Abstract
Background
Combined modality treatment consisting of chemotherapy followed by localised radiotherapy is the standard treatment for patients with early stage Hodgkin lymphoma (HL). However, due to long‐ term adverse effects such as secondary malignancies the role of radiotherapy has been questioned recently and some clinical study groups advocate chemotherapy only for this indication.
Objectives
To assess the effects of chemotherapy alone compared to chemotherapy plus radiotherapy in adults with early stage HL .
Search methods
For the or i ginal version of this review, we searched MEDLINE, Embase and CENTRAL as well as conference proceedings (American Society of Hematology, American Society of Clinical Oncology and International Symposium of Hodgkin Lymphoma) from January 1980 to November 2010 for randomised controlled trials (RCTs) comparing chemotherapy alone versus chemotherapy regimens plus radiotherapy. For the updated review we searched MEDLINE, CENTRAL and conference proceedings to December 2016.
Selection criteria
We included RCTs comparing chemotherapy alone with chemotherapy plus radiotherapy in patients with early stage HL. We excluded trials with more than 20% of patients in advanced stage. As the value of radiotherapy in addition to chemotherapy is still not clear, we also compared to more cycles of chemotherapy in the control arm. In this updated review, we also included a second comparison evaluating trials with varying numbers of cycles of chemotherapy between intervention and control arms, same chemotherapy regimen in both arms assumed. We excluded trials evaluating children only, therefore only trials involving adults are included in this updated review.
Data collection and analysis
Two review authors independently extracted data and assessed the quality of trials. We contacted study authors to obtain missing information. As effect measures we used hazard ratios (HR) for overall survival (OS) and progression‐free survival (PFS) and risk ratios (RR) for response rates. Since not all trials reported PFS according to our definitions, we evaluated all similar outcomes (e.g. event‐free survival) as PFS/tumour control.
Main results
Our search led to 5518 potentially relevant references. From these, we included seven RCTs in the analyses involving 2564 patients. In contrast to the first version of this review including five trials, we excluded trials randomising children. As a result, we excluded one trial from the former analyses and we identified three new trials.
Five trials with 1388 patients compared the combination of chemotherapy alone and chemotherapy plus radiotherapy, with the same number of chemotherapy cycles in both arms. The addition of radiotherapy to chemotherapy has probably little or no difference on OS (HR 0.48; 95% confidence interval (CI) 0.22 to 1.06; P = 0.07, moderate‐ quality evidence), however two included trials had potential other high risk of bias due to a high number of patients not receiving planned radiotherapy. After excluding these trials in a sensitivity analysis, the results showed that the combination of chemotherapy and radiotherapy improved OS compared to chemotherapy alone (HR 0.31; 95% CI 0.19 to 0.52; P <0.00001, moderate‐ quality evidence). In contrast to chemotherapy alone the use of chemotherapy and radiotherapy improved PFS (HR 0.42; 95% CI 0.25 to 0.72; P = 0.001; moderate‐ quality evidence). Regarding infection‐ related mortality (RR 0.33; 95% CI 0.01 to 8.06; P = 0.5; low‐ quality evidence), second cancer‐ related mortality (RR 0.53; 95% CI 0.07 to 4.29; P = 0.55; low‐ quality evidence) and cardiac disease‐ related mortality (RR 2.94; 95% CI 0.31 to 27.55; P = 0.35;low‐ quality evidence), there is no evidence for a difference between the use of chemotherapy alone and chemotherapy plus radiotherapy. For complete response rate (CRR) (RR 1.08; 95% CI 0.93 to 1.25; P = 0.33; low‐ quality evidence), there is also no evidence for a difference between treatment groups.
Two trials with 1176 patients compared the combination of chemotherapy alone and chemotherapy plus radiotherapy, with different numbers of chemotherapy cycles in both arms. OS is reported in one trial only, the use of chemotherapy alone (more chemotherapy cycles) may improve OS compared to chemotherapy plus radiotherapy (HR 2.12; 95% CI 1.03 to 4.37; P = 0.04; low‐ quality evidence). This trial also had a potential other high risk of bias due to a high number of patients not receiving planned therapy. There is no evidence for a difference between chemotherapy alone and chemotherapy plus radiotherapy regarding PFS (HR 0.42; 95% CI 0.14 to 1.24; P = 0.12; low‐ quality evidence). After excluding the trial with patients not receiving the planned therapy in a sensitivity analysis, the results showed that the combination of chemotherapy and radiotherapy improved PFS compared to chemotherapy alone (HR 0.24; 95% CI 0.070 to 0.88; P = 0.03, based on one trial). For infection‐ related mortality (RR 6.90; 95% CI 0.36 to 132.34; P = 0.2; low‐ quality evidence), second cancer‐ related mortality (RR 2.22; 95% CI 0.7 to 7.03; P = 0.18; low‐ quality evidence) and cardiac disease‐related mortality (RR 0.99; 95% CI 0.14 to 6.90; P = 0.99; low‐quality evidence), there is no evidence for a difference between the use of chemotherapy alone and chemotherapy plus radiotherapy. CRR rate was not reported.
Authors' conclusions
This systematic review compared the effects of chemotherapy alone and chemotherapy plus radiotherapy in adults with early stage HL .
For the comparison with same numbers of chemotherapy cycles in both arms, we found moderate‐ quality evidence that PFS is superior in patients receiving chemotherapy plus radiotherapy than in those receiving chemotherapy alone. The addition of radiotherapy to chemotherapy has probably little or no difference on OS . The sensitivity analysis without the trials with potential other high risk of bias showed that chemotherapy plus radiotherapy improves OS compared to chemotherapy alone.
For the comparison with different numbers of chemotherapy cycles between the arms there are no implications for OS and PFS possible, because of the low quality of evidence of the results.
Plain language summary
Treatment of early stage Hodgkin lymphoma
Background
Hodgkin lymphoma (HL) is a malignancy of the lymphatic system. It occurs in children and adults, but it is more common in the third decade of life. It is one of the most curable forms of cancer. There are four stages of HL, stages I and II are considered as early stage HL and stages III and IV as advanced stage. Using risk factors such as presence or absence of bulky disease and presence or absence of B‐symptoms, like night sweats or fever, early stage HL is further classified into early favourable and early unfavourable stages. Treatment options are chemotherapy, radiotherapy or both. Radiotherapy may have, more treatment‐ related side effects than chemotherapy, including second malignancies; this applies at least to the large treatment fields used in the past. However, with modern, very limited treatment fields, the risks of long‐term side effects caused by radiotherapy have been reduced significantly.
Review question
This systematic review compares overall survival (OS) and progression free survival (PFS) in adults with early stage HL after receiving chemotherapy alone or chemotherapy plus radiotherapy.
Study characteristics
We searched important medical databases such as the Cochrane Central Register of Controlled Trials and MEDLINE. Two review authors independently screened, summarised and analysed the results. This led to the inclusion of seven randomised controlled trials involving with 2564 patients.
The evidence provided is current to December 2016.
Key results
For the comparison of chemotherapy alone and chemotherapy plus radiotherapy with the same number of chemotherapy cycles in both arms, this systematic review found no evidence for a difference regarding OS between the interventions, however, two included trials had potential other high risk of bias due to a high number of patients not receiving radiotherapy as planned beforehand. After excluding these trials in a further analysis, OS was superior in adults receiving chemotherapy plus radiotherapy than in those receiving chemotherapy alone. PFS was also superior in adults receiving chemotherapy plus radiotherapy. Most trials reported adverse events (AEs), but in different ways. Because of insufficient comparable data we focused on adverse events considered of particular interest. For infection‐ related mortality, second cancer‐ related mortality and cardiac disease‐ related mortality, there was no evidence for a difference between treatment groups. For complete response rate (CRR) there was no evidence for a difference between treatment groups either.
For the comparison of chemotherapy alone and chemotherapy plus radiotherapy with different numbers of chemotherapy cycles in the arms, OS was reported in one trial only. The use of chemotherapy alone may improve OS compared to chemotherapy plus radiotherapy. There was no evidence for a difference between treatment groups regarding PFS. After excluding one trial with patients not receiving the planned therapy the results showed that chemotherapy plus radiotherapy improved PFS. For infection‐ related mortality, second cancer‐ related mortality and cardiac disease‐ related mortality, there is no evidence for a difference between treatment groups. CR was not reported.
Quality of evidence
For the same number of chemotherapy cycles in both arms, we judged the quality of evidence for OS and PFS as moderate, for AEs and CR as low.
For different numbers of chemotherapy cycles in the arms, we considered the quality of evidence for OS, PFS and AEs to be low.
Conclusion
This systematic review compared the effects of chemotherapy alone and chemotherapy plus radiotherapy in adults with early stage HL .
For the comparison with same numbers of chemotherapy cycles in both arms we found moderate‐ quality evidence that PFS is superior in patients receiving chemotherapy plus radiotherapy than in those receiving chemotherapy alone. The addition of radiotherapy to chemotherapy has probably little or no difference on OS. A further analysis without the trials with potential other high risk of bias showed that chemotherapy plus radiotherapy improves OS (both analyses moderate‐ quality evidence).
For the comparison of chemotherapy alone and chemotherapy plus radiotherapy with different numbers of chemotherapy cycles between the arms there were no implications for OS and PFS possible, because of the low quality of evidence of the results.
Summary of findings
Summary of findings for the main comparison. Same number of chemotherapy cycles in both arms.
| Same number of chemotherapy cycles in both arms | ||||||
| Chemotherapy alone versus chemotherapy plus radiotherapy for adults with early stage Hodgkin lymphoma. | ||||||
| Outcomes | № of participants (studies) Follow‐ up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Comment | |
| Risk with chemotherapy only | Risk with chemotherapy plus radiotherapy | |||||
| Mortality (calculated instead of overall survival) Follow‐up : 5 years The low‐ mortality rate was taken from the EORTC‐GELA H9‐F trial, the high‐ mortality rate was taken from the Mexico B2H031trial |
1388 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | HR 0.48 (0.22 to 1.06) | Low risk to die | ||
| 30 per 1000 | 15 per 1000 (7 to 32) | Number of people who will die | ||||
| High risk to die | ||||||
| 150 per 1000 | 75 per 1000 (35 to 158) | |||||
| Mortality sensitivity analysis (calculated instead of overall survival) ‐ without UK NCRI Rapid
trial and MSKCC trial #90‐44due to high risk of other bias Follow‐up : 5 years The low‐ mortality rate was taken from the EORTC‐GELA H9‐F trial, the high‐ mortality rate was taken from the Mexico B2H031trial |
816 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | HR 0.31 (0.19 to 0.52) | Low risk to die | ||
| 30 per 1000 | 9 per 1000 (6 to 16) | Number of people who will die | ||||
| High risk to die | ||||||
| 150 per 1000 | 49 per 1000 (30 to 81) | |||||
| Relapse, progression or death (calculated instead of PFS) Follow‐up : 5 years |
1351 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | HR 0.42 (0.25 to 0.72) | Low risk of progress, relapse or death | ||
| 100 per 1000 | 43 per 1000 (26 to 73) | Number of people who will have a progress, relapse or die | ||||
| High risk of progress, relapse or death | ||||||
| 300 per 1000 | 139 per 1000 (85 to 226) | |||||
| Infection‐ related mortality | 152 (1 RCT) | ⊕⊕⊝⊝ LOW 4 | RR 0.33 (0.01 to 8.06) | Study population | ||
| 13 per 1000 | 4 per 1000 (0 to 106) | |||||
| Second cancer‐ related mortality | 1199 (3 RCTs) | ⊕⊕⊝⊝ LOW 4 | RR 0.53 (0.07 to 4.29) | Study population | ||
| 9 per 1,000 | 5 per 1000 (1 to 39) | |||||
| Cardiac disease‐ related mortality | 457 (2 RCTs) | ⊕⊕⊝⊝ LOW 4 | RR 2.94 (0.31 to 27.55) | Low risk | ||
| 1 per 1,000 | 3 per 1000 (0 to 28) | |||||
| Complete response rate | 376 (3 RCTs) | ⊕⊕⊝⊝ LOW 5, 6 | RR 1.08 (0.93 to 1.25) | Study population | ||
| 839 per 1,000 | 906 per 1000 (780 to 1,000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; PFS: progre ssion‐free survival | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Substantial heterogeneity, downgraded by 1 point for inconsistency
2 Sensitivity analysis, excluding two trials with potential high risk of other bias. Downgraded by 1 point for imprecision due to low number of included patients and events
3 Definition of PFS varied across trials, downgraded by 1 point for inconsistency
4 Very small number of events, downgraded by 2 points for imprecision
5Statistical heterogeneity (I ² = 67%), downgraded by 1 point for inconsistency
6 Low number of events, downgraded by 1 point for imprecision
Summary of findings 2. Different numbers of chemotherapy cycles in both arms.
| Different numbers of chemotherapy cycles in both arms | ||||||
| Chemotherapy alone versus chemotherapy plus radiotherapy for adults with early stage Hodgkin lymphoma | ||||||
| Outcomes | № of participants (studies) Follow‐ up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Comment | |
| Risk with chemotherapy only | Risk with chemotherapy plus radiotherapy | |||||
| Mortality (calculated instead of overall survival) Follow‐up : 5 years The low‐ mortality rate was taken from the EORTC‐GELA H9‐F trial, the high‐ mortality rate was taken from the Mexico B2H031trial |
276 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | HR 2.12 (1.03 to 4.37) | Low risk to die | ||
| 30 per 1000 | 63 per 1000 (31 to 125) | Number of people who will die | ||||
| High risk to die | ||||||
| 150 per 1000 | 291 per 1000 (154 to 508) | |||||
| Relapse, progression or death (calculated instead of PFS) Follow‐up : 5 years |
1176 (3 RCTs) | ⊕⊕⊝⊝ LOW 2 | HR 0.42 (0.14 to 1.24) | Low risk of progress, death | ||
| 100 per 1000 | 43 per 1000 (15 to 122) | Number of people who will have a progress, relapse or die | ||||
| High risk of progress, death | ||||||
| 300 per 1000 | 139 per 1000 (49 to 357) | |||||
| Relapse, progression or death (calculated instead of PFS) sensitivity analysis ‐ without HD6trial due to high risk of other bias Follow‐up : 5 years |
900 2 (RCTs) |
⊕⊕⊕⊝ MODERATE 3 | HR 0.24 (0.07 to 0.88) | Low risk of progress, death | ||
| 100 per 1000 |
25 per 1000 (7 to 88) |
Number of people who will have a progress, relapse or die | ||||
| High risk of progress, death | ||||||
| 300 per 1000 |
82 per 1000 (25 to 269) |
|||||
| Infection‐ related mortality | 276 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | RR 6.90 (0.36 to 132.34) | Low risk | ||
| 1 per 1000 | 7 per 1000 (0 to 132) | H10F; H10U; HD6 | ||||
| Second cancer‐ related mortality | 276 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | RR 2.22 (0.70 to 7.03) | Study population | ||
| 29 per 1000 | 65 per 1000 (20 to 205) | |||||
| Cardiac disease‐ related mortality | 276 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | RR 0.99 (0.14 to 6.90) | Study population | ||
| 15 per 1000 | 14 per 1000 (2 to 101) | |||||
| Complete response rate | not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR : Hazard ratio ; PFS: progression‐free surviv al | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Very low number of events, downgraded by 2 points for imprecision
2 Serious heterogeneity (I² = 84%), downgraded by 2 points for inconsistency
Background
Description of the condition
Hodgkin lymphoma (HL) is one of the most common malignancies in young adults (Swerdlow 2003; Thomas 2002). It is a malignancy of the lymph nodes and lymphatic system with possible involvement of other organs. The disease is rare with an annual incidence of approximately two to three per 100,000 in most western countries (DeVita 1997; Diehl 2005; Mauch 1999; Parkin 2005), and occurs mostly in young people, the incidence being greatest in the third decade of life (Mueller 1999). Factors associated with HL include family history, viral exposures, and immune suppression (Glaser 1996). HL is one of the most curable form of cancer worldwide, the cure rates are up to 90% (Engert 2010; Engert 2012).
Staging of HL is based on the Ann Arbor system (Carbone 1971), with the addition of a definition of bulky disease (largest tumour diameter > 10 cm), often referred to as the Cotswold modification (Lister 1989). Information about prognostic factors such as mediastinal mass, other bulky nodal disease, and extent of sub‐diaphragmatic disease is included in this classification. Generally, HL is differentiated into early stage HL and advanced stage HL. On the basis of clinical staging and risk factors, patients are usually assigned to early favourable, early unfavourable and advanced stages (Engert 2007; Klimm 2005). However, there are still small differences in the definition of risk factors used and in the classification of certain subgroups of patients among the different study groups in Europe and the USA.
Description of the intervention
Usually patients with early stage HL receive two cycles of ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) in combination with involved‐field radiotherapy (Engert 2010; Rancea 2013). Depending on the intensity and dose of treatment given, long‐ term complications such as secondary malignancies (Franklin 2005), cardiac disease (Adams 2004) and infertility occur more frequently in Hodgkin survivors as compared to the general population. For patients with early stage disease, the 20‐year cumulative secondary malignancy rate is estimated to be between 4% and 20 % (Franklin 2005; Ng 2002a). Risk factors for secondary malignancies (and cardiac disease) are the choice and dose of radiotherapy and chemotherapy (Aleman 2003; Bhatia 2003; Dores 2002; Franklin 2005; Green 2000; Ng 2002a; Ng 2002b; Swerdlow 2000; van Leeuwen 2000). Unfortunately, no long‐term comparison of combined modality treatment, consisting of chemotherapy plus radiotherapy, with chemotherapy alone was possible in cohorts of Hodgkin survivors, in part due to the changes in treatment regimens over time (Ng 2002a). Nonetheless, to avoid additional radiation‐induced toxicity, chemotherapy‐ alone treatment for patients with early stage HL has been advocated (Canellos 2005). This notion was supported by two clinical trials comparing combined modality treatment with chemotherapy alone in which no significant survival disadvantage was observed in patients receiving chemotherapy alone (HD6; MSKCC trial #90‐44). However, one of these trials compared two cycles of chemotherapy plus radiotherapy with four to six cycles of chemotherapy. Data are now emerging on long‐term toxicity also of chemotherapy, including secondary malignancies and cardiac disease (Henderson 2016; Maraldo 2015; Schaapveld 2015).
How the intervention might work
Chemotherpay and radiotherapy act on differentiating cells, prone to damage, and stop their growth and ultimately damage them, as a result the tumour mass shrinks. Along with tumour cure, normal body cells are also affected after treatment resulting in treatment‐ related side effects.
Biologic basis of chemotherapy
The most commonly used chemotherapeutic drugs in the treatment of early stage HL are classified as follows.
Alkylating agents: cyclophosphamide, mechlorethamine, procarbazine, dacarbazine.
Anti‐tumour antibiotics: bleomycin, doxorubicine (adriamycin), epirubicin.
Anti‐mitotic agents: vincristine, vinblastine.
Steroid hormones: prednisone.
Alkylating agents and anti‐tumour antibiotics are phase‐nonspecific chemotherapeutic drugs which can injure DNA at any phase of cell cycle, but appear to then block in S‐phase or G2 at a check point in a cell cycle before cell division (Sausville 2005). Anti‐mitotic agents and steroid hormones are phase‐specific chemotherapeutic drugs. Anti‐mitotic agents act in M‐phase and prevent tumour cell division by destroying mitotic spindle, and anti‐metabolites act in S‐phase and prevent replication of the tumour cell's DNA, stopping tumour cell proliferation. Steroid hormones act in M‐phase by suppressing the mitosis in lymphocytes (Chaber 2006).
Biologic basis of radiotherapy
Injury to DNA is the primary mechanism by which ionising radiation kills cells. This happens largely via the formation of free radicals, which accounts for 65 % or more of the damage to biologic materials. This may cause base damage, single‐strand breaks, double‐strand breaks, sugar damage, and DNA‐DNA and DNA‐protein cross‐links. Lymphoma cells are highly radiosensitive, and they undergo rapid apoptosis in response to DNA damage within a few hours after irradiation to relatively low doses. Because of this high radiosensitivity of lymphoma cells, much lower doses of radiation are necessary than for most solid tumours. Generally, normal cells are capable of repairing some of the radiation damage, whereas lymphoma cells are not (McBride 2008).
Why it is important to do this review
In recent years, a concept of minimal curative therapy with greatest efficacy and least toxicity has emerged in the treatment of early stage HL (Connors 2001; Connors 2005). This concept is based on the assumption that avoidance of radiotherapy would result in fewer deaths from late effects and the long‐term survival would be at least comparable and possibly better for patients treated with chemotherapy alone in early stage HL. To test this assumption, we performed a systematic review with meta‐analysis of randomised controlled trials (RCTs) comparing chemotherapy alone with chemotherapy plus radiotherapy in patients with early stage HL with respect to adverse events, response rate, progression‐free survival or similar outcomes, and overall survival.
Objectives
To assess the effects of chemotherapy alone compared to chemotherapy plus radiotherapy in adults with early stage Hodgkin lymphoma (HL).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing chemotherapy alone with combined modality treatment consisting of chemotherapy plus radiotherapy in newly diagnosed patients with early favourable and early unfavourable stages clinical stage (CS) I and CS II Hodgkin lymphoma (HL). We excluded RCTs comparing chemotherapy alone with chemotherapy plus radiotherapy in patients with all stages of HL if more than 20 % of patients had advanced disease. We used the risk factor definitions as described in the individual trials. The terms "early stage" and "limited stage" were considered equivalent. We excluded quasi‐ randomised trials. We had also planned to exclude trials including fewer than 10 patients per arm, although we did not find such trials.
Types of participants
We included both male and female adults, with newly confirmed diagnosis of early stage HL (CS I and II) without any prior treatment for HL. If there were more than 20 % of the patients with advanced stage, the trial was excluded. In contrast to the first version of this review ( Herbst 2011 ), we excluded trials including children, as their treatment differs from that of adults (Kung 2006; Wolden 2012).
Types of interventions
We compared chemotherapy alone (single agent or multiple agent, regardless of dose, number of cycles and intervention time) and both chemotherapy plus radiotherapy (regardless of dose, field used and intervention time) as primary treatment for people with CS I and CS II HL (early favourable and early unfavourable stages of HL). In the first version of the review, we excluded trials if the chemotherapy regimen was not identical in all study arms. As the value of radiotherapy in addition to chemotherapy is still not clear, also compared to more cycles of chemotherapy in the control arm, we amended the inclusion criteria. In contrast to the first version of the review, we also included trials with varying numbers of cycles of chemotherapy between intervention and control arms, same chemotherapy regimen in both arms assumed. In this update, trials with different numbers of chemotherapy cycles in both arms are presented in a second comparison.
Types of outcome measures
Primary outcomes
We evaluated overall survival (OS) as the primary endpoint. The preferred definition of OS was "time from entry onto the clinical trial until death as a result of any cause" (Cheson 2007).
Secondary outcomes
-
Response rate
Measured as overall response rate (ORR) and complete response (CR).
The definitions of overall response and CR were used as given in the publication. If only CR and partial response were given, the ORR was calculated as CR plus partial response.
-
Progression‐free survival (PFS)
Definded as time to tumour progression, relapse or death.
Because not all trials reported PFS according to our definition, we accepted other tumour control outcomes and evaluated these.
-
Adverse events (AEs)
Most trials reported AEs , but in different ways. Because of insufficient comparable data we focused on AEs considered of particular interest: infection‐ related mortality, second cancer‐ related mortality, cardiac disease‐ related mortality and infertility.
Search methods for identification of studies
We adapted search strategies from the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). No language restriction was applied to reduce the language bias, especially English language bias, as studies showing an intervention to be effective are more likely to be published in English (Dickersin 1993; Egger 1997; Juni 2002). We designed a search strategy with the assistance of the Information Specialist (IM) for health‐related bibliographic databases.
Electronic searches
We searched the following databases and sources.
-
Databases of medical literature:
Cochrane Central Register of Controlled Trials from January 1977 to November 2010 and for the update from December 2010 to December 2016 (for search strategies see Appendix 1);
MEDLINE (Ovid) from January 1977 to November 2010 and for the update from December 2010 to December 2016 (for search strategy see Appendix 2);
Embase from January 1977 to June 2009 (for search strategy see Appendix 3).
-
Conference proceedings of the annual meetings of the following societies for abstracts (2000 to 2015, if not included in CENTRAL):
American Society of Hematology (ASH) (update until 2015);
American Society of Clinical Oncology (ASCO) (update until 2015);
International Symposium on Hodgkin Lymphoma (IHSL) (update until 2013).
-
Databases of ongoing trials:
meta‐register of controlled trials: https://www.controlled‐trials.com/mrct/ ;
EU clinical trials register: https://www.clinicaltrialsregister.eu/ctr‐search/search ;
Clinicaltrials.gov: https://www.clinicaltrials.gov ;
databases and websites of relevant institutions, agencies, organisations, societies and registries.
Searching other resources
-
Handsearching:
We checked the reference lists of all identified trials, relevant review articles and current treatment guidelines for further literature.
