Abstract
Pseudoxanthoma elasticum (PXE) is a heritable disease caused by ABCC6 deficiency. Patients develop ectopic calcification in skin, eyes and vascular tissues. ABCC6, primarily found in liver and kidneys, mediates the cellular efflux of ATP, which is rapidly converted into pyrophosphate (PPi), a potent inhibitor of calcification. PXE patients and Abcc6−/− mice display reduced PPi levels in plasma and peripheral tissues. PXE is currently incurable, although some palliative treatments exist. In recent years, we have successfully developed therapeutic methodologies to compensate the PPi deficit in animal models and humans. Here, we inadvertently discovered that modulating dietary PPi can also be an effective approach to reducing calcification in Abcc6−/− mice. Our findings were prompted by a change in institutional rodent diet. The new chow was enriched in PPi, which increased plasma PPi, and significantly reduced mineralization in Abcc6−/− mice. We also found that dietary PPi is readily absorbed in humans. Our results suggest that the consumption of food naturally or artificially enriched in PPi represents a possible intervention to mitigate calcification progression in PXE, that dietary preferences of patients may explain PXE heterogeneous manifestations and that animal chow has the potential to influence data reproducibility.
INTRODUCTION
Physiological mineralization is a multifactorial metabolic process generally restricted to the extracellular matrix of bones and teeth. The intra- and extracellular mechanisms regulating mineralization rest upon a delicate balance between calcification inhibitors and promoters. In normal circumstances, calcium and inorganic phosphate (Pi) concentrations are near saturation in most soft tissues, which necessitates potent calcification inhibition systems (Atzeni et al., 2006). Our understanding of calcification inhibition processes in soft tissues has evolved in recent years with the identification of mutations in ABCC6 (Bergen et al., 2000; Le Saux et al., 2000; Ringpfeil et al., 2000) and the characterization of the molecular function of this ABC transporter (Jansen et al., 2013). ABCC6 mediates the cellular efflux of ATP, which is rapidly converted into inorganic pyrophosphate (PPi) and adenosine by ENPP1 and CD73 at the cellular surface (Jansen et al., 2014; Jansen et al., 2013; Markello et al., 2011; Miglionico et al., 2014). PPi is a key molecule in the prevention of mineralization in soft tissues (Heinonen, 2001; Orriss et al., 2016). The liver, where ABCC6 is mainly expressed, is one of the main sources of PPi in the circulation and the overall ABCC6 function accounts for ~ 60% of the PPi plasma levels in mice and humans (Jansen et al., 2014; Pomozi et al., 2017b). Inactivating mutations in genes encoding enzymes participating in PPi homeostasis result in inherited disorders characterized by soft tissue calcification. ABCC6 deficiencies underlie the calcification disorders PXE (OMIM #264800) (Bergen et al., 2000; Le Saux et al., 2001; Le Saux et al., 2000), some cases of generalized arterial calcification of infancy (GACI; OMIM #614473), which is otherwise associated with ENPP1 mutations (OMIM #208000) (Le Boulanger et al., 2010; Nitschke et al., 2012) and the dystrophic cardiac calcification (DCC) phenotype of Abcc6-deficient mice (Aherrahrou et al., 2008; Brampton et al., 2014; Meng et al., 2007). The clinical manifestations related to the ABCC6→ENPP1→ CD73 pathway are, in fact, a spectrum of related diseases with similar calcification phenotypes, PXE, GACI, and also calcification of joints and arteries (CALJA also known as ACDC; OMIM #211800). The latter rare disease is associated with CD73 (encoded by NT5E) deficiency (Markello et al., 2011). The explication of these genetic conditions places ABCC6 as an upstream regulator of a purinergic/adenosinergic pathway that notably inhibits mineralization (Kauffenstein et al., 2018; Pomozi et al., 2017b). PXE, GACI, and DCC in Abcc6-deficient mice result from a deficit in PPi production, whereas CALJA (Markello et al., 2011; Miglionico et al., 2014) is caused by enhanced PPi degradation, resulting from the activation of tissue non-specific alkaline phosphatase (TNAP/ALPL) (Miglionico et al., 2014; Ziegler et al., 2017).
We and others have recently shown that PPi supplementation either by injection or oral delivery via drinking water and TNAP inhibition restores mineralization inhibition in Abcc6−/−, Enpp1−/−, and Nt5e−/− animals (Dedinszki et al., 2017; Pomozi et al., 2017b; Ziegler et al., 2017). In this study, we inadvertently tested the hypothesis that dietary PPi can be an effective approach to reducing ectopic calcification associated with ABCC6 deficiency. Polyphosphates and especially PPi, are a common additive in the food industry. Our results suggest that the phenotypic heterogeneity and intra-familial variation in PXE manifestations could be at least partly explained by dietetic preferences. Dietary PPi could be an easy, safe and inexpensive approach to mitigate the progression of symptoms of PXE patients.
