Abstract
There is a growing need to screen multiple infections simultaneously rather than diagnosis of one pathogen at a time in order to improve the quality of healthcare while saving initial screening time and reduce costs. This is the first demonstration of a five-step protein array assay for the multiplexed detection of HIV, HPV and HSV antibodies on an integrated microfluidic system. HIV, HPV and HSV reactive antibodies from both serum and saliva were rapidly detected by acoustic streaming-based mixing and pumping to enable an integrated, rapid and simple-to-use multiplexed assay device. We validated this device with 37 serum and saliva samples to verify reactivity of patient antibodies with HIV, HPV and HSV antigens. Our technology can be adapted with different protein microarrays to detect a variety of other infections, thus demonstrating a powerful platform to detect multiple putative protein biomarkers for rapid detection of infectious diseases. This integrated microfluidic protein array platform is the basis of a potent strategy to delay progression of primary infection, reduce the risk of co-infections and prevent onward transmission of infections by point-of-care detection of multiple pathogens in both serum and oral fluids.
Keywords: acoustic microstreaming, microfluidics, human immunodeficiency virus, herpes simplex virus, human papilloma virus, serum, saliva
Introduction
In 2016, an estimated 36.7 million people were living with human immunodeficiency virus (HIV), including 1.8 million new cases and 1 million deaths worldwide (1). More than 3.7 billion people under the age of 50 – or 67% of the population – are estimated to be infected with herpes simplex virus type 1 (HSV-1), the primary cause of oral herpes, and 417 million people aged 15–49 years are estimated to be infected with HSV-2, the primary cause of genital herpes (2). Human papilloma virus (HPV) infection is also very common with an estimated 11.7% prevalence worldwide or nearly 900 million infected people (3). HIV, HSV and HPV can all be transmitted sexually as well as vertically (4–6). Moreover, HPV and HSV can also be transmitted by more casual contact (5,6).
Clinicians have observed that patients with one sexually transmitted disease (STD) are more likely to have another, either concomitantly or subsequently. Moreover, both HSV-1 and HSV-2, in immunocompromised people such as those with HIV, can have severe symptoms with more frequent outbreaks. Genital lesions by HSV-2 increase the risk of acquiring new HIV infections by approximately three fold (7,8). In females, synergism between HIV and HPV have also been observed, and women who are HIV-seropositive have higher rates of HPV infection than women who are HIV-seronegative (9). Higher rates of hysterectomy among HIV-infected women are persistent due to poor response to treatment and recurrent neoplasia (10). However, with early and appropriate diagnosis, HPV related cervical cancer is a preventable disease. Therefore, we developed a novel multiplexed microfluidic device to diagnose multiple HIV, HSV and HPV antigen reactivity from serum and saliva samples within <20 minutes.
Standard laboratory methods for detection of HIV infection include nucleic acid based viral load assay as well as western blot, and enzyme linked immunosorbent assays (ELISA) for detection of anti-HIV antibodies (11). Clinical diagnosis of HSV or HPV infection is confirmed by viral culture (cytological testing) and DNA detection in fluid from blisters and cervical lesions for the latter. Cytological testing has low sensitivity (45%), and poor specificity as it is highly subjective to the interpretation of the cytologist (12,13). On the other hand, standard nucleic acid based testing is expensive, time-consuming (>2 hours), and needs genomic amplification (14). Bench-top western blot and ELISA take a long time (up to two days) due to inefficient mass transport for immune-agents to move from a solution to the surface, require trained personnel and utilize large sample volumes, which makes them unsuitable for use in resource limited areas (15). With such a high prevalence of these viral infections, integrating and incorporating, rapid (<20 minutes), multiplexed, and low-cost diagnosis is desirable.
