Abstract
The anogenital prevalence of sexually transmitted infections (STIs) and the use of cervico-vaginal self-collected vs. clinician-collected samples were evaluated for the diagnosis of human immunodeficiency virus (HIV)-infected and HIV-uninfected women in the Tapajós region, Amazon, Brazil. We recruited 153 women for a cross-sectional study (112 HIV-uninfected and 41 HIV-infected) who sought health services. Anal and cervical scrapings and cervico-vaginal self-collection samples were collected. Real-time polymerase chain reaction methods were used for Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis and Mycoplasma genitalium. A syphilis test was also performed. Risk factors for STIs were identified by multivariate analysis. The overall prevalence of STIs was 30.4% (34/112) in HIV-uninfected women and 24.4% (10/41) in HIV-infected women. Anogenital Chlamydia trachomatis infection was the most prevalent in both groups of women (20.5% vs 19.5%). There was significant agreement for each STI between self-collected and clinician-collected samples: 91.7%, kappa 0.67, 95% confidence interval (CI) 0.49–0.85 for Chlamydia trachomatis; 99.2%, kappa 0.85, 95% CI 0.57–1.00 for Neisseria gonorrhoeae; 97.7%, kappa 0.39, 95% CI -0.16–0.94 for Trichomonas vaginalis; and 94.7%, kappa 0.51, 95% CI 0.20–0.82 for Mycoplasma genitalium. Women with human papillomavirus had coinfection or multiple infections with other STIs. Risk factors for STIs were being ≤ 25 years old, being employed or a student, reporting a history of STI and having a positive HPV test. A high prevalence of STIs in women in the Tapajós region was found. Cervico-vaginal self-collection is a useful tool for STI screening and can be used in prevention control programs in low-resource settings, such as in northern Brazil.
Introduction
The World Health Organization (WHO) estimates that among adults aged 15–49 years there are 357 million new infections each year of either Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Trichomonas vaginalis (TV) or syphilis. This represents approximately one million new infections every day [1]. Most of these infections are treatable with currently available antibiotics; however, antimicrobial resistance is a growing threat for NG, syphilis and Mycoplasma genitalium (MG) [2]. If untreated, STIs can cause several serious complications and increased risks of human immunodeficiency virus 1 (HIV-1) acquisition and transmission and cancer [3].
Very few population-based studies of STI prevalence exist for low- and middle-income countries [3]. In Brazil, the epidemiological surveillance data of STIs provided by the Ministry of Health are essentially restricted to HIV/AIDS, hepatitis and syphilis [4]. Some isolated studies in Brazil show a varied prevalence of STIs that depends on the infectious agent, population approached, sample size and laboratory techniques used [5,6]. In Brazil, 5.3% of women self-reported having had an STI in 2013. Women appear to carry a greater burden of STIs and self-reported STIs that were associated with increasing age, decreasing socioeconomic status, current or previous drug use, sex with a casual partner in the last 12 months, sex with the same sex partner, nonindigenous status and one or more previous HIV tests [7]. In the Amazon, accessibility, measured as the travel time via local transportation to a public health unit, remains a challenge for health care access, as well as for scientific research in these places [8], and this may be why there are very few studies on STIs in northern Brazil.
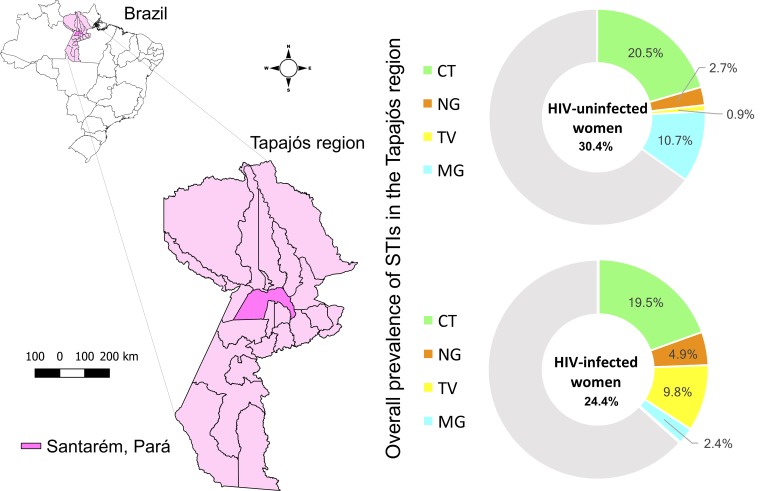
The Tapajós region is located in northern Brazil and comprises two meso-regions of the state of Pará, namely, the Lower Amazon and the Southwest (Fig 1). The main socioeconomic center is Santarém, which supports health services for 22 neighboring municipalities [9,10]; however, travel to Santarém for health services can be challenging and difficult [10]. Increasing knowledge regarding the epidemiology of CT, NG, TV and MG infection in women living in the Tapajós region is essential to provide surveillance data, a key element in the control and management of STIs. Moreover, the advantages of cervico-vaginal self-collection compared to clinician-collected samples include added privacy, convenience and time savings (since a provider appointment is not always necessary). Expanding STI testing options, especially in populations at risk [11], such as those who live in the Tapajós region.
Fig 1. Representative map of the Tapajós region in northern Brazil.
Highlight for Santarém and the 22 neighboring municipalities and the overall prevalence of STIs.
The aim of this study was to determine the anogenital prevalence of STIs and to evaluate the use of cervico-vaginal self-collection versus clinician-collection samples for STI diagnosis in HIV-infected and HIV-uninfected women living in the Tapajós region, Amazon, Brazil.
Materials and methods
Study design and population
The cross-sectional study was conducted from August 2015 to August 2016 and included nonindigenous, HIV-infected and HIV-uninfected women living in the Tapajós region. A total of 186 women were invited to participate in the study, and 153 of those accepted and were eligible to enter the study. To ensure that the study population was representative, the participants were recruited from nine public health units strategically covering the central, semiperipheral and peripheral points of Santarém. The population of other municipalities search for health care in public health units in the outskirts of town, while the population of Santarém search for health care in public health units downtown. The accessibility and infrastructure of the public health units that had health professionals prepared to perform the sample collections were also considered. We also invited all HIV-infected women at the Santarém Counseling and Testing Center, the only referral center for the care and follow-up of people living with HIV in this region, to participate. The HIV-uninfected women voluntarily sought the public health unit to schedule pap smear collection and were then invited to participate in the study. HIV-infected women who either had an appointment with a healthcare provider without a scheduled gynecological examination or who went to the public health unit to get antiretrovirals were then invited to participate in the survey. The HIV diagnostic testing, HIV viral load determination, and CD4+ T-lymphocyte count were conducted according to the manufacturer’s instructions in the published protocol [12].
Inclusion and exclusion criteria
The inclusion criteria were being 18 years of age or older (or consent from the legal guardian if the age was less than 18 years), sexually active and able to sign an informed consent form. The exclusion criteria were samples considered unsatisfactory on the pap smear, pregnancy, and use of antimicrobials in the previous 15 days. All women who met all inclusion criteria were considered eligible to participate in the survey.
Ethical issues and data collection
The study was approved by the Universidade do Estado do Pará Ethics Committee Board (UEPA, protocol no. 1.099.852) and the Instituto Oswaldo Cruz (IOC-FIOCRUZ, protocol no. 1.059.253) Ethics and Research Committees. All women agreed to participate in the study and signed an informed consent form in compliance with the Brazilian human ethical guidelines. This study was supported by a previous study in which we evaluated the acceptability of the cervico-vaginal self-collection, and HIV and HPV tests and sociodemographic data are fully described elsewhere [12]. Participation in this study included the collection of sociodemographic and clinical data using a standardized epidemiological form followed by the collection of specimens. All data are presented in the Tables and Figs. Detailed dataset can be available under request.
Specimen collection
Trained health professionals of the public health unit collected samples with a brush using anal scraping techniques. Similarly, cervical scrapings were collected with an endocervical brush during the pap smear, and women who agreed to perform the cervico-vaginal self-collection received the collection instructions and an individual collection kit. The self-collection was performed immediately after the pap smear in another office where the woman was alone, and at the end of the procedure, the sample was given to the provider to be labeled/identified and stored [12]. The anal and cervical scrapings were clinician-collected samples, and we compared cervical scrapings with the cervico-vaginal self-collection samples.
