Abstract
Background
Bypass surgery is one of the mainstay treatments for patients with critical lower limb ischaemia (CLI). This is the second update of the review first published in 2000.
Objectives
To assess the effects of bypass surgery in patients with chronic lower limb ischaemia.
Search methods
For this update, the Cochrane Vascular Group searched its trials register (last searched October 2016) and the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (last searched Issue 9, 2016).
Selection criteria
We selected randomised controlled trials of bypass surgery versus control or any other treatment. The primary outcome parameters were defined as early postoperative non‐thrombotic complications, procedural mortality, clinical improvement, amputation, primary patency, and mortality within follow‐up.
Data collection and analysis
For the update, two review authors extracted data and assessed trial quality. We analysed data using odds ratio (OR) and 95% confidence intervals (CIs). We applied fixed‐effect or random‐effects models.
Main results
We selected 11 trials reporting a total of 1486 participants. Six trials compared bypass surgery with percutaneous transluminal angioplasty (PTA), and one each with remote endarterectomy, thromboendarterectomy, thrombolysis, exercise, and spinal cord stimulation. The quality of the evidence for the most important outcomes of bypass surgery versus PTA was high except for clinical improvement and primary patency. We judged the quality of evidence for clinical improvement to be low, due to heterogeneity between the studies and the fact that this was a subjective outcome assessment and, therefore, at risk of detection bias. We judged the quality of evidence for primary patency to be moderate due heterogeneity between the studies. For the remaining comparisons, the evidence was limited. For several outcomes, the CIs were wide.
Comparing bypass surgery with PTA revealed a possible increase in early postinterventional non‐thrombotic complications (OR 1.29, 95% CI 0.96 to 1.73; six studies; 1015 participants) with bypass surgery, but bypass surgery was associated with higher technical success rates (OR 2.26, 95% CI 1.49 to 3.44; five studies; 913 participants). Analyses by different clinical severity of disease (intermittent claudication (IC) or CLI) revealed that peri‐interventional complications occurred more frequently in participants with CLI undergoing bypass surgery than PTA (OR 1.57, 95% CI 1.09 to 2.24). No differences in periprocedural mortality were identified (OR 1.67, 95% CI 0.66 to 4.19; five studies; 913 participants). The primary patency rate at one year was higher after bypass surgery than after PTA (OR 1.94, 95% CI 1.20 to 3.14; four studies; 300 participants), but this difference was not shown at four years (OR 1.15, 95% CI 0.74 to 1.78; two studies; 363 participants). No differences in clinical improvement (OR 0.65, 95% CI 0.03 to 14.52; two studies; 154 participants), amputation rates (OR 1.24, 95% CI 0.82 to 1.87; five studies; 752 participants), reintervention rates (OR 0.76, 95% CI 0.42 to 1.37; three studies; 256 participants), or mortality within the follow‐up period (OR 0.94, 95% CI 0.71 to 1.25; five studies; 961 participants) between surgical and endovascular treatment were identified. No differences in subjective outcome parameters, indicated by quality of life and physical and psychosocial well‐being, were reported. The hospital stay for the index procedure was reported to be longer in participants undergoing bypass surgery than in those treated with PTA.
In the single study (116 participants) comparing bypass surgery with remote endarterectomy of the superficial femoral artery, the frequency of early postinterventional non‐thrombotic complications was similar in the treatment groups (OR 1.11, 95% CI 0.53 to 2.34). No mortality within 30 days of the index treatment or during stay in hospital in either group was recorded. No differences were identified in patency (OR 1.66, 95% CI 0.79 to 3.46), amputation (OR 1.70, 95% CI 0.27 to 10.58), and mortality rates within the follow‐up period (OR 1.66, 95% CI 0.61 to 4.48). Information regarding clinical improvement was unavailable.
No differences in major complications (OR 0.66, 95% CI 0.34 to 1.31) or mortality (OR 2.09, 95% CI 0.67 to 6.44) within 30 days of treatment between surgery and thrombolysis (one study, 237 participants) for chronic lower limb ischaemia were identified. The amputation rate was lower after bypass surgery (OR 0.10, 95% CI 0.01 to 0.80). No differences in late mortality were found (OR 1.56, 95% CI 0.71 to 3.44). No data regarding patency rates and clinical improvement were reported.
Technical success resulting in blood flow restoration was higher after bypass surgery than thromboendarterectomy for aorto‐iliac occlusive disease (one study, 43 participants) (OR 0.01, 95% CI 0 to 0.17). The periprocedural mortality (OR 0.33, 95% CI 0.01 to 8.65), follow‐up mortality (OR 3.29, 95% CI 0.13 to 85.44), and amputation rates (OR 0.47, 95% CI 0.08 to 2.91) did not differ between treatments. Clinical improvement and patency rates were not reported.
Comparing surgery and exercise (one study, 75 participants) did not identify differences in early postinterventional complications (OR 7.45, 95% CI 0.40 to 137.76) and mortality (OR 1.55, 95% CI 0.06 to 39.31). The remaining primary outcomes were not reported. There was no difference in maximal walking time between exercise and surgery (1.66 min, 95% CI ‐1.23 to 4.55).
Regarding comparisons of bypass surgery with spinal cord stimulation for CLI, there was no difference in amputation rates after 12 months of follow‐up (OR 4.00, 95% CI 0.25 to 63.95; one study, 12 participants). The remaining primary outcome parameters were not reported.
Authors' conclusions
There is limited high quality evidence for the effectiveness of bypass surgery compared with other treatments; no studies compared bypass to optimal medical treatment. Our analysis has shown that PTA is associated with decreased peri‐interventional complications in participants treated for CLI and shorter hospital stay compared with bypass surgery. Surgical treatment seems to confer improved patency rates up to one year. Endovascular treatment may be advisable in patients with significant comorbidity, rendering them high risk surgical candidates. No solid conclusions can be drawn regarding comparisons of bypass surgery with other treatments because of the paucity of available evidence. Further large trials evaluating the impact of anatomical location and extent of disease and clinical severity are required.
Plain language summary
Bypass surgery for chronic lower limb ischaemia
Background
The most common symptom of chronic lower limb ischaemia (inadequate blood flow to the legs) is claudication, a cramping pain caused by a poor supply of blood to the affected muscle. It often affects the calf muscle, and is typically triggered by exercise and relieved by rest. More severe restriction of the blood supply may produce pain at rest, leg ulcers, or gangrene. These conditions, and severe claudication, may require bypass surgery or other treatments to improve blood flow to the leg.
Key results
This review of eleven trials with a total of 1486 participants (current until October 2016) identified six trials comparing bypass surgery with angioplasty (balloon stretching and/or stent of the narrowed or occluded artery), and one each with remote endarterectomy (a combination of plaque removal and stent), thromboendarterectomy (removal of the plaque and clot), thrombolysis (clot dissolving), exercise, and spinal cord stimulation. In this review, no evidence was found to favour bypass surgery over angioplasty in terms of the effect on death, improvement of symptoms, amputation rate, need for further procedure, or long‐term mortality. Procedural complications occurred more frequently in patients with severe leg ischaemia (rest pain, ulcers, or gangrene) undergoing bypass surgery than those undergoing angioplasty. There was evidence that bypass surgery was more often technically successful, was associated with longer hospital stay, and that the bypass graft remained open (patent) at a higher rate one year after the procedure compared with angioplasty; this difference in patency in favour of surgery disappeared after four years. There was also no clear evidence to favour bypass surgery compared with other treatments, as indicated by procedural complications and deaths, clinical improvement, vessel patency, and long‐term mortality. Comparisons of bypass surgery with thrombolysis showed fewer amputations in patients subjected to bypass surgery, whereas for the rest of the comparisons the amputation rate was similar.
Quality of the evidence
In general, the quality of the evidence was high for all but two of the clinically most important outcomes. Quality of the evidence for clinical improvement was judged to be low as this was a subjective outcome at risk of bias since the outcome assessors were not blinded to the study treatments and because there were differences in results between the studies. Quality of the evidence for patency of the bypass graft was moderate because of differences in results between the studies. Further research including large numbers of participants is needed to investigate the effectiveness of bypass surgery for chronic lower limb ischaemia.
Summary of findings
for the main comparison.
| Bypass surgery compared with angioplasty for chronic lower limb ischaemia | ||||||
|
Patient or population: Individuals with peripheral arterial disease Settings: Hospital Intervention: Bypass surgery Comparison: Percutaneous transluminal angioplasty | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Angioplasty | Bypass surgery | |||||
|
Early postoperative non‐thrombotic complications Follow up: 30 days |
Medium risk population1 | OR 1.29 (0.96 to 1.73) | 1015 (6 studies) | ⊕⊕⊕⊕ high | ||
| 245 per 1000 | 295 per 1000 (238 to 360) | |||||
|
Procedural mortality Follow up: 30 days |
Medium risk population1 | OR 1.67 (0.66 to 4.19) | 913 (5 studies) | ⊕⊕⊕⊕ high | Three studies reported no cases of procedural mortality | |
| 15 per 1000 | 25 per 1000 (10 to 60) | |||||
|
Clinical improvement Follow up: 23‐48 months |
Medium risk population1 | OR 0.65 (0.03 to 14.52) | 154 (2 studies) | ⊕⊕⊝⊝ low2, 3 | Estimate effect based on two studies | |
| 800 per 1000 | 722 per 1000 (107 to 983) | |||||
|
Amputation Follow up: 12‐48 months |
Medium risk population1 | OR 1.24 (0.82 to 1.87) | 752 (5 studies) | ⊕⊕⊕⊕ high | ||
| 126 per 1000 | 152 per 1000 (106 to 213) | |||||
|
Primary patency Follow up: 12 months |
Medium risk population1 | OR 1.94 (1.20 to 3.14) | 300 (4 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 583 per 1000 | 731 per 1000 (627 to 814) | |||||
|
Primary patency Follow up: 4 years |
Medium risk population1 | OR 1.15 (0.74 to 1.78) | 363 (2 studies) | ⊕⊕⊕⊕ high | Estimate effect based on two studies | |
| 633 per 1000 | 665 per 1000 (561 to 755) | |||||
|
Mortality within follow‐up Follow up: 12‐48 months |
Medium risk population1 | OR 0.94 (0.71 to 1.25) | 961 (5 studies) | ⊕⊕⊕⊕ high | ||
| 371 per 1000 | 357 per 1000 (295 to 424) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Based on studies including both claudication and critical ischaemia participants; the assumed risk was calculated by the mean number of events in the control groups of the selected studies for each outcome. 2 Subjective outcome assessment and no blinding. 3 Heterogeneity in treatment effect among studies.
Background
Description of the condition
Even though peripheral arterial disease (PAD) may present with acute limb ischaemia, chronic lower limb ischaemia is the most common presentation of PAD and results from an atherosclerotic process affecting the lower extremity arteries causing a reduction in the blood supply to the leg. Intermittent claudication (IC) is the most common presenting symptom for patients with PAD. It is thought to be produced by an inadequate supply of oxygen to the calf, thigh, or buttock muscles during exercise, resulting in anaerobic metabolism and pain. In its more severe manifestations, PAD may lead to critical limb ischaemia (CLI), which is characterised by intractable rest pain, ischaemic ulceration, or gangrene. Patients with CLI are at significant risk of developing irreversible ischaemic damage to the leg or foot if no appropriate treatment is undertaken, and this may result in amputation of the limb (Norgren 2007). In the National Health and Nutrition Examination Survey (NHANES), the overall prevalence of symptomatic or asymptomatic PAD in individuals aged 40 years or older was 4.3%, with a dramatic increase with age, rising from 0.9% in those younger than 50 years to 14.5% in those 70 years or older (Selvin 2004). As well as having a detrimental impact on functional capacity and quality of life, PAD indicates a more widespread systemic atherosclerotic disease affecting arterial trees in different organ systems, such as the coronary and cerebral circulation.
Description of the intervention
The importance of identifying and appropriately treating patients with PAD lies in both the management of ischaemia, to relieve symptoms or prevent amputation, or both, and the control of atherosclerotic risk factors, life style modifications and optimal medical treatment to mitigate the cardiovascular and cerebrovascular risk. Treatments for PAD range from conservative measures, such as management of cardiovascular risk factors with antiplatelets, statins, and exercise regimens, to interventional therapies, including surgical and endovascular arterial reconstruction.
Surgical bypass of the diseased arterial segment is one of the main treatments for the patient with life‐limiting claudication or CLI. The type of bypass procedure in the lower limbs depends on the extent of disease and involves reconstructions of the aorto‐iliac segment or infra‐inguinal arterial segment or both. The first successful femoro‐popliteal bypass operation was performed in 1950 by William Holden using a section of the patient's own vein; this is called autogenous vein graft (Holden 1950). Since then, a number of synthetic materials have been developed, including Dacron and expanded polytetrafluoroethylene (PTFE), a whole range of collagen tubes derived from human umbilical vein, and bovine carotid artery. Cadaveric homografts have also been used. Autogenous vein is considered the preferred conduit for infra‐inguinal bypass.
Apart from a surgical bypass, endoluminal procedures have been developed for the treatment of PAD. Endovascular techniques for the treatment of patients with lower extremity ischaemia include balloon angioplasty, insertion of stents and stent‐grafts, plaque debulking procedures, thrombolysis, and percutaneous thrombectomy (Tepe 2006). The range of new adjunct or alternative endovascular treatments and techniques is consistent with the constant advent of technological developments and bioengineering.
How the intervention might work
The primary goals of interventional treatment of chronic lower limb ischaemia are to relieve ischaemic pain, heal ischaemic ulcers, prevent limb loss, and improve patient's functional capacity and quality of life. In order to achieve these outcomes, some patients will ultimately require a surgical or endovascular revascularization procedure. Bypass procedures have the advantages of technical success, satisfactory anatomical patency and clinical durability, whereas proponents of endovascular therapies emphasise the minimally invasive nature of the procedures with subsequent reduced morbidity and mortality, enhanced recovery, and improved resource utilisation. A plethora of clinical research provides supporting evidence for the relative merits of approaches and techniques of lower limb revascularization.
Why it is important to do this review
The aging population, the rising prevalence of diabetes in western societies, and continued tobacco abuse worldwide is likely to result in a wide spread increase of PAD and increase in the number of revascularization procedures in the foreseeable future, with resultant socioeconomic implications and consumption of health care resources. As with many surgical interventions, bypass surgery was introduced without formal evaluation. Nowadays, however, patients and doctors are expected to make informed decisions based on evidence from randomised controlled trials, and it is important that the evidence comparing surgery with other treatment modalities is readily available (Antoniou 2013a). This review summarizes all previous trials of bypass surgery and highlights the advantages and disadvantages of surgery compared with other treatments. Furthermore, it identifies areas for future research. The review provides comparisons of bypass surgery to other treatments for symptomatic PAD, but does not assess the effect of any treatment on the natural history of PAD, either claudication or CLI.
Objectives
To assess the effects of bypass surgery in patients with chronic lower limb ischaemia.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials of bypass surgery versus control (no treatment) or any other regimen were eligible for the review. Possible comparisons included endovascular intervention, exercise therapy, and medical treatment. Any method of randomisation was eligible and differences in quality were taken into account in the analysis. Trials that were not analysed on an intention‐to‐treat basis were included provided all randomised participant were accounted for.
Types of participants
Trials in individuals with lower limb ischaemia due to atherosclerotic disease, in which disease was confirmed by objective testing, were eligible for the review (Fontaine stages II, III, and IV) (Fontaine 1954). Trials of individuals with chronic ischaemia were included, defined as the presence of symptoms for more than 14 days. The severity of symptoms did not affect inclusion in the review, but this factor was taken into account in the analysis.
Types of interventions
Any surgical bypass procedure for the treatment of chronic lower limb ischaemia was included, irrespective of the approach, route, or type of graft employed. This was, therefore, likely to focus on individuals undergoing femoro‐popliteal bypass surgery, but other routes such as aorto‐iliac segment surgery were also included, if performed to treat lower limb ischaemia.
Types of outcome measures
Primary outcomes
The primary outcome measures were divided into early perioperative or peri‐interventional outcomes and follow‐up outcomes, as follows:
Early perioperative or peri‐interventional outcomes
early postoperative non‐thrombotic complications
procedural mortality
Follow‐up outcomes
clinical improvement (defined as improvement in Rutherford category) (Rutherford 1997)
amputation
primary patency (vessel or graft patency following initial procedure with no further intervention)
mortality
Secondary outcomes
Similarly, the secondary outcome measures were divided into early perioperative or peri‐interventional outcomes and follow‐up outcomes, as follows:
Early peri‐operative or peri‐interventional outcomes
technical success (defined as technical accomplishment of the intended intervention)
Follow‐up outcomes
assisted primary patency (patency not lost but maintained with prophylactic intervention)
secondary patency (restored patency after occlusion)
vessel or graft occlusion
reinterventions
walking distance (time to onset of pain and maximal walking distance)
success in ulcer healing assessed by complete healing
Subjective measures included:
quality of life scores as reported in the included studies
use of resources (such as length of hospital stay)
Technical success, clinical improvement, vessel or graft patency, and reinterventions are additional outcomes to those included in the initial review. The selected outcome parameters were thought to provide valuable additional information related to the comparative effectiveness of bypass surgery for the treatment of PAD.
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Vascular Clinical Information Specialist (CIS) searched the following databases for relevant trials:
The Cochrane Vascular Specialised Register (October 2016);
The Cochrane Central Register of Controlled Trials (CENTRAL (2016, Issue 9)) via The Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals, and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
In addition, the CIS searched the following trial registries (October 2016) for details of ongoing and unpublished studies;
ClinicalTrials.gov (www.clinicaltrials.gov)
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch)
ISRCTN Register (www.isrctn.com/).
Searching other resources
The reference lists of relevant articles retrieved by electronic searches were searched for additional citations.
Data collection and analysis
Selection of studies
For the present update, eligibility assessment of the reports provided by the Cochrane Vascular CIS was performed independently by two review authors (GAA and GSG). Disagreements were discussed with a third review author (FT), who acted as an adjudicator in the event of disagreement. We contacted the principal investigators of trials that were potentially included but terminated early and no published results could be found, to check availability of additional information.
Data extraction and management
For this update, two review authors (GSG and SAA) independently extracted data using a prespecified data collection form based on the Cochrane Vascular data extraction template. Disagreements were resolved by discussion with the contact author (GAA).
Assessment of risk of bias in included studies
The Cochrane Collaboration's risk of bias tool was applied to assess the risk of bias of the selected trials according to Higgins 2011. This tool evaluates six main domains: random sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias. We completed a risk of bias table for each eligible study. For each individual domain, we classified studies into low, unclear, or high risk of bias. We considered blinding and incomplete outcome data separately for each outcome.Two review authors (RRM, JDS) independently assessed the methodological quality of the trials. The contact author (GAA) acted as an adjudicator in the event of disagreement. FT performed the risk of bias assessment of an article written in the Italian language and discussed the results with the contact author (GAA).
Measures of treatment effect
Analysis of dichotomous variables, such as mortality and the occurrence of postoperative complications, was carried out using the odds ratio (OR) with 95% confidence interval (CI) as the summary statistic. The total numbers of participants and numbers of events for each outcome parameter were entered into RevMan 5.3 to calculate the effect measure. Patency rates were transformed into a dichotomous outcome for specific time periods. For continuous variables, such as length of hospital stay, we aimed to calculate mean differences (MDs) using means and standard deviations (SD). If different scales were used in the different studies, the results were standardised, where possible, and then combined to form a standardised mean difference (SMD). Where these data were unavailable, we reported medians and interquartile range, but did not include these results in a meta‐analysis.
Unit of analysis issues
We did not identify any cluster‐randomised or cross‐over trials; therefore, no special issues with regard to analyses of studies with non‐standard designs existed. Each participant was counted as the unit of analysis for the defined outcome measures (e.g. primary patency).
Dealing with missing data
We planned to contact authors of selected studies to clarify any missing or unclear outcome data. Quantitative analyses were performed on an intention‐to‐treat basis where possible.
Assessment of heterogeneity
We anticipated that there might be considerable heterogeneity among the studies because of differences in severity of chronic lower limb ischaemia, anatomical level of disease, and methods of surgical or other treatments applied. In‐between study heterogeneity was examined with the combination of the Cochrane Q (Chi2) test and the I2 statistic. Important heterogeneity (Chi2 P < 0.05 and I2 ≥ 75%) was investigated, where possible, by subgroup analyses.
Assessment of reporting biases
For each study, the effect by the inverse of its standard error was plotted. If 10 or more studies were included in any single meta‐analysis, we planned to assess publication bias both visually evaluating the symmetry of such funnel plots and using the Egger’s regression intercept.
Data synthesis
Pooled ORs with 95% CIs were calculated using the Mantel–Haenszel fixed‐effect model, unless evidence of between study heterogeneity (Chi2 P < 0.05 and I2 ≥ 75%) existed, in which case random‐effects models of DerSimonian and Laird were applied.
Subgroup analysis and investigation of heterogeneity
Where sufficient information was available, we planned to investigate the following subgroups, which could account for heterogeneity among studies: individuals undergoing arterial reconstruction at different anatomical levels (e.g. aorto‐iliac or infra‐inguinal reconstruction) and individuals with different disease severity (e.g. IC or CLI).
