Abstract
Background
The treatment of people with acute abdominal pain differs if they have acute pancreatitis. It is important to know the diagnostic accuracy of serum amylase, serum lipase, urinary trypsinogen‐2, and urinary amylase for the diagnosis of acute pancreatitis, so that an informed decision can be made as to whether the person with abdominal pain has acute pancreatitis. There is currently no Cochrane review of the diagnostic test accuracy of serum amylase, serum lipase, urinary trypsinogen‐2, and urinary amylase for the diagnosis of acute pancreatitis.
Objectives
To compare the diagnostic accuracy of serum amylase, serum lipase, urinary trypsinogen‐2, and urinary amylase, either alone or in combination, in the diagnosis of acute pancreatitis in people with acute onset of a persistent, severe epigastric pain or diffuse abdominal pain.
Search methods
We searched MEDLINE, Embase, Science Citation Index Expanded, National Institute for Health Research (NIHR HTA and DARE), and other databases until March 2017. We searched the references of the included studies to identify additional studies. We did not restrict studies based on language or publication status, or whether data were collected prospectively or retrospectively. We also performed a 'related search' and 'citing reference' search in MEDLINE and Embase.
Selection criteria
We included all studies that evaluated the diagnostic test accuracy of serum amylase, serum lipase, urinary trypsinogen‐2, and urinary amylase for the diagnosis of acute pancreatitis. We excluded case‐control studies because these studies are prone to bias. We accepted any of the following reference standards: biopsy, consensus conference definition, radiological features of acute pancreatitis, diagnosis of acute pancreatitis during laparotomy or autopsy, and organ failure. At least two review authors independently searched and screened the references located by the search to identify relevant studies.
Data collection and analysis
Two review authors independently extracted data from the included studies. The thresholds used for the diagnosis of acute pancreatitis varied in the trials, resulting in sparse data for each index test. Because of sparse data, we used ‐2 log likelihood values to determine which model to use for meta‐analysis. We calculated and reported the sensitivity, specificity, post‐test probability of a positive and negative index test along with 95% confidence interval (CI) for each cutoff, but have reported only the results of the recommended cutoff of three times normal for serum amylase and serum lipase, and the manufacturer‐recommended cutoff of 50 mg/mL for urinary trypsinogen‐2 in the abstract.
Main results
Ten studies including 5056 participants met the inclusion criteria for this review and assessed the diagnostic accuracy of the index tests in people presenting to the emergency department with acute abdominal pain. The risk of bias was unclear or high for all of the included studies. The study that contributed approximately two‐thirds of the participants included in this review was excluded from the results of the analysis presented below due to major concerns about the participants included in the study. We have presented only the results where at least two studies were included in the analysis.
Serum amylase, serum lipase, and urinary trypsinogen‐2 at the standard threshold levels of more than three times normal for serum amylase and serum lipase, and a threshold of 50 ng/mL for urinary trypsinogen‐2 appear to have similar sensitivities (0.72 (95% CI 0.59 to 0.82); 0.79 (95% CI 0.54 to 0.92); and 0.72 (95% CI 0.56 to 0.84), respectively) and specificities (0.93 (95% CI 0.66 to 0.99); 0.89 (95% CI 0.46 to 0.99); and 0.90 (95% CI 0.85 to 0.93), respectively). At the median prevalence of 22.6% of acute pancreatitis in the studies, out of 100 people with positive test, serum amylase (more than three times normal), serum lipase (more than three times normal), and urinary trypsinogen (more than 50 ng/mL), 74 (95% CI 33 to 94); 68 (95% CI 21 to 94); and 67 (95% CI 57 to 76) people have acute pancreatitis, respectively; out of 100 people with negative test, serum amylase (more than three times normal), serum lipase (more than three times normal), and urinary trypsinogen (more than 50 ng/mL), 8 (95% CI 5 to 12); 7 (95% CI 3 to 15); and 8 (95% CI 5 to 13) people have acute pancreatitis, respectively. We were not able to compare these tests formally because of sparse data.
Authors' conclusions
As about a quarter of people with acute pancreatitis fail to be diagnosed as having acute pancreatitis with the evaluated tests, one should have a low threshold to admit the patient and treat them for acute pancreatitis if the symptoms are suggestive of acute pancreatitis, even if these tests are normal. About 1 in 10 patients without acute pancreatitis may be wrongly diagnosed as having acute pancreatitis with these tests, therefore it is important to consider other conditions that require urgent surgical intervention, such as perforated viscus, even if these tests are abnormal.
The diagnostic performance of these tests decreases even further with the progression of time, and one should have an even lower threshold to perform additional investigations if the symptoms are suggestive of acute pancreatitis.
Plain language summary
Blood and urine tests for the diagnosis of acute pancreatitis (sudden inflammation of pancreas)
Background
The pancreas is an organ in the abdomen (tummy) that secretes several digestive enzymes (substances that break down the food we eat) into the pancreatic ductal system, which empties into the small bowel. The pancreas also contains the islets of Langerhans, which secrete several hormones such as insulin (which helps regulate blood sugar). Acute pancreatitis is sudden inflammation of the pancreas, which can lead to damage of the heart, lungs, and kidneys and cause them to fail. Acute pancreatitis usually manifests as upper abdominal pain radiating to the back. However, there are several potential causes of upper abdominal pain. It is important to determine if someone with abdominal pain has acute pancreatitis or another illness in order to start appropriate treatment. Blood tests such as serum amylase and serum lipase, as well as urine tests such as urinary trypsinogen‐2 and urinary amylase, can be used to determine if someone with abdominal pain has acute pancreatitis. It is usually the case that a patient is considered to have acute pancreatitis only when amylase or lipase levels are three times the upper limit of normal. With regard to urinary trypsinogen‐2, a level of more than 50 ng/mL of trypsinogen‐2 in the urine is considered an indication of acute pancreatitis. With regard to urinary amylase, there is no clear‐cut level beyond which someone with abdominal pain is considered to have acute pancreatitis. At present it is unclear whether these tests are equally effective or if one of the tests is better than the other in the diagnosis of acute pancreatitis in people with sudden‐onset abdominal pain. We determined to resolve this question by performing a literature search for studies reporting the accuracy of the above mentioned blood and urine tests. We included studies reported until 20 March 2017.
Study characteristics
We identified 10 studies reporting information on 5056 people with abdominal pain that started suddenly. The studies included pancreatitis due to all causes.
Quality of evidence
All of the studies were of unclear or low methodological quality, which may result in arriving at false conclusions. We excluded the study that contributed approximately two‐thirds of the participants included in this review from the results of the analysis presented below due to concerns about whether the participants included in the study are typical of those seen in the emergency department.
Key results
The accuracy of serum amylase, serum lipase, and urinary trypsinogen‐2 in making the diagnosis of acute pancreatitis was similar. About a quarter of people with acute pancreatitis fail to be diagnosed as having acute pancreatitis with these tests. The patient should be admitted and treated as having acute pancreatitis, even if these tests are normal, if there is a suspicion of acute pancreatitis. As about 1 in 10 patients without acute pancreatitis may be wrongly diagnosed as having acute pancreatitis with these tests, it is important to consider other conditions that require urgent surgery, even if these tests are abnormal. The diagnostic performance of these tests decreases even further with the progression of time, and additional investigations should be performed if there is a suspicion of acute pancreatitis.
Summary of findings
Summary of findings'. 'Serum amylase and lipase and urinary trypsinogen and amylase for diagnosis of acute pancreatitis.
| Population | People with abdominal pain seen in emergency care | ||||||||||
| Setting | Secondary care in various countries | ||||||||||
| Target condition | Acute pancreatitis | ||||||||||
| Reference standard |
|
||||||||||
| Pre‐test probability (prevalence of acute pancreatitis) | 22.6% | ||||||||||
| Index test | Sensitivity | Specificity | Post‐test probability of a positive test1 | Post‐test probability of a negative test1 | Number of false positives per 100 people having a positive test | Number of false negatives per 100 people having a negative test | Number of studies | Number of participants | Risk of bias | Applicability concerns | Inconsistency |
| Serum amylase (threshold: > 3 times normal) (on admission)2 | 0.72 (95% CI 0.59 to 0.82) | 0.93 (95% CI 0.66 to 0.99) | 74.0% (95% CI 33.4% to 94.1%) | 8.1% (95% CI 5.4% to 12.1%) | 26 (95% CI 6 to 67) | 8 (95% CI 5 to 12) | 3 | 605 | Unclear | Low | Moderate |
| Serum lipase (threshold: > 3 times normal) (on admission)2 | 0.79 (95% CI 0.54 to 0.92) | 0.89 (95% CI 0.46 to 0.99) | 68.1% (95% CI 21.4% to 94.3%) | 6.6% (95% CI 2.7% to 15.1%) | 32 (95% CI 6 to 79) | 7 (95% CI 3 to 15) | 4 | 678 | Unclear | Low | Moderate |
| Urinary trypsinogen‐2 (threshold: Actim Pancreatitis ‐ all studies; > 50 ng/mL) (on admission) | 0.72 (95% CI 0.56 to 0.84) | 0.90 (95% CI 0.85 to 0.93) | 67.2% (95% CI 57.3% to 75.7%) | 8.4% (95% CI 5.2% to 13.3%) | 33 (95% CI 24 to 43) | 8 (95% CI 5 to 13) | 5 | 841 | High | Unclear | Moderate |
| Urinary trypsinogen‐2 (quantitative) (threshold: > 50 ng/mL) (on admission) | 0.71 (95% CI 0.63 to 0.78) | 0.89 (95% CI 0.84 to 0.92) | 65.6% (95% CI 57.0% to 73.3%) | 8.7% (95% CI 6.9% to 10.9%) | 34 (95% CI 27 to 43) | 9 (95% CI 7 to 11) | 1 | 412 | High | Low | Not applicable |
| Urinary trypsinogen‐2 (threshold: only + or most positive ‐ the threshold for this was not available) (on admission) | 0.60 (95% CI 0.51 to 0.67) | 0.92 (95% CI 0.88 to 0.95) | 69.1% (95% CI 59.0% to 77.6%) | 11.4% (95% CI 9.6% to 13.5%) | 31 (95% CI 22 to 41) | 11 (95% CI 10 to 13) | 1 | 412 | High | Low | Not applicable |
| Urinary amylase (quantitative) (threshold: above normal) (on admission) | 0.83 (95% CI 0.65 to 0.94) | 0.86 (95% CI 0.77 to 0.91) | 62.8% (95% CI 50.8% to 73.5%) | 5.4% (95% CI 2.5% to 11.3%) | 37 (95% CI 27 to 49) | 5 (95% CI 2 to 11) | 1 | 134 | Unclear | Unclear | Not applicable |
| Urinary amylase (qualitative) (threshold: 1 plus) (on admission) | 0.66 (95% CI 0.49 to 0.79) | 0.94 (95% CI 0.90 to 0.97) | 77.3% (95% CI 64.2% to 86.6%) | 9.6% (95% CI 6.5% to 14.0%) | 23 (95% CI 13 to 36) | 10 (95% CI 6 to 14) | 1 | 218 | High | High | Not applicable |
| Urinary amylase (qualitative) (threshold: 2 plus) (on admission) | 0.44 (95% CI 0.29 to 0.60) | 0.99 (95% CI 0.96 to 1.00) | 95.8% (95% CI 75.8% to 99.4%) | 14.2% (95% CI 11.2% to 17.8%) | 4 (95% CI 1 to 24) | 14 (95% CI 11 to 18) | 1 | 218 | High | High | Not applicable |
CI: confidence interval
1The post‐test probabilities were calculated at the median pre‐test probability. At the lower quartile of pre‐test probability of 16.3%, the post‐test probabilities of positive test for serum amylase (more than three times normal), serum lipase (more than three times normal), and urinary trypsinogen (more than 50 ng/mL) were 65.5% (95% CI 25.1% to 91.5%); 58.7% (95% CI 15.4% to 91.7%); and 57.7% (95% CI 47.2% to 67.5%), respectively. At the same pre‐test probability of 16.3%, the post‐test probabilities of negative test for serum amylase (more than three times normal), serum lipase (more than three times normal), and urinary trypsinogen (more than 50 ng/mL) were 5.6% (95% CI 3.7% to 8.4%); 4.5% (95% CI 1.8% to 10.6%); and 5.8% (95% CI 3.5% to 9.3%), respectively. At the upper quartile of pre‐test probability of 47.3%, the post‐test probabilities of positive test for serum amylase (more than three times normal), serum lipase (more than three times normal), and urinary trypsinogen (more than 50 ng/mL) were 89.7% (95% CI 60.7% to 98.0%); 86.7% (95% CI 45.6% to 98.1%); and 86.3% (95% CI 80.5% to 90.6%), respectively. At the same pre‐test probability of 47.3%, the post‐test probabilities of negative test for serum amylase (more than three times normal), serum lipase (more than three times normal), and urinary trypsinogen (more than 50 ng/mL) were 21.4% (95% CI 14.9% to 29.7%); 17.8% (95% CI 7.9% to 35.4%); and 22.0% (95% CI 14.4% to 32.0%), respectively. 2The results do not include one study for which there was high concern about applicability.
Background
The pancreas is an abdominal organ that secretes several digestive enzymes into the pancreatic ductal system, which empties into the small bowel. It also houses the islets of Langerhans, which secrete several hormones including insulin (NCBI 2014). Acute pancreatitis is a sudden inflammatory process in the pancreas, with variable involvement of adjacent organs or other organ systems (Bradley 1993). The annual incidence of acute pancreatitis ranges from 5 to 30 per 100,000 population (Roberts 2013; Yadav 2006). In the last one to two decades there has been an increase in the incidence of acute pancreatitis in the UK and USA (Roberts 2013; Yang 2008). Acute pancreatitis is the most common gastrointestinal (digestive tract) cause of hospital admission in the USA (Peery 2012). Gallstones and alcohol are the two main causes of acute pancreatitis. Approximately 50% to 70% of cases of acute pancreatitis are caused by gallstones (Roberts 2013; Yadav 2006). Increasing age, male gender, and lower socioeconomic class are associated with a higher incidence of acute pancreatitis (Roberts 2013).
According to a consensus conference on the classification of acute pancreatitis, the diagnosis of acute pancreatitis is generally made when at least two of the following three features are present (Banks 2013).
Acute onset of a persistent, severe epigastric pain often radiating to the back.
Serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal.
Characteristic findings of acute pancreatitis on contrast‐enhanced computed tomography (CECT) and, less commonly, magnetic resonance imaging (MRI) or transabdominal ultrasonography.
Acute pancreatitis can be classified into interstitial oedematous pancreatitis (diffuse or occasionally localised enlargement of the pancreas due to inflammatory oedema as seen on CECT) or necrotising pancreatitis (necrosis involving either the pancreas or peripancreatic tissues, or both) (Banks 2013). Approximately 90% to 95% of people with acute pancreatitis have interstitial oedematous pancreatitis, while the remainder have necrotising pancreatitis (Banks 2013). Necrotising pancreatitis may be sterile or infected (Banks 2013). Various theories exist as to how pancreatic and peripancreatic tissues become infected, including spreading of the infection from blood circulation, lymphatics, bile, from the small bowel (duodenum) through the pancreatic duct, and migration through the large bowel wall (translocation) (Schmid 1999).
Local complications of acute pancreatitis include acute peripancreatic fluid collection, pancreatic pseudocyst, acute necrotic collection, and walled‐off necrosis (Banks 2013). The systemic complications of acute pancreatitis include worsening of pre‐existing illnesses, such as heart or chronic lung disease (Banks 2013). The mortality rate following an attack of acute pancreatitis is between 6% and 20% (Roberts 2013; Yadav 2006). The mortality rate depends upon the severity of acute pancreatitis and the presence of infection. Acute pancreatitis can be classified as mild, moderate, or severe, depending upon the presence of local or systemic complications, transient organ failure involving one of more of lungs, kidneys, and cardiovascular system (heart and blood vessels) lasting up to 48 hours, or persistent failure of the same organs mentioned above lasting beyond 48 hours. In mild pancreatitis, there are no local complications, systemic complications, or organ failure. In moderately severe acute pancreatitis, there may be local or systemic complications or transient organ failure. In severe acute pancreatitis, there is persistent organ failure (Banks 2013). (See summary in Table 2.) Acute severe pancreatitis carries the worst prognosis in terms of mortality, while mild pancreatitis has the best prognosis (Banks 2013). Infected necrotising pancreatitis carries a significantly worse prognosis than sterile necrotising pancreatitis, with an average in‐hospital mortality of more than 30% for people with infected necrotising pancreatitis, which increases to more than 40% in the subgroup of people with organ failure in addition to infection (Petrov 2010).
1. Acute pancreatitis classification.
| Mild acute pancreatitis | Moderate acute pancreatitis | Severe acute pancreatitis |
|
|
|
See Appendix 1 for a glossary of terms.
Target condition being diagnosed
Acute pancreatitis in people with acute epigastric pain or diffuse abdominal pain.
Index test(s)
All of the index tests evaluated in this review are performed by the laboratory technician and interpreted by the clinician.
Serum amylase
Amylase is an enzyme secreted by the pancreas. Various other tissues including salivary glands, small intestine, ovaries, adipose tissue, and skeletal muscles secrete amylase. There are two major isoforms of amylase: pancreatic amylase and salivary amylase. The normal range of amylase varies between laboratories, but is usually between 100 international units (IU)/L to 300 IU/L (Vissers 1999). Acute pancreatitis is one cause of increased amylase (hyperamylasaemia). The reason for this elevation is unclear, although capillary leakage due to obstruction of venous and lymphatic drainage of pancreatic and peripancreatic tissues, and transperitoneal absorption of amylase may be responsible (Vissers 1999). In acute pancreatitis, serum amylase levels usually rise within 6 to 24 hours, peak at 48 hours, and decrease to normal or near normal levels over the next 5 to 7 days (Vissers 1999). A common threshold used is three times the normal limit (Banks 2013).
Serum lipase
Lipase is another enzyme secreted by the pancreas. Acute pancreatitis is the main reason for an increase in lipase, although a number of other conditions such as chronic pancreatitis, acute cholecystitis, and bowel obstruction can increase lipase activity (Vissers 1999). In acute pancreatitis, serum lipase levels usually rise within 4 to 8 hours, peak at 24 hours, and decrease to normal or near normal levels over the next 8 to 14 days. Serum lipase remains elevated for a longer period of time compared to the period of elevation of serum amylase after acute pancreatitis (Vissers 1999). A common threshold used is three times the normal limit (Banks 2013).
Urinary trypsinogen level
Autodigestion because of trypsinogen activation is one of the mechanisms believed to result in acute pancreatitis. Since trypsinogen levels are elevated in acute pancreatitis, measurement of urinary trypsinogen‐2 (an isoenzyme of trypsinogen) has been proposed as a test for diagnosing pancreatitis (Hedstrom 1994; Hedstrom 1996; Hedstrom 1996c). In acute pancreatitis, urinary trypsinogen levels usually rise to high levels within a few hours and decrease in three days (Matull 2006). A common threshold used is 50 ng/mL (Chang 2012).
Urinary amylase
Urinary amylase above 2000 IU/L is considered abnormal. Measurement of urinary amylase has been proposed as a test for the diagnosis of pancreatitis (Hedstrom 1996c; Kemppainen 1997c).
Clinical pathway
For people with acute onset of a persistent, severe epigastric pain or with diffuse abdominal pain starting in the epigastric region (or if the person is unsure about the region in which diffuse abdominal pain began), clinical examination including recording of blood pressure, pulse rate, and oxygen saturations (when available) are performed. Routine blood tests such as full blood count, urea, creatinine, and electrolytes are also performed. Blood tests such as amylase and lipase (index tests being evaluated in this review) are performed to confirm (or rule out) the diagnosis of acute pancreatitis. Radiological findings of acute pancreatitis evolve over a few days and the radiological features may not be apparent in the early stages, or may even be normal (Banks 2013; Vissers 1999), thus one cannot rely on radiological tests to diagnose acute pancreatitis, at least in the early stages. Radiological examination with CT scan or MRI is not routinely performed if a diagnosis of acute pancreatitis is suspected. If acute pancreatitis can be ruled out, other causes of acute epigastric pain should be considered. Peptic ulcer, functional dyspepsia, and gallstones can present with acute epigastric pain (Gurusamy 2014; Moayyedi 2006). All of these alternative causes of epigastric pain are generally investigated and treated after discharge of the patient unless there is a strong suspicion of perforated peptic ulcer, usually because of features of peritonitis or because pain control could not be achieved. In such instances, either a plain X‐ray of the abdomen or emergency CT scan, or both may be performed to identify the presence of free‐intraperitoneal gas (Ghekiere 2007; Grassi 2004). The usual treatment for perforated peptic ulcer is emergency surgical closure, which can be performed by open or laparoscopic surgery (Sanabria 2013).
If a diagnosis of acute pancreatitis can be established, usually based on the consensus criteria, the patient is admitted to hospital and the severity of pancreatitis is assessed. The treatment of acute pancreatitis is generally supportive treatment, that is maintenance of fluid and electrolyte imbalance. Despite various pharmacological interventions being evaluated in acute pancreatitis, none is currently recommended as treatment. Abdominal ultrasound and magnetic resonance cholangiopancreatography or endoscopic ultrasound may be performed to investigate the aetiology of acute pancreatitis. In the presence of gallstones, cholecystectomy is performed. The timing of cholecystectomy in acute pancreatitis is controversial, and different factors must be considered depending upon the severity of acute pancreatitis (Gurusamy 2013). Endoscopic sphincterotomy or common bile duct exploration may have to be performed in the presence of common bile duct stones (Ayub 2004; Larson 2006). In the absence of gallstones, investigation of other causes of acute pancreatitis is required. Patients are generally monitored clinically. If the patient improves clinically with supportive treatment, the patient with gallstone pancreatitis is discharged after cholecystectomy or after scheduling a cholecystectomy or on a planned list, within two weeks. For those patients with severe acute pancreatitis, cholecystectomy is undertaken when clinically appropriate after resolution of pancreatitis. If the patient deteriorates clinically, the patient undergoes a CT scan and may require high‐dependency or intensive care in the presence of organ failure or infected pancreatic necrosis.
In the presence of organ failure, patients undergo a CT scan or MRI to identify any local complications. C‐reactive protein, procalcitonin, and lactate dehydrogenase might distinguish between oedematous and necrotising pancreatitis (Alfonso 2003; Khanna 2013; Rau 1998), and could potentially be used as a triage test to identify patients who need further radiological tests in those without organ failure (Alfonso 2003). Some centres use C‐reactive protein routinely to determine whether patients require radiological investigations to diagnose necrotising pancreatitis. Frequently, the rising trend in C‐reactive protein, procalcitonin, or lactate dehydrogenase, rather than a single test, may be used to determine whether patients require radiological investigations to diagnose necrotising pancreatitis. It must be noted that CT scan or MRI is not routinely performed during the initial stages of acute pancreatitis, but usually in the presence of organ failure or because of the results of the serum C‐reactive protein. The various treatment strategies for acute necrotising pancreatitis include non‐surgical (conservative) treatment, percutaneous drainage, endoscopic transluminal drainage, early surgical debridement (necrosectomy, which can be performed by open surgery or by minimally invasive retroperitoneal debridement), delayed necrosectomy (delaying the surgery by about four weeks), or a step‐up approach that consists of endoscopic or percutaneous drainage followed by laparoscopic necrosectomy if required, and non‐surgical (conservative) treatment (Bakker 2012; Mouli 2013; Tenner 2013; van Brunschot 2014; van Santvoort 2010; van Santvoort 2011). A recent Cochrane systematic review found that a step‐up approach may be preferable to direct surgery in participants with acute necrotising pancreatitis (Gurusamy 2016). All of these treatments are supported by appropriate fluid therapy and nutritional support. This is in comparison with severe acute oedematous pancreatitis, where the main treatment is supportive treatment for systemic complications, including organ failure and treatment of local complications such as pseudocyst if symptomatic (Cannon 2009; Cheruvu 2003; Johnson 2009; Varadarajulu 2008; Varadarajulu 2013). If patients have infected pancreatic necrosis, appropriate antibiotics are administered in addition to the treatment outlined above for non‐infected pancreatic necrosis. If patients have acute peripancreatic collections or pseudocysts on the radiological tests, clinical and radiological follow‐up are required to ensure resolution of these collections.
If the diagnosis of acute pancreatitis cannot be ruled out on the basis of the clinical presentation and serum amylase or lipase, the patient is admitted to hospital and the evolution of signs and symptoms is noted. Serum amylase and lipase may be repeated or radiological examinations may be performed to establish or rule out acute pancreatitis with a reasonable amount of certainty. Tests for organ failure (e.g. urea and creatinine for identifying renal failure, blood pressure, pulse rate, respiratory rate, urine output, and arterial blood gases) may also be performed to ensure that the patient does not have moderately severe or severe pancreatitis irrespective of the results of serum amylase and lipase. The possible clinical pathway in the diagnosis and management of acute pancreatitis is shown in Figure 1.
1.
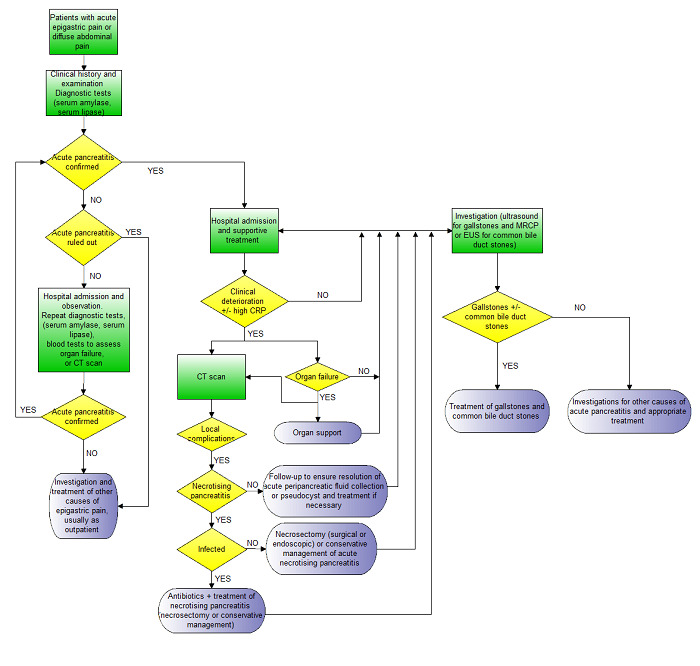
Clinical pathway.
Footnotes:
- Acute pancreatitis is usually confirmed by consensus criteria (Banks 2013).
- Irrespective of the CT scan findings and presence or absence of necrosis, patients with organ failure will require organ support and will receive a CT scan.
- CT scan may also be performed in people without organ failure if there is clinical deterioration (not amounting to organ failure) or in some centres based on an elevated CRP.
- Necrotising pancreatitis is usually confirmed by the findings on the CT scan and by histopathological examination of the biopsy obtained during necrosectomy if early necrosectomy is performed.
- Infected necrotising pancreatitis is usually confirmed by the findings on the CT scan and by microbiological examination of fluid aspirated under radiological guidance or from the tissue biopsy obtained during necrosectomy if early necrosectomy is performed.
- Organ failure is diagnosed on the basis of clinical examination and blood tests (urea, creatinine, blood pressure, pulse rate, respiratory rate, arterial blood gas analysis).
Abbreviations:
CRP: C‐reactive protein CT: computed tomography EUS: endoscopic ultrasound MRCP: magnetic resonance cholangiopancreatography
Prior test(s)
The minimum prior test that is performed before these tests are conducted is clinical history and clinical examination, which includes obtaining the body temperature, heart rate, blood pressure, respiratory rate, and pulse oximetry (when available).
Role of index test(s)
The index tests are used for the diagnosis of acute pancreatitis in people with acute onset of a persistent, severe epigastric pain or with diffuse abdominal pain that started in the epigastric region (or if the person is unsure about the region in which diffuse abdominal pain began). The current tests used are serum amylase and serum lipase. Urinary trypsinogen and urinary amylase are being evaluated as replacement tests for serum amylase and serum lipase.
Alternative test(s)
Other tests used in the diagnosis of acute pancreatitis include serum trypsinogen‐2 (Hedstrom 1994), and radiological tests such as contrast‐enhanced computed tomography (CECT), magnetic resonance imaging (MRI), or transabdominal ultrasonography (Banks 2013). Other biomarkers such as serum trypsin‐2‐alpha1‐antitrypsin complex, carboxypeptidase B activation peptide (CAPAP), and urinary trypsinogen activation peptide (TAP) have been evaluated as diagnostic tests for acute pancreatitis (Hedstrom 1996d; Saez 2005), but these are not in routine use for the diagnosis of this condition.
Rationale
In addition to acute pancreatitis, there are several other causes of epigastric pain including peptic ulcer, functional dyspepsia, and gallstones (Gurusamy 2014; Moayyedi 2006). Of these various causes, people with acute pancreatitis and perforated peptic ulcer need emergency admission and treatment, while others may be discharged if pain control can be achieved. It is thus important to make a diagnosis of acute pancreatitis. Radiological findings of acute pancreatitis evolve over a few days, and the radiological examination may not demonstrate characteristic features in the early stages, or may even be normal (Banks 2013; Vissers 1999), thus radiological tests are not routinely performed for diagnosing this condition. In addition, acute pancreatitis can mimic perforated peptic ulcer (Kuzmich 2012), which is usually treated by surgery. Correct diagnosis of acute pancreatitis can avoid unnecessary surgery. Hence, an accurate diagnostic test for the diagnosis of acute pancreatitis is essential in people with suspected acute pancreatitis. Serum amylase and lipase are the tests most commonly used in the diagnosis of acute pancreatitis. It is important to understand the diagnostic accuracy of these tests. Urinary trypsinogen and amylase have been investigated as alternate tests, and it is important to understand whether they can replace serum amylase and lipase in the diagnosis of acute pancreatitis. If one or more of the tests being assessed has a high degree of accuracy, patients with acute pancreatitis can be identified and managed appropriately. At the same time, unnecessary hospital admission for observation can be avoided in patients without acute pancreatitis, resulting in considerable resource savings. There has been no systematic review and meta‐analysis of the diagnostic accuracy of serum lipase and amylase activity or urinary amylase in the diagnosis of acute pancreatitis. The current consensus criteria about diagnosis of acute pancreatitis included serum lipase or amylase activity at least three times greater than the upper limit of normal as one of the criteria for diagnosis of acute pancreatitis (two of the three criteria must be met, the other two being acute abdominal pain and imaging characteristic of acute pancreatitis) (Banks 2013). However, these criteria are based on consensus rather than on systematic reviews. In addition, the threshold for amylase or lipase may need to be revised from three times normal to a different threshold if these tests are accurate at different thresholds. If this systematic review found that urinary amylase or trypsinogen‐2 were better than serum amylase or lipase, the criteria for the diagnosis of acute pancreatitis would need to be altered. There have been two systematic reviews on the diagnostic test accuracy of urinary trypsinogen‐2 in the diagnosis of acute pancreatitis (Chang 2012; Jin 2013). In both reviews, language restrictions (English and Chinese) were present. The searches were performed in 2011 and 2012, respectively. Only one of the reviews used appropriate statistical analysis (Chang 2012). There has been no Cochrane review on the role of urinary trypsinogen‐2 in the diagnosis of acute pancreatitis. The change in diagnostic accuracy of these tests with different time intervals from presentation has not been previously assessed in a systematic review. A Cochrane systematic review of the diagnostic test accuracy of serum and urine tests in the diagnosis of acute pancreatitis was, therefore, necessary.
Objectives
To compare the diagnostic accuracy of serum amylase, serum lipase, urinary trypsinogen‐2, and urinary amylase, either alone or in combination, in the diagnosis of acute pancreatitis in people with acute onset of a persistent, severe epigastric pain or diffuse abdominal pain.
Secondary objectives
We planned to explore the following sources of heterogeneity.
Studies at low risk of bias in all of the domains versus those at unclear or high risk of bias (as assessed by the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool, recommended by the Cochrane Diagnostic Test Accuracy Group) (Whiting 2006; Whiting 2011).
Prospective studies versus retrospective studies (to determine whether there is a difference in diagnostic accuracy between prospective and retrospective studies).
Full‐text publications versus abstracts (this can be indicative of publication bias since there may be an association between the results of the study and the study reaching full publication status) (Eloubeidi 2001).
Previous history of acute pancreatitis.
Different aetiology for acute pancreatitis (gallstone versus alcohol versus other aetiology). The accuracy of the test may depend upon the aetiology of the acute pancreatitis.
Presence of organ failure. The accuracy of the test may depend upon the presence of organ failure.
Average time to performance of the test. The accuracy of the test may depend upon the interval between the onset of clinical symptoms and the performance of the test.
Different test manufacturers.
Methods
Criteria for considering studies for this review
Types of studies
We included studies that evaluated the accuracy of the index tests mentioned above in the appropriate patient population (see below). We included relevant studies irrespective of language or publication status (i.e. published as full text or abstract), whether the data were collected prospectively or retrospectively, and whether there was a comparison between the tests. However, we excluded case reports (which describe how the diagnosis of acute pancreatitis was made on an individual patient or a group of patients and which do not provide sufficient diagnostic test accuracy data, i.e. true positive, false positive, false negative, and true negative). We also excluded case‐control studies because they are prone to bias (Whiting 2011).
Participants
Adults with acute epigastric or diffuse abdominal pain (with or without previous history of acute pancreatitis and with or without systemic signs and symptoms of acute pancreatitis), presenting to the hospital within three days of the onset of symptoms, irrespective of the interval between onset of symptoms and the time at which the test was performed.
Index tests
Serum amylase, serum lipase, urinary trypsinogen, and urinary amylase either alone or in combination. A variety of kits are available for measuring these tests. We included kits from all manufacturers, and included studies irrespective of the threshold used. Although we did not plan to include repeat tests, the diagnostic test accuracy of these index tests on later days might give some indication of the performance of these tests in patients with a prolonged period of symptoms before going to the hospital. We have therefore analysed and reported this information separately from the tests conducted on admission.
Target conditions
Acute pancreatitis (regardless of severity: mild, moderately severe, or severe)
Reference standards
While inflammation of the pancreas confirmed by biopsy can be considered to be the gold standard for the diagnosis of acute pancreatitis, for ethical reasons it is unlikely to be performed in any participant. As a result, different study authors may use different reference standards such as radiological features of acute pancreatitis or the presence of organ failure. However, such reference standards may miss some cases of mild acute pancreatitis, which will result in an underestimation of diagnostic test accuracy of the index tests. We also accepted the consensus conference definition of acute pancreatitis, that is when at least two of the following three features are present (Banks 2013).
Acute onset of a persistent, severe epigastric pain often radiating to the back.
Serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal.
Characteristic findings of acute pancreatitis on CECT, and less commonly on MRI or transabdominal ultrasonography.
We also accepted any of the following reference standards, used alone or in combination: biopsy, radiological features of acute pancreatitis (CT or MRI), diagnosis of acute pancreatitis during laparotomy or autopsy, organ failure, or the consensus conference definition (including or excluding the index test being evaluated). In terms of ranking the reference standards, we considered biopsy to be the best reference standard (although for ethical reasons it is unlikely to have been performed in any participant) followed by the consensus definition of acute pancreatitis; radiological, laparotomy, or autopsy features of acute pancreatitis; or the presence of organ failure, in that order. However, we anticipated that the authors would exclude the test being assessed to be incorporated into the reference standard. For example, if serum amylase was being evaluated, the final diagnosis of acute pancreatitis would not depend upon the levels of serum amylase; this was not the case, as described below. If the test being assessed was incorporated into the reference standard, the diagnostic accuracy of the test would be overestimated.
Search methods for identification of studies
We included all studies irrespective of the language of publication and publication status. We obtained translations for non‐English language articles.
Electronic searches
We searched the following databases.
MEDLINE (In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R)) via OvidSP (January 1946 to 20 March 2017) (Appendix 2).
Embase via OvidSP (January 1947 to 20 March 2017) (Appendix 3).
Science Citation Index Expanded via Web of Knowledge (January 1980 to 20 March 2017) (Appendix 4).
Conference Proceedings Citation Index‐Science (CPCI‐S) via Web of Knowledge (January 1990 to 20 March 2017) (Appendix 4).
National Insitute for Health Research (NIHR HTA and DARE) via Centre for Reviews and Dissemination (20 March 2017) (Appendix 5).
Zetoc via British Library (20 March 2017) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/) (20 March 2017) (Appendix 7).
ClinicalTrials.gov (clinicaltrials.gov/) (20 March 2017) (Appendix 8).
We used the same strategy for this review and another review on the diagnosis of pancreatic necrosis in people with established acute pancreatitis (Gurusamy 2015).
Searching other resources
We searched the references of the included studies to identify additional studies. We also searched for articles related to the included studies by performing the 'related search' function in MEDLINE (OvidSP) and Embase (OvidSP) and a 'citing reference' search (by searching the articles that cite the included articles) in these databases (Sampson 2008).
Data collection and analysis
Selection of studies
Two review authors (KSG and OK) independently searched the references to identify relevant studies. We obtained the full texts of references considered to be relevant by at least one of the review authors. Two review authors (KSG and GR or AH) independently screened the full‐text papers against the inclusion criteria. Any disagreements in study selection were resolved by discussion. We planned to contact the study authors if there were any doubts about study eligibility.
Data extraction and management
Two review authors (KSG and GR or AH) independently extracted the following data from each included study using a data extraction form designed and piloted by KSG. Any differences were resolved by discussion.
First author.
Year of publication.
Study design (prospective or retrospective cohort studies; cross‐sectional studies or randomised controlled trials).
