Abstract
Background
Current guidelines recommend screening of people with oesophageal varices via oesophago‐gastro‐duodenoscopy at the time of diagnosis of hepatic cirrhosis. This requires that people repeatedly undergo unpleasant invasive procedures with their attendant risks, although half of these people have no identifiable oesophageal varices 10 years after the initial diagnosis of cirrhosis. Platelet count, spleen length, and platelet count‐to‐spleen length ratio are non‐invasive tests proposed as triage tests for the diagnosis of oesophageal varices.
Objectives
Primary objectives
To determine the diagnostic accuracy of platelet count, spleen length, and platelet count‐to‐spleen length ratio for the diagnosis of oesophageal varices of any size in paediatric or adult patients with chronic liver disease or portal vein thrombosis, irrespective of aetiology. To investigate the accuracy of these non‐invasive tests as triage or replacement of oesophago‐gastro‐duodenoscopy.
Secondary objectives
To compare the diagnostic accuracy of these same tests for the diagnosis of high‐risk oesophageal varices in paediatric or adult patients with chronic liver disease or portal vein thrombosis, irrespective of aetiology.
We aimed to perform pair‐wise comparisons between the three index tests, while considering predefined cut‐off values.
We investigated sources of heterogeneity.
Search methods
The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Hepato‐Biliary Group Diagnostic Test Accuracy Studies Register, the Cochrane Library, MEDLINE (OvidSP), Embase (OvidSP), and Science Citation Index ‐ Expanded (Web of Science) (14 June 2016). We applied no language or document‐type restrictions.
Selection criteria
Studies evaluating the diagnostic accuracy of platelet count, spleen length, and platelet count‐to‐spleen length ratio for the diagnosis of oesophageal varices via oesophago‐gastro‐duodenoscopy as the reference standard in children or adults of any age with chronic liver disease or portal vein thrombosis, who did not have variceal bleeding.
Data collection and analysis
Standard Cochrane methods as outlined in the Cochrane Handbook for Diagnostic Test of Accuracy Reviews.
Main results
We included 71 studies, 67 of which enrolled only adults and four only children. All included studies were cross‐sectional and were undertaken at a tertiary care centre. Eight studies reported study results in abstracts or letters. We considered all but one of the included studies to be at high risk of bias. We had major concerns about defining the cut‐off value for the three index tests; most included studies derived the best cut‐off values a posteriori, thus overestimating accuracy; 16 studies were designed to validate the 909 (n/mm3)/mm cut‐off value for platelet count‐to‐spleen length ratio. Enrolment of participants was not consecutive in six studies and was unclear in 31 studies. Thirty‐four studies assessed enrolment consecutively. Eleven studies excluded some included participants from the analyses, and in only one study, the time interval between index tests and the reference standard was longer than three months.
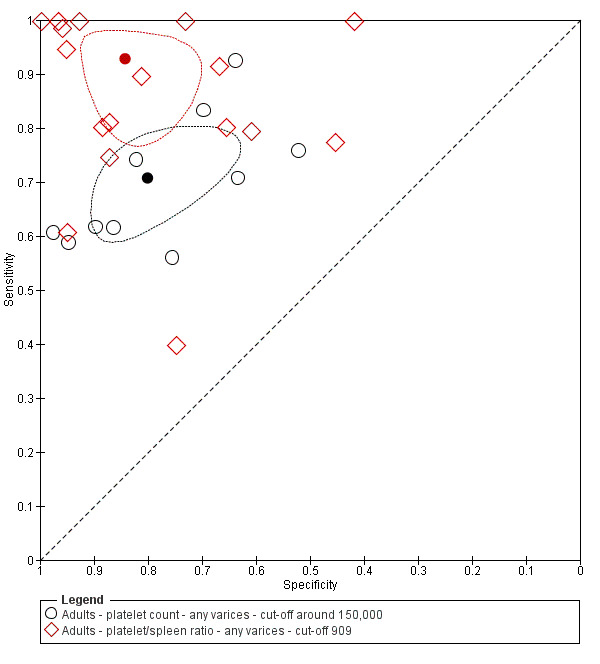
Diagnosis of varices of any size. Platelet count showed sensitivity of 0.71 (95% confidence interval (CI) 0.63 to 0.77) and specificity of 0.80 (95% CI 0.69 to 0.88) (cut‐off value of around 150,000/mm3 from 140,000 to 150,000/mm3; 10 studies, 2054 participants). When examining potential sources of heterogeneity, we found that of all predefined factors, only aetiology had a role: studies including participants with chronic hepatitis C reported different results when compared with studies including participants with mixed aetiologies (P = 0.036). Spleen length showed sensitivity of 0.85 (95% CI 0.75 to 0.91) and specificity of 0.54 (95% CI 0.46 to 0.62) (cut‐off values of around 110 mm, from 110 to 112.5 mm; 13 studies, 1489 participants). Summary estimates for detection of varices of any size showed sensitivity of 0.93 (95% CI 0.83 to 0.97) and specificity of 0.84 (95% CI 0.75 to 0.91) in 17 studies, and 2637 participants had a cut‐off value for platelet count‐to‐spleen length ratio of 909 (n/mm3)/mm. We found no effect of predefined sources of heterogeneity. An overall indirect comparison of the HSROCs of the three index tests showed that platelet count‐to‐spleen length ratio was the most accurate index test when compared with platelet count (P < 0.001) and spleen length (P < 0.001).
Diagnosis of varices at high risk of bleeding. Platelet count showed sensitivity of 0.80 (95% CI 0.73 to 0.85) and specificity of 0.68 (95% CI 0.57 to 0.77) (cut‐off value of around 150,000/mm3 from 140,000 to 160,000/mm3; seven studies, 1671 participants). For spleen length, we obtained only a summary ROC curve as we found no common cut‐off between studies (six studies, 883 participants). Platelet count‐to‐spleen length ratio showed sensitivity of 0.85 (95% CI 0.72 to 0.93) and specificity of 0.66 (95% CI 0.52 to 0.77) (cut‐off value of around 909 (n/mm3)/mm; from 897 to 921 (n/mm3)/mm; seven studies, 642 participants). An overall indirect comparison of the HSROCs of the three index tests showed that platelet count‐to‐spleen length ratio was the most accurate index test when compared with platelet count (P = 0.003) and spleen length (P < 0.001).
DIagnosis of varices of any size in children. We found four studies including 277 children with different liver diseases and or portal vein thrombosis. Platelet count showed sensitivity of 0.71 (95% CI 0.60 to 0.80) and specificity of 0.83 (95% CI 0.70 to 0.91) (cut‐off value of around 115,000/mm3; four studies, 277 participants). Platelet count‐to‐spleen length z‐score ratio showed sensitivity of 0.74 (95% CI 0.65 to 0.81) and specificity of 0.64 (95% CI 0.36 to 0.84) (cut‐off value of 25; two studies, 197 participants).
Authors' conclusions
Platelet count‐to‐spleen length ratio could be used to stratify the risk of oesophageal varices. This test can be used as a triage test before endoscopy, thus ruling out adults without varices. In the case of a ratio > 909 (n/mm3)/mm, the presence of oesophageal varices of any size can be excluded and only 7% of adults with varices of any size would be missed, allowing investigators to spare the number of oesophago‐gastro‐duodenoscopy examinations. This test is not accurate enough for identification of oesophageal varices at high risk of bleeding that require primary prophylaxis. Future studies should assess the diagnostic accuracy of this test in specific subgroups of patients, as well as its ability to predict variceal bleeding. New non‐invasive tests should be examined.
Plain language summary
Platelet count, spleen length, and platelet‐to‐spleen length ratio for the diagnosis of oesophageal varices in people with liver disease
Background
Hepatic cirrhosis is a severe disease with scars and nodules on the liver tissue. As a result, the normal function of the liver is impaired. Whatever the cause of cirrhosis, changes in the structure of and blood flow within the liver increase pressure in the portal vein (called portal vein hypertension), which is the vein that drains blood from the bowels to the liver. Portal hypertension induces dilatation (extension) of veins within the wall of the oesophagus (food pipe or gullet), which often rupture (break) with severe bleeding. Thus, when liver cirrhosis is diagnosed, an oesophago‐gastro‐duodenoscopy (OGD) is recommended to detect the presence of oesophageal varices (areas of abnormal dilatation of veins). During OGD, a small camera at the end of a tube is inserted down the oesophagus from the mouth and pictures are relayed back to a screen. Large varices or red signs on even small varices show high risks of rupture and bleeding. If high‐risk varices are found, treatment with beta‐blockers is effective in reducing the risk of bleeding. Three simple non‐invasive tests could be used to identify people with liver diease at high risk of having oesophageal varices: platelet count ‐ a simple laboratory test on a blood sample by which the number of platelets (a blood element ensuring coagulation) is measured; length (maximal diameter) of the spleen measured during ultrasound examination of the abdomen; and ratio of platelet count to spleen length.
Study characteristics
We searched scientific databases for clinical studies comparing platelet count, spleen length, or platelet count‐to‐spleen length ratio versus oesophago‐gastro‐duodenoscopy in detecting the presence of varices in children or adults with chronic liver disease or portal vein thrombosis (narrowing of the portal vein). The evidence is current to June 2016.
Key results
We found 25 studies with 5096 participants assessing the use of platelet count to diagnose the presence of varices and grade the risk of bleeding, and comparing platelet count versus oesophago‐gastro‐duodenoscopy in adults with cirrhosis: 13 studies with 1489 participants assessed the diagnostic ability of spleen length, and 38 studies with 5235 participants assessed the diagnostic ability of platelet count‐to‐spleen length ratio. Platelet count‐to‐spleen length ratio was the most accurate and could be used to identify people with liver disease who were at high risk of having oesophageal varices. Particularly, in people with hepatic cirrhosis among whom 580 out of 1000 people are expected to have oesophageal varices, only 41 (7% of 580) people will be missed as having varices and will have no appropriate preventive treatment or follow‐up. Thus, if platelet count‐to‐spleen length ratio is lower than 909 (n/mm3)/mm (the most used threshold), the presence of oesophageal varices can be excluded. Thus, it is possible to reduce the number of endoscopic examinations needed to find a person with oesophageal varices. On the contrary, this ratio is not accurate enough to replace endoscopy for identification of high risk of bleeding oesophageal varices.
Quality of the evidence
All but one study had problems of risk of bias involving mainly the definition of positive or negative index tests (platelet count, spleen length, and their ratio), which should be defined before and not after data analyses, and blinding of test results to the endoscopists who performed oesophago‐gastro‐duodenoscopy. Hence, these problems could impair the accuracy estimates of the three tests.
Summary of findings
Background
Oesophageal varices in portal hypertension
Portal hypertension commonly accompanies advanced liver disease and often gives rise to life‐threatening complications, including haemorrhage from oesophageal and gastrointestinal varices. Prevalence of cirrhosis in high‐income countries ranges from 0.4% to 1.1% of the population (Bellentani 1994; Quinn 1997); up to two thirds of people with cirrhosis will develop gastro‐oesophageal varices (Pagliaro 1992; D'Amico 1999; Jensen 2002). The incidence of oesophageal varices among people with compensated cirrhosis is around 5% per year (Merli 2003; Groszmann 2005), and the cumulative incidence among people with well‐defined compensated cirrhosis seems lower: 44% at 10 years and 53% at 20 years (D'Amico 2014). Gastro‐oesophageal varices are an extension of oesophageal varices; isolated gastric varices in the absence of oesophageal varices are rare and usually are associated with splenic vein thrombosis (Garcia‐Tsao 2007). As varices grow larger, they become more likely to rupture and bleed (Lebrec 1980; NIEC 1988). Haemorrhage from ruptured oesophageal varices is one of the most common causes of gastrointestinal bleeding and is the most common cause of death among individuals with cirrhosis (D'Amico 2006 a; Garcia‐Tsao 2007). Studies conducted by the Northern Italian Endoscopic Club have shown that bleeding over two years occurs at a frequency of up to 30% from large varices compared with 5% to 18% from small varices (NIEC 1988; Zoli 1996; D'Amico 1999). Variceal bleeding is a medical emergency that, in spite of recent progress, is associated with mortality of 10% to 20% at six weeks. Up to 30% of initial bleeding episodes are fatal, and bleeding recurs among 70% of survivors (Graham 1981; NIEC 1988; Sharara 2001; D'Amico 2003; Bambha 2008). However, primary prophylaxis with non‐selective beta blockers or endoscopic variceal banding lowers the incidence of first variceal haemorrhage, especially from medium to large varices (Garcia‐Tsao 2008; de Franchis 2015). Detection of oesophageal varices allows one to define the bleeding risk and to identify progression to decompensated cirrhosis associated with further complications and a poor prognosis requiring more intense follow‐up (D'Amico 2006 b; D'Amico 2014).
Current North American European and Asian Pacific guidelines for detection and management of oesophageal varices recommend performance of oesophago‐gastro‐duodenoscopy to screen for oesophageal varices at the time hepatic cirrhosis is diagnosed (Garcia‐Tsao 2007; Sarin 2008; ASGE Standards of Practice Committee 2012). However, the point prevalence of oesophageal varices requiring prophylaxis is only about 15% to 25%, and most people undergoing screening oesophago‐gastro‐duodenoscopy do not have varices or have varices that do not require treatment. Moreover, oesophago‐gastro‐duodenoscopy is an invasive procedure that often requires sedation and may be associated with serious, even rare, complications and with frequent unexpected hospital admissions (Silvis 1976; Wolfsen 2004; Geraci 2009; Leffler 2010). Therefore, a cost‐effective triage pathway must be developed to select people who will benefit from oesophago‐gastro‐duodenoscopy screening. A recent consensus conference (de Franchis 2015) identified individuals with chronic liver disease who could safely avoid screening endoscopy because their risk of oesophageal varices was very low when liver stiffness was measured by transient elastography < 20 kPa and a platelet count > 150,000 per mm3. However no systematic review supports this recommendation (de Franchis 2015).
A non‐invasive test can play the role of a triage test if it can serve to accurately rule out the presence of varices without missing effective treatments, and hence to reduce the use of endoscopy, reserving its use for people with positive results. A non‐invasive test may even be more accurate than the reference standard, that is, oesophago‐gastro‐duodenoscopy, which is limited by interobserver reliability, which is poor even for the definition of the presence of varices and for assessment of their size and volume (Winkfield 2003). In such a case, the non‐invasive test could replace the reference standard. However, for a non‐invasive test to replace oesophago‐gastro‐duodenoscopy as the preferred diagnostic test for varices, it should accurately demonstrate the presence of varices while providing qualitative information that currently can be gained only from endoscopy. It is important to note that the non‐invasive test should be able to predict the risk of variceal bleeding with as much or greater accuracy than oesophago‐gastro‐duodenoscopy
Many non‐invasive tests have been proposed for the diagnosis of oesophageal varices. This systematic review is one of five that have examined the diagnostic utility of these tests (Gana 2010a; Gana 2010b; Gana 2010c; Colli 2014b).
Target condition being diagnosed
Oesophageal varices
Oesophageal varices of any size were diagnosed. Oesophageal varices are dilated blood vessels within the wall of the oesophagus that develop when resistance to blood flow through the liver is increased as the result of cirrhosis or portal vein obstruction. Large oesophageal varices are associated with greater risk of bleeding than are smaller varices. Red marks (or red signs) on varices diagnosed during oesophago‐gastro‐duodenoscopy have also been associated with increased bleeding risk (JSPH 1980; NIEC 1988; Garcia‐Tsao 2007; Garcia‐Tsao 2008). Medium varices were classified as large varices, as suggested by the American Association for the Study of Liver Diseases, because recommendations for management of medium‐sized varices are the same as for large varices (Garcia‐Tsao 2007).
Index test(s)
Platelet count, spleen length, and platelet count‐to‐spleen length ratio
If non‐invasive tests predict the presence of oesophageal varices with sufficient accuracy, then oesophago‐gastro‐duodenoscopy can be limited to patients identified to be at high risk of varices. Certain blood tests and imaging modalities and calculations based on their results have shown a promising correlation with oesophageal varices. Of these, the most frequently studied non‐invasive tests are platelet count and ultrasound measurements of spleen length. Increased spleen length in patients with chronic liver disease is almost always caused by increased portal pressure (Pockros 2002; Liangpunsakul 2003). Thrombocytopenia may be the result of splenic pooling of platelets due to portal hypertension, immune‐mediated mechanisms, or reduced thrombopoietin synthesis (Peck‐Radosavljevic 2000; Giannini 2003a; Peck‐Radosavljevic 2007). Integrating platelet count and spleen length in a ratio provides a measure of the degree of thrombocytopenia that may result from hypersplenism. This review aims to evaluate the diagnostic accuracy of platelet count, spleen length, or platelet count‐to‐spleen length ratio in predicting the presence of oesophageal varices.
Clinical pathway
At the time of diagnosis of hepatic cirrhosis of whatever aetiology, an oesophago‐gastro‐duodenoscopy is recommended to detect the presence of oesophageal varices and to define the risk of their rupture and bleeding while providing an overall prognostic assessment. In the case of high‐risk varices (large varices or presence of red marks), primary prophylaxis with a non‐selective beta‐blocker or endoscopic banding ligation of varices has been demonstrated to be effective and hence is recommended (D'Amico 1999; Imperiale 2001; Gluud 2007; Gluud 2012). If oesophago‐gastro‐duodenoscopy reveals no varices, a repeated examination is recommended in three years. If low‐risk varices are seen (small varices without red marks), then oesophago‐gastro‐duodenoscopy should be repeated in two years. If small varices are associated with red signs or with Child‐Pugh score B‐C (Pugh 1973), non‐selective beta‐blocker prophylaxis is recommended (Garcia‐Tsao 2007; Garcia‐Tsao 2008; ASGE Standards of Practice Committee 2012; de Franchis 2015).
Prior test(s)
The diagnosis of liver cirrhosis usually is based on clinical judgement derived from history, laboratory testing, physical examination, imaging, liver histology, or a combination of these. No prior test is recommended by the guidelines before screening with oesophago‐gastro‐duodenoscopy of oesophageal varices when the diagnosis of cirrhosis is made.
Role of index test(s)
The possible role of platelet count, spleen length, and platelet count‐to‐spleen length ratio involves screening people with a diagnosis of cirrhosis for the presence of varices, sparing oesophago‐gastro‐duodenoscopy in people with negative results. Furthermore, these non‐invasive tests could even be so accurate in detecting high‐risk varices (large varices or presence of red marks) for which primary prophylaxis is recommended that they could replace oesophago‐gastro‐duodenoscopy.
Alternative test(s)
Some non‐invasive tests other than platelet count, spleen length, and platelet count‐to‐spleen length ratio have been proposed for the diagnosis of oesophageal varices, such as serum markers for liver fibrosis, transient elastography, or imaging with ultrasound computed tomography, magnetic resonance, or capsule endoscopy (Colli 2014b).
We will examine some of these tests in future planned reviews (Gana 2010a; Gana 2010b; Gana 2010c).
Rationale
Effective prevention of the first variceal haemorrhage (primary prophylaxis) in adults with medium or large varices can be achieved via non‐selective beta‐blockers or endoscopic variceal ligation (D'Amico 1999; Imperiale 2001; Gluud 2007). Therefore, both North American (Grace 1998; Adams 2004; Garcia‐Tsao 2007; Garcia‐Tsao 2008) and European guidelines (Jalan 2000; Garcia‐Tsao 2008; EASL 2011; Tripathi 2015; NICE 2016) recommend endoscopy at the time of diagnosis of cirrhosis and at intervals thereafter to identify at‐risk patients who might benefit from prophylactic treatment. These guidelines require that patients repeatedly undergo an unpleasant invasive procedure with its attendant risks, although half have no identifiable oesophageal varices 10 years after the initial diagnosis of cirrhosis. Oesophago‐gastro‐duodenoscopy requires appropriate sedation and analgesia (Cotton 2006) and is associated with an overall complication rate of 0.13% and a mortality rate of 0.004% (Silvis 1976).
Two cost‐effectiveness studies suggested avoidance of surveillance oesophago‐gastro‐duodenoscopy and treatment with non‐selective beta‐blockers for all people with cirrhosis, irrespective of the presence or size of varices (Saab 2003; Spiegel 2003). A third cost‐effectiveness analysis suggested that this non‐selective strategy should be reserved for people with decompensated liver disease (Arguedas 2002). Those conflicting cost‐effectiveness recommendations do not recognise that non‐selective beta‐blockers do not prevent the development of oesophageal varices (Groszmann 2005). Therefore, oesophago‐gastro‐duodenoscopy remains the recommended test for the diagnosis and prognosis of oesophageal varices (Garcia‐Tsao 2007; Garcia‐Tsao 2008).
In view of the invasive nature and costs of oesophago‐gastro‐duodenoscopy, a non‐invasive test with adequate accuracy could serve as a screening test. Such a test would assist in triaging people before oesophago‐gastro‐duodenoscopy, and, if varices of sufficient risk of bleeding are present, primary prophylaxis will be recommended to prevent variceal haemorrhage. Non‐invasive tests for varices, if sufficiently accurate in detecting high‐risk varices, could even replace oesophago‐gastro‐duodenoscopy, which is still the preferred test for diagnosing oesophageal varices. For these reasons, we aimed (1) to assess the ability of platelet count, spleen length, and platelet count‐to‐spleen length ratio to triage people for oesophago‐gastro‐duodenoscopy investigation, and (2) to determine whether this approach could replace oesophago‐gastro‐duodenoscopy.
Objectives
Primary objectives
To determine the diagnostic accuracy of platelet count, spleen length, and platelet count‐to‐spleen length ratio for the diagnosis of oesophageal varices of any size in paediatric or adult patients with chronic liver disease or portal vein thrombosis, irrespective of their aetiology. To investigate the accuracy of these non‐invasive tests as triage or replacement of oesophago‐gastro‐duodenoscopy. We considered separately studies with adult participants and studies with paediatric participants.
Secondary objectives
To compare the diagnostic accuracy of platelet count, spleen length, and platelet count‐to‐spleen length ratio for the diagnosis of high‐risk oesophageal varices in paediatric or adult patients with chronic liver disease or portal vein thrombosis, irrespective of aetiology.
We aimed to perform pair‐wise comparisons between the three index tests, while considering predefined cut‐off values, as reported in the 'Index test' section.
We investigated the following sources of heterogeneity.
Chronic liver disease compared with portal vein thrombosis.
Prevalence of oesophageal varices in the study group (≥ 50% versus < 50% for any varices; > 25% versus ≤ 25% for high‐risk varices).
Severity of liver disease Child A (> 50% versus ≤ 50%).
Different aetiologies (hepatitis C virus (HCV)‐associated cirrhosis versus cirrhosis of all causes).
Methods
Criteria for considering studies for this review
Types of studies
We aimed to include studies that, irrespective of publication status and language, evaluated the diagnostic accuracy of platelet count, spleen length, and platelet count‐to‐spleen length ratio for the diagnosis of oesophageal varices with oesophago‐gastro‐duodenoscopy as the reference standard. We considered studies of cross‐sectional cohort design including people with clinical suspicion of portal hypertension as well as studies of participant‐control design that compared people with oesophageal varices versus matched controls (Colli 2014a). We excluded studies that analysed data only per varix rather than per participant unless participant data were made available by study authors.
Participants
Participants included paediatric or adult patients of any age with chronic liver disease or portal vein thrombosis, irrespective of aetiology, severity of disease, and duration of illness, in whom the presence or absence of varices was confirmed by oesophago‐gastro‐duodenoscopy. The review focused on diagnostic questions related to patients who have not yet suffered gastrointestinal bleeding from oesophageal varices. Patients with a previous surgical portal‐systemic shunt procedure or insertion of a transjugular intrahepatic portal‐systemic shunt (TIPS), previous ligation or sclerotherapy of oesophageal varices, previous history of upper gastrointestinal portal hypertensive bleeding, or previous primary prophylactic therapy of variceal haemorrhage make up a distinct group for whom the diagnosis or natural history of oesophageal varices has been modified. These patients were not the focus of this review, hence we excluded studies that included such patients unless investigators presented data in such a way as to allow this patient group to be isolated from other included patients.
Index tests
Platelet count is obtained from a complete blood count, a readily available automated clinical test. A platelet count cut‐off value less than 150,000/mm3 is considered thrombocytopenia.
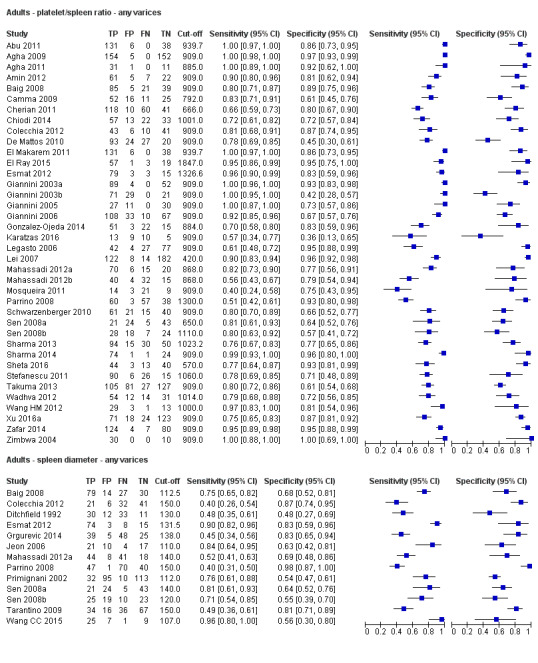
Spleen length is usually obtained through evaluation of the patient's abdomen by ultrasound scan (USS). Interobserver agreement when spleen length is determined with USS is considered excellent. For adults, the upper limit of spleen length is 130 mm, beyond which the spleen is generally considered enlarged. Spleen length of 110 mm is regarded as a sensitive cut‐off for exclusion of splenomegaly (Grover 1993). For children, spleen length is expressed as a standard deviation score relative to normal values for both age and sex (spleen length z‐score) (Megremis 2004).
