Abstract
The (pro)renin receptor (PRR) is a multifunctional protein that is expressed in multiple organs. Binding of prorenin/renin to the PRR activates angiotensin-II dependent and independent pathways. The PRR is also involved in autophagy and Wnt/ß catenin signaling, functions that are not contingent on prorenin binding. Emerging evidence suggests that the PRR plays an important role in blood pressure regulation and glucose and lipid metabolism. Herein, we review PRR function in health and disease, with particular emphasis on hypertension and the metabolic syndrome.
Keywords: Prorenin receptor, (pro)renin receptor, PRR, hypertension, insulin resistance, obesity
The renin angiotensin system (RAS) plays a vital role in the maintenance of blood pressure (BP) and sodium homeostasis. In this system, circulating angiotensinogen is cleaved sequentially by renin and angiotensin converting enzyme to generate angiotensin-II (Ang-II) which then modulates BP through a multitude of effects including vasoconstriction, activation of the sympathetic nervous system, increased aldosterone synthesis, and antinatriuresis 1. A number of organs contain their own RAS, wherein Ang-II can exert highly localized effects 2. Additional components of the RAS have now been identified 3; amongst these, the (pro)renin receptor (PRR) has received much attention 4. Substantial efforts have been made to understand the localization, regulation and function of the PRR both at a molecular and system level. Further, the recent development of PRR antagonists 5,6 has advanced our understanding of the complex functions of this pleiotropic protein. Herein, we review current knowledge on the biological roles of the PRR, focusing on its involvement in hypertension and the metabolic syndrome.
Basic biology of the (pro)renin receptor
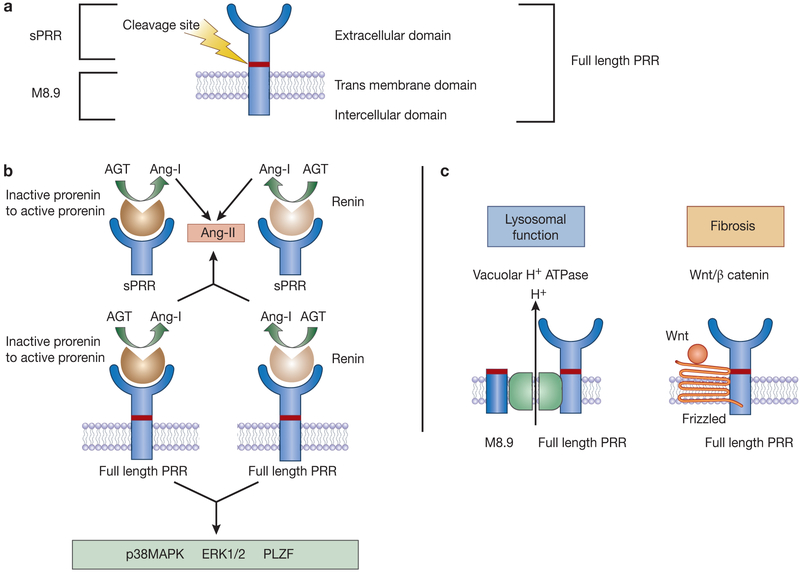
The PRR, encoded by the ATP6AP2 gene on the X chromosome, was first cloned in 2002 4 and is highly conserved across species 7. The full-length 350-amino acid protein localizes to the plasma membrane and encompasses a large extracellular domain that can be cleaved to form the soluble PRR (sPRR) and a smaller transmembrane and cytoplasmic domain (M8.9) 8 (Figure 1). The PRR has been localized to several organs including the kidney, heart, vascular smooth, brain, adipose tissue, liver, eye and placenta 4.
Figure 1.
Basic biology of the (pro)renin receptor (PRR). (A) Simplified schematic of the PRR protein; (B) Prorenin/renin dependent PRR function; and (C) Prorenin/renin independent PRR function. Binding of the prorenin to the full length or sPRR induces non-proteolytic activation of prorenin to cleave angiotensinogen while binding of renin to full length or sPRR increases catalytic efficiency. Independent of Ang-II, binding of prorenin/renin to the PRR activates intra-cellular signaling pathways. AGT – angiotensinogen; Ang-I – angiotensin-I; Ang-II – angiotensin-II; PRR – (pro)renin receptor; sPRR – soluble (pro)renin receptor; p38MAPK – mitogen activated prorenin kinase; ERK1/2 – extracellular signal-regulated kinase; PI3K – phosphoinositide 3-kinase; PLZF – promyelocytic leukemia zinc finger; Wnt/ß catenin.
The PRR serves a multitude of functions that depend, at least in part, on whether it is intact or cleaved into soluble and membrane/cytoplasmic components (Figure 1). Binding of prorenin to full-length PRR induces non-proteolytic activation of prorenin-mediated angiotensinogen cleavage, while renin bound to the PRR has four-fold higher catalytic efficiency as compared to unbound renin 4. The soluble fragment (sPRR) also binds and activates prorenin and renin. Independent of Ang-II generation, prorenin/renin binding to membrane-bound full-length PRR activates intra-cellular signaling pathways such as p38 MAPK and ERK1/2 4,9-11. PRR (M8.9) can function as an accessory subunit of the vacuolar H+-ATPase and is involved in lysosomal acidification 12; this function of the PRR (M8.9) appears to be independent of prorenin/renin binding 13. More recently, the PRR has been reported to be involved in the Wnt/ß catenin signaling cascade with an essential role in embryonic development, cell differentiation and metabolism 14.
Challenges in studying the (pro)renin receptor
Establishing the physiological role of the PRR has been challenging since global or cell-specific deletion of PRR often causes early lethality or organ malformation associated with abnormal lysosomal acidification 7,15-18. Cardiomyocyte-specific PRR ablation results in lethal cardiac failure within 3 weeks after birth 15. Podocyte- specific PRR knockout (KO) mice develop severe proteinuria and die of renal failure in the first month after birth 16,17. Collecting duct (CD) specific PRR KO mice have pronounced apoptosis, marked renal hypoplasia, and a malformed CD system 19. Loss of neuronal or adipose tissue PRR does not appear to affect organ structure or function 20-22, suggesting that the lysosomal function of the PRR is tissue-specific. A recent study attempted to induce global PRR inactivation in adult mice using ROSA26-creERT2; although this model was not efficient in targeting brain, kidney, aorta or white adipose tissue, the inducible PRR KO mice displayed early lethality, marked weight loss, hypoglycemia and hypercholesterolemia associated with pathologic changes in the colon, bone marrow and liver 18.
Antagonists that block prorenin from the binding to the PRR have also been developed 5,6. The first PRR blocker was directed toward the handle region peptide (HRP) and prevented the binding of prorenin to the PRR; despite initial promising results 5, HRP has now fallen out of favor due to partial agonistic properties 23. A newer agent, PRO20, acts as a competitive antagonist, is identical to the first 20 amino acids of the prorenin segment and contains all of the PRR binding sites 6. As discussed later, several groups have validated the specificity of PRO20 in preventing prorenin-mediated PRR function 6,24,25.
Despite the challenges using gene targeting and PRR antagonism, as discussed in the following sections, substantial progress has been made in uncovering the physiological and pathophysiological roles of the PRR.
