Abstract
Traumatic brain injury (TBI) is a common cause of death and acquired disability in adults and children. Identifying biomarkers for mild TBI (mTBI) that can predict functional impairments on neuropsychiatric and neurocognitive testing after head trauma is yet to be firmly established. Extracellular vesicles (EVs) are known to traffic from the brain to the oral cavity and can be detected in saliva. We hypothesize the genetic profile of salivary EVs in patients who have suffered head trauma will differ from normal healthy controls, thus constituting a unique expression signature for mTBI. We enrolled a total of 54 subjects including for saliva sampling, 23 controls with no history of head traumas, 16 patients enrolled from an outpatient concussion clinic, and 15 patients from the emergency department who had sustained a head trauma within 24 hr. We performed real‐time PCR of the salivary EVs of the 54 subjects profiling 96 genes from the TaqMan Human Alzheimer's disease array. Real‐time PCR analysis revealed 57 (15 genes, p < 0.05) upregulated genes in emergency department patients and 56 (14 genes, p < 0.05) upregulated genes in concussion clinic patients when compared with controls. Three genes were upregulated in both the emergency department patients and concussion clinic patients: CDC2, CSNK1A1, and CTSD ( p < 0.05). Our results demonstrate that salivary EVs gene expression can serve as a viable source of biomarkers for mTBI. This study shows multiple Alzheimer's disease genes present after an mTBI.
Keywords: biomarkers, extracellular vesicles (EVs), real‐time PCR, traumatic brain injury (TBI)
1. INTRODUCTION
Traumatic brain injury (TBI) occurs when a head impact, penetration or rapid movement causes the brain to move rapidly within the skull leading to damage (Prins, Greco, Alexander, & Giza, 2013). Each year, approximately 1.7 million TBIs occur. This results in 1,365,000 (80.7%) emergency department visits, 275,000 (16.3%) hospitalizations, and 52,000 (3.0%) deaths (Taylor, Greenspan, Xu, & Kresnow, 2015). TBI based on clinical symptoms is classified according to the Glasgow Coma Scale: mild (score 13–15), moderate (score 9–10), and severe (score <9; Prins et al., 2013). Multiple neurochemical processes and cellular pathways are involved in response to the initial insult, including neuron and oligodendrocyte death (Raghupathi, 2004). Secondary injuries can occur from cellular and molecular mechanisms responding to the initial injury and can continue long‐term (Prins et al., 2013). Repeated TBI is associated with chronic and sometimes progressive clinical symptoms and neuro‐pathological loss of function. In addition, evidence is growing that moderate to severe or repeated mild TBI (mTBI) incidents could lead to increased risk for Alzheimer's Disease (Heneka et al., 2015; Prins et al., 2013), and chronic traumatic encephalopathy (CTE; McKee et al., 2009; Mez et al., 2017; Prins et al., 2013), which is specifically described in patients that have a history of repeated head impacts (Gavett, Stern, Cantu, Nowinski, & McKee, 2010).
Objective and quantifiable biomarkers are needed to aid in acute TBI diagnosis and help predict those at risk for long‐term effects (Rogg et al., 2014). Recent reviews evaluating moderate to severe TBI highlight the importance of candidate protein biomarkers abundant within neuronal and glial cells (Dash, Zhao, Hergenroeder, & Moore, 2010; Kochanek et al., 2008; Svetlov et al., 2009; Yokobori et al., 2013). However, this strategy has not produced relevant and clinically useful results when applied to mTBI. Although numerous works are focused on biomarkers to identify complicated or hemorrhagic mTBI, there is a paucity of similar studies on uncomplicated mTBI (Papa et al., 2016), which is a more prevalent pathology.
Extracellular vesicles (EVs) were described as a mechanism of cell‐to‐cell communication. EVs are released by cells, including stem cells and progenitors, and interact with target cells by surface‐expressed ligands in the transfer of surface receptors, proteins, mRNA, and bioactive lipids (Michael et al., 2010; Papa et al., 2016; Svetlov et al., 2009; Yokobori et al., 2013). Clinically EVs can be isolated easily and quickly in a noninvasive fashion from multiple bodily fluids including urine and blood (Lakkaraju & Rodriguez‐Boulan, 2008). Because of the distinctive cargo, EVs can shuttle, as well as the fact that they are tissue specific, they may have a strong clinical application as biomarkers (S. Hu et al., 2008; Skog et al., 2008; Zhong, Taylor, & Whittington, 2010). In addition, because EVs are membrane‐bound, they are not subject to the same degradation that conventional serum biomarkers face. While most studies investigate disease processes with EVs isolated from serum, those that focus on noninvasive EVs biomarkers, such as those present in urine as seen in renal disease (Gonzales et al., 2009; S. Hu et al., 2008) and prostate cancer (Mitchell et al., 2009) or saliva (Gonzalez‐Begne et al., 2009; Kapsogeorgou, Abu‐Helu, Moutsopoulos, & Manoussakis, 2005), as seen in brain cancer, poses an exciting avenue to painlessly diagnose disease.
In the present study, we report the isolation and characterization of EVs from saliva and for the first time profiled the expression of Alzheimer disease genes in three groups of patients: acutely head injured emergency department (ED) patients, patients diagnosed with a concussion from an outpatient concussion clinic, and controls. Given the literature surrounding head injury and Alzheimer's disease (Becker, Kapogiannis, & Greig, 2018; Grinberg et al., 2016; Julien et al., 2017; Mendez, Paholpak, Lin, Zhang, & Teng, 2015; Ramos‐Cejudo et al., 2018), we hypothesized that patients with mTBI would express Alzheimer's disease genes at significantly greater levels than controls. Our aim is to determine whether those gene expression profiles changed after mTBI and if the changes of the biomarkers could be potentially used to diagnose mTBI to prognosticate future development of postconcussion syndrome (PCS) or CTE, a disease characterized by tau protein deposition and amyloid beta plagues similar to those seen in Alzheimer's disease (Gavett et al., 2010).
2. MATERIALS AND METHODS
All participants and/or their relatives in addition to normal healthy control subjects gave written informed consent. The study was approved by the Rhode Island Hospital IRB. All clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki and have been carried out according to the international Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) standard.
2.1. Patient selection
The study enrolled 54 participants: 15 patients with acute head trauma from the Rhode Island Hospital Level 1 Trauma Center Emergency Department (Emergency department patients; EDPT), 23 controls, and 16 patients with a diagnosis of concussion evaluated at an outpatient concussion clinic patients (CCPT). The patients and control subjects were randomly selected, and not matched for age, sex, or ethnicity. Controls were screened and denied a history of mild, moderate, or severe TBI. The patient demographic data are summarized (Table 1).
Table 1.
