Abstract
Small heat shock proteins (sHSPs) comprise an important protein family that is ubiquitously expressed, is highly conserved among species, and has emerged as a critical regulator of protein folding. While these proteins are functionally important for a variety of tissues, an emerging field of cardiovascular research reveals sHSPs are also extremely important for maintaining normal cardiac function and regulating the cardiac stress response. Notably, numerous mutations in genes encoding sHSPs have been associated with multiple cardiac diseases. sHSPs (HSPB5, HSPB6, and HSPB8) have been described as mediating chaperone functions within the heart by interacting with the cochaperone protein BCL-2–associated anthanogene 3 (BAG3); however, recent reports indicate that sHSPs (HSPB7) can perform other BAG3-independent functions. Here, we summarize the cardiac functions of sHSPs and present the notion that cardiac sHSPs function via BAG3-dependent or -independent pathways.
Introduction
The small heat shock proteins (sHSPs), also referred to as HSP family B (HSPB), belong to the superfamily of HSPs and play critical roles within the cell. sHSPs are characterized by their small molecular weight, ranging from 15 to 40 kDa, as well as by a highly conserved domain of approximately 80 amino acids, the α-crystallin domain (ACD) (1, 2). Although most sHSPs can be assembled into large, multimeric complexes that vary in size and contain up to 24 to 40 subunits, they can also exist in the form of monomers and dimers (1). The human genome encodes 10 sHSPs (HSPB1–HSPB10) (3), all of which exhibit distinct tissue-specific expression profiles. Four sHSP members (HSPB5–8) are highly expressed in the heart (4). Mutations in sHSP-encoding genes have been associated with human cardiac disease (5–18), highlighting the important role of sHSPs in maintaining cardiac function.
The sHSPs were initially recognized as a first line of defense against protein aggregation to maintain protein homeostasis (19, 20). sHSPs are ATP-independent chaperones, which function as “holdases” to bind to and stabilize denatured or non-native proteins against aggregation. Additionally, they facilitate subsequent protein renaturation in cooperation with “foldases,” the ATP-dependent, high–molecular-weight chaperones (19, 21). Thus far, BCL-2–associated anthanogene 3 (BAG3), a cochaperone protein, is the only protein known to bridge ATP-independent sHSPs and the ATP-dependent HSP70 family to form ternary complexes (22–26). Recent studies also suggest that BAG3 is not a passive scaffolding factor, in that the chaperone-like functions of cardiac sHSPs are highly dependent on BAG3. Among the 4 cardiac sHSPs, HSPB5, HSPB6, and HSBP8, but not HSPB7, interact with BAG3 (27). Moreover, the protein stability of HSPB5, HSPB6, and HSP8, but not HSPB7, is dependent on BAG3 (28, 29). Recent evidence also reveals that HSPB7 carries a unique BAG3-independent function. In this Review, we summarize recent findings from studies focused on understanding the diverse functions of cardiac sHSPs as well as the importance of their interaction with BAG3.
BAG3 and its interacting sHSPs
BAG3 is a chaperone protein that is highly expressed in the heart and is associated with the development of human cardiomyopathy (30–36). The conserved BCL-2–associated anthanogene domain binds to the nucleotide-binding domain of members of the HSP70 family and helps release ADP from the chaperone to facilitate nucleotide cycling (26). BAG3 also contains multiple unique domains and motifs that facilitate interactions with different proteins, including a WW (Trp-Trp) domain at the amino terminus, a central proline-rich (PxxP) region, with 2 IIe-Pro-Val (IPV) motifs between the WW domain and the PxxP region (37, 38). These multifaceted interactions enable BAG3 to assemble large multichaperone complexes (22–24) that play essential roles in cardiac protein homeostasis, cardiac structure, and cardiac function (39, 40).
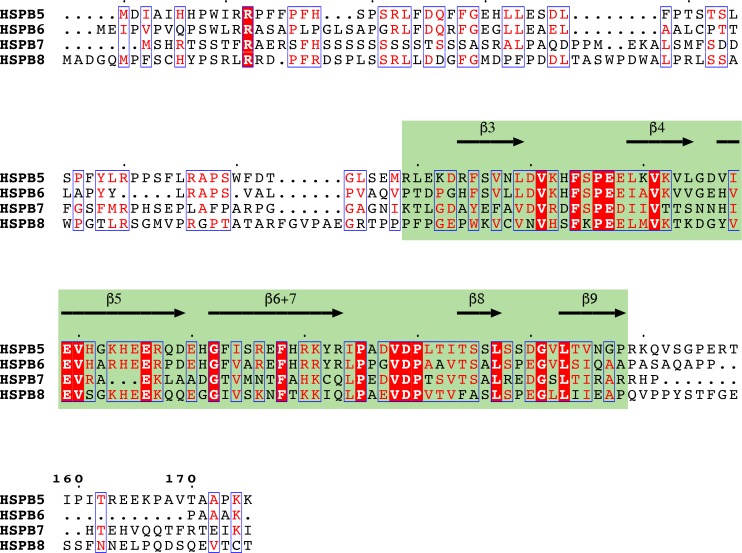
BAG3 physically and functionally links HSP70 and sHSPs.
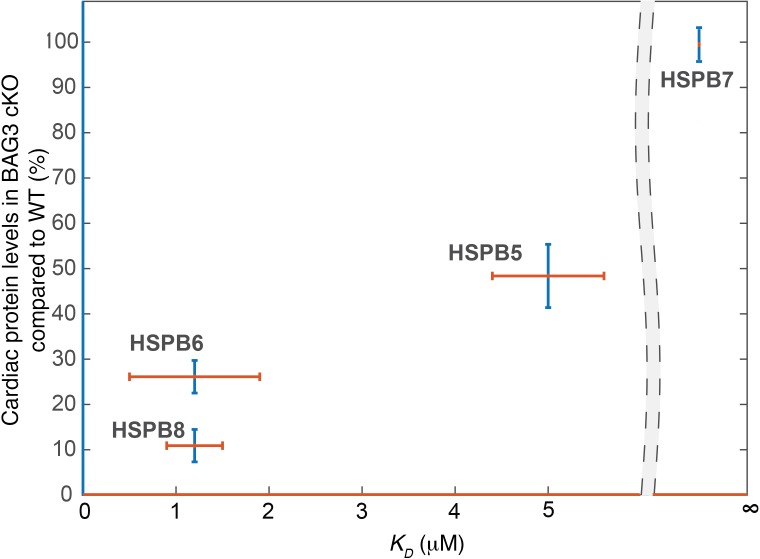
BAG3 has been shown to directly interact with sHSPs, including HSPB5, HSPB6, and HSPB8, in cells overexpressing BAG3 and via glutathione-S-transferase (GST) pull-down assays (22), isothermal titration calorimetry assays (23), and coimmunoprecipitation experiments (24, 41). The 2 IPV motifs in the intermediate region of BAG3 have been shown to directly bind 2 sHSP molecules (22–24), whereas the BAG, WW, and PxxP domains are not essential for this interaction. However, the sHSP-binding site for BAG3 has not been equivocally identified. All mammalian sHSPs consist of a highly conserved central β-sheet containing an α-crystallin domain and highly variable C- and N-terminal extensions (Figure 1). One study mapped the BAG3-binding region of HSPB8 to residues 149–169, encompassing the last 2 β-strands of the α-crystallin domain by generating HSPB8-HSPB1 chimeras (41). This experiment was based on the premise that HSPB1 does not bind BAG3 in pull-down assays when overexpressed in mammalian cells (42). However, more quantitative isothermal calorimetry titration experiments reported an HSPB1-BAG3 interaction, but this affinity was weaker than what was previously measured between BAG3 and other sHSPs (23). Therefore, future studies are required to identify the sHSP-binding sites for BAG3 and to understand the physiologically relevant variation in binding affinities between BAG3 and cardiac sHSPs. Binding with BAG3 promotes the deoligomerization of sHSPs, likely because BAG3 competes with the self-interactions among sHSPs, which normally stabilize large oligomers (23). Forming a complex with HSP70 through BAG3 is essential to the chaperone function of sHSPs because sHSPs lack the enzymatic activity that appears to be required for active remodeling or refolding by the other categories of chaperones (23, 39, 43, 44). Thus, the sHSPs-BAG3-HSP70 complex is essential for denatured proteins to refold to protect against protein aggregation. Our lab recently discovered that without BAG3, or when the BAG domain carries a glutamic acid to lysine (E455K) mutation, which decreases the interaction between BAG3 and HSP70, HSPB5, HSPB6, and HSPB8 become unstable, consequently leading to accumulation of insoluble proteins and dilated cardiomyopathy (DCM) (28). However, the levels of HSPB7, which does not interact with BAG3 (27), were not changed in BAG3-deficient or E455K-mutant mice (28). Interestingly, the binding affinity between specific sHSPs and BAG3 (23, 27) correlates with the magnitude of protein reduction we observed in mice with cardiomyocyte-specific knockout (cKO) of BAG3 or mice expressing the E455K mutation (28, 29) (Figure 2), suggesting that the cardiac functions of HSPB5, HSPB6, and HSPB8, but not HSPB7, are largely dependent on BAG3. In the following sections (as well as summarized in Table 1), we will discuss the cardiac-specific roles of sHSPs, which we have grouped based upon their dependence on BAG3.