Recent advances in microfluidic technologies hold promise for the development of rapid, portable diagnostic tests for HIV, HPV and HSV (16,17). Microfluidic devices for detection of infectious diseases generally use either nucleic acids or immunoassays based on antigen-antibody interactions for detecting viral load. Nucleic acid based microfluidic devices, however, require additional sample pre-treatment steps such as cell lysis, nucleic acid extraction and amplification which adds complexity (18,19). Lateral flow immunoassays, on the other hand, use capillary forces for fluid actuation on paper based microfluidic devices (20,21), however, they generally have low sensitivity (μM-mM), are qualitative, rely on user interpretation and are unable to detect multiple targets at once (22). Microfluidic devices for capturing and detecting intact HIV-1 using antibody conjugated quantum dots, magnetic beads, and photonic crystals (23–25) are promising but they suffer from added expense and typically more bulky instrumentation needs.
ELISA or western blot based microfluidic devices have been developed with chemiluminescent (26,27) fluorescent (28) plasmonic (29) or colorimetric (30) detection to measure the antibody concentration in bodily fluids. However, chemiluminescence, fluorescence and plasmonic detection of antibodies require expensive instrumentation and are not suitable for resource-limited settings. Quantitative colorimetric detection which can rapidly generate the diagnosis will be vital. Recently, a microfluidic chip has been developed to capture antibodies against HIV-1 glycoprotein 41 and glycoprotein 36 using silver ions on gold for signal amplification (31–33). Although, the optical detection was inexpensive and simple to use, the system was limited to detection of a handful of antigens. Here, we demonstrate a quick, portable, multiplexed immunoassay for detection of infection with three viruses simultaneously. Moreover, our assay utilized multiple antigens from each virus to increase both sensitivity (≤20 pM) and specificity.
Here we integrate a protein microarray printed on a nitrocellulose coated glass slide (8,34,35) with an acoustic microstreaming based microfluidic device to pump and mix the ELISA reagents (36,37). The reagent flow is achieved with lateral cavity acoustic transducers (LCATs) and the mixing is achieved with vertical cavity acoustic transducers (VCATs). LCATs are the dead-end side channels arrayed in the X-Y plane while VCATs lead to the formation of the air pockets in the Z direction (normal to the plane) (Fig. 1a). An activated piezoelectric transducer (PZT) transmits acoustic energy to the air-liquid interfaces in both LCATs and VCATs, causing them to vibrate and produce streaming patterns in the nearby fluid. Net bulk fluid flow is generated by the LCAT vortices and fluid mixing is created in the direction perpendicular to fluid flow by two vortices in opposite directions due to the activity of VCATs. This transitions the biomolecular transport from diffusion dominated to convective, thereby reducing the detection time by more rapid antigen-antibody binding (38). In this work, we show the diagnosis from serum samples of HIV, HPV, HSV positive patients and normal donors within 17.5 minutes in comparison with the benchtop assay on the same microarrays which takes two hours up to overnight. We found antigens that showed significant reactivity for anti-HIV, anti-HPV and anti-HSV antibodies from serum samples; these are candidates for the diagnosis of these viral infections. To further demonstrate the device capability in a doctor’s or dentist’s office or where access to healthcare and phlebotomists is limited, we operate the device using saliva samples from HIV, HPV and HSV positive people. Overall, this work demonstrates a rapid and portable diagnostic platform for the detection of multiple biomarkers as a result of single or co-infection with HIV, HSV and HPV to monitor infections, reduce resistance and improve overall healthcare.