All collected specimens from scraping and self-collection were immediately stored in ThinPrep Pap Test (Hologic, Inc., San Diego, California, USA) at 5°C and transported to the Universidade Federal do Oeste do Pará in Santarém, Pará, for storage and, afterwards, were shipped to the Instituto Oswaldo Cruz, Fundação Oswaldo Cruz in Rio de Janeiro, Brazil. The transportation in Brazil was performed in appropriate polystyrene foam-insulated dry ice boxes with strict temperature monitoring and replenishment of dry ice on every shipment of the blood samples (-20°C to -80°C). All scraping and self-collection DNA samples were transported later to Johns Hopkins University in Baltimore, Maryland, United States of America, in appropriate packaging with strict temperature monitoring (-20°C to -80°C) and replacement of dry ice. All flight transport procedures in Brazil and the USA followed the International Air Transport Association (IATA) international requests for biological materials and were performed by a specialized commercial company (World Courier).
DNA extraction and STI molecular detection
DNA extraction from the samples was performed with the QIAamp DNA Mini Kit (Qiagen, Valencia, California, USA) and quantified by spectrophotometry using a NanoDrop (ND 1000, Fisher Scientific, Wilmington, Delaware, USA).
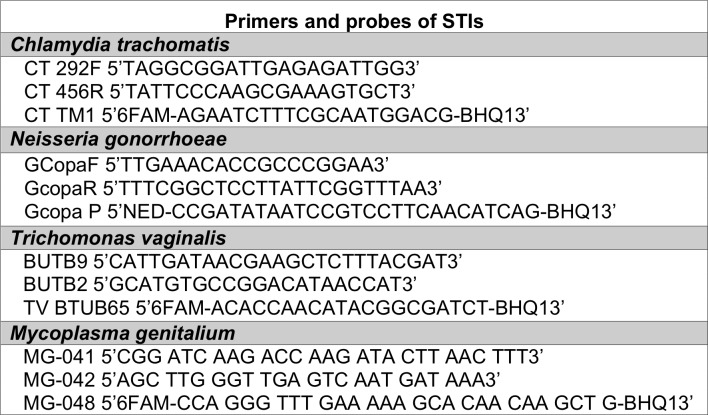
A duplex real-time PCR was performed for CT and NG, which was validated at the International Chlamydia Research Laboratory at Johns Hopkins University, utilizing primers and probes from an unpublished assay with sensitivity and specificity of 93.8% and 99.5% for CT and 100% and 100% for NG, respectively, that was validated by comparison with the Roche Amplicor and Hologic Aptima Combo 2 assays. The NG primers were adapted from a previously published protocol [13]. Real-time PCR was performed to detect TV with 90.1% sensitivity and 100% specificity [14], and real-time pdhD PCR was performed to detect MG with 80% sensitivity and 95% specificity [15]. The primer and probe sequences used to detect CT, NG, TV, and MG are shown below (Fig 2).
Fig 2. Primers and probes used to detect each STI.
All reactions included 40 μl of master mix (25 μl Taqman Fast Advance Master Mix (2X), 2 μl MgCl2 (25 mM), 2 μl each of the primers and probes (10 μM) and completed with dH2O) and 10 μl of sample DNA to a final volume of 50 μl of PCR reaction mixture. All reactions were performed in a QuantStudio 12K Flex Real-Time PCR System (Applied BioSystems by Life Technologies, Carlsbad, California, USA), and the parameters were 95°C for 20 s and 40 cycles of 95°C for 20 s and 60°C for 1 min.
HPV detection and genotyping
A nested PCR was performed to increase the specificity of HPV DNA detection, with PGMY09 and PGMY11 primers for the first round and GP5+ and GP6+ primers for the second round, as previously described [16]. HPV genotyping was performed according to a previously published protocol [12].
Syphilis test
The Alere DetermineTM Syphilis TP test (Delaware, USA) was used as a point-of-care screening test to detect antibodies to Treponema pallidum (TP) in the serum of HIV-infected women; the test has 100% sensitivity and 100% specificity, according to the manufacturer’s instructions [17].
Statistical analysis
Descriptive statistics of the qualitative variables were determined by frequency distribution and the quantitative variables by medians and interquartile ranges (IQR) or means and standard deviation (SD). The chi-square test was used to compare the proportions between the groups for categorical variables and the Mann-Whitney U test for continuous variables, both at 95% CI and P-value ≤ 0.05. For risk factors, the univariate analysis and odds ratio (OR) were calculated, considering the P-value ≤ 0.20. In the multivariable model, only the variables with a P-value ≤ 0.05 remained in the model, and the adjusted odds ratio (aOR) was calculated. Spearman's ρ test was performed to evaluate the linear relationship between the CD4+ T-cell count and STIs. We considered 39 copies/ml for the HIV viral load below the limit of detection (40 copies/ml) for statistical purposes. The positive agreement between cervico-vaginal self-collection and cervical scraping was calculated using the Kappa test with 95% CI and P-value ≤ 0.05. All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) software version 19.0 (IBM Corp., Armonk, USA).
Results
Sociodemographic characteristics of the women participants
A total of 153 women agreed to participate in the study, and overall, they provided 439 samples. Of these, 306 were collected by health professionals using anal and cervical scraping techniques, and 133 were self-collected by participants. The participants were split into two groups: HIV-uninfected women (n = 112) and HIV-infected women (n = 41). Overall, 53.1% had three or more pregnancies, 43.8% did not use hormonal contraception, 68.4% had her first sexual intercourse at or before 17 years of age, 54.7% reported four or more sexual partners in her life, 85.2% had pap smears regularly, 58.6% practiced anal sex, only 16.4% used condoms, and 30.5% reported a history of STIs. Additionally, 22.2% had abnormal pap smears, 49.7% had HPV DNA detected in cervical scrapings, and 86.9% performed the cervico-vaginal self-collection (Table 1). Some results have been reported previously in [12].
Table 1. Epidemiological variables of HIV-uninfected and HIV-infected women.
| Variables | HIV-uninfected n = 112 (%) |
HIV-infected n = 41 (%) |
Total n = 153 (%) |
Chi-square (P-value) | |
|---|---|---|---|---|---|
| Age (years) (n = 153) | 0.506 | ||||
| ≤20 | 08 (7.1) | 05 (12.2) | 13 (8.5) | ||
| 21–30 | 36 (32.1) | 08 (19.5) | 44 (28.8) | ||
| 31–40 | 26 (23.2) | 12 (29.3) | 38 (24.8) | ||
| 41–50 | 23 (20.5) | 10 (24.4) | 33 (21.6) | ||
| >50 | 19 (17.0) | 06 (14.6) | 25 (16.3) | ||
| Employment status (n = 153) | 0.585 | ||||
| Unemployed | 60 (53.6) | 24 (58.5) | 84 (54.9) | ||
| Employed or student | 52 (46.4) | 17 (41.5) | 69 (45.1) | ||
| Marital status (n = 150) | 0.006 | ||||
| Single | 36 (32.7) | 23 (57.5) | 59 (39.3) | ||
| Married/living together | 74 (67.3) | 17 (42.5) | 91 (60.7) | ||
| Education (years) (n = 152) | 0.146 | ||||
| Up to 14 | 34 (30.6) | 18 (43.9) | 52 (34.2) | ||
| 15–17 | 57 (51.4) | 20 (48.8) | 77 (50.7) | ||
| 18+ | 20 (18.0) | 03 (7.3) | 23 (15.1) | ||
| City of birth (n = 153) | 0.191 | ||||
| Santarém, Pará | 78 (69.6) | 25 (61.0) | 103 (67.3) | ||
