Abstract
Background
Progress in early labour is usually slow and may include painful uterine contractions. Women may feel distressed and lose their confidence during this phase. Support and assessment interventions have been assessed in two previous Cochrane Reviews. This review updates and replaces these two reviews.
Objectives
To investigate the effect of assessment and support interventions for women during early labour on the duration of labour, the rate of obstetric interventions, and on other maternal and neonatal outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (31 October 2016) and reference lists of retrieved studies.
Selection criteria
Randomised controlled and cluster randomised trials of any assessment or support intervention in the latent phase of labour.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, and extracted data. We resolved any disagreement by discussion or by involving a third assessor. The quality of the evidence was assessed using the GRADE approach.
Main results
We included five trials including 10,421 pregnant women and a cluster randomised trial with 2183 women. Trials were conducted in the UK, Canada and America and compared interventions in early labour versus usual care. We examined four comparisons: early labour assessment versus immediate admission to hospital; home visits by midwives versus usual care (telephone triage); one‐to‐one structured midwifery care versus usual care and hospital assessment using an algorithm for labour diagnosis versus usual assessment. Trials were at moderate‐ risk of bias mainly because blinding women and staff to these interventions is not generally feasible. For important outcomes we assessed evidence using GRADE; we downgraded evidence for study design limitations, imprecision, and where we carried out meta‐analysis, for inconsistency.
One trial with 209 women compared early labour assessment with direct admission to hospital. Duration of labour from hospital admission was reduced for women in the assessment group (mean difference (MD) ‐5.20 hours, 95% confidence interval (CI) ‐7.06 to ‐3.34; 209 women, low‐quality evidence). There were no clear differences between groups for caesarean section or instrumental vaginal birth (risk ratio (RR) 0.72, 95% CI 0.30 to 1.72, very low quality evidence; and, RR 0.86, 95% CI 0.58 to 1.26, very low quality evidence, respectively). Serious maternal morbidity was not reported. Women in the early assessment group were slightly less likely to have epidural or oxytocin for labour augmentation (RR 0.87, 95% CI 0.78 to 0.98, low‐quality evidence; RR 0.57, 95% CI 0.37 to 0.86, respectively) and increased satisfaction with their care (MD 16.00, 95% CI 7.53 to 24.47). No babies were born before admission to hospital and only one infant had a low Apgar score at five minutes after the birth (very low quality evidence). Admission to neonatal intensive care (NICU) was not reported.
Three studies examined home assessment and midwifery support versus telephone triage. One trial reported the duration of labour; home visits did not have any clear impact compared with usual care (MD 0.29 hours, 95% CI ‐0.14 to 0.72; 1 trial, 3474 women, low‐quality evidence). There were no clear differences for the rate of caesarean section (RR 1.05, 95% CI 0.95 to 1.17; 3 trials, 5170 women; I² = 0%; moderate‐quality evidence) or instrumental vaginal birth (average RR 0.95, 95% CI 0.79 to 1.15; 2 trials, 4933 women; I² = 69%; low‐quality evidence). One trial reported birth before arrival at hospital; there was no clear difference between the groups (RR 1.33, 95% CI 0.30 to 5.95; 1 trial, 3474 women). No clear differences were identified for serious maternal morbidity (RR 0.93, 95% CI 0.61 to 1.42; 1 trial, 3474 women; low‐quality evidence), or use of epidural (average RR 0.95, 95% CI 0.87 to 1.05; 3 trials, 5168 women; I² = 60%; low‐quality evidence). There were no clear differences for NICU admission (average RR 0.84, 95% CI 0.50 to 1.42; 3 trials, 5170 infants; I² = 71%; very low quality evidence), or for low Apgar score at five minutes (RR 1.19, 95% CI 0.71 to 1.99; 3 trials, 5170 infants; I² = 0%; low‐quality evidence).
One study (5002 women) examined one‐to‐one structured care in early labour versus usual care. Length of labour was not reported. There were no clear differences between groups for caesarean section (RR 0.93, 95% CI 0.84 to 1.02; 4996 women, high‐quality evidence) instrumental vaginal birth (RR 0.94, 95% CI 0.82 to 1.08; 4996 women, high‐quality evidence), or serious maternal morbidity (RR 1.13, 95% CI 0.84 to 1.52; 4996 women, moderate‐quality evidence). Use of epidural was similar in the two groups (RR 1.00, 95% CI 0.99 to 1.01; 4996 women, high‐quality evidence). For infant outcomes, there were no clear differences between groups (admission to NICU: RR 0.98, 95% CI 0.80 to 1.21; 4989 infants, high‐quality evidence; low Apgar score at five minutes: RR 1.07, 95% CI 0.64 to 1.79; 4989 infants, moderate‐quality evidence).
A cluster randomised trial with 2183 women examined a labour diagnosis tool used by midwives compared with usual assessment. There were no clear differences between groups for most of the outcomes measured. Interventions in labour (augmentation with oxytocin (RD 0.3, 95% CI ‐9.2 to 9.8), epidural (RD 2.1, 95% CI ‐8.0 to 12.2), instrumental or caesarean birth (spontaneous vertex birth RD ‐3.2, 95% CI ‐15.1 to 8.7)) were similar between groups after adjustment for baseline differences between maternity units. Women in the intervention group were less likely to be admitted to hospital at first presentation. There were no clear differences between groups for infant outcome.
Authors' conclusions
Assessment and support in early labour does not have a clear impact on rate of caesarean section or instrumental birth, or birth before arrival at hospital. However, some evidence suggested that interventions may have an impact on reducing the use of epidural, and on increasing maternal satisfaction with care. Evidence on the use of oxytocin for labour augmentation was mixed. Evidence about the effectiveness of early labour assessment versus immediate admission was very limited and more research is needed in this area.
Plain language summary
Assessment and support during early labour for improving birth outcomes
What is the issue?
Progress in early labour may be slow. Women identify onset of labour from various signs including painful contractions and blood‐stained vaginal loss and may seek advice from health professionals about progress of their labour and for reassurance. Women may be advised to stay at home for as long as possible, or be sent home from hospital because their labour is not established. However, if progress in labour is more rapid than expected, delayed admission may result in an unplanned home birth.
Why is this important?
Women may feel anxious or distressed in early labour and lose confidence; this may slow progress and women may be less likely to experience a normal birth. In this review we evaluated whether assessment and providing support to women during early labour affected the duration of labour, the need for interventions and other outcomes.
What evidence did we find?
We searched the medical literature (31 October 2016). We included five randomised controlled trials, involving 10,421 women from Canada, the USA, and the UK and a trial where maternity units were randomised in Scotland UK with 2183 women. The quality of the evidence ranged from very low to high for different outcomes.
One trial (209 women) compared assessment with direct admission for women arriving at hospital. Women in the assessment group had shorter labours in hospital (low‐quality evidence). There were no clear differences between groups for caesarean or instrumental vaginal birth (i.e. forceps or ventouse) (very low quality evidence). Serious complications were not reported. Women in the assessment group were slightly less likely to have an epidural (low‐quality evidence), or labour augmentation with oxytocin, and had increased satisfaction with their care. No babies were born before admission to hospital. Admission to neonatal special care was not reported.
Three studies examined home midwifery support versus telephone triage. Home visits did not appear to have any clear impact on the length of labour in one trial (low‐quality evidence). There was no clear difference between groups for caesarean (three trials, moderate‐quality evidence) or instrumental vaginal birth (two trials, low‐quality evidence). One trial reported birth before hospital arrival; there was no clear difference for this outcome or for serious maternal morbidity (low‐quality evidence), or use of epidural (three trials, low‐quality evidence). There were no clear differences for neonatal admission to special care (very low quality evidence), or for low Apgar score at five minutes after birth (low‐quality evidence).
One‐to‐one structured care in early labour versus usual care was examined in one study with 5002 women. Length of labour was not reported. There were no clear differences between groups for the rate of caesarean, instrumental vaginal birth (high‐quality evidence), or serious maternal morbidity (moderate‐quality evidence). Use of epidural was similar in the two groups (high‐quality evidence). For infant outcomes, there were no clear differences between groups for admission to special care (high‐quality evidence) or low Apgar score (moderate‐quality evidence).
A trial with 2183 women where maternity units were randomised examined very strict criteria for labour diagnosis compared with usual midwifery assessment. There were no clear differences between women and babies in the two groups for most outcomes. Interventions in labour (augmentation with oxytocin, epidural, instrumental or caesarean birth) were similar once baseline differences between maternity units had been taken into account. Women in the intervention group were less likely to be admitted to hospital in labour at first presentation. There were no clear differences between groups for infant outcomes.
What does this mean?
Assessment and support in early labour does not have a clear impact on rate of caesarean or instrumental vaginal birth, or whether babies are born before arrival at hospital. However, some evidence showed that these interventions may have an impact on reducing the use of epidural, the need to augment labour with oxytocin and on increasing maternal satisfaction. Evidence about the effectiveness of early labour assessment versus immediate admission was very limited and more research is needed on this.
Summary of findings
Background
Description of the condition
In clinical practice, the first stage of labour is usually the longest stage and consists of two phases: the latent phase (early labour) and the active phase. The latent phase has been described as "a period of time when there are painful contractions, and there is some cervical change, including cervical dilatation up to 4 cm" (NICE 2007).
The progress of labour in the latent phase is usually slow and may include painful uterine contractions. Women may feel distressed and lose their confidence during this phase (Austin 1999). Distressed feelings, loneliness, or anxiety may theoretically trigger the secretion of catecholamines that counteract the effect of oxytocin (Lederman 1979; Simkin 2011), and slow down the progress of labour (Alehagen 2005). Therefore, maternal distress could be associated with prolongation of the latent and active phases and the second stage of labour. Emotional distress in the latent phase increases the likelihood of instrumental vaginal birth, and women with higher levels of pain in the latent phase may be less likely to experience spontaneous vaginal delivery (Wuitchik 1989).
Although it is difficult to determine exactly when labour begins, it is usually thought to start at the point where regular uterine contractions are perceptible to women (Friedman 1972). Gross 2003 investigated how women experienced the onset of labour and found that women identified onset from various signs and symptoms including recurrent or non‐recurrent pain, rupture of the amniotic membranes, the appearance of blood stained vaginal discharge, gastrointestinal symptoms, altered sleep patterns, and emotional upheaval. Some women reported that labour began several days before the baby was born (Gross 2006).
Cheyne 2007 found that labouring women decided to go to hospital because of painful contractions, the need for reassurance, or when following their partner's advice; the combination of pain, uncertainty and anxiety influenced women's decisions in the latent phase of labour. Women admitted to the labour ward during early labour tended to show an urgency to place the responsibility for the labour into the hands of professionals (Carlsson 2009).
Because of an association between early admission and subsequent interventions (including caesarean section, labour augmentation, and epidural analgesia) (Bailit 2005; Hemminki 1986; Holmes 2001; Klein 2004; Petersen 2013; Rahnama 2006), women are advised by midwives to stay at home as long as possible during the latent phase, or are sent home because their labour is not established. Flamm 1998 recommended the avoidance of hospital admissions for 'false' labour in order to reduce the rates of caesarean section. However, it is unclear whether avoiding admission or being sent home during the latent phase might result in better outcomes. The effect of deferred admission on the rate of caesarean sections has not been clearly established in randomized trials, but negative effects of deferring admission have been highlighted in observational studies, including confusion, anger, and resentment (Jackson 2003), as well as stress and feelings of being neglected among both women and their partners (Baxter 2007). Barnett 2008 interviewed six nulliparous women in Scotland who were sent home in the latent phase of labour. The women reported that they had felt unsupported and their anxiety had increased after being sent home. If the progress of labour is more rapid than expected, the policy of delayed admission might result in an unplanned home birth and a baby born before arrival (at hospital), which increases the risk of both maternal and neonatal complications (Loughney 2006).
Description of the intervention
In this review, support interventions are defined as non‐pharmacological interventions that support pregnant women during early labour, including: relaxation or stress management training and education; professional or lay visits at home, telephone‐based peer support; educational counselling; non‐directive counselling; comfort measures and various other supportive interventions. Assessment interventions are interventions delivered at home or in hospital to determine the stage of labour and progress in labour, and to assess how well women are coping, as well as their physical and psychological well‐being, in order to plan labour management (including immediate or delayed admission to hospital). Intervention providers are health professionals such as nurses, midwives, childbirth educators, physicians, or psychologists, or lay people, who deliver interventions at hospital, at home, or via telephone (see Types of interventions).
How the intervention might work
During early labour, women are encouraged to keep active, to walk about and to eat and drink as usual in order to prevent prolonged labour. Some women are advised to stay at home for as long as possible. This advice, or being asked to return home, may cause some women to feel unsupported and more anxious, and such feelings might affect the progress of their labour adversely (Wuitchik 1989), and also their satisfaction with childbirth. In the latent phase of labour, women seek out the advice of health professionals to address their need for information about the progress of their labour and reassurance that what they are experiencing is normal. Professional home visits, lay home visits, or telephone‐based peer support during early labour may give assurance to women and relieve their anxiety and distress. Early labour assessment by midwives or doctors may result in the reduction of unnecessary admissions during the latent phase, which in turn might improve obstetric outcomes. Educational information about the patterns of spontaneous labour may influence women's decision making about when to go to hospital. Support and encouragement, relaxation or stress management techniques might improve women's ability to cope with labour (Hodnett 1996). Provision of emotional support, comfort measures, information and advice, advocacy and support of the woman's partner by healthcare providers might encourage women to cope with their labour. This might relieve their anxiety, fear and stress and so avoid an unnecessary cascade of obstetric interventions, and improve obstetric and neonatal outcomes. Overall, the rates of prolonged labour, caesarean section, or instrumental vaginal birth might decrease.
