Abstract
The deactivated CRISPR/Cas9 (dCas9) is now the most widely used gene activator. However, current dCas9-based gene activators are still limited by their unsatisfactory activity. In this study, we developed a new strategy, the CRISPR-assisted trans enhancer, for activating gene expression at high efficiency by combining dCas9-VP64/sgRNA with the widely used strong CMV enhancer. In this strategy, CMV enhancer DNA was recruited to target genes in trans by two systems: dCas9-VP64/csgRNA-sCMV and dCas9-VP64-GAL4/sgRNA-UAS-CMV. The former recruited trans enhancer by annealing between two short complementary oligonucleotides at the ends of the sgRNA and trans enhancer. The latter recruited trans enhancer by binding between GAL4 fused to dCas9 and UAS sequence of trans enhancer. The trans enhancer activated gene transcription as the natural looped cis enhancer. The trans enhancer could activate both exogenous reporter genes and variant endogenous genes in various cells, with much higher activation efficiency than that of current dCas9 activators.
Research organism: Human
Introduction
Clustered regularly interspaced short palindromic repeats (CRISPR) was originally identified in the immune system of bacteria, with the function of destroying the invading microphage DNA by enzymatic digestion. The system has been developed into a highly efficient gene editing tool (Doudna and Charpentier, 2014; Mali et al., 2013c), and also into new gene activators. For example, the dead Cas9 (dCas9) and its associated single guide RNA (sgRNA) have been widely used to regulate gene expression in recent years (Dominguez et al., 2016; Hilton et al., 2015; Jinek et al., 2012; Kiani et al., 2015; Mali et al., 2013b; Radzisheuskaya et al., 2016; Wang et al., 2016). Both dCas9 and sgRNA have been engineered for activating or repressing gene expression. For instance, the dCas9 protein has been fused with various gene activation or repression domains, such as VP48 (Cheng et al., 2013), VP160 (Perrin et al., 2017), VP64 (Maeder et al., 2013; Perez-Pinera et al., 2013), VPR (VP64-p65-Rta) (Chavez et al., 2015), and KRAB (Zheng et al., 2018). Additionally, the dCas9 protein has been fused with other functional domains with transcriptional regulatory functions, such as p300 (Hilton et al., 2015), LSD1 (Kearns et al., 2015), Dnmt3a (Liu et al., 2016a; Saunderson et al., 2017), and Tet1 (Choudhury et al., 2016; Liu et al., 2016a). Based on these domains, more elaborate activators have been developed for more potent activation of target genes in mammalian cells, such as SunTag (dcas9-GCN4/sgRNA plus scFV-VP64) (Tanenbaum et al., 2014) and SPH (dCas9-GCN4/sgRNA plus scFV-p65-HSF1) (Zhou et al., 2018). Furthermore, some inducible dCas9 systems have been developed to control activity of dCas9 activators in cells, such as light-activated CRISPR/Cas9 effector (Nihongaki et al., 2015; Polstein and Gersbach, 2015), hybrid drug inducible CRISPR/Cas9 technology (HIT) (Lu et al., 2018), and CRISPR activator gated by human antibody-based chemically induced dimerizers (AbCIDs) (Liu et al., 2018). However, it is difficult to package most of these dCas9 fusion proteins into adeno-associated virus (AAV) for in vivo application.
sgRNA has also been engineered to develop new dCas9-based activators. Compared with dCas9 engineering, sgRNA is more simple, flexible, and efficient to redesign. Moreover, the engineered sgRNA is more helpful for the in vivo application of dCas9-based activators because of its limited length for virus packaging. The most widely used sgRNA-based gene activator is the synergistic activation mediator (SAM) system, in which MS2 loops are fused to the 3′ end of sgRNA (Konermann et al., 2015; Liao et al., 2017). Similarly, Pumilio/FBF (PUF), modular scaffold RNAs (MS2, PP7, and com), and riboswitches have been fused to sgRNA (Cheng et al., 2016; Liu et al., 2016b; Zalatan et al., 2015). However, these chimeric sgRNA-based strategies were limited by their complicated RNA aptamers and the cognate RNA-binding fusion proteins.
Although variant dCas9-based activators have been developed (Chen and Qi, 2017), the current dCas9-based transcriptional activators are relatively inefficient in endogenous gene activation and cell reprogramming (Gao et al., 2014). By a systematic comparison of relative potency and effectiveness across various cell types and species (human, mouse, and fly) (Chavez et al., 2016), it was found that the majority of second-generation activators had higher activity than that of dCas9-VP64, with the three most potent activators being VPR, SAM, and Suntag. The three activators were consistently better than VP64 across a range of target genes and cellular environments. Moreover, the three activators showed a similar level of activity, and fusing their elements did not yield more potent activators (Chavez et al., 2016). Novel, more potent dCas9-based activators might be built by creating other architectures.
Almost three decades ago, the human cytomegalovirus (CMV) enhancer/promoter (referred to as CMV enhancer hereafter) was found. It is a natural mammalian promoter with high transcriptional activity (Boshart et al., 1985). Later studies showed the CMV enhancer to be a strong enhancer in various mammalian cells (Boshart et al., 1985; Foecking and Hofstetter, 1986; Ho et al., 2015; Kim et al., 1990). This enhancer has been widely used to drive ectopic expression of various genes in a wide range of mammalian cells, and to drive ectopic expression of exogenous genes in broad tissues in transgenic animals (Furth et al., 1991; Schmidt et al., 1990), protein production by gene engineering, and gene therapy. We have recently improved the transcriptional activity of the CMV enhancer by changing the natural NF-κB binding sites into artificially selected NF-κB binding sequences with high binding affinity (Wang et al., 2018). Therefore, we conceived that a unique architecture may be constructed to improve dCas9-based activators using the CMV enhancer.
In this study, mimicking the natural enhancer activating gene expression by a loop structure (Carter et al., 2002; Tolhuis et al., 2002), we developed a new dCas9-based activator by combining dCas9/sgRNA with CMV enhancer. The 3′ end of sgRNA was redesigned to contain a short capture sequence complementary to a stick-end of a double-stranded CMV enhancer. The CMV enhancer was anchored to the promoter region of a target gene by dCas9/sgRNA. The dCas9/sgRNA-recruited CMV enhancer thus functioned like a natural looped cis enhancer in a trans form. We found that the new activator could efficiently activate exogenous and endogenous genes in various cells. More importantly, the CMV enhancer could be also recruited to a target gene in trans using another system consisting of dCas9-VP64-GAL4/sgRNA and UAS-CMV.
Results
Principle of gene activation by a CRISPR-assisted trans enhancer
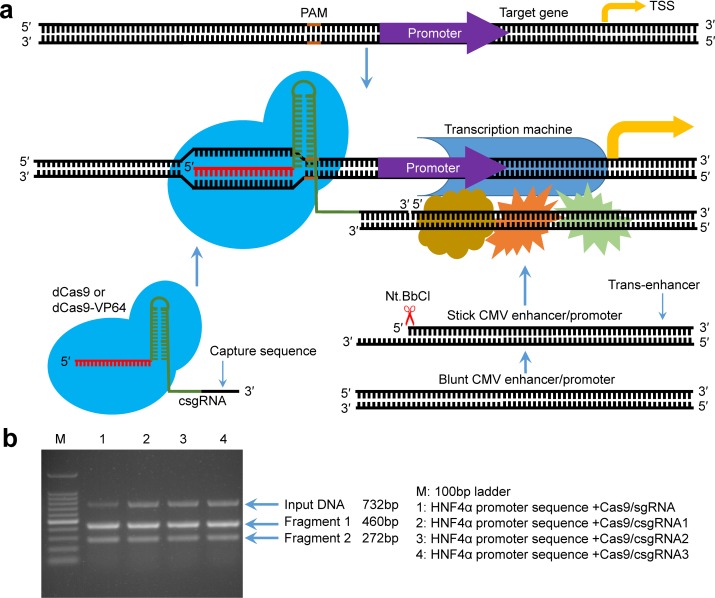
The principle of activating gene expression by a CRISPR-assisted trans enhancer is schematically illustrated in Figure 1a. A capture sgRNA (csgRNA) was produced by adding a capture sequence to the 3′ end of a normal sgRNA sequence. A linear stick-end CMV (sCMV) enhancer was produced by adding a 3′ end single-strand overhang. The overhang can anneal with the csgRNA capture sequence. When dCas9 protein was guided to the promoter of the target gene by csgRNA, sCMV could be recruited by csgRNA. The recruited sCMV may activate the transcription of the target gene like a natural looped cis enhancer. Because the dCas9/csgRNA-anchored sCMV functions as a transcription factors in trans, we named it a trans enhancer to distinguish it from the natural cis enhancer.
Figure 1. Principle of gene expression activation by the CRISPR-assisted trans enhancer and evaluation of designed csgRNAs.
(a) Schematic illustration of the principle of gene expression activation by the CRISPR-assisted trans enhancer. A capture sequence is added to the 3′ end of sgRNA, which is used to capture a trans CMV enhancer with a single-stranded overhang that can anneal with the capture sequence of sgRNA. The captured trans CMV enhancer may function like the natural looped cis enhancer to activate transcription of the gene of interest, including exogenous and endogenous genes. (b) In vitro target DNA cutting by the Cas9-csgRNA complex. DNA fragments (732 bp) amplified from the HNF4α promoter region were, respectively, cut by the Cas9/csgRNA and Cas9/sgRNA complexes. csgRNA1, csgRNA2 and csgRNA3 had the same target sequence but different capture sequences.
