Abstract
Idiopathic uric acid nephrolithiasis is characterized by an overly acidic urine pH caused by the combination of increased acid production and inadequate buffering of urinary protons by ammonia. A large proportion of uric acid stone formers exhibit features of the metabolic syndrome. We previously demonstrated that thiazolidinediones improved the urinary biochemical profile in an animal model of the metabolic syndrome. In this proof-of-concept study, we examined whether the thiazolidinedione pioglitazone can also ameliorate the overly acidic urine in uric acid stone formers. 36 adults with idiopathic uric acid nephrolithiasis were randomized to pioglitazone 30 mg/day or matching placebo for 24 weeks. At baseline and study end, participants underwent collection of blood and 24-hour urine in an inpatient research unit while consuming a fixed metabolic diet, followed by assessment of the ammoniagenic response to an acute oral acid load. 28 participants completed the study. Pioglitazone treatment improved features of the metabolic syndrome. Pioglitazone also reduced net acid excretion and increased urine pH (5.37 to 5.59), the proportion of net acid excreted as ammonium, and ammonium excretion in response to an acute acid load, whereas these parameters were unchanged with placebo. Treatment of patients with idiopathic uric acid nephrolithiasis with pioglitazone for 24 weeks led to a reduction in the acid load presented to the kidney and a more robust ammoniagenesis and ammonium excretion, resulting in significantly higher urine pH. Future studies should consider the impact of this targeted therapy on uric acid stone formation.
Keywords: Uric Acid, Nephrolithiasis, Metabolic Syndrome, Insulin Resistance, Pioglitazone, Thiazolidinedione
Introduction:
The prevalence of kidney stones has increased globally in recent decades1,2. Idiopathic uric acid nephrolithiasis (IUAN) comprises an increasing fraction of kidney stones3,4. Unduly acid urine pH (typically urine pH ≤5.5), is an invariable feature of IUAN that leads to precipitation of the sparingly soluble uric acid, raising the risk of uric acid stone formation5,6. In IUAN, the abnormally acidic urine pH is caused by the combination of increased load from non-dietary derived acid coupled with inadequate buffering of urinary protons by the major urinary buffer ammonia (NH3) excreted as ammonium (NH4+)6–11.
Both observational and metabolic studies have demonstrated that patients with obesity, type 2 diabetes (T2DM), and/or the metabolic syndrome (MS) are at an increased risk for IUAN, primarily because of a low urine pH6,10,12–15. We observed similar findings in the Zucker Diabetic Fatty (ZDF) rats, an established rodent model of the MS that displays several biochemical features of IUAN including renal steatosis, low urinary pH and NH4+6,16.
Thiazolidinediones (TZDs) are selective ligands of the nuclear transcription factor peroxisome-proliferator-activated receptor γ (PPARγ). TZDs were first introduced to address the basic problem of insulin resistance in patients with type 2 diabetes17,18. These pharmacological agents exert their effects in part by promoting fatty acid uptake and storage in adipose tissue, but also by altering the release of adipokines, and/or through modulation of insulin sensitivity outside the adipose tissue by relieving the ectopic fat load in organs such as the liver, heart and skeletal muscle19,20. We have shown that TZD treatment of ZDF rats reversed the renal fat deposition and urinary acidification defect caused by reduced ammonium excretion, resulting in an increase in urine pH to levels comparable to lean control rats21. However, the intermediary steps that mediate this phenotypic improvement, and whether this finding can be extrapolated to humans are all unknown.
The treatment of IUAN in humans thus far has been restricted to empirical manipulation of urinary chemistry by oral tripotassium citrate22 which provides base equivalents to neutralize the increased acid production of yet-to-be-determined origin, and direct elevation of urinary pH which decreases the physico-chemical risk of uric acid precipitation. No attempts have been made to design therapy directed at the underlying pathophysiology. The current study seeks to test whether findings of our previous study of TZDs in ZDF rats can be generalized to IUAN patients in a proof-of-concept interventional study, to escalate treatment of IUAN from empiric manipulation of urinary chemistry, and further to examine whether TZDs targets the increased net acid generation, defective ammonia synthesis, or both.
Results:
Baseline Characteristics
Thirty-six IUAN patients were included in this study. Table 1 is a summary of general and metabolic characteristics of the study population at baseline. The majority of participants were men (64%), and of White race (86%) and non-Hispanic ethnicity (81%). The gender, ethnicity and racial compositions were similar in both groups. Five subjects in the placebo group and ten subjects in the pioglitazone group had previously diagnosed type 2 diabetes. Serum electrolyte composition, albumin and BUN levels were similar between the two groups.
Table 1.
