised treatment should be made on a case‐by‐case basis, taking into account the characteristics of patients, and social factors (i.e. employment status and social relationships).
Implications for research.
More randomised trials with larger samples sizes are still needed to further explore the current available evidence for supervised or take‐home strategies for the management of opioid dependence in long‐acting opioid medication.
The efficacy of the intervention needs further exploration of replication and applicability to other settings and extended population (i.e. of heroin dependents as well as of prescription‐opioid dependents). Future high‐quality trials should designed and conducted and address long‐term outcomes to further investigate delayed effects of OST. In particular, there is a need for studies assessing in more detail the risk of diversion and safety outcomes of using supervised OST to manage opioid dependence.
At present, the results of our review do not allow us to answer the most relevant clinical question, that is which is the most effective type of treatment in terms of supervised or take‐away dosing?
Acknowledgements
Laura Amato and Zuzana Mitrova.
Appendices
Appendix 1. MEDLINE Complete search strategy (via EBSCO)
MH "Opioid‐Related Disorders+")
(MH "Prescription Drug Misuse+")
MH "Drug Prescriptions+")
TX "prescription drug" OR TX "prescription drugs"
AB (heroin OR analgesic* OR opiate* OR opioid OR morphin* OR morfin* OR methadone OR oxycodone OR oxycontin OR narcotic OR hydrocodone OR hydromorphone OR codeine OR fentanyl OR meperidine OR oxymorphone OR propoxyphene OR tramadol OR buprenorphine) N6 (abuse OR abusing OR abuses OR addict* OR dependen* OR maintenance)
TI (heroin OR analgesic* OR opiate* OR opioid OR morphin* OR morfin* OR methadone OR oxycodone OR oxycontin OR narcotic OR hydrocodone OR hydromorphone OR codeine OR fentanyl OR meperidine OR oxymorphone OR propoxyphene OR tramadol OR buprenorphine) N6 (abuse OR abusing OR abuses OR addict* OR dependen* OR maintenance)
S1 OR S2 OR S3 OR S4 OR S5 OR S6
TX supervision OR TX supervised
TX(medication AND assisted)
(MH "Opiate Substitution Treatment")
TX OST
S8 OR S9 OR S10 OR S11
MH animals
MH humans
S13 NOT S14
S7 AND S12
S16 NOT S15
Appendix 2. CENTRAL
MeSH descriptor: [Opioid‐Related Disorders] explode all trees
MeSH descriptor: [Prescription Drug Misuse] explode all trees
MeSH descriptor: [Drug Prescriptions] explode all trees
"prescription drug":ti,ab
"prescription drugs":ti,ab
(heroin OR analgesic* OR opiate* OR opioid OR morphin* OR morfin* OR methadone OR oxycodone OR oxycontin OR narcotic OR hydrocodone OR hydromorphone OR codeine OR fentanyl OR meperidine oxymorphone OR propoxyphene OR tramadol OR buprenorphine) near (abuse OR abusing OR abuses OR addict* OR dependen* OR maintenance):ti,ab
#1 OR #2 OR #3 OR #4 OR #5 OR #6
supervision:ti,ab
supervised:ti,ab
observ*:ti,ab
MeSH descriptor: [Opiate Substitution Treatment] explode all trees
OST:ti,ab
#8 OR #9 OR #10 OR #11 OR #12
#7 and #13
Appendix 3. Embase search
addiction/exp
((drug OR substance) NEAR/ 5 (abuse* OR depend* OR addict*)):ab,ti
narcotic dependence/exp
'prescription drug'/exp
(prescription NEAR/3 drug*):ab,ti
((heroin:ab,ti OR methadone:ab,ti OR opioid*:ab,ti OR opiate*:ab,ti OR morphin*:ab,ti OR morfin*:ab,ti OR narcot*:ab,ti OR buprenorphine:ab,ti OR oxycodone:ab,ti OR oxycontin:ab,ti OR hydrocodone:ab,ti OR hydromorphone:ab,ti OR codeine:ab,ti OR fentanyl:ab,ti OR meperidine:ab,ti OR oxymorphone:ab,ti OR propoxyphene:ab,ti OR tramadol:ab,ti) NEAR/3 (abuse:ab,ti OR abusing:ab,ti OR abuses:ab,ti OR addict*:ab,ti OR dependen*:ab,ti OR maintenance:ab,ti))
#1 or #2 or #3 or #4 or #5 or #6
supervision:ab,ti OR supervised:ab,ti or observ*:ab,ti
OST:ti,ab
'opiate substitution treatment'/exp
(Medication NEAR/3 assist*):ab,ti
#8 OR #9 OR #10 OR #11
#7 AND #12
#13 AND human/de
Appendix 4. Web of Science
TS=((drug or substance*) NEAR/3 (misuse or abuse* or addict*))) OR TS=((heroin OR methadone OR opioid* OR opiate* OR morphin* OR morfin* OR narcot* OR buprenorphine OR oxycodone OR oxycontin OR hydrocodone OR hydromorphone OR codeine OR fentanyl OR meperidine OR oxymorphone OR propoxyphene OR tramadol) NEAR/3 (abuse OR abusing OR abuses OR addict* OR dependen* OR maintenance)) AND TI=(supervision or supervised))
Appendix 5. Criteria for risk of bias in RCTs and cohort studies
| Item | Judgment | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation. |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention. | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk. | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes. |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement. | |
| 3. Blinding of participants and providers (performance bias) Objective outcomes |
Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding. Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 4. Blinding of participants and providers (performance bias) Subjective outcomes |
Low risk | Blinding of participants and providers ensured and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding. Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 5. Blinding of outcome assessor (detection bias) Objective outcomes |
Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding. Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding. Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 6.Blinding of outcome assessor (detection bias) Subjective outcomes |
Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding. Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding. Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 7. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop out |
Low risk | No missing outcome data. Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias). Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size. Missing data have been imputed using appropriate methods. All randomised patients are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention‐to‐treat). |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size. ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of dropouts not reported for each group). | |