Effect of capture sequence on the function of sgRNA
To determine whether the capture sequence affects the function of sgRNA, we prepared a normal sgRNA and three csgRNAs targeting the same site of the HNF4α promoter. The three csgRNAs had different capture sequences. We used these sgRNAs to associate with the Cas9 endonuclease to cut a 732 bp HNF4α promoter fragment. The results indicated that the target DNA could be digested by all sgRNAs (Figure 1b), indicating that the capture sequence did not affect the sgRNA function.
Activation of exogenous reporter gene by trans enhancer
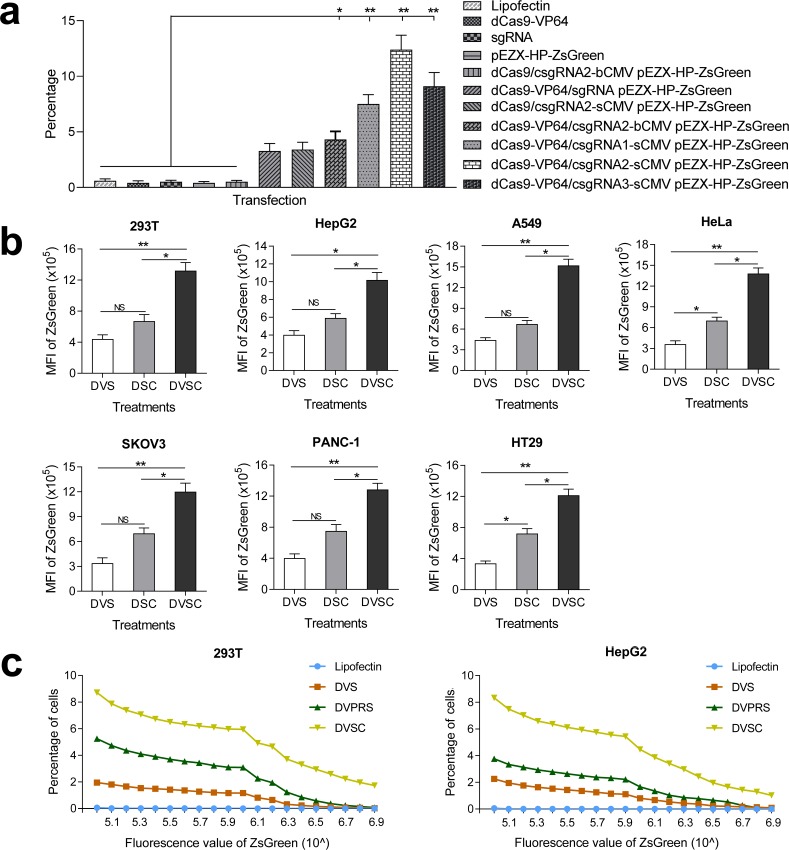
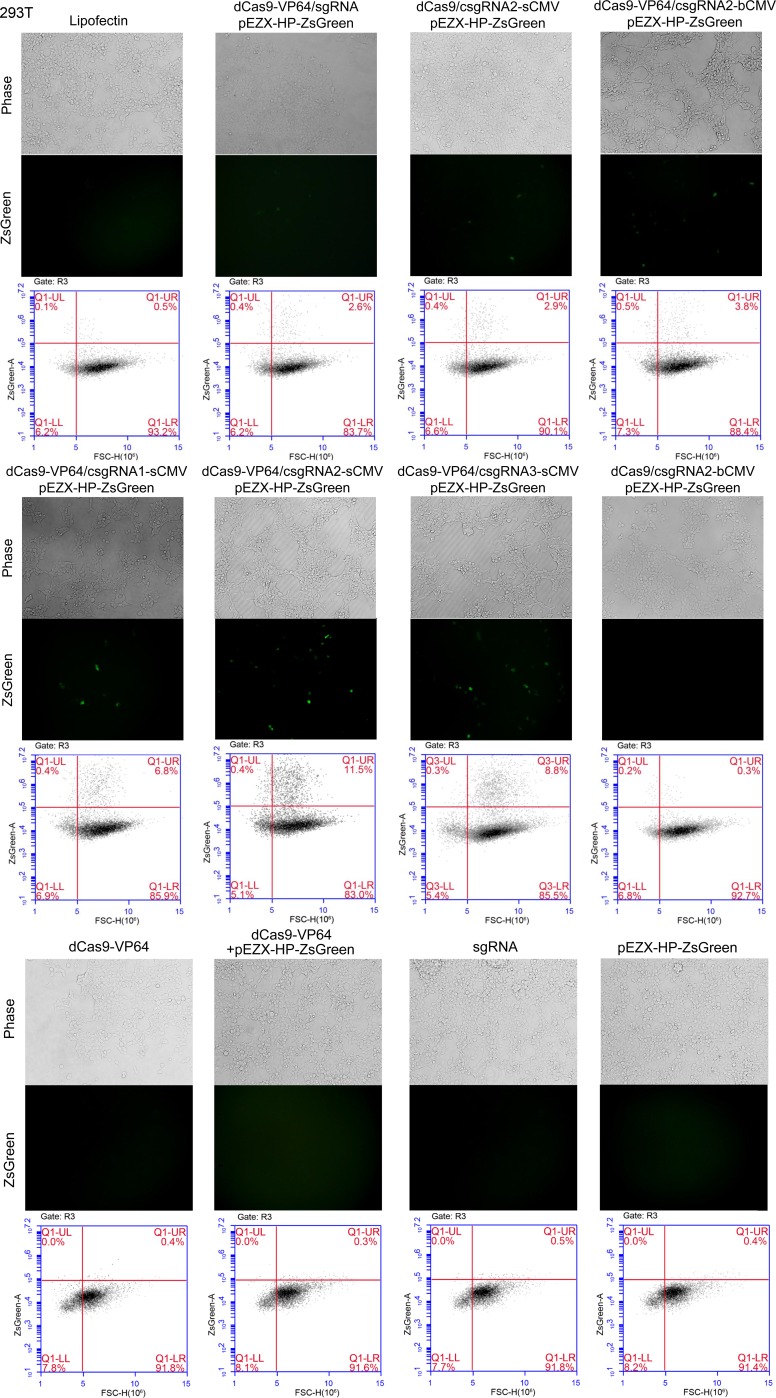
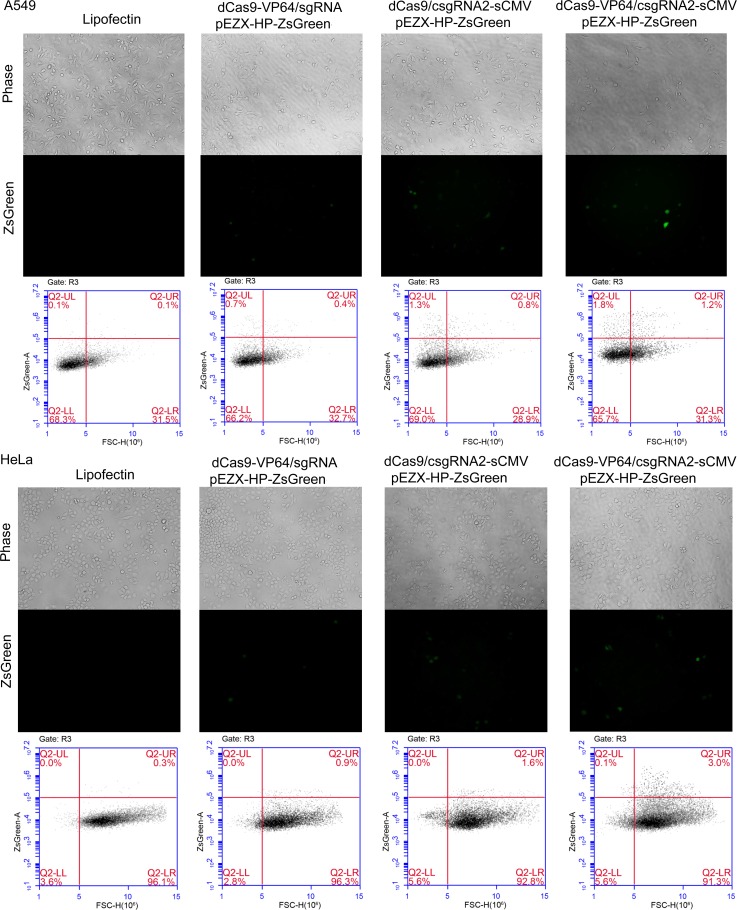
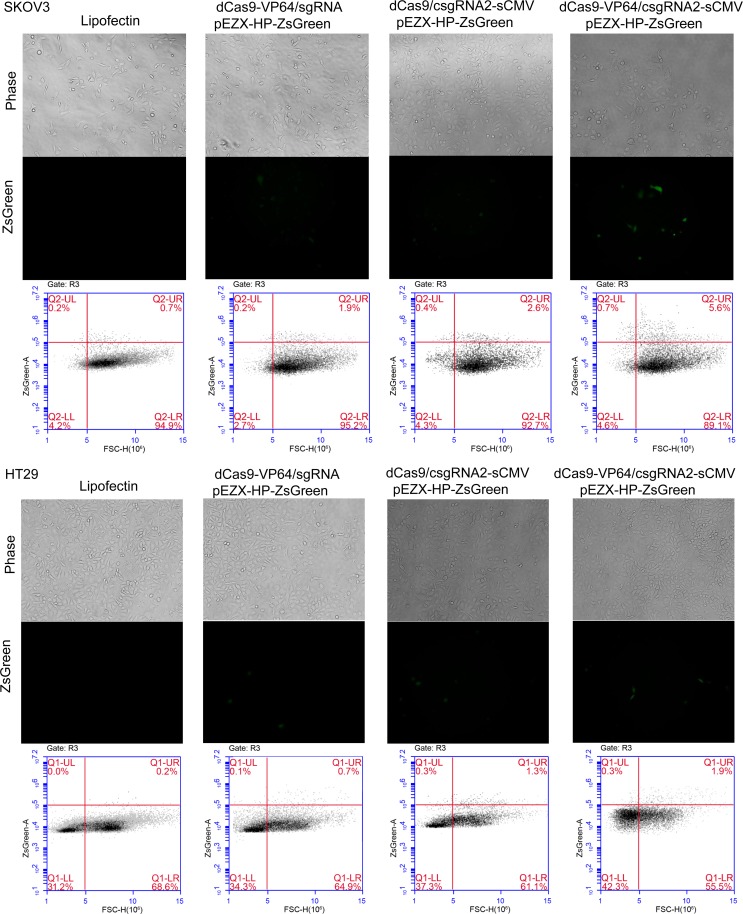
To determine whether the CRISPR-assisted trans enhancer activates gene expression, we constructed a reporter construct of HNF4α promoter (pEZX-HP-ZsGreen). 293 T cells were then transfected with various vectors (Figure 2a, Figure 2—figure supplement 1). The transfection indicated that ZsGreen expression could be successfully activated by dCas9/csgRNA2-sCMV but not activated by dCas9/csgRNA2-blunt CMV (bCMV). Although the dCas9/csgRNA2-sCMV showed a similar activation level to Cas9-VP64/sgRNA, it was far inferior to cis CMV enhancer. To improve the performance of trans CMV, we tried transfecting 293 T cells with dCas9-VP64/csgRNA2-sCMV. The results indicated that ZsGreen expression was highly activated by the transfection. In contrast, the dCas9-VP64/csgRNA2-bCMV showed a similar activation level to dCas9-VP64/sgRNA. These data revealed that the trans CMV not only truly functioned in trans via dCas9/csgRNA, but also synergistically interacted with dCas9-fused VP64. Subsequent transfections indicated that ZsGreen expression could also be highly activated by combination of dCas9-VP64, sCMV and other two csgRNAs, csgRNA1 and csgRNA3.
