Abstract
Introduction
Measurement of ventilatory efficiency, defined as minute ventilation per unit carbon dioxide production (VE/VCO2), by cardiopulmonary exercise testing (CPET) has been proposed as a screen for hyperventilation syndrome (HVS). However, increased VE/VCO2 may be associated with other disorders which need to be distinguished from HVS. A more specific marker of HVS by CPET would be clinically useful. We hypothesized ventilatory control during exercise is abnormal in patients with HVS.
Methods
Patients who underwent CPET from years 2015 through 2017 were retrospectively identified and formed the study group. HVS was defined as dyspnea with respiratory alkalosis (pH >7.45) at peak exercise with absence of acute or chronic respiratory, heart or psychiatric disease. Healthy patients were selected as controls. For comparison the Student t-test or Mann-Whitney U test were used. Data are summarized as mean ± SD or median (IQR); p<0.05 was considered significant.
Results
Twenty-nine patients with HVS were identified and 29 control subjects were selected. At rest, end-tidal carbon dioxide (PETCO2) was 27 mmHg (25–30) for HVS patients vs. 30 mmHg (28–32); in controls (p = 0.05). At peak exercise PETCO2 was also significantly lower (27 ± 4 mmHg vs. 35 ± 4 mmHg; p<0.01) and VE/VCO2 higher ((38 (35–43) vs. 31 (27–34); p<0.01)) in patients with HVS. In contrast to controls, there were minimal changes of PETCO2 (0.50 ± 5.26 mmHg vs. 6.2 ± 4.6 mmHg; p<0.01) and VE/VCO2 ((0.17 (-4.24–6.02) vs. -6.6 (-11.4-(-2.8)); p<0.01)) during exercise in patients with HVS. The absence of VE/VCO2 and PETCO2 change during exercise was specific for HVS (83% and 93%, respectively).
Conclusion
Absence of VE/VCO2 and PETCO2 change during exercise may identify patients with HVS.
Introduction
Hyperventilation syndrome (HVS) is characterized as episodic dyspnea with inappropriately high alveolar ventilation exceeding metabolic requirements [1,2]. HVS is highly prevalent in patients with psychological pathologies [3]. However, it is not clear if psychological pathologies are a cause of HVS [4,5].
There are no clear diagnostic criteria or screening tools for HVS [6] and the diagnosis is typically made by exclusion of other cardiopulmonary diseases characterized by symptoms of dyspnea including heart failure, asthma or chronic obstructive pulmonary disease [7]. Previously, the hyperventilation provocation test was used to identify patients with HVS [1]. However, this test has been considered invalid and is no longer used in clinical practice [8,9]. Questionnaires (especially the Nijmegen questionnaire [10]) have also been used to assess symptoms typical for HVS. However, it has been recommended that the Nijmegen questionnaire no longer be used as the sole criterion for HVS and a multidimensional diagnostic approach is advised [11].
Markers of hyperventilation including increased minute ventilation per unit of carbon dioxide production (VE/VCO2) and low partial pressure of end-tidal carbon dioxide (PETCO2) during exercise have been shown to be associated with HVS [12,13]. The slope of VE/VCO2 is increased in other conditions which need to be distinguished from HVS [14] which may limit this marker as a screening tool for HVS. However, cardiopulmonary exercise testing (CPET) is useful for evaluation of the differential diagnosis of dyspnea [15] suggesting patients with HVS may benefit from this evaluation. A more specific marker for HVS would be clinically useful.
We hypothesized that ventilatory control is abnormal at rest and during exercise in patients with HVS. Accordingly, the aim of the study was to compare CPET of subjects with HVS and healthy controls in order to identify rest or exercise CPET parameters which may be useful for the diagnosis of HVS.
Methods
Subject selection
Medical records of all individuals that underwent CPET at the Department of Respiratory Diseases, University Hospital Brno between January 1st, 2015 and December 31st, 2017 were retrospectively analyzed. HVS was defined similar as in previous studies [6,7]; episodes of dyspnea with documented respiratory alkalosis (pH >7.45) by arterial blood gas analysis at peak exercise and the absence of known acute or chronic respiratory, heart or psychiatric disease. Controls matched by pulmonary function testing were selected from healthy subjects that completed CPET at the Department of Respiratory Diseases, University Hospital Brno between January 1st, 2017 and December 31st, 2017. The study was approved by the institutional Ethics Committee of the University Hospital Brno, Czech Republic (study approval code 01–070318).
Cardiopulmonary exercise testing
All subjects underwent symptom-limited CPET on an electronically braked cycle ergometer (Ergoline, Ergometrics 800, Germany) with a 12-channel electrocardiography unit (Schiller AG, AT-104, Switzerland) using a ramp protocol with linear increase of workload of 25 watts per minute. Expired gases were collected and analyzed by the PowerCube-Ergo system (Ganshorn Medizin Electronic GmbH, Germany). Arterial blood gases were examined at rest and at peak exercise. The measured spiroergometric variables included oxygen consumption (VO2), output of carbon dioxide (VCO2), partial pressure of end-tidal carbon dioxide (PETCO2), tidal volume (VT), breathing frequency (fb) and minute ventilation (VE). The data were recorded continuously. The variables were reported as average values obtained during the final 30 seconds of each workload. The derived parameters included respiratory exchange ratio (RER), defined as the ratio of VCO2 and VO2, dead space volume to tidal volume ratio (VD/VT), VE/VCO2 slope and VE/VCO2 ratio for rest and peak exercise [16]. VT was indexed to body surface area (BSA) [17] to allow comparison between groups (there was a significant difference in sex between both groups).