Platelet count‐to‐spleen length ratio is a derivative mathematical model shown to increase the accuracy of both non‐invasive tests for the diagnosis of oesophageal varices. The cut‐off value used most often for adults is 909 (n/mm3)/mm. In children, platelet count‐to‐spleen length ratio is calculated using the spleen length z‐score.
Target conditions
The presence of any oesophageal varices (independent of size) was detected by oesophago‐gastro‐duodenoscopy. For secondary analyses, the target condition considered was the presence of oesophageal varices at high risk of bleeding. High‐risk varices were defined as medium or large varices or small varices with red marks, or in patients with decompensated cirrhosis, as assessed by a B‐C Child‐Pugh score (Garcia‐Tsao 2007). Studies will require at least one of two target conditions to be identified: the presence of any oesophageal varices, or the presence of high‐risk varices.
Reference standards
Oesophago‐gastro‐duodenoscopy is the clinical reference standard test for the diagnosis of oesophageal varices in which the presence of varices in the oesophagus is directly observed through the endoscope. The size and appearance of oesophageal varices are graded at the time of endoscopy according to one of the systems described below, and the largest varix identified is used to classify the patient. Severity of cirrhosis, which is the other factor that defines bleeding risk, is assessed by Child‐Pugh score, with three classes ‐ A, B, and C ‐ indicating increasing severity (Pugh 1973). Patients whose largest varix is medium or large or who are included in class B‐C are considered for prophylactic therapy.
The Baveno Consensus system differentiates small from large oesophageal varices (de Franchis 1992), defining small oesophageal varices as varices that flatten with insufflation during endoscopy or that minimally protrude into the oesophageal lumen, and large oesophageal varices as varices that protrude into the oesophageal lumen and touch each other, or that fill at least 50% of the oesophageal lumen.
The Japanese Research Society for Portal Hypertension used three grades for variceal size (JSPH 1980). Grade 1 varices collapse with insufflation during endoscopy, grade 2 varices do not collapse with insufflation and do not occlude the lumen, and grade 3 varices occlude the lumen. For this review, we will consider grade 2 as equivalent to medium, and grade 3 as large.
The Japanese classification was revised by the Italian Liver Cirrhosis Project (ILCP) Group (Pagliaro 1988; Zoli 1996), which describes variceal size as the percentage of the radius of the oesophageal lumen that is occupied by the largest varix. A small or grade 1 varix is said to occupy less than 25%, a medium or grade 2 varix occupies 25% to 50%, and a large or grade 3 varix occupies greater than 50% of the radius of the lumen of the oesophagus.
The Cales criteria define varices as small if they flatten with insufflation during endoscopy, medium if they do not flatten with insufflation, and large if they do not flatten with insufflation during endoscopy and are confluent (Cales 1990).
We will include studies applying alternate classifications if adequately described and logically defined.
Red marks are usually noted as present or absent and may be described according to different classifications. Even small varices showing red marks are classified as ’at high risk of bleeding’. The interval between index tests and oesophago‐gastro‐duodenoscopy has to be less than 3 months to avoid possible evolution of the target condition. When a study reported longer time intervals, we included the study but considered it to be at risk of bias. Clinically, patients with medium or large oesophageal varices or with red marks are at greatest risk of haemorrhage; therefore, we confined secondary analyses to two subgroups: patients with no varices and small varices compared with patients at high risk.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2016), the Cochrane Hepato‐Biliary Group Diagnostic Test of Accuracy Studies Register (Gluud 2016), the Cochrane Library, MEDLINE (OvidSP), Embase (OvidSP), and Science Citation Index ‐ Expanded (Web of Science) (Royle 2003). We have presented in Appendix 1 search strategies along with time spans of the searches. .We applied no language or document‐type restrictions.
Searching other resources
We identified additional references by manually searching the references of articles retrieved from computerised databases and relevant review articles. We sought information on unpublished studies by contacting experts in the field. In addition, we handsearched abstract books from meetings of the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) held over the past 10 years.
Data collection and analysis
We followed available guidelines as provided in the Cochrane Handbook for Diagnostic Test of Accuracy Reviews (DTA Handbook 2010).
Selection of studies
We retrieved publications if they were potentially eligible for inclusion on the basis of abstract review, or if they were relevant review articles for a manual reference search. Two review authors independently reviewed publications for eligibility. To determine eligibility, we assessed each publication to determine whether participants met the inclusion criteria detailed above. We included abstracts only if they provided sufficient data for analysis. We resolved disagreements by consensus.
Data extraction and management
Review authors, working in pairs (JCG and JY or AC and GC), completed a data extraction form for each included study. AC and GC completed extraction forms for studies retrieved during the last search (from 2009 to 2016). Each review author independently retrieved study data. In cases of discordance, we reached consensus through discussion. We retrieved the following data.
General information: title, journal, year, publication status, and study design (prospective vs retrospective).
Sample size: number of participants meeting the criteria and total number of participants screened.
Baseline characteristics: baseline diagnosis, age, sex, race, and disease severity, and medications used concurrently. We considered severity of liver disease among the studied population by using the Child‐Pugh score (Pugh 1973) and the model for end‐stage liver disease (MELD) in adults (Kamath 2001), and by using the Child‐Pugh score and paediatric end‐stage liver disease (PELD) scores in children (McDiarmid 2002).
We reported index tests with all cut‐off values.
We used the following as clinical reference standard tests: variceal size, type of classification used, number of endoscopists, and handling of interobserver error on oesophago‐gastro‐duodenoscopy.
Numbers of true positive (TP), true negative (TN), false positive (FP), and false negative (FN) findings. We extracted these data for each presented cut‐off value and for the two target conditions.
We summarised data from each study in 2 × 2 tables (FP, FN, TP, TN) according to the two target conditions and entered the data into Review Manager 5 software.
Missing data
We contacted primary authors by email to ask for missing data that we needed to build the 2 × 2 tables. When we received no reply, we sent a second email two weeks later. When we still received no reply, we excluded the study.
Assessment of methodological quality
Two review authors independently assessed the risk of bias of included studies using QUADAS‐2 (revised tool for quality assessment of diagnostic accuracy studies) domains (Whiting 2011). In cases of discordance, we reached a consensus through discussion. We adopted the domains in Appendix 2 to address aspects of study quality involving the participant spectrum, index tests, target conditions, reference standards, and flow and timing. We did not plan to consider blinding of the index test to results of the reference standard for cases in which platelet count is obtained by an automated counter. We classified a study as having high risk of bias if we judged study to have high risk of bias or unclear risk of bias in at least one of the domains of QUADAS‐2.
Statistical analysis and data synthesis
We carried out statistical analyses according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (DTA Handbook 2010).
We built 2 × 2 tables (TP, TN, FP, FN) for each primary study for the three index tests for the two target conditions (any varices and high‐risk varices). We considered studies with adult participants and studies with paediatric participants separately, as we retrieved only studies that included only adult or paediatric participants.
For all combinations of index test/target condition/participants, we followed the following strategy of analysis. First, we performed a graphical descriptive analysis of the included studies: We reported forest plots (sensitivity and specificity separately, with their 95% confidence intervals (CIs)), and we provided a graphical representation of studies in the receiver operating characteristic (ROC) space (sensitivity against 1 ‐ specificity). Second, we performed a meta‐analysis. When primary studies reported accuracy estimates of an index test using different cut‐off points, we used the hierarchical summary ROC model (HSROC) to pool data (sensitivities and specificities) and to plot a summary ROC (SROC) curve (Rutter 2001). When considering studies with a common cut‐off value, we used the bivariate model and provided estimates of summary sensitivity and specificity. We used pooled estimates obtained from the fitted models to calculate summary estimates of positive and negative likelihood ratios (LR+ and LR‐, respectively).
For primary studies that reported accuracy results for more than one cut‐off point, we reported sensitivities and specificities for all cut‐off points, but we used a single cut‐off point for each study in HSROC (or bivariate) analysis.
We made pair‐wise comparisons between tests by adding a covariate for the index test to the HSROC (for comparisons of SROC curves) or bivariate (for comparisons of sensitivity and specificity at fixed cut‐off value) model. We assessed the significance of differences in test accuracy by using the log‐likelihood ratio test for comparison of models with and without the index test covariate term. We performed both indirect and direct comparisons, if sufficient data were available.
We considered P values less than 0.05 as two‐sided and statistically significant.
We performed all statistical analyses using SAS statistical software, release 9.4 (SAS Institute Inc., Cary, NC, USA) and macro METADAS (DTA Handbook 2010).
Investigations of heterogeneity
We investigated effects of the following predefined sources of heterogeneity.
Chronic liver disease compared with portal vein thrombosis.
Prevalence of oesophageal varices in the study group (≥ 50% versus < 50% for any varices; > 25% versus ≤ 25% for high‐risk varices).
Severity of liver disease Child A (> 50% versus ≤ 50%).
Different aetiologies (HCV‐associated cirrhosis versus all aetiologies),
by adding covariates to the bivariate or to the HSROC. We assessed the statistical significance of the covariate effect by using the log‐likelihood ratio test for comparison of models with and without the covariate term.
To limit the number of statistical analyses, we investigated sources of heterogeneity by considering only studies with the cut‐off value defined in the "Index test" section.
Sensitivity analyses
We attempted to assess effects of risk of bias of included studies on diagnostic accuracy by performing a sensitivity analysisfrom which we excluded studies with the following characteristics.
-
Studies classified at high risk of bias. We classified a study as having high risk of bias if we judged study to have high risk of bias or unclear risk of bias in at least one of the domains of QUADAS‐2 (Appendix 2). In addition, we identified the two following signalling questions as most relevant, and we decided to assess them in separate sensitivity analyses.
"Was a case‐control design avoided?"
"If a threshold was used, was it prespecified?"
Studies published only in abstract/letter form.
To limit the number of statistical analyses, we performed sensitivity analyses by considering only studies with the cut‐off value defined in the "Index test" section.
Results
Results of the search
We ran the search on 14 June 2016. We identified 3832 references by searching the following databases: the Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 17), the Cochrane Hepato‐Biliary Group Diagnostic Test Accuracy Register (n = 8), the Cochrane Library (n = 73), MEDLINE (OvidSP) (n = 943), Embase (OvidSP) (n = 2188), and Science Citation Index ‐ Expanded (Web of Science) (n = 603). After exclusion of 1172 duplicates, 2660 references remained for possible eligibility. We retrieved five additional references through handsearching. After reading the title and the abstract of these 2665 references, we excluded 2566 of them, as they did not meet the inclusion criteria. We retrieved full texts of the remaining 99 records, and after reading the full texts, we excluded 34 studies for various reasons (see Characteristics of excluded studies). Finally, we included in our review 65 references reporting data on 71 studies (Figure 1).
1.
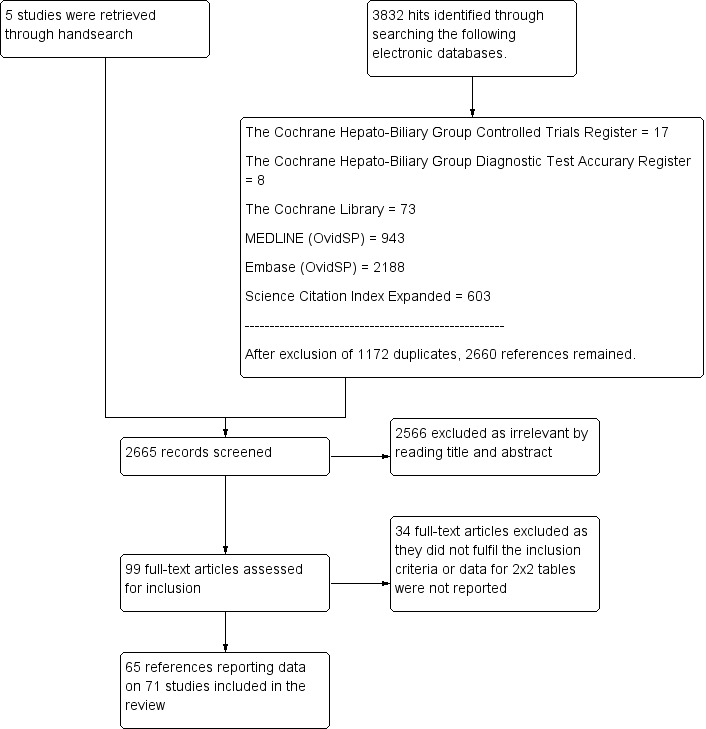
Study flow diagram.
We reported in the Characteristics of included studies tables the main characteristics of the 71 included studies. Investigators reported five studies (Primignani 2002; Lei 2007; Aqodad 2011; El Ray 2015; Wang CC 2015) only in abstract form and three (Zimbwa 2004; Sen 2008a; Sen 2008b) as letters. Four studies (Colecchia 2011; Gana 2011; Alcantara 2012; Adami 2013) included only paediatric participants, and the other 67 studies included only adult participants. All included studies were cross‐sectional studies, prospective or retrospective, conducted at tertiary referral centres. Sixteen studies (Madhotra 2002; Baig 2008; Parrino 2008; Sen 2008a; Sen 2008b; Sarangapani 2010; Schwarzenberger 2010; Cherian 2011; Colecchia 2011; Colecchia 2012; Esmat 2012; Mahassadi 2012a; Mahassadi 2012b; Adami 2013; Chiodi 2014; Grgurevic 2014) assessed the accuracy of more than one index test on the same participants. The number of participants enrolled in each of the 71 included studies ranged from 31 to 1016 (median = 111). Eight studies included only participants in Child‐Pugh class A, three studies did not include any participant in Child‐Pugh class A, and 26 studies did not report Child‐Pugh classification.
Methodological quality of included studies
We have reported in detail results of the quality assessment of included studies in the Characteristics of included studies tables, and we have summarised this information in Figure 2 and Figure 3.
2.
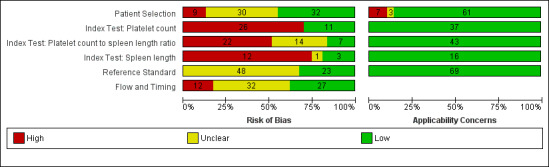
Methodological quality of the 71 included studies.
3.
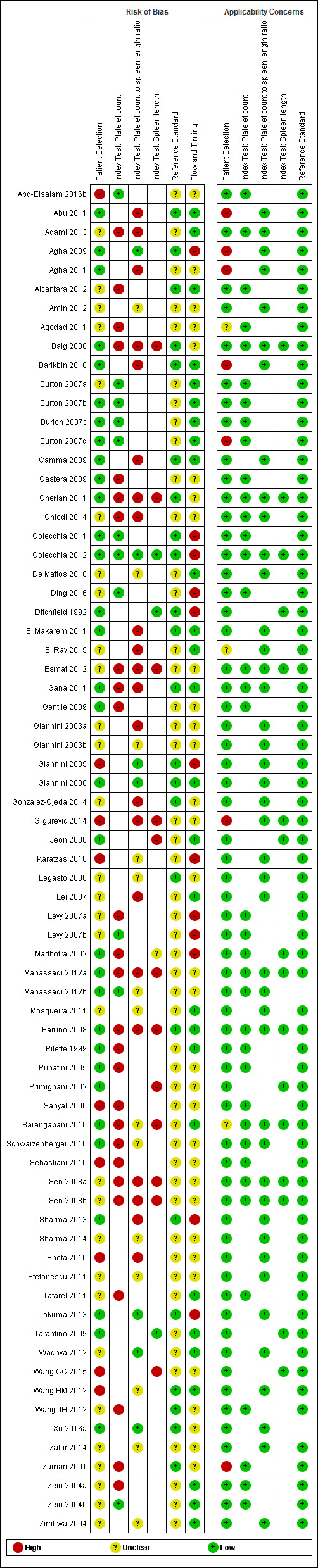
Quality assessment summary: review authors' judgements about each risk of bias item for each included study.
Not all of the included studies considered all three index tests. Cells are empty when an index test was not considered in a study.
Patient selection
All 71 studies were cross‐sectional: 29 studies were prospective, 21 were retrospective, and, in 21 studies, it was not clear whether a prospective or retrospective design was adopted. Thirty‐four studies reported that they enrolled consecutive participants; six studies reported non‐consecutive enrolment of participants (Sebastiani 2010; Wang HM 2012; Grgurevic 2014; Wang CC 2015; Abd‐Elsalam 2016b; Sheta 2016); for the remaining 31 studies, this information was unclear. The authors of three studies did not avoid inappropriate exclusions (Giannini 2005; Sanyal 2006; Karatzas 2016): One study included only people with previous negative screening for oesophageal varices who regularly attended an outpatient clinic and excluded the others (Giannini 2005); one study excluded patients with contraindications for computerised tomography, which was one of the index tests considered in that study (Karatzas 2016); one study included only participants from an interventional randomised clinical trial according to the exclusion criteria of this trial (Sanyal 2006). In eight other studies, information about exclusions was unclear. In summary, we classified nine studies as having high risk of bias, 30 studies unclear risk of bias, and 32 low risk of bias for the patient selection domain.
We had high concern regarding patient selection in seven studies, as they included mainly participants with advanced and decompensated disease (Zaman 2001; Burton 2007d; Agha 2009; Barikbin 2010; Abu 2011; Agha 2011; Grgurevic 2014); we had unclear concern about three studies that did not report a definition for severity of liver disease (Sarangapani 2010; Aqodad 2011; El Ray 2015).
Index tests
Platelet count: We considered 11 studies to have low risk of bias, and 26 to have high risk of bias.
Spleen length: We considered three studies to have low risk of bias, and 12 to have high risk of bias as the threshold value was not predefined and/or blind interpretation of results was not ensured (Primignani 2002; Jeon 2006; Baig 2008; Parrino 2008; Sen 2008a; Sen 2008b; Sarangapani 2010; Cherian 2011; Esmat 2012; Mahassadi 2012a; Grgurevic 2014; Wang CC 2015). One study provided a predefined cut‐off value but blinding presented unclear risk of bias (Madhotra 2002).
Platelet count‐to‐spleen length ratio: We considered seven studies to have low risk of bias, 22 high risk of bias, and 14 unclear risk of bias as the threshold value was not predefined and/or blind interpretation of results was not clearly ensured.
We had no applicability concerns.
Reference standards
All studies used an acceptable reference standard: gastrointestinal endoscopy with varices graded according to a recognised common scoring system. We had some concerns regarding blinded (without knowledge of results of the index tests) interpretation of the reference standard. Investigators in 23 studies reported that reference standard results were interpreted without knowledge of the results, and 48 studies provided unclear information on this. On the basis of these results, we classified 48 studies as having unclear risk of bias and 23 as having low risk of bias for the reference standard domain. We had no concerns regarding applicability.
Flow and timing
All participants underwent the same reference standard in all studies. The time interval between the index test and the reference standard execution was appropriate (i.e. < 3 months) in 34 studies, was inappropriate in one study (Ding 2016; time interval < 6 months), and was not reported in the remaining 36 studies.
Eleven studies excluded some participants from the analysis. Reasons reported by study authors included incomplete information, participants lost to follow‐up, and participants who did not undergo the reference standard or the index test. On the basis of these results, we classified 12 studies as having high risk of bias, 32 unclear risk of bias, and 27 low risk of bias for the flow and timing domain.
Overall assessment
Only one study was at low risk of bias in all four QUADAS‐2 domains (Giannini 2006). We classified 52 studies as having high risk of bias in at least one domain. We judged the remaining 18 studies as having unclear risk of bias.
Funding
Sebastiani 2010 reported under "Financial support" that the first study author "... is funded by an unrestricted grant from Roche‐Italia". Sanyal 2006 reported under "Disclosures" that "This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, the National Center for Minority Health and Health Disparities, and by General Clinical Research Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). Additional funding to conduct this study was supplied by Hoffmann‐La Roche, Inc, through a Cooperative Research and Development Agreement with the National Institutes of Health".
Eighteen studies reported that they received no funding. The remaining 51 studies provided no information on funding.
Findings
Adult participants ‐ any varices
Platelet count for any varices
Any cut‐off value
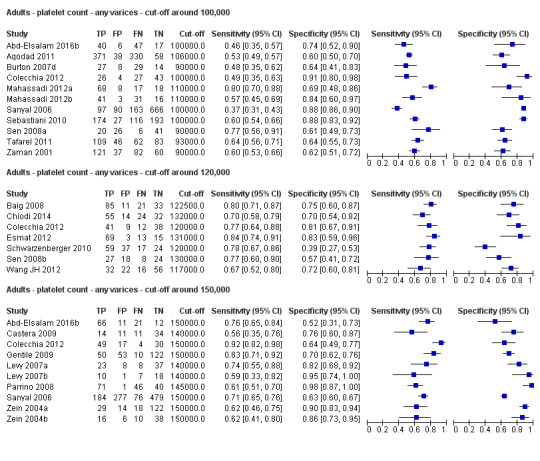
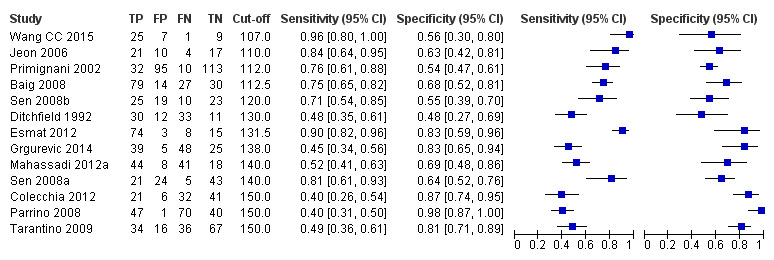
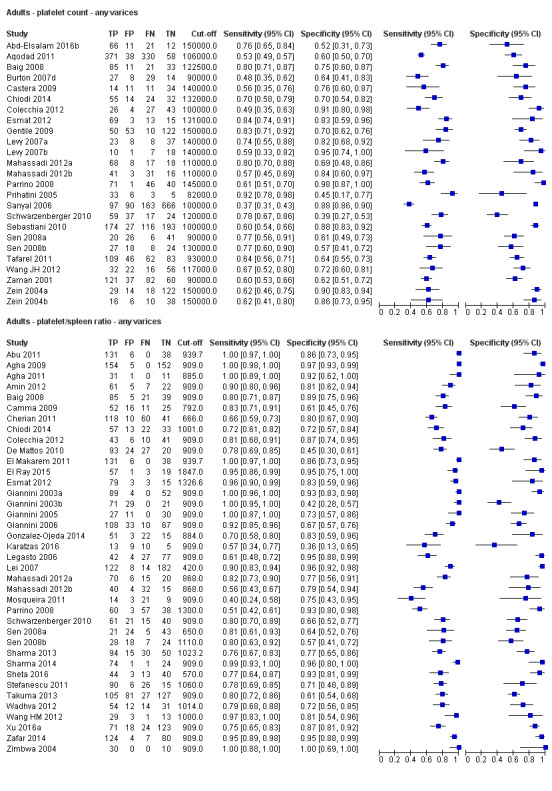
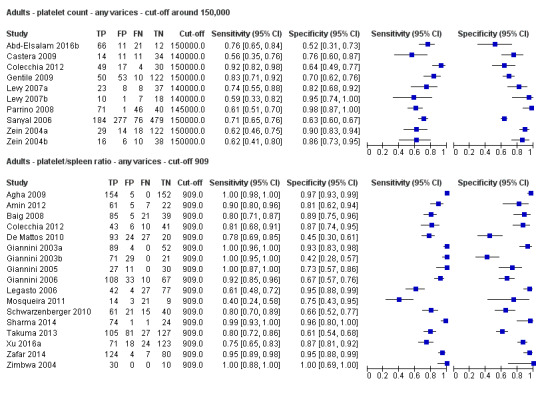
Twenty‐five studies with 5096 participants provided data assessing platelet count for the presence of any varices. The median prevalence of the target disease was 57% (range 26% to 88%). Cut‐off values ranged from 82,000 to 150,000/mm3. Sensitivity of platelet count for the diagnosis of oesophageal varices of any size ranged from 0.37 to 0.92, and specificity ranged from 0.39 to 0.98 (Figure 4).
4.
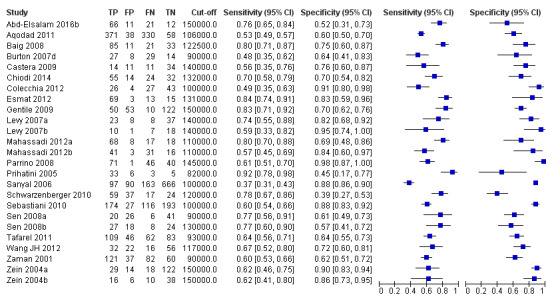
Forest plot. Adult participants ‐ platelet count ‐ any varices.
We then carried out three meta‐analyses that included only studies that reported a cut‐off value of around 100,000/mm3, around 120,000/mm3, and around 150,000/mm3.