Participant demographics of all subjects
| All participants | Normal healthy controls | Concussion clinic patients | ED Head trauma patients | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample size | 54 | 23 | 16 | 15 | |||||
| Age range | 21–63 | 21–59 | 6–69 | ||||||
| Median age | 25 | 33 | 27 | ||||||
| Gender | |||||||||
| Male | 19 | 7 | 6 | 6 | |||||
| Female | 35 | 16 | 10 | 9 | |||||
| Concussion clinic Patients ID | Gender | Date of Injury | Age at collection | Days from injury to collection | Medications prescribed at time of sampling | ||||
|---|---|---|---|---|---|---|---|---|---|
| CCPT1 | M | 2‐Mar‐16 | 48 | 56 | None | ||||
| CCPT2 | F | 3‐Dec‐15 | 51 | 145 | Amitriptyline 10 mg, melatonin 3 mg, voltaren 1% topical gel | ||||
| CCPT3 | F | 5‐Nov‐15 | 52 | 173 | None | ||||
| CCPT4 | F | 28‐Mar‐16 | 31 | 30 | Fioricet with codeine | ||||
| CCPT5 | M | 17‐Mar‐16 | 46 | 41 | None | ||||
| CCPT6 | F | 10‐Nov‐15 | 50 | 168 | Meclizine 25 mg | ||||
| CCPT7 | M | 1‐Oct‐15 | 28 | 207 | Amitripyline 50 mg, amitriptyline 75 mg | ||||
| CCPT8 | F | 3‐Mar‐16 | 59 | 55 | None | ||||
| CCPT9 | M | 1‐May‐16 | 33 | 107 | None | ||||
| CCPT10 | F | 26‐Jul‐16 | 33 | 22 | None | ||||
| CCPT11 | M | 19‐Jul‐16 | 21 | 29 | None | ||||
| CCPT12 | F | 14‐May‐16 | 29 | 94 | Trazodone 50 mg | ||||
| CCPT13 | M | 21‐Jul‐16 | 49 | 27 | None | ||||
| CCPT15 | F | 6‐Jun‐16 | 33 | 72 | None | ||||
| CCPT17 | F | 9‐Jun‐16 | 22 | 69 | None | ||||
| CCPT19 | F | 16‐May‐16 | 26 | 92 | None | ||||
| ED patient ID | Age at injury | Gender | Diagnosis of concussion in ED | Reason for ED visit | CT imaging | ||||
|---|---|---|---|---|---|---|---|---|---|
| EDPT01 | 43 | M | U | Complex neuro history | None documented | ||||
| EDPT02 | 36 | M | U | tree limb fell on head | None documented | ||||
| EDPT03 | 25 | F | U | motocycle crash | None documented | ||||
| EDPT04 | 31 | M | U | Recent head injury, assaulted | Small subarachnoid hemorrhage | ||||
| EDPT05 | 21 | M | U | Motocycle crash | None documented | ||||
| EDPT06 | 26 | F | N | Collision with bus | None documented | ||||
| EDPT07 | 6 | F | N | Acute pharyngitis | None documented | ||||
| EDPT08 | 67 | F | N | Fell on ice, hit head, contusion Occipital area | CT head, negative | ||||
| EDPT09 | 31 | F | Y | Motocycle crash head and neck | None documented | ||||
| EDPT10 | 24 | F | Y | Fell on ice, struck left side of head and elbow | None documented | ||||
| EDPT11 | 27 | F | Y | Car accident | CT head/cervical spine negative | ||||
| EDPT12 | 45 | M | U | Hit head snowboarding with helmet | None documented | ||||
| EDPT13 | 43 | M | U | Fell off minibike yesterday | None documented | ||||
| EDPT14 | 20 | F | U | Hit head during car accident | None documented | ||||
| EDPT15 | 19 | F | U | Head collision against another player | Head CT, negative | ||||
Note. CCPT: concussion clinic patients; ED: emergency department; EDPT: ED head trauma patients; N: no; M: male; F: female; Y: yes; U: unknown. Number of participants in control group, Concussion clinic patient group, and ED head trauma patients, with age range, median age, and gender.
2.2. Saliva sample collection
According to established protocols (Navazesh, 1993), subjects were directed to orally rinse with cup of water before saliva collection. Subjects were directed to spit saliva into the test tube every 60 s. At least 5 ml of saliva was collected. One sample was collected per patient. Patients recruited from the ED had their head injury within 24 hr of saliva collection. EVs were isolated via differential ultracentrifugation, and the size and concentration of the EV were analyzed using the NanoSight NS500 instrument (Nanosight, Malvern, UK), transmission electron microscopy (TEM), and western blot analysis.
2.3. Salivary EVs isolation
The protocol was adapted and modified from a previously reported method for salivary EVs isolation (Michael et al., 2010). Saliva samples were stored at −80°C until they were ready to be analyzed. The samples were subsequently thawed and centrifuged at 1,500 g for 10 min at 4°C. The supernatant was collected and centrifuged at 17,000 g for 15 min at 4°C. The supernatant was transferred and underwent ultracentrifugation at 120,000 g for 1 hr at 4°C. The remaining pellet was washed with phosphate buffered saline (PBS) and centrifuged at 120,000 g for 1 hr at 4°C. EVs were then resuspended in 500 µl PBS.
2.4. Measurement of particle size and concentration distribution with NanoSight
Nanoparticles in the saliva EVs suspensions were analyzed using the NanoSight NS500 instrument (Nanosight, Malvern, UK). The analysis settings were optimized and kept constant between samples, and each video was analyzed to give the mean, mode, median and estimated concentration for each particle size. Samples were measured at 1:20 dilution, yielding particle concentrations in the region of 1 × 108 particles ml−1 as per the manufacturer's recommendations. All samples were analyzed in triplicate.
2.5. Transmission electron microscopy
TEM was performed on isolated salivary EVs resolved in PBS, placed on 200 mesh nickel formvar‐carbon coated grids (Electron Microscopy Science (Electron Microscopy Sciences, Hatfield, PA)) and left to adhere for 20 min. Grids were incubated with 2.5% glutaraldehyde/2% sucrose. EVs were negatively stained with NanoVan (Nanoprobes, Yaphank, NY) and observed by Jeol JEM 1010 electron microscope (Jeol).
2.6. Western blot analysis
EVs were lysed with radioimmunoprecipitation assay buffer (Sigma‐Aldrich, St. Louis, MO). Protein content was measured by the Bradford method (Bio‐Rad). EVs lysates (30 μg) were separated by 4–15% gradient polyacrylamide gel electrophoresis (Bio‐Rad, Hercules, CA) and then immunoblotted with antibody anti‐CD63 (Santa Cruz, biotechnology, Santa Cruz, CA). The protein bands were visualized with an enhanced chemiluminescence detection kit and ChemiDoc™ XRS‐System (Bio‐Rad, Hercules, CA).
2.7. PCR profiling
RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. RNA quality and quantification was done using Nanodrop 1000 (Thermo Fisher Scientific, Waltham, MA). Complementary DNA (cDNA) was synthesized from the RNA with the High Capacity cDNA transcription kit (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA) in a final volume of 20 µl. Amplification reactions consisted of one cycle for 10 min at 25°C, one cycle for 120 min at 37°C, and one cycle for 5 min at 85°C using a 9800 Fast Thermal Cycler (Applied Biosystems, Waltham, MA). Preamplification reactions were performed in a final volume of 50 µl: 12.5 µl of diluted 96 TaqMan gene assay mix, 25 µl of TaqMan Preamp Master mix (Applied Biosystems), and 12.5 µl of cDNA. The reaction consisted of 10 min at 95°C followed by 14 cycles consisted of 15 s at 95°C then 60°C at 4 min. TaqMan® Human Alzheimer's Array (Applied Biosystems, Waltham, MA), (Supporting Information Table S1, list of genes on the array) has 93 genes (3 endogenous controls) known to be altered in Alzheimer's disease and three endogenous controls. Cards were loaded with cDNA and TaqMan® Universal PCR master mix (Applied Biosystems, Waltham, MA) and run on the Viia7 Real‐Time PCR System (Life Technologies, Carlsbad, CA) using Relative Quantification settings. The cycle threshold (Ct) readings were used to determine fold change (FC) of gene expression. Samples with a Ct of <35 were considered for calculating the FC in expression. The method was used to calculate the relative expression of each target gene. Mean Ct value of target genes in each sample was normalized to its averaged housekeeping gene (GAPDH) Ct value to give a delta Ct value. This was then normalized to control sample (delta delta Ct), and the value was obtained and converted to FC.