RESULTS
Elevated levels of PPi in the standard rodent diet
In the last two years, we have initiated follow-up studies of the DCC phenotype using Abcc6−/− mice (Brampton et al., 2014; Pomozi et al., 2017a). However, we were quickly confronted with the inability to reproduce the level of cardiac calcification that we previously characterized and used (Fig. 1). In search of plausible solutions to these inexplicable results, we hypothesized that the absence of dystrophic calcification post-cryoinjuries to cardiac tissues could be related to exogenous factors. The possibility of genetic changes or breeding errors in Abcc6−/− mice was quickly ruled out with a review of our systematic genotyping records. As we have shown that PPi supplementation, in small or intermittent injection or oral delivery through water was very effective at suppressing DCC (Dedinszki et al., 2017; Pomozi et al., 2017b), we evaluated the possibility of PPi contamination in water and/or diet. For this purpose, we developed a protocol to measure the presence of PPi in standard chow (cf. methods section).
Figure 1: The effect of dietary pyrophosphate (PPi) on the acute dystrophic cardiac calcification (DCC) phenotype of Abcc6−/− mice.
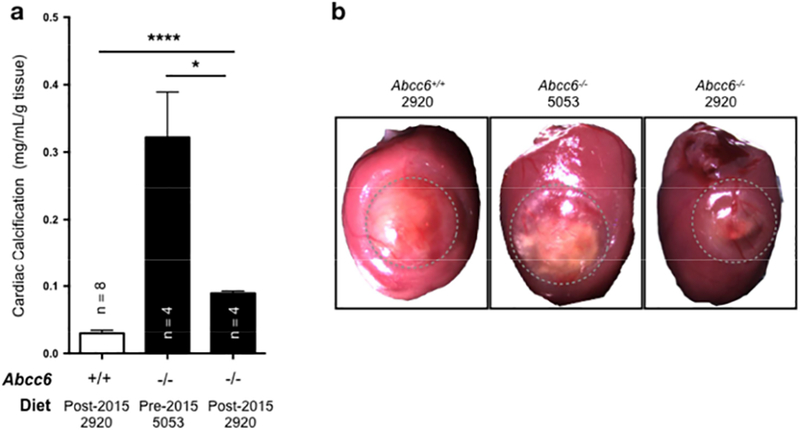
A: Abcc6−/− mice consuming the post-2015 diet (2920) with high PPi levels developed limited DCC as compared to animals that were fed the pre-2015 diet (5053) with low PPi. The level of calcification was measured as total ventricular calcium and normalized to the weight of the tissue. The number of mice per group is shown and results are +/− SEM. * p<0.05, **** p<0.0001. B: Representative images of the surface lesions (outlined) and calcification in wild type and Abcc6−/− mice fed either the post-2015 diet (2920) with high PPi levels or the pre-2015 diet (5053) with low PPi. The calcified deposits (white) were visibly reduced with post-2015 diet (2920).
The PPi concentration of the water supply was negligible (Fig. 2). However, we found a relatively high PPi content (0.30μmol/g dry weight) in the standard chow provided by the Animal Veterinary Services (AVS) of the University of Hawaii. This diet is manufactured by Envigo/Teklad (Madison, WI) and designated 2920 (Supplemental Table 1). If one assumes a daily consumption of about 4g of food for an average mouse of 25g, and a PPi bioavailability of 0.5% (estimate from (Pomozi et al., 2017b)), the 2920 standard chow would provide a cumulative daily intake of PPi of about 6nmol/day. This value largely exceeds (~4.3 fold) the minimal PPi effective dose to suppress DCC (Fig. 2, red line) we previously estimated at ~1.4nmol (Pomozi et al., 2017b). Remarkably, the University of Hawaii AVS had changed supplier of animal chow in the course of 2015 after our first experiments using DCC (Brampton et al., 2014; Pomozi et al., 2017a). We suspected that the chow formulation used prior to 2015 probably had less PPi. To verify this assumption, we obtained a sample of this animal chow from the manufacturer (designated 5053) and determined its PPi content. Even though the food formulations of the pre- and post-2015 diets (5053 vs 2920) were comparable (Supplementary Table 1), our results showed that indeed the 2920 diet had 7.1 times more PPi than the previous 5053 diet (0.30μmol/g vs 0.042μmol/g dry weight). To confirm these results, samples of both 5053 and 2920 chow were sent to one of the authors’ laboratory (AV) at the Hungarian Academy of Sciences, in Budapest. Similar PPi results were obtained which validated our initial results (Fig. 2). As control, the standard rodent chow used at the Hungarian Academy of Sciences was measured and showed low PPi concentrations similar to the 5053 diet (Fig. 2).
Figure 2: Estimated daily consumption of PPi from the pre-2015 (5053) and post-2015 (2920) chow in Hawaii (HI), the Hungarian chow in Budapest (Hu) and drinking water (HI).