Inclusion and exclusion criteria for individual studies.
Total number of participants.
Number of females.
Average age of the participants.
Average time between onset of symptoms and index test.
Aetiology of acute pancreatitis.
Proportion of participants with organ failure.
Description of the index test.
Threshold used for index test.
Reference standard.
Number of true positives, false positives, false negatives, and true negatives.
If the same study reported multiple index tests, we extracted the number of true positives, false positives, false negatives, and true negatives for each index test at each threshold. If the same study reported the number of true positives, false positives, false negatives, and true negatives for each index test at different thresholds, we extracted this information for each threshold. If the study reported the results for a combination of tests, we planned to extract the number of true positives, false positives, false negatives, and true negatives for each different combination of tests.
We defined a combination of tests as positive in two ways: 'at least one test positive' or 'all tests positive'. We planned to extract the number of true positives, false positives, false negatives, and true negatives for both the scenarios. If the study reported the test at multiple time points, we planned to use the results of the first test in the diagnosis of acute pancreatitis to calculate the true positives, false positives, false negatives, and true negatives, since the aim of this review was to assess the diagnostic accuracy in people with acute epigastric pain and abdominal pain who have not undergone any prior tests other than routine clinical examination. However, the diagnostic test accuracy of these index tests on later days of hospital might give some indication on the performance of these tests in patients with a prolonged period of symptoms before going to hospital. We have therefore analysed and reported this information separately from the tests conducted on admission.
We planned to exclude patients with uninterpretable index test results (whatever the reason given for lack of interpretation), since in clinical practice, uninterpretable index test results will result in additional tests for the diagnosis of acute pancreatitis. However, we planned to record the number of uninterpretable index test results, as this would provide information on the applicability of the test in clinical practice and may affect the cost‐effectiveness of a test. (Although cost‐effectiveness is outside the scope of this review, cost‐effectiveness studies may use data from this review). If there was an overlap of participants between multiple reports, as suggested by common authors and centres, we planned to contact the study authors to seek clarification about the overlap. If we were unable to contact the authors, we planned to extract the maximum possible information from all of the reports. We sought further information from study authors where necessary.
Assessment of methodological quality
Two review authors (KSG and GR or AH) independently assessed study quality using the QUADAS‐2 assessment tool (Whiting 2006; Whiting 2011). We resolved any differences by discussion and using the criteria to classify the different studies published in the protocol and available in Table 3. We considered studies classified as 'low risk of bias' and 'low concern' in all of the domains as studies with high methodological quality. We have presented the results in a 'Risk of bias' summary and graphs in addition to a narrative summary.
2. QUADAS‐2 classification (acute pancreatitis).
| Domain 1: Participant selection | Patient sampling | Adult patients with acute epigastric or diffuse abdominal pain. |
| Was a consecutive or random sample of patients enrolled? | Yes: If a consecutive sample or a random sample of patients with acute epigastric or diffuse abdominal pain was included in the study. No: If a consecutive sample or a random sample of patients with acute epigastric or diffuse abdominal pain was not included in the study. Unclear: If this information was not available. | |
| Did the study avoid inappropriate exclusions? | Yes: If all patients with acute epigastric or diffuse abdominal pain suspected to be acute pancreatitis were included. No: If the study excluded patients based on high or low probability of acute pancreatitis (e.g. those with organ failure). Unclear: If this information was not available. | |
| Could the selection of participants have introduced bias? | Low risk of bias: If 'yes' classification for both of the above two questions. High risk of bias: If 'no' classification for either of the above two questions. Unclear risk of bias: If 'unclear' classification for either of the above two questions but without a 'no' classification for either of the above two questions. |
|
| Participant characteristics and setting | Yes: If all patients with acute epigastric or diffuse abdominal pain suspected to be acute pancreatitis were included. No: If a proportion of patients with acute epigastric or diffuse abdominal pain were excluded on the basis of the results of another diagnostic test (e.g. an arterial blood gas analysis performed after the index test). Unclear: If it is not clear whether the patients have been included on the basis of the results of another diagnostic test (e.g. an arterial blood gas analysis performed after the index test). | |
| Are there concerns that the included participants and setting do not match the review question? | Low concern: If the participant characteristics and setting is classified as 'yes'. Unclear concern: If the participant characteristics and setting is classified as 'unclear'. High concern: If the participant characteristics and setting is classified as 'no'. |
|
| Domain 2: Index test | Index test(s) | Serum amylase, serum lipase, urinary trypsinogen‐2, urinary amylase |
| Were the index test results interpreted without knowledge of the results of the reference standard? | The index test would always be conducted, though not interpreted before the reference standard. Yes: If the index test was conducted and interpreted without knowledge of the results of the reference standard. No: If the index test was interpreted with knowledge of the results of the reference standard. Unclear: If it was not clear whether the index test was interpreted without knowledge of the results of the reference standard. |
|
| If a threshold was used, was it prespecified? | Yes: If a prespecified threshold was used. No: If a prespecified threshold was not used. Unclear: If it was not clear whether the threshold used was prespecified. |
|
| Could the conduct or interpretation of the index test have introduced bias? | Low risk of bias: If 'yes' classification for both of the above two questions. High risk of bias: If 'no' classification for either of the above two questions. Unclear risk of bias: If 'unclear' classification for either of the above two questions but without a 'no' classification for either of the above two questions. |
|
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern: If the criteria for positive index test are clearly stated. High concern: If the criteria for positive index test are not stated. |
|
| Domain 3: Target condition and reference standard | Target condition and reference standard(s) | Target condition: acute pancreatitis (mild, moderately severe, or severe). While inflammation of the pancreas confirmed by biopsy can be considered to be the gold standard for the diagnosis of acute pancreatitis, for ethical reasons it is unlikely to performed in any participant. As a result, different study authors may use different reference standards such as radiological features of acute pancreatitis or the presence of organ failure. However, such reference standards can miss cases of mild acute pancreatitis, resulting in an underestimation of diagnostic test accuracy of the index tests. We also accepted the consensus conference definition of acute pancreatitis, i.e. when at least two of the following three features are present (Banks 2013).
We accepted any of the following used alone or in combination as reference standards: biopsy, radiological features of acute pancreatitis, laparotomy, autopsy, organ failure, or the consensus conference definition (including or excluding the index test being evaluated). In terms of ranking the reference standards, we considered biopsy as the best reference standard (although for ethical reasons it is unlikely to have been performed in any participant) followed by the consensus definition of acute pancreatitis, radiological, surgical, or autopsy features of acute pancreatitis, or the presence of organ failure, in that order. |
| Is the reference standard likely to correctly classify the target condition? | Yes: If histological confirmation of acute pancreatitis is obtained or the consensus definition of acute pancreatitis is used. No: If the reference standard is radiological confirmation or organ failure. Unclear: If the reference standard was not adequately described. | |
| Is the reference standard independent of the index test? | Yes: If the index test was not part of the reference standard. No: If the index test was part of the reference standard. Unclear: If it was not clear whether the index test was part of the reference standard. As anticipated, we classified all studies included in the review as 'yes' or 'no' for this item. | |
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes: If the reference standard was interpreted without knowledge of the results of the index test. No: If the reference standard was interpreted with knowledge of the results of the index test. Unclear: If it was not clear if the reference standard was interpreted without knowledge of the results of the index test. | |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk of bias: If 'yes' classification for all of the above three questions. High risk of bias: If 'no' classification for any of the above three questions. Unclear risk of bias: If 'unclear' classification for any of the above three questions but without a 'no' classification for any of the above three questions. |
|
| Are there concerns that the target condition as defined by the reference standard does not match the question? | As anticipated, we classified all of the included studies as 'low concern' based on the inclusion criteria for this review. | |
| Domain 4: Flow and timing | Flow and timing | Patients may have complete resolution of acute pancreatitis if they had acute pancreatitis, or may have an episode of acute pancreatitis if they did not have acute pancreatitis if the interval between the index test and reference standard is long. |
| Was there an appropriate interval between index test and reference standard? | Yes: If the time interval between index test and reference standard was less than one week. No: If the time interval between index test and reference standard was more than one week. Unclear: If the time interval between index test and reference standard was unclear. | |
| Did all participants receive a reference standard? | Yes: If all participants received a reference standard.
No: If some participants did not receive a reference standard. Such studies were excluded.
Unclear: If it was not clear whether all participants received a reference standard. Such studies were excluded. As anticipated, we classified all studies included in the review as 'yes' for this item. |
|
| Did all participants receive the same reference standard? | Yes: If all participants received the same reference standard (we anticipate that all studies will be classified as 'yes').
No: If different participants received different reference standards. Unclear: If this information was not clear. |
|
| Were all participants included in the analysis? | Yes: If all participants were included in the analysis irrespective of whether the results were interpretable. No: If some participants were excluded from the analysis due to uninterpretable results. Unclear: If this information was not clear. | |
| Could the patient flow have introduced bias? | Low risk of bias: If 'yes' classification for all of the above four questions. High risk of bias: If 'no' classification for any of the above four questions. Unclear risk of bias: If 'unclear' classification for any of the above four questions but without a 'no' classification for any of the above four questions. |
CECT: contrast‐enhanced computed tomography MRI: magnetic resonance imaging
Statistical analysis and data synthesis
We have reported the reference standards in each study included in the analysis and have analysed the studies at different threshold levels separately. We plotted study estimates of sensitivity and specificity on forest plots and in receiver operating characteristic (ROC) space to explore between‐study variation in the performance of each test stratified by the threshold. To estimate the summary sensitivity and specificity of each test at each threshold level, we attempted to perform the meta‐analysis by fitting the bivariate model (Chu 2006; Reitsma 2005). This model accounts for between‐study variability in estimates of sensitivity and specificity through the inclusion of random effects for the logit sensitivity and logit specificity parameters of the bivariate model. However, because of sparse data, we used simpler models described by Takwoingi 2015 (random‐effects model ignoring the inverse correlation between sensitivities and specificities in the different studies due to intrinsic threshold effect, and the fixed‐effect model for either sensitivity or specificity, or both). We based the choice between the different models on the ‐2 log likelihood ratio and the distribution of sensitivities and specificities as noted in the forest plots or ROC space (Takwoingi 2015).
We performed the meta‐analysis using the NLMIXED command in SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). We calculated the summary likelihood ratios and their confidence intervals from the functions of the parameter estimates from the bivariate model or other models that were fitted to estimate the summary sensitivities and specificities. We calculated the post‐test probability using the median pre‐test probability. Post‐test probability associated with a positive test is the probability of having the target condition (acute pancreatitis) on the basis of a positive test result, and is the same as the term 'positive predictive value' used in a single diagnostic accuracy study. Post‐test probability associated with a negative test is the probability of having the target condition (acute pancreatitis) on the basis of a negative test result and is 1 ‐ 'negative predictive value'. Negative predictive value is the term used in a single diagnostic accuracy study to indicate the chance that the patient has no target condition when the test is negative.
Investigations of heterogeneity
Of the eight sources of heterogeneity mentioned in the Secondary objectives section, we planned to use risk of bias, publication status, prospective or retrospective studies, and different test manufacturers as categorical covariates, and proportion of participants with a previous history of acute pancreatitis, proportion of participants with different aetiologies, proportion of participants with organ failure, and the average time to performance of the test as continuous covariates in the regression model. We planned to include one covariate at a time in the regression model. We planned to use the likelihood ratio test to determine whether the covariate was statistically significant. However, because of the paucity of data, we did not perform any of the above analyses.
Sensitivity analyses
We did not plan any sensitivity analyses except when the data available from the studies were ambiguous (e.g. the numbers in the text differed from the numbers in the figures), in which case we planned to assess the impact of different data used by a sensitivity analysis. However, we performed three post hoc sensitivity analyses.
There was incorporation bias (index test was a part of the reference standard) in many of the studies that reported on the diagnostic accuracy of serum amylase and lipase. We performed a sensitivity analysis by excluding these studies.
There was high risk of bias and applicability concerns in one retrospective study that contributed to most of the effect estimate (Chang 2011). We performed a sensitivity analysis by excluding this study.
For urinary trypsinogen‐2, the authors of one study appeared to have used the threshold suggested by the manufacturer (Aysan 2008); however, this was not stated clearly. We performed a sensitivity analysis by excluding this study.
Assessment of reporting bias
We planned to investigate whether the summary sensitivity and specificity differed between studies published as full texts and those that were available only as abstracts (at least two years prior to the search date) using the methods described in the Investigations of heterogeneity section. We did not perform this since all of the included studies were full texts.
Results
Results of the search
We identified a total of 23,660 references through the electronic searches of MEDLINE (n = 7326), Embase (n = 11,502), Science Citation Index Expanded (n = 4293), National Institute for Health Research (NIHR HTA and DARE) (n = 142), Zetoc (n = 360), WHO ICTRP (n = 1), and ClinicalTrials.gov (n = 36). We excluded 10,657 duplicates and 12,547 clearly irrelevant references through reading the titles or abstracts, or both. We sought full‐text articles for 456 references, but were unable to obtain the full texts for six references (Anand 1956; Cherry 1953; Coppola 1954; Do Prado 1952; Lippi 2013; Stimac 1995). We retrieved full‐text articles of 450 references for further assessment against our review protocol inclusion criteria. Of these 450 references, we excluded 440 references for the reasons provided in the Characteristics of excluded studies section. The reasons for exclusion were: case‐control study: 102; inappropriate population: 195; inappropriate reference standard: 48; inappropriate target condition: 2; no diagnostic test accuracy data: 33; not a primary research study: 60; could not be obtained: 6. Ten studies (10 references) fulfilled the inclusion criteria and provided the diagnostic accuracy data for the review (Abraham 2011; Aysan 2008; Burkitt 1987; Chang 2011; Keim 1998; Mayumi 2012; Patt 1966; Saez 2005; Viel 1990; Wu 2009). We have shown the reference flow in Figure 2.
2.
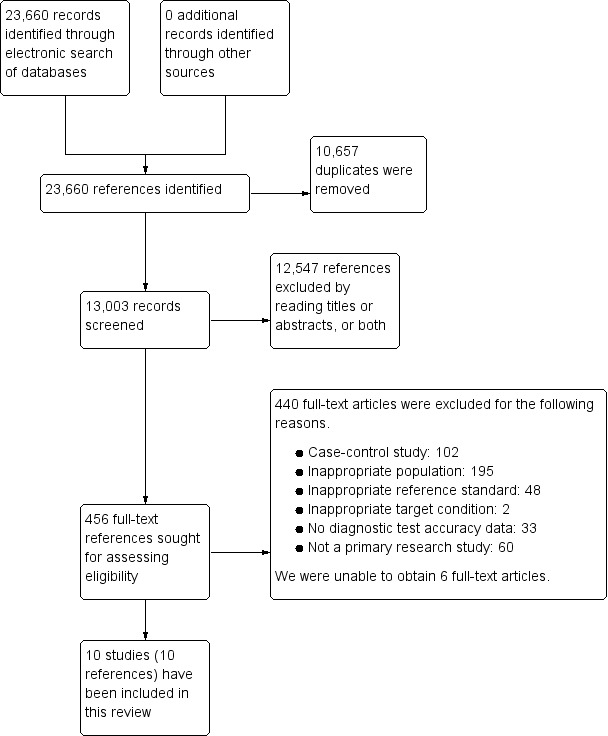
Study flow diagram.
Included studies
Ten studies including 5056 participants met the inclusion criteria for this review and assessed the diagnostic accuracy of the index tests in people presenting to the hospital emergency department with acute abdominal pain. The average age of participants in the studies ranged from 37 years to 59 years in the five studies that reported this information (Aysan 2008; Keim 1998; Mayumi 2012; Saez 2005; Wu 2009). About 45% of participants (442/970 participants) were females in the five studies that reported this information (Aysan 2008; Keim 1998; Mayumi 2012; Saez 2005; Wu 2009). Six studies were prospective studies (Abraham 2011; Aysan 2008; Burkitt 1987; Keim 1998; Mayumi 2012; Saez 2005); one study was a retrospective study (Chang 2011); and it was unclear whether three studies were prospective or retrospective (Patt 1966; Viel 1990; Wu 2009). All of the included studies were full‐text publications. The studies did not report whether people with previous history of acute pancreatitis were included. Two studies clearly stated that they included patients with gallstone pancreatitis and alcoholic pancreatitis (Mayumi 2012; Saez 2005). None of the studies reported any restriction of inclusion criteria based on aetiology or provided diagnostic accuracy information separately for people with gallstone and alcoholic pancreatitis. None of the studies included only people with organ failure or excluded all people with organ failure. One study excluded people with renal failure, but there was no restriction on the basis of other organ failures (Chang 2011). None of the studies reported data separately for people with and without organ failure. Only one study reported that they included only people with less than 24 hours since onset of symptoms (Saez 2005). None of the other trials restricted participants based on the duration of symptoms. However, since all of the studies included participants with acute abdominal pain, it is likely that the onset of pain was less than two to three days prior to hospital admission.
The studies measured the diagnostic accuracy on admission and used different thresholds for diagnosis of acute pancreatitis. Eight studies contributed to two or more analyses (Abraham 2011; Burkitt 1987; Chang 2011; Keim 1998; Mayumi 2012; Patt 1966; Saez 2005; Wu 2009). However, none of the studies reported the diagnostic accuracy of a combination of tests.
Methodological quality of included studies
The methodological quality of the included studies is shown in Characteristics of included studies, and summaries of the methodological quality are shown in Figure 3 and Figure 4.
3.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
4.
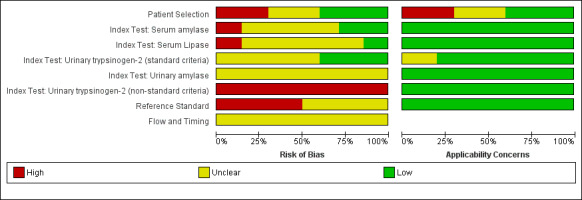
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
Participant selection
A total of four studies were at low risk of bias in the participant selection domain (Abraham 2011; Mayumi 2012; Saez 2005; Viel 1990). A total of three studies were at high risk of bias in the participant selection domain (Burkitt 1987; Chang 2011; Patt 1966). In one study, some participants who had normal urinary amylase were excluded from analysis (Burkitt 1987); in another study, participants with parotid disease and end‐stage renal failure were excluded (Chang 2011); and in a third study, only participants who underwent laparotomy or autopsy were included, that is only people with severe symptoms were included (Patt 1966). A total of three studies were at unclear risk of bias in the participant selection domain (Aysan 2008; Keim 1998; Wu 2009).
There was low concern in the participant selection domain in four studies (Abraham 2011; Mayumi 2012; Saez 2005; Viel 1990). There was high concern in the participant selection domain in three studies (Burkitt 1987; Chang 2011; Patt 1966). The reasons for high concern were the same as those for high risk of bias (Burkitt 1987). There was unclear concern in the participant selection domain in three studies (Aysan 2008; Keim 1998; Wu 2009).
Index test
One study was at low risk of bias for all index tests other than one threshold (urinary trypsinogen positive or most positive) (Mayumi 2012); for that threshold the study was at high risk of bias since the threshold was not prespecified (Mayumi 2012). One study was at high risk of bias for all index tests since the threshold was not prespecified (Keim 1998). The remaining trials were at unclear risk of bias since it was not clear whether the threshold was prespecified and whether blinded interpretation of the index tests was performed (Abraham 2011; Aysan 2008; Burkitt 1987; Chang 2011; Patt 1966; Saez 2005; Viel 1990; Wu 2009). All of the index tests reported in eight studies were at low concern about applicability (Abraham 2011; Burkitt 1987; Chang 2011; Keim 1998; Patt 1966; Saez 2005; Viel 1990; Wu 2009). One study was at unclear concern about applicability since the threshold used was not clearly reported by the authors (the authors appear to have used the manufacturer's suggested threshold, but this was not entirely clear) (Aysan 2008). One study was at high concern about applicability for all index tests except for one threshold level (positive or most positive), since this is not a standard threshold recommended by the manufacturer (Mayumi 2012).
Reference standard
Five studies were at high risk of bias in the reference standard domain because they did not use a biopsy or consensus definition (Aysan 2008; Chang 2011; Keim 1998; Viel 1990; Wu 2009). One of these studies also included the index test as part of the reference standard despite not using consensus definition (Wu 2009). Five studies were at unclear risk of bias about the reference standard since they did not report whether the people interpreting the reference standards were blinded to the index test results (Abraham 2011; Burkitt 1987; Mayumi 2012; Patt 1966; Saez 2005). However, it should be noted that three studies were at high risk of bias for the index tests serum amylase and serum lipase, which were part of the reference standards, but were at low risk of bias for urinary trypsinogen‐2 (Abraham 2011; Mayumi 2012; Saez 2005). As we included only studies in which the reference standard was adequately described, the applicability concern was low in all studies.
Flow and timing
All of the studies were at unclear risk of bias in the flow and timing domain since the studies either did not report whether any participants with uninterpretable results were excluded or did not state the time interval between the index test and reference standard.
Findings
The included studies reported the diagnostic test accuracy of the different tests in the diagnosis of acute pancreatitis at different test thresholds and on different days. Due to sparse data, we performed the meta‐analysis using different models described by Takwoingi 2015. The data and the SAS code used are shown in Appendix 9. The model fit (‐2 log likelihood ratios) for various analyses is reported in Appendix 10. The median pre‐test probability of acute pancreatitis (proportion of people with acute pancreatitis out of the total number of included participants) was 22.6% with a minimum of 0.6% and a maximum of 69.4%. The lower and upper quartiles were 16.3% and 47.3%, respectively. The sensitivity and specificity along with the 95% confidence interval (CI) for each of the main analyses are shown in a forest plot (Figure 5) and ROC space (Figure 6). The sensitivities, specificities, post‐test probabilities of a positive test, and post‐test probabilities of a negative test at the median pre‐test probability for the main analyses are presented in the Table 1 and for all of the tests are presented in Table 4, Table 5, and Table 6.
5.

Forest plot of serum amylase, serum lipase, and urinary trypsinogen at different thresholds. There was reasonable overlap of 95% confidence intervals except specificity for serum amylase > 3 times normal, sensitivity and specificity of serum lipase > 3 times normal, and specificity of urinary trypsinogen‐2.
6.
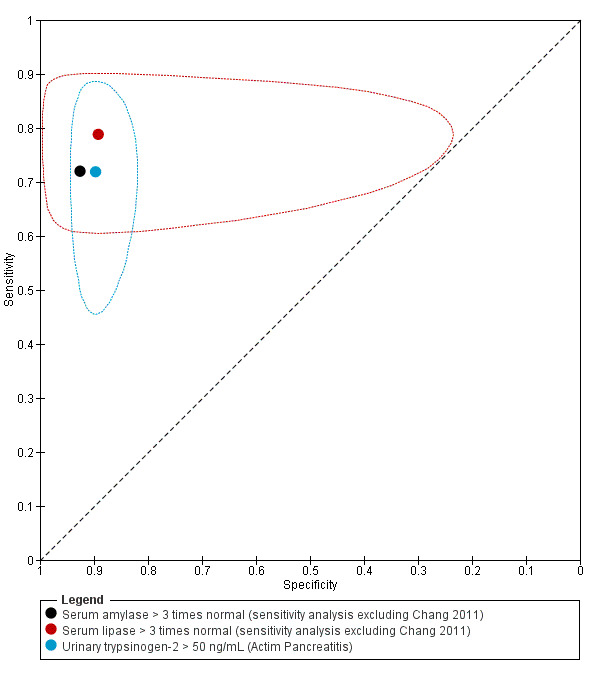
Summary estimates and 95% confidence region (ellipses) of the three main meta‐analyses showing similar diagnostic test accuracies. No confidence regions could be computed for serum amylase due to small numbers of studies.
3. Serum amylase at different thresholds and different times.
| Index test | Sensitivity | Specificity | Post‐test probability of a positive test1 | Post‐test probability of a negative test1 | Number of false positives per 100 people having a positive test | Number of false negatives per 100 people having a negative test | Number of studies (Number of participants) | Risk of bias / Applicability concerns / Inconsistency |
| Serum amylase (threshold: > 3 times normal) (on admission) | 0.71 (95% CI 0.65 to 0.77) | 0.99 (95% CI 0.99 to 0.99) | 95.4% (95% CI 93.4% to 96.8%) | 7.8% (95% CI 6.4% to 9.5%) | 5 (95% CI 3 to 7) | 8 (95% CI 6 to 9) | 4 (4056) | High / High / No |
| Serum amylase (threshold: > 3 times normal) (on admission (excluding Chang 2011)) | 0.72 (95% CI 0.59 to 0.82) | 0.93 (95% CI 0.66 to 0.99) | 74.0% (95% CI 33.4% to 94.1%) | 8.1% (95% CI 5.4% to 12.1%) | 26 (95% CI 6 to 67) | 8 (95% CI 5 to 12) | 3 (605) | Unclear / Low / Moderate |
| Serum amylase (threshold: > 3 times normal) (on admission (excluding studies with incorporation bias)) | 0.64 (95% CI 0.41 to 0.82) | 0.99 (95% CI 0.99 to 1.00) | 97.1% (95% CI 95.1% to 98.3%) | 9.7% (95% CI 5.8% to 15.7%) | 3 (95% CI 2 to 5) | 10 (95% CI 6 to 16) | 1 (3451) | High / High / Not applicable |
| Serum amylase (threshold: > twice normal) (on admission) | 0.76 (95% CI 0.57 to 0.88) | 0.99 (95% CI 0.98 to 0.99) | 95.3% (95% CI 92.6% to 97.1%) | 6.6% (95% CI 3.5% to 12.2%) | 5 (95% CI 3 to 7) | 7 (95% CI 4 to 12) | 2 (3704) | High / High / No |
| Serum amylase (threshold: > twice normal) (on admission (excluding Chang 2011)) | 0.72 (95% CI 0.53 to 0.86) | 0.98 (95% CI 0.95 to 0.99) | 92.1% (95% CI 81.1% to 96.9%) | 7.7% (95% CI 4.6% to 12.7%) | 8 (95% CI 3 to 19) | 8 (95% CI 5 to 13) | 1 (253) | High / Unclear / Not applicable |
| Serum amylase (threshold: > twice normal) (2 to 3 days after admission) | 0.25 (95% CI 0.12 to 0.44) | 0.97 (95% CI 0.93 to 0.99) | 69.8% (95% CI 47.3% to 85.6%) | 18.5% (95% CI 15.6% to 21.7%) | 30 (95% CI 14 to 53) | 18 (95% CI 16 to 22) | 1 (253) | High / Unclear / Not applicable |
| Serum amylase (threshold: > twice normal) (4 to 5 days after admission) | 0.06 (95% CI 0.01 to 0.22) | 0.93 (95% CI 0.89 to 0.96) | 21.2% (95% CI 6.1% to 52.9%) | 22.7% (95% CI 21.1% to 24.5%) | 79 (95% CI 47 to 94) | 23 (95% CI 21 to 24) | 1 (253) | High / Unclear / Not applicable |
| Serum amylase (threshold: > normal) (on admission) | 0.88 (95% CI 0.77 to 0.94) | 0.88 (95% CI 0.84 to 0.91) | 68.7% (95% CI 61.6% to 75.1%) | 3.8% (95% CI 1.9% to 7.3%) | 31 (95% CI 25 to 38) | 4 (95% CI 2 to 7) | 3 (587) | High / Unclear / No |
| Serum amylase (threshold: > normal) (on admission (excluding studies with incorporation bias)) | 0.89 (95% CI 0.72 to 0.96) | 0.88 (95% CI 0.83 to 0.92) | 69.3% (95% CI 60.0% to 77.2%) | 3.5% (95% CI 1.3% to 9.0%) | 31 (95% CI 23 to 40) | 4 (95% CI 1 to 9) | 2 (453) | High / Unclear / No |
| Serum amylase (threshold: > normal) (2 to 3 days after admission) | 0.66 (95% CI 0.47 to 0.81) | 0.83 (95% CI 0.77 to 0.87) | 52.8% (95% CI 43.2% to 62.1%) | 10.8% (95% CI 7.0% to 16.4%) | 47 (95% CI 38 to 57) | 11 (95% CI 7 to 16) | 1 (253) | High / Unclear / Not applicable |
| Serum amylase (threshold: > normal) (4 to 5 days after admission) | 0.34 (95% CI 0.19 to 0.53) | 0.86 (95% CI 0.81 to 0.90) | 41.8% (95% CI 28.7% to 56.1%) | 18.3% (95% CI 14.7% to 22.4%) | 58 (95% CI 44 to 71) | 18 (95% CI 15 to 22) | 1 (253) | High / Unclear / Not applicable |
CI: confidence interval
1The post‐test probabilities were calculated at the median pre‐test probability of 22.6%.
4. Serum lipase at different thresholds and different times.
| Index test | Sensitivity | Specificity | Post‐test probability of a positive test1 | Post‐test probability of a negative test1 | Number of false positives per 100 people having a positive test | Number of false negatives per 100 people having a negative test | Number of studies (Number of participants) | Risk of bias / Applicability concerns / Inconsistency |
| Serum lipase (threshold: > 3 times normal) (on admission) | 0.80 (95% CI 0.73 to 0.86) | 0.93 (95% CI 0.00 to 1.00) | 78.3% (95% CI 0.0% to 100.0%) | 5.9% (95% CI 2.3% to 14.4%) | 22 (95% CI 0 to 100) | 6 (95% CI 2 to 14) | 5 (4129) | High / High / Moderate |
| Serum lipase (threshold: > 3 times normal) (on admission (excluding Chang 2011)) | 0.79 (95% CI 0.54 to 0.92) | 0.89 (95% CI 0.46 to 0.99) | 68.1% (95% CI 21.4% to 94.3%) | 6.6% (95% CI 2.7% to 15.1%) | 32 (95% CI 6 to 79) | 7 (95% CI 3 to 15) | 4 (678) | Unclear / Low / Moderate |
| Serum lipase (threshold: > 3 times normal) (on admission (excluding studies with incorporation bias)) | 0.88 (95% CI 0.02 to 1.00) | 0.94 (95% CI 0.00 to 1.00) | 81.2% (95% CI 0.0% to 100.0%) | 3.7% (95% CI 0.0% to 99.2%) | 19 (95% CI 0 to 100) | 4 (95% CI 0 to 99) | 2 (3534) | High / High / High |
| Serum lipase (threshold: > twice normal) (on admission) | 0.96 (95% CI 0.78 to 0.99) | 0.98 (95% CI 0.98 to 0.99) | 94.3% (95% CI 92.1% to 96.0%) | 1.1% (95% CI 0.2% to 7.0%) | 6 (95% CI 4 to 8) | 1 (95% CI 0 to 7) | 2 (3704) | High / High / No |
| Serum lipase (threshold: > twice normal) (on admission (excluding Chang 2011)) | 0.94 (95% CI 0.78 to 0.99) | 0.95 (95% CI 0.91 to 0.97) | 84.6% (95% CI 75.5% to 90.8%) | 1.9% (95% CI 0.5% to 6.9%) | 15 (95% CI 9 to 25) | 2 (95% CI 1 to 7) | 1 (253) | High / Unclear / Not applicable |
| Serum lipase (threshold: > twice normal) (2 to 3 days after admission) | 0.69 (95% CI 0.50 to 0.83) | 0.91 (95% CI 0.86 to 0.94) | 69.0% (95% CI 57.9% to 78.2%) | 9.1% (95% CI 5.7% to 14.4%) | 31 (95% CI 22 to 42) | 9 (95% CI 6 to 14) | 1 (253) | High / Unclear / Not applicable |
| Serum lipase (threshold: > twice normal) (4 to 5 days after admission) | 0.41 (95% CI 0.24 to 0.59) | 0.84 (95% CI 0.79 to 0.89) | 42.9% (95% CI 30.9% to 55.7%) | 17.1% (95% CI 13.4% to 21.7%) | 57 (95% CI 44 to 69) | 17 (95% CI 13 to 22) | 1 (253) | High / Unclear / Not applicable |
| Serum lipase (threshold: > normal) (on admission) | 0.96 (95% CI 0.00 to 1.00) | 0.83 (95% CI 0.47 to 0.96) | 62.5% (95% CI 21.7% to 90.9%) | 1.3% (95% CI 0.0% to 100.0%) | 38 (95% CI 9 to 78) | 1 (95% CI 0 to 100) | 2 (453) | High / Unclear / Hight |
| Serum lipase (threshold: > normal) (2 to 3 days after admission) | 0.97 (95% CI 0.82 to 1.00) | 0.79 (95% CI 0.73 to 0.84) | 57.7% (95% CI 51.1% to 64.0%) | 1.1% (95% CI 0.2% to 7.4%) | 42 (95% CI 36 to 49) | 1 (95% CI 0 to 7) | 1 (253) | High / Unclear / Not applicable |
| Serum lipase (threshold: > normal) (4 to 5 days after admission) | 0.59 (95% CI 0.41 to 0.76) | 0.70 (95% CI 0.64 to 0.76) | 36.8% (95% CI 29.1% to 45.2%) | 14.5% (95% CI 10.0% to 20.6%) | 63 (95% CI 55 to 71) | 14 (95% CI 10 to 21) | 1 (253) | High / Unclear / Not applicable |
CI: confidence interval
1The post‐test probabilities were calculated at the median pre‐test probability of 22.6%.
5. Urinary tests.
| Index test | Sensitivity | Specificity | Post‐test probability of a positive test1 | Post‐test probability of a negative test1 | Number of false positives per 100 people having a positive test | Number of false negatives per 100 people having a negative test | Number of studies (Number of participants) | Risk of bias / Applicability concerns / Inconsistency |
| Urinary trypsinogen‐2 (threshold: Actim Pancreatitis ‐ all studies; > 50 ng/mL) (on admission) | 0.72 (95% CI 0.56 to 0.84) | 0.90 (95% CI 0.85 to 0.93) | 67.2% (95% CI 57.3% to 75.7%) | 8.4% (95% CI 5.2% to 13.3%) | 33 (95% CI 24 to 43) | 8 (95% CI 5 to 13) | 5 (841) | High / Unclear / Moderate |
| Urinary trypsinogen‐2 (threshold: Actim Pancreatitis ‐ sensitivity analysis; > 50 ng/mL) (on admission) | 0.74 (95% CI 0.56 to 0.87) | 0.89 (95% CI 0.84 to 0.93) | 66.9% (95% CI 55.4% to 76.7%) | 7.7% (95% CI 4.3% to 13.5%) | 33 (95% CI 23 to 45) | 8 (95% CI 4 to 14) | 4 (742) | High / Unclear / Moderate |
| Urinary trypsinogen‐2 (quantitative) (threshold: > 50 ng/mL) (on admission) | 0.71 (95% CI 0.63 to 0.78) | 0.89 (95% CI 0.84 to 0.92) | 65.6% (95% CI 57.0% to 73.3%) | 8.7% (95% CI 6.9% to 10.9%) | 34 (95% CI 27 to 43) | 9 (95% CI 7 to 11) | 1 (412) | High / Low / Not applicable |
| Urinary trypsinogen‐2 (threshold: only + or most positive ‐ the threshold for this was not available) (on admission) | 0.60 (95% CI 0.51 to 0.67) | 0.92 (95% CI 0.88 to 0.95) | 69.1% (95% CI 59.0% to 77.6%) | 11.4% (95% CI 9.6% to 13.5%) | 31 (95% CI 22 to 41) | 11 (95% CI 10 to 13) | 1 (412) | High / Low / Not applicable |
| Urinary amylase (quantitative) (threshold: above normal) (on admission) | 0.83 (95% CI 0.65 to 0.94) | 0.86 (95% CI 0.77 to 0.91) | 62.8% (95% CI 50.8% to 73.5%) | 5.4% (95% CI 2.5% to 11.3%) | 37 (95% CI 27 to 49) | 5 (95% CI 2 to 11) | 1 (134) | Unclear / Unclear / Not applicable |
| Urinary amylase (qualitative) (threshold: 1 plus) (on admission) | 0.66 (95% CI 0.49 to 0.79) | 0.94 (95% CI 0.90 to 0.97) | 77.3% (95% CI 64.2% to 86.6%) | 9.6% (95% CI 6.5% to 14.0%) | 23 (95% CI 13 to 36) | 10 (95% CI 6 to 14) | 1 (218) | High / High / Not applicable |
| Urinary amylase (qualitative) (threshold: 2 plus) (on admission) | 0.44 (95% CI 0.29 to 0.60) | 0.99 (95% CI 0.96 to 1.00) | 95.8% (95% CI 75.8% to 99.4%) | 14.2% (95% CI 11.2% to 17.8%) | 4 (95% CI 1 to 24) | 14 (95% CI 11 to 18) | 1 (218) | High / High / Not applicable |
CI: confidence interval
1The post‐test probabilities were calculated at the median pre‐test probability of 22.6%.
Serum amylase
More than three times normal on admission
A total of four studies (4056 participants) were included in this analysis (Abraham 2011; Chang 2011; Mayumi 2012; Saez 2005). It should be noted that except for Chang 2011, all of the studies suffered from incorporation bias (i.e. index test was part of reference standard). Based on the ‐2 log likelihood ratio, fixed‐effect model for both sensitivity and specificity was used for meta‐analysis. The summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.71 (95% CI 0.65 to 0.77) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.99 (95% CI 0.99 to 0.99).
Excluding three studies with incorporation bias, Abraham 2011, Mayumi 2012, and Saez 2005 (studies that used consensus definition as the reference standard) resulted in the inclusion of only Chang 2011, which used radiology as the reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.64 (95% CI 0.41 to 0.82) and the estimate of specificity for diagnosis of acute pancreatitis was 0.99 (95% CI 0.99 to 1.00).
