Abstract
Background
Changing population demographics have led to an increasing number of functionally dependent older people who require care and medical treatment. In many countries, government policy aims to shift resources into the community from institutional care settings with the expectation that this will reduce costs and improve the quality of care compared.
Objectives
To assess the effects of long‐term home or foster home care versus institutional care for functionally dependent older people.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Library, MEDLINE, Embase, CINAHL, and two trials registers to November 2015.
Selection criteria
We included randomised and non‐randomised trials, controlled before‐after studies and interrupted time series studies complying with the EPOC study design criteria and comparing the effects of long‐term home care versus institutional care for functionally dependent older people.
Data collection and analysis
Two reviewers independently extracted data and assessed the risk of bias of each included study. We reported the results narratively, as the substantial heterogeneity across studies meant that meta‐analysis was not appropriate.
Main results
We included 10 studies involving 16,377 participants, all of which were conducted in high income countries. Included studies compared community‐based care with institutional care (care homes). The sample size ranged from 98 to 11,803 (median N = 204). There was substantial heterogeneity in the healthcare context, interventions studied, and outcomes assessed. One study was a randomised trial (N = 112); other included studies used designs that had potential for bias, particularly due lack of randomisation, baseline imbalances, and non‐blinded outcome assessment. Most studies did not select (or exclude) participants for any specific disease state, with the exception of one study that only included patients if they had a stroke. All studies had methodological limitations, so readers should interpret results with caution.
It is uncertain whether long‐term home care compared to nursing home care decreases mortality risk (2 studies, N = 314, very‐low certainty evidence). Estimates ranged from a nearly three‐fold increased risk of mortality in the homecare group (risk ratio (RR) 2.89, 95% confidence interval (CI) 1.57 to 5.32) to a 62% relative reduction (RR 0.38, 95% CI 0.17 to 0.61). We did not pool data due to the high degree of heterogeneity (I2 = 94%).
It is uncertain whether the intervention has a beneficial effect on physical function, as the certainty of evidence is very low (5 studies, N = 1295). Two studies reported that participants who received long‐term home care had improved activities of daily living compared to those in a nursing home, whereas a third study reported that all participants performed equally on physical function.
It is uncertain whether long‐term home care improves happiness compared to nursing home care (RR 1.97, 95% CI 1.27 to 3.04) or general satisfaction because the certainty of evidence was very low (2 studies, N = 114).
The extent to which long‐term home care was associated to more or fewer adverse health outcomes than nursing home care was not reported.
It is uncertain whether long‐term home care compared to nursing home care decreases the risk of hospital admission (very low‐certainty evidence, N = 14,853). RR estimates ranged from 2.75 (95% CI 2.59 to 2.92), showing an increased risk for those receiving care at home, to 0.82 (95% CI 0.72 to 0.93), showing a slightly reduced risk for the same group. We did not pool data due to the high degree of heterogeneity (I2 = 99%).
Authors' conclusions
There are insufficient high‐quality published data to support any particular model of care for functionally dependent older people. Community‐based care was not consistently beneficial across all the included studies; there were some data suggesting that community‐based care may be associated with improved quality of life and physical function compared to institutional care. However, community alternatives to institutional care may be associated with increased risk of hospitalisation. Future studies should assess healthcare utilisation, perform economic analysis, and consider caregiver burden.
Plain language summary
Home or foster home alternatives to institutional long‐term care for functionally dependent older people
What is the aim of this review?
The aim of this Cochrane Review was to assess the effects of home or foster home alternatives to institutional care for older people who depend on others for their care.
Key messages
The studies included different participants and healthcare settings, as well as different interventions. Some of the studies were poorly conducted, which means we have to be careful when interpreting our results.
At present, there is insufficient evidence to support recommendations for home‐based alternatives to institutional long‐term care for frail older people.
What we studied in the review
In many countries, frail older adults with different illnesses may receive long‐term care in nursing homes or other institutions. Due to the increasing number of older adults and the costs associated with care homes, other ways of providing care are necessary, including extra care in a person's own home. We assessed studies that provided care at home versus care in an institution.
What are the main results of the review?
We included 10 studies that took place in five different countries (USA, Taiwan, Sweden, the UK, and Canada). The studies included 16,377 older people thought to be in need of care services. All studies compared some form of home care setting with long‐term institutional care. Most studies involved people with several different conditions, with the exception of one study that only included participants who had a stroke.
We are uncertain whether long‐term home care compared to nursing home care decreases the risk of mortality or hospital admission as the evidence was very low‐certainty. Likewise, we are uncertain whether the intervention increases physical function or quality of life, as again the evidence was considered to be low‐certainty. We could not find papers that reported adverse health outcomes.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to November 2015.
Summary of findings
for the main comparison.
| Home or foster home versus institutional long‐term care for functionally dependent older people | ||||
|
Patient or population: older adults with functional dependence Settings: long‐term care Intervention: long‐term home care Comparison: long‐term institutional care | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Certainty of evidence (GRADE) | Comments |
| Mortality (6 months) | It is uncertain whether long‐term home care compared to nursing home care decreases mortality risk Estimates ranged from a relative increase in risk of mortality of RR 2.89 (95% CI 1.57 to 5.32) to a relative reduction in risk of RR 0.38 (95% CI 0.17 to 0.61). |
314* (2 studies) | ⊕⊝⊝⊝ Very lowa,b,c | Data were not pooled due to the high degree of statistical heterogeneity for this outcome (I2 = 94%) |
|
Physical function (3‐6 months) |
It is uncertain whether long‐term home care compared to nursing home care improves physical function Estimates ranged from and improvement in activities of daily living of MD −0.25 points (95% CI −0.44 to −0.06) to MD −1.90 (95% CI −2.18 to −1.62) |
1295* (5 studies) | ⊕⊝⊝⊝ Very lowa,b,c | 3 studies reporting data used the Katz Index of ADLs or a variation of this measure 2 studies did not provide usable post or change‐score data. 1 study reported no between‐group difference in change in ADLs. 2 studies reported improvements in ADLs for participants receiving home LTC compared to nursing home LTC |
|
Quality of life (3‐6 months) |
It is uncertain whether long‐term home care compared to nursing home care improves happiness (RR 1.97, 95% CI 1.27 to 3.04) or general satisfaction | 114 (2 studies) | ⊕⊝⊝⊝ Very lowa,b,c | Both studies used proxy items for this outcome. While both variables were assessed using continuous measures, authors further dichotomised the outcome for reporting, precluding meta‐analysis. |
| Hospital admissions | It is uncertain whether long‐term home care compared to nursing home care decreases hospital admissions Estimates ranged from a relative increase in risk of a hospitalisation of RR 2.75 (95% CI 2.59 to 2.92) to a relative reduction in risk of RR 0.82 (95% CI 0.72 to 0.93). |
14,853 (3 studies) | ⊕⊝⊝⊝ Very lowa,b,c | This outcome described the number of participants having at least one hospital admission. Data were not pooled due to the high degree of statistical heterogeneity for this outcome (I2 = 99%) |
| Number of adverse health outcomes | The extent to which long‐term home care was associated to more or fewer adverse health outcomes than nursing home care was not reported. | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| ADL: activities of daily living; CI: confidence interval; LTC: long‐term care; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
aDowngraded due to study design. bDowngraded due to risk of bias. cDowngraded due to inconsistency.
* For Chuang 2005, only participants receiving institutional or home/community‐based care were included; participants receiving family care were not included.
Background
Changing population demographics have led to an increasing number of functionally dependent older people who require care and medical treatment (World Population Aging 2015). In many countries, government policy aims to shift resources into the community from care homes with the expectation that this will reduce costs and improve the quality of care compared to institutional care settings.
Description of the condition
Long‐term care of chronically dependent older people has become an increasingly important issue for both policy makers and healthcare providers.
For older adults, their place of residence and the quality of care they receive can influence their quality of life. Costs, consumer preference, and growing demand for long‐term care have led to increased interest in alternative care models for the elderly and a shift in resources from long‐term institutional care towards home‐ and community‐based care (Iwarsson 2007). For example, investment from the public and private sector has created housing schemes for older people that combine independent living with relatively high levels of care. Home and community care services aim to help older people live independently in their homes and to maintain or enhance their quality of life for as long as possible.
The two main options for providing formal long‐term support for older people who become functionally dependent are enhanced domiciliary support services (home care) or care home placement (institutional care).
Description of the intervention
Enhanced long‐term home care services can include a number of different elements, such as formal personal care (including bathing, toileting, feeding, dressing, transfers, meal preparation, shopping), adapted environments (including within the older person's own home, or in a specifically adapted residence), day care (planned regular care given in day care centres to patients otherwise living at home), or respite care (care given primarily at home, but where patients receive planned regular respite within an institution).
How the intervention might work
In theory, enhanced long‐term home care services should favour maintenance of independence and personal autonomy in the home environment, a reduction in institutionalisation, and most likely an increased level of satisfaction and quality of life for the person. This can help maintain and support seniors' relationships to their caregivers and avoid separation from them.
Why it is important to do this review
A previously published (and now withdrawn) Cochrane Review, Mottram 2002, included one randomised trial that compared foster care through a community care programme, where caregivers had been trained and closely followed up by health professionals, versus nursing home care, concluding that there was insufficient evidence on the likely benefit and harms of institutional versus home care for functionally dependent older people (Oktay 1987).
It is not clear whether in practice, enhanced home care can provide an alternative that satisfies both the functionally dependent older person and their informal caregivers, without causing increased caregiver stress or increasing the burden on primary and secondary care.
There have been several studies describing home care versus institutional care (e.g. Braun 1991), and by offering an up‐to‐date synthesis of the data, we aim to clarify whether or not home care for the functionally dependent older person is a viable alternative to long‐term institutional care.
Objectives
To assess the effects of long‐term home or foster home care versus institutional care for functionally dependent older people, with a particular focus on mortality, physical function, quality of life, and caregiver outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We anticipated finding few randomised trials given the logistical difficulties of conducting them in this area. Thus, in addition to including randomised trials, we also included non‐randomised trials, controlled before‐after studies and interrupted time series studies that compared the effects of institutional versus home care for functionally dependent older people.
We excluded controlled before‐after studies that did not meet the EPOC Group study design criteria resources (EPOC 2016).
The timing of the periods of study for the control and intervention groups should be comparable (that is, the pre‐ and postintervention periods of measurement for the control and intervention groups should be the same).
The intervention and control groups should be comparable on key characteristics.
We excluded interrupted time series studies that did not meet the EPOC Group study design criteria resources (EPOC 2016).
There should be a clearly defined point in time when the intervention occurred.
There should be at least three data points before and three after the intervention.
Types of participants
We included elderly participants (aged 65 years or older) with long‐term functional dependency, who were considered as potentially requiring care home placement (from hospital or the community). We defined functional dependence as the need for assistance in one or more activities of daily living (ADLs).
We excluded studies if they recruited the following participants.
Participants under the age of 65 years.
Participants who had become acutely functionally impaired and who were likely to require only a period of rehabilitation.
Participants who predominantly required palliative care.
Types of interventions
We included studies comparing enhanced long‐term homecare services versus long‐term institutional care.
Enhanced long‐term homecare services included the following:
-
Formal personal care provided by trained staff (including bathing, toileting, feeding, dressing, transfers, meal preparation, shopping). Where possible we categorised this as follows.
Regular care: personal care received regularly, but less often than daily, for specific activities of daily living.
Daily care: personal care received daily for specific activities of daily living, but not completely dependent on others for all activities of daily living and participation.
Continuous care: personal care for fully dependent participants.
Adapted environments, including within the older person's own home or in a specifically adapted residence.
Day care, where participants received planned regular care given in day care centres but were otherwise living at home.
Respite care, where participants receive planned regular care within an institution.
We defined Institutional long‐term care as care given to a participant in a day‐and‐night institution from which he or she could be discharged according to the rules applying to the institution.
We defined home care as care given to people in their own home, in a foster care setting, or in a group living setting.
Types of outcome measures
We included the following outcome measures.