Baseline Characteristics
| Placebo | Pioglitazone | |
|---|---|---|
| Number of subjects | 18 | 18 |
| Age, years | 55.2 ± 11.2 | 58.9 ± 9.1 |
| Gender (F/M) | 6 / 12 | 7 / 11 |
| Race (Black/White) | 3 / 15 | 2 / 16 |
| Ethnicity (Hispanic/Non-Hispanic) | 5 / 13 | 2 / 16 |
| Type 2 Diabetes (Yes/No) | 5 / 13 | 10 / 8 |
| Serum chemistry | ||
| Na, mmoles/L | 138 ± 2 | 137 ± 2 |
| K, mmoles/L | 4.4 ± 0.3 | 4.4 ± 0.4 |
| Cl, mmoles/L | 105 ± 2 | 104 ± 3 |
| CO2, mmoles/L | 25 ± 3 | 24 ± 3 |
| Ca, mmoles/L | 2.31 ± 0.05 | 2.32 ± 0.10 |
| Albumin, g/L | 413 ± 19 | 414 ± 27 |
| BUN, mmoles/L | 5.6 ± 1.8 | 6.0 ± 1.8 |
| Creatinine, μmoles/L | 94 ± 33 | 92 ± 27 |
Data shown as mean ± standard deviation
Six participants in the placebo group and 2 participants in the pioglitazone dropped out after completing the initial evaluation and randomization. Reasons for dropout included withdrawal of consent due to inability to commit time to study procedures (4 participants in the placebo group and 2 in the pioglitazone group), intercurrent illness (1 participant in placebo group), and loss of contact with study team (1 participant in placebo group). Twenty-eight subjects completed the week 24 evaluation.
Glucose and Lipid Metabolism
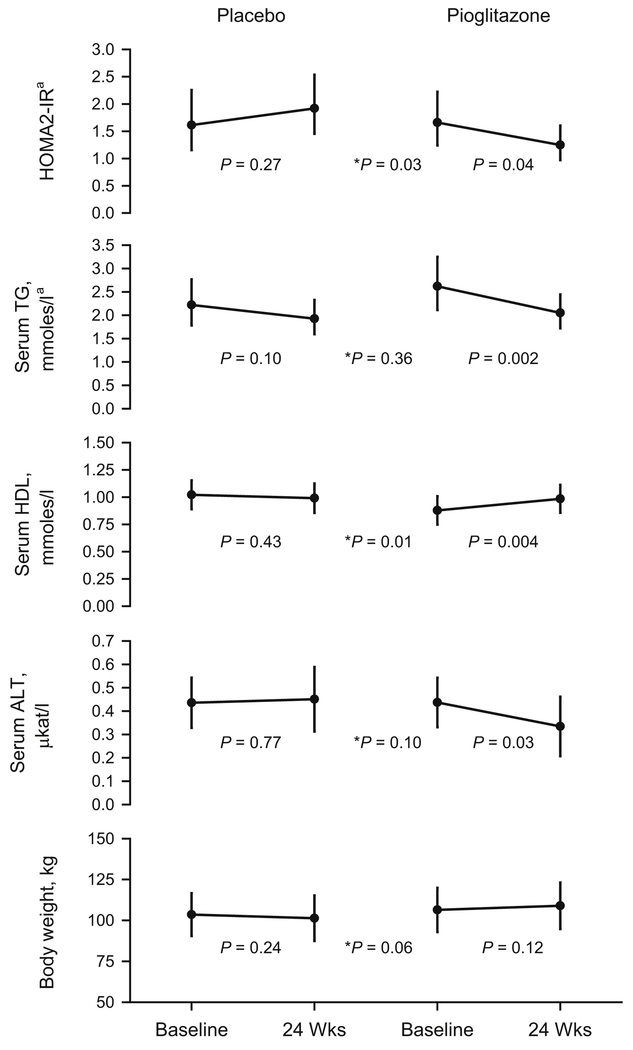
Pioglitazone treatment resulted in a robust decline in peripheral insulin resistance as shown by a reduction in HOMA2-IR (p=0.04), accompanied by declines serum alanine aminotransferase (ALT, p=0.03), and TG (p=0.002), and an increase in HDL-cholesterol (p=0.004), while none of these parameters changed in the placebo group (Figure 1). Treatment effects (week 24 – week 0 in the pioglitazone group vs. placebo group) were significant for HOMA2-IR (p=0.03) and HDL (p=0.01), but not for TG (p=0.36), or ALT (p=0.10). Serum glucose declined significantly with pioglitazone (p=0.01), but did not change with placebo (Table 2). Treatment with pioglitazone was well tolerated and did not result in hypoglycemia in non-diabetics. Serum insulin decreased with pioglitazone (p=0.06) without reaching statistical significance, and did not change with placebo (p=0.19), but a significant treatment effect was detected (p=0.03). Changes in non-esterified free fatty acids (NEFFA), alkaline phosphatase, Aspartate Aminotransferase (AST), and total cholesterol were not significantly different between the two groups. BMI did not change significantly in either group or between groups during the 24 weeks.
Figure 1. Glucose and lipid metabolism.
Data shown as Least Square Means or ageometric means ± 95% confidence intervals. P values indicate difference between Baseline and 24 Weeks, * indicates the treatment and visit interaction P value for comparison between placebo and pioglitazone.
Table 2.
