Abstract
Background
Hypertensive nephrosclerosis is the second most common cause of end-stage renal failure in the United States. The mechanism by which hypertension produces renal failure is incompletely understood. Recent evidence demonstrated that an unscheduled and inappropriate increase in apoptosis occurred in the Dahl/Rapp rat, an inbred strain of rat that uniformly develops hypertension and hypertensive nephrosclerosis; early correction of the hypertension prevents the renal injury. The present study examined the role of the Fas/FasL pathway in this process.
Methods
Young male Dahl/Rapp salt-sensitive (S) and Sprague-Dawley rats were fed diets that contained 0.3% or 8.0% NaCl diets. Kidneys were examined at days 7 and 21 of the study.
Results
An increase in Fas and FasL expression was observed in glomerular and tubular compartments of kidneys of hypertensive S rats, whereas dietary salt did not change expression of either of these molecules in normotensive Sprague-Dawley rats. Associated with this increase was cleavage of Bid and activation of caspase-8, the initiator caspase in this apoptotic pathway, by day 21 of the study.
Conclusions
Augmented expression of apoptotic signaling by the Fas/FasL pathway occurred during development of end-stage renal failure in this model of hypertensive nephrosclerosis.
Background
Hypertension is a common medical problem in the United States, occurring in as many as 43 million individuals [1]. While end-stage renal failure represents the second most common cause of end-stage renal disease [2], perhaps only 1 in 2,500 hypertensive patients develops clinically important hypertensive nephrosclerosis. This low frequency of end-organ renal damage suggests a potential predisposition to this complication. The inbred Dahl/Rapp rat is a genetic model of salt-sensitive hypertension [3,4]. On a diet containing 8.0% NaCl, young Dahl/Rapp salt-sensitive (S) rats rapidly and uniformly manifest low-renin hypertension and die from hypertensive nephrosclerosis within weeks of institution of the high-salt diet [5]. The pathology is identical to human hypertensive renal disease and consists of arteriolosclerosis, glomerulosclerosis, and interstitial scarring with tubular cell dropout. While other animal models of hypertension can be employed to study the pathogenesis of hypertension, because of the rapid and reproducible development of renal failure, S rats provide a unique means to investigate the pathogenesis of hypertensive nephrosclerosis. In this respect, this model is very useful in understanding hypertensive nephrosclerosis that occurs in human low-renin hypertension, especially in defined, more homogeneous populations such as the black patients described by Grim and associates [6].
Recent studies have shown that apoptosis was accentuated in kidneys of S rats during development of nephrosclerosis [7]. These studies demonstrated increased numbers of TUNEL-positive cells, increased cytoplasmic nucleosome content and caspase-3 activation in kidneys of hypertensive S rats, compared to normotensive Sprague-Dawley control rats on the same high-salt diet. An increase in apoptosis rates in glomeruli and tubules was observed, confirming a process that involved several different cell types in the kidney. Apoptotic bodies were noted particularly in areas of glomerular sclerosis and in dilated tubules. An increase in the number of PCNA-stained cells was also demonstrated during the 21-day time frame, but because renal function deteriorated, renal failure correlated therefore positively with apoptosis and inversely with proliferation rates. Thus, the apoptotic process appeared to be a strong determinant of outcome in this model.
The Fas/FasL system transmits apoptotic signals from the surrounding environment into the cell. Fas contains a single transmembrane domain and belongs to the tumor necrosis factor (TNF)/nerve growth factor family [8]. FasL contains a single transmembrane domain and is also a member of the same TNF family [9]. A soluble form of FasL has been described, but appears to be less capable of inducing apoptosis, when compared with the bound form [10,11]. The binding of FasL with Fas initiates receptor oligomerization, which recruits Fas-associated death domain (FADD) [12]. FADD binds procaspase-8 and permits activation of caspase-8 through self-cleavage [13]. Caspase-8 activates the effector caspases, which commits the cell to the orderly process of apoptosis [14,15]. In addition, caspase-8 cleaves Bcl-2-interactive-death-agonist (Bid) [16]. Truncated Bid localizes to the mitochondria and promotes cytochrome c release; this process also serves as a major apoptotic-signaling pathway for Fas [16-18]. Depending upon the amount of caspase-8 that is activated, the predominant pathway can be either Bid cleavage with subsequent mitochondrial release of cytochrome c or activation of the effector caspase pathway [18]. Resident cells of the kidney express both Fas and FasL and Fas/FasL signaling is functional in these cells [19-24]. A recent review by Ortiz and associates [25] noted that the combined literature demonstrating participation of Fas/FasL pathway in renal injury essentially fulfilled Koch's postulates.
In kidneys of S rats, associated with apoptosis was a temporal increase in expression of Fas, which was demonstrated using Western analysis of lysates of kidney cortex [7]. The purpose of the present study was to demonstrate the site of expression and potential functional significance of Fas and FasL in the kidneys of S rats during development of hypertensive nephrosclerosis.