Role of the (pro)renin receptor in blood pressure regulation
Kidney
The PRR has been localized to mesangial cells, podocytes, proximal tubule, distal convoluted tubule, the luminal membrane of intercalated cells, and principal cells with the highest renal expression in intercalated cells 17,26,27. The CD PRR may be particularly important since prorenin is luminally secreted by the CD where it may act on the luminal PRR to stimulate sodium reabsorption and elevate blood pressure (BP) 28. Relevant to this, renal medullary PRR expression is enhanced in hypertensive rats (Ang-II infusion and 2-kidney and 1 clip Goldblatt hypertension) 29,30. Renal medullary infusion of PRO20 reduced the hypertensive response and renal injury to Ang-II infusion in rats 25. Similarly, infusion of PRR shRNA in the renal medulla decreased expression of the epithelial sodium channel (ENaC) in rats (although the effect on BP was not examined) 31. In order to delineate the role of nephron PRR in BP regulation, we developed an inducible renal tubule PRR knockout (KO) mouse 32 to avoid the deleterious effects of PRR deletion on organ development. Renal tubule PRR KO mice had similar BP as controls under varying sodium intake but had an attenuated hypertensive response with reduced renal ENaC expression following Ang-II infusion 33. Further, prorenin stimulated ENaC activity in acutely isolated cortical CD in control mice but not in renal tubule PRR KO mice 33. Two other mouse models with CD specific PRR deletion recapitulated the protective effects of PRR deletion on Ang-II induced hypertension and ENaC expression 34,35. In contrast, Trepiccione et al observed no differences in BP, sodium excretion or ENaC expression following Ang-II infusion in their nephron wide PRR KO mouse model compared to controls 36. Instead, the authors observed reduced ability to excrete an acid load, blunted vacuolar H+-ATPase activity and expression, and increased renal medullary lysosomal protein and autophagosome markers 36. One explanation for the discordant findings may be related to the high dose of Ang-II used to induce hypertension (1000 ng/kg/min in the latter study vs 300-600 ng/kg/min in others) as well as the timing of PRR deletion (prenatal in the Trepiccione study versus during adulthood in other studies). Renal tubular PRR also modulates water reabsorption through Ang-II dependent and independent mechanisms 32,36-38. Recent studies have shown that the PRR regulates vasopressin-stimulated AQP2 expression via activation of prostaglandin EP4 receptor 38 and the Wnt/β-catenin pathway 37.
A fundamental question is if the intercalated and/or principal cell PRR modulates renal sodium and water reabsorption. To address this, we developed mice with principal or intercalated cell specific deletion of the PRR 39. Although loss of the PRR selectively in intercalated cells resulted in lower body weight, there was no effect on renal histology, medullary ENaC or aquaporin-2 expression, urine concentrating ability, or prorenin-stimulated ENaC activity by isolated CD. In contrast, principal cell specific PRR deletion reduced ENaC and AQP2 expression in the renal medulla, decreased urine concentrating ability, and abolished prorenin-stimulated ENaC activity in isolated CD. Thus, these studies indicate that principal, but not intercalated, cell PRR modulates renal sodium and water transport.
The macula densa PRR also appears to regulate BP and systemic renin release 40. Using a combination of cell culture and in-vivo studies, Riquier-Brison et al demonstrated that the macula densa PRR amplifies renin/prorenin-stimulated renin release via ERK1/2 signaling in a short-loop feed forward mechanism 40. Consistent with this, mice with macula densa specific PRR ablation have reduced BP and plasma renin levels; these effects were more prominent following treatment with low salt diet and renin-angiotensin system (RAS) blockers 40.
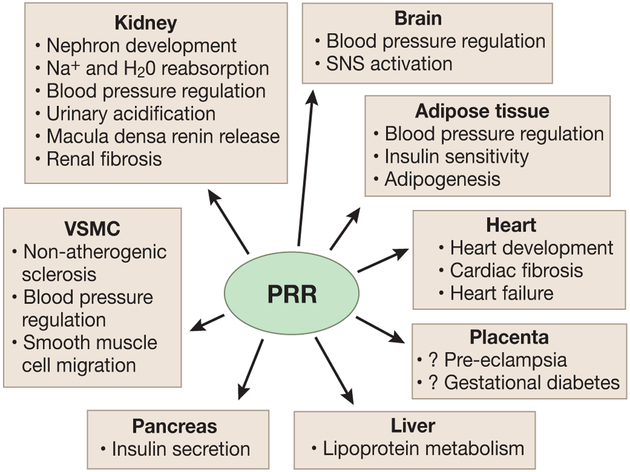
The renal PRR may also contribute to development of hypertension in kidney disease. Mice heterozygous for PRR deletion in nephron progenitor cells (homozygous deletion leads to early neonatal death) have fewer glomeruli, altered glomerular basement membrane ultrastructure, and develop hypertension at 2 months of age 41, suggesting that early loss of PRR may be involved in developmental programming of hypertension. Recently, Xu et al found increased renal PRR expression, activation of the intra-renal RAS, and salt sensitive hypertension in rats fed a high fructose diet; treatment with PRO20 reduced high fructose induced sodium retention, BP and intra-renal RAS activation 42. Polycystic kidney disease is associated with early onset of hypertension; an animal model of polycystic kidney disease had increased renin expression in cysts and mis-localization of the PRR from the luminal membrane of intercalated cells to the basolateral membrane of principal cells 43. However, despite growing evidence for the renal PRR in BP regulation and renal function, some inconsistencies exist. Overexpression of the PRR under the control of cytomegalovirus early enhances/chicken ß-actin (CAG) promoter (expressed in embryonic stem cells) in mice does not alter BP or albuminuria despite a 25-80-fold increase in renal PRR expression 44. Conversely, transgenic rats with ubiquitous human PRR overexpression remain normotensive despite proteinuria and progressive nephropathy 45. Taken together, these studies raise the possibility that while general PRR overexpression may not affect BP, specific renal cell (particularly the CD) PRR is involved in BP regulation particularly under pathophysiological conditions (Figure 2).
Figure 2.
Organ specific functions of the (pro)renin receptor (PRR).VSMC – vascular smooth muscle cells.
Cardiovascular system
Cardiac myocyte PRR is closely linked to the vacuolar H+-ATPase as well as the ryanodine receptor 46. Cardiac PRR expression is increased in diabetes 46, myocardial infarction 47 and heart failure 48. In addition, high sodium intake and severe sodium restriction modulate cardiac PRR expression 49. Rats fed a high salt diet (8.9% NaCl) had elevated BP and cardiac fibrosis associated with enhanced cardiac expression of prorenin/renin and the PRR 49. Similarly, very low salt intake (0.01% sodium) in rats increased cardiac expression of prorenin/renin and the PRR, and accelerated cardiac and perivascular fibrosis despite normal blood pressure 50.
The in vivo role of cardiac PRR in hypertension and cardiac function has been difficult to determine since, as mentioned above, constitutive deletion of the PRR in the cardiomyocytes leads to lethal heart failure as a result of impaired lysosomal function 15. Global or cardio-specific overexpression of the PRR did not alter BP or cardiac structure or function at baseline 45,47 or in response to stress and ischemic injury 47. Similarly, mice with constitutive overexpression of PRR under the control of CAG promoter had no changes in BP or cardiac function despite a 400-fold increase in cardiac PRR expression 44. In contrast, over-expression of PRR in adult rat hearts via adenovirus mediated gene delivery for 2 weeks caused reduced left ventricular ejection fraction and resulted in Ang-II independent activation of cell signaling pathways 51. Taken together, these studies suggest that either the cardiac PRR does not play a role in BP regulation or compensatory mechanisms counter any effects exerted by the increased PRR expression (Figure 2).
The PRR is also found in vascular smooth cells where it localizes to the cell surface and interacts with the vacuolar H+-ATPase 52. Vascular smooth muscle cell specific loss of PRR in mice caused non-atherogenic sclerosis of the abdominal aorta in the face of unchanged BP 52. Rats with vascular smooth cell-specific overexpression of the human PRR develop hypertension and tachycardia at 6 months of age 53. Vascular smooth cell PRR also mediates prorenin-stimulated smooth muscle migration in cultured aorta 54, induction of inflammatory mediators such as plasminogen activator inhibitor- 1, and activation of ERK1/2 signaling pathways 54. Thus, the vascular smooth cell PRR, seemingly more than the cardiac PRR, may play a role in BP regulation; such an effect occurs via both Ang-II dependent and -independent pathways (Figure 2).
Brain
The central nervous system PRR may play a vital role in the local RAS, particularly given that brain renin levels are thought to be too low to generate significant local Ang-II 55. Within the brain, the PRR has been localized to the supraoptic nucleus, nucleus of the solitary tract, the subfornical organ (SFO) and the paraventricular nucleus, all of which are vital regulators of cardiovascular function and volume homeostasis. As in other tissues, binding of prorenin/renin to the neuronal PRR enhances Ang-II synthesis and activates intracellular signaling pathways such as ERK1/2 56.
