Abstract
Evidence of popular interest in the interrelationships between mind, body, and heart disease dates back to Grecian times and paved the way for modern day scientific inquiry into the relationships between psychological comorbidities in coronary heart disease. While the systematic evidence suggests an association of poor medical prognosis and lower quality of life among patients with coronary heart disease with comorbid psychological conditions, the mechanisms are less well understood. In this selective review article, the epidemiology, mechanisms, screening, and treatment recommendations for four common psychological conditions (depression, anxiety, stress, and insomnia) comorbid with coronary heart disease will be presented. We focus on the grand challenges and unprecedented opportunities for research in this area in light of the methodological and technological innovations of the 21st century.
Keywords: depression, anxiety, stress, insomnia, coronary heart disease, psychosocial factors
The interrelationships between mind, body, and heart disease have long piqued the curiosity of philosophers, doctors, scientists, laypeople, and even emperors and their personal physicians. Indeed, Napoleon’s favorite physician, Corvisart, wrote that heart disease was due to “the passions of the mind” (Rosch, 2004). Evidence of this popular interest in the role of emotion, and later psychological disorder, in cardiovascular disease dates back to ancient Grecian times and it is this interest that paved the way for the establishment of a new scientific area of inquiry that has grown exponentially since its early beginnings.
As early as 1937, clinical scientists documented a correlational link between psychological disorder and coronary heart disease, observing an increased risk of cardiovascular death among those with “involution melancholia” or late-life depression (Malzberg, 1937). In the nearly 80 years since that first observation, much has been gleaned about the relationship between specific psychological disorders, psychological vulnerabilities, and cardiovascular morbidity and mortality. The systematic observational evidence overwhelmingly suggests a heightened risk of poor medical prognosis and lower quality of life among patients with coronary heart disease and comorbid psychological conditions. The pathophysiological and behavioral mechanisms driving these associations are starting to be elucidated, and the scientific advances and technological innovations of the 21st century hold promise for even deeper mechanistic insights and for novel treatment approaches that could then be tested. For this selective review, we have chosen to focus on depression, anxiety, stress, and insomnia in those with already existing coronary heart disease because of higher prevalence of these conditions among patients with CHD compared to the general adult population, the significant public health burden and economic toll associated with these comorbid conditions, and the existence of established psychological interventions. We also focus on the major challenges and novel grand opportunities for future research directions at this complex intersection of mental and physical disease.
Definition of Coronary Heart Disease
Coronary heart disease (CHD) is a type of cardiovascular disease (CVD) or a class of diseases that involve the heart or blood vessels (Fuster, 2004). CHD’s primary clinical manifestations are myocardial infarction (MI) and unstable angina. Stable angina, or short bouts of chest discomfort with exertion, is also a common manifestation of CHD, though it is less studied in the psychosocial literature. CHD is the leading cause of years of life lost worldwide (GBD 2013 Mortality and Causes of Death Collaborators, 2015) and in the United States.
Common Comorbid Psychological Disorders in Patients with Coronary Heart Disease
The prevalence of depression, anxiety, stress, and insomnia is estimated to be higher among patients with CHD compared to the general adult population (Table), and the association with excess medical prognostic risk is reviewed for each psychological condition, below. We note at the outset that almost all of the evidence linking psychological disorders to prognostic outcomes derives from observational studies. Although most of the studies we review carefully adjust for plausible confounders, the reader should keep in mind this work still does not provide a strong basis for making causal inferences. As we discuss later in the paper, this is a major challenge for research going forward, as the history of medicine is replete with examples of treatments being adopted based on observational data, and subsequent in more rigorous evaluations being proven ineffective.
Table.
Prevalence of Selected Psychological Disorders in the General Population and in the Coronary Heart Disease Population
| Target Psychological Disorder |
Twelve-Month Prevalence in General Population |
Prevalence in Coronary Heart Disease Population |
|---|---|---|
| Depression | 117.0% | 20% |
| GAD | 2.9% | 5.5% |
| PTSD | 3.0% | 32% |
| Insomnia | 10% | Unknown |
Note. GAD= Generalized Anxiety Disorder; PTSD = Posttraumatic Stress Disorder
Depression
Depression is quickly becoming the leading cause of years of life lived with disability world-wide (Lopez, Mathers, Ezzati, Jamison, & Murray, 2006). The twelve-month prevalence of depression in the general population is 7% (Association, 2013). In contrast, in patients with CHD, up to 20% meet criteria for a Major Depressive Disorder, and up to 47% have significant patient-reported depression symptoms (Bush et al., 2005) (Table). In observational studies, both clinically diagnosed depression and elevated depressive symptoms predict increased cardiac recurrence and early mortality risk (Nicholson, Kuper, & Hemingway, 2006). Thus, as many as 7 million of Americans living with CHD are also suffering with either clinically diagnosed depression or quality-of-life impairing depression symptoms, and we add half of a million new cases to this public health burden annually.
Specific depressive disorders and vulnerabilities.
There is considerable debate in the field about the extent to which specific subtypes of depression, namely cognitive/affective, somatic/affective (de Miranda Azevedo, Roest, Hoen, & de Jonge, 2014) and anhedonic depression subtypes (K.W. Davidson et al., 2010) account for the depression−CHD prognostic association. Recent research in the past decade has started to more explicitly grapple with this question. A recent systematic review found that the somatic/affective depression subtype relative to the cognitive/affective depression subtype was more strongly and consistently linked with mortality and cardiac recurrence in patients with heart disease in models that adjusted for each subtype (de Miranda Azevedo et al., 2014). Relatedly, there has been some support for the differential prognostic association of anhedonic depression subtype versus depressed mood subtype and cardiac prognosis (Damen et al., 2013; K. W. Davidson et al., 2010; Diamond et al., 2014; Doyle, Conroy, & McGee, 2012).
Anxiety
Although anxiety in the context of CHD is less well-studied than depression, the evidence suggests that anxiety is also highly prevalent and comorbid with CHD (Moser et al., 2011). Between 13.4% to 59.5% of CHD patients report elevated levels of anxiety symptoms following their cardiac event (A. M. Roest, Martens, Denollet, & de Jonge, 2010). Elevated anxiety levels have been independently associated with increased prognostic risk in CHD patients, specifically with a 36% increased risk of an adverse cardiac event, including a 71% increased risk of a cardiac event recurrence and a 23% increased risk of cardiac death (A. M. Roest et al., 2010); though the models did not account for CHD severity or other psychological factors that often covary with anxiety, such as depression. However, other studies that accounted for CHD risk factors and depression have corroborated the results of this meta- analysis. There is also evidence from studies of otherwise healthy adults that anxiety and depression are differentially associated with cardiac outcomes. In these studies, anxiety symptom scores were negatively associated with CHD mortality and electrocardiographic (ECG) T-wave inversions, whereas depressive symptom scores were positively associated with odds of CHD mortality and ECG T-wave inversions (Mykletun et al., 2007; Whang et al., 2014).
Specific anxiety disorders and vulnerabilities.