Figure 2. Activation of an exogenous reporter gene ZsGreen under the control of a HNF4α promoter by the CRISPR-assisted trans enhancer in multiple cells.
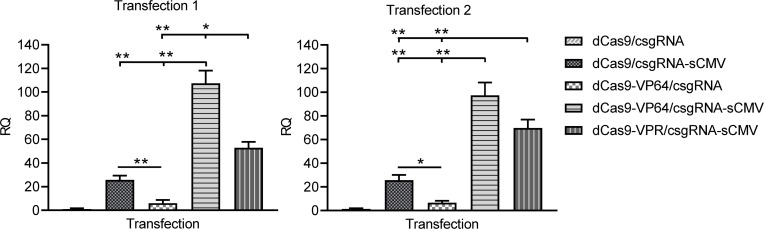
(a) Transcriptional activation of reporter gene ZsGreen in various cells transfected by different vectors. The florescence intensity of cells was analyzed by flow cytometry and is shown as the mean fluorescence intensity (MFI). Transfections: DVS, dCas9-VP64/sgRNA; DSC, dCas9/csgRNA-sCMV; DVSC, dCas9-VP64/csgRNA-sCMV. (b) Comparison between trans enhancer and VPR. Cells were transfected with three different transcriptional activation systems to activate reporter gene ZsGreen. The florescence intensity of cells was analyzed by flow cytometry and the number of cells with certain fluorescence intensity was counted. Transfections: Lipo, lipofectin; DVS, dCas9-VP64/sgRNA; DVPRS; dCas9-VPR/csgRNA; DVSC, dCas9-VP64/csgRNA-sCMV.
Figure 2—figure supplement 1. Activation of an exogenous reporter gene ZsGreen under the control of a HNF4α promoter by the CRISPR-assisted trans enhancer in 293 T cells.
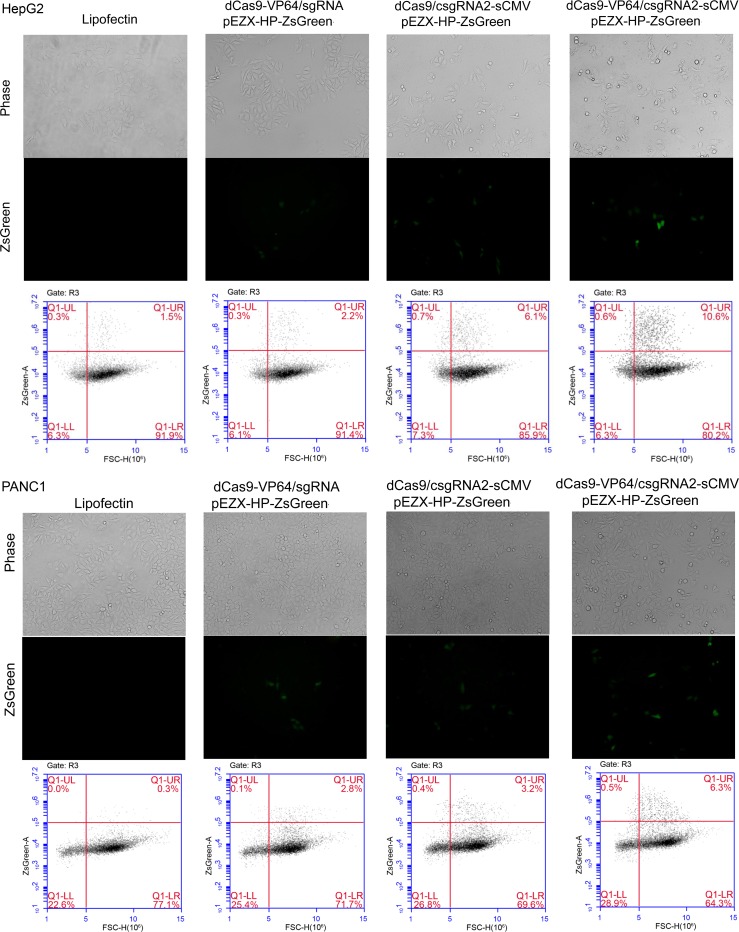
Figure 2—figure supplement 2. Activation of an exogenous reporter gene ZsGreen under the control of a HNF4α promoter by the CRISPR-assisted trans enhancer in HepG2 and PANC1 cells.
Figure 2—figure supplement 3. Activation of an exogenous reporter gene ZsGreen under the control of a HNF4α promoter by the CRISPR-assisted trans enhancer in A549 and HeLa cells.
Figure 2—figure supplement 4. Activation of an exogenous reporter gene ZsGreen under the control of a HNF4α promoter by the CRISPR-assisted trans enhancer in SKOV3 and HT29 cells.
To further verify the function of CRISPR-assisted trans enhancer, we transfected six different cell lines with reporter construct and dCas9-VP64/csgRNA2-sCMV, dCas9/csgRNA2-sCMV, or dCas9-VP64/sgRNA (Figure 2b; Figure 2—figure supplement 2–4). The results revealed that dCas9-VP64/csgRNA2-sCMV always showed the highest gene activation efficiency in all cell lines. Additionally, dCas9/csgRNA2-sCMV always showed higher activity than dCas9-VP64/sgRNA. These results indicate that genes could be activated by the CRISPR-assisted trans enhancer. Importantly, the trans sCMV could synergistically function with dCas9-fused VP64 in gene activation.
Comparison of trans CMV enhancer with VPR
Having shown that VPR is a more potent transcriptional activation domain than VP64, we next compared the trans enhancer with VPR. 293T and HepG2 cells were, respectively, transfected with reporter construct and dCas9-VP64/csgRNA, dCas9-VPR/csgRNA, or dCas9-VP64/csgRNA-sCMV (Figure 2c). The results showed that dCas9-VPR/csgRNA had better activity than dCas9-VP64/csgRNA as previously reported. However, the dCas9-VP64/csgRNA-sCMV always showed significantly higher activity than dCas9-VPR/csgRNA.