-
Personal contacts:
We contacted experts in the field in order to retrieve information on unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (OB, NS) independently screened the results of the search for eligibility for this review by reading the abstracts. In the case of disagreement, we obtained the full‐ text publication. If no consensus could be reached, we asked a third review author for final decision (Higgins 2011).
We documented the study selection process in a flow chart, as recommended in the PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analysis) statement (Moher 2009), showing the total numbers of retrieved references and the numbers of included and excluded studies (see Figure 1).
1.
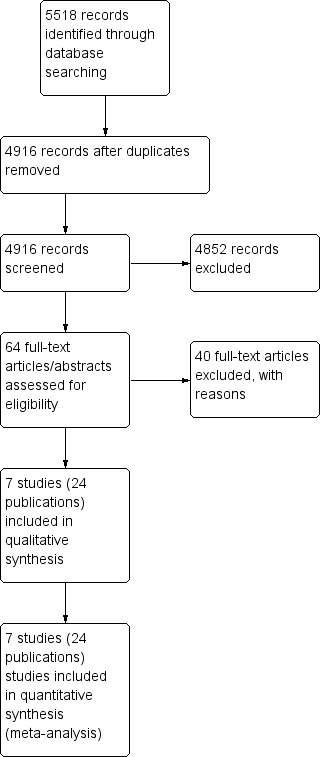
Study flow diagram.
Data extraction and management
Two review authors (OB, NS) extracted data as specified in the guidelines of Cochrane . If required, we contacted the authors of particular studies for supplementary information (Higgins 2011b).
For the data extraction we used a standardised form containing the following items:
general information: author, title, source, publication date, country, language, duplicate publications;
quality assessment: (as specified in the 'Assessment of risk of bias in included studies' section);
study characteristics: trial design, aims, setting and dates, source of patients, inclusion/exclusion criteria, comparability of groups, subgroup analysis, statistical methods, power calculations, treatment cross‐overs, compliance with assigned treatment, length of follow‐ up, time point of randomisation;
patient characteristics: age, gender, ethnicity, number of patients recruited/allocated/evaluated, patients lost to follow‐ up, additional diagnoses, stage of disease;
interventions: setting, type of (multi‐agent) chemotherapy (intensity of regimen, number of cycles), field and dose of radiotherapy, duration of follow‐ up;
outcomes: OS, PFS, response rate (CR, ORR), AEs .
Assessment of risk of bias in included studies
Two review authors (OB, NS) independently assessed the risk of bias in each study using the following criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b):
sequence generation;
allocation concealment;
blinding (patients, personnel, outcome assessors);
incomplete outcome data;
selective outcome reporting;
other sources of bias.
For every criterion we made a judgement using one of three categories:
'low risk': if the criterion was adequately fulfilled in the study (i.e. the study was at a low risk of bias for the given criterion);
'high risk': if the criterion was not fulfilled in the study (i.e. the study was at high risk of bias for the given criterion);
'unclear risk': if the study report did not provide sufficient information to allow for a judgement of 'low risk' or 'high risk', or if the risk of bias was unknown for one of the criteria listed above.
Measures of treatment effect
Time‐to‐event data
For treatment effect measures of individual trials estimated as hazard ratios (HRs) for OS and PFS/tumour control from survival analysis, we used methods described by Parmar 1998 and Tierney 2007. As no HRs were reported, we used logrank statistics through reported P values and numbers of events in comparison arm and estimated the HRs for OS and PFS/tumour control indirectly. When P values were not reported, we estimated HRs for OS and PFS/tumour control using survival curves data. Finally, we calculated and entered log HRs with standard errors (SEs) in RevMan 5 (RevMan 2014) for analysis.
Dichotomous data
We calculated effect measures of individual trials for ORR, CR and AEs as risk ratios (RRs).
Dealing with missing data
A number of potential sources for missing data are suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), which need to be taken into account: at study level, at outcome level and at summary data level. In the first instance, it is of the utmost importance to differentiate between data 'missing at random' and 'not missing at random'.
If data were missing, we intended in the next step, to request this from the original investigators. If, after this, data were still missing, we would have made explicit assumptions of any methods used: for example, that the data were assumed to be missing at random or that missing values were assumed to have a particular value, such as a poor outcome.
Additionally, we intended to perform sensitivity analyses to estimate how sensitive the results were to reasonable changes in the assumptions that we had made.
Assessment of heterogeneity
We assessed heterogeneity of treatment effects between trials using a Chi2 test with a significance level at P value < 0.05. We used the I2 statistic to quantify possible heterogeneity (I2 > 30 % moderate heterogeneity, I2 > 75 % considerable heterogeneity) (Deeks 2011). We intended to explore potential causes of heterogeneity through sensitivity and subgroup analyses.
Assessment of reporting biases
In meta‐analyses involving at least 10 trials, we intended to explore potential publication bias by generating a funnel plot and statistically testing this by conducting a linear regression test (Sterne 2011). We would have considered a P value of < 0.1 as significant for this test. However, as we included only seven trials in the review, this test was not conducted (Sterne 2011).
Data synthesis
We performed analyses according to the recommendations of Cochrane (Deeks 2011). We used the Cochrane statistical software Review Manager (RevMan) 5 (RevMan 2014) for analyses. We analysed same numbers and different numbers of chemotherapy cycles in each arm separately (e.g. three cycles in the experimental arm versus four cycles in the standard arm). Had the data been considered sufficiently similar to be combined, we intended to pool the results using a fixed‐effect model. Due to the clinical heterogeneity of the trials (e.g. different type of chemotherapy, starting points in different decades), we used a random‐effects model.
We used the GRADE (Grading of Recommendations Assessment, Development and Evaluation) profiler to create 'Summary of findings' tables, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We prioritised outcomes according to their relevance to patients. The most important outcome was OS, followed by PFS, AEs , CR and ORR.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses to investigate the potential causes of heterogeneity with different treatment effects in different groups.
Proportion of patients with early favourable stage HL versus early unfavourable stage HL.
Bulky versus non‐bulky disease e.g. I) with mediastinal mass versus without mediastinal mass II) with > 3 involved nodal areas versus < 3 involved nodal areas.
Different sequence of interventions e.g. chemotherapy + radiotherapy versus radiotherapy + chemotherapy versus chemotherapy‐radiotherapy‐chemotherapy.
Different radiotherapy treatment regimens e.g. involved‐field (IF)‐radiotherapy versus extended‐ field (EF)‐radiotherapy.
Different chemotherapy regimens e.g. ABVD ( adriamycin, bleomycin, vinblastine, and dacarbazine ) versus CVPP (cyclophosphamide, vinblastine, procarbazine, prednisone) versus EBVP (epirubicin, bleomycin, vinblastine, prednisone).
We assessed subgroup differences using the test for subgroup differences in RevMan5 (RevMan 2014).
Sensitivity analysis
We performed a sensitivity analysis to assess the robustness of the overall result with respect to quality and trial design. Using sensitivity analysis we explored the following .
Measures of study quality (intention‐t o ‐treat (ITT) analysis, > 10 % of patients not evaluated versus ≤ 10 % not evaluated).
Measures of OS, PFS, CR and ORR while excluding three trials (HD6; MSKCC trial #90‐44; UK NCRI Rapid), because of potential other high risk of bias (high number of patients did not receive the intended therapy and no per‐protocol results available).
Additional measures of PFS with per‐protocol results of one trial (UK NCRI Rapid).
Results
Description of studies
Results of the search
Our updated literature search led to 5518 potentially relevant references related to the treatment of patients with early stage Hodgkin lymphoma (HL). Of these, we identified 602 as duplicates and excluded 4852 at the initial stage of screening because they did not fulfil our predefined inclusion criteria. The remaining 64 publications we retrieved as full‐ text publications or abstract publications for detailed evaluation. Of these 64 trials, we excluded 40 trials. So finally we formally included seven trials (24 publications) with 2564 patients in the main analyses of this review.
The search in 2010 yielded 2800 references. Of these, we excluded 2749 at the initial stage. Of the remaining 51 trials, we excluded 41 and so we included five trials with 10 publications in the analyses of the review.
We documented the overall number of trials screened, identified, selected, excluded and included in a PRISMA flow diagram (Figure 1).
Included studies
See also the 'Characteristics of included studies' tables and Table 3.
1. Overview of study characteristics.
| CALGB 7751 | H10F | H10U | HD6 | EORTC‐GELA H9‐F | Mexico B2H031 | MSKCC trial #90‐44 | UK NCRI Rapid | |
| Number of patients evaluated | 18: chemotherapy 19: chemotherapy plus radiotherapy |
193: chemotherapy 188: chemotherapy plus radiotherapy |
268: chemotherapy 251: chemotherapy plus radiotherapy |
137: chemotherapy 139: chemotherapy plus radiotherapy |
130: chemotherapy 448: chemotherapy plus radiotherapy |
99: chemotherapy 102: chemotherapy plus radiotherapy |
76: chemotherapy 76: chemotherapy plus radiotherapy |
211: chemotherapy 209: chemotherapy plus radiotherapy |
| Chemotherapy and radiotherapy | 6 cycles of CVPP +/‐ involved‐field radiotherapy (dosage unknown) | 4 cycles of ABVD vs 3 cycles of ABVD + 30 Gy (+6 Gy) involved node radiotherapy | 6 cycles of ABVD vs 4 cycles of ABVD + 30 Gy (+6 Gy) involved node radiotherapy | 4 cycles of ABVD or 2 cycles of ABVD + 35 Gy subtotal nodal radiotherapy | 6 cycles of EBVP +/‐ IF radiotherapy | 6 cycles of ABVD +/‐ EF‐radiotherapy | 6 cycles of ABVD +/‐ EF or IF radiotherapy | 3 cycles of ABVD +/‐ 30 Gy IF‐radiotherapy |
| Median duration of follow‐up | 1.8 years | 1.1 years | 1.1 years | 11.3 years | 4.3 years | 11.4 years | 5.6 years | 60 months |
We included seven trials (CALGB 7751; EORTC‐GELA H9‐F; H10F/H10U; HD6; Mexico B2H031; MSKCC trial #90‐44; UK NCRI Rapid) in this review. Five trials had the same number of chemotherapy cycles in each arm (CALGB 7751; EORTC‐GELA H9‐F; Mexico B2H031; MSKCC trial #90‐44; UK NCRI Rapid), two with different numbers of chemotherapy cycles in the experimental arm and the standard arm (H10F/H10U; HD6). The earliest trial recruited in the 1970s and the latest between 2003 and 2010. We extracted the data from full‐ text publications for six trials and for one trial (EORTC‐GELA H9‐F), from the abstract. For the H10 trial we analysed the two subgroups H10F and H10U separately.
Design
Of the seven included trials, three are two‐armed randomised controlled trials (RCTs) (CALGB 7751; MSKCC trial #90‐44; UK NCRI Rapid); four are three‐armed RCTs :
One trial (H10F/H10U) divided patients into two main groups (favourable (F) and unfavourable (U)) prior to randomisation, and then used a three‐armed design for each group: one standard arm (chemotherapy plus radiotherapy), one positron emission tomography (PET)‐negative (chemotherapy alone) and one PET‐positive arm (chemotherapy plus radiotherapy) in each group. We excluded the PET‐positive arm of each group in this systematic review.
Mexico B2H031 randomised patients to radiotherapy alone, chemotherapy plus radiotherapy or chemotherapy alone. We excluded the radiotherapy arm in this systematic review.
EORTC‐GELA H9‐F randomised patients to chemotherapy alone, chemotherapy plus radiotherapy with 36 Gy or chemotherapy plus radiotherapy with 20 Gy. We evaluated the two radiotherapy dosages together in this review.
HD6 randomised patients to chemotherapy alone versus radiotherapy alone or chemotherapy plus radiotherapy. In the experimental arm people with an unfavourable risk profile received chemotherapy plus radiotherapy, whereas people with a favourable risk profile received radiotherapy only. In the standard arm, people with unfavourable and favourable risk profile both received chemotherapy only. We considered in this review only people with an unfavourable risk profile and the comparison chemotherapy alone versus chemotherapy plus radiotherapy.
Two trials compared different numbers of chemotherapy cycles in the arms (H10F/H10U; HD6).
Sample sizes
The smallest trial included 55 (37 analysed) patients (CALGB 7751) and the largest trial 1137 (900 PET‐negative patients analysed) patients (H10F/H10U).
Location
The included trials came from a range of research groups from different countries: one trial in USA (CALGB 7751); one trial in USA and Canada (MSKCC trial #90‐44); one trial in USA, Canada and Italy (HD6); two trials in different institutions of European countries (EORTC‐GELA H9‐F; H10F/H10U); one trial in Mexico (Mexico B2H031); and one trial in UK (UK NCRI Rapid).
Participants
This review included a total of 2564 male and female adults, with a newly confirmed diagnosis of clinical stage (CS) I and II or pathologic stage (PS) I and II HL and without previous treatment.
Interventions
Chemotherapy cycles used:
six cycles of chemotherapy alone or six cycles of same chemotherapy plus radiotherapy in four trials (CALGB 7751; EORTC‐GELA H9‐F; Mexico B2H031; MSKCC trial #90‐44);
three cycles of chemotherapy alone or three cycles of same chemotherapy plus radiotherapy in one trial (UK NCRI Rapid);
six cycles of chemotherapy alone or four cycles of same chemotherapy plus radiotherapy in a subgroup of one trial (H10U);
four cycles of chemotherapy alone or three cycles of same chemotherapy plus radiotherapy in a subgroup of one trial (H10F);
four cycles of chemotherapy alone or two cycles of same chemotherapy plus radiotherapy in one trial (HD6).
Chemotherapy regimens used:
ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) in five trials (H10F/H10U; HD6; Mexico B2H031; MSKCC trial #90‐44; UK NCRI Rapid);
CVPP (cyclophosphamide, vinblastine, procarbazine, prednisone) in one trial (CALGB 7751);
EBVP (epirubicin, bleomycin, vinblastine, prednisone) in one trial (EORTC‐GELA H9‐F).
Size of radiation fields used for the delivery of radiotherapy:
involved‐field radiotherapy in three trials (CALGB 7751; EORTC‐GELA H9‐F; UK NCRI Rapid);
extended‐field radiotherapy in one trial (Mexico B2H031);
mixed radiotherapy (extended‐field or involved‐field radiotherapy) in one trial (MSKCC trial #90‐44);
subtotal nodal radiotherapy in one trial (HD6);
One trial (Mexico B2H031) administrated three cycles of chemotherapy before and after radiotherapy (sandwich technique); in the other trials chemotherapy was administered prior to radiotherapy.
Outcomes
Primary outcome measure
Six of the seven trials analysed overall survival (CALGB 7751; EORTC‐GELA H9‐F; HD6; Mexico B2H031; MSKCC trial #90‐44; UK NCRI Rapid).
Secondary outcome measures
Not all of the included trials reported p rogression‐free survival (PFS) data according to our definition (time to progression or death of any cause). All trials except CALGB 7751 reported some type of progression outcome (see Table 4). Three trials (CALGB 7751; Mexico B2H031; MSKCC trial #90‐44) reported response rate. Most trials reported adverse events (AEs), but in different ways. Two trials reported infection‐ related mortality (HD6; MSKCC trial #90‐44), four trials second cancer‐ related mortality (EORTC‐GELA H9‐F; HD6; Mexico B2H031; UK NCRI Rapid), and three cardiac disease‐ related mortality (CALGB 7751; Mexico B2H031; UK NCRI Rapid). No trial reported infertility.
2. Definitions of progression outcomes.
| Trial | Definition of progression outcome. |
| EORTC‐GELA H9‐F | Definition of disease‐free survival not reported (Note all patients are in CR at the time of randomisation). |
| H10F/H10U | From the date of random assignment to date of progression—as relapse after previous complete remission or progression after reaching partial remission (>= 50% decrease and resolution of B symptoms and no new lesions) or progressive disease (50% increase from nadir of any previous partial remission lesions or appearance of new lesions) on computed tomography scan measurements during protocol treatment or death resulting from any cause, whichever occurred first. |
| HD6 | Measured as event‐free survival from the date of randomisation until the date of disease progression or death from any cause. |
| Mexico B2H031 | Contradictory definitions. In the methods section: “Disease free survival was calculated for CR patients from the beginning of treatment until clinically or radiologically and biopsy proven relapse.” In the results section the percentage disease free were calculated based on the full population. |
| MSKCC trial #90‐44 | Time from enrolment until any progression of disease. |
| UK NCRI Rapid | Time from the date of randomisation to first progression, relapse, or death, whichever occurred first. |
Conflict of interest
No trial reported information with respect to conflict of interest.
Excluded studies
After the screening of abstracts we excluded 4852 trials that clearly did not match our inclusion criteria.
We excluded a total of 40 articles after detailed evaluation of full text publications with the following main reasons:
eight trials were non‐randomised comparisons or reviews (Cimino 1990; Cosset 1992; Kim 2003; Körholz 2004; Longo 1992; Meyer 2013; Reinartz 2013; Specht 1992);
16 trials not chemotherapy alone versus chemotherapy plus radiotherapy (Andrieu 1999; Brusamolino 1994; Cheveresan 1998; Desablens 1999; Dionet 1988; Ferme 2005; Horning 2007; Noordijk 2006; Pavlovsky 1997; Radford 2002; Rüffer 1996; Rüffer 1998; Rüffer 1999; Straus 1989; Thistlethwaite 2007; Thomas 2004);
eight trials involved < 80% early stage patients (Bonnet 2007; Horning 1996; Kung 1993; Kung 2006; Laskar 2004; O'Dwyer 1984; O'Dwyer 1985; Picardi 2007);
six trials did not include adults (Friedmann 2014; Lemerle 1986; Nachman 2002; Pavlovsky 1988; Weiner 1997; Wolden 2012);
two publications were of "one trial" (Hirsch 1994; Hirsch 1996), where MSKCC patients were randomised to chemotherapy alone versus chemotherapy plus radiotherapy, or different chemotherapy plus differing radiotherapy schemes were followed for pulmonary function for approximately one year. The 45 patients with a relevant comparison to this review are presumably included in the MSKCC trial #90‐44.
These publications we described under Characteristics of excluded studies.
Risk of bias in included studies
See 'Risk of bias' tables sections in the 'Characteristics of included studies' tables and to Figure 2: this 'Risk of bias' summary figure presents all our judgements in a cross‐tabulation of study by entry. Overall, we considered the quality of included trials to be moderate.
2.
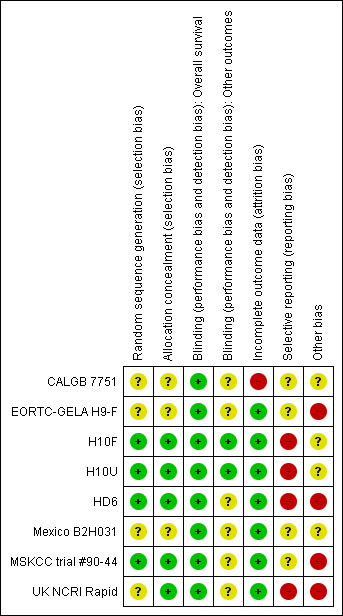
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
A random component in the sequence generation process described in three trials (H10F/H10U; HD6; MSKCC trial #90‐44), we judged the risk of selection bias as low. The other trials are described as "randomised trials" without further information about the process of randomisation (CALGB 7751; EORTC‐GELA H9‐F; Mexico B2H031; UK NCRI Rapid), so we judged the risk of bias as unclear.
Four trials performed treatment allocation of patients at a central trial office (H10F/H10U; HD6; MSKCC trial #90‐44; UK NCRI Rapid), we judged these to be at low risk of potential bias. We judged the risk of selection bias for the other three trials to be unclear (CALGB 7751; EORTC‐GELA H9‐F; Mexico B2H031), because of no available information regarding the allocation concealment.
Blinding
As radiotherapy is difficult to blind, one does not expect the patients to be blinded. However, one trial reported information about blinding of outcome assessors or statisticians (H10F/H10U), so we judged the risk of bias as low. As blinding of the outcome assessors is considered important for this review, we judged the other trials as unclear for the question of blinding.
Although the trials were not blinded, this does not affect the outcome overall survival (OS). Therefore, we judged the risk of blinding for OS as low for all trials.
Incomplete outcome data
Four trials described missing outcome data in detail or included all randomised patients in the analysis without reporting any missing data for this outcome (EORTC‐GELA H9‐F; H10F/H10U; MSKCC trial #90‐44; UK NCRI Rapid); hence we judged the risk of bias as low.
In the Mexico B2H031 trial 20/327 patients were missing from the analyses without further information (this information was not available only for the two arms included in this review). As the number of missing patients is less than 10%, we judged the risk of bias as low. Similarly for the HD6 trial with 6/405 patients not analysed. The CALGB 7751 trial did not analyse a high proportion of patients (18/55 patients), therefore we judged the risk of bias as high.
Selective reporting
Most of the trials reported little information about which outcomes were primary outcomes and how these were defined, so we judged the risk of bias as unclear for these trials (CALGB 7751; EORTC‐GELA H9‐F; Mexico B2H031; MSKCC trial #90‐44). Three trials did not report all of the study’s pre‐specified secondary outcomes (H10F/H10U; HD6; UK NCRI Rapid), hence we judged the risk of bias as high. We found study protocols for four trials only (EORTC‐GELA H9‐F; H10F/H10U; HD6; UK NCRI Rapid).
Other potential sources of bias
Three trials did not provide sufficient information to assess whether an important risk of bias exists, therefore we judged the risk of other potential sources of bias as unclear (CALGB 7751; H10F/H10U; Mexico B2H031). One trial ended early due to predefined stopping rule. The hazard ratio (HR) estimate is based on the full group receiving additional radiotherapy and not only those patients up to the time the no radiotherapy arm was stopped (EORTC‐GELA H9‐F). This is known to increase the effect estimate of trials. In addition, the data are preliminary. So we judged the risk of bias as high for this trial. In the UK NCRI Rapid trial 28 of 420 patients did not receive treatment as randomised: two received radiotherapy in the chemotherapy alone arm and 26 did not receive radiotherapy in the chemotherapy plus radiotherapy arm. Five of the eight patients who died in the chemotherapy plus radiotherapy arm, received no radiotherapy. These patients were still included in the analysis. Because this could effect the results, we judged the risk of bias as high. In the MSKCC trial #90‐44 trial, 11 patients randomised to the chemotherapy plus radiotherapy arm never received radiotherapy (six refused, four progressed on chemotherapy prior to receiving radiotherapy, one never received radiotherapy because of bleomycin‐ induced toxicity to radiotherapy). In the HD6 trial, a total of 41 patients did not re c eive the assigned therapy. Among the patients in the chemotherapy arm, 16 of 196 patients without any further subdivision into favourable and unfavourable risk profile, in the radiation‐therapy group, 11 of the 64 patients in the cohort with a favourable risk profile (radiotherapy alone) and 14 of the 139 in the cohort with an unfavourable risk profile (chemotherapy plus radiotherapy) did not receive the assigned therapy. For both these trials, we judged the risk of bias as high.
Effects of interventions
1. Same number of chemotherapy cycles in both arms
Five of the seven trials evaluated the same number of chemotherapy cycles in each arm (CALGB 7751; EORTC‐GELA H9‐F; Mexico B2H031; MSKCC trial #90‐44; UK NCRI Rapid).
Primary outcome Overall survival (OS)
Patients
Five trials with 1388 patients reported OS (CALGB 7751; EORTC‐GELA H9‐F; Mexico B2H031; MSKCC trial #90‐44; UK NCRI Rapid). In the UK NCRI Rapid trial, 12% of the patients in the chemotherapy plus radiotherapy arm did not receive treatment as randomised: 26 did not receive radiotherapy. Five of the eight patients who died in the chemotherapy plus radiotherapy arm received no radiotherapy. These patients were included in the published analysis. In the MSKCC trial #90‐44 trial, 11 patients randomised to radiotherapy never received radiotherapy. Because this affects the results and no per‐protocol results were available for both trials, we excluded the trials from the meta‐analysis for a sensitivity analysis and performed subgroup analyses without these trials as well (three trials with 816 patients).
Results
The addition of radiotherapy to chemotherapy has probably little or no difference on OS (hazard ratio (HR) = 0.48; 95% confidence interval (CI) 0.22 to 1.06; P = 0.07, see Analysis 1.1), with a moderate grade of heterogeneity between trials (I2 = 52%). One reason for the heterogeneity could be the results of the UK NCRI Rapid and MSKCC trial #90‐44 trials. In the UK NCRI Rapid trial 28 of 420 patients did not receive treatment as randomised: two received radiotherapy in the chemotherapy alone arm and 26 did not receive radiotherapy in the chemotherapy plus radiotherapy arm. Five of the eight patients who died in the chemotherapy plus radiotherapy arm received no radiotherapy. These patients were included in the published analysis of the trial. In the MSKCC trial #90‐44 trial, 11 patients randomised to the chemotherapy plus radiotherapy arm never received radiotherapy (six refused, fo ur progressed on chemotherapy prior to receiving radiotherapy, one never received radiotherapy because of bleomycin‐ induced toxicity to radiotherapy).