Changes in body mass index and selected serum parameters during the trial.
| Placebo | Pioglitazone | Interaction | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 24 Weeks | P | Baseline | 24 Weeks | P | P | |
| BMI, kg/m2 | 34.9 (31.0 to 38.7) | 34.0 (30.1 to 37.9) | 0.18 | 35.1 (31.1 to 39.0) | 35.8 (31.9 to 39.8) | 0.19 | 0.07 |
| Serum | |||||||
| Insulin, mU/La | 9.0 (6.2 to 13.2) | 12.0 (7.9 to 18.4) | 0.19 | 11.8 (8.0 to 17.3) | 8.1 (5.5 to 11.9) | 0.06 | 0.03 |
| NEFFA, mEq/L | 0.52 (0.43 to 0.62) | 0.57 (0.44 to 0.70) | 0.48 | 0.61 (0.51 to 0.70) | 0.56 (0.46 to 0.66) | 0.45 | 0.31 |
| Glucose, mmoles/L | 5.4 (4.7 to 6.1) | 5.4 (4.8 to 6.0) | 0.99 | 6.4 (5.6 to 7.3) | 5.4 (4.9 to 6.0) | 0.01 | 0.09 |
| Phosphorus, mmoles/L | 1.13 (1.06 to 1.20) | 1.09 (1.01 to 1.18) | 0.30 | 1.12 (1.05 to 1.19) | 1.10 (1.02 to 1.18) | 0.45 | 0.77 |
| Magnesium, mmoles/L | 0.89 (0.85 to 0.93) | 0.87 (0.83 to 0.91) | 0.30 | 0.85 (0.81 to 0.89) | 0.82 (0.78 to 0.85) | 0.05 | 0.56 |
| Alkaline Phosphatase, μkat/L | 1.30 (1.12 to 1.48) | 1.17 (1.00 to 1.33) | 0.004 | 1.24 (1.06 to 1.42) | 1.13 (0.98 to 1.29) | 0.009 | 0.63 |
| AST, μkat/L | 0.36 (0.30 to 0.43) | 0.38 (0.30 to 0.46) | 0.67 | 0.39 (0.32 to 0.46) | 0.33 (0.25 to 0.40) | 0.07 | 0.13 |
| Cholesterol, mmoles/La | 5.26 (4.70 to 5.90) | 5.07 (4.45 to 5.79) | 0.36 | 5.25 (4.69 to 5.87) | 4.99 (4.40 to 5.65) | 0.15 | 0.80 |
Data presented as Least Square Means or geometric means (95% confidence intervals). Interaction P value corresponds to the treatment and visit interaction for comparison between placebo and pioglitazone groups
Uric Acid Metabolism
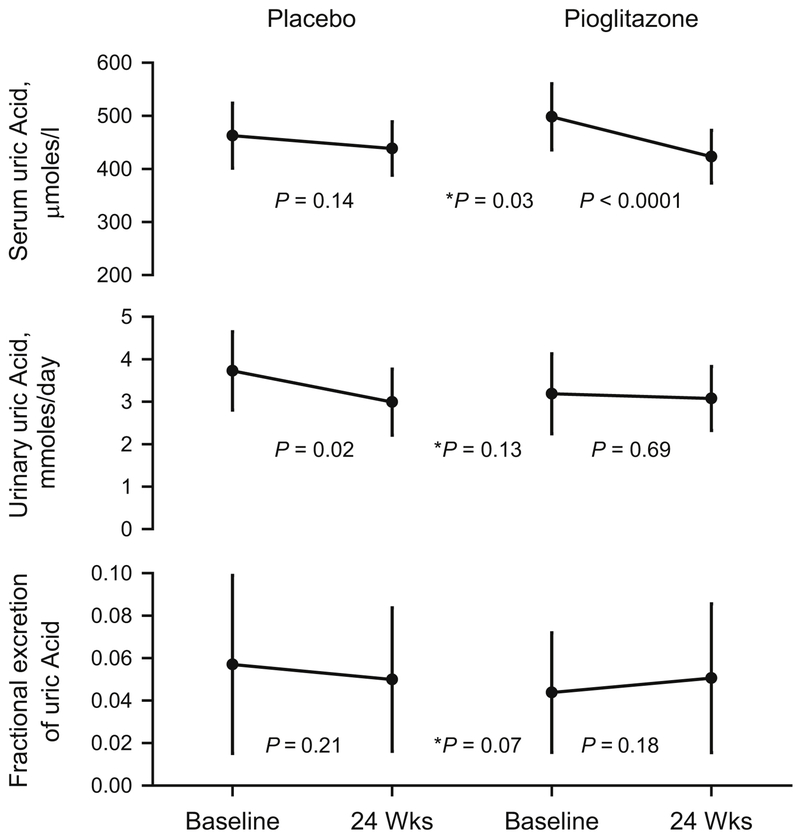
Serum uric acid decreased in the pioglitazone group (p<0.0001), but showed no change with placebo (p=0.14), with a significant treatment effect (p=0.03, Figure 2). The pioglitazone group showed no changes in urinary uric acid (p=0.69), or fractional excretion of uric acid (p=0.18). The placebo group showed a significant decrease in urinary uric acid (p=0.02) with no change in fractional excretion of uric acid (p=0.21), (Figure 2).
Figure 2. Uric acid metabolism and excretion.
Data shown as Least Square Means ± 95% confidence intervals. P values indicate difference between Baseline and 24 Weeks, * indicates the treatment and visit interaction P value for comparison between placebo and pioglitazone.
Other Serum and Urine Parameters
There was no effect between groups for serum magnesium (p=0.56) and phosphorus (p=0.77), however the serum magnesium showed a small, but statistically significant decline with pioglitazone (p=0.05, Table 2). No significant changes were detected in urinary total volume (TV), magnesium, chloride, phosphate or sulfate within or between groups. While urine Ca increased and urine Ox declined with pioglitazone treatment (p=0.04 and p=0.01, respectively), these parameters were unchanged in the placebo group, and there were no significant treatment effects (p=0.58 and p=0.43, respectively).
