Abstract
Background
Increasing numbers of incidental pancreatic lesions are being detected each year. Accurate characterisation of pancreatic lesions into benign, precancerous, and cancer masses is crucial in deciding whether to use treatment or surveillance. Distinguishing benign lesions from precancerous and cancerous lesions can prevent patients from undergoing unnecessary major surgery. Despite the importance of accurately classifying pancreatic lesions, there is no clear algorithm for management of focal pancreatic lesions.
Objectives
To determine and compare the diagnostic accuracy of various imaging modalities in detecting cancerous and precancerous lesions in people with focal pancreatic lesions.
Search methods
We searched the CENTRAL, MEDLINE, Embase, and Science Citation Index until 19 July 2016. We searched the references of included studies to identify further studies. We did not restrict studies based on language or publication status, or whether data were collected prospectively or retrospectively.
Selection criteria
We planned to include studies reporting cross‐sectional information on the index test (CT (computed tomography), MRI (magnetic resonance imaging), PET (positron emission tomography), EUS (endoscopic ultrasound), EUS elastography, and EUS‐guided biopsy or FNA (fine‐needle aspiration)) and reference standard (confirmation of the nature of the lesion was obtained by histopathological examination of the entire lesion by surgical excision, or histopathological examination for confirmation of precancer or cancer by biopsy and clinical follow‐up of at least six months in people with negative index tests) in people with pancreatic lesions irrespective of language or publication status or whether the data were collected prospectively or retrospectively.
Data collection and analysis
Two review authors independently searched the references to identify relevant studies and extracted the data. We planned to use the bivariate analysis to calculate the summary sensitivity and specificity with their 95% confidence intervals and the hierarchical summary receiver operating characteristic (HSROC) to compare the tests and assess heterogeneity, but used simpler models (such as univariate random‐effects model and univariate fixed‐effect model) for combining studies when appropriate because of the sparse data. We were unable to compare the diagnostic performance of the tests using formal statistical methods because of sparse data.
Main results
We included 54 studies involving a total of 3,196 participants evaluating the diagnostic accuracy of various index tests. In these 54 studies, eight different target conditions were identified with different final diagnoses constituting benign, precancerous, and cancerous lesions. None of the studies was of high methodological quality. None of the comparisons in which single studies were included was of sufficiently high methodological quality to warrant highlighting of the results. For differentiation of cancerous lesions from benign or precancerous lesions, we identified only one study per index test. The second analysis, of studies differentiating cancerous versus benign lesions, provided three tests in which meta‐analysis could be performed. The sensitivities and specificities for diagnosing cancer were: EUS‐FNA: sensitivity 0.79 (95% confidence interval (CI) 0.07 to 1.00), specificity 1.00 (95% CI 0.91 to 1.00); EUS: sensitivity 0.95 (95% CI 0.84 to 0.99), specificity 0.53 (95% CI 0.31 to 0.74); PET: sensitivity 0.92 (95% CI 0.80 to 0.97), specificity 0.65 (95% CI 0.39 to 0.84). The third analysis, of studies differentiating precancerous or cancerous lesions from benign lesions, only provided one test (EUS‐FNA) in which meta‐analysis was performed. EUS‐FNA had moderate sensitivity for diagnosing precancerous or cancerous lesions (sensitivity 0.73 (95% CI 0.01 to 1.00) and high specificity 0.94 (95% CI 0.15 to 1.00), the extremely wide confidence intervals reflecting the heterogeneity between the studies). The fourth analysis, of studies differentiating cancerous (invasive carcinoma) from precancerous (dysplasia) provided three tests in which meta‐analysis was performed. The sensitivities and specificities for diagnosing invasive carcinoma were: CT: sensitivity 0.72 (95% CI 0.50 to 0.87), specificity 0.92 (95% CI 0.81 to 0.97); EUS: sensitivity 0.78 (95% CI 0.44 to 0.94), specificity 0.91 (95% CI 0.61 to 0.98); EUS‐FNA: sensitivity 0.66 (95% CI 0.03 to 0.99), specificity 0.92 (95% CI 0.73 to 0.98). The fifth analysis, of studies differentiating cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) provided six tests in which meta‐analysis was performed. The sensitivities and specificities for diagnosing cancer (high‐grade dysplasia or invasive carcinoma) were: CT: sensitivity 0.87 (95% CI 0.00 to 1.00), specificity 0.96 (95% CI 0.00 to 1.00); EUS: sensitivity 0.86 (95% CI 0.74 to 0.92), specificity 0.91 (95% CI 0.83 to 0.96); EUS‐FNA: sensitivity 0.47 (95% CI 0.24 to 0.70), specificity 0.91 (95% CI 0.32 to 1.00); EUS‐FNA carcinoembryonic antigen 200 ng/mL: sensitivity 0.58 (95% CI 0.28 to 0.83), specificity 0.51 (95% CI 0.19 to 0.81); MRI: sensitivity 0.69 (95% CI 0.44 to 0.86), specificity 0.93 (95% CI 0.43 to 1.00); PET: sensitivity 0.90 (95% CI 0.79 to 0.96), specificity 0.94 (95% CI 0.81 to 0.99). The sixth analysis, of studies differentiating cancerous (invasive carcinoma) from precancerous (low‐grade dysplasia) provided no tests in which meta‐analysis was performed. The seventh analysis, of studies differentiating precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) from precancerous (low‐grade dysplasia) provided two tests in which meta‐analysis was performed. The sensitivity and specificity for diagnosing cancer were: CT: sensitivity 0.83 (95% CI 0.68 to 0.92), specificity 0.83 (95% CI 0.64 to 0.93) and MRI: sensitivity 0.80 (95% CI 0.58 to 0.92), specificity 0.81 (95% CI 0.53 to 0.95), respectively. The eighth analysis, of studies differentiating precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) from precancerous (low‐grade dysplasia) or benign lesions provided no test in which meta‐analysis was performed.
There were no major alterations in the subgroup analysis of cystic pancreatic focal lesions (42 studies; 2086 participants). None of the included studies evaluated EUS elastography or sequential testing.
Authors' conclusions
We were unable to arrive at any firm conclusions because of the differences in the way that study authors classified focal pancreatic lesions into cancerous, precancerous, and benign lesions; the inclusion of few studies with wide confidence intervals for each comparison; poor methodological quality in the studies; and heterogeneity in the estimates within comparisons.
Plain language summary
Accuracy of different imaging techniques for determining whether a pancreatic tumour is cancerous
Background
The pancreas is an organ in the abdomen that secretes pancreatic juice, which aids digestion and contains cells that produce important hormones such as insulin. Increasingly, abnormalities in the pancreas are noted in people undergoing routine scans, such as ultrasound or computed tomography (CT) scans, in the form of what are known as 'shadows', which may be described as focal pancreatic lesion, pancreatic mass, pancreatic tumour, pancreatic cyst, or pancreatic nodule. A significant proportion of focal pancreatic lesions are benign (non‐cancerous) lesions requiring no treatment. Surgical removal of the tumour is the main method of treatment for precancerous (i.e. focal pancreatic lesions that are not full‐blown cancer and do not have the ability to spread like cancer, but can turn into cancer) and cancerous focal pancreatic lesions. New methods are being developed for treating precancerous lesions, such as using heat to destroy the tumour. Surgical removal remains the only potentially curative treatment for people with limited pancreatic cancer. It is thus important to characterise whether a focal pancreatic lesion is non‐cancerous, precancerous, or cancerous. A number of scans are available for characterising the nature of the focal pancreatic lesion, which include the following.
• Computed tomography (CT) scan: a series of X‐rays taken from different angles, which are then reconstructed using a computer.
• Magnetic resonance imaging (MRI): the use of a powerful magnet to produce images of different tissues of the body.
• Positron emission tomography (PET): the use of a small amount of radioactive glucose (sugar) to differentiate between different tissues. It takes advantage of the tendency of cancer cells to use more glucose than normal cells.
• Endoscopic ultrasound (also known as endosonography or EUS): the use of an endoscope, a camera introduced into a body cavity to view the inside of the body. An ultrasound (high‐energy sound waves) probe at the end of the endoscope is used to differentiate tissues.
• EUS elastography: this measures the stiffness of the lesion, which is used to identify whether the lesion is cancerous.
• EUS‐guided biopsy: the removal of cells or tissues for examination under a microscope or to perform other tests on the cells or tissue.
At present it is unclear how effective different scans are in characterising focal pancreatic lesions.
Study characteristics
We performed a thorough literature search for studies reporting the accuracy of different scans until 19 July 2016. We identified 54 studies reporting information on 3196 people with focal pancreatic lesions. These studies evaluated one or more of the above tests and compared these test results with the eventual diagnosis provided by surgical removal of the lesion and examination under microscope. There were no diagnostic test accuracy studies of EUS elastography or studies that looked at multiple scans rather than single scans.
Key results
Variations in how studies defined precancerous and cancerous lesions meant that we were not able to combine the data to provide the overall results for many tests. We were unable to arrive at any firm conclusions for the following reasons.
• The way that study authors classified focal pancreatic lesions into cancerous, precancerous, and benign lesions was not consistent in different studies.
• The studies included few participants, leading to significant uncertainty in the results.
• The studies were of poor methodological quality, which introduced additional uncertainty in the results.
• Even among the studies that classified focal pancreatic lesions into cancerous, precancerous, and benign lesions in a similar manner, the results were not consistent.
Quality of evidence
All of the studies were of low methodological quality, which may result in arriving at false conclusions.
Summary of findings
Summary of findings'. 'Imaging modalities for characterising focal pancreatic lesions.
| Name of test | Number of studies (number of participants) | Sensitivity (95% CI) | Specificity (95% CI) | Post‐test probability of positive test* (95% CI) | Post‐test probability of negative test* (95% CI) | Number of false positives per 100 positive index test results (95% CI) | Number of false negatives per 100 negative index test results (95% CI) | Risk of bias | Applicability concerns | Uncertainty (due to inconsistency or inability to assess inconsistency, and random errors because of overall small sample size) |
| Cancerous versus benign or precancerous (median pre‐test probability: 63%) | ||||||||||
| EUS‐FNA (cytology) | 1 (45) | 0.79 (0.60 to 0.91) | 1.00 (0.85 to 1.00) | 98% (79% to 100%) | 26% (14% to 43%) | 2 (0 to 21) | 26 (14 to 43) | Unclear | High | High |
| EUS‐FNA (CEA > 500 ng/mL) | 1 (24) | 0.93 (0.70 to 0.99) | 0.33 (0.12 to 0.65) | 70% (59% to 79%) | 25% (4% to 73%) | 30 (21 to 41) | 25 (4 to 73) | High | High | High |
| PET (criteria unspecified) | 1 (76) | 0.85 (0.73 to 0.92) | 0.91 (0.72 to 0.97) | 94% (81% to 98%) | 21% (12% to 34%) | 6 (2 to 19) | 21 (12 to 34) | Unclear | High | High |
| Cancerous versus benign (median pre‐test probability: 70%) | ||||||||||
| EUS | 2 (133) | 0.95 (0.84 to 0.99) | 0.53 (0.31 to 0.74) | 82% (74% to 88%) | 18% (6% to 45%) | 18 (12 to 26) | 18 (6 to 45) | Unclear or high | High | High |
| EUS‐FNA (cytology) | 3 (147) | 0.79 (0.07 to 1.00) | 1.00 (0.91 to 1.00) | 99% (90% to 100%) | 32% (2% to 92%) | 0 (0 to 9) | 32 (2 to 92) | High | High | High |
| PET (criteria unspecified) | 3 (99) | 0.92 (0.80 to 0.97) | 0.65 (0.39 to 0.85) | 86% (75% to 92%) | 22% (9% to 44%) | 14 (8 to 25) | 22 (9 to 44) | High | High | High |
| PET (SUVmax > 3.5) | 1 (80) | 0.96 (0.87 to 0.99) | 0.62 (0.43 to 0.78) | 85% (78% to 90%) | 12% (3% to 36%) | 15 (10 to 22) | 12 (3 to 36) | High | High | High |
| CT | 2 (123) | 0.98 (0.00 to 1.00) | 0.76 (0.02 to 1.00) | 90% (17% to 100%) | 6% (0% to 100%) | 10 (0 to 83) | 6 (0 to 100) | Unclear or high | High | High |
| MRI | 1 (29) | 0.80 (0.58 to 0.92) | 0.89 (0.57 to 0.98) | 94% (72% to 99%) | 34% (17% to 56%) | 6 (1 to 28) | 34 (17 to 56) | High | High | High |
| Precancerous or cancerous versus benign (median pre‐test probability: 71%) | ||||||||||
| EUS | 1 (34) | 0.92 (0.74 to 0.98) | 0.60 (0.31 to 0.83) | 85% (72% to 92%) | 25% (7% to 58%) | 15 (8 to 28) | 25 (7 to 58) | High | High | High |
| EUS‐FNA (cytology) | 2 (52) | 0.73 (0.01 to 1.00) | 0.94 (0.15 to 1.00) | 97% (25% to 100%) | 41% (1% to 98%) | 3 (0 to 75) | 41 (1 to 98) | Unclear or high | High | High |
| EUS‐FNA (CEA > 50 ng/mL) | 1 (11) | 0.29 (0.08 to 0.64) | 0.25 (0.05 to 0.70) | 48% (20% to 77%) | 87% (54% to 98%) | 52 (23 to 80) | 87 (54 to 98) | High | High | High |
| PET (SUVmax 2.4) | 1 (32) | 0.94 (0.74 to 0.99) | 0.93 (0.69 to 0.99) | 97% (83% to 100%) | 13% (2% to 49%) | 3 (0 to 17) | 13 (2 to 49) | High | High | High |
| CT | 1 (48) | 0.62 (0.45 to 0.76) | 0.64 (0.39 to 0.84) | 81% (66% to 90%) | 59% (44% to 72%) | 19 (10 to 34) | 59 (44 to 72) | Unclear | High | High |
| MRI | 1 (27) | 0.93 (0.69 to 0.99) | 0.85 (0.58 to 0.96) | 94% (80% to 98%) | 17% (3% to 58%) | 6 (2 to 20) | 17 (3 to 58) | High | High | High |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) (median pre‐test probability: 27%) | ||||||||||
| EUS | 5 (156) | 0.78 (0.45 to 0.94) | 0.91 (0.61 to 0.98) | 75% (37% to 94%) | 8% (3% to 22%) | 25 (6 to 63) | 8 (3 to 22) | Unclear or high | High | High |
| EUS‐FNA (cytology) | 3 (158) | 0.66 (0.03 to 0.99) | 0.92 (0.73 to 0.98) | 75% (29% to 95%) | 12% (1% to 69%) | 25 (5 to 71) | 12 (1 to 69) | Unclear or high | High | High |
| EUS‐FNA (CEA > 200 ng/mL) | 1 (41) | 1.00 (0.57 to 1.00) | 0.64 (0.48 to 0.78) | 51% (40% to 61%) | Not estimable | 49 (39 to 60) | Not estimable | High | High | High |
| CT | 6 (326) | 0.72 (0.50 to 0.87) | 0.92 (0.81 to 0.97) | 78% (57% to 91%) | 10% (5% to 18%) | 22 (9 to 43) | 10 (5 to 18) | Unclear or high | High | High |
| MRI | 1 (32) | 0.75 (0.30 to 0.95) | 0.93 (0.77 to 0.98) | 80% (48% to 94%) | 9% (2% to 35%) | 20 (6 to 52) | 9 (2 to 35) | High | High | High |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) (median pre‐test probability: 45%) | ||||||||||
| EUS | 4 (196) | 0.86 (0.74 to 0.92) | 0.91 (0.83 to 0.96) | 89% (80% to 94%) | 11% (7% to 19%) | 11 (6 to 20) | 11 (7 to 19) | High | High | High |
| EUS‐FNA (cytology) | 3 (310) | 0.47 (0.24 to 0.70) | 0.91 (0.32 to 1.00) | 81% (19% to 99%) | 32% (22% to 45%) | 19 (1 to 81) | 32 (22 to 45) | Unclear or high | High | High |
| EUS‐FNA (CEA > 200 ng/mL) | 3 (160) | 0.58 (0.28 to 0.83) | 0.51 (0.19 to 0.81) | 49% (28% to 70%) | 40% (19% to 65%) | 51 (30 to 72) | 40 (19 to 65) | High | High | High |
| EUS‐FNA (CA 19‐9 > 1000 U/mL) | 1 (41) | 0.90 (0.60 to 0.98) | 0.42 (0.26 to 0.59) | 56% (47% to 65%) | 16% (3% to 57%) | 44 (35 to 53) | 16 (3 to 57) | High | High | High |
| EUS‐FNA (CEA > 692.8 ng/mL) | 1 (20) | 0.80 (0.49 to 0.94) | 0.90 (0.60 to 0.98) | 87% (50% to 98%) | 15% (5% to 39%) | 13 (2 to 50) | 15 (5 to 39) | Unclear | High | High |
| PET (SUVmax > 2 to 2.5) | 4 (124) | 0.90 (0.79 to 0.96) | 0.94 (0.81 to 0.99) | 93% (78% to 98%) | 8% (4% to 16%) | 7 (2 to 22) | 8 (4 to 16) | High | High | High |
| CT | 3 (139) | 0.87 (0.00 to 1.00) | 0.96 (0.00 to 1.00) | 95% (0% to 100%) | 10% (0% to 100%) | 5 (0 to 100) | 10 (0 to 100) | Unclear or high | High | High |
| MRI | 3 (189) | 0.69 (0.44 to 0.86) | 0.93 (0.43 to 1.00) | 89% (35% to 99%) | 21% (12% to 36%) | 11 (1 to 65) | 21 (12 to 36) | High | High | High |
| Cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia) (median pre‐test probability: 21%) | ||||||||||
| EUS | 1 (51) | 0.77 (0.50 to 0.92) | 0.89 (0.76 to 0.96) | 67% (43% to 84%) | 7% (3% to 16%) | 33 (16 to 57) | 7 (3 to 16) | Unclear | High | High |
| CT | 1 (46) | 0.50 (0.22 to 0.78) | 0.95 (0.83 to 0.99) | 72% (36% to 92%) | 13% (7% to 22%) | 28 (8 to 64) | 13 (7 to 22) | High | High | High |
| Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) (median pre‐test probability: 59%) | ||||||||||
| CT | 3 (106) | 0.83 (0.68 to 0.92) | 0.83 (0.64 to 0.93) | 89% (56% to 98%) | 33% (18% to 52%) | 11 (2 to 44) | 33 (18 to 52) | High | High | High |
| MRI | 2 (71) | 0.80 (0.58 to 0.92) | 0.81 (0.53 to 0.95) | 86% (67% to 95%) | 27% (13% to 47%) | 14 (5 to 33) | 27 (13 to 47) | High | High | High |
| Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) or benign (median pre‐test probability: 43%) | ||||||||||
| EUS | 1 (70) | 0.97 (0.83 to 0.99) | 0.40 (0.26 to 0.55) | 55% (48% to 61%) | 6% (1% to 31%) | 45 (39 to 52) | 6 (1 to 31) | High | High | High |
*Post‐test probability was calculated at the median pre‐test probability.
Abbreviations:
CA 19‐9: carbohydrate antigen 19‐9 CEA: carcinoembryonic antigen CI: confidence interval CT: computed tomography EUS: endoscopic ultrasound FNA: fine‐needle aspiration MRI: magnetic resonance imaging PET: positron emission tomography SUVmax: maximum standardised uptake values
Background
(Please see the glossary in Appendix 1 for terms that have not been described in the main text.)
A 'shadow’ identified in the pancreas on imaging may be variously described as a focal pancreatic lesion, pancreatic mass, pancreatic tumour, pancreatic cyst, or pancreatic nodule. This phrasing refers to focal lesions, as opposed to diffuse changes of the pancreas, and includes solid and cystic lesions of the pancreas. In the Western world, the prevalence of focal pancreatic lesions is approximately 1.2% and is increasing steadily (by approximately 8%) each year, with smaller and asymptomatic lesions being identified more frequently (Gaujoux 2011; Spinelli 2004). An incidental pancreatic lesion is one that is detected in the pancreas of a patient who undergoes radiological investigations for an unrelated medical condition (Sachs 2009). Such asymptomatic incidental lesions represent 55% to 60% of pancreatic tumours (Gaujoux 2011; Spinelli 2004). Some focal pancreatic lesions may be associated with symptoms, depending upon their size and nature. The symptoms of pancreatic cancer, which generally refers to pancreatic adenocarcinoma, can include obstructive jaundice (yellowish discolouration of the skin and the whites of the eyes with dark urine and pale stool due to blockage of bile duct (National Cancer Institute 2011a), a tube that transports the bile from the liver), loss of appetite, and abdominal pain (Holly 2004). The symptoms of pancreatic neuroendocrine tumours (tumours arising from cells that secrete hormones), some of which may be cancer, are related to the excessive secretion of hormones (by the tumour) such as insulin, glucagon, gastrin, somatostatin, and vasoactive peptide resulting in hypoglycaemia (decreased blood sugar), hyperglycaemia (increased blood sugar, a rare cause of diabetes), and gastrointestinal disturbances such as peptic ulcer and diarrhoea (Batcher 2011). The symptoms of chronic pancreatitis (chronic inflammation of the pancreas that can result in alteration in the structure and function of the pancreas) are abdominal and back pain and those symptoms related to pancreatic insufficiency, which include steatorrhoea, malabsorption, vitamin deficiency, diabetes, or weight loss (Braganza 2011; Nair 2007). About 40% of people with focal pancreatic lesions have chronic pancreatitis (Spinelli 2004). In the remaining 60% of people with focal pancreatic lesions, the remaining pancreas is normal.
Focal pancreatic lesions can be benign (serous pancreatic cystadenoma, acinar cell cystadenoma, papillary cysts, lymphoepithelial cysts, simple cysts), precancerous (intraductal papillary mucinous neoplasm (IPMN) with dysplasia but without invasive cancer, mucinous cystic neoplasm (MCN), benign neuroendocrine tumours), or cancer (ductal adenocarcinoma, acinar cell carcinoma, IPMN with invasive carcinoma, cystadenocarcinoma, pancreatoblastoma, solid pseudo‐papillary neoplasm, cancer neuroendocrine tumours) (Luttges 2011; Sachs 2009; Spinelli 2004; WHO 2016). Dysplasia can be low grade, intermediate grade, or high grade (WHO 2016). About 80% of benign lesions, 50% of precancerous lesions, and 20% of cancerous lesions are asymptomatic (Spinelli 2004). Focal pancreatic lesions can be solid or cystic or mixed solid and cystic tumours (Cho 2011).
Surgical resection is generally considered to be the only curative treatment for pancreatic cancer. Worldwide, only 15% to 20% of people with pancreatic cancer undergo potentially curative resection (Conlon 1996; Engelken 2003; Katz 2009; Michelassi 1989; Shahrudin 1997; Smith 2008). In the remaining patients, the cancers are not resected because of infiltration of local structures or disseminated disease. Early diagnosis of pancreatic cancer might enable resection of the pancreatic cancer before it is too late to resect. Pancreatic resection is a major surgery, with an approximately 1% to 25% risk of perioperative death reported worldwide (Conlon 1996; Katz 2009; Michelassi 1989; Shahrudin 1997; van Oost 2006). High‐volume centres show a lower perioperative mortality of less than 5% compared to low‐volume centres, which are associated with a perioperative mortality of up to 25% (Gurusamy 2013; van Oost 2006). Pancreatic resection is also associated with an about 40% morbidity rate (Gurusamy 2013; van der Gaag 2010). Only 5% to 25% of patients survive for five years (Conlon 1996; Katz 2009; Michelassi 1989; Shahrudin 1997). Surgery is generally offered if there are features suggestive of precancerous or cancerous lesions (Lee 2005c), although some clinicians prefer sequential follow‐up (by imaging) of precancerous lesions to surgical resection (Irie 2004). Surgery is offered when there is an increase in the size or morphology (the way the lesion appears) of the lesion in sequential imaging (Gaujoux 2011). Surgery is also offered when there is considerable uncertainty as to the nature of the lesion. In some ways, surgery can be considered as a diagnostic test for characterisation of the lesion and as a treatment for people with cancerous and precancerous lesions. Histological confirmation of the lesion by percutaneous biopsy is generally not performed because of difficulty in accessing the lesion percutaneously and because of dissemination of cancer cells.
Target condition being diagnosed
Cancerous versus benign or precancerous lesions.
Precancerous or cancerous (including the type of cancerous lesion) versus benign lesions.
Index test(s)
Computed tomography (CT) scan
This involves a series of X‐rays taken from different angles, which are then reconstructed using a computer (National Cancer Institute 2011a). Morphological features of the lesion, such as density, regularity of margins, vascularity, and the diameter of the pancreatic duct, are taken into account to characterise the lesion. The main side effect of CT scan is the ionising radiation (radioactivity) associated with it. Everyone is exposed to very small amounts of radiation (background radiation). One CT scan of the abdomen is equivalent to approximately three years of background radiation (Fred 2004). In addition, the contrast material (dye used to view the structures better) can cause allergic reactions, such as difficulty breathing, or kidney damage, particularly in people with pre‐existing kidney disease (Namasivayam 2006).
Magnetic resonance imaging (MRI)
This involves the use of a powerful magnet to produce images of different tissues of the body. Magnetic resonance imaging is also known as nuclear magnetic resonance imaging (NMRI) (National Cancer Institute 2011b). Similar features as those employed in CT scan are used to characterise the lesion. While MRI does not use radiation, it is contraindicated in people with metallic implants such as artificial joints, those with cardiac pacemakers (devices used to control heart rhythm), and those with claustrophobia (fear of closed spaces) (Dill 2008). Some of the contrasts used can also cause kidney damage (Dill 2008).
Positron emission tomography (PET)
This involves the use of a small amount of radioactive glucose (sugar) to differentiate between different tissues. It takes advantage of the tendency of cancer cells to use more glucose than normal cells. Positron emission tomography is also known as PET scan (National Cancer Institute 2011c). Cancerous lesions appear as areas of increased uptake. Positron emission tomography also uses ionising radiation (Leide‐Svegborn 2010). The radiation exposure to one PET scan is similar to that in one CT scan of abdomen (Fred 2004; Leide‐Svegborn 2010).
Endoscopic ultrasound (EUS)
This involves the use of an endoscope, a camera introduced into a body cavity to view the inside of the body. An ultrasound (high‐energy sound waves) probe at the end of the endoscope is used to differentiate tissues. Endoscopic ultrasound is also known as endosonography (National Cancer Institute 2011d). Features such as echogenicity and regularity of margins are taken into account and used to characterise the lesion. Complications following EUS are rare and include perforation (Benson 2010; Niv 2011).
EUS elastography
This measures the stiffness of the lesion, which can be used to identify whether the lesion is benign or cancerous (Iglesias‐Garcia 2010). The complications associated with EUS elastography are the same as with EUS.
EUS‐guided biopsy
This is the removal of cells or tissues for examination by a pathologist. The pathologist may study the tissue under a microscope or perform other tests on the cells or tissue. There are many different types of biopsy procedures. The most common types include:
incisional biopsy, in which only a sample of tissue is removed;
excisional biopsy, in which an entire lump or suspicious area is removed; and
needle biopsy, in which a sample of tissue or fluid is removed with a needle. When a wide needle is used the procedure is called a core biopsy. When a thin needle is used the procedure is called a fine‐needle aspiration biopsy (FNAB) (National Cancer Institute 2011e).
Because of the risk of dissemination from cancer, EUS‐guided biopsy is preferable to percutaneous (image‐guided) biopsy (Micames 2003). The examinations under the microscope used may include the routine haemotoxylin and eosin stain for core biopsy and special staining for FNAB (Mehta 2010). Immunocytochemistry and proteomic profiling to identify the presence of biomarkers in the tissue may also be used in the diagnosis (Cui 2009; Mehta 2010). A positive core biopsy can confirm cancer, but a negative core biopsy cannot rule out cancer. Cytology results are not quite as reliable as core biopsy as false‐positive cytology has been reported (Hancke 1984).
Complications associated with EUS‐guided biopsy include those associated with EUS as well as bleeding (Benson 2010; Niv 2011).
Of these index tests, the commonly available tests are CT scan and MRI. The remaining tests (PET, EUS, EUS elastography, and EUS‐guided biopsy) are available in major tertiary centres only.
Clinical pathway
There is no standard algorithm in the diagnosis or management of focal pancreatic lesions. The algorithm may vary from one centre to another and even within the same centre (Gaujoux 2011; Goh 2006b). One possible diagnostic clinical pathway is shown in Figure 1. As noted in Figure 1, an increase in the size of or change of morphological features is one of the reasons that surgeons recommend surgical excision, as this may indicate that the lesion was malignant in the first instance (without features suggestive of malignancy in the original scan) or has transformed into a malignant lesion. The interval for sequential scans is variable. Our local protocol advises sequential scanning in one year in the absence of malignant features. It is important to distinguish whether the focal pancreatic lesion is benign with no cancer potential so that unnecessary surgery and anxiety can be avoided. It is also important to know whether the lesion is precancerous or cancerous so that an informed decision about surgery can be made after weighing the potential benefits and harms. In addition, new alternative treatments such as radiofrequency ablation (destruction of tissue using radiofrequency waves) are being evaluated for precancerous lesions (Pereira 2015). It is also necessary to differentiate the different types of cancer, since different malignancies carry different prognoses (Klempnauer 1995). Some surgeons follow the single‐test strategy, that is making decisions based on the features of the lesion in a single test, while others follow repeated testing (repeating the imaging modality or using a different imaging modality), particularly if the nature of the lesion is indeterminate. The optimal interval between the tests in the repeated‐testing strategy is unknown.
1.
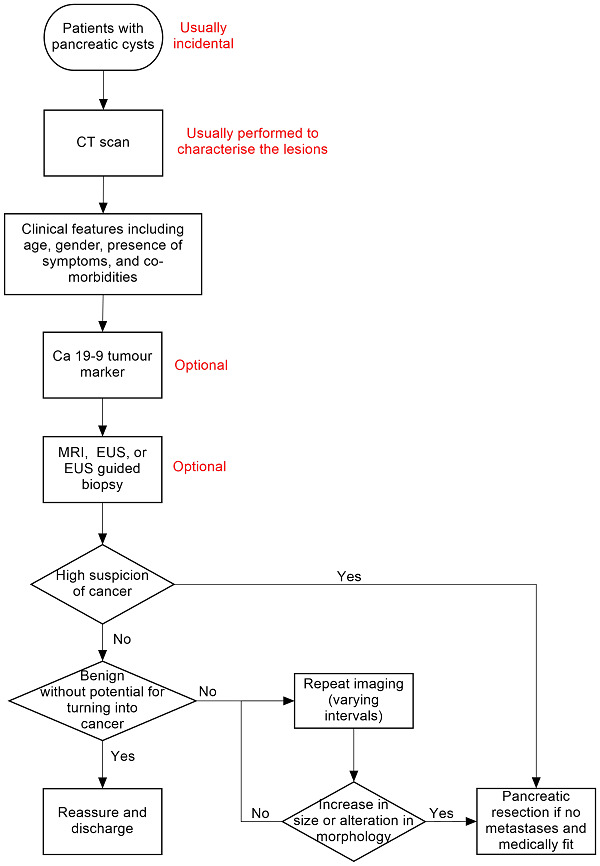
Clinical pathway.
Abbreviations:
Ca 19‐9: carbohydrate antigen 19‐9 CT: computed tomography EUS: endoscopic ultrasound MRI: magnetic resonance imaging PET: positron emission tomography
Prior test(s)
The tests that occur prior to pancreatic imaging depend on how the patient presents. The investigation may be targeted if the patient presents with abdominal symptoms, however it is equally possible that the pancreatic lesion is an incidental finding on an abdominal scan for an alternative reason. As pancreatic cancer is relatively late presenting (Porta 2005), the number of incidental lesions found will be high comparative to other cancers where symptoms will primarily drive discovery. Whilst CT, MRI, and PET may identify incidental lesions, EUS and EUS‐guided fine‐needle aspiration (EUS‐FNA) are the likely second test for known lesions of symptomatic individuals.
Role of index test(s)
All of the index tests described are used primarily to characterise pancreatic lesions as either benign or cancerous, or more importantly as needing significant or more conservative treatment. The location of the pancreas makes percutaneous biopsy dangerous because of the risk of cancer spread, therefore determination of cancer stage and consequently required treatment must be made non‐invasively by the imaging techniques and by EUS‐FNA.
Alternative test(s)
Computed tomography is usually part of a standard algorithm for assessing focal pancreatic lesions (Gaujoux 2011). If the incidental lesion is detected on CT scan, then CT scan can be the only investigation, since the added value of the other tests is not known. One or more of the above tests may be used in addition to, or instead of, CT scan. Diagnostic laparoscopy and laparoscopic ultrasound are other tests that may be used in the differential diagnosis of focal pancreatic lesions; however, these tests are not used routinely. Serum carbohydrate antigen 19‐9 (CA 19‐9) is a substance released into the bloodstream by both cancer cells and normal cells and is used as a type of tumour marker (National Cancer Institute 2011f). Excessive CA 19‐9 in the blood can be a sign of pancreatic cancer or other types of cancer or conditions. The amount of CA 19‐9 in the blood can be used to measure how effective cancer treatments are or if cancer has returned. It can be used in conjunction with other imaging modalities in the assessment of the focal pancreatic lesion.
Rationale
The various imaging modalities use different methods to differentiate normal and diseased tissues. Endoscopic ultrasound is closer to the tissues and therefore high‐frequency ultrasound waves can be used, which have better resolution but poorer penetration than an external ultrasound. Image‐guided biopsy can be performed and the tissue can be examined under the microscope to differentiate between types of focal pancreatic lesion.
Accurate characterisation of lesions will help in patient management. Patients with cancerous lesions will be offered surgery if there is no distant spread of cancer and assuming they are fit for major surgery. Patients with cancerous lesions who are not eligible for surgery because of distant spread of cancer or lack of fitness for major surgery will be offered other treatments such as chemotherapy. Patients with precancerous lesions may also undergo surgery or ablation depending upon the clinician and patient preferences. Unnecessary major surgery can be avoided in patients with benign lesions.
There is currently no Cochrane review of studies assessing the diagnostic accuracy of different imaging modalities in the assessment of focal pancreatic lesions.
Objectives
To determine and compare the diagnostic accuracy of various imaging modalities in detecting cancerous and precancerous lesions in people with focal pancreatic lesions.
Secondary objectives
We planned to explore the following sources of heterogeneity.
Studies at low risk of bias versus those at unclear or high risk of bias (as assessed by the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool as recommended by the Cochrane Screening and Diagnostic Tests Methods Group) (Whiting 2006). In particular, we considered the studies classified as 'yes' for the items differential verification, un‐interpretable results, and withdrawals as the most important sources of heterogeneity.
Full‐text publications versus abstracts (this might be indicative of publication bias, since there may be an association between the results of the study and the study reaching full publication) (Eloubeidi 2001).
Prospective studies versus retrospective studies.
Different types of reference standard.
Symptomatic versus asymptomatic lesions (the presence of symptoms may increase the pre‐test probability).
Solid versus cystic lesions (as the diagnostic accuracy of the imaging modalities may vary depending upon whether the lesion is solid or cystic).
Participants with chronic pancreatitis versus those without chronic pancreatitis.
Different criteria used by the authors to classify the lesions.
Single imaging versus sequential imaging (repeated imaging).
Different intervals of sequential imaging (e.g. imaging every six months versus annual review).
Methods
Criteria for considering studies for this review
Types of studies
We included studies reporting on cross‐sectional information of the index test and reference test in the appropriate patient population (see below), irrespective of language or publication status or whether the data were collected prospectively or retrospectively. However, we excluded case series in which only true‐positive results or true‐negative results were reported without any information on the other participants who underwent the test.
Participants
Adults with focal pancreatic lesions.
Index tests
CT scan, MRI scan, PET scan, EUS, EUS elastography, and EUS‐guided biopsy either alone or in combination as replacement for major surgery for diagnostic purposes.
We accepted the criteria stated by the authors to classify the lesion as benign, precancerous, and cancerous for different imaging modalities.
There is no standard algorithm in the diagnosis or management of focal pancreatic lesions. Other tests that may be used in the diagnosis of focal pancreatic lesions include diagnostic laparoscopy, laparoscopic ultrasound, serum levels of CA 19‐9, and surgical resection (surgical resection may be considered diagnostic when the diagnosis is uncertain after all other diagnostic modalities have been attempted).
Target conditions
Benign versus precancerous and cancerous lesions (including the type of cancerous lesion).
Benign and precancerous versus cancerous lesions.
Reference standards
We accepted the following reference standards.
Histopathological examination of the entire lesion by surgical resection (gold standard). This classified the lesion as benign, precancerous, or cancerous.
Histopathological examination (irrespective of how the tissues were obtained for histopathological examination) in people with positive test (for cancerous or precancerous lesions) and clinical follow‐up by a doctor (with or without sequential follow‐up with imaging but using appropriate criteria such as metastases or confirmation of cancer by biopsy or death of participants due to cancer) of all participants with negative test for a period of at least six months and for a maximum period of 24 months. Until a definitive diagnosis is available, percutaneous biopsy is generally avoided because of the fear of seeding of cancer cells in potentially resectable cancers. As anticipated, the tissues obtained for histopathological examination were obtained from surgical resection. It is unlikely that patients with a low likelihood for cancer based on clinical symptoms and signs and test results (may include the results of index test) are subject to surgery or biopsy. Even if a biopsy is performed in such patients, a cancerous or precancerous lesion cannot be ruled out because of sampling error. Consequently, such patients are usually followed up clinically with sequential imaging. Pancreatic adenocarcinoma will cause clinical deterioration or increase in tumour size during a period of six months, and so we accepted clinical follow‐up or sequential follow‐up imaging (irrespective of the modality of the imaging) of all participants with a negative biopsy or no biopsy for at least six months as one of the reference standards. However, we accepted clinical follow‐up as a reference standard only when the criteria used for diagnosis were appropriate (e.g. identification of metastases, later biopsy of the lesion confirming the nature of the lesion, and death of participants due to cancer). The choice of a maximum period of 24 months was an arbitrary choice based on the low probability of precancerous lesions becoming cancerous during 24 months. Clinical follow‐up of patients is unlikely to classify precancerous lesions correctly since patients are unlikely to develop metastases or die within this interval.
Search methods for identification of studies
Electronic searches
We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 7, 2016) (Appendix 2).
MEDLINE via PubMed (January 1946 to 19 July 2016) (Appendix 3).
Embase via OvidSP (January 1947 to 19 July 2016) (Appendix 4).
Science Citation Index Expanded via ISI Web of Knowledge (January 1980 to 19 July 2016) (Appendix 5).
Searching other resources
We searched the references of included studies to identify further studies (Horsley 2011). We also searched for additional articles related to the included studies by performing the 'related search' function in MEDLINE (PubMed) and Embase (OvidSP) and 'citing reference' search (search the articles that cited the included articles) in Science Citation Index Expanded and Embase (OvidSP) (Sampson 2008).
Data collection and analysis
Selection of studies
Two review authors independently searched the references to identify relevant studies. We obtained the full text of references that at least one of the review authors consider relevant and used these full texts to further exclude irrelevant references. We selected references to studies that met the inclusion criteria for data extraction. Any differences in study selection were arbitrated by review author BR Davidson.
Data extraction and management
Two review authors independently extracted the following data from each included study.
First author of report.
Year of publication of report.
Study design (prospective or retrospective; cross‐sectional studies or randomised clinical trials).
Inclusion and exclusion criteria for individual studies.
Total number of participants.
Number of females.
Mean age of the participants.
Criteria used for classification of lesions.
Preoperative tests carried out prior to index test.
Index test.
Reference standard.
True positive (TP), false positive (FP), true negative (TN), and false negative (FN) data.
Main analysis
The unit of analysis was the participant. We extracted the TP, FP, TN, and FN information for each index test for the following situations (when data were available).
Precancerous or cancerous lesions (positive test) versus benign lesions with no cancer potential (negative test) (this helps determine whether the patient needs further follow‐up).
Cancerous lesions (positive test) versus non‐cancerous lesions (negative test) (this helps determine whether the patient needs immediate surgery).
In the group of participants with precancerous or cancerous lesions (i.e. those with positive test in the analysis of benign lesions with no cancer potential (negative test) versus precancerous or cancerous lesions (positive test)), we extracted the TP, FP, TN, and FN information for cancerous lesions (positive test) versus precancerous lesions (negative test) (this helps in assessing whether or not surgery is appropriate; surgery is the only curative option for cancerous lesions, while follow‐up may be an option for precancerous lesions).
We extracted the information on indeterminate results separately from the TP, FP, TN, and FN data. There is no standard algorithm of management of patients with indeterminate results in the first scan. Some surgeons may recommend surgical resection for indeterminate lesions, while others may advise additional scans or sequential follow‐up imaging. We therefore planned to perform a sensitivity analysis as described in Sensitivity analyses.
For tests performed for sequential follow‐up imaging (repeated‐testing strategy), we planned to extract the TP, FP, TN, and FN data for the strategy as a whole. We considered increase in size or change in the lesion on sequential follow‐up imaging (performed within 12 months) a positive index test. If the lesion remained static (or decreased in size) without any change in the characteristics of the lesion, we considered this a negative index test. The majority of surgeons will recommend further follow‐up imaging or no follow‐up if the sequential follow‐up image shows no change in the lesion, and there is no clinical deterioration for the comparison between precancerous and cancerous lesions. We therefore considered indeterminate results on sequential follow‐up imaging as negative results for this comparison.
We sought further information from study authors where necessary. Any differences between the review authors were resolved by discussion.
Assessment of methodological quality
Two review authors independently assessed the quality of the studies using the QUADAS‐2 assessment tool (Whiting 2006; Whiting 2011). We resolved any differences in assessment using the QUADAS‐2 assessment algorithm published in the protocol. We sought further information from the authors of the studies in order to accurately assess the methodological quality of the studies.
We assessed the quality items derived from the QUADAS‐2 tool using the methodology stated in Table 2.
1. QUADAS‐2 classification.
| Domain | Signalling question | Signalling question | Signalling question | Risk of bias | Concerns for applicability |
| 1: Patient sampling | Was a consecutive or random sample of patients enrolled? | Was a case‐control design avoided? | Did the study avoid inappropriate exclusions? | Could the selection of participants have introduced bias? | Are there concerns that the included participants and setting do not match the review question? |
| Yes: all consecutive patients or random sample of patients with focal pancreatic lesions were enrolled No: selected patients were enrolled Unclear: this was not clear from the report |
Yes: case‐control design was avoided No: case‐control design was not avoided Unclear: this was not clear from the report |
Yes: the study avoided inappropriate exclusions (i.e. difficult‐to‐diagnose patients) No: the study excluded patients inappropriately Unclear: this was not clear from the report |
Low risk: 'yes' for all signalling questions High risk: 'no' or 'unclear' for at least 1 signalling question |
Low concern: the selected participants represent the patients in whom the tests will be used in clinical practice (please see diagnostic pathway (Figure 1)) High concern: there is high concern that participant selection was performed in such a way that the included participants did not represent the patients in whom the tests will be used in clinical practice |
|
| 2: Index test(s) | Were the index test results interpreted without knowledge of the results of the reference standard? | If a threshold was used, was it prespecified? | — | Could the conduct or interpretation of the index test have introduced bias? | Are there concerns that the index test, its conduct, or its interpretation differ from the review question? |
| Yes: index test results were interpreted without knowledge of the results of the reference standard No: index test results were interpreted with knowledge of the results of the reference standard Unclear: this was not clear from the report |
Yes: if the criteria for a positive test were prespecified No: if the criteria for a positive test were not prespecified Unclear: this was not clear from the report |
— | Low risk: 'yes' for all signalling questions High risk: 'no' or 'unclear' for at least 1 of the 2 signalling questions |
High concern: there is high concern that the conduct or interpretation of the index test differs from the way it is likely to be used in clinical practice Low concern: there is low concern that the conduct or interpretation of the index test differs from the way it is likely to be used in clinical practice |
|
| 3: Target condition and reference standard(s) | Is the reference standard likely to classify the target condition correctly? | Were the reference standard results interpreted without knowledge of the results of the index tests? | — | Could the reference standard, its conduct, or its interpretation have introduced bias? | Are there concerns that the target condition as defined by the reference standard does not match the review question? |
| Yes: histopathological examination of the entire lesion by surgical resection No: histopathological examination (irrespective of how the tissues were obtained for histopathological examination) in patients with positive test (for cancerous or precancerous lesions) and clinical follow‐up by a doctor (with or without sequential follow‐up with imaging) of all patients with negative test for a period of at least 6 months and for a maximum period of 24 months Unclear: this was not clear from the report. Such studies will be excluded Yes: reference standard results were interpreted without knowledge of the results of the index test No: reference standard results were interpreted with knowledge of the results of the index test Unclear: this was not clear from the report |
— | Low risk: 'yes' for all signalling questions High risk: 'no' or 'unclear' for at least 1 of the 2 signalling questions |
Low concern: histopathological examination of the entire lesion by surgical resection High concern: histopathological examination (irrespective of how the tissues were obtained for histopathological examination) in patients with positive test (for cancerous or precancerous lesions) and clinical follow‐up by a doctor (with or without sequential follow‐up with imaging) of all patients with negative test for a period of at least 6 months and for a maximum period of 24 months |
— | |
| 4: Flow and timing | Was there an appropriate interval between index test and reference standard? | Did all patients receive the same reference standard? | Were all patients included in the analysis? | Could the patient flow have introduced bias? | — |
| Yes: histopathological examination of the entire lesion (gold standard) ‐ performed within 2 months (chosen arbitrarily). Histopathological examination (irrespective of how the tissues were obtained for histopathological examination) in patients with positive test (for cancerous or precancerous lesions) performed within 2 months and clinical follow‐up (including sequential follow‐up with imaging) of all patients with negative test for a period of at least 6 months No: the histopathological examination was performed beyond 2 months of the index tests. The clinical follow‐up (including sequential follow‐up imaging) was performed less than 6 months after the index test, because some tumours may be slow‐growing Unclear: this was not clear from the report |
Yes: histopathological examination of the entire lesion by surgical resection No: histopathological examination (irrespective of how the tissues were obtained for histopathological examination) in patients with positive test (for cancerous or precancerous lesions) and clinical follow‐up by a doctor (with or without sequential follow‐up with imaging) of all patients with negative test for a period of at least 6 months and for a maximum period of 24 months Unclear: this was not clear from the report. Such studies will be excluded |
Yes: all patients meeting the selection criteria (selected participants) were included in the analysis, or data on all of the selected participants were available so that a 2 x 2 table including all selected participants could be constructed No: not all patients meeting the selection criteria were included in the analysis, or the 2 x 2 table could not be constructed using data on all selected participants Unclear: this was not clear from the report |
Low risk: 'yes' for all signalling questions High risk: 'no' or 'unclear' for at least 1 signalling question |
— |
Statistical analysis and data synthesis
We have plotted study estimates of sensitivity and specificity on forest plots and in receiver operating characteristic (ROC) space to explore between‐study variation in the performance of each test. To estimate the summary sensitivity and specificity of each test, we planned to perform the meta‐analysis by fitting the bivariate model (Chu 2006; Reitsma 2005), which accounts for between‐study variability in estimates of sensitivity and specificity through the inclusion of random effects for the logit sensitivity and logit specificity parameters of the bivariate model. As there was lack of convergence due to sparse data, we tried other alternate models suggested by Takwoingi 2015 and colleagues. These included the random‐effects model, ignoring the inverse correlation between sensitivities and specificities in the different studies due to intrinsic threshold effect, and the fixed‐effect model for either sensitivity or specificity or both after visualising the forest plots and summary receiver operating characteristics (SROC) plots (Takwoingi 2015). We based our choice between the different models on the distribution of sensitivities and specificities as noted in the forest plots or ROC space. We also used the model fit as indicated by the ‐2 log likelihood and considered the model with the lower ‐2 log likelihood to be the better model.
We planned to compare the diagnostic accuracy of the tests by including covariate terms for test type (CT scan, MRI, PET, EUS, EUS‐FNA, EUS elastography) in the bivariate model to estimate differences in the sensitivity and specificity of the tests. We planned to allow both the sensitivity and specificity to vary by covariate. In addition, we planned to permit the variances of the random effects and their covariance to also depend on test type, thus allowing the variances to differ between tests. We planned to use likelihood ratio tests to compare the model with and without covariate (test type). We planned to use a P value of less than 0.05 for the likelihood ratio test to indicate differences in the diagnostic accuracy between the tests. If studies that reported different tests in the same study population were available from at least four studies, we planned to perform a direct head‐to‐head comparison by limiting the test comparison to such studies. We planned to calculate the relative sensitivities and specificities for each pair‐wise comparison of tests.
We performed the meta‐analysis using the NLMIXED command in SAS version 9.4 (SAS Institute Inc, Cary, North Carolina, USA) (Takwoingi 2012). The post‐test probabilities were calculated using these pre‐test probabilities and the summary positive and negative likelihood ratios. We calculated the summary likelihood ratios and their confidence intervals from the functions of the parameter estimates from the model that we fitted to estimate the summary sensitivities and specificities. Post‐test probability associated with a positive test is the probability of having the target condition (e.g. precancer or cancer) on the basis of a positive test result (e.g. positive CT) and is the same as the term 'positive predictive value' used in a single diagnostic accuracy study. Post‐test probability associated with a negative test is the probability of having the target condition (e.g. precancer or cancer) on the basis of a negative test result (e.g. negative CT) and is 1 ‐ 'negative predictive value'. 'Negative predictive value' is the term used in a single diagnostic accuracy study to indicate the chance that the participant has no target condition when the test is negative. We have reported the summary sensitivity, specificity, and post‐test probabilities for the median pre‐test probabilities whenever possible.
Investigations of heterogeneity
We visually inspected forest plots of sensitivity and specificity and the ROC curve to identify heterogeneity. We planned to explore heterogeneity by using the different sources of heterogeneity as covariates in the METADAS macro (Takwoingi 2012), but due to the sparseness of the data we were unable to do this. We planned to assess whether there was a statistically significant difference in the likelihood ratios in order to identify heterogeneity. Although we did not formally compare the diagnostic test accuracy of different index tests between solid and cystic lesions, we have presented a subgroup analysis of solid and cystic lesions, since some clinicians consider the diagnostic test accuracies to differ between the two.
Sensitivity analyses
In the presence of indeterminate results (for any reason) for the initial test, we planned to consider two scenarios: the participants with indeterminate results as positive for the test, as some surgeons will recommend surgical resection for indeterminate lesions; and the indeterminate results as negative for the test, as some surgeons will recommend sequential follow‐up imaging. We planned to assess the diagnostic accuracy in both of these scenarios. However, due to sparse data and few studies reporting indeterminate results we did not perform the above.
We also planned to assess the comparative performance of tests by direct comparison (i.e. the tests performed in the same participant) versus indirect comparison (the tests performed in different participants across studies).
Results
Results of the search
We identified 33,795 references through electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and Science Citation Index. We were left with 24,799 references after removing duplicate references. We excluded 23,879 clearly irrelevant references through reading the abstracts. We sought the full text for 920 references for further assessment. We did not identify any additional references to studies through other searches. We excluded 866 references for the reasons described in the Characteristics of excluded studies tables. Fifty‐two studies (54 references) met the inclusion criteria. Two studies reported the diagnostic test data on solid and cystic lesions separately (Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid; Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid)therefore, we considered them as separate studies. We thus included a total of 54 studies in the review (Brand 2000; Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid; Cellier 1998; Choi 2003; Correa‐Gallego 2009; de Jong 2012; Doi 2002; Erkan 2012; Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid; Fisher 2008; Grieser 2010; Harrison 1999; Higashi 1997; Hong 2010; Hu 2013; Jafarimehr 2010; Jang 2014a; Jang 2014b; Jin 2013a; Jin 2015; Kalha 2003; Kamata 2016a; Kato 1995; Kim 2015; Klau 2011; Kobayashi 2012; Kubo 2001; Kucera 2012; Le Baleur 2011a; Lee 2014; Maire 2008; McHenry 2002; Nakagawa 2009; Nara 2009; Ogawa 2008; Ogawa 2014; Otomi 2014; Pais 2007; Sahani 2006; Saito 2013; Salla 2007; Sedlack 2002; Smith 2016; Takanami 2011; Takeshita 2008; Tan 2009; Taouli 2000; Tomimaru 2010; Yamao 2001; Zhan 2011; Zhan 2013). The reference flow diagram is shown in Figure 2.
2.
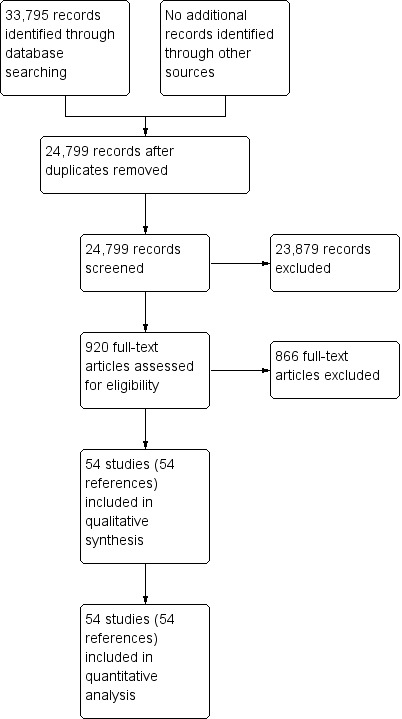
Study flow diagram.
Characteristics of included studies
For a summary of the characteristics of included studies see the Characteristics of included studies table.
We included a total of 54 studies involving 31,196 participants in this systematic review. The studies reported investigation of eight different target conditions:
cancerous versus benign or precancerous lesions;
cancerous versus benign lesions;
precancerous or cancerous lesions versus benign lesions;
cancerous (invasive carcinoma) versus precancerous (dysplasia) lesions;
cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) lesions;
cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia) lesions;
precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) lesions; and
precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) or benign lesions.
The variation in target condition was due to different definitions of what constitutes a benign, precancerous, and cancerous lesion. For example, the World Health Organization pancreatic tumour classification system classifies intraductal papillary mucinous neoplasms' (IPMNs) as precancerous tumours regardless of dysplasia (Luttges 2011). However, many of the included studies considered IPMNs to be benign lesions or even classified them as benign or cancerous based on the grade of dysplasia. This meant that the index tests were actually used for differentiating between very different populations of cancerous and benign tumours, and therefore the combination of all studies as simply cancer versus benign would have been inappropriate. In addition, different surgeons will have different thresholds for recommending surgery. Consequently, we have presented the results for all of the various definitions used by authors to classify a lesion as benign, precancerous, or cancerous.
Three studies reported data on tests differentiating cancerous from benign or precancerous lesions. Of these three studies, one reported the performance of EUS‐FNA using cytology (McHenry 2002); another reported the performance of EUS‐FNA using a carcinoembryonic antigen (CEA) threshold of 500 ng/mL (Kalha 2003); and the third reported the performance of PET to differentiate between benign or precancerous and cancerous lesions (Jafarimehr 2010). The median pre‐test probability of a cancerous lesion in these studies was 0.625 or 62.5% (minimum 0.533, maximum 0.711).
Twelve studies reported data on tests differentiating cancerous from benign lesions. Of these 12 studies, two reported the performance of EUS (Brand 2000; Harrison 1999); three reported the performance of EUS‐FNA (Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid; Cherian 2010); three reported the performance of PET (Erkan 2012; Higashi 1997; Kato 1995); one reported the performance of PET with a standard uptake value (SUV) maximum of greater than 3.5 as its threshold for positivity (Hu 2013); two reported the performance of CT (Grieser 2010; Harrison 1999); and one reported the performance of MRI to differentiate between cancerous and benign lesions (Klau 2011). The median pre‐test probability of a cancerous lesion in these studies was 0.697 or 69.7% (minimum 0.231, maximum 0.889).
Six studies reported data on tests differentiating precancerous or cancerous from benign lesions, with one study providing data for multiple imaging modalities (Sedlack 2002). One study reported the performance of EUS (Sedlack 2002); three studies reported the performance of EUS‐FNA (Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid; Sedlack 2002); one study reported the performance of EUS‐FNA using a CEA threshold of 50 ng/mL (Sedlack 2002); one study reported the performance of PET with an SUV maximum threshold of greater than 2.4 as its threshold for positivity (Otomi 2014); one study reported the performance of CT (Fisher 2008); and one study reported the performance of MRI (Jang 2014a). The median pre‐test probability of a precancerous or cancerous lesion in these studies was 0.706 or 70.6% (minimum 0.519, maximum 0.75).
Twelve studies reported data on tests differentiating cancerous invasive carcinomas from precancerous dysplastic lesions, with some studies reporting the diagnostic test accuracy or more than one index test. Five studies reported the performance of EUS (Cellier 1998; de Jong 2012; Nakagawa 2009; Yamao 2001; Zhan 2011); three studies reported the performance of EUS‐FNA (Jin 2015; Pais 2007; Salla 2007); and one study reported the performance of EUS‐FNA using a CEA threshold of 200 ng/mL (Maire 2008). Six studies reported the performance of CT (Cellier 1998; Nakagawa 2009; Nara 2009; Ogawa 2008; Taouli 2000; Yamao 2001), and one study reported the performance of MRI (de Jong 2012). The median pre‐test probability of a cancerous invasive carcinoma was 0.270 or 27% (minimum 0.122, maximum 0.618).
Eighteen studies reported data on tests differentiating cancerous lesions defined by high‐grade dysplasia or invasive carcinoma from precancerous lesions with a low or intermediate grade of dysplasia, with some studies reporting the diagnostic test accuracy or more than one index test. Four studies reported the performance of EUS (Doi 2002; Kobayashi 2012; Lee 2014; Yamao 2001). Three studies reported the performance of EUS‐FNA (Jin 2013a; Smith 2016; Zhan 2013). Three studies reported the performance of EUS‐FNA using a CEA threshold of 200 ng/mL (Correa‐Gallego 2009; Kucera 2012; Maire 2008). One study reported the performance of EUS‐FNA using a carbohydrate antigen 19‐9 threshold of greater than 1000 U/mL (Maire 2008). One study reported the performance of EUS‐FNA using a CEA threshold of 692.8 ng/mL (Zhan 2013). Four studies reported the performance of PET with an SUVmax value between 2 and 2.5 as their threshold for positivity (Hong 2010; Saito 2013; Takanami 2011; Tomimaru 2010). Three studies reported the performance of CT (Hong 2010; Le Baleur 2011a; Yamao 2001). Three studies reported the performance of MRI (Jang 2014b; Kim 2015; Ogawa 2014). The median pre‐test probability of a cancerous lesion defined by high‐grade dysplasia or invasive carcinoma in these studies was 0.449 or 44.9% (minimum 0.167, maximum 0.875).
Two studies reported data on tests differentiating cancerous invasive carcinomas from precancerous lesions with a low grade of dysplasia. One study reported the performance of EUS (Kubo 2001), and one study reported the performance of CT (Takeshita 2008). The median pre‐test probability of cancerous invasive carcinoma in these studies was 0.214 or 21.4% (minimum 0.174, maximum 0.255).
Five studies reported data on tests differentiating precancerous or cancerous lesions that may be moderately or highly dysplastic or invasive carcinomas from precancerous lesions with a low grade of dysplasia. Three studies reported the performance of CT (Ogawa 2008; Sahani 2006; Tan 2009), and two studies reported the performance of MRI (Choi 2003; Sahani 2006). None of the studies reported the diagnostic accuracy of EUS elastography or sequential testing. The median pre‐test probability of a cancerous lesion that may be moderately or highly dysplastic or an invasive carcinoma in these studies was 0.593 or 59.3% (minimum 0.574, maximum 0.68).
One study reported data on tests differentiating precancerous or cancerous lesions that may be moderately or highly dysplastic or invasive carcinomas from benign or precancerous lesions with a low grade of dysplasia. This study reported the performance of EUS. The median pre‐test probability of a cancerous lesion that may be moderately or highly dysplastic or an invasive carcinoma in this study was 0.429 or 42.9%.
Forty‐six studies were full‐text publications (Brand 2000; Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid; Cellier 1998; Cherian 2010; Choi 2003; Correa‐Gallego 2009; de Jong 2012; Doi 2002; Fisher 2008; Grieser 2010; Harrison 1999; Higashi 1997; Hong 2010; Hu 2013; Jang 2014a; Jang 2014b; Jin 2015; Kamata 2016a; Kato 1995; Kim 2015; Klau 2011; Kobayashi 2012; Kubo 2001; Kucera 2012; Le Baleur 2011a; Lee 2014; Maire 2008; Nakagawa 2009; Nara 2009; Ogawa 2008; Ogawa 2014; Otomi 2014; Pais 2007; Sahani 2006; Saito 2013; Salla 2007; Sedlack 2002; Smith 2016; Takanami 2011; Takeshita 2008; Tan 2009; Taouli 2000; Tomimaru 2010; Yamao 2001; Zhan 2013). The remaining studies were abstracts (Erkan 2012; Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid; Jafarimehr 2010; Jin 2013a; Kalha 2003; McHenry 2002; Zhan 2011). Three studies were prospective (Brand 2000; de Jong 2012; Erkan 2012); 39 were retrospective (Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid; Cellier 1998; Cherian 2010; Correa‐Gallego 2009; Doi 2002; Fisher 2008; Grieser 2010; Harrison 1999; Hong 2010; Hu 2013; Jafarimehr 2010; Jang 2014a; Jang 2014b; Jin 2013a; Jin 2015; Kalha 2003; Kamata 2016a; Kim 2015; Klau 2011; Kobayashi 2012; Kubo 2001; Kucera 2012; Lee 2014; Maire 2008; McHenry 2002; Nakagawa 2009; Ogawa 2008; Otomi 2014; Pais 2007; Sahani 2006; Saito 2013; Salla 2007; Sedlack 2002; Smith 2016; Takanami 2011; Taouli 2000; Zhan 2011; Zhan 2013); and 12 did not state whether they were prospective or retrospective (Choi 2003; Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid; Higashi 1997; Kato 1995; Le Baleur 2011a; Nara 2009; Ogawa 2014; Takeshita 2008; Tan 2009; Tomimaru 2010; Yamao 2001).
None of the studies reported data on symptomatic and asymptomatic participants separately. Forty‐two studies (2086 participants) reported on cystic pancreatic lesions (Brandwein 2001 ‐ Cystic; Cellier 1998; Choi 2003; Correa‐Gallego 2009; de Jong 2012; Doi 2002; Fischer 2009 ‐ Cystic; Fisher 2008; Hong 2010; Hu 2013; Jang 2014a; Jang 2014b; Jin 2013a; Jin 2015; Kalha 2003; Kamata 2016a; Kim 2015; Kobayashi 2012; Kubo 2001; Kucera 2012; Le Baleur 2011a; Lee 2014; Maire 2008; McHenry 2002; Nakagawa 2009; Nara 2009; Ogawa 2008; Ogawa 2014; Pais 2007; Sahani 2006; Saito 2013; Salla 2007; Sedlack 2002; Smith 2016; Takanami 2011; Takeshita 2008; Tan 2009; Taouli 2000; Tomimaru 2010; Yamao 2001; Zhan 2011; Zhan 2013). Four studies reported on solid pancreatic lesions (Brandwein 2001 ‐ Solid; Cherian 2010; Fischer 2009 ‐ Solid; Klau 2011). The remaining eight studies either did not mention whether the lesions were cystic or solid, or did not report this information separately (Brand 2000; Erkan 2012; Grieser 2010; Harrison 1999; Higashi 1997; Jafarimehr 2010; Kato 1995; Otomi 2014). None of the studies reported data on people with chronic pancreatitis separately.
Overall, 12 studies reported data on EUS results (Brand 2000; Cellier 1998; de Jong 2012; Doi 2002; Harrison 1999; Kamata 2016a; Kobayashi 2012; Kubo 2001; Lee 2014; Nakagawa 2009; Sedlack 2002; Yamao 2001); 19 studies reported data on EUS‐FNA (Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid; Cherian 2010; Correa‐Gallego 2009; Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid; Fisher 2008; Jin 2013a; Jin 2015; Kalha 2003; Kucera 2012; Maire 2008; McHenry 2002; Pais 2007; Salla 2007; Sedlack 2002; Smith 2016; Zhan 2011; Zhan 2013); 10 studies reported data on PET (Erkan 2012; Higashi 1997; Hong 2010; Hu 2013; Jafarimehr 2010; Kato 1995; Otomi 2014; Saito 2013; Takanami 2011; Tomimaru 2010); 13 studies reported data on CT (Cellier 1998; Grieser 2010; Harrison 1999; Hong 2010; Le Baleur 2011a; Nakagawa 2009; Nara 2009; Ogawa 2008; Sahani 2006; Takeshita 2008; Tan 2009; Taouli 2000; Yamao 2001); and eight studies reported data on MRI (Choi 2003; de Jong 2012; Jang 2014a; Jang 2014b; Kim 2015; Klau 2011; Ogawa 2014; Sahani 2006).
The criteria for a positive test result varied widely by study and are described in detail in Characteristics of included studies. The reference standards in all of the included studies was surgical excision.
Methodological quality of included studies
The methodological quality of the included studies is summarised in Figure 3 and Figure 4. None of the included studies was of high methodological quality.
3.
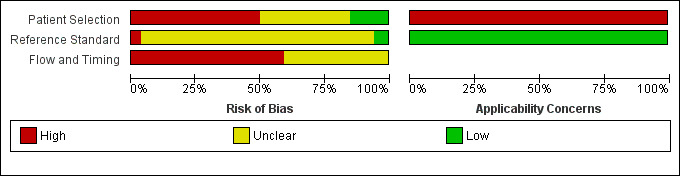
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
4.
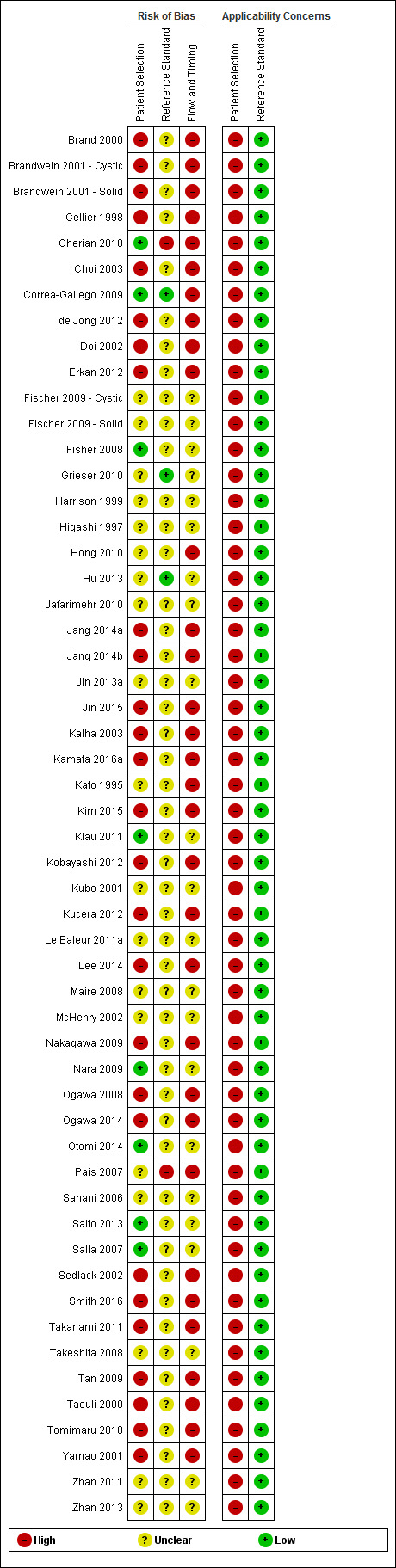
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
Participant selection domain
In the participant selection domain, nine studies had a low risk of bias (Cherian 2010; Correa‐Gallego 2009; Fisher 2008; Kamata 2016a; Klau 2011; Nara 2009; Otomi 2014; Saito 2013; Salla 2007). All of the studies had high applicability concerns because of concerns that the participants did not match the review question. The review question was to find out the diagnostic accuracy of these index tests in people with focal lesions. However, all of the studies meeting the inclusion criteria for this review except Cherian 2010 used surgical excision as the reference standard, suggesting that the surgeons considered these patients to be at high risk of malignancy based on the results of the index tests or the tests that patients had prior to or subsequent to the index test. Cherian 2010 was also at high risk of applicability concern because it excluded participants with resectable lesions on CT scan and included only those equivocal lesions on CT scan.
Index test domain
In the index test domain, nine studies were at low risk of bias (Correa‐Gallego 2009; Hong 2010; Jang 2014b; Kim 2015; Kubo 2001; Nara 2009; Ogawa 2014; Tan 2009; Taouli 2000). Of the remaining studies, 31 were at unclear risk of bias because it was unclear whether the index test results were interpreted without knowledge of the results of the reference standard (Brand 2000; Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid; Cellier 1998; Choi 2003; Cherian 2010; de Jong 2012; Doi 2002; Erkan 2012; Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid; Fisher 2008; Harrison 1999; Jafarimehr 2010; Jin 2013a; Jin 2015; Kalha 2003; Kamata 2016a; Kato 1995; Kobayashi 2012; Kucera 2012; Le Baleur 2011a; McHenry 2002; Ogawa 2008; Pais 2007; Salla 2007; Sedlack 2002; Smith 2016; Yamao 2001; Zhan 2011; Zhan 2013). Fifteen studies were at high risk of bias because the threshold for the index test was not prespecified (Grieser 2010; Higashi 1997; Hu 2013; Jang 2014a; Klau 2011; Lee 2014; Maire 2008; Nakagawa 2009; Otomi 2014; Sahani 2006; Saito 2013; Takanami 2011; Takeshita 2008; Tomimaru 2010; Zhan 2013). Twenty‐eight studies had low applicability concerns (Brand 2000; Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid; Cellier 1998; Choi 2003; Cherian 2010; Correa‐Gallego 2009; de Jong 2012; Doi 2002; Hong 2010; Hu 2013; Jang 2014b; Jin 2013a; Jin 2015; Kalha 2003; Kamata 2016a; Kim 2015; Kubo 2001; Le Baleur 2011a; Nara 2009; Ogawa 2008; Ogawa 2014; Pais 2007; Sahani 2006; Sedlack 2002; Smith 2016; Yamao 2001; Zhan 2013), and the remaining 27 studies had high applicability concerns because of concerns that the index test, its conduct, or interpretation differed from the review question (Erkan 2012; Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid; Fisher 2008; Grieser 2010; Harrison 1999; Higashi 1997; Jafarimehr 2010; Jang 2014a; Kato 1995; Klau 2011; Kobayashi 2012; Kucera 2012; Lee 2014; Maire 2008; McHenry 2002; Nakagawa 2009; Otomi 2014; Saito 2013; Salla 2007; Sedlack 2002; Takanami 2011; Takeshita 2008; Tan 2009; Taouli 2000; Tomimaru 2010; Zhan 2011).
Reference standard domain
In the reference standard domain, three studies were at low risk of bias (Correa‐Gallego 2009; Grieser 2010; Hu 2013). Two studies were at high risk of bias because the reference standard results were not interpreted without knowledge of the index test results (Pais 2007), or because radiological and clinical follow‐up was used in some of the participants as the reference standard. The remaining 49 studies were at unclear risk of bias as it was unclear if the reference standard was interpreted without knowledge of the results of index tests (Brand 2000; Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid; Cellier 1998; Choi 2003; de Jong 2012; Doi 2002; Erkan 2012; Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid; Fisher 2008; Harrison 1999; Higashi 1997; Hong 2010; Jafarimehr 2010; Jang 2014a; Jang 2014b; Jin 2013a; Jin 2015; Kalha 2003; Kamata 2016a; Kato 1995; Kim 2015; Klau 2011; Kobayashi 2012; Kubo 2001; Kucera 2012; Le Baleur 2011a; Lee 2014; Maire 2008; McHenry 2002; Nakagawa 2009; Nara 2009; Ogawa 2008; Ogawa 2014; Otomi 2014; Sahani 2006; Saito 2013; Salla 2007; Sedlack 2002; Smith 2016; Takanami 2011; Takeshita 2008; Tan 2009; Taouli 2000; Tomimaru 2010; Yamao 2001; Zhan 2011; Zhan 2013). All studies were at low concern for applicability, as we considered the definition of the target condition by the reference standard to match the review question.
Flow and timing domain
None of the studies were at low risk of bias in the flow and timing domain. Thirty studies were at high risk of bias because not all of the participants were included in the analysis, or there was an inappropriate interval between the index test and reference standard (Brand 2000; Brandwein 2001 ‐ Cystic; Cellier 1998; Choi 2003; Correa‐Gallego 2009; de Jong 2012; Doi 2002; Erkan 2012; Hong 2010; Jang 2014a; Jang 2014b; Jin 2015; Kalha 2003; Kamata 2016a; Kato 1995; Kim 2015; Kobayashi 2012; Kucera 2012; Lee 2014; Nakagawa 2009; Ogawa 2008; Ogawa 2014; Pais 2007; Sedlack 2002; Smith 2016; Takanami 2011; Tan 2009; Taouli 2000; Tomimaru 2010; Yamao 2001). One study was at high risk of bias because the reference standards that participants received were dependent on the index test results. The remaining 23 studies were at unclear risk of bias because it was either unclear if there was an appropriate interval between the index test and reference standard or if all participants were included in the analysis, or both (Brandwein 2001 ‐ Solid; Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid; Fisher 2008; Grieser 2010; Harrison 1999; Higashi 1997; Hu 2013; Jafarimehr 2010; Jin 2013a; Klau 2011; Kubo 2001; Le Baleur 2011a; Maire 2008; McHenry 2002; Nara 2009; Otomi 2014; Sahani 2006; Saito 2013; Salla 2007; Takeshita 2008; Zhan 2011; Zhan 2013).
Findings
The results are summarised in the Table 1. The overall sensitivities and specificities for different tests for different target conditions are tabulated in Table 3. A detailed description is given below.
2. Summary sensitivity and specificity of different tests for different target conditions.
| Comparison | Name of test | Sensitivity | Specificity |
| Cancerous versus benign or precancerous | EUS‐FNA (cytology) | 0.79 (95% CI 0.60 to 0.91) | 1.00 (95% CI 0.85 to 1.00) |
| Cancerous versus benign or precancerous | EUS‐FNA (CEA > 500 ng/mL) | 0.93 (95% CI 0.70 to 0.99) | 0.33 (95% CI 0.12 to 0.65) |
| Cancerous versus benign or precancerous | PET (criteria unspecified) | 0.85 (95% CI 0.73 to 0.92) | 0.91 (95% CI 0.72 to 0.97) |
| Cancerous versus benign | EUS | 0.95 (95% CI 0.84 to 0.99) | 0.53 (95% CI 0.31 to 0.74) |
| Cancerous versus benign | EUS‐FNA (cytology) | 0.79 (95% CI 0.07 to 1.00) | 1.00 (95% CI 0.91 to 1.00) |
| Cancerous versus benign | PET (criteria unspecified) | 0.92 (95% CI 0.80 to 0.97) | 0.65 (95% CI 0.39 to 0.85) |
| Cancerous versus benign | PET (SUVmax > 3.5) | 0.96 (95% CI 0.87 to 0.99) | 0.62 (95% CI 0.43 to 0.78) |
| Cancerous versus benign | CT | 0.98 (95% CI 0.00 to 1.00) | 0.76 (95% CI 0.02 to 1.00) |
| Cancerous versus benign | MRI | 0.80 (95% CI 0.58 to 0.92) | 0.89 (95% CI 0.57 to 0.98) |
| Precancerous or cancerous versus benign | EUS | 0.92 (95% CI 0.74 to 0.98) | 0.60 (95% CI 0.31 to 0.83) |
| Precancerous or cancerous versus benign | EUS‐FNA (cytology) | 0.73 (95% CI 0.01 to 1.00) | 0.94 (95% CI 0.15 to 1.00) |
| Precancerous or cancerous versus benign | EUS‐FNA (CEA > 50 ng/mL) | 0.29 (95% CI 0.08 to 0.64) | 0.25 (95% CI 0.05 to 0.70) |
| Precancerous or cancerous versus benign | PET (SUVmax 2.4) | 0.94 (95% CI 0.74 to 0.99) | 0.93 (95% CI 0.69 to 0.99) |
| Precancerous or cancerous versus benign | CT | 0.62 (95% CI 0.45 to 0.76) | 0.64 (95% CI 0.39 to 0.84) |
| Precancerous or cancerous versus benign | MRI | 0.93 (95% CI 0.69 to 0.99) | 0.85 (95% CI 0.58 to 0.96) |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) | EUS | 0.78 (95% CI 0.45 to 0.94) | 0.91 (95% CI 0.61 to 0.98) |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) | EUS‐FNA (cytology) | 0.66 (95% CI 0.03 to 0.99) | 0.92 (95% CI 0.73 to 0.98) |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) | EUS‐FNA (CEA > 200 ng/mL) | 1.00 (95% CI 0.57 to 1.00) | 0.64 (95% CI 0.48 to 0.78) |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) | CT | 0.72 (95% CI 0.50 to 0.87) | 0.92 (95% CI 0.81 to 0.97) |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) | MRI | 0.75 (95% CI 0.30 to 0.95) | 0.93 (95% CI 0.77 to 0.98) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | EUS | 0.86 (95% CI 0.74 to 0.92) | 0.91 (95% CI 0.83 to 0.96) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | EUS‐FNA (cytology) | 0.47 (95% CI 0.24 to 0.70) | 0.91 (95% CI 0.32 to 1.00) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | EUS‐FNA (CEA > 200 ng/mL) | 0.58 (95% CI 0.28 to 0.83) | 0.51 (95% CI 0.19 to 0.81) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | EUS‐FNA (CA 19‐9 > 1000 U/mL) | 0.90 (95% CI 0.60 to 0.98) | 0.42 (95% CI 0.26 to 0.59) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | EUS‐FNA (CEA > 692.8 ng/mL) | 0.80 (95% CI 0.49 to 0.94) | 0.90 (95% CI 0.60 to 0.98) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | PET (SUVmax > 2 to 2.5) | 0.90 (95% CI 0.79 to 0.96) | 0.94 (95% CI 0.81 to 0.99) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | CT | 0.87 (95% CI 0.00 to 1.00) | 0.96 (95% CI 0.00 to 1.00) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | MRI | 0.69 (95% CI 0.44 to 0.86) | 0.93 (95% CI 0.43 to 1.00) |
| Cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia) | EUS | 0.77 (95% CI 0.50 to 0.92) | 0.89 (95% CI 0.76 to 0.96) |
| Cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia) | CT | 0.50 (95% CI 0.22 to 0.78) | 0.95 (95% CI 0.83 to 0.99) |
| Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) | CT | 0.83 (95% CI 0.68 to 0.92) | 0.83 (95% CI 0.64 to 0.93) |
| Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) | MRI | 0.80 (95% CI 0.58 to 0.92) | 0.81 (95% CI 0.53 to 0.95) |
| Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) or benign | EUS | 0.97 (95% CI 0.83 to 0.99) | 0.40 (95% CI 0.26 to 0.55) |
| Cystic lesion subgroup analysis | Cancerous versus benign ‐ EUS‐FNA (cytology) | 0.50 (95% CI 0.19 to 0.81) | 1.00 (95% CI 0.84 to 1.00) |
| Cystic lesion subgroup analysis | Cancerous versus benign ‐ PET (SUVmax > 3.5) | 0.96 (95% CI 0.87 to 0.99) | 0.62 (95% CI 0.43 to 0.78) |
| Cystic lesion subgroup analysis | Precancerous or cancerous versus benign ‐ EUS‐FNA (cytology) | 0.43 (95% CI 0.19 to 0.71) | 1.00 (95% CI 0.74 to 1.00) |
CA 19‐9: carbohydrate antigen 19‐9 CEA: carcinoembryonic antigen CI: confidence interval CT: computed tomography EUS: endoscopic ultrasound FNA: fine‐needle aspiration MRI: magnetic resonance imaging PET: positron emission tomography SUVmax: maximum standardised uptake values
Cancerous versus benign or precancerous
EUS‐FNA cytology: We included one study reporting data on 45 participants for this test (McHenry 2002). The sensitivity and specificity for diagnosing cancer were 0.79 (95% confidence interval (CI) 0.60 to 0.91) and 1.00 (95% CI 0.85 to 1.00), respectively.
EUS‐FNA (CEA > 500 ng/mL): We included one study reporting data on 24 participants for this test (Kalha 2003). The sensitivity and specificity for diagnosing cancer were 0.93 (95% CI 0.70 to 0.99) and 0.33 (95% CI 0.12 to 0.65), respectively.
PET (criteria: not specified): We included one study reporting data on 76 participants for this test (Jafarimehr 2010). The sensitivity and specificity for diagnosing cancer were 0.85 (95% CI 0.73 to 0.92) and 0.91 (95% CI 0.72 to 0.97), respectively.
The results including sensitivities and specificities and post‐test probabilities at median pre‐test probabilities are summarised in Table 1. A forest plot summarising all of the sensitivity and specificity data for the 'cancerous versus benign or precancerous' studies is shown in Figure 5.
5.
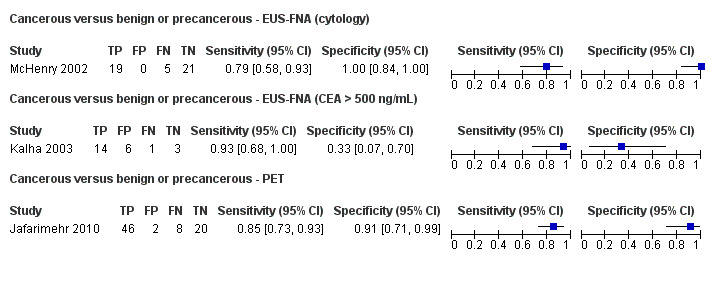
Forest plot ‐ Cancerous versus benign or precancerous.
Cancerous versus benign
EUS: Two studies reporting data on 133 participants were included for this test, allowing meta‐analysis to be performed (Brand 2000; Harrison 1999). The summary sensitivity and summary specificity for diagnosing cancer were 0.95 (95% CI 0.84 to 0.99) and 0.53 (95% CI 0.31 to 0.74), respectively.
EUS‐FNA cytology: Three studies reporting data on 147 participants were included for this test (Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid; Cherian 2010). The sensitivity and specificity for diagnosing cancer were 0.79 (95% CI 0.07 to 1.00) and 1.00 (95% CI 0.91 to 1.00), respectively.
PET (criteria: not specified): Three studies reporting data on 99 participants were included for this test, allowing meta‐analysis to be performed (Erkan 2012; Higashi 1997; Kato 1995). The summary sensitivity and summary specificity for diagnosing cancer were 0.92 (95% CI 0.80 to 0.97) and 0.65 (95% CI 0.39 to 0.85), respectively.
PET (SUVmax > 3.5): We included one study reporting data on 80 participants for this test (Hu 2013). The sensitivity and specificity for diagnosing cancer were 0.96 (95% CI 0.87 to 0.99) and 0.62 (95% CI 0.43 to 0.78), respectively.
CT: Two studies reporting data on 123 participants were included for this test, allowing meta‐analysis to be performed (Grieser 2010; Harrison 1999). The summary sensitivity and summary specificity for diagnosing cancer were 0.98 (95% CI 0.00 to 1.00) and 0.76 (95% CI 0.02 to 1.00), respectively.
MRI: We included one study reporting data on 29 participants for this test (Klau 2011). The sensitivity and specificity for diagnosing cancer were 0.80 (95% CI 0.58 to 0.92) and 0.89 (95% CI 0.57 to 0.98), respectively.
The results including sensitivities and specificities and post‐test probabilities at median pre‐test probabilities are summarised in Table 1. A forest plot summarising all of the sensitivity and specificity data for the 'cancerous versus benign' studies is shown in Figure 6.
6.
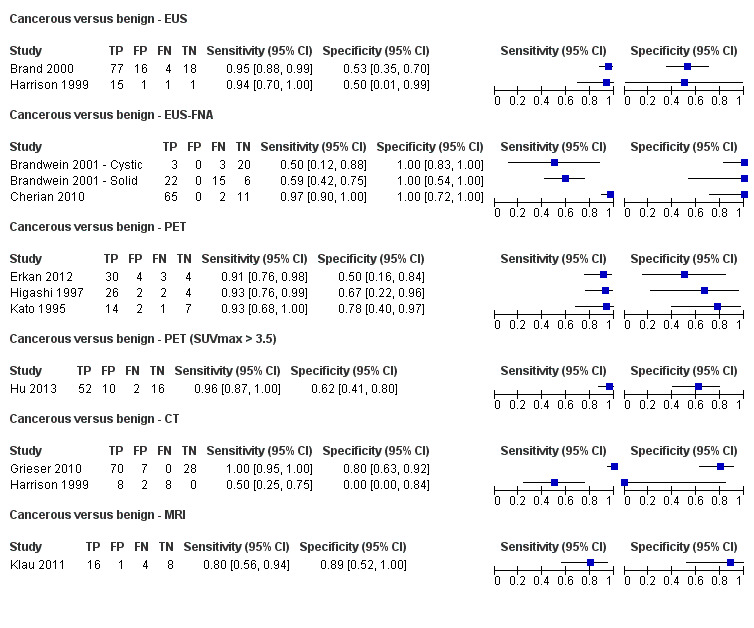
Forest plot ‐ Cancerous versus benign.
Precancerous or cancerous versus benign
EUS: We included one study reporting data on 34 participants for this test (Sedlack 2002). The sensitivity and specificity for diagnosing cancer or precancer were 0.92 (95% CI 0.74 to 0.98) and 0.60 (95% CI 0.31 to 0.83), respectively.
EUS‐FNA cytology: We included three studies, reporting data on 52 participants for this test (Fischer 2009 ‐ Cystic; Fischer 2009 ‐ Solid; Sedlack 2002). The summary sensitivity and summary specificity for diagnosing cancer or precancer were 0.73 (95% CI 0.01 to 1.00) and 0.94 (95% CI 0.15 to 1.00), respectively.
EUS‐FNA (CEA > 50 ng/mL): We included one study reporting data on 11 participants for this test (Sedlack 2002). The sensitivity and specificity for diagnosing cancer or precancer were 0.29 (95% CI 0.08 to 0.64) and 0.25 (95% CI 0.05 to 0.70), respectively.
PET (SUVmax > 2.4): We included one study reporting data on 32 participants for this test (Otomi 2014). The sensitivity and specificity for diagnosing cancer or precancer were 0.94 (95% CI 0.74 to 0.99) and 0.93 (95% CI 0.69 to 0.99), respectively.
CT: We included one study reporting data on 48 participants for this test (Fisher 2008). The sensitivity and specificity for diagnosing cancer or precancer were 0.62 (95% CI 0.45 to 0.76) and 0.64 (95% CI 0.39 to 0.84), respectively.
MRI: We included one study reporting data on 27 participants for this test (Jang 2014a). The sensitivity and specificity for diagnosing cancer or precancer were 0.93 (95% CI 0.69 to 0.99) and 0.85 (95% CI 0.58 to 0.96), respectively.
The results including sensitivities and specificities and post‐test probabilities at median pre‐test probabilities are summarised in Table 1. A forest plot summarising all of the sensitivity and specificity data for the 'precancerous or cancerous versus benign' studies is shown in Figure 7.
7.
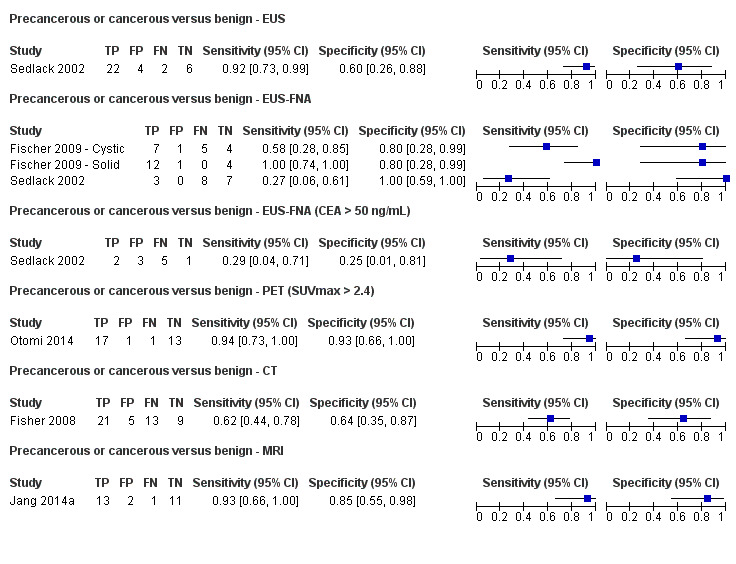
Forest plot ‐ Precancerous or cancerous versus benign.
Cancerous (invasive carcinoma) versus precancerous (dysplasia)
EUS: Five studies reporting data on 156 participants were included for this test, allowing meta‐analysis to be performed (Cellier 1998; de Jong 2012; Nakagawa 2009; Yamao 2001; Zhan 2011). The summary sensitivity and summary specificity for diagnosing invasive cancer were 0.78 (95% CI 0.45 to 0.94) and 0.91 (95% CI 0.61 to 0.98), respectively.
EUS‐FNA cytology: Three studies reporting data on 158 participants were included for this test, allowing meta‐analysis to be performed (Jin 2013a; Pais 2007; Salla 2007). The summary sensitivity and summary specificity for diagnosing invasive cancer were 0.66 (95% CI 0.03 to 0.99) and 0.92 (95% CI 0.73 to 0.98), respectively.
EUS‐FNA (CEA > 200 ng/mL): We included one study reporting data on 41 participants for this test (Maire 2008). The sensitivity and specificity for diagnosing invasive cancer were 1.00 (95% CI 0.57 to 1.00) and 0.64 (95% CI 0.48 to 0.78), respectively.
CT: Six studies reporting data on 326 participants were included for this test, allowing meta‐analysis to be performed (Cellier 1998; Nakagawa 2009; Nara 2009; Ogawa 2008; Taouli 2000; Yamao 2001). The summary sensitivity and summary specificity for diagnosing invasive cancer were 0.72 (95% CI 0.50 to 0.87) and 0.92 (95% CI 0.81 to 0.97), respectively.
MRI: We included one study reporting data on 32 participants for this test (de Jong 2012). The sensitivity and specificity for diagnosing invasive cancer were 0.75 (95% CI 0.30 to 0.95) and 0.93 (95% CI 0.77 to 0.98), respectively.
The results including sensitivities and specificities and post‐test probabilities at median pre‐test probabilities are summarised in Table 1. A forest plot summarising all of the sensitivity and specificity data for the 'cancer (invasive carcinoma) versus precancerous (dysplasia)' studies is shown in Figure 8.
8.
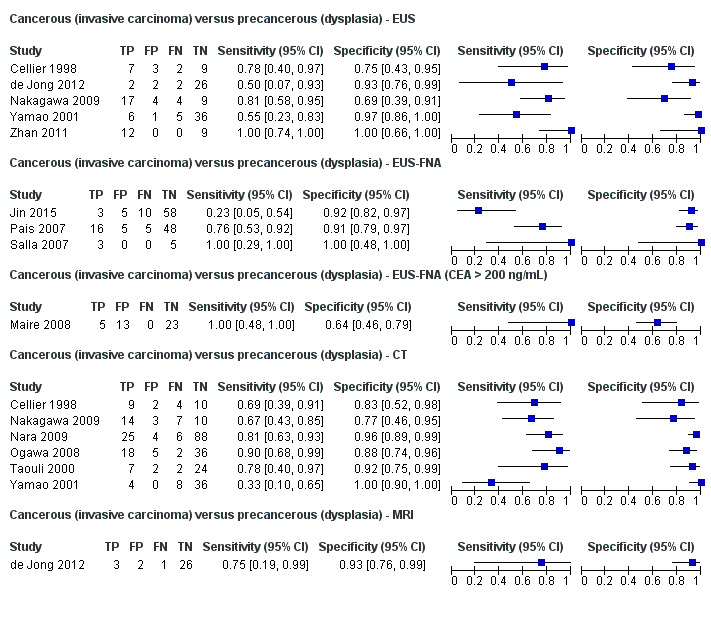
Forest plot ‐ Cancerous (invasive carcinoma) versus precancerous (dysplasia).
Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia)
EUS: Four studies reporting data on 196 participants were included for this test, allowing meta‐analysis to be performed (Doi 2002; Kobayashi 2012; Lee 2014; Yamao 2001). The summary sensitivity and summary specificity for diagnosing high‐grade dysplasia or invasive cancer were 0.86 (95% CI 0.74 to 0.92) and 0.91 (95% CI 0.83 to 0.96), respectively.
EUS‐FNA cytology: Three studies reporting data on 310 participants were included for this test, allowing meta‐analysis to be performed (Jin 2013a; Smith 2016; Zhan 2013). The summary sensitivity and summary specificity for diagnosing high‐grade dysplasia or invasive cancer were 0.47 (95% CI 0.24 to 0.70) and 0.91 (95% CI 0.32 to 1.00), respectively.
EUS‐FNA (CEA > 200 ng/mL): Three studies reporting data on 160 participants were included for this test, allowing meta‐analysis to be performed (Correa‐Gallego 2009; Kucera 2012; Maire 2008). The summary sensitivity and summary specificity for diagnosing high‐grade dysplasia or invasive cancer were 0.58 (95% CI 0.28 to 0.83) and 0.51 (95% CI 0.19 to 0.81), respectively.
EUS‐FNA (carbohydrate antigen 19‐9 > 1000 U/mL): We included one study reporting data on 41 participants for this test (Maire 2008). The sensitivity and specificity for diagnosing high‐grade dysplasia or invasive cancer were 0.90 (95% CI 0.60 to 0.98) and 0.42 (95% CI 0.26 to 0.59), respectively.
EUS‐FNA (CEA > 692.8 ng/mL): We included one study reporting data on 20 participants for this test (Zhan 2013). The sensitivity and specificity for diagnosing high‐grade dysplasia or invasive cancer were 0.80 (95% CI 0.49 to 0.94) and 0.90 (95% CI 0.60 to 0.98), respectively.
PET (SUVmax 2 to 2.5): Four studies reporting data on 124 participants were included for this test, allowing meta‐analysis to be performed (Hong 2010; Saito 2013; Takanami 2011; Tomimaru 2010). The summary sensitivity and summary specificity for diagnosing high‐grade dysplasia or invasive cancer were 0.90 (95% CI 0.79 to 0.96) and 0.94 (95% CI 0.81 to 0.99), respectively.
CT: Three studies reporting data on 139 participants were included for this test, allowing meta‐analysis to be performed (Hong 2010; Le Baleur 2011a; Yamao 2001). The summary sensitivity and summary specificity for diagnosing high‐grade dysplasia or invasive cancer were 0.87 (95% CI 0.00 to 1.00) and 0.96 (95% CI 0.00 to 1.00), respectively.
MRI: Three studies reporting data on 189 participants were included for this test, allowing meta‐analysis to be performed (Jang 2014b; Kim 2015; Ogawa 2014). The summary sensitivity and summary specificity for diagnosing high‐grade dysplasia or invasive cancer were 0.69 (95% CI 0.44 to 0.86) and 0.93 (95% CI 0.43 to 1.00), respectively.
The results including sensitivities and specificities and post‐test probabilities at median pre‐test probabilities are summarised in Table 1. A forest plot summarising all of the sensitivity and specificity data for the 'cancer (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia)' studies is shown in Figure 9.
9.
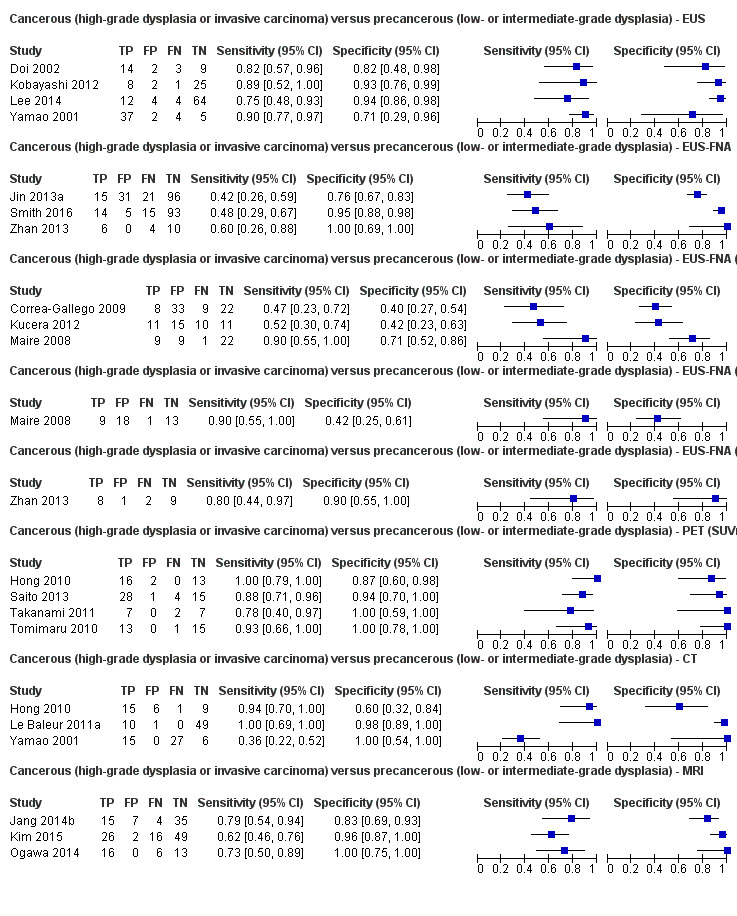
Forest plot ‐ Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia).
Cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia)
EUS: We included one study reporting data on 51 participants for this test (Kubo 2001). The sensitivity and specificity for diagnosing invasive cancer were 0.77 (95% CI 0.50 to 0.92) and 0.89 (95% CI 0.76 to 0.96), respectively.
CT: We included one study reporting data on 46 participants for this test (Takeshita 2008). The sensitivity and specificity for diagnosing invasive cancer were 0.50 (95% CI 0.22 to 0.78) and 0.95 (95% CI 0.83 to 0.99), respectively.
The results including sensitivities and specificities and post‐test probabilities at median pre‐test probabilities are summarised in Table 1. A forest plot summarising all of the sensitivity and specificity data for the 'cancer (invasive carcinoma) versus precancerous (low‐grade dysplasia)' studies is shown in Figure 10.
10.
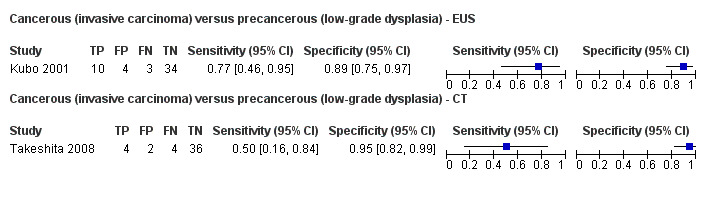
Forest plot ‐ Cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia).
Precancerous or cancer (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia)
CT: Three studies reporting data on 106 participants were included for this test, allowing meta‐analysis to be performed (Ogawa 2008; Sahani 2006; Tan 2009). The summary sensitivity and summary specificity for diagnosing intermediate‐ or high‐grade dysplasia or invasive cancer were 0.83 (95% CI 0.68 to 0.92) and 0.83 (95% CI 0.64 to 0.93), respectively.
MRI: Two studies reporting data on 71 participants were included for this test, allowing meta‐analysis to be performed (Choi 2003; Takeshita 2008). The summary sensitivity and specificity for diagnosing intermediate‐ or high‐grade dysplasia or invasive cancer were 0.80 (95% CI 0.58 to 0.92) and 0.81 (95% CI 0.53 to 0.95), respectively.
The results including sensitivities and specificities and post‐test probabilities at median pre‐test probabilities are summarised in Table 1. A forest plot summarising all of the sensitivity and specificity data for the 'precancerous or cancer (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia)' studies is shown in Figure 11.
11.
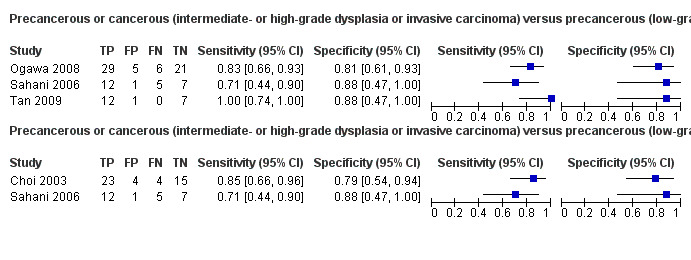
Forest plot ‐ Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia).
Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) or benign
EUS: We included one study reporting data on 70 participants for this test (Kamata 2016a). The sensitivity and specificity for diagnosing intermediate‐ or high‐grade dysplasia or invasive carcinoma were 0.97 (95% CI 0.83 to 0.99) and 0.40 (95% CI 0.26 to 0.55), respectively.
The results including sensitivity and specificity and post‐test probability at median pre‐test probability are summarised in Table 1. A forest plot summarising the sensitivity and specificity data for the 'precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) or benign' study is shown in Figure 12.
12.