Excluding Chang 2011, for which there was major concern about applicability, resulted in the inclusion of a total of three studies (605 participants) (Abraham 2011; Mayumi 2012; Saez 2005). Based on the ‐2 log likelihood ratio, the fixed‐effect model for sensitivity and random‐effects model for specificity were used for meta‐analysis. The summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.72 (95% CI 0.59 to 0.82) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.93 (95% CI 0.66 to 0.99).
More than twice normal
On admission
A total of two studies (3704 participants) were included in this analysis (Chang 2011; Keim 1998). There was no incorporation bias in either study, as both studies used radiology as a reference standard. Based on the ‐2 log likelihood ratio, fixed‐effect model for both sensitivity and specificity was used for meta‐analysis. The summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.76 (95% CI 0.57 to 0.88) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.99 (95% CI 0.98 to 0.99). Excluding Chang 2011, for which there was major concern about applicability, resulted in the inclusion of one study (253 participants) in this analysis (Keim 1998). The estimate of sensitivity for diagnosis of acute pancreatitis was 0.72 (95% CI 0.53 to 0.86) and the estimate of specificity for diagnosis of acute pancreatitis was 0.98 (95% CI 0.95 to 0.99).
Two to three days after admission
One study (253 participants) was included in this analysis (Keim 1998). There was no incorporation bias in this study as this study used radiology as a reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.25 (95% CI 0.12 to 0.44) and the estimate of specificity for diagnosis of acute pancreatitis was 0.97 (95% CI 0.93 to 0.99).
Four to five days after admission
One study (253 participants) was included in this analysis (Keim 1998). There was no incorporation bias in this study as this study used radiology as a reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.06 (95% CI 0.01 to 0.22) and the estimate of specificity for diagnosis of acute pancreatitis was 0.93 (95% CI 0.89 to 0.96).
More than normal
On admission
A total of three studies (587 participants) were included in this analysis (Keim 1998; Patt 1966; Wu 2009). There was no incorporation bias in two studies: Keim 1998 used radiology and Patt 1966 used laparotomy or autopsy as the reference standard. Based on the ‐2 log likelihood ratio, fixed‐effect model for both sensitivity and specificity was used for meta‐analysis. The summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.88 (95% CI 0.77 to 0.94) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.88 (95% CI 0.84 to 0.91). Excluding Wu 2009, a study that had incorporation bias (although the authors did not use the consensus definition, they used a combination of pain, radiology, and raised amylase), resulted in a summary sensitivity and specificity for diagnosis of acute pancreatitis of 0.89 (95% CI 0.72 to 0.96) and 0.88 (95% CI 0.83 to 0.92), respectively. This was based on fixed‐effect model for both sensitivity and specificity, the only model that converged.
Two to three days after admission
One study (253 participants) was included in this analysis (Keim 1998). There was no incorporation bias in this study, as this study used radiology as the reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.66 (95% CI 0.47 to 0.81) and the estimate of specificity for diagnosis of acute pancreatitis was 0.83 (95% CI 0.77 to 0.87).
Four to five days after admission
One study (253 participants) was included in this analysis (Keim 1998). There was no incorporation bias in this study, as this study used radiology as the reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.34 (95% CI 0.19 to 0.53) and the estimate of specificity for diagnosis of acute pancreatitis was 0.86 (95% CI 0.81 to 0.90).
Serum lipase
More than three times normal on admission
A total of five studies (4129 participants) were included in this analysis (Abraham 2011; Chang 2011; Mayumi 2012; Saez 2005; Viel 1990). Of these, there was no incorporation bias in two studies: Chang 2011 used radiology as the reference standard, while Viel 1990 used 3‐fold increase of serum amylase and evidence of pancreatitis in radiology, endoscopic retrograde cholangiopancreatography (ERCP), or surgery as the reference standard. Based on the ‐2 log likelihood ratio, fixed‐effect model for sensitivity and random‐effects model for specificity were used for meta‐analysis. The summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.80 (95% CI 0.73 to 0.86) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.93 (95% CI 0.00 to 1.00).
Excluding three studies with incorporation bias (these studies used consensus definition as the reference standard) (Abraham 2011; Mayumi 2012; Saez 2005), the summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.88 (95% CI 0.02 to 1.00) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.94 (95% CI 0.00 to 1.00). Only two models, fixed‐effect model for sensitivity and random‐effects model for specificity and fixed‐effect models for both sensitivity and specificity, converged. Based on the ‐2 log likelihood ratio, fixed‐effect model for sensitivity and random‐effects model for specificity were used for meta‐analysis.
Excluding Chang 2011, for which there was major concern about applicability, resulted in the inclusion of four studies (678 participants) in this analysis (Abraham 2011; Mayumi 2012; Saez 2005; Viel 1990). Based on the ‐2 log likelihood ratio, random‐effects model for both sensitivity and specificity was used for meta‐analysis. The summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.79 (95% CI 0.54 to 0.92) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.89 (95% CI 0.46 to 0.99).
More than twice normal
On admission
A total of two studies (3704 participants) were included in this analysis (Chang 2011; Keim 1998). There was no incorporation bias in either study, as both studies used radiology as the reference standard. Based on the ‐2 log likelihood ratio, fixed‐effect model for both sensitivity and specificity was used for meta‐analysis. The summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.96 (95% CI 0.78 to 0.99) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.98 (95% CI 0.98 to 0.99). Excluding Chang 2011, for which there was major concern about applicability, resulted in the inclusion of one study (253 participants) in this analysis (Keim 1998). The estimate of sensitivity for diagnosis of acute pancreatitis was 0.94 (95% CI 0.78 to 0.99) and the estimate of specificity for diagnosis of acute pancreatitis was 0.95 (95% CI 0.91 to 0.97).
Two to three days after admission
One study (253 participants) was included in this analysis (Keim 1998). There was no incorporation bias in this study, as this study used radiology as the reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.69 (95% CI 0.50 to 0.83) and the estimate of specificity for diagnosis of acute pancreatitis was 0.91 (95% CI 0.86 to 0.94).
Four to five days after admission
One study (253 participants) was included in this analysis (Keim 1998). There was no incorporation bias in this study, as this study used radiology as the reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.41 (95% CI 0.24 to 0.59) and the estimate of specificity for diagnosis of acute pancreatitis was 0.84 (95% CI 0.79 to 0.89).
More than normal
On admission
A total of two studies (453 participants) were included in this analysis (Keim 1998; Patt 1966). There was no incorporation bias in either study, as Keim 1998 used radiology as the reference standard, while Patt 1966 used laparotomy or autopsy as the reference standard. Based on the ‐2 log likelihood ratio, random‐effects model for sensitivity and fixed‐effect model for specificity were used for meta‐analysis. The summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.96 (95% CI 0.00 to 1.00) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.83 (95% CI 0.47 to 0.96).
Two to three days after admission
One study (253 participants) was included in this analysis (Keim 1998). There was no incorporation bias in this study, as this study used radiology as the reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.97 (95% CI 0.82 to 1.00) and the estimate of specificity for diagnosis of acute pancreatitis was 0.79 (95% CI 0.73 to 0.84).
Four to five days after admission
One study (253 participants) was included in this analysis (Keim 1998). There was no incorporation bias in this study, as this study used radiology as the reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.59 (95% CI 0.41 to 0.76) and the estimate of specificity for diagnosis of acute pancreatitis was 0.70 (95% CI 0.64 to 0.76).
Urinary trypsinogen‐2
Actim Pancreatitis (Medix Biochemica) test (threshold: > 50 ng/mL)
A total of five studies (841 participants) were included in this analysis (Abraham 2011; Aysan 2008; Mayumi 2012; Saez 2005; Wu 2009). Of these, three studies used the consensus definition as the reference standard (Abraham 2011; Mayumi 2012; Saez 2005); Aysan 2008 used radiology as the reference standard; and Wu 2009 used pain, radiology, and amylase as the reference standard.
Based on the ‐2 log likelihood ratio, random‐effects model for sensitivity and fixed‐effect model for specificity were used for meta‐analysis. The summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.72 (95% CI 0.56 to 0.84) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.90 (95% CI 0.85 to 0.93). While four studies clearly stated the threshold (Abraham 2011; Mayumi 2012; Saez 2005; Wu 2009), in one study the threshold was unclear. Based on the authors' description, it appears the manufacturer's threshold was used (Aysan 2008). Consequently, we included this study in the primary analysis, but performed a sensitivity analysis excluding this study. Based on the ‐2 log likelihood ratio, random‐effects model for sensitivity and fixed‐effect model for specificity were used for meta‐analysis. The summary estimate of sensitivity for diagnosis of acute pancreatitis was 0.74 (95% CI 0.56 to 0.87) and the summary estimate of specificity for diagnosis of acute pancreatitis was 0.89 (95% CI 0.84 to 0.93), that is there was only a minor change in summary sensitivity and specificity by excluding this study.
Quantitative urinary trypsinogen (threshold: > 50 ng/mL)
One study (412 participants) was included in this analysis (Mayumi 2012). The reference standard used in this study was the consensus definition. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.71 (95% CI 0.63 to 0.78) and the estimate of specificity for diagnosis of acute pancreatitis was 0.89 (95% CI 0.84 to 0.92).
Urinary trypsinogen: only positive or most positive (threshold not reported)
One study (412 participants) was included in this analysis (Mayumi 2012). The reference standard used in this study was the consensus definition. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.60 (95% CI 0.51 to 0.67) and the estimate of specificity for diagnosis of acute pancreatitis was 0.92 (95% CI 0.88 to 0.95).
Urinary amylase
Above normal (quantitative test)
One study (134 participants) was included in this analysis (Wu 2009). This study used pain, radiology, and amylase as the reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.83 (95% CI 0.65 to 0.94) and the estimate of specificity for diagnosis of acute pancreatitis was 0.86 (95% CI 0.77 to 0.91).
One plus (qualitative test: 1+ (threshold level for 1+ not stated))
One study (218 participants) was included in this analysis (Burkitt 1987). This study used pain and amylase greater than 1000 (normal = 300 IU) as the reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.66 (95% CI 0.49 to 0.79) and the estimate of specificity for diagnosis of acute pancreatitis was 0.94 (95% CI 0.90 to 0.97).
Two plus (qualitative test: 2+ (threshold level for 2+ not stated))
One study (218 participants) was included in this analysis (Burkitt 1987). This study used pain and amylase greater than 1000 (normal = 300 IU) as the reference standard. The estimate of sensitivity for diagnosis of acute pancreatitis was 0.44 (95% CI 0.29 to 0.60) and the estimate of specificity for diagnosis of acute pancreatitis was 0.99 (95% CI 0.96 to 1.00).
Comparison of different tests
Although we attempted to perform hierarchical summary receiver operating characteristics curve (HSROC) analysis using test as a covariate in order to compare the accuracy of the tests, the models did not converge. We were therefore unable to formally compare the diagnostic performance of the different tests.
Investigation of heterogeneity
Because of sparse data, we did not investigate heterogeneity.
Discussion
Summary of main results
Ten studies including 5056 participants met the inclusion criteria for this review and assessed the diagnostic accuracy of the index tests in people presenting to the emergency department with acute abdominal pain (Abraham 2011; Aysan 2008; Burkitt 1987; Chang 2011; Keim 1998; Mayumi 2012; Patt 1966; Saez 2005; Viel 1990; Wu 2009). These 10 studies reported the diagnostic test accuracy of the index tests at different thresholds. For the currently recommended threshold of above three times normal values, the summary sensitivities and specificities of admission serum amylase and admission serum lipase were as follows.
Serum amylase: sensitivity 0.71 (95% CI 0.65 to 0.77) and specificity 0.99 (95% CI 0.99 to 0.99).
Serum lipase: sensitivity 0.80 (95% CI 0.73 to 0.86) and specificity 0.93 (95% CI 0.00 to 1.00).
However, one retrospective study excluded people with parotid tumours and renal impairment; the inclusion of such patients will decrease the diagnostic accuracy (Chang 2011). After excluding this trial, which had high applicability concern in the participant selection domain, the summary sensitivities and specificities of admission serum amylase and admission serum lipase were as follows.
Serum amylase: sensitivity 0.72 (95% CI 0.59 to 0.82) and specificity 0.93 (95% CI 0.66 to 0.99).
Serum lipase: sensitivity 0.79 (95% CI 0.54 to 0.92) and specificity 0.89 (95% CI 0.46 to 0.99).
In comparison, the admission urinary trypsinogen‐2 was associated with a sensitivity of 0.72 (95% CI 0.56 to 0.84) and a specificity of 0.90 (95% CI 0.85 to 0.93). Thus, the tests appear to have similar diagnostic test accuracy. While we could not perform a formal comparison of the diagnostic test accuracy because of sparse data, it is unlikely to demonstrate statistical significance given the significant overlap of confidence intervals of sensitivities (and specificities) in these three tests.
At the median prevalence of 22.6% of acute pancreatitis in the studies, out of 100 people with positive test, serum amylase (more than three times normal), serum lipase (more than three times normal), and urinary trypsinogen (more than 50 ng/mL), 74 (95% CI 33 to 94); 68 (95% CI 21 to 94); and 67 (95% CI 57 to 76) people have acute pancreatitis, respectively; out of 100 people with negative test, serum amylase (more than three times normal), serum lipase (more than three times normal), and urinary trypsinogen (more than 50 ng/mL), 8 (95% CI 5 to 12); 7 (95% CI 3 to 15); and 8 (95% CI 5 to 13) people have acute pancreatitis, respectively. This means that although negative index test result decreases the probability of a person having acute pancreatitis, a significant proportion of people with acute pancreatitis have negative results and require further investigations to rule out acute pancreatitis, depending upon the nature and intensity of their pain.
The diagnostic accuracy reported at other thresholds and times was based on even less data and is subject to significant systematic and random errors. The results presented should therefore be considered as exploratory information rather than information based on which conclusions can be made. They indicate that a threshold of twice the normal limit of both serum amylase and lipase should be explored as an alternative to thrice the normal limit for the diagnosis of acute pancreatitis. The diagnostic accuracy also appears to be less for both serum amylase and lipase as the time interval between admission and the performance of the test increases, with the diagnostic accuracy of serum amylase decreasing more than serum lipase. Even the diagnostic accuracy of serum lipase in the later days is not sufficiently accurate to have any clinical role. Unless future studies show a major improvement in diagnostic accuracy, it appears that these tests do not have major clinical roles, that is a patient who has clinical symptoms of acute pancreatitis with a long time interval between the onset of symptoms and performance of the test should undergo radiological tests directly to confirm or rule out acute pancreatitis. One could also explore urinary amylase as a potential triage test prior to radiological tests for later days, as amylase gets excreted mainly by urine.
Strengths and weaknesses of the review
One of the main strengths of this review was that we searched the literature thoroughly, without any publication or language restrictions. We did not use any diagnostic test accuracy filters in our literature search because such filters could have led us to exclude some relevant studies (Doust 2005). Inclusion of abstracts and non‐English articles may decrease the impact of publication bias to a certain extent, although the determinants and the extent of publication bias and selective reporting are not well known for diagnostic accuracy studies. We also planned to exclude case‐control studies because these studies are prone to bias (Whiting 2011). Two review authors (KSG and OK, GR, or AH) independently searched the references produced by the search to identify relevant studies; screened the full‐text papers against the inclusion criteria; and extracted data. Data extractions by two review authors potentially reduced the chance of errors related to data extraction by a single review author (Buscemi 2006). Another strength of this review was that we used the recommended methodological quality methods to assess the risk of bias and applicability concerns in the included studies and took these into consideration while interpreting the evidence.
Weaknesses
There were several shortcomings in our review. Firstly, the studies included in the review had several methodological deficiencies. We had to interpret the results of serum amylase and lipase without a study that included approximately two‐thirds of the included participants because of major concerns about applicability. Another major methodological deficiency was that three of the four studies, Abraham 2011, Mayumi 2012, Saez 2005, and four of the five studies, Abraham 2011, Mayumi 2012, Saez 2005, Wu 2009, that contributed to the assessment of diagnostic accuracy of serum amylase and lipase used serum amylase and lipase as part of the reference standard, which might have overestimated their diagnostic test accuracy. Exclusion of these studies in a post hoc sensitivity analysis left only a few studies at high risk of bias and with applicability concerns to be included in the main analysis, and increased the unreliability of the evidence. None of the studies reported whether the index tests and reference standards were interpreted independently of one another. If they were not interpreted independently of each other, the accuracy of the tests would have been overestimated. Only four studies reported that they included all participants attending the emergency department with acute abdominal pain (Abraham 2011; Mayumi 2012; Saez 2005; Viel 1990). In three studies there was inappropriate exclusion of participants (Burkitt 1987; Chang 2011; Patt 1966). Of particular concern was the exclusion of people with parotid disease and end‐stage renal failure in one study (Chang 2011), which can overestimate the diagnostic accuracy. Because of the large number of participants included in this study, this study could have significantly influenced the overall results and resulted in poor estimation of diagnostic test accuracy. Future meta‐analyses on this topic should exclude Chang 2011 and other significantly biased studies that use a very large number of participants because of the influence of such studies on the result of the meta‐analysis. It was unclear whether inappropriate exclusions were avoided in the remaining three studies (Aysan 2008; Keim 1998; Wu 2009). It was unclear whether some of the participants had indeterminate values in any of these six studies in which there were inappropriate exclusions or those that did not report participant flow. Exclusion of people with borderline values close to the threshold used or those with other causes of elevation of these tests will overestimate the diagnostic test accuracy of these tests.
Secondly, there was significant heterogeneity in some of the comparisons. In the analysis of lipase more than three times normal on admission with inclusion of Chang 2011, the confidence intervals covered the entire range of possible specificities. While we could have used the fixed‐effect model to overcome these wide confidence intervals, this would have meant that we would have ignored heterogeneity that was evident in lack of overlap of confidence intervals. This model would also have had a poorer fit than the one we reported, leading us to make wrong conclusions as a result of ignoring heterogeneity. The various reasons for heterogeneity included different reference standards.
Thirdly, the sample sizes of the studies were small after the large study was excluded due to major concerns about applicability in the participant selection domain (Chang 2011), resulting in wide confidence intervals. We found a large number of studies using case‐control study designs that compared the diagnostic performance of the tests between people with acute pancreatitis and healthy controls. While the inclusion of such studies would have improved precision, it would have resulted in significant overestimation of the results. This would have been meaningless in the context of how these tests are used in clinical practice, that is diagnose acute pancreatitis in people with acute abdominal pain. We therefore accepted inclusion of fewer studies to provide a reasonably reliable diagnostic test accuracy estimate. However, this trade‐off resulted in sparse data and prevented us from formally comparing the different index tests and investigating heterogeneity. In particular, we were not able to assess whether the diagnostic performance changes with a time interval between onset of clinical symptoms and the performance of the test. As the half lifes of the different tests are different, this is of great clinical significance.
Comparison with other reviews
In the systematic review by Chang 2012, the summary estimate of urinary trypsinogen‐2 was 0.82 (95% CI 0.79 to 0.85) and the specificity was 0.94 (95% CI 0.92 to 0.95). These results show greater diagnostic accuracy and a more precise estimate of the diagnostic accuracy compared to what we have found in this review. The likely reason for this is the inclusion of studies with an inadequate reference standard, which would have resulted in improved precision and may improve the diagnostic accuracy. The results of the other systematic review by Jin 2013 were similar to those of Chang 2012. Jin 2013 reported the diagnostic test accuracy of serum amylase, serum lipase, and urinary trypsinogen‐2, and stated that the three tests had similar diagnostic test accuracies. We agree with the inference that the diagnostic accuracies of serum amylase, serum lipase, and urinary trypsinogen‐2 are similar.
Applicability of findings to the review question
Generalisability of the results
The studies did not restrict the participants to specific aetiologies of acute pancreatitis, therefore the findings of this review are applicable to all aetiologies of acute pancreatitis. Most studies used the test in the same way that it is used in clinical practice, that is in people with acute abdominal pain. Consequently, the results are applicable in people with acute abdominal pain. None of the studies reporting the diagnostic accuracy of tests post‐endoscopic retrograde cholangiopancreatography met the criteria for the reference standards used in this review, therefore the findings of this review may not be applicable in patients undergoing endoscopic retrograde cholangiopancreatography.
Use of the test in the clinical setting
The main role of the index test is diagnosis in clinical practice. Use of a test with good diagnostic accuracy makes it possible to decide on admission and appropriate management of patients with acute abdominal pain. The post‐test probabilities of positive and negative test depend upon the pre‐test probabilities of the test. The median pre‐test probability observed in the trials included in this review was 22.6%. Depending upon the type of people arriving at the emergency department, this can vary; this pre‐test probability seems to be higher than that routinely seen in clinical practice. This might be because the clinicians may have included only patients whom they suspect to have acute pancreatitis rather than any patients with abdominal pain. At the median pre‐test probability of 22.6%, the post‐test probabilities suggest that a significant proportion of people with negative tests, that is an average of 7% to 9%, have pancreatitis. Even at the lower quartile of pre‐test probability of 16.3%, an average of 5% to 6% of people with negative tests have acute pancreatitis. People with severe abdominal pain suggestive of acute pancreatitis may therefore require admission for observation, pain control, and supportive treatment even if one or more of the index tests is negative, as the implications of missing acute pancreatitis in severe abdominal pain are high.
Authors' conclusions
Implications for practice.
About a quarter of people with acute pancreatitis fail to be diagnosed as having acute pancreatitis with the tests evaluated in this review. Consequently, one should have a low threshold to admit the patient and treat as acute pancreatitis if the symptoms are suggestive of acute pancreatitis, even if these tests are normal. As about 1 in 10 patients without acute pancreatitis may be wrongly diagnosed as having acute pancreatitis with these tests, it is important to consider other conditions that require urgent surgical intervention such as perforated viscus even if these tests are abnormal.
The diagnostic performance of these tests decreases even further with the progression of time, and one should have an even lower threshold to perform additional investigations if the symptoms are suggestive of acute pancreatitis.
Implications for research.
Further well‐designed diagnostic test accuracy studies with prespecified index test threshold of serum amylase, serum lipase, and urinary trypsinogen‐2 are required. Such studies should avoid including the index test in the reference standard. Further well‐designed diagnostic test accuracy studies with prespecified index test threshold of urinary amylase and urinary trypsinogen‐2 are required to investigate the potential of these tests to diagnose acute pancreatitis when there is a delay between onset of symptoms and performance of the test.
Feedback
Definition of reference standard, 8 February 2018
Summary
This diagnostic test accuracy review addresses a relevant clinical problem: the role of some laboratory tests for the diagnosis of acute pancreatitis. The review seems to have some problems in trying to answer the questions and may therefore have difficulties to support clinical decisions and future clinical research (Colli 2014).
The major problem is regarding the definition of the reference standard. The review authors have chosen to use a consensus definition of acute pancreatitis (Banks 2013), based on the presence of at least two of three features:
acute onset of a persistent, severe epigastric pain often radiating to the back;
serum lipase activity or amylase activity at least three times greater than the upper limit of normal;
characteristic findings of acute pancreatitis on contrast enhanced computed tomography or magnetic resonance imaging or trans‐abdominal ultrasound.
The three criteria do not all seem adequate to us as reference standard for diagnostic studies and reviews. The first criterion is a definition of participants and an inclusion criterion for studies. Thus, the participants in all included studies should satisfy this criterion and then the three criteria become two criteria (2 and 3); the second criterion, for most included studies, corresponds to the index test (which may create incorporation bias and is likely to lead to overestimated diagnostic accuracy). The third criterion, radiological features, is quite inaccurate as the authors are well aware as they stated: “Radiological findings of acute pancreatitis evolve over a few days and the radiological features may not be apparent in the early stages, or may even be normal (Banks 2013; Vissers 1999), thus one cannot rely on radiological tests to diagnose acute pancreatitis, at least in the early stages”.
Despite this imperfect reference standard and the high risk of bias of all included studies, the review authors have produced some meta‐analysis estimates which are problematic, suggesting that pooling might not be appropriate. For instance, the summary estimate of specificity for serum amylase was 0.99 (95% CI 0.99 to 0.99) and for serum lipase 0.93 (95% CI 0.00 to 1.00). Moreover, in the abstract the review authors did not report the main analysis results but only those of planned and post hoc sensitivity analyses.
As a consequence of these problems both the discussion and the authors conclusions may be misleading and seem to reflect a circular reasoning. “As about a quarter of people with acute pancreatitis fail to be diagnosed as having acute pancreatitis with the evaluated tests, one should have a low threshold to admit the patient and treat them for acute pancreatitis if the symptoms are suggestive of acute pancreatitis, even if these tests are normal. About 1 in 10 patients without acute pancreatitis may be wrongly diagnosed as having acute pancreatitis with these tests, therefore it is important to consider other conditions that require urgent surgical intervention, such as perforated viscus, even if these tests are abnormal.” We think this could be expressed more shortly: do not do these tests; they are useless, unable to change your decision which could be based only on symptoms. The review results actually do not support any conclusions except that the retrieved studies are at high risk of bias and use an inadequate and inappropriate reference standard. But, unfortunately, this is not sufficiently emphasised in the authors’ conclusions.
We find that in the absence of an accurate reference standard, the accuracy of serum amylase and lipase cannot be estimated (Chang 2011). Further research could explore whether different diagnostic pathways using different testing might produce different clinical outcomes.
In the case of a new test, such as urinary trypsinogen, a reference standard including serum amylase or lipase could have been acceptable avoiding at least the incorporation bias. Anyway, an imperfect reference standard, such as the three consensus criteria, would misclassify some results, impairing the accuracy estimates.
We noted a large number of studies excluded for “inappropriate reference standard”. Due to the above mentioned problems of imperfectness of the reference standard, we wonder if the exclusion of these studies cannot be somehow arbitrary. We assessed one of them, a large Finnish study (Kemppainen 1997c) excluded for “inappropriate reference standard”. The definition of reference standard reported in the paper is: “The study group consisted of 500 consecutive patients (306 men and 194 women) with acute abdominal pain. After the patients had undergone clinical evaluation, serum amylase concentrations were determined in all patients except 12 with diagnoses other than acute pancreatitis. In patients with marginally elevated serum amylase concentrations (300 to 900 U per liter) and abdominal pain, pancreatitis was ruled out or confirmed on the basis of repeated serum and urinary amylase measurements and clinical follow‐up, as well as abdominal CT and ultrasonographic studies”. It seems the review authors used the three consensus criteria and therefore we think this study should have been included in the review.
In conclusion, we think that in the discussion and in the conclusions of this systematic review, the methodological problems related to the imperfect reference standard should be emphasised and the reader should be warned against the use of the results of this review in the clinical practice. Regarding the “Implications for research”, one cannot obviously disagree that well diagnostic test accuracy studies are needed, but in the actual absence of an adequate reference standard we think that different study designs exploring whether different diagnostic pathways using different testing might produce different clinical outcomes could be more informative.
Reply
Thank you for your interest in this review. When there is an inadequate reference standard, one has to use the best available reference standard. Therefore, we used a consensus definition of acute pancreatitis. We are fully aware of the issues around this definition as we mention in the review. The alternative is to biopsy the pancreas and have a histopathological confirmation. As we highlight in the review, this is neither performed routinely nor ethical to perform.
We have highlighted the problem with Chang 2011, a retrospective study including 3451 participants and excluding difficult to diagnose patients. This is the reason for not presenting the Chang 2011 as our main analysis as it is clinically not useful to present these results. We have fully acknowledged the incorporation bias in some of the studies and performed a sensitivity analysis excluding the studies with incorporation bias and reported this in the review.
Our conclusions are that one should not overly rely on these tests rather than avoid these tests completely. This is in line with what happens clinically, where patients with normal amylase levels/lipase levels with severe abdominal pain are admitted in hospital and investigated further.
Regarding the Kemppainen 1997c study, the author team felt that there were inadequate details to consider the reference standard to be adequate, but we will review this further in the next update.
We fully agree that the current reference standards are not adequate. In the absence of a universally accepted definition of acute pancreatitis, there is certainly potentially for research in making diagnosis of acute pancreatitis more accurately and/or arriving at a better consensus definition. Such studies could use adequately described clinical follow‐up as ways of confirming or ruling out pancreatitis.
Contributors
Commentators
Agostino Colli, The Cochrane Hepato‐Biliary Group, Copenhagen Trial Unit, Center for Clinical Intervention Research, Department 7812, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; and Department of Internal Medicine, Ospedale A. Manzoni, Lecco, Italy
Giovanni Casazza, The Cochrane Hepato‐Biliary Group, Copenhagen Trial Unit, Center for Clinical Intervention Research, Department 7812, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; and Department of Biomedical and Clinical Sciences, “ L. Sacco,” Universit a degli Studi di Milano, Milan, Italy;
Christian Gluud, The Cochrane Hepato‐Biliary Group, Copenhagen Trial Unit, Center for Clinical Intervention Research, Department 7812, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark;
Replying author
Kurinchi Selvan Gurusamy, Department of Surgery, Royal Free Campus, UCL Medical School, London, UK
Feedback Editor
Javier Gisbert, Gastroenterology Unit, Hospital Universitario de la Princesa, Instituto de Investigación Sanitaria Princesa (IIS‐IP), and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Spain
What's new
| Date | Event | Description |
|---|---|---|
| 2 March 2018 | Amended | Feedback and author's response included. |
Acknowledgements
We thank the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group, the United Kingdom Support Unit for Diagnostic Test Accuracy (DTA) Reviews, and the DTA editorial team for their advice in the preparation of this review. In particular, we want to thank Y Takwoingi for providing the SAS codes for the different types of analysis for a different review. We have used the same codes with the data modified.
We thank the copy‐editing team for improving the readability of the review.
Appendices
Appendix 1. Glossary of terms
Acute: sudden onset.
Adipose: fat.
Aetiology: cause.
Autodigestion: breaking down of the same organ that secretes the substance.
Cholecystectomy: removal of the gallbladder.
Cholecystitis: inflammation of the gallbladder.
Debridement: surgical removal of damaged, dead, or infected tissue; in this context, identical with necrosectomy.
Dyspepsia: discomfort in the upper abdomen or chest that may be described as gas, a feeling of fullness, or burning.
Endoscopic: using an endoscope, a flexible tube with a light and camera attached to it, to view the inner aspects of the food pipe, stomach, and upper small intestine.
Epigastric: upper central abdomen.
Gastrointestinal: relating to the stomach and the intestines.
Heterogeneity: differences between studies.
Histological: by examination of the tissue under a microscope.
Hyperamylasaemia: excess amylase in circulation.
Interstitial: small, narrow spaces between tissues or parts of an organ.
Intraperitoneal: inside the abdominal cavity.
Isoforms: two or more functionally similar proteins that have a similar but not identical composition.
Laparotomy: surgical incision into the abdominal cavity, for diagnosis or treatment of intra‐abdominal diseases.
Lymphatics: vessels carrying lymph in the body.
Magnetic resonance cholangiopancreatography: medical imaging technique that uses magnetic resonance imaging (use of magnetic field to differentiate between different structures) to visualise the biliary and pancreatic ducts in a non‐invasive manner.
Methodological: related to methods by which the study was conducted (in this context).
Mortality rate: death rate.
Necrosectomy: removal of dead tissue.
Necrosis: death and decomposition of living tissue usually caused by lack of blood supply, but can be the result of other pathological insult.
Necrotising: presence of necrosis.
Oedema: swelling.
Oedematous: tissue with an excess of interstitial fluid.
Pancreatic ductal system: tubular system that transports the pancreatic juice secreted by the pancreatic cells to the small intestine.
Pancreatic pseudocysts: fluid collections in the pancreas or the tissues surrounding the pancreas, enclosed by a well‐defined wall and containing only fluid with little or no solid material.
Parenchyma: functional parts of an organ.
Percutaneous: through the skin.
Percutaneous drainage: drainage carried out by insertion of drain from the external surface of the body, usually guided by an ultrasound or computed tomography (CT) scan.
Peripancreatic tissues: tissues surrounding the pancreas.
Peritonitis: inflammation of the peritoneum (the inner lining of the abdominal wall).
Prognosis: health outcome.
Pulse oximetry: non‐invasive method of measuring the oxygen level (oxygen saturation) of the blood, usually using infrared.
Radiating to the back: pain in front going to the back (in this context).
Retroperitoneal: behind the abdominal cavity.
SAS code: set of instructions for using an 'SAS' program to perform statistical analysis.
Sphincterotomy: partial division of the sphincter of Oddi, a circular band of muscle at the junction of the biliary tree (tubes that conduct bile from the liver to the small intestine) and pancreatic duct (tubes that conduct pancreatic juice into the second part of the duodenum).
Transabdominal: through the abdominal cavity.
Transluminal: through the lumen (inner cavity of a tubular structure).
Transperitoneal: through the abdominal cavity.
Triage: determining whether the patient requires further tests (in this context).
Ultrasonography: using high‐frequency sound to view internal structures of the body (in this context).
Appendix 2. MEDLINE search strategy
1. Pancreatitis, Acute Necrotizing/
2. Pancreatitis/et
3. Pancreas/ab, pa, pp
4. (acute adj3 pancrea*).mp.
5. (necro* adj3 pancrea*).mp.
6. (inflam* adj3 pancrea*).mp.
7. ((interstitial or edema* or oedema*) adj2 pancrea*).mp.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. exp Amylases/ or exp Lipase/ or exp Trypsinogen/
10. (amylase or lipase or trypsinogen or hyperamylasaemia or hyperamylasemia).mp.
11. exp C‐Reactive Protein/
12. ("c‐reactive protein" or "c reactive protein" or CRP).mp.
13. procalcitonin.mp.
14. exp L‐Lactate Dehydrogenase/
15. ("lactate dehydrogenase" or LDH).mp.
16. 9 or 10 or 11 or 12 or 13 or 14 or 15
17. 8 and 16
Appendix 3. Embase search strategy
1. acute hemorrhagic pancreatitis/
2. Pancreatitis/et
3. acute pancreatitis/
4. (acute adj3 pancrea*).mp.
5. (necro* adj3 pancrea*).mp.
6. (inflam* adj3 pancrea*).mp.
7. ((interstitial or edema* or oedema*) adj2 pancrea*).mp.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. exp amylase/
10. exp triacylglycerol lipase/
11. exp trypsinogen/
12. (amylase or lipase or trypsinogen or hyperamylasaemia or hyperamylasemia).mp.
13. exp C reactive protein/
14. ("c‐reactive protein" or "c reactive protein" or CRP).mp.
15. exp procalcitonin/
16. procalcitonin.mp.
17. exp lactate dehydrogenase/
18. ("lactate dehydrogenase" or LDH).mp.
19. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20. 8 and 19
Appendix 4. Science Citation Index and Conference Proceedings Citation Index‐Science search strategy
# 1 TS=((acute or necro* or inflam* or interstitial or edema* or oedema*) near/3 pancrea*)
# 2 TS=(amylase or lipase or trypsinogen or hyperamylasaemia or hyperamylasemia or "c‐reactive protein" or "c reactive protein" or CRP or procalcitonin or "lactate dehydrogenase" or LDH)
# 3 #2 AND #1
Appendix 5. National Institute for Health Research ‐ HTA and DARE search strategy
acute pancreatitis
Appendix 6. Zetoc search strategy
Each of the following lines will be searched separately. since the Boolean operator 'or' is not available for searching Zetoc database.