1.1. Analysis.
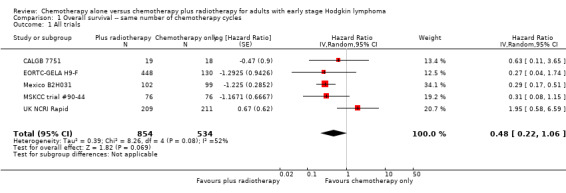
Comparison 1 Overall survival ‐‐ same number of chemotherapy cycles, Outcome 1 All trials.
In a sensitivity analysis without UK NCRI Rapid and MSKCC trial #90‐44, the addition of radiotherapy to chemotherapy significantly improved OS (HR 0.31; 95% CI 0.19 to 0.52; P < 0.00001). We found no evidence of heterogeneity across the trials in the meta‐analysis (P value of the homogeneity test = 0.72; I² = 0%), see Figure 3.
3.
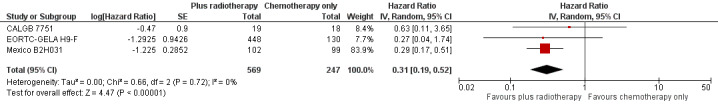
Forest plot of comparison: 2 Overall survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44 , outcome: 2.1 Sensitivity analysis ‐ without UK NCRI Rapid and MSKCC trial #90‐44.
Subgroup analyses
The subgroup analyses showed no statistically significant differences between the subgroups examined (early favourable or unfavourable disease, bulky or non‐bulky disease, timing and type of radiation therapy, type of chemotherapy). See Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6.
1.2. Analysis.
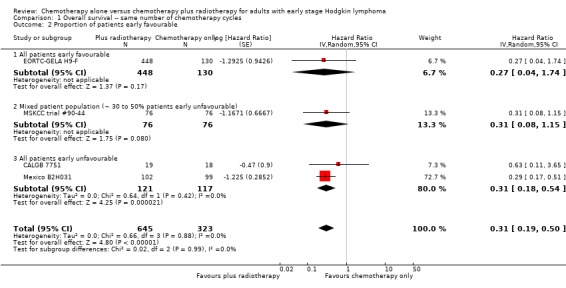
Comparison 1 Overall survival ‐‐ same number of chemotherapy cycles, Outcome 2 Proportion of patients early favourable.
1.3. Analysis.
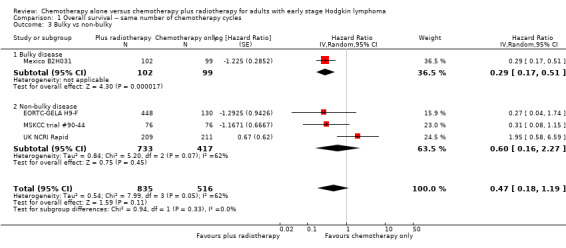
Comparison 1 Overall survival ‐‐ same number of chemotherapy cycles, Outcome 3 Bulky vs non‐bulky.
1.4. Analysis.
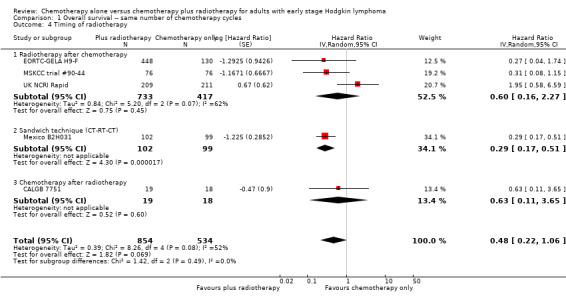
Comparison 1 Overall survival ‐‐ same number of chemotherapy cycles, Outcome 4 Timing of radiotherapy.
1.5. Analysis.
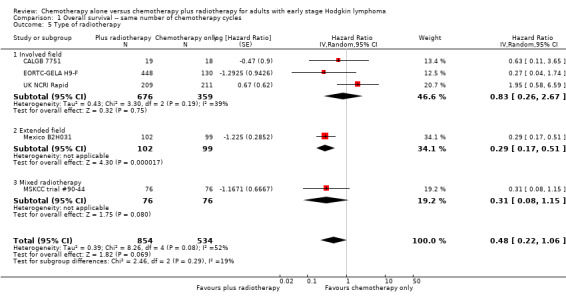
Comparison 1 Overall survival ‐‐ same number of chemotherapy cycles, Outcome 5 Type of radiotherapy.
1.6. Analysis.
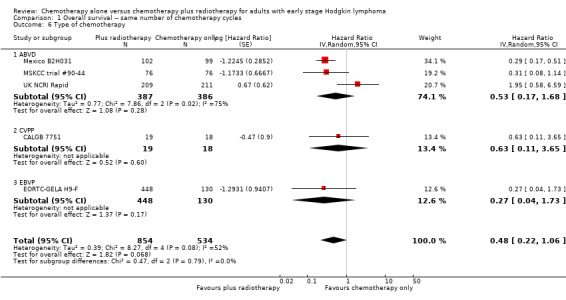
Comparison 1 Overall survival ‐‐ same number of chemotherapy cycles, Outcome 6 Type of chemotherapy.
The P value for the intention‐to‐teat (ITT)‐analysis is P = 0.78, so there are no statistically significant differences between the subgroups in the performed sensitivity analysis, see Analysis 1.7.
1.7. Analysis.
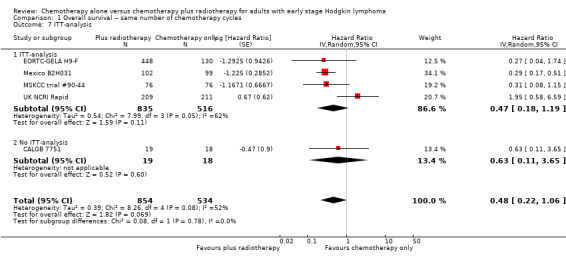
Comparison 1 Overall survival ‐‐ same number of chemotherapy cycles, Outcome 7 ITT‐analysis.
Subgroup analyses withoutUK NCRI RapidandMSKCC trial #90‐44
The subgroup analyses without UK NCRI Rapid and MSKCC trial #90‐44 showed no statistically significant differences between the subgroups examined (early favourable or unfavourable disease, bulky or non‐bulky disease, timing of radiotherapy, type of radiotherapy and type of chemotherapy. See Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6.
2.2. Analysis.
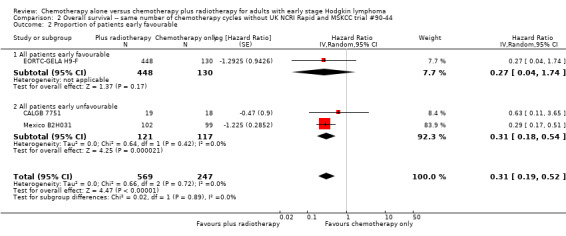
Comparison 2 Overall survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 2 Proportion of patients early favourable.
2.3. Analysis.
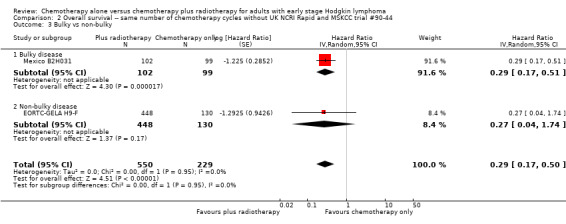
Comparison 2 Overall survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 3 Bulky vs non‐bulky.
2.4. Analysis.
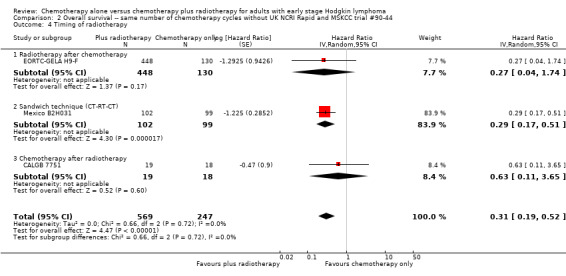
Comparison 2 Overall survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 4 Timing of radiotherapy.
2.5. Analysis.
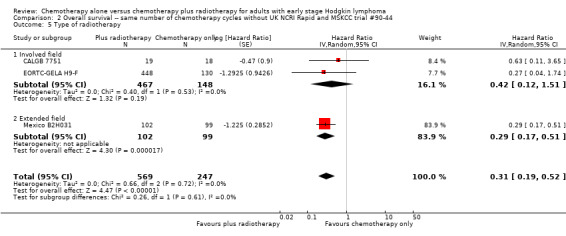
Comparison 2 Overall survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 5 Type of radiotherapy.
2.6. Analysis.

Comparison 2 Overall survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 6 Type of chemotherapy.
There are no statistically significant subgroup differences in the ITT‐analysis as well (P = 0.42), see Analysis 2.7.
2.7. Analysis.
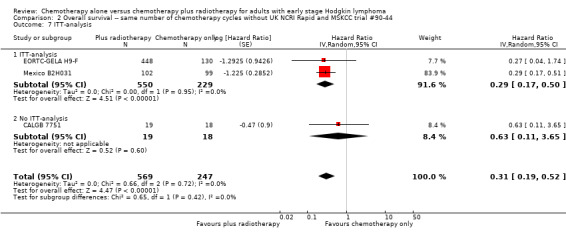
Comparison 2 Overall survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 7 ITT‐analysis.
Secondary outcomes
Progression‐free survival (PFS)
Patients
Four trials with 1351 patients reported PFS (EORTC‐GELA H9‐F; Mexico B2H031; MSKCC trial #90‐44; UK NCRI Rapid).
Results
Not all trials reported PFS according to the definition in the protocol (time to progression or death from any cause). However, all trials in the main analysis reported some progression endpoint, such as event‐free survival, time to treatment failure and time to progression, and were evaluated as tumour control. We have provided exact given definitions in Table 4.
The combination of chemotherapy and radiotherapy probably improved PFS statistically significantly (HR 0.42; 95% CI 0.25 to 0.72); P = 0.001; see Figure 4), with a clear statistical heterogeneity between trials (I2 = 69%), which may in part be due to the different definitions used. For example, some trials examined progression or freedom from treatment failure in all patients, while others examined disease‐free survival, which is restricted to patients who reached a complete response (CR).
4.
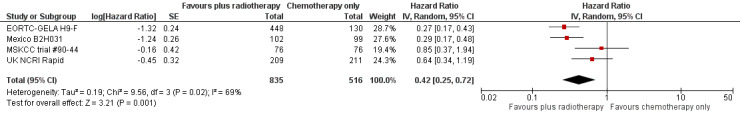
Forest plot of comparison: 2 Progression‐free survival, outcome: 2.1 All trials.
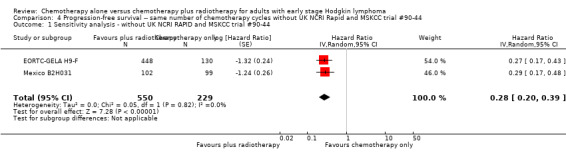
In a sensitivity analysis without the UK NCRI Rapid and MSKCC trial #90‐44 trials, the addition of radiotherapy to chemotherapy significantly improved PFS (HR 0.28, 95% CI 0.20 to 0.39; P < 0.00001), see Analysis 4.1.
4.1. Analysis.
Comparison 4 Progression‐free survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 1 Sensitivity analysis ‐ without UK NCRI RAPID and MSKCC trial #90‐44.
Subgroup analyses
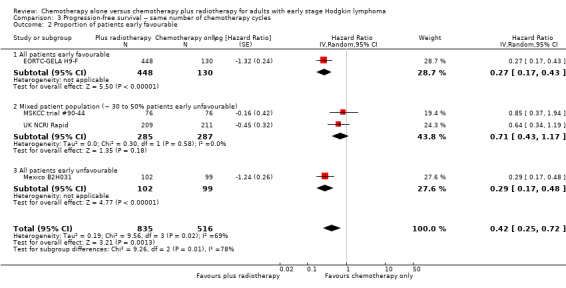
The subgroup analysis including the UK NCRI Rapid and MSKCC trial #90‐44 trials by proportion of patients with early favourable disease (P = 0.01, see Analysis 3.2), which showed statistically significant differences. In trials with early favourable patients (EORTC‐GELA H9‐F, 578 patients, HR 0.27; 95% CI 0.17 to 0.43; P < 0.00001) and unfavourable patients only (Mexico B2H031, 201 patients, HR 0.29; 95% CI 0.17 to 0.48; P < 0.00001), the addition of radiotherapy to chemotherapy significantly improved PFS. The trials with mixed patient population (MSKCC trial #90‐44; UK NCRI Rapid, 572 patients) showed no evidence for a difference between treatments (HR 0.71; 95% CI 0.43 to 1.17; P = 0.18).
3.2. Analysis.
Comparison 3 Progression‐free survival ‐‐ same number of chemotherapy cycles, Outcome 2 Proportion of patients early favourable.
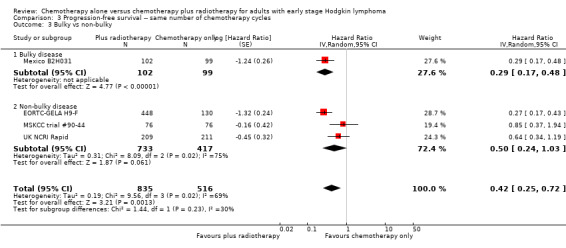
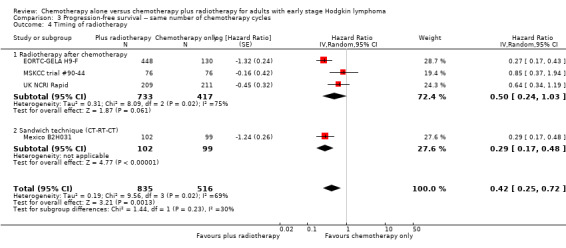
The other subgroup analyses showed no statistically significant differences between the subgroups examined (bulky or non‐bulky disease, timing of radiotherapy, type of radiotherapy, type of chemotherapy), see Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6.
3.3. Analysis.
Comparison 3 Progression‐free survival ‐‐ same number of chemotherapy cycles, Outcome 3 Bulky vs non‐bulky.
3.4. Analysis.
Comparison 3 Progression‐free survival ‐‐ same number of chemotherapy cycles, Outcome 4 Timing of radiotherapy.
3.5. Analysis.
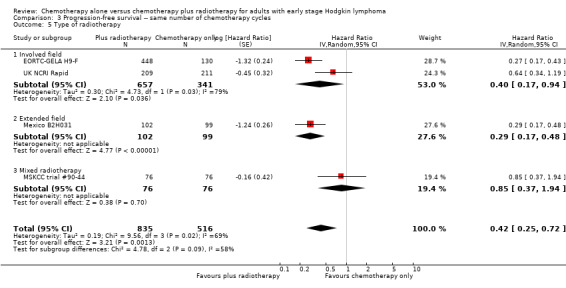
Comparison 3 Progression‐free survival ‐‐ same number of chemotherapy cycles, Outcome 5 Type of radiotherapy.
3.6. Analysis.
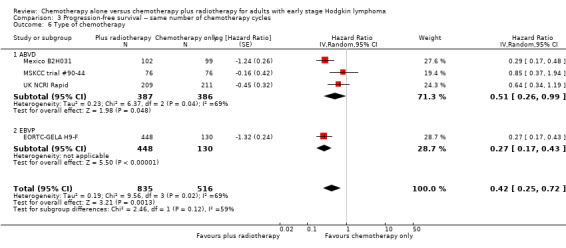
Comparison 3 Progression‐free survival ‐‐ same number of chemotherapy cycles, Outcome 6 Type of chemotherapy.
Because of the potential other high risk of bias of the UK NCRI Rapid trial, we used the per‐protocol results of this trial for another sensitivity analysis. We excluded the MSKCC trial #90‐44 trial because no per‐protocol results were available. The results of the analysis agreed with the main results that the combination of chemotherapy and radiotherapy probably improved PFS in a statistically significant way (HR 0.30; 95% CI 0.22 to 0.41; P < 0.00001), see Analysis 3.7.
3.7. Analysis.
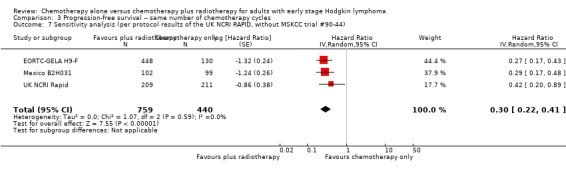
Comparison 3 Progression‐free survival ‐‐ same number of chemotherapy cycles, Outcome 7 Sensitivity analysis (per protocol results of the UK NCRI RAPID, without MSKCC trial #90‐44).
Subgroup analyses withoutUK NCRI RapidandMSKCC trial #90‐44
The subgroup analyses without the UK NCRI Rapid and MSKCC trial #90‐44 trials showed no statistically significant differences between the subgroups examined (early favourable or unfavourable disease, bulky or non‐bulky disease, timing of radiotherapy, type of radiotherapy and type of chemotherapy, see Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6).
4.2. Analysis.
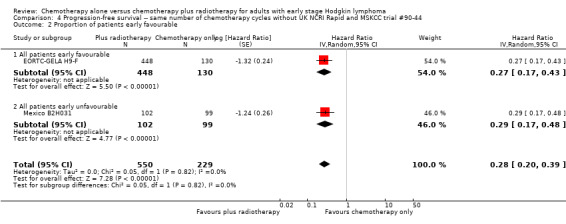
Comparison 4 Progression‐free survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 2 Proportion of patients early favourable.
4.3. Analysis.
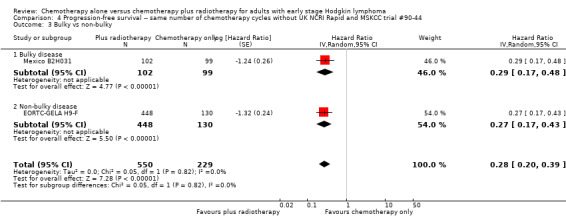
Comparison 4 Progression‐free survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 3 Bulky vs non‐bulky.
4.4. Analysis.
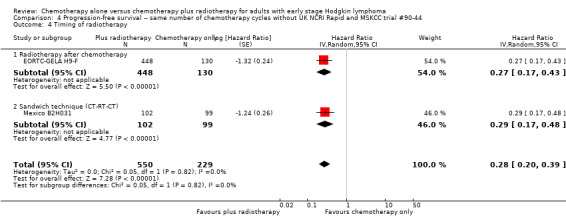
Comparison 4 Progression‐free survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 4 Timing of radiotherapy.
4.5. Analysis.
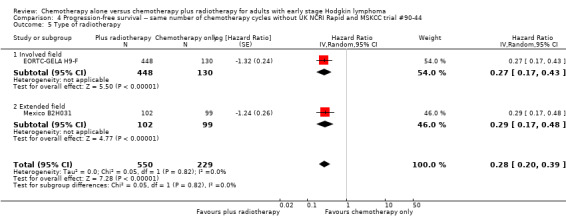
Comparison 4 Progression‐free survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 5 Type of radiotherapy.
4.6. Analysis.
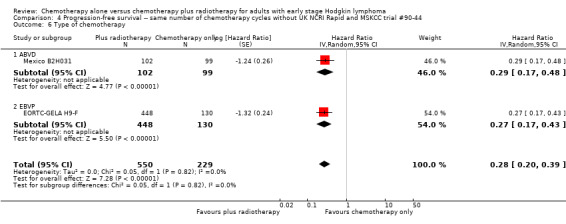
Comparison 4 Progression‐free survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 6 Type of chemotherapy.
Complete response (CR)
Patients
Three trials including 376 patients reported the CR rate (CALGB 7751; Mexico B2H031; MSKCC trial #90‐44).
Results
We found no evidence of an improvement in CR in favour of the chemotherapy plus radiotherapy group (risk ratio (RR) 1.08; 95% CI 0.93 to 1.25; P = 0.33), with a clear statistical heterogeneity between trials (I2 = 67%), without explainable reasons (Figure 5). In a sensitivity analysis without the MSKCC trial #90‐44 trial, the results do not change (RR 1.22; 95% CI 0.84 to 1.78; P = 0.28, see Analysis 6.1).
5.
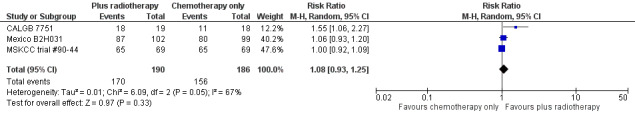
Forest plot of comparison: 3 Complete response rate, outcome: 3.1 All trials.
6.1. Analysis.
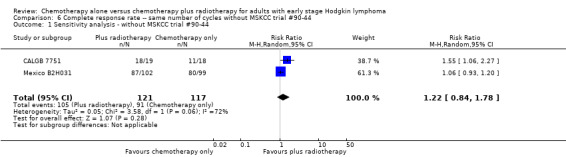
Comparison 6 Complete response rate ‐‐ same number of cycles without MSKCC trial #90‐44, Outcome 1 Sensitivity analysis ‐ without MSKCC trial #90‐44.
Subgroup analyses
A subgroup analysis by type of chemotherapy showed statistically significant differences (P = 0.03), see Analysis 5.6. In one trial (CALGB 7751, 37 patients), chemotherapy plus radiotherapy showed a statistically significant improved CR by administering CVPP (RR 1.55; 95% CI 1.06 to 2.27; P = 0.03), the use of ABVD showed no evidence for differences between chemotherapy alone and chemotherapy plus radiotherapy regarding CR (Mexico B2H031; MSKCC trial #90‐44, 339 patients, RR 1.02; 95% CI 0.95 to 1.09; P = 0.64).
5.6. Analysis.
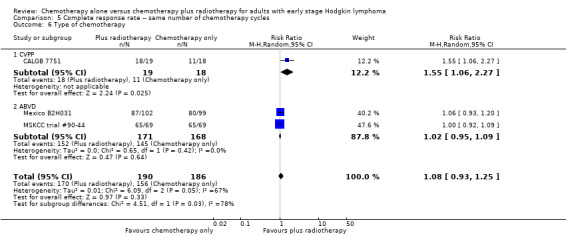
Comparison 5 Complete response rate ‐‐ same number of chemotherapy cycles, Outcome 6 Type of chemotherapy.
The other subgroup and sensitivity analyses showed no statistically significant differences between the subgroups (early favourable or unfavourable disease, bulky or non‐bulky disease, timing and type of radiotherapy, see Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5.
5.2. Analysis.
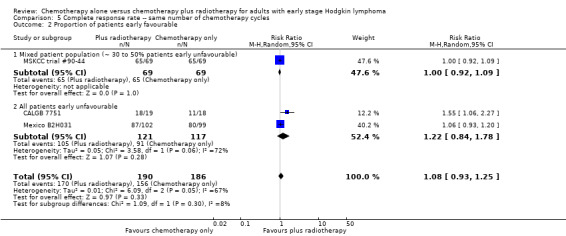
Comparison 5 Complete response rate ‐‐ same number of chemotherapy cycles, Outcome 2 Proportion of patients early favourable.
5.3. Analysis.
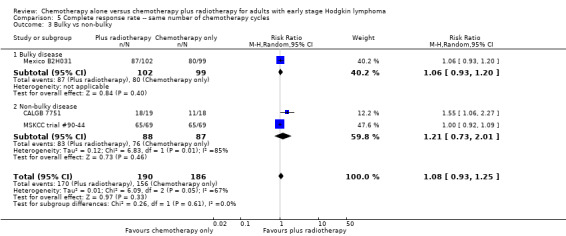
Comparison 5 Complete response rate ‐‐ same number of chemotherapy cycles, Outcome 3 Bulky vs non‐bulky.
5.4. Analysis.
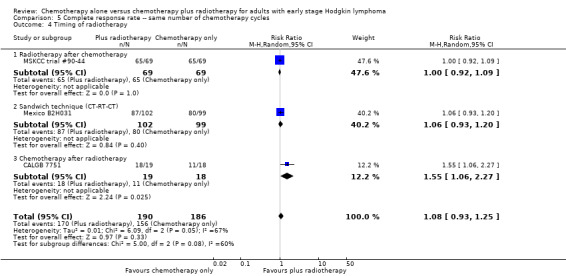
Comparison 5 Complete response rate ‐‐ same number of chemotherapy cycles, Outcome 4 Timing of radiotherapy.
5.5. Analysis.
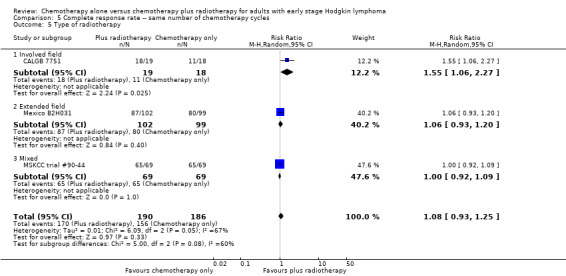
Comparison 5 Complete response rate ‐‐ same number of chemotherapy cycles, Outcome 5 Type of radiotherapy.
