Abstract
Background
Pelvic inflammatory disease (PID) is an infection that affects 4% to 12% of young women, and is one of the most common causes of morbidity in this age group. The main intervention for acute PID is the use of broad‐spectrum antibiotics which cover Chlamydia trachomatis, Neisseria gonorrhoeae, and anaerobic bacteria, administered intravenously, intramuscularly, or orally. In this review, we assessed the optimal treatment regimen for PID.
Objectives
To assess the effectiveness and safety of antibiotic regimens used to treat pelvic inflammatory disease.
Search methods
We searched the Cochrane Sexually Transmitted Infections Review Group's Specialized Register, which included randomized controlled trials (RCTs) from 1944 to 2016, located through electronic searching and handsearching; the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid platform (1991 to July 2016); MEDLINE (1946 to July 2016); Embase (1947 to July 2016); LILACS, iAHx interface (1982 to July 2016); World Health Organization International Clinical Trials Registry Platform (July 2016); Web of Science (2001 to July 2016); OpenGrey (1990, 1992, 1995, 1996, and 1997); and abstracts in selected publications.
Selection criteria
We included RCTs comparing the use of antibiotics with placebo or other antibiotics for the treatment of PID in women of reproductive age, either as inpatient or outpatient treatment. We limited our review to comparison of drugs in current use that are recommended for consideration by the 2015 US Centers for Disease Control and Prevention (CDC) guidelines for treatment of PID.
Data collection and analysis
At least two review authors independently selected trials for inclusion, extracted data, and assessed risk of bias. We contacted investigators to obtain missing information. We resolved disagreements by consensus or by consulting a fourth review author if necessary. We assessed the quality of the evidence using GRADE criteria, classifying it as high, moderate, low, or very low. We calculated Mantel‐Haenszel risk ratios (RR), using either random‐effects or fixed‐effect models and number needed to treat for an additional beneficial outcome or for an additional harmful outcome, with their 95% confidence interval (CI), to measure the effect of the treatments. We conducted sensitivity analyses limited to studies at low risk of bias, for comparisons where such studies were available.
Main results
We included 37 RCTs (6348 women). The quality of the evidence ranged from very low to high, the main limitations being serious risk of bias (due to poor reporting of study methods and lack of blinding), serious inconsistency, and serious imprecision.
Azithromycin versus doxycycline
There was no clear evidence of a difference between the two drugs in rates of cure for mild‐moderate PID (RR 1.18, 95% CI 0.89 to 1.55, I2 = 72%, 2 RCTs, 243 women, very low‐quality evidence), severe PID (RR 1.00, 95% CI 0.96 to 1.05, 1 RCT, 309 women, low‐quality evidence), or adverse effects leading to discontinuation of treatment (RR 0.71, 95% CI 0.38 to 1.34, 3 RCTs, 552 women, I2 = 0%, low‐quality evidence). In a sensitivity analysis limited to a single study at low risk of bias, azithromycin was superior to doxycycline in achieving cure in mild‐moderate PID (RR 1.35, 95% CI 1.10 to 1.67, 133 women, moderate‐quality evidence).
Quinolone versus cephalosporin
There was no clear evidence of a difference between the two drugs in rates of cure for mild‐moderate PID (RR 1.04, 95% CI 0.98 to 1.10, 3 RCTs, 459 women, I2 = 5%, low‐quality evidence), severe PID (RR 1.06, 95% CI 0.91 to 1.23, 2 RCTs, 313 women, I2 = 7%, low‐quality evidence), or adverse effects leading to discontinuation of treatment (RR 2.24, 95% CI 0.52 to 9.72, 5 RCTs, 772 women, I2 = 0%, very low‐quality evidence).
Nitroimidazole versus no use of nitroimidazole
There was no conclusive evidence of a difference between the nitroimidazoles (metronidazole) group and the group receiving other drugs with activity over anaerobes (e.g. amoxicillin‐clavulanate) in rates of cure for mild‐moderate PID (RR 1.01, 95% CI 0.93 to 1.10, 5 RCTs, 2427 women, I2 = 60%, moderate‐quality evidence), severe PID (RR 0.96, 95% CI 0.92 to 1.01, 11 RCTs, 1383 women, I2 = 0%, moderate‐quality evidence), or adverse effects leading to discontinuation of treatment (RR 1.00, 95% CI 0.63 to 1.59; participants = 3788; studies = 16; I2 = 0% , low‐quality evidence). In a sensitivity analysis limited to studies at low risk of bias, findings did not differ substantially from the main analysis (RR 1.06, 95% CI 0.98 to 1.15, 2 RCTs, 1201 women, I2 = 32%, high‐quality evidence).
Clindamycin plus aminoglycoside versus quinolone
There was no evidence of a difference between the two groups in rates of cure for mild‐moderate PID (RR 0.88, 95% CI 0.69 to 1.13, 1 RCT, 25 women, very low‐quality evidence), severe PID (RR 1.02, 95% CI 0.87 to 1.19, 2 studies, 151 women, I2 = 0%, low‐quality evidence), or adverse effects leading to discontinuation of treatment (RR 0.21, 95% CI 0.02 to 1.72, 3 RCTs, 163 women, very low‐quality evidence).
Clindamycin plus aminoglycoside versus cephalosporin
There was no clear evidence of a difference between the two groups in rates of cure for mild‐moderate PID (RR 1.02, 95% CI 0.95 to 1.09, 2 RCTs, 150 women, I2 = 0%, low‐quality evidence), severe PID (RR 1.00, 95% CI 0.95 to 1.06, 10 RCTs, 959 women, I2 = 21%, moderate‐quality evidence), or adverse effects leading to discontinuation of treatment (RR 0.78, 95% CI 0.18 to 3.42, 10 RCTs, 1172 women, I2 = 0%, very low‐quality evidence).
Authors' conclusions
We found no conclusive evidence that one regimen of antibiotics was safer or more effective than any other for the cure of PID, and there was no clear evidence for the use of nitroimidazoles (metronidazole) compared to use of other drugs with activity over anaerobes. Moderate‐quality evidence from a single study at low risk of bias suggested that a macrolide (azithromycin) may be more effective than a tetracycline (doxycycline) for curing mild‐moderate PID. Our review considered only the drugs that are currently used and mentioned by the CDC.
Treatment for pelvic inflammatory disease
Review question
We assessed the effectiveness and safety of different treatments for pelvic inflammatory disease (PID) that are recommended for consideration by current clinical guidelines for treatment of PID (the 2015 US Centers for Disease Control and Prevention guidelines for treatment of PID).
Background
PID is an infection of the upper part of the woman's reproductive system (womb, fallopian tubes (tube connecting the womb and ovary that the egg travels along), ovaries (which make eggs), and inside of the pelvis). It is a common condition affecting women of childbearing age. Symptoms of PID vary, ranging from no symptoms to severe symptoms. If effective treatment is not started promptly, the consequences of the condition can be infertility (unable to have children), pregnancies outside of the womb, and chronic pelvic pain (pain in the lower tummy). There is a wide range of treatment options, the choice of which is based on severity of symptoms, experience of the doctor, national/international guidelines, and rate of side effects. We wanted to learn if there is a better antibiotic (used to treat bacterial infections) therapy with higher rates of cure and lower side effects to treat PID.
Trial characteristics
We searched the available literature up to 11 July 2016 and included 37 studies with 6348 women with an average of 14 days of treatment and follow‐up (monitoring after treatment). These trials included women of childbearing age with mild to severe PID. Trials mostly used a combination of antibiotics with different administration routes: intravenous (into a blood vessel), intramuscular (into the muscle), and oral (as a tablet). In mild‐moderate cases, intramuscular and oral treatments were prescribed, and in moderate‐severe cases, treatments were usually started in hospital and were completed at home.
Key results
We found no conclusive evidence that one treatment was safer or more effective than any other for the cure of PID, and there was no clear evidence for the use of nitroimidazoles (a type of antibiotic; metronidazole) compared to use of other antibiotics. Moderate‐quality evidence from a single study at low risk of bias suggested that a macrolide (a type of antibiotic; azithromycin) may be more effective than a tetracycline (a type of antibiotic; doxycycline) for curing mild‐moderate PID.
Quality of evidence
The quality of the evidence ranged from very low to high, the main problems being serious risk of bias (poor reporting of study methods; doctors and women may have known which medicine was given), and results differed across studies.
Summary of findings
Summary of findings for the main comparison.
Macrolides (azithromycin) compared to tetracycline (doxycycline) for pelvic inflammatory disease (PID)
| Azithromycin compared to doxycycline for pelvic inflammatory disease (PID) | ||||||
|
Population: women with PID Setting: hospital ward or outpatient clinic Intervention: azithromycin Comparison: doxycycline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with doxycycline | Risk with azithromycin | |||||
|
Clinical cure according to criteria established by study authors Mild‐moderate PID Follow‐up: median 14 days |
84/122 750 per 1000 |
99/121 818 per 1000 (740 to 876) |
RR 1.18 (0.89 to 1.55) NNTB 13 to NNTH 3 |
243 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 | ‐ |
|
Clinical cure according to criteria established by study authors Mild‐moderate PID Follow‐up: median 14 days Sensitivity analysis restricted to study at low risk of bias |
42/67 627 per 1000 |
56/66 848 per 1000 (743 to 921) |
RR 1.35 (1.10 to 1.67) NNTB 5 (3 to 14) |
133 (1 RCT) |
⊕⊕⊕⊝ Moderate5 | ‐ |
|
Clinical cure according to criteria established by study authors Severe PID Follow‐up: range 13‐18 days |
93/96 969 per 1000 |
207/213 971 per 1000 (940 to 987) |
RR 1.00 (0.96 to 1.05) NNTB 16 to NNTH 29 |
309 (1 RCT) | ⊕⊕⊝⊝ Low4 | ‐ |
|
Adverse events: any antibiotic‐related adverse event leading to discontinuation of therapy. Follow‐up: range 13‐18 days |
17/217 78 per 1000 |
16/335 47 per 1000 (29 to 76) |
RR 0.71 (0.38 to 1.34) NNTB 13 to NNTH 101 |
552 (3 RCTs) | ⊕⊕⊝⊝ Low1,3 | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; PID: pelvic inflammatory disease; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for serious risk of bias (poor reporting of methods and high risk of performance and detection bias in one or more studies).
2 Downgraded one level for serious inconsistency (I2 = 72%).
3 Downgraded one level for serious imprecision: confidence intervals compatible with benefit in one or both groups, or with no difference between the groups.
4 Downgraded two levels for very serious risk of bias: single unblinded study with poor reporting of methods.
5 Downgraded one level for serious imprecision: single study with only 98 events.
Summary of findings 2.
Quinolone compared to cephalosporins for pelvic inflammatory disease
| Quinolone compared to cephalosporins for pelvic inflammatory disease | ||||||
|
Population: women with PID Setting: hospital ward or outpatient clinic Intervention: quinolone Comparison: cephalosporins | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cephalosporins | Risk with quinolone | |||||
|
Clinical cure according to criteria established by study authors Mild‐moderate PID Follow‐up: range 14‐28 days |
198/225 880 per 1000 |
214/234 915 per 1,000 (871 to 944) |
RR 1.04 (0.98 to 1.10) NNTB 11 to NNTH 46 |
459 (3 RCTs) | ⊕⊕⊝⊝ Low1,2 | ‐ |
|
Clinical cure according to criteria established by study authors Severe PID Follow‐up: range 14‐28 days |
106/160 643 per 1000 |
107/153 700 per 1000 (622 to 766) |
RR 1.06 (0.91 to 1.23) NNTB 7 to NNTH 15 |
313 (2 RCTs) | ⊕⊕⊝⊝ Low1,2 | ‐ |
|
Adverse events: any antibiotic‐related adverse event leading to discontinuation of therapy Follow‐up: mean 14 days |
2/384 5 per 1000 |
5/388 12 per 1000 (5 to 29) |
RR 2.24 (0.52 to 9.72) NNTB 40 to NNTH 129 |
772 (5 RCTs) | ⊕⊝⊝⊝ Very low1,3 | 2 of the 5 RCTs (502 women) did not contribute to this analysis because the authors reported no events. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; PID: pelvic inflammatory disease; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for serious risk of bias (poor reporting of methods and high or unclear risk of performance and detection bias in one or more studies).
2 Downgraded one level for serious imprecision: confidence intervals compatible with benefit in one or both groups, or with no difference between the groups.
3 Downgraded two levels for very serious imprecision: confidence intervals compatible with benefit in one or both groups, or with no difference between the groups, only seven events overall.
Summary of findings 3.
Nitroimidazole compared to no nitroimidazole for pelvic inflammatory disease
| Nitroimidazole compared to no nitroimidazole for pelvic inflammatory disease | ||||||
|
Population: women with PID Setting: hospital ward or outpatient clinic Intervention: nitroimidazole Comparison: no use of nitroimidazole | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no use of nitroimidazole | Risk with nitroimidazole | |||||
|
Clinical cure according to criteria established by study authors Mild‐moderate PID Follow‐up: range 14‐28 days |
925/1214 762 per 1000 |
949/1213 782 per 1000 (758 to 804) |
RR 1.01 (0.93 to 1.10) NNTB 19 to NNTH 77 |
2427 (5 RCTs) | ⊕⊕⊕⊝ Moderate1,2 | ‐ |
|
Clinical cure according to criteria established by study authors Mild‐moderate PID Follow‐up: range 14‐28 days Sensitivity analysis restricted to studies at low risk of bias |
452/606 746 per 1000 |
470/595 790 per 1000 (755 to 820) |
RR 1.06 (0.98 to 1.15) NNTB 11 to NNTH 266 |
1201 (2 RCTs) |
⊕⊕⊕⊕ High |
‐ |
|
Clinical cure according to criteria established by study authors Severe PID Follow‐up: range 14‐28 days |
573/689 832 per 1000 |
558/694 804 per 1000 (772 to 831) |
RR 0.96 (0.92 to 1.01) NNTB 15 to NNTH 76 |
1383 (11 RCTs) | ⊕⊕⊕⊝ Moderate1 | ‐ |
|
Adverse events: any antibiotic‐related adverse event leading to discontinuation of therapy Follow‐up: mean 14 days |
34/1911 18 per 1000 |
33/1910 17 per 1000 (12 to 24) |
RR 1.00 (0.63 to 1.59) NNTB 110 to NNTH 125 |
3821 (16 RCTs) | ⊕⊕⊝⊝ Low1,3 | 10/16 studies (1088 women) did not contribute data to the analysis because the authors reported no events. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; PID: pelvic inflammatory disease; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for serious risk of bias (poor reporting of methods and high or unclear risk of performance and detection bias in one or more studies).
2 Substantial inconsistency (I2 = 50%). Not downgraded because all inconsistency related to a single small study (30 women) which barely influenced the overall estimate.
3 Downgraded one level for serious imprecision: confidence intervals compatible with benefit in one or both groups, or with no difference between the groups, only 68 events overall.
Summary of findings 4.
Clindamycin plus aminoglycoside compared to quinolone for pelvic inflammatory disease
| Clindamycin plus aminoglycoside compared to quinolone for pelvic inflammatory disease | ||||||
|
Population: women with PID Setting: hospital ward or outpatient clinic Intervention: clindamycin + aminoglycoside Comparison: quinolone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with quinolone | Risk with clindamycin + aminoglycoside | |||||
|
Clinical cure according to criteria established by authors Mild‐moderate PID Follow‐up: median 14 days |
10/10 1000 per 1000 |
13/15 867 per 1000 (621 to 962) |
RR 0.88 (0.69 to 1.13) NNTB 3 to NNTH 6 |
25 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | ‐ |
|
Clinical cure according to criteria established by authors Severe PID Follow‐up: median 14 days |
60/75 800 per 1000 |
62/76 816 per 1000 (714 to 887) |
RR 1.02 (0.87 to 1.19) NNTB 7 to NNTH 9 |
151 (2 RCTs) | ⊕⊕⊝⊝ Low3,4 | ‐ |
|
Adverse events: any antibiotic‐related adverse event leading to discontinuation of therapy Follow‐up: mean 14 days |
4/80 50 per 1000 |
0/83 0 per 1000 (0 to 44) |
RR 0.21 (0.02 to 1.72) NNTB 8 to NNTH 273 |
163 (3 RCTs) | ⊕⊝⊝⊝ Very low1,2 | 1/3 RCTs (25 women) did not contribute data to the analysis because the authors reported no events. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; PID: pelvic inflammatory disease; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded two levels for very serious risk of bias: single unblinded study with poor reporting of methods.
2 Downgraded two levels for serious imprecision (though further downgrading not possible): confidence intervals compatible with benefit in one or both groups, or with no difference between the groups, very few events overall.
3 Downgraded one level for serious risk of bias (poor reporting of methods and high or unclear risk of performance and detection bias in both studies).
4 Downgraded one level for serious imprecision: confidence intervals compatible with benefit in one or both groups, or with no difference between the groups.
Summary of findings 5.
Clindamycin plus aminoglycoside compared to cephalosporin for pelvic inflammatory disease
| Clindamycin plus aminoglycoside compared to cephalosporin for pelvic inflammatory disease | ||||||
|
Population: women with PID Setting: hospital ward or outpatient clinic Intervention: clindamycin + aminoglycoside Comparison: cephalosporin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of women (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cephalosporin | Risk with clindamycin plus aminoglycoside | |||||
|
Clinical cure according to criteria established by authors Mild‐moderate PID Follow‐up: median 14 days |
69/72 958 per 1000 |
76/78 974 per 1000 (911 to 993) |
RR 1.02 (0.95 to 1.09) NNTB 11 to NNTH 19 |
150 (2 RCTs) | ⊕⊕⊝⊝ Low1,2 | ‐ |
|
Clinical cure according to criteria established by authors Severe PID Follow‐up: median 14 days |
441/525 840 per 1000 |
364/434 838 per 1000 (801 to 870) |
RR 1.0 (0.95 to 1.06) NNTB 21 to NNTH 22 |
959 (10 RCTs) | ⊕⊕⊕⊝ Moderate1 | ‐ |
|
Adverse events: any antibiotic‐related adverse event leading to discontinuation of therapy Follow‐up: mean 14 days |
3/670 4 per 1000 |
3/502 6 per 1000 (2 to 17) |
RR 0.78 (0.18 to 3.42) NNTB 75 to NNTH 126 |
1172 (10 RCTs) | ⊕⊝⊝⊝ Very low1,3 | 7/10 RCTs (617 women) did not contribute data to the analysis reported because the authors reported no events. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95 CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; PID: pelvic inflammatory disease; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level for serious risk of bias (poor reporting of methods and high or unclear risk of performance and detection bias in one or more studies).
2 Downgraded one level for serious imprecision, small overall sample size.
3 Downgraded two levels for serious imprecision (though further downgrading not possible): confidence intervals compatible with benefit in one or both groups, or with no difference between the groups, only six events overall.
Background
Description of the condition
Pelvic inflammatory disease (PID) in women describes inflammation of the upper genital tract and surrounding structures as a result of ascending infection from the lower genital tract ‐ bacteria spread directly from the cervix to the endometrium and on to the upper genital tract (Soper 2010). The signs and symptoms of PID are not specific and may range from asymptomatic to serious illness. PID can produce endometritis, parametritis (infection of the structures near the uterus), salpingitis (infection of the fallopian tubes), oophoritis (infection of the ovary), and tubo‐ovarian abscess (Workowski 2015). Peritonitis (infection inside the peritoneum, the thin layer of tissue lining the abdomen) and perihepatitis (infection around the liver) can also occur. Peritonitis, tubo‐ovarian abscess, and severe systemic illness (e.g. fever and malaise) are considered severe forms of PID; the other forms of presentation are considered mild or moderate according to the subjective opinion of the examining doctor or nurse (Soper 2010).
The most common complaint of PID is lower abdominal pain, with or without vaginal discharge. Specific grading of the clinical presentation using symptom scores has been described (e.g. McCormack 1977; Hager 1989), but has not been validated, and use of these scores is inconsistent. PID does not have a diagnostic gold standard. The most commonly used diagnostic criteria are based on those from the Centers for Disease Control and Prevention (CDC) (Workowski 2015), namely sexually active young women and other women at risk for sexually transmitted disease (STD) who are experiencing recent pelvic or lower abdominal pain where no cause other than PID can be identified, and one or more of the following minimum criteria are present on pelvic examination: cervical motion tenderness, uterine tenderness, or adnexal tenderness. The requirement for all three minimum criteria to be present increases the specificity of the diagnosis but reduces sensitivity.
Two sexually transmitted infections (Chlamydia trachomatis and Neisseria gonorrhoeae) have been strongly implicated in the aetiology of PID; however, based on the pattern of organisms isolated from the upper genital tract, the infection may often be polymicrobial (caused by more than one type of bacteria) (Eschenbach 1975; Arredondo 1997; Baveja 2001; Haggerty 2006). This suggests that initial damage produced by C trachomatis or N gonorrhoeae may permit the opportunistic entry of other bacteria, including anaerobes (bacteria that do not need oxygen to grow) (Ross 2014b).
The public health importance of PID can be estimated from the frequency of chlamydial and gonococcal infections. In 2012, among women aged 15 to 49 years, the estimated global prevalence of chlamydia was 4.2% (95% confidence interval (CI) 3.7% to 4.7%), gonorrhoea (0.8%, 95% CI 0.6% to 1.0%), and trichomoniasis (5.0%, 95% CI 4.0% to 6.4%) (Newman 2015). In a prospective study of 1170 women with elevated risk for having chlamydial cervicitis, 8.6% developed PID within three years; among women with chlamydia, the risk ratio (RR) for developing PID was 2.5 (95% CI 1.5 to 4.0) (Ness 2006). In the UK, the prevalence of PID is around 2% among women between 16 and 46 years old (Simms 1999; Datta 2012; Ross 2014b). However, in some other countries, the rates of chlamydia infection are lower, for example, in Jordan it is 0.6 and 0.5% in symptomatic and asymptomatic women, respectively (Mahafzah 2008). Among women with PID, 10% to 20% may become infertile, 40% will develop chronic pelvic pain, and 10% of those who conceive will have an ectopic pregnancy (Blanchard 1998; Ness 2002; Ness 2005; Mahafzah 2008).
The morbidity associated with PID relates to the acute inflammatory process, which can cause abdominal pain, vaginal discharge, dyspareunia (pain during sexual intercourse), and abnormal menstrual bleeding. In addition, long‐term complications secondary to tubal damage occur and include chronic pelvic pain, ectopic pregnancy, and infertility (Workowski 2015). PID has a prevalence of between 2% and 12%, and it cannot be diagnosed reliably from clinical symptoms and signs, which have a positive predictive value for salpingitis of only 65% to 90% compared with laparoscopy (Workowski 2015). Direct visualization of the fallopian tubes via laparoscopy has a higher sensitivity, but there is considerable inter‐ and intra‐observer reproducibility (Molander 2003). Endometrial biopsy may have some utility (Ross 2004), but is not performed routinely and is of uncertain diagnostic and prognostic value, since endometritis (infection of the inner mucosal lining of the uterus) can persist despite the resolution of clinical symptoms (Ness 2002; Savaris 2007).
The financial cost of pelvic infection has been estimated to exceed USD 2.4 billion in the USA, and the mean total cost per episode is around USD 5000 (Trent 2011). In the UK, the mean cost of a non‐complicated episode of PID is GBP 163 (Aghaizu 2011).
Description of the intervention
The main intervention for acute PID is the use of broad‐spectrum antibiotics which cover C trachomatis, N gonorrhoeae, and anaerobic bacteria. There are three routes of administration (intravenous, intramuscular, or oral). These routes of administration (oral and intramuscular versus intravenous) have been considered effective (Ness 2002; Walker 2007). In refractory cases, surgery to drain an abscess or hydrosalpinx may be necessary. When parenteral treatment is used, it is usually discontinued 24 hours after a woman improves clinically (Workowski 2015).
The optimal treatment strategy is unclear. A variety of antibiotic regimens have been used, with marked geographical variation. Current practice generally involves the use of multiple agents to cover C trachomatis,N gonorrhoeae, and anaerobic bacteria, but the best combination of agents is unknown. The background prevalence and antimicrobial resistance patterns of bacterial pathogens in different regions may influence the choice of empirical therapy.
Guidelines have been produced in the USA (Workowski 2015), and in Europe (Judlin 2010b; Ross 2014a), to guide therapy, but these have not been based on a formal systematic review. In addition, to choose an antibiotic for PID treatment, it is necessary to consider its spectrum, cost, adverse effects, and posology (i.e. dosage, interval) to achieve the best balance between compliance and efficacy. There are no current systematic reviews on this topic.
The different antibiotic regimens proposed to treat PID vary in cost, reported efficacy, and adverse effects. Potential adverse effects of therapy for PID include allergic reactions and gastrointestinal symptoms, which can lead to discontinuation of therapy. Lack of evidence is revealed in the current CDC and British Association for Sexual Health and HIV (BASHH) guidelines, where the authors state that there is limited evidence for the need to eradicate anaerobes and for the use of alternative regimens, such as azithromycin (Workowski 2015), and the comparison between clindamycin plus aminoglycoside and fluoroquinolones (Ross 2014a). Likewise, if the prevalence of N gonorrhoeae is low, the use of fluoroquinolones can be considered if allergy to cephalosporin is an issue (Workowski 2015).
How the intervention might work
It is likely that the intervention works by eradicating bacterial pathogens and reducing the associated inflammation which leads to scarring. Necrotic tissue and pus present in an abscess may prevent antibiotics reaching the infected area. Mechanical drainage of the abscess through open surgery, laparoscopy, or aspiration through a large bore needle is likely to work by removing infected material which antibiotics are unable to treat (Workowski 2015). Clinical cure without surgery in women with a tubo‐ovarian abscess is around 75% (DeWitt 2010). The rationale for using broad‐spectrum antibiotics is to cover the wide variety of pathogens found in PID, which include Gram‐positive (e.g. Streptococcus), Gram‐negative (e.g. Chlamydia, Klebsiella, Escherichia coli, Neisseria), and anaerobic bacteria (Gram positive or negative: Peptostreptococcus, Bacteroides).
Why it is important to do this review
PID is a common disease (4.4%) that is accompanied by high rates of morbidity in young women (Ness 2002; Morris 2014). It requires effective treatment to reduce the incidence of chronic pelvic pain, infertility, and transmitted STDs. A variety of different antibiotic regimens have been proposed to treat PID, which vary in cost, reported efficacy, and adverse effects, but the optimal treatment strategy is unclear.
Currently there are no systematic reviews of this subject and the optimal treatment strategy is unclear. This review will address clinical questions raised by current guidelines on the treatment of PID (Ross 2014a; Workowski 2015), regarding the effectiveness and safety of nitroimidazole, the relative benefits of azithromycin versus doxycycline, the use of quinolones, and the relative benefits of cephalosporins compared to the most‐used regimen of clindamycin plus aminoglycoside, to inform future guideline development and clinical practice.
Objectives
To assess the effectiveness and safety of antibiotic regimens used to treat pelvic inflammatory disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), including those which did not describe their method of randomization (i.e. where the authors stated that treatment was randomized without providing further details). We chose randomized trials as providing the strongest evidence for evaluating the efficacy of therapy (Higgins 2011). We included studies irrespective of publication status or language.
We excluded quasi‐randomized trials because they produce effects estimates indicating more extreme benefits when compared with RCTs (Higgins 2011). We also excluded cross‐over and cluster trials.
Types of participants
Women of reproductive age (14 years of age or older) diagnosed as having acute PID (symptoms for less than six weeks) based on clinical findings, laparoscopy, endometrial biopsy, or detectable gonorrhoea or chlamydia in the upper genital tract.
We divided women into two groups: mild‐moderate (e.g. absence of tubo‐ovarian abscess) and severe (e.g. systemically unwell, presence of tubo‐ovarian abscess).
Types of interventions
We limited our review to comparison of drugs in current use that are recommended for consideration by the 2015 US CDC guidelines for treatment of PID (Workowski 2015).
We included trials comparing the following treatments for PID:
azithromycin versus doxycycline;
quinolone versus cephalosporin;
nitroimidazole versus no nitroimidazole;
clindamycin plus aminoglycoside versus quinolone;
clindamycin plus aminoglycoside versus cephalosporin.
Types of outcome measures
Primary outcomes
Effectiveness: clinical cure according to the criteria defined by the treating physician (e.g. resolution or improvement of signs and symptoms related to PID).
Adverse events: any antibiotic‐related adverse event leading to discontinuation of therapy.
Secondary outcomes
Microbiological clearance of chlamydia from either the upper or lower genital tract, according to the method provided by the authors.
Microbiological clearance of gonorrhoea from either the upper or lower genital tract, according to the method provided by the authors.
Laparoscopic evidence of resolution of PID based on physician opinion.
Length of stay (for inpatient care).
Rate of fertility based on at least one participant‐reported live birth following PID treatment in women not using effective contraception.
Where studies included women with various types of pelvic infection, we considered only women with endometritis, salpingitis, parametritis, or oophoritis (not related to labour, delivery, cancer, or surgery).
Where studies reported multiple time points, we considered the period between 14 and 28 days after initiation of treatment.
Search methods for identification of studies
We identified relevant RCTs of 'antibiotic therapy' for 'PID', irrespective of their language of publication, publication date, and publication status (published, unpublished, in press, and in progress). We used both electronic searching in bibliographic databases and handsearching, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Electronic searches
We contacted the Information Specialist of the Cochrane Sexually Transmitted Infections Review Group to implement a comprehensive search strategy capturing as many relevant RCTs as possible in electronic databases. We used a combination of controlled vocabulary (MeSH, Emtree, DeCS, including exploded terms) and free‐text terms (considering spelling variants, synonyms, acronyms, and truncation) for 'pelvic inflammatory disease (PID)' and 'antibiotic therapy', with field labels, proximity operators, and Boolean operators. We have presented the search strategies in Appendix 1. Specifically, we searched the following electronic databases.
The Cochrane Sexually Transmitted Infections Review Group's Specialized Register, which includes RCTs from 1944 to 2016 located through electronic searching and handsearching. The electronic databases searched for the register are CENTRAL, MEDLINE, and Embase.
The Cochrane Central Register of Controlled Trials (CENTRAL), Ovid platform (1991 to 11 July 2016).
MEDLINE, Ovid platform (1946 to 11 July 2016).
MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid platform (1946 to 11 July 2016).
MEDLINE Daily Update, Ovid platform (1946 to 11 July 2016).
Embase (1947 to 11 July 2016).
LILACS, iAHx interface (1982 to 11 July 2016).
Web of Science (2001 to July 2016).
In MEDLINE, we used the Cochrane Highly Sensitive Search Strategy for identifying RCTs: sensitivity and precision maximizing version (2008 revision), Ovid format (Higgins 2011). The LILACS search strategy combined RCTs filter of iAHx interface.
Searches were updated to within 12 months of publication of the review.
Searching other resources
We attempted to identify other relevant RCTs using the methods below.
We searched the following trials registers:
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/);
ClinicalTrials.gov (ClinicalTrials.gov/).
We searched for grey literature in OpenGrey (www.opengrey.eu/) (1990, 1992, 1995, 1996, and 1997). We contacted authors of all RCTs identified by other methods as well as pharmaceutical companies producing 'antibiotic therapy' for 'pelvic inflammatory disease (PID).'
We handsearched conference proceeding abstracts in the following publications: Indian Journal of Sexually Transmitted Diseases (2007 to July 2016), Sexually Transmitted Diseases (1974 to July 2016), Sexually Transmitted Infections (1996 to July 2016), Journal of Sexual Medicine (2004 to July 2016), Sexual and Relationship Therapy (2000 to July 2016), and the Society for the Scientific Study of Sexuality's Sexual Science Newsletter (2000 to July 2016).
We handsearched previous systematic reviews on similar topics identified from:
the Cochrane Library (www.thecochranelibrary.com/);
Epistemonikos (www.epistemonikos.org/).
We handsearched the reference lists of all identified RCTs.
Data collection and analysis
Selection of studies
Two review authors (DGF and RVD) performed an initial screen of titles and abstracts retrieved by the search, and we retrieved the full text of all potentially eligible studies. Two review authors (DGF and RVD) independently examined these studies for compliance with the inclusion criteria and selected studies that met these criteria. We resolved disagreements regarding eligibility by discussion or by consulting a third review author (JR). We documented the selection process in a PRISMA flow chart (Figure 1). We excluded pelvic infection related to obstetric or surgical procedures. Where a study contained both 'eligible' and 'ineligible' participants, we included a subset of data relating to the 'eligible' participants if sufficient details were provided for analysis.
Figure 1.
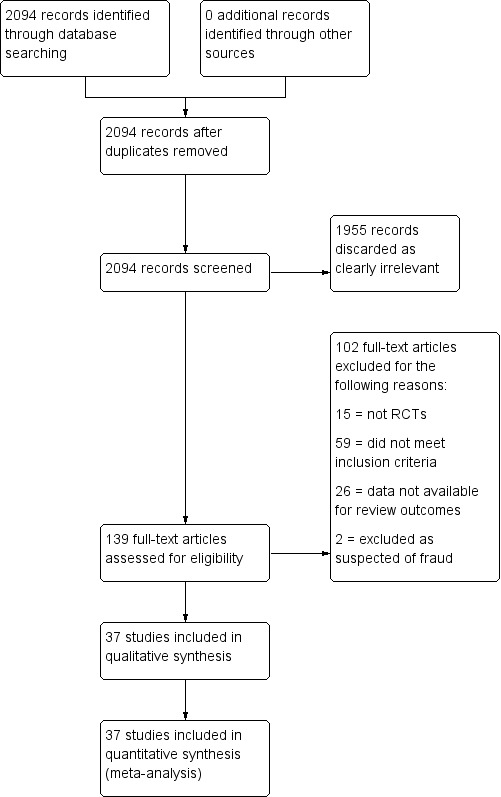
Study flow diagram.
