Abstract
Introduction:
Thoracic endovascular aortic repair(TEVAR) has become a mainstay of therapy for acute(AD) and chronic(CD) type B dissection(TBAD). Dynamic aortic morphologic changes, untreated dissected aorta and persistent false lumen(FL) perfusion have significant consequences on re-intervention after TEVAR for TBAD. However, few reports contrast differences in secondary aortic intervention(SAI) after TEVAR for TBAD or describe their influence on mortality. This analysis examines incidence, timing, and types of SAI after TEVAR for AD/CD TBAD and determines their impact on survival.
Methods:
All TEVAR procedures for AD/CD(2005–2016) were retrospectively reviewed. Patients with staged(<30-days) or concomitant ascending aortic arch repair/replacement were excluded. Acuity was defined by symptom onset[0–30 days(AD);>30-days(CD)]. SAI was grouped into open(intended treatment zone or remote aortic site), major endovascular(TEVAR extension or endograft implanted at non-contiguous site) or minor endovascular(side-branch/FL embolization) categories. Kaplan-Meier methodology was used to estimate freedom from SAI and survival. Cox proportional hazards were used to identify SAI predictors.
Results:
TEVAR for TBAD was performed in 258 patients[AD, 49%(n=128);CD, 51%(n=130)]. Mean follow-up was 17±22 months with an overall SAI rate of 27%[n=70; AD, 22%(28) vs. CD, 32%(42);OR 1.7, 95%CI 0.9–2.9;p=.07]. Median time to SAI was significantly less after AD[0.7(0–12) vs. CD, 7(0–91)months;p<.001]; however, freedom from SAI was not different(1-year:AD, 67±4% vs. CD, 68±5%;3-year: AD, 65±7% vs. CD, 52±8%;p=.7). Types of SAI were similar(AD/CD:open, 61% vs. 55%;p=.6;major endovascular, 36% vs. 38%;p=.8;minor endovascular 21% vs. 21%;p=1). The open conversion rate(either partial or total endograft explant)[AD:10%(13 of 128) vs. CD:15%(20 of 130);p=.2] and incidence of retrograde dissection[AD:6%(7 of 128) vs. CD:4%(5 of 130);p=.5] was similar.
There was no difference in survival for SAI patients(5-year: AD+SAI, 55±9% vs. AD without SAI, 67±8%;p=.3;5-year: CD+SAI, 72±6% vs. CD without SAI, 72±7%;p=.7). Factors associated with SAI included younger age, AD with larger maximal aortic diameter at presentation, Marfan syndrome, and use of arch vessel adjunctive procedures with the index TEVAR. Indication for the index TEVAR(aneurysm, malperfusion, rupture, or pain/hypertension) or remote preoperative history of proximal arch procedure were not predictive of SAI.
Conclusion:
SAI after TEVAR for TBAD is common. AD has a higher proportion of early SAI, however CD appears to have on-going risk of remediation after the first postoperative year. SAI types are similar between groups and the occurrence of aorta related re-intervention does not impact survival. Patient features and anatomy predict need for SAI. These data should be taken into consideration for patient selection, device design and surveillance strategies after TEVAR for TBAD.
Introduction
Acute type B aortic dissection(TBAD) is a challenging clinical problem that is associated with a risk of catastrophic complications such as rupture and/or end-organ malperfusion. Since the advent of thoracic endovascular aortic repair(TEVAR), several reports have highlighted the efficacy of this technology in the management of complicated, acute TBAD1–9. For uncomplicated, acute TBAD patients who are managed medically, a significant proportion develop chronic false lumen(FL) aneurysms and have similarly been increasingly treated with TEVAR6, 8, 10–12. The adoption of TEVAR for treatment of both acute and chronic TBAD(AD/CD) has been associated with high technical success, low complication rates, positive short-term aortic remodeling, and mid-term survival benefits13–15.
However, the acute and chronic phases of TBAD are characterized by a dynamic aortic remodeling environment with differences in dissection septum compliance, persistent FL perfusion, branch vessel perfusion status/location and aortic wall integrity, which all have implications on the incidence, timing and type of re-intervention that may occur after TEVAR16,17. Although population based studies have shown increasing utilization of TEVAR for TBAD, they rarely provide detailed information about secondary aortic related intervention(SAI). Importantly, the nature of the re-intervention can be quite variable depending on the underlying failure mechanism. A variety of different and potentially complex remedial endovascular, open, and/or hybrid techniques may be needed to address the failure modes after TEVAR for TBAD. Moreover, differentiation of the factors influencing re-intervention after TEVAR for AD/CD, as well as the implications of SAI on survival have not been well established.
The purpose of this study is to review our experience with SAI after TEVAR for acute and chronic TBAD.
Methods
The University of Florida Institutional Review Board approved this study(#1621–2017). A waiver of informed consent was granted, as all collected data pre-existed in medical records and no subject contact or study-related interventions occurred.
Study cohort and database.
A prospectively maintained endovascular aortic registry of all consecutive TEVAR procedures from 2005–2016 was reviewed. A total of 1025 TEVAR procedures were performed, with 294 for AD or CD related pathology. Patients with thoracoabdominal FL aneurysm managed with chimney, renal-mesenteric fenestrated/branched grafts or visceral debranching with TEVAR were omitted. Patients who underwent simultaneous open arch vessel replacement and/or debranching with TEVAR were also excluded(n=36). While acute proximal dissections(Stanford A18 or Debakey I/II19) were not included in the analysis, patients previously treated(>30-days) for Type A dissection with residual, chronic TBAD with aneurysm formation were included, as were cases with concurrent arch vessel intervention(e.g. left carotid-subclavian bypass, left carotid/left subclavian chimney and/or fenestrated stent placement) to augment proximal landing zones. After applying these inclusion/exclusion criteria, a total of 258 TEVAR cases for acute and chronic TBAD were available for analysis.
Patient demographics, comorbidities, and surgical history of prior aortic procedures were abstracted. Computed tomographic arteriograms(CTA) were reviewed for anatomic detail and operative data was recorded. Proximal and distal(proximal to celiac, proximal to SMA, or infrarenal) contiguous coverage zones were documented, as well as use of adjunctive procedures(e.g. arch vessel bypass/embolization/stent, visceral stenting, or infrarenal EVAR). The prospectively maintained endovascular aortic registry and electronic medical records were reviewed for post-TEVAR aortic diameter, presence of endoleak, and occurrence of SAI. A retrospective chart review was subsequently performed for additional data collection to ascertain SAI details.
Clinical practice.
