Abstract
BACKGROUND
Multiple health problems have been reported in survivors of Ebola virus disease (EVD). Attribution of these problems to the disease without a control group for analysis is difficult.
METHODS
We enrolled a cohort of EVD survivors and their close contacts and prospectively collected data on symptoms, physical examination findings, and laboratory results. A subset of participants underwent ophthalmologic examinations. Persistence of Ebola virus (EBOV) RNA in semen samples from survivors was determined.
RESULTS
A total of 966 EBOV antibody–positive survivors and 2350 antibody-negative close contacts (controls) were enrolled, and 90% of these participants were followed for 12 months. At enrollment (median time to baseline visit, 358 days after symptom onset), six symptoms were reported significantly more often among survivors than among controls: urinary frequency (14.7% vs. 3.4%), headache (47.6% vs. 35.6%), fatigue (18.4% vs. 6.3%), muscle pain (23.1% vs. 10.1%), memory loss (29.2% vs. 4.8%), and joint pain (47.5% vs. 17.5%). On examination, more survivors than controls had abnormal abdominal, chest, neurologic, and musculoskeletal findings and uveitis. Other than uveitis (prevalence at enrollment, 26.4% vs. 12.1%; at year 1, 33.3% vs. 15.4%), the prevalence of these conditions declined during follow-up in both groups. The incidence of most symptoms, neurologic findings, and uveitis was greater among survivors than among controls. EBOV RNA was detected in semen samples from 30% of the survivors tested, with a maximum time from illness to detection of 40 months.
CONCLUSIONS
A relatively high burden of symptoms was seen in all participants, but certain symptoms and examination findings were more common among survivors. With the exception of uveitis, these conditions declined in prevalence during follow-up in both groups. Viral RNA in semen persisted for a maximum of 40 months.
The 2014–2016 Ebola virus disease (EVD) epidemic in West Africa was unprecedented, with more than 11,000 deaths and more than 28,000 infections. Among the three countries with the highest number of cases and deaths (Sierra Leone, Liberia, and Guinea), 5852 survivors are registered.1 Cross-sectional studies from this and previous Ebola outbreaks suggest that patients who survive EVD can have a myriad of health complications.2–12 In addition, there is evidence that survivors may be the source of new infections because they can harbor and intermittently shed Ebola virus (EBOV) in semen, resulting in the infection of sex partners.13–16
To more reliably determine the long-term consequences of EVD, in June 2015 the Partnership for Research on Ebola Virus in Liberia (PREVAIL) launched a cohort study (PREVAIL III) involving survivors of EVD as well as their close contacts, who would serve as controls, with 5 years of planned follow-up. Here, we compare the prevalence of symptoms and abnormal findings among survivors and controls at study entry and at 6- and 12-month follow-up examinations and report the incidence of new findings for these groups during the first year of follow-up. We also report findings regarding the persistence of EBOV RNA in semen in a subset of survivors.
METHODS
STUDY DESIGN
We designed and implemented the study through a partnership between the Ministry of Health in Liberia and the National Institute of Allergy and Infectious Diseases (NIAID). The protocol, available with the full text of this article at NEJM.org, was approved by the National Research Ethics Board of Liberia and the NIAID Institutional Review Board at the National Institutes of Health. Written informed consent (or assent) was obtained from all participants. The members of the writing group, who were responsible for the study design, the collection and analysis of the data, and the preparation and submission of the manuscript for publication, vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
PARTICIPANTS
Survivors of EVD of any age who were in the registry of the Ministry of Health in Liberia, which documented that they had a diagnosis of EVD and had been treated at an Ebola treatment unit, were enrolled at three sites. At the time of enrollment, survivors identified up to nine close contacts (up to five nonsexual contacts and four sexual contacts) who lived with them at the time of diagnosis or after recovery or were sex partners after their discharge from the Ebola treatment unit. These contacts were invited to participate in the study as close-contact controls solely on the basis of their exposure to a survivor. Close contacts with no history of EVD were enrolled without regard for their current health status and did not need to be among the nine contacts identified by the survivor. Close contacts were not age- or sex-matched to survivors.
BASELINE AND FOLLOW-UP EXAMINATIONS
Survivors and close contacts underwent the same medical examinations at study entry and every 6 months during follow-up (see Section 1.1 in the Supplementary Appendix, available at NEJM.org). The baseline and follow-up examinations included past, interim, and current medical history, a checklist regarding symptoms the patient was having at the time of examination (the questionnaires are provided in the Supplementary Appendix), a physical examination, and collection of blood for the determination of anti-EBOV antibody levels, routine chemical analyses, and hematologic assessment. Detailed eye examinations were conducted by an ophthalmologist in an ophthalmology clinic at the JFK Medical Center in a subset of 564 survivors and 635 close contacts who enrolled at the JFK Medical Center site (see Section 1.3 in the Supplementary Appendix).