2.8. Statistical analysis methods
All statistical analysis was done on STATA software. One‐way analysis of variance statistical test was performed on participant ages. Wilcoxon sum test was performed on the gender differences in each group. Wilcoxon sum test was used to compare the delta Ct values of each gene between two groups: ED patients (EDPT) versus controls, CCPT versus controls, and EDPT versus CCPT. A p value of <0.05 was used for statistical significance.
3. RESULTS
3.1. Sample comparison of patient groups to healthy controls
The mean ages of the outpatient concussion clinic patients are significantly older than the average age of controls (38.1 vs. 29.5, p = 0.045), but not so with the EDPT (30.9 vs. 29.52, p = 0.76; Table 1).
3.2. Characterization, quantification, and size distribution of human salivary EVs
TEM was performed on purified EVs characterizing their spheroid morphology and size (Figure 1a) and protein marker CD63 (Figure 1b). The diameter of the particles ranged from 20 to 1000 nm. The individual patient salivary EVs sample size distribution (Figure 2a) and concentration of EVs (Figure 2b) from controls, ED patients, and concussion clinic patients are displayed with standard deviation. Both the mean size (Figure 2c) of the EVs as well as the concentration of the EVs (Figure 2d) increased in ED patients compared with controls.
Figure 1.
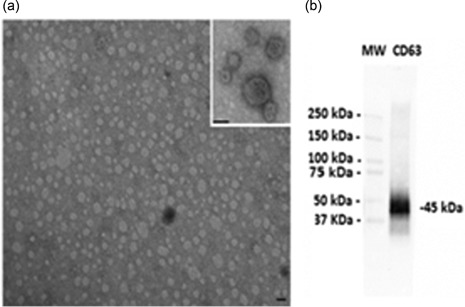
Transmission electron microscope and western blot images and NanoSight images. (a) Representative transmission electron microscopy of EVs isolated from saliva. EVs were viewed by JEOL Jem 1010 electron microscope (original magnification ×100,000; inset original magnification ×150,000; black lines = 100 nm). (b) Representative western blot analysis of CD63 from saliva EVs. (MW = standard molecular weight markers). EVs: extracellular vesicles
Figure 2.
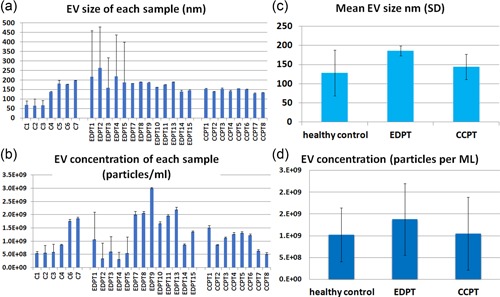
Transmission electron microscope and western blot images and NanoSight images. (a) Salivary EVs size distribution in nanometer (nm) of each control (n = 7), concussion clinic patients (CCPT) (n = 8), and ED head trauma patients (EDPT) (n = 13). (b) EVs concentration for each patient by NanoSight analysis showing the number of EVs per milliliter of saliva derived from controls (n = 7), CCPT (n = 8), and EDPT (n = 13). (c) Mean salivary EVs size with standard deviation by NanoSight analysis showing the mode size of EVs in 1 ml of saliva derived from controls (n = 7), CCPT (n = 8), and EDPT (n = 13). (d) Mean EVs concentration with standard deviation by NanoSight analysis showing the mode size of EVs in 1 ml of saliva derived from controls (n = 7), CCPT (n = 8), and EDPT (n = 13). ED: emergency department; EVs: extracellular vesicles [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Comparison between control and acute ED patients
To assess whether the salivary EVs gene expression profiles in response to head trauma, we used Alzheimer's disease array analysis in salivary EVs. Of the 93 genes from the array, 57 genes were upregulated with an FC higher than two between EDPT and controls. Wilcoxon sum test shows a statistically different expression between the two groups in 15 genes (Table 2a), including ABCA1, AGER, APLP2, CDC2, CSNK1A1, CSNKID, CTSD, GSK3B, IL1B, LRPAP1, MAPT, PRKCB1, PSEN1, SOAT1, and SOD2. Each individual EDPT with the number of genes with FC above 50, 20, or 2 (FC above 2 considered biologically relevant) is represented in Figure 3a. The 57 genes that were upregulated in EDPT compared with controls is shown in Figure 3b. Gene upregulation (FC > 2) ranged from 45 genes in EDPT5 and EDPT8 to only eight genes in EDPT12.
Table 2.