| Other municipalities in the Tapajós region | 16 (14.3) | 11 (26.8) | 27 (17.6) | ||
| Outside the Tapajós region | 18 (16.1) | 05 (12.2) | 23 (15.0) | ||
| City of residence (n = 152) | 0.005 | ||||
| Santarém, Pará | 111 (99.1) | 35 (87.5) | 146 (96.1) | ||
| Other municipalities in the Tapajós region | 01 (0.9) | 05 (12.5) | 06 (3.9) | ||
| Number of pregnancies (n = 145) | 0.595 | ||||
| None | 14 (13.0) | 03 (8.1) | 17 (11.7) | ||
| Up to 2 | 39 (36.1) | 12 (32.4) | 51 (35.2) | ||
| 3+ | 55 (50.9) | 22 (59.5) | 77 (53.1) | ||
| Hormonal contraception (n = 153) | 0.004 | ||||
| Yes | 39 (34.8) | 07 (17.1) | 46 (30.1) | ||
| No | 40 (35.7) | 27 (65.9) | 67 (43.8) | ||
| No information | 33 (29.5) | 07 (17.1) | 40 (26.1) | ||
| History of cancer (n = 132) | 0.167 | ||||
| Yes | 15 (16.5) | 11 (26.8) | 26 (19.7) | ||
| No | 76 (83.5) | 30 (73.2) | 106 (80.3) | ||
| Alcohol use (n = 152) | 0.447 | ||||
| Yes | 32 (28.6) | 14 (35.0) | 46 (30.3) | ||
| No | 80 (71.4) | 26 (65.0) | 106 (69.7) | ||
| Tobacco use (n = 152) | 0.973 | ||||
| Yes | 16 (14.4) | 06 (14.6) | 22 (14.5) | ||
| No | 95 (85.6) | 35 (85.4) | 130 (85.5) | ||
| Illicit drug use (n = 151) | 0.124 | ||||
| Yes | 02 (1.8) | 03 (7.3) | 05 (3.3) | ||
| No | 108 (98.2) | 38 (92.7) | 146 (96.7) | ||
| Age of first sexual intercourse (years) (n = 152) | 0.052 | ||||
| Up to 17 | 71 (64.0) | 33 (80.5) | 104 (68.4) | ||
| 18+ | 40 (36.0) | 08 (19.5) | 48 (31.6) | ||
| Number of sex partners (n = 150) | 0.012 | ||||
| 1–3 | 57 (51.4) | 11 (28.2) | 68 (45.3) | ||
| 4+ | 54 (48.6) | 28 (71.8) | 82 (54.7) | ||
| Regular pap smear (n = 135) | 0.310 | ||||
| Yes | 82 (87.2) | 33 (80.5) | 115 (85.2) | ||
| No | 12 (12.8) | 08 (19.5) | 20 (14.8) | ||
| Condoms use (n = 152) | 0.100 | ||||
| Yes | 13 (11.7) | 12 (29.3) | 25 (16.4) | ||
| No | 98 (88.3) | 29 (70.7) | 127 (83.6) | ||
| Practiced anal sex (n = 152) | 0.457 | ||||
| Yes | 67 (60.4) | 22 (53.7) | 89 (58.6) | ||
| No | 44 (39.6) | 19 (46.3) | 63 (41.4) | ||
| History of STIa (n = 151) | 0.126 | ||||
| No | 81 (73.0) | 24 (60.0) | 105 (69.5) | ||
| Yes | 30 (27.0) | 16 (40.0) | 46 (30.5) | ||
| Types of STI: | |||||
| Anogenital warts | 22 (19.6) | 09 (22.0) | 31 (20.3) | ||
| Herpes | 05 (4.5) | 01 (2.4) | 06 (3.9) | ||
| Gonorrhea | 01 (0.9) | 01 (2.4) | 02 (1.3) | ||
| Syphilis | 01 (0.9) | 04 (9.8) | 05 (3.3) | ||
| Hepatitis B | 01 (0.9) | 0 (0.0) | 01 (0.7) | ||
| No information | 01 (0.9) | 01 (2.4) | 02 (1.3) | ||
| Pap smear result (n = 153) | b | ||||
| Negative | 104 (92.9) | 15 (36.6) | 119 (77.8) | ||
| Inflammation | 08 (7.1) | 21 (51.2) | 29 (19.0) | ||
| LSIL | 0 (0.0) | 03 (7.3) | 03 (2.0) | ||
| HSIL | 0 (0.0) | 02 (4.9) | 02 (1.3) | ||
| HPV test result (n = 153) | <0.0001 | ||||
| Negative | 75 (67.0) | 02 (4.9) | 77 (50.3) | ||
| Positive | 37 (33.0) | 39 (95.1) | 76 (49.7) | ||
| Cervico-vaginal self-collection (n = 153) | 0.069 | ||||
| Yes | 94 (83.9) | 39 (95.1) | 133 (86.9) | ||
| No | 18 (16.1) | 02 (4.9) | 20 (13.1) | ||
| HIV viral load (n = 41) | c | - | |||
| <40 copies/ml | 31 (75.6) | - | |||
| ≥40 copies/ml | 10 (24.4) | - | |||
| CD4+ T-cell count (N = 41) | c | - | |||
| <200 cells/mm3 | 08 (19.5) | - | |||
| 200–500 cells/mm3 | 20 (48.8) | - | |||
| >500 cells/mm3 | 13 (31.7) | - | |||
a The same person may have had more than one prior STI.
b Since the number of HIV-infected women in the Hepatitis B category was zero, the P-value could not be calculated. Since the number of HIV-uninfected women in the LSIL and HSIL categories was zero, the P-value could not be calculated.
c HIV-uninfected women did not perform CD4+ T-cell counts and HIV viral load testing.
LSIL: low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion.
Some of the data in Table 1 have been previously presented in [12].
Overall anogenital prevalence of STIs according to the sample type
A high prevalence of STIs was identified in the population studied, with occurrences in 30.4% (34/112) of HIV-uninfected and 24.4% (10/41) of HIV-infected women based on positivity in at least one of the three clinical sample types, although no statistically significant difference was observed (P = 0.470). Women diagnosed with cervical lesions and inflammation due to bacterial vaginosis were referred for medical care follow-up.
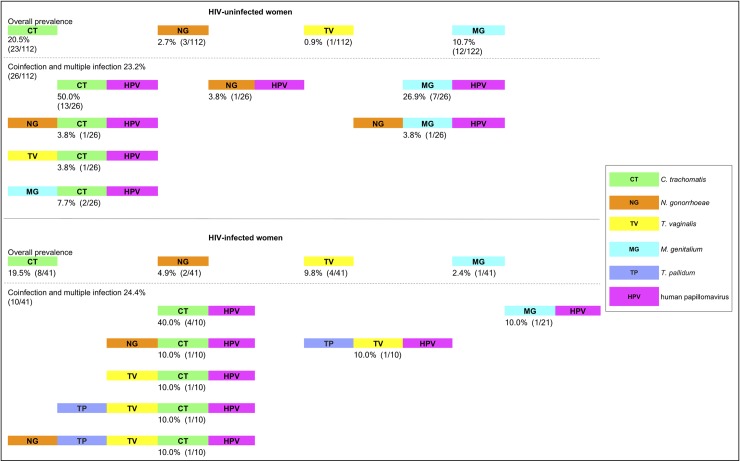
HIV-uninfected women had the highest anogenital prevalences of CT (20.5%) and MG (10.7%), while in HIV-infected women, the prevalences for CT (19.5%) and TV (9.8%) were highest, based on positivity in at least one of the three types of clinical specimens (Fig 3). The STI prevalence according to the sample type among HIV-uninfected and HIV-infected women is shown in Table 2.
Fig 3. Overall prevalence of each STI and the percentage of coinfection and multiple infection.
Coinfection is defined as a sample testing positive for two different microorganisms, for example, CT and HPV, while multiple infection is defined as a sample testing positive for three or more different microorganisms, for example, NG and CT and HPV. The number of HPV types was not used to define either coinfection or multiple infection.
Table 2. Prevalence of STIs in cervico-vaginal self-collection, cervical scraping and anal scraping samples.
| Sample type | CT | NG | TV | MG | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) |
N | % (95% CI) |
n | % (95% CI) |
n | % (95% CI) |
||
| HIV-uninfected women (n = 112) | |||||||||
| Self-collection (n = 94) | 13 | 13.8 (7.1–21.7) |
2 | 2.1 (0.0–5.6) |
1 | 1.1 (0.0–3.3) |
9 | 9.6 (4.1–15.5) |
|
| Cervical scraping (n = 112) | 15 | 13.4 (7.5–20.4) |
1 | 0.9 (0.0–2.9) |
1 | 0.9 (0.0–2.9) |
4 | 3.6 (0.9–7.6) |
|
| Anal scraping (n = 112) | 12 | 10.7 (5.3–16.8) |
2 | 1.8 (0.0–4.5) |
0 | 0 | 3 | 2.7 (0.0–6.1) |
|
| Total women* | 23 | 20.5 (13.4–28.7) |
3 | 2.7 (0.0–5.8) |
1 | 0.9 (0.0–2.9) |
12 | 10.7 (5.3–16.7) |
|
| HIV-infected women (n = 41) | |||||||||
| Self-collection (n = 39) | 6 | 15.4 (5.1–27.5) |
2 | 5.1 (0.0–13.3) |
1 | 2.6 (0.0–8.6) |
1 | 2.6 (0.0–8.6) |
|
| Cervical scraping (n = 41) | 7 | 17.1 (6.3–30.3) |
2 | 4.9 (0.0–12.5) |
3 | 7.3 (0.0–16.2) |
1 | 2.4 (0.0–8.1) |
|
| Anal scraping (n = 41) | 1 | 2.4 (0.0–8.1) |
2 | 4.9 (0.0–12.5) |
0 | 0 | 0 | 0 | |
| Total women* | 8 | 19.5 (7.7–32.4) |
2 | 4.9 (0.0–12.5) |
4 | 9.8 (0.0–19.5) |
1 | 2.4 (0.0–8.1) |
|
Self-collection: Cervico-vaginal self-collection, CT: Chlamydia trachomatis, NG: Neisseria gonorrhoeae, TV: Trichomonas vaginalis, MG: Mycoplasma genitalium, CI: confidence interval.
