Abstract
Background
Soft tissue sarcomas (STS) are a highly heterogeneous group of rare malignant solid tumors. Nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) comprise all STS except rhabdomyosarcoma. In people with advanced local or metastatic disease, autologous hematopoietic stem cell transplantation (HSCT) applied after high‐dose chemotherapy (HDCT) is a planned rescue therapy for HDCT‐related severe hematologic toxicity. The rationale for this update is to determine whether any randomized controlled trials (RCTs) have been conducted and to clarify whether HDCT followed by autologous HSCT has a survival advantage.
Objectives
To assess the efficacy and safety of high‐dose chemotherapy (HDCT) followed by autologous hematopoietic stem cell transplantation (HSCT) for all stages of nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in children and adults.
Search methods
For this update, we revised the search strategy to improve the precision and reduce the number of irrelevant hits. We searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8), PubMed from 2012 to 6 September 2016, and Embase from 2012 to 26 September 2016. We searched online trial registries and congress proceedings from 2012 to 26 September 2016.
Selection criteria
Terms representing STS and autologous HSCT were required in the title or abstract. We restricted the study design to RCTs. We included studies if at least 80% of participants had a diagnosis listed in any version of the World Health Organization (WHO) classification and classified as malignant. The search included children and adults with no age limits.
Data collection and analysis
We used standard methodologic procedures expected by Cochrane. The primary outcomes were overall survival and treatment‐related mortality.
Main results
We identified 1549 records; 85 items from electronic databases, 45 from study registries, and 1419 from congress proceedings. The revised search strategy did not identify any additional RCTs. In the previous version of the review, we identified one RCT comparing HDCT followed by autologous HSCT versus standard‐dose chemotherapy (SDCT). The trial randomized 87 participants who were considerably heterogeneous with respect to 19 different tumor entities. The data from 83 participants were available for analysis.
In the single included trial, overall survival at three years was 32.7% in the HDCT arm versus 49.4% in the SDCT arm and there was no difference between the treatment groups (hazard ratio (HR) 1.26, 95% confidence interval (CI) 0.70 to 2.29, P = 0.44; 1 study, 83 participants; high quality evidence). In a subgroup of participants who had a complete response before HDCT, overall survival was higher in both treatment groups and overall survival at three years was 42.8% in the HDCT arm versus 83.9% in the SDCT arm and favored the SDCT group (HR 2.92, 95% CI 1.1 to 7.6, P = 0.028; 1 study, 39 participants).
In the single included trial, the authors reported one treatment‐related leukemia death two years after HDCT. They also evaluated severe adverse events WHO grade 3 to 4 in 22 participants in the HDCT arm and in 51 participants in the SDCT arm. The authors reported 11 events concerning digestive‐, infection‐, pain‐, or asthenia‐related toxicity in the HDCT arm and one event in the SDCT arm (moderate quality evidence). The development of secondary neoplasia was not addressed. We judged the study to have an overall unclear risk of bias as three of seven items had unclear and four items had low risk of bias. For GRADE, we judged three items as high quality and three items were not reported.
Authors' conclusions
The limited data of a single RCT with an unclear risk of bias and moderate to high quality evidence showed no survival advantage for HDCT. If this treatment is offered it should only be given after careful consideration on an individual person basis and possibly only as part of a well‐designed RCT.
Keywords: Adult; Aged; Humans; Middle Aged; Antineoplastic Combined Chemotherapy Protocols; Antineoplastic Combined Chemotherapy Protocols/administration & dosage; Antineoplastic Combined Chemotherapy Protocols/adverse effects; Hematopoietic Stem Cell Transplantation; Hematopoietic Stem Cell Transplantation/methods; Hematopoietic Stem Cell Transplantation/mortality; Salvage Therapy; Salvage Therapy/methods; Salvage Therapy/mortality; Sarcoma; Sarcoma/drug therapy; Sarcoma/mortality; Transplantation, Autologous
Plain language summary
High‐dose chemotherapy followed by autologous hematopoietic stem cell transplantation for nonrhabdomyosarcoma soft tissue sarcomas
Review question
We reviewed the evidence about the effect of high‐dose chemotherapy (medicines to kill the cancer) followed by autologous hematopoietic stem cell transplantation compared to standard‐dose chemotherapy on overall survival (time from cancer diagnosis, or treatment, to death from any cause) in people with nonrhabdomyosarcoma soft tissue sarcomas. We found one randomized controlled trial (RCT; a clinical study where people are randomly put into one of two or more treatment groups) comparing both treatments.
Background
Nonrhabdomyosarcoma soft tissue sarcomas are a group of rare cancers. People with inoperable (cannot be removed during an operation) or metastatic (where the cancer has spread to other parts of the body) disease have a poor prognosis (outcome). It was believed that higher doses of chemotherapy might improve people's survival. However, high doses of chemotherapy stop the production of blood cells in the bone marrow and can be harmful. Stem cells (cells that can form into many cell types) collected from people before high‐dose chemotherapy can be transplanted back to the person if the blood cell count gets too low; this is called autologous hematopoietic stem cell transplantation. Due to a lack of research studies, it has not been proven that people treated like this live any longer than people treated with standard chemotherapy. We wanted to determine whether using high‐dose chemotherapy followed by autologous hematopoietic stem cell transplantation was better or worse than standard‐dose chemotherapy.
Study characteristics
The evidence is current to 6 September 2016. We found one RCT that compared 38 people in the high‐dose chemotherapy and transplantation group versus 45 people in the chemotherapy‐only group and was judged to have mainly a low risk of bias (as it was well designed). The participants were 18 to 65 years old, had various types of nonrhabdomyosarcoma soft tissue sarcomas and were monitored for about 55 months. The treatment period ranged from 2000 to 2008. The single RCT was funded by a nonprofit organization (the funder did not benefit if the trial found good results).
Key results
The results of the RCT did not favor either of the two treatment arms with respect to overall survival. There was one death related to treatment in the transplantation group and none in the chemotherapy‐only group. There were eight cases of severe nonhematologic (not related to the blood) side effects in the transplantation group and one in the chemotherapy‐only group.
Quality of evidence
The overall quality of the data was unclear and based on only one RCT. Currently, research evidence is limited for the use of high‐dose chemotherapy followed by autologous hematopoietic stem cell transplantation for people with non‐rhabdomyosarcoma soft tissue sarcomas. Further evidence is needed through well‐designed clinical trials.
Summary of findings
Summary of findings for the main comparison. Autologous hematopoietic stem cell transplantation following high‐dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcoma.
| Autologous hematopoietic stem cell transplantation following high‐dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcoma | ||||||
|
Patient or population: people with non‐rhabdomyosarcoma soft tissue sarcoma Settings: specialized hospital Intervention: autologous hematopoietic stem cell transplantation following HDCT Comparison: SDCT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| SDCT | Autologous HSCT following HDCT | |||||
|
Overall survival Follow‐up: median 55 months |
489 per 1000 | 571 per 1000 (375 to 785) | HR 1.26 (0.7 to 2.29) | 83 (1 study) | ⊕⊕⊕⊕ High | ‐ |
|
Treatment‐related mortality Follow‐up: 24 months |
See comment | See comment | Not estimable | 83 (1 study) | ⊕⊕⊕⊕ High | 1 event 2 years after HDCT and 0 events after SDCT |
| Disease‐free survival Follow‐up: 3 years | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
|
Progression‐free survival Follow‐up: median 55 months |
756 per 1000 | 849 per 1000 (681 to 955) | HR 1.34 (0.81 to 2.2) | 83 (1 study) | ⊕⊕⊕⊕ High | ‐ |
| Non‐hematologic toxicity grade 3 to 4 | See comment | See comment | Not estimable | ‐ | See comment | Not adequately reported, people from within and without the randomization were mixed in the control arm. |
| Health‐related quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HDCT: high‐dose chemotherapy; HR: hazard ratio; HSCT: hematopoietic stem cell transplantation; SDCT: standard‐dose chemotherapy. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Soft tissue sarcomas (STS) are a highly heterogeneous group of rare malignant solid tumors of nonepithelial extraskeletal body tissue and are classified on a histogenetic basis (Weiss 2001). STS have a significant risk of distant metastasis in addition to the potential for locally destructive growth and recurrence. Nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) comprise all STS except rhabdomyosarcoma, which primarily affects children and young adults. In this review, we investigated NRSTS which are categorized as malignant according to the World Health Organization (WHO) classification and included in any of the 2002 first (Fletcher 2002) or the 2013 updated second version (Fletcher 2013). Rhabdomyosarcoma was addressed in the Cochrane Review by Admiraal 2010.
NRSTS usually originate de novo and rarely from benign tumors. In most cases, the pathogenesis is unknown; however, some factors are associated with the development of NRSTS (Weiss 2001). These include exposure to ionizing radiation, environmental carcinogenic substances, oncogenic viruses, and immunologic factors. Genetic factors can also play a role since some inherited diseases such as neurofibromatosis type 1 are associated with a higher risk of NRSTS (Tsao 2000). NRSTS are rare in both children and adults and the distribution of NRSTS differs significantly between children and adults according to Spunt 2006. In the USA, the yearly incidence of STS is 1 per 100,000 population for people 20 years of age or younger and about 7 per 100,000 population for people 20 years of age or older (NCI snapshot 2014). Between 2009 and 2013, the median age at diagnosis of STS, including tumors of the heart, was 59 years (Howlader 2016). Rhabdomyosarcoma represents about 50% of STS in children (Gurney 1997; Miller 1995).
Disease progression may be dichotomized into the two categories of limited and extensive disease. Limited disease is typically localized, small‐sized, low‐grade, and operable and is an accessible tumor that has no regional lymph node involvement and no distant metastases. Extensive disease can also be denoted as advanced disease, defined as a localized, large‐sized, and high‐grade tumor that may not be completely removed by surgery, may be invasive, and may have regional lymph node involvement or distant metastases. Both categories differ significantly in terms of prognosis and treatment. Many people with limited disease may be cured by surgery whereas extensive disease is associated with a poor outcome and many people may receive chemotherapy as palliative therapy.
The Tumor, Node, Metastasis (TNM) staging system is developed and maintained by the Union for International Cancer Control (UICC 2009). It combines grade, depth, and size of the tumor as well as regional lymph node involvement and distant metastases and describes the extent of a cancer's spread from stage 0 to IV. It is used by other organizations (AJCC 2016; NCI staging 2015) and combines grade, depth and size of the tumor as well as regional lymph node involvement and distant metastases, and describes the extent of a cancer's spread from stage 0 to IV. According to statistics from the National Cancer Institute, the overall five‐year survival is around 50% (ACS 2016). The overall survival (OS) varies by stage and was estimated at 16% for sarcomas with distant spread and 83% for localized sarcomas (ACS 2016).
The location of the primary tumor can involve any area of the body. The distribution is 40% lower limb and girdle, 20% upper limb and girdle, 20% abdominal sites, 10% trunk, and 10% head and neck (Clark 2005). NRSTS can involve any type of tissue and typically affect muscles, tendons, adipose tissue, blood vessels, and joints (Sondak 2001), and commonly present as a painless mass. The symptoms depend on the anatomical site of origin, the size of the mass, and other aspects. Retroperitoneal sarcomas are most often asymptomatic, until the mass grows large enough to be clinically obvious or presses on vital organs and causes pain (Dileo 2005). People who relapse or experience progressive disease after therapy or metastasis are commonly called high‐risk people because these signs are associated with shorter survival time. Spontaneous recovery from NRSTS is unknown.
Description of the intervention
Surgery is the standard treatment for localized NRSTS (ESMO 2014), and can be curative if distant dissemination is not present (Kotilingam 2006). Chemotherapy is a standard treatment for people with distant metastasis (ESMO 2014), and is regarded mainly as a palliative treatment for high‐risk people who are characterized by inoperable, locally advanced and metastatic disease. Doxorubicin, ifosfamide, gemcitabine, dacarbazine, docetaxel, and trabectedin are used in monotherapy or in combinations (ESMO 2014). Riedel 2012 provides an overview of current systemic therapies and discusses possible novel therapeutic agents and treatment strategies.