| 8. Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk | Not all of the study’s pre‐specified primary outcomes have been reported. One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified. One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect). One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 9. Free of other bias: Comparability of cohorts for baseline characteristics and outcome measures on the basis of the design or analysis |
Low risk | Exposed and non‐exposed individuals are matched in the design for most important confounding factors. Authors demonstrated balance between group for the confounders. Analyses are adjusted for most important confounding factors and imbalance. Randomised controlled trial. |
| High risk | No matching or no adjustment for most important confounding factor. | |
| Unclear risk | No information about comparability of cohort. | |
| 10. Free of other bias: Selection of the non‐exposed cohort | Low risk | The sample has been drawn from the same community as the exposed cohort. |
| High risk | The sample has been drawn from a different source. | |
| Unclear risk | No description of the derivation of the non‐exposed cohort. | |
| 11. Free of other bias: protection against contamination | Low risk | Allocation was by community, institution or practice and it is unlikely that the control group received the intervention. |
| High risk | It is likely that the control group received the intervention. | |
| Unclear risk | It is possible that communication between intervention and control groups could have occurred. | |
| 12. Ascertainment of exposure | Low risk | Information in the study was obtained from a secure record (e.g. clinical records or structured interview). |
| High risk | Self‐report. | |
| Unclear risk | No description. |
Data and analyses
Comparison 1. supervised vs unsupervised.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retention | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 retention at any duration | 4 | 716 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.12] |
| 1.2 retention at 3 months | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 2 Adverse serious events (any) | 2 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.10, 3.86] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bell 2007.
| Methods |
Study design: randomised controlled multicentre trial setting: outpatients (specialist outpatient drug treatment centres) Duration of study: 3 months Setting: outpatient drug treatment centres Unit of analysis: individual Intention‐to‐treat (ITT) analysis |
|
| Participants |
Country: Australia N = 119 adults Diagnosis: Participants were people who were seeking treatment for heroin addiction at four specialist outpatient drug treatment centres in Australia Age: 34.7 (mean) (SD 8.8) Gender: 75% male (n = 89) Employment: 38% (n = 45) History: Mean days used opioid, (in previous 28 days): 24.2 (SD 5.9*); years opioid dependent: 8.8 (SD 7.3); previous opioid treatment, n = 96 (81%) Inclusion criteria: opioid dependent, with a history of at least 12 months’ opioid use Exclusion criteria: contraindication to buprenorphine, pregnancy , unstable medical or psychiatric illness, dependent on alcohol, benzodiazepines or stimulants; risk of incarceration, not having stable accommodation |
|
| Interventions |
Intervention: buprenorphine–naloxone in observed dosing (n = 61) Control: buprenorphine–naloxone in unobserved dosing (n = 58) |
|
| Outcomes |
Primary outcomes: ‐retention in treatment ‐heroin use at 3 months ‐Costs of treatment were measured (in Australian dollars, AU$) and cost‐effectiveness Secondary outcomes: ‐quality of life ‐psychological symptoms ‐use of non‐opioid drugs |
|
| Notes |
Study period: not reported Study funding: Authors received funding support from NSW Health Centre for Drugs and Alcohol (Government Department) and manufacturers of buprenorphine–naloxone Competing interests: no information reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A pharmacist, not involved in the study and off‐site to the study sites, generated a randomization list prior to study commencement". |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence was generated in blocks of 66 by drawing cards marked ‘observed’ or ‘unobserved’ from a container". |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Quote: "Subjects and clinicians delivering treatment could not be blinded to group assignment" Comment: No blinding or incomplete blinding, but the review authors judge that this is likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "Subjects and clinicians delivering treatment could not be blinded to group assignment" Comment: No blinding or incomplete blinding, but the review authors judge that this is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "Outcomes were assessed at research interview by four research assistants, who were trained to administer the questionnaires and were blind to randomization". Comment: Probably done but authors reported that during follow‐up interviews participants frequently revealed which treatment group they had been in. |
| Blinding of outcome assessment (detection bias) subjective outcomes | High risk | Quote: "Outcomes were assessed at research interview by four research assistants, who were trained to administer the questionnaires and were blind to randomization". Comment: Probably done but authors reported that during follow‐up interviews participants frequently revealed which treatment group they had been in. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All analyses were performed on an intention‐to‐treat basis". Comment: 6 protocol violations after randomisation balanced in two groups. |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was no identified but results were reported for all the declared outcomes in the methods section. |
| Other bias | Low risk | On almost all variables, the groups were well‐matched—with the exception of their reported heroin use in the month prior to commencing treatment, with participants randomised to observed treatment reporting a mean of 3 days’ more heroin use, a difference that was statistically significant. |
Cousins 2016.
