Abstract
Gordonia bronchialis is an aerobic actinomycetes that rarely causes infections in humans. Few reports describe Gordonia spp. causing eye-related infections. We report a case of chronic infectious endophthalmitis in Oregon, USA, associated with infection by G. bronchialis.
Keywords: Gordonia bronchialis, bacteria, actinomycetes, endophthalmitis, eye, eye-related infections, uveitis, Oregon, United States
Gordonia bronchialis is an aerobic bacteria that rarely causes infections in humans. We report a case of chronic infectious endophthalmitis caused by infection with G. bronchialis.
A 63-year-old woman was referred to a uveitis service in Portland, Oregon, USA, because of a 10-month history of decreased vision in the left eye caused by 3 recurrences of anterior and intermediate uveitis that was refractory to topical corticosteroids. Her ocular history included cataract surgery with intraocular lens implants placed bilaterally 2.5 years before symptom onset. Her medical history included chronic obstructive pulmonary disease, diabetes mellitus type 2, and hypertension. A complete review of systems, including fevers, chills, night sweats, weight loss, or history of contacts with ill persons, was unremarkable.
Her visual acuity was 20/20, and she had hand motion vision at 2 feet in the right and left eyes. No afferent pupillary defect was appreciated, and intraocular pressures were normal bilaterally. In the left eye, anterior segment examination showed 4+ anterior chamber cell with a hypopyon and a diffuse white granular sheet along the posterior face of the intraocular lens implant. Visualization of the left fundus was precluded by 4+ vitreous cell and haze. B-scan ultrasonography showed extensive vitreous opacities and an attached retina (Figure).
Figure.
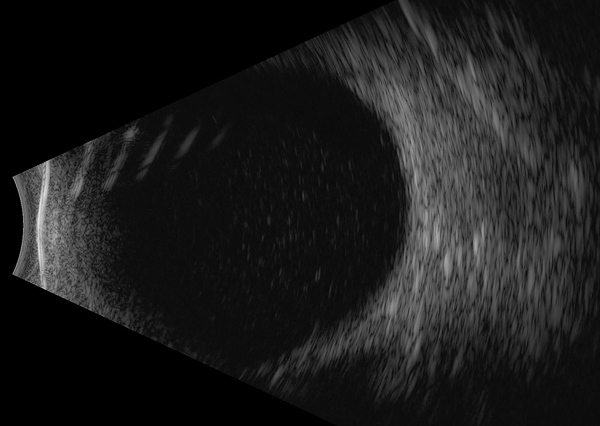
B-scan ultrasonography of the left eye of a 63-year-old woman with Gordonia bronchialis–associated endophthalmitis, Oregon, USA, showing dense opacities in the vitreous space.
Laboratory test results were negative for rapid plasma reagin, fluorescent treponemal antibody absorption, tuberculosis (Quantiferon-Gold TB; Quest Diagnostics, https://www.questdiagnostics.com), angiotensin-converting enzyme, rheumatoid factor, antinuclear antibody, and human leukocyte antigen B27. We obtained standard results for a complete blood count with differential counts, erythrocyte sedimentation rate, complete metabolic panel, and chest radiography.
The patient was given a diagnosis of presumptive chronic infectious endophthalmitis and underwent a pars plana vitrectomy and intraocular lens implant removal of the left eye. A vitreous sample was sent for pan-culture, cytologic analysis, flow cytometry, and broad-range PCR analyses with 16S rRNA gene sequencing. Results of cytology analysis and flow cytometry were negative for neoplastic processes. Culture analysis showed gram-positive bacilli but did not identify the species.
The 16S rRNA gene sequencing yielded positive results for G. bronchialis. We confirmed the bacterium to be G. bronchialis by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Antimicrobial susceptibility testing showed favorable MICs for amikacin, ceftriaxone, amoxicillin/clavulanic acid, and ciprofloxacin. Subsequently, the patient was treated with an intravitreal injection of 400 µg of amikacin to the left eye.
Two weeks after this intervention, visual acuity had improved to 20/100 in the left eye and ocular inflammation had resolved. However, 3 weeks later, the patient returned because of worsening symptoms, hand motion vision, and severe symptomatic recurrent anterior chamber and vitreous inflammation in the left eye. Intravitreal ceftazidime (2.25 mg) was administered to the left eye, and a 21-day course of oral moxifloxacin (400 mg/d) was prescribed. One week after completing her moxifloxacin regimen, her symptoms had improved, her best corrected visual acuity had improved to 20/40 in the left eye, and intraocular inflammation had resolved.