Sensitivity analysis
We prespecified several additional analyses to assess the robustness of our results; we tested the effect of removing one study at a time on the pooled effect measure. We also undertook analyses to explore the contribution of risk of bias by excluding the trials that were found to be at high risk of bias in one or more domains.
Summary of findings table
We constructed a table compiling and summarizing the best evidence of relevant outcomes for the comparison of bypass surgery with PTA. We considered study populations consisting of individuals with disease severity ranging form claudication to severe limb ischaemia. We selected the most important and clinically relevant outcomes (both desirable and undesirable) that were thought to be essential for decision‐making for the Table 1. We calculated assumed control intervention risks by the mean number of events in the control groups of the selected studies for each outcome. We used the system developed by the Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE working group) for grading the quality of evidence as high, moderate, low and very low, based on within‐study risk of bias, directness of evidence, heterogeneity, precision of effects estimates, and risk of population bias (GRADE 2004).
Results
Description of studies
See Figure 1.
1.
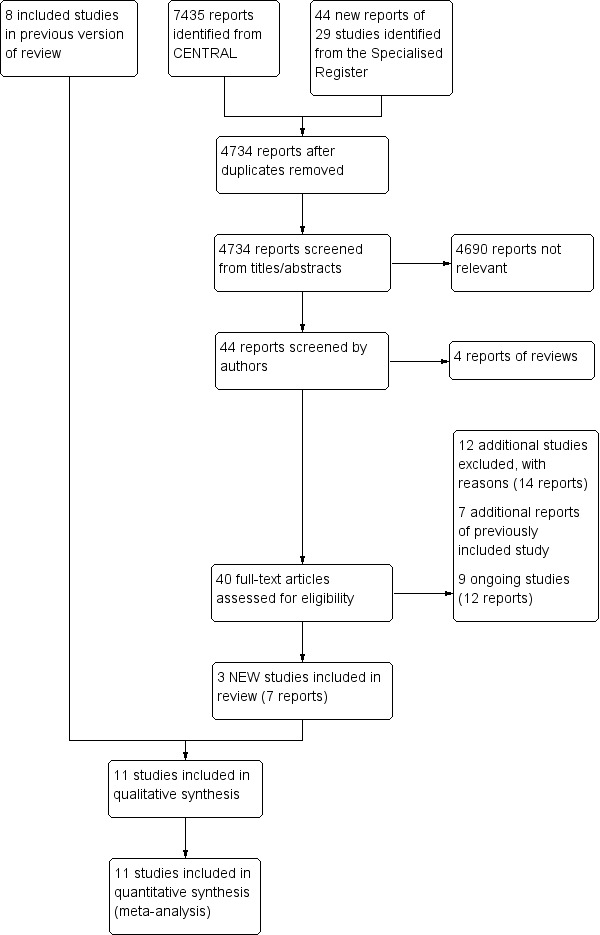
Study flow diagram.
Results of the search
The search of CENTRAL and the Specialist Register identified 4734 reports, after duplicates were removed. Irrelevant reports were discarded and we assessed the full text of 40 articles for eligibility. Of these, 12 additional studies (14 reports) were excluded and the reasons for exclusion are provided in the Characteristics of excluded studies table, seven reports were additional publications of a previously included study (BASIL study), and nine (12 reports) were ongoing trials. Three new studies (seven reports) were identified, which along with the eight studies included in the previous version of this review made a total of 11 studies included in the qualitative synthesis and meta‐analysis.
Included studies
Three additional studies were included in this update (Lepantalo 2009; McQuade 2010; REVAS Trial). There were also seven additional publications added for one study (BASIL study). Eleven studies reporting a total of 1486 participants fulfilled our inclusion criteria and were selected for analysis (BAESIC study; BASIL study; Gaspard 1972; Guarnera 1994; Holm 1991; Lepantalo 2009; Lundgren 1989; McQuade 2010; REVAS Trial; STILE Trial; Veterans Study). They are summarized in the Characteristics of included studies table. Approximately half of these studies (five trials) were published in 2000s; the first trial investigating the effects of bypass surgery in individuals with chronic lower limb ischaemia was published in the early 1970s (Gaspard 1972). The number of participants in the included trials ranged from 12 to 452. The largest trial is the BASIL study, which assigned individuals with severe lower limb ischaemia secondary to infra‐inguinal arterial disease to receive bypass surgery or balloon angioplasty. The eleven included trials were conducted in six different countries (seven trials in Europe and four in North America).
There was also some variation in the types of participant included in the eleven trials. Most trials included both men and women, except the Veterans Study which involved men only. Seven trials included individuals with a range of disease severity (both IC and CLI), but two were restricted to individuals with claudication only (BAESIC study; Lundgren 1989) and two included only those with CLI (BASIL study; Guarnera 1994). The proportion of claudicants in the trials with mixed groups ranged from 34% in the STILE Trial to 89% in the Lepantalo 2009 trial. In addition, the STILE Trial included a mixture of individuals with native artery disease and individuals with existing grafts, but only the subset with native artery disease has been included in this review.
There were no trials that compared bypass surgery with a placebo, no intervention, or medical management. Six trials compared bypass surgery with PTA (BAESIC study; BASIL study; Holm 1991; Lepantalo 2009; McQuade 2010; Veterans Study). In the remaining trials, bypass surgery was compared with: remote endarterectomy of the superficial femoral artery (REVAS Trial); thromboendarterectomy (Gaspard 1972); thrombolysis (STILE Trial); spinal cord stimulation (Guarnera 1994); and exercise, in which the control group performed dynamic leg exercises that were supervised by a physiotherapist (Lundgren 1989).
The type of bypass procedure performed in each trial was similar in most studies. Vein grafts were generally used for distal reconstructions, and synthetic prostheses for aorto‐iliac or ilio‐femoral bypasses and some femoro‐popliteal bypasses above the knee. In the REVAS Trial, the type of graft for the femoro‐popliteal bypass above the knee was either prosthetic (PTFE) or vein (great saphenous vein), and separate analyses were performed for the two types of graft. There was also some variation in the types of surgery performed. In four trials, unfortunately, a number of participant underwent endarterectomy rather than bypass surgery (BASIL study; Holm 1991; Lundgren 1989; STILE Trial) and these different groups were not separated in the analysis. Furthermore, the type of endovascular procedure varied among trials comparing effects of bypass surgery with those of endovascular treatment for chronic lower limb ischaemia. Four trials (BAESIC study; BASIL study, Holm 1991; Veterans Study) used PTA without stenting or with stenting at the discretion of the treating physician, whereas two trials (Lepantalo 2009; McQuade 2010) used an endograft (covered stent) in all their endovascular procedures.
Nine of the eleven trials included mortality and procedural (or technical) success as outcome measures (BAESIC study; BASIL study; Gaspard 1972; Holm 1991;Lepantalo 2009; McQuade 2010; REVAS Trial; STILE Trial; Veterans Study), and most of these also reported complications, patency rates, and need for amputation. Three trials included subjective measures (BASIL study; Guarnera 1994; Veterans Study); one included only treadmill testing and measures of lower limb blood flow (Lundgren 1989).
Excluded studies
For this update, an additional 12 studies were excluded (ABC 2010; CLEVER study; Djoric 2011; Gavrilenko 2008; IRONIC Trial; Matyas 2008; Nordanstig 2011; PROOF 2007; Stanisic 2009; TECCO Trial; Tiek 2009; Tiek 2012). This made a total of 23 excluded studies (ABC 2010; CLEVER study; de Donato 2002; Devine 2004; Djoric 2011; Gavrilenko 2008; Gelin 2001; Hamsho 1999; IRONIC Trial; Jensen 2007; Linhart 1991; Matyas 2008; McCollum 2003; Mohammadi 2007; Nordanstig 2011; Panneton 2004; PROOF 2007; Stanisic 2009; Taft 2004; TECCO Trial; Tiek 2009; Tiek 2012; Vukobratov 2006) These studies are summarised in the Characteristics of excluded studies table. Most of the excluded studies compared different techniques of bypass surgery or different types of bypass grafts (de Donato 2002; Devine 2004; Hamsho 1999; Gavrilenko 2008; Jensen 2007; Matyas 2008; McCollum 2003; Mohammadi 2007; Panneton 2004; Stanisic 2009; Tiek 2012; Vukobratov 2006). Three studies did not have a bypass group (CLEVER study; Djoric 2011; Tiek 2009), and in another study there is no mention of randomisation and the two different treatment options (surgery and medical therapy) were not compared in the analysis (Linhart 1991). Two of the excluded trials (PROOF 2007; ABC 2010) potentially fulfilled the inclusion criteria, as they were randomised controlled trials comparing bypass surgery with plaque excision (Silverhawk Plaque Excision) or angioplasty for the treatment of participants with CLI and IC, respectively. Unfortunately, both trials were terminated and no published or presented results could be found. The principal investigators either confirmed the absence of published results (ABC 2010) or did not respond to our request (PROOF 2007). Another four trials (Gelin 2001; IRONIC Trial; Nordanstig 2011; Taft 2004) comparing invasive with non‐invasive treatment for lower limb ischaemia were excluded because the enrolled participants were randomised to any invasive treatment (including surgical or endovascular) rather than bypass surgery. The CLEVER study is a randomised controlled trial comparing optimal medical management, stent placement, supervised exercise rehabilitation, and combined stenting with supervised exercise rehabilitation for aorto‐iliac occlusive disease in individuals suffering from IC. It was excluded from our review and analysis because bypass surgery was not included in the treatment arms. Furthermore, even though the TECCO Trial compared surgery with endovascular treatment for common femoral artery disease, a minority of participants underwent bypass surgery in the surgical treatment arm and therefore, this study was excluded.
Ongoing studies
Nine ongoing trials were identified through searches of clinical trials databases. BASIL 2 is a multi‐centre randomised controlled trial conducted in the UK comparing the clinical and cost effectiveness of a "vein bypass first" with an "endovascular first" revascularization strategy for severe limb ischaemia due to infra‐geniculate arterial disease. Best endovascular treatment involves balloon angioplasty and possibly the use of stents. Participant recruitment started in May 2014 and the anticipated end date is October 2019. The BEST‐CLI trial is a pragmatic, multicentre, open label, randomised trial that compares best endovascular therapy with best open surgical treatment in individuals with CLI eligible for both treatments. This trial is funded by the National Lung Heart and Blood Institute of the National Institutes of Health and aims to enrol 2100 participants with CLI at 120 sites in North America . Participant recruitment started in August 2014 and the anticipated end date is December 2018. FINNPTX is a Finnish multicentre randomised clinical trial comparing paclitaxel‐eluting stent with femoro‐popliteal bypass using PTFE graft for the treatment of long superficial femoral artery occlusion in individuals with life‐limiting IC or CLI. The trial commenced in October 2011 and is estimated to be completed in 2017 with an enrolment of 400 participants. ROBUST is a single‐centre randomised clinical trial comparing bypass surgery with angioplasty and stenting for TASC II B and C lesions of the superficial femoral artery. It was launched in 2009 and aims to enrol 320 individuals with IC that does not respond to medical management or with CLI. SUPERB is a randomised controlled trial comparing heparin‐bonded endoluminal with surgical femoro‐popliteal bypass in individuals with symptomatic PAD. This trial, which is currently recruiting participants in the Netherlands, commenced in October 2010 and the estimated date of completion is December 2019. The ZILVERPASS trial is another randomised controlled trial comparing the Cook Zilver PTX drug‐eluting stent with bypass surgery for the treatment of femoro‐popliteal TASC C and D lesions in individuals with symptomatic PAD. This study is being conducted in Belgium, commenced in August 2014, and is anticipated to enrol 220 participants by November 2017. The Optimized Strategy for Diabetic Patients with Critical Limb Ischaemia study (NCT01171703) randomises diabetic individuals with chronic long occlusion of the superficial femoral artery to receive a femoro‐popliteal PTFE bypass above the knee or stenting. Another ongoing randomised controlled trial (ISRCTN18315574) compares bypass surgery with ipsilateral great saphenous vein with percutaneous transluminal angioplasty with stent placement in individuals with IC or CLI and TASC C/D femoropopliteal disease. The NCT02580084 trial is the only trial comparing aorto‐femoral bypass with hybrid intervention consisting of common femoral endarterectomy and iliac balloon angioplasty and stenting. The study is being conducted in Russia and the estimated completion date is August 2020.
Risk of bias in included studies
Our risk of bias assessments for each included study are summarized in Figure 2 and as percentages across all studies in Figure 3. Details and reasons for each assessment are listed in the Characteristics of included studies table.
2.
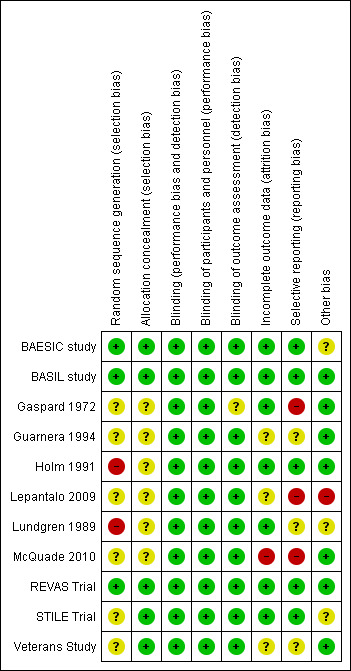
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
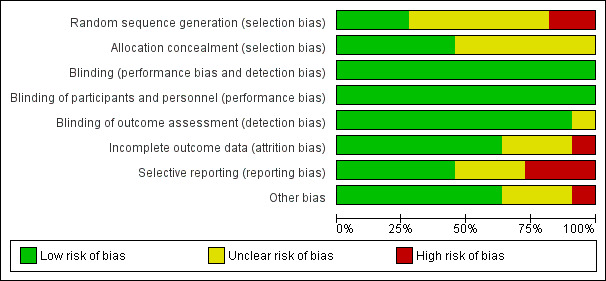
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In three trials, the allocation sequence was adequately generated (BAESIC study; BASIL study; REVAS Trial). A computerised random‐number generator or a central telephone number in a permuted‐block sequence was applied to generate the allocation sequence. A sequential treatment assignment, with balancing for prognostic factors, which was applied in two trials (Holm 1991; Lundgren 1989), was not considered an appropriate method of sequence generation. Unfortunately, the rest of the selected trials provided insufficient information about the sequence generation process to permit judgement (Gaspard 1972; Guarnera 1994; Lepantalo 2009; McQuade 2010; STILE Trial; Veterans Study).
Appropriate methods of allocation concealment were used in five trials (BAESIC study; BASIL study; REVAS Trial; STILE Trial; Veterans Study). Either a central computerised allocation or sealed envelopes were used to conceal allocation.
Blinding
Inevitably, in trials of a surgical intervention, blinding was not possible, but as there were no comparison groups that received no treatment this may be less significant. None of the reports stated that those taking measurements were blinded to the treatment group and, therefore, it must be assumed that they were not. However, in several trials there was a set protocol for follow‐up assessment, and objective measures for assessment of main outcomes, such as Duplex ultrasound or angiographic imaging for the assessment of patency, were used. Therefore, we judged that the outcome and the outcome measurement were not likely to be influenced by lack of blinding.
Incomplete outcome data
There were either no or minimal losses to follow up in most trials. No issues with incompleteness of data were identified for six of the trials (BAESIC study; BASIL study; Gaspard 1972; Holm 1991; Lundgren 1989; REVAS Trial). Missing outcome data were balanced in numbers across intervention groups (BAESIC study; Holm 1991; Lundgren 1989) or the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate (BASIL study; REVAS Trial). The STILE Trial reported a transparent process of dealing with missing or incomplete data and was therefore considered to be of low risk of bias. In the McQuade 2010 trial, six (12%) and 15 participants (30%) were lost to follow‐up in the stent and bypass group, respectively, and this trial was therefore considered to be high risk of attrition bias because of the imbalance in numbers across intervention groups. The remaining studies provided insufficient information to permit judgment.
Selective reporting
Reporting bias was judged to be present in three trials (Gaspard 1972; Lepantalo 2009; McQuade 2010). In the Gaspard 1972 trial, the outcome measures were not clearly defined and the study failed to include key outcomes. In the Lepantalo 2009 trial, even though costs were prespecified as a secondary outcome parameter, they were not reported. Insufficient information was available concerning whether amputation and mortality were prespecified outcomes in the McQuade 2010 trial. For the remaining trials, either no issues with regard to reporting bias existed or insufficient information to permit judgment was provided.
Other potential sources of bias
Two trials, the BAESIC study and the Lepantalo 2009 trial, were terminated prematurely because of recruitment issues and lack of benefit of endoluminal stent‐graft placement in the superficial femoral artery over bypass surgery in the Lepantalo 2009 trial. Furthermore, there might be a risk of bias in relation to participant compliance with exercise treatment in the Lundgren 1989 trial, but insufficient evidence that this problem would introduce bias was available. The 237 participants with native artery disease in the STILE Trial were a subset of a larger trial of 393 participants, which included both native artery and graft disease, and this may have biased the results. Reinterventions affecting assisted primary patency are potentially subject to intervention use bias, unless the criteria for reintervention are prespecified and applied equally to both interventions. Of the trials providing data for assisted primary patency (Lepantalo 2009; REVAS Trial), the REVAS Trial only defined criteria for reintervention in both groups, whereas the Lepantalo 2009 trial did not provide specific reintervention criteria to maintain primary patency and is, therefore, subject to reintervention bias.
Effects of interventions
See: Table 1
Bypass surgery compared with angioplasty
Six trials compared bypass surgery with PTA (BAESIC study; BASIL study; Holm 1991; Lepantalo 2009; McQuade 2010; Veterans Study). Participant groups in these trials included participants with IC, CLI, or both. The follow‐up period for each trial varied from 12 months (Holm 1991; Lepantalo 2009), 23 months (BAESIC study), 36 months (BASIL study), 48 months (McQuade 2010) to 49 months (Veterans Study).
Early postoperative non‐thrombotic complications
Early postoperative non‐thrombotic complications were reported in all trials comparing bypass surgery with angioplasty for chronic lower limb ischaemia (BAESIC study; BASIL study; Holm 1991; Lepantalo 2009; McQuade 2010; Veterans Study). Early complications occurred either within 30 days of the index treatment or during the initial hospital stay, and were local or systemic. Most trials reported major complications significantly affecting the participant's postoperative course or requiring intervention (e.g. surgical treatment of groin infection). Even though early non‐thrombotic complications occurred more frequently in participants undergoing bypass surgery, the difference did not reach statistical significance (OR 1.29, 95% CI 0.96 to 1.73; six studies; 1015 participants; Analysis 1.1). See Figure 4. Heterogeneity among the trials was I2 = 55%.
1.1. Analysis.
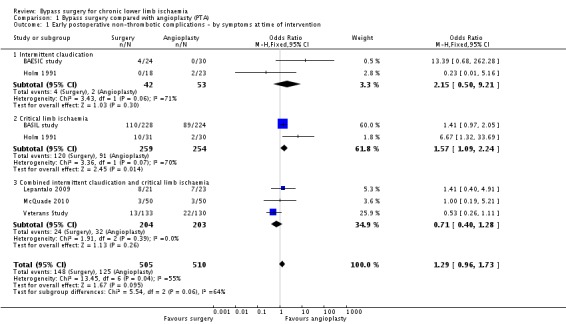
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 1 Early postoperative non‐thrombotic complications ‐ by symptoms at time of intervention.
4.
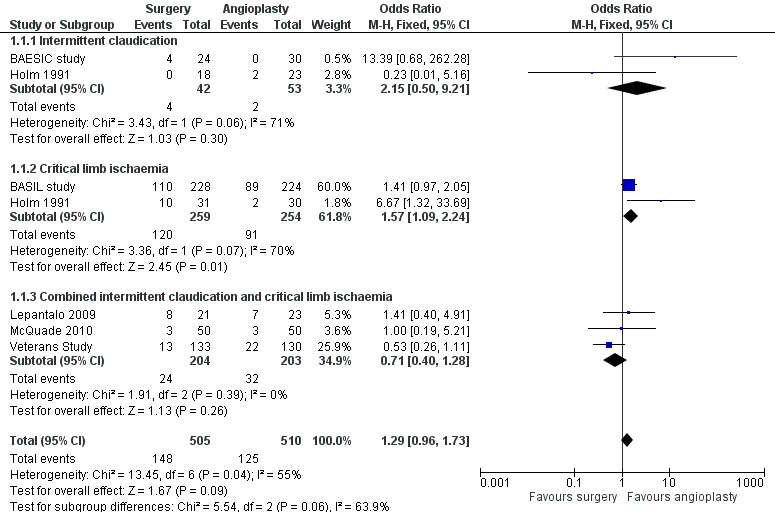
Forest plot of comparison: 1 Bypass surgery compared with angioplasty (PTA), outcome: 1.1 Early postoperative non‐thrombotic complications ‐ by symptoms at time of intervention.