Data extraction and management
Three review authors (SF, DGF, RVD) independently extracted data from each study using a data extraction form that the review authors designed and pilot tested. We collected data from the included studies in sufficient detail to complete the Characteristics of included studies table. We also extracted detailed numerical outcome data in duplicate to allow calculation of Mantel‐Haenszel RRs for each comparison. We examined data for errata, retraction, fraud, and inconsistencies. We resolved disagreements by consensus or by consulting a fourth review author (JR or RFS).
If a study had more than two intervention arms, we included or combined only those that met the predefined inclusion criteria. For instance, if the study compared azithromycin (group A) versus azithromycin plus metronidazole (group B) versus metronidazole plus cefoxitin or doxycycline (group C), and the analysis was between the use or not of metronidazole, we combined groups B and C versus group A. The treatment effect was expressed as rate of cure (%) and its magnitude and direction checked in forest plots to ensure consistency with the original study. Where studies had multiple publications, we used the main trial report as the reference and derived additional details from secondary papers. We corresponded with study investigators for further data as required.
Data collected with the data extraction form were piloted‐tested and included:
-
Study factors:
author, date of publication, journal;
date of study;
study design;
location;
setting;
quality of randomization, treatment allocation, and blinding;
method of PID diagnosis;
sample size.
-
Participant factors:
age, ethnicity;
pregnancy;
presence of intrauterine device (IUD);
duration of symptoms;
presence of abscess (pyosalpinx, tubo‐ovarian abscess).
-
Outcome measured:
method of assessment of pelvic pain and score;
timing of assessment;
adverse events;
additional assessments of outcome: laparoscopy, microbiology, fertility.
-
Intervention factors:
antibiotic given: dose, route, length of therapy;
comparator regimen: dose, route, length of therapy;
additional treatment given.
-
Additional data:
whether contact tracing was performed.
Assessment of risk of bias in included studies
Three review authors (SF, DGF, RVD) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements between two review authors by consensus or by involving a third review author (JR or RFS). We assessed risk of bias using the Cochrane 'Risk of bias' tool provided in Review Manager 5 (RevMan 2014). We provided justification for risk of bias (high, low, unclear) in the 'Risk of bias' table by direct reference to the relevant report. We requested missing information from the study investigators using open‐ended questions.
1. Random sequence generation (checking for possible selection bias)
For each included study, we verified the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
unclear risk of bias (e.g. authors stated that women were randomized to one of the treatments, without further explanation).
2. Allocation concealment (checking for possible selection bias)
For each included study, we verified the method used to conceal allocation to interventions prior to assignment, and we assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered, sealed, opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or
unclear risk of bias (allocation was mentioned without further details).
3.1. Blinding of participants and personnel (checking for possible performance bias)
For each included study, we verified the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at:
low risk of bias if participants and personnel were blinded, or if we judged that the lack of blinding would be unlikely to affect results (e.g. culture for N gonorrhoeae);
high risk if participants and personnel were not blinded;
unclear risk of bias if no further details were provided.
3.2. Blinding of outcome assessment (checking for possible detection bias)
For each included study, we verified the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed methods used to blind outcome assessment as:
low risk of bias if assessors were blinded, or if we judged that the lack of blinding would be unlikely to affect results (e.g. culture for N gonorrhoeae);
high risk if assessors were not blinded;
unclear risk of bias if no further details were provided.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we verified the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomization); or
unclear risk of bias: no further details were provided.
We used a cutoff point of 20% missing data in determining if a study was at low or high risk of bias (Fewtrell 2008).
5. Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study did not include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias: no further details were provided.
6. Other bias (checking for bias due to problems not covered by 1. to 5. above)
For each included study, we described any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of bias;
high risk of bias; or
unclear whether there is risk of bias.
7. Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to 1. to 6. above, we assessed the likely magnitude and direction of the bias and whether we considered that it was likely to impact on the findings. We explored the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
For dichotomous data, we used number of events in the control and intervention groups to calculate Mantel‐Haenszel risk ratios (RR). We presented 95% confidence intervals (CI) for all outcomes. For number need to treat for an additional beneficial (NNTB) or harmful (NNTH) outcome, we followed the recommendation given by Altman (Altman 1998). When we observed a treatment effect we reported the NNTH or NNTB were given with their 95% CIs.
When possible, we performed analysis based on intention to treat (ITT). When information for an ITT analysis was not available, we used the results provided by the authors.
We performed meta‐analysis separately for mild‐moderate PID and severe PID. We defined severe PID as the presence of tubo‐ovarian abscess, being systemically unwell, or the presence of peritonitis, and mild‐moderate PID as no presence of tubo‐ovarian abscess. We further analyzed cases in these two groups across different classes of antibiotics.
Unit of analysis issues
The primary unit of analysis was an event per woman randomized, which was used to calculate the percentage response rate (e.g. clinical cure).
Dealing with missing data
We analyzed the data on an ITT basis to the greatest degree possible and made attempts to obtain missing data from the original trials. Where we were unable to obtain these data, we considered cases that were lost to follow‐up as treatment failure (worst‐case scenario) in the primary analysis. For other outcomes, we analyzed the available data. We did not analyze data from other reported outcomes (e.g. pooled rates of cure of different diseases, including PID).
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by measure of the I2 statistic as follows: low (l2 value below 40%), moderate (l2 value of 40% to 75%), or high (l2 value above 75%) (Sutton 2008; Higgins 2011). We also assessed statistical heterogeneity in each meta‐analysis using the t2 and Chi2 statistics.
We regarded heterogeneity as substantial if I2 was greater than 40% and either t2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity. If we detected substantial heterogeneity, we explored possible explanations for it in subgroup analyses (see Subgroup analysis and investigation of heterogeneity). We took statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect. If so, we used random‐effects analysis, instead of fixed‐effect analysis.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Some types of reporting bias (e.g. publication bias, multiple publication bias, language bias, etc.) reduce the likelihood that all studies eligible for a review are retrieved (Higgins 2011). If all eligible studies are not retrieved, the review would be biased. In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimize their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there were 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We performed statistical analyses using Review Manager 5 (RevMan 2014). We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that trials were estimating the same underlying treatment effect (i.e. where trials were examining the same intervention, and the trials' populations and methods were sufficiently similar). We conducted separate analyses for mild‐moderate and severe PID.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity (I2 40% or greater), we used a random‐effects meta‐analysis to produce an overall summary if a mean treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the mean range of possible treatment effects, and discussed the clinical implications of treatment effects differing between trials.
If the mean treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, we presented the results as the mean treatment effect with 95% CIs, and the estimates of the t2 and I2 statistics.
Subgroup analysis and investigation of heterogeneity
Where data were available, we performed the following prespecified subgroup analyses:
route of antibiotic administration (oral, intramuscular, or intravenous);
length of therapy (less or more than seven continuous days receiving antibiotics);
detection of chlamydia;
detection of gonorrhoea;
site of initiation of treatment (inpatient or outpatient).
We avoided selective reporting of a particular subgroup by not performing multiple subgroup analysis. If we identified substantial heterogeneity (I2 40% or greater), we used a random‐effects analysis as the primary statistical analysis.
Where there was substantial heterogeneity, we explored possible reasons for this finding by stratifying results according to the characteristics of the study population (e.g. method of PID diagnosis), the intervention (e.g. class of antibiotics used, dose of antibiotic, route of administration), or methodological characteristics (e.g. length of time to outcome measurement).
Sensitivity analysis
We undertook the following sensitivity analysis to investigate whether our conclusions were robust to methodological decisions made by review authors:
risk of bias (restricting analysis to blinded studies at low risk of selection bias).
Grading the quality of evidence
We used GRADEpro software (GRADEpro GDT 2014) to produce 'Summary of findings' tables. The GRADE approach considers the following criteria: risk of bias, inconsistency, indirectness of evidence, imprecision, and publication bias, and specifies four levels of quality: high, moderate, low, and very low, starting from high for RCTs. If there was a flaw in the RCT, we downgraded the quality of the evidence by one or two levels.
Two review authors independently applied a consistent grading for GRADE in the 'Summary of findings' tables. We resolved any disagreements by consensus. We downgraded for risk of bias due to crucial risk of bias for two or more criteria (as it was graded in Figure 2). The evidence was downgraded further if there was inconsistency. Inconsistency was based on statistical test for heterogeneity and how much variation there was in the findings of the studies that contributed to the outcome. We also considered imprecision, and downgraded if the CIs were compatible with benefit in one or both groups, or with no difference between the groups. In each domain, we downgraded one level for serious risk of bias and two levels for very serious risk of bias.
Figure 2.
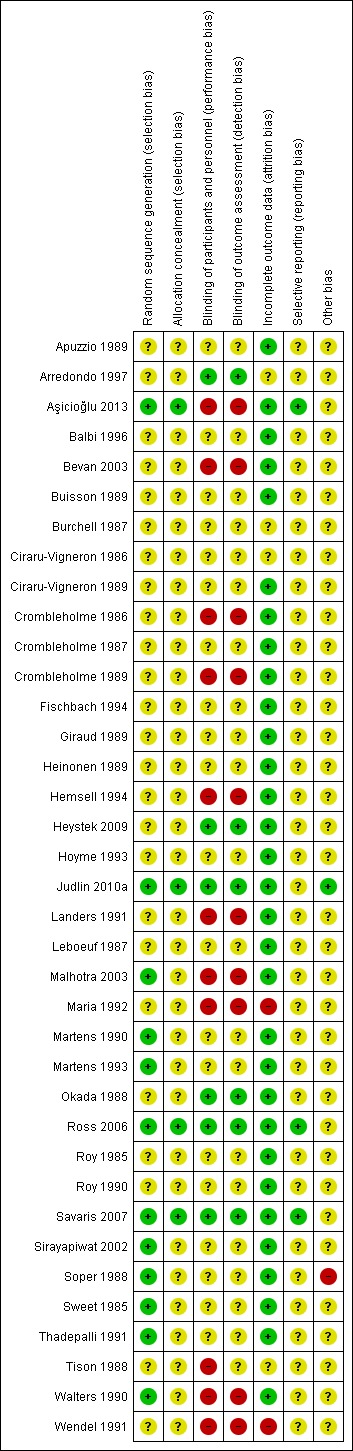
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Results
Description of studies
Results of the search
We retrieved 2133 references and screened 2094 records after removing duplicated references. We discarded 1955 records as clearly irrelevant and considered 139 full‐text articles. After full‐text review, 37 studies met our inclusion criteria and we excluded 102 studies (Figure 1). The reasons for exclusion of the 102 full‐text studies are explained in the Characteristics of excluded studies table. We extracted data from the full‐text article for each study.
Included studies
The 37 included trials had 6348 women, with a sample size ranging from 25 (Apuzzio 1989), to 1156 (Aşicioğlu 2013). Retrieved studies came from a wide range of inpatient and outpatient settings from different continents (Americas, Europe, Asia, Oceania, and Africa) and were written in English, German, French, Japanese, and Italian.
Population
The included trials recruited women aged 14 years and over with diagnosis of PID according to CDC criteria (pelvic or lower abdominal pain and one or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness) (Workowski 2015). Studies varied in degree of disease severity of participants, treatment location (i.e. inpatient or outpatient), and countries and continents. PID was considered severe in the presence of systemically unwell women, peritonitis, or pelvic abscess.
Interventions
The 37 RCTs yielded 6348 women and made the following comparisons.
For mild‐moderate PID:
azithromycin versus doxycycline (Malhotra 2003; Savaris 2007);
cephalosporin versus quinolone (Wendel 1991; Martens 1993; Arredondo 1997);
nitroimidazole versus no nitroimidazole (Burchell 1987; Tison 1988; Hoyme 1993; Ross 2006; Judlin 2010a; Aşicioğlu 2013);
clindamycin plus aminoglycoside versus quinolone (Apuzzio 1989);
clindamycin plus aminoglycoside versus cephalosporin (Walters 1990).
For severe PID:
azithromycin versus doxycycline (Bevan 2003);
quinolone versus cephalosporin (Okada 1988; Martens 1993; Fischbach 1994);
nitroimidazole versus no nitroimidazole (Ciraru‐Vigneron 1986; Crombleholme 1986; Crombleholme 1987; Leboeuf 1987; Buisson 1989; Ciraru‐Vigneron 1989; Giraud 1989; Heinonen 1989; Fischbach 1994; Sirayapiwat 2002; Heystek 2009);
clindamycin plus aminoglycoside versus quinolone (Crombleholme 1989; Thadepalli 1991);
clindamycin plus aminoglycoside versus cephalosporin (Roy 1985; Sweet 1985; Soper 1988; Martens 1990; Roy 1990; Walters 1990; Landers 1991; Maria 1992; Hemsell 1994; Balbi 1996).
Outcomes
The main outcome was clinical cure, and 5147 women were reported as clinically cured. We defined clinical cure according to the authors' definitions, which ranged from absence of symptoms for 24 hours (Apuzzio 1989), to a 60% or greater reduction in total pain score at day 21 combined with an absence of pelvic discomfort/tenderness, temperature less than 37.8 °C, and white blood cell level less than 10,000/mm3 on day 21 (Aşicioğlu 2013). Most of the trials used clinical parameters for cure, that is, defervesce, reduction, or absence of pain at different time points after treatment. We identified adverse effects leading to discontinuation of treatment as those related to suspension of therapeutic regimen.
Excluded studies
We excluded 102 studies. The most common reason for exclusion was that the study did not report a comparison of interest to this review (47 studies). Other common reasons for exclusion were that PID cases were not distinguished from other pelvic infectious conditions (21 studies) or the studies was not randomized (14 studies) (see Characteristics of excluded studies table).
Risk of bias in included studies
We performed a full risk of bias assessment on all included studies. We classified those studies where authors stated that women were randomized to one of two treatments, without further details, as at unclear risk of selection bias. Many of these studies were performed before the year 2000, and predated the introduction of CONSORT guideline.
We have summarized the risk of bias in Figure 2 and Figure 3. Additional details of the included trials are provided in the Characteristics of included studies table.
Figure 3.
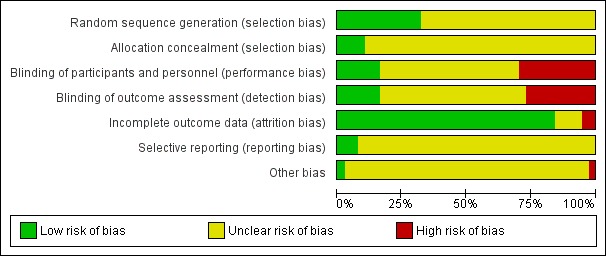
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Twelve trials adequately reported a truly random process of sequence generation, for example, random number table or computer random number generator, making selection bias at entry unlikely (Sweet 1985; Soper 1988; Martens 1990; Walters 1990; Thadepalli 1991; Martens 1993; Sirayapiwat 2002; Malhotra 2003; Ross 2006; Savaris 2007; Judlin 2010a; Aşicioğlu 2013). The remaining included trials did not report the random sequence generation methods, making the risk of selection bias at entry unclear.
For allocation concealment, four trials implemented sequentially numbered drug containers as a concealment allocation method, making selection bias at entry unlikely (Ross 2006; Savaris 2007; Judlin 2010a; Aşicioğlu 2013). The remaining included trials did not report the method used to conceal allocation to interventions prior to assignment, making the risk of selection bias at entry unclear.
Blinding
Six trials used placebo with identical appearance for the control group to blind trial participants and personnel, making performance and detection bias unlikely (Figure 2) (Okada 1988; Arredondo 1997; Ross 2006; Savaris 2007; Heystek 2009; Judlin 2010a). In 10 studies, investigators and participants were not blinded to the allocation, making them at high risk of bias (Crombleholme 1986; Crombleholme 1989; Walters 1990; Landers 1991; Wendel 1991; Maria 1992; Hemsell 1994; Bevan 2003; Malhotra 2003; Aşicioğlu 2013). The remaining included trials did not specify how the participants and the personnel were blinded from knowledge of which intervention a participant received, making the risk of performance and detection bias unclear.
Incomplete outcome data
Completeness of data was adequate (i.e. less than 20% of data missing) for 28 of the studies (Figure 2) (Roy 1985; Sweet 1985; Crombleholme 1987; Leboeuf 1987; Okada 1988; Soper 1988; Apuzzio 1989; Ciraru‐Vigneron 1989; Crombleholme 1989; Giraud 1989; Heinonen 1989; Martens 1990; Roy 1990; Walters 1990; Landers 1991; Thadepalli 1991; Hoyme 1993; Martens 1993; Fischbach 1994; Hemsell 1994; Balbi 1996; Sirayapiwat 2002; Bevan 2003; Malhotra 2003; Ross 2006; Savaris 2007; Heystek 2009; Judlin 2010a).
The remaining studies had more than 20% of data missing, with an associated high risk of attrition bias.
Selective reporting
For most of the included studies, the trial protocol was not available, and it was unclear whether the published reports included all the expected outcomes, including those that were prespecified. The reports had insufficient information to permit judgement of 'yes' or 'no', and were therefore rated as at unclear risk of bias. For three trials, the trial protocol was available, and it was clear that the published reports included all the expected outcomes, including those that were prespecified, making reporting bias unlikely (Ross 2006; Savaris 2007; Aşicioğlu 2013).
Other potential sources of bias
One study had imbalances in baseline characteristics and was at high risk of potential bias (Soper 1988). Out of 37 trials, 13 had some form of funding by pharmaceutical companies (Arredondo 1997;Crombleholme 1989; Hemsell 1994; Heystek 2009; Judlin 2010a; Landers 1991; Martens 1993; Ross 2006; ;Roy 1990; Savaris 2007; Thadepalli 1991; Walters 1990; Wendel 1991); however, there is no clear evidence that trial methods are more likely to be flawed if a trial is industry funded (Sterne 2013), therefore these trials were rated as at unclear risk. The remaining trials provided insufficient information to permit a judgement of 'yes' or 'no' and were therefore rated as at unclear risk of bias.
Assessment of reporting bias
We explored publication bias through visual assessment of funnel plot asymmetry when there were data from 10 or more trials in the same analysis. We constructed a funnel plot for Analysis 3.3 (Figure 4) and noted some asymmetry in the plot, suggestive of potential publication bias. There are five possible causes for the asymmetric funnel plot: reporting bias, poor methodological quality, true heterogeneity, artefactual, and chance (Egger 1997). Only through formal statistical analysis or using 'contour‐enhanced' funnel plot is an explication for these asymmetrical funnel plots possible. Although it is usually impossible to know the precise mechanism for funnel plot asymmetry, publication bias could explain the presence of an asymmetrical funnel plot.
Analysis 3.3.
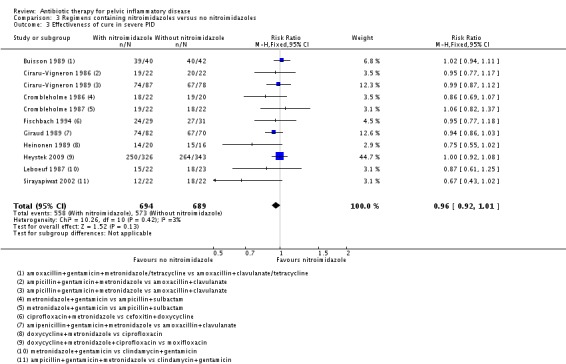
Comparison 3 Regimens containing nitroimidazoles versus no nitroimidazoles, Outcome 3 Effectiveness of cure in severe PID.
Figure 4.
Funnel plot of comparison: 3.2 Effectiveness of cure in severe PID in regimens containing nitroimidazoles versus without nitroimidazoles.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
We analyzed effectiveness of clinical cure and adverse effects leading to discontinuation of treatment in five scenarios based on drug class comparison:
regimens containing macrolides (azithromycin) versus tetracycline (doxycycline);
regimens containing quinolones versus cephalosporin;
regimens containing nitroimidazoles versus without nitroimidazoles;
regimens containing clindamycin plus aminoglycoside versus quinolone;
regimens containing clindamycin plus aminoglycoside versus cephalosporin.
We analyzed the efficacy of therapy in these five comparisons in women with mild‐moderate PID and women with severe PID. We also compared adverse events leading to discontinuation of the therapy. Lack of further analysis is discussed in the Differences between protocol and review section.
1. Regimens containing macrolides (azithromycin) versus tetracycline (doxycycline)
Three studies compared azithromycin versus doxycycline in mild‐moderate (Malhotra 2003; Savaris 2007) or severe (Bevan 2003) PID.
Primary outcomes
1.1. Effectiveness
1.1a. Clinical cure in mild‐moderate pelvic inflammatory disease
We included two trials in the analysis of clinical cure in mild‐moderate PID (Malhotra 2003; Savaris 2007). There was no clear evidence of a difference between azithromycin and doxycycline regimens (RR 1.18, 95% CI 0.89 to 1.55; 243 women; 2 studies; I2 = 72%; very low‐quality evidence) (Analysis 1.1; Figure 5).
Analysis 1.1.
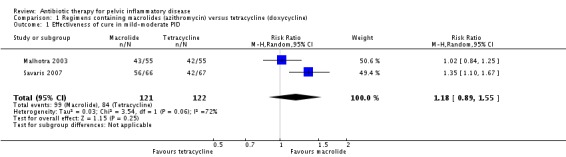
Comparison 1 Regimens containing macrolides (azithromycin) versus tetracycline (doxycycline), Outcome 1 Effectiveness of cure in mild‐moderate PID.
Figure 5.
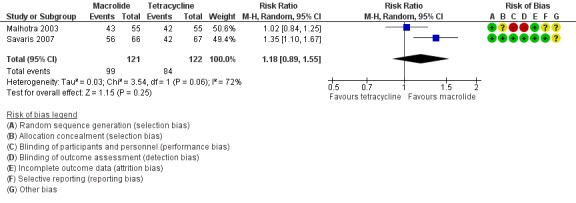
1.1 Effectiveness of cure in mild‐moderate PID on regimens containing macrolides (azithromycin) versus tetracycline (doxycycline) using ITT.
In a sensitivity analysis limited to the study at low risk of bias, azithromycin was superior to doxycycline in achieving cure in mild‐moderate PID (RR 1.35, 95% CI 1.10 to 1.67, 133 women, moderate‐quality evidence) (Analysis 1.2)
Analysis 1.2.
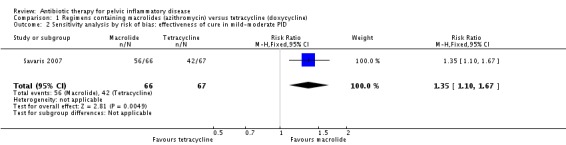
Comparison 1 Regimens containing macrolides (azithromycin) versus tetracycline (doxycycline), Outcome 2 Sensitivity analysis by risk of bias: effectiveness of cure in mild‐moderate PID.
1.1b. Clinical cure in severe pelvic inflammatory disease
One trial reported clinical cure in severe PID (Bevan 2003). There was no clear evidence of a difference in rates of cure between regimens using azithromycin or doxycycline to treat severe PID (RR 1.00, 95% CI 0.96 to 1.05; 309 women; 1 study; low‐quality evidence) (Analysis 1.3; Figure 6.
Analysis 1.3.
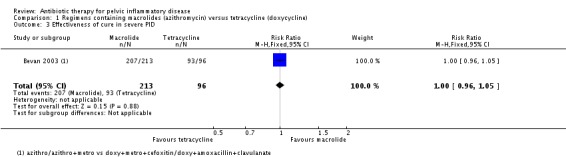
Comparison 1 Regimens containing macrolides (azithromycin) versus tetracycline (doxycycline), Outcome 3 Effectiveness of cure in severe PID.
Figure 6.
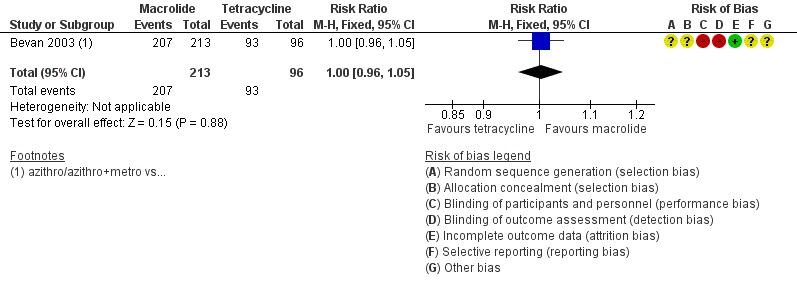
1.2 Effectiveness of cure in severe PID in regimens containing macrolides (azithromycin) versus tetracycline (doxycycline).
1.2. Adverse events
1.2.1. Antibiotic‐related adverse effects leading to discontinuation of therapy
We included three trials in the analysis of antibiotic‐related adverse effects leading to discontinuation of therapy (Bevan 2003; Malhotra 2003; Savaris 2007). There was no clear evidence of a difference between the groups (RR 0.71, 95% CI 0.38 to 1.34; 552 women; 3 studies; I2 = 0%, low‐quality evidence) (Analysis 1.4).
Analysis 1.4.
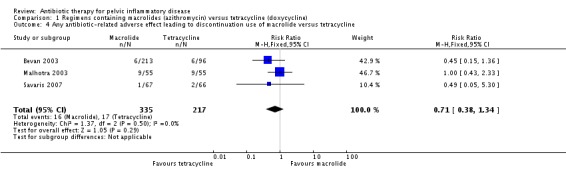
Comparison 1 Regimens containing macrolides (azithromycin) versus tetracycline (doxycycline), Outcome 4 Any antibiotic‐related adverse effect leading to discontinuation use of macrolide versus tetracycline.
Data are depicted in Table 1.
Secondary outcomes
1.3. Microbiological clearance of chlamydia
Two studies reported chlamydia clearance (Bevan 2003; Savaris 2007) and cure was obtained in 49/50 women in the azithromycin group (cure 98%, 95% CI 89% to 100%) and 33/33 women in the doxycycline group (cure 100%, 95% CI 90% to 100%).
1.4. Microbiological clearance of gonorrhoea
Two studies reported gonorrhoea clearance (Bevan 2003; Savaris 2007), but only one found evidence of N gonorrhoeae (Bevan 2003). In the azithromycin group, there was cure in 11/11 cases (100%, 95% CI 74% to 100%). Cure was also 100% in the doxycycline group (5/5 cases; 100%, 95% CI 57% to 100%).
1.5. Laparoscopic evidence of resolution of pelvic inflammatory disease based on physician opinion
We found no data for laparoscopic evidence of resolution of PID.
1.6. Length of stay (for inpatient care)
One study reported length of hospital stay (Bevan 2003). Women were kept in the hospital, per protocol, for 13 to 18 days.
1.7. Rate of fertility
We found no data for rate of fertility.
2. Regimens containing quinolones versus cephalosporins
Six studies compared quinolones versus cephalosporins in mild‐moderate PID (Wendel 1991; Martens 1993; Arredondo 1997) or severe PID (Okada 1988; Martens 1993; Fischbach 1994).
Primary outcomes
2.1. Effectiveness
2.1a. Clinical cure in mild‐moderate pelvic inflammatory disease
We included three trials in the analysis of clinical cure in mild‐moderate PID (Wendel 1991; Martens 1993; Arredondo 1997). There was no clear evidence of a difference between the groups (RR 1.04, 95% CI 0.98 to 1.10; 459 women; 3 studies; I2 = 5%; low‐quality evidence) (Analysis 2.1).
Analysis 2.1.
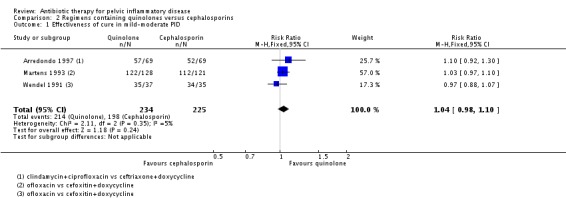
Comparison 2 Regimens containing quinolones versus cephalosporins, Outcome 1 Effectiveness of cure in mild‐moderate PID.
2.1b. Clinical cure in severe pelvic inflammatory disease
We included two trials in the analysis of clinical cure in severe PID (Okada 1988; Fischbach 1994). There was no clear evidence of a difference in rates of cure between regimens using quinolones or cephalosporins to treat severe PID (RR 1.06, 95% CI 0.91 to 1.23; 313 women; 2 studies; I2 = 7%; low‐quality evidence) (Analysis 2.2).
Analysis 2.2.
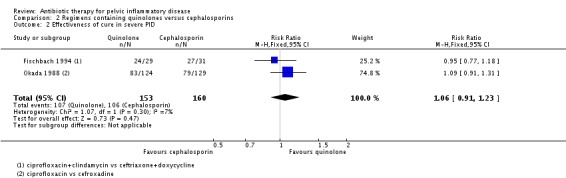
Comparison 2 Regimens containing quinolones versus cephalosporins, Outcome 2 Effectiveness of cure in severe PID.
2.2. Adverse events
2.2.1. Antibiotic‐related adverse effect leading to discontinuation of therapy
The five trials reported few adverse effects leading to discontinuation of treatment (quinolones: 1.2%, 95% CI 0.5 to 2.9 versus cephalosporin: 0.5%, 95% CI 0.1 to 1.9%), with no clear evidence of a difference in rates of discontinuation (RR 2.24, 95% CI 0.52 to 9.72; 772 women; 5 studies; I2 = 0%, very low‐quality evidence) (Okada 1988; Wendel 1991; Martens 1993; Fischbach 1994; Arredondo 1997) (Analysis 2.3).
Analysis 2.3.
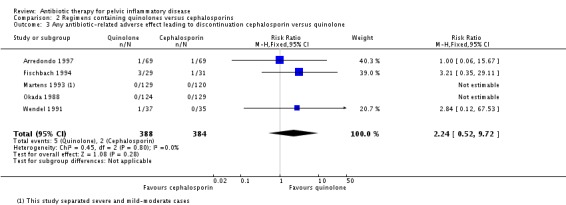
Comparison 2 Regimens containing quinolones versus cephalosporins, Outcome 3 Any antibiotic‐related adverse effect leading to discontinuation cephalosporin versus quinolone.
Data are depicted in Table 2.
Secondary outcomes
2.3. Microbiological clearance of chlamydia
Five studies reported chlamydia clearance (Martens 1990; Wendel 1991; Martens 1993; Fischbach 1994; Arredondo 1997), and three of which reported clearance of chlamydia (Wendel 1991; Fischbach 1994; Arredondo 1997). Cure occurred in 20/21 women in the quinolone group (95.2%, 95% CI 77% to 99%) and 25/25 women in the cephalosporin group (100%, 95% CI 86 to 100%).
2.4. Microbiological clearance of gonorrhoea
Five studies reported gonorrhoea clearance (Martens 1990; Wendel 1991; Martens 1993; Fischbach 1994; Arredondo 1997), and three of which reported clearance of chlamydia (Wendel 1991; Fischbach 1994; Arredondo 1997). Cure occurred in 27/30 women in the quinolone group (90%, 95% CI 74 to 96%) and 20/21 women in the cephalosporin group (95.2%, 95% CI 77 to 99%).
2.5. Laparoscopic evidence of resolution of pelvic inflammatory disease based on physician opinion
We found no data for laparoscopic evidence of resolution of PID.
2.6. Length of stay (for inpatient care)
We found no data suitable for analysis for length of hospital stay. Fischbach and colleagues admitted women for intravenous treatment for two to five days, followed by seven to 12 days of oral therapy, without further details (Fischbach 1994).
2.7. Rate of fertility
We found no data for rate of fertility.
3. Regimens containing nitroimidazoles versus no nitroimidazoles
Sixteen studies compared nitroimidazoles versus without nitroimidazoles in mild‐moderate PID (Burchell 1987; Tison 1988; Ross 2006; Judlin 2010a; Aşicioğlu 2013) or severe PID (Ciraru‐Vigneron 1986; Crombleholme 1986; Crombleholme 1987; Leboeuf 1987; Buisson 1989; Ciraru‐Vigneron 1989; Giraud 1989; Heinonen 1989; Fischbach 1994; Sirayapiwat 2002; Heystek 2009).
Primary outcomes
3.1. Effectiveness
3.1a. Clinical cure in mild‐moderate pelvic inflammatory disease
We included five studies in the analysis of clinical cure in mild‐moderate PID (Burchell 1987; Tison 1988; Ross 2006; Judlin 2010a; Aşicioğlu 2013). In all the studies the nitroimidazole used was metronidazole. There was no conclusive evidence of a difference in effectiveness between metronidazole versus no use of metronidazole in mild‐moderate PID (RR 1.01, 95% CI 0.93 to 1.10; 2427 women; 5 studies; I2 = 60%; moderate‐quality evidence) (Analysis 3.1; Figure 7).
