Abstract
Background
This is an updated version of the original Cochrane review published in 2013, Issue 4.
Low‐grade gliomas (LGG) constitute a class of slow‐growing primary brain neoplasms. Patients with clinically and radiographically suspected LGG have two initial surgical options, biopsy or resection. Biopsy can provide a histological diagnosis with minimal risk but does not offer a direct treatment. Resection may have additional benefits such as increasing survival and delaying recurrence, but is associated with a higher risk for surgical morbidity. There remains controversy about the role of biopsy versus resection and the relative clinical outcomes for the management of LGG.
Objectives
To assess the clinical effectiveness of biopsy compared to surgical resection in patients with a new lesion suspected to be a LGG.
Search methods
The following electronic databases were searched in 2012 for the first version of the review: Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 11), MEDLINE (1950 to November week 3 2012), Embase (1980 to Week 46 2012). For this updated version, the following electronic databases were searched: Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 5), MEDLINE (Nov 2012 to June week 3 2016), Embase (Nov 2012 to 2016 week 26). All relevant articles were identified on PubMed and by using the ’related articles’ feature. We also searched unpublished and grey literature including ISRCTN‐metaRegister of Controled Trials, Physicians Data Query and ClinicalTrials.gov for ongoing trials.
Selection criteria
We planned to include patients of any age with a suspected intracranial LGG receiving biopsy or resection within a randomized clinical trial (RCT) or controlled clinical trial (CCT). Patients with prior resections, radiation therapy, or chemotherapy for LGG were excluded. Outcome measures included overall survival (OS), progression‐free survival (PFS), functionally independent survival (FIS), adverse events, symptom control, and quality of life (QoL).
Data collection and analysis
A total of 1375 updated citations were searched and critically analyzed for relevance. This was undertaken independently by two review authors. The original electronic database searches yielded a total of 2764 citations. In total, 4139 citations have been critically analyzed for this updated review.
Main results
No new RCTs of biopsy or resection for LGG were identified. No additional ineligible non‐randomized studies (NRS) were included in this updated review. Twenty other ineligible studies were previously retrieved for further analysis despite not meeting the pre‐specified criteria. Ten studies were retrospective or were literature reviews. Three studies were prospective, however they were limited to tumor recurrence and volumetric analysis and extent of resection. One study was a population‐based parallel cohort in Norway, but not an RCT. Four studies were RCTs, however patients were randomized with respect to varying radiotherapy regimens to assess timing and dose of radiation. One RCT was on high‐grade gliomas (HGGs) and not LGG. Finally, one RCT evaluated diffusion tensor imaging (DTI)‐based neuro‐navigation for surgical resection.
Authors' conclusions
Since the last version of this review, no new studies have been identified for inclusion and currently there are no RCTs or CCTs available on which to base definitive clinical decisions. Therefore, physicians must approach each case individually and weigh the risks and benefits of each intervention until further evidence is available. Some retrospective studies and non‐randomized prospective studies do seem to suggest improved OS and seizure control correlating to higher extent of resection. Future research could focus on RCTs to determine outcomes benefits for biopsy versus resection.
Plain language summary
Surgical sampling or removal of low‐grade glioma brain tumors
The issue: Low‐grade gliomas (LGGs) are slow growing, less aggressive brain tumors. The most optimal surgical management is under debate.
The aim of the review: There are two surgical management strategies (treatments) for a person with a suspected LGG. These are biopsy, the surgical sampling of a small amount of tumor tissue, or resection, where as much as possible of the tumor is surgically removed. Tissues from both operations are then histologically examined to give a definitive diagnosis of the type and grade (severity) of the tumor. The aim of the review is to determine if biopsy or resection is the best treatment strategy.
The main findings: There is much debate about which of these surgical techniques is the best for patient survival. We searched the literature up to June 2016. However, currently there are no randomized controlled trials (RCTs) which have looked at which is the better procedure, only retrospective research studies looking at how people have responded to procedures that have happened in the past. Therefore, in the future, more RCTs are needed to try and answer this question.
Quality of the evidence: We were unable to determine this as no studies were included and only low‐quality evidence from non‐RCTs is available.
Conclusions: There are no randomized clinical trials on this topic; some institutional, non‐clinical trials studies have suggested improved overall survival and seizure control with higher extent of resection. However, physicians should approach each case individually and weigh the risks and benefits of biopsy versus surgical resection, as well as incorporate patient preference into their clinical decision‐making. Prognostic factors such as patient age, tumor size, and tumor location as well as potential implications for quality of life should be taken into account.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (2013, Issue 4)(Veeravagu 2013).
Description of the condition
Gliomas are a group of central nervous system (CNS) neoplasms consisting of neuroglial cells. Low‐grade gliomas (LGGs) are rare and constitute approximately one fifth of all CNS glial tumors, and affect 1800 to 3000 new patients annually in the USA (Pouratian 2010). The incidence of low‐grade astrocytoma has not been shown to vary significantly with nationality and has an incidence of 0.8 cases per 100,000 population (Epidemiology of LGG). Compared to high‐grade gliomas (HGGs), LGG are heterogeneous, slower growing, less aggressive lesions, with patients typically living with the disease for five to 20 years. Efforts have been underway to consolidate our current understanding of the clinical behavior for these tumors in order to optimize management and to provide a basis for future randomized clinical trials.
Establishing a diagnosis of LGG is important in order to differentiate a lesion from a more aggressive tumor type. The differential diagnosis should also include non‐neoplastic lesions, which must be ruled out. A presumptive diagnosis of LGG can be made based on clinical presentation and imaging characteristics. A patient with transient neurological symptoms consistent with seizure and a non‐enhancing hemispheric mass lesion on magnetic resonance imaging (MRI) or computed tomography (CT), or both, that produces little mass effect are suggestive of LGG. Focal neurological deficits are rare but can occur. These tumors are best seen on T2‐weighted and fluid attenuated inversion recovery (FLAIR) MRI sequences and are frequently non‐enhancing on T1‐gadolinium sequences. Currently it is unclear what the diagnostic accuracy of MRI is for suspected LGG. Clinically, studies have shown that among 1028 patients with brain tumor, the prevalence of seizures was higher in patients with LGGs (85%) than in patients with anaplastic glioma (69%) or glioblastoma (49%) (Lote 1998). Furthermore, in a series of 831 consecutive patients, tumor contrast enhancement was present on CT in 21% of cases with LGG compared with 57% to 96% of those with HGGs (Lote 1998b).
Although suspected LGG can be diagnosed clinically and radiographically, a definitive diagnosis must be made based on two histopathologic characteristics of a surgical specimen. First, the cell type that constitutes the bulk of the tumor is of glial origin, which can be further subdivided as astrocytoma, oligodendroglioma, or mixed oligoastrocytoma. Second, the grade of the tumor is rated based on the World Health Organization (WHO) classification scheme (WHO Tumor Grading Classification). For the purpose of this review, we restricted our definition of LGG to hemispheric WHO Grade II astrocytomas, oligodendroglioma, or mixed oligoastrocytoma. The controversy regarding biopsy versus resection lies specifically in the management of these diffuse hemispheric LGGs. A large proportion of LGGs, and virtually all diffuse LGGs, fall in the above mentioned three histologies.