In the last decade, considerable research has started to focus on ascertaining the prognostic risks associated with specific types of anxiety disorders, rather than non-specific anxiety symptoms. For example, in a prospective study of CHD patients, the prevalence of generalized anxiety disorder (GAD) was 5.5% (compared to 2.9% in the general adult population, Table), and those with GAD had an 81–95% increased risk of cardiovascular event recurrence or all-cause mortality than those without a GAD diagnosis (American Psychiatric Association, 2013; A.M. Roest, Zuidersma, & de Jonge, 2012). Additional research has also started to focus on understanding the independent role of specific dimensions of anxiety or cognitive vulnerabilities of anxiety and their relation to CHD prognosis (A. M. Roest, Heideveld, Martens, de Jonge, & Denollet, 2014).
Stress
There are numerous psychosocial factors other than depression or anxiety that have been proposed as markers for cardiovascular recurrence risk in CHD patients. Many of these constructs fall under the umbrella of chronic stress (Alan Rozanski, 2014). Chronic stress can result from numerous life circumstances where a person feels on an ongoing basis that environmental demands outstrip coping sources, and have been operationalized in the literature as lasting social isolation, lack of control or autonomy at work, conflictual spousal and family relationships, impoverished economic circumstances, and war-related upheaval. Interested readers are encouraged to consult reviews linking these experiences with CVD incidence and progression (Everson-Rose & Lewis, 2005; A. Rozanski, Blumenthal, Davidson, Saab, & Kubzansky, 2005)Smith & Baucom, 2017; Steptoe & Kivimaki, 2012).
Specific stress disorders and vulnerabilities.
In addition to general chronic stress, observational studies in the past decade have indicated a consistent association between posttraumatic stress disorder (PTSD), a specific stress disorder that is now classified as a Trauma and Stressor-Related Disorder, incident CHD, and also poor CHD prognosis (Cohen, Edmondson, & Kronish, 2015; Force, 2009; Marrelli, Tondora, & Hoge, 2005). While far less research has focused on the association of PTSD and medical prognosis in CHD patients, several recent meta-analytic studies have found that CHD-induced PTSD, or PTSD that is due to an acute life-threatening CHD event such as an acute coronary syndrome event, is prevalent in as many as 32% of CHD patients (Edmondson et al., 2012)Table), compared to 3% in the general adult population (Association, 2013). Further, CHD-induced PTSD relative to no PTSD is associated with a doubled risk of an adverse clinical outcome such as re-hospitalization, recurrent cardiac event, or death in CHD patients (Cohen et al., 2015; Marrelli et al., 2005; Shemesh et al., 2004).
Insomnia
The prevalence of sleep disturbances in CHD patients is markedly higher than in the general population. For example, between 50–74% of ACS survivors report poor sleep quality or sleeping less than 7 hours per night after hospitalization, compared to 33% of the general population (Alcantara, Peacock, Davidson, Hiti, & Edmondson, 2014; Leineweber, Kecklund, Janszky, Akerstedt, & Orth-Gomer, 2003; Luyster, Strollo, Zee, & Walsh, 2012; “Unhealthy sleep-related behaviors−−12 States, 2009,” 2011). Further, sleep disturbances such as sleeping less than 7 hours in the month after a CHD event has been associated with a 52% increased risk of an adverse cardiac event or death among CHD patients (Alcantara et al., 2014).
Specific sleep disorders and vulnerabilities.
While the overwhelming evidence base has focused on sleep and the incidence of CHD and cardiovascular risk factors, diagnosable sleep disorders (and not just sleep disturbances or deficits) such as obstructive sleep apnea (OSA), are also highly prevalent in patients with CHD and associated with adverse prognostic outcomes (readers are encouraged to consult with the “Sleep and Circadian Rhythms” article in this special issue for more detailed coverage of sleep disorders and cardiovascular disease).
Of note for psychological science, a disproportionate amount of research on sleep and CHD has focused on OSA and other sleep disordered breathing conditions, leaving significant gaps in our understanding of the association of insomnia and CHD. Yet, insomnia is the most prevalent sleep disorder in the United States, most commonly treated with psychological interventions, and characterized by autonomic hyperarousal, a pathogenic mechanism associated with increased cardiovascular risk. Indeed, in the general population, 10% of adults meet diagnostic criteria for insomnia, and a third exhibit insomnia symptoms (Association, 2013). Among patients with CHD, the exact prevalence of clinical insomnia is unknown, though insomnia symptoms are endorsed between 3.3% to 36.2% in those at risk of CHD (Laugsand, Vatten, Platou, & Janszky, 2011) Table). A recent meta-analysis found that insomnia was associated with a 45% increased risk of developing or dying from cardiovascular disease (Sofi et al., 2014).
Mechanisms Linking Psychological Comorbidities to Coronary Heart Disease Prognosis
There has been considerable interest in understanding why psychological comorbidities are associated with adverse prognosis in patients with CHD. Because of page constraints, we do not provide a detailed treatment of this voluminous literature here. However, we encourage interested readers to seek out any of several excellent reviews focused explicitly on mechanisms (Burg et al., 2013; Everson-Rose & Lewis, 2005; J. H. Lichtman et al., 2008; Matthews & Gallo, 2011; Steptoe & Kivimaki, 2012).
Unidirectional Hypotheses.
Much of the extant research is grounded in unidirectional hypotheses about causality, wherein comorbidities are thought to give rise to behavioral and/or biological processes that hasten the recurrence or progression of disease (Figure). This body of work can be loosely categorized into studies that have focused on iatrogenic, behavioral, or biological hypotheses. With regard to iatrogenesis, some of the earliest research in this literature considered whether prognostic associations were simply artifacts of toxicity related to the use of anti-depressant medications (Spurious Model, see Figure). Prior to the advent of serotonin reuptake inhibitors (SRIs), depressed patients were commonly prescribed monoamine oxidase inhibitors and tricyclic anti-depressants. Both of these classes of drugs have side-effect profiles that can include cardiac toxicities (Sheline, Freedland, & Carney, 1997). Nonetheless, studies consistently found elevated rates of morbidity and mortality among CHD patients who were depressed but free of anti-depressants, illustrating that mood’s association with prognosis was not simply a product of iatrogenesis (Glassman & Shapiro, 1998; Sheline et al., 1997).
Figure.