| Methods |
Study design: prospective cohort study Duration of study: 6 years Setting: outpatients Unit of analysis: individual |
|
| Participants |
Country: Ireland N = 6983 people registered on the Central Treatment List (CTL) who were prescribed and dispensed at least one prescription for methadone in primary care between 1 August 2004 and 31 December 2010. Age: range 16‐65 Gender: 68.7% male n = 4796 Total length of time on treatment = 1090 days (median) Inclusion criteria: opioids users who were prescribed and dispensed at least one prescription for methadone in primary care between 1 August 2004 and 31 December 2010 |
|
| Interventions |
Intervention: regularly supervised consumption of methadone, more than 50% of their prescriptions supervised (n = 2823) Control: < 50% prescriptions of methadone supervised (n = 4160) |
|
| Outcomes |
Primary outcomes: Drug‐related mortality Secondary outcomes: All‐cause mortality |
|
| Notes |
Study period: 1 August 2004 ‐ 31 December 2010 Study funding: Authors received funding support Health Research Board of Ireland through the HRB Centre for Primary Care Research Competing interests: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: cohort study. |
| Allocation concealment (selection bias) | High risk | Comment: cohort study. |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Comment: no blinding or incomplete blinding, but the review authors judge that, for the nature of outcome, this is not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Comment: no blinding or incomplete blinding, but the review authors judge that, for the nature of outcome, this is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not mentioned but unlikely to have been done based on study design and for the type of outcome, this is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Comment: no subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: record linkage with the National Drug Related Death Index (NDRDI), census of drug‐related deaths and deaths among drug users. in Ireland |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes mentioned in methods section have been reported. |
| Other bias | Low risk |
Baseline outcome measurements Comment: unclear risk: information not reported. Baseline characteristics similar Comment: substantive imbalance in age and gender but in the multivariate analysis the authors adjusted for these covariates, thus we judged low risk of bias. Protection against contamination Quote: "Patients with more than 50% of their prescriptions supervised were classed as being supervised regularly" Comment: we judged low risk of bias because prescriptions of methadone were used to determine the frequency of supervised methadone consumption. |
Gerra 2011.
| Methods |
Study design: prospective observational non‐randomised multicentre trial Duration of study: 12 months Setting: outpatients treatment facility Unit of analysis: individual |
|
| Participants |
Country: Italy Diagnosis:heroin‐dependent for at least 4 years (DSM IV) and positive for urine morphine metabolites at recruitment. Daily intake of heroin ranged from 1.5 g to 3.0 g. of street heroin N = 300 patients Mean age: 28, 28 ± 1.58 (mean of mean) Gender: 83 % males (n = 249) (mean of percentage in three groups) Employment: 45% (n = 135) (mean of the percentage) Psychiatric co‐morbidity: 36 showed depression (12%), 4% displayed schizophrenia (9) or schizotypal disorder (3), and 28 showed bipolar disorder (9.3%). Fifteen patients were diagnosed with borderline personality disorder (5%), 80 with antisocial personality disorder (25.1%). 17 with obsessive–compulsive disorder (5.6%) and 14 with unspecified personality disorders (4.6%) following Axis II DSM IV criteria. Exclusion criteria: drug use other than heroin for long periods (3 consecutive months or more) or had prolonged alcohol dependence (6 consecutive months or more), severe chronic liver illness, renal disease, other chronic medical disorders, recent significant weight loss or obesity, endocrinopathy or immunodeficiency |
|
| Interventions |
Group A Strictly supervised daily consumption 6 days a week and take‐home methadone only on Sunday, (n =100) Group B Take‐home methadone programme in a behavioural/incentive perspective (n = 100) Group C: Early non‐contingent take‐home methadone (n =100) |
|
| Outcomes | 1) Retention in treatment (checked every few weeks) 2) Drug‐free urine tests (these were considered positive when at least one the latest four weekly urinalyses showed the presence of one or more of the following: amphetamines, methamphetamines, morphine, methadone, cannabis, cocaine, barbiturates, benzodiazepines) 3) Improvements in psychiatric symptoms (based on the reduction of SCL 90 scores) 4) Reduction of unlawful episodes (involving crime or violence) 5) Reduction of methadone diversion to the black market (self‐reported) |
|
| Notes |
Study period: June 2006 to June 2008 Study funding: information not reported Competing interests: the authors report no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote "No MMT protocol randomization was used in the three centres..." |
| Allocation concealment (selection bias) | High risk | Quote: "To address concerns about the potential bias introduced by the lack of randomization, as indicated above, measures were taken to reduce differences among the three recruiting centres and standardize patient recruitment protocols. More specifically, the three treatment centres used the same protocols, including a common counselling therapy which consisted in one thirty‐minute counselling session per week for each of the three groups of patients". Comment: observational prospective study |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Comment: no blinding or incomplete blinding, but the review authors judge that this is likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Comment: no blinding or incomplete blinding, but the review authors judge that this is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: information not reported, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) subjective outcomes | High risk | Comment: information not reported, but the review authors judge that the outcome is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A small number of patients (n=5) moved to other centres during the study, and were therefore excluded from the investigation" |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was not identified, but results were reported for all the declared outcomes in the methods section. |
| Other bias | Low risk |
Baseline outcome measurements Quote: "the three treatment centres used the same protocols, including a common counselling therapy which consisted in one thirty‐minute counselling session per week for each of the three groups of patients. Moreover, the urinalysis tests and other protocols took an identical form in the three treatment centres" Comment: we judged low risk of bias. Baseline characteristics similar Quote" The models include checks on gender and age of individuals as possible confounding factors" Comment: analyses are adjusted for most important confounding factors (gender and age) and "no significant differences were found between the three patient groups with respect to age, gender, quality of interpersonal relationships, employment rate, legal problems, previous methadone treatments, average methadone doses or previous residential treatments". Comment: the sample has been drawn from the same community as the exposed cohort, so were judged at low risk of bias. Protection against contamination Quote: "The centres operated with similar modalities and numbers of professionals, in line with public health‐care national standards. The only difference was their methadone administration protocol" Comment:the study had a potential source of bias related to the specific study design used. None of the authors have conflicts of interest or received funding. |
Holland 2012.