Figure 1. Comparison of cardiac sHSP sequences.
Reference sequences of human sHSPs were retrieved from Uniprot database and aligned using Kalign (129). Secondary structure of the α-crystallin domain (green box) was annotated based on NMR structure of αB-crystallin (PDB ID: 2KLR) (130).
Figure 2. Reduction of cardiac sHSP levels in BAG3-cKO mice correlates with their affinity for BAG3.
The x axis (red lines) shows the dissociation constant (KD) between sHSP and BAG3. A lower KD value indicates a stronger interaction between the respective sHSP and BAG3. KD values between sHSP and BAG3 were obtained from Raush et al. (23), and Vos et al. (27) reported the absence of affinity (infinite KD) between HSPB7 and BAG3. The y axis (blue lines) shows cardiac levels of sHSP protein in BAG3-cKO mice relative to WT mice. Cardiac levels of sHSPs in BAG3-cKO mice were obtained from Fang et al. (28). Infinite KD indicates no binding.
Table 1. Summary of BAG3 and sHSPs.
HSPB8.
HSPB8 (also known as protein kinase H11, αC-crystallin, and HSP22) is a ubiquitous 22-kDa sHSP that is predominantly expressed in the heart and skeletal muscles (45, 46). Elevated expression of HSPB8 in the heart has been observed in animal models and patients with various forms of cardiac disease, including both acute and chronic myocardial ischemic injuries, as well as pressure overload–induced cardiac hypertrophy (45, 47, 48). Most cardiac HSPB8 has been found in complexes of high molecular masses, ranging from 25 kDa up to 670 kDa and greater (49). Structure and biochemistry studies determined that, similar to other sHSPs, HSPB8 interacts with itself and/or with other sHSPs to form homo- and hetero-oligomeric complexes (49, 50). Although HSPB8 was initially reported to have protein kinase activity, a series of thorough experiments demonstrated that it lacks kinase activity. In fact, HSPB8 functions as a molecular chaperone to regulate protein quality control (reviewed in ref. 51). Given the predominant expression of this sHSP in the heart under basal conditions and its elevated expression in various forms of cardiac disease (45–48), researchers have long believed that HSPB8 is important for maintaining cardiac function at baseline and in response to cardiac stress. Initial reports sought to overexpress HSPB8 in cardiomyocytes and analyze the cardiac phenotype at baseline and in response to myocardial infarction. A 6- to 7-fold increase in HSPB8 expression in cardiomyocytes causes a compensated cardiac hypertrophy phenotype, with preserved contractile function at 2 to 3 months of age under basal conditions (45, 52). These results are consistent with observations of a dose-dependent prohypertrophic effect of HSPB8 overexpression in cultured cardiomyocytes (53, 54). In response to myocardial infarction stress, HSPB8 overexpression is as powerful as ischemic preconditioning at preventing myocardial infarction and thereby offers preemptive cytoprotection by defending the myocardium against cell death and by promoting the metabolic switch through the IP3K/AKT, PKCε, AMPK, and hypoxia-inducible factor 1-α pathways (52). Loss-of-function studies reveal that HSPB8 is not essential for normal cardiac development or baseline cardiac function; however, the loss of HSPB8 accelerates cardiac dysfunction and remodeling and leads to heart failure in the pressure overload–induced model (55). Mechanistic studies point out that HSPB8 deletion impairs the translocation and phosphorylation of STAT3, as well as gene expression downstream of STAT3 signaling (55).
K141N and K141E, 2 dominant missense mutations that target the same lysine 141 residue in the highly conserved α-crystallin domain of HSPB8, are associated with autosomal dominant distal hereditary motor neuropathy and axonal Charcot-Marie-Tooth disease (56–58). Interestingly, the K141N and K141E mutations in HSPB8 reduce its ability to bind to BAG3 (59) but promote binding with its homo- and hetero-oligomeric partners (56). HSPB8K141N-knockin mice display late-onset muscle atrophy and severe axonal degeneration. This phenotype is likely because of a toxic gain of function of the mutant protein, because Hspb8-knockout mice do not have a discernable phenotype at baseline (57). Notably, the levels of HSPB8 and BAG3 are significantly reduced in the sciatic nerve of 2-month-old HSPB8K141N-expressing mice (57). Although there are no reports regarding the cardiac function of HSPB8K141N mutation in patients or HSPB8K141N-knockin mice, cardiac-specific expression of HSPB8K141N in Tg mice results in mild hypertrophy and a slight reduction of cardiac function (60).
HSPB6.
Kato et al. first identified HSPB6 (also known as HSP20 and P20) in the skeletal muscles of rats and humans (61). Similar to HSPB8, HSPB6 is ubiquitously expressed but highly enriched in cardiac, skeletal, and smooth muscle tissues (61–63). It is well accepted that HSPB6 forms homo- or hetero-oligomeric complexes that may undergo stress-induced dissociation accompanied by increased chaperone activity (61). In addition to a conserved α-crystallin domain, HSPB6 contains a homology sequence of troponin I, a domain that inhibits platelet aggregation, and a consensus motif (RRAS) for PKA/PKG-dependent phosphorylation at Ser16 (reviewed in ref. 64). The expression levels of HSPB6 and its phosphorylation at Ser16 are increased in β-adrenergic agonist–stimulated cardiomyocytes (65), postinfarcted hearts (66, 67), and failing hearts (67, 68). Reports from in vivo and in vitro studies indicate that HSPB6 protects cardiomyocytes against apoptosis, and phosphorylation at the Ser16 site enhances HSPB6-mediated cardioprotection (63, 64). Two comprehensive reviews have summarized these studies of the cardiac-specific roles of HSPB6 (please refer to reviews in refs. 63 and 64). Here, we discuss HSPB6 mutation(s) associated with human cardiomyopathy, as well as several genetic mouse models involved in studying the cardiac function of HSPB6.