Forest plot of 33 Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) or benign ‐ EUS.
Subgroup analyses
We assessed the performance of the tests excluding any studies investigating participants with solid lesions and those in which information for solid and cystic lesions was not reported separately. All of the studies assessing the ability of different imaging modalities to differentiate precancerous versus cancerous lesions regardless of the definitions used by authors for precancer and cancer (Analysis 4 to Analysis 8) included participants with cystic focal pancreatic lesions only, therefore all the results reported are for cystic focal pancreatic lesions only.
In the analysis assessing the ability of different imaging modalities to differentiate benign or precancerous versus cancerous lesions, we excluded one study because if did not specify the type (solid or cystic) of lesions for which included participants were investigated (Jafarimehr 2010). However, as this study did not contribute to a meta‐analysis, there were no changes to the analysis.
Cancerous versus benign
In the analysis assessing the ability of different imaging modalities to differentiate benign versus cancerous lesions, we excluded eight studies because they did not explicitly include participants with cystic lesions (Brand 2000; Erkan 2012; Grieser 2010; Harrison 1999; Higashi 1997; Hu 2013; Kato 1995; Klau 2011), and one study that only had one component included (Brandwein 2001 ‐ Cystic; Brandwein 2001 ‐ Solid). This left two remaining studies, which did not contribute to a meta‐analysis due to the exclusion of the other studies (Brandwein 2001 ‐ Cystic; Hu 2013). We therefore performed no meta‐analyses for this group. The new findings for benign versus cancerous lesions are described below.
EUS‐FNA: We included one study reporting data on 26 participants for this test (Brandwein 2001 ‐ Cystic). The sensitivity and specificity for diagnosing cancer were 0.50 (95% CI 0.19 to 0.81) and 1.00 (95% CI 0.84 to 1.00), respectively.
PET: We included one study reporting data on 80 participants for this test (Hu 2013). The sensitivity and specificity for diagnosing cancer were 0.96 (95% CI 0.87 to 0.99) and 0.62 (95% CI 0.43 to 0.78), respectively.
A forest plot summarising all the sensitivity and specificity data for the cystic subgroup analysis of 'cancerous versus benign lesion' studies is shown in Figure 13.
13.
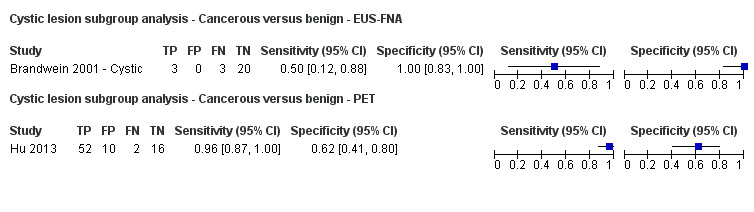
Forest plot ‐ Cystic lesion subgroup analysis: Cancerous versus benign.
Precancerous or cancerous versus benign
In the analysis assessing the ability of different imaging modalities to differentiate precancerous or cancerous versus benign lesions, we excluded a component of one study because the participants had solid pancreatic lesions (Fischer 2009 ‐ Solid). We therefore re‐performed the meta‐analysis for precancerous or cancerous versus benign lesions ‐ EUS‐FNA without these data. The remaining tests for this target condition did not have any studies excluded and were therefore not redone. The new findings are described below.
EUS‐FNA: Two studies reporting data on 34 participants were included for this test, allowing meta‐analysis to be performed (Fischer 2009 ‐ Cystic; Sedlack 2002). The summary sensitivity and summary specificity for diagnosing precancer or cancer were 0.43 (95% CI 0.19 to 0.71) and 1.00 (95% CI 0.74 to 1.00), respectively.
A forest plot summarising the sensitivity and specificity data for the cystic subgroup analysis of 'precancerous or cancerous versus benign lesion' study is shown in Figure 14.
14.

Forest plot ‐ Cystic lesion subgroup analysis: Precancerous or cancerous versus benign ‐ EUS‐FNA.
Discussion
Summary of main results
The results are summarised in Table 1.
We included 54 studies involving a total of 3196 participants that evaluated the diagnostic accuracy of various imaging modalities (EUS, EUS‐FNA, PET, CT, and MRI) for characterising focal pancreatic lesions. We identified eight different target conditions in these studies, with the studies using imaging modalities to differentiate: cancerous versus benign or precancerous lesions; cancerous versus benign lesions; precancerous or cancerous lesions versus benign lesions; cancerous (invasive carcinoma) versus precancerous (dysplasia) lesions; cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) lesions; cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia) lesions; precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) lesions; and precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) or benign lesions. The wide variety of tumour types that constituted benign and cancerous lesions within the studies meant that only a few meaningful meta‐analyses could be performed. None of the comparisons in which single studies were included were of sufficiently high methodological quality to warrant highlighting of the results.
For differentiation of cancerous lesions from benign or precancerous lesions, only single studies were included and therefore meta‐analysis was not performed. Overall, EUS‐FNA (cytology) had a sensitivity of 0.79 (95% CI 0.60 to 0.91) and specificity of 1.00 (95% CI 0.85 to 1.00); EUS‐FNA (CEA > 500 ng/mL) had a sensitivity of 0.93 (95% CI 0.70 to 0.99) and specificity of 0.33 (95% CI 0.12 to 0.65); and PET had a sensitivity of 0.85 (95% CI 0.73 to 0.92) and specificity of 0.91 (95% CI 0.72 to 0.97).
The second analysis, of studies differentiating cancerous versus benign lesions, provided three tests in which meta‐analysis could be performed, however the data were sparse: one of these tests contained three studies, and the remaining two tests contained two studies, meaning the meta‐analysis was of limited value. There was little difference in the diagnostic test accuracy between the imaging techniques. EUS‐FNA achieved very high specificity (of 1.00, i.e. no false negatives) but modest sensitivity (0.79; 95% CI 0.07 to 1.00). A high specificity of EUS‐FNA can be expected, since this involves physically sampling the lesion. However, the modest sensitivity may reflect that the sampling methods were inadequate. Additional guidance such as identifying the location most likely to yield the correct results or additional guidance using optical endoscopy techniques such as confocal laser microendoscopy may overcome this problem and improve the sensitivity of EUS (Giovannini 2012), but there are major challenges, such as knowing the area within the lesion that is being examined by confocal laser microendoscopy, that must be addressed before such methods can be used routinely.
The third analysis, of studies differentiating precancerous or cancerous and benign lesions, only provided one test (EUS‐FNA) for which meta‐analysis was performed. The results were unreliable due to significant heterogeneity in the results between the studies.
The fourth analysis, of studies differentiating cancerous (invasive carcinoma) versus precancerous (dysplasia) lesions, provided three tests in which meta‐analysis was performed, with one test containing five studies (EUS), one test containing three studies (EUS‐FNA), and the third test containing six studies (CT). All five of the tests included in the analysis had a similar level of accuracy according to their respective ROC curves. EUS and CT showed the highest (and similar) accuracy estimates (EUS = sensitivity 0.78 and specificity 0.91; CT = sensitivity 0.72 and specificity 0.92) and included the largest number of studies (five and six, respectively) among all comparisons.
The fifth analysis, of studies differentiating cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) lesions, provided six tests in which meta‐analysis was performed, with two tests containing four studies (EUS and PET SUVmax 2 to 2.5), one test containing two studies (EUS‐FNA), three tests containing three studies (EUS‐FNA > 200, CT, and MRI), and the remaining two tests providing single studies. PET performed with the highest accuracy (sensitivity 0.90 (95% CI 0.79 to 0.96) and specificity 0.94 (95% CI 0.81 to 0.99)).
The sixth analysis, of studies differentiating cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia) lesions, provided no tests in which meta‐analysis was performed.
The seventh analysis, of studies differentiating precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) lesions, provided two tests in which meta‐analysis was performed. The meta‐analysis results for CT (sensitivity 0.83 (95% CI 0.68 to 0.92) and specificity 0.83 (95% CI 0.64 to 0.93)) were similar to those of MRI (sensitivity 0.80 (95% CI 0.58 to 0.92) and specificity 0.81 (95% CI 0.53 to 0.95)), however lack of significant data means little can be inferred from this.
The eighth analysis, of studies differentiating precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) or benign lesions, provided no tests in which meta‐analysis was performed.
We performed a subgroup analysis to investigate the performance of imaging modalities for cystic pancreatic lesions. This only resulted in alterations to the 'cancerous versus benign or precancerous', 'cancerous versus benign', and 'precancerous or cancerous versus benign' groups, however when re‐performed in these groups, the analysis did not result in any significant changes.
Overall, none of the tests assessed had sufficient overall diagnostic accuracy to be considered a definitive diagnostic modality. High sensitivity of the test is required so that precancer or cancer is not missed. High specificity is required to avoid major surgery. Sensitivity and specificity in excess of 90% are required to recommend the particular modality over other modalities. Only PET in differentiating precancerous (low‐ or intermediate‐grade dysplasia) versus cancer (high‐grade dysplasia or invasive carcinoma) approaches this level of accuracy. Overall, modalities other than EUS‐FNA had moderate to high sensitivity but moderate specificity, while EUS‐FNA had high specificity with moderate sensitivity in distinguishing the nature of focal pancreatic lesions.
Strengths and weaknesses of the review
We conducted a thorough literature search and included full‐text publications and abstracts without any language restrictions. Two review authors independently identified and extracted data from the studies, potentially reducing the chance of error that would be associated with one person performing the data extraction. We used strict reference standards that are likely to diagnose the target condition with a high degree of accuracy. These were the major strengths of the review.
We included EUS‐FNA as part of the review. Strictly speaking, EUS‐FNA cannot be considered an imaging modality since it uses cytology criteria or levels of tumour markers in the aspirate rather than imaging features to make the diagnosis. We had mentioned at the protocol stage that we would include EUS‐FNA in this review, as the searches for EUS return EUS‐FNA as well and because EUS‐FNA along with the imaging modalities included in this review are the most widely used tests for characterising focal pancreatic lesions. Our review provides the most important information about the tests performed to characterise focal lesions in one location and hence is probably more useful for clinicians, who would otherwise have to search for another review for information on EUS‐FNA.
The major limitation in the review process was the diverse nature of the collected data, with a wide variety of definitions of benign, precancerous, and cancerous lesions. This limited the possible analysis of the data and the conclusions that could be made from our analyses. While some authors defined precancerous lesions as lesions with low‐ and intermediate‐grade dysplasia, others defined it as low‐grade dysplasia only, while yet others considered any form of dysplasia as precancerous lesions. In the comparison 'cancer versus benign', it is unclear how the study authors dealt with precancerous lesions, that is whether they included precancerous lesions in the 'cancer' group or the 'benign' group, or whether they simply excluded them, consequently undermining any conclusions that could be made for this comparison.
We could not perform a bivariate random‐effects model that takes correlation between sensitivity and specificity into account and were unable to compare the diagnostic test accuracy of index tests using formal statistical methods due to the sparseness of data for each comparison. As a result, we performed the analysis using simpler models suggested by Takwoingi 2015 and colleagues. We reported the model with the lowest ‐2 log likelihood and also visualised the forest plots and ROC plots in deciding the model to be reported. The confidence intervals were extremely wide for the following analyses: benign versus cancer: CT; benign versus cancer: EUS‐FNA; benign versus precancer or cancer: EUS‐FNA; and precancer (low‐ or intermediate‐grade dysplasia) versus cancer (high‐grade dysplasia or invasive carcinoma): CT. While fixed‐effect model provided narrower confidence intervals for some of the above analyses, such models were inappropriate for these data because of the poor overlap of confidence intervals on the forest plot. This observation (i.e. that fixed‐effect models were not appropriate) was supported by the ‐2 log likelihoods, which were higher for the univariate fixed‐effect model than those of the models presented. The alternative was not to perform a meta‐analysis at all, which is even more difficult to interpret. At least the current results allowed us to interpret that the sensitivity or specificity or both could not be estimated reliably. There was reasonable overlap of confidence intervals in the other meta‐analyses performed. With regard to the tests for which meta‐analysis could not be performed, the diagnostic test accuracy from single studies needs confirmation by other studies to assess whether the results are reproducible. Hence, we are unable to arrive at any major conclusions based on information by a single study.
A high proportion of studies were at high risk of bias and with high concern regarding applicability in all four domains of the QUADAS‐2 tool. This makes the validity of the results questionable. Of particular concern was the type of people who underwent these tests. Because of the strict but appropriate reference standard, all of the participants in all of the studies included in this review except Cherian 2010 underwent surgical resection. This suggested that the surgeons thought that these participants had high probability of having high‐grade dysplasia or cancer, either because of the results of this test or other tests performed alongside the index tests. Since most of the studies were retrospective studies, if participants were operated on on the basis of the index test, and only participants who underwent surgery were included, participants with negative index tests but who had cancer would have been excluded inappropriately. This would have resulted in overestimation of sensitivity. The studies did not report the proportion of people in whom the different tests were feasible. This is particularly important for EUS and EUS‐FNA, since the participants may have been selected to undergo EUS or EUS‐FNA based on the proximity to the stomach or duodenum. This increases the concern regarding applicability. The studies did not report the complications associated with the index test. While this is unlikely to influence the diagnostic accuracy of the index test, it may have implications in determining the balance of benefits and harms in choosing a test.
Another limitation of this review was that we have included sensitivity‐maximising diagnostic filters for searching MEDLINE and Embase databases (Haynes 2004; Wilczynski 2005), and also used terms to limit the searches in Science Citation Index. We did this because the original searches without the filters retrieved more than 60,000 references. We had to balance the possibility of missing some studies against the risk of not being able to complete the review. We decided that it is useful to have evidence from major studies rather than having no information at all. However, it must be noted that the diagnostic filters we used have a sensitivity of 98.6% for MEDLINE and 100% for Embase. Consequently, the chances that we missed some relevant diagnostic studies are extremely low. This was further reduced by performing a 'related search' and 'citing reference search', in which we found no studies that could be included in this review.
We identified six other systematic reviews on the topics included in this systematic review (Banafea 2016; Chen 2012; Fuccio 2013; Gillis 2015; Hewitt 2012; Mei 2013). These included the role of EUS‐FNA (cytology), K‐ras gene mutation analysis of FNA aspirate, and EUS elastography in focal pancreatic lesions. The diagnostic test accuracy in four of the studies showed that EUS cytology and K‐ras gene mutation analysis of FNA aspirate had a reasonably high sensitivity (0.80 to 0.86) and very high specificity (96% to 98%) in solid pancreatic lesions (Banafea 2016; Chen 2012; Fuccio 2013; Hewitt 2012). These studies accepted cytology and clinical follow‐up (without specifying the exact nature of acceptable clinical follow‐up) in addition to histopathology as reference standards (Chen 2012; Fuccio 2013; Hewitt 2012). It is likely that this methodological difference was responsible for the major differences between our observations and these systematic reviews. In addition, these systematic reviews restricted participants to those with solid pancreatic lesions (Banafea 2016; Chen 2012; Fuccio 2013; Hewitt 2012), which could be another explanation for the differences between our observations and these systematic reviews. One systematic review evaluated EUS elastography in focal pancreatic lesions and reported a high sensitivity of 0.95 and a specificity of 0.67 (Mei 2013). We did not identify any study evaluating EUS elastography that met our inclusion criteria with respect to our reference standard, therefore we are unable to comment on the observation by Mei 2013. The last systematic review evaluated the role of EUS‐FNA and EUS‐FNA molecular analysis (i.e. check for abnormal genes) in people with cystic pancreatic lesions. The authors found poor sensitivity and high specificity of EUS‐FNA, which is similar to our findings (Gillis 2015).
Applicability of findings to the review question
All studies had high applicability concerns, making the applicability of findings to the target patient population of all incidental lesions questionable. The findings are applicable only for people who are suspected to be at high risk of high‐grade dysplasia or cancer. The review question was to find out the diagnostic accuracy of these index tests in people with focal pancreatic lesions, usually detected incidentally. However, all of the studies that met the inclusion criteria for this review used surgical excision as the reference standard, suggesting that the surgeons considered these patients to have a high risk of malignancy based on the results of the index tests or any additional tests. In terms of current availability of these tests, CT scan and MRI are likely to be available in most secondary centres. EUS is likely to be available in limited secondary centres and most tertiary centres that treat pancreatic lesions. PET is likely to be available only in limited tertiary centres, although the tertiary centres are likely to have access to a PET scan. However, based on the observations in this review, there do not appear to be any major differences between the different imaging modalities. The improved sensitivity of EUS‐FNA compared to other imaging modalities is compensated by a corresponding decrease in sensitivity, consequently there do not appear to be major advantages to using EUS for characterising focal pancreatic lesions compared to other non‐invasive methodologies.
Authors' conclusions
Implications for practice.
We were unable to arrive at any firm conclusions because of the differences in the way that study authors classified focal pancreatic lesions into cancerous, precancerous, and benign lesions; the inclusion of few studies with wide confidence intervals for each comparison; poor methodological quality in the studies; and heterogeneity in the estimates within comparisons.
Implications for research.
Further studies of high methodological quality are necessary. Future research should be conducted in a prospective manner, however most importantly the definition of benign and cancerous lesions in the analysis of studies should be standardised according to World Health Organization (WHO) classification. The threshold for positivity of endoscopic ultrasound‐guided fine‐needle aspiration cancer markers should be prespecified. Future studies should avoid any inappropriate exclusions to ensure that true diagnostic accuracy can be determined. Long‐term follow‐up of participants with negative tests will help in understanding the implications of false‐negative results and will aid clinical decision‐making.
Acknowledgements
Cochrane Upper Gastrointestinal and Pancreatic Diseases and Diagnostic Test Accuracy Reviews groups for their advice in the preparation of this review.
Appendices
Appendix 1. Glossary of terms
Ablation: destruction of tissue.
Adenocarcinoma: cancer arising from cells that secrete digestive enzymes (proteins that help with the breakdown of food into simple substances that the gut can absorb).
Algorithm: order in which diagnostic tests are performed and actions taken depending upon the results of the tests (in this context).
Asymptomatic: not showing any signs of disease or illness.
Benign: non‐cancerous (in this context).
Biomarkers: substances in an organism that indicate disease or illness.
Chemotherapy: medication used to treat or control cancer (in this context).
Contraindication: something that causes a specific treatment or procedure to be withheld because it would cause harm.
Cystic: related to an abnormal enclosed sac found within the body that is filled with a fluid or semifluid substance.
Cytology: the study of cells obtained from a tissue to determine whether the cell is cancerous (in this context).
Density: the measure of how compact something is (in this context).
Diffuse: spread out.
Disseminated: spread of cancer (in this context).
Dysplasia: abnormal growth or development of cell; precancerous (in this context).
Focal: characterised as being a specific or limited area of disease (in this context).
Gastrointestinal: related to the stomach and intestines.
Histological: examination of tissues under a microscope.
Histopathological: examination of tissues under a microscope to determine the changes related to a disease or illness.
Hormone: a chemical substance secreted by the body's cells that acts on other cells of the body, stimulating them to perform their role or suppressing the functions of the cells. Hormones are generally transported in the blood or other body fluids (e.g. stomach juice) from the cell that secretes the hormones to the cell on which they act.
Immunocytochemistry: examination of tissues under a microscope using special stains that bind to specific types of cells or tissues.
Ionising radiation: radiation consisting of particles, X‐rays, or gamma rays with sufficient energy to cause ionisation in the medium through which it passes, thereby damaging cells (in this context).
Laparoscopy: a surgical procedure in which an instrument is inserted through a small incision in the abdomen to view the organs or permit a surgical procedure using small instruments.
Lesions: abnormal changes in the structure of all or part of an organ due to disease (in this context).
Malignancies: cancers.
Metastases: the spread of cancer beyond its original source.
Modality: method.
Morphological: related to structure.
Mortality: death.
Peptic: related to stomach or the upper part of the intestine.
Percutaneous: performed through the skin.
Perioperative: around the time of surgery.
Prognosis: outcomes resulting from disease or illness or related to the treatment of disease or illness.
Proteomic: related to the study of proteins.
Radiological: related to X‐rays or ultrasound.
Resection: removal of all or part of an organ.
Steatorrhoea: excessive fat in stools.
Surveillance: close observation.
Vascularity: the degree of vessels (tubes that carry blood in humans).
Appendix 2. Cochrane search strategy
#1 (pancreas OR pancreatic) #2 (CT OR tomodensitometry OR PET OR MRI OR NMRI OR zeugmatogra* OR ((computed OR computerised OR computerized OR emission OR positron OR magneti* OR MR OR NMR OR proton OR acoustic OR ARFI) AND (tomogra* OR scan OR scans OR imaging)) OR endosonogra* OR EUS OR ((echogra* OR ultrason* OR ultrasound) AND endoscop*) OR elastogr* OR sonoelastogr* OR acoustogra*) #3 #1 AND #2
Appendix 3. MEDLINE search strategy
1. exp Pancreas/
2. exp Pancreatic Neoplasms/di [Diagnosis]
3. exp Pancreatitis, Chronic/di [Diagnosis]
4. exp Pancreatic Cyst/di [Diagnosis]
5. (pancreas or pancreatic).ti,ab.
6. 1 or 2 or 3 or 4 or 5
7. (sensitiv: or diagnos:).mp. or di.fs.
8. 6 and 7
9. (CT or tomodensitometry or PET or MRI or NMRI or zeugmatogra* or ((computed or computerised or computerized or emission or positron or magneti* or MR or NMR or proton or acoustic or ARFI) and (tomogra* or scan or scans or imaging))).ti,ab.
10. exp Tomography, X‐Ray Computed/ or Positron‐Emission Tomography/ or exp Magnetic Resonance Imaging/
11. 9 or 10
12. exp Endosonography/
13. (endosonogra* or EUS).ti,ab.
14. 12 or 13
15. exp Ultrasonography/
16. (echogra* or ultrason* or ultrasound).ti,ab.
17. 15 or 16
18. exp Endoscopy, Gastrointestinal/
19. endoscop*.ti,ab.
20. 18 or 19
21. 17 and 20
22. 14 or 21
23. exp Elasticity Imaging Techniques/
24. (elastogr* or sonoelastogr* or acoustogra*).ti,ab.
25. 23 or 24
26. 11 or 22 or 25
27. 8 and 26
Appendix 4. Embase search strategy
1. exp pancreas/
2. exp pancreas tumor/di [Diagnosis]
3. exp chronic pancreatitis/di [Diagnosis]
4. exp pancreas cyst/di [Diagnosis]
5. (pancreas or pancreatic).ti,ab.
6. 1 or 2 or 3 or 4 or 5
7. sensitiv:.tw. or diagnostic accuracy.sh. or diagnostic.tw.
8. 6 and 7
9. (CT or tomodensitometry or PET or MRI or NMRI or zeugmatogra* or ((computed or computerised or computerized or emission or positron or magneti* or MR or NMR or proton or acoustic or ARFI) and (tomogra* or scan or scans or imaging))).ti,ab.
10. exp computer assisted tomography/ or positron emission tomography/ or exp nuclear magnetic resonance imaging/
11. 9 or 10
12. endoscopic echography/
13. (endosonogra* or EUS).ti,ab.
14. 12 or 13
15. exp ultrasound/
16. (echogra* or ultrason* or ultrasound).ti,ab.
17. 15 or 16
18. exp gastrointestinal endoscopy/
19. endoscop*.ti,ab.
20. 18 or 19
21. 17 and 20
22. 14 or 21
23. exp elastography/
24. (elastogr* or sonoelastogr* or acoustogra*).ti,ab.
25. 23 or 24
26. 11 or 22 or 25
27. 8 and 26
Appendix 5. Science Citation Index Expanded search strategy
#1 TS=(pancreas OR pancreatic) #2 TS=(CT OR tomodensitometry OR PET OR MRI OR NMRI OR zeugmatogra* OR ((computed OR computerised OR computerized OR emission OR positron OR magneti* OR MR OR NMR OR proton OR acoustic OR ARFI) AND (tomogra* OR scan OR scans OR imaging)) OR endosonogra* OR EUS OR ((echogra* OR ultrason* OR ultrasound) AND endoscop*) OR elastogr* OR sonoelastogr* OR acoustogra*) #3 TS=(sensitiv* or "predictive value" or diagnostic or accuracy) #4 #1 AND #2 AND #3
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.

Cancerous versus benign or precancerous ‐ EUS‐FNA (cytology).
2. Test.

Cancerous versus benign or precancerous ‐ EUS‐FNA (CEA > 500 ng/mL).
3. Test.

Cancerous versus benign or precancerous ‐ PET.
4. Test.
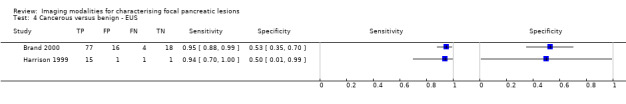
Cancerous versus benign ‐ EUS.
5. Test.
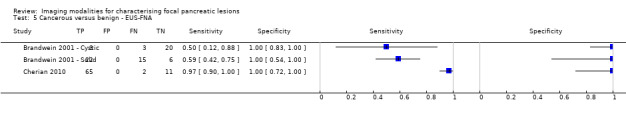
Cancerous versus benign ‐ EUS‐FNA.
6. Test.
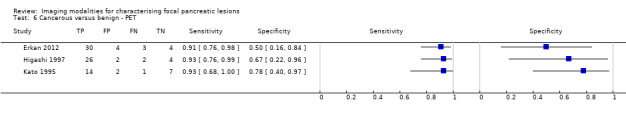
Cancerous versus benign ‐ PET.
7. Test.

Cancerous versus benign ‐ PET (SUVmax > 3.5).
8. Test.
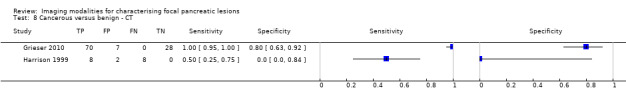
Cancerous versus benign ‐ CT.
9. Test.

Cancerous versus benign ‐ MRI.
10. Test.

Precancerous or cancerous versus benign ‐ EUS.
11. Test.
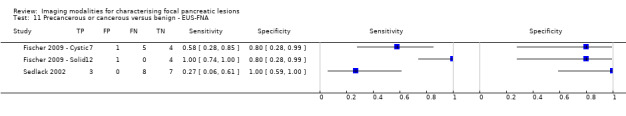
Precancerous or cancerous versus benign ‐ EUS‐FNA.
12. Test.
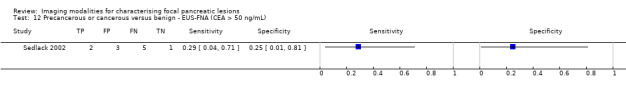
Precancerous or cancerous versus benign ‐ EUS‐FNA (CEA > 50 ng/mL).
13. Test.

Precancerous or cancerous versus benign ‐ PET (SUVmax > 2.4).
14. Test.

Precancerous or cancerous versus benign ‐ CT.
15. Test.