We performed separate meta‐analyses for participants treated for CLI and those treated for claudication. No significant difference in the frequency of early postoperative non‐thrombotic complications between surgery and angioplasty in participants with claudication was found (OR 2.15, 95% CI 0.50 to 9.21), whereas in those with CLI, complications occurred more frequently in the bypass group (OR 1.57, 95% CI 1.09 to 2.24; test for subgroup differences P = 0.06; Analysis 1.1). Furthermore, the risk of complications in the surgery and angioplasty group was similar when separate meta‐analyses were performed for participants with iliac disease (OR 0.62, 95% CI 0.24 to 1.58) and those treated for femoro‐popliteal disease (OR 1.34, 95% CI 0.97 to 1.86; test for subgroup differences P = 0.13; Analysis 1.2).
1.2. Analysis.
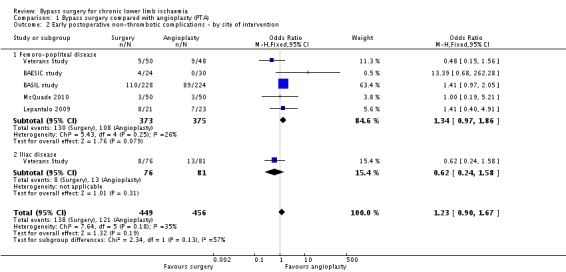
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 2 Early postoperative non‐thrombotic complications ‐ by site of intervention.
Sensitivity analysis removing one study at a time showed an effect in favour of angioplasty when the Veterans Study was removed (OR 1.55, 95% CI 1.12 to 2.15; I2 = 30%). Sensitivity analysis excluding the trials that were found to be at high risk of bias in one or more domains (Holm 1991; Lepantalo 2009; McQuade 2010) found no difference between the groups (OR 1.17, 95% CI 0.43 to 3.19; I2 = 75%).
Procedural mortality
Mortality occurring within 30 days of treatment or during the hospital stay for the index procedure was reported in five trials (BAESIC study; BASIL study; Lepantalo 2009; McQuade 2010; Veterans Study). No significant difference in procedural mortality between surgical and endovascular treatment for chronic lower limb ischaemia was identified (OR 1.67, 95% CI 0.66 to 4.19; 913 participants; Analysis 1.3). The heterogeneity among the studies was I2 = 0%. Three studies reported no cases of procedural mortality (BAESIC study; Lepantalo 2009; McQuade 2010).
1.3. Analysis.
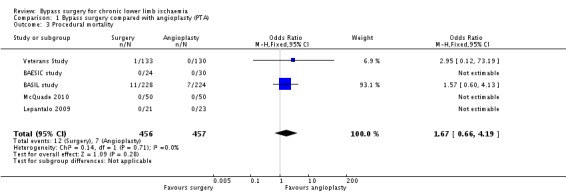
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 3 Procedural mortality.
Repeating the analysis after removing one study at a time and after excluding the high risk of bias trials showed no difference between treatments.
Clinical improvement
Improvement in the clinical grade of the Rutherford classification was reported in two trials (BAESIC study; McQuade 2010). Our analyses revealed similar clinical improvement after bypass surgery and PTA (OR 0.65, 95% CI 0.03 to 14.52; 154 participants; Analysis 1.4). Heterogeneity was I2 = 75%.
1.4. Analysis.
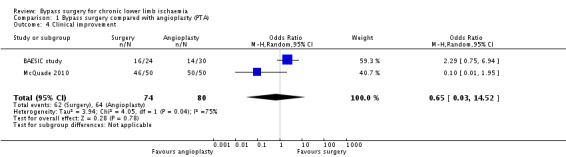
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 4 Clinical improvement.
Sensitivity analysis removing one study at a time showed no difference between the treatment groups.
Amputation
Five trials reported the numbers of participants who had an amputation of the treated limb during the follow‐up period (BAESIC study; BASIL study; Holm 1991; Lepantalo 2009; McQuade 2010). Participants treated with bypass surgery had a similar rate of progression to amputation of the treated limb to participants treated with PTA (OR 1.24, 95% CI 0.82 to 1.87; 752 participants; Analysis 1.5). Heterogeneity among the included studies was I2 = 31%.
1.5. Analysis.
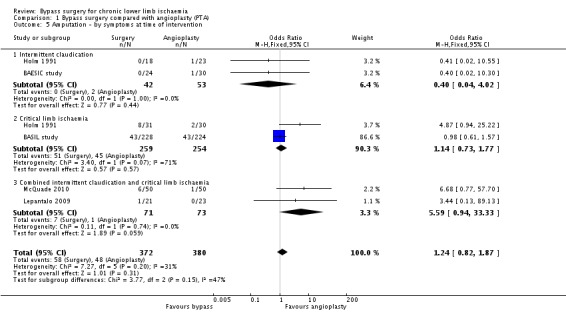
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 5 Amputation ‐ by symptoms at time of intervention.
We created separate meta‐analysis models to involve trials reporting outcome data for participants with claudication and those with CLI. Such analyses found no difference in amputation rates between surgery and angioplasty in participants treated for claudication (OR 0.40, 95% CI 0.04 to 4.02) and those treated for critical ischaemia (OR 1.14, 95% CI 0.73 to 1.77) (test for subgroup differences P = 0.15; Analysis 1.5).
Sensitivity analysis removing one study at a time showed an effect in favour of angioplasty when the BASIL study was excluded (OR 2.91, 95% CI 1.13, 7.48; I2 = 0%). Repeating the analysis after excluding trials at high risk of bias (Holm 1991; Lepantalo 2009; McQuade 2010) revealed no difference between the treatment groups (OR 0.96, 95% CI 0.60 to 1.52; I2 = 0%).
Primary patency
Primary patency rates were reported in five trials (BAESIC study; Holm 1991; Lepantalo 2009; McQuade 2010; Veterans Study). The primary patency at one year was found to be significantly higher in participants treated with bypass surgery than those receiving endovascular treatment (OR 1.94, 95% CI 1.20 to 3.14; four studies; 300 participants; Analysis 1.6). See Figure 5. The statistical heterogeneity among the studies was I2 = 71%. However, two of four trials (Holm 1991; McQuade 2010) found no effect and the other two (BAESIC study; Lepantalo 2009), which were relatively small trials, found a benefit. When applying a random‐effects model, there was no longer a combined benefit in favour of bypass surgery (OR 2.47, 95% CI 0.92 to 6.61). Sensitivity analysis removing one study at a time showed no difference when the BAESIC study (OR 1.53, 95% CI 0.90 to 2.61; I2 = 66%) and the Lepantalo 2009 trial were removed (OR 1.58, 95% CI 0.94 to 2.67; I2 = 67%). Repeating the analysis after excluding trials that were found to be at high risk of bias (Holm 1991; Lepantalo 2009; McQuade 2010) showed a difference in favour of bypass surgery (OR 6.54, 95% CI 1.79 to 23.84).
1.6. Analysis.
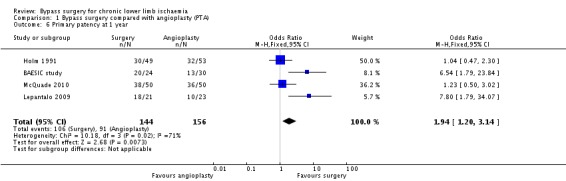
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 6 Primary patency at 1 year.
5.
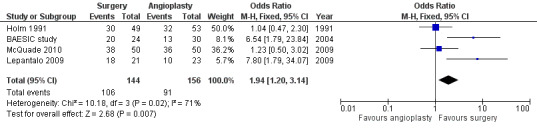
Forest plot of comparison: 1 Bypass surgery compared with angioplasty (PTA), outcome: 1.6 Primary patency at 1 year.
At four years, the primary patency was not found to be different between surgery and angioplasty (OR 1.15, 95% CI 0.74 to 1.78; two studies; 363 participants; Analysis 1.7). See Figure 6. The statistical heterogeneity was I2 = 0%. The Veterans Study provided specific four‐year patency information for participants with claudication and critical ischaemia, as well as for participants with iliac and femoro‐popliteal disease. Meta‐analyses found no significant differences in primary patency at four years between surgical and endovascular treatment in participants with claudication (OR 1.44, 95% CI 0.77 to 2.69) or critical ischaemia (OR 0.95, 95% CI 0.37 to 2.43; test for subgroup differences P = 0.63; Analysis 1.7), and in participants with femoro‐popliteal disease (OR 0.91, 95% CI 0.41 to 2.01) or iliac disease (OR 1.57, 95% CI 0.78 to 3.14; test for subgroup differences P = 0.31; Analysis 1.8). Sensitivity analysis removing one study at a time revealed no difference between the treatment groups.
1.7. Analysis.
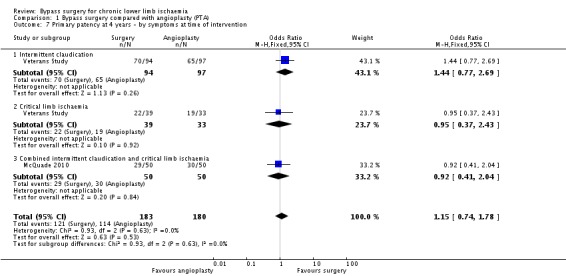
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 7 Primary patency at 4 years ‐ by symptoms at time of intervention.
6.
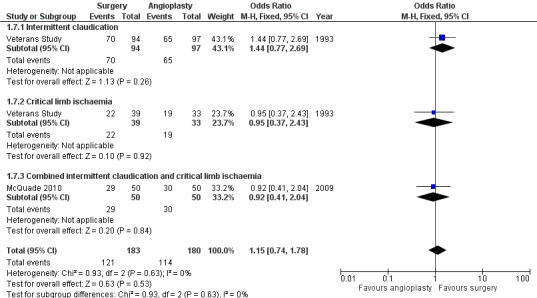
Forest plot of comparison: 1 Bypass surgery compared with angioplasty (PTA), outcome: 1.7 Primary patency at 4 years ‐ by symptoms at time of intervention.
1.8. Analysis.
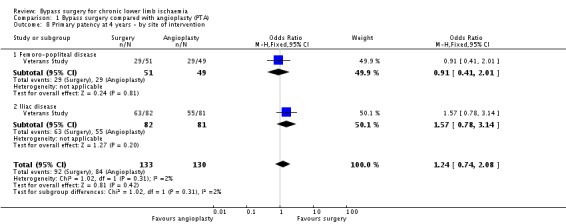
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 8 Primary patency at 4 years ‐ by site of intervention.
Mortality within follow‐up
Five out of the six trials reported mortality of the study populations within the follow‐up period (BASIL study; Holm 1991; Lepantalo 2009; McQuade 2010; Veterans Study). No significant difference in mortality between the treatment modalities was identified (OR 0.94, 95% CI 0.71 to 1.25; 961 participants; Analysis 1.9). Between‐study heterogeneity was I2 = 0%.
1.9. Analysis.
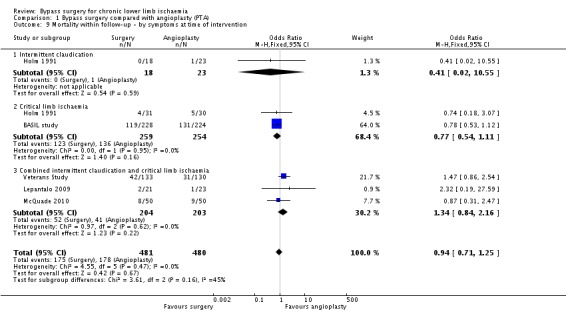
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 9 Mortality within follow‐up ‐ by symptoms at time of intervention.
When separate analyses for participants with claudication and those with CLI were performed, no differences in mortality were identified (OR 0.41, 95% CI 0.02 to 10.55; and OR 0.77, 95% CI 0.54 to 1.11, respectively; test for subgroup differences P = 0.16 Analysis 1.9).
Sensitivity analysis removing one study at a time revealed no significant difference between the groups. Similarly, repeating the analysis without the trials that were found to be at high risk of bias (Holm 1991; Lepantalo 2009; McQuade 2010) showed no difference in mortality within follow‐up (OR 0.95, 95% CI 0.70 to 1.29; I2 = 73%).
Technical success
Technical success rates were reported in five trials (BAESIC study; BASIL study; Lepantalo 2009; McQuade 2010; Veterans Study). Technical success was either inconsistently defined by the study authors or a clear definition was not provided. Bypass surgery was found to be associated with a higher technical success rate than PTA (OR 2.26, 95% CI 1.49 to 3.44; 913 participants; Analysis 1.10). The statistical heterogeneity was I2 = 65%.
1.10. Analysis.
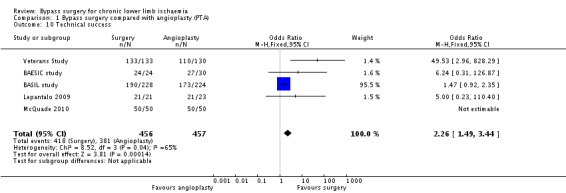
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 10 Technical success.
Sensitivity analysis removing one study at a time confirmed higher technical success with bypass surgery. However, excluding the high risk of bias trials (Lepantalo 2009; McQuade 2010) showed no significant difference between treatments (OR 5.91, 95% CI 0.51 to 69.01; I2 = 75%).
Assisted primary patency
Assisted primary patency rates were provided by one trial only (Lepantalo 2009). Assisted primary patency at one year was found to be significantly higher after bypass surgery than PTA (OR 8.71, 95% CI 1.64 to 46.31; 44 participants; Analysis 1.11). However, this result should be cautiously interpreted because of the very wide CI.
1.11. Analysis.
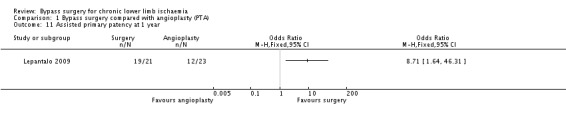
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 11 Assisted primary patency at 1 year.
Secondary patency
Secondary patency rates were reported in three trials (Holm 1991; Lepantalo 2009; McQuade 2010). Pooled analysis revealed that the secondary patency at one year was similar in the bypass surgery and the angioplasty group (OR 1.28, 95% CI 0.71 to 2.34; 246 participants; Analysis 1.12). The statistical heterogeneity was I2 = 74%. Repeating the analysis after removing one study at a time showed no difference between treatments. All three trials were found to be of high risk of bias.
1.12. Analysis.
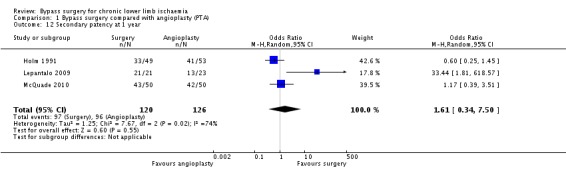
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 12 Secondary patency at 1 year.
Similar to primary patency, no difference in secondary patency rates at four years between the treatment groups was identified (OR 0.90, 95% CI 0.37 to 2.19; one study; 100 participants; Analysis 1.13).
1.13. Analysis.
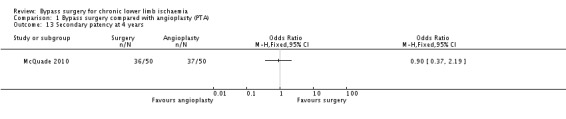
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 13 Secondary patency at 4 years.
Vessel or graft occlusion
Two studies reported vessel or graft occlusion within the follow‐up period (BAESIC study; McQuade 2010). Even though the incidence of vessel or graft occlusion was higher in the angioplasty group, no statistically significant difference between the treatment groups was found (OR 0.56, 0.27 to 1.15; 154 participants; Analysis 1.14). The statistical heterogeneity was I2 = 46%.
1.14. Analysis.
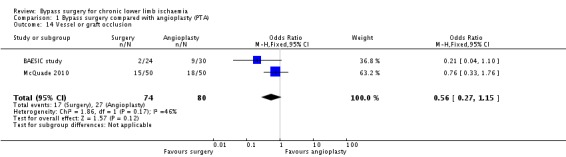
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 14 Vessel or graft occlusion.
Repeating the analysis after removing one study at a time revealed no difference between the treatment groups.
Reinterventions within follow‐up
Three trials reported numbers of reinterventions within the follow‐up period (BAESIC study; Holm 1991; McQuade 2010). Bypass surgery was associated with a lower reintervention rate, but the difference between surgery and angioplasty was not statistically significant (OR 0.76, 95% CI 0.42 to 1.37; 256 participants; Analysis 1.15). Heterogeneity among the trials was I2 = 0%.
1.15. Analysis.
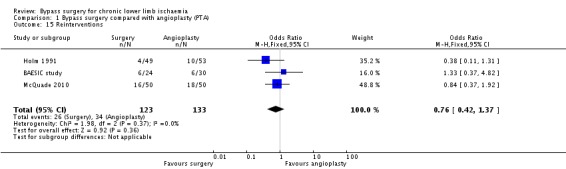
Comparison 1 Bypass surgery compared with angioplasty (PTA), Outcome 15 Reinterventions.
Sensitivity analysis removing one study at a time revealed no difference between the treatment groups. Similarly, repeating the analysis after excluding the trials that were found to be at high risk of bias (Holm 1991; McQuade 2010) showed no difference between the treatments (OR 1.33, 95% CI 0.37 to 4.82).
Walking distance
Not reported.
Ulcer healing
Not reported.
Subjective measures
In the Veterans Study, the Sickness Impact Profile (SIP) was used to evaluate health status. This instrument provides a score of physical and psychosocial well‐being, measured on an interval scale from zero (no impairment) to 100 (maximum impairment), with an average score of 5.2 in healthy controls. Mean SIP scores after 12 months were 10.6 in the surgery group and 10.8 in the angioplasty group compared with 15.8 and 15.6 at baseline, respectively. Both groups showed a significant improvement in scores compared with baseline, without any significant differences between the two groups at any point in the follow‐up. In the BASIL study, self reported health‐related quality of life (HRQOL) was measured using the Vascular Quality of Life Questionaire (VascuQol), the EuroQoL (EQ‐5D) health outcome measure, and the Short Form 36 (SF‐36) up to three years from randomisation. These generic measures were recorded at baseline and at three, six, 12, 24, and 36 months after randomisation. No significant differences in HRQOL indices from baseline scores were found in either treatment group. There were no subjective measures of health status reported in the BAESIC study; Holm 1991; Lepantalo 2009; and McQuade 2010 trials.
Use of resources
The BASIL study reported that over three years, the use of inpatient hospital services was broadly similar in the treatment arms, as measured by the number of hospital admissions and total days in the hospital. Over three years, both groups had an average of three hospital stays. Furthermore, by three years, there was an insignificant difference in the mean length of hospital stay between the two groups, with 60 days (16 to 82 days) for the bypass group and 57 days (8 to 73 days) for the angioplasty group. Three more studies mention resource utilisation (Holm 1991; Lepantalo 2009; McQuade 2010), where the length of stay in hospital is reported. In the Holm 1991 trial, the post‐treatment stay was significantly shorter in the angioplasty group. The median lengths of stay for the IC and CLI groups, respectively, were 8.6 days and 15.0 days for the surgery group, and 2.6 and 5.0 days for the angioplasty group. In the Lepantalo 2009 trial, the hospital stay was longer for participants assigned bypass surgery (mean 4.5 days, range 2 to 10 days) than for participants randomised to endovascular treatment (mean 1.7 days, range 0 to 7 days). Similarly, in the McQuade 2010 trial, the length of hospital stay was significantly longer in the bypass group than that in the endovascular treatment group (mean 3.1 days, SD 1.8 days versus mean 0.9 days, SD 0.8 days; P < 0.01). Unfortunately, data from the Holm 1991 and Lepantalo 2009 trials were not available in a form suitable for inclusion in a statistical meta‐analysis.
Bypass surgery compared with remote endarterectomy
Only one trial with 116 participants (REVAS Trial) compared bypass surgery with remote endarterectomy for the treatment of lower limb ischaemia. This trial enrolled participants with severe claudication or CLI treated with supra‐geniculate bypass surgery with long saphenous vein or PTFE graft, or remote endarterectomy of the superficial femoral artery. The median duration of follow‐up for the whole study population was 37 months.
Early postoperative non‐thrombotic complications
The frequency of early postoperative non‐thrombotic complications was similar in the treatment groups (OR 1.11, 95% CI 0.53 to 2.34; Analysis 2.1).
2.1. Analysis.
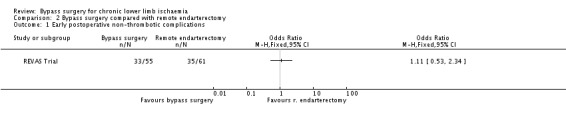
Comparison 2 Bypass surgery compared with remote endarterectomy, Outcome 1 Early postoperative non‐thrombotic complications.
Procedural mortality
No mortality within 30 days of the index treatment or during stay in hospital was recorded in either group.
Clinical improvement
Not reported.
Amputation
Three participants in the bypass group (55 participants) and two participants in the remote endarterectomy group (61 participants) progressed to major amputation of the treated limb, and the difference between the treatment groups was insignificant (OR 1.70, 95% CI 0.27 to 10.58; Analysis 2.2).
2.2. Analysis.
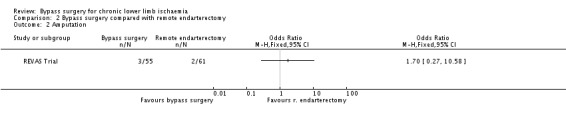
Comparison 2 Bypass surgery compared with remote endarterectomy, Outcome 2 Amputation.