The role of the brain PRR in hypertension has been studied extensively over the last several years 56 (Figure 2). Supraoptic nuclear specific knock-down of the PRR in spontaneously hypertensive rats attenuated age-related increases in BP 57. Intracerebroventricular (ICV) infusion of PRR shRNA in mice overexpressing both human renin and angiotensinogen reduced BP, lowered cardiac and vasomotor sympathetic tone, and improved baroreflex sensitivity 58. These findings suggested that brain Ang-II was generated by PRR; to test this hypothesis, Li et al generated neuron-specific PRR KO mice and observed reduced brain Ang-II levels and an attenuated hypertensive response to ICV infusion of mouse prorenin 20. Further, neuron-specific PRR deletion or ICV infusion of PRO20 diminished deoxycorticosterone acetate-salt-induced neuronal PRR expression, Ang-II formation and hypertension 6,20. Post-mortem analysis of human brain tissues showed higher neuronal PRR expression in the SFO in hypertensive vs. normotensive patients, and this significantly correlated with BP 59. Finally, recent studies have demonstrated that the neuronal PRR exerts Ang-II independent functions by stimulating sympathetic outflow via reactive oxygen species and inducible nitric oxide synthase signaling along with activation of ERK/NADPH oxidase 4 pathways 60.
As mentioned earlier, unlike PRR deletion in other organs, neuronal PRR deletion or pharmacological blockade with PRO20 within the brain does not detectably alter lysosomal function, metabolism or survival 6,20 since the neuronal PRR does not appear to modulate vacuolar H+-ATPase activity or the Wnt/β-catenin signaling pathway. This relatively unique feature of the neuronal PRR may be helpful in developing novel PRR antagonists that specifically block prorenin dependent functions.
Adipose tissue
Adipose tissue is increasingly recognized as an endocrine organ and expresses many components of the RAS, including the PRR 61. Adipose tissue PRR expression increases with obesity 62-64 and, similar to other tissues, binding of renin/prorenin to adipocyte PRR results in Ang-II generation as well as ERK1/2 activation 62-64. Using constitutive adipocyte-specific PRR deletion, Wu et al observed elevated systolic BP and increased plasma sPRR levels in KO compared to control mice under varying fat intake without observable differences in systemic RAS components 21 (Figure 2). The increase in sPRR, which may have contributed to the observed hypertension, was unexpected. Notably, these mice had aberrant glucose and lipid homeostasis, so it is possible that sPRR production by other organs or sPRR metabolism was secondarily affected. As discussed in more detail below, adipocyte PRR is also an important modulator of glucose and lipid homeostasis and may contribute to insulin resistance in obesity 62-64.
Placenta
The placenta PRR may regulate BP although the evidence is limited. Among gestational tissues (amnion, chorion, decidua and placenta), the PRR is most highly expressed in the placenta where it is thought to play a role in prorenin-stimulated prostaglandin synthesis 65.
The first study to describe an association between placental RAS and pre-eclampsia noted a 2-fold increase in plasma and placental prorenin levels and increased placental PRR immunostaining in a rat model of pre-eclampsia 66. This study also reported increased plasma prorenin levels in preeclamptic women compared to normotensive pregnant women 66. A subsequent study found higher placental PRR expression and plasma sPRR levels in preeclamptic women; systolic BP positively correlated with placental PRR but not plasma sPRR 67. Although plasma prorenin/renin levels were not measured, these findings raise the possibility of placental PRR-mediated RAS activation in preeclampsia. Recently, Suggule et al examined maternal blood samples for prorenin, renin and sPRR in a group of preeclamptic, diabetic and healthy pregnant women 68. While no differences were observed in plasma prorenin or renin levels among the study groups, plasma sPRR levels were increased in diabetic pregnancies but not preeclamptic pregnancies. These findings raise several questions about the role of placental PRR in altered BP regulation (Figure 2), including the source of the plasma sPRR in healthy and complicated pregnancies, whether plasma sPRR is an indicator of local RAS activation within the placenta, and the biological function of the placental PRR.
Clinical studies
Single nucleotide polymorphisms (SNPs) in the ATP6AP2 gene have been linked to BP and vascular disease. Using linkage-disequilibrium analyses, Hirose et al identified three SNPs in the ATP6AP2 gene (located in the promoter region, intron 5 and the 3’ untranslated region) and examined the association with BP in a Japanese cohort of 1100 participants 69. T-allele carriers of the IVS5+169C > T polymorphism (found in intron 5) had higher ambulatory BP in men but not women 69. These results were replicated in a separate study of Caucasian men where higher office and ambulatory systolic BP was observed in T-allele than C-allele carriers 70. In contrast, the association of lacunar infarction and left ventricular hypertrophy was higher in women with the +1513A>G polymorphism (located in the 3’ translated region) with no difference in prevalence of lacunar infarction or left ventricular hypertrophy among the three SNPs in men 71. While these studies lend credence to the notion that the PRR contributes to BP regulation and cardiovascular disease, they need to be validated in larger clinical studies as well as determining the phenotypic effects of these mutations. ATP6AP2 gene mutations have also been associated with X-linked mental retardation, epilepsy and parkinsonism 72-74 but these SNPs are limited to single families of 2-7 affected individuals.
Alterations in plasma sPRR levels may be associated with hypertension as well as other cardiovascular and renal conditions. Plasma sPRR levels did not change with age, sex, posture, hormonal status or circadian rhythm, but are 25% lower in black than white participants 75. Plasma sPRR levels increase as the severity of kidney disease worsens 76,77. High serum sPRR levels have been described in hemodialysis patients and are associated with atherosclerosis risk 78. Plasma sPRR levels are elevated in patients with chronic heart failure with reduced ejection fraction and are even greater in patients with concomitant heart failure and renal dysfunction 79. Finally, elevated plasma sPRR levels occur in pre-eclampsia 67 and obstructive sleep apnea 80. Thus, plasma sPRR levels may be of pathological significance and/or could be of value as a biomarker in the development and progression of kidney and cardiovascular disease. A summary of all current experimental and clinical studies of the PRR in BP regulation is described in Table 1.
Table 1.
Experimental and clinical models of the (pro)renin receptor in blood pressure regulation
| Experimental studies |
Species | Promoter/Cre | Blood pressure (BP) |
Phenotypic characteristics |
Organ specific effects |
Reference |
|---|---|---|---|---|---|---|
| Global | ||||||
| Over-expression of PRR | Mice | CAG promoter (embryonic stem cells) | No change | Renal expression ↑ 25-80 fold Cardiac expression ↑ 400 fold |
No proteinuria No cardiac dysfunction |
44 |
| Over-expression of human PRR | Rats | CAG promoter (embryonic stem cells) | No change | No change | Proteinuria Glomerulosclerosis |
45 |
| PRR KO | Mice | ROSA26-creERT2 | Not reported | Early lethality and weight loss | Pathology in colon, bone marrow and liver | 18 |
| Kidney | ||||||
| Podocyte PRR KO | Mice | podocin-Cre | Not reported | Nephrotic syndrome, early post-natal lethality | Severe renal injury | 16,17 |
| HRP in 2-kidney 1-clip Goldblatt | Rat | - | No change | HRP did not change plasma renin or aldosterone levels | No change in cardiac or renal injury | 107 |
| PRO20 infusion in inner medulla | Rat | - | ↓ Ang-II infused hypertension (100 ng/kg/min) | - | ↓ proteinuria and glomerulosclerosis following Ang-II | 25 |
| PRR shRNA injected to renal medulla | Rat | - | No change | - | Reduced α-ENaC abundance | 31 |
| Renal tubule PRR KO | Mice | Pax8-rtTA/LC1- Cre (inducible) | ↓ Ang-II infused hypertension (600 ng/kg/min) | Urinary concentration defect | Reduced α-ENaC abundance | 33 |