Acid-Base Parameters
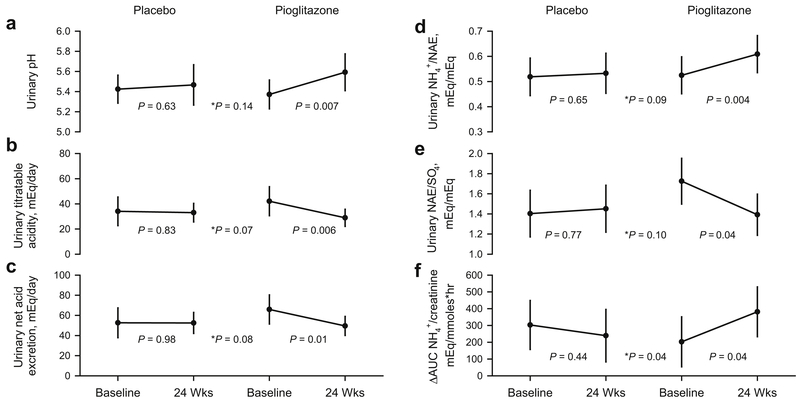
While urinary NH4+ excretion rate did not change with either placebo (p=0.44) or pioglitazone (p=0.15), pioglitazone treatment significantly increased urinary pH (5.37 to 5.59, p=0.007, Figure 3A), and the fraction of net acid excreted as NH4+ (NH4+/NAE, 0.52 to 0.61 mEq/mEq, p=0.004, Figure 3B). TA and NAE both significantly decreased with pioglitazone use (p=0.006 and p=0.01, respectively, Figure 3C and 3D). Furthermore, the ratio NAE/SO4 decreased significantly with pioglitazone treatment (p=0.04, Figure 3E). No changes were detected in any of the acid-base parameters with placebo treatment (Figure 3A-3E).
Figure 3. Acid-base profile.
Data shown as Least Square Means ± 95% confidence intervals. P values indicate difference between Baseline and 24 Weeks, * indicates the treatment and visit interaction P value for comparison between placebo and pioglitazone. Figure 3A-3E refer to 24hr urine studies. Figure 3F refers to acute response to an NH4Cl load.
Finally, chronic TZD treatment resulted in a significant improvement in the acute ammoniagenic response following an NH4Cl load (ΔAUC NH4+/Creatinine, 203 to 381 mEq/mmoles·hr, p=0.04, Figure 3F).
Discussion:
This comprehensive metabolic study is the first to assess the underlying pathophysiology of IUAN with an interventional approach. In this randomized double-blind, placebo-controlled proof-of-concept trial, we evaluated the impact of the TZD pioglitazone in IUAN patients on a fixed metabolic diet with two seminal and novel findings in this study.
First, pioglitazone modestly, but significantly, increased urine pH, the fraction of net acid excretion carried by NH4+ (NH4+/NAE) and the ammoniagenic response (Δ NH4+/creatinine) following an acid load (Figure 3). The results are consistent with an improvement in the synthesis and/or excretion of ammonium, a high capacity buffer previously demonstrated to be impaired in untreated IUAN and a major pathophysiologic factor in lithogenicity6,8,11.
Second, both TA and NAE decreased with TZD treatment, indicating that total H+ presented to the kidneys is reduced, and less H+ is excreted with non-NH3 buffers. This was not related to changes in dietary acid precursors, since the NAE/SO4 ratio decreased significantly with pioglitazone, indicating a reduction in the diet-independent acid generation that was previously found to be higher in IUAN and non-treated obese and T2DM non-stone forming subjects under a fixed metabolic diet8,10. These improvements occurred contemporaneously with improvement in systemic metabolic disturbances in insulin sensitivity (HOMA-IR), hepatic (ALT), lipid (TG & HDL) (Figure 1), and uric acid metabolism (serum UA) (Figure 2), without a significant change in body weight or body mass index.
Therefore, with the dual change of less acid presented to the kidney, and more robust ammoniagenesis and excretion, urinary pH is higher, and the biochemical risk for UA precipitation may be reduced. The results of this study prompt a significant shift in understanding the pathophysiology of UA urolithiasis, and could have a clinical impact on the treatment of uric acid formers potentially shifting from the traditional empiric urinary alkalization22,23 to a potential specific pathophysiology-based targeted treatment.
The improvement in acid-base profile with TZD may be mediated by several mechanisms18: Improvement in insulin resistance, redistribution of fat away from the kidney and liver with reduction in hepatic and renal fat, reduction in circulating fatty acid presented as a substrate to the kidney, and/or increased circulating adiponectin.
Insulin stimulates both renal ammoniagenesis (from L-glutamine)24 and excretion 25–28 There is a known association between peripheral insulin resistance (hyperinsulinemic euglycemic clamp) and defective NH4+ excretion and low urine pH in IUAN7. IUAN patients exhibit significantly greater insulin resistance and lower urinary pH when compared to healthy subjects, and acute hyperinsulinemia was associated with higher urinary pH and urinary ammonium excretion, suggesting that low urinary ammonium and pH may be a renal manifestation of insulin resistance7. In the present study, pioglitazone resulted a significant improvement in peripheral insulin resistance (assessed by HOMA2-IR29,30, Figure 1), and increases in urine pH and NH4 excretion (Figure 3).