The UF/Health Aorta Center (https://ufhealth.org/uf-health-aorta-center/welcome) is constituted by a multi-disciplinary group of vascular and cardiovascular surgeons, as well as cardiovascular anesthesia and intensive care specialists. This serves as a major referral center of acute and chronic aortic disease for the Southeastern United States. Patients with ruptured TBAD and/or subjects presenting with end-organ ischemia(e.g. spinal, renal, bowel, lower extremity) were immediately treated with TEVAR while stable patients were admitted to a cardiovascular designated surgical intensive care unit(SICU) for serial evaluation, blood pressure management, and pain control. Patients with chronic TBAD with FL aneurysm were typically offered repair if the maximal thoracic aneurysm diameter exceeded ≥ 6.0cm or had a documented growth rate ≥ 1.0cm on serial centerline reconstructions of CTA imaging over 12 months14.
Need and timing for TEVAR was discussed among the group in all cases. Device selection and implantation sequence was left to the operating surgeon’s choice. Anatomic suitability was generally determined based on reference aortic(between left common carotid-left subclavian artery) inner(intimal) diameter ≤ 42mm, and a centerline distance from the left common carotid artery to the dominant primary entry tear ≥ 20mm. Aortic device oversizing for AD was typically 1–10% and intravascular ultrasound(IVUS) was used in all cases with digital subtraction arteriography to determine true lumen(TL) cannulation, landing zone morphology, and branch vessel anatomy.
Subclavian revascularization was performed routinely for AD/CD TBAD patients needing subclavian coverage, as per practice guidelines20. TEVAR length for AD without rupture was at the discretion of the operating surgeon, with the goal being coverage of the primary entry tear and FL depressurization/restoration of TL flow. Success was guided by IVUS appearance of TL/FL mobility and assessment of FL perfusion on completion arteriography. In cases of AD with rupture, TEVAR coverage length routinely went to the level of the celiac origin. Compliant ballooning was avoided unless there was evidence of significant endoleak.
Endograft sizing, aortic coverage length and compliant ballooning principles that governed the care of chronic TBAD with TEVAR have been previously published by our group14. Additional details highlighting spinal drain protocol usage and postoperative TEVAR management has also been previously published21. Post-discharge CTA surveillance was similar for AD/CD TEVAR cases and typically consisted of imaging at 1-month, 3-months, 6-months, 12-months, 18-months, 24-months and annually, thereafter. The need, timing and type of SAI was based upon the multi-disciplinary group’s judgement.
End-points and definitions.
The primary end-point was mortality after SAI. Secondary end-points included complications and out of hospital survival after SAI. AD was defined by diagnosis within 30-days of symptom onset whereas CDs were those repaired at greater than 30-days after the index diagnosis. Primary technical success for the index AD TEVAR procedure was defined as coverage of the proximal entry tear,22 endograft deployment without type Ia/III endoleak, and absence of open conversion and/or death within 24-hours postoperatively.23 Technical success for the index CD TEVAR procedure was defined as deployment of the endograft at the intended aortic segment with absence of antegrade flow into the false lumen/aneurysm at case completion. Comorbidities and aortic coverage zones were defined based on previously published definitions from the Society of Vascular Surgery.24, 25
Re-intervention was defined as any aorta-related endovascular or open surgical procedure that occurred after the index TEVAR either at the intended treatment zone or at remote aorticsites. Re-intervention was performed for multiple indications including: retrograde dissection, persistent FL flow into the treated segment with further aneurysmal degeneration, Type I, II or III endoleak, stent-graft induced new re-entry tear(SINE) causing pain or recurrent FL flow/aneurysm, device failure(such as endograft infolding), and aneurysmal degeneration of the untreated proximal or distal aorta. Endovascular SAI procedures were sub-classified as major if they included an additional aortic endograft at the intended treatment zone or remote aortic site, and defined as being minor if a side branch or false lumen embolization. Patient mortality was verified utilizing the Social Security Death Masterfile.
Statistics.
Categorical and continuous variables were compared between patients with and without SAI using Chi-square and Student T-tests where appropriate. Similarly, AD and CD SAI events were compared to determine if there were differences in the timing, type and outcome of these events. Kaplan-Meier methodology was used to estimate freedom from SAI and mid-term(3 and 5-year) survival. Cox proportional hazards modeling was used to identify predictors of SAI. All statistical analysis was performed using STATA 12 software(StataCorp, College Station, Texas). A P-value < .05 was considered significant.
Results
Patient cohort.
A total of 258 TEVAR procedures were performed for acute(n=128;49%) and chronic(n=130;51%) TBAD during the study interval. Demographics and comorbidities are depicted in Table I. Over a quarter(28%) of the patients had a remote preoperative history of a prior aortic repair. Although most features were similar between the two cohorts, there were important differences for patients who ultimately underwent SAI. Specifically, there was a higher proportion of Marfan syndrome patients who ultimately underwent SAI(11% vs. 4%;p=.02). As expected, the aortic diameter prior to the index TEVAR procedure was larger for CD patients compared to AD subjects(63±11mm vs. 47± 11mm;p< .001). AD subjects who experienced SAI were more likely to have had a prior aortic repair(AD-29% vs. CD-12%;p=.03). Other demographics and comorbidity relationships were similar when stratifying AD and CD patients with and without SAI(Table IIa and IIb).
Table I.
Patient demographics, comorbidities, and indications for all patients with or without secondary aortic intervention after TEVAR of type B dissection
| Feature, % (No.) |
All patients N=258 |
Secondary Aortic Intervention(+) 27%(N=70) |
Secondary Aortic Intervention(−) 73%(N=188) |
p-value |
|---|---|---|---|---|
| Age (years±SD) | 61.5±12.9 | 60.2±12.0 | 62.0±13.3 | .3 |
| BMI | 28.8±5.8 | 29.2±5 | 28.6±6 | .5 |
| Male | 79(203) | 86(60) | 77(144) | .1 |
| Acute (days) | 50(128) | 40(28) | 53(100) | .06 |
| -days from presentation | 4.8±6 | 5.2±7.5 | 4.6±5.5 | .6 |
| Chronic (mos) | 50(130) | 60(42) | 47(88) | .06 |
| -months from presentation | 46.9±59 | 45.8±43 | 47.5±65 | .9 |
| Prior aortic repair | 28(73) | 37(26) | 25(47) | .05 |
| Time since prior aortic repair (mos) | 196 | 302 | 132 | .1 |
| ASA 2 | 0.4(1) | 0 | 0.5(1) | .5 |
| 3 | 17(45) | 21(15) | 16(30) | .3 |
| 4 | 80(207) | 79(55) | 81(152) | .7 |
| 5 | 2(5) | 0 | 3(5) | .2 |
| COPD | 13(34) | 16(11) | 12(23) | .5 |
| Smoking history | 39(101) | 37(26) | 40(75) | .7 |
| End stage renal disease | 2(5) | 1(1) | 2(4) | .7 |
| Chronic renal insufficiency | 31(80) | 24(17) | 34(63) | .2 |
| Hypertension | 92(236) | 90(63) | 92(173) | .6 |
| Dyslipidemia | 44(114) | 47(33) | 43(81) | .6 |
| Diabetes mellitus | 12(32) | 16(11) | 11(21) | .3 |
| Congestive heart failure | 5(13) | 4(3) | 5(10) | .7 |
| Peripheral vascular disease | 4(10) | 3(2) | 4(8) | .6 |
| Cerebrovascular disease | 7(17) | 7(5) | 6(12) | .8 |
| Arrhythmia | 8(20) | 10(7) | 7(13) | .4 |
| Coronary artery disease | 18(47) | 19(13) | 18(34) | .9 |
| Marfan syndrome | 6(15) | 11(8) | 4(7) | .02 |
| Indication for TEVAR | ||||
| -Refractory hypertension/pain | 7(18) | 4(3) | 8(15) | .3 |
| -Aneurysm | 60(155) | 73(51) | 55(104) | .01 |
| -Malperfusion | 24(62) | 17(12) | 27(50) | .1 |
| -Rupture | 9(23) | 6(4) | 10(19) | .3 |
BMI, body mass index; ASA, American Society of Anesthesia; COPD, chronic obstructive pulmonary disease; TEVAR, thoracic endovascular aortic repair
Table IIa.