Why it is important to do this review
Several Cochrane Reviews have assessed the effectiveness of support or psychosocial interventions for women at high risk of complications, such as women undergoing treatment for alcohol abuse during pregnancy (Lui 2008), antenatal depression (Dennis 2007b), postpartum depression (Dennis 2007a; Dennis 2013), and maternal smoking cessation (Chamberlain 2013). More generally, supportive interventions have been found to be effective for reducing emotional distress and improving coping abilities in people who have been treated for cancer, HIV/AIDS and cardiovascular disease (Fekete 2007; Vos 2006).
Existing Cochrane Reviews have also assessed educational interventions such as self‐diagnosis of the onset of active labour at term (Lauzon 1998), and delayed admission until active labour (Lauzon 2001). This review will update and replace the Lauzon 2001 review which is now out of date. In addition, in this review we aim to investigate systematically whether assessment, support and educational interventions in the early stages of labour (latent phase) are effective in improving maternal and neonatal outcomes.
Objectives
To investigate the effect of assessment and support interventions for women during early labour on the duration of labour, the rate of obstetric interventions, and on other maternal and neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomized controlled trials and cluster randomised trials that evaluated the efficacy of early labour assessment interventions and any support intervention for women in the latent phase of labour. We included studies published in abstract form only, if the abstract contained sufficient information to assess eligibility and risk of bias, and if the results were described in detail. We did not include quasi‐randomised trials.
Types of participants
We included pregnant women in this review. However, we excluded trials with participants who were high‐risk pregnant women, such as those with mental health conditions (Fenwick 2015; Jesse 2015; Toohill 2014).
Types of interventions
We included trials examining assessment programmes in early labour that aimed to assess physical and emotional well‐being and progress in labour with a view to planning hospital admission, along with support interventions in early labour to optimise outcomes for women and babies.
We included interventions that were provided either by healthcare professionals caring for labouring women (e.g. physicians, nurses, or midwives), or by a trained female companion (e.g. doula). Both individual or group interventions were included. We included interventions that were administered at the maternity unit, the woman's home, over the telephone, online (e.g. websites or social media), or via electronic devices.
Examples of assessment in early labour include:
home or hospital physical examination by health professional to assess stage of labour;
home, hospital or telephone assessment of progress in labour (by maternal report);
home, hospital or telephone assessment of psychological well‐being.
Each of these types of assessment might include advice to women about when to seek hospital admission.
Examples of support that encompass psychosocial interventions in early labour include:
psychosocial supportive interventions (e.g. emotional support for the labouring woman and her birth companions, advice and guidance about her labour, attention to physical comfort, non‐directive counselling, maintaining conversation, telephone‐based peer support, counselling visits at home);
cognitive behavioural therapy (CBT), cognitive and behavioural interventions (e.g. mental image training, stress reduction program, relaxation training program);
exercise therapies (e.g. exercise program, fitness, physical activity);
non‐pharmacological alternative strategies (e.g. acupuncture, Reiki, hypnosis, guided imagery, meditation);
comfort measures (e.g. massage, aromatherapy, or music therapy).
Support also encompasses educational interventions. These aim to distribute new knowledge or promote coping skills to pregnant women, such as information about the progress of labour, managing the latent phase, or when to go to the labour ward. Examples of educational interventions include:
information about relaxation;
information about coping with labour pain;
information about labour progress.
Interventions were compared to no intervention, other interventions or usual care. Usual care was defined as the care that might be provided to pregnant women if they were not included in the clinical trial.
We included combined interventions that consisted of two or more types of interventions in this review (for example, interventions including both assessment and support). We excluded studies that included support interventions combined with pharmacological treatments. We excluded any educational interventions that provided women with information without any personal contact and communication, e.g. giving women a booklet. We did not apply any language restrictions.
Types of outcome measures
Primary outcomes
Maternal outcomes
Length of labour
Rate of caesarean section or instrumental vaginal birth
Neonatal outcomes
Baby born before arrival at hospital or in an unplanned home birth
Secondary outcomes
Maternal outcomes
Serious maternal morbidity (e.g. uterine rupture, admission to intensive care unit, septicaemia, postpartum haemorrhage (defined by trialist))
Augmentation of labour
Use of epidural or any regional anaesthesia
Prolonged labour (defined by trialist)
Duration of hospital stay (antenatal, postnatal)
Maternal satisfaction (intrapartum, postpartum) with the childbirth (defined by trialist)
Postpartum depression (defined by trialist)
Neonatal outcomes
Perinatal death (stillbirth or early neonatal death)
Neonatal admission to special care and/or intensive care unit
Apgar score of less than seven at five minutes
Exclusive breastfeeding at discharge
Exclusive breastfeeding at three months
Search methods for identification of studies
The methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth's Trials Register by contacting their Information Specialist (31 October 2016).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth's Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals, plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set, which has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (31 Octoer 2016) (see: Appendix 1).
Searching other resources
We contacted key personnel and organisations in the relevant field for published and unpublished references.
We also searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review was based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Review authors S Kobayashi (SK) and K Takehara (KT), independently assessed full text of all potential studies identified as a result of the search strategy for inclusion. We resolved any disagreement through discussion or, if required, we consulted the third review author, H Sasaki (HS).
We created a study flow diagram to map out the number of records identified, included and excluded (Figure 1).
1.
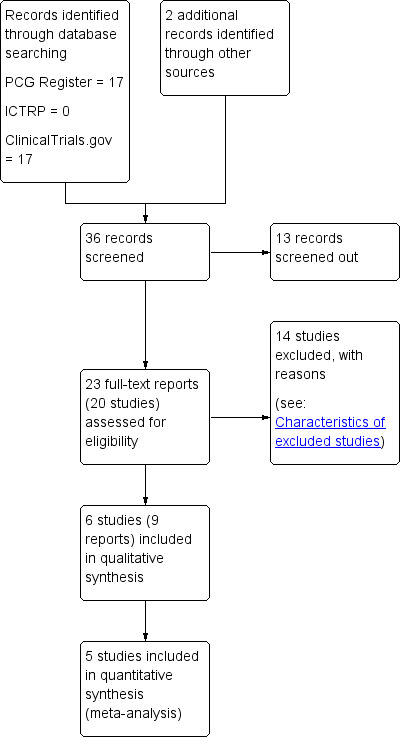
Study flow diagram.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager 5.3 software (RevMan 2014), and checked for accuracy.
When information regarding any of the above was unclear, for example only an abstract was available, we attempted to contact the authors of the original reports to ask them to provide further details.
Assessment of risk of bias in included studies
We (SK, KT) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor (HS).
(1) Random sequence generation (checking for possible selection bias)
For each included study we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
low risk of bias;
high risk of bias;
unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to have an impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update, we assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook. We assessed the quality of the body of evidence relating to the following outcomes for the main comparisons (i.e. assessment versus direct admission in labour; home support versus telephone triage, and one‐to‐one structured care versus usual care).
Length of labour
Rate of caesarean section
Rate of instrumental vaginal birth
Serious maternal morbidity (e.g. postpartum haemorrhage)
Use of epidural or any regional anaesthesia
Neonatal admission to special care or intensive care unit, or both
Apgar score of less than seven at five minutes
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create 'Summary of findings' tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented pooled results as summary risk ratios with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between pooled trials. In future updates, if appropriate, we will use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We included a cluster‐randomised trials in the analyses along with individually‐randomised trials. In future updates, we had intended to adjust the event rates and sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions. However, we were unable to obtain an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial, or from a similar trial or a study of a similar population. We have therefore presented unadjusted figures in our data and analyses which do not take into account the cluster design effect.
Multi‐armed trials
If we identify eligible multi‐armed trials in future updates, we will include them in the analyses. We will combine all relevant interventions into a single group and incorporate all relevant control groups into a single group so that we create single pair‐wise comparisons as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.5.4) (Higgins 2011).
Dealing with missing data
We noted the levels of attrition of included studies. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by prespecified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5.3 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, that is, where trials were examining the same intervention, and the trials’ populations and methods were judged to be sufficiently similar.
If clinical heterogeneity was sufficient to lead us to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, where we considered an average treatment effect across trials to be clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects, and we have discussed the clinical implications of treatment effects differing between trials. If we considered that the average treatment effect was not clinically meaningful, we planned not to combine trials. When we have used random‐effects analyses, the results have been presented as the average treatment effect with 95% confidence intervals.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses and to consider whether an overall summary was meaningful. If it was, we planned to use random‐effects analysis to produce it.
We planned the following subgroup analyses:
the use of epidural or any regional anaesthesia by parity (nulliparity versus multiparity).
We were unable to carry out this planned subgroup analysis due to insufficient data.
In future updates if subgroup analysis is carried out, we will assess subgroup differences using interaction tests available within the current version of Review Manager. We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We did not carry out a sensitivity analysis. However, in future updates of this review, we will perform sensitivity analysis to assess the affect of results due to the high risk of bias of some of the included trials. For the purpose of this sensitivity analysis, 'high quality' will be defined as a trial having a low risk of bias for random sequence generation and allocation concealment, and missing less than 20% of the data, given the stated importance of attrition as a quality measure (Tierney 2005). We will include only the primary outcome in the sensitivity analyses. If statistical heterogeneity is evident, we will carry out the sensitivity analysis to explore the effects of fixed‐effect or random‐effects analyses. Furthermore, if we made any assumptions for ICC values used in cluster‐randomised trials, we will perform a sensitivity analysis using a range of ICC values.
Results
Description of studies
Results of the search
See: Figure 1.
The search identified 36 reports. We screened out 13 (not randomised controlled trials (RCTs) or not within the scope of the review). As a result of reviewing the 23 remaining full texts, we included six trials (nine reports) in the analysis and excluded 14.
Included studies
The included trials were published between 1996 and 2008. Three trials had two reports each (Cheyne 2008; Janssen 2006; McNiven 1996). One trial was not a journal publication but a report for a national centre (ISRCTN11168521).
Design
Five studies were designed as RCTs. Four of these trials were multi‐centre, randomised trials (Hodnett 2008; ISRCTN11168521; Janssen 2003; Janssen 2006). One study was a cluster randomised trial; 14 maternity units in Scotland (UK) were randomised.
Sample sizes
The number of women randomised in the RCTs ranged from 209 to 5002. In the cluster randomised trial the sample included 2183 women (Cheyne 2008).
Setting
All trials were conducted in hospital settings. Three trials were conducted in Canada (Janssen 2003; Janssen 2006; McNiven 1996). The other trials were from the USA and UK (Hodnett 2008; ISRCTN11168521). The cluster randomised trial was conducted in Scotland (UK) (Cheyne 2008).
Participants
The participants of all studies were pregnant women. All trials focused on women in early labour (Cheyne 2008; Hodnett 2008; ISRCTN11168521; Janssen 2003; Janssen 2006; McNiven 1996). Five trials included only nulliparous women (Cheyne 2008; Hodnett 2008; ISRCTN11168521; Janssen 2006; McNiven 1996), and five trials clearly stated that they included only women who had a singleton fetus (Cheyne 2008; Hodnett 2008; ISRCTN11168521; Janssen 2003; Janssen 2006). One trial did not have an age limit for participation, and informed consent was obtained from a parent or guardian; participants as young as 14 years of age were included in the trial (Hodnett 2008). One trial did not describe eligibility regarding age (McNiven 1996). The other trials had a lower age limit, which was 16 years of age (Cheyne 2008; ISRCTN11168521; Janssen 2003; Janssen 2006).
Interventions and comparisons
The six trials assessed the impact of methods of assessment or special care for women in early labour (Cheyne 2008; Hodnett 2008; ISRCTN11168521; Janssen 2003; Janssen 2006; McNiven 1996). Of these, three trials reported the impact of an intervention for home visit assessment by health professionals versus telephone triage (Janssen 2003; Janssen 2006), while ISRCTN11168521 evaluated an intervention that involved a home visit by a community midwife that included advice, support, and encouragement versus usual care (telephone triage and direct admission). Hodnett 2008 evaluated structured early labour care by a midwife (one‐to‐one care) with support and encouragement versus usual care; McNiven 1996 compared early labour assessment with individually planned care versus immediate admission to hospital. In the cluster randomised trial midwives caring for women in the intervention group used an algorithm with strict criteria for labour diagnosis compared with women receiving routine care (Cheyne 2008).
Outcomes
The included trials focused on mode of delivery, analgesia, length of labour, and maternal and neonatal outcomes. One trial also assessed self‐diagnosis for onset of labour (Janssen 2006). Three trials evaluated women’s satisfaction and perception of care programs (Hodnett 2008; ISRCTN11168521; Janssen 2003).
Excluded studies
We excluded a total of 14 studies. Of these studies, five trials were not RCTs (Dowding 2011; IRCT138903063078N4; Janssen 2013; Lumluk 2011; Memon 2015), one trial included participants who were only at risk for depression (Werner 2016), one trial included participants who were not pregnant women but healthcare providers (Cheyne 2008a), and seven trials were excluded because the intervention or participants did not match our inclusion criteria (Bonovich 1990; Fenwick 2015; Jesse 2015; Karp 2013; Khooshide 2015; Magriples 2015; Toohill 2014; Zocco 2007).
