1. Introduction
Neurofibromatosis Type 1 (NF1) is an autosomal dominant disorder affecting 1 in 3000 to 4000 people, caused by mutations in the NF1 gene on chromosome 17 in humans [97]. Diagnosis of NF1 is made on a clinical basis when two or more of the following diagnostic criteria are present: six or more café au lait spots; freckling in axillary or groin areas; presence of plexiform neurofibromas or more than one cutaneous or subcutaneous neurofibroma; bony dysplasia with or without pseudoarthrosis [35]. NF1 is a multisystem disorder, affecting not only cognitive development, but also muscle, bone, and nerve constitution [91]. Major disease complications affect the nervous system, skin, and bones, manifesting among other symptoms, in café au lait patches, skin fold freckling, Lisch Nodules, orthopedic complications, cutaneous and subcutaneous neurofibromas with approximately 10% chance of malignant transformation [91], malignant peripheral nerve sheath tumors, cognitive impairment and learning deficits, arterial stenosis, epilepsy, macrocephaly, optic pathway obstruction, gastrointestinal complications and short stature [35]. NF1 patients exhibit neurological deficits such as sensory loss, weakness or tingling due to tumor-dependent neuropathy [91]. In addition to these commonly described symptoms of NF1, patients also report worsened mental health, sleep, and pain as a result of the disorder; however, chronic pain is a severely understudied phenomenon of NF1 [14; 34; 44; 89]. The prevalence of pain in NF1 patients is unknown but quality of life-based questionnaires in Australia, South America, Europe, and North America consistently identify both the intensity and quality of pain as having a major impact on NF1 patients [8; 21; 55; 90; 111; 112].
Of the handful of studies reporting NF1-related pain demographics, there is no consensus in terms of percentages of NF1 patients experiencing pain. While some studies report that 29% of NF1 patients report pain [22], others report that upwards of ~70% do so [36]. One in 4 persons with NF1 experience chronic pain that can persist for months to years and characterized as a painful peripheral sensory neuropathy [64]. While ~70% of children and adults with NF1 use prescription pain medications [22], pain is often overlooked [22; 111]. Many studies reported pain as a key symptom of NF1 patients affecting their quality of life [22; 65; 111]. There are no approved treatments for NF1 pain. In fact, NF1 patients report that opioids were not beneficial and increased their ongoing pain levels [15; 98]. It has been reported that children with NF1 taking pain medication rated their pain higher than those not on pain medications [84]. A survey from the National Cancer Institute reported 63% of the patient’s families hope for more clinical trials aiming at the management of the pain related to NF1 [111].
The etiology of NF1 pain is unknown. Pain as a symptom of NF1 plays a role in creating not just physical agony but psychological and social distress that accompanies the NF1 diagnosis, subsequently lowering the quality of life of these patients [1; 2; 21; 66; 112]. In the physical domain of the NF1 disease, pain is considered a significant issue [8]; again however, this symptom is often overlooked. While most of the research on NF1 focuses on the tumor component of the disease, pain remains largely understudied. Even organizations created for raising NF1 awareness make rare mention of this obviously present symptom of the disease; the Children’s Tumor Foundation (CTF) for example, organizes an annual meeting spearheading the future of NF1 research. The published annual reports from the NF conference have only sporadic mentions of pain in NF1. This review is aimed at increasing the awareness of pain in NF1 patients by synthesizing an overview of the most important topics in basic and applied pain research as it relates to NF1.
2. NF1-induced pain and headache: Anatomy and Location
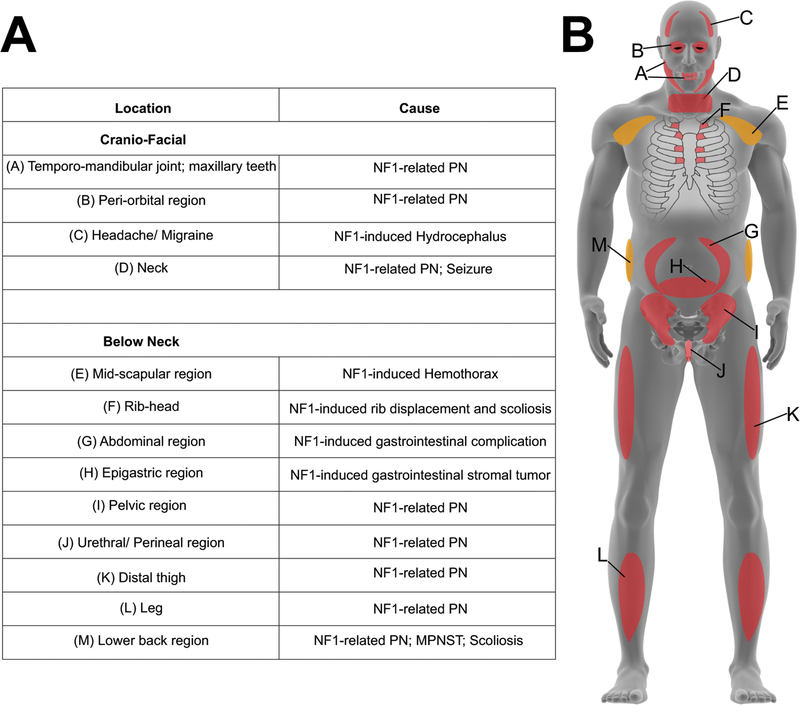
Symptoms of NF1 known to cause chronic pain include plexiform and subcutaneous neurofibromas, gastrointestinal complications such as gastrointestinal stromal tumors, and orthopedic symptoms like scoliosis and pseudoarthrosis [35; 97]. Benign causes of NF1 pain can become life-threatening with the manifestation of malignant peripheral nerve sheath tumors (MPNSTs), derived from benign plexiform neurofibromas or subcutaneous neurofibromas [22; 79]. Cutaneous and subcutaneous neurofibromas have an ~10% chance of malignant transformation [91]. As a result of these symptoms, NF1 pain can arise throughout the body. NF1 case reports have shown NF1-related pain in the abdominal [44; 92], midscapular [89], rib head [17], lower back [34; 37], appendage [48], epigastric [60], ocular [57], craniofacial [97], neck [14; 99], temporomandibular and maxillary [97], distal thigh and leg [29; 68], pelvic [88], perineal, and urethral areas [6] (Figure 1). From the clinical cases alluded to in this review, it can be gathered that NF1-pain is most commonly associated with tumors, specifically in the form of nerve sheath tumors, gastrointestinal stromal tumors, or optic gliomas, and orthopedic problems such as bone deformations – these complications are among the most common of NF1 symptoms. Few cases of polyneuropathies in NF1 have been described, and in most of these instances the pain results from involvement of multiple sites of origin. These clinical data unequivocally identify the most common cause of NF1 pain to be tumor-related or related to bone deformations, and therefore, the heterogenous nature of these tumors as well as the diverse painful experiences of these NF1 patients must be considered in pain-centered NF1 research.
Figure 1. Anatomical indication of reported NF1 pain.
(A) Table showing body regions indicated in NF1 case reports as having NF1-related pain. (B) Human model representing anatomy of implicated in NF1 pain reports. Red color represents anterior regions; orange color represents posterior regions.
Additionally, NF1 patients have reported sciatica [16], and very commonly, headaches [1; 25; 97]. These included tension-type headaches, chronic idiopathic headaches, analgesic-abuse headaches and migraines. In comparison to the 14% of non-NF1 children with migraines, 54% of NF1 children report migraines [85] and in adults, ~70–80% report headaches [1; 36]. Of these individuals, 83% report migraines, and 11% report chronic daily headaches [1]. Equally important, NF1-related neuropathy and similarly neuropathic pain is present in these patients, and thus research into NF1-neuropathic pain pathophysiology is needed for effective pain therapies [91]. NF1-pain is comorbid with sleep, gastrointestinal health, and overall life satisfaction [36]. Apart from case reports demonstrating physical pain location, not much has been reported as to how NF1 pain arises in these locations.
3. NF1 pain from a biopsychosocial approach
It is apparent that NF1 patients suffer psychological consequences of the disease in the form of anxiety, sleep disorders, and a lower overall quality of life [53; 66; 82]. NF1 pain worsens this already present social hinderance. Crawford et. al demonstrated the effects of NF1 pain in heightening emotional symptoms such as anxiety, stress, and low mood in Australian adults between 18 and 40 years of age [21]. Ninety-four total interviews varying in length between 30 and 80 minutes anecdotally revealed that from NF1-related pain, patients had both low mood and interference with daily functioning. In terms of pain-NF1 comorbidities, it was shown that in NF1 patients, there is an inverse relationship between pain and life satisfaction (−0.30), a positive correlation between pain and sleep problems (0.48), and a positive correlation between pain and gastrointestinal problems (0.35) [36], again stressing the importance of a better understanding of pain in the NF1 population. Pain emotional symptoms were especially prevalent in women, indicating a gender dimorphism in the prevalence and experience of NF1 pain. In fact, in a study of 142 NF1-patients (88 women and 54 men), Fjermestad and colleagues reported that women with NF1 have more mental health, sleep, and pain-related complications, including bloating, than men with NF1 [36]. Furthermore, women with NF1 experienced more musculoskeletal pain than controls; however, there was no difference in musculoskeletal pain felt by men with NF1 and controls. Additional demographic evaluation showed a negative correlation between socioeconomic status and bodily pain experienced by children with NF1 [56]. Pain specifically as a result of plexiform neurofibromas impairs daily functioning (pain interference), including social-emotional functioning, in children with NF1, even with consumption of pain medications, and greater pain interference was associated with a variety of psychosocial tendencies such as internalizing problems, depression, anxiety, and socialization difficulties, reiterating the social aspect of pain symptomology [112]. Furthermore, the manner in which parents of children with NF1 react to their child’s physical complaints can contribute additional social factors to the NF1 pain experience [9]. It is also known that patients with depressive symptoms show a higher prevalence and severity of pain, portraying the psychological connection with the physical symptom of pain [4]. Specifically, Allen et. al, investigated the relationship between heart rate variability (HRV), the variability in time elapsed between heartbeats that measures autonomic nervous system function, and pain symptoms. It was found that NF1 patients with chronic pain exhibit lower HRV, which is linked to poor adaptability and psychological flexibility, leading to greater pain interference [2; 22]. This data suggests a link between chronic pain seen in NF1 patients and enhanced pain interference, a social hinderance. Thus, a biopsychosocial approach to NF1 pain is necessary to understand this symptom of NF1.