Patient demographics, comorbidities, and indications for acute dissection patients with or without secondary aortic intervention after TEVAR for type B dissection
| Acute, %(No.) |
Acute patients N=128 |
Secondary Aortic Intervention(+) 22%(N=28) |
Secondary Aortic Intervention(−) 78%(N=100) |
p-value |
|---|---|---|---|---|
| Age (years) | 60.6±14.0 | 59.5±14.8 | 60.9±13.8 | .6 |
| BMI | 28.3±5.6 | 27.7±5.3 | 28.5±5.7 | .5 |
| Male | 74(95) | 82(23) | 72(72) | .3 |
| Timing of TEVAR (days) | 4.8±6 | 5.2±7 | 4.6±5 | .6 |
| Prior aortic repair | 16(20) | 29(8) | 12(12) | .03 |
| Time since prior aortic repair (mos) | 38.5±46 | 32.8±5 4 | 20.3±38 | .1 |
| ASA | ||||
| 3 | 16(21) | 11(3) | 18(18) | .4 |
| 4 | 80(102) | 89(25) | 77(77) | .2 |
| 5 | 4(5) | 0 | 5(5) | .2 |
| COPD | 10(13) | 18(5) | 8(8) | .1 |
| Smoking history | 34(44) | 25(7) | 37(37) | .2 |
| End stage renal disease | 0 | 0 | 0 | 1 |
| Chronic renal insufficiency | 30(39) | 29(8) | 31(31) | .8 |
| Hypertension | 89(114) | 86(24) | 90(90) | .5 |
| Dyslipidemia | 34(44) | 32(9) | 35(35) | .8 |
| Diabetes mellitus | 11(14) | 11(3) | 11(11) | 1 |
| Congestive heart failure | 2(3) | 0 | 3(3) | .4 |
| Peripheral vascular disease | 2(3) | 4(1) | 2(2) | .6 |
| Cerebrovascular disease | 5(6) | 7(2) | 4(4) | .5 |
| Arrhythmia | 7(9) | 11(3) | 6(6) | .4 |
| Coronary artery disease | 14(18) | 18(5) | 13(13) | .5 |
| Marfan syndrome | 5(7) | 14(4) | 3(3) | .02 |
| TAAA diameter (mm) | 47.4±11 | 50.4±10 | 46.5±11 | .1 |
BMI, body mass index; TEVAR, thoracic aortic endovascular repair; COPD, chronic obstructive pulmonary disease; TAAA, thoracoabdominal aortic false lumen aneurysm
Table IIb.
Patient demographics, comorbidities, and indications for chronic dissection patients with or without secondary aortic intervention after TEVAR for type B dissection
| Chronic, %(No.) |
Chronic patients N=130 |
Secondary Aortic Intervention(+) 32%(N=42) |
Secondary Aortic Intervention(−) 68%(N=88) |
p-value |
|---|---|---|---|---|
| Age (years±SD) | 62.5±12 | 60.6±10 | 63.3±13 | .2 |
| BMI | 29.2±6 | 30.1±5 | 28.7±6 | .2 |
| Male | 84(109) | 88(37) | 82(72) | .4 |
| Chronic (months) | ||||
| -months from presentation | 46.9±59 | 45.8±43 | 47.5±65 | .9 |
| Prior aortic repair | 41(53) | 43(18) | 40(35) | .7 |
| Time since prior aortic repair (mos) |
38.5±46 | 32.8±54 | 20.3±38 | .6 |
| ASA 2 | 1(1) | 0 | 1(1) | .5 |
| 3 | 18(24) | 29(12) | 14(12) | .04 |
| 4 | 81(105) | 71(30) | 85(75) | .06 |
| 5 | 0 | |||
| COPD | 16(21) | 14(6) | 17(15) | .7 |
| Smoking history | 44(57) | 45(19) | 43(38) | .8 |
| End stage renal disease | 4(5) | 2(1) | 5(4) | .5 |
| Chronic renal insufficiency | 32(41) | 21(9) | 36(32) | .09 |
| Hypertension | 94(122) | 93(39) | 94(83) | .7 |
| Dyslipidemia | 14(18) | 19(8) | 11(10) | .2 |
| Diabetes mellitus | ||||
| Congestive heart failure | 8(10) | 7(3) | 8(7) | .9 |
| Peripheral vascular disease | 5(7) | 2(1) | 7(6) | .3 |
| Cerebrovascular disease | 8(11) | 7(3) | 9(8) | .7 |
| Arrhythmia | 8(11) | 10(4) | 8(7) | .8 |
| Coronary artery disease | 6(9) | 10(4) | 5(4) | .3 |
| Marfan syndrome | 6(8) | 10(4) | 5(4) | .3 |
| TAAA diameter (mm) | 62.8±11 | 65.0±11 | 61.7±11 | .1 |
BMI, body mass index; TEVAR, thoracic aortic endovascular repair; COPD, chronic obstructive pulmonary disease; TAAA, thoracoabdominal aortic false lumen aneurysm
Anatomic detail.
A majority of patients received TEVAR coverage within zone 2(64%) or 3(26%) but there was no difference for patients who did or did not undergo SAI(Table III). Only 4% had coverage extending below the celiac artery. An arch vessel adjunct to augment the proximal landing zone was required in 29% including carotid-subclavian bypass(22%), subclavian embolization(20%), and/or arch vessel stent(graft)(4%). Despite no difference in the overall proximal landing zone distribution, adjunctive arch vessel procedures during the index TEVAR were significantly more frequent for patients who subsequently underwent SAI(p=.02). Distal extent and visceral or infrarenal adjuncts were similar. Initial aortic diameter was larger in patients who ultimately underwent SAI(60±13 vs. 55± 14mm, p=.02). There was no difference in graft manufacturer or number of components used between patients with and without SAI.