Pulmonary function tests
Spirometry was performed in all subjects before CPET. All measurements were performed in accordance with the American Thoracic Society standards [18] using the ZAN 100 spirometer (nSpire Health, Inc., Longmont, CO, USA). The following variables were considered for further assessment: forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1) and the FEV1/FVC ratio. The values of FEV1 and FVC were expressed as a percentage of predicted value.
Statistics
The Shapiro-Wilk test was used to evaluate normality. Student t-test or Mann-Whitney U test were used for comparison of subjects with HVS and controls. ANOVA and Tukey's HSD post-hoc test was used to evaluate changes of VE/VCO2 and PETCO2 during exercise. Linear regression was performed to evaluate the relationship between PETCO2 or VD/VT and VE/VCO2 ratio at peak exercise. Differences in proportions were tested by two-tailed Fisher exact test. Logistic regression analysis adjusted for potential confounders (subject characteristic parameters which were significantly different between both groups–gender and BMI) was used to evaluate the difference of (peak-rest) PETCO2 and difference of (peak-rest) VE/VCO2 association with HVS. Results were expressed as the odds ratios (OR) with 95% confidence intervals (CI). Decision statistics (2x2 tables) were calculated for several cut-off values of the difference of (peak-rest) PETCO2 and the difference of (peak-rest) VE/VCO2. Data are summarized as mean ± SD or median (inter-quartile range) with p values <0.05 considered statistically significant. Statistical analysis was performed using Statistica 12.0 (StatSoft, Prague, Czech Republic).
Results
Fifty-eight patients were included in this retrospective study. Twenty-nine patients were diagnosed with HVS and comprised the study group and 29 patients served as healthy controls. Group comparison of subject characteristics, pulmonary function test parameters and arterial blood gases are shown in Table 1. Subjects with HVS were mostly women with significantly lower BMI. There were no differences in pulmonary function test parameters or arterial blood gas analysis at rest. At peak exercise patients with HVS exhibited higher PaO2 and by definition higher pH and lower PaCO2.
Table 1. Group comparison.
| HVS (n = 29) | control (n = 29) | p | |
|---|---|---|---|
| male No. (%) | 4 (14) | 14 (48) | <0.01 |
| age (years) | 56 (43–61) | 61 (41–65) | 0.77 |
| height (cm) | 168 (165–172) | 170 (164–179) | 0.23 |
| BMI (kg/m2) | 27.2 ± 5.8 | 30.5 ± 5.2 | 0.02 |
| Pulmonary function test | |||
| FEV1 (%) | 95 (90–104) | 100 (91–108) | 0.30 |
| FVC (%) | 95 ± 11 | 97 ± 11 | 0.47 |
| FEV1/FVC (%) | 86 (83–93) | 84 (81–89) | 0.09 |
| Arterial blood gas analysis at rest | |||
| PaO2 (mmHg) | 84 ± 9 | 83 ± 11 | 0.82 |
| PaCO2 (mmHg) | 36 (33–37) | 36 (34–38) | 0.44 |
| BE | 0.4 (-0.8–1.4) | 0.1 (-0.5–0.9) | 0.50 |
| pH | 7.45 (7.43–7.47) | 7.44 (7.43–7.45) | 0.07 |
| Arterial blood gas analysis at peak exercise | |||
| PaO2 (mmHg) | 96 (93–109) | 88 (83–93) | <0.01 |
| PaCO2 (mmHg) | 29 ± 4 | 35 ± 3 | <0.01 |
| BE | -2.0 ± 1.8 | -2.9 ± 1.7 | 0.05 |
| pH | 7.47 (7.46–7.50) | 7.40 (7.37–7.42) | <0.01 |
Data shown as mean ± SD or median (IQR). BE = base excess; cm = centimeter; FEV1 = forced expiratory volume-one second; FVC = forced vital capacity; HVS = hyperventilation syndrome; kg = kilogram; m2 = square meter; min = minute; ml = milliliter; mmHg = millimeter of mercury; PaCO2 = partial pressure of arterial carbon dioxide; PaO2 = partial pressure of arterial oxygen; VO2 = oxygen consumption
Rest and peak exercise ventilatory parameter comparison is shown in Table 2. At rest, the only difference between the study groups was in PETCO2 which was significantly lower in patients with HVS. At peak exercise, patients with HVS also had significantly lower VO2, VCO2, VT (including after correction for BSA) and PETCO2 and higher fb, VD/VT, RER and VE/VCO2 ratio and slope.
Table 2. Cardiopulmonary exercise testing.