Materials and Methods
Animal preparation
Studies were conducted using 40 male Dahl/Rapp salt-sensitive (SS/Jr, termed S) and 40 male Sprague-Dawley (SD) rats, initially 28 days of age, obtained from Harlan Sprague Dawley, Indianapolis, IN. The rats were housed under standard conditions and given a formulated diet (AIN-76A, Dyets, Inc., Bethlehem, PA) that contained either 0.3% or 8.0% NaCl. These diets were prepared specifically to be identical in protein and electrolyte composition and differed only in NaCl and sucrose content. Groups of S and SD rats on 0.3% and 8.0% NaCl were maintained contemporaneously for each of the experiments performed. On days 7 and 21 of study, rats from all the groups were anesthetized by intraperitoneal injection of sodium pentobarbital (Abbott Laboratories, North Chicago, IL), 50 mg/kg. Laparotomy was performed and the kidneys were perfused in situ through the aorta with cold heparinized 0.9% NaCl solution until blanching occurred, which generally required infusion of 50 to 60 ml saline over 2 min. Both kidneys were harvested under sterile conditions to obtain protein for Western analysis and for light microscopy and histochemistry after fixation in 4% paraformaldehyde in PBS.
In situ detection of DNA fragmentation using TUNEL
In situ detection of DNA fragmentation was performed by incorporation of fluorescein-12-dUTP at the 3'-OH ends of DNA using Terminal deoxynucleotidyl Transferase (TUNEL assay) (Apoptosis Detection System, Fluorescein, Promega, Madison, WI), as performed previously by this laboratory [7,26]. Nuclei were counterstained using propidium iodide (Sigma Chemical Co., St. Louis, MO). For fluorescein, the excitation and barrier filters were set at 450–490 and 515–560 nm, respectively. Red fluorescence of propidium iodide was observed using excitation and barrier filters of 515–560 and 580 nm, respectively.
Immunohistochemistry
After embedding in paraffin, sections 5 μm in thickness were immersed twice into xylene for 5 min each, followed by immersion twice for 3 min each in 100% ethanol and then 95% ethanol. Slides were rinsed for 30 sec using deionized water and then immersed twice in deionized water for 5 min. To detect Fas, tissue sections were initially treated with 1% SDS in TBS (100 mM Tris-HCl, pH 7.4, 138 mM NaCl and 27 mM KCl) for 5 min at room temperature, followed by three 5-min washes in TBS alone. Slides were covered in 0.1–1.0% H2O2 for 5 min at room temperature and incubated in goat serum for 30 minutes at room temperature. Slides were incubated for 30 minutes at room temperature in PBS containing monoclonal mouse anti-human Fas/CD95/APO-1 (Transduction Laboratories, Lexington, Kentucky), 0.5–1.0 μg/ml, and 10% goat serum. Slides were rinsed and covered with TBS containing 10% rat serum and peroxidase-labeled polymer conjugated to goat anti-mouse IgG (DAKO EnVision™+ System, DAKO Corporation, Carpinteria, CA) for 30 minutes at room temperature and color was developed using 3,3'-diaminobenzidine (DAB) Chromogen solution (DAKO). Cells were counterstained using hematoxylin and the slides were mounted in standard fashion. As a negative control, the primary antibody was omitted from the reaction. As an additional control, the anti-Fas antibody was pre-incubated with recombinant soluble human Fas (PharMingen, San Diego, CA), 0.5 μg/ml, for 1 h at room temperature prior to use as the primary antibody to stain the specimens. To detect FasL, paraffin-embedded sections were initially heated twice at 95°C for 5 min in 10-mM sodium citrate buffer, pH 6.0. Staining then proceeded in standard fashion using goat polyclonal anti-FasL antibody (goat polyclonal antibody IgG raised against FasL(N-20), Santa Cruz Biotechnology, Inc., Santa Cruz, CA), 1–4 μg/ml, followed by biotinylated donkey anti-goat IgG. Color was developed using the avidin-biotin detection system (ImmunoCruz Staining Systems, Santa Cruz Biotechnology, Inc.).
Immunoenzyme double staining of kidney tissue was performed using DAKO EnVision Doublestain System (DAKO). The sections were initially heated twice at 95°C for 5 min in 10-mM sodium citrate buffer, pH 6.0, and then incubated for 30 min with the mouse anti-Fas IgG (Transduction Laboratories), 1:500 dilution in PBS containing 1% BSA. The sections were incubated in horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse immunoglobulins for 30 min and developed with 3,3'-diaminobenzidine (DAB) Chromogen solution (DAKO). After incubation for 10 min in the Doublestain Block (DAKO) at room temperature, the specimens were incubated for 30 min with the goat anti-FasL IgG (Santa Cruz Biotechnology, Inc.), 1:50 dilution in PBS containing 1% BSA. The samples were then incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG and goat anti-mouse IgG for 30 min. The slides were developed using alkaline-phosphatase substrate to produce the red color. The sections were then counterstained using hematoxylin before study.
The specimens were examined using a light microscope (Leica, Germany) equipped with a digital camera (Model C5810, Hamamatsu Photonics K.K.). Semi-quantification of stained tubular epithelial cells was determined at ×20 magnification by projecting the image and counting the number of positive cells; a total of six different fields were analyzed for each kidney and the results were averaged to produce a final count for each kidney. Quantification of staining of glomerular compartment was determined at ×20 magnification by counting the number of stained cells in twenty-five glomeruli.