1. acute pancreatitis amylase
2. acute pancreatitis lipase
3. acute pancreatitis trypsinogen
4. acute pancreatitis hyperamylasaemia
5. acute pancreatitis hyperamylasemia
6. acute pancreatitis "c‐reactive protein"
7. acute pancreatitis "c reactive protein"
8. acute pancreatitis CRP
9. acute pancreatitis procalcitonin
10. acute pancreatitis "lactate dehydrogenase"
11. acute pancreatitis LDH
Appendix 7. WHO ICTRP search strategy
Title: (amylase or lipase or trypsinogen or hyperamylasaemia or hyperamylasemia or "c‐reactive protein" or "c reactive protein" or CRP or procalcitonin or "lactate dehydrogenase" or LDH)
Condition: acute pancreatitis
Appendix 8. ClinicalTrials.gov search strategy
amylase OR lipase OR trypsinogen OR hyperamylasaemia OR hyperamylasemia OR "c‐reactive protein" OR "c reactive protein" OR CRP OR procalcitonin OR "lactate dehydrogenase" OR LDH | acute pancreatitis
Appendix 9. SAS code used for fitting different models
data DiagnosticTestMetaAnalysis; input Study_id TP FP FN TN; /* Modify the data for the different tests*/
datalines; 1 52 7 17 48 2 14 19 8 3410 3 109 9 47 244 4 37 3 13 19 run; /* Modify the dataset for the bivariate analysis */ data dt; set DiagnosticTestMetaAnalysis; sens=1; spec=0; true=tp; n=tp+fn; output; sens=0; spec=1; true=tn; n=tn+fp; output; run; /* Ensure that both records for a study are clustered together */ proc sort data=dt; by study_id ; run; /* MODEL 1 */ /* Save NLMIXED output in the following datasets*/ ods output ParameterEstimates=pet1 FitStatistics=fitt1 additionalestimates=addest1 CovMatParmEst=covparmestt1 ConvergenceStatus=convgstatt1; /* Run the bivariate random effects logistic regression model for sensitivity and specificity */ /* The cov option requests that a covariance matrix is printed for all model parameter estimates.*/ proc nlmixed data=dt cov tech=quanew lis=5; parms msens=2 mspec=1 s2usens=0 s2uspec=0 covsesp=0; logitp=(msens+usens)*sens+(mspec+uspec)*spec; p = exp(logitp)/(1+exp(logitp)); model true ˜ binomial(n,p); random usens uspec ˜ normal([0,0],[s2usens,covsesp,s2uspec]) subject=study_id out=randeffs; estimate 'logLR+' log((exp(msens)/(1+exp(msens)))/(1‐(exp(mspec)/(1+exp(mspec))))); estimate 'logLR‐' log((1‐(exp(msens)/(1+exp(msens))))/(exp(mspec)/(1+exp(mspec)))); run; /* Obtain summary sens and spec from the model 1*/ /* change the number if this is for a different model*/ data summary1; set pet1; if parameter = 'msens' then name = 'Sensitivity'; else if parameter = 'mspec' then name = 'Specificity'; if parameter = 'msens' or parameter ='mspec' then summary=100 * exp(estimate)/(1 + exp(estimate)); if parameter = 'msens' or parameter ='mspec' then summlower=100 * exp(lower)/(1 + exp(lower)); if parameter = 'msens' or parameter ='mspec' then summupper=100 *exp(upper)/(1 + exp(upper)); output; run; /* Obtain summary LR from the model 1 */ data summaryLR1; set addest1; summary=exp(estimate); summlower=exp(lower); summupper=exp(upper); output; run; PROC EXPORT DATA= WORK.SUMMARY1
/* Modify the path for this outfile and the other outfiles */
OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Summary1.csv" DBMS=CSV REPLACE; RUN; /* Export parameter estimates table */ PROC EXPORT DATA= WORK.pet1 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Parameter estimates1.csv" DBMS=CSV REPLACE; RUN; /* Export the summary LR as an Excel .csv file */ PROC EXPORT DATA= WORK.SUMMARYLR1 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\SummaryLR1.csv" DBMS=CSV REPLACE; RUN; /* Export Fit statistics table */ PROC EXPORT DATA= WORK.fitt1 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Fit statistics1.csv" DBMS=CSV REPLACE; RUN; /* Export covariance parameter estimates table */ PROC EXPORT DATA= WORK.covparmestt1 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Covariance parameter estimates1.csv" DBMS=CSV REPLACE; RUN; /* MODEL 2 */ ods output ParameterEstimates=pet2 FitStatistics=fitt2 additionalestimates=addest2 CovMatParmEst=covparmestt2 ConvergenceStatus=convgstatt2; /* Run univariate random effects logistic regression models for sensitivity and specificity, i.e., ignore the correlation */ proc nlmixed data=dt cov tech=quanew lis=5; parms msens=2 mspec=1 s2usens=0 s2uspec=0 ; logitp=(msens+usens)*sens+(mspec+uspec)*spec; p = exp(logitp)/(1+exp(logitp)); model true ˜ binomial(n,p); random usens uspec ˜ normal([0,0],[s2usens,0,s2uspec]) subject=study_id out=randeffs; estimate 'logLR+' log((exp(msens)/(1+exp(msens)))/(1‐(exp(mspec)/(1+exp(mspec))))); estimate 'logLR‐' log((1‐(exp(msens)/(1+exp(msens))))/(exp(mspec)/(1+exp(mspec)))); run; /* Obtain summary sens and spec from the model 2*/ /* change the number if this is for a different model*/ data summary2; set pet2; if parameter = 'msens' then name = 'Sensitivity'; else if parameter = 'mspec' then name = 'Specificity'; if parameter = 'msens' or parameter ='mspec' then summary=100 * exp(estimate)/(1 + exp(estimate)); if parameter = 'msens' or parameter ='mspec' then summlower=100 * exp(lower)/(1 + exp(lower)); if parameter = 'msens' or parameter ='mspec' then summupper=100 *exp(upper)/(1 + exp(upper)); output; run; /* Obtain summary LR from the model 2 */ data summaryLR2; set addest2; summary=exp(estimate); summlower=exp(lower); summupper=exp(upper); output; run; PROC EXPORT DATA= WORK.SUMMARY2 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Summary2.csv" DBMS=CSV REPLACE; RUN; /* Export parameter estimates table */ PROC EXPORT DATA= WORK.pet2 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Parameter estimates2.csv" DBMS=CSV REPLACE; RUN; /* Export the summary LR as an Excel .csv file */ PROC EXPORT DATA= WORK.SUMMARYLR2 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\SummaryLR2.csv" DBMS=CSV REPLACE; RUN; /* Export Fit statistics table */ PROC EXPORT DATA= WORK.fitt2 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Fit statistics2.csv" DBMS=CSV REPLACE; RUN; /* Export covariance parameter estimates table */ PROC EXPORT DATA= WORK.covparmestt2 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Covariance parameter estimates2.csv" DBMS=CSV REPLACE; RUN; /* MODEL 3 */ ods output ParameterEstimates=pet3 FitStatistics=fitt3 additionalestimates=addest3 CovMatParmEst=covparmestt3 ConvergenceStatus=convgstatt3 additionalestimates=addest3; /* Run random effects logistic regression model for sensitivity and fixed model for specificity */ proc nlmixed data=dt cov tech=quanew lis=5 qpoints=10; parms msens=2 mspec=1 s2usens=0 ; logitp=(msens+usens)*sens+(mspec)*spec; p = exp(logitp)/(1+exp(logitp)); model true ˜ binomial(n,p); random usens ˜ normal([0],[s2usens]) subject=study_id out=randeffs; estimate 'logLR+' log((exp(msens)/(1+exp(msens)))/(1‐(exp(mspec)/(1+exp(mspec))))); estimate 'logLR‐' log((1‐(exp(msens)/(1+exp(msens))))/(exp(mspec)/(1+exp(mspec)))); run; /* Obtain summary sens and spec from the model 3*/ /* change the number if this is for a different model*/ data summary3; set pet3; if parameter = 'msens' then name = 'Sensitivity'; else if parameter = 'mspec' then name = 'Specificity'; if parameter = 'msens' or parameter ='mspec' then summary=100 * exp(estimate)/(1 + exp(estimate)); if parameter = 'msens' or parameter ='mspec' then summlower=100 * exp(lower)/(1 + exp(lower)); if parameter = 'msens' or parameter ='mspec' then summupper=100 *exp(upper)/(1 + exp(upper)); output; run; /* Obtain summary LR from the model 3 */ data summaryLR3; set addest3; summary=exp(estimate); summlower=exp(lower); summupper=exp(upper); output; run; PROC EXPORT DATA= WORK.SUMMARY3 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Summary3.csv" DBMS=CSV REPLACE; RUN; /* Export parameter estimates table */ PROC EXPORT DATA= WORK.pet3 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Parameter estimates3.csv" DBMS=CSV REPLACE; RUN; /* Export the summary LR as an Excel .csv file */ PROC EXPORT DATA= WORK.SUMMARYLR3 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\SummaryLR3.csv" DBMS=CSV REPLACE; RUN; /* Export Fit statistics table */ PROC EXPORT DATA= WORK.fitt3 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Fit statistics3.csv" DBMS=CSV REPLACE; RUN; /* Export covariance parameter estimates table */ PROC EXPORT DATA= WORK.covparmestt3 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Covariance parameter estimates3.csv" DBMS=CSV REPLACE; RUN; /* MODEL 4 */ ods output ParameterEstimates=pet4 FitStatistics=fitt4 additionalestimates=addest4 CovMatParmEst=covparmestt4 ConvergenceStatus=convgstatt4; /* Run fixed effect logistic regression model for sensitivity and random effects model for specificity */ proc nlmixed data=dt cov tech=quanew lis=5 qpoints=10; parms msens=2 mspec=1 s2uspec=0 ; logitp=(msens)*sens+(mspec+uspec)*spec; p = exp(logitp)/(1+exp(logitp)); model true ˜ binomial(n,p); random uspec ˜ normal([0],[s2uspec]) subject=study_id out=randeffs; estimate 'logLR+' log((exp(msens)/(1+exp(msens)))/(1‐(exp(mspec)/(1+exp(mspec))))); estimate 'logLR‐' log((1‐(exp(msens)/(1+exp(msens))))/(exp(mspec)/(1+exp(mspec)))); run; /* Obtain summary sens and spec from the model 4*/ /* change the number if this is for a different model*/ data summary4; set pet4; if parameter = 'msens' then name = 'Sensitivity'; else if parameter = 'mspec' then name = 'Specificity'; if parameter = 'msens' or parameter ='mspec' then summary=100 * exp(estimate)/(1 + exp(estimate)); if parameter = 'msens' or parameter ='mspec' then summlower=100 * exp(lower)/(1 + exp(lower)); if parameter = 'msens' or parameter ='mspec' then summupper=100 *exp(upper)/(1 + exp(upper)); output; run; /* Obtain summary LR from the model 4 */ data summaryLR4; set addest4; summary=exp(estimate); summlower=exp(lower); summupper=exp(upper); output; run; PROC EXPORT DATA= WORK.SUMMARY4 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Summary4.csv" DBMS=CSV REPLACE; RUN; /* Export parameter estimates table */ PROC EXPORT DATA= WORK.pet4 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Parameter estimates4.csv" DBMS=CSV REPLACE; RUN; /* Export the summary LR as an Excel .csv file */ PROC EXPORT DATA= WORK.SUMMARYLR4 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\SummaryLR4.csv" DBMS=CSV REPLACE; RUN; /* Export Fit statistics table */ PROC EXPORT DATA= WORK.fitt4 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Fit statistics4.csv" DBMS=CSV REPLACE; RUN; /* Export covariance parameter estimates table */ PROC EXPORT DATA= WORK.covparmestt4 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Covariance parameter estimates4.csv" DBMS=CSV REPLACE; RUN; /* MODEL 5 */ ods output ParameterEstimates=pet5 FitStatistics=fitt5 additionalestimates=addest5 CovMatParmEst=covparmestt5 ConvergenceStatus=convgstatt5; /* Run fixed effect logistic regression model for sensitivity and specificity */ proc nlmixed data=dt cov tech=quanew lis=5 qpoints=10; parms msens=2 mspec=1; logitp=(msens)*sens+(mspec)*spec; p = exp(logitp)/(1+exp(logitp)); model true ˜ binomial(n,p); estimate 'logLR+' log((exp(msens)/(1+exp(msens)))/(1‐(exp(mspec)/(1+exp(mspec))))); estimate 'logLR‐' log((1‐(exp(msens)/(1+exp(msens))))/(exp(mspec)/(1+exp(mspec)))); run; /* Obtain summary sens and spec from the model 5*/ /* change the number if this is for a different model*/ data summary5; set pet5; if parameter = 'msens' then name = 'Sensitivity'; else if parameter = 'mspec' then name = 'Specificity'; if parameter = 'msens' or parameter ='mspec' then summary=100 * exp(estimate)/(1 + exp(estimate)); if parameter = 'msens' or parameter ='mspec' then summlower=100 * exp(lower)/(1 + exp(lower)); if parameter = 'msens' or parameter ='mspec' then summupper=100 *exp(upper)/(1 + exp(upper)); output; run; /* Obtain summary LR from the model 5 */ data summaryLR5; set addest5; summary=exp(estimate); summlower=exp(lower); summupper=exp(upper); output; run; PROC EXPORT DATA= WORK.SUMMARY5 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Summary5.csv" DBMS=CSV REPLACE; RUN; /* Export parameter estimates table */ PROC EXPORT DATA= WORK.pet5 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Parameter estimates5.csv" DBMS=CSV REPLACE; RUN; /* Export the summary LR as an Excel .csv file */ PROC EXPORT DATA= WORK.SUMMARYLR5 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\SummaryLR5.csv" DBMS=CSV REPLACE; RUN; /* Export Fit statistics table */ PROC EXPORT DATA= WORK.fitt5 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Fit statistics5.csv" DBMS=CSV REPLACE; RUN; /* Export covariance parameter estimates table */ PROC EXPORT DATA= WORK.covparmestt5 OUTFILE = "C:\Users\kurinchi2k\Downloads\Acute pancreatitis DTAR\SA_3\SASFile\IndeterminatesExcluded\Covariance parameter estimates5.csv" DBMS=CSV REPLACE; RUN;
Appendix 10. Model fit for index tests for which meta‐analysis was possible
| Model fit | Bivariate random‐effects model taking correlation into account | Bivariate random‐effects model ignoring correlation | Random‐effects univariate logistic regression model for sensitivity and fixed‐effect model for specificity | Fixed‐effect model for sensitivity and random‐effects univariate logistic regression model for specificity | Fixed‐effect model for both sensitivity and specificity |
| Serum amylase (threshold: > 3 times normal) (on admission) | No convergence | No convergence | No convergence | No convergence | 86.4 |
| Serum amylase (threshold: > 3 times normal) (on admission; excluding studies with incorporation bias) | No convergence | No convergence | No convergence | 30.9 | 33.4 |
| Serum amylase (threshold: > 3 times normal) (on admission; excluding Chang 2011) | 89.9 | 91.9 | 178 | 30.9 | 33.4 |
| Serum amylase (threshold: > twice normal) (on admission) | No convergence | No convergence | No convergence | 30.8 | 17.1 |
| Serum amylase (threshold: > normal) (on admission) | No convergence | No convergence | No convergence | 30.8 | 24.9 |
| Serum amylase (threshold: > normal) (on admission) | No convergence | No convergence | No convergence | No convergence | 17.3 |
| Serum lipase (threshold: > 3 times normal) (on admission) | 89.9 | 91.9 | 178 | 89.3 | 183.5 |
| Serum lipase (threshold: > 3 times normal) (on admission; excluding studies with incorporation bias) | No convergence | No convergence | 125.7 | 33.2 | 125.7 |
| Serum lipase (threshold: > 3 times normal) (on admission; excluding Chang 2011) | 89.9 | 49.6 | 178 | 53.8 | 84.4 |
| Serum lipase (threshold: > twice normal) (on admission) | 89.9 | 91.9 | 178 | 30.6 | 25 |
| Serum lipase (threshold: > normal) (on admission) | 89.9 | 91.9 | 20.6 | 30.6 | 26.9 |
| Urinary trypsinogen‐2 (threshold: Actim Pancreatitis ‐ all studies; > 50 ng/mL) (on admission) | 89.9 | 91.9 | 57.4 | 59.2 | 59.4 |
| Urinary trypsinogen‐2 (threshold: Actim Pancreatitis ‐ sensitivity analysis; > 50 ng/mL excluding Aysan 2008) (on admission) | 89.9 | 91.9 | 45.5 | 45.9 | 46 |
| For each test with at least 2 studies, simpler models were fitted because of sparse data (Takwoingi 2015). The ‐2 log likelihood for the different models for each meta‐analysis is shown. The lowest ‐2 log likelihood ratio for each test is shown in bold italic font. The corresponding model was used for meta‐analysis. | |||||
Appendix 11. Statistical methods that were planned but not performed because of paucity of data
The statistical analysis and data synthesis below were planned but could not be performed because of the paucity of data.
We planned to stratify the analysis by the different reference standards (i.e. we planned to use different reference standards as different index tests). However, because of paucity of data, we did not stratify the studies based on reference standards.
We planned to compare the diagnostic accuracy of the different tests by including a single covariate term for test type in the bivariate model to estimate differences in the sensitivity and specificity of the tests. We planned to consider a combination of tests for each of the scenarios (any test positive or all tests positive) as different index tests. We planned to allow the variances of the random effects and their covariance to also depend on test type, thus allowing the variances to differ between tests. We planned to use the hierarchical summary receiver operating characteristics curve (HSROC) to test hypotheses about whether one test is superior to another and to investigate heterogeneity (Rutter 2001). For this purpose, we planned to combine tests irrespective of the thresholds and reference standards. We used the HSROC model to compare whether one test is superior to another since the HSROC model allows combining tests regardless of the thresholds and might overcome the problem of a limited number of studies included under each threshold. In case the study reported results at multiple thresholds, we used the threshold used by the authors for primary analysis for inclusion in the HSROC model. We planned to use likelihood ratio tests to compare the model with and without covariate (test type). We planned to use a P value of less than 0.05 for the likelihood ratio test to indicate differences in diagnostic accuracy between the tests. We also planned to compare the estimates of sensitivity and specificity between models to check the robustness of our assumptions about the variances of the random effects. If at least four studies that evaluated different tests in the same study population were available (e.g. in studies that perform more than one index test in all of the participants, individual index tests and combination of index tests in all of the participants, or randomised controlled trials in which participants have been randomised to the different index tests), we planned to perform a direct head‐to‐head comparison by limiting the test comparison to such studies. We also planned to present the relative sensitivities and relative specificities of the index tests from the direct comparisons in a table.
We planned to create a graph of pre‐test probabilities (using the observed median and range of prevalence from the included studies) against post‐test probabilities for each test stratified by different thresholds and reference standards. We planned to calculate the post‐test probabilities using these pre‐test probabilities and the summary positive and negative likelihood ratios. We planned to report the summary sensitivity, specificity, positive and negative likelihood ratios, and post‐test probabilities for the median, lower quartile, and upper quartile of the pre‐test probabilities. However, because of paucity of data, we did not present the pre‐test probability versus post‐test probability graph. We have not presented the likelihood ratios, as we had to provide the most important information in the table for a number of comparisons.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
Serum amylase > 3 times normal.
2. Test.
Serum amylase > 3 times normal (sensitivity analysis excluding studies with incorporation bias).
3. Test.
Serum amylase > 3 times normal (sensitivity analysis excluding Chang 2011).
4. Test.
Serum amylase > twice normal.
5. Test.
Serum amylase > twice normal (sensitivity analysis excluding Chang 2011).
6. Test.
Serum amylase > twice normal (2 to 3 days).
7. Test.
Serum amylase > twice normal (4 to 5 days).
8. Test.
Serum amylase > normal.
9. Test.
Serum amylase > normal (sensitivity analysis excluding studies with incorporation bias).
10. Test.
Serum amylase > normal (2 to 3 days).
11. Test.
Serum amylase > normal (4 to 5 days).
12. Test.
Serum lipase > 3 times normal.
13. Test.
Serum lipase > 3 times normal (sensitivity analysis excluding studies with incorporation bias).
14. Test.
Serum lipase > 3 times normal (sensitivity analysis excluding Chang 2011).
15. Test.
Serum lipase > twice normal.
16. Test.
Serum lipase > twice normal (sensitivity analysis excluding Chang 2011).
17. Test.
Serum lipase > twice normal (2 to 3 days).
18. Test.
Serum lipase > twice normal (4 to 5 days).
19. Test.
Serum lipase > normal.
20. Test.
Serum lipase > normal (2 to 3 days).
21. Test.
Serum lipase > normal (4 to 5 days).
22. Test.
Urinary trypsinogen‐2 > 50 ng/mL (Actim Pancreatitis).
23. Test.
Urinary trypsinogen‐2 > 50 ng/mL (Actim Pancreatitis ‐ sensitivity analysis).
24. Test.
Urinary trypsinogen‐2 > 50 ng/mL (quantitative method).
25. Test.
Urinary trypsinogen‐2 only positive or most positive (threshold for this not available).
26. Test.
Urinary amylase > normal (quantitative).
27. Test.
Urinary amylase 1+ (qualitative).
28. Test.
Urinary amylase 2+ (qualitative).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abraham 2011.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 124. Females: not stated. Median or median age: not stated. Presentation: Patients with acute abdominal pain. Setting: secondary care, India. | ||
| Index tests | Index test: serum amylase.
Further details:
Technical specifications: not stated.
Performed by: not stated.
Criteria for positive diagnosis: > 3 times normal. Index test: serum lipase. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: > 3 times normal. Index test: urinary trypsinogen‐2. Further details: Technical specifications: Actim Pancreatitis (Medix Biochemica). Performed by: not stated. Criteria for positive diagnosis: > 50 ng/mL. |
||
| Target condition and reference standard(s) | Target condition: acute pancreatitis.
Reference standard: consensus definition.
Further details:
Technical specifications: not applicable.
Performed by: not stated.
Criteria for positive diagnosis: consensus definition. For the index tests serum amylase and serum lipase, the answer for the signalling question 'Is the reference standard independent of the index test?' is 'No' and the risk of bias is 'High risk'. |
||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | The index tests serum amylase and lipase were not independent of the reference standard, but urinary trypsinogen‐2 was independent of the reference standard. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serum amylase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Serum Lipase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Urinary trypsinogen‐2 (standard criteria) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Is the reference standard independent of the index test? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Aysan 2008.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 99. Females: 46 (46.5%). Median or median age: 37 years. Presentation: Patients with abdominal pain. Exclusion criteria: Patients with trauma or who required emergency surgical intervention. Setting: secondary care, Turkey. | ||
| Index tests | Index test: urinary trypsinogen‐2. Further details: Technical specifications: Medix Biochemica. Performed by: not stated. Criteria for positive diagnosis: not clearly stated (probably used the manufacturer's level of > 50 ng/mL). | ||
| Target condition and reference standard(s) | Target condition: acute pancreatitis. Reference standard: CT scan. Further details: Technical specifications: not stated. Performed by: radiologists. Criteria for positive diagnosis: at least 1 of the following:
|
||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Urinary trypsinogen‐2 (standard criteria) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Is the reference standard independent of the index test? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Burkitt 1987.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 306.
Females: not stated.
Median or median age: not stated.
Presentation:
People with abdominal pain. Note: 88 patients who had normal urinary amylase were excluded from analysis. Setting: seconday care, UK. |
||
| Index tests | Index test: urinary amylase.
Further details:
Technical specifications: Rapignost‐Amylase test.
Performed by: not stated.
Criteria for positive diagnosis: 1 plus. Second criteria for positive diagnosis: 2 plus. |
||
| Target condition and reference standard(s) | Target condition: acute pancreatitis. Reference standard: Phadebas Amylase > 1000 (normal limit 300) + abdominal pain. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: amylase > 1000 (normal limit 300) + abdominal pain. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: 88 (28.8%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test Urinary amylase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Is the reference standard independent of the index test? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Chang 2011.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 3451. Females: not stated. Median or median age: not stated. Presentation: Patients with acute abdominal pain and undergoing blood tests. Exclusion criteria Patients with parotid disease, intracranial haemorrhage, end‐stage renal failure. Setting: secondary care, Hong Kong, China. | ||
| Index tests | Index test: serum amylase.
Further details:
Technical specifications: Beckman Coulter chemistry analyser.
Performed by: not stated.
Criteria for positive diagnosis: > 3 times normal. Second criteria for positive diagnosis: > twice normal. Index test: serum lipase. Further details: Technical specifications: Beckman Coulter chemistry analyser. Performed by: not stated. Criteria for positive diagnosis: > 3 times normal. Second criteria for positive diagnosis: > twice normal. |
||
| Target condition and reference standard(s) | Target condition: acute pancreatitis. Reference standard: radiology (ultrasound or CT). Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Serum amylase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Serum Lipase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Is the reference standard independent of the index test? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Keim 1998.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 253. Females: 108 (42.7%). Median or median age: 56 years. Presentation: Patients with acute abdominal pain and undergoing blood tests. Setting: secondary care, Germany. | ||
| Index tests | Index test: serum amylase.
Further details:
Technical specifications: Boehringer Mannheim.
Performed by: not stated. Criteria for positive diagnosis: > twice normal. Second criteria for positive diagnosis: > normal. Index test: serum lipase. Further details: Technical specifications: Boehringer Mannheim. Performed by: not stated. Criteria for positive diagnosis: > twice normal. Second criteria for positive diagnosis: > normal. Serum amylase and lipase were measured at the 2 specified thresholds for each test at 3 different time points (on admission, 2 to 3 days later, and 4 to 5 days later). |
||
| Target condition and reference standard(s) | Target condition: acute pancreatitis. Reference standard: radiology (ultrasound or CT). Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serum amylase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Serum Lipase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Is the reference standard independent of the index test? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Mayumi 2012.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 412. Females: 186 (45.1%). Median or median age: 55 years. Presentation: Adult patients with acute abdominal pain. Setting: secondary care, Japan. | ||
| Index tests | Index test: serum amylase.
Further details:
Technical specifications: BioMajesty JCA‐BM or LABOSPECT.
Performed by: study collaborators.
Criteria for positive diagnosis: > 3 times normal. Index test: serum lipase. Further details: Technical specifications: BioMajesty JCA‐BM or LABOSPECT. Performed by: study collaborators. Criteria for positive diagnosis: > 3 times normal. Index test: urinary trypsinogen‐2. Further details: Technical specifications: Actim Pancreatitis (Medix Biochemica). Performed by: study collaborators. Criteria for positive diagnosis: > 50 ng/mL. Second criteria for positive diagnosis: only + or most positive. Index test: urinary trypsinogen‐2. Further details: Technical specifications: quantitative immunoenzymometric assay trypsinogen‐2 test (Medix Biochemica). Performed by: study collaborators. Criteria for positive diagnosis: > 50 ng/mL. |
||
| Target condition and reference standard(s) | Target condition: acute pancreatitis. Reference standard: consensus definition. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | The index tests serum amylase and lipase were not independent of the reference standard, but urinary trypsinogen‐2 was independent of the reference standard. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serum amylase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serum Lipase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urinary trypsinogen‐2 (standard criteria) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Urinary trypsinogen‐2 (non‐standard criteria) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Is the reference standard independent of the index test? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Patt 1966.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 200. Females: not stated. Median or median age: not stated. Presentation:
Note: This indicates that only people with severe symptoms have been included. Setting: secondary care, USA. |
||
| Index tests | Index test: serum amylase.
Further details:
Technical specifications: not stated.
Performed by: not stated.
Criteria for positive diagnosis: > normal. Index test: serum lipase. Further details: Technical specifications: calorimetric method. Performed by: not stated. Criteria for positive diagnosis: > normal. |
||
| Target condition and reference standard(s) | Target condition: acute pancreatitis. Reference standard: laparotomy or autopsy. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Serum amylase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Serum Lipase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Is the reference standard independent of the index test? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Saez 2005.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 72. Females: not stated. Median or median age: not stated. Presentation: Patients with acute abdominal pain. Setting: secondary care, Spain. | ||
| Index tests | Index test: serum amylase.
Further details:
Technical specifications: Amyl, Boehringer Mannheim Systems.
Performed by: not stated.
Criteria for positive diagnosis: > 3 times normal. Index test: serum lipase. Further details: Technical specifications: Lip, Boehringer Mannheim Systems. Performed by: not stated. Criteria for positive diagnosis: > 3 times normal. Index test: urinary trypsinogen‐2. Further details: Technical specifications: Actim Pancreatitis test strip. Performed by: not stated. Criteria for positive diagnosis: > 50 ng/mL. |
||
| Target condition and reference standard(s) | Target condition: acute pancreatitis.
Reference standard: 3‐fold increase of serum amylase and evidence of pancreatitis in radiology or surgery.
Further details:
Technical specifications: not stated.
Performed by: not stated.
Criteria for positive diagnosis: not stated. For the index test serum amylase, the answer for the signalling question 'Is the reference standard independent of the index test?' is 'No' and the risk of bias is 'High risk'. |
||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | The index test serum amylase was not independent of the reference standard, but serum lipase and urinary trypsinogen‐2 were independent of the reference standard. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serum amylase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serum Lipase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Urinary trypsinogen‐2 (standard criteria) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Is the reference standard independent of the index test? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Viel 1990.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 83. Females: not stated. Median or median age: not stated. Presentation: Patients with acute abdominal pain. Setting: secondary care, France. | ||
| Index tests | Index test: serum lipase. Further details: Technical specifications: Boehringer Mannheim. Performed by: not stated. Criteria for positive diagnosis: > 3 times normal. | ||
| Target condition and reference standard(s) | Target condition: acute pancreatitis. Reference standard: 3‐fold increase of serum amylase and evidence of pancreatitis in radiology, endoscopic retrograde cholangiopancreatography, or surgery. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Serum Lipase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Is the reference standard independent of the index test? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Wu 2009.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 134. Females: 66 (49.3%). Median or median age: 48 years. Presentation: Patients with acute abdominal pain. Setting: secondary care, China. | ||
| Index tests | Index test: serum amylase. Further details: Technical specifications: Beckman automatic biochemical analyzer. Performed by: not stated. Criteria for positive diagnosis: > normal. | ||
| Target condition and reference standard(s) | Target condition: acute pancreatitis.