| HVS (n = 29) | control (n = 29) | p | |
|---|---|---|---|
| rest | |||
| VO2 (l/min) | 0.32 (0.25–0.40) | 0.38 (0.29–0.50) | 0.29 |
| VO2 (ml/kg/min) | 4.2 (3.4–5.8) | 4.3 (2.9–6.4) | 0.91 |
| VCO2 (l/min) | 0.26 (0.19–0.31) | 0.27 (0.21–0.36) | 0.61 |
| VE (l/min) | 10 (8–12) | 11 (7–13) | 0.96 |
| VT (l) | 0.49 (0.41–0.65) | 0.55 (0.46–0.76) | 0.53 |
| VT/BSA (l/m2) | 0.27 (0.21–0.37) | 0.29 (0.22–0.35) | 0.91 |
| fb (bpm) | 19 ± 5 | 18 ± 5 | 0.42 |
| VD/VT | 0.21 ± 0.11 | 0.22 ± 0.10 | 0.77 |
| PETCO2 (mmHg) | 27 (25–30) | 30 (28–32) | 0.05 |
| VE/VCO2 ratio | 38 (33–44) | 37 (32–40) | 0.31 |
| RER | 0.76 (0.67–0.90) | 0.69 (0.65–0.75) | 0.08 |
| HR (beat/min) | 97 ± 13 | 82 ± 13 | <0.01 |
| peak exercise | |||
| Workload (W) | 135 (111–142) | 163 (137–186) | <0.01 |
| VO2 (l/min) | 1.37 (1.30–1.72) | 2.00 (1.59–2.47) | <0.01 |
| VO2 (ml/kg/min) | 18.7 (15.8–21.6) | 24.2 (19.4–29.2) | 0.01 |
| VCO2 (l/min) | 1.38 (1.24–1.57) | 1.81 (1.56–2.15) | <0.01 |
| VE (l/min) | 55 (46–62) | 53 (48–63) | 0.69 |
| VT (l) | 1.25 (1.16–1.64) | 1.87 (1.55–2.29) | <0.01 |
| VT/BSA (l/m2) | 0.72 (0.61–0.87) | 1.01 (0.77–1.07) | <0.01 |
| fb (bpm) | 39 (34–46) | 30 (27–33) | <0.01 |
| VD/VT | 0.18 ± 0.05 | 0.13 ± 0.06 | <0.01 |
| PETCO2 (mmHg) | 27 ± 4 | 35 ± 4 | <0.01 |
| VE/VCO2 ratio | 38 (35–43) | 31 (27–34) | <0.01 |
| RER | 0.96 ± 0.1 | 0.89 ± 0.12 | 0.03 |
| HR (beat/min) | 142 ± 17 | 147 ± 20 | 0.30 |
| VE/VCO2 slope | 37 (33–43) | 27 (24–30) | <0.01 |
Data shown as mean ± SD or median (IQR). bpm = breaths per minute; BSA = body surface area; fb = breathing frequency; HR = hear rate; HVS = hyperventilation syndrome; kg = kilogram; l = liter; m2 = square meter; min = minute; ml = milliliter; mmHg = millimeters of mercury; PETCO2 = partial pressure of end-tidal carbon dioxide; RER = respiratory exchange ratio; VCO2 = carbon dioxide output; VD = dead space volume; VE = minute ventilation; VE/VCO2 = ventilatory efficiency; VO2 = oxygen consumption; VT = tidal volume; W = watts
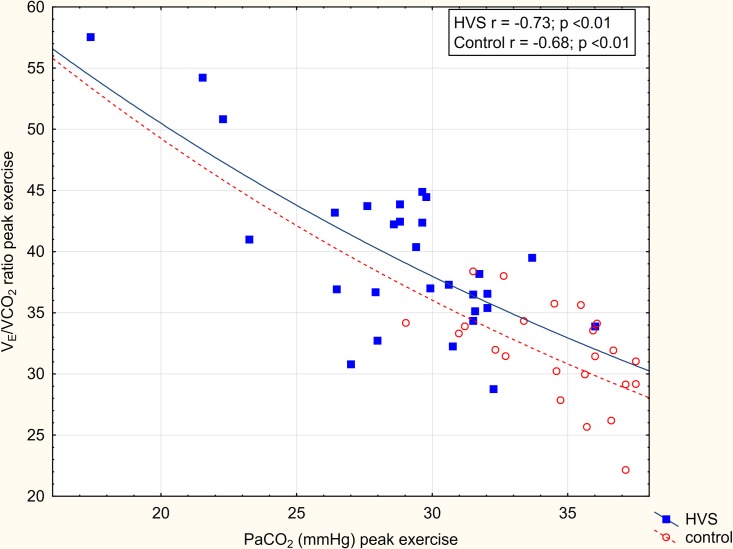
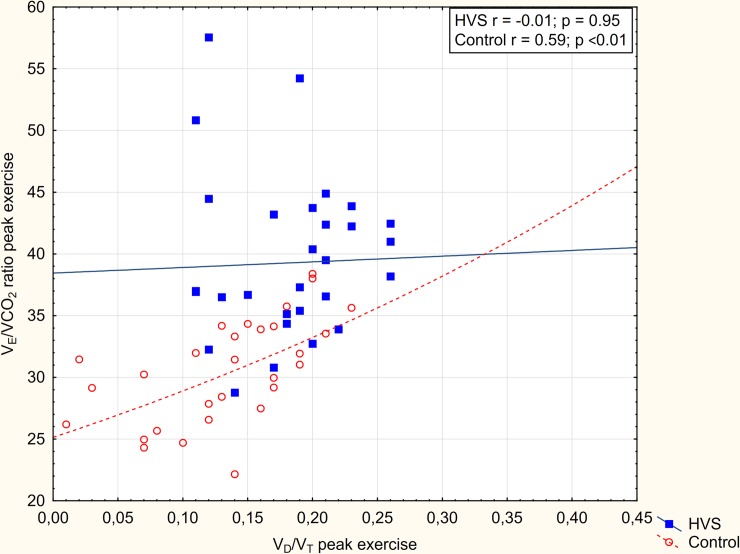
The relation of VE/VCO2 ratio and PaCO2 at peak exercise in patients with HVS and in controls is shown in Fig 1. In both groups, there was a significant correlation of PaCO2 and the VE/VCO2 ratio. However, a shift of the slope of this relation was observed in patients with HVS. The shift is consistent with a significantly higher VD/VT ratio (ventilation-perfusion mismatch) in patients with HVS. Similarly, Fig 2 shows the relation of VE/VCO2 ratio and VD/VT at peak exercise in patients with HVS and in controls. The VE/VCO2 ratio correlated significantly with VD/VT only for control subjects. In HVS subjects, the correlation was not observed and the slope shifted upwards. The shift of slope corresponds with the observed significantly lower PaCO2 consistent with increased ventilatory drive in patients with HVS.