Activation of endogenous genes by trans enhancer
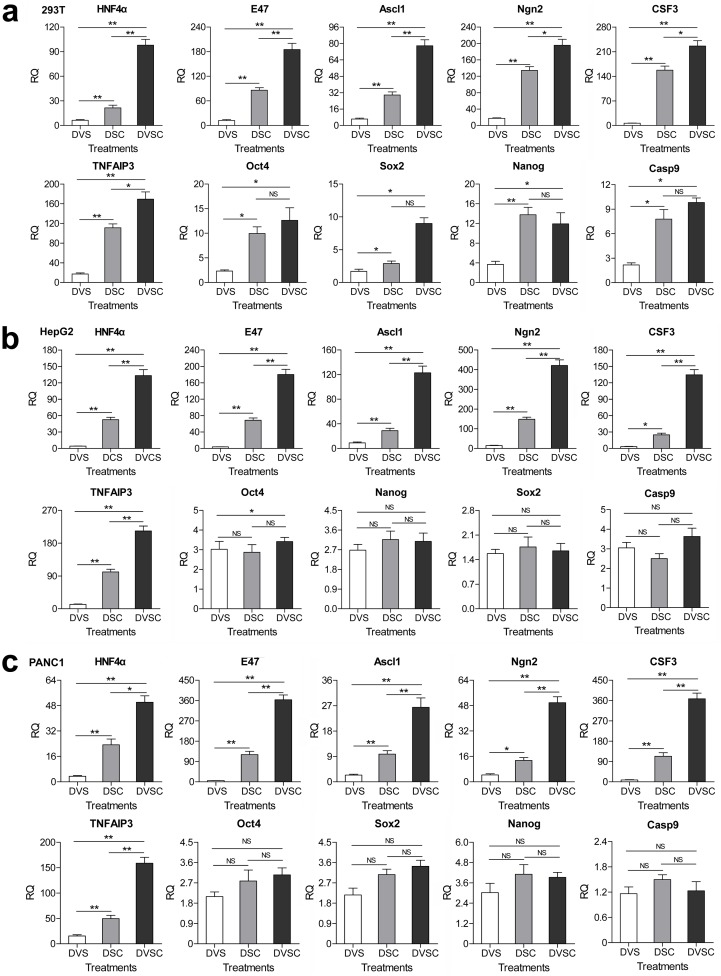
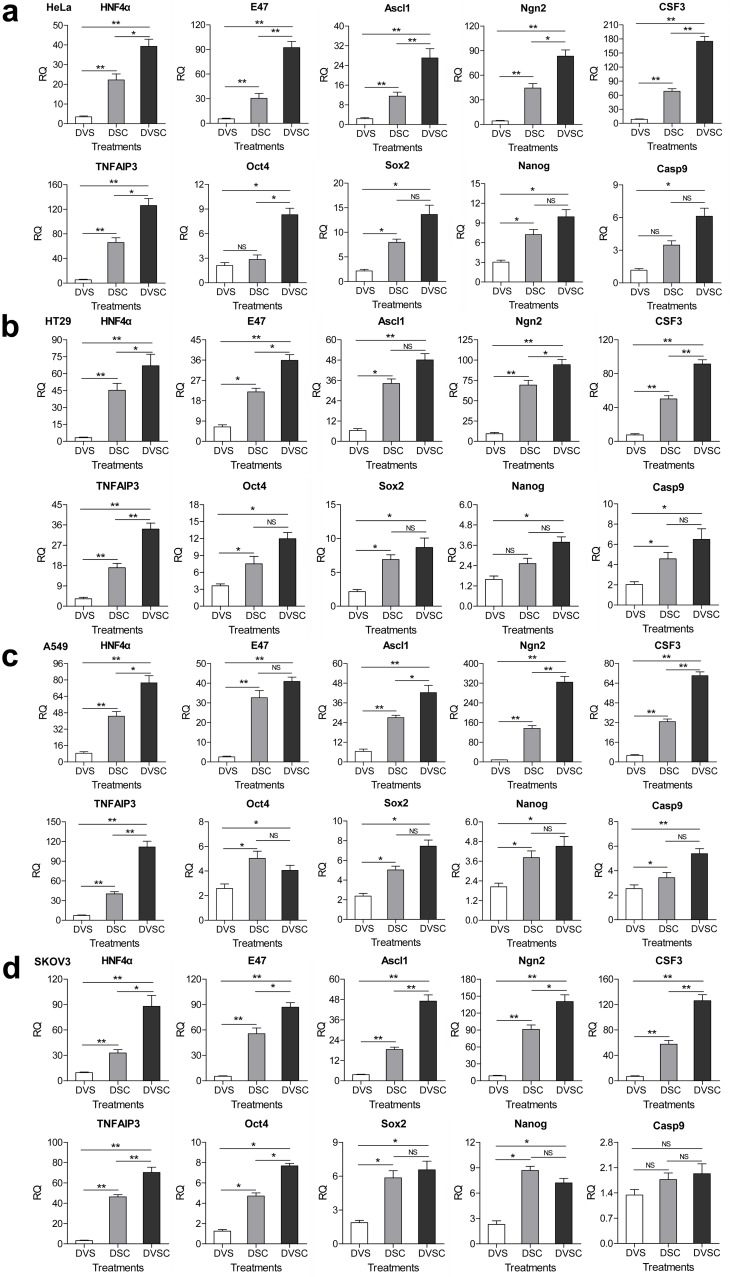
To further evaluate the activity of CRISPR-assisted trans enhancer, we activated endogenous genes with trans sCMV. csgRNAs targeting ten different genes was designed and their linear expression vectors were produced. Seven different cell lines were transfected with dCas9-VP64/csgRNA2-sCMV, dCas9-VP64/sgRNA and dCas9/csgRNA2-sCMV (Figure 3—figure supplement 1). The quantitative PCR (qPCR) detection of gene expression revealed that almost all genes were most significantly activated by dCas9-VP64/csgRNA2-sCMV in all cells. Moreover, most genes were more significantly activated by dCas9/csgRNA-sCMV than dCas9-VP64/sgRNA in all cells. These results suggest that the CRISPR-assisted trans enhancer could be used to activate variant endogenous genes in various cells. In addition, by activating the HNF4α gene in 293 T cells, we found that dCas9-VP64/csgRNA-sCMV had better activity than dCas9-VPR/csgRNA-sCMV in activating endogenous genes (Figure 3—figure supplement 2).
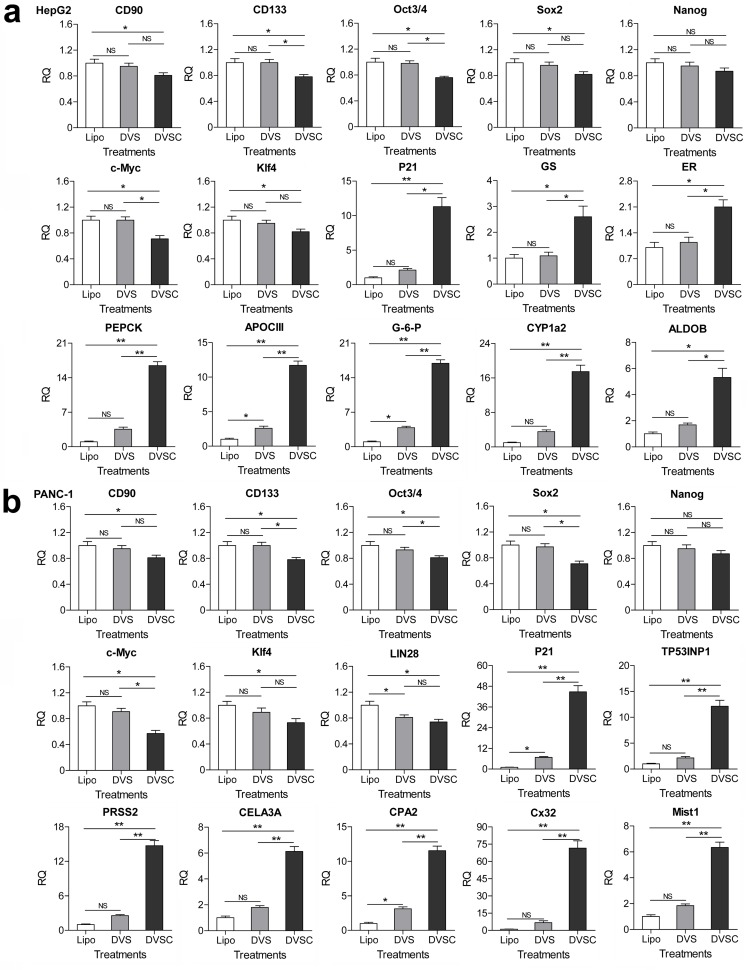
It has been reported that the cancer cells HepG2 and PANC1 can be differentiated into normal liver- and pancreas-like cells by exogenously expressing transcription factor HNF4α and E47. In the above assays, we found that the endogenous HNF4α and E47 genes were highly activated by the CRISPR-assisted trans enhancer in HepG2 and PANC1 cells (Figure 3). To further confirm the cellular effects of HNF4α and E47 activation, we detected expressions of other genes related to the differentiation of the two cancer cells (Figure 4). The results indicated that the genes related to stemness (CD133 and CD90) and pluripotency (Oct3/4, Sox2, Nanog, c-Myc, LIN28, and Klf4) were down-regulated, but those related to normal liver (GS, BR, ALDOB, CYP1a2, PEPCK, APOCIII, G-6-P, and HPD) and pancreas (MIST1, PRSS2, CELA3A, and CPA2) functions were highly up-regulated in HepG2 and PANC1 cells. Additionally, the cell cycle arrest-related gene p21 (HepG2 and PANC1) and TP53INP1 (PANC1) were highly up-regulated.
Figure 3. Transcriptional activation of endogenous genes by the CRISPR-assisted trans enhancer.
294T, HepG2 and PANC1 cells were transfected with three different transcriptional activation systems to activate expression of 10 endogenous genes. Gene transcription was detected by qPCR and the expression level is shown as the relative RNA expression fold to house-keeping gene GAPDH. Data are shown as mean ± SD, n = 3. The statistical difference was analyzed using the Student’s t test. *, p<0.05; **, p<0.01; NS, no significant statistical difference. Transfection: DVS, dCas9-VP64/sgRNA; DSC, dCas9/csgRNA2-sCMV; DVSC, dCas9-VP64/csgRNA2-sCMV.
Figure 3—figure supplement 1. Transcriptional activation of endogenous genes by the CRISPR-assisted trans enhancer.
Figure 3—figure supplement 2. Activation of endogenous HNF4α gene in 293 T cell with trans enhancers based on dCas9-VP64 and dCas9-VPR.
Figure 4. Changes of gene expression in the HNF4α-activated HepG2 cells and E47-activated PANC-1 cells.
(a and b) Changes of gene expression in the HNF4α-activated HepG2 cells (a) and E47-activated PANC-1 cells (b). The gene transcription was detected by qPCR and the expression level is shown as the relative RNA expression fold to house-keeping gene GAPDH. Data are shown as mean ± SD, n = 3. The statistical difference was analyzed by Student’s t test. *, p<0.05; **, p<0.01; NS, no significant statistical difference. Transfection: Lipo, lipofectin; DVS, dCas9-VP64/sgRNA; DVSC, dCas9-VP64/csgRNA2-sCMV.