Autologous hematopoietic stem cell transplantation (HSCT) is defined as the transplantation of stem cells that have been collected previously from bone marrow or peripheral blood of the same person. High‐dose chemotherapy (HDCT) uses higher doses of chemotherapeutic agents than are usually applied in standard‐dose chemotherapy (SDCT). HDCT may ablate the person's bone marrow reserves and create an absolute requirement for stem cell rescue. Autologous HSCT applied after HDCT or high‐dose radiation is a planned rescue therapy for HDCT‐related severe hematologic toxicity (Banna 2007).
HDCT and autologous HSCT are not standard treatment options; they are an experimental approach mainly used to treat high‐risk people with an unfavorable prognosis (stage IV with distant metastases). HDCT and autologous HSCT may be used in special cases after careful consideration, usually for people who respond well to standard chemotherapy according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria (Therasse 2000). Independent of the disease status, HDCT and autologous HSCT are hazardous interventions that carry the risk of life‐threatening organ failure. Hematologic adverse events as a result of autologous HSCT are usually manageable but life‐threatening consequences of pancytopenia. They generally affect all patients and include, for example, graft failure, severe infections, and bleeding. Of 23,883 autologous HSCTs that were registered in Europe in 2014 by the European Group for Blood and Marrow Transplantation (EBMT), 19 were undertaken for STS (Passweg 2016).
How the intervention might work
HDCT followed by autologous HSCT was adopted to treat high‐risk people because it was believed that escalating doses in chemotherapy might increase survival by capturing putatively remnant malignant cells and might overcome resistance to SDCT (Banna 2007). HDCT may cause severe hematologic and nonhematologic toxicity and autologous HSCT is a planned rescue therapy for the HDCT‐related demise of hematopoietic stem cells.
Why it is important to do this review
Several authors stated a lack of evidence and the need to conduct RCTs to clarify the relevance of HDCT followed by autologous HSCT in high‐risk people with STS (Blay 2000; Carvajal 2005; Dumontet 1992; Ek 2006; Elias 1998; Kasper 2007; Ladenstein 1997; Pinkerton 1986; Reichardt 2002; Rosti 2002; Schlemmer 2006; Seeger 1991; Woods 1999). Some authors have warned against the use of HDCT followed by autologous HSCT, indicating the possibility of repositioning of malignant cells (Woods 1999). Others have questioned the use of HDCT with reference to the potential existence of refractory cancer stem cells (Banna 2007; Bonnet 1997; Sanchez‐Garcia 2007). In the previous version of this review, we identified and included one RCT (Peinemann 2013). The rationale for this update is to clarify whether additional RCTs have been published or are ongoing.
Objectives
To assess the efficacy and safety of high‐dose chemotherapy (HDCT) followed by autologous hematopoietic stem cell transplantation (HSCT) for all stages of nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Inclusion criteria
We adopted WHO classification of soft tissue tumors to define the population of people with malignant soft tissue tumors. This classifies soft tissue tumors as benign, intermediate (locally aggressive), intermediate (rarely metastasizing), and malignant. We included all tumor entities classified as malignant in any of the two published versions (Fletcher 2002; Fletcher 2013). This means that an entity is included in the present review, although it was listed in the 2002 version (Fletcher 2002) but not any longer listed in the 2013 (Fletcher 2013) version. This means also that an entity is included in the present review if it was not listed in the 2002 version but was introduced in the 2013 version. Studies were included if least 80% of participants had a diagnosis listed in any version of the WHO classification and classified as malignant, though we did not apply this limitation to rhabdomyosarcoma. We included children and adults with no age limits. Participants were included regardless of the severity of the disease and the clinical staging information, if they received autologous (from either a peripheral or bone marrow source, or both) HSCT.
Exclusion criteria
While the WHO classification of NRSTS includes the Ewing family of tumors, that is extraosseous tumor types, we excluded these as they are primarily bone sarcomas. Because extraosseous types are rarely diagnosed and share common features, they were regarded as one entity with osseous types and were excluded.
Types of interventions
Autologous hematopoietic stem cell transplantation (HSCT), stem cells from a peripheral source or the bone marrow, serving as a rescue therapy usually applied after high‐dose chemotherapy (HDCT) versus standard‐dose chemotherapy (SDCT), which is defined as chemotherapy at a lower dose than HDCT without the need for stem cell rescue.
Types of outcome measures
Primary outcomes
Overall survival (OS): the event was death by any cause, from diagnosis or start of HDCT and autologous HSCT.
Treatment‐related mortality (TRM): incidence of deaths that were classified as treatment related or the participants died of treatment complications.
Secondary outcomes
Disease‐free survival (DFS): time free of disease after diagnosis or start of HDCT and autologous HSCT.
Progression‐free survival (PFS): time staying free of disease progression after diagnosis or start of HDCT and autologous HSCT. We provided the definitions if reported in the studies.
Event‐free survival (EFS): time staying free of any of a particular group of defined events after diagnosis or start of HDCT and autologous HSCT.
Nonhematologic toxicity grade 3 to 4 affecting organs such as gastrointestinal tract, kidney, liver, nervous system, and heart according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE 2016).
Secondary neoplasia: as classified by the study authors.
Health‐related quality of life measured by validated questionnaires.
Search methods for identification of studies
Electronic searches
We conducted an electronic search in the following medical literature databases. We carefully revised the search strategies to improve precision.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8). The search strategy is shown in Appendix 1. The search dates were limited from 1 January 2012 to 6 September 2016. The search dates of the previous version included references from inception to 5 December 2012.
PubMed. The search strategy is shown in Appendix 2. The search dates were limited from 1 January 2012 to 6 September 2016. The search dates of the previous version in MEDLINE included references from inception to 5 December 2012.
Embase. We used the search term "soft tissue sarcoma". The search dates were limited from 01 January 2012 to 29 September 2016. The search dates of the previous version in Embase included references from inception to 05 December 2012.
We searched for ongoing trials by scanning the following online registries on 26 September 2016.
ClinicalTrials.gov (ClinicalTrials.gov). Search: diagnosis 'soft tissue sarcoma'; intervention 'stem cell transplantation'. Limits: year '2012 to 2016'; study type 'interventional studies'; phase '2' or '3'.
WHO International Clinical Trials Registry Platform (ICTRP). Search: diagnosis 'sarcoma'; intervention 'transplantation'. Limits: date of registration '1 January 2012 to 26 September 2016'.
We searched abstracts of annual meeting proceedings issued by the following societies on 26 September 2016:
American Society of Clinical Oncology (ASCO): ASCO meetings in 2012 to 2016.
American Society of Hematology (ASH): ASH meetings in 2013 to 2015. Search: 'autologous'.
Bone Marrow Transplantation (BMT) Tandem Meetings of the American Society for Blood and Marrow Transplantation (ASBMT) and the Center for International Blood and Marrow Transplant Research (CIBMTR) (BMT Tandem Meeting 2012; BMT Tandem Meeting 2013; BMT Tandem Meeting 2014; BMT Tandem Meeting 2015).
European Society for Blood and Marrow Transplantation (EBMT) (EBMT Meeting 2014; EBMT Meeting 2015; EBMT Meeting 2016). Search: 'sarcoma'.
EBMT current study list (EBMT Studies 2016).
International Society of Paediatric Oncology (SIOP) (SIOP Meeting 2012; SIOP Meeting 2013). Search: 'sarcoma'.
The search strategies used have been developed and executed by the author team.
Searching other resources
We planned to locate information about trials not registered in electronic databases by searching the reference lists of recently published relevant articles and review articles.
Data collection and analysis
Selection of studies
We endorsed the PRISMA statement, adhered to its principles, and conformed to its checklist (Moher 2009). We retrieved all titles and abstracts by electronic searching, downloaded them, and transferred the bibliographical data into an Excel spreadsheet. We removed duplicates and two review authors (FP, HE) examined the remaining references independently. We excluded those studies that clearly did not meet the inclusion criteria and we documented the reasons for the exclusion of studies. We resolved disagreement by discussion and it was not necessary to consult a third review author (LAS). We considered studies written in languages other than English and asked peers familiar with the particular language and with the principles of study evaluation to translate major methodologic issues. We planned to use the Google Translate 2016 program if required, but this was not necessary.
Data extraction and management
We extracted the following data as in the previous version.
General information on author, title, source, publication date.
Study characteristics: trial design, setting, inclusion and exclusion criteria, comparability of participants' characteristics between groups, treatment allocation, blinding, subgroup analysis, length of follow‐up.
Participant characteristics: age; gender; number of participants recruited, allocated, affected, analyzed; additional diagnoses; participants lost to follow‐up.
Interventions: type of HDCT, source of stem cells, and type of SDCT.
Outcomes: OS, TRM, DFS, PFS, EFS including type of event, toxicity, secondary neoplasia, health‐related quality of life.
Assessment of risk of bias in included studies
Two review authors (FP, LAS) independently checked the risk of bias in the included studies using the standard criteria to assess RCTs according to the Cochrane 'Risk of bias' tool (Higgins 2011a). With respect to the previous version, we removed the risk of bias items specifically aimed at checking the risk of bias in nonrandomized studies.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting such as not reporting prespecified outcomes (reporting bias).
Other bias.
We applied Cochrane criteria for judging risk of bias (Higgins 2011a). In general, there was a 'low risk' of bias if the bias was unlikely to seriously alter the results, for example, participants and investigators enrolling participants could not have foreseen assignment. There was a 'high risk' of bias if the bias seriously weakened confidence in the results, for example, participants or investigators enrolling participants could possibly have foreseen assignments. There was 'unclear' risk of bias if the bias raised some doubt about the results, for example, the method of concealment was not described or not described in sufficient detail to allow a definite judgment.
Measures of treatment effect
The primary effect measure was the hazard ratio (HR) for time‐to‐event data. If the HR was not directly given in the publication, we planned to estimate HRs according to methods proposed by Tierney 2007, but this was not necessary. We planned to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for dichotomous outcomes, but this was not applicable. In the case of rare events, we planned to use Peto OR instead, but this was not applicable. We planned to analyze continuous data and to present them as mean differences, if all results were measured on the same scale (e.g. length of hospital stay), but this was not applicable. If this was not the case (e.g. pain or quality of life), we planned to use standardized mean differences, but this was not applicable.
Dealing with missing data
We conformed to Cochrane's principal options for dealing with missing data and analyzed only the available data (Higgins 2011b). If data were missing or only imputed data were reported, we planned to contact trial authors to request data on the outcomes among participants who were assessed.
In the previous version of the review, we contacted the authors of the study by Bui‐Nguyen 2012 by e‐mail (1 December 2012) to ask for missing data about the histologic types that were combined as 'others'. The authors responded and as a consequence we could base the inclusion or exclusion of participant data on the additional data (Table 2). In the current version of the review (September 2016), we sent e‐mail inquiries as shown in Appendix 3 to two authors (Binh Bui‐Nguyen, 3 October 2016; Jean‐Yves Blay, 5 October 2016) of the included study (Bui‐Nguyen 2012) regarding clarification of survival data and Food and Drug Administration (FDA) warning letter on objectionable conditions and inadequate responses (FDA 2015). We did not receive any reply by 7 March 2017.
1. Tumor entities reported by Bui‐Nguyen 2012.
| Sarcoma type | Sarcoma type 'Others' | Both arms | HDCT arm | SDCT arm |
| Leiomyosarcoma | ‐ | 16 | 7 | 9 |
| Liposarcoma | ‐ | 10 | 6 | 4 |
| Synovial sarcoma | ‐ | 9 | 2 | 7 |
| Angiosarcoma | ‐ | 6 | 2 | 4 |
| Malignant peripheral nerve sheath tumor | ‐ | 2 | 1 | 1 |
| Clear cell sarcoma | ‐ | 1 | 1 | 0 |
| Desmoplastic small round cell sarcoma | ‐ | 1 | 0 | 1 |
| Rhabdomyosarcoma | ‐ | 9 | 4 | 5 |
| Malignant fibrous histiocytoma | ‐ | 16 | 8 | 8 |
| Extraskeletal osteosarcoma | ‐ | 1 | 0 | 1 |
| Melanoma* | ‐ | 1 | 1 | 0 |
| 'Others' | Leiomyosarcoma | 1 | 1 | 0 |
| Fibrosarcoma | 1 | 1 | 0 | |
| Myofibrosarcoma | 1 | 0 | 1 | |
| Undifferentiated sarcoma | 2 | 1 | 1 | |
| Desmoplastic small round cell sarcoma | 2 | 2 | 0 | |
| Gastrointestinal stromal tumor | 1 | 0 | 1 | |
| Malignant Triton tumor | 1 | 0 | 1 | |
| Unclassified sarcoma | 1 | 1 | 0 | |
| Myoepithelioma* | 2 | 2 | 0 | |
| Endometrial stromal sarcoma* | 3 | 1 | 2 | |
| Total | 87 | 41 | 46 | |
| Not listed in the WHO classification | 6 | 4 | 2 | |
HDCT: high‐dose chemotherapy; SDCT: standard‐dose chemotherapy; WHO: World Health Organization.