There are statistically significant subgroup differences in the ITT‐analysis (P = 0.03), see Analysis 5.7. In one trial without ITT‐analysis (CALGB 7751, 37 patients), chemotherapy plus radiotherapy showed a statistically significant improved CR. The trials with ITT‐analysis (≤ 10 % of patients not evaluated; Mexico B2H031; MSKCC trial #90‐44, 339 patients, RR 1.02; 95% CI 0.95 to 1.09; P = 0.64) showed no evidence for a difference between the trials.
5.7. Analysis.

Comparison 5 Complete response rate ‐‐ same number of chemotherapy cycles, Outcome 7 ITT‐analysis.
Subgroup analyses withoutMSKCC trial #90‐44
Without the MSKCC trial #90‐44 trial, the subgroup analyses showed no statistically significant differences between the subgroups examined (bulky or non‐bulky disease, timing and type of radiation therapy, type of chemotherapy, see Analysis 6.2; Analysis 6.3; Analysis 6.4; Analysis 6.5) and the ITT‐analysis, see Analysis 6.6.
6.2. Analysis.
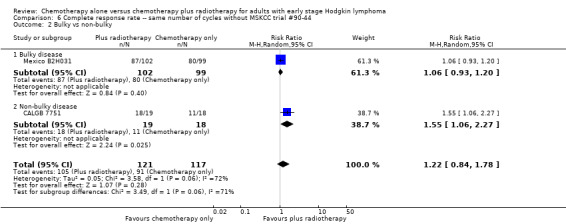
Comparison 6 Complete response rate ‐‐ same number of cycles without MSKCC trial #90‐44, Outcome 2 Bulky vs non‐bulky.
6.3. Analysis.
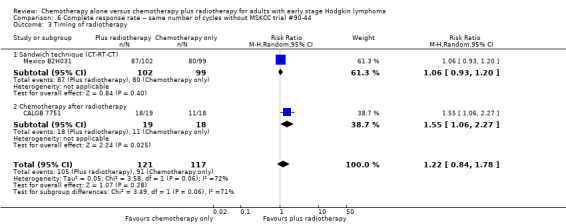
Comparison 6 Complete response rate ‐‐ same number of cycles without MSKCC trial #90‐44, Outcome 3 Timing of radiotherapy.
6.4. Analysis.
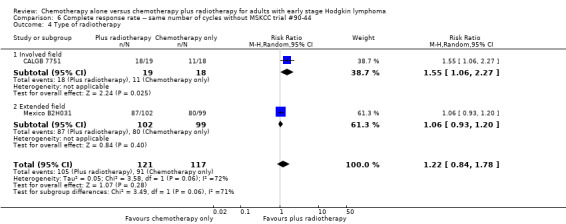
Comparison 6 Complete response rate ‐‐ same number of cycles without MSKCC trial #90‐44, Outcome 4 Type of radiotherapy.
6.5. Analysis.
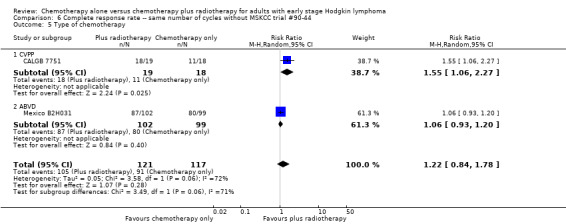
Comparison 6 Complete response rate ‐‐ same number of cycles without MSKCC trial #90‐44, Outcome 5 Type of chemotherapy.
6.6. Analysis.
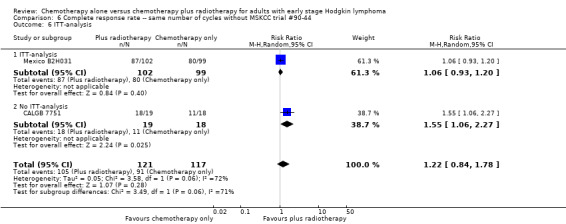
Comparison 6 Complete response rate ‐‐ same number of cycles without MSKCC trial #90‐44, Outcome 6 ITT‐analysis.
Overall response rate (ORR)
Patients
Two trials including 339 patients reported ORR (Mexico B2H031; MSKCC trial #90‐44).
Results
We found no evidence of a statistically significant difference regarding ORR between the chemotherapy alone group and the chemotherapy plus radiotherapy group (RR 1.00; 95% CI 0.94 to 1.07; P = 0.98), with a fixed‐effect analysis. We found no evidence of heterogeneity across the trials in the meta‐analysis (P value of the homogeneity test = 0.37; I² = 0%) (Figure 6). Without the MSKCC trial #90‐44 trial, only one trial reported ORR and the results do not change (RR 1.04; 95% CI 0.92 to 1.18; P = 0.51), see Analysis 8.1.
6.
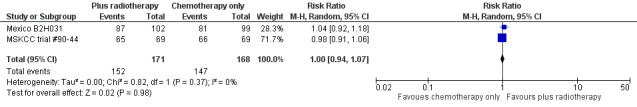
Forest plot of comparison: 4 Overall Response Rate, outcome: 4.1 All Trials.
8.1. Analysis.
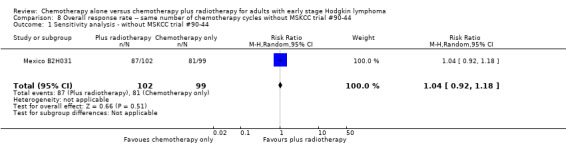
Comparison 8 Overall response rate ‐‐ same number of chemotherapy cycles without MSKCC trial #90‐44, Outcome 1 Sensitivity analysis ‐ without MSKCC trial #90‐44.
Subgroup analyses
No subgroup analysis showed statistically significant differences (P = 0.44 for early favourable or unfavourable disease, bulky or non‐bulky disease, timing and type of radiotherapy, see Analysis 7.2; Analysis 7.3; Analysis 7.4; Analysis 7.5).
7.2. Analysis.
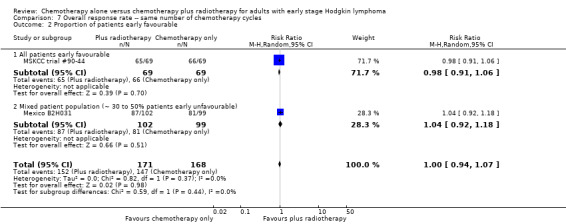
Comparison 7 Overall response rate ‐‐ same number of chemotherapy cycles, Outcome 2 Proportion of patients early favourable.
7.3. Analysis.
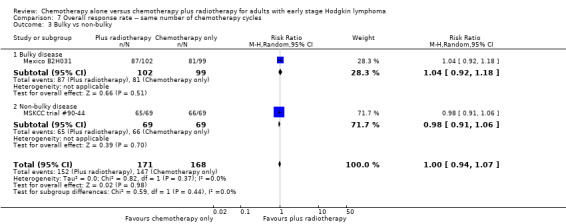
Comparison 7 Overall response rate ‐‐ same number of chemotherapy cycles, Outcome 3 Bulky vs non‐bulky.
7.4. Analysis.
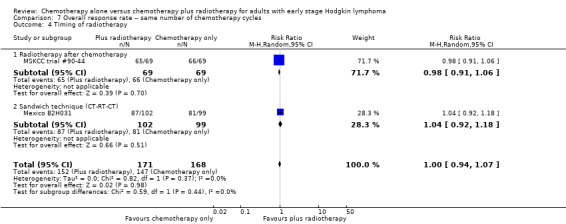
Comparison 7 Overall response rate ‐‐ same number of chemotherapy cycles, Outcome 4 Timing of radiotherapy.
7.5. Analysis.
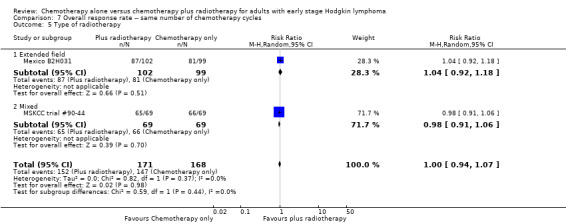
Comparison 7 Overall response rate ‐‐ same number of chemotherapy cycles, Outcome 5 Type of radiotherapy.
Subgroup analyses withoutMSKCC trial #90‐44
Without the MSKCC trial #90‐44 trial, only one trial reported ORR (Mexico B2H031), therefore we did not perform subgroup analyses.
Adverse events (AEs)
Most adverse events reported in the trials seem to be similar in both groups and are typical for the chemotherapy received (e.g. haematological effects, bleomycin‐ induced lung disease). Only a few trials reported AEs considered of particular interest (secondary malignancies, cardiac disease). Regarding infection‐ related mortality, (one trial (MSKCC trial #90‐44), 152 patients, RR 0.33; 95% CI 0.01 to 8.06; P = 0.5), second cancer‐ related mortality (three trials (EORTC‐GELA H9‐F; Mexico B2H031; UK NCRI Rapid), 1199 patients, RR 0.53; 95% CI 0.07 to 4.29; P = 0.55), and cardiac disease‐ related mortality (two trials (CALGB 7751; UK NCRI Rapid), 457 patients, RR 2.94; 95% CI 0.31 to 27.55; P = 0.35), there are no statistically significant differences between the use of chemotherapy alone and chemotherapy plus radiotherapy (see Analysis 9.1; Analysis 9.2; Analysis 9.3). No trial reported data regarding infertility.
9.1. Analysis.
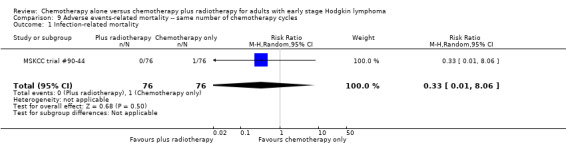
Comparison 9 Adverse events‐ related mortality ‐‐ same number of chemotherapy cycles, Outcome 1 Infection‐ related mortality.
9.2. Analysis.
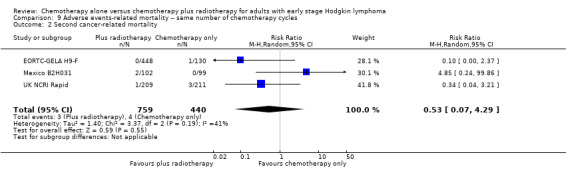
Comparison 9 Adverse events‐ related mortality ‐‐ same number of chemotherapy cycles, Outcome 2 Second cancer‐ related mortality.
9.3. Analysis.
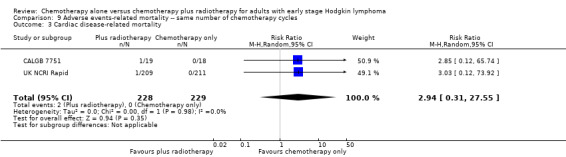
Comparison 9 Adverse events‐ related mortality ‐‐ same number of chemotherapy cycles, Outcome 3 Cardiac disease‐ related mortality.
In a sensitivity analysis without the UK NCRI Rapid and MSKCC trial #90‐44 trials, the results do not change : there are no statistically significant differences between the use of chemotherapy alone and chemotherapy plus radiotherapy regarding second cancer‐ related mortality (two trials (EORTC‐GELA H9‐F; Mexico B2H031), 779 patients, RR 0.71; 95% CI 0.02 to 33.60; P = 0.86, see Analysis 10.1) and cardiac disease‐ related mortality (one trial (CALGB 7751), 37 patients, RR 2.85; 95% CI 0.12 to 65.74; P = 0.51, see Analysis 10.2). Without MSKCC trial #90‐44, no trial reported infection‐ related mortality.
10.1. Analysis.
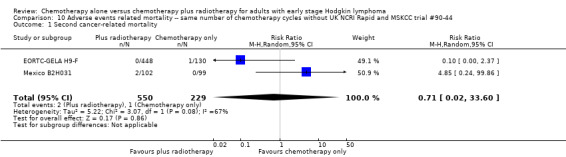
Comparison 10 Adverse events related mortality ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 1 Second cancer‐ related mortality.
10.2. Analysis.
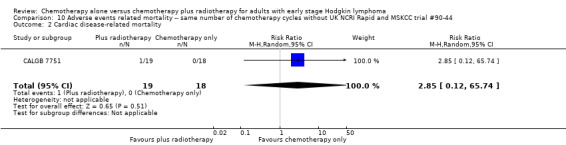
Comparison 10 Adverse events related mortality ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 2 Cardiac disease‐ related mortality.
2. Different numbers of chemotherapy cycles in both arms
Two of the seven trials evaluated different numbers of chemotherapy cycles in each arm (H10F/H10U; HD6)
Primary outcome Overall survival (OS)
Patients
Only one trial with 276 patients reported OS (HD6), therefore we did not perform subgroup analyses. In this trial, 41 of 399 patients did not receive therapy as randomised, therefore a sensitivity analysis without the HD6 trial could no be performed for the same reasons.
Results
The use of chemotherapy alone significantly improved OS (HR 2.12; 95% CI 1.03 to 4.37; P = 0.04), see Analysis 11.1.
11.1. Analysis.
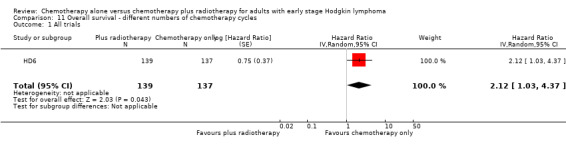
Comparison 11 Overall survival ‐ different numbers of chemotherapy cycles, Outcome 1 All trials.
Secondary outcomes
Progression‐free survival (PFS)
Patients:
Both trials with 1176 patients reported PFS (H10F/H10U; HD6).
Results
The addition of radiotherapy to chemotherapy did not improve PFS in a statistically significant way (HR 0.42; 95% CI 0.14 to 1.24; P = 0.12, see Analysis 12.1 ) with a clear statistical heterogeneity between trials (I2 = 84%), which may in part be due to the different definitions used.
12.1. Analysis.
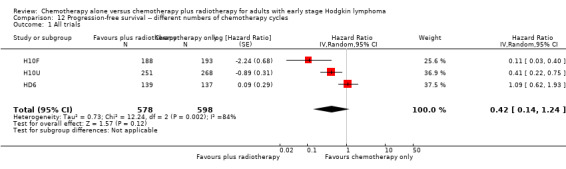
Comparison 12 Progression‐free survival ‐‐ different numbers of chemotherapy cycles, Outcome 1 All trials.
In a sensitivity analysis without the HD6 trial, the addition of radiotherapy to chemotherapy significantly improved PFS (HR 0.24; 95% CI 0.07 to 0.88; P = 0.03, based on one trial, 900 partic i pants), see Analysis 13.1.
13.1. Analysis.
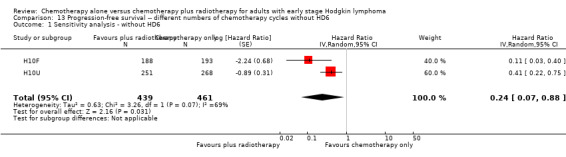
Comparison 13 Progression‐free survival ‐‐ different numbers of chemotherapy cycles without HD6, Outcome 1 Sensitivity analysis ‐ without HD6.
Subgroup analyses
The subgroup analyses by proportion of patients with early favourable disease (P = 0.03, see Analysis 12.2) and type of radiotherapy (P = 0.04, see Analysis 12.4) showed statistically significant differences.
12.2. Analysis.
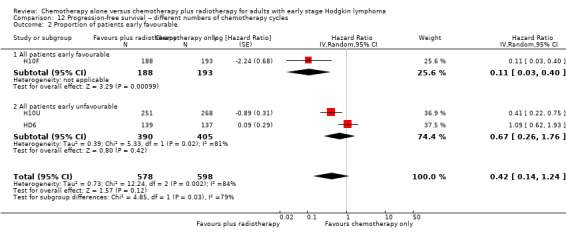
Comparison 12 Progression‐free survival ‐‐ different numbers of chemotherapy cycles, Outcome 2 Proportion of patients early favourable.
12.4. Analysis.
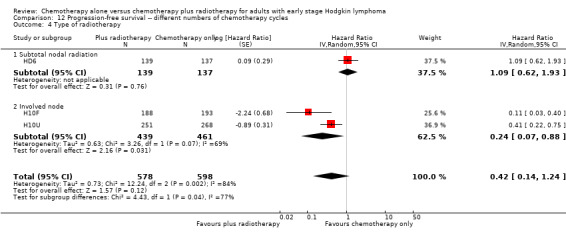
Comparison 12 Progression‐free survival ‐‐ different numbers of chemotherapy cycles, Outcome 4 Type of radiotherapy.
In the part of the trial with early favourable patients (H10F, 381 patients), the addition of radiotherapy to chemotherapy significantly improved PFS (HR 0.11; 95% CI 0.03 to 0.40; P = 0.001). In the trials with only unfavourable patients (H10U; HD6, 795 patients), the addition of radiotherapy to chemotherapy showed no evidence for a difference regarding PFS (HR 0.67; 95% CI 0.26 to 1.76; P = 0.42).
Chemotherapy plus radiotherapy improved PFS in a statistically significant way by the use of involved‐ field radiotherapy (H10F/H10U, 900 patients, HR 0.24; 95% CI 0.07 to 0.88; P = 0.03). No evidence for a difference between chemotherapy alone and chemotherapy plus radiotherapy was shown by the use of subtotal nodal radiation (HD6, 276 patients, HR 1.09; 95% CI 0.62 to 1.93; P = 0.76).
The other subgroup analysis showed no statistically significant differences between the subgroups examined (bulky or non‐bulky disease P = 0.93, see Analysis 12.3).
12.3. Analysis.

Comparison 12 Progression‐free survival ‐‐ different numbers of chemotherapy cycles, Outcome 3 Bulky vs non‐bulky.
As data of all patients were analysed, we did not perform sensitivity/ITT‐analysis.
Subgroup analyses withoutHD6
No subgroup analysis showed statistically significant differences (P = 0.07 for early favourable or unfavourable disease and bulky or non‐bulky disease, see Analysis 13.2; Analysis 13.3).
13.2. Analysis.
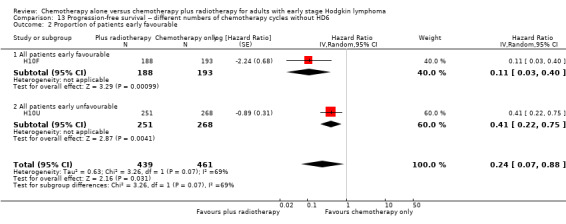
Comparison 13 Progression‐free survival ‐‐ different numbers of chemotherapy cycles without HD6, Outcome 2 Proportion of patients early favourable.
13.3. Analysis.
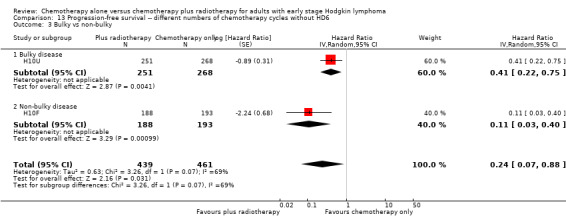
Comparison 13 Progression‐free survival ‐‐ different numbers of chemotherapy cycles without HD6, Outcome 3 Bulky vs non‐bulky.
Complete response (CR)
Not reported.
Overall response rate (ORR)
Not reported.
Adverse events ( AEs)
Only one trial (HD6) reported adverse events considered of particular interest, but there is no evidence for differences between the use of chemotherapy alone and chemotherapy plus radiotherapy regarding infection‐ related mortality (RR 6.90; 95% CI 0.36 to 132.34; P = 0.20), second cancer‐ related mortality (RR 2.22; 95% CI 0.70 to 7.03; P = 0.18), and cardiac disease‐ related mortality (RR 0.99; 95% CI 0.14 to 6.90; P = 0.99), see Analysis 14.1; Analysis 14.2; Analysis 14.3. The trial did not report data regarding infertility. A sensitivity analysis without the HD6 trial could no be performed.
14.1. Analysis.
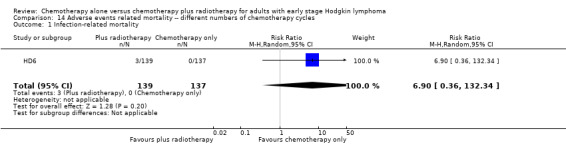
Comparison 14 Adverse events related mortality ‐‐ different numbers of chemotherapy cycles, Outcome 1 Infection‐ related mortality.
14.2. Analysis.
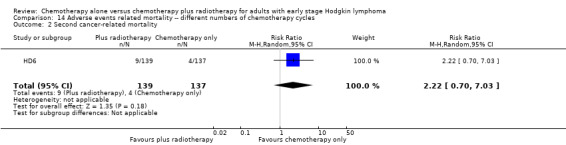
Comparison 14 Adverse events related mortality ‐‐ different numbers of chemotherapy cycles, Outcome 2 Second cancer‐ related mortality.
14.3. Analysis.
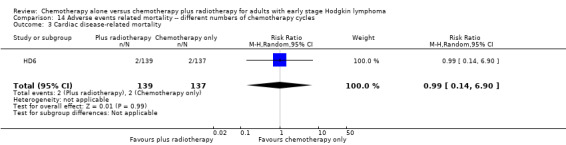
Comparison 14 Adverse events related mortality ‐‐ different numbers of chemotherapy cycles, Outcome 3 Cardiac disease‐ related mortality.
Discussion
Summary of main results
The following findings emerge from this updated Cochrane review and meta‐analysis evaluating the effects of chemotherapy alone compared to chemotherapy plus radiotherapy in adults with early‐stage Hodgkin lymphoma (HL).
Same number of chemotherapy cycles in both arms
The addition of radiotherapy to chemotherapy has probably little or no difference on OS. Without inclusion of two trials from the main analysis because of potential other high risk of bias chemotherapy plus radiotherapy improves overall survival (OS) compared to chemotherapy alone (results of the sensitivity analysis).
Chemotherapy plus radiotherapy probably improves progression‐free survival (PFS) /tumour control compared to chemotherapy alone.
Regarding complete response (CR), there are no significant statistical differences between chemotherapy alone and chemotherapy plus radiotherapy.
Regarding overall response rate (ORR), there are no significant statistical differences between chemotherapy alone and chemotherapy plus radiotherapy.
There are no significant statistical differences between chemotherapy alone and chemotherapy plus radiotherapy regarding adverse events‐ related mortality considered of particular interest (infection‐ related mortality, second cancer‐ related mortality and cardiac disease‐ related mortality).
Different numbers of chemotherapy cycles in both arms
Chemotherapy alone may improve slightly OS compared to chemotherapy plus radiotherapy, based on one trial with potential other high risk of bias.
Regarding PFS/ tumour control, there are no significant statistical differences between chemotherapy alone and chemotherapy plus radiotherapy. Without inclusion of one trial from the main analysis becaus of potential other high risk of bias, chemotherapy plus radiotherapy improves PFS compared to chemotherapy alone (results of the sensitivity analysis, based on one trial).
There are no significant statistical differences between chemotherapy alone and chemotherapy plus radiotherapy regarding adverse events‐ related mortality considered of particular interest (infection‐ related mortality, second cancer‐ related mortality and cardiac disease‐ related mortality), based on one trial.
When interpreting these results, it is important to consider that results lacking statistical significance do not necessarily rule out differences which may be clinically relevant for some patients.
Overall completeness and applicability of evidence
There are seven published r a ndomised controlled trials (RCTs) dealing with a comparison of chemotherapy alone and chemotherapy plus radiotherapy in previously untreated adults with early stage HL .
Six of the included studies were published as full‐text articles, providing sufficient information about the design, participants, methods and outcomes (CALGB 7751; H10F/H10U; HD6; Mexico B2H031; MSKCC trial #90‐44; UK NCRI Rapid). The remaining trial was published as abstract and therefore lacked information on relevant data (EORTC‐GELA H9‐F).
Five of the seven trials evaluated the same number of chemotherapy cycles in each arm (CALGB 7751; EORTC‐GELA H9‐F; Mexico B2H031; MSKCC trial #90‐44; UK NCRI Rapid), and two, different numbers of cycles (H10F/H10U; HD6).
The primary endpoint of this review was OS, due to its prime clinical relevance and its importance for patients. Moreover, it is a commonly accepted direct measure of the benefit of cancer treatment, as well as an endpoint that is not subject to bias by the outcome assessor. Five trials with 1388 patients evaluated the same number of chemotherapy cycles in each arm included in this meta‐analysis, the addition of radiotherapy to chemotherapy has no evidence for a difference in OS. With the exception of two trials from the main analysis because of potential other high risk of bias, OS is significantly better in patients receiving chemotherapy plus radiotherapy compared to chemotherapy alone (results of the sensitivity analysis, two trials were excluded from the main analysis because of potential other high risk of bias). Only one trial with different cycles of chemotherapy in the arms reported OS and found a benefit for the use of chemotherapy alone. This trial has a potential other high risk of bias as well.
In addition to these published trials, we are aware of three ongoing trials (GHSG HD16; GHSG HD17; HD0801) comparing chemotherapy alone and chemotherapy plus radiotherapy in adults with the same number of cycles of chemotherapy in both arms. These trials included 2720 patients with early‐stage HL, as dealt with in this systematic review. The publication of the results of these trials could deliver clearly results regarding OS and will necessitate an update to this review. We identified no ongoing trials comparing chemotherapy alone and chemotherapy plus radiotherapy with different numbers of chemotherapy cycles in the arms.