There is general consensus that Grade I tumors such as pilocytic astrocytomas, subependymal giant cell astrocytoma, pleomorphic xanthoastrocytoma, subependymoma, etc. are managed with surgical resection, which is preferred as a cure; while other therapies are less effective. The diagnosis for WHO I tumors can frequently be suspected on imaging and surgical resection is then procured, thus the controversy regarding biopsy versus resection does not exist in these types of tumors.
In summary, LGGs constitute a class of slow‐growing primary brain neoplasms. Optimal clinical management has been debated, with general agreement that a combination of surgical, radiation therapeutic, and chemotherapeutic approaches are necessary for optimal survival and outcome. The goals of treatment for patients with LGGs include prolonging overall survival (OS) and progression‐free survival (PFS) and minimizing morbidity.
Description of the intervention
Management of LGGs differs from that of higher‐grade lesions. If a LGG is suspected based on clinical presentation and radiographical characteristics, the most definitive diagnosis will require a histopathological and molecular diagnosis by obtaining a tissue sample (via either biopsy or resection).
There are many treatment dilemmas and one major discussion involves the role of surgery. Surgical biopsy includes all procedures that aim to obtain enough diagnostic tissue to make a definitive pathological diagnosis. The procedure may be performed open or with a needle with a freehand, stereotactic, or image‐guided technique. Surgical resection includes all procedures where the pre‐operative aim is to remove more tumor than is necessary for making a pathological diagnosis.
There remains controversy about the role of biopsy versus resection in the initial management of patients with LGG. The main aim of surgical resection is to improve survival, or at least delay the need for subsequent therapy. Resection may also improve neurological deficits and seizure control. However, gross total resection is often not possible without a significant risk of neurological sequelae due to the diffuse infiltrative nature of LGG. Biopsy can confirm diagnosis and carries fewer risks, but may not extend survival or improve symptoms, and may have an associated sampling error. Retrospective studies indicate that a maximally safe resection at the time of diagnosis may be linked with improved survival (Keles 2001; Pignatti 2002; Shaw 2002). In contrast, there is also evidence that a more conservative approach (delaying resection therapy until radiographic evidence of tumor growth, transformation, or impairment) may be more appropriate for patients with small, minimally symptomatic LGG (Olson 2000; Recht 1992; van Veelen 1998). More recently, the European Federation of Neurological Societies ‐ European Association for Neuro‐Oncology (EFNS‐EANO) Task Force released guidelines which suggest that younger age, normal neurological examination, oligodendroglial histology, and chromosome 1p loss are favorable prognostic factors. Total or near total resection can improve seizure control, PFS and OS, all while reducing the risk of malignant transformation (Soffietti 2010).
How the intervention might work
Surgical resection in the management of LGG decreases tumor burden and may decrease the rate of progression or recurrence, or both, of the tumor. Biopsy of LGG provides a pathological or histological diagnosis but does not achieve debulking of the tumor. Usually resection involves an open procedure in which a craniotomy must be performed, whereas a biopsy may be performed through the use of a stereotactic needle.
Why it is important to do this review
The current treatment approach for patients with LGG includes operative management (biopsy or resection) followed by either immediate or delayed postoperative radiation therapy. The relatively long survival times of patients with LGG have made secondary outcomes including quality of life (QoL) measures and cognitive performance a significant component of management decisions. Thus, we have undertaken a systematic review of clinical trials that address optimal long‐term outcomes in patients with LGG managed by surgical biopsy versus resection.
Specific to this study, it was hypothesized that resection may provide a clinical advantage over biopsy, which only provides histological confirmation. However, the current literature is conflicting on the relative merits of each procedure and it is not readily apparent whether the more invasive procedure of resection confers any long‐term benefits in OS or PFS. Fairly unique among those with CNS malignancy, patients with LGG have long expected survival times. Thus, it is crucial to develop therapeutic approaches that optimize patient survival as well as cognitive performance and quality of life. The authors performed a systematic review of the literature on this topic to inform future clinical decisions on the initial surgical management of patients with LGG.
Objectives
To assess the clinical effectiveness of biopsy compared to surgical resection in patients with a new lesion suspected to be a low‐grade glioma (LGG).
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and controlled clinical trials (CCTs) meeting the selection criteria, such as LGG patients randomized to initial surgery versus biopsy. Studies which randomized patients to receive a particular radiotherapy or chemotherapy regimen and subsequently stratified patients according to degree of surgery were not accepted. Cases of suspected Grade III or IV gliomas on imaging were also excluded.
Types of participants
Adult patients of any age with a suspected intracranial LGG, as evidenced by clinical and radiographic evaluation, who have not received radiotherapy or chemotherapy. LGG included WHO Grade II astrocytoma, oligodendroglioma, and mixed oligoastrocytoma. Patients were excluded for the following criteria.
Patient had received previous cranial radiation.
Patient had undergone previous surgery for the suspected intracranial LGG.
Types of interventions
Surgical biopsy included all procedures that aim to obtain enough diagnostic tissue to make a definitive pathological diagnosis. The procedure may have been performed open or with a needle with a freehand, stereotactic, or image‐guided technique.
Surgical resection included all procedures where the preoperative aim was to remove more tumor than is necessary for making a pathological diagnosis. Resection confirmed by postoperative imaging and classified as attempted gross total, near gross total (great than 90%), or partial. Extent of resection evaluation on postoperative imaging is detailed in a review by Henson and colleagues, who discussed measurement approaches, response criteria, selection of lesions for measurement, technical imaging considerations, interval between tumor measurements and response confirmation, and validity of imaging as a measure of efficacy (Henson 2008).
Types of outcome measures
Primary outcomes
Overall survival (OS), defined as survival until death from all causes from time of randomization
Secondary outcomes
Progression‐free survival (PFS), defined as survival until evidence of tumor recurrence is documented by computed tomography (CT) or magnetic resonance imaging (MRI) scan
Functionally independent survival (FIS) using the Karnofsky Performance Scale (KPS) Index, which allows patients to be classified according to their functional impairment. A lower KPS reduces the likelihood of survival for most serious illnesses (Appendix 1)
Time to progression (TTP) using the updated MacDonald criteria (MacDonald 1990; Wen 2010). The new criteria acknowledge that contrast enhancement may be non‐specific and not necessarily a true surrogate of tumor control (due to effect of chemotherapy and anti‐angiogenic therapy)
Symptom control, improvements in symptoms or a maintenance of symptoms without deterioration, including seizure control
Quality of Life (QoL), as measured using a scale validated through reporting of norms in a peer‐reviewed publication, such as the European Organization for Research and Treatment of Cancer Quality of Life Questionnaires QLQ‐C30 (Aaronson 1993) and QLQ‐BN20 (Taphoorn 2010), Functional Assessment of Cancer Therapy ‐ General (FACT‐G) or Functional Assessment of Cancer Therapy ‐ Brain (FACT‐Br) (Weitzner 1995), or MD Anderson Symptom Inventory ‐ Brain Tumor Module (MDASI‐BT) (Armstrong 2006)
Adverse effects of surgical resection as defined by the Medical Dictionary for Regulatory authorities. These include new neurologic deficit, headache, nausea and vomiting, hematoma, wound complications, infection (and site), cerebrospinal fluid (CSF) leak, edema, seizure, and general medical complications
* The Karnofsky Performance Scale (KPS) Index allows patients to be classified according to their functional impairment. A lower KPS reduces the likelihood of survival for most serious illnesses.