| Methods |
Study design: randomised controlled trial Duration of study: 3 months Setting: outpatients in treatment centres Unit of analysis: individual |
|
| Participants |
Country: Scotland Diagnosis: opiate‐dependent patients who had received methadone treatment for 3 months N = 60 Age: 34 (median) Gender: 70.2% males (n = 42) Employment: 31.9% Exclusion criteria: pregnant or breastfeeding; under 16 years of age; incapable of providing informed consent; on methadone doses >120 mg; severe a medical or psychiatric condition |
|
| Interventions |
Intervention: a) methadone ‘twice weekly supervision’ were dispensed daily, but supervised on only 2 days per week, (n = 21) b) methadone daily supervised consumption, attended their pharmacy where their pharmacist dispensed their medication to be swallowed under their supervision, (n = 20) Daily meant 6 days per week (occasional clients attended 5 or 7 days per week) depending on usual pharmacy opening hours Control : daily methadone ‘no supervision’ discontinued supervision on entry to the study (i.e. after completing 3 months of supervised consumption), but collected their medication daily. (n =19) |
|
| Outcomes |
Primary end‐points were: ‐retention in treatment ‐ illicit heroin use (self‐reported, using the Maudsley Addiction Profile (MAP)) at follow‐up Secondary outcomes ‐urine drug results(collected from patient notes) ‐other illicit drug use (self‐report using MAP) ‐alcohol use measured by the brief Alcohol Use Disorders Identification Test (brief AUDIT) ‐addiction severity and social functioning measured by the MAP ‐changes in psychological functioning (MAP) ‐changes in quality of life (Short Form‐12, SF‐12) ‐changes in criminal behaviour (MAP) ‐treatment satisfaction (Treatment Satisfaction Questionnaire) ‐adverse events (e.g. drug overdose/death) |
|
| Notes |
Study period: not reported Study funding: Chief Scientist Office Competing interests: information not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using an automated telephone randomisation system, stratified by site" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All clients were dispensed methadone by a community pharmacy" |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Quote: "Participants and researchers were not ‘blinded’ to the group allocation" Comment: no blinding or incomplete blinding, but the review authors judge that this is likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "Participants and researchers were not ‘blinded’ to the group allocation" Comment: no blinding or incomplete blinding, but the review authors judge that this is not likely to be influenced by lack of blinding; |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "future larger study could blind researchers to the participants’ allocation when collecting follow‐up data" Comment: no blinding but the review authors judge that this is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) subjective outcomes | High risk | Quote: "future larger study could blind researchers to the participants’ allocation when collecting follow‐up data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: after randomisation 2 patients moved to supervised group. ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was not identified, but results were reported for all the declared outcomes in the methods section. |
| Other bias | Low risk | None identified. |
Holland 2014.
| Methods |
Study design: pragmatic randomised controlled multicentre trial Duration of study: 3 months Setting: outpatients Unit of analysis:individual Intention‐to‐treat (ITT) analysis |
|
| Participants |
Country: UK Diagnosis: opiate‐dependent patients who had received methadone treatment for 3 months N = 298 Age:34.2 (mean) Gender: 77.1% males (n = 226) Employment: 31.7% Exclusion criteria included recent opioid treatment (<14 days); under 16 years; refusing oral therapy; severe medical condition; incapacity to give consent; or transfer to service not requiring re‐titration |
|
| Interventions |
Intervention Daily (Monday to Saturday/Sunday depending on pharmacy availability) methadone or buprenorphine supervised consumption under pharmacist supervision. Those not supervised on Sunday were dispensed with Sunday’s supply on Saturday.(n = 145) Control: supervision therapy for between 7 and 28 days, then no supervision, collecting their medication daily, (n = 148) |
|
| Outcomes |
Primary end‐points Retention in treatment after 3 months. Secondary outcomes Retention in treatment after 6 months (from clinic records) ‐illicit opioid use (self‐report using Maudsley Addiction Profile (MAP) ‐use of other drugs and alcohol use (MAP) ‐addiction severity and social functioning measured by the Christo Inventory for Substance Services (CISS) ‐changes in psychological functioning (MAP) ‐changes in quality of life (Short Form 12 Health Survey ‐SF12) ‐criminal behaviour (MAP) ‐self‐reported drug compliance and diversion |
|
| Notes |
Study period: not reported
Trial registration: ISRCTN 61294249: http://isrctn.com/ Study funding: The National Institute for Health Research (NIHR), the Norfolk and Suffolk Comprehensive Local Research Network Competing interests: the authors report no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"The Norwich Clinical Trials unit randomized participants (1 : 1) using an automated telephone system with a random block size of 4–6." |
| Allocation concealment (selection bias) | Low risk | Quote: "As an RCT, this study was conducted carefully with concealed allocation" |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Comment: no blinding or incomplete blinding, but the review authors judge that this is likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Comment: no blinding or incomplete blinding, but the review authors judge that this is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "researchers recruiting participants often collected follow‐up data, preventing blinding of outcome data collection" Quote: "objective data (e.g. toxicology data) confirmed questionnaire results, suggesting that the data were unbiased" Comment: the review authors judge that it is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) subjective outcomes | High risk | Quote: "researchers recruiting participants often collected follow‐up data, preventing blinding of outcome data collection" Comment: the review authors judge that for these outcomes is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT analysis and description of reasons of dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes were reported as planned in the protocol (http://www.isrctn.com/ISRCTN61294249). |
| Other bias | Low risk | Quote: "..supervised group had a greater proportion of women (27 versus 19%), fewer supervised had criminal convictions (66 versus 80%), previous methadone scripts (55 versus 64%), a current physical diagnosis (33 versus 43%) or used alcohol (55 versus 63%). ... in order to minimize potential confounding, we fully adjusted analyses alongside our reported results" |
Schwartz 2012.