Gordonia spp. are gram-positive, weakly acid-fast aerobic actinomycetes that are ubiquitous in the environment; 29 species have been identified (1). Human infections are rare, although a few case reports of sternal wound infections, bloodstream and intravascular catheter related infections, and skin abscesses have been published (2). Optimal antimicrobial drug treatment for infection with Gordonia spp. is unknown. However, the organisms are generally believed to be susceptible to cephalosporins, aminoglycosides, and fluoroquinolones (3).
One previous case of Gordonia spp. playing a pathogenic role in an eye-related infection in a case of traumatic endophthalmitis secondary to infection with G. sputi has been reported (4). In contrast, the peculiarity of our case was the lack of obvious mode of transmission for G. bronchialis into the intraocular space. Seeding of the intraocular lens implant at the time of cataract surgery is possible, but unlikely, given the time lapse of 2.5 years between surgery and onset of symptoms. The patient also had no risk factors or history suggesting seeding from the bloodstream.
The course of our patient was of particular interest because the eye initially improved after vitrectomy and administration of intravitreal amikacin. However, a robust recurrence of inflammation occurred 3 weeks later, probably caused by incomplete treatment during initial therapy.
Although previously accurate identification of Gordonia spp. by using traditional culturing techniques has been challenging, advent of molecular biology techniques, such as 16S rRNA sequencing and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, has improved identification of aerobic actinomycetes (3–8). Because the organism was partially identified by culture and fully identified by multiple molecular techniques and responded clinically to targeted antimicrobial drugs, we believe this case was a pathogenic infection and not a nonpathogenic contaminant/colonizer.
We report a case of chronic infectious endophthalmitis caused by infection with G. bronchialis. Molecular methods have enabled successful identification of this organism, which is generally considered to have low virulence and be highly susceptible to antimicrobial drugs. A combination approach of pars plana vitrectomy and intraocular lens explantation with intravitreal and oral antimicrobial drugs might lead to a successful outcome.
Acknowledgments
This study was supported by core grant P30 EY010572 (to the Casey Eye Institute) from the National Institutes of Health and an unrestricted grant to the Casey Eye Institute from Research to Prevent Blindness (New York, NY)
Biography
Dr. Choi is a vitreoretinal surgery fellow at the Casey Eye Institute at Oregon Health and Science University, Portland, OR. His primary research interest is in elucidating the mechanisms that govern infectious and noninfectious causes of uveitis.
Footnotes
Suggested citation for this article: Choi R, Strnad L, Flaxel CJ, Lauer AK, Suhler EB. Gordonia bronchialis–associated endophthalmitis, Oregon, USA. Emerg Infect Dis. 2019 May [date cited]. https://doi.org/10.3201/eid2505.180340
References
- 1.Sukackiene D, Rimsevicius L, Kiveryte S, Marcinkeviciene K, Bratchikov M, Zokaityte D, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2017. [DOI] [PubMed]
- 2.Ramanan P, Deziel PJ, Wengenack NL. Gordonia bacteremia. J Clin Microbiol. 2013;51:3443–7. 10.1128/JCM.01449-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaschke AJ, Bender J, Byington CL, Korgenski K, Daly J, Petti CA, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483–6. 10.1086/520018 [DOI] [PubMed] [Google Scholar]
- 4.Fang W, Li J, Cui HS, Jin X, Zhai J, Dai Y, et al. First identification of Gordonia sputi in a post-traumatic endophthalmitis patient - a case report and literatures review. BMC Ophthalmol. 2017;17:190. 10.1186/s12886-017-0573-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma P, Brown JM, Nunez VH, Morey RE, Steigerwalt AG, Pellegrini GJ, et al. Native valve endocarditis due to Gordonia polyisoprenivorans: case report and review of literature of bloodstream infections caused by Gordonia species. J Clin Microbiol. 2006;44:1905–8. 10.1128/JCM.44.5.1905-1908.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gil-Sande E, Brun-Otero M, Campo-Cerecedo F, Esteban E, Aguilar L, García-de-Lomas J. Etiological misidentification by routine biochemical tests of bacteremia caused by Gordonia terrae infection in the course of an episode of acute cholecystitis. J Clin Microbiol. 2006;44:2645–7. 10.1128/JCM.00444-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T, Kong F, Chen S, Xiao M, Sorrell T, Wang X, et al. Improved identification of Gordonia, Rhodococcus and Tsukamurella species by 5′-end 16S rRNA gene sequencing. Pathology. 2011;43:58–63. 10.1097/PAT.0b013e328340e431 [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, Rodríguez-Fernández A, Ruiz de Alegria C, Martinez-Martinez L, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28348789&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]