Activation of genes by other trans enhancers
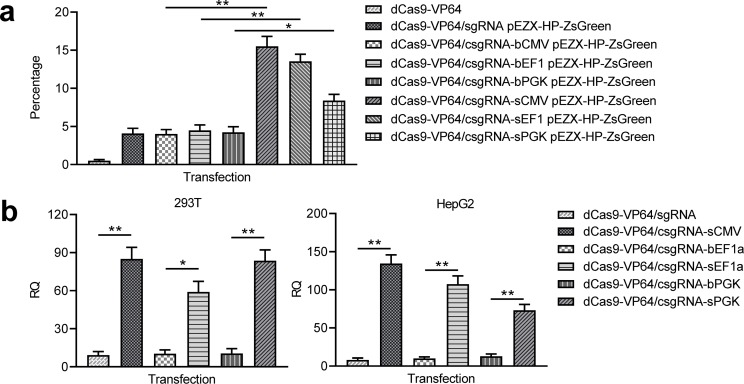
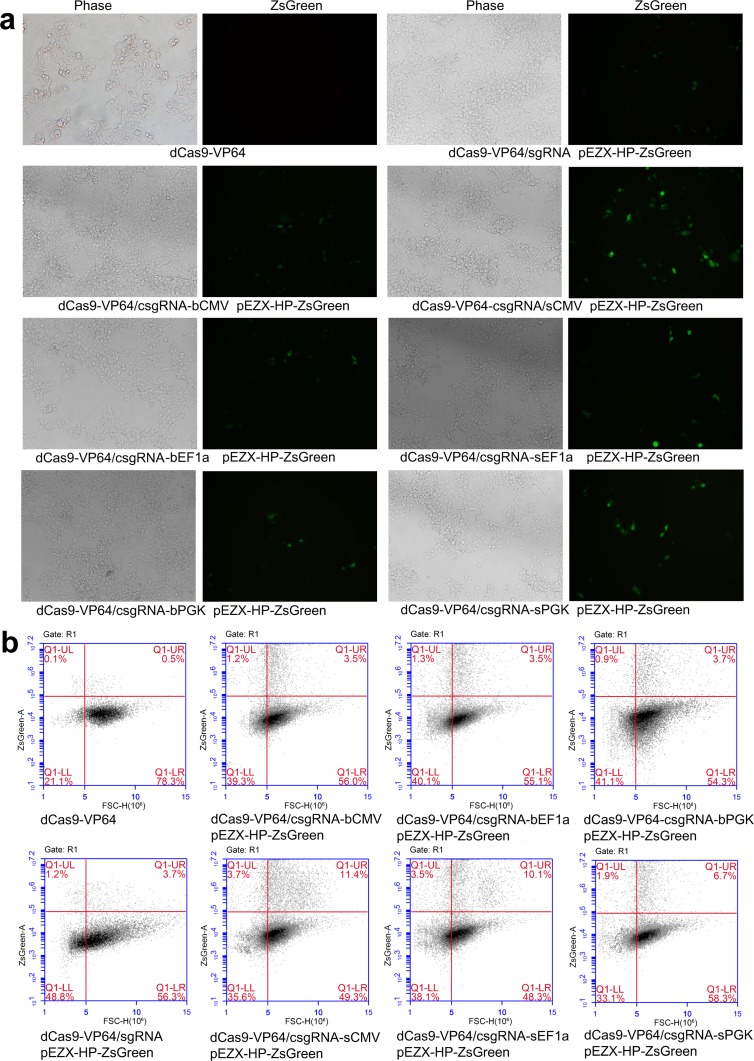
To explore whether other enhancers could be also used as trans enhancers, we fabricated the blunt- and stick-end versions of two other widely used promoters EF1a and PGK (bEF1a, bPGK, sEF1a, and sPGK). 293 T cells were co-transfected with these trans enhancers and dCas9-VP64/csgRNA and reporter construct. The results indicated that the ZsGreen expression was also activated by the two trans enhancers; however, the activation levels were lower than that of sCMV (Figure 5a, Figure 5—figure supplement 1). All other transfections as controls did not activate ZsGreen expression (Figure 5a, Figure 5—figure supplement 1). The qPCR detection of HNF4α expression in the same transfected 293 T cells revealed that the endogenous HNF4α gene expression was also significantly activated by three stick-end trans enhancers, but not activated by all blunt-ended trans enhancers (Figure 5b). Subsequent HepG2 cell transfections with the same trans enhancers and dCas9-VP64/csgRNA indicated that the endogenous HNF4α gene expression could also be significantly activated by all stick-ended trans enhancers, but not activated by all blunt-ended trans enhancers (Figure 5b). These results indicate that the variant enhancers could be used as the CRISPR-assisted trans enhancer.
Figure 5. Activation of exogenous and endogenous genes with other trans enhancers.
(a) Activation of exogenous reporter gene ZsGreen. The fluorescence intensity of cells was analyzed with flow cytometry. (b) Activation of endogenous HNF4α gene in 293T and HepG2 cells with two new trans enhancers, sEF1a and sPGK. The sCMV was used as a positive control for comparison. The blunt-end trans enhancers (bEF1a and bPGK) were also used as controls. Data are shown as mean ± SD, n = 3. The statistical difference was analyzed using Student’s t test. *, p<0.05; **, p<0.01.
Figure 5—figure supplement 1. Activation of exogenous and endogenous genes with other CRISPR-assisted trans enhancers.
Activation of genes by the GAL4/UAS-based trans enhancer
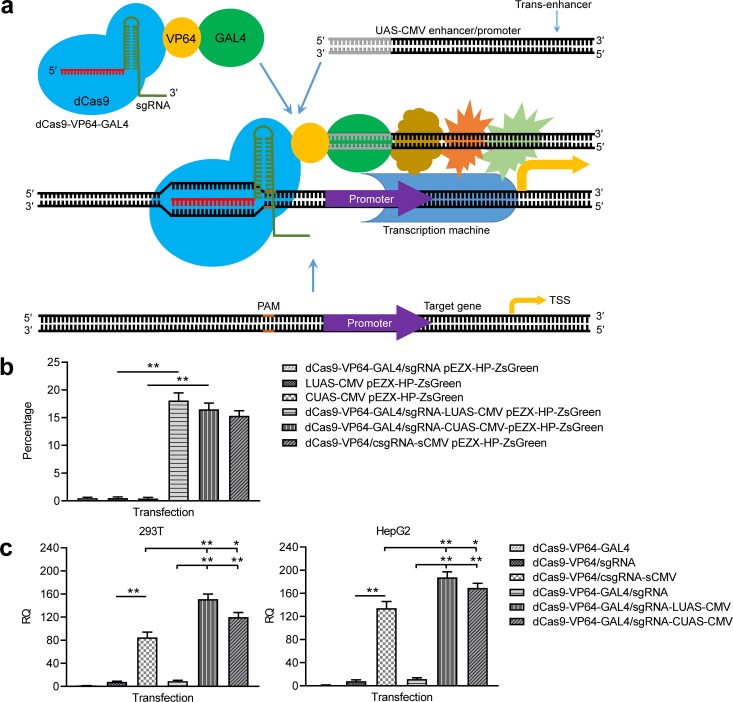
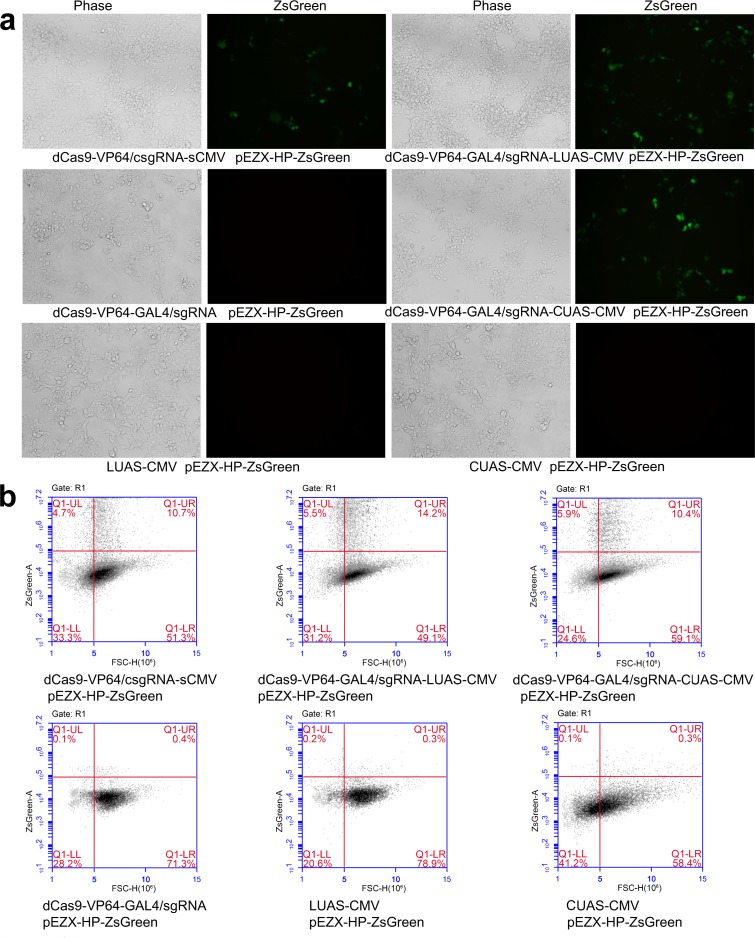
To further improve in vivo application of the CRISPR-assisted trans enhancer, we tried realizing the trans enhancer with the GAL4-UAS system. A dCas9-VP64-GAL4 expression vector and a UAS-CMV trans enhancer was constructed. Two forms of trans UAS-CMV enhancers, linear UAS-CMV (LUAS-CMV) and circular UAS-CMV (CUAS-CMV), were expected to be recruited to the target gene by the dCas9-VP64-fused GAL4 (Figure 6a). By transfecting 293 T cells with dCas9-VP64-GAL4/sgRNA-LUAS-CMV/CUAS-CMV and reporter construct, the ZsGreen expression of the exogenous reporter gene was significantly activated by both LUAS-CMV and CUAS-CMV, but not activated by all transfections as controls (Figure 6b, Figure 6—figure supplement 1). By transfecting 293T and HepG2 cells with dCas9-VP64-GAL4/sgRNA-LUAS-CMV/CUAS-CMV, the expression of endogenous HNF4α gene was highly activated in the two cells (Figure 6c). More importantly, both trans LUAS-CMV and CUAS-CMV enhancers showed significantly higher activity than the trans sCMV (Figure 6c). In contrast, all transfections as controls did not activate the expression of endogenous HNF4α gene in the two cells (Figure 6c). These results reveal that the CRISPR-assisted trans enhancer could be better realized with the GAL4-UAS system.