4. Mechanisms of Nociception – NF1 Pain
Surprisingly, what still remains largely uncertain is the molecular basis of NF1 pain signaling. Current research has proposed several animal models for studying NF1-related pain, including those in rodents, mouse and recently in miniswine [78; 80; 106].
4.1. Mouse Models of NF1
Several mouse models of NF1 have been developed and characterized for among other effects, musculoskeletal and developmental defects. As mice with homozygous deletion of the Nf1 gene died during embryonic development due to heart failure and edema secondary to developmental cardiac vessel defects, the haploinsufficient Nf1+/− mouse model has become the standard mouse model for biochemical, electrophysiological, and behavioral testing [10; 51]. These heterozygous Nf1+/− mice have already been characterized for some non-pain symptoms of NF1. Silva and colleagues investigated the effects of the heterozygous Nf1 mutation on learning and memory via testing in the Morris water maze [95]. After 10 days of training, in comparison to the wildtype mice, the Nf1+/− mice spent less time in the training quadrant and wild type mice crossed the exact site where the platform had been more often than the Nf1+/− mice. Silva and group concluded that spatial learning and memory is impaired in Nf1+/− mice. Since neuropsychological studies show that NF1 patients can improve learning via remedial training, Silva and group repeated these learning and memory studies via Morris water maze test with 14 days of training instead of 10. They showed that this augmented training was able to rescue the learning deficits seen in the NF1+/− mice with only ten days of training [95].
Apart from mice exhibiting Nf1 heterozygosity via targeted deletion, mice with heterozygosity for Nf1 and p53 [19], another tumor suppressor gene, have been generated. These mice are known to express MPNSTs, linked to pain as a result of nerve obstruction by NF1-related tumors [24; 87]. Additional mouse models include those with exon specific knockout mice for both exon 23a [20] and exon 9a [41] of the Nf1 gene; Nf1 chimeric mice produced via adoptive transfer [58] or embryonic injection of a small number of Nf1−/− cells [19]; and conditional Nf1 knockout mice produced via Cre-LoxP technology [41; 113]. Cre-LoxP technology has also been used to achieve cell-specific knockout of Nf1 in astrocytes, Schwann cells, and CNS neurons [41; 113], but never specifically in dorsal root ganglia neurons to our knowledge. It is known that dorsal root ganglia play a key role in nociceptive signaling [40], and thus, this presents an unexplored area of NF1 pain research. Of the available mouse models, only traditional Nf1+/− mouse models as described by Brannan and Jacks [10; 51] have ever been studied for implications on NF1-pain.
4.2. Evaluation of pain-related behaviors in the Nf1+/− heterozygous model
In Nf1+/− mice, O’Brien and colleagues investigated the effects of NF1 heterozygosity [10] on pain and itch behaviors [80]. Aside from a slight inhibition of formalin-induced nocifensive behavior, Nf1 heterozygosity did not enhance pain or itch behaviors induced by capsaicin or nerve growth factor; nor did it alter histamine-dependent or histamine-independent scratching behaviors, despite evidence for hyperexcitability of neurons. Furthermore, both development and maintenance of cold allodynia were not altered by NF1 heterozygosity in this mouse model of NF1.
Using the same strain of Nf1+/− mice, White and colleagues investigated the role of the Nf1+/− genotype for its ability to exhibit a nociceptive phenotype in male and female mice, and additionally in the presence and absence of an exogenous inflammatory agent [107]. In the absence of injury, there were no differences in thermal paw withdrawal latencies by genotype or by gender. In terms of mechanical sensitivity, female Nf1+/− mice exhibited a slight increase in sensitivity compared with their wild type counterparts; however, this trend did not persist in the male mice. In terms of heat hyperalgesia evoked by exogenous agents, capsaicin significantly increased heat hyperalgesia in both female Nf1+/− mice and wildtype mice, and significantly increased heat hyperalgesia in male Nf1+/− mice but not in the wildtype male mice. However, post-capsaicin paw withdrawal latencies in all of these mice were comparable, and thus, capsaicin-induced heat hyperalgesia is comparable in both genotypes and genders. Calcitonin gene related peptide (CGRP)-induced heat hyperalgesia exhibited genotype-specific behavior. While CGRP injection produced heat hyperalgesia in both genotypes and genders, both male and female Nf1+/− mice had significantly greater decreases in paw withdrawal latencies compared to their wildtype counterparts. In terms of mechanical sensitivity, although female Nf1+/− and wildtype mice experienced equivalent mechanical sensitivity post-capsaicin injection, male Nf1+/− mice experienced less mechanical sensitivity than male wild type mice post-capsaicin injection. CGRP injection yielded gender-based differences in resulting mechanical sensitivity. While female Nf1+/− mice experienced less mechanical hypersensitivity than female wildtypes, male Nf1+/− mice experienced more mechanical hypersensitivity than male wildtypes [107].
White and group also measured spontaneous pain behavior as a result of injection of capsaicin or formalin in female and male Nf1+/− wild type mice [107]. In the case of capsaicin, both females and males exhibited comparable spontaneous behavior in duration of licking of the hind paw between genotypes. In the case of formalin, female Nf1+/− mice experienced a lower duration of licking in the second phase in comparison to the female wildtype mice. However, there was a difference in formalin-induced spontaneous behavior between genotypes in male mice. In regard to other formalin-induced behaviors, such as guarding, unweighting, and flinching, female Nf1+/− mice exhibited more of these behaviors than their wildtype counterparts, but again, there was no difference for these behaviors between genotypes in male mice. Furthermore, White and group quantified levels of CGRP mRNA and receptor activity-modifying protein-1 mRNA, known to be rate-limiting for the CGRP receptor. However, there were no statistically significant differences in either transcript level across genders or genotypes.
While the Nf1+/− mice have been instructive in illustrating pain behaviors accompanying heterozygosity of Nf1, they are nevertheless encumbered by the finding that the mice used in both pain studies [80; 107] did not present tumors at the time that pain testing was done. These mice models exhibit a large discordance in comparison to the human NF1 phenotype with the lack of visible tumor masses during pain testing. Additionally, while the Brannan NF1 mouse exhibits leukemia-related tumors, these are not the plexiform neurofibromas, malignant peripheral nerve sheath tumors, or optic gliomas that are present in patients with NF1. Similarly, Jacks and group too describe a Nf1 mouse predisposed to pheochromocytomas and myeloid leukemia tumors, also present in NF1 patients. Again, these mouse models fail to exhibit the malignant peripheral nerve sheath tumors or optic gliomas very commonly present in human NF1 patients. This is a big limitation of the models since in humans neurofibromas and the presence of MPNSTs seems to have an important role in the development of pain. Apart from these two studies, other Nf1 mouse models have not been used for pain behavior testing. Since pain is a significant debilitating symptom of NF1, further investigations of pain behaviors in mice used in these above studies and all available mouse models is imperative.
4.3. Nf1 haploinsufficient mice exhibit increased excitability, ion channel remodeling, and have increased neuropeptide release.
From a molecular mechanistic perspective, not much is known about the molecular signaling responsible for the onset and potentiation of NF1 pain. In an attempt to test the effect of heterozygous loss of the Nf1 gene in sensory neurons, which are known to be involved in nociceptive signaling, Wang and group isolated sensory neurons found to be capsaicin-sensitive from Nf1+/− and wild type mice, finding a greater number of action potentials in the neurons from Nf1+/− mice (14 APs) than the wild type mice (5 APs)[103; 104]. The firing threshold – the membrane voltage at which the first AP is generated, was significantly decreased in neurons from Nf1+/− mice, indicating that neurons from these mice are capable of generating action potentials in a more hyperpolarized state than their wild type counterparts. Similarly, the firing latency, or time from onset of current injection to the initiation of the action potential was greatly lowered in neurons from Nf1+/− mice. Important too, the minimum amount of current required to elicit an action potential (i.e. the rheobase) was three times lower in neurons from Nf1+/− mice than in those from wild type mice [103; 104]. However, neither input resistance, nor average resting membrane potential was different between the two genotypes. These data show that capsaicin-sensitive sensory neurons from Nf1+/− mice have greater excitability compared to their wild type counterparts.
Since the protein neurofibromin is a known guanosine triphosphatase activating protein, for Ras, Wang and group hypothesized that the overactivation of the Ras pathway in the absence of functional neurofibromin was a likely cause for this sensory neuronal excitability; because nerve growth factor (NGF) is a neurotrophic factor that activates the Ras transduction cascade, Wang and group investigated the effects of NGF on capsaicin-sensitive sensory neurons from Nf1+/− mice and wild type mice. It is hypothesized that transcription-dependent or post translational modification of the Ras pathway may be responsible for the hyperexcitability of Nf1+/− neurons [39]. In wild type mice, NGF elicited a concentration-dependent increase in the number of action potentials fired by neurons; however, this effect did not persist in neurons from Nf1+/− mice, as there was no significant difference in the number of action potentials fired by neurons from Nf1+/− mice in the presence or absence of NGF. Again, both firing latency and rheobase were lowered as a result of treatment with NGF in neurons from wild type mice, but this was not the case in neurons from Nf1+/− mice. Wang and colleagues thus concluded that NGF treatment alters excitability in capsaicin-sensitive neurons of wild type mice in a way that mimics the inherent hyperexcitability present in neurons from Nf1+/− mice.
