Abstract
While children with Prader-Willi Syndrome (PWS), a rare genetic disease with an incidence of 1:15,000, typically present with hypotonia and hyperphagia, their lives are made more difficult by an ever-present sleepiness as well as multiple neuro-cognitive dysfunctions, including cognitive defects. We describe a case series of 3 children who were treated with the histamine 3 receptor inverse agonist pitolisant. While this first-in-class inverse agonist is approved for another orphan disease (i.e., narcolepsy with or without cataplexy), we have observed that pediatric patients with PWS prescribed pitolisant demonstrate decreased daytime sleepiness and improved cognition, as evidenced by increased processing speed and improved mental clarity. Pitolisant may represent a novel therapeutic option that might relieve substantial PWS disease burden, including cognitive disability, excessive daytime sleepiness, and poor-quality nighttime sleep.
Keywords: cataplexy, cognition, narcolepsy, pitolisant, Prader-Willi Syndrome, sleep
Introduction
Prader-Willi Syndrome (PWS) is a rare genetic disease that exerts a tremendous multifaceted burden on the daily life of patients and their families.1 Patients experience imbalances in hunger/satiety, sleep/wake states, and body composition. According to a 2014 survey2 of caregivers of individuals with PWS, 98% have experienced hypotonia or weak muscles, 88% have experienced developmental delay or intellectual disability, and 65% have experienced sleep problems, such as apnea or daytime sleepiness. In many cases, patients with PWS present with clinical symptoms that are consistent with the 5th Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for narcolepsy with cataplexy.3,4
In 2016, the European Medicines Agency (EMA) approved pitolisant (Wakix, Bioprojet Pharma, Paris, France) for the treatment of narcolepsy with or without cataplexy.5,6 In spring 2018, the US Food and Drug Administration (FDA) granted “Breakthrough Therapy” and “Fast Track” designations to pitolisant for the treatment of narcolepsy with or without cataplexy. Pitolisant is a first-in-class histamine H3 receptor inverse agonist that enhances the activity of histaminergic neurons. It has been shown to provide effective treatment for excessive daytime sleepiness and to be well tolerated when compared to the standard-of-care drug modafinil.7 Since individuals with PWS are known to suffer from excessive daytime sleepiness, cataplexy-like symptoms, and cognitive impairments,8 we concluded that pitolisant might not only be effective as a treatment of excessive daytime sleepiness in patients with PWS but might also help to relieve some of the fundamental symptoms of PWS.9
A 14-year-old male with PWS was, to the best of our knowledge, the first individual with PWS to begin treatment with pitolisant. This patient, who has genetically confirmed PWS by deletion, received approval from the FDA to personally import pitolisant from the European Union (EU) to treat his narcolepsy with cataplexy. He currently receives 54 mg of pitolisant each morning. Treatment with pitolisant not only relieved his symptoms of narcolepsy and cataplexy but also resulted in dramatic improvement in 5 aspects of cognition, as documented by online training for cognitive function and speed (Lumosity, www.lumosity.com). Moreover, an objective neurological assessment confirmed his parents' subjective impression that his tone had normalized, an outcome that is atypical for patients with PWS. The patient's carbohydrate tolerance also appeared to normalize, as did his relationship to food. Indeed, it was his success with pitolisant treatment that prompted other families to request personal importation, resulting in this current case series of 3 patients with PWS who have successfully initiated treatment with pitolisant.
Methods
Parents obtained a prescription for pitolisant from their personal physicians, purchased the medication in Germany, and imported it to the United States via personal importation allowed at the FDA's discretion. The families agreed to document their experience on the TREND Community platform in order to benefit the larger PWS community.
The parents of the 3 children in the case series provided informed consent and then responded to a weekly questionnaire that asked for quantitative outcomes, such as weight and nap frequency, as well as subjective qualitative outcomes, such as mental acuity and behavioral issues. Data were systematically collected through an online system named TREND Community (https://trend.community/). TREND Community was created to gather patient experience data in response to the Patient-Focused Drug Development Initiative expanded by the US 21st Century Cures Act. The TREND Community allows parents to document and quantify real-world data in a manner that can be used to inform the FDA and medical community of patient experience.
TREND Community is an invitation-only network of patients living with rare disease and their caregivers who have formally consented and are engaged in community-powered science. Since the gathering of patient experience data is about understanding a human perspective, TREND Community uses data collection techniques from the social sciences. These techniques stand in contrast to the biochemical and medical techniques typically employed by pharmaceutical researchers.
Every week parents used a Likert scale (with scores ranging from 1 to 5) to answer a standard short questionnaire identifying the frequency/intensity of multiple PWS clinical features. Data collection began prior to the initiation of treatment with pitolisant in order to establish a baseline. Questions covered the topics of cognition, behavior, and sleepiness.