Risk of bias in included studies
A risk‐of‐bias graph and summary can be found in Figure 2 and Figure 3.
2.
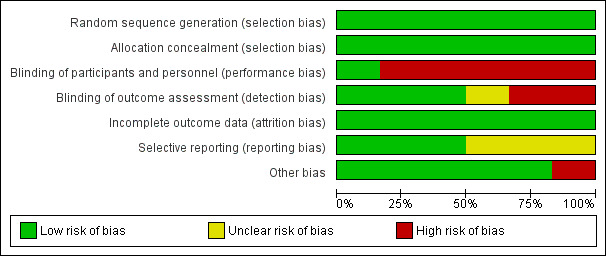
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Allocation sequence generation
We judged all five RCTs to be at a low risk of bias because they used appropriate methods for randomisation of participants, such as computer‐generated randomisation and sealed opaque envelopes (Hodnett 2008; ISRCTN11168521; Janssen 2003; Janssen 2006; McNiven 1996). In the cluster randomised trial methods of randomisation were also appropriate (Cheyne 2008).
Allocation concealment
We judged all RCTs to be at a low risk of bias because allocation of participants and investigators was concealed by use of sealed opaque envelopes and central allocation systems (Hodnett 2008; ISRCTN11168521; Janssen 2003; Janssen 2006; McNiven 1996). In the cluster trial the randomisation process was also judged to be low risk of bias (Cheyne 2008).
Blinding
Participants and personnel
Blinding of participants and care providers in these types of interventions was not easy because caregivers such as nurses, midwives, and physicians provided interventions or usual care to participants as required by the results of allocation. However, one trial had low risk of bias for this domain because objective data were collected as a part of routine practice (Hodnett 2008). We evaluated the remaining five studies as being at high risk (Cheyne 2008; ISRCTN11168521; Janssen 2003; Janssen 2006; McNiven 1996).
Outcome assessment
We assessed three trials as being at a low risk of bias because the outcome was not likely to be influenced by lack of blinding (Hodnett 2008; ISRCTN11168521; Janssen 2006). Two trials were not blinded and trialists highlighted that outcomes may have been influenced by lack of blinding (Janssen 2003; McNiven 1996). In the cluster randomised trial the effect of lack of blinding was not clear (Cheyne 2008).
Incomplete outcome data
We assessed all studies as being at a low risk of attrition bias as overall sample attrition was low and balanced across randomised groups (Cheyne 2008; Hodnett 2008; ISRCTN11168521; Janssen 2003; Janssen 2006; McNiven 1996). For the cluster randomised trial we were unable to obtain the information we needed to be able to adjust the results for cluster design effect and so we have presented raw data. The likely effect of adjustment would be to widen 95% CIs, so the results we have presented for this study should be interpreted with caution (Cheyne 2008).
Selective reporting
We assessed only three of the studies as being at a low risk of reporting bias (Cheyne 2008; Hodnett 2008; ISRCTN11168521). We judged the others to be at an unclear risk of bias because the reports provided insufficient information for us to make an informed decision.
Other potential sources of bias
We judged only one trial to have a high risk of bias for this domain because the compliance in the intervention group was very low (25.5%) (ISRCTN11168521). In the cluster randomised trial there was some imbalance between clusters at baseline but this was appropriately accounted for in the analysis (Cheyne 2008).The remaining trials did not have any apparent source of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Assessment compared to direct admission in early labour for improving birth outcomes.
| Assessment compared with direct admission in early labour for improving birth outcomes | ||||||
| Patient or population: healthy pregnant women Setting: large hospital in Canada (high resource setting), study published 1996 Intervention: assessment Comparison: direct admission to hospital in early labour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with direct admission in early labour | Risk with assessment | |||||
| Length of labour (hours) | The mean length of labour (hours) was 8.3 hours in the intervention group and 13.5 hours in the control group | MD 5.2 lower (7.06 lower to 3.34 lower) | ‐ | 209 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | This outcome relates to the length of labour in hospital |
| Rate of caesarean section | Study population | RR 0.72 (0.30 to 1.72) | 209 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 3 | ||
| 106 per 1000 | 76 per 1000 (32 to 182) | |||||
| Rate of instrumental vaginal birth | Study population | RR 0.86 (0.58 to 1.26) | 209 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 3 | ||
| 356 per 1000 | 306 per 1000 (206 to 448) | |||||
| Serious maternal morbidity | Study population | ‐ | (0 RCTs) | ‐ | Serious maternal morbidity was not reported | |
| see comment | See comment | |||||
| Use of epidural or any regional anaesthesia | Study population | RR 0.87 (0.78 to 0.98) | 209 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | ||
| 904 per 1000 | 786 per 1000 (705 to 886) | |||||
| Neonatal admission to special care | Study population | |||||
| see comment | See comment | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 2.97 (0.12 to 72.12) | 209 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 4 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Single trial with design limitations (lack of blinding) (‐1)
2 Single trial with small sample size (‐1)
3 Wide 95% CI crossing the line of no effect and small sample size (‐2)
4 Wide 95% CI crossing the line of no effect, small sample size and low event rate (‐2)
Summary of findings 2. Home support compared to telephone triage for improving birth outcomes.
| Home support compared with telephone triage for improving birth outcomes | ||||||
| Patient or population: healthy pregnant women Setting: studies in Canada (2 multi‐centre studies) and the UK (1 study) (high resource settings); studies published 2003‐2008 Intervention: home support Comparison: telephone triage | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with telephone triage | Risk with home support | |||||
| Length of labour (hours) | The mean length of labour (hours) was 9.66 in the intervention group and 9.37 in the control group | MD 0.29 higher (0.14 lower to 0.72 higher) | ‐ | 3474 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | Criteria for start of labour were not clearly described |
| Rate of caesarean section | Study population | RR 1.05 (0.95 to 1.17) | 5170 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ||
| 215 per 1000 | 226 per 1000 (204 to 252) | |||||
| Rate of instrumental vaginal birth | Study population | RR 0.95 (0.79 to 1.15) | 4933 (2 RCTs) | ⊕⊕⊝⊝ LOW 3, 4 | ||
| 233 per 1000 | 222 per 1000 (184 to 268) | |||||
| Serious maternal morbidity | Study population | RR 0.93 (0.61 to 1.42) | 3474 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | ||
| 25 per 1000 | 23 per 1000 (15 to 35) | |||||
| Use of epidural or any regional anaesthesia | Study population | RR 0.95 (0.87 to 1.05) | 5168 (3 RCTs) | ⊕⊕⊝⊝ LOW 3, 5 | ||
| 505 per 1000 | 480 per 1000 (439 to 530) | |||||
| Neonatal admission to special care | Study population | RR 0.84 (0.50 to 1.42) | 5170 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2, 3, 6 | ||
| 58 per 1000 | 49 per 1000 (29 to 82) | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 1.19 (0.71 to 1.99) | 5170 (3 RCTs) | ⊕⊕⊝⊝ LOW 2, 3 | ||
| 10 per 1000 | 12 per 1000 (7 to 20) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Study with design limitations (lack of blinding) (‐1)
2 Wide 95% CI crossing line of no effect (‐1)
3 All studies contributing data had design limitations (lack of blinding) (‐1)
4 High heterogeneity (I2 69%) (‐1)
5 High heterogeneity (I2 60%) (‐1)
6 High heterogeneity (I2 71%) (‐1)
Summary of findings 3. One‐to‐one structured care compared to usual care for improving birth outcomes.
| One‐to‐one structured care compared to usual care for improving birth outcomes | ||||||
| Patient or population: healthy pregnant women Setting: multi‐centre study in North American and UK hospitals (high resource settings). Study published 2008 Intervention: one‐to‐one structured care Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with one‐to‐one structured care | |||||
| Length of labour (hours) | ‐ | See comment | ‐ | (0 study) | ‐ | Not reported |
| Rate of caesarean section | Study population | RR 0.93 (0.84 to 1.02) | 4996 (1 RCT) | ⊕⊕⊕⊕ HIGH | ||
| 242 per 1000 | 225 per 1000 (203 to 247) | |||||
| Rate of instrumental vaginal birth | Study population | RR 0.94 (0.82 to 1.08) | 4996 (1 RCT) | ⊕⊕⊕⊕ HIGH | ||
| 145 per 1000 | 136 per 1000 (119 to 156) | |||||
| Serious maternal morbidity | Study population | RR 1.13 (0.84 to 1.52) | 4996 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 32 per 1000 | 36 per 1000 (27 to 48) | |||||
| Use of epidural or any regional anaesthesia | Study population | RR 1.00 (0.99 to 1.01) | 4996 (1 RCT) | ⊕⊕⊕⊕ HIGH | ||
| 955 per 1000 | 955 per 1000 (946 to 965) | |||||
| Neonatal admission to special care | Study population | RR 0.98 (0.80 to 1.21) | 4989 (1 RCT) | ⊕⊕⊕⊕ HIGH | ||
| 69 per 1000 | 67 per 1000 (55 to 83) | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 1.07 (0.64 to 1.79) | 4989 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 11 per 1000 | 12 per 1000 (7 to 20) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Wide 95% CI crossing line of no effect (‐1)
Assessment in early labour versus direct admission (one trial with 209 women)
One trial with 209 participants is included in this comparison (McNiven 1996). In this trial women attending hospital with contractions were randomised to either assessment or direct admission to hospital. Women who were assessed were either admitted if labour was diagnosed, advised to go home or to go for a walk (with encouragement and education about when to return), or were advised to remain on the hospital premises and were reassessed at a later period.
Primary outcomes
Length of labour
Length of labour from the point of hospital admission was reported. Women in the early assessment group had a shorter time labouring in hospital (mean difference (MD) ‐5.20 hours, 95% confidence interval (CI) ‐7.06 to ‐3.34; 209 women; low‐quality evidence;Analysis 1.1). (Women in this study were also asked to estimate the duration of labour at home; women in the early assessment group estimated a longer period in labour at home. Data not shown.)
1.1. Analysis.
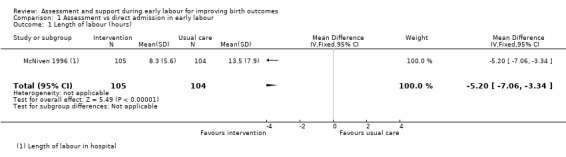
Comparison 1 Assessment vs direct admission in early labour, Outcome 1 Length of labour (hours).
Rate of caesarean section or instrumental vaginal birth
There were no clear differences between groups for the number of women undergoing caesarean section (risk ratio (RR) 0.72, 95% CI 0.30 to 1.72; very low quality evidence; Analysis 1.2) or instrumental vaginal birth (RR 0.86, 95% CI 0.58 to 1.26, very low quality evidence;Analysis 1.3).
1.2. Analysis.
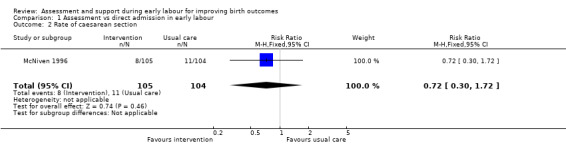
Comparison 1 Assessment vs direct admission in early labour, Outcome 2 Rate of caesarean section.
1.3. Analysis.
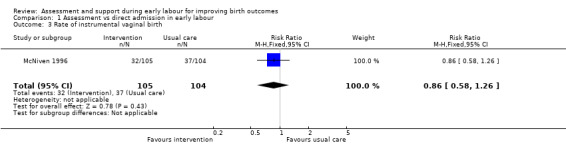
Comparison 1 Assessment vs direct admission in early labour, Outcome 3 Rate of instrumental vaginal birth.
Baby born before arrival at hospital or unplanned home birth
No babies were born before hospital admission (Analysis 1.4).
1.4. Analysis.
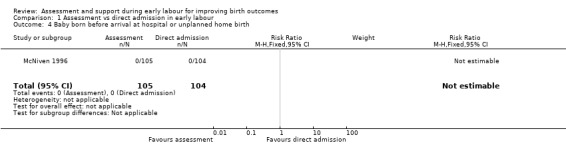
Comparison 1 Assessment vs direct admission in early labour, Outcome 4 Baby born before arrival at hospital or unplanned home birth.
Secondary outcomes
McNiven 1996 did not report serious maternal morbidity such as postpartum haemorrhage (loss of more than 1000 mL of blood), postnatal fever, blood transfusion and maternal death.
Women who had early labour assessment were less likely to receive oxytocin for labour augmentation (RR 0.57, 95% CI 0.37 to 0.86; Analysis 1.5), and were slightly less likely to have epidural anaesthesia (RR 0.87, 95% CI 0.78 to 0.98; low‐quality evidence;Analysis 1.6). Women in this group were also more satisfied with their care than women in the direct admission group (MD 16.00, 95% CI 7.53 to 24.47; Analysis 1.7).
1.5. Analysis.
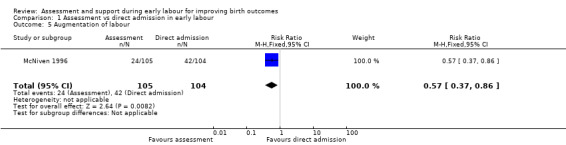
Comparison 1 Assessment vs direct admission in early labour, Outcome 5 Augmentation of labour.
1.6. Analysis.
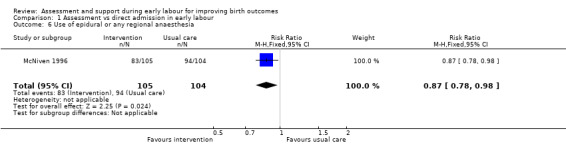
Comparison 1 Assessment vs direct admission in early labour, Outcome 6 Use of epidural or any regional anaesthesia.