Analysis 3.1.
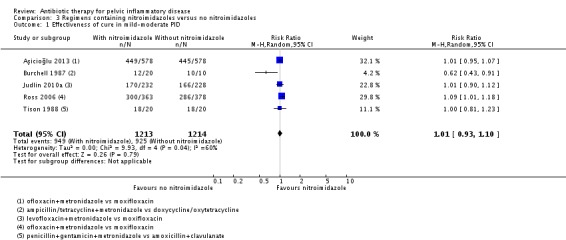
Comparison 3 Regimens containing nitroimidazoles versus no nitroimidazoles, Outcome 1 Effectiveness of cure in mild‐moderate PID.
Figure 7.
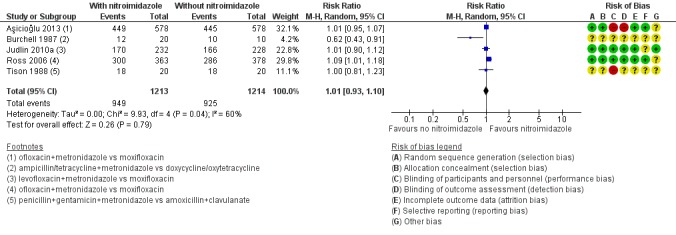
3.1 Effectiveness of cure in mild‐moderate PID in regimens containing nitroimidazoles versus without nitroimidazoles.
Sensitivity analysis restricted to the two studies at low risk of bias did not substantially change the main findings (RR 1.06, 95% CI 0.98 to 1.15; 1201 women; 2 studies; I2 = 32%; high‐quality evidence) (Ross 2006; Judlin 2010a) (Analysis 3.2).
Analysis 3.2.
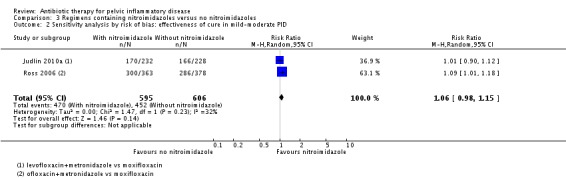
Comparison 3 Regimens containing nitroimidazoles versus no nitroimidazoles, Outcome 2 Sensitivity analysis by risk of bias: effectiveness of cure in mild‐moderate PID.
3.1b. Clinical cure in severe pelvic inflammatory disease
Eleven studies evaluated nitroimidazole in severe PID; and all studies used metronidazole (Ciraru‐Vigneron 1986; Crombleholme 1986; Crombleholme 1987; Leboeuf 1987; Buisson 1989; Ciraru‐Vigneron 1989; Giraud 1989; Heinonen 1989; Fischbach 1994; Sirayapiwat 2002; Heystek 2009). There was no evidence of a difference in clinical cure rates between women treated with metronidazole and women not treated with it (RR 0.96, 95% CI 0.92 to 1.01; 1383 women; 11 studies; I2 = 3%; moderate‐quality evidence) (Analysis 3.3; Figure 8).
Figure 8.
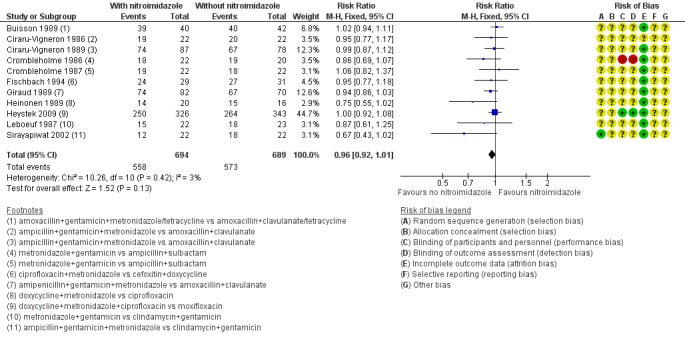
3.2 Effectiveness of cure in severe PID in regimens containing nitroimidazoles versus without nitroimidazoles.
3.2. Adverse events
3.2.1. Antibiotic‐related adverse effects leading to discontinuation of therapy
We analyzed 16 studies pf antibiotic‐related adverse effects leading to discontinuation of therapy (Ciraru‐Vigneron 1986; Crombleholme 1986; Burchell 1987; Crombleholme 1987; Leboeuf 1987; Tison 1988; Buisson 1989; Ciraru‐Vigneron 1989; Giraud 1989; Heinonen 1989; Fischbach 1994; Sirayapiwat 2002; Ross 2006; Heystek 2009; Judlin 2010a; Aşicioğlu 2013). There was no clear evidence of a difference between groups in rates of discontinuation of treatment due to adverse effects (RR 1.00, 95% CI 0.63 to 1.59; 3788 women; 16 studies; I2 = 0%; low‐quality evidence). Of note, out of 16 RCTs, 10 studies did not contribute data to the analysis because the authors reported no adverse effects (Ciraru‐Vigneron 1986; Crombleholme 1986; Burchell 1987; Leboeuf 1987; Tison 1988; Ciraru‐Vigneron 1989; Giraud 1989; Heinonen 1989; Sirayapiwat 2002; Heystek 2009). Only six studies reported adverse effects leading to discontinuation of treatment, yielding a rate of 1.8% of severe adverse effects (95% CI 1.2% to 2.5%) in the group not treated with nitroimidazole, and a rate of 1.7% (95% CI 1.2% to 2.4%) in the group treated with nitroimidazole.
Sensitivity analysis restricted to the two studies at low risk of bias did not substantially change the main findings (RR 1.06, 95% CI 0.98 to 1.15; 1201 women; 2 studies; I2 = 32%; high‐quality evidence) (Ross 2006; Judlin 2010a).
Data are depicted in Table 3.
Secondary outcomes
3.3. Microbiological clearance of chlamydia
This is not applicable, since nitroimidazoles do not have activity against chlamydia.
3.4. Microbiological clearance of gonorrhoea
This is not applicable, since nitroimidazoles do not have activity against gonorrhoea.
3.5. Laparoscopic evidence of resolution of pelvic inflammatory disease based on physician opinion
We found no data for laparoscopic evidence of resolution of PID.
3.6. Length of stay (for inpatient care)
Burchell and colleagues did not give details of the length of hospital stay: the authors mentioned that "ampicillin plus metronidazole in group II began with four 1 g intravenous doses given at 6‐hourly intervals and then 400 mg 8‐hourly orally for 14 days" and in Table III stated "patient response after 4 days treatment" (Burchell 1987).
Ciraru‐Vigneron and colleagues reported that women treated with amoxicillin plus clavulanate had a stay of 3.6 days and with ampicillin plus gentamicin plus metronidazole had a stay of 3.7 days (Ciraru‐Vigneron 1986).
Buisson and colleagues reported that the mean treatment for the amoxicillin plus clavulanate group was four days followed by a mean of 17 days of oral therapy, and for the triple treatment (ampicillin plus gentamicin plus metronidazole) it was seven days per protocol, due to the use of gentamicin, followed by ampicillin plus metronidazole until clinical improvement (Buisson 1989).
Ciraru‐Vigneron and colleagues reported that the mean duration of intravenous therapy was 7.6 ± 2.1 days in amoxicillin plus clavulanate and 7.7 ± 2.2 days in the ampicillin plus gentamicin plus metronidazole group. They did not specify if the oral treatment, followed intravenous treatment, was performed in hospital, but both were similar (11.2 ± 4.8 days with amoxicillin plus clavulanate and 11.1 ± 6.6 days with ampicillin plus gentamicin plus metronidazole) (Ciraru‐Vigneron 1989).
Crombleholme and colleagues report that treatment was for 14 days, starting with intravenous infusion and switched to oral, but they did not provide details if oral therapy was in hospital (Crombleholme 1989). In their previous study, intravenous treatment length was mentioned as at least five days, without further details (Crombleholme 1986).
Fischbach and colleagues admitted women for intravenous treatment for two to five days, followed by seven to 12 days of oral therapy, without further details (Fischbach 1994).
3.7. Rate of fertility
We found no data for rate of fertility.
4. Regimens containing clindamycin plus aminoglycoside versus quinolone
Three studies compared clindamycin plus aminoglycoside versus quinolone in mild‐moderate PID (Apuzzio 1989) or severe PID (Crombleholme 1989; Thadepalli 1991).
Primary outcomes
4.1. Effectiveness
4.1a. Clinical cure in mild‐moderate pelvic inflammatory disease
Only one study compared clindamycin with an aminoglycoside (gentamicin) versus a quinolone (ciprofloxacin) (Apuzzio 1989). It was unclear whether there was any difference between the regimens (RR 0.88, 95% CI 0.69 to 1.13; 25 women; 1 study; I2 = 0%; very low‐quality evidence) (Analysis 4.1).
Analysis 4.1.
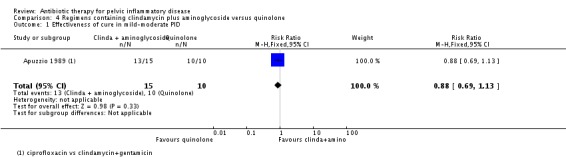
Comparison 4 Regimens containing clindamycin plus aminoglycoside versus quinolone, Outcome 1 Effectiveness of cure in mild‐moderate PID.
4.1b. Clinical cure in severe pelvic inflammatory disease
We included two studies in the analysis of clinical cure in severe PID (Crombleholme 1989; Thadepalli 1991). There was no clear evidence of a difference between these regimens in rates of cure for severe PID (RR 1.02, 95% CI 0.87 to 1.19; 151 women; 2 studies; I2 = 0%; low‐quality evidence) (Analysis 4.2).
Analysis 4.2.
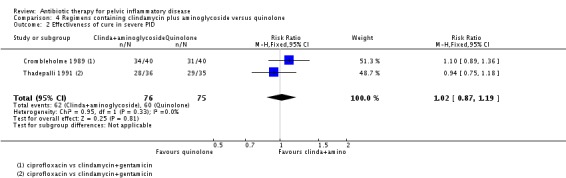
Comparison 4 Regimens containing clindamycin plus aminoglycoside versus quinolone, Outcome 2 Effectiveness of cure in severe PID.
4.2. Adverse events
4.2.1. Antibiotic‐related adverse effects leading to discontinuation of therapy
The incidence of antibiotic‐related adverse effects leading to discontinuation of therapy with clindamycin with aminoglycoside was 0% (95% CI 0% to 4.4%) and with quinolone was 5% (95% CI 1.9% to 12%). There was no clear evidence of a difference between regimens (RR 0.21, 95% CI 0.02 to 1.72; 163 women; 3 studies; I2 = 0%; very low‐quality evidence) (Analysis 4.3).
Analysis 4.3.
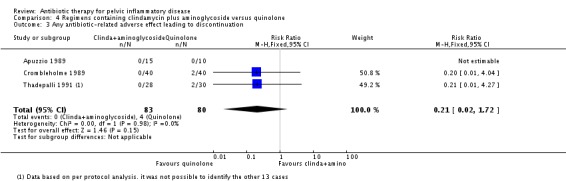
Comparison 4 Regimens containing clindamycin plus aminoglycoside versus quinolone, Outcome 3 Any antibiotic‐related adverse effect leading to discontinuation.
Data are depicted in Table 4.
Secondary outcomes
4.3. Microbiological clearance of chlamydia
Three studies reported chlamydia clearance (Apuzzio 1989; Crombleholme 1989; Thadepalli 1991), and cure occurred in 10/10 women in the clindamycin plus aminoglycoside group (100%, 95% CI 72% to 100%) and in 11/12 women in the quinolone group (92%, 95% CI 65% to 99%).
4.4. Microbiological clearance of gonorrhoea
Three studies reported gonorrhoea clearance (Apuzzio 1989; Crombleholme 1989; Thadepalli 1991), and cure occurred in 44/44 women in the clindamycin plus aminoglycoside group (100%, 95% CI 92% to 100%) and in 41/41 women in the quinolone group (100%, 95% CI 91% to 100%).
4.5. Laparoscopic evidence of resolution of pelvic inflammatory disease based on physician opinion
We found no data for laparoscopic evidence of resolution of PID.
4.6. Length of stay (for inpatient care)
We found no data suitable for analysis for length of hospital stay. Crombleholme and colleagues reported that treatment was for 14 days, starting with intravenous infusion and then switched to oral, but they did not provide details if oral therapy was in hospital (Crombleholme 1989). Appuzio and colleagues did not give many details of the length of stay. They mentioned that women received intravenous antibiotics for three to five days until they were asymptomatic for 24 hours (Apuzzio 1989). Likewise, Thadepalli reported that women received intravenous ciprofloxacin for three or more days, followed by oral ciprofloxacin for about one week (Thadepalli 1991).
4.7. Rate of fertility
We found no data for rate of fertility.
5. Regimens containing clindamycin plus aminoglycoside versus cephalosporin
Ten studies compared clindamycin plus aminoglycoside versus cephalosporin in mild‐moderate PID (Sweet 1985; Walters 1990) or severe PID (Roy 1985; Sweet 1985; Soper 1988; Martens 1990; Roy 1990; Walters 1990; Landers 1991; Maria 1992; Hemsell 1994; Balbi 1996). Studies from Sweet 1985 and Walters 1990 had both populations with mild‐moderate and severe PID, thus they were used in both analyses.
Primary outcomes
5.1. Effectiveness
5.1a. Clinical cure in mild‐moderate pelvic inflammatory disease
We analyzed two studies for clinical cure in mild‐moderate PID (Sweet 1985; Walters 1990). There was no clear evidence of a difference between these regimens in rates of cure for mild‐moderate PID (RR 1.02, 95% CI 0.95 to 1.09; 150 women; 2 studies; I2 = 0%, low‐quality evidence) (Analysis 5.1; Figure 9).
Analysis 5.1.
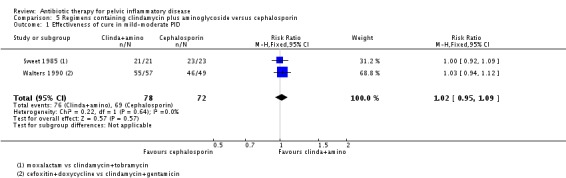
Comparison 5 Regimens containing clindamycin plus aminoglycoside versus cephalosporin, Outcome 1 Effectiveness of cure in mild‐moderate PID.
Figure 9.
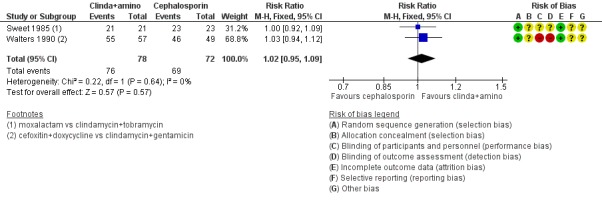
Forest plot of comparison: 5 Regimens containing clindamycin plus aminoglycoside versus cephalosporin, outcome: 5.1 Effectiveness of cure in mild‐moderate PID.
5.1b. Clinical cure in severe pelvic inflammatory disease
We included 10 studies in the analysis of clinical cure in severe PID (Roy 1985; Sweet 1985; Soper 1988; Martens 1990; Roy 1990; Walters 1990; Landers 1991; Maria 1992; Hemsell 1994; Balbi 1996). There was no clear evidence of a difference between these regimens in rates of cure of severe PID (RR 1.00, 95% CI 0.95 to 1.06; 959 women; 10 studies; I2 = 21%; moderate‐quality evidence) (Analysis 5.2; Figure 10).
Analysis 5.2.
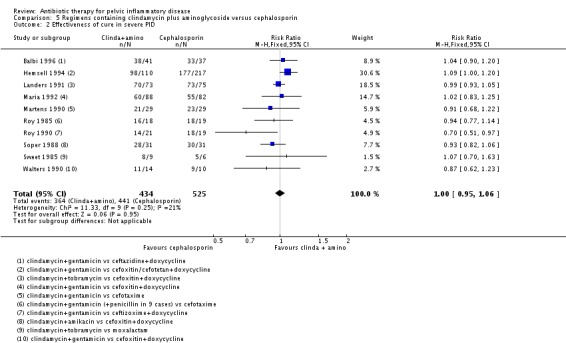
Comparison 5 Regimens containing clindamycin plus aminoglycoside versus cephalosporin, Outcome 2 Effectiveness of cure in severe PID.
Figure 10.
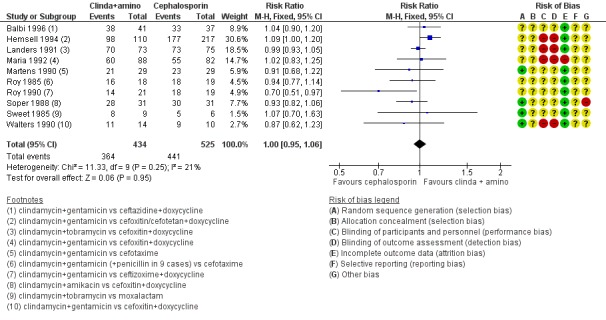
5.2 Effectiveness of cure in severe PID in regimens containing clindamycin plus aminoglycoside versus cephalosporin.
5.2. Adverse events
5.2.1. Antibiotic‐related adverse effects leading to discontinuation of therapy
We included 10 studies in the analysis of antibiotic‐related adverse effects leading to discontinuation of therapy (Roy 1985; Sweet 1985; Soper 1988; Martens 1990; Roy 1990; Walters 1990; Landers 1991; Maria 1992; Hemsell 1994; Balbi 1996). There was no clear evidence of a difference between groups in adverse effects leading to discontinuation of treatment (RR 0.78, 95% CI 0.18 to 3.42; 1172 women; 10 studies; I2 = 0%; very low‐quality evidence) (Analysis 5.3).
Analysis 5.3.
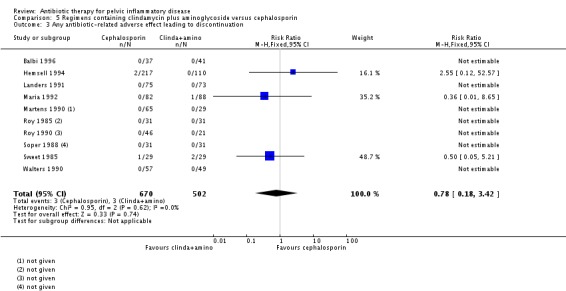
Comparison 5 Regimens containing clindamycin plus aminoglycoside versus cephalosporin, Outcome 3 Any antibiotic‐related adverse effect leading to discontinuation.
Data are depicted in Table 5.
Secondary outcomes
5.3. Microbiological clearance of chlamydia
Five studies reported chlamydia clearance (Sweet 1985; Roy 1990; Walters 1990; Maria 1992; Balbi 1996). Cure occurred in 53/56 women in the cephalosporin group (94.6%, 95% CI 85% to 98%) and in 75/78 women in the clindamycin plus aminoglycoside group (96%, 95% CI 89% to 99%).
5.4. Microbiological clearance of gonorrhoea
Five studies reported gonorrhoea clearance (Sweet 1985; Roy 1990; Walters 1990; Maria 1992; Balbi 1996). Cure occurred in 96/96 women in the clindamycin plus aminoglycoside group (100%, 95% CI 96% to 100%) and 115/117 women in the cephalosporin group (98%, 95% CI 94% to 99%).
5.5. Laparoscopic evidence of resolution of pelvic inflammatory disease based on physician opinion
We found no data for laparoscopic evidence of resolution of PID.
5.6. Length of stay (for inpatient care).
Seven studies did not provide enough data for analysis of length of hospital stay (Roy 1985; Sweet 1985; Roy 1990; Walters 1990; Landers 1991; Maria 1992; Balbi 1996). Three studies provided the mean and standard deviations of hospital stay, or range of days (Soper 1988; Martens 1990; Hemsell 1994). The mean length of stay for the cephalosporin group varied from 5.8 days to 9.6 days (range 3 days to 18 days) in the clindamycin plus aminoglycoside group and the mean days of in hospital days varied from 5.8 days to 9.8 days (range from 2 days to 25 days).
5.7. Rate of fertility
We found no data for rate of fertility.
Other analyses
We were unable to conduct our planned subgroup analyses due to insufficient data in the included studies.
Discussion
Summary of main results
We included 37 trials with 6348 women in the review. We found no clear evidence of a difference between any of the regimens studied in terms of effectiveness or safety.
The only comparison that clearly suggested a difference between the interventions was the sensitivity analysis of cases of mild‐moderate PID for the comparison macrolide (azithromycin) versus tetracycline (doxycycline). When we limited analysis to the single study at low risk of bias, moderate‐quality evidence suggested that azithromycin was superior to doxycycline in achieving clinical cure (Table 1).
Some guidelines have recommended the use of nitroimidazoles for treating PID (Ross 2007; Workowski 2015). We found no conclusive evidence of a difference between the use or not of nitroimidazoles (metronidazole) in rates of cure in either mild‐moderate or severe PID. There was also no clear evidence of a difference in rates of adverse effects.
Overall completeness and applicability of evidence
Although we conducted comprehensive searches to identify all published and unpublished RCTs, this systematic review included trials at high risk of bias and consequently with low confidence in the estimate of effect (see Table 1; Table 2; Table 3; Table 4; Table 5). When we used ITT analysis, there was substantial heterogeneity in the azithromycin trial results, which may limit our conclusions.
Data were lacking for several of our secondary outcomes. None of the included studies reported data on fertility or laparoscopic evidence of PID resolution, and data were very scant on length of stay.
The applicability of the evidence to the target population (women of reproductive age diagnosed with PID) was broad because the included trials were conducted in different clinical settings and implemented varying diagnostic approaches. Additionally, the interventions analyzed in the review are available in various clinical settings and represent the most frequently used therapeutic schemes in current clinical practice. Given these factors, we consider that the evidence identified applies to a wide range of women with PID varying in disease severity, age, geographical location, and diagnostic criteria, which provides external validity.
PID can be life or fertility threatening, and treatment is routinely started before laboratory cultures or laparoscopic confirmation. In some cases, women were subsequently diagnosed with another condition; however, we included all women in our ITT analysis, in accordance with our review protocol.
Quality of the evidence
Most of the 37 included studies had unclear or high risk of bias in most domains, and only three were at low risk of bias in most domains (Ross 2006; Savaris 2007; Judlin 2010a). The remaining studies had a number of limitations, for instance, 25 trials did not clearly define randomization, and there was potential performance and detection bias in 20 studies (Figure 3).
The overall quality of the evidence ranged from very low to high, the main limitations being serious risk of bias (due to poor reporting of study methods and lack of blinding), serious inconsistency, and serious imprecision. There was substantial heterogeneity in the comparison between azithromycin and doxycycline (I2 = 72%; Figure 5) and in the comparison of nitroimidazole versus no nitroimidazole (I2 = 60%; Figure 7). Imprecision was related to suboptimal sample sizes and the low number of studies for some comparisons.
The quality of evidence for the outcomes analyzed is provided in the 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4; Table 5). The only high‐quality evidence was for the sensitivity analysis regarding the use (or not) of nitroimidazole. There was moderate‐quality evidence in the sensitivity analysis regarding the use of azithromycin in mild‐moderate cases of PID, in comparisons between the use or not of nitroimidazole for curing mild‐moderate or severe PID, and in comparisons between clindamycin plus aminoglycoside versus cephalosporins for curing severe PID.
For all other comparisons, the evidence was low or very low quality.
Potential biases in the review process
An important limitation of this systematic review was the potential for measurement bias introduced by using the investigators' definitions of cure. This approach was necessary because of the wide variation in methods used and lack of a widely accepted objective outcome measure. The short‐term follow‐up of most of the studies prevented the identification of long‐term sequelae. In addition, the inaccuracy of clinical diagnosis for PID and the wide variety of assessment criteria used for clinical cure may have reduced the power of the analysis to detect a significant effect.
Some studies identified PID and endometritis separately but these were pooled for our analysis.
Agreements and disagreements with other studies or reviews
One previous meta‐analysis, published in 1993, formed the basis for the CDC guidelines (Walker 1993). The authors reported pooled clinical cure rates ranging from 75% to 94%, which is similar to our updated review with overall rate of cure of 81%. Therefore, our results support the updated 2015 CDC guidelines (Workowski 2015) and the International Union against Sexually Transmitted Infections (IUSTI)/BASHH (Judlin 2010b; Ross 2014a), but not the inclusion of nitroimidazoles in treatment regimens.
Authors' conclusions
We found no conclusive evidence that one regimen is safer or more effective than any other for the cure of PID, and there is no clear evidence for the use of nitroimidazoles (metronidazole) compared to use of other drugs with activity over anaerobes. Moderate‐quality evidence from a single study at low risk of bias suggests that a macrolide (azithromycin) may be more effective than a tetracycline (doxycycline) for curing mild‐moderate PID. Our review considers only the drugs that are currently used and mentioned by the US Centers for Disease Control and Prevention (CDC).
There is a need for high‐quality randomized controlled trials following CONSORT guidance to assess treatments for women with PID, particularly further trials comparing oral azithromycin versus oral doxycycline and the use of nitroimidazoles. The lack of a consistent outcome measure to assess response to therapy is a major limitation and there is a clear need for core outcome measures to be developed.
Acknowledgements
We acknowledge the support of Helen Nagels, Motokazu Yanagi, Kensuke Takaoka, and Chen Hengxi with the non‐English language studies.
Appendices
Appendix 1. Electronic search strategies
CENTRAL (Ovid platform)
1 exp Pelvic Inflammatory Disease/ (418)
2 (pelvic adj5 inflammatory adj5 disease$).tw. (282)
3 pid.tw. (273)
4 pelvic inflammat$.tw. (310)
5 pelvic peritonitis.tw. (0)
6 adnexitis.tw. (10)
7 exp Pelvic Infection/ (196)
8 (pelvic adj5 infection$).tw. (208)
9 (ovar$ adj5 inflammation).tw. (16)
10 exp Oophoritis/ (7)
11 oophoriti$.tw. (7)
12 exp Endometritis/ (216)
13 endometritis.tw. (356)
14 endomyometritis.tw. (56)
15 exp Salpingitis/ (42)
16 salpingiti$.tw. (56)
17 exp Parametritis/ (9)
18 parametriti$.tw. (2)
19 (celluliti$ adj5 pelvic).tw. (26)
20 exp Fallopian Tube Diseases/ (134)
21 (fallopian adj5 tube adj5 disease$).tw. (5)
22 (tubal adj5 obstruction$).tw. (19)
23 (uterine adj5 tube adj5 disease$).tw. (0)
24 (tuboovarian adj5 abscess).tw. (5)
25 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 (1286)
26 exp Anti‐Bacterial Agents/ (20463)
27 (anti adj5 bacterial$).tw. (90)
28 antibacterial$.tw. (1562)
29 antibiotic$.tw. (13603)
30 bacteriocid$.tw. (13)
31 bactericide$.tw. (5)
32 exp Anti‐Infective Agents/ (48142)
33 (anti adj5 infective$).tw. (127)
34 antiinfective$.tw. (21)
35 microbicid$.tw. (257)
36 antimicrobial$.tw. (3551)
37 (anti adj5 microbial$).tw. (93)
38 exp Aminoglycosides/ (6672)
39 aminoglycosides.tw. (211)
40 exp Amoxicillin‐Potassium Clavulanate Combination/ (461)
41 amoxicillin.tw. (2645)
42 exp Azithromycin/ (702)
43 azithromycin.tw. (1283)
44 exp Cefotaxime/ (1574)
45 cefotaxime.tw. (598)
46 exp Cefotetan/ (105)
47 cefotetan.tw. (155)
48 exp Cefoxitin/ (274)
49 cefoxitin.tw. (410)
50 exp Ceftizoxime/ (164)
51 ceftizoxime.tw. (125)
52 exp Ceftriaxone/ (565)
53 ceftriaxone.tw. (940)
54 exp Ciprofloxacin/ (941)
55 ciprofloxacin.tw. (1597)
56 exp Clindamycin/ (664)
57 clindamycin.tw. (1082)
58 exp Doxycycline/ (731)
59 doxycycline.tw. (1120)
60 exp Gentamicins/ (1044)
61 gentamicin.tw. (1284)
62 exp Metronidazole/ (1688)
63 metronidazole.tw. (2693)
64 moxifloxacin.tw. (667)
65 exp Ofloxacin/ (752)
66 ofloxacin.tw. (824)
67 levofloxacin.tw. (730)
68 sultamicillin.tw. (44)
69 (ampicillin adj5 sulbactam).tw. (229)
70 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 (63988)
71 25 and 70 (611)
MEDLINE (Ovid platform)
1 exp Pelvic Inflammatory Disease/ (10207)
2 (pelvic adj5 inflammatory adj5 disease$).tw. (4083)
3 pid.tw. (3492)
4 pelvic inflammat$.tw. (4283)
5 pelvic peritonitis.tw. (105)
6 adnexitis.tw. (496)
7 exp Pelvic Infection/ (5135)
8 (pelvic adj5 infection$).tw. (1833)
9 (ovar$ adj5 inflammation).tw. (279)
10 exp Oophoritis/ (499)
11 oophoriti$.tw. (407)
12 exp Endometritis/ (3599)
13 endometritis.tw. (3169)
14 endomyometritis.tw. (200)
15 exp Salpingitis/ (1994)
16 salpingiti$.tw. (1513)
17 exp Parametritis/ (198)
18 parametriti$.tw. (112)
19 (celluliti$ adj5 pelvic).tw. (97)
20 exp Fallopian Tube Diseases/ (7297)
21 (fallopian adj5 tube adj5 disease$).tw. (84)
22 (tubal adj5 obstruction$).tw. (423)
23 (uterine adj5 tube adj5 disease$).tw. (3)
24 (tuboovarian adj5 abscess).tw. (143)
25 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 (23104)
26 exp Anti‐Bacterial Agents/ (613243)
27 (anti adj5 bacterial$).tw. (2879)
28 antibacterial$.tw. (52343)
29 antibiotic$.tw. (261141)
30 bacteriocid$.tw. (544)
31 bactericide$.tw. (658)
32 exp Anti‐Infective Agents/ (1415302)
33 (anti adj5 infective$).tw. (3071)
34 antiinfective$.tw. (417)
35 microbicid$.tw. (5363)
36 antimicrobial$.tw. (111599)
37 (anti adj5 microbial$).tw. (2944)
38 exp Aminoglycosides/ (140356)
39 aminoglycosides.tw. (8449)
40 exp Amoxicillin‐Potassium Clavulanate Combination/ (2250)
41 amoxicillin.tw. (11786)
42 exp Azithromycin/ (4124)
43 azithromycin.tw. (6083)
44 exp Cefotaxime/ (12415)
45 cefotaxime.tw. (7263)
46 exp Cefotetan/ (485)
47 cefotetan.tw. (734)
48 exp Cefoxitin/ (1763)
49 cefoxitin.tw. (3821)
50 exp Ceftizoxime/ (1101)
51 ceftizoxime.tw. (887)
52 exp Ceftriaxone/ (5094)
53 ceftriaxone.tw. (8422)
54 exp Ciprofloxacin/ (11440)
55 ciprofloxacin.tw. (20547)
56 exp Clindamycin/ (5195)
57 clindamycin.tw. (8787)
58 exp Doxycycline/ (8330)
59 doxycycline.tw. (10686)
60 exp Gentamicins/ (17804)
61 gentamicin.tw. (21225)
62 exp Metronidazole/ (11543)
63 metronidazole.tw. (13091)
64 moxifloxacin.tw. (3450)
65 exp Ofloxacin/ (6176)
66 ofloxacin.tw. (5988)
67 levofloxacin.tw. (5700)
68 sultamicillin.tw. (141)
69 (ampicillin adj5 sulbactam).tw. (1564)
70 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 (1632933)
71 randomized controlled trial.pt. (425167)
72 controlled clinical trial.pt. (91285)
73 randomized.ab. (355829)
74 placebo.ab. (174189)
75 clinical trials as topic.sh. (178323)
76 randomly.ab. (254885)
77 trial.ti. (155344)
78 71 or 72 or 73 or 74 or 75 or 76 or 77 (1039211)
79 exp animals/ not humans.sh. (4280378)
80 78 not 79 (957018)
81 25 and 70 and 80 (684)
Embase
#1 'pelvic inflammatory disease'/exp (13,726)
#2 'pelvic inflammatory disease':ti,ab (4326)
#3 p.i.d.:ti,ab (55)
#4 pid:ti,ab (5,216)
#5 'pelvic infection':ti,ab (731)
#6 (pelvi? NEAR/5 inflammat*):ti,ab (5,453)
#7 'pelvic peritonitis':ti,ab (137)
#8 'ovary inflammation'/exp (695)
#9 (ovar* NEAR/5 inflammatio*):ti,ab (377)
#10 oophoriti*:ti,ab (471)
#11 ovaritis:ti,ab (48)
#12 'endometritis'/exp (5,280)
#13 endometritis:ti,ab (3,647)
#14 'salpingitis'/exp (2,633)
#15 salpingitis:ti,ab (1,657)
#16 'uterine tube disease'/exp (9,070)
#17 'uterine tube disease':ti,ab (1)
#18 'uterine tube inflammation':ti,ab (1)
#19 'fallopian tube diseases':ti,ab (3)
#20 'fallopian tube inflammation':ti,ab (2)
#21 'adnexitis'/exp (1,019)
#22 adnexitis:ti,ab (552)
#23 'adnex inflammation uterine':ti,ab (0)
#24 'adnexa infection':ti,ab (0)
#25 'adnexa inflammation':ti,ab (0)
#26 parametriti*:ti,ab (112)
#27 (para NEAR/5 metriosis):ti,ab (0)
#28 'tuboovarian abscess':ti,ab (190)
#29 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16
OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 (31,956)
#30 'antiinfective agent'/exp (2,566,913)
#31 'antiinfective agent':ti,ab (25)
#32 antibacterial*:ti,ab (70,680)
#33 'anti bacterial':ti,ab (3,425)
#34 'anti infective':ti,ab (3,462)
#35 antimicrobial*:ti,ab (147,222)
#36 bacteriocid*:ti,ab (660)
#37 bactericide*:ti,ab (1,159)
#38 microbicid*:ti,ab (6,092)
#39 'antibiotic agent'/exp (1,121,485)
#40 antibiotic*:ti,ab (338,040)
#41 'aminoglycoside'/exp (13,112)
#42 aminoglycoside:ti,ab (12,338)
#43 'amoxicillin plus clavulanic acid'/exp (29,766)
#44 'amoxicillin':ti,ab (16,673)
#45 'azithromycin'/exp (26,174)
#46 azithromycin:ti,ab (8,812)
#47 'cefotaxime'/exp (35,191)
#48 cefotaxime:ti,ab (9,344)
#49 'cefotetan'/exp (3,075)
#50 cefotetan:ti,ab (977)
#51 'cefoxitin'/exp (15,495)
#52 cefoxitin:ti,ab (4,658)
#53 'ceftizoxime'/exp (3,860)
#54 ceftizoxime:ti,ab (1,234)
#55 'ceftriaxone'/exp (45,306)
#56 ceftriaxone:ti,ab (12,087)
#57 'ciprofloxacin'/exp (77,874)
#58 ciprofloxacin:ti,ab (27,319)
#59 'clindamycin'/exp (42,275)
#60 clindamycin:ti,ab (11,122)
#61 'doxycycline'/exp (41,407)
#62 doxycycline:ti,ab (14,273)
#63 'gentamicin'/exp (90,658)
#64 gentamicin:ti,ab (26,194)
#65 'metronidazole'/exp (56,425)
#66 metronidazole:ti,ab (17,513)
#67 'moxifloxacin'/exp (13,514)
#68 moxifloxacin:ti,ab (4,833)
#69 'ofloxacin'/exp (22,915)
#70 ofloxacin:ti,ab (7,905)
#71 'levofloxacin'/exp (25,916)
#72 levofloxacin:ti,ab (8,786)
#73 'sultamicillin'/exp (8,641)
#74 sultamicillin:ti,ab (277)
#75 'ampicillin sulbactam':ti,ab (1,567)
#76 #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 (2,684,527)
#77 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti NOT ('animal'/de OR 'animal experiment'/de OR 'nonhuman'/de NOT ('animal'/de OR 'animal experiment'/de OR 'nonhuman'/de AND 'human'/de)) (1,286,106)
#78 #29 AND #76 AND #77 (812)
LILACS (IAHx interface)
((mh:"Enfermedad Inflamatoria Pélvica" OR tw:"enfermedad inflamatoria pélvica" OR tw:"pelvic inflammatory disease" OR tw:"doença inflamatória pélvica" OR tw:"doença pélvica inflamatória" OR tw:"doença inflamatória da pelve" OR tw:anexit$ OR tw:adnexitis OR tw:epi OR tw:eip OR tw:"inflamación pélvica" OR tw:"inflamación del ovario" OR tw:"peritonitis pélvica" OR tw:"absceso tubo ovárico" OR mh:Endometritis OR tw:endometrit$ OR tw:endomiometrit$ OR tw:endomyometritis OR mh:Ooforitis OR tw:ooforit$ OR tw:oophoritis OR mh:Parametritis OR tw:parametrit$ OR tw:"celulitis pélvica" OR tw:"cellulitis pelvic" OR tw:"celulite pélvica" OR mh:Salpingitis OR tw:salpingit$ OR mh:"Infección Pélvica" OR tw:"infección pélvica" OR tw:"pelvic infection" OR tw:"infecção pélvica" OR mh:"Enfermedades de las Trompas Uterinas" OR tw:"enfermedades de las trompas uterinas" OR tw:"fallopian tube diseases" OR tw:"doenças das tubas uterinas" OR tw:"enfermedades de las trompas de falopio" OR tw:"doenças das trompas de falópio") AND (mh:Antibacterianos OR tw:antibacterian$ OR tw:"anti bacterial" OR tw:antibacterial$ OR tw:antibiótic$ OR tw:antibiotic$ OR tw:bactericid$ OR mh:Antiinfecciosos OR tw:antiinfeccioso$ OR tw:"anti infective" OR tw:microbicid$ OR tw:antimicrobian$ OR mh:Aminoglicósidos OR tw:aminoglicósidos OR tw:aminoglycosides OR tw:aminoglicosídeos OR mh:"Combinación Amoxicilina‐Clavulanato de Potasio" OR tw:"combinación amoxicilina clavulanato de potasio" OR tw:"amoxicillin potassium clavulanate combination" OR tw:"combinação amoxicilina clavulanato de potássio" OR mh:Azitromicina OR tw:azitromicina OR tw:azithromycin OR mh:Cefotaxima OR tw:cefotaxim$ OR mh:Cefotetán OR tw:cefotetán OR tw:cefotetan OR mh:Cefoxitina OR tw:cefoxitin$ OR mh:Ceftizoxima OR tw:ceftizoxim$ OR mh:Ceftriaxona OR tw:ceftriaxon$ OR mh:Ciprofloxacino OR tw:ciprofloxacin$ OR mh:Clindamicina OR tw:clindamicina OR tw:clindamycin OR mh:Doxiciclina OR tw:doxiciclina OR tw:doxycycline OR mh:Gentamicinas OR tw:gentamicin$ OR mh:Metronidazol OR tw:metronidazol$ OR tw:moxifloxacina OR mh:Ofloxacino OR tw:ofloxacin$ OR tw:levofloxacino OR tw:"ampicilina sulbactam"))
RCTs filter:
((PT:"ensayo clinico controlado aleatorio" OR PT:"ensayo clinico controlado" OR PT:"estudio multicéntrico" OR MH:"ensayos clinicos controlados aleatorios como asunto" OR MH:"ensayos clinicos controlados como asunto" OR MH:"estudios multicéntricos como asunto" OR MH:"distribución aleatoria" OR MH:"método doble ciego" OR MH:"metodo simple‐ciego") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animales OR MH:conejos OR MH:ratones OR MH:ratas OR MH:primates OR MH:perros OR MH:gatos OR MH:porcinos OR PT:"in vitro")
Data and analyses
Comparison 1.