Fig 1. Relation of VE/VCO2 and PaCO2 in patients with HVS and controls.
Slopes of ventilatory efficiency (VE/VCO2) to partial pressure of arterial oxygen (PaCO2) at peak exercise are compared in patients with HVS and controls. The shift of the slope of this relationship in patients with HVS is consistent with the observed higher VD/VT ratio (i.e. higher ventilation-perfusion mismatch).
Fig 2. Relation of VE/VCO2 and VD/VT in patients with HVS and controls.
Slopes of VE/VCO2 and ratio of tidal volume to dead space (VD/VT) at peak exercise are compared in patients with HVS and controls. The shift of the slope of this relationship in patients with HVS is consistent with the observed lower PaCO2 (i.e. increased ventilatory drive).
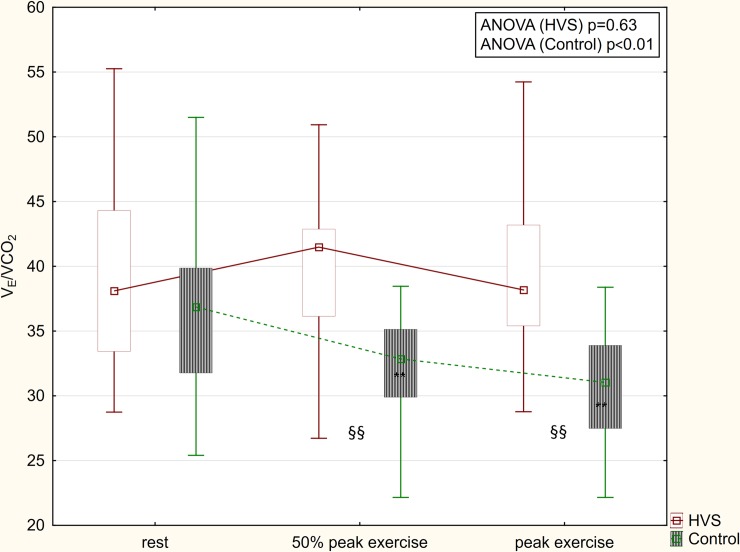
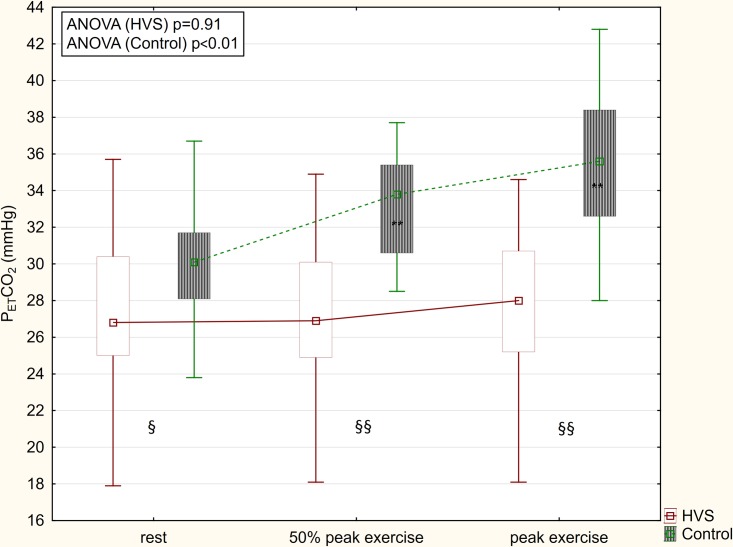
Differences from peak exercise to rest of ventilatory parameters are summarized in Table 3. In patients with HVS, the increase of VO2, VCO2, VT and heart rate (HR) during exercise was significantly lower than in controls. Breathing frequency increased significantly more in patients with HVS. In contrast to controls, VE/VCO2 and PETCO2 did not change significantly during exercise in patients with HVS (Figs 3 and 4).
Table 3. Change of ventilatory parameters (peak-rest).