Search methods for identification of studies
Papers in all languages were sought and translations carried out if necessary.
Electronic searches
For the first version of the review, we searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 11), MEDLINE (1950 to November week 3 2012), Embase (1980 to Week 46 2012). All relevant articles were identified on PubMed and by using the ’related articles’ feature. For the current version of the review, we searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 5), MEDLINE (Nov 2012 to June week 3 2016), Embase (Nov 2012 to 2016 Week 26). The search strategies can be found in Appendix 2, Appendix 3 and Appendix 4.
Searching other resources
Unpublished and grey literature
We searched Metaregister, Physicians Data Query, www.controlled-trials.com/rct, www.clinicaltrials.gov, and www.cancer.gov/clinicaltrials for ongoing trials. We contacted the main investigators of the relevant ongoing trials for further information, as well as the major co‐operative trials groups active in this area.
Handsearching
We handsearched the reference lists of all relevant trials obtained by the above search for further trials.
Correspondence
We planned to contact the authors of RCTs (if any) for any unpublished data.
Data collection and analysis
Selection of studies
Two of the review authors (BJ, KC) sifted the search results and no RCTs were identified for inclusion in this review.
Data extraction and management
There were no included studies with data suitable for extraction or meta‐analysis.
For future versions of this review, please see the planned data extraction and management if any RCTs are identified for inclusion in the Differences between protocol and review section.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The original electronic database searches yielded a total of 2764 citations: • MEDLINE ‐ 884; • CENTRAL ‐ 316; • Embase ‐ 1564.
In addition, the checking of reference lists, handsearching and personal communications failed to reveal any relevant trials.
The updated electronic database yielded a total of 1375 additional citations: • MEDLINE ‐ 196; • CENTRAL ‐ 153; • Embase ‐ 1026.
No ongoing studies were found in a search of Metaregister, Physicians Data Query, http://www.controlled‐trials.com/ rct, www.clinicaltrials.gov, and www.cancer.gov/clinicaltrials.
Please refer to Figure 1 for the PRIMSA flow diagram of the process.
1.
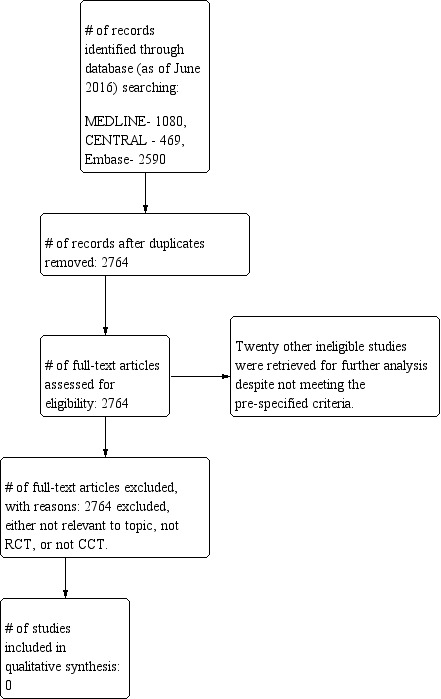
Flow diagram for study inclusion and exclusion.
Included studies
No new randomized controlled trials (RCTs) of biopsy or resection for low‐grade glioma (LGG) were identified.
Excluded studies
There are no additional excluded studies in the updated review. In the previous review, 20 other ineligible studies were retrieved for further analysis despite not meeting the pre‐specified criteria. Ten studies were retrospective (Ahmadi 2009; Claus 2005; Laws 1984; Philippon 1993; Recht 1992; Scerrati 1996; Smith 2008; van Veelen 1998) or were literature reviews (Sanai 2008; Whittle 2010). Three studies were prospective, however they were limited to tumor recurrence (Shaw 2008) and volumetric analysis and extent of resection (Jung 2011; Majchrzak 2012). One study was a population‐based parallel cohort study in Norway, but not an RCT (Jakola 2012). Four studies were RCTs, however patients were randomized with respect to varying radiotherapy regimens to assess timing (van den Bent 2005) and dose of radiation (Karim 1996; Shaw 2002). One RCT was on high‐grade gliomas (HGGs) and not LGG (Vuorinen 2003). One last RCT evaluated diffusion tensor imaging (DTI)‐based neuro‐navigation for surgical resection (Wu 2007).
Risk of bias in included studies
Not applicable as there were no included studies.
Effects of interventions
Not applicable as there were no included studies.
Discussion
The literature surrounding low‐grade gliomas (LGGs) is far more limited than that for high‐grade gliomas (HGGs), possibly due to the fact that the major cause of mortality of LGGs is the advancement to HGG. Fortunately, the literature continues to expand and comprehensive reviews have recently been published on the treatment options for LGGs (Omay 2012; Ruiz 2009).
Our thorough examination of the literature via this Cochrane review did not identify any studies that met pre‐defined inclusion criteria. At present, there remains a significant need for randomized controlled trials (RCTs) evaluating resection versus biopsy for LGGs. This area of study may benefit patients who maintain poor preoperative morbidity and are thus more likely to undergo radiotherapy rather than surgical resection. Such an RCT has been performed to evaluate resection versus biopsy in patients diagnosed with HGGs (Vuorinen 2003).
Pathological diagnosis via biopsy or resection is important for LGGs as malignancy may be underestimated using radiographic and clinical characteristics alone (Lote 1998). If a patient presents with a lesion that appears to be an LGG based on radiographic and clinical evidence, it remains unclear in the clinical trials literature whether definitive diagnosis via biopsy or resection will result in increased progression‐free survival (PFS) and quality of life (QoL). However, it is well known that the larger sample obtained from surgical resection presents greater opportunity for revealing heterogeneity (Revesz 1993).
Furthermore, several recent institutional studies have demonstrated the advantages of maximal resection for patient outcomes. In a prospective, volumetric analysis of extent of resection (EOR) of 68 consecutive patients with hemispheric LGGs, smaller preoperative tumor volume and greater EOR were found to be associated with longer overall survival (OS), PFS and malignant degeneration‐free survival (MFS) (Majchrzak 2012). In another prospective study on 86 patients with LGGs, both univariate and multivariate analysis demonstrated a statistical correlation between gross total removal and longer PFS (Jung 2011). Overall, Sanai and Berger reviewed every major glioma publication from 1990 to 2008, which included 10 LGG articles, and concluded that more extensive surgical resection is associated with longer life expectancy (Sanai 2008).