Primary outcomes
Participant outcomes
Mortality at the end of scheduled follow‐up
Physical function (activities of daily living scales, such as the Barthel (Mahoney 1965) or the Katz (Katz 1963) Indexes of Daily Living)
Quality of life measures (e.g. WHO Quality of life assessment, WHOQOL Group 1995)
Secondary outcomes
Participant outcomes
Satisfaction with care
Number of adverse health outcomes, including incidence of infection (chest and urinary) over the period of the study
Hospital admissions
Informal caregivers of functionally dependent older people
Satisfaction with care (of the caregiver)
Perceived stress
Perceived burden
Search methods for identification of studies
We identified primary studies by searching the following bibliographic databases up to November 2015. We identified related systematic reviews by searching the Cochrane Database of Systematic Reviews (CDSR; 2015, Issue 11), the Database of Abstracts of Reviews of Effectiveness (DARE; 2015, Issue 2). EPOC Information Specialists (IS) developed search strategies in consultation with the authors. We used two methodological search filters, the Cochrane RCT Sensitivity/Precision Maximizing Filter (cf.Cochrane Handbook for Systematic Reviews of Interventions 6.4d; Higgins 2011) and the EPOC Filter, to limit retrieval to appropriate study designs. We restricted this review to studies published in English. The MEDLINE strategy is in Appendix 1.
Electronic searches
We undertook a comprehensive search covering the following databases on 11 November 2015, except where specified otherwise.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2015, issue 10), including the EPOC Trials Register, in the Cochrane Library.
Health Technology Assessment Database (2015, Issue 4) in the Cochrane Library.
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE (1946 to 10 November 2015).
Embase Ovid (1974 to 10 November 2015)
CINAHL EBSCO (from 1981 to 10 November 2015).
WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch).
The Grey Literature Report (The New York Academy of Medicine) (www.greylit.org).
OpenGrey (www.opengrey.eu).
Agency for Healthcare Research and Quality (AHRQ) (from inception to July 2012).
ClinicalTrials.gov (clinicaltrials.gov).
Association of Gerentology & Geriatrics (IAGG) (from inception to July 2012).
Searching other resources
We identified additional information as follows.
We conducted cited reference searches for studies selected for inclusion in our review.
We reviewed reference lists of relevant systematic reviews and other relevant publications.
If/when required, we contacted authors of relevant studies/reviews to clarify reported information or seek unpublished results/data.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to the reference management database and removed duplicates. Two review authors (CY, DS) independently examined the remaining unique records, excluding studies that clearly did not meet the inclusion criteria and obtaining full‐text copies of potentially relevant references. Two review authors (CY, DS) independently applied the eligibility criteria and resolved disagreements by discussion.
Data extraction and management
Two review authors (CY, DS) independently extracted details of study design, participants, intervention and comparison intervention, and outcome data from included articles using a specially designed data extraction form based on the EPOC data collection sheet (EPOC 2013). We resolved any disagreements by discussion and consensus.
Assessment of risk of bias in included studies
Two review authors (CY, AH) assessed the risk of bias of each included study. We used the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the guidance from the EPOC group (EPOC 2015). We assessed nine domains: sequence generation, allocation concealment, blinding of participants, blinding of outcome assessments, incomplete outcome data, selective outcome reporting, baseline measures, freedom from contamination, and 'other issues'. The ninth domain, 'other issues', included a baseline assessment (do the groups differ in fundamental ways?). We assessed baseline measures in all studies by considering if there were differences on key variables including age, sex and function. We assessed if studies were free from contamination by looking for crossover between intervention arms, if cross over was not reported it was judged to be low.
We used the overall 'Risk of bias' assessment to inform the certainty of the evidence, for which we used GRADE methodology.
Measures of treatment effect
We estimated the effect of the intervention using risk ratio for dichotomous data and mean difference and standardised mean difference for continuous data, together with the appropriate associated 95% confidence interval. We ensured that an increase in scores for continuous outcomes could be interpreted in the same way for each outcome.
Dealing with missing data
Whenever possible, we tried to contact authors for the primary studies to request missing data; however it was not always possible to find contacts for the authors, as some of the studies were published more than two decades ago.
Assessment of heterogeneity
We examined statistical heterogeneity among trials using the I2 statistic for mortality and hospital admissions.
Assessment of reporting biases
We did not assess reporting biases as planned using the Risk Correlation test and funnel plot, as there were too few studies to give a meaningful result.
Data synthesis
We conducted Mantel‐Haenszel fixed‐effects meta‐analyses for two outcome measures (mortality and hospital admissions), as the remaining collected data, identified interventions, and outcome measurements were not comparable. One of the studies included in the meta‐analyses was multi‐arm. Chuang 2005 had two control groups, home or community‐based and family care, and we included the former in the analysis as the services provided to the participants were compatible with the types of interventions we defined a priori, whereas the latter group received care from relatives, without training or additional services, and thus was not eligible. Where it was not possible to meta‐analyse the data due to diversity of interventions and outcomes, we reported the results using a narrative summary.
Sensitivity analysis
In order to determine how robust and consistent the results were, we planned to conduct sensitivity analyses, based upon study design (randomised trial versus other) or overall risk of bias in study (high, medium, low, according to the EPOC quality checklists; EPOC 2015).
Summary of findings
We created a 'Summary of findings' table for our primary comparison of long‐term home care versus institutional care, including all outcomes deemed important to decision makers, including patient outcomes and healthcare use (mortality, physical function, quality of life, hospital admissions, number of adverse health outcomes). We did not present secondary outcomes related to caregivers in the 'Summary of findings' table but did report them in the text. Similarly, we reported results for our secondary comparison of foster care and blended care in text only.
We assessed the overall certainty of evidence for each outcome using the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and EPOC (EPOC 2013a). Factors that may decrease the certainty of evidence are: study design and risk of bias (downgraded if more than 25% of the participants were from studies at a high risk of bias); inconsistency of results (downgraded if considerable heterogeneity was apparent in visual inspection or if the I2 value was greater than 50%); indirectness (generalisability of the findings; downgraded if more than 50% of the participants were outside the target group); imprecision (downgraded if fewer than 400 participants were included in the comparison for continuous data and there were fewer than 300 events for dichotomous data (Mueller 2007) and other factors (e.g. reporting bias, publication bias). As suggested previously, if a study included fewer than 400 participants we assessed its outcomes as inconsistent and imprecise and downgraded two levels to 'low certainty evidence', downgrading it further to 'very low certainty evidence' if there were other limitations. We reduced the certainty of evidence for a specific outcome by one level, according to the performance of the studies against these five factors, and we described the evidence as follows.
High‐certainty evidence: there are consistent findings among at least 75% of trials with low risk of bias; consistent, direct, and precise data; and no known or suspected publication biases. Further research is unlikely to change either the estimate or our confidence in the results.
Moderate‐certainty evidence: one of the domains is inadequate. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low‐certainty evidence: two of the domains are inadequate. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low‐certainty evidence: we are very uncertain about the estimate.
No evidence: we did not find any studies that addressed this outcome.
Results
Description of studies
Results of the search
We retrieved 14,671 unique records from the electronic database search and excluded 14,638 based on title and abstract. We assessed the full text 33 records, identifying 10 studies that were eligible for inclusion in this review. Figure 1 shows the study selection process.
1.
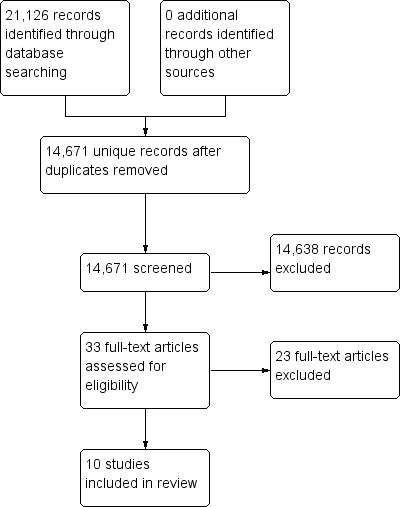
Study flow diagram.
Included studies
See Characteristics of included studies.
Design and country
Of the 10 included studies, there was 1 randomised trial (Oktay 1987), 4 non‐randomised trials (Braun 1991; Challis 1991; Chuang 2005; Mitchell 1978), 4 observational cohort studies (Condelius 2010; Sherwood 1986; Wilson 2005; Wysocki 2014) and 1 nested case‐control study (Braun 1987). The studies took place in five different countries, including six studies in the USA (Braun 1987; Braun 1991; Mitchell 1978; Oktay 1987; Sherwood 1986; Wysocki 2014), one in Taiwan (Chuang 2005), one in Sweden (Condelius 2010), one in the UK (Challis 1991), and one in Canada (Wilson 2005). All studies except one reported sources of funding (Challis 1991).
Participants
The 10 studies included 16,377 total participants. Sample sizes ranged from 98 to 11,803, and mean participant age ranged from 65 years to 82 years. All studies excluded participants who were younger than 65 except Mitchell 1978, which included participants from age 26 but had a mean cohort age of 65.6 years. Most participants in all studies were considered to be functionally dependent older people in need of long‐term care services. Most studies did not select (or exclude) participants for any specific co‐morbidities, with the exception of Chuang 2005, which only included patients if they had had a stroke.
Description of the interventions
Home care
See Table 2.
1. Description of long‐term home care interventions.
| Study | Service Location (home / community) | Type of services | Dose (how many services and their frequency of provision) | Provider |
| Condelius 2010 | Home | Help with laundry, shopping, cleaning, and personal care. Excluded meals on wheels or transport services | ≥ 4 home visits per month | Not reported |
| Mitchell 1978 | Home | Medical care and ancillary services in participant's own home. A potential caregiver (friend, relative, or hired caretaker) must be living in the patient's home and able to assume responsibility for care. | Not reported | Home care team (physician, nurse, dietician, social worker) |
| Challis 1991 | Home | Darlington Care Project: case management service, which could include any number of medical services based on client needs (speech therapy, stoma care, catheter care, change of dressing). Personal care (bathing, dressing, toileting, feeding, hand/nail care), physical care, (assist with walking, lifting/transferring). Social and recreational activities and therapeutic exercises | Not reported | Case manager likely a nurse? Other HCPs as needed |
| Wilson 2005 | Home | Not reported | Not reported | Not reported |
| Wysocki 2014 | Home or community | Not reported | Not reported | Not reported |
| Chuang 2005 | Home or community | Not reported | Not reported | Not reported |
| Braun 1987 | Home or community | Nursing Home Without Walls (NHWW) provides an array of services including case management, skilled nursing, personal care, adult day health, home delivered meals, nutritional counselling, transportation, respite, emergency alarms, moving assistance, rehabilitation, home maintenance, environmental modifications, homemakers | Not reported | Nurse |
| Sherwood 1986 | Community | Counselling, transportation, meals, recreational activities, information/referral, and monitoring services. Based on a patient assessment, the programme could provide medication monitoring, and/or arrange for various types of therapies | Not reported | Social worker/nurse |
The intervention in eight studies was provision of home care services to participants, mostly living in their own home (Braun 1987; Challis 1991; Chuang 2005; Condelius 2010; Mitchell 1978; Wilson 2005; Wysocki 2014), but also in the community (Sherwood 1986). Three studies did not report intervention details (Chuang 2005; Wilson 2005; Wysocki 2014). The home care services provided in four studies tended to include both medical and ancillary services, consisting of personal care, household chores, or both (Braun 1987; Challis 1991; Mitchell 1978; Sherwood 1986). Two of these studies provided a detailed list of the services offered, including a case management service that provided referrals for medical services (e.g. speech therapy, stoma care, catheter care, change of dressing), personal care (e.g. bathing, dressing, toileting, feeding, hand/nail care), physical care (e.g. assistance with walking, lifting/transferring, and therapeutic exercises) and social and recreational activities (Braun 1987; Challis 1991). The intervention in Condelius 2010 provided household and personal care, but authors did not report any provision of medical services. Lastly, the intervention in Sherwood 1986 provided community‐based services from a geriatric day hospital where participants attended and received an assessment by a nurse or social worker, along with any other services based on their needs, including counselling, transportation, meals, recreational activities, information/referral, medication monitoring, or other various types of therapies.