Primary patency
The primary patency rate at three years was similar after bypass surgery and remote endarterectomy (OR 1.66, 95% CI 0.79 to 3.46; Analysis 2.3).
2.3. Analysis.
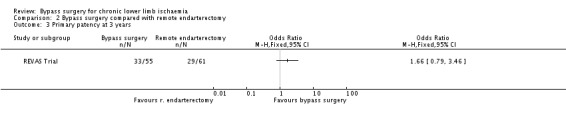
Comparison 2 Bypass surgery compared with remote endarterectomy, Outcome 3 Primary patency at 3 years.
Mortality within follow‐up
No differences in late mortality during the follow‐up period between the treatment arms were found (OR 1.66, 95% CI 0.61 to 4.48; Analysis 2.4).
2.4. Analysis.
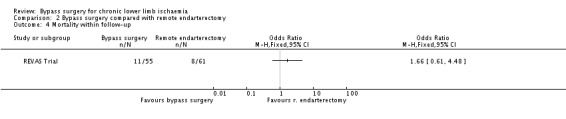
Comparison 2 Bypass surgery compared with remote endarterectomy, Outcome 4 Mortality within follow‐up.
Technical success
The technical success rate was higher in the bypass surgery group, but the difference between bypass surgery and remote endarterectomy was not significant (OR 10.81, 95% CI 0.58 to 200.08; Analysis 2.5). However, this result should be interpreted with caution because of the very wide CI.
2.5. Analysis.
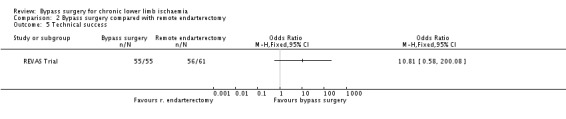
Comparison 2 Bypass surgery compared with remote endarterectomy, Outcome 5 Technical success.
Assisted primary patency
No differences in assisted primary patency at three years between treatments were identified (OR 1.35, 95% CI 0.63 to 2.93; Analysis 2.6).
2.6. Analysis.
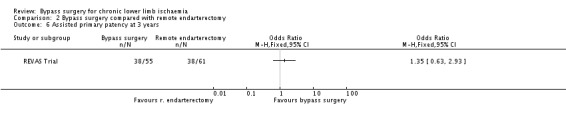
Comparison 2 Bypass surgery compared with remote endarterectomy, Outcome 6 Assisted primary patency at 3 years.
Secondary patency
The secondary patency rate at three years was similar in the bypass and remote endarterectomy group (OR 1.21, 95% CI 0.54 to 2.69; Analysis 2.7).
2.7. Analysis.
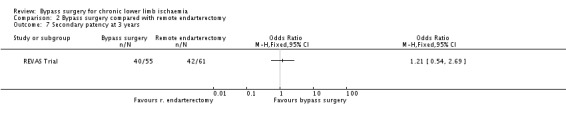
Comparison 2 Bypass surgery compared with remote endarterectomy, Outcome 7 Secondary patency at 3 years.
Vessel or graft occlusion
Similar episodes of graft or native vessel occlusion occurred in the bypass group and the remote endarterectomy group within the follow‐up period (OR 0.97, 95% CI 0.43 to 2.19; Analysis 2.8).
2.8. Analysis.
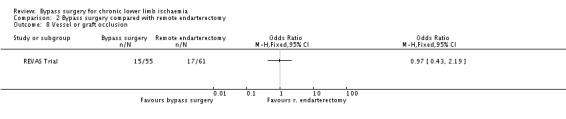
Comparison 2 Bypass surgery compared with remote endarterectomy, Outcome 8 Vessel or graft occlusion.
Reinterventions within follow‐up
Not reported.
Walking distance
Not reported.
Ulcer healing
Not reported.
Subjective measures
Not reported.
Use of resources
The only information about resource utilisation provided by the REVAS Trial was the length of stay in hospital for the index procedure. Participants with chronic lower limb ischaemia treated with bypass surgery stayed in hospital for a significantly longer period (median 6 days, range 3 to 28 days) than those undergoing remote endarterectomy (median 4 days, range 1 to 21; P = 0.004).
Bypass surgery compared with thrombolysis
Bypass surgery was compared with thrombolysis in the STILE Trial only (237 participants). As mentioned in the Characteristics of included studies table, these results were included with the proviso that only 86% of the surgery group had a bypass procedure and 20% of the participants had acute rather than chronic ischaemia.
Early postoperative non‐thrombotic complications
In the Weaver 1996 report of the STILE Trial for results in native arteries, complications were combined into a measure of "major morbidity". This category included: life‐threatening haemorrhage requiring resuscitation; perioperative complications, for example myocardial infarction or stroke; renal failure requiring dialysis; serious anaesthesia‐related complications; vascular complications, for example, dissection; and postoperative wound complications. No significant difference in major morbidity within 30 days of treatment between surgery and thrombolysis was identified (OR 0.66, 95% CI 0.34 to 1.31; Analysis 3.1). Data were not provided for major morbidity excluding those participants with acute ischaemia or separated according to symptoms at presentation. Analysis by site of lesion also showed no significant difference between surgery and thrombolysis within 30 days of intervention (test for subgroup differences P = 0.19; Analysis 3.1).
3.1. Analysis.
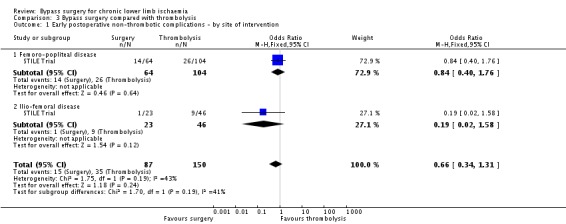
Comparison 3 Bypass surgery compared with thrombolysis, Outcome 1 Early postoperative non‐thrombotic complications ‐ by site of intervention.
Procedural mortality
There was no significant difference in 30‐day mortality between the surgery group and the thrombolysis group (OR 2.09, 95% CI 0.67 to 6.44; Analysis 3.2). No separate data for participants treated for claudication or CLI were provided. The site of the lesion also did not significantly affect mortality (test for subgroup differences P = 0.50; Analysis 3.2).
3.2. Analysis.
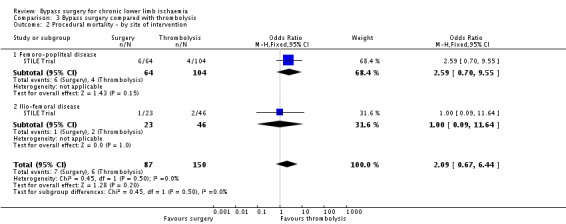
Comparison 3 Bypass surgery compared with thrombolysis, Outcome 2 Procedural mortality ‐ by site of intervention.
Clinical improvement
Not reported.
Amputation
After one year, there were significantly fewer amputations in the surgery group compared with the thrombolysis group (OR 0.10, 95% CI 0.01 to 0.80; Analysis 3.3). The significantly lower amputation rate in the surgery group at one year persisted when those with acute symptoms were excluded from the analysis. This significant difference was also present in the group of participants presenting with CLI (OR 0.06, 95% CI 0 to 1.02), but not in the group presenting with claudication (OR 0.44, 95% CI 0.02 to 11.12; test for subgroup differences P = 0.36; Analysis 3.3). Amputation rates at one year were also affected by the site of the lesion: there were significantly fewer amputations in those participants with femoro‐popliteal occlusions who received surgery compared with thrombolysis (OR 0.05, 95% CI 0 to 0.83), but there was no difference in the group with ilio‐femoral occlusions (OR 0.65, 95% CI 0.03 to 16.46; test for subgroup differences P = 0.24; Analysis 3.4). Analysis in the original article demonstrated that both diabetes and critical ischaemia were significant prognostic factors for amputation (P = 0.03). Diabetes as a risk factor alone did not reach statistical significance (OR 1.97, 95% CI 0.99 to 3.93; Weaver 1996).
3.3. Analysis.
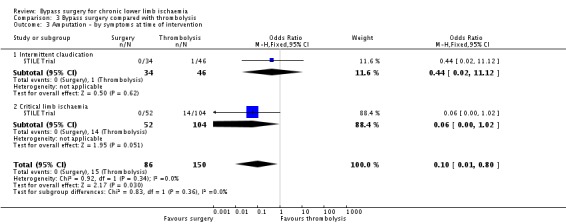
Comparison 3 Bypass surgery compared with thrombolysis, Outcome 3 Amputation ‐ by symptoms at time of intervention.
3.4. Analysis.
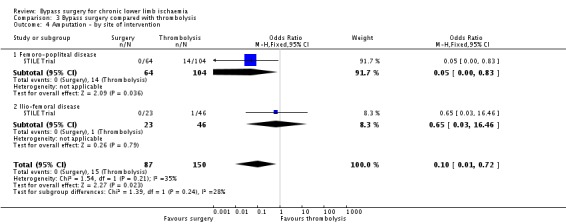
Comparison 3 Bypass surgery compared with thrombolysis, Outcome 4 Amputation ‐ by site of intervention.
Primary patency
Not reported.
Mortality within follow‐up
There was no significant difference in mortality at one year between the surgery group and the thrombolysis group (OR 1.56, 95% CI 0.71 to 3.44; Analysis 3.5). Mortality rates also did not differ significantly between surgery and thrombolysis when the analysis was performed excluding those participants with acute limb ischaemia. When the data were split by symptoms at the time of intervention (IC and CLI), there were also no significant differences between surgery and thrombolysis, although mortality tended to be less in the CLI group treated with thrombolysis (test for subgroup differences P = 0.53; Analysis 3.5). The site of the lesion also did not significantly affect mortality (OR 1.45, 95% CI 0.66 to 3.18) (test for subgroup differences P = 0.17; Analysis 3.6).
3.5. Analysis.
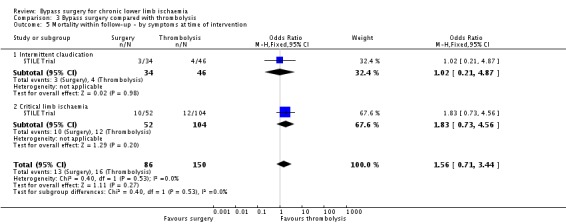
Comparison 3 Bypass surgery compared with thrombolysis, Outcome 5 Mortality within follow‐up ‐ by symptoms at time of intervention.
3.6. Analysis.
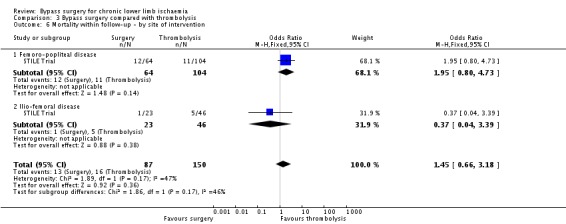
Comparison 3 Bypass surgery compared with thrombolysis, Outcome 6 Mortality within follow‐up ‐ by site of intervention.
Technical success
Not reported.
Assisted primary patency
Not reported.
Secondary patency
Not reported.
Vessel or graft occlusion
Not reported.
Reinterventions within follow‐up
Not reported.
Walking distance
Not reported.
Ulcer healing
Not reported.
Subjective measures
Not reported.
Use of resources
Not reported.
Bypass surgery compared with thromboendarterectomy
Bypass surgery was compared with thromboendarterectomy in the Gaspard 1972 trial only. The results from this trial were unfortunately limited because it included only 43 participants, the follow‐up period was short (approximately six weeks), and relatively few outcome measures were included.
Early postoperative non‐thrombotic complications
The only complication reported was blood loss during surgery, which was reported by the study authors to be significantly greater in the thromboendarterectomy group (an average of 3.6 units per participant were required compared with 2.7 units required in the bypass group).
Procedural mortality
One participant in the thromboendarterectomy group died during hospital stay for the index procedure, whereas no in‐hospital death was recorded in the bypass group (OR 0.33, 95% CI 0.01 to 8.65; Analysis 4.1).
4.1. Analysis.
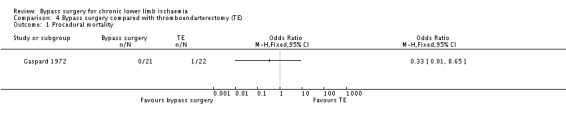
Comparison 4 Bypass surgery compared with thromboendarterectomy (TE), Outcome 1 Procedural mortality.
Clinical improvement
Not reported.
Amputation
Six weeks after intervention, there were two amputations in the bypass surgery group and four in the thromboendarterectomy group; this difference was not statistically significant (OR 0.47, 95% CI 0.08 to 2.91; Analysis 4.2).
4.2. Analysis.

Comparison 4 Bypass surgery compared with thromboendarterectomy (TE), Outcome 2 Amputation.
Primary patency
Not reported.
Mortality within follow‐up
During follow up, there was one death in the bypass group due to a disrupted aortic suture line, whereas no death in the thromboendarterectomy group was recorded (OR 3.29, 95% CI 0.13 to 85.44; Analysis 4.3). Caution is required when interpreting this result because of the wide CI.
4.3. Analysis.
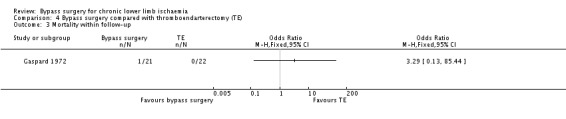
Comparison 4 Bypass surgery compared with thromboendarterectomy (TE), Outcome 3 Mortality within follow‐up.
Technical success
Technical success was achieved in all participants in the bypass group, whereas the thromboendarterectomy was unsuccessful in six participants (OR 0.01, 95% CI 0 to 0.17; Analysis 4.4).
4.4. Analysis.
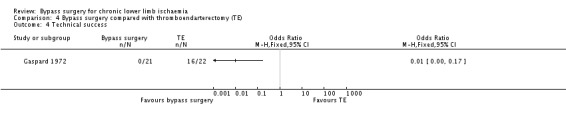
Comparison 4 Bypass surgery compared with thromboendarterectomy (TE), Outcome 4 Technical success.
Assisted primary patency
Not reported.
Secondary patency
Not reported.
Vessel or graft occlusion
Not reported.
Reinterventions within follow‐up
Not reported.
Walking distance
Not reported.
Ulcer healing
Not reported.
Subjective measures
Not reported.
Use of resources
This was reported as the average time to complete the procedure. The time was an hour longer in the thromboendarterectomy group (5.8 hours compared with 4.6 hours), but no statistical tests were reported.
Bypass surgery compared with exercise
Bypass surgery was compared with exercise in only one trial (Lundgren 1989). This was a small trial enrolling only 75 participants, with a relatively short follow‐up period (eight to nine months). Twenty five participants were randomised to surgery, 25 participants to exercise, and another 25 participants were randomised to combined treatment with surgery and exercise therapy.
Early postoperative non‐thrombotic complications
Comparisons of the complication rates in the surgery and exercise group produced very wide CI (OR 7.45, 95% CI 0.40 to 137.76; Analysis 5.1). In the total of 50 participants randomised to surgery (surgery alone and surgery plus exercise), three developed a wound haematoma, two developed a myocardial infarction, and one suffered a pulmonary embolus. There were no direct complications of exercise.
5.1. Analysis.
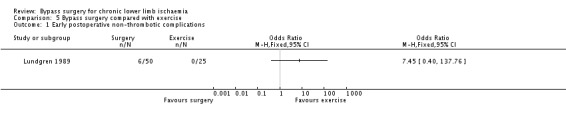
Comparison 5 Bypass surgery compared with exercise, Outcome 1 Early postoperative non‐thrombotic complications.
Procedural mortality
One participant died in the surgery group and, similar to the comparison of early postoperative non‐thrombotic complications, comparisons of procedural mortality produced a wide CI (OR 1.55, 95% CI 0.06 to 39.31; Analysis 5.2).
5.2. Analysis.
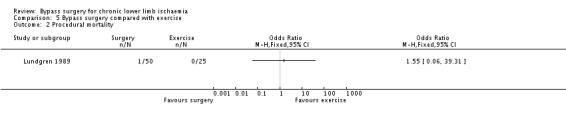
Comparison 5 Bypass surgery compared with exercise, Outcome 2 Procedural mortality.
Clinical improvement
Not reported.
Amputation
No amputations were reported in either group.
Primary patency
Not reported.
Mortality within follow‐up
Within the follow‐up period, there were two deaths in the surgery group, but this result should be cautiously interpreted because of the very wide CI (OR 2.63, 95% CI 0.12 to 56.86; Analysis 5.3).
5.3. Analysis.
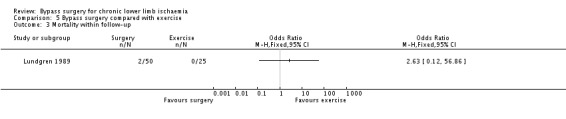
Comparison 5 Bypass surgery compared with exercise, Outcome 3 Mortality within follow‐up.
Technical success
Not reported.
Assisted primary patency
Not reported.
Secondary patency
Not reported.
Vessel or graft occlusion
Not reported.
Reinterventions within follow‐up
The requirement for further intervention did not differ significantly between the two groups (OR 2.19, 95% CI 0.43 to 11.19; Analysis 5.4). In the total of 50 participants randomised to surgery, three participants required thrombectomy and five required a second reconstruction. In two of the participants randomised to exercise, limb‐threatening ischaemia developed and bypass surgery was performed.
5.4. Analysis.
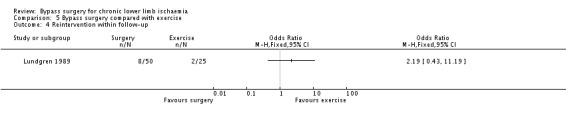
Comparison 5 Bypass surgery compared with exercise, Outcome 4 Reintervention within follow‐up.
Walking distance
There was no significant difference in maximal walking time between the exercise and surgery group at the end of the trial (1.66 min, 95% CI ‐1.23 to 4.55; Analysis 5.5), although improvement was slightly less in the exercise group (150% compared with 173%). There was no significant difference between the surgery group and a third group receiving both surgery and exercise.
5.5. Analysis.
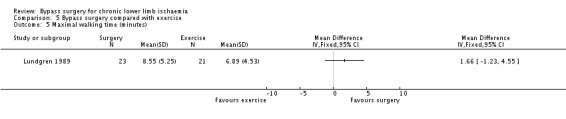
Comparison 5 Bypass surgery compared with exercise, Outcome 5 Maximal walking time (minutes).
Ulcer healing
Not relevant (the trial included only participants with IC).
Subjective measures
Not reported.
Use of resources
Not reported.
Bypass surgery compared with spinal cord stimulation
Bypass surgery was compared with spinal cord stimulation in one small trial of 12 participants in which very few outcome measures were reported (Guarnera 1994). This small sample size did not allow the demonstration of any statistically significant differences.
Early postoperative non‐thrombotic complications
Not reported.
Procedural mortality
No procedural deaths were reported in either group.
Clinical improvement
Clinical improvement defined as improvement in Rutherford category was not reported. However, therapeutic success was reported as good or fair where either complete or evident pain regression and trophic ulcer healing were obtained; otherwise, the result was considered poor. At 12 months, the results were poor in 60% of the bypass group and in 28% of the cord stimulation group; this difference was not statistically significant (OR 3.75, 95% CI 0.33 to 42.47; Analysis 6.1). However, this result should be cautiously interpreted because of the very wide CI.
6.1. Analysis.
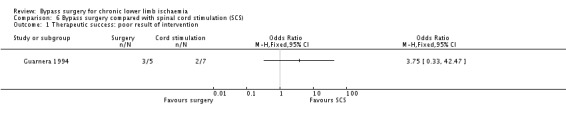
Comparison 6 Bypass surgery compared with spinal cord stimulation (SCS), Outcome 1 Therapeutic success: poor result of intervention.
Amputation
There was no significant difference in amputation rates between surgery and spinal cord stimulation after 12 months of follow‐up (OR 4.00, 95% CI 0.25 to 63.95; Analysis 6.2). Again, the result should be interpreted with caution because of the very wide CI.
6.2. Analysis.
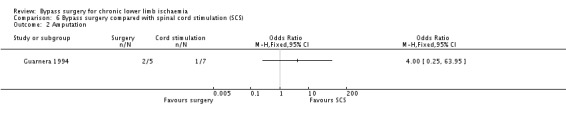
Comparison 6 Bypass surgery compared with spinal cord stimulation (SCS), Outcome 2 Amputation.
Primary patency
Not reported.
Mortality within follow‐up
Not reported.
Technical success
Not reported.
Assisted primary patency
Not reported.
Secondary patency
Not reported.
Vessel or graft occlusion
Not reported.
Reinterventions within follow‐up
Not reported.
Walking distance
Not reported.
Ulcer healing
Ulcer healing was included in the overall measure of therapeutic success (see above), but was not reported separately.
Subjective measures
Not reported.
Use of resources
Not reported.
Discussion
Summary of main results
No studies comparing bypass surgery with no intervention or medical treatment were identified. In the trials selected in this review, bypass surgery was the de facto "gold standard" for the management of chronic lower extremity ischaemia of sufficient symptomatic severity to require treatment. The review provides comparisons of bypass surgery to other treatments for symptomatic PAD, but does not assess the effect of any treatment on the natural history of PAD, either claudication or CLI.