Visceral fat deposition has previously been suggested to mediate the metabolic complications of obesity to a larger extent than subcutaneous fat31,32. TZD use was shown in human studies to redistribute ectopic fat deposited in organs such as the liver, heart, and skeletal muscle33 to adipocytes and the bone marrow19,34. We have shown that obesity is associated with fat deposition in the kidney, with a predominant localization in proximal tubule cells35, the major site of renal ammonia production from its precursor glutamine36. TZD treatment also reduces renal triglyceride accumulation and restores urinary acidification parameters in ZDF rats to levels comparable to their lean littermates21.
Furthermore, functional abnormalities induced by fat loading in a cell culture model of proximal tubule steatosis and lipotoxicity could be reversed by fat removal but not by TZD alone, suggesting a causative role of renal steatosis in the pathogenesis of urinary acidification defects21. It is plausible that pioglitazone improved the urinary acidification defects in the present study by redistributing fat from the renal proximal tubule of IUAN participants, although this was not directly assessed in our study. A reduction in hepatic fat with pioglitazone use may improve hepatic metabolism of organic anions delivered to the liver, thus resulting in lowering NAE/SO4 (from the reduced non-dietary proton generation) (Figure 3).
Pioglitazone may have also ameliorated the acid-base profile in IUAN through an increase in serum adiponectin, as adiponectin receptors in the kidney have been linked to the improvement in albuminuria and diabetic nephropathy37,38. Alternatively, pioglitazone use may be related to local reduction in cytokine activity within the kidney39. Both of the above may affect renal acidification.
There are several clinical implications to our findings: Our group and others have identified type 2 diabetes and insulin resistance as major risk factors for low urine pH and uric acid nephrolithiasis9,11–13,40. We only examined an intermediate dose of pioglitazone (30 mg/day), and only for a short duration. It is not known whether a longer treatment duration and/or use of the highest clinically recommended pioglitazone dose of 45 mg/day can result in even greater improvement in acid-base parameters. Still, pioglitazone use in IUAN stone formers with insulin resistance may be advantageous to raise urine pH (and potential allow the use of lower doses of alkali) while improving insulin sensitivity. On the other hand, in a previous single-center cross-sectional study of type 2 diabetic patients with stone disease41, thiazolidinedione use was not associated with higher urine pH than use of other oral anti-hyperglycemic agents. Differences in study design (cross-sectional vs. placebo-control trial), study population (all stone formers with type 2 diabetes vs. IUAN with and without diabetes), diet, glycemic control, and duration of therapy preclude definitive conclusions.
One limitation of our study was the lack of direct assessment of hepatic and renal fat content that should be addressed in future control studies. Additional limitations of our study include moderate sample size (18 patients per group), study of participants while on a metabolic diet (instead of their usual home diet), and testing of a single dose of pioglitazone (30 mg/day), and the relatively short duration of only 24 weeks.
In conclusion, the result of this double blind proof-of-concept study indicates that pharmacological intervention using pioglitazone could be a paradigm shift in the understanding and management of uric acid stones. This targeted treatment is promising given that it not only improves the urinary biochemical profile (although it did not result in a change in uric acid supersaturation), but also targets the underlying pathobiology in this population. This work must be supplemented by a larger scale multi-center clinical trial.
Methods:
This randomized, double-blind, placebo-controlled study was approved by the UT Southwestern Institutional Review Board and registered with the United States National Library of Medicine (NCT 00904046; www.clinicaltrials.gov).
Study Participants:
Study participants were recruited from the Mineral Metabolism Clinic of the Center of Mineral Metabolism and Clinical Research, and the Urology Clinic at the University of Texas Southwestern Medical Center. Included were participants age >21 years of either gender and any ethnicity, who had a documented diagnosis of uric acid nephrolithiasis based on the most recent available stone analysis showing 100% uric acid in composition determined by x-ray crystallography. Excluded were participants with body weight > 157 kg (350 lbs), those with chronic diarrhea, impaired renal function (endogenous creatinine clearance <60 mL/min), history of recurrent urinary tract infection, or a contra-indication to TZD use (liver disease, congestive heart failure NYHA Class III or IV, significant pedal edema, or those unwilling to practice effective contraception for the duration of the study). Subjects who were receiving insulin, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, or non-steroidal anti-inflammatory agents on a chronic basis, or who had received any TZD in the preceding 18 months were excluded. Participants receiving alkali, thiazides and/or allopurinol were instructed to discontinue these medications for 2 weeks before the baseline visit and again 2 weeks before the final evaluation of the study. The University of Texas Southwestern Institutional Review Board approved the study and informed consent was obtained from each of the participating subjects.
Participants were enrolled either by the research nurse or by one of the study investigators. Randomization was performed utilizing a blocked randomization with variable block sizes of 4 to 6 by the study statistician. All personnel with study participant contact were blinded from the study drug allocations. Upon participant enrollment, study drugs were dispensed by designated research personnel in accordance with the randomization table. The last study participant completed the last visit in November 2017.