Immunoblot analysis
Samples of renal cortex from S and SD rats on the 0.3% and 8.0% NaCl diets for 7 and 21 days were diced into small pieces and chilled in 3 ml of ice cold RIPA buffer per gram of tissue, then homogenized (Omni-Mixer 17105; Omni, Waterbury, CT) in standard fashion. Reagents were from Sigma Chemical Co. The solutions were mixed gently and incubated on ice for 30 min, then homogenized by passage through 21-gauge needle. Thirty μl of phenylmethylsulfonyl fluoride (PMSF), 10 mg/ml, was added per gram of tissue and incubated on ice for 30 min. Samples were centrifuged at 15,000 ×g for 20 min at 4°C and the supernatant was harvested. Total protein of each sample was determined using a kit (Micro BCA protein assay reagent kit, Pierce, Rockford, IL.). For each experiment that examined a protein of interest, all samples were processed simultaneously. Samples containing 60 μg of total protein were mixed with an equal volume of 2x SDS-Laemmli sample buffer (100 mM Tris-HCl, pH 6.8, 20% glycerol, 200 mM dithiothreitol, 4% SDS, and 0.2% bromphenol blue) and boiled for 5 min, then resolved using either 12% SDS-polyacrylamide gel electrophoresis. Proteins were transferred on to nitrocellulose membrane using an electroblotting apparatus (Bio-Rad, Richmond, CA). Following incubation in blocking buffer (10 mM Tris-HCl, pH 7.5, containing 10% non-fat dry milk, 100 mM NaCl, and 0.1% Tween 20), the membranes were probed with an affinity-purified rabbit polyclonal IgG directed against caspase-8 (Caspase-8 p20, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), 1:200 dilution in blocking buffer, overnight at 4°C. The membranes were then washed five times with TBS-Tween and were incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG (Bio-Rad, Hercules, CA), 1:5,000 dilution. After three additional washes using TBS-Tween, the membrane was developed using ECL Western blotting system and Hyperfilm (Amersham Pharmacia Biotech, Piscataway, NJ). The antibody used in these studies recognized bands of the expected size for pro-caspase-8 (about 55 kDa) and active caspase-8 (about 20 kDa). In other experiments, samples containing 80 μg total protein underwent SDS-PAGE using 15% polyacrylamide gels. The samples were transferred on to nitrocellulose membranes, which were incubated in blocking buffer (TBS, pH 7.5, containing 10% dried milk and 0.1% Tween-20) and then rabbit anti-Bid antiserum (BD PharMingen, San Diego, CA), 1:2000 dilution in the same blocking buffer, at 4°C, overnight. Following incubation with donkey anti-rabbit IgG conjugated with horseradish peroxidase (Santa Cruz Biotechnology Inc. Santa Cruz, CA), 1:6000 dilution, for 1 h at room temperature, the membrane was developed as described above.
Statistical analysis
All data were presented as mean ± standard error. Significant differences were determined using one-way analysis of variance with standard post-hoc testing (Statview, version 5.0, SAS Institute, Cary, NC). A P value of < 0.05 assigned statistical significance.
Results
Kidneys of S and SD rats on both 0.3% and 8.0% NaCl diets were examined on days 7 and 21. The blood pressure responses to dietary salt intake of the Dahl/Rapp S rat, an experimental model of hypertensive nephrosclerosis, and of the SD strain of rat have been published. After seven days on the high salt diet, S rats were hypertensive (146 ± 2 mm Hg) but had preserved renal morphology [5]. By day 21 on the 8.0% NaCl diet, the mean blood pressure increased to 169 ± 7 mm Hg [7,27,28]. Mean blood pressures of SD rats were not influenced by intake of salt over this time frame of study (118 ± 4 mm Hg on the 8.0% NaCl diet, compared to 115 ± 4 mm Hg on the 0.3% NaCl diet). In addition, SD animals were healthy, the intake of either diet was similar, and they demonstrated no renal abnormalities [27-29]. In the present study, by day 21 the kidneys of S rats demonstrated significant vascular, glomerular and tubulo-interstitial injury. TUNEL demonstrated apoptosis in glomerular and tubular compartments (Fig 1). SD rats on both diets and S rats on 0.3% NaCl diet demonstrated by light microscopy no renal morphological alterations over the same period of study. These findings were similar to previous publications from this laboratory [5,7].
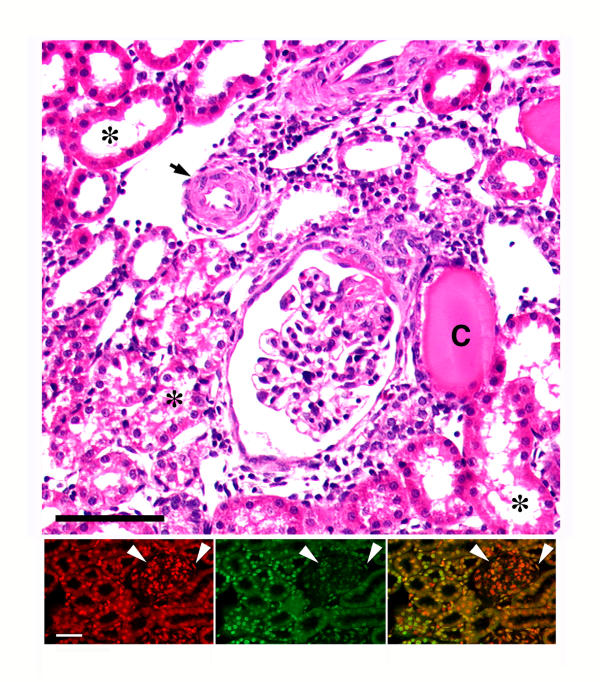
Figure 1.