Cut‐off value around 100,000/mm3
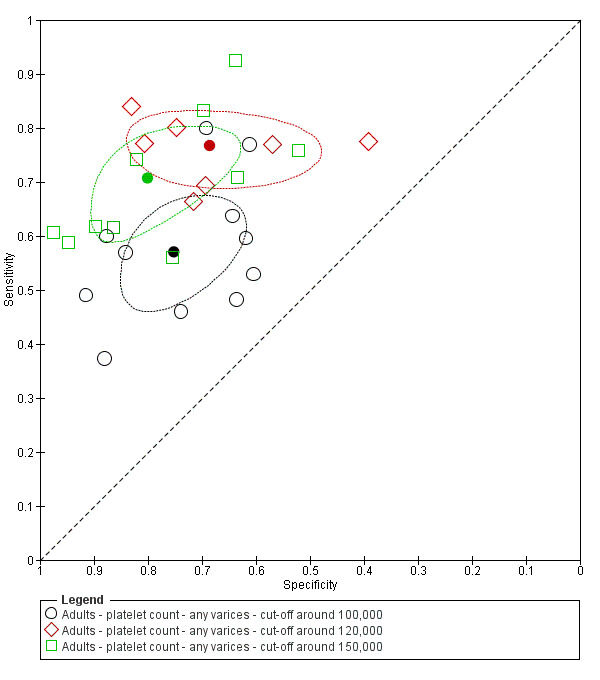
Eleven studies with 3506 participants provided data using a cut‐off value of around 100,000/mm3 (range 90,000 to 110,000/mm3). Sensitivity of the 11 studies varied from 0.37 to 0.80, and specificity from 0.60 to 0.91 (Figure 5). By using the bivariate model, we obtained the following estimates: sensitivity 0.57 (95% CI 0.50 to 0.64), specificity 0.75 (95% CI 0.67 to 0.82), LR+ 2.3 (95% CI 1.7 to 3.1), and LR‐ 0.57 (95% CI 0.49 to 0.67) (Figure 6).
5.
Forest plots. Adult participants ‐ platetelet count ‐ various cut‐off values ‐ any varices.
6.
Studies in the ROC space. Adult participants ‐ platelet count ‐ various cut‐off values ‐ any varices.
Cut‐off value around 120,000/mm3
Seven studies with 815 participants provided data using a cut‐off value of around 120,000/mm3 (range 117,000 to 132,000/mm3). Sensitivity of the seven studies varied from 0.67 to 0.84, and specificity from 0.39 to 0.83 (Figure 5). By using the bivariate model, we obtained the following estimates: sensitivity 0.77 (95% CI 0.72 to 0.81), specificity 0.69 (95% CI 0.57 to 0.78), LR+ 2.4 (95% CI 1.7 to 3.5), and LR‐ 0.34 (95% CI 0.26 to 0.44) (Figure 6).
Cut‐off value around 150,000/mm3
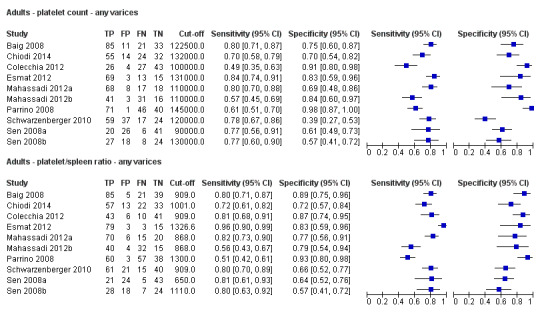
Ten studies with 2054 participants provided data using a cut‐off value of around 150,000/mm3 (range 140,000 to 150,000/mm3). Sensitivity of the 10 studies varied from 0.56 to 0.92, and specificity from 0.52 to 0.98 (Figure 5). By using the bivariate model, we obtained the following estimates: sensitivity 0.71 (95% CI 0.63 to 0.77), specificity 0.80 (95% CI 0.69 to 0.88), LR+ 3.6 (95% CI 2.4 to 5.4), and LR‐ 0.37 (95% CI 0.30 to 0.45) (Figure 6).
Heterogeneity analysis
We investigated heterogeneity while considering only studies with a cut‐off value of around 150,000/mm3 ‐ the predefined cut‐off value. We found no effect of prevalence of varices (≤ 50% vs > 50%) or Child A on accuracy. We found an effect of aetiology (P = 0.036). Sensitivity and specificity were 0.76 (95% CI 0.60 to 0.86) and 0.63 (0.59 to 0.67) for the four studies that included only participants with HCV. Sensitivity and specificity were 0.71 (95% CI 0.66 to 0.76) and 0.88 (95% CI 0.83 to 0.91) for the four studies that included participants with mixed aetiology.
Sensitivity analysis
When considering Zein 2004b, Levy 2007b, Colecchia 2012, and Abd‐Elsalam 2016b, with a prespecified cut‐off value among all studies of around 150,000/mm3, we obtained sensitivity of 0.74 (95% CI 0.57 to 0.86) and specificity of 0.78 (95% CI 0.57 to 0.90). We could not perform the remaining sensitivity analyses, as all studies were cross‐sectional, all were at high/unclear risk of bias, and all were published as full text.
Spleen length for any varices
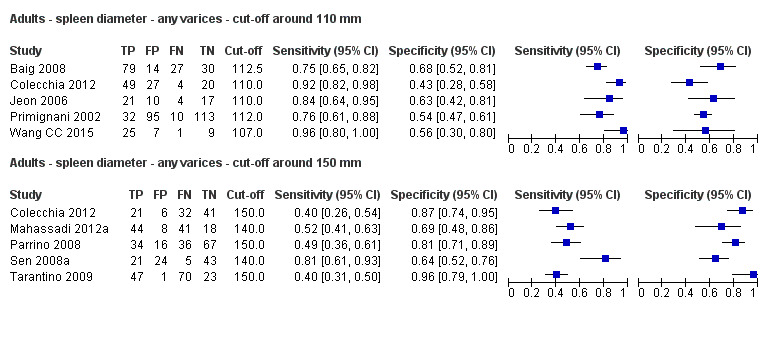
Any cut‐off value
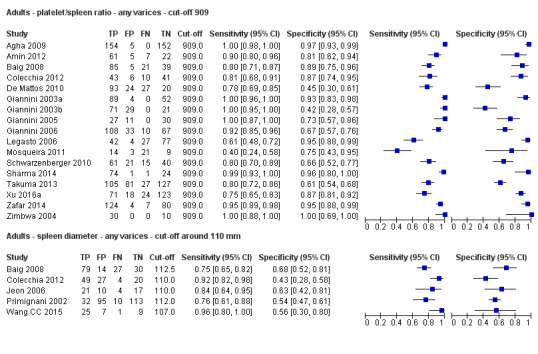
Thirteen studies with 1489 participants provided data on assessment of spleen length for the presence of any varices. The median prevalence of the target disease was 62% (range 17% to 82%). Sensitivity of the 13 studies varied from 0.40 to 0.96, and specificity from 0.48 to 0.98. Cut‐off values ranged from 107 to 150 mm (Figure 7). We included in this analysis one study reporting data on two cut‐offs (110 mm and 150 mm) by using only the cut‐off of 150 mm (Colecchia 2012).
7.
Forest plot. Adult participants ‐ spleen length ‐ any varices.
Cut‐off value around 110 mm
Five studies with 594 participants reported data using a cut‐off value of around 110 mm (range 110 to 112.5 mm). Sensitivity of the five studies varied from 0.75 to 0.96, and specificity from 0.43 to 0.68 (Figure 8). By using the bivariate model, we obtained the following estimates: sensitivity 0.85 (95% CI 0.75 to 0.91), specificity 0.54 (95% CI 0.46 to 0.62), LR+ 1.8 (95% CI 1.6 to 2.1), and LR‐ 0.28 (95% CI 0.17 to 0.44) (Figure 9).
8.
Forest plots. Adult participants ‐ spleen length ‐ any varices: various cut‐off values.
9.
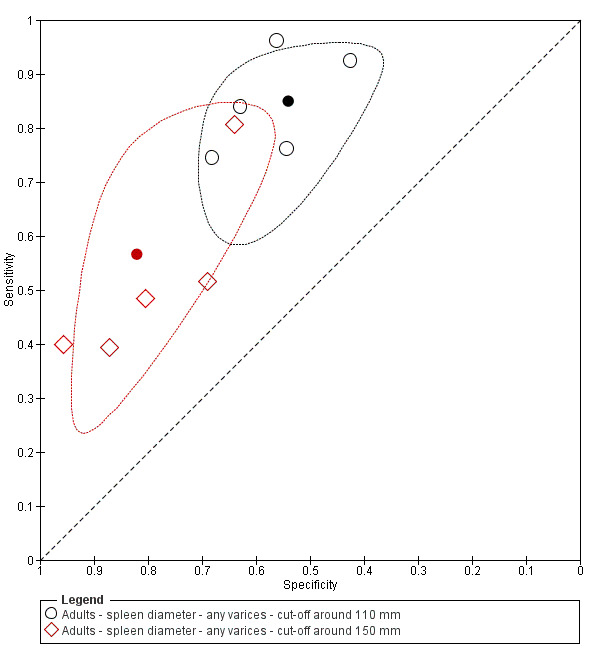
Studies in the ROC space. Adult participants ‐ spleen length ‐ any varices: various cut‐off values.
Cut‐off value around 150 mm
FIve studies with 598 participants reported data using a cut‐off value of around 150 mm (range 140 to 150 mm). Sensitivity of the five studies varied from 0.40 to 0.81, and specificity from 0.64 to 0.96 (Figure 8). By using the bivariate model, we obtained the following estimates: sensitivity 0.57 (95% CI 0.41 to 0.71), specificity 0.82 (95% CI 0.72 to 0.89), LR+ 3.2 (95% CI 2.3 to 4.4), and LR‐ 0.53 (95% CI 0.39 to 0.72) (Figure 9).
Heterogeneity analysis
We could not assess effects of sources of heterogeneity among studies with a cut‐off value around 110 mm, as the models failed to converge owing to the small number of studies.
Sensitivity analysis
In considering studies with a cut‐off value of around 110 mm, when we excluded the two studies reported only in abstract form, we obtained sensitivity of 0.84 (95% CI 0.71 to 0.92) and specificity of 0.58 (95% CI 0.43 to 0.71) (Primignani 2002; Wang CC 2015). We could not perform the remaining sensitivity analyses because all studies were cross‐sectional and were at high/unclear risk of bias, and all but one of the studies used a prespecified cut‐off value.
Platelet count‐to‐spleen length ratio for any varices
Any cut‐off value
Thirty‐eight studies with 5235 participants provided data on assessment of platelet count to spleen length for the presence of varices of any size. The median prevalence of varices was 65% (range 28% to 85%). Sensitivity of the 38 studies varied from 0.40 to 1.00, and specificity from 0.36 to 1.00. Cut‐off values ranged from 420 to 1847 (n/mm3)/mm (Figure 10).
10.
Forest plot. Adult participants ‐ platelet count‐to‐spleen length ratio ‐ any varices.
We then carried out a meta‐analysis including only studies that reported a cut‐off value of 909 (n/mm3)/mm.
Cut‐off value of 909 (n/mm3)/mm
Seventeen studies with 2637 participants provided data using a cut‐off value of 909 (n/mm3)/mm. Sensitivity of the 17 studies varied from 0.40 to 1.00, and specificity from 0.42 to 1.00. By using the bivariate model, we obtained the following estimates: sensitivity 0.93 (95% CI 0.83 to 0.97), specificity 0.84 (95% CI 0.75 to 0.91), LR+ 5.9 (95% CI 3.5 to 9.9), and LR‐ 0.09 (95% CI 0.03 to 0.22) (Figure 11).
11.
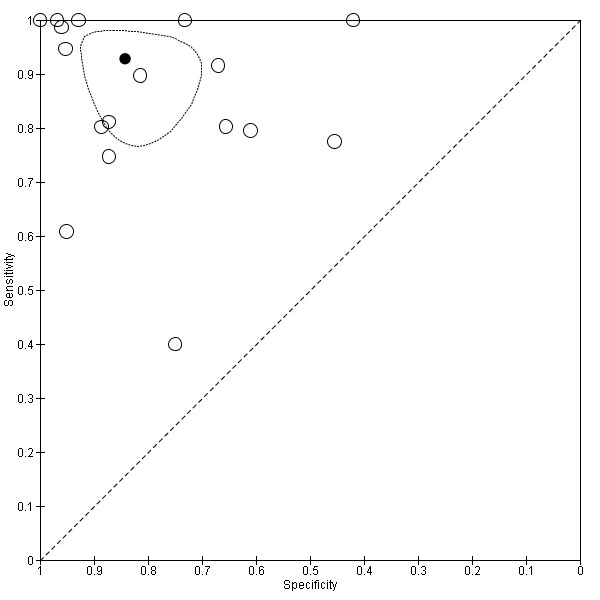
Studies in the ROC space. Adult participants ‐ platelet count‐to‐spleen length ratio. Only studies with a cut‐off value of 909 (n/mm3)/mm ‐ any varices.
Heterogeneity analysis
We investigated effects of sources of heterogeneity among studies using a cut‐off value of 909 (n/mm3)/mm. We found no effect of prevalence of varices, of prevalence of Child A participants, or of aetiology.
Sensitivity analysis
We could not perform the remaining sensitivity analyses, as all studies were cross‐sectional, all but one were at high/unclear risk of bias (Giannini 2006), all but one were published as full text (Zimbwa 2004), and all but one used a prespecified cut‐off value (Giannini 2003a).
Comparative analysis of tests for any varices
Platelet count compared with spleen length
We compared the accuracy of platelet count (25 studies) and spleen length (13 studies) for the presence of any varices (Figure 12) among all included studies (indirect comparisons) using varying cut‐off values. The HSROC model analysis showed a statistically significant result (P = 0.001), suggesting higher overall accuracy of the platelet count test.
12.
Indirect comparison. Forest plot. Adult participants ‐ platelet count compared with spleen length ‐ any varices.
When we compared the 10 studies that reported a cut‐off value of 150,000/mm3 for platelet count with the five studies that reported a cut‐off value of around 110 mm for spleen length (indirect comparison; Figure 13), we observed higher accuracy of platelet count (P = 0.021; Figure 14; Table 6).
13.
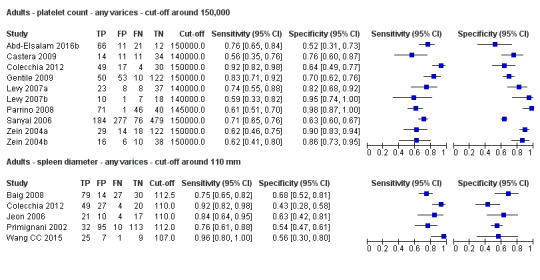
Forest plot. Indirect comparison. Adult participants ‐ platelet count (cut‐off around 150,000) compared with spleen length (cut‐off around 110 mm) ‐ any varices.
14.
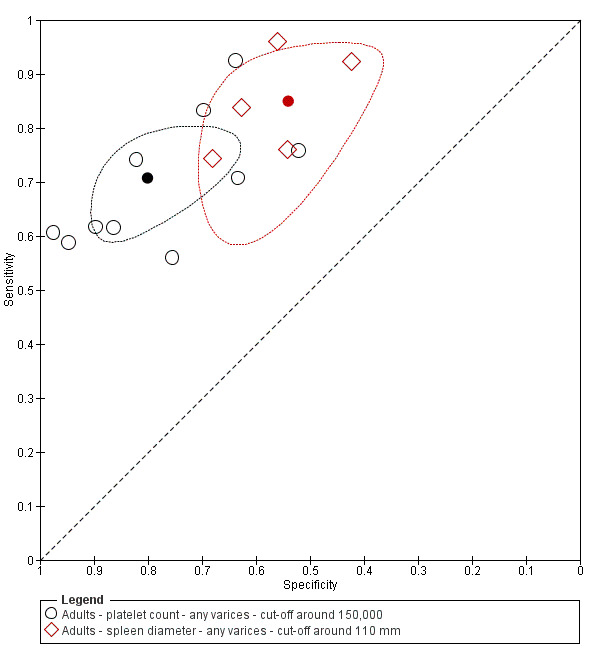
Indirect comparison. Studies in the ROC space. Adult participants ‐ platelet count (cut‐off around 150,000) compared with spleen length (cut‐off around 110 mm) ‐ any varices.
1. Any varices ‐ comparisons between tests.
| Any varices ‐ indirect comparisons | |||||
| Index test | No. of studies | Cut‐off value |
Sensitivity (95% CI) |
Specificity (95% CI) |
P value* |
| Platelet count | 10 | Around 150,000/mm3 | 0.71 (0.63 to 0.77) |
0.80 (0.69 to 0.88) |
0.252 |
| Platelet count‐to‐spleen length ratio | 17 | 909 (n/mm3)/mm | 0.93 (0.83 to 0.97) |
0.84 (0.75 to 0.91) |
|
| Platelet count | 9 | Around 150,000/mm3 | 0.71 (0.63 to 0.77) |
0.80 (0.69 to 0.88) |
0.021 |
| Spleen length | 5 | Around 110 mm | 0.85 (0.75 to 0.91) |
0.54 (0.46 to 0.62) |
|
| Platelet count‐to‐spleen length ratio | 17 | 909 (n/mm3)/mm | 0.93 (0.83 to 0.97) |
0.84 (0.75 to 0.91) |
<0.001 |
| Spleen length | 5 | Around 110 mm | 0.85 (0.75 to 0.91) |
0.54 (0.46 to 0.62) |
|
* Pair‐wise comparisons between index tests performed by adding the index test as covariate to the bivariate model. P values were obtained by comparing the ‐2 log likelihood of the model with the covariate with the ‐2 log likelihood of the model without the covariate.
Platelet count compared with platelet count‐to‐spleen length ratio
We compared the accuracy of platelet count (25 studies) and platelet count‐to‐spleen length ratio (38 studies) for the presence of any varices among all included studies (indirect comparisons; Figure 15) using varying cut‐off values. The HSROC model analysis showed a statistically significant result (P < 0.001), suggesting higher overall accuracy of the platelet count‐to‐spleen length ratio test. We performed HSROC analysis that was limited to the 10 studies reporting data on both index tests (Figure 16); we again found a statistically significant result favouring the ratio (P = 0.007; direct comparisons).
15.
Indirect comparison. Forest plots. Adult participants ‐ platelet count compared with platelet count‐to‐spleen length ratio ‐ any varices.
16.
Direct comparison. Forest plots. Adult participants ‐ platelet count compared with platelet count‐to‐spleen length ratio ‐ any varices.
On the contrary, when we compared the 17 studies that reported a cut‐off value of 909 (n/mm3)/mm for platelet count‐to‐spleen length ratio with the 10 studies that reported a cut‐off value of around 150,000/mm3 for platelet count (indirect comparison; Figure 17), we observed a non‐statistically significant result (P = 0.252; Figure 18; Table 6). Only one study (Colecchia 2012) provided data for direct comparison.
17.
Indirect comparison. Forest plots. Adult participants ‐ platelet count (cut‐off around 150.000/mm3) compared with platelet count‐to‐spleen length ratio (cut‐off 909 (n/mm3)/mm) ‐ any varices.
18.
Indirect comparison. Studies in the ROC space. Adult participants ‐ platelet count (cut‐off around 150.000) compared with platelet count‐to‐spleen length ratio (cut‐off 909 (n/mm3)/mm) ‐ any varices.
Platelet count‐to‐spleen length ratio compared with spleen length
We compared the accuracy of platelet count‐to‐spleen length ratio (38 studies) and spleen length (13 studies) for the presence of any varices among all included studies (indirect comparisons; Figure 19) using varying cut‐off values. The HSROC model analysis showed a statistically significant result (P < 0.001), suggesting higher overall accuracy of the platelet count‐to‐spleen length ratio test .
19.
Indirect comparison. Forest plots. Adult participants ‐ platelet count‐to‐spleen length ratio compared with spleen length ‐ any varices.
When we compared the 17 studies that reported a cut‐off value of 909 (n/mm3)/mm for platelet count‐to‐spleen length ratio with the five studies that reported a cut‐off value of around 110 mm for spleen length (indirect comparison; Figure 20), we observed higher accuracy of platelet count‐to‐spleen length ratio (P < 0.001; Figure 21; Table 6).
20.
Indirect comparison. Forest plots. Adult participants ‐ platelet count‐to‐spleen length ratio (cut‐off 909 (n/mm3)/mm) compared with spleen length (cut‐off around 110) ‐ any varices.
21.
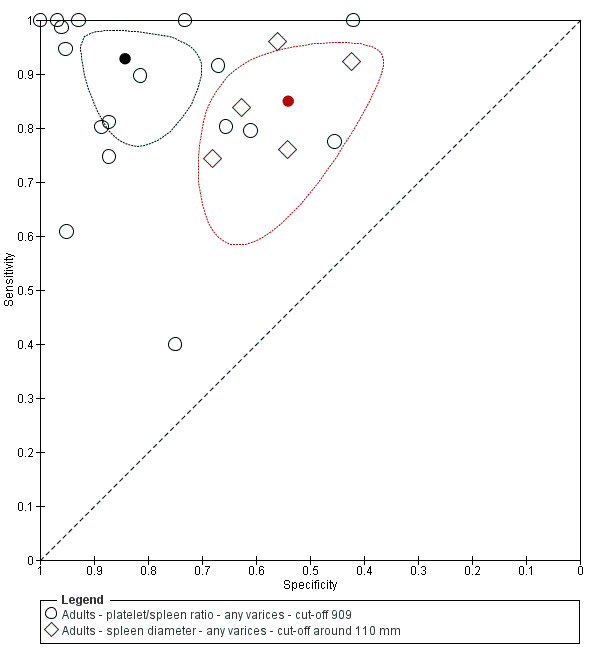
Indirect comparison. Studies in the ROC space. Adult participants ‐ platelet count‐to‐spleen length ratio (cut‐off 909 (n/mm3)/mm) compared with spleen length (cut‐off around 110 mm) ‐ any varices.
Adult participants ‐ high‐risk varices
Platelet count for high‐risk varices
Twenty‐one studies with 4266 participants provided data on assessment of platelet count for the presence of high‐risk varices. The median prevalence of high‐risk varices was 20% (range 4% to 70%). Sensitivity of the 21 studies varied from 0.33 to 1.00, and specificity from 0.39 to 0.87. Cut‐off values ranged from 68,000/mm3 to 160,000/mm3 (Figure 22). We fitted the HSROC model to the 21 studies, and we obtained an estimate of the SROC curve.
22.
Forest plot. Adult participants ‐ platelet count ‐ high‐risk varices.
We carried out two meta‐analyses including only studies that reported a cut‐off value of around 90,000/mm3 and around 150,000/mm3 (Figure 23).
23.
Forest plots. Adult participants ‐ platelet count ‐ various cut‐off values ‐ high‐risk varices.
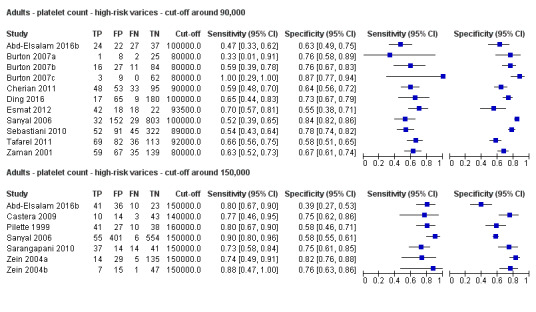
Cut‐off value of around 90,000/mm3
Eleven studies with 3084 participants provided data using a cut‐off value of around 90,000/mm3 (range 80,000 to 100,000/mm3). Sensitivity of the 11 studies varied from 0.33 to 1.00, and specificity from 0.55 to 0.87. By using the bivariate model, we obtained the following estimates: sensitivity 0.59 (95% CI 0.54 to 0.64), specificity 0.72 (95% CI 0.66 to 0.78), LR+ 2.1 (95% CI 1.8 to 2.6), and LR‐ 0.57 (95% CI 0.52 to 0.63) (Figure 24).
24.
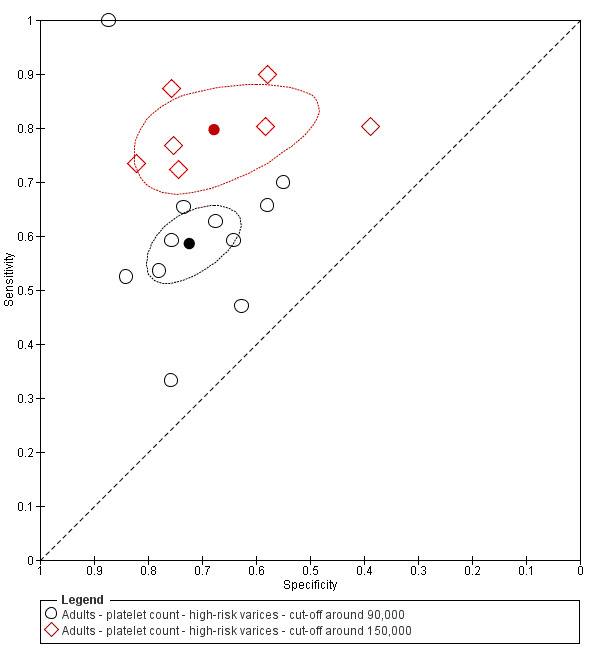
Studies in the ROC space. Adult participants ‐ platelet count ‐ various cut‐off values ‐ high‐risk varices.
