Abstract
An important step in many pathological conditions, particularly tissue and organ fibrosis, is the conversion of relatively quiescent cells into active myofibroblasts. These are highly specialized cells that participate in normal wound healing but also contribute to pathogenesis. These cells possess characteristics of smooth muscle cells and fibroblasts, have enhanced synthetic activity secreting abundant extracellular matrix components, cytokines and growth factors and are capable of generating contractile force. As such, these cells have become potential therapeutic targets in a number of disease settings. TGF-β is a potent stimulus of fibrosis and myofibroblast formation and likewise is an important therapeutic target in several disease conditions. The plant-derived isothiocyanate sulforaphane has been shown to have protective effects in several pathological models including diabetic cardiomyopathy, carcinogenesis and fibrosis. These studies suggest that sulforaphane may be an attractive preventive agent against disease progression, particularly in conditions involving alterations of the extracellular matrix and activation of myofibroblasts. However, few studies have evaluated the effects of sulforaphane on cardiac fibrobroblast activation and their interactions with the extracellular matrix. The present studies were carried out to determine the potential effects of sulforaphane on the conversion of quiescent cardiac fibroblasts to an activated myofibroblast phenotype and associated alterations in signaling, expression of extracellular matrix receptors and cellular physiology following stimulation with TGF-β1. These studies demonstrate that sulforaphane attenuates TGF-β1-induced myofibroblast formation and contractile activity. Sulforaphane also reduces expression of collagen-binding integrins and inhibits canonical and non-canonical TGF-β signaling pathways.
Keywords: Fibroblast, Extracellular matrix, Sulforaphane, TGF-β1
Introduction
Fibrosis resulting from an imbalance between deposition and degradation of extracellular matrix (ECM) components plays an important role in the progression of organ dysfunction in many pathological conditions. Excessive deposition of ECM alters the structural and biomechanical properties of tissues and interferes with organ function and homeostasis. An important step in fibrosis is the activation of quiescent fibroblasts or other cell types into an active myofibroblast phenotype. Myofibroblasts are highly specialized cells that participate in normal wound healing but also contribute to pathological conditions including fibrosis and cancer (for recent reviews see Gerarduzzi and Di Battista, 2017, Carthy, 2018; Pakshir and Hinz, 2018). These cells possess characteristics of smooth muscle cells and fibroblasts, have enhanced synthetic activity secreting abundant extracellular matrix components, cytokines and growth factors and are capable of generating contractile force. Accompanying differentiation of myofibroblasts are alterations in integrin expression and interactions of the cells with the extracellular matrix (Talior-Volodarsky et al., 2012; Fausther and Dranoff, 2014).
Members of the TGF-β superfamily are among the most potent stimuli of fibrosis and myofibroblast formation described to date and have become attractive therapeutic targets for fibrotic conditions (Carthy, 2018). Activation of the SMAD-2/3 canonical TGF-β signaling pathway regulates the expression of a number of profibrotic genes and promotes the differentiation or activation of quiescent fibroblasts into a myofibroblast phenotype. In addition, TGF-β stimulates epithelial-to-mesenchymal transformation, which contributes to increased myofibroblast population and the profibrotic response (Weng et al., 2018). Our understanding of the interactions between TGF-β signaling and oxidative stress in mediation of fibroblast activation and fibrosis are evolving. TGF-β enhances the production of NADPH oxidases and generation of reactive oxygen species (ROS) (Abe et al., 2013; Hagler et al., 2013). Several studies have illustrated a role for NADPH oxidases and ROS in mediating the effects of TGF-β on fibroblast activation and fibrosis (Albright et al., 2003).
Nrf2 is a member of the cap ‘n’ collar family of basic leucine zipper transcription factors that controls the expression of over two hundred genes via the antioxidant response element. Nrf2 has broad-ranging functions via the regulation of the expression of diverse genes including phase II detoxifying enzymes, scavenger receptors, chaperone proteins and others. Nrf2 has been shown to be cardioprotective in animal models and to alleviate diabetic cardiomyopathy (Qu et al., 2015; He et al., 2018). The potential interactions between Nrf2 and TGF-β are complex and the modalities whereby they interact are not well known (Arfmann-Knubel et al., 2015). A number of studies have illustrated antagonistic interactions between Nrf2 and TGF-β (Huang et al., 2017; Tang et al., 2017). In contrast, both TGF-β and Nrf2 appear to promote a migratory phenotype in some cell types including cancerous cells. Recent studies have illustrated an additive effect of Nrf2 and TGF-β in wound healing and epithelial-to-mesenchymal transition (Arfmann-Knubel et al., 2015). These studies illustrate the complexity between Nrf2 and TGF-β interactions and likely illustrate cell type and physiologically-specific effects.
Sulforaphane is an isothiocyanate found in cruciferous plants that has been used as an anti-cancer drug and acts in part through activation of Nrf2 (Lan et al., 2016). This compound has also been demonstrated to protect the heart from doxorubicin-induced toxicity and to alleviate diabetic cardiomyopathy (Zhang et al., 2014; Singh et al., 2015). While sulforaphane has been show to be anti-fibrotic in animal models (Sun et al., 2016), little is known about the effects of sulforaphane on activation of cardiac fibroblasts or their interactions with the ECM. The present studies were carried out to assess the effects of sulforaphane on TGF-β-induced fibroblast activation, ECM remodeling and expression of genes involved in these processes.
Materials and Methods
Cell isolation and culture –
Fibroblasts were isolated from adult (8–10 weeks of age) male Sprague Dawley rat hearts as previously described (Carver et al., 1995). Briefly, rats were received from Harlan Laboratories and allowed to acclimate in the University of South Carolina School of Medicine animal facility for 24 to 48 hours. Animals were euthanized by cervical dislocation while under anesthesia from isoflurane inhalation. Hearts were removed and rinsed in sterile saline. Cardiac tissue was minced and digested with Liberase TM (Sigma-Adrich). Fibroblasts from the resulting tissue digestion were purified by differential adhesion to tissue culture plastic. Fibroblasts were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum and antibiotics. At approximately 80 percent confluence, fibroblasts were passaged following incubation in a solution containing trypsin/ethylenediaminetetraacetic acid. Cells were used in bioassays prior to the fifth passage as cardiac fibroblast phenotype has been shown to be preserved at low passage numbers. Prior to utilization in bioassays, cells were rinsed in Moscona’s saline solution and culture continued in DMEM containing 1.5% fetal bovine serum (low serum medium) for 24 hours. Cells were cultured in low serum concentrations prior to bioassays to reduce spontaneous conversion to a myofibroblast phenotype in response to serum components.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) –
Fibroblasts were cultured for 48 hours in the presence of 0 or 5 ng/ml TGF-β1 (R&D Systems, catalog number 101B1001) with 0, 10 or 20 μM sulforaphane (Sigma-Aldrich, catalog number S4441), concentrations that have previously been shown to be effective and non-toxic (Higgins et al., 2009). Cells were extracted in TriZol reagent (Invitrogen) and RNA purified using RNeasy MiniPrep columns (Qiagen). cDNA was produced and qRT-PCR carried out using primers specific to rat heme oxygenase and superoxide dismutase. Expression was normalized to expression of acidic ribosomal binding protein (ARBP) and data presented as the expression of the target mRNA following TGF-β1 and/or sulforaphane treatment versus culture with only vehicle (dimethylsulfoxide). Specific primers used are provided in Table 1.