Analysis of Gene Expression of Participant Populations
| A Controls vs ED head trauma patients | ||||
|---|---|---|---|---|
| Gene | ΔCT±SD controls | ΔCT±SD EDPT | p value ΔCT | FC±SD EDPT |
| ABCA1 | 9·51 ± 3·54 | 7·99 ± 1·80 | 0·0239 | 5·27 ± 6·73 |
| AGER | 8·60 ± 4·02 | 5·92 ± 2·95 | 0·0203 | 20·5 ± 32·75 |
| APLP2 | 7·36 ± 3·24 | 5·33 ± 2·34 | 0·0008 | 17·20 ± 26·99 |
| CDC2 | 9·62 ± 3·11 | 11·48 ± 3·70 | 0·055 | 6·90 ± 18·48 |
| CSNK1A1 | 6·25 ± 2·57 | 3·92 ± 2·01 | 0·0071 | 15·9 ± 15·2 |
| CSNK1D | 4·80 ± 2·03 | 3·33 ± 1·58 | 0·004 | 5·34 ± 5·6 |
| CTSD | 4·68 ± 3·53 | 2·95 ± 2·27 | 0·0354 | 8·06 ± 5·09 |
| GSK3B | 6·44 ± 2·74 | 3·91 ± 1·25 | 0·0027 | 6·89 ± 5·87 |
| IL1B | −0·28 ± 5·95 | −1·63 ± 1·39 | 0·055 | 2·72 ± 2·35 |
| LRPAP1 | 7·67 ± 2·53 | 6·27 ± 1·12 | 0·0102 | 3·77 ± 4·39 |
| MAPT | 8·15 ± 2·81 | 10·93 ± 3·93 | 0·0477 | 3·0 ± 7·15 |
| PRKCB1 | 5·8 ± 3·24 | 3·27 ± 2·04 | 0·0049 | 10·599 ± 15·49 |
| PSEN1 | 6·94 ± 2·66 | 6·15 ± 2·05 | 0·0259 | 5·1 ± 7·64 |
| SOAT1 | 8·67 ± 4·59 | 7·31 ± 2·09 | 0·044 | 7·51 ± 14·87 |
| SOD2 | 1·24 ± 4·43 | −0·74 ± 1·30 | 0·027 | 4·29 ± 3·22 |
| B Controls versus Concussion clinic patients | ||||
|---|---|---|---|---|
| ACHE | 10·84 ± 3·86 | 12·8 ± 2·4 | 0·054 | 3·57 ± 14·31 |
| APPBP1 | 9·54 ± 3·57 | 11·62 ± 1·71 | 0·0141 | 0·42 ± 0·65 |
| CAPNS2 | 2·80 ± 3·05 | −0·32 ± 2·03 | 0·0002 | 19·30 ± 167 |
| CASP6 | 7·90 ± 2·88 | 10·41 ± 3·5 | 0·0811 | 2·11 ± 3·41 |
| CDC2 | 9·62 ± 3·11 | 12·42 ± 2·73 | 0·006 | 1·83 ± 5·88 |
| CDK5R1 | 3·90 ± 3·55 | −0·69 ± 1·78 | 0·009 | 42·25 ± 41·46 |
| CHRM1 | 3·99 ± 3·13 | 0·44 ± 1·80 | 0·0011 | 23·7 ± 30·48 |
| CHRM3 | 5·22 ± 4·54 | 2·63 ± 4·7 | 0·011 | 34·97 ± 38·18 |
| CHRNA7 | 10·91 ± 4·11 | 12·87 ± 2·9 | 0·051 | 16·89 ± 67·59 |
| CSNK1A1 | 6·25 ± 2·57 | 3·4 ± 1·10 | 0·0002 | 14·0 ± 9·25 |
| CTSD | 4·68 ± 3·53 | 2·8 ± 2·69 | 0·0195 | 8·59 ± 6·55 |
| GJB1 | 3·74 ± 3·44 | −0·23 ± 1·74 | 0·0002 | 22·03 ± 18·3 |
| GRIN2A | 10·93 ± 3·79 | 13·05 ± 2·3 | 0·051 | 2·4 ± 9·8 |
| SLC18A3 | 4·18 ± 4·97 | 1·71 ± 3·36 | 0·0153 | 15·71 ± 15·92 |
| C Concussion clinic patients compared with ED head trauma patients | |||||
|---|---|---|---|---|---|
| Gene | ΔCT±SD CCPT | FC±SD CCPT | ΔCT±SD EDPT | FC±SD EDPT | EDPT vs CCPT p value |
| AGER | 8·32 ± 3·37 | 5·65 ± 7·7 | 5·92 ± 2·95 | 20·17 ± 32·9 | 0·0243 |
| APH1B | 8·79 ± 2·8 | 1·2 ± 1·22 | 6·78 ± 2·07 | 3·23 ± 4·54 | 0·0398 |
| APLP2 | 8·3 ± 3·46 | 5·5 ± 10·44 | 5·33 ± 2·34 | 17·0 ± 27·12 | 0·0159 |
| BACE2 | 5·58 ± 1·81 | 3·97 ± 7·8 | 7·10 ± 1·95 | 0·65 ± 0·75 | 0·015 |
| CAPNS2 | −0·32 ± 2·03 | 19·30 ± 16·77 | 5·45 ± 4·31 | 1·36 ± 2·22 | 0·0001 |
| CDK5R1 | −0·69 ± 1·78 | 42·2 ± 41·46 | 2·21 ± 3·10 | 10·35 ± 15·76 | 0·003 |
| CHRM1 | 0·44 ± 1·80 | 23·72 ± 30·48 | 5·42 ± 4·60 | 4·4 ± 7·5 | 0·0009 |
| CHRM3 | 2·63 ± 4·7 | 34·9 ± 38·18 | 6·18 ± 4·03 | 6·09 ± 14·2 | 0·0044 |
| CSNK1D | 4·1 ± 0·90 | 2·2 ± 1·3 | 3·33 ± 1·58 | 5·2 ± 5·7 | 0·0398 |
| GJB1 | −0·23 ± 1·74 | 22·0 ± 18·33 | 4·57 ± 3·95 | 3·23 ± 6·36 | 0·0001 |
| GSK3B | 5·92 ± 2·4 | 2·94 ± 4·39 | 3·91 ± 1·25 | 6·82 ± 5·95 | 0·0034 |
| IL1B | −0·60 ± 1·36 | 1·4 ± 1·7 | −1·63 ± 1·39 | 2·72 ± 2·35 | 0·0197 |
| IL6 | 12·5 ± 2·71 | 1·66 ± 5·13 | 9·89 ± 2·62 | 4·37 ± 14·77 | 0·0143 |
| MAPK1 | 5·48 ± 1·7 | 1·32 ± 0·95 | 4·62 ± 2·41 | 3·22 ± 2·57 | 0·0297 |
| MME | 6·64 ± 1·52 | 2·04 ± 1·86 | 5·58 ± 2·79 | 8·68 ± 16·2 | 0·0481 |
| NCSTN | 8·857 ± 1·95 | 0·84 ± 1·20 | 6·92 ± 1·62 | 2·17 ± 2·89 | 0·0023 |
| PRKCB1 | 5·44 ± 2·31 | 1·98 ± 1·92 | 3·27 ± 2·04 | 10·46 ± 15·58 | 0·0012 |
| PSEN1 | 7·72 ± 2·46 | 1·5 ± 1·09 | 6·15 ± 2·05 | 4·98 ± 7·77 | 0·0481 |
| SLC18A3 | 1·71 ± 3·36 | 15·7 ± 15·9 | 6·04 ± 4·48 | 3·13 ± 5·87 | 0·002 |
| SOAT1 | 8·82 ± 2·5 | 3·27 ± 4·9 | 7·31 ± 2·09 | 7·18 ± 15·03 | 0·0481 |
| SOD2 | 0·94 ± 1·4 | 1·33 ± 0·87 | −0·74 ± 1·30 | 4·29 ± 3·22 | 0·0018 |
| ST6GAL1 | 10·69 ± 2·84 | 1·18 ± 1·85 | 8·62 ± 3·81 | 4·40 ± 7·08 | 0·0398 |
| TNF | 9·37 ± 4·13 | 2·4 ± 3·4 | 5·95 ± 3·23 | 7·76 ± 8·04 | 0·0114 |
Note. CT: cycle threshold; FC: fold change; SD: standard deviation.
(a) Comparison between controls (n = 23) and EDPT (n = 15), columns: gene, controls average ± SD delta CT, EDPT average ± SD delta CT, p value, and average ± SD FC.
(b) Comparison between controls (n = 23) and CCPT (n = 16), Columns: gene, controls average ± SD delta CT, CCPT average ± SD delta CT, p value, and average ± SD FC.
(c) Comparison of gene expression between CCPT and EDPT. Columns: gene, CCPT average ± SD delta CT, CCPT average ± SD FC, EDPT average ± SD delta CT, EDPT average ± SD FC, and the p value. CCPT: concussion clinic patients; EDPT: ED head trauma patients.
Figure 3.