Representative light micrograph (top panel) of a kidney from an S rat that had been on 8.0% NaCl diet for three weeks. The figure shows typical hypertensive renal lesions, which include thickening of the arteriolar wall (arrow), glomerular sclerosis, and dilatation of tubules with tubular atrophy (asterisks) and presence of cast material (C) in some tubules. Expansion of the interstitium, indicated by an increase in the distance between the tubules, was also evident. H/E stain. Black bar represented 50 μm. The dual immunofluorescent studies at the bottom show how exuberant the apoptotic process can be in this model. The bottom left panel represents a section that was counterstained with propidium iodide to label the nuclei (red color). The middle panel is the same section that was stained using the TUNEL assay, which fluorescein-labels the 3'-OH termini of DNA. Several nuclei in the tubular and glomerular compartments demonstrate the green fluorescence. The bottom right panel is a combined image; the yellow color is the result of overlapping propidium iodide and fluorescein labeling. White bar represented 50 μm. Arrowheads denote glomerulus.
Using immunohistochemistry, expression of Fas was increased specifically in kidneys of S rats on 8.0% NaCl diet by day 7 (Fig. 2). Staining was inhibited when the anti-Fas antibody was pre-incubated with recombinant soluble Fas (Fig. 2E and 2J). Quantification analysis comparing the four groups of rats in the study showed that expression of Fas in glomerular and tubular cells was greatest (P < 0.05) in kidneys of S rats exposed to the 8.0% NaCl diet for 7 and 21 days (Fig. 3). Comparing animals on the two diets for the same duration, dietary salt did not alter Fas expression in kidneys of SD rats. The increase in Fas expression in kidneys of S rats on the 8.0% NaCl diet was observed in cells in the glomeruli, tubules and arteries (Fig. 4A,B,C). Cytoplasmic staining was particularly apparent in these samples.
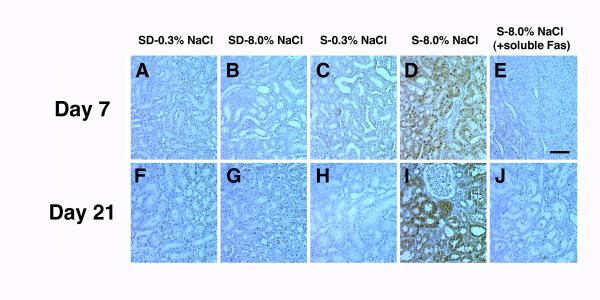
Figure 2.
Representative images of immunohistochemical stains of kidney sections from each of the group of rats in the study. While Fas staining was evident in kidneys from SD rats on both diets (panels A, B, F, and G) and S rats on 0.3% NaCl (panels C and H), an increase in staining was observed in kidneys from S rats on 8.0% NaCl diet (panels D and I). Staining was diminished when the antibody was pre-incubated with soluble Fas (panels E and J). The bar represented 50 μm.
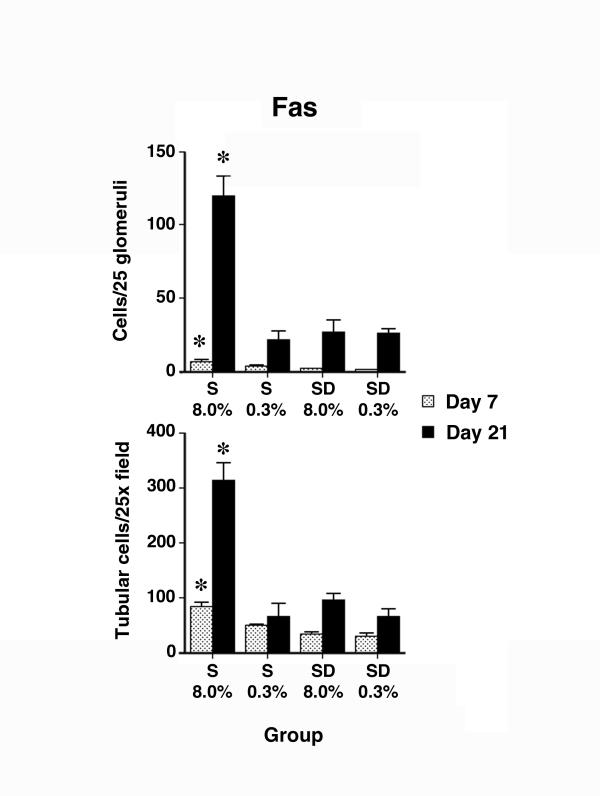
Figure 3.
Semi-quantitative analysis of Fas staining showed an increase (P < 0.05) in expression of Fas by glomerular and tubular cells from rats on 8.0% NaCl diet for 7 and 21 days (n = 6 rats in each group), compared to the other groups of rats examined at the same time. Asterisks denote the group that was different (P < 0.05) than the other three groups from the same day of study. The increase was prominent on day 21 of the diet.