Quality of the evidence
Overall, we judged the potential risk of bias of the seven included trials as moderate. All the included trials were reported as randomised studies, but only three of the trials reported the sequence generation process. In three trials, the treatment allocation of patients is unclear. As radiotherapy is difficult to blind, in the included trials blinding of patients as well as blinding of physicians was impossible. Blinding of the outcome assessor would have made no difference to the primary outcome, OS, and therefore we judged the risk of performance and detection bias to be low for all three trials. For the other reported outcomes, most studies did not report whether outcome assessors were blinded with the exception of one trial. As it is not feasible to blind the intervention exercise, we judged the risk of detection bias for these trials as unclear. For four trials, we judged the potential risk of other bias as high. In one trial, one arm was stopped early and data were not available for these patients. In the other three trials, patients did not receive treatment as randomised, but were included in the analysis. Because this could effect the results, we excluded the trials from the meta‐analysis for a sensitivity analysis and performed subgroup analyses without these trials. Three trials did not report all of the study’s pre‐specified secondary outcomes, hence we judged the risk of selective reporting bias as high for these trials. For the same number of chemotherapy cycles in both arms, we judged the quality of evidence for OS as moderate. We downgraded one point for substantial heterogeneity. The inclusion of two trials in which several patients did not receive planned radiotherapy, could lead to increased heterogeneity. The quality of evidence for the sensitivity analysis without these trials is moderate, as the results are more imprecise, as less patients have been included and less events happened, but homogenous. The quality of the evidence for PFS (definition of PFS varied across trials), we considered to be moderate as well. Because of the very small number of adverse events (AEs), we considered the quality of evidence for AEs as low (imprecision). For CR, we judged the quality of evidence as low because of th e low number of events (imprecision) and heterogeneity (inconsistency) between the trials, see Table 1.
For different numbers of chemotherapy cycles in the arms, we judged the quality of evidence for OS as low, because only one trial provided data with a very low number of events (imprecision) and the trial have potential other high risk of bias because 41 of 399 patients did not receive therapy as randomised. For PFS, we considered the quality of evidence as low because of the strong heterogeneity of data (inconsistency) and for AEs as low, because only one trial provided data with a very low number of events (high imprecision), see Table 2.
Potential biases in the review process
To prevent bias within the review, we considered only RCTs and performed all relevant processes in duplicate. We developed a sensitive search strategy, and searched all relevant data from international cancer congresses and study registries to minimise potential publication bias. We are not aware of any obvious deficiencies in our review process. We generated no funnel plot to explore potential publication bias because the number of included trials was too low (<10 trials). However, in our intensive search in trial registries we found no completed but unpublished trials, therefore we judge potential risk of publication bias as low.
Agreements and disagreements with other studies or reviews
To our knowledge this is the first comprehensive review with meta‐analysis focusing on patients with early stage HL in adults that compared chemotherapy alone with chemotherapy plus radiotherapy.
The main analysis according to the strict inclusion criteria of our review protocol included seven randomised controlled trials with 2564 patients of both early favourable and early unfavourable HL . The literature search revealed a number of trials with more than 20% of patients in advanced stages (Bonnet 2007; Horning 1996; Kung 1993; Kung 2006; Laskar 2004; O'Dwyer 1984; O'Dwyer 1985; Picardi 2007) or trials including children (Friedmann 2014; Lemerle 1986; Nachman 2002; Pavlovsky 1988; Weiner 1997; Wolden 2012, but wh i ch do not match the inclusion criteria.
In the UK NCRI Rapid trial, patients with positive positron emission tomography (PET findings) received a fourth cycle of ABVD and radiotherapy. No results are stated for this arm. However, this comparison did not match the inclusion criteria. Similar ly for the patients with positive PET findings of the H10F/H10U trial. These patients did not receive received chemotherapy only, thus no comparison was possible. In PET positive patients irradiation is probably more important than in PET negative patients, and PET positive patients have a higher risk of dying, thus survival in the trials with PET negative patients is higher. If that is the case, then the restriction of omitting irradiation for PET negative patients in UK NCRI Rapid and H10F/H10U probably caused the need of irradiation in the analysis regarding PFS/tumour control is underestimated.
Crump and c olleagues considered in a review the role of radiation therapy in the treatment of early stage HL and matched relevant RCTs to different topics without performing a meta‐analysis (Crump 2015). Because they included only RCTs published from 2003 onward or systematic reviews published from 2011 onward they identified only one trial (HD6), which dealt with the comparison of chemotherapy plus radiotherapy versus chemotherapy alone. The other trials did not refer to this comparison including two trials from this review (H10F/H10U; UK NCRI Rapid), which presented a comparison of FDG‐PET scanning versus direct therapy.
Sickinger and colleagues meta‐analysed the effects of PET‐adapted therapy (chemotherapy only) or chemotherapy followed by radiotherapy in patients with HL in a systematic review (Sickinger 2015). They found moderate‐ quality evidence that PFS is better in individuals with early stage HL and a negative PET scan receiving chemotherapy and additional radiotherapy in contrast to those receiving chemotherapy only, based on results of three trials (H10F/H10U; Picardi 2007; UK NCRI Rapid), without a differentiation between same and different numbers of chemotherapy cycles in the arms.
Authors' conclusions
Implications for practice.
This systematic review compared the effects of chemotherapy alone and chemotherapy plus additional radiotherapy in adults with early stage Hodgkin lymphoma (HL).
For the comparison with same numbers of chemotherapy cycles in both arms, we found moderate‐ quality evidence that progression‐free survival (PFS) /tumour control is superior in patients receiving chemotherapy plus additional radiotherapy compared to chemotherapy alone. The addition of radiotherapy to chemotherapy has probably little or no difference on overall survival (OS). The sensitivity analysis without two trials at potential other high risk of bias showed that chemotherapy plus radiotherapy improves OS compared to chemotherapy alone. There are no significant statistical differences between chemotherapy alone and chemotherapy plus radiotherapy regarding adverse events‐ related mortality considered of particular interest (infection‐ related mortality, second cancer‐ related mortality and cardiac disease‐ related mortality).
For different numbers of chemotherapy cycles in the arms, there are no implications for OS and PFS/tumour control possible, because of the low quality of evidence of the results. There are no significant statistical differences between chemotherapy alone and chemotherapy plus radiotherapy regarding adverse events‐ related mortality considered of particular interest (infection‐ related mortality, second cancer‐ related mortality and cardiac disease‐ related mortality).
Implications for research.
Since adding radiotherapy may result in more secondary malignancies or cardiac disease and deaths, long‐term follow‐up (more than 15 years) of clinical trials examining treatment options in early stage HL would be helpful. In addition, clear definitions of outcomes that examine PFS/tumour control would be useful in order to reduce heterogeneity. We recommend measuring PFS , i.e. time to progression, relapse or death of any cause. There is a need for further research concerning the comparison of chemotherapy alone and chemotherapy plus radiotherapy with different numbers of chemotherapy cycles in the arms because of the low quality of evidence.
What's new
| Date | Event | Description |
|---|---|---|
| 22 December 2016 | New citation required and conclusions have changed | Inclusion criteria amended: now without studies in children, but with studies evaluating different number of chemotherapy cycles in both arms (separate comparison) |
| 20 December 2016 | New search has been performed | Update |
Notes
Parts of the review matched the templates of the Cochrane Haematological Malignancies Group, especially the methods.
Acknowledgements
We are grateful to the following persons for their comments and improving the first version of the review: Dr. Sue Richards and Prof. Benjamin Djulbegovic, Editors of the Cochrane Haematological Malignancies Group, and Sabine Kluge and Dr. Kathrin Bauer from Cochrane Haematological Malignancies Group.
We are grateful to the following persons for publishing the first version of the review: Rehan F, Brillant C, Schulz H, Bohlius J, Herbst C.
Appendices
Appendix 1. CENTRAL search strategy
CENTRAL search strategy (January 1977 to November 2010)
1. (favourable or unfavourable)
2. ((earl* or low* or limit*) near/3 (stag* or grad*))
3. (intermediate*)
4. (bulky)
5. (#1 OR #2 OR #3 OR #4)
6. MeSH descriptor LYMPHOMA, this term only
7. MeSH descriptor HODGKIN DISEASE explode all trees
8. (hodgkin* near/2 (disease* or granulom*))
9. (reticulolymphosarcom* or germinoblastom*)
10. (malignan* near/2 (lymphogranulom* or granulom*))
11. (#6 OR #7 OR #8 OR #9 OR #10)
12. MeSH descriptor ANTINEOPLASTIC AGENTS explode all trees
13. MeSH descriptor REMISSION INDUCTION explode all trees
14. MeSH descriptor ANTINEOPLASTIC PROTOCOLS explode all trees
15. ((consolidat* or induct* or maintenance or conditioning*) and (therap* or treat* or regimen* or patient*))
16. ((therap* or induc*) near/3 remission*)
17. (chemotherap* or chemo‐therap*)
18. (Antineoplast* or anti‐neoplast*)
19. ((cytosta* or cytotox*) near/2 (therap* or treat* or regimen*))
20. MeSH descriptor RADIOTHERAPY explode all trees
21. (radiotherap* or radio‐therap*)
22. (chemoradiotherap* or chemo‐radio‐therap*)
23. MeSH descriptor COMBINED MODALITY THERAPY explode all trees
24. ((multimodal* or multi‐modal*) near/3 (treat* or therap*))
25. MeSH descriptor LYMPHATIC IRRADIATION explode all trees
26. (combi* near/3 modalit*)
27. (#13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26)
28. (#5 AND #11 AND #27)
CENTRAL search strategy (December 2010 to December 2016)
1. MeSH descriptor: [Lymphoma] explode all trees
2. MeSH descriptor: [Hodgkin Disease] explode all trees
3. germinoblastom*
4. reticulolymphosarcom*
5. hodgkin* or hogkin* or hodkin* or hodgin*
6. malignan* near/2 lymphogranulom*
7. malignan* near/2 granulom*
8. #1 or #2 or #3 or #4 or #5 or #6 or #7
9. MeSH descriptor: [Antineoplastic Agents] explode all trees
10. MeSH descriptor: [Remission Induction] explode all trees
11. MeSH descriptor: [Antineoplastic Protocols] explode all trees
12. ((consolidat* or induct* or maintenance or conditioning*) and (therap* or treat* or regimen* or patient*))
13. ((therap* or induc*) near/3 remission*)
14. (chemotherap* or chemo‐therap*)
15. (Antineoplast* or anti‐neoplast*)
16. ((cytosta* or cytotox*) near/2 (therap* or treat* or regimen*))
17. #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
18. MeSH descriptor: [Vinblastine] explode all trees
19. vinblastin* or vincaleukoblastin*
20. (lemblastin* or velban* or velbe* or cellblastin*)
21. #18 or #19 or #20
22. MeSH descriptor: [Dacarbazine] explode all trees
23. decarbazin* or dacarbazin*
24. NSC45388 or NSC 45388
25. DTIC* or ICDT* or DIC*
26. asercit* or deticen* or biocarbazin* or dacatic* or detimedac* or fauldetic*
27. WR‐139007 or WR139007
28. imidazol* carboxamid*
29. #22 or #23 or #24 or #25 or #26 or #27 or #28
30. MeSH descriptor: [Epirubicin] explode all trees
31. epirubicin*
32. (farmorubicin* or pharmorubicin* or epidoxorubicin* or epiadriamycin*)
33. (epi‐cell* or epicell* or ebew* or ellenc*)
34. #30 or #31 or #32 or #33
35. MeSH descriptor: [Doxorubicin] explode all trees
36. doxorubi*
37. (adriamycin* or adriablastin*)
38. hydroxydaunorubincin*
39. (doxo cell or doxo‐cell or dox sl or dox‐sl)
40. (doxotec* or doxolem* or doxil*)
41. (rubex* or ribodox* or onkodox*)
42. (myeocet* or caelyx*)
43. farmiblastin*
44. #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43
45. MeSH descriptor: [Bleomycin] explode all trees
46. bleomy* or bleomi*
47. (blenoxan* or blanoxan* or bleolem*)
48. (bleo cell or bleocell)
49. #45 or #46 or #47 or #48
50. MeSH descriptor: [Prednisone] explode all trees
51. predniso*
52. (winpred* or sterapred* or prednidib* or predniment* pronisone*)
53. (cartancyl* or cortan* or kortancyl* or encorton* or enkortolon* or decortisyl* orrectodelt*)
54. (panafcort* or panasol* or meticorten* or dacortin* or orason* or cutason*)
55. #51 or #52 or #53 or #54
56. ebvp*
57. #34 and #49 and #21 and #55
58. abvd*
59. #44 and #49 and #21 and #29
60. ebvd*
61. #34 and #49 and #21 and #29
62. #56 or #57 or #58 or #59 or #60 #61
63. MeSH descriptor: [Radiotherapy] explode all trees
64. (radiotherap* or radio‐therap*)
65. radiation*
66. MeSH descriptor: [Lymphatic Irradiation] explode all trees
67. #63 or #64 or #65 or #66
68. #67 and (#17 or #62)
69. (chemoradiotherap* or chemo‐radio‐therap*)
70. MeSH descriptor: [Combined Modality Therapy] explode all trees
71. ((multimodal* or multi‐modal*) near/3 treat*)
72. ((multimodal* or multi‐modal*) near/3 therap*)
73. (combi* adj3 modalit*)
74. #69 or #70 or #71 or #72 or #73
75. #8 and (#68 or #74) in Trials
Appendix 2. MEDLINE search strategy
MEDLINE search strategy (January 1977 to November 2010)
1. (favourable or unfavourable).tw,kf,ot.
2. ((earl$ or low$ or limit$) adj3 (stag$ or grad$)).tw,kf,ot.
3. intermediate$.tw,kf,ot.
4. bulky.tw,kf,ot.
5. or/1‐4
6. *LYMPHOMA/
7. exp HODGKIN DISEASE/
8. Germinoblastom$.tw,kf,ot.
9. Reticulolymphosarcom$.tw,kf,ot.
10. Hodgkin$.tw,kf,ot.
11. (malignan$ adj2 (lymphogranulom$ or granulom$)).tw,kf,ot.
12. or/6‐11
13. exp ANTINEOPLASTIC AGENTS/
14. REMISSION INDUCTION/
15. exp ANTINEOPLASTIC PROTOCOLS/
16. ((consolidat$ or induct$ or maintenance or conditioning$) and (therap$ or treat$ or regimen$ or patient$)).tw,kf,ot.
17. ((therap$ or induc$) adj3 remission$).tw,kf,ot.
18. (chemotherap$ or chemo‐therap$).tw,kf,ot.
19. (Antineoplast$ or anti‐neoplast$).tw,kf,ot.
20. ((cytosta$ or cytotox$) adj2 (therap$ or treat$ or regimen$)).tw,kf,ot.
21. exp RADIOTHERAPY/
22. (radiotherap$ or radio‐therap$).tw,kf,ot.
23. (chemoradiotherap$ or chemo‐radio‐therap$).tw,kf,ot.
24. exp COMBINED MODALITY THERAPY/
25. ((multimodal$ or multi‐modal$) adj3 (treat$ or therap$)).tw,kf,ot.
26. exp LYMPHATIC IRRADIATION/
27. (combi$ adj3 modalit$).tw,kf,ot.
28. or/13‐27
29. randomized controlled trial.pt.
30. controlled clinical trial.pt.
31. RANDOMIZED CONTROLLED TRIALS/
32. RANDOM ALLOCATION/
33. DOUBLE BLIND METHOD/
34. SINGLE BLIND METHOD/
35. or/29‐34
36. (ANIMALS not HUMANS).sh.
37. 35 not 36
38. clinical trial.pt.
39. exp CLINICAL TRIALS/
40. (clin$ adj25 trial$).ti,ab.
41. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
42. PLACEBOS/
43. placebo$.ti,ab.
44. random$.ti,ab.
45. RESEARCH DESIGN/
46. or/38‐45
47. 46 not 36
48. 47 not 37
49. COMPARATIVE STUDY/
50. exp EVALUATION STUDIES/
51. FOLLOW UP STUDIES/
52. PROSPECTIVE STUDIES/
53. (control$ or prospectiv$ or volunteer$).ti,ab.
54. or/49‐53
55. 54 not 36
56. 55 not (37 or 48)
57. 37 or 48 or 56
58. 5 and 12 and 28 and 57
MEDLINE search strategy (December 2010 to December 2016)
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomi?ed.ab.
4. placebo.ab.
5. clinical trials as topic.sh.
6. randomly.ab.
7. trial.ti.
8. or/1‐7
9. humans.sh.
10. 8 and 9
11.*LYMPHOMA/
12. exp HODGKIN DISEASE/
13. Germinoblastom$.tw,kf,ot.
14. Reticulolymphosarcom$.tw,kf,ot.
15. Hodgkin$.tw,kf,ot.
16. (malignan$ adj2 (lymphogranulom$ or granulom$)).tw,kf,ot.
17. or/11‐16
18. exp ANTINEOPLASTIC AGENTS/
19. REMISSION INDUCTION/
20. exp ANTINEOPLASTIC PROTOCOLS/
21. ((consolidat$ or induct$ or maintenance or conditioning$) and (therap$ or treat$ or regimen$ or patient$)).tw,kf,ot.
22. ((therap$ or induc$) adj3 remission$).tw,kf,ot.
23. (chemotherap$ or chemo‐therap$).tw,kf,ot.
24. (Antineoplast$ or anti‐neoplast$).tw,kf,ot.
25. ((cytosta$ or cytotox$) adj2 (therap$ or treat$ or regimen$)).tw,kf,ot.
26. or/18‐25
27. VINBLASTINE/
28. vinblastin$.tw,kf,ot,nm.
29. vincaleukoblastin$.tw,kf,ot.
30. (lemblastin$ or velban$ or velbe$ or cellblastin$).tw,kf,ot.
31. or/27‐30
32. DACARBAZINE/
33. (decarbazin$ or dacarbazin$).tw,kf,ot.
34. (NSC45388 or NSC 45388).tw,kf,ot,nm.
35. (DTIC$ or ICDT$ or DIC$).tw,kf,ot.
36. (asercit$ or deticen$ or biocarbazin$ or dacatic$ or detimedac$ or fauldetic$).tw,kf,ot.
37. (WR‐139007 or WR139007).tw,kf,ot.
38. imidazol$ carboxamid$.tw,kf,ot.
39. or/32‐38
40. EPIRUBICIN/
41. epirubicin$.tw,kf,ot,nm.
42. (farmorubicin$ or pharmorubicin$ or epidoxorubicin$ or epiadriamycin$).tw,kf,ot.
43. (epi‐cell$ or epicell$ or ebew$ or ellenc$).tw,kf,ot.
44. or/40‐43
45. exp DOXORUBICIN/
46. doxorubi?in$.tw,kf,ot,nm.
47. (adriamycin$ or adriablastin$).tw,kf,ot.
48. hydroxydaunorubincin$.tw,kf,ot.
49. (doxo cell or doxo‐cell or dox sl or dox‐sl).tw,kf,ot.
50. (doxotec$ or doxolem$ or doxil$).tw,kf,ot.
51. (rubex$ or ribodox$ or onkodox$).tw,kf,ot.
52. (myeocet$ or caelyx$).tw,kf,ot.
53. Farmiblastin$.tw,kf,ot.
54. or/45‐53
55. exp BLEOMYCIN/
56. bleomy?in$.tw,kf,ot,nm.
57. bleomicin$.tw,kf,ot.
58. (blenoxan$ or blanoxan$ or bleolem$).tw,kf,ot.
59. (bleo cell or bleocell).tw,kf,ot.
60. or/55‐59
61. PREDNISONE/
62. predniso$.tw,kf,ot,nm.
63. (winpred$ or sterapred$ or prednidib$ or predniment$ pronisone$).tw,kf,ot.
64. (cartancyl$ or cortan$ or kortancyl$ or encorton$ or enkortolon$ or decortisyl$ orrectodelt$).tw,kf,ot.
65. (panafcort$ or panasol$ or meticorten$ or dacortin$ or orason$ or cutason$).tw,kf,ot.
66. or/61‐65
67. ebvp$.tw,kf,ot,nm,ps.
68. 44 and 60 and 31 and 66
69. abvd$.tw,kf,ot,nm,ps.
70. 31 and 39 and 54 and 60
71. ebvd$.tw,kf,ot,nm,ps.
72. 31 and 39 and 44 and 60
73. or/67‐72
74. exp RADIOTHERAPY/
75. (radiotherap$ or radio‐therap$).tw,kf,ot.
76. radiation*.tw,kf,ot.
77. exp Lymphatic Irradiation/
78. or/74‐77
79. 78 and (73 or 26)
80. (chemoradiotherap$ or chemo‐radio‐therap$).tw,kf,ot.
81. exp Combined Modality Therapy/
82. ((multimodal$ or multi‐modal$) adj3 (treat$ or therap$)).tw,kf,ot.
83. (combi$ adj3 modalit$).tw,kf,ot.
84. or/80‐83
85. 17 and (79 or 84)
86. 17 and (79 or 84) and 10
Appendix 3. Embase search strategy
1. (favourable or unfavourable or favorable or unfavorable).tw,kf,ot.
2. ((earl$ or low$ or limit$) adj3 (stag$ or grad$)).tw,kf,ot.
3. intermediate$.tw,kf,ot.
4. bulky.tw,kf,ot.
5. or/1‐4
6. *LYMPHOMA/
7. exp HODGKIN DISEASE/
8. Germinoblastom$.tw,kf,ot.
9. Reticulolymphosarcom$.tw,kf,ot.
10. Hodgkin$.tw,kf,ot.
11. (malignan$ adj2 (lymphogranulom$ or granulom$)).tw,kf,ot.
12. or/6‐11
13. exp ANTINEOPLASTIC AGENT/
14. REMISSION/
15. exp CLINICAL PROTOCOL/
16. ((consolidat$ or induct$ or maintenance or conditioning$) and (therap$ or treat$ or regimen$ or patient$)).tw,kf,ot.
17. ((therap$ or induc$) adj3 remission$).tw,kf,ot.
18. (chemotherap$ or chemo‐therap$).tw,kf,ot.
19. (Antineoplast$ or anti‐neoplast$).tw,kf,ot.
20. ((cytosta$ or cytotox$) adj2 (therap$ or treat$ or regimen$)).tw,kf,ot.
21. exp RADIOTHERAPY/
22. (radiotherap$ or radio‐therap$).tw,kf,ot.
23. (chemoradiotherap$ or chemo‐radio‐therap$).tw,kf,ot.
24. exp MULTIMODALITY CANCER THERAPY/
25. ((multimodal$ or multi‐modal$) adj3 (treat$ or therap$)).tw,kf,ot.
26. exp LYMPH NODE IRRADIATION/
27. (combi$ adj3 modalit$).tw,kf,ot.
28. or/13‐27
29. CLINICAL TRIAL/
30. RANDOMIZED CONTROLLED TRIALS/
31. RANDOM ALLOCATION/
32. SINGLE‐BLIND METHOD/
33. DOUBLE‐BLIND METHOD/
34. CROSS‐OVER STUDIES/
35. PLACEBOS/
36. Randomi?ed controlled trial$.tw.
37. RCT.tw.
38. Random allocation.tw.
39. Randomly allocated.tw.
40. Allocated randomly.tw.
41. (allocated adj2 random).tw.
42. Single blind$.tw.
43. Double blind$.tw.
44. ((treble or triple) adj blind$).tw.