Comparisons of bypass surgery with angioplasty is the area in which most evidence is currently available. The main results are outlined in the Table 1. Six of the eleven randomised trials reported comparisons of bypass surgery with endovascular therapy for chronic lower limb ischaemia in a total of 1015 participants (BAESIC study; BASIL study; Holm 1991; Lepantalo 2009; McQuade 2010; Veterans Study). In terms of the peri‐interventional outcomes, pooled analysis revealed that bypass surgery was associated with greater technical success than PTA. However, when interpreting this finding, one should take into account that technical success was either inconsistently defined among the trials included in the meta‐analysis or not defined at all. Furthermore, it was not possible to assess haemodynamic parameters as a measure of technical success, and no distinction was made between procedural success measures, such as improved blood flow to the foot and increased ankle brachial index (ABI), and technical success of merely accomplishing the intended intervention. Even though a trend towards reduced early postinterventional non‐thrombotic complications and procedural mortality in the endovascular treatment group was demonstrated, such differences did not reach statistical significance. Regarding the follow‐up outcomes, an interesting finding of our analysis is that the primary patency rate at one year was higher after bypass surgery, but no difference was found at four years of follow up. Furthermore, the assisted primary patency was higher in the bypass group, but this finding should be cautiously interpreted in the presence of a wide CI. One should also take into account that primary patency is a measure of the effectiveness of the procedure itself, while assisted primary patency is a function of the postprocedure surveillance process and willingness to intervene and is, therefore, subject to surveillance, observer and intervention bias. Modern objective performance comparisons consider the number and types of interventions needed to maintain patency, but data on this were not available. No differences were identified in the rest of the clinical outcome parameters for which data were available for meta‐analyses, including clinical improvement, amputation and reintervention rates, and late mortality. Similar results were found when subgroup analyses, comprising of participants with similar clinical severity of disease and anatomical level of reconstruction, were performed, except that bypass surgery was associated with a higher early non‐thrombotic complication rate compared with angioplasty in participants with CLI but not in those with claudication. No difference in subjective outcome parameters, indicated by quality of life and physical and psychosocial well‐being, was reported. The hospital stay for the index procedure was reported to be longer in participants undergoing bypass surgery than those treated with angioplasty. It is unknown, however, why participants undergoing bypass surgery stayed in the hospital for a longer period, and whether there were more minor amputations, debridement of pedal wounds or gangrene in one group versus the other, or the difference was entirely due to recovery from the index revascularization procedure itself.
Limited literature information was identified regarding comparisons of bypass surgery with other treatment modalities for chronic lower limb ischaemia. The recent REVAS Trial compared outcomes of bypass surgery and remote endarterectomy for femoro‐popliteal arterial disease. In this trial cohort of 116 patients, femoro‐popliteal bypass above the knee demonstrated similar outcomes with remote endarterectomy of the superficial femoral artery, expressed by technical success, perioperative morbidity and mortality, vessel or graft patency, progression to amputation, and late mortality.
The only clinical trial evaluating the comparative effectiveness of bypass surgery and thrombolysis for the treatment of chronic lower limb ischaemia is the STILE Trial. The results of this trial suggest some benefit of surgery compared with thrombolysis; the amputation rate was lower in the bypass group, particularly in patients with CLI and femoro‐popliteal lesions. Furthermore, the frequency of ongoing or recurrent ischaemia was lower after bypass surgery than after thrombolysis. There were no differences in the mortality rate or complications of the intervention between the two groups.
One trial only compared bypass surgery with thromboendarterectomy in a total of 43 participants with chronic limb ischaemia (Gaspard 1972). Despite the small size of the trial, a difference in restoration of blood flow and blood loss in favour of bypass surgery was found. Mortality and amputation rates did not differ significantly between the treatment groups. Unfortunately, information on the rest of the clinical outcome parameters was not provided.
Surgery was compared with exercise in a small trial (Lundgren 1989), and these results may, therefore, also have limited relevance. The only clear statistically significant difference was in the ABI, which was higher in the surgery group following intervention.
Bypass was compared with spinal cord stimulation in one trial of only 12 participants (Guarnera 1994). No differences were reported between the two interventions. Due to the small sample size of the study, analyses produced very wide CIs, so the finding of no difference should be interpreted with caution.
Overall completeness and applicability of evidence
Our search of bibliographic databases identified limited clinical research validating bypass surgery for the treatment of chronic lower limb ischaemia. The eleven trials included in this review varied in size, quality, and in the intervention served as control. There were no trials in which bypass surgery was compared with a placebo or no intervention, undoubtedly for ethical reasons. Furthermore, no trials comparing surgery with medical treatment were identified. Most existing evidence provides comparative information about bypass surgery versus endovascular treatment for chronic lower limb ischaemia (six trials). For the comparisons of bypass surgery with other treatment modalities, the evidence is limited, being provided by only one trial in each comparison. Most trials reported important outcome parameters, such as mortality, morbidity, patency and amputation rates, but other clinical information, such as walking distance in claudicants and ulcer healing in patients with CLI, was inadequately reported. Furthermore, amputation free survival and major adverse limb events, which are important outcomes and part of modern recommendations for studies of comparative effectiveness, were not reported in the studies included in review. Similarly, limited information was provided regarding subjective measures, such as quality of life and resource utilization.
Unfortunately, the existing results of bypass surgery versus PTA do not provide an overall clear picture favouring one treatment over the other. This may be because the existing trials were too small, because the effects really are similar, or because differences will appear only in defined subgroups of patients. It must also be remembered that these trials include only a small subset of patients with lower limb ischaemia, as those patients with multilevel disease requiring extensive or hybrid surgical and endovascular arterial reconstruction (Antoniou 2009) or those requiring tibial or pedal artery bypass may not be eligible for angioplasty. Extrapolation of our findings to the entire population suffering from PAD should be judiciously performed, because a great proportion of patients presenting with chronic lower limb ischaemia may be unsuitable for intervention due to the presence of comorbid conditions and, therefore, treated conservatively. Furthermore, these trials only included patients deemed suitable for either surgery or PTA. Patients with extensive lesions could have been excluded from receiving angioplasty and, therefore, participating in the trial. It is possible that these patients with more severe lesions receive more benefits from a surgical approach. This remains to be investigated. The long‐term (< 5 years) effects or bypass surgery in comparison to endovascular therapy remain unknown. The BASIL study found that for those patients who survived for at least two years after randomisation, a bypass‐first revascularization strategy was associated with a significant increase in overall survival and a trend towards improved amputation‐free survival compared to a balloon angioplasty‐first revascularization strategy. The trials also did not give detailed descriptions of how the angioplasties were performed and whether stents were used as an adjunct. A systematic review and meta‐analysis supports the use of primary stenting as opposed to balloon angioplasty alone, mainly for long lesions, as a first‐line endovascular treatment for symptomatic disease in the femoro‐popliteal segment (Acin 2012). None of the trials mentioned subintimal angioplasty, which may have produced different results (Chang 2013). Lastly, balloon angioplasty and stent technology is constantly evolving. Application of modern endovascular therapies, such as drug‐eluting balloons and stents and bioabsorbable stents for the treatment of anatomically "difficult" lesions, may reveal subtle differences in outcomes between treatments (Antoniou 2014). Furthermore, the introduction of endovascular tools, such as atherectomy, chronic total occlusion and re‐entry devices, may play an important role in improving procedural success and limb salvage.
All the evidence comparing bypass surgery with thrombolysis came from the STILE Trial. This was a large high quality trial, but was included in the review with some reservations (see Characteristics of included studies table). Some of these problems were mitigated by the randomisation method, which ensured that those with native artery disease were balanced across the interventions, and by the analysis, which was performed after excluding those patients with acute ischaemia. The third problem, relating to the use of endarterectomy in 14% of the surgery group, could not be addressed.
The rest of the trials comparing bypass surgery with remote endarterectomy (REVAS Trial), thromboendarterectomy (Gaspard 1972), exercise (Lundgren 1989), and spinal cord stimulation (Guarnera 1994) were relatively small, with a short follow‐up period; therefore, the results must be viewed with caution. Lack of statistical significance may result from the small sample size and not from any true absence of difference. The trials Gaspard 1972 and Guarnera 1994 are old studies, involving extensive aorto‐iliac endarterectomy and spinal cord stimulation, respectively, procedures which are currently rarely used in clinical practice.
Quality of the evidence
See Table 1
The majority of the evidence investigating effects of bypass surgery for chronic lower limb ischaemia derives from randomised trials of surgical versus endovascular treatment for PAD affecting the lower limbs. Six such trials were identified, reporting a total of 1015 participants. Most of these studies had an adequate design and were executed well. No significant methodological constraints were identified. The available information is limited, however, by the fact that most of these studies recruited patients with a wide range of disease severity and/or anatomical location and extent of disease. According to current guidelines, the extent of atherosclerotic disease has a great impact on decision making and the selection of type of treatment (Norgren 2007). We attempted to circumvent such limitations by performing subgroup analyses, but the numbers of participants included in such analyses were relatively small. Furthermore, the type of endovascular treatment varied among studies, ranging from balloon angioplasty with bare stent placement at the discretion of the treating physician to routine use of covered stents. Despite the heterogenous nature of study populations and treatments, the trials consistently reported no great differences in main outcomes between surgical and endovascular treatment of chronic lower limb ischaemia. No solid conclusions can be drawn regarding comparisons of bypass surgery with other treatments because of the paucity of available evidence; only one study for each comparison was identified. When assessing bypass surgery versus PTA, we judged the quality of the evidence to be high for all primary outcomes except for clinical improvement and primary patency. We judged the quality of the evidence for clinical improvement to be low due to due to heterogeneity between the studies and the fact this was a subjective outcome assessment and therefore at risk of detection bias. We judged the quality of the evidence for primary patency moderate due to heterogeneity between the studies. Furthermore, the CI for this outcome and for several outcomes in the comparisons of bypass surgery with other treatments was large, which might be due to either a lack of studies, small participant numbers, or low number of events for some of the outcomes. For the comparisons of bypass surgery with other treatment modalities, the evidence is also limited by being provided by only one trial in each comparison.
Potential biases in the review process
We were unable to undertake funnel plots or to assess publication bias because we identified fewer than 10 studies for any outcome. We used participants as the unit of analysis, but one study (McQuade 2010) used limbs and this unit was used in the analysis. Sensitivity analysis excluding this study revealed no effect on the outcomes.
Agreements and disagreements with other studies or reviews
A related systematic review and meta‐analysis of surgical versus endovascular reconstruction of femoro‐popliteal arterial disease was recently conducted by our evidence synthesis research group (Antoniou 2013b). This review identified four randomised trials and six observational studies comprising a total of 2817 patients. Pooled analysis revealed that endovascular treatment was accompanied by lower 30‐day morbidity (OR 2.93, 95% CI 1.34 to 6.41) and higher technical failure than bypass surgery (OR 0.10, 95% CI 0.05 to 0.22). Similar to the results of the present review, analyses of follow‐up outcomes demonstrated higher primary patency rates in the surgical treatment arm one (OR 2.42, 95% CI 1.37 to 4.28), two (OR 2.03, 95% CI 1.20 to 3.45), and three years following intervention (OR 1.48, 95% CI 1.12 to 1.97), with this difference favouring surgery disappearing at four years (OR 1.09, 95% CI 0.74 to 1.60). Limb loss rates within two and three years of intervention was found to be higher in the endovascular group; however, at the end of the fourth year, the benefit in limb salvage in favour bypass surgery was eliminated. The study concluded that an endovascular‐first approach may be advisable in patients with significant comorbidity, whereas for fit patients with a longer‐term perspective, a bypass procedure may be offered as a first line interventional treatment.
A systematic review of nine studies (3071 subjects) investigating the comparative effectiveness of bypass surgery versus endovascular treatment for severe or CLI found no difference in mortality (OR 0.72, 95% CI 0.44 to 1.16) or amputation (OR 1.2, 95% CI 0.87 to 1.65), but higher primary patency (OR 2.5, 95% CI 1.25 to 4.99) and assisted primary patency (OR 3.39, 95% CI 1.53 to 7.51) (Abu Dabrh 2016). Another recent meta‐analysis found that angioplasty was not inferior to bypass surgery in patients with CLI, as indicated by amputation‐free survival, revascularisation, leg amputation, and overall mortality (Fu 2015).
No systematic reviews and analyses of bypass surgery versus other treatment modalities for chronic lower limb ischaemia were identified. A systematic review of the literature undertaken by our research group (Antoniou 2008) demonstrated that remote endarterectomy of the superficial femoral artery had acceptable outcomes, as indicated by technical success, procedure‐related complications, and patency rates. However, it was limited by the fact that it included single‐arm observational studies only and no comparisons with other therapeutic modalities, such as bypass surgery, were performed, as no related information was available. A recent systematic review in patients with acute lower limb ischaemia (< 14 days) found that thrombolysis may be associated with a higher risk of ongoing limb ischaemia and haemorrhagic complications, including stroke, than surgery (Berridge 2013).
Authors' conclusions
Implications for practice.
Evidence for the effectiveness of bypass surgery is limited and, therefore, in many comparisons, no clear implications for practice can be drawn. Our analysis has shown that angioplasty is associated with decreased peri‐interventional complications, especially in patients with CLI, and shorter hospital stays compared with bypass surgery. Surgical treatment, on the other hand, seems to confer improved patency rates within a year of treatment, whereas comparative long‐term effects (> 5 years) of bypass surgery and endoluminal therapy are unknown. Interestingly, the BASIL study found that for those participants who survived for at least two years after randomisation, a bypass‐first revascularization strategy was associated with a significant increase in overall survival compared to a balloon angioplasty‐first revascularization strategy. In view of these findings, endovascular treatment may be advisable in patients with significant co‐morbid conditions, rendering them high risk surgical candidates, whereas bypass surgery may be preferred for young and fit patients. No solid conclusions can be drawn regarding comparisons of bypass surgery with other treatments because of the presence of one study only in each comparison. The available evidence is limited by wide CIs for several outcomes.
Implications for research.
One of the challenges for the vascular specialist is to identify which treatment is most appropriate for which patient, and this question should be addressed in future research. Trials should be large enough to ensure that any impact of potentially important factors (e.g. site and extent of disease, symptoms, and risk factor status) on outcome can be determined in the analysis. These features should, therefore, be balanced at randomisation to prevent bias. One of the limitations of published research is that whilst most arterial lesions can be treated by surgery, there is no agreement on what is suitable for angioplasty (Bradbury 2004). This limits the validity of trials' results, as individual centres' suitability may differ substantially from that of trials'. The same problem affects future research. Large, pragmatic, scientifically robust randomised trials investigating long‐term (> 5 years) outcomes are needed to elucidate therapeutic dilemmas in the management of the patient with severe limb ischaemia and produce the answers needed to make nation‐wide decisions about the most appropriate treatment in specific patient categories. Furthermore, technological achievements and the constantly evolving endovascular techniques, such as drug‐eluting balloons and stents and bioabsorbable stents, should be incorporated in clinical research and their efficacy assessed in clinical trials. Best medical therapy accompanied by exercise regimens has not been adequately evaluated in patients presenting with claudication. Assessment of quality of life is also of prime importance in patients with chronic lower limb ischaemia. Utilization of resources, patient satisfaction, and cost‐effectiveness of interventional treatments for chronic lower limb ischaemia also constitute areas of future research.
What's new
| Date | Event | Description |
|---|---|---|
| 5 October 2016 | New search has been performed | Search re‐run. Three new studies included, 12 new studies excluded and nine ongoing studies identified |
| 5 October 2016 | New citation required but conclusions have not changed | Search re‐run. Three new studies included, 12 new studies excluded and nine ongoing studies identified. Risk of bias and Summary of Findings table added. Text updated in keeping with current Cochrane guidelines. No changes to conclusions |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 7 May 2008 | Amended | Converted to new review format. |
| 13 December 2007 | New citation required and conclusions have changed | Submitted for publication in Issue 2, 2008.Substantive amendment. New contact author added. New trials included and excluded; more evidence offered and conclusions modified. Plain Language Summary authored by the Cochrane Consumer Network |
| 27 June 2005 | Amended | Contact details edited. Submitted for publication in Issue 3, 2005. |
| 17 November 2004 | Amended | Edited. Submitted for publication in Issue 1, 2005. |
| 29 May 2001 | New search has been performed | Minor update. Submitted for publication in Issue 4, 2001. |
| 26 May 2000 | New citation required and conclusions have changed | First version of the review submitted for publication in Issue 3, 2000. |
Acknowledgements
We are grateful to Dr Marlene Stewart, Managing Editor, and Dr Cathryn Broderick, Assistant Managing Editor, whose support in preparing high quality work has been invaluable. We also thank Dr Karen Welch, CIS for Cochrane Vascular, for assistance in identifying relevant trials. The contribution of Fowkes F, Leng GC, Davis M and Baker D, authors of the previous versions of this review, is also acknowledged and greatly appreciated.
Appendices
Appendix 1. CENTRAL search strategy
| Search run on Wed Oct 5 2016 | ||
| #1 | MESH DESCRIPTOR Arteriosclerosis | 868 |
| #2 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #3 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 71 |
| #4 | MESH DESCRIPTOR Atherosclerosis | 610 |
| #5 | MESH DESCRIPTOR Arterial Occlusive Diseases | 721 |
| #6 | MESH DESCRIPTOR Intermittent Claudication | 710 |
| #7 | MESH DESCRIPTOR Ischemia | 785 |
| #8 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2193 |
| #9 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 8845 |
| #10 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 7613 |
| #11 | (peripheral near3 dis*):TI,AB,KY | 3257 |
| #12 | (claudic* or IC):TI,AB,KY | 2944 |
| #13 | (isch* or CLI):TI,AB,KY | 22904 |
| #14 | arteriopathic:TI,AB,KY | 7 |
| #15 | dysvascular*:TI,AB,KY | 10 |
| #16 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 89 |
| #17 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 133 |
| #18 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 75 |
| #19 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1103 |
| #20 | MESH DESCRIPTOR Iliac Artery | 144 |
| #21 | MESH DESCRIPTOR Popliteal Artery | 276 |
| #22 | MESH DESCRIPTOR Femoral Artery | 807 |
| #23 | MESH DESCRIPTOR Tibial Arteries | 33 |
| #24 | (femor* or *femoral or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial):TI,AB,KY | 13857 |
| #25 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 | 53365 |
| #26 | MESH DESCRIPTOR Vascular Grafting EXPLODE ALL TREES | 5756 |
| #27 | MESH DESCRIPTOR Limb Salvage | 57 |
| #28 | MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES | 408 |
| #29 | MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES | 398 |
| #30 | bypass*:TI,AB,KY | 12695 |
| #31 | revascul*:TI,AB,KY | 6332 |
| #32 | graft*:TI,AB,KY | 16762 |
| #33 | reconstruct*:TI,AB,KY | 4984 |
| #34 | revasculari*:TI,AB,KY | 6329 |
| #35 | #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 | 32232 |
| #36 | #25 AND #35 | 7435 |
Data and analyses
Comparison 1. Bypass surgery compared with angioplasty (PTA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early postoperative non‐thrombotic complications ‐ by symptoms at time of intervention | 6 | 1015 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.96, 1.73] |
| 1.1 Intermittent claudication | 2 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.50, 9.21] |
| 1.2 Critical limb ischaemia | 2 | 513 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.09, 2.24] |
| 1.3 Combined intermittent claudication and critical limb ischaemia | 3 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.40, 1.28] |
| 2 Early postoperative non‐thrombotic complications ‐ by site of intervention | 5 | 905 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.90, 1.67] |
| 2.1 Femoro‐popliteal disease | 5 | 748 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.97, 1.86] |
| 2.2 Iliac disease | 1 | 157 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.24, 1.58] |
| 3 Procedural mortality | 5 | 913 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.66, 4.19] |
| 4 Clinical improvement | 2 | 154 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.03, 14.52] |
| 5 Amputation ‐ by symptoms at time of intervention | 5 | 752 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.82, 1.87] |
| 5.1 Intermittent claudication | 2 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.04, 4.02] |
| 5.2 Critical limb ischaemia | 2 | 513 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.73, 1.77] |
| 5.3 Combined intermittent claudication and critical limb ischaemia | 2 | 144 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.94, 33.33] |
| 6 Primary patency at 1 year | 4 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.20, 3.14] |
| 7 Primary patency at 4 years ‐ by symptoms at time of intervention | 2 | 363 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.74, 1.78] |
| 7.1 Intermittent claudication | 1 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.77, 2.69] |
| 7.2 Critical limb ischaemia | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.37, 2.43] |
| 7.3 Combined intermittent claudication and critical limb ischaemia | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.41, 2.04] |
| 8 Primary patency at 4 years ‐ by site of intervention | 1 | 263 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.74, 2.08] |
| 8.1 Femoro‐popliteal disease | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.41, 2.01] |
| 8.2 Iliac disease | 1 | 163 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.78, 3.14] |
| 9 Mortality within follow‐up ‐ by symptoms at time of intervention | 5 | 961 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.25] |
| 9.1 Intermittent claudication | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.02, 10.55] |
| 9.2 Critical limb ischaemia | 2 | 513 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.11] |
| 9.3 Combined intermittent claudication and critical limb ischaemia | 3 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.84, 2.16] |
| 10 Technical success | 5 | 913 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.49, 3.44] |
| 11 Assisted primary patency at 1 year | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Secondary patency at 1 year | 3 | 246 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [0.34, 7.50] |
| 13 Secondary patency at 4 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Vessel or graft occlusion | 2 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.15] |
| 15 Reinterventions | 3 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.42, 1.37] |
Comparison 2. Bypass surgery compared with remote endarterectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early postoperative non‐thrombotic complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Primary patency at 3 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Mortality within follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Technical success | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Assisted primary patency at 3 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Secondary patency at 3 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Vessel or graft occlusion | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Bypass surgery compared with thrombolysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early postoperative non‐thrombotic complications ‐ by site of intervention | 1 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.34, 1.31] |
| 1.1 Femoro‐popliteal disease | 1 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.40, 1.76] |
| 1.2 Ilio‐femoral disease | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.58] |
| 2 Procedural mortality ‐ by site of intervention | 1 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.67, 6.44] |
| 2.1 Femoro‐popliteal disease | 1 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.59 [0.70, 9.55] |
| 2.2 Ilio‐femoral disease | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.09, 11.64] |
| 3 Amputation ‐ by symptoms at time of intervention | 1 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.80] |
| 3.1 Intermittent claudication | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.02, 11.12] |
| 3.2 Critical limb ischaemia | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 1.02] |
| 4 Amputation ‐ by site of intervention | 1 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.72] |
| 4.1 Femoro‐popliteal disease | 1 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.83] |
| 4.2 Ilio‐femoral disease | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.03, 16.46] |
| 5 Mortality within follow‐up ‐ by symptoms at time of intervention | 1 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.71, 3.44] |
| 5.1 Intermittent claudication | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.21, 4.87] |
| 5.2 Critical limb ischaemia | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.73, 4.56] |
| 6 Mortality within follow‐up ‐ by site of intervention | 1 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.66, 3.18] |
| 6.1 Femoro‐popliteal disease | 1 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.80, 4.73] |
| 6.2 Ilio‐femoral disease | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.39] |
Comparison 4. Bypass surgery compared with thromboendarterectomy (TE).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Procedural mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mortality within follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Technical success | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Bypass surgery compared with exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early postoperative non‐thrombotic complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Procedural mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mortality within follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Reintervention within follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Maximal walking time (minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Bypass surgery compared with spinal cord stimulation (SCS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Therapeutic success: poor result of intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
BAESIC study.