Foster care
See Table 3.
2. Description of long‐term geriatric foster care interventions.
| Study | Service Location | Type of services | Provider |
| Oktay 1987 | Room in a foster home | Caregivers provided the patient with meals, laundry, assistance with personal and instrumental ADLS, 24‐hour supervision and nursing tasks as needed (e.g. monitoring medication, injections and behavioural modification) | Caregivers were trained by the Johns Hopkins Hospital |
| Sherwood 1986 | Foster home care | Caregivers provided personal care services, 24‐hour supervision, and meal, laundry and household services | Caregivers were a part of a certification and monitoring programme |
| Braun 1987 | Foster care home | Community Care Program: families provide 24‐hour supervision, room and board, homemaker services, personal care including assistance with ADLs, medication, range of motion and other exercises, and in some cases, tube feeding, dressing changes, insulin injections, catheter irrigations, transportation to medical and social outings | Families are trained and supervised by social worker/nurse teams to adopt and care for 1‐2 patients |
In addition to interventions provided to participants in their home (as above), we identified studies that provided geriatric foster care within a home environment as an alternative to nursing home care. Oktay 1987 used this type of intervention exclusively, and two other studies used it as an additional comparison arm (Braun 1987; Sherwood 1986). All three studies described the foster care intervention as including a foster caregiver/family member providing 24‐hour supervision, room and board, homemaker services, personal care including assistance with activities of daily living, medication monitoring, physical exercise, and in some cases, tube feeding, dressing changes, insulin injections, catheter irrigations, and transportation to medical and social outings.
Blended (mix of different intervention types)
Lastly, in one study, the intervention included participants who were receiving either care in a foster home or in their own home (Braun 1991).
While this blended model fulfilled our protocol criteria by offering the participant an alternative to nursing home care within a domestic home environment, the service provision type and intensity were different. In foster care, the person resides in the home of a trained caregiver who provides 24‐hour monitoring and performs an extended role, often including nursing‐type support. This contrasts with what is generally more limited homecare services provided within the person's own home environment.
Control group: nursing home
Participants in the control group were residents in a long‐term care facility where they received 24‐hour monitoring and care.
Outcomes
Primary outcomes
Patient level outcomes reported in the included studies were mortality (4 studies), physical function (4 studies) and quality of life (3 studies).
Investigators reported mortality at 6 months in Braun 1991, Challis 1991, and Chuang 2005, and at 12 months in Challis 1991 and Oktay 1987.
Six studies measured physical function using a version of the Katz Index of Activities of Daily Living (ADL) at 6 months (Braun 1987; Braun 1991; Chuang 2005), 9 months (Sherwood 1986), and 12 months (Condelius 2010; Oktay 1987), while one used the Functional Status Index at 3 months (Mitchell 1978).
Secondary outcomes
Three studies assessed hospital admissions (Condelius 2010; Wilson 2005; Wysocki 2014).
Excluded studies
We excluded 23 studies after full‐text review. The most common reasons for exclusion were ineligible study design or lack of reporting on an eligible outcome (Characteristics of excluded studies).
Risk of bias in included studies
See Figure 2.
2.
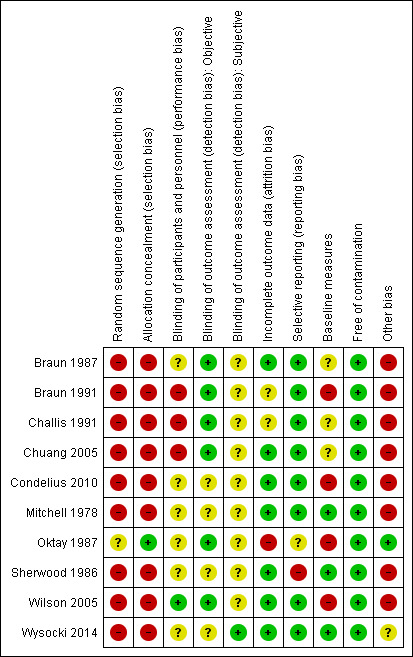
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Sequence generation
Nine studies were not randomised, so we assigned a high risk of bias rating on this item. The single randomised trial did not report the method of randomisation, leading us to rate it as being at unclear risk of bias (Oktay 1987).
Allocation
As above, nine studies were not randomised and did not use allocation methods, so we rated them as being at high risk of bias. The single randomised trial reported adequate allocation concealment, and we rated it as being at low risk (Oktay 1987).
Blinding
Blinding of participants and personnel
With the exception of one study (Wilson 2005), participants and personnel were either unblinded and rated as being at high risk of bias (Braun 1987; Challis 1991; Chuang 2005), or there was insufficient information to make a judgment.
Blinding of outcome assessors
Only two studies blinded outcome assessors (Braun 1987, Wilson 2005).
Incomplete outcome data
We rated seven studies as being at low risk of bias on this item. We considered one other study to be at high risk (Oktay 1987), while two were at unclear risk (Braun 1991; Challis 1991).
Selective reporting
In the absence of a protocol, it is difficult to judge if authors report outcomes as planned. We assessed whether the Methods and Results sections reported the same outcomes. Consequently, we rated eight studies as being at low risk of bias on this item, one at high risk (Sherwood 1986), and one at unclear risk (Oktay 1987).
Other potential sources of bias
We judged four studies to have between‐group similarity at baseline (Chuang 2005; Mitchell 1978; Sherwood 1986; Wysocki 2014). We considered that four studies had between‐group differences at baseline that could influence outcome (Braun 1991; Condelius 2010; Oktay 1987; Wilson 2005). Baseline similarity was unclear in two studies.
The possibility of reverse causality was high in the included non‐randomised trials.
Effects of interventions
See: Table 1
Main comparison: home care versus nursing home care
Mortality
Two studies reported the number of participants in each group who had died at six months (Challis 1991; Chuang 2005). It is uncertain whether long‐term home care decreases risk of mortality at six months compared to nursing home care; RR ranged from 0.38 (95% CI 0.17 to 0.61) to 2.89 (95% CI 1.57 to 5.32) (2 studies, N = 314, very low‐certainty evidence). When combined in a meta‐analysis, there was high heterogeneity (I2 = 94%), and thus we did not retain the pooled estimate. Challis 1991 also assessed mortality at 12 months; the effect on mortality is uncertain (RR 1.28, 0.89 to 1.84). See Table 4; Analysis 1.1.
3. Mortality: data for all included studies reporting this outcome.
| Study | Time‐point | Type of long‐term care | Sample size | Results | Relative effect RR (95% CI) | |
| Home care | Nursing home | |||||
| Braun 1991 | 6 months | Blended | 352 | 8% (18/221) | 16% (21/131) | 0.51 (0.28 to 0.92) |
| Challis 1991 | 6 months | Home care | 214 | 34% (31/101) | 11% (12/113) | 2.89 (1.57 to 5.32) |
| Chuang 2005 | 6 months | Home care | 474 poststroke |
6% (24/408) | 18% (12/66) | 0.38 (0.17 to 0.61) |
| Challis 1991 | 12 months | Home care | 214 | 40% (40/101) | 31% (35/113) | 1.28 (0.89 to 1.84) |
| Oktay 1987 | 12 months | Foster care | 112 | 29% (17/59) | 32% (17/53) | 0.90 (0.51 to 1.57) |
| CI: confidence interval; RR: risk ratio. | ||||||
RR: risk ratio.
1.1. Analysis.
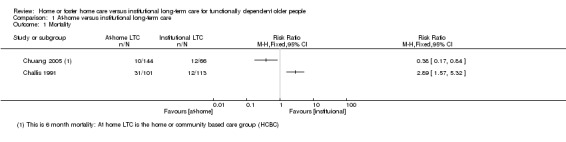
Comparison 1 At‐home versus institutional long‐term care, Outcome 1 Mortality.
Physical function
Five studies assessed function; four studies used the Katz ADL index, either in its original version (Braun 1987; Chuang 2005) or a modified variation (Condelius 2010; Sherwood 1986). One study used the Function Status Index (FSI) (Mitchell 1978). We are uncertain whether the intervention improves physical function (5 studies, N = 1295; very low‐certainty evidence). Three studies did not provide post‐test or change score data (Braun 1987; Chuang 2005; Mitchell 1978). For the three studies that did provide usable data on this outcome, two studies provided very low‐certainty evidence for a beneficial effect of long‐term home care on ADLs (MD −1.90, 95% CI −2.18 to −1.62, Condelius 2010; MD −0.25 points, 95% CI −0.44 to −0.06, Sherwood 1986). In both of these studies, a lower score indicated better ADL performance; the former scale ranged from 0 (independent in all activities) to 4 (dependent in all activities), whereas the latter ranged between 1 (good) to 2 (severely impaired). See Table 5.
4. Physical function: data for all included studies reporting this outcome.
| Study | Time point | Sample size | Measure | D/Ca | Results | Relative effectb (95% CI) | |
| Home care | Nursing home | ||||||
| Home care | |||||||
| Mitchell 1978c | 3 months | 195 | ADLd (change) | — | — | — | — |
| Braun 1987 | 3 months | 98 | ADLe | C | Post: 13.02 | Post: 13.16 | — |
| Braun 1987 | 3 months | 98 | ADL (mobility)e | C | −1.02 | −1.05 | — |
| Chuang 2005 | 6 months | 210 | ADLe | — | — | — | — |
| Sherwood 1986 | 9 months | 98 | ADL performance assessment |
? | — | — | −0.25 (−0.44 to −0.06) |
| Condelius 2010f | Unclear | 694 | ADLg | C | 3.0 (1.2) | 3.9 (0.4) | −0.9 (−1.02 to −0.78) |
| Condelius 2010f | Unclear | 694 | ADLh | C | 1.4 (1.7) | 3.4 (1.9) | −1.90 (−2.18 to −1.62) |
| Foster care | |||||||
| Oktay 1987 | 12 months | 53 | ADLd (improved/maintained) | D | 79% (22/28) | 60% (15/25) | 0.19 (−0.07 to 0.43) |
| Oktay 1987 | 12 months | 53 | ADLg (improved/maintained) |
D | 75% (21/28) | 68% (17/25) | 0.07 (−0.17 to 0.31) |
| Sherwood 1986 | 9 months | 62 | ADL performance assessment |
— | — | — | — |
| Braun 1987 | Unclear | — | — | — | — | — | — |
| Blended | |||||||
| Braun 1991 | 6 months | 352 | ADLe (pre‐post) | C | Pre: 12.87 Post: 12.16 |
Pre: 14.43 Post: 13.78 |
— |
| Braun 1991 | 6 months | 352 | ADLe (change) | 0.71 | 0.65 | — | |
| ADL: activities of daily living; CI: confidence interval; RR: risk ratio. | |||||||
aD: dichotomous outcome; C: continuous outcome. bFor dichotomous outcomes, the relative effect is reported as a risk ratio (RR). cNo post‐test data provided for this study. Pre‐test Functional Status Index mean (SD): home care (HC) 8.12 (3.9), institution (hospital): 10.48 (3.3). dADLs were assessed with theFunction Status Index (FSI). This measure evaluates people on the extent to which they can perform everyday activities and socially defined roles. The self‐care dimension was expanded to include an item (continence) not in the original FSI. Continence used in ADL‐ Katz total FSI scores ranges from a 0 to 17. Higher score worse. eADLs assessed with theKatz Index of ADLs (Katz 1963). This measures function in eight activities: bathing, dressing, transfer, toileting, continence, feeding, ambulation, house confinement. ADL is first 6 items summed for total score (range: 6 to 18). Mobility is final 2 items summed for total score (range: 2 to 9) We are using the ADL score. Higher scores worse. fIn this study, there were no pre‐post measures reported; it appears these data are cross‐sectional, and it is unclear at what time point they were taken. gADL: Activities of daily living were assessed with the Instrumental Activities of Daily Living (IADL) including cooking, transportation, cleaning and shopping. The IADL sum score ranges from 0 (independent in all activities) to 6 (dependent in all activities). hADL: Activities of daily living were assessed with thePersonal Activities of Daily Living (PADL) including bathing, dressing, going to the toilet, transferring, continence and feeding. The PADL sum score ranges from 0 (independent in all activities) to 4 (dependent in all activities).