To determine the mechanism underpinning the hyperexcitability of sensory neurons of the Nf1+/− genotype, Wang and group examined differences in specific membrane currents, with relation to modulation of sensory neurons [103] because ion channel modulation can affect the firing pattern of sensory neurons. For potassium currents, the current density-voltage relations and biophysical properties of activation and inactivation for both peak and steady-state total potassium currents were comparable across genotypes. To then compare the contribution of IA-like potassium currents to these phenomenon, Wang and group subtracted the slowly inactivating IK trace from the more rapidly inactivating trace to obtain a rapidly inactivating current that has many of the hallmarks of IA. Although not every small-diameter sensory neuron of either genotype exhibited this A-type current, peak A-type current values as well as activation/inactivation relations were again similar in neurons from both genotypes. Thus, Wang and group concluded that rapidly inactivating delayed-rectifier (IA) like potassium currents are likely not responsible for the hyperexcitability in sensory neurons from Nf1+/− mice. It should be noted however that generalized hyperexcitability in this mouse model would imply a generalized pain condition that does not match typical clinical presentation in NF1 patients. Nonetheless, these data may begin to uncover the mechanism of heightened pain sensation in NF1 patients.
Since modulation of sodium channels can influence the firing patterns of sensory neurons, Wang and group measured total, TTX-sensitive (TTX-S), and TTX-resistant (TTX-R) sodium currents in sensory neurons from Nf1+/− and wild type mice [103]. The peak value for total sodium current in neurons from Nf1+/− mice was higher in comparison to neurons from wildtype mice. Current density for TTX-S sodium current was also significantly larger in Nf1+/− neurons than in wildtype neurons. Although the voltage dependence for activation of TTX-S sodium current did not differ between genotypes, the half-maximal voltage for steady state inactivation of these currents was shifted to more depolarized values; this shift, could be responsible for the hyperexcitability of the neurons from Nf1+/− mice. Along these lines, current density for TTX-R sodium current was also significantly larger in Nf1+/− neurons than in wildtype neurons. Again, the voltage dependence for activation of TTX-R sodium current did not differ between genotypes, but the half-maximal voltage for steady state inactivation of these currents was shifted to more depolarized values [103].
To examine this phenomenon in the genotype-related difference in the inactivation of both TTX-S and TTX-R sodium currents, Wang and group measured the persistent sodium current under control recording conditions and with treatment of TTX, thereby measuring TTX-R sodium currents. While persistent sodium current values in control conditions of neurons from Nf1+/− mice were larger, this trend did not persist with TTX-R sodium currents, with comparable values across genotypes. Taken together, their data suggested that the Nf1+/− genotype could alter the inactivation properties of sodium channels responsible for total sodium current, and this phenomenon could explain the hyperexcitability of sensory neurons from Nf1+/− mice.
Since sodium channels were implicated in the hyperexcitability of sensory neurons from NF1 haploinsufficient mice, Hodgdon et. al investigated whether this was due to increased levels of sodium channel mRNAs [47]. A relative gene expression analysis approach indicated that mRNAs for NaV1.1, NaV1.3, NaV1.7, and NaV1.8 were elevated significantly in sensory neurons from Nf1+/− mice in comparison to the wildtype, while expression of mRNAs of NaV1.2, NaV1.5, NaV1.6, and NaV1.9 was not.
While these studies highlight ion channel remodeling and heightened excitability of sensory neurons, the mechanisms that promote NF1 pain remain unclear, due in part, to the relative lack of suitable animal models. Work with Nf1+/− transgenic mice led to the hypothesis that sensitization of small-diameter nociceptive sensory neurons [72] may explain pain in NF1 patients. Consistent with this possibility, small-diameter capsaicin-sensitive sensory neurons isolated from these mice showed increased peak current densities for both TTX-S and TTX-R sodium channels [103] and N-type voltage-gated Ca2+ (CaV2.2) channel [26; 102], which resulted in augmented excitability [103; 104] and increased stimulus-evoked release of the nociceptive neuropeptide calcitonin gene-related peptide (CGRP) [46]. In line with the latter observations, sensory neurons from Nf1+/− mice were reported to have elevated stimulus-evoked release of neuropeptides, substance P and CGRP, in comparison to wildtype neurons [45]. While these findings were encouraging in suggesting a significant contribution of sensitization of nociceptors between neurofibromin expression and pain, behavioral studies with Nf1+/− mice were inconsistent with reports of both increased and decreased pain sensitivity in male mice (summarized above), and without changes in sensitivity to acute nociceptive stimuli, or in models of inflammatory, or neuropathic pain [80; 107]. An additional complication was an apparent sex-dependence in which female, but not male, Nf1+/− mice were reported to be hyperalgesic [64]. These disparate results have prevented clear understanding of the possible contribution of NF1 to pain.
4.4. Rat Models of NF1
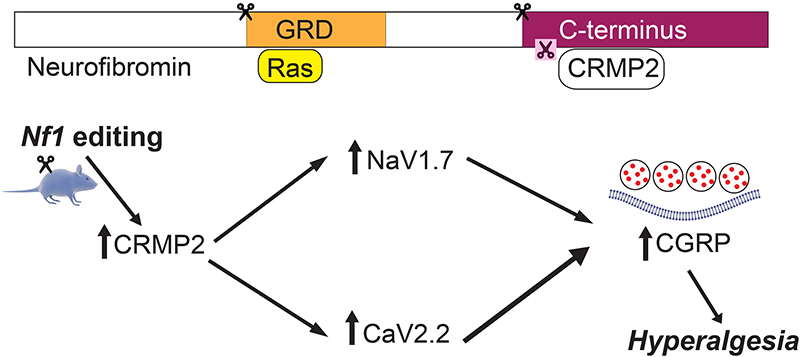
Over 3000 pathogenic allelic variants have been reported in Nf1 [69; 110] with at least 80% of NF1 patients expressing a C-terminal truncated neurofibromin [32; 43; 94; 101; 109]. We hypothesized that modifying the Nf1 gene rather than deleting one allele would recapitulate a pain phenotype allowing for mechanistic investigation of pain relevant to NF1 patients [78]. Since the current mouse models have shown less than consistent results, we proposed an alternative model for NF1 pain in rats. In this model, truncation of neurofibromin was achieved by acute clustered regularly interspaced short palindromic repeats (CRISPR) associated protein-9 nuclease (Cas9) editing of the Nf1 gene in adult rats. Specifically, in rats, the CRISPR/Cas 9 gene editing system to intrathecally deliver guide RNA Cas 9 nuclease plasmid to achieve targeted truncation of the neurofibromin protein’s C terminal. Increases in voltage-gated sodium and calcium channel currents in sensory neurons of rats with truncated neurofibromin and subsequent neuronal hyperexcitability and behavioral hyperalgesia were noted, thereby setting up this as a suitable model to study Nf1-related pain in rats [78]. Moutal and group found a unique link between cytosolic protein collapsin response mediator protein 2 (CRMP2), responsible for regulating the trafficking and activity of voltage-gated ion channels (CaV2.2; NaV1.7) [18; 27] and neurofibromin [77]. As altered expression of α subunits of CaV2.2 had not been observed in Nf1+/− mice [26], Moutal and colleagues examined neurofibromin-dependent pathways that might regulate CaV2.2 activity. CRMP2 binds directly with CaV2.2 leading to increased Ca2+ current density and increased neurotransmitter release in sensory neurons [18]. Wild type neurofibromin inhibits this function of CRMP2 and inhibits calcium influx through voltage-gated calcium channels, and subsequent calcium-driven nociceptive neurotransmission. Truncated neurofibromin fails to do so, and thus results in nociception. CRMP2, also interacts with the C-terminal domain of neurofibromin [61; 83] so that loss of neurofibromin increases CRMP2 phosphorylation [83], which in turn, increases its association with CaV2.2 [13; 75]. Additionally, CRMP2 was reported to control the tetrodotoxin-sensitive (TTX-S) Na+ voltage gated sodium channel NaV1.7 [28], a major determinant of nociception [59] whose activity is increased in NF1 [103]. In contrast to the increased TTX-R sodium currents observed in DRGs from Nf1+/− mice [103], DRGs from the CRISPR/Cas9 Nf1 editing model did not exhibit any changes in TTX-R currents [78]. While the lack of congruence in the rat CRISPR-Cas9 induced Nf1 modification and the heterozygous mouse model has not been explored, it has been previously demonstrated that TTX-R sodium currents are not under the direct regulation of CRMP2 [27]. Nevertheless, this mismatch between the models remains an unexplored weakness and which scenario predominates in the NF1 patients is also not currently known.
In the absence of functional neurofibromin, Moutal and group showed that CRMP2 is available to bind snare protein syntaxin 1A and facilitates greater release of pro-nociceptive neurotransmission via release of calcitonin gene-related peptide [76]. Moutal and colleagues’ data suggest an important role for CRMP2 in the development of NF1-related pain, and even posit that CRMP2 is necessary for the onset of NF1 pain [71]. Small interfering RNA knockdown of CRMP2 is sufficient to reverse both voltage-gated ion channel dysregulation and neurotransmitter release induced by NF1 gene editing [73]. Using this new rat model of NF1, they established CRMP2 as a central protein contributing, in consort with neurofibromin, to CaV2.2 and NaV1.7 ion channel dysregulation and to hyperalgesia. Given that over 70% of children with NF1 use pain medications [22] and NF1-related pain appears to be resistant to opioids, we propose the use of rat models of Nf1-editing to test the translational targeting of CRMP2, CaV2.2, NaV1.7 and CGRP as novel strategies to manage NF1-related pain (Figure 2).
Figure 2. Convergent signaling involving CRMP2 and Neurofibromin controls CaV2.2 and NaV1.7 activity and NF1-related pain.
Our previous work identified a direct binding between CaV2.2 and CRMP2 leading to a CRMP2-mediated increase in Ca2+ current density and increased CGRP release in sensory neurons [11; 12]. Loss of CRMP2/neurofibromin interaction increases CRMP2 phosphorylation and binding to CaV2.2 and NaV1.7 [77; 83].