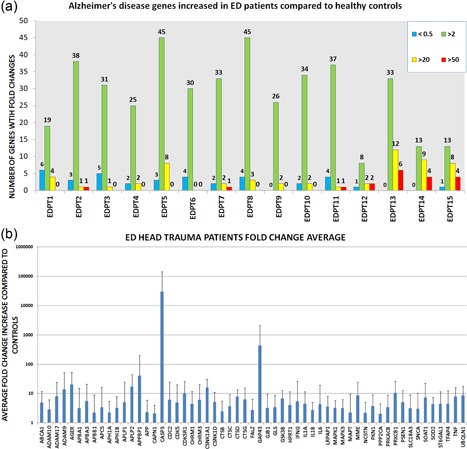
Gene expression information of ED patients. (a) Number of genes upregulated and downregulated. Upregulated gene expression in three tiers: fold increase 50‐fold higher than controls in red, 20‐fold higher in yellow, and fold change of two in green. Downregulated gene expression <0.5 in blue. (b) Genes with a two‐fold increase in gene expression or higher. Error bars represent standard deviation. ED: emergency department [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Comparison between control and chronic concussion clinic patients
Gene expression profile in outpatient clinic patients (CCPT) showed 56 genes upregulated compared with controls. Wilcoxon rank sum test identified 14 genes with a significant difference between the two groups (Table 2b), including APBB3, ACHE, CAPNS2, CDC2, CDK5R1, CHRM1, CHM3, CSNK1A1, CTSD, GJB1, IFNG, IL6, CHRNA7, GRIN2A, and SLC18A3. The number of genes upregulated in each CCPT compared with controls is demonstrated in Figure 3a. The genes of the individual patients with FC above 50, 20, or 2 is shown in Figure 4. Gene upregulation (FC > 2) ranged from 54 genes with CCPT4 to 17 genes in CCPT6. The level in gene expression from each Alzheimer's disease gene was averaged (Figure 4b).
Figure 4.
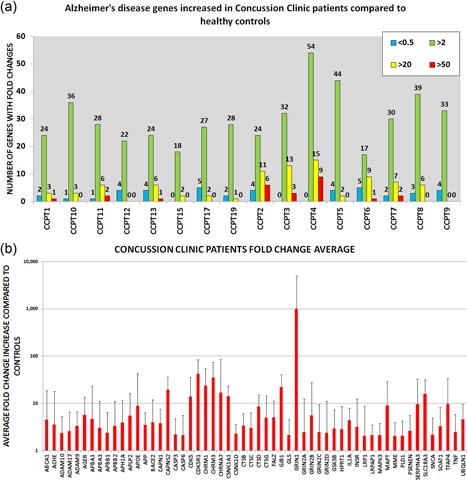
Gene expression information of concussion clinic patients. (a) Number of genes upregulated and downregulated. Upregulated genes are shown in three tiers: fold increase 50‐fold higher than controls in red, 20‐fold higher in yellow, and a fold change of two in green. Downregulated gene expression <0.5 shown in blue. (b) All the genes that had a two‐fold increase in expression or higher. Error bars represent standard deviation [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Comparison between ED patients and outpatient concussion clinic patients
Wilcoxon sum test of delta CT values shows that 23 Alzheimer's disease genes have a statistically significant difference between the two patient groups (Table 2c), including: AGER, APH1B, APLP2, BACE2, CAPNS2, CDK5R1, CHRM1, CHRM3, CSNK1D, GJB1, GSK3B, IL1B, IL6, MAPK1, MME, NCSTN, PRKCB1, PSEN1, SLC18A3, SOAT1, SOD2, ST6GAL1, and TNF. The average FC in each gene is shown in graph (Figure 5a) comparing EDPT and CCPT. Concussion clinic patients have higher upregulation of genes BACE2, CAPNS2, CDK5R1, CHRM1, CHRM3, GJB1, and SLC18A3, whereas emergency department patients have higher gene upregulation of AGER, APH1B, APLP2, CSNK1D, GSK3B, ILIB, IL6, MAPK1, MME, NCSTN, PRKCB1, PSEN1, SOAT1, SOD2, ST6GAL1, and TNF. Of the 14 genes of the CCPT and 15 genes of the EDPT that had statistically significant changes compared with controls, three were found in both group CDC2, CSNK1A1, and CTSD. Comparing the genes among the individual patients from the emergency department using the Wilcoxon test showed no statistical significance, p > 0.05. EDPT FC was CDC2 (6.1 ± 18.75), CSNK1A1 (15.78 ± 15.84), and CTSD (7.86 ± 5.39). CCPT FC was CDC2 (1.83 ± 5.88), CSNK1A1 (14.0 ± 9.25), and CTSD (8.5 ± 6.5) (Figure 5b).
Figure 5.
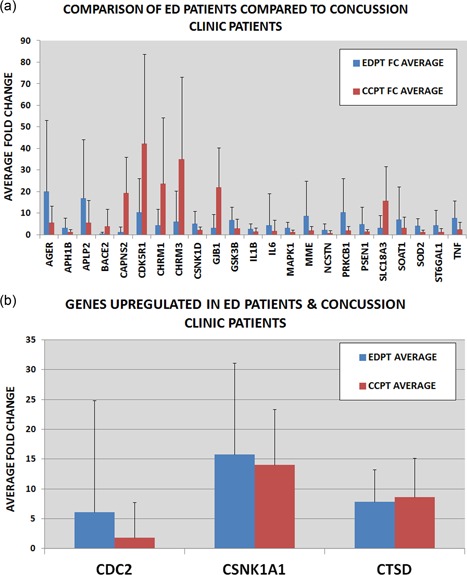
Upregulated Genes in experimental groups. Wilcoxon analysis was done comparing delta CT of EDPT and CCPT. Twenty‐three Alzheimer's disease genes significantly (p < 0.05) changed in EDPT (n = 15) compared with CCPT (n = 16). (a) Fold change of significant genes of each patient is compared. (b) Three genes found in both patient groups. No statistical difference of three genes between EDPT and CCPT (p > 0.05) [Color figure can be viewed at wileyonlinelibrary.com]
4. CONCLUSIONS
There are no biomarkers to help diagnose mTBI or that can predict poor sequelae, such as PCS or CTE. Current biomarkers of brain injuries are obtained from serum or CSF, which is not easily accessible, and focus on more severe head injuries that have associated radiographic abnormalities. On the other hand, identifying a noninvasive biomarker, such as saliva, that can diagnose a concussion or that can identify those at risk for sequelae such as a prolonged recovery or postconcussive syndrome, Alzheimer's disease, or CTE is an exciting possibility. This is the first study isolating EVs from saliva to identify potential biomarkers for mTBI. In this study, we successfully isolated and identified EVs from saliva as confirmed by TEM and NanoSight analysis of EVs morphology and size and we were able to detect the EVs size distribution and concentration through NanoSight (Figure 2a–2d). Circulating EVs contain proteins and RNAs, such as mRNA and miRNA (Quesenberry, Aliotta, Deregibus, & Camussi, 2015). Several studies have suggested using exosome biomarkers for disease diagnosis (Lau & Wong, 2012; Michael et al., 2010; Valadi, Ekstrӧm, Bossios, Sjӧstrand, & Lee, 2007). The use of exosome cargo as possible markers for disease is a new area of research and EVs to diagnose dementia has been explored previously by Schneider et al. (2018), and Goetzl et al. (2016).