Reference standard:
Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. |
||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: not stated. Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | The index test serum amylase was not independent of the reference standard, but serum lipase and urinary amylase were independent of the reference standard. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Serum amylase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Urinary trypsinogen‐2 (standard criteria) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Urinary amylase | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Is the reference standard independent of the index test? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
CT: computed tomography
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abascal 1982 | No diagnostic test accuracy data |
| Abate 1979 | Inappropriate population |
| Acero 1982 | Case‐control study |
| Adam 1986 | No diagnostic test accuracy data |
| Adams 1968 | No diagnostic test accuracy data |
| Adler 1985 | Inappropriate reference standard |
| Ahmed 2009 | Inappropriate population |
| Aho 1988 | No diagnostic test accuracy data |
| Aho 1989 | No diagnostic test accuracy data |
| Alvarez 1998 | Not a primary research study |
| Anand 1956 | Inappropriate population |
| Andersen 2010 | Case‐control study |
| Andre 1967 | Inappropriate population |
| Andren‐Sandberg 1997 | Not a primary research study |
| Andriushchenko 1998 | Inappropriate population |
| Anonymous 1966 | Not a primary research study |
| Anonymous 2012 | Not a primary research study |
| Aparisi 1987 | Not a primary research study |
| Apple 1991 | Inappropriate reference standard |
| Arzoglou 1983 | Inappropriate population |
| Arzoglou 1986 | Case‐control study |
| Bacchini 1980 | Inappropriate population |
| Bachmann 1979 | Case‐control study |
| Baillie 1997 | Not a primary research study |
| Baillie 1998 | Not a primary research study |
| Bang 2016 | Inappropriate population |
| Banks 1996 | Inappropriate population |
| Barbado 1977 | Case‐control study |
| Barbieri 2016 | Not a primary research study |
| Bargum 1983 | No diagnostic test accuracy data |
| Barnett 1986 | Inappropriate population |
| Batra 2015 | Inappropriate population |
| Batsakis 1965 | Not a primary research study |
| Benini 1987 | Inappropriate reference standard |
| Benini 1987a | Inappropriate population |
| Benini 1992 | Inappropriate reference standard |
| Berger 1976 | Case‐control study |
| Bernard 1959 | No diagnostic test accuracy data |
| Bernard 1964 | Not a primary research study |
| Bernard 1964a | Not a primary research study |
| Bernard 1964b | Not a primary research study |
| Berry 1982 | Inappropriate population |
| Blamey 1983 | Inappropriate population |
| Bluskina 1966 | Case‐control study |
| Bode 1987 | Not a primary research study |
| Borda 1978 | Case‐control study |
| Borgstrom 1984 | Inappropriate population |
| Borgstrom 2002 | Not a primary research study |
| Bowen 1983 | Inappropriate population |
| Brailski 1975 | Not a primary research study |
| Branford 1948 | No diagnostic test accuracy data |
| Brault 1985 | Inappropriate population |
| Brisinda 1999 | Inappropriate population |
| Brkic 1966 | Not a primary research study |
| Brodie 1977 | Inappropriate population |
| Brohee 1980 | Inappropriate population |
| Brohee 1981 | Not a primary research study |
| Brohee 1987 | Not a primary research study |
| Brunner 1980 | Not a primary research study |
| Buchler 1986 | Inappropriate population |
| Budd 1959 | Inappropriate population |
| Bunodiere 1975 | Inappropriate population |
| Butler 2000 | Not a primary research study |
| Caillens 1980 | Inappropriate target condition |
| Calkins 1968 | Inappropriate population |
| Cameron 1973 | Inappropriate population |
| Campbell 1979 | Inappropriate population |
| Caputo 1983 | Inappropriate population |
| Cases 1988 | Inappropriate population |
| Cevik 2010 | Case‐control study |
| Chase 1996 | Inappropriate reference standard |
| Chen 1994 | Case‐control study |
| Chen 2004 | Not a primary research study |
| Chen 2005 | Inappropriate reference standard |
| Cheng 2004 | Inappropriate population |
| Cheung 2015 | No diagnostic test accuracy data |
| Choi 2009 | Case‐control study |
| Choudhary 2012 | Not a primary research study |
| Christoforidis 2002 | Inappropriate population |
| Chylinski 1972 | Inappropriate population |
| Chylinski 1978 | Inappropriate population |
| Cintra 1952 | Inappropriate population |
| Cintra 1953 | Inappropriate population |
| Clave 1995 | Inappropriate reference standard |
| Close 1987 | Not a primary research study |
| Coffey 2014 | Inappropriate population |
| Coffey 2014a | Inappropriate population |
| Collins 1982 | Case‐control study |
| Concepcion Martin 2013 | Inappropriate population |
| Concepcion‐Martin 2016 | No diagnostic test accuracy data |
| Corfield 1984 | Inappropriate population |
| Cornett 2010 | No diagnostic test accuracy data |
| Corsetti 1993 | Inappropriate reference standard |
| Cote 1979 | Inappropriate population |
| Courtois 1986 | Inappropriate population |
| Dalgat 1986 | Inappropriate population |
| Dankner 1951 | Inappropriate population |
| Dati 1988 | Not a primary research study |
| de Boer 1986 | Not a primary research study |
| De Leo 1954 | Not a primary research study |
| Dehesa 1979 | Case‐control study |
| Delcourt 1977 | Not a primary research study |
| Deril 1989 | Case‐control study |
| Deril 1992 | Not a primary research study |
| Devanath 2009 | Inappropriate population |
| Diaz 2009 | Inappropriate population |
| Distefano 1952 | No diagnostic test accuracy data |
| Domenech 1999 | No diagnostic test accuracy data |
| Donaldson 1977 | No diagnostic test accuracy data |
| Dreiling 1974 | Inappropriate population |
| Dreiung 1954 | Inappropriate population |
| Dronov 2009 | Inappropriate population |
| Drozdov 2003 | Not a primary research study |
| Durr 1977 | Inappropriate population |
| Durr 1983 | No diagnostic test accuracy data |
| Eckfeldt 1985 | No diagnostic test accuracy data |
| Elman 1942 | Not a primary research study |
| Engel 1977 | No diagnostic test accuracy data |
| Ermini 1964 | Not a primary research study |
| Esber 1995 | Inappropriate population |
| Esperov 1972 | Inappropriate population |
| Fabris 1976 | No diagnostic test accuracy data |
| Farkas 1967 | No diagnostic test accuracy data |
| Farrar 1978 | Inappropriate population |
| Finke 1978 | Inappropriate population |
| Fiocca 1983 | Inappropriate population |
| Fiorucci 1986 | Inappropriate population |
| Fishman 1955 | Inappropriate population |
| Flamion 1987 | Inappropriate population |
| Forell 1959 | Inappropriate population |
| Forest 1990 | Inappropriate reference standard |
| Fridhandler 1972 | Inappropriate population |
| Frost 1978 | Inappropriate population |
| Fruchart 1974 | Inappropriate population |
| Fruchart 1980 | Inappropriate population |
| Fujiki 1980 | Inappropriate population |
| Fujita 1989 | Inappropriate population |
| Fukumoto 1981 | Inappropriate population |
| Gambill 1975 | Not a primary research study |
| Garden 1985 | No diagnostic test accuracy data |
| Gilbert 1955 | No diagnostic test accuracy data |
| Gluskina 1965 | Inappropriate population |
| Gomez 2012 | Inappropriate population |
| Gonzalez 1978 | Case‐control study |
| Grinblatt 1997 | Not a primary research study |
| Grosberg 1979 | Case‐control study |
| Gullo 2005 | Not a primary research study |
| Gumaste 1991 | Inappropriate population |
| Gumaste 1992 | Case‐control study |
| Gumaste 1993 | Case‐control study |
| Gumaste 1993a | Not a primary research study |
| Gungor 2011 | Inappropriate population |
| Gunn 1986 | Inappropriate population |
| Guth 1960 | Inappropriate population |
| Gwozdz 1990 | Inappropriate reference standard |
| Haas 1985 | Inappropriate population |
| Haffter 1981 | Case‐control study |
| Haffter 1983 | Case‐control study |
| Hale 2015 | Inappropriate population |
| Hathaway 1983 | Case‐control study |
| Hayakawa 1985 | Case‐control study |
| Hayakawa 1989 | Case‐control study |
| Hedstroem 1998 | Inappropriate reference standard |
| Hedstrom 1994 | Case‐control study |
| Hedstrom 1996 | Case‐control study |
| Hedstrom 1996a | Case‐control study |
| Hedstrom 1996b | Case‐control study |
| Hedstrom 1996c | Case‐control study |
| Hedstrom 2001 | Case‐control study |
| Heer 1983 | Case‐control study |
| Hegewald 1998 | Inappropriate population |
| Hegewald 1999 | Inappropriate population |
| Hegewald 2001 | Inappropriate population |
| Hemingway 1988 | Case‐control study |
| Hendry 1987 | Inappropriate population |
| Henry 1957 | Inappropriate population |
| Hoferichter 1964 | Inappropriate population |
| Hoffman 1991 | Inappropriate reference standard |
| Hofmeyr 2014 | Inappropriate population |
| Holdsworth 1984 | Case‐control study |
| Holmes 2011 | Inappropriate population |
| Horanyi 1984 | Inappropriate population |
| Hostein 1976 | Inappropriate population |
| Hostein 1977 | Inappropriate population |
| Hostein 1978 | Inappropriate population |
| Houry 1985 | Inappropriate reference standard |
| Houry 1989 | Inappropriate reference standard |
| Huang 2010 | Inappropriate population |
| Huguet 1993 | Inappropriate reference standard |
| Husain 2004 | Inappropriate population |
| Hwang 2004 | Case‐control study |
| Ignjatovic 1997 | Inappropriate reference standard |
| Ignjatovic 2000 | Inappropriate reference standard |
| Im 2010 | Inappropriate population |
| Imrie 1979 | Inappropriate population |
| Ito 2007 | Inappropriate population |
| Jacobson 1982 | Not a primary research study |
| Jam 1978 | Inappropriate population |
| Jang 2007 | Inappropriate population |
| Jensen 1970 | Inappropriate population |
| Jin 2012 | Not a primary research study |
| Jin 2013 | Not a primary research study |
| Jin 2013a | Not a primary research study |
| Johnson 2004 | Inappropriate population |
| Jordanov 2009 | Case‐control study |
| Joshi 2008 | Inappropriate reference standard |
| Junge 1982 | Case‐control study |
| Kaiser 1987 | Inappropriate reference standard |
| Kamer 2007 | Case‐control study |
| Kameya 1985 | Case‐control study |
| Kameya 1986 | Case‐control study |
| Kapetanos 2007 | Inappropriate population |
| Karlsson 1979 | Inappropriate population |
| Kaw 2001 | Inappropriate population |
| Kazmierczak 1991 | Inappropriate reference standard |
| Kehl 1985 | Inappropriate population |
| Keim 2003 | Case‐control study |
| Kemppainen 1997 | Inappropriate reference standard |
| Kemppainen 1997a | Inappropriate population |
| Kemppainen 1997b | Inappropriate population |
| Kemppainen 1997c | Inappropriate reference standard |
| Kemppainen 1997d | Not a primary research study |
| Kerlin 1986 | Inappropriate population |
| Khrapach 1992 | Inappropriate population |
| Khvatova 1973 | Inappropriate population |
| Kim 2015 | Inappropriate population |
| King 1995 | Inappropriate population |
| Kirchner 1976 | Inappropriate population |
| Kitterer 2015 | Inappropriate population |
| Kobayashi 2011 | Inappropriate population |
| Koehler 1982 | Inappropriate population |
| Kolars 1982 | Inappropriate population |
| Kolars 1984 | Inappropriate population |
| Kopacova 2010 | Inappropriate population |
| Kubo 1975 | Not a primary research study |
| Kulikovsky 2014 | Inappropriate population |
| Kurti 2011 | Inappropriate reference standard |
| Kusama 1956 | Inappropriate population |
| Kutter 1983 | Inappropriate population |
| Kylanpaa‐Back 1999 | Inappropriate reference standard |
| Kylanpaa‐Back 2000 | Inappropriate reference standard |
| Kylanpaa‐Back 2000a | Inappropriate reference standard |
| Kylanpaa‐Back 2002 | Inappropriate reference standard |
| Lacher 1986 | Inappropriate population |
| Lankisch 1977 | Case‐control study |
| Lankisch 1977a | Case‐control study |
| Lankisch 1994 | Inappropriate population |
| Lankisch 1994a | Inappropriate population |
| Lankisch 2006 | Inappropriate population |
| Lankisch 2012 | No diagnostic test accuracy data |
| Laurent‐Puig 1992 | Inappropriate population |
| Lauschke 1963 | Inappropriate population |
| Leclerc 1983 | Inappropriate population |
| Lee 1995 | Case‐control study |
| Lee 1996 | Not a primary research study |
| Lempinen 2001 | Inappropriate population |
| Lempinen 2003 | Inappropriate population |
| Lessinger 1994 | Case‐control study |
| Levitt 1975 | Not a primary research study |
| Lifton 1974 | Inappropriate population |
| Lifton 1974a | Inappropriate population |
| Ligny 1987 | Case‐control study |
| Lin 1989 | Case‐control study |
| Lindahl 1979 | No diagnostic test accuracy data |
| Liyanage 2012 | Inappropriate population |
| Logrono 2000 | Inappropriate reference standard |
| Long 1976 | Case‐control study |
| Loo 1992 | Not a primary research study |
| Lott 1985 | Not a primary research study |
| Lott 1985a | Not a primary research study |
| Lott 1986 | Inappropriate population |
| Lott 1991 | Not a primary research study |
| Lott 1991a | Case‐control study |
| Luengo 1996 | Inappropriate population |
| Lunghi 1984 | Inappropriate population |
| MacArthur 2013 | Inappropriate population |
| Macgregor 1976 | Case‐control study |
| Maekelae 1997 | Inappropriate population |
| Majkicsingh 1986 | Inappropriate population |
| Malfertheiner 1989 | Inappropriate population |
| Mangano 1990 | Inappropriate target condition |
| Marten 1976 | Case‐control study |
| Masoero 1978 | Inappropriate population |
| Masoero 1980 | Case‐control study |
| Massey 1985 | Inappropriate reference standard |
| Mayer 1985 | Inappropriate reference standard |
| McCulloch 1984 | Case‐control study |
| McIntosh 1976 | Not a primary research study |
| McMahon 1981 | Inappropriate population |
| McMahon 1982 | Inappropriate population |
| Merina 1957 | Inappropriate population |
| Millat 1999 | Not a primary research study |
| Miller 1973 | Inappropriate population |
| Millson 1998 | Inappropriate reference standard |
| Mimoz 1993 | Inappropriate population |
| Mingxin 2001 | Inappropriate reference standard |
| Mirmiranyazdy 1995 | Inappropriate population |
| Mohamed 1989 | Case‐control study |
| Moller‐Petersen 1983 | Inappropriate reference standard |
| Moller‐Petersen 1985 | Inappropriate reference standard |
| Moller‐Petersen 1986 | Inappropriate reference standard |
| Morel 1981 | Case‐control study |
| Murray 1976 | Inappropriate reference standard |
| Murray 1977 | Inappropriate reference standard |
| Murray 1980 | Inappropriate reference standard |
| Navarro 1984 | Not a primary research study |
| Navarro 1987 | Case‐control study |
| Nechai 1973 | Inappropriate population |
| Nechiporuk 1982 | Inappropriate population |
| Neoptolemos 1990 | No diagnostic test accuracy data |
| Neoptolemos 1993 | Not a primary research study |
| Neoptolemos 2000 | Inappropriate population |
| Neovius 1984 | Inappropriate reference standard |
| Neves 1985 | Inappropriate population |
| Newland 2002 | Inappropriate population |
| Oellerich 1983 | Case‐control study |
| Orda 1982 | Inappropriate reference standard |
| Orda 1984 | Case‐control study |
| Orebaugh 1994 | Inappropriate population |
| Osipov 1970 | Inappropriate population |
| Ostrovskii 2012 | Inappropriate population |
| Otsuki 1995 | Case‐control study |
| Pace 1985 | Inappropriate population |
| Pacheco 2003 | Case‐control study |
| Pakkala 2012 | Inappropriate population |
| Panteghini 1989 | Inappropriate population |
| Panteghini 1990 | Inappropriate population |
| Panteghini 1992 | Inappropriate population |
| Papaioannou 1996 | Case‐control study |
| Papp 1969 | Case‐control study |
| Parodi 1983 | Case‐control study |
| Pereiaslov 1999 | Inappropriate population |
| Peromingo 2009 | Inappropriate population |
| Pezzilli 1992 | Case‐control study |
| Pezzilli 1992a | Case‐control study |
| Pezzilli 1994 | Case‐control study |
| Pezzilli 1997 | Inappropriate population |
| Pezzilli 1998 | Case‐control study |
| Pezzilli 1999 | Case‐control study |
| Pezzilli 1999a | Case‐control study |
| Pezzilli 2000 | Case‐control study |
| Pezzilli 2001 | Inappropriate population |
| Pezzilli 2004 | Case‐control study |
| Phillip 2013 | Inappropriate population |
| Pirolla 2015 | Inappropriate population |
| Ponseti‐Bosch 1977 | Case‐control study |
| Ponteziere 2001 | Inappropriate reference standard |
| Popivanov 1963 | No diagnostic test accuracy data |
| Protsenko 1966 | No diagnostic test accuracy data |
| Raju 2003 | Case‐control study |
| Raty 2007 | Inappropriate population |
| Reilly 2011 | Inappropriate population |
| Rick 1968 | Inappropriate population |
| Roberts 1985 | Case‐control study |
| Roberts 1987 | Case‐control study |
| Rodriguez‐Cuartero 2000 | No diagnostic test accuracy data |
| Rokicki 1976 | Not a primary research study |
| Rosenblum 1991 | Not a primary research study |
| Rosenburg 1957 | No diagnostic test accuracy data |
| Rudis 2014 | Inappropriate population |
| Ruzena 1989 | No diagnostic test accuracy data |
| Sacchetti 1988 | Inappropriate population |
| Sacchetti 1989 | Inappropriate population |
| Sadowski 1992 | Inappropriate population |
| Sainio 1995 | Case‐control study |
| Sankaralingam 2007 | Inappropriate population |
| Satz 1989 | Case‐control study |
| Satz 1990 | Case‐control study |
| Satz 1990a | Case‐control study |
| Saxon 1957 | Case‐control study |
| Schmidt 2004 | Inappropriate population |
| Scholz 1979 | No diagnostic test accuracy data |
| Schultis 1969 | No diagnostic test accuracy data |
| Schultis 1969a | Inappropriate reference standard |
| Schultis 1973 | Case‐control study |
| Schwokowski 1979 | Not a primary research study |
| Scottolini 1977 | Not a primary research study |
| Serra 2011 | Inappropriate population |
| Siede 1969 | Not a primary research study |
| Singh 2002 | Inappropriate population |
| Singh 2004 | Inappropriate population |
| Smith 2005 | Inappropriate population |
| Solomon 1978 | Not a primary research study |
| Steinberg 1983 | Inappropriate reference standard |
| Steinberg 1985 | Case‐control study |
| Sternby 1996 | Inappropriate population |
| Strebel 1970 | Inappropriate population |
| Su 2010 | Inappropriate reference standard |
| Suehiro 1984 | Case‐control study |
| Sutton 2009 | Inappropriate population |
| Szalaj 1973 | Case‐control study |
| Testoni 1999 | Inappropriate population |
| Testoni 1999a | Inappropriate population |
| Testoni 2001 | Inappropriate population |
| Thomson 1987 | Inappropriate reference standard |
| Ticktin 1965 | Inappropriate population |
| Tietz 1986 | Inappropriate population |
| Tomaszewski 1984 | Inappropriate population |
| Torrens 1998 | No diagnostic test accuracy data |
| Tournut 1978 | Inappropriate population |
| Treacy 2001 | Inappropriate population |
| Tsai 1988 | Inappropriate population |
| Tseng 2011 | Inappropriate population |
| Tvorogova 1991 | Not a primary research study |
| Uhl 1992 | Case‐control study |
| Uminska 1985 | Case‐control study |
| Van Hee 1979 | Case‐control study |
| Van Ingen 1992 | Case‐control study |
| Varas 1994 | Inappropriate population |
| Vega 1981 | Inappropriate reference standard |
| Ventrucci 1983 | Case‐control study |
| Ventrucci 1985 | Inappropriate reference standard |
| Ventrucci 1986 | Case‐control study |
| Ventrucci 1989 | Case‐control study |
| Ventrucci 1992 | Case‐control study |
| Ventrucci 1994 | Case‐control study |
| Wajda 1978 | Inappropriate population |
| Walker 2013 | Inappropriate population |
| Waller 1971 | Inappropriate population |
| Wang 2009 | Inappropriate population |
| Warshaw 1975 | Case‐control study |
| Weaver 1985 | Inappropriate population |
| Werner 1989 | Case‐control study |
| Wilson 2005 | Case‐control study |
| Winslet 1990 | Inappropriate population |
| Wyatt 1974 | No diagnostic test accuracy data |
| Wyllie 1979 | Inappropriate population |
| Xu 2008 | Case‐control study |
| Xu 2010 | Inappropriate reference standard |
| Yang 1987 | Case‐control study |
| Yang 2005 | Case‐control study |
| Zakrzewska 1982 | Case‐control study |
| Zakrzewska 1985 | Case‐control study |
| Zaninotto 1990 | Case‐control study |
| Zastrow 1973 | Inappropriate population |
| Zeng 2010 | Not a primary research study |
| Zeze 1975 | Case‐control study |
| Zhang 2010 | Case‐control study |
| Zharkovskaia 1978 | Inappropriate population |
| Zheltvai 1969 | Not a primary research study |
Characteristics of studies awaiting classification [ordered by study ID]
Anand 1956a.
| Study characteristics | |||
| Patient sampling | Awaiting full text | ||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Cherry 1953.
| Study characteristics | |||
| Patient sampling | Awaiting full text | ||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Coppola 1954.
| Study characteristics | |||
| Patient sampling | Awaiting full text | ||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Do Prado 1952.
| Study characteristics | |||
| Patient sampling | Awaiting full text | ||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Lippi 2013.
| Study characteristics | |||
| Patient sampling | Awaiting full text | ||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Stimac 1995.
| Study characteristics | |||
| Patient sampling | Awaiting full text | ||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Differences between protocol and review
Although we did not plan to include repeat tests in this review, the diagnostic test accuracy of these index tests on later days of hospital might indicate the performance of these tests in patients with a prolonged period of symptoms. We have therefore analysed and reported this information separately from the tests conducted on admission.
We have accepted visual inspection of pancreas during laparotomy or autopsy as a reference standard. This is at least as good as radiological examination for the diagnosis of acute pancreatitis.
The other methods that we planned but could not perform because of paucity of data are listed in Appendix 11.
Contributions of authors
Gianluca Rompianesi, Angus Hann, and Oluyemi Komolafe were involved in study selection and data extraction. Angus Hann entered data into the Characteristics of studies tables. Stephen Pereira and Brian Davidson commented critically on the review. Kurinchi Selvan Gurusamy selected studies, extracted data, analysed the data, and wrote the review.
Sources of support
Internal sources
-
University College London, UK.
The source supports salary, consumables (including printing, photocopying), and equipment (including laptop, scanner, printer, and any other equipment) necessary to complete the review.
External sources
-
National Institute for Health Research, UK.
The source supports salary, consumables (including printing, photocopying), and equipment (including laptop, scanner, printer, and any other equipment) necessary to complete the review in addition to payment for editorial support.
Declarations of interest
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
GR: none known.
AH: none known.
OK: none known.
SPP: none known.
BRD: none known.
KSG: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Abraham 2011 {published data only}
- Abraham P. Point‐of‐care urine trypsinogen‐2 test for diagnosis of acute pancreatitis. Journal of the Association of Physicians of India 2011;59(4):231‐2. [PubMed] [Google Scholar]
Aysan 2008 {published data only}
- Aysan E, Sevinc M, Basak E, Tardu A, Erturk T. Effectivity of qualitative urinary trypsinogen‐2 measurement in the diagnosis of acute pancreatitis: A randomized, clinical study. Acta Chirurgica Belgica 2008;108(6):696‐8. [DOI] [PubMed] [Google Scholar]
Burkitt 1987 {published data only}
- Burkitt DS. The Rapignost‐Amylase test in acute pancreatitis. British Journal of Surgery 1987;74(11):1063. [DOI] [PubMed] [Google Scholar]
Chang 2011 {published data only}
- Chang JWY, Chung CH. Diagnosing acute pancreatitis: Amylase or lipase?. Hong Kong Journal of Emergency Medicine 2011;18(1):20‐5. [Google Scholar]
Keim 1998 {published data only}
- Keim V, Teich N, Fiedler F, Hartig W, Thiele G, Mossner J. A comparison of lipase and amylase in the diagnosis of acute pancreatitis in patients with abdominal pain. Pancreas 1998;16(1):45‐9. [DOI] [PubMed] [Google Scholar]
Mayumi 2012 {published data only}
- Mayumi T, Inui K, Maetani I, Yokoe M, Sakamoto T, Yoshida M, et al. Validity of the urinary trypsinogen‐2 test in the diagnosis of acute pancreatitis. Pancreas 2012;41(6):869‐75. [DOI] [PubMed] [Google Scholar]
Patt 1966 {published data only}
- Patt HH, Kramer SP, Woel G, Zeitung D, Seligman AM. Serum lipase determination in acute pancreatitis. Clinical appraisal of a new method. Archives of Surgery 1966;92(5):718‐23. [DOI] [PubMed] [Google Scholar]
Saez 2005 {published data only}
- Saez J, Martinez J, Trigo C, Sanchez‐Paya J, Company L, Laveda R, et al. Clinical value of rapid urine trypsinogen‐2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis. World Journal of Gastroenterology 2005;11(46):7261‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viel 1990 {published data only}
- Viel JF, Foucault P, Bureau F, Albert A, Drosdowsky MA. Combined diagnostic value of biochemical markers in acute pancreatitis. Clinica Chimica Acta 1990;189(2):191‐8. [DOI] [PubMed] [Google Scholar]
Wu 2009 {published data only}
- Wu L, Li P, Ling M. The diagnostic value of urinary trypsinogen‐2 screening acute pancreatitis. Chinese Journal of Gastroenterology and Hepatology 2009;18(7):665‐7. [Google Scholar]
References to studies excluded from this review
Abascal 1982 {published data only}
- Abascal J, Acero J, Acero E, Engel M, Gea T. Amylase creatinine clearance ratio modifications in acute pancreatitis and after different types of surgery. Langenbeck's Archives of Surgery 1982;357(3):186. [Google Scholar]
Abate 1979 {published data only}
- Abate S, Marzano LA, Ferulano GP, Fasano S. The nature and significance of amylase‐creatinine clearance in acute pancreatitis. (pre‐ and postoperative results). Chirurgia Gastroenterologica 1979;13(2):277‐89. [Google Scholar]
Acero 1982 {published data only}
- Acero Sanz J, Abascal Morte J, Engel M. Amylase clearance with respect to creatinine clearance in acute pancreatitis. Gastroenterologia y Hepatologia 1982;5(8):416‐21. [Google Scholar]
Adam 1986 {published data only}
- Adam A, Boulanger J, Chapelle JP, Ers P, Reynaert M, Roeseler J. The immunochemical determinations of serum lipase in acute pancreatitis: Further results. Clinical Chemistry 1986;32(10):1987. [PubMed] [Google Scholar]
Adams 1968 {published data only}
- Adams JT, Libertino JA, Schwartz SI. Significance of an elevated serum amylase. Surgery 1968;63(6):877‐84. [PubMed] [Google Scholar]
Adler 1985 {published data only}
- Adler G, Bauerfeind U, Dati F. Rapid diagnosis of acute pancreatitis by an immunochemical latex test for serum pancreatic lipase. Medizinische Klinik 1985;80(18):490‐4. [Google Scholar]
Ahmed 2009 {published data only}
- Ahmed A, Begum I, Aquil N, Atif S, Hussain T, Vohra EA. Hyperamylasemia and acute pancreatitis following organophosphate poisoning. Pakistan Journal of Medical Sciences 2009;25(6):957‐61. [Google Scholar]
Aho 1988 {published data only}
- Aho HJ, Sternby B, Kallajoki M, Nevalainen TJ. Carboxyl ester lipase in human acute pancreatitis. Digestion 1988;40(2):68. [DOI] [PubMed] [Google Scholar]
Aho 1989 {published data only}
- Aho HJ, Sternby B, Kallajoki M, Nevalainen TJ. Carboxyl ester lipase in human tissues and in acute pancreatitis. International Journal of Pancreatology 1989;5(2):123‐34. [DOI] [PubMed] [Google Scholar]
Alvarez 1998 {published data only}
- Alvarez F, Dominguez‐Munoz J. Clinical usefulness of the lipase/amylase ratio and the PMN elastase in acute pancreatitis. Revista Espanola De Enfermedades Digestivas 1998;90(2):126‐7. [PubMed] [Google Scholar]
Anand 1956 {published data only}
- Anand SS, Sawhney CP. Urinary diastase in health and disease; with special reference to its value in acute pancreatitis. Indian Journal of Surgery 1956;18(3):191‐6. [Google Scholar]
Andersen 2010 {published data only}
- Andersen AM, Novovic S, Ersboll AK, Jorgensen LN, Hansen MB. Urinary trypsinogen‐2 dipstick in acute pancreatitis. Pancreas 2010;39(1):26‐30. [DOI] [PubMed] [Google Scholar]
Andre 1967 {published data only}
- Andre R, Leclerc JL, Govaerts JP, Kiekens R, Geertruyden J. The diagnosis of postoperative acute pancreatitis. Acta Chirurgica Belgica 1967;66(3):239‐46. [PubMed] [Google Scholar]
Andren‐Sandberg 1997 {published data only}
- Andren‐Sandberg A, Borgstrom A. Laboratory diagnosis of acute pancreatitis. Amylase analysis is still the leading one, but a "urinary strip" is on its way to be marketed. Lakartidningen 1997;94(26‐27):2451‐3. [PubMed] [Google Scholar]
Andriushchenko 1998 {published data only}
- Andriushchenko VP, Lysiuk IS, Barvins'ka AS. Diagnostic and prognostic significance of urinary amylase in acute biliary pancreatitis. Klinichna Khirurhiia 1998, (3):17‐8. [PubMed] [Google Scholar]
Anonymous 1966 {published data only}
- The Editors of JAMA. Pancreatic lipase in acute pancreatitis. JAMA 1966;196(7):657. [Google Scholar]
Anonymous 2012 {published data only}
- Pancreatitis: Most cases of acute pancreatitis can be diagnosed or ruled out by urinary trypsinogen‐2 dipstick test. Nature Reviews Gastroenterology and Hepatology 2012;9(6):302. [Google Scholar]
Aparisi 1987 {published data only}
- Aparisi Quereda L. Value of pancreatic enzymes in the diagnosis of acute pancreatitis. Medicina Clinica 1987;89(19):829‐34. [PubMed] [Google Scholar]
Apple 1991 {published data only}
- Apple F, Benson P, Preese L, Eastep S, Bilodeau L, Heiler G. Lipase and pancreatic amylase activities in tissues and in patients with hyperamylasemia. American Journal of Clinical Pathology 1991;96(5):610‐4. [DOI] [PubMed] [Google Scholar]
Arzoglou 1983 {published data only}
- Arzoglou PL, Metais P, Ferard G. Diagnostic value of plasma lipase concentrations in pancreatic diseases. Comparison with plasma amylase concentrations. Nouvelle Presse Medicale 1983;12(5):273‐5. [PubMed] [Google Scholar]
Arzoglou 1986 {published data only}
- Arzoglou PL, Lessinger JM, Ferard G. Plasma lipase properties as related to pancreatic condition. Clinical Chemistry 1986;32(1 Pt 1):50‐2. [PubMed] [Google Scholar]
Bacchini 1980 {published data only}
- Bacchini I, Mantovani R, Viti M, Martino G, Sammartano C, Falaschi CF. Study of postoperative amylasemia. Its diagnostic value in postoperative acute pancreatitis (PAP). Minerva Chirurgica 1980;35(6):429‐38. [PubMed] [Google Scholar]
Bachmann 1979 {published data only}
- Bachmann C, Colombo JP, Lorenz E. Determination of alpha‐amylase in acute pancreatitis: Choice of reference parameter?. Schweizerische Medizinische Wochenschrift 1979;109(34):1256‐9. [PubMed] [Google Scholar]
Baillie 1997 {published data only}
- Baillie J. Rapid measurement of urinary trypsinogen‐2 as a screening test for acute pancreatitis. Gastrointestinal Endoscopy 1997;46(4):385‐6. [PubMed] [Google Scholar]
Baillie 1998 {published data only}
- Baillie J. Increased serum trypsinogen 2 and trypsin 2‐1 antitrypsin complex values identify endoscopic retrograde cholangiopancreatography (ERCP)‐induced pancreatitis with high accuracy. Gastrointestinal Endoscopy 1998;47(6):554‐5. [PubMed] [Google Scholar]
Bang 2016 {published data only}
- Bang CS, Kim JB, Park SH, Baik GH, Su KT, Yoon JH, et al. Clinical efficacy of serum lipase subtype analysis for the differential diagnosis of pancreatic and non‐pancreatic lipase elevation. Korean Journal of Internal Medicine 2016;31(4):660‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banks 1996 {published data only}
- Banks PA, Carr‐Locke DL, Slivka A, Dam J, Lichtenstein DR, Hughes M. Urinary trypsinogen activation peptides (tap) are not increased in mild ERCP‐induced pancreatitis. Pancreas 1996;12(3):294‐7. [DOI] [PubMed] [Google Scholar]
Barbado 1977 {published data only}
- Barbado Hernandez FJ, Valdes Canedo M, Vazquez Rodriguez JJ, Gil Aguado A, Ortiz Vazquez J. Value of the amylase/creatinine ratio in the diagnosis of acute pancreatitis. Revista Espanola De Las Enfermedades Del Aparato Digestivo 1977;51(5):547‐56. [PubMed] [Google Scholar]
Barbieri 2016 {published data only}
- Barbieri JS, Riggio JM, Jaffe R. Amylase testing for abdominal pain and suspected acute pancreatitis. Journal of Hospital Medicine 2016;11(5):366‐8. [DOI] [PubMed] [Google Scholar]
Bargum 1983 {published data only}
- Bargum R, Larsen KE, Trostmann AF, Rohr N, Boesby S. The value of pancreas iso‐amylase and lipase in serum in the diagnosis of acute pancreatitis. Scandinavian Journal of Gastroenterology Supplement 1983;18(86):6. [Google Scholar]
Barnett 1986 {published data only}
- Barnett JL, Wilson JA. Alcoholic pancreatitis and parotitis: Utility of lipase and urinary amylase clearance determinations. Southern Medical Journal 1986;79(7):832‐5. [PubMed] [Google Scholar]
Batra 2015 {published data only}
- Batra H, Kumar A, Saha T, Misra P, Ambade V. Comparative study of serum amylase and lipase in acute pancreatitis patients. Indian Journal of Clinical Biochemistry 2015;30(2):230‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batsakis 1965 {published data only}
- Batsakis JG, Stiles DE, Boeve CA, Briere RO. Clinical enzymology. 3. Serum and urinary amylase and lipase in acute pancreatitis. Medical Bulletin 1965;31:117‐20. [PubMed] [Google Scholar]
Benini 1987 {published data only}
- Benini L, Bevilacqua D, Cavallini G, Rizzotti P, Brocco G, Caliari S, et al. Lipase latex test in acute pancreatitis ‐ comparison with lipase‐EIA, trypsin and elastase‐1. Digestive Diseases and Sciences 1987;32(10):1160. [Google Scholar]
Benini 1987a {published data only}
- Benini L, Rizzotti P, Vaona B, Sembenini C, Brocco G, Micciolo R, et al. Elastase‐1 vs trypsin, lipase and amylase serum levels in pancreatic diseases. International Journal of Pancreatology 1987;2(5‐6):361‐70. [DOI] [PubMed] [Google Scholar]
Benini 1992 {published data only}
- Benini L, Bevilacqua D, Brocco G, Pilati S, Bardelli E, Vantini I, et al. Lipase latex test for acute abdominal pain: Comparison with serum lipase, trypsin, elastase and amylase. Italian Journal of Gastroenterology 1992;24(2):61‐4. [PubMed] [Google Scholar]
Berger 1976 {published data only}
- Berger GMB, Cowlin J, Turner TJ. Amylase‐creatinine clearance ratio and urinary excretion of lysozyme in acute pancreatitis and acute duodenal perforation. South African Medical Journal 1976;50(40):1559‐61. [PubMed] [Google Scholar]
Bernard 1959 {published data only}
- Bernard HR, Criscione JR, Moyer CA. The pathologic significance of the serum amylase concentration. An evaluation with special reference to pancreatitis and biliary lithiasis. Archives of Surgery 1959;79(2):311‐8. [DOI] [PubMed] [Google Scholar]
Bernard 1964 {published data only}
- Bernard A. Value of amylase tests in acute pancreatitis. Semaine Des Hopitaux 1964;40:727‐9. [PubMed] [Google Scholar]
Bernard 1964a {published data only}
- Bernard A, Lamelin P, Delattre A. Amylase behavior in acute pancreatitis. Archives des Maladies de l'Appareil Digestif et des Maladies de la Nutrition 1964;53:359‐64. [PubMed] [Google Scholar]
Bernard 1964b {published data only}
- Bernard A, Lamelin P, Delattre A. Research on the behavior of amylase in acute pancreatitis. Journal des Sciences Medicales de Lille 1964;82:73‐94. [PubMed] [Google Scholar]
Berry 1982 {published data only}
- Berry AR, Taylor TV, Davies GC. Diagnostic tests and prognostic indicators in acute pancreatitis. Journal of the Royal College of Surgeons of Edinburgh 1982;27(6):345‐52. [PubMed] [Google Scholar]
Blamey 1983 {published data only}