Using CRISPR/Cas9 to induce neurofibromin truncations allows gene editing in adult animals and only in regions relevant to pain. This approach exclusively models the nociceptive phenotype of NF1 patients without the cognitive impairment described in the transgenic mouse model [70; 95]. A methodological weakness of this model is that it assumes that Nf1 single guide RNA plasmids injected intrathecally would exert effects solely on DRG neurons which in turn influences pain behavior. However, it is well established that neurofibromin is also abundantly expressed in the rodent spinal cord and thus it is conceivable that the effects of Nf1 sgRNA plasmid injection intrathecally could have exerted effects not only on DRG neurons but also second order sensory neurons in the spinal cord [23; 38; 42; 63].
Another limitation is that Nf1 gene editing is likely to impact both alleles of the gene [86]. While NF1 patients only have one allele of the gene mutated, inactivation or loss of the second allele (loss of heterozygosity, LOH) was confirmed as the second hit necessary for the development of NF1-related symptoms (neurofibromas [7; 93], café au lait macules [30], MPNST [33; 96]). Put another way, while the studies by Moutal and colleagues [76] represent an intriguing use of genomic editing to model an important rare disease involving a dominant allele, the homozygous condition of the CRISPR edited NF1 in mice will result in a doubling of the dominant “antimorphic” functions of which the consequence to the pathology in mice may not be representative of the patient pathology. A more exact model would be single copy gene truncation possibly via cre-loxp introduction of a stop codon in a cell-type specific manner. However, homozygous editing of Nf1 using CRISPR/Cas9 may still be a relevant strategy to model neuron related NF1 symptoms (such as pain) as it was done before in a mouse model of NF1 cognitive disorder [81].
4.5. Porcine Models of NF1
Most recently, White et. al characterizes a novel porcine model of NF1 with heterozygous deletion of exon 42 to study NF1-dervied pain [106]. In conjunction with dysregulation of voltage-gated ion channels involved in the pain phenotype of NF1, this porcine model phenocopies the human NF1 disease with many of the clinical manifestations of NF1 including café au lait spots, neurofibromas, axillary freckling, and even neurological deficits in learning and memory, and will perhaps serve as a tool to understand the molecular signaling of pain in human NF1 patients. White and group use histopathology to show the increase in pigmentation of the transgenic miniswine. In addition to this, White and group highlight the deletion’s effect on perturbing the Ras pathway, by demonstrating an increase in Ras signaling by way of upregulation of both p53 and p21 in NF1 +/ex42del cells. In learning and memory analyses, White and group utilized a T-maze test, similar to tests used for learning and memory in rodents, to identify learning deficits in the miniswine. At 9–10 months of age, the miniswine exhibited learning deficits by less accurately choosing the correct reward arm in comparison to their wildtype counterparts. Additionally, swine were observed to have anxious and hyperactive tendencies, and this was displayed by their reticence in interacting with unfamiliar objects. Use of histopathology again showed results similar to those seen in NF1 patients in the formation of cutaneous neurofibromas in the swine. Furthermore, examination of both calcium and sodium signaling in NF1 mutant swine neurons showed increased depolarization-evoked calcium influx and increased sodium current densities in the mutant neurons. These data suggest a dysregulation of both calcium and sodium signaling in the NF1 mutant swine neurons, recapitulating what was reported in the rodent models of Nf1.
Yet another porcine model of NF1, developed by Isakson and colleagues [50], offers similar phenotypic findings in relation to NF1. These minipigs too exhibit phenotypic similarities to NF1 patients including café au lait macules, neurofibromas, and optic pathway gliomas [50]. This minipig model was generated with a mimicked recurrent nonsense mutation (R1947*) in the swine NF1 gene sharing 100% amino acid identity with human exon 39. Transcription activator-like effector nucleases flanking NF1R1947* were transfected into fetal Ossbaw minipig fibroblasts. Colonies derived from single cells were then isolated and genotyped for the NF1R1947* mutation and the heterozygous clones were subjected to chromatin transfer resulting in two viable pregnancies with eight F0 male piglets heterozygous for the NF1R1947* mutation. These mutant F0 piglets were bred with wildtype sows and exhibited germline transmission of the mutant Nf1 allele. From birth, all NF1 piglets demonstrated café-colored skin patches with hyperpigmentation resembling café au lait macules present in human NF1 patients; in contrast, this was not present in wildtype pigs. NF1 minipigs exhibited superficial tumors resembling neurofibromas by four months of age; these tumors had a Ki67 proliferative index and showed mast cell infiltration sharing again the classic features of human neurofibromas. A symptom present in 15–20% of children with NF1, is optic gliomas. One of 7 NF1 minipigs exhibited this phenotype. Finally, administration of Mek-inhibitor PD0325901 at a 0.79mgkg−1 oral dose was detected to a great extent in the plasma of NF1 minipigs over their wildtype counterparts proving that these minipigs could serve as a preclinical model for pharmacokinetic and pharmacodynamic analysis of targeted NF1 therapies. Although data from these pig models offer unique insights into the signaling involved in NF1-derived pain, the miniswine have not been yet tested for pain. But since miniswine are more similar to humans than mice/rats in anatomy, physiology, and genome, there is an urgent need for further investigation of NF1 pain in this porcine model. It is our expectation that this miniswine NF1 model will serve as a link for the development of effective pre-clinical therapies to transition to the clinical setting to manage NF1-related pain.
5. Current therapies for NF1-derived pain
The complex pathology of NF1 pain calls for targeted therapies for NF1-derived pain; however, specific treatments for NF1 pain are scarce, again supporting the need to understand NF1-related pain and current strategies to alleviate this symptom of NF1. Current therapies include over-the-counter (OTC) medications such as ibuprofen and acetaminophen, but these do not substantially counteract symptoms such as the pain interference due to NF1 pain [112]. Wolters and group reported that of 60 youth with NF1, 33% were taking pain medication, and of these, 10% took only OTC medication for their pain. 90% took prescription pain medication or a combination of OTC and prescription medication. Prescription pain medication included opioids, such as morphine, codeine, hydrocodone, and Vicodin; anticonvulsants, such as Gabapentin, Pregabalin, Neurontin, Tegretol, Topiramate; antidepressants, such as Amitriptyline, Rizatriptan, Zolmitriptan; and even topical treatments, such as a lidocaine patch. Despite taking pain medication, pain interference in daily functioning was rated high by patients; 93% of adolescents rated pain as interfering with functioning.
Other non-OTC pharmacotherapies for NF1 pain include removal of plexiform neurofibromas and inhibition of MPNSTs, known to cause pain, with targeting of the mTOR pathway with drugs, Sirolimus and Everolimus, both currently in phase II clinical trials [31; 105]. In assessing the use of targeting the AKT/mTOR pathway for relieving NF1 pain, Endo and group found that Everolimus had a dose-dependent inhibitory effect on MPNST cell line proliferation. In-vitro administration of Everolimus slowed growth in both MPNST cell lines derived from NF1 patients, and sporadic MPNST cell lines. A 30 nmol/L concentration of Everolimus, a dose that can be safely given orally to humans, inhibited wound healing in both MPNST cell lines and inhibited invasion of the MPNST cell lines through the Matrigel matrix, relative to control-treated cells. Hua and group demonstrated the effectiveness of Sirolimus in 3 patient case reports. In patient 1, a 17-year old adolescent, a daily 1 mg dose of Sirolimus, which was subsequently increased to 3 mg after three months, and 4 mg at 6 months, decreased both pain intensity and frequency, and after one year of treatment, abdominal pain due to NF1 had ceased, and remained absent for three years post-treatment. In patient 2, a 16-year old adolescent, a daily 1 mg dose of Sirolimus decreased NF1-related PN-derived neuropathic pain in four limbs, and after two years, the patient exhibited almost no pain. Patient 3, an 8-year old girl with extensive PNs in the right thigh and pelvis, resulting in neuropathic pain, was given a maximum dose of 2 mg of Sirolimus per day, to have pain intensity decrease of 10/10 to complete disappearance. This loss of pain persisted one-year post-treatment with Sirolimus. Thus, it appears that Sirolimus both increases time to progression of plexiform neurofibromas into MPNSTs and improves pain in NF1 patients [48; 105].
Other potential drug therapies for NF1 pain include MEK inhibitors, Selumetinib, Trametinib, and PD-0325901, currently in phase 2 clinical trials [3; 49; 54]. Preclinically tested in mouse models, PD-0325901 delays neurofibroma development in mice and at very low doses, 0.5mg/kg/day, may shrink already developed neurofibromas, [54]. In a Nf1 flox/flox; Dhh-Cre mouse model, 17 mice treated for 90 days with1.5mg/kg/day of PD-0325901 exhibited smaller neurofibroma volumes. Ki-67 staining for neurofibroma cell proliferation showed decreased proliferation as a result of drug treatment. Notably, PD-0325901 did not accelerate tumor development after stopping treatment. Selumetinib, another phase II clinical trial Mek-inhibitor also yields promising results for NF1 pain, with reported decreases in tumor volume, and subsequent alleviation of pain due to these tumors [49]. Pre-clinically, 12 of 18 mice treated with Selumetinib experienced decreased neurofibroma volume, compared to increases in neurofibroma volume in 14 of 15 vehicle-treated mice. In phase 1 trials, 71% (17 of 24) children reported efficacy of Selumetinib to establish a maximum dose of 25 mg/m2 and these results were comparable to those seen in adults. Ameratunga and group demonstrated the effectiveness of MEK-inhibitor Trametinib in a 24-year old male with NF1-related optic glioma-induced hydrocephalus, presenting with headaches drowsiness, and ataxia. Within three weeks of treatment with Trametinib, this patient showed decreases in glioma mass volume, and subsequent reduction of resulting symptoms. Taken together, these data show the promise of MEK inhibitors in NF1-related pain relief. It is important to note regarding these pharmacotherapies is that their mechanism of action is essentially through the reduction of tumor size, as this is a large cause of NF1-related pain. However, these drug therapies might not help a small percentage of patients suffering from non-tumor related pain, thus again reinforcing the notion to identify new treatments aimed at relief of non-tumor related NF1 pain.