| CD specific PRR KO | Mice | AQP2-Cre | ↓ Ang-II infused hypertension (300 ng/kg/min) | - | Reduced α-ENaC abundance | 34 |
| CD specific PRR KO | Mice | Hoxb7-Cre | ↓ basal systolic BP ↓ Ang-II infused hypertension (400 ng/kg/min) |
Renal hypoplasia and ↓ renal function | Reduced cleaved α-ENaC and γ-ENaC abundance | 35 |
| Renal tubule PRR KO | Mice | Pax8-rtTA/LC1- Cre (inducible) | No change in BP with Ang-II infusion (1000 ng/kg/min) | ↑ lysosomal proteins and autophagosome markers | ↓ ability to excrete acid load | 36 |
| CD cell specific PRR KO | Mice | AQP2-Cre Principal cell specific | No change in BP | Urinary concentration defect | Reduced α-ENaC abundance | 39 |
| CD cell specific PRR KO | Mice | B1-ATPase-Cre Intercalated cell specific | No change in BP | Lower body weight | No change in ENaC abundance | 39 |
| Macula densa specific PRR KO | Mice | nNOS/CreERT2 | ↓ BP at baseline and following low Na+ + RAS blocker | ↓ plasma renin concentration | ↓ cyclooxygenase-2 and prostaglandin E synthase | 40 |
| Nephron progenitor cells | Mice | Six2GFPCre | Hypertension at 2 months of age | Homozygous deletion – early lethality | ↓ glomeruli number and thickening of basement membrane | 41 |
| Cardiovascular system | ||||||
| Cardiomyocyte PRR KO | Mice | α MHC-Cre | Not reported | Lethal heart failure within 3 weeks of birth | Autophagic vacuoles in cardiomyocytes | 15 |
| Cardiomyocyte specific PRR overexpression | Mice | α MHC promoter | Not reported | 170-fold ↑ PRR mRNA and 5-fold ↑ PRR protein | No change in cardiac function at baseline and following injury | 47 |
| Cardiomyocyte specific PRR overexpression | Rats | Adenovirus mediated gene delivery | Not reported | 2-3 fold ↑ PRR protein | ↓ left ventricular ejection fraction | 51 |
| Vascular smooth muscle cell PRR KO | Mice | No change in BP | Non-atherogenic sclerosis of aorta | VSMC autophagic cell death | 52 | |
| Vascular smooth muscle cell PRR overexpression | Rats | Smooth muscle cell myosin heavy chain gene | Hypertension at 6 months of age | Tachycardia | - | 53 |
| Brain | ||||||
| Supra-optic nucleus PRR knock-down | Rats (SHR) | Adenovirus mediated gene delivery | ↓ age associated hypertension | ↓ heart rate | ↓ plasma vasopressin levels | 57 |
| ICV PRR shRNA infusion | Mice | Overexpressing human renin and angiotensinogen | ↓ BP | ↓ sympathetic tone ↑ baroreflex sensitivity |
↓ Ang-II type 1 receptor and vasopressin levels in brain | 58 |
| Neuron specific PRR KO | Mice | Nefh-Cre | ↓ BP in ICV infusion of prorenin/ DOCA-salt | ↓ sympathetic tone ↑ baroreflex sensitivity |
↓ brain Ang-II levels | 20 |
| ICV PRO20 infusion | Mice | - | ↓ BP Ang-II and DOCA-salt hypertension | ↓ sympathetic tone ↑ baroreflex sensitivity |
↓ brain hypothalamic Ang- II levels with DOCA-salt | 6 |
| Adipose tissue | ||||||
| Adipose tissue PRR KO | Mice | Adiponectin-Cre | ↑ BP at baseline and following high fat diet | Lipodystrophy | ↑ plasma sPRR | 21 |
| Clinical Studies | Species | Controls | Blood pressure | Other pertinent findings | ||
| Pre-eclamptic women | Human | Normotensive pregnant women | ↑ BP | ↑ placental PRR and ↑ plasma sPRR BP correlated with placental PRR but not plasma sPRR | 67 | |
| Pre-eclamptic and diabetic women | Human | Healthy pregnant controls | Not reported | ↑ plasma sPRR in diabetes but not preeclamptic or healthy controls | 68 | |
| Japanese cohort of 1100 participants | Human | - | ↑ BP in men with IVS5+169C > T polymorphism | - | 69 | |
| Caucasian cohort | Human | - | ↑ BP in men with IVS5+169C > T polymorphism | - | 70 | |
KO - knock out; Ang-II – Angiotensin-II; ENaC – epithelial Na channel; RAS – renin angiotensin system blocker; ICV – intracerebroventricular; sPRR- soluble PRR; DOCA-salt – Deoxycorticosterone acetate;
Role of the (pro)renin receptor in glucose and lipid metabolism
Diabetes and insulin resistance
Soon after the discovery of the PRR, a decoy peptide termed ‘handle region peptide’ (HRP) was developed to block prorenin binding to the PRR 5. Early studies demonstrated that HRP markedly inhibited the development of diabetic nephropathy and reduced renal Ang-I and Ang-II levels in streptozotocin (STZ) induced Type 1 diabetic rats despite no changes in body weight, hyperglycemia and BP 5. Subsequently, HRP treatment was reported to be protective against development of diabetic nephropathy in Type 1 and 2 diabetic rodent models 81-83,84,85 and in reversing established diabetic nephropathy in STZ-induced diabetes 86. However, recent studies have shown that HRP increases inflammatory markers and cardiac fibrosis in diabetes 23,87 and may even have partial agonistic effects, questioning the specificity of HRP with regards to PRR function 88. Still, enhanced PRR expression is reported in whole kidney extracts in diabetic rats 83,89 and cultured renal cell lines exposed to high glucose 27,90,91. This is of particular significance since intrarenal, and particularly CD, prorenin/renin levels are markedly elevated in diabetes 92 and may contribute to the development of diabetic nephropathy via interaction with the renal PRR.
Other studies have provided indirect evidence for enhanced renal medullary PRR expression in insulin resistance. Mice with a null mutation of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam 1) had insulin resistance, visceral obesity and postprandial hyperglycemia associated with increased expression of medullary PRR and activation of tubular RAS components 93; all of these abnormalities were exacerbated by high fat intake 94. The same group demonstrated that high fat intake per se augments renal medullary PRR expression and Ang-II levels leading to hypertension, while renal tubular PRR KO mice were protected from high-fat diet induced hypertension 95. Thus, the renal PRR might play a role in the development and progression of renal injury in diabetes and insulin resistance.
Several studies have examined the role of adipocyte PRR in obesity and insulin resistance. Mice fed a high fat diet for 10 weeks had increased adipose tissue PRR expression without changes in heart, kidney and liver PRR levels 64. HRP treatment decreased weight gain, adipose tissue and circulating leptin levels, adipocyte inflammatory markers, plasma glucose and insulin, and triglyceride levels in obese mice 64. These effects appear to be due to redistribution of fat storage by increased adipogenesis, decreased lipogenesis and activation of triglyceride/free fatty acid cycling, resulting in reduced visceral adipose tissue and increased subcutaneous fat deposition 96. Consistent with the above studies, PRR mRNA was modestly increased in subcutaneous fat obtained from insulin resistant obese women compared to insulin sensitive obese women 64.
Recently, adipose tissue-specific PRR KO mice have been developed which show lower body weights compared to controls 21,22. When adipose tissue PRR is deleted using the adipocyte protein 2-Cre (which reportedly reduces PRR gene expression by 50-80%), hemizygous male KO mice have lower total fat mass, higher total lean mass and increased basal metabolic rate 22. Male KO mice and high fat-fed female KO mice had lower plasma insulin levels compared to WT mice although glucose tolerance test results were similar between KO and control mice. Further, circulating adiponectin levels were elevated in both male and female mice with no differences in circulating lipids or systemic renin levels 22. In contrast, adipocyte PRR deletion induced by adiponectin-Cre (with higher gene targeting efficiency) results in elevated plasma insulin levels likely due to hepatic steatosis and impaired insulin clearance 21; these mice also demonstrate improved insulin sensitivity under high fat intake 21. Collectively, these studies highlight the importance of adipose tissue PRR in obesity and insulin resistance. Further studies are needed to determine the molecular mechanisms involved in adipocyte PRR modulation of insulin signaling, particularly under diabetic conditions.
Finally, PRR expression is described in both human and mouse pancreatic islet cells and can modulate GLP1R signaling and insulin processing to affect insulin secretion 97. Additionally, PRR expression was reduced in islet cells from human diabetics compared to healthy controls, raising the possibility of a role for the PRR in insulin processing and/or secretion.