Study Protocol
Study participants were randomized to take pioglitazone 30 mg or a matching placebo once daily with breakfast for 24 weeks. Study tablets were provided by Takeda Pharmaceuticals North America, Inc. (Deerfield, IL). Compliance was ensured via telephone reminders at 2, 8, 16, and 20 weeks and with two outpatient visits at 4 and 12 weeks of the study. Detailed metabolic evaluations were performed before and after 24 weeks of treatment. These evaluations consisted of an outpatient 3-day stabilization period followed by a 3-day inpatient admission. During the 3-day stabilization period, participants were maintained on a frozen isocaloric metabolic diet [30% fat, 55% carbohydrate, 15% protein, and 300mg cholesterol, 400mg (10 mmoles) calcium, 800 mg (26 mmoles) phosphorus, 100 mEq sodium, 40 mEq potassium] with a fixed acid ash content and sufficient fluid (distilled water) to ensure 2 liters of urine per day. After 3 days of stabilization, participants were admitted to the inpatient research unit for 3 days and provided with a diet of similar composition. 24-hour urine specimen were collected under mineral oil and maintained refrigerated on days 4 and 5. On the morning of study days 4 and 5, fasting blood was obtained for free fatty acids (FFA), insulin, complete metabolic panels (CMP), uric acid, and lipid profiles. On the evening of day 5, subjects fasted except for 300 ml distilled water at bedtime. On the morning of day 6, breakfast was withheld and an oral ammonium chloride (NH4Cl) test was performed. Urine collected hourly from 7:00 am to 12:00pm under mineral oil. At 8:00am, 50 mEq NH4Cl in gelatin capsules was administered orally with 250 mL of distilled water. To ensure adequate urine output, 250 mL distilled water was drunk at 7:00, 8:00, 9:00, 10:00, and 11:00 am. Hourly urine samples were analyzed for total volume pH, creatinine, NH4+, TA and bicarbonate. Arterialized venous blood was obtained (venous blood drawn without stasis from an antecubital vein after 30 minutes of application of electric warm pack to the forearm) for basic metabolic profile, pH and blood gas at 7:00, 10:00am and 12:00pm.
Analytical Procedures and Calculations
Serum insulin and NEFFA were measured by ELISA kits (Kamiya Biomedical Company, Seattle, WA and Wako Pure Chemical Corporation, Mountain View, CA). Serum calcium (Ca), magnesium (Mg), uric acid (UA), glucose, and triglycerides (TG) were measured by Synchron CX9ALX auto analyzer (Beckman Coulter Bera, California). The 24-hour urine samples were analyzed for total urine volume (TV), creatinine (Cr) by the picric acid method, NH4+ by glutamate dehydrogenase, and phosphorus by an Olympus AU400 chemistry. Urinary pH was assessed by digital pH electrode, urinary sodium (Na) and potassium (K) by a flame photometer (BWB Technologies, Newbury, United Kingdom), urinary calcium and magnesium by an atomic absorption spectrophotometry (Varian-Agilent Technologies, Palo Alto, California), UA by uricase, urinary sulfate (SO4) by ion chromatography (Dionex Sunnyvale, California), urinary citrate by citrate lyase (Cobas Fara, Roche, New Jersey) and urinary chloride (Cl) by chloridometer. Urinary titratable acidity (TA) was measured directly using automated burette end-point titration system (Radiometer, Copenhagen, Denmark). The milliequivalents of OH− required to bring the original pH to pH 7.4 yielded TA.
Net acid excretion (NAE) was calculated as (NH4+ + TA) − (HCO3− + Ionized citrate2−/3−); all in milliequivalents. Urinary HCO3− was calculated from urinary pH and PCO2 and milliequivalents of ionized citrate was calculated from urinary pH and pKa of citrate2−/citrate3− of 5.6. The arterialized venous plasma HCO3− concentration was calculated by blood pH and PCO2 (Henderson-Hasselbalch equation). Net GI alkali absorption (NGIA) was calculated as previously described42. The updated homeostasis model assessment of insulin resistance (HOMA2-IR) was calculated using online HOMA calculator version 2.2.3 for specific insulin at available websitewww.dtu.ox.ac.uk/homacalculator29.
Statistical Analysis
The original sample size was powered to detect changes in renal fat content with pioglitazone use. However, renal fat content was too low to reliably detect by magnetic resonance spectroscopy. An intention-to-treat analysis was performed for the current analysis of urinary biochemistry. Treatment group comparisons and changes from baseline over time (study visits) of the primary outcome (urine pH) and continuous secondary outcome variables were analyzed with mixed-effects model repeated analysis. Fixed factors for the treatment group, visit, and the interaction between treatment and visit were included in the models with the study participant modeled as a random effect. The difference in response between pioglitazone and placebo was tested by the treatment × visit interaction factor. Statistical analysis was conducted with SAS 9.4 (SAS Institute, Cary NC). A two-sided alpha of 0.05 was considered statistically significant. Data are mean ± standard deviation or least square means (95%CI) unless otherwise noted.
Table 3.