The widely accepted method to remove plexiform neurofibromas is surgery [52]; however, the infiltration of the neurofibromas and their high vascularity and size often lend to an inability to perform complete resection of the tumor [48]. Therefore, other methods of NF1 pain management are essential. Previously, pain relief by electrodessication to remove painful cutaneous neurofibromas has been proposed [62]. In short, electrosurgery, in which tissue is desiccated via dehydration and denaturation of the dermis, allows for removal of several neurofibromas simultaneously; with low complications, this technique, although less understood, is perhaps more promising than surgical removal of NF1-related tumors. Additionally, Bardo-Brouard and group investigated the use of topical capsaicin as a therapy for NF1-related neuropathic pain [5]. A capsaicin patch was applied to the painful area on NF1 patients for 60 minutes; ~38% of patients had an average relief rate beyond the threshold of 30% as a result of the treatment. However, patients reported a transitory burning sensation while wearing the patch and experienced a slight increase in heart rate and blood pressure.
As the psychosocial symptoms of pain do inevitably exist, it is additionally important to find methods of pain treatment that address these effects of pain as well. It is well known that psychotherapy is valuable for chronic pain therapy [100]. Martin et. al proposes acceptance and commitment therapy (ACT), in which children with NF1 pain and their parents re-focus on valued relationships and activities, leading to a decline in both pain interference and pain intensity, but not in mood [67]. Separate workshops were tailored specifically for patients and parents focused on helping patients cope with pain effectively in 3 two-hour sessions over the course of two days. Patient feedback was then gathered via mail-in questionnaire three months post-ACT workshop training. Patient workshops started with the physiological description of pain and progressed with practice of mindfulness techniques, such as mindful breathing, and diffusion techniques, such as physicalizing pain and picturing it in a form or shape; finally, facilitators helped set short and long-term goals for pain management. Parent workshops focused on how to support children with NF1 pain and how to cope with their own feelings about their child’s symptoms. All participants were given exercises post-workshop session. As a result of these ACT workshops, parents reported less pain interference in their children’s’ lives, and patients reported decreased pain intensity three months after ACT workshop training. Six of ten patients were taking less pain medication, and 60 percent of patients were using mindfulness techniques; 60 percent were using diffusion techniques. However, three patients reported increases in pain medication, suggesting that the efficacy of ACT workshop training for NF1 pain relief needs further refining.
5.1. Pre-clinical treatments for NF1-derived pain
Preclinical pharmacotherapies for NF1 pain are important for developing future effective treatments for NF1 pain. In 2012, Wilson and colleagues demonstrated the efficacy of 15-amino acid peptides derived from the C-terminus of CaV2.2 and CaV1.2 (Ct-dis) to disrupt CRMP2-CaV2.2 interaction and inhibit resulting NF1-pain [108]. Specifically, a 10μM concentration of tat-fused Ct-dis decreased (~ 70%) 50mM KCl-evoked calcitonin gene-related peptide (CGRP) release, neuropeptide signaling involved in pain, in Nf+/− mice. In 2017, Moutal and group implicated (S)-Lacosamide ((S)-LCM), an enantiomer of the clinically approved anti-epileptic drug, (R)-Lacosamide, in targeting NF1 pain by regulating activity of protein CRMP2 [78]. Using CRISPR/Cas 9 gene editing system, Moutal and group characterized a new model for NF1 pain in which they identified increases in voltage-gated sodium and calcium channel currents for rats with truncated neurofibromin. Since cytosolic protein CRMP2 modulates activity of both these channels and the neurofibromin protein, using (S)-LCM to prevent phosphorylation of CRMP2 and thus rescuing activity of these channels allowed for inhibition of nociception in this novel NF1 pain model. This highlights (S)-LCM as a promising therapy for patients with NF1 pain. Furthermore, migraines are clearly more prevalent in the NF1 population, and yet, few therapies exist for this symptom of NF1 [85]. For this too, (S)-LCM has proven effective in preclinical testing [74], inhibiting both neurotransmitter release and periorbital withdrawal threshold in rats. Along similar lines, Moutal and group targeted the interaction between CRMP2 and neurofibromin with a 15-amino acid CRMP2-derived peptide, tat-CRMP2/neurofibromin regulating peptide 1 (tat-CNRP1) [77]. Treating rat neurons with this peptide again exhibited inhibition of nociceptive signaling and thus, tat-CNRP1 too exhibits potential for efficacy for treating NF1-derived pain. Table 1 summarizes the currently available therapies at various clinical and preclinical stages.
Table 1.
The preclinical and clinical landscape of therapies for NF1-related pain.
| Compound | Clinical Trial Stage | Target/mechanism of action | Reference |
|---|---|---|---|
| Everolimus | Phase 2 | mTOR | [30] |
| Sirolimus | Phase 2 | mTOR | [47] |
| Selumetnib | Phase 2 | MEK | [48] |
| Trametinib | Phase 2 | MEK | [3] |
| PD-0325901 | Phase 2 | MEK | Clinicaltrials.gov |
| Pembrolizumab | Phase 2 | PD-1 | Clinicaltrials.gov |
| Capsaicin | Phase 1 | TRPV1 | [5] |
| (S)-Lacosamide | Pre-clinical | CRMP2 phosphorylation | [75] |
| tat-CNRP1 | Pre-clinical | CRMP2-Neurofibromin Complex | [73] |
| tat-Ct-dis | Pre-clinical | CRMP2-Ca2+ Channel Complex | [103] |
6. Conclusion
Our current understanding of NF1-related is at a very early stage. Although there are several reports of NF1-related pain in various locations of the body, the causes of all of these cases remain unclear, and whether they are a result of NF1-related tumors or another symptom of the disease itself is unknown. Additionally, more research needs to be done on the effects of NF1 pain on a social and emotional level. As pain is subjective phenotype, a biopsychosocial approach to its understanding would provide the most benefit to the characterization and treatment of this symptom of the NF1 disease. Current treatments specifically targeting NF1-pain are scarce; although recent technological advances are making more thorough removal of tumors possible via techniques such as electrodessication, pharmacotherapies seem largely neglected. Few drugs have made it to the clinical setting and are somewhere between the preclinical (S. Lacosamide; tat-CNRP1) and clinical trial (Sirolimus, Selumetinib) abyss. Other treatment options include general over the counter medication and surgery for removal of painful neurofibromas, neither of which result in complete attenuation of NF1-pain. Furthermore, a biopsychosocial approach for pain treatment posits the use of acceptance and commitment therapy as an effective tool to alleviate the pain hindrance and intensity that results from NF1 pain, but more investigation into psychosocial treatments that could be effective for mood related effects of NF1 pain remains necessary. In developing effective therapies for NF1-related pain, understanding of the mechanism of pain signaling involved in the development and maintenance of this pain is required. While studies of NF1-rat models highlight CRMP2 as a key player in the development of NF1 pain, mice models and the novel porcine model remain understudied. Even though at least 7 mouse models of Nf1 exist, only 1 of them has been tested for presence of pain behaviors. NF1 pain research is especially intriguing in the NF1 porcine model because of the stark phenotypic similarities seen in these miniswine. Finally, a large deficit in the understanding of NF1-related pain studies is in the gender dimorphisms involved in NF1 pain. While Crawford et. al demonstrates a greater effect of NF1 pain on mood in women, this one of very few studies that investigates these gender-based differences, exhibiting a gap in our understanding of the disease. With increasing prevalence of pain-NF1 comorbidities, as shown by Fjermestad and group, the importance of recognizing and exploring pain in the context of NF1 is heightened. Understanding pain in the context of NF1 will advance clinical care and improve the lifestyle of NF1 patients.
Supplementary Material
Acknowledgements -
This work was supported by National Institutes of Health awards (R01NS098772 from the National Institute of Neurological Disorders and Stroke and R01DA042852 from the National Institute on Drug Abuse to R.K.); and a Neurofibromatosis New Investigator Award from the Department of Defense Congressionally Directed Military Medical Research and Development Program (NF1000099); and a Children’s Tumor Foundation NF1 Synodos award (2015-04-009A) to R.K. S.S.B. was supported by funds from the University of Arizona Vice President of Research’s office through the Undergraduate Biology Research Program.
Footnotes
Conflict of interest – There is no conflict of interest for any of the authors.