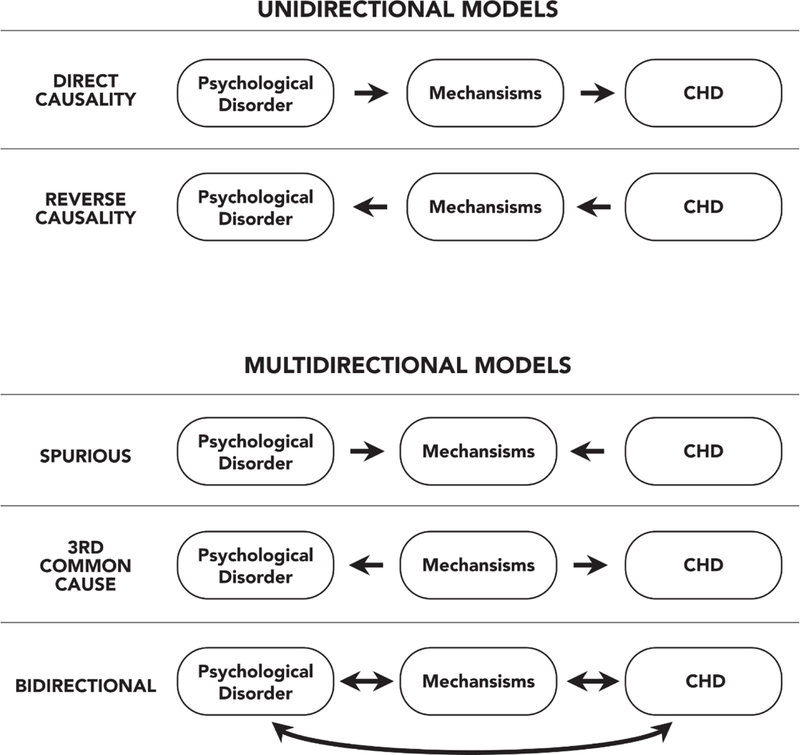
Unidirectional and Multidirectional Models of Mechanisms Linking Psychological Comorbidities to Coronary Heart Disease
Research on putative behavioral mechanisms focuses on cigarette smoking, alcohol use, physical inactivity, poor nutrition, and non-adherence with cardiac treatment and rehabilitation, all of which are more prevalent in depressed patients with CHD, compared to non-depressed patients (A. Rozanski et al., 2005). Despite these associations, research has struggled to document formal evidence of mediation, wherein the candidate behavioral mechanism explains a substantial proportion of the excess morbidity and mortality that is attributable to the association with depression. Further, in CHD patients with other psychosocial comorbidities, little is known about the distribution of presumptive behavioral and lifestyle mediators. This is a key area for future research.
The same themes characterize the literature on candidate biological mechanisms. Research shows that among CHD patients, depression is associated with a variety of biological processes known to contribute to recurrence, progression, and mortality. These processes include cardiac autonomic dysfunction, impaired endothelial functioning, low-grade inflammation, platelet hyperactivity and increased coagulation, and a tendency to experience stress-induced myocardial ischemia (Carney & Freedland, 2009; Poole, Dickens, & Steptoe, 2011). This is methodologically challenging work to do, and the most appropriate conclusion at this point is probably “the jury is still out.” With biological mediators, mechanistic research is further complicated by the dynamic nature of CHD’s underlying pathophysiology (Burg et al., 2013). Most of the extant research has assessed presumptive mediators on a single occasion, and as a consequence likely to have missed the key biological transitions and/or trajectories that underlie differential prognosis.
Individuals with high levels of chronic stress, anxiety, and sleep problems also show many of the same CHD-relevant biological patterns noted above, including cardiac autonomic dysfunction, impaired endothelial functioning, low-grade inflammation, and platelet hyperactivity (Davies & Allgulander, 2013; Edmondson & Cohen, 2013; Everson-Rose & Lewis, 2005; Motivala, 2011; Steptoe & Kivimaki, 2012). However, much of the evidence regarding these associations derives from studies of otherwise healthy individuals. Thus, it remains unclear whether similar patterns are evident in CHD patients, and what role(s) they play in prognosis.
Multidirectional Hypotheses.
Other mechanistic research is rooted in a multidirectional framework. Unlike the research described above, which generally assumes a linear chain of events (comorbidity to mediator to prognosis for direct causality or the opposite for reverse causality, Figure), this line of thinking emphasizes the role that common underlying processes play in driving both comorbidities and prognosis. One version of this hypothesis revolves around atherosclerosis itself (Taylor, Aizenstein, & Alexopoulos, 2013), and postulates that greater systemic plaque burden reduces blood flow to the brain (causing depression) and the heart (worsening prognosis). Another version focuses on genetic liabilities that predispose individuals to both mood disorders and cardiac diseases (see Figure for 3rd common cause model). There are numerous categories of such genes, including those encoding proteins involved with serotonergic neurotransmission, adrenergic signaling, the renin-angiotensin system, platelet aggregation, and inflammatory activity, among others (McCaffery et al., 2006). A third version focuses on the possibility of a common pathogenic role for inflammation (G.E. Miller & Blackwell, 2006; Shimbo, Davidson, Haas, Fuster, & Badimon, 2005; Steptoe & Kivimaki, 2012) in both psychological comorbidity and CHD prognosis (see Figure for Bidirectionality model). Higher inflammatory biomarkers are found in conjunction with depressive and anxious symptoms, PTSD, insomnia, and SDB, as well as chronic stressors related to work, family, and socioeconomic status (Howren, Lamkin, & Suls, 2009; G. E. Miller, Chen, & Parker, 2011; Motivala, 2011).
Although there is preliminary evidence to support each of these hypotheses (McCaffery et al., 2006; Motivala, 2011; Poole et al., 2011; Taylor et al., 2013), logistical, financial, and technical roadblocks have precluded definitive studies. With the advent of methods to noninvasively monitor inflammatory activity in plaque (Tarkin, Joshi, & Rudd, 2014), and technical advances that enable deep, cheap, and rapid sequencing of large segments of the genome (O’Donnell & Nabel, 2011), future research should have the tools needed to glean deeper insights about these pathways. Nonetheless, formidable logistical and financial challenges will remain, as definitive studies will require much larger cohorts of patients and more follow-up assessments than are typical in this field.
Screening for Psychological Comorbidities in Coronary Heart Disease
The strength of the associations between depression and CHD has led many to advise universal depression screening for all CHD patients and referral for treatment when indicated. This approach is endorsed by European cardiology societies (Graham et al., 2007), the British health care system (National Institute for & Clinical, 2009), and has been incorporated into a recent advisory from the American Heart Association (Judith H. Lichtman et al., 2014), and endorsed by the American Psychiatric Association. Specifically, these advisories recommend administering a depression screening questionnaire to patients with CHD and referring those who screen positive to a professional qualified to diagnose and manage depression. The universal screening recommendation has been controversial (Carney, Freedland, & Jaffe, 2009; Thombs, Jewett, Knafo, Coyne, & Ziegelstein, 2009; Whang & Davidson, 2009; Whooley, 2009; Ziegelstein, Thombs, Coyne, & de Jonge, 2009). Partly in response to it, Thombs et al conducted a systematic review of the literature (Thombs et al., 2008) and found no trial that actually tested whether depression screening was beneficial in patients with CHD. This is a pressing question for the field to answer, with significant clinical and economic importance. Given the stakes, we believe a large-scale randomized screening trial is warranted. It will also be important to determine whether screening and referral to treatment benefits CHD patients with anxiety, stress, PTSD, or sleep problems.