Quality of life
Two studies reported on participants' quality of life (Braun 1987; Challis 1991). Neither study used a standardised assessment of health‐related quality of life such as the Short Form 36‐item Health Survey (SF‐36) or EurQoL; rather, both studies used proxy outcomes. Braun 1987 used level of happiness, and Challis 1991 used general satisfaction reporting. Investigators measured happiness using a single‐item question: "Are you happy here?" with a three‐point response: 1 = rarely; 2 = sometimes; 3 = often. They assessed satisfaction using a single item but did not describe it. While investigators assessed both outcomes using continuous measures, authors further dichotomised happiness into 'happy' or 'not happy' and reported the results for general satisfaction as within‐group mean change scores without standard deviations, precluding meta‐analysis for this outcome. It is uncertain whether long‐term home care compared to nursing home care improves happiness (RR 1.97, 95% CI 1.27 to 3.04) or general satisfaction (2 studies, N = 114, very‐low certainty evidence). See Table 6.
5. Quality of Life: data for all included studies reporting this outcome.
| Study | Time point | Type of long‐term care | Sample Size | Measure | D/Ca | Results |
Relative effectb (95% CI) |
Favours | |
| Home care | Nursing home | ||||||||
| Home care | |||||||||
| Braun 1987 | 3 months | Community care | 132 | Reported level of happinessc | D | 67% (59/88) | 34% (15/44) | 1.97 (1.27 to 3.04) | Home care |
| Challis 1991 | 6 months | Community care | 214 | General satisfactiond (change) | C | 0.79 | 0.08 | — | Home care |
| Challis 1991 | 6 months | Community care | 214 | Well‐being – moraled (change) | C | 0.79 | 0.21 | — | Home care |
| Challis 1991 | 6 months | Community care | 214 | Well‐being – depressiond (change) | C | 0.33 | −1.05 | — | Home care |
| Foster care | |||||||||
| Oktay 1987 | 12 months | Foster care | 53 | Life satisfaction (improved/maintained) | D | 46% (13/28) | 72% (18/25) | RR: 0.64 (0.40 to 1.03) | Nursing |
| Oktay 1987 | 12 months | Foster care | 53 | Perceived health (improved/maintained) | D | 68% (19/28) | 84% (21/25) | RR: 0.81 (0.59 to 1.10) | Nursing |
| Oktay 1987 | 12 months | Foster care | 53 | Mental status (improved/maintained) | D | 64% (18/28) | 60% (15/25) | RR: 1.07 (0.70 to 1.64) | |
| CI: confidence interval; RR: risk ratio. | |||||||||
aD: dichotomous outcome; C: continuous outcome. bFor dichotomous outcomes, the relative effect is reported as a risk ratio (RR). cA single item question, "Are you happy here?" with a 3 point response: 1 = rarely, 2 = sometimes, 3 = often. They report raw response data on each point and then dichotomise data as happy or not. Higher score = better. dA single item was used but not described. Mean change score at 6 month is reported. SD were not provided. Higher scores are assumed to be better.
Satisfaction with care
We did not find studies reporting on satisfaction with care.
Number of adverse health outcomes
We did not find studies reporting on adverse health outcomes.
Hospital admissions
Three studies assessed the number of patients admitted to hospital during the study period using observational datasets (Condelius 2010; Wilson 2005; Wysocki 2014). It is uncertain whether home care decreases the risk of hospitalisation compared to nursing home care (3 studies, N = 14,853, very‐low certainty evidence). Studies reported both increased risk of hospitalisation (RR 2.75, 95% CI 2.59 to 2.92; Wilson 2005; and RR 1.41, 95% CI 1.25 to 1.60; Wysocki 2014), and reduced risk of hospitalisation (RR 0.82, 95% CI 0.72 to 0.93; Condelius 2010). See Table 7; Analysis 1.2.
6. Hospital admissions: data for all included studies reporting this outcome.
| Study | Time‐point | Type of long‐term care | Sample Size | Measure | Results | Relative effect: RR (95% CI) | Favours | |
| Home care |
Nursing home |
|||||||
| Wilson 2005 | Observational (data set) |
Home care | 11,803 | Hospital admissiona | 55% (3880/7029) |
20% (958/4774) |
2.75 (2.59 to 2.92) | Nursing home? |
| Wilson 2005 | Observational (dataset) | Home care | 11,803 | Emergency room visitsb | 70.1% (4992/70290 |
34.8% (1662/4774) |
— | Nursing home? |
| Wysocki 2014 | Observational (dataset) |
Home/community care | 2338 | Preventable hospitalisationc |
11.4% (133/1169) |
9.7% (113/1169) |
— | Nursing home? |
| Wysocki 2014 | Observational (dataset) |
Home/community care | 2338 | Any hospitalisationd | 35.8% (419/1169) |
25.4% (297/1169) |
1.41 (1.25, 1.60) |
Nursing home? |
| Condelius 2010e | unclear | Home care | 694 | Hospital staysf | 53.6% (228/425) |
65.4% (176/269) |
0.82 (0.72, 0.93) |
Home care? |
| CI: confidence interval; RR: risk ratio. | ||||||||
aNumber admitted to hospital at least once. bNumber of patients having ≥ 1 emergency room visits. cNumber of patients with at least one potentially preventable hospitalisation. dNumber of patients with at least one any type hospitalisation. eIn this study, there were no pre‐post measures reported; it appears these data are cross‐sectional, and it is unclear at what time point they were taken. fNumber of patients with 1 hospital stay.
1.2. Analysis.
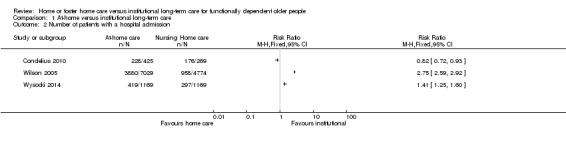
Comparison 1 At‐home versus institutional long‐term care, Outcome 2 Number of patients with a hospital admission.
Caregiver outcomes
We did not find studies reporting on caregiver outcomes, including satisfaction with care, stress and burden.
Secondary comparison: foster care versus nursing home care
Mortality
One study assessed the effect of foster home care compared to nursing home care on mortality at 12 months (Oktay 1987), generating low‐certainty evidence showing no effect for this care model on mortality (OR 0.90, 95% CI 0.51 to 1.57). See Table 4.
Physical function
Three studies assessed the effect of foster home care compared to nursing home care on changes in function measured by ADLs (Braun 1987; Oktay 1987; Sherwood 1986). Braun 1987 reported the mean pre‐ and post‐test scores but did not report standard deviations; from the scores provided, those in the foster care group did have greater improvements in ADLs than those in the nursing home group. Sherwood 1986 reported that participants allocated to a geriatric day hospital were less impaired than those allocated to a nursing home (MD ‐ 0.25, 95% CI ‐ 0.44 to ‐ 0.06). Oktay 1987 used the Katz Index of ADLs reporting a dichotomised estimate of the participants who either improved or maintained ADLs (RR 0.19, 95% CI ‐ 0.07 to 0.43). It is uncertain whether long‐term foster care compared to nursing home care improves or maintains ADLs because the certainty of evidence is very low. See Table 5.
Quality of life
One study assessed the effect of foster home care versus nursing home care on quality of life (Oktay 1987). Similar to other included studies, Oktay 1987 did not use a standardised assessment of quality of life. Instead, the study used the life satisfaction scale, which aims to assess psychological well‐being using five items: zest, resolution and fortitude, goal achievement, positive self‐concept, and mood. Assessors administered the test by telephone at baseline and at 3, 6, 9, and 12 months; however, authors reported only the 12 month scores. While the scale is continuous, with total scores ranging from 5 to 25, the authors dichotomised the total score as either improved/maintained or not. It is uncertain whether long‐term foster care compared to nursing home care improves satisfaction with life because the certainty of evidence is very low (RR 0.64, 95% CI 0.40 to 1.03). See Table 6.
Satisfaction with care
We did not find studies reporting on satisfaction with care.
Number of adverse health outcomes
We did not find studies reporting on adverse health outcomes.
Hospital admissions
We did not find studies reporting on hospital admissions.
Caregiver outcomes
Data were incomplete for this outcome, and we cannot draw any conclusion.
Discussion
Summary of main results
We attempted to establish whether dependent older people benefit from enhanced long‐term home care services compared with placement in a long‐term care institution. We also aimed to determine whether enhanced home care affects informal caregivers.
Our literature search yielded relevant data on a wide range of interventions of interest, but the certainty of evidence was poor with a lack of randomised trial data and substantial between‐study heterogeneity. For example, we were very inclusive regarding the types of studies eligible for the review to demonstrate the types of study design used in this area.
The studies reviewed here were heterogeneous in their inclusion criteria, design, sample, and methods of delivery. There was variability not just in the choice of instruments to measure outcomes, but the outcomes measured. The health and social care systems in which the studies took place differ significantly. The UK offers universal health and social care, whereas people in the USA receive care from multiple providers (for profit, not‐for‐profit, and state) and reimbursement from insurers as well as state and federal funds.
Therefore, we deemed meta‐analysis of the available data inappropriate, and we offered a narrative overview that we hope will stimulate thought and debate about options for long‐term care for frail elderly people.
The inconsistencies in outcomes between studies are notable.
Effects on mortality varied greatly, from a reduction to an increase in risk of death with enhanced care in a community setting compared to long‐term care home. None of the data for this outcome came from randomised trials, and these results are highly likely to be subject to allocation bias, with frailer participants at higher baseline risk of death more likely to be admitted to an institutional care home than to enhanced home care. There was little or no difference in mortality at 12 months between people allocated to foster home care compared to care homes (Oktay 1987). However, foster care is not a standard type of care for the elderly in most countries, so the results are hard to generalise. The certainty of evidence was very low.
Changes in physical function varied between studies, although results showed benefits for enhanced home care versus institutional care in Condelius 2010 and Sherwood 1986 and little or no difference in Braun 1987. Oktay 1987 noted improvement in physical function in participants allocated to foster home care. The certainty of evidence was very low.
Two studies described a measure of quality of life, reporting that this was better in those allocated to enhanced home care (Braun 1987; Challis 1991). However, patients receiving foster care were less likely to report improved or maintained life satisfaction compared to institutional care (Oktay 1987). The certainty of evidence was very low.
Overall completeness and applicability of evidence
We used a sensitive search strategy, and we believe we have a comprehensive overview of all studies that address our question of interest. However, the studies included in our review evaluated complex interventions involving various different patient groups with different medical diagnoses and different cultural, ethnic, and socioeconomic backgrounds. The included studies were from multinational locations (the USA, Sweden, Taiwan, and the UK) and were published over a prolonged period (around 40 years), during which long‐term care health and social care policies and practice have changed. Furthermore, ethnic and cultural aspects are relevant in the care of frail dependent elderly people, with large variations internationally in the use of long‐term care facilities. It was not possible to group the studies by geographic location or ethnicity.
While all studies included some form of home care, the level of care varied between studies. There was variability not just in the choice of instruments to measure outcomes, but in the outcomes measured and the time points of assessment. The wide diversity of outcome measures used prevented pooling of the data.
The incomplete and inconsistent reporting of data, including age, and unusable formats limited our ability to synthesise the evidence.
Certainty of evidence
A total of 16,377 people participated in 10 studies of long‐term care for functionally dependent older people. The sample size in the included studies ranged from 98 to 11,803. We elected not to offer summary analyses using meta‐analysis for most outcomes. In presenting results at individual study level, we must be mindful that some studies were modest in size and likely underpowered to answer their primary question, and that the quality of studies varied considerably.
We determined that the certainty of evidence across all the included studies was very low with high risk of bias for all outcomes. We are thus uncertain whether the intervention decreases the risk of mortality or hospital admission, or increases physical function and quality of life. Various study designs included one randomised trial, four non‐randomised trials, four observational cohort studies and one nested case‐control study.