Finally, a recent study from two Norwegian universities examined survival in population‐based parallel cohorts involving 153 patients with diffuse LGGs; 66 patients were enrolled at hospital A (47 received biopsy, 19 received initial resection) while 87 were enrolled at hospital B (12 biopsy, 75 resection). OS was better with early resection, at a rate of 74% at five years compared to 60% in the biopsy group (Jakola 2012). Regional practice variation played an important role in the type of treatment offered; at hospital A, resection of suspected LGG was only offered if a safe total resection was feasible based on preoperative planning, while at Hospital B, due to availability of neuro‐navigation with three dimensional (3D) ultrasound‐based intra‐operative imaging, the majority of LGGs were preferentially resected. There were no differences in the tumor stratification, with WHO II oligodendrogliomas and oligoastrocytomas found in hospital B and WHO II astrocytomas found in hospital A. Other variations between centers included tumor characteristics (maximal diameter, eloquent location) and postoperative treatments, such as chemotherapy and radiotherapy. The authors further explored whether these factors or other variables might have contributed to the demonstrated survival differences. In a multivariate analysis, the relative hazard ratio (HR) was 1.8 (95% confidence interval (CI), 1.1 to 2.9, P value 0.03) for patients treated at a center favoring biopsy and watchful waiting, thereby confirming the survival benefit of early resection even without possible confounders (Jakola 2012).
In terms of QoL, Englot and colleagues performed a quantitative, comprehensive systematic literature review of seizure‐control outcomes in 1181 patients with epilepsy across 41 studies after surgical resection of low‐grade temporal lobe gliomas and glioneuronal tumors. Again, no RCTs were identified, however based on observational case series, gross total lesionectomy of temporal lobe LGGs resulted in improved seizure control over subtotal resection (Englot 2012). Nevertheless, these non‐randomized studies (NRSs) are limited by selection bias. Patients who received total resection often have other desirable prognostic factors such as small non‐eloquent tumors in otherwise healthy patients. Conversely, those undergoing subtotal resection or biopsy, or both, often have larger eloquent tumors and are less healthy patients. These confounding effects on survival comparisons can only be reliably obviated through randomization and proper trial methodology.
Historically, biopsy has been utilized for lesions located within or adjacent to eloquent parenchyma. Though surgical biopsy is a relatively safe procedure, some studies have suggested the risks of morbidity and inaccurate diagnosis may not outweigh the benefits. In a retrospective review of 81 patients with radiographic evidence of glioma who underwent stereotactic biopsy followed by surgical resection (within 60 days) between 1993 and 1998, diagnosis based on biopsy or resection differed in 40 cases (49%) (Jackson 2001). This was reduced to 30 cases (38%) when biopsy slides were reviewed preoperatively by three neuropathologists. The diagnostic accuracy in the Jackson study was lower compared to previously reports in the literature (63% to 95%). The authors attributed the high rates of diagnostic discrepancy to tumor heterogeneity, as there was a strong trend towards higher‐grade malignancy on openly resected specimens. Following stereotactic biopsy, three patients (3.7%) had major complications and one (1.2%) had minor neurologic complications. Complications included cerebral hemorrhages, persistent hemiparesis, and temporary unilateral leg weakness. The authors concluded that stereotactic biopsy may actually be an unnecessary procedure in the management of suspected LGG, due to the high risk of inaccurate diagnosis subsequently delaying or negatively hindering appropriate surgical management.
In the absence of robust RCTs evaluating the initial steps of glioma diagnosis, physicians should consider prognostic factors when determining diagnostic and treatment methodologies for patients with LGGs. In one study by Pignatti, Cox analysis of 288 adults with LGGs demonstrated that patient age greater than 40 years, astrocytoma histology, large tumor diameter greater than 6 cm, midline extension, and preoperative neurologic deficit are unfavorable prognostic factors for survival (Pignatti 2002). Chang and colleagues has proposed a preoperative scoring system to prognosticate degree of lesion resectability, PFS, and OS in patients with LGG based on location, the Karnofsky Performance Scale (KPS), patient's age, and tumor diameter (Chang 2008). In clinical practice, patients can be divided into low‐ and high‐risk subgroups based on their total number of unfavorable prognostic factors. One possibility yet to be studied is the hypothesis that immediate resection may decrease mortality in the high‐risk subgroup, while conservative management with initial biopsy may be more appropriate in the low‐risk subgroup. However, whether patients with low‐ or high‐risk status would benefit from histological diagnosis via biopsy versus resection has yet to be definitively established.
Though not examined within the scope of this review, an alternative expectant management may improve QoL measures by eliminating the risks associated with biopsy, resection, or additional interventional therapy. In one small clinical trial, Recht and colleagues studied 26 patients who presented with a transient event (often seizures) and radiographic evidence suggestive of a low‐grade primary supratentorial neoplasm who chose to withhold from all therapy until deemed necessary (Recht 1992). This group was compared to a similar group of 20 patients who received immediate intervention. With a median follow‐up of 46 months, no identifiable difference between the groups in terms of survival or QoL was found. This evidence supports a personalized approach in determining a treatment plan for each patient presenting with LGG. While some patients may benefit from early knowledge of their histological diagnosis via biopsy or resection, others may prefer to postpone intervention unless it becomes necessary to maintain QoL. A larger clinical trial could be performed to provide more definitive evidence for observational treatment.
Importantly, the excluded studies did provide evidence in a non‐randomized series that extensive resection yielded better outcomes (Ahmadi 2009; Claus 2005; Smith 2008). Region‐of‐interest analysis of 216 adults who underwent surgical resection of LGG showed that patients with at least a 90% extent of resection (EOR) had five‐ and eight‐year OS rates of 97% and 91%, respectively (Smith 2008). In contrast, patients with less than 90% EOR had five‐ and eight‐year OS rates of 76% and 60%, respectively. Analysis of 130 cases of adult supratentorial LGGs similarly demonstrated that both extended surgery and re‐surgery were found to prolong OS and PFS (Claus 2005). Though there is evidence that increased EOR yields increased OS rates, it is still unknown whether biopsy without resection may similarly yield increased OS rates in certain cases.
Finally, there are recent data suggesting that an extended resection with a margin beyond MRI‐defined abnormalities, a 'supratotal' resection, might improve outcomes in patients with LGG. Yordanova and colleagues enrolled 15 right‐handed patients with a total of 17 WHO Grade II gliomas involving non‐functional areas within the left dominant hemisphere all of whom underwent awake craniotomy with resection extended until cortical or subcortical "eloquent" areas as defined by intra‐operative electrical mapping. Supratotal resection was achieved in 15/17 tumors based on postoperative MRI (with resection cavity > 10 cc larger than tumor volume). At mean 36 months follow‐up, 4/15 patients experienced recurrence, 0/15 experienced anaplastic transformation, while a control group of 29 patients who underwent only complete resection had anaplastic transformation, seen in 7/29 cases (Yordanova 2011). These results resonated with the work by Duffau, Lang, and Bello, among others, and will continue to be an area of focus going forward.
Overall, this review demonstrated the continued need for RCTs to be performed in the area of biopsy versus resection for management of LGGs. Such a study will provide survival and QoL outcome data to guide management of patients with LGGs. Specifically, cases where an extensive resection can be completed safely may not necessarily need to be the focus of an RCT since the controversy in the field mainly relates to cases of subtotal resection.
Authors' conclusions
Implications for practice.