MEASUREMENT OF SERUM ANTIBODY AND SEMEN VIRAL RNA
Levels of IgG antibody against the EBOV surface glycoprotein in serum samples collected from survivors and close contacts at the baseline examination were measured with the use the Filovirus Animal Nonclinical Group assay at the Liberian Institute for Biomedical Research, as previously described.17 A level of 548 enzyme-linked immunosorbent assay units (EU) per milliliter was used as the cutoff for positivity. To characterize the diagnostic performance of this cutoff value, we constructed a receiver operating characteristic curve using presumed EBOV antibody–negative and EBOV antibody–positive specimens. When 548 EU per milliliter was used as the cutoff, the sensitivity was 94.4% and the specificity was 96.7% (see Section 1.2 in the Supplementary Appendix). Semen testing for EBOV RNA with the use of the modified GeneXpert Ebola reverse-transcriptase polymerase chain reaction (RT-PCR) was performed as previously described.18
STATISTICAL ANALYSIS
Differences in the prevalence of symptoms and abnormal findings between survivors and close contacts at study entry were determined with generalized estimating equations to account for the relationships between survivors and close contacts. To reduce the risk of a type I error, the analyses described in this report were largely focused on symptoms and physical examination findings that were evaluated in both sexes at study entry for which the P value for the difference between survivors and close contacts was less than 0.0001. For symptoms, we also required the difference in prevalence to exceed 10 percentage points. We refer to these symptoms and abnormal findings as targeted conditions. For these targeted conditions, the relative differences in the prevalence of symptoms and abnormal findings at each examination (at baseline and at 6 and 12 months) are summarized with odds ratios (survivors vs. close contacts) that have been adjusted for age, sex, site, and relationship between survivors and close contacts. The incidences of targeted conditions during follow-up (number of participants and rate per 1000 person-years) among survivors and close contacts are also reported with similarly adjusted odds ratios and 95% confidence intervals.
In addition to these summaries of the examinations in all participants, the differences between survivors and close contacts with regard to uveitis, cataracts, and visual acuity based on an ophthalmologic examination at baseline and at 12 months, as well as the decrease in the frequency of positive tests for EBOV RNA in semen after the acute illness, were examined (with the use of the strict significance criteria used for the targeted conditions) in subsets of participants. Although strict criteria (P<0.0001) were used for the determination of significance in comparisons of the prevalence of symptoms and physical examination findings, ophthalmologic findings, and EBOV positivity in semen, the criterion for significance in all other analyses (described in detail in Section 1.5 in the Supplementary Appendix) was a P value of less than 0.01.
Supplementing these preplanned analyses, other, more exploratory associations, such as those between uveitis and the detection of viral RNA in semen, were examined. The exploratory analyses should be interpreted with caution, because they were not adjusted for type I error.
All statistical analyses were performed with SAS software, version 9.3 (SAS Institute), or R software, version 3.2.3 (R Foundation for Statistical Computing). All P values are two-sided.
RESULTS
PARTICIPANTS
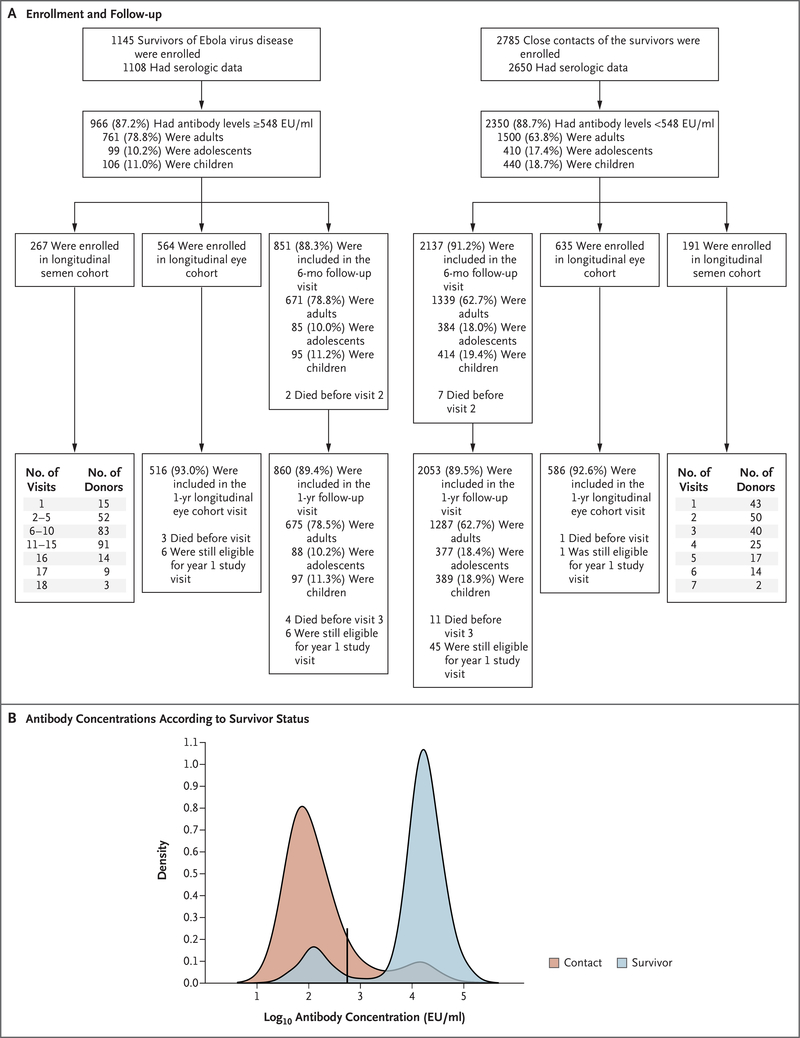
From June 2015 through June 2017, three sites in Liberia enrolled 1145 of the approximately 1500 EVD survivors listed in the Ministry of Health registry and 2785 close contacts with no history of EVD. Among the survivors, the median time from the onset of EVD to the baseline protocol visit was 358 days (interquartile range, 313 to 405) (Table 1). Of the 1108 Ministry of Health–reported survivors who underwent serologic testing, 966 (87.2%) had EBOV-specific antibodies detected at levels equal to or above 548 EU per milliliter; 2350 (88.7%) of the survivor-reported close contacts with serologic test results had antibody levels below 548 EU per milliliter (Fig. 1, and Section 2.1 in the Supplementary Appendix). Antibody-positive EVD survivors and antibody-negative close contacts make up the analysis cohort for this study. These groups are referred to as survivors and controls, respectively, when comparisons are made between these two groups.