Treatment with pitolisant, commercially available (since its EMA approval in the EU in 2016) in dosages of 4.5-mg and 18-mg tablets, was initiated at the lowest dosage of 4.5 mg/day and slowly increased stepwise in consultation with the patient's physician. Patient A gradually increased his dose from 4.5 mg to 27 mg over the course of 15 months. Patient B gradually increased her dose from 4.5 mg to 27 mg over the course of 16 months. Patient C gradually increased her dose from 4.5 mg to 31.5 mg over the course of 14 months. Pitolisant is taken in the morning. All patients are US citizens. Patient details are shown in the Table.
Table.
Patient Details
| Patient* | PWS | Sex (Race) | Age (yr) | Body Mass Index, kg/m2 (% Body Fat) | Pitolisant Dosage (mg/day) | |
|---|---|---|---|---|---|---|
| Before Pitolisant | After Pitolisant | |||||
| A | Deletion | Male (Caucasian) | 12 | 16.4 (18.5) | 15.2 (19.5) | 4.5–27 |
| B | Uniparental disomy | Female (Caucasian) | 10 | 20 (30.2) | 22.5 (31.4) | 4.5–27 |
| C† | Deletion | Female (Caucasian) | 15 | 23.1 (not available) | 23.6 (not available) | 4.5–31.5 |
*All children received nightly growth hormone injections.
† At initiation of treatment with pitolisant, Patient C also received escitalopram, buspirone, risperidone, levoxythyroxine, and estradiol.
Case Series
Patient demographics are depicted in the Table, and parental responses to a weekly questionnaire are summarized in the Figure.
Figure.
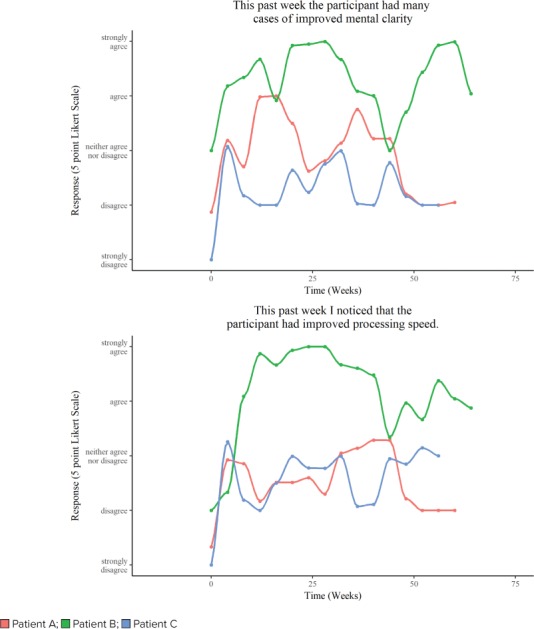
Improved mental clarity and processing speed in response to pitolisant. The figure illustrates the Likert scale indicating parental responses to the weekly questionnaire. 1 = strongly disagree, 2 = disagree, 3 = neither agree nor disagree, 4 = agree, and 5 = strongly agree
Patient A. During 15 months of treatment with pitolisant (4.5–27 mg), the parents of Patient A noted improved processing speed. The child also had a slight decrease in daily tantrums (data not shown). Shortly after initiation of treatment with pitolisant, Patient A's occupational therapist noted an improved ability to follow complex directions and to self-correct when he became distracted. “He was super speedy and we high-fived for that and he got lots of congratulatory hugs from peers. He's in good spirits, alert, following conversation and contributing appropriate responses and clarification to my questions,” wrote his resource teacher 6 weeks after the patient started pitolisant treatment. However, reports of Patient A's behavioral issues still dominated teacher communications both before and after beginning treatment with pitolisant. Patient A is on a non-graduation track and is not given standardized examinations through the school system.
Patient B. Patient B's mother scored her as having slow processing speed and average mental clarity prior to initiating pitolisant. Following 16 months of treatment with pitolisant (4.5–27 mg), the mother agreed that the child had improved processing speed and agreed that her daughter had improved mental clarity. The procognitive effects of pitolisant were reflected in the classroom as well as in standardized test scores. In an informal assessment, the teacher commented on Patient B's focus, noting that, “[She] has struggled somewhat to switch gears when we transition from one subject area or task to another. In the past week, she has been able to transition better and appears to be attending to the lessons better.” The counselor noted improvements in validated assessments, writing that, “currently [Patient B] performed within the average range on all working memory and processing speed tasks presented.”
Patient C. Patient C also experienced cognitive gains on pitolisant. The patient's mother noted improvements in processing speed and mental clarity. Her assessment of her daughter's intelligence also improved after 14 months of treatment with pitolisant (data not shown). Patient C had fewer tantrums after starting pitolisant, and her mother noted that the child was slower coming to the table for meals. Visiting family members have also noticed that since starting pitolisant, Patient C appears to be more in touch with others and aware of events happening around her. Patient C was weaned off of metformin prior to the initiation of pitolisant. Patient C's parents wonder if the removal of metformin combined with underdosing of levoxythyroxine may have contributed to Patient C's weight gain (from a body mass index [BMI] of 23.1 to a BMI of 23.6) at this time.