Multiple studies have shown that S10F and P20L substitutions in HSPB6 are associated with human DCM (6, 7). Computational structure analysis indicates that the S10F mutation may change the content of the helix, strand, and loop in the secondary structure of HSPB6, which may consequently affect its function (7). Liu et al. (7) generated HSPB6S10F-Tg mice, with 10-fold overexpression of mutant HSPB6 in the heart, to investigate cardiac-specific effects. Compared with non-Tg controls, male HSPB6S10F-Tg mice developed DCM at 6 months of age, and their survival rate declined rapidly by 11 months (7). Interestingly, cardiac overexpression of HSPB6S10F induces lethal peripartum cardiomyopathy in female Tg mice (8). The P20L mutation is located 4 amino acids downstream of the PKA/PKG-dependent Ser16 phosphorylation site (6). In vitro studies reveal diminished phosphorylation and the loss of cytoprotective properties in HSPB6P20L-expressing cells (6). Qian et al. studied a cardiac-specific Tg model with 7-fold overexpression of a phospho-null, mutant HSPB6 (HSPB6S16A, referred to as S16A-Tg), and their findings supported a cardioprotective role for the phosphorylated form of HSPB6. In comparison with non-Tg controls, S16A-Tg mice are more sensitive to I/R injury, as evidenced by a lower contractile function recovery rate, increased necrosis and apoptosis, and decreased autophagy (67). Moreover, Edwards et al. reported that overexpression of the phosphomimetic form of HSPB6 (HSPB6S16D) in vivo confers protection from β-agonist–induced apoptosis in the heart (69). In addition, overexpression of WT HSPB6 in the heart protects against I/R injury as well as cardiac stress from LPS or ISO treatment (70–72). Together, these studies suggest that HSPB6 may constitute a new therapeutic target for ischemic heart diseases; however, analysis of knockout and knockin mouse models is required to unequivocally study the cardiac role of HSPB6.
HSPB5.
HSPB5 (also referred to as αB-crystallin) was initially identified as an abundant component of ocular lenses and is thus referred to as αB-crystallin (73, 74). However, further studies have demonstrated that αB-crystallin expression is not restricted to the eye. Various tissues, including the cardiac muscle, express αB-crystallin at a level that is equal to 3% to 5% of the total soluble protein (73, 75, 76). During cardiac ischemia stress, αB-crystallin rapidly translocates from the cytosol to the myofibrils (77–79). An important binding partner of αB-crystallin in cardiac myofibrils is titin (77, 80, 81). αB-crystallin interacts with the extensible N2B region of titin (77, 80, 81), which is exclusively found in cardiac isoforms and regulates cardiac elasticity and diastolic function (82–85). αB-crystallin can also translocate to the mitochondria, where it inhibits mitochondrial permeability transition pore opening, thus stabilizing mitochondrial membrane potential and inhibiting apoptosis (86–89). In the heart, αB-crystallin undergoes at least 2 types of posttranslational modification, phosphorylation and O-GlcNAcylation (an O-linked attachment of the monosaccharide β-N-acetyl-glucosamine), which potentially regulate αB-crystallin translocation and cellular function (88, 90–95). In vitro studies of adenovirus-mediated overexpression of αB-crystallin in neonatal cardiomyocytes, as well as in vivo Tg models of αB-crystallin overexpression, have elucidated a cardioprotective role of αB-crystallin under myocardial ischemia (96, 97).
The αB-crystallin–encoding and HSPB2-encoding genes are in close proximity to one another; therefore, generation of αB-crystallin–knockout mice also disrupts HSPB2 (98, 99). At baseline, mice lacking both αB-crystallin and HSPB2 (double-knockout [DKO] mice) do not have an obvious phenotype in the early stages of their lives; however, these animals begin to die at 30 weeks of age, and less than 20% of the DKO mice survive to 40 weeks due to skeletal muscle defects (82, 98–100). In-depth analysis revealed that αB-crystallin and HSPB2 deficiency activates the nuclear factor of activated T cells/calcineurin (NFAT/calcineurin) pathway, leading to the development of cardiac hypertrophy during stress-free or minimal-stress conditions, and exacerbates cardiac dysfunction in response to pressure overload. Additionally, αB-crystallin overexpression attenuates the hypertrophic response to pressure overload via suppression of the NFAT/calcineurin pathway (101). In ex vivo Langendorff-perfused experiments, DKO hearts exhibit lower contractile recovery along with an increase in necrosis and apoptosis after I/R (99, 100). In contrast, Benjamin et al. reported smaller infarct sizes in DKO hearts compared with those from WT controls in both in vivo and ex vivo I/R injury models (102). Thus far, no single αB-crystallin cKO model has been generated.
Numerous mutations in the αB-crystallin–encoding gene have been associated with cardiomyopathy (5, 9–18). The first αB-crystallin mutation to be identified was the R120G mutation, which was discovered in a large multigenerational French family. This mutation causes cataracts, hypertrophic cardiomyopathy, and DRMs, in which the formation of intracellular aggregates containing both desmin and αB-crystallin are observed in muscle fibers (5). A D109H mutation in αB-crystallin was identified in a 2-generation family, with 5 affected individuals who displayed a very similar clinical phenotype to those described in the R120G family, suggesting a similar pathogenic mechanism (11). Additionally, Q151X and 464delCT mutations are linked to DRM (18), and R157H and G154S mutations have been reported in patients with late-onset DCM (12, 13). However, these 2 mutations have incomplete penetrance of the cardiac phenotype, indicating that unknown genetic modifier(s) may be required to manifest a cardiomyopathy phenotype in patients carrying R157H or G154S mutations (13, 14). Although the characterization of the effects of most αB-crystallin mutations is limited, the functional consequences of the R120G mutation have been extensively investigated. The R120G mutation resides in the ACD of αB-crystallin, and protein structure studies reveal that this mutation results in an irregular structure that stabilizes a closed-groove dimer, forms large oligomers, and is prone to aggregation (11, 103–105). In vitro assays have shown that the chaperone function of R120G-mutant αB-crystallin is significantly reduced or abolished (104, 106). The R120G mutation also markedly enhances the binding affinity between αB-crystallin and desmin filaments (107). The combined loss of chaperone function and the strong binding with desmin leads to aggregation of intermediate filament proteins with the mutant αB-crystallin, as well as the formation of inclusion bodies in cells (104, 107, 108).
Cardiac-specific expression of αB-crystallin R120G recapitulates desmin-related cardiomyopathy in Tg mice (109). These animals display severe cardiomyopathy, early death, aberrant desmin expression, and αB-crystallin aggregation in a gene dosage–dependent manner (109). Tg mice with high expression of the R120G mutation exhibit 100% mortality by early adulthood, and those with modest expression of this mutation exhibit phenotypes that appear at a later stage but are strikingly similar to clinical features of desmin-related cardiomyopathies, including cardiac hypertrophy, overtly affected desmin filaments, and impaired myofibril alignment (109). These observations suggest a dosage- and time-dependent process in αB-crystallin R120G mutation–associated cardiomyopathy. However, the αB-crystallin R120G–knockin mouse model does not exhibit premature lethality like the R120G-Tg mice (110). Although R120G-knockin mice have been reported to develop skeletal muscle defects and cataracts similar to humans with the homologous mutation, no careful characterization of the heart in this mouse model has been carried out (110). Therefore, it is unclear whether the aberrant desmin and αB-crystallin aggregation observed in the skeletal muscle of R120G-knockin mice also occurs in the cardiac muscle or whether a late-onset cardiomyopathy would develop in the aged R120G-knockin mice (110).