Regimens containing macrolides (azithromycin) versus tetracycline (doxycycline)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness of cure in mild‐moderate PID | 2 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.89, 1.55] |
| 2 Sensitivity analysis by risk of bias: effectiveness of cure in mild‐moderate PID | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.10, 1.67] |
| 3 Effectiveness of cure in severe PID | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.05] |
| 4 Any antibiotic‐related adverse effect leading to discontinuation use of macrolide versus tetracycline | 3 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.38, 1.34] |
Comparison 2.
Regimens containing quinolones versus cephalosporins
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness of cure in mild‐moderate PID | 3 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.10] |
| 2 Effectiveness of cure in severe PID | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.23] |
| 3 Any antibiotic‐related adverse effect leading to discontinuation cephalosporin versus quinolone | 5 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.52, 9.72] |
Comparison 3.
Regimens containing nitroimidazoles versus no nitroimidazoles
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness of cure in mild‐moderate PID | 5 | 2427 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.93, 1.10] |
| 2 Sensitivity analysis by risk of bias: effectiveness of cure in mild‐moderate PID | 2 | 1201 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.15] |
| 3 Effectiveness of cure in severe PID | 11 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.92, 1.01] |
| 4 Any antibiotic‐related adverse effect leading to discontinuation | 16 | 3788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.59] |
Analysis 3.4.
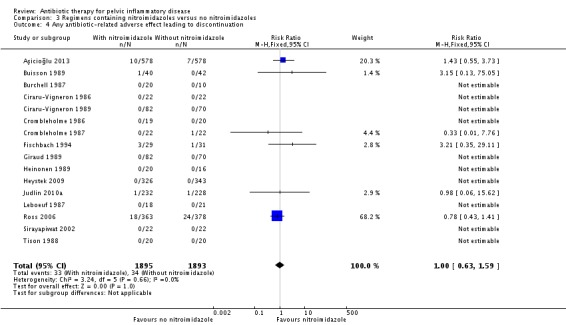
Comparison 3 Regimens containing nitroimidazoles versus no nitroimidazoles, Outcome 4 Any antibiotic‐related adverse effect leading to discontinuation.
Comparison 4.
Regimens containing clindamycin plus aminoglycoside versus quinolone
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness of cure in mild‐moderate PID | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 2 Effectiveness of cure in severe PID | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.87, 1.19] |
| 3 Any antibiotic‐related adverse effect leading to discontinuation | 3 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.02, 1.72] |
Comparison 5.
Regimens containing clindamycin plus aminoglycoside versus cephalosporin
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness of cure in mild‐moderate PID | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.09] |
| 2 Effectiveness of cure in severe PID | 10 | 959 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.06] |
| 3 Any antibiotic‐related adverse effect leading to discontinuation | 10 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.18, 3.42] |
Differences between protocol and review
We made the following changes to the protocol dated from 5 September 2013.
Criteria for considering studies
Types of interventions
In our protocol, we stated: "Trials comparing any antibiotics regimen compared to an alternative regimen or placebo will be eligible for inclusion."
After a long debate involving discussion with editorial board and reviewers, we decided to focus on the most clinically relevant interventions and comparisons; as identified in current clinical guidelines for treatment of PID.
Types of outcomes
The protocol listed a secondary outcome of "If the percentage of pain is available, a treatment will be considered effective when the reduction is 50% or more." However, from the clinical point of view, a reduction of pain over 50% is not relevant. For instance, if a woman with PID has an initial pain level of 10 and after treatment, the pain level was 4, this woman had a 60% reduction. However, in another case, a women with PID had an initial pain level of 5, and after treatment, her pain level was 3. In these two cases, if the 50% reduction in pain score criteria was used, the first woman would have obtained clinical cure while the second woman would not, despite the second woman having a lower pain level. Therefore, we did not perform this analysis, and used the clinical cure according to the criteria defined by the treating physician (e.g. resolution or improvement of signs and symptoms related to PID).
We amended the wording under secondary outcomes to clarify that where studies reported multiple time points, we considered the period between 14 and 28 days after initiation of treatment.
Search
In our protocol, we planned that searches would be updated within six months of publication in the review. Due to a lengthy publication process, we have extended this to 12 months
Data collection and analysis
Data extraction and management
In our protocol, we did not plan to conduct separate analyses for differing severity of disease. However, while preparing our review, we determined that severe PID (i.e. with tubo‐ovarian abscess) is a distinct condition from mild‐moderate PID (i.e. without tubo‐ovarian abscess). Therefore, we decided to present separate analyses for these two groups.
Statistical model
The protocol planned fixed‐effect models. However, we decided to use a random‐effects model where analyses had substantial heterogeneity (I2 40% or greater), to present a more conservative effect estimate.
Effect measure
We stated in the protocol that we would calculate Peto odds ratios for dichotomous outcomes. However, we noted that the Cochrane Handbook for Systematic Reviews of Interventions suggests avoiding using the Peto method as a default method of analysis because it may cause bias unless events are not particularly common and there are similar numbers in the intervention and control groups (Higgins 2011). As these criteria were not fulfilled in the review, we therefore, used Mantel‐Haenszel risk ratios for all dichotomous outcomes,
Subgroup analysis
In our protocol, we planned to undertake a subgroup analysis by PID severity, but in view of our decision to present separate analyses for mild‐moderate and severe PID, the subgroup analysis was no longer appropriate.
Sensitivity analyses
In our protocol, we planned to undertake sensitivity analyses for the following factors: risk of bias, heterogeneity (I2 40% or greater), length of time to measurement of outcomes, and method of PID diagnosis.
In the review, we listed variables that would be used to explore heterogeneity under the heading "Investigation of heterogeneity", and we included length of time to outcome measurement and method of PID diagnosis as two of these variables.
For the sensitivity analysis by risk of bias, we added a definition of low risk of bias (which we defined as blinded, and at low risk of selection bias).
Quality assessment
We added the reporting of quality of evidence following MECIR guidelines.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women admitted to the University Hospital of the University of Medicine and Dentistry of New Jersey from February 1987 to October 1988 with the diagnosis of either postpartum endometritis or acute salpingitis. Diagnosis of uncomplicated PID was based on the clinical criteria described by Hager and colleagues (Hager 1989). Exclusion criteria: history of allergy to the study drugs or received any antibiotic in the 2‐week period prior to the study (exclusive of prophylactic antibiotics for caesarean delivery). Number of women randomized: 25 for PID Number of women analyzed: group A: 10; group B: 15. Number of withdrawals/exclusions/loss to follow‐up and reasons: .none Number of centres: 1. Age (years): group A: 23.2; group B: 23.2. Country: US. |
|
| Interventions |
Group A: ciprofloxacin 300 mg IV every 12 h; treatment continued for 3‐5 days until the woman was asymptomatic for 24 h. Upon discharge from hospital, women received oral antibiotics to complete 10‐14 days of ciprofloxacin 750 mg PO twice daily. Group B: clindamycin 900 mg IV every 8 h + gentamicin 1.5 mg/kg IV every 8 h. Upon discharge from the hospital, the women received oral antibiotics to complete 10‐14 days of clindamycin 450 mg PO every 6 h. |
|
| Outcomes | Primary outcome: treatment success, defined as women asymptomatic for 24 h. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women randomly assigned to receive clindamycin + gentamicin or ciprofloxacin intravenously. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | 25 women with uncomplicated PID randomized. 10 women received ciprofloxacin and 15 women received clindamycin + gentamicin. 1 woman prescribed clindamycin + gentamicin was not available because triple therapy was initially started. This woman was included in the analysis as treatment success. The authors did not clarify that. |
| Methods | Randomized, double‐blind, comparative study. | |
| Participants |
Inclusion criteria: women aged 18‐55 years with clinical diagnosis of mild or moderate PID, as confirmed by laparoscopy graded according to the methods of Hager and colleagues (Hager 1983); and Soper (Soper 1991). Exclusion criteria: observable pelvic mass; presented with laparoscopic evidence of severe PID; pregnant or breastfeeding; allergic to clindamycin, ciprofloxacin, ceftriaxone, or doxycycline; required other antibiotic therapy for non‐protocol reasons; had had pelvic or abdominal surgery in the 30 days prior to admission (except emergency exploratory laparoscopy resulting in a primary diagnosis of PID that did not require pelvic cleanup); had concomitant disease that could have affected the evaluation of response to protocol therapy (such as inflammatory bowel disease or significant renal or hepatic disease); had a history of colitis; were known to have frequent sexual contacts with multiple partners; were seropositive for syphilis; had severe medical condition(s) (e.g. neoplasms or haematological malignancy); had taken ≥ 2 antibiotics within 72 h before evaluation for enrolment in the study; had received any investigational drug 30 days before evaluation for enrolment; or had been previously enrolled in the study. Number of women randomized: 69 in each group. Number of women analyzed: .69 in each group Number of withdrawals/exclusions/loss to follow‐up and reasons: 2 in the clindamycin+ciprofloxacin group; 1 chose to discontinue because she was asymptomatic and 1 withdrew because of a side effect. In the ceftriaxone+doxycycline group, 1 withdrew because of a side effect, 1 was lost to follow‐up and 1 was withdrawn because of receiving additional antibiotic treatment for syphilis after initiation of study medication. Number of centres: 6; Chile (1 centre), Peru (2 centres), Colombia (2 centres), and Mexico (1 centre). Age (mean) (years): group A: 28.9; group B: 30.7. Country: Mexico |
|
| Interventions |
Group A: clindamycin 300 mg (2 capsules 3 times daily) + ciprofloxacin (250 mg, 1 tablet twice daily) for 14 days and placebo IM (for an equivalent of 1 dose of ceftriaxone). Group B: ceftriaxone 250 mg IM (as a single dose) + doxycycline 100 mg (1 capsule twice daily) and placebo (2 capsules 3 times daily for equivalent doses of clindamycin) for 14 days. |
|
| Outcomes | Clinical cure defined by the absence of, or minimal, pelvic tenderness, temperature < 37.5 °C, and a WBC count of 10,000/mm3, if a minimum of 4 days of treatment had been completed. Clinical improvement defined as resolution of 2 of these 3 symptoms. Failure when 1 of the following circumstances was noted after at least 48 h of protocol therapy: signs and symptoms remained unchanged or worsened (during the first 72 h of therapy). Microbiological cure defined as eradication of N gonorrhoeae or C trachomatis (or both) from clinically cured women. Failure defined as persistence of 1 or both of these 2 organisms or, in the case of clinical improvement or failure, the presence of endocervical pathogens. Superinfection defined as the isolation of ≥ 1 new pathogens. Adverse effects leading to discontinuation of treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | All eligible women randomized to 1 of the treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo medication was added as necessary to complete the double‐blind design. All oral medication was encapsulated to ensure blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo medication was added as necessary to complete the double‐blind design. All oral medication was encapsulated to ensure blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized, parallel‐group study. | |
| Participants |
Inclusion criteria: women aged 14‐45 years with acute uncomplicated PID based on presence of all the following symptoms and signs: pelvic discomfort, direct lower abdominal tenderness, adnexal and cervical motion tenderness on bimanual vaginal examination, and pelvic pain for < 30 days, as well as ≥ 1 of the following signs: pyrexia (rectal, tympanic, or oral temperature > 38.8 °C or axillary temperature > 37.5 °C), elevated CRP > 6 mg/L, WBC > 10,500/mm3, and a normal ultrasonographic scan. Exclusion criteria: urinary tract infection; complicated PID (such as tubo‐ovarian abscess); endometriosis; pelvic pain > 30 days; history of antibiotic therapy within last week; previous failure to adhere to antibiotic treatment; other causes of abdominopelvic pain such as appendicitis, diverticulitis, or ovarian cysts; oral intolerance, defined as 1 episode of vomiting after the first oral medication administration; and delivery, abortion, or surgery within the last month. Number of women randomized: 1156. Number of women analyzed: group A: 578; group B: 578. Number of withdrawals/exclusions/loss to follow‐up and reasons: group A: 18 women lost (oral intolerance n = 2; non‐compliance n = 5; worsening of pain n = 3; tubo‐ovarian abscess n = 2; urinary tract infection n = 2; other n = 4); group B: 35 women lost (oral intolerance n = 4; non‐compliance n = 18; worsening of pain n = 2; tubo‐ovarian abscess n = 2; urinary tract infection n = 4; other n = 5). Number of centres: 4. Age (mean ± SD) (years): group A: 30.3 ± 3.7; group B: 29.3 ± 3.5. Country: Turkey. |
|
| Interventions |
Group A: moxifloxacin 400 mg once daily for 14 days. Group B: ofloxacin 400 mg twice daily + metronidazole 500 mg PO twice daily. |
|
| Outcomes | Clinical cure, microbiological cure, adverse effects. Primary outcome: clinical cure, defined as a ≥ 60% reduction in the total pain score at day 21 compared with baseline and the absence of pelvic discomfort and tenderness, temperature < 37.8 °C, and WBC < 10,000/mm3 on day 21. |
|
| Notes |
Ethical approval: yes. Informed consent: yes, women gave written informed consent. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random‐numbers table. |
| Allocation concealment (selection bias) | Low risk | Assigned treatments written on cards and sealed in secure opaque envelopes numbered in sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators not blinded to the procedure allocation. Moxifloxacin group received just 1 tablet, whereas ofloxacin + metronidazole group received 4 tablets daily. No placebo was added to mask groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 7 days after drug treatment at secondary visits (day 21), all women underwent a secondary evaluation by the same physician who allocated women. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From 1156 women, 53 lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Trial registered in ClinicalTrial.gov (NCT01799356). |
| Other bias | Unclear risk | Risk of potential bias unclear. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women with PID diagnosis based on all the following criteria: pelvic pain, either spontaneous or at palpation; cervical motion tenderness; and adnexal pain. Exclusion criteria: aged < 16 year, current pregnancy, allergy to 1 of the medications used in study or to penicillin, serum creatinine > 1.5 mg/dL, previous or current hepatic disease, use of antibiotics in the last 7 days, in situ IUD. Number of women randomized: 78. Number of women analyzed: 76; group A: 40; group B: 36. Number of withdrawals/exclusions/loss to follow‐up and reasons: 2, intolerance to penicillin. Number of centres: 1. Age (mean ± SD) (years): group A: 25.3 ± 7.7.4; group B: 29.4 ± 7.8. Country: Italy. |
|
| Interventions |
Group A: gentamicin, 2 mg/kg IV (attack dose), followed by 1.5 mg/kg IV every 8 h + clindamycin 900 mg IV every 8 h for 4 days, followed by clindamycin 450 mg PO every 6 h for a total of 14 days of treatment. Group B: ceftazidime 1 g IV every 8 h + doxycycline 100 mg PO every 12 h for 4 days, followed by doxycycline 100 mg PO every 12 h for a total of 14 days of treatment. |
|
| Outcomes |
Primary outcome: clinical recovery, defined as: body temperature < 37 °C per 48 h, disappearance of pelvic pain, no increase of eventual adnexal mass after 7 days of the end of treatment. Secondary outcome: follow‐up performed 30 days after treatment finished; endocervical culture for N gonorrhoeae and C trachomatis and endometrial culture for C Trachomatis performed in all positive cases at admission. |
|
| Notes |
Ethical approval: not stated. Informed consent: not stated. Funding source: no funding stated or declaration of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly allocated into 2 treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/78 women excluded from analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized, open‐label, comparative, multicentre, and multinational trial. | |
| Participants |
Inclusion criteria: aged ≥ 18 years, fulfilling Hager's criteria for a clinical diagnosis of acute PID (Hager 1983), and requiring hospitalization for treatment. Diagnosis of acute PID confirmed by laparoscopy unless impossible due to need for immediate treatment. Exclusion criteria: palpable tubo‐ovarian abscess (i.e. ultrasound diameter ≥ 5 cm); use of additional antimicrobial therapy for a concurrent infection; use of antibiotic therapy during the preceding 2 weeks; terminal illness; immunosuppression; impaired gastrointestinal function or absorption (or both); hepatic or renal impairment; known allergy to macrolides, tetracycline, metronidazole, penicillin, cephalosporins, or clavulanic acid; use of oral hypoglycaemic drugs, ergot, dicoumarin anticoagulants, carbamazepine, ciclosporin, digoxin, or theophylline; and known drug addiction, alcoholism, or taking of recreational drugs. Number of women randomized: 310. Number of women analyzed: 309; group A: 106; group B: 107; group C: 96. Number of withdrawals/exclusions/loss to follow‐up and reasons: 10 patients were excluded from the analysis at end of treatment because of the following protocol violations: did not receive study medication (n = 1); inappropriate primary diagnosis (n = 2); concomitant antibacterial treatment (n = 1); no signs/symptoms recorded at day evaluation (n = 1); and missing data or mistimed evaluation (n = 5). Number of centres: multiple, but the authors did not specify how many. Age (mean (range)) (years): group A: 28.4 (18‐54); group B: 27.6 (18‐46); group C: 27.6 (17‐54). Country: UK and others that were not stated. |
|
| Interventions |
Group A: azithromycin 500 mg IV single dose, days 2‐7: 250 mg PO once daily or days 1‐2: azithromycin 500 mg IV once daily; days 3‐7: 250 mg PO once daily. Group B: as group A + day 1: metronidazole 500 mg IV 3 times daily or 400 mg PO 3 times daily, days 2‐12: metronidazole 400 mg PO 3 times daily or days 1‐2: azithromycin 500 mg IV once daily; days 3‐7: 250 mg PO once daily plus day 1‐2: metronidazole 500 mg IV 3 times daily, or 500 mg PO 3 times daily. Days 3‐12: metronidazole 500 mg PO 3 times daily or days 1‐21: metronidazole 500 mg PO 3 times daily. Group C: day 1: metronidazole 500 mg IV 3 times daily, or metronidazole 400 mg PO 3 times daily; day 2‐12: metronidazole 400 mg PO 3 times daily + days 1‐14: cefoxitin 2 g IV or IM 4 times daily + day 1: probenecid 1 g PO single dose or day 1‐21: doxycycline 100 mg PO twice daily + day 1‐5: amoxicillin/clavulanate 1 g IV + times daily; day 6‐21 amoxicillin/clavulanate 500 mg PO 3 times daily. |
|
| Outcomes | Clinical response to treatment: cure, resolution of all baseline signs and symptoms; improvement, lessening of the baseline signs and symptoms or absence of ≥ 1, but not all, of the baseline findings; or failure, no improvement or deterioration of baseline condition. Successful clinical outcome defined as cure or improvement. Assessment on day 15 (9‐26 inclusive) and at follow‐up (day 35‐44). Microbiological outcome: eradication, absence of the baseline isolate(s); persistence, presence of baseline isolate(s); or superinfection, presence of a micro‐organism different from that found at baseline. |
|
| Notes |
Ethical approval: yes "European Ethical Review Committee and by local hospital ethical committees." Informed consent: yes, prior to entry into either study, written informed or witnessed oral consent was obtained from each woman. Source of funding: not stated, but 1 the authors was from Pfizer Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women randomized to 1 of 3 treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate (< 20%). |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Trial had support of pharmaceutical industry. 1 case missing from the analysis and not specified in the report. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women with PID associated or not associated with endometritis confirmed by laparoscopy according to the clinical criteria described by Hager and colleagues (Hager 1989). Exclusion criteria: known allergy to betalactamics, pregnant, renal and liver insufficiency. Number of women randomized: 82. Number of women analyzed: group A: 42; group B: 40. Number of withdrawals/exclusions/loss to follow‐up and reasons: 1 abandoned the amoxicillin‐clavulanic acid after knowing that the bacteria (K. pneumoniae) was resistant to the antibiotic. One stopped amoxicillin‐clavulanic acid after 6 days of treatment due to no clinical improvement and she was switched to group B; In group B, one patient stopped the treatment due to side‐effect (Quincke edema) Number of centres: 8. Age (mean (range)) (years): group A: 27.7 (18‐46); group B: 28.7 (15‐49). Country: France. |
|
| Interventions |
Group A: amoxicillin‐clavulanic acid 1 g IV every 8 h for at least 48 h, then 1.5‐2 g PO twice daily. Mean length of treatment 19 days, never less than 14 days. Group B: amoxicillin 3‐4 g IV per 24 h, mean 4 days, then 1.5‐2 g PO daily. Mean length of treatment 17 days + aminoside (chosen by researcher's preference) 3‐5 mg/kg IM per 24 h 2 or 3 times daily depending of the aminoside, mean length of treatment 7 days + metronidazole 1 or 1.5 g IV or suppository daily. For each case, a secondary prescription for a tetracycline 200 mg per 24 h was given, either immediately if results of laparoscopy or other investigations justified it, or later on positive chlamydia serology. Length of this treatment 3‐4 weeks as decided by the researcher. |
|
| Outcomes |
Primary outcomes: clinical cure; defined as absence of fever, pain, and previously observed adnexal masses at 5‐8 weeks' follow‐up; adverse events leading to discontinuation of treatment. Secondary outcome: none reported. |
|
| Notes |
Ethical approval: not stated. Informed consent: not stated. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The choice of treatment fixed by randomization. |
| Allocation concealment (selection bias) | Unclear risk | The choice of treatment fixed by randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/82 women lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: 40 women with PID established by blood sampling, laparoscopic, follow‐up clinical evaluations, and taking of microbiological specimens for culture done by same physician to ensure uniformity. Women had laparoscopic examination and microbiological cultures to confirm the clinical diagnosis of acute PID. Exclusion criteria: general peritonitis or with abdominal distension and absent bowel sounds; large pelvic masses extending into the abdomen; toxic; in poor general condition; pregnant (e.g. postabortal); and antibiotics in the 14 days before presentation. Number of women randomized: 30 women, 10 in each group. Number of women analyzed: 30 women, 10 in each group. Number of withdrawals/exclusions/loss to follow‐up and reasons: 10 women excluded because laparoscopic examination and microbiological cultures did not confirm the clinical diagnosis of acute PID. Number of centres: not stated. Age (years): not stated. Country: South Africa. |
|
| Interventions |
Group A: doxycycline infusion 200 mg in 200 mL 5% dextrose over 2 h + doxycycline 100 mg after 24 h. The course was completed with oxytetracycline 250 mg every 6 h for 14 days. Group B: ampicillin + metronidazole every 6 h 1 g IV and then 500 mg PO every 6 h for 14 days. Group C: tetracycline + metronidazole with 3 × 1 g suppositories every 8 h and then 400 mg PO every 8 h for 14 days. |
|
| Outcomes | Primary outcome: clinical cure. | |
| Notes |
Ethical approval: not stated. Informed consent: yes, informed consent was obtained from all women. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | No study group characteristics reported. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: severe PID, defined as signs of fever, pain. Local signs (adnexal mass), discovery of pathogen, leukocytosis, elevated ESR, pelvic ultrasound, and possibly laparoscopy. Exclusion criteria: not stated. Number of women randomized: 44; 22 in each group. Number of women analyzed: 44; 22 in each group. Number of withdrawals/exclusions/loss to follow‐up and reasons: only 20% of women seen. Number of centres: 1. Age (years): not stated. Country: France. |
|
| Interventions |
Group A: amoxicillin‐clavulanic acid while in hospital, 4 g per 24 h, first by IV, then PO once symptoms improved. At discharge, amoxicillin‐clavulanic acid PO if chlamydia serology was negative; if positive for chlamydia, doxycycline prescribed for 3 weeks (dose not stated). Group B: ampicillin 6 g per 24 h IV + gentamicin 160 mg per 24 h IM + metronidazole 1.5 g per 24 h IV; when switched to oral administration, ampicillin replaced by amoxicillin 3 g per 24 h + metronidazole 1.5 g per 24 h PO and gentamicin 160 mg per 24 h IM for minimum of 7 days. At discharge, amoxicillin‐clavulanic acid PO if chlamydia serology negative; if positive for chlamydia, doxycycline for 3 weeks (dose not stated). |
|
| Outcomes |
Primary outcomes: clinical cure, defined by no fever, reduction of pain, and normal WBC count at discharge and in 30 days; adverse events leading to discontinuation of treatment. Secondary outcomes: microbial cure of N gonorrhoeae and C trachomatis; length of hospital stay. |
|
| Notes |
Ethical approval: not stated. Informed consent: not stated. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized study. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Assumed that assessment of 'cure', 'improvement,' and 'failure' performed during hospitalization or at discharge. Follow‐up at 30 days; only 20% in each group seen. In those women, no secondary adverse events seen. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: not stated. Exclusion criteria: required systemic or local antibiotic therapy other than those specified in the protocol; pregnant or likely to become pregnant; or allergic to penicillins or cephalosporins. Number of women randomized: 165 women; group A: 78; group B: 87. Number of women analyzed: 152 women; group A: 70; group B: 82. Number of withdrawals/exclusions/loss to follow‐up and reasons: group A: 8; group B: 5 due to poor compliance. Number of centres: 8. Age (mean) (years): group A: 26.9; group B: 25.1. Country: France. |
|
| Interventions |
Group A: amoxicillin 1 g IV + clavulanic acid 200 mg (Augmentin 1.2 g), 3 or 4 times daily until clinical and laboratory findings improved, after which 4‐6 tablets containing amoxicillin 500 mg + clavulanic acid 125 mg (Augmentin 625 mg) per tablet. Group B: amoxicillin or ampicillin 3‐4 g daily, with an aminoglycoside (gentamicin 160 mg, dibekacin 150 mg, or tobramycin 150 mg) + metronidazole 1.5 g daily parenterally. Subsequent conversion was to oral combination of amoxicillin or ampicillin 2‐3 g + metronidazole 1‐1.5 g daily. |
|
| Outcomes | Primary outcomes: excellent response defined as resolution of physical findings with continued improvements in laboratory values; favourable response equate with a favourable course, allowing the persistence of ≥ 1 clinical signs or abnormal laboratory values (or both); failure defined as absence of therapeutic efficacy or of a favourable course after at least 6 days of therapy that a change in management, either surgery or an alternative antibiotic treatment was warranted. | |
| Notes |
Ethical approval: not stated. Informed consent: not stated. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 152/165 women included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: severe acute PID with peritonitis, tubo‐ovarian abscess, endometritis, and pelvic cellulitis. Exclusion criteria: not stated. Number of women randomized: 39; group A: 20; group B: 19. Number of women analyzed: 39; group A: 20; group B: 19. Number of withdrawals/exclusions/loss to follow‐up and reasons: not stated. Number of centres: not stated. Age (years): not stated. Country: not stated. |
|
| Interventions |
Group A: sulbactam 1 g + ampicillin 2 g IV every 6 h. Group B: metronidazole 15 mg/kg loading followed by 7.5 mg/kg IV every 6 h and gentamicin 1.5 mg/kg IV every 8 h. Antibiotics in both groups continued for minimum of 5 days. |
|
| Outcomes | No relevant outcomes reported for our analysis because the outcomes were not separately reported for the different diagnoses within the study groups. Authors stated clinical cure occurred in 19/20 women in group A and 16/19 women in group B. | |
| Notes |
Ethical approval: not stated. Informed consent: not stated. Source of funding: not stated, and no conflicts of interest reported. 2 cases of posthysterectomy pelvic cellulitis were included in the analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: hospitalized women aged ≥ 18 years with a documented or clinical diagnosis of mixed aerobic‐anaerobic PID. Exclusion criteria: pregnant or lactating; allergy to penicillins, cephalosporins, aminoglycosides, or metronidazole; impaired renal function (serum creatinine > 1.8 mg/100 mL); family history of glycogen storage disease or recurrent hypoglycaemia; history of unstable cardiovascular, hepatic, renal, or neurological disease. Number of women randomized: 44; 22 in each group. Number of women analyzed: 42; 21 in each group. Number of withdrawals/exclusions/loss to follow‐up and reasons: 1 women in group A with a clinical diagnosis of PID and a right adnexal mass excluded. Laparoscopy revealed a right ovarian cyst, no visual evidence of infection, and essentially no growth from cervical and endometrial cultures. 1 woman in group B with a clinical diagnosis of PID excluded; she left the hospital against medical advice on the third hospital day. Number of centres: 1. Age (mean ± SD) (years): group A: 27.7 ± 6.9; group B: 29.0 ± 12.1. Country: US. |
|
| Interventions |
Group A: ampicillin 2 g + sulbactam 1 g IV every 6 h. Group B: metronidazole 15 mg/kg IV every 6 h + gentamicin 1.5 mg/kg IV every 8 h. Therapy continued until women became afebrile and were without clinical signs of infection for 48 h or until clinical judgement dictated cessation of therapy. Range of treatment 3‐11 days. |
|
| Outcomes | Primary outcome: clinical cure defined as absence of fever, without clinical signs of infections for 48 h or until clinical judgement. | |
| Notes |