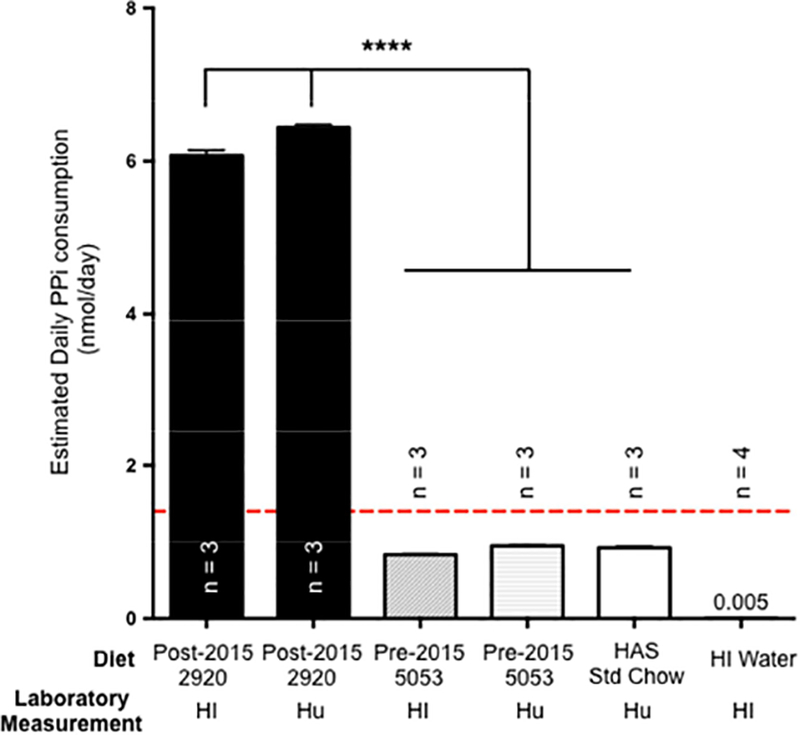
The chow used in Hawaii (HI) post-2015 (2920 diet) and in Hungary had significantly higher PPi content (7.1 folds) than the chow used before 2015 (5053 diet) which led to a superior cumulative daily intake. These results were confirmed by the authors located at the Hungarian Academy of Sciences, Budapest, Hungary. The PPi content of the drinking water was comparatively negligible. The dashed red line represents the minimal effective amount of PPi to prevent the development of dystrophic cardiac calcification as previously determined (Pomozi et al., 2017b). The results are +/− SEM. **** p<0.0001.
Remarkably, the formulations of the 2920 and 5053 diets were similar (Supplemental Table 1) notably the phosphorus content. The main source of phosphorus is from dicalcium phosphate whereas corn and wheat provide additional sources in this diet. The manufacturer has indicated that heat treatment to dry their product is insufficient to generate PPi. As the manufacturer does not specifically add or even account for PPi, we tested whether the irradiation could be the cause of the elevated pyrophosphate using a sample of the same diet but non-irradiated (2020x). The PPi content was found to be similarly elevated (0.28μmol/g dry weight) suggesting that one or more of the basic ingredients is enriched in PPi.
PPi in rodent diet is sufficient to suppress ectopic calcification and raise plasma PPi
To further test the hypothesis that the elevated PPi content of the standard 2920 diet is indeed capable of inhibiting ectopic calcification development in Abcc6−/− mice, we compared calcification data in vibrissae from Abcc6−/− animals on the current 2920 diet and results obtained prior to 2015 with the 5053 chow. In a previous study, we quantified the development of vibrissae mineralization over the life span of Abcc6−/− mice (Brampton et al., 2011). This work notably established that vibrissae calcification increased rapidly within the first 6 months of age and slows thereafter, reaching a plateau in 12 months old mice. Therefore, we compared data from two age groups that reflects these critical phases in calcification development. The results shown in Fig. 3 revealed a significant decrease of 57% in calcification at 6 months of age and a slightly more modest decline (44%) in 1-year old mice (p<0.0001). This confirmed that dietary PPi efficiently counteracts the development of chronic mineralization. Further, we verified that the presence of inorganic pyrophosphate in the diet raised plasma PPi significantly, which is consistent with results previously published (Dedinszki et al., 2017; Pomozi et al., 2017b).
Figure 3. The effect of dietary pyrophosphate (PPi) on the chronic (PXE-like) calcification phenotype of Abcc6−/− mice.
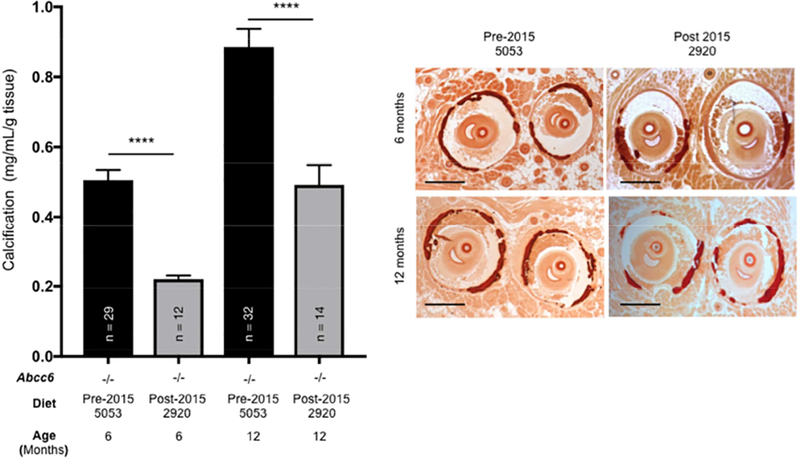
A: Vibrissae calcification from Abcc6−/− mice fed either low (5053) or high (2920) PPi chow was quantified. The data was collected from 6- and 12-months old animals when whiskers calcification is optimal. Results pre- and post-dating the change of institutional rodent chow in 2015 were compiled to generate these results. The number of mice per group is shown and results are +/− SEM. **** p<0.0001. B: Representative images of vibrissae calcification (chronic PXE-like phenotype) of Abcc6−/− mice visualized by Alizarin Red S staining (red to dark red color) are shown. Scale bar: 250μm.