1.7. Analysis.
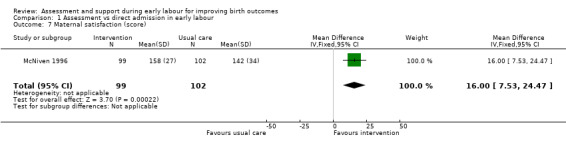
Comparison 1 Assessment vs direct admission in early labour, Outcome 7 Maternal satisfaction (score).
Only one infant had a low Apgar score at five minutes after the birth (RR 2.97, 95% CI 0.12 to 72.12; very low quality evidence;Analysis 1.8).
1.8. Analysis.
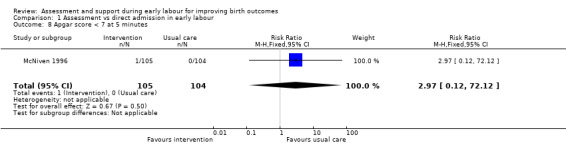
Comparison 1 Assessment vs direct admission in early labour, Outcome 8 Apgar score < 7 at 5 minutes.
Our other secondary outcomes (i.e. duration of hospital stay, postpartum depression, perinatal death, neonatal admission to special care, breastfeeding at discharge or at three months postpartum) were not reported.
Home assessment and support versus telephone triage (three studies with 5210 women)
Three studies are included in this comparison (ISRCTN11168521; Janssen 2003; Janssen 2006).
Primary outcomes
Length of labour
One trial reported the duration of labour (although it was not clear exactly how this outcome was assessed). Home visiting and assessment in early labour by midwives did not appear to have any clear impact on the length of labour compared with usual care (MD 0.29 hours, 95% CI ‐0.14 to 0.72; 1 trial, 3474 women; low‐quality evidence; Analysis 2.1).
2.1. Analysis.
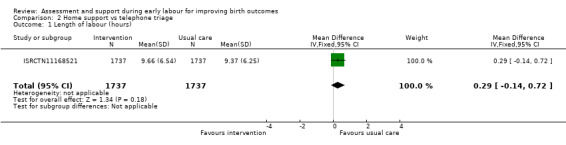
Comparison 2 Home support vs telephone triage, Outcome 1 Length of labour (hours).
Rate of caesarean section or instrumental vaginal birth
Three trials reported rate of caesarean section (ISRCTN11168521; Janssen 2003; Janssen 2006), and two reported instrumental vaginal birth (ISRCTN11168521; Janssen 2006). There was no clear difference for the rate of caesarean section (RR 1.05, 95% CI 0.95 to 1.17; 3 trials, 5170 women; I² = 0%; moderate‐quality evidence; Analysis 2.2), or for the rate of instrumental vaginal birth (average RR 0.95, 95% CI 0.79 to 1.15; 2 trials, 4933 women; I² = 69%; low‐quality evidence; Analysis 2.3).
2.2. Analysis.
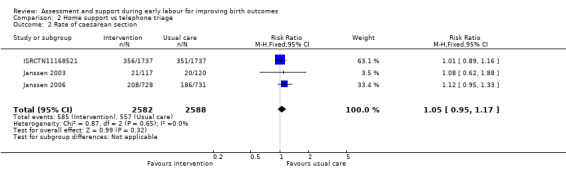
Comparison 2 Home support vs telephone triage, Outcome 2 Rate of caesarean section.
2.3. Analysis.
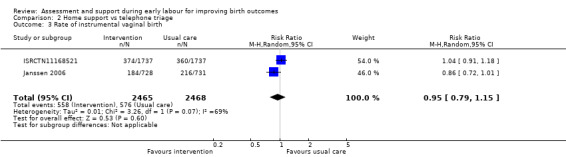
Comparison 2 Home support vs telephone triage, Outcome 3 Rate of instrumental vaginal birth.
Baby born before arrival at hospital or unplanned home birth
Only one trial evaluated the effect on the number of babies born before arrival at hospital or unplanned home births (ISRCTN11168521), and reported that there was no clear difference between groups (RR 1.33, 95% CI 0.30 to 5.95; 1 trial, 3474 women; Analysis 2.4).
2.4. Analysis.
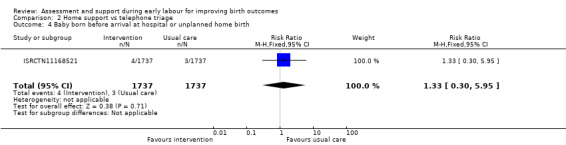
Comparison 2 Home support vs telephone triage, Outcome 4 Baby born before arrival at hospital or unplanned home birth.
Secondary outcomes
No clear differences were reported for serious maternal morbidity such as postpartum haemorrhage of more than 1000 mL, postnatal fever, blood transfusion and maternal death (RR 0.93, 95% CI 0.61 to 1.42; 1 trial, 3474 women; low‐quality evidence;Analysis 2.5); augmentation of labour (RR 0.96, 95% CI 0.88 to 1.04; 2 trials, 1694 women; I² = 0%; Analysis 2.6); use of epidural or any regional anaesthesia (average RR 0.95, 95% CI 0.87 to 1.05; 3 trials, 5168 women; I² = 60%; low‐quality evidence; Analysis 2.7); duration of hospital stay (postpartum stay in hospital more than five days) (RR 1.15, 95% CI 0.83 to 1.60; 1 trial, 3474 women; Analysis 2.8), and postpartum depression using Edinburgh Post‐natal Depression Scale (EPDS) (RR 1.08, 95% CI 0.82 to 1.42; 1 trial, 2584 women; Analysis 2.10). There was a slight increase in maternal satisfaction among women in the intervention group (MD 3.47, 95% CI 1.00 to 5.94; 1 trial, 423 women; Analysis 2.9).
2.5. Analysis.
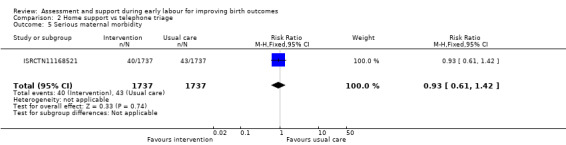
Comparison 2 Home support vs telephone triage, Outcome 5 Serious maternal morbidity.
2.6. Analysis.
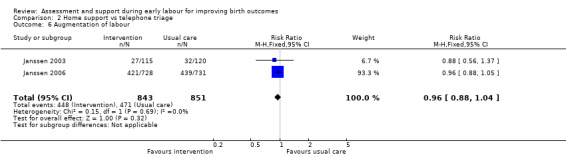
Comparison 2 Home support vs telephone triage, Outcome 6 Augmentation of labour.
2.7. Analysis.
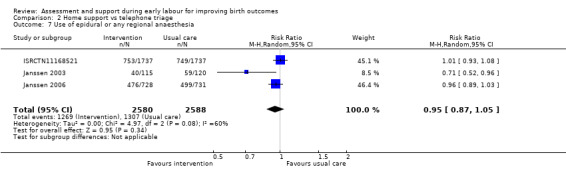
Comparison 2 Home support vs telephone triage, Outcome 7 Use of epidural or any regional anaesthesia.
2.8. Analysis.
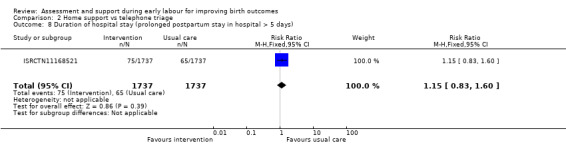
Comparison 2 Home support vs telephone triage, Outcome 8 Duration of hospital stay (prolonged postpartum stay in hospital > 5 days).
2.10. Analysis.
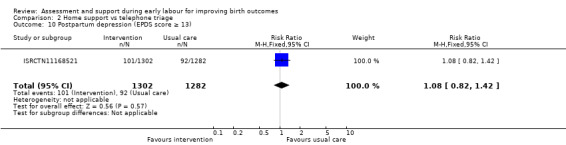
Comparison 2 Home support vs telephone triage, Outcome 10 Postpartum depression (EPDS score ≥ 13).
2.9. Analysis.
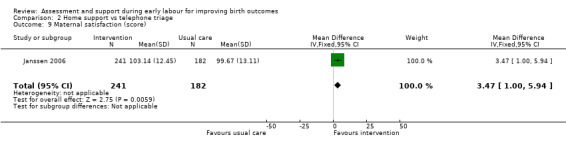
Comparison 2 Home support vs telephone triage, Outcome 9 Maternal satisfaction (score).
One trial evaluated perinatal death showing no clear difference between groups (RR 1.00, 95% CI 0.42 to 2.40; 3474 infants; Analysis 2.11). Other neonatal outcomes that also showed no clear difference between groups included; neonatal admission to special care (average RR 0.84, 95% CI 0.50 to 1.42; 3 trials, 5170 infants; I² = 71%; very low quality evidence; Analysis 2.12), and Apgar score of less than seven at five minutes after birth (RR 1.19, 95% CI 0.71 to 1.99; 3 studies, 5170 infants; I² = 0%; low‐quality evidence; Analysis 2.13). ISRCTN11168521 reported that exclusive breastfeeding both at discharge and at six weeks did not show any differences: exclusive breastfeeding at discharge (RR 1.00, 95% CI 0.96 to 1.04; 3474 women; Analysis 2.14), and exclusive breastfeeding at six weeks postpartum (RR 1.05, 95% CI 0.97 to 1.14; 3474 women; Analysis 2.15).
2.11. Analysis.
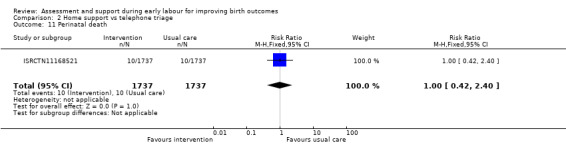
Comparison 2 Home support vs telephone triage, Outcome 11 Perinatal death.
2.12. Analysis.
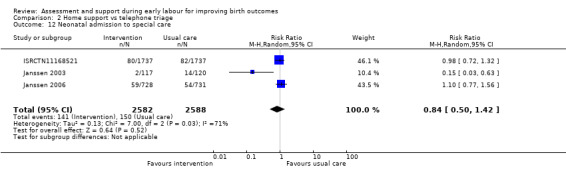
Comparison 2 Home support vs telephone triage, Outcome 12 Neonatal admission to special care.
2.13. Analysis.
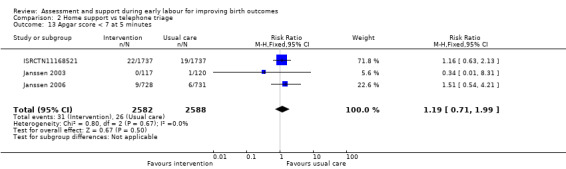
Comparison 2 Home support vs telephone triage, Outcome 13 Apgar score < 7 at 5 minutes.
2.14. Analysis.
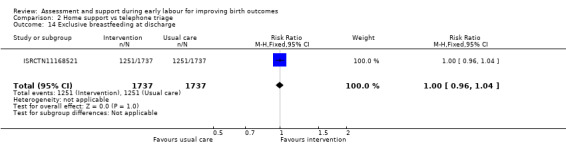
Comparison 2 Home support vs telephone triage, Outcome 14 Exclusive breastfeeding at discharge.
2.15. Analysis.
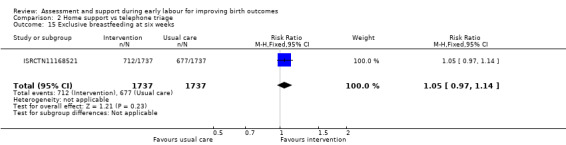
Comparison 2 Home support vs telephone triage, Outcome 15 Exclusive breastfeeding at six weeks.
One‐to‐one structured care in early labour versus usual care (one study with 5002 women)
One study with a large sample size was included in this comparison (Hodnett 2008).
Primary outcomes
Length of labour
Length of labour was not reported.
Rate of caesarean section or instrumental vaginal birth
There were no clear differences between women receiving one‐to‐one structured midwifery care and usual care for the rate of caesarean section (RR 0.93, 95% CI 0.84 to 1.02; 4996 women, high‐quality evidence; Analysis 3.1), or for instrumental vaginal birth (RR 0.94, 95% CI 0.82 to 1.08; 4996 women, high‐quality evidence; Analysis 3.2).
3.1. Analysis.
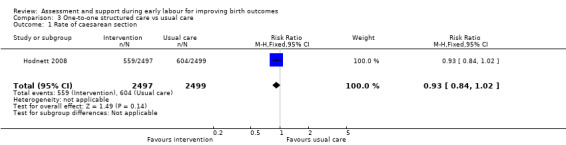
Comparison 3 One‐to‐one structured care vs usual care, Outcome 1 Rate of caesarean section.
3.2. Analysis.
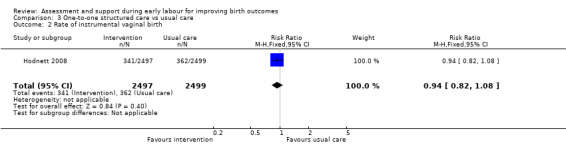
Comparison 3 One‐to‐one structured care vs usual care, Outcome 2 Rate of instrumental vaginal birth.
Baby born before arrival at hospital or unplanned home birth
The number of babies born before arrival at hospital or unplanned home births was not reported.