Table 1.
Baseline Clinical Measurements among Survivors and Close Contacts*
| Characteristic | <12 Yr of Age | 12–17 Yr of Age | ≥18 Yr of Age | Overall† | ||||
|---|---|---|---|---|---|---|---|---|
| Survivors (N = 106) | Contacts (N = 440) | Survivors (N = 99) | Contacts (N = 410) | Survivors (N = 761) | Contacts (N = 1500) | Survivors (N = 966) | Contacts (N = 2350) | |
| Age (yr) | ||||||||
| Median | 8 | 7 | 14 | 14 | 33 | 31 | 29 | 23 |
| IQR | 6–9 | 5–9 | 13–16 | 13–16 | 25–42 | 24– 41 | 19–40 | 13–34 |
| Female sex (%) | 43.4 | 53.4 | 58.6 | 53.2 | 55.7 | 57.1 | 54.7 | 55.7 |
| Pregnant at entry (%) | NA | NA | 8.5 | 6.9 | 13.2 | 9.0 | 12.6 | 8.5 |
| Body-mass index‡ | ||||||||
| Median | 15.7 | 15.6 | 18.6 | 19.3 | 23.7 | 23.1 | 22.5 | 21.3 |
| IQR | 14.6–16.8 | 14.8–16.5 | 17.4–20.6 | 17.5–21.1 | 21.3–27.1 | 21–26.8 | 19.5–26.3 | 18.2–24.6 |
| HIV infection (%) | NA | NA | 0 | 0.5 | 1.6 | 3.3 | 1.4 | 2.7 |
| Syphilis (%) | NA | NA | 2.1 | 1.0 | 4.5 | 4.9 | 4.2 | 4.1 |
| Antibody concentration (EU/ml) | ||||||||
| Median | 28,017 | 63 | 22,564 | 84 | 18,288 | 90 | 19,242 | 83 |
| IQR | 16,127–52,837 | 34–133 | 16,939–35,884 | 47–162 | 11,860–29,855 | 52–165 | 12,550–33,201 | 48–160 |
| Time since symptom onset (days) | ||||||||
| Median | 410 | NA | 357 | NA | 351 | NA | 358 | NA |
| IQR | 378–438 | NA | 322–423.75 | NA | 303–398 | NA | 313–405 | NA |
| Contact relationship to survivor (%) | ||||||||
| Sexual contact | NA | NA | NA | 1.2 | NA | 20.8 | NA | 13.5 |
| Household member | NA | 77.7 | NA | 73.9 | NA | 64.5 | NA | 68.6 |
EU denotes enzyme-linked immunosorbent assay units, HIV human immunodeficiency virus, IQR interquartile range, and NA not applicable.
Age, body-mass index, and frequency of pregnancy differed significantly between survivors and contacts (P<0.01).
Body-mass index is the weight in kilograms divided by the square of the height in meters.
Figure 1. Enrollment, Follow-up, and Antibody Measurements in the Study Population.
Panel A shows a flow diagram for the generation of the analysis cohort. Panel B shows a kernel-density plot of the distribution of log10 antibody concentrations among the Ministry of Health–reported survivors and the survivor-reported close contacts. The black vertical line indicates the antibody concentration cutoff for determining Ebola virus seropositivity or seronegativity. EU denotes enzyme-linked immunosorbent assay units.
ANTIBODY-NEGATIVE SURVIVORS AND ANTIBODY-POSITIVE CONTACTS
There were 142 survivors reported by the Ministry of Health who had Ebola antibody levels below 548 EU per milliliter (median antibody level, 98 EU per milliliter) (Fig. 1). Antibody-negative survivors were less likely than antibody-positive survivors to have a positive EBOV RT-PCR result reported during their acute illness (31% vs. 76%, P<0.001).