| Methods |
Study design: randomised controlled trial Duration of study :12 months Setting: outpatients Unit of analysis: individual |
|
| Participants |
Country: USA Diagnosis: meeting US MTP admission criteria Individuals being placed on awaiting list for publicly subsidised treatment availability at one of two methadone treatment programmes (MTPs) N = 230 Mean age: 43.2 (SD 8.0) Gender: 70.0% males (n = 161) Employment: Employed in last 30 days, n = 75 (32.6%) Exclusion criteria: (i) pregnancy or (ii) acute medical or psychiatric conditions |
|
| Interventions |
‐Intervention: a) interim methadone (IM; supervised methadone with emergency counselling only for the first 4 months of treatment) (n = 108) b) standard methadone treatment (SM; with routine counselling)( n = 107) ‐Control : restored methadone treatment (RM: routine counselling with smaller case‐loads) (n = 29) |
|
| Outcomes |
Primary end‐point/ Secondary outcomes: Addiction Severity Index and a supplemental questionnaire at baseline, 4 and 12 months post‐ baseline. Measurements included retention in treatment, self‐reported days of heroin and cocaine use, criminal behavior and arrests and urine tests for heroin and cocaine metabolites. |
|
| Notes | Study period: May 2008 to January 2010 Study funding NIDA grant 2R01 DA 13636 and by the Abell Foundation, which supported the counsellor in the Restored condition. Competing interests: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"participants were block‐randomized to study condition using a computer‐generated procedure". |
| Allocation concealment (selection bias) | Low risk | Quote:"At study onset, the project manager used this list to create a series of numbered cards with the study ID and assigned condition, inserting them into numbered, opaque envelopes." |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | No blinding or incomplete blinding, but the review authors judge that this is not likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No blinding or incomplete blinding, but the review authors judge that this is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Participants were assessed at baseline by research assistants (RAs) at MTP admission (in blinded fashion, prior to random assignment) and in an unblinded fashion at 4 and 12 months post‐baseline" Comment: no blinding or incomplete blinding, but the review authors judge that this is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) subjective outcomes | High risk | Quote: "Participants were assessed at baseline by research assistants (RAs) at MTP admission (in blinded fashion, prior to random assignment) and in an unblinded fashion at 4 and 12 months post‐baseline" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced across the group. Comment: ITT analysis |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was not identified, but results were reported for all the declared outcomes in the methods section. |
| Other bias | Low risk | None identified. |
DSM IV: Diagnostic and Statistical Manual, 4th edition ITT: intention‐treat MAP: Maudsley Addiction Profile MMT: methadone maintenance treatment MTP: methadone treatment programme SCL: Symptom checklist SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amass 2001 | Excluded because type of interventions were not in the inclusion criteria. The participants received the same intervention with two different intensity: "Thrice‐weekly supervised dosing with the combination buprenorphine‐naloxone tablet is preferred to daily supervised dosing by opioid‐dependent" |
| Auriacombe 2004 | Excluded because type of interventions were not in the inclusion criteria. 202 patients were assigned quasi‐randomly to daily supervised dosing for either two weeks, three months, or six months. |
| Bell 2008 | Extention study of Bell 2007. |
| Carrieri 2014 | Excluded because type of interventions were not in the inclusion criteria. Comparison of two different setting/intensities 1) Methaville model for Primary Care (PC): "During induction, methadone intake is delivered and supervised daily at the pharmacy (with take‐home doses only for the weekend). Supervision is compulsory during induction". 2) Current Methadone model for specialised care (SC): "During induction, methadone is delivered daily at the center by the physician, the pharmacist or the nurse is delivered at the pharmacy (with take‐home doses only for the weekend). Supervision is compulsory during induction". |
| Groshkova 2013 | Excluded because type of interventions were not in the inclusion criteria. Comparison of "supervised" in three different intensities and in relation to three different medications: supervised injectable (heroin or methadone) treatment or optimised oral maintenance treatment at supervised injectable maintenance clinics in London. |
| Haasen 2007 | Excluded because type of interventions were not in the inclusion criteria. Comparison of prescribed heroin (supervised) compared to oral methadone. |
| Hutchinson 2000 | Excluded because type of interventions were not in the inclusion criteria. All participants received methadone treatment at GP‐centred programme. |
| Kakko 2003 | Excluded because type of interventions were not in the inclusion criteria. Comparison of daily buprenorphine versus placebo. |
| Krook 2002 | Excluded because type of interventions were not in the inclusion criteria. Comparison of daily buprenorphine (supervised daily administration for a least 6 months) versus placebo. |
| Lintzeris 2006 | Excluded because it is a protocol without results. |
| Lintzeris 2013 | Excluded because compared the same intervention "supervised daily dosing" with two different formulation (tablets vs film). |
| Strang 2010 | Excluded because type of interventions were not in the inclusion criteria. Comparison of heroin vs injectable methadone vs oral methadone, all treatments on nursing supervision. |
| Suzuki 2014 | Excluded because type of interventions were not in the inclusion criteria. Comparison of the same intervention in opioid‐dependent patients or patients with chronic pain using opioids non‐medically (patients were treated with buprenorphine and managed by a supervising psychiatrist, pharmacist care manager, and health coaches). |
| van den Brink 2003 | Excluded because type of interventions were not in the inclusion criteria. Comparison of methadone plus heroin (supervised) compared to methadone alone. |
Differences between protocol and review
We have changed the Objectives of this review so that this is more consistent with the inclusion criteria. In particular, we removed “abstinence and retention in treatment” as this review wants to be more comprehensive and focus also on interventions that can prevent diversion (a relevant problem with opioid substitution treatment), and adverse effects such as mortality and aberrant opioid‐related behaviours. We amended the Types of studies deleting "retrospective controlled cohort studies". We only considered prospective cohort studies as due to the type of interventions, we did not expect to find many randomised controlled trials that answered our review question.