Figure 6. Activation of exogenous and endogenous genes with the GAL4-UAS-based trans enhancer.
(a) Schematic show of gene activation using the GAL4-UAS-based CRISPR-assisted trans enhancer. (b) Activation of exogenous reporter gene ZsGreen. The fluorescence intensity of cells was analyzed with flow cytometry. (c) Activation of endogenous HNF4α gene in 293T and HepG2 cells with the GAL4-UAS-based CRISPR-assisted trans enhancer. The sCMV was used as a positive control for comparison. Three other transfections were used as controls. LUAS-CMV, linear UAS-CMV; CUAS-CMV, circular UAS-CMV. Data are shown as mean ± SD, n = 3. The statistical difference was analyzed by Student’s t-test. *, p<0.05; **, p<0.01.
Figure 6—figure supplement 1. Activation of exogenous and endogenous genes with CRISPR-assisted trans enhancer using the GAL4-UAS system.
Discussion
In this study, we developed a new dCas9-based gene activation strategy, the CRISPR-assisted trans enhancer, in which a trans enhancer could be recruited to target promoters by dCas9-VP64/csgRNA or dCas9-VP64-GAL4/sgRNA. The results revealed that expression of variant exogenous and endogenous genes could be highly activated by CRISPR-assisted trans enhancers in various mammalian cells, more efficiently than with current widely used dCas9-VP64 and dCas9-VPR. This strategy has unique advantages over the current dCas9-based gene activation systems.
First, only one csgRNA was used in activating all target genes in various cells with the CRISPR-assisted trans enhancer. However, in current dCas9-based gene activations, multiple sgRNAs are used. In general, three or more sgRNAs are used to activate a gene of interest (Cheng et al., 2013; Maeder et al., 2013; Mali et al., 2013a; Perez-Pinera et al., 2013). In many assays with various numbers of sgRNAs, one sgRNA often produced very low or undetectable expression. Using multiple sgRNAs, each sgRNA has to be independently transcribed by a long U6 promoter. Second, csgRNA is the simplest sgRNA used in dCas9-based gene activators, which only extended a 24 bp short sequence at the 3′ end of normal sgRNA. However, current dCas9/sgRNA activators often use long complex chimeric sgRNAs that harbor multiple tandem aptamers of various RNA-binding proteins, such as SAM sgRNA (MS2) (Konermann et al., 2015; Liao et al., 2017), Casilio sgRNA (Pumilio/FBF) (Cheng et al., 2016), and scaffold RNAs (MCP, PCP, and Com) (Zalatan et al., 2015).
The capture sequences of csgRNA can be easily designed. We originally designed three different capture sequences. All functioned in the CRISPR-assisted trans enhancer; however, csgRNA2 showed the best performance. The capture sequences were artificially designed short sequences, they have no complementary sequences in human cells, which is important for their specific annealing with sCMV at high efficiency. This study demonstrated that sCMV could be efficiently captured by csgRNA in the nucleus of human cells. To our knowledge, this is the first report of a gene being activated by an artificial DNA in trans.
In this study, we realized the CRISPR-assisted trans enhancer with two forms: csgRNA-sCMV and GAL4-UAS. Two forms can be easily used to activate genes in in vitro cultivated cells. As to the in vivo applications, the csgRNA-sCMV-based trans enhancer can be used via nanoparticle gene carriers, while the GAL4-UAS-based trans enhancer can be easily transferred by virus vectors such as AAV, with AAV already being approved for use as a gene vector in clinics. We found that the GAL4-UAS-based trans enhancer had better performance than the csgRNA-sCMV-based trans enhancer, especially the linear UAS-CMV. In in vivo applications, the linear UAS-CMV can be easily transferred by AAV vector.
As a typical application, dCas9-based transcriptional activators are used to reprogram cells in vitro and in vivo for biomedical applications by activating endogenous genes. For example, fibroblasts were reprogramed into induced pluripotent stem (iPS) cells by endogenous activation of the Oct4 and Sox2 genes with dCas9-SunTag-VP64 (Liu et al., 2018). Mouse embryonic fibroblasts were converted into neuronal cells by endogenous activation of the Brn2, Ascl1, and Myt1l genes with VP64dCas9VP64 (Black et al., 2016). In vivo target genes were activated by MPH to ameliorate disease phenotypes in mouse models of type I diabetes, acute kidney injury, and muscular dystrophy (Liao et al., 2017). Brain astrocytes were converted into functional neurons in vivo by activating the Ascl1, Neurog2 and Neurod1 genes with SPH (Zhou et al., 2018). These studies make CRISPR therapies the grade not the cut (Burgess, 2018).
In this study, we selected 10 endogenous genes to be activated by the CRISPR-assisted trans enhancer. Most of these genes code transcription factors, including HNF4α, E47, Ascl1, Ngn2, Sox2, Oct4, and Nanog. Ascl1, Ngn2, and Sox2 are used to directly reprogram fibroblasts into nerve cells (Zhao et al., 2015). Oct4, Sox2, and Nanog are widely used to reprogram fibroblasts into iPS cells (Takahashi et al., 2007; Takahashi and Yamanaka, 2016; Yu et al., 2007). HNF4α and E47 are used to differentiate liver and panaceas cancer cells into normal cells (Kim et al., 2015; Yin et al., 2008). TNFAIP3 is a well-known natural NF-κB inhibitor (Cooper et al., 1996), having the potential to treat NF-κB-overactivated diseases such as inflammation and cancers. Caspase9 is a key gene making cell apoptosis (Li et al., 2017). CSF3 codes granulocyte-colony stimulating factor (G-CSF), a glycoprotein that stimulates the bone marrow to produce granulocytes and stem cells and release them into bloodstream (Cetean et al., 2015), and is widely used in chemotherapy to enhance the immunity of cancer patients. We selected these genes for exploring the future in vitro and in vivo applications of the CRISPR-assisted trans enhancer.
Materials and methods
Vector construction
A lac operon fragment was amplified from pEASY-Blunt-simple (Transgen) using primers Lac-px-F and Lac-px-R. The product was ligated into px458 (Addgene) to construct px458-lac using BbsI (NEB) and BsaI (NEB). The U6-sgRNA-lac fragment was amplified from px458-lac using primers U6-F and U6-R/U6-1-R/U6-2-R/U6-3-R. The products were cloned into the pEASY-Blunt-simple to produce pEASY-sgRNA and pEASY-csgRNA (Supplementary file 2), which were used to construct particular sgRNA/csgRNA expressing plasmids. The sgRNAs targeting genes of interest were designed by CHOPCHOP. Chemically synthesized complementary oligonucleotides containing sgRNA/csgRNA targets were annealed and ligated into pEASY-sgRNA/pEASY-csgRNA. The ligation reaction consisted of 10 U BbsI (NEB), 120 U T4 DNA ligase (NEB), 1 × T4 DNA ligase buffer, 0.1 mg/mL bovine serum albumin, and 50 ng plasmid pEASY-csgRNA. The reaction was run as follows: 10 rounds of 37 °C5 min and 16°C 10 min, 37°C 30 min, and 80°C 5 min. The reaction was then used to transfect DH5α competent cells. The white clones were screened by blue-white screening on LB agar plates with 100 µg/mL ampicillin, 40 µL of 20 mg/mL X-gal, and 40 µL of 0.1 M IPTG. The vectors were validated by sequencing, then the linear sgRNA/csgRNA expression vectors were amplified from the validated pEASY-sgRNA/pEASY-csgRNAs using primers U6-F and U6-R/U6-1-R/U6-2-R/U6-3-R. The primer U6-R was used to amplify the normal sgRNA expression template (named as U6-sgRNA) from pEASY-sgRNA. The primer U6-1/2/3 R was used to amplify the csgRNA expression template (named as U6-csgRNA) from pEASY-csgRNA. The PCR products were purified with PCR clean kit (Axygen) and used to transfect cells as sgRNA/csgRNA expression vector.
The CMV enhancer fragment was amplified from pEGFP-N1 using primers CMV-F and CMV-1-R/CMV-2-R/CMV-3-R. The PCR products were purified with PCR clean kit and used as linear blunt-end CMV (bCMV). To prepare stick-end CMV (sCMV), the PCR products were firstly digested with Nt.BbvCI and then added with complementary oligonucleotide CS-1-R/CS-2-R/CS-3-R. The PCR products were denatured at 85°C for 10 min and then naturally cooled to room temperature. The PCR products was purified with PCR clean kit and used as linear sCMV. The blunt-end EF1α/PGK promoters were all amplified from pEF1a-FB-dCas9-puro (Addgene) using primers EF1-a-F/R and PGK-F/R. The stick-end EF1α/PGK promoters were similarly prepared by treating blunt-end EF1α/PGK promoters.