Secondary outcomes
No clear differences between groups were reported for serious maternal morbidity such as postpartum haemorrhage of more than 1000 mL, postnatal fever, blood transfusion and maternal death (RR 1.13, 95% CI 0.84 to 1.52; 4996 women, moderate‐quality evidence; Analysis 3.3). Use of epidural or any regional anaesthesia was similar in the two groups (RR 1.00, 95% CI 0.99 to 1.01; 4996 women, high‐quality evidence' Analysis 3.4). Other maternal outcomes including augmentation of labour, duration of hospital stay, postpartum depression and breastfeeding were not reported.
3.3. Analysis.
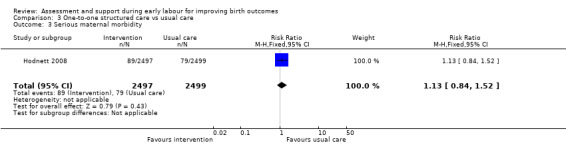
Comparison 3 One‐to‐one structured care vs usual care, Outcome 3 Serious maternal morbidity.
3.4. Analysis.
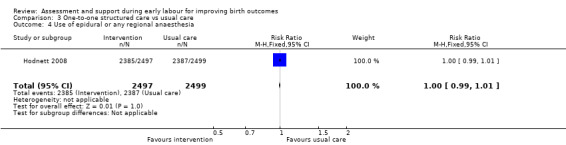
Comparison 3 One‐to‐one structured care vs usual care, Outcome 4 Use of epidural or any regional anaesthesia.
For infant outcomes, there were no cases of perinatal death in this study and there were no clear differences between groups for neonatal outcomes (neonatal intensive care unit admission: RR 0.98, 95% CI 0.80 to 1.21; 4989 infants, high‐quality evidence; Analysis 3.6; Apgar score of less than seven at five minutes: RR 1.07, 95% CI 0.64 to 1.79; 4989 infants, moderate‐quality evidence; Analysis 3.7).
3.6. Analysis.
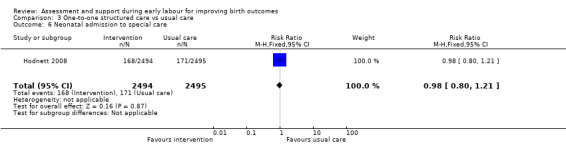
Comparison 3 One‐to‐one structured care vs usual care, Outcome 6 Neonatal admission to special care.
3.7. Analysis.
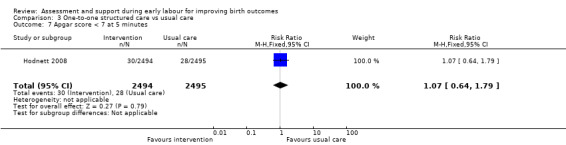
Comparison 3 One‐to‐one structured care vs usual care, Outcome 7 Apgar score < 7 at 5 minutes.
Labour diagnosis by algorithm versus routine midwifery care (one cluster randomised trial, 14 maternity units, 2183 women)
One cluster randomised trial was included in this comparison (Cheyne 2008). The trial was assessed as low risk of bias for most domains except blinding. The trial compared women attending units where assessment by midwives was carried out either by using strict criteria for labour diagnosis using an algorithm or by routine assessment to decide whether women were in labour.
We were unable to enter data from this trial into RevMan 2014 data and analysis tables or carry out assessment using GRADEpro. Results are set out in additional tables (Table 4; Table 5) using data from the main trial report (Cheyne 2008). Adjustment was carried out by the trial statistician taking account of differences between clusters (maternity units) at baseline. Results are expressed as differences between intervention and control groups (adjusted). For some outcomes (e.g. unplanned birth outside hospital or postpartum haemorrhage) there were insufficient data to carry out meaningful adjustment and for these outcomes the event data are set out in Table 4. The number of women included in the analysis following the intervention for all outcomes was 2171.
1. Labour diagnosis algorithm versus routine care (Cheyne 2008).
| OUTCOME |
Intervention (after ) n = 892 |
Control (after) n = 1279 |
Difference between groups adjusted for baseline differences between clusters with 95% CI | P value |
| Spontaneous vertex delivery | 526 | 785 | ‐3.2 (‐15.1 to 8.7) | 0.6 |
| ARM | 401 | 500 | 5.6 (‐2.2 to 13.4) | 0.1 |
| Electronic fetal monitoring | 557 | 820 | ‐0.1 (‐14.2 to 14.1) | 1.0 |
| Assisted vaginal delivery | 241 | 323 | ||
| Caesarean section | 123 | 168 | ||
| 3rd or 4th degree tear | 7 | 8 | ||
| Epidural | 290 | 441 | 2.1 (‐8.0 to 12.2) | 0.7 |
| Additional analgesia required Opiate |
532 | 649 | 1.5 (‐4.6 to 7.6) | 0.6 |
| Additional analgesia required Epidural and opiate |
177 | 225 | 4.4 (‐2.8 to 11.7) | 0.2 |
| Any maternal complication | 439 | 596 | 3.9 (‐9.4 to 17.2) | 0.5 |
| PP haemorrhage (specify) Intrapartum Post partum |
5 10 |
7 20 |
||
| Labour augmentation with oxytocin | 343 | 484 | 0.3 (‐9.2 to 9.8) | 0.9 |
| Unplanned birth out of hospital | 11 | 11 | ||
| Fetal distress | 166 | 242 | 2.4 (‐6.6 to 11.3) | 0.6 |
| Meconium stained liquor | 133 | 211 | ‐0.5 (‐7.2 to 6.3) | 0.9 |
| Neonatal resuscitation | 106 | 145 | ‐0.9 (‐6.4 to 4.7) | 0.7 |
| Admission to special care | 29 | 60 | ‐0.4 (‐2.6 to 1.8) | 0.7 |
| Apgar score < 7 at 5 minutes | 9 | 13 | ||
| Admission to hospital at first presentation (1 admission) One presentations before admission in labour Two presentations before admission in labour Three or more presentations before admission in labour |
398 305 149 35 |
795 366 88 20 |
‐19.2 (‐29.9 to ‐8.6) | 0.002 |
| Failure to progress 1st stage Failure to progress 2nd stage |
42 142 |
59 119 |
‐3.4 (‐15.3 to 8.6) 15.2 (‐4.5 to 34.9) |
0.5 0.1 |
2. Labour diagnosis algorithm versus routine care (Cheyne 2008) (Continuous data).
| OUTCOME | Intervention n =892 Mean |
SD | Control n=1279 Mean |
SD | Difference between groups adjusted for baseline differences between clusters | P value |
| Duration of labour from admission to labour ward to delivery | 9.6 | 11.29 | 8.06 | 5.41 | 0.75 (‐0.55 to 2.05) | 0.2 |
| Mean number of vaginal examinations | 3.67 | Range 0‐11 | 3.46 | Range 0‐11 | 0.2 (‐0.3 to 0.7) | 0.3 |
Primary outcomes
Length of labour
There was no clear difference between the women assessed by midwives using a labour diagnosis algorithm compared with routine midwife assessment for duration of labour from admission to the labour ward until delivery (adjusted difference between means (hours) 0.75, 95% CI ‐0.55 to 2.05).
Rate of caesarean section or instrumental vaginal birth
After adjustment for baseline differences between units there was no clear difference between the two types of assessment for instrumental vaginal birth or caesarean section (see Table 4) (spontaneous vertex birth, risk difference (RD) ‐3.2, 95% CI ‐15.1 to 8.7).
Baby born before arrival at hospital or unplanned home birth
There were 11 babies in each group born before arrival in hospital.
Secondary outcomes
No clear differences between groups were reported for intrapartum or postpartum haemorrhage (Table 4). Adjusted data showed no clear differences in the use of epidural (RD 2.1, 95% CI ‐8.0 to 12.2) opioid analgesia (RD 1.5, 95% CI ‐4.6 to 7.6) or both (RD 4.4, 95% CI ‐2.8 to 11.7).
The primary outcome in the trial was labour augmentation with oxytocin; there was no clear evidence that the intervention reduced labour augmentation (RD 0.3, 95 % CI ‐9.2 to 9.8). There were similar rates of severe perineal trauma in both groups (7 versus 8). Other maternal outcomes including postpartum depression and breastfeeding were not reported.
A review non‐prespecified outcome that was reported in this trial was the number of times women presented at hospital before they were admitted to the labour ward. It was less likely that women would be admitted at the first presentation in the intervention group (RD ‐19.2, 95% CI ‐29.9 to ‐8.6). Some women attended hospital three or more times before admission (Table 4).
For infant outcomes, there were no clear differences between groups for neonatal outcomes (neonatal intensive care unit admission: RD ‐0.4, 95% CI ‐2.6 to 1.8; Apgar score of less than seven at five minutes: 9 versus 13 babies; and need for neonatal resuscitation RD ‐0.9, 95% CI ‐6.4 to 4.7).
Discussion
Summary of main results
This review included five randomised controlled trials involving 10,421 women and their babies, and a cluster randomised trial with 2183 women. We assessed non‐pharmacological assessment and support interventions that were administered in early labour.
A trial with a small sample size (209 women) that examined the impact of early labour assessment versus immediate admission showed some differences between groups, but many outcomes of interest to this review were not reported. Women in the early assessment group had a shorter time in labour in hospital (low‐quality evidence), had increased satisfaction with their care, were less likely to have labour augmentation and were slightly less likely to have an epidural (low‐quality evidence). There were similar rates of other labour interventions between the groups, including caesarean section and instrumental birth (very low quality evidence). Only one infant had a low Apgar score at five minutes (very low quality evidence).
Three trials examined home midwifery assessment and support versus telephone triage. For this comparison, results should be interpreted with caution because of the statistical heterogeneity of the trials combined in the meta‐analysis.
One trial reported the duration of labour, but the intervention did not appear to have any clear impact on the length of labour (low‐quality evidence). There was no clear difference between groups for the rate of caesarean section (reported in three trials, moderate‐quality evidence) or the rate of instrumental vaginal birth (reported in two trials, low‐quality evidence). One trial reported serious maternal morbidity, where there was no clear difference between groups (low‐quality evidence). Use of epidural in intervention and control groups was not clearly different in the three studies (low‐quality evidence), and neonatal outcomes also showed no clear differences (neonatal admission to special care, very low quality evidence, and Apgar score of less than seven at five minutes, low‐quality evidence).
One large trial compared one‐to‐one midwifery care in early labour with usual care. Length of labour was not reported and there were few clear differences between groups for other outcomes, including caesarean section and instrumental vaginal birth (both high‐quality evidence), serious maternal morbidity (moderate‐quality evidence), or use of epidural (high‐quality evidence). There were no cases of perinatal death in this study and there were no clear differences between groups for neonatal outcomes (neonatal intensive care unit admission, high‐quality evidence; Apgar score of less than seven at five minutes, moderate‐quality evidence).
A cluster randomised trial examined the impact of a labour diagnosis tool used by midwives compared with usual midwifery assessment when women attended hospital. There were no clear differences in outcomes for women and babies in the two groups for most of the outcomes measured. Interventions in labour (augmentation, epidural, instrumental or caesarean birth) were similar after adjustment for baseline differences between maternity units. Women in the intervention group were less likely to be admitted to hospital in labour at first presentation. There were no clear differences between groups for the infant outcome reported.
Overall completeness and applicability of evidence
The interventions examined in this review were very varied and results were mixed. The interventions included assessment of pregnant women at home by health workers before hospital admission (ISRCTN11168521; Janssen 2003; Janssen 2006), assessment versus immediate hospital admission, and structured care including physical assessment of pain and emotional support by nurses or midwives after admission to hospital (Hodnett 2008). The outcome data from these trials were limited.
Only two of the five trials examined the length of labour as a main outcome in this review (ISRCTN11168521; McNiven 1996).
All the trials were conducted in Western, high‐income countries including Canada, the USA and the UK, therefore, the applicability of evidence to low‐income and middle‐income countries is limited.
Quality of the evidence
We judged the overall risk of bias in the individually randomised trials as being low to unclear for most of the included studies, although lack of blinding was a source of bias.
We assessed the quality of the evidence for the outcomes presented in the 'Summary of findings' tables using GRADE. Overall, the evidence ranged from very low to high quality. We downgraded the evidence in four of the five studies included in the review due to study design limitations, and for many outcomes the effect estimates were imprecise. Where data were pooled in meta‐analysis, results from trials were inconsistent and we downgraded the evidence for high statistical heterogeneity.
The cluster randomised trial was at risk of bias from lack of blinding but otherwise the trial was at low risk of bias. All data were appropriately adjusted for baseline differences between clusters.
Potential biases in the review process
We tried to reduce bias to a minimum during the review process. Two authors assessed the eligibility of studies and risk of bias, and extracted data independently. Although we followed Cochrane Pregnancy and Childbirth search strategies and its recommended review process, there may be relevant unpublished trials that we were unable to find. It is also possible that a different review team may have made different judgments when assessing study eligibility and risks of bias.
Agreements and disagreements with other studies or reviews
We identified no other studies or reviews that addressed this area. However, one systematic review demonstrated that walking and maintaining an upright position in the first stage of labour reduces the length of labour (Lawrence 2013). Only one trial adapted walking and keeping an upright position into an intervention (McNiven 1996). In future research, interventions should include not only support, encouragement, and advice, but also instructions to walk around and maintain an upright position in early labour (Lawrence 2013; McNiven 1996).