Table III.
Operative description, anatomic detail and outcomes for all patients with or without secondary aortic intervention after TEVAR for type B dissection
| Feature, % (No.) |
All patients N=258 |
Secondary Aortic Intervention(+) 27%(N=70) |
Secondary Aortic Intervention(−) 73%(N=188) |
p-value |
|---|---|---|---|---|
| Device Type | ||||
| -Cook | 46(119) | 41(29) | 48(90) | .4 |
| -W.L. Gore | 36(94) | 36(25) | 37(69) | .9 |
| -Medtronic | 17(45) | 23(16) | 15(29) | .2 |
| # stent-graft components (±SD) | 2.1±0.9 | 2.1±0.9 | 2.1± 0.9 | .6 |
| Aortic diameter (mm±SD) | 56.6±13.1 | 59.9±13 | 54.9±14 | .02 |
| Proximal coverage zone | ||||
| -0 | 3(7) | 4(3) | 2(4) | .3 |
| -1 | 3(9) | 6(4) | 3(5) | .2 |
| -2 | 64(164) | 64(45) | 63(119) | .9 |
| -3 | 26(66) | 21(15) | 27(51) | .4 |
| -4 | 5(12) | 4(3) | 5(9) | .9 |
| Distal extent below celiac | 4(11) | 1(1) | 5(9) | .3 |
| Arch vessel adjunct | 29(75) | 40(28) | 25(47) | .02 |
| -C-SC bypass | 22(58) | 29(20) | 20(38) | .2 |
| -SCA embolization | 20(51) | 26(18) | 18(33) | .1 |
| -Arch vessel stent | 4(10) | 9(6) | 2(4) | .02 |
| EVAR | 8(20) | 7(5) | 8(15) | .8 |
| Visceral/renal adjunct | 17(45) | 11(8) | 20(37) | .1 |
| Elective | 43(111) | 49(34) | 41(77) | .3 |
| Urgent-symptomatic | 37(95) | 39(27) | 36(68) | .7 |
| Emergent-rupture | 20(52) | 13(9) | 23(43) | .08 |
| 30-day mortality | 7(19) | 1(1) | 10(18) | .03 |
| Any complication | 39(101) | 44(31) | 37(70) | .3 |
| Length of stay (days±SD) | 10.2±11 | 11.9±14 | 9.5±9 | .1 |
| Any postoperative SCI | 10(26) | 4(3) | 12(23) | .07 |
| Permanent SCI | 6(16) | 1(1) | 8(15) | .1 |
| Endoleak ever in follow-up | 61(137) | 92(59) | 49(78) | <.0001 |
| Endoleak within 1st postop year | 53(130) | 85(57) | 41(73) | <.0001 |
SD, standard deviation; C-SC, carotid subclavian bypass; SCA, subclavian artery; EVAR, infrarenal endovascular aortic stent repair; SCI, spinal cord ischemia; permanent deficits include partial or complete paralysis
As expected, CD patients most commonly had the initial TEVAR performed for aneurysm(95%) and less commonly malperfusion(5%) with only one rupture(0.8%). Conversely, AD patients had more variable indications for TEVAR; malperfusion(44%), aneurysm(25%), rupture(17%), and intractable pain/hypertension(14%).
Postoperative outcomes after index TEVAR.
Length of stay was 10±11 days. 30-day mortality was 7%(19) overall and significantly higher with AD than CD(13% vs. 2%;p=.002). The overall rate of any postoperative complication occurrence was 39%(AD 52% vs. CD 27%;p=.001). When examining differences between patients with or without SAI, no difference in elective or non-elective presentation for index TEVAR was noted, however SAI patients did have a lower 30-day mortality with their index TEVAR(1% vs. No SAI, 10%;p=.03). Initial aortic diameter was larger in patients who ultimately underwent SAI(60±13 vs. 55±14mm, p=.015).
Secondary aortic interventions.
Over a mean follow up of 17±22 months, SAIs occurred in 27%(70) of patients with a trend toward a higher rate for CD patients[CD, 32%(n=42) vs. 22%(28) for AD;p=.06). The distribution of the type of SAIs were similar among the AD and CD cohorts(Table IV). SAIs were evenly distributed between open and endovascular procedures. Likewise, the overall open conversion rate(including partial or total endograft explant) was comparable between AD and CD patients[10% vs. 15%;p=.22], as was the incidence of retrograde dissection resulting in SAI[6%(7 of 128) vs. 4%(5 of 130);p=.5].
Table IV.
Types of secondary aorta related re-intervention after TEVAR for acute and chronic type B dissection patients
| Feature, % (No.) |
Acute dissection SAI N=28 |
Chronic dissection SAI N=42 |
p-value .06 |
|---|---|---|---|
| Open Procedure | 61(17) | 23(55) | .3 |
| -Open Conversion | 46(13) | 48(20) | .2 |
| Major Endovascular | 39(11) | 38(16) | .3 |
| Minor Endovascular | 25(7) | 24(10) | .5 |
| Other | 32(9) | 10(4) | .1 |
Among all SAIs, 26% (n=18) occurred emergently and was not different among the two cohorts. Freedom from SAI was similar for both AD and CD patients (3-year: 65±7% vs. 52±8%;p=.7)(Figure 1). Median time to initial SAI, however, was significantly shorter for acute dissection subjects[0.7(0–12) vs. CD, 7(0–91)months;p<.001].
Figure 1.
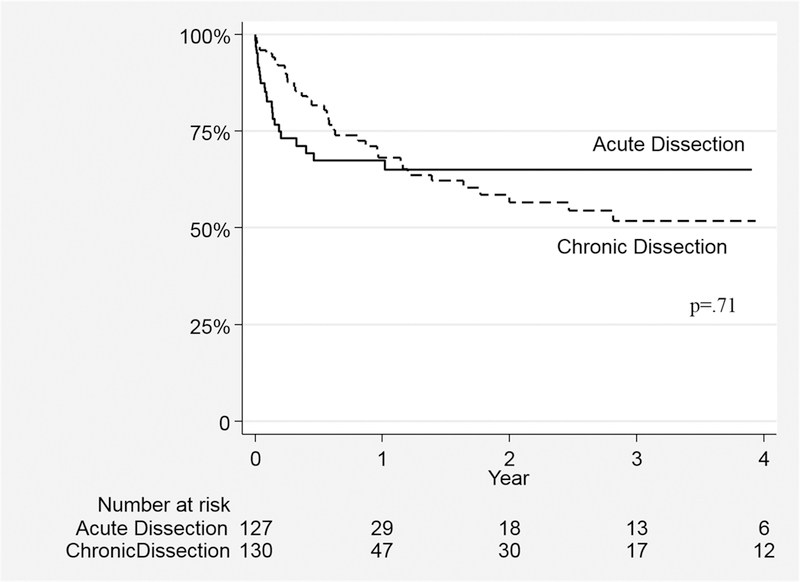
Freedom from Secondary Aorta Related Re-intervention after TEVAR for Acute and Chronic Type B Dissection
Survival Impact and Predictors of SAI.