45. Placebo$.tw.
46. PROSPECTIVE STUDIES/
47. or/29‐46
48 CASE STUDY/
49. Case report.tw.
50. ABSTRACT REPORT/ or LETTER/
51. or/48‐50
52. 47 not 51
53. ANIMAL/
54. HUMAN/
55. 53 not 54
56. 52 not 55
57. 5 and 12 and 28 and 56
Data and analyses
Comparison 1. Overall survival ‐‐ same number of chemotherapy cycles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All trials | 5 | 1388 | Hazard Ratio (Random, 95% CI) | 0.48 [0.22, 1.06] |
| 2 Proportion of patients early favourable | 4 | 968 | Hazard Ratio (Random, 95% CI) | 0.31 [0.19, 0.50] |
| 2.1 All patients early favourable | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.04, 1.74] |
| 2.2 Mixed patient population (˜ 30 to 50% patients early unfavourable) | 1 | 152 | Hazard Ratio (Random, 95% CI) | 0.31 [0.08, 1.15] |
| 2.3 All patients early unfavourable | 2 | 238 | Hazard Ratio (Random, 95% CI) | 0.31 [0.18, 0.54] |
| 3 Bulky vs non‐bulky | 4 | 1351 | Hazard Ratio (Random, 95% CI) | 0.47 [0.18, 1.19] |
| 3.1 Bulky disease | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.51] |
| 3.2 Non‐bulky disease | 3 | 1150 | Hazard Ratio (Random, 95% CI) | 0.60 [0.16, 2.27] |
| 4 Timing of radiotherapy | 5 | 1388 | Hazard Ratio (Random, 95% CI) | 0.48 [0.22, 1.06] |
| 4.1 Radiotherapy after chemotherapy | 3 | 1150 | Hazard Ratio (Random, 95% CI) | 0.60 [0.16, 2.27] |
| 4.2 Sandwich technique (CT‐RT‐CT) | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.51] |
| 4.3 Chemotherapy after radiotherapy | 1 | 37 | Hazard Ratio (Random, 95% CI) | 0.63 [0.11, 3.65] |
| 5 Type of radiotherapy | 5 | 1388 | Hazard Ratio (Random, 95% CI) | 0.48 [0.22, 1.06] |
| 5.1 Involved field | 3 | 1035 | Hazard Ratio (Random, 95% CI) | 0.83 [0.26, 2.67] |
| 5.2 Extended field | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.51] |
| 5.3 Mixed radiotherapy | 1 | 152 | Hazard Ratio (Random, 95% CI) | 0.31 [0.08, 1.15] |
| 6 Type of chemotherapy | 5 | 1388 | Hazard Ratio (Random, 95% CI) | 0.48 [0.22, 1.06] |
| 6.1 ABVD | 3 | 773 | Hazard Ratio (Random, 95% CI) | 0.53 [0.17, 1.68] |
| 6.2 CVPP | 1 | 37 | Hazard Ratio (Random, 95% CI) | 0.63 [0.11, 3.65] |
| 6.3 EBVP | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.04, 1.73] |
| 7 ITT‐analysis | 5 | 1388 | Hazard Ratio (Random, 95% CI) | 0.48 [0.22, 1.06] |
| 7.1 ITT‐analysis | 4 | 1351 | Hazard Ratio (Random, 95% CI) | 0.47 [0.18, 1.19] |
| 7.2 No ITT‐analysis | 1 | 37 | Hazard Ratio (Random, 95% CI) | 0.63 [0.11, 3.65] |
Comparison 2. Overall survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis ‐ without UK NCRI RAPID and MSKCC trial #90‐44 | 3 | 816 | Hazard Ratio (Random, 95% CI) | 0.31 [0.19, 0.52] |
| 2 Proportion of patients early favourable | 3 | 816 | Hazard Ratio (Random, 95% CI) | 0.31 [0.19, 0.52] |
| 2.1 All patients early favourable | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.04, 1.74] |
| 2.2 All patients early unfavourable | 2 | 238 | Hazard Ratio (Random, 95% CI) | 0.31 [0.18, 0.54] |
| 3 Bulky vs non‐bulky | 2 | 779 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.50] |
| 3.1 Bulky disease | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.51] |
| 3.2 Non‐bulky disease | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.04, 1.74] |
| 4 Timing of radiotherapy | 3 | 816 | Hazard Ratio (Random, 95% CI) | 0.31 [0.19, 0.52] |
| 4.1 Radiotherapy after chemotherapy | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.04, 1.74] |
| 4.2 Sandwich technique (CT‐RT‐CT) | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.51] |
| 4.3 Chemotherapy after radiotherapy | 1 | 37 | Hazard Ratio (Random, 95% CI) | 0.63 [0.11, 3.65] |
| 5 Type of radiotherapy | 3 | 816 | Hazard Ratio (Random, 95% CI) | 0.31 [0.19, 0.52] |
| 5.1 Involved field | 2 | 615 | Hazard Ratio (Random, 95% CI) | 0.42 [0.12, 1.51] |
| 5.2 Extended field | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.51] |
| 6 Type of chemotherapy | 3 | 816 | Hazard Ratio (Random, 95% CI) | 0.31 [0.19, 0.52] |
| 6.1 ABVD | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.51] |
| 6.2 CVPP | 1 | 37 | Hazard Ratio (Random, 95% CI) | 0.63 [0.11, 3.65] |
| 6.3 EBVP | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.04, 1.73] |
| 7 ITT‐analysis | 3 | 816 | Hazard Ratio (Random, 95% CI) | 0.31 [0.19, 0.52] |
| 7.1 ITT‐analysis | 2 | 779 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.50] |
| 7.2 No ITT‐analysis | 1 | 37 | Hazard Ratio (Random, 95% CI) | 0.63 [0.11, 3.65] |
2.1. Analysis.
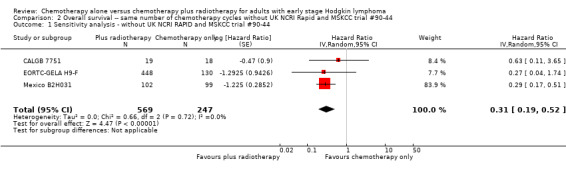
Comparison 2 Overall survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44, Outcome 1 Sensitivity analysis ‐ without UK NCRI RAPID and MSKCC trial #90‐44.
Comparison 3. Progression‐free survival ‐‐ same number of chemotherapy cycles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All trials | 4 | 1351 | Hazard Ratio (Random, 95% CI) | 0.42 [0.25, 0.72] |
| 2 Proportion of patients early favourable | 4 | 1351 | Hazard Ratio (Random, 95% CI) | 0.42 [0.25, 0.72] |
| 2.1 All patients early favourable | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.17, 0.43] |
| 2.2 Mixed patient population (˜ 30 to 50% patients early unfavourable) | 2 | 572 | Hazard Ratio (Random, 95% CI) | 0.71 [0.43, 1.17] |
| 2.3 All patients early unfavourable | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 3 Bulky vs non‐bulky | 4 | 1351 | Hazard Ratio (Random, 95% CI) | 0.42 [0.25, 0.72] |
| 3.1 Bulky disease | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 3.2 Non‐bulky disease | 3 | 1150 | Hazard Ratio (Random, 95% CI) | 0.50 [0.24, 1.03] |
| 4 Timing of radiotherapy | 4 | 1351 | Hazard Ratio (Random, 95% CI) | 0.42 [0.25, 0.72] |
| 4.1 Radiotherapy after chemotherapy | 3 | 1150 | Hazard Ratio (Random, 95% CI) | 0.50 [0.24, 1.03] |
| 4.2 Sandwich technique (CT‐RT‐CT) | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 5 Type of radiotherapy | 4 | 1351 | Hazard Ratio (Random, 95% CI) | 0.42 [0.25, 0.72] |
| 5.1 Involved field | 2 | 998 | Hazard Ratio (Random, 95% CI) | 0.40 [0.17, 0.94] |
| 5.2 Extended field | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 5.3 Mixed radiotherapy | 1 | 152 | Hazard Ratio (Random, 95% CI) | 0.85 [0.37, 1.94] |
| 6 Type of chemotherapy | 4 | 1351 | Hazard Ratio (Random, 95% CI) | 0.42 [0.25, 0.72] |
| 6.1 ABVD | 3 | 773 | Hazard Ratio (Random, 95% CI) | 0.51 [0.26, 0.99] |
| 6.2 EBVP | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.17, 0.43] |
| 7 Sensitivity analysis (per protocol results of the UK NCRI RAPID, without MSKCC trial #90‐44) | 3 | 1199 | Hazard Ratio (Random, 95% CI) | 0.30 [0.22, 0.41] |
3.1. Analysis.
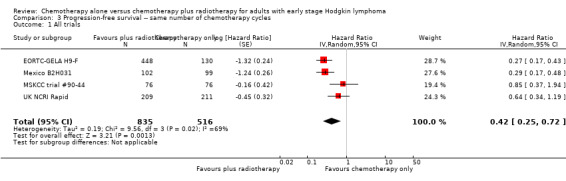
Comparison 3 Progression‐free survival ‐‐ same number of chemotherapy cycles, Outcome 1 All trials.
Comparison 4. Progression‐free survival ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis ‐ without UK NCRI RAPID and MSKCC trial #90‐44 | 2 | 779 | Hazard Ratio (Random, 95% CI) | 0.28 [0.20, 0.39] |
| 2 Proportion of patients early favourable | 2 | 779 | Hazard Ratio (Random, 95% CI) | 0.28 [0.20, 0.39] |
| 2.1 All patients early favourable | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.17, 0.43] |
| 2.2 All patients early unfavourable | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 3 Bulky vs non‐bulky | 2 | 779 | Hazard Ratio (Random, 95% CI) | 0.28 [0.20, 0.39] |
| 3.1 Bulky disease | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 3.2 Non‐bulky disease | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.17, 0.43] |
| 4 Timing of radiotherapy | 2 | 779 | Hazard Ratio (Random, 95% CI) | 0.28 [0.20, 0.39] |
| 4.1 Radiotherapy after chemotherapy | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.17, 0.43] |
| 4.2 Sandwich technique (CT‐RT‐CT) | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 5 Type of radiotherapy | 2 | 779 | Hazard Ratio (Random, 95% CI) | 0.28 [0.20, 0.39] |
| 5.1 Involved field | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.17, 0.43] |
| 5.2 Extended field | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 6 Type of chemotherapy | 2 | 779 | Hazard Ratio (Random, 95% CI) | 0.28 [0.20, 0.39] |
| 6.1 ABVD | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 6.2 EBVP | 1 | 578 | Hazard Ratio (Random, 95% CI) | 0.27 [0.17, 0.43] |
Comparison 5. Complete response rate ‐‐ same number of chemotherapy cycles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All trials | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 2 Proportion of patients early favourable | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 2.1 Mixed patient population (˜ 30 to 50% patients early unfavourable) | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.09] |
| 2.2 All patients early unfavourable | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.78] |
| 3 Bulky vs non‐bulky | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 3.1 Bulky disease | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 3.2 Non‐bulky disease | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.73, 2.01] |
| 4 Timing of radiotherapy | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 4.1 Radiotherapy after chemotherapy | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.09] |
| 4.2 Sandwich technique (CT‐RT‐CT) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 4.3 Chemotherapy after radiotherapy | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.06, 2.27] |
| 5 Type of radiotherapy | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 5.1 Involved field | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.06, 2.27] |
| 5.2 Extended field | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 5.3 Mixed | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.09] |
| 6 Type of chemotherapy | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 6.1 CVPP | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.06, 2.27] |
| 6.2 ABVD | 2 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.09] |
| 7 ITT‐analysis | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 7.1 ITT‐analysis | 2 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.09] |
| 7.2 No ITT‐analysis | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.06, 2.27] |
5.1. Analysis.
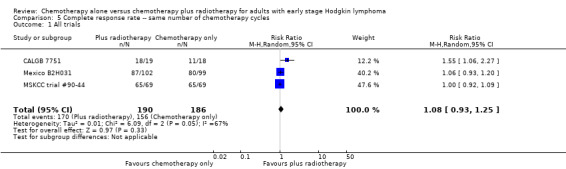
Comparison 5 Complete response rate ‐‐ same number of chemotherapy cycles, Outcome 1 All trials.
Comparison 6. Complete response rate ‐‐ same number of cycles without MSKCC trial #90‐44.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis ‐ without MSKCC trial #90‐44 | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.78] |
| 2 Bulky vs non‐bulky | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.78] |
| 2.1 Bulky disease | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 2.2 Non‐bulky disease | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.06, 2.27] |
| 3 Timing of radiotherapy | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.78] |
| 3.1 Sandwich technique (CT‐RT‐CT) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 3.2 Chemotherapy after radiotherapy | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.06, 2.27] |
| 4 Type of radiotherapy | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.78] |
| 4.1 Involved field | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.06, 2.27] |
| 4.2 Extended field | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 5 Type of chemotherapy | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.78] |
| 5.1 CVPP | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.06, 2.27] |
| 5.2 ABVD | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 6 ITT‐analysis | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.78] |
| 6.1 ITT‐analysis | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 6.2 No ITT‐analysis | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.06, 2.27] |
Comparison 7. Overall response rate ‐‐ same number of chemotherapy cycles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All trials | 2 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.07] |
| 2 Proportion of patients early favourable | 2 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.07] |
| 2.1 All patients early favourable | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.06] |
| 2.2 Mixed patient population (˜ 30 to 50% patients early unfavourable) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 3 Bulky vs non‐bulky | 2 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.07] |
| 3.1 Bulky disease | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 3.2 Non‐bulky disease | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.06] |
| 4 Timing of radiotherapy | 2 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.07] |
| 4.1 Radiotherapy after chemotherapy | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.06] |
| 4.2 Sandwich technique (CT‐RT‐CT) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 5 Type of radiotherapy | 2 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.07] |
| 5.1 Extended field | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 5.2 Mixed | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.06] |
7.1. Analysis.
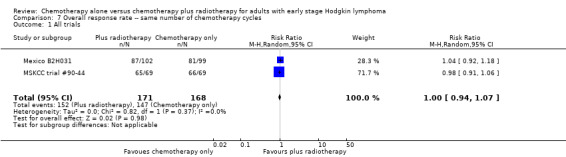
Comparison 7 Overall response rate ‐‐ same number of chemotherapy cycles, Outcome 1 All trials.
Comparison 8. Overall response rate ‐‐ same number of chemotherapy cycles without MSKCC trial #90‐44.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis ‐ without MSKCC trial #90‐44 | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
Comparison 9. Adverse events‐ related mortality ‐‐ same number of chemotherapy cycles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection‐ related mortality | 1 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.06] |
| 2 Second cancer‐ related mortality | 3 | 1199 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.07, 4.29] |
| 3 Cardiac disease‐ related mortality | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [0.31, 27.55] |
Comparison 10. Adverse events related mortality ‐‐ same number of chemotherapy cycles without UK NCRI Rapid and MSKCC trial #90‐44.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Second cancer‐ related mortality | 2 | 779 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.02, 33.60] |
| 2 Cardiac disease‐ related mortality | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 2.85 [0.12, 65.74] |
Comparison 11. Overall survival ‐ different numbers of chemotherapy cycles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All trials | 1 | 276 | Hazard Ratio (Random, 95% CI) | 2.12 [1.03, 4.37] |
Comparison 12. Progression‐free survival ‐‐ different numbers of chemotherapy cycles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All trials | 3 | 1176 | Hazard Ratio (Random, 95% CI) | 0.42 [0.14, 1.24] |
| 2 Proportion of patients early favourable | 3 | 1176 | Hazard Ratio (Random, 95% CI) | 0.42 [0.14, 1.24] |
| 2.1 All patients early favourable | 1 | 381 | Hazard Ratio (Random, 95% CI) | 0.11 [0.03, 0.40] |
| 2.2 All patients early unfavourable | 2 | 795 | Hazard Ratio (Random, 95% CI) | 0.67 [0.26, 1.76] |
| 3 Bulky vs non‐bulky | 3 | 1176 | Hazard Ratio (Random, 95% CI) | 0.42 [0.14, 1.24] |
| 3.1 Bulky disease | 1 | 519 | Hazard Ratio (Random, 95% CI) | 0.41 [0.22, 0.75] |
| 3.2 Non‐bulky disease | 2 | 657 | Hazard Ratio (Random, 95% CI) | 0.37 [0.04, 3.61] |
| 4 Type of radiotherapy | 3 | 1176 | Hazard Ratio (Random, 95% CI) | 0.42 [0.14, 1.24] |
| 4.1 Subtotal nodal radiation | 1 | 276 | Hazard Ratio (Random, 95% CI) | 1.09 [0.62, 1.93] |
| 4.2 Involved node | 2 | 900 | Hazard Ratio (Random, 95% CI) | 0.24 [0.07, 0.88] |
Comparison 13. Progression‐free survival ‐‐ different numbers of chemotherapy cycles without HD6.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis ‐ without HD6 | 2 | 900 | Hazard Ratio (Random, 95% CI) | 0.24 [0.07, 0.88] |
| 2 Proportion of patients early favourable | 2 | 900 | Hazard Ratio (Random, 95% CI) | 0.24 [0.07, 0.88] |
| 2.1 All patients early favourable | 1 | 381 | Hazard Ratio (Random, 95% CI) | 0.11 [0.03, 0.40] |
| 2.2 All patients early unfavourable | 1 | 519 | Hazard Ratio (Random, 95% CI) | 0.41 [0.22, 0.75] |
| 3 Bulky vs non‐bulky | 2 | 900 | Hazard Ratio (Random, 95% CI) | 0.24 [0.07, 0.88] |
| 3.1 Bulky disease | 1 | 519 | Hazard Ratio (Random, 95% CI) | 0.41 [0.22, 0.75] |
| 3.2 Non‐bulky disease | 1 | 381 | Hazard Ratio (Random, 95% CI) | 0.11 [0.03, 0.40] |
Comparison 14. Adverse events related mortality ‐‐ different numbers of chemotherapy cycles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection‐ related mortality | 1 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 6.9 [0.36, 132.34] |
| 2 Second cancer‐ related mortality | 1 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [0.70, 7.03] |
| 3 Cardiac disease‐ related mortality | 1 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.90] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CALGB 7751.
| Methods | Randomised controlled trial with two arms:
Recruitment period:
Baseline patient's characteristics described Median follow‐up time:
No ITT analysis; more than 10% of the enrolled patients not evaluated Conducted by the Cancer and Leukemia Group B (CALGB), USA |
|
| Participants | Inclusion criteria:
Exclusion criteria:
PS I, II:
Prognostic features: not reported Mean age:
Gender:
Baseline patient's characteristics: more male patients in chemotherapy plus radiotherapy arm; more patients with mediastinal mass in chemotherapy alone arm Histopathologic diagnosis: according to Rye modification of Lukes and Butler classification Country:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Patients not blinded. No information about blinding of the assessor. This is judged not to be a source of bias for overall survival. |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Patients not blinded. No information about blinding of the assessor. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22 months OS and response outcome: 18/55 missing from the outcome analysis; no information per study arm. This trial was considered not to have performed an ITT analysis in the subgroup analysis. |
| Selective reporting (reporting bias) | Unclear risk | Dates of relapse and deaths are given. Dates of progression not given nor information about censoring. No time‐ to‐ event outcomes calculated. No study protocol identified, therefore unclear if all the planned outcomes are reported. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
EORTC‐GELA H9‐F.
| Methods | Randomised controlled trial with three arms:
Recruitment period
Baseline patient's characteristics not reported (abstract publication) Median follow‐up:
ITT analysis Conducted by EORTC (European Organization for Research and Treatment of Cancer) and GELA (Groupe d'Etude des Lymphomes de l'Adulte); 111 institutions from 10 European countries involved |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Mean age (range):
Gender:
CS: patients with CS I‐II without bulky disease Prognostic features: all included patients with favourable risk factors Histopathologic diagnosis: not reported Country:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly allocated. No further information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available from the publications. |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Patients not blinded (not expected due to the treatment with radiotherapy). No information about blinding of the assessor. This is judged not to be a source of bias for overall survival. |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Patients and physicians not blinded (not expected due to the treatment with radiotherapy). No information about blinding of the assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals and protocol violations after randomisation reported. Analysis was performed on ITT basis and all randomised patients were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Rationale for the use of disease‐free survival not described. However all patients are in CR at the time of randomisation. Disease‐free survival should therefore be equivalent to progression‐free survival. Other progression outcomes that are more prone to bias are not used and not reported. Study protocol available, no planed outcomes stated. |
| Other bias | High risk | The chemotherapy alone arm ended early due to stopping rules. Unfortunately it was not possible to receive the data on patients receiving additional radiotherapy only up to the date the chemotherapy alone arm was stopped. This is known to increase the effect estimate of trials. In addition the data are preliminary. |
H10F.
| Methods | See H10U | |
| Participants | See H10U | |
| Interventions | See H10U | |
| Outcomes | See H10U | |
| Notes | See H10U | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Minimization technique was used...". |
| Allocation concealment (selection bias) | Low risk | "Centrally randomly assigned to receive either...". |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Although the study is likely not to be blinded, this does not affect the outcome OS. |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | The study did not address blinding of participants or physicians. Regarding the study design it is likely that there was no blinding. However, the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Study protocol available. Not all of the study’s pre‐specified secondary outcomes reported:
|
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
H10U.
| Methods | Randomised controlled trial with two main groups (favourable (F) vs unfavourable (U) disease), each with two subgroups, one consisting of two arms, the other of one arm. Comparison of three treatment models in total. PET measurement after randomisation Subgroups: experimental arm (PET‐adapted therapy) vs standard treatment
The PET‐positive arms are not considered in this review Recruitment period:
Favourable:
Unfavourable:
Baseline patient's characteristics described Median follow‐up time:
No ITT analysis Conducted by the European Organisation for Research and Treatment of Cancer (EORTC) |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Mean age (range):
Gender:
CS I, II:
Country:
|
|
| Interventions | Experimental:
Standard treatment:
FDG‐PET scans:
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Minimization technique was used...". |
| Allocation concealment (selection bias) | Low risk | "Centrally randomly assigned to receive either...". |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Although the study is likely not to be blinded, this does not affect the outcome OS. |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | The study did not address blinding of participants or physicians. Regarding the study design it is likely that there was no blinding. However, the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Study protocol available. Not all of the study’s pre‐specified secondary outcomes reported:
|
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
HD6.
| Methods | Randomised controlled trial with three arms: Chemotherapy alone arm and chemotherapy plus radiotherapy or radiotherapy alone arm
Recruitment period:
Baseline patient characteristics described Median follow‐up time:
ITT‐Analysis Conducted by National Cancer Institute of Canada Clinical Trials Group (NCIC‐CTG) in 1994. Collaboration with the Eastern Cooperative Oncology Group (ECOG) in 1996 |
|
| Participants | Inclusion criteria:
Exclusion criteria:
399 patients included in the analyses
Patients not receiving therapy as randomised (41 of 399):
Patients excluded before randomisation: 6 Mean age:
Gender:
Country:
|
|
| Interventions | Chemotherapy alone:
Chemotherapy plus radiotherapy:
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The HD.6 trial was a [...] randomized, controlled trial". "The process for randomization was concealed and was performed by means of a computer‐generated random‐number sequence that was held at the central office of the NCIC Clinical Trials Group". |
| Allocation concealment (selection bias) | Low risk | "The process for randomization was concealed and was performed by means of a computer‐generated random‐number sequence that was held at the central office of the NCIC Clinical Trials Group". |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Patients not blinded. No information about blinding of the assessor. This is judged not to be a source of bias for overall survival. |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Patients not blinded. No information about blinding of the assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All primary analyses were performed on data from the modified intention‐to‐treat population". 6 of 405 randomised patients were "subsequently considered to be ineligible on the basis of prerandomization data". |
| Selective reporting (reporting bias) | High risk | Study protocol available. Not all of the study’s pre‐specified secondary outcomes reported. Not all of the study’s pre‐specified secondary outcomes reported:
|
| Other bias | High risk | 41 of 399 patients not received therapy as randomised. |
Mexico B2H031.
| Methods | Randomised controlled trial with three arms:
Recruitment period:
Median follow‐up time:
No ITT analysis; less than 10% of enrolled patients not evaluated Conducted at Oncology Hospital, National Medical Center, Mexico |
|
| Participants | Inclusion criteria:
Exclusion criteria:
CS I, II:
Prognostic features not reported Mean age (range):
Gender:
Country:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "a prospective randomised trial" No further information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Patients not blinded. No information about blinding of the assessor. This is judged not to be a source of bias for overall survival. |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Patients and physicians not blinded. No information about blinding of the assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 years OS and tumour control outcome: 20/327 missing from the outcome analysis; no information per study arm. The authors do not give any further information about the method of analysis (e.g. ITT ) We do not believe that these few missing patients induced large bias in the analysis, the information is not available by study arm. For subgroup analysis this trial was considered to have no ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | In the methods section: "Disease‐free survival was calculated for CR patients from the beginning of treatment until clinical or radiological and biopsy proven relapse." No information about patients who did not achieve CR. However, the denominator in the results section is the full population, not only patients in CR. Both disease‐free survival and relapse‐free survival were calculated but only disease‐free survival was reported. Due to the information given about toxic deaths, overall survival and disease‐free survival, we assumed that relapse‐free survival would also have been statistically significant and possibly similar to disease‐free survival, thus not resulting in any bias. In addition, disease‐free survival is preferable to relapse‐free survival as it includes deaths. For these reasons, we choose "unclear" and not "no". There is no information about progression‐free survival. No study protocol available. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
MSKCC trial #90‐44.
| Methods | Randomised controlled with two arms:
Recruitment period:
Median follow‐up time:
ITT analysis for overall survival; no ITT analysis for response outcomes Conducted by MSKCC (Memorial Sloan‐Kettering Cancer Center), USA |
|
| Participants | Inclusion criteria:
Exclusion criteria:
CS I, II:
CS III:
Prognostic features not reported Median age:
Gender:
Country:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was not reported. "Randomisation was performed after a check for eligibility. Patients were stratified according to clinical stage (IA or IIA, IIIA, I B or IIB)." Presumably the randomisation was adequate. |
| Allocation concealment (selection bias) | Low risk | "Patients were enrolled by telephone call or fax to the MSKCC Clinical Trials Office". |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Patients and physicians not blinded. No information about blinding of the assessor. This is judged not to be a source of bias for overall survival. |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Patients and physicians not blinded. No information about blinding of the assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | OS: all patients included in the analysis, ITT‐analysis Tumour control: all patients included in the analysis, ITT‐analysis Response rates: 7/76 excluded from chemotherapy alone arm and 7/76 excluded from chemotherapy plus radiotherapy arm; three lost to follow‐up before completion of six cycles of chemotherapy and 11 stage IA patients with no measurable disease prior to treatment. |
| Selective reporting (reporting bias) | Unclear risk | Choice of progression outcome not described ‐ both disease‐free survival and freedom from progression evaluated; freedom from progression was closer to our definition of PFS and was thus used in the analyses. No study protocol available. |
| Other bias | High risk | 11 patients randomised to radiotherapy never received radiotherapy: 6 refused, 4 progressed on chemotherapy prior to receiving radiotherapy, 1 never received radiotherapy because of bleomycin induced toxicity to radiotherapy. |
UK NCRI Rapid.