| HVS (n = 29) | control (n = 29) | p | |
|---|---|---|---|
| VO2 (l/min) | 1.10 (0.98–1.38) | 1.65 (1.24–2.02) | <0.01 |
| VO2 (ml/kg/min) | 15.2 ± 6.0 | 20.4 ± 6.7 | <0.01 |
| VCO2 (l/min) | 1.1 (1.0–1.3) | 1.5 (1.3–1.8) | <0.01 |
| VE (l/min) | 44 ± 14 | 43 (39–51) | 0.70 |
| fb (bpm) | 22 ± 8 | 12.2 ± 7.1 | <0.01 |
| VT (l) | 0.88 ± 0.36 | 1.36 ± 0.54 | <0.01 |
| VD/VT | -0.03 ± 0.09 | -0.09 ± 0.11 | 0.04 |
| PETCO2 (mmHg) | 0.50 ± 5.26 | 6.2 ± 4.6 | <0.01 |
| VE/VCO2 ratio | 0.17 (-4.24–6.02) | -6.6 (-11.4-(-2.8)) | <0.01 |
| RER | 0.18 (0.04–0.31) | 0.2 (0.14–0.28) | 0.69 |
| HR (beat/min) | 45 ± 14 | 65 ± 15 | <0.01 |
Data shown as mean ± SD or median (IQR). bpm = breaths per minute; fb = breathing frequency; FiO2 = fraction of inspired oxygen; HR = hear rate; HVS = hyperventilation syndrome; kg = kilogram; l = liter; min = minute; ml = milliliter; mmHg = millimeters of mercury; PaCO2 = partial pressure of arterial carbon dioxide; VCO2 = carbon dioxide output; VD = dead space volume; VE = minute ventilation; VE/VCO2 = ventilatory efficiency; VO2 = oxygen consumption; VT = tidal volume
Fig 3. VE/VCO2 changes during exercise.
In contrast to patients with HVS, VE/VCO2 decreased during exercise in controls. ** = p<0.01 compared to rest; §§ = p<0.01 HVS vs. control.
Fig 4. PETCO2 changes during exercise.
In contrast to patients with HVS, PETCO2 increased during exercise in controls. ** = p<0.01 compared to rest; § = p<0.05 HVS vs. control; §§ = p<0.01 HVS vs. control.
Logistic regression adjusted for gender and BMI showed the change of VO2 (OR 1.21; 95%CI 1.05–1.38; p = 0.01; ROC AUC = 0.84), VCO2 (OR = 24; 95%CI 1.8–318; p = 0.02; ROC AUC = 0.81), fb (OR = 0.83; 95%CI 0.74–0.93; p<0.01; ROC AUC = 0.87), VT (OR = 6.7; 95%CI 1.3–36; p = 0.03; ROC AUC = 0.79), HR (OR = 1.14; 95%CI 1.05–1.23; p<0.01; ROC AUC = 0.90), PETCO2 (OR 1.26; 95%CI 1.08–1.48; p<0.01; ROC AUC = 0.83) and change of VE/VCO2 (OR 0.88; 95%CI 0.80–0.97; p = 0.01; ROC AUC = 0.81) to be independently associated with the presence of HVS. Decision statistics for several cut-off values of the change of VE/VCO2 and change of PETCO2 and the diagnosis of HVS are shown in Table 4. The absence of VE/VCO2 or PETCO2 changes during exercise was highly specific for HVS (83% and 93%, respectively). Specificity further increased with VE/VCO2 increase (up to 97%) and PETCO2 decrease (up to 100%) during exercise.
Table 4. Decision statistics for the change of VE/VCO2 and PETCO2 cut-off values and HVS.
| VE/VCO2 ratio | ||||||
| Δ (peak-rest) | sensitivity | specificity | +LR | -LR | PPV | NPV |
| +5 | 31 (15–51) | 97 (82–100) | 9 (1.2–67) | 0.7 (0.6–0.9) | 90 (55–99) | 58 (52–64) |
| 0 | 52 (33–71) | 83 (64–94) | 3 (1.3–7.2) | 0.6 (0.4–0.9) | 75 (56–88) | 63 (53–72) |
| -5 | 83 (64–94) | 59 (39–76) | 2 (1.3–3.2) | 0.3 (0.1–0.7) | 67 (56–76) | 77 (59–89) |
| -10 | 97 (82–100) | 34 (18–54) | 1.5 (1.1–1.9) | 0.1 (0.01–0.73) | 60 (53–66) | 91 (58–99) |
| PETCO2 (mmHg) | ||||||
| Δ (peak-rest) | sensitivity | specificity | +LR | -LR | PPV | NPV |
| -5 | 21 (8–40) | 100 (88–100) | - | 0.8 (0.7–1) | 100 | 56 (51–60) |
| 0 | 55 (36–74) | 93 (77–99) | 8 (2–31) | 0.5 (0.3–0.7) | 89 (67–97) | 68 (58–76) |
| +5 | 83 (64–94) | 62 (42–79) | 2.2 (1.3–3.6) | 0.3 (0.1–0.7) | 69 (57–78) | 78 (61–89) |
| +10 | 93 (77–99) | 14 (4–32) | 1.1 (0.9–1.3) | 0.5 (0.1–2.5) | 52 (48–56) | 67 (28–91) |
Δ = delta; +LR = positive likelihood ratio; -LR = negative likelihood ratio; NPV = negative predictive value; PETCO2 = partial pressure of end-tidal carbon dioxide; PPV = positive predictive value; VE/VCO2 = ventilatory efficiency
Discussion
The major finding of this study was that in patients with HVS both the VE/VCO2 and PETCO2 remained relatively unchanged from rest to peak exercise in patients with HVS consistent with abnormal ventilatory control throughout exercise.”
In our study, subjects with HVS were mostly women with lower BMI. This is in agreement with previous studies showing HVS to be more prevalent in women [6,19]. There were no significant differences in rest arterial blood gases between HVS subjects and controls. However, there was a nonsignificant trend towards higher pH in subjects with HVS. As there was no difference in PaCO2 at rest, we speculate higher pH may correspond with metabolic compensation of chronic episodes of hyperventilation in patients with HVS. Indeed, base excess tended to be higher in patients with HVS. At peak exercise, PaO2 was significantly higher and pH was significantly higher which exceeded 7.45 as per HVS definition and PaCO2 was significantly lower in patients with HVS compared to controls. Peak exercise arterial blood gases were very similar to values showed in a larger previous study [6].