Use of epidural or any regional anaesthesia and labour augmentation were reduced in intervention groups in some of the trials, however, use of these interventions was likely to be influenced by lack of blinding.
Authors' conclusions
Implications for practice.
The effect of interventions in early labour is mixed. It is not clear that any of the assessment or support interventions included in this review was associated with a reduction in the rate of caesarean or instrumental birth although evidence comparing early labour assessment versus immediate hospital admission showed that early assessment may have an impact on reducing the use of epidural, labour augmentation and on increasing maternal satisfaction. Three trials reported whether the baby was born before arrival at hospital or in an unplanned home birth and the interventions did not appear to affect these outcomes.
Most of the included studies in this review had unclear risk of bias for selective reporting, which should be taken into account when interpreting the results. The included trials reported only some of the primary outcomes of interest to this review. Therefore, future studies should address these outcomes.
Concepts of and tools for assessing maternal satisfaction may vary depending on aspects of the study setting, such as culture, language, and health systems.
Implications for research.
The included trials in this review examined interventions in early labour. However, the interventions varied and evidence for all interventions was limited. Further high‐quality randomised control trials are required. Further trials including both primiparous and multiparous women are required to examine the effect of parity, and further studies are recommended to investigate the effectiveness of support and assessment interventions on longer‐term outcomes, such as infant development. The main limitation of this review is the lack of data from a large number of trials, which made it difficult to assess the various bias domains clearly. Therefore, we may have produced a false estimate of the underlying truth. Another limitation was heterogeneity in the type and timing of interventions, outcome measurement, and characteristics of participants. In addition, as the included trials were conducted in Western and high‐income countries, generalisability of the findings to different ethnic groups and countries may not be feasible. In order to make results of future studies generalisable, it would be helpful to harmonise measurements and reporting practices. Taking into account the nature of the interventions and outcomes investigated in this review, it would be possible to consider a broad range of study designs, including randomised controlled trials, in future updates, as these may provide us with a better understanding of the effects and behavioural nature of assessment and support interventions.
What's new
| Date | Event | Description |
|---|---|---|
| 17 August 2017 | Amended | This review has been amended since the last published version in April 2017. The overall conclusions remain unchanged. Two reports of a trial previously excluded have now been included (Cheyne 2008). This was a cluster‐randomised trial. The trial compared women attending units where assessment by midwives was carried out either using strict criteria for labour diagnosis using an algorithm or by routine assessment to decide whether women were in labour. |
History
Protocol first published: Issue 2, 2015 Review first published: Issue 4, 2017
| Date | Event | Description |
|---|---|---|
| 23 June 2017 | Amended | Clarified the reason for exclusion for Cheyne 2008a. |
| 21 April 2017 | Amended | Added citation for the review protocol. |
Acknowledgements
We thank Ms Emma Barber for her editorial support.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIIHR, NHS or the Department of Health.
Rintaro Mori's and Chie Nagata's institution receives government funding from the Clinical Research Program for Child Health and Development, AMED, Japan to provide support for the Pregnancy and Childbirth Satellite in Japan.
As part of the prepublication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), members of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search terms
ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP)
(Labor onset OR labour onset OR latent phase OR early labor OR early labour OR perinatal) AND (educational OR education OR assess OR assessment OR psychological OR psychosocial OR support OR supportive)
Data and analyses
Comparison 1. Assessment vs direct admission in early labour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of labour (hours) | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | ‐5.20 [‐7.06, ‐3.34] |
| 2 Rate of caesarean section | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.30, 1.72] |
| 3 Rate of instrumental vaginal birth | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.58, 1.26] |
| 4 Baby born before arrival at hospital or unplanned home birth | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Augmentation of labour | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.86] |
| 6 Use of epidural or any regional anaesthesia | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.98] |
| 7 Maternal satisfaction (score) | 1 | 201 | Mean Difference (IV, Fixed, 95% CI) | 16.0 [7.53, 24.47] |
| 8 Apgar score < 7 at 5 minutes | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.12, 72.12] |
Comparison 2. Home support vs telephone triage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of labour (hours) | 1 | 3474 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.14, 0.72] |
| 2 Rate of caesarean section | 3 | 5170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.95, 1.17] |
| 3 Rate of instrumental vaginal birth | 2 | 4933 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.15] |
| 4 Baby born before arrival at hospital or unplanned home birth | 1 | 3474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.30, 5.95] |
| 5 Serious maternal morbidity | 1 | 3474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.61, 1.42] |
| 6 Augmentation of labour | 2 | 1694 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.04] |
| 7 Use of epidural or any regional anaesthesia | 3 | 5168 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.05] |
| 8 Duration of hospital stay (prolonged postpartum stay in hospital > 5 days) | 1 | 3474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.83, 1.60] |
| 9 Maternal satisfaction (score) | 1 | 423 | Mean Difference (IV, Fixed, 95% CI) | 3.47 [1.00, 5.94] |
| 10 Postpartum depression (EPDS score ≥ 13) | 1 | 2584 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.42] |
| 11 Perinatal death | 1 | 3474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.42, 2.40] |
| 12 Neonatal admission to special care | 3 | 5170 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.50, 1.42] |
| 13 Apgar score < 7 at 5 minutes | 3 | 5170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.71, 1.99] |
| 14 Exclusive breastfeeding at discharge | 1 | 3474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.96, 1.04] |
| 15 Exclusive breastfeeding at six weeks | 1 | 3474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.97, 1.14] |
Comparison 3. One‐to‐one structured care vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of caesarean section | 1 | 4996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.02] |
| 2 Rate of instrumental vaginal birth | 1 | 4996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 3 Serious maternal morbidity | 1 | 4996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.84, 1.52] |
| 4 Use of epidural or any regional anaesthesia | 1 | 4996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.99, 1.01] |
| 5 Perinatal death | 1 | 4989 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal admission to special care | 1 | 4989 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 7 Apgar score < 7 at 5 minutes | 1 | 4989 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.64, 1.79] |
3.5. Analysis.
Comparison 3 One‐to‐one structured care vs usual care, Outcome 5 Perinatal death.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cheyne 2008.
| Methods | Cluster randomised trial. Maternity units in Scotland with at least 800 annual births were randomised.14 units participated in this study; each of seven units were allocated the experimental and control group. |
|
| Participants | 2183 women included. Inclusion criteria: Primiparous women presenting for admission in spontaneous labour with singleton pregnancy, cephalic presentation, 37‐42 weeks’ gestation and uncomplicated pregnancy. Exclusion criteria: girls under the age of 16, women with learning difficulties, severe illness or important medical problems, or mental health problems or drug or alcohol abuse. Women with essential hypertension, cardiac, renal or endocrine disease, epilepsy or a history of thromboembolism or asthma or women with complications in the current pregnancy including ante[partum haemorrhage, pregnancy induced hypertension, anaemia or low maternal weight at booking. |
|
| Interventions | All women: Maternity units encouraged women to contact their maternity unit, by telephone, for advice when they thought that they were in labour and if appropriate to attend the maternity unit for admission assessment. Labour assessment was done in either the labour ward or a designated assessment area. During the trial, women in both groups contacted the hospital and then attended for assessment in a similar way. i.e. telephone advice before attending INTERVENTION: Algorithm for labour diagnosis 7 Maternity units (1029 women, median cluster size 162 women at baseline; 896 women followed up after the intervention with data for 892 included in the analysis) In the experimental group, midwives who admitted women in labour were invited to attend workshops on the intervention and received a training manual on how to use the algorithm for labour diagnosis. The admitting midwife identified eligible women on admission to the labour suite and provided written and verbal explanations of the study and asked for consent. Women received a physical examination and midwives then used a strict process for diagnosing labour. Active labour was diagnosed when women were having regular, painful moderate or strong uterine contractions, spontaneous rupture of membranes or “show”, cervix effacing and at least 3cm dilated. After admission assessment women in both groups received standard care. Women identified as not yet in active labour were encouraged to return home if appropriate or were admitted to an antenatal area, depending on local maternity unit policy. Control Group: 7 maternity units (1291 women, median cluster size 199 at baseline; 1287 women followed up with data for 1297) No special intervention. Women phoned for advice when they thought they were in labour. Once they presented at the study hospital they were assessed by midwives (without using the labour diagnosis algorithm) and then women were managed according to local maternity unit policy. Women in the control group were asked for consent in the postnatal wards. |
|
| Outcomes | Primary outcome: oxytocin for labour augmentation. Secondary outcomes were interventions in labour (artificial rupture of membranes, vaginal examination, continuous electronic fetal monitoring, and use of analgesia), admission management (number of admissions before labour, time spent in labour ward, and duration of active labour), and labour outcomes (mode of delivery, intrapartum complications, neonatal outcome, and unplanned out of hospital births). |
|
| Notes | The trial took place between April 2005 and June 2007 with a 10 month data collection period in each maternity hospital. Funding: This work was supported by the Scottish Executive Chief Scientist Office Health Service Research Committee (CZH/4/245). The research was independent of the funders. Competing interests: None declared The unit of randomisation in this study was the maternity hospital, midwives were participants at the level of the intervention, and outcomes were measured for women receiving maternity care. Maternity units in Scotland UK with at least 800 births were eligible for randomisation. All hospitals had facilities for labour augmentation.The trial authors kindly provided the original data for this study. Pending further analysis we have included data from this trial in additional tables; the trial statistician carried out appropriate analysis for this cluster randomised trials which accounted for cluster effect within maternity units. Data were collected before the intervention period (baseline) and after the intervention had been introduced. "The primary analysis used multiple regression of maternity unit level data adjusted for baseline". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trialists used minimisation to allocate maternity units to experimental or control groups. The first unit, was randomly allocated then clusters were allocated to maximise balance between groups. Presence or absence of an on‐site midwife managed birth unit was the balancing variable, as these units had a policy of lower intervention in labour. |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out by the trial statistician. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Midwives were not blind to the intervention and women in the intervention group would be aware of the intervention. Although the cluster design effect may have reduced contamination the staff in all units may have been aware of study allocation. Trialists reported that staff in the control units received minimal information about the study algorithm. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | A clinical midwife in each unit was responsible for facilitating study implementation and collecting trial outcome data from case records. It was not clear whether this midwife was also involved in providing care or obtaining consent. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 units participated in this study; seven units were allocated the experimental and control groups. 896 women were in the intervention group and 1287 women were in the control group. Lost to follow‐up: experimental group (n=4), control group (n=8). No loss of clusters from the analysis. It was not clear if there were missing data for any outcomes. |
| Selective reporting (reporting bias) | Low risk | Trial registration and appropriate power calculation. Pre‐specified outcomes reported the paper. Reported adjusting results for cluster design effect. |
| Other bias | Low risk | There were baseline differences between maternity units randomised but baseline differences between clusters were accounted for in the analyses. We did not enter raw data from this trial in our data and analysis tables. Rather, we have presented results from the period following the intervention and reported the difference between groups and the P value; the results take account of baseline differences between maternity units. |
Hodnett 2008.
| Methods | Multicentre, randomized controlled trial | |
| Participants | Enrolled 5002 nulliparous women experiencing contractions but not in active labour arriving at hospital. Women who were of less than 34 weeks' gestation were excluded. Immediately after randomisation the appropriate form of care was provided to 2412 women (96.6%) in the structured care group (intervention group) and to 2497 women (99.8%) in the usual care group (control group). The women were nulliparous, had a live singleton fetus in the cephalic position, and had no contraindications to labour. The study was conducted with pregnant women from 20 North American and UK hospitals. |
|
| Interventions | Both groups received care in hospital. Experimental group (structured care): immediately after randomisation, women received structured care from a nurse or midwife trained in this type of care for a minimum of 1 hour. Components of structured care: palpate to assess fetal position; encourage maternal positions that promote fetal head rotation or relieve pain; assess labour pain, both contraction pain and backache; demonstrate cognitive, behavioural, and sensory interventions to manage labour pain; assess maternal emotional status; use interventions to reduce emotional distress Control group: usual care was provided by a nurse or midwife who had not been trained in structured care. Each nurse or midwife often provided care to more than 1 woman. Usual care depended on many factors. |
|
| Outcomes |
Primary outcome Spontaneous vaginal birth Secondary outcomes Number of women:
Other immediate outcomes Labour onset Oxytocin started after active labour Analgesia or anaesthesia Continuous electronic fetal heart rate monitoring Method of delivery Maternal death Health problems during postnatal stay Neonatal outcomes Alive at birth Birthweight Apgar score Neonatal death High level of care |
|
| Notes | Funding source: Canadian Institutes of Health Research (grant No MCT59614) Study dates: women were enrolled between 1 May 2003 and 6 March 2007. Declarations of interest of trial authors: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as follows: "Randomisation was centrally controlled and concealed, using an Internet based service". |
| Allocation concealment (selection bias) | Low risk | Randomisation was centrally controlled and concealed, using an internet‐based service. The nurse or midwife accessed the trial website to obtain the participants' study group allocation. Used the central allocation system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Nurses' and midwives' study allocation was not blinded. Incomplete blinding, but the outcome was not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Incomplete blinding, but the outcome was not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sample attrition was low and balanced between the groups (lost to follow‐up: intervention group 4; control group 2; major congenital anomaly: intervention group 3; control group 4). There was increased loss to follow up for the questionnaire survey at 6‐8 weeks postpartum (intervention 82.7% response rate versus control 82.6%). |
| Selective reporting (reporting bias) | Low risk | Most outcomes specified in the review were reported. |
| Other bias | Low risk | No apparent source of other bias. |
ISRCTN11168521.