* Total number women considering positivity in at least one of the three types of clinical specimens.
Self-collected versus clinician-collected samples
The cervical scraping samples were used as clinician-collected samples for comparisons with cervico-vaginal self-collection using the Kappa test, with 95% CI, and P ≤ 0.05. There was significant agreement for each STI detected in the cervico-vaginal self-collection and cervical scraping groups. The comparisons of the CT DNA, NG DNA, TV DNA and MG DNA evaluations for both collection methods are shown in Table 3.
Table 3. Concordance between cervico-vaginal self-collection and cervical scraping collection calculated using the Kappa test.
| STIs | CT | NG | TV | MG |
|---|---|---|---|---|
| All participants (n = 133) | ||||
| Agreement | 91.7% | 99.2% | 97.7% | 94.7% |
| Positive | 122/133 | 132/133 | 130/133 | 126/133 |
| kappa (95% CI) | 0.67 (0.49–0.85) | 0.85 (0.57–1.00) | 0.39 (-0.16–0.94) | 0.51 (0.20–0.82) |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| HIV-uninfected (n = 94) | ||||
| Agreement | 91.5% | 98.9% | 100% | 92.6% |
| Positive | 86/94 | 93/94 | 94/94 | 87/94 |
| kappa (95% CI) | 0.64 (0.42–0.87) | 0.66 (0.04–1.00) | 1.00 | 0.43 (0.09–0.77) |
| P-value | <0.0001 | <0.0001 | <0,0001 | <0.0001 |
| HIV-infected (n = 39) | ||||
| Agreement | 92.3% | 100% | - | 100% |
| Positive | 36/39 | 39/39 | - | 39/39 |
| kappa (95% CI) | 0.72 (0.43–1.00) | 1.00 | - | 1.00 |
| P-value | <0.0001 | <0.0001 | 0.814 | <0.0001 |
CT: Chlamydia trachomatis, NG: Neisseria gonorrhoeae, TV: Trichomonas vaginalis, MG: Mycoplasma genitalium, CI: confidence interval.
It is important to note that substantial agreement (kappa> 0.60) was observed for comparisons of CT or NG DNA for all participants, HIV infected women, and HIV uninfected women. MG DNA showed substantial to moderate agreement among HIV-infected and non-HIV-infected women, respectively. The TV DNA presented the worst agreement for the two collection methods (Table 3).
Coinfections and multiple infections and association with HPV in HIV-uninfected and HIV-infected women
The prevalence of each STI and the percentage of coinfections and multiple infections in both groups was 23.2% (26/112) in HIV-uninfected women and 24.4% (10/41) in HIV-infected women (Fig 3). No statistically significant differences were observed (P = 0.879). In Fig 3, when we described the prevalence of each STI (CT, NG, TV and MG DNA) and coinfections and multiple infections (STI-HPV and STIs-HPV), "coinfection" is defined when the woman was positive for two microorganisms of different classes, for example CT and HPV, while "multiple infection" is defined when the woman was positive for three or more microorganisms of different classes, for example NG and CT and HPV. In this way, the criterion of coinfections and multiple infections was based on the quantity and diversity of microorganisms that the woman had, independent of the number of HPV types she was infected with.
For the HIV-uninfected women, the coinfection rate by decreasing order of prevalence was 50.0% (13/26) for CT-HPV, 26.9% (7/26) for MG-HPV, 7.7% (2/26) for MG-CT-HPV, and 3.8% (1/26) for NG-HPV, for NG-CT-HPV, TV-CT-HPV and for NG-MG-HPV. The group of HIV-infected women had a higher diversity of STIs, and the seroprevalence of syphilis was 9.8% (4/41). Coinfections by decreasing order of prevalence were 40.0% (4/10) for CT-HPV, and 10.0% (1/21) for MG-HPV, for NG-CT-HPV, TV-CT-HPV, TV-HPV, TP-TV-CT-HPV and NG-TP-TV-CT-HPV (Fig 3).
HPV infection was detected in all cases of coinfection and multiple infections, and women with positive HPV DNA tests were compared with those with negative tests. Women with positive HPV tests had coinfection or multiple infections with other STIs, and a statistically significant difference was observed (P = 0.004).
Additionally, using sequencing, for the HIV-uninfected women with coinfections and multiple infections, 61.5% (16/26) had only one HPV type and 38.5% (10/26) had multiples HPV types; all HIV-infected women positive for any of the four STIs had multiples HPV types (100%; 10/10). HPV types and classification by high oncogenic risk identified per woman are detailed below (Table 4).
Table 4. Number and HPV types identified per woman with coinfection and multiple infections.
| HIV-uninfected women N = 26 (23.2%) | |||
| Patient ID | Coinfection (STI-HPV) | Patient ID | Multiple infection (STIs-HPV) |
| 44 | CT-hrHPV58 | 70 | CT-NG-HPV6 |
| 88 | CT-hrHPV16 | 85 | CT-MG-HPV6-hrHPV16 |
| 120 | CT-HPV34 | 12 | CT-TV-hrHPV18-hrHPV45 |
| 47 | CT-hrHPV16 | 106 | CT-MG-hrHPV18-HPV54-HPV82 |
| 29 | CT-HPV62 | 73 | NG-MG-hrHPV16-hrHPV33-HPV53 |
| 42 | CT-HPV83 | ||
| 108 | CT-hrHPV18 | ||
| 74 | NG-hrHPV33 | ||
| 82 | MG-HPV6 | ||
| 92 | MG-hrHPV31 | ||
| 93 | MG-HPV6 | ||
| 109 | MG-HPV83 | ||
| 110 | MG-hrHPV16 | ||
| 65 | MG-hrHPV16 | ||
| 105 | MG-hrHPV31 | ||
| 16 | CT-hrHPV33-HPV87 | ||
| 21 | CT-hrHPV16-HPV83 | ||
| 41 | CT-hrHPV45-HPV70 | ||
| 86 | CT-HPV6-hrHPV16 | ||
| 103 | CT-HPV72-HPV84 | ||
| 111 | CT-HPV54-HPV69 | ||
| HIV-infected women n = 10 (24.4%) | |||
| Patient ID | Multiple infection (STI-HPV) | Patient ID | Multiple infection (STIs-HPV) |
| 112 | CT-HPV35-HPV40-HPV42- HPV44-hrHPV45-hrHPV56-hrHPV59-HPV61-HPV68-HPV69-HPV82 | 130 | CT-TV-HPV11-hrHPV31-HPV35-HPV40-HPV42-hrHPV51-hrHPV58-HPV68-HPV73-HPV82-HPV83 |
| 118 | CT-hrHPV33-HPV40-HPV42-HPV43-HPV44-hrHPV51-hrHPV56-hrHPV58-hrHPV59-HPV68-HPV70-HPV73-HPV82 | 116 | CT-NG-HPV11-hrHPV16-hrHPV31-HPV53-HPV54-hrHPV51-HPV82 |
| 153 | CT-HPV42-HPV70-hrHPV45 | 146 | TV-TP-HPV6-hrHPV16-hrHPV18-HPV40-HPV42-HPV43-hrHPV51-HPV53-hrHPV56-hrHPV59-HPV68-HPV82 |
| 135 | MG-HPV44-hrHPV56-HPV68-hrHPV16-HPV42-HPV53-HPV61-HPV82-HPV83 | 136 | CT-TV-TP-HPV44-hrHPV56-HPV82-hrHPV59 |
| 140 | CT-HPV11-HPV39-hrHPV45-HPV53-HPV69-HPV81-hrHPV31-HPV54 | 129 | CT-NG-TV-TP-HPV11-HPV40-HPV42-hrHPV51-HPV68-hrHPV31-HPV39-hrHPV58-HPV70-HPV73-HPV52 |
CT: Chlamydia trachomatis, NG: Neisseria gonorrhoeae, TV: Trichomonas vaginalis, MG: Mycoplasma genitalium, hrHPV: high-risk HPV, TP: Treponema pallidum.