Precancerous or cancerous versus benign ‐ MRI.
16. Test.
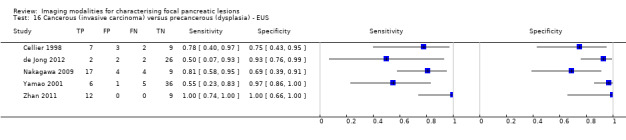
Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS.
17. Test.
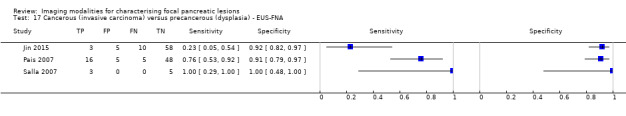
Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS‐FNA.
18. Test.

Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS‐FNA (CEA > 200 ng/mL).
19. Test.
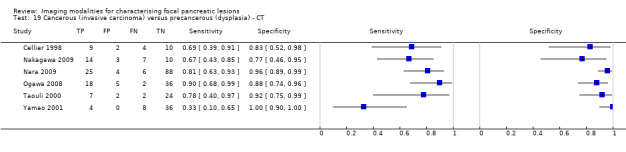
Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ CT.
20. Test.
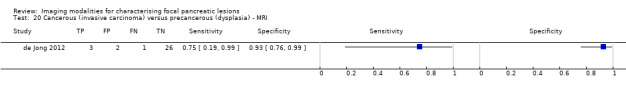
Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ MRI.
21. Test.

Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) ‐ EUS.
22. Test.

Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) ‐ EUS‐FNA.
23. Test.
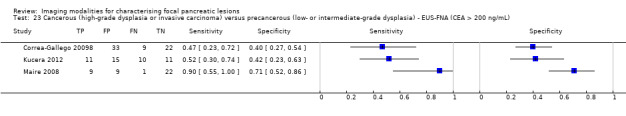
Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) ‐ EUS‐FNA (CEA > 200 ng/mL).
24. Test.

Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) ‐ EUS‐FNA (CA 19‐9 > 1000 U/mL).
25. Test.

Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) ‐ EUS‐FNA (CEA > 692.8 ng/mL).
26. Test.

Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) ‐ PET (SUVmax 2 to 2.5).
27. Test.
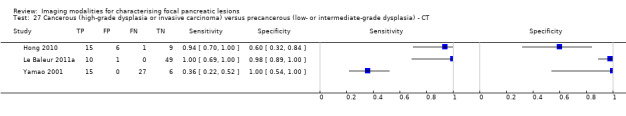
Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) ‐ CT.
28. Test.

Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) ‐ MRI.
29. Test.

Cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia) ‐ EUS.
30. Test.
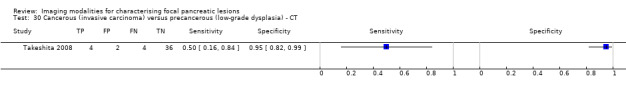
Cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia) ‐ CT.
31. Test.
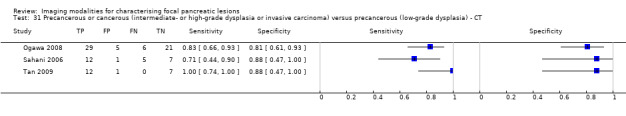
Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) ‐ CT.
32. Test.
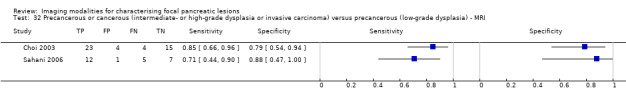
Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) ‐ MRI.
33. Test.

Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) or benign ‐ EUS.
34. Test.
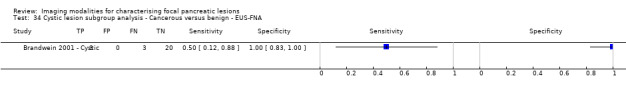
Cystic lesion subgroup analysis ‐ Cancerous versus benign ‐ EUS‐FNA.
35. Test.