Ethical approval: not stated. Informed consent: not stated. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman missing in each group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women aged ≥ 18 years admitted to San Francisco General hospital with a diagnosis of acute PID according to established criteria: history of lower abdominal pain and direct lower abdominal tenderness with or without rebound; tenderness with motion of the cervix and uterus; and adnexal tenderness. ≥ 1 of the following: endocervix positive for Gram‐negative intracellular diplococci or direct fluorescent antibody test revealing C trachomatis; elevated ESR; temperature > 38 °C; leukocytosis > 10,500 white blood cells/mm3; purulent material (WBCs and bacteria) from the peritoneal cavity by culdocentesis; or a pelvic abscess or inflammatory complex on bimanual examination or by sonography. Exclusion criteria: history of allergy to any of the study drugs; mild infections not requiring parenteral antimicrobial therapy; pregnancy or lactation; severe underlying terminal illness; need for concomitant antimicrobial with a spectrum of activity similar to that of study drug; or severe impairment of renal function (creatinine level > 2 mg/dL or creatinine clearance < 50 mL/minute). Number of women randomized: 80; 40 in each group. Number of women analyzed: 80; 40 in each group. Number of withdrawals/exclusions/loss to follow‐up and reasons: none reported. Number of centres: 1. Age (mean ± SD) (years): group A: 25.7 ± 4.8; group B: 25.3 ± 6.2. Country: US. |
|
| Interventions |
Group A: ciprofloxacin 300 mg IV every 12 h for 2‐5 days, with ≥ 2 days of parenteral therapy and 48 h without fever before switching to ciprofloxacin 750 mg PO every 12 h, to complete a 14‐day course. Group B: clindamycin 600 mg IV every 6 h + 1 mg/kg IV every 8 h after ≥ 4 days of parenteral gentamicin and 48 h without fever before switching to clindamycin 300 mg PO every 6 h, to complete a 14‐day course. |
|
| Outcomes |
Primary outcome: clinical cure at 3 days: improvement in clinical signs and symptoms. Secondary outcomes: microbial cure of C trachomatis and N gonorrhoeae. |
|
| Notes |
Ethical approval: yes, women gave written informed consent as approved by the Committee of Human Research of the University of California, San Francisco. Informed consent: yes, women gave written informed consent as approved by the Committee of Human Research of the University of California, San Francisco. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women aged > 18 years, acute PID (endometriosis, salpingitis, pyosalpinx, adnexitis, pelvic peritonitis, tubo‐ovarian abscess). Diagnosis by manual examination, echography, laparoscopy, and clinical parameters such as fever, leukocytosis, ESR, and positive bacterial culturing (anaerobe, aerobe, chlamydia). Exclusion criteria: aged < 18 years; pregnancy or lactation; antibiotic therapy up to 3 days before inclusion; known allergies against gyrase‐inhibitors, cephalosporine, metronidazole, and tetracycline; serious impaired renal and liver function; and other serious comorbidities. Number of women randomized: 60. Number of women analyzed: 57; group A: 26; group B: 31. Number of withdrawals/exclusions/loss to follow‐up and reasons: group A: 3 women excluded from analysis due to adverse effects. Number of centres: not stated. Age (average (range)) (years): group A: 27 (30 ± 10); group B: 22 (26 ± 7). Country: Germany. |
|
| Interventions |
Group A: ciprofloxacin 2 × 0.2 g IV daily + metronidazole 3 × 0.5 g IV daily. Group B: cefoxitin 3 × 2 g IV daily + doxycycline 2 × 0.1 g IV daily. After 2‐5 days of treatment, ciprofloxacin, metronidazole, and doxycycline given PO. Both groups treated for 7‐14 days. |
|
| Outcomes | Primary outcome: clinical cure defined as subjective lack of symptoms, improvement upon gynaecological examination, normal leukocytes count, declining ESR, no fever, and elimination of bacteria. | |
| Notes |
Ethical approval: not mentioned. Informed consent: yes, women gave written informed consent. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 57/60 women were available for analysis. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women with severe PID. Exclusion criteria: pregnant or likely to be pregnant, allergic to penicillin or to cephalosporines, receiving concomitant treatment with allopurinol, needing to receive a different antibiotic during hospitalization, infectious mononucleosis. Number of women randomized: 152. Number of women analyzed: group A: 70; group B: 82. Number of withdrawals/exclusions/loss to follow‐up and reasons: 0. Number of centres: unclear. Age (mean) (years): group A: 26.9; group B: 25.1. Country: France. |
|
| Interventions |
Group A: amoxicillin‐clavulanic acid; while in hospital, 3 or 4 g per 24 h, by IV or perfusion, then 2 or 3 g per 24 h PO. Group B: parenteral: ampicillin 3 or 4 g IV per 24 h, OR amoxicillin 3 g IV per 24 h + gentamicin 160 mg IM per 24 h OR dibekacin 150 g IM per 24 h + metronidazole 1.5 g IV per 24 h. Oral administration: ampicillin 3 g per 24 h OR amoxicillin 2 g/24 h + metronidazole 1.5 or 2 g per 24 h. Additional treatments were limited, but could include: surgical or laparoscopic drainage of pus, local antibiotic for treatment of trichomonacides, non‐steroidal anti‐inflammatory drugs, corticoids. |
|
| Outcomes |
Primary outcomes: clinical cure at 10th day; clinical progress and improvement of biological parameters. Secondary outcome: length of hospital stay. |
|
| Notes |
Ethical approval: not stated. Informed consent: not stated. Source of funding: not stated. No conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how women were randomized. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 20% attrition. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants | Women referred to Department of Obstetrics and Gynecology with suspected PID with different degrees of severity (none, mild, moderate, and severe); 53% with mild degree of salpingitis. Inclusion criteria: history of lower abdominal pain < 3 weeks' duration, and presence of cervical motion tenderness, uterine and adnexal tenderness on bimanual examination. Exclusion criteria: use of antibiotics, had any gynaecological operation or instrumentation of the upper genital tract in the preceding month, had systemic disease or epilepsy, pregnant, suspected allergy to any of the drugs used, or puerperal infection. Number of women randomized: 40. Number of women analyzed: group A: 16; group B: 20. Number of withdrawal/exclusions/loss to follow‐up and reasons: 4 due to other diagnosis: toxoplasmosis, urinary infection, periappendicular abscess, no pathological findings. Number of centres: 1. Age (mean ± SD (range)) (years): group A: 29 ± 8 (18‐43); group B: 29 ± 1 (16‐50). Country: Finland. |
|
| Interventions |
Group A: ciprofloxacin 200 mg IV every 12 h for 2 days followed by 750 mg PO every 12 h to complete 14‐day course. Group B: doxycycline 100 mg IV every 12 h + metronidazole 500 mg IV every 8 h for the first 2 days, followed by doxycycline 150 mg PO every 24 h + metronidazole 400 mg PO every 8 h to complete a 14‐day course. |
|
| Outcomes |
Primary outcomes: clinical response based on a scale from 0 (absent or normal) to 3+ (severe) assessed on days 3, 6, 14, and 21 after the antimicrobial treatment was started. Failure defined as the presence of ≥ 1 of following criteria: no improvement in the clinical severity score at day 3 after the microbial treatment was started; CRP > 20 mg/L or a decline < 50% in the initial CRP level at day 6; positive cervical culture of N gonorrhoeae or C trachomatis at days 14 or 21; or the need for additional antimicrobial agents or surgical intervention. Secondary outcomes: adverse reactions and effects. |
|
| Notes |
Ethical approval: not stated. Informed consent: signed consent obtained. Source of funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated how randomization was done. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated if all women were assessed 21 days after the antimicrobial treatment was started. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: clinical diagnosis of acute PID hospitalized at 1 of 6 sites for treatment. Acute PID diagnosed in women with lower abdominal and pelvic pain and lower abdominal and cervical motion and adnexal tenderness in addition to ≥ 1 of the following: oral temperature ≥ 38.0 °C; leukocyte count ≥ 10,500/mm3; elevated ESR; endocervical specimen positive for Gram‐negative intracellular diplococci; endocervical or endometrial culture positive for N gonorrhoeae or C trachomatis; ultrasound findings consistent with an adnexal inflammatory mass; or purulence in or a positive culture of intraperitoneal material obtained at either culdocentesis or optional laparoscopy. Exclusion criteria: ruptured tubo‐ovarian or pelvic abscess; history of hypersensitivity to penicillin, cephalosporins, clindamycin, aminoglycosides, or tetracycline; among women with IUD in place, those who permitted the removal of the device within 48 h were included and those who did not were excluded; pregnancy or lactation; renal impairment (a serum creatinine level about 2 mg/dL), neutropenia (< 1000 neutrophils/mm3), receipt of another investigational drug or another antibiotic up to 2 weeks before the time of anticipated enrolment; and known or suspected active bacterial infection other than acute PID that might subsequently require concomitant therapy. Number of women randomized: 344. Number of women analyzed: group A: 109; group B: 110; group C: 108. Number of withdrawal/exclusions/loss to follow‐up and reasons: 52 women (15%) were not evaluable, 21 enrolled incorrectly (i.e. despite a violation of inclusion or exclusion criteria, or both), aII given an incorrect first dose of study drug or were subsequently given incorrect doses, 10 left hospital against medical advice, 5 treated for < 48 h, 2 withdrew, 2 had adverse reactions resulting in the cessation of treatment, and 1 given penicillin for the treatment of syphilis. Of the 2 women with adverse reactions, 1 developed blisters on her lips after the third dose of cefotetan + doxycycline, and the other developed hives after the initial dose of cefoxitin. All 5 women who were not evaluable because they received < 48 h of therapy had ≥ 1 abscesses, and 3/5 women underwent surgery because of worsening symptoms. 2/5 women were in group A, 2 were in group B, and 1 was in group 3. 20 of the unevaluable women were in group A, 20 were in group B, and 12 were in group C. Number of centres: 6. Age (mean ± SD) (years): success group: 24.7 ± 4.9; failure group: 26.2 ± 5.7. Country: US. |
|
| Interventions |
Group A: cefoxitin 2 g IV every 6 h + doxycycline 100 mg every 12 h; followed by doxycycline 100 mg PO twice daily for a total of 10‐14 days. Group B: clindamycin 900 mg IV + gentamicin 1.5 mg/kg every 8 h after an initial gentamicin loading dose calculated at 2 mg/kg followed by clindamycin 450 mg PO 4 times daily for a total of 10‐14 days. Group C: cefotetan 2 g IV + doxycycline 100 mg every 12 h, followed by doxycycline 100 mg PO twice daily for a total of 10‐14 days. |
|
| Outcomes |
Primary outcome: clinical cure defined as reduction of the severity score by ≥ 70%, with a normal temperature and leukocyte count. Secondary outcome: none reported. |
|
| Notes |
Ethical approval: consent forms were approved by the local institutional review board at each centre. Informed consent: women aged 16‐18 years old had to have parental consent, and women aged ≥19 years had to give their own consent. Source of funding: ICI Pharmaceuticals Group (now ZENECA, Inc.), Wilmington, Delaware. No conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described how randomization codes were generated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 292/343 women were analyzed (15% were not evaluable). |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women aged ≥ 18 years and receiving either hospital or outpatient care for uncomplicated acute PID and using a reliable form of contraception. Exclusion criteria: positive serological test for syphilis or evidence of a pelvic abscess on sonographic or laparoscopic examination. Number of women randomized: 686. Number of women analyzed: 669; group A: 343; group B: 326. Number of withdrawals/exclusions/loss to follow‐up and reasons: 17; 2 never randomized and in 15 it was impossible to confirm if they received study medication. Number of centres: 43. Age (mean ± SD) (years): group A: 29.0 ± 7.3; group B: 28.2 ± 7.2. Country: 14 countries. |
|
| Interventions |
Group A: moxifloxacin 400 mg PO once daily for 14 days. Group B: doxycycline 100 mg PO twice daily + metronidazole 400 mg PO 3 times daily for 14 days + ciprofloxacin 500 mg PO once. |
|
| Outcomes |
Primary outcome (modified ITT): clinical cure at 2‐14 days' post treatment: clinical success defined as cure (severity score reduced by ≥70%plus normal temperature and leukocyte count) or improvement (severity score reduced <70% but >30% plus normal temperature and leukocyte count). Therapy considered to have failed if symptoms and signs of infection persisted or worsened, as shown by persistent fever, leukocytosis, a reduction in severity score of ≤30%, or a combination of these. Clinical efficacy was 'unevaluable' when a woman could not be assessed. Secondary outcome (PP): microbiological clearance of chlamydia, microbiological clearance of gonorrhoea. |
|
| Notes |
Ethical approval: yes, study protocol prepared in accordance with the European Guidelines for Good Clinical Practice (1991) and National Rules and Regulations. Study conducted in accordance with the Declaration of Helsinki. Informed consent: not stated. Source of funding: Bayer Schering Pharma, Germany, provided financial and logistical support. No conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate (< 20%). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women with laparoscopically verified salpingitis. Exclusion criteria: none stated. Number of women randomized: 33. Number of women analyzed: 33; group A: 15; group B: 18. Number of withdrawals/exclusions/loss to follow‐up and reasons: 0. Number of centres: 1. Age (years): not stated. Country: Germany. |
|
| Interventions |
Group 1: ofloxacin 2 × 200 mg + metronidazole 2 × 500 mg, first IV and then PO for 10 days in total. Group 2: gentamicin 3 × 80 mg + clindamycin 4 × 600 mg (initially 1200 mg IV) for 10 days in total. |
|
| Outcomes | Primary outcome: clinical cure, no raw data reported for outcomes. | |
| Notes |
Ethical approval: judged by the ethics committee. Informed consent: yes, women gave written informed consent. Source of funding: not stated, and no conflicts of interest reported. All women hospitalized for the whole treatment. Only percentages and no numbers reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Prospective, randomized, double‐blind, double‐dummy, parallel‐group study. | |
| Participants | No significant differences between treatment groups in demographic characteristics. Inclusion criteria: diagnosis of uncomplicated PID based on presence of all following symptoms and signs: pelvic discomfort; direct lower abdominal tenderness; adnexal and cervical motion tenderness on bimanual vaginal examination; and ≥ 1 of the following signs: pyrexia (rectal, tympanic, or oral temperature > 38 °C or axillary temperature > 37.5 °C), CRP > 6 mg/L, WBC > 10,500/mm3, laparoscopic evidence of PID, cervical infection including mucopurulent cervical discharge or positive stain for Gram‐negative intracellular diplococci from the endocervix, and untreated, recent (< 14 days) documented gonococcal or chlamydial cervicitis. Exclusion criteria: pregnant or lactating; complicated PID (pelvic or tubo‐ovarian abscess ruled out by pelvic ultrasonography or laparoscopic examination within 48 h before or 24 h after the start of therapy) or any condition likely to require surgical intervention within 24 h of the start of treatment (or both); hypersensitivity to any study drug, related compound, or excipient; a history of tendon disorders associated with quinolones; history of clinically relevant cardiovascular abnormalities; history of epilepsy; defect in glucose‐6‐phosphate dehydrogenase; receipt of systemic antibacterial therapy ≤ 7 days before enrolment; history of uterine or pelvic or abdominal surgery ≤ 30 days before treatment; intolerance to or inability to follow an oral antibiotic regimen; impaired liver function (Child‐Pugh C) or transaminase levels > 5 times the upper limit of normal (or both); impaired renal function (creatinine clearance ≤ 50 mL/minute); neutropenia (< 1000/mm3); infection with HIV and CD4 count < 200/mm3; AIDS; and active antiretroviral therapy. Number of women randomized: 460. Number of women analyzed: ITT: group A: 228; group B: 232; PP: group A: 194; group B: 190. Number of withdrawals/exclusions/loss to follow‐up and reasons: group A: 34 (violations of inclusion/exclusion criteria (n = 11); not treated with study drug (n = 3), non‐compliance with study drug (n = 4), insufficient duration of therapy (n = 9), violation of time schedule (n = 5), informed consent withdrawn (n = 2), essential data missing/invalid (n = 14), lost to follow‐up (n = 2), use of prohibited concomitant medication (n = 4); group B: 42 (violations of inclusion/exclusion criteria (n = 16); not treated with study drug (n = 2), non‐compliance with study drug (n = 2), insufficient duration of therapy (n = 9), violation of time schedule (n = 6), informed consent withdrawn (n = 2), essential data missing/invalid (n = 18), lost to follow‐up (n = 2), use of prohibited concomitant medication (n = 6). Number of centres: 7. Age (mean ± SD) (years): group A: 35.2 ± 8.4; group B: 35.4 ± 8.7. Countries: China, Indonesia, South Korea, The Philippines, Pakistan, Thailand, Taiwan. |
|
| Interventions |
Group A: moxifloxacin 400 mg PO once daily for 14 days. Group B: levofloxacin 500 mg (2 x 250 mg tablets) PO once daily + metronidazole 500 mg (1 tablet) PO twice daily for 14 days. |
|
| Outcomes | Efficacy: primary efficacy variable was clinical response at test‐of‐cure in the PP population:
Secondary efficacy variables:
Safety: occurrence of adverse events (ITT population), including most commonly occurring drug‐related treatment‐emergent events seen in > 2% of either group (ITT/safety population). Safety evaluations included a physical examination (including vital signs) at enrolment, during treatment, at the test‐of‐cure visit and at follow‐up. Clinical laboratory assessments (for blood chemistry and haematological parameters) performed on blood samples taken within 48 h of the first dose of the study drug, and repeated at test‐of‐cure and follow‐up (in case abnormalities arose on or after the test‐of‐cure visit). |
|
| Notes | Study funded by Bayer HealthCare AG, Leverkusen, Germany. 1 author (PJ) received travel grants and consulting fees from GlaxoSmithKline and Sanofi‐Pasteur‐MSD, and consulting fees from Bayer HealthCare. 2 authors (QL and ZL) declared no conflicts of interest. 1 author (PR) was an employee of Bayer Vital GmbH; 2 authors (PA and BH) were employees of Bayer Schering Pharma. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random code provided by Bayer Biometry (Wuppertal, Germany); numbers assigned in sequential ascending order; no numbers left out or substituted. |
| Allocation concealment (selection bias) | Low risk | Provided by the investigator in sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel administering and assessing the outcome blinded to the treatment given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and personnel administering and assessing the outcome blinded to the treatment given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate (< 20%). |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Low risk | No other source of potential bias found for ITT analysis. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women with either laparoscopically confirmed salpingitis or histologically confirmed plasma cell endometritis or they met previously published criteria for non‐invasive diagnosis of salpingitis. Criteria included direct abdominal tenderness, cervical motion tenderness, and adnexal tenderness, plus ≥ 1 of the following: temperature ≥ 38 °C, peripheral blood leukocytosis WBC > 10,500/mm3, purulent material or culdocentesis, evidence of pelvic abscess on ultrasonography or pelvic examination, evidence of gonococcal or chlamydial cervicitis (by positive monoclonal antibody test or by Gram's stain showing gram‐negative intracellular diplococcic), or mucopurulent cervicitis as previously defined. Exclusion criteria: allergy to any of the 4 antibiotics involved in trial; pregnancy; or history of pelvic surgery, abortion, uterine curettage, or delivery within 6 weeks of admission. Number of women randomized: 162. Number of women analyzed: 148, group A: 75; group B: 73. Number of withdrawals/exclusions/loss to follow‐up and reasons: 14; reasons for exclusion: incorrect diagnosis that was discovered at laparoscopy or laparotomy, refusal of the woman to remain hospitalized long enough to complete treatment. Number of centres: 2. Age (mean ± SD) (years): total: 23.5 ± 6.1; group A: 23.3 ± 5.3; group B: 23.8 ± 6.0. Country: US. |
|
| Interventions |
Group A: cefoxitin 2 g IV every 6 h + doxycycline 100 mg IV every 12 h for minimum 4 days and at least 48 h after disappearance of fever. Women without fever on admission treated for minimum of 4 days in the hospital for at least 24 h beyond the adequate relief of pain and tenderness to a normal lifestyle without surgical intervention. After discharge from the hospital, doxycycline 100 mg PO twice daily was continued to complete a total of 14 days of treatment. Group B: clindamycin 600 mg IV every 6 h + tobramycin 2 mg/kg IV for 1 dose, followed by 1.5 mg/kg IV every 8 h for minimum of 4 days and for at least 48 h after disappearance of fever. Women without fever on admission treated for minimum of 4 days in hospital for at least 24 h beyond adequate relief of pain and tenderness to a normal lifestyle without surgical intervention. After discharge from hospital, clindamycin 450 mg PO 4 times daily continued to complete a total of 14 days of treatment. |
|
| Outcomes |
Primary outcome (ITT): clinical response for a satisfactory initial clinical response defined as an improvement of admitting signs and symptoms, included abdominal‐pelvic pain, fever, and pelvic tenderness. Follow‐up evaluation performed at hospital discharge and at 2‐6 weeks after initial enrolment. Secondary outcomes (PP): microbiological clearance of C trachomatis and reduction in tenderness score. |
|
| Notes |
Ethical approval: yes, study reviewed and approved by institutional review board at both hospitals. Informed consent: yes, written informed consent obtained from all women before enrolment. Source of funding: supported in part by National Institutes of Health grants AI12192 and 1PO1 AI24768 and by Merck, Sharp & Dohme, Westpoint, PA. No conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women aged ≥ 18 years hospitalized with 1 of the following conditions: endometritis, myometritis, postoperative infections in pelvic region, pelvic peritonitis, acute adnexal infections such as salpingitis or pyosalpinx. Exclusion criteria: allergy to clindamycin, lincomycin, metronidazole, or gentamicin; colitis when taking antibiotics; taken antibiotics in the 48 h before entering study; pregnant or lactating; vestibular or cochlear lesion; renal insufficiency (creatinine > 12 mg/L; WBC < 2000/mm2; platelets < 100,000/mm3; history of thrombopathy; peripheral neuropathy; participation in another clinical trial. Number of women randomized: 45; group A: 23; group B: 22. Number of women analyzed: 39; group A: 21; group B: 18. Number of withdrawals/exclusions/loss to follow‐up and reasons: group A: 2; group B: 4. All unexplained. Number of centres: 2. Age (mean ± SD) (years): group A: 27.9 ± 5.2; group B: 29.1 ± 9.5. Country: France. |
|
| Interventions |
Group A: clindamycin 900 mg IV every 8 h diluted in 150 mL (minimum volume) saline slow perfusion (30‐60 minutes) + gentamicin 1 mg/kg IM every 8 h (with minimum dose prescribed of 60 mg every 8 h, according to bodyweight). Treatment given for minimum of 5 days in hospital. Clindamycin perfusion not stopped until 48 consecutive h with temperature below 37.5 °C; at that point treatment could be PO. Maximum length of treatment at the discretion of the therapist. If also treated with a tetracycline, this was not prescribed < 48 h after the end of the treatment protocol (either clindamycin + gentamicin or metronidazole + gentamicin). Group B: metronidazole 500 mg every 8 h in slow IV perfusion (30‐60 minutes) + gentamicin 1 mg/kg IM every 8 h (with minimum dose prescribed of 60 mg every 8 h, according to bodyweight). Treatment given for 6 weeks. |
|
| Outcomes |
Primary outcome: clinical cure: absence of infection in the days following cessation of treatment according to clinical observations, microbe eradicated during or after treatment. Secondary outcome: length of hospital stay. |
|
| Notes |
Ethical approval: not stated. Informed consent: not stated. Source of funding: not stated, and no conflicts of interest reported. Mean days of hospitalization: 11.17 days. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition < 20%. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women with first episode of PID. Exclusion criteria: women with recurrent PID or with previous antibiotic therapy. Number of women randomized: 165. Number of women analyzed: 153 women; group A: 52; group B: 48; group C: 53. Number of withdrawals/exclusions/loss to follow‐up and reasons: group A: 3 women excluded from analysis; group B: 7; group C: 2. Number of centres: 1. Age (mean ±SD) (years): group A without women excluded from analysis: 25.6 ± 3.9; group B without women excluded from analysis: 25.8 ± 3.2; group C without women excluded from analysis: 25.5 ± 4.9. Country: India. |
|
| Interventions |
Group A: ciprofloxacin 500 mg + tinidazole 600 mg (Table Brakke, Franko India Pharma, Mumbai, India) twice daily for 7 days. Group B: fluconazole 150 mg (1 tablet) + azithromycin 1 g (1 tablet) + secnidazole 2 g (2 tablets) (Fas‐3 kit Lyka, Mumbai, India). Advised to take azithromycin on empty stomach in the morning, secnidazole with or after food and fluconazole in the evening. Group C: doxycycline 100 mg twice daily + metronidazole 200 mg 3 times daily for 1 week. The 2 drugs were available in the hospital pharmacy free of cost. |
|
| Outcomes |
Primary outcome: clinical cure: women assessed at the first visit by a severity score (Modified Severity Score of Soper 1988) the findings at the first examination and severity score noted in the Performa filled for every woman. The women were asked to report after 1 week and 4 weeks. Repeat gynaecological examination and severity score were performed and recorded in the Performa. All women were advised to report within 3 days if there was no improvement in symptoms, any deterioration in their condition, or inability to carry on with the oral therapy when they were hospitalized. Clinical cure defined as at least 70% reduction in severity score, no more than mild abdominal pain, and no recurrence of symptoms or signs of PID within 4 weeks of therapy. Treatment failure defined as < 20% decrease in tenderness score. Modified ITT (cases that were not PID were excluded). Group A: 52. Group B: 48. Group C: 53. |
|
| Notes |
Ethical approval: yes, the departmental ethical committee approved the study. Informed consent: yes, verbal informed consent taken from all the women. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated number, all women were randomized to 1 of 3 treatment groups initially, and later it was a block randomization to equalize the group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported by whom allocation was done and how. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Unfortunately we could not get similar looking packs from the market so the assignment was not concealed from the investigator." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Unfortunately we could not get similar looking packs from the market so the assignment was not concealed from the investigator." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12/165 women excluded from analysis. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women aged 15‐51 years who required hospitalization for treatment of acute PID based on clinical and laboratory evidence. All women had abdominal, parametrial, and cervical motion tenderness. The additional finding of fever, leukocytosis, pelvic mass, or purulent material in the peritoneal cavity confirmed the diagnosis. Exclusion criteria: allergies to study drugs, requirement for concomitant therapy with other antibiotics, and > 2 doses of antibiotics in the 7 days prior to admission. Number of women randomized: 170 women; group A: 88; group B: 82. Number of women analyzed: 170 women; group A: 88; group B: 82. Number of withdrawals/exclusions/loss to follow‐up and reasons: group A: 28; group B: 27 due to failure to follow randomization scheme, protocol deviation, and incorrect diagnoses. Number of centres: 10. Age (mean) (years): whole study group: 28; age per group not stated. Country: 9 in Europe and 1 Africa. |
|
| Interventions |
Group A: clindamycin 900 mg IV every 8 h + gentamicin 2 mg/kg IV, followed by 1.5 mg/kg IV every 8 h for minimum of 4 days. At the end of the period of IV therapy, clindamycin 450 mg PO every 6 h was given to complete 14 days of treatment. Group B: cefoxitin 2 g IV every 6 h + doxycycline 100 mg IV every 12 h were given for at least 4 days. At the end of IV therapy, doxycycline 100 mg PO was given every 12 h to complete a total of 14 days of treatment. |
|
| Outcomes |
Primary outcomes: clinical failure, minimum of 48 h of protocol therapy and characterized by signs and symptoms as unchanged or worsened during the first 48‐72 h of treatment, or worsening later, failure to improve further; need of additional antibiotics or need for surgery considered as failure; adverse events leading to discontinuation of therapy. Secondary outcomes: microbial cure of C trachomatis and N gonorrhoeae. |
|
| Notes |
Ethical approval: not stated. Informed consent: yes, informed consent obtained from all women prior to entry into the trial. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated how randomization sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated by whom allocation concealment was done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 115/170 women analyzed (68%). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: diagnosis of PID based upon the following signs and symptoms: oral body temperature ≥ 38.3 °C, lower abdominal tenderness, cervical or uterine tenderness on palpation and motion, and tenderness on palpation of adnexa. In addition, the following may have been present: purulent endocervical discharge, WBC ≥ 14,000/mm3, adnexal mass or abscess, nausea, and vomiting. Women selected for the uncomplicated PID group if the above criteria were met, but no adnexal mass was noted on palpation, ultrasonography, or at the time of surgery. In the complicated PID group, women with tubo‐ovarian complex did not meet the above criteria and had evidence of a unilateral or bilateral adnexal mass on pelvic examination, not confirmed by a radiolucent area on ultrasound examination or surgery. Women with tubo‐ovarian abscess had the above findings including a radiolucent area on ultrasound, consistent with an abscess or pus‐filled cavity noted at surgery. Exclusion criteria: not stated. Number of women randomized: 99. Number of women analyzed: 94. Number of withdrawals/exclusions/loss to follow‐up and reasons: 5 women were excluded from evaluation due to protocol violations. Number of centres: not stated. Age (mean ± SD) (years): group A: 26 ± 7; group B: 25 ± 7; group C: 26 ± 7. Country: not stated. |
|
| Interventions |
Group A: cefoxitin 2 g every 6 h for minimum of 4 days and continued until the woman was apyrexial with improvement of symptoms for at least 48 h. Group B: cefotaxime 2 g every 8 h for minimum of 4 days and continued until the woman was apyrexial with improvement of symptoms for at least 48 h. Group C: clindamycin 900 mg every 8 h + gentamicin at initial loading dose of 120 mg, followed by maintenance doses of 80 mg every 8 h. Subsequent maintenance doses determined by evaluating trough and peak serum aminoglycoside concentrations. Antibiotics given for minimum of 4 days, and continued until the woman was apyrexial with improvement of symptoms for at least 48 h. |
|