Table 1.
qRT-PCR primer sequences
| RNA Target | Primer Sequence |
|---|---|
| Heme oxygenase 1 | 5’ – ACAGCACTACGTAAAGCGTCTCCA – 3’ |
| 5’ – CATGGCCTTCTGCGCAATCTTCTT – 3’ | |
| Superoxide dismutase 1 | 5’ – GGTGTGGCCAATGTGTCCATTGAA – 3’ |
| 5’ – CGGCTTCCAGCATTTCCAGTCTT – 3’ | |
| Acidic ribosomal binding protein | 5’ – TAGAGGGTGTCCGCAATG – 3’ |
| 5’ – GAAGGTGTAGTCAGTCTC – 3’ |
Collagen gel contraction –
Fibroblasts were cultured for 24 hours in medium containing 1.5% fetal bovine serum and antibiotics (low serum medium) as indicated above. Fibroblasts were subsequently trypsinized, centrifuged and resuspended in low serum medium. Cells were added to bovine collagen type I (PureCol; Advanced BioMatrix, Inc., catalog number 5005001ML) at a ratio of 100,000 cells per milliliter of collagen and a final collagen concentration of 1.2 milligrams per milliliter. This mixture was allowed to polymerize at 37oC for one hour, then the 3-dimensional collagen scaffolds were dislodged from the plastic wells by addition of 1 milliliter of low serum medium containing 0 or 5 ng/ml TGF-β1 with varying concentrations of sulforaphane (0, 10 or 20 μM). Culture was continued for 48 hours after which the perimeter of the collagen gels was measured as an indicator of the ability of cells to remodel and contract the 3-dimensional scaffolds.
Immunoblotting –
For analysis of integrin and α-smooth muscle actin expression by immunoblotting, fibroblasts were cultured for 24 hours in low serum medium. Culture was then continued for 48 hours in the presence of 0 or 5 ng/ml TGF-β1 with varying concentrations of sulforaphane (0, 10 or 20 μM) in low serum medium. Cells were rinsed with phosphate-buffered saline and incubated in RIPA solution (150 mM sodium chloride, 1% Triton × 100, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 1.5 mM ethylenediaminetetraacetic acid, 50 mM Tris, pH 8.0) containing Complete Mini Protease Inhibitor Cocktail (Sigma-Aldrich, catalog number 11836153001) for 5 minutes. Lysates were centrifuged to remove cellular debris and total protein concentration determined with the bicinchronic acid (BCA) protein assay (Thermo Scientific). Proteins were separated by sodium dodecyl sulfate – polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose. Nitrocellulose was rinsed in Tris-buffered saline containing TWEEN 20 (TBS-T) and blocked in TBS-T containing 5% powdered milk. Nitrocellulose was rinsed in TBS-T and incubated in primary antiserum overnight at 4oC. Specific antisera utilized are listed in Table 2. Nitrocellulose was rinsed and incubated 1–2 hours in horse radish peroxidase-conjugated secondary antibodies. Following additional rinses in TBS-T, immunoblots were developed with the SuperSignal West Pico ECL Substrate (Thermo Scientific, catalog number 34578) and exposed to x-ray film. Images were captured using a BioRad GelDoc system and quantified with Alpha Innotech AlphaView software. Signals with proteins of interest were normalized to signals with anti-glyceraldehyde phosphate dehydrogenase and data presented as fold of vehicle-treated controls.
Table 2.
Antibody information
| Antibody | Company and Catalog Number |
|---|---|
| β1 integrin | Millipore, AB1952 |
| α1 integrin | Millipore, AB1934 |
| α2 integrin | Millipore, AB 1936 |
| α11 integrin | Millipore, AB6031 |
| Type I collagen | Santa Cruz, sc-8784 |
| Type III collagen | Santa Cruz, sc-28888 |
| Fibronectin | Santa Cruz, sc-6953 |
| Periostin | Santa Cruz, sc-49480 |
| Glyceraldehyde phosphate dehydrogenase | Santa Cruz, sc-20357 |
| Matrix metalloproteinase 2 | Santa Cruz, sc-10736 |
| Matrix metalloproteinase 9 | Millipore, AB-19016 |
| SMAD 2 | Cell Signaling, 5339S |
| p-SMAD 2 | Cell Signaling, 3108S |
| Erk 1/2 | Cell Signaling, 4370S |
| p-Erk 1/2 | Cell Signaling, 4695S |
| p38 | Cell Signaling, 8690S |
| p-p38 | Cell Signaling, 4511S |
| JNK | Cell Signaling, 9252S |
| p-JNK | Cell Signaling, 4668S |
For analysis of TGF-β-related signaling pathways, fibroblasts were cultured for 24 hours in low serum medium as indicated above. Due to the rapidity of signal transduction pathway activation in response to TGF-β1, cells were pretreated for 24 hours with varying doses of sulforaphane (0, 10 or 20 μM). At the end of the pretreatment period, fibroblasts were treated with 5 ng/ml of TGF-β1 for 1 hour in the continued presence of sulforaphane. Fibroblasts were collected by centrifugation and the cell pellet rinsed in PBS. The pelleted cells were then dissolved in M-PER mammalian protein extraction reagent (Thermo Scientific, catalog number 78501) with Complete Mini Protease Inhibitor Cocktail (Sigma-Aldrich, catalog number 11836153001) at room temperature. The cell lysate was centrifuged at 15,000 rpm, the supernatant collected and total protein concentration determined using the BCA protein assay. Protein samples were resolved by SDS-PAGE using 4–20% gradient gels (BioRad Laboratories). Proteins were transferred electrophoretically to nitrocellulose membranes and subsequently probed with primary antisera to SMAD2, pSMAD2, p38, pp38, JNK, pJNK, ERK1/2 or pERK1/2. Membranes were extensively rinsed and incubated in horseradish peroxidase-conjugated anti-rabbit or anti-mouse serum as appropriate. Immunoblots were developed with Clarity Western ECL detection reagent (BioRad Laboratories) and exposed to X-OMAT AR films (Eastman Kodak). Immunoblots were also incubated with anti-β-actin monoclonal serum as a loading control. Data are presented as the intensity of signal with antiserum to the phosphorylated protein relative to its respective non-phosphorylated form.
Immunohistochemical Staining –
As an indicator of myofibroblast formation, cells were stained with antisera to α-smooth muscle actin and percentage of α-smooth muscle actin-positive cells determined. Cells were cultured on laminin-coated coverslips and treated as indicated above in low serum medium with 0 or 5 ng/ml TGF-β1 and 0, 10 or 20 mM sulforaphane. Following 48 hours of treatment, cells were rinsed in phosphate-buffered saline (PBS) and fixed in 2% paraformaldehyde for 10 minutes. Cells were rinsed in PBS, blocked in normal serum and incubated in anti-α-smooth muscle actin overnight at 4oC. Cells were rinsed again in PBS and incubated in fluorescein isothiocyanate-conjugated secondary antiserum. Coverslips were rinsed in PBS and cells incubated with DAPI to visualize nuclei. Cells were analyzed with a Nikon E600 microscope equipped for epifluorescence. Cells that stained positive for α-smooth muscle actin cytoskeletal filaments was determined and data presented as a percentage of total cell number (DAPI-positive).