Crystalline PPi added to plain food is readily absorbed in humans
Because the elevated level of PPi present in our standard animal chow was readily absorbed and lowered the susceptibility to ectopic calcification in Abcc6−/− mice, we tested whether a dry crystallin form of inorganic pyrophosphate could also be absorbed in healthy human volunteers consuming regular food. The food chosen was plain boiled potatoes to which 50mg/kg body weight of disodium PPi was added. Blood samples were taken before to establish a baseline and at 30, 60, 120 and 240 minutes after the start of the meal (Fig. 4). The average plasma PPi rose significantly above baseline levels and peaked at 60 minutes (differential of 1.52±0.30μmol/L or ~2.3 folds) with an average total concentration of 2.70±0.29μmol/L (p<0.001, n=8). The concentration decreased progressively thereafter but was still significantly elevated 4hrs (p<0.01) post ingestion. The average absorption of PPi from solid food was estimated at 0.05% and we found no significant difference between male and female volunteers (p=0.31, n=4).
Figure 4: Uptake of dietary pyrophosphate (PPi) in plasma of Abcc6−/− mice and healthy human volunteer.
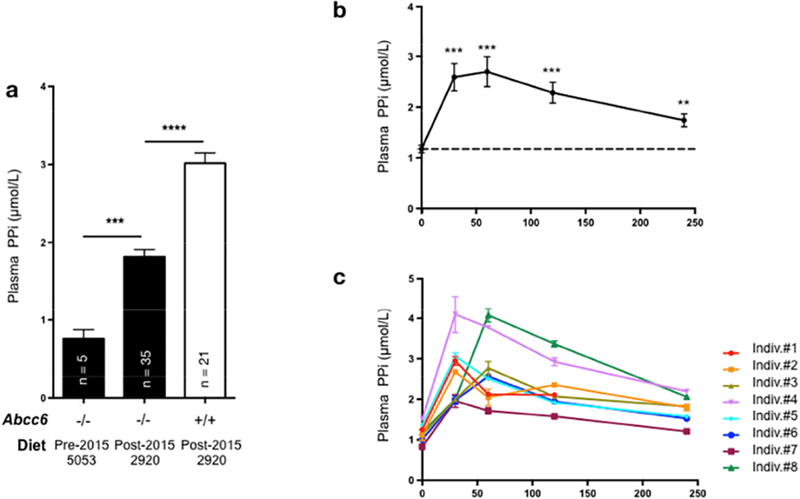
A: Plasma PPi concentration was determined in 3- to 6-month old Abcc6−/− (−/−) and wild type mice (+/+) that were fed standard chow with low PPi content (5053) prior to 2015 or high PPi levels (2920) after 2015. The number of mice per group is shown and results are +/− SEM. *** p<0.001; **** p<0.0001. B: Oral uptake of dietary PPi in humans. Volunteers ingested boiled potatoes mixed with 50mg/kg (body weight) of disodium pyrophosphate. The average plasma PPi level was determined before (0 min), and 30, 60, 120 and 240min after food ingestion. Results are +/− SEM, n=8: ** p<0.01; *** p<0.001. C: Individual variations of plasma PPi levels after oral uptake. Results are +/− SEM.
DISCUSSION
The present study shows that dietary PPi is readily absorbed in humans and is also an effective approach to reducing ectopic calcification associated with ABCC6 deficiency in a recognized mouse model of PXE. The clinical manifestations of PXE are highly varied and the heterogeneity in symptoms, occurrence, presentation and severity is compounded by the absence of clear-cut genotype-phenotype correlation (Le Saux et al., 2001; Legrand et al., 2017; Pfendner et al., 2007). This phenotypic heterogeneity of PXE and the lack of correlation complicates diagnosis and prognosis. The inadvertent discovery of substantial amounts of PPi in our standard animal chow and its consequences on ectopic calcification has three main implications.
PPi as a possible treatment
PXE is an incurable disease. Some symptomatic treatment exists for patients (Finger et al., 2011) and a recent clinical trial with a PPi analog (etidronate) only reported mixed results (Kranenburg et al., 2018). In recent years, we have developed and demonstrated therapeutic methodologies that could be applied to treat PXE patients. In a series of studies with a humanized mouse model, we have shown that 4-phenylbutyrate, a drug used to treat urea cycle disorders, can be repurposed to restore function to ABCC6 proteins having specific amino acid substitutions (Le Saux et al., 2011; Pomozi et al., 2014; Pomozi et al., 2017a). The other approach addressed the root problem of PXE (Jansen et al., 2014; Jansen et al., 2013), by compensating the PPi deficit through various methods of delivery and was proven to be effective in animals with limited testing done in humans (Dedinszki et al., 2017; Pomozi et al., 2017b). Both 4-phenylbutyrate and PPi methods have limitations with the former being restricted to certain genotypes, whereas the latter lacks approved formulation and clinical guidelines for PPi administration. Although gene therapy has encountered many obstacles from its inception, it has now matured enough along with emerging genome editing technologies that clinical applications can be envisaged in the not so distant future (Dunbar et al., 2018). Indeed, gene therapy would only need to target a few tissues (liver, kidneys) in PXE. However, as these therapeutic concepts are slowly coming of age, defining biomarkers and criteria to determine treatment efficacy in PXE is becoming the next priority in the field.