Risk factors for any STI or CT infection in women in the Tapajós region
All HIV-infected women were on antiretroviral therapy (ART). The median HIV-1 viral loads among women with negative and positive test results for any of the four STIs were 39 (IQR, 39–39) and 39 (IQR, 39–833.75) copies/ml, respectively; the CD4 + T-cell counts were 382 (IQR, 223–567) and 416 (IQR, 217–551) cells/mm3, respectively. No statistically significant correlations were found (P = 0.286; P = 0.846) (S1 Table). Considering the CD4+ T-cell count as a proxy of the immunosuppression levels, Spearman's ρ test showed no significant correlation between the CD4+ T-cell count and the number of STIs (rô = -0.043, P = 0.790).
The identification of risk factors for STIs was determined by univariate and multivariate analyses. In the multivariate analysis, all variables were adjusted, and in the last model, the following variables remained in the model as risk factors: age ≤ 25 years increased the chance of having any of the four STIs by 5.6 (aOR = 5.60, 95% CI 2.22–14.13) and being employed or a student (aOR = 2.17, 95% CI 0.99–4.78), reporting a history of an STI (aOR = 2.37, 95% CI 1.01–5.55) and having a positive HPV DNA test (aOR = 2.82, 95% CI 1.27–6.25) significantly increased the risk of having any of the four STIs (Table 5). The multivariable analysis showed the following variables as risk factors for having a CT infection (P ≤ 0.05): age ≤ 25 years (aOR = 4.04, 95% CI 1.58–10.35), single status (aOR = 2.86, 95% CI 1.20–6.82) and alcohol use (aOR = 2.34, 95% CI 0.97–5.65) (Table 5).
Table 5. Univariate and multivariate analyses of risk factors for any STI or CT infection in HIV-uninfected and HIV-infected women.
| Any of the four STIs | CT infection | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Total (153) |
N (%) | P-value | OR (95% CI) |
Adjusted OR (95% CI) | N (%) | P-value | OR (95% CI) |
Adjusted OR (95% CI) |
| HIV status | 0.470 | 0.889 | |||||||
| HIV-uninfected | 112 | 34 (30.4) | - | - | 23 (20.5) | - | - | ||
| HIV-infected | 41 | 10 (24.4) | - | - | 08 (19.5) | - | - | ||
| Age (years) | <0.001 | 0.005 | |||||||
| ≤25 years | 31 | 18 (58.1) | 5.06 (2.20–11.66) |
5.60 (2.22–14.13) |
12 (38.7) | 3.39 (1.42–8.12) |
4.04 (1.58–0.35) |
||
| 26+ | 121 | 26 (21.5) | 1.00 | 1.00 | 19 (15.7) | 1.00 | 1.00 | ||
| Employment status | 0.027 | 0.222 | |||||||
| Unemployed | 84 | 18 (21.4) | 1.00 | 1.00 | 14 (16.7) | - | - | ||
| Employed or student | 69 | 26 (37.7) | 2.22 (1.09–4.52) |
2.17 (0.99–4.78) |
17 (24.6) | - | - | ||
| Marital status | 0.085 | 0.017 | |||||||
| Single | 59 | 22 (37.3) | 1.87 (0.91–3.81) |
- | 18 (30.5) | 2.63 (1.18–5.91) |
2.86 (1.20–6.82) |
||
| Married/ living together | 91 | 22 (24.2) | 1.00 | - | 13 (14.3) | 1.00 | 1.00 | ||
| Education (years) | 0.503 | 1.000 | |||||||
| Up to 17 | 129 | 36 (27.9) | - | - | 27 (20.9) | - | - | ||
| 18+ | 23 | 08 (34.8) | - | - | 04 (17.4) | - | - | ||
| Number of pregnancies | 0.001 | 0.015 | |||||||
| Up to 1 | 39 | 19 (48.7) | 3.62 (1.65–7.94) |
- | 13 (33.3) | 2.81 (1.20–6.59) |
- | ||
| 2+ | 106 | 22 (20.8) | 1.00 | - | 16 (15.1) | 1.00 | - | ||
| Hormonal contraception | 0.825 | 0.572 | |||||||
| No | 67 | 20 (29.9) | - | - | 14 (20.9) | - | - | ||
| Yes | 46 | 14 (30.4) | - | - | 11 (23.9) | - | - | ||
| No information | 40 | 10 (25.0) | - | - | 06 (15.0) | - | - | ||
| History of cancer | 0.550 | 0.707 | |||||||
| No | 106 | 35 (33.0) | - | - | 24 (22.6) | - | - | ||
| Yes | 26 | 07 (26.9) | - | - | 5 (19.2) | - | - | ||
| Alcohol use | 0.242 | 0.029 | |||||||
| No | 106 | 27 (25.5) | - | - | 16 (15.1) | 1.00 | 1.00 | ||
| Yes | 46 | 16 (34.8) | - | - | 14 (30.4) | 2.46 (1.08–5.60) |
2.34 (0.97–5.65) |
||
| Tobacco use | 0.851 | 0.777 | |||||||
| No | 130 | 38 (29.2) | - | - | 26 (20.0) | - | - | ||
| Yes | 22 | 06 (27.3) | - | - | 05 (22.7) | - | - | ||
| Age of first sexual intercourse (y. o.) | 0.265 | 0.227 | |||||||
| Up to 17 | 104 | 33 (31.7) | - | - | 24 (23.1) | - | - | ||
| 18+ | 48 | 11 (22.9) | - | - | 07 (14.6) | - | - | ||
| Lifetime number sex partners | 0.585 | 0.870 | |||||||
| 1–3 | 68 | 21 (30.9) | - | - | 14 (20.6) | - | - | ||
| 4+ | 82 | 22 (26.8) | - | - | 16 (19.5) | - | - | ||
| Regular pap smear | 0.012 | 0.140 | |||||||
| No | 20 | 11 (55.0) | 3.31 (1.25–8.76) |
- | 07 (35.0) | 2.28 (0.83–6.37) |
- | ||
| Yes | 115 | 31 (27.0) | 1.00 | - | 22 (19.1) | 1.00 | - | ||
| Condoms use | 0.909 | 0.957 | |||||||
| No | 127 | 37 (29.1) | - | - | 26 (20.5) | - | - | ||
| Yes | 25 | 07 (28.0) | - | - | 05 (20.0) | - | - | ||
| History of STI | 0.162 | 0.120 | |||||||
| No | 105 | 27 (25.7) | 1.00 | 1.00 | 18 (17.1) | 1.00 | - | ||
| Yes | 46 | 17 (37.0) | 1.69 (0.81–3.56) |
2.37 (1.01–5.55) |
13 (28.3) | 1.90 (0.84–4.32) |
- | ||
| Pap smear result | 0.210 | -* | |||||||
| Negative | 119 | 31 (26.1) | - | - | 21 (17.6) | 1.00 | - | ||
| Inflammation (BV) | 29 | 11 (37.9) | - | - | 10 (34.5) | 2.46 (1.00–6.04) |
- | ||
| LSIL | 3 | 02 (66.7) | - | - | 0 (0.0) | - | - | ||
| HSIL | 2 | 0 (0.0) | - | - | 0 (0.0) | - | - | ||
| HPV test | 0.004 | 0.008 | - | ||||||
| Negative | 77 | 14 (18.2) | 1.00 | 1.00 | 09 (11.7) | 1.00 | - | ||
| Positive | 76 | 30 (39.5) | 2.94 (1.40–6.15) |
2.82 (1.27–6.27) |
22 (28.9) | 3.08 (1.31–7.23) |
- | ||
*Since the number of CT-infected women in the LSIL and HSIL categories was zero, the P-value could not be calculated; since the number of Mycoplasma genitalium-infected women in the HSIL category was zero, the P-value could not be calculated.
CT: Chlamydia trachomatis, OR: odds ratio, CI: confidence interval, aOR: adjusted odds ratio, BV: bacterial vaginosis, LSIL: low-grade squamous intraepithelial lesion, HSIL: high-grade squamous intraepithelial lesion.
Discussion
This is the first study of STI prevalence detected by molecular testing in self-collected samples compared to clinician-collected samples in women living in the Tapajós region, Amazon, Brazil. In the present study, a high STI prevalence was identified, namely, 30.4% in HIV-uninfected women and 24.4% in HIV-infected women, as assessed by positivity in at least one of the three types of clinical specimens. This prevalence is much higher than the 2.1% detected in a previous multicenter study with HIV-infected women [5] and the 13.6% of women in the general population in Peru [18], but the prevalence was less than the 36.5% observed in HIV-infected pregnant women in northeastern Brazil [6] and the 60.6% observed in female sex workers in Peru [18]. In this study, we found an overall prevalence of STIs slightly higher in HIV-uninfected women than in HIV-infected women, although this difference was not statistically significant. We believe that this can be explained by the differences in sample size distribution between the groups, which is a limitation of our study and is discussed more below. However, we believe that the overall prevalence of STIs in HIV-infected women should be higher because these women had a higher diversity of STIs, coinfections and multiple infections, and the seroprevalence of syphilis was also high in this study.