Cystic lesion subgroup analysis ‐ Cancerous versus benign ‐ PET.
36. Test.
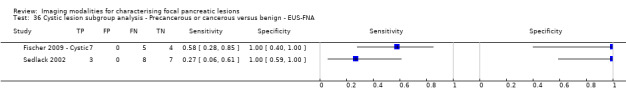
Cystic lesion subgroup analysis ‐ Precancerous or cancerous versus benign ‐ EUS‐FNA.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brand 2000.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 179. Females: 47 (26.3%). Age: 61 years. Presentation: Patients with pancreatic lesions who had undergone EUS and surgical resection with histological confirmation. Setting: secondary care, Germany. | ||
| Index tests | Index test: EUS. Further details: Technical specifications: Olympus GF‐UM 3, GF‐UM 20, and GF‐UM 200. Performed by: gastroenterologist. Criteria for positive diagnosis: a mass lesion with irregular borders, non‐homogeneous echotexture, and/or loss of vascular interface or obvious vascular involvement, without any signs of chronic pancreatitis in the lesion or the rest of the gland. However, in the presence of obvious chronic pancreatitis, an associated malignancy was suspected if the EUS morphology of the focal lesion suggested involvement of the adjacent structures. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 64 (35.8%). | ||
| Comparative | |||
| Notes | Possible overlap with Binmoeller 1998a and Binmoeller 1998b; out of 179 patients, only 115 patients with histologically confirmed diagnosis were included. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Brandwein 2001 ‐ Cystic.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 26. Females: not stated. Age: not stated. Presentation: Patients with cystic and solid pancreatic lesions who had undergone surgical resection; only patients with cystic lesions included in our analysis. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: Pentax echoendoscope (model not stated); 22‐gauge needle. Performed by: endoscopist and cytologist. Criteria for positive diagnosis: a malignant mass was defined as a focal hypoechoic heterogeneous lesion within the pancreatic parenchyma and cytology reported stated malignancy. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cystic lesion subgroup analysis ‐ Cancerous versus benign ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Brandwein 2001 ‐ Solid.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 43. Females: not stated. Age: not stated. Presentation: Patients with cystic and solid pancreatic lesions who had undergone surgical resection. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: Pentax echoendoscope (model not stated); 22‐gauge needle. Performed by: endoscopist and cytologist. Criteria for positive diagnosis: a malignant mass was defined as a focal hypoechoic heterogeneous lesion within the pancreatic parenchyma and cytology reported stated malignancy. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Cellier 1998.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 46. Females: not stated. Age: not stated. Presentation: Patients with IPMN undergoing surgery. Setting: secondary care, France. | ||
| Index tests | Index test: EUS.
Further details:
Technical specifications: Olympus GFUM3 or GF UM20.
Performed by: endoscopist.
Criteria for positive diagnosis:
Index test: CT. Further details: Technical specifications: conventional CT (further details not available). Performed by: not stated. Criteria for positive diagnosis:
|
||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 22 (46.8%). | ||
| Comparative | |||
| Notes | A number of patients were excluded from the analysis. The reasons were not reported. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Cherian 2010.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 78. Females: not stated. Age: not stated. Presentation:
Setting: secondary care, UK. |
||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: Olympus GF‐UCT240‐AL5. Performed by: endoscopist and cytologist. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: benign versus malignant. Reference standard: surgical excision and histology in people who had undergone surgery and clinical follow‐up, defined as serial imaging at 12 months that demonstrated progression of disease or patients had clinical deterioration or death. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Choi 2003.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 64. Females: 14 (21.9%). Age: 61 years. Presentation: Patients with IPMN undergoing surgical resection. Setting: secondary care, Korea. | ||
| Index tests | Index test: MRI. Further details: Technical specifications: 1.5‐T MR system (Magnetom Vision; Siemens, Erlangen, Germany). Performed by: radiologist. Criteria for positive diagnosis: presence of mural nodules. | ||
| Target condition and reference standard(s) | Target condition: precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: 0 (0%). Number of patients who were excluded from the analysis: 18 (28.1%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test Precancerous or cancerous (intermediate or high grade dysplasia or invasive carcinoma) versus precancerous (low grade dysplasia) ‐ MRI | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Correa‐Gallego 2009.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 72. Females: not stated. Age: not stated. Presentation: Patients with IPMN who had undergone surgical resection. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: cyst CEA fluid >= 200 ng/mL. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS FNA (CEA > 200 ng/ml) | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
de Jong 2012.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 32. Females: 19 (59.4%). Age: 62 years. Presentation: Inclusion criteria
Exclusion criteria
Setting: secondary care, Netherlands. |
||
| Index tests | Index test: EUS.
Further details:
Technical specifications: Olympus GF‐UC(T)140(P).
Performed by: endoscopists.
Criteria for positive diagnosis: diffuse main duct dilatation (> 10 mm), and/or mural nodes were present, and/or a solid component was seen outside the cyst. Index test: MRI. Further details: Technical specifications: Avanto 1.5 Tesla MR. Performed by: radiologist. Criteria for positive diagnosis: diffuse main duct dilatation (> 10 mm), and/or mural nodes were present, and/or a solid component was seen outside the cyst. |
||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Interval between index test and reference standard varied, with a median of 78 days. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ MRI | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Doi 2002.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 38. Females: 12 (31.6%). Age: 60 years. Presentation: Patients with IPMN who had undergone a pancreatic resection. Setting: secondary care, Japan. | ||
| Index tests | Index test: EUS. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: presence of mural nodule or papillary projection. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Out of 38 participants included in the study, only 28 underwent EUS. We obtained diagnostic accuracy information from the discussion. The tables provide information on the number of participants who underwent EUS and the diagnostic accuracy of EUS in identifying the presence of the lesion. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Erkan 2012.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 46. Females: not stated. Age: not stated. Presentation: Patients undergoing PET/CT scan for suspected pancreatic lesions and surgical resection. Setting: secondary care, Germany. | ||
| Index tests | Index test: PET. Further details: Technical specifications: model and manufacturer not stated. Performed by: radiologist. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 5 (10.9%). | ||
| Comparative | |||
| Notes | FLT‐PET was also available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ PET | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Fischer 2009 ‐ Cystic.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 33. Females: not stated. Age: not stated. Presentation: Patients with pancreatic lesions undergoing EUS‐FNA. Setting: secondary care, country not stated. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: precancerous or cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Precancerous or cancerous versus benign ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cystic lesion subgroup analysis ‐ Precancerous or cancerous versus benign ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Fischer 2009 ‐ Solid.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 33. Females: not stated. Age: not stated. Presentation: Patients with pancreatic lesions undergoing EUS‐FNA. Setting: secondary care, country not stated. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: precancerous or cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Precancerous or cancerous versus benign ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Fisher 2008.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 48. Females: 33 (68.8%). Age: 60 years. Presentation: Inclusion criteria
Exclusion criteria
Setting: secondary care, USA. |
||
| Index tests | Index test: CT. Further details: Technical specifications: not stated. Performed by: radiologist. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: precancerous or cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Precancerous or cancerous versus benign ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Grieser 2010.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 105. Females: 32 (30.5%). Age: 58 years. Presentation: Patients undergoing surgical exploration or resection for pancreatic mass and CT scan. Setting: secondary care, Germany. | ||
| Index tests | Index test: CT. Further details: Technical specifications: Siemens Somatom Plus 4; GE Healthcare LightSpeed Ultra, LightSpeed 16/Pro16, LightSpeed VCT. Performed by: radiologist. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign. Reference standard: surgical excision or biopsy during exploratory laparotomy for non‐resectable cancers. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Another radiologist has a lower specificity. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ CT | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Harrison 1999.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 18. Females: 10 (55.6%). Age: 62 years. Presentation: Patients undergoing surgery for suspected pancreatic cancer. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS.
Further details:
Technical specifications: Olympus UM20.
Performed by: endoscopist.
Criteria for positive diagnosis: not stated. Index test: CT. Further details: Technical specifications: not stated. Performed by: endoscopist. Criteria for positive diagnosis: not stated. |
||
| Target condition and reference standard(s) | Target condition: cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Higashi 1997.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 34. Females: 16 (47.1%). Age: 61 years. Presentation: Patients with suspected pancreatic tumours undergoing PET and surgery. Setting: secondary care, Japan. | ||
| Index tests | Index test: PET. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ PET | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Hong 2010.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 31. Females: 16 (51.6%). Age: 65 years. Presentation: Patients with IPMN who had undergone CT/PET. Setting: secondary care, Korea. | ||
| Index tests | Index test: PET.
Further details:
Technical specifications: DSTe (GE Healthcare).
Performed by: radiologist.
Criteria for positive diagnosis: SUVmax > 2.5. Index test: CT. Further details: Technical specifications: LightSpeed Plus (GE Healthcare) or Somatom Sensation 64 (Siemens Healthcare). Performed by: radiologist. Criteria for positive diagnosis:
|
||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision, open laparotomy biopsy or biopsy of metastases. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ PET (SUV max 2‐2.5) | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
Hu 2013.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 80. Females: 36 (45.0%). Age: 57 years. Presentation: Inclusion criteria
Exclusion criteria
Setting: secondary care, China. |
||
| Index tests | Index test: PET. Further details: Technical specifications: Biograph 16 HR PET/CT scanner (Siemens). Performed by: radiologist. Criteria for positive diagnosis: SUVmax > 3.5. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cystic lesion subgroup analysis ‐ Cancerous versus benign ‐ PET | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ PET (SUV max > 3.5) | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Jafarimehr 2010.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 76. Females: 40 (52.6%). Age: not stated. Presentation: Patients with pancreatic lesions with PET or PET/CT. Setting: secondary care, USA. | ||
| Index tests | Index test: PET. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign or precancerous. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous versus benign or precancerous ‐ PET | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Jang 2014a.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 34. Females: 21 (61.8%). Age: 52 years. Presentation: Patients with neuroendocrine pancreatic lesions who had undergone MRI and surgery. Setting: secondary care, Korea. | ||
| Index tests | Index test: MRI. Further details: Technical specifications: Intera Achieva 3.0‐T. Performed by: radiologist. Criteria for positive diagnosis: apparent diffusion coefficient: 1.09 x 103 mm2/s. | ||
| Target condition and reference standard(s) | Target condition: precancerous or cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 7 (20.6%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Precancerous or cancerous versus benign ‐ MRI | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Jang 2014b.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 65. Females: 27 (41.5%). Age: not stated. Presentation: Patients with IPMN undergoing MRI and surgery. Setting: secondary care, Korea. | ||
| Index tests | Index test: MRI. Further details: Technical specifications: Intera Achieva 3.0‐T. Performed by: radiologist. Criteria for positive diagnosis: signal intensity of normal pancreatic parenchyma at the mural nodule, septum, cystic wall, ductal wall, and solid lesion of the IPMNs. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 4 (6.2%). | ||
| Comparative | |||
| Notes | A second observer with 1 more false positive (with correspondingly 1 less true negative) was also available. The sensitivity and specificity are for combined conventional‐ and diffusion‐weighted scan. The accuracy was lower with conventional scan. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ MRI | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Jin 2013a.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 162. Females: 99 (61.1%). Age: 64 years. Presentation: Patients with pancreatic cysts who underwent surgery. Setting: secondary care, further details not available. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: model and manufacturer not stated. Performed by: endoscopist. Criteria for positive diagnosis: cellular atypia. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Jin 2015.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 86. Females: not stated. Age: not stated. Presentation: Patients with mucinous pancreatic cysts undergoing operative resection. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: EUS model not stated; 22‐gauge needle. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: 1 (1.3%). Number of patients who were excluded from the analysis: 9 (10.6%). | ||
| Comparative | |||
| Notes | Results were reported for only 76 out of 77 participants with mucinous cysts. The final results were possible only for these participants. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Kalha 2003.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 24. Females: not stated. Age: not stated. Presentation: Patients undergoing EUS‐FNA and surgery for cystic pancreatic lesions. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: EUS model or needle size not stated. Performed by: not stated. Criteria for positive diagnosis: cyst CEA fluid >= 500 ng/mL. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign or precancerous. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Of 84 participants, 60 who were observed were excluded because the reference standard was not adequate for these participants. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous versus benign or precancerous ‐ EUS FNA (cytology) | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Kamata 2016a.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 70. Females: 39 (55.7%). Age: 62 years. Presentation: Inclusion criteria
Exclusion criteria
Setting: secondary care, Japan. |
||
| Index tests | Index test: EUS. Further details: Technical specifications: GF‐UCT260; Olympus Medical Systems, Tokyo, Japan. Performed by: endoscopist. Criteria for positive diagnosis: presence of mural nodules. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) or benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: 0 (0%). Number of patients who were excluded from the analysis: 419 (85.7%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) or benign ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Kato 1995.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 24. Females: not stated. Age: not stated. Presentation: Patients with pancreatic masses. Setting: secondary care, Japan. | ||
| Index tests | Index test: PET. Further details: Technical specifications: HEADTOME‐IV (Shimadzu Corporation). Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign. Reference standard: surgical excision, open laparotomy biopsy or clinical follow‐up for at least 3 years. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ PET | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
Kim 2015.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 123. Females: not stated. Age: not stated. Presentation: Patients with surgically proven and histopathologically confirmed IPMN and who had undergone MRI examinations with diffusion‐weighted imaging before surgery. Setting: secondary care, Korea. | ||
| Index tests | Index test: MRI. Further details: Technical specifications: Verio or Trio (Siemens Medical Solutions), Signa HDTx (GE Medical Systems), Achieva (Philips Healthcare). Performed by: radiologist. Criteria for positive diagnosis: signal intensity of normal pancreatic parenchyma at the mural nodule, septum, cystic wall, ductal wall, and solid lesion of the IPMNs. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 30 (24.4%). | ||
| Comparative | |||
| Notes | 25 participants were excluded due to lack of diffusion‐weighed MRI or subquality MRI. The sensitivities and specificities reported by other radiologists were lower. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ MRI | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Klau 2011.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 29. Females: 10 (34.5%). Age: 61 years. Presentation: Patients with solid focal pancreatic lesions who had undergone surgery. Setting: secondary care, Germany. | ||
| Index tests | Index test: MRI. Further details: Technical specifications: 1.5 T Magnetom Avanto, Siemens. Performed by: radiologist. Criteria for positive diagnosis: perfusion fraction < 0.1105. | ||
| Target condition and reference standard(s) | Target condition: cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Sensitivities and specificities for other cut‐off values were lower. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Cancerous versus benign ‐ MRI | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Kobayashi 2012.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 36. Females: 15 (41.7%). Age: 66 years. Presentation: Patients with IPMN who had undergone surgical resection. Setting: secondary care, Japan. | ||
| Index tests | Index test: EUS. Further details: Technical specifications: UM20, UM2000; Olympus. Performed by: endoscopists. Criteria for positive diagnosis: lateral spread of the nodule > 15 mm. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Of 238 participants with IPMN, only 46 who had undergone surgical resection were included in the analysis; another criterion for diagnosis with lower diagnostic test accuracy was also available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Kubo 2001.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 51. Females: 19 (37.3%). Age: 67 years. Presentation: Patients with IPMN who had undergone surgical resection. Setting: secondary care, Japan. | ||
| Index tests | Index test: EUS.
Further details:
Technical specifications: GF‐UM2, UM3, UM20; Olympus.
Performed by: endoscopists.
Criteria for positive diagnosis:
|
||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (low grade dysplasia) ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Kucera 2012.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 47. Females: 15 (31.9%). Age: 66 years. Presentation: Patients with IPMN who had undergone EUS‐FNA with cyst fluid analysis and surgical resection. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: GF‐UC30P and GF‐UC140P, Olympus. Performed by: endoscopist and cytologist. Criteria for positive diagnosis: CEA > 200 ng/mL. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Of 87 participants who had undergone surgical resection for IPMN, 40 were excluded because they had not undergone EUS‐FNA. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS FNA (CEA > 200 ng/ml) | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Le Baleur 2011a.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 60. Females: 59 (98.3%). Age: 43 years. Presentation: Patients with MCN who had undergone surgical resection. Setting: secondary care, France. | ||
| Index tests | Index test: CT. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: presence of mural nodule. | ||
| Target condition and reference standard(s) | Target condition: pre‐malignant (low‐ or intermediate‐grade dysplasia) versus malignant (high‐grade dysplasia or invasive carcinoma). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Sensitivity and specificity for other parameters related to size of tumour were available and were lower. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Lee 2014.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 84. Females: 29 (34.5%). Age: 65 years. Presentation: Patients with branch duct IPMN who had undergone surgical resection. Setting: secondary care, Korea. | ||
| Index tests | Index test: EUS. Further details: Technical specifications: not stated. Performed by: endoscopists. Criteria for positive diagnosis: an EUS score composed of cyst size, mural nodule height, associated main pancreatic duct dilation, thick septum, and patulous papilla >= 7. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
Maire 2008.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 41. Females: 27 (65.9%). Age: 64 years. Presentation: Patients with IPMN who had undergone surgical resection. Setting: secondary care, France. | ||
| Index tests | Index test: EUS‐FNA.
Further details:
Technical specifications: Pentax‐FG 32 UA 120°.
Performed by: endoscopists.
Criteria for positive diagnosis: CEA > 200 ng/mL. Second criteria for positive diagnosis: carbohydrate antigen 19‐9 > 1000 U/mL. |
||
| Target condition and reference standard(s) | Target conditions:
Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. |
||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | 6 different criteria for diagnosis were used. All 6 are listed. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS FNA (CEA > 200 ng/ml) | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS FNA (CEA > 200 ng/ml) | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS FNA (Ca 19.9 > 1000 U/ml) | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
McHenry 2002.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 45. Females: not stated. Age: not stated. Presentation: Patients with cystic pancreatic lesion who had undergone surgical resection. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: Pentax echoendoscope. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: precancerous or cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cystic lesion subgroup analysis ‐ Precancerous or cancerous versus benign ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Nakagawa 2009.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 34. Females: not stated. Age: not stated. Presentation: Patients with cystic pancreatic lesion who had undergone surgical resection and EUS. Setting: secondary care, Japan. | ||
| Index tests | Index test: EUS.
Further details:
Technical specifications: GF‐UMP230 or GF‐UC2000P (Olympus).
Performed by: endoscopists.
Criteria for positive diagnosis: height of protruding lesion > 4.1 mm. Index test: CT. Further details: Technical specifications: GE LightSpeed Ultra, GE LightSpeed 16; GE Medical Systems. Performed by: radiologists. Criteria for positive diagnosis: height of protruding lesion > 4.1 mm. |
||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Nara 2009.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 123. Females: 53 (43.1%). Age: 65 years. Presentation: Patients with IPMN who had undergone surgical resection. Setting: secondary care, Japan. | ||
| Index tests | Index test: CT. Further details: Technical specifications: single‐slice helical CT or MDCT. Performed by: radiologist. Criteria for positive diagnosis: irregularly shaped hypoattenuating solid mass detected adjacent to or surrounding an IPMN on contrast‐enhanced CT. | ||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Ogawa 2008.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 64. Females: 20 (31.3%). Age: 65 years. Presentation: Patients with surgically proven and histopathologically confirmed IPMN. Setting: secondary care, Japan. | ||
| Index tests | Index test: CT. Further details: Technical specifications: Aquilion; Toshiba. Performed by: radiologist. Criteria for positive diagnosis: main pancreatic duct ‐ the maximum diameter, the presence of a septum, and the presence of a mural nodule and its maximum size (length of major axis); the type (unilocular or multilocular) of lesion, the maximum size of the lesion, the presence of wall thickness. | ||
| Target condition and reference standard(s) | Target conditions:
Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. |
||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 5 (7.8%). | ||
| Comparative | |||
| Notes | Only analysis at lesion level was available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Precancerous or cancerous (intermediate or high grade dysplasia or invasive carcinoma) versus precancerous (low grade dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Ogawa 2014.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 38. Females: 12 (31.6%). Age: 68 years. Presentation: Patients with IPMN undergoing surgery and MRI. Setting: secondary care, Japan. | ||
| Index tests | Index test: MRI. Further details: Technical specifications: EXCELART Vantage, Toshiba. Performed by: radiologist. Criteria for positive diagnosis: presence of positive signal in diffusion‐weighted imaging. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 3 (7.9%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ MRI | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Otomi 2014.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 32. Females: 17 (53.1%). Age: 63 years. Presentation: Patients with pancreatic lesions undergoing PET/CT prior to surgery other than pancreatic adenocarcinoma. Setting: secondary care, Japan. | ||
| Index tests | Index test: PET. Further details: Technical specifications: F100 & CYPRIS (Sumitomo Heavy Industries) and Aquido (Toshiba) CT scanner. Performed by: radiologist. Criteria for positive diagnosis: SUVmax > 2.4. | ||
| Target condition and reference standard(s) | Target condition: precancerous or cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Sensitivity and specificity for other parameters such as visualisation and SUVmean were available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Precancerous or cancerous versus benign ‐ PET (SUV max > 2.4) | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Pais 2007.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 74. Females: 36 (48.6%). Age: 65 years. Presentation: Patients with IPMN undergoing surgery and EUS‐FNA. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: Olympus GF‐UM20, GF‐UM130, or GF‐UM160; 22‐gauge needle. Performed by: endoscopist and cytologist. Criteria for positive diagnosis: presence of hyperchromasia, nuclear crowding, and loss of nuclear uniformity, nucleolar prominence, or chromatin abnormalities. | ||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Sahani 2006.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 25. Females: 12 (48.0%). Age: 69 years. Presentation: Patients with IPMN undergoing surgery. Setting: secondary care, USA. | ||
| Index tests | Index test: CT.
Further details:
Technical specifications: LightSpeed QX/I (GE Medical Systems).
Performed by: radiologist.
Criteria for positive diagnosis: presence of mural nodules, papillary projections, or a solid mass in the dilated duct or within the cystic lesion. Index test: MRI. Further details: Technical specifications: 1.5‐T system Signa (GE Medical Systems). Performed by: radiologist. Criteria for positive diagnosis: presence of mural nodules, papillary projections, or a solid mass in the dilated duct or within the cystic lesion. |
||
| Target condition and reference standard(s) | Target condition: precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Precancerous or cancerous (intermediate or high grade dysplasia or invasive carcinoma) versus precancerous (low grade dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Precancerous or cancerous (intermediate or high grade dysplasia or invasive carcinoma) versus precancerous (low grade dysplasia) ‐ MRI | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Saito 2013.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 48. Females: 16 (33.3%). Age: 69 years. Presentation: Patients with IPMN who had undergone surgical resection. Setting: secondary care, Japan. | ||
| Index tests | Index test: PET. Further details: Technical specifications: Aquiduo (Toshiba Medical Systems), Advance NXi (GE Healthcare), and Discovery ST (GE Healthcare). Performed by: radiologist. Criteria for positive diagnosis: SUVmax > 2 and retention index < ‐10. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Sensitivity and specificity for SUVmax > 2 are also available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ PET (SUV max 2‐2.5) | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Salla 2007.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 8. Females: 3 (37.5%). Age: 63 years. Presentation: Patients with IPMN who had undergone surgical resection. Setting: secondary care, Greece. | ||
| Index tests | Index test: EUS‐FNA. Further details: Technical specifications: equipment not stated; 22‐gauge needle. Performed by: endoscopist. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Sedlack 2002.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 34. Females: 18 (52.9%). Age: 55 years. Presentation: Patients with cystic lesions of pancreas who had undergone EUS and surgical resection. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS. Further details: Technical specifications: GFU‐130, Olympus. Performed by: endoscopist. Criteria for positive diagnosis: If 1 or more of the following EUS criteria were met.
Index test: EUS‐FNA. Further details: Technical specifications: GFUC‐30P, Olympus; 22‐gauge needle. Performed by: endoscopist. Criteria for positive diagnosis: CEA >= 50 ng/mL. Second criteria for positive diagnosis: not stated. |
||
| Target condition and reference standard(s) | Target condition: precancerous or cancerous versus benign. Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Precancerous or cancerous versus benign ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Precancerous or cancerous versus benign ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Precancerous or cancerous versus benign ‐ EUS FNA (CEA > 50 ng/ml) | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cystic lesion subgroup analysis ‐ Precancerous or cancerous versus benign ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Smith 2016.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 138. Females: 99 (71.7%). Age: 62 years. Presentation: Patients with IPMN or MCN. Setting: secondary care, USA. | ||
| Index tests | Index test: EUS‐FNA.
Further details:
Technical specifications: not stated.
Performed by: not stated.
Criteria for positive diagnosis: high‐grade atypia or worse. Second criteria for positive diagnosis: abnormal cytology. |
||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: 0 (0%). Number of patients who were excluded from the analysis: 11 (8%). | ||
| Comparative | |||
| Notes | Diagnostic accuracy was also available for another threshold (abnormal cytology) with lower diagnostic accuracy. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Takanami 2011.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 59. Females: 3 (5.1%). Age: 66 years. Presentation: Patients with IPMN with mural nodules who had undergone PET/CT and surgical resection. Setting: secondary care, Japan. | ||
| Index tests | Index test: PET. Further details: Technical specifications: Biograph LSO DUO PET/CT scanner, Siemens. Performed by: radiologist. Criteria for positive diagnosis: SUVmax > 2.3. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 43 (72.9%). | ||
| Comparative | |||
| Notes | Only 16 of 43 people with IPMN were included in the analysis. Sensitivity was also available for SUVmax 2.0 and 2.5. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ PET (SUV max 2‐2.5) | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Takeshita 2008.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 53. Females: 25 (47.2%). Age: 65 years. Presentation: Patients with IPMN who had undergone surgery. Setting: secondary care, Japan. | ||
| Index tests | Index test: CT. Further details: Technical specifications: LightSpeed QX/I; GE Medical Systems. Performed by: radiologist. Criteria for positive diagnosis: presence of mural nodule and main ductal dilatation (> 5 mm) or presence of mural nodule and cystic tumour size > 3 cm. | ||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 7 (13.2%). | ||
| Comparative | |||
| Notes | Sensitivity and specificity for other parameters were available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (low grade dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Tan 2009.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 20. Females: 9 (45.0%). Age: 62 years. Presentation: Patients with IPMN who had undergone surgical resection. Setting: secondary care, China. | ||
| Index tests | Index test: CT. Further details: Technical specifications: LightSpeed QX/I or LightSpeed 16; GE Medical Systems. Performed by: radiologist. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Precancerous or cancerous (intermediate or high grade dysplasia or invasive carcinoma) versus precancerous (low grade dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Taouli 2000.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 36. Females: 17 (47.2%). Age: 61 years. Presentation: Patients with IPMN who had undergone surgical resection. Setting: secondary care, France. | ||
| Index tests | Index test: CT. Further details: Technical specifications: Elscint CT Twin; Elscint. Performed by: radiologist. Criteria for positive diagnosis: dilatation of MPD > 10 mm. | ||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Sensitivity and specificity for other parameters were available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Tomimaru 2010.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 29. Females: 13 (44.8%). Age: 65 years. Presentation: Patients with IPMN who had undergone surgical resection. Setting: secondary care, Japan. | ||
| Index tests | Index test: PET. Further details: Technical specifications: Headtome/Set 2400W; Shimadzu Corporation. Performed by: radiologist. Criteria for positive diagnosis: SUVmax > 2.5. | ||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ PET (SUV max 2‐2.5) | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Yamao 2001.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 49. Females: 18 (36.7%). Age: 63 years. Presentation: Patients with IPMN undergoing surgical resection. Setting: secondary care, Japan. | ||
| Index tests | Index test: CT.
Further details:
Technical specifications: CT9200 (Yokogawa), HiSpeed Advantage (GE).
Performed by: radiologist.
Criteria for positive diagnosis: wall‐thickening, presence of nodule, and heterogenous pattern. Index test: EUS. Further details: Technical specifications: JF‐UM20 and GF‐UM240 (Olympus). Performed by: endoscopist. Criteria for positive diagnosis: wall‐thickening, presence of nodule, and heterogenous pattern. |
||
| Target condition and reference standard(s) | Target conditions:
Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. |
||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: 1 (2%). Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | The study reported 3 x 3 table for CT scan and EUS. 1 patient was excluded from analysis, but this differed between CT and EUS. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ CT | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Zhan 2011.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 21. Females: 3 (14.3%). Age: not stated. Presentation: Patients with MCN undergoing operative resection. Setting: secondary care, China. | ||
| Index tests | Index test: EUS. Further details: Technical specifications: model and manufacturer not stated. Performed by: endoscopist. Criteria for positive diagnosis: different criteria were reported for IPMN and MCN without any information on how these were distinguished prior to FNA. | ||
| Target condition and reference standard(s) | Target condition: cancerous (invasive carcinoma) versus precancerous (dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Other criteria with lower sensitivity and specificity were available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous (invasive carcinoma) versus precancerous (dysplasia) ‐ EUS | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Zhan 2013.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 20. Females: 6 (30.0%). Age: 59 years. Presentation: Patients with MCN undergoing operative resection. Setting: secondary care, China. | ||
| Index tests | Index test: EUS‐FNA.
Further details:
Technical specifications: GF‐UCT‐2000‐OL5 (Olympus).
Performed by: endoscopist.
Criteria for positive diagnosis: cytology Second criteria for positive diagnosis: CEA > 692.8 ng/mL. |
||
| Target condition and reference standard(s) | Target condition: cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia). Reference standard: surgical excision. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS FNA | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Cancerous (high grade dysplasia or invasive carcinoma) versus precancerous (low or intermediate grade dysplasia) ‐ EUS FNA (CEA > 692.8 ng/ml) | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
CEA: carcinoembryonic antigen CT: computed tomography EUS: endoscopic ultrasound FNA: fine‐needle aspiration IPMN: intraductal pancreatic mucinous neoplasm MCN: mucinous cystic neoplasm MDCT: multidetector computed tomography MPD: main pancreatic duct MRI: magnetic resonance imaging PET: positron emission tomography SUV: standard uptake value
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aburime 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Adamek 2000 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Adimoolam 2011 | There was no comparison of whether cancer was present or not. |
| Afify 2003 | There was no comparison of whether cancer was present or not. |
| Agarwal 2004 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Agarwal 2008a | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Agarwal 2008b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Agarwal 2008c | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Aguilar‐Saavedra 2011 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Ahmad 2001 | There was no comparison of whether cancer was present or not. |
| Ahmad 2003 | There was no comparison of whether cancer was present or not. |
| Ainsworth 2010 | There was no comparison of whether cancer was present or not. |
| Aithal 2001 | There was no comparison of whether cancer was present or not. |
| Aithal 2002 | There was no comparison of whether cancer was present or not. |
| Akahoshi 1998 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Akwei 2011 | There was no comparison of whether cancer was present or not. |
| Al‐Haddad 2007 | There was no comparison of whether cancer was present or not. |
| Al‐Haddad 2010a | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Al‐Haddad 2010b | There was no comparison of whether cancer was present or not. |
| Al‐Haddad 2014 | There was no comparison of whether cancer was present or not. |
| Al‐Jebreen 2004 | There was no comparison of whether cancer was present or not. |
| Al‐Najami 2015 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Alizadeh 2014 | There was no comparison of whether cancer was present or not. |
| Aljebreen 2007 | Inadequate reference standard (nature of follow‐up not stated) |
| Alsohaibani 2008 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Alsohaibani 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Alston 2014 | There was no comparison of whether cancer was present or not. |
| Amin 2006 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Andersen 1994 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Antonini 2015 | There was no comparison of whether cancer was present or not. |
| Arabul 2012 | There was no comparison of whether cancer was present or not. |
| Ardengh 2007a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities were used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ardengh 2007b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ardengh 2008a | There was no comparison of whether cancer was present or not. |
| Ardengh 2008b | There was no comparison of whether cancer was present or not. |
| Ardengh 2013 | Inadequate reference standards |
| Argimak 2009 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Arikawa 2007 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Arlt 2013 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Asagi 2013 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Aslanian 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Asnacios 2003 | There was no comparison of whether cancer was present or not. |
| Atef 2013 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Attasaranya 2007 | There was no comparison of whether cancer was present or not. |
| Awadallah 2008 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Azizi 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Baba 2004 | Although this study provides diagnostic accuracy data for pancreatic lesions, it presents information on branch type and non‐branch type first, then presents the diagnostic test accuracy only for branch type and not for the overall cohort. This is therefore not a representative population. |
| Baek 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Baghbanian 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Baiocchi 2008 | Overlap with Baiocchi 2012 |
| Baiocchi 2010 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Baiocchi 2012 | Inadequate reference standard (criteria for diagnosing malignancy during clinical follow‐up not stated) |
| Bali 2011 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Bang 2012a | There was no comparison of whether cancer was present or not. |
| Bang 2012b | There was no comparison of whether cancer was present or not. |
| Bang 2013a | There was no comparison of whether cancer was present or not. |
| Bang 2013b | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Bang 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Barber 2011 | There was no comparison of whether cancer was present or not. |
| Bares 1994 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Barkin 1977 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Baron 1997 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Barral 2013a | There was no comparison of whether cancer was present or not. |
| Barral 2013b | There was no comparison of whether cancer was present or not. |
| Barresi 2014 | There was no comparison of whether cancer was present or not. |
| Barron 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Bartsch 1998 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Basir 2003 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Bassi 2003 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Beal 2015a | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Beal 2015b | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Becker 2001 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Beliao 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Bentz 1998 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Bergeron 2015 | There was no comparison of whether cancer was present or not. |
| Bernstein 2013 | There was no comparison of whether cancer was present or not. |
| Berzosa 2015 | There was no comparison of whether cancer was present or not. |
| Bhutani 1995 | There was no comparison of whether cancer was present or not. |
| Bhutani 1997 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Bick 2015 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Bighi 1989 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Binmoeller 1998a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Binmoeller 1998b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Bluen 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Bournet 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Bournet 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Bournet 2012 | There was no comparison of whether cancer was present or not. |
| Bournet 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Boutros 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Brand 2002 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Brand 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Brenin 1995 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Brimiene 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Brugge 2000 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Brugge 2004a | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Brugge 2004b | There was no comparison of whether cancer was present or not. |
| Bruno 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Buchholz 2005 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Buchs 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Butt 2015a | There was no comparison of whether cancer was present or not. |
| Butt 2015b | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Caglar 2013 | There was no comparison of whether cancer was present or not. |
| Cahn 1996 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Caldelari 2011 | There was no comparison of whether cancer was present or not. |
| Camellini 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Cantley 2014 | There was no comparison of whether cancer was present or not. |
| Carbognin 2006 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Carlinfante 2014 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Carroll 1997 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Casneuf 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Catanzaro 2003 | There was no comparison of whether cancer was present or not. |
| Catanzaro 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Cermak 2012 | There was no comparison of whether cancer was present or not. |
| Chai 2013 | There was no comparison of whether cancer was present or not. |
| Chang 1994 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Chang 1997 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Chang 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Chaudhari 2007 | There was no comparison of whether cancer was present or not. |
| Chaudhari 2008 | There was no comparison of whether cancer was present or not. |
| Chaya 2006 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Chebib 2014 | There was no comparison of whether cancer was present or not. |
| Chen 2001 | There was no comparison of whether cancer was present or not. |
| Chen 2003 | There was no comparison of whether cancer was present or not. |
| Chen 2007 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Chen 2014 | There was no comparison of whether cancer was present or not. |
| Cheng 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Cheng 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Chiu 2005 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Chiu 2006 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Cho 2005 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Cho 2013 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Choi 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Choi 2013 | There was no comparison of whether cancer was present or not. |
| Choi 2016 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Chung 2009 | Inappropriate index test |
| Cizginer 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Clave 1999 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Cocieru 2011 | There was no comparison of whether cancer was present or not. |
| Collins 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Collins 2013 | There was no comparison of whether cancer was present or not. |
| Collins 2015 | There was no comparison of whether cancer was present or not. |
| Cone 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Corominas‐Cishek 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Cosgrove 2015 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Crippa 2010 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Cuillerier 1996 | There was no comparison of whether cancer was present or not. |
| D'Onofrio 2007 | There was no comparison of whether cancer was present or not. |
| D'Onofrio 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Dadabhai 2005 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Dadds 2012 | There was no comparison of whether cancer was present or not. |
| Dani 2000 | There was no comparison of whether cancer was present or not. |
| Dawwas 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Dawwas 2013 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| De Jong 2010 | Should be included under de Jong 2012 |
| de Jong 2011 | There was no comparison of whether cancer was present or not. |
| De Tejada 2008 | There was no comparison of whether cancer was present or not. |
| Decalan 1995 | There was no comparison of whether cancer was present or not. |
| Del Vecchio 2016 | Inadequate reference standards |
| Delbeke 1999 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| DelMaschio 1991 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Deng 2008 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Deshpande 2008 | There was no comparison of whether cancer was present or not. |
| DeWitt 2004 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| DeWitt 2005 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| DeWitt 2008 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Di Cataldo 2014 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Diederichs 2000 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Diehl 1999 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Dietrich 2008 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Dim 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| DiMagno 1977 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Dinkel 1990 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Do 2014 | There was no comparison of whether cancer was present or not. |
| Draganov 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Eguia 2013 | There was no comparison of whether cancer was present or not. |
| Elmas 1996 | Inadequate reference standards |
| Eloubeidi 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Eloubeidi 2003a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Eloubeidi 2003b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Eloubeidi 2005 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Eloubeidi 2006a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Eloubeidi 2006b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Eloubeidi 2006c | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Eloubeidi 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Eloubeidi 2008a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Eloubeidi 2008b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Eloubeidi 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Ergul 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Erickson 1997 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Erickson 2000 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Erickson 2001 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Ernst 1998 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Erturk 2006a | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Erturk 2006b | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Fabbri 2013 | There was no comparison of whether cancer was present or not. |
| Fabbri 2015a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Fabbri 2015b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Faigel 1997 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Fan 2013 | There was no comparison of whether cancer was present or not. |
| Fan 2015 | There was no comparison of whether cancer was present or not. |
| Fanning 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Faravelli 1990 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Felgueroso 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Fernandez‐Esparrach 2007a | There was no comparison of whether cancer was present or not. |
| Fernandez‐Esparrach 2007b | There was no comparison of whether cancer was present or not. |
| Figueiredo 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Fischer 2002 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Fischer 2009 | There was no comparison of whether cancer was present or not. |
| Fisher 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Fisher 2011 | There was no comparison of whether cancer was present or not. |
| Frampton 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Friess 1995 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Fritscher‐Ravens 1998 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Fritscher‐Ravens 1999 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Fritscher‐Ravens 2000 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Fritscher‐Ravens 2001a | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Fritscher‐Ravens 2001b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Fritscher‐Ravens 2002 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Frossard 2003 | There was no comparison of whether cancer was present or not. |
| Fugazzola 1991 | There was no comparison of whether cancer was present or not. |
| Furuhashi 2015 | There was no comparison of whether cancer was present or not. |
| Furuhata 2012 | There was no comparison of whether cancer was present or not. |
| Fusari 2010 | There was no comparison of whether cancer was present or not. |
| Fusaroli 2010 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Fusaroli 2014 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Gaa 1999 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Gambitta 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Ganc 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ganc 2015 | Inadequate reference standards |
| Gaspar 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Gill 2008 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Gimeno‐Garcia 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Giorgetti 2010 | Inadequate reference standard (nature of follow‐up not stated) |
| Giovannini 1995 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Giovannini 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Glasbrenner 2000 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Goh 2006a | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Goh 2008 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Gomez 2006 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Gomez 2008 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Gong 2004 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Gordon 2014 | Inadequate reference standards |
| Gowland 1981 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Green 2002 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Grenacher 2004 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Gress 1997 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Gress 2001 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Grieser 2013 | There was no comparison of whether cancer was present or not. |
| Guo 2008 | There was no comparison of whether cancer was present or not. |
| Gupta 1995 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Gupta 2008 | There was no comparison of whether cancer was present or not. |
| Haba 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Haba 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Hammel 1995 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Hammel 1998 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Han 2016 | Inadequate reference standards |
| Hanada 2009 | There was no comparison of whether cancer was present or not. |
| Hanninen 2002 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Hanninen 2005 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Harewood 2001a | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Harewood 2001b | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Harewood 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Hasan 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Hasenberg 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Hasyagar 2004 | There was no comparison of whether cancer was present or not. |
| Hayashi 2013 | There was no comparison of whether cancer was present or not. |
| Hebert‐Magee 2015 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Heinrich 2005 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Henkes 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Heo 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Herman‐Sucharska 1999 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Hernandez 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Herrmann 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Higashi 2002a | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Higashi 2002b | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Higashi 2003 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Hijioka 2014 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Hikichi 2009 | Inadequate reference standard (details of clinical follow‐up not stated) |
| Hilendarov 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Hilendarov 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Hilendarov 2012 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Hilendarov 2013 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Ho 1996 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ho 2004 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Hocke 2006 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Hocke 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Hollerbach 2004 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Holt 2008 | There was no comparison of whether cancer was present or not. |
| Holt 2014 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Hong 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Honselmann 2016 | Inadequate reference standards |
| Horatagis 2003 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Horwhat 2004 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Horwhat 2006 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Hou 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Hu 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Huang 2010 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Huang 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Hunt 2009 | There was no comparison of whether cancer was present or not. |
| Hussain 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Hwang 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Hwang 2011 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Ibrahim 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ichikawa 2001 | There was no comparison of whether cancer was present or not. |
| Iftimia 2012 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Iglesias 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Iglesias‐Garcia 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Iglesias‐Garcia 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Iglesias‐Garcia 2009a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Iglesias‐Garcia 2009b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Iglesias‐Garcia 2010 | There was no comparison of whether cancer was present or not. |
| Iglesias‐Garcia 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Iglesias‐Garcia 2013a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Iglesias‐Garcia 2013b | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Iguchi 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ikeura 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Ikeura 2015a | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Ikeura 2015b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Imazu 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Imdahl 1999 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Inokuma 1995 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Iordache 2016 | Inadequate reference standards |
| Ippolito 2015 | There was no comparison of whether cancer was present or not. |
| Irie 2002 | There was no comparison of whether cancer was present or not. |
| Ironside 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ishigami 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Ishii 2012 | There was no comparison of whether cancer was present or not. |
| Ishikawa 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Itoh 2005 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Itoi 2005a | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Itoi 2005b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Itoi 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Itokawa 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Itokawa 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Iwashita 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Iwashita 2015 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Izuishi 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Jabbar 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Jadvar 2001 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Jahng 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Jahromi 2014 | There was no comparison of whether cancer was present or not. |
| Jang 2012 | There was no comparison of whether cancer was present or not. |
| Jang 2015 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Jani 2006 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Jani 2008 | There was no comparison of whether cancer was present or not. |
| Janssen 2007 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Jayasekeran 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Jeong 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Jhala 2007 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Jin 2013b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Jing 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Johnson 1999 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kadayifci 2014 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Kadayifci 2016 | Inadequate reference standards |
| Kaffes 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kaimakliotis 2015 | Inappropriate index test |
| Kalb 2013 | There was no comparison of whether cancer was present or not. |
| Kalra 2003 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Kamata 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kamata 2016b | Inadequate reference standards |
| Kamin 1980 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kamisawa 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kanazawa 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kang 2013 | Reference to be included under Kim 2015. |
| Kang 2014 | There was no comparison of whether cancer was present or not. |
| Kang 2016 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Katanuma 2013 | There was no comparison of whether cancer was present or not. |
| Katz 2007 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Kauhanen 2009a | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kauhanen 2009b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kauhanen 2015 | Inadequate reference standards |
| Kawada 2012 | There was no comparison of whether cancer was present or not. |
| Kawada 2014 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Kawada 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kawada 2016 | Inappropriate target condition |
| Kawamoto 2006 | There was no comparison of whether cancer was present or not. |
| Keil 2008 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Keswani 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Khalid 2005 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Khalid 2006 | Same as Kim 2015 |
| Khan 2010 | There was no comparison of whether cancer was present or not. |
| Khashab 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Khashab 2013 | There was no comparison of whether cancer was present or not. |
| Khodadadian 2001 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Khurana 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Khurana 2014 | There was no comparison of whether cancer was present or not. |
| Kida 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kim 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kim 2009 | There was no comparison of whether cancer was present or not. |
| Kim 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kim 2012a | There was no comparison of whether cancer was present or not. |
| Kim 2012b | There was no comparison of whether cancer was present or not. |
| Kim 2012c | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kim 2013a | There was no comparison of whether cancer was present or not. |
| Kim 2013b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kim 2013c | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kim 2013d | There was no comparison of whether cancer was present or not. |
| Kim 2014a | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kim 2014b | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Kim 2014c | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Kim 2014d | There was no comparison of whether cancer was present or not. |
| Kim 2015a | Inappropriate index test |
| Kin 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kitano 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Klapman 2003 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Klapman 2004 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Klapman 2005 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kliment 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kliment 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kokhanenko 2001 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kongkam 2015 | Inadequate reference standards |
| Kopelman 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Koranda 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Korenblit 2016 | Inadequate reference standards |
| Koyama 2001 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kriger 2011 | This study was not included as the index test was not performed to distinguish between cancerous, precancerous, and benign lesions. |
| Krishna 2009a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Krishna 2009b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Krishna 2009c | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Krishna 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Krishna 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Krishna 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Krishnan 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kubiliun 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kubo 2009 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Kudo 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kula 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kumon 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kumon 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kumon 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kung 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Kursawa 1991 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kwong 2015 | Inadequate reference standards |
| Kyokane 1996 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Kysucan 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Lackner 1980 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Larghi 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Larino‐Noia 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Le Baleur 2009 | There was no comparison of whether cancer was present or not. |
| Le Baleur 2011b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| LeBlanc 2004 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| LeBlanc 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lee 2005a | There was no comparison of whether cancer was present or not. |
| Lee 2005b | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Lee 2006 | There was no comparison of whether cancer was present or not. |
| Lee 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lee 2008a | There was no comparison of whether cancer was present or not. |
| Lee 2008b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lee 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Lee 2010a | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Lee 2010b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Lee 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lee 2013a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lee 2013b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lee 2013c | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lee 2013d | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Lee 2014b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lee 2014c | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lee 2014d | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Leeds 2013 | There was no comparison of whether cancer was present or not. |
| Legmann 1998 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Lehmann 1998 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Lemke 2004 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Levy 1995 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Levy 2005 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Levy 2007 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Levy 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Lightdale 1994 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Lim 2005 | There was no comparison of whether cancer was present or not. |
| Lim 2013 | There was no comparison of whether cancer was present or not. |
| Lin 2003 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lin 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lin 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Linder 2006 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Liu 2010a | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Liu 2010b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Liu 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lopez 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lozano 2011 | There was no comparison of whether cancer was present or not. |
| Lu 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lu 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Lytras 2005 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Mackie 1979 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Madan 2012 | There was no comparison of whether cancer was present or not. |
| Madura 1997 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Maguchi 2006 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Maire 2003 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Makaiova 2005 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Malak 2016 | Inadequate reference standards |
| Malleo 2012 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Mallery 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Maluf 2005 | There was no comparison of whether cancer was present or not. |
| Maluf‐Filho 2007 | There was no comparison of whether cancer was present or not. |
| Mamoon 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Manfredi 2009 | There was no comparison of whether cancer was present or not. |
| Mansoor 2012 | There was no comparison of whether cancer was present or not. |
| Mansour 2006 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Mao 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Marchevsky 2003 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Marotta 1991 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Martin 1998 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Martinez 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Marzioni 2015 | Inadequate reference standards; details of clinical follow‐up not available |
| Matsubara 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Matsubayashi 2015 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Matsuda 2012 | There was no comparison of whether cancer was present or not. |
| Matsumoto 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Matsumoto 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Matsumoto 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Maurea 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Mavrogenis 2015 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Mayerle 2016 | Inadequate reference standards |
| McClellan 2003 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| McDowell 1997 | There was no comparison of whether cancer was present or not. |
| Mehan 2009 | There was no comparison of whether cancer was present or not. |
| Mehmood 2015 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Meijer 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Meijer 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Mera 1999 | There was no comparison of whether cancer was present or not. |
| Mertz 2000 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Mesihovic 2005 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Micames 2007 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Michaels 2006 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Midwinter 1999 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Mishra 2006 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Mitsuhashi 2006 | There was no comparison of whether cancer was present or not. |
| Miyabe 2015 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Moehler 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Moparty 2007 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Moris 2016 | Inappropriate target population |
| Morozova 2014 | There was no comparison of whether cancer was present or not. |
| Morozova 2015 | Inappropriate target population |
| Murayama 2011 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Nadig 2012 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Nagamachi 2013 | Inadequate reference standards |
| Nagula 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Nakai 2015 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Nakamoto 2000 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Nakamoto 2003 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Napoleon 2010a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Napoleon 2010b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Napoleon 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Nattermann 1995 | There was no comparison of whether cancer was present or not. |
| Nayar 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Nayar 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Nguyen 1998 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Nguyen 2007 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Nguyen 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Nicaud 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Nieto 2007 | Details of clinical follow‐up not available |