Cut‐off value of around 150,000/mm3
Seven studies with 1671 participants provided data using a cut‐off value of around 150,000/mm3 (range 140,000 to 160,000/mm3). Sensitivity of the seven studies varied from 0.73 to 0.90, and specificity from 0.39 to 0.82. By using the bivariate model, we obtained the following estimates: sensitivity 0.80 (95% CI 0.73 to 0.85), specificity 0.68 (95% CI 0.57 to 0.77), LR+ 2.5 (95% CI 1.8 to 3.3), and LR‐ 0.30 (95% CI 0.23 to 0.39) (Figure 24).
Heterogeneity analysis
We could not assess effects of sources of heterogeneity among studies with a cut‐off value of around 150,000/mm3, as the models failed to converge owing to the small number of studies.
Sensitivity analysis
For studies with a cut‐off value of around 150,000/mm3, we could not perform the sensitivity analysis, as all studies were cross‐sectional, all were at high/unclear risk of bias, all were published as full text, and only two reported a prespecified cut‐off value.
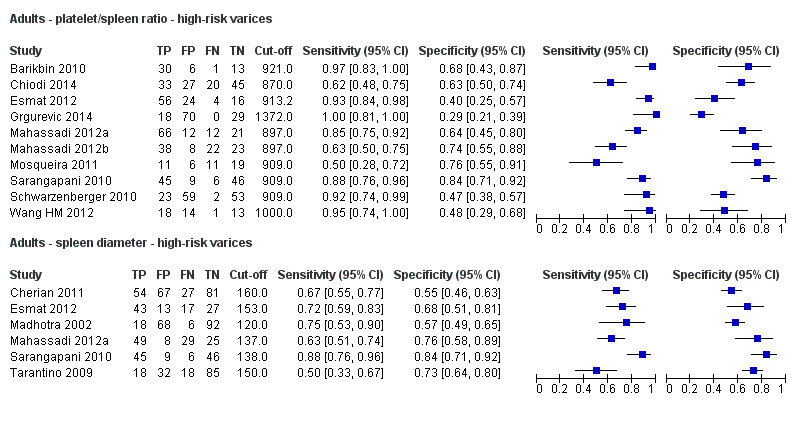
Spleen length for high‐risk varices
Six studies with 883 participants provided data on assessmentof spleen length for the presence of high‐risk varices. The median prevalence of high‐risk varices was 42% (range 13% to 70%). Sensitivity of the six studies varied from 0.50 to 0.88, and specificity from 0.55 to 0.84. Cut‐off values ranged from 120 mm to 160 mm (Figure 25). We used the HSROC model to obtain an estimate of the SROC curve.
25.
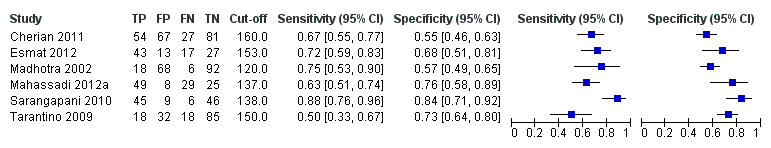
Forest plot. Adult participants ‐ spleen length ‐ high‐risk varices.
Heterogeneity analysis
We found no effects of aetiology. We could not assess effects of Child A and of prevalence of varices, as the models failed to converge owing to the small number of studies.
Sensitivity analysis
We could not perform sensitivity analyses because all studies were cross‐sectional, all were at high/unclear risk of bias, all were published as full text, and only two reported a prespecified cut‐off value.
Platelet count‐to‐spleen length ratio for high‐risk varices
Ten studies with 930 participants provided data for assessment of platelet count‐to‐spleen length ratio for the presence of high‐risk varices. The median prevalence of high‐risk varices was 47% (range 15% to 70%). Sensitivity of the 10 studies varied from 0.50 to 1.00, and specificity from 0.29 to 0.84. Cut‐off values ranged from 870 to 1372 (n/mm3)/mm.(Figure 26).
26.
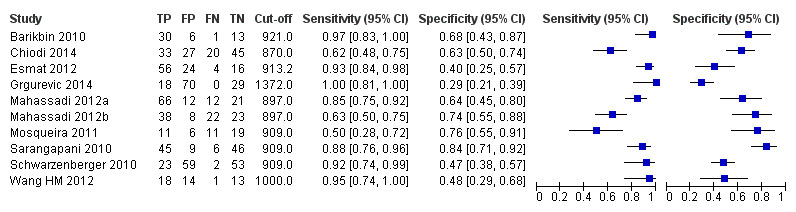
Forest plot. Adult participants ‐ platelet count‐to‐spleen length ratio ‐ high‐risk varices.
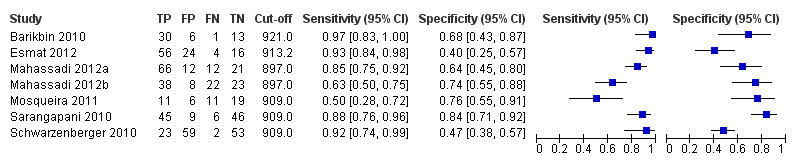
Cut‐off value of around 909 (n/mm3)/mm
Seven studies with 642 participants provided data with a cut‐off value of around 909 (n/mm3)/mm (range 897 to 921 n/mm3/mm; Figure 27). Sensitivity of the seven studies varied from 0.50 to 0.97, and specificity from 0.40 to 0.84. By using the bivariate model, we obtained the following estimates: sensitivity 0.85 (95% CI 0.72 to 0.93), specificity 0.66 (95% CI 0.52 to 0.77), LR+ 2.5 (95% CI 1.8 to 3.4), and LR‐ 0.22 (95% CI 0.12 to 0.42) (Figure 28).
27.
Forest plot. Adult participants ‐ platelet count‐to‐spleen length ratio ‐ cut‐off around 909 (n/mm3)/mm ‐ high‐risk varices.
28.
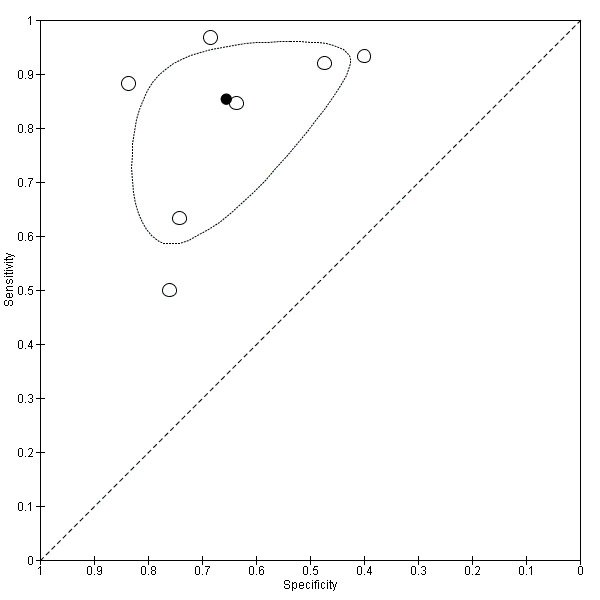
Studies in the ROC space. Adult participants ‐ platelet count‐to‐spleen length ratio ‐ cut‐off around 909 (n/mm3)/mm ‐ high‐risk varices.
Heterogeneity analysis
We investigated effects of sources of heterogeneity among studies with a cut‐off value of 909 (n/mm3)/mm. We found no effect of prevalence of varices nor of aetiology. We could not assess the effect of Child A (≤ 50% vs > 50%), as the models failed to converge owing to the small number of studies.
Sensitivity analysis
Among studies with a cut‐off value of around 909 (n/mm3)/mm, and when considering only those that reported a prespecified cut‐off value, we obtained sensitivity of 0.82 (95% CI 0.55 to 0.94) and specificity of 0.71 (95% CI 0.49 to 0.86). We could not perform the remaining sensitivity analyses because all studies were cross‐sectional, all were at high/unclear risk of bias, and all were published as full text.
Comparative analysis of tests for high‐risk varices
Platelet count compared with spleen length
We fitted the HSROC model to compare the accuracy of platelet count (21 studies) and spleen length (six studies) for the presence of high‐risk varices among all included studies (indirect comparisons; Figure 29), irrespective of the cut‐off value. We observed a non‐statistically significant result (P = 0.304).
29.
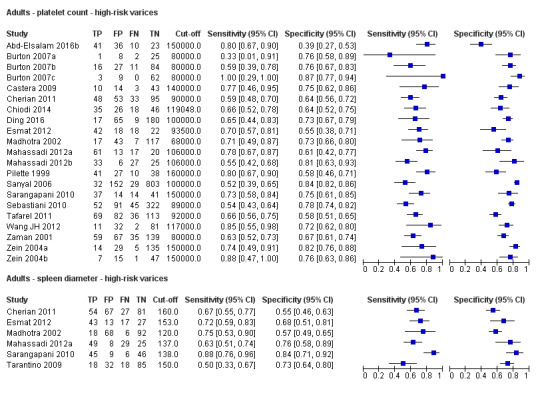
Indirect comparison. Forest plot. Adult participants ‐ platelet count compared with spleen length ‐ high‐risk varices.
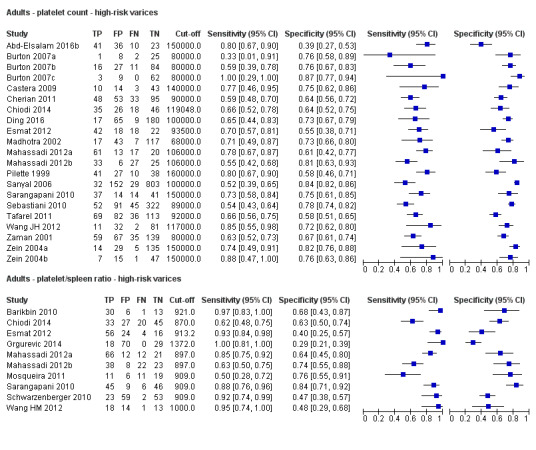
Platelet count compared with platelet count‐to‐spleen length ratio
We compared the accuracy of platelet count (21 studies) and platelet count‐to‐spleen length ratio (10 studies) for the presence of high‐risk varices among all included studies (indirect comparisons; Figure 30). The HSROC model analysis showed a statistically significant result (P = 0.003), suggesting higher overall accuracy of platelet count‐to‐spleen length ratio. We confirmed this result when we performed HSROC analysis limited to the five studies reporting data on both index tests (direct comparisons; P = 0.034) (Figure 31).
30.
Indirect comparison. Forest plots. Adult participants ‐ platelet count compared with platelet count‐to‐spleen length ratio ‐ high‐risk varices.
31.
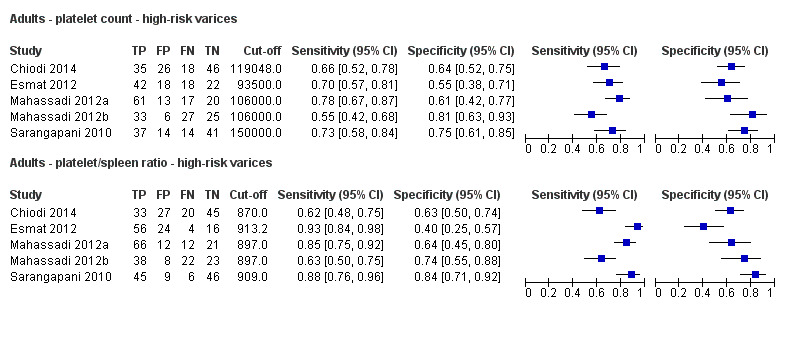
Direct comparison. Forest plots. Adult participants ‐ platelet count compared with platelet count‐to‐spleen length ratio ‐ high‐risk varices.
When we compared the seven studies that reported a cut‐off value of 909 (n/mm3)/mm for platelet count‐to‐spleen length ratio with the seven studies that reported a cut‐off value of 150,000/mm3 for platelet count, we observed a non‐statistically significant result (indirect comparison, bivariate model; P = 0.638) (Figure 32; Figure 33). Only one study reported data on both tests (Sarangapani 2010).
32.
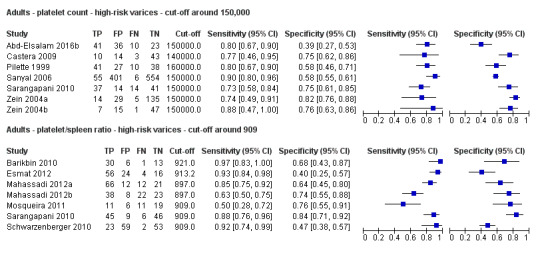
Indirect comparison. Forest plots. Adult participants ‐ platelet count (cut‐off around 150.000/mm3) compared with platelet count‐to‐spleen length ratio (cut‐off 909 (n/mm3)/mm) ‐ high‐risk varices.
33.
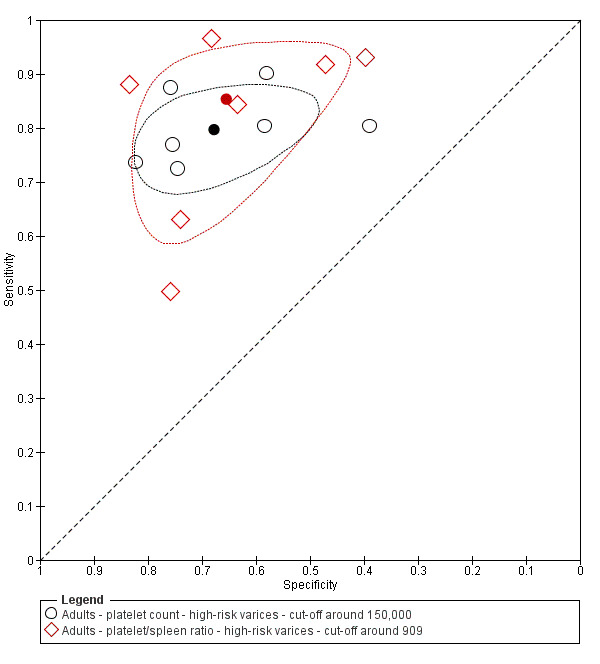
Indirect comparison. Studies in the ROC space. Adult participants ‐ platelet count (cut‐off around 150,000/mm3) compared with platelet count‐to‐spleen length ratio (cut‐off 909 (n/mm3)/mm) ‐ high‐risk varices.
Platelet count‐to‐spleen length ratio compared with spleen length
Finally, when we compared the accuracy of spleen length (six studies) and platelet count‐to‐spleen length ratio (10 studies) for the presence of high‐risk varices among all included studies (indirect comparisons; Figure 34), we observed a statistically significant difference between the two tests (P < 0.001), suggesting higher accuracy of platelet count‐to‐spleen length ratio.
34.
Indirect comparison. Forest plots. Adult participants ‐ platelet count‐to‐spleen length ratio compared with spleen length ‐ high‐risk varices.
Paediatric participants ‐ any varices
We found four studies including 277 paediatric participants with different types of liver disease and/or portal vein thrombosis (Colecchia 2011; Gana 2011; Alcantara 2012; Adami 2013).
Platelet count for any varices
Four studies with 277 paediatric participants provided data on assessment of platelet count for the presence of any varices. Cut‐off values used by the four studies were 115,000/mm3 (three studies) and 119,000/mm3 (one study). Sensitivity of platelet count for diagnosis of oesophageal varices of any size ranged from 0.53 to 0.81, and specificity from 0.71 to 0.94 (Figure 35). We fitted the bivariate model to the four studies, and we obtained the following estimates: sensitivity 0.71 (95% CI 0.60 to 0.80), specificity 0.83 (95% CI 0.70 to 0.91), LR+ 4.2 (95% CI 2.4 to 7.3), and LR‐ 0.35 (95% CI 0.25 to 0.48).
35.
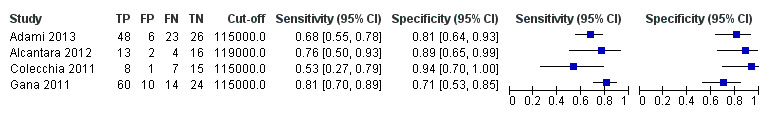
Forest plot. Paediatric participants ‐ platelet count ‐ any varices.
Spleen length z‐score for any varices
We found no studies reporting results of spleen length z‐score for any varices.
Platelet count‐to‐spleen length z‐score ratio for any varices
Two studies with 197 paediatric participants provided data on assessment of platelet count‐to‐spleen length ratio for the presence of any varices. Cut‐off values used by the two studies were 24 and 25. Sensitivities reported by the two studies were 0.69 and 0.82, and specificities 0.79 and 0.53 (Figure 36). We fitted the bivariate model, and we obtained the following estimates: sensitivity 0.74 (95% CI 0.65 to 0.81), specificity 0.64 (95% CI 0.36 to 0.85), LR+ 2.0 (95% CI 1.0 to 4.0), and LR‐ 0.41 (95% CI 0.27 to 0.61).
36.

Forest plot. Paediatric participants ‐ platelet count‐to‐spleen length z‐score ratio ‐ any varices.
Discussion
Summary of main results
We included 71 studies, 67 of which enrolled only adults and four only children. We considered and analysed these four paediatric studies separately because they enrolled only paediatric patients with a different spectrum of the liver disease.
For adults, all included studies were undertaken in a secondary/tertiary care setting, and studies reported a wide range of prevalences of oesophageal varices ‐ both varices of any size and high‐risk varices. We considered all but one of the included studies to be at high risk of bias. We had major concerns about the predefinition of the cut‐off value for the three index tests: Most included studies derived a posteriori the best cut‐off values, overestimating accuracy. Only 10 studies assessed a predefined cut‐off value of platelet count, and only 16 were designed to validate the 909 (n/mm3)/mm cut‐off value for platelet count‐to‐spleen length ratio.
Platelet count‐to‐spleen length ratio seems the most accurate test ‐ more accurate than simple platelet count or spleen length measurement for the diagnosis of varices of any size or high‐risk varices. As expected, combining two measurements in a ratio improved accuracy: For portal hypertension, platelet count (numerator) decreases and spleen length (denominator) increases.
Estimates of sensitivity and specificity obtained by the bivariate model are reported in the 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4; Table 5).
Summary of findings 1. Adult participants ‐ platelet count.
| Review question | What is the diagnostic accuracy of platelet count for the diagnosis of oesophageal varices in adults with liver disease or portal vein thrombosis? | |||||
| Population | Adults with diagnosis of chronic liver disease or portal vein thrombosis. Age ≥ 18 years | |||||
| Settings | Outpatients and inpatients in secondary/tertiary care setting | |||||
| Study design | Prospective and retrospective cross‐sectional studies. No case‐control studies were found | |||||
| Index tests | Platelet count | |||||
| Reference standards | Upper endoscopy | |||||
| Target condition | Summary accuracy (95% CI) | No. of participants (studies) |
Prevalence, Median (range ) |
Implications in a hypothetical cohort of 1000 people | Post‐test probability | Quality and comments |
|
Any varices Cut‐off value: around 150,000 /mm3 (range 140,000 to 150,000/mm3) |
Sensitivity 0.71 (0.63 to 0.77) Specificity 0.80 (0.69 to 0.88) LR+ 3.6 (2.4 to 5.4) LR‐ 0.37 (0.30 to 0.45) |
2054 participants (10) | 38% (25% to 79%) | With a prevalence of 38%, 380 out of 1000 people will have varices of any size. Of these 380 people, 110 (29% of 380) people with varices will receive misdiagnosis and will not received appropriate prophylaxis or follow‐up The remaining 620 people will have no varices. 124 people (20% of 620) will receive false diagnosis of varices and will undergo an unnecessary endoscopy |
Assuming a pretest probability of 38% Post‐test probabilities:
|
Most studies are at high risk of bias No predefinition of cut‐off value of the index test for most studies Median prevalence of any varices is lower than that reported by most guidelines (around 50%) . |
|
High risk varices Cut‐off value: around 150,000 /mm3 (range 140,000 to 160,000/mm3) |
Sensitivity 0.80 (0.73 to 0.85) Specificity 0.68 (0.57 to 0.77) LR+ 2.5 (1.8 to 3.3) LR‐ 0.30 (0.23 to 0.39) |
1671 participants (7) | 20% (6% to 48%) | With a prevalence of 20%, 200 out of 1000 people will have varices at high risk of bleeding. Of these 200 people, 40 (20% of 200) people with high‐risk varices will receive misdiagnosis and will not receive effective prophylaxis The remaining 800 people will not have high‐risk varices. 256 people (32% of 800) will receive false diagnosis of high‐risk varices and will undergo an unnecessary endoscopy |
Assuming a pretest probability of 20% Post‐test probabilities:
|
Most or all studies at high risk of bias No predefinition of cut‐off value of the index test for most studies |
Summary of findings 2. Adult participants ‐ spleen length.
| Review question | What is the diagnostic accuracy of spleen length for the diagnosis of oesophageal varices in adult people with liver disease or portal vein thrombosis? | |||||
| Population | Adults with diagnosis of chronic liver disease or portal vein thrombosis. Age ≥ 18 years | |||||
| Settings | Outpatients and inpatients in secondary/tertiary care setting | |||||
| Study design | Prospective and retrospective cross‐sectional studies. No case‐control studies were found | |||||
| Index tests | Spleen length | |||||
| Reference standards | Upper endoscopy | |||||
| Target condition | Summary accuracy (95% CI) | No. of participants (studies) |
Prevalence, Median (range ) |
Implications in a hypothetical cohort of 1000 people | Post‐test probability | Quality and comments |
|
Any varices Cut‐off value: around 110 mm (range 110 to 112.5 mm) |
Sensitivity 0.85 (0.75 to 0.91) Specificity 0.54 (0.46 to 0.62) LR+ 1.8 (1.6 to 1.21) LR‐ 0.28 (0.17 to 0.44) |
594 participants (5) | 53% (17% to 71%) | With a prevalence of 53%, 530 out of 1000 people will have varices of any size. Of these 530 people, 80 (15% of 530) people with varices will receive misdiagnosis and will not receive appropriate prophylaxis or follow‐up The remaining 470 people will have no varices. 216 people (46% of 470) will receive false diagnosis of varices and will undergo an unnecessary endoscopy |
Assuming a pretest probability of 53% Post‐test probabilities:
|
Most or all studies at high risk of bias |
|
High‐risk varices Cut‐off value: no common cut‐off value. Range 120 to 160 mm |
Sensitivity ranged from 0.50 to 0.88 and specificity from 0.55 to 0.84 | 883 participants (6) | 42% (13% to 70%) | Inconsistency of results (no common cut‐off value) prevents any conclusions . |
Most or all studies at high risk of bias | |
Summary of findings 3. Adult participants ‐ platelet count‐to‐spleen length ratio.
| What is the diagnostic accuracy of platelet count‐to‐spleen length ratio? | ||||||
| Review question | What is the diagnostic accuracy of platelet count‐to‐spleen length ratio for the diagnosis of oesophageal varices in adult people with liver disease or portal vein thrombosis? | |||||
| Population | Adults with diagnosis of chronic liver disease or portal vein thrombosis. Age ≥ 18 years | |||||
| Settings | Outpatients and inpatients in secondary/tertiary care setting | |||||
| Study design | Prospective and retrospective cross‐sectional studies. No case‐control studies were found | |||||
| Index tests | Platelet count‐to‐spleen length ratio | |||||
| Reference standards | Upper endoscopy | |||||
| Target condition | Summary accuracy (95% CI) | No. of participants (studies) |
Prevalence, Median (range ) |
Implications in a hypothetical cohort of 1000 people | Post‐test probability | Quality and comments |
|
Any varices Cut‐off value: 909 (n/mm3)/mm |
Sensitivity 0.93 (0.93 to 0.87) Specificity 0.84 (0.75 to 0.91) LR+ 5.9 (3.5 to 9.9) LR‐ 0.09 (0.03 to 0.22) |
2637 participants (17) | 58% (38% to 75%) | With a prevalence of 58%, 580 out of 1000 people will have varices of any size. Of these 580 people, 41 (7% of 580) people with varices will receive misdiagnosis and will not receive appropriate prophylaxis or follow‐up The remaining 420 people will have no varices. 67 people (16% of 420) will receive false diagnosis of varices and will undergo an unnecessary endoscopy |
Assuming a pretest probability of 58% Post‐test probabilities:
|
Most studies are at high risk of bias |
|
High‐risk varices Cut‐off value: around 909 (n/mm3)/mm (range 897 to 921 (n/mm3)/mm) |
Sensitivity 0.85 (0.72 to 0.93) Specificity 0.66 (0.52 to 0.77) LR+ 2.5 (1.8 to 3.4) LR‐ 0.22 (0.12 to 0.42) |
642 participants (7) | 60% (18% to 70%) | With a prevalence of 60%, 600 out of 1000 people will have varices at high risk of bleeding. Of these 2600 people, 90 (15% of 600) people with high‐risk varices will receive misdiagnosis and will not receive effective prophylaxis The remaining 400 people will not have high‐risk varices. 136 people (34% of 400) will receive false diagnosis of high‐risk varices and will undergo an unnecessary endoscopy |
Assuming a pre‐test probability of 60% Post‐test probabilities:
|
Most studies are at high risk of bias Median prevalence of any varices is higher than that reported by most guidelines (around 25%) |
Summary of findings 4. Paediatric participants ‐ platelet count.
| What is the diagnostic accuracy of platelet count? | ||||||
| Review question | What is the diagnostic accuracy of platelet count for the diagnosis of oesophageal varices in paediatric people with liver disease or portal vein thrombosis? | |||||
| Population | Children with diagnosis of chronic liver disease or portal vein thrombosis. Age < 18 years | |||||
| Settings | Outpatients and inpatients in secondary/tertiary care setting | |||||
| Study design | Prospective and retrospective cross‐sectional studies. No case‐control studies were found | |||||
| Index tests | Platelet count | |||||
| Reference standards | Upper endoscopy | |||||
| Target condition | Summary accuracy (95% CI) | No. of participants (studies) |
Prevalence, Median (range ) |
Implications in a hypothetical cohort of 1000 people | Post‐test probability | Quality and comments |
|
Any varices Cut‐off value: around 120,000 /mm3 (range 115,000 to 119,000/mm3) |
Sensitivity 0.71 (0.60 to 0.80) Specificity 0.83 (0.70 to 0.91) LR+ 4.2 (2.4 to 7.3) LR‐ 0.35 (0.25 to 0.48) |
277 participants (4) | 58% (48% to 69%) | With a prevalence of 58%, 580 out of 1000 children will have varices of any size. Of these 580 children, 168 (29% of 580) children with varices will receive misdiagnosis and will not receive appropriate. prophylaxis or follow‐up The remaining 420 children will have no varices. 71 children (17% of 420) will receive false diagnosis of varices and will undergo an unnecessary endoscopy |
Assuming a pretest probability of 58% Post‐test probabilities:
|
Studies were at high risk of bias |
Summary of findings 5. Paediatric participants ‐ platelet count‐to‐spleen length ratio.
| What is the diagnostic accuracy of platelet count‐to‐spleen length ratio? | ||||||
| Review question | What is the diagnostic accuracy of platelet count‐to‐spleen length ratio for the diagnosis of oesophageal varices in paediatric people with liver disease or portal vein thrombosis? | |||||
| Population | Children with diagnosis of chronic liver disease or portal vein thrombosis. Age < 18 years | |||||
| Settings | Outpatients and inpatients in secondary/tertiary care setting | |||||
| Study design | Prospective and retrospective cross‐sectional studies. No case‐control studies were found | |||||
| Index tests | Platelet count‐to‐spleen length ratio | |||||
| Reference standards | Upper endoscopy | |||||
| Target condition | Summary accuracy (95% CI) | No. of participants (studies) | Prevalences | Implications in a hypothetical cohort of 1000 people | Post‐test probability | Quality and comments |
|
Any varices Cut‐off value: around 1000 (n/mm3)/mm |
Sensitivity 0.74 (0.65 to 0.81) Specificity 0.64 (0.36 to 0.85) LR+ 2.0 (1.0 to 4.0) LR‐ 0.41 (0.27 to 0.61) |
197 participants (2) | 72% and 73% | With a prevalence of 50%, 500 out of 1000 children will have varices of any size. Of these 500 children, 130 (26% of 500) children with varices will receive misdiagnosis and will not receive appropriate prophylaxis or follow‐up The remaining 500 children will have no varices. 180 children (36% of 500) will receive false diagnosis of varices and will undergo an unnecessary endoscopy |
Assuming a pretest probability of 50% Post‐test probabilities:
|
Limited evidence. Only 2 studies were found. These 2 studies were at high risk of bias |
For the 17 studies assessing ratio of platelet count to spleen length using the cut‐off value of 909 (n/mm3)/mm for the diagnosis of varices of any size, sensitivity was 0.93 and specificity 0.84 (Table 7), whereas for high‐risk varices, accuracy was lower: sensitivity 0.85 and specificity 0.66. We found some heterogeneity of results that was not due to a threshold effect, as the same cut‐off value was used. Moreover, we found no effect of other explored factors: aetiology, severity of liver disease (Child class), and prevalence of the target disease.