We amended Types of participants to state: People "diagnosed as opioid dependent and receiving opioid substitution treatment with either buprenorphine or methadone". At the protocol stage we included Types of interventions: supervised long‐acting opiate medication (methadone, buprenorphine) alone or combined with psychosocial intervention aimed at maintenance or detoxification compared with non‐supervised opiate therapy. In the full review, we amended this part considering supervised opioid substitution intervention (methadone, buprenorphine) and clarifying the definition of "no supervised treatment".
We renamed the primary outcome dropout from treatment to retention in treatment on the basis of Cochrane Drugs and Alcohol Group guidance. Other published Cochrane Drugs and Alcohol Group reviews usually consider the positive outcome (retention) instead of the negative one (dropout).
We changed the secondary outcome of "Overdoses and hospitalisation" to "Overdoses". We considered hospital admission as a serious adverse event.
For the primary outcome of retention we made a post‐protocol decision to report it in the 'Summary of findings' table, stratifying according to the duration of treatment: any duration, and retention at three months.
Contributions of authors
Searched for studies: SV
Obtained copies of studies: RS, SV
Selected which studies to include (two review authors plus one arbiter): RS, SV, LG.
Extracted data from studies (two review authors): RS, SV.
Entered data into RevMan: RS
Carried out the analysis: RS, SV.
Interpreted the analysis: RS, SV, LG
Drafted the final review: RS, SV.
Edited the final review: RS, SV, LG.
Sources of support
Internal sources
Department of Epidemiology Lazio Region‐ASL Roma1, Italy.
External sources
No sources of support supplied
Declarations of interest
RS: no conflict of interest.
SV: no conflict of interest.
LG: no conflict of interest.
New
References
References to studies included in this review
Bell 2007 {published data only}
- Bell J, Shanahan M, Mutch C, Rea F, Ryan A, Batey R, et al. A randomized trial of effectiveness and cost‐effectiveness of observed versus unobserved administration of buprenorphine‐naloxone for heroin dependence. Addiction (Abingdon, England) 2007;102(12):1899‐907. [DOI] [PubMed] [Google Scholar]
Cousins 2016 {published data only}
- Cousins G, Boland F, Courtney B, Barry J, Lyons S, Fahey T. Risk of mortality on and off methadone substitutiontreatment in primary care: a national cohort study. Addiction 2016;111(1):73‐82. [DOI] [PubMed] [Google Scholar]
Gerra 2011 {published data only}
- Gerra G, Saenz E, Busse A, Maremmani I, Ciccocioppo R, Zaimovic A, et al. Supervised daily consumption, contingent take‐home incentive and non‐contingent take‐home in methadone maintenance. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2011;35(2):483‐9. [DOI] [PubMed] [Google Scholar]
Holland 2012 {published data only}
- Holland R, Matheson C, Anthony G, Roberts K, Priyardarshi S, Macrae A, et al. A pilot randomised controlled trial of brief versus twice weekly versus standard supervised consumption in patients on opiate maintenance treatment. Drug and Alcohol Review 2012;31(4):483‐91. [DOI] [PubMed] [Google Scholar]
Holland 2014 {published data only}
- Holland R, Maskrey V, Swift L, Notley C, Robinson A, Nagar J, et al. Treatment retention, drug use and social functioning outcomes in those receiving 3 months versus 1 month of supervised opioid maintenance treatment. Results from the Super C randomized controlled trial. Addiction (Abingdon, England) 2014;109(4):596‐607. [DOI] [PubMed] [Google Scholar]
Schwartz 2012 {published data only}
- Schwartz RP, Kelly SM, O'Grady KE, Gandhi D, Jaffe JH. Randomized trial of standard methadone treatment compared to initiating methadone without counseling: 12‐month findings. Addiction (Abingdon, England) 2012;107(5):943‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Amass 2001 {published data only}
- Amass L, Kamien JB, Mikulich SK. Thrice‐weekly supervised dosing with the combination buprenorphine‐naloxone tablet is preferred to daily supervised dosing by opioid‐dependent humans. Drug and Alcohol Dependence 2001;61(2):173‐81. [DOI] [PubMed] [Google Scholar]
Auriacombe 2004 {published data only}
- Auriacombe M, Fatseas M, Dubernet J, Daulouede JP, Tignol J. French field experience with buprenorphine. American Journal on Addiction 2004;13(S1):17‐28. [DOI] [PubMed] [Google Scholar]
Bell 2008 {published data only}
- Bell JR, Ryan A, Mutch C, Batey R, Rea F. Optimising the benefits of unobserved dose administration for stable opioid maintenance patients: Follow‐up of a randomised trial. Drug and Alcohol Dependence 2008;96:183–6. [DOI] [PubMed] [Google Scholar]
Carrieri 2014 {published data only}
- Carrieri PM, Michel L, Lions C, Cohen J, Vray M, Mora M, et al. Methadone induction in primary care for opioid dependence: a pragmatic randomized trial (ANRS Methaville). PLoS One 2014;9(11):e112328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groshkova 2013 {published data only}