The consumption of solid food, naturally or artificially enriched in PPi might be one day recommended as part of a treatment strategy to slow or mitigate progression of ectopic calcification and resulting signs and symptoms. The World Health Organization (WHO) considers PPi as a nontoxic physiological metabolite with a high maximal tolerable daily intake value (MTDI) of 70mg/kg (http://www.inchem.org/documents/jecfa/jeceval/jec_2259.htm) which compares favorably to the LD50 of 2,600mg/kg reported in rodents (Seo et al., 2011). In the US, PPi is classified as Generally Recognized As Safe (GRAS) by the Food and Drug Administration and is designated as additive E450(a) in Europe. PPi is thus widely used in the food industry as a preservative in canned seafood, in baking soda, in cured meat, etc. Given the level of oral absorption we observed with human volunteers which is comparable to absorption from water (Dedinszki et al., 2017), PPi derived from food sources or even from toothpaste might have broader application towards other conditions that also manifest ectopic calcification, such as atherosclerosis (Alexopoulos and Raggi, 2009) diabetes (Chen and Moe, 2003) (Kreines et al, 1985), chronic renal insufficiency (Schlieper et al., 2016), b-thalassemia (Aessopos et al., 2002), or heterotopic ossification of traumatized muscle (Jackson et al., 2009).
Dietary PPi as a modulator of the PXE phenotype
The role of ABCC6 in the pathogenesis of PXE, GACI and DCC is now well established in humans (Le Saux et al., 2012) and several independent animal models (Li et al., 2014; Li et al., 2017). The development of ectopic calcification is clearly related to PPi deficit (Dedinszki et al., 2017; Jansen et al., 2013; Pomozi et al., 2017b; Ziegler et al., 2017). However, many facets of the phenotypes associated with ABCC6 dysfunction remain obscure, notably the heterogeneous presentation and severity of calcification in PXE (Hosen et al., 2015; Plomp et al., 2009). Indeed, in addition to producing considerable inter- and intra-familial phenotypical variability (Uitto et al., 2014), identical mutations can also lead in humans to the relatively mild PXE phenotype or severe GACI (Nitschke et al., 2012) and mice (Le Corre et al., 2012). The presence of modifier genes modulating calcification in PXE has been investigated but results are thus far inconclusive (Hendig et al., 2013; Hosen et al., 2015; Vanakker et al., 2013). Given the significant influence of elevated PPi in the institutional diet fed to our Abcc6−/− mice, the widespread presence of PPi in processed food, and its efficient oral absorption in humans (our data and (Dedinszki et al., 2017)), we suggest that dietary preference is a plausible explanation for the heterogeneous manifestations of PXE.
Animal diet as an important variable in laboratory animal research
The third and last consideration regards the relatively recent raised awareness and concern about animal research reproducibility and translation (Jilka, 2016; Mogil, 2017). The National Institutes of Health, as a funding agency, is addressing this issue specifically with considerations on statistical analysis, scientific rigor, sex as a biological variable and validation of key biological resources. There are many other factors affecting research reproducibility, such as investigator practices, institutional review panels (Silverman et al., 2017), veterinarians and animal facilities. Practical solutions are now being proposed (Smith et al., 2017) to mitigate this problem and the use of multi-laboratory design to increase coverage probability is seen as an important step (Voelkl et al., 2018). Although the authors of the present study have used the latter approach on several occasions(Brampton et al., 2014; Brampton et al., 2011; Le Saux et al., 2011; Pomozi et al., 2014; Pomozi et al., 2017a; Pomozi et al., 2017b; Pomozi et al., 2013), the potential effects of variation in “normal” animal diet is not often considered in biomedical research. The discovery that an apparently innocuous change in the supplier of normal standard chow had such a profound effect on our results is good illustration of actual variability between “identical” chow formulations. Beside the fact that PPi is not a reported ingredient in chow, our data suggest that animal diet could have a significant impact on data reproducibility between laboratories at different institutions and that more consideration should be given to what animals eat.
The unexpected results of this study have not only provided a fresh insight into how the immediate environment affects the progression of this rare disease in an animal model, but also further the prospect of dietary intervention as a possible treatment for PXE. Importantly, the collective findings that PPi treatment does not reverse established calcification (Dedinszki et al., 2017; Pomozi et al., 2017b) and that the extent of skin lesions correlates with severe cardiovascular and/or ophthalmological complications in PXE (Navasiolava et al., 2018) suggests that any PPi-based intervention should be initiated as early as possible, at diagnosis or soon thereafter.
MATERIAL & METHODS
Animals
C57BL/6J mice, designated herein as wild type, were derived from mice purchased from Jackson Laboratories (Bar Harbor, ME). Abcc6tm1Aabb mice were generated on 129/Ola background (Gorgels et al., 2005) and backcrossed into a C57BL/6J >10 times. These mice are herein designated Abcc6−/−. Both male and female, age-matched Abcc6−/− and wild type mice were used, as gender had no significant impact on results. All animals were housed in approved animal facilities at the University of Hawaii School of Medicine. Mice were kept under routine laboratory conditions with 12-hour light-dark cycle with ad libitum access to water and chow. The University of Hawaii Institutional Animal Care and Use Committees approved these studies. Experiments were conducted according to the NIH Guide for the Care and Use of Laboratory Animals.