Since the last version of this review there are no new relevant studies to include that provide additional information to change the conclusions. Currently there are no RCTs or controlled clinical trials to base recommendations for the diagnostic and surgical management of patients with LGGs. The key controversy exists as it relates to the case of subtotal resection, in which a large residual remains, and the risks of surgical adverse events must be balanced by the possible advantages of biopsy alone. There are retrospective studies and non‐randomized prospective studies which do seem to suggest improved OS and seizure control correlating to higher extent of resection, however physicians should approach each case individually and weigh the risks and benefits of biopsy versus surgical resection, as well as incorporate patient preference into their clinical decision‐making. Prognostic factors such as patient age, tumor size, and tumor location as well as potential implications for QoL should be taken into account.
Implications for research.
To provide evidence‐based medicine in the diagnosis and treatment of LGGs, a large‐scale RCT of biopsy versus resection for LGG needs to be conducted. Such a study will maximize patient outcome, minimize risk, and optimize healthcare costs. Specifically, cases where an extensive resection can be completed safely may not necessarily need to be the focus of an RCT, since the controversy in the field mainly relates to cases of subtotal resection. A large‐scale RCT may aid to confirm the current retrospective, institution‐based, and parallel cohort studies which suggest that maximal surgical resection is associated with improved survival, delayed time to recurrence, and seizure control. In the event that an RCT is not forthcoming, controlled trials on the extent of resection based on new technologies such as intra‐operative MRI may be more plausible.
What's new
| Date | Event | Description |
|---|---|---|
| 10 June 2020 | Review declared as stable | Not currently for update as no studies identified in most recent search in September 2019. Future studies into the extent of resection in LGG in development. |
History
Protocol first published: Issue 9, 2011 Review first published: Issue 4, 2013
| Date | Event | Description |
|---|---|---|
| 29 March 2017 | New citation required but conclusions have not changed | No studies identified for inclusion. |
| 3 February 2017 | New search has been performed | The searches were re‐run to June 2016; 1202 additional references were screened. |
| 27 June 2013 | Amended | We critically reviewed a large number of papers in this manuscript, including a JAMA 2012 article by Jakola et al. Recently, we were asked by Dr. Jakola to re‐visit our analysis of his paper; it appears our interpretation of his data in the Cochrane review is not congruent with the post‐hoc analysis done by his group. We re‐examined our analysis and found Dr. Jakola's argument convincing and have thus updated the review accordingly. |
Acknowledgements
The author team would like to thank Clare Jess and the editorial team for the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group for their enthusiasm, support and guidance throughout the process. Peer reviewers Kathie Godfrey, Ruth Garside, Robin Grant, Andy Bryant, and Florence L‐D are also commended for their insightful comments. We would like to thank Cassie Ludwig for her contribution to literature search, review, and appraisal in the original review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Functionally independent survival scales
Karnofsky Performance Scale (KPS)
The KPS score runs from 100 to 0, where 100 is perfect health and 0 is death (Karnofsky 1949; Karnofsky 1951):
100% ‐ normal, no complaints, no signs of disease
90% ‐ capable of normal activity, few symptoms or signs of disease
80% ‐ normal activity with some difficulty, some symptoms or signs
70% ‐ caring for self, not capable of normal activity or work
60% ‐ requiring some help, can take care of most personal requirements
50% ‐ requires help often, requires frequent medical care
40% ‐ disabled, requires special care and help
30% ‐ severely disabled, hospital admission indicated but no risk of death
20% ‐ very ill, urgently requiring admission, requires supportive measures or treatment
10% ‐ moribund, rapidly progressive fatal disease processes
0% ‐ death
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Glioma explode all trees #2 glioma* #3 astrocytoma* #4 oligodendroglioma* #5 oligoastrocytoma* #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Surgical Procedures, Operative explode all trees #8 surg* #9 Any MeSH descriptor with qualifier: SU #10 biopsy #11 resect* #12 (#7 OR #8 OR #9 OR #10 OR #11) #13 (#6 AND #12)
Appendix 3. MEDLINE Ovid search strategy
1 exp Glioma/ 2 glioma*.mp. 3 astrocytoma*.mp. 4 oligodendroglioma*.mp. 5 oligoastrocytoma*.mp. 6 1 or 2 or 3 or 4 or 5 7 exp Surgical Procedures, Operative/ 8 surg*.mp. 9 surgery.fs. 10 biopsy.mp. 11 resect*.mp. 12 7 or 8 or 9 or 10 or 11 13 randomized controlled trial.pt. 14 controlled clinical trial.pt. 15 randomized.ab. 16 placebo.ab. 17 clinical trials as topic.sh. 18 randomly.ab. 19 trial.ti. 20 13 or 14 or 15 or 16 or 17 or 18 or 19 21 6 and 12and 20 Key: mp=title, original title, abstract, name of substance word, subject heading word, unique identifier fs=floating subheading ab=abstract sh=subject heading ti=title
Appendix 4. Embase Ovid search strategy
1 exp glioma/ 2 glioma*.mp. 3 astrocytoma*.mp. 4 oligodendroglioma*.mp. 5 oligoastrocytoma*.mp. 6 1 or 2 or 3 or 4 or 5 7 exp surgery/ 8 surg*.mp. 9 su.fs. 10 biopsy.mp. 11 resect*.mp. 12 7 or 8 or 9 or 10 or 11 13 exp controlled clinical trial/ 14 crossover procedure/ 15 randomized controlled trial/ 16 single blind procedure/ 17 random*.mp. 18 factorial*.mp. 19 (crossover* or cross over* or cross‐over).mp. 20 placebo*.mp. 21 (doubl* adj blind*).mp. 22 (singl* adj blind*).mp. 23 assign*.mp. 24 allocat*.mp. 25 volunteer*.mp. 26 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27 6 and 12 and 26
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmadi 2009 | Retrospective review of 130 cases of adult supratentorial LGGs. Cox analysis was used to correlate extent of resection to OS and progression‐free survival (PFS). Both extended surgery and re‐surgery were found to prolong OS and PFS. |
| Claus 2005 | Retrospective review of 156 patients who underwent surgical resection of supratentorial LGG using intraoperative MRI guidance. Studied the association between extent of resection and risk of recurrence or death using a postoperatively performed volumetric analysis. Patient age and histology were adjusted for survival time, but adjuvant therapy was not. Follow‐up time was 36 months. Patients who did not receive gross total resection were at 1.4 times the risk of disease recurrence and 4.9 times the risk of death. |
| Englot 2012 | Quantitative systematic literature review of seizure control outcomes in 1181 patients with epilepsy across 41 studies after surgical resection of low‐grade temporal lobe gliomas and glioneuronal tumors. Gross total lesionectomy of low‐grade temporal lobe tumors resulted in significantly improved seizure control over subtotal resection. |
| Jakola 2012 | Population‐based parallel cohorts of LGGs from 2 Norwegian university hospitals that utilized different surgical treatment strategies. 66 patients received biopsy and watchful waiting while 87 underwent early resection. The early resection cohort was associated with better OS compared to the biopsy group. |
| Jung 2011 | Prospective study on 86 patients with LGGs, both univariate and multivariate analysis demonstrated a statistical correlation between gross total removal and longer PFS. |
| Karim 1996 | RCT of postoperative irradiation for low‐grade cerebral gliomas at doses of 45 Gy in 5 weeks or 59.4 Gy in 6.6 weeks. 379 adult patients were randomized at the European Organization for Research and Treatment of Cancer (EORTC) data center. No difference in OS or PFS was found, but tumor size was found to be an important prognostic factor. |