Psychological and Behavioral Interventions for Psychological Comorbidities in Coronary Heart Disease
As reviewed above, adults with CHD are quite likely to have comorbid psychological conditions such as depression, anxiety, stress, and insomnia, each of which are associated with compromised quality of life, increased health care and societal costs, increased recurrence of cardiac events, and shortened life. Once a case has been detected, there are several evidence-based therapies, namely cognitive behavioral therapy (CBT), interpersonal psychotherapy (IPT), and problem solving therapy (PST) that have been shown to be efficacious at treating psychological disorders in CHD patients. The majority of the randomized controlled trials (RCTs) that provide the evidence base for these psychological and behavioral therapies have focused on the treatment of depression, though a few RCTs have also focused on psychological treatments for anxiety and stress reduction. The literature is simply sparse on the use of psychological treatments for either PTSD or insomnia in adults with CHD.
Among adults with CHD, a recent systematic review supports prior studies indicating that psychological interventions for depression (including CBT, IPT, as well as supportive therapy) compared to usual care are beneficial for the treatment of depression, though the effect size is small and the trials too small to test for mortality or recurrent cardiac event differences (Baumeister, Hutter, & Bengel, 2011; Whalley, Thompson, & Taylor, 2014). Comparative studies of the differential effect sizes of varying psychological treatments for depression in CHD patients have found that PST and CBT exhibit the largest reductions in depression and depressive symptoms (standardized mean differences=0.34, 0.23, respectively), though the highest quality randomized controlled trials suggest superior effects for CBT (Dickens et al., 2013). In contrast, other studies find no differences among psychological approaches in depression outcomes for CHD patients (Baumeister et al., 2011). Importantly, though most of these meta-analytic findings have not found improved medical prognostic risks associated with the treatment of depression, recent results from the IMPACT trial indicate that among those without baseline CHD, collaborative treatment for depression (psychotherapy and antidepressants) resulted in 48% reduced risk of the first CHD event compared to those in usual care, though this was not found for the CHD patients for a second event (Stewart, Perkins, & Callahan, 2014).
Few studies have focused on the treatment of anxiety and stress in the context of CHD, (Whalley et al., 2014). Indeed, in most treatment studies to date, anxiety and stress have been treated as secondary outcomes, and effect sizes for these conditions were not routinely reported. Nonetheless, pooled analyses of RCTs testing the effectiveness of psychological interventions for anxiety (including cognitive techniques, relaxation training, and social support) indicate effect sizes for anxiety that are of equal magnitude to those observed for depression among patients with CHD (standardized mean differences=0.25) (Whalley et al., 2014). Although there are some small, single trials for stress reduction alone in patients with CHD (Gulliksson et al., 2011; Schneider et al., 2012), or the combination of stress management and cardiac rehabilitation (Blumenthal et al., 2016), there are no systematic reviews of this evidence base. To our knowledge there are also no published empirical studies on the treatment of PTSD among patients with CHD.
While CBT for insomnia (CBT-I) is the gold standard psychological treatment for insomnia among adults without CHD (M. Irwin, Cole, & Nicassio, 2006), to our knowledge, there are no published RCTs that have examined the effectiveness of CBT-I for insomnia symptom reduction and cardiac prognosis in adults with CHD. Recent studies have shown that CBT-I was associated with improved sleep quality and cardiovascular disease risk factor reduction among patients with chronic medical conditions, some of whom had CHD. For example, participation in CBT-I or Tai Chi relative to a sleep seminar control was associated with improved sleep quality and reduced biomarker disease risk (high-density lipoprotein, low-density lipoprotein, triglycerides, hemoglobinA1c, glucose, insulin, C-reactive protein, and fibrinogen) in a sample of older adults with comorbid medical conditions including CHD (Carroll et al., 2015; M. R. Irwin et al., 2014). Similarly, in a sample of patients with chronic insomnia and comorbid medical conditions including CHD, those randomized to the CBT-I treatment condition versus those randomized to the stress management curriculum condition exhibited improved subjective sleep quality (Rybarczyk et al., 2005).
Behavioral and Lifestyle Interventions for Psychological Comorbidities in Coronary Heart Disease
Approximately 36 percent of all deaths that occurred in the United States in 2000 were in turn attributable to behavioral or lifestyle factors, including tobacco use, poor diet, physical inactivity, and alcohol (Mokdad, Marks, Stroup, & Gerberding, 2004). In the context of cardiovascular disease, 2009 estimates suggest that high blood pressure accounts for 45% of all deaths, followed by overweight–obesity, physical inactivity, high LDL cholesterol, smoking, high dietary salt, high dietary trans fatty acids, and low dietary omega-3 fatty acids. Some have argued that we should target the mechanism that is implicated in both the psychological comorbidity and the behavioral/lifestyle risk factors in patients with CHD or at risk for CHD, to improve both their mental and physical well-being. For example, in one trial, participants randomized to a Mediterranean lifestyle program compared to usual care exhibited greater improvement in both psychological well-being and reduction in multiple lifestyle risk factors (diet, physical activity, stress management) in women at risk for CVD (Toobert, Strycker, Glasgow, Barrera Jr, & Angell, 2005). Systematic reviews of cardiac rehabilitation, and exercise improvement specifically, have also shown reductions in cardiovascular mortality, and improvements in depression in patients with CHD (Anderson et al., 2016; Rutledge, Redwine, Linke, & Mills, 2013). Thus, while this is a relatively new field of intervention research, to target multiple behaviors alongside the psychological comorbidity, much work has to be done in this area to understand timing or order of the treatments offered, and how much synergy can be expected by targeting many issues at the same time in patients with CHD.
Challenges and Potential Grand Opportunities for Future Research
Challenges
While the field has grown substantially since the early writing of Corvisant, three formidable challenges remain that impede its progress.
First, most of the empirical research in support of a link between psychological conditions and CHD recurrence risk is based on observational research where causality is difficult to ascertain and the relationships observed might be spurious or epiphenomenal. In other words, though there may be systematic evidence of a prospective association between psychological conditions and CHD prognosis, many of these studies cannot rule out other intervening or third variables. In fact, we have many situations in which observational and biomechanistic reasoning has resulted in the adoption of treatments that, when rigorously tested by clinical trials, do not improve disease outcomes (Prasad, 2013). We also have situations in which no known mechanism exists to understand an observation, and yet treatments are effective when tested in clinical trials—such as aspirin for CHD recurrence (Miner & Hoffhines, 2007). Both of these potential errors suggest we need treatment trials to truly test the causal association among our psychological comorbidities, hypothesized mehanisms, and CHD recurrence.
Second, there is substantial construct and measurement overlap among many of the psychological conditions comorbid in coronary heart disease, yet most studies have assessed each psychological condition in isolation, ignoring the issue of measurement and construct overlap. Similar issues have been raised in parallel discussions of negative affect in psychopathology research and assessment (Smith, 2010). For example, in relation to CHD, one comprehensive literature review concluded that the considerable co-variation between depression and anxiety, both in its measurement and as risk factors for CHD, may contribute to the inconsistent epidemiological findings on the association of psychological conditions and CHD prognosis in clinical samples (Suls & Bunde, 2005). Indeed, measures of anxiety and depression often include items that assess both constructs and the two are themselves highly comorbid (Suls & Bunde, 2005). Additionally, sleep disturbances are also often included as single items in measures of anxiety, depression, and stress, thereby further contributing to the measurement and construct overlap, and making it difficult to disentangle the independent versus the shared association of sleep disturbances and other psychological conditions, on CHD prognosis. To address these measurement and construct concerns, scholars have suggested a focus on underlying psychological vulnerabilities such as a general predisposition towards negative affectivity instead of purported discrete psychological conditions (Suls & Bunde, 2005). However, most of the contemporary empirical research continues to study psychological conditions in isolation, at one time point, and without assessment of the underlying vulnerabilities, with some exceptions (Alcantara et al., 2015; Burg et al., 2013).