References
- [1].Afridi SK, Leschziner GD, Ferner RE. Prevalence and clinical presentation of headache in a National Neurofibromatosis 1 Service and impact on quality of life. American journal of medical genetics Part A 2015;167A(10):2282–2285. [DOI] [PubMed] [Google Scholar]
- [2].Allen TM, Struemph KL, Toledo-Tamula MA, Wolters PL, Baldwin A, Widemann B, Martin S. The Relationship Between Heart Rate Variability, Psychological Flexibility, and Pain in Neurofibromatosis Type 1. Pain practice : the official journal of World Institute of Pain 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ameratunga M, McArthur G, Gan H, Cher L. Prolonged disease control with MEK inhibitor in neurofibromatosis type I-associated glioblastoma. Journal of clinical pharmacy and therapeutics 2016;41(3):357–359. [DOI] [PubMed] [Google Scholar]
- [4].Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Archives of internal medicine 2003;163(20):2433–2445. [DOI] [PubMed] [Google Scholar]
- [5].Bardo-Brouard P, Luizard C, Valeyrie-Allanore L, Goujon C, Do B, Colin A, Wolkenstein P, Paul M. High-concentration topical capsaicin in the management of refractory neuropathic pain in patients with neurofibromatosis type 1: a case series. Current medical research and opinion 2018;34(5):887–891. [DOI] [PubMed] [Google Scholar]
- [6].Batta AG, Gundian JC, Myers RP. Neurofibromatosis presenting as perineal pain and urethral burning. Urology 1989;33(2):138–140. [DOI] [PubMed] [Google Scholar]
- [7].Beert E, Brems H, Renard M, Ferreiro JF, Melotte C, Thoelen R, De Wever I, Sciot R, Legius E, Debiec-Rychter M. Biallelic inactivation of NF1 in a sporadic plexiform neurofibroma. Genes Chromosomes Cancer 2012;51(9):852–857. [DOI] [PubMed] [Google Scholar]
- [8].Bicudo NP, de Menezes Neto BF, da Silva de Avo LR, Germano CM, Melo DG. Quality of Life in Adults with Neurofibromatosis 1 in Brazil. J Genet Couns 2016;25(5):1063–1074. [DOI] [PubMed] [Google Scholar]
- [9].Blount RL, Cohen LL, Frank NC, Bachanas PJ, Smith AJ, Manimala MR, Pate JT. The Child-Adult Medical Procedure Interaction Scale-Revised: an assessment of validity. Journal of pediatric psychology 1997;22(1):73–88. [DOI] [PubMed] [Google Scholar]
- [10].Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW, Buchberg AM, Jenkins NA, Parada LF, Copeland NG. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes & development 1994;8(9):1019–1029. [DOI] [PubMed] [Google Scholar]
- [11].Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, Fehrenbacher JC, Fitz SD, Khanna M, Park CK, Schmutzler BS, Cheon BM, Due MR, Brustovetsky T, Ashpole NM, Hudmon A, Meroueh SO, Hingtgen CM, Brustovetsky N, Ji RR, Hurley JH, Jin X, Shekhar A, Xu XM, Oxford GS, Vasko MR, White FA, Khanna R. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2)(+) channel complex. Nature medicine 2011;17(7):822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brittain JM, Piekarz AD, Wang Y, Kondo T, Cummins TR, Khanna R. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. The Journal of biological chemistry 2009;284(45):31375–31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brittain JM, Wang Y, Eruvwetere O, Khanna R. Cdk5-mediated phosphorylation of CRMP-2 enhances its interaction with CaV2.2. FEBS letters 2012;586(21):3813–3818. [DOI] [PubMed] [Google Scholar]
- [14].Buckley M, Kuan S, Barton D. An unusual cause of collapse and neck pain. Emergency medicine journal : EMJ 2008;25(12):857–858. [DOI] [PubMed] [Google Scholar]
- [15].Burns KM, Wolters PL, Martin S, Baldwin A, Dombi E, Kurwa A, Gillespie A, Salzer W, Wildemann B. Parent and self reports of pain in children and adolescents with NF1 and plexiform neurofibromas: relation to quality of life, social-emotional functioning, and physical manifestations. National Conference in Pediatric Psychology; San Antonio, TX, 2011. [Google Scholar]
- [16].Charpin C, Berbis P, Schmitz J, Boudinet H, Mattei JP, Balandraud N, Chagnaud C, Roudier J, Guis S. Neurofibromatosis type 1 with sciatica. Joint, bone, spine : revue du rhumatisme 2007;74(3):300–301. [DOI] [PubMed] [Google Scholar]
- [17].Chen AM, Neustadt JB, Kucera JN. Rib head dislocation causing spinal canal stenosis in a child with neurofibromatosis, type 1. Journal of radiology case reports 2017;11(8):8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chi XX, Schmutzler BS, Brittain JM, Wang Y, Hingtgen CM, Nicol GD, Khanna R. Regulation of N-type voltage-gated calcium channels (Cav2.2) and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. Journal of cell science 2009;122(Pt 23): 4351–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science 1999;286(5447):2172–2176. [DOI] [PubMed] [Google Scholar]
- [20].Costa RM, Yang T, Huynh DP, Pulst SM, Viskochil DH, Silva AJ, Brannan CI. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nature genetics 2001;27(4):399–405. [DOI] [PubMed] [Google Scholar]
- [21].Crawford HA, Barton B, Wilson MJ, Berman Y, McKelvey-Martin VJ, Morrison PJ, North KN. The Impact of Neurofibromatosis Type 1 on the Health and Wellbeing of Australian Adults. J Genet Couns 2015;24(6):931–944. [DOI] [PubMed] [Google Scholar]
- [22].Creange A, Zeller J, Rostaing-Rigattieri S, Brugieres P, Degos JD, Revuz J, Wolkenstein P. Neurological complications of neurofibromatosis type 1 in adulthood. Brain 1999;122(Pt 3): 473–481. [DOI] [PubMed] [Google Scholar]
- [23].Daston MM, Scrable H, Nordlund M, Sturbaum AK, Nissen LM, Ratner N. The protein product of the neurofibromatosis type 1 gene is expressed at highest abundance in neurons, Schwann cells, and oligodendrocytes. Neuron 1992;8(3):415–428. [DOI] [PubMed] [Google Scholar]
- [24].del Carmen Baena-Ocampo L, Reyes-Sanchez A, Alpizar-Aguirre A, Rosales-Olivares LM. Malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1: report of two clinical cases. Cirugia y cirujanos 2009;77(5):391–395. [PubMed] [Google Scholar]
- [25].DiMario FJ Jr., Langshur S. Headaches in patients with neurofibromatosis-1. Journal of child neurology 2000;15(4):235–238. [DOI] [PubMed] [Google Scholar]
- [26].Duan JH, Hodgdon KE, Hingtgen CM, Nicol GD. N-type calcium current, Cav2.2, is enhanced in small-diameter sensory neurons isolated from Nf1+/− mice. Neuroscience 2014;270:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dustrude ET, Moutal A, Yang X, Wang Y, Khanna M, Khanna R. Hierarchical CRMP2 posttranslational modifications control NaV1.7 function. Proceedings of the National Academy of Sciences of the United States of America 2016;113(52):E8443–E8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dustrude ET, Wilson SM, Ju W, Xiao Y, Khanna R. CRMP2 protein SUMOylation modulates NaV1.7 channel trafficking. The Journal of biological chemistry 2013;288(34):24316–24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Edmiston J Leg pain in a teenager with a history of neurofibromatosis. JAAPA : official journal of the American Academy of Physician Assistants 2013;26(2):63–64. [DOI] [PubMed] [Google Scholar]
- [30].Eisenbarth I, Assum G, Kaufmann D, Krone W. Evidence for the presence of the second allele of the neurofibromatosis type 1 gene in melanocytes derived from cafe au laitmacules of NF1 patients. Biochemical and biophysical research communications 1997;237(1):138–141. [DOI] [PubMed] [Google Scholar]
- [31].Endo M, Yamamoto H, Setsu N, Kohashi K, Takahashi Y, Ishii T, Iida K, Matsumoto Y, Hakozaki M, Aoki M, Iwasaki H, Dobashi Y, Nishiyama K, Iwamoto Y, Oda Y. Prognostic significance of AKT/mTOR and MAPK pathways and antitumor effect of mTOR inhibitor in NF1-related and sporadic malignant peripheral nerve sheath tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19(2):450–461. [DOI] [PubMed] [Google Scholar]
- [32].Esposito T, Piluso G, Saracino D, Uccello R, Schettino C, Dato C, Capaldo G, Giugliano T, Varriale B, Paolisso G, Di Iorio G, Melone MA. A novel diagnostic method to detect truncated neurofibromin in neurofibromatosis 1. Journal of neurochemistry 2015;135(6):1123–1128. [DOI] [PubMed] [Google Scholar]
- [33].Fang Y, Elahi A, Denley RC, Rao PH, Brennan MF, Jhanwar SC. Molecular characterization of permanent cell lines from primary, metastatic and recurrent malignant peripheral nerve sheath tumors (MPNST) with underlying neurofibromatosis-1. Anticancer Res 2009;29(4):1255–1262. [PubMed] [Google Scholar]
- [34].Fernandez-Codina A, Aranda-Rodriguez S, Romagosa C, Deu-Martin M, Parra-Farinas C, Bujan-Rivas S. A 40-Year-Old Woman With Back Pain. Chest 2016;150(6):e159–e165. [DOI] [PubMed] [Google Scholar]
- [35].Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet neurology 2007;6(4):340–351. [DOI] [PubMed] [Google Scholar]
- [36].Fjermestad KW, Nyhus L, Kanavin OJ, Heiberg A, Hoxmark LB. Health Survey of Adults with Neurofibromatosis 1 Compared to Population Study Controls. J Genet Couns 2018. [DOI] [PubMed] [Google Scholar]
- [37].Flavin K, Mitra R. Unknown case: Low back pain in a patient with cafe au lait spots. Spine 2011;36(13):1069. [DOI] [PubMed] [Google Scholar]
- [38].Geist RT, Gutmann DH. Expression of a developmentally-regulated neuron-specific isoform of the neurofibromatosis 1 (NF1) gene. Neuroscience letters 1996;211(2):85–88. [DOI] [PubMed] [Google Scholar]
- [39].Gereau RWt. Neurofibromatosis pain is in the membrane. Focus on “sensory neurons from Nf1 haploinsufficient mice exhibit increased excitability”. Journal of neurophysiology 2005;94(6):3659–3660. [DOI] [PubMed] [Google Scholar]