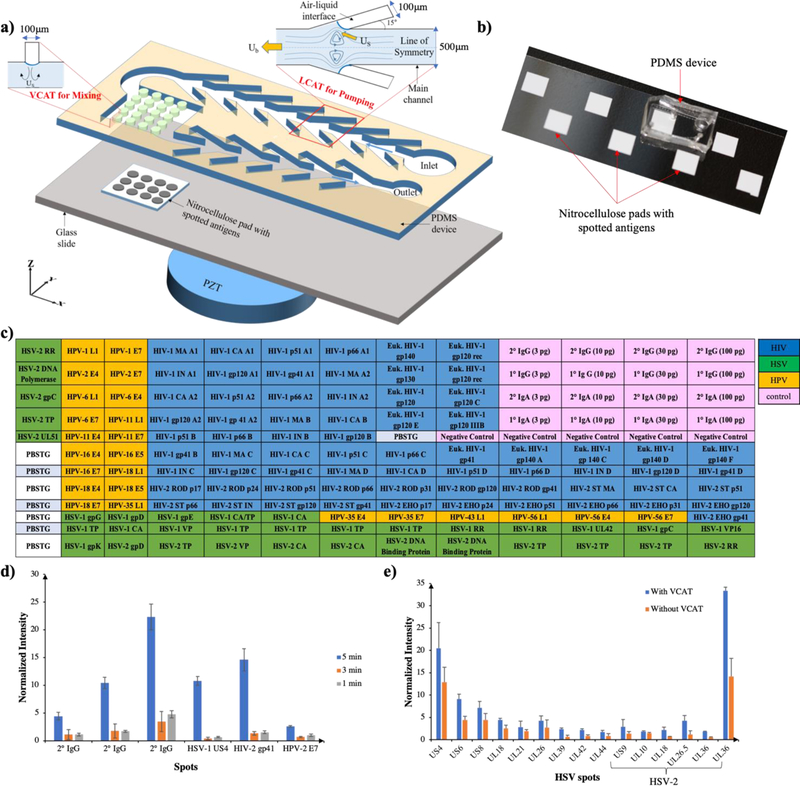
Figure 1: Schematic and layoutof an integrated microfluidic device for multiplexed detection of viral infection.
a) Device schematic showing LCAT’s and VCAT’s for pumping and mixing of patient sample containing antibodies aligned on the nitrocellulose pad. b) PDMS device bonded on top of the nitrocellulose pad, ready for use. c) Layout of HSV, HIV, HPV antigens produced by IVTT and HIV antigens produced in eukaryotic cells as well as primary and secondary IgG and IgA and negative controls. d) Plot of normalized spot intensity for reactivity against various spots at different sample incubation times. e) Plot of normalized intensity for reactivity against HSV+ spots for devices with and without VCAT mixers.
Materials and Methods
Microfluidic Chip Fabrication
To fabricate the microfluidic device, we patterned SU-8 photoresist 2050 onto a 3-inch silicon wafer following the manufacturer’s protocol for a height of 100 µm to fabricate the first layer with LCATs. Following that we aligned the second layer (50 µm height) with MA-56 aligner (Karl Suss) to fabricate VCATs. Polydimethylsiloxane (PDMS) was cast onto the wafer at a ratio of 1:11.5 (curing agent: base). Following the punching of inlet and outlet holes, the PDMS device was reversibly bonded to a microscope slide bearing nitrocellulose pads using a solution of Rain-X.
Protein Expression and Microarray Printing
Mature HIV, HSV and HPV proteins were amplified by PCR, inserted into plasmid expression vectors, expressed by coupled E. coli based in vitro transcription and translation (IVTT) and printed on nitrocellulose coated slides. Approximately 1 nL (~0.4 ng protein of interest) of each IVTT reaction was spotted onto 16-pad nitrocellulose coated Oncyte Avid Slides (Grace Bio-Labs) using an OmniGrid Accent microarray printer (Digilab) equipped with a 946 Printhead and 946 MP4 Spotting Pins (ArrayIt). This array contains 56 of the most sero-reactive HIV proteins produced by IVTT, from HIV-1 subtypes A1, A2, B, C and D as well as HIV-2 groups A and B, plus 13 HIV glycoproteins produced in mammalian cells obtained from the NIH AIDS Reagent Repository, 34 of the most seroreactive HSV-1 and HSV-2 proteins and 24 of the most sero-reactive HPV proteins from nine publicly available subtypes: HPV-1, 2, 6, 11, 16, 18, 35, 43 and 56. All HSV and HPV proteins used were produced by IVTT.