At rest, PETCO2 was significantly lower in subjects with HVS. However, logistic regression adjusted for confounders (gender and BMI) failed to show rest PETCO2 to be significantly associated with the presence of HVS (OR 1.12; 95% CI 0.99–1.28; p = 0.08). In the Hammo et al. study [13], no difference was found in rest PETCO2 in patients with HVS and controls. However, only 10 patients with HVS were included in this study [13], suggesting the study may have been underpowered. In contrast to our study, Kinnula et al. [12] showed significantly higher VE/VCO2 ratio at rest in patients with HVS. The VE/VCO2 ratio at rest was extremely high in the Kinnula et al. study (59±9.8) [12], suggesting a highly selected cohort. In contrast, a larger number of consecutive subjects with HVS included in our study may explain this apparent discrepancy.
At peak exercise, subjects with HVS exhibited lower VO2 which is in agreement with a previous study [20]. There was no difference in VE between subjects with HVS and controls. However, the VCO2 was significantly lower in patients with HVS, suggesting an inadequately increased VE for metabolic demand. Moreover, the breathing pattern was significantly different; fb was significantly higher and VT was significantly lower (even after correction for BSA) in subjects with HVS. Moreover, PETCO2 was lower while VD/VT and peak VE/VCO2 ratio were significantly higher in patients with HVS compared to controls. Our observation of increased peak VE/VCO2 ratio in patients with HVS is consistent with previous reports [12,20]. By the alveolar gas equation, VE/VCO2 is increased by either an increase of VD/VT or by a decrease of PaCO2 [16]. In our subjects, we showed both an increase of VD/VT (ventilation-perfusion mismatch) and a decrease of PaCO2 (increased ventilatory drive) contribute to the elevated VE/VCO2 ratio at peak exercise (Figs 1 and 2). We thereby confirm and extend the previous observations [12,20].
An increased VE/VCO2 ratio has been proposed as a diagnostic screen for HVS [12]. However, increased VE/VCO2 and exertional dyspnea are common in several conditions which need to be distinguished from HVS including heart failure [21], COPD [14], asthma [20], restrictive lung disease [22] and pulmonary artery hypertension (PAH) [23]. Therefore, we aimed to find a more specific marker of HVS. However, most of the other gas exchange and ventilatory parameters found to be significantly different in our HVS patients may also be associated with other conditions characterized by exertional dyspnea. Significant increase of fb with diminished increase of VT (i.e. rapid shallow breathing pattern) is frequent in heart failure and in both COPD and restrictive lung disease patients [24,25]. Diminished increase of VO2 during exercise may be seen in heart failure patients, especially in those with central sleep apnea (i.e., in those HF patients with the highest ventilatory drive) [24]. Only the (absence of changes) of VE/VCO2 and PETCO2 during exercise were specific.
Physiologically, VE/VCO2 decreases and PETCO2 increases from rest to peak exercise [26]. This physiological pattern may also be observed in patients with heart failure [24,27], COPD [28] and restrictive lung disease [28]. In contrast, in our subjects with HVS, the VE/VCO2 ratio and PETCO2 did not change significantly from rest to peak exercise (Figs 3 and 4). Moreover, an inverse trend for increased VE/VCO2 and decreased PETCO2 was highly specific for the presence of HVS (97% and 100%, respectively). Therefore, we speculate an increased VE/VCO2 combined with an inverse trend of VE/VCO2 and PETCO2 during exercise may be helpful in the identification of subjects with HVS and in distinguishing these patients from other patients with exertional dyspnea caused by chronic heart failure, COPD and restrictive lung diseases. In asthma, the response to exercise may vary between patients and over time [29]. Therefore, making comparisons with asthmatic patients may be problematic.
PAH is also associated with exercise dyspnea and increased VE/VCO2 [23]. In patients with moderate-severe PAH, VE/VCO2 increases [30] and PETCO2 decreases because of poor pulmonary perfusion during exercise [22]. In contrast to obstructive and restrictive lung diseases (heart failure is also characterized by both pulmonary restriction and obstruction [31]), in patients with PAH the ventilatory response to exercise seems to be more closely related to ventilatory-perfusion mismatch and increased ventilatory drive than to pulmonary mechanics [32]. Indeed, we have observed a similar inverse trend of VE/VCO2 and PETCO2 in some of our HVS patients and showed its association with both ventilatory-perfusion mismatch and increased ventilatory drive, suggesting the ventilatory response to exercise in patients with PAH and HVS may be similar. However, it also suggests the inverse trend of ventilatory response to exercise may not allow discrimination of patients with HVS from those with PAH.