Bui‐Nguyen: the table lists the sarcoma types assigned to each individual of all randomized participants of the study by Bui‐Nguyen 2012.
*Soft tissue sarcomas: tumor entities not listed in either versions of the WHO classification (Fletcher 2002; Fletcher 2013), or soft tissue tumors not categorized as malignant are italicized. Myoepithelioma is categorized as an intermediate soft tissue tumor. Melanoma and endometrial stromal sarcoma are not listed in the classification.
Assessment of heterogeneity
We had planned to assess heterogeneity between studies by visual inspection of forest plots; by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (I2 statistic) (Higgins 2003); by a formal statistical test of the significance of the heterogeneity (Cochran's Q) (Deeks 2011); and, if possible, by subgroup analyses (see Subgroup analysis and investigation of heterogeneity). We had planned to investigate and report possible reasons if there was evidence of substantial heterogeneity. We had planned to use the random‐effects model with inverse variance weighting for statistical pooling (DerSimonian 1986). We did not pool estimates.
Assessment of reporting biases
In addition to the evaluation of reporting bias as described in the Assessment of risk of bias in included studies section, we had planned to assess reporting bias (such as publication bias, time lag bias, multiple (duplicate) publication bias, location bias, citation bias, language bias) by constructing a funnel plot if there were a sufficient number of included studies (i.e. at least 10 studies included in a meta‐analysis otherwise the power of the tests would be too low to distinguish chance from real asymmetry (Sterne 2011)). We did not assess reporting bias because of the low number of identified studies.
Data synthesis
We analyzed data using Review Manager 5 (RevMan 2014). This was done by one review author (FP) and checked by another review author (LAS). If sufficient, clinically similar studies were available, we had planned to pool their results in meta‐analyses if they used comparable outcome definitions. As we included one RCT, we presented the results descriptively. We had planned to use random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986), but this was not applicable.
For each comparison, we prepared a 'Summary of findings' table using the GRADE profiler software (GRADEpro 2014), in which we presented the following primary outcomes: OS, TRM, DFS, PFS, non‐hematologic toxicity grade 3 to 4 and health‐related quality of life . For each outcome, two review authors (FP, LAS) independently assessed the quality of the evidence by using the five GRADE considerations, that is, study limitations, inconsistency, indirectness, imprecision, and publication bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
We had planned subgroup analyses based on age, stage, and time period of treatment. However, we found no appropriate data to conduct these analyses.
Sensitivity analysis
We had planned sensitivity analyses to compare the results of studies with low versus high risk of bias. As we included only one study, a sensitivity analysis was not applicable.
Results
Description of studies
Clinical heterogeneity was substantial because tumor entities varied considerably between participants.
Results of the search
For this update we revised the search strategy to improve the precision and reduce the number of irrelevant hits. We identified a total of 1549 items including 85 items from electronic databases, 45 items from study registries, and 1419 items from congress proceedings (Figure 1).
1.
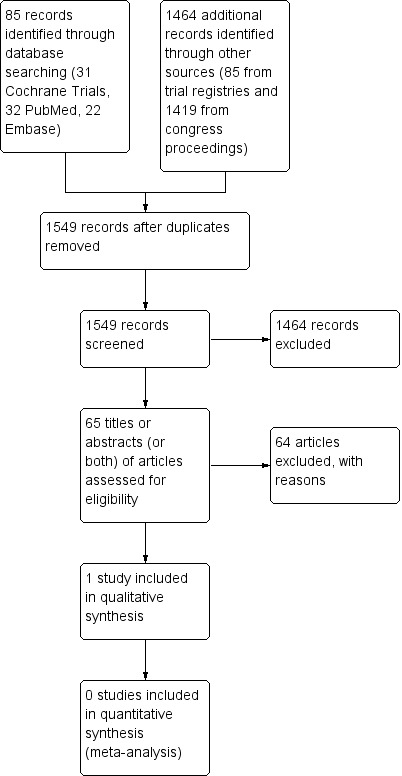
Study flow diagram.
We retrieved 85 records from the electronic literature databases CENTRAL, PubMed and Embase and screened 65 different articles after removal of duplicates. The titles, abstracts or both of all 65 articles did not fulfil the inclusion criteria and we excluded the articles with reasons (Figure 1).
We identified 45 records from study registries and all 45 items did not fulfill the inclusion criteria. We retrieved 39 studies from ClinicalTrials.gov Studies 2016 and 6 studies from ICTRP Studies 2016. We identified a total of 1419 potentially relevant meeting abstracts and all 1419 items did not fulfil the inclusion criteria. We identified 830 abstracts in ASCO Meetings 2012 to 2016, 128 abstracts in ASH Meetings 2013 to 2015, 21 relevant abstracts in BMT Tandem Meeting 2012, BMT Tandem Meeting 2013, BMT Tandem Meeting 2014, BMT Tandem Meeting 2015, and BMT Tandem Meeting 2016, 149 abstracts in EBMT Meeting 2014, EBMT Meeting 2015, EBMT Meeting 2016, 52 current EBMT Studies 2016, and 239 abstracts from SIOP Meeting 2012, SIOP Meeting 2013, SIOP Meeting 2014, SIOP Meeting 2015, and SIOP Meeting 2016.
For the update, we did not identify any additional studies and it was not necessary to contact authors to for missing information.
Included studies
As the updated classification of STS included some changes, we rechecked the extracted data from the previously included RCT which remains included in the this updated version of the review. Two review authors (FP, LAS) independently checked data for study characteristics, participants and interventions, duration of follow‐up, outcomes, and deviations from the protocol. In addition, two review authors (FP, LAS) independently checked the risk of bias. We had planned to resolve differences between review authors by discussion or by appeal to a third review author, but it was not necessary. The update search did not identify any additional RCTs. Therefore, we included one RCT in this update (Bui‐Nguyen 2012). Bui‐Nguyen 2012 randomized 87 participants and included 83 participants in a modified intention‐to‐treat analysis. A detailed description of the study is shown in the Characteristics of included studies table.
Design
Bui‐Nguyen 2012 reported an RCT with two parallel treatment groups, HDCT followed by autologous HDCT versus SDCT. It was an open, multicenter, randomized phase III study. All participants received the same baseline treatment. Participants were eligible for randomization if they had responded to chemotherapy or, for stable disease, if a complete surgical resection of all disease sites could be carried out. Randomization was carried out centrally.
Sample sizes
The trial authors modified the intention‐to‐treat analysis to exclude the data for four participants who were initially randomized but found to be ineligible at central histology review (Bui‐Nguyen 2012). Initially, 87 participants were randomized: 41 in the HDCT arm versus 46 in the SDCT arm but only 83 participants were analyzed in a modified intention‐to‐treat‐analysis: 38 in the HDCT arm versus 45 in the SDCT arm.
Setting
The single included RCT was a French multicenter trial set in 16 different centers (Bui‐Nguyen 2012).
Participants
Bui‐Nguyen 2012 reported a median age of 45.8 years in the HDCT arm and 43.3 years in the SDCT arm. The proportion of males was 58.5% in the HDCT arm and 50% in the SDCT arm. A total of 19 different diagnoses were assigned to the 87 participants. In Table 2, we provide a list of all diagnoses and their incidence among the participants. We clarified the category 'Others' by contacting the trial author.
Interventions
In the study by Bui‐Nguyen 2012, 87 participants received courses one to five of SDCT. Forty‐one participants were randomized to receive HDCT and transplantation of autologous peripheral stem cells as course six in the HDCT arm. Of these, 38 participants were analyzed in a modified intention‐to‐treat analysis. Forty‐six participants were randomized to again receive SDCT as course six. Of these, 45 participants were analyzed in a modified intention‐to‐treat analysis.
Primary outcome
OS was the primary outcome of the review and the study (Bui‐Nguyen 2012). TRM was the primary outcome of the review but was a secondary outcome among adverse events of the study.
Secondary outcomes
PFS and adverse events were secondary outcomes of the review and the study (Bui‐Nguyen 2012). The trial authors evaluated complete remission as a secondary outcome, which was not considered as an outcome in this review.
Excluded studies
We excluded 65 references of the potentially relevant articles with the following reasons (Figure 1):
not study type of interest (14);
not population of interest (34);
not intervention of interest (16).
Excluded studies are described in the Characteristics of excluded studies table.
Risk of bias in included studies
An overview of the risk of bias of Bui‐Nguyen 2012 is shown in Figure 2.
2.
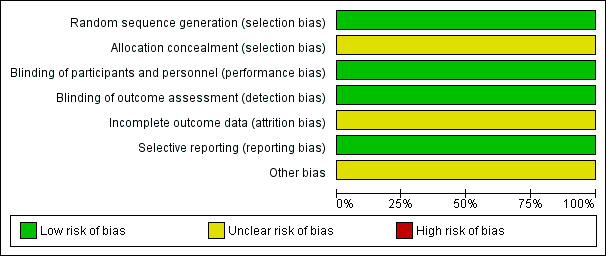
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Allocation
Reporting appeared to be compatible with an adequate random sequence generation and we judged it at low risk of bias. Allocation concealment was not described and it is unclear whether it was carried out adequately, therefore, we judged it at unclear risk of bias.
Blinding
The study did not address blinding of participants and blinding of outcome assessment. Nevertheless, blinding is not relevant for OS and TRM. Therefore, we judged it at low risk of bias. The previous version of this review did not judge blinding of participants and we added it to the present version. The result of the judgment of blinding of outcome assessment was changed from high risk (blinding not reported) to low risk (blinding not relevant for the primary outcomes).
Incomplete outcome data
The previous version of this review did not judge incomplete outcome data and we added it to this update. From the 87 participants, the data of four participants had not been included in the analysis. At 36 months from randomization (HDCT versus SDCT), 51 participants had died (24 in HDCT arm versus 27 in SDCT arm) and 25 were alive (eight in HDCT arm versus 17 in SDCT arm). Of the 83 participants (38 in HDCT arm versus 45 in SDCT arm) included in the modified intention‐to‐treat survival analysis, 76 participants (32 in HDCT arm versus 44 in SDCT arm) were accounted for but seven participants (six in HDCT arm versus one in SDCT arm) were not adequately explained.
Figure 1 in Bui‐Nguyen 2012 showed that 41 participants were randomized to the HDCT arm, but only 22 participants of these received HDCT and were evaluated. Also 46 participants were randomized to the SDCT arm and 40 participants received SDCT and were evaluated.
Table 2 of Bui‐Nguyen 2012 showed WHO grades 3/4 toxicity for all randomized participants, 22 in the HDCT arm and 51 in the SDCT arm. There was an inconsistency in the number of randomized and evaluated participants between Figure 1 and Table 2. It appeared conflicting that 51 participants were reported to be randomized to the SDCT arm in Table 2, although, according to Figure 1, only 46 participants were randomized and only 40 participants actually received SDCT treatment. Therefore, it appears that only 40 participants were actually eligible to evaluate adverse events after SDCT. As we were unable to contact the trial authors, we could not clarify this issue.
The potential influence of the reported missing information was unclear, therefore we judged it at unclear risk of bias.
Selective reporting
We did not identify any selective reporting and judged it at low risk of bias.
Other potential sources of bias
The previous version of this review did not judge other potential sources of bias and we added it to this update. The FDA sent a warning letter (Reference 15‐HFD‐45‐05‐01) addressed to the first author of the trial on 4 May 2015 to inform of objectionable conditions observed during an inspection at the clinical site between 17 and 19 September 2014 (FDA 2015). The inspection happened after conclusion of the study and may not be related to the risk of bias. The potential influence of this information was unclear, therefore we judged it at unclear risk of bias.