| Laws 1984 | Retrospective review of 461 cases of supratentorial low‐grade astrocytoma. Studied prognostic indicators of survival time following diagnosis. Variables correlating with increased survival time were lower age at time of surgery, gross total surgical removal, lack of major preoperative neurological deficit, long duration of symptoms prior to surgery, seizures as a presenting symptom, lack of major postoperative neurological deficit and surgery performed in recent decades. |
| Majchrzak 2012 | Prospective volumetric analysis of extent of resection in adults diagnosed with hemispheric LGGs. Smaller preoperative tumor volume and greater extent of resection were significantly associated with longer OS, PFS and MFS. |
| Philippon 1993 | Retrospective review of 179 cases of supratentorial low‐grade astrocytoma. Studied prognostic indicators of survival time following diagnosis. Best prognostic factors found were age, preoperative neurological status and histological grade. |
| Recht 1992 | Retrospective review of 46 patients who presented with radiographic evidence of suspected LGG. Compared 26 patients for whom all therapy was withheld until deemed necessary to 20 patients for whom immediate intervention was elected. No difference in survival or quality of life was found. |
| Sanai 2008 | Literature review not an RCT. |
| Scerrati 1996 | Retrospective review of 171 cases of hemispheric WHO grade II gliomas. Studied the effect of age, Karnofsky score, histology, tumor extension, extent of surgical resection and radiotherapy on survival. |
| Shaw 2002 | RCT of low‐dose (50.4 Gy/28 fractions) versus high‐dose (64.8 Gy/36 fractions). Compared survival and toxicity in 203 adult patients with supratentorial LGG. Survival at 2 and 5 years was non‐significantly better with low‐dose radiotherapy as opposed to high‐dose radiotherapy. Also, there was a higher incidence of radiation necrosis in the high‐dose radiotherapy group. Histological subtype, tumor size and age were consistently and significantly associated with OS. |
| Shaw 2008 | Prospective study of surgery considering primarily tumor recurrence only. |
| Smith 2008 | Retrospective review of 216 cases of adults who underwent surgical resection of LGG. Studied the association between extent of resection (EOR) and long‐term outcome. Patients with at least 90% EOR had 5‐ and 8‐year OS rates of 97% and 91%, respectively. In contrast, patients with less than 90% EOR had 5‐ and 8‐year OS rates of 76% and 60%, respectively. Furthermore, predicted OS was negatively influenced by residual tumor volumes in the order of 10 cm3 and increased EOR was not associated with additional morbidity. |
| van den Bent 2005 | RCT of early radiotherapy compared with delayed radiotherapy for LGGs. 157 patients were assigned early and 157 delayed radiotherapy. Median PFS was 5.3 years in the early and 3.4 years in the delayed radiotherapy group. Furthermore, seizures were better controlled at 1 year in the early group. However, OS was similar in both groups. The main flaws of this study were that the surgeon estimated the extent of resection during surgery with no postoperative CT scanning and that 26% of patients for whom pathology review was available were subsequently diagnosed with a high‐grade tumors. Furthermore, no quality of life study was performed. |
| van Veelen 1998 | Retrospective review of 90 cases of supratentorial low‐grade astrocytoma. Studied the effect of age, preoperative neurological condition, epilepsy as the single sign, extent of surgery, and histology on survival. Also looked at de‐differentiation and early versus delayed surgery in a subgroup of patients presenting with epilepsy as their only symptom. Survival in both groups was identical and better than the whole group overall. |
| Vuorinen 2003 | RCT on HGG not LGG. |
| Whittle 2010 | Literature review not an RCT. |
| Wu 2007 | RCT of diffusion tensor imaging (DTI)‐based functional neuro‐navigation versus routine 3‐D navigational MRI data‐based neuro‐navigation in surgery on cerebral gliomas involving the pyramidal tract. 238 patients were evaluated, 129 of whom were diagnosed with LGGs and 85 of whom were diagnosed with HGGs involving the pyramidal tract. No significant difference was found for the resection of LGGs. However, less postoperative motor deficits and higher 6‐month Karnofsky Performance Scores were associated with the study group for LGGs. |
CT: computed tomography HGG:high‐grade glioma LGG: low‐grade glioma MFS: malignant degeneration‐free survival MRI: magnetic resonance imaging OS: overall survival PFS: progression‐free survival RCT: randomized controlled trial
Differences between protocol and review
Data extraction and management
For future versions of this review, if identified data from new randomized controlled trials (RCTs) are available, they will be extracted onto a pre‐designed data collection sheet. We will also record the following information, as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Author, year of publication and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
-
Study population
total number enrolled
patient characteristics
age
sex
co‐morbidities
previous treatment
neurological performance
primary cancer type
-
Tumor details at diagnosis
size of tumor
location of tumor
tumor histology
-
Intervention details
-
details of surgery
extent of biopsy or resection
-
Risk of bias in study (assessment of risk of bias in included studies)
Duration of follow‐up
Outcomes including overall survival (OS), progression‐free survival (PFS), functionally independent survival (FIS), local tumor control, cause of death, steroid requirement and adverse events
Assessment of risk of bias in included studies
Selection bias: random sequence generation and allocation concealment
Performance bias: blinding of participants and personnel (patients and treatment providers)
Detection bias: blinding of outcome assessment
Attrition bias: incomplete outcome data
Reporting bias: selective reporting of outcomes
Other possible sources of bias
Data on outcomes will be extracted as below
For time to event (e.g. OS, PFS, and time to tumor progression) data, we will extract the log of the hazard ratio [log(HR)] and its standard error from trial reports; if these are not reported, we will attempt to estimate them from other reported statistics using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events or deaths if it is not possible to use a HR), we will extract the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at the endpoint, in order to estimate a risk ratio (RR).
For continuous outcomes (e.g. QoL measures), we will extract the final value and standard deviation (SD) of the outcome of interest and the number of patients assessed at the endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference (MD) (if trials measured outcomes on the same scale) or standardized mean difference (SMD) (if trials measured outcomes on different scales) between treatment arms and its standard error.
Where possible, all data extracted will be those relevant to an intention‐to‐treat (ITT) analysis, in which participants are analyzed in the groups to which they are assigned. The time points at which outcomes were collected and reported will be noted. New data will be abstracted independently by two review authors (AV, BJ) onto a data abstraction form specially designed for the review. Differences between review authors will be resolved by discussion or by appeal to a third review author, if necessary.
Risk of bias in future RCTs and CCTs will be assessed using the following questions and criteria (see Chapter 8 of Higgins 2011). Funnel plots corresponding to meta‐analysis of the primary outcomes will assess the potential for small‐study effects such as publication bias. If these plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random‐effects model, we plan to perform further meta‐analyses using fixed‐effect models.
Sequence generation
Was the allocation sequence adequately generated?