| Methods | A multi‐centre, randomized controlled trial | |
| Participants | 3514 pregnant women were randomly allocated between the 2 arms of the trial: home group = 1759; hospital group = 1755. Allocation between home and hospital groups remained equivalent at the centre level. Eligible participants were at 34 weeks of pregnancy or more, pregnant with a live, single fetus, nulliparous, at least 16 years of age at the time of consent, and were planning a hospital birth. The study took place in the UK. | |
| Interventions | Experimental (home) group: community midwives supported and assessed nulliparous women at home in early labour. Women received assessment of maternal and fetal condition and labour progress, according to midwives' existing responsibilities when providing care during labour. They supported women with coping strategies, including breathing and relaxation techniques, advice on keeping upright and mobile, guidance on when to go to the hospital, advice on hydration, nutrition and bladder care, the involvement of the woman's birth companion in providing support. Control (hospital) group: standard care was given directly, which usually included telephone advice to attend the hospital delivery suite to determine whether labour was established. |
|
| Outcomes |
Primary outcomes The proportion of women delivered by:
Secondary outcomes Labour: interventions, duration and complications:
Maternal complications:
Improving care at the primary/secondary interface:
Neonatal complications:
Breastfeeding rates:
|
|
| Notes | Funding source: NHS Service Delivery and Organisation R&D Programme. Ref: SDO/40/2003/UK Study dates: recruitment commenced 16 August 2004 and closed 26 June 2006 Declarations of interest of trial authors: no declaration found in trial reports |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial computing staff at an external unit produced the randomisation code and they were the only people with access to this code. |
| Allocation concealment (selection bias) | Low risk | The unit of randomisation was the individual woman (p35). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Midwives were expected at all times to follow the policies, guidelines and group protocols of their employer, the NHS Trust. Participants and investigators were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, and the outcome was likely to be influenced by lack of blinding because most participants knew the group to which they were allocated. However, most caregivers may not have been aware of a woman's group allocation from informal discussion and so on. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4263 women were assigned a study number. 789 women (18.5%) were excluded from the study. 1737 women were allocated to the home visit care group and 1737 women were allocated to the hospital group. All participants were included in the analysis of primary outcomes in this study. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but the published reports include expected outcomes in tables. |
| Other bias | High risk | Compliance in the intervention group was very low (447/1759). |
Janssen 2003.
| Methods | Non‐blinded, multicentre, randomized trial | |
| Participants | A total of 237 eligible women who had completed 37 to 41 weeks of gestation, and were aged between 15 and 42 years old, participated in this study. 117 participants were randomized to receive home care and 120 to receive telephone triage. 1 person in each group withdrew from the study, but outcomes for both women were retained and analyzed according to intention‐to‐treat. The study took place at the BC Women's Hospital in Canada. | |
| Interventions | Experimental group (home visit): the time spent by nurses at participants' homes ranged between 60 and 90 minutes. The nursing assessment at home was identical to a routine admission to hospital and included a brief history, assessment of maternal vital signs etc. The women were instructed in comfort measures such as positioning, relaxation techniques, and standard advice about when to proceed to the hospital. Control group (telephone triage): women in the telephone triage group made their own decision about when to come to hospital, based on their telephone conversation with a nurse from the triage or assessment unit. The decision to come to hospital was made without a clinical assessment. |
|
| Outcomes |
Primary outcome Epidural analgesia Caesarean delivery Secondary outcomes Labour augmentation Use of electronic fetal monitoring Use of analgesia Cervical dilatation on admission Time from admission to delivery Apgar scores Admission to the neonatal care nursery |
|
| Notes | Funding source: British Columbia (BC) Health Research Foundation, the BC Medical Services Foundation, the BC Women's Foundation, and BC Women's Hospital Study dates: not reported in trial report Declarations of interest of trial authors: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was accomplished by means of opening consecutively numbered opaque envelopes containing treatment allocation." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was accomplished by means of opening consecutively numbered opaque envelopes containing treatment allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of nurses and physicians caring for participants after hospital admission was not feasible, as women were likely to discuss their early labour experience with their caregivers. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment, and some outcome measurements such as 'use of electronic fetal monitoring' and 'use of analgesia' were likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 237 eligible women participated in this study, 117 of whom were randomized to receive home care and 120 to receive telephone triage. 1 person in each group withdrew from the study, but outcomes for both women were retained and analyzed according to intention‐to‐treat. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information provided to enable us to make this judgement. |
| Other bias | Low risk | No apparent source of other bias. |
Janssen 2006.
| Methods | Multicentre, randomized trial | |
| Participants | This study took place at 7 hospitals in the City of Vancouver, Canada. 2347 eligible women were assessed in this study, 1459 women were randomized (home visit n = 728; telephone support n = 731). No loss to follow‐up in either group. Inclusion criteria of this study were that women lived within a 30‐minute drive of the hospital, were between the ages of 16 and 42 years, had completed 37–41 weeks of gestation, were nulliparous, and were carrying a singleton fetus in the vertex position. | |
| Interventions | Experimental (home visit) group: the nursing assessment at home was identical to that over the telephone, but also included maternal vital signs, abdominal palpation, auscultation of the fetal heart rate, assessment of contractions and examination of the cervix. After the assessment, nurses contacted the primary physician by telephone. Control (telephone support/triage) group: study nurses asked women about their contractions (frequency, duration, and strength), and their own assessment of how they were coping over the phone. Both groups of women were given the same advice. |
|
| Outcomes |
Primary outcome Rate of caesarean delivery Secondary outcomes Rates of admission to hospital in the latent phase of labour (≤ 3 cm cervical dilatation) Number of visits to hospital that did not result in admission Ability to cope with pain on arrival as assessed by the admitting nurse Rates of intrapartum interventions including:
Newborn outcomes:
|
|
| Notes | Funding source: Canadian Institute of Health Research Study dates: women were enrolled between 14 August 2001 to 30 October 2004 Declarations of interest of trial authors: not reported in trial report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation was achieved using a centralised randomisation service. Randomisation was stratified within participating hospitals, with randomly generated block sizes of 6, 8, and 10. |
| Allocation concealment (selection bias) | Low risk | The computer‐generated randomisation was achieved using a centralised randomisation service. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nurses and physicians were not blinded to study allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were ascertained from study data collected prospectively and from reviews of hospital charts within 24 hours of discharge. Charts were reviewed by trained nursing research assistants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1459 women participated in this study; 728 women were allocated to the home visit group and 731 women were allocated to the telephone support group. Lost to follow‐up: 0, received allocated intervention: home visit 654 (89.8%), telephone 725 (99.2%). |
| Selective reporting (reporting bias) | Unclear risk | Not enough information provided for us to make this judgement. |
| Other bias | Low risk | No apparent source of other bias. |
McNiven 1996.
| Methods | Randomised control, parallel trial | |
| Participants | 209 low‐risk nulliparous women at 37 weeks' or more gestation recruited from a large teaching hospital in Ontario, Canada. Pregnant women who were booked for induction of labour or caesarean section were excluded. | |
| Interventions | Experimental (early labour assessment) group: women received the usual assessments of fetal and maternal well‐being, such as fetal heart rate, blood pressure, and urine tests. They were also instructed when to return to the hospital. The assessment area nurse transferred women in the experimental group to the labour and delivery unit when they had progressed to the active phase of labour. Control group: direct admission to the labour and delivery unit. No instructions or advice were given regarding labour before admission to the labour ward. |
|
| Outcomes | Oxytocin Amniotomy Anaesthesia Percentage of caesarean deliveries percentage of instrumental deliveries Labor Agentry Scale (LAS) Length of labour Apgar at 1 min < 7 Apgar at 5 min < 7 Expectations |
|
| Notes | Funding source: a grant from the Perinatal Nursing Research Unit, University of Toronto, Ontario, Canada Study dates: recruitment took place from February 1994 to January 1995. Declarations of interest of trial authors: Patricia McNiven was a faculty member of the McMaster University Midwifery Education Programme and had a part‐time midwifery practice in Hamiltion; Jack Williams was a Professor at the University of Toronto and the Deputy Director‐Research, Institute for Clinical Evaluative Sciences, Toronto; Ellen Hodnett was a Professor at the University of Toronto and Heather Reisman Chair in Perinatal Nursing Research, Toronto; Karyn Kaufman was Professor and Chair of the McMaster Midwifery Education Programme, Hamilton; Mary Hannah was the Director of the University of Toronto Maternal, Infant and Reproductive Health Research Unit, Toronto, Ontario, Canada. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component in the sequence generation process to open numbered, sealed, and opaque envelopes sequentially. |
| Allocation concealment (selection bias) | Low risk | Using sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Incomplete blinding. Investigators knew the outcome, so it was influenced. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, and the outcome was likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 209 low‐risk nulliparous women were recruited from a large teaching hospital. 105 women were randomly allocated to the early labour assessment group and 104 to the direct admission group. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information provided for us to make this judgement. |
| Other bias | Low risk | No apparent source of other bias. |
Abbreviations
GA: general anaesthetic NHS: National Health Service SCBU: special care baby unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bonovich 1990 | This trial examined an intervention in the antenatal period. |
| Dowding 2011 | This study focused on the design and evaluation of an algorithm for the diagnosis of labour. Not a psychosocial or educational intervention. Not a randomized control trial. |
| Fenwick 2015 | This intervention aimed is to reduce women's fear of birth. The scope of this review is specifically about teaching women how to recognise and cope with the latent phase of labour. This study did not match those criteria. |
| IRCT138903063078N4 | Not a randomized control trial. |
| Janssen 2013 | This study focused on the development of the Early Labour Experience Questionnarie (ELEQ). No investigation effect of psychosocial and educational interventions. |
| Jesse 2015 | This study used the cognitive behavioral interventions to reduce risk of antepartum depression. |
| Karp 2013 | This study assessed breastfeeding initiation. The timing of the intervention was not onset of labour nor antepartum: "the nurse home visits and augmented standard prenatal care through 48 hours postpartum". |
| Khooshide 2015 | This trial examined an intervention in the antenatal period. |
| Lumluk 2011 | Not a randomized control trial. The study design was a quasi‐randomised trial. Participants were selected by their date of attendance according to weekly scheduled visits. |
| Magriples 2015 | This study assessed the effects of pregnancy and postpartum weight trajectories. Women completed interventions at 4 time points: during pregnancy in the second and third trimester as well as postpartum at 6 months and 12 months. |
| Memon 2015 | Not a randomized control trial. The study followed an exploratory quasi‐experimental design. The overall population of 283,324 comprising 35,641 households located in the study was allocated to intervention and control areas based on geographical proximity. |
| Toohill 2014 | The intervention aimed to review women's current expectations and feelings around fear of childbirth, and to encourage women to express their feelings. This program focused explicitly on the fear of childbirth. This study did not match the criteria or outcomes for this review. |
| Werner 2016 | The goal of this study was to examine the effectiveness of a new protocol to prevent postpartum depression. This intervention did not help women during the latent phase of labour. The participants received assessments and interventions between 18 and 36 hours after giving birth, at 2 weeks, 6 weeks, 10 weeks and 16 weeks postpartum. |
| Zocco 2007 | This study assessed the obstetric triage system. The intervention was to assign women to the triage room, and did not include any support intervention. |
Differences between protocol and review
In our protocol (Hanada 2015), the types of participants were defined as pregnant women. We decided to focus on healthy pregnant women and redefined the types of participants as 'healthy pregnant women'. We may need to revise this further.
The title has changed to ‘Assessment and support in early labour for improving birth outcomes’.
The scope has changed slightly to early labour interventions only (five included studies, two excluded).
The background has been revised to include labour assessment as an intervention and the psychosocial aspect has been toned down.
Contributions of authors
Nobutsugu Hanada (NH) drafted the protocol with support from Erika Ota (EO), Masayo Matsuzaki (MM) and Rintaro Mori (RM). NH, Shinobu Kobayashi (SK) and Kenji Takehara (KT) selected studies and extracted data. SK, KT, Hatoko Sasaki (HS) and EO conducted analysis and interpretation. EO, SK, KT, HS, MM, and Chie Nagata (CN) drafted the review.
Sources of support
Internal sources
National Center for Child Health and Development, Japan.
External sources
-
Ministry of Health, Labour and Welfare, Japan.
Health Labour Sciences Research Grant (No.13800128)
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Nobutsugu Hanada: none known. Masayo Matsuzaki: none known. Erika Ota: none known. Rintaro Mori: none known. Shinobu Kobayashi: none known. Kenji Takehara: none known. Hatoko Sasaki: none known. Chie Nagata: Chie Nagata's institution receives government funding from the Clinical Research Program for Child Health and Development, AMED, Japan to provide support for the Cochrane Pregnancy and Childbirth Satellite.
Edited (no change to conclusions)
References
References to studies included in this review
Cheyne 2008 {published data only}
- Cheyne H. A cluster randomised trial to investigate the use of a decision aid for the diagnosis of active labour in term pregnancy. National Research Register (www.nrr.nhs.uk) (accessed 6 July 2006) 2006.
- Cheyne H, Hundley V, Dowding D, Bland JM, McNamee P, Greer I, et al. Effects of algorithm for diagnosis of active labour: cluster randomised trial. BMJ 2008;337:a2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodnett 2008 {published data only}
- Hodnett ED, Stremler R, Willan AR, Weston JA, Lowe NK, Simpson KR, et al. Effect on birth outcomes of a formalised approach to care in hospital labour assessment units: international, randomised controlled trial. BMJ 2008;337:a1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN11168521 {published data only}
- ISRCTN11168521. Improving care at the primary/secondary interface: a trial of community‐based support in early labour. isrctn.com/ISRCTN11168521 Date first received 5 November 2004.