Effects of interventions
See: Table 1
Primary outcome
Overall survival
The HR between the survival functions of the HDCT and the SDCT arms in Bui‐Nguyen 2012 was reported as 1.26 (95% CI 0.70 to 2.29; P = 0.44; 1 study, 83 participants; high quality evidence). Therefore, the data did not favor either treatment arm with respect to OS. The trial authors reported the probability of OS at three years postrandomization as 32.7% in the HDCT arm versus 49.4% in the SDCT arm. The trial authors conducted a subgroup analysis of participants who had achieved a complete response before HDCT. The estimated HR for OS of 2.92 (95% CI 1.1 to 7.6; P = 0.028; 1 study, 39 participants) favored the SDCT arm.
Treatment‐related mortality
The trial authors reported a single treatment‐related leukemia death two years after HDCT.
Secondary outcomes
Disease‐free survival
The study did not address DFS.
Progression‐free survival
The HR between the survival functions of the HDCT and the SDCT arms in Bui‐Nguyen 2012 was reported as 1.34 (95% CI 0.81 to 2.20; P = 0.25; 1 study, 83 participants). Therefore, the data did not favor either treatment with respect to PFS. The trial authors reported the probability of PFS at the time point of three years postrandomization of 9.3% in the HDCT arm versus 21.6% in the SDCT arm. The trial authors conducted a subgroup analysis of participants who had achieved a complete response before HDCT. The estimated HR for PFS of 2.87 (95% CI 1.3 to 6.3; P = 0.009; 1 study, 39 participants) favored the SDCT arm.
Event‐free survival
The study did not address event‐free survival.
Nonhematologic toxicity grade 3 to 4
The study evaluated severe adverse events in 22 participants in the HDCT arm and 51 participants in the SDCT arm according to Table 2 in Bui‐Nguyen 2012. The trial authors reported 11 events including digestive‐, infection‐, pain‐, or asthenia‐related toxicity in 22 participants of the HDCT arm and one event in 51 participants of the SDCT arm. However, the study also stated that 40 participants had been randomized to the SDCT arm. We can only assume that the authors mixed participants who were part of the randomization process and other people who were not. As it was not possible to continue a communication with the authors, we could not clarify this issue.
Secondary neoplasia
The study did not address secondary neoplasia.
Health‐related quality of life
The study did not address health‐related quality of life.
Discussion
Summary of main results
In this update, we identified no additional RCTs other than the one RCT that was included in the previous version of this review (Bui‐Nguyen 2012). The data did not favor the HDCT with respect to OS, PFS, or adverse events. The considerable heterogeneity of the tumor entities included in the study may be an important factor as OS may differ between the entities.
Overall completeness and applicability of evidence
The search was comprehensive and we considered the risk of not detecting an RCT (either published or ongoing) to be very small. The participants included in the trial were recruited from 2000 to 2008 and, considering the advancement in medicine, the results may not be applicable to the current treatment of people with NRSTS.
Quality of the evidence
Using the 'Risk of bias' tool for randomized studies we judged an overall unclear risk of bias. We judged a low risk of bias for four items (selection bias, performance bias, detection bas, and reporting bias) and unclear for the remaining three items. Each tumor entity may carry an individual risk profile and, therefore, ideally should be evaluated separately. However, the frequency of the population and the intervention of interest is tiny. In 2014, only 19 autologous HSCTs indicated for STS were registered by the European Group for Blood and Marrow Transplantation (Passweg 2016). We presented estimates in the Table 1 for outcomes that mainly constitute death as the endpoint. These outcomes included OS, TRM, and PFS. The lack of blinding did not result in judging a high risk of bias. In Table 1, we assigned high quality with respect to those outcomes using GRADE criteria. Other outcomes were not reported or were not adequately reported. The authors reported a secondary analysis carried out to investigate the effects of surgery. According to the authors, "Overall, there were no survival differences observed (HR = 0.63, 95% CI 0.35‐1.12), according to the performance of surgery (54.3%) or not (33.2%)." We were unable to determine the number of participants in this subgroup. Therefore, we did not include the information in the 'Results' section.
Potential biases in the review process
Strengths: the search strategy was broad and it is very likely that the search identified all relevant studies. We contacted authors to request additional data.
Limitations: heterogeneity of the tumor entities and the time period of treatment may limit the conclusions that may be drawn from the data.
Agreements and disagreements with other studies or reviews
We agree with Kasper 2005 and would like to extend the views that the use of HDCT followed by autologous HSCT for locally advanced or metastatic adult STS is highly experimental, might be even be less effective than SDCT, and should not be performed outside of RCTs.
Authors' conclusions
Implications for practice.
The evidence base does not support the use of high‐dose chemotherapy (HDCT) followed by autologous hematopoietic stem cell transplantation (HSCT) in high‐risk people with non‐rhabdomyosarcoma soft tissue sarcomas (NRSTS). If this treatment is offered, it should only be after careful consideration on an individual person basis and possibly only as part of a well‐designed randomized controlled trial (RCT).
Implications for research.
It is doubtful whether further studies are necessary to clarify the relevance of HDCT followed by autologous HSCT in people with NRSTS. However, if appropriate, any future studies would need to be methodologically well‐designed RCTs with a low risk of bias as nonrandomized studies are not beneficial in addressing this topic. Criteria for the included tumor types in any future trial should adhere to the WHO classification.
What's new
| Date | Event | Description |
|---|---|---|
| 17 July 2018 | Amended | Next expected date amended |
| 28 June 2018 | Review declared as stable | Intervention not in general use for solid tumours. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 2, 2011
| Date | Event | Description |
|---|---|---|
| 19 April 2017 | Amended | Minor corrections for figures. |
| 8 March 2017 | New citation required but conclusions have not changed | No additional RCTs identified. One new author added and one removed and acknowledged. WHO classification extended to include recently updated version. |
| 8 March 2017 | New search has been performed | Search strategy revised and study design limited to RCTs. Risk of bias assessment revised; overall assessment changed from low to unclear risk of bias. |
| 10 June 2013 | New citation required and conclusions have changed | We identified a single randomized controlled trial and judged it to have a low risk of bias. The results did not support high‐dose chemotherapy followed by autologous hematopoietic stem cell transplantation compared to standard‐dose chemotherapy in patients with non‐rhabdomyosarcoma soft tissue sarcoma. |
| 5 December 2012 | New search has been performed | Searches re‐run and one new study included. |
Acknowledgements
We thank the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Editorial Team for their assistance during the preparation of the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
| ID | Search | Hits |
| #1 | MeSH descriptor: [Sarcoma] explode all trees | 704 |
| #2 | MeSH descriptor: [Carcinoma, Small Cell] explode all trees | 748 |
| #3 | MeSH descriptor: [Hemangioendothelioma] explode all trees | 2 |
| #4 | MeSH descriptor: [Mesenchymoma] explode all trees | 2 |
| #5 | MeSH descriptor: [Perivascular Epithelioid Cell Neoplasms] explode all trees | 13 |
| #6 | MeSH descriptor: [Rhabdoid Tumor] explode all trees | 1 |
| #7 | MeSH descriptor: [Gastrointestinal Stromal Tumors] explode all trees | 112 |
| #8 | alveolar soft part sarcoma* | 8 |
| #9 | alveolar soft tissue sarcoma* | 12 |
| #10 | angiosarcoma* | 21 |
| #11 | hemangiosarcoma* | 9 |
| #12 | clear cell sarcoma* | 130 |
| #13 | clear cell tumor* or clear cell tumour* | 1228 |
| #14 | desmoplastic small round cell tumor* or desmoplastic small round cell tumor* | 6 |
| #15 | epithel* sarcoma* | 55 |
| #16 | fibrosarcoma* | 34 |
| #17 | myxofibrosarcoma* | 3 |
| #18 | hemangioendothelioma* | 7 |
| #19 | hemangioendotheliosarcoma* | 1 |
| #20 | intimal sarcoma* | 1 |
| #21 | leiomyosarcoma* | 111 |
| #22 | liposarcoma* | 58 |
| #23 | malignant glomus tumor* or malignant glomus tumour* | 2 |
| #24 | malignant mesenchymoma* | 2 |
| #25 | perivascular epithelioid cell tumor* or perivascular epithelioid cell tumour* | 1 |
| #26 | rhabdoid tumor* or rhabdoid tumour* | 15 |
| #27 | rhabdoid sarcoma* | 7 |
| #28 | synovial sarcoma* | 31 |
| #29 | gastrointestinal stromal tumor* or gastrointestinal stromal tumour* | 263 |
| #30 | malignant peripheral nerve sheath tumour* | 8 |
| #31 | undifferentiated pleomorphic sarcoma* | 10 |
| #32 | MeSH descriptor: [Stem Cell Transplantation] explode all trees | 1861 |
| #33 | MeSH descriptor: [Bone Marrow Transplantation] explode all trees | 1421 |
| #34 | MeSH descriptor: [Transplantation, Autologous] explode all trees | 1528 |
| #35 | MeSH descriptor: [Consolidation Chemotherapy] explode all trees | 41 |
| #36 | autologous transplant* | 4038 |
| #37 | bone marrow rescue | 203 |
| #38 | bone marrow support | 2824 |
| #39 | bone marrow cell | 3820 |
| #40 | stem cell rescue | 211 |
| #41 | stem cell support | 2117 |
| #42 | peripheral blood stem cell | 1487 |
| #43 | high dose chemotherapy | 5860 |
| #44 | intensified chemotherapy | 373 |
| #45 | intensive chemotherapy | 2053 |
| #46 | myeloablative chemotherapy | 234 |
| #47 | dose intensive treatment | 4499 |
| #48 | high dose combination | 11065 |
| #49 | MeSH descriptor: [Randomized Controlled Trial] explode all trees | 157 |
| #50 | randomized controlled trial or randomised controlled trial | 562380 |
| #51 | randomized controlled study or randomised controlled study | 495206 |
| #52 | randomized trial or randomised trial | 564855 |
| #53 | randomized study or randomised study | 498484 |
| #54 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 | 3040 |
| #55 | #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 #42 or #43 or #44 or #45 or #46 or #47 or #48 | 25564 |
| #56 | #49 or #50 or #51 or #52 or #53 | 612136 |
| #57 | #54 and #55 and #56 | 928 |
Limits: publication date from 01 January 2012 to 06 September 2016.
Appendix 2. PubMed search strategy
| ID | Search |
| #1 | "Sarcoma"[Mesh] |
| #2 | "Carcinoma, Small Cell"[Mesh] |
| #3 | "Hemangioendothelioma"[Mesh] |
| #4 | "Mesenchymoma"[Mesh] |
| #5 | "Perivascular Epithelioid Cell Neoplasms"[Mesh] |
| #6 | "Rhabdoid Tumor"[Mesh] |
| #7 | "Gastrointestinal Stromal Tumors"[Mesh] |
| #8 | alveolar soft part sarcoma* |
| #9 | alveolar soft tissue sarcoma* |
| #10 | angiosarcoma* |
| #11 | hemangiosarcoma* |
| #12 | clear cell sarcoma* |
| #13 | clear cell tumor* or clear cell tumour* |
| #14 | desmoplastic small round cell tumor* or desmoplastic small round cell tumor* |
| #15 | epithel* sarcoma* |
| #16 | fibrosarcoma* |
| #17 | myxofibrosarcoma* |
| #18 | hemangioendothelioma* |
| #19 | hemangioendotheliosarcoma* |
| #20 | intimal sarcoma* |
| #21 | leiomyosarcoma* |
| #22 | liposarcoma* |
| #23 | malignant glomus tumor* or malignant glomus tumour* |
| #24 | malignant mesenchymoma* |
| #25 | perivascular epithelioid cell tumor* or perivascular epithelioid cell tumour* |
| #26 | rhabdoid tumor* or rhabdoid tumour* |
| #27 | rhabdoid sarcoma* |
| #28 | synovial sarcoma* |
| #29 | gastrointestinal stromal tumor* or gastrointestinal stromal tumour* |
| #30 | malignant peripheral nerve sheath tumor* or malignant peripheral nerve sheath tumour* |
| #31 | undifferentiated pleomorphic sarcoma* |
| #32 | "Stem Cell Transplantation"[Mesh] |
| #33 | "Bone Marrow Transplantation"[Mesh] |
| #34 | "Transplantation, Autologous"[Mesh] |
| #35 | "Consolidation Chemotherapy"[Mesh] |
| #36 | autologous transplant* |
| #37 | bone marrow rescue |
| #38 | bone marrow support |
| #39 | bone marrow cell |
| #40 | stem cell rescue |
| #41 | stem cell support |
| #42 | peripheral blood stem cell |
| #43 | high dose chemotherapy |
| #44 | intensified chemotherapy |
| #45 | intensive chemotherapy |
| #46 | myeloablative chemotherapy |
| #47 | dose intensive treatment |
| #48 | high dose combination |
| #49 | "Randomized Controlled Trial" [Publication Type] |
| #50 | randomized controlled trial or randomised controlled trial |
| #51 | randomized controlled study or randomised controlled study |
| #52 | randomized trial or randomised trial |
| #53 | randomized study or randomised study |
| #54 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 |
| #55 | #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 #42 or #43 or #44 or #45 or #46 or #47 or #48 |
| #56 | #49 or #50 or #51 or #52 or #53 |
| #57 | #54 and #55 and #56 |
Limits: publication date from 01 January 2012 to 06 September 2016.