Median survival time for the series was 48.7(68) months. The overall survival with or without SAI for the entire cohort was 81.3±4.9% vs. 70.5±3.4% at 3 and 69.6±6.1% vs. 63.0±3.8% at 5 years, respectively(log-rank p=.18)(Figure 2). Survival was similar regardless of the need for SAI for both AD(5-year: with SAI 67.1±9.9% vs. 55.0±5.5%;p=.24) and CD(5-year: 71.7±7.7% vs. 71.8±5.1%;p=.72)(Figure 3). Notably, survival was not predicted by type of SAI. Specifically, when examining the survival impact of open conversion events including repairs of retrograde type A dissection after TEVAR for TBAD, no difference in survival is noted compared to subjects not experiencing these events(p=.35;Figure 4). In an attempt to further understand the influence of SAI on survival beyond the early postoperative interval for both AD and CD, 30-day death events were censored and surviving subjects who subsequently went on to experience SAI were analyzed. As depicted in Figure 5, no significant difference in 3 or 5 year survival is noted for AD or CD with or without SAI among this subset of patients (AD;p=.76, CD:p=.99).
Figure 2.
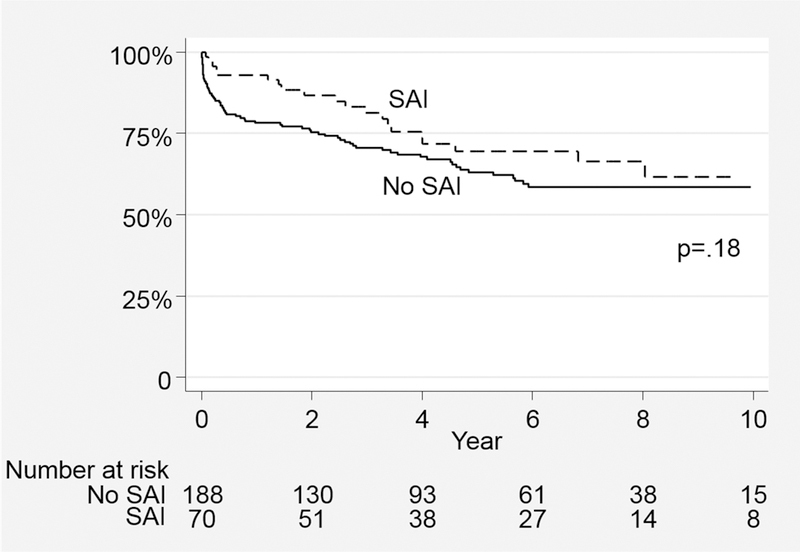
Survival with or without Secondary Aorta Related Re-intervention after TEVAR for Type B Dissection
Figure 3.
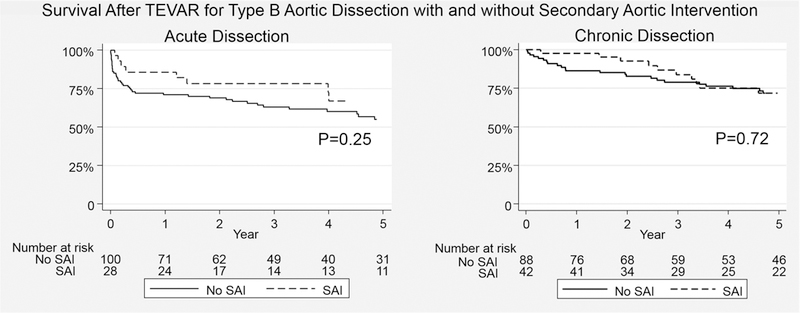
Survival Impact of Secondary Aorta Related Re-intervention after TEVAR for Acute and Chronic Type B Dissection
Figure 4.
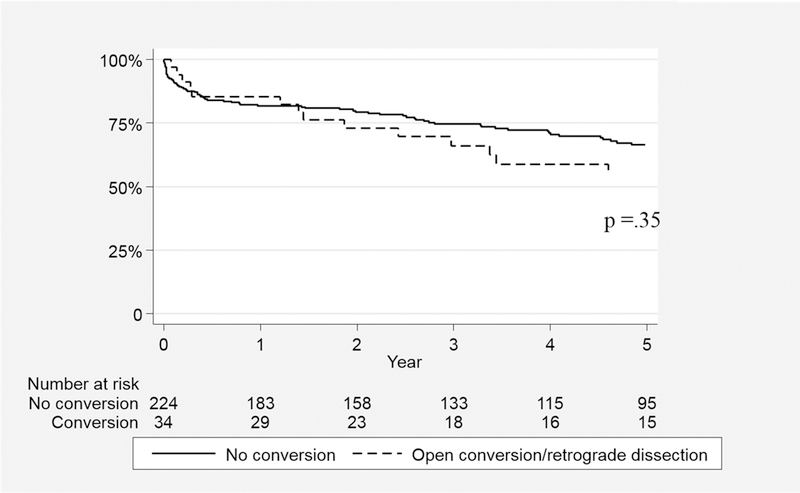
Survival Impact of Open Conversion and/or Repair of Retrograde Type A Dissection after TEVAR for Acute and Chronic Type B Dissection
Figure 5.
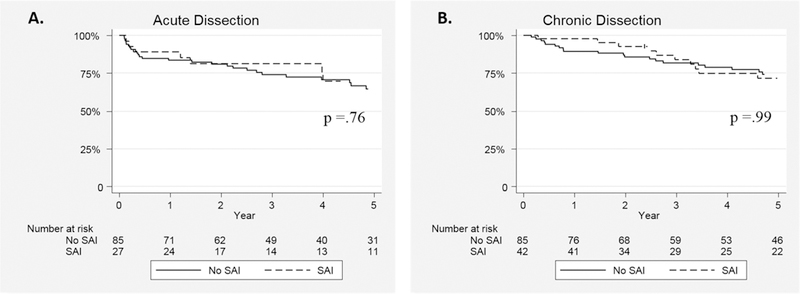
Survival Impact of Secondary Aorta Related Re-intervention Excluding Early (<30-day) Mortality Events after TEVAR for Acute and Chronic Type B Dissection
Univariate predictors of SAI included larger maximal aortic diameter, patients with Marfan syndrome, and those with an arch vessel adjunctive procedure performed with index TEVAR (Table Va). In multivariable analysis, there was an interaction between maximal aortic diameter and dissection acuity that was identified, demonstrating a stronger relationship for increasing diameter with an AD indication compared to chronic TBAD. The effect of Marfan syndrome and arch vessel adjunctive procedures were no longer significant when including all predictive variables. Importantly, indication for index procedure(aneurysm, malperfusion, rupture, or pain), remote (>30-day) history of a preoperative aortic procedure, number of stent grafts used during the index TEVAR, or proximal/distal landing zone coverage was not predictive of SAI (Table Vb).