Among the 300 close contacts with EBOV antibody levels of at least 548 EU per milliliter, the median antibody level was significantly lower than that among the antibody-positive survivors (3979 EU per milliliter vs. 19,242 EU per milliliter, P<0.001). Among the antibody-positive close contacts, 47% reported having symptoms consistent with EVD within 3 weeks after their linked survivor’s acute illness, and most symptoms were reported with at least twice the frequency among the antibody-positive close contacts than among the antibody-negative close contacts (Table S1 in the Supplementary Appendix). In the antibody-negative close-contact group, 31% of participants recalled symptoms suggestive of EVD within a 3-week period after their linked survivor’s acute illness (P<0.001 for the difference) (Table S1 in the Supplementary Appendix).
ANTIBODY-POSITIVE SURVIVORS AND ANTIBODY-NEGATIVE CONTACTS (CONTROLS)
Both survivors and controls reported a wide range of symptoms. A complete list of the symptoms reported by survivors and controls is provided in Tables S4 and S5 in the Supplementary Appendix. Table 2 lists the prevalence of the targeted symptoms and findings. Six symptoms met the criteria for significance (i.e., P<0.0001): urinary frequency (14.7% vs. 3.4%), headache (47.6% vs. 35.6%), fatigue (18.4% vs. 6.3%), muscle pain (23.1% vs. 10.1%), memory loss (29.2% vs. 4.8%), and joint pain (47.5% vs. 17.5%) were reported significantly more often by survivors than by controls at enrollment. Child and adolescent participants in both the survivor group and the control group reported fewer symptoms than adults (Fig. S2 in the Supplementary Appendix). Among antibody-positive survivors, there was no association of antibody levels with the symptoms shown in Table 2 (see Section 2.5 in the Supplementary Appendix).
Table 2.
Selected Symptoms and Findings on Physical Examination among Survivors and Close Contacts over Time.*
| Symptom or Finding | Baseline Visit |
6-Month Visit |
12-Month Visit |
P Value† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survivors (N = 966) | Contacts (N =2350) | Odds Ratio (95% Cl) | Survivors (N = 851) | Contacts (N = 2137) | Odds Ratio (95% Cl) | Survivors (N = 860) | Contacts (N = 2053) | Odds Ratio (95% Cl) | ||
| no. (%) | no. (%) | no. (%) | ||||||||
| Urinary frequency | 142 (14.7) | 81 (3.4) | 5.9 (3.9–7.8) | 21 (2.5) | 35 (1.6) | 1.7 (0.7–2.6) | 18 (2.1) | 18 (0.9) | 2.7 (0.9–4.4) | <0.001 |
| Headache | 460 (47.6) | 837 (35.6) | 1.5 (1.2–1.7) | 322 (37.8) | 498 (23.3) | 1.8 (1.5–2.1) | 280 (32.6) | 262 (12.8) | 3.0 (2.4–3.6) | <0.001 |
| Fatigue | 178 (18.4) | 149 (6.3) | 3.1 (2.4–3.8) | 75 (8.8) | 61 (2.9) | 3.0 (1.9–4.1) | 44 (5.1) | 23 (1.1) | 4.3 (2.2–6 5) | 0.41 |
| Muscle pain | 223 (23.1) | 238 (10.1) | 3.4 (2.6–4.1) | 118 (13.9) | 205 (9.6) | 1.8 (1.3–2.2) | 110 (12.8) | 154 (7.5) | 2.1 (1.6–2.7) | <0.001 |
| Memory loss | 282 (29.2) | 113 (4.8) | 8.2 (6.4–10.5) | 89 (10.5) | 12 (0.6) | 19.7 (10.5–36.9) | 40 (4.7) | 3 (0.1) | 31.2 (9.5–102.6) | 0.009 |
| Joint pain | 459 (47.5) | 411 (17.5) | 4.0 (3.3–4.8) | 298 (35.0) | 253 (11.8) | 3.6 (2.9–4.3) | 237 (27.6) | 182 (8.9) | 3.5 (2.6–4.3) | 0.50 |
| Chest findings | 41 (4.2) | 48 (2.0) | 2.3 (1.3–3.2) | 16 (1.9) | 35 (1.6) | 1.2 (0.5–1.9) | 12 (1.4) | 13 (0.6) | 2.4 (0.5–4.2) | 0.16 |
| Joint findings | 42 (4.3) | 46 (2.0) | 2.1 (1.2–3.1) | 21 (2.5) | 26 (1.2) | 1.9 (0.8–3.0) | 20 (2.3) | 21 (1.0) | 2.1 (0.8–3.4) | 0.91 |
| Neurologic findings | 43 (4.5) | 35 (1.5) | 2.7 (1.4–4.0) | 23 (2.7) | 16 (0.7) | 3.2 (1.1–5.2) | 13 (1.5) | 13 (0.6) | 2.0 (0.4–3.7) | 0.51 |
| Muscle findings | 44 (4.6) | 29 (1.2) | 4.6 (2.8–7 6) | 15 (1.8) | 8 (0.4) | 5.6 (2.3–13.7) | 5 (0.6) | 8 (0.4) | 1.7 (0.5–5.3) | 0.14 |
| Abdominal findings | 100 (10.4) | 150 (6.4) | 1.9 (1.3–2.4) | 56 (6.6) | 99 (4.6) | 1.6 (1.0–2.1) | 44 (5.1) | 68 (3.3) | 1.7 (1.0–2.4) | 0.67 |
| Eye examination findings‡ | ||||||||||
| Uveitis | 149 (26.4) | 77 (12.1) | 2.5 (1.7–3.2) | — | — | — | 172 (33.3) | 90 (15.4) | 2.5 (1.8–3.2) | 0.77 |
| Cataracts | 78 (13.8) | 81 (12.8) | 0.9 (0.6–1.2) | — | — | — | 71 (13.8) | 67 (11.4) | 0.9 (0.6–1.2) | 0.52 |
| Moderate-to-severe visual loss | 14 (2.5) | 12 (2.0) | 1.2 (0.2–2.2) | 16 (3.1) | 11(1.9) | 1.2 (0.3–2.2) | 0.54 | |||
| Blind | 10 (1.8) | 9(1.5) | 1.1 (0.1–2.2) | — | — | — | 12 (2.3) | 9(1.6) | 1.2 (0.1–2.2) | 0.46 |
All estimates are adjusted for age, sex, and relationship between contacts and survivors.