Appendix 3. Inquiry to trial authors
For this review update, we sent e‐mail inquiries to two authors (Binh Bui‐Nguyen, Jean‐Yves Blay) of the included study (Bui‐Nguyen 2012) regarding clarification of survival data and the US Food and Drug Administration (FDA) warning letter on objectionable conditions and inadequate responses. The warning letter sent by the FDA was addressed to the first author Binh Bui‐Nguyen (Reference 15‐HFD‐45‐05‐01). It informs of objectionable conditions observed during an inspection at the clinical site between 17 and 19 September 2014 (FDA 2015).
E‐mail sent to Binh Bui‐Nguyen on 3 October 2016, quote: "I am conducting an update of my Cochrane Review and I would like to add some questions. At the moment, I am working on judging the Incomplete outcome data (attrition bias). On page 781, I extracted the following information from section 'Survival outcomes' and from Figure 2: At 36 months from randomization (HDCT versus SDCT), 51 patients had died (24 versus 27) and 25 are at risk (8 versus 17). Of 83 patients included in the modified ITT survival analysis, 76 are accounted for but 7 patients may not be explained. My question: Did I extract correctly? Does 'at the time of analysis' correspond to 36 months after randomization? Do the figures that I extracted or deduced correspond to 36 months after randomization?"
E‐mail sent to Jean‐Yves Blay on 5 October 2016, which contains the above quoted text sent to Binh Bui‐Nguyen and the following additional text, quote: "I also would like to ask about the importance of the attached FDA warning letter on objectionable conditions and inadequate responses. Is there any connection or conflict with the study of Bui‐Nguyen 2012?"
We did not receive any reply by 7 March 2017.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bui‐Nguyen 2012.
| Methods | Duration: 2000 to 2008 Study design: randomized controlled trial: "This open, multicenter, randomized phase III study [...]". "All patients eligible for preenrollment received the same baseline treatment [...]". "[...] eligible for randomization if they had responded to chemotherapy or, for stable disease, if a complete surgical resection of all disease sites could be carried out. Patients were ineligible for randomization if they had progressed or had only stable disease with no possibility for complete resection of the primary and/or metastatic tumor". "Randomization was stratified by center using a blocked method with block size of four and was carried out centrally". "The intention to treat (ITT)‐modified population included all randomly assigned patients excluding patients found to be ineligible at central histology review." Treatment: number of arms: 2 Follow‐up time: time to event analysis at 3 years with a median follow‐up of 55 months for survivors |
|
| Participants | Setting: multicenter trial in 16 centers in France Eligibility criteria: people aged 18 to 65 years with histologically confirmed, inoperable locally advanced or metastatic soft tissues sarcomas; Eastern Cooperative Oncology Group performance status of 0 or 1; normal cardiac, hepatic, and renal function; adequate bone marrow reserve; participants had received no prior chemotherapy or concurrent therapy Exclusions: people for whom it was possible to perform potentially curative locoregional treatments and people with uterine, bone, or digestive tumors Number of participants: 264 participants pre‐enrolled; 207 participants received first 4 of 6 chemotherapy courses:
Age
Gender
|
|
| Interventions | All participants received 5 courses of SDCT: doxorubicin 60 mg/m2, ifosfamide 7500 mg/m2, dacarbazine 900 mg/m2, total doses; the 6th course was different between HDCT + autologous HSCT arm and SDCT arm: HDCT + autologous HSCT arm, 6th course:
SDCT arm, 6th course:
|
|
| Outcomes | Primary outcomes as defined by the study
Secondary outcomes as defined by the study
|
|
| Notes | Financial support: Programme Hospitalier de Recherche Clinique, French Health Ministry (nonprofit organization); French National Federation for Comprehensive Cancer Centers (nonprofit organization). Information about the histologic type of sarcoma designated as "Others" in the article were communicated by personal contact with the first author and listed in Table 2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified by center using a blocked method with block size of four and was carried out centrally". We assumed an adequate random sequence generation and judged at low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was carried out centrally, though masking of allocation was not described in full detail. We assumed an adequate allocation concealment. However, we missed a clarifying statement. Therefore, we judged at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not reported; very likely not possible and not relevant for the reported outcomes of overall survival, treatment‐related mortality, and progression‐free survival. Blinding of participants has no influence on overall survival and treatment‐related mortality, which are defined as primary outcomes of the present review. Therefore, we judged at low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported; very likely not possible and not relevant for the reported outcomes overall survival, treatment‐related mortality, and progression‐free survival. The study was denoted as an "open, multicenter, randomized phase III study". Blinding of outcome assessment has no influence on overall survival and treatment‐related mortality, which are defined as primary outcomes of the present review. Therefore, we judged at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At 36 months from randomization (HDCT versus SDCT), 51 participants had died (24 versus 27) and 25 were at risk (8 versus 17). Of 83 participants (38 versus 45) included in the modified intention‐to‐treat survival analysis, 76 participants (32 versus 44) are accounted for but 7 participants (6 versus 1) may not be explained. The number of participants with missing information was small. The potential influence of this missing information was unclear, therefore we judged at unclear risk of bias. Figure 1 of Bui‐Nguyen 2012 showed that 41 participants were randomized to the HDCT arm, but 22 participants actually received high dose and were evaluated. Figure 1 also showed that 46 participants were randomized to the SDCT arm, but 40 participants actually received standard dose and were evaluated. The potential influence of this missing information was unclear, therefore we judged atn unclear risk of bias. Table 2 of Bui‐Nguyen 2012 showed WHO grades 3/4 toxicity for all randomized participants, 22 in the HDCT arm and 51 in the SDCT arm. There was an inconsistency concerning the number of randomized and evaluated participants between Figure 1 and Table 2. It appeared conflicting that 51 participants were reported to be randomized to the SDCT arm in Table 2, although, according to Figure 1, only 46 participants were randomized and only 40 participants actually received SDCT. Thus, instead of 51 participants, it appeared that only 40 participants were actually eligible to evaluate adverse events after SDCT. |
| Selective reporting (reporting bias) | Low risk | We did not identify any selective outcome reporting. |
| Other bias | Unclear risk | The US Food and Drug Administration sent a warning letter (Reference 15‐HFD‐45‐05‐01) addressed to the first author on 4 May 2015 to inform of objectionable conditions observed during an inspection at the clinical site between 17 and 19 September 2014 (FDA 2015). The inspection happened after the conclusion of the study included in the present review and may not have been related to the risk of bias. The potential influence of this information was unclear, therefore we judged it at unclear risk of bias. |
HDCT: high‐dose chemotherapy; HSCT: hematopoietic stem cell transplantation; SDCT: standard‐dose chemotherapy; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Araki 2016 | Not intervention of interest |
| Benesch 2014 | Not study design of interest |
| Brana 2014 | Not population of interest |
| Calabro 2015 | Not population of interest |
| Cohen 2012 | Not intervention of interest |
| Conter 2013 | Not population of interest |
| Czarnecka 2014 | Not population of interest |
| Davis 2015 | Not intervention of interest |
| Delannes 2013 | Not intervention of interest |
| Demetri 2016 | Not intervention of interest |
| Diez‐Tejedor 2014 | Not population of interest |
| Drabko 2012 | Not population of interest |
| Ehlert 2012 | Not study design of interest |
| Engelhard 2013 | Not population of interest |
| Friedman 2014 | Not population of interest |
| Froeb 2012 | Not population of interest |
| Gaspar 2012 | Not population of interest |
| Grignani 2015 | Not population of interest |
| Gronchi 2012 | Not intervention of interest |
| Halland 2012 | Not population of interest |
| Hartmann 2013 | Not study design of interest |
| Hensley 2013 | Not population of interest |
| Infante 2015 | Not population of interest |
| Ishida 2014 | Not study design of interest |
| Ishida 2016 | Not study design of interest |
| Joensuu 2012 | Not intervention of interest |
| Judson 2014 | Not intervention of interest |
| Kawai 2015 | Not intervention of interest |
| Khmelevsky 2015 | Not population of interest |
| Lanza 2015 | Not study design of interest |
| Laws 2014 | Not population of interest |
| Le Deley 2014 | Not population of interest |
| Liu 2012 | Not population of interest |
| Liu 2013a | Not study design of interest |
| Liu 2013b | Not population of interest |
| Maher 2014 | Not population of interest |
| Merker 2015 | Not intervention of interest |
| Meyers 2015 | Not population of interest |
| Munir 2016 | Not population of interest |
| Narumi 2012 | Not study design of interest |
| Oberlin 2012 | Not population of interest |
| Palassini 2015 | Not intervention of interest |
| Pavlyk 2013 | Not intervention of interest |
| Peinemann 2013 | Not study design of interest |
| Peinemann 2014 | Not study design of interest |
| Porta 2014 | Not population of interest |
| Rettinger 2012a | Not population of interest |
| Rettinger 2012b | Not population of interest |
| Schmidinger 2012 | Not population of interest |
| Schoffski 2016 | Not intervention of interest |
| Shvarova 2012 | Not study design of interest |
| Smolen 2014 | Not population of interest |
| Stahel 2015 | Not population of interest |
| Teichert von Luettichau 2014 | Not intervention of interest |
| Teppo 2016 | Not study design of interest |
| Uehara 2015 | Not study design of interest |
| Vecsei 2014 | Not population of interest |
| Verweij 2013 | Not intervention of interest |
| Woll 2012 | Not intervention of interest |
| Womer 2012 | Not population of interest |
| Yamada 2012 | Not study design of interest |
| Yuan 2015 | Not population of interest |
| Zhang 2013 | Not population of interest |
| Zhou 2013 | Not population of interest |
| Zhu 2014 | Not population of interest |
Differences between protocol and review
Differences between the previous version and the current version of the review
We revised the criteria for considering studies for this review. First, we confined the types of studies to RCTs. Therefore, we removed non‐randomized studies and associated data from the review. The single RCT included in the previous version was carried over to the current version of the review. Second, we extended the previous WHO classification of soft tissue sarcomas by adding information from the recently updated version of the WHO classification of soft tissue sarcomas. We revised the search strategies to improve precision and reported the results of the update search.
We changed the items of the 'Risk of bias' tool. We removed the items that were designed to judge the risk of bias of non‐randomized studies. We extended the rest of the items to complete all items of the risk of bias for RCTs. Thus, we included the judgment of some items of risk of bias that were not part of the previous version. Subsequently, the risk of bias was different between the previous and the current version of the review and the judgment changed from low to unclear risk of bias.
We identified additional inconsistencies in the reporting of the included study and sent inquiries to two authors of that study, but we did not receive a response. We identified a published warning letter sent by the FDA to the first author. We do not know if the cause of this letter was associated with conducting the study.