Yes (low risk of bias), e.g. a computer‐generated random sequence or a table of random numbers
No (high risk of bias), e.g. date of birth, clinic identity (ID)‐number or surname
Unclear (uncertain risk of bias), e.g. not reported
Blinding
Assessment of blinding will be restricted to blinding of outcome assessors, since it would not be possible to blind participants and treatment providers to the different interventions.
Was knowledge of the allocated interventions adequately prevented during the study?
Yes (low risk of bias)
No (high risk of bias)
Unclear (uncertain risk of bias)
Performance
Was similar care provided to patients in the treatment and control groups other than the intervention of interest?
Yes (low risk of bias), e.g. both groups were followed on similar schedules of neurologic exam and brain imaging
No (high risk of bias), e.g. each group was followed according to different schedules
Unclear (uncertain risk of bias), e.g. not reported
Incomplete reporting of outcome data
We will record the proportion of participants whose outcomes were not reported at the end of the study.
Were incomplete outcome data adequately addressed?
Yes (low risk of bias), if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms
No (high risk of bias), if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms
Unclear (uncertain risk of bias) if loss to follow‐up was not reported
Selective reporting of outcomes
Are reports of the study free of suggestion of selective outcome reporting?
Yes (low risk of bias), e.g. if review reported all outcomes specified in the protocol
No (high risk of bias), otherwise
Unclear (uncertain risk of bias), if insufficient information available
Other potential threats to validity
Was the study apparently free of other problems that could put it at a high risk of bias?
Yes (low risk of bias)
No (high risk of bias)
Unclear (uncertain risk of bias)
Measurement of treatment effect will be done with HR and RR, and with QoL measures; we will use the MD between treatment arms.
If future data are sufficient and clinically similar studies are available, the results will be pooled in meta‐analyses. For time‐to‐event data, HRs will be pooled using the generic inverse variance facility of RevMan 5. For dichotomous outcomes, the RR will be calculated for each study and then pooled. For continuous outcomes, the MD between the treatment arms at the end of follow‐up will be pooled if all trials measured the outcome on the same scale, otherwise standardized MD will be pooled. Random‐effects models with inverse variance weighting will be used for all meta‐analyses (DerSimonian 1986).
Measures of treatment effect
We will use the following measures of the effect of treatment.
For time‐to‐event data, we will use the HR, where possible.
For dichotomous outcomes, we will use the RR.
For continuous outcomes (e.g. QoL measures), we will use the MD between treatment arms.
Unit of analysis issues
Unit of analysis issues will be reviewed by two authors (AV, BJ) according to Higgins 2011 and differences will be resolved by discussion. These include reports where:
groups of individuals were randomized together to the same intervention (i.e. cluster‐randomized trials);
individuals undergo more than one intervention (e.g. in a cross‐over trial, or simultaneous treatment of multiple sites on each individual); or
there are multiple observations for the same outcome (e.g. repeated measurements, recurring events, measurements on different body parts).
Dealing with missing data
We will not impute missing outcome data. For the primary outcome, if data were missing or only imputed data were reported, we will contact trial authors to request data on the outcomes among participants who were assessed.
Assessment of heterogeneity
Heterogeneity between studies will be assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there is evidence of substantial heterogeneity, the possible reasons for this will be investigated and reported.
Assessment of reporting biases
Reporting biases will be reviewed and recorded by two review authors (AV, BJ).
Data synthesis
if sufficient, clinically similar studies are available, the results will be pooled in meta‐analyses.
For time‐to‐event data, HRs will be pooled using the generic inverse variance facility of RevMan 5.
For dichotomous outcomes, the RR will be calculated for each study and then pooled.
For continuous outcomes, the MD between the treatment arms at the end of follow‐up will be pooled if all trials measured the outcome on the same scale, otherwise SMD will be pooled.
Random‐effects models with inverse variance weighting will be used for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
Factors such as age, tumor size, tumor histology, extent of tumor resection, and length of follow‐up will be considered in interpretation of any heterogeneity.
Sensitivity analysis
Determination of whether sensitivity analysis will be required will be determined by two review authors (AV, BJ) and differences resolved through discussion according to Higgins 2011. If sensitivity analyses were required, one will be performed that will exclude trials which do not report adequate concealment of allocation or blinding of outcome assessor.
Contributions of authors
CP had the original idea for the protocol and helped review initial drafts of the protocol. AV, KC and BJ wrote the protocol, search, and final review. SC and KB provided senior mentorship and reviewed final drafts.
Sources of support
Internal sources
None, Other
External sources
None, Other
Declarations of interest
Bowen Jiang ‐ none known Anand Veeravagu ‐ none known Steven D Chang ‐ none known Keith L Black ‐ relevant financial activities outside the submitted work include employment with Cedars‐Sinai Medical Center. Chirag G Patil ‐ none known Kaisorn Chaichana ‐ none known
Stable (no update expected for reasons given in 'What's new')
References
References to studies excluded from this review
Ahmadi 2009 {published data only}
- Ahmadi R, Dictus C, Hartmann C, Zurn O, Edler L, Hartmann M, et al. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochirurgica 2009;151(11):1359-65. [DOI] [PubMed] [Google Scholar]
Claus 2005 {published data only}
- Claus EB, Horlacher A, Hsu L, Schwartz RB, Dello-Iacono D, Talos F, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer 2005;103(6):1227-33. [DOI] [PubMed] [Google Scholar]
Englot 2012 {published data only}
- Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery 2012;70(4):921-8. [DOI] [PubMed] [Google Scholar]
Jakola 2012 {published data only}
- Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgard G, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade glioma. JAMA 2012;308(18):1881-8. [DOI] [PubMed] [Google Scholar]
Jung 2011 {published data only}
- Jung TY, Jung S, Moon JH, Kim IY, Moon KS, Jang WY. Early prognostic factors related to progression and malignant transformation of low-grade gliomas. Clinical Neurology and Neurosurgery 2011;113(9):752-7. [DOI] [PubMed] [Google Scholar]
Karim 1996 {published data only}
- Karim ABMF, Maat B, Hiitlevoll R, Menten J, Rutten EHJM, Thomas DGT, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. International Journal of Radiation Oncology, Biology, Physics 1996;36(3):549-56. [DOI] [PubMed] [Google Scholar]
Laws 1984 {published data only}
- Laws ER, Taylor WF, Clifton MB, Okazaki H. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. Journal of Neurosurgery 1984;61(4):665-73. [DOI] [PubMed] [Google Scholar]
Majchrzak 2012 {published data only}
- Majchrzak K, Kaspera W, Bobek-Billewicz B, Hebda A, Stasik-Pres G, Majchrzak H, et al. The assessment of prognostic factors in surgical treatment of low-grade gliomas: A prospective study. Clinical Neurology and Neurosurgery 2012;114(8):1135-44. [DOI] [PubMed] [Google Scholar]