P value is for the interaction between follow-up visit and survivor status and indicates whether the odds ratio changed over time (P<0.01 was considered to indicate statistical significance).
Only a subset of participants underwent eye examinations. For the evaluation of uveitis and cataracts, data were available at baseline for 564 survivors and 635 controls, and data were available at 12 months for 516 survivors and 586 controls. For the evaluation of visual acuity, data were available at baseline for 556 survivors and 611 controls, and data were available at 12 months for 514 survivors and 573 controls.
Objective abnormalities on physical examination were less common than reported symptoms. Survivors had significantly (P<0.0001) higher percentages of abnormal findings on abdominal, chest, neurologic, muscle, and joint examination than did controls (Table 2, and Table S8 in the Supplementary Appendix).
Only a small percentage of survivors had physical findings suggestive of inflammatory arthritis or myositis, with the most common abnormal musculoskeletal findings being muscle tenderness (4.1% of survivors and 0.9% of controls), decreased joint range of motion (2.7% and 1.4%), and joint swelling (0.8% and 0.5%). The prevalence of muscle tenderness on examination was significantly higher among survivors than among controls (odds ratio, 4.35; 95% confidence interval [CI], 2.58 to 7.34; P<0.001), whereas joint swelling (odds ratio, 1.46; 95% CI, 0.58 to 3.65; P = 0.42) and decreased range of motion (odds ratio, 1.75; 95% CI, 1.04 to 2.93; P = 0.04) did not meet the criteria for significance imposed in this study. The most common abnormalities found on neurologic examination among survivors and controls were abnormal reflexes (1.4% and 0.7%, respectively), tremor (0.9% and 0.2%), gait or balance abnormalities (0.7% and 0.9%), speech abnormalities (0.7% and 0.2%), and cranial nerve abnormalities (0.7% and 0.1%). On abdominal examination, the most common abnormal findings noted among survivors and controls were tenderness (5.6% and 3.7%, respectively), mass (2.4% and 2.0%), and distension (1.9% and 1.4%). The most common abnormal chest findings on examination among survivors and controls were irregular pulse (1.0% and 0.5%, respectively), decreased breath sounds (0.9% and 0.4%), and heart murmur (0.9% and 0.3%). A complete list of the frequencies of individual abnormal findings is provided in Table S8 in the Supplementary Appendix. Evaluations of renal, hepatic, and hematopoietic laboratory measurements did not reveal any clinically relevant differences between survivors and controls at baseline (Table S9 in the Supplementary Appendix).
CLINICAL AND LABORATORY FINDINGS AT FOLLOW-UP VISITS
The prevalence of all targeted symptoms decreased at the 6- and 12-month follow-up visits among both survivors and controls (Table 2); however, the odds ratio (survivor vs. control) increased over time for headache and decreased for muscle pain. To assess the rate at which targeted symptoms and findings developed after the baseline visit, data from the 6- and 12-month visits were used to calculate the incidence of targeted symptoms and findings during the first year of follow-up. The incidence of new urinary frequency symptoms and abnormal chest and abdominal findings did not differ significantly between survivors and controls. The incidence of the other targeted symptoms and findings (other than joint and muscle) was higher among survivors than among controls (Table 3), although the overall prevalence decreased after baseline. Follow-up laboratory evaluations did not reveal any emerging differences between survivors and controls (Table S9 in the Supplementary Appendix).
Table 3.