Statistical Analysis –
All experiments were repeated at least in triplicate with independent sets of fibroblasts as indicated in figure legends. Quantitative data were plotted in GraphPad Prism and statistical analyses carried out by Anova with multiple comparisons.
Results
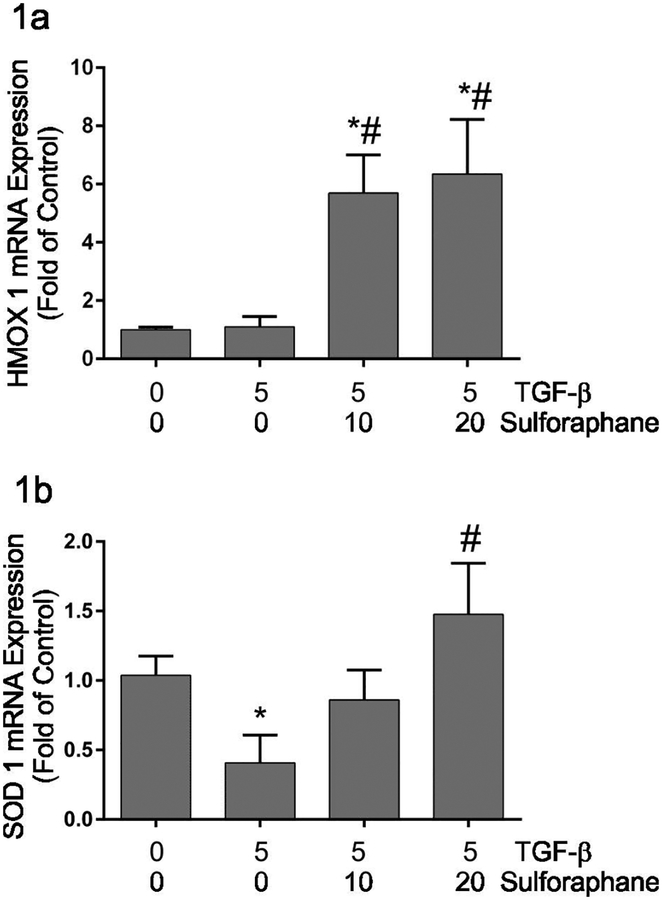
Sulforaphane is an active isothiocyanate with wide-ranging effects mediated at least in part through the activation of Nrf2. Nrf2 regulates transcription of numerous endogenous antioxidant enzymes via the antioxidant response element (ARE). The transcript levels of heme oxygenase 1 and superoxide dismutase were assayed to validate that sulforaphane treatment of adult cardiac fibroblasts activated transcription of Nrf2-target genes. These studies illustrated no significant change in heme oxygenase 1 (HMOX 1) mRNA levels (Figure 1a) and a decrease in superoxide dismutase 1 (SOD1) mRNA levels (Figure 1b) following 48 hours of TGF-β1 treatment alone. Sulphoraphane treatment resulted in significant increases in HMOX 1 and SOD1 mRNA expression relative to TGF-β treatment alone.
Figure 1.
This graphically illustrates the effects of TGF-β1 and sulforaphane on heme oxygenase I (Figure 1a) and superoxide dismutase I (Figure 1b) mRNA expression as analyzed by qRT-PCR. Expression of heme oxygenase I and superoxide dismutase were normalized to acidic ribosomal binding protein and are expressed as fold of untreated control. Significant differences (p ≤ 0.05) from untreated control (*) or TGF-β1 treatment alone (#) were determined by Anova with multiple comparisons. n = 4 independent sets of experiments.
Extracellular matrix expression –
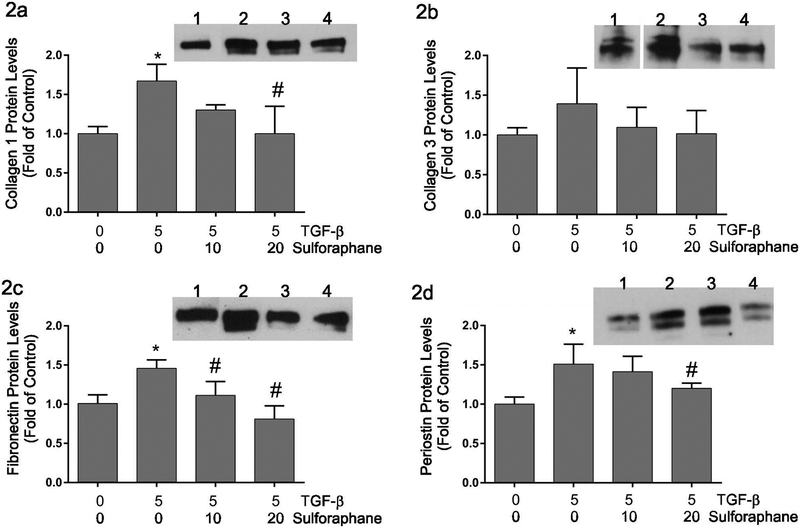
Sulforaphane has been shown to reduce collagen type I and type III mRNA expression in skin fibroblasts (Kawarazaki et al., 2017) and to protect against fibrosis in an animal model of diabetic nephropathy (Shang et al., 2015). To evaluate the effects of sulforaphane on TGF-β-induced ECM expression, cardiac fibroblasts were treated for 48 hours with TGF-β1 (5 ng/ml) and varying concentrations of sulforaphane (0, 10 or 20 μM) followed by examination of collagen type I (Figure 2a), collagen type III (Figure 2b), fibronectin (Figure 2c) and periostin (Figure 2d) protein levels in the conditioned medium by immunoblot analysis. These experiments illustrated significant increases in the accumulation of collagen type I, fibronectin and periostin in response to TGF-β1 treatment. The levels of these proteins were reduced to approximately baseline by the highest concentration of sulforaphane. While there was a similar trend in the levels of collagen type III, the effects did not reach statistical significance.
Figure 2.
This graphically illustrates relative levels of collagen type I (Figure 2a), collagen type III (Figure 2b), fibronectin (Figure 2c) and periostin (Figure 2d) proteins in conditioned medium of adult cardiac fibroblasts following TGF-β1 and/or sulforaphane treatment for 48 hours. The insets show representative immunoblots with the respective primary antisera. Lanes on the insets are: 1) untreated controls, 2) treatment with TGF-β1 alone, 3) treatment with TGF-β1 and 10 μM sulforaphane and 4) treatment with TGF-β1 and 20 μM sulforaphane. Quantitative data are presented relative to untreated control cells. Significance (p ≤ 0.05) was determined by Anova comparison to untreated control cells (*) or to TGF-β1 treatment alone (#). n = 3 independent sets of experiments.