CTE is a constellation of cognitive, mood, personality, and behavioral alterations that can develop following a single incident or repeated episodes of mTBI (Gavett et al., 2011; Jordan, 2000; Mendez, 1995). CTE currently can only be diagnosed at autopsy and in vivo biomarker studies are lacking as are longitudinal studies (Asken, Sullan, DeKosky, Jaffee, & Bauer, 2017). CTE features include extensive tau neurofibrillary tangles, amyloid beta plaques, and some macroscopic abnormalities, such as cerebral atrophy and enlarged ventricles as seen in Alzheimer's disease (Gavett et al., 2010). Although the clinical definition is much debated, there is clear overlap between CTE and TBI‐induced dementia as experienced by professional boxers (Zetterberg, Smith, & Blennow, 2013), retired football players, soccer players, hockey, and wrestlers (Gavett et al., 2010). On this same spectrum of cognitive decline, one could also include Alzheimer's disease, and its cardinal findings of neurofibrillary tangles, tau and amyloid plaques—a striking pathology often found in professional boxers with CTE (Roberts, Allsop, & Bruton, 1990; Tokuda, Ikeda, Yanagisawa, Ihara, & Glenner, 1991). Studies of individuals who died after a TBI event had amyloid plaques present in all age groups (Johnson, Stewart, & Smith, 2010; Ramos‐Cejudo et al., 2018). However, among patients who died from nonneurological causes, plaques were only seen in elderly individuals (Johnson et al., 2010; Ramos‐Cejudo et al., 2018). Therefore, TBI or repeated head injuries (mTBI) is a strong risk factor for both CTE and Alzheimer's disease (Gavett et al., 2010; Sivanandam & Thakur, 2012). Because of this association, the TaqMan® human Alzheimer's disease array was used to profile gene expressions in our patient samples. The selected genes that were identified are involved in amyloid precursor protein (APP) processing and are implicated in multiple secondary steps of Aβ aggregation, tau hyperphosphorylation, excitotoxicity, inflammation, apoptosis, oxidation, and microglial activation.
We identified 15 Alzheimer's disease‐associated mRNAs that had significant expression changes in salivary EVs isolated from ED patients when compared with controls and 14 Alzheimer's disease‐associated mRNAs in outpatient concussion clinic patients compared with controls (Table 2a,b). There were three genes that were common in both patient groups CDC2, cathepsin D (CTSD), and CSNK1A1. CTSD is associated with pathways involved in plaque formation and APP metabolism and was present in 12 out of the 15 ED patients and 15 out of 16 concussion clinic patients with an FC higher than two when compared with controls (p < 0.05). CSNK1A1 was present in 13 out of 15 ED patients and 16 out of 16 concussion clinic patients with an FC higher than two when compared with controls (p < 0.05). This is a casein kinase which is involved in the phosphorylation state of tau, a component of neurofibrillary tangles and plays a key role in the pathology to Alzheimer's disease and cell death. Both CTSD and casein kinase (CSNK1A1) are potential candidates for determination of head trauma and likely concussion. Future studies will correlate the levels of these two candidate biomarkers with neurocognitive testing.
Genes associated with Alzheimer's disease have also been associated with other cerebral/neuronal injury (Ramos‐Cejudo et al., 2018; White et al., 2016). We assayed a number of Alzheimer's disease‐related genes that also play a role in neuronal injury, including CAPN1 (Saatman, Creed, & Raghupathi, 2010), CDK5R1 (Dekker et al., 2014), CDK5 (Yousuf et al., 2016), MAPT (Raghupathi, 2004), GSK3B (White et al., 2016), and CASP3 (Raghupathi, 2004; White et al., 2016), which were all upregulated (FC > 2) in the patient populations. These genes are involved in the formation of neurofibrillary tangles and cell death associated with Alzheimer's disease. Also, of interest was the portion of the Alzheimer's disease pathway that is involved in CDK5 deregulation. Many genes involved in the deregulation of CDK5 aspect of the Alzheimer's disease pathway were upregulated in both subacute patients from the concussion clinic and acutely head injured patients from the ED. The genes involved in the deregulation of CDK5 within the Alzheimer's disease pathway that were upregulated (FC > 2) in the concussion clinic patients: CDK5R1 (FC = 44.9) CDK5 (FC = 27.83), GSK3B (FC = 5.54), CAPN1 (FC = 5.13), CAPNS2 (FC = 20.5), CSNK1A1 (FC = 14.0), and MAPT (FC = 23.85). The genes involved in the deregulation of CDK5 that were upregulated (FC > 2) in the ED patient group are CDK5(FC = 18.58), CDK5R1(FC = 12.83), GSK3B(FC = 7.72), CAPN1(FC = 3.81), CSNK1A1(FC = 18.21), and CSNK1D(FC = 6.62). Current research has demonstrated aberrant CDK5 expression with TBI. CDK5 knockout mice subjected to controlled cortical impact show significantly less injury compared to wild type mice (Yousuf et al., 2016). CDK5 is consistently elevated in mice subjected to cortical impact (Yousuf et al., 2016) and in hypoxia/ischemic brain injury in rats (Tan et al., 2015). This pathway becomes of interest for human TBI diagnosis and for possible therapeutic targets.
The salivary markers we have identified have established physiological roles in the pathogenesis of neurodegenerative disease, such as Alzheimer's disease, a disease with a multitude of pathophysiological, and clinical correlations with TBI (Y. S. Hu, Xin, Hu, Zhang, & Wang, 2017). Clinically, collecting salivary EVs is a simple and noninvasive process. In addition, EVs are membrane bound, and are therefore not subject to the same degradation that conventional serum biomarkers face. Salivary EVs, in particular, can be isolated based on tissue specificity and have well‐established roles in the detection of numerous other disease states, including oral squamous cell carcinoma (Tang, Wu, Zhang, & Su, 2013). Grading and stratifying TBI severity are routinely based on very subtle examination and neuroimaging findings, which are increasingly difficult to identify acutely (Papa, Edwards, & Ramia, 2015). Salivary EVs may circumvent this and ultimately allow for early diagnosis, as well as stratification of TBI at a time when intervention may dictate prognosis. Another clinical potential of salivary EVs would be to isolate their chemical cargo and monitor therapeutic responses to interventions by scanning for signals associated with neural regeneration or neural degeneration; thus, alerting clinicians to patients that warrant more aggressive therapy earlier in the course of recovery.
Limitations of this study include the cross‐sectional design. While samples were collected only once in each patient group, the use of the acute ED head injury and a subacute/chronic symptomatic concussed group provided inferential data on the longitudinal course of mTBI. A prospective study of patients with mTBI, obtaining repeated samples over weeks and months could provide data on intrasubject patterns of post‐TBI gene expression. Another limitation is that it has not been definitively shown that a single mTBI can be a precursor to CTE. Although the Alzheimer's disease panel appears to be a potential marker for mTBI and PCS, whether the Alzheimer's disease panel is an indicator of potential future CTE is not known or addressed in our study.