Incidence of New Symptoms and Physical Examination Findings among Survivors and Controls.
| Symptom or Finding | New Cases | Incidence | Adjusted Odds Ratio | |||
|---|---|---|---|---|---|---|
| Survivors | Contacts | Survivors | Contacts | Estimate | P Value* | |
| number | cases/1000 person-yr | |||||
| Urinary frequency | 17 | 43 | 18.76 | 20.20 | 1.26 | 0.42 |
| Headache | 148 | 291 | 163.29 | 136.68 | 1.58 | <0.001 |
| Fatigue | 69 | 71 | 76.13 | 33.35 | 2.59 | <0.001 |
| Muscle pain | 126 | 234 | 139.02 | 109.91 | 2.25 | <0.001 |
| Memory loss | 48 | 5 | 52.96 | 2.35 | 23.82 | <0.001 |
| Joint pain | 120 | 202 | 132.40 | 94.88 | 2.34 | <0.001 |
| Chest findings | 18 | 39 | 19.86 | 18.32 | 1.35 | 0.30 |
| Joint findings | 21 | 21 | 23.17 | 9.86 | 1.86 | 0.04 |
| Neurologic findings | 18 | 9 | 19.86 | 4.23 | 3.55 | .0002 |
| Muscle findings | 10 | 9 | 11.03 | 4.23 | 3.49 | 0.01 |
| Abdominal findings | 57 | 115 | 62.89 | 54.02 | 1.28 | 0.18 |
| Uveitis† | 85 | 52 | 177.82 | 101.32 | 2.22 | <0.001 |
| Acute uveitis† | 18 | 10 | 37.66 | 19.48 | 3.04 | 0.02 |
| Chronic uveitis† | 79 | 48 | 165.27 | 93.53 | 2.00 | 0.001 |
The P values were adjusted for age, sex, site, and relationships between survivors and contacts (P<0.01 was considered to indicate statistical significance).
Uveitis results should be treated as a separate family of tests; between-group differences in eye sequelae were analyzed separately from differences in general symptoms and findings.
OPHTHALMOLOGIC FINDINGS
For participants in the longitudinal eye cohort, no significant differences in corrected visual acuity or age-adjusted prevalence of cataracts were noted between survivors and controls either at baseline or at the 12-month visit (Table 2). The median corrected visual acuity was 20/20 (interquartile range, 20/20 to 20/25) for both survivors and controls. On the basis of the World Health Organization criteria for visual impairment,19 the prevalence of moderate-to-severe visual impairment did not differ significantly between survivors and controls at baseline or at the 12-month follow-up visit (Table 2).
At baseline, 149 survivors (26.4%) had evidence of uveitis in at least one eye on ophthalmologic examination, as compared with 77 controls (12.1%) (P<0.0001). Among these participants, 30 survivors (5.3%) and 13 controls (2.0%) had active uveitis (P = 0.003). The prevalence of uveitis increased among both survivors and controls between baseline and the 12-month follow-up visit (Table 2). The incidence of new uveitis was significantly higher among survivors than among controls (Table 3).
ANALYSIS OF SEMEN FOR EBOV RNA
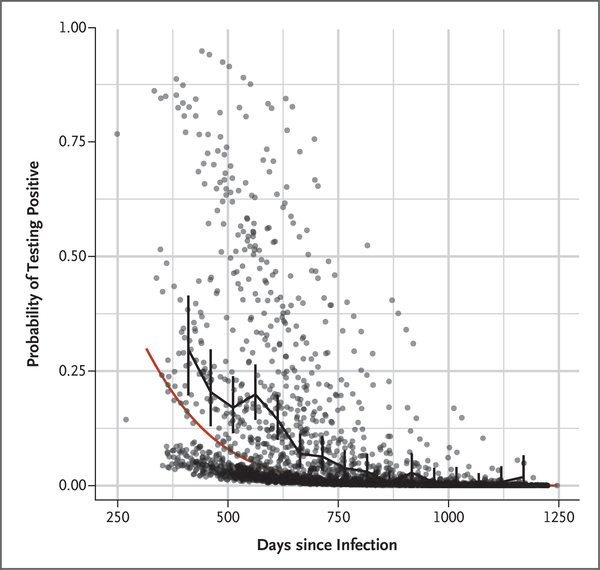
A total of 267 antibody-positive male survivors (median age, 33 years; interquartile range, 26 to 41) provided 2416 semen samples with which an EBOV RNA determination was successfully conducted (see Section 1.4 in the Supplementary Appendix). Viral RNA was detected in one or more semen samples in 81 men (30%) (Table S3 in the Supplementary Appendix). Among the 252 men who provided more than one semen sample, detection of viral RNA in semen was intermittent in 78 (31%), with 36 men having two negative tests followed by a positive test at least once. The time from acute EVD illness to first semen sampling ranged from 233 to 1178 days (median, 551 days [19 months]). A significant (P<0.0001) decline in the frequency of positive results over time was observed (Fig. 2). To date, the longest time from acute EVD illness to the detection of viral RNA in a semen sample is 40 months.
Figure 2. Frequency of Semen Samples Testing Positive for Ebola Virus RNA since the Time of Acute Infection.
Data points represent model-based estimates of the probability of testing positive for individual samples; vertical bars represent 95% confidence intervals for the probability of a sample being positive, based on samples grouped into 25-day bins; and the piecewise linear black curve shows the sample proportions in these bins. The red curve represents a model-based population trend for the probability of a sample testing positive for Ebola virus RNA.
We found no correlation between the persistence of viral RNA in semen and the symptoms shown in Table 2 (see Section 2.6 in the Supplementary Appendix). There was a positive association between the presence of uveitis and the detection of viral RNA in at least one semen sample (odds ratio, 2.62; 95% CI, 1.32 to 5.22).