| Nijhawan 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Nikiforova 2013 | There was no comparison of whether cancer was present or not. |
| Nishihara 1996 | Inappropriate target condition |
| Nitzsche 2002 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Nobrega 1994 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Noda 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Noma 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Noone 2004 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Norton 2001 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Nougaret 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| O'Toole 2004 | There was no comparison of whether cancer was present or not. |
| Ogawa 2008b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ogura 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Oguz 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ohno 2009 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Ohta 2012 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Ohtsuka 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Okada 1979 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Okada 1981 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Okasha 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Okasha 2015 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Olson 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Ooi 1998 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Ootaki 2012 | There was no comparison of whether cancer was present or not. |
| Opacic 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Oppong 2015 | Inadequate reference standards |
| Osman 2016 | Inadequate reference standards |
| Othman 2011 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Ozkan 2016 | Inadequate reference standards |
| Paik 2015 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Palacios‐Gerona 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Palaniappan 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Palazzo 1993 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Palazzo 2011 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Pan 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Panaro 1978 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Papanikolaou 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Papos 1999 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Papos 2002a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Papos 2002b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Park 2014a | There was no comparison of whether cancer was present or not. |
| Park 2014b | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Park 2016a | Inadequate reference standards |
| Park 2016b | Inadequate reference standards |
| Pasanen 1992 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Pasanen 1993 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Patoureaux 2013 | There was no comparison of whether cancer was present or not. |
| Paye 2000 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Pedrazzoli 2005 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Pellise 2003 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Perri 2012 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Perrone 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Petrone 2012 | There was no comparison of whether cancer was present or not. |
| Pezzilli 2013 | There was no comparison of whether cancer was present or not. |
| Pitman 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Pitman 2013a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Pitman 2013b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Pitman 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Pomerri 1991 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Pongpornsup 2011 | There was no comparison of whether cancer was present or not. |
| Qian 2003 | There was no comparison of whether cancer was present or not. |
| Qian 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Qin 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Quentin 2005 | There was no comparison of whether cancer was present or not. |
| Qureshi 2013 | There was no comparison of whether cancer was present or not. |
| Raddaoui 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Rajput 1998 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Raman 2013 | There was no comparison of whether cancer was present or not. |
| Ramesh 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ramesh 2015 | There was no comparison of whether cancer was present or not. |
| Ramesh 2016 | Inadequate reference standards |
| Ramirez‐Luna 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Rana 2011 | There was no comparison of whether cancer was present or not. |
| Ranney 2012 | There was no comparison of whether cancer was present or not. |
| Rao 2011 | There was no comparison of whether cancer was present or not. |
| Rasmussen 2001 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Rasmussen 2004 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Raut 2002 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Raut 2003 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Redelman 2014 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Reicher 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Repak 2009 | There was no comparison of whether cancer was present or not. |
| Ribeiro 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Richter 1996 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Richter 2001 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Ridtitid 2015 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Rocca 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Roch 2014 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Rodriguez 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Rodriguez 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Rodriguez‐D'Jesus 2013 | There was no comparison of whether cancer was present or not. |
| Rogart 2011 | There was no comparison of whether cancer was present or not. |
| Romagnuolo 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Rong 2012 | There was no comparison of whether cancer was present or not. |
| Rosch 1990a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Rosch 1990b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Rosch 1991a | There was no comparison of whether cancer was present or not. |
| Rosch 1991b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Rosch 2000 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Rose 1999 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Rosique 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Rudolph 2001 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Ruf 2006 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Ryozawa 2005 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Saftoiu 2006 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Saftoiu 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Saftoiu 2010a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Saftoiu 2010b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Saftoiu 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Saftoiu 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Saftoiu 2013 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Saftoiu 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sahai 2012 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Sahani 2006b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sahani 2011 | There was no comparison of whether cancer was present or not. |
| Sai 2003 | There was no comparison of whether cancer was present or not. |
| Sakamoto 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sakamoto 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Salvia 2012 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Sandrasegaran 2011 | There was no comparison of whether cancer was present or not. |
| Santhosh 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sarbia 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sariya 2003 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Savides 2006 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Savides 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Savoy 2007 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Saxena 2014 | There was no comparison of whether cancer was present or not. |
| Schick 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Schima 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Schmidt 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Schneider 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Schrader 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Schraibman 2011 | There was no comparison of whether cancer was present or not. |
| Scott 2000 | There was no comparison of whether cancer was present or not. |
| Seicean 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Seicean 2016 | Inadequate reference standards |
| Sendino 2010 | There was no comparison of whether cancer was present or not. |
| Sendler 2000 | Inadequate reference standards |
| Serikawa 2006 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Shah 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Shen 2013 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Shimizu 2010 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Shimizu 2013a | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Shimizu 2013b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Shimizu 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Shimizu 2015 | Inappropriate index test |
| Shin 2002 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Shin 2010 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Siddiqui 2009 | There was no comparison of whether cancer was present or not. |
| Siddiqui 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Siddiqui 2011 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Siddiqui 2012 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Siddiqui 2013 | There was no comparison of whether cancer was present or not. |
| Siech 1998 | There was no comparison of whether cancer was present or not. |
| Simon 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sina 2014 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Singer 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Singhi 2014 | There was no comparison of whether cancer was present or not. |
| Singhi 2016 | Inadequate reference standards |
| Singu 2008 | There was no comparison of whether cancer was present or not. |
| Soares 2015a | Inadequate reference standards |
| Soares 2015b | Inadequate reference standards |
| Sole 2005 | There was no comparison of whether cancer was present or not. |
| Song 2007 | There was no comparison of whether cancer was present or not. |
| Song 2010 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sperti 1994 | There was no comparison of whether cancer was present or not. |
| Sperti 2001 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sperti 2005 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sperti 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sreenarasimhaiah 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sreenarasimhaiah 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sreenarasimhaiah 2013 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Sreenarasimhaiah 2015 | There was no comparison of whether cancer was present or not. |
| Staib 1997 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Starkov 2008 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Stelow 2003 | There was no comparison of whether cancer was present or not. |
| Storch 2006 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Storch 2007 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Story 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Strand 2014 | There was no comparison of whether cancer was present or not. |
| Strauss 2016 | Inadequate reference standards |
| Strobel 2013 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Strohm 1984 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Su 2007 | There was no comparison of whether cancer was present or not. |
| Sugimoto 2015 | Inadequate reference standards |
| Sugiyama 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Suits 1999 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Sun 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sur 2015 | Inadequate reference standards |
| Suzuki 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Suzuki 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Sverko 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Swobodnik 1983 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Szafranska 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Tada 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Tadic 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Takahashi 2005 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Talar‐Wojnarowska 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Tallini 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Taouli 2002 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Tarantino 2014a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Tarantino 2014b | There was no comparison of whether cancer was present or not. |
| Tarantino 2014c | There was no comparison of whether cancer was present or not. |
| Tatsumi 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Tatsuta 1985 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Taylor 2007 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Tervahartiala 1997 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Tessler 2006 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Theruvath 2010 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Thomas 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Thomas 2010a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Thomas 2010b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Tlostanova 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Togliani 2015 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Touchefeu 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Trifunovic 2004 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Tummala 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Turowska 2007 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Uehara 2011 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Uehara 2015 | Inadequate reference standards |
| Uekitani 2016 | Inadequate reference standards |
| Valinas 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| van Gulik 1999 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| van Kouwen 2004 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| van Kouwen 2005 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Vanbiervliet 2014 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Varadarajulu 2004a | There was no comparison of whether cancer was present or not. |
| Varadarajulu 2004b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Varadarajulu 2014a | There was no comparison of whether cancer was present or not. |
| Varadarajulu 2014b | There was no comparison of whether cancer was present or not. |
| Vasile 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Verzola 2000 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Vilgrain 1989 | There was no comparison of whether cancer was present or not. |
| Vilgrain 1995 | There was no comparison of whether cancer was present or not. |
| Vilmann 1995 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Virtue 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Visser 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Visser 2008 | There was no comparison of whether cancer was present or not. |
| Voss 2000 | There was no comparison of whether cancer was present or not. |
| Votrubova 2005 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Vullierme 2007 | There was no comparison of whether cancer was present or not. |
| Wachs 2010 | There was no comparison of whether cancer was present or not. |
| Wakabayashi 2008 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Wakatsuki 2004 | There was no comparison of whether cancer was present or not. |
| Wakatsuki 2005 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Walter 2015 | Inadequate reference standards |
| Wang 2005 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Wang 2007a | There was no comparison of whether cancer was present or not. |
| Wang 2007b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Wang 2009 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Wang 2011a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Wang 2011b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Wang 2012 | There was no comparison of whether cancer was present or not. |
| Wani 2011 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Wani 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Warda 2015 | Inadequate reference standards |
| Watanabe 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Waters 2008 | There was no comparison of whether cancer was present or not. |
| Waxman 2001 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Wegener 1995 | There was no comparison of whether cancer was present or not. |
| Wiersema 1994 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Wiersema 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Wiesenauer 2003 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Will 2007 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Will 2008 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Will 2010 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Williams 1999 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Wilson 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Winner 2015 | There was no comparison of whether cancer was present or not. |
| Wittmann 2006 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Woolf 2013 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Wright 2014 | There was no comparison of whether cancer was present or not. |
| Wu 2007a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Wu 2007b | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Wu 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Wu 2014 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Wyse 2009 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Xiao 2009 | There was no comparison of whether cancer was present or not. |
| Xu 2012 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Xu 2013 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Xu 2014 | There was no comparison of whether cancer was present or not. |
| Yamada 2010a | There was no comparison of whether cancer was present or not. |
| Yamada 2010b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Yamaguchi 1990 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Yamao 2003 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Yamashita 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Yan 2014 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Yang 2014 | There was no comparison of whether cancer was present or not. |
| Yang 2015a | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Yang 2015b | Inadequate reference standards |
| Yantiss 2008 | There was no comparison of whether cancer was present or not. |
| Yao 2012 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Yeh 1999 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Yim 2005 | There was no comparison of whether cancer was present or not. |
| Yin 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Yin 2015 | Inadequate reference standards |
| Ylagan 2002 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Yoshioka 2015 | There was no comparison of whether cancer was present or not. |
| Yuan 2007 | There was no comparison of whether cancer was present or not. |
| Yun 2007 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Yusuf 2009 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Zamboni 2012 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Zaruba 2013 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Zdanyte 2004 | There was no comparison of whether cancer was present or not. |
| Zeiderman 1991 | This study did not use an index test such as CT, MRI, PET, EUS‐FNA, and EUS elastography. |
| Zhang 2010a | There was no comparison of whether cancer was present or not. |
| Zhang 2010b | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Zhang 2010c | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Zhang 2010d | There was no comparison of whether cancer was present or not. |
| Zhang 2011 | Diagnostic accuracy data were not available for this study. We classed any of the following as acceptable forms of data: (2 x 2 table OR sensitivity + specificity + number with and without disease OR positive + negative predictive values + number of positive and negative tests) OR total test positive + total test negative + total disease positive + total disease present + total disease absent + accuracy percentage. |
| Zhang 2012 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Zhang 2015 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Zhong 2012 | There was no comparison of whether cancer was present or not. |
| Zhu 2008 | There was no comparison of whether cancer was present or not. |
| Zhu 2013 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Ziak 2011 | There was no comparison of whether cancer was present or not. |
| Zimny 1997 | This study was not included as no pancreatic mass was present in the patient(s) tested. |
| Zimny 1998 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Zimny 1999 | The study was not classed as primary research (i.e. not a review or editorial or comment). |
| Zubarik 2004 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
| Zyrek‐Betts 2008 | This study included an inadequate reference standard. Our criteria for a reference standard included the following: all surgery, or with negative follow‐up of at least 6 months; if EUS‐FNA or other imaging modalities used for reference standard (either positive or negative), not accepted unless all patients (positive and negative) were followed up for at least 6 months. Clear evidence of metastases during follow‐up in patients with positive diagnosis (an increase in size is not sufficient). Biopsy (but not EUS‐FNA cytology) is acceptable for positive results (< 6 months) but not for negative results – if negative, patients must have been followed for at least 6 months. |
CT: computed tomography EUS: endoscopic ultrasound FNA: fine‐needle aspiration MRI: magnetic resonance imaging PET: positron emission tomography
Differences between protocol and review
We have included sensitivity‐maximising diagnostic filters for searching MEDLINE and Embase databases because the original searches without the filters retrieved more than 50,000 references (Haynes 2004; Wilczynski 2005). We also made some modifications to the search strategy because we needed to balance the possibility of missing some studies against the risk of not being able to complete the review. We decided that it is useful to have evidence from major studies rather than having no information at all.
We did not search the Cochrane Register of Diagnostic Test Accuracy Studies, as we believe it is no longer maintained.
We have performed the related search function through MEDLINE (OvidSP) rather than MEDLINE (PubMed) and also performed a cited reference search in MEDLINE (via OvidSP).
We have reworded the Statistical analysis and data synthesis section to bring this in line with our recent reviews. There were no material differences to the plan of statistical analysis except that we also planned to perform a bivariate analysis, which takes into account the correlation between sensitivity and specificity for tests with explicit thresholds as well. We did this because the summary sensitivity and specificity (and hence the positive likelihood ratio and negative likelihood ratio from which the post‐test probabilities can be calculated) are available from the bivariate model.
We have simplified the analysis in the presence of sparse data based on the article by Takwoingi and colleagues (Takwoingi 2015).
We have presented the post‐test probabilities only for the median prevalence in the comparison to avoid presenting readers with an overwhelming amount of data.
Contributions of authors
L Best, K Gurusamy, and V Rawji wrote sections of the review. SP Pereira and BR Davidson critically commented on the review.
Sources of support
Internal sources
None, Other.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant, or Cochrane Incentive funding to the Upper Gastrointestinal and Pancreatic Diseases Group and Cochrane Hepato‐Biliary Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Declarations of interest
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure, Cochrane Programme Grant, or Cochrane Incentive funding to the Upper Gastrointestinal and Pancreatic Diseases Group and Cochrane Hepato‐Biliary Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
LMJB: none known.
VR: none known.
SPP: none known.
BRD: none known.
KSG: none known.
New
References
References to studies included in this review
Brand 2000 {published data only}
- Brand B, Pfaff T, Binmoeller KF, Sriram PV, Fritscher‐Ravens A, Knofel WT, et al. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scandinavian Journal of Gastroenterology 2000;35(11):1221‐8. [DOI] [PubMed] [Google Scholar]
Brandwein 2001 ‐ Cystic {published data only}
- Brandwein SL, Farrell JJ, Centeno BA, Brugge WR. Detection and tumor staging of malignancy in cystic, intraductal, and solid tumors of the pancreas by EUS. Gastrointestinal Endoscopy 2001;53(7):722‐7. [DOI] [PubMed] [Google Scholar]
Brandwein 2001 ‐ Solid {published data only}
- Brandwein SL, Farrell JJ, Centeno BA, Brugge WR. Detection and tumor staging of malignancy in cystic, intraductal, and solid tumors of the pancreas by EUS. Gastrointestinal Endoscopy 2001;53(7):722‐7. [DOI] [PubMed] [Google Scholar]
Cellier 1998 {published data only}
- Cellier C, Cuillerier E, Palazzo L, Rickaert F, Flejou JF, Napoleon B, et al. Intraductal papillary and mucinous tumors of the pancreas: Accuracy of preoperative computed tomography, endoscopic retrograde pancreatography and endoscopic ultrasonography, and long‐term outcome in a large surgical series. Gastrointestinal Endoscopy 1998;47(1):42‐9. [DOI] [PubMed] [Google Scholar]
Cherian 2010 {published data only}
- Cherian PT, Mohan P, Douiri A, Taniere P, Hejmadi RK, Mahon BS. Role of endoscopic ultrasound‐guided fine‐needle aspiration in the diagnosis of solid pancreatic and peripancreatic lesions: Is onsite cytopathology necessary?. HPB 2010;12(6):389‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2003 {published data only}
- Choi BS, Kim TK, Kim AY, Kim KW, Park SW, Kim PN, et al. Differential diagnosis of benign and malignant intraductal papillary mucinous tumors of the pancreas: MR cholangiopancreatography and MR angiography. Korean Journal of Radiology 2003;4(3):157‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Correa‐Gallego 2009 {published data only}
- Correa‐Gallego C, Warshaw AL, Fernandez‐del Castillo C. Fluid CEA in ipmns: A useful test or the flip of a coin?. American Journal of Gastroenterology 2009;104(3):796‐7. [DOI] [PubMed] [Google Scholar]
de Jong 2012 {published data only}
- Jong K, Nio CY, Gouma DJ, Bruno MJ, Fockens P. Accuracy of MRI and EUS in a prospective cohort of patients with histological proven pancreatic cystic lesions of the pancreas. Gastroenterology 2010;138(5 Suppl 1):S547. [Google Scholar]
- Jong K, Hooft JE, Nio CY, Gouma DJ, Dijkgraaf MG, Bruno MJ, et al. Accuracy of preoperative workup in a prospective series of surgically resected cystic pancreatic lesions. Scandinavian Journal of Gastroenterology 2012;47(8‐9):1056‐63. [DOI] [PubMed] [Google Scholar]
Doi 2002 {published data only}
- Doi R, Fujimoto K, Wada M, Imamura M. Surgical management of intraductal papillary mucinous tumor of the pancreas. Surgery 2002;132(1):80‐5. [DOI] [PubMed] [Google Scholar]
Erkan 2012 {published data only}
- Erkan M, Herrmann K, Dobritz M, Schmid RM, Friess H, Schwaiger M, et al. Comparison of 3'‐deoxy‐3'‐[18f] fluorothymidine positron emission tomography (flt‐pet) and FDG‐PET/CT for the detection and characterization of pancreatic tumors. HPB 2012;14:207. [DOI] [PubMed] [Google Scholar]
Fischer 2009 ‐ Cystic {published data only}
- Fischer CP, Fahy BN, Aloia TA, Raijman I, Barroso AO, Schwarz PJ, et al. Fine needle aspiration cytology from pancreatic cysts ‐ limited utility in surgical decision making. Gastroenterology 2009;136(5 Suppl 1):A886. [Google Scholar]
Fischer 2009 ‐ Solid {published data only}
- Fischer CP, Fahy BN, Aloia TA, Raijman I, Barroso AO, Schwarz PJ, et al. Fine needle aspiration cytology from pancreatic cysts ‐ limited utility in surgical decision making. Gastroenterology 2009;136(5 Suppl 1):A886. [Google Scholar]
Fisher 2008 {published data only}
- Fisher WE, Hodges SE, Yagnik V, Moron FE, Wu MF, Hilsenbeck SG, et al. Accuracy of CT in predicting malignant potential of cystic pancreatic neoplasms. HPB 2008;10(6):483‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grieser 2010 {published data only}
- Grieser C, Steffen IG, Grajewski L, Stelter L, Streitparth F, Schnapauff D, et al. Preoperative multidetector row computed tomography for evaluation and assessment of resection criteria in patients with pancreatic masses. Acta Radiologica 2010;51(10):1067‐77. [DOI] [PubMed] [Google Scholar]
Harrison 1999 {published data only}
- Harrison JL, Millikan KW, Prinz RA, Zaidi S. Endoscopic ultrasound for diagnosis and staging of pancreatic tumors. American Surgeon 1999;65(7):659‐64; discussion 664‐5. [DOI] [PubMed] [Google Scholar]
Higashi 1997 {published data only}
- Higashi T, Tamaki N, Honda T, Torizuka T, Kimura T, Inokuma T, et al. Expression of glucose transporters in human pancreatic tumors compared with increased FDG accumulation in pet study. Journal of Nuclear Medicine 1997;38(9):1337‐44. [PubMed] [Google Scholar]
Hong 2010 {published data only}
- Hong HS, Yun M, Cho A, Choi JY, Kim MJ, Kim KW, et al. The utility of f‐18 FDG PET/CT in the evaluation of pancreatic intraductal papillary mucinous neoplasm. Clinical Nuclear Medicine 2010;35(10):776‐9. [DOI] [PubMed] [Google Scholar]
Hu 2013 {published data only}
- Hu SL, Yang ZY, Zhou ZR, Yu XJ, Ping B, Zhang YJ. Role of SUV(max) obtained by 18f‐FDG PET/CT in patients with a solitary pancreatic lesion: Predicting malignant potential and proliferation. Nuclear Medicine Communications 2013;34(6):533‐9. [DOI] [PubMed] [Google Scholar]
Jafarimehr 2010 {published data only}
- Jafarimehr E, Lilien DL, Zibari GB, Shokouh‐Amiri H. Role of 18 f‐fluorodeoxyglucose positron emission tomography in detecting pancreatic lesion. HPB 2010;12:412. [Google Scholar]
Jang 2014a {published data only}
- Jang KM, Kim SH, Lee SJ, Choi D. The value of gadoxetic acid‐enhanced and diffusion‐weighted MRI for prediction of grading of pancreatic neuroendocrine tumors. Acta Radiologica 2014;55(2):140‐8. [DOI] [PubMed] [Google Scholar]
Jang 2014b {published data only}
- Jang KM, Kim SH, Min JH, Lee SJ, Kang TW, Lim S, et al. Value of diffusion‐weighted MRI for differentiating malignant from benign intraductal papillary mucinous neoplasms of the pancreas. AJR American Journal of Roentgenology 2014;203(5):992‐1000. [DOI] [PubMed] [Google Scholar]
Jin 2013a {published data only}
- Jin DX, Jhala N, Shah PM, Bernstein GR, Chandrasekhara V, Ginsberg GG, et al. Cytological atypia does not predict malignancy in pancreatic cystic lesions. Gastroenterology 2013;1:S795. [Google Scholar]
Jin 2015 {published data only}
- Jin DX, Small AJ, Vollmer CM, Jhala N, Furth EE, Ginsberg GG, et al. A lower cyst fluid CEA cut‐off increases diagnostic accuracy in identifying mucinous pancreatic cystic lesions. Journal of the Pancreas 2015;16(3):271‐7. [Google Scholar]
Kalha 2003 {published data only}
- Kalha I, Kaw M, Singh S, Patel M, Cohen D, Morns J, et al. Diagnostic accuracy of endoscopic ultrasound‐guided fine‐needle aspiration of cystic lesions of the pancreas. Gastroenterology 2003;124(4):A188‐A. [Google Scholar]
Kamata 2016a {published data only}
- Kamata K, Kitano M, Omoto S, Kadosaka K, Miyata T, Yamao K, et al. Contrast‐enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy 2016;48(1):35‐41. [DOI] [PubMed] [Google Scholar]
Kato 1995 {published data only}
- Kato T, Fukatsu H, Ito K, Tadokoro M, Ota T, Ikeda M, et al. Fluorodeoxyglucose positron emission tomography in pancreatic cancer: An unsolved problem. European Journal of Nuclear Medicine 1995;22(1):32‐9. [DOI] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kang KM, Lee JM, Shin CI, Baek JH, Kim SH, Yoon JH, et al. Added value of diffusion‐weighted imaging to MR cholangiopancreatography with unenhanced MR imaging for predicting malignancy or invasiveness of intraductal papillary mucinous neoplasm of the pancreas. Journal of Magnetic Resonance Imaging 2013;38(3):555‐63. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee JM, Lee ES, Baek JH, Kim JH, Han JK, et al. Intraductal papillary mucinous neoplasms of the pancreas: Evaluation of malignant potential and surgical resectability by using MR imaging with MR cholangiography. Radiology 2015;274(3):723‐33. [DOI] [PubMed] [Google Scholar]
Klau 2011 {published data only}
- Klau M, Lemke A, Grunberg K, Simon D, Re TJ, Wente MN, et al. Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Investigative Radiology 2011;46(1):57‐63. [DOI] [PubMed] [Google Scholar]
Kobayashi 2012 {published data only}
- Kobayashi N, Sugimori K, Shimamura T, Hosono K, Watanabe S, Kato S, et al. Endoscopic ultrasonographic findings predict the risk of carcinoma in branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology 2012;12(2):141‐5. [DOI] [PubMed] [Google Scholar]
Kubo 2001 {published data only}
- Kubo H, Chijiiwa Y, Akahoshi K, Hamada S, Harada N, Sumii T, et al. Intraductal papillary‐mucinous tumors of the pancreas: Differential diagnosis between benign and malignant tumors by endoscopic ultrasonography. American Journal of Gastroenterology 2001;96(5):1429‐34. [DOI] [PubMed] [Google Scholar]
Kucera 2012 {published data only}
- Kucera S, Centeno BA, Springett G, Malafa MP, Chen YA, Weber J, et al. Cyst fluid carcinoembryonic antigen level is not predictive of invasive cancer in patients with intraductal papillary mucinous neoplasm of the pancreas. JOP: Journal of the Pancreas [Electronic Resource] 2012;13(4):409‐13. [DOI] [PubMed] [Google Scholar]
Le Baleur 2011a {published data only}
- Baleur Y, Couvelard A, Vullierme MP, Sauvanet A, Hammel P, Rebours V, et al. Mucinous cystic neoplasms of the pancreas: Definition of preoperative imaging criteria for high‐risk lesions. Pancreatology 2011;11(5):495‐9. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee KH, Lee SJ, Lee JK, Ryu JK, Kim EY, Kim TH, et al. Prediction of malignancy with endoscopic ultrasonography in patients with branch duct‐type intraductal papillary mucinous neoplasm. Pancreas 2014;43(8):1306‐11. [DOI] [PubMed] [Google Scholar]
Maire 2008 {published data only}
- Maire F, Voitot H, Aubert A, Palazzo L, O'Toole D, Couvelard A, et al. Intraductal papillary mucinous neoplasms of the pancreas: Performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. American Journal of Gastroenterology 2008;103(11):2871‐7. [DOI] [PubMed] [Google Scholar]
McHenry 2002 {published data only}
- McHenry L, DeWitt J, Sherman S, LeBlanc J, McGreedy K, Chriswell M. Mucinous cystic neoplasms of the pancreas: Accuracy of cytology and mucin stain at endoscopic ultrasound‐guided fine‐needle aspiration. Gastrointestinal Endoscopy 2002;56(4 Suppl):S118. [Google Scholar]
Nakagawa 2009 {published data only}
- Nakagawa A, Yamaguchi T, Ohtsuka M, Ishihara T, Sudo K, Nakamura K, et al. Usefulness of multidetector computed tomography for detecting protruding lesions in intraductal papillary mucinous neoplasm of the pancreas in comparison with single‐detector computed tomography and endoscopic ultrasonography. Pancreas 2009;38(2):131‐6. [DOI] [PubMed] [Google Scholar]
Nara 2009 {published data only}
- Nara S, Onaya H, Hiraoka N, Shimada K, Sano T, Sakamoto Y, et al. Preoperative evaluation of invasive and noninvasive intraductal papillary‐mucinous neoplasms of the pancreas: Clinical, radiological, and pathological analysis of 123 cases. Pancreas 2009;38(1):8‐16. [DOI] [PubMed] [Google Scholar]
Ogawa 2008 {published data only}
- Ogawa H, Itoh S, Ikeda M, Suzuki K, Naganawa S. Intraductal papillary mucinous neoplasm of the pancreas: Assessment of the likelihood of invasiveness with multisection CT. Radiology 2008;248(3):876‐86. [DOI] [PubMed] [Google Scholar]
Ogawa 2014 {published data only}
- Ogawa T, Horaguchi J, Fujita N, Noda Y, Kobayashi G, Ito K, et al. Diffusion‐weighted magnetic resonance imaging for evaluating the histological degree of malignancy in patients with intraductal papillary mucinous neoplasm. Journal of Hepato‐Biliary‐Pancreatic Sciences 2014;21(11):801‐8. [DOI] [PubMed] [Google Scholar]
Otomi 2014 {published data only}
- Otomi Y, Otsuka H, Terazawa K, Nose H, Kubo M, Matsuzaki K, et al. Comparing the performance of visual estimation and standard uptake value of f‐18 fluorodeoxyglucose positron emission tomography/computed tomography for detecting malignancy in pancreatic tumors other than invasive ductal carcinoma. Journal of Medical Investigation 2014;61(1‐2):171‐9. [DOI] [PubMed] [Google Scholar]
Pais 2007 {published data only}
- Pais SA, Attasaranya S, Leblanc JK, Sherman S, Schmidt CM, DeWitt J. Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: Correlation with surgical histopathology. Clinical Gastroenterology & Hepatology 2007;5(4):489‐95. [DOI] [PubMed] [Google Scholar]
Sahani 2006 {published data only}
- Sahani DV, Kadavigere R, Blake M, Castillo CFD, Lauwers GY, Hahn PF. Intraductal papillary mucinous neoplasm of pancreas: Multi‐detector row CT with 20 curved reformations ‐ correlation with MRCP. Radiology 2006;238(2):560‐9. [DOI] [PubMed] [Google Scholar]
Saito 2013 {published data only}
- Saito M, Ishihara T, Tada M, Tsuyuguchi T, Mikata R, Sakai Y, et al. Use of f‐18 fluorodeoxyglucose positron emission tomography with dual‐phase imaging to identify intraductal papillary mucinous neoplasm. Clinical Gastroenterology & Hepatology 2013;11(2):181‐6. [DOI] [PubMed] [Google Scholar]
Salla 2007 {published data only}
- Salla C, Chatzipantelis P, Konstantinou P, Karoumpalis I, Sakellariou S, Pantazopoulou A, et al. Endoscopic ultrasound‐guided fine‐needle aspiration cytology in the diagnosis of intraductal papillary mucinous neoplasms of the pancreas. A study of 8 cases. JOP: Journal of the Pancreas 2007;8(6):715‐24. [PubMed] [Google Scholar]
Sedlack 2002 {published data only}
- Sedlack R, Affi A, Vazquez‐Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointestinal Endoscopy 2002;56(4):543‐7. [DOI] [PubMed] [Google Scholar]
Smith 2016 {published data only}
- Smith AL, Abdul‐Karim FW, Goyal A. Cytologic categorization of pancreatic neoplastic mucinous cysts with an assessment of the risk of malignancy: A retrospective study based on the Papanicolaou Society of Cytopathology guidelines. Cancer Cytopathology 2016;124(4):285‐93. [DOI] [PubMed] [Google Scholar]
Takanami 2011 {published data only}
- Takanami K, Hiraide T, Tsuda M, Nakamura Y, Kaneta T, Takase K, et al. Additional value of FDG PET/CT to contrast‐enhanced CT in the differentiation between benign and malignant intraductal papillary mucinous neoplasms of the pancreas with mural nodules. Annals of Nuclear Medicine 2011;25(7):501‐10. [DOI] [PubMed] [Google Scholar]
Takeshita 2008 {published data only}
- Takeshita K, Kutomi K, Takada K, Haruyama T, Fukushima J, Aida R, et al. Differential diagnosis of benign or malignant intraductal papillary mucinous neoplasm of the pancreas by multidetector row helical computed tomography: Evaluation of predictive factors by logistic regression analysis. Journal of Computer Assisted Tomography 2008;32(2):191‐7. [DOI] [PubMed] [Google Scholar]
Tan 2009 {published data only}
- Tan L, Zhao YE, Wang DB, Wang QB, Hu J, Chen KM, et al. Imaging features of intraductal papillary mucinous neoplasms of the pancreas in multi‐detector row computed tomography. World Journal of Gastroenterology 2009;15(32):4037‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taouli 2000 {published data only}
- Taouli B, Vilgrain V, Vullierme MP, Terris B, Denys A, Sauvanet A, et al. Intraductal papillary mucinous tumors of the pancreas: Helical CT with histopathologic correlation. Radiology 2000;217(3):757‐64. [DOI] [PubMed] [Google Scholar]
Tomimaru 2010 {published data only}
- Tomimaru Y, Takeda Y, Tatsumi M, Kim T, Kobayashi S, Marubashii S, et al. Utility of 2‐[f‐18] fluoro‐2‐deoxy‐d‐glucose positron emission tomography in differential diagnosis of benign and malignant intraductal papillary‐mucinous neoplasm of the pancreas. Oncology Reports 2010;24(3):613‐20. [DOI] [PubMed] [Google Scholar]
Yamao 2001 {published data only}
- Yamao K, Ohashi K, Nakamura T, Suzuki T, Watanabe Y, Shimizu Y, et al. Evaluation of various imaging methods in the differential diagnosis of intraductal papillary‐mucinous tumor (IPMT) of the pancreas. Hepato‐Gastroenterology 2001;48(40):962‐6. [PubMed] [Google Scholar]
Zhan 2011 {published data only}
- Zhan XB, Xie B, Ma D, Li ZS. Value of endoscopic ultrasonographic features in predicting the malignancy of pancreatic mucinous cystic neoplasms and intraductal papillary mucinous neoplasms. Gut 2011;60:A202. [Google Scholar]
Zhan 2013 {published data only}
- Zhan XB, Wang B, Liu F, Ye XF, Jin ZD, Li ZS. Cyst fluid carcinoembryonic antigen concentration and cytology by endosonography‐guided fine needle aspiration in predicting malignant pancreatic mucinous cystic neoplasms. Journal of Digestive Diseases 2013;14(4):191‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aburime 2014 {published data only}
- Aburime E, Jafri M, Afzal A, Gress FG. Use of real time EUS elastography in targeting EUS‐FNA biopsy of suspicious pancreatic masses: A pilot study. Gastrointestinal Endoscopy 2014;1:AB333. [Google Scholar]
Adamek 2000 {published data only}
- Adamek HE, Albert J, Breer H, Weitz M, Schilling D, Riemann JF. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: A prospective controlled study. Lancet 2000;356(9225):190‐3. [DOI] [PubMed] [Google Scholar]
Adimoolam 2011 {published data only}
- Adimoolam V, Sanchez MJ, Siddiqui UD, Yu S, Dzuira JD, Padda MS, et al. Endoscopic ultrasound identifies synchronous pancreas cystic lesions not seen on initial cross‐sectional imaging. Pancreas 2011;40(7):1070‐2. [DOI] [PubMed] [Google Scholar]
Afify 2003 {published data only}
- Afify AM, al‐Khafaji BM, Kim B, Scheiman JM. Endoscopic ultrasound‐guided fine needle aspiration of the pancreas. Diagnostic utility and accuracy. Acta Cytologica 2003;47(3):341‐8. [DOI] [PubMed] [Google Scholar]
Agarwal 2004 {published data only}
- Agarwal B, Abu‐Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound‐guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. American Journal of Gastroenterology 2004;99(5):844‐50. [DOI] [PubMed] [Google Scholar]
Agarwal 2008a {published data only}
- Agarwal B, Krishna NB, Labundy JL, Safdar R, Akduman EI. EUS and/or EUS‐guided FNA in patients with CT and/or magnetic resonance imaging findings of enlarged pancreatic head or dilated pancreatic duct with or without a dilated common bile duct. Gastrointestinal Endoscopy 2008;68(2):237‐42. [DOI] [PubMed] [Google Scholar]
Agarwal 2008b {published data only}
- Agarwal B, Ludwig OJ, Collins BT, Cortese C. Immunostaining as an adjunct to cytology for diagnosis of pancreatic adenocarcinoma. Clinical Gastroenterology and Hepatology 2008;6(12):1425‐31. [DOI] [PubMed] [Google Scholar]
Agarwal 2008c {published data only}
- Agarwal B, Reddy AV, Labundy JI, Krishna NB. Diagnostic accuracy of EUS/EUS‐FNA for pancreatic cancer in patients with or without associated chronic pancreatitis. Gastrointestinal Endoscopy 2008;67(5):AB213. [Google Scholar]
Aguilar‐Saavedra 2011 {published data only}
- Aguilar‐Saavedra JR, Lentz G, Chalikonda S, Vogt D, Walsh M. Pancreatic serous cystic neoplasms ‐ is size an indication for surgery?. Gastroenterology 2011;140(5 Suppl 1):S1038. [Google Scholar]
Ahmad 2001 {published data only}
- Ahmad NA, Kochman ML, Lewis JD, Ginsberg GG. Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas?. American Journal of Gastroenterology 2001;96(12):3295‐300. [DOI] [PubMed] [Google Scholar]
Ahmad 2003 {published data only}
- Ahmad NA, Kochman ML, Brensinger C, Brugge WR, Faigel DO, Gress FG, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non‐neoplastic pancreatic cystic lesions. Gastrointestinal Endoscopy 2003;58(1):59‐64. [DOI] [PubMed] [Google Scholar]
Ainsworth 2010 {published data only}
- Ainsworth AP, Hansen T, Fristrup CW, Mortensen MB. Indications for and clinical impact of repeat endoscopic ultrasound. Scandinavian Journal of Gastroenterology 2010;45(4):477‐82. [DOI] [PubMed] [Google Scholar]
Aithal 2001 {published data only}
- Aithal GP, Cunningham JT, Kim EY, Wallace MB, Patel RS, Hawes RH, et al. Accuracy of endoscopic ultrasound in suspecting the diagnosis of intraductal papillary mucinous tumor of the pancreas. Gastrointestinal Endoscopy 2001;53(5):AB166. [DOI] [PubMed] [Google Scholar]
Aithal 2002 {published data only}
- Aithal GP, Chen RY, Cunningham JT, Durkalski V, Kim EY, Patel RS, et al. Accuracy of EUS for detection of intraductal papillary mucinous tumor of the pancreas. Gastrointestinal Endoscopy 2002;56(5):701‐7. [DOI] [PubMed] [Google Scholar]
Akahoshi 1998 {published data only}
- Akahoshi K, Chijiiwa Y, Nakano I, Nawata H, Ogawa Y, Tanaka M, et al. Diagnosis and staging of pancreatic cancer by endoscopic ultrasound. British Journal of Radiology 1998;71(845):492‐6. [DOI] [PubMed] [Google Scholar]
Akwei 2011 {published data only}
- Akwei S, Al‐Khyatt W, Malhotra R, Whiting F, Boyce T, Hammouche D, et al. The value of endoscopic ultrasound and endoscopic ultrasound guided fine needle aspiration in the diagnosis and assessment of resectability of mass lesions in the head of pancreas. HPB 2011;13:104. [Google Scholar]
Al‐Haddad 2007 {published data only}
- Al‐Haddad M, Raimondo M, Woodward T, Krishna M, Pungpapong S, Noh K, et al. Safety and efficacy of cytology brushings versus standard FNA in evaluating cystic lesions of the pancreas: A pilot study. Gastrointestinal Endoscopy 2007;65(6):894‐8. [DOI] [PubMed] [Google Scholar]
Al‐Haddad 2010a {published data only}
- Al‐Haddad M, Hajj II, Eloubeidi MA. Endoscopic ultrasound for the evaluation of cystic lesions of the pancreas. Journal of the Pancreas 2010;11(4):299‐309. [PubMed] [Google Scholar]
Al‐Haddad 2010b {published data only}
- Al‐Haddad MA, Dewitt JM, Sherman S, Schmidt CM, Juliar BE, Stuart JS, et al. Diagnostic accuracy of pancreatic cyst fluid tumor markers obtained by EUS‐guided sampling. Gastrointestinal Endoscopy 2010;71(5):AB283. [Google Scholar]
Al‐Haddad 2014 {published data only}
- Al‐Haddad M, Dewitt J, Sherman S, Max Schmidt C, Leblanc JK, McHenry L, et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointestinal Endoscopy 2014;79(1):79‐87. [DOI] [PubMed] [Google Scholar]
Alizadeh 2014 {published data only}
- Alizadeh AHM, Hadizadeh M, Padashi M, Shahbaazi S, Molaee M, Shariatpanahi ZV. Comparison of two techniques for endoscopic ultrasonography fine‐needle aspiration in solid pancreatic mass. Endoscopic Ultrasound 2014;3(3):174‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Jebreen 2004 {published data only}
- Al‐Jebreen AM, Perini R, Sutherland F, Romagnuolo J. Accuracy of endoscopic ultrasound, fine needle aspiration, cytology, and cyst fluid tumor markers in patients with pancreatic cystic lesions: Comparison with surgical pathology and 12‐month follow‐up. Gastrointestinal Endoscopy 2004;59(5):P232. [Google Scholar]
Aljebreen 2007 {published data only}
- Aljebreen AM, Romagnuolo J, Perini R, Sutherland F. Utility of endoscopic ultrasound, cytology and fluid carcinoembryonic antigen and CA 19‐9 levels in pancreatic cystic lesions. World Journal of Gastroenterology 2007;13(29):3962‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Najami 2015 {published data only}
- Al‐Najami I, Ainsworth AP. Endoscopic ultrasonography is a valuable diagnostic tool in patients with incidental findings in the pancreas or bile ducts. Danish Medical Journal 2015;62(3):A5039. [PubMed] [Google Scholar]
Alsohaibani 2008 {published data only}
- Alsohaibani F, Bigam D, Kneteman N, Shapiro AMJ, Sandha GS. The impact of preoperative endoscopic ultrasound on the surgical management of pancreatic neuroendocrine tumours. Canadian Journal of Gastroenterology 2008;22(10):817‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alsohaibani 2009 {published data only}
- Alsohaibani F, Girgis S, Sandha GS. Does onsite cytotechnology evaluation improve the accuracy of endoscopic ultrasound‐guided fine‐needle aspiration biopsy?. Canadian Journal of Gastroenterology 2009;23(1):26‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alston 2014 {published data only}
- Alston E, Bae S, Eltoum IA. Atypical cytologic diagnostic category in EUS‐FNA of the pancreas: Follow‐up, outcomes, and predictive models. Cancer Cytopathology 2014;122(6):428‐34. [DOI] [PubMed] [Google Scholar]
Amin 2006 {published data only}
- Amin Z, Theis B, Russell RC, House C, Novelli M, Lees WR. Diagnosing pancreatic cancer: the role of percutaneous biopsy and CT. Clinical Radiology 2006;61(12):996‐1002. [DOI] [PubMed] [Google Scholar]
Andersen 1994 {published data only}
- Andersen HB, Pihl HE, Tjalve E, Burcharth F. CT diagnosis of pancreatic or periampullary cancer. Ugeskrift for Laeger 1994;156(50):7534‐7. [PubMed] [Google Scholar]
Antonini 2015 {published data only}
- Antonini F, Belfiori V, Minicis S, Lo Cascio M, Marraccini B, Piergallini S, et al. Is "wet suction" technique useful to improve the diagnostic accuracy of endoscopic ultrasound guided fine‐needle biopsy of pancreatic masses without rapid on‐site cytologic evaluation? Preliminary results of a comparative study. Digestive and Liver Disease 2015;47(Suppl 2):e149. [Google Scholar]
Arabul 2012 {published data only}
- Arabul M, Karakus F, Alper E, Kandemir A, Celik M, Karakus V, et al. Comparison of multidetector CT and endoscopic ultrasonography in malignant pancreatic mass lesions. Hepato‐Gastroenterology 2012;59(117):1599‐603. [DOI] [PubMed] [Google Scholar]
Ardengh 2007a {published data only}
- Ardengh JC, Lopes CV, Campos AD, Pereira de Lima LF, Venco F, Modena JL. Endoscopic ultrasound and fine needle aspiration in chronic pancreatitis: Differential diagnosis between pseudotumoral masses and pancreatic cancer. JOP: Journal of the Pancreas [Electronic Resource] 2007;8(4):413‐21. [PubMed] [Google Scholar]
Ardengh 2007b {published data only}
- Ardengh JC, Lopes CV, Lima LF, Oliveira JR, Venco F, Santo GC, et al. Diagnosis of pancreatic tumors by endoscopic ultrasound‐guided fine‐needle aspiration. World Journal of Gastroenterology 2007;13(22):3112‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ardengh 2008a {published data only}
- Ardengh JC, Lopes CV, Lima LF, Venco F, Santo GC, Begnami MD, et al. Cell block technique and cytological smears for the differential diagnosis of pancreatic neoplasms after endosonography‐guided fine‐needle aspiration. Acta Gastroenterologica Latinoamericana 2008;38(4):246‐51. [PubMed] [Google Scholar]
Ardengh 2008b {published data only}
- Ardengh JC, Paulo GA, Nakao FS, Venco F, Santo GC, Geocze S. Endoscopic ultrasound guided fine‐needle aspiration core biopsy: Comparison between an automatic biopsy device and two conventional needle systems. Acta Gastroenterologica Latinoamericana 2008;38(2):105‐15. [PubMed] [Google Scholar]
Ardengh 2013 {published data only}
- Ardengh JC, Lopes CV, Kemp R, Venco F, Lima‐Filho ER, dos Santos JS. Accuracy of endoscopic ultrasound‐guided fine‐needle aspiration in the suspicion of pancreatic metastases. BMC Gastroenterology 2013;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Argimak 2009 {published data only}
- Argimak Y, Temizoz O, Tosun A, Cakir B. Evaluation of pancreatic malign tumours with mangafodipir trisodium. Trakya Universitesi Tip Fakultesi Dergisi 2009;26(1):53‐8. [Google Scholar]
Arikawa 2007 {published data only}
- Arikawa S, Uchida M, Shinagawa M, Uozumi J, Hayabuchi N, Okabe Y, et al. The role of multi‐detector‐row computed tomograph in the diagnosis of intraductal papillary‐mucinous tumors of the pancreas in comparison to endoscopic retrograde pancreatography, endoscopic ultrasonography, magnetic resonance cholangiopancreatography. Nippon Shokakibyo Gakkai Zasshi ‐ Japanese Journal of Gastroenterology 2007;104(3):373‐80. [PubMed] [Google Scholar]
Arlt 2013 {published data only}
- Arlt A, Ellrichmann M, Schreiber S, Monig H. Diagnosis and differential diagnosis of panceatic lesions. Deutsche Medizinische Wochenschrift 2013;138(49):2539‐42. [DOI] [PubMed] [Google Scholar]
Asagi 2013 {published data only}
- Asagi A, Ohta K, Nasu J, Tanada M, Nadano S, Nishimura R, et al. Utility of contrast‐enhanced FDG‐PET/CT in the clinical management of pancreatic cancer: Impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas 2013;42(1):11‐9. [DOI] [PubMed] [Google Scholar]
Aslanian 2011 {published data only}
- Aslanian HR, Estrada JD, Rossi F, Dziura J, Jamidar PA, Siddiqui UD. Endoscopic ultrasound and endoscopic retrograde cholangiopancreatography for obstructing pancreas head masses: Combined or separate procedures?. Journal of Clinical Gastroenterology 2011;45(8):711‐3. [DOI] [PubMed] [Google Scholar]
Asnacios 2003 {published data only}
- Asnacios A, Buscail L, Selves J, Nogueira E, Fourtanier G, Suc B, et al. Predictive diagnostic value of endoscopic ultrasound‐guided fine needle aspiration of unilocular macrocystic lesions of the pancreas. Gastroenterology 2003;124(4):A440. [Google Scholar]
Atef 2013 {published data only}
- Atef E, Nakeeb A, Hanafy E, Hemaly M, Hamdy E, El‐Geidie A. Pancreatic cystic neoplasms: Predictors of malignant behavior and management. Saudi Journal of Gastroenterology 2013;19(1):45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Attasaranya 2007 {published data only}
- Attasaranya S, Pais S, LeBlanc J, McHenry L, Sherman S, DeWitt JM. Endoscopic ultrasound‐guided fine needle aspiration and cyst fluid analysis for pancreatic cysts. JOP: Journal of the Pancreas [Electronic Resource] 2007;8(5):553‐63. [PubMed] [Google Scholar]
Awadallah 2008 {published data only}
- Awadallah NS, Shroyer KR, Langer DA, Torkko KC, Chen YK, Bentz JS, et al. Detection of b7‐h4 and p53 in pancreatic cancer: Potential role as a cytological diagnostic adjunct. Pancreas 2008;36(2):200‐6. [DOI] [PubMed] [Google Scholar]
Azizi 2014 {published data only}
- Azizi A, Kumbhari V, Gill R, Pavey D, Leong RWL, Merrett N, et al. Diagnostic yield of surepath in solid pancreatic and gist lesions. Journal of Gastroenterology and Hepatology 2014;29(Suppl S2):48. [Google Scholar]
Baba 2004 {published data only}
- Baba T, Yamaguchi T, Ishihara T, Kobayashi A, Oshima T, Sakaue N, et al. Distinguishing benign from malignant intraductal papillary mucinous tumors of the pancreas by imaging techniques. Pancreas 2004;29(3):212‐7. [DOI] [PubMed] [Google Scholar]
Baek 2015 {published data only}
- Baek HW, Park MJ, Rhee YY, Lee KB, Kim MA, Park IA. Diagnostic accuracy of endoscopic ultrasound‐guided fine needle aspiration cytology of pancreatic lesions. Journal of Pathology & Translational Medicine 2015;49(1):52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baghbanian 2012 {published data only}
- Baghbanian M, Shabazkhani B, Ghofrani H, Forutan H, Dariani N, Farahvash M, et al. Efficacy of endoscopic ultrasound guided fine needle aspiration in patients with solid pancreatic neoplasms. Saudi Journal of Gastroenterology 2012;18(6):358‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baiocchi 2008 {published data only}
- Baiocchi GL, Portolani N, Bertagna F, Gheza F, Pizzocaro C, Giubbini R, et al. Possible additional value of 18FDG‐PET in managing pancreas intraductal papillary mucinous neoplasms: Preliminary results. Journal of Experimental and Clinical Cancer Research 2008;27(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baiocchi 2010 {published data only}
- Baiocchi GL, Portolani N, Missale G, Baronchelli C, Gheza F, Cantu M, et al. Intraductal papillary mucinous neoplasm of the pancreas (IPMN): Clinico‐pathological correlations and surgical indications. World Journal of Surgical Oncology 2010;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baiocchi 2012 {published data only}
- Baiocchi GL, Bertagna F, Gheza F, Grazioli L, Calanducci D, Giubbini R, et al. Searching for indicators of malignancy in pancreatic intraductal papillary mucinous neoplasms: the value of 18FDG‐PET confirmed. Annals of Surgical Oncology 2012;19(11):3574‐80. [DOI] [PubMed] [Google Scholar]
Bali 2011 {published data only}
- Bali MA, Metens T, Denolin V, Delhaye M, Demetter P, Closset J, et al. Tumoral and nontumoral pancreas: Correlation between quantitative dynamic contrast‐enhanced MR imaging and histopathologic parameters. Radiology 2011;261(2):456‐66. [DOI] [PubMed] [Google Scholar]
Bang 2012a {published data only}
- Bang JY, Hebert‐Magee S, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the 22‐gauge aspiration and 22‐gauge biopsy needles for EUS‐guided sampling of solid pancreatic mass lesions. Gastrointestinal Endoscopy 2012;76(2):321‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bang 2012b {published data only}
- Bang JY, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the fanning and standard techniques for EUS‐guided FNA of solid pancreatic mass lesions. Gastrointestinal Endoscopy 2012;1:AB445‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bang 2013a {published data only}
- Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound‐guided fine needle aspiration of solid pancreatic mass lesions. Journal of Digestive Endoscopy 2013;4(2):55‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bang 2013b {published data only}
- Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound‐guided fine‐needle aspiration of solid pancreatic mass lesions. Endoscopy 2013;45(6):445‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bang 2015 {published data only}
- Bang JY, Krall K, Hebert‐Magee S, Hasan MK, Navaneethan U, Hawes R, et al. Needle changes during EUS‐FNA: An attempt to improve outcomes. Gastrointestinal Endoscopy 2015;1:AB431. [Google Scholar]
Barber 2011 {published data only}
- Barber TW, Kalff V, Cherk MH, Yap KSK, Evans P, Kelly MJ. 18f‐FDG PET/CT influences management in patients with known or suspected pancreatic cancer. Internal Medicine Journal 2011;41(11):776‐83. [DOI] [PubMed] [Google Scholar]
Bares 1994 {published data only}
- Bares R, Klever P, Hauptmann S, Hellwig D, Fass J, Cremerius U, et al. F‐18 fluorodeoxyglucose PET in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology 1994;192(1):79‐86. [DOI] [PubMed] [Google Scholar]
Barkin 1977 {published data only}
- Barkin J, Vining D, Miale A, Gottlieb S, Redlhammer DE, Kalser MH. Computerized tomography, diagnostic ultrasound, and radionuclide scanning ‐ comparison of efficacy in diagnosis of pancreatic carcinoma. JAMA 1977;238(19):2040‐2. [PubMed] [Google Scholar]
Baron 1997 {published data only}
- Baron PL, Aabakken LE, Cole DJ, LeVeen MB, Baron LF, Daniel DM, et al. Differentiation of benign from malignant pancreatic masses by endoscopic ultrasound. Annals of Surgical Oncology 1997;4(8):639‐43. [DOI] [PubMed] [Google Scholar]
Barral 2013a {published data only}
- Barral M, Sebbag‐Sfez D, Hoeffel C, Chaput U, Dohan A, Eveno C, et al. Characterization of focal pancreatic lesions by the measurement of apparent diffusion coefficient in MRI 1.5 Tesla: Preliminary experience. Journal De Radiologie Diagnostique Et Interventionnelle 2013;94(6):636‐44. [DOI] [PubMed] [Google Scholar]
Barral 2013b {published data only}
- Barral M, Sebbag‐Sfez D, Hoeffel C, Chaput U, Dohan A, Eveno C, et al. Characterization of focal pancreatic lesions using normalized apparent diffusion coefficient at 1.5‐Tesla: Preliminary experience. Diagnostic and Interventional Imaging 2013;94(6):619‐27. [DOI] [PubMed] [Google Scholar]
Barresi 2014 {published data only}
- Barresi L, Tarantino I, Traina M, Granata A, Curcio G, Azzopardi N, et al. Endoscopic ultrasound‐guided fine needle aspiration and biopsy using a 22‐gauge needle with side fenestration in pancreatic cystic lesions. Digestive & Liver Disease 2014;46(1):45‐50. [DOI] [PubMed] [Google Scholar]
Barron 2014 {published data only}
- Barron MR, Roch AM, Waters JA, Parikh JA, DeWitt JM, Al‐Haddad MA, et al. Does preoperative cross‐sectional imaging accurately predict main duct involvement in intraductal papillary mucinous neoplasm?. Journal of Gastrointestinal Surgery 2014;18(3):447‐55; discussion 455‐6. [DOI] [PubMed] [Google Scholar]
Bartsch 1998 {published data only}
- Bartsch D, Bastian D, Barth P, Schudy A, Nies C, Kisker O, et al. K‐ras oncogene mutations indicate malignancy in cystic tumors of the pancreas. Annals of Surgery 1998;228(1):79‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basir 2003 {published data only}
- Basir Z, Pello N, Dayer AM, Shidham VB, Komorowski RA. Accuracy of cytologic interpretation of pancreatic neoplasms by fine needle aspiration and pancreatic duct brushings. Acta Cytologica 2003;47(5):733‐8. [DOI] [PubMed] [Google Scholar]
Bassi 2003 {published data only}
- Bassi C, Salvia R, Molinari E, Biasutti C, Falconi M, Pederzoli P. Management of 100 consecutive cases of pancreatic serous cystadenoma: Wait for symptoms and see at imaging or vice versa?. World Journal of Surgery 2003;27(3):319‐23. [DOI] [PubMed] [Google Scholar]
Beal 2015a {published data only}
- Beal H, Shea JE, Witt B, Adler DG, Mulvihill SJ, Downs‐Kelly E, et al. Accuracy of diagnosing pancreatic ductal adenocarcinoma by EUS‐FNA at a single institution. Journal of Clinical Oncology 2015;33(3 Suppl):258. [Google Scholar]
Beal 2015b {published data only}
- Beal HL, Shea JE, Witt BL, Adler DG, Mulvihill SJ, Downs‐Kelly E, et al. Accuracy of diagnosing PDA, neuroendocrine tumors, and IPMN by EUS‐FNA at a single institution. Journal of Gastroenterology and Hepatology Research 2015;4(12):1844‐9. [Google Scholar]
Becker 2001 {published data only}
- Becker D, Strobel D, Bernatik T, Hahn EG. Echo‐enhanced color‐ and power‐doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointestinal Endoscopy 2001;53(7):784‐9. [DOI] [PubMed] [Google Scholar]
Beliao 2012 {published data only}
- Beliao S, Ferreira A, Vierasu I, Blocklet D, Goldman S, Metens T, et al. MR imaging versus PET/CT for evaluation of pancreatic lesions. European Journal of Radiology 2012;81(10):2527‐32. [DOI] [PubMed] [Google Scholar]
Bentz 1998 {published data only}
- Bentz JS, Kochman ML, Faigel DO, Ginsberg GG, Smith DB, Gupta PK. Endoscopic ultrasound‐guided real‐time fine‐needle aspiration: Clinicopathologic features of 60 patients. Diagnostic Cytopathology 1998;18(2):98‐109. [DOI] [PubMed] [Google Scholar]
Bergeron 2015 {published data only}
- Bergeron JP, Perry KD, Houser PM, Yang J. Endoscopic ultrasound‐guided pancreatic fine‐needle aspiration: Potential pitfalls in one institution's experience of 1212 procedures. Cancer Cytopathology 2015;123(2):98‐107. [DOI] [PubMed] [Google Scholar]
Bernstein 2013 {published data only}
- Bernstein GR, Jin DX, Fontaine NN, Shah PM, Chandrasekhara V, Kochman ML, et al. Pancreatic cyst size does not have an impact on diagnostic yield of endosonographic ultrasound‐guided fine needle aspiration. Gastrointestinal Endoscopy 2013;1:AB408. [Google Scholar]
Berzosa 2015 {published data only}
- Berzosa M, Villa N, El‐Serag HB, Sejpal, Patel KV. Comparison of endoscopic ultrasound guided 22‐gauge core needle with standard 25‐gauge fine‐needle aspiration for diagnosing solid pancreatic lesions. Endoscopic Ultrasound 2015;4(1):28‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutani 1995 {published data only}
- Bhutani M, Hoffman B, Vanvelse A, Sanderscliette A, Hawes R. Endoscopic ultrasound (EUS) guided fine‐needle aspiration (FNA) of malignant pancreatic lesions ‐ accuracy, safety and clinical utility. Gastrointestinal Endoscopy 1995;41(4):298. [Google Scholar]
Bhutani 1997 {published data only}
- Bhutani MS, Hawes RH, Baron PL, Sanders‐Cliette A, Velse A, Osborne JF, et al. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy 1997;29(9):854‐8. [DOI] [PubMed] [Google Scholar]
Bick 2015 {published data only}
- Bick BL, Enders FT, Levy MJ, Zhang L, Henry MR, Dayyeh BKA, et al. The string sign for diagnosis of mucinous pancreatic cysts. Endoscopy 2015;47(7):626‐31. [DOI] [PubMed] [Google Scholar]
Bighi 1989 {published data only}
- Bighi S, Cervi P, Lupi L, Cardona P, Zappi M, Trevisani L. The diagnostic significance of computer tomography in pancreas cancer: 3‐year experience. Italian Current Radiology 1989;8(4):249‐52. [Google Scholar]
Binmoeller 1998a {published data only}
- Binmoeller KF, Brand B, Thul R, Rathod V, Soehendra N. EUS‐guided, fine‐needle aspiration biopsy using a new mechanical scanning puncture echoendoscope. Gastrointestinal Endoscopy 1998;47(5):335‐40. [DOI] [PubMed] [Google Scholar]
Binmoeller 1998b {published data only}
- Binmoeller KF, Thul R, Rathod V, Henke P, Brand B, Jabusch HC, et al. Endoscopic ultrasound‐guided, 18‐gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointestinal Endoscopy 1998;47(2):121‐7. [DOI] [PubMed] [Google Scholar]
Bluen 2012 {published data only}
- Bluen BE, Lachter J, Khamaysi I, Kamal Y, Malkin L, Keren R, et al. Accuracy and quality assessment of EUS‐FNA: A single‐center large cohort of biopsies. Diagnostic & Therapeutic Endoscopy 2012;2012:139563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bournet 2007 {published data only}
- Bournet B, Souque A, Barthet M, Senesse P, Lesavre N, O'Toole D, et al. Diagnostic performances of oncogene k‐ras mutation analysis coupled to fine needle aspiration biopsy under endoscopic ultrasound (EUS‐FNA) for the diagnosis of solid pancreatic masses: A French multicenter prospective study. Gastroenterology 2007;132(4 Suppl 2):A690‐A. [Google Scholar]
Bournet 2009 {published data only}
- Bournet B, Souque A, Senesse P, Assenat E, Barthet M, Lesavre N, et al. Endoscopic ultrasound‐guided fine‐needle aspiration biopsy coupled with kras mutation assay to distinguish pancreatic cancer from pseudotumoral chronic pancreatitis. Endoscopy 2009;41(6):552‐7. [DOI] [PubMed] [Google Scholar]
Bournet 2012 {published data only}
- Bournet B, Pointreau A, Souque A, Oumouhou N, Muscari F, Lepage B, et al. Gene expression signature of advanced pancreatic ductal adenocarcinoma using low density array on endoscopic ultrasound‐guided fine needle aspiration samples. Pancreatology 2012;12(1):27‐34. [DOI] [PubMed] [Google Scholar]
Bournet 2015 {published data only}
- Bournet B, Selves J, Grand D, Danjoux M, Hanoun N, Cordelier P, et al. Endoscopic ultrasound‐guided fine‐needle aspiration biopsy coupled with a kras mutation assay using allelic discrimination improves the diagnosis of pancreatic cancer. Journal of Clinical Gastroenterology 2015;49(1):50‐6. [DOI] [PubMed] [Google Scholar]
Boutros 2010 {published data only}
- Boutros C, Genova E, Haniff M, Toubia N, Somasundar P, Espast NJ. Single institution experience of 215 patients comparing accuracy of endoscopic ultrasound with subsequent pancreatic surgery. HPB 2010;12:413‐4. [Google Scholar]
Brand 2002 {published data only}
- Brand B, Ponnudurai R, Ryozawa S, Mendes KL, Yang AM, Bohnacker S, et al. A new radial mechanical puncture echoendoscope: Prospective comparison with standard linear and radial echoendoscopes in assessment of focal pancreatic lesions. Gastrointestinal Endoscopy 2002;55(2):249‐54. [DOI] [PubMed] [Google Scholar]
Brand 2014 {published data only}
- Brand RE, Adai AT, Centeno BA, Lee LS, Rateb G, Vignesh S, et al. A microRNA‐based test improves endoscopic ultrasound‐guided cytologic diagnosis of pancreatic cancer. Clinical Gastroenterology and Hepatology 2014;12(10):1717‐23. [DOI] [PubMed] [Google Scholar]
Brenin 1995 {published data only}
- Brenin DR, Talamonti MS, Yang EY, Sener SF, Haines GK, Joehl RJ, et al. Cystic neoplasms of the pancreas. A clinicopathologic study, including DNA flow cytometry. Archives of Surgery 1995;130(10):1048‐54. [DOI] [PubMed] [Google Scholar]
Brimiene 2011 {published data only}
- Brimiene V, Brimas G, Strupas K. Differential diagnosis between chronic pancreatitis and pancreatic cancer: A prospective study of 156 patients. Medicina (Kaunas, Lithuania) 2011;47(3):154‐62. [PubMed] [Google Scholar]
Brugge 2000 {published data only}
- Brugge WR. The role of EUS in the diagnosis of cystic lesions of the pancreas. Gastrointestinal Endoscopy 2000;52(6):S18‐22. [DOI] [PubMed] [Google Scholar]
Brugge 2004a {published data only}
- Brugge WR. Evaluation of pancreatic cystic lesions with EUS. Gastrointestinal Endoscopy 2004;59(6):698‐707. [DOI] [PubMed] [Google Scholar]
Brugge 2004b {published data only}
- Brugge WR, Lewandrowski K, Lee‐Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology 2004;126(5):1330‐6. [DOI] [PubMed] [Google Scholar]
Bruno 2009 {published data only}
- Bruno M, Bosco M, Carucci P, Pacchioni D, Repici A, Mezzabotta L, et al. Preliminary experience with a new cytology brush in EUS‐guided FNA. Gastrointestinal Endoscopy 2009;70(6):1220‐4. [DOI] [PubMed] [Google Scholar]
Buchholz 2005 {published data only}
- Buchholz M, Kestler HA, Bauer A, Bock W, Rau B, Leder G, et al. Specialized DNA arrays for the differentiation of pancreatic tumors. Clinical Cancer Research 2005;11(22):8048‐54. [DOI] [PubMed] [Google Scholar]
Buchs 2011 {published data only}
- Buchs NC, Buhler L, Bucher P, Willi JP, Frossard JL, Roth AD, et al. Value of contrast‐enhanced 18f‐fluorodeoxyglucose positron emission tomography/computed tomography in detection and presurgical assessment of pancreatic cancer: A prospective study. Journal of Gastroenterology & Hepatology 2011;26(4):657‐62. [DOI] [PubMed] [Google Scholar]
Butt 2015a {published data only}
- Butt MA, Papasavvas S, Sangwaiya A, Westaby J, Bansi D, Westaby D, et al. Modelling of suspicious and high risk endosonographic morphology, cytopathology and cyst biochemistry highlights the efficacy of endosonography alone to predict operative histological outcome in pancreatic cystic tumours. Gastrointestinal Endoscopy 2015;81(5 Suppl):AB562‐3. [Google Scholar]
Butt 2015b {published data only}
- Butt MA, Papasavvas S, Sangwaiya A, Westaby J, Kim JU, Bansi D, et al. Modelling of suspicious and high risk endosonographic morphology, cytopathology and cyst biochemistry highlights the accuracy of endosonography to predict operative histological outcome in pancreatic cystic tumours. United European Gastroenterology Journal 2015;3(5 Suppl):A537. [Google Scholar]
Caglar 2013 {published data only}
- Caglar E, Senturk H, Atasoy D, Sisman G, Canbakan BI, Tuncer M. The role of EUS and EUS‐FNA in the management of pancreatic masses: Five‐year experience. Hepato‐Gastroenterology 2013;60(124):896‐9. [DOI] [PubMed] [Google Scholar]
Cahn 1996 {published data only}
- Cahn M, Chang K, Nguyen P, Butler J. Impact of endoscopic ultrasound with fine‐needle aspiration on the surgical management of pancreatic cancer. American Journal of Surgery 1996;172(5):470‐2. [DOI] [PubMed] [Google Scholar]
Caldelari 2011 {published data only}
- Caldelari ACA, Miquel R, Bombi JA, Gines A, Fernandez‐Esparrach G, Ayuso JR, et al. Malignancy predictive factors in pancreatic intraductal papillary mucinous neoplasm. Medicina Clinica 2011;137(14):631‐6. [DOI] [PubMed] [Google Scholar]
Camellini 2011 {published data only}
- Camellini L, Carlinfante G, Azzolini F, Iori V, Cavina M, Sereni G, et al. A randomized clinical trial comparing 22g and 25g needles in endoscopic ultrasound‐guided fine‐needle aspiration of solid lesions. Endoscopy 2011;43(8):709‐15. [DOI] [PubMed] [Google Scholar]
Cantley 2014 {published data only}
- Cantley RL, Li W, Ahmad U, Rafiq E, Molnar S, Las Casas LE. Endoscopic ultrasound‐guided fine needle aspiration with cytopathologist guidance and rapid on‐site evaluation is highly accurate for diagnosis of pancreatic masses. Laboratory Investigation 2014;94:98A. [Google Scholar]
Carbognin 2006 {published data only}
- Carbognin G, Zamboni G, Pinali L, Chiara ED, Girardi V, Salvia R, et al. Branch duct IPMTs: Value of cross‐sectional imaging in the assessment of biological behavior and follow‐up [erratum appears in Abdominal Imaging 2013;38(6):1466]. Abdominal Imaging 2006;31(3):320‐5. [DOI] [PubMed] [Google Scholar]
Carlinfante 2014 {published data only}
- Carlinfante G, Baccarini P, Berretti D, Cassetti T, Cavina M, Conigliaro R, et al. Ki‐67 cytological index can distinguish well‐differentiated from poorly differentiated pancreatic neuroendocrine tumors: A comparative cytohistological study of 53 cases. Virchows Archiv 2014;465(1):49‐55. [DOI] [PubMed] [Google Scholar]
Carroll 1997 {published data only}
- Carroll N, Quirk D, Centeno B, Warshaw A, Brugge W. Accuracy of EUS‐guided pancreatic biopsies. Gastrointestinal Endoscopy 1997;45(4):AB169. [Google Scholar]
Casneuf 2007 {published data only}
- Casneuf V, Delrue L, Kelles A, Damme N, Huysse J, Berrevoet F, et al. Is combined 18f‐fluorodeoxyglucose‐positron emission tomography/computed tomography superior to positron emission tomography or computed tomography alone for diagnosis, staging and restaging of pancreatic lesions?. Acta Gastroenterologica Belgica 2007;70(4):331‐8. [PubMed] [Google Scholar]
Catanzaro 2003 {published data only}
- Catanzaro A, Richardson S, Veloso H, Isenberg GA, Wong RC, Sivak MV, et al. Long‐term follow‐up of patients with clinically indeterminate suspicion of pancreatic cancer and normal EUS. Gastrointestinal Endoscopy 2003;58(6):836‐40. [DOI] [PubMed] [Google Scholar]
Catanzaro 2013 {published data only}
- Catanzaro R, Arona S, Sapienza C, Giangreco E, Magnano A, Palermo F, et al. Endoscopic ultrasound‐guided fine needle aspiration role in preoperative diagnosis of pancreatic tumors. Digestive and Liver Disease 2013;45:S163. [Google Scholar]
Cermak 2012 {published data only}
- Cermak TS, Wang B, DeBrito P, Carroll J, Haddad N, Sidawy MK. Does on‐site adequacy evaluation reduce the nondiagnostic rate in endoscopic ultrasound‐guided fine‐needle aspiration of pancreatic lesions?. Cancer Cytopathology 2012;120(5):319‐25. [DOI] [PubMed] [Google Scholar]
Chai 2013 {published data only}
- Chai SM, Herba K, Kumarasinghe MP, Boer WB, Amanuel B, Grieu‐Iacopetta F, et al. Optimizing the multimodal approach to pancreatic cyst fluid diagnosis: Developing a volume‐based triage protocol. Cancer Cytopathology 2013;121(2):86‐100. [DOI] [PubMed] [Google Scholar]
Chang 1994 {published data only}
- Chang KJ, Katz KD, Durbin TE, Erickson RA, Butler JA, Lin F, et al. Endoscopic ultrasound‐guided fine‐needle aspiration. Gastrointestinal Endoscopy 1994;40(6):694‐9. [PubMed] [Google Scholar]
Chang 1997 {published data only}
- Chang KJ, Nguyen P, Erickson RA, Durbin TE, Katz KD. The clinical utility of endoscopic ultrasound‐guided fine‐needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointestinal Endoscopy 1997;45(5):387‐93. [DOI] [PubMed] [Google Scholar]
Chang 2009 {published data only}
- Chang YH, Sang SL, Tae JS, Moon SH, Lee D, Do HP, et al. Endoscopic ultrasound guided fine needle aspiration biopsy in diagnosis of pancreatic and peripancreatic lesions: A single center experience in Korea. Gut & Liver 2009;3(2):116‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaudhari 2007 {published data only}
- Chaudhari VV, Raman SS, Vuong NL, Zimmerman P, Farrell J, Reber H, et al. Pancreatic cystic lesions: Discrimination accuracy based on clinical data and high resolution CT features. Journal of Computer Assisted Tomography 2007;31(6):860‐7. [DOI] [PubMed] [Google Scholar]
Chaudhari 2008 {published data only}
- Chaudhari VV, Raman SS, Vuong NL, Zimmerman P, Farrell J, Reber H, et al. Pancreatic cystic lesions: Discrimination accuracy based on clinical data and high‐resolution computed tomographic features. Journal of Computer Assisted Tomography 2008;32(5):757‐63. [DOI] [PubMed] [Google Scholar]
Chaya 2006 {published data only}
- Chaya C, Nealon WH, Bhutani MS. EUS or percutaneous CT/US‐guided FNA for suspected pancreatic cancer: When tissue is the issue. Gastrointestinal Endoscopy 2006;63(7):976‐8. [DOI] [PubMed] [Google Scholar]
Chebib 2014 {published data only}
- Chebib I, Yaeger K, Mino‐Kenudson M, Pitman MB. The role of cytopathology and cyst fluid analysis in the preoperative diagnosis and management of pancreatic cysts > 3 cm. Cancer Cytopathology 2014;122(11):804‐9. [DOI] [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen CH, Tseng LJ, Yang CC, Yeh YH. Preoperative evaluation of periampullary tumors by endoscopic sonography, transabdominal sonography, and computed tomography. Journal of Clinical Ultrasound 2001;29(6):313‐21. [DOI] [PubMed] [Google Scholar]