HSPB7- and BAG3-independent sHSP function
Besides collaborating with BAG3 and HSP70, some sHSPs have been shown to have diverse functions beyond their classically defined chaperone activity. These functions are independent of the HSP70-BAG3 complex (4, 27, 111–115) and include cytoskeleton assembly (111, 112), RNA splicing (113, 114), and HSP70 machinery–independent aggregation suppression (4, 27, 115). The most typical BAG3-independent sHSP is HSPB7, which is highly expressed in the heart (27, 116). Unlike HSPB5, HSPB6, and HSPB8, HSPB7 does not interact with BAG3 (27), and its stability is not impaired in BAG3-deficient cardiomyocytes (28). Upon overexpression in cells, HSPB7 does not form large oligomers (27). The ability of HSPB7 to prevent aggregation of polyglutamine (polyQ) proteins and the parkin (PARK2) C289G mutant is not dependent on the expression of heat shock factor 1 or HSP70. (27, 115). These phenomena all point out that the HSP70-BAG3 complex functions independently of HSPB7.
Many intronic SNPs have been identified in the HSPB7-encoding gene and have been found to be highly associated with HF, DCM, and idiopathic DCM in human patients (117–122), highlighting an important role for HSPB7 in maintaining cardiac function. Global depletion of Hspb7 in zebrafish disrupts normal cardiac morphogenesis (123, 124), and the essential role of HSPB7 in cardiomyocytes is further supported by the discovery that the global and cKO of HSPB7 in mice results in embryonic lethality between embryonic days 11.5 and 12.5 (E11.5–12.5) (29). Using biochemical assays, our laboratory recently revealed that HSPB7 plays a critical role in directly modulating actin filament length by binding to monomeric actin and limiting its availability for polymerization (125). Data from inducible, cardiomyocyte-specific–knockout HSPB7 (HSPB7-icKO) mice demonstrated that HSPB7 is also essential for adult cardiac function (126). HSPB7-icKO mice develop cardiac arrhythmia, and several cardiac intercalated disc proteins, such as connexin 43, desmoplakin, and N-cadherin, are downregulated, while filamin C protein aggregates are enriched in HSPB7-icKO murine hearts (126). It has also recently been reported that loss of HSPB7 in zebrafish or human cardiomyocytes leads to enhanced autophagic pathways (127). Inhibition of autophagy results in filamin C aggregation in HSPB7-null human cardiomyocytes and developmental cardiomyopathy in hspb7-null zebrafish embryos, suggesting that HSPB7 may be involved in regulating the autophagic processing of specific proteins within cardiomyocytes, but this remains to be fully explored (127).
Conclusion and future prospects
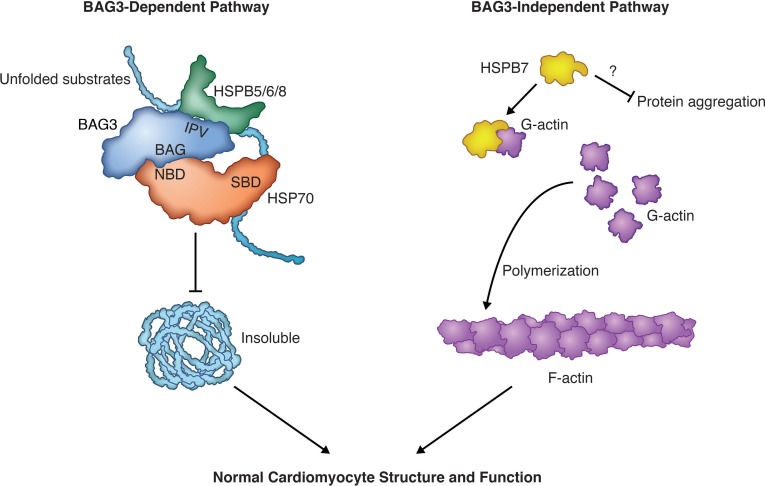
In this Review, we have summarized the diverse functions of sHSPs in the heart (Table 1) and have divided cardiac sHSPs into 2 distinct groups: BAG3-dependent (HSPB5, HSPB6, and HSPB8) and -independent (HSPB7) pathways (Figure 3). In BAG3-dependent pathways, HSPB5, HSPB6, and HSPB8 physically and functionally interact with BAG3 and form a multichaperone complex (22–26). These sHSPs are destabilized in cardiomyocytes lacking BAG3 function, with the magnitude of protein loss correlating with the binding affinity between the respective sHSP and BAG3 (refs. 23, 27–29, and Figure 2). Although almost all HSPB8 protein is lost upon BAG3 deletion, HSPB6 and HSPB5 levels are preserved at about 20% and 50%, respectively, in BAG3-deficient cardiomyocytes, implying that HSPB6 and HSPB5 may also carry BAG3-independent functions. To further elucidate potential BAG3-dependent roles of cardiac sHSPs, it will be interesting to investigate mice harboring genetically engineered mutations that disrupt interactions between BAG3 and sHSPs. Meanwhile, lack of basal phenotypes in mice harboring global deletions of HSPB8 (55), and HSPB5 and HSPB2 (82, 98–100), suggests potential genetic redundancy among HSPB5, HSPB6, and HSPB8. The generation of mice lacking all 3 BAG3-dependent sHSPs (HSPB5, HSPB6, and HSPB8) will be important to avoid potential compensation issues when addressing essential functions of these proteins in the heart.
Figure 3. The BAG3-dependent and -independent pathways of cardiac sHSPs.
HSPB5/6/8 represent the BAG3-dependent sHSPs (left), in which HSPB5/6/8 physically and functionally interact with BAG3 to form a multichaperone complex that prevents unfolded substrates from becoming insoluble in cardiomyocytes. HSPB7 represents the BAG3-independent cardiac sHSP (right) that binds to monomeric actin and limits its availability for polymerization. However, it is still unknown whether HSPB7 carries out chaperone activity in vivo. SBD, substrate-binding domain; NBD, nucleotide-binding domain. Illustrated by Rachel Davidowitz.
Although these BAG3-dependent sHSPs mediate critical roles within the heart, we also summarized the impact HSPB7 has in the heart independent of BAG3. Three key pieces of evidence support the premise that HSPB7 exhibits BAG3-independent roles in cardiomyocytes. First, HSPB7 does not interact with BAG3 (27). Second, protein stability of HSPB7 is not dependent on BAG3 (27–29). Third, HSPB7-knockout mice die between E11.5 and E12.5 because of heart defects (29), a phenotype that is much severer than that of BAG3-knockout mice, which die immediately after weaning at 21 days after birth (27, 30, 128). HSPB7 directly modulates actin thin filament length in developing cardiomyocytes by binding monomeric actin and limiting its availability for polymerization (29). However, it remains unclear why or how loss of HSPB7 in adult cardiomyocytes results in accumulation of proteins, such as filamin C. Although Mymrikov et al. showed that HSPB7 does not exhibit chaperone activity in vitro (19), it is still unknown whether HSPB7 carries out chaperone activity in vivo or whether any other cochaperone proteins, analogous to BAG3, interact with HSPB7 to facilitate its potential protein quality control functions. Future studies of HSPB7-interacting partners may reveal novel functions of HSPB7 in adult cardiomyocytes.
Acknowledgments
XF is supported by NIH grant K99HL143210. JC is funded by grants from the National Heart, Lung, and Blood Institute and the Fondation Leducq (TNE-13CVD04) and holds an American Heart Association Endowed Chair in Cardiovascular Research. JB is supported by the European Commission’s Marie Sklodowska-Curie Individual Fellowship (Titin Signals, 656636).
Version 1. 02/21/2019
Electronic publication
Version 2. 02/22/2019
The authorship note was added.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Published: February 21, 2019
Reference information: JCI Insight. 2019;4(4):e126464. https://doi.org/10.1172/jci.insight.126464.
Contributor Information
Xi Fang, Email: xifang@ucsd.edu.
Julius Bogomolovas, Email: jbogomolovas@ucsd.edu.
Ju Chen, Email: juchen@ucsd.edu.