DISCUSSION
Unlike other studies in which symptoms and conditions in the survivors of the West African Ebola outbreak have been described,3,5–7,9–11 our study was designed with a control group made up of participants who did not have EVD and who shared environmental exposures similar to those of the survivors. For our primary aim of estimating the difference in prevalence and incidence of symptoms and physical findings between survivors and controls, we were able to compare survivors who had serologic evidence of previous EBOV infection with survivor-reported close contacts who did not have serologic evidence of past infection, in order to minimize the potential effect of the uncertainty of the clinical and laboratory EVD diagnosis.
For survivors and controls, abnormal findings identified on general physical examination and laboratory evaluation were much less frequent than reported symptoms. The constellation of subjective symptoms that were reported more frequently by survivors was similar to what has been described in a number of non-EVD illnesses, including post-traumatic stress disorder (PTSD) in combat veterans20 and postinfection syndromes described after resolution of certain viral and nonviral infections.21–24 The pathogenesis of the symptoms reported by survivors of EVD is unclear and requires further study.
During the first year of follow-up, the prevalence of targeted conditions declined in both groups, and no new symptoms or findings emerged that met our criteria for targeted conditions. Several possible factors that are likely to have contributed to this decline include regression toward the mean, resolution of PTSD over time,25 interaction with a health care system, and resolution of tissue damage sustained during the acute EVD.
The 26% prevalence of uveitis is consistent with what has been reported in three previous studies of the West African outbreak.5,10,11 Our study differed in that we found a 12% prevalence of uveitis at baseline among controls and an increase in the prevalence of uveitis over time in both groups. The unexpectedly high incidence of uveitis in the control group highlights the importance of including population controls in a geographic region in which non-Ebola causes of uveitis are present26 and suggests that some cases of uveitis identified in EVD survivors may have been due to other diseases.
We found prolonged shedding of viral RNA in semen, a finding consistent with observations in other studies.15 However, our findings suggest that shedding of EBOV RNA in semen may be more frequent, intermittent, and persistent than previously reported.27 The semen testing protocol that we used did not stipulate discontinuation of testing after two negative results. Thus, we were able to show that shedding of viral RNA was frequently intermittent, with more than 30 men having two consecutive negative tests followed by one or more positive PCR tests for EBOV RNA. This finding suggests that current guidelines recommending discontinuation of semen testing in survivors after two negative results28 may need to be reevaluated.
We found a positive correlation between viral persistence in semen and detection of uveitis on baseline ophthalmologic examination. A previous study showed that a higher virus load during acute EVD was associated with the development of uveitis.5 This suggests that a high virus load during the acute illness may also be associated with persistence of EBOV RNA in semen. We are unable to directly address this hypothesis in our study because we have incomplete data on EBOV load at the time of acute illness. The biologic significance of the persistence of EBOV RNA in semen remains unclear. Transmission of Ebola infection through semen has been implicated in a small number of cases.14 However, the presence of viral RNA in semen does not necessarily indicate the presence of infectious virus.16
Our study had some limitations. Functional disability associated with the reported symptoms was not assessed; thus, we cannot define how these symptoms affected the lives of participants. General medical providers performed the physical examinations (with the exception of the ophthalmologic examination), and the examiners were aware of whether the participant was a survivor or a close contact. To partially address this limitation, we have initiated a neurology substudy within the PREVAIL III cohort to better evaluate possible neurologic sequelae. The lack of records from Ebola treatment units documenting the clinical manifestations during acute EVD prevents evaluation of associations between the severity of the acute illness and outcomes during convalescence. Close contacts were largely identified by survivors. This may have led to some bias in the identification of contacts with medical problems who hoped to benefit from the medical evaluations that were performed as part of the study. Finally, although we focused on targeted conditions and used strict criteria, many tests of significance were performed, including exploratory analyses, which increases the risk of type I error.
In summary, observations of survivors of EVD and close-contact controls in Liberia provided insights into the nature and severity of medical conditions specific to survivors of acute EVD. A relatively high burden of symptoms was seen in both survivors and close contacts, which emphasizes the importance of evaluating a control population.
Supplementary Material
Acknowledgments
Supported by the National Institute of Allergy and Infectious Diseases and the National Eye Institute.
Funded by the National Institute of Allergy and Infectious Diseases and the National Eye Institute; PREVAIL III ClinicalTrials.gov number, NCT02431923.
Footnotes
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A complete list of members of the PREVAIL III Study Group is provided in the Supplementary Appendix.
APPENDIX
The affiliations of the members of the writing committee are as follows: National Institutes of Health, Bethesda (M.C.S., R.J.B., L.E.H., E.S.H., A.N., K.T., J.V., K.S.J., B.D.-K., H.C.L.), and Johns Hopkins University, Ophthalmology, Baltimore (A.O.E.) — both in Maryland; Division of Biostatistics, University of Minnesota, Minneapolis (C.R., J.D.N.); and Liberian Ministry of Health (M.B., M.P.F.) and John F. Kennedy Medical Center (S.J.M.), Monrovia, the Duport Road Clinic, Paynesville (D.G.-D.), and C.H. Rennie Hospital, Kakata (K.L.J.) — all in Liberia.