Patient C's teachers noted after 3 months of treatment with pitolisant that she had improved ability to articulate thoughts and improved mental flexibility. The teachers also commented on increased processing speed, reading ability, and improved interactions with others. A teacher report stated that while Patient C was still having a few behavioral issues a day, they were not as lengthy as they had been before treatment.
Discussion
We report here a case series of 3 patients and their experience with pitolisant. The children in our case series experienced improved cognition in the form of faster processing speed and improved mental clarity following treatment with pitolisant. The data are primarily subjective and reflect the inherent biases of individual patient experience. All 3 children in our series appeared to do best with dosing of pitolisant similar to that recommended for adult patients with narcolepsy.
Narcolepsy. In the EU, pitolisant is approved for the treatment of narcolepsy with or without cataplexy.5 Interestingly, while the children in this case series seemed less sleepy, none of the parents noted marked changes in nap frequency. This may be because, in general, this small group of children did not frequently nap prior to initiating pitolisant. While they were frequently sleepy during the day and all had global low tone, they did not present with typical adult symptoms of narcolepsy, possibly reflecting the lack of consistent presentation of narcolepsy/cataplexy in children, and particularly in children with PWS.8 Despite their excessive sleepiness and global low tone, none of the children in this case series had received a diagnosis of narcolepsy with cataplexy.
Although the children had not received a diagnosis of narcolepsy, parents reported that upon treatment with pitolisant, the children were not only less sleepy when awake but they were also more active and more engaged. Specifically, the children appeared to sleep longer and more thoroughly during the night and to be more awake during the day. Moreover, some had their first active report of dream experiences. This would be consistent with the concept that pitolisant is not a stimulant but exerts its impact through normalization of the patient's sleep-wake cycles.6
Metabolism. The children generally tolerated pitolisant well. Patient A's BMI decreased while on pitolisant, and the BMI of Patients B and C increased while on pitolisant. Our experience would suggest that pitolisant is not a weight loss drug, although it will be interesting to see its effect in children with PWS who are obese. Parents did feel that their children were undergoing metabolic changes in response to pitolisant. These included changes in patient odor and carbohydrate tolerance. Unfortunately, the data are too preliminary, the cohort too small, and the development of the children during adolescent phases too unpredictable to make any firm conclusions.
Parents reported that pitolisant seemed to change their children's relationship to food. While hyperphagia is a hallmark symptom of PWS, experienced by approximately half of patients,2 our cohort comprises children whose lives are not dominated by hyperphagia. They are thus not the appropriate group in which to evaluate an intervention targeted at hyperphagia. Nevertheless, prior to initiating treatment with pitolisant, the children in our case series had an altered relationship to food that was characterized by an increased interest and focus on food relative to that of their siblings. Overall, parents agreed that following treatment with pitolisant, children were able to go longer periods of time without feeling an urgent need to eat.
Adverse Effects. Parents of Patient A and Patient C described them as anxious prior to initiation of pitolisant. That anxiety decreased (but did not completely resolve) over time on pitolisant. Patient B did not exhibit anxiety prior to initiating pitolisant, and there was no change in anxiety upon exposure to pitolisant. All parents noted some increased anxiety with dose increases, but this anxiety faded after approximately 2 days.
Two of the 3 children occasionally noted headaches that lasted for 2 days following initiation of pitolisant and with each dose increase. Headaches did not impact daily function and resolved without any additional treatment. One child also described feeling more tired on the first 2 days following an increase in dose. All parents noted that establishing a proper dose for pitolisant was challenging, particularly because dosing changes were sometimes associated with transient increases in anxiety, behavioral issues, and an increased desire for sensory input.
Although this series is limited by a small sample size, we now know of 10 children in the United States (including the 3 described in this case series) who are taking pitolisant obtained through personal importation from the EU at the FDA's discretion. Nine of these children had similar positive outcomes and have experienced limited adverse effects. The parents of one child decided to stop treatment as a result of an increase in behavioral issues.
Pitolisant has been suggested10 to have positive impact on cognition, and our observations are consistent with these previous reports. All of the parents have noted that it can be challenging for the children and the family members to adapt to the changes that occur in children taking pitolisant. The children often express an increased desire for sensory input, activity, and social interactions. We speculate that the increased sensory input from learning, movement, and social interactions may also be driving neurological development. We also note that the cognitive gains in our case series are different from those described in previous reports of the use of the stimulant modafinil in the PWS population. These previous reports10,11 have described improvements in excessive daytime sleepiness but have not documented any cognitive gains.