Contributions of authors
FP: designed and co‐ordinated the review, collected data, designed search strategies, undertook searches, screened search results, organized retrieval of papers, screened retrieved papers against eligibility criteria, appraised quality of papers, extracted data from papers, wrote to authors of papers for additional information, managed data, entered data into Review Manager 5, analyzed data, interpreted data, wrote manuscript.
HE: screened retrieved papers against eligibility criteria, reviewed manuscript.
LAS: provided methodologic advice, appraised quality of papers, reviewed manuscript.
Sources of support
Internal sources
-
University of Cologne, Germany.
Provision of full texts
External sources
No sources of support supplied
Declarations of interest
FP: none known.
HE: none known.
LAS: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bui‐Nguyen 2012 {published data only}
- Binh NN, Chevreau C, Penel N, Bay J, Coindre J, Mathoulin‐Pelissier S, et al. Consolidation with high‐dose chemotherapy for responding patients to standard chemotherapy in advanced, metastatic soft tissue sarcoma (STS): a randomized trial from FNCLCC‐French Sarcoma Group. Journal of Clinical Oncology 2009;27(15 Suppl):10505. [Google Scholar]
- Bui‐Nguyen B, Ray‐Coquard I, Chevreau C, Penel N, Bay JO, Coindre JM, et al. High‐dose chemotherapy consolidation for chemosensitive advanced soft tissue sarcoma patients: an open‐label, randomized controlled trial. Annals of Oncology 2012;23(3):777‐84. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Araki 2016 {published data only}
- Araki N, Takahashi S, Sugiura H, Ueda T, Yonemoto T, Takahashi M, et al. Retrospective inter‐ and intra‐patient evaluation of trabectedin after best supportive care for patients with advanced translocation‐related sarcoma after failure of standard chemotherapy. European Journal of Cancer 2016;56:122‐30. [DOI] [PubMed] [Google Scholar]
Benesch 2014 {published data only}
- Benesch M, Bartelheim K, Fleischhack G, Gruhn B, Schlegel PG, Witt O, et al. High‐dose chemotherapy (HDCT) with auto‐SCT in children with atypical teratoid/rhabdoid tumors (AT/RT): a report from the European Rhabdoid Registry (EU‐RHAB). Bone Marrow Transplantation 2014;49(3):370‐5. [DOI] [PubMed] [Google Scholar]
Brana 2014 {published data only}
- Brana I, Berger R, Golan T, Haluska P, Edenfield J, Fiorica J, et al. A parallel‐arm phase I trial of the humanised anti‐IGF‐1R antibody dalotuzumab in combination with the AKT inhibitor MK‐2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK‐0752, in patients with advanced solid tumours. British Journal of Cancer 2014;111(10):1932‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calabro 2015 {published data only}
- Calabro L, Morra A, Fonsatti E, Cutaia O, Fazio C, Annesi D, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy‐resistant malignant mesothelioma: an open‐label, single‐arm, phase 2 study. Lancet Respiratory Medicine 2015;3(4):301‐9. [DOI] [PubMed] [Google Scholar]
Cohen 2012 {published data only}
- Cohen MH, Johnson JR, Justice R, Pazdur R. Approval summary: imatinib mesylate for one or three years in the adjuvant treatment of gastrointestinal stromal tumors. Oncologist 2012;17(7):992‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conter 2013 {published data only}
- Conter HJ. Ten‐year follow‐up of patients (pts) with metastatic renal cell carcinoma (mRCC) treated with interferon alfa‐2b (IFN) as first‐line therapy: results from a randomized trial. Journal of Clinical Oncology 2013;31(Suppl 6):Abstr 365. [Google Scholar]
Czarnecka 2014 {published data only}
- Czarnecka AM, Szczylik C, Rini B. The use of sunitinib in renal cell carcinoma: where are we now?. Expert Review of Anticancer Therapy 2014;14(9):983‐99. [DOI] [PubMed] [Google Scholar]
Davis 2015 {published data only}
- Davis EJ, Chugh R, Zhao L, Lucas DR, Biermann JS, Zalupski MM, et al. A randomised, open‐label, phase II study of neo/adjuvant doxorubicin and ifosfamide versus gemcitabine and docetaxel in patients with localised, high‐risk, soft tissue sarcoma. European Journal of Cancer 2015;51(13):1794‐802. [DOI] [PubMed] [Google Scholar]
Delannes 2013 {published data only}
- Delannes M, Thomas L, Brun T, David I, Ducassou A. Brachytherapy for extremity soft tissue sarcomas [Curietherapie des sarcomes des tissus mous des membres]. Cancer Radiotherapie 2013;17(2):151‐4. [DOI] [PubMed] [Google Scholar]
Demetri 2016 {published data only}
- Demetri GD, Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. Journal of Clinical Oncology 2016;34(8):786‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diez‐Tejedor 2014 {published data only}
- Diez‐Tejedor E, Gutierrez‐Fernandez M, Martinez‐Sanchez P, Rodriguez‐Frutos B, Ruiz‐Ares G, Lara ML, et al. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double‐blind, placebo‐controlled, single‐center, pilot clinical trial. Journal of Stroke and Cerebrovascular Diseases 2014;23(10):2694‐700. [DOI] [PubMed] [Google Scholar]
Drabko 2012 {published data only}
- Drabko K, Raciborska A, Bilska K, Styczynski J, Ussowicz M, Choma M, et al. Consolidation of first‐line therapy with busulphan and melphalan, and autologous stem cell rescue in children with Ewing's sarcoma. Bone Marrow Transplantation 2012;47(12):1530‐4. [DOI] [PubMed] [Google Scholar]
Ehlert 2012 {published data only}
- Ehlert K, Rossig C, Groll A, Waeltermann M, Froehlich B, Juergens H. Toxicity of treosulfan in paediatric high‐dose chemotherapy regimens. Bone Marrow Transplantation 2012;47:S380. [Google Scholar]
Engelhard 2013 {published data only}
- Engelhard M, Allgauer M, Amela‐Neuschwander S, Brand HU, Brandes A, Bucker R, et al. Follicular lymphoma: final results of the randomized evaluation of curative radiotherapy in limited stage nodal disease. Strahlentherapie und Onkologie 2013;189(Suppl 1):1‐152. [Google Scholar]
Friedman 2014 {published data only}
- Friedman DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, et al. Dose‐intensive response‐based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate‐risk Hodgkin lymphoma: a report from the Children's Oncology Group Study AHOD0031. Journal of Clinical Oncology 2014;32(32):3651‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Froeb 2012 {published data only}
- Froeb D, Ranft A, Boelling T, Paulussen M, Klco‐Brosius S, Jurgens H, et al. Ewing sarcoma of the hand or foot. Klinische Padiatrie 2012;224(6):348‐52. [DOI] [PubMed] [Google Scholar]
Gaspar 2012 {published data only}
- Gaspar N, Rey A, Berard PM, Michon J, Gentet JC, Tabone MD, et al. Risk adapted chemotherapy for localised Ewing's sarcoma of bone: the French EW93 study. European Journal of Cancer 2012;48(9):1376‐85. [DOI] [PubMed] [Google Scholar]
Grignani 2015 {published data only}
- Grignani G, Palmerini E, Ferraresi V, D'Ambrosio L, Bertulli R, Asaftei SD, et al. Sorafenib and everolimus for patients with unresectable high‐grade osteosarcoma progressing after standard treatment: a non‐randomised phase 2 clinical trial. Lancet Oncology 2015;16(1):98‐107. [DOI] [PubMed] [Google Scholar]
Gronchi 2012 {published data only}
- Gronchi A, Frustaci S, Mercuri M, Martin J, Lopez‐Pousa A, Verderio P, et al. Short, full‐dose adjuvant chemotherapy in high‐risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Journal of Clinical Oncology 2012;30(8):850‐6. [DOI] [PubMed] [Google Scholar]
Halland 2012 {published data only}
- Halland AM. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate at one year ‐ the LITHE study the use of radiographs as a measure of structural damage. European Musculoskeletal Review 2012;7(4):eprint. [Google Scholar]
Hartmann 2013 {published data only}
- Hartmann JT, Horger M, Kluba T, Konigsrainer A, Zwart P, Weyhern CH, et al. A non‐comparative phase II study of dose intensive chemotherapy with doxorubicin and ifosfamide followed by high dose ICE consolidation with PBSCT in non‐resectable, high grade, adult type soft tissue sarcomas. Investigational New Drugs 2013;31(6):1592‐601. [DOI] [PubMed] [Google Scholar]
Hensley 2013 {published data only}
- Hensley ML, Wathen JK, Maki RG, Araujo DM, Sutton G, Priebat DA, et al. Adjuvant therapy for high‐grade, uterus‐limited leiomyosarcoma: results of a phase 2 trial (SARC 005). Cancer 2013;119(8):1555‐61. [DOI] [PubMed] [Google Scholar]
Infante 2015 {published data only}
- Infante JR, Ramanathan RK, George D, Tan E, Quinlan M, Liu A, et al. A randomized, crossover phase 1 study to assess the effects of formulation (capsule vs tablet) and meal consumption on the bioavailability of dovitinib (TKI258). Cancer Chemotherapy and Pharmacology 2015;75(4):729‐37. [DOI] [PubMed] [Google Scholar]
Ishida 2014 {published data only}
- Ishida Y, Qiu D, Maeda M, Fujimoto J, Kigasawa H, Kobayashi R, et al. Secondary cancers after cancer diagnosis in childhood: a hospital‐based retrospective cohort study in Japan. Pediatric Blood and Cancer 2014;61:S198. [DOI] [PubMed] [Google Scholar]
Ishida 2016 {published data only}
- Ishida Y, Qiu D, Maeda M, Fujimoto J, Kigasawa H, Kobayashi R, et al. Secondary cancers after a childhood cancer diagnosis: a nationwide hospital‐based retrospective cohort study in Japan. International Journal of Clinical Oncology 2016;21(3):506‐16. [DOI] [PubMed] [Google Scholar]
Joensuu 2012 {published data only}
- Joensuu H. Adjuvant therapy for high‐risk gastrointestinal stromal tumour: considerations for optimal management. Drugs 2012;72(15):1953‐63. [DOI] [PubMed] [Google Scholar]
Judson 2014 {published data only}
- Judson I, Verweij J, Gelderblom H, Hartmann JT, Schoffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first‐line treatment of advanced or metastatic soft‐tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncology 2014;15(4):415‐23. [DOI] [PubMed] [Google Scholar]
Kawai 2015 {published data only}
- Kawai A, Araki N, Sugiura H, Ueda T, Yonemoto T, Takahashi M, et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation‐related sarcoma: a randomised, open‐label, phase 2 study. Lancet Oncology 2015;16(4):406‐16. [DOI] [PubMed] [Google Scholar]
Khmelevsky 2015 {published data only}
- Khmelevsky EV, Bychkova NM. Radiosensitivity of bone metastases from tumors in different primary sites [Russian]. Voprosy Onkologii 2015;61(1):77‐84. [PubMed] [Google Scholar]
Lanza 2015 {published data only}
- Lanza F, Pedrazzoli P, Ravelli A, Brambilla P. Hematopoietic stem cell transplantation in solid tumors. 41st Annual Meeting of the European Society for Blood and Marrow Transplantation, EBMT 2015. Istanbul Turkey. Bone Marrow Transplantation 2015;50:S114. [Google Scholar]
Laws 2014 {published data only}
- Laws DA, Kraft AS, Leddy LR, LaRue AC. The role of circulating fibroblast precursors in promoting metastatic sarcoma. Cancer Research 2014;75(1 Suppl 1):A46. [Google Scholar]
Le Deley 2014 {published data only}
- Deley MC, Paulussen M, Lewis I, Brennan B, Ranft A, Whelan J, et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard‐risk Ewing sarcoma: results of the randomized noninferiority Euro‐EWING99‐R1 trial. Journal of Clinical Oncology 2014;32(23):2440‐8. [DOI] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu CJ, Chen KW, Hu YW, Hong YC, Huang YC, Chiou TJ, et al. Hematopoietic stem cell transplantation and secondary primary malignancies in Taiwan. Blood 2012;120(21):4219. [Google Scholar]