2. Summary of diagnostic accuracy results.
| Pooled results | |||||
| Cut‐off |
Sensitivity (95% CI) |
Specificity (95% CI) |
LR+ (95% CI) |
LR‐ (95% CI) |
|
| Any varices | |||||
| Platelet count | Around 100,000 | 0.57 (0.50 to 0.64) |
0.75 (0.67 to 0.82) |
2.3 (1.7 to 3.1) |
0.57 (0.49 to 0.67) |
| Around 120,000 | 0.77 (0.72 to 0.81) |
0.69 (0.57 to 0.78) |
2.4 (1.7 to 3.5) |
0.34 (0.26 to 0.44) |
|
| Around 150,000 | 0.71 (0.63 to 0.77) |
0.80 (0.69 to 0.88) |
3.6 (2.4 to 5.4) |
0.37 (0.30 to 0.45) |
|
| Spleen length | Around 110 mm |
0.85 (0.75 to 0.91) |
0.54 (0.46 to 0.62) |
1.8 (1.6 to 2.1) |
0.28 (0.17 to 0.44) |
| Around 150 mm |
0.57 (0.41 to 0.71) |
0.82 (0.72 to 0.89) |
3.2 (2.3 to 4.4) |
0.53 (0.39 to 0.72) |
|
| Platelet count‐to‐spleen length ratio | 909 (n/mm3)/mm | 0.93 (0.83 to 0.97) |
0.84 (0.75 to 0.91) |
5.9 (3.5 to 9.9) |
0.09 (0.03 to 0.22) |
| High‐risk varices | |||||
| Platelet count | Around 90,000 | 0.59 (0.54 to 0.64) |
0.72 (0.66 to 0.78) |
2.1 (1.8 to 2.6) |
0.57 (0.52 to 0.63) |
| Around 150,000 | 0.80 (0.73 to 0.85) |
0.68 (0.57 to 0.77) |
2.5 (1.8 to 3.3) |
0.30 (0.23 to 0.39) |
|
| Spleen length | ‐ | ‐ | ‐ | ‐ | ‐ |
| Platelet count‐to‐spleen length ratio | Around 909 (n/mm3)/mm |
0.85 (0.72 to 0.93) |
0.66 (0.52 to 0.77) |
2.5 (1.8 to 3.4) |
0.22 (0.12 to 0.42) |
For platelet count, accuracy estimates varied according to the different cut‐off values used in the included studies (Table 7). A low platelet count is associated with portal hypertension, and hence with oesophageal varices. As expected, with use of 120,000/mm3 instead of 100,000/mm3 as a cut‐off value, sensitivity increased and specificity decreased. In contrast, when the highest value of 150,000/mm3 was used, sensitivity decreased and specificity increased unexpectedly. Furthermore, we found an effect of aetiology of liver disease (chronic hepatitis C vs other or mixed aetiologies), but other factors such as prevalence of varices or severity of liver disease (proportion of Child A) showed no effect on accuracy.
A large spleen is associated with portal hypertension, and a higher cut‐off value (150 mm vs 110 mm) showed, as expected, lower sensitivity and higher specificity (Table 7). We found no effect of the other explored sources of heterogeneity.
Platelet count‐to‐spleen length ratio is a simple and inexpensive test that is available for all patients with cirrhosis at the moment of diagnosis and at any follow‐up control. Its accuracy allows the clinician to identify a patient with low risk of oesophageal varices. With assumption of prevalence of 58%, which is the median of the included studies and is close to the expected value of 50% in compensated cirrhosis (Garcia‐Tsao 2007), only 10% will be false negative (Table 3). These patients, in the case of varices of any size, would miss an adequate follow‐up, and, in the case of high risk of bleeding varices, would miss an effective prophylaxis. As the proportion of high‐risk varices at the moment of first detection in compensated cirrhosis is lower than 30%, only about 3% of these patients should actually lose the opportunity of receiving effective treatment. When a non‐invasive test is used for screening oesophageal varices, a recent consensus conference defined as acceptable and safe a proportion of less than 5% of false negative results in the case of high‐risk varices requiring prophylaxis (de Franchis 2015; Abraldes 2016). On the other hand, as shown in Table 3, in the case of prevalence of high‐risk varices of 60%, platelet count‐to‐spleen length ratio seems inadequate for ruling out or ruling in the presence of high‐risk varices, as 15% of patients with high‐risk varices would be missed and 21% of patients with a positive test result would be false positive and consequently overtreated. Finally, if this test is used as a triage test, 394 out of 1000 adults could avoid upper endoscopy, and only 10% would be false negatives for the diagnosis of varices of any size.
Assessment of any new non‐invasive test should take into account that platelet count‐to‐spleen length ratio is an accurate and widely available test not requiring additional costs at the moment of diagnosis of cirrhosis. It can also be combined with other tests such as liver stiffness or spleen stiffness measurement by transient elastography, or other techniques. Liver stiffness is widely used and, at least in cases of chronic hepatitis C, can replace histology for the diagnosis of cirrhosis, with high values predicting the presence of portal hypertension. Its accuracy can be further increased by combining liver stiffness measurements with platelet count (Abraldes 2016; de Franchis 2015), or hypothetically with platelet count‐to‐spleen length ratio.
Finally, from the four paediatric studies that considered platelet count (including 294 paediatric participants with different types of liver disease and/or portal vein thrombosis), we obtained estimates of sensitivity 0.71 and specificity of 0.83. These four studies used similar, not predefined, cut‐off values (range 115,000 to 119,000/mm3). Given that spleen length in paediatric patients changes with age, we included and analysed for the index tests of spleen length and platelet count‐to‐spleen length ratio only studies that expressed spleen size in a way that corrects for expected changes for age (z‐score). We found two studies with 197 paediatric participants that assessed the platelet count‐to‐spleen length z‐score ratio, using cut‐off values of 24 and 25, and we obtained estimates of sensitivity (0.74) and specificity (0.64). We found no studies assessing the accuracy of spleen length z‐score.
Strengths and weaknesses of the review
We aimed to assess the accuracy of three index tests for the diagnosis of oesophageal varices and included 71 studies that were conducted in many countries, showed widespread implementation globally of the index tests, and confirmed the clinical relevance of this review question. We identified four studies through manual searching of non‐indexed journals and are confident that we have included most, if not all, of the includable published studies. We also assessed the accuracy of the index tests to detect varices that are at high risk of bleeding, which provide the main clinical reason for screening cirrhotic patients with endoscopy. Moreover, the included studies allowed comparison of the accuracies of the three index tests.
An overall quality assessment of the studies showed several common methodological weaknesses, and we considered only one study to have low risk of bias. Most studies derived "a posteriori" the optimal cut‐off value with consequent overestimation of accuracy. Furthermore, in many instances, study reporting was incomplete, and investigators provided no information about consecutive enrolment and blinding of the reference standard. Prevalence of the target disease varied widely, suggesting different inclusion criteria, with participants enrolled not only at the time of diagnosis of cirrhosis, but also during follow‐up; and non‐consecutive enrolment, with retrospective selection based on available data. Anyway, the median prevalence of varices of any size was close to the expected value of around 50%. In contrast, prevalence of high‐risk varices was much higher than expected, suggesting that the index test was used not at the time of diagnosis of cirrhosis, but later, to monitor the development of complications.
Despite the large numbers of included studies and participants, estimates of accuracy were imprecise, and results of included studies were not consistent. This heterogeneity could be explained only in part by the use of different cut‐off values. Sources of this heterogeneity remained unexplained, even after inspection of the most likely explanatory variables, such as different severity and aetiology of liver disease and different prevalence of oesophageal varices. However, for the index test platelet count‐to‐spleen length ratio, we found 17 studies (with 2637 participants) that used the same cut‐off value: one derivation study and 16 validation studies. Through meta‐analysis of the results of these studies, we obtained consistent estimates of sensitivity and specificity, which could support the use of platelet count‐to‐spleen length ratio with this cut‐off to rule out the presence of varices in adults with cirrhosis.
Available data prevent proper comparison of accuracy through direct comparison of the three index tests, each with the same predefined cut‐off value. The included studies mainly allowed indirect comparisons, and in the case of direct comparisons, different cut‐off values were used across studies, preventing clear interpretation of results.
Another possible limitation of the review is that the reference standard for diagnosis and staging of oesophageal varices is not perfect. In fact, interobserver agreement in interpretation of oesophago‐gastro‐duodenoscopy findings is unfortunately well below that desired for an ideal reference standard (Cales 1989; Bendtsen 1990; Winkfield 2003). This poor reproducibility of the reference standard could impair the accuracy estimation of the index tests. Furthermore, included studies assessed the accuracy of index tests in diagnosing varices of any size or large oesophageal varices or both, but they did not directly assess bleeding risk by measuring actual bleeding outcomes. Thus, these studies could not answer directly the question of whether these index tests can predict bleeding or can properly indicate which people might benefit from primary prophylactic treatment.
We found two reviews on the same topic, both assessing the accuracy of platelet count‐to‐spleen length ratio (Chawla 2012; Ying 2012). One of these reviews considered only studies assessing the accuracy of the ratio with the predefined cut‐off value of 909 (n/mm3)/mm and included only eight studies (Chawla 2012). We found and included nine additional studies that validated this cut‐off. The other review (Ying 2012) included 20 studies assessing the accuracy of the ratio on the basis of all cut‐off values. In our review, we found 18 additional studies. Furthermore, in both reviews, the statistical approach was not the most appropriate, as neither bivariate nor hierarchical summary receiver operating characteristic (HSROC) models were used.
Applicability of findings to the review question
The accuracy of platelet count, spleen length, and platelet count‐to‐spleen length ratio in detecting the presence of oesophageal varices has been, with the limitations noted above, addressed in a tertiary care setting and in adult patients with suspected cirrhosis mainly due to chronic viral hepatitis or alcoholic liver disease. It is uncertain how applicable these results may be to other specific patient groups, such as those with cholestatic disease or portal vein thrombosis, children with liver disease, or patients in other settings.
Authors' conclusions
Implications for practice.
Although current guidelines recommend use of oesophago‐gastro‐duodenoscopy to screen for varices in all adults with suspected cirrhosis, poor uptake of this recommendation has occurred because oesophago‐gastro‐duodenoscopy is invasive and unpleasant, and has a low diagnostic yield when applied to all adults with cirrhosis (Garcia‐Tsao 2007; Garcia‐Tsao 2008; de Franchis 2010). Therefore, a pressing need exists for a non‐invasive test that enables oesophago‐gastro‐duodenoscopy to be avoided or applied to a higher‐risk patient group (de Franchis 2015; Garcia‐Tsao 2017). This review shows that a simple test such as platelet count‐to‐spleen length ratio could be used to stratify the risk of oesophageal varices, particularly as a triage test before endoscopy to rule out people without varices. In fact, in the case of a ratio greater than 909 (n/mm3)/mm, only 7% of patients with varices of any size would be missed and would not receive appropriate prophylaxis or follow‐up. If prevalence of varices of 58% is assumed, the negative predictive value of the test is 90% and about 40% of esophago‐gastro‐duodenoscopy examinations for screening people with cirrhosis would be spared. However, most studies were at high risk of bias and estimates of sensitivity and specificity were imprecise, limiting the strength of this conclusion. Furthermore, prevalence of the target condition widely varied, suggesting differences in study design or participant selection.
For detection of high risk of bleeding varices, included studies reported prevalence of 60%, which is higher than expected, especially if the test is used at the time of diagnosis of cirrhosis. In this context, the test is not accurate enough to replace endoscopy, with 15% of patients missing a correct diagnosis and the consequent primary prophylaxis. In fact, a proportion of less than 5% for missed diagnosis is regarded by experts as acceptable and safe (de Franchis 2015; Abraldes 2016).
Implications for research.
To better define the role of platelet count‐to‐spleen size ratio in clinical practice, future studies should explore the following areas.
Diagnostic accuracy of these non‐invasive tests when used in specific subgroups of patients, such as patients with different causes of portal hypertension, with different severity of liver disease, or of different age groups (paediatric patients), or those for whom different classification systems for varices are used.
Diagnostic accuracy of platelet count‐to‐spleen size ratio in predicting variceal bleeding and real‐world effectiveness and cost‐effectiveness of management strategies that employ platelet count‐to‐spleen size ratio to identify patients for primary prophylaxis of variceal bleeding, compared with the currently recommended approach using oesophago‐gastro‐duodenoscopy alone.
Assessment of new non‐invasive tests for detection of oesophageal varices should also include comparison with platelet count‐to‐spleen size ratio.
When diagnostic strategies have been refined, these ought to be assessed for benefits and harms in randomised clinical trials (Colli 2014a).
Acknowledgements
Dimitrinka Nikolova for continuous help during the review process. Sarah Louise Klingenberg for assistance with the search strategy.
Contact Editor: Gennaro D'Amico, Italy. Sign‐off Editor: Christian Gluud, Denmark.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: The views and opinions expressed in this review are those of the review authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | June 2016 | ((((platelet* or thrombocyt*) near (count or distribution or volume)) or PLT or PDW or MPV) OR (((splenic* or spleen*) near3 (enlarg* or hypertroph or length or palpable or size or diamet* or index or examin*)) or splenomegal*)) AND (*esophag* near3 (varic* or varix*)) |
| Cochrane Hepato‐Biliary Diagnostic Test of Accuracy Studies Register | June 2016 | ((((platelet* or thrombocyt*) near (count or distribution or volume)) or PLT or PDW or MPV) OR (((splenic* or spleen*) near3 (enlarg* or hypertroph or length or palpable or size or diamet* or index or examin*)) or splenomegal*)) AND (*esophag* near3 (varic* or varix*)) |
| The Cochrane Library | 2016, Issue 6 | #1 MeSH descriptor: [Platelet Count] explode all trees #2 ((platelet* or thrombocyt*) near (count or distribution or volume)) or PLT or PDW or MPV #3 #1 or #2 #4 MeSH descriptor: [Splenomegaly] explode all trees #5 ((splenic* or spleen*) near/3 (enlarg* or hypertroph or length or palpable or size or diamet* or index or examin*)) or splenomegal* #6 #4 or #5 #7 MeSH descriptor: [Esophageal and Gastric Varices] explode all trees #8 *esophag* near/3 (varic* or varix*) #9 #7 or #8 #10 (#3 or #6) and #9 |
| MEDLINE (OvidSP) | 1946 to June 2016. | 1. exp Platelet Count/ 2. (((platelet* or thrombocyt*) adj (count or distribution or volume)) or PLT or PDW or MPV).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 3. 1 or 2 4. exp Splenomegaly/ 5. (((splenic* or spleen*) adj3 (enlarg* or hypertroph or length or palpable or size or diamet* or index or examin*)) or splenomegal*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 6. 4 or 5 7. (Esophageal and Gastric Varices).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 8. ((esophag* or oesophag*) adj3 (varic* or varix*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 9. 7 or 8 10. (3 or 6) and 9 |
| Embase (OvidSP) | 1974 to June 2016 | 1. exp thrombocyte count/ 2. (((platelet* or thrombocyt*) adj (count or distribution or volume)) or PLT or PDW or MPV).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp splenomegaly/ 5. (((splenic* or spleen*) adj3 (enlarg* or hypertroph or length or palpable or size or diamet* or index or examin*)) or splenomegal*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. 4 or 5 7. exp esophagus varices/ 8. ((esophag* or oesophag*) adj3 (varic* or varix*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 9. 7 or 8 10. (3 or 6) and 9 |
| Science Citation Index ‐ Expanded | 1900 to June 2016 | #5 #4 AND #3 #4 TS=(*esophag* NEAR/3 (varic* or varix*)) #3 #2 OR #1 #2 TS=(((splenic* or spleen*) NEAR/3 (enlarg* or hypertroph or length or palpable or size or diamet* or index or examin*)) or splenomegal*) #1 TS=(((platelet* or thrombocyt*) NEAR (count or distribution or volume)) or PLT or PDW or MPV) |
Appendix 2. QUADAS‐2
| Domain | 1. Participant selection | 2. Index test | 3. Reference standard | 4. Flow and timing |
| Signalling questions and criteria | Q.1: "Was a consecutive or random sample of participants enrolled?" Yes ‐ If the study reports on a consecutive or a random selection of participants. No ‐ if the study reports on another form of selection of participants. Unclear ‐ if the study does not report on how the participants were enrolled. Q.2: "Was a case‐control design avoided?" Yes ‐ if the case‐control design was avoided. No ‐ if the study was a case‐control. Unclear ‐ if the study design was not clear. Q.3: "Did the study avoid inappropriate exclusions?" Yes ‐ if the study definitions of exclusion criteria are appropriate (i.e. previous bleeding or treatment for oesophageal varices) and all exclusions are reported. No ‐ if exclusion criteria are inappropriate and exclusions are not reported. Unclear ‐ if the study does not report causes of exclusions. |
Q.1: "Were the index test results interpreted without knowledge of the results of the reference standard?" Yes ‐ if the study reports that results of the index test were interpreted without the knowledge of results of the reference standard. No ‐ if the study reports that results of the index test were interpreted with results of the reference standard. Unclear ‐ if the study does not report information about blinding of results of the index test and reference standard. Q.2: "If a threshold was used, was it prespecified?" Yes ‐ if the threshold used was reported in the methods section. No ‐ if the study reports that the threshold was chosen during the data analysis stage (e.g. maximum of Youden index). Unclear ‐ if the study does not report information about threshold selection. |
Q.1: "Is the reference standard likely to correctly classify the target condition?" Yes ‐ if the reference standard correctly classifies oesophageal varices (according to common grading scores or systems detailed in "Reference Standard" section). No ‐ if there is some doubt whether the reference standard classifies oesophageal varices. Unclear ‐ if the study does not report on the reference standard used. Q.2: "Were the reference standard results interpreted without knowledge of results of the index test?" Yes ‐ if the study reports that results of the reference standard were interpreted without knowledge of results of the index test. No ‐ if the study reports that results of the reference standard were interpreted with results of the test index. Unclear ‐ if the study does not report information about blinding of results of the reference standard and the index test. |
Q.1: "Was there an appropriate interval between the index test and the reference standard?" Yes ‐ if the interval between the index test and the reference standard was less than 3 months. No ‐ if the interval was longer than 3 months. Unclear ‐ if the study does not report the interval between the index test and the reference standard. Q.2: "Did all participants receive the same reference standard?" Yes ‐ if the study has only one reference standard for all participants (OGD with appropriate classification of oesophageal varices). No ‐ if the study has more than one reference standard. Unclear‐ if the study is not clear about the reference standard used. Q.3 "Were all participants included in the analysis?" Answer: Yes ‐ if all enrolled participants were included in the analysis (even in the case of uninterpretable index test result). No ‐ if any participant was excluded from the analysis for any reason. Unclear ‐ if it is not clear about exclusions of participants from the analysis. |
| Risk of bias |
Could the selection of participants have introduced bias? Low risk: "Yes" for all signalling questions. High risk: "No" or "Unclear" for at least one signalling question. |
Could the conduct or interpretation of the index test have introduced bias? Low risk: "Yes" for the signalling question. High risk: "No" or "Unclear" for the signalling question. |
Could the reference standard, its conduct, or its interpretation have introduced bias? Low risk: "Yes" for all signalling questions. High risk: "No" or "Unclear" for at least one signalling question. |
Could the participant flow have introduced bias? Low risk: "Yes" for all signalling questions. High risk: "No" or "Unclear" for at least one signalling question. |
| Concerns about applicability |
Are there concerns that the included participants and setting do not match the review question? Low concern: Participants included in the review represent participants for whom the test is used in clinical practice. High concern: Participants included in the review differ from participants for whom the test is used in clinical practice. |
Are there concerns that the index test, its conduct, or interpretation differ from the review question? High concern: The index test, its conduct, or interpretation of the index test differs from the way it is used in clinical practice. Low concern: The index test, its conduct, or interpretation of the index test does not differ from the way it is used in clinical practice. |
Are there concerns that the target condition as defined by the reference standard does not match the question? | ‐ |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
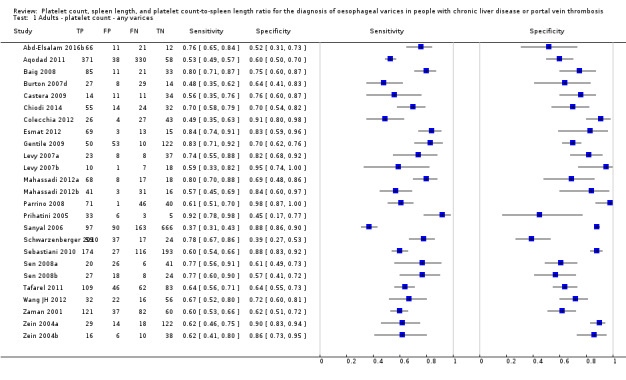
Adults ‐ platelet count ‐ any varices.
2. Test.
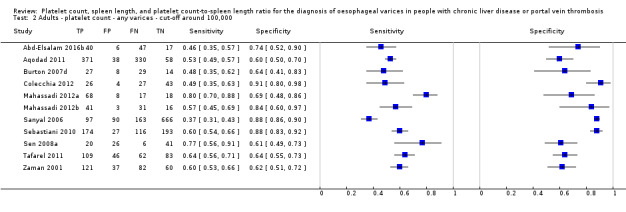
Adults ‐ platelet count ‐ any varices ‐ cut‐off around 100,000.
3. Test.
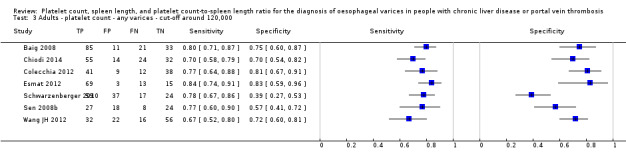
Adults ‐ platelet count ‐ any varices ‐ cut‐off around 120,000.