Human volunteers
To confirm that crystalline PPi can be readily absorbed from solid food by humans, eight healthy subjects (four males, four females) consumed 2g/kg of boiled potato. Disodium pyrophosphate salt was added to the levels of 50mg/kg of body weight to mashed potatoes, stirred and consumed within 2 minutes. Blood samples were obtained before food ingestion and at 30, 60, 120 and 240 min thereafter. Blood samples were collected in three separate tubes. Plasma samples were divided in three aliquots and frozen at −80˚C until use. The human uptake studies were approved by the National Review Board of the Ministry of Health, Hungary (ETT TUKEB). Written informed consent was obtained from each volunteer prior to the study and experiments conformed to the principles of the Declaration of Helsinki, which was indicated in the informed consent. All human samples were de-identified at collection time.
Myocardial cryoinjury
At 72 hours post tail vein injection, cardiac injury was instilled through trans-diaphragm cryoinjury as previously described by one of us (Aherrahrou et al., 2004; Ivandic et al., 2001). Briefly, 10 seconds freeze-thaw injuries using a liquid nitrogen cooled probe are applied to the heart through the diaphragm from a 10–12mm incision on the abdomen. This approach limits the area of cardiac injury to a single cardiac location and offers a relative uniform size of the necrotic tissue and a very high survival rate (>90%). Sham-operated Abcc6−/− mice underwent the same surgical procedure using a room-temperature probe. Mice were sacrificed by CO2 asphyxiation 7 days after injury to ensure that the cardiac calcification phenotype was fully developed. Hearts were quickly removed, rinsed in PBS, minced and placed into 0.15N HCl for 48 hours and then the calcium content of the supernatant was determined by a colorimetric assay (Calcium LiquiColor Test, Stanbio Laboratory, TX).
Histochemistry and calcification measurements
Direct histological visualization of calcium deposition following Alizarin Red S staining on paraffin-embedded sections was carried out on the left muzzle skin of each mouse as previously described (Brampton et al., 2011). The level of mineralization in whiskers or hearts (minus the atria) was quantified following several methodologies. We first used the Calcium LiquiColor colorimetric assay (McGee-Russell, 1958) that measures directly the amount of excess calcium, which is normalized to the weight of the excised tissues, as previously described (Brampton et al., 2014) and expressed in μg/dL per milligram of tissue.
Inorganic pyrophosphate.
Food grade PPi in disodium form was generously provided by FOSFA Life Science (Břeclav, Czech Republic, https://web.fosfa.cz/en/). The dosages referenced therein for human volunteers was based on this disodium form.
The concentration of PPi in plasma (whether human or animal) was measured as described previously (Jansen et al., 2013). The levels of PPi in rodent chow was determined with the following method: 1.25 g of ground chow was dissolved in 7.5mL of sterile Milli-Q water. The sample was agitated at 4˚C overnight. After low-g centrifugation to pellet debris, PPi concentration was measured from the supernatant using the same procedure as described (Jansen et al., 2013). To validate our results, coded samples of the diet were sent to the Hungarian Academy of Sciences (AV) to perform identical but blind measurements.
Data analysis
Data were compared by the Student t test. Values are expressed as mean +/− standard error of the mean (SEM). A p value <0.05 was considered statistically significant. Animal numbers used for individual sets of data varied and are shown on the figures.
Supplementary Material
ACKNOWLEDGEMENTS
Financial support to OLS came from National Institutes of Health HL108249, P20GM113134, G12 MD007601, Victoria S. And Bradley L. Geist Fund of the Hawaii Community Foundation (18CON-90814) and PXE International Inc. The Hungarian grants OTKA 114136, 104227 and OTKA K111625 provided support to AV. The funding agencies were not involved in the design or execution of this study. The authors also acknowledge the essential assistance that E. Kozák and D. Dedinszki (Institute of Enzymology, Budapest, Hungary) provided for PPi assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
A.V. filed a patent untitled “Oral pyrophosphate for use in reducing tissue calcification” to the Netherland patient office (P32885NL00/RKI).