| Methods | Study design: randomised, no statement of blinding Method of randomisation: computer Exclusions postrandomisation: still on waiting list for surgery, refusal of surgery, refusal of participation Power calculation: 80% power, alpha = 0.05 Losses to follow up: 3 Intention‐to‐treat analysis: states yes but did not | |
| Participants | Country: Netherlands and UK
Setting: 18 centres (16 Netherlands, 2 UK)
Number of participants: 56
Age: 42 to 84 years Sex: male and female Inclusion criteria: IC not responding to therapy for 3 months and stenosis/occlusion of SFA (length 5 to 15 cm). Exclusion criteria: haemodynamically significant stenosis of the aorto‐iliac tract, absence of patent crural arteries, previous treatment of femoro‐popliteal segment, life expectancy < 1 year, contraindication for PTA |
|
| Interventions | Treatment: vein bypass ‐ in situ or reversed autogenous vein graft (4 participants received prosthetic graft), aspirin 100 mg daily for 3 months Control: PTA ‐ conventional balloon dilation, with stenting performed at the discretion of the treating physician, aspirin 100 mg daily for 3 months Duration: median 703 days (range 39 to 1430) | |
| Outcomes | Primary: re‐occlusion of femoral artery Secondary: clinical improvement; assisted primary patency; SVS/ISCVS classification; mortality and adverse events | |
| Notes | No source of funding/sponsorship was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to PTA or vein bypass by computer randomisation, stratified for each centre". Comment: A computer random‐number generator was used, therefore appropriate method of randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation as described above. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Probably no blinding because of the nature of surgical intervention. However, the outcome is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated. However, outcome assessments were following a set protocol. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants were lost to follow up, 2 from the PTA group and 1 from the bypass group, but missing outcome data were balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes as predefined. |
| Other bias | Unclear risk | Terminated prematurely because of recruitment issues. |
BASIL study.
| Methods | Study design: randomised, no statement of blinding
Method of randomisation: by computer, random numbers, sealed envelope Power calculation performed: 90% power, alpha = 0.05 Losses to follow up: 4 Intention‐to‐treat analysis: yes |
|
| Participants | Country: UK
Setting: 27 centres
Number of participants: 452 Age: median age 75 years (interquartile range 67 to 82) Sex: male and female Inclusion criteria: severe limb ischaemia defined as rest pain or tissue loss of presumed arterial aetiology for more than 2 weeks and treatable by bypass or balloon angioplasty Exclusion criteria: supra‐inguinal disease, pre‐existing medical condition that makes revascularization inappropriate |
|
| Interventions | Treatment: infra‐inguinal bypass surgery Control: balloon angioplasty Duration: all participants were monitored for 3 years and more than half for > 5 years | |
| Outcomes | Primary outcomes: time to amputation of trial leg or death (whichever occurred first) Secondary outcomes: all‐cause mortality, 30‐day morbidity and mortality, reinterventions, health‐related quality of life, use of hospital resources | |
| Notes | This trial was funded by the UK NHS Research and Development Health Technology Assessment programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation sequences were generated by a computerised random‐number generator in the University of Edinburgh Medical Statistics Unit (Edinburgh, UK) and supplied to the coordinating centre in identical, sealed envelopes”. Comment: Appropriate method of randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation, as described above. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcomes unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No mention that the personnel measuring the outcome were blinded. However, the primary outcome measures were objective and unlikely to be affected. The final data were checked using NHS sources, hospital and GP records. Endpoint data for death and amputation were also collected via the national audit mechanism. Postoperative complications and reinterventions data were collected by 4 dedicated research nurses during the first year, travelling to all centres; thereafter, yearly by the trial coordinator visiting each centre. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Apart from 4 participants lost to follow up, there was a three‐year complete follow‐up for all participants. |
| Selective reporting (reporting bias) | Low risk | The study predefined primary and secondary outcomes were reported. |
| Other bias | Low risk | No other source of bias was identified. |
Gaspard 1972.
| Methods | Study design: randomised, not blinded
Method of randomisation: states random, method unknown Losses to follow up: no losses to follow up |
|
| Participants | Country: USA Number of participants: 43 (29 participants with IC and 14 with rest pain) Sex: male and female Age: mean age 56.5 years Inclusion criteria: individuals requiring aorto‐ilio‐femoral reconstruction Exclusion criteria: individuals with disease confined to the aorto‐iliac segment | |
| Interventions | Treatment: bypass graft using a Dacron bifurcated prosthesis, either woven or knitted Control: thromboendarterectomy Duration: postoperative period only | |
| Outcomes | Mortality Treatment failure Complications of surgery/intervention | |
| Notes | No source of funding/sponsorship was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “randomly assigned”. Comment: Insufficient information about the random generation process. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Probably no blinding because of the nature of the operative procedure, but the review authors judged that the outcome and the outcome measurement were not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data. |
| Selective reporting (reporting bias) | High risk | Outcome measures not clearly defined and the study failed to include key outcomes. |
| Other bias | Low risk | No other source of bias was identified. |
Guarnera 1994.
| Methods | Study design: states random, method unknown, not blinded Losses to follow up: no losses to follow up | |
| Participants | Country: Italy Number of participants: 12 Sex: male and female Age: mean age 71 years Inclusion criteria: CLI (Fontaine stage IV), plus multi‐level distal lesions on angiogram Exclusion criteria: individuals with diabetes and compromising medical conditions | |
| Interventions | Treatment: distal surgical bypass, ideally using a vein (prosthetic graft was used if this was not possible) Control: spinal cord stimulation, with the electrode introduced under local anaesthesia; treatment was generally continued for a period of 7 to 14 days Duration: 12 months | |
| Outcomes | Amputation rate Subjective improvement: good/fair (complete or evident pain relief and trophic lesion healing); or poor | |
| Notes | No source of funding/sponsorship was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation method. Allocation by randomisation only mentioned in 1 sentence in abstract but not in main text. |
| Allocation concealment (selection bias) | Unclear risk | See above. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding performed, but lack of blinding unlikely to influence outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding described, but the review authors judged that the outcome and the outcome measurement were not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Low risk | No other source of bias was identified. |
Holm 1991.
| Methods | Study design: randomised, not blinded
Method of randomisation: using a method of stratification to balance 4 factors: symptoms (IC vs CLI); diabetes (present vs absent); age (greater vs less than 62 years); and level of disease (above vs below inguinal ligament) One randomised participant died prior to planned PTA |
|
| Participants | Country: Sweden Number of participants: 102 Sex: male and female Age: mean age 70 years Inclusion criteria: individuals with CLI or severe IC who had not benefited from exercise, and with an occlusion or significant stenosis (> 75% narrowing of lumen) 6 cm or shorter in the common iliac, external iliac, femoral, or popliteal artery Exclusion criteria: any concomitant disease contraindicating surgery, a mental disorder indicating treatment or follow‐up could not be performed properly, or unwilling to give consent | |
| Interventions | Treatment: bypass graft, using a synthetic graft above the inguinal ligament and a vein graft below the inguinal ligament, or endarterectomy Control: PTA Duration: 1 year | |
| Outcomes | Mortality Treatment failure Complications of surgery/intervention Primary and secondary patency ABI Amputation rate | |
| Notes | The study was supported by grants from the Swedish Medical Research Council. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "A sequential treatment assignment, with balancing for prognostic factors according to Pocock and Simon, was performed to ensure that the two treatment groups should be comparable". Comment: Not appropriate method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding because of the nature of surgical/interventional treatment, but the outcome and the outcome measurement were not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Probably no blinding of outcome assessment. However, there was a set protocol for follow‐up assessment, reducing the risk of differential behaviours by the assessors and, therefore, the outcome measures are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Low risk | Most expected outcomes are reported. |
| Other bias | Low risk | No other source of bias was identified. |
Lepantalo 2009.
| Methods | Study design: randomised controlled multicenter study, no statement of blinding Method of randomisation: closed envelopes Power calculation performed: no Losses to follow up: 13 participants Intention‐to‐treat analysis: yes |
|
| Participants | Country: Finland Setting: 15 vascular centres in Scandinavia Number of participants: 44 Age: mean age in the endovascular group 64 years (range 48 to 79), mean age in the bypass group 66 years (range 53 to 80) Sex: male and female Inclusion criteria: SFA occlusion ranging from 5 to 25 cm in length, adjacent inflow and outflow segments close to normal, vessel diameter between 4.8 and 6.5 mm, at least one patent distal run off vessel, at least 1 cm of healthy SFA below and above the lesion Exclusion criteria: allergy or contraindications to contrast medium, adjuvant antithrombotic medication or bleeding diathesis, presence of one or several previously placed endografts or stents in the SFA segment, other planned endovascular therapy of the same segment, evolving malignancy and any other illness posing an immediate threat to life, life‐expectancy less than 2 years, noncompliance, participation in another vascular clinical study less than 30 days prior to inclusion |
|
| Interventions | Treatment: femoro‐popliteal bypass, preferably with a 6 mm non‐coated expanded PTFE graft, with inflow from the common femoral artery to the popliteal artery above the knee Control: Viabahn endograft in the SFA Duration: the study was terminated when recruiting had continued for 42 months and one year follow‐up data were available for 28 participants |
|
| Outcomes | Primary outcomes: primary patency Secondary outcomes: functional success, complications, costs |
|
| Notes | No source of funding/sponsorship was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation was stratified by the centre and by the severity of ischaemia". Comment: Unclear whether an appropriate method of randomisation was used. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomisation was made using closed envelopes". Comment: It remains unclear whether envelopes were sequentially numbered. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study report did not state blinding. Both participants and personnel were probably not blinded. However, the lack of blinding was not likely to influence the outcome and the outcome measures. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “Completion angiogram was mandatory to reveal possible technical errors and for verification of the morphological result in both groups” and “Patency had to be demonstrated by duplex ultrasound or other imaging modalities at every control visit”. Comment: Blinding of outcome assessment not stated, but most probably no blinding existed. The assessment of the main outcomes is by imaging and is therefore unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgment. |
| Selective reporting (reporting bias) | High risk | Costs were prespecified as a secondary outcome parameter, but no outcomes reported. |
| Other bias | High risk | Terminated prematurely because of recruitment issues and lack of benefit of endoluminal stent‐graft placement in the SFA over bypass surgery. Did not provide specific reintervention criteria to maintain primary patency and is, therefore, subject to reintervention bias. |
Lundgren 1989.
| Methods | Study design: randomised Method of randomisation: randomised, balanced by age, sex and presence of diabetes; not blinded Losses to follow up: surgery not performed in 2 participants; exercise not carried out in 4 participants | |
| Participants | Country: Sweden Number of participants: 75 Age: 40 to 80 years Sex: male and female Inclusion criteria: IC > 6 months; MWD < 600 m; BP in first toe > 30 mm Hg Exclusion criteria: rest pain or ulcer | |
| Interventions | Treatment: surgical intervention, including thromboendarterectomy, bypass with synthetic y‐graft, saphenous vein or PTFE graft Control: dynamic leg exercises beyond appearance of pain, supervised by physiotherapist, 30 min sessions 3 times each week; encouraged to exercise at leisure Duration: 12 to 15 months | |
| Outcomes | Treadmill test: pain‐free and MWD (4 km/h at 0 degree slope to maximum of 1000 m) ABI | |
| Notes | A third group of 25 participants received surgery combined with an exercise regimen; these results were discussed but not included in formal meta‐analysis. No source of funding/sponsorship was reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The patients were randomized to one of the three treatment groups with help of an algorithm described by Pocock and Simon, accounting for the distribution of sex, age, and diabetes". Comment: Not appropriate randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No information provided. Most probably blinding was not obtained given the nature of the study. However, the lack of blinding was not likely to influence the outcome and the outcome measures. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information provided. Probably no blinding existed, but a set protocol for assessment of follow‐up outcomes was defined, which minimises the risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients never underwent operations, and complete data at the follow‐up were not available for four patients of Op group. Five patients of the Op + Train group were not treated according to the protocol, and complete follow‐up data were not available for three of these patients. Four patients of the Train group never started their treatment according to the protocol, and follow‐up data for these patients are incomplete." Comment: Missing outcome data balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Unclear risk | A potential risk of bias in relation to participant compliance with exercise treatment, but insufficient evidence that this problem would introduce bias was available. |
McQuade 2010.
| Methods | Study design: single‐centre randomised trial, no statement of blinding Method of randomisation: not stated Power calculation performed: 80% power, alpha < 0.05 Losses to follow‐up: 6 participants in the endovascular group and 15 participants in the surgical group Intention‐to‐treat analysis: not stated |
|
| Participants | Country: USA Setting: single private institution Number of participants: 86 (100 limbs randomised) Age: mean age in the endovascular group 71.8 years (SD 9.9), mean age in the surgical group 66.9 years (SD 10.7) Sex: males and females Inclusion criteria: atherosclerotic stenotic or occlusive lesions of the SFA with no significant aorto‐iliac disease, patent infra‐popliteal segment, at least 1 vessel run‐off to the ankle, suitability for surgical treatment Exclusion criteria: not stated |
|
| Interventions | Treatment: surgical femoro‐popliteal bypass above the knee with synthetic graft Control: percutaneous endovascular treatment of the SFA with stent‐graft Duration: 48‐month follow‐up was available in 64% of limbs in the endovascular group and 52% of limbs in the surgical group |
|
| Outcomes | Primary patency Secondary patency Amputation rate |
|
| Notes | The study was funded by grants provided by W.L. Gore & Associates, Flagstaff, Arizona. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Prospective randomised study". Comment: No information about the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Insufficient information to permit judgement, but most probably absence of blinding given the nature of interventions. The review authors judged that the outcome and the outcome measurement were not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "follow‐up at 3, 6, 9, and 12 months included clinical exam, color flow Doppler ultrasound imaging, and determination of the ABI". Comment: Blinding of outcome assessment most probably was not obtained, but objective assessment measures were used, which minimises the risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Six patients (12%) were lost to follow‐up" in the stent group; "Fifteen (30%) patients were lost to follow up" in the bypass group. Comment: missing outcome data not balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | High risk | Insufficient information available concerning whether amputation and mortality were prespecified outcomes. |
| Other bias | Low risk | No other source of bias was identified. |
REVAS Trial.
| Methods | Study design: randomised, surgeons blinded on the sequence of the randomisation list Method of randomisation: central telephone number using sealed envelopes in a permuted‐block sequence Power calculation performed: 80% power, alpha <0.05 Losses to follow up: 3 participants Intention‐to‐treat analysis: yes |
|
| Participants | Country: Netherlands Setting: 1 university medical centre and 3 major teaching hospitals Number of participants: 116 Age: mean age of the remote endarterectomy group 68 years (range 50 to 84), mean age of the bypass group 68 years (range 44 to 86) Sex: males and females Inclusion criteria: severe IC, CLI, or tissue loss (Rutherford category 3 to 5), TASC C or D lesion of the SFA, patent popliteal P1 segment with at least 1 crural run off vessel, chronic complaints originating from atherosclerotic disease Exclusion criteria: previous surgery of PTA with additional stent placement of the target SFA, SFA diameter < 4 mm |
|
| Interventions | Treatment: femoro‐popliteal bypass above the knee with long saphenous vein or PTFE graft Control: remote endarterectomy of the SFA Duration: median follow‐up 37 months |
|
| Outcomes | Primary outcomes: primary patency Secondary outcomes: assisted primary patency, secondary patency, limb salvage, operation time, postoperative complications, hospital stay |
|
| Notes | It was stated that "this study was not supported financially by a medical device company or the pharmaceutical industry". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done at a central telephone number, using sealed envelopes, in a permuted‐block sequence". Comment: appropriate method of randomisation. |
| Allocation concealment (selection bias) | Low risk | Appropriate method of allocation concealment (see quote above). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Insufficient information to make a judgment, but the outcomes are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No mention, but objective measures were used for main outcome assessment (patency) and therefore not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three patients (4.2%) were lost to follow‐up". Comment: The proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | The published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | No other source of bias was identified. |
STILE Trial.
| Methods | Study design: randomised, not blinded Method of randomisation: randomised by telephone and stratified for native artery occlusions, bypass graft occlusions, or unreconstructable vascular disease; randomised into three groups to test two different methods of thrombolysis; analysed as intention‐to‐treat | |
| Participants | Country: USA and Canada.