In our study, the HIV-uninfected and HIV-infected women were able to easily understand how to perform adequate self-collection, considering the substantial and significant overall agreement of detection for each STI compared to the cervical scraping (Table 3). This finding is in agreement with results of studies conducted in other countries, where the self-collected samples were found to be a valid and reliable method for STI testing in women, with high sensitivity and high specificity and moderate to substantial agreement in comparison with clinician-collected samples [19–22]. In Brazil, we found only two studies reporting self-collected samples for STI detection performed with women in São Paulo but with different designs and objectives. In a 2006 study focusing on low-income women (n = 818), the objective was to compare the immediate response rate to the acceptability of self-collected vaginal swabs for CT, NG and TV DNA detection and rapid self-test for trichomoniasis in women participating in home-based screening and women in clinic-based screening; that is, they basically assessed the same techniques in two groups of different women. The home group had 80%, the clinic group had 76% of the immediate response rate, and no significant differences were observed in the overall prevalence of STI between groups [23]. The second study was in 2013, as part of the study previously cited, with women (n = 625) also participating in home-and clinic-based screenings that self-collected two vaginal swabs, aiming to evaluate specificity and sensitivity of the rapid self-test for trichomoniasis in both settings, having a PCR as the gold standard [24]. In spite of the importance of the scientific data reported in both studies, there is a comparative limitation because in those two studies, there was no analysis of agreement of STI detection between self-collected and clinician collected samples of the same woman and with the two specimens collected at the same time, as in our study; this approach allowed us to state that women routinely attending health clinics, either HIV-infected women or HIV-uninfected women that often do not attend or infrequently attend health clinics, were able to perform a self-collection as well as clinician-collected samples. To the best of our knowledge, this is a pioneering study conducted in Brazil on the prevalence of STI, including unpublished data on MG prevalence in the Brazilian Amazon, and comparative performance of the molecular detection of STI using scientifically validated methods among self-collected and clinician-collected samples produced by the same woman and at the same time. We preserved the scenario of care in the public health units of the region to make it more feasible to include this methodology in the screening of STI in the reality of the most vulnerable women. The unavailability of laboratory STI testing and inaccessible medical appointments were barriers to the early diagnosis of STIs of bacterial etiology that have not been well explored; however, this can be improved by less invasive and patient-centered methods, such as cervico-vaginal self-collection, which has proven to be an efficient alternative, not only for STI screening but also for cervical cancer screening [20,25].
Anogenital CT was the most common STI detected, with an overall prevalence of 20.5% for HIV-uninfected women and 19.5% for HIV-infected women. The prevalence of anogenital CT infection was higher than that found in multicenter and isolated studies conducted with HIV-uninfected and HIV-infected women in other low- and middle-income countries [26], but variable findings exist in previous studies from Brazil [5,27,28]. In the Brazilian Amazon, the prevalence of genital infection by CT ranges from ~4.8% to ~11.0% in nonindigenous women [29–31]. It is believed that, in the Tapajós region, the prevalence of CT is high in HIV-infected and HIV-uninfected women. This hypothesis is reinforced by a high seroprevalence of specific serotypes of CT (48.1%) detected in blood samples from women in Santarém, Pará [32].
Anogenital prevalence of NG identified in the two groups of participants (2.7% vs 4.9%) is in agreement with the range of 0.3% to 0.9% of NG reported for women in Brazil [5,31]; however, the prevalence of TV identified in our study (0.9 vs 9.8%) was smaller than the 18.04% identified by another study in Amazonian women [29].
The seroprevalence of syphilis (9.8%) observed in HIV-infected women was higher than that in high-risk populations for STIs in eastern Africa (8.4%) [3]. The magnitude of syphilis worldwide has been challenging to address and is a source of great concern among health authorities since the number of cases has increased dramatically and the risk of mother-to-child transmission of HIV has become an emerging threat [1,33]. Efforts have been made to improve the coverage of rapid diagnostic testing in pregnant women at antenatal care clinics in Brazil [34]. Additionally, trials for the implementation of point-of-care syphilis testing have been performed with women living in high-risk areas in the Brazilian Amazon [35].
There have been very few studies of MG prevalence, and those have assessed specific groups of women [36]. Our findings provide the first report of anogenital MG infection in northern Brazil. We identified a prevalence of 10.7% of MG infection in HIV-uninfected women without cervical lesions but with inflammation due to bacterial vaginosis, compared to 2.4% in HIV-infected women with cervical dysplasia and inflammation. This prevalence was lower than that detected in 28.1% of women living in the northeastern region of Brazil [37]. This finding was notable because HIV-uninfected women had a higher prevalence of MG infection and a total of 30.7% of coinfection and multiple infections with MG, while HIV-infected women had only 10% prevalence of coinfection and multiple infections with MG. Further epidemiological studies are needed to understand MG infection in women living in the Tapajós region.
There was no significant correlation in the univariate or multivariate analysis of HIV infection status and STI positivity and no statistically significant difference between the groups. It is important to note that all HIV-infected women in this study were on cART, and most of them were clinically and immunologically well, with virological suppression. Fortunately, this can be attributed to the efficient public health strategies implemented in Brazil to combat HIV/AIDS, such as the provision of regular medical appointments and treatment for HIV free-of-charge by the Brazilian Ministry of Health [38,39]. This study suggests that both HIV-infected and HIV-uninfected women in the Tapajós region have a high prevalence of STIs with coinfections.
Interestingly, in all cases of coinfections, there was an HPV infection; a statistically significant difference and association in the uni- and multi-variate analyses were observed, suggesting that women with HPV infection are at an increased risk of having another STI. Other studies have shown an association between HPV and other STIs and the development of cervical and anal lesions, with an increased risk for HPV-CT and HPV-MG [40–42].
One limitation of our study was the small sample size in the group of HIV-infected women, which may have been influenced by difficulties with accessibility and the infrastructure of the health units in Santarém, as well as the lack of data in the literature on HIV and STI prevalence in the population studied. To maintain homogeneity between the two groups of women, we decided to match them based on age and the date of diagnosis and survey. We established the criterion of including only HIV-infected women who had been diagnosed with HIV infection during the same study period and in the same age groups as HIV-uninfected women (36.9 ± 13.1). The WHO recognizes that one of the current challenges of STI control strategies is to improve the quality of future estimates and supporting countries to generate their own national estimates. This should be done with more data from studies conducted on the general population with low risk and on key populations. WHO is also exploring alternative methods for generating STI prevalence and incidence estimates, including approaches that use data collected from studies conducted in key populations and the use of new technologies to facilitate diagnosis [33,43]. The strength of this study is that the unpublished epidemiological findings of the prevalence of STIs in a population of HIV-infected and HIV-uninfected women and the significant agreement in the STI detection in self-collected compared to clinician-collected samples obtained meet the needs noted by the WHO. Accurate estimates are crucial for guiding broader STI prevention and control efforts, primarily in limited-resource settings at higher risk and with very little scientific data available. The methods used in this study, as well as the implications of the findings, may be applied to new studies conducted among similar populations in isolated and low-resource settings. Further epidemiological studies are needed to elucidate STIs, with attention to the ascent of MG infection due to the increase in the prevalence and the high capacity of resistance aggravated by the lack of knowledge of the population and the unfamiliarity of health professionals.
STI surveillance strategies in the Tapajós region should be targeted to the general population of women, independent of HIV-infected status, with a focus on young women and adolescents with a history of STI or HPV infection who are single and use alcohol, especially during their reproductive life. The risk factors found are well established in the literature [27,28,40,41]. Cross-sectional population-based studies indicate that not only geographic but also political isolation are responsible for unequal access to health services and maternal and child care in more urbanized areas of the Amazon [44,45]. The Tapajós region is geographically isolated, and people have difficulty accessing the public health units in peripheral areas of Santarém.
Efforts should aim to reduce the burden of STIs for all women, especially at a time when cART reduces the burden of HIV infection, and women can have the ability to talk more about reproductive choices. The mobilization of resources for the implementation of cervico-vaginal self-collection as an alternative for the detection of STIs by rapid diagnostic tests (RDTs) based on nucleic acid amplification assays (NAATs) should be seriously evaluated as a strategy to improve screening programs [33], considering the insufficient coverage of neglected women living in isolated areas with high incidence and mortality of cervical cancer and high prevalence of STI, as in the Tapajós region.