- Blamey SL, Osborne DH, Gilmour WH. The early identification of patients with gallstone associated pancreatitis using clinical and biochemical factors only. Annals of Surgery 1983;198(5):574‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bluskina 1966 {published data only}
- Bluskina VM. The dextrination method of determining amylase activity in the urine of healthy persons and patients with acute pancreatitis. Laboratornoe Delo 1966;12:744‐6. [PubMed] [Google Scholar]
Bode 1987 {published data only}
- Bode JC, Bode C. Laboratory diagnosis of acute pancreatitis. Deutsche Medizinische Wochenschrift 1987;112(31‐32):1220‐2. [DOI] [PubMed] [Google Scholar]
Borda 1978 {published data only}
- Borda F, Burusco MJ, Garcia Carasusan M. Clinic‐biological correlations of the amylase/creatinine clearance's quotient in acute pancreatitis. Revista Espanola De Las Enfermedades Del Aparato Digestivo 1978;52(3):339‐48. [PubMed] [Google Scholar]
Borgstrom 1984 {published data only}
- Borgstrom A, Lasson A. Trypsin‐alpha 1‐protease inhibitor complexes in serum and clinical course of acute pancreatitis. Scandinavian Journal of Gastroenterology 1984;19(8):1119‐22. [PubMed] [Google Scholar]
Borgstrom 2002 {published data only}
- Borgstrom A, Appelros S, Muller CA, Uhl W, Buchler MW. Role of activation peptides from pancreatic proenzymes in the diagnosis and prognosis of acute pancreatitis. Surgery 2002;131(2):125‐8. [DOI] [PubMed] [Google Scholar]
Bowen 1983 {published data only}
- Bowen M, Cooper EH, McMahon MJ. The diagnosis of acute pancreatitis: Enhanced sensitivity from an ELISA assay of lipase. Biomedicine & Pharmacotherapy 1983;37(8):395‐8. [PubMed] [Google Scholar]
Brailski 1975 {published data only}
- Brailski KH. Acute pancreatitis (etiology, pathogenesis and pathobiochemistry). Vutreshni Bolesti 1975;14(4):1‐11. [PubMed] [Google Scholar]
Branford 1948 {published data only}
- Branford WV. Acute epigastric pain and blood amylase activity. Southern Medicine and Surgery 1948;110(2):41‐4. [PubMed] [Google Scholar]
Brault 1985 {published data only}
- Brault D, Bonnefoy A, Houry S. Acute pancreatitis and biochemical markers. Isoamylase. Pathologie Biologie 1985;33(3):195‐9. [PubMed] [Google Scholar]
Brisinda 1999 {published data only}
- Brisinda G, Maria G, Ferrante A, Civello IM. Evaluation of prognostic factors in patients with acute pancreatitis. Hepato‐Gastroenterology 1999;46(27):1990‐7. [PubMed] [Google Scholar]
Brkic 1966 {published data only}
- Brkic D, Glisic L. Significance of lipase determination in the diagnosis of acute pancreatitis. Medicinski Glasnik 1966;20(8):298. [PubMed] [Google Scholar]
Brodie 1977 {published data only}
- Brodie MJ, Donaldson LA, McIntosh W, Joffe SN. Amylase thermolability ‐ application in diagnosis of acute pancreatitis and pancreatic pseudocysts. Gut 1977;18(5):A415‐6. [PubMed] [Google Scholar]
Brohee 1980 {published data only}
- Brohee D, Rondelez L, Maertelaer V. The effect of age on amylasemia and amylasuria related to creatinuria, using a chromogenic amylase assay. Acta Gastro‐Enterologica Belgica 1980;43(11‐12):493‐501. [PubMed] [Google Scholar]
Brohee 1981 {published data only}
- Brohee D. Reliability and interpretation of the amylase to creatinine clearance ratio. Revue Francaise de Gastro‐Enterologie 1981;168:27‐34. [Google Scholar]
Brohee 1987 {published data only}
- Brohee D, Vanhaeverbeek M. Value of the simultaneous determination of alpha‐amylase and pancreatic lipase for the diagnosis of attacks of acute pancreatitis. Revue Medicale de Bruxelles 1987;8(2):92‐3. [PubMed] [Google Scholar]
Brunner 1980 {published data only}
- Brunner H. The diagnosis of acute pancreatitis. Acta Medica Austriaca 1980;7(1):7‐10. [PubMed] [Google Scholar]
Buchler 1986 {published data only}
- Buchler M, Malfertheiner P, Schoetensack C, Uhl W, Scherbaum W, Beger HG. Value of biochemical and imaging procedures for the diagnosis and prognosis of acute pancreatitis ‐ results of a prospective clinical study. Zeitschrift Fur Gastroenterologie 1986;24(2):100‐9. [PubMed] [Google Scholar]
Budd 1959 {published data only}
- Budd JJ Jr, Walter KE, Harris ML, Knight WA Jr. Urine diastase in the evaluation of pancreatic disease. Gastroenterology 1959;36(3):333‐53. [PubMed] [Google Scholar]
Bunodiere 1975 {published data only}
- Bunodiere M, Soria P, Mathey JC. The value of blood lipase assay in the diagnosis of acute pancreatitis. Annales De Chirurgie 1975;29(6):571‐5. [PubMed] [Google Scholar]
Butler 2000 {published data only}
- Butler J, Harrison M. Urinary trypsinogen to rule out acute pancreatitis in patients with abdominal pain. Journal of Accident & Emergency Medicine 2000;17(5):366‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caillens 1980 {published data only}
- Caillens H, Jaffray P, Benoit MO, Ekindjian OG, Souciet C, Leger L. A study of the specificity of amylases. Measurement of pancreatic isoamylases. Nouvelle Presse Medicale 1980;9(41):3079‐81. [PubMed] [Google Scholar]
Calkins 1968 {published data only}
- Calkins WG. A study of urinary amylase excretion in patients with acute pancreatitis. American Journal of Gastroenterology 1968;49(5):415‐24. [PubMed] [Google Scholar]
Cameron 1973 {published data only}
- Cameron JL, Capuzzi DM, Zuidema GD, Margolis S. Acute pancreatitis with hyperlipemia: The incidence of lipid abnormalities in acute pancreatitis. Annals of Surgery 1973;177(4):483‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1979 {published data only}
- Campbell FC, Imrie CW, Gordon DA, McKay AJ, Oneill J. Re‐examination of the amylase‐to‐creatinine clearance ratio (ACCR) in acute pancreatitis. South African Journal of Surgery 1979;17(3):142. [Google Scholar]
Caputo 1983 {published data only}
- Caputo G, Micali B, Luciano G, Fabiano V, Venuti A. Validity and limits of the relation between amylase and creatinine clearance in the diagnosis of acute postoperative pancreatitis. Annali Italiani Di Chirurgia 1983;55(6):585‐92. [PubMed] [Google Scholar]
Cases 1988 {published data only}
- Cases A, Navarro S, Elena M, Munoz Ruiz I, Lopez Pedret J, Revert L. Usefulness of serum concentrations of pancreatic enzymes in the diagnosis of acute pancreatitis in hemodialyzed patients. Nefrologia 1988;8(4):345‐50. [Google Scholar]
Cevik 2010 {published data only}
- Cevik Y, Kavalci C, Ozer M, Das M, Kiyak G, Ozdogan M. The role of urine trypsinogen‐2 test in the differential diagnosis of acute pancreatitis in the emergency department. Ulusal Travma Ve Acil Cerrahi Dergisi 2010;16(2):125‐9. [PubMed] [Google Scholar]
Chase 1996 {published data only}
- Chase CW, Barker DE, Russell WL, Burns RP. Serum amylase and lipase in the evaluation of acute abdominal pain. American Surgeon 1996;62(12):1028‐33. [PubMed] [Google Scholar]
Chen 1994 {published data only}
- Chen CC, Wang SS, Chao Y, Chen SJ. Serum pancreas‐specific protein in acute pancreatitis. Its clinical utility in comparison with serum amylase. Scandinavian Journal of Gastroenterology 1994;29(1):87. [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen CC. Serum markers in the early assessment of severity of acute pancreatitis: Which is the most useful?. Journal of the Chinese Medical Association 2004;67(9):439‐41. [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Chen YT, Chen CC, Wang SS, Chang FY, Lee SD. Rapid urinary trypsinogen‐2 test strip in the diagnosis of acute pancreatitis. Pancreas 2005;30(3):243‐7. [DOI] [PubMed] [Google Scholar]
Cheng 2004 {published data only}
- Cheng L, Wang X. Changes in etiology, diagnosis and therapeutics in patients with acute pancreatitis during the past ten years: A retrospective study of 725 cases. Chinese Journal of Gastroenterology 2004;9(5):280‐3. [Google Scholar]
Cheung 2015 {published data only}
- Cheung T, Clifford R, Siew S, Gunasekera R. Efficiency of the completion of diagnostic serum amylase for patients presenting with acute abdominal pain. International Journal of Surgery 2015;23:S118. [Google Scholar]
Choi 2009 {published data only}
- Choi JH, Kang NL, Choi SD. Lipase/amylase ratio distinguishes mild acute biliary pancreatitis from nonpancreatitis. Central European Journal of Medicine 2009;4(3):293‐8. [Google Scholar]
Choudhary 2012 {published data only}
- Choudhary RK, Choudhary M. A useful diagnostic and prognostic tool for acute appendicitis and acute pancreatitis. BMJ 2012;344:e1404. [DOI] [PubMed] [Google Scholar]
Christoforidis 2002 {published data only}
- Christoforidis E, Goulimaris I, Kanellos I, Tsalis K, Demetriades C, Betsis D. Post‐ERCP pancreatitis and hyperamylasemia: Patient‐related and operative risk factors. Endoscopy 2002;34(4):286‐92. [DOI] [PubMed] [Google Scholar]
Chylinski 1972 {published data only}
- Chylinski J. The role of the glucose amylase test in the diagnosis of pancreatic diseases. Polish Archives of Internal Medicine 1972;49(6):535‐9. [PubMed] [Google Scholar]
Chylinski 1978 {published data only}
- Chylinski J, Adrich Z. 1‐hour urinary amylase excretion in acute pancreatitis. Polski Tygodnik Lekarski 1978;33(15):581‐2. [PubMed] [Google Scholar]
Cintra 1952 {published data only}
- Cintra Do Prado F, Bove P. Study of the amylase content of the blood in acute pancreatitis. La Prensa Medica Argentina 1952;39(46):2764‐9. [PubMed] [Google Scholar]
Cintra 1953 {published data only}
- Cintra Do Prado F, Bove P. A study of amylase activity of the blood in acute pancreatitis. Resenha Clinica‐Cientifica 1953;22(2):39‐43. [PubMed] [Google Scholar]
Clave 1995 {published data only}
- Clave P, Guillaumes S, Blanco I, Nabau N. Amylase, lipase, pancreatic isoamylase, and phospholipase A in diagnosis of acute pancreatitis. Clinical Chemistry 1995;41(8):1129. [PubMed] [Google Scholar]
Close 1987 {published data only}
- Close P. Simultaneous determination of alpha‐amylase and pancreatic lipase in acute episodes of pancreatitis. Revue Medicale de Bruxelles 1987;8(3):159‐60. [PubMed] [Google Scholar]
Coffey 2014 {published data only}
- Coffey MJ, Nightingale S, Ooi CY. Diagnosing acute pancreatitis in children: What is the diagnostic yield and concordance for serum pancreatic enzymes and imaging within 96 h of presentation?. Pancreatology 2014;14(4):251‐6. [DOI] [PubMed] [Google Scholar]
Coffey 2014a {published data only}
- Coffey MJ, Nightingale S, Ooi CY. Diagnosing acute pancreatitis in children: What is the diagnostic yield and concordance for serum pancreatic enzymes and imaging?. Gastroenterology 2014;146(5 Suppl):S‐625. [DOI] [PubMed] [Google Scholar]
Collins 1982 {published data only}
- Collins RE, Frost SJ, Spittlehouse KE. The p3 iso‐enzyme of serum amylase in the management of patients with acute pancreatitis. British Journal of Surgery 1982;69(7):373‐5. [DOI] [PubMed] [Google Scholar]
Concepcion Martin 2013 {published data only}
- Concepcion Martin M, Guarner‐Argente C, Gemez‐Oliva C, Juanes Borrego A, Torras Colell X, Sainz Saenz‐Torre S, et al. Early blood predictors for post‐ERCP pancreatitis. Pancreatology 2013;13(4 Suppl 1):e8. [Google Scholar]
Concepcion‐Martin 2016 {published data only}
- Concepcion‐Martin M, Gomez‐Oliva C, Juanes A, Mora J, Vidal S, Diez X, et al. Il‐6, Il‐10 and TNFα do not improve early detection of post‐endoscopic retrograde cholangiopancreatography acute pancreatitis: A prospective cohort study. Scientific Reports 2016;6:33492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corfield 1984 {published data only}
- Corfield AP, Cooper MJ, Thompson MH, Mountford R, Whicher JT, Williamson RCN. Hyperamylasemia after ERCP ‐ a sub‐clinical acute pancreatitis. Digestion 1984;30(2):115‐6. [Google Scholar]
Cornett 2010 {published data only}
- Cornett D, Spier BJ, Pfau P. The causes and outcome of pancreatitis associated with serum lipase exceeding 10,000. Gastroenterology 2010;138(5 Suppl):S239‐40. [Google Scholar]
Corsetti 1993 {published data only}
- Corsetti JP, Cox C, Schulz TJ, Arvan DA. Combined serum amylase and lipase determinations for diagnosis of suspected acute pancreatitis. Clinical Chemistry 1993;39(12):2495. [PubMed] [Google Scholar]
Cote 1979 {published data only}
- Cote J, Forest JC, Lacerte M, Rousseau B. Diagnostic value of the amylase/creatinine clearance ratio in the diagnosis of pancreatitis. Union Medicale du Canada 1979;108(5):569‐72. [PubMed] [Google Scholar]
Courtois 1986 {published data only}
- Courtois P, Gnat D, Wenders G, Vertongen F, Franckson JR. Value of simultaneous determinations of alpha‐amylase and pancreatic lipase in the diagnosis of acute pancreatitis. Revue Medicale de Bruxelles 1986;7(9):527‐32. [PubMed] [Google Scholar]
Dalgat 1986 {published data only}
- Dalgat DM, Magomaev M, Medzhidov RT, Kurbanov KM. Diagnosis and treatment of acute pancreatitis. Vestnik Khirurgii Imeni i ‐ i ‐ Grekova 1986;136(4):29‐33. [PubMed] [Google Scholar]
Dankner 1951 {published data only}
- Dankner A, Heifetz CJ. The interrelationship of blood and urine diastase during transient acute pancreatitis. Gastroenterology 1951;18(2):207‐17. [PubMed] [Google Scholar]
Dati 1988 {published data only}
- Dati F, Malfertheiner P. Acute pancreatitis: New diagnostic possibilities. Ricerca in Clinica e in Laboratorio 1988;18(Suppl 1):81‐94. [DOI] [PubMed] [Google Scholar]
de Boer 1986 {published data only}
- Boer HH. Diagnosis of acute pancreatitis. Nederlands Tijdschrift voor Geneeskunde 1986;130(47):2122‐4. [PubMed] [Google Scholar]
Dehesa 1979 {published data only}
- Dehesa M, Segovia E. Amylase test in the diagnosis of acute pancreatitis. Revista de Gastroenterologia de Mexico 1979;44(2):57‐62. [PubMed] [Google Scholar]
Delcourt 1977 {published data only}
- Delcourt A. Amylase‐creatinine clearance in diagnosis of acute pancreatitis. Acta Clinica Belgica 1977;32(5):346‐7. [Google Scholar]
De Leo 1954 {published data only}
- Leo F. Amylase in blood and urine in diagnosis of acute pancreatitis; importance and clinical significance of excess amylase in the blood in acute abdominal disorders of extra‐pancreatic origin. Policlinico ‐ Sezione Pratica 1954;61(36):1173‐9. [PubMed] [Google Scholar]
Deril 1989 {published data only}
- Deril GM, Bosoni T, Lesi C. Pancreatic amylase in serum for differential diagnosis of acute pancreatitis and acute abdominal diseases. Clinical Chemistry 1989;35(10):2142‐3. [PubMed] [Google Scholar]
Deril 1992 {published data only}
- Deril GVM, Ventrucci M. Persistence of increased amylase and lipase concentrations in acute pancreatitis. Clinical Chemistry 1992;38(4):609. [PubMed] [Google Scholar]
Devanath 2009 {published data only}
- Devanath A, Kumari J, Joe J, Peter S, Rajan S, Sabu L, et al. Usefulness of lipase/amylase ratio in acute pancreatitis in South Indian population. Indian Journal of Clinical Biochemistry 2009;24(4):361‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaz 2009 {published data only}
- Diaz Peromingo JA, Alban A, Pesqueira P, Molinos S, Gayol MC. Usefulness of determining urinary trypsinogen‐2 in diagnosis and prognosis of patients with acute pancreatitis. Anales Del Sistema Sanitario De Navarra 2009;32(3):343‐50. [DOI] [PubMed] [Google Scholar]
Distefano 1952 {published data only}
- Distefano G. The glucose and amylase content of the blood in acute abdomen of obscure origin. Rivista di Patologia e Clinica 1952;7(8):164‐6. [Google Scholar]
Domenech 1999 {published data only}
- Domenech Calvet J, Sanchez Cano JJ, Sanchez Marin A, Sanchez Perez J, Guspi Saiz F, Bertran Llusa N, et al. Macroamylasemia in the differential diagnosis of acute pancreatitis. Revista Clinica Espanola 1999;199(7):440‐1. [PubMed] [Google Scholar]
Donaldson 1977 {published data only}
- Donaldson LA, McIntosh W. Cause of misleading serum amylase concentrations in acute pancreatitis. Scottish Medical Journal 1977;22(2):151‐3. [DOI] [PubMed] [Google Scholar]
Dreiling 1974 {published data only}
- Dreiling DA, Leichtling JJ, Janowitz HD. The amylase creatinine clearance ratio. Diagnostic parameter or physiologic phenomenon?. American Journal of Gastroenterology 1974;61(4):290‐6. [PubMed] [Google Scholar]
Dreiung 1954 {published data only}
- Dreiung DA, Greenspan EM, Sanders M. A correlative study of the external pancreatic secretion, the plasma antithrombin titer, the blood amylase concentration, and the serum mucoprotein level in patients with and without pancreatic disease. Gastroenterology 1954;27(6):755‐65. [PubMed] [Google Scholar]
Dronov 2009 {published data only}
- Dronov OI, Koval's'ka IO, Kovalenko AP, Lubenets TV. Outcome of clinical application for medical test: Actim Pancreatitis test for acute pancreatitis diagnostics and control test. Klinichna Khirurhiia 2009, (7‐8):23‐4. [PubMed] [Google Scholar]
Drozdov 2003 {published data only}
- Drozdov VN, Noskova KK. Laboratory diagnostics of acute pancreatitis. Eksperimental'Naia i Klinicheskaia Gastroenterologiia 2003, (6):106‐8. [PubMed] [Google Scholar]
Durr 1977 {published data only}
- Durr HK, Bindrich D, Bode JC. The frequency of marcroamylasemia and the diagnostic value of the amylase to creatinine clearance ratio in patients with elevated serum amylase activity. Scandinavian Journal of Gastroenterology 1977;12(6):701‐5. [DOI] [PubMed] [Google Scholar]
Durr 1983 {published data only}
- Durr GH, Bode C. Diagnostic value of lipase and isoamylase determination. Monitoring studies in patients with proven and suspected pancreatitis. Deutsche Medizinische Wochenschrift 1983;108(49):1876‐80. [DOI] [PubMed] [Google Scholar]
Eckfeldt 1985 {published data only}
- Eckfeldt JH, Kolars JC, Elson MK. Serum tests for pancreatitis in patients with abdominal pain. Archives of Pathology and Laboratory Medicine 1985;109(4):316‐9. [PubMed] [Google Scholar]
Elman 1942 {published data only}
- Elman R. Surgical aspects of acute pancreatitis ‐ with special reference to its frequency as revealed by the serum amylase test. Journal of the American Medical Association 1942;118(15):1265‐8. [Google Scholar]
Engel 1977 {published data only}
- Engel JJ, Rermudez FG, Spellberg MA. Acute pancreatitis, hyperamylasemia and carbohydrate intolerance. GEN 1977;31(3):203‐8. [PubMed] [Google Scholar]
Ermini 1964 {published data only}
- Ermini M. Humoral diagnosis of acute pancreatitis. Minerva Medica 1964;55:3675‐7. [PubMed] [Google Scholar]
Esber 1995 {published data only}
- Esber E, Druzina I, Blum J, Marshall J. Prospective evaluation of the lipase amylase ratio in the diagnosis of acute pancreatitis. Gastroenterology 1995;108(4 Suppl):A352. [Google Scholar]
Esperov 1972 {published data only}
- Esperov BN, Mavrodi VM. The diagnosis and treatment of acute pancreatitis. Khirurgiya 1972;48(7):28‐35. [PubMed] [Google Scholar]
Fabris 1976 {published data only}
- Fabris G, Farini R, Fasolo GF. Acute pancreatitis and hyperlipaemia. Acta Chirurgica Italica 1976;32(4):557‐63. [Google Scholar]
Farkas 1967 {published data only}
- Farkas L, Bak R, Veress O. A rapid and simple method for serum amylase determination in the diagnosis of acute pancreatitis. Orvosi Hetilap 1967;108(3):112‐4. [PubMed] [Google Scholar]
Farrar 1978 {published data only}
- Farrar WH, Calkins G. Sensitivity of the amylase‐creatinine clearance ratio in acute pancreatitis. Archives of Internal Medicine 1978;138(6):958‐62. [PubMed] [Google Scholar]
Finke 1978 {published data only}
- Finke E. Significance of the determination of multiple forms of urinary amylases for the diagnosis of exocrine pancreas diseases. Zeitschrift fur die Gesamte Innere Medizin und Ihre Grenzgebiete 1978;33(24):887‐91. [PubMed] [Google Scholar]
Fiocca 1983 {published data only}
- Fiocca F, Mutiis C, Quondamcarlo C. Acute post‐operative pancreatitis. A study of enzymatic activity of pancreatic origin. Chirurgia Gastroenterologica 1983;17(4):738‐46. [Google Scholar]
Fiorucci 1986 {published data only}
- Fiorucci S, Cassetta C, Fiorucci G, Bassotti G, Pelli MA, Narducci F, et al. Diagnostic effectiveness of immunoreactive serum trypsinogen, pancreatic iso‐amylase and lipase in the diagnosis of acute pancreatic injury in hyperamylasemic patients. Recenti Progressi in Medicina 1986;77(1):1‐6. [PubMed] [Google Scholar]
Fishman 1955 {published data only}
- Fishman L, Doubilet H. A rapid serum amylase test. Journal of the American Medical Association 1955;157(11):908‐9. [DOI] [PubMed] [Google Scholar]
Flamion 1987 {published data only}
- Flamion B, Delhaye M, Horanyi Z. Comparison of elastase‐1 with amylase, lipase, and tryspin‐like immunoreactivity in the diagnosis of acute pancreatitis. American Journal of Gastroenterology 1987;82(6):532‐5. [PubMed] [Google Scholar]
Forell 1959 {published data only}
- Forell MM, Dobovicnik W. On possibilities and limitations of the diagnosis of acute and chronic pancreas diseases on the basis of diastase, lipase and trypsin determinations. Klinische Wochenschrift 1959;37(19):1018‐24. [DOI] [PubMed] [Google Scholar]
Forest 1990 {published data only}
- Forest JC, Turcotte G, Nadeau L, Bergeron J, Deslauriers D, Fruteau B, et al. Serum lipase, total and pancreatic amylases in the diagnosis of acute pancreatitis and other acute abdominal diseases in an emergency room setting. Clinical Chemistry 1990;36(6):1124. [Google Scholar]
Fridhandler 1972 {published data only}
- Fridhandler L, Berk JE, Ueda M. Isolation and measurement of pancreatic amylase in human serum and urine. Clinical Chemistry 1972;18(12):1493‐7. [PubMed] [Google Scholar]
Frost 1978 {published data only}
- Frost SJ. A simple quantitative index of the p3 amylase isoenzyme in the diagnosis of acute pancreatitis. Clinica Chimica Acta 1978;87(1):23‐8. [DOI] [PubMed] [Google Scholar]
Fruchart 1974 {published data only}
- Fruchart JC, Dewailly P, Jaillard J, Sezille G. An automated colorimetric determination of fatty acids. Application to measurements of plasma free fatty acids, of serum lipase and triglyceride lipases in post heparin plasma. Annales De Biologie Clinique 1974;32(3):237‐44. [PubMed] [Google Scholar]
Fruchart 1980 {published data only}
- Fruchart JC, Sezille G, Ghisbain H. Serum lipase assay for clinical use. Critical study. Nouvelle Presse Medicale 1980;9(8):509‐12. [PubMed] [Google Scholar]
Fujiki 1980 {published data only}
- Fujiki T. Clinical study on amylase. Japanese Journal of Gastroenterology 1980;77(9):1444‐53. [PubMed] [Google Scholar]
Fujita 1989 {published data only}
- Fujita C, Kasai C, Kosuge H, Ogata K, Oshima I, Shima K. Radioimmunoassay of human pancreatic amylase with monoclonal antibody. Annals of Clinical Biochemistry 1989;26(Pt 5):416‐21. [DOI] [PubMed] [Google Scholar]
Fukumoto 1981 {published data only}
- Fukumoto K, Wakabayashi A, Takeda Y, Saeki M, Saitoh S, Amatsu T, et al. The amylase clearance/creatinine clearance ratios in acute pancreatitis. Bulletin of the Osaka Medical School 1981;27(1):55‐63. [PubMed] [Google Scholar]
Gambill 1975 {published data only}
- Gambill EE. Hot pancreas, hot belly. Diagnosing pancreatitis. Emergency Medicine (Parsippany) 1975;7(4):179‐85. [ISSN: 0013‐6654] [Google Scholar]
Garden 1985 {published data only}
- Garden OJ, Dominiczak MH, Shenkin A, Carter DC. The diagnosis of acute pancreatitis in the presence of hyperlipaemia. Scottish Medical Journal 1985;30(4):235‐6. [DOI] [PubMed] [Google Scholar]
Gilbert 1955 {published data only}
- Gilbert M. Evaluation of a rapid serum amylase test. Journal of the American Medical Association 1955;159(8):775‐6. [PubMed] [Google Scholar]
Gluskina 1965 {published data only}
- Gluskina VM. The diagnostic value of determining the amylase content in blood in acute pancreatitis. Vestnik Khirurgii Imeni i ‐ i ‐ Grekova 1965;95(10):51‐6. [PubMed] [Google Scholar]
Gomez 2012 {published data only}
- Gomez D, Addison A, Rosa A, Brooks A, Cameron IC. Retrospective study of patients with acute pancreatitis: Is serum amylase still required?. BMJ Open 2012;2(5):e001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez 1978 {published data only}
- Gonzalez Espinoza G, Garcia Garduno JR, Esquivel Lopez A, Gutierrez Samperio C. Amylase/creatinine clearance in the differential diagnosis of acute pancreatitis. La Prensa Medica Mexicana 1978;43(1‐2):21‐4. [PubMed] [Google Scholar]
Grinblatt 1997 {published data only}
- Grinblatt JA. Measurement of urinary trypsinogen‐2 as a screening test for acute pancreatitis. New England Journal of Medicine 1997;337(19):1394‐5. [DOI] [PubMed] [Google Scholar]
Grosberg 1979 {published data only}
- Grosberg SJ, Wapnick S, Purow E, Purow JR. Specificity of serum amylase and amylase creatinine clearance ratio in the diagnosis of acute and chronic pancreatitis. American Journal of Gastroenterology 1979;72(1):41‐5. [PubMed] [Google Scholar]
Gullo 2005 {published data only}
- Gullo L. Acute pancreatitis: Diagnostic gold standard ‐ new perspectives?. In: Ammann RW, Adler G, Buchler MW, Dimagio EP, Sarner M editor(s). Pancreatitis: Advances in pathobiology, diagnosis and treatment. 2005 Falk symposium 143. New York: Springer‐Verlag, 2006:45‐52. [Google Scholar]
Gumaste 1991 {published data only}
- Gumaste VV, Dave PB, Weissman D, Messer J. Lipase/amylase ratio. A new index that distinguishes acute episodes of alcoholic from nonalcoholic acute pancreatitis. Gastroenterology 1991;101(5):1361‐6. [PubMed] [Google Scholar]
Gumaste 1992 {published data only}
- Gumaste V, Dave P, Sereny G. Serum lipase: A better test to diagnose acute alcoholic pancreatitis. American Journal of Medicine 1992;92(3):239‐42. [DOI] [PubMed] [Google Scholar]
Gumaste 1993 {published data only}
- Gumaste V. Serum amylase levels and acute pancreatitis. Gut 1993;34(3):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gumaste 1993a {published data only}
- Gumaste VV, Roditis N, Mehta D, Dave PB. Serum lipase levels in nonpancreatic abdominal pain versus acute pancreatitis. American Journal of Gastroenterology 1993;88(12):2051. [PubMed] [Google Scholar]
Gungor 2011 {published data only}
- Gungor B, Caglayan K, Polat C, Seren D, Erzurumlu K, Malazgirt Z. The predictivity of serum biochemical markers in acute biliary pancreatitis. ISRN Gastroenterology Print 2011;2011:279607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunn 1986 {published data only}
- Gunn IR, Faye S, Clayton MGG. Prospective evaluation of urinary amylase test strip. Lancet 1986;1(8490):1161. [DOI] [PubMed] [Google Scholar]
Guth 1960 {published data only}
- Guth PH. Evaluation of phototurbidimetrictechnics for the determination of serum amylase, lipase and esterase. American Journal of Gastroenterology 1960;33(3):319‐34. [PubMed] [Google Scholar]
Gwozdz 1990 {published data only}
- Gwozdz GP, Steinberg WM, Werner M, Henry JP, Pauley C. Comparative evaluation of the diagnosis of acute pancreatitis based on serum and urine enzyme assays. Clinica Chimica Acta 1990;187(3):243‐54. [DOI] [PubMed] [Google Scholar]
Haas 1985 {published data only}
- Haas GE, Segal LB. Hyperamylasemia and pancreatitis following biliary tract surgery: A prospective study. Journal of the American Osteopathic Association 1985;85(8):519‐27. [PubMed] [Google Scholar]
Haffter 1981 {published data only}
- Haffter D, Reichlin B, Gyr K. Ratio of amylase clearance and creatinine clearance in the diagnosis of acute pancreatitis. Schweizerische Medizinische Wochenschrift Journal Suisse de Medecine 1981;111(22):806‐8. [PubMed] [Google Scholar]
Haffter 1983 {published data only}
- Haffter D, Meyer N, Scholer A, Gyr K. Diagnostic value of serum amylase and serum lipase determination in suspected acute episode of acute or chronic pancreatitis. Schweizerische Medizinische Wochenschrift 1983;113(5):184‐8. [PubMed] [Google Scholar]
Hale 2015 {published data only}
- Hale MF, Sanders DS, Sidhu R. Hyperamylasaemia and acute pancreatitis after double balloon enteroscopy: A prospective study. Gut 2015;64:A83‐4. [Google Scholar]
Hathaway 1983 {published data only}
- Hathaway JA, Kitt D, Wingate B. A comparison of currently used serum lipase and amylase procedures in the serial detection of enzyme elevations in acute pancreatitis. Clinica Chimica Acta 1983;133(3):327‐30. [DOI] [PubMed] [Google Scholar]
Hayakawa 1985 {published data only}
- Hayakawa T, Noda A, Kondo T. Diagnostic usefulness of serum lipase activity measured by enzyme immunoassay in pancreatic diseases. Japanese Journal of Gastroenterology 1985;82(11):2809‐15. [PubMed] [Google Scholar]
Hayakawa 1989 {published data only}
- Hayakawa T, Kondo T, Shibata T, Kitagawa M, Ono H, Sakai Y, et al. Enzyme immunoassay for serum pancreatic lipase in the diagnosis of pancreatic diseases. Gastroenterologia Japonica 1989;24(5):556‐60. [DOI] [PubMed] [Google Scholar]
Hedstroem 1998 {published data only}
- Hedstroem J, Svens E, Kenkimaeki P, Kemppainen E, Puolakkainen P, Haapiainen R, et al. Evaluation of a new urinary amylase test strip in the diagnosis of acute pancreatitis. Scandinavian Journal of Clinical and Laboratory Investigation 1998;58(8):611‐6. [DOI] [PubMed] [Google Scholar]
Hedstrom 1994 {published data only}
- Hedstrom J, Leinonen J, Sainio V, Stenman UH. Time‐resolved immunofluorometric assay of trypsin‐2 complexed with alpha 1‐antitrypsin in serum. Clinical Chemistry 1994;40(9):1761‐5. [PubMed] [Google Scholar]
Hedstrom 1996 {published data only}
- Hedstrom J, Korvuo A, Kenkimaki P, Tikanoja S, Haapiainen R, Kivilaakso E, et al. Urinary trypsinogen‐2 test strip for acute pancreatitis. Lancet 1996;347(9003):729‐31. [DOI] [PubMed] [Google Scholar]
Hedstrom 1996a {published data only}
- Hedstrom J, Puolakkainen P, Sainio V, Kemppainen E, Haapiainen R, Kivilaakso E, et al. Urinary trypsinogen‐2 test strip, a new rapid test for the early diagnosis of acute pancreatitis. Gastroenterology 1996;110(4):A397. [Google Scholar]
Hedstrom 1996b {published data only}
- Hedstrom J, Sainio V, Kemppainen E, Haapiainen R, Kivilaakso E, Schroder T, et al. Serum complex of trypsin 2 and alpha(1) antitrypsin as diagnostic and prognostic marker of acute pancreatitis: Clinical study in consecutive patients. BMJ 1996;313(7053):333‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedstrom 1996c {published data only}
- Hedstrom J, Sainio V, Kemppainen E, Puolakkainen P, Haapiainen R, Kivilaakso E, et al. Urine trypsinogen‐2 as marker of acute pancreatitis. Clinical Chemistry 1996;42(5):685‐90. [PubMed] [Google Scholar]
Hedstrom 2001 {published data only}
- Hedstrom J, Kemppainen E, Andersen J, Jokela H, Puolakkainen P, Stenman UH. A comparison of serum trypsinogen‐2 and trypsin‐2‐alpha 1‐antitrypsin complex with lipase and amylase in the diagnosis and assessment of severity in the early phase of acute pancreatitis. American Journal of Gastroenterology 2001;96(2):424‐30. [DOI] [PubMed] [Google Scholar]
Heer 1983 {published data only}
- Heer M, Pei P, Streuli R. Pancreatitis diagnosis at the bedside with urinary amylase test strip. Schweizerische Medizinische Wochenschrift 1983;113(51):1950‐2. [PubMed] [Google Scholar]
Hegewald 1998 {published data only}
- Hegewald M, Isenberg G, Sterling R, Chak G, Cooper GS, Sivak MY. Evaluation of a rapid urine amylase test in acute pancreatitis. Gastrointestinal Endoscopy 1998;47(4):AB137. [Google Scholar]
Hegewald 1999 {published data only}
- Hegewald MJ, Isenberg G, Sterling RS, Chak A, Cooper GS, Sivak MV. Evaluation of a rapid urine amylase test in acute pancreatitis. Gastrointestinal Endoscopy 1999;49(4):AB72. [Google Scholar]
Hegewald 2001 {published data only}
- Hegewald MJ, Isenberg G, Sterling RK, Cooper GS, Chak A, Sivak MV Jr. Evaluation of a rapid urine amylase test using post‐ERCP hyperamylasemia as a model. American Journal of Gastroenterology 2001;96(9 Suppl):2640‐5. [DOI] [PubMed] [Google Scholar]
Hemingway 1988 {published data only}
- Hemingway DM, Johnson I, Tuffnell DJ, Croton RS. The value of immunoreactive lipase in acute pancreatitis. Annals of the Royal College of Surgeons of England 1988;70(4):195‐6. [PMC free article] [PubMed] [Google Scholar]
Hendry 1987 {published data only}
- Hendry WS, Thomson SR, Scott ST, Davidson AI. Significant hyperamylasaemia in conditions other than acute pancreatitis. Journal of the Royal College of Surgeons of Edinburgh 1987;32(4):213‐5. [PubMed] [Google Scholar]
Henry 1957 {published data only}
- Henry RJ, Sobel C, Berkman S. On the determination of 'pancreatitis lipase' in serum. Clinical Chemistry 1957;3(2):77‐89. [PubMed] [Google Scholar]
Hoferichter 1964 {published data only}
- Hoferichter J. The value of serum enzyme determinations in acute pancreatic diseases. An experimental study. Bruns Beitrage fur Klinischen Chirurgie 1964;208:255‐64. [PubMed] [Google Scholar]
Hoffman 1991 {published data only}
- Hoffman JR, Jaber AJ, Schriger DL. Serum amylase determination in the emergency department evaluation of abdominal pain. Journal of Clinical Gastroenterology 1991;13(4):401‐6. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2014 {published data only}
- Hofmeyr S, Meyer C, Warren BL. Serum lipase should be the laboratory test of choice for suspected acute pancreatitis. South African Journal of Surgery 2014;52(3):72‐4. [DOI] [PubMed] [Google Scholar]
Holdsworth 1984 {published data only}
- Holdsworth PJ, Mayer AD, Wilson DH, Flowers MW, McMahon MJ. A simple screening test for acute pancreatitis. British Journal of Surgery 1984;71(12):958‐9. [DOI] [PubMed] [Google Scholar]
Holmes 2011 {published data only}
- Holmes J, Bhatt A, Lopez R, Stevens T. Serum amylase and lipase level and trend does not correlate with acute pancreatitis severity markers. American Journal of Gastroenterology 2011;106:S69. [Google Scholar]
Horanyi 1984 {published data only}
- Horanyi Z, Delange A, Delhaye M, Demanet H, Flamion B, Quenon M, et al. Comparison of elastase‐1 with alpha‐amylase, lipase and trypsin‐like immunoreactivity during the course of acute pancreatitis ‐ preliminary results. Digestion 1984;30(2):109. [Google Scholar]
Hostein 1976 {published data only}
- Hostein J, Fournet J, Morel F, Denis MC, Bonnet‐Eymard J. Amylase clearance compared to creatinine clearance in diagnosis of acute pancreatitis [Clairance de l'amylase, rapportee a la clairance de la creatinine, dans le diagnostic des pancreatites aigues]. Nouvelle Presse Medicale 1976;5(31):1979‐80. [PubMed] [Google Scholar]
Hostein 1977 {published data only}
- Hostein J, Fournet J, Bonneteymard J. Value of amylase clearance in relation of creatinine clearance ‐ diagnostic significance in acute pancreatitis ‐ 191 cases. Gastroenterologie Clinique Et Biologique 1977;1(4):405. [Google Scholar]
Hostein 1978 {published data only}
- Hostein J, Fournet J, Letoublon C, Roche J, Aubert H, Rachail M, et al. Increase in amylase clearance in relation to creatinin clearance during acute pancreatitis. Digestion 1978;18(1‐2):70‐6. [DOI] [PubMed] [Google Scholar]
Houry 1985 {published data only}
- Houry S, Brault D, Huguier M, Bonnefoy E, Bunodiere M. Diagnostic accuracy of serum lipase and isoamylase isoenzymes in acute pancreatitis. Annales De Medecine Interne 1985;136(6):528. [Google Scholar]
Houry 1989 {published data only}
- Houry S, Brault D, Huguier M. Diagnostic accuracy of serum lipase and pancreatic amylase isoenzyme in acute pancreatitis. Digestive Surgery 1989;6(4):195‐8. [Google Scholar]
Huang 2010 {published data only}
- Huang QL, Qian ZX, Li H. A comparative study of the urinary trypsinogen‐2, trypsinogen activation peptide, and the computed tomography severity index as early predictors of the severity of acute pancreatitis. Hepato‐Gastroenterology 2010;57(102‐103):1295‐9. [PubMed] [Google Scholar]
Huguet 1993 {published data only}
- Huguet J, Castineiras MJ, Fuentesarderiu X. Diagnostic accuracy evaluation using ROC curve analysis. Scandinavian Journal of Clinical & Laboratory Investigation 1993;53(7):693‐9. [DOI] [PubMed] [Google Scholar]
Husain 2004 {published data only}
- Husain L, Oommen, Lord, Cooper JC. Identifying patients with acute pancreatitis in whom serum amylase was normal (< 300 u/dl) using urinary trypsinogen‐2 testing. British Journal of Surgery 2004;91:126‐7. [Google Scholar]