Number of participants: 237
Sex: males and females Age: median age 66 years (80 participants with IC, 83 with rest pain and 74 with ischaemic necrosis) Inclusion criteria: individuals aged 18 to 90 years with signs or symptoms of worsening limb ischaemia within the past 6 months requiring intervention, and those with angiographically documented nonembolic arterial occlusion Exclusion criteria: individuals with acute embolism, active internal bleeding, or a history of cerebrovascular accident, intracranial bleed, transient ischaemic attack, recent intracranial or intraspinal surgery or trauma, central nervous system neoplasm, arterio‐venous malformation or aneurysm, severe bleeding diathesis, uncontrolled hypertension, suspected pregnancy, recent eye surgery, contraindication to surgery |
|
| Interventions | Treatment: optimal surgical revascularization as determined by attending surgeon and documented before randomisation (86% had a bypass graft); autogenous material was used for infra‐inguinal occlusions, and prosthetics grafts for aorto‐iliac or ilio‐femoral occlusions Control: thrombolysis using either rt‐PA at 0.05 mg/kg/hr for up to 12 hours (56%); or urokinase as a bolus of 250,000 units followed by 4000 units/min for 4 hours, then 2000 units/min for 36 hours (44%) Duration: 1 year | |
| Outcomes | Mortality Treatment failure Complications of surgery/intervention Primary patency Amputation rate | |
| Notes | This trial has been included with the following reservations: participants with native artery disease were a subset of a larger study that included individuals with graft occlusion (total number 393), although the stratified randomisation should have balanced native arterial disease with graft disease; only 86% had bypass grafts, and presumably the remainder underwent thrombectomy or thromboendarterectomy; and 20% of the participants had acute (less than 14 days) ischaemia. This study was supported by a research grant from Genentech, Inc., South San Francisco, California. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information of method of randomisation provided. |
| Allocation concealment (selection bias) | Low risk | Quote: "The investigators and study coordinators telephoned a 24‐hour/day, 7‐day/week randomization center". Comment: Appropriate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No information provided, but most probably no blinding obtained given the nature of interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information, but probably no blinding existed. However, the outcome and the outcome measurement were not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A detailed case report form was completed for each patient. The accuracy of the case report forms were verified by study monitors, who checked them with the patients' medical records. The case report forms then were forwarded to the data coordinating center (Collaborative Studies Coordinating Center, Department of Biostatistics, University of North Carolina, Chapel Hill, NC) for evaluation and the generation of queries about missing or inconsistent data and subsequent data entry". Comment: Transparent process of dealing with missing or incomplete data. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all prespecified outcomes reported. |
| Other bias | Unclear risk | The 237 participants with native artery disease were a subset of a larger trial of 393 participant, which included both native artery and graft disease which may have biased the results. |
Veterans Study.
| Methods | Study design: randomised, not blinded Method of randomisation: using a method of stratification to balance two factors: symptoms (claudication vs rest pain); and level of disease requiring treatment (iliac vs femoro‐distal) Exclusions postrandomisation: eight randomised participants in the surgery group were not treated because of an intervening event, and 2 randomised to PTA refused to give consent and were not treated. In addition, eight treated participants withdrew Losses to follow up: 20 | |
| Participants | Country: USA Number of participants: 263 Sex: male Age: mean age 61.5 years (191 participants with IC and 72 CLI; 163 had iliac disease and 100 femoro‐popliteal disease) Inclusion criteria: 1) angiographically demonstrated significant stenosis (≥ 80%) or occlusion, < 10 cm in length in the iliac, superficial femoral, or popliteal arteries; 2) ABI of affected leg 0.9 or less at rest; 3) symptoms in the affected leg of either claudication (less than 2 blocks and preventing daily activities), rest pain, or impending gangrene; 4) considered suitable for treatment by both the vascular surgeon and the radiologist Exclusion criteria: contraindication to a short course of heparin, life expectancy of less than 3 years, medical contraindications to major surgery or unwilling to participate | |
| Interventions | Treatment: bypass surgery (details of the technical performance of the intervention were left to the discretion of the individual surgeon) Control: PTA Duration: median 4.1 years (range 2 to 6 years) | |
| Outcomes | Mortality Treatment failure Primary and secondary patency ABI Amputation rate Subjective measure (Sickness Impact Profile scores) | |
| Notes | This study was supported by the Cooperative Studies Program of the Medical Research Service, Department of Veteran Affairs Central Office, Washington, DC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After consent was obtained, the participating hospital contacted the study biostatistician by phone for treatment assignment. Randomization was stratified by center and for each of four disease categories". Comment: Unclear method for randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation (see quote above). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Most probably no blinding, but the nature of interventions rendered blinding not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment, however outcome measures were not likely to be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | No other source of bias was identified. |
ABI: ankle brachial index BP: blood pressure CLI: critical limb ischaemia GP: General Practitioner IC: intermittent claudication m: meter MWD: maximal walking distance NHS: National Health Service PTA: percutaneous transluminal angioplasty PTFE: polytetrafluoroethylene rt‐PT: recombinant tissue plasminogen activator SD: standard deviation SFA: superficial femoral artery SVS/ISCVS: Society for Vascular Surgery/International Society for Cardiovascular Surgery TASC: Trans‐Atlantic Inter‐Society Consensus Document on Management of Peripheral Arterial Disease
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ABC 2010 | The ABC trial is a RCT comparing bypass surgery with angioplasty for the treatment of individuals suffering from IC caused by complex atherosclerotic lesions of the superficial femoral artery. As of 31/01/2012, this study was stopped because of participant recruitment issues. The principal investigator responded to our request of study results confirming that no results have ever been published or presented. The study protocol that was published in the European Journal of Vascular and Endovascular Surgery was withdrawn at the request of the study author(s) and/or editor. |
| CLEVER study | This is a RCT evaluating outcomes of four treatment strategies for individuals with aorto‐iliac arterial disease manifesting with IC: (1) optimal medical care (claudication pharmacotherapy); (2) primary stent placement; (3) supervised exercise rehabilitation; and (4) combined stenting with supervised exercise rehabilitation. It was excluded because there was no bypass surgery group in the treatment arms. |
| de Donato 2002 | This is a RCT comparing minimally invasive direct aortic surgery with conventional transperitoneal laparotomy for aorto‐bifemoral bypass. It has, therefore, been excluded from this review because bypass was included in both arms of the trial and, therefore, there was no true control group. |
| Devine 2004 | This is a RCT comparing heparin‐bonded Dacron prostheses or PTFE graft for use in femoro‐distal bypass. It has, therefore, been excluded from this review because bypass was included in both arms of the trial and, therefore, there was no true control group. |
| Djoric 2011 | This is a RCT that compared distal venous arterialisation with conservative management using antiplatelet agents in individuals with CLI. This trial was excluded because there was no treatment group of individuals undergoing bypass surgery. |
| Gavrilenko 2008 | This study evaluated results of the combination of vascular reconstructive surgery with gene‐engineering technologies in individuals with chronic lower limb ischaemia and was, therefore, excluded. |
| Gelin 2001 | This is a RCT of individuals with IC. The objective of the study was to compare the effect of surgery, exercise, and observation on maximum exercise power. Participant were randomised to control, supervised exercise, or intervention group. Participants in the intervention group underwent an endovascular or open surgical procedure, but it is not specified which open surgical procedure(s) were used in the intervention group. |
| Hamsho 1999 | This is a RCT of femoro‐distal bypass using PTFE grafts with and without the addition of adjuvant arterio‐venous fistula. It has, therefore, been excluded from this review because bypass was included in both arms of the trial and, therefore, there was no true control group. |
| IRONIC Trial | This RCT compares invasive treatment (including endovascular and/or open revascularization) with best medical treatment in individuals with significant aorto‐iliac and/or femoro‐popliteal disease suffering from IC. This trial was excluded from our review and analysis because participants were randomised to any invasive treatment (surgical or endovascular) rather than bypass surgery. |
| Jensen 2007 | This multicentre RCT had all 427 participants receiving bypass in both arms of the trial and was primarily a study comparing graft material. |
| Linhart 1991 | This study was not clearly described, but appears to be a follow‐up study of two different methods of treatment. There is no mention of randomisation and the two different treatment options (surgery and medical therapy) were not compared in the analysis. Further information was sought from the study authors, but no reply was received. |
| Matyas 2008 | This RCT was excluded because it compared variants of a surgical technique (bioresorbable paclitaxel‐eluting wrap implanted with a synthetic vascular graft versus the graft implanted alone) for femoro‐popliteal bypass. |
| McCollum 2003 | Both arms of the trial underwent bypass, so there was no true control group. Furthermore, the study sought to compare types of graft material. |
| Mohammadi 2007 | This 3‐year trial on 103 above‐knee femoro‐popliteal bypass graft operations in 85 individuals compared types of grafts. Additionally, all participants received bypass surgery, so there was no true control group. |
| Nordanstig 2011 | This is a RCT comparing invasive (endovascular or surgical) and non‐invasive treatment for IC. This trial was excluded because enrolled participants were randomised to any invasive treatment (including surgical or endovascular) rather than bypass surgery. |
| Panneton 2004 | This is a RCT comparing a pre‐cuffed PTFE graft with a vein‐cuffed PTFE graft for infrageniculate arterial bypass. It has, therefore, been excluded from this review because bypass was included in both arms of the trial and, therefore, there was no true control group. |
| PROOF 2007 | This is a RCT comparing bypass surgery with plaque excision (Silverhawk Plaque Excision) for treatment of CLI. This trial has been terminated and no published results were found. The principal investigator was contacted to see whether any published or unpublished results could be obtained, but no response was received. |
| Stanisic 2009 | This study evaluated laparoscopic techniques in individuals with CLI secondary to aorto‐iliac occlusive disease. It was excluded as it compares variants of a surgical technique. |
| Taft 2004 | This is a RCT of individuals with IC who were randomised to control, supervised exercise, or intervention group. The objective of the study was to identify predictors of treatment outcome. Participants in the intervention group underwent an endovascular or open surgical procedure, but it was not specified which open surgical procedure(s) were used in the intervention group. |
| TECCO Trial | This RCT compared endovascular with surgical reconstruction of common femoral artery disease. It was excluded from the present review because a minority only (18%) of the participant in the surgical treatment arm underwent bypass surgery for chronic lower limb ischaemia. |
| Tiek 2009 | This is a nonrandomised study assessing the role of thrombolysis in acute infra‐inguinal bypass occlusion and was, therefore, excluded from the present review and analysis. |
| Tiek 2012 | This is a RCT comparing a laparoscopic with an open surgical approach for the treatment of aorto‐iliac occlusive disease. It was excluded because it compared variants of a bypass surgical technique. |
| Vukobratov 2006 | This study on 118 participants compared two methods of bypass. As all participants received bypass, there was no true control group. |
CLI: critical limb ischaemia IC: intermittent claudication PTFE: polytetrafluoroethylene RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
BASIL 2.
| Trial name or title | BASIL‐2: Bypass versus Angioplasty in Severe Ischaemia of the Leg‐2 |
| Methods | Randomised multicentre pragmatic two‐arm open trial |
| Participants | This study aims to recruit 600 adult individuals with severe limb ischaemia due to infra‐geniculate arterial disease from the participating hospitals |
| Interventions | Participants will be randomly allocated to receive either vein bypass surgery or the best endovascular treatment |
| Outcomes | Amputation‐free survival, defined as the time to major limb amputation of the index limb or death from any cause |
| Starting date | The study runs from 30/05/2014 and the anticipated end date is 15/10/2019 |
| Contact information | Prof Andrew Bradbury, Heart of England NHS Foundation Trust, Netherwood House, Lode Lane, Solihull Hospital, Solihull, B91 2JL, UK. Tel: +44 121 415 8011, fax: +44 121 415 9135, email: researchgovernance@contacts.bham.ac.uk |
| Notes | Sources of funding: NIHR Health Technology Assessment Programme (UK); ref. 12/35/45 |
BEST‐CLI trial.
| Trial name or title | Best Endovascular Versus Best Surgical Therapy in Patients With Critical Limb Ischemia |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Individuals with PAD presenting with CLI |
| Interventions | Open surgical revascularization versus endovascular revascularization |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | August 2014 |
| Contact information | Angela S Knox, MS, PMP aknox@neriscience.com |
| Notes |
NCT02060630 Sponsors and Collaborators: New England Research Institutes; Brigham and Women's Hospital; Massachusetts General Hospital; Boston Medical Center; National Heart, Lung, and Blood Institute (NHLBI) |
FINNPTX.
| Trial name or title | Paclitaxel Eluting Stent in Long Superficial Femoral Artery Obstruction: a Prospective, Randomized Comparison With Bypass Surgery Using PTFE Graft in a Finnish Multicenter Study |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Individuals suffering from severe lifestyle limiting IC indicating revascularisation as well as individuals suffering from CLI and having de novo SFA obstruction with a total length ranging from 7 to 20 cm |
| Interventions | Femoro‐popliteal artery bypass operation by using synthetic PTFE graft versus placement of paclitaxel‐eluting stent in long SFA obstruction |
| Outcomes | Primary patency, amputation‐free survival |
| Starting date | October 2011 |
| Contact information | Hannu Manninen hannu.manninen@kuh.fi, Marja‐Liisa Sutinen marja‐liisa.sutinen@kuh.fi |
| Notes | NCT0145722 Sponsors and Collaborators: Kuopio University Hospital; Helsinki University Central Hospital; Turku University Hospital; Tampere University Hospital; Oulu University Hospital; Finnish Society of Interventional Radiology; North Karelia Central Hospital; Paijat‐Hame Hospital District |
ISRCTN18315574.
| Trial name or title | Minimal invasive balloon expansion versus bypass operation to treat complicated occlusions and stenoses of the femoral and popliteal arteries |
| Methods | Study design: prospective randomised parallel trial Primary study design: interventional Secondary study design: randomised controlled trial Trial setting: hospitals Trial type: treatment |
| Participants | Individuals with PAD of the lower extremity (disabling claudication or CLI) |
| Interventions | Bypass operation with ipsilateral greater saphenous vein Percutaneous transluminal angioplasty with stent placement |
| Outcomes | Primary outcome measures: technical success, primary and secondary patency Secondary outcome measures: local complications, systemic complications, limb salvage rate, survival rate, costs |
| Starting date | March 2016 |
| Contact information | Dr Klaus Linni Paracelsus Medical University Müllner Hauptstraße 48 Salzburg 5020 Austria +43 662 4482 53201 k.linni@salk.at |
| Notes | Sponsor: Paracelsus Medical University (PMU) |
NCT01171703.
| Trial name or title | Optimized strategy for diabetic patients with critical limb ischaemia: a multi‐center, randomised controlled trial and registration study (Part 1) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Diabetic individuals with CLI |
| Interventions | Femoro‐popliteal bypass above the knee with PTFE graft Stenting of the SFA |
| Outcomes | Primary outcome measures: occlusion of the stent or bypass Secondary outcome measures: mortality, rate of limb salvage, procedural complications, quality of life assessment, re‐stenosis |
| Starting date | November 2010 |
| Contact information | Liu Chang‐wei, Vascular Surgery, Peking Union Medical College Hospital, Beijing, China, 100032 |
| Notes |
NCT01171703 Sponsors and Collaborators: Peking Union Medical College Hospital; Beijing Tongren Hospital; Xuanwu Hospital; Beijing |
NCT02580084.
| Trial name or title | Prospective randomized clinical study of the aorta‐femoral bypass and hybrid intervention and the iliac arteries with stenting and plasty of the common femoral artery effectiveness in patients with the iliac segment and femoral artery occlusive disease (TASC C, D) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: single group assignment Masking: single blind (subject) Primary purpose: treatment |
| Participants | Individuals with chronic lower limb ischaemia (Rutherford classification 4‐6) |
| Interventions | Aorto‐femoral bypass Hybrid intervention (common femoral endarterectomy and iliac balloon angioplasty or stenting) |
| Outcomes | Primary outcome measures: 30‐day mortality Secondary outcome measures: primary patency, secondary patency, preservation of the operated limb, lymphorrhoea in the area of intervention, infection/septic complications, haematoma, myocardial infarction |
| Starting date | August 2015 |
| Contact information | Vyacheslav Mitrofanov, v_mitrofanov@meshalkin.ru |
| Notes |
NCT02580084 Sponsors and Collaborators: Meshalkin Research Institute of Pathology of Circulation |
ROBUST.
| Trial name or title | Revascularization with Open Bypass versUs angioplasty and STenting of the lower extremity trial (ROBUST) |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Symptomatic individuals with IC or CLI; individuals with TASC II B and C lesions of the SFA |
| Interventions | Open bypass surgery with autogenous vein or PTFE graft versus angioplasty and stenting of the SFA with nitinol stent |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | July 2009 |
| Contact information | Mahmoud B Malas, M.D., MHS: bmalas1@jhmi.edu; Umair M Qazi, M.D., MPH: uqazi1@jhmi.edu |
| Notes |
NCT01602159 Sponsors and Collaborators: Johns Hopkins University |
SUPERB.
| Trial name or title | Heparin‐bonded edoluminal versus surgical femoropopliteal bypass; a multicentre randomized controlled trial |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Individuals with symptomatic PAD of the SFA with a Rutherford category 3 to 6 |
| Interventions | Heparin‐bonded ePTFE endoluminal femoro‐popliteal bypass versus surgical femoro‐popliteal bypass |
| Outcomes | Primary outcome measures: primary (and assisted) patency; QoL Secondary outcome measures: secondary patency; complications; clinical improvement; reintervention; target lesion revascularization |
| Starting date | October 2010 |
| Contact information | MMPJ Reijnen ‐ Tel.:+31260058888 ext 3154, email: mreijnen@alysis.nl MMA Lensvelt ‐ Tel.: +31641266258, email: superbtrial@gmail.com |
| Notes |
NCT01220245 Sponsors and Collaborators: Rijnstate Hospital |
ZILVERPASS.
| Trial name or title | The Cook ZILVER PTX Drug‐eluting Stent Versus ByPASS surgery of femoropopliteal TASC C & D lesions (ZILVERPASS) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Individuals presenting with lifestyle‐limiting claudication, rest pain, or minor tissue loss (Rutherford classification from 2 to 5) |
| Interventions | Zilver PTX paclitaxel‐eluting stent versus prosthetic bypass for TASC C and D femoro‐popliteal lesions |
| Outcomes | Primary outcome measures: primary patency at 12 months Secondary outcome measures: proportion of subjects who experience device malfunction or serious device‐related or serious adverse events within 30 days postprocedure Other outcome measures: technical success; infection rate/haematoma at puncture site or at incision sites requiring intervention; haemodynamic primary patency rate; primary assisted patency rate; secondary patency rate; target lesion revascularization; clinical success; serious adverse events |
| Starting date | August 2014 |
| Contact information | Bavo Van Puyvelde ‐ Tel.: +32 52 25 28 22, email: office@fmrp.be |
| Notes |
NCT01952457 Sponsors and Collaborators: Flanders Medical Research Program |
CLI: critical limb ischaemia IC: intermittent claudication NIHR: National Institute for Health Research PAD: peripheral arterial disease PTFE: polytetrafluoroethylene QoL: quality of life SFA: superficial femoral artery TASC: Trans‐Atlantic Inter‐Society Consensus Document on Management of Peripheral Arterial Disease
Differences between protocol and review
For this update, a Table 1 has been added. Methodological quality has been assessed using the risk of bias tool according to Higgins 2011. This is in keeping with current Cochrane policy. Additional outcomes for the main comparisons to those included in the initial review were defined. Technical success, clinical improvement, vessel or graft patency, and reinterventions are additional outcomes to those included in the initial review. The selected outcome parameters were thought to provide valuable additional information related to the comparative effectiveness of bypass surgery for the treatment of chronic lower limb ischaemia.