References
- 1.Carra S, et al. The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperones. 2017;22(4):601–611. doi: 10.1007/s12192-017-0787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12(10):842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- 3.Kappé G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8(1):53–61. doi: 10.1379/1466-1268(2003)8<53:THGECS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos MJ, Kanon B, Kampinga HH. HSPB7 is a SC35 speckle resident small heat shock protein. Biochim Biophys Acta. 2009;1793(8):1343–1353. doi: 10.1016/j.bbamcr.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Vicart P, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20(1):92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 6.Nicolaou P, et al. Human mutation in the anti-apoptotic heat shock protein 20 abrogates its cardioprotective effects. J Biol Chem. 2008;283(48):33465–33471. doi: 10.1074/jbc.M802307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu GS, et al. Regulation of BECN1-mediated autophagy by HSPB6: Insights from a human HSPB6S10F mutant. Autophagy. 2018;14(1):80–97. doi: 10.1080/15548627.2017.1392420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu GS, et al. A novel human S10F-Hsp20 mutation induces lethal peripartum cardiomyopathy. J Cell Mol Med. 2018;22(8):3911–3919. doi: 10.1111/jcmm.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fardeau M, et al. [A new familial muscular disorder demonstrated by the intra-sarcoplasmic accumulation of a granulo-filamentous material which is dense on electron microscopy (author’s transl)] Rev Neurol (Paris) 1978;134(6–7):411–425. [PubMed] [Google Scholar]
- 10.Sacconi S, et al. A novel CRYAB mutation resulting in multisystemic disease. Neuromuscul Disord. 2012;22(1):66–72. doi: 10.1016/j.nmd.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Bagnéris C, et al. Crystal structures of alpha-crystallin domain dimers of αB-crystallin and Hsp20. J Mol Biol. 2009;392(5):1242–1252. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki N, et al. αB-crystallin mutation in dilated cardiomyopathy. Biochem Biophys Res Commun. 2006;342(2):379–386. doi: 10.1016/j.bbrc.2006.01.154. [DOI] [PubMed] [Google Scholar]
- 13.Pilotto A, Marziliano N, Pasotti M, Grasso M, Costante AM, Arbustini E. αB-crystallin mutation in dilated cardiomyopathies: low prevalence in a consecutive series of 200 unrelated probands. Biochem Biophys Res Commun. 2006;346(4):1115–1117. doi: 10.1016/j.bbrc.2006.05.203. [DOI] [PubMed] [Google Scholar]
- 14.Reilich P, et al. The p.G154S mutation of the αB crystallin gene (CRYAB) causes late-onset distal myopathy. Neuromuscul Disord. 2010;20(4):255–259. doi: 10.1016/j.nmd.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Del Bigio MR, et al. Infantile muscular dystrophy in Canadian aboriginals is an αB-crystallinopathy. Ann Neurol. 2011;69(5):866–871. doi: 10.1002/ana.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrest KM, et al. Infantile onset myofibrillar myopathy due to recessive CRYAB mutations. Neuromuscul Disord. 2011;21(1):37–40. doi: 10.1016/j.nmd.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Lacson AG, et al. Autosomal recessive, fatal infantile hypertonic muscular dystrophy among Canadian Natives. Can J Neurol Sci. 1994;21(3):203–212. doi: 10.1017/S0317167100041172. [DOI] [PubMed] [Google Scholar]
- 18.Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative αB-crystallin mutations. Ann Neurol. 2003;54(6):804–810. doi: 10.1002/ana.10767. [DOI] [PubMed] [Google Scholar]
- 19.Mymrikov EV, Daake M, Richter B, Haslbeck M, Buchner J. The chaperone activity and substrate spectrum of human small heat shock proteins. J Biol Chem. 2017;292(2):672–684. doi: 10.1074/jbc.M116.760413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang X, Trexler C, Chen J. Ushering in the cardiac role of Ubiquilin1. J Clin Invest. 2018;128(12):5195–5197. doi: 10.1172/JCI124567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giffard RG, Macario AJ, de Macario EC. The future of molecular chaperones and beyond. J Clin Invest. 2013;123(8):3206–3208. doi: 10.1172/JCI70799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hishiya A, Salman MN, Carra S, Kampinga HH, Takayama S. BAG3 directly interacts with mutated αB-crystallin to suppress its aggregation and toxicity. PLoS One. 2011;6(3):e16828. doi: 10.1371/journal.pone.0016828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauch JN, et al. BAG3 is a modular, scaffolding protein that physically links heat shock protein 70 (Hsp70) to the small heat shock proteins. J Mol Biol. 2017;429(1):128–141. doi: 10.1016/j.jmb.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carra S, Seguin SJ, Landry J. HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy. 2008;4(2):237–239. doi: 10.4161/auto.5407. [DOI] [PubMed] [Google Scholar]
- 25.Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274(2):781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- 26.Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3(10):E237–E241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- 27.Vos MJ, et al. HSPB7 is the most potent polyQ aggregation suppressor within the HSPB family of molecular chaperones. Hum Mol Genet. 2010;19(23):4677–4693. doi: 10.1093/hmg/ddq398. [DOI] [PubMed] [Google Scholar]
- 28.Fang X, et al. Loss-of-function mutations in co-chaperone BAG3 destabilize small HSPs and cause cardiomyopathy. J Clin Invest. 2017;127(8):3189–3200. doi: 10.1172/JCI94310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Judge LM, et al. A BAG3 chaperone complex maintains cardiomyocyte function during proteotoxic stress. JCI Insight. 2017;2(14):94623. doi: 10.1172/jci.insight.94623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol. 2006;169(3):761–773. doi: 10.2353/ajpath.2006.060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizushima W, Sadoshima J. BAG3 plays a central role in proteostasis in the heart. J Clin Invest. 2017;127(8):2900–2903. doi: 10.1172/JCI95839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arimura T, Ishikawa T, Nunoda S, Kawai S, Kimura A. Dilated cardiomyopathy-associated BAG3 mutations impair Z-disc assembly and enhance sensitivity to apoptosis in cardiomyocytes. Hum Mutat. 2011;32(12):1481–1491. doi: 10.1002/humu.21603. [DOI] [PubMed] [Google Scholar]
- 33.Chami N, et al. Nonsense mutations in BAG3 are associated with early-onset dilated cardiomyopathy in French Canadians. Can J Cardiol. 2014;30(12):1655–1661. doi: 10.1016/j.cjca.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 34.Feldman AM, et al. Decreased levels of BAG3 in a family with a rare variant and in idiopathic dilated cardiomyopathy. J Cell Physiol. 2014;229(11):1697–1702. doi: 10.1002/jcp.24615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norton N, et al. Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am J Hum Genet. 2011;88(3):273–282. doi: 10.1016/j.ajhg.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villard E, et al. A genome-wide association study identifies two loci associated with heart failure due to dilated cardiomyopathy. Eur Heart J. 2011;32(9):1065–1076. doi: 10.1093/eurheartj/ehr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosati A, Graziano V, De Laurenzi V, Pascale M, Turco MC. BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis. 2011;2:e141. doi: 10.1038/cddis.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doong H, Vrailas A, Kohn EC. What’s in the ‘BAG’? — A functional domain analysis of the BAG-family proteins. Cancer Lett. 2002;188(1-2):25–32. doi: 10.1016/S0304-3835(02)00456-1. [DOI] [PubMed] [Google Scholar]