| Methods | Randomised controlled trial with two arms:
Recruitment period:
Median follow‐up time:
Information about not randomised patients provided ITT analysis for OS and PFS |
|
| Participants | Inclusion criteria:
Exclusion criteria:
420 patients randomised:
Patients not receiving therapy as randomised (28 of 420)
Patients not randomised (182):
Mean age of all 602 patients registered into the RAPID trial:
Gender of all 602 patients registered into the RAPID trial:
Country:
|
|
| Interventions | Induction chemotherapy (all patients):
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation progress. ("This is an ongoing randomized, controlled, non‐inferiority trial (...)"). |
| Allocation concealment (selection bias) | Low risk | "Block randomization was performed at the Cancer Research UK and University College London Cancer Trials Centre; no stratification factors were used". |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Although the study is likely not to be blinded, this does not affect the outcome OS. |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | The study did not address blinding of participants or physicians. Regarding the study design it is likely that there was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in the analysis, ITT‐analysis |
| Selective reporting (reporting bias) | High risk | Study protocol available. Not all of the study’s pre‐specified secondary outcomes reported:
|
| Other bias | High risk | 28 of 420 patients did not received treatment as randomised: 2 received radiotherapy in chemotherapy alone arm and 26 did not received radiotherapy in the chemotherapy plus radiotherapy arm. These patients were still included in the analysis. In the chemotherapy plus radiotherapy arm 5 of the 8 deaths occurred in patients who received no radiotherapy. |
ABVD: adriamycin, bleomycin, vinblastine, and dacarbazine CS: clinical stage CVPP: cyclophosphamide, vinblastine , procarbazine , prednisone EBVP: epirubicin , bleomycin , vinblastine , prednisone EF: extended‐field radiotherapy ESR: erythrocyte sedimentation rate IF: involved‐field radiotherapy ITT: intent i on‐ to‐treat MF: mantle‐field radiotherapy OS: overall survival PET: positron emission tomography PS: pathologic stage
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrieu 1999 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; all included patients received chemotherapy plus radiotherapy. Less than 80% of the patients had early stage Hodgkin lymphoma; only 25% of the included patients had early stage Hodgkin lymphoma. |
| Bonnet 2007 | Less than 80% of the patients had early stage Hodgkin lymphoma; only 6 of the 576 included patients had Hodgkin lymphoma. |
| Brusamolino 1994 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; compared interventions radiotherapy alone versus chemotherapy plus radiotherapy. |
| Cheveresan 1998 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; all included patients received chemotherapy plus radiotherapy. |
| Cimino 1990 | Not a randomised controlled trial; a review article. |
| Cosset 1992 | Not a randomised controlled trial; a review article. |
| Desablens 1999 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; all patients received chemotherapy plus radiotherapy. |
| Dionet 1988 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy and different chemotherapy regimens used in comparison arms. |
| Ferme 2005 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; all patients received radiotherapy. Unfavourable patients of the EORTC‐GELA H9 trial. |
| Friedmann 2014 | Only children are included in this trial. |
| Hirsch 1994 | Evaluation of pulmonary symptoms in patients randomised to MSKCC trials 1989 to 1993. Not a report of one specific trial Relevant patients presumably analysed in MSKCC trial #90‐44 (recruitment 1990‐2000) Only 45 patients with the relevant comparison included 30: 6 X ABVD 15: 6 X ABVD plus EF radiotherapy No mortality data given Adverse events included only pulmonary function and included 15 patients not in the relevant randomised comparison. During chemotherapy 53% of patients had symptoms of cough or dyspnoea on exertion At the end of follow‐up (˜ 1 year after treatment), 18% (chemotherapy alone) vs. 30% (chemotherapy plus radiotherapy) reported persistent symptoms (P = 0.36).(See also Hirsch 1996). |
| Hirsch 1996 | Evaluation of pulmonary symptoms in patients randomised to MSKCC trials 1989 to 1993. Not a report of one specific trial Relevant patients presumably analysed in MSKCC trial #90‐44 (recruitment 1990‐2000) Only 45 patients with the relevant comparison included 30: 6 X ABVD 15: 6 X ABVD plus EF radiotherapy No mortality data given Adverse events included only pulmonary function and included 15 patients not in the relevant randomised comparison. During chemotherapy 53% of patients had symptoms of cough or dyspnoea on exertion At the end of follow‐up (˜ 1 year after treatment), 18% (chemotherapy alone) vs. 30% (chemotherapy plus radiotherapy) reported persistent symptoms (P = 0.36). |
| Horning 1996 | Less than 80% of the patients had early stage Hodgkin lymphoma; only 42% of the included patients had early stage Hodgkin lymphoma. |
| Horning 2007 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; compared interventions radiotherapy alone versus chemotherapy plus radiotherapy. |
| Kim 2003 | Not a randomised controlled trial; a retrospective data analysis of patients' records with Hodgkin lymphoma. |
| Kung 1993 | Less than 80% of the patients had early stage Hodgkin lymphoma; 69% of the included patients had early stage Hodgkin lymphoma. No subgroup information available. (See also Kung 2006). |
| Kung 2006 | Less than 80% of the patients had early stage Hodgkin lymphoma; 69% of the included patients had early stage Hodgkin lymphoma. No subgroup information available. |
| Körholz 2004 | Not a randomised controlled trial. |
| Laskar 2004 | Less than 80% of the patients had early stage Hodgkin lymphoma; 55% of the included patients had early stage Hodgkin lymphoma. |
| Lemerle 1986 | Only children are included in this trial. |
| Longo 1992 | Not a randomised controlled trial; a review article about the trials (Pavlovsky 1988; O'Dwyer 1985). |
| Meyer 2013 | Not a randomised controlled trial. |
| Nachman 2002 | Only children and adolescents are included in this trial. No subgroup information regarding age available. Less than 80% of the patients had early stage Hodgkin lymphoma; 72% of the included patients had early stage Hodgkin lymphoma. |
| Noordijk 2006 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; compared interventions radiotherapy alone versus chemotherapy plus radiotherapy. |
| O'Dwyer 1984 | Less than 80% of the patients with early stage Hodgkin lymphoma; 69% of the evaluable patients with early stage Hodgkin lymphoma. Duplicate publication (see also O'Dwyer 1985). |
| O'Dwyer 1985 | Less than 80% of the patients had early stage Hodgkin lymphoma; 69% of the evaluable patients had early stage Hodgkin lymphoma. |
| Pavlovsky 1988 | The GATLA 9‐H‐77 trial was included in the first version of the review. The trial did not include a large enough proportion of adults (124 patients (45%) are children < 16 years) and data for this subgroup were not available. |
| Pavlovsky 1997 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy. |
| Picardi 2007 | Less than 80% of the patients had early stage Hodgkin lymphoma; 66% of the included patients had early stage Hodgkin lymphoma. No subgroup information available. |
| Radford 2002 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; compared interventions radiotherapy alone versus chemotherapy plus radiotherapy. |
| Reinartz 2013 | Not a randomised controlled trial; a review article about the trial Wolden 2012. |
| Rüffer 1996 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; compared interventions radiotherapy versus radiotherapy. |
| Rüffer 1998 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; all patients received chemotherapy plus radiotherapy. |
| Rüffer 1999 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; all patients received chemotherapy plus radiotherapy. Duplicate publication (see also Rüffer 1998); all patients received chemotherapy plus radiotherapy. |
| Specht 1992 | Not a randomised controlled trial; a review article. |
| Straus 1989 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; all patients received chemotherapy plus radiotherapy. |
| Thistlethwaite 2007 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; compared interventions radiotherapy alone versus chemotherapy plus radiotherapy. |
| Thomas 2004 | Comparison arms not treated with chemotherapy alone or chemotherapy plus radiotherapy; all patients received radiotherapy. Unfavourable patients of the EORTC‐GELA H9 trial. |
| Weiner 1997 | Only children and adolescents are included in this trial. No subgroup information regarding age available. |
| Wolden 2012 | Only children and adolescents are included in this trial. No subgroup information regarding age available. Less than 80% of the patients had early stage Hodgkin lymphoma; 72% of the included patients had early stage Hodgkin lymphoma. Duplicate publication (see also Nachman 2002). |
ABVD: adriamycin, bleomycin, vinblastine, and dacarbazine EF: e xtended‐field radio therapy
Characteristics of ongoing studies [ordered by study ID]
GHSG HD16.
| Trial name or title | Official title: HD16 for early stages ‐ treatment optimization trial in the first‐line treatment of early stage Hodgkin lymphoma; treatment stratification by means of FDG‐PET |
| Methods | Randomised controlled trial, non‐inferiority design |
| Participants | 18 years to 75 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm 1: 2 cycles ABVD followed by 30 Gy IF‐radiotherapy irrespective of FDG‐PET results after chemotherapy Arm 2: 2 cycles ABVD followed by 30 Gy IF‐radiotherapy if FDG‐PET is positive after chemotherapy; 2 cycles ABVD and treatment stop if FDG‐PET is negative after chemotherapy |
| Outcomes |
|
| Starting date | unclear |
| Contact information | Michael Fuchs; GHSG@uk‐koeln.de |
| Notes | clinicaltrials.gov identifier NCT00736320; 1100 patients to be enrolled |
GHSG HD17.
| Trial name or title | Official title: HD17 for intermediate stages treatment optimization trial in the firstline treatment of intermediate stage Hodgkin lymphoma |
| Methods | Randomised controlled trial |
| Participants | 18 years to 60 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm 1: 2 cycles BEACOPP escalated plus 2 cycles ABVD followed by 30 Gy IF‐RT irrespective of FDG‐PET results after chemotherapy Arm 2: 2 cycles BEACOPP escalated plus 2 cycles ABVD followed by 30 Gy IN‐RT if FDG‐PET is positive after chemotherapy; 2 cycles BEACOPP escalated plus 2 cycles ABVD and treatment stop if FDG‐PET is negative after chemotherapy |
| Outcomes |
|
| Starting date | December 2011 |
| Contact information | Michael Fuchs; GHSG@uk‐koeln.de |
| Notes | clinicaltrials.gov identifier NCT01356680; 1100 patients to be enrolled |
HD0801.
| Trial name or title | Official title: Early salvage with high dose chemotherapy and stem cell transplantation in advanced stage Hodgkin's lymphoma patients with positive PET after two courses of ABVD (PET‐2 positive) and comparison of RT versus no RT in PET‐2 negative patients |
| Methods | Randomised controlled trial |
| Participants | 18 years to 70 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm 1: 2 courses of ABVD. Early restaging with FDG‐PET scan (PET‐2). The subsequent treatment will be as it follows:
Arm 2: 2 courses of ABVD. Early restaging with FDG‐PET scan (PET‐2). The subsequent treatment will be as it follows:
|
| Outcomes |
|
| Starting date | September 2008 |
| Contact information | Fondazione Italiana Linfomi ONLUS Centro di Riferimento per l'Epidemiologia e la Prev. Oncologica Piemonte |
| Notes | clinicaltrials.gov identifier NCT00784537; 520 patients to be enrolled |
ABVD: adriamycin, bleomycin, vinblastine, and dacarbazine A LT: alani ne transaminase AST: aspartate transaminase BEACOPP: Bleomycin, Etoposide, Adriamycin, Cyclophosphamide, Oncovin, Procarbazine, Prednisolone CS: clinical stage CT: computed tomography ESR: erythrocyte sedimentation rate FDG‐PET : fluorodeoxyglucose positron emission tomography IF ‐RT : involved‐field radiotherapy
Differences between protocol and review
Data synthesis
Because of the clinical heterogeneity of the trials (e.g. different types of chemotherapy, starting points in different decades) we used a random‐effects model.
Assessment of risk of bias in included studies
For quality assessment we preferred to use a "domain‐based evaluation" as described in Cochrane's tool for assessing risk of bias (Higgins 2011b), since it was more compatible to the 'Risk of bias' table included in the RevMan 5. We replaced the following quality questions.
Was treatment allocation concealed?
Were outcome assessors blind to treatment assigned?
Were numbers of withdraws, dropouts, lost to follow‐up and protocol violations in each group stated and were there less than 10% in each arm?
Were patients included in the analyses as part of the group to which they were allocated (intention‐to‐treat analyses)?
Were the baseline characteristics similar in both groups?
Progression‐free survival
Because not all trials reported progression‐free survival (PFS) according to our definition (time to progress or relapse or death of any cause in all randomised patients), we accepted other progression outcomes and evaluated these as tumour control.
'Summary of findings' tables
We included 'Summary of findings' tables using the GRADE approach.
Differences between review and review update
-
In accordance with Methodological Expectations of Cochrane Intervention Reviews (MECIR), we additionally searched the following clinical trial registers:
EU clinical trials register: https://www.clinicaltrialsregister.eu/ctr‐search/search;
Clinicaltrials.gov: https://clinicaltrials.gov/.
No post‐hoc analyses: in the updated review we did not search explicitly for patients in advanced stages, therefore it is doubtful that all trials are identified and post‐hoc analyses could be biased.
In contrast to the first version of this review, we excluded trials randomising children. We considered only trials with adults. So we excluded the GATLA 9‐H‐77 trial (Pavlovsky 1988) from the analyses, because the trial did not include a large enough proportion of adults and that data for this subgroup were not available.
In the first version of the review, we excluded trials if the number of cycles of chemotherapy was not identical in both study arms. In contrast to the first version, we included these trials in the update and added a second comparison with trials evaluating different numbers of chemotherapy cycles in both arms.
In this update we excluded the sensitivity analysis regarding the influence of a single large study on the overall result because the data situation changed. However we excluded three trials (HD6; MSKCC trial #90‐44; UK NCRI Rapid) from a sensitivity analysis because we found potential other high risk of bias regarding overall survival (OS), and we did not find per‐protocol results. Because of the published per‐protocol results regarding progression‐free survival (PFS) of the UK NCRI Rapid trial, we completed a sensitivity analysis with these results. For the other trials no per‐protocol results for PFS were available.
To reduce the number of the subgroup analyses, we removed some of the clinically less relevant subgroups (median length of follow‐up and four ‐year survival in the chemotherapy alone group), or of these where no data are available (gender, age, clinical stage). We will consider the subgroup analyses regarding gender, age and clinical stage for future updates if more data allow such analyses.
We examined the trials regarding adverse events. Because of insufficient comparable data we focused on adverse events leading to death: infection‐ related mortality, second cancer‐ related mortality, cardiac disease‐ related mortality.
Contributions of authors
Blank O: Abstract screening, data extraction, quality assessment (risk of bias ), data analysis and interpretation, drafting of the review, 'Summary of findings' tables , adverse events.
Skoetz N: Data checking (third author), communication between authors, proofreading, update screening.
Monsef I: Search strategy, electronic search, handsearching for trials.
Specht L: Clinical expertise, advice for the protocol.
Engert A: Clinical expertise, content input.
von Tresckow B: Clinical expertise, content input.
Sources of support
Internal sources
University Hospital of Cologne, Department I of Internal Medicine, Germany.
External sources
-
BMBF, Germany.
For the first version of the review: Project grant application NO 01KG0815, Federal Ministry of Education and Research (BMBF)
Declarations of interest
Blank O: no known conflict of interest.
Monsef I: no known conflict of interest.
Specht L: no known conflict of interest.
Engert A: no known conflict of interest.
Skoetz N: no known conflict of interest.
von Tresckow B: no known conflict of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
CALGB 7751 {published data only}
- Bloomfield CD, Pajak TF, Glicksman AS, Gottlieb AJ, Coleman M, Nissen NI, et al. Chemotherapy and combined modality therapy for Hodgkin's disease: a progress report on Cancer and Leukemia Group B studies. Cancer Treatment Reports 1982; Vol. 66, issue 4:835‐46. [PubMed]
EORTC‐GELA H9‐F {published data only}
- Eghbali H, Brice P, Creemers GY, Kooji MM, Carde P, Van't Veer MB, et al. Comparson of three radiation dose levels after EBVP regimen in favourable supradiaphragmatic clinical stages (CS) I‐II Hodgkin's lymphoma (HL): Preliminary results of EORTC‐GELA H9‐F trial. Blood 2005;106(11 suppl):abstract 814. [Google Scholar]
- Noordijk EM, Thomas J, Ferme C, 't Veer MB, Brice P, Divine M, et al. First results of EORTC‐GELA H9 randomized trials: the H9‐F trial (comparing 3 radiation dose levels) and H9‐U trial (comparing 3 chemotherapy schemes) in patients with favourable or unfavourable early stage Hodgkin's lymphoma (HL). Journal of Clinical Oncology 2005; Vol. 23, issue suppl:abstract 6505.
- Noordijk EM, Thomas J, Fermé C, Van't Veer MB, Brice P, Divine M, et al. First results of the EORTC‐GELA H9 randomized trials: the H9‐F trial (comparing 3 radiation dose levels) and H9‐U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage Hodgkin's lymphoma (HL). ASCO Annual Meeting Presentation Slides YR:2005.
- Thomas J, Ferme C, Noordijk EM, Rieux C, Hennequin C, Lybeert MLM, et al. Six cycles of EBVP followed by 36 Gy involvedfield irradiation vs. no irradiation in favourable supradiaphragmatic clinical stages III Hodgkin's lymphoma: the EORTCGELA strategy in 771 patients (H9‐F trial‐20982) [Abstract AE11a]. European Journal of Haematology 2004;73(Supp. 65):40. [Google Scholar]
- Thomas J, Fermé C, Noordijk EM, Eghbali H, Henry‐Amar M. The EORTC‐GELA treatment strategy in clinical stages I‐II HL: Results of the H9‐F and H9‐U trials. International Symposium on Hodgkin Lymphoma, Cologne, Presentation YR:2007.
- Thomas J, Fermé C, Noordijk EM, 't Veer MB, Brice P, Divine M, et al. Results of the EORTC‐GELA H9 randomized trials: The H9‐F trial (comparing 3 radiation dose levels) and H9‐U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage Hodgkin's lymphoma (HL). Haematologica 2007;92(suppl. 5):27. [Google Scholar]
H10F {published data only}
- Andre MPE. An update on the EORTC / LYSA / FIL H10 trial. 9th International Symposium on Hodgkin Lymphoma Cologne, Germany 2013.
- Andre MPE, Reman O, Federico M, Girinski T, Brice P, Brusamolino E, et al. Interim analysis of the randomized EORTC/LYSA/FIl Intergroup H10 trial on early PET‐scan driven treatment adaptation in stage I/II Hodgkin lymphoma. Blood 2012;120:549. [Google Scholar]
- Raemaekers JM, André MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography‐negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology 2014;32(12):1188‐94. [DOI] [PubMed] [Google Scholar]
H10U {published data only}
- * Raemaekers JM, André MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography‐negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology 2014;32(12):1188‐94. [DOI] [PubMed] [Google Scholar]
- Andre MPE. An update on the EORTC / LYSA / FIL H10 trial. 9th International Symposium on Hodgkin Lymphoma Cologne, Germany 2013.
- Andre MPE, Reman O, Federico M, Girinski T, Brice P, Brusamolino E, et al. Interim analysis of the randomized EORTC/LYSA/FIl Intergroup H10 trial on early PET‐scan driven treatment adaptation in stage I/II Hodgkin lymphoma. Blood 2012;120:549. [Google Scholar]
HD6 {published data only}
- Meyer R, Gospodarowicz M, Connors J, Pearcey R, Bezjak A, Wells W. A randomized phase III comparison of single‐modality ABVD with a strategy that includes radiation therapy in patients with early‐stage Hodgkin's Disease: the HD‐6 trial of the National Cancer Institute of Canada Clinical Trials Group (Eastern Cooperative Oncology Group Trial HD06). Blood. 2003; Vol. 11, issue 11:26a. [DOI] [PubMed]
- Meyer RM, Gospodarowicz M, Connors JM, Pearcey RG, Wells WA, Winter JN. Final analysis of a randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy (RT) in patients with limited‐stage Hodgkin lymphoma (HL): NCIC CTG/ECOG HD.6. Blood. 2011; Vol. 118, issue 21.
- Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Bezjak A, Wells WA, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited‐stage Hodgkin's lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2005;23(21):4634‐42. [DOI] [PubMed] [Google Scholar]
- Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, et al. ABVD alone versus radiation‐based therapy in limited‐stage Hodgkin's lymphoma. New England Journal of Medicine 2012;366(5):399‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portlock CS. Clinical trials report. Comparison of ABVD chemotherapy and a regimen including radiation therapy in patients with limited‐stage non‐Hodgkin's lymphoma. Current Oncology Reports 2006;8(5):354‐7. [PubMed] [Google Scholar]
Mexico B2H031 {published data only}
- Aviles A, Delgado S. A prospective clinical trial comparing chemotherapy, radiotherapy and combined therapy in the treatment of early stage Hodgkin's disease with bulky disease. Clinical & Laboratory Haematology 1998;20(2):95‐9. [DOI] [PubMed] [Google Scholar]
MSKCC trial #90‐44 {published data only}
- Straus DJ, Portlock CS, Qin J, Myers J, Zelenetz AD, Moskowitz C, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood 2004;104(12):3483‐9. [DOI] [PubMed] [Google Scholar]
UK NCRI Rapid {published data only}
- Radford J. Update on the NCRI RAPID trial. 9th International Symposium on Hodgkin Lymphoma, Cologne, Germany 2013.
- Radford J, Barrington S, Counsell N, Pettengell R, Johnson P, Wimperis J, et al. Involved field radiotherapy versus no further treatment in patients with clinical stages IA and IIA Hodgkin lymphoma and a ‘negative’ PET scan after 3 cycles ABVD. Results of the UK NCRI RAPID trial. Blood. 2012; Vol. 120, issue 21.