Our study has several limitations. First, it was a retrospective study. Second, only subjects with HVS and healthy controls were included. This study design allowed us to describe the HVS phenotype though it also prevents the generalization of these findings to the broader population. Third, there was a significant difference in RER between subject groups at peak exercise (RER was lower in controls). Lower RER may suggest lower exercise intensity in controls. However, the workload at peak exercise was significantly higher in controls, which further supports the concept of impaired ventilatory control resulting as inappropriately high ventilation in patients with HVS during exercise. Moreover, there was a trend towards lower RER at rest in controls also (p = 0.08) and there was no difference in the change of RER during exercise between both groups. We believe the lower RER might have been caused by inclusion of trained individuals (former athletes) as controls, as trained subjects have been shown to exhibit lower RER compared to untrained subjects at the same workload [33]. Fourth, PETCO2 was lower than normal in both groups at rest. Resting values were obtained while sitting on the cycle ergometer with a facemask on. This might have caused a stress-related increase in minute ventilation and decrease of PETCO2 in both groups. Indeed, psychological stress might have caused further increase in ventilation and may explain the even lower PETCO2 in our subjects with HVS at rest (HR was significantly higher in the HVS group at rest) [4]. Fifth, HVS was defined as respiratory alkalosis–i.e., low PaCO2 by arterial blood gas analysis at peak exercise (similar to previous studies [6,7]). The use of low PaCO2 as a selection criterion for HVS patients may make the comparison of other peak exercise parameters related to PaCO2 (like PETCO2 and VE/VCO2 ratio) difficult. However, VE/VCO2 has been shown to be influenced not only by PaCO2 (increased ventilatory drive), but also nearly equally by VD/VT (ventilation-perfusion mismatch) [16]. Contributors to low PETCO2 may also include both increased ventilatory drive and ventilation-perfusion mismatch. Indeed, peak exercise VD/VT was significantly higher in our patients with HVS as was the alveolar-arterial difference of CO2 ((0 (-1.7–1.3) vs. -1.8 (-3.0–0.6); p = 0.03)). Therefore, as these two parameters (peak PETCO2 and peak VE/VCO2 ratio) are not solely influenced by peak PaCO2, we believe the comparison is justified. Moreover, we suggest submaximal parameters (which were not used to define HVS) like rest to peak exercise changes of VE/VCO2 and PETCO2 may be used to detect and discriminate HVS from other conditions with exertional dyspnea like chronic heart failure, COPD or restrictive lung diseases.
Conclusion
In subjects with HVS, both VE/VCO2 and PETCO2 remained unchanged from rest to peak exercise in patients with HVS suggesting abnormal ventilatory control. These findings may promote recognition of the HVS phenotype by evaluation of patients by CPET.
Supporting information
Minimal dataset.
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
IC Jr. was supported by project no. LQ1605 from the National Program of Sustainability II (MEYS CR) and by the project FNUSA-ICRC no. CZ.1.05/1.1.00/02.0123 (OP VaVpI). KB was supported by Ministry of Health of the Czech Republic (FNBr 65269705) and by the Czech Pneumological and Phthisiological Society (publication fee grant). BDJ was supported by NIH Grant HL71478. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Malmberg LP, Tamminen K, Sovijärvi AR. Orthostatic increase of respiratory gas exchange in hyperventilation syndrome. Thorax. 2000;55: 295–301. 10.1136/thorax.55.4.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chenivesse C, Similowski T, Bautin N, Fournier C, Robin S, Wallaert B, et al. Severely impaired health-related quality of life in chronic hyperventilation patients: exploratory data. Respir Med. 2014;108: 517–523. 10.1016/j.rmed.2013.10.024 [DOI] [PubMed] [Google Scholar]
- 3.Cowley DS, Roy-Byrne PP. Hyperventilation and panic disorder. Am J Med. 1987;83: 929–937. [DOI] [PubMed] [Google Scholar]
- 4.Jack S, Rossiter HB, Pearson MG, Ward SA, Warburton CJ, Whipp BJ. Ventilatory responses to inhaled carbon dioxide, hypoxia, and exercise in idiopathic hyperventilation. Am J Respir Crit Care Med. 2004;170: 118–125. 10.1164/rccm.200207-720OC [DOI] [PubMed] [Google Scholar]
- 5.Ritz T, Meuret AE, Bhaskara L, Petersen S. Respiratory muscle tension as symptom generator in individuals with high anxiety sensitivity. Psychosom Med. 2013;75: 187–195. 10.1097/PSY.0b013e31827d1072 [DOI] [PubMed] [Google Scholar]
- 6.Pfortmueller CA, Pauchard-Neuwerth SE, Leichtle AB, Fiedler GM, Exadaktylos AK, Lindner G. Primary Hyperventilation in the Emergency Department: A First Overview. PLoS ONE. 2015;10: e0129562 10.1371/journal.pone.0129562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis RA, Howell JB. Definition of the hyperventilation syndrome. Bull Eur Physiopathol Respir. 1986;22: 201–205. [PubMed] [Google Scholar]
- 8.Hornsveld HK, Garssen B, Dop MJ, van Spiegel PI, de Haes JC. Double-blind placebo-controlled study of the hyperventilation provocation test and the validity of the hyperventilation syndrome. Lancet. 1996;348: 154–158. [DOI] [PubMed] [Google Scholar]
- 9.Hornsveld H, Garssen B. Hyperventilation syndrome: an elegant but scientifically untenable concept. Neth J Med. 1997;50: 13–20. [DOI] [PubMed] [Google Scholar]