Lipid homeostasis
A novel, Ang-II independent function attributed to the PRR is regulation of lipoprotein metabolism. In a series of elegantly designed studies, Lu et al identified sortilin-1 (SORT1) as a key PRR interacting protein by mapping the PRR-interactome in HEK293 cells 98. They determined that the PRR was a post-transcriptional regulator of SORT1 that in turn modulates low-density lipoprotein (LDL) metabolism. In cultured hepatocytes, knock-down of the PRR reduced SORT1 and LDL receptor abundance leading to severely attenuated cellular LDL uptake which could be reversed by treatment with lysosomotropic agents such as bafilomycin A1 98, suggesting that PRR regulation of LDL receptor and SORT1 was independent of vacuolar H+-ATPase activity. The authors then demonstrated that mice injected with antisense oligonucleotides specifically targeting hepatic PRR had increased plasma LDL levels with an unexpected decrease in plasma triglyceride levels on a normal fat diet and during early stages of high fat intake 99. Further, hepatic PRR knock-down attenuated diet-induced obesity and hepatosteatosis in chronic high fat diet fed mice and reduced both plasma cholesterol and triglyceride levels in LDL-receptor deficient mice regardless of fat intake 99. These effects appear to be mediated via reduced lipid synthesis and increased fatty acid oxidation via acetyl-CoA carboxylase and pyruvate dehydrogenase.
Similarly, a recent study described an association between missense mutations in the extracellular domain of the PRR with a ‘congenital glycosylation disorder’ manifested by liver disease, immunodeficiency and psychomotor impairment in humans 100. The authors studied this in a mouse model with liver-specific knockdown of PRR and proposed that the clinical symptoms of the glycosylation disorder were likely due to impaired vacuolar H+-ATPase assembly leading to defects in autophagy 100. Taken together, these studies highlight a novel role of the PRR in lipid metabolism and liver function.
Clinical studies
Limited clinical data exists on the role of the PRR in glucose and lipid metabolism. Mutations in the ATP6AP2 gene were associated with disorders of glycosylation and autophagy in a small group of patients 100,101. Elevated plasma sPRR levels were described in several studies of gestational diabetes mellitus 68,102,103 yet plasma sPRR levels in diabetic patients were similar to those in healthy controls 75. Ongoing research on the tissue-specific role of the PRR in glucose and lipid metabolism will help clarify these conflicting results and determine if the PRR is a reasonable therapeutic target. Table 2 summarizes current evidence for the PRR in glucose and lipid metabolism.
Table 2.
Experimental and clinical models of the PRR in glucose and lipid metabolism
| Experimental studies | Species | Model studied | Phenotypic characteristics | Organ specific effects | Reference |
|---|---|---|---|---|---|
| Handle region peptide (HRP) from 4 weeks to 28 weeks of age (0.1 mg/kg) | Rat | STZ induced Type 1 diabetes | No change in body weight or hyperglycemia | ↓ proteinuria and development of diabetic nephropathy | 5 |
| HRP from 4 weeks to 16 weeks of age (1mg/kg/day) | Mice | db/db mice | No change in body weight or hyperglycemia | ↓ proteinuria and development of diabetic nephropathy | 81 |
| HRP (0.1 mg/kg) or Imidapril (0.015% in water) from 8 weeks to 24 weeks of age | Mice | STZ induced Type 1 diabetes in AT1-receptor KO mice | No change in body weight or hyperglycemia but BP lower in ATKO mice and after Imidapril treatment | HRP prevented proteinuria and development of diabetic nephropathy while Imidapril ↓ proteinuria | 82 |
| HRP (0.2 mg/kg) or Valsartan (2 mg/kg/day) or both for 2 weeks | Rats | STZ induced Type 1 diabetes | No change in body weight, kidney weight or hyperglycemia | Both treatments ↓ proteinuria & inflammatory cytokines | 83 |
| HRP (0.1 mg/kg) or Imidapril (0.015% in water) from 17 weeks to 29 weeks of age | Rats | STZ induced Type 1 diabetes | No change in body weight or hyperglycemia but BP lower after Imidapril treatment | HRP reverses diabetic glomerulosclerosis while Imidapril ↓ renal further damage | 86 |
| HRP (0.1 mg/kg/day) for 10 weeks | Mice | High fat diet | HRP ↓ weight gain, plasma glucose, insulin and triglyceride levels | ↓ plasma and adipocyte leptin levels and adipocyte inflammatory markers | 64 |
| HRP (1mg/kg/day) or Aliskiren (10mg/kg/day) or combined treatment for 3 weeks | Rats | STZ induced Type 1 diabetes in heterozygousRen2 rats | Aliskiren alone decreased BP | Aliskiren alone ↓ proteinuria and glomerulosclerosis while HRP counteracts the effects of Aliskiren | 23 |
| HRP (1mg/kg/day) or Aliskiren (10mg/kg/day) or combined treatment for 3 weeks | Rats | STZ induced Type 1 diabetes in heterozygousRen2 rats | No change in blood glucose but BP lower in HRP and Aliskiren treatments | Aliskiren alone improved vascular dysfunction in diabetic rats and addition of HRP diminished the effects | 87 |
| HRP (0.1 mg/kg/day) or Imidapril (0.015% or 0.060% in water) or combined treatment for 4 weeks | Rats | STZ induced Type 1 diabetes stroke prone SHR | HRP had no effect on BP while Imidapril & combined treatment lowered BP, no change in blood glucose | Combined treatment ↓ cardiac weight, proteinuria & glomerulosclerosis than HRP or Imidapril alone | 85 |
| HRP (0.1 mg/kg/day) or Quinapril (50 mg/kg/day) or combined treatment for 8 weeks | Rats | STZ induced Type 1 diabetes | No change in blood glucose or body weight, blood pressure not measured | Combined treatment and Quinapril ↓ proteinuria and tubular injury but not HRP;HRP & Quinapril ↓ glomerular but no additive effects | 84 |
| Ceacam1 KO mice (model of insulin resistance) | Mice | Normal fat diet | ↑ blood pressure | ↑ expression of medullary PRR and activation of tubular RAS components | 93 |
| Ceacam1 KO mice (model of insulin resistance) | Mice | High fat diet for 8 weeks | Visceral obesity and postprandial hyperglycemia | ↑ expression of medullary PRR and activation of tubular RAS components | 94 |
| Renal tubule PRR KO | Mice | High fat diet for 10 weeks | ↓ obesity induced hypertension | ↓ α ENaC abundance in the kidney | 95 |
| Adipocyte PRR KO (Adiponectin-Cre) | Mice | High fat diet for 16 weeks | Prevented development of obesity | ↑ plasma insulin levels and improved insulin sensitivity | 21 |
| Adipocyte PRR KO (Adipocyte protein 2-Cre) | Mice | High fat diet for 9 weeks | ↓ weight gain, ↑ basal metabolic rate | ↑ plasma insulin and adiponectin levels and improved insulin sensitivity | 22 |
| Antisense oligonucleotide to block hepatic PRR | Mice | Normal or high fat diet | ↓ diet induced obesity and hepatosteatosis | ↑ plasma LDL levels, ↑ triglyceride levels | 99 |
| Adeno-Cre injection to reduce liver PRR | Mice | Floxed PRR mice | ↑ plasma cholesterol | ↑ liver injury and autophagy defects | 100 |
| Clinical studies | Species | Mutations | Predominant characteristics | Other features | |
| Missense mutations in PRR (N=3) | Human | c.293T>C c.212G>A |
Liver cirrhosis and immunodeficiency | Intellectual disability, ataxia, and cutis laxa | 100,101 |
STZ - streptozotocin; ENaC – epithelial Na channel; RAS – renin angiotensin system blocker; AT1 – angiotensin-II Type 1 receptor; LDL- low density lipoprotein
Role of the (pro)renin receptor in fibrosis
As described earlier, the PRR is involved in the Wnt/β-catenin signaling pathway and plays an important role in kidney development and cell differentiation. New research suggests that PRR activation of Wnt/β-catenin signaling could contribute to renal fibrosis 104. Renal PRR expression is up-regulated in mice with chronic kidney disease following unilateral ureteral obstruction, adriamycin-induced renal injury or following chronic Ang-II infusion 104. In these conditions, the renal PRR, via activation of the Wnt/β-catenin pathway, enhances expression of downstream profibrotic markers such as fibronectin, plasminogen activator inhibitor 1 and α– smooth muscle actin (α-SMA). Consistent with this, PRR immunostaining was markedly enhanced in human kidney biopsy specimens with diabetic nephropathy, membranous nephropathy or lupus nephritis compared to control normal kidney biopsies 104. While PRR activation of the Wnt/β-catenin signaling was independent of prorenin in the above study 104, others report that prorenin treatment in cultured renal tubular epithelial cells, albeit at higher doses than seen in vivo, enhances expression of the PRR, vacuolar H+-ATPase and profibrotic markers 105,106. Further, inhibition of the PRR or vacuolar H+-ATPase activity partially reduced prorenin-induced fibronectin and α-SMA expression, suggesting that prorenin, via the PRR, promotes renal fibrosis 105. However, mice with constitutive global overexpression of PRR did not manifest cardiac or renal fibrosis despite marked increases in renal and cardiac PRR expression 44. Interpretation of these findings is difficult; however, the bulk of evidence suggests that the PRR, at least under some conditions, may play a pro-fibrotic role.