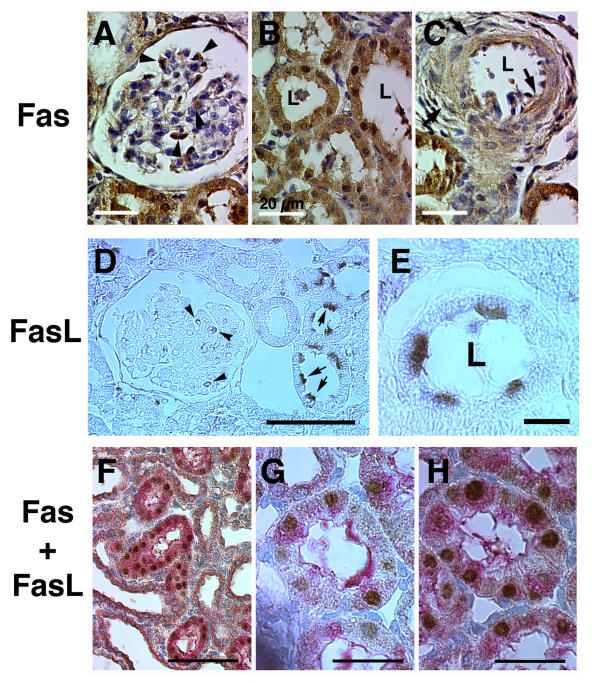
Figure 4.
Representative higher magnification images of immunohistochemical stain using antibodies to Fas and FasL. Fas stain was noted in cells in glomeruli (arrowheads, panel A), tubules (panel B) and arterial smooth muscle (arrows, panel C). White bar represented 20 μm; L, lumen. FasL expression was observed in glomerular (panel D) and tubular (panel E) cells, where expression was prominent particularly on the lumenal side. Black bars represented 50 and 10 μm, respectively. Co-expression of both Fas (brown color) and FasL (red color) was identified in some tubular cells (panels F, G and H). Bar in panel F represented 100 μm and represented 50 μm in panels G and H.
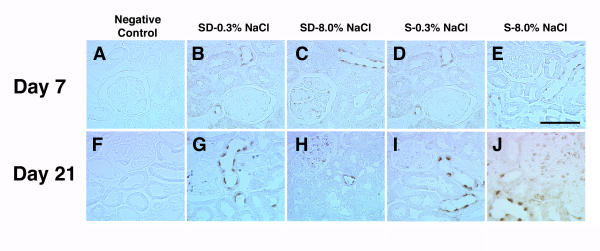
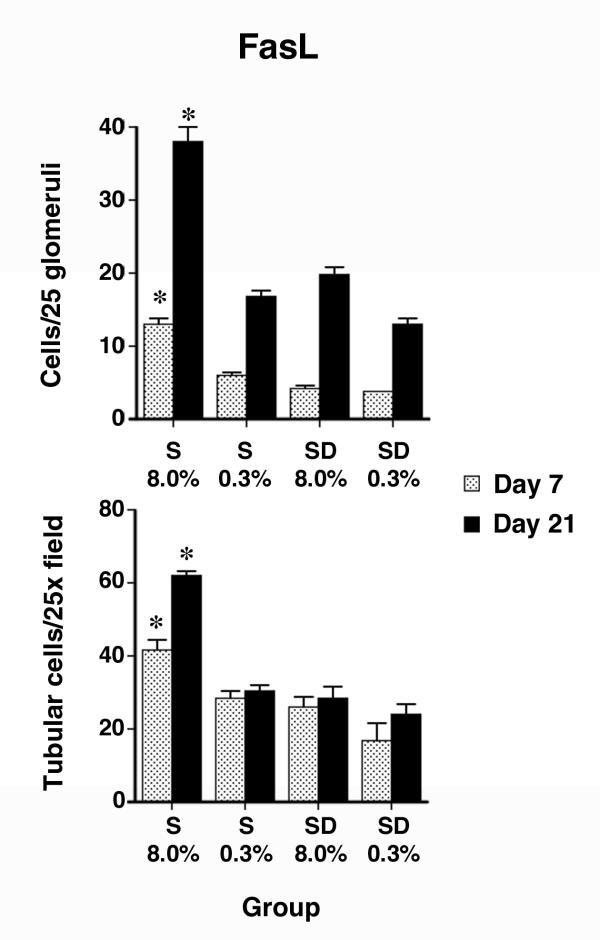
Over the same time period, FasL expression was also examined (Fig. 5A,B,C,D,E,F,G,H,I,J). The anti-FasL antibody labeled cells in both glomerular and tubular compartments, but expression was more subtle than Fas, particularly in the glomeruli. Quantification of these observations showed an increase in expression of FasL in both glomerular and tubular compartments of kidneys of S rats on the 8.0% NaCl diet, compared with the other three groups (Fig. 6). Again, dietary salt did not alter glomerular and tubular expression of FasL in kidneys of SD rats. Tubular epithelial cell expression of FasL appeared to be predominantly apical (Fig. 4D and 4E), although cytoplasmic staining was also observed, particularly in cells of S rats on the 8.0% NaCl diet. In addition, some tubular epithelial cells of kidneys from S rats on 8.0% NaCl diet for 21 days appeared to express both Fas and FasL (Fig. 4F,G and 4H).
Figure 5.
Representative images of immunohistochemical stains using antibody to FasL. Panels A and F represent negative controls, where the primary antibody was omitted from the reaction. Expression of FasL did not appear to be altered by dietary salt in the SD rats, but increased in S rats on the 8.0% NaCl. The effect was pronounced by the third week of study. Expression was observed in both glomerular and tubular compartments of the kidney. Black bar represented 50 μm.