Table Va.
Age-adjusted univariate predictors of secondary aortic intervention after TEVAR for acute and chronic type B aortic dissection
| Predictor | HR | 95% CI | P-value |
|---|---|---|---|
| Aortic diameter | 1.02 | 1.0–1.04 | .04 |
| Acute dissection* | 1.03 | .6–1.7 | .9 |
| Marfan syndrome | 2.3 | 1.1–4.9 | .03 |
| Arch vessel adjunct | 1.7 | 1.03–2.7 | .04 |
Table Vb.
Multivariable predictors of secondary aortic intervention after TEVAR for acute and chronic type B aortic dissection
| Predictor | HR | 95% CI | P-value |
|---|---|---|---|
| Age (by 10 year interval) | .78 | .61–1.0 | .05 |
| Aortic diameter | 1.03 | 1.01–1.06 | .007 |
| Diameter*acute dissection interaction | 1.01 | 1.00–1.03 | .04 |
| Marfan syndrome | 2.1 | .9–5.1 | .1 |
| Arch vessel adjunct | 1.5 | .8–2.6 | .2 |
Discussion
The study demonstrates that the need for SAI is common after TEVAR for both acute and chronic TBAD. Importantly, remediation procedures do not appear to adversely impact survival. Despite acute and chronic TBAD having significantly different indications and goals of treatment with the index TEVAR procedure, the likelihood of subsequent SAI is similar. Notably, the timing of SAI after TEVAR for AD typically occurred within the first postoperative year, which was significantly different than CD subjects who frequently underwent SAI beyond the first postoperative year. Notwithstanding these differences, the types of remedial procedures that occurred were comparable between groups. The significance of the study is highlighted by the fact that it is one of the largest series to date that provides granular details of SAI after TEVAR and directly compares acute and chronic TBAD patients.
There are several proposed mechanisms of TEVAR failure after treatment of TBAD including: 1) persistent retrograde FL perfusion from distal re-entry tears, 2) kinetic motion of the dissection flap and variable aortic-wall compliance 3) continued pressurization from the distal lumen despite thoracic FL aneurysm thrombosis 4) intimal-medial erosion26 from the interaction of the self-expanding stent-graft with the fragile aortic intima 5) endograft collapse due to a non-compliant dissection flap and 6) visceral vessel ischemia(if branch vessels receive dual or sole perfusion from the FL).27 Frequently, the extent of the dissection (e.g. extension into the visceral aortic segment; Debakey IIIb) has been reported to impact long-term aortic remodeling13, 27 which could also possibly contribute to the likelihood of subsequent aorta related re-intervention.
The incidence of SAI after TEVAR for TBAD has been reported by trials and registries demonstrating the efficacy of this technology in the management of acute, complicated presentations, including patients with rupture and/or end-organ malperfusion1, 2, 5, 6, 8, 11, 13, 28–30. In contrast, the indications for treatment of chronic TBAD remains poorly defined due to a lack of consensus about treatment goals, definitions of clinical success, as well as concerns about treatment failure.15, 27, 31 A variety of methods have been described to define successful clinical and anatomical outcome related to aortic remodeling after TEVAR for TBAD such as evaluation of false: true lumen ratios, false lumen volumetry, and total aortic/aneurysm diameter changes over time10, 13, 32, 33. Unlike degenerative aneurysms, complete exclusion of the FL(aneurysm) in dissection patients is often difficult to attain due to secondary distal septal tears. This distinction is notable since one could speculate that the lack of harmonization between AD and CD TBAD treatment indications and goals of care could lead to significant variability in reported rates of SAI.
Interestingly, despite these differences, a similar proportion of patients undergoing TEVAR for acute (7–35%) and chronic TBAD (5–47%) undergo subsequent re-intervention compared to 12–25% for degenerative aneurysm patients13, 14, 17, 34–36. A majority of these patients achieve FL thrombosis parallel to the stent graft and have good overall survival after re-intervention13, 36. Correspondingly, the incidence of SAI and overall survival within our study was similar to these series. Given the fact that AD is an acute aortic pathology that results in long-term vulnerability of secondary aortic complications, diligent follow-up and judicious re-intervention should be an expected part of the management algorithm.
The types of re-interventions that occurred in this series were similar between AD and CD patients. The relatively high proportion of open operations is notable since other reports have highlighted that endovascular re-interventions usually dominate. For example, a review of 41 patients who underwent TEVAR for AD had 14 subjects who experienced SAI and 13(93%) were endovascular re-interventions29. Remediation strategies after TEVAR for TBAD are variable and depend upon the failure mechanism, however the moderate incidence of open repair events(which include hybrid procedures) in our experience including open conversions reflects the anatomic complexity and selection bias within our own multi-disciplinary group practice. While the rate of open SAI operations was different than some other reports, some of these procedures were planned staged repairs, in whom the initial TEVAR procedure was performed to decrease the extent/complexity of the second operation, and/or decrease the risk of spinal cord ischemia by staging the repair. These types of complicated patients are common in our practice and may not be completely representative of all practices.
Virtually all SAIs performed after TEVAR for AD occurred within the first year, whereas those after CD happened at more variable time points and much later during the follow-up period. The variable timing of re-interventions after TEVAR for TBAD has been previously reported8, 14–16, 29, 37, 38. Device oversizing >10%, bare-spring stents, and early endoleaks with incomplete FL thrombosis have all been implicated as drivers of late re-interventions29. In our experience, the need for SAI was most strongly associated with initial aortic diameter, use of arch vessel adjuncts to augment a proximal landing zone, and history of Marfan syndrome. Interestingly, initial diameter was an important predictor only in AD cases. The use of TEVAR for patients with known connective tissue disorders is controversial due concerns about durability, however in a patient with AD, it can offer a bridge to definitive open surgical therapy after stabilization39. These risk factors may be markers for poor aortic wall integrity and indicate that the aorta is inherently more vulnerable to poor remodeling after intervention.
Perhaps the most dreaded complication from these interventions is precipitation of retrograde type A dissection(RTAD) that has been reported to occur in up to 2–6% of cases13, 26, 40. In our series, the overall RTAD rate was 5% and occurred with similar frequency after TEVAR for acute or chronic TBAD. In a systematic review of 8969 patients, the pooled estimate for RTAD was 2.5%(95%CI 2–3.1) with a 37.1%(95%CI 23.7–51.6%) mortality rate; however, this included all indications. Specific to aortic dissection, the relative risk of RTAD was 1.81(95% CI 1.04–3.14) for AD(relative to CD) a nd 5.33(95% CI, 2.70–10.51) for AD (relative to a degenerative aneurysm). Notably in that analysis, the incidence of RTAD was significantly different in patients with proximal bare stent and non-bare stent endografts (RR=2.06; 95% CI 1.22–3.50) 40.