Liu 2013a {published data only}
- Liu SL, Chen G, Zhao YP, Wu WM, Zhang TP. Optimized dose of imatinib for treatment of gastrointestinal stromal tumors: a meta‐analysis. Journal of Digestive Diseases 2013;14(1):16‐21. [DOI] [PubMed] [Google Scholar]
Liu 2013b {published data only}
- Liu J, Yu H, Shang Q, Yan C, Jiang P, Wang X. The effects of low‐dose splenic irradiation and radiotherapy on immune system of patients with locally advanced non‐small cell lung cancer. Chinese‐German Journal of Clinical Oncology 2013;12(2):51‐5. [Google Scholar]
Maher 2014 {published data only}
- Maher O, Cooper L, Tarek N, Worth LL, Petropoulis D, Lee DA, et al. Successful treatment for secondary AML and MDS following myeloablative hematopoetic stem cell transplantation for children, adolescents and young adults. Bone Marrow Transplantation 2014;49:S376. [Google Scholar]
Merker 2015 {published data only}
- Merker M, Rettinger E, Oelsner S, Pfirrmann V, Ullrich E, Wels W, et al. ErbB2‐chimeric antigen receptor (CAR) modified cytokine induced killer (CIK) cell intervention for refractory solid tumors after allogeneic stem cell transplantation. Bone Marrow Transplantation 2015;50:S93. [Google Scholar]
Meyers 2015 {published data only}
Munir 2016 {published data only}
- Munir T, Cohen D, Pocock C, Rawstron A, Collet L, McParland L, et al. Physical examination and MRD assessment rather than CT‐scans is effective to assess responses in patients treated with fludarabine‐based chemoimmunotherapy. Analysis of patients in the UK NCRI ADMIRE and ARCTIC trials. Leukemia & Lymphoma 2015;56:26‐7. [Google Scholar]
Narumi 2012 {published data only}
- Narumi K, Udagawa T, Suzuki K, Aida K, Makimoto A, Aoki K. Syngeneic hematopoietic stem cell transplantation enhances the antitumor immunity of intratumoral type I interferon gene transfer for sarcoma. Molecular Therapy 2012;20:S263. [DOI] [PubMed] [Google Scholar]
Oberlin 2012 {published data only}
- Oberlin O, Rey A, Sanchez de Toledo J, Martelli H, Jenney ME, Scopinaro M, et al. Randomized comparison of intensified six‐drug versus standard three‐drug chemotherapy for high‐risk nonmetastatic rhabdomyosarcoma and other chemotherapy‐sensitive childhood soft tissue sarcomas: long‐term results from the International Society of Pediatric Oncology MMT95 study. Journal of Clinical Oncology 2012;30(20):2457‐65. [DOI] [PubMed] [Google Scholar]
Palassini 2015 {published data only}
- Palassini E, Ferrari S, Verderio P, Paoli A, Martin Broto J, Quagliuolo V, et al. Feasibility of preoperative chemotherapy with or without radiation therapy in localized soft tissue sarcomas of limbs and superficial trunk in the Italian Sarcoma Group/Grupo Espanol de Investigacion en Sarcomas randomized clinical trial: three versus five cycles of full‐dose epirubicin plus ifosfamide. Journal of Clinical Oncology 2015;33(31):3628‐34. [DOI] [PubMed] [Google Scholar]
Pavlyk 2013 {published data only}
- Pavlyk S, Shaida E, Grigoriy K. Metronomic application of cyclophosphamide and vinorelbine for treatment of soft‐tissue sarcoma in children. Pediatric Blood and Cancer 2013;60:37. [Google Scholar]
Peinemann 2013 {published data only}
- Peinemann F, Smith LA, Bartel C. Autologous hematopoietic stem cell transplantation following high dose chemotherapy for non‐rhabdomyosarcoma soft tissue sarcomas. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD008216.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peinemann 2014 {published data only}
- Peinemann F, Labeit AM. Autologous haematopoietic stem cell transplantation following high‐dose chemotherapy for non‐rhabdomyosarcoma soft tissue sarcomas: a Cochrane systematic review. BMJ Open 2014;4(7):e005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porta 2014 {published data only}
- Porta C, Giglione P, Lombardo F, Paglino C. First‐line medical therapy in advanced metastatic RCC (conference abstract). Anticancer Research 2014;34(10):6117‐8. [Google Scholar]
Rettinger 2012a {published data only}
- Rettinger E, Meyer V, Kreyenberg H, Volk A, Kuci S, Willasch A, et al. Cytotoxic capacity of IL‐15‐stimulated cytokine‐induced killer cells against human acute myeloid leukemia and rhabdomyosarcoma in humanized preclinical mouse models. Frontiers in Oncology 2012;2:00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rettinger 2012b {published data only}
- Rettinger E, Meyer V, Kreyenberg H, Kuci S, Willasch A, Fulda S, et al. Clinical scale generation and functional assessment of cytokine‐induced killer cells against acute leukaemia and soft tissue sarcoma. Bone Marrow Transplantation 2012;47:S361. [Google Scholar]
Schmidinger 2012 {published data only}
- Schmidinger M, Szczylik C, Sternberg CN, Kania M, Kelly CS, Decker R, et al. Dose escalation and pharmacokinetics study of enzastaurin and sunitinib versus placebo and sunitinib in patients with metastatic renal cell carcinoma. American Journal of Clinical Oncology 2012;35(5):493‐7. [DOI] [PubMed] [Google Scholar]
Schoffski 2016 {published data only}
- Schoffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open‐label, multicentre, phase 3 trial. Lancet 2016;387(10028):1629‐37. [DOI] [PubMed] [Google Scholar]
Shvarova 2012 {published data only}
- Shvarova A, Ivanova N, Dolgopolov I. The survivorship of children with primary metastatic bone and soft tissue sarcomas. 2012 International MASCC/ISOO Symposium. New York City, NY. Supportive Care in Cancer 2012;20:S86‐7. [Google Scholar]
Smolen 2014 {published data only}
- Smolen JS, Weinblatt ME, Sheng S, Zhuang Y, Hsu B. Sirukumab, a human anti‐interleukin‐6 monoclonal antibody: a randomised, 2‐part (proof‐of‐concept and dose‐finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Annals of the Rheumatic Diseases 2014;73(9):1616‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stahel 2015 {published data only}
- Stahel RA, Riesterer O, Xyrafas A, Opitz I, Beyeler M, Ochsenbein A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncology 2015;16(16):1651‐8. [DOI] [PubMed] [Google Scholar]
Teichert von Luettichau 2014 {published data only}
- Teichert von Luettichau I, Salat C, Wawer A, Noessner E, Eckl J, Multhoff G, et al. Targeting the graft‐versus‐tumor effect of DLI by regional hyperthermia to the tumor compartment. Bone Marrow Transplantation 2014;49:S499. [Google Scholar]
Teppo 2016 {published data only}
- Teppo E, Penttinen J, Myohanen O, Vettenranta K, Lohi O. Single‐centre study reports a 84% five‐year overall survival rate for paediatric solid tumours. Acta Paediatrica 2016;105(8):952‐8. [DOI] [PubMed] [Google Scholar]
Uehara 2015 {published data only}
- Uehara S, Oue T, Nakahata K, Nara K, Ueno T, Owari M, et al. Perioperative management after high‐dose chemotherapy with autologous or allogeneic hematopoietic stem cell transplantation for pediatric solid tumors. European Journal of Pediatric Surgery 2015;25(1):118‐22. [DOI] [PubMed] [Google Scholar]
Vecsei 2014 {published data only}
- Vecsei A, Engert Z, Bardi E. Primary inoperable pelvic embryonal rhabdomyosarcoma became operable and curable due to complex therapy. EAU 14th Central European Meeting, CEM 2014. Cracow Poland. European Urology, Supplements 2014;13(6):e1365. [Google Scholar]
Verweij 2013 {published data only}
- Verweij J, Sleijfer S. Pazopanib, a new therapy for metastatic soft tissue sarcoma. Expert Opinion on Pharmacotherapy 2013;14(7):929‐35. [DOI] [PubMed] [Google Scholar]
Woll 2012 {published data only}
- Woll PJ, Reichardt P, Cesne A, Bonvalot S, Azzarelli A, Hoekstra HJ, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft‐tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncology 2012;13(10):1045‐54. [DOI] [PubMed] [Google Scholar]
Womer 2012 {published data only}
- Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, et al. Randomized controlled trial of interval‐compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. Journal of Clinical Oncology 2012;30(33):4148‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamada 2012 {published data only}
- Yamada K, Inoue M, Kondo O, Sato M, Sawada A, Yasui M, et al. Issues associated with cancer in adolescents and young adults. 10th Annual Meeting of the Japanese Society of Medical Oncology. Osaka Japan. Annals of Oncology 2012;23:xi167. [Google Scholar]
Yuan 2015 {published data only}
- Yuan J, Liu T, Zhang X, Si Y, Ye Y, Zhao C, et al. Intensive versus conventional glycemic control in patients with diabetes during enteral nutrition after gastrectomy. Journal of Gastrointestinal Surgery 2015;19(8):1553‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang X, Wang Z, Wang Z, Zhang Y, Jia Q, Wu L, et al. Impact of acetylsalicylic acid on tumor angiogenesis and lymphangiogenesis through inhibition of VEGF signaling in a murine sarcoma model. Oncology Reports 2013;29(5):1907‐13. [DOI] [PubMed] [Google Scholar]
Zhou 2013 {published data only}
- Zhou A, Zhang W, Chang C, Chen X, Zhong D, Qin Q, et al. Phase I study of the safety, pharmacokinetics and antitumor activity of famitinib. Cancer Chemotherapy and Pharmacology 2013;72(5):1043‐53. [DOI] [PubMed] [Google Scholar]
Zhu 2014 {published data only}
- Zhu HD, Guo JH, Mao AW, Lv WF, Ji JS, Wang WH, et al. Conventional stents versus stents loaded with (125)iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. Lancet Oncology 2014;15(6):612‐9. [DOI] [PubMed] [Google Scholar]
Additional references
ACS 2016
- American Cancer Society. Survival by stage of soft tissue sarcoma. www.cancer.org/cancer/sarcoma‐adultsofttissuecancer/detailedguide/sarcoma‐adult‐soft‐tissue‐cancer‐survival‐rates (accessed prior to 28 March 2017).
Admiraal 2010
- Admiraal R, Paardt M, Kobes J, Kremer LC, Bisogno G, Merks JH. High‐dose chemotherapy for children and young adults with stage IV rhabdomyosarcoma. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD006669.pub2] [DOI] [PubMed] [Google Scholar]
AJCC 2016
- American Joint Committee on Cancer. Soft tissue sarcoma. In: Amin MB, Edge S, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. editor(s). AJCC Cancer Staging Manual. 8th Edition. New York: Springer, 2016. [Google Scholar]
ASCO Meetings 2012 to 2016
- Meeting Library Search Abstracts 2012 to 2016. American Society of Clinical Oncology (ASCO), Alexandria, Virginia, U.S.A. 2016, issue http://meetinglibrary.asco.org/abstracts.
ASH Meetings 2013 to 2015
- ASH Annual Meeting Abstracts 2013 to 2015. American Society of Hematology (ASH), Washington, District of Columbia, U.S.A. 2016, issue http://www.bloodjournal.org/page/ash‐annual‐meeting‐abstracts.
Banna 2007
- Banna GL, Simonelli M, Santoro A. High‐dose chemotherapy followed by autologous hematopoietic stem‐cell transplantation for the treatment of solid tumors in adults: a critical review. Current Stem Cell Research and Therapy 2007;2(1):65‐82. [DOI] [PubMed] [Google Scholar]
Blay 2000
- Blay JY, Bouhour D, Ray‐Coquard I, Dumontet C, Philip T, Biron P. High‐dose chemotherapy with autologous hematopoietic stem‐cell transplantation for advanced soft tissue sarcoma in adults. Journal of Clinical Oncology 2000;18(21):3643‐50. [DOI] [PubMed] [Google Scholar]
BMT Tandem Meeting 2012
- Abstracts from the 2012 BMT Tandem Meetings. Biology of Blood and Marrow Transplantation. 2012; Vol. 18, issue 2 Suppl:S201‐406.