The external validity/generalisability of included studies to an unselected frail, older adult population was generally good, although one of the included studies only included people who had had a stroke.
Potential biases in the review process
Identifying relevant studies in this broad topic area was challenging. We searched a wide variety of databases, including trial registers.
Two review authors, working independently, carried out study identification and data extraction. Although we were very careful not to discard relevant studies, we cannot discount the possibility that we may have missed some. It is also possible that limiting the review to English‐language studies might have biased our results, although recent data seems to suggest little or no evidence of systematic bias associated with language restriction in systematic reviews in conventional medicine (Morrison 2012).
Agreements and disagreements with other studies or reviews
Our review adds new data to the previous Cochrane review on this topic (Mottram 2002), albeit we could not find new randomised trials. Our more inclusive approach to studies gave us a larger pool of evidence to work with, but our final conclusions are similar to those of the previous review, with insufficient high quality data to allow a definitive statement on the utility of home care versus institutional care. We did not find any non‐Cochrane systematic reviews in this area.
Authors' conclusions
Implications for practice.
We are unable to offer definitive evidence to support or refute the benefit of home care versus institutional care, and any conclusions are limited by the relatively small amount of data available and the methodological problems outlined above. Results from studies were inconsistent, with studies suggesting positive, negative, and null effects of home care on key outcome measures such as mortality and quality of life. The data would suggest that doctors, patients, and caregivers should make no assumptions on the universal benefits of home care. There are some data to suggest enhanced home care may put additional strain on other parts of the healthcare system, with the possibility of increased risk of hospitalisation compared to institutional care. We note the heterogeneity in included participant populations, and it is feasible that home care may be more suited to certain groups.
Implications for research.
Study design
The lack of randomised trials in the area allude to the difficulty in performing this kind of research according to the classical randomised trial paradigm. Participants may be unhappy to let their future residence depend on randomisation, and resulting high participant selection may result in limited external validity. The Medical Research Council offers a framework for evaluating complex interventions and highlights the potential utility of approaches such as cluster randomisation (Craig 2008); however, the framework does not overcome the difficulties in obtaining informed consent.
Given the difficulty of performing traditional randomised trials, well‐performed observational research – planned realistically as health services evaluations (e.g. interrupted time series, step wedge designs with phased roll out of new services) – are more likely to be feasible and provide useful data to inform service planning.
However, simple observational studies will be prone to selection and intervention biases and are unlikely to advance the evidence base beyond where we are now.
Interventions
There was substantial heterogeneity in the interventions used to support older adults in the home environment. This complicates any attempts at comparative or summary analysis. Ideally, researchers would work together to study similar interventions. We recognise that this approach may not always be feasible or appropriate, and we would encourage future studies to at least describe the intervention and its delivery in sufficient detail to allow replication and comparison with other studies.
Outcomes
There was heterogeneity in the outcomes assessed, and we would encourage greater consistency in outcomes included for future studies of home care. Mortality, physical function and quality of life are important and should be included, but other outcomes are of interest and were less commonly assessed in the studies that inform our review, for example measures of caregiver burden.
Future studies should consider the potential for enhanced home care to put additional strain on other parts of health and social care system, and researchers should collect robust metrics on health and social care utilisation and economic burden.
Notes
This review was first published in the Cochrane Library in 2002 (Mottram 2002), but in 2007 it was withdrawn, as it was determined to be out‐of‐date at that time and contained possibly misleading evidence. A revised protocol was published in 2012 (Young 2012).
Acknowledgements
Supported by an unrestricted grant from the Age‐related Diseases Trust, and the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding and a Cochrane programme grant to the EPOC Group; the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Search Strategies
Medline (OVID)
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to 10 November 2015
| No. | Search terms |
| 1 | Aged/ or "aged, 80 and over"/ or Frail elderly/ |
| 2 | elderly.ti,ab. |
| 3 | (community adj4 (elder? or geriatric? or old* adult? or senior?)).ab. |
| 4 | geriatric patient?.ti,ab. |
| 5 | (older adult? or older person? or older people or older patient?).ti,ab. |
| 6 | Geriatrics/ or *Geriatric Dentistry/ or *Geriatric Nursing/ or Geriatric Psychiatry/ |
| 7 | Geriatric Assessment/ |
| 8 | "Health Services for the Aged"/ |
| 9 | ((geriatric? or elder? or senior?) adj2 (care or health service? or healthcare)).ti,ab. |
| 10 | or/1‐9 |
| 11 | long‐term care/ |
| 12 | long‐term care.ti,ab. |
| 13 | (long stay adj2 (care or healthcare or service? or treatment? or patient? or resident?)).ti,ab. |
| 14 | (function* adj2 (dependen* or independen* or limit* or decline* or status or impair*)).ti,ab. |
| 15 | (candidate? adj3 (institution* or deinstitution* or home or place*)).ti,ab. |
| 16 | or/11‐15 |
| 17 | home care services, hospital‐based/ |
| 18 | ((home or domicil*) adj3 (care or healthcare or nurs* or rehabilit* or service or services or treatment? or therapy or therapies or therapist? or visiting or visit?)).ti,ab. |
| 19 | (residential adj3 (care or healthcare or facilit*)).ti,ab. |
| 20 | Day care/ |
| 21 | (day hospital? or ((adult? or elder* or geriatric?) adj2 (day care or daycare))).ti,ab. |
| 22 | residential facilities/ |
| 23 | assisted living facilities/ |
| 24 | group homes/ |
| 25 | (group? adj (home? or living)).ti,ab. |
| 26 | halfway houses/ |
| 27 | halfway hous*.ti,ab. |
| 28 | homes for the aged/ |
| 29 | exp nursing homes/ |
| 30 | home care services/ |
| 31 | home nursing/ |
| 32 | respite care/ |
| 33 | respite care.ti,ab. |
| 34 | home health nursing/ |
| 35 | or/17‐34 |
| 36 | 10 and 16 and 35 |
| 37 | randomized controlled trial.pt. |
| 38 | controlled clinical trial.pt. |
| 39 | multicenter study.pt. |
| 40 | pragmatic clinical trial.pt. |
| 41 | (randomis* or randomiz* or randomly).ti,ab. |
| 42 | groups.ab. |
| 43 | (trial or multicenter or multi center or multicentre or multi centre).ti. |
| 44 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. |
| 45 | non‐randomized controlled trials as topic/ |
| 46 | interrupted time series analysis/ |
| 47 | controlled before‐after studies/ |
| 48 | or/37‐47 |
| 49 | exp animals/ |
| 50 | humans/ |
| 51 | 49 not (49 and 50) |
| 52 | review.pt. |
| 53 | meta analysis.pt. |
| 54 | news.pt. |
| 55 | comment.pt. |
| 56 | editorial.pt. |
| 57 | cochrane database of systematic reviews.jn. |
| 58 | comment on.cm. |
| 59 | (systematic review or literature review).ti. |
| 60 | or/51‐59 |
| 61 | 48 not 60 |
| 62 | 36 and 61 |
Embase (OVID)
Embase 1974 to 10 November 2015
| No. | Search terms |
| 1 | aged/ not (*adolescent/ or *adult/ or child/ or embryo/ or fetus/ or *middle aged/ or newborn/) |
| 2 | *aged/ or frail elderly/ |
| 3 | elderly.ti,ab. |
| 4 | (older adult? or older patient? or older people or older person?).ti,ab. |
| 5 | geriatric patient?.ti,ab. |
| 6 | *geriatric patient/ |
| 7 | (community adj4 (elder? or geriatric? or old* adult? or senior?)).ab. |
| 8 | ((geriatric? or elder? or senior?) adj2 (care or health service? or healthcare)).ti,ab. |
| 9 | *elderly care/ |
| 10 | exp *geriatrics/ |
| 11 | exp *geriatric nursing/ |
| 12 | *geriatric care/ |
| 13 | *geriatric rehabilitation/ |
| 14 | *geriatric assessment/ |
| 15 | or/1‐14 |
| 16 | *long term care/ |
| 17 | long‐term care.ti,ab. |
| 18 | (long stay adj2 (care or healthcare or service? or treatment? or patient? or resident?)).ti,ab. |
| 19 | (function* adj2 (dependen* or independen* or limit* or decline* or status or impair*)).ti,ab. |
| 20 | (candidate? adj3 (institution* or deinstitution* or home or place*)).ti,ab. |
| 21 | or/16‐20 |
| 22 | ((home or domicil*) adj3 (care or healthcare or nurs* or rehabilit* or service or services or treatment? or therapy or therapies or therapist? or visiting or visit?)).ti,ab. |
| 23 | (residential adj3 (care or healthcare or facilit*)).ti,ab. |
| 24 | (day hospital? or ((adult? or elder* or geriatric?) adj2 (day care or daycare))).ti,ab. |
| 25 | (group? adj (home? or living)).ti,ab. |
| 26 | halfway hous*.ti,ab. |
| 27 | respite care.ti,ab. |
| 28 | *respite care/ |
| 29 | *home rehabilitation/ |
| 30 | *home care/ |
| 31 | *day hospital/ |
| 32 | *nursing home/ |
| 33 | *residential home/ |
| 34 | *assisted living facility/ |
| 35 | *halfway house/ |
| 36 | *home for the aged/ |
| 37 | *institutional care/ |
| 38 | or/22‐37 |
| 39 | 15 and 21 and 38 |
| 40 | randomized controlled trial/ |
| 41 | controlled clinical trial/ |
| 42 | quasi experimental study/ |
| 43 | pretest posttest control group design/ |
| 44 | time series analysis/ |
| 45 | experimental design/ |
| 46 | multicenter study/ |
| 47 | (randomis* or randomiz* or randomly).ti,ab. |
| 48 | groups.ab. |
| 49 | (trial or multicentre or multicenter or multi centre or multi center).ti. |
| 50 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. |
| 51 | or/40‐50 |
| 52 | (systematic review or literature review).ti. |
| 53 | "cochrane database of systematic reviews".jn. |
| 54 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ |
| 55 | human/ or normal human/ or human cell/ |
| 56 | 54 not (54 and 55) |
| 57 | 52 or 53 or 56 |
| 58 | 51 not 57 |
| 59 | 39 and 58 |
The Cochrane Library
| No. | Search terms |
| #1 | [mh Aged] |
| #2 | [mh "aged, 80 and over"] |
| #3 | [mh "Frail elderly"] |
| #4 | [mh Geriatrics] |
| #5 | [mh "*Geriatric Dentistry"] |
| #6 | [mh "*Geriatric Nursing"] |
| #7 | [mh "Geriatric Psychiatry"] |
| #8 | [mh "Geriatric Assessment"] |
| #9 | [mh "Health Services for the Aged"] |
| #10 | elderly:ti,ab |
| #11 | (community near/4 (elder? or geriatric? or old* adult? or senior?)):ab |
| #12 | geriatric patient?:ti,ab |
| #13 | (older adult? or older person? or older people or older patient?):ti,ab |
| #14 | ((geriatric? or elder? or senior?) near/2 (care or health service? or healthcare)):ti,ab |
| #15 | {or #1‐#14} |
| #16 | [mh "long‐term care"] |
| #17 | long‐term care:ti,ab |
| #18 | (long stay near/2 (care or healthcare or service? or treatment? or patient? or resident?)):ti,ab |
| #19 | (function* near/2 (dependen* or independen* or limit* or decline* or status or impair*)):ti,ab |
| #20 | (candidate? near/3 (institution* or deinstitution* or home or place*)):ti,ab |
| #21 | {or #16‐#20} |
| #22 | [mh "home care services, hospital‐based"] |
| #23 | [mh "day care"] |
| #24 | [mh "residential facilities"] |
| #25 | [mh "assisted living facilities"] |
| #26 | [mh "group homes"] |
| #27 | [mh "halfway houses"] |
| #28 | [mh "homes for the aged"] |
| #29 | [mh "nursing homes"] |
| #30 | [mh "home care services"] |
| #31 | [mh "home nursing"] |
| #32 | [mh "respite care"] |
| #33 | [mh "home health nursing"] |
| #34 | ((home or domicil*) near/3 (care or healthcare or nurs* or rehabilit* or service or services or treatment? or therapy or therapies or therapist? or visiting or visit?)):ti,ab |
| #35 | (residential near/3 (care or healthcare or facilit*)):ti,ab |
| #36 | (day hospital? or ((adult? or elder* or geriatric?) near/2 (day care or daycare))):ti,ab |
| #37 | (group? next (home? or living)):ti,ab |
| #38 | halfway hous*:ti,ab |