Perspectives and future studies
The PRR exerts a multitude of effects involving numerous tissues; these have physiological and pathophysiological consequences. The PRR may be of pathological significance in hypertension, metabolic syndrome, diabetes and other disorders. While clearly additional studies are necessary, it is instructive to speculate on what our current knowledge of the PRR might imply with regard to clinical relevance and, in particular, its utility as a clinical marker and/or therapeutic target. Several studies raise the interesting possibility of using sPRR as a clinical marker; however, it remains to be seen whether circulating and/or urinary sPRR, possibly together with prorenin, will have prognostic or diagnostic utility. With regard to therapeutic potential, it is first important to distinguish targeting PRR from effects elicited by other RAS antagonists: angiotensin receptor blockers, angiotensin converting enzyme inhibitors or direct renin inhibitors. None of these agents interfere with prorenin or renin binding to, or activating of, the PRR, i.e., the Ang-II independent effects of the PRR would not be targeted. Since Ang-II independent effects of PRR activation include stimulation of profibrogenic pathways and, as described herein, targeting the PRR reduces end organ pathology independent of targeting Ang-II, it is appropriate to further investigate the potential for using PRR antagonists in disease. However, such agents would have to block prorenin/renin-stimulated PRR signaling without interfering with PRR modulation of the vacuolar H+-ATPase. Since PRR regulation of the vacuolar H+-ATPase is independent of prorenin/renin, such agents may be possible; clearly, much additional work is needed in this area.
Acknowledgements
This research was supported in part by research grants from National Kidney Foundation of Utah and Idaho to N.R., and NIH (DK097007) and Veterans Affairs Merit Review to D.E.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All the authors declared no competing interests.
References
- 1.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical Renin-Angiotensin system in kidney physiology. Compr Physiol. 2014;4(3):1201–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86(3):747–803. [DOI] [PubMed] [Google Scholar]
- 3.Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res. 2012;318(9):1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109(11):1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichihara A, Hayashi M, Kaneshiro Y, et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the "handle" region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114(8):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Sullivan MN, Zhang S, et al. Intracerebroventricular infusion of the (Pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension. 2015;65(2):352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burckle C, Bader M. Prorenin and its ancient receptor. Hypertension. 2006;48(4):549–551. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen G, Muller DN. The biology of the (pro)renin receptor. J Am Soc Nephrol. 2010;21(1):18–23. [DOI] [PubMed] [Google Scholar]
- 9.Feldt S, Batenburg WW, Mazak I, et al. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension. 2008;51(3):682–688. [DOI] [PubMed] [Google Scholar]
- 10.Saris JJ, t Hoen PA, Garrelds IM, et al. Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension. 2006;48(4):564–571. [DOI] [PubMed] [Google Scholar]
- 11.Schefe JH, Neumann C, Goebel M, et al. Prorenin engages the (pro)renin receptor like renin and both ligand activities are unopposed by aliskiren. J Hypertens. 2008;26(9):1787–1794. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig J, Kerscher S, Brandt U, et al. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem. 1998;273(18):10939–10947. [DOI] [PubMed] [Google Scholar]
- 13.Lu X, Garrelds IM, Wagner CA, Danser AH, Meima ME. (Pro)renin receptor is required for prorenin-dependent and -independent regulation of vacuolar H(+)-ATPase activity in MDCK.C11 collecting duct cells. Am J Physiol Renal Physiol. 2013;305(3):F417–425. [DOI] [PubMed] [Google Scholar]
- 14.Cruciat CM, Ohkawara B, Acebron SP, et al. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327(5964):459–463. [DOI] [PubMed] [Google Scholar]
- 15.Kinouchi K, Ichihara A, Sano M, et al. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res. 2010;107(1):30–34. [DOI] [PubMed] [Google Scholar]
- 16.Oshima Y, Kinouchi K, Ichihara A, et al. Prorenin receptor is essential for normal podocyte structure and function. J Am Soc Nephrol. 2011;22(12):2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riediger F, Quack I, Qadri F, et al. Prorenin receptor is essential for podocyte autophagy and survival. J Am Soc Nephrol. 2011;22(12):2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wendling O, Champy MF, Jaubert S, et al. Atp6ap2 ablation in adult mice impairs viability through multiple organ deficiencies. Sci Rep. 2017;7(1):9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song R, Preston G, Ichihara A, Yosypiv IV. Deletion of the prorenin receptor from the ureteric bud causes renal hypodysplasia. PloS One. 2013;8(5):e63835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Peng H, Mehaffey EP, et al. Neuron-specific (pro)renin receptor knockout prevents the development of salt-sensitive hypertension. Hypertension. 2014;63(2):316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu CH, Mohammadmoradi S, Thompson J, et al. Adipocyte (Pro)Renin-Receptor Deficiency Induces Lipodystrophy, Liver Steatosis and Increases Blood Pressure in Male Mice. Hypertension. 2016;68(1):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shamansurova Z, Tan P, Ahmed B, Pepin E, Seda O, Lavoie JL. Adipose tissue (P)RR regulates insulin sensitivity, fat mass and body weight. Mol Metab. 2016;5(10):959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.te Riet L, van den Heuvel M, Peutz-Kootstra CJ, et al. Deterioration of kidney function by the (pro)renin receptor blocker handle region peptide in aliskiren-treated diabetic transgenic (mRen2)27 rats. Am J Physiol Renal Physiol. 2014;306(10):F1179–1189. [DOI] [PubMed] [Google Scholar]
- 24.Lu X, Wang F, Liu M, et al. Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: involvement of Nox4-derived hydrogen peroxide. Am J Physiol Renal Physiol. 2016;310(11):F1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Lu X, Liu M, Feng Y, Zhou SF, Yang T. Renal medullary (pro)renin receptor contributes to angiotensin II-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Med. 2015;13:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Advani A, Kelly DJ, Cox AJ, et al. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009;54(2):261–269. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Siragy HM. Glucose promotes the production of interleukine-1beta and cyclooxygenase-2 in mesangial cells via enhanced (Pro)renin receptor expression. Endocrinology. 2009;150(12):5557–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramkumar N, Kohan DE. Role of the Collecting Duct Renin Angiotensin System in Regulation of Blood Pressure and Renal Function. Curr Hypertens Rep. 2016;18(4):29. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension. 2011;57(4):859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prieto MC, Botros FT, Kavanagh K, Navar LG. Prorenin receptor in distal nephron segments of 2-kidney, 1-clip goldblatt hypertensive rats. Ochsner J. 2013;13(1):26–32. [PMC free article] [PubMed] [Google Scholar]