Conclusions
In summary, we identified a high prevalence of STIs and a significant positive agreement rate between self-collected and clinician-collected samples in this study. Considering these findings, we suggest the implementation of STI screening and prevention control programs in groups at high risk of acquiring and transmitting STIs, such as young women and HIV-infected women, especially in resource-limited settings and isolated regions of the country. In addition, further epidemiological studies are warranted to investigate the role of HPV with other STIs and the impact of CT and MG on HIV-infected and HIV-uninfected women living in the Tapajós region, Amazon, and other regions of Brazil.
Supporting information
(DOCX)
Acknowledgments
The authors would like to thank all of the women who agreed to participate in this study. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. We would like to recognize the Postgraduation Program in Tropical Medicine at the Oswaldo Cruz Institute, Oswaldo Cruz Foundation for support of Luana L. S. Rodrigues’s doctorate. We would also like to recognize Dr. Charlotte A. Gaydos for mentorship during Luana L. S. Rodrigues’s visitation as a doctoral student and all in members of the STD lab in the Division of Infectious Diseases, Johns Hopkins University. We would like to thank the Secretaria Municipal de Saúde and Centro de Testagem e Aconselhamento of Santarém, Pará, for having authorized the recruitment of the women in the health units. Finally, we thank Jonas Aguiar for the excellent figure quality in the Tapajós region map.
Data Availability
Data underlying the study cannot be made publicly available as the participants did not consent to have their full transcripts made publicly available. The original data set is available to researchers who meet the criteria for accessing sensitive data, upon request to the corresponding author (luana.robrigues@ufopa.edu.br) or the Ethics Committee of the Oswaldo Cruz Institute (cepfiocruz@ioc.fiocruz.br).
Funding Statement
L. L. S. R. was supported a grant, process number 88881.131796/2016-01, from CAPES Foundation - Brazil. C. A. G. was supported by, grant number U54EB007958, of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and, grant number U-01068613, of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). A. F. N. was supported by, grant number 401810/2015-1, the Programa Estratégico de Apoio a Pesquisa em Saúde (PAPES VII), Fundação Oswaldo Cruz (FIOCRUZ)/ Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization—WHO. Report on global sexually transmitted infection surveillance 2015 [Internet]. Geneva, Switzerland; 2016. Available: http://www.who.int
- 2.Workowski Kimberly A., Bolan GA. Sexually Transmitted Diseases Treatment Guidelines [Internet]. Morbidity and Mortality Weekly Report (MMWR). 2015. 10.1097/00008480-200308000-00006 [DOI] [PubMed] [Google Scholar]
- 3.Torrone EA, Morrison CS, Chen P-L, Kwok C, Francis SC, Hayes RJ, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies [Internet]. PLOS Medicine. 2018. 10.1371/journal.pmed.1002511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministério da Saúde do Brasil. Boletins Epidemiológicos–Linha do tempo. In: Ministério da Saúde do Brasil. Secretaria de Vigilância em Saúde. Departamento de Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais. [Internet]. 2018 [cited 5 May 2018]. Available: http://www.aids.gov.br/pt-br/centrais-de-conteudos/boletins-epidemiologicos-vertical
- 5.Miranda AE, Silveira MF, Travassos AGÁ, Tenório T, Val ICC, Lannoy L de, et al. Prevalence of Chlamydia trachomatis and Neisseria gonorrhea and associated factors among women living with Human Immunodeficiency Virus in Brazil: a multicenter study. Brazilian J Infect Dis. Sociedade Brasileira de Infectologia; 2017;21: 402–407. 10.1016/j.bjid.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travassos AGÁ, Brites C, Netto EM, Fernandes S de A, Rutherford GW, Queiroz CM. Prevalence of sexually transmitted infections among HIV-infected women in Brazil. Brazilian J Infect Dis. 2012;16: 581–585. 10.1016/j.bjid.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 7.De Queiroz MJ, Sabidó M, De Barros CHD, Pascom ARP, Galbán E, Benzaken AS. P3. 148 Reported sexually transmitted infections in brazil: prevalence and risk factors These include: Email alerting service. Sex Transm Infect. 2017;93: 2017–2018. 10.1136/sextrans-2017-053264.383 [DOI] [Google Scholar]
- 8.Ruffinen CZ, Sabidó M, Díaz-Bermúdez XP, Lacerda M, Mabey D, Peeling RW, et al. Point-of-care screening for syphilis and HIV in the borderlands: Challenges in implementation in the Brazilian Amazon. BMC Health Serv Res. BMC Health Services Research; 2015;15: 1–10. 10.1186/s12913-014-0652-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Instituto Brasileiro de Geografia e Estatistica. Uso da terra no Estado do Pará: relatório técnico [Internet]. 95892nd ed. Rio de Janeiro; 2013. 95892
- 10.Cissa Loyola. Conheça a estrutura do estado do Tapajós. In: Tapajos meu estado [Internet]. 2018 [cited 2 Jun 2018]. Available: http://www.tapajosmeuestado.com.br/2018/02/conheca-estrutura-do-estado-do-tapajos.html
- 11.Gaydos CA. Let’s take a “selfie”: Self-collected samples for sexually transmitted infections. Sex Transm Dis. 2018;45: 278–279. 10.1097/OLQ.0000000000000785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues LLS, Morgado MG, Sahasrabuddhe VV, De Paula VS, Oliveira NS, Chavez-Juan E, et al. Cervico-vaginal self-collection in HIV-infected and uninfected women from Tapajós region, Amazon, Brazil: High acceptability, hrHPV diversity and risk factors. Gynecol Oncol. 2018;151: 102–110. 10.1016/j.ygyno.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabrizi SN, Chen S, Tapsall J, Garland SM. Evaluation of opa-based real-time PCR for detection of Neisseria gonorrhoeae. Sex Transm Dis. 2005;32: 199–202. 10.1097/01.olq.0000154495.24519.bf [DOI] [PubMed] [Google Scholar]
- 14.Hardick J, Yang S, Lin S, Duncan D, Gaydos C. Use of the Roche LightCycler Instrument in a Real-Time PCR for Trichomonas vaginalis in Urine Samples from Females and Males Use of the Roche LightCycler Instrument in a Real-Time PCR for Trichomonas vaginalis in Urine Samples from Females and Males. J Clin Microbiol. 2003;41: 5619–5622. 10.1128/JCM.41.12.5619-5622.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller EE, Venter JME, Magooa MP, Morrison C, Lewis DA, Mavedzenge SN. Development of a rotor-gene real-time PCR assay for the detection and quantification of Mycoplasma genitalium. J Microbiol Methods. Elsevier B.V.; 2012;88: 311–315. 10.1016/j.mimet.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 16.Oliveira NS, Andrade C V., Grinsztejn B, Friedman RK, Cunha CB, Veloso VG, et al. In situ FoxP3+ and IL-10 over-expression is associated with high grade anal lesions in HIV infected patients. Int J Clin Exp Pathol. 2016;9: 1520–1532. [Google Scholar]
- 17.ALERE DETERMINETM SYPHILIS. Alere Determine Syphilis Brochure—Global (English). In: ALERETM [Internet]. 2013 [cited 12 Dec 2018] p. 4. Available: https://www.alere.com/en/home/product-details/determine-syphilis.html
- 18.Cárcamo CP, Campos PE, García PJ, Hughes JP, Garnett GP, Holmes KK. Prevalences of sexually transmitted infections in young adults and female sex workers in Peru: A national population-based survey. Lancet Infect Dis. Elsevier Ltd; 2012;12: 765–773. 10.1016/S1473-3099(12)70144-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lockhart A, Psioda M, Ting J, Campbell S, Mugo N, Kwatampora J, et al. Prospective Evaluation Of Cervico-Vaginal Self And Cervical Physician- Collection For The Detection Of Chlamydia Trachomatis, Neisseria Gonorrhoeae, Trichomonas Vaginalis, And Mycoplasma Genitalium Infections. Sex Transm Dis. 2018;45: 488–493. 10.1097/OLQ.0000000000000778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reisner SL, Deutsch MB, Peitzmeier SM, White Hughto JM, Cavanaugh T, Pardee DJ, et al. Comparing self- and provider-collected swabbing for HPV DNA testing in female-to-male transgender adult patients: A mixed-methods biobehavioral study protocol. BMC Infect Dis. BMC Infectious Diseases; 2017;17: 1–10. 10.1186/s12879-016-2122-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canadian Agency for Drugs and Technologies in Health. Self-Collected versus Clinician Collected Samples for Sexually Transmitted Infection Testing in Women: A Review of Comparative Clinical Effectiveness Cost-Effectiveness, and Guidelines. CADTH Rapid Response Reports. 2016; https://www.ncbi.nlm.nih.gov. Available: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0088794/pdf/PubMedHealth_PMH0088794.pdf [PubMed]