| #39 | respite care:ti,ab |
| #40 | {or #22‐#39} |
| #41 | #15 and #21 and #40 |
Cinahl (EBSCO)
| No. | Search terms |
| S1 | MH aged OR MH ( "aged 80 and over" ) OR MH frail elderly |
| S2 | TI elderly OR AB elderly |
| S3 | AB community N4 (elder? or geriatric? or old* adult? or senior?) |
| S4 | TI geriatric patient? OR AB geriatric patient? |
| S5 | TI ( older adult? or older person? or older people or older patient? ) OR AB ( older adult? or older person? or older people or older patient? ) |
| S6 | MH geriatrics OR MH Dental care for aged OR MH Gerontologic Nursing OR MH Geriatric psychiatry |
| S7 | MH geriatric assessment |
| S8 | MH "Health Services for the Aged" |
| S9 | TI ((geriatric? or elder? or senior?) N2 (care or health service? or healthcare)) OR AB ((geriatric? or elder? or senior?) N2 (care or health service? or healthcare)) |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 |
| S11 | (MH "Long Term Care") |
| S12 | TI long‐term care or AB long‐term care |
| S13 | TI (long‐stay N2 (care or healthcare or service? or treatment? or patient? or resident?)) OR AB (long‐stay N2 (care or healthcare or service? or treatment? or patient? or resident?)) |
| S14 | TI (function* N2 (dependen* or independen* or limit* or decline* or status or impair*)) or AB (function* N2 (dependen* or independen* or limit* or decline* or status or impair*)) |
| S15 | TI (candidate? N3 (institution* or deinstitution* or home or place*)) OR AB (candidate? N3 (institution* or deinstitution* or home or place*)) |
| S16 | S11 OR S12 OR S13 OR S14 OR S15 |
| S17 | TI ((home or domicil*) N3 (care or healthcare or nurs* or rehabilit* or service or services or treatment? or therapy or therapies or therapist? or visiting or visit?)) OR AB ((home or domicil*) N3 (care or healthcare or nurs* or rehabilit* or service or services or treatment? or therapy or therapies or therapist? or visiting or visit?)) |
| S18 | TI (residential N3 (care or healthcare or facilit*)) OR AB (residential N3 (care or healthcare or facilit*)) |
| S19 | TI (day hospital? or ((adult? or elder* or geriatric?) N2 (day care or daycare))) OR AB (day hospital? or ((adult? or elder* or geriatric?) N2 (day care or daycare))) |
| S20 | TI (group? N (home? or living)) OR AB (group? N (home? or living)) |
| S21 | TI halfway hous* OR AB halfway hous* |
| S22 | TI respite care OR AB respite care |
| S23 | MH Housing for the Elderly |
| S24 | MH Home Nursing |
| S25 | (MH "Home Rehabilitation") OR (MH "Homemaker Services") OR (MH "Home Health Care") |
| S26 | MH Day care |
| S27 | (MH "Nursing Home Patients") |
| S28 | MH "Residential Facilities+" |
| S29 | MH "Residential Care+" |
| S30 | MH "Assisted Living" |
| S31 | (MH "Halfway Houses") |
| S32 | (MH "Nursing Homes+") |
| S33 | MH "Home Nursing, Professional" |
| S34 | MH "Home Nursing" |
| S35 | S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 |
| S36 | S10 AND S16 AND S35 |
| S37 | PT randomized controlled trial |
| S38 | PT clinical trial |
| S39 | PT research |
| S40 | (MH "Randomized Controlled Trials") |
| S41 | (MH "Clinical Trials") |
| S42 | (MH "Intervention Trials") |
| S43 | (MH "Nonrandomized Trials") |
| S44 | (MH "Experimental Studies") |
| S45 | (MH "Pretest‐Posttest Design+") |
| S46 | (MH "Quasi‐Experimental Studies+") |
| S47 | (MH "Multicenter Studies") |
| S48 | MH "Health Services Research" |
| S49 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) |
| S50 | TI (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) OR AB (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) |
| S51 | S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 |
| S52 | S36 AND S51 |
| S53 | S52 Limiters ‐ Exclude MEDLINE records |
ClinicalTrials.gov
elderly AND "long term care"
WHO International Clinical Trials Registry Platform (ICTRP)
elderly AND "long term care"
Web searches
| Website name/organisation | URL | Date | Notes |
| Grey Lit (New York Academy of Medicine) | www.greylit.org | 11 November 2015 | elderly AND "long term care" |
| Open Grey | www.opengrey.eu | 11 November 2015 | elderly AND "long term care" |
Data and analyses
Comparison 1. At‐home versus institutional long‐term care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients with a hospital admission | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Braun 1987.
| Methods | Study design: nested case‐control study | |
| Participants |
Participants: N = 98 (Control N = 49; Intervention: N = 49) Mean age: Control: 79.9 years, Intervention: 81.6 years Gender: Control: 57% female, intervention: 80% female Setting: Hawaii |
|
| Interventions |
Type of intervention: care in participant's own home Description of the intervention: participants receiving care in their own home from the Nursing Home Without Walls Program. Nursing Home Without Walls (NHWW) provides an array of services including case management, skilled nursing, personal care, adult day health, home delivered meals, nutritional counselling, transportation, respite, emergency alarms, moving assistance, rehabilitation, home maintenance, environmental modifications, homemakers Control: nursing home care |
|
| Outcomes | Happiness at 3 months; physical function at 3 months (ADL) | |
| Notes | Study supported by the Hawaii Department of Social Services and Housing; and the Henry J Kaiser Foundation (California, USA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial. Notes from text: since agency procedures did not permit random assignment to placement settings, sample groups were selected. Intermediate Care Facility patients ≥ 55 years of age who staying in one of the three settings for 3 months were considered. They were then matched on ADL, mobility and orientation scores. |
| Allocation concealment (selection bias) | High risk | No allocation concealment used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear if the staff delivering care or the participants were aware of the study. |
| Blinding of outcome assessment (detection bias) Objective | Low risk | N/A ‐ no objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective | Unclear risk | Data obtained from self‐report surveys |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up rate was either 100% or 88% for all outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Baseline measures | Unclear risk | The participants in each group were matched based on ADL, mobility and orientation. Across groups, age, ADL and mobility are similar |
| Free of contamination | Low risk | No crossover |
| Other bias | High risk | Possible reverse causality |
Braun 1991.
| Methods | Study design: non‐randomised trial | |
| Participants |
Participants: N = 352 (Control: 131; Intervention: 221 [foster care N = 138, home care N = 83]) Mean age: Control: 79.85 years, Intervention: 78.83 years Gender: Control: 62% female, Intervention: 63% female. Setting: Hawaii |
|
| Interventions |
Type of intervention: foster home care or care at home Description of the intervention: care provided in a foster home setting with comprehensive Intermediate Care Facility home services. Control: nursing home care |
|
| Outcomes | Mortality at 6 months, change in ADL at 6 months using Katz Index of ADLs | |
| Notes | Baseline imbalances include reduced prevalence of dementia in those allocated to care in community. Note: patients received foster care or care in their own home. Care in their own home was provided by Nursing Without Walls. Case managers arrange a package of services for the family, most commonly personal care, chore service, meals, home modifications, and transportation. In all, the study involved 131 patients in 10 nursing homes, 138 patients in 98 foster homes, and 83 patients in their own homes, for a total of 352 patients. In this case the intervention arm data combined both foster and home care, with most data coming from foster care and thus, we could not include the results for analysis in the comparison of home care with nursing care. Study supported by the Hawaii Department of Social Services and Housing; and the Henry J Kaiser Foundation (California, USA) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is not a randomised trial. As described, the study uses a 2‐step Heckman procedure to further control for selection bias before comparing outcomes of 352 patients in nursing homes and community care. |
| Allocation concealment (selection bias) | High risk | No allocation concealment used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants had to provide informed consent, so they were likely unblinded. Unclear if personnel were aware of study |
| Blinding of outcome assessment (detection bias) Objective | Low risk | Data obtained from medical records |
| Blinding of outcome assessment (detection bias) Subjective | Unclear risk | Data obtained from self‐report survey Unclear if the personnel were aware of the study purpose or if this was similar to routinely collected data. All data were collected by 2 hospital staff members (one fluent in Japanese) within a few days of admission to the long‐term care setting by observing and interviewing patients, reviewing charts, and discussing patient physical function with formal and informal caregivers. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | For mortality this would be low as data is available for all participants; however, it would be high for physical function. Timing of and reason for discharge were available for all 352 members of the sample. Six‐month ADL and mobility scores were available for the 220 patients still in placement at the end of 6 months: 54 (65%) in Nursing Home Without Walls, 80 (58%) in foster care, and 86 (65%) in nursing homes. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Baseline measures | High risk | Groups differed at baseline on several factors, including ethnicity and comorbidities such as dementia, cancer and musculoskeletal diagnoses, as well as baseline levels of medications used, ADL function and mobility levels. |
| Free of contamination | Low risk | No crossover |
| Other bias | High risk | Possibility of reverse casualty |
Challis 1991.
| Methods | Study design: non‐randomised trial | |
| Participants |
Participants: N = 214 (Control: 113; Intervention: 101) Mean age: Control: 81 years; Control: 80 years Gender: Control: 65% female; Intervention: 64% female Setting: UK |
|
| Interventions |
Type of intervention: care in own home Description of the intervention: people receiving care in their own home from the Darlington Care Project. Darlington Care Project: case management service, which could include any number of medical services based on client needs (speech therapy, stoma care, catheter care, change of dressing). Personal care (bathing, dressing, toileting, feeding, hand/nail care), physical care, (assist with walking, lifting/transferring). Social and recreational activities and therapeutic exercises Control: long‐stay care wards |
|
| Outcomes | Mortality at 6 and 12 months, subjective well‐being at 6 months | |
| Notes | Increased social disturbance in participants allocated to nursing home care, increased length of stay for control patients No information about funding sources. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial. The study compared individual cases receiving services from the project group with a similar group of patients in LTC in adjacent health districts. Groups were not randomly allocated to receive a particular intervention. |
| Allocation concealment (selection bias) | High risk | No allocation to conceal |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel and participants in the home care group were likely unblinded as part of the Darlington Care Project. |
| Blinding of outcome assessment (detection bias) Objective | Low risk | Data obtained from medical records |
| Blinding of outcome assessment (detection bias) Subjective | Unclear risk | Data obtained from self‐report surveys |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 100% of data participant data available on mortality; however, likely high risk for quality of life with less than 60% of cases providing data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Baseline measures | Unclear risk | Age and disability appear similar at baseline; however, there may be difference in social disturbance and length of stay indicating a possible selection effect |
| Free of contamination | Low risk | No crossover |
| Other bias | High risk | Potential for baseline confounding |
Chuang 2005.