Chen 2003 {published data only}
- Chen SL, Venegas R, French S, Lee T, Lee H, Feng J, et al. Does immunohistochemical staining improve the diagnostic yield of endoscopic ultrasonography‐guided fine needle pancreatic aspirates?. Gastrointestinal Endoscopy 2003;57(5):AB237. [Google Scholar]
Chen 2007 {published data only}
- Chen GE, Bai L, Long Y, Yang G, Dang YT, Li L. Diagnosis value of pathological changes in the pancreas under computed tomography guidance after skin puncture. World Chinese Journal of Digestology 2007;15(24):2657‐9. [Google Scholar]
Chen 2014 {published data only}
- Chen S, Lin J, Wang X, Wu HH, Cramer H. EUS‐guided FNA cytology of pancreatic neuroendocrine tumour (PanNET): A retrospective study of 132 cases over an 18‐year period in a single institution. Cytopathology 2014;25(6):396‐403. [DOI] [PubMed] [Google Scholar]
Cheng 2012 {published data only}
- Cheng M, Wang H, Liu K, Yen R, Tzen K, Wu Y. Prospective comparison of 18f‐FDG and 3'‐deoxy‐3'‐[18f]fluorothymidine PET/CT in the differentiation and characterization of periampullary tumors. European Journal of Nuclear Medicine and Molecular Imaging 2012;39:S582. [Google Scholar]
Cheng 2013 {published data only}
- Cheng MF, Wang HP, Tien YW, Liu KL, Yen RF, Tzen KY, et al. Usefulness of PET/CT for the differentiation and characterization of periampullary lesions. Clinical Nuclear Medicine 2013;38(9):703‐8. [DOI] [PubMed] [Google Scholar]
Chiu 2005 {published data only}
- Chiu YC, Tsai TL, Changchien CS, Chiu KW, Hu TH, Chen YS, et al. Clinical application of endoscopic ultrasonography in the diagnosis of periampullary lesions. Journal of Medical Ultrasound 2005;13(2):67‐73. [Google Scholar]
Chiu 2006 {published data only}
- Chiu SS, Lim JH, Lee WJ, Chang KT, Oh DK, Lee KT, et al. Intraductal papillary mucinous tumour of the pancreas: Differentiation of malignancy and benignancy by CT. Clinical Radiology 2006;61(9):776‐83. [DOI] [PubMed] [Google Scholar]
Cho 2005 {published data only}
- Cho SG, Lee DH, Lee KY, Ji B, Lee KH, Ros PR, et al. Differentiation of chronic focal pancreatitis from pancreatic carcinoma by in vivo proton magnetic resonance spectroscopy. Journal of Computer Assisted Tomography 2005;29(2):163‐9. [DOI] [PubMed] [Google Scholar]
Cho 2013 {published data only}
- Cho CS, Russ AJ, Loeffler AG, Rettammel RJ, Oudheusden G, Winslow ER, et al. Preoperative classification of pancreatic cystic neoplasms: The clinical significance of diagnostic inaccuracy. Annals of Surgical Oncology 2013;20(9):3112‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2011 {published data only}
- Choi ER, Jang TH, Chung YH, Jang KT, Park SM, Lee JK, et al. A prospective comparison of liquid‐based cytology and traditional smear cytology in pancreatic endoscopic ultrasound‐guided fine needle aspiration. Acta Cytologica 2011;55(5):401‐7. [DOI] [PubMed] [Google Scholar]
Choi 2013 {published data only}
- Choi JH, Lee KH, Kim KM, Shin JU, Lee JK, Lee KT, et al. A prospective, comparative trial to optimize sampling techniques in EUS‐guided FNA of solid pancreatic masses. Gastrointestinal Endoscopy 2013;77(5):745‐51. [DOI] [PubMed] [Google Scholar]
Choi 2016 {published data only}
- Choi TW, Lee JM, Kim JH, Yu MH, Han JK, Choi BI. Comparison of multidetector CT and gadobutrol‐enhanced MR imaging for evaluation of small, solid pancreatic lesions. Korean Journal of Radiology 2016;17(4):509‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2009 {published data only}
- Chung YE, Kim MJ, Choi JY, Lim JS, Hong HS, Kim YC, et al. Differentiation of benign and malignant solid pseudopapillary neoplasms of the pancreas. Journal of Computer Assisted Tomography 2009;33(5):689‐94. [DOI] [PubMed] [Google Scholar]
Cizginer 2011 {published data only}
- Cizginer S, Turner B, Bilge AR, Karaca C, Pitman MB, Brugge WR. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 2011;40(7):1024‐8. [DOI] [PubMed] [Google Scholar]
Clave 1999 {published data only}
- Clave P, Boadas J, Gonzalez‐Carro P, Mora J, Perez C, Martinez A, et al. Accuracy of imaging techniques and tumor markers in the diagnosis of pancreatic cancer. Gastroenterologia y Hepatologia 1999;22(7):335‐41. [PubMed] [Google Scholar]
Cocieru 2011 {published data only}
- Cocieru A, Brandwein S, Saldinger PF. The role of endoscopic ultrasound and cyst fluid analysis in the initial evaluation and follow‐up of incidental pancreatic cystic lesions. HPB 2011;13(7):459‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2007 {published data only}
- Collins BI, Agarwal B, Krishna NB, LaBundy J, Saripalli S, Safdar R. Diagnostic value of endoscopic ultrasound fine needle aspiration in suspected pancreatic cancer patients with focal lesion on CT/MRI without obstructive jaundice. Cancer Cytopathology 2007;111(5):418‐9. [Google Scholar]
Collins 2013 {published data only}
- Collins BT, Murad FM, Wang JF, Bernadt CT. Rapid on‐site evaluation for endoscopic ultrasound‐guided fine‐needle biopsy of the pancreas decreases the incidence of repeat biopsy procedures. Cancer Cytopathology 2013;121(9):518‐24. [DOI] [PubMed] [Google Scholar]
Collins 2015 {published data only}
- Collins BT, Adhikari LJ, Bernadt CT, Wang JF. Correlation of liver and pancreas endoscopic ultrasonography‐guided fine‐needle aspiration biopsy in patients with a primary pancreatic lesion. Journal of the American Society of Cytopathology 2015;4(2):74‐8. [DOI] [PubMed] [Google Scholar]
Cone 2011 {published data only}
- Cone MM, Rea JD, Diggs BS, Billingsley KG, Sheppard BC. Endoscopic ultrasound may be unnecessary in the preoperative evaluation of intraductal papillary mucinous neoplasm. HPB 2011;13(2):112‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corominas‐Cishek 2014 {published data only}
- Corominas‐Cishek A, Perez A, Barturen A, Casado I, Alvarez JA, Mosteiro L. Diagnostic usefulness and limitations of endoscopic ultrasound fine needle aspiration cytology (EUS‐FNAc) in a series of 428 pancreatic lesions. Laboratory Investigation 2014;94:521A. [PubMed] [Google Scholar]
Cosgrove 2015 {published data only}
- Cosgrove ND, Yan L, Siddiqui A. Preoperative endoscopic ultrasound‐guided fine needle aspiration for diagnosis of pancreatic cancer in potentially resectable patients: Is this safe?. Endoscopic Ultrasound 2015;4(2):81‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crippa 2010 {published data only}
- Crippa S, Falconi M. Improving cytological diagnosis of pancreatic cysts: Is it clinically necessary or just the latest fashion?. Digestive and Liver Disease 2010;42(12):844‐5. [DOI] [PubMed] [Google Scholar]
Cuillerier 1996 {published data only}
- Cuillerier E, Cellier C, Palazzo L, Deviere L, Napoleon B, VanGansbeke D, et al. Comparison of the accuracy of CT scan (CT), endoscopic retrograde cholangio‐pancreatography (ERCP) and endoscopic ultrasonography (EUS) in preoperative staging of intraductal papillary mucinous tumors of the pancreas. Gastroenterology 1996;110(4 Suppl):A384. [Google Scholar]
D'Onofrio 2007 {published data only}
- D'Onofrio M, Megibow AJ, Faccioli N, Malago R, Capelli P, Falconi M, et al. Comparison of contrast‐enhanced sonography and MRI in displaying anatomic features of cystic pancreatic masses. AJR: American Journal of Roentgenology 2007;189(6):1435‐42. [DOI] [PubMed] [Google Scholar]
D'Onofrio 2013 {published data only}
- D'Onofrio M, Crosara S, Signorini M, Robertis R, Canestrini S, Principe F, et al. Comparison between CT and cEUS in the diagnosis of pancreatic adenocarcinoma. Ultraschall in Der Medizin 2013;34(4):377‐81. [DOI] [PubMed] [Google Scholar]
Dadabhai 2005 {published data only}
- Dadabhai A, Chen SL, Tsai S, Venegas R, French S, Arora S, et al. The utility of immunohistochemical staining with CEA, ca19‐9, p53, and MIB to improve the diagnostic yield of endoscopic ultrasonography guided fine needle pancreatic aspirates. Gastrointestinal Endoscopy 2005;61(5):AB276. [Google Scholar]
Dadds 2012 {published data only}
- Dadds HR, Keane G, Huggett MT, Pereira SP. Utility of EUS‐FNA for identifying (pre‐)malignant pancreatic cysts. Pancreatology 2012;12 (3):e3. [Google Scholar]
Dani 2000 {published data only}
- Dani R, Cundari AM, Nogueira CE, Reis GM, Silva LD. Magnetic resonance cholangiopancreatography in cystic lesions of the pancreas. Pancreas 2000;20(3):313‐8. [DOI] [PubMed] [Google Scholar]
Dawwas 2012 {published data only}
- Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: A prospective, single‐center study. Gastrointestinal Endoscopy 2012;76(5):953‐61. [DOI] [PubMed] [Google Scholar]
Dawwas 2013 {published data only}
- Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Differential diagnosis of focal pancreatic masses by semiquantitative EUS elastography: Between strain ratios and strain histograms response. Gastrointestinal Endoscopy 2013;78(1):189‐90. [DOI] [PubMed] [Google Scholar]
Decalan 1995 {published data only}
- Decalan L, Levard H, Hennet H, Fingerhut A. Pancreatic cystadenoma and cystadenocarcinoma ‐ diagnostic‐value of preoperative morphological investigations. European Journal of Surgery 1995;161(1):35‐40. [PubMed] [Google Scholar]
De Jong 2010 {published data only}
- Jong K, Nio CY, Gouma DJ, Bruno MJ, Fockens P. Accuracy of MRI and EUS in a prospective cohort of patients with histological proven pancreatic cystic lesions of the pancreas. Gastroenterology 2010;138(5 Suppl 1):S547. [Google Scholar]
de Jong 2011 {published data only}
- Jong K, Poley JW, Hooft JE, Visser M, Bruno MJ, Fockens P. Endoscopic ultrasound‐guided fine‐needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: Initial results from a prospective study. Endoscopy 2011;43(7):585‐90. [DOI] [PubMed] [Google Scholar]
Delbeke 1999 {published data only}
- Delbeke D, Rose DM, Chapman WC, Pinson CW, Wright JK, Beauchamp RD, et al. Optimal interpretation of FDG PET in the diagnosis, staging and management of pancreatic carcinoma. Journal of Nuclear Medicine 1999;40(11):1784‐91. [PubMed] [Google Scholar]
DelMaschio 1991 {published data only}
- DelMaschio A, Vanzulli A, Sironi S, Castrucci M, Mellone R, Staudacher C, et al. Pancreatic cancer versus chronic pancreatitis: Diagnosis with CA 19‐9 assessment, US, CT, and CT‐guided fine‐needle biopsy. Radiology 1991;178(1):95‐9. [DOI] [PubMed] [Google Scholar]
Del Vecchio 2016 {published data only}
- Vecchio Blanco G, Paoluzi OA, Mannisi E, Bevivino G, Formica V, Portarena I, et al. Repetition or simultaneous sampling of primary and metastatic lesions improve diagnostic accuracy of EUS‐FNA in the assessment of suspected neoplastic pancreatic mass. Digestive and Liver Disease 2016;48:e141. [Google Scholar]
Deng 2008 {published data only}
- Deng HB, Shi JH, Wilkerson M, Meschter S, Dupree W, Lin F. Usefulness of s100p in diagnosis of adenocarcinoma of pancreas on fine‐needle aspiration biopsy specimens. American Journal of Clinical Pathology 2008;129(1):81‐8. [DOI] [PubMed] [Google Scholar]
Deshpande 2008 {published data only}
- Deshpande AR, Szabo D, Rocha‐Lima C, Levi JU, Ganjei‐Azar P, Jorda M, et al. Sensitivity of EUS‐guided biopsy of malignant pancreatic solid masses in biopsy‐naive vs rescue patients. Gastrointestinal Endoscopy 2008;67(5):AB212‐AB. [Google Scholar]
De Tejada 2008 {published data only}
- Tejada AH, Chennat JS, Cislo BM, Muchu J, Lin SP, Stearns L, et al. Does early needle exchange in EUS‐FNA improve the diagnostic yield of cytopathological evaluation in pancreatic adenocarcinoma? Preliminary results from an ongoing randomized trial. Gastrointestinal Endoscopy 2008;67(5):AB219. [Google Scholar]
DeWitt 2004 {published data only}
- DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer [summary for patients in Annals of Internal Medicine 2004;141(10):I46; pmid: 15545671]. Annals of Internal Medicine 2004;141(10):753‐63. [DOI] [PubMed] [Google Scholar]
DeWitt 2005 {published data only}
- DeWitt J, Devereaux B, Chriswell M, Kane S. Is endoscopic ultrasound really better than multidetector CT for pancreatic cancer?. Evidence‐Based Gastroenterology 2005;6(2):50. [Google Scholar]
DeWitt 2008 {published data only}
- DeWitt J, McGreevy K, Sherman S, LeBlanc J. Utility of a repeated EUS at a tertiary‐referral center. Gastrointestinal Endoscopy 2008;67(4):610‐9. [DOI] [PubMed] [Google Scholar]
Di Cataldo 2014 {published data only}
- Cataldo A, Palmucci S, Latino R, Trombatore C, Cappello G, Amico A, et al. Cystic pancreatic tumors: Should we resect all of them?. European Review for Medical and Pharmacological Sciences 2014;18(2 Suppl):16‐23. [PubMed] [Google Scholar]
Diederichs 2000 {published data only}
- Diederichs CG, Staib L, Vogel J, Glasbrenner B, Glatting G, Brambs HJ, et al. Values and limitations of 18f‐fluorodeoxyglucose‐positron‐emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas 2000;20(2):109‐16. [DOI] [PubMed] [Google Scholar]
Diehl 1999 {published data only}
- Diehl SJ, Lehmann KJ, Gaa J, Meier‐Willersen HJ, Wendl K, Georgi M. The value of magnetic resonance tomography (MRT), magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP) in the diagnosis of pancreatic tumors. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 1999;170(5):463‐9. [DOI] [PubMed] [Google Scholar]
Dietrich 2008 {published data only}
- Dietrich CF, Ignee A, Braden B, Barreiros AP, Ott M, Hocke M. Improved differentiation of pancreatic tumors using contrast‐enhanced endoscopic ultrasound. Clinical Gastroenterology and Hepatology 2008;6(5):590‐7.e1. [DOI] [PubMed] [Google Scholar]
Dim 2014 {published data only}
- Dim DC, Jiang F, Qiu Q, Li T, Darwin P, Rodgers WH, et al. The usefulness of s100p, mesothelin, fascin, prostate stem cell antigen, and 14‐3‐3 sigma in diagnosing pancreatic adenocarcinoma in cytological specimens obtained by endoscopic ultrasound guided fine‐needle aspiration. Diagnostic Cytopathology 2014;42(3):193‐9. [DOI] [PubMed] [Google Scholar]
DiMagno 1977 {published data only}
- DiMagno EP, Malagelada JR, Taylor WF, Go VL. A prospective comparison of current diagnostic tests for pancreatic cancer. New England Journal of Medicine 1977;297(14):737‐42. [DOI] [PubMed] [Google Scholar]
Dinkel 1990 {published data only}
- Dinkel E, Helwig A, Jager B, Ruckauer K, Scholmerich J, Hauenstein KH, et al. Computed tomographic‐guided fine‐needle biopsy of the pancreas for histology determination. Radiologe 1990;30(9):420‐4. [PubMed] [Google Scholar]
Do 2014 {published data only}
- Do RK, Katz SS, Gollub MJ, Li J, LaFemina J, Zabor EC, et al. Interobserver agreement for detection of malignant features of intraductal papillary mucinous neoplasms of the pancreas on MDCT. AJR: American Journal of Roentgenology 2014;203(5):973‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Draganov 2010 {published data only}
- Draganov PV, Nicaud M, Hou W, Collins D, Wagh MS, Chauhan S. The utility of repeat endoscopic ultrasound‐guided fine needle aspiration for suspected pancreatic cancer. Gastroenterology Research and Practice 2010;2010:268290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eguia 2013 {published data only}
- Eguia V, Chiang AL, Doukides TP, Sethi A, Poneros JM, Allendorf JD, et al. Potential risks and benefits of preoperative endosonographic evaluation of resectable pancreatic masses. Gastrointestinal Endoscopy 2013;1:AB412‐3. [Google Scholar]
Elmas 1996 {published data only}
- Elmas N, Oran I, Oyar O, Ozer H. A new criterion in differentiation of pancreatitis and pancreatic carcinoma: Artery‐to‐vein ratio using the superior mesenteric vessels. Abdominal Imaging 1996;21(4):331‐3. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2002 {published data only}
- Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, et al. Endoscopic ultrasound‐guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: Diagnostic accuracy and acute and 30‐day complication assessment. American Journal of Gastroenterology 2002;97(9 Suppl):S65‐6. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2003a {published data only}
- Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, et al. Endoscopic ultrasound‐guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: Diagnostic accuracy and acute and 30‐day complications. American Journal of Gastroenterology 2003;98(12):2663‐8. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2003b {published data only}
- Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, et al. Yield of endoscopic ultrasound‐guided fine‐needle aspiration biopsy in patients with suspected pancreatic carcinoma ‐ emphasis on atypical, suspicious, and false‐negative aspirates. Cancer Cytopathology 2003;99(5):285‐92. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2005 {published data only}
- Eloubeidi MA, Tamhane A. EUS‐guided FNA of solid pancreatic masses: A learning curve with 300 consecutive procedures. Gastrointestinal Endoscopy 2005;61(6):700‐8. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2006a {published data only}
- Eloubeidi M, Varadarajulu S, Desai S, Wilcox CM. The negative predictive value of EUS‐guided FNA in patients with suspected pancreatic cancer. Gastrointestinal Endoscopy 2006;63(5):AB279. [Google Scholar]
Eloubeidi 2006b {published data only}
- Eloubeidi MA, Tamhane A, Jhala N, Chhieng D, Jhala D, Crowe DR, et al. Agreement between rapid onsite and final cytologic interpretations of EUS‐guided FNA specimens: Implications for the endosonographer and patient management. American Journal of Gastroenterology 2006;101(12):2841‐7. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2006c {published data only}
- Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS‐guided FNA of solid pancreatic masses: A prospective evaluation. Gastrointestinal Endoscopy 2006;63(4):622‐9. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2007 {published data only}
- Eloubeidi MA, Varadarajulu S, Desai S, Shirley R, Heslin MJ, Mehra M, et al. A prospective evaluation of an algorithm incorporating routine preoperative endoscopic ultrasound‐guided fine needle aspiration in suspected pancreatic cancer. Journal of Gastrointestinal Surgery 2007;11(7):813‐9. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2008a {published data only}
- Eloubeidi MA, Tamhane A. Prospective assessment of diagnostic utility and complications of endoscopic ultrasound‐guided fine needle aspiration. Results from a newly developed academic endoscopic ultrasound program. Digestive Diseases 2008;26(4):356‐63. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2008b {published data only}
- Eloubeidi MA, Varadarajulu S, Desai S, Wilcox CM. Value of repeat endoscopic ultrasound‐guided fine needle aspiration for suspected pancreatic cancer. Journal of Gastroenterology and Hepatology 2008;23(4):567‐70. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2013 {published data only}
- Eloubeidi MA, Luz LP, Tamhane A, Khan M, Buxbaum JL. Ratio of pancreatic duct caliber to width of pancreatic gland by endosonography is predictive of pancreatic cancer. Pancreas 2013;42(4):670‐9. [DOI] [PubMed] [Google Scholar]
Ergul 2014 {published data only}
- Ergul N, Gundogan C, Tozlu M, Toprak H, Kadioglu H, Aydin M, et al. Role of (18)f‐fluorodeoxyglucose positron emission tomography/computed tomography in diagnosis and management of pancreatic cancer; comparison with multidetector row computed tomography, magnetic resonance imaging and endoscopic ultrasonography. Revista Espanola de Medicina Nuclear e Imagen Molecular 2014;33(3):159‐64. [DOI] [PubMed] [Google Scholar]
Erickson 1997 {published data only}
- Erickson RA, Sayage‐Rabie L, Avots‐Avotins A. Clinical utility of endoscopic ultrasound‐guided fine needle aspiration. Acta Cytologica 1997;41(6):1647‐53. [DOI] [PubMed] [Google Scholar]
Erickson 2000 {published data only}
- Erickson RA, Sayage‐Rabie L, Beissner RS. Factors predicting the number of EUS‐guided fine‐needle passes for diagnosis of pancreatic malignancies. Gastrointestinal Endoscopy 2000;51(2):184‐90. [DOI] [PubMed] [Google Scholar]
Erickson 2001 {published data only}
- Erickson RA. EUS/EUS‐FNA diagnostic data supports pancreatic adenocarcinoma being a sex‐hormone sensitive tumor. Gastrointestinal Endoscopy 2001;53(5):AB137. [Google Scholar]
Ernst 1998 {published data only}
- Ernst OJ, Sergent GF, Meunier B, Chaveron C, Spilliaert B, L'Hermine C. Dynamic MR imaging of the pancreas: Enhancement patterns and diagnostic value in pancreatitis and malignant tumors. Radiology 1998;209(2 Suppl):371. [Google Scholar]
Erturk 2006a {published data only}
- Erturk SM. Value of the single‐phase technique in MDCT assessment of pancreatic tumors. AJR: American Journal of Roentgenology 2006;186(1):266‐7; author reply 7. [DOI] [PubMed] [Google Scholar]
Erturk 2006b {published data only}
- Erturk SM, Mortele KJ, Tuncali K, Saltzman JR, Lao R, Silverman SG. Fine‐needle aspiration biopsy of solid pancreatic masses: Comparison of CT and endoscopic sonography guidance. AJR: American Journal of Roentgenology 2006;187(6):1531‐5. [DOI] [PubMed] [Google Scholar]
Fabbri 2013 {published data only}
- Fabbri C, Maimone A, Tarantino I, Baccarini P, Luigiano C, Liotta R, et al. EUS‐guided biopsy of solid pancreatic lesions less than 2 cm using a new 22‐gauge needle device. Digestive and Liver Disease 2013;45:S161. [Google Scholar]
Fabbri 2015a {published data only}
- Fabbri C, Fornelli A, Fuccio L, Maimone A, Antonini F, Baccarini P, et al. Comparison of the yield and diagnostic accuracy of endoscopic ultrasound‐guided fine‐needle core biopsy with and without rapid on‐site evaluation. Digestive and Liver Disease 2015;47(Suppl 2):e104. [Google Scholar]
Fabbri 2015b {published data only}
- Fabbri C, Luigiano C, Maimone A, Tarantino I, Baccarini P, Fornelli A, et al. Endoscopic ultrasound‐guided fine‐needle biopsy of small solid pancreatic lesions using a 22‐gauge needle with side fenestration. Surgical Endoscopy 2015;29(6):1586‐90. [DOI] [PubMed] [Google Scholar]
Faigel 1997 {published data only}
- Faigel DO, Ginsberg GG, Bentz JS, Gupta PK, Smith DB, Kochman ML. Endoscopic ultrasound‐guided real‐time fine‐needle aspiration biopsy of the pancreas in cancer patients with pancreatic lesions. Journal of Clinical Oncology 1997;15(4):1439‐43. [DOI] [PubMed] [Google Scholar]
Fan 2013 {published data only}
- Fan Z, Li Y, Yan K, Wu W, Yin S, Yang W, et al. Application of contrast‐enhanced ultrasound in the diagnosis of solid pancreatic lesions ‐ a comparison of conventional ultrasound and contrast‐enhanced CT. European Journal of Radiology 2013;82(9):1385‐90. [DOI] [PubMed] [Google Scholar]
Fan 2015 {published data only}
- Fan Z, Yan K, Wang Y, Qiu J, Wu W, Yang L, et al. Application of contrast‐enhanced ultrasound in cystic pancreatic lesions using a simplified classification diagnostic criterion. BioMed Research International 2015;2015:974621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fanning 2010 {published data only}
- Fanning S, Kwok A, Jones B, Leong R. EUS aspiration needle size: Smaller is better?. Gastrointestinal Endoscopy 2010;72(4):904‐5. [DOI] [PubMed] [Google Scholar]
Faravelli 1990 {published data only}
- Faravelli A, Barisoni L, Parolini D, Carlucci M, Vanzulli A, Maschio A. Fine needle aspiration of pancreatic masses. A study of 81 cases. Pathologica 1990;82(1082):695‐705. [PubMed] [Google Scholar]
Felgueroso 2014 {published data only}
- Felgueroso MM, Wallace M, Raimondo M, Woodward T, Skinner V, Arcidiacono P, et al. International multicenter intraductal papillary mucinous neoplasm (IPMN) registry: Role of EUS‐FNA cytology, CEA, and amylase in the diagnosis of IPMNs. American Journal of Gastroenterology 2014;109:S664. [Google Scholar]
Fernandez‐Esparrach 2007a {published data only}
- Fernandez‐Esparrach G, Gines A, Garcia P, Pellise M, Sole M, Cortes P, et al. Incidence and clinical significance of hyperamylasemia after endoscopic ultrasound‐guided fine‐needle aspiration (EUS‐FNA) of pancreatic lesions: A prospective and controlled study. Endoscopy 2007;39(8):720‐4. [DOI] [PubMed] [Google Scholar]
Fernandez‐Esparrach 2007b {published data only}
- Fernandez‐Esparrach G, Pellise M, Sole M, Soria MT, Miquel R, Mata A, et al. EUS FNA in intraductal papillary mucinous tumors of the pancreas. Hepato‐Gastroenterology 2007;54(73):260‐4. [PubMed] [Google Scholar]
Figueiredo 2012 {published data only}
- Figueiredo FAF, Silva PM, Monges G, Bories E, Pesenti C, Caillol F, et al. Yield of contrast‐enhanced power doppler endoscopic ultrasonography and strain ratio obtained by EUS‐elastography in the diagnosis of focal pancreatic solid lesions. Endoscopic Ultrasound 2012;1(3):143‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 2002 {published data only}
- Fischer U, Vosshenrich R, Horstmann O, Becker H, Salamat B, Baum F, et al. Preoperative local MRI‐staging of patients with a suspected pancreatic mass. European Radiology 2002;12(2):296‐303. [DOI] [PubMed] [Google Scholar]
Fischer 2009 {published data only}
- Fischer CP, Fahy BN, Aloia TA, Raijman I, Barroso AO, Schwarz PJ, et al. Fine needle aspiration cytology from pancreatic cysts ‐ limited utility in surgical decision making. Gastroenterology 2009;136(5 Suppl 1):A886. [Google Scholar]
Fisher 2009 {published data only}
- Fisher J, Gordon S, Gardner T. The impact of prior biliary stenting on the accuracy and complication rate of EUS‐FNA for diagnosing pancreatic adenocarcinoma. American Journal of Gastroenterology 2009;104:S63. [DOI] [PubMed] [Google Scholar]
Fisher 2011 {published data only}
- Fisher JM, Gordon SR, Gardner TB. The impact of prior biliary stenting on the accuracy and complication rate of endoscopic ultrasound fine‐needle aspiration for diagnosing pancreatic adenocarcinoma. Pancreas 2011;40(1):21‐4. [DOI] [PubMed] [Google Scholar]
Frampton 2013 {published data only}
- Frampton A, Krell J, Gall T, Castellano L, Vlavianos P, Stebbing J, et al. Prospective validation of microRNA signatures for detecting pancreatic malignant transformation in endoscopic‐ultrasound guided fine‐needle aspiration biopsies. Pancreatology 2013;1:S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friess 1995 {published data only}
- Friess H, Langhans J, Ebert M, Beger HG, Stolfuss J, Reske SN, et al. Diagnosis of pancreatic cancer by 2[18f]‐fluoro‐2‐deoxy‐d‐glucose positron emission tomography. Gut 1995;36(5):771‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fritscher‐Ravens 1998 {published data only}
- Fritscher‐Ravens A, Petrasch S, Reinacher‐Schick A, Schmiegel W. Comparison of diagnostic value and therapeutic implication of endosonographically guided fine needle aspiration cytology of mediastinal masses and pancreatic lesions. Gastroenterology 1998;114(4 Suppl 1):A598. [Google Scholar]
Fritscher‐Ravens 1999 {published data only}
- Fritscher‐Ravens A, Schirrow L, Atay Z, Petrasch S, Brand B, Bohnacker S, et al. Endosonographically controlled fine needle aspiration cytology ‐ indications and results in routine diagnosis. Zeitschrift fur Gastroenterologie 1999;37(5):343‐51. [PubMed] [Google Scholar]
Fritscher‐Ravens 2000 {published data only}
- Fritscher‐Ravens A, Izbicki JR, Sriram PV, Krause C, Knoefel WT, Topalidis T, et al. Endosonography‐guided, fine‐needle aspiration cytology extending the indication for organ‐preserving pancreatic surgery. American Journal of Gastroenterology 2000;95(9):2255‐60. [DOI] [PubMed] [Google Scholar]
Fritscher‐Ravens 2001a {published data only}
- Fritscher‐Ravens A, Brand L, Koefel WT, Bobrowski C, Ponnudurai R, Topalidis T, et al. EUS‐FNA in focal pancreatic lesions ‐ a reliable diagnostic tool?. Gastrointestinal Endoscopy 2001;53(5):AB174. [Google Scholar]
Fritscher‐Ravens 2001b {published data only}
- Fritscher‐Ravens A, Topalidis T, Bobrowski C, Krause C, Thonke E, Jackle S, et al. Endoscopic ultrasound‐guided fine‐needle aspiration in focal pancreatic lesions: A prospective intraindividual comparison of two needle assemblies. Endoscopy 2001;33(6):484‐90. [DOI] [PubMed] [Google Scholar]
Fritscher‐Ravens 2002 {published data only}
- Fritscher‐Ravens A, Brand L, Knofel WT, Bobrowski C, Topalidis T, Thonke F, et al. Comparison of endoscopic ultrasound‐guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. American Journal of Gastroenterology 2002;97(11):2768‐75. [DOI] [PubMed] [Google Scholar]
Frossard 2003 {published data only}
- Frossard JL, Amouyal P, Amouyal G, Palazzo L, Amaris J, Soldan M, et al. Performance of endosonography‐guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. American Journal of Gastroenterology 2003;98(7):1516‐24. [DOI] [PubMed] [Google Scholar]
Fugazzola 1991 {published data only}
- Fugazzola C, Procacci C, Andreis IAB, Iacono C, Portuese A, Dompieri P, et al. Cystic tumors of the pancreas: Evaluation by ultrasonography and computed tomography. Gastrointestinal Radiology 1991;16(1):53‐61. [DOI] [PubMed] [Google Scholar]
Furuhashi 2015 {published data only}
- Furuhashi N, Suzuki K, Sakurai Y, Ikeda M, Kawai Y, Naganawa S. Differentiation of focal‐type autoimmune pancreatitis from pancreatic carcinoma: Assessment by multiphase contrast‐enhanced CT. European Radiology 2015;25(5):1366‐74. [DOI] [PubMed] [Google Scholar]
Furuhata 2012 {published data only}
- Furuhata A, Shirahase H, Shirai T, Hirata M, Yoshizawa A, Haga H, et al. Utility of rapid on‐site cytology in endoscopic ultrasonography‐guided fine needle aspiration of pancreatic masses. Rinsho byori 2012;60(5):429‐34. [PubMed] [Google Scholar]
Fusari 2010 {published data only}
- Fusari M, Maurea S, Imbriaco M, Mollica C, Avitabile G, Soscia F, et al. Comparison between multislice CT and MR imaging in the diagnostic evaluation of patients with pancreatic masses. Radiologia Medica 2010;115(3):453‐66. [DOI] [PubMed] [Google Scholar]
Fusaroli 2010 {published data only}
- Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo‐endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clinical Gastroenterology and Hepatology 2010;8(7):629‐34.e1‐2. [DOI] [PubMed] [Google Scholar]
Fusaroli 2014 {published data only}
- Fusaroli P, Eloubeidi MA. Diagnosis of pancreatic cancer by contrast‐harmonic endoscopic ultrasound (EUS): Complementary and not competitive with EUS‐guided fine‐needle aspiration. Endoscopy 2014;46(5):380‐1. [DOI] [PubMed] [Google Scholar]
Gaa 1999 {published data only}
- Gaa J, Wendl K, Tesdal IK, Meier‐Willersen HJ, Lehmann KJ, Bohm C, et al. Combined use of MRI and MR cholangiopancreatography and contrast enhanced dual phase 3‐D MR angiography in diagnosis of pancreatic tumors: Initial clinical results. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 1999;170(6):528‐33. [DOI] [PubMed] [Google Scholar]
Gambitta 2014 {published data only}
- Gambitta P, Armellino A, Forti E, Vertemati M, Colombo PE, Aseni P. Endoscopic ultrasound‐guided fine‐needle aspiration for suspected malignancies adjacent to the gastrointestinal tract. World Journal of Gastroenterology 2014;20(26):8599‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganc 2014 {published data only}
- Ganc RL, Carbonari AP, Colaiacovo R, Rocha H, Silva RA, Pacheco AM, et al. EUS‐FNA of solid pancreatic lesions: A prospective, randomized, single blinded, comparative study using the 22‐gauge EchoTip ProCore HD and the 22‐gauge EchoTip Ultra HD endoscopic ultrasound needles. Gastrointestinal Endoscopy 2014;1:AB320‐1. [PMC free article] [PubMed] [Google Scholar]
Ganc 2015 {published data only}
- Ganc RL, Carbonari APC, Colaiacovo R, Araujo J, Filippi S, Silva RA, et al. Rapid on‐site cytopathological examination (ROSE) performed by endosonagraphers and its improvement in the diagnosis of pancreatic solid lesions. Acta Cirurgica Brasileira 2015;30(7):503‐8. [DOI] [PubMed] [Google Scholar]
Gaspar 2012 {published data only}
- Gaspar AMR, Alberto EML, Tamaris JV, Gonzalez FAC. Evaluation of neoplastic pancreatic focal lesions multidetector computed tomography. Gaceta Medica de Mexico 2012;148(4):358‐70. [PubMed] [Google Scholar]
Gill 2008 {published data only}
- Gill KRS, Wallace MB. EUS elastography for pancreatic mass lesions: Between image and FNA?. Gastrointestinal Endoscopy 2008;68(6):1095‐7. [DOI] [PubMed] [Google Scholar]
Gimeno‐Garcia 2014 {published data only}
- Gimeno‐Garcia AZ, Elwassief A, Paquin SC, Gariepy G, Sahai AV. Randomized controlled trial comparing stylet‐free endoscopic ultrasound‐guided fine‐needle aspiration with 22‐g and 25‐g needles. Digestive Endoscopy 2014;26(3):467‐73. [DOI] [PubMed] [Google Scholar]
Giorgetti 2010 {published data only}
- Giorgetti A, Genovesi D, Bottoni A, Filidei E, Porciello C, Magagnini F, et al. Different SUVmax cut‐off values improve the diagnostic accuracy of 18‐FDG PET/TC in patients with pancreatic lesions. European Journal of Nuclear Medicine and Molecular Imaging 2010;37:S431. [Google Scholar]
Giovannini 1995 {published data only}
- Giovannini M, Seitz JF, Monges G, Perrier H, Rabbia I. Fine‐needle aspiration cytology guided by endoscopic ultrasonography: Results in 141 patients. Endoscopy 1995;27(2):171‐7. [DOI] [PubMed] [Google Scholar]
Giovannini 2009 {published data only}
- Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: A multicenter study. World Journal of Gastroenterology 2009;15(13):1587‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasbrenner 2000 {published data only}
- Glasbrenner B, Schwarz M, Pauls S, Preclik G, Beger HG, Adler G. Prospective comparison of endoscopic ultrasound and endoscopic retrograde cholangiopancreatography in the preoperative assessment of masses in the pancreatic head. Digestive Surgery 2000;17(5):468‐74. [DOI] [PubMed] [Google Scholar]
Goh 2006a {published data only}
- Goh BKP. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost‐effectiveness [5]. Annals of Surgery 2006;243(5):709‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goh 2008 {published data only}
- Goh BK, Tan YM, Thng CH, Cheow PC, Chung YF, Chow PK, et al. How useful are clinical, biochemical, and cross‐sectional imaging features in predicting potentially malignant or malignant cystic lesions of the pancreas? Results from a single institution experience with 220 surgically treated patients. Journal of the American College of Surgeons 2008;206(1):17‐27. [DOI] [PubMed] [Google Scholar]
Gomez 2006 {published data only}
- Gomez D, Rahman SH, Wong LF, Verbeke CS, McMahon MJ, Menon KV. Characterization of malignant pancreatic cystic lesions in the background of chronic pancreatitis. Journal of the Pancreas 2006;7(5):465‐72. [PubMed] [Google Scholar]
Gomez 2008 {published data only}
- Gomez D, Rahman SH, Wong LF, Verbeke CS, Menon KV. Predictors of malignant potential of cystic lesions of the pancreas. European Journal of Surgical Oncology 2008;34(8):876‐82. [DOI] [PubMed] [Google Scholar]
Gong 2004 {published data only}
- Gong JS, Xu JM. Role of curved planar reformations using multidetector spiral CT in diagnosis of pancreatic and peripancreatic diseases. World Journal of Gastroenterology 2004;10(13):1943‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2014 {published data only}
- Gordon H, Lloyd D, Higginson A, McCrudden R, Bent C, Shek F, et al. The sensitivity of EUS FNA of solid pancreatic lesions, working from a regional MDT and within a regional network. Gut 2014;63:S252‐3. [Google Scholar]
Gowland 1981 {published data only}
- Gowland M, Kalantzis N, Warwick F, Braganza J. Relative efficiency and predictive value of ultrasonography and endoscopic retrograde pancreatography in diagnosis of pancreatic disease. Lancet 1981;2(8239):190‐3. [DOI] [PubMed] [Google Scholar]
Green 2002 {published data only}
- Green JA, Barkin JS. Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas?. American Journal of Gastroenterology 2002;97(11):2918‐9. [DOI] [PubMed] [Google Scholar]
Grenacher 2004 {published data only}
- Grenacher L, Klauss M, Dukic L, Delorme S, Knaebel HP, Dux M, et al. Diagnosis and staging of pancreatic carcinoma: MRI versus multislice‐CT ‐ a prospective study. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2004;176(11):1624‐33. [DOI] [PubMed] [Google Scholar]
Gress 1997 {published data only}
- Gress FG, Hawes RH, Savides TJ, Ikenberry SO, Lehman GA. Endoscopic ultrasound‐guided fine‐needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointestinal Endoscopy 1997;45(3):243‐50. [DOI] [PubMed] [Google Scholar]
Gress 2001 {published data only}
- Gress F, Gottlieb K, Sherman S, Lehman G. Endoscopic ultrasonography‐guided fine‐needle aspiration biopsy of suspected pancreatic cancer. Annals of Internal Medicine 2001;134(6):459‐64. [DOI] [PubMed] [Google Scholar]
Grieser 2013 {published data only}
- Grieser C, Heine G, Stelter L, Steffen IG, Rothe JH, Walter TC, et al. Morphological analysis and differentiation of benign cystic neoplasms of the pancreas using computed tomography and magnetic resonance imaging. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2013;185(3):219‐27. [DOI] [PubMed] [Google Scholar]
Guo 2008 {published data only}
- Guo CG, Tian YT, Liu Q, Wang CF, Zhao P. Diagnosis and treatment of pancreatic cystic tumors: An analysis of 34 cases. World Chinese Journal of Digestology 2008;16(5):544‐7. [Google Scholar]
Gupta 1995 {published data only}
- Gupta RK. Value of image guided fine‐needle aspiration cytology in the diagnosis of pancreatic malignancies. Diagnostic Cytopathology 1995;13(2):120‐3. [DOI] [PubMed] [Google Scholar]
Gupta 2008 {published data only}
- Gupta R, Mortele KJ, Tatli S, Girshman J, Glickman JN, Levy AD, et al. Pancreatic intraductal papillary mucinous neoplasms: Role of CT in predicting pathologic subtypes [erratum appears in AJR: American Journal of Roentgenology 2008;191(6):1876]. AJR: American Journal of Roentgenology 2008;191(5):1458‐64. [DOI] [PubMed] [Google Scholar]
Haba 2011 {published data only}
- Haba S, Yamao K, Mizuno N, Hara K, Hijioka S, Imaoka H, et al. Diagnostic ability of endoscopic ultrasound‐guided fine needle aspiration for pancreatic solid lesions: A large single center experience. Journal of Gastroenterology and Hepatology 2011;26:240. [DOI] [PubMed] [Google Scholar]
Haba 2013 {published data only}
- Haba S, Yamao K, Bhatia V, Mizuno N, Hara K, Hijioka S, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound‐guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. Journal of Gastroenterology 2013;48(8):973‐81. [DOI] [PubMed] [Google Scholar]
Hammel 1995 {published data only}
- Hammel P, Levy P, Voitot H, Levy M, Vilgrain V, Zins M, et al. Preoperative cyst fluid analysis is useful for the differential‐diagnosis of cystic lesions of the pancreas. Gastroenterology 1995;108(4):1230‐5. [DOI] [PubMed] [Google Scholar]
Hammel 1998 {published data only}
- Hammel P, Voitot H, Vilgrain V, Levy P, Ruszniewski P, Bernades P. Diagnostic value of ca 72‐4 and carcinoembryonic antigen determination in the fluid of pancreatic cystic lesions. European Journal of Gastroenterology & Hepatology 1998;10(4):345‐8. [DOI] [PubMed] [Google Scholar]
Han 2016 {published data only}
- Han C, Lin R, Zhang Q, Liu J, Ding Z, Hou X. Role of endoscopic ultrasound‐guided fine needle aspiration in the diagnosis of mass lesions. Experimental and Therapeutic Medicine 2016;12(2):1085‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanada 2009 {published data only}
- Hanada K. Diagnostic value of EUS‐FNA in cases with t1 (less than 20 mm) pancreatic solid tumor lesion. Gastrointestinal Endoscopy 2009;69(2):S244. [Google Scholar]
Hanninen 2002 {published data only}
- Hanninen EL, Amthauer H, Hosten N, Ricke J, Bohmig M, Langrehr J, et al. Prospective evaluation of pancreatic tumors: Accuracy of MR imaging with MR cholangiopancreatography and MR angiography. Radiology 2002;224(1):34‐41. [DOI] [PubMed] [Google Scholar]
Hanninen 2005 {published data only}
- Hanninen EL, Ricke J, Amthauer H, Rottgen R, Bohmig M, Langrehr J, et al. Magnetic resonance cholangiopancreatography: Image quality, ductal morphology, and value of additional t2‐ and t1‐weighted sequences for the assessment of suspected pancreatic cancer [erratum appears in Acta Radiologica 2005;46(3):Following 331]. Acta Radiologica 2005;46(2):117‐25. [DOI] [PubMed] [Google Scholar]
Harewood 2001a {published data only}
- Harewood GC, Wiersema MJ. Diagnosis of pancreatic cancer ‐ EUS/FNA to the rescue?. American Journal of Gastroenterology 2001;96(8):2501‐2. [DOI] [PubMed] [Google Scholar]
Harewood 2001b {published data only}
- Harewood GC, Wiersema MJ, Halling AC, Keeney GL, Salamao DR, Wiersema LM. Prospective blinded assessment of the effect of experience and pathology interpretation on accuracy of endoscopic ultrasound guided fine needle aspiration biopsy of pancreatic masses. Gastrointestinal Endoscopy 2001;53(5):AB77. [Google Scholar]
Harewood 2002 {published data only}
- Harewood GC, Wiersema MJ. Endosonography‐guided fine needle aspiration biopsy in the evaluation of pancreatic masses. American Journal of Gastroenterology 2002;97(6):1386‐91. [DOI] [PubMed] [Google Scholar]
Hasan 2014 {published data only}
- Hasan MK, Bang JY, Varadarajulu S. Diagnostic value of priming the endoscopic ultrasound‐guided fine‐needle aspiration needle with heparin to improve specimen quality. Digestive Endoscopy 2014;26(3):491. [DOI] [PubMed] [Google Scholar]
Hasenberg 2009 {published data only}
- Hasenberg F, Heinzow H, Berssenbrugge C, Opitz JP, Domschke W, Meister T. Endoscopic ultrasound‐guided fine needle aspiration of pancreatic lesions: A useful preoperative diagnostic tool?. Scandinavian Journal of Gastroenterology 2009;44:38. [Google Scholar]
Hasyagar 2004 {published data only}
- Hasyagar CYP, Andersen DK, Tada H, Fischer A, Mehta S, Wassef W. Accuracy of endoscopic ultrasound‐guided fine‐needle aspiration (FNA) in pancreatic cancer tissue acquisition ‐ umass experience. American Journal of Gastroenterology 2004;99(10):S293. [Google Scholar]
Hayashi 2013 {published data only}
- Hayashi T, Ishiwatari H, Yoshida M, Ono M, Sato T, Miyanishi K, et al. Rapid on‐site evaluation by endosonographer during endoscopic ultrasound‐guided fine needle aspiration for pancreatic solid masses. Journal of Gastroenterology and Hepatology 2013;28(4):656‐63. [DOI] [PubMed] [Google Scholar]
Hebert‐Magee 2015 {published data only}
- Hebert‐Magee S. Is there a role for endoscopic ultrasound‐guided fine‐needle biopsy in pancreatic cancer?. Endoscopy 2015;47(4):291‐2. [DOI] [PubMed] [Google Scholar]
Heinrich 2005 {published data only}
- Heinrich S, Goerres GW, Schafer M, Sagmeister M, Bauerfeind P, Pestalozzi BC, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost‐effectiveness. Annals of Surgery 2005;242(2):235‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henkes 2013 {published data only}
- Henkes DN, Patel SN, Rosenkranz LA, Escobedo JL. The utility of urovysion fluorescence in situ hybridization in pancreatic fine‐needle aspiration samples directed and obtained by endoscopic ultrasonography. Archives of Pathology & Laboratory Medicine 2013;137(1):64‐71. [DOI] [PubMed] [Google Scholar]
Heo 2013 {published data only}
- Heo J, Jung MK, Jeon S, Cho CM. The comparison of specimen adequacy for diagnosis of solid lesion between EUS‐FNA with suction and EUS‐FNA without suction: Prospective randomized paired crossover study. Gastrointestinal Endoscopy 2013;77(5 Suppl):AB399. [Google Scholar]
Herman‐Sucharska 1999 {published data only}
- Herman‐Sucharska I, Hoe L, Sucharski P, Tomaszewska R, Urbanik A, Chrzan R, et al. Tumour associated trypsin inhibitor as a marker helpful in CT differentiation between carcinoma pancreatis and chronic pancreatitis. Polski Przeglad Radiologii 1999;64(2):117‐9. [Google Scholar]
Hernandez 2002 {published data only}
- Hernandez LV, Mishra G, Forsmark C, Draganov PV, Petersen JM, Hochwald SN, et al. Role of endoscopic ultrasound (EUS) and EUS‐guided fine needle aspiration in the diagnosis and treatment of cystic lesions of the pancreas. Pancreas 2002;25(3):222‐8. [DOI] [PubMed] [Google Scholar]
Herrmann 2012 {published data only}
- Herrmann K, Erkan M, Dobritz M, Schuster T, Siveke JT, Beer AJ, et al. Comparison of 3'‐deoxy‐3'‐[18f]fluorothymidine positron emission tomography (FLT PET) and FDG PET/CT for the detection and characterization of pancreatic tumours. European Journal of Nuclear Medicine & Molecular Imaging 2012;39(5):846‐51. [DOI] [PubMed] [Google Scholar]
Higashi 2002a {published data only}
- Higashi T, Saga T, Ishimori T, Konishi J. Diagnostic value of FDG‐PET in patients with pancreatic lesions. Japanese Journal of Clinical Radiology 2002;47(9):1113‐20. [Google Scholar]
Higashi 2002b {published data only}
- Higashi T, Saga T, Ishimori T, Mamede MH, Maetani Y, Ito K, et al. Diagnostic value of f‐18‐FDG PET in patients with cystic pancreatic tumors, including intraductal papillary mucinous tumor (IPMT). Journal of Nuclear Medicine 2002;43(5 Suppl):298P‐9P. [Google Scholar]
Higashi 2003 {published data only}
- Higashi T, Saga T, Nakamoto Y, Ishimori T, Fujimoto K, Doi R, et al. Diagnosis of pancreatic cancer using fluorine‐18 fluorodeoxyglucose positron emission tomography (FDG PET) ‐ usefulness and limitations in "clinical reality". Annals of Nuclear Medicine 2003;17(4):261‐79. [DOI] [PubMed] [Google Scholar]
Hijioka 2014 {published data only}
- Hijioka S, Shimizu Y, Mizuno N, Hara K, Imaoka H, Mekky MA, et al. Can long‐term follow‐up strategies be determined using a nomogram‐based prediction model of malignancy among intraductal papillary mucinous neoplasms of the pancreas?. Pancreas 2014;43(3):367‐72. [DOI] [PubMed] [Google Scholar]
Hikichi 2009 {published data only}
- Hikichi T, Irisawa A, Bhutani MS, Takagi T, Shibukawa G, Yamamoto G, et al. Endoscopic ultrasound‐guided fine‐needle aspiration of solid pancreatic masses with rapid on‐site cytological evaluation by endosonographers without attendance of cytopathologists. Journal of Gastroenterology 2009;44(4):322‐8. [DOI] [PubMed] [Google Scholar]
Hilendarov 2010 {published data only}
- Hilendarov A, Velkova K. Imaging controlled diagnostic interventions of cystic pancreatic lesions: When and why we prefer US or CT guidance. Rentgenologiya i Radiologiya 2010;49(3):206‐9. [Google Scholar]
Hilendarov 2011 {published data only}
- Hilendarov A, Velkova K, Deenichin G, Anavi B. Imaging‐based evaluation and guidelines in the therapeutic behavior of pancreatic cystic lesions. Rentgenologiya i Radiologiya 2011;50(3):202‐6. [Google Scholar]
Hilendarov 2012 {published data only}
- Hilendarov AD, Deenichin GP, Velkova KG. Imaging investigation of pancreatic cystic lesions and proposal for therapeutic guidelines. World Journal of Radiology 2012;4(8):372‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hilendarov 2013 {published data only}
- Hilendarov A, Simova E, Deenichin G, Petrova A, Traikova N. Unusual cystic pancreatic neoplasms ‐ image‐pathological correlations. Rentgenologiya i Radiologiya 2013;52(2):124‐8. [Google Scholar]
Ho 1996 {published data only}
- Ho CL, Dehdashti F, Griffeth LK, Buse PE, Balfe DM, Siegel BA. FDG‐PET evaluation of indeterminate pancreatic masses. Journal of Computer Assisted Tomography 1996;20(3):363‐9. [DOI] [PubMed] [Google Scholar]
Ho 2004 {published data only}
- Ho S, Bonasera R, Michael H, Pollack B, Grendell J, Gupta M, et al. The accuracy of endoscopic ultrasound (EUS)‐guided fine needle aspiration (FNA) for diagnosing solid pancreatic lesions using a new 25‐gauge needle system. Gastrointestinal Endoscopy 2004;59(5):P222. [Google Scholar]
Hocke 2006 {published data only}
- Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast‐enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World Journal of Gastroenterology 2006;12(2):246‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hocke 2012 {published data only}
- Hocke M, Ignee A, Dietrich CF. Advanced endosonographic diagnostic tools for discrimination of focal chronic pancreatitis and pancreatic carcinoma ‐ elastography, contrast enhanced high mechanical index (CEHMI) and low mechanical index (CELMI) endosonography in direct comparison. Zeitschrift fur Gastroenterologie 2012;50(2):199‐203. [DOI] [PubMed] [Google Scholar]
Hollerbach 2004 {published data only}
- Hollerbach S, Brasch F, Junker K, Kosciesza S, Reiser M, Topalidis T, et al. Accuracy of histology, cytology, and immunohistochemistry (dpc‐4, muc‐1) in EUS‐guided pancreatic biopsies. Gastrointestinal Endoscopy 2004;59(5):P95. [Google Scholar]
Holt 2008 {published data only}
- Holt EW, Macklin EA, Brugge WR. Variables affecting the accuracy of EUS‐guided FNA in the diagnosis of focal pancreatic masses. Gastrointestinal Endoscopy 2008;67(5):AB218‐9. [Google Scholar]
Holt 2014 {published data only}
- Holt B, Varadarajulu S. Endoscopic ultrasound‐guided fine needle aspiration or fine needle biopsy: The beauty is in the eye of the beholder. Endoscopy 2014;46(12):1046‐8. [DOI] [PubMed] [Google Scholar]
Hong 2012 {published data only}
- Hong SKS, Loren DE, Rogart JN, Siddiqui AA, Sendecki JA, Bibbo M, et al. Targeted cyst wall puncture and aspiration during EUS‐FNA increases the diagnostic yield of precancerous and malignant pancreatic cysts. Gastrointestinal Endoscopy 2012;75(4):775‐82. [DOI] [PubMed] [Google Scholar]
Honselmann 2016 {published data only}
- Honselmann KC, Krauss T, Geserick S, Wellner UF, Wittel U, Hopt UT, et al. Cystic lesions of the pancreas ‐ is radical surgery really warranted?. Langenbecks Archives of Surgery 2016;401(4):449‐56. [DOI] [PubMed] [Google Scholar]
Horatagis 2003 {published data only}
- Horatagis APE, Fayad LM, Holland GA, Loren DE, Mitchell DG, Kowalski TE. Positive predictive value of MRI for the diagnosis of mucinous cystic lesions of the pancreas. Gastroenterology 2003;124(4):A187. [Google Scholar]
Horwhat 2004 {published data only}
- Horwhat JD, McGrath K, Enns RA, Stiffler H, Branch MS, Baillie J, et al. EUS FNA is more sensitive than CT/US FNA for diagnosing pancreatic malignancy: A randomized controlled trial. Gastrointestinal Endoscopy 2004;59(5):P222. [Google Scholar]
Horwhat 2006 {published data only}
- Horwhat JD, Paulson EK, McGrath K, Branch MS, Baillie J, Tyler D, et al. A randomized comparison of EUS‐guided FNA versus CT or US‐guided FNA for the evaluation of pancreatic mass lesions. Gastrointestinal Endoscopy 2006;63(7):966‐75. [DOI] [PubMed] [Google Scholar]
Hou 2015 {published data only}
- Hou X, Jin Z, Xu C, Zhang M, Zhu J, Jiang F, et al. Contrast‐enhanced harmonic endoscopic ultrasound‐guided fine‐needle aspiration in the diagnosis of solid pancreatic lesions: A retrospective study. PLoS ONE 2015;10(3):e0121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2014 {published data only}
- Hu C, Qian HF, Li F, Hu C. Comparison of enhanced magnetic resonance imaging and magnetic resonance cholangiopancreatography in the differential diagnosis of benign and malignant intraductal papillary mucinous neoplasms of the pancreas. Acta Academiae Medicinae Sinicae 2014;36(1):98‐101. [DOI] [PubMed] [Google Scholar]
Huang 2010 {published data only}
- Huang ES, Turner BG, Fernandez‐Del‐Castillo C, Brugge WR, Hur C. Pancreatic cystic lesions: Clinical predictors of malignancy in patients undergoing surgery. Alimentary Pharmacology & Therapeutics 2010;31(2):285‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2011 {published data only}
- Huang WC, Sheng J, Chen SY, Lu JP. Differentiation between pancreatic carcinoma and mass‐forming chronic pancreatitis: Usefulness of high b value diffusion‐weighted imaging. Journal of Digestive Diseases 2011;12(5):401‐8. [DOI] [PubMed] [Google Scholar]
Hunt 2009 {published data only}
- Hunt G, Master S, Yang SJ, Savides T. Determining diagnostic yield of EUS‐FNA for solid pancreatic masses at a newly established EUS center. Gastrointestinal Endoscopy 2009;69(2):S244. [Google Scholar]
Hussain 2009 {published data only}
- Hussain T, Salamat A, Farooq MA, Hassan F, Hafeez M. Indications for endoscopic ultrasound and diagnosis on fine‐needle aspiration and cytology. JCPSP: Journal of the College of Physicians and Surgeons Pakistan 2009;19(4):223‐7. [PubMed] [Google Scholar]
Hwang 2009 {published data only}
- Hwang CY, Lee SS, Song TJ, Moon SH, Lee D, Park DH, et al. Endoscopic ultrasound guided fine needle aspiration biopsy in diagnosis of pancreatic and peripancreatic lesions: A single center experience in Korea. Gut & Liver 2009;3(2):116‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hwang 2011 {published data only}
- Hwang DW, Jang JY, Lim CS, Lee SE, Yoon YS, Ahn YJ, et al. Determination of malignant and invasive predictors in branch duct type intraductal papillary mucinous neoplasms of the pancreas: A suggested scoring formula. Journal of Korean Medical Science 2011;26(6):740‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 2014 {published data only}
- Ibrahim AA, Cramer HM, Wu HH. Endoscopic ultrasound‐guided fine‐needle aspiration of the pancreas: A retrospective study of 1000 cases. Journal of the American Society of Cytopathology 2014;3(5):227‐35. [DOI] [PubMed] [Google Scholar]
Ichikawa 2001 {published data only}
- Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K, et al. Duct‐penetrating sign at MRCP: Usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology 2001;221(1):107‐16. [DOI] [PubMed] [Google Scholar]
Iftimia 2012 {published data only}
- Iftimia N, Yoon WJ, Brugge WR. Cystic lesions of the pancreas: More reliable differentiation with in situ high‐resolution optical imaging?. Expert Review of Gastroenterology & Hepatology 2012;6(2):125‐7. [DOI] [PubMed] [Google Scholar]
Iglesias 2013 {published data only}
- Iglesias Garcia J, Larino Noia J, Dominguez Munoz JE. Differential diagnosis of solid pancreatic masses by quantitative endoscopic ultrasound elastography (q EUS e): A validation study. Pancreatology 2013;13(4 Suppl 1):e9. [Google Scholar]
Iglesias‐Garcia 2007 {published data only}
- Iglesias‐Garcia J, Dominguez‐Munoz E, Lozano‐Leon A, Abdulkader I, Larino‐Noia J, Antunez J, et al. Impact of endoscopic ultrasound‐guided fine needle biopsy for diagnosis of pancreatic masses. World Journal of Gastroenterology 2007;13(2):289‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iglesias‐Garcia 2008 {published data only}
- Iglesias‐Garcia J, Larino‐Noia J, Eugenyeva E, Abdulkader I, Lozano‐Leon A, Vieites B, et al. Accuracy of endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA) for the cytological diagnosis of solid pancreatic masses and clinical impact of on‐site cytophatological evaluation. Gastroenterology 2008;134(4 Suppl 1):A697. [Google Scholar]
Iglesias‐Garcia 2009a {published data only}
- Iglesias‐Garcia J, Lanno J, Dominguez‐Munoz E. Accuracy of second‐generation endoscopic ultrasound (EUS) elastography for the differential diagnosis of solid pancreatic masses: A quantitative analysis of tissue stiffness. Gastroenterology 2009;136(5 Suppl 1):A130. [Google Scholar]
Iglesias‐Garcia 2009b {published data only}
- Iglesias‐Garcia J, Larino‐Noia J, Abdulkader I, Forteza J, Dominguez‐Munoz JE. EUS elastography for the characterization of solid pancreatic masses. Gastrointestinal Endoscopy 2009;70(6):1101‐8. [DOI] [PubMed] [Google Scholar]
Iglesias‐Garcia 2010 {published data only}
- Iglesias‐Garcia J, Larino‐Noia J, Abdulkader I, Forteza J, Dominguez‐Munoz JE. Quantitative endoscopic ultrasound elastography: An accurate method for the differentiation of solid pancreatic masses. Gastroenterology 2010;139(4):1172‐80. [DOI] [PubMed] [Google Scholar]
Iglesias‐Garcia 2011 {published data only}
- Iglesias‐Garcia J, Dominguez‐Munoz JE, Abdulkader I, Larino‐Noia J, Eugenyeva E, Lozano‐Leon A, et al. Influence of on‐site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA) of solid pancreatic masses. American Journal of Gastroenterology 2011;106(9):1705‐10. [DOI] [PubMed] [Google Scholar]
Iglesias‐Garcia 2013a {published data only}
- Iglesias‐Garcia J. Endoscopic ultrasound‐guided fine needle aspiration for solid pancreatic masses. Optimizing the diagnostic yield. Journal of Gastrointestinal and Liver Diseases 2013;22(2):127‐8. [PubMed] [Google Scholar]
Iglesias‐Garcia 2013b {published data only}
- Iglesias‐Garcia J, Lindkvist B, Cruz‐Soares JB, Lopes LM, Marra‐Lopez C, Larino‐Noia J, et al. Differential diagnosis of solid pancreatic masses: Contrast‐enhanced harmonic EUS (cehEUS), quantitative EUS‐elastography (qe‐EUS) or both?. Pancreatology 2013;13(2):e35. [Google Scholar]
Iguchi 2010 {published data only}
- Iguchi H, Oota K, Tanada M, Asagi A, Nasu J, Takeji S, et al. Differential diagnosis of IPMNs (benign or malignant) by contrast‐enhanced PET/CT. Pancreatology 2010;10(Suppl 1):79‐80. [Google Scholar]
Ikeura 2014 {published data only}
- Ikeura T, Detlefsen S, Zamboni G, Manfredi R, Negrelli R, Amodio A, et al. Retrospective comparison between preoperative diagnosis by international consensus diagnostic criteria and histological diagnosis in patients with focal autoimmune pancreatitis who underwent surgery with suspicion of cancer. Pancreas 2014;43(5):698‐703. [DOI] [PubMed] [Google Scholar]
Ikeura 2015a {published data only}
- Ikeura T, Takaoka M, Uchida K, Shimatani M, Miyoshi H, Kato K, et al. Fluorescence cytology with 5‐aminolevulinic acid in EUS‐guided FNA as a method for differentiating between malignant and benign lesions. Gastrointestinal Endoscopy 2015;81(6):1457‐62. [DOI] [PubMed] [Google Scholar]
Ikeura 2015b {published data only}
- Ikeura T, Takaoka M, Uchida K, Shimatani M, Miyoshi H, Okazaki K. Photodynamic diagnosis using 5‐aminolevulinic acid during endoscopic ultrasound‐guided fine needle aspiration for pancreatobiliary lesions. Gastrointestinal Endoscopy 2015;1:AB536. [Google Scholar]
Imazu 2009 {published data only}
- Imazu H, Uchiyama Y, Kakutani H, Ikeda K, Sumiyama K, Kaise M, et al. A prospective comparison of EUS‐guided FNA using 25‐gauge and 22‐gauge needles. Gastroenterology Research & Practice 2009;2009:546390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imdahl 1999 {published data only}
- Imdahl A, Nitzsche E, Krautmann F, Hogerle S, Boos S, Einert A, et al. Evaluation of positron emission tomography with 2‐[18f]fluoro‐2‐deoxy‐d‐glucose for the differentiation of chronic pancreatitis and pancreatic cancer. British Journal of Surgery 1999;86(2):194‐9. [DOI] [PubMed] [Google Scholar]
Inokuma 1995 {published data only}
- Inokuma T, Tamaki N, Torizuka T, Magata Y, Fujii M, Yonekura Y, et al. Evaluation of pancreatic tumors with positron emission tomography and f‐18 fluorodeoxyglucose: Comparison with CT and US. Radiology 1995;195(2):345‐52. [DOI] [PubMed] [Google Scholar]
Iordache 2016 {published data only}
- Iordache S, Costache MI, Popescu CF, Streba CT, Cazacu S, Saftoiu A. Clinical impact of EUS elastography followed by contrast‐enhanced EUS in patients with focal pancreatic masses and negative EUS‐guided FNA. Medical Ultrasonography 2016;18(1):18‐24. [DOI] [PubMed] [Google Scholar]
Ippolito 2015 {published data only}
- Ippolito D, Allegranza P, Bonaffini PA, Talei Franzesi C, Leone F, Sironi S. Diagnostic accuracy of 256‐detector row computed tomography in detection and characterization of incidental pancreatic cystic lesions. Gastroenterology Research & Practice 2015;2015:707546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irie 2002 {published data only}
- Irie H, Honda H, Kuroiwa T, Yoshimitsu K, Aibe H, Shinozaki K, et al. Measurement of the apparent diffusion coefficient in intraductal mucin‐producing tumor of the pancreas by diffusion‐weighted echo‐planar MR imaging. Abdominal Imaging 2002;27(1):82‐7. [DOI] [PubMed] [Google Scholar]
Ironside 2010 {published data only}
- Ironside A, Young M. Diagnostic accuracy of endoscopic ultrasound guided fine needle aspiration of pancreatic lesions. Journal of Pathology 2010;222:S45. [Google Scholar]
Ishigami 2010 {published data only}
- Ishigami K, Tajima T, Nishie A, Kakihara D, Fujita N, Asayama Y, et al. Differential diagnosis of groove pancreatic carcinomas vs. groove pancreatitis: Usefulness of the portal venous phase. European Journal of Radiology 2010;74(3):e95‐100. [DOI] [PubMed] [Google Scholar]
Ishii 2012 {published data only}
- Ishii H, Taniguchi H, Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, et al. Use of multidetector‐row computed tomography to evaluate branch duct type intraductal papillary mucinous neoplasms of the pancreas: Influence on surgical decision‐making. Hepato‐Gastroenterology 2012;59(115):884‐8. [DOI] [PubMed] [Google Scholar]
Ishikawa 2010 {published data only}
- Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, et al. Usefulness of EUS combined with contrast‐enhancement in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors. Gastrointestinal Endoscopy 2010;71(6):951‐9. [DOI] [PubMed] [Google Scholar]
Itoh 2005 {published data only}
- Itoh T, Hirooka Y, Itoh A, Hashimoto S, Kawashima H, Hara K, et al. Usefulness of contrast‐enhanced transabdominal ultrasonography in the diagnosis of intraductal papillary mucinous tumors of the pancreas. American Journal of Gastroenterology 2005;100(1):144‐52. [DOI] [PubMed] [Google Scholar]
Itoi 2005a {published data only}
- Itoi T, Itokawa F, Sofuni A, Nakamura K, Tsuchida A, Yamao K, et al. Puncture of solid pancreatic tumors guided by endoscopic ultrasonography: A pilot study series comparing trucut and 19‐gauge and 22‐gauge aspiration needles. Endoscopy 2005;37(4):362‐6. [DOI] [PubMed] [Google Scholar]
Itoi 2005b {published data only}
- Itoi T, Takei K, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, et al. Immunohistochemical analysis of p53 and mib‐1 in tissue specimens obtained from endoscopic ultrasonography‐guided fine needle aspiration biopsy for the diagnosis of solid pancreatic masses. Oncology Reports 2005;13(2):229‐34. [PubMed] [Google Scholar]
Itoi 2011 {published data only}
- Itoi T, Tsuchiya T, Itokawa F, Sofuni A, Kurihara T, Tsuji S, et al. Histological diagnosis by EUS‐guided fine‐needle aspiration biopsy in pancreatic solid masses without on‐site cytopathologist: A single‐center experience. Digestive Endoscopy 2011;23 Suppl 1:34‐8. [DOI] [PubMed] [Google Scholar]
Itokawa 2010 {published data only}
- Itokawa F, Itoi T, Sofuni A, Kurihara T, Ishii K. EUS elastography for the differentiation of solid pancreatic masses. Pancreatology 2010;10(Suppl 1):122‐3. [Google Scholar]
Itokawa 2011 {published data only}
- Itokawa F, Itoi T, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, et al. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. Journal of Gastroenterology 2011;46(6):843‐53. [DOI] [PubMed] [Google Scholar]
Iwashita 2013 {published data only}
- Iwashita T, Nakai Y, Samarasena JB, Park DH, Zhang Z, Gu M, et al. High single‐pass diagnostic yield of a new 25‐gauge core biopsy needle for EUS‐guided FNA biopsy in solid pancreatic lesions. Gastrointestinal Endoscopy 2013;77(6):909‐15. [DOI] [PubMed] [Google Scholar]
Iwashita 2015 {published data only}
- Iwashita T, Yasuda I, Mukai T, Doi S, Nakashima M, Uemura S, et al. Macroscopic on‐site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS‐guided FNA using a 19‐gauge needle for solid lesions: A single‐center prospective pilot study (MOSE study). Gastrointestinal Endoscopy 2015;81(1):177‐85. [DOI] [PubMed] [Google Scholar]
Izuishi 2010 {published data only}
- Izuishi K, Yamamoto Y, Sano T, Takebayashi R, Masaki T, Suzuki Y. Impact of 18‐fluorodeoxyglucose positron emission tomography on the management of pancreatic cancer. Journal of Gastrointestinal Surgery 2010;14(7):1151‐8. [DOI] [PubMed] [Google Scholar]
Jabbar 2014 {published data only}
- Jabbar KS, Verbeke C, Hyltander AG, Sjovall H, Hansson GC, Sadik R. Proteomic mucin profiling for the identification of cystic precursors of pancreatic cancer. Journal of the National Cancer Institute 2014;106(2):djt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadvar 2001 {published data only}
- Jadvar H, Fischman AJ. Evaluation of pancreatic carcinoma with FDG PET. Abdominal Imaging 2001;26(3):254‐9. [DOI] [PubMed] [Google Scholar]
Jahng 2010 {published data only}
- Jahng AW, Reicher S, Chung D, Varela D, Chhablani R, Dev A, et al. Staining for p53 and ki‐67 increases the sensitivity of EUS‐FNA to detect pancreatic malignancy. World Journal of Gastrointestinal Endoscopy 2010;2(11):362‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jahromi 2014 {published data only}
- Jahromi AH, Fallahzadeh MK, Takalkar A, Sheng J, Zibari G, Amiri HS. Impact of plasma glucose level at the time of fluorodeoxyglucose administration on the accuracy of FDG‐PET/CT in the diagnosis of pancreatic lesions. International Journal of Endocrinology and Metabolism 2014;12(4):e16429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jang 2012 {published data only}
- Jang KM, Kim SH, Kim YK, Park MJ, Lee MH, Hwang J, et al. Imaging features of small (< 3 cm) pancreatic solid tumors on gadoxetic‐acid‐enhanced MR imaging and diffusion‐weighted imaging: An initial experience. Magnetic Resonance Imaging 2012;30(7):916‐25. [DOI] [PubMed] [Google Scholar]
Jang 2015 {published data only}
- Jang DK, Song BJ, Ryu JK, Chung KH, Lee BS, Park JK, et al. Preoperative diagnosis of pancreatic cystic lesions: The accuracy of endoscopic ultrasound and cross‐sectional imaging. Pancreas 2015;44(8):1329‐33. [DOI] [PubMed] [Google Scholar]
Jani 2006 {published data only}
- Jani N, McGrath K. What is the negative predictive value of endoscopic ultrasonography in patients with suspected pancreatic cancer?. Nature Clinical Practice Gastroenterology & Hepatology 2006;3(6):308‐9. [Google Scholar]
Jani 2008 {published data only}
- Jani N, Dewitt J, Eloubeidi M, Varadarajulu S, Appalaneni V, Hoffman B, et al. Endoscopic ultrasound‐guided fine‐needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: A multicenter experience. Endoscopy 2008;40(3):200‐3. [DOI] [PubMed] [Google Scholar]
Janssen 2007 {published data only}
- Janssen J, Schlorer E, Greiner L. EUS elastography of the pancreas: Feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointestinal Endoscopy 2007;65(7):971‐8. [DOI] [PubMed] [Google Scholar]
Jayasekeran 2012 {published data only}
- Jayasekeran V, Hazeldine S, Chong A. A five year experience on the performance of the 22g EUS‐FNA needle with on site cytological analysis in the evaluation of solid lesions. Journal of Gastroenterology and Hepatology 2012;27(Suppl S4):57‐8. [Google Scholar]
Jeong 2012 {published data only}
- Jeong HS, Kim H, Tae Kim J, Han JH, Youn SJ, Chae HB, et al. Comparing liquid based cytology (Cellprep) methods with conventional smear methods for EUS or ERCP cytology of pancreas or bile duct. Journal of Gastroenterology and Hepatology 2012;27:371‐2. [Google Scholar]
Jhala 2007 {published data only}
- Jhala NC, Eltoum IA, Eloubeidi MA, Meara R, Chhieng DC, Crowe DR, et al. Providing on‐site diagnosis of malignancy on endoscopic‐ultrasound‐guided fine‐needle aspirates: Should it be done?. Annals of Diagnostic Pathology 2007;11(3):176‐81. [DOI] [PubMed] [Google Scholar]
Jin 2013b {published data only}
- Jin DX, Small AJ, Bernstein GR, Shah PM, Ginsberg GG, Kochman ML, et al. A low CEA cut‐off identifies mucinous pancreatic cystic lesions with increased diagnostic accuracy. Gastroenterology 2013;1:S796. [Google Scholar]
Jing 2009 {published data only}
- Jing X, Wamsteker EJ, Li H, Pu RT. Combining fine needle aspiration with brushing cytology has improved yields in diagnosing pancreatic ductal adenocarcinoma. Diagnostic Cytopathology 2009;37(8):574‐8. [DOI] [PubMed] [Google Scholar]
Johnson 1999 {published data only}
- Johnson PT, Outwater EK. Pancreatic carcinoma versus chronic pancreatitis: Dynamic MR imaging. Radiology 1999;212(1):213‐8. [DOI] [PubMed] [Google Scholar]
Kadayifci 2014 {published data only}
- Kadayifci A, Brugge WR. Endoscopic ultrasound‐guided fine‐needle aspiration for the differential diagnosis of intraductal papillary mucinous neoplasms and size stratification for surveillance. Endoscopy 2014;46(4):357. [DOI] [PubMed] [Google Scholar]
Kadayifci 2016 {published data only}
- Kadayifci A, Al‐Haddad M, Atar M, Dewitt JM, Forcione DG, Sherman S, et al. The value of KRAS mutation testing with CEA for the diagnosis of pancreatic mucinous cysts. Endoscopy International Open 2016;4(4):E391‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaffes 2012 {published data only}
- Kaffes AJ, Chen RYM, Tam W, Norton I, Cho S, Devereaux B, et al. A prospective multicenter evaluation of a new side‐port endoscopic ultrasound‐fine‐needle aspiration in solid upper gastrointestinal lesions. Digestive Endoscopy 2012;24(6):448‐51. [DOI] [PubMed] [Google Scholar]
Kaimakliotis 2015 {published data only}
- Kaimakliotis P, Riff B, Pourmand K, Chandrasekhara V, Furth EE, Siegelman ES, et al. Sendai and Fukuoka consensus guidelines identify advanced neoplasia in patients with suspected mucinous cystic neoplasms of the pancreas. Clinical Gastroenterology and Hepatology 2015;13(10):1808‐15. [DOI] [PubMed] [Google Scholar]
Kalb 2013 {published data only}
- Kalb B, Martin DR, Sarmiento JM, Erickson SH, Gober D, Tapper EB, et al. Paraduodenal pancreatitis: Clinical performance of MR imaging in distinguishing from carcinoma. Radiology 2013;269(2):475‐81. [DOI] [PubMed] [Google Scholar]
Kalra 2003 {published data only}
- Kalra MK, Maher MM, Boland GW, Saini S, Fischman AJ. Correlation of positron emission tomography and CT in evaluating pancreatic tumors: Technical and clinical implications. American Journal of Roentgenology 2003;181(2):387‐93. [DOI] [PubMed] [Google Scholar]
Kamata 2014 {published data only}
- Kamata K, Kitano M, Kudo M, Sakamoto H, Kadosaka K, Miyata T, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms [erratum appears in Endoscopy 2014;46(4):358]. Endoscopy 2014;46(1):22‐9. [DOI] [PubMed] [Google Scholar]
Kamata 2016b {published data only}
- Kamata K, Kitano M, Yasukawa S, Kudo M, Chiba Y, Ogura T, et al. Histologic diagnosis of pancreatic masses using 25‐gauge endoscopic ultrasound needles with and without a core trap: A multicenter randomized trial. Endoscopy 2016;48(7):632‐8. [DOI] [PubMed] [Google Scholar]
Kamin 1980 {published data only}
- Kamin PD, Bernardino ME, Wallace S, Jing BS. Comparison of ultrasound and computed tomography in the detection of pancreatic malignancy. Cancer 1980;46(11):2410‐2. [DOI] [PubMed] [Google Scholar]
Kamisawa 2008 {published data only}
- Kamisawa T, Imai M, Chen PY, Tu YY, Egawa N, Tsuruta K, et al. Strategy for differentiating autoimmune pancreatitis from pancreatic cancer. Pancreas 2008;37(3):E62‐7. [DOI] [PubMed] [Google Scholar]
Kanazawa 2012 {published data only}
- Kanazawa K, Imazu H, Mori N, Ikeda K, Kakutani H, Sumiyama K, et al. A comparison of electronic radial and curvilinear endoscopic ultrasonography in the detection of pancreatic malignant tumor. Scandinavian Journal of Gastroenterology 2012;47(11):1313‐20. [DOI] [PubMed] [Google Scholar]
Kang 2013 {published data only}
- Kang KM, Lee JM, Shin CI, Baek JH, Kim SH, Yoon JH, et al. Added value of diffusion‐weighted imaging to MR cholangiopancreatography with unenhanced MR imaging for predicting malignancy or invasiveness of intraductal papillary mucinous neoplasm of the pancreas. Journal of Magnetic Resonance Imaging 2013;38(3):555‐63. [DOI] [PubMed] [Google Scholar]
Kang 2014 {published data only}
- Kang KM, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI. Intravoxel incoherent motion diffusion‐weighted MR imaging for characterization of focal pancreatic lesions. Radiology 2014;270(2):444‐53. [DOI] [PubMed] [Google Scholar]
Kang 2016 {published data only}
- Kang HJ, Lee JM, Joo I, Hur BY, Jeon JH, Jang JY, et al. Assessment of malignant potential in intraductal papillary mucinous neoplasms of the pancreas: Comparison between multidetector CT and MR imaging with MR cholangiopancreatography. Radiology 2016;279(1):128‐39. [DOI] [PubMed] [Google Scholar]
Katanuma 2013 {published data only}
- Katanuma A, Maguchi H, Yane K, Hashigo S, Kin T, Kaneko M, et al. Factors predictive of adverse events associated with endoscopic ultrasound‐guided fine needle aspiration of pancreatic solid lesions. Digestive Diseases and Sciences 2013;58(7):2093‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2007 {published data only}
- Katz DS, Friedel DM, Kho D, Georgiou N, Hines JJ. Relative accuracy of CT and MRI for characterization of cystic pancreatic masses. AJR: American Journal of Roentgenology 2007;189(3):657‐61. [DOI] [PubMed] [Google Scholar]
Kauhanen 2009a {published data only}
- Kauhanen SP, Komar G, Seppanen MP, Dean KI, Minn HR, Kajander SA, et al. A prospective diagnostic accuracy study of 18f‐fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Annals of Surgery 2009;250(6):957‐63. [DOI] [PubMed] [Google Scholar]
Kauhanen 2009b {published data only}
- Kauhanen SP, Komar G, Seppanen MP, Dean KI, Minn HR, Kajander SA, et al. A prospective diagnostic accuracy study of f‐18‐fluorodeoxyglucose positron emission tomography/computed tomography (FDG‐PET/CT), multidetector row computed tomography (MDCT) and magnetic resonance imaging (MRI) in primary diagnosis and staging of pancreatic cancer. Pancreas 2009;38(8):1014. [DOI] [PubMed] [Google Scholar]
Kauhanen 2015 {published data only}
- Kauhanen S, Rinta‐Kiikka I, Kemppainen J, Gronroos J, Kajander S, Seppanen M, et al. Accuracy of 18F‐FDG PET/CT, multidetector CT, and MR imaging in the diagnosis of pancreatic cysts: A prospective single‐center study. Journal of Nuclear Medicine 2015;56(8):1163‐8. [DOI] [PubMed] [Google Scholar]
Kawada 2012 {published data only}
- Kawada N, Uehara H, Katayama K, Takakura R, Ioka T, Tanaka S, et al. Diagnostic clues and subsequent examinations that detected small pancreatic cancer. Hepato‐Gastroenterology 2012;59(118):1665‐9. [DOI] [PubMed] [Google Scholar]
Kawada 2014 {published data only}
- Kawada N, Uehara H, Nagata S, Tsuchishima M, Tsutsumi M, Tomita Y. Predictors of malignancy in branch duct intraductal papillary mucinous neoplasm of the pancreas. Journal of the Pancreas 2014;15(5):459‐64. [DOI] [PubMed] [Google Scholar]
Kawada 2015 {published data only}
- Kawada N, Uehara H, Hosoki T, Takami M, Shiroeda H, Arisawa T, et al. Usefulness of dual‐phase 18f‐FDG PET/CT for diagnosing small pancreatic tumors. Pancreas 2015;44(4):655‐9. [DOI] [PubMed] [Google Scholar]
Kawada 2016 {published data only}
- Kawada N, Uehara H, Nagata S, Tsuchishima M, Tsutsumi M, Tomita Y. Mural nodule of 10 mm or larger as predictor of malignancy for intraductal papillary mucinous neoplasm of the pancreas: Pathological and radiological evaluations. Pancreatology 2016;16(3):441‐8. [DOI] [PubMed] [Google Scholar]
Kawamoto 2006 {published data only}
- Kawamoto S, Lawler LP, Horton KM, Eng J, Hruban RH, Fishman EK. MDCT of intraductal papillary mucinous neoplasm of the pancreas: Evaluation of features predictive of invasive carcinoma. AJR: American Journal of Roentgenology 2006;186(3):687‐95. [DOI] [PubMed] [Google Scholar]
Keil 2008 {published data only}
- Keil R, Hrdlicka L, Leffler J, Sims J, Pafko P, Kodetova D. The role of endoscopic ultrasound with fine needle aspiration biopsy in the differential diagnosis of focal pancreatic lesions. Ceska a Slovenska Gastroenterologie a Hepatologie 2008;62(3):142‐6. [Google Scholar]
Keswani 2014 {published data only}
- Keswani RN, Krishnan K, Wani S, Keefer L, Komanduri S. Addition of endoscopic ultrasound (EUS)‐guided fine needle aspiration and on‐site cytology to EUS‐guided fine needle biopsy increases procedure time but not diagnostic accuracy. Clinical Endoscopy 2014;47(3):242‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalid 2005 {published data only}
- Khalid A, McGrath KM, Zahid M, Wilson M, Brody D, Swalsky P, et al. The role of pancreatic cyst fluid molecular analysis in predicting cyst pathology. Clinical Gastroenterology and Hepatology 2005;3(10):967‐73. [DOI] [PubMed] [Google Scholar]
Khalid 2006 {published data only}
- Khalid A, Nodit L, Zahid M, Bauer K, Brody D, Finkelstein SD, et al. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. American Journal of Gastroenterology 2006;101(11):2493‐500. [DOI] [PubMed] [Google Scholar]
Khan 2010 {published data only}
- Khan EA, Cuka N, Madan R, Olyaee M, Thomas PA, Fan F. Endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA) of pancreatic cystic lesions without overt cytologic atypia: Proposed diagnostic categories with utilization of fluid carcinoembryonic (CEA) level. Laboratory Investigation 2010;90:97A. [DOI] [PubMed] [Google Scholar]
Khashab 2010 {published data only}
- Khashab M, Mokadem M, DeWitt J, Emerson R, Sherman S, LeBlanc J, et al. Endoscopic ultrasound‐guided fine‐needle aspiration with or without flow cytometry for the diagnosis of primary pancreatic lymphoma ‐ a case series. Endoscopy 2010;42(3):228‐31. [DOI] [PubMed] [Google Scholar]
Khashab 2013 {published data only}
- Khashab MA, Kim K, Lennon AM, Shin EJ, Tignor AS, Amateau SK, et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas 2013;42(4):717‐21. [DOI] [PubMed] [Google Scholar]
Khodadadian 2001 {published data only}
- Khodadadian E, Grendell J, Gress FG. The diagnostic yield of endoscopic ultrasound‐guided fine needle aspiration biopsy of suspected pancreatic cancer in patients with negative cytology/biopsies from CT FNA and/or ERCP: A prospective evaluation of a large single center experience. Gastrointestinal Endoscopy 2001;53(5):AB177. [Google Scholar]
Khurana 2012 {published data only}