Serum and Saliva Sample Collection and Processing
Anonymous blood and saliva samples were collected from normal volunteer donors at UCI with IRB approval and informed consent. Blood samples were centrifuged at 1300 rpm for 10 minutes to separate serum from cells. Saliva was collected using Sarstedt Salivette devices and processed according to the manufacturer’s recommendations. Deidentified HIV+ sera and saliva samples were kindly provided by the Consortium for the Evaluation and Performance of HIV Incidence Assays. One-half percent Triton X-100 was added to the samples to inactivate viruses.
Assay Details
After priming, the microfluidic device was placed onto a piezoelectric transducer coated with ultrasound gel, subjected to a square wave at 49.8 kHz, 7 Vpp and sample (diluted 1:25) was added to the inlet port (39). The sample was loaded continuously for five minutes and remaining sample was removed from the inlet. Blocking buffer was then pumped for one minute to remove unbound antibodies from the pad. Next, 25 µL of alkaline phosphatase conjugated goat anti-human IgG or goat anti-human IgA was added and pumped for five minutes. After a second wash with blocking buffer for one minute to remove unbound secondary antibodies, 25 µL of substrate (NBT/BCIP) was pumped and incubated for 3.5 minutes to generate the colored product on antibody-bound antigen spots. The pad was then washed with blocking buffer and DI water (one minute each) to remove excess substrate and prevent color intensity saturation. The pad was finally dried at room temperature after de-laminating the PDMS device and imaged using a USB microscope (Koolertron). All experiments were performed in accordance with the Institutional Biosafety Committee Guidelines (protocol number: 2011–1383) and approved by the Institutional Review Board (Study number: 2012–8675) at University of California Irvine. Study participants were fully informed regarding the purposes of the study and consent was obtained.
Data collection and Analysis
Spot intensity values were exported for each array as CSV files. The individual CSV files were compiled and organized using R into “raw” data files. The raw data was normalized by dividing the IVTT protein spot intensity by the sample specific median of the IVTT negative control spots (no DNA added to IVTT) printed throughout the chip, then taking the base-2 logarithm of the ratio. The normalized data provides a relative measure of specific antibody binding to the non-specific antibody binding to the IVTT negative controls. Normalized data was imported into R statistical software and analyzed for antigen reactivity and significance. Multi-group comparisons were made using ANOVA. All measures of significance were adjusted for multiple comparisons by the false discovery rate (FDR).
Results
We previously utilized acoustic microstreaming to demonstrate pumping, mixing, sorting and enrichment of target cells/particles from complex biofluids such as blood (40–42). For the present study, fluid pumping was accomplished by 100 μm wide LCATs arranged at a 15° angle to the main channel (500 μm) and spaced 350 μm that were designed and fabricated (Fig. 1a). For fluid mixing, VCATs with 100 μm diameter and spacing, oriented at 90° relative to the main fluid channel were aligned on the top wall of the microarray assay chambers (Fig. 1a). These dead-end side channels trap air and generate air-liquid interfaces when primed with an aqueous solution. The vibration of these air-liquid interfaces by a PZT cause first-order periodic flow in the liquid phase. This induces a steady second-order flow within the boundary layer and the slip condition then drives the steady streaming in the bulk of the fluid. The cavities at 15° generate a net force parallel to the flow direction. However, at 90°, two equal and opposite force components parallel to the flow direction cancel each other and the perpendicular component recirculates the fluid in the vertical direction, thereby causing mixing. Fig. 1b shows the molded PDMS microfluidic device bonded to a glass slide with eight nitrocellulose pads bonded to it which can aid in running eight patient samples in parallel.