In this study, we have provided evidence that salivary EVs serve as a minimally invasive and reliable source for human mTBI‐biomarkers. The patterns of candidate biomarkers might indicate current risk factors for PCS, and their expression might be an indication of symptomatic and neurophysiologic recovery after mTBI. Delineating the evolution of salivary EVs gene expression after head trauma or diagnosis of concussion will be necessary for a fuller understanding of the significance to these elevated gene expression patterns.
We assert that the determination of mRNA expression on the Taqman® Alzheimer's disease array may be a valid measure of concussion risk. Larger, longitudinal studies over time will be necessary to determine their overall value in patients with mTBI.
FUNDING INFORMATION
This project was supported by the National Heart, Lungs, and Blood Institute Grant #T32HL116249. Additional support from the National Institute of General Medical Sciences of the NIH through grant (COBRE) #P20GM103468 Flow Cytometry Core, NIH Commonfund grant #5UH3TROOO880‐05 and institutional support through the Division of Hematology/oncology, Rhode Island Hospital, Providence, RI.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Y.C. conducted the study conception and design, data acquisition, analysis, and interpretation, drafting and revision of the manuscript, accountability for accuracy and integrity of the data. M.P., M.D., T.B., L.G., M.D. and G.C. were responsible for data acquisition, analysis, and interpretation, revision, and approval of the manuscript. N.R. and J.R. were responsible for subject enrollment, data acquisition, revision and approval of the manuscript. W.L., M.Q. and B.R. performed data interpretation, revision and approval of the manuscript. P.J.Q. was responsible for study conception and design; data acquisition, analysis, and interpretation, revision of the manuscript; accountability for accuracy and integrity of the data.
Supporting information
Supporting Information
Cheng Y, Pereira M, Raukar N, et al. Potential biomarkers to detect traumatic brain injury by the profiling of salivary extracellular vesicles. J Cell Physiol. 2018;234:14377–14388. 10.1002/jcp.28139
References
REFERENCES
- Asken, B. M. , Sullan, M. J. , DeKosky, S. T. , Jaffee, M. S. , & Bauer, R. M. (2017). Research gaps and controversies in chronic traumatic encephalopathy a review. JAMA Neurol, 74(10), 1255–1262. [DOI] [PubMed] [Google Scholar]
- Becker, R. E. , Kapogiannis, D. , & Greig, N. H. (2018). Does traumatic brain injury hold the key to the Alzheimer's disease puzzle? Alzheimer's & Dementia, 14, 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash, P. K. , Zhao, J. , Hergenroeder, G. , & Moore, A. N. (2010). Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics, 7, 100–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker, S. E. , Bambakidis, T. , Sillesen, M. , Liu, B. , Johnson, C. N. , Jin, G. , … Alam, H. B. (2014). Effect of pharmacologic resuscitation on the brain gene expression profiles in a swine model of traumatic brain injury and hemorrhage. Journal of Trauma and Acute Care Surgery, 77(6), 906–912. [DOI] [PubMed] [Google Scholar]
- Gavett, B. E. , Stern, R. A. , Cantu, R. C. , Nowinski, C. J. , & McKee, A. C. (2010). Mild traumatic brain injury: A risk factor for neurodegeneration. Alzheimer's research & therapy, 2(3), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett, B. E. , Cantu, R. C. , Shenton, M. , Lin, A. P. , Nowinski, C. J. , McKee, A. C. , & Stern, R. A. (2011). Clinical appraisal of chronic traumatic encephalopathy: Current perspectives and future directions. Current Opinion in Neurology, 24, 525–531. [DOI] [PubMed] [Google Scholar]
- Goetzl, E. J. , Mustapic, M. , Kapogiannis, D. , Eitan, E. , Lobach, I. V. , Goetzl, L. , … Miller, B. L. (2016). Cargo proteins of plasma astrocyte‐derived exosomes in Alzheimer's disease. The FASEB Journal, 30(11), 3853–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales, P. A. , Pisitkun, T. , Hoffert, J. D. , Tchapyjnikov, D. , Star, R. A. , Kleta, R. , … Knepper, M. A. (2009). Large‐scale proteomics and phosphoproteomics of urinary exosomes. Journal of the American Society of Nephrology, 20(2), 363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Begne, M. , Lu, B. , Han, X. , Hagen, F. K. , Hand, A. R. , Melvin, J. E. , & Yates, J. R. (2009). Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT). Journal of Proteome Research, 8, 1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg, L. T. , Anghinah, R. , Nascimento, C. F. , Amaro, E. , Leite, R. P. , Martin Mda, G. , … Nitrini, R. (2016). Chronic Traumatic Encephalopathy Presenting as Alzheimer's Disease in a Retired Soccer Player. Journal of Alzheimer's Disease, 54, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka, M. T. , Carson, M. J. , El Khoury, J. , Landreth, G. E. , Brosseron, F. , Feinstein, D. L. , … Herrup, K. (2015). Neuroinflammation in Alzheimer's disease. The Lancet Neurology, 14(4), 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S. , Arellano, M. , Boontheung, P. , Wang, J. , Zhou, H. , Jiang, J. , … Wong, D. T. (2008). Salivary proteomics for oral cancer biomarker discovery. Clinical Cancer Research, 14(19), 6246–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y. S. , Xin, J. , Hu, Y. , Zhang, L. , & Wang, J. (2017). Analyzing the genes related to Alzheimer's disease via a network and pathway‐based approach. Alzheimer's research & therapy, 9(1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, V. E. , Stewart, W. , & Smith, D. H. (2010). Traumatic brain injury and amyloid‐β pathology: A link to Alzheimer's disease? Nature Reviews Neuroscience, 11(5), 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, B. D. (2000). Chronic traumatic brain injury associated with boxing. Seminars in Neurology, 20, 179–185. [DOI] [PubMed] [Google Scholar]
- Julien, J. , Joubert, S. , Ferland, M. C. , Frenette, L. C. , Boudreau‐Duhaime, M. M. , Malo‐Véronneau, L. , & De Guise, E. (2017). Association of traumatic brain injury and Alzheimer disease onset: A systematic review. Annals of pHysical Aand Rehabilitation Medicine, 60(5), 347–356. [DOI] [PubMed] [Google Scholar]
- Kapsogeorgou, E. K. , Abu‐Helu, R. F. , Moutsopoulos, H. M. , & Manoussakis, M. N. (2005). Salivary gland epithelial cell exosomes: A source of autoantigenic ribonucleoproteins. Arthtitis and Rheumatism, 52, 1517–1521. [DOI] [PubMed] [Google Scholar]
- Kochanek, P. M. , Berger, R. P. , Bayir, H. , Wagner, A. K. , Jenkins, L. W. , & Clark, R. S. (2008). Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: Diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Current Opinion in Critical Care, 14, 135–141. [DOI] [PubMed] [Google Scholar]
- Lakkaraju, A. , & Rodriguez‐Boulan, E. (2008). Itinerant exosomes: Emerging roles in cell and tissue polarity. Trends in Cell Biology, 18, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, C. S. , & Wong, D. T. (2012). Breast cancer exosome‐like microvesicles and salivary gland cells interplay alters salivary gland cell‐derived exosome‐like microvesicles in vitro. PLoS One, 7, e33037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee, A. C. , Cantu, R. C. , Nowinski, C. J. , Hedley‐Whyte, E. T. , Gavett, B. E. , Budson, A. E. , … Stern, R. A. (2009). Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. Journal of Neuropathology & Experimental Neurology, 68(7), 709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez, M. F. (1995). The neuropsychiatric aspects of boxing. International Journal of Psychiatry in Medicine, 25, 249–262. [DOI] [PubMed] [Google Scholar]