Changes in 24-hour urinary parameters during the trial.
| Placebo | Pioglitazone | Interaction | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 24 Weeks | P | Baseline | 24 Weeks | P | P | |
| Total Volume, L/d | 2.56(2.29 to 2.84) | 2.48(2.10 to 2.86) | 0.59 | 2.50(2.22 to 2.78) | 2.40(2.04 to 2.75) | 0.42 | 0.90 |
| Na, mmoles/d | 94(75 to 112) | 83(58 to 109) | 0.44 | 89(69 to 108) | 100(77 to 123) | 0.33 | 0.23 |
| K, mmoles/d | 38(33 to 44) | 35(29 to 40) | 0.13 | 39(34 to 44) | 37(32 to 42) | 0.37 | 0.58 |
| Creatinine, mmoles/d | 13.6(11.8 to 15.4) | 13.5(11.5 to 15.5) | 0.89 | 14.3(12.4 to 16.1) | 13.7(11.8 to 15.7) | 0.30 | 0.56 |
| Ca, mmoles/d | 2.41(1.80 to 3.02) | 2.69(1.81 to 3.56) | 0.26 | 2.67(2.05 to 3.30) | 3.13(2.27 to 3.99) | 0.038 | 0.58 |
| Mg, mmoles/d | 3.93(3.26 to 4.59) | 3.70(3.02 to 4.37) | 0.38 | 3.40(2.71 to 4.08) | 3.15(2.51 to 3.79) | 0.29 | 0.96 |
| Cl, mmoles/d | 88(66 to 110) | 78(55 to 102) | 0.49 | 97(74 to 119) | 95(75 to 116) | 0.91 | 0.66 |
| Ammonium, mEq/d | 26(22 to 30) | 28(24 to 32) | 0.44 | 31(27 to 36) | 29(25 to 33) | 0.19 | 0.15 |
| Citrate, mmoles/d | 2.50(1.52 to 3.48) | 2.58(1.62 to 3.54) | 0.87 | 2.68(1.68 to 3.68) | 2.61(1.72 to 3.49) | 0.86 | 0.81 |
| Citrate, mEq/d | 5.80(3.65 to 7.96) | 6.07(3.52 to 8.61) | 0.82 | 5.95(3.74 to 8.17) | 6.27(3.94 to 8.60) | 0.76 | 0.98 |
| Phosphorus, mmoles/d | 20.6(16.8 to 24.4) | 20.0(16.0 to 24.0) | 0.67 | 23.3(19.4 to 27.2) | 21.7(17.9 to 25.6) | 0.22 | 0.16 |
| Oxalate, μmoles/d | 319(279 to 359) | 286(246 to 326) | 0.17 | 336(295 to 377) | 278(243 to 314) | 0.01 | 0.43 |
| Sulfate, mEq/d | 37(32 to 42) | 35(30 to 40) | 0.31 | 38(33 to 43) | 37(32 to 42) | 0.45 | 0.79 |
| NGIA, mEq/d | 35.3(16.0 to 54.5) | 18.3(9.9 to 26.7) | 0.13 | 4.1(−15.3 to 23.4) | 15.5(8.1 to 22.9) | 0.29 | 0.07 |
Data presented as Least Square Means (95% confidence intervals). Interaction P value corresponds to the treatment and visit interaction for comparison between placebo and pioglitazone groups
Acknowledgements:
The authors are grateful for the assistance of the research nursing and technical staff at the Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, the Mineral Metabolism Laboratory, and the Clinical & Translational Research Center at UT Southwestern. This work was supported by NIDDK grant R01-DK081423 (to K.S. and O.W.M.). The UT Southwestern Clinical Research Unit is supported in part by NIH grant UL1-TR001105. Study tablets were kindly provided by Takeda Pharmaceuticals North America, Inc. (Deerfield, IL). Takeda Pharmaceuticals had no input in the design or conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors disclose no competing interests.
References
- 1.Scales CD Jr., Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. European urology. 2012;62(1):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoag J, Tasian GE, Goldfarb DS, et al. The new epidemiology of nephrolithiasis. Advances in chronic kidney disease. 2015;22(4):273–278. [DOI] [PubMed] [Google Scholar]
- 3.Xu LHR, Adams-Huet B, Poindexter JR, et al. Temporal Changes in Kidney Stone Composition and in Risk Factors Predisposing to Stone Formation. The Journal of urology. 2017;197(6):1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daudon M, Knebelmann B. [Epidemiology of urolithiasis]. La Revue du praticien. 2011. ;61 (3):372–378. [PubMed] [Google Scholar]
- 5.Maalouf NM, Cameron MA, Moe OW, et al. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens. 2004;13(2):181–189. [DOI] [PubMed] [Google Scholar]
- 6.Sakhaee K, Adams-Huet B, Moe OW, et al. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62(3):971–979. [DOI] [PubMed] [Google Scholar]
- 7.Abate N, Chandalia M, Cabo-Chan AV, Jr., et al. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65(2):386–392. [DOI] [PubMed] [Google Scholar]
- 8.Maalouf NM, Cameron MA, Moe OW, et al. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol. 2010;5(7):1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maalouf NM, Sakhaee K, Parks JH, et al. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65(4):1422–1425. [DOI] [PubMed] [Google Scholar]
- 10.Cameron MA, Maalouf NM, Adams-Huet B, et al. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17(5):1422–1428. [DOI] [PubMed] [Google Scholar]
- 11.Bobulescu IA, Maalouf NM, Capolongo G, et al. Renal ammonium excretion after an acute acid load: blunted response in uric acid stone formers but not in patients with type 2 diabetes. American journal of physiology Renal physiology. 2013;305(10):F1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daudon M, Lacour B, Jungers P. High prevalence of uric acid calculi in diabetic stone formers. Nephrol Dial Transplant. 2005;20(2):468–469. [DOI] [PubMed] [Google Scholar]
- 13.Lieske JC, de la Vega LS, Gettman MT, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis. 2006;48(6):897–904. [DOI] [PubMed] [Google Scholar]