4. Test.
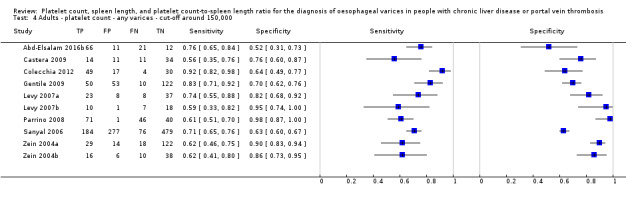
Adults ‐ platelet count ‐ any varices ‐ cut‐off around 150,000.
5. Test.
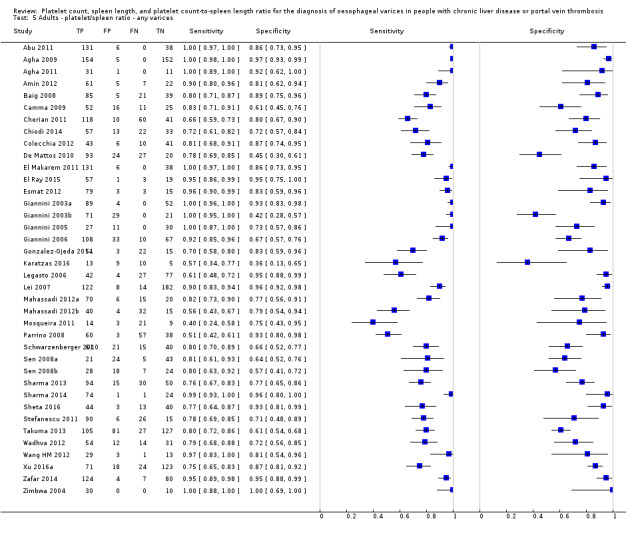
Adults ‐ platelet/spleen ratio ‐ any varices.
6. Test.
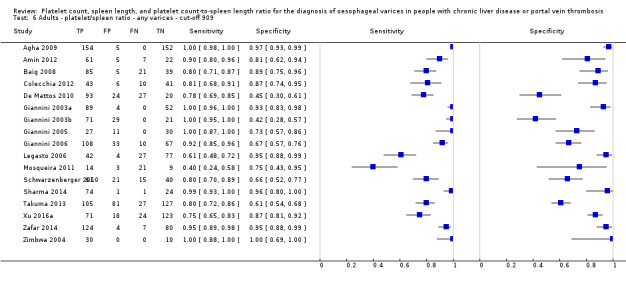
Adults ‐ platelet/spleen ratio ‐ any varices ‐ cut‐off 909.
7. Test.
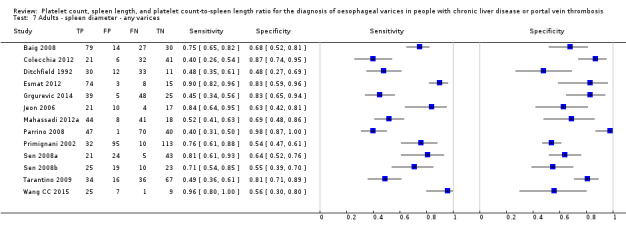
Adults ‐ spleen diameter ‐ any varices.
8. Test.
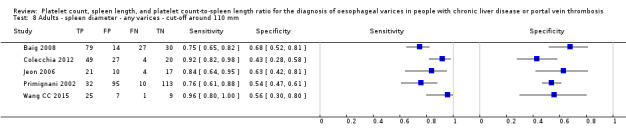
Adults ‐ spleen diameter ‐ any varices ‐ cut‐off around 110 mm.
9. Test.
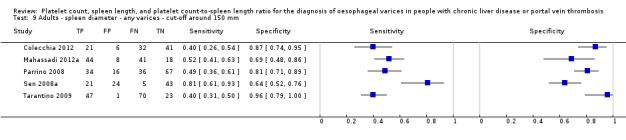
Adults ‐ spleen diameter ‐ any varices ‐ cut‐off around 150 mm.
10. Test.
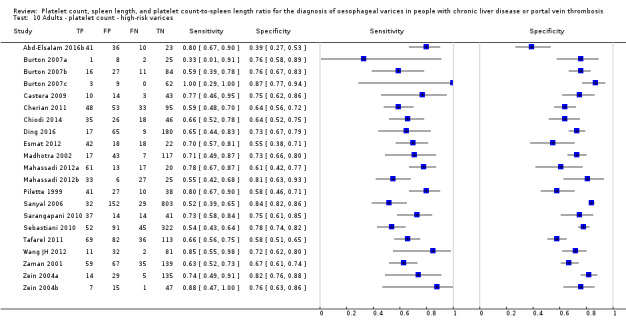
Adults ‐ platelet count ‐ high‐risk varices.
11. Test.
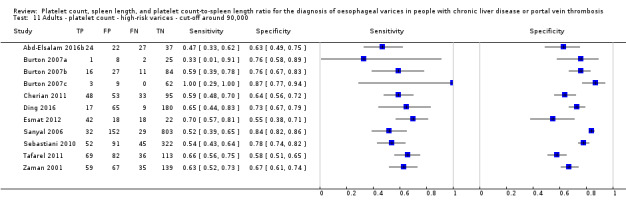
Adults ‐ platelet count ‐ high‐risk varices ‐ cut‐off around 90,000.
12. Test.
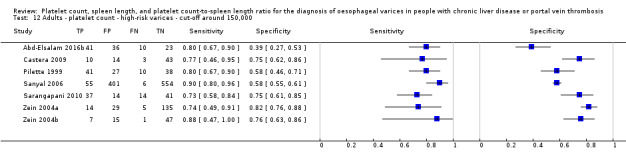
Adults ‐ platelet count ‐ high‐risk varices ‐ cut‐off around 150,000.
13. Test.
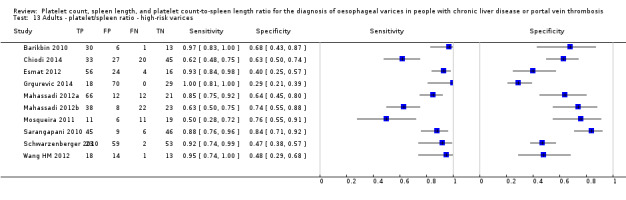
Adults ‐ platelet/spleen ratio ‐ high‐risk varices.
14. Test.
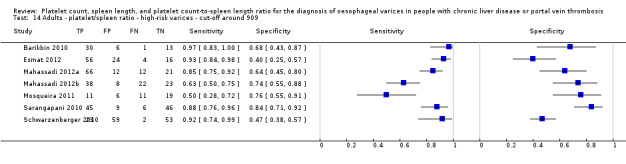
Adults ‐ platelet/spleen ratio ‐ high‐risk varices ‐ cut‐off around 909.
15. Test.
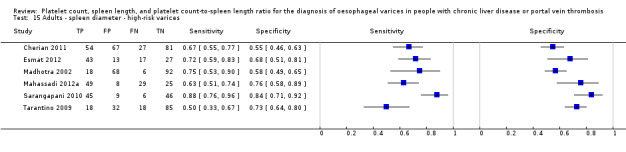
Adults ‐ spleen diameter ‐ high‐risk varices.
16. Test.
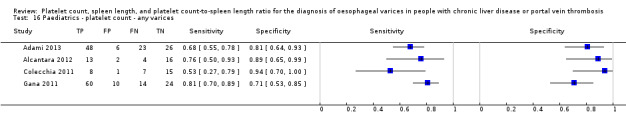
Paediatrics ‐ platelet count ‐ any varices.
17. Test.

Paediatrics ‐ platelet/spleen ratio z‐score ‐ any varices.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abd‐Elsalam 2016b.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | 110 adult patients with cirrhosis due to hepatitis C virus. Child A 49.1%. Setting: tertiary referral centre in Egypt | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Abu 2011.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | 175 consecutive adult patients with cirrhosis due to hepatitis C virus. Child A only 26%. Histological diagnosis of cirrhosis in 26% of patients. Setting: tertiary referral centre in Egypt | ||
| Index tests | Platelet count‐to‐spleen length ratio | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Adami 2013.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional | ||
| Patient characteristics and setting | 103 paediatric patients (98 with chronic liver disease, 5 with extrahepatic portal vein obstruction). 55% Child A. Setting: tertiary referral centre in Brazil | ||
| Index tests | Platelet count, platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Paediatric | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Agha 2009.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional. | ||
| Patient characteristics and setting | 316 consecutive adult patients with hepatitis C‐related liver cirrhosis. Child A: 25.8%. Setting: tertiary referral centre in Pakistan | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Any oesophageal varices. Upper endoscopy | ||
| Flow and timing | 5 patients did not complete the clinical workup | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Agha 2011.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | 43 consecutive adult patients with evidence of schistosomal infection (based on seropositivity for Schistosoma mansonii) and periportal hepatic fibrosis confirmed on abdominal ultrasound. Setting: referral tertiary centre in Kingdom of Saudi Arabia | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Alcantara 2012.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional | ||
| Patient characteristics and setting | 53 paediatric patients. 35 with chronic liver disease and 18 with extrahepatic portal obstruction. Child A: 82.4%. Setting: tertiary referral centre in Brazil | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Paediatric | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Amin 2012.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | 95 adult patients with HCV cirrhosis. Child A: 30%. Setting: tertiary referral centre in Pakistan | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Aqodad 2011.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional | ||
| Patient characteristics and setting | 797 adult patients. Setting: tertiary referral centre in Morocco | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Abstract | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Baig 2008.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | 150 consecutive adult patients. Child A: 64.7%. Setting: tertiary referral centre in India | ||
| Index tests | Platelet count; spleen diameter; platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Barikbin 2010.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | 50 adult patients with cirrhosis. Child A: 10%. Setting: tertiary referral centre in Iran | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | High‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Burton 2007a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional | ||
| Patient characteristics and setting | 101 adult patients. Accuracy data reported only for 36 Child A patients. Child A: 100%. Setting: tertiary referral centre in Italy | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | High‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Burton 2007b.
| Study characteristics | |||
| Patient sampling | Cross‐sectional | ||
| Patient characteristics and setting | 252 consecutive adult patients. Accuracy data reported only for 138 Child A patients. Child A: 100%. Setting: tertiary referral centre in USA | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | High‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Burton 2007c.
| Study characteristics | |||
| Patient sampling | Cross‐sectional | ||
| Patient characteristics and setting | 152 consecutive adult patients. Accuracy data reported only for 74 Child A patients. Child A: 100%. Setting: tertiary referral centre in USA | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | High‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Burton 2007d.
| Study characteristics | |||
| Patient sampling | Cross‐sectional | ||
| Patient characteristics and setting | 152 consecutive adult patients. Accuracy data reported only for 78 Child B/CA patients. Child A: 0%. Setting: tertiary referral centre in USA | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Camma 2009.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | 104 consecutive adult patients. Child A: 100%. Setting: tertiary referral centre in Italy | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Castera 2009.
| Study characteristics | |||
| Patient sampling | Cross‐sectional | ||
| Patient characteristics and setting | 70 consecutive adult patients with histologically proven cirrhosis HCV related. Child A: 100%. Setting: tertiary referral centrer in France. Multi‐centre | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Cherian 2011.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | 229 consecutive adult patients. Child A: 18.3%. Setting: tertiary referral centre in India | ||
| Index tests | Platelet count, platelet count‐to‐spleen diameter ratio, spleen diameter | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Chiodi 2014.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional | ||
| Patient characteristics and setting | 125 adult patients with cirrhosis. Child A: not reported. Tertiary referring centres in Uruguay | ||
| Index tests | Platelet count, platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Colecchia 2011.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | 33 paediatric patients who had undergone Kasai portoenterostomy. Child A: 77%. Tertiary referring centre in Italy | ||
| Index tests | Platelet count, platelet count‐to‐spleen length ratio, spleen length | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Individual patient data available ‐ paediatric | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Colecchia 2012.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 113 consecutive adult patients. Child A: 68%. Setting: tertiary referral centre in Italy | ||
| Index tests | Platelet count, platelet count‐to‐spleen diameter ratio, spleen diameter | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | 13 patients excluded from the analysis | ||
| Comparative | |||
| Notes | Individual patient data available | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
De Mattos 2010.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 160 adult patients. Child A: 57.6%. Setting: tertiary referral centre in Brazil | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ding 2016.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional | ||
| Patient characteristics and setting | 271 adult patients with cirrhosis. Child A: 100%. Tertiary referral centres in Australia | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Ditchfield 1992.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study | ||
| Patient characteristics and setting | 118 adult patients. Child A: not reported. Setting: tertiary referral centre in Australia | ||
| Index tests | Spleen size | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | Only 86/118 patients underwent endoscopy | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
El Makarem 2011.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 175 adult patients. Child A: 26.3. Setting: tertiary referral centre in Egypt | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
El Ray 2015.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study | ||
| Patient characteristics and setting | 80 adult patients | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Abstract | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Esmat 2012.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 100 adult patients. Child A: 20%. Etiology: all patients with HCV. Setting: tertiary referral centre in Egitto | ||
| Index tests | Platelet count, platelet count‐to‐spleen diameter ratio, spleen diameter | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Gana 2011.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 108 paediatric patients. Child A: 78%. Setting: tertiary referral centres ‐ multi‐centre | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Paediatric | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Gentile 2009.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 235 adult patients. Child A: not reported. Setting: tertiary referral centre in Italy | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Giannini 2003a.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 145 adult patients. Child A: 37%. Setting: tertiary referral centre in Italy | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Giannini 2003b.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 121 adult patients. Child A: 41%. Setting: tertiary referral centre in Italy | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Giannini 2005.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 106 adult patients. Child A: 59%. Setting: tertiary referral centre in Italy | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | 31 patients lost to follow‐up, 6 deaths, 1 OLT | ||
| Comparative | |||
| Notes | Study included only patients with previous (24 months) negative endoscopy | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Giannini 2006.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 218 adult patients. Child A: 51%. Setting: tertiary referral centres in Europe and USA | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Gonzalez‐Ojeda 2014.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 91 adult patients. Child A: 19%. Setting: tertiary referral centre in Mexico | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Grgurevic 2014.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 117 adult patients with alcoholic cirrhosis. Patients with previous variceal bleeding have been included. Child A: 14.6%. Setting: tertiary referral centre in Croatia | ||
| Index tests | Spleen diameter and platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Jeon 2006.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 52 adult patients. Child A: 51%. Setting: tertiary referral centres in Korea | ||
| Index tests | Spleen diameter | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Karatzas 2016.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 38 adult patients. Child A: 55%. Setting: tertiary referral centre in Greece | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Legasto 2006.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 150 adult patients. Child A: not reported. Setting: tertiary referral centre in Philippines | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Lei 2007.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 326 adult patients. Child A: not reported. Setting: tertiary referral centre in China | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Abstract | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Levy 2007a.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 113 adult patients with PBC. Setting: tertiary referral centre in USA | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | Only 91/113 underwent endoscopy. Only 76/91 had platelet count | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Levy 2007b.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 92 adult patients with PBC. Setting: tertiary referral centre in USA | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | Only 36/92 underwent endoscopy | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Madhotra 2002.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 192 consecutive adult patients. Child A: 43.5%. Setting: tertiary referral centre in USA | ||
| Index tests | Platelet count, spleen diameter | ||
| Target condition and reference standard(s) | Presence of high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | 8 patients with incomplete records were excluded from the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Mahassadi 2012a.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 111 consecutive adult patients (training set). Child A: 22.5%. Setting: tertiary referral centres in Ivory Coast | ||
| Index tests | Platelet count, spleen diameter, and platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Mahassadi 2012b.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 91 consecutive adult patients (validation set). Child A: 19.8%. Setting: tertiary referral centre in Ivory Coast | ||
| Index tests | Platelet count, spleen diameter, and platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | |||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Mosqueira 2011.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 47 adult patients. Child A: not reported. Setting: tertiary referral centre in Peru | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Parrino 2008.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 158 consecutive adult patients. Child A: 64%. Setting: tertiary referral centre in Italy | ||
| Index tests | Platelet count, platelet count‐to‐spleen diameter ratio, spleen diameter | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Pilette 1999.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 116 consecutive adult patients. Child A: 50%. Setting: tertiary referral centre in France | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Prihatini 2005.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 47 consecutive adult patients. Child A: 59.6%. Setting: tertiary referral centre in Indonesia | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Primignani 2002.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 250 consecutive adult patients. Child A: 91.6%. Setting: tertiary referral centre in Italy | ||
| Index tests | Spleen diameter | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | Upper gastrointestinal endoscopy performed within 6 months of liver biopsy. Time interval between endoscopy and execution of the index test was not reported | ||
| Comparative | |||
| Notes | Abstract | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Sanyal 2006.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 1016 consecutive adult patients. Child A: 100%. Setting: tertiary referral centres in USA | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | This report included all randomised patients at all clinical centres participating in the HALT‐C trial | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Sarangapani 2010.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 106 consecutive adult patients. Child A: not reported. Setting: tertiary referral centre in India | ||
| Index tests | Platelet count, platelet count‐to‐spleen diameter ratio, spleen diameter | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Schwarzenberger 2010.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 137 consecutive adult patients. Child A: 48%. Setting: tertiary referral centre in USA | ||
| Index tests | Platelet count, platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Sebastiani 2010.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 510 non‐consecutive adult patients. Child A: 79%. Setting: 5 tertiary referral centres in Italy and France | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Sen 2008a.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 93 adult patients with HCV. Child A: not reported. Setting: tertiary referral centre in UK | ||
| Index tests | Platetet count, spleen diameter, and platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Research letter | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Sen 2008b.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 77 adult patients with alcoholic cirrhosis. Child A: not reported. Setting: tertiary referral centre in UK | ||
| Index tests | Platetet count, spleen diameter, and platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Research letter | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Sharma 2013.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 200 consecutive adult patients. Child A: 79%. Setting: not reported | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | 26 patients excluded owing to inconclusive spleen stiffness and/or liver stiffness measurement | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Sharma 2014.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study | ||
| Patient characteristics and setting | 100 adult patients. Child A: 18%. Setting: tertiary referral centre in India | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Sheta 2016.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 100 adult patients. Child A: not reported. Setting: tertiary referral centre in Egypt | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Stefanescu 2011.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 137 adult patients. Child A: 64.9%. Setting: tertiary referral centre in Romania | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Tafarel 2011.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 300 adult patients. Child A: 71%. Setting: not reported | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Takuma 2013.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 340 consecutive adult patients. Child A: 67%. Setting: tertiary referral centres in Japan | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | 19 patients excluded for unsuccessful transient elastography measurements | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Tarantino 2009.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 153 consecutive adult patients. Child A: 42%. Setting: tertiary referral centre in Italy | ||
| Index tests | Spleen diameter | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Wadhva 2012.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 111 adult patients. Child A: 41%. Setting: tertiary referral centre in Pakistan | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Wang CC 2015.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study | ||
| Patient characteristics and setting | 42 adult patients. Child A: not reported. Setting: tertiary referral centre in Taiwan | ||
| Index tests | Spleen diameter | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Abstract | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test Spleen length | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Wang HM 2012.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 46 adult patients. Child A: not reported. Setting: tertiary referral centre in Taiwan | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Wang JH 2012.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 126 adult patients. Child A: 100%. Setting: tertiary referral centre in Taiwan | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Xu 2016a.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 236 adult patients. Child A: not reported. Setting: tertiary referral centre in China | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | |||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Zafar 2014.
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional study | ||
| Patient characteristics and setting | 215 adult patients. Child A: not reported. Setting: tertiary referral centre in Pakistan | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Zaman 2001.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 300 adult patients. Child A: 22%. Setting: tertiary referral centre in USA | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Study authors wrote, "This was an unmatched case‐control study, with cases and controls selected from patients undergoing liver transplantation evaluation at the OHSU/PVAMC Liver Transplantation Program between January 1, 1995, and September 1, 1999"
This sentence might suggest a case‐control design However, careful reading of the paper reveals that it is clear that the study design is not case‐control but retrospective cross‐sectional based on registry data "This study presents the results from the entire cohort of liver transplantation patients undergoing liver transplantation evaluation at the OHSU/PVAMC Liver Transplant Department between January 1, 1995, and September 1, 1999" "629 cirrhotic patients underwent liver transplantation evaluation. Of these, 300 patients did not have a history of variceal hemorrhage (the study group)" . |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Zein 2004a.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 183 adult patients. Child A: not applicable (only PSC participants). Setting: tertiary referral centre in USA | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Zein 2004b.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 72 adult patients. Child A: not applicable (only PSC participants). Setting: tertiary referral centre in USA | ||
| Index tests | Platelet count | ||
| Target condition and reference standard(s) | Presence of any and high‐risk oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Zimbwa 2004.
| Study characteristics | |||
| Patient sampling | Retrospective cross‐sectional study | ||
| Patient characteristics and setting | 40 adult patients. Child A: not reported. Setting: tertiary referral centre in UK | ||
| Index tests | Platelet count‐to‐spleen diameter ratio | ||
| Target condition and reference standard(s) | Presence of any oesophageal varices. Upper endoscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Letter (Abstract) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Platelet count to spleen length ratio | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
HALT‐C = hepatitis C antiviral long‐term treatment against cirrhosis; HCV = hepatitis C virus; OLT = orthotopic liver transplantation; PBC = primary biliary cholangitis; PSC = primary sclerosing cholangitis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albreedy 2015 | No data for 2 × 2 table |
| Amarapurkar 1994 | In only a minority of patients, spleen length was assessed by ultrasound |
| Barrera 2009 | No data for 2 × 2 table |
| Chalasani 1999 | Study provides data only for combination of splenomegaly and platelet count as predictors of oesophageal varices. Individual 2 × 2 tables for platelet count or presence/absence of splenomegaly are not extractable from manuscript |
| Cho 2015 | No data for 2 × 2 table (Table S3 provides unreliable data) |
| El Guindi 2008 | No data for 2 × 2 table |
| El‐Sherif 2008 | No data for 2 × 2 table. Published data not consistent. Study authors contacted by email. They did not respond |
| Fagundes 2008 | Not acceptable reference standard |
| Gana 2010 | No data on index tests of interest |
| Giannini 2007 | Same data as Agha 2009 (n = 311 patients) |
| Hong 2009 | Different index test (spleen width) |
| Koncoro 2014 | No definition of the reference standard used |
| Lee 2009 | No data for 2 × 2 table |
| Malik 2015 | No data for 2 × 2 table |
| Nashaat 2010 | No data for 2 × 2 table. Published data not consistent |
| Nazish 2011 | No data for 2 × 2 table |
| Ng 1999 | Study identified patients with oesophagogastric varices |
| Park 2015 | No data for 2 × 2 table |
| Qamar 2008 | No data for 2 × 2 table |
| Rockey 2016 | Only patients wih acute upper gastrointestinal bleeding were included. |
| Sebastiani 2008 | Preliminary data (Sebastiani 2010) |
| Sethar 2006 | No data for 2 × 2 table |
| Shah 2011 | No data for 2 × 2 table |
| Sharma 2007 | Platelet data for large oesophageal varices were not extractable from the text. Study proposes predictor function model derived from multi‐variate analysis as better model to predict large oesophageal varices |
| Takuma 2016 | No data for 2 × 2 table |
| Tao 2008 | No data for 2 × 2 table. Published data not consistent |
| Thayumanavan 2012 | No data for 2 × 2 table |
| Thomopoulos 2003 | No data for 2 × 2 table |
| Treeprasertsuk 2010 | No data for 2 × 2 table |
| Valente | No data for 2 × 2 table |
| Yu 2008 | Different definition of the target condition (data reported only for severe vs moderate small and no varices) |
| Zaman 1999 | Overlap with Zaman 2001 |
| Zhang 2013 | Reference standard different from endoscopy |
| Zhang 2016 | No data for 2 × 2 table. Published data not consistent (see Table 3: sensitivity and specificity not consistent with positive and negative predictive values) |
Differences between protocol and review
At the review stage, we decided to analyse paediatric and adult patients separately, as we found only studies enrolling only adult people or only paediatric patients. Furthermore, transitivity of results to children is unknown. Analyses of sources of heterogeneity were added as secondary objectives, in accordance with recommendations provided in the Cochrane Handbook for Diagnostic Test of Accuracy Reviews. The QUADAS‐2 tool was used instead of the original QUADAS tool.
Contributions of authors
AC: completed the search for studies, performed data extraction and quality assessment, drafted parts of the review, provided methodological and statistical analyses and expert hepatology opinion, and reviewed the final version of the manuscript. JCG: formulated the research question, searched the articles, performed data extraction and quality assessment, drafted the manuscript, and reviewed the final version of the manuscript. JY: searched for articles, performed data extraction and quality assessment, and reviewed the final version of the manuscript. TAW: implemented search strategies, and reviewed the final version of the manuscript. NR: searched for articles and reviewed the final version of the manuscript. SL: formulated the research question, provided hepatology expert opinion, drafted the manuscript, and reviewed the final version of the manuscript. GC: completed the search for studies, performed data extraction and quality assessment, drafted parts of the manuscript, conducted statistical analyses, provided methodological expertise, and reviewed the final version of the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Canadian Institutes of Health Research (CIHR), Canada.
Synthesis Grant: Knowledge Translation, 2008
-
Canadian Association for the Study of the Liver, Canada.
CASL/Schering Victor Feinman Fellowship for the period of one year (2007), for Dr. Juan Cristobal Gana
Declarations of interest
None known.