A 1000 bp HNF4α promoter sequence was amplified from the genomic DNA of HepG2 cells using primers HNF4α-P-F and HNF4α-P-R. The amplified promoter fragment was ligated into pEZX-ZsGreen, producing an HNF4α promoter reporter construct pEZX-HP-ZsGreen. The VP64 sequence was deleted from pcDNA-dCas9-VP64 (Addgene) to construct pcDNA-dCas9. The VPR sequence was cloned into pcDNA-dCas9 to construct pcDNA-dCas9-VPR.
The GAL4 fragment was amplified from pGBKT7 (MiaoLing Plasmid Sharing Platform) using primers GAL4-BsiEW-F and GAL4-BspE-R, and was then ligated into pcDNA-dCas9-VP64 using BsiWI and BspEI to prepare pcDNA-dCas9-VP64-GAL4. A chemically synthesized 5 × UAS fragment was ligated into pEASY-Blunt using BglII and HindIII to obtain pEASY-Blunt-UAS. A CMV fragment was amplified from pEGFP-C1 using primers UAS-CMV-Bgl-F and UAS-CMV-Hind-R. The CMV fragment was then ligated into pEASY-Blunt-UAS to obtain pEASY-Blunt-UAS-CMV, which was used as circular UAS-CMV (CUAS-CMV). The linear UAS-CMV (LUAS-CMV) fragment was amplified from pEASY-Blunt-UAS-CMV using primers CMV-UAS-Bgl-F and UAS-CMV-R.
The sequences of all PCR primers used in the above vector construction are shown in the Supplementary file 1–Table 1. The chemically synthesized complementary oligonucleotides used to construct pEASY-sgRNA/pEASY-csgRNA are shown in the Supplementary file 1–Table 2. The functional sequences of all linear and plasmid vectors are provided as Supplementary file 3.
DNA cutting with Cas9-csgRNA
A sgRNA targeting the HNF4α promoter sequence was selected. The sgRNAs were prepared by in vitro transcription using T7 RNA polymerase (NEB). The sgRNA transcription template was amplified from pEASY-csgRNA using primers HNF4α-T7-F and U6-R/U6-1-R/U6-2-R/U6-3-R. A normal sgRNA (HNF4α-sgRNA) and three csgRNAs (HNF4α-csgRNAs) were prepared. A 732 bp HNF4α promoter fragment was amplified from pEZX-HP-ZsGreen using primers HNF4α-sP-F and HNF4α-sP-R. The sequences of PCR primers are shown in the Supplementary file 1-Table 1. The Cas9 digestion reaction (30 μL) consisted of 1 × Cas9 Nuclease Reaction Buffer, 1 µM Cas9 Nuclease (NEB), and 300 nM HNF4α-sgRNA or HNF4α-csgRNA. The reaction was incubated at 25°C for 10 min. Then 400 ng of purified 732 bp HNF4α promoter fragment was added to the reaction and incubated at 37°C for 15 min. Finally, the Cas9 nuclease was inactivated at 65°C for 10 min. The reaction was run with 1.5% agarose gel electrophoresis.
Cell lines
All cells including 293T, HepG2, PANC1, A549, HeLa, SKOV3, and HT29 were obtained from the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. The identity was authenticated by STR profiling. Mycoplasma contamination testing was performed and no mycoplasma contamination was ensured.
Cell culture and transfection
Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) or Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% FBS, 100 units/mL penicillin, and 100 µg/mL streptomycin. Cells at >70% confluence in each well of a 12-well plate were transfected with various combinations (see figures) of linear or plasmid vectors, including pcDNA-dCas9, pcDNA-dCas9-VP64, pcDNA-dCas9-VPR, U6-sgRNA, U6-csgRNA, sCMV, bCMV, and pEZX-HP-ZsGreen, using Lipofectamine 2000 (ThermoFisher Scientific) according to the manufacturer’s instructions. The transfected cells were incubated at 37°C and 5% CO2 for 36 h. Cells were then imaged with a fluorescence microscope (Olympus) at 200 × magnification.
Flow cytometry
The fluorescence intensity of cells was quantified with flow cytometry (Calibur). Ten-thousand cells were measured for each transfection. Flow cytometry data analysis and figure preparation were done using BD software.
Quantitative PCR
The total RNA was extracted from cells using TRIzol (Invitrogen). The complementary DNA (cDNA) was synthesized with 3 μg of total RNA using the Hifair III SuperMix (Yeasen). Gene transcription was detected with quantitative PCR (qPCR) using the Hieff qPCR SYBR Green Master Mix (Yeasen) according to the manufacturer’s instructions. GAPDH was used as an internal reference to analyze the relative mRNA expression of different genes. The sequences of PCR primers are shown in the Supplementary file 1-Table 3 and 4. The qPCR programs were run on StepOne Plus (Applied Biosystems). Each qPCR detection was performed in at least three technical replicates. Melting curve analysis was performed. Data analysis was performed using the Applied Biosystems StepOne software v2.3, and Ct values were normalized with that of GAPDH. The relative expression level of target mRNAs was calculated as relative quantity (RQ) according to the equation: RQ = 2-ΔΔCt.
Statistical analyses
Each cell transfection for detecting gene expression activation by trans enhancer was performed in three biological replicates. In each biological replicate, at least three technical replicates (three replicate wells) were performed. In qPCR detection of gene expression, the mean RQ value of technical replicates was used as the RQ value of one biological replicate. The mean RQ value of three biological replicates was used to calculate the final mean and standard deviation (SD). Data were analyzed by Student’s t test when comparing two groups. Data are shown as mean ± SD, and differences were considered significant at p<0.05.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Jinke Wang, Email: wangjinke@seu.edu.cn.
Irwin Davidson, Institut de Génétique et de Biologie Moléculaire et Cellulaire, France.
Kevin Struhl, Harvard Medical School, United States.
Funding Information
This paper was supported by the following grant:
National Natural Science Foundation of China 61571119 to Jinke Wang.
Additional information
Competing interests
No competing interests declared.
Author contributions
Methodology, Writing—original draft, Writing—review and editing, Performed the csgRNA-sCMV-based experiments.
Methodology, Writing—review and editing, Performed the GAL4-UAS-based experiments.
Methodology, Writing—review and editing, Helped to prepare solutions and culture cells.
Methodology, Writing—review and editing, Helped to construct vectors.
Data curation, Software, Validation, Writing—review and editing, Helped to analyze data.
Conceptualization, Supervision, Funding acquisition, Writing—original draft, Writing—review and editing, Conceptualized, designed and supervised the research, Wrote the manuscript and provided financial support for the project.
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
References
- Black JB, Adler AF, Wang HG, D'Ippolito AM, Hutchinson HA, Reddy TE, Pitt GS, Leong KW, Gersbach CA. Targeted epigenetic remodeling of endogenous loci by CRISPR/Cas9-Based transcriptional activators directly converts fibroblasts to neuronal cells. Cell Stem Cell. 2016;19:406–414. doi: 10.1016/j.stem.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M, Weber F, Jahn G, Dorsch-Häsler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41:521–530. doi: 10.1016/S0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Burgess DJ. Translational genetics: CRISPR therapies - making the grade not the cut. Nature Reviews Genetics. 2018;19:63. doi: 10.1038/nrg.2017.112. [DOI] [PubMed] [Google Scholar]
- Carter D, Chakalova L, Osborne CS, Dai YF, Fraser P. Long-range chromatin regulatory interactions in vivo. Nature Genetics. 2002;32:623–626. doi: 10.1038/ng1051. [DOI] [PubMed] [Google Scholar]
- Cetean S, Căinap C, Constantin AM, Căinap S, Gherman A, Oprean L, Hangan A, Oprean R. The importance of the granulocyte-colony stimulating factor in oncology. Medicine and Pharmacy Reports. 2015;88:468–472. doi: 10.15386/cjmed-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez A, Scheiman J, Vora S, Pruitt BW, Tuttle M, P R Iyer E, Lin S, Kiani S, Guzman CD, Wiegand DJ, Ter-Ovanesyan D, Braff JL, Davidsohn N, Housden BE, Perrimon N, Weiss R, Aach J, Collins JJ, Church GM. Highly efficient Cas9-mediated transcriptional programming. Nature Methods. 2015;12:326–328. doi: 10.1038/nmeth.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez A, Tuttle M, Pruitt BW, Ewen-Campen B, Chari R, Ter-Ovanesyan D, Haque SJ, Cecchi RJ, Kowal EJK, Buchthal J, Housden BE, Perrimon N, Collins JJ, Church G. Comparison of Cas9 activators in multiple species. Nature Methods. 2016;13:563–567. doi: 10.1038/nmeth.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Qi LS. Repurposing CRISPR system for transcriptional activation. Advances in Experimental Medicine and Biology. 2017;983:147–157. doi: 10.1007/978-981-10-4310-9_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AW, Wang H, Yang H, Shi L, Katz Y, Theunissen TW, Rangarajan S, Shivalila CS, Dadon DB, Jaenisch R. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Research. 2013;23:1163–1171. doi: 10.1038/cr.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AW, Jillette N, Lee P, Plaskon D, Fujiwara Y, Wang W, Taghbalout A, Wang H. Casilio: a versatile CRISPR-Cas9-Pumilio hybrid for gene regulation and genomic labeling. Cell Research. 2016;26:254–257. doi: 10.1038/cr.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury SR, Cui Y, Lubecka K, Stefanska B, Irudayaraj J. CRISPR-dCas9 mediated TET1 targeting for selective DNA demethylation at BRCA1 promoter. Oncotarget. 2016;7:46545–46556. doi: 10.18632/oncotarget.10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JT, Stroka DM, Brostjan C, Palmetshofer A, Bach FH, Ferran C. A20 blocks endothelial cell activation through a NF-kappaB-dependent mechanism. Journal of Biological Chemistry. 1996;271:18068–18073. doi: 10.1074/jbc.271.30.18068. [DOI] [PubMed] [Google Scholar]