| Outcomes | Primary outcome: clinical failure: evaluated on a daily basis. Women who had not demonstrated signs of improvement after 48‐72 h of antibiotic therapy were considered an unsuccessful result. | |
| Notes |
Ethical approval: not stated. Informed consent: not stated. Source of funding: not stated, and no conflicts of interest declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | There were 2 randomization codes, 1 for each diagnosis group. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94/99 women included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: non‐pregnant, non‐lactating women using a reliable form of contraception and who fulfilled the diagnostic criteria for uncomplicated PID. Diagnostic inclusion criteria for uncomplicated PID were all the following: direct lower abdominal tenderness with or without rebound tenderness, cervical motion tenderness, and adnexal tenderness. Plus ≥ 1 of the following: recent positive endocervical culture for N gonorrhoeae or C trachomatis, temperature > 38 °C), WBC count > 10,000/mm3, leukocytic endocervical discharge. Exclusion criteria: pelvic infection severe enough to require parenteral antimicrobial therapy or if surgical intervention within the next 24 h was anticipated; evidence of a pelvic abscess by ultrasonography or clinical examination; if pain had been present for > 2 weeks; allergy to study medications; major gastrointestinal, renal, or hepatic disorders; used other antimicrobial agents within the previous 2 weeks; IUD in place; or alcohol or drug abusers. Number of women randomized: total: 295; group A: 150; group B: 145. Number of women analyzed: total: 249; group A: 128; group B: 121. Number of withdrawals/exclusions/loss to follow‐up and reasons: 46 women excluded from analysis due to protocol violations or loss to follow‐up. Number of centres: 16. Age (mean ± SD) (years): group A: 25.9 ± 5.8; group B: 26.0 ± 6.8. Country: US. |
|
| Interventions |
Group A: ofloxacin 400 mg PO every 12 h for 10 days. Group B: cefoxitin 2 g IM + probenecid 1 g PO, followed by doxycycline 100 mg PO every 12 h for 10 days. |
|
| Outcomes |
Primary outcomes: clinical cure: complete resolution of tenderness; clinical improvement: partial resolution of tenderness without the need for additional antibiotic therapy. Secondary outcomes: microbial cure of C trachomatis, microbial cure of N gonorrhoeae. |
|
| Notes |
Ethical approval: yes, "with approval from their respective institutional review boards." Informed consent: yes, "the subjects were enrolled after giving informed consent." Source of funding: supported in part by a grant from Ortho Pharmaceutical Corporation, Raritan, New Jersey. No conflicts of interest declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After obtaining informed consent, women given a computer‐generated randomization code number and assigned to either treatment regimen using 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46/295 women excluded from analysis due to protocol violations or loss to follow‐up (< 20%). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women aged ≥ 16 years, inpatients and outpatients; intrauterine infection, uterine adnexitis, bartholinitis, or bartholin's abscess. Exclusion criteria: age < 16 years; premedication with ciprofloxacin or cefroxadine; women improving with other treatments; allergy to cephem or pyridonecarboxylic acid; taking theophylline or fenbufen, severe problems in the heart, liver, or kidneys; women with epilepsy; pregnant or breastfeeding; rejection by the doctor. Number of women randomized: total: 253; group A: 124; group B: 129. Number of women analyzed: total: 209; group A: 104; group B: 105. Number of withdrawals/exclusions/loss to follow‐up and reasons: total: 44; group A: 20; group B: 24. No reasons presented. Number of centres: 55. Age (years): only presented as frequencies within age groups. Country: Japan. |
|
| Interventions |
Group A: ciprofloxacin 200 mg PO + dummy cefroxadine placebo, 3 times daily for 7 consecutive days. Group B: cefroxadine 250 mg PO + dummy ciprofloxacin placebo, 3 times daily for 7 consecutive days. |
|
| Outcomes |
Primary outcomes: clinical cure at 7th day of treatment: excellent: clinical marked improvement and clearance of bacteria; good: clear clinical improvement; poor: no clear clinical improvement; adverse events. Secondary outcome: local tenderness around uterus. |
|
| Notes |
Ethical approval: not stated. Informed consent: not stated. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 2 ciprofloxacin and 2 cefroxadine each with dummy placebo were arranged for 7‐day‐use and numbered. Numbers randomly assigned. 4 series of numbered medicine packed in a box. Then, some boxes were delivered to every institution. |
| Allocation concealment (selection bias) | Unclear risk | Having used numbered series of medicine to women consecutively. Block size of 4 and might be too small for concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo method. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind method. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition < 20%. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women with uncomplicated PID, confirmed by absence of pelvic or tubo‐ovarian abscess on transabdominal/transvaginal pelvic ultrasound or laparoscopy (within 2 days before or 1 day after the start of treatment), or both ultrasound and laparoscopy. Diagnosis of PID was based on the presence of all the following: pelvic discomfort, direct lower abdominal tenderness with or without rebound tenderness, and adnexal/cervical motion tenderness on bimanual vaginal examination. In addition, ≥ 1 of the following signs: raised temperature (> 37.5 °C); ESR > 15 mm in the first hour; CRP value above the upper limit of the normal range; WBC count > 10,500/mm3; laparoscopic evidence of PID; signs suggestive of cervical infection (e.g. mucopurulent cervical discharge); or untreated, documented gonococcal or chlamydial cervicitis within the previous 14 days. Exclusion criteria: contraindications to study drugs; required surgery within the next 24 h or had a history of uterine or pelvic or abdominal surgery within the past 30 days; or previous treatment with systemic antibiotic therapy in the last 7 days. Number of women randomized: 749. Number of women analyzed: group A: 384; group B: 365. Number of withdrawals/exclusions/loss to follow‐up and reasons: group A: 51 (24 due to adverse events; 14 consent withdrawn; 7 lost to follow‐up; 2 non‐compliant; 1 protocol violation; 2 insufficient therapeutic effect; 1 investigator decision); group B: 38 (17 due to adverse events; 9 consent withdrawn; 5 lost to follow‐up; 4 non‐compliant; 3 protocol violation). Number of centres: 13. Age (mean ± SD) (years): group A: 30.1 ± 8.4; group B: 30.5 ± 8.5. Countries: Denmark; Finland; France; Germany; Greece; Hungary; Italy; Lithuania; Poland; Russia; South Africa; Sweden; UK. |
|
| Interventions |
Group A: moxifloxacin 400 mg PO once daily for 14 days. Group B: ofloxacin 400 mg PO twice daily + metronidazole 500 mg PO twice daily for 14 days. |
|
| Outcomes |
Primary outcome: clinical cure (5‐24 days post‐therapy): reduction of the pelvic pain score by > 70% (McCormack score, table A) + apyrexia (rectal/tympanic/oral temperature < 38.0 °C or axillary/cutaneous temperature < 37.5 °C) + WBC count < 10,500/mm3. Secondary outcomes: microbial cure of C trachomatis; microbial cure of N gonorrhoeae. |
|
| Notes |
Ethical approval: study protocol prepared in accordance with the Declaration of Helsinki and ethical approval obtained for each centre. Informed consent: written, informed consent obtained from each woman. Source of funding: grant from Bayer HealthCare. No competing interests declared for 4 authors (HC, TP, IR, or DV). 1 author (JR) received payment as a consultant and lecturer, and sponsorship to attend medical conferences from Bayer HealthCare. 1 author (JR) was an associate editor of Sexually Transmitted Infections. 4 authors (AK, MA, PA, and PR) were employees of Bayer HealthCare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No description of how randomization was generated. Author retrieved information from full report. |
| Allocation concealment (selection bias) | Low risk | No description if allocation was concealed. After consulting the full report, the author stated that allocation was made by the pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding achieved by dispensing medication with identical packaging (blister packs) and appearance (all drugs and placebo tablets were encapsulated). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition < 20%. |
| Selective reporting (reporting bias) | Low risk | Trial protocol was published in www.stijournal.com/supplemental. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: not stated. Exclusion criteria: not stated. Number of women randomized: 46 (36 with acute PID). Number of women analyzed: group A: 19; group B: 9; group C: 9. Number of withdrawals/exclusions/loss to follow‐up and reasons: all 46 women completed the study; however, only 36 had acute PID. Number of centres: 1. Age (years): not stated. Country: US. |
|
| Interventions |
Group A: cefotaxime 2 g IV or IM every 8 h. Group B: clindamycin 600 mg IV every 6 h + gentamicin 1.5 mg/kg lean bodyweight IV or IM every 8 h. Group C: clindamycin 600 mg IV every 6 h + gentamicin 1.5 mg/kg lean bodyweight IV or IM every 8 h + penicillin G 5 million units IM every 4 h. |
|
| Outcomes |
Primary outcomes: clinical cure: antibiotic change was made for treatment failure from an assigned regimen based upon persistence or worsening of signs and symptoms after 48 h. Secondary outcomes: none reported. |
|
| Notes |
Ethical approval: not stated. Informed consent: yes, enrolled after informed consent obtained. Source of funding: not stated, and no conflicts of interest reported. Range of hospital stay: 3‐11 days. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomized completed the study. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: lower abdominal pain and tenderness, cervical motion or adnexal tenderness, and 1 of the following: oral temperature > 38 °C, leukocytosis > 10,500/mm3, or presence of a suspected inflammatory pelvic mass on examination or by ultrasound. Exclusion criteria: allergy to cephalosporins or penicillins; had taken antibiotics in the previous 3 days; had received any investigational drugs in the previous 30 days; pregnant or breastfeeding; rapidly progressive underlying disease that could preclude evaluation of therapy; or required other systemic antibiotics on admission. Number of women randomized: 67. Number of women analyzed: total: 67; group A: 13; group B: 14; group C: 19; group D: 21. Number of withdrawals/exclusions/loss to follow‐up and reasons: 3 women, 1 because of protocol violation and 2 left the study before completion of therapy. Number of centres: 1. Age (mean ± SEM) (years): group A: 26.6 ± 1.9; group B: 28.9 ± 1.7; group C: 27.1 ± 1.3; group D: 28.3 ± 1.0. Country: US. |
|
| Interventions |
Group A: ceftizoxime 2 g IV every 12 h + doxycycline 100 mg IV twice daily. Group B: ceftizoxime 2 g IV every 6 h + doxycycline 100 mg IV twice daily. Group C: ceftizoxime 2 g IV every 8 h + doxycycline 100 mg IV twice daily. Group D: clindamycin 900 mg IV every 8 h + gentamicin 2 mg/kg loading dose followed by 1.5 mg/kg IV every 8 h with adjustments if necessary. |
|
| Outcomes |
Primary outcomes: clinical cure: adequate response to therapy: clinically improved and afebrile for 48 h at the time of discharge; 8‐24 before discharge; no pelvic tenderness; adverse events leading to discontinuation of therapy. Secondary outcomes: microbial cure of C trachomatis, microbial cure of N gonorrhoeae, and length of hospital stay. |
|
| Notes |
Ethical approval: not stated. Informed consent: yes, women signed informed consent forms previously approved by institutional review board. Source of funding: financial assistance, in part, provided by Smith Kline & French Laboratories, Philadelphia, PA. No conflicts of interest reported. Follow‐up: 10‐14 days. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women withdrew from the study or were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: history of pelvic discomfort for < 30 days, with findings of pelvic organ tenderness (uterine or adnexal) on bimanual examination, and leukorrhoea or mucopurulent cervicitis. Exclusion criteria: current urinary infection; pregnancy; presence of tubo‐ovarian abscess, endometriosis, appendicitis, diverticulitis, haemorrhagic ovarian cysts or torsion; abdominal hernia; homelessness; fever > 38 °C; abdominal rebound tenderness; pelvic pain > 30 days' duration; allergy to ceftriaxone, azithromycin, or doxycycline; history of antimicrobial therapy within 7 days of recruitment; delivery, abortion, or gynaecological surgery within 30 days; prior hysterectomy or bilateral salpingectomy; and oral intolerance for the antibiotics. Number of women randomized: 133. Number of women analyzed: group A: 66; group B: 67. Number of withdrawals/exclusions/loss to follow‐up and reasons: group A: 4 lost to follow‐up, 2 discontinued intervention (1 oral intolerance, 1 worsening of the pain); group B: 7 lost to follow‐up: 9 discontinued intervention (2 oral intolerance, 7 worsening of pain). Number of centres: 1. Age (mean ± SD) (years): group A: 28.3 ± 0.8; group B: 29.27 ± 1.1. Country: Brazil. |
|
| Interventions |
Group A: ceftriaxone IM 250 mg + azithromycin 1 g PO single dose and repeated after 7 days. Group B: ceftriaxone IM 250 mg + doxycycline 200 mg PO for 14 days. |
|
| Outcomes |
Primary outcome: clinical cure defined as ≥ 70% reduction in the total tenderness score at day 14 compared with baseline, for both visual analogue scale and McCormack pain scale. Secondary outcome: microbial cure of C trachomatis. |
|
| Notes |
Ethical approval: study protocol approved by ethics committee of Hospital de Clínicas de Porto Alegre, and registered at isrctn.org under ISRCTN46117662. Informed consent: enrolled in study after signing the informed consent. Source of funding: supported by Grupo de Pesquisa e Pos‐Graduacão Do Hospital de Clínicas de Porto Alegre under grant # 03/006. GenProbe (San Diego, CA) donated the kits to run the bacteriological analysis, and Pfizer (New York, NY) donated azithromycin to the Global Program to Eliminate Trachoma (Dr Schachter had a National Institutes of Health grant to do operational research on azithromycin treatment of trachoma, and the company donated the drug). The drug used in this study was obtained independently in Brazil, without Pfizer support. The other authors had no potential conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated table for allocation sequence. Women allocated in blocks of 4. |
| Allocation concealment (selection bias) | Low risk | To avoid bias, both medications were manipulated by the hospital pharmacy and put in identically coded blisters and capsules. Because of the difference in the number of capsules in each treatment, the empty azithromycin blisters were filled with placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and assessors blinded to group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women and assessors blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate (< 20%). |
| Selective reporting (reporting bias) | Low risk | Trial protocol published (ISRCTN46117662). |
| Other bias | Unclear risk | The drug used in this study was obtained independently in Brazil, without Pfizer support. Analysis was provided as modified ITT, where conditions others than PID were excluded from analysis after randomization. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: aged > 16 years old; give written consent prior to entry; clinical diagnosis of acute PID made when woman had all following: lower abdominal tenderness, adnexal tenderness, and cervical motion tenderness; ≥ 1 of following: oral temperature ≥ 38.3 °C, abnormal cervical discharge, elevated ESR or CRP, and endocervical specimen positive for N gonorrhoeae or C trachomatis. Exclusion criteria: pregnant or lactation; history of hypersensitivity to penicillin, aminoglycoside, clindamycin, or metronidazole; severe hepatic disease; renal impairment (serum creatinine level > 2 mg/dL), or evidence of ruptured tubo‐ovarian abscess. Number of women randomized: 44; 22 in each group. Number of women analyzed: 44; 22 in each group. Number of withdrawals/exclusions/loss to follow‐up and reasons: 0 reported as lost to follow‐up. Number of centres: 1. Age (mean ± SD) (years): group A: 31.2 ± 9.1; group B: 24.9 ± 7.6. Country: Thailand. |
|
| Interventions |
Group A: (triple therapies) received intravenous therapy of 1 gm of ampicillin every 6 hours plus 5 mg/kg. (not exceed 240 mg) of gentamicin once daily and 500 mg of Metronidazole every 8 hours. Group B: clindamycin 600 mg IV every 8 h + gentamicin 5 mg/kg not exceeding a maximum dose of 240 mg once daily. In both groups, parenteral therapies continued until women were afebrile for minimum of 48 h then all women received a regimen of doxycycline 100 mg PO every 12 h to complete a 14‐day course. |
|
| Outcomes |
Primary outcomes: clinical cure; adverse events leading to discontinuation of therapy. Secondary outcome: length of hospital stay. Visual analogue scale on day 3 of treatment. |
|
| Notes |
Ethical approval: yes, approval for study obtained from the Ethical Committee of the Faculty of Medicine, Chulalongkorn University before trial was started. Informed consent: yes, all women gave written consent before entering into study. Source of funding: not stated, and no conflicts of interest reported. There were significant baseline imbalances between the 2 groups. Age, infertility, and severity of pain prior to this study significantly lower in group B (clindamycin/gentamicin). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization codes computer‐generated and sealed in envelope, then women were randomly assigned to 1 of the 2 regimens depending on their codes (A or B). |
| Allocation concealment (selection bias) | Unclear risk | Not stated by whom allocation concealment was done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: written informed consent; met criteria for diagnosis of PID with the following: lower abdominal pain and bilateral adnexal tenderness on bimanual pelvic examination; microscopy of a wet mount of the vaginal contents revealing marked increase in the number of leukocytes (i.e. leukocytes outnumbered all other cellular elements in the smear); ≥ 2 of the following: temperature > 38 °C, leukocytosis (> 11,000/mm3), purulent material from the peritoneal cavity by culdocentesis, inflammatory complex on bimanual examination or sonography, ESR > 20 mm/h; uncomplicated salpingitis limited to tube(s) or ovary(ies) (or both) without pelvic peritonitis; complicated (inflammatory mass or abscess involving tube(s) or ovary(ies) (or both) with or without pelvic peritonitis. Exclusion criteria: pregnant; history of allergy to 1 of study drugs. Number of women randomized: 62; 31 in each group. Number of women analyzed: 62; 31 in each group. Number of withdrawals/exclusions/loss to follow‐up and reasons: 0 reported as lost to follow‐up. Number of centres: 1. Age (mean ± SD) (years): group A: 23.4 ± 5.8; group B: 21.9 ± 3.7. Country: US. |
|
| Interventions |
Group A: cefoxitin 2 g IV every 6 h + doxycycline 100 mg IV every 12 h. Women discharged with doxycycline 100 mg PO twice daily to complete a 10‐day course. Group B: clindamycin 600 mg IV every 6 h + amikacin 7.5 mg/kg IV every 12 h. Women discharged with clindamycin 300 mg PO 4 times daily to complete a 10‐day course. |
|
| Outcomes |
Primary outcome: clinical failure: persistence of fever (> 38 °C), elevated WBC (> 11,000/mm3), moderate‐severe pelvic organ tenderness despite 96 h of antibiotic therapy, need of laparotomy. Secondary outcome: length of hospital stay. |
|
| Notes |
Ethical approval: not stated. Informed consent: yes, enrolled after obtaining informed consent according to guidelines of the Human Research Committee. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women admitted to hospital and assigned randomly in a double‐blind fashion, to 1 of 2 treatment regimens by sealed envelope, generated from a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation reported to have taken place in sealed envelopes, but it was not reported who distributed them. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated who was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | High risk | Significant baseline imbalances between the 2 groups. Age, infertility, and severity of pain prior to this study was significant lower in group B (clindamycin/gentamicin). |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: none stated. Exclusion criteria: none stated. Number of women randomized: 60; 30 in each group. Number of women analyzed: 60; 30 in each group. Number of withdrawals/exclusions/loss to follow‐up and reasons: 0 reported as lost to follow‐up. Number of centres: 1. Age (mean ± SD) (years): group A: 24.2 ± 5.1; group B: 24.7 ± 7.2. Country: US. |
|
| Interventions |
Group A: moxalactam 2 g IV every 8 h. Group B: clindamycin 600 mg IV every 6 h + tobramycin 1.5 mg/kg every 8 h. |
|
| Outcomes |
Primary outcome: microbiological cure. Secondary outcome: length of hospital stay. |
|
| Notes |
Ethical approval: not stated. Informed consent: yes, all women gave informed consent approved by the University of California (San Francisco) Committee on Human Research. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women randomized using a computer‐generated randomized schedule. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women of reproductive age who had clinical features of PID, acute salpingitis, or suspected pelvic abscess, such as lower abdominal pain associated with fever and chills, and cervical motion tenderness with or without signs of adnexal masses; required hospitalization for IV antibiotic therapy. In all cases, CDC criteria for PID satisfied. Exclusion criteria: suspected or known to be pregnant; breastfeeding. Number of women randomized: 71; group A: 35; group B: 36. Number of women analyzed: 61; group A: 31; group B: 30. Number of withdrawals/exclusions/loss to follow‐up and reasons: 10 women; group A: 4; group B: 6 later excluded from evaluation due to wrong diagnosis or protocol violations. Number of centres: not stated. Age (mean (range)) (years): group A: 27.5 (15‐37); group B: 26.5 (19‐36). Country: not stated. |
|
| Interventions |
Group A: ciprofloxacin 300 mg IV twice daily for ≥ 3 days followed by ciprofloxacin 500 mg PO twice daily for about 1 week. Group B: clindamycin 600 mg IV every 6 h + gentamicin 80 mg IV every 8 h, administered separately. Clindamycin by IV route for 3 days, followed by oral administration for about 1 week. Gentamicin dose adjusted based on serum creatinine and gentamicin levels. |
|
| Outcomes |
Primary outcomes: clinical cure: when there was resolution or clearing (or both) of signs of infection as evidenced by defervescence, reversal of leukocytosis, and abatement of abdominal pain and cervical motion tenderness; adverse events leading to discontinuation of therapy. Secondary outcome: length of hospital stay. |
|
| Notes |
Ethical approval: study protocol approved by the Human Rights Committee of their institution. Informed consent: yes, all women entered in study were informed in full and required to read and sign a consent form. Source of funding: supported by Miles Laboratory, New Haven, CT, a subsidiary of Bayer, Leverkusen, West Germany. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated randomization scheme. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10/71 women not analyzed (14%). |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: upper genital tract infection, pelvic pain (bilateral, unilateral), fever > 37.5 °C, leukorrhoea, bleeding, digestive and urinary signs, pain in right hypochondrium. Diagnosis confirmed by laparoscopy. Exclusion criteria: pregnant or likely to be; allergy to penicillin or cephalosporins; receiving concomitant treatment with allopurinol; already received an antibiotic before admission; renal insufficiency. Number of women randomized: 40; 20 in each group. Number of women analyzed: 40; 20 in each group. Number of withdrawals/exclusions/loss to follow‐up and reasons: 1 woman withdrawn during hospital admission; 13 women lost to follow‐up. Number of centres: 1. Age (mean (range)) (years): group A: 27.5 (17‐39); group B: 22.5 (17‐34). Country: France. |
|
| Interventions |
Group A: amoxicillin‐clavulanate 1 g slow IV perfusion every 8 h. PO once temperature normal for 48 h, 4 tablets of 500 mg, 2 at a time as long as hospitalized. After discharge, treatment continued with 3 tablets daily of amoxicillin‐clavulanate for 3 weeks. Group B: penicillin G 10 million units/24 h IV, followed by penicillin V PO + gentamicin 160 mg/24 h IM for 10 days + metronidazole 500 mg IV 3 times daily followed by metronidazole 500 mg PO 3 times daily. Duration of treatment unclear. |
|
| Outcomes | Effectiveness: cure: absence of pelvic pain on examination and referred by women, normal body temperature at discharge and 3 weeks later. Adverse events: any antibiotic‐related adverse event leading to discontinuation of therapy during hospital stay and after 30 days. |
|
| Notes |
Ethical approval: not stated. Informed consent: not stated. Source of funding: not stated, and no conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Not possible to identify. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: women hospitalized from July 1986 to December 1988 with clinical diagnosis of acute PID diagnosed using the criteria proposed by Hager and colleagues (Hager 1983), and candidates for therapy. Exclusion criteria: aged < 15 years; pregnant; allergic to 1 of the study drugs or penicillin; concomitant infection requiring another antibiotic; serum creatinine level > 1.5 mg/dL; received any antibiotic therapy during the past 7 days; or pelvic or abdominal surgery in the past 30 days. Number of women randomized: 147; unclear how many in each group. Number of women analyzed: group A: 63; group B: 67. PP analysis only. Number of withdrawals/exclusions/loss to follow‐up and reasons: 17 women received < 48 h of protocol therapy and were removed from the study. Of these exclusions, diagnosis was made in error in 11 women; 2 did not meet the inclusion criteria; 2 required emergency surgery for ruptured tubo‐ovarian abscess within 24 h of admission and 2 left hospital against medical advice. Number of centres: 1. Age (mean ± SD) (years): total age not stated; group A: 25.4 ± 7.7; group B: 24.5 ± 7.8. Country: US. |
|
| Interventions |
Group A: gentamicin 2.0 mg/kg IV as loading dose, then 1.5 mg/kg IV every 8 h + clindamycin 900 mg IV every 8 h for minimum 4 days. After discharge, clindamycin 450 mg PO every 6 h for total 14 days. Group B: cefoxitin 2 g IV every 6 h + doxycycline 100 mg IV every 12 h for minimum 4 days. After discharge, doxycycline 100 mg PO every 12 h for total 14 days. |
|
| Outcomes |
Primary outcomes: clinical cure defined as oral temperature < 38 °C for 48 h, resolution of pain and tenderness, and no increase in the size of any pelvic mass after 21 days of initiation of treatment. Secondary outcomes: microbiological clearance of chlamydia; microbiological clearance of gonorrhoea after 21 days of initiation of treatment. |
|
| Notes |
Ethical approval: not stated. Informed consent: informed consent obtained and approved by Institutional Review Board of The University of Texas Health Science Center at San Antonio. Source of funding: supported by grant from the Upjohn Co., Kalamazoo, MI. No conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 147 women randomized, 17 received < 48 h of protocol therapy and were removed from the study. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
| Methods | Randomized controlled trial. | |
| Participants |
Inclusion criteria: non‐pregnant, non‐lactating women using a reliable form of contraception who met the inclusion criteria for acute salpingitis. Exclusion criteria: pelvic infection severe enough to require parenteral antimicrobial therapy (diffuse peritonitis) or if surgical intervention within the next 24 h was anticipated; evidence of a pelvic abscess on sonographic or clinical examination. Number of women randomized: 96; unclear how many to each group. Number of women analyzed: group A: 35; group B: 37. PP analysis only. Number of withdrawals/exclusions/loss to follow‐up and reasons: 24 women considered unevaluable because of total non‐compliance with study drug (1 woman) or lack of attendance at any follow‐up visits (23 women). Number of centres: 1. Age (mean) (years): total age not stated; group A: 24.7; group B: 23.3. Country: US. |
|
| Interventions |
Group A: cefoxitin 2 g IM + probenecid 1 g PO followed by doxycycline 100 mg PO every 12 h for 10 days. Group B: ofloxacin 400 mg PO every 12 h for 10 days. |
|
| Outcomes |
Primary outcomes: clinical cure defined as complete resolution of tenderness (> 65% decrease in clinical score); adverse events leading to discontinuation of therapy. Secondary outcomes: microbiological clearance of chlamydia; microbiological clearance of gonorrhoea. |
|
| Notes |
Ethical approval: unclear. Study approved by Texas Southwestern Medical Center Institutional Review Board. Informed consent: women enrolled after they gave informed consent. Source of funding: supported in part by a grant from Ortho Pharmaceutical Corporation, Raritan, NJ. No conflicts of interest reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24/96 women considered unevaluable. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol found. |
| Other bias | Unclear risk | Not stated. |
CDC: Centers for Disease Control and Prevention; ESR: erythrocyte sedimentation rate; h: hour; IM: intramuscular; ITT: intention to treat; IV: intravenous; n: number of women; PID: pelvic inflammatory disease; PO: per os; PP: per protocol; SD: standard deviation; IUD: intrauterine device.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acar 1989 | Results not separate for PID. |
| Andersson 1980 | Quasi‐RCT and not randomized to doxycycline. |
| Bartlett 1982 | Not an RCT. |
| Berkeley 1986 | Results not separate for PID. |
| Blanco 1983 | Results not separate for PID. |
| Brihmer 1988 | Did not report effectiveness or safety outcomes. |
| Brihmer 1989 | Not a comparison of interest. Group A: doxycycline 200 mg IV the first day, followed by 100 mg PO daily for 10 days + benzylpenicillin‐procaine 2.25 millions IM twice daily for 2 days. Group B: trimethoprim 160 mg + sulfamethoxazole 800 mg IV twice daily the first day, followed by 800 mg PO for 10 days. |
| Bruhat 1986 | Not a comparison of interest. Group A: sulbactam 2 g daily IV + ampicillin 4 g daily for 5 days, followed by 15 days of sulbactam IM + ampicillin 2 g daily (n = 9) Group B: cefoxitin 6 g daily IV for 5 days, followed by 15 days of cefoxitin 2 g daily IM (n = 11) In both groups, if there was evidence of chlamydial infection, doxycycline 200 mg PO daily for 30 days given. |
| Bruhat 1989 | Not a comparison of interest. Group A: sulbactam 2 g daily IV + ampicillin 4 g daily for 5 days, followed by 15 days of sulbactam IM + ampicillin 2 g daily (n = 20) Group B: cefoxitin 6 g daily IV for 5 days, followed by 15 days of cefoxitin 2 g daily IM (n = 20) |
| Bruno 1985 | Not an RCT |
| Carty 1973 | Not antibiotic |
| Chatwani 1997 | Not a comparison of interest. Group A: trospectomycin 500 mg IV every 8 h for at least 4 days of inpatient treatment and had achieved complete resolution of sign and symptoms of acute PID, followed by 10 days of doxycycline PO (n = 26) Group B: cefoxitin 2 g every 6 h + doxycycline 100 mg PO or IV for at least 4 days of inpatient treatment and had achieved complete resolution of sign and symptoms of acute PID, followed by 10 days of doxycycline PO (n = 13) |
| Confino 1988 | Did not report effectiveness or safety outcomes |
| De Beer 1983 | Not a comparison of interest. Group A: ampicillin 2 g IV on admission and then 1 g IV every 4 h thereafter. If the response was good, ampicillin was administered PO after 48 h. Group B: cefoxitin 2 g IV followed by 1 g IV every 8 h. This was continued for at least 72 h. |