- 14.Daudon M, Traxer O, Conort P, et al. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17(7):2026–2033. [DOI] [PubMed] [Google Scholar]
- 15.Pak CY, Sakhaee K, Peterson RD, et al. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int. 2001;60(2):757–761. [DOI] [PubMed] [Google Scholar]
- 16.Bobulescu IA, Dubree M, Zhang J, et al. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. American journal of physiology Renal physiology. 2008;294(6):F1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazaki Y, Mahankali A, Matsuda M, et al. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes care. 2001. ;24(4):710–719. [DOI] [PubMed] [Google Scholar]
- 18.Yki-Jarvinen H. Thiazolidinediones. The New England journal of medicine. 2004;351(11):1106–1118. [DOI] [PubMed] [Google Scholar]
- 19.Zib I, Jacob AN, Lingvay I, et al. Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2007;55(5):230–236. [DOI] [PubMed] [Google Scholar]
- 20.Tonelli J, Li W, Kishore P, et al. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53(6):1621–1629. [DOI] [PubMed] [Google Scholar]
- 21.Bobulescu IA, Dubree M, Zhang J, et al. Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. American journal of physiology Renal physiology. 2009;297(5):F1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cy Pak, Sakhaee K, Fuller C. Successful management of uric acid nephrolithiasis with potassium citrate. Kidney Int. 1986;30(3):422–428. [DOI] [PubMed] [Google Scholar]
- 23.Sakhaee K, Nicar M, Hill K, et al. Contrasting effects of potassium citrate and sodium citrate therapies on urinary chemistries and crystallization of stone-forming salts. Kidney Int. 1983;24(3):348–352. [DOI] [PubMed] [Google Scholar]
- 24.Chobanian MC, Hammerman MR. Insulin stimulates ammoniagenesis in canine renal proximal tubular segments. The American journal of physiology. 1987;253(6 Pt 2):F1171–1177. [DOI] [PubMed] [Google Scholar]
- 25.Fuster DG, Bobulescu IA, Zhang J, et al. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. American journal of physiology Renal physiology. 2007;292(2):F577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klisic J, Hu MC, Nief V, et al. Insulin activates Na(+)/H(+) exchanger 3: biphasic response and glucocorticoid dependence. American journal of physiology Renal physiology. 2002;283(3):F532–539. [DOI] [PubMed] [Google Scholar]
- 27.Gesek FA, Schoolwerth AC. Insulin increases Na(+)-H+ exchange activity in proximal tubules from normotensive and hypertensive rats. The American journal of physiology. 1991. ;260(5 Pt 2):F695–703. [DOI] [PubMed] [Google Scholar]
- 28.Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest. 1988;81(1):159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghasemi A, Tohidi M, Derakhshan A, et al. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta diabetologica. 2015;52(5):905–915. [DOI] [PubMed] [Google Scholar]
- 30.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes care. 2000;23(1):57–63. [DOI] [PubMed] [Google Scholar]
- 31.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Molecular aspects of medicine. 2013;34(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasouli N, Raue U, Miles LM, et al. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. American journal of physiology Endocrinology and metabolism. 2005;288(5):E930–934. [DOI] [PubMed] [Google Scholar]
- 34.Lm Pop, Lingvay I, Yuan Q, et al. Impact of pioglitazone on bone mineral density and bone marrow fat content. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2017;28(11):3261–3269. [DOI] [PubMed] [Google Scholar]
- 35.Bobulescu IA, Lotan Y, Zhang J, et al. Triglycerides in the human kidney cortex: relationship with body size. PloS one. 2014;9(8):e101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagami GT. Ammonia production and secretion by the proximal tubule. Am J Kidney Dis. 1989;14(4):258–261. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y, Lim JH, Kim MY, et al. The Adiponectin Receptor Agonist AdipoRon Ameliorates Diabetic Nephropathy in a Model of Type 2 Diabetes. J Am Soc Nephrol. 2018;29(4):1108–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zha D, Wu X, Gao P. Adiponectin and Its Receptors in Diabetic Kidney Disease: Molecular Mechanisms and Clinical Potential. Endocrinology. 2017;158(7):2022–2034. [DOI] [PubMed] [Google Scholar]
- 39.Hu YY, Ye SD, Zhao LL, et al. Hydrochloride pioglitazone decreases urinary cytokines excretion in type 2 diabetes. Clinical endocrinology. 2010;73(6):739–743. [DOI] [PubMed] [Google Scholar]
- 40.Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2(5):883–888. [DOI] [PubMed] [Google Scholar]
- 41.Torricelli FC, De S, Gebreselassie S, et al. Type-2 diabetes and kidney stones: impact of diabetes medications and glycemic control. Urology. 2014;84(3):544–548. [DOI] [PubMed] [Google Scholar]
- 42.Oh MS. A new method for estimating G-I absorption of alkali. Kidney Int. 1989;36(5):915–917. [DOI] [PubMed] [Google Scholar]