- Dominguez AA, Lim WA, Qi LS. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nature Reviews Molecular Cell Biology. 2016;17:5–15. doi: 10.1038/nrm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E. Genome editing. the new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Foecking MK, Hofstetter H. Powerful and versatile enhancer-promoter unit for mammalian expression vectors. Gene. 1986;45:101–105. doi: 10.1016/0378-1119(86)90137-X. [DOI] [PubMed] [Google Scholar]
- Furth PA, Hennighausen L, Baker C, Beatty B, Woychick R. The variability in activity of the universally expressed human cytomegalovirus immediate early gene 1 enhancer/promoter in transgenic mice. Nucleic Acids Research. 1991;19:6205–6208. doi: 10.1093/nar/19.22.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Tsang JC, Gaba F, Wu D, Lu L, Liu P. Comparison of TALE designer transcription factors and the CRISPR/dCas9 in regulation of gene expression by targeting enhancers. Nucleic Acids Research. 2014;42:e155. doi: 10.1093/nar/gku836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton IB, D'Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, Gersbach CA. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nature Biotechnology. 2015;33:510–517. doi: 10.1038/nbt.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SC, Mariati, Yeo JH, Fang SG, Yang Y. Impact of using different promoters and matrix attachment regions on recombinant protein expression level and stability in stably transfected CHO cells. Molecular biotechnology. 2015;57:138–144. doi: 10.1007/s12033-014-9809-2. [DOI] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns NA, Pham H, Tabak B, Genga RM, Silverstein NJ, Garber M, Maehr R. Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nature Methods. 2015;12:401–403. doi: 10.1038/nmeth.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani S, Chavez A, Tuttle M, Hall RN, Chari R, Ter-Ovanesyan D, Qian J, Pruitt BW, Beal J, Vora S, Buchthal J, Kowal EJ, Ebrahimkhani MR, Collins JJ, Weiss R, Church G. Cas9 gRNA engineering for genome editing, activation and repression. Nature Methods. 2015;12:1051–1054. doi: 10.1038/nmeth.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Uetsuki T, Kaziro Y, Yamaguchi N, Sugano S. Use of the human elongation factor 1 alpha promoter as a versatile and efficient expression system. Gene. 1990;91:217–223. doi: 10.1016/0378-1119(90)90091-5. [DOI] [PubMed] [Google Scholar]
- Kim S, Lahmy R, Riha C, Yang C, Jakubison BL, van Niekerk J, Staub C, Wu Y, Gates K, Dong DS, Konieczny SF, Itkin-Ansari P. The basic helix-loop-helix transcription factor E47 reprograms human pancreatic cancer cells to a quiescent acinar state with reduced tumorigenic potential. Pancreas. 2015;44:718–727. doi: 10.1097/MPA.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, Nureki O, Zhang F. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhou L, Zhao T, Liu X, Zhang P, Liu Y, Zheng X, Li Q. Caspase-9: structure, mechanisms and clinical application. Oncotarget. 2017;8:23996–24008. doi: 10.18632/oncotarget.15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HK, Hatanaka F, Araoka T, Reddy P, Wu MZ, Sui Y, Yamauchi T, Sakurai M, O'Keefe DD, Núñez-Delicado E, Guillen P, Campistol JM, Wu CJ, Lu LF, Esteban CR, Izpisua Belmonte JC. In Vivo Target Gene Activation via CRISPR/Cas9-Mediated Trans-epigenetic Modulation. Cell. 2017;171:1495–1507. doi: 10.1016/j.cell.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, Shu J, Dadon D, Young RA, Jaenisch R. Editing DNA methylation in the mammalian genome. Cell. 2016a;167:233–247. doi: 10.1016/j.cell.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhan Y, Chen Z, He A, Li J, Wu H, Liu L, Zhuang C, Lin J, Guo X, Zhang Q, Huang W, Cai Z. Directing cellular information flow via CRISPR signal conductors. Nature Methods. 2016b;13:938–944. doi: 10.1038/nmeth.3994. [DOI] [PubMed] [Google Scholar]
- Liu P, Chen M, Liu Y, Qi LS, Ding S. CRISPR-Based chromatin remodeling of the endogenous Oct4 or Sox2 locus enables reprogramming to pluripotency. Cell Stem Cell. 2018;22:252–261. doi: 10.1016/j.stem.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhao C, Zhao Y, Zhang J, Zhang Y, Chen L, Han Q, Ying Y, Peng S, Ai R, Wang Y. Multimode drug inducible CRISPR/Cas9 devices for transcriptional activation and genome editing. Nucleic Acids Research. 2018;46:e25. doi: 10.1093/nar/gkx1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, Joung JK. CRISPR RNA-guided activation of endogenous human genes. Nature Methods. 2013;10:977–979. doi: 10.1038/nmeth.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nature Biotechnology. 2013a;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nature Methods. 2013b;10:957–963. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013c;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihongaki Y, Yamamoto S, Kawano F, Suzuki H, Sato M. CRISPR-Cas9-based photoactivatable transcription system. Chemistry & Biology. 2015;22:169–174. doi: 10.1016/j.chembiol.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Perez-Pinera P, Kocak DD, Vockley CM, Adler AF, Kabadi AM, Polstein LR, Thakore PI, Glass KA, Ousterout DG, Leong KW, Guilak F, Crawford GE, Reddy TE, Gersbach CA. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nature Methods. 2013;10:973–976. doi: 10.1038/nmeth.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin A, Rousseau J, Tremblay JP. Increased expression of laminin subunit alpha 1 chain by dCas9-VP160. Molecular Therapy - Nucleic Acids. 2017;6:68–79. doi: 10.1016/j.omtn.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polstein LR, Gersbach CA. A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nature Chemical Biology. 2015;11:198–200. doi: 10.1038/nchembio.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzisheuskaya A, Shlyueva D, Müller I, Helin K. Optimizing sgRNA position markedly improves the efficiency of CRISPR/dCas9-mediated transcriptional repression. Nucleic Acids Research. 2016;44:e141. doi: 10.1093/nar/gkw583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunderson EA, Stepper P, Gomm JJ, Hoa L, Morgan A, Allen MD, Jones JL, Gribben JG, Jurkowski TP, Ficz G. Hit-and-run epigenetic editing prevents senescence entry in primary breast cells from healthy donors. Nature Communications. 2017;8:1450. doi: 10.1038/s41467-017-01078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EV, Christoph G, Zeller R, Leder P. The cytomegalovirus enhancer: a pan-active control element in transgenic mice. Molecular and Cellular Biology. 1990;10:4406–4411. doi: 10.1128/MCB.10.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nature Reviews Molecular Cell Biology. 2016;17:183–193. doi: 10.1038/nrm.2016.8. [DOI] [PubMed] [Google Scholar]
- Tanenbaum ME, Gilbert LA, Qi LS, Weissman JS, Vale RD. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 2014;159:635–646. doi: 10.1016/j.cell.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Molecular Cell. 2002;10:1453–1465. doi: 10.1016/S1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Wang H, La Russa M, Qi LS. CRISPR/Cas9 in genome editing and beyond. Annual Review of Biochemistry. 2016;85:227–264. doi: 10.1146/annurev-biochem-060815-014607. [DOI] [PubMed] [Google Scholar]
- Wang D, Dai W, Wu J, Wang J. Improving transcriptional activity of human cytomegalovirus major immediate-early promoter by mutating NF-κB binding sites. Protein Expression and Purification. 2018;142:16–24. doi: 10.1016/j.pep.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Yin C, Lin Y, Zhang X, Chen YX, Zeng X, Yue HY, Hou JL, Deng X, Zhang JP, Han ZG, Xie WF. Differentiation therapy of hepatocellular carcinoma in mice with recombinant adenovirus carrying hepatocyte nuclear factor-4alpha gene. Hepatology. 2008;48:1528–1539. doi: 10.1002/hep.22510. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zalatan JG, Lee ME, Almeida R, Gilbert LA, Whitehead EH, La Russa M, Tsai JC, Weissman JS, Dueber JE, Qi LS, Lim WA. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell. 2015;160:339–350. doi: 10.1016/j.cell.2014.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Zhu T, Lu X, Zhu J, Li L. Neurogenin 2 enhances the generation of patient-specific induced neuronal cells. Brain Research. 2015;1615:51–60. doi: 10.1016/j.brainres.2015.04.027. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Shen W, Zhang J, Yang B, Liu YN, Qi H, Yu X, Lu SY, Chen Y, Xu YZ, Li Y, Gage FH, Mi S, Yao J. CRISPR interference-based specific and efficient gene inactivation in the brain. Nature Neuroscience. 2018;21:447–454. doi: 10.1038/s41593-018-0077-5. [DOI] [PubMed] [Google Scholar]
- Zhou H, Liu J, Zhou C, Gao N, Rao Z, Li H, Hu X, Li C, Yao X, Shen X, Sun Y, Wei Y, Liu F, Ying W, Zhang J, Tang C, Zhang X, Xu H, Shi L, Cheng L, Huang P, Yang H. In vivo simultaneous transcriptional activation of multiple genes in the brain using CRISPR-dCas9-activator transgenic mice. Nature Neuroscience. 2018;21:440–446. doi: 10.1038/s41593-017-0060-6. [DOI] [PubMed] [Google Scholar]