New
References
References to studies included in this review
Abd‐Elsalam 2016b {published data only}
- Abd‐Elsalam S, Habba E, Elkhalawany W, Tawfeek S, Elbatea H, El‐Kalla F, et al. Correlation of platelet count with endoscopic findings in a cohort of Egyptian patients with liver cirrhosis. Medicine (Baltimore) 2016;95:e3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habba E, Abd‐Elsalam S, Ibrahim S, Dwidar A. Correlation of platelet count with endoscopic findings in Egyptian patients with liver cirrhosis. Hepatology International 2016;10:S365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Abu 2011 {published data only}
- Abu El Makarem MA, Shatat ME, Shaker Y, Abdel Aleem AA, Sherif AM, Moaty MA, et al. Platelet count/bipolar spleen diameter ratio for the prediction of esophageal varices: the special Egyptian situation. Hepatitis Monthly 2011;11(4):278‐84. [PMC free article] [PubMed] [Google Scholar]
Adami 2013 {published data only}
- Adami MR, Ferreira CT, Kieling CO, Hirakata V, Vieira SMG. Noninvasive methods for prediction of esophageal varices in pediatric patients with portal hypertension. World Journal of Gastroenterology 2013;19(13):2053‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agha 2009 {published data only}
- Agha A, Anwar E, Bashir K, Savarino V, Giannini EG. External validation of the platelet count/spleen diameter ratio for the diagnosis of esophageal varices in hepatitis C virus‐related cirrhosis. Digestive Diseases and Sciences 2009;54(3):654‐60. [PUBMED: 18594972] [DOI] [PubMed] [Google Scholar]
Agha 2011 {published data only}
- Agha A, Abdulhadi MM, Marenco S, Bella A, Alsaudi D, Haddad A, et al. Use of the platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices in patients with schistosomiasis. Saudi Journal of Gastroenterology 2011;17(5):307‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alcantara 2012 {published data only}
- Alcantara RV, Yamada RM, Tommaso A, Bellomo‐Brandão MA, Hessel G. Non‐invasive predictors of esophageal varices in children and adolescents with chronic liver disease or extrahepatic portal venous obstruction. Jornal de Pediatria 2012;88(4):341‐6. [DOI] [PubMed] [Google Scholar]
Amin 2012 {published data only}
- Amin K, Muhammad D, Anjum A, Jamil K, Hassan A. Platelet count to spleen diameter ratio as a predictor of esophageal varices in patients of liver cirrhosis due to hepatitis C virus. Journal of University Medical Dental College 2012;3:6‐11. [Google Scholar]
Aqodad 2011 {published data only}
- Aqodad N, Ighoudane H, Elyousfi M, Mellouki I, Elabkari M, Benajeh D, et al. Correlation of platelet count with grading of esophageal varices in cirrhotic patients. Hepatology International 2011;5(1):362. [Google Scholar]
Baig 2008 {published data only}
- Baig WW, Nagaraja MV, Varma M, Prabhu R. Platelet count to spleen diameter ratio for the diagnosis of esophageal varices: is it feasible?. Canadian Journal of Gastroenterology 2008;22(10):825‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barikbin 2010 {published data only}
- Barikbin R, Hekmatnia A, Omidifar N, Farghadani M, Adibi P. Prediction severity of esophageal varices: a new cutoff point for platelet count/spleen diameter ratio. Minerva Gastroenterologica e Dietologica 2010;56(1):1‐6. [PubMed] [Google Scholar]
Burton 2007a {published data only}
- Burton JR Jr, Liangpunsakul S, Lapidus J, Giannini E, Chalasani N, Zaman A. Validation of a multivariate model predicting presence and size of varices. Journal of Clinical Gastroenterology 2007;41(6):609‐15. [DOI] [PubMed] [Google Scholar]
Burton 2007b {published data only}
- Burton JR Jr, Liangpunsakul S, Lapidus J, Giannini E, Chalasani N, Zaman A. Validation of a multivariate model predicting presence and size of varices. Journal of Clinical Gastroenterology 2007;41(6):609‐15. [DOI] [PubMed] [Google Scholar]
Burton 2007c {published data only}
- Burton JR Jr, Liangpunsakul S, Lapidus J, Giannini E, Chalasani N, Zaman A. Validation of a multivariate model predicting presence and size of varices. Journal of Clinical Gastroenterology 2007;41(6):609‐15. [DOI] [PubMed] [Google Scholar]
Burton 2007d {published data only}
- Burton JR Jr, Liangpunsakul S, Lapidus J, Giannini E, Chalasani N, Zaman A. Validation of a multivariate model predicting presence and size of varices. Journal of Clinical Gastroenterology 2007;41(6):609‐15. [DOI] [PubMed] [Google Scholar]
Camma 2009 {published data only}
- Camma C, Petta S, Marco V, Bronte F, Ciminnisi S, Licata G, et al. Insulin resistance is a risk factor for esophageal varices in hepatitis C virus cirrhosis. Hepatology (Baltimore, Md) 2009;49(1):195‐203. [PUBMED: 19065558] [DOI] [PubMed] [Google Scholar]
Castera 2009 {published data only}
- Castera L, Bail B, Roudot‐Thoraval F, Bernard PH, Foucher J, Merrouche W, et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non‐invasive scores. Journal of Hepatology 2009;50(1):59‐68. [DOI] [PubMed] [Google Scholar]
Cherian 2011 {published data only}
- Cherian JV, Deepak N, Ponnusamy RP, Somasundaram A, Jayanthi V. Non‐invasive predictors of esophageal varices. Saudi Journal of Gastroenterology 2011;17(1):64‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiodi 2014 {published data only}
- Chiodi D, Hernandez N, Saona G, Sanchez A, Berrueta J, Mescia G, et al. [Noninvasive diagnosis of esophageal varices in cirrhotic patients] [Spanish]. Acta Gastroenterologica Latinoamericana 2014;44(2):108‐13. [PubMed] [Google Scholar]
Colecchia 2011 {published data only}
- Colecchia A, Biase AR, Scaioli E, Predieri B, Iughetti L, Reggiani MLB, et al. Non‐invasive methods can predict oesophageal varices in patients with biliary atresia after a Kasai procedure. Digestive and Liver Disease 2011;43(8):659‐63. [DOI] [PubMed] [Google Scholar]
Colecchia 2012 {published data only}
- Colecchia A, Montrone L, Scaioli E, Bacchi‐Reggiani ML, Colli A, Casazza G, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV‐related cirrhosis. Gastroenterology 2012;143(3):646‐54. [DOI] [PubMed] [Google Scholar]
De Mattos 2010 {published data only}
- Mattos AZ, Mattos AA, Vianna FF, Musskopf MI, Pereira‐Lima JC, Maciel AC. Platelet count/spleen diameter ratio: analysis of its capacity as a predictor of the existence of esophageal varices. Arquivos de Gastroenterologia 2010;47(3):275‐8. [DOI] [PubMed] [Google Scholar]
Ding 2016 {published data only}
- Ding NS, Nguyen T, Iser DM, Hong T, Flanagan E, Wong A, et al. Liver stiffness plus platelet count can be used to exclude high‐risk oesophageal varices. Liver International 2016;36(2):240‐5. [CRS: 6800131000104399; EMBASE: 20151018144] [DOI] [PubMed] [Google Scholar]
Ditchfield 1992 {published data only}
- Ditchfield MR, Gibson RN, Donlan JD, Gibson PR. Duplex Doppler ultrasound signs of portal hypertension: relative diagnostic value of examination of paraumbilical vein, portal vein and spleen. Australasian Radiology 1992;36(2):102‐5. [PUBMED: 1520164] [DOI] [PubMed] [Google Scholar]
El Makarem 2011 {published data only}
- Abu El Makarem MA, Shatat ME, Shaker Y, Abdel Aleem AA, Sherif AM, Moaty MA, et al. Platelet count/bipolar spleen diameter ratio for the prediction of esophageal varices: the special Egyptian situation. Hepatitis Monthly 2011;11:278‐84. [PMC free article] [PubMed] [Google Scholar]
El Ray 2015 {published data only}
- Ray A, Azab MM, El‐Aziz IM, El‐Aleem AA, El‐Talkawy MD, El‐Badea MA, et al. Non‐invasive predictors for the presence, grade and risk of bleeding from esophageal varices in patients with post‐hepatic cirrhosis. Journal of the Egyptian Society of Parasitology 2015;45:421‐8. [DOI] [PubMed] [Google Scholar]
Esmat 2012 {published data only}
- Esmat S, Omarn D, Rashid L. Can we consider the right hepatic lobe size/albumin ratio a noninvasive predictor of oesophageal varices in hepatitis C virus‐related liver cirrhotic Egyptian patients?. European Journal of Internal Medicine 2012;23(3):267‐72. [DOI] [PubMed] [Google Scholar]
Gana 2011 {published data only}
- Gana JC, Turner D, Mieli‐Vergani G, Davenport M, Miloh T, Avitzur Y, et al. A clinical prediction rule and platelet count predict esophageal varices in children. Gastroenterology 2011;141(6):2009‐16. [DOI] [PubMed] [Google Scholar]
Gentile 2009 {published data only}
- Gentile I, Viola C, Graf M, Liuzzi R, Quarto M, Cerini R. A simple noninvasive score predicts gastroesophageal varices in patients with chronic viral hepatitis. Journal of Clinical Gastroenterology 2009;43(1):81‐7. [DOI] [PubMed] [Google Scholar]
Giannini 2003a {published data only}
- Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, et al. Platelet count/spleen diameter ratio: proposal and validation of a non‐invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut 2003;52(8):1200‐5. [PUBMED: 12865282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giannini 2003b {published data only}
- Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, et al. Platelet count/spleen diameter ratio: proposal and validation of a non‐invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut 2003;52(8):1200‐5. [PUBMED: 12865282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giannini 2005 {published data only}
- Giannini EG, Botta F, Borro P, Dulbecco P, Testa E, Mansi C, et al. Application of the platelet count/spleen diameter ratio to rule out the presence of oesophageal varices in patients with cirrhosis: a validation study based on follow‐up. Digestive and Liver Disease 2005;37(10):779‐85. [PUBMED: 15996912] [DOI] [PubMed] [Google Scholar]
Giannini 2006 {published data only}
- Giannini EG, Zaman A, Kreil A, Floreani A, Dulbecco P, Testa E, et al. Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation study. American Journal of Gastroenterology 2006;101(11):2511‐9. [PUBMED: 17029607] [DOI] [PubMed] [Google Scholar]
Gonzalez‐Ojeda 2014 {published data only}
- Gonzalez‐Ojeda A, Cervantes‐Guevara G, Chavez‐Sanchez M, Davalos‐Cobian C, Ornelas‐Cazares S, Macias‐Amezcua MD, et al. Platelet count/spleen diameter ratio to predict esophageal varices in Mexican patients with hepatic cirrhosis. World Journal of Gastroenterology 2014;20(8):2079‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grgurevic 2014 {published data only}
- Grgurevic I, Jukic I, Sokol S, Banic M, Bilic B, Gunjaca I, et al. Low specificity of platelet to spleen ratio for noninvasive prediction and characterization of esophageal varices in patients with alcoholic liver cirrhosis [Omjer broja trombocita i velicine slezene ima nisku specificnost u neinvazivnoj predikciji i karakterizaciji varikoziteta jednjaka kod bolesnika s alkoholnom cirozom jetre]. Acta Medica Croatica 2014;68(4‐5):353‐60. [CRS: 6800131000062413; EMBASE: 2015400928; JC‐‐NLM: Journal ID:bh2, 9208249; PUBMED: 26285468] [PubMed] [Google Scholar]
Jeon 2006 {published data only}
- Jeon SW, Cho CM, Tak WY, Ryeom HK, Kweon YO, Kim SK, et al. The value of Doppler‐ultrasonography and laboratory tests as non‐invasive predictors of the presence of esophageal varices in patients with chronic liver disease. Korean Journal of Gastroenterology 2006;48(3):180‐7. [PUBMED: 17047433] [PubMed] [Google Scholar]
Karatzas 2016 {published data only}
- Karatzas A, Triantos C, Kalafateli M, Marzigie M, Labropoulou‐Karatza C, Thomopoulos K, et al. Multidetector computed tomography versus platelet/spleen diameter ratio as methods for the detection of gastroesophageal varices. Annals of Gastroenterology 2016;29(1):71‐8. [CRS: 6800131000104406; EMBASE: 20160031721] [PMC free article] [PubMed] [Google Scholar]
Legasto 2006 {published data only}
- Legasto JMA, Sevilla J, Balay A, Tan JA, Cham LV, Vitug A, et al. Platelet count/spleen diameter ratio: a noninvasive parameter to predict the presence of esophageal varices. Philippine Journal of Gastroenterology 2006;2:33‐8. [Google Scholar]
Lei 2007 {published data only}
- Lei JB, Huang TZ. Value of the platelet count/spleen diameter ratio on predicting esophageal varices of liver cirrhosis. Practical Clinical Medicine 2007;8:25‐7. [Google Scholar]
Levy 2007a {published data only}
- Levy C, Zein CO, Gomez J, Soldevila‐Pico C, Firpi R, Morelli G, et al. Prevalence and predictors of esophageal varices in patients with primary biliary cirrhosis. Clinical Gastroenterology and Hepatology 2007;5(7):803‐8. [PUBMED: 17544879] [DOI] [PubMed] [Google Scholar]
Levy 2007b {published data only}
- Levy C, Zein CO, Gomez J, Soldevila‐Pico C, Firpi R, Morelli G, et al. Prevalence and predictors of esophageal varices in patients with primary biliary cirrhosis. Clinical Gastroenterology and Hepatology 2007;5(7):803‐8. [PUBMED: 17544879] [DOI] [PubMed] [Google Scholar]
Madhotra 2002 {published data only}
- Madhotra R, Mulcahy HE, Willner I, Reuben A. Prediction of esophageal varices in patients with cirrhosis. Journal of Clinical Gastroenterology 2002;34(1):81‐5. [PUBMED: 11743252] [DOI] [PubMed] [Google Scholar]
Mahassadi 2012a {published data only}
- Mahassadi AK, Bathaix FY, Assi C, Bangoura AD, Allah‐Kouadio E, Kissi HY, et al. Usefulness of noninvasive predictors of oesophageal varices in black African cirrhotic patients in Cote d'Ivoire (West Africa). Gastroenterology Research and Practice 2012;2012:(no pagination). [CRS: 6800131000104543; EMBASE: 2012476143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahassadi 2012b {published data only}
- Mahassadi AK, Bathaix FY, Assi C, Bangoura AD, Allah‐Kouadio E, Kissi HY, et al. Usefulness of noninvasive predictors of oesophageal varices in black African cirrhotic patients in Cote d'Ivoire (West Africa). Gastroenterology Research and Practice 2012;2012:(no pagination). [CRS: 6800131000104543; EMBASE: 2012476143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosqueira 2011 {published data only}
- Mosqueira JR, Montiel JB, Rodriguez D, Monge E. Evaluation of the diagnostic test of index platelet counts/size spleen, as a predictor of the presence of esophageal varices in cirrhosis [Spanish]. Revista de Gastroenterologia del Peru 2011;31(1):11‐6. [PubMed] [Google Scholar]
Parrino 2008 {published data only}
- Parrino A, Gesaro V, Terranova A, Sesti R, Corsale S, Patti AM, et al. Non‐invasive diagnosis of esophageal varices in cirrhotic patients: endoscopic vs ultrasonographic findings [Italian]. Acta Medica Mediterranea 2008;24(1):11‐8. [Google Scholar]
Pilette 1999 {published data only}
- Pilette C, Oberti F, Aube C, Rousselet MC, Bedossa P, Gallois Y, et al. Non‐invasive diagnosis of esophageal varices in chronic liver diseases. Journal of Hepatology 1999;31(5):867‐73. [PUBMED: 10580584] [DOI] [PubMed] [Google Scholar]
Prihatini 2005 {published data only}
- Prihatini J, Lesmana LA, Manan C, Gani RA. Detection of esophageal varices in liver cirrhosis using non‐invasive parameters. Acta Medica Indonesiana 2005;37(3):126‐31. [PUBMED: 16110174] [PubMed] [Google Scholar]
Primignani 2002 {published data only}
- Primignani M, Savojardo V, Dell'Era A, Fazzini L, Zatelli S, Pedotti P, et al. Presence of esophageal varices in patients with a recent histologic diagnosis of cirrhosis of the liver: evaluation of clinical predictors. Gastroenterology 2002; Vol. 122, issue Suppl 4:A354.
Sanyal 2006 {published data only}
- Sanyal AJ, Fontana RJ, Bisceglie AM, Everhart JE, Doherty MC, Everson GT, et al. The prevalence and risk factors associated with esophageal varices in subjects with hepatitis C and advanced fibrosis. Gastrointestinal Endoscopy 2006;64(6):855‐64. [PUBMED: 17140886] [DOI] [PubMed] [Google Scholar]
Sarangapani 2010 {published data only}
- Sarangapani A, Shanmugam C, Kalyanasundaram M, Rangachari B, Thangavelu P, Subbarayan JK. Noninvasive prediction of large esophageal varices in chronic liver disease patients. Saudi Journal of Gastroenterology 2010;16(1):38‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarzenberger 2010 {published data only}
- Schwarzenberger E, Meyer T, Golla V, Sahdala NP, Min AD. Utilization of platelet count spleen diameter ratio in predicting the presence of esophageal varices in patients with cirrhosis. Journal of Clinical Gastroenterology 2010;44(2):146‐50. [DOI] [PubMed] [Google Scholar]
Sebastiani 2010 {published data only}
- Sebastiani G, Tempesta D, Fattovich G, Castera L, Halfon P, Bourliere M, et al. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non‐invasive markers: results of a multicenter, large‐scale study. Journal of Hepatology 2010;53(4):630‐8. [DOI] [PubMed] [Google Scholar]
Sen 2008a {published data only}
- Sen S, Griffiths WJH. Non‐invasive prediction of oesophageal varices in cirrhosis. World Journal of Gastroenterology 2008;14:2454‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sen 2008b {published data only}
- Sen S, Griffiths WJH. Non‐invasive prediction of oesophageal varices in cirrhosis. World Journal of Gastroenterology 2008;14:2454‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2013 {published data only}
- Sharma P, Kirnake V, Tyagi P, Bansal N, Singla V, Kumar A, et al. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. American Journal of Gastroenterology 2013;108(7):1101‐7. [DOI] [PubMed] [Google Scholar]
Sharma 2014 {published data only}
- Sharma J, Yadav MK, Gupta A, Arya TS. A study of role of platelet count/spleen diameter ratio as a predictor of esophageal varices in patient with chronic liver disease. National Journal of Medical Research 2014;4(3):232‐4. [Google Scholar]
- Sharma J, Yadav MK, Gupta A, Arya TS. A study of the role of platelet count/spleen diameter ratio as a predictor of esophageal varices in patients with chronic liver disease. Indian Journal of Gastroenterology 2012;31(1 Suppl):A136‐7. [Google Scholar]
Sheta 2016 {published data only}
- Abd‐Elsalam SM, Sheta EA, Yousef M, Mohamed REE, Amer I, Ismail A. Non invasive diagnosis of esophageal varices: can it replace screening endoscopy?. Hepatology International 2016;10(1 Suppl):S496. [CRS: 6800131000104388; EMBASE: 72201392] [Google Scholar]
- Sheta EAE, Yousef M, Abd‐Elsalam S, Mohamed REE, Ismail A, El‐Kalla F, et al. Non invasive diagnosis of esophageal varices: can it replace screening endoscopy?. International Journal of Current Microbiology and Applied Sciences 2016;5:701‐16. [Google Scholar]
Stefanescu 2011 {published data only}
- Stefanescu H, Grigorescu M, Lupsor M, Procopet B, Maniu A, Badea R. Spleen stiffness measurement using fibroscan for the noninvasive assessment of esophageal varices in liver cirrhosis patients. Journal of Gastroenterology and Hepatology 2011;26:164‐70. [DOI] [PubMed] [Google Scholar]
Tafarel 2011 {published data only}
- Tafarel JR, Tolentino LHL, Correa LM, Bonilha DR, Piauilino P, Martins FP, et al. Prediction of esophageal varices in hepatic cirrhosis by noninvasive markers. European Journal of Gastroenterology & Hepatology 2011;23(9):754‐8. [DOI] [PubMed] [Google Scholar]
Takuma 2013 {published data only}
- Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, et al. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology 2013;144(1):92‐101. [DOI] [PubMed] [Google Scholar]
Tarantino 2009 {published data only}
- Tarantino G, Citro V, Esposito P, Giaquinto S, Leone A, Milan G, et al. Blood ammonia levels in liver cirrhosis: a clue for the presence of portosystemic collateral veins. BMC Gastroenterology 2009;9:21. [PUBMED: 19292923] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wadhva 2012 {published data only}
- Wadhva R, Abbas Z, Luck NH, Hassan SM, Abubakr M. Platelet count to splenic diameter ratio and splenoportal index for predicting esophageal varices among patients with cirrhosis. Hepatology International 2012;6(1):294. [Google Scholar]
Wang CC 2015 {published data only}
- Wang C‐C, Tsai M‐C, Yang T‐W, Lin C‐B, Chen T‐Y, Lin C‐C. Acoustic radiation force impulse sonography‐based non‐invasive prediction of esophageal varices. Journal of Gastroenterology and Hepatology 2015;30(Suppl S4):367‐8. [CRS: 6800131000104411; EMBASE: 72135359] [Google Scholar]
Wang HM 2012 {published data only}
- Wang H‐M, Lo G‐H, Chen W‐C, Hsu P‐I, Yu H‐C, Lin C‐K, et al. Efficacy of transient elastography in screening for large esophageal varices in patients with suspicious or proven liver cirrhosis. Journal of Digestive Diseases 2012;13(8):430‐8. [DOI] [PubMed] [Google Scholar]
Wang JH 2012 {published data only}
- Wang J‐H, Chuah S‐K, Lu S‐N, Hung C‐H, Chen C‐H, Kee K‐M. Transient elastography and simple blood markers in diagnosis of oesophageal varices for compensated patients with hepatitis B virus‐related cirrhosis. Hepatology International 2012;6(1):6. [DOI] [PubMed] [Google Scholar]
Xu 2016a {published data only}
- Xu X‐D, Xu C‐F, Dai J‐J, Qian J‐Q, Pin X. Ratio of platelet count/spleen diameter predicted the presence of esophageal varices in patients with schistosomiasis liver cirrhosis. European Journal of Gastroenterology & Hepatology 2016;28(5):588‐91. [CRS: 6800131000104363; EMBASE: 20160122451] [DOI] [PubMed] [Google Scholar]
Zafar 2014 {published data only}
- Zafar S, Latif MM. Diagnostic accuracy of platelet count/spleen diameter ratio for detection of esophageal varices in cirrhotic patients taking endoscopy as gold standard. Pakistan Journal of Medical and Health Sciences 2014;8(4):951‐4. [Google Scholar]
Zaman 2001 {published data only}
- Zaman A, Becker T, Lapidus J, Benner K. Risk factors for the presence of varices in cirrhotic patients without a history of variceal hemorrhage. Archives of Internal Medicine 2001;161(21):2564‐70. [DOI] [PubMed] [Google Scholar]
Zein 2004a {published data only}
- Zein CO, Lindor KD, Angulo P. Prevalence and predictors of esophageal varices in patients with primary sclerosing cholangitis. Hepatology (Baltimore, Md) 2004;39(1):204‐10. [PUBMED: 14752839] [DOI] [PubMed] [Google Scholar]
Zein 2004b {published data only}
- Zein CO, Lindor KD, Angulo P. Prevalence and predictors of esophageal varices in patients with primary sclerosing cholangitis. Hepatology (Baltimore, Md) 2004;39(1):204‐10. [PUBMED: 14752839] [DOI] [PubMed] [Google Scholar]
Zimbwa 2004 {published data only}
- Zimbwa TA, Blanshard C, Subramaniam A. Platelet count/spleen diameter ratio as a predictor of oesophageal varices in alcoholic cirrhosis. Gut 2004; Vol. 53, issue 7:1055. [PUBMED: 15194662] [PMC free article] [PubMed]
References to studies excluded from this review
Albreedy 2015 {published data only}
- Albreedy AM. Platelet count to spleen diameter ratio and to spleen area ratio as predictors for esophageal varices in chronic hepatitis C patients with liver cirrhosis. Journal of the Egyptian Society of Parasitology 2015;45:485‐92. [DOI] [PubMed] [Google Scholar]
Amarapurkar 1994 {published data only}
Barrera 2009 {published data only}
- Barrera F, Riquelme A, Soza A, Contreras A, Barrios G, Padilla O, et al. Platelet count/spleen diameter ratio for non‐invasive prediction of high risk esophageal varices in cirrhotic patients. Annals of Hepatology 2009;8(4):325‐30. [PubMed] [Google Scholar]
Chalasani 1999 {published data only}
- Chalasani N, Imperiale TF, Ismail A, Sood G, Carey M, Wilcox CM, et al. Predictors of large esophageal varices in patients with cirrhosis. American Journal of Gastroenterology 1999;94(11):3285‐91. [PUBMED: 10566731] [DOI] [PubMed] [Google Scholar]
Cho 2015 {published data only}
- Cho EJ, Kim MY, Lee JH, Lee IY, Lim YL, Choi DH, et al. Diagnostic and prognostic values of noninvasive predictors of portal hypertension in patients with alcoholic cirrhosis. PloS One 2015;10(7):e0133935. [CRS: 6800131000104272; JC‐‐NLM: Journal ID:101285081; OI:SOURCE:: NLM. PMC4511411; PUBMED: 26196942] [DOI] [PMC free article] [PubMed] [Google Scholar]
El Guindi 2008 {published data only}
- Guindi M, Arabi H, Henawy I, Basiouny H. Non‐invasive parameters as predictors for esophageal varices in children with chronic liver disease. Hepatology (Baltimore, Md) 2008; Vol. 48, issue 4 Suppl:1040A.