- Khurana KK, Rong R, Wang D, Roy A. Dynamic telecytopathology for on‐site preliminary diagnosis of endoscopic ultrasound‐guided fine needle aspiration of pancreatic masses. Journal of Telemedicine & Telecare 2012;18(5):253‐9. [DOI] [PubMed] [Google Scholar]
Khurana 2014 {published data only}
- Khurana KK, Graber B, Wang DL, Roy A. Telecytopathology for on‐site adequacy evaluation decreases the nondiagnostic rate in endoscopic ultrasound‐guided fine‐needle aspiration of pancreatic lesions. Telemedicine and E‐Health 2014;20(9):822‐7. [DOI] [PubMed] [Google Scholar]
Kida 2011 {published data only}
- Kida M, Araki M, Miyazawa S, Ikeda H, Takezawa M, Kikuchi H, et al. Comparison of diagnostic accuracy of endoscopic ultrasound‐guided fine‐needle aspiration with 22‐ and 25‐gauge needles in the same patients. Journal of Interventional Gastroenterology 2011;1(3):102‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim JK, Altun E, Elias J Jr, Pamuklar E, Rivero H, Semelka RC. Focal pancreatic mass: Distinction of pancreatic cancer from chronic pancreatitis using gadolinium‐enhanced 3D‐gradient‐echo MRI. Journal of Magnetic Resonance Imaging 2007;26(2):313‐22. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim SH, Lim JH, Lee WJ, Lim HK. Macrocystic pancreatic lesions: Differentiation of benign from precancerous and malignant cysts by CT. European Journal of Radiology 2009;71(1):122‐8. [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim YC, Choi JY, Chung YE, Bang S, Kim MJ, Park MS, et al. Comparison of MRI and endoscopic ultrasound in the characterization of pancreatic cystic lesions. AJR: American Journal of Roentgenology 2010;195(4):947‐52. [DOI] [PubMed] [Google Scholar]
Kim 2012a {published data only}
- Kim JH, Eun HW, Park HJ, Hong SS, Kim YJ. Diagnostic performance of MRI and EUS in the differentiation of benign from malignant pancreatic cyst and cyst communication with the main duct. European Journal of Radiology 2012;81(11):2927‐35. [DOI] [PubMed] [Google Scholar]
Kim 2012b {published data only}
- Kim JH, Hong SS, Kim YJ, Kim JK, Eun HW. Intraductal papillary mucinous neoplasm of the pancreas: Differentiate from chronic pancreatits by MR imaging. European Journal of Radiology 2012;81(4):671‐6. [DOI] [PubMed] [Google Scholar]
Kim 2012c {published data only}
- Kim JH, Kim MH, Byun JH, Lee SS, Lee SJ, Park SH, et al. Diagnostic strategy for differentiating autoimmune pancreatitis from pancreatic cancer: Is an endoscopic retrograde pancreatography essential?. Pancreas 2012;41(4):639‐47. [DOI] [PubMed] [Google Scholar]
Kim 2013a {published data only}
- Kim JH, Eun HW, Kim KW, Lee JY, Lee JM, Han JK, et al. Intraductal papillary mucinous neoplasms with associated invasive carcinoma of the pancreas: Imaging findings and diagnostic performance of MDCT for prediction of prognostic factors. AJR: American Journal of Roentgenology 2013;201(3):565‐72. [DOI] [PubMed] [Google Scholar]
Kim 2013b {published data only}
- Kim JH, Lee JM, Park JH, Kim SC, Joo I, Han JK, et al. Solid pancreatic lesions: Characterization by using timing bolus dynamic contrast‐enhanced MR imaging assessment ‐ a preliminary study. Radiology 2013;266(1):185‐96. [DOI] [PubMed] [Google Scholar]
Kim 2013c {published data only}
- Kim TH, Choi KH, Song HS, Kim JW, Jeon BJ. Histology combined with cytology by endoscopic ultrasound‐guided fine needle aspiration for the diagnosis of solid pancreatic mass and intra‐abdominal lymphadenopathy. Gut & Liver 2013;7(5):605‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2013d {published data only}
- Kim YI, Woo SM, Lee WJ, Han SS, Park SJ, Kim TH, et al. Appropriate indications of initial endoscopic ultrasound evaluation for detecting mural nodules in branch duct intraductal papillary mucinous neoplasms of the pancreas. Scandinavian Journal of Gastroenterology 2013;48(5):610‐6. [DOI] [PubMed] [Google Scholar]
Kim 2014a {published data only}
- Kim J, Ryu JK, Park JM, Paik WH, Song BJ, Kim YT, et al. Clinical factors associated with accuracy of EUS‐FNA for pancreatic or peripancreatic solid mass without on‐site cytopathologists. Journal of Gastroenterology and Hepatology 2014;29(4):887‐92. [DOI] [PubMed] [Google Scholar]
Kim 2014b {published data only}
- Kim JH, Lee SJ, Moon SH, Kim HJ, Kim HJ, Song IH, et al. Incremental value of cell block preparations over conventional smears alone in the evaluation of EUS‐FNA for pancreatic masses. Hepato‐Gastroenterology 2014;61(135):2117‐22. [PubMed] [Google Scholar]
Kim 2014c {published data only}
- Kim TH, Kim MH, Hwang JH, Yoo KS, Lee WJ, Lee JK, et al. Predictors of malignancy in pure branch duct intraductal papillary mucinous neoplasm of the pancreas: A multicenter study in Korea. Gastroenterology 2014;146(5 Suppl 1):S483‐4. [Google Scholar]
Kim 2014d {published data only}
- Kim YI, Kim SK, Paeng JC, Lee HY. Comparison of f‐18‐FDG PET/CT findings between pancreatic solid pseudopapillary tumor and pancreatic ductal adenocarcinoma. European Journal of Radiology 2014;83(1):231‐5. [DOI] [PubMed] [Google Scholar]
Kim 2015a {published data only}
- Kim JR, Jang JY, Kang MJ, Park T, Lee SY, Jung W, et al. Clinical implication of serum carcinoembryonic antigen and carbohydrate antigen 19‐9 for the prediction of malignancy in intraductal papillary mucinous neoplasm of pancreas. Journal of Hepato‐Biliary‐Pancreatic Sciences 2015;22(9):699‐707. [DOI] [PubMed] [Google Scholar]
Kin 2015 {published data only}
- Kin T, Katanuma A, Yane K, Takahashi K, Osanai M, Takaki R, et al. Diagnostic ability of EUS‐FNA for pancreatic solid lesions with conventional 22‐gauge needle using the slow pull technique: A prospective study. Scandinavian Journal of Gastroenterology 2015;50(7):900‐7. [DOI] [PubMed] [Google Scholar]
Kitano 2012 {published data only}
- Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, et al. Characterization of small solid tumors in the pancreas: The value of contrast‐enhanced harmonic endoscopic ultrasonography. American Journal of Gastroenterology 2012;107(2):303‐10. [DOI] [PubMed] [Google Scholar]
Klapman 2003 {published data only}
- Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on‐site cytopathology interpretation on endoscopic ultrasound‐guided fine needle aspiration. American Journal of Gastroenterology 2003;98(6):1289‐94. [DOI] [PubMed] [Google Scholar]
Klapman 2004 {published data only}
- Klapman JB, Chang KJ, Nguyen PT. The negative predictive value (NPV) of endoscopic ultrasound (EUS) in a large series of patients with suspected pancreatic cancer. Gastrointestinal Endoscopy 2004;59(5):P223. [Google Scholar]
Klapman 2005 {published data only}
- Klapman JB, Chang KJ, Lee JG, Nguyen P. Negative predictive value of endoscopic ultrasound in a large series of patients with a clinical suspicion of pancreatic cancer. American Journal of Gastroenterology 2005;100(12):2658‐61. [DOI] [PubMed] [Google Scholar]
Kliment 2010 {published data only}
- Kliment M, Urban O, Cegan M, Fojtik P, Falt P, Dvorackova J, et al. Endoscopic ultrasound‐guided fine needle aspiration of pancreatic masses: The utility and impact on management of patients. Scandinavian Journal of Gastroenterology 2010;45(11):1372‐9. [DOI] [PubMed] [Google Scholar]
Kliment 2013 {published data only}
- Kliment M, Urban O, Lovecek M, Straka M, Ziak D, Falt P, et al. Endosonography‐guided fine needle aspiration biopsy of solid pancreatic masses ‐ accuracy and impact on the treatment of 358 patients. Gastroenterologie a Hepatologie 2013;67(5):431‐7. [Google Scholar]
Kokhanenko 2001 {published data only}
- Kokhanenko N, Amosov VI, Nikonchuk NP, Mosiagina SG, Bryzgalova SV, Dundukov NN, et al. Significance of ultrasound examination and computed tomography in the diagnosis of pancreatic cancer. Vestnik Khirurgii Imeni i ‐ i ‐ Grekova 2001;160(5):61‐5. [PubMed] [Google Scholar]
Kongkam 2015 {published data only}
- Kongkam P, Lakananurak N, Navicharern P, Chantarojanasiri T, Aye K, Ridtitid W, et al. Combination of EUS‐FNA and elastography (strain ratio) to exclude malignant solid pancreatic lesions: A prospective single‐blinded study. Journal of Gastroenterology and Hepatology 2015;30(11):1683‐9. [DOI] [PubMed] [Google Scholar]
Kopelman 2011 {published data only}
- Kopelman Y, Marmor S, Ashkenazi I, Fireman Z. Value of EUS‐FNA cytological preparations compared with cell block sections in the diagnosis of pancreatic solid tumours. Cytopathology 2011;22(3):174‐8. [DOI] [PubMed] [Google Scholar]
Koranda 2010 {published data only}
- Koranda P, Buriankova E, Formanek R, Kysucan J, Havlik R, Myslivecek M. 18f‐FDG PET/CT in pancreatic carcinoma: Diagnosis and staging. Ceska Radiologie 2010;64(3):185‐91. [Google Scholar]
Korenblit 2016 {published data only}
- Korenblit J, Tholey DM, Tolin J, Loren D, Kowalski T, Adler DG, et al. Effect of the time of day and queue position in the endoscopic schedule on the performance characteristics of endoscopic ultrasound‐guided fine‐needle aspiration for diagnosing pancreatic malignancies. Endoscopic Ultrasound 2016;5(2):78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koyama 2001 {published data only}
- Koyama K, Okamura T, Kawabe J, Nakata B, Chung K, Ochi H, et al. Diagnostic usefulness of FDG PET for pancreatic mass lesions. Annals of Nuclear Medicine 2001;15(3):217‐24. [DOI] [PubMed] [Google Scholar]
Kriger 2011 {published data only}
- Kriger AG, Karmazanovskii GG, Kochatkov AV, Gorin DS, Solodinina EN, Kozlov IA, et al. Intraductal papillary‐mucinous tumor of the pancreas: Difficulties and mistakes of diagnostics and treatment. Khirurgiia 2011, (8):24‐32. [PubMed] [Google Scholar]
Krishna 2009a {published data only}
- Krishna N, Meehan C, Agarwal B. Prevalence of neoplasia in small and potentially resectable focal pancreatic "mass" lesions noted on CT/MRI in non‐jaundiced patients. Pancreas 2009;38(8):1019‐20. [Google Scholar]
Krishna 2009b {published data only}
- Krishna NB, LaBundy JL, Saripalli S, Safdar R, Agarwal B. Diagnostic value of EUS‐FNA in patients suspected of having pancreatic cancer with a focal lesion on CT scan/MRI but without obstructive jaundice. Pancreas 2009;38(6):625‐30. [DOI] [PubMed] [Google Scholar]
Krishna 2009c {published data only}
- Krishna NB, Mehra M, Reddy AV, Agarwal B. EUS/EUS‐FNA for suspected pancreatic cancer: Influence of chronic pancreatitis and clinical presentation with or without obstructive jaundice on performance characteristics. Gastrointestinal Endoscopy 2009;70(1):70‐9. [DOI] [PubMed] [Google Scholar]
Krishna 2012 {published data only}
- Krishna NB, Tummala P, Mehan CD, Reddy AV, Hartman JA, Agarwal B. Small and potentially resectable focal pancreatic lesions noted on CT/MRI scans in nonjaundiced patients: Likelihood of neoplasia and utility of EUS. Journal of Gastrointestinal Surgery 2012;16(4):793‐800. [DOI] [PubMed] [Google Scholar]
Krishna 2013 {published data only}
- Krishna SG, Bhattacharya A, Ladha HS, Singh A, Ross WA, Bhutani MS, et al. Pretest probability and diagnostic accuracy of endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA) for pancreatic adenocarcinoma at a tertiary care oncology center: A 12 year experience. Gastrointestinal Endoscopy 2013;77(5 Suppl):AB403. [Google Scholar]
Krishna 2015 {published data only}
- Krishna SG, Li F, Bhattacharya A, Ladha H, Porter K, Singh A, et al. Differentiation of pancreatic ductal adenocarcinoma from other neoplastic solid pancreatic lesions: A tertiary oncology center experience. Gastrointestinal Endoscopy 2015;81(2):370‐9. [DOI] [PubMed] [Google Scholar]
Krishnan 2013 {published data only}
- Krishnan K, Dalal S, Nayar R, Keswani RN, Keefer L, Komanduri S. Rapid on‐site evaluation of endoscopic ultrasound core biopsy specimens has excellent specificity and positive predictive value for gastrointestinal lesions. Digestive Diseases and Sciences 2013;58(7):2007‐12. [DOI] [PubMed] [Google Scholar]
Kubiliun 2011 {published data only}
- Kubiliun N, Ribeiro A, Fan YS, Rocha‐Lima CM, Sleeman D, Merchan J, et al. EUS‐FNA with rescue fluorescence in situ hybridization for the diagnosis of pancreatic carcinoma in patients with inconclusive on‐site cytopathology results. Gastrointestinal Endoscopy 2011;74(3):541‐7. [DOI] [PubMed] [Google Scholar]
Kubo 2009 {published data only}
- Kubo H, Nakamura K, Itaba S, Yoshinaga S, Kinukawa N, Sadamoto Y, et al. Differential diagnosis of cystic tumors of the pancreas by endoscopic ultrasonography. Endoscopy 2009;41(8):684‐9. [DOI] [PubMed] [Google Scholar]
Kudo 2014 {published data only}
- Kudo T, Kawakami H, Hayashi T, Yasuda I, Mukai T, Inoue H, et al. High and low negative pressure suction techniques in EUS‐guided fine‐needle tissue acquisition by using 25‐gauge needles: A multicenter, prospective, randomized, controlled trial. Gastrointestinal Endoscopy 2014;80(6):1030‐7.e1. [DOI] [PubMed] [Google Scholar]
Kula 2008 {published data only}
- Kula Z, Malkowski B, Pietrzak T, Szefer J. The clinical value of PET/CT imaging in differential diagnosis of pancreatic tumours ‐ analysis of 52 cases. Przeglad Gastroenterologiczny 2008;3(4):185‐91. [Google Scholar]
Kumon 2009 {published data only}
- Kumon RE, Pollack MJ, Faulx AL, Olowe K, Farooq FT, Chen VK, et al. Characterization of pancreatic cancer and intra‐abdominal lymph node malignancy using spectrum analysis of endoscopic ultrasound imaging. IEEE Engineering in Medicine and Biology Society 2009;2009:1949‐52. [DOI] [PubMed] [Google Scholar]
Kumon 2010 {published data only}
- Kumon RE, Pollack MJ, Faulx AL, Olowe K, Farooq FT, Chen VK, et al. In vivo characterization of pancreatic and lymph node tissue by using EUS spectrum analysis: A validation study. Gastrointestinal Endoscopy 2010;71(1):53‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumon 2012 {published data only}
- Kumon RE, Repaka A, Atkinson M, Faulx AL, Wong RC, Isenberg GA, et al. Characterization of the pancreas in vivo using EUS spectrum analysis with electronic array echoendoscopes. Gastrointestinal Endoscopy 2012;75(6):1175‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kung 2014 {published data only}
- Kung JS, Lopez OA, McCoy EE, Reicher S, Eysselein VE. Fluid genetic analyses predict the biological behavior of pancreatic cysts: Three‐year experience. JOP: Journal of the Pancreas [Electronic Resource] 2014;15(5):427‐32. [DOI] [PubMed] [Google Scholar]
Kursawa 1991 {published data only}
- Kursawa R, Luning M, Mai A, Menzel A. Computed tomographic criteria and diagnostic accuracy in differentiating space occupying lesions of the pancreas (carcinoma ‐ chronic pancreatitis). Radiologia Diagnostica 1991;32(3):182‐8. [Google Scholar]
Kwong 2015 {published data only}
- Kwong WT, Lawson RD, Hunt G, Fehmi SM, Proudfoot JA, Xu R, et al. Rapid growth rates of suspected pancreatic cyst branch duct intraductal papillary mucinous neoplasms predict malignancy. Digestive Diseases and Sciences 2015;60(9):2800‐6. [DOI] [PubMed] [Google Scholar]
Kyokane 1996 {published data only}
- Kyokane T, Furukawa H, Takayasu K, Mukai K, Shimada K, Kosuge T, et al. CT diagnosis of intraductal papillary neoplasm of the pancreas in comparison with histopathologic findings. International Journal of Pancreatology 1996;20(3):163‐7. [DOI] [PubMed] [Google Scholar]
Kysucan 2010 {published data only}
- Kysucan J, Lovecek M, Klos D, Tozzi I, Koranda P, Buriankova E, et al. Benefit of PET/CT in the preoperative staging in pancreatic carcinomas. Rozhledy V Chirurgii 2010;89(7):433‐40. [PubMed] [Google Scholar]
Lackner 1980 {published data only}
- Lackner K, Frommhold H, Grauthoff H. The value of computer tomography and sonography in the investigation of the pancreas. Fortschritte auf den Gebiete der Rontgenstrahlen und der Nuklearmedizin 1980;132(5):509‐13. [DOI] [PubMed] [Google Scholar]
Larghi 2013 {published data only}
- Larghi A, Iglesias‐Garcia J, Poley JW, Monges G, Petrone MC, Rindi G, et al. Feasibility and yield of a novel 22‐gauge histology EUS needle in patients with pancreatic masses: A multicenter prospective cohort study. Surgical Endoscopy 2013;27(10):3733‐8. [DOI] [PubMed] [Google Scholar]
Larino‐Noia 2013 {published data only}
- Larino‐Noia J, Iglesias‐Garcia J, Dominguez‐Munoz JE. Quantitative endoscopic ultrasound elastography (q‐EUS‐e) is an accurate method for the differential diagnosis of solid pancreatic masses: A validation study. Pancreatology 2013;1:S4. [Google Scholar]
Le Baleur 2009 {published data only}
- Baleur Y, Couvelard A, Vullierme MP, Sauvanet A, Rebours V, Hentic O, et al. CT scan allows accurate preoperative diagnosis of malignancy in patients with pancreatic mucinous cystadenomas. Pancreatology 2009;9(4):522. [Google Scholar]
Le Baleur 2011b {published data only}
- Baleur Y, Couvelard A, Vullierme MP, Sauvanet A, Hammel P, Rebours V, et al. Mucinous cystic neoplasms of the pancreas: Definition of preoperative imaging criteria for high‐risk lesions. Pancreatology 2011;11(5):495‐9. [DOI] [PubMed] [Google Scholar]
LeBlanc 2004 {published data only}
- LeBlanc JK, Ciaccia D, Al‐Assi MT, McGrath K, Imperiale T, Tao LC, et al. Optimal number of EUS‐guided fine needle passes needed to obtain a correct diagnosis. Gastrointestinal Endoscopy 2004;59(4):475‐81. [DOI] [PubMed] [Google Scholar]
LeBlanc 2010 {published data only}
- LeBlanc JK, Emerson RE, Dewitt J, Symms M, Cramer HM, McHenry L, et al. A prospective study comparing rapid assessment of smears and thinprep for endoscopic ultrasound‐guided fine‐needle aspirates. Endoscopy 2010;42(5):389‐94. [DOI] [PubMed] [Google Scholar]
Lee 2005a {published data only}
- Lee LS, Saltzman JR, Bounds BC, Poneros JM, Brugge WR, Thompson CC. EUS‐guided fine needle aspiration of pancreatic cysts: A retrospective analysis of complications and their predictors. Clinical Gastroenterology and Hepatology 2005;3(3):231‐6. [DOI] [PubMed] [Google Scholar]
Lee 2005b {published data only}
- Lee SY, Lee KT, Lee JK, Jeon YH, Choi D, Lim JH, et al. Long‐term follow up results of intraductal papillary mucinous tumors of pancreas. Journal of Gastroenterology and Hepatology (Australia) 2005;20(9):1379‐84. [DOI] [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee CH, Pan KT, Hung CF, Tseng JH, Yeh TS, Liu NJ. Magnetic resonance imaging and magnetic resonance cholangiopancreatography of intraductal papillary mucinous tumor of the pancreas. Chinese Journal of Radiology 2006;31(6):267‐74. [Google Scholar]
Lee 2007 {published data only}
- Lee H, Jong KL, Seok SK, Choi D, Jang KT, Jeong HK, et al. Is there any clinical or radiologic feature as a preoperative marker for differentiating mass‐forming pancreatitis from early‐stage pancreatic adenocarcinoma?. Hepato‐Gastroenterology 2007;54(79):2134‐40. [PubMed] [Google Scholar]
Lee 2008a {published data only}
- Lee SE, Kwon Y, Jang JY, Kim YH, Hwang DW, Kim MA, et al. The morphological classification of a serous cystic tumor (SCT) of the pancreas and evaluation of the preoperative diagnostic accuracy of computed tomography. Annals of Surgical Oncology 2008;15(8):2089‐95. [DOI] [PubMed] [Google Scholar]
Lee 2008b {published data only}
- Lee SS, Byun JH, Park BJ, Park SH, Kim N, Park B, et al. Quantitative analysis of diffusion‐weighted magnetic resonance imaging of the pancreas: Usefulness in characterizing solid pancreatic masses. Journal of Magnetic Resonance Imaging 2008;28(4):928‐36. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee TY, Kim MH, Park DH, Seo DW, Lee SK, Kim JS, et al. Utility of (18)f‐FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. AJR: American Journal of Roentgenology 2009;193(2):343‐8. [DOI] [PubMed] [Google Scholar]
Lee 2010a {published data only}
- Lee JH, Lee KT, Park J, Bae SY, Lee KH, Lee JK, et al. Predictive factors associated with malignancy of intraductal papillary mucinous pancreatic neoplasms. World Journal of Gastroenterology 2010;16(42):5353‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2010b {published data only}
- Lee SH, Ozden N, Pawa R, Hwangbo Y, Pleskow DK, Chuttani R, et al. Periductal hypoechoic sign: An endosonographic finding associated with pancreatic malignancy. Gastrointestinal Endoscopy 2010;71(2):249‐55. [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee HJ, Kim MJ, Choi JY, Hong HS, Kim KA. Relative accuracy of CT and MRI in the differentiation of benign from malignant pancreatic cystic lesions. Clinical Radiology 2011;66(4):315‐21. [DOI] [PubMed] [Google Scholar]
Lee 2013a {published data only}
- Lee JK, Choi JH, Lee KH, Kim KM, Shin JU, Lee JK, et al. A prospective, comparative trial to optimize sampling techniques in EUS‐guided FNA of solid pancreatic masses. Gastrointestinal Endoscopy 2013;77(5):745‐51. [DOI] [PubMed] [Google Scholar]
Lee 2013b {published data only}
- Lee JK, Lee KT, Choi ER, Jang TH, Jang KT, Lee JK, et al. A prospective, randomized trial comparing 25‐gauge and 22‐gauge needles for endoscopic ultrasound‐guided fine needle aspiration of pancreatic masses. Scandinavian Journal of Gastroenterology 2013;48(6):752‐7. [DOI] [PubMed] [Google Scholar]
Lee 2013c {published data only}
- Lee LS, Szafranska‐Schwarzbach AE, Wylie D, Bellizzi AM, Doyle LA, Kadiyala V, et al. Investigation of microRNA (miRNA) in pancreatic cystic tumors (impact) study: Differential expression observed among pancreatic cystic neoplasms. Pancreatology 2013;13(2):e47‐8. [Google Scholar]
Lee 2013d {published data only}
- Lee TY, Cheon YK, Shim CS. Clinical role of contrast‐enhanced harmonic endoscopic ultrasound in differentiating solid lesions of the pancreas: A single‐center experience in Korea. Gut & Liver 2013;7(5):599‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014b {published data only}
- Lee LS, Wu BU, Banks PA, Kadiyala V, Mehta S, Saltzman JR, et al. Utility of commercial DNA analysis in detecting malignancy within pancreatic cysts. Journal of the Pancreas 2014;15(2):182‐8. [DOI] [PubMed] [Google Scholar]
Lee 2014c {published data only}
- Lee YN, Moon JH, Kim HK, Choi HJ, Choi MH, Kim DC, et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound‐guided sampling of solid pancreatic masses: A randomized parallel‐group study. Endoscopy 2014;46(12):1056‐62. [DOI] [PubMed] [Google Scholar]
Lee 2014d {published data only}
- Lee YN, Moon JH, Kim HK, Choi HJ, Lee SH, Choi MH, et al. A triple approach for diagnostic assessment of endoscopic ultrasound‐guided fine needle aspiration in pancreatic solid masses and lymph nodes. Digestive Diseases and Sciences 2014;59(9):2286‐93. [DOI] [PubMed] [Google Scholar]
Leeds 2013 {published data only}
- Leeds JS, Nayar MN, Dawwas M, Scott J, Anderson K, Haugk B, et al. Comparison of endoscopic ultrasound and computed tomography in the assessment of pancreatic cyst size using pathology as the gold standard. Pancreatology 2013;13(3):263‐6. [DOI] [PubMed] [Google Scholar]
Legmann 1998 {published data only}
- Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, et al. Pancreatic tumors: Comparison of dual‐phase helical CT and endoscopic sonography. AJR: American Journal of Roentgenology 1998;170(5):1315‐22. [DOI] [PubMed] [Google Scholar]
Lehmann 1998 {published data only}
- Lehmann KJ, Diehl SJ, Lachmann R, Georgi M. Value of dual‐phase‐helical CT in the preoperative diagnosis of pancreatic cancer ‐ a prospective study. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 1998;168(3):211‐6. [DOI] [PubMed] [Google Scholar]
Lemke 2004 {published data only}
- Lemke AJ, Niehues SM, Hosten N, Amthauer H, Boehmig M, Stroszczynski C, et al. Retrospective digital image fusion of multidetector CT and 18f‐FDG PET: Clinical value in pancreatic lesions ‐ a prospective study with 104 patients. Journal of Nuclear Medicine 2004;45(8):1279‐86. [PubMed] [Google Scholar]
Levy 1995 {published data only}
- Levy M, Levy P, Hammel P, Zins M, Vilgrain V, Amouyal G, et al. Diagnosis of cystadenomas and cystadenocarcinomas of the pancreas. Study of 35 cases. Gastroenterologie Clinique et Biologique 1995;19(2):189‐96. [PubMed] [Google Scholar]
Levy 2005 {published data only}
- Levy MJ, Smyrk TC, Reddy RP, Clain JE, Harewood GC, Kendrick ML, et al. Endoscopic ultrasound‐guided trucut biopsy of the cyst wall for diagnosing cystic pancreatic tumors. Clinical Gastroenterology and Hepatology 2005;3(10):974‐9. [DOI] [PubMed] [Google Scholar]
Levy 2007 {published data only}
- Levy MJ, Clain JE, Clayton A, Halling KC, Kipp BR, Rajan E, et al. Preliminary experience comparing routine cytology results with the composite results of digital image analysis and fluorescence in situ hybridization in patients undergoing EUS‐guided FNA. Gastrointestinal Endoscopy 2007;66(3):483‐90. [DOI] [PubMed] [Google Scholar]
Levy 2012 {published data only}
- Levy MJ, Oberg TN, Campion MB, Clayton AC, Halling KC, Henry MR, et al. Comparison of methods to detect neoplasia in patients undergoing endoscopic ultrasound‐guided fine‐needle aspiration. Gastroenterology 2012;142(5):1112‐21.e2. [DOI] [PubMed] [Google Scholar]
Lightdale 1994 {published data only}
- Lightdale CJ. Endosonography for evaluating pancreatic mass lesions. International Journal of Pancreatology 1994;16(2‐3):261‐4. [Google Scholar]
Lim 2005 {published data only}
- Lim SJ, Alasadi R, Wayne JD, Rao S, Rademaker A, Bell R, et al. Preoperative evaluation of pancreatic cystic lesions: Cost‐benefit analysis and proposed management algorithm. Surgery 2005;138(4):672‐9; discussion 9‐80. [DOI] [PubMed] [Google Scholar]
Lim 2013 {published data only}
- Lim LG, Lakhtakia S, Ang TL, Vu CK, Dy F, Chong VH, et al. Factors determining diagnostic yield of endoscopic ultrasound guided fine‐needle aspiration for pancreatic cystic lesions: A multicentre Asian study. Digestive Diseases and Sciences 2013;58(6):1751‐7. [DOI] [PubMed] [Google Scholar]
Lin 2003 {published data only}
- Lin F, Staerkel G. Cytologic criteria for well differentiated adenocarcinoma of the pancreas in fine‐needle aspiration biopsy specimens. Cancer 2003;99(1):44‐50. [DOI] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin JL, Barthel JS, Keshishian J, Eikman EA, Klapman JB. Negative predictive value of positron emission tomography/computed tomography in patients with a clinical suspicion of pancreatic cancer. Pancreas 2011;40(5):653‐6. [DOI] [PubMed] [Google Scholar]
Lin 2014 {published data only}
- Lin M, Hair CD, Green LK, Vela SA, Patel KK, Qureshi WA, et al. Endoscopic ultrasound‐guided fine‐needle aspiration with on‐site cytopathology versus core biopsy: A comparison of both techniques performed at the same endoscopic session. Endoscopy International Open 2014;2(4):E220‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linder 2006 {published data only}
- Linder JD, Geenen JE, Catalano MF. Cyst fluid analysis obtained by EUS‐guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: A prospective single‐center experience. Gastrointestinal Endoscopy 2006;64(5):697‐702. [DOI] [PubMed] [Google Scholar]
Liu 2010a {published data only}
- Liu Y, Lin X, Upadhyaya M, Song Q, Chen K. Intraductal papillary mucinous neoplasms of the pancreas: Correlation of helical CT features with pathologic findings. European Journal of Radiology 2010;76(2):222‐7. [DOI] [PubMed] [Google Scholar]
Liu 2010b {published data only}
- Liu Y, Xu XQ, Lin XZ, Song Q, Chen KM. Prospective study comparing two iodine concentrations for multidetector computed tomography of the pancreas. Radiologia Medica 2010;115(6):898‐905. [DOI] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu K, Xie P, Peng W, Zhou Z. Assessment of dynamic contrast‐enhanced magnetic resonance imaging in the differentiation of pancreatic ductal adenocarcinoma from other pancreatic solid lesions. Journal of Computer Assisted Tomography 2014;38(5):681‐6. [DOI] [PubMed] [Google Scholar]
Lopez 2002 {published data only}
- Lopez Hanninen E, Amthauer H, Hosten N, Ricke J, Bohmig M, Langrehr J, et al. Prospective evaluation of pancreatic tumors: Accuracy of MR imaging with MR cholangiopancreatography and MR angiography. Radiology 2002;224(1):34‐41. [DOI] [PubMed] [Google Scholar]
Lozano 2011 {published data only}
- Lozano MD, Subtil JC, Miravalles TL, Echeveste JI, Prieto C, Betes M, et al. Echobrush may be superior to standard EUS‐guided FNA in the evaluation of cystic lesions of the pancreas. Cancer Cytopathology 2011;119(3):209‐14. [DOI] [PubMed] [Google Scholar]
Lu 2013 {published data only}
- Lu Q, Wang PJ, Shao ZH, Ni J, Wang GL. CT enhancement scanning and diffusion‐weighted magnetic resonance imaging for differential diagnosis between chronic mass‐forming pancreatitis and pancreatic carcinoma. Academic Journal of Second Military Medical University 2013;34(9):974‐9. [Google Scholar]
Lu 2014 {published data only}
- Lu X. The diagnostic value of EUS in pancreatic cystic neoplasms compared with CT and MRI. Journal of Digestive Diseases 2014;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lytras 2005 {published data only}
- Lytras D, Connor S, Bosonnet L, Jayan R, Evans J, Hughes M, et al. Positron emission tomography does not add to computed tomography for the diagnosis and staging of pancreatic cancer. Digestive Surgery 2005;22(1‐2):55‐61; discussion 62. [DOI] [PubMed] [Google Scholar]
Mackie 1979 {published data only}
- Mackie CR, Cooper MJ, Lewis MH, Moossa AR. Non‐operative differentiation between pancreatic cancer and chronic pancreatitis. Annals of Surgery 1979;189(4):480‐7. [PMC free article] [PubMed] [Google Scholar]
Madan 2012 {published data only}
- Madan R, Khan E, Cuka N, Olyaee M, Tawfik O, Fan F. Pancreatic cystic lesions without overt cytologic atypia: Proposed diagnostic categories for endoscopic ultrasound‐guided fine‐needle aspiration cytology with utilization of fluid carcinoembryonic antigen level. Acta Cytologica 2012;56(1):34‐40. [DOI] [PubMed] [Google Scholar]
Madura 1997 {published data only}
- Madura JA, Wiebke EA, Howard TJ, Cummings OW, Hull MT, Sherman S, et al. Mucin‐hypersecreting intraductal neoplasms of the pancreas: A precursor to cystic pancreatic malignancies. Surgery 1997;122(4):786‐92; discussion 92‐3. [DOI] [PubMed] [Google Scholar]
Maguchi 2006 {published data only}
- Maguchi H, Takashi K, Osanai M, Katanuma A. Small pancreatic lesions: Is there need for EUS‐FNA preoperatively? What to do with the incidental lesions?. Endoscopy 2006;38(Suppl 1):S53‐6. [DOI] [PubMed] [Google Scholar]
Maire 2003 {published data only}
- Maire F, Couvelard A, Hammel P, Ponsot P, Palazzo L, Aubert A, et al. Intraductal papillary mucinous tumors of the pancreas: The preoperative value of cytologic and histopathologic diagnosis. Gastrointestinal Endoscopy 2003;58(5):701‐6. [DOI] [PubMed] [Google Scholar]
Makaiova 2005 {published data only}
- Makaiova I, Kovacova S, Vesely J, Durkovsky A, Kausitz J, Salek T, et al. Evaluation of the role of FDG‐PET and PET/CT in diagnostics of a suspected pancreatic cancer. European Journal of Nuclear Medicine and Molecular Imaging 2005;32:S89‐90. [Google Scholar]
Malak 2016 {published data only}
- Malak M, Masuda D, Ogura T, Imoto A, Abdelaal UM, Sabet EA, et al. Yield of endoscopic ultrasound‐guided fine needle aspiration and endoscopic retrograde cholangiopancreatography for solid pancreatic neoplasms. Scandinavian Journal of Gastroenterology 2016;51(3):360‐7. [DOI] [PubMed] [Google Scholar]
Malleo 2012 {published data only}
- Malleo G, Marchegiani G, Pennacchio S, Paiella S, Paini M, Pea A, et al. Pancreatic resections for cystic neoplasms: From the surgeon's presumption to the pathologist's reality. Pancreatology 2012;12(6):587‐8. [DOI] [PubMed] [Google Scholar]
Mallery 2002 {published data only}
- Mallery JS, Centeno BA, Hahn PF, Chang Y, Warshaw AL, Brugge WR. Pancreatic tissue sampling guided by EUS, CT/US, and surgery: A comparison of sensitivity and specificity. Gastrointestinal Endoscopy 2002;56(2):218‐24. [DOI] [PubMed] [Google Scholar]
Maluf 2005 {published data only}
- Maluf F, Kubrusly MS, Cunha JEM, Sakai P, Ishioka S, Machado MC, et al. Detection of k‐ras point mutation increases the sensitivity of EUS‐guided FNA for the diagnosis of pancreatic adenocarcinoma. Gastrointestinal Endoscopy 2005;61(5):AB291. [Google Scholar]
Maluf‐Filho 2007 {published data only}
- Maluf‐Filho F, Kumar A, Gerhardt R, Kubrusly M, Sakai P, Hondo F, et al. Kras mutation analysis of fine needle aspirate under EUS guidance facilitates risk stratification of patients with pancreatic mass. Journal of Clinical Gastroenterology 2007;41(10):906‐10. [DOI] [PubMed] [Google Scholar]
Mamoon 2011 {published data only}
- Mamoon N, Mushtaq S, Rathore MU. Endoscopic ultrasound guided aspiration cytology ‐ a useful diagnostic tool. Journal of the Pakistan Medical Association 2011;61(4):367‐71. [PubMed] [Google Scholar]
Manfredi 2009 {published data only}
- Manfredi R, Graziani R, Motton M, Mantovani W, Baltieri S, Tognolini A, et al. Main pancreatic duct intraductal papillary mucinous neoplasms: Accuracy of MR imaging in differentiation between benign and malignant tumors compared with histopathologic analysis. Radiology 2009;253(1):106‐15. [DOI] [PubMed] [Google Scholar]
Mansoor 2012 {published data only}
- Mansoor M, Omar NA, Kolb B, Kaushik VY. EUS‐guided FNA of pancreatic lesions: Which needle size is appropriate. Pancreatology 2012;12(3):e15. [Google Scholar]
Mansour 2006 {published data only}
- Mansour JC, Schwartz L, Pandit‐Taskar N, D'Angelica M, Fong Y, Larson SM, et al. The utility of f‐18 fluorodeoxyglucose whole body PET imaging for determining malignancy in cystic lesions of the pancreas. Journal of Gastrointestinal Surgery 2006;10(10):1354‐60. [DOI] [PubMed] [Google Scholar]
Mao 2011 {published data only}
- Mao JW, Xu LG, Tang HY, Wang YD. Diagnostic value of endoscopic ultrasound and endoscopic ultrasound‐guided fine needle aspiration biopsy in pancreatic diseases. World Chinese Journal of Digestology 2011;19(5):533‐7. [Google Scholar]
Marchevsky 2003 {published data only}
- Marchevsky AM, Nelson V, Martin SE, Greaves TS, Raza AS, Zeineh J, et al. Telecytology of fine‐needle aspiration biopsies of the pancreas: A study of well‐differentiated adenocarcinoma and chronic pancreatitis with atypical epithelial repair changes. Diagnostic Cytopathology 2003;28(3):147‐52. [DOI] [PubMed] [Google Scholar]
Marotta 1991 {published data only}
- Marotta F, Tajiri H, Yoshimori M, Nakamura K, Ozaki H. A nine year retrospective analysis of resectable pancreatic cancer at the National Cancer Center Hospital in Tokyo: Clues to diagnosis and diagnostic assessment. Italian Journal of Gastroenterology 1991;23(4):197‐201. [PubMed] [Google Scholar]
Martin 1998 {published data only}
- Martin I, Hammond P, Scott A, Redhead D, Carter DC, Garden OJ. Cystic tumours of the pancreas. British Journal of Surgery 1998;85(11):1484‐6. [DOI] [PubMed] [Google Scholar]
Martinez 2014 {published data only}
- Martinez N, Rosenkranz L, Patel S. Yield and safety of pancreatoscopy in main duct intraductal papillary mucinous neoplasms. American Journal of Gastroenterology 2014;109:S87‐8. [Google Scholar]
Marzioni 2015 {published data only}
- Marzioni M, Germani U, Agostinelli L, Bedogni G, Saccomanno S, Marini F, et al. Pdx‐1 mRNA expression in endoscopic ultrasound‐guided fine needle cytoaspirate: Perspectives in the diagnosis of pancreatic cancer. Digestive and Liver Disease 2015;47(2):138‐43. [DOI] [PubMed] [Google Scholar]
Matsubara 2011 {published data only}
- Matsubara H, Itoh A, Kawashima H, Kasugai T, Ohno E, Ishikawa T, et al. Dynamic quantitative evaluation of contrast‐enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas 2011;40(7):1073‐9. [DOI] [PubMed] [Google Scholar]
Matsubayashi 2015 {published data only}
- Matsubayashi H. Role of k‐ras mutation analysis in EUS‐FNA samples obtained from pancreatic solid mass. Journal of Clinical Gastroenterology 2015;49(2):173. [DOI] [PubMed] [Google Scholar]
Matsuda 2012 {published data only}
- Matsuda F, Okabe Y, Nakajima J, Osaki Y, Inayama K, Wakasa T, et al. EUS‐guided FNAb for diagnosis of pancreatic cancer. Pancreas 2012;41(7):1151‐2. [Google Scholar]
Matsumoto 2012 {published data only}
- Matsumoto I, Shinzeki M, Toyama H, Asari S, Goto T, Ajiki T, et al. The clinical role of positron emission tomography with f‐18‐ fluorodeoxyglucose (FDG‐PET) in diagnosis and staging in patients with pancreatic ductal adenocarcinoma. HPB 2012;14:653‐4. [Google Scholar]
Matsumoto 2013 {published data only}
- Matsumoto I, Shirakawa S, Shinzeki M, Asari S, Goto T, Ajiki T, et al. 18‐fluorodeoxyglucose positron emission tomography does not aid in diagnosis of pancreatic ductal adenocarcinoma. Clinical Gastroenterology and Hepatology 2013;11(6):712‐8. [DOI] [PubMed] [Google Scholar]
Matsumoto 2014 {published data only}
- Matsumoto K, Takeda Y, Harada K, Horie Y, Yashima K, Murawaki Y. Effect of pancreatic juice cytology and/or endoscopic ultrasound‐guided fine‐needle aspiration biopsy for pancreatic tumor. Journal of Gastroenterology and Hepatology 2014;29(1):223‐7. [DOI] [PubMed] [Google Scholar]
Maurea 2009 {published data only}
- Maurea S, Caleo O, Mollica C, Imbriaco M, Mainenti PP, Palumbo C, et al. Comparative diagnostic evaluation with MR cholangiopancreatography, ultrasonography and CT in patients with pancreatobiliary disease. Radiologia Medica 2009;114(3):390‐402. [DOI] [PubMed] [Google Scholar]
Mavrogenis 2015 {published data only}
- Mavrogenis G, Weynand B, Sibille A, Hassaini H, Deprez P, Gillain C, et al. 25‐gauge histology needle versus 22‐gauge cytology needle in endoscopic ultrasonography‐guided sampling of pancreatic lesions and lymphadenopathy. Endoscopy International Open 2015;3(1):E63‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayerle 2016 {published data only}
- Mayerle J, Beyer G, Simon P, Dickson EJ, Carter RC, Duthie F, et al. Prospective cohort study comparing transient EUS guided elastography to EUS‐FNA for the diagnosis of solid pancreatic mass lesions. Pancreatology 2016;16(1):110‐4. [DOI] [PubMed] [Google Scholar]
McClellan 2003 {published data only}
- McClellan T, Erickson RA. Factors associated with non‐diagnostic EUS‐guided FNA in pancreatic cancer. Gastrointestinal Endoscopy 2003;57(5):AB238. [Google Scholar]
McDowell 1997 {published data only}
- McDowell RK, Gazelle GS, Murphy BL, Boland GW, Mayo‐Smith WW, Warshaw AL, et al. Mucinous ductal ectasia of the pancreas. Journal of Computer Assisted Tomography 1997;21(3):383‐8. [DOI] [PubMed] [Google Scholar]
Mehan 2009 {published data only}
- Mehan CD, Krishna NB, Reddy AV, Hartman JA, Agarwal B. Small focal non‐cystic pancreatic lesions in patients without obstructive jaundice: How many of these are neoplastic? What is the role of EUS‐FNA in these patients?. Gastrointestinal Endoscopy 2009;69 (5):AB242. [Google Scholar]
Mehmood 2015 {published data only}
- Mehmood S, Jahan A, Loya A, Yusuf MA. Onsite cytopathology evaluation and ancillary studies beneficial in EUS‐FNA of pancreatic, mediastinal, intra‐abdominal, and submucosal lesions. Diagnostic Cytopathology 2015;43(4):278‐86. [DOI] [PubMed] [Google Scholar]
Meijer 2009 {published data only}
- Meijer OLM, Weersma RK, Dullemen HM. The diagnostic value of endoscopic ultrasonography in patients with a clinical suspicion of malignant pancreatic disease and inconclusive or negative CT scan. European Journal of Gastroenterology & Hepatology 2009;21(3):A23‐4. [Google Scholar]
Meijer 2010 {published data only}
- Meijer OLM, Weersma RK, Jagt EJ, Dullemen HM. Endoscopic ultrasonography in suspected pancreatic malignancy and indecisive CT. Netherlands Journal of Medicine 2010;68(9):360‐4. [PubMed] [Google Scholar]
Mera 1999 {published data only}
- Mera K, Tajiri H, Muto M, Ohtsu A, Furuse J, Maru Y, et al. Clinical significance of magnetic resonance cholangiopancreatography for the diagnosis of cystic tumor of the pancreas compared with endoscopic retrograde cholangiopancreatography and computed tomography. Japanese Journal of Clinical Oncology 1999;29(6):294‐8. [DOI] [PubMed] [Google Scholar]
Mertz 2000 {published data only}
- Mertz HR, Sechopoulos P, Delbeke D, Leach SD. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointestinal Endoscopy 2000;52(3):367‐71. [DOI] [PubMed] [Google Scholar]
Mesihovic 2005 {published data only}
- Mesihovic R, Vanis N, Husic‐Selimovic A, Prohic D, Gribajcevic M, Tanovic H, et al. Evaluation of the diagnostic accuracy of the endoscopic ultrasonography results in the patients examined in a period of three years. Medicinski Arhiv 2005;59(5):299‐302. [PubMed] [Google Scholar]
Micames 2007 {published data only}
- Micames CG, Gress FG. EUS elastography: A step ahead?. Gastrointestinal Endoscopy 2007;65(7):979‐81. [DOI] [PubMed] [Google Scholar]
Michaels 2006 {published data only}
- Michaels PJ, Brachtel EF, Bounds BC, Brugge WR, Pitman MB. Intraductal papillary mucinous neoplasm of the pancreas ‐ cytologic features predict histologic grade. Cancer Cytopathology 2006;108(3):163‐73. [DOI] [PubMed] [Google Scholar]
Midwinter 1999 {published data only}
- Midwinter MJ, Beveridge CJ, Wilsdon JB, Bennett MK, Baudouin CJ, Charnley RM. Correlation between spiral computed tomography, endoscopic ultrasonography and findings at operation in pancreatic and ampullary tumours. British Journal of Surgery 1999;86(2):189‐93. [DOI] [PubMed] [Google Scholar]
Mishra 2006 {published data only}
- Mishra G, Zhao Y, Sweeney J, Pineau BC, Case D, Ho C, et al. Determination of qualitative telomerase activity as an adjunct to the diagnosis of pancreatic adenocarcinoma by EUS‐guided fine‐needle aspiration. Gastrointestinal Endoscopy 2006;63(4):648‐54. [DOI] [PubMed] [Google Scholar]
Mitsuhashi 2006 {published data only}
- Mitsuhashi T, Ghafari S, Chang CY, Gu M. Endoscopic ultrasound‐guided fine needle aspiration of the pancreas: Cytomorphological evaluation with emphasis on adequacy assessment, diagnostic criteria and contamination from the gastrointestinal tract. Cytopathology 2006;17(1):34‐41. [DOI] [PubMed] [Google Scholar]
Miyabe 2015 {published data only}
- Miyabe K, Hori Y, Nakazawa T, Hayashi K, Naitoh I, Shimizu S, et al. Locus/chromosome aberrations in intraductal papillary mucinous neoplasms analyzed by fluorescence in situ hybridization. American Journal of Surgical Pathology 2015;39(4):512‐20. [DOI] [PubMed] [Google Scholar]
Moehler 2011 {published data only}
- Moehler M, Voigt J, Kastor M, Heil M, Sengespeick C, Biesterfeld S, et al. Endoscopic ultrasonography‐guided fine‐needle aspiration (EUS‐FNA) as primary diagnostic tool for unclear lesions in the upper gastrointestinal tract. Deutsche Medizinische Wochenschrift 2011;136(7):303‐8. [DOI] [PubMed] [Google Scholar]
Moparty 2007 {published data only}
- Moparty B, Logrono R, Nealon WH, Waxman I, Raju GS, Pasricha PJ, et al. The role of endoscopic ultrasound and endoscopic ultrasound‐guided fine‐needle aspiration in distinguishing pancreatic cystic lesions. Diagnostic Cytopathology 2007;35(1):18‐25. [DOI] [PubMed] [Google Scholar]
Moris 2016 {published data only}
- Moris M, Raimondo M, Woodward TA, Skinner V, Arcidiacono PG, Petrone MC, et al. Diagnostic accuracy of endoscopic ultrasound‐guided fine‐needle aspiration cytology, carcinoembryonic antigen, and amylase in intraductal papillary mucinous neoplasm. Pancreas 2016;45(6):870‐5. [DOI] [PubMed] [Google Scholar]
Morozova 2014 {published data only}
- Morozova TG, Borsukov AV. Clinical relevance of compression elastography in differential diagnosis of pancreatic cystic masses. Sovremennye Tehnologii v Medicine 2014;6(2):103‐7. [Google Scholar]
Morozova 2015 {published data only}
- Morozova T, Borsukov A. Compression elastography at endosonography: Clinical and diagnostical value in complex algorithm of patients with pancreas diseases examination. United European Gastroenterology Journal 2015;3(5 Suppl):A572. [Google Scholar]
Murayama 2011 {published data only}
- Murayama S, Kimura W, Hirai I, Takasu N, Takeshita A, Moriya T. Volumetric and morphological analysis of intraductal papillary mucinous neoplasm of the pancreas using computed tomography and magnetic resonance imaging. Pancreas 2011;40(6):876‐82. [DOI] [PubMed] [Google Scholar]
Nadig 2012 {published data only}
- Nadig SN, Pedrosa I, Goldsmith JD, Callery MP, Vollmer CM. Clinical implications of mucinous nonneoplastic cysts of the pancreas. Pancreas 2012;41(3):441‐6. [DOI] [PubMed] [Google Scholar]
Nagamachi 2013 {published data only}
- Nagamachi S, Nishii R, Wakamatsu H, Mizutani Y, Kiyohara S, Fujita S, et al. The usefulness of 18f‐FDG PET/MRI fusion image in diagnosing pancreatic tumor: Comparison with 18f‐FDG PET/CT. Annals of Nuclear Medicine 2013;27(6):554‐63. [DOI] [PubMed] [Google Scholar]
Nagula 2010 {published data only}
- Nagula S, Kennedy T, Schattner MA, Brennan MF, Gerdes H, Markowitz AJ, et al. Evaluation of cyst fluid CEA analysis in the diagnosis of mucinous cysts of the pancreas. Journal of Gastrointestinal Surgery 2010;14(12):1997‐2003. [DOI] [PubMed] [Google Scholar]
Nakai 2015 {published data only}
- Nakai Y, Iwashita T, Park DH, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS‐guided, through‐the‐needle confocal laser‐induced endomicroscopy and cystoscopy trial: Detect study. Gastrointestinal Endoscopy 2015;81(5):1204‐14. [DOI] [PubMed] [Google Scholar]
Nakamoto 2000 {published data only}
- Nakamoto Y, Higashi T, Sakahara H, Tamaki N, Kogire M, Doi R, et al. Delayed (18)F‐fluoro‐2‐deoxy‐D‐glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer 2000;89(12):2547‐54. [DOI] [PubMed] [Google Scholar]
Nakamoto 2003 {published data only}
- Nakamoto Y, Saga T, Higashi T, Ishimori T, Kobayashi H, Ishizu K, et al. Optimal scan time for evaluating pancreatic disease with positron emission tomography using f‐18‐fluorodeoxyglucose. Annals of Nuclear Medicine 2003;17(5):421‐6. [DOI] [PubMed] [Google Scholar]
Napoleon 2010a {published data only}
- Napoleon B, Alvarez‐Sanchetz MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, et al. Contrast‐enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: Results of a pilot study. Endoskopie Heute 2010;23(3):213‐20. [DOI] [PubMed] [Google Scholar]
Napoleon 2010b {published data only}
- Napoleon B, Alvarez‐Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, et al. Contrast‐enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: Results of a pilot study. Endoscopy 2010;42(7):564‐70. [DOI] [PubMed] [Google Scholar]
Napoleon 2015 {published data only}
- Napoleon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: Needle‐based confocal laser endomicroscopy. Endoscopy 2015;47(1):26‐32. [DOI] [PubMed] [Google Scholar]
Nattermann 1995 {published data only}
- Nattermann C, Goldschmidt AJW, Dancygier H. Endoscopic ultrasonography for distinguishing malignant from benign tumours of the pancreas: Comparison of findings with cancerous and segmental inflammatory changes. Deutsche Medizinische Wochenschrift 1995;120(46):1571‐6. [DOI] [PubMed] [Google Scholar]
Nayar 2011 {published data only}
- Nayar M, Joy D, Wadehra V, Oppong K. Effect of dedicated and supervised training on achieving competence in EUS‐FNA of solid pancreatic lesions. Scandinavian Journal of Gastroenterology 2011;46(7‐8):997‐1003. [DOI] [PubMed] [Google Scholar]
Nayar 2013 {published data only}
- Nayar MK, Chatterjee S, Wadehra V, Cunningham J, Leeds J, Oppong K. Does on‐site adequacy assessment by cytotechnologists improve results of EUS guided FNA of solid pancreaticobiliary lesions?. Journal of the Pancreas 2013;14(1):44‐9. [DOI] [PubMed] [Google Scholar]
Nguyen 1998 {published data only}
- Nguyen P, Ghazourian NS, Hata K, Chang KJ. The negative predictive value of endoscopic ultrasound (EUS) in patients with suspected pancreatic cancer. Gastrointestinal Endoscopy 1998;47(4):AB151. [Google Scholar]
Nguyen 2007 {published data only}
- Nguyen WT, Chen AM, Chen WP, Lee J. Predictive value of pancreatic ductal dilation on endoscopic ultrasound diagnosis of pancreatic cancer. Gastrointestinal Endoscopy 2007;65(5):AB298. [Google Scholar]
Nguyen 2008 {published data only}
- Nguyen TTH, Lee CE, Whang CS, Ashida R, Lee JG, Chang K, et al. A comparison of the diagnostic yield and specimen adequacy between 22 and 25 gauge needles for endoscopic ultrasound guided fine‐needle aspiration (EUS‐FNA) of solid pancreatic lesions (SPL): Is bigger better?. Gastrointestinal Endoscopy 2008;67(5):AB100. [Google Scholar]
Nicaud 2010 {published data only}
- Nicaud M, Hou W, Collins D, Wagh MS, Chauhan S, Draganov PV. The utility of repeat endoscopic ultrasound‐guided fine needle aspiration for suspected pancreatic cancer. Gastroenterology Research & Practice 2010;2010:268290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nieto 2007 {published data only}
- Nieto JM, Alia D, Reicher S, David C, Pham BV, French S, et al. Immunohistochemical staining improves the diagnostic yield of endoscopic ultrasonography guided fine needle aspirates of pancreatic solid lesions. Gastrointestinal Endoscopy 2007;65(5):AB298. [Google Scholar]
Nijhawan 2014 {published data only}
- Nijhawan S, Singh B, Kumar A, Ramrakhiani D, Mathur A, Gupta G. Randomized controlled trial of comparison of the adequacy and diagnostic yield of endoscopic ultrasound guided fine needle aspiration with and without a stylet in Indian patients: A prospective single blind study. Journal of Digestive Endoscopy 2014;5(4):149‐53. [Google Scholar]
Nikiforova 2013 {published data only}
- Nikiforova MN, Khalid A, Fasanella KE, McGrath KM, Brand RE, Chennat JS, et al. Integration of kras testing in the diagnosis of pancreatic cystic lesions: A clinical experience of 618 pancreatic cysts. Modern Pathology 2013;26(11):1478‐87. [DOI] [PubMed] [Google Scholar]
Nishihara 1996 {published data only}
- Nishihara K, Kawabata A, Ueno T, Miyahara M, Hamanaka Y, Suzuki T. The differential diagnosis of pancreatic cysts by MR imaging. Hepato‐Gastroenterology 1996;43(9):714‐20. [PubMed] [Google Scholar]
Nitzsche 2002 {published data only}
- Nitzsche EU, Hoegerle S, Mix M, Brink I, Otte A, Moser E, et al. Non‐invasive differentiation of pancreatic lesions: Is analysis of FDG kinetics superior to semiquantitative uptake value analysis?. European Journal of Nuclear Medicine and Molecular Imaging 2002;29(2):237‐42. [DOI] [PubMed] [Google Scholar]
Nobrega 1994 {published data only}
- Nobrega J, dos Santos G. Aspirative cytology with fine‐needle in the abdomen, retroperitoneum and pelvic cavity: A seven year experience of the Portuguese Institute of Oncology, Center of Porto. European Journal of Surgical Oncology 1994;20(1):37‐42. [PubMed] [Google Scholar]
Noda 2010 {published data only}
- Noda Y, Fujita N, Kobayashi G, Itoh K, Horaguchi J, Takasawa O, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound‐guided fine‐needle aspiration. Journal of Gastroenterology 2010;45(8):868‐75. [DOI] [PubMed] [Google Scholar]
Noma 2014 {published data only}
- Noma Y, Kawamoto H, Kato H, Iwamuro M, Hirao K, Fujii M, et al. The efficacy and safety of single‐session endoscopic ultrasound‐guided fine needle aspiration and endoscopic retrograde cholangiopancreatography for evaluation of pancreatic masses. Hepato‐Gastroenterology 2014;61(134):1775‐9. [PubMed] [Google Scholar]
Noone 2004 {published data only}
- Noone TC, Semelka RC, Chaney DM, Reinhold C. Abdominal imaging studies: Comparison of diagnostic accuracies resulting from ultrasound, computed tomography, and magnetic resonance imaging in the same individual. Magnetic Resonance Imaging 2004;22(1):19‐24. [DOI] [PubMed] [Google Scholar]
Norton 2001 {published data only}
- Norton ID, Zheng Y, Wiersema MS, Greenleaf J, Clain JE, Dimagno EP. Neural network analysis of EUS images to differentiate between pancreatic malignancy and pancreatitis. Gastrointestinal Endoscopy 2001;54(5):625‐9. [DOI] [PubMed] [Google Scholar]
Nougaret 2014 {published data only}
- Nougaret S, Reinhold C, Chong J, Escal L, Mercier G, Fabre JM, et al. Incidental pancreatic cysts: Natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. European Radiology 2014;24(5):1020‐9. [DOI] [PubMed] [Google Scholar]
O'Toole 2004 {published data only}
- O'Toole D, Palazzo L, Hammel P, Ben Yaghlene L, Couvelard A, Felce‐Dachez M, et al. Macrocystic pancreatic cystadenoma: The role of EUS and cyst fluid analysis in distinguishing mucinous and serous lesions. Gastrointestinal Endoscopy 2004;59(7):823‐9. [DOI] [PubMed] [Google Scholar]
Ogawa 2008b {published data only}
- Ogawa H, Itoh S, Ikeda M, Suzuki K, Naganawa S. Intraductal papillary mucinous neoplasm of the pancreas: Assessment of the likelihood of invasiveness with multisection CT. Radiology 2008;248(3):876‐86. [DOI] [PubMed] [Google Scholar]
Ogura 2012 {published data only}
- Ogura T, Yamao K, Sawaki A, Mizuno N, Hara K, Hijioka S, et al. Clinical impact of k‐ras mutation analysis in EUS‐guided FNA specimens from pancreatic masses. Gastrointestinal Endoscopy 2012;75(4):769‐74. [DOI] [PubMed] [Google Scholar]
Oguz 2013 {published data only}
- Oguz D, Oztas E, Kalkan IH, Tayfur O, Cicek B, Aydog G, et al. Accuracy of endoscopic ultrasound‐guided fine needle aspiration cytology on the differentiation of malignant and benign pancreatic cystic lesions: A single‐center experience. Journal of Digestive Diseases 2013;14(3):132‐9. [DOI] [PubMed] [Google Scholar]
Ohno 2009 {published data only}
- Ohno E, Hirooka Y, Itoh A, Ishigami M, Katano Y, Ohmiya N, et al. Intraductal papillary mucinous neoplasms of the pancreas: Differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Annals of Surgery 2009;249(4):628‐34. [DOI] [PubMed] [Google Scholar]
Ohta 2012 {published data only}
- Ohta K, Tanada M, Iguchi H. Differential diagnosis between benign or malignant intraductal papillary mucinous neoplasms (IPMNs) by contrast‐enhanced PET/CT. HPB 2012;14:625‐6. [Google Scholar]
Ohtsuka 2013 {published data only}
- Ohtsuka T, Ideno N, Aso T, Nagayoshi Y, Kono H, Tamura K, et al. Role of ERCP in cytological confirmation for possibly resectable pancreatic ductal adenocancinoma (PDAC) in the era of EUS‐FNA. Pancreatology 2013;13(2):e62‐3. [DOI] [PubMed] [Google Scholar]
Okada 1979 {published data only}
- Okada K, Yagi A, Tamio T. Diagnostic evaluation of CT and ERCP based on the retrospective analysis of operated cases with hepato‐biliary and pancreatic diseases. Japanese Journal of Gastroenterology 1979;76(1):54‐63. [PubMed] [Google Scholar]
Okada 1981 {published data only}
- Okada K, Yagi A, Tamio T. Diagnostic evaluation of CT and ERCP based on a retrospective analysis of hepato‐biliary and pancreatic diseases. Japanese Journal of Surgery 1981;11(4):277‐82. [DOI] [PubMed] [Google Scholar]
Okasha 2013 {published data only}
- Okasha HH, Naga MI, Esmat S, Naguib M, Hassanein M, Hassani M, et al. Endoscopic ultrasound‐guided fine needle aspiration versus percutaneous ultrasound‐guided fine needle aspiration in diagnosis of focal pancreatic masses. Endoscopic Ultrasound 2013;2(4):190‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okasha 2015 {published data only}
- Okasha HH, Ashry M, Imam HM, Ezzat R, Naguib M, Farag AH, et al. Role of endoscopic ultrasound‐guided fine needle aspiration and ultrasound‐guided fine‐needle aspiration in diagnosis of cystic pancreatic lesions. Endoscopic Ultrasound 2015;4(2):132‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olson 2012 {published data only}
- Olson MT, Ali SZ. Cytotechnologist on‐site evaluation of pancreas fine needle aspiration adequacy: Comparison with cytopathologists and correlation with the final interpretation. Acta Cytologica 2012;56(4):340‐6. [DOI] [PubMed] [Google Scholar]
Ooi 1998 {published data only}
- Ooi L, Ho GH, Chew SP, Low CH, Soo KC. Cystic tumours of the pancreas: A diagnostic dilemma. Australian and New Zealand Journal of Surgery 1998;68(12):844‐6. [DOI] [PubMed] [Google Scholar]
Ootaki 2012 {published data only}
- Ootaki C, Stevens T, Vargo J, You J, Shiba A, Foss J, et al. Does general anesthesia increase the diagnostic yield of endoscopic ultrasound‐guided fine needle aspiration of pancreatic masses?. Anesthesiology 2012;117(5):1044‐50. [DOI] [PubMed] [Google Scholar]
Opacic 2015 {published data only}
- Opacic D, Rustemovic N, Kalauz M, Markos P, Ostojic Z, Majerovic M, et al. Endoscopic ultrasound elastography strain histograms in the evaluation of patients with pancreatic masses. World Journal of Gastroenterology 2015;21(13):4014‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oppong 2015 {published data only}
- Oppong KW, Dawwas MF, Charnley RM, Wadehra V, Elamin K, White S, et al. EUS and EUS‐FNA diagnosis of suspected pancreatic cystic neoplasms: Is the sum of the parts greater than the CEA?. Pancreatology 2015;15(5):531‐7. [DOI] [PubMed] [Google Scholar]
Osman 2016 {published data only}
- Osman NM, Mohammad SA, Khalil RM. Diagnostic benefit of MRCP in hepatopancreaticobiliary diseases in children. Egyptian Journal of Radiology and Nuclear Medicine 2016;47(1):291‐5. [Google Scholar]
Othman 2011 {published data only}
- Othman MO, Raimondo M. Endoscopic ultrasound fine needle aspiration of pancreatic lesions: Is a smaller needle safer and better?. Digestive and Liver Disease 2011;43(8):587‐8. [DOI] [PubMed] [Google Scholar]
Ozkan 2016 {published data only}
- Ozkan M, Cakiroglu M, Kocaman O, Kurt M, Yilmaz B, Can G, et al. Age‐based computer‐aided diagnosis approach for pancreatic cancer on endoscopic ultrasound images. Endoscopic Ultrasound 2016;5(2):101‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paik 2015 {published data only}
- Paik WH, Park Y, Park DH, Hong SM, Lee BU, Choi JH, et al. Prospective evaluation of new 22 gauge endoscopic ultrasound core needle using capillary sampling with stylet slow‐pull technique for intra‐abdominal solid masses. Journal of Clinical Gastroenterology 2015;49(3):199‐205. [DOI] [PubMed] [Google Scholar]
Palacios‐Gerona 2012 {published data only}
- Palacios‐Gerona H, Sanchez‐Sanchez R, Lainez MNP, Rodriguez‐Fernandez A, Garrote‐Lara D, Gomez‐Rio M, et al. Usefulness of 18f‐FDG PET/CT in the evaluation of malignant suspected pancreatic lesions: Diagnostic accuracy and clinical impact. European Journal of Nuclear Medicine and Molecular Imaging 2012;39(2 Suppl):S583. [Google Scholar]
Palaniappan 2014 {published data only}
- Palaniappan S, Arvind M, Dinesh J, Melpakam S, Vijaya S, Venkataraman J, et al. Role of endoscopic ultrasound‐guided fine‐needle aspiration in the diagnosis of space occupying lesions of the pancreas. Journal of Digestive Endoscopy 2014;5(3):110‐3. [Google Scholar]
Palazzo 1993 {published data only}
- Palazzo L, Roseau G, Gayet B, Vilgrain V, Belghiti J, Fekete F, et al. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma ‐ results of a prospective‐study with comparison to ultrasonography and CT scan. Endoscopy 1993;25(2):143‐50. [DOI] [PubMed] [Google Scholar]
Palazzo 2011 {published data only}
- Palazzo L, O'Toole D. Endoscopic ultrasound in cystic pancreatic lesions: Operator training needs to be improved, EUS‐guided sampling should be standardized, and decision‐making should be multidisciplinary and evidence‐based. Endoscopy 2011;43(7):557‐9. [DOI] [PubMed] [Google Scholar]
Pan 2014 {published data only}
- Pan SB, Zhao H, Geng X. Diagnostic value of 18f‐FDG PET/CT in pancreatic cancer. HPB 2014;16(Suppl S2):618‐9. [Google Scholar]
Panaro 1978 {published data only}
- Panaro VA, Alker GJ Jr, Oh YS, Leslie EV. Efficacy of computed tomography of the abdomen ‐ early results. Computerized Tomography 1978;2(1):21‐6. [DOI] [PubMed] [Google Scholar]
Papanikolaou 2008 {published data only}
- Papanikolaou IS, Adler A, Wegener K, Al‐Abadi H, Durr A, Koch M, et al. Prospective pilot evaluation of a new needle prototype for endoscopic ultrasonography‐guided fine‐needle aspiration: Comparison of cytology and histology yield. European Journal of Gastroenterology & Hepatology 2008;20(4):342‐8. [DOI] [PubMed] [Google Scholar]
Papos 1999 {published data only}
- Papos M, Takacs T, Tron L, Farkas G, Ambrus E, Lonovics J, et al. The possible place of FDG‐PET investigations in the differential diagnosis of focal pancreatic lesions. Radioactive Isotopes in Clinical Medicine and Research xxiii. Birkhäuser, 1999:471‐4. [Google Scholar]
Papos 2002a {published data only}
- Papos M, Takacs T, Pavics L, Farkas G, Ambrus E, Szakall S Jr, et al. The role of FDG‐PET scan in the diagnosis of pancreatic carcinoma. Orvosi Hetilap 2002;143(21 Suppl 3):1283‐6. [PubMed] [Google Scholar]
Papos 2002b {published data only}
- Papos M, Takacs T, Tron L, Farkas G, Ambrus E, Szakall S Jr, et al. The possible role of f‐18 FDG positron emission tomography in the differential diagnosis of focal pancreatic lesions. Clinical Nuclear Medicine 2002;27(3):197‐201. [DOI] [PubMed] [Google Scholar]
Park 2014a {published data only}
- Park JS, Kim HK, Bang BW, Kim SG, Jeong S, Lee DH. Effectiveness of contrast‐enhanced harmonic endoscopic ultrasound for the evaluation of solid pancreatic masses. World Journal of Gastroenterology 2014;20(2):518‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2014b {published data only}
- Park JW, Jang JY, Kang MJ, Kwon W, Chang YR, Kim SW. Mucinous cystic neoplasm of the pancreas: Is surgical resection recommended for all surgically fit patients?. Pancreatology 2014;14(2):131‐6. [DOI] [PubMed] [Google Scholar]
Park 2016a {published data only}
- Park JK, Kang KJ, Oh CR, Lee JK, Lee KT, Jang KT, et al. Evaluating the minimal specimens from endoscopic ultrasound‐guided fine‐needle aspiration in pancreatic masses. Medicine 2016;95(21):e3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2016b {published data only}
- Park SW, Chung MJ, Lee SH, Lee HS, Lee HJ, Park JY, et al. Prospective study for comparison of endoscopic ultrasound‐guided tissue acquisition using 25‐ and 22‐gauge core biopsy needles in solid pancreatic masses. PLoS ONE 2016;11(5):e0154401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasanen 1992 {published data only}
- Pasanen P, Partanen K, Pikkarainen P, Alhava E, Pirinen A, Janatuinen E. Diagnostic accuracy of ultrasound, computed tomography and endoscopic retrograde cholangiopancreatography in the detection of pancreatic cancer in patients with jaundice or cholestasis. In Vivo 1992;6(3):297‐301. [PubMed] [Google Scholar]
Pasanen 1993 {published data only}
- Pasanen PA, Partanen KP, Pikkarainen PH, Alhava EM, Janatuinen EK, Pirinen AE. A comparison of ultrasound, computed tomography and endoscopic retrograde cholangiopancreatography in the differential diagnosis of benign and malignant jaundice and cholestasis. European Journal of Surgery 1993;159(1):23‐9. [PubMed] [Google Scholar]
Patoureaux 2013 {published data only}
- Patoureaux S, Saint‐Paul MC, Vanbiervliet G, Barthet M, Fabre M. Randomised prospective comparison of endoscopic ultrasound (EUS)‐guided fine needle aspiration (FNA) and core biopsy of solid pancreatic tumours using ProCore and EchoTip 22g needles: The 'Picore' study. Acta Cytologica 2013;57:27‐8. [Google Scholar]
Paye 2000 {published data only}
- Paye F, Sauvanet A, Terris B, Ponsot P, Vilgrain V, Hammel P, et al. Intraductal papillary mucinous tumors of the pancreas: Pancreatic resections guided by preoperative morphological assessment and intraoperative frozen section examination. Surgery 2000;127(5):536‐44. [DOI] [PubMed] [Google Scholar]
Pedrazzoli 2005 {published data only}
- Pedrazzoli S, Pasquali C, Sperti C, Chierichetti F. 18‐fluorodeoxyglucose positron emission tomography and pancreatic cancer. Digestive Surgery 2005;22(6):467‐8. [DOI] [PubMed] [Google Scholar]
Pellise 2003 {published data only}
- Pellise M, Castells A, Gines A, Sole M, Mora J, Castellvi‐Bel S, et al. Clinical usefulness of kras mutational analysis in the diagnosis of pancreatic adenocarcinoma by means of endosonography‐guided fine‐needle aspiration biopsy. Alimentary Pharmacology & Therapeutics 2003;17(10):1299‐307. [DOI] [PubMed] [Google Scholar]
Perri 2012 {published data only}
- Perri F. EUS‐guided FNA of solid pancreatic masses with or without on‐site cytological evaluation: More sample adequacy with less needle passes. American Journal of Gastroenterology 2012;107(3):490. [DOI] [PubMed] [Google Scholar]
Perrone 2012 {published data only}
- Perrone G, Gaeta ML, Brunelli C, Pandolfi M, Borzomati D, Matteo FM, et al. K‐ras mutation analysis in endosonography‐guided fine needle aspiration of pancreatic solid lesions. HPB 2012;14:237‐8. [Google Scholar]
Petrone 2012 {published data only}
- Petrone MC, Arcidiacono PG, Carrara S, Mezzi G, Doglioni C, Testoni PA. Does cytotechnician training influence the accuracy of EUS‐guided fine‐needle aspiration of pancreatic masses?. Digestive and Liver Disease 2012;44(4):311‐4. [DOI] [PubMed] [Google Scholar]
Pezzilli 2013 {published data only}
- Pezzilli R, Serra C, Calculli L, Ferroni F, Iammarino MT, Casadei R. Three‐dimensional contrast‐enhanced ultrasonography of intraductal papillary mucinous neoplasms of the pancreas: A comparison with magnetic resonance imaging. Pancreas 2013;42(7):1164‐8. [DOI] [PubMed] [Google Scholar]
Pitman 2010 {published data only}
- Pitman M, Genevay M, Yaeger K, Chebib I, Turner B, Mino‐Kenudson M, et al. Atypical epithelial cells in EUS‐FNAb samples of pancreatic mucinous cysts correlates with a high risk of malignancy. Cancer Cytopathology 2010;118:381‐2. [DOI] [PubMed] [Google Scholar]
Pitman 2013a {published data only}
- Pitman MB, Centeno BA, Genevay M, Fonseca R, Mino‐Kenudson M. Grading epithelial atypia in endoscopic ultrasound‐guided fine‐needle aspiration of intraductal papillary mucinous neoplasms: An international interobserver concordance study. Cancer Cytopathology 2013;121(12):729‐36. [DOI] [PubMed] [Google Scholar]
Pitman 2013b {published data only}
- Pitman MB, Yaeger KA, Brugge WR, Mino‐Kenudson M. Prospective analysis of atypical epithelial cells as a high‐risk cytologic feature for malignancy in pancreatic cysts. Cancer Cytopathology 2013;121(1):29‐36. [DOI] [PubMed] [Google Scholar]
Pitman 2014 {published data only}
- Pitman MB, Centeno BA, Daglilar ES, Brugge WR, Mino‐Kenudson M. Cytological criteria of high‐grade epithelial atypia in the cyst fluid of pancreatic intraductal papillary mucinous neoplasms. Cancer Cytopathology 2014;122(1):40‐7. [DOI] [PubMed] [Google Scholar]
Pomerri 1991 {published data only}
- Pomerri F, Alfieri P, Pescarini L, Pittarello F, Muzzio PC. Echography, computerized tomography and retrograde cholangiopancreatography for the diagnosis of focal pathology of the exocrine pancreas. Radiologia Medica 1991;81(1‐2):22‐8. [PubMed] [Google Scholar]
Pongpornsup 2011 {published data only}
- Pongpornsup S, Piyapittayanan S, Charoensak A. MDCT imaging findings for characterization pancreatic cystic lesion: Differentiation between benign and malignant pattern. Journal of the Medical Association of Thailand 2011;94(3):369‐78. [PubMed] [Google Scholar]
Qian 2003 {published data only}
- Qian X, Hecht JL. Pancreatic fine needle aspiration. A comparison of computed tomographic and endoscopic ultrasonographic guidance. Acta Cytologica 2003;47(5):723‐6. [DOI] [PubMed] [Google Scholar]
Qian 2014 {published data only}
- Qian HF, Li FQ, Hu CH. Comparison of enhanced magnetic resonance imaging and magnetic resonance cholangiopancreatography in the differential diagnosis of benign and malignant intraductal papillary mucinous neoplasms of the pancreas. Acta Academiae Medicinae Sinicae 2014;36(1):98‐101. [DOI] [PubMed] [Google Scholar]
Qin 2014 {published data only}
- Qin SY, Zhou Y, Li P, Jiang HX. Diagnostic efficacy of cell block immunohistochemistry, smear cytology, and liquid‐based cytology in endoscopic ultrasound‐guided fine‐needle aspiration of pancreatic lesions: A single‐institution experience. PLoS ONE 2014;9(9):e108762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quentin 2005 {published data only}
- Quentin V, Rioux‐Leclercq N, Pagenault M, Olivie D, Campion JP, Gosselin M, et al. Accuracy of preoperative imaging methods in a retrospective series of 14 patients with operated intraductal papillary mucinous tumors of the pancreas. Gastroenterologie Clinique et Biologique 2005;29(2):150‐5. [DOI] [PubMed] [Google Scholar]
Qureshi 2013 {published data only}
- Qureshi A, Hassan U, Loya A, Akhter N, Najam ud D, Yusuf A. Diagnostic utility of endoscopic ultrasound guided aspiration cytology in evaluation of pancreatic masses. Journal of the College of Physicians & Surgeons ‐ Pakistan 2013;23(7):484‐6. [PubMed] [Google Scholar]
Raddaoui 2011 {published data only}
- Raddaoui E. Clinical utility and diagnostic accuracy of endoscopic ultrasound‐guided fine needle aspiration of pancreatic lesions: Saudi Arabian experience. Acta Cytologica 2011;55(1):26‐9. [DOI] [PubMed] [Google Scholar]
Rajput 1998 {published data only}
- Rajput A, Stellato TA, Faulhaber PF, Vesselle HJ, Miraldi F. The role of fluorodeoxyglucose and positron emission tomography in the evaluation of pancreatic disease. Surgery 1998;124(4):793‐8. [DOI] [PubMed] [Google Scholar]
Raman 2013 {published data only}
- Raman SP, Kawamoto S, Blackford A, Hruban RH, Lennon AM, Wolfgang CL, et al. Histopathologic findings of multifocal pancreatic intraductal papillary mucinous neoplasms on CT [erratum appears in AJR: American Journal of Roentgenology 2013;200(5):1174 note: O'Brien‐Lennon, Anne Marie corrected to Lennon, Ann Marie]. AJR: American Journal of Roentgenology 2013;200(3):563‐9. [DOI] [PubMed] [Google Scholar]
Ramesh 2014 {published data only}
- Ramesh J, Reddy K, Eltoum IEA. Correlation between pre‐test risk score and EUS FNA findings in pancreatic ductal carcinoma. Gastrointestinal Endoscopy 2014;1:AB344. [Google Scholar]
Ramesh 2015 {published data only}
- Ramesh J, Bang JY, Hebert‐Magee S, Trevino J, Eltoum I, Frost A, et al. Randomized trial comparing the flexible 19g and 25g needles for endoscopic ultrasound‐guided fine needle aspiration of solid pancreatic mass lesions. Pancreas 2015;44(1):128‐33. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Ramesh 2016 {published data only}
- Ramesh J, Kim H, Reddy K, Eltoum IE. Performance characteristic of endoscopic ultrasound‐guided fine needle aspiration is unaffected by pancreatic mass size. Endoscopy International Open 2016;4(4):E434‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramirez‐Luna 2008 {published data only}
- Ramirez‐Luna MA, Zepeda‐Gomez S, Chavez‐Tapia NC, Tellez‐Avila FI. Diagnostic yield and therapeutic impact of fine‐needle aspiration biopsies guided by endoscopic ultrasound in pancreatic lesions. Revista de Investigacion Clinica 2008;60(1):11‐4. [PubMed] [Google Scholar]
Rana 2011 {published data only}
- Rana SS, Bhasin DK, Srinivisan R, Gupta R, Singh K. Endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) for evaluation of pancreatic masses: Experience from tropics. Pancreatology 2011;11:53‐4. [Google Scholar]
Ranney 2012 {published data only}
- Ranney N, Phadnis M, Trevino J, Ramesh J, Wilcox CM, Varadarajulu S. Impact of biliary stents on EUS‐guided FNA of pancreatic mass lesions. Gastrointestinal Endoscopy 2012;76(1):76‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2011 {published data only}
- Rao SX, Zeng MS, Cheng WZ, Yao XZ, Jin DY, Ji Y. Small solid tumors (< or = 2 cm) of the pancreas: Relative accuracy and differentiation of CT and MR imaging. Hepato‐Gastroenterology 2011;58(107‐8):996‐1001. [PubMed] [Google Scholar]
Rasmussen 2001 {published data only}
- Rasmussen I, Sorensen J, Haglund U. Diagnostic value of positron emission tomography using f‐18‐fluorodeoxyglucose and c‐11‐acetate for the differentiation of chronic pancreatitis and pancreatic adenocarcinoma. Gastroenterology 2001;120(5):A484‐5. [Google Scholar]
Rasmussen 2004 {published data only}
- Rasmussen I, Sorensen J, Langstrom B, Haglund U. Is positron emission tomography using 18f‐fluorodeoxyglucose and 11c‐acetate valuable in diagnosing indeterminate pancreatic masses?. Scandinavian Journal of Surgery 2004;93(3):191‐7. [DOI] [PubMed] [Google Scholar]
Raut 2002 {published data only}
- Raut CP, Pisters PW, Grau A, Kaw M, Staerkel GA, Wolff RA, et al. Diagnostic accuracy of EUS‐FNA in presumed pancreatic cancer. Gastroenterology 2002;123(1 Suppl):6. [http://ssat.com/meetings/abstracts/02ddw/107901.cgi] [Google Scholar]
Raut 2003 {published data only}
- Raut CP, Grau AJ, Staerkel GA, Kaw M, Tavzm EP, Wolff RA, et al. Diagnostic accuracy of endoscopic ultrasound‐guided fine‐needle aspiration in patients with presumed pancreatic cancer. Journal of Gastrointestinal Surgery 2003;7(1):118‐26. [DOI] [PubMed] [Google Scholar]
Redelman 2014 {published data only}
- Redelman M, Cramer HM, Wu HH. Pancreatic fine‐needle aspiration cytology in patients < 35 years of age: A retrospective review of 174 cases spanning a 17‐year period. Diagnostic Cytopathology 2014;42(4):297‐301. [DOI] [PubMed] [Google Scholar]
Reicher 2011 {published data only}
- Reicher S, Boyar FZ, Albitar M, Sulcova V, Agersborg S, Nga V, et al. Fluorescence in situ hybridization and k‐ras analyses improve diagnostic yield of endoscopic ultrasound‐guided fine‐needle aspiration of solid pancreatic masses. Pancreas 2011;40(7):1057‐62. [DOI] [PubMed] [Google Scholar]
Repak 2009 {published data only}
- Repak R, Rejchrt S, Bartova J, Malirova E, Tycova V, Bures J. Endoscopic ultrasonography (EUS) and EUS‐guided fine‐needle aspiration with cyst fluid analysis in pancreatic cystic neoplasms. Hepato‐Gastroenterology 2009;56(91‐2):629‐35. [PubMed] [Google Scholar]
Ribeiro 2014 {published data only}
- Ribeiro A, Peng J, Casas C, Fan YS. Endoscopic ultrasound guided fine needle aspiration with fluorescence in situ hybridization analysis in 104 patients with pancreatic mass. Journal of Gastroenterology and Hepatology 2014;29(8):1654‐8. [DOI] [PubMed] [Google Scholar]
Richter 1996 {published data only}
- Richter GM, Simon C, Hoffmann V, DeBernardinis M, Seelos R, Senninger N, et al. Hydrospiral CT of the pancreas in thin section technique. Radiologe 1996;36(5):397‐405. [DOI] [PubMed] [Google Scholar]
Richter 2001 {published data only}
- Richter A, Gaa J, Niedergethmann M, Georgi M, Trede M, Post S. Ultrafast magnetic resonance tomography changes the standard in pancreas diagnosis. Chirurg 2001;72(6):697‐703. [DOI] [PubMed] [Google Scholar]
Ridtitid 2015 {published data only}
- Ridtitid W, Schmidt CM, Dewitt JM, Roch AM, Stuart JS, Sherman S, et al. Management of branched duct intraductal papillary mucinous neoplasms: A large single center study to assess predictors of malignancy and recurrence on long term follow‐up. Gastrointestinal Endoscopy 2015;1:AB115‐6. [DOI] [PubMed] [Google Scholar]
Rocca 2007 {published data only}
- Rocca R, Angelis C, Daperno M, Carucci P, Ravarino N, Bruno M, et al. Endoscopic ultrasound‐fine needle aspiration (EUS‐FNA) for pancreatic lesions: Effectiveness in clinical practice. Digestive and Liver Disease 2007;39(8):768‐74. [DOI] [PubMed] [Google Scholar]
Roch 2014 {published data only}
- Roch AM, Ceppa EP, DeWitt JM, Al‐Haddad MA, House MG, Nakeeb A, et al. International consensus guidelines parameters for the prediction of malignancy in intraductal papillary mucinous neoplasm are not properly weighted and are not cumulative. HPB 2014;16(10):929‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez 2007 {published data only}
- Rodriguez S, Faigel DO. Improving the predictive value of EUS‐FNA for pancreatic cancer. Gastrointestinal Endoscopy 2007;65(5):AB307. [Google Scholar]
Rodriguez 2010 {published data only}
- Rodriguez S, Faigel D. Absence of a dilated duct predicts benign disease in suspected pancreas cancer: A simple clinical rule. Digestive Diseases and Sciences 2010;55(4):1161‐6. [DOI] [PubMed] [Google Scholar]
Rodriguez‐D'Jesus 2013 {published data only}
- Rodriguez‐D'Jesus A, Fernandez‐Esparrach G, Boadas J, Busquets J, Fernandez‐Cruz L, Ferrer J, et al. Impact of endoscopic ultrasonography (EUS) and EUS‐guided fine needle aspiration (EUSFNA) on the management of pancreatic cystic lesions: Preliminary results. Pancreatology 2013;1:e10. [DOI] [PubMed] [Google Scholar]
Rogart 2011 {published data only}
- Rogart JN, Loren DE, Singu BS, Kowalski TE. Cyst wall puncture and aspiration during EUS‐guided fine needle aspiration may increase the diagnostic yield of mucinous cysts of the pancreas. Journal of Clinical Gastroenterology 2011;45(2):164‐9. [DOI] [PubMed] [Google Scholar]
Romagnuolo 2011 {published data only}
- Romagnuolo J, Hoffman B, Vela S, Hawes R, Vignesh S. Accuracy of contrast‐enhanced harmonic EUS with a second‐generation perflutren lipid microsphere contrast agent. Gastrointestinal Endoscopy 2011;73(1):52‐63. [DOI] [PubMed] [Google Scholar]
Rong 2012 {published data only}
- Rong L, Kida M, Yamauchi H, Okuwaki K, Miyazawa S, Iwai T, et al. Factors affecting the diagnostic accuracy of endoscopic ultrasonography‐guided fine‐needle aspiration (EUS‐FNA) for upper gastrointestinal submucosal or extraluminal solid mass lesions. Digestive Endoscopy 2012;24(5):358‐63. [DOI] [PubMed] [Google Scholar]
Rosch 1990a {published data only}
- Rosch T, Dancygier H, Lorenz R, Braig C, Feuerbach S, Siewert, et al. Endoscopic ultrasonography in diagnosis and differentiation of pancreatic tumors ‐ comparison with other diagnostic methods. Gastrointestinal Endoscopy 1990;36(2):199. [Google Scholar]
Rosch 1990b {published data only}
- Rosch T, Lorenz R, Braig C, Feuerbach S, Siewert JR, Classen M. Endoscopic ultrasound in the diagnosis of pancreatic tumours. Deutsche Medizinische Wochenschrift 1990;115(36):1339‐47. [DOI] [PubMed] [Google Scholar]
Rosch 1991a {published data only}