- 39.Sontake V, et al. Hsp90 regulation of fibroblast activation in pulmonary fibrosis. JCI Insight. 2017;2(4):e91454. doi: 10.1172/jci.insight.91454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su F, et al. Bcl-2-associated athanogene 3 protects the heart from ischemia/reperfusion injury. JCI Insight. 2016;1(19):e90931. doi: 10.1172/jci.insight.90931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs M, et al. Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction. Biochem J. 2009;425(1):245–255. doi: 10.1042/BJ20090907. [DOI] [PubMed] [Google Scholar]
- 42.Carra S, Seguin SJ, Lambert H, Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem. 2008;283(3):1437–1444. doi: 10.1074/jbc.M706304200. [DOI] [PubMed] [Google Scholar]
- 43.Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol. 2015;427(7):1537–1548. doi: 10.1016/j.jmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glazier AA, et al. HSC70 is a chaperone for wild-type and mutant cardiac myosin binding protein C. JCI Insight. 2018;3(11):e99319. doi: 10.1172/jci.insight.99319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Depre C, et al. H11 kinase is a novel mediator of myocardial hypertrophy in vivo. Circ Res. 2002;91(11):1007–1014. doi: 10.1161/01.RES.0000044380.54893.4B. [DOI] [PubMed] [Google Scholar]
- 46.Verschuure P, Tatard C, Boelens WC, Grongnet JF, David JC. Expression of small heat shock proteins HspB2, HspB8, Hsp20 and cvHsp in different tissues of the perinatal developing pig. Eur J Cell Biol. 2003;82(10):523–530. doi: 10.1078/0171-9335-00337. [DOI] [PubMed] [Google Scholar]
- 47.Depre C, et al. Gene program for cardiac cell survival induced by transient ischemia in conscious pigs. Proc Natl Acad Sci U S A. 2001;98(16):9336–9341. doi: 10.1073/pnas.171297498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Depre C, et al. Program of cell survival underlying human and experimental hibernating myocardium. Circ Res. 2004;95(4):433–440. doi: 10.1161/01.RES.0000138301.42713.18. [DOI] [PubMed] [Google Scholar]
- 49.Sun X, Fontaine JM, Rest JS, Shelden EA, Welsh MJ, Benndorf R. Interaction of human HSP22 (HSPB8) with other small heat shock proteins. J Biol Chem. 2004;279(4):2394–2402. doi: 10.1074/jbc.M311324200. [DOI] [PubMed] [Google Scholar]
- 50.Arrigo AP. Human small heat shock proteins: protein interactomes of homo- and hetero-oligomeric complexes: an update. FEBS Lett. 2013;587(13):1959–1969. doi: 10.1016/j.febslet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Li F, Xiao H, Hu Z, Zhou F, Yang B. Exploring the multifaceted roles of heat shock protein B8 (HSPB8) in diseases. Eur J Cell Biol. 2018;97(3):216–229. doi: 10.1016/j.ejcb.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Depre C, et al. H11 kinase prevents myocardial infarction by preemptive preconditioning of the heart. Circ Res. 2006;98(2):280–288. doi: 10.1161/01.RES.0000201284.45482.e8. [DOI] [PubMed] [Google Scholar]
- 53.Hase M, Depre C, Vatner SF, Sadoshima J. H11 has dose-dependent and dual hypertrophic and proapoptotic functions in cardiac myocytes. Biochem J. 2005;388(Pt 2):475–483. doi: 10.1042/BJ20041314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sui X, Li D, Qiu H, Gaussin V, Depre C. Activation of the bone morphogenetic protein receptor by H11kinase/Hsp22 promotes cardiac cell growth and survival. Circ Res. 2009;104(7):887–895. doi: 10.1161/CIRCRESAHA.108.192328. [DOI] [PubMed] [Google Scholar]
- 55.Qiu H, et al. H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of STAT3 and accelerates the transition into heart failure on cardiac overload. Circulation. 2011;124(4):406–415. doi: 10.1161/CIRCULATIONAHA.110.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irobi J, et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet. 2004;36(6):597–601. doi: 10.1038/ng1328. [DOI] [PubMed] [Google Scholar]
- 57.Bouhy D, et al. A knock-in/knock-out mouse model of HSPB8-associated distal hereditary motor neuropathy and myopathy reveals toxic gain-of-function of mutant Hspb8. Acta Neuropathol. 2018;135(1):131–148. doi: 10.1007/s00401-017-1756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang BS, et al. Small heat-shock protein 22 mutated in autosomal dominant Charcot-Marie-Tooth disease type 2L. Hum Genet. 2005;116(3):222–224. doi: 10.1007/s00439-004-1218-3. [DOI] [PubMed] [Google Scholar]
- 59.Carra S, et al. Identification of the Drosophila ortholog of HSPB8: implication of HSPB8 loss of function in protein folding diseases. J Biol Chem. 2010;285(48):37811–37822. doi: 10.1074/jbc.M110.127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanbe A, et al. Phenotype of cardiomyopathy in cardiac-specific heat shock protein B8 K141N transgenic mouse. J Biol Chem. 2013;288(13):8910–8921. doi: 10.1074/jbc.M112.368324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato K, Goto S, Inaguma Y, Hasegawa K, Morishita R, Asano T. Purification and characterization of a 20-kDa protein that is highly homologous to alpha B crystallin. J Biol Chem. 1994;269(21):15302–15309. [PubMed] [Google Scholar]
- 62.Salinthone S, Tyagi M, Gerthoffer WT. Small heat shock proteins in smooth muscle. Pharmacol Ther. 2008;119(1):44–54. doi: 10.1016/j.pharmthera.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan GC, Chu G, Kranias EG. Hsp20 and its cardioprotection. Trends Cardiovasc Med. 2005;15(4):138–141. doi: 10.1016/j.tcm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Fan GC, Kranias EG. Small heat shock protein 20 (HspB6) in cardiac hypertrophy and failure. J Mol Cell Cardiol. 2011;51(4):574–577. doi: 10.1016/j.yjmcc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu G, et al. Phosphoproteome analysis of cardiomyocytes subjected to beta-adrenergic stimulation: identification and characterization of a cardiac heat shock protein p20. Circ Res. 2004;94(2):184–193. doi: 10.1161/01.RES.0000107198.90218.21. [DOI] [PubMed] [Google Scholar]
- 66.Qian J, et al. Small heat shock protein 20 interacts with protein phosphatase-1 and enhances sarcoplasmic reticulum calcium cycling. Circ Res. 2011;108(12):1429–1438. doi: 10.1161/CIRCRESAHA.110.237644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qian J, et al. Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ Res. 2009;105(12):1223–1231. doi: 10.1161/CIRCRESAHA.109.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dohke T, et al. Proteomic analysis reveals significant alternations of cardiac small heat shock protein expression in congestive heart failure. J Card Fail. 2006;12(1):77–84. doi: 10.1016/j.cardfail.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Edwards HV, Scott JD, Baillie GS. PKA phosphorylation of the small heat-shock protein Hsp20 enhances its cardioprotective effects. Biochem Soc Trans. 2012;40(1):210–214. doi: 10.1042/BST20110673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan GC, et al. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111(14):1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- 71.Wang X, et al. Overexpression of Hsp20 prevents endotoxin-induced myocardial dysfunction and apoptosis via inhibition of NF-kappaB activation. J Mol Cell Cardiol. 2009;47(3):382–390. doi: 10.1016/j.yjmcc.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan GC, et al. Small heat-shock protein Hsp20 attenuates beta-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ Res. 2006;99(11):1233–1242. doi: 10.1161/01.RES.0000251074.19348.af. [DOI] [PubMed] [Google Scholar]