El‐Sherif 2008 {published data only}
- Sherif AM, Abou‐Shady MA, Al Bahrawy AM, Bakr RM, Hosny A. Nitric oxide levels in chronic liver disease patients with and without oesophageal varices. Hepatology International 2008;2:341‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagundes 2008 {published data only}
- Fagundes ED, Ferreira AR, Roquete ML, Penna FJ, Goulart EM, Figueiredo Filho PP, et al. Clinical and laboratory predictors of esophageal varices in children and adolescents with portal hypertension syndrome. Journal of Pediatric Gastroenterology and Nutrition 2008;46(2):178‐83. [PUBMED: 18223377] [DOI] [PubMed] [Google Scholar]
Gana 2010 {published data only}
- Gana JC, Turner D, Roberts EA, Ling SC. Derivation of a clinical prediction rule for the noninvasive diagnosis of varices in children. Journal of Pediatric Gastroenterology and Nutrition 2010;50:188‐93. [DOI] [PubMed] [Google Scholar]
Giannini 2007 {published data only}
- Giannini EG, Anwar E, Bashir K, Savarino V, Agha A. Platelet count/spleen diameter ratio and AASLD criterion for screening esophageal varices in patients with hepatitis C virus related compensated cirrhosis. Hepatology (Baltimore, Md) 2007; Vol. 46, issue 4 Suppl 1:568A.
Hong 2009 {published data only}
- Hong WD, Zhu QH, Huang ZM, Chen XR, Jiang ZC, Xu SH, et al. Predictors of esophageal varices in patients with HBV‐related cirrhosis: a retrospective study. BMC Gastroenterology 2009;9:11. [PUBMED: 19196464] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koncoro 2014 {published data only}
- Koncoro H, Mariadi K, Somayana G, Suryadarma GA, Purwadi N, Wibawa DN. Prediction of large esophageal varices among patients with liver cirrhosis in Sanglah Hospital Danpasar. Journal of Gastroenterology and Hepatology 2014;29(Suppl S3):193. [Google Scholar]
Lee 2009 {published data only}
- Lee JH, Yoon JH, Lee CH, Myung SJ, Keam B, Kim BH, et al. Complete blood count reflects the degree of oesophageal varices and liver fibrosis in virus‐related chronic liver disease patients. Journal of Viral Hepatitis 2009;16(6):444‐52. [PUBMED: 19200133] [DOI] [PubMed] [Google Scholar]
Malik 2015 {published data only}
- Malik K, Batool F, Khan SA, ul Amir Z. Platelet count/splenic size ratio as a non‐invasive parameter to predict the presence of esophageal varices in cirrhotics. Rawal Medical Journal 2015;40(4):371‐4. [CRS: 6800131000063484; EMBASE: 2015504984] [Google Scholar]
Nashaat 2010 {published data only}
- Nashaat EH, Abd‐Elaziz H, Sabry M, Ibrahim AA. Non‐endoscopic predictors of esophageal varices and portal hypertensive gastropathy. Nature and Science 2010;8:43‐50. [Google Scholar]
Nazish 2011 {published data only}
- Nazish Z, Inayatullah M, Tanweer S. Non endoscopic predictors of esophageal varices in cirrhotics. Medical Forum Monthly 2011;22:3‐7. [Google Scholar]
Ng 1999 {published data only}
- Ng FH, Wong SY, Loo CK, Lam KM, Lai CW, Cheng CS. Prediction of oesophagogastric varices in patients with liver cirrhosis. Journal of Gastroenterology and Hepatology 1999;14(8):785‐90. [PUBMED: 10482429] [DOI] [PubMed] [Google Scholar]
Park 2015 {published data only}
- Park Y, Kim SU, Park SY, Kim BK, Park JY, Kim DY, et al. A novel model to predict esophageal varices in patients with compensated cirrhosis using acoustic radiation force impulse elastography. PLoS One 2015;10(3):e0121009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qamar 2008 {published data only}
- Qamar AA, Grace ND, Groszmann RJ, Garcia‐Tsao G, Bosch J, Burroughs AK. Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis. Hepatology (Baltimore, Md) 2008;47:153‐9. [DOI] [PubMed] [Google Scholar]
Rockey 2016 {published data only}
- Rockey DC, Elliott A, Lyles T. Prediction of esophageal varices and variceal hemorrhage in patients with acute upper gastrointestinal bleeding. Journal of Investigative Medicine 2016;64(3):745‐51. [CRS: 6800131000104572] [DOI] [PubMed] [Google Scholar]
Sebastiani 2008 {published data only}
Sethar 2006 {published data only}
- Sethar GH, Ahmed R, Rathi SK, Shaikh NA. Platelet count/splenic size ratio: a parameter to predict the presence of esophageal varices in cirrhotics. Journal of the College of Physicians and Surgeons ‐ Pakistan 2006;16(3):183‐6. [PUBMED: 16542615] [PubMed] [Google Scholar]
Shah 2011 {published data only}
- Shah ZH, Sarwar S, Saleem K, Abaidullah S, Mehboob F, Afzal A. Identification of non‐endoscopic predictors of esophageal varices in cirrhosis. Pakistan Journal of Medical and Health Sciences 2011;5:257‐61. [Google Scholar]
Sharma 2007 {published data only}
- Sharma SK, Aggarwal R. Prediction of large esophageal varices in patients with cirrhosis of the liver using clinical, laboratory and imaging parameters. Journal of Gastroenterology and Hepatology 2007;22(11):1909‐15. [PUBMED: 17914969] [DOI] [PubMed] [Google Scholar]
Takuma 2016 {published data only}
- Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Takabatake H, et al. Portal hypertension in patients with liver cirrhosis: diagnostic accuracy of spleen stiffness. Radiology 2016;279(2):609‐19. [CRS: 6800131000104355; EMBASE: 20160357558] [DOI] [PubMed] [Google Scholar]
Tao 2008 {published data only}
- Tao W, Yang SQ, Lv XC, Yang L. Value of the platelet count/spleen diameter ratio on diagnosis of esophageal varices in patients with cirrhosis. Journal of Ningxia Medical College 2008;30:349‐50. [Google Scholar]
Thayumanavan 2012 {published data only}
- Thayumanavan L, Jaisankar P, Ramani R, Kannan M, Sangumani J, Sreeraj V. Validating the usefulness of doppler study of hepatic veins in predicting esophageal varices in cirrhotic patients. Journal of Clinical and Experimental Hepatology 2012;2(1 Suppl):S19. [Google Scholar]
Thomopoulos 2003 {published data only}
- Thomopoulos KC, Labropoulou‐Karatza C, Mimidis KP, Katsakoulis EC, Iconomou G, Nikolopoulou VN. Non‐invasive predictors of the presence of large oesophageal varices in patients with cirrhosis. Digestive and Liver Disease 2003;35(7):473‐8. [PUBMED: 12870732] [DOI] [PubMed] [Google Scholar]
Treeprasertsuk 2010 {published data only}
- Treeprasertsuk S, Kowdley KV, Luketic VAC, Harrison ME, Mccashland T, Befeler AS. The predictors of the presence of varices in patients with primary sclerosing cholangitis. Hepatology (Baltimore, Md) 2010;51:1302‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valente {published data only}
- Valente Pinto M, Castanhinha S, Pereira L, Lopes AI, Barreto C. Liver disease in children with cystic fibrosis ‐ The contribution of ultrasound evaluation. Journal of Cystic Fibrosis 2013;12(Suppl 1):S112. [Google Scholar]
Yu 2008 {published data only}
- Yu JY, Liu XN, Wang D, Cui JJ. Non‐invasive predictive factors of esophageal varices in patients with post‐hepatitis B cirrhosis. Journal of Binzhou Medical University 2008;31:345‐9. [Google Scholar]
Zaman 1999 {published data only}
- Zaman A, Hapke R, Flora K, Rosen HR, Benner K. Factors predicting the presence of esophageal or gastric varices in patients with advanced liver disease. American Journal of Gastroenterology 1999;94(11):3292‐6. [PUBMED: 10566732] [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang J, Tao R, You Z, Dai Y, Fan Y, Cui J, et al. Gamna‐Gandy bodies of the spleen detected with susceptibility weighted imaging: maybe a new potential non‐invasive marker of esophageal varices. PloS One 2013;8(1):e55626. [CRS: 6800131000104538; EMBASE: 2013079030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang C‐X, Xu J‐M, Li J‐B, Kong D‐R, Wang L, Xu X‐Y, et al. Predict esophageal varices via routine trans‐abdominal ultrasound: a design of classification analysis model. Journal of Gastroenterology and Hepatology (Australia) 2016;31(1):194‐9. [CRS: 6800131000104397; EMBASE: 20160106034] [DOI] [PubMed] [Google Scholar]
Additional references
Abraldes 2016
Adams 2004
- Adams PC, Arthur MJ, Boyer TD, DeLeve LD, Bisceglie AM, Hall M, et al. Screening in liver disease: report of an AASLD clinical workshop. Hepatology (Baltimore, Md) 2004;39(5):1204‐12. [PUBMED: 15122748] [DOI] [PubMed] [Google Scholar]
Arguedas 2002
- Arguedas MR, Heudebert GR, Eloubeidi MA, Abrams GA, Fallon MB. Cost‐effectiveness of screening, surveillance, and primary prophylaxis strategies for esophageal varices. American Journal of Gastroenterology 2002;97(9):2441‐52. [PUBMED: 12358270] [DOI] [PubMed] [Google Scholar]
ASGE Standards of Practice Committee 2012
- ASGE Standards of Practice Committee, Ben‐Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, Fisher DA, et al. Adverse events of upper GI endoscopy. Gastrointestinal Endoscopy 2012;76:707‐18. [DOI] [PubMed] [Google Scholar]
Bambha 2008
- Bambha K, Kim WR, Pedersen R, Bida JP, Kremers WK, Kamath PS. Predictors of early re‐bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis. Gut 2008;57(6):814‐20. [PUBMED: 18250126] [DOI] [PubMed] [Google Scholar]
Bellentani 1994
- Bellentani S, Tiribelli C, Saccoccio G, Sodde M, Fratti N, Martin C, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos study. Hepatology (Baltimore, Md) 1994;20(6):1442‐9. [PUBMED: 7982643] [DOI] [PubMed] [Google Scholar]
Bendtsen 1990
- Bendtsen F, Skovgaard LT, Sorensen TI, Matzen P. Agreement among multiple observers on endoscopic diagnosis of esophageal varices before bleeding. Hepatology (Baltimore, Md) 1990;11(3):341‐7. [PUBMED: 2312048] [DOI] [PubMed] [Google Scholar]
Cales 1989
- Cales P, Buscail L, Bretagne JF, Champigneulle B, Bourbon P, Duclos B, et al. Interobserver and intercenter agreement of gastro‐esophageal endoscopic signs in cirrhosis. Results of a prospective multicenter study [Concordance inter‐observateurs inter‐centres des signes endoscopiques gastro‐oesophagiens au cours de la cirrhose. Resultats d'une etude prospective multicentrique]. Gastroenterologie Clinique et Biologique 1989;13(12):967‐73. [PUBMED: 2696663] [PubMed] [Google Scholar]
Cales 1990
- Cales P, Desmorat H, Vinel JP, Caucanas JP, Ravaud A, Gerin P, et al. Incidence of large oesophageal varices in patients with cirrhosis: application to prophylaxis of first bleeding. Gut 1990;31(11):1298‐302. [PUBMED: 2253916] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chawla 2012
- Chawla S, Katz A, Attar BM, Gupta A, Sandhu DS, Agarwal R. Platelet count/spleen diameter ratio to predict the presence of esophageal varices in patients with cirrhosis: a systematic review. European Journal of Gastroenterology and Hepatology 2012;24:431‐6. [DOI] [PubMed] [Google Scholar]
Colli 2014a
- Colli A, Fraquelli M, Casazza G, Conte D, Nikolova D, Duca P, et al. The architecture of diagnostic research: from bench to bedside ‐ research guidelines using liver stiffness as an example. Hepatology (Baltimore, Md) 2014;60:408‐18. [DOI: 10.1002/hep.26948] [DOI] [PubMed] [Google Scholar]
Colli 2014b
- Colli A, Gana JC, Turner D, Yap J, Adams‐Webber T, Ling SC, et al. Capsule endoscopy for the diagnosis of oesophageal varices in people with chronic liver disease or portal vein thrombosis. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD008760.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotton 2006
- Cotton PB, Hawes RH, Barkun A, Ginsberg GG, Amman S, Cohen J, et al. Excellence in endoscopy: toward practical metrics. Gastrointestinal Endoscopy 2006;63:286‐91. [DOI] [PubMed] [Google Scholar]
D'Amico 1999
- D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence‐based approach. Seminars in Liver Disease 1999;19(4):475‐505. [PUBMED: 10643630] [DOI] [PubMed] [Google Scholar]
D'Amico 2003
- D'Amico G, Franchis R. Upper digestive bleeding in cirrhosis. Post‐therapeutic outcome and prognostic indicators. Hepatology (Baltimore, Md) 2003;38(3):599‐612. [PUBMED: 12939586] [DOI] [PubMed] [Google Scholar]
D'Amico 2006 a
- D'Amico G, Garcia‐Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology 2006;131(5):1611‐24. [PUBMED: 17101332] [DOI] [PubMed] [Google Scholar]
D'Amico 2006 b
- D’Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44:217–31. [DOI] [PubMed] [Google Scholar]
D'Amico 2014
- D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25‐year inception cohort study of 494 patients. Alimentary Pharmacology & Therapeutics 2014;39:1180‐93. [DOI] [PubMed] [Google Scholar]
de Franchis 1992
- Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson M, Fleig W, et al. Definitions, methodology and therapeutic strategies in portal hypertension. A Consensus Development Workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. Journal of Hepatology 1992;15(1‐2):256‐61. [PUBMED: 1506645] [DOI] [PubMed] [Google Scholar]
de Franchis 2010
- Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. Journal of Hepatology 2010;53(4):762‐8. [PUBMED: 20638742] [DOI] [PubMed] [Google Scholar]
de Franchis 2015
- Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. Journal of Hepatology 2015;63:743‐52. [DOI] [PubMed] [Google Scholar]
DTA Handbook 2010
- Diagnostic Test Accuracy Working Group. Handbook for DTA Reviews. srdta.cochrane.org/handbook‐dta‐reviews 2010 (accessed 23 September 2016).
EASL 2011
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. Journal of Hepatology 2011;55:245‐64. [DOI] [PubMed] [Google Scholar]
Gana 2010a
- Gana JC, Turner D, Yap J, Adams‐Webber T, Rashkovan N, Ling SC. Magnetic resonance imaging, computer tomography scan, and oesophagography for the diagnosis of oesophageal varices in patients with chronic liver disease or portal vein thrombosis. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008763] [DOI] [Google Scholar]
Gana 2010b
- Gana JC, Turner D, Yap J, Adams‐Webber T, Rashkovan N, Ling SC. Transient ultrasound elastography and magnetic resonance elastography for the diagnosis of oesophageal varices in patients with chronic liver disease or portal vein thrombosis. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008761] [DOI] [Google Scholar]
Gana 2010c
- Gana JC, Turner D, Yap J, Adams‐Webber T, Rashkovan N, Ling SC. Non‐invasive test of liver fibrosis for the diagnosis of oesophageal varices in patients with chronic liver disease or portal vein thrombosis. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008764] [DOI] [Google Scholar]
Garcia‐Tsao 2007
- Garcia‐Tsao G, Sanyal AJ, Grace ND, Carey WD. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. American Journal of Gastroenterology 2007;102(9):2086‐102. [PUBMED: 17727436] [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2008
- Garcia‐Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding ‐ unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single‐topic conference. Hepatology (Baltimore, Md) 2008; Vol. 47, issue 5:1764‐72. [PUBMED: 18435460] [DOI] [PubMed]
Garcia‐Tsao 2017
- Garcia‐Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017;65:310‐35. [DOI] [PubMed] [Google Scholar]
Geraci 2009
- Geraci G, Pisello F, Modica G, Li Volsi F, Arnone E, Sciumè C. Complications of elective esophago‐gastro‐duodenoscopy (EGDS). Personal experience and literature review. Il Giornale di Chirurgia 2009;30:502‐6. [PubMed] [Google Scholar]
Gluud 2007
- Gluud LL, Klingenberg S, Nikolova D, Gluud C. Banding ligation versus beta‐blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. American Journal of Gastroenterology 2007;102(12):2842‐8; quiz 2841, 2849. [PUBMED: 18042114] [DOI] [PubMed] [Google Scholar]
Gluud 2012
- Gluud LL, Krag A. Banding ligation versus beta‐blockers for primary prevention in oesophageal varices in adults. CochraneDatabase of Systematic Reviews 2012;Issue 8:CD004544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2016
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2016, Issue 8. Art. No.: LIVER.
Grace 1998
Graham 1981
- Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology 1981;80(4):800‐9. [PUBMED: 6970703] [PubMed] [Google Scholar]
Groszmann 2005
- Groszmann RJ, Garcia‐Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. Beta‐blockers to prevent gastroesophageal varices in patients with cirrhosis. New England Journal of Medicine 2005;353(21):2254‐61. [PUBMED: 16306522] [DOI] [PubMed] [Google Scholar]
Grover 1993
- Grover SA, Barkun AN, Sackett DL. Does this patient have splenomegaly?. JAMA 1993;270:2218‐21. [PubMed] [Google Scholar]
Imperiale 2001
- Imperiale TF, Chalasani N. A meta‐analysis of endoscopic variceal ligation for primary prophylaxis of esophageal variceal bleeding. Hepatology (Baltimore, Md) 2001;33(4):802‐7. [PUBMED: 11283842] [DOI] [PubMed] [Google Scholar]
Jalan 2000
- Jalan R, Hayes PC. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut 2000;46(Suppl 3‐4):III1‐III15. [PUBMED: 10862604] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2002
- Jensen DM. Endoscopic screening for varices in cirrhosis: findings, implications, and outcomes. Gastroenterology 2002;122(6):1620‐30. [PUBMED: 12016427] [DOI] [PubMed] [Google Scholar]
JSPH 1980
- Japanese Society for Portal Hypertension. The general rules for recording endoscopic findings on esophageal varices. Japanese Journal of Surgery 1980;10(1):84‐7. [DOI] [PubMed] [Google Scholar]
Kamath 2001
- Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology (Baltimore, Md) 2001;33(2):464‐70. [PUBMED: 11172350] [DOI] [PubMed] [Google Scholar]
Lebrec 1980
- Lebrec D, Fleury P, Rueff B, Nahum H, Benhamou JP. Portal hypertension, size of esophageal varices, and risk of gastrointestinal bleeding in alcoholic cirrhosis. Gastroenterology 1980;79(6):1139‐44. [PUBMED: 6969201] [PubMed] [Google Scholar]
Leffler 2010
- Leffler DA, Kheraj R, Garud S, Neeman N, Nathanson LA, Kelly CP, et al. The incidence and cost of unexpected hospital use after scheduled outpatient endoscopy. Archives of Internal Medicine 2010;170:1752‐7. [DOI] [PubMed] [Google Scholar]
Liangpunsakul 2003
- Liangpunsakul S, Ulmer BJ, Chalasani N. Predictors and implications of severe hypersplenism in patients with cirrhosis. American Journal of the Medical Sciences 2003;326(3):111‐6. [PUBMED: 14501224] [DOI] [PubMed] [Google Scholar]
McDiarmid 2002
- McDiarmid SV, Anand R, Lindblad AS. Development of a pediatric end‐stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation 2002;74(2):173‐81. [PUBMED: 12151728] [DOI] [PubMed] [Google Scholar]
Megremis 2004
- Megremis SD, Vlachonikolis IG, Tsilimigaki AM. Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology 2004;231:129‐34. [DOI] [PubMed] [Google Scholar]
Merli 2003
- Merli M, Nicolini G, Angeloni S, Rinaldi V, Santis A, Merkel C, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. Journal of Hepatology 2003;38(3):266‐72. [PUBMED: 12586291] [DOI] [PubMed] [Google Scholar]
NICE 2016
- National Institute for Health and Clinical Excellence (NICE). Cirrhosis in over 16s. Assessment and management. NICE guideline NG50. Methods, evidence and recommendations. National Institute for Health and Care Excellence, 2016. [Google Scholar]
NIEC 1988
- North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. The North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. New England Journal of Medicine 1988;319(15):983‐9. [PUBMED: 3262200] [DOI] [PubMed] [Google Scholar]
Pagliaro 1988
- Pagliaro L, Spina GP, D'Amico G, Brocchi E, Caletti G, Cosentino F, et al. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Journal of Hepatology 1987;4:93‐8. [Google Scholar]
Pagliaro 1992
- Pagliaro L, D’Amico G, Sorenson TIA, Lebrec D, Burroughs AK, Morabito A, et al. Prevention of first bleeding in cirrhosis. A meta‐analysis of randomized clinical trials of nonsurgical treatment. Annals of Internal Medicine 1992;117:59–70. [DOI] [PubMed] [Google Scholar]
Peck‐Radosavljevic 2000
- Peck‐Radosavljevic M. Thrombocytopenia in liver disease. Canadian Journal of Gastroenterology 2000;14(Suppl D):60D‐6D. [PUBMED: 11110614] [DOI] [PubMed] [Google Scholar]
Peck‐Radosavljevic 2007
- Peck‐Radosavljevic M. Review article: coagulation disorders in chronic liver disease. Alimentary Pharmacology & Therapeutics 2007;26(Suppl 1):21‐8. [PUBMED: 17958516] [DOI] [PubMed] [Google Scholar]
Pockros 2002
- Pockros PJ, Duchini A, McMillan R, Nyberg LM, McHutchison J, Viernes E. Immune thrombocytopenic purpura in patients with chronic hepatitis C virus infection. American Journal of Gastroenterology 2002;97(8):2040‐5. [PUBMED: 12190174] [DOI] [PubMed] [Google Scholar]
Pugh 1973
- Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. British Journal of Surgery 1973;60(8):646‐9. [PUBMED: 4541913] [DOI] [PubMed] [Google Scholar]
Quinn 1997
- Quinn PG, Johnston DE. Detection of chronic liver disease: costs and benefits. Gastroenterologist 1997;5(1):58‐77. [PUBMED: 9074920] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Rutter 2001
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [PUBMED: 11568945] [DOI] [PubMed] [Google Scholar]
Saab 2003
- Saab S, DeRosa V, Nieto J, Durazo F, Han S, Roth B. Costs and clinical outcomes of primary prophylaxis of variceal bleeding in patients with hepatic cirrhosis: a decision analytic model. American Journal of Gastroenterology 2003;98(4):763‐70. [PUBMED: 12738453] [DOI] [PubMed] [Google Scholar]
Sarin 2008
- Sarin SK, Kumar A, Angus PV, Baijal SS, Chawla YK, Dhiman RK, et al. Primary prophylaxis of gastroesophageal variceal bleeding: consensus recommendations of the Asian Pacific Association for the Study of the Liver. Hepatology International 2008;2:429‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharara 2001
- Sharara AI, Rockey DC. Gastroesophageal variceal hemorrhage. New England Journal of Medicine 2001;345(9):669‐81. [PUBMED: 11547722] [DOI] [PubMed] [Google Scholar]
Silvis 1976
- Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA 1976;235:928‐30. [DOI] [PubMed] [Google Scholar]
Spiegel 2003
- Spiegel BM, Targownik L, Dulai GS, Karsan HA, Gralnek IM. Endoscopic screening for esophageal varices in cirrhosis: is it ever cost effective?. Hepatology (Baltimore, Md) 2003;37(2):366‐77. [PUBMED: 12540787] [DOI] [PubMed] [Google Scholar]
Tripathi 2015
- Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64:1680‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Winkfield 2003
- Winkfield B, Aube C, Burtin P, Cales P. Inter‐observer and intra‐observer variability in hepatology. European Journal of Gastroenterology & Hepatology 2003;15(9):959‐66. [PUBMED: 12923367] [DOI] [PubMed] [Google Scholar]
Wolfsen 2004
- Wolfsen HC, Hemminger LL, Achem SR, Loeb DS, Stark ME, Bouras EP, et al. Complications of endoscopy of the upper gastrointestinal tract: a single‐center experience. Mayo Clinic Proceedings 2004;79:1264‐7. [DOI] [PubMed] [Google Scholar]
Ying 2012
- Ying L, Lin X, Xie ZL, Hu YP, Shi KQ. Performance of platelet count/spleen diameter ratio for diagnosis of esophageal varices in cirrhosis: a meta‐analysis. Digestive Diseases and Sciences 2012;57:1672‐81. [DOI] [PubMed] [Google Scholar]
Zoli 1996
- Zoli M, Merkel C, Magalotti D, Marchesini G, Gatta A, Pisi E. Evaluation of a new endoscopic index to predict first bleeding from the upper gastrointestinal tract in patients with cirrhosis. Hepatology (Baltimore, Md) 1996;24(5):1047‐52. [PUBMED: 8903373] [DOI] [PubMed] [Google Scholar]