- 31.Quadri S, Siragy HM. (Pro)renin receptor contributes to regulation of renal epithelial sodium channel. J Hypertens. 2016;34(3):486–494; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramkumar N, Stuart D, Calquin M, et al. Nephron-specific deletion of the prorenin receptor causes a urine concentration defect. Am J Physiol Renal Physiol. 2015;309(1):F48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramkumar N, Stuart D, Mironova E, et al. Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am J Physiol Renal Physiol. 2016;311(1):F186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng K, Lu X, Wang F, et al. Collecting duct (pro)renin receptor targets ENaC to mediate angiotensin II-induced hypertension. Am J Physiol Renal Physiol. 2017;312(2):F245–F253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prieto MC, Reverte V, Mamenko M, et al. Collecting duct prorenin receptor knockout reduces renal function, increases sodium excretion, and mitigates renal responses in ANG II-induced hypertensive mice. Am J Physiol Renal Physiol. 2017;313(6):F1243–F1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trepiccione F, Gerber SD, Grahammer F, et al. Renal Atp6ap2/(Pro)renin Receptor Is Required for Normal Vacuolar H+-ATPase Function but Not for the Renin-Angiotensin System. J Am Soc Nephrol. 2016;27(11):3320–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu X, Wang F, Xu C, et al. Soluble (pro)renin receptor via beta-catenin enhances urine concentration capability as a target of liver X receptor. Proc Natl Acad Sci U S A. 2016;113(13):E1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang F, Lu X, Peng K, et al. Antidiuretic Action of Collecting Duct (Pro)Renin Receptor Downstream of Vasopressin and PGE2 Receptor EP4. J Am Soc Nephrol. 2016;27(10):3022–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramkumar N, Stuart D, Mironova E, et al. Collecting Duct Principal, but Not Intercalated, Cell Prorenin Receptor Regulates Renal Sodium and Water Excretion. Am J Physiol Renal Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riquier-Brison ADM, Sipos A, Prokai A, et al. The macula densa prorenin receptor is essential in renin release and blood pressure control. Am J Physiol Renal Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song R, Kidd L, Janssen A, Yosypiv IV. Conditional ablation of the prorenin receptor in nephron progenitor cells results in developmental programming of hypertension. Physiol Rep. 2018;6(7):e13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu C, Lu A, Lu X, et al. Activation of Renal (Pro)Renin Receptor Contributes to High Fructose-Induced Salt Sensitivity. Hypertension. 2017;69(2):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saigusa T, Dang Y, Bunni MA, et al. Activation of the intrarenal renin-angiotensin-system in murine polycystic kidney disease. Physiol Rep. 2015;3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosendahl A, Niemann G, Lange S, et al. Increased expression of (pro)renin receptor does not cause hypertension or cardiac and renal fibrosis in mice. Lab Invest. 2014;94(8):863–872. [DOI] [PubMed] [Google Scholar]
- 45.Kaneshiro Y, Ichihara A, Sakoda M, et al. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18(6):1789–1795. [DOI] [PubMed] [Google Scholar]
- 46.Connelly KA, Advani A, Kim S, et al. The cardiac (pro)renin receptor is primarily expressed in myocyte transverse tubules and is increased in experimental diabetic cardiomyopathy. J Hypertens. 2011;29(6):1175–1184. [DOI] [PubMed] [Google Scholar]
- 47.Mahmud H, Candido WM, van Genne L, et al. Cardiac function and architecture are maintained in a model of cardiorestricted overexpression of the prorenin-renin receptor. PloS One. 2014;9(2):e89929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirose T, Mori N, Totsune K, et al. Gene expression of (pro)renin receptor is upregulated in hearts and kidneys of rats with congestive heart failure. Peptides. 2009;30(12):2316–2322. [DOI] [PubMed] [Google Scholar]
- 49.Hayakawa Y, Aoyama T, Yokoyama C, et al. High salt intake damages the heart through activation of cardiac (pro) renin receptors even at an early stage of hypertension. PloS One. 2015;10(3):e0120453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okamoto C, Hayakawa Y, Aoyama T, et al. Excessively low salt diet damages the heart through activation of cardiac (pro) renin receptor, renin-angiotensin-aldosterone, and sympatho-adrenal systems in spontaneously hypertensive rats. PloS One. 2017;12(12):e0189099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moilanen AM, Rysa J, Serpi R, et al. (Pro)renin receptor triggers distinct angiotensin II-independent extracellular matrix remodeling and deterioration of cardiac function. PloS One. 2012;7(7):e41404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurauchi-Mito A, Ichihara A, Bokuda K, et al. Significant roles of the (pro)renin receptor in integrity of vascular smooth muscle cells. Hypertens Res. 2014;37(9):830–835. [DOI] [PubMed] [Google Scholar]
- 53.Burckle CA, Jan Danser AH, Muller DN, et al. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension. 2006;47(3):552–556. [DOI] [PubMed] [Google Scholar]
- 54.Greco CM, Camera M, Facchinetti L, et al. Chemotactic effect of prorenin on human aortic smooth muscle cells: a novel function of the (pro)renin receptor. Cardiovasc Res. 2012;95(3):366–374. [DOI] [PubMed] [Google Scholar]
- 55.Young CN, Davisson RL. Angiotensin-II, the Brain, and Hypertension: An Update. Hypertension. 2015;66(5):920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Q, Jensen DD, Peng H, Feng Y. The critical role of the central nervous system (pro)renin receptor in regulating systemic blood pressure. Pharmacol Ther. 2016;164:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shan Z, Shi P, Cuadra AE, et al. Involvement of the brain (pro)renin receptor in cardiovascular homeostasis. Circ Res. 2010;107(7):934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li W, Peng H, Cao T, et al. Brain-targeted (pro)renin receptor knockdown attenuates angiotensin II-dependent hypertension. Hypertension. 2012;59(6):1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooper SG, Trivedi DP, Yamamoto R, et al. Increased (pro)renin receptor expression in the subfornical organ of hypertensive humans. Am J Physiol Heart Circ Physiol. 2018;314(4):H796–H804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huber MJ, Basu R, Cecchettini C, Cuadra AE, Chen QH, Shan Z. Activation of the (pro)renin receptor in the paraventricular nucleus increases sympathetic outflow in anesthetized rats. Am J Physiol Heart Circ Physiol. 2015;309(5):H880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cassis LA, Police SB, Yiannikouris F, Thatcher SE. Local adipose tissue renin-angiotensin system. Curr Hypertens Rep. 2008;10(2):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Achard V, Boullu-Ciocca S, Desbriere R, Nguyen G, Grino M. Renin receptor expression in human adipose tissue. Am J Physiol Reg Integr Comp Physiol. 2007;292(1):R274–282. [DOI] [PubMed] [Google Scholar]
- 63.Achard V, Tassistro V, Boullu-Ciocca S, Grino M. Expression and nutritional regulation of the (pro)renin receptor in rat visceral adipose tissue. J Endocrinol Invest. 2011;34(11):840–846. [DOI] [PubMed] [Google Scholar]
- 64.Tan P, Shamansurova Z, Bisotto S, et al. Impact of the prorenin/renin receptor on the development of obesity and associated cardiometabolic risk factors. Obesity. 2014;22(10):2201–2209. [DOI] [PubMed] [Google Scholar]
- 65.Marques FZ, Pringle KG, Conquest A, et al. Molecular characterization of renin-angiotensin system components in human intrauterine tissues and fetal membranes from vaginal delivery and cesarean section. Placenta. 2011;32(3):214–221. [DOI] [PubMed] [Google Scholar]
- 66.Thomason J, Reyes M, Allen SR, et al. Elevation of (Pro)Renin and (Pro)Renin Receptor in Preeclampsia. Am J Hypertens. 2015;28(10):1277–1284. [DOI] [PubMed] [Google Scholar]
- 67.Narita T, Ichihara A, Matsuoka K, et al. Placental (pro)renin receptor expression and plasma soluble (pro)renin receptor levels in preeclampsia. Placenta. 2016;37:72–78. [DOI] [PubMed] [Google Scholar]
- 68.Sugulle M, Heidecke H, Maschke U, et al. Soluble (pro)renin receptor in preeclampsia and diabetic pregnancies. J Am Soc Hypertens. 2017;11(10):644–652. [DOI] [PubMed] [Google Scholar]
- 69.Hirose T, Hashimoto M, Totsune K, et al. Association of (pro)renin receptor gene polymorphism with blood pressure in Japanese men: the Ohasama study. Am J Hypertens. 2009;22(3):294–299. [DOI] [PubMed] [Google Scholar]
- 70.Ott C, Schneider MP, Delles C, Schlaich MP, Hilgers KF, Schmieder RE. Association of (pro)renin receptor gene polymorphism with blood pressure in Caucasian men. Pharmacogenet Genomics. 2011;21(6):347–349. [DOI] [PubMed] [Google Scholar]