- 73.Bhat SP, Nagineni CN. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989;158(1):319–325. doi: 10.1016/S0006-291X(89)80215-3. [DOI] [PubMed] [Google Scholar]
- 74.Ruebsam A, et al. A specific phosphorylation regulates the protective role of αA-crystallin in diabetes. JCI Insight. 2018;3(4):e97919. doi: 10.1172/jci.insight.97919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kato K, Shinohara H, Kurobe N, Inaguma Y, Shimizu K, Ohshima K. Tissue distribution and developmental profiles of immunoreactive αB crystallin in the rat determined with a sensitive immunoassay system. Biochim Biophys Acta. 1991;1074(1):201–208. doi: 10.1016/0304-4165(91)90062-l. [DOI] [PubMed] [Google Scholar]
- 76.Bhat SP, Horwitz J, Srinivasan A, Ding L. Alpha B-crystallin exists as an independent protein in the heart and in the lens. Eur J Biochem. 1991;202(3):775–781. doi: 10.1111/j.1432-1033.1991.tb16432.x. [DOI] [PubMed] [Google Scholar]
- 77.Golenhofen N, Arbeiter A, Koob R, Drenckhahn D. Ischemia-induced association of the stress protein alpha B-crystallin with I-band portion of cardiac titin. J Mol Cell Cardiol. 2002;34(3):309–319. doi: 10.1006/jmcc.2001.1513. [DOI] [PubMed] [Google Scholar]
- 78.Golenhofen N, Htun P, Ness W, Koob R, Schaper W, Drenckhahn D. Binding of the stress protein αB-crystallin to cardiac myofibrils correlates with the degree of myocardial damage during ischemia/reperfusion in vivo. J Mol Cell Cardiol. 1999;31(3):569–580. doi: 10.1006/jmcc.1998.0892. [DOI] [PubMed] [Google Scholar]
- 79.Golenhofen N, Ness W, Koob R, Htun P, Schaper W, Drenckhahn D. Ischemia-induced phosphorylation and translocation of stress protein αB-crystallin to Z lines of myocardium. Am J Physiol. 1998;274(5 pt 2):H1457–H1464. doi: 10.1152/ajpheart.1998.274.5.H1457. [DOI] [PubMed] [Google Scholar]
- 80.Bullard B, et al. Association of the chaperone αB-crystallin with titin in heart muscle. J Biol Chem. 2004;279(9):7917–7924. doi: 10.1074/jbc.M307473200. [DOI] [PubMed] [Google Scholar]
- 81.Zhu Y, Bogomolovas J, Labeit S, Granzier H. Single molecule force spectroscopy of the cardiac titin N2B element: effects of the molecular chaperone αB-crystallin with disease-causing mutations. J Biol Chem. 2009;284(20):13914–13923. doi: 10.1074/jbc.M809743200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Golenhofen N, Redel A, Wawrousek EF, Drenckhahn D. Ischemia-induced increase of stiffness of αB-crystallin/HSPB2-deficient myocardium. Pflugers Arch. 2006;451(4):518–525. doi: 10.1007/s00424-005-1488-1. [DOI] [PubMed] [Google Scholar]
- 83.Radke MH, et al. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci U S A. 2007;104(9):3444–3449. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kötter S, et al. Human myocytes are protected from titin aggregation-induced stiffening by small heat shock proteins. J Cell Biol. 2014;204(2):187–202. doi: 10.1083/jcb.201306077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Franssen C, Kole J, Musters R, Hamdani N, Paulus WJ. αB crystallin reverses high diastolic stiffness of failing human cardiomyocytes. Circ Heart Fail. 2017;10(3):e003626. doi: 10.1161/CIRCHEARTFAILURE.116.003626. [DOI] [PubMed] [Google Scholar]
- 86.Chis R, et al. α-Crystallin B prevents apoptosis after H2O2 exposure in mouse neonatal cardiomyocytes. Am J Physiol Heart Circ Physiol. 2012;303(8):H967–H978. doi: 10.1152/ajpheart.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mitra A, Basak T, Datta K, Naskar S, Sengupta S, Sarkar S. Role of α-crystallin B as a regulatory switch in modulating cardiomyocyte apoptosis by mitochondria or endoplasmic reticulum during cardiac hypertrophy and myocardial infarction. Cell Death Dis. 2013;4:e582. doi: 10.1038/cddis.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whittaker R, Glassy MS, Gude N, Sussman MA, Gottlieb RA, Glembotski CC. Kinetics of the translocation and phosphorylation of αB-crystallin in mouse heart mitochondria during ex vivo ischemia. Am J Physiol Heart Circ Physiol. 2009;296(5):H1633–H1642. doi: 10.1152/ajpheart.01227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin JK, Whittaker R, Glassy MS, Barlow SB, Gottlieb RA, Glembotski CC. Localization of phosphorylated αB-crystallin to heart mitochondria during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294(1):H337–H344. doi: 10.1152/ajpheart.00881.2007. [DOI] [PubMed] [Google Scholar]
- 90.Aggeli IK, Beis I, Gaitanaki C. Oxidative stress and calpain inhibition induce αB-crystallin phosphorylation via p38-MAPK and calcium signalling pathways in H9c2 cells. Cell Signal. 2008;20(7):1292–1302. doi: 10.1016/j.cellsig.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 91.Hoover HE, Thuerauf DJ, Martindale JJ, Glembotski CC. αB-crystallin gene induction and phosphorylation by MKK6-activated p38. A potential role for alpha B-crystallin as a target of the p38 branch of the cardiac stress response. J Biol Chem. 2000;275(31):23825–23833. doi: 10.1074/jbc.M003864200. [DOI] [PubMed] [Google Scholar]
- 92.Shu E, et al. αB-crystallin is phosphorylated during myocardial infarction: involvement of platelet-derived growth factor-BB. Arch Biochem Biophys. 2005;438(2):111–118. doi: 10.1016/j.abb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 93.Morrison LE, Hoover HE, Thuerauf DJ, Glembotski CC. Mimicking phosphorylation of αB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ Res. 2003;92(2):203–211. doi: 10.1161/01.RES.0000052989.83995.A5. [DOI] [PubMed] [Google Scholar]
- 94.Roquemore EP, Chevrier MR, Cotter RJ, Hart GW. Dynamic O-GlcNAcylation of the small heat shock protein αB-crystallin. Biochemistry. 1996;35(11):3578–3586. doi: 10.1021/bi951918j. [DOI] [PubMed] [Google Scholar]
- 95.Krishnamoorthy V, Donofrio AJ, Martin JL. O-GlcNAcylation of αB-crystallin regulates its stress-induced translocation and cytoprotection. Mol Cell Biochem. 2013;379(1–2):59–68. doi: 10.1007/s11010-013-1627-5. [DOI] [PubMed] [Google Scholar]
- 96.Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgene overexpression of αB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 2001;15(2):393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- 97.Martin JL, Mestril R, Hilal-Dandan R, Brunton LL, Dillmann WH. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997;96(12):4343–4348. doi: 10.1161/01.CIR.96.12.4343. [DOI] [PubMed] [Google Scholar]
- 98.Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42(12):2924–2934. [PubMed] [Google Scholar]
- 99.Pinz I, Robbins J, Rajasekaran NS, Benjamin IJ, Ingwall JS. Unmasking different mechanical and energetic roles for the small heat shock proteins CryAB and HSPB2 using genetically modified mouse hearts. FASEB J. 2008;22(1):84–92. doi: 10.1096/fj.07-8130com. [DOI] [PubMed] [Google Scholar]
- 100.Morrison LE, Whittaker RJ, Klepper RE, Wawrousek EF, Glembotski CC. Roles for αB-crystallin and HSPB2 in protecting the myocardium from ischemia-reperfusion-induced damage in a KO mouse model. Am J Physiol Heart Circ Physiol. 2004;286(3):H847–H855. doi: 10.1152/ajpheart.00715.2003. [DOI] [PubMed] [Google Scholar]