| Drasa 2010 | Results not separate for PID. |
| Drusano 1982 | Results not separate for PID |
| Duarte 1995 | Not an RCT. |
| Faro 1988 | Not a comparison of interest. Group A: ceftizoxime 2 g IV every 8 h for at least 5 days (n = 18) Group B: cefotaxime 2 g IV every 8 h for at least 5 days (n = 19) |
| Fedele 1989 | Not PID. |
| Frongillo 1992 | Results not separate for PID. |
| Gall 1981 | Not all participants randomized. |
| Gall 1990a | Not an RCT. |
| Gall 1990b | Not a comparison of interest. Group A: clindamycin 900 mg + tobramycin 80 mg/m2 IV every 8 h for at least 4 days. Group B: clindamycin 900 mg + placebo IV every 8 h for at least 4 days. Both treatment were followed by clindamycin 450 mg PO every 6 h, to complete 14 days of treatment. |
| Gerber 1992 | Not a comparison of interest. Group A: oxytetracycline 4 × 0.5 g PO daily or doxycycline 2 × 0.1 g PO daily, initially IV + metronidazole first 2 days 2 × 0.5 g IV from day 3, 2 × 0.25 g PO daily). Group B: oxytetracycline 4 × 0.5 g PO daily or doxycycline 2 × 0.1 g PO daily, initially IV + metronidazole first 2 days 2 × 0.5 g IV from day 3, 2 × 0.25 g PO daily) + additional bathing therapy. Group C: amoxicillin‐clavulanic acid 1st + 2nd day 1.2 g daily IV, 3rd to 10th day 3 × 0.75 g PO daily. Group D: ciprofloxacin initial 2 × 0.2 g IV then to day 10 2 × 0.5 g PO daily + metronidazole 1st and 2nd day 2 × 0.5 g day IV from day 3, 2 × 0.25 g PO daily. |
| Gerstner 1990 | Results not separate for PID. |
| Ghomian 2012 | Many inconsistencies in the study, author did not respond. |
| Giamarellou 1982 | Results not separate for PID. |
| Gibbs 1980a | Not a comparison of interest. Group A: penicillin G 5 x 106 units IV every 6 h + kanamycin 500 mg IM every 12 h for 4‐7 days after good clinical response (n = 5). Group B: spectinomycin 500 mg IM every 12 h for 4‐7 days after good clinical response (n = 7). |
| Gjonnaess 1981 | Not all participants randomized. |
| Grafford 1980 | Not a comparison of interest. Group A: bacampicillin 400 mg PO 3 times daily for 14 days (n = 39). Group B: bacampicillin 800 mg PO 3 times daily for 14 days (n = 31). |
| Grafford 1981 | Not an RCT. |
| Gunning 1986a | Not a comparison of interest. Group A: piperacillin 250 mg/kg daily IV divided into every 6 h for 5‐11 days (n = 31). Group B: clindamycin 600 mg IV 6/6 h + gentamicin 1.5 mg/kg IV every 8 h for 3‐8 days (n = 33). |
| Gunning 1986b | Not a comparison of interest. Group A: sulbactam 1 g + ampicillin 2 g IV every 6 h until asymptomatic and without clinical signs or pathogen sensitivity testing dictated a change in therapy (n = 21). Group B: clindamycin 600 mg IV 6/6 h + gentamicin 1.5 mg/kg IV every 8 h until asymptomatic and without clinical signs or pathogen sensitivity testing dictated a change in therapy (n = 18). |
| Hager 1989 | Not PID. |
| Harding 1982 | Not PID. |
| Harding 1984 | Not PID. |
| Hemsell 1982 | Results not separate for PID. |
| Hemsell 1987 | Not an RCT. |
| Hemsell 1988a | Authors report data without details of clinical cure |
| Hemsell 1988b | Results not separate for PID. |
| Hemsell 1988c | Not a comparison of interest. Group A: cefoxitin 2 g IV every 6 h + doxycycline 100 mg every 12 h for minimum of 4 days and for at least 48 h after defervescence, followed by doxycycline 100 mg PO when discharged from the hospital to complete 14 days of therapy (n = 32). Group B: ceftizoxime 2 g IV every 12 h + doxycycline 100 mg was given separately in 250 mL diluent and infused over 2 h for minimum of 4 days and for at least 48 h after defervescence, followed by doxycycline 100 mg PO when discharged from the hospital to complete 14 days of therapy (n = 30). Group C: ceftizoxime 2 g IV 3 times daily every 8 h for minimum of 4 days and for at least 48 h after defervescence (n = 29). Group D: ceftizoxime 2 g IV twice daily every 12 h for minimum of 4 days and for at least 48 h after defervescence (n = 30). |
| Hemsell 1988d | Results not separate for PID. |
| Hemsell 1990 | Not a comparison of interest. Group A: ampicillin 2 g + sulbactam 1 g IV every 6 h. Women whose treatment was successful received therapy for a mean (± SD) of 4.5 ± 0.9 days (n = 35). Group B: cefoxitin 2 g IV every 6 h. Women whose treatment was successful received therapy for a mean (± SD) of 4.5 ± 0.9 days (n = 19) |
| Hemsell 1991 | Did not report effectiveness or safety outcomes. |
| Hemsell 1993 | Not a comparison of interest. Group A: ampicillin/sulbactam 3 g IV every 6 h on at least 4 consecutive days and until the woman was afebrile for at least 48 h. Women with C trachomatis at study entry received doxycycline 100 mg PO twice daily for 10‐14 days when discharged from hospital. Women negative for C trachomatis discharged on no oral antibiotic (n = 76). Group B: cefoxitin 2 g IV every 6 h on at least 4 consecutive days and until the woman was afebrile for at least 48 h. Women with C trachomatis at study entry received doxycycline 100 mg PO twice daily for 10‐14 days when discharged from hospital. Women negative for C trachomatis discharged on no oral antibiotic (n = 41). |
| Hemsell 1997 | Not a comparison of interest. Group A: meropenem 500 mg IV given over 20‐30 minutes every 8 h for at least 48 h, for clinical evaluation (suggested treatment duration 4‐10 days, with a maximum duration of 28 days). Group B: clindamycin 900 mg IV + gentamicin 1.5 mg/kg following a loading dose of 2.0 mg/kg every 8 h for at least 48 h, for clinical evaluation (suggested treatment duration 4‐10 days, with a maximum duration of 28 days). |
| Henry 1985 | Not a comparison of interest. Group A: aztreonam 1‐2 g every 8‐12 h. Most women received 1 or 2 g 3 times daily. Clindamycin (usually at a dosage of 600 mg every 8 h) administered concurrently with aztreonam as a means of providing coverage against Gram‐positive and anaerobic organisms (n = 50; 5 with PID). Group B: gentamicin 3‐5 mg/kg daily in 3 equally divided doses + clindamycin 600 mg every 8 h (n = 38; 8 with PID). In both groups, antibiotics usually administered IM or IV; however, PO administration of clindamycin was permitted. |
| Holloway 1988 | Results not separate for PID. |
| Ibrahim 1990 | Not a comparison of interest. Group A: amikacin 14 mg/kg IV infusion in 150 mL saline over 30 minutes once daily. Treatment administered for 7‐9 days. Group B: amikacin 14 mg/kg IV infusion in 150 mL saline over 30 minutes twice daily. Treatment administered for 7‐9 days. Group C: netilmicin 6.6 mg/kg IV infusion in 150 mL saline over 30 minutes once daily. Treatment administered for 7‐9 days. Group D: netilmicin 6.6 mg/kg IV infusion in 150 mL saline over 30 minutes 3 times daily. Treatment administered for 7‐9 days. All 4 groups also received tinidazole 0.8 g once daily + ampicillin 4 g daily. |
| Jemsek 1997 | Not a comparison of interest. Group A: minimum 12 doses = 3 days of ampicillin 2 g/sulbactam 1 g IV every 6 h. Group B: minimum 12 doses = 3 days of cefoxitin 2 g IV every 6 h. Doxycycline 100 mg PO or IV twice daily administered concurrently to women with cultures positive for C trachomatis. Because of the significant possibility of false negatives, women with cultures negative for C trachomatis were empirically treated with 10‐14 days of doxycycline 100 mg PO twice daily after the study ended. |
| Jordheim 1974 | Not a comparison of interest. Group A: pivampicillin 175 mg PO capsule 4 times daily for 0‐4/5‐9/10‐14/15‐19 days (n = 28) Group B: pivampicillin 350 mg PO capsule 4 times daily for 0‐4/5‐9/10‐14/15‐19 days (n = 30) In some cases, duration of therapy was longer or shorter depending on clinical response. |
| Judlin 1995 | Not a comparison of interest. Group A: ofloxacin 400 mg daily in 2 × 200 mg tablets morning and night; amoxicillin‐clavulanic acid 2 g daily, 2 × 500 mg tablets morning and night. Combination given for 3 weeks. Group B: doxycycline 200 mg daily, 1 × 100 mg tablet morning and night; amoxicillin‐clavulanic acid 2 g daily, 2 × 500 mg tablets morning and night. Combination given for 6 weeks. |
| Knupell 1988 | Not a comparison of interest. Group A: cefotetan 2 g IV every 12 h, mean duration of therapy 6.1 days (n = 36). Group B: cefoxitin 2 g IV every 6 or 8 h, mean duration of therapy 6.4 days (n = 17). |
| Kosseim 1991 | Not a comparison of interest. Group A: ampicillin‐sulbactam 750 mg PO twice daily for 10 days (n = 38). Group B: cefoxitin 2 g IM + probenecid 1 g PO, followed by doxycycline 100 mg twice daily for 10 days (n = 37). |
| Kotoulas 1992 | Not an RCT. |
| Kunzig 1990 | Results not separate for PID. |
| Kvile 1980 | Compared same drug with different doses. |
| Larsen 1986 | Results not separate for PID. |
| Larsen 1992 | Not a comparison of interest. Group A: imipenem‐cilastatin 500 mg IV every 6 or 8 h. Treatment continued for at least 3 days or until the woman was afebrile for 24‐48 h (n = 44). Group B: clindamycin 900 mg IV every 8 h + gentamicin 1.5 mg/kg IV or IM for first dose, and 1.0 mg/kg every 8 h for succeeding doses. Treatment continued for at least 3 days or until the woman had been afebrile for 24‐48 h (n = 50). In both treatments, if therapy for Chlamydia spp was indicated, doxycycline 100 mg PO or IV added to either regimen every 12 h. |
| Livengood 1992 | Not a comparison of interest. Group A: clindamycin 600 mg IV every 6 h + cefamandole 2 g every 6 h. Group B: clindamycin 600 mg IV every 6 h + doxycycline 100 mg + 10 mL of 4% sodium bicarbonate alternating with placebo (5% dextrose in water) every 6 h. Protocol antibiotic therapy continued for 48 h after declaration of response and for minimum of 5 days (20 doses), after which women discharge without supplemental oral antibiotic treatment. |
| Ma 2010 | Not a comparison of interest. Group A: levornidazole 0.5 g twice daily for 5‐7 days. Group B: ornidazole 0.5 g twice daily for 5‐7 days. |
| Maggioni 1998 | Results not separate for PID. |
| Maki 1979 | Not PID. |
| Mandell 1993 | Results not separate for PID. |
| Marier 1982 | Not a comparison of interest. Group A: piperacillin 250 mg/kg daily IV every 4‐6 h. Group B: carbenicillin 450 mg/kg daily IV every 4‐6 h. |
| Marshall 1982 | Not PID. |
| Matsuda 1988 | Not a comparison of interest. Group A: cefpodoxime proxetil 100 mg tablet + dummy bacampicillin placebo, twice daily. Alternately, dummy CS‐807 + dummy bacampicillin, twice daily; therefore, women took tablets 4 times daily for 7 consecutive days. Group B: bacampicillin 250 mg tablet + dummy cefpodoxime proxetil placebo, 4 times daily for 7 consecutive days. |
| Matsuda 1989 | Not a comparison of interest. Group A: ceftibuten 100 mg 3 times daily. The shape of 2 tablets (7432‐S, bacampicillin) was different, so women took 8 tablets daily (after meal and before sleep) with placebo. At least 3‐day administration when a primary physician found cure of the infection, or assessed no efficacy and need to change a different drug. Group B: bacampicillin 250 mg 4 times daily. The shape of 2 tablets (7432‐S, bacampicillin) was different, so women took 8 tablets daily (after meal and before sleep) with placebo. At least 3‐day administration when a primary physician found cure of the infection, or assessed no efficacy and need to change a different drug. |
| Moghtadaei 2008a | Risk of fraud, no reply from author. |
| Moghtadaei 2008b | Risk of fraud, no reply from author. |
| Ness 2002 | Compared the same drugs as inpatient versus outpatient. |
| Ness 2005 | Not a comparison of interest. Group A: inpatient strategy cefoxitin 2 g IV every 6 h + doxycycline 100 mg IV or PO twice daily for at least 72 h, followed by doxycycline 100 mg PO twice daily for a total 14‐day course. Group B: outpatient treatment consisted of cefoxitin 2 g IM + probenecid 1 g PO, followed by doxycycline 100 mg PO twice daily for 14 days. |
| Nicolle 1986 | Not PID. |
| Paavonen 1985 | Did not report effectiveness or safety outcomes. |
| Pastorek 1985 | Results not separate for PID. |
| Roy 1998 | Not PID. |
| Ruiz Conde 1999 | Results not separate for PID. |
| Sanfilippo 1989 | Not a comparison of interest. Group A: mezlocillin 250 mg daily every 6 h + appropriate IV solution containing no antibiotic but identical in appearance to tobramycin solution every 8 h. Group B: aqueous crystalline penicillin G 480,000 U/kg every 6 h (maximum of 20 million units daily) + tobramycin 3 mg/kg daily every 8 h. Dose of tobramycin was then adjusted according to peak and rough blood levels to keep tobramycin levels within therapeutic range. Another physician, not directly involved with clinical management, evaluated tobramycin levels and contacted the pharmacy for changes of tobramycin levels. |
| Schnider 1979 | Not a comparison of interest. Group A: penicillin G 30 million units daily + netilmicin 2 mg/kg daily parenterally over 5 days. Group B: penicillin G 30 million units daily + gentamicin 3 mg/kg daily parenterally over 5 days. |
| Senft 1986 | Results not separate for PID. |
| Sesti 1990 | Not an RCT. |
| Sharma 2007 | Not a comparison of interest. Group A: ofloxacin 400 mg/ornidazole 500 mg/Saccharomyces boulardii (2 million spores)/lactic acid bacillus (60 million spores)/serratiopeptidase 10 mg once daily for total of 10 days (n = 98). Group B: doxycycline 100 mg twice daily + metronidazole 400 mg 3 times daily + serratiopeptidase 10 mg once daily for total of 10 days (n = 95). |
| Silva 1990 | Results not separate for PID. |
| Skerk 2003 | Compared same drug with different doses. |
| Spence 1981 | Not a comparison of interest. Group A: ampicillin 2 g IV every 4 h for minimum of 96 h followed by 0.5 g PO every 6 h to complete 10 days of treatment. Group B: doxycycline 200 mg IV initially, then 100 mg IV every 12 h for at least 96 h followed by 100 mg PO every 12 h to complete 10 days of treatment. |
| Stamm 1984 | Not PID. |
| Stiglmayer 1996 | Not a comparison of interest. Group A: sulbactam 1 g + ampicillin 2 g IV every 8 h. Group B: cefoxitin 2 g IV every 8 h. |
| Sweet 1988 | Not a comparison of interest. Group A: cefotetan 2 g IV every 12 h + doxycycline 100 mg IV every 12 h. Group B: cefoxitin 2 g IV every 6 h + doxycycline 100 mg IV every 12 h. Both treatments had a minimum of 4 days, at least for 48 h after disappearance of fever, after hospital discharge, doxycycline 100 mg PO every 12 h to complete 14 days of treatment. |
| Sweet 1994 | Results not separate for PID. |
| Takase 1986 | Not a comparison of interest. Group A: ofloxacin 1 dose 200 mg 3 times daily for 7 days. Group B: amoxicillin 1 dose 250 mg 4 times daily for 7 days. |
| Tellado 2002a | Not an RCT. |
| Tellado 2002b | Not an RCT. |
| Teppler 2002 | Not an RCT. |
| Thompson 1980 | Not a comparison of interest. Group A: aqueous penicillin G, 5 million units IV every 4 h + gentamicin 60 mg or 80 mg IV every 8 h based on weight. Group B: amoxicillin 1 g PO every 4 h. If the woman could not take oral medicines she received ampicillin 1 g IV every 4 h, until she could be changed to oral amoxicillin. She was switched as soon as possible to ampicillin 2 g PO daily if on regimen A, or amoxicillin 2 g PO daily if on regimen B, to complete 10 consecutive days of antibiotic treatment. |
| Thompson 1985 | Not a comparison of interest. Group A: aqueous procaine penicillin G, 4.8 million units IM + probenecid 1 g PO, followed by ampicillin monohydrate 0.5 g PO 4 times daily for 10 days. Group B: tetracycline hydrochloride 1.5 g PO as a single loading dose, followed by tetracycline 0.5 g PO 4 times daily for 10 days. |
| Tulkens 1988 | Not a comparison of interest. Group A: netilmicin 6.6 mg/kg IV once daily + tinidazole 0.8 g daily (once daily) + ampicillin 4 g daily (twice daily) for mean duration of 7 days. Group B: netilmicin 2.2 mg/kg IV 3 times daily + tinidazole 0.8 g daily (once daily) + ampicillin 4 g daily (twice daily) for a mean duration of 7 days. |
| Tulkens 1991 | Not an RCT. |
| Van Gelderen 1987 | Not a comparison of interest. Group A: ceftriaxone 2 g IV once daily for 2 days followed by 1 g once daily for the next 2‐8 days. Group B: penicillin G sodium 4200 mg IV + chloramphenicol 500 mg 4 times daily for 4‐10 days. Additional treatment: because there was a strong suspicion that anaerobic bacteria (which were not isolated specifically) were present, 5 ceftriaxone‐treated women and 8 penicillin/chloramphenicol‐treated women also received nitroimidazole 400‐500 mg every 8 h. |
| Walker 1991 | Not a comparison of interest. Group A: cefotetan 2 g IV every 12 h + doxycycline 100 mg IV every 12 h for minimum of 4 days and for at least 48 h after clinical response, followed by doxycycline 100 mg PO every 12 h was continued to complete a 14‐day course after the woman was discharged (n = 54). Group B: cefoxitin 2 g IV every 6 h + doxycycline 100 mg IV every 12 h for minimum of 4 days and for at least 48 h after clinical response, followed by doxycycline 100 mg PO every 12 h was continued to complete a 14‐day course after the woman was discharged (n = 54). |
| Wikler 1989 | Not a comparison of interest. Group A: ceftizoxime 2 g every 12 h. Group B: ceftizoxime 2 g every 8 h. Group C: ceftizoxime 2 g + doxycycline 100 mg every 12 h. Group D: cefoxitin 2 g every 6 h + doxycycline 100 mg every 12 h. Women receiving IV doxycycline continued to receive doxycycline 100 mg PO every 12 h after discharge from hospital for a total duration of 14 days (IV + PO therapy). |
| Witte 1993 | Not a comparison of interest. Group A: pefloxacin 800 mg daily + metronidazole 500 mg every 8 h. Group B: doxycycline initial dose of 200 mg followed by 100 mg daily + metronidazole 500 mg every 8 h. For both group A and B, the duration of treatment was at least 10 days, and maximal 14 days, unless the clinical response was considered insufficient after 5 days. The minimal duration of therapy to allow efficacy assessment was 5 days. |
| Wynd 1999 | Cost‐effectiveness study comparing ampicillin/sulbactam versus cefoxitin for the treatment of PID |
h: hour; IM: intramuscular; IV: intravenous; PID: pelvic inflammatory disease; PO: per os; RCT: randomized controlled trial; SD: standard deviation.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The Importance of Anti‐anaerobic Therapy for Acute Pelvic Inflammatory Disease (PID). |
| Methods | Randomized placebo‐controlled trial comparing 2 antibiotic treatment regimens for acute PID: group A: ceftriaxone + doxycycline; group B: ceftriaxone + doxycycline + metronidazole for 14 days. |
| Participants | Women aged 15‐40 years. |
| Interventions | Group A: ceftriaxone 250 mg IM single dose + doxycycline 100 mg PO twice daily × 14 days + placebo PO twice daily × 14 days. Group B: ceftriaxone 250 mg IM single dose + doxycycline 100 mg PO twice daily × 14 days + metronidazole 500 mg PO twice daily × 14 days. |
| Outcomes | Clearance of anaerobic organisms from the endometrium (over 30 days) (designated as safety issue: no). Follow‐up: 1 month for clinical outcomes, and assessment for clearance of microorganisms from the upper genital tract. |
| Starting date | November 2010. |
| Contact information | Harold Wiesenfeld, Associate Professor, University of Pittsburgh. |
| Notes |
IM: intramuscular; PID: pelvic inflammatory disease; PO: per os.
Contributions of authors
RFS: co‐ordination, study design, statistical analysis and review, writing the manuscript, grading the evidence in GRADE, and final approval of the manuscript.
DGF: data collection, extraction, grading risk of bias, and final approval of the manuscript.
RVD: data collection, extraction, grading risk of bias, and final approval of the manuscript.
SF: data collection, extraction, grading risk of bias, and final approval of the manuscript.
JR: study design, writing the manuscript, grading the evidence in GRADE, and final approval of the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR’s 2012 Cochrane Review Incentive Scheme Award, UK.
Declarations of interest
All authors certify that they do not have any affiliations with, or involvement in, any organization or entity with a direct financial interest in the subject matter of this review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony). They disclose that two of the authors (RFS and JR) had two publications used in the analysis. RFS and JR did not participate in the process for considering these studies for inclusion, data extraction, and grading for risk of bias.
Notes
New
References
References to studies included in this review
- Apuzzio JJ, Stankiewicz R, Ganesh V, Jain S, Kaminski Z, Louria D. Comparison of parenteral ciprofloxacin with clindamycin‐gentamicin in the treatment of pelvic infection. American Journal of Medicine 1989;87(5A):148S‐51S. [DOI] [PubMed] [Google Scholar]
- Arredondo JL, Diaz V, Gaitan H, Maradiegue E, Oyarzún E, Paz R, et al. Oral clindamycin and ciprofloxacin versus intramuscular ceftriaxone and oral doxycycline in the treatment of mild‐to‐moderate pelvic inflammatory disease in outpatients. Clinical Infectious Diseases 1997;24(2):170‐8. [DOI] [PubMed] [Google Scholar]
- Aşicioğlu O, Gungorduk K, Ozdemir A, Ertas IE, Yildirim G, Sanci M, et al. Single daily dose of moxifloxacin versus ofloxacin plus metronidazole as a new treatment approach to uncomplicated pelvic inflammatory disease: a multicentre prospective randomized trial. European Journal of Obstetrics and Gynecology and Reproductive Biology 2013;171(1):116‐21. [DOI] [PubMed] [Google Scholar]
- Balbi G, Piscitelli V, Grazia F, Martini S, Balbi C, Cardone A. Acute pelvic inflammatory disease: comparison of therapeutic protocols [Malattia infiammatoria pelvica acuta: protocolli terapeutici a confronto]. Minerva Ginecologica 1996;48(1‐2):19‐23. [PubMed] [Google Scholar]
- Bevan CD, Ridgway GL, Rothermel CD. Efficacy and safety of azithromycin as monotherapy or combined with metronidazole compared with two standard multidrug regimens for the treatment of acute pelvic inflammatory disease. Journal of International Medical Research 2003;31(1):45‐54. [DOI] [PubMed] [Google Scholar]
- Buisson P, Mulard C, Baudet J, Bernard P, Mares P, Montero M, et al. Treatment of upper genital infections in women. Multicenter study of the comparative efficacy and tolerance of an amoxicillin‐clavulanic acid combination and of a triple antibiotic combination [Étude multicentrique de l'efficacité et de la tolérance comparées de l'association amoxicilline‐acide clavulanique et d'une triple association antibiotique]. Revue Française de Gynecologie et d'Obstetrique 1989;84(10):699‐703. [PubMed] [Google Scholar]
- Burchell HJ, Cronje HS, Wet JI. Efficacy of different antibiotics in the treatment of pelvic inflammatory disease. South African Medical Journal 1987;72:248‐9. [PubMed] [Google Scholar]
- Ciraru‐Vigneron N, Bercau G, Sauvanet E, Nguyen Tan Lung R, Felten A, et al. The drug combination amoxicillin‐clavulanic acid compared to the triple combination ampicillin‐gentamicin‐metronidazole in the treatment of severe adnexal infections [L'association amoxicilline‐acide clavulanique comparée à la triple association ampicilline‐gentamicine‐métronidazole dans le traitement des infections utéro‐annexielles sévères]. Pathologie Biologie 1986;34(5 Pt 2):665‐8. [PubMed] [Google Scholar]
- Ciraru‐Vigneron N, Barrier J, Becue J, Chartier M, Giraud JR, Landes P, et al. Amoxycillin/clavulanic acid ('Augmentin') compared with a combination of aminopenicillin, aminoglycoside and metronidazole in the treatment of pelvic inflammatory disease. Pharmatherapeutica 1989;5(5):312‐9. [PubMed] [Google Scholar]
- Crombleholme D, Landers D, Ohm‐Smith M, Robbie MO, Hadley WK, DeKay V, et al. Sulbactam/ampicillin versus metronidazole/gentamicin in the treatment of severe pelvic infections. Drugs 1986;31(Suppl 2):11‐3. [DOI] [PubMed] [Google Scholar]
- Crombleholme WR, Ohm‐Smith M, Robbie MO, DeKay V, Sweet RL. Ampicillin/sulbactam versus metronidazole‐gentamicin in the treatment of soft tissue pelvic infections. American Journal of Obstetrics and Gynaecology 1987;156(2):507‐12. [DOI] [PubMed] [Google Scholar]
- Crombleholme WR, Schachter J, Ohm‐Smith M, Luft J, Whidden R, Sweet RL. Efficacy of single‐agent therapy for the treatment of acute pelvic inflammatory disease with ciprofloxacin. American Journal of Medicine 1989;87(Suppl 5A):142S‐7S. [DOI] [PubMed] [Google Scholar]
- Fischbach F, Deckardt R, Graeff H. Ciprofloxacin/metronidazol vs. cefoxitin/doxycyclin: vergleich zweier therapieschemata zur behandlung der akuten pelvinen infektion. Geburtshilfe und Frauenheilkunde 1994;54:337‐40. [DOI] [PubMed] [Google Scholar]
- Giraud JR, Chartier M, Ciraru Vigneron N, Becue J, Landes P, Leng JJ, et al. A comparison of the efficacy of and tolerance to Augmentin used alone and as one of three drugs used to treat acute upper genital tract infections. Results of a multicentre trial 152 cases [Comparaison de l’efficacite et de la tolerance de l’Augmentine en monotherapie versus triple association dans le traitment des infections genitales hautes aigues. Resultats d’une etude multicentrique portant sur 152 cas]. Contraception Fertilite Sexualite 1989;17(10):941‐8. [Google Scholar]
- Heinonen PK, Teisala K, Miettinen A, Aine R, Punnonen R, Grönroos P. A comparison of ciprofloxacin with doxycycline plus metronidazole in the treatment of acute pelvic inflammatory disease. Scandinavian Journal of Infectious Diseases Supplement 1989;60:66‐73. [PubMed] [Google Scholar]
- Hemsell DL, Little BB, Faro S, Sweet RL, Ledger WJ, Berkeley AS, et al. Comparison of three regimens recommended by the Centers for Disease Control and Prevention for the treatment of women hospitalized with acute pelvic inflammatory disease. Clinical Infectious Diseases 1994;19(4):720‐7. [DOI] [PubMed] [Google Scholar]
- Heystek M, Ross JD, PID Study Group. A randomized double‐blind comparison of moxifloxacin and doxycycline/metronidazole/ciprofloxacin in the treatment of acute, uncomplicated pelvic inflammatory disease. International Journal of STD & AIDS 2009;20(10):690‐5. [DOI] [PubMed] [Google Scholar]
- Hoyme UB, Ansorg R, Recklinghausen G, Schindler AE. Quinolones in the treatment of uncomplicated salpingitis: ofloxacin/metronidazole vs gentamicin/clindamycin. Infections in the Gynecology and obstetrics. [Chinolone in der therapie der unkoplizierten salpingitis: ofloxacin/metronidazol vs gentamicin/clindamicin]. Infektionen in der Gynäcologie und geburtshilfe 1993;VII.8a:607‐8. [Google Scholar]
- Judlin P, Liao Q, Liu Z, Reimnitz P, Hampel B, Arvis P. Efficacy and safety of moxifloxacin in uncomplicated pelvic inflammatory disease: the MONALISA study. BJOG 2010;117:1475‐84. [DOI] [PubMed] [Google Scholar]
- Landers DV, Wolner‐Hanssen P, Paavonen J, Thorpe E, Kiviat N, Ohm‐Smith M, et al. Combination antimicrobial therapy in the treatment of acute pelvic inflammatory disease. American Journal of Obstetrics and Gynecology 1991;164(3):849‐58. [DOI] [PubMed] [Google Scholar]
- Leboeuf D, Rousset D, Cacault JA, Engelman P. Prospective randomized trial comparing the efficacy and the tolerance of clindamycin‐gentamicin versus metronidazole‐gentamicin in acute utero‐adnexal infections in hospitalized patients [Essai prospectif randomise comparant l'efficacite et la tolerance de la clindamycine ‐ gentamycine versus metronidazole ‐ gentamycine dans les infections utero‐annexielles aigues chez des patientes hospitalisees]. Revue Française de Gynecologie et d'Obstetrique 1987;82(1):9‐15. [PubMed] [Google Scholar]
- Malhotra M, Sharma JB, Batra S, Arora R, Sharma S. Ciprofloxacin‐tinidazole combination, fluconazole‐azithromycin‐secnidazole‐kit and doxycycline‐metronidazole combination therapy in syndromic management of pelvic inflammatory disease: a prospective randomized controlled trial. Indian Journal of Medical Sciences 2003;57(12):549‐55. [PubMed] [Google Scholar]
- Maria B, Dublanchet A, Strecker J, Dennemark N, Genazzani A, Fioretti P, et al. Comparative evaluation of clindamycin/gentamicin and cefoxitin/doxycycline for treatment of pelvic inflammatory disease: a multi‐center trial. Acta Obstetricia et Gynecologica Scandinavica 1992;71(2):129‐34. [DOI] [PubMed] [Google Scholar]
- Martens MG, Faro S, Hammill H, Maccato M, Riddle GD, LaPread E. Comparison of cefotaxime, cefoxitin and clindamycin plus gentamicin in the treatment of uncomplicated and complicated pelvic inflammatory disease. Journal of Antimicrobial Chemotherapy 1990;26(Suppl A):37‐43. [DOI] [PubMed] [Google Scholar]
- Martens MG, Gordon S, Yarborough DR, Faro S, Binder D, Berkeley A, Ambulatory PID Research Group. Multicenter randomized trial of ofloxacin versus cefoxitin and doxycycline in outpatient treatment of pelvic inflammatory disease. Southern Medical Journal 1993;86(6):604‐10. [DOI] [PubMed] [Google Scholar]
- Okada H, Yamamoto T, Yasuda J, Kanao M, Shimizu T, Yorozu Y, et al. Comparative clinical study on ciprofloxacin and cefroxadine in the treatment of infections in obstetrics and gynecology [in Japanese]. Chemotherapy 1988;36(11):821‐57. [Google Scholar]
- Ross JD, Cronjé HS, Paszkowski T, Rakoczi I, Vildaite D, Kureishi A, et al. MAIDEN Study Group. Moxifloxacin versus ofloxacin plus metronidazole in uncomplicated pelvic inflammatory disease: results of a multicentre, double blind, randomised trial. Sexually Transmitted Infections 2006;82(6):446‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Wilkins J. Cefotaxime in the treatment of female pelvic soft tissue infections. Infection 1985;13(Suppl 1):S56‐61. [DOI] [PubMed] [Google Scholar]
- Roy S, Wilkins J, March CM, Solera NC, Galvan NI, Ressler RL, et al. A comparison of the efficacy and safety of ceftizoxime with doxycycline versus conventional CDC therapies in the treatment of upper genital tract infection with or without a mass. Clinical Therapeutics 1990;12(Suppl C):53‐73. [PubMed] [Google Scholar]
- Savaris RF, Teixeira LM, Torre TG, Edelweiss MIA, Moncada J, Schachter J. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstetrics and Gynecology 2007;110(1):53‐60. [DOI] [PubMed] [Google Scholar]
- Sirayapiwat P, Chaithongwongwatthana S, Tirawatnapong S. Triple therapies versus clindamycin plus gentamicin in the treatment of acute pelvic inflammatory disease: a randomized controlled trial. Thai Journal of Obstetrics and Gynaecology 2002;14:215‐21. [Google Scholar]
- Soper DE, Despres B. A comparison of two antibiotic regiments for treatment of pelvic inflammatory disease. Obstetrics and Gynecology 1988;72(7):7‐12. [PubMed] [Google Scholar]
- Sweet RL, Ohm‐Smith M, Landers DV, Robbie MO. Moxalactam versus clindamycin plus tobramycin in the treatment of obstetric and gynecologic infections. American Journal of Obstetrics and Gynecology 1985;152:808‐17. [DOI] [PubMed] [Google Scholar]
- Thadepalli H, Mathai D, Scottie R, Bansal MB, Savage E. Ciprofloxacin monotherapy for acute pelvic infections: a comparison with clindamycin plus gentamicin. Obstetrics and Gynaecology 1991;78(4):696‐701. [PubMed] [Google Scholar]
- Tison E, Marpeau L, Pigné A, Tessier F, Barrat J. Treatment of acute non‐chlamydial salpingitis. Study of the efficacy and tolerance of a single‐therapy antibiotic: Augmentin [Traitement des salpingites aiguës non chlamydiennes. Etude de l'efficacité et de la tolérance d'un antibiotique en monothérapie: l'Augmentin®]. Journal de Gynécologie Obstétrique et Biologie de la Reproduction 1988;17(4):513‐9. [PubMed] [Google Scholar]
- Walters MD, Gibbs RS. A randomized comparison of gentamicin‐clindamycin and cefoxitin‐doxycycline in the treatment of acute pelvic inflammatory disease. Obstetrics and Gynecology 1990;75(5):867‐72. [PubMed] [Google Scholar]
- Wendel GD Jr, Cox SM, Bawdon RE, Theriot SK, Heard MC, Nobles BJ. A randomized trial of ofloxacin versus cefoxitin and doxycycline in the outpatient treatment of acute salpingitis. American Journal of Obstetrics and Gynecology 1991;164(5 Pt 2):1390‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Acar B, Zissis NP. Piperacillin alone vs triple antibiotic combination in gynecological infections. Journal of chemotherapy 1989;1(6):403‐6. [DOI] [PubMed] [Google Scholar]
- Andersson PO, Hackl H, Jensen P, Larsen KR. A comparison of two different dosages of pivampicillin and doxycycline in patients with gynaecological infections. Current Medical Research and Opinion 1980;6(8):513‐7. [DOI] [PubMed] [Google Scholar]