| Methods | Study design: non‐randomised trial | |
| Participants |
Participants: N = 474 (Control: HCBC, N = 144; FC, N = 264; Intervention: N = 66) Mean age: 71.2 years Gender: 46% female Participants were included if they had had a stroke Setting: Taiwan |
|
| Interventions |
Type of intervention: care in the community Description of the intervention: people receiving care in the community. This was either respite care, day care, home maker services or care provided by a live‐in personal helper Control: nursing home care |
|
| Outcomes | Mortality at 6 months, physical function at 6 months | |
| Notes | This study was longitudinal and classified patients into groups depending on the type of care they had received in the previous 6 months. There were 3 categories of care: institution (INS), home‐ or community‐based care (HCBC), and family care (FC). Since family care was defined as only receiving care solely by a family member with no formal care services, this group was not included for analysis in the main comparison. Study supported by the National Health Research Institute, Taiwan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial. This study used a longitudinal quasi‐experimental study design following stroke patients 6 months after discharge |
| Allocation concealment (selection bias) | High risk | Participants were allocated based on their self‐report of LTC received during the telephone survey |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Hospitals and patients were unblinded to study. Page 67‐68: "formal approvals of the study were obtained from the hospitals. Patients and their families were given a written statements describing the purpose of the study". It is unclear if formal care providers were blinded. |
| Blinding of outcome assessment (detection bias) Objective | Low risk | Data obtained from medical records |
| Blinding of outcome assessment (detection bias) Subjective | Unclear risk | Data obtained from self‐report surveys |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that 100% of data provided for mortality |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Baseline measures | Unclear risk | Unclear |
| Free of contamination | Low risk | No crossover |
| Other bias | High risk | Potential for residual confounding |
Condelius 2010.
| Methods | Study design: observational cohort study, data set | |
| Participants |
Participants: N = 694 (Control: N = 477 [special accommodation: N = 269], Intervention N = 402 [home care: N = 425]) Mean age: 80 years Gender: 65% female Participanst were included if they had one or more hospital admission during the year 2001, were aged 65 years or older and received long‐term care and services from the municipality Setting: Sweden |
|
| Interventions |
Type of intervention: care in own home Description of the intervention: participants receiving at least 4 visits per month in their home from care services. Help with laundry, shopping, cleaning, and personal care. Excluded meals on wheels or transport services Control: nursing home care |
|
| Outcomes | Hospital visits and outpatient usage at 1 year; physical function (assessed using ADL staircase); general health (assessed using a 6‐item health complaints questionnaire); mental health (assessed using the Berger scale) Data were collected by means of a form completed by Registered Nurses, assistant officers, physiotherapists, or occupational therapists. The form comprises questions concerning demographic data, physical function, health complaints, adaptation and standard of housing and formal and informal care. |
|
| Notes | Baseline imbalances included younger age and less dependency in those living at home Study supported by the Vardal Institute (Sweden); the Swedish Institute for Health Sciences; and the Faculty of Medicine, Unit of Caring Sciences, Lund University, Sweden |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not a randomised trial. Using routinely collected data within an existing data set |
| Allocation concealment (selection bias) | High risk | No allocation methods used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if consent was obtained for this study. Since this is just an analysis of an existing data set, it is unlikely that participants are aware of this study. |
| Blinding of outcome assessment (detection bias) Objective | Unclear risk | Data from medical records. Data were collected as part of another national study on ageing ‐ unlikely data collection team were aware of this study aim |
| Blinding of outcome assessment (detection bias) Subjective | Unclear risk | Data obtained from self‐report surveys |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcomes of interest were reported. |
| Baseline measures | High risk | Participant characteristics differed between groups. Participants that received care at home were younger, lived with someone, less dependent and less depressed mood. |
| Free of contamination | Low risk | No crossover |
| Other bias | High risk | Possibility of reverse causality |
Mitchell 1978.
| Methods | Study design: non‐randomised trial | |
| Participants |
Participants N =195. (Control: N = 87; Intervention: N = 108) Mean age: 65.6 years Gender: All patients were male Setting: USA |
|
| Interventions |
Type of intervention: care in own home Description of the intervention: participants receiving care in their own home from a home care team including physician, nurse, dietician and social worker as a minimum. Medical care and ancillary services in participant's own home. A potential caregiver (friend, relative, or hired caretaker) must be living in the patient's home and able to assume responsibility for care. Control: nursing home care |
|
| Outcomes | Disability at 3 months using functional status index | |
| Notes | Partially supported by a doctoral training grant from the Department of Medicine and Surgery, Veterans Administration (USA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial (page 443). As random assignment of participant could not be achieved, it was necessary to control for differences in initial health status; the VA hospitals were not chosen randomly. |
| Allocation concealment (selection bias) | High risk | No allocation methods used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear. it is not reported if participants knew of the study or its aims |
| Blinding of outcome assessment (detection bias) Objective | Unclear risk | N/A ‐ no objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective | Unclear risk | Data from self‐report survey. It is not reported if the social workers collecting the data were blinded to study aims |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The total sample was 195, not one of whom was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Baseline measures | Low risk | Adjustments made, including for initial health status |
| Free of contamination | Low risk | No crossover |
| Other bias | High risk | Potential for residual confounding |
Oktay 1987.
| Methods | Study design: randomised trial | |
| Participants |
Participants: N = 112 (Control: N = 53; Intervention: N = 59) Meand age: Control: 69.8 years, Intervention: 70.2 years Gender: Control 64.2% female, Intervention 64.6% female Setting: USA |
|
| Interventions |
Type of intervention: care in foster home Description of the intervention: people living in a foster home setting. Caregivers provided the patient with meals, laundry, assistance with personal and instrumental ADLS, 24‐hour supervision and nursing tasks as needed (e.g. monitoring medication, injections and behavioural modification) Control: nursing home care |
|
| Outcomes | all outcomes were measured at 12 months, Mortality, attitudes‐life satisfaction, attitudes‐perceived health, physical function, basic ADL and instrumental ADL, mental status | |
| Notes | Study supported by the Robert Wood Johnson Foundation, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how the random sequence was generated |
| Allocation concealment (selection bias) | Low risk | Random sequence was placed in sealed envelopes and opened in sequence by a blinded research assistance once a patient was deemed eligible (page 1506). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants. Unclear if personnel were aware of study aim. |
| Blinding of outcome assessment (detection bias) Objective | Low risk | Data obtained from medical records |
| Blinding of outcome assessment (detection bias) Subjective | Unclear risk | Data obtained from self‐report survey. It is not reported if the social workers collecting the data were blinded to study aims. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported but no means or measures of variability reported for any outcome |
| Baseline measures | High risk | Groups were different at baseline on ethnicity and marital status, which could influence outcome |
| Free of contamination | Low risk | No crossover |
| Other bias | Low risk | Randomised trial. Note: only 20% of the eligible sample agreed to participate in the study |
Sherwood 1986.
| Methods | Study design: observational cohort study | |
| Participants |
Participants: N = 98 (Control: N = 49, Intervention: N = 49) Setting: USA |
|
| Interventions |
Type of intervention: care in foster home Description of the intervention: people living foster homes and receiving services through the Pennsylvania Domiciliary Care Programme. Foster homes provide personal care services for 1‐3 clients. Counselling, transportation, meals, recreational activities, information/referral, and monitoring services. Based on a patient assessment, the programme could provide medication monitoring, and/or arrange for various types of therapies Control: nursing home care |
|
| Outcomes | All outcomes were measured at 9 months. Community integration and feeling of contentment; utilisation of skills for independent living | |
| Notes | Study supported by the Department of Health and Human Services, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial |
| Allocation concealment (selection bias) | High risk | No allocation methods used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear if participants or personnel had knowledge of the study aim. |
| Blinding of outcome assessment (detection bias) Objective | Unclear risk | N/A ‐ no objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective | Unclear risk | Data obtained from self‐report. It is unclear if the trained interviewers collecting data knew of the study aim or patient allocation. Additionally some data on services were collected by a third party. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The sample sizes at baseline and follow‐up appear similar |
| Selective reporting (reporting bias) | High risk | Authors selectively reported results |
| Baseline measures | Low risk | Simulated random group assignment computer procedure used to match groups by large number of variables |
| Free of contamination | Low risk | No crossover |
| Other bias | High risk | Baseline confounding |
Wilson 2005.
| Methods | Study design: observational cohort study | |
| Participants |
Participants: N = 11,803 (Control: N = 4774; Intervention: N = 7029) Mean age: Control: 83 years, Intervention: 81 years Gender: Control 74.3% female, Intervention 76.9% female Setting: Canada |
|
| Interventions |
Type of intervention: care in own home Description of the intervention: people living in own home receiving home care services Control: nursing home care |
|
| Outcomes | Admission to hospital over a 2‐year period | |
| Notes | Study supported by the Health Research Fund of the Alberta Heritage Foundation for Medical Research, Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial |
| Allocation concealment (selection bias) | High risk | No allocation methods used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This study used retrospective, routinely collected data and thus participants or personnel were unaware of the study aim |
| Blinding of outcome assessment (detection bias) Objective | Low risk | Data obtained from medical records |
| Blinding of outcome assessment (detection bias) Subjective | Unclear risk | N/A ‐ no subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It would appear that all data were obtained for the cohort of interest. There was some missing data identified during the data cleaning process but was described as < 1% |
| Selective reporting (reporting bias) | Low risk | All outcomes of interest appear to be reported with point estimates and measures of variance |
| Baseline measures | High risk | Limited data provided on key baseline characteristics with no adjustment for baseline status |
| Free of contamination | Low risk | No crossover |
| Other bias | High risk | Potential multiple baseline confounders |
Wysocki 2014.
| Methods | Study design: observational cohort study, dataset | |
| Participants |
Participants: N = 2338 (Control: N = 1169; Intervention: N = 1169) Age: range 65‐91 Gender: Control: 75% female; Intervention: 73% female Setting: USA |
|
| Interventions |
Type of intervention: care in own home or community Description of the intervention: home‐ and community‐based services provided by Medicaid within the first month after being discharged from a nursing home in which they had received care for at least 90 days (called transitioners) Control: nursing home care (called stayers) |
|
| Outcomes | All outcomes were assessed at 12 months. Primary outcome: potentially preventable hospitalisations: hospitalisations with an ambulatory case‐sensitive condition Secondary: hospitalisations of any type |
|
| Notes | The total populations included 32,504 stayers and 1,942 transitioners. The study sample used a propensity score matching method (page 73). After matching the sample this study included 1169 in each group. Study supported by the Centers for Medicare and Medicaid Services, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial |
| Allocation concealment (selection bias) | High risk | No allocation methods used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This study used data from 7 US states, routinely collected as part of an ongoing initiative to examine progress for rebalancing LTC programs ‐ it is unlikely that participants or personnel were aware of this study aim |
| Blinding of outcome assessment (detection bias) Objective | Unclear risk | Data obtained from medical records |
| Blinding of outcome assessment (detection bias) Subjective | Low risk | N/A ‐ no subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data were available for the included sample |
| Selective reporting (reporting bias) | Low risk | Data were reported for all outcomes of interest with point estimates and measures of variance |
| Baseline measures | Low risk | Groups were matched on key criteria (age, gender, ethnicity, residence, diagnosis, ADLs and cognitive impairment) and appear similar. |
| Free of contamination | Low risk | No crossover |
| Other bias | Unclear risk | Potential multiple baseline confounders |
ADL: activities of daily living; FC: family care; HCBC: home‐ or community‐based care; LTC: long‐term care; N/A: not applicable
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anstey 2007 | Does not look at enhanced domiciliary services |
| Beerens 2014 | Cross‐sectional survey |
| Boggatz 2009 | Looks only at barriers to acceptance of care models |
| Bowling 1991 | Does not include enhanced home care services |
| Boyle 2004 | Groups different at baseline |
| Brajkovic 2009 | Cross‐sectional survey |
| Brennan 2003 | Does not look at functionally dependent older people in the community |
| Chappell 2004 | Only lists economic data |
| Davis 2005 | Looks at assessment of dementia in 3 different settings |
| Hasson 2011 | Cross‐sectional survey |
| Hollander 2007 | No robust outcome data reported |
| Hughes 1988 | Does not include institutional care setting |
| Kane 1991 | Does not include stated primary or secondary outcomes |
| Karakaya 2009 | Does not look at functionally dependent older people in the community |
| Kelley‐Gillespie 2011 | Observational before after study with no control |
| Kuo 2010 | Does not include stated primary or secondary outcomes |
| Lane 2004 | Does not look at functionally dependent older people in the community |
| Marek 2012 | Only lists economic data |
| Nikmat 2013 | Cross‐sectional survey |
| Page 2009 | Does not include stated primary or secondary outcomes |
| Powers 1985 | Cost analysis study |
| Skellie 1982 | Does not include institutional care setting |
| Wilson 2007 | Does not fit defined interventions and outcomes |
Differences between protocol and review
We amended the title of the review to reflect the interventions identified for inclusion. We introduced other changes to comply with Cochrane standards for conducting and reporting a review, namely introduced a Summary of findings table. Two authors left the review team (EMMvdG and LH) and two authors joined the review team (AMH and DCGB).