- 71.Hirose T, Hashimoto M, Totsune K, et al. Association of (pro)renin receptor gene polymorphisms with lacunar infarction and left ventricular hypertrophy in Japanese women: the Ohasama study. Hypertens Res. 2011;34(4):530–535. [DOI] [PubMed] [Google Scholar]
- 72.Gupta HV, Vengoechea J, Sahaya K, Virmani T. A splice site mutation in ATP6AP2 causes X-linked intellectual disability, epilepsy, and parkinsonism. Parkinsonism Relat Disord. 2015;21(12):1473–1475. [DOI] [PubMed] [Google Scholar]
- 73.Korvatska O, Strand NS, Berndt JD, et al. Altered splicing of ATP6AP2 causes X-linked parkinsonism with spasticity (XPDS). Hum Mol Genet. 2013;22(16):3259–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramser J, Abidi FE, Burckle CA, et al. A unique exonic splice enhancer mutation in a family with X-linked mental retardation and epilepsy points to a novel role of the renin receptor. Hum Mol Genet. 2005;14(8):1019–1027. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen G, Blanchard A, Curis E, et al. Plasma soluble (pro)renin receptor is independent of plasma renin, prorenin, and aldosterone concentrations but is affected by ethnicity. Hypertension. 2014;63(2):297–302. [DOI] [PubMed] [Google Scholar]
- 76.Hamada K, Taniguchi Y, Shimamura Y, et al. Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol. 2013;17(6):848–856. [DOI] [PubMed] [Google Scholar]
- 77.Morimoto S, Ando T, Niiyama M, et al. Serum soluble (pro)renin receptor levels in patients with essential hypertension. Hypertens Res. 2014;37(7):642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amari Y, Morimoto S, Nakajima F, Ando T, Ichihara A. Serum Soluble (Pro)Renin Receptor Levels in Maintenance Hemodialysis Patients. PloS One. 2016;11(7):e0158068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gong L, Zhang S, Li L, et al. Elevated plasma soluble (pro)renin receptor levels are associated with left ventricular remodeling and renal function in chronic heart failure patients with reduced ejection fraction. Peptides. 2018. [DOI] [PubMed] [Google Scholar]
- 80.Nishijima T, Tajima K, Takahashi K, Sakurai S. Elevated plasma levels of soluble (pro)renin receptor in patients with obstructive sleep apnea syndrome: association with polysomnographic parameters. Peptides. 2014;56:14–21. [DOI] [PubMed] [Google Scholar]
- 81.Ichihara A, Sakoda M, Kurauchi-Mito A, Nishiyama A, Itoh H. Involvement of receptor-bound prorenin in development of nephropathy in diabetic db/db mice. J Am Soc Hypertens. 2008;2(5):332–340. [DOI] [PubMed] [Google Scholar]
- 82.Ichihara A, Suzuki F, Nakagawa T, et al. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2006;17(7):1950–1961. [DOI] [PubMed] [Google Scholar]
- 83.Matavelli LC, Huang J, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol. 2010;37(3):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kokeny G, Fang L, Revesz C, et al. The Effect of Combined Treatment with the (Pro)Renin Receptor Blocker HRP and Quinapril in Type 1 Diabetic Rats. Kidney Blood Press Res. 2017;42(1):109–122. [DOI] [PubMed] [Google Scholar]
- 85.Seki Y, Ichihara A, Mizuguchi Y, et al. Add-on blockade of (pro)renin receptor in imidapril-treated diabetic SHRsp. Front Biosci. 2010;2:972–979. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi H, Ichihara A, Kaneshiro Y, et al. Regression of nephropathy developed in diabetes by (Pro)renin receptor blockade. J Am Soc Nephrol. 2007;18(7):2054–2061. [DOI] [PubMed] [Google Scholar]
- 87.Batenburg WW, van den Heuvel M, van Esch JH, et al. The (pro)renin receptor blocker handle region peptide upregulates endothelium-derived contractile factors in aliskiren-treated diabetic transgenic (mREN2)27 rats. J Hypertens. 2013;31(2):292–302. [DOI] [PubMed] [Google Scholar]
- 88.Maschke U, Muller DN. The (pro)renin receptor and the mystic HRP--is there a role in cardiovascular disease? Front Biosci. 2010;2:1250–1253. [DOI] [PubMed] [Google Scholar]
- 89.Siragy HM, Huang J. Renal (pro)renin receptor upregulation in diabetic rats through enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp Physiol. 2008;93(5):709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang J, Siragy HM. Regulation of (pro)renin receptor expression by glucose-induced mitogen-activated protein kinase, nuclear factor-kappaB, and activator protein-1 signaling pathways. Endocrinology. 2010;151(7):3317–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li C, Siragy HM. High glucose induces podocyte injury via enhanced (pro)renin receptor-Wnt-beta-catenin-snail signaling pathway. PloS One. 2014;9(2):e89233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51(6):1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang J, Ledford KJ, Pitkin WB, Russo L, Najjar SM, Siragy HM. Targeted deletion of murine CEACAM 1 activates PI3K-Akt signaling and contributes to the expression of (Pro)renin receptor via CREB family and NF-kappaB transcription factors. Hypertension. 2013;62(2):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li C, Culver SA, Quadri S, et al. High-fat diet amplifies renal renin angiotensin system expression, blood pressure elevation, and renal dysfunction caused by Ceacam1 null deletion. Am J Physiol Endocrinol Metab. 2015;309(9):E802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Quadri SS, Culver S, Ramkumar N, Kohan DE, Siragy HM. (Pro)Renin receptor mediates obesity-induced antinatriuresis and elevated blood pressure via upregulation of the renal epithelial sodium channel. PloS One. 2018;13(8):e0202419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan P, Blais C, Nguyen TM, Schiller PW, Gutkowska J, Lavoie JL. Prorenin/renin receptor blockade promotes a healthy fat distribution in obese mice. Obesity. 2016;24(9):1946–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dai FF, Bhattacharjee A, Liu Y, et al. A Novel GLP1 Receptor Interacting Protein ATP6ap2 Regulates Insulin Secretion in Pancreatic Beta Cells. J Biol Chem. 2015;290(41):25045–25061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu X, Meima ME, Nelson JK, et al. Identification of the (Pro)renin Receptor as a Novel Regulator of Low-Density Lipoprotein Metabolism. Circ Res. 2016;118(2):222–229. [DOI] [PubMed] [Google Scholar]
- 99.Ren L, Sun Y, Lu H, et al. (Pro)renin Receptor Inhibition Reprograms Hepatic Lipid Metabolism and Protects Mice From Diet-Induced Obesity and Hepatosteatosis. Circ Res. 2018;122(5):730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rujano MA, Cannata Serio M, Panasyuk G, et al. Mutations in the X-linked ATP6AP2 cause a glycosylation disorder with autophagic defects. J Exp Med. 2017;214(12):3707–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cannata Serio M, Rujano MA, Simons M. Mutations in ATP6AP2 cause autophagic liver disease in humans. Autophagy. 2018;14(6):1088–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonakdaran S, Azami G, Tara F, Poorali L. Soluble (Pro) Renin Receptor is a Predictor of Gestational Diabetes Mellitus. Curr Diabetes Rev. 2017;13(6):555–559. [DOI] [PubMed] [Google Scholar]
- 103.Gokulakrishnan K, Maheswari K, Mahalakshmi MM, et al. Association of Soluble (Pro) Renin Receptor with Gestational Diabetes Mellitus. Endocr Pract. 2015;21(1):7–13. [DOI] [PubMed] [Google Scholar]
- 104.Li Z, Zhou L, Wang Y, et al. (Pro)renin Receptor Is an Amplifier of Wnt/beta-Catenin Signaling in Kidney Injury and Fibrosis. J Am Soc Nephrol. 2017;28(8):2393–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y, Zuo S, Li X, et al. Interaction between V-ATPase B2 and (Pro) renin Receptors in Promoting the progression of Renal Tubulointerstitial Fibrosis. Sci Rep. 2016;6:25035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gonzalez AA, Zamora L, Reyes-Martinez C, et al. (Pro)renin receptor activation increases profibrotic markers and fibroblast-like phenotype through MAPK-dependent ROS formation in mouse renal collecting duct cells. Clin Exp Pharmacol Physiol. 2017:44(11)11134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muller DN, Klanke B, Feldt S, et al. (Pro)renin receptor peptide inhibitor "handle-region" peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension. 2008;51(3):676–681. [DOI] [PubMed] [Google Scholar]