- Bartlett JG. Therapeutic trial of moxalactam in intra‐abdominal sepsis and gynaecological infections. Drugs under Experimental and Clinical Research 1982;VIII(5):483‐6. [Google Scholar]
- Berkeley AS, Freedman KS, Hirsch JC, Ledger WJ. Randomized, comparative trial of imipenem/cilastatin and moxalactam in the treatment of serious obstetric and gynecologic infections. Surgery, Gynecology & Obstetrics 1986;162(3):204‐8. [PubMed] [Google Scholar]
- Blanco JD, Gibbs RS, Duff P, Castaneda YS, Clair PJ. Randomized comparison of ceftazidime versus clindamycin‐tobramycin in the treatment of obstetrical and gynecological infections. Antimicrobial Agents and Chemotherapy 1983;24(4):500‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brihmer C, Brundin J. Second look laparoscopy after treatment of acute salpingitis with doxycycline/benzylpenicillin procaine or trimethoprim‐sulfamethoxazole. Scandinavian Journal of Infectious Diseases 1988;53:63‐9. [PubMed] [Google Scholar]
- Brihmer C, Kallings I, Nord CE, Brundin J. Second look laparoscopy; evaluation of two different antibiotic regimens after treatment of acute salpingitis. European Journal of Obstetrics & Gynecology 1989;30(3):263‐74. [DOI] [PubMed] [Google Scholar]
- Bruhat MA, Pouly JL, Boedec G, Mage G. Treatment of acute salpingitis with sulbactam/ampicillin. Drugs 1986;31 Suppl(2):7‐10. [DOI] [PubMed] [Google Scholar]
- Bruhat MA, Bouedec G, Pouly JL, Mage G, Canis M. Treatment of acute salpingitis with sulbactam/ampicillin. International Journal of Gynecology & Obstetrics 1989;31 Suppl(2):41‐6. [DOI] [PubMed] [Google Scholar]
- Bruno V, Lombardo M, Schimberni M, Torregrossa G, Pepe C, Carenza L. A clinical trial with the new antibiotic combination piperacillin/flucloxacillin used on gynaecological postoperative infections. Current Therapeutic Research 1985;38(3):465‐73. [Google Scholar]
- Carty MJ. The use of oxyphenbutazone ('Tanderil') in acute pelvic inflammatory disease. Current Medical Research and Opinion 1973;1(4):203‐4. [DOI] [PubMed] [Google Scholar]
- Chatwani A, Dandalou V, Harmanli O, Nyirjesy P. Trospectomycin in acute pelvic inflammatory disease: a preliminary report. Infectious Diseases in Obstetrics and Gynecology 1997;5(3):215‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confino E, Friberg J, Vermesh M, Madanes A, Suarez M, Gleicher N. Mezlocillin versus doxycycline in the treatment of acute salpingitis. Mount Sinai Journal of Medicine 1988;55(2):154‐8. [PubMed] [Google Scholar]
- Beer JAA, Ende J, Odendaal HJ. Efficacy of ampicillin and cefoxitin in the treatment of acute pelvic inflammatory disease. A comparative study. South African Medical Journal 1983;64:733‐5. [PubMed] [Google Scholar]
- Drasa K, Koci E. Azithromycin vs. clarithromycin and both combination in treatment of mycoplasma genitalium. European Urology Supplements 2010;9(2):172. [Google Scholar]
- Drusano GL, Warren JW, Saah AJ, Caplan ES, Tenney JH, Hansen S, et al. A prospective randomized controlled trial of cefoxitin versus clindamycin‐aminoglycoside in mixed anaerobic‐aerobic infections. Surgery, Gynecology & Obstetrics 1982;154(5):715‐20. [PubMed] [Google Scholar]
- Duarte G, Quintana SM, Gir E, Marana HR, Cunha SP. Evaluation of doxycycline for the complementary treatment of acute inflammatory pelvic disease. A double‐blind study [Avaliação da doxiciclina como tratamento complementar da doença inflamatória pélvica aguda ‐ Estudo duplo‐cego]. Revista Brasileira de Medicina do Esporte 1995;52(6):651‐6. [Google Scholar]
- Faro S, Martens MG, Phillips LE, Lapread E, Riddle GD, Turner RM. Ceftizoxime versus cefotaxime in the treatment of hospitalized patients with pelvic inflammatory disease. Current Therapeutic Research 1988;43(3):349‐54. [Google Scholar]
- Fedele L, Acaia B, Marchini M, Grassi R, Benzi‐Cipelli R, Bonino S. Treatment of Chlamydia trachomatis endometritis with josamycin. Journal of Chemotherapy 1989;1(4 Suppl):911‐2. [PubMed] [Google Scholar]
- Frongillo RF, Custo GM, Gilardi G, Martella L, Palumbo M. Imipenem versus netilmicin plus chloramphenicol in gynecological upper tract infections: a comparative study. Chemotherapy 1992;5(1):41‐4. [Google Scholar]
- Gall SA, Kohan AP, Ayers OM, Hughes CE, Addison WA, Hill GB. Intravenous metronidazole or clindamycin with tobramycin for therapy of pelvic infections. Obstetrics & Gynecology 1981;57(1):51‐8. [PubMed] [Google Scholar]
- Gall S. Therapeutic dilemmas in the treatment of pelvic infections. Journal of Reproductive Medicine 1990;35(11 Suppl):1091‐4. [PubMed] [Google Scholar]
- Gall SA, Constantine L. Comparative evaluation of clindamycin versus clindamycin plus tobramycin in the treatment of acute pelvic inflammatory disease. Obstetrics & Gynecology 1990;75(2):282‐6. [PubMed] [Google Scholar]
- Gerber B, Wilken H, Zacharias K, Barten G, Splitt G. Treatment of acute salpingitis with tetracycline/metronidazole with or without additional balneotherapy. Augmentan or ciprofloxacin/metronidazole: a second‐look‐laparoscopy study [Zur Behandlung der akuten Salpingitis mit Tetracyclin/Metronidazol mit oder ohne zusätzliche Heilbadkur, Augmentan oder Ciprofloxacin/Metronidazol: Eine second look‐Laparoskopie‐Studie]. Geburtshilfe und Frauenheilkunde 1992;52:165‐70. [DOI] [PubMed] [Google Scholar]
- Gerstner GJ. Comparison of ceftriaxone (1 x 1 g/day) versus cefotaxime (3 x 1 g/day) for gynecologic and obstetric infections. A randomized clinical trial. Gynecologic and Obstetric Investigation 1990;29(4):273‐7. [DOI] [PubMed] [Google Scholar]
- Ghomian N, HafiziI L, Tavassoli F, Ahrari RT. Comparing ceftriaxone plus azithromycin or doxycycline for outpatient treatment in pelvic inflammatory disease [مقايسه سفترياكسون و آزيترومايسين با سفترياكسون و داكسي سيكلين در درمان سرپايي بيماري التهابي لگن]. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;14(8):1‐8. [Google Scholar]
- Giamarellou H, Volanaki M, Avlami A, Tsatsiadis K, Petrochilos E, Daikos GK. Ornidazole versus clindamycin: Comparative evaluation in the treatment of 140 serious anaerobic infections. Chemotherapy 1982;28(6):502‐11. [DOI] [PubMed] [Google Scholar]
- Gibbs RS. A trial of spectinomycin hydrochloride compared with aqueous penicillin G plus kanamycin for treatment of severe pelvic inflammatory disease. Sexually Transmitted Diseases 1980;7(1):21‐3. [DOI] [PubMed] [Google Scholar]
- Gjonnaess H, Dalaker K, Urnes A, Norling B, Kville G, Mardh P, et al. Treatment of pelvic inflammatory disease. Effects of lymecycline and clindamycin. Current Therapeutic Research 1981;29(6):885‐92. [Google Scholar]
- Grafford K, Guttorm E, Kristensen GB, Philipson T, Selnes A. Bacampicillin in the treatment of pelvic inflammatory disease: a study of two dosage regimens. Infection 1980;8(6):290‐2. [Google Scholar]
- Grafford K, Nilsson BS. Twice daily dosage of bacampicillin: a summary of clinical documentation. Journal of Antimicrobial Chemotherapy 1981;8(Suppl C):119‐27. [DOI] [PubMed] [Google Scholar]
- Gunning JE. A comparison of piperacillin and clindamycin plus gentamicin in women with pelvic infections. Surgery, Gynecology & Obstetrics 1986;163:156‐62. [PubMed] [Google Scholar]
- Gunning J. A comparison of parenteral sulbactam/ampicillin versus clindamycin/gentamicin in the treatment of pelvic inflammatory disease. Drugs 1986;31(Suppl 2):14‐7. [DOI] [PubMed] [Google Scholar]
- Hager WD, Pascuzzi M, Vernon M. Efficacy of oral antibiotics following parenteral antibiotics for serious infections in obstetrics and gynecology. Obstetrics & Gynecology 1989;73(3 Pt 1):326‐29. [PubMed] [Google Scholar]
- Harding G, Vincelette J, Rachlis A, Fong I, Mandell L, Feld R, et al. A preliminary report on the use of ceftizoxime versus clindamycin/tobramycin for the therapy of intra‐abdominal and pelvic infections. Journal of Antimicrobial Chemotherapy 1982;10(Suppl C):191‐2. [DOI] [PubMed] [Google Scholar]
- Harding GK, Nicolle LE, Haase DA, Aoki FY, Stiver HG, Blanchard RJ, et al. Prospective, randomized, comparative trials in the therapy for intraabdominal and female genital tract infections. Reviews of Infectious Diseases 1984;6(Suppl 1):S283‐92. [DOI] [PubMed] [Google Scholar]
- Hemsell DL, Cunningham FG, Nolan CM, Miller TT. Clinical experience with cefotaxime in obstetric and gynecologic infections. Reviews of Infectious Diseases 1982;4(Suppl):S432‐8. [DOI] [PubMed] [Google Scholar]
- Hemsell DL, Hemsell PG, Heard MC, Nobles BJ. Piperacillin and a combination of clindamycin and gentamicin for the treatment of hospital and community acquired acute pelvic infections including pelvic abscess. Surgery, Gynecology & Obstetrics 1987;165(3):223‐9. [PubMed] [Google Scholar]
- Hemsell DL, Heard MC, Hemsell PG, Nobles BJ. Sulbactam/ampicillin versus cefoxitin for uncomplicated and complicated acute pelvic inflammatory disease. Drugs 1988;35(Suppl 7):39‐42. [DOI] [PubMed] [Google Scholar]
- Hemsell DL, Wendel GD, Gall SA, Newton ER, Gibbs RS, Knuppel RA, et al. Multicenter comparison of cefotetan and cefoxitin in the treatment of acute obstetric and gynecologic infections. American Journal of Obstetrics & Gynecology 1988;158(3 pt 2):722‐77. [DOI] [PubMed] [Google Scholar]
- Hemsell DL, Nobles BJ, Heard MC, Hemsell PG. Upper and lower reproductive tract bacteria in 126 women with acute pelvic inflammatory disease. Microbial susceptibility and clinical response to four therapeutic regimens. Journal of Reproductive Medicine 1988;33(10):799‐805. [PubMed] [Google Scholar]
- Hemsell DL, Heard MC, Hemsell PG, Nobles BJ. Sulbactam/ampicillin versus cefoxitin for uncomplicated and complicated acute pelvic inflammatory disease. Drugs 1988;35(Suppl 7):39‐42. [DOI] [PubMed] [Google Scholar]
- Hemsell DL, Bawdon RE, Hemsell PG, Nobles BJ, Heard MC. Single‐agent therapy for acute pelvic inflammatory disease: sulbactam/ampicillin versus cefoxitin. Journal of International Medical Research 1990;18(Suppl 4):85D‐9D. [PubMed] [Google Scholar]
- Hemsell DL, Heard MC, Nobles BJ. Comparative bacteriology of parenteral single‐agent vs. combination therapy in salpingitis. Advances in Therapy 1991;8(1):27‐35. [Google Scholar]
- Hemsell DL, Wendel GD, Hemsell PG, Heard ML, Nobles BJ. Inpatient treatment for uncomplicated and complicated acute pelvic inflammatory disease: ampicillin/sulbactam vs. cefoxitin. Infectious Diseases in Obstetrics and Gynecology 1993;1(3):123‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsell DL, Martens MG, Faro S, Gall S, McGregor JA. A multicenter study comparing intravenous meropenem with clindamycin plus gentamicin for the treatment of acute gynecologic and obstetric pelvic infections in hospitalized women. Clinical Infectious Diseases 1997;24(Suppl 2):S222‐30. [DOI] [PubMed] [Google Scholar]
- Henry SA. Overall clinical experience with aztreonam in the treatment of obstetric‐gynecologic infections. Reviews of Infectious Diseases 1985;7(Suppl 4):S703‐8. [DOI] [PubMed] [Google Scholar]
- Holloway WJ. Infection in women. Clinical experience with beta‐lactamase inhibitors. Journal of Reproductive Medicine 1988;33(6 Suppl):595‐7. [PubMed] [Google Scholar]
- Ibrahim S, Derde MP, Kaufman L, Clerckx‐Braun F, Jacqmin P, Brulein V, et al. Safety, pharmacokinetics and efficacy of once‐a‐day netilmicin and amikacin versus their conventional schedules in patients suffering from pelvic inflammatory disease. Renal Failure 1990;12(3):199‐203. [DOI] [PubMed] [Google Scholar]
- Jemsek JG, Harrison F. Ampicillin/sulbactam vs. cefoxitin for the treatment of pelvic inflammatory disease. Infectious Diseases in Obstetrics and Gynecology 1997;5(5):319‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordheim O. Pivampicillin treatment of urogenital infections in gynaecological patients. Clinical evaluation. Infection 1974;2(3):142‐4. [DOI] [PubMed] [Google Scholar]
- Judlin P, Koebele A, Zaccabri A, Walleghen E, Pavis A, Badonnel Y, et al. Comparative study of ofloxacin+amoxicillin‐clavulanic acid versus doxycycline+amoxicillin‐clavulanic acid combination in the treatment of pelvic Chlamydia trachomatis infections [Etude comparative des associations ofloxacine+amoxicilline‐acide clavulanique versus doxycycline+amoxicilline‐acide clavulanique dans le traitment des infections génitales hautes à Chlamydia Trachomatis]. Journal de Gynecologie Obstetrique et Biologie de la Reproduction 1995;24(3):253‐9. [PubMed] [Google Scholar]
- Knuppel RA, O'Bryan D, Lake M. Cefotetan: comparative and non comparative studies in obstetric and gynecologic infections. Southern Medical Journal 1988;81(2):185‐8. [PubMed] [Google Scholar]
- Kosseim M, Rondald A, Plummer FA, D'Costa L, Brunham RC. Treatment of acute pelvic inflammatory disease in the ambulatory setting: trial of cefoxitin and doxycycline versus ampicillin‐sulbactam. Antimicrobial Agents and Chemotherapy 1991;35(8):1651‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoulas IG, Cardamakis E, Michopoulos J, Chronis G, Antoniou S. Comparison of ceftriaxone plus ornidazole, ceftazidime plus ornidazole, and ornidazole in the treatment of pelvic inflammatory disease (PID). Chemotherapy 1992;5(3):159‐64. [Google Scholar]
- Kunzig HJ, Petersen EE, Hoyme UB, Martius J, Busch W. Sequential therapy with ciprofloxacin i.v./p.o. and metronidazole compared with cefotiam and metronidazole in the treatment of gynecological infections. Chemotherapy 1990;3(4):225‐8. [Google Scholar]
- Kvile G, Langeland P, Norling B. Treatment of salpingitis with pivampicillin. A comparison of twice‐daily and thrice‐daily dosages. Infection 1980;8(1):32‐6. [DOI] [PubMed] [Google Scholar]
- Larsen JW Jr, Voise CT, Grossman JH 3rd. Comparison of cefoxitin and clindamycin‐gentamicin for pelvic infections. Clinical Therapeutics 1986;9(1):77‐83. [PubMed] [Google Scholar]
- Larsen JW, Gabel‐Hughes K, Kreter B. Efficacy and tolerability of imipenem‐cilastatin versus clindamycin+gentamicin for serious pelvic infections. Clinical Therapeutics 1992;14(1):90‐6. [PubMed] [Google Scholar]
- Livengood CH 3rd, Hill GB, Addison WA. Pelvic inflammatory disease: findings during inpatient treatment of clinically severe, laparoscopy‐documented disease. American Journal of Obstetrics and Gynecology 1992;166(2):519‐24. [DOI] [PubMed] [Google Scholar]
- Ma L, Zhang YZ, Zheng YL, Wang ZH, Xu YD, Kong LN. Multicenter randomized controlled clinical study on levornidazole and sodium chloride injection in the treatment of pelvic anaerobic infections [in Chinese]. Chinese Journal of Obstetrics & Gynecology 2010;45(10):754‐6. [PubMed] [Google Scholar]
- Maggioni P, Stefano F, Facchini V, Irato S, Mancuso S, Colombo M, et al. Treatment of obstetric and gynecologic infections with meropenem: comparison with imipenem/cilastatin. Journal of Chemotherapy 1998;10(2):114‐21. [DOI] [PubMed] [Google Scholar]
- Maki DG, Craig WA, Agger WA. A comparative clinical trial of sisomicin and gentamicin in major gram‐negative infections. Infection 1979;7(Suppl 3):S298‐300. [Google Scholar]
- Mandell LA, Turgeon PL, Ronalds AR, Canadian Clinical Trials Group. A prospective randomized trial of imipenem‐cilastatin versus clindamycin/tobramycin in the treatment of intra‐abdominal and pelvic infections. Canadian Journal of Infectious Diseases 1993;4(5):279‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marier RL, Sanders CV, Faro S, Janney A, Williams WW, Derks F, et al. Piperacillin v carbenicillin in the therapy for serious infections. Archives of Internal Medicine 1982;142(11):2000‐5. [PubMed] [Google Scholar]
- Marshall JR, Chow AW, Sorrell TC. Effectiveness of mezlocillin in female genital tract infections. Journal of Antimicrobial Chemotherapy 1982;9(Suppl A):149‐58. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Suzuki M, Shimizu T, Ishikawa M, Haga H, Mizoguchi H, et al. Comparative clinical study of CS‐807 (cefpodoxime proxetil) and bacampicillin (BAPC) on the infectious disease in the field of obstetrics and gynecology [in Japanese]. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1988;4(9):1641‐85. [Google Scholar]
- Matsuda S, Shimizu T, Maki M, Chimura T, Yajima A, Takahashi K, et al. Comparative double‐blind clinical trial of ceftibuten (7432‐S) and bacampicillin (BAPC) against gynecological infections [in Japanese]. Chemotherapy 1989;37(Suppl 1):667‐700. [Google Scholar]
- Moghtadaei P, Sardari F, Esmaeilian S. Comparison of ceftriaxone plus weekly azithromycin or daily ofloxacin for outpatient treatment of pelvic inflammatory disease: a randomized clinical trial. Journal of Family and Reproductive Health 2008;2(2):87‐93. [Google Scholar]
- Moghtadaei P, Sardari F, Esmaeilian S. Comparison of ceftriaxone plus weekly azithromycin or daily ofloxacin for outpatient treatment of pelvic inflammatory disease: a randomized clinical trial. Journal of Family and Reproductive Health 2008;2(2):87‐93. [Google Scholar]
- Ness RB, Soper DE, Holley RL, Peipert J, Randall H, Sweet RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. American Journal of Obstetric and Gynecology 2002;186(5):929‐37. [PUBMED: 12015517] [DOI] [PubMed] [Google Scholar]
- Ness RB, Trautmann G, Richter HE, Randall H, Peipert JF, Nelson DB, et al. Effectiveness of treatment strategies of some women with pelvic inflammatory disease a randomized trial. Obstetrics and Gynaecology 2005;106(3):573‐80. [DOI] [PubMed] [Google Scholar]
- Nicolle LE, Harding GK, Louie TJ, Thomson MJ, Blanchard RJ. Cefoxitin plus tobramycin and clindamycin plus tobramycin. A prospective randomized comparison in the therapy of mixed aerobic/anaerobic infections. Archives of Surgery 1986;121(8):891‐6. [DOI] [PubMed] [Google Scholar]
- Paavonen J, Vesterinen E, Aantaa K, Räsänen J. Factors predicting abnormal hysterosalpingographic findings in patients treated for acute pelvic inflammatory disease. International Journal of Gynaecology and Obstetrics 1985;23(3):171‐5. [DOI] [PubMed] [Google Scholar]
- Pastorek JG Jr, Aldridge KE, Cunningham GL, Faro S, Graffeo S, McNeeley GS, et al. Comparison of ticarcillin plus clavulanic acid with cefoxitin in the treatment of female pelvic infection. American Journal of Medicine 1985;29(79):161‐3. [DOI] [PubMed] [Google Scholar]
- Roy S, Koltun W, Chatwani A, Martens MG, Dittrich R, Luke DR. Treatment of acute gynecologic infections with trovafloxacin. Trovafloxacin Surgical Group. American Journal of Surgery 1998;178(6A Suppl):67S‐73S. [DOI] [PubMed] [Google Scholar]
- Ruiz Conde MA, Grupo de Estudio Multicêntrico. Comparative multicenter study between meropenem versus clindamycin with gentamicin for the treatment of inpatient with obstetrics and/or gynecologic infections [Estudio multicéntrico comparativo entre meropenem y la asociación clindamicina‐gentamicina en el tratamentiento de infecciones obstétricas y/o ginecológicas en pacientes hospitalizadas]. Clínica e Investigación en Ginecología y Obstetricia 1999;26(5):202‐7. [Google Scholar]
- Sanfilippo JS, Schikler KN. Mezlocillin versus penicillin and tobramycin in adolescent pelvic inflammatory disease: a prospective study. Internal Pediatrics 1989;4:53‐6. [Google Scholar]
- Schnider G, Birken RA, Poindexter AN. A comparison of netilmicin and gentamicin in the treatment of pelvic infections. Obstetrics & Gynecology 1979;54(5):554‐7. [PubMed] [Google Scholar]
- Senft HH, Stiglmayer R, Eibach HW, Koerner H. Sulbactam/ampicillin versus cefoxitin in the treatment of obstetric and gynaecological infections. Drugs 1986;31(Suppl 2):18‐21. [DOI] [PubMed] [Google Scholar]
- Sesti F, Farnè C, Piccione E. Medical treatment of pelvic inflammatory disease. A clinical study on the therapeutic effectiveness of piperacillin + erythromycin and of piperacillin + clindamycin + gentamycin [Trattamento medico della malattia infiammatoria pelvica. studio clinico sull'efficacia terapeutica de piperacillina + eritromicina e di piperacillina + clindamicina + gentamicina]. La Clinica Terapeutica 1990;132(1):41‐4. [PubMed] [Google Scholar]
- Sharma JB, Chanana C, Sunesh K, Roy K, Malhotra N. Comparison of ofloxacin and ornidazole with probiotic versus doxycycline and metronidazole for the outpatient treatment of pelvic inflammatory disease. JK Science 2007;9(2):66‐9. [Google Scholar]
- Silva JLP, Credidio RMV, Gabiatti JRE, Polli CH, Bedone AJ, Silva JCG, et al. Imipenen/cilastatin in the treatment of pelvic infections [Imipenem/cilastatina no tratamento de infecções pélvicas]. Revista Brasileira de Medicina 1990;47:196‐200. [Google Scholar]
- Skerk V, Puntaric A, Begovac J, Jaksic J, Strapac Z, Vickovic N, et al. Comparative study of intravenous azithromycin alone or intravenous and oral azithromycin for the treatment of pelvic inflammatory disease caused by Chlamydia trachomatis [in Croatian]. Croatian Journal of Infection 2003;23(3):125‐8. [Google Scholar]
- Spence MR, Genadry R, Raffel L. Randomized prospective comparison of ampicillin and doxycycline in the treatment of acute pelvic inflammatory disease in hospitalized patients. Sexually Transmitted Diseases 1981;8(Suppl 2):164‐6. [Google Scholar]
- Stamm WE, Guinan ME, Johnson C, Starcher T, Holmes KK, McCormack WM. Effect of treatment regimens for Neisseria gonorrhoeae on simultaneous infection with Chlamydia trachomatis. New England Journal of Medicine 1984;310(9):545‐9. [DOI] [PubMed] [Google Scholar]
- Stiglmayer R, Senft HH, Eibach HW, Körner J. Sulbactam/ampicillin versus cefoxitin in the treatment of gynaecological infection: an antibiotic therapeutic study. International Journal of Antimicrobial Agents 1996;6:61‐5. [PubMed] [Google Scholar]
- Sweet RL, Schachter J, Landers DV, Ohm‐Smith M, Robbie MO. Treatment of hospitalized patients with acute pelvic inflammatory disease: comparison of cefotetan plus doxycycline and cefoxitin plus doxycycline. American Journal of Obstetrics and Gynecology 1988;158(3):736‐41. [DOI] [PubMed] [Google Scholar]
- Sweet RL, Roy S, Faro S, O'Brien WF, Sanfilippo JS, Seidlin M. Piperacillin and tazobactam versus clindamycin and gentamicin in the treatment of hospitalized women with pelvic infection. Obstetrics & Gynecology 1994;83(2):280‐6. [PubMed] [Google Scholar]
- Takase Z, Komoto K, Katayama M. Comparative clinical study of ofloxacin (OFLX) and amoxicillin (AMPC) of the infectious disease in the field of obstetrics and gynecology [in Japanese]. Chemotherapy 1986;34(1):31‐63. [Google Scholar]
- Tellado J, Woods GL, Gesser R, McCarroll K, Teppler H. Ertapenem versus piperacillin‐tazobactam for treatment of mixed anaerobic complicated intra‐abdominal, complicated skin and skin structure, and acute pelvic infections. Surgical Infection Society 2002;3(4):303‐14. [DOI] [PubMed] [Google Scholar]
- Tellado J, Woods GL, Gesser R, McCarroll K, Teppler H. Ertapenem versus piperacillin‐tazobactam for treatment of mixed anaerobic complicated intra‐abdominal, complicated skin and skin structure, and acute pelvic infections. Surgical Infection Society 2002;3(4):303‐14. [DOI] [PubMed] [Google Scholar]
- Teppler H, McCarroll K, Gesser RM, Woods GL. Surgical infections with enterococcus: outcome in patients treated with ertapenem versus piperacillin‐tazobactam. Surgical Infection Society 2002;3(4):337‐49. [DOI] [PubMed] [Google Scholar]
- Thompson SE III, Hager WD, Wong KH, Lopez B, Ramsey C, Allen SD, et al. The microbiology and therapy of acute pelvic inflammatory disease. American Journal of Obstetrics and Gynecology 1980;136(2):179‐86. [DOI] [PubMed] [Google Scholar]
- Thompson SE, Brooks C, Eschenbach DA, Spence MR, Cheng S, Sweet R, et al. High failure rates in outpatient treatment of salpingitis with either tetracycline alone or penicillin/ampicillin combination. American Journal of Obstetrics and Gynecology 1985;152(6):635‐41. [DOI] [PubMed] [Google Scholar]
- Tulkens PM, Cerckx‐Braun F, Donnez J, Ibrahim S, Kallay Z, Delmee M, et al. Safety and efficacy of aminoglycosides once‐a‐day: experimental data and randomized controlled evaluation in patients suffering from pelvic inflammatory disease. Journal of Drug Development 1988;1(Suppl 3):71‐82. [Google Scholar]
- Tulkens PM. Pharmacokinetic and toxicological evaluation of a once‐daily regimen versus conventional schedules of netilmicin and amikacin. Journal of Antimicrobial Chemotherapy 1991;27(Suppl C):49‐61. [DOI] [PubMed] [Google Scholar]
- Gelderen CJ. A comparative trial of ceftriaxone and a penicillin/chloramphenicol combination in gynaecological infections complicated by peritonitis. South African Medical Journal 1987;Suppl 2:13‐5. [PubMed] [Google Scholar]
- Walker CK, Landers DV, Ohm‐Smith MJ, Robbie MO, Luft J, Schachter J, et al. Comparison of cefotetan plus doxycycline with cefoxitin plus doxycycline in the inpatient treatment of acute salpingitis. Sexually Transmitted Diseases 1991;18(2):119‐23. [PUBMED: 1862460] [DOI] [PubMed] [Google Scholar]
- Wikler MA, Moonsammy GI, Hemsell DL. Comparison of ceftizoxime versus combination therapy in the treatment of pelvic inflammatory disease (PID). Journal of Chemotherapy 1989;1(4 Suppl):882‐3. [PubMed] [Google Scholar]
- Witte EH, Peters AA, Smit IB, Linden MC, Mouton RP, Meer JW, et al. A comparison of pefloxacin/metronidazole and doxycycline/metronidazole in the treatment of laparoscopically confirmed acute pelvic inflammatory disease. European Journal of Obstetrics, Gynecology and Reproductive Biology 1993;50(2):153‐8. [DOI] [PubMed] [Google Scholar]
- Wynd MA, Hemsell DL, Paladino JA. Cost‐effectiveness of ampicillin/sulbactam versus cefoxitin in the treatment of pelvic inflammatory disease. Journal of Infectious Disease Pharmacotherapy 1999;4(1):35‐48. [Google Scholar]
References to ongoing studies
- The Importance of Anti‐anaerobic Therapy for Acute Pelvic Inflammatory Disease (PID).. Ongoing study November 2010..
Additional references
- Aghaizu A, Adams EJ, Turner K, Kerry S, Hay P, Simms I, et al. What is the cost of pelvic inflammatory disease and how much could be prevented by screening for chlamydia trachomatis? Cost analysis of the Prevention of Pelvic Infection (POPI) trial. Sexually Transmitted Infections 2011;87(4):312‐7. [DOI] [PubMed] [Google Scholar]
- Altman DG. Confidence intervals for the number needed to treat. BMJ 1998;317(7168):1309‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baveja G, Saini S, Sangwan K, Arora DR. A study of bacterial pathogens in acute pelvic inflammatory disease. Journal of Communicable Diseases 2001;88:121‐5. [PubMed] [Google Scholar]
- Blanchard JF, Moses S, Greenaway C, Orr P, Hammond GW, Brunham RC. The evolving epidemiology of chlamydial and gonococcal infections in response to control programs in Winnipeg, Canada. American Journal of Public Health 1998;88(10):1496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SD, Torrone E, Kruszon‐Moran D, Berman S, Johnson R, Satterwhite CL, et al. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999‐2008. Sexually Transmitted Diseases 2012;39(2):92‐6. [DOI] [PubMed] [Google Scholar]
- DeWitt J, Reining A, Allsworth JE, Peipert JF. Tuboovarian abscess: is size associated with duration of hospitalization and complications?. Obstetrics and Gynecology International 2010;2010:1‐5. [DOI: 10.1155/2010/847041] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test.. BMJ 1997;315(629):634. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbach DA, Buchanan TM, Pollock HM, Forsyth PS, Alexander ER, Lin JS, et al. Polymicrobial etiology of acute pelvic inflammatory disease. New England Journal of Medicine 1975;293(4):166‐71. [DOI] [PubMed] [Google Scholar]
- Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders‐Algra M, et al. How much loss to follow‐up is acceptable in long‐term randomised trials and prospective studies?. Archives of Disease in Childhood 2008;93(6):458‐61. [DOI: 10.1136/adc.2007.127316] [DOI] [PubMed] [Google Scholar]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to February 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
- Hager WD, Eschenbach DA, Spence MR, Sweet RL. Criteria for diagnosis and grading of salpingitis [editorial].. Obstet Gynecol 1983;61(1):113‐114. [PUBMED: 6218430] [PubMed] [Google Scholar]
- Haggerty CL, Ness RB. Epidemiology, pathogenesis and treatment of pelvic inflammatory disease. Expert Review of Anti‐infective Therapy 2006;4(2):235‐47. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Judlin P. Current concepts in managing pelvic inflammatory disease. Current Opinion in Infectious Diseases 2010;23(1):83. [DOI] [PubMed] [Google Scholar]
- Mahafzah AM, Al‐Ramahi MQ, Asa'd AM, El‐Khateeb MS. Prevalence of sexually transmitted infections among sexually active Jordanian females. Sexually Transmitted Diseases 2008;35(6):607‐10. [DOI] [PubMed] [Google Scholar]
- McCormack WM, Nowroozi K, Alpert S, Sackel SG, Lee YH, Lowe EW, et al. Acute pelvic inflammatory disease: characteristics of patients with gonococcal and nongonococcal infection and evaluation of their response to treatment with aqueous procaine penicillin G and spectinomycin hydrochloride. Sexually Transmitted Diseases 1977;4(4):125‐31. [PubMed] [Google Scholar]
- Molander P, Finne P, Sjoberg J, Sellors J, Paavonen J. Observer agreement with laparoscopic diagnosis of pelvic inflammatory disease using photographs. Obstetrics and Gynecology 2003;101:875‐80. [DOI] [PubMed] [Google Scholar]
- Morris GC, Stewart CM, Schoeman SA, Wilson JD. A cross‐sectional study showing differences in the clinical diagnosis of pelvic inflammatory disease according to the experience of clinicians: implications for training and audit. Sexually Transmitted Infections 2014;90(6):445‐51. [DOI: 10.1136/sextrans-2014-051646] [DOI] [PubMed] [Google Scholar]
- Ness RB, Smith KJ, Chang CC, Schisterman EF, Bass DC, Gynecologic Infection Follow‐Through, GIFT, Investigators. Prediction of pelvic inflammatory disease among young, single, sexually active women. Sexually Transmitted Diseases 2006;33(3):137‐42. [DOI] [PubMed] [Google Scholar]
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015;10(12):e0143304. [DOI: 10.1371/journal.pone.0143304] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Ross JD. What is endometritis and does it require treatment?. Sexually Transmitted Infections 2004;80(4):252‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Judlin P, Nilas L. European guidelines of the management of pelvic inflammatory disease. International Journal of STD and AIDS 2007;18:662‐6. [DOI] [PubMed] [Google Scholar]
- Ross J, Judlin P, Jensen J. 2012 European guideline for the management of pelvic inflammatory disease. International Journal of STD & AIDS 2014;25(1):1‐7. [PUBMED: 24216035] [DOI] [PubMed] [Google Scholar]
- Ross JD. Pelvic inflammatory disease. American Family Physician 2014;90(10):725‐6. [Google Scholar]
- Simms I, Rogers P, Charlett A. The rate of diagnosis and demography of pelvic inflammatory disease in general practice: England and Wales. International Journal of STD and AIDS 1999;10(7):448‐51. [DOI] [PubMed] [Google Scholar]
- Soper DE. Diagnosis and laparoscopic grading of acute salpingitis.. Am J Obstet Gynecol 1991;164(5):1370‐1376. [PUBMED: 1827704] [DOI] [PubMed] [Google Scholar]
- Soper DE. Pelvic inflammatory disease. Obstetrics and Gynecology 2010;116((2 Pt 1)):419‐28. [DOI] [PubMed] [Google Scholar]
- Sterne J. Why the Cochrane risk of bias tool should not include funding source as a standard item [editorial]. Cochrane Database of Systematic Reviews2013; Vol. 12. [10.1002/14651858.ED000076] [DOI] [PMC free article] [PubMed]
- Sutton AJ, Higgins J. Recent developments in meta‐analysis. Statistics in Medicine 2008;27(5):625‐50. [DOI] [PubMed] [Google Scholar]
- Trent M, Ellen JM, Frick KD. Estimating the direct costs of pelvic inflammatory disease in adolescents: a within‐system analysis. Sexually Transmitted Diseases 2011;38(4):326‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CK, Kahn JG, Washington AE, Peterson HB, Sweet RL. Pelvic inflammatory disease: metaanalysis of antimicrobial regimen efficacy. Journal of Infectious Diseases 1993;168(4):969‐78. [DOI] [PubMed] [Google Scholar]
- Walker CK, Wiesenfeld H. Antibiotic therapy for acute pelvic inflammatory disease: the 2006 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clinical Infectious Diseases 2007;28 (Suppl 1):S29‐36. [DOI] [PubMed] [Google Scholar]
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 2015;64(RR‐03):1‐137. [PMC free article] [PubMed] [Google Scholar]