Third, we currently have predominantly non-specific mechanistic hypotheses about the pathophysiology and pathways associated with different psychological comorbidities of CHD. Whether there are common mechanistic pathways in reality, or whether the hypotheses about pathways and mediators were thought to be the same in part because of the method and construct problem listed above, is unknown. The issues of method, measurement, and construct overlap will have to be tackled with more precision before we can begin to address if there are unique, or common mechanisms implicated in the worse prognosis suffered by those patients with both CHD and one of these psychological comorbidities.
Potential Grand Opportunities
Recent transformations in healthcare management along with the scientific advances in psychological science of the 21st century create novel opportunities to address the grand challenges involved in managing CHD patients with depression, anxiety, PTSD, and insomnia. We detail four such opportunities.
First, we simply must conduct a definitive randomized controlled trial of depression screening, and another one on depression treatment in patients with CHD. Without such a trial, powered to mortality and CHD recurrence, with appropriate assessment of depression improvement, and a sample representative of the racial/ethnic diversity of the US, reviews of observational literature will simply continue to speculate about whether depression is casual in the etiology of premature death and impoverished quality of life for all patients with CHD (Davidson, 2017). One might wonder why the ENRICHD randomized trial does not answer this call (Berkman et al., 2003). The ENRICHD trial targeted those with social isolation and/or depression, and was designed to treat both of these risk factors. Much has been learned since the important lessons of ENRICHD about the timing, type, and target of depression treatment needed by depressed patients with CHD, and these lessons should be applied to a definitive depression treatment trial. Tempting as it is to call for additional, simple, prospective observational studies, as these are the most feasible to conduct in this field, we need a definitive trial to disentangle the complex associations that have been noted throughout this article.
Second, technological and conceptual breakthroughs will also provide opportunities for deeper mechanistic insights. For example, thanks to advances in cardiac imaging, researchers can now noninvasively monitor inflammatory activity of atherosclerotic plaques along the cardiac tree (Tarkin et al., 2014). Methods like this will provide unprecedented opportunities for behavioral researchers to peer inside the body, and test mechanistic hypotheses at a level of resolution that until today was only possible in animal models. Many of the culprit pathways will be familiar to biobehavioral scientists, as they involve the sympathetic nervous system and its modulation of inflammatory cells. By following the leads generated by this preclinical research, and taking advantage of new technologies, the field is poised to make large mechanistic strides in the coming decade, and may prove invaluable in discovering the true underpinnings of the association of these psychological comorbidities and CHD, particularly when done as ancillary to a large, definitive trial.
Third, the time may be ripe to start targeting some of the putative common mechanisms for the poor prognosis in these patients, and this may alter the trajectory or the course of either or both of their current diseases. Many interesting common mechanisms have been suggested, and the investigation of these mechanisms warrants early basic behavioral science investigation into their mutability and utility. So, for example, targeting the self-regulation deficits noted in patients with these comorbidities might reveal some interesting insights into the processes by which the psychological and cardiovascular dysregulations are related to each other (Alcantara et al., 2014). Other interesting candidate common mechanisms include some of the psychosocial vulnerabilities, such as anhedonia or poor habit formation tendencies. Stress reactivity is another common mechanism that has long been suspected as linking the psychologic comorbidities with cardiovascular disease, and any experimental manipulation of these, whether in animals or in humans (Grippo & Johnson, 2009), would prove to be very enlightening. Again, however, without a programmatic move into experimental and interventional designs, we will not know if the association of these psychological states with the disease processes involved in CHD is causal or spurious.
Fourth, there are psychological and behavioral interventions that target multiple psychological comorbidities and/or psychological and cardiovascular factors simultaneously and it may be time to shift more vigorously into this type of trial design. For example, in a single-blind, randomized clinical trial of 183 patients hospitalized for acute coronary syndrome, heart failure, or arrhythmia and with symptoms of depression, generalized anxiety, or panic, Huffman and colleagues tested whether telephone-based collaborative mental health care treatment, delivered by a social worker and a team of psychiatrists, could improve mental and physical health across 6 months of treatment compared with usual care (Huffman et al., 2014). They found statistically and clinically significant improvements in mental health–related quality of life—the trial’s primary outcome. Separately, Norlund et al, 2015 is testing whether an online platform of CBT (iCBT) is effective for the treatment of both depression and anxiety in CHD patients (U-CARE Heart) (Norlund, Olsson, Burell, Wallin, & Held, 2015). The recent demonstrated benefits of a unified protocol for transdiagnostic treatment of affective disorders compared to a single-disorder protocol lend support to the potential for multiple-syndrome targeting therapies to confer similar benefits for CHD patients (Barlow et al., 2017). Importantly, these multi-syndrome targeting therapies can now be provided over the telephone, and with remote monitoring of the patient, which is both cost-effective and removes barriers to treatments for patients with mobility or transportation issues.
Conclusion
The significant changes in health care delivery and practice and the great advances in psychological science of the 21st century make this an exciting time for those of us invested in bridging psychology, medicine, medical informatics, public health, and behavioral cardiology to lower the public health burden for adults with CHD and comorbid psychological disorders and improve population health. Although as a field we continue to face grand challenges concerning overlap of psychological disorders and vulnerabilities, non-specific mechanistic hypotheses and designs, and lack of definitive evidence regarding the public health benefits of screening and treatment of psychological comorbidities in the context of CHD, the methodological and technological innovations, as well as the health policy disruptions of the 21st century create unprecedented opportunities for future research in this area. The time is now to move vigorously and purposefully toward more basic behavioral science on this topic, and more interdisciplinary team intervention science conducted with our colleagues from related disciplines to conduct the definitive trials needed to inform the care of these patients with mental and medical life-long disorders. Let us remember that it is our patients, those with both psychological and coronary heart disease, who bear a disproportionate burden of suffering, who will stand to benefit the most from the resolution of the grand challenges identified in this review article.