- Groshkova T, Metrebian N, Hallam C, Charles V, Martin A, Forzisi L, et al. Treatment expectations and satisfaction of treatment‐refractory opioid‐dependent patients in RIOTT, the Randomised Injectable Opiate Treatment Trial, the UK's first supervised injectable maintenance clinics. Drug and Alcohol Review 2013;32(6):566‐73. [DOI] [PubMed] [Google Scholar]
- Metrebian N, Groshkova T, Hellier J, Charles V, Martin A, Forzisi L, et al. Drug use, health and social outcomes of hard‐to‐treat heroin addicts receiving supervised injectable opiate treatment: secondary outcomes from the Randomized Injectable Opioid Treatment Trial (RIOTT). Addiction 2015;110(3):479‐90. [DOI: 10.1111/add.12748] [DOI] [PubMed] [Google Scholar]
Haasen 2007 {published data only}
- Haasen C, Verthein U, Degkwitz P, Berger J, Krausz M, Naber D. Heroin‐assisted treatment for opioid dependence: randomised controlled trial. British Journal of Psychiatry : the journal of mental science 2007;191(1):55‐62. [DOI] [PubMed] [Google Scholar]
Hutchinson 2000 {published data only}
- Hutchinson SJ, Taylor A, Gruer L, Barr C, Mills C, Elliott L, et al. One‐year follow‐up of opiate injectors treated with oral methadone in a GP‐centred programme. Addiction (Abingdon, England) 2000;95(7):1055‐68. [DOI] [PubMed] [Google Scholar]
Kakko 2003 {published data only}
- Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1‐year retention and social function after buprenorphine‐assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo‐controlled trial. Lancet 2003;361(9358):662‐8. [DOI] [PubMed] [Google Scholar]
Krook 2002 {published data only}
- Krook AL, Brors O, Dahlberg J, Grouff K, Magnus P, Roysamb E, et al. A placebo‐controlled study of high dose buprenorphine in opiate dependents waiting for medication‐assisted rehabilitation in Oslo, Norway. Addiction (Abingdon, England) 2002;97(5):533‐42. [DOI] [PubMed] [Google Scholar]
Lintzeris 2006 {published data only}
- Lintzeris N, Strang J, Metrebian N, Byford S, Hallam C, Lee S, et al. Methodology for the Randomised Injecting Opioid Treatment Trial (RIOTT): evaluating injectable methadone and injectable heroin treatment versus optimised oral methadone treatment in the UK. Harm Reduction Journal 2006;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lintzeris 2013 {published data only}
- Lintzeris N, Leung SY, Dunlop AJ, Larance B, White N, Rivas GR, et al. A randomised controlled trial of sublingual buprenorphine‐naloxone film versus tablets in the management of opioid dependence. Drug and Alcohol Dependence 2013;131(1‐2):119‐26. [DOI] [PubMed] [Google Scholar]
Strang 2010 {published data only}
- Strang J, Metrebian N, Lintzeris N, Potts L, Carnwath T, Mayet S, et al. Supervised injectable heroin or injectable methadone versus optimised oral methadone as treatment for chronic heroin addicts in England after persistent failure in orthodox treatment (RIOTT): a randomised trial. Lancet 2010;375(9729):1885‐95. [DOI] [PubMed] [Google Scholar]
Suzuki 2014 {published data only}
- Suzuki J, Matthews ML, Brick D, Nguyen MT, Wasan AD, Jamison RN, et al. Implementation of a collaborative care management program with buprenorphine in primary care: a comparison between opioid‐dependent patients and patients with chronic pain using opioids nonmedically. Journal of Opioid Management 2014;10(3):159‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Brink 2003 {published data only}
- Brink W, Hendriks VM, Blanken P, Koeter MW, Zwieten BJ, Ree JM. Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials. BMJ (Clinical research ed) 2003;327(7410):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Amato 2011
- Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD004147.pub4] [DOI] [PubMed] [Google Scholar]
Bell 2014
- Bell J. Pharmacologicalmaintenance treatments ofopiate addiction. British Journal of ClinicalPharmacology 2014;77(2):253–63. [DOI: ] [Google Scholar]
Cai 2010
- Cai R, Crane, E, Poneleit, K, PaulozzI L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004‐2008. Journal of Pain & Palliative Care Pharmacotherapy 2010;24(3):293‐7. [DOI] [PubMed] [Google Scholar]
Cicero 2005
- Cicero TJ. Diversion and abuse of methadone prescribed for pain management. JAMA 2005;293:297–8. [DOI] [PubMed] [Google Scholar]
Cleeland 2009
- Cleeland CS. The Brief Pain Inventory. User Guide. Houston,Texas: The University of Texas, Anderson Cancer Center, 2009. [Google Scholar]
Dale‐Perera 2015
- Dale‐Perera A, Alam F, Barker P. Opioid‐dependence treatment in the era of recovery: insights from a UK survey of physicians, patients and out‐of‐treatment opioid users. Journal of Substance Use 2015;20(5):354‐62. [Google Scholar]
Dolan 2015
- Dolan K, Mehrjerdi ZA. Medication assisted treatment of opioid dependence: a review of the evidence. Australian National Council on Drugs 2015.
EPOC 2016
- Effective Practice and Organization of Care Cochrane Group. EPOC Resources for Cochrane Authors. http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 22 February, 2016).