- Mendez, M. F. , Paholpak, P. , Lin, A. , Zhang, J. Y. , & Teng, E. (2015). Prevalence of Traumatic Brain Injury in Early Versus Late‐Onset Alzheimer's Disease. Journal of Alzheimer's Disease, 47, 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mez, J. , Daneshvar, D. H. , Kiernan, P. T. , Abdolmohammadi, B. , Alvarez, V. E. , Huber, B. R. , … Cormier, K. A. (2017). Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. Journal of the American Medical Association, 318(4), 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael, A. , Bajracharya, S. D. , Yuen, P. S. , Zhou, H. , Star, R. A. , Illei, G. G. , & Alevizos, I. (2010). Exosomes from human saliva as a source of microRNA biomarkers. Oral Diseases, 16(1), 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, P. J. , Welton, J. , Staffurth, J. , Mason, M. D. , Tabi, Z. , & Clayton, A. (2009). Can urinary exosomes act as treatment response markers in prostate cancer? Journal of Translational Medicine, 7(1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazesh, M. (1993). Methods for collecting saliva. Annals of the New York Academy of Sciences, 694, 72–77. [DOI] [PubMed] [Google Scholar]
- Papa, L. , Edwards, D. , & Ramia, M. (2015). Exploring Serum Biomarkers for Mild Traumatic Brain Injury In Kobeissy F. H. (Ed.), Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Frontiers in Neuroengineering. CRC Press/Taylor & Francis; Chapter 22. [Google Scholar]
- Papa, L. , Brophy, G. M. , Welch, R. D. , Lewis, L. M. , Braga, C. F. , Tan, C. N. , … Silvestri, S. (2016). Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH‐L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA neurology, 73(5), 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins, M. , Greco, T. , Alexander, D. , & Giza, C. C. (2013). The pathophysiology of traumatic brain injury at a glance. Disease Models & Mechanisms, 6(6), 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry, P. J. , Aliotta, J. , Deregibus, M. C. , & Camussi, G. (2015). Role of extracellular RNA‐carrying vesicles in cell differentiation and reprogramming. Stem Cell Research & Therapy, 6, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathi, R. (2004). Cell death mechanisms following traumatic brain injury. Brain Pathology, 14(2), 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos‐Cejudo, J. , Wisniewski, T. , Marmar, C. , Zetterberg, H. , Blennow, K. , de Leon, M. J. , & Fossati, S. (2018). Traumatic brain injury and Alzheimer's disease: The cerebrovascular link. EBioMedicine., 28, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, G. W. , Allsop, D. , & Bruton, C. (1990). The occult aftermath of boxing. Journal of Neurology, Neurosurgery, and Psychiatry, 53, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg, J. , Spader, H. E. , Wilcox, B. J. , Ellermeier, A. , Correia, S. T. , Chodobski, A. D. , & Lafrance, W., Jr (2014). The Brown University Traumatic Brain Injury Research Consortium and the Norman Prince Neurosciences Institute. Rhode Island Medical Journal, 96(3), 22–26. [PubMed] [Google Scholar]
- Saatman, K. E. , Creed, J. , & Raghupathi, R. (2010). Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics, 7(1), 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, R. , McKeever, P. , Kim, T. , Graff, C. , van Swieten, J. C. , Karydas, A. , … Robertson, J. (2018). Downregulation of exosomal miR‐204‐5p and miR‐632 as a biomarker for FTD: A GENFI study. Journal of Neurology, Neurosurgery, and Psychiatry, 89(8), 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanandam, T. M. , & Thakur, M. K. (2012). Traumatic brain injury: A risk factor for Alzheimer's disease. Neuroscience and Biobehavioral Reviews, 36, 1376–1381. [DOI] [PubMed] [Google Scholar]
- Skog, J. , Wurdinger, T. , van Rijn, S. , Meijer, D. , Gainche, L. , Sena‐Esteves, M. , … Breakfield, X. O. (2008). Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature Cell Biology, 10, 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlov, S. I. , Larner, S. F. , Kirk, D. R. , Atkinson, J. , Hayes, R. L. , & Wang, K. K. (2009). Biomarkers of blast‐induced neurotrauma: Profiling molecular and cellular mechanisms of blast brain injury. Journal of Neurotrauma, 26, 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, X. , Chen, Y. , Li, J. , Li, X. , Miao, Z. , Xin, N. , … Xu, X. (2015). The inhibition of Cdk5 activity after hypoxia/ischemia injury reduces infarct size and promotes functional recovery in neonatal rats. Neuroscience, 290, 552–560. [DOI] [PubMed] [Google Scholar]
- Tang, H. , Wu, Z. , Zhang, J. , & Su, B. (2013). Salivary lncRNA as a potential marker for oral squamous cell carcinoma diagnosis. Molecular Medicine Reports, 7, 761–766. [DOI] [PubMed] [Google Scholar]
- Taylor, C. A. , Greenspan, A. I. , Xu, L. , & Kresnow, M. J. (2015). Comparability of national estimates for traumatic brain injury‐related medical encounters. The Journal of Head Trauma Rehabilitation, 30, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda, T. , Ikeda, S. , Yanagisawa, N. , Ihara, Y. , & Glenner, G. G. (1991). Re‐examination of ex‐boxers' brains using immunohistochemistry with antibodies to amyloid beta‐protein and tau protein. Acta Neuropathologica, 82, 280–285. [DOI] [PubMed] [Google Scholar]
- Valadi, H. , Ekstrӧm, K. , Bossios, A. , Sjӧstrand, M. , Lee, J. J. , & Lӧtvall, J. O. (2007). Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9, 654–659. [DOI] [PubMed] [Google Scholar]
- White, T. E. , Surles‐Zeigler, M. C. , Ford, G. D. , Gates, A. S. , Davids, B. , Distel, T. , … Ford, B. D. (2016). Bilateral gene interaction hierarchy analysis of the cell death gene response emphasizes the significance of cell cycle genes following unilateral traumatic brain injury. BMC Genomics, 17(1), 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokobori, S. , Hosein, K. , Burks, S. , Sharma, I. , Gajavelli, S. , & Bullock, R. (2013). Biomarkers for the clinical differential diagnosis in traumatic brain injury‐‐a systematic review. CNS Neuroscience & Therapeutics, 19, 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf, M. A. , Tan, C. , Torres‐Altoro, M. I. , Lu, F. M. , Plautz, E. , Zhang, S. , … Bibb, J. A. (2016). Involvement of aberrant cyclin‐dependent kinase 5/p25 activity in experimental traumatic brain injury. Journal of Neurochemistry, 138(2), 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg, H. , Smith, D. H. , & Blennow, K. (2013). Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nature Reviews Neurology, 9, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, L. , Taylor, D. L. , & Whittington, R. J. (2010). Proteomic profiling of ovine serum by SELDI‐TOF MS: Optimisation, reproducibility and feasibility of biomarker discovery using routinely collected samples. Comparative Immunology, Microbiology and Infectious Diseases, 33, 47–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