Fibroblast activation and collagen hydrogel contraction –
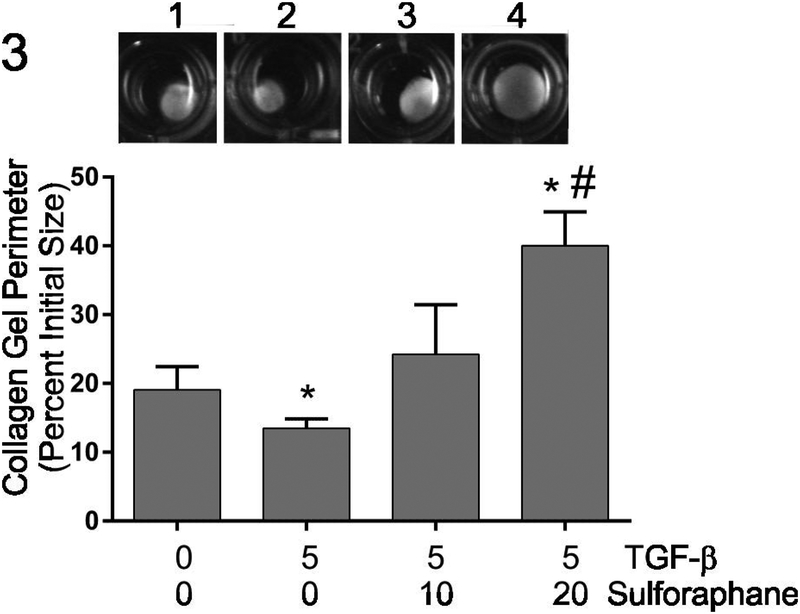
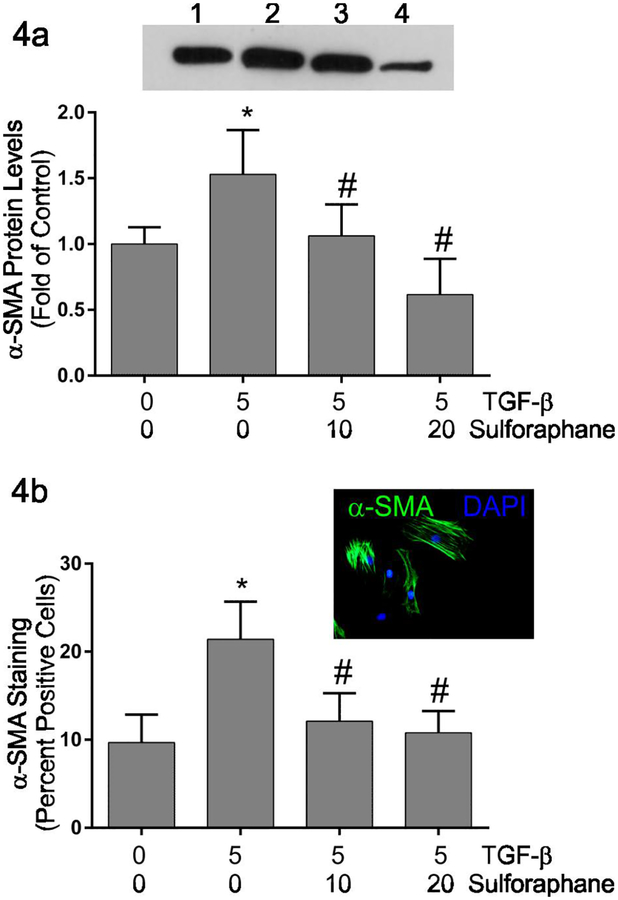
TGF-β1 exposure results in activation of a number of cell types including quiescent fibroblasts yielding a myofibroblast phenotype characterized by enhanced contractile activity (Carthy et al., 2015; Pattarayan et al., 2018). The ability of cardiac fibroblasts to contract 3-dimensional collagen hydrogels was assayed as an indirect measure of fibroblast activation. Similar to previous studies (Tingstrom et al., 1992), TGF-β1 treatment resulted in enhanced contraction of three-dimensional collagen hydrogels by cardiac fibroblasts indicated by reduced collagen hydrogel perimeter (Figure 3). Simultaneous treatment with sulforaphane attenuated TGF-β1-induced collagen hydrogel contraction to levels less than that seen by untreated controls. The expression of α-smooth muscle actin, widely used as a marker of myofibroblast phenotype, was evaluated by immunoblots (Figure 4a) and immunocytochemical staining (Figure 4b) following 48 hours of treatment with 0 or 5 ng/ml TGF-β1 in the presence of 0, 10 or 20 μM sulforaphane. Immunoblot analysis indicated increased levels of α-smooth muscle actin in fibroblast lysates following TGF-β1 treatment (Figure 4a). Simultaneous treatment of fibroblasts with 10 μM sulforaphane and TGF-β1 reduced α-smooth muscle actin levels to the basal level seen in fibroblasts without TGF-β1 nor sulforaphane. At 20 μM, sulforaphane further reduced α-smooth muscle actin expression below baseline. Similar results were obtained with immunocytochemical staining and quantification of α-smooth muscle actin-positive cells (Figure 4b). TGF-β1 treatment alone resulted in an almost twofold increase in the percentage of α-smooth muscle actin-positive cells. Simultaneous treatment with sulforaphane reduced the percentage of positive cells to approximately baseline levels.
Figure 3.
This graphically illustrates the effects of TGF-β1 and sulforaphane on the contraction of collagen hydrogels by cardiac fibroblasts. The inset shows representative collagen hydrogels after 48 hours of treatment. Wells on the inset are: 1) untreated controls, 2) treatment with TGF-β1 alone, 3) treatment with TGF-β1 and 10 μM sulforaphane and 4) treatment with TGF-β1 and 20 μM sulforaphane. Data are presented as the final perimeter of the hydrogels relative to their original size. Statistical significance (p < 0.05) was determined by Anova comparison to untreated controls (*) or TGF-β1 treatment alone (#). n = 4 independent experiments.
Figure 4.
These graphically illustrate α-smooth muscle actin protein levels as determined by immunoblot analysis (Figure 4a) or protein expression determined by immunocytochemical staining (Figure 4b). Immunoblot quantitative data were determined by image analysis of α-smooth muscle actin normalized to glyceraldehyde phosphate dehydrogenase and are expressed as fold of untreated control cells. Immunocytochemical data are presented as the percent of fibroblasts in each condition that are positive for α-smooth muscle actin staining. The immunoblot inset illustrates a representative Western blot with corresponding lanes being: 1) untreated controls, 2) treatment with TGF-β1 alone, 3) treatment with TGF-β1 and 10 μM sulforaphane and 4) treatment with TGF-β1 and 20 μM sulforaphane. The photomicrograph inset illustrates TGF-β1-treated fibroblasts stained with DAPI to visualize all nuclei (blue) and immunocytochemically-stained to visualize α-smooth muscle actin-positive cytoskeletal filaments (green). Statistical significance (p ≤ 0.05) was determined by Anova comparison to untreated control fibroblasts (*) or to TGF-β1 treatment alone (#). n = 5 independent experiments for immunoblots and 4 independent experiments for immunocytochemistry.