- [40].Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nature medicine 2010;16(11):1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gutmann DH, Giovannini M. Mouse models of neurofibromatosis 1 and 2. Neoplasia 2002;4(4):279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hajra A, Martin-Gallardo A, Tarle SA, Freedman M, Wilson-Gunn S, Bernards A, Collins FS. DNA sequences in the promoter region of the NF1 gene are highly conserved between human and mouse. Genomics 1994;21(3):649–652. [DOI] [PubMed] [Google Scholar]
- [43].Heim RA, Kam-Morgan LN, Binnie CG, Corns DD, Cayouette MC, Farber RA, Aylsworth AS, Silverman LM, Luce MC. Distribution of 13 truncating mutations in the neurofibromatosis 1 gene. Hum Mol Genet 1995;4(6):975–981. [DOI] [PubMed] [Google Scholar]
- [44].Heuschkel R, Kim S, Korf B, Schneider G, Bousvaros A. Abdominal migraine in children with neurofibromatosis type 1: a case series and review of gastrointestinal involvement in NF1. J Pediatr Gastroenterol Nutr 2001;33(2):149–154. [DOI] [PubMed] [Google Scholar]
- [45].Hingtgen CM. Neurofibromatosis: the role of guanosine triphosphatase activating proteins in sensory neuron function. Sheng li xue bao : [Acta physiologica Sinica] 2008;60(5):581–583. [PubMed] [Google Scholar]
- [46].Hingtgen CM, Roy SL, Clapp DW. Stimulus-evoked release of neuropeptides is enhanced in sensory neurons from mice with a heterozygous mutation of the Nf1 gene. Neuroscience 2006;137(2):637–645. [DOI] [PubMed] [Google Scholar]
- [47].Hodgdon KE, Hingtgen CM, Nicol GD. Dorsal root ganglia isolated from Nf1+/− mice exhibit increased levels of mRNA expression of voltage-dependent sodium channels. Neuroscience 2012;206:237–244. [DOI] [PubMed] [Google Scholar]
- [48].Hua C, Zehou O, Ducassou S, Minard-Colin V, Hamel-Teillac D, Wolkenstein P, Valeyrie-Allanore L. Sirolimus improves pain in NF1 patients with severe plexiform neurofibromas. Pediatrics 2014;133(6):e1792–1797. [DOI] [PubMed] [Google Scholar]
- [49].Hutchinson L Targeted therapies: Selumetinib MEKing differences in NF1. Nature reviews Clinical oncology 2017;14(3):140. [DOI] [PubMed] [Google Scholar]
- [50].Isakson SH, Rizzardi AE, Coutts AW, Carlson DF, Kirstein MN, Fisher J, Vitte J, Williams KB, Pluhar GE, Dahiya S, Widemann BC, Dombi E, Rizvi T, Ratner N, Messiaen L, Stemmer-Rachamimov AO, Fahrenkrug SC, Gutmann DH, Giovannini M, Moertel CL, Largaespada DA, Watson AL. Genetically engineered minipigs model the major clinical features of human neurofibromatosis type 1. Communications biology 2018;1:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nature genetics 1994;7(3):353–361. [DOI] [PubMed] [Google Scholar]
- [52].Janes LE, Sabino J, Matthews JA, Papadimitriou JC, Strome SE, Singh DP. Surgical management of craniofacial neurofibromatosis type 1 associated tumors. The Journal of craniofacial surgery 2013;24(4):1273–1277. [DOI] [PubMed] [Google Scholar]
- [53].Johnson H, Wiggs L, Stores G, Huson SM. Psychological disturbance and sleep disorders in children with neurofibromatosis type 1. Developmental medicine and child neurology 2005;47(4):237–242. [DOI] [PubMed] [Google Scholar]
- [54].Jousma E, Rizvi TA, Wu J, Janhofer D, Dombi E, Dunn RS, Kim MO, Masters AR, Jones DR, Cripe TP, Ratner N. Preclinical assessments of the MEK inhibitor PD-0325901 in a mouse model of Neurofibromatosis type 1. Pediatric blood & cancer 2015;62(10):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kodra Y, Giustini S, Divona L, Porciello R, Calvieri S, Wolkenstein P, Taruscio D. Health-related quality of life in patients with neurofibromatosis type 1. A survey of 129 Italian patients. Dermatology 2009;218(3):215–220. [DOI] [PubMed] [Google Scholar]
- [56].Krab LC, Oostenbrink R, de Goede-Bolder A, Aarsen FK, Elgersma Y, Moll HA. Health-related quality of life in children with neurofibromatosis type 1: contribution of demographic factors, disease-related factors, and behavior. The Journal of pediatrics 2009;154(3):420–425, 425 e421. [DOI] [PubMed] [Google Scholar]
- [57].Lai M, Flynn S, Cannon TC, Schmucker T, Davis R, Harper R. Case of the month. Neurofibromatosis type 1. The Journal of the Arkansas Medical Society 2003;100(3):100–101. [PubMed] [Google Scholar]
- [58].Largaespada DA, Brannan CI, Jenkins NA, Copeland NG. Nf1 deficiency causes Ras-mediated granulocyte/macrophage colony stimulating factor hypersensitivity and chronic myeloid leukaemia. Nature genetics 1996;12(2):137–143. [DOI] [PubMed] [Google Scholar]
- [59].Lee JH, Park CK, Chen G, Han Q, Xie RG, Liu T, Ji RR, Lee SY. A monoclonal antibody that targets a NaV1.7 channel voltage sensor for pain and itch relief. Cell 2014;157(6):1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [60].Leung VK, Lee SW, Yuen NW, Kung NN, Loke TK. Epigastric pain in a patient with neurofibromatosis type 1. Hong Kong medical journal = Xianggang yi xue za zhi 2005;11(3):213–215. [PubMed] [Google Scholar]
- [61].Lin YL, Hsueh YP. Neurofibromin interacts with CRMP-2 and CRMP-4 in rat brain. Biochemical and biophysical research communications 2008;369(2):747–752. [DOI] [PubMed] [Google Scholar]
- [62].Lutterodt CG, Mohan A, Kirkpatrick N. The use of electrodessication in the treatment of cutaneous neurofibromatosis: A retrospective patient satisfaction outcome assessment. Journal of plastic, reconstructive & aesthetic surgery : JPRAS 2016;69(6):765–769. [DOI] [PubMed] [Google Scholar]
- [63].Mantani A, Wakasugi S, Yokota Y, Abe K, Ushio Y, Yamamura K. A novel isoform of the neurofibromatosis type-1 mRNA and a switch of isoforms during murine cell differentiation and proliferation. Gene 1994;148(2):245–251. [DOI] [PubMed] [Google Scholar]
- [64].Marquez de PB, Hammond DL. Sex dependent enhancement of pain responses in a mouse model of neurofibromatosis., Proceedings of the Society for Neuroscience, 2011. [Google Scholar]
- [65].Martin S, Gillespie A, Wolters PL, Widemann BC. Experiences of families with a child, adolescent, or young adult with neurofibromatosis type 1 and plexiform neurofibroma evaluated for clinical trials participation at the National Cancer Institute. Contemp Clin Trials 2011;32(1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Martin S, Wolters P, Baldwin A, Gillespie A, Dombi E, Walker K, Widemann B. Social-emotional functioning of children and adolescents with neurofibromatosis type 1 and plexiform neurofibromas: relationships with cognitive, disease, and environmental variables. Journal of pediatric psychology 2012;37(7):713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Martin S, Wolters PL, Toledo-Tamula MA, Schmitt SN, Baldwin A, Starosta A, Gillespie A, Widemann B. Acceptance and commitment therapy in youth with neurofibromatosis type 1 (NF1) and chronic pain and their parents: A pilot study of feasibility and preliminary efficacy. American journal of medical genetics Part A 2016;170(6):1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Marushack MM, Merkel KD, Gilula LA. A 30-year-old man with a mass in the distal left thigh and radiating leg pain. Orthopaedic review 1994;23(5):454–460. [PubMed] [Google Scholar]
- [69].Messiaen LM, Callens T, Mortier G, Beysen D, Vandenbroucke I, Van Roy N, Speleman F, Paepe AD. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat 2000;15(6):541–555. [DOI] [PubMed] [Google Scholar]
- [70].Molosh AI, Johnson PL, Spence JP, Arendt D, Federici LM, Bernabe C, Janasik SP, Segu ZM, Khanna R, Goswami C, Zhu W, Park SJ, Li L, Mechref YS, Clapp DW, Shekhar A. Social learning and amygdala disruptions in Nf1 mice are rescued by blocking p21-activated kinase. Nature neuroscience 2014;17(11):1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Moutal A, Cai S, Luo S, Voisin R, Khanna R. CRMP2 is necessary for Neurofibromatosis type 1 related pain. Channels 2018;12(1):47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Moutal A, Dustrude ET, Khanna R. Sensitization of Ion Channels Contributes to Central and Peripheral Dysfunction in Neurofibromatosis Type 1. Molecular neurobiology 2016. [DOI] [PubMed] [Google Scholar]
- [73].Moutal A, Dustrude ET, Largent-Milnes TM, Vanderah TW, Khanna M, Khanna R. Blocking CRMP2 SUMOylation reverses neuropathic pain. Molecular psychiatry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Moutal A, Eyde N, Telemi E, Park KD, Xie JY, Dodick DW, Porreca F, Khanna R. Efficacy of (S)-Lacosamide in preclinical models of cephalic pain. Pain reports 2016;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Moutal A, Francois-Moutal L, Perez-Miller S, Cottier K, Chew LA, Yeon SK, Dai J, Park KD, Khanna M, Khanna R. (S)-Lacosamide Binding to Collapsin Response Mediator Protein 2 (CRMP2) Regulates CaV2.2 Activity by Subverting Its Phosphorylation by Cdk5. Molecular neurobiology 2016;53(3):1959–1976. [DOI] [PubMed] [Google Scholar]
- [76].Moutal A, Sun L, Yang X, Li W, Cai S, Luo S, Khanna R. CRMP2-Neurofibromin Interface Drives NF1-related Pain. Neuroscience 2018;381:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Moutal A, Wang Y, Yang X, Ji Y, Luo S, Dorame A, Bellampalli SS, Chew LA, Cai S, Dustrude ET, Keener JE, Marty MT, Vanderah TW, Khanna R. Dissecting the role of the CRMP2-neurofibromin complex on pain behaviors. Pain 2017;158(11):2203–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Moutal A, Yang X, Li W, Gilbraith KB, Luo S, Cai S, Francois-Moutal L, Chew LA, Yeon SK, Bellampalli SS, Qu C, Xie JY, Ibrahim MM, Khanna M, Park KD, Porreca F, Khanna R. CRISPR/Cas9 editing of Nf1 gene identifies CRMP2 as a therapeutic target in neurofibromatosis type 1-related pain that is reversed by (S)-Lacosamide. Pain 2017;158(12):2301–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nguyen R, Kluwe L, Fuensterer C, Kentsch M, Friedrich RE, Mautner VF. Plexiform neurofibromas in children with neurofibromatosis type 1: frequency and associated clinical deficits. The Journal of pediatrics 2011;159(4):652–655 e652. [DOI] [PubMed] [Google Scholar]