- 22.Lunny C, Taylor D, Hoang L, Wong T, Gilbert M, Lester R, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: A systemic review and meta-analysis. PLoS One. 2015;10: 1–23. 10.1371/journal.pone.0132776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippman SA, Jones HE, Luppi CG, Pinho AA, Veras MAMS, Van De Wijgert JHHM. Home-based self-sampling and self-testing for sexually transmitted infections: Acceptable and feasible alternatives to provider-based screening in low-income women in São Paulo, Brazil. Sex Transm Dis. 2007;34: 421–428. 10.1097/01.olq.0000245958.34961.27 [DOI] [PubMed] [Google Scholar]
- 24.Jones HE, Lippman SA, Caiaffa-Filho HH, Young T, Van De Wijgert JHHM. Performance of a rapid self-test for detection of Trichomonas vaginalis in South Africa and Brazil. J Clin Microbiol. 2013;51: 1037–1039. 10.1128/JCM.01547-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown AJ, Trimble CL. New Technologies for Cervical Cancer Screening. Best Pr Res Clin Obs Gynaecol. 2012;26: 233–242. 10.1016/j.bpobgyn.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph Davey D, Shull H, Billings J, Wang D, Adachi K, Klausner J. Prevalence of Curable Sexually Transmitted Infections in Pregnant Women in Low- and Middle-Income Countries From 2010 to 2015: A Systematic Review. Sex Transm Dis. 2016;43: 450–458. 10.1097/OLQ.0000000000000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travassos AG, Xavier-Souza E, Netto E, Dantas EV, Timbó M, Nóbrega I, et al. Anogenital infection by Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-infected men and women in Salvador, Brazil. Brazilian J Infect Dis. Elsevier Editora Ltda; 2016;20: 569–575. 10.1016/j.bjid.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto VM, Szwarcwald CL, Baroni C, Stringari LL, Inocêncio LA, Miranda AE. Chlamydia trachomatis prevalence and risk behaviors in parturient women aged 15 to 24 in Brazil. Sex Transm Dis. 2011;38: 957–961. 10.1097/OLQ.0b013e31822037fc [DOI] [PubMed] [Google Scholar]
- 29.Costa-Lira E, Jacinto AHVL, Silva LM, Napoleão PFR, Barbosa-Filho RAA, Cruz GJS, et al. Prevalence of human papillomavirus, Chlamydia trachomatis, and Trichomonas vaginalis infections in Amazonian women with normal and abnormal cytology. Genet Mol Res. 2017;16 10.4238/gmr16029626 [DOI] [PubMed] [Google Scholar]
- 30.Brasiliense DM, Borges N, Augusto W, Ferreira S. Genotyping and prevalence of Chlamydia trachomatis infection among women in Belém, Pará, northern Brazil. J Infect Dev Ctries. 2016;10: 134–137. 10.3855/jidc.6474 [DOI] [PubMed] [Google Scholar]
- 31.Benzaken A, Sabidó M, Galban E, Rodrigues Dutra DL, Leturiondo AL, Mayaud P. HIV and sexually transmitted infections at the borderlands: Situational analysis of sexual health in the Brazilian Amazon. Sex Transm Infect. 2012;88: 294–300. 10.1136/sextrans-2011-050309 [DOI] [PubMed] [Google Scholar]
- 32.De Oliveira Guimares Ishak M, Mesquita Costa M, De Costa Almeida NC, Menezes Santiago A, De Brito WB, Vallinoto ACR, et al. Chlamydia trachomatis serotype A infections in the amazon region of Brazil: Prevalence, entry and dissemination. Rev Soc Bras Med Trop. 2015;48: 170–174. 10.1590/0037-8682-0038-2015 [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization—WHO. Global Health Sector Strategy on Sexually Transmitted Infections 2016–2021 [Internet]. World Health Organization; Geneva; 2016. Available: http://apps.who.int/iris/bitstream/10665/183117/1/9789241508841_eng.pdf [Google Scholar]
- 34.Bazzo ML, Rapone L, Rudolf-oliveira RCM, Bigolin A, Golfetto L, Mesquita F, et al. Evaluation of seven rapid tests for syphilis available in Brazil using defibrinated plasma panels. Sex Transm Infect. 2017;93: 46–50. 10.1136/sextrans-2016-052558 [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro LVDC, Sabidó M, Galbán E, De Oliveira Guerra JA, Mabey D, Peeling RW, et al. Home-based counseling and testing for HIV and syphilis—An evaluation of acceptability and quality control, in remote Amazonas State, Brazil. Sex Transm Infect. 2015;91: 94–96. 10.1136/sextrans-2014-051625 [DOI] [PubMed] [Google Scholar]
- 36.Daley GM, Russell DB, Tabrizi SN, McBride J. Mycoplasma genitalium: A review. Int J STD AIDS. 2014;25: 475–487. 10.1177/0956462413515196 [DOI] [PubMed] [Google Scholar]
- 37.Campos GB, Lobão TN, Selis NN, Amorim AT, Martins HB, Barbosa MS, et al. Prevalence of Mycoplasma genitalium and Mycoplasma hominis in urogenital tract of Brazilian women. BMC Infect Dis. 2015;15: 4–11. 10.1186/s12879-014-0732-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camacho-Gonzalez AF, Chernoff MC, Williams PL, Chahroudi A, Oleske JM, Traite S, et al. Sexually transmitted infections in youth with controlled and uncontrolled human immunodeficiency virus infection. J Pediatric Infect Dis Soc. 2017;6: e22–e29. 10.1093/jpids/piw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Souza RP, Abreu ALP de, Ferreira ÉC, Rocha-Brischiliari SC, Carvalho MD de B, Pelloso SM, et al. Simultaneous Detection of Seven Sexually Transmitted Agents in Human Immunodeficiency Virus–Infected Brazilian Women by Multiplex Polymerase Chain Reaction. Am J Trop Med Hyg. 2013;89: 1199–1202. 10.4269/ajtmh.13-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HS, Kim TJ, Lee IH, Hong SR. Associations between sexually transmitted infections, high-risk human papillomavirus infection, and abnormal cervical Pap smear results in OB/GYN outpatients. J Gynecol Oncol. 2016;27: 1–11. 10.3802/jgo.2016.27.e49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellaminutti S, Seraceni S, Seta F De, Gheit T, Tommasino M, Comar M. HPV and Chlamydia trachomatis Co-Detection in Young Asymptomatic Women from High Incidence Area for Cervical Cancer. J Med Virol. 2014;86: 1920–5. 10.1002/jmv.24041 [DOI] [PubMed] [Google Scholar]
- 42.Gesink DC, Mulvad G, Montgomery-andersen R, Poppel U, Montgomery-andersen S, Binzer A, et al. Mycoplasma genitalium presence, resistance and epidemiology in Greenlad. Int J Circumpolar Heal. 2012;1: 1–8. 10.3402/ijch.v71i0.18203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman L, Rowley J, Hoorn S Vander, Wijesooriya NS, Unemo M, Low N, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One. 2015;10: 1–17. 10.1371/journal.pone.0143304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araujo MEA, Silva MT, Galvao TF, Pereira MG. Prevalence of health services usage and associated factors in the Amazon region of Brazil: a population-based cross-sectional study. BMJ Open. 2017;7: e017966 10.1136/bmjopen-2017-017966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guimarães AS, Mantovani SAS, Oliart-Guzmán H, Martins AC, Filgueira-Júnior JA, Santos AP, et al. Prenatal care and childbirth assistance in Amazonian women before and after the Pacific Highway Construction (2003–2011): A cross-sectional study. BMC Womens Health. BMC Women’s Health; 2016;16: 1–13. 10.1186/s12905-015-0282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Data underlying the study cannot be made publicly available as the participants did not consent to have their full transcripts made publicly available. The original data set is available to researchers who meet the criteria for accessing sensitive data, upon request to the corresponding author (luana.robrigues@ufopa.edu.br) or the Ethics Committee of the Oswaldo Cruz Institute (cepfiocruz@ioc.fiocruz.br).