- Radford J, Barrington S, Counsell N, Pettengell R, Johnson P, Wimperis J, et al. Prognostic performance of pre‐treatment EORTC, GHSG and IPI risk factors and post‐chemotherapy PET response in the UK NCRI RAPID TRIAL in early stage Hodgkin lymphoma (HL). Haematologica 2013;98 ( Suppl. 2 ):13. [Google Scholar]
- Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET‐directed therapy for early‐stage Hodgkin’s lymphoma. New England Journal of Medicine 2015;372(17):1598‐607. [DOI] [PubMed] [Google Scholar]
- Radford J, O'Doherty M, Barrington S, Qian W, Patrick P, Coltart S, et al. Results of the 2nd planned interim analysis of the rapid trial (involved field radiotherapy versus no further treatment) in patients with clinical stages 1a and 2a Hodgkin lymphoma and a 'negative' FDG‐PET scan after 3 cycles ABVD. Blood 2008;112(11):143‐4. [Google Scholar]
- Radford J, O'Doherty M, Barrington S, Quian W, Popova B, Pettengell R, et al. Results of the 3rd planned interim analysis of the UK NCRI rapid trial (involved field radiotherapy versus no further treatment) in patients with clinical stages IA/IIA Hodgkin lymphoma and a 'negative' 18fdg‐pet scan after 3 cycles ABVD. Haematologica 2010;95 ( Suppl.4 ):16‐7. [Google Scholar]
- Radford JA, Barrington SF, O'Doherty MJ, Qian W, Mouncey P, Pettengell R, et al. Interim results of a UK NCRI randomized trial comparing involved field radiotherapy with no further treatment after 3 cycles ABVD and a negative pet scan in clinical stages IA/IIA Hodgkin lymphoma. Haematologica 2007;92 ( Suppl. 5 ):32. [Google Scholar]
References to studies excluded from this review
Andrieu 1999 {published data only}
- Andrieu JM, Jais JP, Escoffre‐Barbe M, Delwail V, Desablens B, Kiladjian JJ, et al. Bulky Hodgkin's disease (B‐HD): treatment with an initial 7 drug chemotherapy (CT) delivered over 12 weeks followed by high dose extended field irradiation (EF‐RT). Seven year results of the GOELAMS H90M multicentric randomized trial. Blood 1999;94(10 suppl.):abstract 528a. [Google Scholar]
Bonnet 2007 {published data only}
- Bonnet C, Fillet G, Mounier N, Ganem G, Molina TJ, Thiéblemont C, et al. CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: a study by the Groupe d'Etude des Lymphomes de l'Adulte. Journal of Clinical Oncology 2007;25(7):787‐92. [DOI] [PubMed] [Google Scholar]
Brusamolino 1994 {published data only}
- Brusamolino E, Lazzarino M, Orlandi E, Canevari A, Morra E, Castelli G, et al. Early‐stage Hodgkin's disease: long‐term results with radiotherapy alone or combined radiotherapy and chemotherapy. Annals of Oncology 1994;5(suppl. 2):101‐6. [DOI] [PubMed] [Google Scholar]
Cheveresan 1998 {published data only}
- Cheveresan LF, Roth I, Balan M, Ionita H. Combined modality therapy in early stage Hodgkin's disease ‐ preliminary results of a clinical trial. Leukemia and Lymphoma 1998;29(suppl. 1):72. [Google Scholar]
Cimino 1990 {published data only}
- Cimino G. Chemotherapy alone for the treatment of early‐stage Hodgkin's disease. European Journal of Cancer 1990;26(11‐12):1115‐8. [DOI] [PubMed] [Google Scholar]
Cosset 1992 {published data only}
- Cosset JM, Henry‐Amar M, Meerwaldt JH, Carde P, Noordijk EM, Thomas J, et al. The EORTC trials for limited stage Hodgkin's disease. The EORTC Lymphoma Cooperative Group. European Journal of Cancer 1992;28A(11):1847‐50. [DOI] [PubMed] [Google Scholar]
Desablens 1999 {published data only}
- Desablens B, Jais JP, Lacotter‐Thierry L, Foussard C, Escoffre‐Barbe M, Moreau P, et al. Treatment of CS IA to IIIB non‐bulky Hodgkin's disease (NB‐HD) with 3 cycles of chemotherapy (CT) (ABVD vs EBVM) followed by high dose irradiation (RT). Results of the GOELAMS H90‐NM multicentre randomized trial. Blood 1999;94(10 suppl. 1):abstract 386a. [Google Scholar]
Dionet 1988 {published data only}
- Dionet C, Oberlin O, Habrand JL, Vilcoq J, Madelain M, Dutou L, et al. Initial chemotherapy and low‐dose radiation in limited fields in childhood Hodgkin's disease: results of a joint cooperative study by the French Society of Pediatric Oncology (SFOP) and Hôpital Saint‐Louis, Paris. International Journal of Radiation Oncology, Biology, Physics 1988;15(2):341‐6. [DOI] [PubMed] [Google Scholar]
Ferme 2005 {published data only}
- Ferme C, Diviné M, Vranovsky A, Morschhauser F, Bouabdallah R, Gabarre J, et al. Four ABVD and involvedfield radiotherapy in unfavorable supradiaphragmatic clinical stages (CS) III Hodgkin's lymphoma (HL): preliminary results of the EORTCGELA H9U trial [Abstract]. Blood 2005;106(11):abstract A‐813. [Google Scholar]
Friedmann 2014 {published data only}
- Friedman DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, et al. Dose‐intensive response‐based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate‐risk Hodgkin lymphoma: a report from the Children’s Oncology Group study AHOD0031. Journal of Clinical Oncology 2014;32(32):3651‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirsch 1994 {published data only}
- Hirsch A. The effect of ABVD chemotherapy with and without mediastinal irradiation on pulmonary function and symptoms in early‐stage Hodgkin's disease. International Journal of Radiation Oncology, Biology, Physics 1994;30(suppl. 1):168. [Google Scholar]
Hirsch 1996 {published data only}
- Hirsch A, Vander EN, Straus DJ, Gomez EG, Leung D, Portlock CS, et al. Effect of ABVD chemotherapy with and without mantle or mediastinal irradiation on pulmonary function and symptoms in early‐stage Hodgkin's disease. Journal of Clinical Oncology 1996;14(4):1297‐305. [DOI] [PubMed] [Google Scholar]
Horning 1996 {published data only}
- Horning SJ, Bennett JM, Bartlett NL, Williams J, Neuberg D, Cassileth PA. 12 weeks of chemotherapy (STANFORD V) and involved field radiotherapy (RT) are highly effective for bulky and advanced stage Hodgkin's disease (HD): a limited institution ECOG pilot study. Blood 1996;88(10 Suppl (Pt 1)):673a. [Google Scholar]
Horning 2007 {published data only}
- Horning SJ, Hoppe RT, Advani RH, Breslin S, McCormick E, Allen J, et al. A prospective trial of involved field radiation (IFRT) + chemotherapy vs. extended field radiation (EFRT) for favorable Hodgkin's disease (HD): Long‐term follow‐up and implications for current combined modality. Haematologica 2007;92(Suppl. 5):53. [Google Scholar]
Kim 2003 {published data only}
- Kim HK, Silver B, Li S, Neuberg D, Mauch P. Hodgkin's disease in elderly patients (> or =60): clinical outcome and treatment strategies. International Journal of Radiation Oncology, Biology, Physics 2003;56(2):556‐60. [DOI] [PubMed] [Google Scholar]
Körholz 2004 {published data only}
- Körholz D, Claviez A, Hasenclever D, Kluge R, Hirsch W, Kamprad F, et al. The concept of the GPOH‐HD 2003 therapy study for pediatric Hodgkin's disease: evolution in the tradition of the DAL/GPOH studies. Klinische Pädiatrie 2004;216(3):150‐6. [DOI] [PubMed] [Google Scholar]
Kung 1993 {published data only}
- Kung FH, Behm FG, Cantor A, Falletta J, Ferree CR, Leventhal BG, et al. Abbreviated chemotherapy vs chemoradiotherapy in early stage Hodgkin's disease of childhood. Proceedings of the American Society of Clinical Oncology. 1993; Vol. 12:414.
Kung 2006 {published data only}
- Kung FH. POG 8625: A randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with stages I, IIA, IIIA Hodgkin disease: A report from the children's oncology group. Journal of Pediatric Hematology/Oncology 2006;28(6):362‐8. [DOI] [PubMed] [Google Scholar]
Laskar 2004 {published data only}
- Laskar S, Gupta T, Vimal S, Muckaden MA, Saikia TK, Pai SK, et al. Consolidation radiation after complete remission in Hodgkin's disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: is there a need?. Journal of Clinical Oncology 2004;22(1):62‐8. [DOI] [PubMed] [Google Scholar]
Lemerle 1986 {published data only}
- Lemerle J, Oberlin O, Schaison G, Leverger G, Olive D, Duffilot B. Effectiveness of primary chemotherapy and low‐dose radiation (RT) in childhood Hodgkin's disease (HD) [abstract]. Proceedings of the American Society of Clinical Oncology. 1986.
Longo 1992 {published data only}
- Longo DL, DeVita VT. The use of combination chemotherapy in the treatment of early stage Hodgkin's disease. In: Vita VT, Helman S, Rosenberg SA editor(s). Important Advances in Oncology. Philadelphia: Lippincott Williams & Wilkins, 1992:155‐65. [PubMed] [Google Scholar]
Meyer 2013 {published data only}
- Meyer RM. Radiation in early‐stage Hodgkin lymphoma. Clinical Advances in Hematology & Oncology 2013;11(3):162‐89. [PubMed] [Google Scholar]
Nachman 2002 {published data only}
- Nachman JB, Sposto R, Herzog P, Gilchrist GS, Wolden SL, Thomson J, et al. Randomized comparison of low‐dose involved‐field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy. Journal of Clinical Oncology 2002;20(18):3765‐71. [DOI] [PubMed] [Google Scholar]
Noordijk 2006 {published data only}
- Noordijk EM, Carde P, Dupouy N, Hagenbeek A, Krol AD, Kluin‐Nelemans JC, et al. Combined‐modality therapy for clinical stage I or II Hodgkin's lymphoma: long‐term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. Journal of Clinical Oncology 2006;24(19):3128‐35. [DOI] [PubMed] [Google Scholar]
O'Dwyer 1984 {published data only}
- O'Dwyer PJ, Stewart MB, Wiernik PH. MOPP vs radiotherapy/MOPP for early‐stage Hodgkin's disease (HD) ‐ a six year follow‐up. 2nd International Conference on Malignant Lymphoma, Lugano, Switzerland. June 13, 1984; Vol. 16:46.
O'Dwyer 1985 {published data only}
- O'Dwyer PJ, Wiernik PH, Stewart MB, Slawson RG. Treatment of early stage Hodgkin's disease: a randomized trial of radiotherapy plus chemotherapy versus chemotherapy alone. In: Cavilli F, Bonadonna G, Rozencweig M editor(s). Malignant Lymphomas and Hodgkin's Disease: Experimental and Therapeutic Advances. Boston: Maritinus Nijhoff, 1985:329‐36. [Google Scholar]
Pavlovsky 1988 {published data only}
- Bloomfield CD, Pajak TF, Glicksman AS, Gottlieb AJ, Coleman M, Nissen NI, et al. Chemotherapy and combined modality therapy for Hodgkin's disease: a progress report on Cancer and Leukemia Group B studies. Cancer Treatment Reports 1982;66(4):835‐46. [PubMed] [Google Scholar]
Pavlovsky 1997 {published data only}
- Pavlovsky S, Schvartzman E, Lastiri F, Magnasco H, Corrado C, Raslawski E, et al. Randomized trial of CVPP for three versus six cycles in favorable‐prognosis and CVPP versus AOPE plus radiotherapy in intermediate‐prognosis untreated Hodgkin's disease. Journal of Clinical Oncology 1997;15(7):2652‐8. [DOI] [PubMed] [Google Scholar]
Picardi 2007 {published data only}
- Picardi M, Renzo A, Pane F, Nicolai E, Pacelli R, Salvatore M, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin's lymphoma with post‐chemotherapy negative positron emission tomography scans. Leukemia & Lymphoma 2007;48(9):1721‐7. [DOI] [PubMed] [Google Scholar]
Radford 2002 {published data only}
- Radford JA, Cowan RA, Ryder WD, Johnson RJ, Bannerjee SS, Deakin DP, et al. Four weeks of VAPEC‐B chemotherapy before involved field radiotherapy minimises the relapse rate in early stage low‐risk Hodgkin's disease and is not associated with an excess of second malignancy. Annals of Oncology 2002;13(Suppl. 2):25. [Google Scholar]
Reinartz 2013 {published data only}
- Reinartz G, Eich HT. Does involved field radiotherapy improve survival for children with Hodgkin's lymphoma in complete remission after chemotherapy?. Strahlentherapie und Onkologie 2013 ;189(4):344‐6. [DOI] [PubMed] [Google Scholar]
Rüffer 1996 {published data only}
- Rüffer U, Brosteanu O, Sieber M, Koch T, Löffler M, Pfreundschuh M. Reduction of radiotherapy in early stage Hodgkin's disease: results of a randomized trial in patients ps I/II. Annals of Oncology 1996;7(Suppl. 3):49. [Google Scholar]
Rüffer 1998 {published data only}
- Rüffer U, Sieber M, Pfistner B, Tesch H, Engert A, Bredenfeld H, et al. Reduction of radiotherapy volume in intermediate Hodgkin's disease: Interim analysis of a randomized trial in patients CS I/II of the GHSG. Blood 1998;92(10 Suppl 1 (Pt 1)):abstract 626a. [Google Scholar]
Rüffer 1999 {published data only}
- Rüffer JU, Sieber M, Pfistner B, Tesch H, Engert A, Bredenfeld H, et al. For intermediate stage Hodgkin's disease extended field radiation after effective chemotherapy is obsolete: interim analysis of HD9 trial (GHSG). Blood 1999;94(10 Suppl. 1):528a. [Google Scholar]
Specht 1992 {published data only}
- Specht L, Carde P, Mauch P, Magrini SM, Santarelli MT. Radiotherapy versus combined modality in early stages. Annals of Oncology 1992;3(suppl. 4):77‐81. [DOI] [PubMed] [Google Scholar]
Straus 1989 {published data only}
- Straus DJ, Myers J, Lee BJ, Koziner B, Nisce LZ, Redman J. Limited chemotherapy and radiation therapy (RT) for early clinical stage (CS) Hodgkin's disease (HD). High complete remission (CR) percentage, disease free survival (DFS) and low toxicity. Blood 1989;74(7 Suppl. 1):239a. [Google Scholar]
Thistlethwaite 2007 {published data only}
- Thistlethwaite F, Qian W, Williams MV, Hancock BW, Hoskin P, Sun‐Mynt H, et al. Selection of patients for minimal initial chemotherapy (MIC); the impact of Hasenclever score on outcome in patients receiving MIC and involved field radiotherapy for clinical stage IA/IIA supra‐diaphragmatic Hodgkin lymphoma in the UK NCRI LY07 trial. Haematologica 2007;92(Suppl. 5):52. [Google Scholar]
Thomas 2004 {published data only}
- Thomas J, Ferme C, Noordijk EM, Rieux C, Divine M, Brice P, et al. Six cycles of ABVD + IFRT vs. four cycles of ABVD + IFRT vs. four cycles of BEACOPP + IFRT in unfavourable supradiaphragmatic clinical stages III Hodgkin's lymphoma: the EORTCGELA H9U randomized clinical trial (20982) in 808 patients [Abstract AE12]. European Journal of Haematology 2004;73(Supp 65):40. [Google Scholar]
Weiner 1997 {published data only}
- Weiner MA, Leventhal B, Brecher ML, Marcus RB, Cantor A, Gieser PW, et al. Randomized study of intensive MOPP‐ABVD with or without low‐dose total‐nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin's disease in pediatric patients: a Pediatric Oncology Group study. Journal of Clinical Oncology 1997;15(8):2769‐79. [DOI] [PubMed] [Google Scholar]
Wolden 2012 {published data only}
- Wolden SL, Chen L, Kelly KM, Herzog P, Gilchrist GS, Thomson J, et al. Long‐term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin’s lymphoma—a report from the Children’s Oncology Group. Journal of Clinical Oncology 2012;30(26):3174‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
GHSG HD16 {published data only}
- GHSG. HD16 for early stages ‐ treatment optimization trial in the first‐line treatment of early stage Hodgkin lymphoma; treatment stratification by means of FDG‐PET. www.clinicaltrials.gov NCT00736320.
GHSG HD17 {published data only}
- GHSG. HD17 for intermediate stages treatment optimization trial in the firstline treatment of intermediate stage Hodgkin lymphoma. www.clinicaltrials.gov NCT01356680.
HD0801 {published data only}
- Fondazione Italiana Linfomi ONLUS. High‐dose chemotherapy and stem cell transplantation, in patients PET‐2 positive, after 2 courses of ABVD and comparison of RT versus no RT in PET‐2 negative patients (HD0801). www.clinicaltrials.gov NCT00784537.
Additional references
Adams 2004
- Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long‐term survivors of Hodgkin's disease treated with chest radiotherapy. Journal of Clinical Oncology 2004;22(15):3139‐48. [DOI] [PubMed] [Google Scholar]
Aleman 2003
- Aleman BM, Belt‐Dusebout AW, Klokman WJ, Van't Veer MB, Bartelink H, Leeuwen FE. Long‐term cause‐specific mortality of patients treated for Hodgkin's disease. Journal of Clinical Oncology 2003;21(18):3431‐9. [DOI] [PubMed] [Google Scholar]
Bhatia 2003
- Bhatia S, Yasui Y, Robison LL, Birch JM, Bogue MK, Diller L, et al. High risk of subsequent neoplasms continues with extended follow‐up of childhood Hodgkin's disease: report from the Late Effects Study Group. Journal of Clinical Oncology 2003;21(23):4386‐94. [DOI] [PubMed] [Google Scholar]
Canellos 2005
- Canellos GP. Chemotherapy alone for early Hodgkin's lymphoma: an emerging option. Journal of Clinical Oncology 2005;23(21):4574‐6. [DOI] [PubMed] [Google Scholar]
Carbone 1971
- Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Research 1971;31(11):1860‐1. [PubMed] [Google Scholar]
Chaber 2006
- Chaber BA, Amrein PC, Druker BJ, Michaelson MD, Mitsiades CS, Goss PF, et al. Antineoplastic agents. In: Brunton LL, Lazo JS, Parker KL editor(s). Goodman and Gillman's. The Pharmacological Basis of Therapeutics. 11. McGraw‐Hill, Medical Publishing Division, 2006:1380. [Google Scholar]
Cheson 2007
- Cheson BD, Pfistner B, Juweid ME, Gscoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. Journal of Clinical Oncology 2007;25(5):579‐86. [DOI] [PubMed] [Google Scholar]
Connors 2001
- Connors JM, Noordijk EM, Horning SJ. Hodgkin's lymphoma: basing the treatment on the evidence. Hematology: American Society of Haematology Education Book. American Society of Hematology, 2001:178‐93. [DOI] [PubMed] [Google Scholar]
Connors 2005
- Connors JM. State‐of‐the‐art therapeutics: Hodgkin's lymphoma. Journal of Clinical Oncology 2005;23(26):6400‐8. [DOI] [PubMed] [Google Scholar]
Crump 2015
- Crump M, Herst J, Baldassarre F, Sussman J, MacEachern J, Hodgson D, et al. Evidence‐based focused review of the role of radiation therapy in the treatment of early‐stage Hodgkin lymphoma. Blood 2015;125(11):1708‐16. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DeVita 1997
- DeVita VT Jr, Mauch PM, Harris NL. Hodgkin's disease. In: DeVita VT Jr, Hellmann S, Rosenberg SA editor(s). Cancer Principles and Practice of Oncology. 5th Edition. Philadelphia: Lippincott‐Raven, 1997:2242‐83. [Google Scholar]
Dickersin 1993
- Dickersin K, Min YI. NIH clinical trials and publication bias. Online Journal of Current Clinical Trials 1993;50:4967. [PubMed] [Google Scholar]
Diehl 2005
- Diehl V, Re D, Josting A. Hodgkin's disease: clinical manifestations, staging, and therapy. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ editor(s). Hematology: Basic Principles and Practice. 4th Edition. Churchill Livingstone, 2005:1347‐78. [Google Scholar]
Dores 2002
- Dores GM, Metayer C, Curtis RE, Lynch CF, Clarke EA, Glimelius B, et al. Second malignant neoplasms among long‐term survivors of Hodgkin's disease: a population‐based evaluation over 25 years. Journal of Clinical Oncology 2002;20(16):3484‐94. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Zellweger‐Zahner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350(9074):326‐9. [DOI] [PubMed] [Google Scholar]
Engert 2007
- Engert A, Franklin J, Eich HT, Brillant C, Sehlen S, Cartoni C, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended‐field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. Journal of Clinical Oncology 2007;25(23):3495‐502. [DOI] [PubMed] [Google Scholar]
Engert 2010
- Engert A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, et al. Reduced treatment intensity in patients with early‐stage Hodgkin's lymphoma. The New England Journal of Medicine 2010;363(7):640‐52. [DOI] [PubMed] [Google Scholar]
Engert 2012
- Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced‐intensity chemotherapy and PET‐guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open‐label, phase 3 non‐inferiority trial. Lancet 2012;379(9828):1791‐9. [PUBMED: 22480758] [DOI] [PubMed] [Google Scholar]
Franklin 2005
- Franklin JG, Paus MD, Pluetschow A, Specht L. Chemotherapy, radiotherapy and combined modality for Hodgkin's disease, with emphasis on second cancer risk. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003187] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glaser 1996
- Glaser SL, Jarrett RF. The epidemiology of Hodgkin's disease. Baillière's Clinical Haematology 1996;9(3):401‐16. [DOI] [PubMed] [Google Scholar]
Green 2000
- Green DM, Hyland A, Barcos MP, Reynolds JA, Lee RJ, Hall BC, et al. Second malignant neoplasms after treatment for Hodgkin's disease in childhood or adolescence. Journal of Clinical Oncology 2000;18(7):1492‐9. [DOI] [PubMed] [Google Scholar]
Henderson 2016
- Henderson TO, Moskowitz CS, Chou JF, Bradbury AR, Neglia JP, Dang CT, et al. Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: A report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology 2016;34(9):910‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
Higgins 2011a
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Juni 2002
- Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta‐analyses of controlled trials: empirical study. International Journal of Epidemiology 2002;31(1):115‐23. [DOI] [PubMed] [Google Scholar]
Klimm 2005
- Klimm B, Engert A, Diehl V. First‐line treatment of Hodgkin's lymphoma. Current Hematology Reports 2005;4(1):15‐22. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lister 1989
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. Journal of Clinical Oncology 1989;7(11):1630‐6. [DOI] [PubMed] [Google Scholar]
Maraldo 2015
- Maraldo MV, Giusti F, Vogelius IR, Lundemann M, Kaaij MA, Ramadan S, et al. Cardiovascular disease after treatment for Hodgkin's lymphoma: an analysis of nine collaborative EORTC‐LYSA trials. Lancet Haematology 2015;2(11):e492‐502. [DOI] [PubMed] [Google Scholar]
Mauch 1999
- Mauch PM, Armitage JO, Diehl V, Hoppe RT, Weiss LM. Hodgkin's Disease. 1st Edition. Philadelphia: Lippicott Williams & Wilkins, 1999. [Google Scholar]
McBride 2008
- McBride WH, Withers HR. Biologic basis of radiation therapy. In: Halperin EC, Perez CA, Brady LW editor(s). Principles and Practice of Radiation Oncology. 5th Edition. Philadelphia: Wolthers Kluwer, Lippincott Williams & Wilkin, 2008:76‐108. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [PUBMED: 19631508] [DOI] [PubMed] [Google Scholar]
Mueller 1999
- Mueller NE, Gruffermann S. The epidemiology of Hodgkin's disease. In: Mauch PM, Armitage JO, Diehl V, Hoppe RT, Weiss LM editor(s). Hodgkin's Disease. 1st Edition. Philadelphia: Lippicott Williams & Wilkins, 1999:61‐78. [Google Scholar]
Ng 2002a
- Ng AK, Bernardo MP, Weller E, Backstrand KH, Silver B, Marcus KC, et al. Long‐term survival and competing causes of death in patients with early‐stage Hodgkin's disease treated at age 50 or younger. Journal of Clinical Oncology 2002;20(8):2101‐8. [DOI] [PubMed] [Google Scholar]
Ng 2002b
- Ng AK, Bernardo MV, Weller E, Backstrand K, Silver B, Marcus KC, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long‐term risks and risk factors. Blood 2002;100(6):1989‐96. [DOI] [PubMed] [Google Scholar]
Parkin 2005
- Parkin DM, Bray F, FerlayJ, Pisani P. Global Cancer Statistics, 2002. CA: A Cancer Journal for Clinicians 2005;55(2):74‐108. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Rancea 2013
- Rancea M, Engert A, Tresckow B, Halbsguth T, Behringer K, Skoetz N. Hodgkin's lymphoma in adults: diagnosis, treatment and follow‐up. Deutsches Arzteblatt International 2013;110(11):177‐83, 183e1‐3. [PUBMED: 23555321] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sausville 2005
- Sausville EA, Longo DL. Principles of cancer treatment: surgery, chemotherapy and biologic therapy. In: Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL editor(s). Harrison's Principles of Internal Medicine. 16. New York: McGraw‐Hill, Medical Publishing Division, 2005:464‐83. [Google Scholar]
Schaapveld 2015
- Schaapveld M, Aleman BM, Eggermond AM, Janus CP, Krol AD, Maazen RW, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. New England Journal of Medicine 2015;373(26):2499‐511. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings tables'. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sickinger 2015
- Sickinger MT, Tresckow B, Kobe C, Engert A, Borchmann P, Skoetz N. Positron emission tomography‐adapted therapy for first‐line treatment in individuals with Hodgkin lymphoma. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010533.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Swerdlow 2000
- Swerdlow AJ, Barber JA, Hudson GV, Cunningham D, Gupta RK, Hancock BW, et al. Risk of second malignancy after Hodgkin's disease in a collaborative British cohort: the relation to age at treatment. Journal of Clinical Oncology 2000;18(3):498‐509. [DOI] [PubMed] [Google Scholar]
Swerdlow 2003
- Swerdlow AJ. Epidemiology of Hodgkin's disease and non‐Hodgkin's lymphoma. European Journal of Nuclear Medicine & Molecular Imaging 2003;30 Suppl 1:S3‐12. [DOI] [PubMed] [Google Scholar]
Thomas 2002
- Thomas RK, Re D, Zander T, Wolf J, Diehl V. Epidemiology and etiology of Hodgkin's lymphoma. Annals of Oncology 2002;13 Suppl 4:147‐52. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8(16):1745‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Leeuwen 2000
- Leeuwen FE, Klokman WJ, Veer MB, Hagenbeek A, Krol AD, Vetter UA, et al. Long‐term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. Journal of Clinical Oncology 2000;18(3):487‐97. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Herbst 2011
- Herbst C, Rehan FA, Skoetz N, Bohlius J, Brillant C, Schulz H, et al. Chemotherapy alone versus chemotherapy plus radiotherapy for early stage Hodgkin lymphoma. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD007110.pub2] [DOI] [PubMed] [Google Scholar]
Rehan 2008
- Rehan FA, Bohlius J, Brillant C, Monsef I, Specht L, Engert A. Chemotherapy alone versus chemotherapy plus radiotherapy for early stage Hodgkin Lymphoma . Cochrane Database of Systematic Reviews 2008 , Issue 2 . [DOI: 10.1002/14651858.CD007110] [DOI] [PubMed] [Google Scholar]