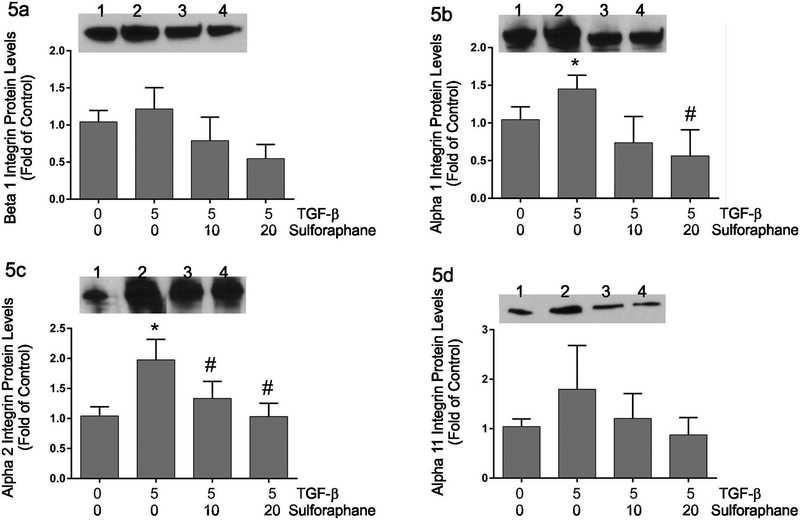
Integrin expression –
Contraction of 3-dimensional collagen hydrogels is dependent upon integrin-mediated interactions between the cells and their collagenous matrix (Gullberg et al., 1989; Carver et al., 1995). The expression of collagen-binding integrins was assessed by immunoblot analysis following 48 hours of treatment with 0 or 5 ng/ml TGF-β1 and varying doses of sulforaphane (Figure 5). TGF-β1 treatment resulted in a slight but insignificant increase in β1 integrin expression (Figure 5a). TGF-β1 alone resulted in significantly increased expression of α1 and α2 integrins and a slight but insignificant increase in α11 integrin levels (Figures 5b, 5c and 5d, respectively). Simultaneous treatment of fibroblasts with sulforaphane and TGF-β1 resulted in a significant decrease in the expression of α1 and α2 integrins compared to TGF-β1 alone. Simultaneous treatment of fibroblasts with sulforaphane and TGF-β1 resulted in slight but statistically insignificant reductions in the levels of β1 and α11 integrin proteins.
Figure 5.
These graphically illustrate immunoblot data for β1 (Figure 5a), α1 (Figure 5b), α2 (Figure 5c) or α11 (Figure 5d) integrin. Image analysis data for each integrin were normalized to data from the same blots probed for glyceraldehyde phosphate dehydrogenase and are presented as fold of untreated controls. The insets show representative immunoblots with each integrin antiserum. Lanes on the insets are: 1) untreated controls, 2) treatment with TGF-β1 alone, 3) treatment with TGF-β1 and 10 μM sulforaphane and 4) treatment with TGF-β1 and 20 μM sulforaphane. Statistical significance (p < 0.05) was determined relative to untreated controls (*) or TGF-β1 treatment alone (#). n = 5 independent experiments.
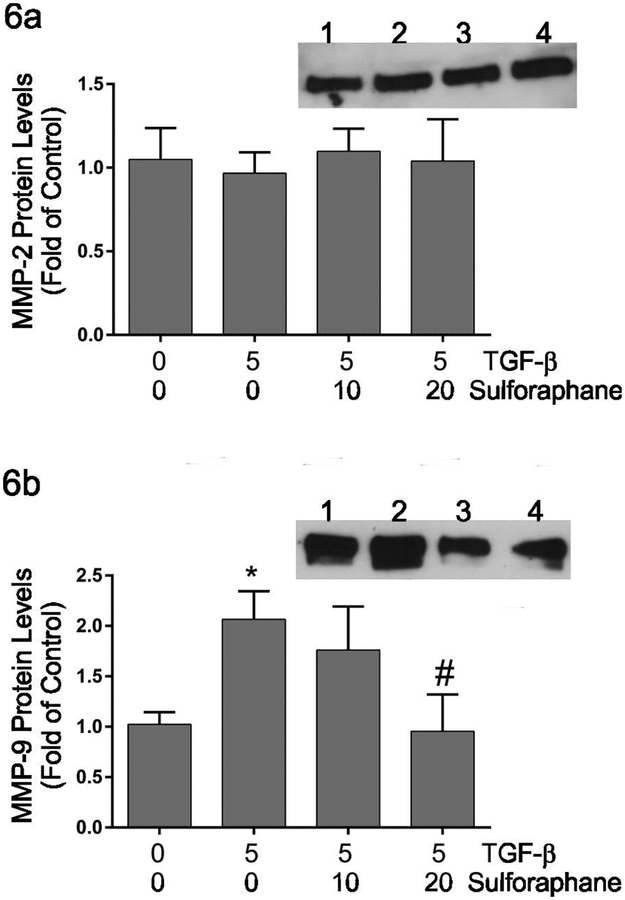
Matrix metalloproteinase expression –
Several studies have illustrated roles for matrix metalloproteinases (MMPs) in migration of fibroblasts and contraction of three-dimensional collagen hydrogels (Kobayashi et al., 2014; Liu et al., 2015). The protein levels of matrix metalloproteinase 9 (MMP9) and matrix metalloproteinase 2 (MMP2), major MMPs produced by fibroblasts were assayed by immunoblot analyses (Figure 6). MMP2 levels were not affected by TGF-β1 alone nor by sulforaphane (Figure 6a). The activation of MMP2 was assessed following TGF-β1 and/or sulforaphane treatment by gelatin zymography. TGF-β1 had no effects on MMP2 activity by adult cardiac fibroblasts (not shown). Similarly, treatment with sulforaphane in had no effect on MMP2 activity. MMP9 protein levels were increased by treatment of cardiac fibroblasts with TGF-β1 alone and this was reduced to approximately baseline levels by concurrent treatment with sulforaphane (Figure 6b).
Figure 6.
This graphically shows relative levels of MMP2 (Figure 6a) or MMP9 (Figure 6b) protein in conditioned medium following treatment of cardiac fibroblasts with TGF-β1 and/or sulforaphane. The insets demonstrate representative immunoblot images with the respective primary antisera. Lanes on the insets are: 1) untreated controls, 2) treatment with TGF-β1 alone, 3) treatment with TGF-β1 and 10 μM sulforaphane and 4) treatment with TGF-β1 and 20 μM sulforaphane. Statistical significance (p < 0.05) was determined relative to untreated controls (*) or TGF-β1 treatment alone (#). Data are presented as the levels of the respective MMP in treated fibroblasts relative to untreated controls. n = 5 independent experiments.