REFERENCES
- Aessopos A, Farmakis D, Loukopoulos D (2002) Elastic tissue abnormalities resembling pseudoxanthoma elasticum in beta thalassemia and the sickling syndromes. Blood 99:30–5. [DOI] [PubMed] [Google Scholar]
- Aherrahrou Z, Axtner SB, Kaczmarek PM, Jurat A, Korff S, Doehring LC, et al. (2004) A locus on chromosome 7 determines dramatic up-regulation of osteopontin in dystrophic cardiac calcification in mice. Am J Pathol 164:1379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aherrahrou Z, Doehring LC, Ehlers EM, Liptau H, Depping R, Linsel-Nitschke P, et al. (2008) An alternative splice variant in Abcc6, the gene causing dystrophic calcification, leads to protein deficiency in C3H/He mice. J Biol Chem 283:7608–15. [DOI] [PubMed] [Google Scholar]
- Alexopoulos N, Raggi P (2009) Calcification in atherosclerosis. Nat Rev Cardiol 6:681–8. [DOI] [PubMed] [Google Scholar]
- Atzeni F, Sarzi-Puttini P, Bevilacqua M (2006) Calcium deposition and associated chronic diseases (atherosclerosis, diffuse idiopathic skeletal hyperostosis, and others). Rheum Dis Clin North Am 32:413–26, viii. [DOI] [PubMed] [Google Scholar]
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. (2000) Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet 25:228–31. [DOI] [PubMed] [Google Scholar]
- Brampton C, Aherrahrou Z, Chen LH, Martin L, Bergen AA, Gorgels TG, et al. (2014) The level of hepatic ABCC6 expression determines the severity of calcification after cardiac injury. Am J Pathol 184:159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brampton C, Yamaguchi Y, Vanakker O, Van Laer L, Chen LH, Thakore M, et al. (2011) Vitamin K does not prevent soft tissue mineralization in a mouse model of pseudoxanthoma elasticum. Cell Cycle 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NX, Moe SM (2003) Arterial calcification in diabetes. Curr Diab Rep 3:28–32. [DOI] [PubMed] [Google Scholar]
- Dedinszki D, Szeri F, Kozak E, Pomozi V, Tokesi N, Mezei TR, et al. (2017) Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol Med 9:1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M (2018) Gene therapy comes of age. Science 359. [DOI] [PubMed] [Google Scholar]
- Finger RP, Charbel Issa P, Schmitz-Valckenberg S, Holz FG, Scholl HN (2011) Long-term effectiveness of intravitreal bevacizumab for choroidal neovascularization secondary to angioid streaks in pseudoxanthoma elasticum. Retina 31:1268–78. [DOI] [PubMed] [Google Scholar]
- Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, et al. (2005) Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet 14:1763–73. [DOI] [PubMed] [Google Scholar]
- Heinonen JK (2001) Biological role of inorganic pyrophosphate. Kluwer Academic Publishers: Boston, viii, 250 p. [Google Scholar]
- Hendig D, Knabbe C, Gotting C (2013) New insights into the pathogenesis of pseudoxanthoma elasticum and related soft tissue calcification disorders by identifying genetic interactions and modifiers. Frontiers in genetics 4:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosen MJ, Van Nieuwerburgh F, Steyaert W, Deforce D, Martin L, Leftheriotis G, et al. (2015) Efficiency of exome sequencing for the molecular diagnosis of pseudoxanthoma elasticum. J Invest Dermatol 135:992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivandic BT, Utz HF, Kaczmarek PM, Aherrahrou Z, Axtner SB, Klepsch C, et al. (2001) New Dyscalc loci for myocardial cell necrosis and calcification (dystrophic cardiac calcinosis) in mice. Physiol Genomics 6:137–44. [DOI] [PubMed] [Google Scholar]
- Jackson WM, Aragon AB, Bulken-Hoover JD, Nesti LJ, Tuan RS (2009) Putative heterotopic ossification progenitor cells derived from traumatized muscle. J Orthop Res 27:1645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Duijst S, Mahakena S, Sommer D, Szeri F, Varadi A, et al. (2014) ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol 34:1985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Kucukosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, et al. (2013) ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci U S A 110:20206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL (2016) The road to reproducibility in animal research. J Bone Miner Res 31:1317–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffenstein G, Yegutkin GG, Khiati S, Pomozi V, Le Saux O, Leftheriotis G, et al. (2018) Alteration of Extracellular Nucleotide Metabolism in Pseudoxanthoma Elasticum. J Invest Dermatol 138:1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg G, de Jong PA, Bartstra JW, Lagerweij SJ, Lam MG, Ossewaarde-van Norel J, et al. (2018) Etidronate for Prevention of Ectopic Mineralization in Patients With Pseudoxanthoma Elasticum. J Am Coll Cardiol 71:1117–26. [DOI] [PubMed] [Google Scholar]
- Le Boulanger G, Labreze C, Croue A, Schurgers LJ, Chassaing N, Wittkampf T, et al. (2010) An unusual severe vascular case of pseudoxanthoma elasticum presenting as generalized arterial calcification of infancy. Am J Med Genet A 152A:118–23. [DOI] [PubMed] [Google Scholar]
- Le Corre Y, Le Saux O, Froeliger F, Libouban H, Kauffenstein G, Willoteaux S, et al. (2012) Quantification of the calcification phenotype of abcc6-deficient mice with microcomputed tomography. Am J Pathol 180:2208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Beck K, Sachsinger C, Silvestri C, Treiber C, Goring HH, et al. (2001) A spectrum of abcc6 mutations is responsible for pseudoxanthoma elasticum. Am J Hum Genet 69:749–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Fulop K, Yamaguchi Y, Ilias A, Szabo Z, Brampton CN, et al. (2011) Expression and in vivo rescue of human ABCC6 disease-causing mutants in mouse liver. PLoS One 6:e24738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Martin L, Aherrahrou Z, Leftheriotis G, Varadi A, Brampton CN (2012) The molecular and physiological roles of ABCC6: more than meets the eye. Frontiers in genetics 3:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, et al. (2000) Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet 25:223–7. [DOI] [PubMed] [Google Scholar]