Figure 6.
Semi-quantitative analysis of FasL expression in the kidney (n = 6 rats in each group). Compared with the other three groups examined at the same time in the study, an increase (P < 0.05) in FasL staining was observed in glomerular and tubular cells of S rats on the 8.0% NaCl diet. Asterisks denote the group that was different (P < 0.05) than the other three groups from the same day of study. The increase was prominent by the third week of study.
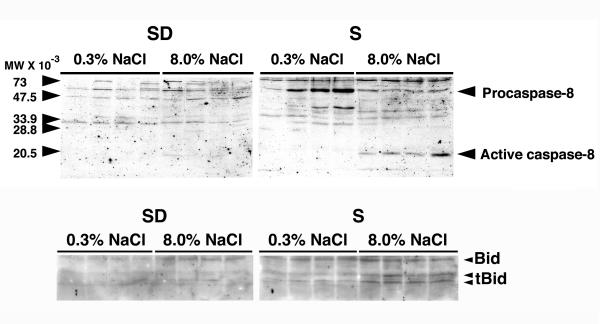
To determine whether the Fas/FasL apoptotic pathway was active, Western blotting of renal cortical lysates was performed using an antibody that recognized both procaspase-8 and the active form of caspase-8. In these experiments, active caspase-8 was demonstrated in lysates from renal cortex of S rats on 8.0% NaCl diet for three weeks (Fig. 7). Active caspase-8 was not demonstrated in samples from rats on the diet for 7 days (data not shown). Tissue was also examined for cleavage of Bid, which was identified specifically in lysates from renal cortex obtained from S rats on 8.0% NaCl diet for three weeks (Fig. 7).
Figure 7.
The top panel showed a Western blot using an anti-caspase-8 polyclonal IgG, which recognized both procaspase-8 and the active enzyme. Active caspase-8 (about 20 kDa) was observed only in kidney cortex of S rats on the 8.0% NaCl diet for 21 days. The bottom panel showed a Western blot that used an anti-Bid polyclonal IgG, which also recognized the 13 and 15 kDa cleaved fragments of Bid (tBid). Fragments of Bid (tBid) were apparent in lysates of renal cortex of S rats on the 8.0% NaCl diet for 21 days. Each lane represented a single rat (n = 4 rats in each group).
Discussion
Recent evidence suggests that apoptosis is involved in several pathological processes in the lung [30-32] and kidney [33-37]. Using the Dahl/Rapp salt-sensitive (S) rat, which develops hypertensive nephrosclerosis that resembles the human disease process [5], this laboratory demonstrated an early and inappropriate apoptotic process that occurred in both glomerular and tubular compartments of the kidneys and correlated with the decline in glomerular filtration rate. Apoptosis can be significant particularly three weeks into the course of hypertensive nephrosclerosis in this model (Figure 1). A previous study used RNase protection assay and Western blotting to demonstrate an increase in Fas expression in kidney cortex of hypertensive S rats. These findings were observed by day 21 of study [7]. The present series of experiments demonstrated the diffuse sites of expression of Fas and FasL in the kidney and explored further the possibility that the Fas/FasL pathway was active during the development of hypertensive renal disease in this rodent model of hypertension. Expression of both Fas and FasL was observed in kidneys of both SD and S rats, but specifically increased over time in hypertensive S rats. Associated with the increased expression was cleavage of procaspase-8 to produce the active enzyme and cleavage of Bid, indicating activation of the Fas/FasL apoptotic pathway in the course of progressive renal failure. These data, along with the previous studies that confirmed morphological evidence of apoptosis and biochemical evidence of caspase-3 activation in these kidneys [7], showed a role for the Fas/FasL pathway in promoting the loss of kidney function that develops in the setting of hypertensive nephrosclerosis in this model.
The mechanism of augmented Fas and FasL expression in kidneys of hypertensive S rats is uncertain. In addition to apoptotic signaling, the Fas/FasL system may also be involved in proliferation. Hueber, et al., showed that Fas might promote T-cell proliferation by modulating release of calcium from intracellular stores [38]. FasL is anti-proliferative by regulating cell-cycle progression [39]. Previous studies demonstrated an increase in cell proliferation rates in kidneys of S rats on the high-salt diet [7], so it is possible that Fas and FasL may play a role in control of proliferation. Another potential explanation is tissue hypoxia, which has been suggested to upregulate Fas in the kidney [36,37]. MDCK cells in culture demonstrated increased expression of Fas, FasL and FADD in response to partial ATP depletion [40]. Fas and FasL expression in the kidney may serve as indicators of cellular stress. Tissue hypoxia therefore remains a possible mechanism of increased expression of Fas and FasL, although it seems less likely that the arteriolosclerosis that developed in S kidneys was severe enough to produce hypoxic conditions sufficient to up-regulate Fas, until the third week of study.