- 101.Kumarapeli AR, et al. αB-crystallin suppresses pressure overload cardiac hypertrophy. Circ Res. 2008;103(12):1473–1482. doi: 10.1161/CIRCRESAHA.108.180117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benjamin IJ, et al. CRYAB and HSPB2 deficiency alters cardiac metabolism and paradoxically confers protection against myocardial ischemia in aging mice. Am J Physiol Heart Circ Physiol. 2007;293(5):H3201–H3209. doi: 10.1152/ajpheart.01363.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clark AR, Naylor CE, Bagnéris C, Keep NH, Slingsby C. Crystal structure of R120G disease mutant of human αB-crystallin domain dimer shows closure of a groove. J Mol Biol. 2011;408(1):118–134. doi: 10.1016/j.jmb.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bova MP, et al. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci U S A. 1999;96(11):6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Michiel M, et al. Abnormal assemblies and subunit exchange of αB-crystallin R120 mutants could be associated with destabilization of the dimeric substructure. Biochemistry. 2009;48(2):442–453. doi: 10.1021/bi8014967. [DOI] [PubMed] [Google Scholar]
- 106.Treweek TM, et al. R120G αB-crystallin promotes the unfolding of reduced α-lactalbumin and is inherently unstable. FEBS J. 2005;272(3):711–724. doi: 10.1111/j.1742-4658.2004.04507.x. [DOI] [PubMed] [Google Scholar]
- 107.Perng MD, Wen SF, van den IJssel P, Prescott AR, Quinlan RA. Desmin aggregate formation by R120G αB-crystallin is caused by altered filament interactions and is dependent upon network status in cells. Mol Biol Cell. 2004;15(5):2335–2346. doi: 10.1091/mbc.e03-12-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perng MD, et al. The cardiomyopathy and lens cataract mutation in αB-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J Biol Chem. 1999;274(47):33235–33243. doi: 10.1074/jbc.274.47.33235. [DOI] [PubMed] [Google Scholar]
- 109.Wang X, et al. Expression of R120G-αB-crystallin causes aberrant desmin andαB-crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89(1):84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- 110.Andley UP, Hamilton PD, Ravi N, Weihl CC. A knock-in mouse model for the R120G mutation of αB-crystallin recapitulates human hereditary myopathy and cataracts. PLoS One. 2011;6(3):e17671. doi: 10.1371/journal.pone.0017671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fujita Y, Ohto E, Katayama E, Atomi Y. alphaB-Crystallin-coated MAP microtubule resists nocodazole and calcium-induced disassembly. J Cell Sci. 2004;117(pt 9):1719–1726. doi: 10.1242/jcs.01021. [DOI] [PubMed] [Google Scholar]
- 112.Ke L, et al. HSPB1, HSPB6, HSPB7, and HSPB8 protect against RhoA GTPase-induced remodeling in tachypaced atrial myocytes. PLoS One. 2011;6(6):e20395. doi: 10.1371/journal.pone.0020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marin-Vinader L, Shin C, Onnekink C, Manley JL, Lubsen NH. Hsp27 enhances recovery of splicing as well as rephosphorylation of SRp38 after heat shock. Mol Biol Cell. 2006;17(2):886–894. doi: 10.1091/mbc.e05-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.den Engelsman J, et al. Mimicking phosphorylation of the small heat-shock protein αB-crystallin recruits the F-box protein FBX4 to nuclear SC35 speckles. Eur J Biochem. 2004;271(21):4195–4203. doi: 10.1111/j.1432-1033.2004.04359.x. [DOI] [PubMed] [Google Scholar]
- 115.Minoia M, Grit C, Kampinga HH. HSPA1A-independent suppression of PARK2 C289G protein aggregation by human small heat shock proteins. Mol Cell Biol. 2014;34(19):3570–3578. doi: 10.1128/MCB.00698-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krief S, et al. Identification and characterization of cvHsp. A novel human small stress protein selectively expressed in cardiovascular and insulin-sensitive tissues. J Biol Chem. 1999;274(51):36592–36600. doi: 10.1074/jbc.274.51.36592. [DOI] [PubMed] [Google Scholar]
- 117.Cappola TP, et al. Common variants in HSPB7 and FRMD4B associated with advanced heart failure. Circ Cardiovasc Genet. 2010;3(2):147–154. doi: 10.1161/CIRCGENETICS.109.898395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garnier S, et al. Involvement of BAG3 and HSPB7 loci in various etiologies of systolic heart failure: Results of a European collaboration assembling more than 2000 patients. Int J Cardiol. 2015;189:105–107. doi: 10.1016/j.ijcard.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 119.Matkovich SJ, et al. Cardiac signaling genes exhibit unexpected sequence diversity in sporadic cardiomyopathy, revealing HSPB7 polymorphisms associated with disease. J Clin Invest. 2010;120(1):280–289. doi: 10.1172/JCI39085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang M, et al. Association between polymorphisms of the HSPB7 gene and Cheyne-Stokes respiration with central sleep apnea in patients with dilated cardiomyopathy and congestive heart failure. Int J Cardiol. 2016;221:926–931. doi: 10.1016/j.ijcard.2016.07.107. [DOI] [PubMed] [Google Scholar]
- 121.Stark K, et al. Genetic association study identifies HSPB7 as a risk gene for idiopathic dilated cardiomyopathy. PLoS Genet. 2010;6(10):e1001167. doi: 10.1371/journal.pgen.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cappola TP, et al. Loss-of-function DNA sequence variant in the CLCNKA chloride channel implicates the cardio-renal axis in interindividual heart failure risk variation. Proc Natl Acad Sci U S A. 2011;108(6):2456–2461. doi: 10.1073/pnas.1017494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lahvic JL, et al. Small heat shock proteins are necessary for heart migration and laterality determination in zebrafish. Dev Biol. 2013;384(2):166–180. doi: 10.1016/j.ydbio.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosenfeld GE, Mercer EJ, Mason CE, Evans T. Small heat shock proteins Hspb7 and Hspb12 regulate early steps of cardiac morphogenesis. Dev Biol. 2013;381(2):389–400. doi: 10.1016/j.ydbio.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu T, et al. HSPB7 is indispensable for heart development by modulating actin filament assembly. Proc Natl Acad Sci U S A. 2017;114(45):11956–11961. doi: 10.1073/pnas.1713763114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liao WC, Juo LY, Shih YL, Chen YH, Yan YT. HSPB7 prevents cardiac conduction system defect through maintaining intercalated disc integrity. PLoS Genet. 2017;13(8):e1006984. doi: 10.1371/journal.pgen.1006984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mercer EJ, Lin YF, Cohen-Gould L, Evans T. Hspb7 is a cardioprotective chaperone facilitating sarcomeric proteostasis. Dev Biol. 2018;435(1):41–55. doi: 10.1016/j.ydbio.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Youn DY, et al. Bis deficiency results in early lethality with metabolic deterioration and involution of spleen and thymus. Am J Physiol Endocrinol Metab. 2008;295(6):E1349–E1357. doi: 10.1152/ajpendo.90704.2008. [DOI] [PubMed] [Google Scholar]
- 129.Lassmann T, Sonnhammer EL. Kalign — an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics. 2005;6:298. doi: 10.1186/1471-2105-6-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jehle S, et al. Solid-state NMR and SAXS studies provide a structural basis for the activation of αB-crystallin oligomers. Nat Struct Mol Biol. 2010;17(9):1037–1042. doi: 10.1038/nsmb.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Inomata Y, et al. Bcl-2-associated athanogene 3 (BAG3) is an enhancer of small heat shock protein turnover via activation of autophagy in the heart. Biochem Biophys Res Commun. 2018;496(4):1141–1147. doi: 10.1016/j.bbrc.2018.01.158. [DOI] [PubMed] [Google Scholar]
- 132.Selcen D, et al. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol. 2009;65(1):83–89. doi: 10.1002/ana.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]