BMT Tandem Meeting 2013
- Abstracts from the 2013 BMT Tandem Meetings. Biology of Blood and Marrow Transplantation. 2013; Vol. 19, issue 2 Suppl:S109‐392.
BMT Tandem Meeting 2014
- Abstracts from the 2014 BMT Tandem Meetings. Biology of Blood and Marrow Transplantation. 2014; Vol. 20, issue 2 Suppl:S1‐326. [PubMed]
BMT Tandem Meeting 2015
BMT Tandem Meeting 2016
Bonnet 1997
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine 1997;3(7):730‐7. [DOI] [PubMed] [Google Scholar]
Carvajal 2005
- Carvajal R, Meyers P. Ewing's sarcoma and primitive neuroectodermal family of tumors. Hematology/Oncology Clinics of North America 2005;19(3):501‐25. [DOI] [PubMed] [Google Scholar]
Clark 2005
- Clark MA, Fisher C, Judson I, Thomas JM. Soft‐tissue sarcomas in adults. New England Journal of Medicine 2005;353(7):701‐11. [DOI] [PubMed] [Google Scholar]
ClinicalTrials.gov Studies 2016
- U.S. National Library of Medicine (NLM), Bethesda, Maryland, U.S.A. ClinicalTrials.gov. clinicaltrials.gov (accessed 26 September 2016).
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dileo 2005
- Dileo P, Demetri GD. Update on new diagnostic and therapeutic approaches for sarcomas. Clinical Advances in Hematology & Oncology 2005;3(10):781‐91. [PubMed] [Google Scholar]
Dumontet 1992
- Dumontet C, Biron P, Bouffet E, Blay JY, Meckenstock R, Chauvin F, et al. High dose chemotherapy with ABMT in soft tissue sarcomas: a report of 22 cases. Bone Marrow Transplantation 1992;10(5):405‐8. [PubMed] [Google Scholar]
EBMT Meeting 2014
EBMT Meeting 2015
- 41st Annual Meeting of the European Society for Blood and Marrow Transplantation, Istanbul, Turkey, 22‐25 March 2015. Bone Marrow Transplantation. 2015; Vol. 50:S1.
EBMT Meeting 2016
- 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation Valencia, Spain 3‐6 April 2016. Bone Marrow Transplantation. 2016; Vol. 51:S1.
EBMT Studies 2016
- European Society for Blood and Marrow Transplantation (EBMT), Leiden, The Netherlands. EBMT Studies Current Research List. www.ebmt.org/Contents/Research/EBMTStudies/CurrentResearch/Pages/CurrentResearch.aspx, (accessed prior to 28 March 2017).
Ek 2006
- Ek ETH, Choong PFM. The role of high‐dose therapy and autologous stem cell transplantation for pediatric bone and soft tissue sarcomas. Expert Review of Anticancer Therapy 2006;6(2):225‐37. [DOI] [PubMed] [Google Scholar]
Elias 1998
- Elias AD. High‐dose therapy for adult soft tissue sarcoma: dose response and survival. Seminars in Oncology 1998;25(2 Suppl 4):19‐23. [PubMed] [Google Scholar]
ESMO 2014
- European Society for Medical Oncology/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2014;25 Suppl 3:iii102‐12. [DOI] [PubMed] [Google Scholar]
FDA 2015
- Kassim SY. Warning letter. Reference: 15‐HFD‐45‐05‐01. www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm452031.htm (accessed prior to 28 March 2017).
Fletcher 2002
- Fletcher CDM, Rydholm A, Singer S, Sundaram M, Coindre JM. Soft tissue tumours: WHO classification, epidemiology, clinical features, histopathological typing and grading. In: Fletcher CDM, Unni KK, Mertens F editor(s). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: International Agency for Research on Cancer, 2002:12‐8. [Google Scholar]
Fletcher 2013
- Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. 4th Edition. Lyon: International Agency for Research on Cancer, 2013. [Google Scholar]
Google Translate 2016 [Computer program]
- Google, Inc. Google Translate. Mountain View: Google, Inc, 2016; translate.google.com/.
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Gurney 1997
- Gurney JG, Young JL, Roffers SD, Smith MA, Bunin GR. Soft tissue sarcomas. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al. editor(s). Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975‐1995 (SEER Pediatric Monograph) NIH Pub. No. 99‐4649. Bethesda: National Cancer Institute, 1997:111‐24. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Howlader 2016
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER Cancer Statistics Review, 1975‐2013. seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER website, April 2016 (accessed prior to 28 March 2017).
ICTRP Studies 2016
- International Clinical Trials Registry Platform (ICTRP) Search Portal. World Health Organization 2016, issue http://apps.who.int/trialsearch/.
Kasper 2005
- Kasper B, Ho AD, Egerer G. Is there an indication for high‐dose chemotherapy in the treatment of bone and soft‐tissue sarcoma?. Oncology 2005;68(2‐3):15‐21. [DOI] [PubMed] [Google Scholar]
Kasper 2007
- Kasper B, Dietrich S, Mechtersheimer G, Ho AD, Egerer G. Large institutional experience with dose‐intensive chemotherapy and stem cell support in the management of sarcoma patients. Oncology 2007;73(1‐2):58‐64. [DOI] [PubMed] [Google Scholar]
Kotilingam 2006
- Kotilingam D, Lev DC, Lazar AJ, Pollock RE. Staging soft tissue sarcoma: evolution and change. CA: A Cancer Journal for Clinicians 2006;56(5):282‐91. [DOI] [PubMed] [Google Scholar]
Ladenstein 1997
- Ladenstein R, Philip T, Gardner H. Autologous stem cell transplantation for solid tumors in children. Current Opinion in Pediatrics 1997;9(1):55‐69. [DOI] [PubMed] [Google Scholar]
Miller 1995
- Miller RW, Young JL Jr, Novakovic B. Childhood cancer. Cancer 1995;75(1 Suppl):395‐405. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCI CTCAE 2016
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC). ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed 2 June 2016).
NCI snapshot 2014
- National Cancer Institute. A snapshot of sarcoma: incidence and mortality. cancer.gov/research/progress/snapshots/sarcoma (accessed 2 June 2016).
NCI staging 2015
- National Cancer Institute. Cancer staging. cancer.gov/cancertopics/factsheet/detection/staging (accessed 2 June 2016).
Passweg 2016
- Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40000 transplants annually. Bone Marrow Transplantation 2016;51(6):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinkerton 1986
- Pinkerton R, Philip T, Bouffet E, Lashford L, Kemshead J. Autologous bone marrow transplantation in paediatric solid tumours. Clinics in Haematology 1986;15(1):187‐203. [DOI] [PubMed] [Google Scholar]
Reichardt 2002
- Reichardt P. High‐dose chemotherapy in adult soft tissue sarcoma. Critical Reviews in Oncology/Hematology 2002;41(2):157‐67. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riedel 2012
- Riedel RF. Systemic therapy for advanced soft tissue sarcomas: highlighting novel therapies and treatment approaches. Cancer 2012;118(6):1474‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosti 2002
- Rosti G, Ferrante P, Ledermann J, Leyvraz S, Ladenstein R, Koscielniak E, et al. High‐dose chemotherapy for solid tumors: results of the EBMT. Critical Reviews in Oncology/Hematology 2002;41(2):129‐40. [DOI] [PubMed] [Google Scholar]
Sanchez‐Garcia 2007
- Sanchez‐Garcia I, Vicente‐Duenas C, Cobaleda C. The theoretical basis of cancer‐stem‐cell‐based therapeutics of cancer: can it be put into practice?. BioEssays 2007;29(12):1269‐80. [DOI] [PubMed] [Google Scholar]
Schlemmer 2006
- Schlemmer M, Wendtner CM, Falk M, Abdel‐Rahman S, Licht T, Baumert J, et al. Efficacy of consolidation high‐dose chemotherapy with ifosfamide, carboplatin and etoposide (HD‐ICE) followed by autologous peripheral blood stem cell rescue in chemosensitive patients with metastatic soft tissue sarcomas. Oncology 2006;71(1‐2):32‐9. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Seeger 1991
- Seeger RC, Reynolds CP. Treatment of high‐risk solid tumors of childhood with intensive therapy and autologous bone marrow transplantation. Pediatric Clinics of North America 1991;38(2):393‐424. [DOI] [PubMed] [Google Scholar]
SIOP Meeting 2012
- International Society of Paediatric Oncology. 44th Congress of the International Society of Paediatric Oncology (SIOP) 2012 International Conference, 2012 Oct 5‐8; London. Pediatric Blood & Cancer 2012;59(6):965‐1152. [DOI] [PubMed] [Google Scholar]
SIOP Meeting 2013
- International Society of Paediatric Oncology. 45th Congress of the International Society of Paediatric Oncology (SIOP) 2013 International Conference, 2013 Sept 25‐28; Hong Kong, China. Pediatric Blood & Cancer 2013;60(Suppl 3):S2‐264. [DOI] [PubMed] [Google Scholar]
SIOP Meeting 2014
- International Society of Paediatric Oncology. 46th Congress of The International Society of Paediatric Oncology (SIOP) 2014 International Conference, 2014 Oct 22‐25; Toronto, Canada. Pediatric Blood & Cancer 2014;61(Suppl 2):S105‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIOP Meeting 2015
- International Society of Paediatric Oncology. 47th Congress of the International Society of Paediatric Oncology (SIOP) 2015 International Conference, 2015 Oct 8‐11; Cape Town, South Africa. Pediatric Blood & Cancer 2015;62(Suppl 4):S143‐418. [Google Scholar]
SIOP Meeting 2016
- International Society of Paediatric Oncology. 48th Congress of the International Society of Paediatric Oncology (SIOP) 2016 International Conference, 2016 Oct 19‐22; Dublin, Ireland. Pediatric Blood & Cancer 2016;63(Suppl 3):S5‐321. [DOI] [PubMed] [Google Scholar]
Sondak 2001
- Sondak VK, Chang AE. Clinical evaluation and treatment of soft tissue tumors. In: Weiss SW, Goldblum JR editor(s). Enzinger and Weiss's Soft Tissue Tumors. 4th Edition. St Louis: Mosby, 2001:21‐44. [Google Scholar]
Spunt 2006
- Spunt SL, Pappo AS. Childhood nonrhabdomyosarcoma soft tissue sarcomas are not adult‐type tumors. Journal of Clinical Oncology 2006;24(12):1958‐9. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. Journal of the National Cancer Institute 2000;92(3):205‐16. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsao 2000
- Tsao H. Update on familial cancer syndromes and the skin. Journal of the American Academy of Dermatology 2000;42(6):939‐69. [PubMed] [Google Scholar]
UICC 2009
- Sobin LH, Gospodarowicz MK, Wittekind C. The TNM Classification of Malignant Tumours. 7th Edition. Geneva: Union for International Cancer Control, 2009. [Google Scholar]
Weiss 2001
- Weiss SW, Goldblum JR. General considerations. In: Weiss SW, Goldblum JR editor(s). Enzinger and Weiss's Soft Tissue Tumors. 4th Edition. St Louis: Mosby, 2001:1‐19. [Google Scholar]
Woods 1999
- Woods WG. Myeloablative therapy followed by stem cell rescue for pediatric solid tumors: a non‐transplanter's perspective. Cancer Research Therapy and Control 1999;9(1‐2):95‐9. [Google Scholar]
References to other published versions of this review
Peinemann 2010
- Peinemann F, Bartel C, Hildebrandt M, Kulig M, Burkhardt‐Hammer T, Kröger N, et al. Autologous stem cell transplantation following high‐dose chemotherapy for non‐rhabdomyosarcoma soft tissue sarcomas. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD008216] [DOI] [PubMed] [Google Scholar]
Peinemann 2011
- Peinemann F, Smith LA, Kromp M, Bartel C, Kröger N, Kulig M. Autologous hematopoietic stem cell transplantation following high‐dose chemotherapy for non‐rhabdomyosarcoma soft tissue sarcomas. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008216.pub3] [DOI] [PubMed] [Google Scholar]
Peinemann 2013
- Peinemann F, Smith LA, Bartel C. Autologous hematopoietic stem cell transplantation following high dose chemotherapy for non‐rhabdomyosarcoma soft tissue sarcomas. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD008216.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