Fischer 2012
- Fischer B, Argento E. Prescription opioid related misuse, harms, diversion and interventions in Canada: a review. Pain Physician 2012;5:ES191–203. [PubMed] [Google Scholar]
Fountain 2000
- Fountain J, Strang J, Gossop M, Farrell M, Griffiths P. Diversion of prescribed drugs by drug users in treatment: analysis of the UK market and new data from London. Addiction 2000;95:393–406. [DOI] [PubMed] [Google Scholar]
Franklin 2009
- Franklin GM, Rahman EA, Turner JA, Daniell WE, Fulton‐Kehoe D. Opioid use for chronic low back pain: A prospective, population‐based study among injured workers in Washington state, 2002‐2005. Clinical Journal of Pain 2009;25(9):743–51. [DOI] [PubMed] [Google Scholar]
Gowing 2014
- Gowing L, Ali R, Dunlop A, Farrell M, Lintzeris N. National Guidelines for Medication‐Assisted Treatment of Opioid Dependence. Print ISBN: 978‐1‐74241‐944‐2; Online ISBN: 978‐1‐74241‐945‐9 2014.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
IOM 2011
- Institute of Medicine 2011. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press. Washington: Ebook: 978‐0‐309‐21488‐9, 2011. [DOI: 10.17226/13172] [DOI] [PubMed] [Google Scholar]
Johannes 2010
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet‐based survey. Journal of Pain 2010;11(11):1230‐9. [DOI: 10.1016/j.jpain.2010.07.002.] [DOI] [PubMed] [Google Scholar]
Kissin 2013
- Kissin I. Long‐term opioid treatment of chronic nonmalignant pain: unproven efficacy and neglected safety?. Journal of Pain Research 2013;6:513‐29. [10.2147/JPR.S47182. Print 2013.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Larance 2011
- Larance B, Degenhardt L, Lintzeris N, Bell J, Winstock A, Dietze P, et al. Post‐marketing surveillance of buprenorphine‐naloxone in Australia: diversion, injectionand adherence with supervised dosing. Drug Alcohol Dependence 2011;1(118(2‐3)):265‐73. [doi: 10.1016/j.drugalcdep.2011.04.002.] [DOI] [PubMed] [Google Scholar]
Ling 2011
- Ling W, Mooney L, Hillhouse M. Prescription opioid abuse, pain and addiction: Clinical issues and implications. Drug and Alcohol Review 2011;30(3):300‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maxwell 2011
- Maxwell JC. The prescription drug epidemic in the United States: A perfect storm. Drug and Alcohol Review 2011;30(3):264‐70. [DOI] [PubMed] [Google Scholar]
McLellan 2000
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 2000;284(13):1689‐9. [DOI] [PubMed] [Google Scholar]
Melzack 1975
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1(3):277‐99. [DOI] [PubMed] [Google Scholar]
NICE 2007
Notley 2013
- Notley CN, Holland R, Nagar J, Maskrey V, Kouimtsidis C. Regaining control: the patient experience of supervised compared to unsupervised consumption in opiate substitution treatment. Drug and Alcohol Review 2014;33(1):64‐70. [10.1111/ dar.12079] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenaghen: The Nordic Cochrane Centre. Review Manager (RevMan) Version 5.3. The Cochrane Collaboration, 2014.
Ritter 2005
- Ritter A, Natalie R. The relationship between take‐away methadone policies and methadone diversion. Drug and Alcohol Review 2005;24(4):347–52. [DOI] [PubMed] [Google Scholar]
Roxburgh 2011
- Roxburgh A, Bruno R, Larance B, Burns L. Prescription of opioid analgesics and related harms in Australia. Medical Journal of Australia 2011;195(5):280‐4. [DOI] [PubMed] [Google Scholar]
Rudd 2016
- Rudd RA, Aleshire N, Zibbell JE, Gladden M. Increases in Drug and Opioid Overdose Deaths‐‐United States, 2000‐2014. MMWR. Morbidity and Mortality Weekly Report 2016;64((50‐51)):1378‐82. [DOI] [PubMed] [Google Scholar]
SAMHSA 2015 a
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug‐Related Emergency Department Visits. Accessed December 10, 2015. Available from: https://www.samhsa.gov/data/sites/default/files/DAWN127/DAWN127/sr127‐DAWN‐highlights.htm accessed December 10, 2015. [PubMed]
SAMSHA 2015 b
- SAMHSA 2015. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. HHS Publication No. SMA 15‐4927, NSDUH Series H‐50. Accessed January 2016.
Schubert 2013
- Schubert I, Ihle P, Sabatowski R. Increase in opiate prescription in Germany between 2000 and 2010: a study based on insurance data. Deutsches Ärzteblatt International 2013;110(4):45‐51. [DOI: 10.3238/arztebl.2013.0045.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seymour 2003
- Seymour A, Black M, Jay J, Cooper G, Weir C, Oliver J. The role of methadone in drug related deaths in the west of Scotland. Addiction 2003;98:995‐1002. [DOI] [PubMed] [Google Scholar]
Sigmon 2006
- Sigmon S. Characterizing the emerging population of prescription opioid abusers. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2006;15:208–12. [DOI] [PubMed] [Google Scholar]
UNODC 2014
- United Nations Office on Drugs and Crime (UNODC). World Drug Report 2015. www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
UNODC 2016
- United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication 2016.
Varenbut 2007
- Varenbut M, Teplin D, Daiter J, Raz B, Worster A, Emadi‐Konjin P, et al. Tampering by office‐based methadone maintenance patients with methadone take home privileges: a pilot study. Harm Reduction Journal 2007;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2004
- World Health Organization. Substitution maintenance therapy in the management of opioid dependence and HIV/AIDS prevention, 2004. WHO/UNODC/UNAIDS position paper 2004.
WHO 2009
- WHO 2009. Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. http://www.who.int/hiv/pub/idu/opioid/en/ 2009; Vol. ISBN 978 92 4 154754 3. [PubMed]
Zin 2014
- Zin CS, Chen LC, Knaggs RD. Changes in trends and pattern of strong opioid prescribing in primary care. European Journal of Pain (London, England) 2014;18(9):1343‐51. [DOI: 10.1002/j.1532-2149.2014.496.x.] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Saulle 2015
- Saulle R, Vecchi S. Supervised dosing with a long acting opioid medication in the management of opioid dependence. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011983] [DOI] [PMC free article] [PubMed] [Google Scholar]