- [80].O’Brien DE, Brenner DS, Gutmann DH, Gereau RWt. Assessment of Pain and Itch Behavior in a Mouse Model of Neurofibromatosis Type 1. The journal of pain : official journal of the American Pain Society 2013;14(6):628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Omrani A, van der Vaart T, Mientjes E, van Woerden GM, Hojjati MR, Li KW, Gutmann DH, Levelt CN, Smit AB, Silva AJ, Kushner SA, Elgersma Y. HCN channels are a novel therapeutic target for cognitive dysfunction in Neurofibromatosis type 1. Mol Psychiatry 2015;20(11):1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pasini A, Lo-Castro A, Di Carlo L, Pitzianti M, Siracusano M, Rosa C, Galasso C. Detecting anxiety symptoms in children and youths with neurofibromatosis type I. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 2012;159B(7):869–873. [DOI] [PubMed] [Google Scholar]
- [83].Patrakitkomjorn S, Kobayashi D, Morikawa T, Wilson MM, Tsubota N, Irie A, Ozawa T, Aoki M, Arimura N, Kaibuchi K, Saya H, Araki N. Neurofibromatosis type 1 (NF1) tumor suppressor, neurofibromin, regulates the neuronal differentiation of PC12 cells via its associating protein, CRMP-2. The Journal of biological chemistry 2008;283(14):9399–9413. [DOI] [PubMed] [Google Scholar]
- [84].Peron SM, Baldwin A. Pain in Neurofibromatosis-1. In: NIoH National Cancer Institute; editor: Midwest NF, 2013. pp. 1–4. [Google Scholar]
- [85].Pinho RS, Fusao EF, Paschoal J, Caran EMM, Minett TSC, Vilanova LCP, Masruha MR. Migraine is frequent in children and adolescents with neurofibromatosis type 1. Pediatrics international : official journal of the Japan Pediatric Society 2014;56(6):865–867. [DOI] [PubMed] [Google Scholar]
- [86].Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, Graham DB, Jhunjhunwala S, Heidenreich M, Xavier RJ, Langer R, Anderson DG, Hacohen N, Regev A, Feng G, Sharp PA, Zhang F. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 2014;159(2):440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Pourtsidis A, Doganis D, Baka M, Bouhoutsou D, Varvoutsi M, Synodinou M, Giamarelou P, Kosmidis H. Malignant peripheral nerve sheath tumors in children with neurofibromatosis type 1. Case reports in oncological medicine 2014;2014:843749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Protopapas A, Sotiropoulou M, Haidopoulos D, Athanasiou S, Loutradis D, Antsaklis A. Ovarian neurofibroma: a rare visceral occurrence of type 1 neurofibromatosis and an unusual cause of chronic pelvic pain. Journal of minimally invasive gynecology 2011;18(4):520–524. [DOI] [PubMed] [Google Scholar]
- [89].Rapp M, Martino J, Allen GB. A 35-year-old patient with midscapular pain and hypertension. Chest 2011;140(1):258–261. [DOI] [PubMed] [Google Scholar]
- [90].Riklin E, Talaei-Kheoi M, Merker VL, Sheridan MR, Jordan JT, Plotkin SR, Vranceanu AM. First report of factors associated with satisfaction in patients with neurofibromatosis. American journal of medical genetics Part A 2017;173(3):671–677. [DOI] [PubMed] [Google Scholar]
- [91].Schulz A, Grafe P, Hagel C, Baumer P, Morrison H, Mautner VF, Farschtschi S. Neuropathies in the setting of Neurofibromatosis tumor syndromes: Complexities and opportunities. Experimental neurology 2018;299(Pt B): 334–344. [DOI] [PubMed] [Google Scholar]
- [92].Schulz M, Schreyer A, Glockner SC, Scholmerich J, Zuber-Jerger I. [69 year-old patient with chronic abdominal pain and anemia in neurofibromatosis]. Der Internist 2011;52(1):81–82, 84–86, 88. [DOI] [PubMed] [Google Scholar]
- [93].Serra E, Puig S, Otero D, Gaona A, Kruyer H, Ars E, Estivill X, Lazaro C. Confirmation of a double-hit model for the NF1 gene in benign neurofibromas. Am J Hum Genet 1997;61(3):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shen MH, Harper PS, Upadhyaya M. Molecular genetics of neurofibromatosis type 1 (NF1). Journal of medical genetics 1996;33(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Silva AJ, Frankland PW, Marowitz Z, Friedman E, Laszlo GS, Cioffi D, Jacks T, Bourtchuladze R. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nature genetics 1997;15(3):281–284. [DOI] [PubMed] [Google Scholar]
- [96].Sohier P, Luscan A, Lloyd A, Ashelford K, Laurendeau I, Briand-Suleau A, Vidaud D, Ortonne N, Pasmant E, Upadhyaya M. Confirmation of mutation landscape of NF1-associated malignant peripheral nerve sheath tumors. Genes Chromosomes Cancer 2017;56(5):421–426. [DOI] [PubMed] [Google Scholar]
- [97].Son C, Park JW. Neurofibromatosis Type 1 Accompanied by Craniofacial Pain: Literature Review and Descriptive Case. Journal of oral & facial pain and headache 2017;31(4):402–409. [DOI] [PubMed] [Google Scholar]
- [98].Struemph K, Wolters P, Martin S, Tonsgard J, MacKenzie C, Schorry E, Manning S, Walsh KS, Kennedy T, Widemann B. Development of Patient-reported Outcomes (PROs) to Assess Pain in Individuals with Neurofibromatosis 1 (NF1) and Plexiform Neurofibromas (PNs) for Clinical Trial Endpoints: Results from Adult Participants. In: CsT Foundation; editor. Conference NF. Washington DC, 2017. [Google Scholar]
- [99].Stuck RM, Johnson RT. Neurofibromatosis producing persistent neck pain and paralysis of right arm following pre-eclampsia. The American surgeon 1955;21(2):166–169. [PubMed] [Google Scholar]
- [100].Sturgeon JA. Psychological therapies for the management of chronic pain. Psychology research and behavior management 2014;7:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Upadhyaya M, Cooper DN. The mutational spectrum in neurofibromatosis type 1 and its underlying mechanisms. In: Upadhyaya MaC, N. D,, editor. Neurofibromatosis Type 1; From Genotype to Phenotype: BIOS Scientific, Oxford, UK,, 1998. pp. pp. 65–82. [Google Scholar]
- [102].Wang Y, Brittain JM, Wilson SM, Hingtgen CM, Khanna R. Altered calcium currents and axonal growth in Nf1 haploinsufficient mice. Translational neuroscience 2010;1(2):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang Y, Duan JH, Hingtgen CM, Nicol GD. Augmented sodium currents contribute to the enhanced excitability of small diameter capsaicin-sensitive sensory neurons isolated from Nf1+/(−) mice. J Neurophysiol 2010;103(4):2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang Y, Nicol GD, Clapp DW, Hingtgen CM. Sensory neurons from Nf1 haploinsufficient mice exhibit increased excitability. JNeurophysiol 2005;94(6):3670–3676. [DOI] [PubMed] [Google Scholar]
- [105].Weiss B, Widemann BC, Wolters P, Dombi E, Vinks A, Cantor A, Perentesis J, Schorry E, Ullrich N, Gutmann DH, Tonsgard J, Viskochil D, Korf B, Packer RJ, Fisher MJ. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: a neurofibromatosis Clinical Trials Consortium phase II study. Neuro-oncology 2015;17(4):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].White KA, Swier VJ, Cain JT, Kohlmeyer JL, Meyerholz DK, Tanas MR, Uthoff J, Hammond E, Li H, Rohret FA, Goeken A, Chan CH, Leidinger MR, Umesalma S, Wallace MR, Dodd RD, Panzer K, Tang AH, Darbro BW, Moutal A, Cai S, Li W, Bellampalli SS, Khanna R, Rogers CS, Sieren JC, Quelle DE, Weimer JM. A porcine model of neurofibromatosis type 1 that mimics the human disease. JCI insight 2018;3(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].White S, Marquez de Prado B, Russo AF, Hammond DL. Heat hyperalgesia and mechanical hypersensitivity induced by calcitonin gene-related peptide in a mouse model of neurofibromatosis. PloS one 2014;9(9):e106767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wilson SM, Schmutzler BS, Brittain JM, Dustrude ET, Ripsch MS, Pellman JJ, Yeum TS, Hurley JH, Hingtgen CM, White FA, Khanna R. Inhibition of transmitter release and attenuation of anti-retroviral-associated and tibial nerve injury-related painful peripheral neuropathy by novel synthetic Ca2+ channel peptides. The Journal of biological chemistry 2012;287(42):35065–35077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wimmer K, Eckart M, Rehder H, Fonatsch C. Illegitimate splicing of the NF1 gene in healthy individuals mimics mutation-induced splicing alterations in NF1 patients. Hum Genet 2000;106(3):311–313. [DOI] [PubMed] [Google Scholar]
- [110].Wimmer K, Yao S, Claes K, Kehrer-Sawatzki H, Tinschert S, De Raedt T, Legius E, Callens T, Beiglbock H, Maertens O, Messiaen L. Spectrum of single- and multiexon NF1 copy number changes in a cohort of 1,100 unselected NF1 patients. Genes Chromosomes Cancer 2006;45(3):265–276. [DOI] [PubMed] [Google Scholar]
- [111].Wolkenstein P, Zeller J, Revuz J, Ecosse E, Leplege A. Quality-of-life impairment in neurofibromatosis type 1: a cross-sectional study of 128 cases. Arch Dermatol 2001;137(11):1421–1425. [DOI] [PubMed] [Google Scholar]
- [112].Wolters PL, Burns KM, Martin S, Baldwin A, Dombi E, Toledo-Tamula MA, Dudley WN, Gillespie A, Widemann BC. Pain interference in youth with neurofibromatosis type 1 and plexiform neurofibromas and relation to disease severity, social-emotional functioning, and quality of life. American journal of medical genetics Part A 2015;167A(9):2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes & development 2001;15(7):859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.