REFERENCES
- 1.Ebola situation report. Geneva: World Health Organization, May 19, 2016. (https://www.who.int/csr/disease/ebola/situation-reports/archive/en/). [Google Scholar]
- 2.Clark DV, Kibuuka H, Millard M, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis 2015; 15:905–12. [DOI] [PubMed] [Google Scholar]
- 3.Etard JF, Sow MS, Leroy S, et al. Multidisciplinary assessment of post-Ebola sequelae in Guinea (Postebogui): an observational cohort study. Lancet Infect Dis 2017;17:545–52. [DOI] [PubMed] [Google Scholar]
- 4.Kibadi K, Mupapa K, Kuvula K, et al. Late ophthalmologic manifestations in survivors of the 1995 Ebola virus epidemic in Kikwit, Democratic Republic of the Congo. J Infect Dis 1999;179:Suppl 1:S13–S14. [DOI] [PubMed] [Google Scholar]
- 5.Mattia JG, Vandy MJ, Chang JC, et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis 2016;16:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanyonga M, Saidu J, Ramsay A, Shindo N, Bausch DG. Sequelae of Ebola virus disease, Kenema District, Sierra Leone. Clin Infect Dis 2016;62:125–6. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi AI, Chughtai M, Loua TO, et al. Study of Ebola virus disease survivors in Guinea. Clin Infect Dis 2015;61:1035–42. [DOI] [PubMed] [Google Scholar]
- 8.Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. J Infect Dis 1999;179:Suppl 1:S28–S35. [DOI] [PubMed] [Google Scholar]
- 9.Scott JT, Sesay FR, Massaquoi TA, Idriss BR, Sahr F, Semple MG. Post-Ebola syndrome, Sierra Leone. Emerg Infect Dis 2016;22:641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shantha JG, Crozier I, Hayek BR, et al. Ophthalmic manifestations and causes of vision impairment in Ebola virus disease survivors in Monrovia, Liberia. Ophthalmology 2017;124:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiffany A, Vetter P, Mattia J, et al. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis 2016;62:1360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wendo C Caring for the survivors of Uganda’s Ebola epidemic one year on. Lancet 2001;358:1350. [DOI] [PubMed] [Google Scholar]
- 13.Deen GF, Broutet N, Xu W, et al. Ebola RNA persistence in semen of Ebola virus disease survivors — final report. N Engl J Med 2017;377:1428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mate SE, Kugelman JR, Nyenswah TG, et al. Molecular evidence of sexual transmission of Ebola virus. N Engl J Med 2015; 373:2448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorson A, Formenty P, Lofthouse C, Broutet N. Systematic review of the literature on viral persistence and sexual transmission from recovered Ebola survivors: evidence and recommendations. BMJ Open 2016;6(1):e0 08859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uyeki TM, Erickson BR, Brown S, et al. Ebola virus persistence in semen of male survivors. Clin Infect Dis 2016;62:1552–5. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy SB, Bolay F, Kieh M, et al. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 2017;377:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettitt J, Higgs E, Fallah M, et al. Assessment and optimization of the GeneXpert diagnostic platform for detection of Ebola virus RNA in seminal fluid. J Infect Dis 2017;215:547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global data on visual impairments 2010. Geneva: World Health Organization, 2012. (http://www.who.int/blindness/GLOBALDATAFINALforweb.pdf). [Google Scholar]
- 20.Baker DG, Mendenhall CL, Simbartl LA, Magan LK, Steinberg JL. Relationship between posttraumatic stress disorder and self-reported physical symptoms in Persian Gulf War veterans. Arch Intern Med 1997;157:2076–8. [PubMed] [Google Scholar]
- 21.Carson PJ, Konewko P, Wold KS, et al. Long-term clinical and neuropsychological outcomes of West Nile virus infection. Clin Infect Dis 2006;43:723–30. [DOI] [PubMed] [Google Scholar]
- 22.Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and nonviral pathogens: prospective cohort study. BMJ 2006;333:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lettinga KD, Verbon A, Nieuwkerk PT, et al. Health-related quality of life and posttraumatic stress disorder among survivors of an outbreak of Legionnaires disease. Clin Infect Dis 2002;35:11–7. [DOI] [PubMed] [Google Scholar]
- 24.Wills AB, Spaulding AB, Adjemian J, et al. Long-term follow-up of patients with Lyme disease: longitudinal analysis of clinical and quality-of-life measures. Clin Infect Dis 2016;62:1546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995;52: 1048–60. [DOI] [PubMed] [Google Scholar]
- 26.Ronday MJ, Stilma JS, Barbe RF, Kijlstra A, Rothova A. Blindness from uveitis in a hospital population in Sierra Leone. Br J Ophthalmol 1994;78:690–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soka MJ, Choi MJ, Baller A, et al. Prevention of sexual transmission of Ebola in Liberia through a national semen testing and counselling programme for survivors: an analysis of Ebola virus RNA results and behavioural data. Lancet Glob Health 2016;4(10):e736–e743. [DOI] [PubMed] [Google Scholar]
- 28.Interim advice on the sexual transmission of Ebola virus disease. Geneva: World Health Organization, 2016. (https://www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/.) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.