References
- Alcantara C, Muntner P, Edmondson D, Safford MM, Redmond N, Colantonio LD, & Davidson KW (2015). Perfect storm: concurrent stress and depressive symptoms increase risk of myocardial infarction or death. Circulation. Cardiovascular Quality and Outcomes, 8(2), 146–154. doi: 10.1161/circoutcomes.114.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara C, Peacock J, Davidson KW, Hiti D, & Edmondson D (2014). The association of short sleep after acute coronary syndrome with recurrent cardiac events and mortality. International Journal of Cardiology, 171(2), e11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara C, & Davidson KW (2014). Mental Disorders and Coronary Heart Disease Risk: Could the Evidence Elude Us While We Sleep?. Circulation, 129(2), 139–141. 10.1161/CIRCULATIONAHA.113.006515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, & Taylor RS (2016). Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews(1), Cd001800. doi: 10.1002/14651858.CD001800.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH, Farchione TJ, Bullis JR, Gallagher MW, Murray-Latin H, Sauer-Zavala S, . . . Cassiello-Robbins C (2017). The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders Compared With Diagnosis-Specific Protocols for Anxiety Disorders: A Randomized Clinical Trial. JAMA Psychiatry, 74(9), 875–884. doi: 10.1001/jamapsychiatry.2017.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister H, Hutter N, & Bengel J (2011). Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database of Systematic Reviews, 9, CD008012. doi: 10.1002/14651858.CD008012.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, . . . Schneiderman N (2003). Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA, 289(23), 3106–3116. doi: 10.1001/jama.289.23.3106 [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, . . . Hinderliter A (2016). Enhancing Cardiac Rehabilitation With Stress Management Training: A Randomized, Clinical Efficacy Trial. Circulation, 133(14), 1341–1350. doi: 10.1161/circulationaha.115.018926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MM, Edmondson D, Whang W, Shaffer JA, Kronish IM, Alcántara C, . . . Davidson KW (2013). The ‘Perfect Storm’ that leads to acute coronary syndrome: Do psychosocial factors play a role? Progress in Cardiovascular Diseases, 55(6), 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, & Freedland KE (2009). Depression and heart rate variability in patients with coronary heart disease. Cleveland Clinic Journal of Medicine, 76 Suppl 2, S13–17. doi:76/Suppl_2/S13 [pii] 10.3949/ccjm.76.s2.03 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, & Jaffe AS (2009). Depression screening in patients with heart disease. JAMA, 301(13), 1337; author reply 1338. doi:301/13/1337[pii]10.1001/jama.2009.408[doi] [DOI] [PubMed] [Google Scholar]
- Carroll JE, Seeman TE, Olmstead R, Melendez G, Sadakane R, Bootzin R, . . . Irwin MR (2015). Improved sleep quality in older adults with insomnia reduces biomarkers of disease risk: Pilot results from a randomized controlled comparative efficacy trial. Psychoneuroendocrinology, 55, 184–192. doi: 10.1016/j.psyneuen.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BE, Edmondson D, & Kronish IM (2015). State of the Art Review: Depression, Stress, Anxiety, and Cardiovascular Disease. American Journal of Hypertension, 28(11),1295–1302. doi: 10.1093/ajh/hpv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen NL, Pelle AJ, Boersma E, Serruys PW, van Domburg RT, & Pedersen SS (2013). Reduced positive affect (anhedonia) is independently associated with 7-year mortality in patients treated with percutaneous coronary intervention: results from the RESEARCH registry. European Journal of Preventive Cardiology, 20(1), 127–134. doi: 10.1177/2047487312436452 [DOI] [PubMed] [Google Scholar]
- Davidson KW (2017). Waiting for Godot: Engaging in Discussions About Depression Care in Patients With Acute Myocardial Infarction While Waiting for a Definitive Trial That Never Appears. Circulation, 135(18), 1690–1692. doi: 10.1161/circulationaha.117.027610 [DOI] [PubMed] [Google Scholar]
- Davidson KW, Burg MM, Kronish IM, Shimbo D, Dettenborn L, Mehran R, . . . Rieckmann N (2010). Association of Anhedonia With Recurrent Major Adverse Cardiac Events and Mortality 1 Year After Acute Coronary Syndrome. Archives of General Psychiatry, 67(5), 480–488. doi: 10.1001/archgenpsychiatry.2010.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, & Allgulander C (2013). Anxiety and cardiovascular disease. Modern Trends in Pharmacopsychiatry, 29, 85–97. doi: 10.1159/000351945 [DOI] [PubMed] [Google Scholar]
- de Miranda Azevedo R, Roest AM, Hoen PW, & de Jonge P (2014). Cognitive/affective and somatic/affective symptoms of depression in patients with heart disease and their association with cardiovascular prognosis: a meta-analysis. Psychological Medicine, 44(13), 2689–2703. doi: 10.1017/s0033291714000063 [DOI] [PubMed] [Google Scholar]
- Diamond L, Chung S, Ferguson W, Gonzalez J, Jacobs EA, & Gany F (2014). Relationship between self-assessed and tested non-English-language proficiency among primary care providers. Medical Care, 52(5), 435–438. doi: 10.1097/mlr.0000000000000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens CP, Cherrington AP, Adeyemi IB, Roughley KM, Bower PP, Garrett CP, . . . Coventry PP (2013). Characteristics of Psychological Interventions That Improve Depression in People With Coronary Heart Disease: A Systematic Review and Meta-Regression. Pyschosomatic Medicine, 75(2), 211–221. [DOI] [PubMed] [Google Scholar]
- Doyle F, Conroy R, & McGee H (2012). Differential predictive value of depressive versus anxiety symptoms in the prediction of 8-year mortality after acute coronary syndrome. Psychosomatic Medicine, 74(7), 711–716. doi: 10.1097/PSY.0b013e318268978e [DOI] [PubMed] [Google Scholar]
- Edmondson D, & Cohen D (2013). Posttraumatic stress disorder and cardiovascular disease. Progress in Cardiovascular Diseases, 55(6), 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, & Neria Y (2012). Posttraumatic Stress Disorder Prevalence and Risk of Recurrence in Acute Coronary Syndrome Patients: A Meta-analytic Review. PLoS ONE, 7(6), e38915. doi: 10.1371/journal.pone.0038915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose SA, & Lewis TT (2005). Psychosocial factors and cardiovascular diseases. Annual Review of Public Health, 26, 469–500. doi: 10.1146/annurev.publhealth.26.021304.144542 [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. (2009). Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: U.S. Preventive Services Task Force reaffirmation recommendation statement. Annals of Internal Medicine, 150(8), 551–555. [DOI] [PubMed] [Google Scholar]
- Fuster V (2004). Hurst’s the Heart. New York, NY: McGraw-Hill, Medical Pub. Division. [Google Scholar]
- GBD 2013 Mortality and Causes of Death Collaborators. (2015). Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet, 385(9963), 117–171. doi: 10.1016/s0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH, & Shapiro PA (1998). Depression and the course of coronary artery disease. American Journal of Psychiatry, 155(1), 4–11. [DOI] [PubMed] [Google Scholar]
- Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, . . . Zampelas A (2007). European guidelines on cardiovascular disease prevention in clinical practice: executive summary. European Heart Journal, 28(19), 2375–2414. doi:ehm316[pii]10.1093/eurheartj/ehm316[doi] [DOI] [PubMed] [Google Scholar]
- Grippo AJ, & Johnson AK (2009). Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress, 12(1), 1–21. doi:907251419[pii]10.1080/10253890802046281[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliksson M, Burell G, Vessby B, Lundin L, Toss H, & Svardsudd K (2011). Randomized Controlled Trial of Cognitive Behavioral Therapy vs Standard Treatment to Prevent Recurrent Cardiovascular Events in Patients With Coronary Heart Disease: Secondary Prevention in Uppsala Primary Health Care Project (SUPRIM). Archives of Internal Medicine, 171(2), 134–140. doi: 10.1001/archinternmed.2010.510 [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, & Suls J (2009). Associations of Depression With C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosomatic Medicine, 71(2), 171–186. doi:PSY.0b013e3181907c1b [pii] 10.1097/PSY.0b013e3181907c1b [doi] [DOI] [PubMed] [Google Scholar]
- Huffman JC, Mastromauro CA, Beach SR, Celano CM, Dubois CM, Healy BC, . . . Januzzi JL (2014). Collaborative Care for Depression and Anxiety Disorders in Patients With Recent Cardiac Events: The Management of Sadness and Anxiety in Cardiology (MOSAIC) Randomized Clinical Trial. JAMA Internal Medicine, 174(6), 927–936. doi: 10.1001/jamainternmed.2014.739 [DOI] [PubMed] [Google Scholar]
- Irwin M, Cole JC, & Nicassio PM (2006). Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in adults and in older adults 55 + years. Health Psychology. Health Psychology, 25(1), 3–14. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, . . . Nicassio P (2014). Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep, 37(9), 1543–1552. doi: 10.5665/sleep.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugsand LE, Vatten LJ, Platou C, & Janszky I (2011). Insomnia and the Risk of Acute Myocardial Infarction: A Population Study. Circulation, 124(19), 2073–2081. doi: 10.1161/circulationaha.111.025858 [DOI] [PubMed] [Google Scholar]
- Leineweber C, Kecklund G, Janszky I, Akerstedt T, & Orth-Gomer K (2003). Poor sleep increases the prospective risk for recurrent events in middle-aged women with coronary disease. The Stockholm Female Coronary Risk Study. Journal of Psychosomatic Research, 54(2), 121–127. [DOI] [PubMed] [Google Scholar]
- Lichtman JH, Bigger JT Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, . . . Froelicher ES (2008). Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation, 118(17), 1768–1775. doi:CIRCULATIONAHA.108.190769 [pii] 10.1161/CIRCULATIONAHA.108.190769 [doi] [DOI] [PubMed] [Google Scholar]
- Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, . . . Wulsin L (2014). Depression as a Risk Factor for Poor Prognosis Among Patients With Acute Coronary Syndrome: Systematic Review and Recommendations: A Scientific Statement From the American Heart Association. Circulation, 129(12), 1350–1369. doi: 10.1161/cir.0000000000000019 [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, & Murray CJ (2006). Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. The Lancet, 367(9524), 1747–1757. doi: 10.1016/s0140-6736(06)68770-9 [DOI] [PubMed] [Google Scholar]
- Luyster FS, Strollo PJ Jr., Zee PC, & Walsh JK (2012). Sleep: a health imperative. Sleep, 35(6), 727–734. doi: 10.5665/sleep.1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malzberg B (1937). Mortality among patients with involution melancholia. American Journal of Psychiatry, 93(5), 1231–1238. doi:doi: 10.1176/ajp.93.5.1231 [DOI] [Google Scholar]
- Marrelli AF, Tondora J, & Hoge MA (2005). Strategies for developing competency models. Administration and Policy in Mental Health, 32(5–6), 533–561. [DOI] [PubMed] [Google Scholar]
- Matthews KA, & Gallo LC (2011). Psychological perspectives on pathways linking socioeconomic status and physical health. Annual Review of Psychology, 62, 501–530. doi: 10.1146/annurev.psych.031809.130711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery JM, Frasure-Smith N, Dube MP, Theroux P, Rouleau GA, Duan Q, & Lesperance F (2006). Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosomatic Medicine, 68(2), 187–200. [DOI] [PubMed] [Google Scholar]
- Miller GE, & Blackwell E (2006). Turning up the Heat: Inflammation as a Mechanism Linking Chronic Stress, Depression, and Heart Disease. Current Directions in Psychological Science, 15(6), 269–272. doi: 10.2307/20183133 [DOI] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–997. doi: 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, & Gerberding JL (2004). Actual causes of death in the United States, 2000. JAMA, 291(10), 1238–1245. doi: 10.1001/jama.291.10.1238 [DOI] [PubMed] [Google Scholar]
- Miner J, & Hoffhines A (2007). The discovery of aspirin’s antithrombotic effects. Texas Heart Institute Journal, 34(2), 179–186. [PMC free article] [PubMed] [Google Scholar]
- Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, . . . Wenger NK (2007). Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Journal of the American College of Cardiology, 49(11), 1230–1250. doi: 10.1016/j.jacc.2007.02.020 [DOI] [PubMed] [Google Scholar]
- Moser DK, McKinley S, Riegel B, Doering LV, Meischke H, Pelter M, . . . Dracup K (2011). Relationship of persistent symptoms of anxiety to morbidity and mortality outcomes in patients with coronary heart disease. Psychosomatic Medicine, 73(9), 803–809. doi: 10.1097/PSY.0b013e3182364992 [DOI] [PubMed] [Google Scholar]
- Motivala S (2011). Sleep and Inflammation: Psychoneuroimmunology in the Context of Cardiovascular Disease. Annals of Behavioral Medicine, 42(2), 141–152. doi: 10.1007/s12160-011-9280-2 [DOI] [PubMed] [Google Scholar]
- Mykletun A, Bjerkeset O, Dewey M, Prince M, Overland S, & Stewart R (2007). Anxiety, Depression, and Cause-Specific Mortality: The HUNT Study. Psychosomatic Medicine, 69(4), 323–331. doi: 10.1097/PSY.0b013e31803cb862 [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence. (2009). Depression in Adults with Chronic Physical Health Problems. Retrieved from: http://guidance.nice.org.uk/CG91
- Nicholson A, Kuper H, & Hemingway H (2006). Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. European Heart Journal, 27(23), 2763–2774. doi: 10.1093/eurheartj/ehl338 [DOI] [PubMed] [Google Scholar]
- Norlund F, Olsson EM, Burell G, Wallin E, & Held C (2015). Treatment of depression and anxiety with internet-based cognitive behavior therapy in patients with a recent myocardial infarction (U-CARE Heart): study protocol for a randomized controlled trial. Trials, 16(1), 154. doi: 10.1186/s13063-015-0689-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell CJ, & Nabel EG (2011). Genomics of cardiovascular disease. New England Journal of Medicine, 365(22), 2098–2109. doi: 10.1056/NEJMra1105239 [DOI] [PubMed] [Google Scholar]