These events highlight the current limitations of stent-graft design in the management of dissection given that treatment must often extend to the origin of the left common carotid artery, requiring the fragile and highly dynamic aortic tissue to interface with a somewhat unforgiving thoracic endograft. Moreover, the endograft frequently must adapt to significant differences in lumen size between the proximal and compressed distal true lumen, especially in CD cases where the septum is less likely to expand immediately after device placement. There may be benefit in the next generation of stent-graft design having pathology-specific device modifications to accommodate these challenges.
There are several limitations of this study. First, this is a retrospective, single center study with a modest sample size which increases risk of type II error. There was no standardized treatment algorithm or guidelines that governed the decisions surrounding SAI so we cannot account for the influence of selection bias on our results. We do not have the ability to compare this TEVAR group to those TBAD patients treated medically. Additionally, it is possible for 16 SAIs to have occurred at other institutions that would impact the results. Follow-up time was relatively short so the longer-term implications of SAI cannot be determined. In many cases, this was due to the nature of a large geographic referral pattern and patient preference to have follow-up closer to home after their index procedure.
Conclusions
SAI after TEVAR for TBAD is common and occurs with similar frequency irrespective if the index procedure was performed for an acute or chronic presentation. Concurrently, there is a similar distribution of the types of open and endovascular remedial procedures that occurs. AD patients have a higher proportion of early SAI, however CD patients appear to have a higher on-going need for remediation after the first year, which may provide some opportunity to tailor follow-up regimens based on presentation. Notably, despite some of the SAI events being complex, the overall survival is not negatively impacted compared to subjects without SAI. Patient features, anatomy and timing of initial presentation predict need for SAI. The relevance of these findings are underscored by the increasing adoption of TEVAR nationally to treat both acute and chronic TBAD which has implications on device design, patient selection, surveillance and anticipated outcomes of aorta related re-intervention.
JVS-D-18-00571R1, Implications of Secondary Aortic Intervention after TEVAR for Acute and Chronic Type B Dissection
Type of Research: Retrospective single center cohort study
Key Findings:
Out of 258 TEVARs performed for acute and chronic Type B aortic dissection 27% required secondary aortic interventions (SAIs ); SAIs did not affect survival.Younger age, acute dissection with large aortic diameter, Marfan syndrome and brachiocephalic adjunctive procedures predicted SAIs.
Take Home Message:
In patients who undergo TEVAR for aortic dissections, younger age, acute dissection with large aortic diameter, Marfan syndrome and brachiocephalic adjunctive procedures will predicted SAIs, but long term survival will not be affected.
Acknowledgements
This work was supported in part by funding from the National Institutes of Health (NIH-NHLBI 5K23HL115673–02) and the Society for Vascular Surgery Foundation Mentored Patient-Oriented Research Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Society for Vascular Surgery Foundation.
Financial Support: This work was supported in part by funding from the National Institutes of Health (NIH-NHLBI 5K23HL115673–02) and the Society for Vascular Surgery Foundation Mentored Patient-Oriented Research Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Fattori R, Montgomery D, Lovato L, Kische S, Di Eusanio M, Ince H, et al. Survival after endovascular therapy in patients with type b aortic dissection: A report from the international registry of acute aortic dissection (irad). JACC Cardiovasc Interv 2013;6:876–882 [DOI] [PubMed] [Google Scholar]
- 2.Hanna JM, Andersen ND, Ganapathi AM, McCann RL, Hughes GC. Five-year results for endovascular repair of acute complicated type b aortic dissection. J Vasc Surg 2014;59:96–106 [DOI] [PubMed] [Google Scholar]
- 3.Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, Shimono T, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340:1546–1552 [DOI] [PubMed] [Google Scholar]
- 4.Fattori R, Cao P, De Rango P, Czerny M, Evangelista A, Nienaber C, et al. Interdisciplinary expert consensus document on management of type b aortic dissection. J Am Coll Cardiol 2013;61:1661–1678 [DOI] [PubMed] [Google Scholar]
- 5.Lombardi JV, Cambria RP, Nienaber CA, Chiesa R, Teebken O, Lee A, et al. Prospective multicenter clinical trial (stable) on the endovascular treatment of complicated type b aortic dissection using a composite device design. J Vasc Surg 2012;55:629–640 e622 [DOI] [PubMed] [Google Scholar]
- 6.Moulakakis KG, Mylonas SN, Dalainas I, Kakisis J, Kotsis T, Liapis CD. Management of complicated and uncomplicated acute type b dissection. A systematic review and meta-analysis. Ann Cardiothorac Surg 2014;3:234–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, et al. Endovascular repair of type b aortic dissection: Long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407–416 [DOI] [PubMed] [Google Scholar]
- 8.Singh M, Hager E, Avgerinos E, Genovese E, Mapara K, Makaroun M. Choosing the correct treatment for acute aortic type b dissection. J Cardiovasc Surg (Torino) 2015;56:217–229 [PubMed] [Google Scholar]
- 9.Zimmerman KP, Oderich G, Pochettino A, Hanson KT, Habermann EB, Bower TC, et al. Improving mortality trends for hospitalization of aortic dissection in the national inpatient sample. J Vasc Surg 2016;64:606–615 e601 [DOI] [PubMed] [Google Scholar]
- 10.Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, et al. Randomized comparison of strategies for type b aortic dissection: The investigation of stent grafts in aortic dissection (instead) trial. Circulation 2009;120:2519–2528 [DOI] [PubMed] [Google Scholar]
- 11.Kische S, Ehrlich MP, Nienaber CA, Rousseau H, Heijmen R, Piquet P, et al. Endovascular treatment of acute and chronic aortic dissection: Midterm results from the talent thoracic retrospective registry. J Thorac Cardiovasc Surg 2009;138:115–124 [DOI] [PubMed] [Google Scholar]
- 12.Conway AM, Qato K, Mondry LR, Stoffels GJ, Giangola G, Carroccio A. Outcomes of thoracic endovascular aortic repair for chronic aortic dissections. J Vasc Surg 2017 [DOI] [PubMed]