Contributions of authors
For this update, George A Antoniou (GAA) served as contact author. He selected trials with George S Georgiadis (GSG), and disagreements were resolved by and advice obtained from Franceso Torella (FT). Stavros A Antoniou (SAA) and GSG collected all available information from the selected studies and extracted data for analysis. Ragai R Makar (RRM) and Jonathan D Smout (JDS) assessed the methodological quality of the selected trials and discussed the results with GAA. GAA performed the statistical analyses, wrote the article, and had the overall responsibility of this work. All review authors critically reviewed and revised the article.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Governement Health Directorates, Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
GAA: none known GSG: none known SAA: received travel and accommodation expenses relating to the duties of the Journal and Publication Committee Members of the European Association for Endoscopic Surgery (EAES); travel, accommodation and congress participation expenses for the 21st International Congress of the EAES; travel and accommodation expenses for presentation of part of the Guidelines on the Closure of Abdominal Wall Incisions at the 36th International Congress of the European Hernia Society; travel and accommodation expenses for the development of the Guidelines on the Closure of Abdominal Wall Incisions RRM: none known JDS: received educational sponsorship (accommodation and course fee) from Cook Ltd for attendance at 2015 Leipzig Interventional Course (GORE Ltd). No personal payments were made. FT: received educational sponsorship (travel, accommodation and meeting‐related expenses) from Endologix Inc. This company produces medical devices. Such devices are not used for treatment of peripheral arterial disease and do not bear any direct relevance to the topic of this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
BAESIC study {published data only}
- Zaag ES, Legemate DA, Prins MH, Reekers JA, Jacobs MJ. Angioplasty or bypass for superficial femoral artery disease? A randomised controlled trial. European Journal of Vascular and Endovascular Surgery 2004;28(2):132‐7. [DOI] [PubMed] [Google Scholar]
BASIL study {published data only}
- Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366(9501):1925‐34. [DOI] [PubMed] [Google Scholar]
- Bell J, Papp L, Bradbury AW. Bypass or angioplasty for severe ischaemia of the leg: the BASIL trial. In: Greenhalgh RM, Powell JT, Mitchell AW editor(s). Vascular and endovascular opportunities. London: WB Saunders, 2000:485‐94. [Google Scholar]
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: A description of the severity and extent of disease using the Bollinger angiogram scoring method and the TransAtlantic Inter‐Society Consensus II classification. Journal of Vascular Surgery 2010;51(5 Supplement 1):32S‐42S. [DOI] [PubMed] [Google Scholar]
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: A survival prediction model to facilitate clinical decision making. Journal of Vascular Surgery 2010;51(5 Suppl 1):52S‐68S. [DOI] [PubMed] [Google Scholar]
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: An intention‐to‐treat analysis of amputation‐free and overall survival in patients randomized to a bypass surgery‐first or a balloon angioplasty‐first revascularization strategy. Journal of Vascular Surgery 2010;51(5 Supplement 1):5S‐17S. [DOI] [PubMed] [Google Scholar]
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Analysis of amputation free and overall survival by treatment received. Journal of Vascular Surgery 2010;51(5 Supplement 1):18S‐31S. [DOI] [PubMed] [Google Scholar]
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I. Erratum: Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) Trial: a survival prediction model to facilitate clinical decision making (Journal of Vascular Surgery (2010) 51:5 Suppl (52S‐68S)). Journal of Vascular Surgery 2010;52(6):1751. [DOI] [PubMed] [Google Scholar]
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al. Multicentre randomised controlled trial of the clinical and cost‐effectiveness of a bypass‐surgery‐first versus a balloon‐angioplasty‐first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technology Assessment 2010;14(14):1‐236. [DOI] [PubMed] [Google Scholar]
- Forbes JF, Adam DJ, Bell J, Fowkes FG, Gillespie I, Raab GM, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Health‐related quality of life outcomes, resource utilization, and cost‐effectiveness analysis. Journal of Vascular Surgery 2010;51(5 Supplement 1):43S‐51S. [DOI] [PubMed] [Google Scholar]
Gaspard 1972 {published data only}
- Gaspard DJ, Cohen JL, Gaspar MR. Aortoiliofemoral thromboendarterectomy vs bypass graft. Archives of Surgery 1972;105(6):898‐901. [DOI] [PubMed] [Google Scholar]
Guarnera 1994 {published data only}
- Guarnera G, Furgiuele S, Camilli S. Spinal cord electric stimulation vs femoro‐distal bypass in critical ischaemia of the legs. Preliminary results of a prospective randomised study [Elettrostimolazione midollare vs bypass femoro‐distale nell'ischemia critica arti inferiori. Risultati preliminari di uno prospettico randomizzato]. Minerva Cardioangiologica 1994;42(5):223‐7. [PubMed] [Google Scholar]
Holm 1991 {published data only}
- Holm J, Arfvidsson B, Jivegard L, Lundgren F, Lundholm K, Schersten T, et al. Chronic lower limb ischaemia. A prospective randomised controlled study comparing the 1‐year results of vascular surgery and percutaneous transluminal angioplasty (PTA). European Journal of Vascular Surgery 1991;5(5):517‐22. [DOI] [PubMed] [Google Scholar]
Lepantalo 2009 {published data only}
- Lepäntalo M, Laurila K, Roth WD, Rossi P, Lavonen J, Mäkinen K, et al. PTFE bypass or thrupass for superficial femoral artery occlusion? A randomised controlled trial. European Society for Vascular Surgery Annual Meeting; 2008 Sep 4‐7, Nice, France. 2008. [DOI] [PubMed]
- Lepäntalo M, Laurila K, Roth WD, Rossi P, Lavonen J, Mäkinen K, et al. Scandinavian Thrupass Study Group. PTFE bypass or thrupass for superficial femoral artery occlusion? A randomised controlled trial. European Journal of Vascular and Endovascular Surgery 2009;37:578‐84. [DOI] [PubMed] [Google Scholar]
Lundgren 1989 {published data only}
- Lundgren F, Dahllof AG, Lundholm K, Schersten T, Volkmann R. Intermittent claudication ‐ surgical reconstruction or physical training? A prospective randomized trial of treatment efficiency. Annals of Surgery 1989;209(3):346‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren F, Dahllof AG, Schersten T, Bylund‐Fellenius AC. Muscle enzyme adaptation in patients with peripheral arterial insufficiency: spontaneous adaptation, effect of different treatments and consequences on walking performance. Clinical Science 1989;77(5):485‐93. [DOI] [PubMed] [Google Scholar]
McQuade 2010 {published data only}
- Kedora J, Hohmann S, Garrett W, Munschaur C, Theune B, Gable D. Randomized comparison of percutaneous Viabahn stent grafts vs prosthetic femoral‐popliteal bypass in the treatment of superficial femoral arterial occlusive disease. Journal of Vascular Surgery 2007;45:10‐16. [DOI] [PubMed] [Google Scholar]
- McQuade K, Gable D, Hohman S, Pearl G, Theune B. Randomized comparison of ePTFE/nitinol self‐expanding stent graft vs prosthetic femoral‐popliteal bypass in the treatment of superficial femoral artery occlusive disease. Journal of Vascular Surgery 2009;49:109‐16. [DOI] [PubMed] [Google Scholar]
- McQuade K, Gable D, Pearl G, Theune B, Black S. Four‐year randomized prospective comparison of percutaneous ePTFE/nitinol self‐expanding stent graft versus prosthetic femoral‐popliteal bypass in the treatment of superficial femoral artery occlusive disease. Journal of Vascular Surgery 2010;52:584‐91. [DOI] [PubMed] [Google Scholar]
REVAS Trial {published data only}
- Gisbertz SS, Ramzan M, Tutein Nolthenius RP, Laan L, Overtoom TT, Moll FL, et al. Short‐term results of a randomized trial comparing remote endarterectomy and supragenicular bypass surgery for long occlusions of the superficial femoral artery (the REVAS Trial). European Journal of Vascular and Endovascular Surgery 2009;37(1):68‐76. [DOI] [PubMed] [Google Scholar]
- Gisbertz SS, Tutein Nolthenius RP, Borst GJ, Laan L, Overtoom TT, Moll FL, et al. Remote endarterectomy versus supragenicular bypass surgery for long occlusions of the superficial femoral artery: medium‐term results of a randomized controlled trial (the REVAS trial). Annals of Vascular Surgery 2010;24(8):1015‐23. [DOI] [PubMed] [Google Scholar]
STILE Trial {published data only}
- STILE Investigators. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Annals of Surgery 1994;220(3):251‐66, discussion 266‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver FA, Comerota AJ, Youngblood M, Froehlich J, Hosking JD, Papanicolaou G, STILE Investigators. Surgical revascularization versus thrombolysis for nonembolic lower extremity native artery occlusions: results of a prospective randomized trial. The STILE Investigators. Surgery versus Thrombolysis for Ischemia of the Lower Extremity. Journal of Vascular Surgery 1996;24(4):513‐23. [DOI] [PubMed] [Google Scholar]
Veterans Study {published data only}
- Bergan JJ, Wilson SE, Wolf G, Deupree RH. Unexpected, late cardiovascular effects of surgery for peripheral artery disease. Archives of Surgery 1992;127(9):1119‐23, discussion 1123‐4. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Wolf GL, Cross AP. Percutaneous transluminal angioplasty versus operation for peripheral arteriosclerosis. Journal of Vascular Surgery 1989;9(1):1‐9. [PubMed] [Google Scholar]
- Wolf GL, Wilson SE, Cross AP, Deupree RH, Stason WB. Surgery or balloon angioplasty for peripheral vascular disease: a randomised clinical trial. Journal of Vascular and Interventional Radiology 1993;4(5):639‐48. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ABC 2010 {published data only}
- NCT01177033. Angioplasty or Bypass surgery in intermittent Claudication (ABC Trial). http://clinicaltrials.gov/show/NCT01177033 (accessed 24 September 2014).
CLEVER study {published data only}
- Murphy TP, Hirsch AT, Cutlip DE, Regensteiner JG, Comerota AJ, Mohler E, et al. Claudication: Exercise Vs Endoluminal Revascularization (CLEVER) study update. Journal of Vascular Surgery 2009;50:942‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TP, Hirsch AT, Ricotta JJ, Cutlip DE, Mohler E, Regensteiner JG, et al. The Claudication: Exercise Vs. Endoluminal Revascularization (CLEVER) study: rationale and methods. Journal of Vascular Surgery 2008;47(6):1356‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TPC, Massaro MR, Jaff S, Collins ME. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: Six‐month outcomes from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study. Circulation 2012;125(1):130‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Donato 2002 {published data only}
- Donato G, Weber G, Donato G. Minimally invasive or conventional aorto‐bifemoral by‐pass. A randomised study. European Journal of Vascular and Endovascular Surgery 2002;24(6):485‐91. [DOI] [PubMed] [Google Scholar]
Devine 2004 {published data only}
- Devine C, McCollum C, North West Femoro‐Popliteal Trial Participants. Heparin‐bonded Dacron or polytetrafluorethylene for femoropopliteal bypass: five‐year results of a prospective randomized multicenter clinical trial. Journal of Vascular Surgery 2004;40(5):924‐31. [DOI] [PubMed] [Google Scholar]
Djoric 2011 {published data only}
- Djoric P. Early individual experience with distal venous arterialization as a lower limb salvage procedure. American Surgeon 2011;77(6):726‐30. [PubMed] [Google Scholar]
Gavrilenko 2008 {published data only}
- Gavrilenko AV, Voronov DA, Konstantinov BA, Bochkov NP. Combination of reconstructive vascular operations with gene‐engineering technologies of angiogenesis stimulation: a present‐day policy aimed at improving the remote results of treating patients with lower limb chronic ischaemia. [Russian]. Angiologiia i Sosudistaia Khirurgiia/Angiology & Vascular Surgery 2008;14(4):49‐53. [PubMed] [Google Scholar]
Gelin 2001 {published data only}
- Gelin J, Jivegard L, Taft C, Karlsson J, Sullivan M, Dahllöf AG, et al. Treatment efficacy of intermittent claudication by surgical intervention, supervised physical exercise training compared to no treatment in unselected randomised patients I: one year results of functional and physiological improvements. European Journal of Vascular and Endovascular Surgery 2001;22(2):107‐13. [DOI] [PubMed] [Google Scholar]
Hamsho 1999 {published data only}
- Hamsho A, Nott D, Harris PL. Prospective randomised trial of distal arteriovenous fistula as an adjunct to femoro‐infrapopliteal PTFE bypass. European Journal of Vascular and Endovascular Surgery 1999;17(3):197‐201. [DOI] [PubMed] [Google Scholar]
IRONIC Trial {published data only}
- NCT01219842. Invasive revascularization or not in intermittent claudication ‐ a randomised controlled trial IRONIC. http://clinicaltrials.gov/show/NCT01219842 (accessed 24 September 2014).
Jensen 2007 {published data only}
- Jensen LP, Lepantalo M, Fossdal JE, Roder OC, Jensen BS, Madsen MS, et al. Dacron or PTFE for above‐knee femoropopliteal bypass. A multicenter randomised study. European Journal of Vascular and Endovascular Surgery 2007;34(1):44‐9. [DOI] [PubMed] [Google Scholar]
Linhart 1991 {published data only}
- Linhart J, Vanek I. Treatment of intermittent claudication: two different mechanisms. International Angiology 1991;10(1):6‐9. [PubMed] [Google Scholar]
Matyas 2008 {published data only}
- Mátyás L, Berry M, Menyhei G, Tamás L, Acsády G, Cuypers P, et al. The safety and efficacy of a paclitaxel‐eluting wrap for preventing peripheral bypass graft stenosis: a 2‐year controlled randomized prospective clinical study. European Journal of Vascular and Endovascular Surgery 2008;35:715‐22. [DOI] [PubMed] [Google Scholar]
McCollum 2003 {published data only}
- McCollum CN, Devine CM, the North West Femoro‐Popliteal Trial Participants. Heparin bonded dacron or polytetrafluoroethylene for femoro‐popliteal bypass: Five year results of a prospective randomised multi‐centre clinical trial. The Vascular Surgical Society of Great Britain and Ireland Yearbook 2003. 2003:67.
Mohammadi 2007 {published data only}
- Mohammadi TA, Warnier de WG, Rhissassi B. Comparing vein with collagen impregnated woven polyester prosthesis in above‐knee femoropopliteal bypass grafting. International Journal of Surgery 2007;5(2):109‐13. [DOI] [PubMed] [Google Scholar]
Nordanstig 2011 {published and unpublished data}
- Nordanstig J, Gelin J, Hensater M, Taft C, Osterberg K, Jivegard L. Walking performance and health‐related quality of life after surgical or endovascular invasive versus non‐invasive treatment for intermittent claudication ‐ a prospective randomised trial. European Journal of Vascular and Endovascular Surgery 2011;42(2):220‐7. [DOI] [PubMed] [Google Scholar]
Panneton 2004 {published data only}
- Panneton JM, Hollier LH, Hofer JM. Multicenter randomized prospective trial comparing a pre‐cuffed polytetrafluoroethylene graft to a vein cuffed polytetrafluoroethylene graft for infragenicular arterial bypass. Annals of Vascular Surgery 2004;18(2):199‐206. [DOI] [PubMed] [Google Scholar]
PROOF 2007 {published data only}
- NCT00504088. Plaque removal versus open bypass surgery for critical limb ischemia (PROOF). http://clinicaltrials.gov/show/NCT00504088 (accessed 24 September 2014).
Stanisic 2009 {published data only}
- Stanisic M, Bucko W, Majewski W. Hand‐assisted laparoscopic aortic surgery as an initial step toward totally laparoscopic techniques in patients with aorto‐iliac occlusion in critical limb ischaemia. Wideochirurgia I Inne Techniki Maloinwazyjne 2009;4:67‐71. [Google Scholar]
Taft 2004 {published data only}
- Taft C, Sullivan M, Lundholm K, Karlsson J, Gelin J, Jivegard L. Predictors of treatment outcome in intermittent claudication. European Journal of Vascular and Endovascular Surgery 2004;27(1):24‐32. [DOI] [PubMed] [Google Scholar]
TECCO Trial {published data only}
- Gouëffic Y. The TECCO trial: results of the French multicentric randomized clinical trial comparing endovascular vs open surgery for the treatment of common femoral artery de novo lesions. Leipzig Interventional Course 2014. 2014.
Tiek 2009 {published data only}
- Tiek J, Fourneau I, Daenens K, Nevelsteen A. The role of thrombolysis in acute infrainguinal bypass occlusion: a prospective nonrandomized controlled study. Annals of Vascular Surgery 2009;23:179‐85. [DOI] [PubMed] [Google Scholar]
Tiek 2012 {published data only}
- Tiek J, Remy P, Sabbe T, D'hont C, Houthoofd S, Daenens K, et al. Laparoscopic versus open approach for aortobifemoral bypass for severe aorto‐iliac occlusive disease ‐ a multicentre randomised controlled trial. European Journal of Vascular and Endovascular Surgery 2012;43(6):711‐5. [DOI] [PubMed] [Google Scholar]
Vukobratov 2006 {published data only}
- Vukobratov V, Kaanski M, Pasternak J, Nikoli D, Popovi V, Obradovi J, et al. Femoro‐popliteal reconstructions: "in situ" versus "reversed" technique: comparative results. Medicinski Pregled 2006;59(7‐8):360‐4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
BASIL 2 {published data only}
- Bradbury A. BASIL 2 randomised trial launched to address need for data for endovascular interventions for severe limb ischaemia. Charing Cross Symposium. 2014.
- ISRCTN27728689. BASIL 2 randomised trial launched to address need for data for endovascular interventions for severe limb ischaemia. ISRCTN Register 2014.
- Popplewell MA, Davies H, Jarrett H, Bate G, Grant M, Patel S, et al. Bypass versus angio plasty in severe ischaemia of the leg ‐ 2 (BASIL‐2) trial: study protocol for a randomised controlled trial. Trials 2016;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
BEST‐CLI trial {published data only}
- Farber A, Rosenfield K, Menard M. The BEST‐CLI trial: A multidisciplinary effort to assess which therapy is best for patients with critical limb ischemia. Techniques in Vascular and Interventional Radiology 2014;17(3):221‐4. [DOI] [PubMed] [Google Scholar]
FINNPTX {published data only}
- NCT01450722. Paclitaxel eluting stent in long SFA obstruction: a prospective, randomized comparison with bypass surgery (finnptx). https://clinicaltrials.gov/ct2/show/NCT01450722 (accessed October 2016).
ISRCTN18315574 {published data only}
- ISRCTN18315574. Minimal invasive balloon expansion versus bypass operation to treat complicated occlusions and stenoses of the femoral and popliteal arteries. www.isrctn.com/ISRCTN18315574 (accessed October 2016).
NCT01171703 {published data only}
- NCT01171703. Optimized strategy for diabetic patients with critical limb ischemia (Part I) (DCLI‐I). clinicaltrials.gov/ct2/show/NCT01171703 (accessed October 2016).
NCT02580084 {published data only}
- NCT02580084. Clinical study of the aorta‐femoral bypass and hybrid intervention and the iliac arteries with stenting and plasty of the common femoral artery effectiveness in patients with the iliac segment and femoral artery occlusive disease (TASC C, D). clinicaltrials.gov/ct2/show/NCT02580084 (accessed October 2016).
ROBUST {published data only}
- Malas MBQ, Freischlag BA. Design of the revascularization with open bypass vs angioplasty and stenting of the lower extremity trial (ROBUST): a randomized clinical trial. JAMA Surgery 2014;149(12):1289‐95. [NCT01602159] [DOI] [PubMed] [Google Scholar]
- NCT01602159. Revascularization with Open Bypass versUs angioplasty and STenting of the lower extremity Trial (ROBUST). https://clinicaltrials.gov/ct2/show/NCT01602159 (accessed October 2016). [DOI] [PubMed]
SUPERB {published data only}
- Lensvelt MM, Holewijn S, Fritschy WM, Wikkeling OR, Walraven LA, Wallis de Vries BM, et al. SUrgical versus PERcutaneous Bypass: SUPERB‐trial; Heparin‐bonded endoluminal versus surgical femoro‐popliteal bypass: study protocol for a randomized controlled trial. Trials 2011;12:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
ZILVERPASS {published data only}
- NCT01952457. The Cook Zilver PTX drug‐eluting stent versus bypass surgery for the treatment the Cook Zilver PTX drug‐eluting stent versus bypass surgery of femoropopliteal TASC C&D Lesions (ZILVERPASS). http://clinicaltrials.gov/ct2/show/NCT01952457?term=NCT01952457&rank=1 (accessed October 2016).
Additional references
Abu Dabrh 2016
Acin 2012
- Acin F, Haro J, Bleda S, Varela C, Esparza L. Primary nitinol stenting in femoropopliteal occlusive disease: a meta‐analysis of randomized controlled trials. Journal of Endovascular Therapy 2012;19:585‐95. [DOI] [PubMed] [Google Scholar]
Antoniou 2008
- Antoniou GA, Koutsias S, Antoniou SA, Giannoukas AD. Remote endarterectomy for long segment superficial femoral artery occlusive disease. A systematic review. European Journal of Vascular and Endovascular Surgery 2008;36:310‐8. [DOI] [PubMed] [Google Scholar]
Antoniou 2009
- Antoniou GA, Sfyroeras GS, Karathanos C, Achouhan H, Koutsias S, Vretzakis G, et al. Hybrid endovascular and open treatment of severe multilevel lower extremity arterial disease. European Journal of Vascular and Endovascular Surgery 2009;38:616‐22. [DOI] [PubMed] [Google Scholar]
Antoniou 2013a
- Antoniou GA, Mavroforou A, Antoniou SA, Murray D, Kuhan G, Giannoukas AD. Evidence‐based medicine in vascular and endovascular practice. Journal of Endovascular Therapy 2013;20:678‐83. [DOI] [PubMed] [Google Scholar]
Antoniou 2013b
- Antoniou GA, Chalmers N, Georgiadis GS, Lazarides MK, Antoniou SA, Serracino‐Inglott F, et al. A meta‐analysis of endovascular versus surgical reconstruction of femoropopliteal arterial disease. Journal of Vascular Surgery 2013;57:242‐53. [DOI] [PubMed] [Google Scholar]
Antoniou 2014
- Antoniou GA, Georgakarakos EI, Antoniou SA, Georgiadis GS. Does endovascular treatment of infra‐inguinal arterial disease with drug‐eluting stents offer better results than angioplasty with or without bare metal stents?. Interactive Cardiovascular and Thoracic Surgery 2014;19:282‐5. [DOI] [PubMed] [Google Scholar]
Berridge 2013
- Berridge DC, Kessel DO, Robertson I. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD002784.pub2] [DOI] [PubMed] [Google Scholar]
Bradbury 2004
- Bradbury A, Wilmink T, Lee AJ, Bell J, Prescott R, Gillespie I, et al. Bypass versus angioplasty to treat severe limb ischemia: factors that affect treatment preferences of UK surgeons and interventional radiologists. Journal of Vascular Surgery 2004;39:1026‐32. [DOI] [PubMed] [Google Scholar]
Chang 2013
- Chang ZH, Liu ZY. Subintimal angioplasty for chronic lower limb arterial occlusion. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD009418.pub2] [DOI] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disease [Die chirurgische Behandlung der peripheren Durchblutungsstorungen]. Helvetica Chirurgica Acta 1954;21(5‐6):499‐533. [PubMed] [Google Scholar]
Fu 2015
- Fu X, Zhang Z, Liang K, Shi S, Wang G, Zhang K, et al. Angioplasty versus bypass surgery in patients with critical limb ischemia ‐ a meta‐analysis. International Journal of Clinical and Experimental Medicine 2015;8:10595‐602. [PMC free article] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1491‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holden 1950
- Holden WD. Reconstruction of the femoral artery for arteriosclerotic thrombosis. Surgery 1950;27:417. [Google Scholar]
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group. Inter‐society consensus for the management of peripheral arterial disease (TASC II). Journal of Vascular Surgery 2007;45 Suppl S:S5‐67. [DOI] [PubMed] [Google Scholar]
Rutherford 1997
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. Journal of Vascular Surgery 1997;26:517‐38. [DOI] [PubMed] [Google Scholar]
Selvin 2004
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999‐2000. Circulation 2004;110:738‐43. [DOI] [PubMed] [Google Scholar]
Tepe 2006
- Tepe G, Schmehl J, Heller S, Wiesinger B, Claussen CD, Duda SH. Superficial femoral artery: current treatment options. European Radiology 2006;16:1316‐22. [DOI] [PubMed] [Google Scholar]
Weaver 1996
- Weaver FA, Comerota AJ, Youngblood M, Froehlich J, Hosking JD, Papanicolaou G, STILE Investigators. Surgical revascularization versus thrombolysis for nonembolic lower extremity native artery occlusions: results of a prospective randomized trial. The STILE Investigators. Surgery versus Thrombolysis for Ischemia of the Lower Extremity. Journal of Vascular Surgery 1996;24(4):513‐23. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fowkes 2008
- Fowkes F, Leng GC. Bypass surgery for chronic lower limb ischaemia. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD002000.pub2] [DOI] [PubMed] [Google Scholar]
Leng 2000
- Leng GC, Davis M, Baker D. Bypass surgery for chronic lower limb ischaemia. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002000.pub2] [DOI] [PubMed] [Google Scholar]