- Poole L, Dickens C, & Steptoe A (2011). The puzzle of depression and acute coronary syndrome: Reviewing the role of acute inflammation. Journal of Psychosomatic Research, 71(2), 61–68. doi: 10.1016/j.jpsychores.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad V (2013). Why randomized controlled trials are needed to accept new practices: 2 medical worldviews. Mayo Clinic Proceedings, 88(10), 1046–1050. doi: 10.1016/j.mayocp.2013.04.026 [DOI] [PubMed] [Google Scholar]
- Roest AM, Heideveld A, Martens EJ, de Jonge P, & Denollet J (2014). Symptom dimensions of anxiety following myocardial infarction: associations with depressive symptoms and prognosis. Health Psychology, 33(12), 1468–1476. doi: 10.1037/a0034806 [DOI] [PubMed] [Google Scholar]
- Roest AM, Martens EJ, Denollet J, & de Jonge P (2010). Prognostic association of anxiety post myocardial infarction with mortality and new cardiac events: a meta-analysis. Psychosomatic Medicine, 72(6), 563–569. doi: 10.1097/PSY.0b013e3181dbff97 [DOI] [PubMed] [Google Scholar]
- Roest AM, Zuidersma M, & de Jonge P (2012). Myocardial infarction and generalised anxiety disorder: 10-year follow-up. British Journal of Psychiatry, 200(4), 324–329. doi: 10.1192/bjp.bp.111.103549 [DOI] [PubMed] [Google Scholar]
- Rosch, P. J. (2004). Type A and Coronary Disease Part 1: Separating Fact From Fiction. An interview with Ray H. Rosenman, M.D. Retrieved from http://www.stress.org/type-a-and-coronary-disease-part-1/
- Rozanski A (2014). Behavioral Cardiology: Current Advances and Future Directions. Journal of the American College of Cardiology, 64(1), 100–110. doi: 10.1016/j.jacc.2014.03.047 [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Davidson KW, Saab PG, & Kubzansky L (2005). The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. Journal of the American College of Cardiology, 45(5), 637–651. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Redwine LS, Linke SE, & Mills PJ (2013). A Meta-Analysis of Mental Health Treatments and Cardiac Rehabilitation for Improving Clinical Outcomes and Depression Among Patients With Coronary Heart Disease. Psychosomatic Medicine, 75(4), 335–349. doi: 10.1097/PSY.0b013e318291d798 [DOI] [PubMed] [Google Scholar]
- Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, & Davis A (2005). A Placebo-Controlled Test of Cognitive-Behavioral Therapy for Comorbid Insomnia in Older Adults. Journal of Consulting and Clinical Psychology, 73(6), 1164–1174. doi: 10.1037/0022-006X.73.6.1164 [DOI] [PubMed] [Google Scholar]
- Schneider RH, Grim CE, Rainforth MV, Kotchen T, Nidich SI, Gaylord-King C, . . . Alexander CN (2012). Stress Reduction in the Secondary Prevention of Cardiovascular Disease: Randomized, Controlled Trial of Transcendental Meditation and Health Education in Blacks. Circulation. Cardiovascular Quality and Outcomes, 5(6), 750–758. doi: 10.1161/circoutcomes.112.967406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Freedland KE, & Carney RM (1997). How safe are serotonin reuptake inhibitors for depression in patients with coronary heart disease? American Journal of Medicine, 102(1), 54–59. [DOI] [PubMed] [Google Scholar]
- Shemesh E, Yehuda R, Milo O, Dinur I, Rudnick A, Vered Z, & Cotter G (2004). Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction. Psychosomatic Medicine, 66(4), 521–526. [DOI] [PubMed] [Google Scholar]
- Shimbo D, Davidson KW, Haas DC, Fuster V, & Badimon JJ (2005). Negative impact of depression on outcomes in patients with coronary artery disease: Mechanisms, treatment considerations, and future directions. Journal of Thrombosis and Haemostasis, 3(5), 897–908. [DOI] [PubMed] [Google Scholar]
- Smith T, & Baucom BRW (2017). Intimate relationships, indivdidual adjustment, and coronary heart disease: Implications of Overlapping Associations in Psychosocial Risk. American Psychologist, 72(6), 578–589. [DOI] [PubMed] [Google Scholar]
- Smith T (2010). Conceptualization, measurement, and analysis of negative affective risk factors In Steptoe A & Freedland KE (Eds.), Handbook of Behavioral Medicine (pp. 155–168). New York: Springer. [Google Scholar]
- Steptoe A, & Kivimaki M (2012). Stress and cardiovascular disease. Nature Reviews Cardiology, 9(6), 360–370. doi: 10.1038/nrcardio.2012.45 [DOI] [PubMed] [Google Scholar]
- Stewart JC, Perkins AJ, & Callahan CM (2014). Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial. Psychosomatic Medicine, 76(1), 29–37. doi: 10.1097/psy.0000000000000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suls J, & Bunde J (2005). Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychological Bulletin, 131(2), 260–300. [DOI] [PubMed] [Google Scholar]
- Tarkin JM, Joshi FR, & Rudd JH (2014). PET imaging of inflammation in atherosclerosis. Nature Reviews Cardiology, 11(8), 443–457. doi: 10.1038/nrcardio.2014.80 [DOI] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, & Alexopoulos GS (2013). The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular Psychiatry, 18(9), 963–974. doi: 10.1038/mp.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, . . . Ziegelstein RC (2008). Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA, 300(18), 2161–2171. doi: 10.1001/jama.2008.667 [DOI] [PubMed] [Google Scholar]
- Thombs BD, Jewett LR, Knafo R, Coyne JC, & Ziegelstein RC (2009). Learning from history: a commentary on the American Heart Association Science Advisory on depression screening. American Heart Journal, 158(4), 503–505. doi: 10.1016/j.ahj.2009.07.032 [DOI] [PubMed] [Google Scholar]
- Toobert DJ, Strycker LA, Glasgow RE, Barrera M Jr, & Angell K (2005). Effects of the mediterranean lifestyle program on multiple risk behaviors and psychosocial outcomes among women at risk for heart disease. Annals of Behavioral Medicine, 29(2), 128–137. doi: 10.1207/s15324796abm2902_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley B, Thompson DR, & Taylor RS (2014). Psychological Interventions for Coronary Heart Disease: Cochrane Systematic Review and Meta-analysis. International Journal of Behavioral Medicine, 21(1), 109–121. doi: 10.1007/s12529-012-9282-x [DOI] [PubMed] [Google Scholar]
- Whang W, & Davidson KW (2009). Is it time to treat depression in patients with cardiovascular disease? Circulation, 120(2), 99–100. doi: 10.1161/circulationaha.109.881987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang W, Peacock J, Soliman EZ, Alcantara C, Nazarian S, Shah AJ, . . . Shimbo D (2014). Relations between Depressive Symptoms, Anxiety, and T Wave Abnormalities in Subjects without Clinically-apparent Cardiovascular Disease (from the Multi-Ethnic Study of Atherosclerosis [MESA]). American Journal of Cardiology, 114(12), 1917–1922. doi: 10.1016/j.amjcard.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whooley MA (2009). To screen or not to screen? Depression in patients with cardiovascular disease. Journal of the American College of Cardiology, 54(10), 891–893. doi:S0735-1097(09)01953-6 [pii] 10.1016/j.jacc.2009.05.034 [doi] [DOI] [PubMed] [Google Scholar]
- Ziegelstein RC, Thombs BD, Coyne JC, & de Jonge P (2009). Routine screening for depression in patients with coronary heart disease never mind. Journal of the American College of Cardiology, 54(10), 886–890. doi:S0735-1097(09)01950-0[pii]10.1016/j.jacc.2009.01.082[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]