Study Limitations. The children in our case series are of different ages and in different disease stages. They are growing and developing, and therefore it is possible, but we believe unlikely, that the changes described here would have occurred without treatment. In addition, most of our data are patient responses to weekly questionnaires. The data are thus subject to response bias and reporter bias. This is especially the case since PWS is a highly burdensome disease and these parents are motivated to see changes in their children. The parents were also in communication with each other within social networks, which could possibly have reinforced perceptions of the positive effect of pitolisant.
Moreover, the subjective Likert scaling reported here was not validated, and parent reports are inherently subjective. The TREND platform, however, had the advantage of facilitating systematic collection of patient experience data under normal living conditions. These parental observations (combined with teacher observations and objective academic testing) suggest that there are neurologic benefits of pitolisant in the PWS population beyond the expected reduction in daytime sleepiness. In particular, Patient B's improvement from the low average to the average range on assessments is relevant and impressive.
Conclusion
The benefits of pitolisant treatment for individuals with PWS appear to extend beyond the expected improvements in excessive daytime sleepiness. Parents and teachers report that, in addition to improved cognition, initiation of treatment with pitolisant was associated with fewer problem behaviors. It may be that a clearer brain and faster processing speed can together translate into an improved ability to respond to environmental stimuli and, consequently, may result in better behavior and improved learning. One of the most interesting and thought-provoking comments from a patient, when questioned on her own perception of pitolisant treatment, was: “I feel happy and laugh a lot now.”
Pitolisant is currently being considered for approval by the FDA for the treatment of narcolepsy with or without cataplexy. We hope that our data will be helpful in the drug approval process, specifically in the design of a clinical trial to determine if pitolisant can improve narcolepsy, cataplexy, cognition, and behavior in patients with PWS.
Acknowledgments
Acknowledgments We thank the families who participated in the exploration and we thank Liyang Huang for graphing support. The observations in this case report describe the experiences of 3 subjects and include data collected on the TREND Community platform. As such, the data were collected without institutional review board approval. All data were contributed by the parents of the patients, and consent was obtained from all participants.
ABBREVIATIONS
- BMI
body mass index
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders
- EMA
European Medicines Agency
- EU
European Union
- FDA
Food and Drug Administration
- PWS
Prader-Willi Syndrome
Footnotes
Disclosure Dr Holger Stark is the co-inventor of pitolisant. Maria Picone is the founder of Formed, Inc, a digital health technology company that is developing the TREND Community platform. TREND Community and Chion Foundation have received unrestricted grants from Harmony Biosciences. The other authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all patient information in this report and take responsibility for the integrity and accuracy of the report.
REFERENCES
- 1.Driscoll DJ, Miller JL, Schwartz S . Prader-Willi Syndrome. In: Adam MP, Ardinger HH, Pagon RA, editors. GeneReviews. Seattle, WA: University of Washington, Seattle; 2016. , eds. [PubMed] [Google Scholar]
- 2.Strong TV. Foundation for Prader-Willi Research Scientific Day—PWS patient voices. https://www.fpwr.org/wp-content/uploads/2014/04/PatientVoices_presentationWebinarFinal.pdf Accessed February 19, 2019.
- 3.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 4.Glaze D, Patel AA. Personal Communication 2018.
- 5.European Medicines Agency Wakix. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002616/human_med_001955.jsp&mid=WC0b01ac058001d124#about Accessed February 19, 2019.
- 6.Schwartz JC. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol. 2011;163(4):713–721. doi: 10.1111/j.1476-5381.2011.01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dauvilliers Y, Bassetti C, Lammers GJ et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068–1075. doi: 10.1016/S1474-4422(13)70225-4. [DOI] [PubMed] [Google Scholar]
- 8.Pillen S, Pizza F, Dhondt K et al. Cataplexy and its mimics: clinical recognition and management. Curr Treat Options Neurol. 2017;19(6):23. doi: 10.1007/s11940-017-0459-0. doi:10.1007/s11940-017-0459-0. [DOI] [PubMed] [Google Scholar]
- 9.Stark H. Histamine H3 receptor antagonists on sleep disturbance. 9th International Prader-Willi-Syndrome Organisation Conference (IPWSO) Scientific Abstract Booklet. 2017;1:26. [Google Scholar]
- 10.Sadek B, Saad A, Sadeq A et al. Histamine H3 receptor as a potential target for cognitive symptoms in neuropsychiatric diseases. Behav Brain Res. 2016;312:415–430. doi: 10.1016/j.bbr.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 11.Weselake SV, Foulds JL, Couch R et al. Prader-Willi syndrome, excessive daytime sleepiness, and narcoleptic symptoms: a case report. J Med Case Rep. 2014;8:127. doi: 10.1186/1752-1947-8-127. doi:10.1186/1752-1947-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]