Increased expression of Fas and FasL appeared to increase the propensity to undergo apoptosis, but was not the sole determinant of this process. Despite the increase in expression of Fas and FasL at day 7 of study, caspase-8 activation and Bid cleavage were not observed. Fas/FasL-mediated apoptotic signaling is modulated by other intracellular factors. Induction of inhibitor of apoptosis proteins (IAP) is one such mechanism [41-43]. In vascular smooth muscle cells, surface expression of Fas [44] and molecules involved in signaling the apoptotic process, such as caspase-8 and caspase-3, appear to be regulated and provide another level of cellular control of apoptosis [45]. However, the potential anti-apoptotic mechanisms present early in the course were ineffective by day 21, when caspase-8 activation was evident. A previous study suggested that the apoptotic process occurred early in the development of hypertension in this model [26], so the data suggested that an apoptotic mechanism in addition to activation of the Fas/FasL pathway is also involved in development of hypertensive nephrosclerosis in S rats.
In conclusion, kidneys of S rats have been shown to be very sensitive to hypertensive injury, which is mediated at least in part by the apoptotic mechanism controlled by Fas. Augmented expression of both Fas and FasL by glomerular and tubular cells resulted in caspase-8 activation. The pronounced increases in Fas and FasL expression and activation of this pathway by the third week of study also correlated with previous evidence that documented irreversibility of the renal injury if the hypertension was not corrected by this time [5]. These data supported the view that the Fas/FasL pathway promoted nephrosclerosis in this model. Activation of the Fas/FasL pathway may also serve as a mechanism of tubular epithelial cell loss and interstitial fibrosis, which are also prominent renal features of hypertension. The role of the Fas/FasL pathway in human hypertensive nephrosclerosis remains to be determined.
Abbreviations
SD, Sprague-Dawley rat
S, Dahl/Rapp salt-sensitive rat
Fas, Fas antigen (or CD95 and APO-1)
FasL, Fas antigen ligand (or CD95L and APO-1L)
PBS, phosphate-buffered saline
RIPA, radioimmunoprecipitation assay
SDS, sodium dodecyl sulfate
TBS, Tris-buffered saline
Bid, Bcl-2-interactive-death-agonist.
Competing interests
None declared
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgments
National Institutes of Health grant (R01 DK46199) and the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, supported this work. The authors thank Ms. Karen Lewis for the excellent technical support.
Contributor Information
Paul W Sanders, Email: psanders@uab.edu.
Pei-Xuan Wang, Email: bwang@nrtc.dom.uab.edu.
References
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the third national health and nutrition examination survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- U.S Renal Data System. USRDS 2001 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, Bethesda, MD.
- Rapp JP. Dahl salt-susceptible and salt-resistant rats. Hypertension. 1982;4:753–763. doi: 10.1161/01.hyp.4.6.753. [DOI] [PubMed] [Google Scholar]
- Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension. 1985;7:340–349. [PubMed] [Google Scholar]
- Chen PY, St. John PL, Kirk KA, Abrahamson DR, Sanders PW. Hypertensive nephrosclerosis in the Dahl/Rapp rat: initial sites of injury and effect of dietary L-arginine administration. Lab Invest. 1993;68:174–184. [PubMed] [Google Scholar]
- Grim CE, Wilson TW, Nicholson GD, Hassell TA, Fraser HS, Grim CM. Blood pressure in blacks. Twin studies in Barbados. Hypertension. 1990;15:803–809. doi: 10.1161/01.hyp.15.6.803. [DOI] [PubMed] [Google Scholar]
- Ying W-Z, Wang P-X, Sanders PW. Induction of apoptosis during development of hypertensive nephrosclerosis. Kidney Int. 2000;58:2007–2017. doi: 10.1046/j.1523-1755.2000.00373.x. [DOI] [PubMed] [Google Scholar]
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S-I, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045–2050. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Holler N, Bodmer J-L, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/S0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science (Wash DC) 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Salveson GS, Dixit VM. Caspases: Intracellular signaling by proteolysis. Cell. 1998;91:443–446. doi: 10.1016/S0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu C-j, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin K-M, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cuadrado S, Lorz C, del Moral RG, O'Valle F, Alonso C, Ramiro F, Ortiz-Gonzalez A, Egido J, Ortiz A. Agonistic anti-Fas antibodies induce glomerular cell apoptosis in mice in vivo. Kidney Int. 1997;51:1739–1746. doi: 10.1038/ki.1997.239. [DOI] [PubMed] [Google Scholar]
- González-Cuadrado S, López-Armada M-J, Gómez-Guerrero C, Subirá D, Garcia-Sahuquillo A, Ortiz-Gonzalez A, Neilson EG, Egido J, Ortiz A. Anti-Fas antibodies induce cytolysis and apoptosis in cultured human mesangial cells. Kidney Int. 1996;49:1064–1070. doi: 10.1038/ki.1996.155. [DOI] [PubMed] [Google Scholar]