Hwang 2004 {published data only}
- Hwang SJ, Chung JP, Kim YG, Song DH, Lee JS, Baek SS, et al. Usefulness of urinary trypsinogen‐2 dipstick test for diagnosis of acute pancreatitis. Korean Journal of Gastroenterology 2004;43(6):364‐9. [PubMed] [Google Scholar]
Ignjatovic 1997 {published data only}
- Ignjatovic S, Majkic‐Singh N, Mitrovic M, Gvozdenovic M, Todorovic M. Lipase, total and pancreatic amylases as markers of acute pancreatitis identified by ROC curve analysis. European Journal of Laboratory Medicine 1997;5(3):145‐7. [Google Scholar]
Ignjatovic 2000 {published data only}
- Ignjatovic S, Majkic‐Singh N, Mitrovic M, Gvozdenovic M. Biochemical evaluation of patients with acute pancreatitis. Clinical Chemistry and Laboratory Medicine 2000;38(11):1141‐4. [DOI] [PubMed] [Google Scholar]
Im 2010 {published data only}
- Im GY, Squillace BA, Sidhu‐Buonocore S, Grendell JH. Elevated amylase and lipase levels in hyperemesis gravidarum: Accurate markers of acute pancreatitis?. Gastroenterology 2010;138(Supp 1):S‐240. [Google Scholar]
Imrie 1979 {published data only}
- Imrie CW, Campbell FC, Gordon DA, McKay AJ, O'Neill J. Re‐examination of the amylase to creatinine clearance ratio (ACCR) in acute pancreatitis. British Journal of Surgery 1979;66(5):364. [Google Scholar]
Ito 2007 {published data only}
- Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, et al. Relationship between post‐ERCP pancreatitis and the change of serum amylase level after the procedure. World Journal of Gastroenterology 2007;13(28):3855‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobson 1982 {published data only}
- Jacobson G. The amylase to creatinine clearance ratio. Is it a suitable test for the diagnosis of acute pancreatitis?. Scandinavian Journal of Gastroenterology 1982;17(7):833‐7. [DOI] [PubMed] [Google Scholar]
Jam 1978 {published data only}
- Jam I, Shoham M, Wolf RO, Mishkin S. Elevated serum amylase activity in the absence of clinical pancreatic or salivary gland disease: Possible role of acute hypoxemia. American Journal of Gastroenterology 1978;70(5):480‐8. [PubMed] [Google Scholar]
Jang 2007 {published data only}
- Jang T, Uzbielo A, Sineff S, Naunheim R, Scott MG, Lewis LM. Point‐of‐care urine trypsinogen testing for the diagnosis of pancreatitis. Academic Emergency Medicine 2007;14(1):29‐34. [DOI] [PubMed] [Google Scholar]
Jensen 1970 {published data only}
- Jensen HE, Nielsen J, Amdrup E. A clinical evaluation of elevated serum and urine amylase. Scandinavian Journal of Gastroenterology 1970;5(6):543‐7. [PubMed] [Google Scholar]
Jin 2012 {published data only}
- Jin T, Huang W, Javed MA, Xiong JJ, Jiang K, Yang XN, et al. Urinary trypsinogen‐2 is as effective as serum amylase but not serum lipase in the diagnosis of acute pancreatitis. Pancreas 2012;41(8):1372. [Google Scholar]
Jin 2013 {published data only}
- Jin T, Huang W, Javed MA, Xiong JJ, Jiang K, Yang XN, et al. Urinary trypsinogen‐2 is as effective as serum amylase but not serum lipase in the diagnosis of acute pancreatitis. Pancreatology 2013;13(2):e40. [Google Scholar]
Jin 2013a {published data only}
- Jin T, Huang W, Jiang K, Xiong JJ, Xue P, Javed MA, et al. Urinary trypsinogen‐2 for diagnosing acute pancreatitis: A meta‐analysis. Hepatobiliary & Pancreatic Diseases International 2013;12(4):355‐62. [DOI] [PubMed] [Google Scholar]
Johnson 2004 {published data only}
- Johnson CD, Lempinen M, Imrie CW, Puolakkainen P, Kemppainen E, Carter R, et al. Urinary trypsinogen activation peptide as a marker of severe acute pancreatitis. British Journal of Surgery 2004;91(8):1027‐33. [DOI] [PubMed] [Google Scholar]
Jordanov 2009 {published data only}
- Jordanov P, Grigorov G, Todorova S, Angov R, Hristov V, Vukov M. Application of an express urinary trypsinogen‐2 test for the diagnosis of acute pancreatitis. Point of Care 2009;8(1):21‐4. [Google Scholar]
Joshi 2008 {published data only}
- Joshi N, Kumar A, Rani M, Reddy TV, Chandra N. Diagnostic utility of rapid urinary trypsinogen‐2 strip test in acute pancreatitis. Journal of Gastroenterology and Hepatology 2008;23:A133‐4. [Google Scholar]
Junge 1982 {published data only}
- Junge W, Leybold K. Detection of colipase in serum and urine of pancreatitis patients. Clinica Chimica Acta 1982;123(3):293‐302. [DOI] [PubMed] [Google Scholar]
Kaiser 1987 {published data only}
- Kaiser C, Janatsch G, Krusejarres JD. Likelihood calculation by means of different alpha‐amylase and lipase tests in inflammatory pancreatitis. Clinical Chemistry 1987;33(6):998. [Google Scholar]
Kamer 2007 {published data only}
- Kamer E, Unalp HR, Derici H, Tansug T, Onal MA. Early diagnosis and prediction of severity in acute pancreatitis using the urine trypsinogen‐2 dipstick test: A prospective study. World Journal of Gastroenterology 2007;13(46):6208‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kameya 1985 {published data only}
- Kameya A, Hayakawa T, Noda A, Kondo T. Differential determination of serum isoamylase using an amylase inhibitor and its clinical application. American Journal of Gastroenterology 1985;80(1):54‐9. [PubMed] [Google Scholar]
Kameya 1986 {published data only}
- Kameya S, Hayakawa T, Kameya A, Watanabe T. Clinical value of routine isoamylase analysis of hyperamylasemia. American Journal of Gastroenterology 1986;81(5):358‐64. [PubMed] [Google Scholar]
Kapetanos 2007 {published data only}
- Kapetanos D, Kokozidis G, Kinigopoulou P, Xiarchos P, Antonopoulos Z, Progia E, et al. The value of serum amylase and elastase measurements in the prediction of post‐ERCP acute pancreatitis. Hepato‐Gastroenterology 2007;54(74):556‐60. [PubMed] [Google Scholar]
Karlsson 1979 {published data only}
- Karlsson FA, Jacobson G. Renal handling of beta‐2‐microglobulin, amylase and albumin in acute pancreatitis. Acta Chirurgica Scandinavica 1979;145(1):59‐63. [PubMed] [Google Scholar]
Kaw 2001 {published data only}
- Kaw M, Singh S. Serum lipase, C‐reactive protein, and interleukin‐6 levels in ERCP‐induced pancreatitis. Gastrointestinal Endoscopy 2001;54(4):435‐40. [DOI] [PubMed] [Google Scholar]
Kazmierczak 1991 {published data only}
- Kazmierczak SC, Vanlente F, Hodges ED. Diagnostic and prognostic utility of phospholipase A activity in patients with acute pancreatitis ‐ comparison with amylase and lipase. Clinical Chemistry 1991;37(3):356‐60. [PubMed] [Google Scholar]
Kehl 1985 {published data only}
- Kehl O, Buhler H, Munch R. Value of immunoreactive lipase in pancreatic diagnosis. Schweizerische Medizinische Wochenschrift 1985;115(33):1135‐9. [PubMed] [Google Scholar]
Keim 2003 {published data only}
- Keim V, Teich N, Bodeker H, Mossner J. Evaluation of Pankrin (TM), a new serum test for diagnosis of acute pancreatitis. Clinica Chimica Acta 2003;332(1‐2):45‐50. [DOI] [PubMed] [Google Scholar]
Kemppainen 1997 {published data only}
- Kemppainen E, Hedstroem J, Puolakkainen P, Sainio V, Haapiainen R, Perhoniemi V, et al. Simple urinary trypsinogen‐2 test strip for screening acute pancreatitis. Gastroenterology 1997;112(4):A452. [Google Scholar]
Kemppainen 1997a {published data only}
- Kemppainen E, Hedstrom J, Puolakkainen P, Halttunen J, Sainio V, Haapiainen R, et al. Increased serum trypsinogen 2 and trypsin 2‐alpha(1) antitrypsin complex values identify endoscopic retrograde cholangiopancreatography induced pancreatitis with high accuracy. Gut 1997;41(5):690‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemppainen 1997b {published data only}
- Kemppainen E, Hedstrom J, Puolakkainen P, Halttunen J, Sainio V, Haapiainen R, et al. Urinary trypsinogen‐2 test strip in detecting ERCP‐induced pancreatitis. Endoscopy 1997;29(4):247‐51. [DOI] [PubMed] [Google Scholar]
Kemppainen 1997c {published data only}
- Kemppainen EA, Hedstrom JI, Puolakkainen PA, Sainio VS, Haapiainen RK, Perhoniemi V, et al. Rapid measurement of urinary trypsinogen‐2 as a screening test for acute pancreatitis. New England Journal of Medicine 1997;336(25):1788‐93. [DOI] [PubMed] [Google Scholar]
Kemppainen 1997d {published data only}
- Kemppainen EA, Puolakkainen PA, Stenman UK. Measurement of urinary trypsinogen‐2 as a screening test for acute pancreatitis ‐ reply. New England Journal of Medicine 1997;337(19):1394‐5. [DOI] [PubMed] [Google Scholar]
Kerlin 1986 {published data only}
- Kerlin P, Wong L, Harris B, Harris O, Furey L. The role of serum isoamylase and lipase determinations in clinical practice. Australian & New Zealand Journal of Surgery 1986;56(3):215‐9. [DOI] [PubMed] [Google Scholar]
Khrapach 1992 {published data only}
- Khrapach VV, Valetskii VL, Balaban OV. Informativeness of the methods of early clinical laboratory diagnosis of acute pancreatitis. Klinicheskaia Khirurgiia 1992, (4):11‐3. [PubMed] [Google Scholar]
Khvatova 1973 {published data only}
- Khvatova EA. Role of certain enzymatic systems in different forms of acute pancreatitis. Klinicheskaia Khirurgiia 1973;2:48‐53. [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim JH, Yang MJ, Hwang JC, Yoo BM. Usefulness of 4‐h post‐ERCP serum amylase and lipase levels for prediction of post‐ERCP pancreatitis. Journal of Gastroenterology and Hepatology 2015;30:241. [Google Scholar]
King 1995 {published data only}
- King LG, Seelig CB, Ranney JE. The lipase to amylase ratio in acute pancreatitis. American Journal of Gastroenterology 1995;90(1):67. [PubMed] [Google Scholar]
Kirchner 1976 {published data only}
- Kirchner R. Diagnostic significance of alpha‐amylase determination in the serum and urine in acute and chronic pancreatitis. Medizinische Welt 1976;27(38):1771‐3. [PubMed] [Google Scholar]
Kitterer 2015 {published data only}
- Kitterer D, Artunc F, Segerer S, Alscher MD, Braun N, Latus J. Evaluation of lipase levels in patients with nephropathia epidemica ‐ no evidence for acute pancreatitis. BMC Infectious Diseases 2015;15:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobayashi 2011 {published data only}
- Kobayashi K, Sasaki T, Serikawa M, Inoue M, Itsuki H, Chayama K. Assessment of trypsinogen‐2 levels as an early diagnostic for post‐endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas 2011;40(8):1206‐10. [DOI] [PubMed] [Google Scholar]
Koehler 1982 {published data only}
- Koehler DF, Eckfeldt JH, Levitt MD. Diagnostic value of routine isoamylase assay of hyperamylasemic serum. Gastroenterology 1982;82(5 I):887‐90. [PubMed] [Google Scholar]
Kolars 1982 {published data only}
- Kolars J, Ellis C, Levitt MD. Sensitivity of serum total amylase, pancreatic isoamylase lipase measurements in the diagnosis of acute pancreatitis. Gastroenterology 1982;82(5):1104. [Google Scholar]
Kolars 1984 {published data only}
- Kolars JC, Ellis CJ, Levitt MD. Comparison of serum amylase pancreatic isoamylase and lipase in patients with hyperamylasemia. Digestive Diseases and Sciences 1984;29(4):289‐93. [DOI] [PubMed] [Google Scholar]
Kopacova 2010 {published data only}
- Kopacova M, Rejchrt S, Tacheci I, Bures J. No influence of learning curve on increased amylase and lipase and/or acute pancreatitis after oral double balloon enteroscopy. A single‐centre prospective study on 138 consecutive procedures. Gastrointestinal Endoscopy 2010;71(5):AB149. [Google Scholar]
Kubo 1975 {published data only}
- Kubo K. Diagnosis and treatment of acute pancreatitis. Gastroenterologia Japonica 1975;10(3):240. [Google Scholar]
Kulikovsky 2014 {published data only}
- Kulikovsky VF, Iarosh AL, Soloshenko AV, Karpachev AA, Francev SP, Nikolaev SB, et al. Diagnostics and treatment of acute biliary pancreatitis. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(6):1366‐9. [Google Scholar]
Kurti 2011 {published data only}
- Kurti F, Basho J, Zaimi E. Urinary trypsinogen test in diagnosing acute pancreatitis. Pancreatology 2011;11:14. [Google Scholar]
Kusama 1956 {published data only}
- Kusama J, Matsuoka S, Nakamura Y, Takeda S. Significance of urinary amylase determination in surgical abdominal diseases. Shinshu Medical Journal 1956;5(1):44‐7. [Google Scholar]
Kutter 1983 {published data only}
- Kutter D, Humbel R, Piergiovanni C, Szody A. Determination of urinary amylase using a test strip. Contribution to the emergency diagnosis of acute pancreatitis. Zfa Zeitschrift fur Allgemeinmedizin 1983;59(23):1271‐4. [PubMed] [Google Scholar]
Kylanpaa‐Back 1999 {published data only}
- Kylanpaa‐Back L, Kemppainen E, Hedstrom J, Haapiainen R. Reliable screening for acute pancreatitis with rapid urine trypsinogen‐2 test strip. Gastroenterology 1999;116(2):G4946. [DOI] [PubMed] [Google Scholar]
Kylanpaa‐Back 2000 {published data only}
- Kylanpaa‐Back ML, Kemppainen E, Puolakkainen P, Hedstrom J, Haapiainen R, Perhoniemi V, et al. Reliable screening for acute pancreatitis with rapid urine trypsinogen‐2 test strip. British Journal of Surgery 2000;87(1):49‐52. [DOI] [PubMed] [Google Scholar]
Kylanpaa‐Back 2000a {published data only}
- Kylanpaa‐Back ML, Kemppainen E, Puolakkainen PA, Hedstrom J, Haapiainen R, Stenman UH. Comparison of urine trypsinogen‐2 test strip with serum lipase in the diagnosis of acute pancreatitis. Gastroenterology 2000;118(4):A162. [PubMed] [Google Scholar]
Kylanpaa‐Back 2002 {published data only}
- Kylanpaa‐Back ML, Kemppainen E, Puolakkainen P, Hedstrom J, Haapiainen R, Korvuo A, et al. Comparison of urine trypsinogen‐2 test strip with serum lipase in the diagnosis of acute pancreatitis. Hepato‐Gastroenterology 2002;49(46):1130‐4. [PubMed] [Google Scholar]
Lacher 1986 {published data only}
- Lacher DA, Harize MB. Determination of amylase activity in serum by using a wheat germ inhibitor with the DuPont ACA. Clinical Chemistry 1986;32(8):1539‐41. [PubMed] [Google Scholar]
Lankisch 1977 {published data only}
- Lankisch PG, Koop H, Otto J. Specificity of increased amylase to creatinine clearance ratio in acute pancreatitis. Digestion 1977;16(1‐2):160‐4. [DOI] [PubMed] [Google Scholar]
Lankisch 1977a {published data only}
- Lankisch PG, Wolfrum DI, Koop H, Winckler K. Amylase‐creatinine clearance ratio (CAM‐CCR) and renal tubular function in acute pancreatitis (AP) ‐ lack of specificity. Irish Journal of Medical Science 1977;146(Suppl 1):28. [Google Scholar]
Lankisch 1994 {published data only}
- Lankisch PG, Haseloff M, Becher R. No parallel between the biochemical course of acute pancreatitis and morphologic findings. Pancreas 1994;9(2):240‐3. [DOI] [PubMed] [Google Scholar]
Lankisch 1994a {published data only}
- Lankisch PG, Petersen M, Gottesleben F. High, not low, amylase and lipase levels indicate severe acute pancreatitis. Zeitschrift Fur Gastroenterologie 1994;32(4):213‐5. [PubMed] [Google Scholar]
Lankisch 2006 {published data only}
- Lankisch PG, Weber‐Dany B, Doobe C, Finger T, Maisonneuve P, Lowenfels AB, et al. Pankrin: A new parameter for the diagnosis of acute pancreatitis in cases of late clinical presentation. Pancreas 2006;32(3):330‐1. [DOI] [PubMed] [Google Scholar]
Lankisch 2012 {published data only}
- Lankisch G. Increased lipase levels. Deutsche Medizinische Wochenschrift 2012;137(24):1317. [DOI] [PubMed] [Google Scholar]
Laurent‐Puig 1992 {published data only}
- Laurent‐Puig P, Boutron A, Briantais MJ, Vahedi K, Fritsch J, Choury AD, et al. Lipase/amylase ratio in pancreatitis: An etiologic index?. Gastroenterology 1992;103(1):353‐4. [DOI] [PubMed] [Google Scholar]
Lauschke 1963 {published data only}
- Lauschke G, Schmechel C. Simple laboratory studies in the diagnosis of acute pancreatitis. Medizinische Monatsschrift 1963;17:497‐9. [PubMed] [Google Scholar]
Leclerc 1983 {published data only}
- Leclerc P, Forest JC. Variations in amylase isoenzymes and lipase during acute pancreatitis, and in other disorders causing hyperamylasemia. Clinical Chemistry 1983;29(6):1020‐3. [PubMed] [Google Scholar]
Lee 1995 {published data only}
- Lee MH, Chen CF, Ng JL, Chung SY, Lin CY. Serum lipase is a better test in the diagnosis of acute episodes of alcoholic pancreatitis. Chinese Journal of Gastroenterology 1995;12(1):1‐8. [Google Scholar]
Lee 1996 {published data only}
- Lee C, Zenilman ME. Use of biochemical markers for prognosis and diagnosis in patients with acute pancreatitis. Problems in General Surgery 1996;13(4):37‐45. [Google Scholar]
Lempinen 2001 {published data only}
- Lempinen M, Kylanpaa‐Back ML, Stenman UH, Puolakkainen P, Haapiainen R, Finne P, et al. Predicting the severity of acute pancreatitis by rapid measurement of trypsinogen‐2 in urine. Clinical Chemistry 2001;47(12):2103‐7. [PubMed] [Google Scholar]
Lempinen 2003 {published data only}
- Lempinen M, Stenman UH, Finne P, Puolakkainen P, Haapiainen R, Kemppainen E. Trypsinogen‐2 and trypsinogen activation peptide in urine of patients with acute pancreatitis. Journal of Surgical Research 2003;111(2):267‐73. [DOI] [PubMed] [Google Scholar]
Lessinger 1994 {published data only}
- Lessinger JM, Ferard G. Plasma pancreatic lipase activity: From analytical specificity to clinical efficiency for the diagnosis of acute pancreatitis. European Journal of Clinical Chemistry and Clinical Biochemistry 1994;32(5):377. [DOI] [PubMed] [Google Scholar]
Levitt 1975 {published data only}
- Levitt MD, Cooperband SR. Increased renal clearance of amylase in pancreatitis. New England Journal of Medicine 1975;292(7):364‐5. [DOI] [PubMed] [Google Scholar]
Lifton 1974 {published data only}
- Lifton LJ, Slickers KA, Katz LA, Pragay DA. Amylase vs lipase in diagnosis of acute pancreatitis. Clinical Chemistry 1974;20(7):880. [Google Scholar]
Lifton 1974a {published data only}
- Lifton LJ, Slickers KA, Pragay DA, Katz LA. Pancreatitis and lipase. A re‐evaluation with a five minute turbidimetric lipase determination. JAMA 1974;229(1):47‐50. [DOI] [PubMed] [Google Scholar]
Ligny 1987 {published data only}
- Ligny G, Meunier JC, Hayard P, Ligny C, Dehout F, Eukem P, et al. Sensitivity and specificity of blood amylase, of the ratio of amylase and creatinine clearance, and of the ratio of urinary amylase and creatinine in the diagnosis of acute pancreatitis. Revue Medicale de Bruxelles 1987;8(3):125‐32. [PubMed] [Google Scholar]
Lin 1989 {published data only}
- Lin XZ, Wang SS, Tsai YT, Lee SD, Shiesh SC, Pan HB, et al. Serum amylase, isomylase, and lipase in the acute abdomen. Their diagnostic value for acute pancreatitis. Journal of Clinical Gastroenterology 1989;11(1):47‐52. [DOI] [PubMed] [Google Scholar]
Lindahl 1979 {published data only}
- Lindahl F, Siemssen OJ, Egedorf J. Value of the amylase/creatinine clearance ratio in the diagnosis of acute pancreatitis. Ugeskrift for Laeger 1979;141(51):3516‐8. [PubMed] [Google Scholar]
Liyanage 2012 {published data only}
- Liyanage C, Nawaratne NMM, Fernandopulle N, Wijesundara DMGC, Nawaratne NJ. 'Amylase levels in diagnosing post ERCP pancreatitis'; is it a significant indicator without abdominal pain?. HPB 2012;14:349. [Google Scholar]
Logrono 2000 {published data only}
- Logrono JR, Grijalba A, Berrueta C, Allue JA, Zugarramurdi MP, Garcia‐Merlo S. Urinary trypsinogen‐2 as biochemical marker of acute pancreatitis. Revista De Diagnostico Biologico 2000;49(4):208‐12. [Google Scholar]
Long 1976 {published data only}
- Long WB, Grider JR. Amylase isoenzyme clearances in normal subjects and in patients with acute pancreatitis. Gastroenterology 1976;71(4):589‐93. [PubMed] [Google Scholar]
Loo 1992 {published data only}
- Loo LK, Charles‐Marcel ZL, Fisher F, Richardson T, Castro D, Haddad M, et al. Diagnosing a diagnostic study ‐ lipase/amylase ratio [7]. Gastroenterology 1992;102(5):1827‐8. [DOI] [PubMed] [Google Scholar]
Lott 1985 {published data only}
- Lott JA, Ellison EC. Amylase assay and diagnosis of pancreatic disease. Clinical Chemistry 1985;31(8):1263. [PubMed] [Google Scholar]
Lott 1985a {published data only}
- Lott JA, Speicher CE, Nemesanszky E. Is serum amylase an obsolete test in the diagnosis of acute pancreatitis?. Archives of Pathology and Laboratory Medicine 1985;109(4):314‐5. [PubMed] [Google Scholar]
Lott 1986 {published data only}
- Lott JA, Patel ST, Sawhney AK. Assays of serum lipase: Analytical and clinical considerations. Clinical Chemistry 1986;32(7):1290‐302. [PubMed] [Google Scholar]
Lott 1991 {published data only}
- Lott JA. The value of clinical laboratory studies in acute pancreatitis. Archives of Pathology & Laboratory Medicine 1991;115(4):325‐6. [PubMed] [Google Scholar]
Lott 1991a {published data only}
- Lott JA, Lu CJ. Lipase isoforms and amylase isoenzymes: Assays and application in the diagnosis of acute pancreatitis. Clinical Chemistry 1991;37(3):361‐8. [PubMed] [Google Scholar]
Luengo 1996 {published data only}
- Luengo L, Castellote M, Ros S, Feliu F, Vadillo J, Olona C. Clinical usefulness of the determination of lipase/amylase ratio and polymorphonuclear elastase in patients with acute pancreatitis. Revista Espanola De Enfermedades Digestivas 1996;88(8):551‐4. [PubMed] [Google Scholar]
Lunghi 1984 {published data only}
- Lunghi C, Berizzi GF, Prestipino F. Limitation of amylase creatinine clearance ratio in early diagnosis of postoperative acute pancreatitis. Giornale di Chirurgia 1984;5(5):461‐4. [Google Scholar]
MacArthur 2013 {published data only}
- MacArthur KL, Martin CR, Berzin TM, Shapiro NI, Sheth S, Sawhney M, et al. Sa1377 lipase: A poor diagnostic marker for acute pancreatitis in the intensive care unit. Gastroenterology 2013;144(Suppl 1):S‐278. [Google Scholar]
Macgregor 1976 {published data only}
- Macgregor IL, Zakim D. Studies on serum amylase in normal man and in acute pancreatitis. Australian and New Zealand Journal of Medicine 1976;6(6):551‐6. [DOI] [PubMed] [Google Scholar]
Maekelae 1997 {published data only}
- Maekelae A, Kuusi T, Schroeder T. Serum phospholipase a˜2, amylase, lipase, and urinary amylase activities in relation to the severity of acute pancreatitis. European Journal of Surgery 1997;163(12):915‐22. [PubMed] [Google Scholar]
Majkicsingh 1986 {published data only}
- Majkicsingh N, Popovic B, Spasic S, Popovic D, Ivanovic I. The significance of lipase enzyme‐immunoassay for diagnosis of acute pancreatitis. Acta Pharmaceutica Jugoslavica 1986;36(3):319‐27. [Google Scholar]
Malfertheiner 1989 {published data only}
- Malfertheiner P, Nevalainen T, Uhl W, Schadlich H, Buchler M. Diagnostic value of immunoreactive phospholipase a2 in acute pancreatitis. Klinische Wochenschrift 1989;67(3):183‐5. [DOI] [PubMed] [Google Scholar]
Mangano 1990 {published data only}
- Mangano MM, Theurer HA, Horowitz GL. Lack of added value of lipase in diagnosis of acute pancreatitis. Clinical Chemistry 1990;36(6):1123. [Google Scholar]
Marten 1976 {published data only}
- Marten A, Elias E, Summerfield JA, Scott J, Sherlock S. Proceedings: Specificity of the renal amylase: Creatinine clearance ratio in the diagnosis of acute pancreatitis and in detecting the incidence of pancreatitis after ERCP. Gut 1976;17(5):385. [PubMed] [Google Scholar]
Masoero 1978 {published data only}
- Masoero G, Andriulli A, Pellegrino S. Amylase to creatinine clearance ratio: Clinical value in the diagnosis of pancreatic disorders. Italian Journal of Gastroenterology 1978;10(4):232‐4. [Google Scholar]
Masoero 1980 {published data only}
- Masoero G, Andriulli A, Recchia S, Marchetto M, Benitti V, Verme G. Trypsin‐like immunoreactivity in the diagnosis of acute pancreatitis. Scandinavian Journal of Gastroenterology ‐ Supplement 1980;62:21‐5. [PubMed] [Google Scholar]
Massey 1985 {published data only}
- Massey TH. Efficiency in the diagnosis of acute pancreatitis increased by improved electrophoresis of amylase isoenzyme‐p3 on cellulose‐acetate. Clinical Chemistry 1985;31(1):70‐5. [PubMed] [Google Scholar]
Mayer 1985 {published data only}
- Mayer AD, McMahon MJ, Holdsworth PJ. Screening for acute pancreatitis: A rapid assay for plasma lipase. British Journal of Surgery 1985;72(6):436‐7. [DOI] [PubMed] [Google Scholar]
McCulloch 1984 {published data only}
- McCulloch PG, Oates S, Campbell R, Imrie CW. Serum lipase, a better screening test for acute pancreatitis. Gut 1984;25(5):A575‐6. [Google Scholar]
McIntosh 1976 {published data only}
- McIntosh W, Donaldson LA, Joffe SN. Amylase thermolability and its application in diagnosis of acute pancreatitis. European Surgical Research 1976;8:72‐3. [PubMed] [Google Scholar]
McMahon 1981 {published data only}
- McMahon MJ, Hodgson J. Diagnosis of acute pancreatitis ‐ have we improved on the plasma amylase. Gut 1981;22(5):A435. [Google Scholar]
McMahon 1982 {published data only}
- McMahon MJ, Bowen M, Cooper EH. A new immunoreactive lipase assay (ELISA) ‐ a more sensitive diagnostic test for acute pancreatitis. British Journal of Surgery 1982;69(11):684. [Google Scholar]
Merina 1957 {published data only}
- Merina VM, Volkova LM. Diagnostic significance of diastasuria in acute pancreatitis. Vestnik Khirurgii Imeni I 1957;79(7):36‐42. [PubMed] [Google Scholar]
Millat 1999 {published data only}
- Millat B. Acute pancreatitis. Etiology, diagnosis, prognosis. Revue du Praticien 1999;49(3):311‐9. [PubMed] [Google Scholar]
Miller 1973 {published data only}
- Miller SF, Whitaker JR Jr, Snyder RD. Incidence of elevated serum amylase levels and pancreatitis after upper abdominal surgery. American Journal of Surgery 1973;125(5):535‐7. [DOI] [PubMed] [Google Scholar]
Millson 1998 {published data only}
- Millson CE, Charles K, Poon P, MacFie J, Mitchell CJ. A prospective study of serum pancreatic elastase‐1 in the diagnosis and assessment of acute pancreatitis. Scandinavian Journal of Gastroenterology 1998;33(6):664‐8. [DOI] [PubMed] [Google Scholar]
Mimoz 1993 {published data only}
- Mimoz O, Laurent‐Puig P, Benhajhmida R, Briantais MJ. Lipase: Amylase ratio in acute pancreatitis: An aetiological index?. European Journal of Gastroenterology & Hepatology 1993;5(5):361. [Google Scholar]
Mingxin 2001 {published data only}
- Mingxin F, Xiong MA, Cuihua X. Clinical value of rapid measurement of urinary trypsinogen‐2 in screening acute pancreatitis. Chinese Journal of Gastroenterology 2001;6(3):139‐40. [Google Scholar]
Mirmiranyazdy 1995 {published data only}
- Mirmiranyazdy A, Bank S, Pan CQ, Stark B. Changes of serum amylase and symptoms in ERCP induced acute pancreatitis. Gastroenterology 1995;108(4):A376. [Google Scholar]
Mohamed 1989 {published data only}
- Mohamed AH, Danilewitz MD, Becker PJ, Jeppe C. The value of routine pancreatic iso‐amylase measurements in the diagnosis of pancreatitis. South African Medical Journal [Suid‐Afrikaanse Tydskrif Vir Geneeskunde] 1989;76(Sep):258‐62. [PubMed] [Google Scholar]
Moller‐Petersen 1983 {published data only}
- Moller‐Petersen J, Delikaris PG, Kloerke M, Dati F. Comparison of cathodic trypsin‐like immunoreactivity, pancreatic lipase and pancreatic isoamylase in the diagnosis of acute pancreatitis in patients with acute abdominal pain. Digestion 1983;28(1):48‐9. [DOI] [PubMed] [Google Scholar]
Moller‐Petersen 1985 {published data only}
- Moller‐Petersen J, Klaerke M, Dati F, Toth T. Immunochemical qualitative latex agglutination test for pancreatic lipase in serum evaluated for use in diagnosis of acute pancreatitis. Clinical Chemistry 1985;31(7):1207‐10. [PubMed] [Google Scholar]
Moller‐Petersen 1986 {published data only}
- Moller‐Petersen J, Klaerke M, Dati F. Evaluation and comparison of cathodic trypsin‐like immunoreactivity, pancreatic lipase and pancreatic isoamylase in the diagnosis of acute pancreatitis in 849 consecutive patients with acute abdominal pain. Clinica Chimica Acta 1986;157(2):151‐65. [DOI] [PubMed] [Google Scholar]
Morel 1981 {published data only}
- Morel C, Ferraina P, Valente A, Cervio R. Correlation of the clearance of amylase and creatinine in acute pancreatitis and other pathologies. Acta Gastroenterologica Latinoamericana 1981;11(1):171‐93. [PubMed] [Google Scholar]
Murray 1976 {published data only}
- Murray WR, Mackay C. Amylase creatinine clearance ratio in acute pancreatitis. European Surgical Research 1976;8:71. [DOI] [PubMed] [Google Scholar]
Murray 1977 {published data only}
- Murray WR, Mackay C. The amylase creatinine clearance ratio in acute pancreatitis. British Journal of Surgery 1977;64(3):189‐91. [DOI] [PubMed] [Google Scholar]
Murray 1980 {published data only}
- Murray RN, Fung WP, Masarei JRL, Tan EGC. P3 amylase isoenzyme in the diagnosis of pancreatitis. Annals of the Academy of Medicine Singapore 1980;9(4):525‐8. [PubMed] [Google Scholar]
Navarro 1984 {published data only}
- Navarro Colas S. Value of the various pancreatic enzymes in the diagnosis of acute pancreatitis. Medicina Clinica 1984;82(20):893‐5. [PubMed] [Google Scholar]
Navarro 1987 {published data only}
- Navarro S, Aused R, Elena M, Casals E, Garciapuges AM, Adrian MJ, et al. The importance of late determinations of serum amylase, lipase and p3 isoamylase in the diagnosis of acute pancreatitis. Revista Clinica Espanola 1987;181(7):368‐71. [PubMed] [Google Scholar]
Nechai 1973 {published data only}
- Nechai AI, Ivanov VA. Diagnostic value of the determination of amylase in the urine in diagnosis of postoperative pancreatic necrosis. Vestnik Khirurgii Imeni I 1973;110(3):117‐8. [PubMed] [Google Scholar]
Nechiporuk 1982 {published data only}
- Nechiporuk VM, Ostrovskii VP, Boliukh BA. Diagnosis and treatment of acute pancreatitis. Klinicheskaia Khirurgiia 1982, (11):21‐4. [PubMed] [Google Scholar]
Neoptolemos 1990 {published data only}
- Neoptolemos JP, London NJM. Serum enzymes and other laboratory tests in acute pancreatitis (i). British Journal of Surgery 1990;77(6):715. [DOI] [PubMed] [Google Scholar]
Neoptolemos 1993 {published data only}
- Neoptolemos JP. Serum amylase levels and acute pancreatitis ‐ reply. Gut 1993;34(3):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neoptolemos 2000 {published data only}
- Neoptolemos JP, Kemppainen EA, Mayer JM, Fitzpatrick JM, Raraty MG, Slavin J, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: A multicentre study. Lancet 2000;355(9219):1955‐60. [DOI] [PubMed] [Google Scholar]
Neovius 1984 {published data only}
- Neovius G, Schain M, Tryding N. A study of lipase, amylase and isoamylase in acute pancreatitis. Lakartidningen 1984;81(25):2514‐6. [PubMed] [Google Scholar]
Neves 1985 {published data only}
- Neves MM, Michelson N, Borges DR. The relationship between amylase and creatinine clearance is useful in the evaluation of acute pancreatitis, but it is not specific. Revista Paulista de Medicina 1985;103(5):265‐6. [PubMed] [Google Scholar]
Newland 2002 {published data only}
- Newland KD, Chan SB. Serum lipase and amylase in the emergency department diagnosis of acute pancreatitis in elderly patients. Annals of Emergency Medicine 2002;40(4 Suppl):S83. [Google Scholar]
Oellerich 1983 {published data only}
- Oellerich M, Seiler D, Nagel D. Test‐strips for the rapid determination of alpha‐amylase in urine: A multi‐centre study. Deutsche Medizinische Wochenschrift 1983;108(35):1308‐11. [PubMed] [Google Scholar]
Orda 1982 {published data only}
- Orda R, Orda S, Baron J, Wiznitzer T. Diagnosis of acute pancreatitis using the amylase‐creatinine clearance ratio and radionuclide hepatobiliary and pancreas imaging. World Journal of Surgery 1982;6(3):347‐51. [DOI] [PubMed] [Google Scholar]
Orda 1984 {published data only}
- Orda R, Orda S, Baron J, Wiznitzer T. Lipase turbidimetric assay and acute pancreatitis. Digestive Diseases and Sciences 1984;29(4):294‐6. [DOI] [PubMed] [Google Scholar]
Orebaugh 1994 {published data only}
- Orebaugh SL. Normal amylase levels in the presentation of acute pancreatitis. American Journal of Emergency Medicine 1994;12(1):21. [DOI] [PubMed] [Google Scholar]
Osipov 1970 {published data only}
- Osipov BK, Shimeliovich LB, Elkonin BL. Differential diagnosis of acute pancreatitis and myocardial infarct. Khirurgiia 1970;46(4):85‐9. [PubMed] [Google Scholar]
Ostrovskii 2012 {published data only}
- Ostrovskii VK, Rodionov PN, Makarov SV. Some of criteria in the evaluation of severity and prognosis with different forms of acute pancreatitis. Anesteziologiia i Reanimatologiia 2012, (3):56‐9. [PubMed] [Google Scholar]
Otsuki 1995 {published data only}
- Otsuki M. Usefulness of amylase isoenzyme determination for the diagnosis of pancreatic diseases. Nippon Rinsho 1995;53(5):1184‐91. [PubMed] [Google Scholar]
Pace 1985 {published data only}
- Pace BW, Bank S, Wise L, Burson LC, Borrero E. Amylase isoenzymes in the acute abdomen: An adjunct in those patients with elevated total amylase. American Journal of Gastroenterology 1985;80(11):898‐901. [PubMed] [Google Scholar]
Pacheco 2003 {published data only}
- Pacheco RC, Nishioka Sde A, Oliveira LC. Validity of serum amylase and lipase in the differential diagnosis between acute/acutized chronic pancreatitis and other causes of acute abdominal pain. Arquivos De Gastroenterologia 2003;40(4):233‐8. [DOI] [PubMed] [Google Scholar]
Pakkala 2012 {published data only}
- Pakkala AK, Mohan R. The utility of serum amylase as a marker for acute pancreatitis. Australasian Medical Journal 2012;5(1):90‐1. [Google Scholar]
Panteghini 1989 {published data only}
- Panteghini M, Pagani F. Diagnostic value of measuring pancreatic lipase and the p3 isoform of the pancreatic amylase isoenzyme in serum of hospitalized hyperamylasemic patients. Clinical Chemistry 1989;35(3):417‐21. [PubMed] [Google Scholar]
Panteghini 1990 {published data only}
- Panteghini M, Pagani F. Diagnostic value of measuring pancreatic isoamylase with a double‐monoclonal antibody immunoassay in serum of hospitalized hyperamylasemic patients. Journal of Clinical Laboratory Analysis 1990;4(6):449‐52. [DOI] [PubMed] [Google Scholar]
Panteghini 1992 {published data only}
- Panteghini M. Electrophoretic fractionation of pancreatic lipase. Clinical Chemistry 1992;38(9):1712‐6. [PubMed] [Google Scholar]
Papaioannou 1996 {published data only}
- Papaioannou P, Fytili C, Tsitamidou R, Foca Z, Nitsas B, Progia E. Evaluation of total and pancreatic amylase determination methods for the diagnosis of acute pancreatitis. Chimika Chronika 1996;25(3):146. [Google Scholar]
Papp 1969 {published data only}
- Papp M, Horvath JE, Nemeth PE. Pancreatic lymph flow and lipase activity in acute pancreatitis (preliminary report). Orvosi Hetilap 1969;110(6):295‐6. [PubMed] [Google Scholar]
Parodi 1983 {published data only}
- Parodi HC, Colombato LA, Balsamo N. Amylase‐creatinine ratio in the diagnosis of acute pancreatic and biliary pathology. Medicina 1983;43(4):375‐82. [PubMed] [Google Scholar]
Pereiaslov 1999 {published data only}
- Pereiaslov AA. Prognostic role trypsinogen‐activating peptide in an acute pancreatitis. Klinichna Khirurhiia 1999, (10):9‐10. [PubMed] [Google Scholar]
Peromingo 2009 {published data only}
- Peromingo JAD, Alban A, Pesqueira P, Molinos S, Gayol MC. Usefulness of determining urinary trypsinogen‐2 in diagnosis and prognosis of patients with acute pancreatitis. Anales Del Sistema Sanitario De Navarra 2009;32(3):343‐50. [DOI] [PubMed] [Google Scholar]
Pezzilli 1992 {published data only}
- Pezzilli R, Billi P, Fiocchi M, Ossani M, Sprovieri G, Fontana G. Serum lipase assay. A test of choice in acute pancreatitis. Panminerva Medica 1992;34(1):30‐4. [PubMed] [Google Scholar]
Pezzilli 1992a {published data only}
- Pezzilli R, Billi P, Gullo L, Motta R, Fontana G. Comparison of three serum lipase assays in acute pancreatitis. European Journal of Gastroenterology & Hepatology 1992;4(4):307‐10. [Google Scholar]
Pezzilli 1994 {published data only}
- Pezzilli R, Billi P, Plate L, Bongiovanni F, Labate AMM, Miglioli M. Human pancreas‐specific protein procarboxypeptidase‐b ‐ a useful serum marker of acute pancreatitis. Digestion 1994;55(2):73‐7. [DOI] [PubMed] [Google Scholar]
Pezzilli 1997 {published data only}
- Pezzilli R, Billi P, Barakat B, Melandri R, Miglio F. Serum amylase, pancreatic isoamylase and lipase in acute alcohol intoxication and in acute alcoholic pancreatitis. Alcologia 1997;9(3):177‐80. [Google Scholar]