- Rosch T, Lorenz R, Braig C, Dancygier H, Classen M. Endoscopic ultrasound in small pancreatic tumors. Zeitschrift fur Gastroenterologie 1991;29(3):110‐5. [PubMed] [Google Scholar]
Rosch 1991b {published data only}
- Rosch T, Lorenz R, Braig C, Feuerbach S, Siewert JR, Schusdziarra V, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointestinal Endoscopy 1991;37(3):347‐52. [DOI] [PubMed] [Google Scholar]
Rosch 2000 {published data only}
- Rosch T, Schusdziarra V, Born P, Bautz W, Baumgartner M, Ulm K, et al. Modern imaging methods versus clinical assessment in the evaluation of hospital in‐patients with suspected pancreatic disease. American Journal of Gastroenterology 2000;95(9):2261‐70. [DOI] [PubMed] [Google Scholar]
Rose 1999 {published data only}
- Rose DM, Delbeke D, Beauchamp RD, Chapman WC, Sandler MP, Sharp KW, et al. 18fluorodeoxyglucose‐positron emission tomography in the management of patients with suspected pancreatic cancer. Annals of Surgery 1999;229(5):729‐37; discussion 37‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosique 2002 {published data only}
- Rosique C, Winant C, Deviere J, Matos C. Intraductal papillary mucinous tumor of the pancreas demonstrated by MR cholangiopancreatography. JBR‐BTR: Organe de la Societe Royale Belge de Radiologie 2002;85(1):44. [PubMed] [Google Scholar]
Rudolph 2001 {published data only}
- Rudolph J, Nahrig J, Werner M, Maurer J. Cystic mass in the pancreas ‐ invasive mucinous ‐ cystic carcinoma of the cauda pancreatis. Radiologe 2001;41(10):923‐6. [DOI] [PubMed] [Google Scholar]
Ruf 2006 {published data only}
- Ruf J, Hanninen EL, Bohmig M, Koch I, Denecke T, Plotkin M, et al. Impact of FDG‐PET/MRI image fusion on the detection of pancreatic cancer. Pancreatology 2006;6(6):512‐9. [DOI] [PubMed] [Google Scholar]
Ryozawa 2005 {published data only}
- Ryozawa S, Kitoh H, Gondo T, Urayama N, Yamashita H, Ozawa H, et al. Usefulness of endoscopic ultrasound‐guided fine‐needle aspiration biopsy for the diagnosis of pancreatic cancer. Journal of Gastroenterology 2005;40(9):907‐11. [DOI] [PubMed] [Google Scholar]
Saftoiu 2006 {published data only}
- Saftoiu A, Popescu C, Cazacu S, Dumitrescu D, Georgescu CV, Popescu M, et al. Power doppler endoscopic ultrasonography for the differential diagnosis between pancreatic cancer and pseudotumoral chronic pancreatitis. Journal of Ultrasound in Medicine 2006;25(3):363‐72. [DOI] [PubMed] [Google Scholar]
Saftoiu 2008 {published data only}
- Saftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, et al. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointestinal Endoscopy 2008;68(6):1086‐94. [DOI] [PubMed] [Google Scholar]
Saftoiu 2010a {published data only}
- Saftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Belciug S. Endoscopic ultrasound elastography in the diagnosis of pancreatic cancer. Annals of Gastroenterology 2010;23(3):200‐1. [Google Scholar]
Saftoiu 2010b {published data only}
- Saftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Iglesias‐Garcia J, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of chronic pancreatitis and pancreatic cancer: A multicentric study. Gastrointestinal Endoscopy 2010;71(5):AB120. [Google Scholar]
Saftoiu 2011 {published data only}
- Saftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: A multicenter study. Endoscopy 2011;43(7):596‐603. [DOI] [PubMed] [Google Scholar]
Saftoiu 2012 {published data only}
- Saftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, et al. Efficacy of an artificial neural network‐based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clinical Gastroenterology and Hepatology 2012;10(1):84‐90.e1. [DOI] [PubMed] [Google Scholar]
Saftoiu 2013 {published data only}
- Saftoiu A, Vilmann P. Differential diagnosis of focal pancreatic masses by semiquantitative EUS elastography: Between strain ratios and strain histograms. Gastrointestinal Endoscopy 2013;78(1):188‐9. [DOI] [PubMed] [Google Scholar]
Saftoiu 2015 {published data only}
- Saftoiu A, Vilmann P, Dietrich CF, Iglesias‐Garcia J, Hocke M, Seicean A, et al. Quantitative contrast‐enhanced harmonic EUS in differential diagnosis of focal pancreatic masses (with videos). Gastrointestinal Endoscopy 2015;82(1):59‐69. [DOI] [PubMed] [Google Scholar]
Sahai 2012 {published data only}
- Sahai AV. Is EUS here to stay? Accuracy is not an indication. Endoscopic Ultrasound 2012;1(3):117‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahani 2006b {published data only}
- Sahani DV, Saokar A, Hahn PF, Brugge WR, Fernandez‐Del Castillo C. Pancreatic cysts 3 cm or smaller: How aggressive should treatment be?. Radiology 2006;238(3):912‐9. [DOI] [PubMed] [Google Scholar]
Sahani 2011 {published data only}
- Sahani DV, Sainani NI, Blake MA, Crippa S, Mino‐Kenudson M, del‐Castillo CF. Prospective evaluation of reader performance on MDCT in characterization of cystic pancreatic lesions and prediction of cyst biologic aggressiveness. AJR: American Journal of Roentgenology 2011;197(1):W53‐61. [DOI] [PubMed] [Google Scholar]
Sai 2003 {published data only}
- Sai JK, Suyama M, Kubokawa Y, Yamanaka K, Tadokoro H, Iida Y, et al. Management of branch duct‐type intraductal papillary mucinous tumor of the pancreas based on magnetic resonance imaging. Abdominal Imaging 2003;28(5):694‐9. [DOI] [PubMed] [Google Scholar]
Sakamoto 2008 {published data only}
- Sakamoto H, Kitano M, Suetomi Y, Maekawa K, Takeyama Y, Kudo M. Utility of contrast‐enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound in Medicine & Biology 2008;34(4):525‐32. [DOI] [PubMed] [Google Scholar]
Sakamoto 2009 {published data only}
- Sakamoto H, Kitano M, Komaki T, Noda K, Chikugo T, Dote K, et al. Prospective comparative study of the EUS guided 25‐gauge FNA needle with the 19‐gauge trucut needle and 22‐gauge FNA needle in patients with solid pancreatic masses. Journal of Gastroenterology and Hepatology 2009;24(3):384‐90. [DOI] [PubMed] [Google Scholar]
Salvia 2012 {published data only}
- Salvia R, Malleo G, Marchegiani G, Pennacchio S, Paiella S, Paini M, et al. Pancreatic resections for cystic neoplasms: From the surgeon's presumption to the pathologist's reality. Surgery 2012;152(3 Suppl 1):S135‐42. [DOI] [PubMed] [Google Scholar]
Sandrasegaran 2011 {published data only}
- Sandrasegaran K, Akisik FM, Patel AA, Rydberg M, Cramer HM, Agaram NP, et al. Diffusion‐weighted imaging in characterization of cystic pancreatic lesions. Clinical Radiology 2011;66(9):808‐14. [DOI] [PubMed] [Google Scholar]
Santhosh 2013 {published data only}
- Santhosh S, Mittal BR, Bhasin D, Srinivasan R, Rana S, Das A, et al. Role of (18)f‐fluorodeoxyglucose positron emission tomography/computed tomography in the characterization of pancreatic masses: Experience from tropics. Journal of Gastroenterology and Hepatology 2013;28(2):255‐61. [DOI] [PubMed] [Google Scholar]
Sarbia 2007 {published data only}
- Sarbia M, Moller K. Endoscopic ultrasound‐guided fine needle aspiration of the pancreas. Diagnostic utility and accuracy. Pathology Research and Practice 2007;203(5):354. [Google Scholar]
Sariya 2003 {published data only}
- Sariya DR, Siddiqui NH, Liu LH, Lamba A. Diagnostic value of endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA) of pancreatic tumors: A 3‐year experience. Laboratory Investigation 2003;83(1):80A. [Google Scholar]
Savides 2006 {published data only}
- Savides T, Hunt G, Al‐Haddad M, Aslanian H, Ben‐Menachem T, Chen V, et al. EUS FNA diagnostic yield of malignancy in solid pancreatic mass: A benchmark for quality performance measurement. Gastrointestinal Endoscopy 2006;63(5):AB258. [DOI] [PubMed] [Google Scholar]
Savides 2007 {published data only}
- Savides TJ, Donohue M, Hunt G, Al‐Haddad M, Aslanian H, Ben‐Menachem T, et al. EUS‐guided FNA diagnostic yield of malignancy in solid pancreatic masses: A benchmark for quality performance measurement. Gastrointestinal Endoscopy 2007;66(2):277‐82. [DOI] [PubMed] [Google Scholar]
Savoy 2007 {published data only}
- Savoy AD, Raimondo M, Woodward TA, Noh K, Pungpapong S, Jones AD, et al. Can endosonographers evaluate on‐site cytologic adequacy? A comparison with cytotechnologists. Gastrointestinal Endoscopy 2007;65(7):953‐7. [DOI] [PubMed] [Google Scholar]
Saxena 2014 {published data only}
- Saxena P, Kumbhari V, Zein M, Abdelgelil A, Besharati S, Mesallam A, et al. Comparison of two needle aspiration techniques for endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA) in solid pancreatic lesions: A prospective multicentre study. Journal of Gastroenterology and Hepatology 2014;29:59. [Google Scholar]
Schick 2008 {published data only}
- Schick V, Franzius C, Beyna T, Oei ML, Schnekenburger J, Weckesser M, et al. Diagnostic impact of 18f‐FDG PET‐CT evaluating solid pancreatic lesions versus endosonography, endoscopic retrograde cholangio‐pancreatography with intraductal ultrasonography and abdominal ultrasound. European Journal of Nuclear Medicine & Molecular Imaging 2008;35(10):1775‐85. [DOI] [PubMed] [Google Scholar]
Schima 2002 {published data only}
- Schima W, Fugger R, Schober E, Oettl C, Wamser P, Grabenwoger F, et al. Diagnosis and staging of pancreatic cancer: Comparison of mangafodipir trisodium‐enhanced MR imaging and contrast‐enhanced helical hydro‐CT. AJR: American Journal of Roentgenology 2002;179(3):717‐24. [DOI] [PubMed] [Google Scholar]
Schmidt 2015 {published data only}
- Schmidt C, Besser A, Petersen I, Stallmach A. Increased accuracy of FNA based cytological diagnosis of solid pancreatic lesions by use of an ethanol based fixative system. Gastrointestinal Endoscopy 2015;1:AB537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2015 {published data only}
- Schneider AR, Nerlich A, Topalidis T, Schepp W. Specialized clinical cytology may improve the results of EUS (endoscopic ultrasound)‐guided fine‐needle aspiration (FNA) from pancreatic tumors. Endoscopy International Open 2015;3(2):E134‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schrader 2012 {published data only}
- Schrader H, Wiese M, Ellrichmann M, Belyaev O, Uhl W, Tannapfel A, et al. Diagnostic value of quantitative EUS elastography for malignant pancreatic tumors: Relationship with pancreatic fibrosis. Ultraschall in der Medizin 2012;33(7):E196‐201. [DOI] [PubMed] [Google Scholar]
Schraibman 2011 {published data only}
- Schraibman V, Goldman SM, Ardengh JC, Goldenberg A, Lobo E, Linhares MM, et al. New trends in diffusion‐weighted magnetic resonance imaging as a tool in differentiation of serous cystadenoma and mucinous cystic tumor: A prospective study. Pancreatology 2011;11(1):43‐51. [DOI] [PubMed] [Google Scholar]
Scott 2000 {published data only}
- Scott J, Martin I, Redhead D, Hammond P, Garden OJ. Mucinous cystic neoplasms of the pancreas: Imaging features and diagnostic difficulties. Clinical Radiology 2000;55(3):187‐92. [DOI] [PubMed] [Google Scholar]
Seicean 2010 {published data only}
- Seicean A, Badea R, Stan‐Iuga R, Mocan T, Gulei I, Pascu O. Quantitative contrast‐enhanced harmonic endoscopic ultrasonography for the discrimination of solid pancreatic masses. Ultraschall in der Medizin 2010;31(6):571‐6. [DOI] [PubMed] [Google Scholar]
Seicean 2016 {published data only}
- Seicean A, Gheorghiu M, Zaharia T, Calinici T, Samarghitan A, Marcus B, et al. Performance of the standard 22g needle for endoscopic ultrasound‐guided tissue core biopsy in pancreatic cancer. Journal of Gastrointestinal and Liver Diseases 2016;25(2):213‐8. [DOI] [PubMed] [Google Scholar]
Sendino 2010 {published data only}
- Sendino O, Fernandez‐Esparrach G, Sole M, Colomo L, Pellise M, Llach J, et al. Endoscopic ultrasonography‐guided brushing increases cellular diagnosis of pancreatic cysts: A prospective study. Digestive and Liver Disease 2010;42(12):877‐81. [DOI] [PubMed] [Google Scholar]
Sendler 2000 {published data only}
- Sendler A, Avril N, Helmberger H, Stollfuss J, Weber W, Bengel F, et al. Preoperative evaluation of pancreatic masses with positron emission tomography using 18F‐fluorodeoxyglucose: Diagnostic limitations. World Journal of Surgery 2000;24(9):1121‐9. [DOI] [PubMed] [Google Scholar]
Serikawa 2006 {published data only}
- Serikawa M, Sasaki T, Fujimoto Y, Kuwahara K, Chayama K. Management of intraductal papillary‐mucinous neoplasm of the pancreas ‐ treatment strategy based on morphologic classification. Journal of Clinical Gastroenterology 2006;40(9):856‐62. [DOI] [PubMed] [Google Scholar]
Shah 2008 {published data only}
- Shah SM, Ribeiro A, Levi J, Jorda M, Rocha‐Lima C, Sleeman D, et al. EUS‐guided fine needle aspiration with and without trucut biopsy of pancreatic masses. Journal of the Pancreas 2008;9(4):422‐30. [PubMed] [Google Scholar]
Shen 2013 {published data only}
- Shen X, Lu D, Xu X, Wang J, Wu J, Yan S, et al. A novel distinguishing system for the diagnosis of malignant pancreatic cystic neoplasm. European Journal of Radiology 2013;82(11):e648‐54. [DOI] [PubMed] [Google Scholar]
Shimizu 2010 {published data only}
- Shimizu Y, Kanemitsu Y, Sano T, Senda Y, Mizuno N, Yamao K. A nomogram for predicting the probability of carcinoma in patients with intraductal papillary‐mucinous neoplasm. World Journal of Surgery 2010;34(12):2932‐8. [DOI] [PubMed] [Google Scholar]
Shimizu 2013a {published data only}
- Shimizu Y, Yamaue H, Maguchi H, Yamao K, Hirono S, Osanai M, et al. Predictors of malignancy in branch duct intraductal papillary mucinous neoplasm of the pancreas (BD‐IPMN) ‐ analysis of 202 pancreatic resection patients at multiple high volume centers. Gastroenterology 2013;144(5 Suppl):S799. [DOI] [PubMed] [Google Scholar]
Shimizu 2013b {published data only}
- Shimizu Y, Yamaue H, Maguchi H, Yamao K, Hirono S, Osanai M, et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: Analysis of 310 pancreatic resection patients at multiple high‐volume centers. Pancreas 2013;42(5):883‐8. [DOI] [PubMed] [Google Scholar]
Shimizu 2014 {published data only}
- Shimizu Y, Yamaue H, Maguchi H, Yamao K, Hirono S, Osanai M, et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas (IPMN) ‐ analysis of 310 pancreatic resection patients at multiple high volume centers. HPB 2014;16:256. [DOI] [PubMed] [Google Scholar]
Shimizu 2015 {published data only}
- Shimizu Y, Yamaue H, Maguchi H, Yamao K, Hirono S, Osanai M, et al. Validation of a nomogram for predicting the probability of carcinoma in patients with intraductal papillary mucinous neoplasm in 180 pancreatic resection patients at 3 high‐volume centers. Pancreas 2015;44(3):459‐64. [DOI] [PubMed] [Google Scholar]
Shin 2002 {published data only}
- Shin HJC, Lahoti S, Sneige N. Endoscopic ultrasound‐guided fine‐needle aspiration in 179 cases ‐ the M.D. Anderson Cancer Center experience. Cancer Cytopathology 2002;96(3):174‐80. [DOI] [PubMed] [Google Scholar]
Shin 2010 {published data only}
- Shin SH, Han DJ, Park KT, Kim YH, Park JB, Kim SC. Validating a simple scoring system to predict malignancy and invasiveness of intraductal papillary mucinous neoplasms of the pancreas. World Journal of Surgery 2010;34(4):776‐83. [DOI] [PubMed] [Google Scholar]
Siddiqui 2009 {published data only}
- Siddiqui UD, Rossi F, Rosenthal LS, Padda MS, Murali‐Dharan V, Aslanian HR. EUS‐guided FNA of solid pancreatic masses: A prospective, randomized trial comparing 22‐gauge and 25‐gauge needles. Gastrointestinal Endoscopy 2009;70(6):1093‐7. [DOI] [PubMed] [Google Scholar]
Siddiqui 2010 {published data only}
- Siddiqui AA, Lyles T, Avula H, Davila R. Endoscopic ultrasound‐guided fine needle aspiration of pancreatic masses in a veteran population: Comparison of results with 22‐ and 25‐gauge needles. Pancreas 2010;39(5):685‐6. [DOI] [PubMed] [Google Scholar]
Siddiqui 2011 {published data only}
- Siddiqui AA, Brown LJ, Hong SKS, Draganova‐Tacheva RA, Korenblit J, Loren DE, et al. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound‐guided fine needle aspiration. Digestive Diseases and Sciences 2011;56(11):3370‐5. [DOI] [PubMed] [Google Scholar]
Siddiqui 2012 {published data only}
- Siddiqui AA, Fein M, Kowalski TE, Loren DE, Eloubeidi MA. Comparison of the influence of plastic and fully covered metal biliary stents on the accuracy of EUS‐FNA for the diagnosis of pancreatic cancer. Digestive Diseases and Sciences 2012;57(9):2438‐45. [DOI] [PubMed] [Google Scholar]
Siddiqui 2013 {published data only}
- Siddiqui AA, Kowalski TE, Kedika R, Roy A, Loren DE, Ellsworth E, et al. EUS‐guided pancreatic fluid aspiration for DNA analysis of kras and gnas mutations for the evaluation of pancreatic cystic neoplasia: A pilot study. Gastrointestinal Endoscopy 2013;77(4):669‐70. [DOI] [PubMed] [Google Scholar]
Siech 1998 {published data only}
- Siech M, Tripp K, Schmidt‐Rohlfing B, Mattfeldt T, Widmaier U, Gansauge F, et al. Cystic tumours of the pancreas: Diagnostic accuracy, pathologic observations and surgical consequences. Langenbecks Archives of Surgery 1998;383(1):56‐61. [DOI] [PubMed] [Google Scholar]
Simon 2009 {published data only}
- Simon P, Dickson E, Lerch M, Carter R, McKay C. Endoscopic ultrasound guided elastography compared to EUS and EUS guided FNA in solid mass lesions of the pancreas. A prospective study in 30 patients. Pancreatology 2009;9(4):528‐9. [Google Scholar]
Sina 2014 {published data only}
- Sina M, Cote GA, Korc M. Improving the diagnostic accuracy of endoscopic ultrasound‐guided fine‐needle aspiration using microRNAs. Gastroenterology 2014;147(4):930‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singer 2007 {published data only}
- Singer E, Gschwantler M, Plattner D, Kriwanek S, Armbruster C, Schueller J, et al. Differential diagnosis of benign and malign pancreatic masses with 18f‐fluordeoxyglucose‐positron emission tomography recorded with a dual‐head coincidence gamma camera. European Journal of Gastroenterology & Hepatology 2007;19(6):471‐8. [DOI] [PubMed] [Google Scholar]
Singhi 2014 {published data only}
- Singhi AD, Nikiforova MN, Fasanella KE, McGrath KM, Pai RK, Ohori NP, et al. Preoperative gnas and kras testing in the diagnosis of pancreatic mucinous cysts. Clinical Cancer Research 2014;20(16):4381‐9. [DOI] [PubMed] [Google Scholar]
Singhi 2016 {published data only}
- Singhi AD, Zeh HJ, Brand RE, Nikiforova MN, Chennat JS, Fasanella KE, et al. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: A clinicopathologic study of 225 patients with supporting molecular data. Gastrointestinal Endoscopy 2016;83(6):1107‐17. [DOI] [PubMed] [Google Scholar]
Singu 2008 {published data only}
- Singu BS, Loren DE, Kowalski TE. Diagnostic yield and safety of cyst wall puncture during EUS‐guided fine needle aspiration of pancreatic cystic lesions. Gastrointestinal Endoscopy 2008;67(5):AB211. [Google Scholar]
Soares 2015a {published data only}
- Soares JB, Iglesias‐Garcia J, Goncalves B, Lindkvist B, Larino‐Noia J, Bastos P, et al. Interobserver agreement of contrast‐enhanced harmonic endoscopic ultrasonography in the evaluation of solid pancreatic lesions. Endoscopy International Open 2015;3(3):E205‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soares 2015b {published data only}
- Soares JB, Iglesias‐Garcia J, Goncalves B, Lindkvist B, Larino‐Noia J, Bastos P, et al. Interobserver agreement of EUS elastography in the evaluation of solid pancreatic lesions. Endoscopic Ultrasound 2015;4(3):244‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sole 2005 {published data only}
- Sole M, Iglesias C, Fernandez‐Esparrach G, Colomo L, Pellise M, Gines A. Fine‐needle aspiration cytology of intraductal papillary mucinous tumors of the pancreas. Cancer 2005;105(5):298‐303. [DOI] [PubMed] [Google Scholar]
Song 2007 {published data only}
- Song SJ, Lee JM, Kim YJ, Kim SH, Lee JY, Han JK, et al. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: Comparison of multirow‐detector CT and MR imaging using ROC analysis. Journal of Magnetic Resonance Imaging 2007;26(1):86‐93. [DOI] [PubMed] [Google Scholar]
Song 2010 {published data only}
- Song TJ, Kim JH, Lee SS, Eum JB, Moon SH, Park DH, et al. The prospective randomized, controlled trial of endoscopic ultrasound‐guided fine‐needle aspiration using 22g and 19g aspiration needles for solid pancreatic or peripancreatic masses. American Journal of Gastroenterology 2010;105(8):1739‐45. [DOI] [PubMed] [Google Scholar]
Sperti 1994 {published data only}
- Sperti C, Pasquali C, Prima F, Rugge M, Petrin P, Costantino V, et al. Percutaneous CT‐guided fine needle aspiration cytology in the differential diagnosis of pancreatic lesions. Italian Journal of Gastroenterology 1994;26(3):126‐31. [PubMed] [Google Scholar]
Sperti 2001 {published data only}
- Sperti C, Pasquali C, Chierichetti F, Liessi G, Ferlin G, Pedrazzoli S. Value of 18‐fluorodeoxyglucose positron emission tomography in the management of patients with cystic tumors of the pancreas. Annals of Surgery 2001;234(5):675‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sperti 2005 {published data only}
- Sperti C, Pasquali C, Decet G, Chierichetti F, Liessi G, Pedrazzoli S. F‐18‐fluorodeoxyglucose positron emission tomography in differentiating malignant from benign pancreatic cysts: A prospective study. Journal of Gastrointestinal Surgery 2005;9(1):22‐8; discussion 28‐9. [DOI] [PubMed] [Google Scholar]
Sperti 2007 {published data only}
- Sperti C, Bissoli S, Pasquali C, Frison L, Liessi G, Chierichetti F, et al. 18‐fluorodeoxyglucose positron emission tomography enhances computed tomography diagnosis of malignant intraductal papillary mucinous neoplasms of the pancreas. Annals of Surgery 2007;246(6):932‐7; discussion 937‐9. [DOI] [PubMed] [Google Scholar]
Sreenarasimhaiah 2008 {published data only}
- Sreenarasimhaiah J. Efficacy of endoscopic ultrasound in characterizing mass lesions in chronic pancreatitis. Journal of Clinical Gastroenterology 2008;42(1):81‐5. [DOI] [PubMed] [Google Scholar]
Sreenarasimhaiah 2009 {published data only}
- Sreenarasimhaiah J, Lara LF, Jazrawi SF, Barnett CC, Tang SJ. A comparative analysis of pancreas cyst fluid CEA and histology with DNA mutational analysis in the detection of mucin producing or malignant cysts. Journal of the Pancreas 2009;10(2):163‐8. [PubMed] [Google Scholar]
Sreenarasimhaiah 2013 {published data only}
- Sreenarasimhaiah J. EUS for pancreas cysts: What should we be sampling?. Digestive Diseases and Sciences 2013;58(6):1457‐8. [DOI] [PubMed] [Google Scholar]
Sreenarasimhaiah 2015 {published data only}
- Sreenarasimhaiah J. Does pancreas cyst size correlate with the risk for malignancy?. International Archives of Medicine 2015;8(1):94. [Google Scholar]
Staib 1997 {published data only}
- Staib L, Diederichs CG, Reske SN, Beger HG. Positron emission tomography (PET): Diagnostic benefit in pancreatic tumors?. European Journal of Cancer 1997;33(Suppl 8):S274. [Google Scholar]
Starkov 2008 {published data only}
- Starkov IG, Solodinina EN, Shishin KV, Plotnikova LS. Endoscopic ultrasonography in diagnosis of surgical treatment of pancreas. Khirurgiia 2008, (1):47‐52. [PubMed] [Google Scholar]
Stelow 2003 {published data only}
- Stelow EB, Stanley MW, Bardales RH, Mallery S, Lai R, Linzie BM, et al. Intraductal papillary‐mucinous neoplasm of the pancreas: The findings and limitations of cytologic samples obtained by endoscopic ultrasound‐guided fine‐needle aspiration. American Journal of Clinical Pathology 2003;120(3):398‐404. [DOI] [PubMed] [Google Scholar]
Storch 2006 {published data only}
- Storch I, Jorda M, Thurer R, Raez L, Rocha‐Lima C, Vernon S, et al. Advantage of EUS trucut biopsy combined with fine‐needle aspiration without immediate on‐site cytopathologic examination. Gastrointestinal Endoscopy 2006;64(4):505‐11. [DOI] [PubMed] [Google Scholar]
Storch 2007 {published data only}
- Storch IM, Sussman DA, Jorda M, Ribeiro A. Evaluation of fine needle aspiration vs. fine needle capillary sampling on specimen quality and diagnostic accuracy in endoscopic ultrasound‐guided biopsy. Acta Cytologica 2007;51(6):837‐42. [DOI] [PubMed] [Google Scholar]
Story 2009 {published data only}
- Story B, Olowe K, Jiranek G, Ross A, Irani S, Schembre D. EUS‐FNA vs. CT‐guided core biopsy for presumed pancreatic cancer: A comparison of accuracy, risk and cost. American Journal of Gastroenterology 2009;104:S509. [Google Scholar]
Strand 2014 {published data only}
- Strand DS, Jeffus SK, Sauer BG, Wang AY, Stelow EB, Shami VM. EUS‐guided 22‐gauge fine‐needle aspiration versus core biopsy needle in the evaluation of solid pancreatic neoplasms. Diagnostic Cytopathology 2014;42(9):751‐8. [DOI] [PubMed] [Google Scholar]
Strauss 2016 {published data only}
- Strauss A, Birdsey M, Fritz S, Schwarz‐Bundy BD, Bergmann F, Hackert T, et al. Intraductal papillary mucinous neoplasms of the pancreas: Radiological predictors of malignant transformation and the introduction of bile duct dilation to current guidelines. British Journal of Radiology 2016;89(1061):20150853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strobel 2013 {published data only}
- Strobel O, Buchler MW. Pancreatic cancer: FDG‐PET is not useful in early pancreatic cancer diagnosis. Nature Reviews Gastroenterology and Hepatology 2013;10(4):203‐5. [DOI] [PubMed] [Google Scholar]
Strohm 1984 {published data only}
- Strohm WD, Kurtz W, Hagenmuller F, Classen M. Diagnostic efficacy of endoscopic ultrasound tomography in pancreatic cancer and cholestasis. Scandinavian Journal of Gastroenterology ‐ Supplement 1984;102:18‐23. [PubMed] [Google Scholar]
Su 2007 {published data only}
- Su JS, Jeong ML, Young JK, Se HK, Jae YL, Joon KH, et al. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: Comparison of multirow‐detector CT and MR imaging using ROC analysis. Journal of Magnetic Resonance Imaging 2007;26(1):86‐93. [DOI] [PubMed] [Google Scholar]
Sugimoto 2015 {published data only}
- Sugimoto M, Takagi T, Hikichi T, Suzuki R, Watanabe K, Nakamura J, et al. Conventional versus contrast‐enhanced harmonic endoscopic ultrasonography‐guided fine‐needle aspiration for diagnosis of solid pancreatic lesions: A prospective randomized trial. Pancreatology 2015;15(5):538‐41. [DOI] [PubMed] [Google Scholar]
Sugiyama 2012 {published data only}
- Sugiyama Y, Kadoya M, Hamano H, Fujinaga Y, Ueda K, Kawa S, et al. Characteristic magnetic resonance features of focal autoimmune pancreatitis useful for differentiation from pancreatic cancer. Japanese Journal of Radiology 2012;30(4):296‐309. [DOI] [PubMed] [Google Scholar]
Suits 1999 {published data only}
- Suits J, Frazee R, Erickson RA. Endoscopic ultrasound and fine needle aspiration for the evaluation of pancreatic masses. Archives of Surgery 1999;134(6):639‐42; discussion 642‐3. [DOI] [PubMed] [Google Scholar]
Sun 2014 {published data only}
- Sun B, Yang X, Ping B, He Y, Zhang Z. Impact of inconclusive endoscopic ultrasound‐guided fine‐needle aspiration results in the management and outcome of patients with solid pancreatic masses. Digestive Endoscopy 2014;27(1):130‐6. [DOI] [PubMed] [Google Scholar]
Sur 2015 {published data only}
- Sur YK, Kim YC, Kim JK, Lee JH, Yoo BM, Kim YB. Comparison of ultrasound‐guided core needle biopsy and endoscopic ultrasound‐guided fine‐needle aspiration for solid pancreatic lesions. Journal of Ultrasound in Medicine 2015;34(12):2163‐9. [DOI] [PubMed] [Google Scholar]
Suzuki 2012 {published data only}
- Suzuki R, Irisawa A, Bhutani MS, Hikichi T, Takagi T, Sato A, et al. Prospective evaluation of the optimal number of 25‐gauge needle passes for endoscopic ultrasound‐guided fine‐needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Digestive Endoscopy 2012;24(6):452‐6. [DOI] [PubMed] [Google Scholar]
Suzuki 2013 {published data only}
- Suzuki R, Lee JH, Krishna SG, Ramireddy S, Qiao W, Weston B, et al. Repeat endoscopic ultrasound‐guided fine needle aspiration for solid pancreatic lesions at a tertiary referral center will alter the initial inconclusive result. Journal of Gastrointestinal and Liver Diseases 2013;22(2):183‐7. [PubMed] [Google Scholar]
Sverko 2011 {published data only}
- Sverko A, Tripalo‐Batos A, Marotti M, Mustapic M, Beslin MB, Kruslin B. Correlation between magnetic resonance imaging and histopathology in differentiation of pancreatic diseases. Acta Clinica Croatica 2011;50(2):137‐44. [PubMed] [Google Scholar]
Swobodnik 1983 {published data only}
- Swobodnik W, Meyer W, Brecht‐Kraus D, Wechsler JG, Geiger S, Malfertheiner P, et al. Ultrasound, computed tomography and endoscopic retrograde cholangiopancreatography in the morphologic diagnosis of pancreatic disease. Klinische Wochenschrift 1983;61(6):291‐6. [DOI] [PubMed] [Google Scholar]
Szafranska 2008 {published data only}
- Szafranska AE, Doleshal M, Edmunds HS, Gordon S, Luttges J, Munding JB, et al. Analysis of microRNAs in pancreatic fine‐needle aspirates can classify benign and malignant tissues. Clinical Chemistry 2008;54(10):1716‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tada 2002 {published data only}
- Tada M, Komatsu Y, Kawabe T, Sasahira N, Isayama H, Toda N, et al. Quantitative analysis of k‐ras gene mutation in pancreatic tissue obtained by endoscopic ultrasonography‐guided fine needle aspiration: Clinical utility for diagnosis of pancreatic tumor. American Journal of Gastroenterology 2002;97(9):2263‐70. [DOI] [PubMed] [Google Scholar]
Tadic 2008 {published data only}
- Tadic M, Kujundzic M, Stoos‐Veic T, Kaic G, Vukelic‐Markovic M. Role of repeated endoscopic ultrasound‐guided fine needle aspiration in small solid pancreatic masses with previous indeterminate and negative cytological findings. Digestive Diseases 2008;26(4):377‐82. [DOI] [PubMed] [Google Scholar]
Takahashi 2005 {published data only}
- Takahashi K, Yamao K, Okubo K, Sawaki A, Mizuno N, Ashida R, et al. Differential diagnosis of pancreatic cancer and focal pancreatitis by using EUS‐guided FNA. Gastrointestinal Endoscopy 2005;61(1):76‐9. [DOI] [PubMed] [Google Scholar]
Talar‐Wojnarowska 2012 {published data only}
- Talar‐Wojnarowska R, Pazurek M, Durko L, Degowska M, Rydzewska G, Smigielski J, et al. A comparative analysis of k‐ras mutation and carcinoembryonic antigen in pancreatic cyst fluid. Pancreatology 2012;12(5):417‐20. [DOI] [PubMed] [Google Scholar]
Tallini 2014 {published data only}
- Tallini G. Next generation sequencing improves the accuracy of kras mutation analysis in endoscopic ultrasound fine needle aspiration pancreatic lesions. PLoS ONE 2014;9(2):e87651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taouli 2002 {published data only}
- Taouli B, Vilgrain V, O'Toole D, Vullierme MP, Terris B, Menu Y. Intraductal papillary mucinous tumors of the pancreas: Features with multimodality imaging. Journal of Computer Assisted Tomography 2002;26(2):223‐31. [DOI] [PubMed] [Google Scholar]
Tarantino 2014a {published data only}
- Tarantino I, Mitri R, Fabbri C, Pagano N, Barresi L, Granata A, et al. Is diagnostic accuracy of fine needle aspiration on solid pancreatic lesions aspiration‐related? A multicentre randomised trial. Digestive and Liver Disease 2014;46(6):523‐6. [DOI] [PubMed] [Google Scholar]
Tarantino 2014b {published data only}
- Tarantino I, Mitri R, Fabbri C, Pagano N, Barresi L, Granata A, et al. Diagnostic accuracy of FNA (fine needle aspiration) on solid pancreatic lesions: Is aspiration‐related?. Digestive and Liver Disease 2014;46:S37. [DOI] [PubMed] [Google Scholar]
Tarantino 2014c {published data only}
- Tarantino I, Fabbri C, Mitri R, Pagano N, Barresi L, Mocciaro F, et al. Complications of endoscopic ultrasound fine needle aspiration on pancreatic cystic lesions: Final results from a large prospective multicenter study. Digestive and Liver Disease 2014;46(1):41‐4. [DOI] [PubMed] [Google Scholar]
Tatsumi 2011 {published data only}
- Tatsumi M, Isohashi K, Onishi H, Hori M, Kim T, Higuchi I, et al. 18f‐FDG PET/MRI fusion in characterizing pancreatic tumors: Comparison to PET/CT. International Journal of Clinical Oncology 2011;16(4):408‐15. [DOI] [PubMed] [Google Scholar]
Tatsuta 1985 {published data only}
- Tatsuta M, Yamamura H, Iishi H. Values of ca 19‐9 in the serum, pure pancreatic juice, and aspirated pancreatic material in the diagnosis of malignant pancreatic tumor. Cancer 1985;56(11):2669‐73. [DOI] [PubMed] [Google Scholar]
Taylor 2007 {published data only}
- Taylor M, Warnock GL, Powell J, Lillemoe K, McKenzie M. Canadian Association of General Surgeons and American College of Surgeons evidence based reviews in surgery. 22. The use of PET/CT scanning on the management of resectable pancreatic cancer. Canadian Journal of Surgery 2007;50(5):400‐2. [PMC free article] [PubMed] [Google Scholar]
Tervahartiala 1997 {published data only}
- Tervahartiala P, Kivisaari L, Lamminen A, Maschek A, Wohling H, Standertskjold‐Nordenstam CG. Dynamic fast‐gradient echo MR imaging of pancreatic tumours. European Journal of Radiology 1997;25(1):74‐80. [DOI] [PubMed] [Google Scholar]
Tessler 2006 {published data only}
- Tessler DA, Catanzaro A, Velanovich V, Havstad S, Goel S. Predictors of cancer in patients with suspected pancreatic malignancy without a tissue diagnosis. American Journal of Surgery 2006;191(2):191‐7. [DOI] [PubMed] [Google Scholar]
Theruvath 2010 {published data only}
- Theruvath TP, Morgan KA, Adams DB. Mucinous cystic neoplasms of the pancreas: How much preoperative evaluation is needed?. American Surgeon 2010;76(8):812‐7. [PubMed] [Google Scholar]
Thomas 2009 {published data only}
- Thomas T, Kaye PV, Ragunath K, Aithal G. Efficacy, safety, and predictive factors for a positive yield of EUS‐guided trucut biopsy: A large tertiary referral center experience. American Journal of Gastroenterology 2009;104(3):584‐91. [DOI] [PubMed] [Google Scholar]
Thomas 2010a {published data only}
- Thomas Cherian P, Mohan P, Douiri A, Taniere P, Hejmadi RK, Mahon BS. Role of endoscopic ultrasound‐guided fine‐needle aspiration in the diagnosis of solid pancreatic and peripancreatic lesions: Is onsite cytopathology necessary?. HPB 2010;12(6):389‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2010b {published data only}
- Thomas T, Bebb J, Mannath J, Ragunath K, Kaye PV, Aithal GP. EUS‐guided pancreatic cyst brushing: A comparative study in a tertiary referral centre. Journal of the Pancreas 2010;11(2):163‐9. [PubMed] [Google Scholar]
Tlostanova 2008 {published data only}
- Tlostanova MS, Tiutin LA, Ryzhkova DV, Pavlovskii AV, Popov SA. Role of 18f‐FDG PET scan in the differential diagnosis of large pancreatic masses. Voprosy Onkologii 2008;54(4):439‐44. [PubMed] [Google Scholar]
Togliani 2015 {published data only}
- Togliani T, Mantovani N, Savioli A, Troiano L, Vitetta E, Pilati S. The easy EUS‐FNA. Digestive and Liver Disease 2015;47(Suppl):e151. [Google Scholar]
Touchefeu 2009 {published data only}
- Touchefeu Y, Rhun M, Coron E, Alamdari A, Heymann MF, Mosnier JF, et al. Endoscopic ultrasound‐guided fine‐needle aspiration for the diagnosis of solid pancreatic masses: The impact on patient‐management strategy. Alimentary Pharmacology & Therapeutics 2009;30(10):1070‐7. [DOI] [PubMed] [Google Scholar]
Trifunovic 2004 {published data only}
- Trifunovic J, Muzikravic L, Prvulovic M, Salma S, Nikolin B, Kukic B. Evaluation of imaging techniques and ca 19‐9 in differential diagnosis of carcinoma and other focal lesions of pancreas. Archive of Oncology 2004;12(2):104‐8. [Google Scholar]
Tummala 2013 {published data only}
- Tummala P, Rao S, Agarwal B. Differential diagnosis of focal non‐cystic pancreatic lesions with and without proximal dilation of pancreatic duct noted on CT scan. Clinical and Translational Gastroenterology 2013;4:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turowska 2007 {published data only}
- Turowska A, Lebkowska U, Kubas B, Janica JR, Ladny RJ, Kordecki K. The role of magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP) in the diagnosis and assessment of resectability of pancreatic tumors. Medical Science Monitor 2007;13 Suppl 1:90‐7. [PubMed] [Google Scholar]
Uehara 2011 {published data only}
- Uehara H, Ikezawa K, Kawada N, Fukutake N, Katayama K, Takakura R, et al. Diagnostic accuracy of endoscopic ultrasound‐guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. Journal of Gastroenterology and Hepatology 2011;26(8):1256‐61. [DOI] [PubMed] [Google Scholar]
Uehara 2015 {published data only}
- Uehara H, Sueyoshi H, Takada R, Fukutake N, Katayama K, Ashida R, et al. Optimal number of needle passes in endoscopic ultrasound‐guided fine needle aspiration for pancreatic lesions. Pancreatology 2015;15(4):392‐6. [DOI] [PubMed] [Google Scholar]
Uekitani 2016 {published data only}
- Uekitani T, Kaino S, Harima H, Suenaga S, Sen‐yo M, Sakaida I. Efficacy of contrast‐enhanced harmonic endoscopic ultrasonography in the diagnosis of pancreatic ductal carcinoma. Saudi Journal of Gastroenterology 2016;22(3):198‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valinas 2002 {published data only}
- Valinas R, Barrier A, Montravers F, Houry S, Talbot JN, Huguier M. 18 f‐fluorodeoxyglucose positron emission tomography for characterization and initial staging of pancreatic tumors. Gastroenterologie Clinique et Biologique 2002;26(10):888‐92. [PubMed] [Google Scholar]
Vanbiervliet 2014 {published data only}
- Vanbiervliet G, Napoleon B, Saint Paul MC, Sakarovitch C, Wangermez M, Bichard P, et al. Core needle versus standard needle for endoscopic ultrasound‐guided biopsy of solid pancreatic masses: A randomized crossover study. Endoscopy 2014;46(12):1063‐70. [DOI] [PubMed] [Google Scholar]
van Gulik 1999 {published data only}
- Gulik TM, Moojen TM, Geenen R, Rauws EAJ, Obertop H, Gouma DJ. Differential diagnosis of focal pancreatitis and pancreatic cancer. Annals of Oncology 1999;10(Suppl 4):S85‐8. [PubMed] [Google Scholar]
van Kouwen 2004 {published data only}
- Kouwen MC, Oyen WJ, Nagengast FM, Jansen JB, Drenth JP. FDG‐PET scanning in the diagnosis of gastrointestinal cancers. Scandinavian Journal of Gastroenterology ‐ Supplement 2004;39(241):85‐92. [DOI] [PubMed] [Google Scholar]
van Kouwen 2005 {published data only}
- Kouwen MC, Jansen JB, Goor H, Castro S, Oyen WJ, Drenth JP. FDG‐PET is able to detect pancreatic carcinoma in chronic pancreatitis. European Journal of Nuclear Medicine & Molecular Imaging 2005;32(4):399‐404. [DOI] [PubMed] [Google Scholar]
Varadarajulu 2004a {published data only}
- Varadarajulu S, Eloubeidi MA. Frequency and significance of acute intracystic hemorrhage during EUS‐FNA of cystic lesions of the pancreas. Gastrointestinal Endoscopy 2004;60(4):631‐5. [DOI] [PubMed] [Google Scholar]
Varadarajulu 2004b {published data only}
- Varadarajulu S, Fraig M, Schmulewitz N, Roberts S, Wildi S, Hawes RH, et al. Comparison of EUS‐guided 19‐gauge trucut needle biopsy with EUS‐guided fine‐needle aspiration. Endoscopy 2004;36(5):397‐401. [DOI] [PubMed] [Google Scholar]
Varadarajulu 2014a {published data only}
- Varadarajulu S, Bang JY, Holt BA, Hasan MK, Logue A, Hawes RH, et al. The 25‐gauge EUS‐FNA needle: Good for on‐site but poor for off‐site evaluation? Results of a randomized trial. Gastrointestinal Endoscopy 2014;80(6):1056‐63. [DOI] [PubMed] [Google Scholar]
Varadarajulu 2014b {published data only}
- Varadarajulu S, Holt BA, Hasan M, Bang JY, Logue AL, Hawes R, et al. The 25g FNA needle: Good for onsite but poor for offsite evaluation? Results of a randomized trial. Gastrointestinal Endoscopy 2014;79(5):AB427. [DOI] [PubMed] [Google Scholar]
Vasile 2012 {published data only}
- Vasile TA, Feier D, Socaciu M, Anton OM, Seicean A, Iancu C, et al. Contrast enhanced ultrasound and computer tomography diagnosis of solid and mixed pancreatic tumors ‐ analysis of confounders. Journal of Gastrointestinal and Liver Diseases 2012;21(3):285‐92. [PubMed] [Google Scholar]
Verzola 2000 {published data only}
- Verzola ED, Smalley WE, Sechopoulos P, Mertz HR. Accuracy and implications of endoscopic ultrasound staging in pancreatic adenocarcinoma. Gastrointestinal Endoscopy 2000;51(4):AB170. [Google Scholar]
Vilgrain 1989 {published data only}
- Vilgrain V, Ponsot P, Menu Y, Nahum H. Pancreatic cystadenomas ‐ diagnostic limitations of echography and tomodensitometry. Gastroenterologie Clinique et Biologique 1989;13(2BIS):A57. [Google Scholar]
Vilgrain 1995 {published data only}
- Vilgrain V, Kazerouni F, Anglade M, Agostini SE, Mathieu DG, Valette P. Interobserver agreement and diagnostic‐accuracy of cystic pancreatic lesions at CT. Radiology 1995;197(2 Suppl):378. [Google Scholar]
Vilmann 1995 {published data only}
- Vilmann P, Giovannini M, Siemsen M, Wiersema M. EUS‐guided biopsy of pancreatic lesions suspected of malignancy. A multicentre study. Acta Endoscopica 1995;25(5):465‐71. [Google Scholar]
Virtue 2008 {published data only}
- Virtue MA, Mallery S, Li R, Sielaff TD. Clinical utility of endoscopic ultrasound in solid pancreatic mass lesions deemed resectable by computer tomography. Journal of the Pancreas 2008;9(2):167‐71. [PubMed] [Google Scholar]
Visser 2007 {published data only}
- Visser BC, Yeh BM, Qayyum A, Way LW, McCulloch CE, Coakley FV. Characterization of cystic pancreatic masses: Relative accuracy of CT and MRI. AJR: American Journal of Roentgenology 2007;189(3):648‐56. [DOI] [PubMed] [Google Scholar]
Visser 2008 {published data only}
- Visser BC, Muthusamy VR, Yeh BM, Coakley FV, Way LW. Diagnostic evaluation of cystic pancreatic lesions. HPB 2008;10(1):63‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Voss 2000 {published data only}
- Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O'Toole D, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut 2000;46(2):244‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Votrubova 2005 {published data only}
- Votrubova J, Belohlavek O, Jaruskova M. Evaluation of the role of FDG‐PET and PET/CT in diagnostics of a suspected pancreatic cancer. European Journal of Nuclear Medicine & Molecular Imaging 2005;32:S89. [Google Scholar]
Vullierme 2007 {published data only}
- Vullierme MP, Giraud‐Cohen M, Hammel P, Sauvanet A, Couvelard A, O'Toole D, et al. Malignant intraductal papillary mucinous neoplasm of the pancreas: In situ versus invasive carcinoma surgical resectability. Radiology 2007;245(2):483‐90. [DOI] [PubMed] [Google Scholar]
Wachs 2010 {published data only}
- Wachs S, Faigel D, Morgan T. Improved diagnostic accuracy for pancreatic cystic lesions: The OHSU experience. Cancer Cytopathology 2010;118:380‐1. [Google Scholar]
Wakabayashi 2008 {published data only}
- Wakabayashi H, Nishiyama Y, Otani T, Sano T, Yachida S, Okano K, et al. Role of 18f‐fluorodeoxyglucose positron emission tomography imaging in surgery for pancreatic cancer. World Journal of Gastroenterology 2008;14(1):64‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wakatsuki 2004 {published data only}
- Wakatsuki T, Irisawa A, Hikichi T, Shibukawa G, Takagi TJ, Yamamoto G, et al. A comparative study of diagnostic value of cytologic sampling by EUS‐guided fine needle aspiration and that by ERP for the management of pancreatic mass without biliary stricture. Gastrointestinal Endoscopy 2004;59(5):P230. [DOI] [PubMed] [Google Scholar]
Wakatsuki 2005 {published data only}
- Wakatsuki T, Irisawa A, Bhutani MS, Hikichi T, Shibukawa G, Takagi T, et al. Comparative study of diagnostic value of cytologic sampling by endoscopic ultrasonography‐guided fine‐needle aspiration and that by endoscopic retrograde pancreatography for the management of pancreatic mass without biliary stricture. Journal of Gastroenterology and Hepatology 2005;20(11):1707‐11. [DOI] [PubMed] [Google Scholar]
Walter 2015 {published data only}
- Walter TC, Steffen IG, Stelter LH, Maurer MH, Bahra M, Faber W, et al. Implications of imaging criteria for the management and treatment of intraductal papillary mucinous neoplasms ‐ benign versus malignant findings. European Radiology 2015;25(5):1329‐38. [DOI] [PubMed] [Google Scholar]
Wang 2005 {published data only}
- Wang SE, Shyr YM, Chen TH, Su CH, Hwang TL, Jeng KS, et al. Comparison of resected and non‐resected intraductal papillary mucinous neoplasms of the pancreas. World Journal of Surgery 2005;29(12):1650‐7. [DOI] [PubMed] [Google Scholar]
Wang 2007a {published data only}
- Wang Y, Gao J, Li ZS, Jin ZD, Gong YF, Man XH. Diagnostic value of mucins (MUC1, MUC2 and MUC5AC) expression profile in endoscopic ultrasound‐guided fine‐needle aspiration specimens of the pancreas. International Journal of Cancer 2007;121(12):2716‐22. [DOI] [PubMed] [Google Scholar]
Wang 2007b {published data only}
- Wang ZQ, Lu GM, Chen YX, Wu J, Quan ZF, Li JS. Value of CT enhancement degree in differential diagnosis between pancreatic carcinoma and inflammatory pancreatic mass. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2007;87(16):1120‐2. [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang ZQ, Yang B, Wu J, Liu ZJ, Wu ZC, Liu YX, et al. The study of CT features in pancreatic carcinoma and inflammatory pancreatic mass. Chinese Journal of Radiology 2009;43(6):621‐4. [Google Scholar]
Wang 2011a {published data only}
- Wang DL, Yu LJ, Wang X, Liang XY, Lu PO, Wang WZ. 18f‐FDG PET/CT imaging and diagnosis method of pancreatic carcinoma. Chinese Journal of Medical Imaging Technology 2011;27(1):103‐7. [Google Scholar]
Wang 2011b {published data only}
- Wang X, Gao J, Ren Y, Gu J, Du Y, Chen J, et al. Detection of kras gene mutations in endoscopic ultrasound‐guided fine‐needle aspiration biopsy for improving pancreatic cancer diagnosis. American Journal of Gastroenterology 2011;106(12):2104‐11. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang KX, Sun SY, Sheng J, Zhan XB, Yang AM, Yang XJ, et al. Incidence of hyperamylasemia after endoscopic ultrasound‐guided fine needle aspiration of pancreatic lesions: a multicenter study from China. Pancreas 2012;41(5):712‐6. [DOI] [PubMed] [Google Scholar]
Wani 2011 {published data only}
- Wani S, Gupta N, Gaddam S, Singh V, Ulusarac O, Romanas M, et al. A comparative study of endoscopic ultrasound guided fine needle aspiration with and without a stylet. Digestive Diseases and Sciences 2011;56(8):2409‐14. [DOI] [PubMed] [Google Scholar]
Wani 2012 {published data only}
- Wani S, Early D, Kunkel J, Leathersich A, Hovis CE, Hollander TG, et al. Diagnostic yield of malignancy during EUS‐guided FNA of solid lesions with and without a stylet: A prospective, single blind, randomized, controlled trial. Gastrointestinal Endoscopy 2012;76(2):328‐35. [DOI] [PubMed] [Google Scholar]
Warda 2015 {published data only}
- Warda MHA, Hasan DI, Elteeh OA. Differentiation of pancreatic lesions using diffusion‐weighted MRI. Egyptian Journal of Radiology and Nuclear Medicine 2015;46(3):563‐8. [Google Scholar]
Watanabe 2012 {published data only}
- Watanabe T, Ito T, Yoneda S, Maruyama M, Kodama R, Muraki T, et al. Yields of endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA) for pancreatic lesion, lymph node and gastrointestinal submucosal tumor. Endoscopic Forum for Digestive Disease 2012;28(1):8‐15. [Google Scholar]
Waters 2008 {published data only}
- Waters JA, Schmidt CM, Pinchot JW, White PB, Cummings OW, Pitt HA, et al. CT vs MRCP: Optimal classification of IPMN type and extent. Journal of Gastrointestinal Surgery 2008;12(1):101‐9. [DOI] [PubMed] [Google Scholar]
Waxman 2001 {published data only}
- Waxman I. Endosonography for differentiating benign from malignant intraductal mucinous tumors of the pancreas: Is the jury out?. American Journal of Gastroenterology 2001;96(5):1323‐5. [DOI] [PubMed] [Google Scholar]
Wegener 1995 {published data only}
- Wegener M, Pfaffenbach B, Adamek RJ. Endosographically guided transduodenal and transgastral fine‐needle aspiration puncture of focal pancreatic lesions. Bildgebung [Imaging] 1995;62(2):110‐5. [PubMed] [Google Scholar]
Wiersema 1994 {published data only}
- Wiersema MJ, Kochman ML, Cramer HM, Tao LC, Wiersema LM. Endosonography‐guided real‐time fine‐needle aspiration biopsy. Gastrointestinal Endoscopy 1994;40(6):700‐7. [PubMed] [Google Scholar]
Wiersema 2002 {published data only}
- Wiersema MJ, Jondal ML, Schwartz DA, Clain JE, Levy MJ, Vazquez‐Sequeiros E. Prospective comparison of a 19‐ versus 22‐gauge needle for performing EUS‐FNA of pancreas mass lesions: Assessment of accuracy and factors influencing safety. Gastrointestinal Endoscopy 2002;55(5):AB240. [Google Scholar]
Wiesenauer 2003 {published data only}
- Wiesenauer CA, Schmidt CM, Cummings OW, Yiannoutsos CT, Howard TJ, Wiebke EA, et al. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Archives of Surgery 2003;138(6):610‐8. [DOI] [PubMed] [Google Scholar]
Will 2007 {published data only}
- Will U, Mueller AK, Meyer F. Value of endoscopic ultrasonography (EUS)‐guided fine needle puncture (FNP) in the diagnostic of neoplastic pancreatic tumor lesions. Gastrointestinal Endoscopy 2007;65(5):AB310. [Google Scholar]
Will 2008 {published data only}
- Will U, Mueller AK, Meyer F. Impact of endoscopic ultrasonography (EUS)‐guided fine needle aspiration (FNA) to the diagnostic of suspicious tumor lesions of the pancreas and peripancreatic lymph nodes. Onkologie 2008;31(Suppl 1):83‐4. [Google Scholar]
Will 2010 {published data only}
- Will U, Mueller A, Topalidis T, Meyer F. Value of endoscopic ultrasonography‐guided fine needle aspiration (FNA) in the diagnosis of neoplastic tumor(‐like) pancreatic lesions in daily clinical practice. Ultraschall in der Medizin 2010;31(2):169‐74. [DOI] [PubMed] [Google Scholar]
Williams 1999 {published data only}
- Williams DB, Sahai AV, Aabakken L, Penman ID, Velse A, Webb J, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut 1999;44(5):720‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2009 {published data only}
- Wilson JL, Kalade A, Prasad S, Cade R, Thomson B, Banting S, et al. Diagnosis of solid pancreatic masses by endoscopic ultrasound‐guided fine‐needle aspiration. Internal Medicine Journal 2009;39(1):32‐7. [DOI] [PubMed] [Google Scholar]
Winner 2015 {published data only}
- Winner M, Sethi A, Poneros JM, Stavropoulos SN, Francisco P, Lightdale CJ, et al. The role of molecular analysis in the diagnosis and surveillance of pancreatic cystic neoplasms. Journal of the Pancreas 2015;16(2):143‐9. [DOI] [PubMed] [Google Scholar]
Wittmann 2006 {published data only}
- Wittmann J, Kocjan G, Sgouros SN, Deheragoda M, Pereira SP. Endoscopic ultrasound‐guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: A prospective study. Cytopathology 2006;17(1):27‐33. [DOI] [PubMed] [Google Scholar]
Woolf 2013 {published data only}
- Woolf KM, Liang H, Sletten ZJ, Russell DK, Bonfiglio TA, Zhou Z. False‐negative rate of endoscopic ultrasound‐guided fine‐needle aspiration for pancreatic solid and cystic lesions with matched surgical resections as the gold standard: One institution's experience. Cancer Cytopathology 2013;121(8):449‐58. [DOI] [PubMed] [Google Scholar]
Wright 2014 {published data only}
- Wright GP, Morrow JB, Shaheen M, Goslin BJ, Baatenburg L, Chung MH. Accuracy of endoscopic ultrasound in the evaluation of cystic pancreatic neoplasms: A community hospital experience. Pancreas 2014;43(3):465‐9. [DOI] [PubMed] [Google Scholar]
Wu 2007a {published data only}
- Wu H, Cheng NS, Zhang YG, Luo HZ, Yan LN, Li J. Improved early diagnosis of cystadenocarcinoma of the pancreas. Hepatobiliary & Pancreatic Diseases International 2007;6(1):87‐91. [PubMed] [Google Scholar]
Wu 2007b {published data only}
- Wu H, Yan LN, Cheng NS, Zhang YG, Ker CG. Role of cystic fluid in diagnosis of the pancreatic cystadenoma and cystadenocarcinoma. Hepato‐Gastroenterology 2007;54(79):1915‐8. [PubMed] [Google Scholar]
Wu 2013 {published data only}
- Wu WC, Yao XZ, Jin DY, Wang DS, Lou WH, Qin XY. Clinical strategies for differentiating autoimmune pancreatitis from pancreatic malignancy to avoid unnecessary surgical resection. Journal of Digestive Diseases 2013;14(9):500‐8. [DOI] [PubMed] [Google Scholar]
Wu 2014 {published data only}
- Wu BU, Sampath K, Berberian CE, Kwok KK, Lim BS, Kao KT, et al. Prediction of malignancy in cystic neoplasms of the pancreas: A population‐based cohort study. American Journal of Gastroenterology 2014;109(1):121‐9; quiz 130. [DOI] [PubMed] [Google Scholar]
Wyse 2009 {published data only}
- Wyse JM, Paquin SC, Joseph L, Sahai A. EUS‐FNA without the stylet: The yield is comparable to that with the stylet and sampling of multiple sites during the same pass may improve sample quality and yield. Gastrointestinal Endoscopy 2009;69(5):AB330‐1. [Google Scholar]
Xiao 2009 {published data only}
- Xiao GQ. Fine‐needle aspiration of cystic pancreatic mucinous tumor: Oncotic cell as an aiding diagnostic feature in paucicellular specimens. Diagnostic Cytopathology 2009;37(2):111‐6. [DOI] [PubMed] [Google Scholar]
Xu 2012 {published data only}
- Xu K, Xu P, Ren DB, Li QH, Yang J, Yu HB. EUS elastography for the differential diagnosis of pancreatic masses. World Chinese Journal of Digestology 2012;20(5):425‐9. [Google Scholar]
Xu 2013 {published data only}
- Xu B, Ding WX, Jin DY, Wang DS, Lou WH. Decision making for pancreatic resection in patients with intraductal papillary mucinous neoplasms. World Journal of Gastroenterology 2013;19(9):1451‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2014 {published data only}
- Xu P, Xu M. Clinical feature, diagnosis and treatment of pancreatic cystic lesion. Chinese Journal of Gastroenterology 2014;19(1):40‐2. [Google Scholar]
Yamada 2010a {published data only}
- Yamada Y, Mori H, Matsumoto S, Hijiya N, Hongo N, Moriyama M. Invasive carcinomas originating from intraductal papillary mucinous neoplasms of the pancreas: Conspicuity and primary sites of the solid masses on triple‐phase dynamic CT imaging. Abdominal Imaging 2010;35(2):181‐8. [DOI] [PubMed] [Google Scholar]
Yamada 2010b {published data only}
- Yamada Y, Mori H, Matsumoto S, Kiyosue H, Hori Y, Hongo N. Pancreatic adenocarcinoma versus chronic pancreatitis: Differentiation with triple‐phase helical CT. Abdominal Imaging 2010;35(2):163‐71. [DOI] [PubMed] [Google Scholar]
Yamaguchi 1990 {published data only}
- Yamaguchi K, Hirakata R, Kitamura K. Mucinous cystic neoplasm of the pancreas. Estimation of grade of malignancy with imaging techniques and its surgical implications. Acta Chirurgica Scandinavica 1990;156(8):553‐64. [PubMed] [Google Scholar]
Yamao 2003 {published data only}
- Yamao K, Nakamura T, Suzuki T, Sawaki A, Hara K, Kato T, et al. Endoscopic diagnosis and staging of mucinous cystic neoplasms and intraductal papillary‐mucinous tumors. Journal of Hepato‐Biliary‐Pancreatic Surgery 2003;10(2):142‐6. [DOI] [PubMed] [Google Scholar]
Yamashita 2015 {published data only}
- Yamashita Y, Kato J, Ueda K, Nakamura Y, Kawaji Y, Abe H, et al. Contrast‐enhanced endoscopic ultrasonography for pancreatic tumors. BioMed Research International 2015;2015:491782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2014 {published data only}
- Yan L, Chen Y, Zhang W, Huang X, Chen M, Li Y, et al. Clinicopathological and CT features of mucinous cystic neoplasms of the pancreas. Zhonghua zhong liu za zhi 2014;36(6):446‐50. [PubMed] [Google Scholar]
Yang 2014 {published data only}
- Yang D, MoezArdalan K, Collins DP, Chauhan SS, Draganov PV, Forsmark CE, et al. Predictors of malignancy in patients with suspicious or indeterminate cytology on pancreatic endoscopic ultrasound‐guided fine‐needle aspiration: A multivariate model. Pancreas 2014;43(6):922‐6. [DOI] [PubMed] [Google Scholar]
Yang 2015a {published data only}
- Yang SS, Liao SC, Ko CW, Tung CF, Peng YC, Lien HC, et al. The clinical efficacy and safety of EUS‐FNA for diagnosis of mediastinal and abdominal solid tumors ‐ a single center experience. Advances in Digestive Medicine 2015;2(2):61‐6. [Google Scholar]
Yang 2015b {published data only}
- Yang MJ, Yim H, Hwang JC, Lee D, Kim YB, Lim SG, et al. Endoscopic ultrasound‐guided sampling of solid pancreatic masses: 22‐gauge aspiration versus 25‐gauge biopsy needles. BMC Gastroenterology 2015;15:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yantiss 2008 {published data only}
- Yantiss RK, Cosar E, Fischer AH. Use of IMP3 in identification of carcinoma in fine needle aspiration biopsies of pancreas. Acta Cytologica 2008;52(2):133‐8. [DOI] [PubMed] [Google Scholar]
Yao 2012 {published data only}
- Yao J, Gan G, Farlow D, Laurence JM, Hollands M, Richardson A, et al. Impact of f18‐fluorodeoxyglycose positron emission tomography/computed tomography on the management of resectable pancreatic tumours. ANZ Journal of Surgery 2012;82(3):140‐4. [DOI] [PubMed] [Google Scholar]
Yeh 1999 {published data only}
- Yeh TS, Cheng AJ, Chen TC, Jan YY, Hwang TL, Jeng LB, et al. Telomerase activity is a useful marker to distinguish malignant pancreatic cystic tumors from benign neoplasms and pseudocysts. Journal of Surgical Research 1999;87(2):171‐7. [DOI] [PubMed] [Google Scholar]
Yim 2005 {published data only}
- Yim HB, Yap WM, Chong PY. Clinical usefulness of endoscopic ultrasonography with or without fine needle aspiration in the diagnosis and staging of pancreatic carcinoma. Annals of the Academy of Medicine, Singapore 2005;34(1):124‐9. [PubMed] [Google Scholar]
Yin 2012 {published data only}
- Yin QH, Wang ML, Wang CS, Wu ZY, Yuan F, Chen K, et al. Differentiation between benign and malignant solid pseudopapillary tumor of the pancreas by MDCT. European Journal of Radiology 2012;81(11):3010‐8. [DOI] [PubMed] [Google Scholar]
Yin 2015 {published data only}
- Yin QH, Zou XN, Zai XD, Wu ZY, Wu QY, Jiang XY, et al. Pancreatic ductal adenocarcinoma and chronic mass‐forming pancreatitis: Differentiation with dual‐energy MDCT in spectral imaging mode. European Journal of Radiology 2015;84(12):2470‐6. [DOI] [PubMed] [Google Scholar]
Ylagan 2002 {published data only}
- Ylagan LR, Edmundowicz S, Kasal K, Walsh D, Lu DW. Endoscopic ultrasound guided fine‐needle aspiration cytology of pancreatic carcinoma ‐ a 3‐year experience and review of the literature. Cancer Cytopathology 2002;96(6):362‐9. [DOI] [PubMed] [Google Scholar]
Yoshioka 2015 {published data only}
- Yoshioka M, Uchinami H, Watanabe G, Sato T, Shibata S, Kume M, et al. F‐18 fluorodeoxyglucose positron emission tomography for differential diagnosis of pancreatic tumors. SpringerPlus 2015;4:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuan 2007 {published data only}
- Yuan D, Yu W, Ren XB, Pan WD, Zhang LH. Characterization and diagnostic accuracy of serous cystadenomas and mucinous neoplasms of the pancreas with multi‐slice helical computed tomography. Chung‐Kuo i Hsueh Ko Hsueh Yuan Hsueh Pao [Acta Academiae Medicinae Sinicae] 2007;29(2):232‐7. [PubMed] [Google Scholar]
Yun 2007 {published data only}
- Yun SS, Remotti H, Vazquez MF, Crapanzano JP, Saqi A. Endoscopic ultrasound‐guided biopsies of pancreatic masses: Comparison between fine needle aspirations and needle core biopsies. Diagnostic Cytopathology 2007;35(5):276‐82. [DOI] [PubMed] [Google Scholar]
Yusuf 2009 {published data only}
- Yusuf TE, Ho S, Pavey DA, Michael H, Gress FG. Retrospective analysis of the utility of endoscopic ultrasound‐guided fine‐needle aspiration (EUS‐FNA) in pancreatic masses, using a 22‐gauge or 25‐gauge needle system: A multicenter experience [erratum appears in Endoscopy 2009;41(6):509]. Endoscopy 2009;41(5):445‐8. [DOI] [PubMed] [Google Scholar]
Zamboni 2012 {published data only}
- Zamboni GA, Bernardin L, Mucelli RP. Dynamic MDCT of the pancreas: Is time‐density curve morphology useful for the differential diagnosis of solid lesions? A preliminary report. European Journal of Radiology 2012;81(3):E381‐5. [DOI] [PubMed] [Google Scholar]
Zaruba 2013 {published data only}
- Zaruba P, Dvorakova T, Zavada F, Belina F, Ryska M. Is accurate preoperative assessment of pancreatic cystic lesions possible?. Rozhledy V Chirurgii 2013;92(12):708‐14. [PubMed] [Google Scholar]
Zdanyte 2004 {published data only}
- Zdanyte E, Strupas K, Bubnys A, Stratilatovas E. Difficulties of differential diagnosis of pancreatic pseudocysts and cystic neoplasms. Medicina (Kaunas, Lithuania) 2004;40(12):1180‐8. [PubMed] [Google Scholar]
Zeiderman 1991 {published data only}
- Zeiderman MR, Wyman A, Euinton HA, Simms JM, Rogers K. Diagnostic difficulties in patients with a pancreatic mass. BMJ 1991;302(6789):1395‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2010a {published data only}
- Zhang HY, Cunningham J, Bhattacharyya A. Diagnostic accuracy of endoscopic ultrasound‐guided fine needle aspiration of pancreatic lesions: A 7‐year experience at one institution. Cancer Cytopathology 2010;118(5):383. [Google Scholar]
Zhang 2010b {published data only}
- Zhang J, Wang PJ, Yuan XD. Correlation between CT patterns and pathological classification of intraductal papillary mucinous neoplasm. European Journal of Radiology 2010;73(1):96‐101. [DOI] [PubMed] [Google Scholar]
Zhang 2010c {published data only}
- Zhang MM, Yang H, Jin ZD, Yu JG, Cai ZY, Li ZS. Differential diagnosis of pancreatic cancer from normal tissue with digital imaging processing and pattern recognition based on a support vector machine of EUS images. Gastrointestinal Endoscopy 2010;72(5):978‐85. [DOI] [PubMed] [Google Scholar]
Zhang 2010d {published data only}
- Zhang S, Defrias DV, Alasadi R, Nayar R. Endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA): Experience of an academic centre in the USA. Cytopathology 2010;21(1):35‐43. [DOI] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang HM, Yao F, Liu GF, Wang XB, Xiu DH, Gen I. The differences in imaging features of malignant and benign branch duct type of intraductal papillary mucinous tumor. European Journal of Radiology 2011;80(3):744‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang Y, Frampton AE, Martin JL, Kyriakides C, Bong JJ, Habib NA, et al. 18f‐fluorodeoxyglucose positron emission tomography in management of pancreatic cystic tumors. Nuclear Medicine & Biology 2012;39(7):982‐5. [DOI] [PubMed] [Google Scholar]
Zhang 2015 {published data only}
- Zhang J, Zuo CJ, Jia NY, Wang JH, Hu SP, Yu ZF, et al. Cross‐modality PET/CT and contrast‐enhanced CT imaging for pancreatic cancer. World Journal of Gastroenterology 2015;21(10):2988‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhong 2012 {published data only}
- Zhong N, Zhang L, Takahashi N, Shalmiyev V, Canto MI, Clain JE, et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clinical Gastroenterology and Hepatology 2012;10(2):192‐8, 8.e1‐2. [DOI] [PubMed] [Google Scholar]
Zhu 2008 {published data only}
- Zhu LC, Grieco V. Diagnostic value of unusual gross appearance of aspirated material from endoscopic ultrasound‐guided fine needle aspiration of pancreatic and peripancreatic cystic lesions. Acta Cytologica 2008;52(5):535‐40. [DOI] [PubMed] [Google Scholar]
Zhu 2013 {published data only}
- Zhu ML, Xu C, Yu JG, Wu YJ, Li CG, Zhang MM, et al. Differentiation of pancreatic cancer and chronic pancreatitis using computer‐aided diagnosis of endoscopic ultrasound (EUS) images: A diagnostic test. PLoS ONE 2013;8(5):e63820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziak 2011 {published data only}
- Ziak D, Dvorackova J, Uvirova M, Polaskova J, Cegan M, Ceganova L, et al. Endoscopic ultrasound‐guided fine needle aspiration cytological diagnosis of cystic lesions of the pancreas. Cytopathology 2011;22:174‐5. [Google Scholar]
Zimny 1997 {published data only}
- Zimny M, Bares R, Fass J, Adam G, Cremerius U, Dohmen B, et al. Fluorine‐18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: A report of 106 cases. European Journal of Nuclear Medicine 1997;24(6):678‐82. [DOI] [PubMed] [Google Scholar]
Zimny 1998 {published data only}
- Zimny M, Buell U, Diederichs CG, Reske SN. False‐positive FDG PET in patients with pancreatic masses: An issue of proper patient selection? [1]. European Journal of Nuclear Medicine 1998;25(9):1352. [PubMed] [Google Scholar]
Zimny 1999 {published data only}
- Zimny M, Buell U. 18FDG‐positron emission tomography in pancreatic cancer. Annals of Oncology 1999;10(Suppl 4):S28‐32. [PubMed] [Google Scholar]
Zubarik 2004 {published data only}
- Zubarik R. Predictors of accuracy in patients undergoing endoscopic ultrasound (EUS) guided fine needle aspirate (FNA) of the pancreas. Gastrointestinal Endoscopy 2004;59(5):P226. [Google Scholar]
Zyrek‐Betts 2008 {published data only}
- Zyrek‐Betts J, Husain M, Giorgadze T, Miac, Dhar R, Feng J. Non‐diagnostic EUS‐FNA of the pancreas: An institutional study. Cancer Cytopathology 2008;114(5):425‐6. [Google Scholar]
Additional references
Banafea 2016
- Banafea O, Mghanga FP, Zhao J, Zhao R, Zhu L. Endoscopic ultrasonography with fine‐needle aspiration for histological diagnosis of solid pancreatic masses: a meta‐analysis of diagnostic accuracy studies. BMC Gastroenterology 2016;16:108. [PUBMED: 27580856] [DOI] [PMC free article] [PubMed] [Google Scholar]
Batcher 2011
- Batcher E, Madaj P, Gianoukakis AG. Pancreatic neuroendocrine tumors. Endocrine Research 2011;36(1):35‐43. [DOI] [PubMed] [Google Scholar]
Benson 2010
- Benson ME, Byrne S, Brust DJ, Manning B 3rd, Pfau PR, Frick TJ, et al. EUS and ERCP complication rates are not increased in elderly patients. Digestive Diseases and Sciences 2010;55(11):3278‐83. [DOI] [PubMed] [Google Scholar]
Braganza 2011
- Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet 2011;377(9772):1184‐97. [DOI] [PubMed] [Google Scholar]
Chen 2012
- Chen J, Yang R, Lu Y, Xia Y, Zhou H. Diagnostic accuracy of endoscopic ultrasound‐guided fine‐needle aspiration for solid pancreatic lesion: a systematic review. Journal of Cancer Research and Clinical Oncology 2012;138(9):1433‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2011
- Cho HW, Choi JY, Kim MJ, Park MS, Lim JS, Chung YE, et al. Pancreatic tumors: emphasis on CT findings and pathologic classification. Korean Journal of Radiology 2011;12(6):731‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta‐analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331‐2. [DOI] [PubMed] [Google Scholar]
Conlon 1996
- Conlon KC, Klimstra DS, Brennan MF. Long‐term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5‐year survivors. Annals of Surgery 1996;223(3):273‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cui 2009
- Cui Y, Tian M, Zong M, Teng M, Chen Y, Lu J, et al. Proteomic analysis of pancreatic ductal adenocarcinoma compared with normal adjacent pancreatic tissue and pancreatic benign cystadenoma. Pancreatology 2009;9(1‐2):89‐98. [DOI] [PubMed] [Google Scholar]
Dill 2008
- Dill T. Contraindications to magnetic resonance imaging: non‐invasive imaging. Heart 2008;94(7):943‐8. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2001
- Eloubeidi MA, Wade SB, Provenzale D. Factors associated with acceptance and full publication of GI endoscopic research originally published in abstract form. Gastrointestinal Endoscopy 2001;53(3):275‐82. [DOI] [PubMed] [Google Scholar]
Engelken 2003
- Engelken FJ, Bettschart V, Rahman MQ, Parks RW, Garden OJ. Prognostic factors in the palliation of pancreatic cancer. European Journal of Surgical Oncology 2003;29(4):368‐73. [DOI] [PubMed] [Google Scholar]
Fred 2004
- Fred HL. Drawbacks and limitations of computed tomography: views from a medical educator. Texas Heart Institute Journal 2004; Vol. 31, issue 4:345‐8. [PMC free article] [PubMed]
Fuccio 2013
- Fuccio L, Hassan C, Laterza L, Correale L, Pagano N, Bocus P, et al. The role of K‐ras gene mutation analysis in EUS‐guided FNA cytology specimens for the differential diagnosis of pancreatic solid masses: a meta‐analysis of prospective studies. Gastrointestinal Endoscopy 2013;78(4):596‐608. [DOI] [PubMed] [Google Scholar]
Gaujoux 2011
- Gaujoux S, Brennan MF, Gonen M, D'Angelica MI, Dematteo R, Fong Y, et al. Cystic lesions of the pancreas: changes in the presentation and management of 1,424 patients at a single institution over a 15‐year time period. Journal of the American College of Surgeons 2011;212(4):590‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillis 2015
- Gillis A, Cipollone I, Cousins G, Conlon K. Does EUS‐FNA molecular analysis carry additional value when compared to cytology in the diagnosis of pancreatic cystic neoplasm? A systematic review. HPB 2015;17(5):377‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giovannini 2012
- Giovannini M, Caillol F, Poizat F, Bories E, Pesenti C, Monges G, et al. Feasibility of intratumoral confocal microscopy under endoscopic ultrasound guidance. Endoscopic Ultrasound 2012;1(2):80‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goh 2006b
- Goh BK, Tan YM, Cheow PC, Chung YF, Chow PK, Wong WK, et al. Cystic lesions of the pancreas: an appraisal of an aggressive resectional policy adopted at a single institution during 15 years. American Journal of Surgery 2006;192(2):148‐54. [DOI] [PubMed] [Google Scholar]
Gurusamy 2013
- Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008370.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hancke 1984
- Hancke S, Holm HH, Koch F. Ultrasonically guided puncture of solid pancreatic mass lesions. Ultrasound in Medicine and Biology 1984;10(5):613‐5. [DOI] [PubMed] [Google Scholar]
Haynes 2004
- Haynes RB, Wilczynski NL. Optimal search strategies for retrieving scientifically strong studies of diagnosis from MEDLINE: Analytical survey. BMJ 2004;328(7447):1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hewitt 2012
- Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS‐guided FNA for diagnosis of solid pancreatic neoplasms: a meta‐analysis. Gastrointestinal Endoscopy 2012;75(2):319‐31. [DOI] [PubMed] [Google Scholar]
Holly 2004
- Holly EA, Chaliha I, Bracci PM, Gautam M. Signs and symptoms of pancreatic cancer: a population‐based case‐control study in the San Francisco Bay Area. Clinical Gastroenterology and Hepatology 2004;2(6):510‐7. [DOI] [PubMed] [Google Scholar]
Horsley 2011
- Horsley T, Dingwall O, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.MR000026] [DOI] [PMC free article] [PubMed] [Google Scholar]
Irie 2004
- Irie H, Yoshimitsu K, Aibe H, Tajima T, Nishie A, Nakayama T, et al. Natural history of pancreatic intraductal papillary mucinous tumor of branch duct type: follow‐up study by magnetic resonance cholangiopancreatography. Journal of Computer Assisted Tomography 2004;28(1):117‐22. [14716244] [DOI] [PubMed] [Google Scholar]
Katz 2009
- Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Long‐term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Annals of Surgical Oncology 2009;16(4):836‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klempnauer 1995
- Klempnauer J, Ridder GJ, Pichlmayr R. Prognostic factors after resection of ampullary carcinoma: multivariate survival analysis in comparison with ductal cancer of the pancreatic head. British Journal of Surgery 1995;82(12):1686‐91. [DOI] [PubMed] [Google Scholar]
Lee 2005c
- Lee SY, Lee KT, Lee JK, Jeon YH, Choi D, Lim JH, et al. Long‐term follow up results of intraductal papillary mucinous tumors of pancreas. Journal of Gastroenterology and Hepatology 2005;20(9):1379‐84. [DOI] [PubMed] [Google Scholar]
Leide‐Svegborn 2010
- Leide‐Svegborn S. Radiation exposure of patients and personnel from a PET/CT procedure with 18F‐FDG. Radiation Protection Dosimetry 2010;139(1‐3):208‐13. [DOI] [PubMed] [Google Scholar]
Luttges 2011
- Luttges J. What's new? The 2010 WHO classification for tumours of the pancreas. Pathologe 2011;32 Suppl 2:332‐6. [DOI] [PubMed] [Google Scholar]
Mehta 2010
- Mehta N, Modi L, Patel T, Shah M. Study of cytomorphology of solid pseudopapillary tumor of pancreas and its differential diagnosis. Journal of Cytology 2010;27(4):118‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mei 2013
- Mei M, Ni J, Liu D, Jin P, Sun L. EUS elastography for diagnosis of solid pancreatic masses: a meta‐analysis. Gastrointestinal Endoscopy 2013;77(4):578‐89. [DOI] [PubMed] [Google Scholar]
Micames 2003
- Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS‐guided FNA vs. percutaneous FNA. Gastrointestinal Endoscopy 2003;58(5):690‐5. [DOI] [PubMed] [Google Scholar]
Michelassi 1989
- Michelassi F, Erroi F, Dawson PJ, Pietrabissa A, Noda S, Handcock M, et al. Experience with 647 consecutive tumors of the duodenum, ampulla, head of the pancreas, and distal common bile duct. Annals of Surgery 1989;210(4):544‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2007
- Nair RJ, Lawler L, Miller MR. Chronic pancreatitis. American Family Physician 2007;76(11):1679‐88. [PubMed] [Google Scholar]
Namasivayam 2006
- Namasivayam S, Kalra MK, Torres WE, Small WC. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emergency Radiology 2006;12(5):210‐5. [DOI] [PubMed] [Google Scholar]
National Cancer Institute 2011a
- National Cancer Institute (U.S. National Institutes of Health). Dictionary of cancer terms. CT scan. www.cancer.gov/dictionary?CdrID=46033 (accessed 25 September 2012).
National Cancer Institute 2011b
- National Cancer Institute (U.S. National Institutes of Health). Dictionary of cancer terms. Magnetic resonance imaging. www.cancer.gov/dictionary?CdrID=45997 (accessed 25 September 2012).
National Cancer Institute 2011c
- National Cancer Institute (U.S. National Institutes of Health). Dictionary of cancer terms. Positron emission tomography. www.cancer.gov/dictionary?CdrID=46218 (accessed 25 September 2012).
National Cancer Institute 2011d
- National Cancer Institute (U.S. National Institutes of Health). Dictionary of cancer terms. Endoscopic ultrasound. www.cancer.gov/dictionary?CdrID=46602 (accessed 25 September 2012).
National Cancer Institute 2011e
- National Cancer Institute (U.S. National Institutes of Health). Dictionary of cancer terms. Biopsy. www.cancer.gov/dictionary?CdrID=335081 (accessed 25 September 2012).
National Cancer Institute 2011f
- National Cancer Institute (U.S. National Institutes of Health). Dictionary of cancer terms. CA 19‐9. www.cancer.gov/dictionary?CdrID=633729 (accessed 25 September 2012).
Niv 2011
- Niv Y, Gershtansky Y, Kenett RS, Tal Y, Birkenfeld S. Complications in endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS): analysis of 7‐year physician‐reported adverse events. Drug, Healthcare and Patient Safety 2011;3:21‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pereira 2015
- Pereira SP. A phase II multicentre trial of endoscopic ultrasound guided radiofrequency ablation of cystic tumours of the pancreas (RADIOCYST01). clinicaltrials.gov/ct2/show/NCT02343692 (first received 12 January 2015).
Porta 2005
- Porta M, Fabregat X, Malats N, Guarner L, Carrato A, Miguel A, et al. Exocrine pancreatic cancer: Symptoms at presentation and their relation to tumour site and stage. Clinical and Translational Oncology 2005;7(5):189‐97. [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
Sachs 2009
- Sachs T, Pratt WB, Callery MP, Vollmer CM Jr. The incidental asymptomatic pancreatic lesion: nuisance or threat?. Journal of Gastrointestinal Surgery 2009;13(3):405‐15. [DOI] [PubMed] [Google Scholar]
Sampson 2008
- Sampson M, Shojania KG, McGowan J, Daniel R, Rader T, Iansavichene AE, et al. Surveillance search techniques identified the need to update systematic reviews. Journal of Clinical Epidemiology 2008;61(8):755‐62. [DOI] [PubMed] [Google Scholar]
Shahrudin 1997
- Shahrudin MD. Carcinoma of the pancreas: resection outcome at the University Hospital Kuala Lumpur. International Surgery 1997;82(3):269‐74. [PubMed] [Google Scholar]
Smith 2008
- Smith RA, Bosonnet L, Ghaneh P, Sutton R, Evans J, Healey P, et al. The platelet‐lymphocyte ratio improves the predictive value of serum CA19‐9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery 2008;143(5):658‐66. [DOI] [PubMed] [Google Scholar]
Spinelli 2004
- Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, et al. Cystic pancreatic neoplasms: observe or operate. Annals of Surgery 2004;239(5):651‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takwoingi 2012
- Takwoingi Y, Deeks JJ. Software for meta‐analysis of DTA studies. methods.cochrane.org/sdt/software‐meta‐analysis‐dta‐studies 2010 (accessed 8 April 2016).
Takwoingi 2015
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta‐analysis of diagnostic test accuracy with few studies or sparse data. Statistical methods in medical research 2015 Jun 26 [Epub ahead of print]. [DOI: 10.1177/0962280215592269] [DOI] [PMC free article] [PubMed]
van der Gaag 2010
- Gaag NA, Rauws EA, Eijck CH, Bruno MJ, Harst E, Kubben FJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. New England Journal of Medicine 2010;362(2):129‐37. [DOI] [PubMed] [Google Scholar]
van Oost 2006
- Oost FJ, Luiten EJ, Poll‐Franse LV, Coebergh JW, Eijnden‐van Raaij AJ. Outcome of surgical treatment of pancreatic, peri‐ampullary and ampullary cancer diagnosed in the south of the Netherlands: a cancer registry based study. European Journal of Surgical Oncology 2006;32(5):548‐52. [DOI] [PubMed] [Google Scholar]
Whiting 2006
- Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Medical Research Methodology 2006;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
WHO 2016
- World Health Organization. WHO Classification of Tumours of the Digestive System: C25 ‐ Pancreas. www.pubcan.org/searchresults.php?topo=c25&action=search (accessed 8 April 2016).
Wilczynski 2005
- Wilczynski NL, Haynes RB. Embase search strategies for identifying methodologically sound diagnostic studies for use by clinicians and researchers. BMC Medicine 2005;3(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]