Contributions of authors
Two review authors (CY and DS) independently selected studies, assessed the methodological quality of the studies and extracted data.CY, AH, DGB, and DS drafted the review, and all authors read and commented on drafts and approved the final version. All authors made substantial contributions to the conception and design of the work.
Sources of support
Internal sources
No sources of support supplied
External sources
Supported by an unrestricted grant from the Age‐related Diseases Trust, TJQ is supported by a joint Stroke Association/Chief Scientist Office Senior Clinical Lecturer Fellowship, UK.
NIHR Cochrane Programme grant, UK.
Declarations of interest
CY: the Age Related Diseases Trust provided grant support to the University of Glasgow for this review
AMH: none known
DGB: none known
TJQ: none known
LH: none known
BVM: none known
DS: the Age Related Diseases Trust provided grant support to the University of Glasgow for this review
New
References
References to studies included in this review
Braun 1987 {published data only}
- Braun KL, Rose CL. Geriatric patient outcomes and costs in three settings: nursing home, foster family, and own home. Journal of the American Geriatrics Society 1987;35(5):387‐97. [DOI] [PubMed] [Google Scholar]
Braun 1991 {published data only}
- Braun KL, Rose CL, Finch MD. Patient characteristics and outcomes in institutional and community long term care. Gerontologist 1991;31(5):648‐56. [DOI] [PubMed] [Google Scholar]
Challis 1991 {published data only}
- Challis D, Darton R, Johnson L, et al. An evaluation of an alternative to long stay hospital care for frail elderly patients: II Costs and effectiveness. Age and Ageing 1991;20(4):245‐54. [DOI] [PubMed] [Google Scholar]
Chuang 2005 {published data only}
- Chuang KY, Wu SC, Yeh MC, et al. Exploring the associations between long term care and mortality rates among stroke patients. Journal of Nursing Research 2005;13(1):66‐73. [PubMed] [Google Scholar]
Condelius 2010 {published data only}
- Condelius A, Hallberg IR, Jakobsson U. Medical healthcare utilization as related to long term care at home or in special accomodation. Archives of Gerontology and Geriatrics 2010;51(2):250‐6. [DOI] [PubMed] [Google Scholar]
Mitchell 1978 {published data only}
- Mitchell JB. Patient outcomes in alternative long term care settings. Medical Care 1978;16(6):439‐52. [DOI] [PubMed] [Google Scholar]
Oktay 1987 {published data only}
- Oktay JS, Volland PJ. Foster home care for the frail elderly as an alternative to nursing home care: an experimental evaluation. American Journal of Public Health 1987;77(12):1505‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherwood 1986 {published data only}
- Sherwood S, Morris JN, Ruchlin HS. Alternative paths to long term care: nursing home, geriatric day hospital, senior centre, and domiciliary care options. American Journal of Public Health 1986;76(1):38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2005 {published data only}
- Wilson D, Truman C. Comparing the health service utilization of long term care residents, home care recipients, and the well elderly. Canadian Journal of Nursing Research 2005;37(4):138‐54. [PubMed] [Google Scholar]
Wysocki 2014 {published data only}
- Wysocki A, Kane RL, Dowd B, Golberstein E, Lum T, Shippee T. Hospitalization of elderly Medicaid long‐term care users who transition from nursing homes. Journal of the American Geriatrics Society 2014;62(1):71‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anstey 2007 {published data only}
- Anstey KJ, Sanden C, Sargent‐Cox K, Luszcz M. Prevalence and risk factors for depression in a longitudinal, population based study including individuals in the community and residential care. American Journal of Psychiatry 2007;15:497‐505. [DOI] [PubMed] [Google Scholar]
Beerens 2014 {published data only}
- Beerens HC, Sutcliffe C, Renom‐Guiteras A, Soto ME, Suhonen R, Zabalegui A, et al. RightTimePlaceCare Consortium. Quality of life and Quality of care for people with dementia receiving long term institutional care or professional home care: the European RightTimePlaceCare Study. Journal of the American Medical Directors Association 2014;15:54‐61. [DOI] [PubMed] [Google Scholar]
Boggatz 2009 {published data only}
- Boggatz T, Farid T, Mohammedin A, Dassen T. Factors related to the acceptance of home care and nursing homes among older Egyptians: a cross‐sectional study. International Journal of Nursing Studies 2009;46:1585‐94. [DOI] [PubMed] [Google Scholar]
Bowling 1991 {published data only}
- Bowling A, Formby J, Grant K, Ebrahim S. A randomised controlled trial of nursing home and long term stay geriatric ward care for elderly people. Age and Aging 1991;20:316‐24. [DOI] [PubMed] [Google Scholar]
Boyle 2004 {published data only}
- Boyle G. Facilitating choice and control for older people in long term care. Health and Social Care in the Community 2004;12:212‐20. [DOI] [PubMed] [Google Scholar]
Brajkovic 2009 {published data only}
- Brajkovic L, Godan A, Godan L. Quality of life after stroke in old age: comparison of persons living in nursing home and those living in their own home. Croatian Medical Journal 2009;50:182‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brennan 2003 {published data only}
- Brennan J, Johansen A, Butler J, Stone M, Richmond P, Jones S, et al. Place of residence and risk of fracture in older people: a population‐based study of over 65‐year‐olds in Cardiff. Osteoporosis International 2003;14:515‐9. [DOI] [PubMed] [Google Scholar]
Chappell 2004 {published data only}
- Chappell NL, Dlitt BH, Hollander MJ, Miller JA, McWilliam C. Comparative costs of home care and residential care. Gerontologist 2004;44:389‐400. [DOI] [PubMed] [Google Scholar]
Davis 2005 {published data only}
- Davis LA, Hoppes S, Chesbro SR. Cognitive‐communicative and independent living skills assessment in individuals with dementia ‐ a pilot study of environmental impact. Topics in Geriatric Rehabilitation 2005;21:136‐43. [Google Scholar]
Hasson 2011 {published data only}
- Hasson H, Arnetz JE. Care recipients and family members perceptions of quality of older people care: a comparison of home based care and nursing homes. Journal of Clinical Nursing 2011;20:1423‐35. [DOI] [PubMed] [Google Scholar]
Hollander 2007 {published data only}
- Hollander MJ, Chappell NL. A comparative analysis of costs to government for home care and long‐term residential care services, standardized for client care needs. Canadian Journal on Aging 2007;26:149‐61. [DOI] [PubMed] [Google Scholar]
Hughes 1988 {published data only}
- Hughes SL, Conrad KJ, Manheim LM, Edelman PL. Impact of long‐term home care on mortality, functional status, and unmet needs. Health Services Research 1988;23:269‐94. [PMC free article] [PubMed] [Google Scholar]
Kane 1991 {published data only}
- Kane RA, Kane RL, Illston LH, Nyman JA, Finch MD. Adult foster care for the elderly in Oregon: a mainstream alternative to nursing homes?. American Journal of Public Health 1991;81:1113‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karakaya 2009 {published data only}
- Karakaya MG, Bilgin SC, Ekici G, Kose N, Otman AS. Functional mobility, depressive symptoms, level of independence, and quality of life of the elderly living at home and in the nursing home. Journal of the American Medical Directors Association 2009;10:662‐6. [DOI] [PubMed] [Google Scholar]
Kelley‐Gillespie 2011 {published data only}
- Kelley‐Gillespie N. A secondary analysis of perceptions of quality of life of older adults residing in a nursing home and assisted living setting using an integrated conceptual model of measurement. Applied Research Quality Life 2012;7:137‐54. [Google Scholar]
Kuo 2010 {published data only}
- Kuo YC, Lan CF, Chen LK, Lan VM. A secondary analysis of perceptions of quality of life of older adults residing in a nursing home and assisted living setting using an integrated conceptual model of measurement. Applied Research in Quality of Life 2010;7:159‐63. [Google Scholar]
Lane 2004 {published data only}
- Lane CJ, Bronskill SE, Sykora K, Dhalla IA, Anderson GM, Mamdani MM, et al. Potentially inappropriate prescribing in Ontario community‐dwelling older adults and nursing home residents. Journal of the American Geriatrics Society 2004;52:861‐6. [DOI] [PubMed] [Google Scholar]
Marek 2012 {published data only}
- Marek KD, Stetzer F, Adams SJ, Popejoy LL, Rantz M. Aging in place versus nursing home care: comparison of costs to Medicare and Medicaid. Research in Gerontological Nursing 2012;5:123‐9. [DOI] [PubMed] [Google Scholar]
Nikmat 2013 {published data only}
- Nikmat AW, Hawthorne G, Al‐Mashoor SH. The comparison of quality of life among people with mild dementia in nursing home and home care: a preliminary report. Dementia 2015;14:114‐25. [DOI] [PubMed] [Google Scholar]
Page 2009 {published data only}
- Page C, Conner T, Prokhorov A, Fang Y, Post L. The effect of care setting on elder abuse: results from a Michigan survey. Journal of Elder Abuse & Neglect 2009;21:239‐52. [DOI] [PubMed] [Google Scholar]
Powers 1985 {published data only}
- Powers JS, Burger C. Home health care vs nursing home care for the elderly. Journal of the Tennessee Medical Association 1995;78:227‐30. [PubMed] [Google Scholar]
Skellie 1982 {published data only}
- Skellie FA, Mobley GM, Coan RE. Cost‐effectiveness of community‐based long‐term care: current findings of Georgia's alternative health services project. American Journal of Public Health 1982;72:353‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2007 {published data only}
- Wilson RS, McCann JJ, Li Y, Aggarwal NT, Gilley DW, Evans DA. Nursing home placement, day care use, and cognitive decline in Alzheimer's disease. American Journal of Psychiatry 2007;164:910‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Craig 2008
- Craig P, Diepp P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2013
- Effective Practice, Organisation of Care (EPOC). Data collection form. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. 2013 Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
EPOC 2013a
- Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for review authors. 2013. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services, 2015. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
EPOC 2016
- Effective Practice, Organisation of Care (EPOC). What study designs should be included in an EPOC review? EPOC Resources for review authors. 2016. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. (updated March 2011). The Cochrane Collaboration, 2011. Available from cochrane‐handbook.org.
Iwarsson 2007
- Iwarsson S1, Wahl HW, Nygren C, Oswald F, Sixsmith A, Sixsmith J, et al. Importance of the home environment for healthy aging: conceptual and methodological background of the European ENABLE–AGE Project. Gerentologist 2007;47(1):78‐84. [DOI] [PubMed] [Google Scholar]
Katz 1963
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185(12):914‐9. [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel D. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:56‐61. [PubMed] [Google Scholar]
Morrison 2012
- Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English‐language restriction on systematic review‐based meta‐analyses: a systematic review of empirical studies. International Journal of Technology Assessment in Health Care 2012;28(2):138‐144. [DOI: 10.1017/S0266462312000086] [DOI] [PubMed] [Google Scholar]
Mueller 2007
- Mueller PS, Montori VM, Bassler D, Koenig BA, Guyatt GH. Ethical issues in stopping randomized trials early because of apparent benefit. Annals of Internal Medicine 2007;146(12):878‐881. [DOI: 10.7326/0003-4819-146-12-200706190-00009] [DOI] [PubMed] [Google Scholar]
WHOQOL Group 1995
- The WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): position paper from the World Health Organization. Social Science & Medicine 1995;41(10):1403‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mottram 2002
- Mottram P, Pitkala K, Lees C. Institutional versus at‐home long term care for functionally dependent older people. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003542] [DOI] [PubMed] [Google Scholar]
Young 2012
- Young C, Glind EMM, Quinn TJ, Hooft L, Legg LA, Munster BC, et al. At‐home versus institutional long‐term‐care for chronic functionally dependent older people. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009844] [DOI] [PMC free article] [PubMed] [Google Scholar]