Philippon 1993 {published data only}
- Philippon JH, Clemenceau SH, Fauchon FH, Foncin JF. Supratentorial low-grade astrocytomas in adults. Neurosurgery 1993;32(4):554-9. [DOI] [PubMed] [Google Scholar]
Recht 1992 {published data only}
- Recht LD, Lew R, Smith TW. Suspected low-grade glioma: Is deferring treatment safe? Annals of Neurology 1992;31(4):431-6. [DOI] [PubMed] [Google Scholar]
Sanai 2008 {published data only}
- Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008;62(4):753-66. [DOI] [PubMed] [Google Scholar]
Scerrati 1996 {published data only}
- Scerrati M, Roselli R, Lacoangeli M, Pompucci A, Rossi GF. Prognostic factors in low grade (WHO grade II) gliomas of the cerebral hemispheres: the role of surgery. Journal of Neurology, Neurosurgery, and Psychiatry 1996;61(3):291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2002 {published data only}
- Shaw E, Arusell R, Scheithauer B, O'Fallon J, O'Neill B, Dinapoli R, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. Journal of Clinical Oncology 2002;20(9):2267-76. [DOI] [PubMed] [Google Scholar]
Shaw 2008 {published data only}
- Shaw EG, Berkey B, Coons SW, Bullard D, Brachman D, Buckner JC, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. Journal of Neurosurgery 2008;109(5):835-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2008 {published data only}
- Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. Journal of Clinical Oncology 2008;26(8):1338-45. [DOI] [PubMed] [Google Scholar]
van den Bent 2005 {published data only}
- den Bent MJ, Hassel MB, Schraub S, Hoang-Xuan K, Malmstrom PO, Collette L, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 2005;366(9490):985-90. [DOI] [PubMed] [Google Scholar]
van Veelen 1998 {published data only}
- Veelen MLC, Avezaat C, Kros J, Putten W, Vecht C. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. Journal of Neurology, Neurosurgery, and Psychiatry 1998;64(5):581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vuorinen 2003 {published data only}
- Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochirurgica 2003;145(1):5-10. [DOI] [PubMed] [Google Scholar]
Whittle 2010 {published data only}
- Whittle IR. What is the place of conservative management for adult supratentorial low-grade glioma? Advances and Technical Standards in Neurosurgery 2010;35:65-79. [DOI] [PubMed] [Google Scholar]
Wu 2007 {published data only}
- Wu JS, Mao Y, Shou LF, Tang WJ, Hu J, Song YY, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery 2007;61(5):935-48. [DOI] [PubMed] [Google Scholar]
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365-76. [DOI] [PubMed] [Google Scholar]
Armstrong 2006
- Armstrong TS, Mendoza T, Gning I, Coco C, Cohen MZ, Eriksen L, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). Journal of Neuro-Oncology 2006;80(1):27-35. [DOI] [PubMed] [Google Scholar]
Chang 2008
- Chang EF, Smith JS, Chang SM. Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. Journal of Neurosurgery 2008;109:817-24. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323(7305):157-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Henson 2008
- Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR: American Journal of Neuroradiology 2008;29(3):419-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane-handbook.org.
Jackson 2001
- Jackson RJ, Fuller GN, Abi-Said D, Lang FF, Gokaslan ZL, Shi WM, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro-Oncology 2001;3(3):193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karnofsky 1949
- Karnofsky DA, Burchenal JH. Evaluation of chemotherapeutic agents. In: CM Macleod, editors(s). The Clinical Evaluation of Chemotherapeutic Agents in Cancer. New York: Columbia University Press, 1949:191-205. [Google Scholar]
Karnofsky 1951
- Karnofsky DA, Burchenal JH, Armistead GC Jr, Southam CM, Bernstein JL, Craver LF, et al. Triethylene melamine in the treatment of neoplastic disease; a compound with nitrogen-mustardlike activity suitable for oral and intravenous use. Archives of Internal Medicine 1951;87:477-516. [DOI] [PubMed] [Google Scholar]
Keles 2001
- Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. Journal of Neurosurgery 2001 Nov;95(5):735-45. [DOI] [PubMed] [Google Scholar]
Lote 1998
- Lote K, Stenwig AE, Skullerud K, Hirschberg H. Prevalence and prognostic significance of epilepsy in patients with gliomas. European Journal of Cancer 1998 Jan;34(1):98-102. [DOI] [PubMed] [Google Scholar]
Lote 1998b
- Lote K, Egeland T, Hager B, Skullerud K, Hirschberg H. Prognostic significance of CT contrast enhancement within histological subgroups of intracranial glioma. Journal of Neuro-Oncology 1998;40(2):161-70. [DOI] [PubMed] [Google Scholar]
MacDonald 1990
- MacDonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. Journal of Clinical Oncology 1990;8(7):1277-80. [DOI] [PubMed] [Google Scholar]
Olson 2000
- Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology 2000 Apr 11;54(7):1442-8. [DOI] [PubMed] [Google Scholar]
Omay 2012
- Omay SB, Piepmeier JM, Knisely JPS. Low-grade gliomas: When and how to treat. Hematology/Oncology Clinics of North America 2012;26(4):797-809. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Pignatti 2002
- Pignatti F, den Bent M, Curran D, Debruyne C. Prognostic factors for survival in adult patients with cerebral low-grade glioma. Journal of Clinical Oncology 2002;20(8):2076-84. [DOI] [PubMed] [Google Scholar]
Pouratian 2010
- Pouratian N, Schiff D. Management of low-grade glioma. Current Neurology and Neuroscience Reports 2010;10(3):224-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Revesz 1993
- Revesz T, Scaravilli F, Coutinho L, Cockburn H, Sacares P, Thomas DGT. Reliability of histological diagnosis including grading in gliomas biopsied by image-guided stereotactic technique. Brain 1993;116(4):781-93. [DOI] [PubMed] [Google Scholar]
Ruiz 2009
- Ruiz J, Lesser GJ. Low-grade gliomas. Current Treatment Options in Oncology 2009;10(3):231-42. [DOI] [PubMed] [Google Scholar]
Soffietti 2010
- Soffietti R, Baumert BG, Bello L, Deimling A, Duffau H, Frénay M, et al: European Federation of Neurological Societies. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. European Journal of Neurology 2010 Sept;17(9):1124-33. [DOI] [PubMed] [Google Scholar]
Taphoorn 2010
- Taphoorn MJ, Claassens L, Aaronson NK, Coens C, Mauer M, Osoba D, et al, EORTC Quality of Life Group, and Brain Cancer, NCIC and Radiotherapy Groups. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. European Journal of Cancer 2010;46(6):1033-40. [DOI] [PubMed] [Google Scholar]
Weitzner 1995
- Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 1995;75(5):1151-61. [DOI] [PubMed] [Google Scholar]
Wen 2010
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. Journal of Clinical Oncology 2010;28(11):1963-72. [DOI] [PubMed] [Google Scholar]
Yordanova 2011
- Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within "noneloquent" areas in the left dominant hemisphere: toward a "supratotal" resection. Clinical article. Journal of Neurosurgery Aug 2011;115(2):232-9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Veeravagu 2011
- Veeravagu A, Jiang B, Chang SD, Black KL, Patil. Biopsy versus resection for the management of low-grade gliomas. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD009319] [DOI] [Google Scholar]
Veeravagu 2013
- Veeravagu A, Jiang B, Ludwig C, Chang SD, Black KL, Patil CG. Biopsy versus resection for the management of low-grade gliomas. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD009319.pub2] [DOI] [PubMed] [Google Scholar]