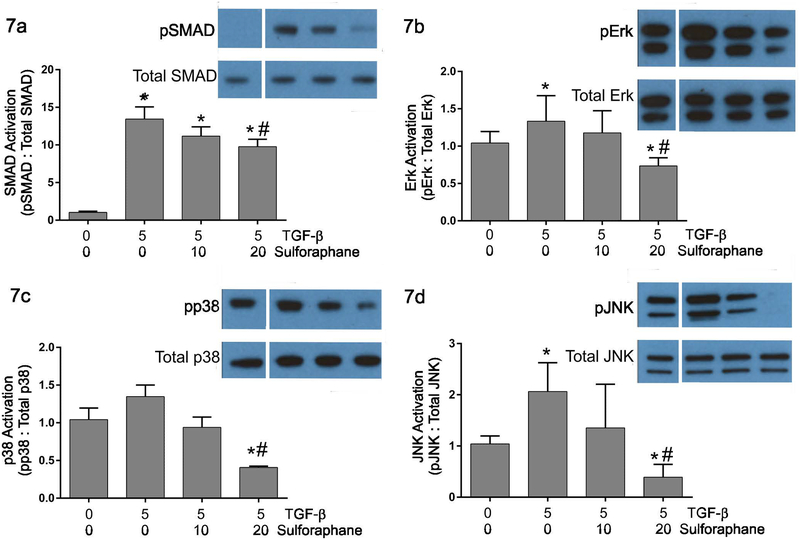
Activation of TGF-β-related signaling pathways –
TGF-β1 activates a number of signaling pathways including the canonical SMAD2/3 pathway and non-canonical p38, JNK and ERK 1/2 pathways. Immunoblots were performed to assess the effects of sulforaphane on activation of signaling pathways by TGF-β1 in adult cardiac fibroblasts. These studies demonstrated that 5 ng/ml treatment of cardiac fibroblasts with TGF-β1 resulted in enhanced activation of the canonical TGF-β signaling pathway indicated by increased phosphorylation of SMAD2 (Figure 7a). This was significantly but incompletely inhibited by pretreatment of fibroblasts with sulforaphane. TGF-β1 treatment also activates several non-canonical signaling pathways in cardiac fibroblasts indicated by significantly increased levels of phosphorylated forms of Erk 1/2 (Figures 7b) and JNK (Figures 7d) and a slight but insignificant increase in p38 phosphorylation (Figures 7c). Pretreatment of cardiac fibroblasts with the highest concentration of sulforaphane (20 μM) attenuated the activation of all of these pathways to below baseline levels.
Figure 7.
These graphically illustrate relative levels of phosphorylated SMAD 2 (Figure 7a), Erk 1/2 (Figure 7b), p38 (Figure 7c) and JNK (Figure 7d) in response to TGF-β1 and/or sulforaphane treatment. Data are presented as the relative levels of phosphorylated protein to the levels of the total respective protein. The insets illustrate representative immunoblots with antisera against phosphorylated SMAD2, total SMAD2, phosphorylated Erk 1/2, total Erk 1/2, phosphorylated p38, total p38, phosphorylated JNK and total JNK. Lanes on the images correspond to: 1) untreated controls, 2) treatment with TGF-β1 alone, 3) treatment with TGF-β1 and 10 μM sulforaphane and 4) treatment with TGF-β1 and 20 μM sulforaphane. Note that all samples within a comparison group were run on the same immunoblot. For the images in this figure, additional irrelevant samples were removed between lane 1 and lane 2. Statistical significance (p ≤ 0.05) was determined by Anova comparison to untreated control fibroblasts (*) or to TGF-β1 treatment alone (#). n = 3 independent experiments.
Discussion
Sulforaphane is a plant-derived isothiocyante particularly abundant in cruciferous vegetables. It has been shown to be protective against a variety of pathological conditions including carcinogenesis (Gamet-Payrastre et al 2000), chronic inflammation (Yehuda et al 2012), tumorigenesis (Bergantin et al 2014) and fibrosis (Artaud-Macari et al 2013; Kawarazaki et al., 2017). Intake of cruciferous vegetables high in sulforaphane has been correlated to decreased risk of cardiovascular disease in epidemiological studies (Mukherjee et al., 2010). Sulforaphane has also been shown to be protective in a number of animal models of cardiovascular disease including doxorubicin-induced heart failure (Singh et al., 2015; Bai et al., 2017) diabetic cardiomyopathy (Zhang et al 2014; Gu 2017) and ischemic injury (Ho et al., 2012). While sulforaphane treatment reduced fibrosis associated with myocardial infarction and doxorubicin-induced toxicity in animal models (Bai et al., 2017), no studies have evaluated the direct effect of this compound on fibroblast-induced ECM remodeling. The present studies were carried out to assess the direct effects of sulforaphane on TGF-β-induced fibroblast activation, collagen remodeling, collagen receptor expression and signal transduction.
TGF-β is a multi-functional cytokine and one of the most potent stimuli of fibroblast activation and fibrosis identified to date. Sulforaphane has recently been demonstrated to inhibit TGF-β-induced epithelial-to-mesenchymal transition of hepatocellular carcinoma cells (Wu et al., 2016) and to inhibit muscle fibrosis in mdx mice via inhibition of TGF-β signaling (Sun et al. 2016). Sulforaphane treatment reduced basal collagen type I and type III mRNA and protein expression in normal and keloid fibroblasts in vitro while also inhibiting Smad 3 signaling (Kawarazaki et al., 2017). Consistent with these data, the present studies illustrated that sulforaphane treatment attenuated induction of ECM fibrotic markers collagen type I and fibronectin. Sulforaphane also attenuated TGF-β1-induced expression of periostin, a matricellular protein associated with myocardial fibrosis (Zhao et al., 2014). In addition to inhibiting TGF-β-induced ECM expression, sulforaphane reduced remodeling and contraction of three-dimensional collagen hydrogels by isolated cardiac fibroblasts. This three-dimensional culture system has been widely used as a model of wound healing and fibroblast contractile activity (Bell et al., 1979; Guidry and Grinnell, 1987). Enhanced contraction of collagen hydrogels by TGF-β treatment is thought to be, at least in part, due to the conversion of some cell types to a myofibroblast phenotype. Treatment of cardiac fibroblasts with TGF-β1 resulted in an increased myofibroblast transition as indicated by enhanced expression of α-smooth muscle actin. Similar to previous reports (Artaud-Macli et al., 2013), simultaneous treatment of the cells with sulforaphane resulted in diminished α-smooth muscle actin expression and fewer α-smooth muscle actin-positive cells. These studies indicate that sulforaphane is capable of attenuating TGF-β-induced fibroblast activation as well as ECM production and contraction associated with the myofibroblast phenotype. It is not possible to determine from the experimental design utilized here whether sulforaphane is capable of reversing myofibroblast conversion, which would be very intriguing from a therapeutic perspective.
Many of the effects of sulforaphane are thought to involve the activation of Nrf2 (Higgins et al., 2009; Cui et al., 2012); however, Nrf2-independent effects have also been described (Davidson et al., 2013). Nrf2 is a redox-sensitive transcription factor that plays a key role in orchestrating cellular antioxidant defenses and maintaining redox homeostasis. Under basal conditions, Nrf2 interacts with Keap1 and is targeted for proteasomal degradation. Under conditions of enhanced stress, Nrf2 is activated via modification of redox-sensitive cysteine residues on Keap1. Upon activation, Nrf2 is translocated to the nucleus where it binds to antioxidant-response elements in promoters of genes encoding antioxidant enzymes and phase II cytoprotective proteins. Approximately two hundred Nrf2 target genes have been identified (Thimmulappa et al 2002; Kwak et al., 2003; Cho et al., 2005). Nrf2 knockout mice are more sensitive to many harmful substances including bacterial lipopolysaccharide, bleomycin, carrageenan, cigarette smoke, UVA irradiation and others (Hong et al 2005; Higgins et al 2009). Similar to previous studies with other cell types, treatment of cardiac fibroblasts with sulforaphane resulted in enhanced expression of Nrf2 target genes including heme oxygenase 1 and superoxide dismutase. While this suggests that Nrf2 induction of antioxidant-response element-containing genes may be involved in the effects of sulforaphane, further molecular or pharmacological perturbation studies will be needed to definitely determine this.