- Legrand A, Cornez L, Samkari W, Mazzella JM, Venisse A, Boccio V, et al. (2017) Mutation spectrum in the ABCC6 gene and genotype-phenotype correlations in a French cohort with pseudoxanthoma elasticum. Genet Med 19:909–17. [DOI] [PubMed] [Google Scholar]
- Li Q, Guo H, Chou DW, Berndt A, Sundberg JP, Uitto J (2014) Mouse models for pseudoxanthoma elasticum: genetic and dietary modulation of the ectopic mineralization phenotypes. PLoS One 9:e89268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kingman J, van de Wetering K, Tannouri S, Sundberg JP, Uitto J (2017) Abcc6 knockout rat model highlights the role of liver in PPi homeostasis in pseudoxanthoma elasticum. J Invest Dermatol 137:1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markello TC, Pak LK, St Hilaire C, Dorward H, Ziegler SG, Chen MY, et al. (2011) Vascular pathology of medial arterial calcifications in NT5E deficiency: implications for the role of adenosine in pseudoxanthoma elasticum. Mol Genet Metab 103:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Russell SM (1958) Histochemical methods for calcium Journal of Histochemistry & Cytochemistry 6:22–42. [DOI] [PubMed] [Google Scholar]
- Meng H, Vera I, Che N, Wang X, Wang SS, Ingram-Drake L, et al. (2007) Identification of Abcc6 as the major causal gene for dystrophic cardiac calcification in mice through integrative genomics. Proc Natl Acad Sci U S A 104:4530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglionico R, Armentano MF, Carmosino M, Salvia AM, Cuviello F, Bisaccia F, et al. (2014) Dysregulation of gene expression in ABCC6 knockdown HepG2 cells. Cell Mol Biol Lett 19:517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS (2017) Laboratory environmental factors and pain behavior: the relevance of unknown unknowns to reproducibility and translation. Lab Anim (NY) 46:136–41. [DOI] [PubMed] [Google Scholar]
- Navasiolava N, Gnanou M, Douillard M, Saulnier P, Aranyi T, Ebran JM, et al. (2018) The extent of PXE skin changes is related to cardiovascular complications and visual loss: a cross-sectional study. Br J Dermatol. [DOI] [PubMed] [Google Scholar]
- Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, et al. (2012) Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet 90:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orriss IR, Arnett TR, Russell RG (2016) Pyrophosphate: a key inhibitor of mineralisation. Curr Opin Pharmacol 28:57–68. [DOI] [PubMed] [Google Scholar]
- Pfendner EG, Vanakker OM, Terry SF, Vourthis S, McAndrew PE, McClain MR, et al. (2007) Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet 44:621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp AS, Bergen AA, Florijn RJ, Terry SF, Toonstra J, van Dijk MR, et al. (2009) Pseudoxanthoma elasticum: Wide phenotypic variation in homozygotes and no signs in heterozygotes for the c.3775delT mutation in ABCC6. Genet Med 11:852–8. [DOI] [PubMed] [Google Scholar]
- Pomozi V, Brampton C, Fulop K, Chen LH, Apana A, Li Q, et al. (2014) Analysis of pseudoxanthoma elasticum-causing missense mutants of ABCC6 in vivo; pharmacological correction of the mislocalized proteins. J Invest Dermatol 134:946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomozi V, Brampton C, Szeri F, Dedinszki D, Kozak E, van de Wetering K, et al. (2017a) Functional Rescue of ABCC6 Deficiency by 4-Phenylbutyrate Therapy Reduces Dystrophic Calcification in Abcc6(−/−) Mice. J Invest Dermatol 137:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomozi V, Brampton C, van de Wetering K, Zoll J, Calio B, Pham K, et al. (2017b) Pyrophosphate Supplementation Prevents Chronic and Acute Calcification in ABCC6-Deficient Mice. Am J Pathol 187:1258–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomozi V, Le Saux O, Brampton C, Apana A, Ilias A, Szeri F, et al. (2013) ABCC6 is a basolateral plasma membrane protein. Circ Res 112:e148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J (2000) Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A 97:6001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlieper G, Schurgers L, Brandenburg V, Reutelingsperger C, Floege J (2016) Vascular calcification in chronic kidney disease: an update. Nephrol Dial Transplant 31:31–9. [DOI] [PubMed] [Google Scholar]
- Seo DS, Kwon M, Sung HJ, Park CB (2011) Acute Oral or Dermal and Repeated Dose 90-Day Oral Toxicity of Tetrasodium Pyrophosphate in Spraque Dawley (SD) Rats. Environ Health Toxicol 26:e2011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J, Macy J, Preisig PA (2017) The role of the IACUC in ensuring research reproducibility. Lab Anim (NY) 46:129–35. [DOI] [PubMed] [Google Scholar]
- Smith MM, Clarke EC, Little CB (2017) Considerations for the design and execution of protocols for animal research and treatment to improve reproducibility and standardization: “DEPART well-prepared and ARRIVE safely”. Osteoarthritis Cartilage 25:354–63. [DOI] [PubMed] [Google Scholar]
- Uitto J, Jiang Q, Varadi A, Bercovitch LG, Terry SF (2014) Pseudoxanthoma Elasticum: Diagnostic Features, Classification, and Treatment Options. Expert Opin Orphan Drugs 2:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanakker OM, Hosen MJ, Paepe AD (2013) The ABCC6 transporter: what lessons can be learnt from other ATP-binding cassette transporters? Frontiers in genetics 4:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkl B, Vogt L, Sena ES, Wurbel H (2018) Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLoS Biol 16:e2003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SG, Ferreira CR, MacFarlane EG, Riddle RC, Tomlinson RE, Chew EY, et al. (2017) Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