- 10.van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res. 1985;29: 199–206. [DOI] [PubMed] [Google Scholar]
- 11.van Dixhoorn J, Folgering H. The Nijmegen Questionnaire and dysfunctional breathing. ERJ Open Res. 2015;1 10.1183/23120541.00001-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinnula VL, Sovijärvi AR. Elevated ventilatory equivalents during exercise in patients with hyperventilation syndrome. Respiration. 1993;60: 273–278. 10.1159/000196215 [DOI] [PubMed] [Google Scholar]
- 13.Hammo AH, Weinberger MM. Exercise-induced hyperventilation: a pseudoasthma syndrome. Ann Allergy Asthma Immunol. 1999;82: 574–578. 10.1016/S1081-1206(10)63169-9 [DOI] [PubMed] [Google Scholar]
- 14.Barron A, Francis DP, Mayet J, Ewert R, Obst A, Mason M, et al. Oxygen Uptake Efficiency Slope and Breathing Reserve, Not Anaerobic Threshold, Discriminate Between Patients With Cardiovascular Disease Over Chronic Obstructive Pulmonary Disease. JACC Heart Fail. 2016;4: 252–261. 10.1016/j.jchf.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.TOMA N, BICESCU G, ENACHE R, DRAGOI R, CINTEZA M. Cardiopulmonary exercise testing in differential diagnosis of dyspnea. Maedica (Buchar). 2010;5: 214–218. [PMC free article] [PubMed] [Google Scholar]
- 16.Woods PR, Olson TP, Frantz RP, Johnson BD. Causes of breathing inefficiency during exercise in heart failure. J Card Fail. 2010;16: 835–842. 10.1016/j.cardfail.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aitken ML, Franklin JL, Pierson DJ, Schoene RB. Influence of body size and gender on control of ventilation. J Appl Physiol. 1986;60: 1894–1899. 10.1152/jappl.1986.60.6.1894 [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. European Respiratory Journal. 2005;26: 153–161. 10.1183/09031936.05.00034505 [DOI] [PubMed] [Google Scholar]
- 19.Gardner WN. The pathophysiology of hyperventilation disorders. Chest. 1996;109: 516–534. [DOI] [PubMed] [Google Scholar]
- 20.Kinnula VL, Sovijärvi AR. Hyperventilation during exercise: independence on exercise-induced bronchoconstriction in mild asthma. Respir Med. 1996;90: 145–151. [DOI] [PubMed] [Google Scholar]
- 21.SHEN Y, ZHANG X, MA W, SONG H, GONG Z, WANG Q, et al. VE/VCO2 slope and its prognostic value in patients with chronic heart failure. Exp Ther Med. 2015;9: 1407–1412. 10.3892/etm.2015.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Donnell DE, Elbehairy AF, Berton DC, Domnik NJ, Neder JA. Advances in the Evaluation of Respiratory Pathophysiology during Exercise in Chronic Lung Diseases. Front Physiol. 2017;8 10.3389/fphys.2017.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rausch CM, Taylor AL, Ross H, Sillau S, Ivy DD. Ventilatory efficiency slope correlates with functional capacity, outcomes, and disease severity in pediatric patients with pulmonary hypertension. Int J Cardiol. 2013;169: 445–448. 10.1016/j.ijcard.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cundrle I, Somers VK, Johnson BD, Scott CG, Olson LJ. Exercise end-tidal CO2 predicts central sleep apnea in patients with heart failure. Chest. 2015;147: 1566–1573. 10.1378/chest.14-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scano G, Innocenti-Bruni G, Stendardi L. Do obstructive and restrictive lung diseases share common underlying mechanisms of breathlessness? Respiratory Medicine. 2010;104: 925–933. 10.1016/j.rmed.2010.02.019 [DOI] [PubMed] [Google Scholar]
- 26.Faisal A, Webb KA, Guenette JA, Jensen D, Neder JA, O’Donnell DE, et al. Effect of age-related ventilatory inefficiency on respiratory sensation during exercise. Respir Physiol Neurobiol. 2015;205: 129–139. 10.1016/j.resp.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto A, Itoh H, Eto Y, Kobayashi T, Kato M, Omata M, et al. End-tidal CO2 pressure decreases during exercise in cardiac patients: association with severity of heart failure and cardiac output reserve. J Am Coll Cardiol. 2000;36: 242–249. [DOI] [PubMed] [Google Scholar]
- 28.Faisal A, Alghamdi BJ, Ciavaglia CE, Elbehairy AF, Webb KA, Ora J, et al. Common Mechanisms of Dyspnea in Chronic Interstitial and Obstructive Lung Disorders. Am J Respir Crit Care Med. 2015;193: 299–309. 10.1164/rccm.201504-0841OC [DOI] [PubMed] [Google Scholar]
- 29.Giacco SRD, Firinu D, Bjermer L, Carlsen K-H. Exercise and asthma: an overview. Eur Clin Respir J. 2015;2 10.3402/ecrj.v2.27984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun XG, Hansen JE, Oudiz RJ, Wasserman K. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation. 2001;104: 429–435. [DOI] [PubMed] [Google Scholar]
- 31.Johnson BD, Beck KC, Olson LJ, O’Malley KA, Allison TG, Squires RW, et al. Ventilatory constraints during exercise in patients with chronic heart failure. Chest. 2000;117: 321–332. [DOI] [PubMed] [Google Scholar]
- 32.Aguggini G, Clement MG, Widdicombe JG. Lung reflexes affecting the larynx in the pig, and the effect of pulmonary microembolism. Q J Exp Physiol. 1987;72: 95–104. [DOI] [PubMed] [Google Scholar]
- 33.Ramos-Jiménez A, Hernández-Torres RP, Torres-Durán PV, Romero-Gonzalez J, Mascher D, Posadas-Romero C, et al. The Respiratory Exchange Ratio is Associated with Fitness Indicators Both in Trained and Untrained Men: A Possible Application for People with Reduced Exercise Tolerance. Clin Med Circ Respirat Pulm Med. 2008;2: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Minimal dataset.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.