The effects of sulforaphane on cellular signal transduction have been somewhat contradictory and may be cell-type specific. Several studies have demonstrated that sulforaphane activates specific signaling pathways. For instance, treatment of isolated neonatal rat myocytes with sulforaphane resulted in the activation of Akt and Erk 1/2 (Leoncini 2011). Simultaneous treatment of the myocytes with pharmacological inhibitors demonstrated that the PI3 kinase/Akt pathway is essential for sulforaphane-induced increase in antioxidant enzyme production. In the same light, simultaneous treatment of chondrocytes with sulforaphane and interleukin-1 resulted in prolonged activation of JNK and p38 compared to interleukin-1 alone (Davidson et al 2013). In contrast, sulforaphane treatment resulted in attenuated interleukin-1-induced NF-KB activation. Sulforaphane has also been shown to reduce basal activation of the Smad3 pathway (Kawa et al., 2017) and angiotensin II-induced activation of Akt, mTOR and NF-KB in the H9C2 cardiomyocyte cell line (Wu et al., 2014). The present studies are consistent with these latter ones in that the data presented herein indicate that sulforaphane pretreatment of cardiac fibroblasts attenuates both canonical and non-canonical TGF-β signaling pathways. The functional consequences of the inhibition of specific pathways by sulforaphane remain to be elucidated.
Myofibroblasts have become a potential therapeutic target, particularly in disorders involving tissue and organ fibrosis. The present studies illustrate that sulforaphane, a plant-derived isothiocyante, is capable of inhibiting TGF-β-induced conversion of quiescent cardiac fibroblasts into activated myofibroblasts. This includes attenuation of collagen remodeling and collagen receptor expression as well as signaling pathways associated with TGF-β. These and other studies indicate that sulforaphane may possess important protective effects; however, few studies have evaluated the potential of sulforaphane to reverse pathogenesis. Future studies will be needed to evaluate this paradigm and to further elucidate the molecular mechanisms of sulforaphane’s protective effects.
Acknowledgements:
This work was funded in part by grants from the National Institutes of Health (R01HL126705 and R01CA218578) and the American Heart Association (17GRNT33650018) and funding from the University of South Carolina School of Medicine. None of the authors have conflicts of interest with the research contained in this manuscript.
References
- Abe Y, Sakairi T, Beeson C, Kopp JB (2013) TGF-β1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway. Am J Physiol Renal Physiol 305:F1477–F1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright CD, Salganik RI, Craciunescu CN, Mar MH, Zeisel SH (2003) Mitochondrial and microsomal derived reactive oxygen species mediate apoptosis induced by transforming growth factor-beta1 in immortalized rat hepatocytes. J Cell Biochem 89:254–261. [DOI] [PubMed] [Google Scholar]
- Arfmann-Knubel S, Struck B, Genrich G, Helm O, Sipos B, Sebens S, Schafer H (2015) The crosstalk between Nrf2 and TGF-β1 in the epithelial-mesenchymal transition of pancreatic duct epithelial cells. PLoS One 10:e0132978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artaud-Macari E, Goven D, Braver S, Hamimi A, Besnard V, Marchal-Somme J, Ali ZE, Crestani B, Kerdine-Romer S, Boutten A, Bonay M (2013) Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal 18:66–79. [DOI] [PubMed] [Google Scholar]
- Bai Y, Chen Q, Sun YP, Wang X, Lv L, Zhang LP, Liu JS, Zhao S, Wang XL (2017) Sulforaphane protection against the development of doxorubicin-induced chronic heart failure is associated with Nrf2 upregulation. Cardiovasc Ther Oct; 35(5). [DOI] [PubMed] [Google Scholar]
- Bell E, Ivarsson B, Merrill C (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A 76:1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergantin E, Quarta C, Nanni C, Fanti S, Pession A, Cantelli-Forti G, Tonelle R, Hrelia P (2014) Sulforaphane induces apoptosis in rhabdomyosarcoma and restores TRAIL-sensitivity in the aggressive aveolar subtype leading to tumor elimination in mice. Cancer Biol Ther 15: 1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthy JM (2018) TGFβ signaling and the control of myofibroblast differentiation: implications for chronic inflammatory disorders. J Cell Physiol 233:98–106. [DOI] [PubMed] [Google Scholar]
- Carthy JM, Sundqvist A, Heldin A, van Dam H, Kletsas D, Heldin CH, Moustakas A (2015) Tamoxifen inhibits TGF-β-mediated activation of myofibroblasts by blocking non-Smad signaling through ERK1/2. J Cell Physiol 230:3084–3092. [DOI] [PubMed] [Google Scholar]
- Carver W, Molano I, Reaves TA, Borg TK, Terracio L (1995) Role of the alpha 1 beta 1 integrin complex in collagen gel contraction in vitro by fibroblasts. J Cell Physiol 165:425–437. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Debiase A, Yamamoto M, Kleeberger SR (2005) Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic Biol Med 38:325–343. [DOI] [PubMed] [Google Scholar]
- Cui W, Bai Y, Miao X, Luo P, Chen Q, Tan Y, Rane MJ, Miao L, Cai L (2012) Prevention of diabetic nephropathy by sulforaphane: possible role of Nrf2 upregulation and activation. Oxid Med Cell Longev 2012:821936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BA, Vethanayagam RR, Grimm MJ, Mullan BA, Raghavendran K, Blackwell TS, Freeman ML, Ayyasamy V, Singh KK, Sporn MB, Itagaki K, Hauser CJ, Knight PR, Segal BH (2013) NADPH oxidase and Nrf2 regulate gastric aspiration-induced inflammation and acute lung injury. J Immunol 190:1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausther M, Dranoff JA (2014) Integrins, myofibroblasts, and organ fibrosis. Hepatology 60:756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Tercé F (2000) Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res 60:1426–1433. [PubMed] [Google Scholar]
- Gerarduzzi C, di Battista JA (2017) Myofibroblast repair mechanisms post-inflammatory response: a fibrotic perspective. Inflamm Res 66: 451–465. [DOI] [PubMed] [Google Scholar]
- Gu J, Cheng Y, Wu H, Kong L, Wang S, Xu Z, Zhang Z, Tan Y, Keller BB, Zhou H, Wang Y, Xu Z, Cai L (2017) Metallothionein is downstream of Nrf2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes 66:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry C, Grinnell F (1987) Contraction of hydrated collagen gels by fibroblasts: evidence for two mechanisms by which collagen fibrils are stabilized. Coll Relat Res 6:515–529. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Terracio L, Borg TK, Rubin K (1989) Identification of integrin-like matrix receptors with affinity for interstitial collagens. J Biol Chem 264:12686–12694. [PubMed] [Google Scholar]
- Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV, Suri RM, Miller JD (2013) TGF-β signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res 99:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Su Q, Liang J, Sun Y, Wang X, Li L (2018) The protective effect of nicorandil on cardiomyocyte apoptosis after coronary microembolization by activating Nrf2/HO-1 signaling pathway in rats. Biochem Biophys Res Commun 496:1296–1301. [DOI] [PubMed] [Google Scholar]
- Higgins LG, Kelleher MO, Eggleston IM, Itoh K, Yamamoto M, Hayes JD (2009) Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol Appl Pharmacol 237:267–280. [DOI] [PubMed] [Google Scholar]
- Ho JN, Yoon HG, Park CS, Kim S, Jun W, Choue R, Lee J (2012) Isothiocyanates ameliorate the symptom of heart dysfunction and mortality in a murine AIDS model by inhibiting apoptosis in the left ventricle. J Med Food 15:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F, Sekhar KR, Freeman ML, Liebler DC (2005) Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J Biol Chem 280:31768–31775. [DOI] [PubMed] [Google Scholar]
- Huang K, Gao X, Wei W (2017) The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-β1 expressions in rat glomerular mesangial cells. Exp Cell Res 361:63–72. [DOI] [PubMed] [Google Scholar]
- Kawarazaki A, Horinaka M, Yasuda S, Numajiri T, Nishino K, Sakai T (2017) Sulforaphane suppresses cell growth and collagen expression of keloid fibroblasts. Wound Repair Regen 25:224–233. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kim H, Liu X, Sugiura H, Kohyama T, Fang Q, Wen FQ, Abe S, Wang X, Atkinson JJ, Shipley JM, Senior RM, Rennard SI (2014) Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am J Physiol Lung Cell Mol Physiol 306:L1006–L1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW (2003) Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem 278:8135–8145. [DOI] [PubMed] [Google Scholar]
- Lan A, Li W, Liu Y, Xiong Z, Zhang X, Zhou S, Palko O, Chen H, Kapita M, Prigge JR, Schmidt EE, Chen X, Sun Z, Chen XL (2016) Chemoprevention of oxidative stress-associated oral carcinogenesis by sulforaphane depends on NRF2 and the isothiocyanate moiety. Oncotarget 7:53502–53514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini E, Malaguti M, Angeloni C, Motori E, Fabbri D, Hrelia S (2011) Cruciferous vegetable phytochemical sulforaphane affects phase II enzyme expression and activity in rat cardiomyocytes through modulation of Akt signaling pathway. J Food Sci 76:H175–H181. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kimura K, Orita T, Teranishi S, Suzuki K, Sonoda KH (2015) All-trans-retinoic acid inhibition of transforming growth factor-β-induced collagen gel contraction mediated by human Tenon fibroblasts: role of matrix metalloproteinases. Br J Ophthalmol 99:561–565. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Lekli I, Ray D, Gangopadhyay H, Raychaudhuri U, Das DK (2010) Comparison of the protective effects of steamed and cooked broccolis on ischaemia-reperfusion-induced cardiac injury. Br J Nutr 103:815–823. [DOI] [PubMed] [Google Scholar]
- Pakshir P, Hinz B (2018) The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics and miscommunication. Matrix Biol 68–69: 81–93. [DOI] [PubMed] [Google Scholar]
- Pattarayan D, Sivanantham A, Krishnaswami V, Loganathan L, Palanichamy R, Natesan S, Muthusamy K, Rajasekaran S (2018) Tannic acid attenuates TGF-β1-induced epithelial-to-mesenchymal transition by effectively intervening TGF-β signaling in lung epithelial cells. J Cell Physiol 233:2513–2525. [DOI] [PubMed] [Google Scholar]
- Qu C, Li B, Lai Y, Li H, Windust A, Hofseth LJ, Nagarkatti M, Nagarkatti P, Wang XL, Tang D, Janicki JS, Tian X, Cui T (2015) Identifying panaxynol, a natural activator of nuclear factor erythroid-2 related factor 2 (Nrf2) from American ginseng as a suppressor of inflamed macrophage-induced cardiomyocyte hypertrophy. J Ethnopharmacol 168:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang G, Tang X, Gao P, Guo F, Liu H, Zhao Z, Chen Q, Jiang T, Zhang N, Li H (2015) Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2 signaling pathway. J Nutr Biochem 26:596–606. [DOI] [PubMed] [Google Scholar]
- Singh P, Sharma R, McElhanon K, Allen CD, Megyesi JK, Beneš H, Singh SP (2015) Sulforaphane protects the heart from doxorubicin-induced toxicity. Free Radic Biol Med 86:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Li S, Li D (2016) Sulforaphane mitigates muscle fibrosis in mdx mice via Nrf2-mediated inhibition of TGF-β/Smad signaling. J Appl Physiol (1985) 120:377–390. [DOI] [PubMed] [Google Scholar]
- Talior-Volodarsky I, Connelly KA, Arora PD, Gullberg D, McCulloch CA (2012) α11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc Res 96:265–275. [DOI] [PubMed] [Google Scholar]
- Tang J, Li B, Liu C, Li Y, Li Q, Wang L, Min J, Hu M, Hong S, Hong L (2017) Mechanism of mechanical trauma-induced extracellular matrix remodeling of fibroblasts in association with Nrf2/ARE signaling suppression mediating TGF-β1/Smad3 signaling inhibition. Oxid Med Cell Longev 2017:8524353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S (2002) Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62:5196–5203. [PubMed] [Google Scholar]
- Tingström A, Heldin CH, Rubin K (1992) Regulation of fibroblast-mediated collagen gel contraction by platelet-derived growth factor, interleukin-1 alpha and transforming growth factor-beta 1. J Cell Sci 102:315–322. [DOI] [PubMed] [Google Scholar]
- Weng D, Chen JX, Li HH, Liu F, Zhou LD, Liu HP, Zheng RJ, Jiang Y, Liu ZH, Ge B (2018) 2-aminopurine suppresses the TGF-β1-induced epithelial-mesenchymal transition and attenuates bleomycin-induced pulmonary fibrosis. Cell Death Discov 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Han J, Hou B, Deng C, Wu H, Shen L (2016) Sulforaphane inhibits TGF-β-induced epithelial-mesenchymal transition of hepatocellular carcinoma cells via the reactive oxygen species-dependent pathway. Oncol Rep 35:2977–2983. [DOI] [PubMed] [Google Scholar]
- Wu QQ, Zong J, Gao L, Dai J, Yang Z, Xu M, Fang Y, Ma ZG, Tang QZ (2014) Sulforaphane protects H9c2 cardiomyocytes from angiotensin II-induced hypertrophy. Herz 39:390–396. [DOI] [PubMed] [Google Scholar]
- Yehuda H, Soroka Y, Zlotkin-Frušić M, Gilhar A, Milner Y, Tamir S (2012) Isothiocyanates inhibit psoriasis-related proinflammatory factors in human skin. Inflamm Res 61:735–742. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang S, Zhou S, Yan X, Wang Y, Chen J, Mellen N, Kong M, Gu J, Tan Y, Zheng Y, Cai L (2014) Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol 77:42–52. [DOI] [PubMed] [Google Scholar]
- Zhao S, Wu H, Xia W, Chen X, Zhu S, Zhang S, Shao Y, Ma W, Yang D, Zhang J (2014) Periostin expression is upregulated and associated with fibrosis in human failing hearts. J Cardiol 63:373–378. [DOI] [PubMed] [Google Scholar]