Pezzilli 1998 {published data only}
- Pezzilli R, Morselli‐Labate AM, Barakat B, Fiocchi M, Cappelletti O. Is the association of serum lipase with á˜2‐microglobulin or C‐reactive protein useful for establishing the diagnosis and prognosis of patients with acute pancreatitis?. Clinical Chemistry and Laboratory Medicine 1998;36(12):963‐8. [DOI] [PubMed] [Google Scholar]
Pezzilli 1999 {published data only}
- Pezzilli R, Morselli‐Labate AM, Miniero R, Barakat B, Cappelletti O, Marrano N, et al. The association of serum lipase with interleukin 6 is useful in simultaneously establishing both the diagnosis and prognosis of patients with acute pancreatitis. Gastroenterology 1999;116(4):A1155. [Google Scholar]
Pezzilli 1999a {published data only}
- Pezzilli R, Morselli‐Labate AM, Miniero R, Barakat B, Fiocchi M, Cappelletti O. Simultaneous serum assays of lipase and interleukin‐6 for early diagnosis and prognosis of acute pancreatitis. Clinical Chemistry 1999;45(10):1762‐7. [PubMed] [Google Scholar]
Pezzilli 2000 {published data only}
- Pezzilli R, D'Alessandro A, Morselli‐Labate AM. Time course and clinical value of urine trypsinogen‐2 in acute pancreatitis. Gastroenterology 2000;118(4):A163. [DOI] [PubMed] [Google Scholar]
Pezzilli 2001 {published data only}
- Pezzilli R, Morselli‐Labate AM, D'Alessandro A, Barakat B. Time‐course and clinical value of the urine trypsinogen‐2 dipstick test in acute pancreatitis. European Journal of Gastroenterology & Hepatology 2001;13(3):269‐74. [DOI] [PubMed] [Google Scholar]
Pezzilli 2004 {published data only}
- Pezzilli R, Venturi M, Morselli‐Labate AM, Ceciliato R, Lamparelli MG, Rossi A, et al. Serum trypsinogen activation peptide in the assessment of the diagnosis and severity of acute pancreatic damage ‐ a pilot study using a new determination technique. Pancreas 2004;29(4):298‐305. [DOI] [PubMed] [Google Scholar]
Phillip 2013 {published data only}
- Phillip V, Schuster T, Hagemes F, Lorenz S, Matheis U, Preinfalk S, et al. Time period from onset of pain to hospital admission and patients' awareness in acute pancreatitis. Pancreas 2013;42(4):647‐54. [DOI] [PubMed] [Google Scholar]
Pirolla 2015 {published data only}
- Pirolla EH, Barros Filho TE, Godoy‐Santos AL, Fregni F. Association of acute pancreatitis or high level of serum pancreatic enzymes in patients with acute spinal cord injury: A prospective study. Spinal Cord 2015;53(6):495. [DOI] [PubMed] [Google Scholar]
Ponseti‐Bosch 1977 {published data only}
- Ponseti‐Bosch JM, Chacon‐Castro P, Maganamorera P, Schwartz‐Riera S, Gomez‐Perez J. Amylase‐creatinine clearance ratio in diagnosis of acute pancreatitis. Medicina Clinica 1977;68(8):369‐73. [Google Scholar]
Ponteziere 2001 {published data only}
- Ponteziere C, Bugugnani MJ. Clinical evaluation of urinary trypsinogen‐2 by rapid Actim Pancreatitis test strip in pancreatic diseases. Immuno‐Analyse et Biologie Specialisee 2001;16(4):246‐50. [Google Scholar]
Popivanov 1963 {published data only}
- Popivanov I, Kovacheva N. Diagnostic and prognostic significance of diastase in acute pancreatitis. Suvremenna Meditsina 1963;14(2):28‐36. [PubMed] [Google Scholar]
Protsenko 1966 {published data only}
- Protsenko VA, Kolesnikov Iu N, Toskin KD. Enzyme activity of blood and urine in acute pancreatitis. Laboratornoe Delo 1966;6:337‐8. [PubMed] [Google Scholar]
Raju 2003 {published data only}
- Raju S, Roberts IM, Templeton D. Trypsinogen‐2 testing is a superior predictor for the diagnosis of acute pancreatitis: Comparison with amylase and lipase. Gastroenterology 2003;124(4):A401. [Google Scholar]
Raty 2007 {published data only}
- Raty S, Sand J, Nordback I. Detection of postoperative pancreatitis after pancreatic surgery by urine trypsinogen strip test. British Journal of Surgery 2007;94(1):64‐9. [DOI] [PubMed] [Google Scholar]
Reilly 2011 {published data only}
- Reilly R, Wu M, Chan SB. Serum lipase and amylase in the diagnosis of acute pancreatitis in elderly patients. Annals of Emergency Medicine 2011;58(4):S232. [Google Scholar]
Rick 1968 {published data only}
- Rick WI. Determination of the serum lipase in pancreatic diseases. Verhandlungen der Deutschen Gesellschaft fur Innere Medizin 1968;74:230‐3. [PubMed] [Google Scholar]
Roberts 1985 {published data only}
- Roberts IM, Mercer D. Radioimmunoassay (RIA) for pancreatic lipase in acute pancreatitis. Gastroenterology 1985;88(5):1557. [Google Scholar]
Roberts 1987 {published data only}
- Roberts IM, Mercer D. Radioimmunoassay for human pancreatic lipase in acute pancreatitis. Digestive Diseases and Sciences 1987;32(4):388‐92. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Cuartero 2000 {published data only}
- Rodriguez‐Cuartero A. High amylasaemia: Is not always acute pancreatitis. Revista Espanola De Enfermedades Digestivas 2000;92(2):110‐1. [PubMed] [Google Scholar]
Rokicki 1976 {published data only}
- Rokicki M, Rokicki W. Clinical value of determination of some enzymes and isoenzymes in acute pancreatitis. Wiadomosci Lekarskie 1976;29(20):1823‐7. [PubMed] [Google Scholar]
Rosenblum 1991 {published data only}
- Rosenblum JL. Serum lipase activity is increased in disease states other than acute pancreatitis: Amylase revisited. Clinical Chemistry 1991;37(3):315‐6. [PubMed] [Google Scholar]
Rosenburg 1957 {published data only}
- Rosenburg SA, Akgun S. Serum amylase test in differential diagnosis of freely perforated ulcer and acute pancreatitis; a re‐evaluation. Archives of Surgery 1957;75(1):41‐3. [DOI] [PubMed] [Google Scholar]
Rudis 2014 {published data only}
- Rudis J, Ryska M. Pancreatic leakage and acute postoperative pancreatitis after proximal pancreatoduodenectomy. Rozhledy V Chirurgii 2014;93(7):380‐5. [PubMed] [Google Scholar]
Ruzena 1989 {published data only}
- Ruzena S. Normal serum amylase in acute pancreatitis. Digestive Diseases and Sciences 1989;34(6):960‐1. [DOI] [PubMed] [Google Scholar]
Sacchetti 1988 {published data only}
- Sacchetti L, Cavalcanti E, Cerasuolo D, Visconti M, Rabitti PG, Uomo G, et al. Serum p‐amylase isoenzyme immunoassay as compared to electrophoresis in acute pancreatitis. Clinical Chemistry 1988;34(6):1162. [Google Scholar]
Sacchetti 1989 {published data only}
- Sacchetti L, Cavalcanti E, Cerasuolo D, Oriani G, Uomo G, Rabitti PG, et al. Evaluation of pancreatic amylase immunoassay in acute pancreatitis. Clinica Chimica Acta 1989;183(1):95‐100. [DOI] [PubMed] [Google Scholar]
Sadowski 1992 {published data only}
- Sadowski DC, Sutherland LR, Gumaste V, Dave P, Weissman D, Messer J. The lipase/amylase ratio: Sensitive but not specific [3]. Gastroenterology 1992;103(1):352‐3. [DOI] [PubMed] [Google Scholar]
Sainio 1995 {published data only}
- Sainio V, Puolakkainen P, Kemppainen E, Hedstrom J, Haapiainen R, Kivisaari L, et al. Serum trypsinogen‐2, a new reliable assay for the early diagnosis and prediction of outcome in acute necrotizing pancreatitis. Gastroenterology 1995;108(4):A387. [DOI] [PubMed] [Google Scholar]
Sankaralingam 2007 {published data only}
- Sankaralingam S, Wesen C, Barawi M, Galera R, Lloyd L. Use of the urinary trypsinogen‐2 dip stick test in early diagnosis of pancreatitis after endoscopic retrograde cholangiopancreatography. Surgical Endoscopy 2007;21(8):1312‐5. [DOI] [PubMed] [Google Scholar]
Satz 1989 {published data only}
- Satz N, Fuhrer I, Inabnit K, Ott A, Knoblauch M. Diagnostic value of a diagnostic strip for determining urinary amylase. Schweizerische Rundschau fur Medizin Praxis 1989;78(13):368‐71. [PubMed] [Google Scholar]
Satz 1990 {published data only}
- Satz N, Jacot des Combes A, Meier L, Hollinger A, Knoblauch M. Semiquantitative lipase determination ‐ a useful screening test for pancreatitis?. Schweizerische Rundschau fur Medizin Praxis 1990;79(11):314‐7. [PubMed] [Google Scholar]
Satz 1990a {published data only}
- Satz N, Jacot des Combes A, Schmid E, Ott A, Knoblauch M. Diagnostic value of various laboratory parameters in acute pancreatitis. Zeitschrift Fur Gastroenterologie 1990;28(4):198‐201. [PubMed] [Google Scholar]
Saxon 1957 {published data only}
- Saxon EI, Hinkley WC, Vogel WC, Zieve L. Comparative value of serum and urinary amylase in the diagnosis of acute pancreatitis. Archives of Internal Medicine 1957;99(4):607‐21. [DOI] [PubMed] [Google Scholar]
Schmidt 2004 {published data only}
- Schmidt LE, Dalhoff K. Hyperamylasaemia and acute pancreatitis in paracetamol poisoning. Alimentary Pharmacology and Therapeutics 2004;20(2):173‐9. [DOI] [PubMed] [Google Scholar]
Scholz 1979 {published data only}
- Scholz HG, Appel W. Long‐time observations of serum‐lipase for controlling of acute and chronic recurrent pancreatitis. Medizinische Klinik 1979;74(5):145‐8. [PubMed] [Google Scholar]
Schultis 1969 {published data only}
- Schultis K, Wagner E. Comparative studies with a rapid lipase test and quantitative determination of serum lipase in the diagnosis of acute pancreatitis. Munchener Medizinische Wochenschrift 1969;111(12):684. [PubMed] [Google Scholar]
Schultis 1969a {published data only}
- Schultis K, Wagner E, Vosskohler E. Experiences with the determination in the serum for the diagnosis of acute and chronic pancreatic diseases. Schweizerische Medizinische Wochenschrift Journal Suisse de Medecine 1969;99(16):603‐6. [PubMed] [Google Scholar]
Schultis 1973 {published data only}
- Schultis K, Wagner E, Vosskohler E. Significance of serum lipase measurement for the diagnosis of acute and chronic pancreatic disease. Deutsche Medizinische Wochenschrift 1973;98(8):364‐74. [DOI] [PubMed] [Google Scholar]
Schwokowski 1979 {published data only}
- Schwokowski CF, Poege AW. Rapid lipase method for diagnosis of acute pancreatitis. Zentralblatt Fur Chirurgie 1979;104(17):1129‐31. [PubMed] [Google Scholar]
Scottolini 1977 {published data only}
- Scottolini AG, Bhagavan NV. Amylase‐creatinine clearance ratio in the diagnosis of acute pancreatitis. Hawaii Medical Journal 1977;36(12):391‐2. [PubMed] [Google Scholar]
Serra 2011 {published data only}
- Serra CE, Lopez Mingorance FN, Uccell NA, Sord JA, Negri G, Carlo MB. Biochemical markers in acute biliary pancreatitis. Clinical Chemistry and Laboratory Medicine 2011;49:S590. [Google Scholar]
Siede 1969 {published data only}
- Siede W, Leinweber W. Diagnosis of acute and chronic pancreatitis. Lebensversicherungs Medizin 1969;21(1):1‐5. [PubMed] [Google Scholar]
Singh 2002 {published data only}
- Singh P, Davidoff S, Pooran N, Nozad V, Kasmin FE, Lee TH, et al. Accuracy of serum amylase measured four hours after ERCP in predicting post‐procedure acute pancreatitis. Gastrointestinal Endoscopy 2002;55(5):AB153. [Google Scholar]
Singh 2004 {published data only}
- Singh R, Mugal T, Madhoun M, Singh P, Pooran N, Stark B, et al. Urinary trypsinogen ‐ a predictor of post‐ERCP acute pancreatitis. Gastroenterology 2004;126(4):A229‐30. [Google Scholar]
Smith 2005 {published data only}
- Smith RC, Southwell‐Keely J, Chesher D. Should serum pancreatic lipase replace serum amylase as a biomarker of acute pancreatitis?. ANZ Journal of Surgery 2005;75(6):399‐404. [DOI] [PubMed] [Google Scholar]
Solomon 1978 {published data only}
- Solomon AR Jr. The value of the amylase/creatinine clearance ratio in the diagnosis of acute pancreatitis. Critical Reviews in Clinical Laboratory Sciences 1978;9(4):367‐80. [DOI] [PubMed] [Google Scholar]
Steinberg 1983 {published data only}
- Steinberg WM, Goldstein SS, Davis ND, Shamaa JM, Anderson KK, Korman LY. Prospective study comparing the serum amylase and trypsinogen in the diagnosis of acute pancreatitis. Gastroenterology 1983;84(5):1322. [Google Scholar]
Steinberg 1985 {published data only}
- Steinberg WM, Goldstein SS, Davis ND. Diagnostic assays in acute pancreatitis. A study of sensitivity and specificity. Annals of Internal Medicine 1985;102(5):576‐80. [DOI] [PubMed] [Google Scholar]
Sternby 1996 {published data only}
- Sternby B, O'Brien JF, Zinsmeister AR, DiMagno EP. What is the best biochemical test to diagnose acute pancreatitis? A prospective clinical study. Mayo Clinic Proceedings 1996;71(12):1138‐44. [DOI] [PubMed] [Google Scholar]
Strebel 1970 {published data only}
- Strebel HM, Ehrengruber H, Stirnemann H. Diagnostic value of amylase determination and early laparotomy in acute pancreatitis. Schweizerische Medizinische Wochenschrift Journal Suisse de Medecine 1970;100(28):1207‐9. [PubMed] [Google Scholar]
Su 2010 {published data only}
- Su Y, Zhao Y, Wang Q. Clinical value of urine trypsinogen‐2 test in the diagnosis of acute pancreatitis in the emergency department. Guoji Jianyan Yixue Zazhi 2010;31(12):1388. [Google Scholar]
Suehiro 1984 {published data only}
- Suehiro I, Otsuki M, Ohki A. Amylase inhibitor from wheat: Its action and clinical application. Gastroenterologia Japonica 1984;19(4):313‐9. [DOI] [PubMed] [Google Scholar]
Sutton 2009 {published data only}
- Sutton PA, Humes DJ, Purcell G, Smith JK, Whiting F, Wright T, et al. The role of routine assays of serum amylase and lipase for the diagnosis of acute abdominal pain. Annals of the Royal College of Surgeons of England 2009;91(5):381‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szalaj 1973 {published data only}
- Szalaj W, Gabryelewicz A. Value of determinations of the circadian rhythm of amylase secretion in the diagnosis of pancreatic diseases. Polskie Archiwum Medycyny Wewnetrznej 1973;50(7):667‐71. [PubMed] [Google Scholar]
Testoni 1999 {published data only}
- Testoni PA, Bagnolo F, Caporuscio S, Lella F. Serum amylase measured four hours after endoscopic sphincterotomy is a reliable predictor of postprocedure pancreatitis. American Journal of Gastroenterology 1999;94(5):1235‐41. [DOI] [PubMed] [Google Scholar]
Testoni 1999a {published data only}
- Testoni PA, Caporuscio S, Bagnolo F, Lella F. Twenty‐four‐hour serum amylase predicting pancreatic reaction after endoscopic sphincterotomy. Endoscopy 1999;31(2):131‐6. [DOI] [PubMed] [Google Scholar]
Testoni 2001 {published data only}
- Testoni PA, Bagnolo F. Pain at 24 hours associated with amylase levels greater than 5 times the upper normal limit as the most reliable indicator of post‐ERCP pancreatitis. Gastrointestinal Endoscopy 2001;53(1):33‐9. [DOI] [PubMed] [Google Scholar]
Thomson 1987 {published data only}
- Thomson HJ, Obekpa PO, Smith AN, Brydon WG. Diagnosis of acute pancreatitis: A proposed sequence of biochemical investigations. Scandinavian Journal of Gastroenterology 1987;22(6):719‐24. [DOI] [PubMed] [Google Scholar]
Ticktin 1965 {published data only}
- Ticktin HE, Trujillo NP, Evans PF, Roe JH. Diagnostic value of a new serum lipase method. Gastroenterology 1965;48(1):12‐7. [PubMed] [Google Scholar]
Tietz 1986 {published data only}
- Tietz NW, Huang WY, Rauh DF, Shuey DF. Laboratory tests in the differential diagnosis of hyperamylasemia. Clinical Chemistry 1986;32(2):301‐7. [PubMed] [Google Scholar]
Tomaszewski 1984 {published data only}
- Tomaszewski L, Uminska H, Konarska L, Szmajda D, Kulesza A, Oledzki J. Usefulness of determining amylase isoenzymes in the blood and urine in acute pancreatitis. Polski Tygodnik Lekarski 1984;39(19):629‐33. [PubMed] [Google Scholar]
Torrens 1998 {published data only}
- Torrens JK, McWhinney PH. Acute pancreatitis. Normal serum amylase does not exclude severe acute pancreatitis. BMJ (Clinical research ed) 1998;316(7149):1982‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tournut 1978 {published data only}
- Tournut R, Allan BJ, White TT. Cancer, pancreatitis, and the detection of the isoenzymes of DNAase, RNAase and amylase. Clinica Chimica Acta 1978;88(2):345‐53. [DOI] [PubMed] [Google Scholar]
Treacy 2001 {published data only}
- Treacy J, Williams A, Bais R, Willson K, Worthley C, Reece J, et al. Evaluation of amylase and lipase in the diagnosis of acute pancreatitis. ANZ Journal of Surgery 2001;71(10):577‐82. [DOI] [PubMed] [Google Scholar]
Tsai 1988 {published data only}
- Tsai LY, Chiang CH. The diagnostic value of amylase isoenzymes. Kaohsiung Journal of Medical Sciences 1988;4(12):655‐9. [PubMed] [Google Scholar]
Tseng 2011 {published data only}
- Tseng CW, Chen CC, Lin SZ, Chang FY, Lin HC, Lee SD. Rapid urinary trypsinogen‐2 test strip in the diagnosis of pancreatitis after endoscopic retrograde cholangiopancreatography. Pancreas 2011;40(8):1211‐4. [DOI] [PubMed] [Google Scholar]
Tvorogova 1991 {published data only}
- Tvorogova MG, Titov VN. Biochemical studies in the diagnosis of acute pancreatitis. Terapevticheskii Arkhiv 1991;63(2):144‐7. [PubMed] [Google Scholar]
Uhl 1992 {published data only}
- Uhl W, Malfertheiner P, Drosdat H, Martini M, Buchler M. Determination of pancreatic lipase by immunoactivation technology. A rapid test system with high sensitivity and specificity. International Journal of Pancreatology 1992;12(3):253‐61. [PubMed] [Google Scholar]
Uminska 1985 {published data only}
- Uminska H, Tomaszewski L, Konarska L. Clinical significance of blood and urine amylase isoenzymes in patients with cholelithiasis without acute pancreatitis. Polski Tygodnik Lekarski 1985;40(11):333‐6. [PubMed] [Google Scholar]
Van Hee 1979 {published data only}
- Hee R, Hubens A. The screening value of the amylase‐creatinine clearance ratio in acute pancreatitis. Acta Chirurgica Belgica 1979;78(5):301‐8. [PubMed] [Google Scholar]
Van Ingen 1992 {published data only}
- Ingen HE, Sanders GTB. Clinical evaluation of a pancreatic lipase mass concentration assay. Clinical Chemistry 1992;38(11):2310‐3. [PubMed] [Google Scholar]
Varas 1994 {published data only}
- Varas MJ. The ratio lipase/amylase and trypsin/amylase in the etiologic diagnosis of acute pancreatitis. Gastroenterologia y Hepatologia 1994;17(6):346. [Google Scholar]
Vega 1981 {published data only}
- Vega Franco L, Garcia Aranda A, Meza Camacho C, Gonzalez R. Pancreatitis in infants with a clinical diagnosis of septicemia. Boletin Medico del Hospital Infantil de Mexico 1981;38(1):131‐42. [PubMed] [Google Scholar]
Ventrucci 1983 {published data only}
- Ventrucci M, Gullo L, Daniele C, Bartolucci C, Priori P, Plate L, et al. Comparative study of serum pancreatic isoamylase, lipase, and trypsin‐like immunoreactivity in pancreatic disease. Digestion 1983;28(2):114‐21. [DOI] [PubMed] [Google Scholar]
Ventrucci 1985 {published data only}
- Ventrucci M, Pezzilli R, Naldoni P, Montone L, Priori P, Gullo L. Evaluation of a new rapid serum lipase assay in the diagnosis of acute pancreatitis. Digestive Diseases and Sciences 1985;30(10):992. [Google Scholar]
Ventrucci 1986 {published data only}
- Ventrucci M, Pezzilli R, Naldoni P, Montone L, Gullo L. A rapid assay for serum immunoreactive lipase as a screening test for acute pancreatitis. Pancreas 1986;1(4):320‐3. [DOI] [PubMed] [Google Scholar]
Ventrucci 1989 {published data only}
- Ventrucci M, Pezzilli R, Gullo L, Plate L, Sprovieri G, Barbara L. Role of serum pancreatic enzyme assays in diagnosis of pancreatic disease. Digestive Diseases and Sciences 1989;34(1):39‐45. [DOI] [PubMed] [Google Scholar]
Ventrucci 1992 {published data only}
- Ventrucci M, Pezzilli R, Montone L, Plate L, Buonamici L, Bergami R, et al. Serum pancreatic enzyme assays in acute abdomen: A comparative prospective study. Italian Journal of Gastroenterology 1992;24(3):115‐8. [PubMed] [Google Scholar]
Ventrucci 1994 {published data only}
- Ventrucci M, Pezzilli R, Garulli L, Liguori L, Moratti R, D'Eril GVM. Clinical evaluation of a new rapid assay for serum lipase determination. Italian Journal of Gastroenterology 1994;26(3):132‐6. [PubMed] [Google Scholar]
Wajda 1978 {published data only}
- Wajda Z, Sledzinski Z. Value of amylase‐creatinine clearance index in the diagnosis of acute pancreatitis. Polski Przeglad Chirurgiczny 1978;50(4):285‐9. [PubMed] [Google Scholar]
Walker 2013 {published data only}
- Walker T, Pullan N, Hewes J. The use and abuse of serum lipase testing in the diagnosis of acute pancreatitis. British Journal of Surgery 2013;100:16. [Google Scholar]
Waller 1971 {published data only}
- Waller SL, Ralston AJ. The hourly rate of urinary amylase excretion, serum amylase, and serum lipase. Part II Patients with gastrointestinal and pancreatic disorders. Gut 1971;12(11):884‐90. [PMC free article] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang L, Luo H, Yan J. The value of serum amylase and lipase concentrations and lipase/amylase ratio in diagnosis of acute pancreatitis. Chinese Journal of Gastroenterology and Hepatology 2009;18(8):695‐7. [Google Scholar]
Warshaw 1975 {published data only}
- Warshaw AL, Fuller AF. Specificity of increased renal clearance of amylase in diagnosis of acute pancreatitis. New England Journal of Medicine 1975;292(7):325‐8. [DOI] [PubMed] [Google Scholar]
Weaver 1985 {published data only}
- Weaver DW, Busuito MJ, Bouwman DL, Wilson RF. Interpretation of serum amylase levels in the critically ill patient. Critical Care Medicine 1985;13(7):532‐3. [DOI] [PubMed] [Google Scholar]
Werner 1989 {published data only}
- Werner M, Steinberg WM, Pauley C. Strategic use of individual and combined enzyme indicators for acute pancreatitis analyzed by receiver‐operator characteristics. Clinical Chemistry 1989;35(6):967‐71. [PubMed] [Google Scholar]
Wilson 2005 {published data only}
- Wilson RB, Warusavitarne J, Crameri DM, Alvaro F, Davies DJ, Merrett N. Serum elastase in the diagnosis of acute pancreatitis: A prospective study. ANZ Journal of Surgery 2005;75(3):152‐6. [DOI] [PubMed] [Google Scholar]
Winslet 1990 {published data only}
- Winslet MC, Hall C, London N, Neoptolemos JP. Serum amylase estimation in the diagnosis of acute pancreatitis. Gut 1990;31(10):A1201. [Google Scholar]
Wyatt 1974 {published data only}
- Wyatt AP. Diagnosis and management of acute pancreatitis. Annals of the Royal College of Surgeons of England 1974;54(5):229‐35. [PMC free article] [PubMed] [Google Scholar]
Wyllie 1979 {published data only}
- Wyllie FJ, Gunn AA. Diagnosis of acute pancreatitis. Journal of the Royal College of Surgeons of Edinburgh 1979;24(6):363‐9. [PubMed] [Google Scholar]
Xu 2008 {published data only}
- Xu S, Han X. Clinical application of pancreatic amylase and lipase in acute pancreatitis. Guoji Jianyan Yixue Zazhi 2008;29(11):980‐2. [Google Scholar]
Xu 2010 {published data only}
- Xu X, Wu J. Diagnostic value of urinary trypsinogen‐2 for acute pancreatitis in children. Journal of Applied Clinical Pediatrics 2010;25(19):1492‐3. [Google Scholar]
Yang 1987 {published data only}
- Yang LY, Huang YS, Ji QD. Diagnostic value of serum pancreatic lipase for acute pancreatitis. Chinese Journal of Surgery 1987;25(9):522‐4, 56. [PubMed] [Google Scholar]
Yang 2005 {published data only}
- Yang RW, Shao ZX, Chen YY, Yin Z, Wang WJ. Lipase and pancreatic amylase activities in diagnosis of acute pancreatitis in patients with hyperamylasemia. Hepatobiliary & Pancreatic Diseases International 2005;4(4):600‐3. [PubMed] [Google Scholar]
Zakrzewska 1982 {published data only}
- Zakrzewska I, Prokopowicz J. Usefulness of determining alpha‐amylase thermolability in the diagnosis of acute pancreatitis. Polskie Archiwum Medycyny Wewntrznej 1982;67(5‐6):243‐7. [PubMed] [Google Scholar]
Zakrzewska 1985 {published data only}
- Zakrzewska I, Kuzma L. The amylase and creatinine clearance coefficient in acute and chronic pancreatitis and diabetes mellitus. Wiadomosci Lekarskie 1985;38(17):1199‐203. [PubMed] [Google Scholar]
Zaninotto 1990 {published data only}
- Zaninotto M, Bertorelle R, Secchiero S, Plebani M, Burlina A. Pancreatic amylase determination by immunological and electrophoretic methods. Clinical Chemistry and Enzymology Communications 1990;3(5):261‐6. [Google Scholar]
Zastrow 1973 {published data only}
- Zastrow F. Significance of diastase and amylase in the diagnosis of acute pancreatitis. Medizinische Welt 1973;24(48):1897‐8. [PubMed] [Google Scholar]
Zeng 2010 {published data only}
- Zeng F, Wang Q, Liu J. Evidence‐based evaluation of trypsinogen‐2 and serum amylase in the early diagnosis of acute pancreatitis. Guoji Jianyan Yixue Zazhi 2010;31(11):1203‐6. [Google Scholar]
Zeze 1975 {published data only}
- Zeze F, Ishii K, Nakamura K. Clinical studies on amylase isoenzyme (2nd report). Japanese Journal of Gastroenterology 1975;72(2):127‐35. [PubMed] [Google Scholar]
Zhang 2010 {published data only}
- Zhang H, Chen C, Wang Y. One‐hour urinary discharge volume of amylase for diagnosis and prognostic evaluation of acute pancreatitis. Chinese Journal of Hepatobiliary Surgery 2010;16 Pt 4:310. [Google Scholar]
Zharkovskaia 1978 {published data only}
- Zharkovskaia A. Differential diagnosis of acute biliary tract diseases and acute pancreatitis by means of laboratory tests [Differentsial'naia diagnostika ostrykh zabolevanii zhelchnykh putei i ostrykh pankreatitov s pomoshch'iu laboratornykh]. Laboratornoe Delo 1978, (2):92‐4. [PubMed] [Google Scholar]
Zheltvai 1969 {published data only}
- Zheltvai VV. On the laboratory diagnosis of the functional state of pancreas. Laboratornoe Delo 1969;4:250‐1. [PubMed] [Google Scholar]
References to studies awaiting assessment
Anand 1956a {published data only}
- Anand SS, Sawhney CP. Evaluation of serum amylase test as a diagnostic aid. Indian Journal of Surgery 1956;18(3):185‐90. [Google Scholar]
Cherry 1953 {published data only}
- Cherry JW. Diagnosis of acute pancreatitis. Proc Clin Honolulu 1953;19(5):73‐7. [Google Scholar]
Coppola 1954 {published data only}
- Coppola W. The plasma amylase test in the diagnosis of acute pancreatitis. Atti dell'Accademia medico‐chirurgica di Perugia e annali della Facolta di medicina 1954;5(4):327‐36. [Google Scholar]
Do Prado 1952 {published data only}
- Do Prado FC, Bove P. Studies on blood amylases in acute pancreatitis. Prensa Medica Argentina 1952;39(46):2764‐9. [PubMed] [Google Scholar]
Lippi 2013 {published data only}
- Lippi G, Aloe R, Musa R, Avanzini P, Cugini A. Evaluation of sentinel pancreatic amylase assay on Beckman Coulter AU5800 clinical chemistry analyzer. Biochimica Clinica 2013;37:S668. [Google Scholar]
Stimac 1995 {published data only}
- Stimac D, Rubinic M, Lenac T. Diagnostic value of lipase/amylase (l/a) ratio in acute alcoholic pancreatitis. In: Papastamatiou L editor(s). European IHPBA Congress Athens. Athens: Monduzzi ed, 1995:583‐6. [2‐345‐67890‐1] [Google Scholar]
Additional references
Alfonso 2003
- Alfonso V, Gomez F, Lopez A, Moreno‐Osset E, Valle R, Anton MD, et al. Value of C‐reactive protein level in the detection of necrosis in acute pancreatitis. Gastroenterologia y Hepatologia 2003;26(5):288‐93. [DOI] [PubMed] [Google Scholar]
Ayub 2004
- Ayub K, Slavin J, Imada R. Endoscopic retrograde cholangiopancreatography in gallstone‐associated acute pancreatitis. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003630.pub2] [DOI] [PubMed] [Google Scholar]
Bakker 2012
- Bakker OJ, Santvoort HC, Brunschot S, Geskus RB, Besselink MG, Bollen TL, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012;307(10):1053‐61. [DOI] [PubMed] [Google Scholar]
Banks 2013
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis ‐ 2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62(1):102‐11. [DOI] [PubMed] [Google Scholar]
Bradley 1993
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis; 1992 September 11‐13; Atlanta, GA. Archives of Surgery 1993;128(5):586‐90. [DOI] [PubMed] [Google Scholar]
Buscemi 2006
- Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. Journal of Clinical Epidemiology 2006;59(7):697‐703. [DOI] [PubMed] [Google Scholar]
Cannon 2009
- Cannon JW, Callery MP, Vollmer CM Jr. Diagnosis and management of pancreatic pseudocysts: What is the evidence?. Journal of the American College of Surgeons 2009;209(3):385‐93. [DOI] [PubMed] [Google Scholar]
Chang 2012
- Chang K, Lu W, Zhang K, Jia S, Li F, Wang F, et al. Rapid urinary trypsinogen‐2 test in the early diagnosis of acute pancreatitis: a meta‐analysis. Clinical Biochemistry 2012;45(13‐14):1051‐6. [DOI] [PubMed] [Google Scholar]
Cheruvu 2003
- Cheruvu CV, Clarke MG, Prentice M, Eyre‐Brook IA. Conservative treatment as an option in the management of pancreatic pseudocyst. Annals of the Royal College of Surgeons of England 2003;85(5):313‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta‐analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331‐2. [DOI] [PubMed] [Google Scholar]
Colli 2014
- Colli A, Fraquelli M, Casazza G Conte D, Nikolova D, Duca P, et al. The architecture of diagnostic research: from bench to bedside. Research guidelines using liver stiffness as an example. Hepatology 2014;60:408‐18. [DOI] [PubMed] [Google Scholar]
Doust 2005
- Doust JA, Pietrzak E, Sanders S, Glasziou PP. Identifying studies for systematic reviews of diagnostic tests was difficult due to the poor sensitivity and precision of methodologic filters and the lack of information in the abstract. Journal of Clinical Epidemiology 2005;58(5):444‐9. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2001
- Eloubeidi MA, Wade SB, Provenzale D. Factors associated with acceptance and full publication of GI endoscopic research originally published in abstract form. Gastrointestinal Endoscopy 2001;53(3):275‐82. [DOI] [PubMed] [Google Scholar]
Ghekiere 2007
- Ghekiere O, Lesnik A, Hoa D, Laffargue G, Uriot C, Taourel P. Value of computed tomography in the diagnosis of the cause of nontraumatic gastrointestinal tract perforation. Journal of Computer Assisted Tomography 2007;31(2):169‐76. [DOI] [PubMed] [Google Scholar]
Grassi 2004
- Grassi R, Romano S, Pinto A, Romano L. Gastro‐duodenal perforations: conventional plain film, US and CT findings in 166 consecutive patients. European Journal of Radiology 2004;50(1):30‐6. [DOI] [PubMed] [Google Scholar]
Gurusamy 2013
- Gurusamy KS, Nagendran M, Davidson BR. Early versus delayed laparoscopic cholecystectomy for acute gallstone pancreatitis. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010326.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2014
- Gurusamy KS, Davidson BR. Gallstones. BMJ 2014;348:g2669. [DOI] [PubMed] [Google Scholar]
Gurusamy 2016
- Gurusamy KS, Belgaumkar AP, Haswell A, Pereira SP, Davidson BR. Interventions for necrotising pancreatitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011383.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedstrom 1996d
- Hedstrom J, Sainio V, Kemppainen E, Haapiainen R, Kivilaakso E, Schroder T, et al. Serum complex of trypsin 2 and alpha 1 antitrypsin as diagnostic and prognostic marker of acute pancreatitis: clinical study in consecutive patients. BMJ 1996;313(7053):333‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2009
- Johnson MD, Walsh RM, Henderson JM, Brown N, Ponsky J, Dumot J, et al. Surgical versus nonsurgical management of pancreatic pseudocysts. Journal of Clinical Gastroenterology 2009;43(6):586‐90. [DOI] [PubMed] [Google Scholar]
Khanna 2013
- Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE‐II, CTSI scores, IL‐6, CRP, and procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surgery 2013;2013:367581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuzmich 2012
- Kuzmich S, Harvey CJ, Fascia DT, Kuzmich T, Neriman D, Basit R, et al. Perforated pyloroduodenal peptic ulcer and sonography. AJR: American Journal of Roentgenology 2012;199(5):W587‐94. [DOI] [PubMed] [Google Scholar]
Larson 2006
- Larson SD, Nealon WH, Evers BM. Management of gallstone pancreatitis. Advances in Surgery 2006;40:265‐84. [DOI] [PubMed] [Google Scholar]
Matull 2006
- Matull WR, Pereira SP, O'Donohue JW. Biochemical markers of acute pancreatitis. Journal of Clinical Pathology 2006;59(4):340‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moayyedi 2006
- Moayyedi P, Talley NJ, Fennerty MB, Vakil N. Can the clinical history distinguish between organic and functional dyspepsia?. JAMA 2006;295(13):1566‐76. [DOI] [PubMed] [Google Scholar]
Mouli 2013
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta‐analysis. Gastroenterology 2013;144(2):333‐40 e2. [DOI] [PubMed] [Google Scholar]
NCBI 2014
- NCBI. MeSH. NLM Controlled Vocabulary. Pancreas. www.ncbi.nlm.nih.gov/mesh/68010179 2014 (accessed 4 July 2014).
Peery 2012
- Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143(5):1179‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrov 2010
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010;139(3):813‐20. [DOI] [PubMed] [Google Scholar]
Rau 1998
- Rau B, Cebulla M, Uhl W, Schoenberg MH, Beger HG. The clinical value of human pancreas‐specific protein procarboxypeptidase B as an indicator of necrosis in acute pancreatitis: comparison to CRP and LDH. Pancreas 1998;17(2):134‐9. [DOI] [PubMed] [Google Scholar]
Reitsma 2005
Roberts 2013
- Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Alimentary Pharmacology and Therapeutics 2013;38(5):539‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutter 2001
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [DOI] [PubMed] [Google Scholar]
Sampson 2008
- Sampson M, Shojania KG, McGowan J, Daniel R, Rader T, Iansavichene AE, et al. Surveillance search techniques identified the need to update systematic reviews. Journal of Clinical Epidemiology 2008;61(8):755‐62. [DOI] [PubMed] [Google Scholar]
Sanabria 2013
- Sanabria A, Villegas MI, Morales Uribe CH. Laparoscopic repair for perforated peptic ulcer disease. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD004778.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmid 1999
- Schmid SW, Uhl W, Friess H, Malfertheiner P, Buchler MW. The role of infection in acute pancreatitis. Gut 1999;45(2):311‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takwoingi 2015
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta‐analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Medical Research 2015 June 26 [Epub ahead of print]. [DOI: 10.1177/0962280215592269] [DOI] [PMC free article] [PubMed]
Tenner 2013
- Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. American Journal of Gastroenterology 2013;108(9):1400‐15. [DOI] [PubMed] [Google Scholar]
van Brunschot 2014
- Brunschot S, Fockens P, Bakker OJ, Besselink MG, Voermans RP, Poley JW, et al. Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surgical Endoscopy 2014;28(5):1425‐38. [DOI] [PubMed] [Google Scholar]
van Santvoort 2010
- Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step‐up approach or open necrosectomy for necrotizing pancreatitis. New England Journal of Medicine 2010;362(16):1491‐502. [DOI] [PubMed] [Google Scholar]
van Santvoort 2011
- Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011;141(4):1254‐63. [DOI] [PubMed] [Google Scholar]
Varadarajulu 2008
- Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointestinal Endoscopy 2008;68(6):1102‐11. [DOI] [PubMed] [Google Scholar]
Varadarajulu 2013
- Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology 2013;145(3):583‐90. [DOI] [PubMed] [Google Scholar]
Vissers 1999
- Vissers RJ, Abu‐Laban RB, McHugh DF. Amylase and lipase in the emergency department evaluation of acute pancreatitis. Journal of Emergency Medicine 1999;17(6):1027‐37. [DOI] [PubMed] [Google Scholar]
Whiting 2006
- Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Medical Research Methodology 2006; Vol. 6:9. [DOI] [PMC free article] [PubMed]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Yadav 2006
- Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas 2006;33(4):323‐30. [DOI] [PubMed] [Google Scholar]
Yang 2008
- Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol‐related liver and pancreatic disease in the United States. Archives of Internal Medicine 2008;168(6):649‐56. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2015
- Gurusamy KS, Davidson BR. Serum amylase and lipase and urinary trypsinogen and amylase for diagnosis of acute pancreatitis and serum C‐reactive protein, procalcitonin and lactate dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD012010] [DOI] [Google Scholar]