Janssen 2003 {published data only}
- Janssen PA, Iker CE, Carty EA. Early labour assessment and support at home: a randomized controlled trial. Journal of Obstetrics and Gynaecology Canada: JOGC 2003;25(9):734‐41. [DOI] [PubMed] [Google Scholar]
Janssen 2006 {published data only}
- Janssen PA, Desmarais SL. Women's experience with early labour management at home vs. in hospital: a randomised controlled trial. Midwifery 2013;29(3):190‐4. [DOI] [PubMed] [Google Scholar]
- Janssen PA, Still DK, Klein MC, Singer J, Carty EA, Liston RM, et al. Early labor assessment and support at home versus telephone triage: a randomized controlled trial. Obstetrics & Gynecology 2006;108(6):1463‐9. [DOI] [PubMed] [Google Scholar]
McNiven 1996 {published data only}
- McNiven P. Feasibility Study for a Multi‐centre Randomized Trial of the Effect of an Early Labour Assessment Program on the Rate of Cesarean Delivery. [thesis]. Toronto: University of Toronto, 1996. [Google Scholar]
- McNiven PS, Williams JI, Hodnett E, Kaufman K, Hannah ME. An early labor assessment program: a randomized, controlled trial. Birth 1998;25(1):5‐10. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bonovich 1990 {published data only}
- Bonovich L. Recognizing the onset of labor. Journal of Obstetric, Gynecologic & Neonatal Nursing 1990;19:2:141‐5. [DOI] [PubMed] [Google Scholar]
Dowding 2011 {published data only}
- Dowding DW, Cheyne HL, Hundley V. Complex interventions in midwifery care: reflections on the design and evaluation of an algorithm for the diagnosis of labour. Midwifery 2011;27:654‐9. [DOI] [PubMed] [Google Scholar]
Fenwick 2015 {published data only}
- Fenwick J, Toohill J, Gamble J, Creedy DK, Buist A, Turkstra E, et al. Effects of a midwife psycho‐educational intervention to reduce childbirth fear on women's birth outcomes and postpartum psychological wellbeing. BMC Pregnancy and Childbirth 2015;15:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT138903063078N4 {published data only}
- IRCT138903063078N4. The effects of consultation with mother in third trimester of pregnancy on pregnancy outcomes. en.search.irct.ir/view/3199 Date first received: 27 May 2010.
Janssen 2013 {published data only}
- Janssen PA, Desmarais SL. Development and psychometric properties of the Early Labour Experience Questionnaire (ELEQ). Midwifery 2013;29(3):181‐9. [DOI] [PubMed] [Google Scholar]
Jesse 2015 {published data only}
- Jesse DE, Gaynes BN, Feldhousen E, Newton ER, Bunch S, Hollon SD. Performance of a culturally tailored cognitive behavioral intervention (CBI) integrated in a public health setting to reduce risk of antepartum depression: a randomized clinical trial. Journal of Midwifery & Women's Health 2015;60(5):578‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karp 2013 {published data only}
- Karp SM, Howe‐Heyman A, Dietrich MS, Lutenbacher M. Breastfeeding initiation in the context of a home intervention to promote better birth outcomes. Breastfeeding Medicine 2013;8(4):381‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khooshide 2015 {published data only}
- Khooshide M, Mirzarahimi T, Akhavan Akbari G. The impact of physiologic and non‐physiologic delivery on the mother and neonate outcomes: a comparative study on the primi gravid mothers. Journal of Family and Reproductive Health 2015;9(1):13‐8. [PMC free article] [PubMed] [Google Scholar]
Lumluk 2011 {published data only}
- Lumluk T, Kovavisarach E. Effect of antenatal education for better self‐correct diagnosis of true labor: a randomized control study. Journal of the Medical Association of Thailand 2011;94(7):772‐4. [PubMed] [Google Scholar]
Magriples 2015 {published data only}
- Magriples U, Boynton MH, Kershaw TS, Lewis J, Rising SS, Tobin JN, et al. The impact of group prenatal care on pregnancy and postpartum weight trajectories. American Journal of Obstetrics and Gynecology 2015;213(5):688.e1–688.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Memon 2015 {published data only}
- Memon ZA, Khan GN, Soofi SB, Baig IY, Bhutta ZA. Impact of a community‐based perinatal and newborn preventive care package on perinatal and neonatal mortality in a remote mountainous district in Northern Pakistan. BMC Pregnancy and Childbirth 2015;15:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toohill 2014 {published data only}
- Toohill J, Fenwick J, Gamble J, Creedy DK, Buist A, Turkstra E, et al. A randomized controlled trial of a psycho‐education intervention by midwives in reducing childbirth fear in pregnant women. Birth 2014;41(4):384‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Werner 2016 {published data only}
- Werner EA, Gustafsson HC, Lee S, Feng T, Jiang N, Desai P, et al. PREPP: postpartum depression prevention through the mother–infant dyad. Archives of Women's Mental Health 2016;19:229‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zocco 2007 {published data only}
- Zocco J, Williams MJ, Longobucco DB, Bernstein B. A systems analysis of obstetric triage. Journal of Perinatal & Neonatal Nursing 2007;21(4):315‐22. [DOI] [PubMed] [Google Scholar]
Additional references
Alehagen 2005
- Alehagen S, Wijma B, Lundberg U, Wijma K. Fear, pain and stress hormones during childbirth. Journal of Psychosomatic Obstetrics & Gynecology 2005;26(3):153‐65. [PUBMED: 16295513] [DOI] [PubMed] [Google Scholar]
Austin 1999
- Austin DA, Calderon L. Triaging patients in the latent phase of labor. Journal of Nurse‐Midwifery 1999;44(6):585‐91. [PUBMED: 10634015] [DOI] [PubMed] [Google Scholar]
Bailit 2005
- Bailit JL, Dierker L, Blanchard MH, Mercer BM. Outcomes of women presenting in active versus latent phase of spontaneous labor. Obstetrics & Gynecology 2005;105(1):77‐9. [PUBMED: 15625145] [DOI] [PubMed] [Google Scholar]
Barnett 2008
- Barnett C, Hundley V, Cheyne H, Kane F. ‘Not in labour’: impact of sending women home in the latent phase. British Journal of Midwifery 2008;16:144‐53. [Google Scholar]
Baxter 2007
- Baxter J. Care during the latent phase of labour: supporting normal birth. British Journal of Midwifery 2007;15(12):765. [Google Scholar]
Carlsson 2009
- Carlsson IM, Hallberg LR, Odberg Pettersson K. Swedish women's experiences of seeking care and being admitted during the latent phase of labour: a grounded theory study. Midwifery 2009;25(2):172‐80. [PUBMED: 17600602] [DOI] [PubMed] [Google Scholar]
Chamberlain 2013
- Chamberlain C, O'Mara‐Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001055.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheyne 2007
- Cheyne H, Terry R, Niven C, Dowding D, Hundley V, McNamme P. 'Should I come in now?' : a study of women's early labour experiences. British Journal of Midwifery 2007;15(10):604‐9. [Google Scholar]
Dennis 2007a
- Dennis CL, Hodnett E. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006116.pub2] [DOI] [PubMed] [Google Scholar]
Dennis 2007b
- Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006309.pub2] [DOI] [PubMed] [Google Scholar]
Dennis 2013
- Dennis CL, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD001134.pub3] [DOI] [PubMed] [Google Scholar]
Fekete 2007
- Fekete EM, Antoni MH, Schneiderman N. Psychosocial and behavioral interventions for chronic medical conditions. Current Opinion in Psychiatry 2007;20(2):152‐7. [PUBMED: 17278914] [DOI] [PubMed] [Google Scholar]
Flamm 1998
- Flamm BL, Berwick DM, Kabcenell A. Reducing cesarean section rates safely: lessons from a "breakthrough series" collaborative. Birth 1998;25(2):117‐24. [PUBMED: 9668746] [DOI] [PubMed] [Google Scholar]
Friedman 1972
- Friedman EA. An objective approach to the diagnosis and management of abnormal labor. Bulletin of the New York Academy of Medicine 1972;48(6):842‐58. [PUBMED: 4504890] [PMC free article] [PubMed] [Google Scholar]
Gross 2003
- Gross MM, Haunschild T, Stoexen T, Methner V, Guenter HH. Women's recognition of the spontaneous onset of labor. Birth 2003;30(4):267‐71. [PUBMED: 14992158] [DOI] [PubMed] [Google Scholar]
Gross 2006
- Gross MM, Hecker H, Matterne A, Guenter HH, Keirse MJ. Does the way that women experience the onset of labour influence the duration of labour?. BJOG: an international journal of obstetrics and gynaecology 2006;113(3):289‐94. [PUBMED: 16487200] [DOI] [PubMed] [Google Scholar]
Hemminki 1986
- Hemminki E, Simukka R. The timing of hospital admission and progress of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1986;22(1‐2):85‐94. [PUBMED: 3721051] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hodnett 1996
- Hodnett E. Nursing support of the laboring woman. Journal of Obstetric, Gynecologic & Neonatal Nursing 1996;25(3):257‐64. [PUBMED: 8683362] [DOI] [PubMed] [Google Scholar]
Holmes 2001
- Holmes P, Oppenheimer LW, Wen SW. The relationship between cervical dilatation at initial presentation in labour and subsequent intervention. BJOG: an international journal of obstetrics and gynaecology 2001;108(11):1120‐4. [PUBMED: 11762649] [DOI] [PubMed] [Google Scholar]
Jackson 2003
- Jackson DJ, Lang JM, Ecker J, Swartz WH, Heeren T. Impact of collaborative management and early admission in labor on method of delivery. Journal of Obstetric, Gynecologic & Neonatal Nursing 2003;32(2):147‐57; discussion 158‐60. [PUBMED: 12685666] [DOI] [PubMed] [Google Scholar]
Klein 2004
- Klein MC, Kelly A, Kaczorowski J, Grzybowski S. The effect of family physician timing of maternal admission on procedures in labour and maternal and infant morbidity. Journal of Obstetrics and Gynaecology Canada: JOGC 2004;26(7):641‐5. [PUBMED: 15248933] [DOI] [PubMed] [Google Scholar]
Lawrence 2013
- Lawrence A, Lewis L, Hofmeyr GJ, Styles C. Maternal positions and mobility during first stage labour. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD003934.pub4] [DOI] [PubMed] [Google Scholar]
Lederman 1979
- Lederman RP, Lederman E, Work BA Jr, McCann DS. Relationship of psychological factors in pregnancy to progress in labor. Nursing Research 1979;28(2):94‐7. [PUBMED: 254068] [PubMed] [Google Scholar]
Loughney 2006
- Loughney A, Collis R, Dastgir S. Birth before arrival at delivery suite: associations and consequences. British Journal of Midwifery 2006;14(4):204‐8. [Google Scholar]
Lui 2008
- Lui S, Terplan M, Smith EJ. Psychosocial interventions for women enrolled in alcohol treatment during pregnancy. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006753.pub2] [DOI] [PubMed] [Google Scholar]
NICE 2007
- National Collaborating Centre for Women's and Children's Health. Normal labour: first stage. Intrapartum care, Care of Healthy Women and their Babies During Childbirth. London: RCOG Press at the Royal College of Obstetricians and Gynaecologists, 2007:138‐55. [PubMed] [Google Scholar]
Petersen 2013
- Petersen A, Penz SM, Gross MM. Women's perception of the onset of labour and epidural analgesia: a prospective study. Midwifery 2013;29(4):284‐93. [PUBMED: 23079870] [DOI] [PubMed] [Google Scholar]
Rahnama 2006
- Rahnama P, Ziaei S, Faghihzadeh S. Impact of early admission in labor on method of delivery. International Journal of Gynecology & Obstetrics 2006;92(3):217‐20. [PUBMED: 16434043] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Simkin 2011
- Simkin P, Ancheta R. The Labor Progress Handbook. Third Edition. Oxford: Wiley‐Blackwell, 2011. [Google Scholar]
Tierney 2005
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta‐analysis. International Journal of Epidemiology 2005;34(1):79‐87. [PUBMED: 15561753] [DOI] [PubMed] [Google Scholar]
Vos 2006
- Vos PJ, Visser AP, Garssen B, Duivenvoorden HJ, Haes HC. Effects of delayed psychosocial interventions versus early psychosocial interventions for women with early stage breast cancer. Patient Education & Counselling 2006;60(2):212‐9. [PUBMED: 16442463] [DOI] [PubMed] [Google Scholar]
Wuitchik 1989
- Wuitchik M, Bakal D, Lipshitz J. The clinical significance of pain and cognitive activity in latent labor. Obstetrics & Gynecology 1989;73(1):35‐42. [PUBMED: 2909041] [PubMed] [Google Scholar]
References to other published versions of this review
Hanada 2015
- Hanada N, Matsuzaki M, Ota E, Mori R. Psychosocial and educational interventions in latent phase or early labour for improving birth outcomes. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauzon 1998
- Lauzon L, Hodnett ED. Antenatal education for self‐diagnosis of the onset of active labour at term. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD000935] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauzon 2001
- Lauzon L, Hodnett ED. Labour assessment programs to delay admission to labour wards. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000936] [DOI] [PubMed] [Google Scholar]