- 13.Andacheh ID, Donayre C, Othman F, Walot I, Kopchok G, White R. Patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type b aortic dissection. J Vasc Surg 2012;56:644–650; discussion 650 [DOI] [PubMed] [Google Scholar]
- 14.Scali ST, Feezor RJ, Chang CK, Stone DH, Hess PJ, Martin TD, et al. Efficacy of thoracic endovascular stent repair for chronic type b aortic dissection with aneurysmal degeneration. J Vasc Surg 2013;58:10–17 e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohlffs F, Tsilimparis N, Diener H, Larena-Avellaneda A, Von Kodolitsch Y, Wipper S, et al. Chronic type b aortic dissection: Indications and strategies for treatment. J Cardiovasc Surg (Torino) 2015;56:231–238 [PubMed] [Google Scholar]
- 16.Leshnower BG, Duwayri YM, Chen EP, Li C, Zehner CA, Binongo JN, et al. Aortic remodeling after endovascular repair of complicated acute type b aortic dissection. Ann Thorac Surg 2017;103:1878–1885 [DOI] [PubMed] [Google Scholar]
- 17.Kamman AV, de Beaufort HW, van Bogerijen GH, Nauta FJ, Heijmen RH, Moll FL, et al. Contemporary management strategies for chronic type b aortic dissections: A systematic review. PLoS One 2016;11:e0154930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coady MA, Ikonomidis JS, Cheung AT, Matsumoto AH, Dake MD, Chaikof EL, et al. Surgical management of descending thoracic aortic disease: Open and endovascular approaches: A scientific statement from the american heart association. Circulation 2010;121:2780–2804 [DOI] [PubMed] [Google Scholar]
- 19.Debakey ME, Henly WS, Cooley DA, Morris GC Jr., Crawford ES, Beall AC Jr. Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg 1965;49:130–149 [PubMed] [Google Scholar]
- 20.Matsumura JS, Lee WA, Mitchell RS, Farber MA, Murad MH, Lumsden AB, et al. The society for vascular surgery practice guidelines: Management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg 2009;50:1155–1158 [DOI] [PubMed] [Google Scholar]
- 21.Feezor RJ, Martin TD, Hess PJ Jr., Beaver TM, Klodell CT, Lee WA. Early outcomes after endovascular management of acute, complicated type b aortic dissection. J Vasc Surg 2009;49:561–566; discussion 566–567 [DOI] [PubMed] [Google Scholar]
- 22.Melissano G, Bertoglio L, Rinaldi E, Civilini E, Tshomba Y, Kahlberg A, et al. Volume changes in aortic true and false lumen after the “petticoat” procedure for type b aortic dissection. J Vasc Surg 2012;55:641–651 [DOI] [PubMed] [Google Scholar]
- 23.Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL, Standards SfVSAHCoTR. Reporting standards for thoracic endovascular aortic repair (tevar). J Vasc Surg 2010;52:1022–1033, e1015 [DOI] [PubMed] [Google Scholar]
- 24.Chaikof EL, Fillinger MF, Matsumura JS, Rutherford RB, White GH, Blankensteijn JD, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg 2002;35:1061–1066 [DOI] [PubMed] [Google Scholar]
- 25.Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Reporting standards for thoracic endovascular aortic repair (tevar). J Vasc Surg 2010;52:1022–1033, e1015 [DOI] [PubMed] [Google Scholar]
- 26.Yang CP, Hsu CP, Chen WY, Chen IM, Weng CF, Chen CK, et al. Aortic remodeling after endovascular repair with stainless steel-based stent graft in acute and chronic type b aortic dissection. J Vasc Surg 2012;55:1600–1610 [DOI] [PubMed] [Google Scholar]
- 27.Thrumurthy SG, Karthikesalingam A, Patterson BO, Holt PJ, Hinchliffe RJ, Loftus IM, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (tevar) of chronic type b aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632–647 [DOI] [PubMed] [Google Scholar]
- 28.Ameli-Renani S, Das R, Morgan RA. Thoracic endovascular aortic repair for the treatment of aortic dissection: Post-operative imaging, complications and secondary interventions. Cardiovasc Intervent Radiol 2015;38:1391–1404 [DOI] [PubMed] [Google Scholar]
- 29.Faure EM, Canaud L, Agostini C, Shaub R, Boge G, Marty-ane C, et al. Reintervention after thoracic endovascular aortic repair of complicated aortic dissection. J Vasc Surg 2014;59:327–333 [DOI] [PubMed] [Google Scholar]
- 30.Ramdass M Tevar for symptomatic stanford b dissection: A systematic review of 30-day mortality and morbidity. Thorac Cardiovasc Surg 2015;63:97–112 [DOI] [PubMed] [Google Scholar]
- 31.Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1–41 [DOI] [PubMed] [Google Scholar]
- 32.Stanley GA, Murphy EH, Knowles M, Ilves M, Jessen ME, Dimaio JM, et al. Volumetric analysis of type b aortic dissections treated with thoracic endovascular aortic repair. J Vasc Surg 2011;54:985–992; discussion 992 [DOI] [PubMed] [Google Scholar]
- 33.Watanabe Y, Shimamura K, Yoshida T, Daimon T, Shirakawa Y, Torikai K, et al. Aortic remodeling as a prognostic factor for late aortic events after thoracic endovascular aortic repair in type b aortic dissection with patent false lumen. J Endovasc Ther 2014;21:517–525 [DOI] [PubMed] [Google Scholar]
- 34.Eggebrecht H, Nienaber CA, Neuhauser M, Baumgart D, Kische S, Schmermund A, et al. Endovascular stent-graft placement in aortic dissection: A meta-analysis. Eur Heart J 2006;27:489–498 [DOI] [PubMed] [Google Scholar]
- 35.Atkins MD Jr., Black JH 3rd, Cambria RP Aortic dissection: Perspectives in the era of stent-graft repair. J Vasc Surg 2006;43 Suppl A:30A–43A [DOI] [PubMed] [Google Scholar]
- 36.Parsa CJ, Schroder JN, Daneshmand MA, McCann RL, Hughes GC. Midterm results for endovascular repair of complicated acute and chronic type b aortic dissection. Ann Thorac Surg 2010;89:97–102; discussion 102–104 [DOI] [PubMed] [Google Scholar]
- 37.Nozdrzykowski M, Luehr M, Garbade J, Schmidt A, Leontyev S, Misfeld M, et al. Outcomes of secondary procedures after primary thoracic endovascular aortic repairdagger. Eur J Cardiothorac Surg 2016;49:770–777 [DOI] [PubMed] [Google Scholar]
- 38.Patel HJ, Williams DM, Meerkov M, Dasika NL, Upchurch GR Jr., Deeb GM Long-term results of percutaneous management of malperfusion in acute type b aortic dissection: Implications for thoracic aortic endovascular repair. J Thorac Cardiovasc Surg 2009;138:300–308 [DOI] [PubMed] [Google Scholar]
- 39.Waterman AL, Feezor RJ, Lee WA, Hess PJ, Beaver TM, Martin TD, et al. Endovascular treatment of acute and chronic aortic pathology in patients with marfan syndrome. J Vasc Surg 2012;55:1234–1240; disucssion 1240–1231 [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Zhang S, Liu L, Lu Q, Zhang T, Jing Z. Retrograde type a aortic dissection after thoracic endovascular aortic repair: A systematic review and meta-analysis. J Am Heart Assoc 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]