- Schelling JR, Nkemere N, Kopp JB, Cleveland RP. Fas-dependent fratricidal apoptosis is a mechanism of tubular epithelial cell deletion in chronic renal failure. Lab Invest. 1998;78:813–824. [PubMed] [Google Scholar]
- Schelling JR, Cleveland RP. Involvement of Fas-dependent apoptosis in renal tubular epithelial cell deletion in chronic renal failure. Kidney Int. 1999;56:1313–1316. doi: 10.1046/j.1523-1755.1999.00684.x. [DOI] [PubMed] [Google Scholar]
- Lorz C, Ortiz A, Justo P, González-Cuadrado S, Duque N, Gómez-Guerrero C, Egido J. Proapoptotic Fas ligand is expressed by normal kidney tubular epithelium and injured glomeruli. J Am Soc Nephrol. 2000;11:1266–1277. doi: 10.1681/ASN.V1171266. [DOI] [PubMed] [Google Scholar]
- Takemura T, Murakami K, Miyazato H, Yagi K, Yoshioka K. Expression of Fas antigen and Bcl-2 in human glomerulonephritis. Kidney Int. 1995;48:1886–1892. doi: 10.1038/ki.1995.487. [DOI] [PubMed] [Google Scholar]
- Ortiz A, Lorz C, Egido J. The Fas ligand/Fas system in renal injury. Nephrol Dial Transplant. 1999;14:1831–1834. doi: 10.1093/ndt/14.8.1831. [DOI] [PubMed] [Google Scholar]
- Ying W-Z, Sanders PW. Cytochrome c mediates apoptosis in hypertensive nephrosclerosis in Dahl/Rapp rats. Kidney Int. 2001;59:662–668. doi: 10.1046/j.1523-1755.2001.059002662.x. [DOI] [PubMed] [Google Scholar]
- Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W-Z, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-β;1 in rat aortic endothelium. Am J Physiol. 1999;277:H1293–H1298. doi: 10.1152/ajpheart.1999.277.4.H1293. [DOI] [PubMed] [Google Scholar]
- Ying W-Z, Sanders PW. Expression of Tamm-Horsfall glycoprotein is regulated by dietary salt in rats. Kidney Int. 1998;54:1150–1156. doi: 10.1046/j.1523-1755.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, Hara N. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest. 1999;104:13–19. doi: 10.1172/JCI5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrao KL, Fortenberry JD, Owens ML, Harris FL, Brown LAS. Neutrophils induce apoptosis of lung epithelial cells via release of soluble Fas ligand. Am J Physiol Lung Cell Mol Physiol. 2001;280:L298–L305. doi: 10.1152/ajplung.2001.280.2.L298. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Kuwano K, Hagimoto N, Matsuba T, Kunitake R, Tanaka T, Maeyama T, Hara N. Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor. Am J Pathol. 2000;157:597–603. doi: 10.1016/S0002-9440(10)64570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H, Kashihara N, Makino H, Yamasaki Y, Ota Z. Apoptosis in glomerular sclerosis. Kidney Int. 1996;49:103–111. doi: 10.1038/ki.1996.14. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Andoh TF, Pichler RH, Shankland SJ, Couser WG, Bennett WM, Johnson RJ. Accelerated apoptosis characterizes cyclosporine-associated interstitial fibrosis. Kidney Int. 1998;53:897–908. doi: 10.1046/j.1523-1755.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- Savill J, Mooney A, Hughes J. Apoptosis and renal scarring. Kidney Int. 1996;49:S-14–S-17. [PubMed] [Google Scholar]
- Nogae S, Miyazaki M, Kobayashi N, Saito T, Abe K, Saito H, Nakane PK, Nakanishi Y, Koji T. Induction of apoptosis in ischemia-reperfusion model of mouse kidney: Possible involvement of Fas. J Am Soc Nephrol. 1998;9:620–631. doi: 10.1681/ASN.V94620. [DOI] [PubMed] [Google Scholar]
- Khan S, Cleveland RP, Koch CJ, Schelling JR. Hypoxia induces renal tubular epithelial cell apoptosis in chronic renal disease. Lab Invest. 1999;79:1089–1099. [PubMed] [Google Scholar]
- Hueber A-O, Zörnig M, Bernard A-M, Chautan M, Evan G. A dominant negative Fas-associated death domain protein mutant inhibits proliferation and leads to impaired calcium mobilization in both T-cells and fibroblasts. J Biol Chem. 2000;275:10453–10462. doi: 10.1074/jbc.275.14.10453. [DOI] [PubMed] [Google Scholar]
- Desbarats J, Duke RC, Newell MK. Newly discovered role for Fas ligand in the cell-cycle arrest of CD4+ cells. Nature Med. 1998;4:1377–1382. doi: 10.1038/3965. [DOI] [PubMed] [Google Scholar]
- Feldenberg LR, Thevananther S, del Rio M, de Leon M, Devarajan P. Partial ATP depletion induces Fas- and caspase-mediated apoptosis in MDCK cells. Am J Physiol. 1999;276:F837–F846. doi: 10.1152/ajprenal.1999.276.6.F837. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature (Lond) 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- Erl W, Hansson GK, de Martin R, Draude G, Weber KSC, Weber C. Nuclear factor-κB regulates induction of apoptosis and inhibitor of apoptosis protein-1 expression in vascular smooth muscle cells. Circ Res. 1999;84:668–677. doi: 10.1161/01.res.84.6.668. [DOI] [PubMed] [Google Scholar]
- Wang C-Y, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-κB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science (Wash DC) 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Bennett M, Macdonald K, Chan S-W, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science (Wash) 1998;282:290–293. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- Chan S-W, Hegyi L, Scott S, Cary NRB, Weissberg PL, Bennett MR. Sensitivity to Fas-mediated apoptosis is determined below receptor level in human vascular smooth muscle cells. Circ Res. 2000;86:1038–1046. doi: 10.1161/01.res.86.10.1038. [DOI] [PubMed] [Google Scholar]