Analysis of HSV, HIV and HPV positive serum samples
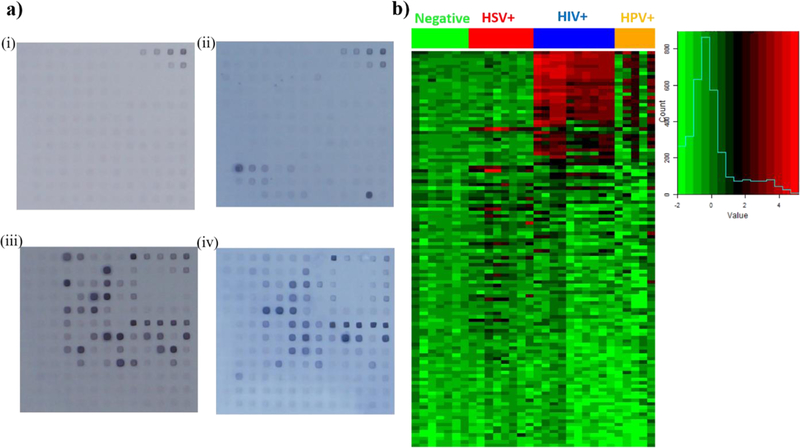
We printed a 12 by 13 array (pitch: 300μm) of immune-reactive HIV, HPV and HSV proteins on nitrocellulose pads bonded to microscope slides. The layout of the spotted antigens is shown in Fig. 1c. Each spot was printed with ~20 ng total protein (~0.4 ng protein of interest). We first analyzed the reactivity of pooled purified anti-HIV-IgG from the NIH AIDS Reagent Program and then five serum samples each from HIV+, HIV and HPV double+ and HSV+ individuals as well as five HIV, HPV and HSV triple negative samples using the assay demonstrated before. HIV-Ig and secondary antibody (AP conjugated goat anti-human IgG) were diluted at 1:50 and utilized for sample incubation time optimization from 1 minute to 5 minutes. We observed the maximum normalized intensity at 5minute sample incubation as shown in Fig. 1d and to reduce the noise level (intensity of negative control spots) and reduce the total assay time, we decided to incubate the antibody samples for 5 min. Subsequently, patient sera and secondary antibodies were diluted 1:25 in blocking buffer with 20% and 2% E. coli lysate, respectively and substrate incubation was optimized to 3.5 minutes after analyzing the HIV-Ig positive signal to blank signal intensity ratio. Serum samples were then tested for reactivity with the antigen spots using the 17.5minute assay. After quantification using ImageJ, the spotted pads (Fig. 2a (i)-(iv) for triple negative, HSV+, HIV+ as well as HIV and HPV double + samples respectively) were analyzed using R software and the heatmap is shown in Fig. 2b.
Figure 2: Serum antibody reactivity with HSV, HIV and HPV proteins on microarray.
a) Microarrays following 17.5minute assay with (i) triple negative donor sera (n=4 patient samples, two repeats), (ii) HSV+ donor sera (n=4 patient samples, two repeats), (iii) HIV+ donor sera (n=5 patient samples, two repeats), and (iv) HIV+, HPV+ sera (n=5 patient samples). b) Heat map of serum antibody reactivity with the color histogram on right indicating intensity on a log base 2 scale.
We used the rapid microfluidic assay we developed to test the reactivity of HSV+ sera with HSV antigens on the array. We found significant reactivity, compared to negative control sera, with nine antigens having ~4x intensity differences (p<<0.05) (Fig. 3a). Seven of these are HSV-1 proteins including glycoproteins G (US4), E (US8), D (US6), and C (UL42), viral capsid proteins encoded by genes UL26, UL18 and UL26.5 and tegument protein UL21. Furthermore, virion membrane glycoprotein D (US6) and tegument protein US9 showed significant positive reactivity among HSV-2 antigens. In addition, we assessed the importance of VCATs for mixing by analyzing the HSV+ sera in an LCAT-only device. As shown in Fig. 1d, we observed higher signal-to-noise ratio of the antibodies with spotted antigens when VCATs were utilized for mixing. This is due to efficient fluid displacement, especially during washing of non-specific antibodies, which resulted in lower intensity (noise) of negative controls.
Figure 3: Most significantly reactive proteins with positive serum antibodies in comparison to triple negative serum samples:
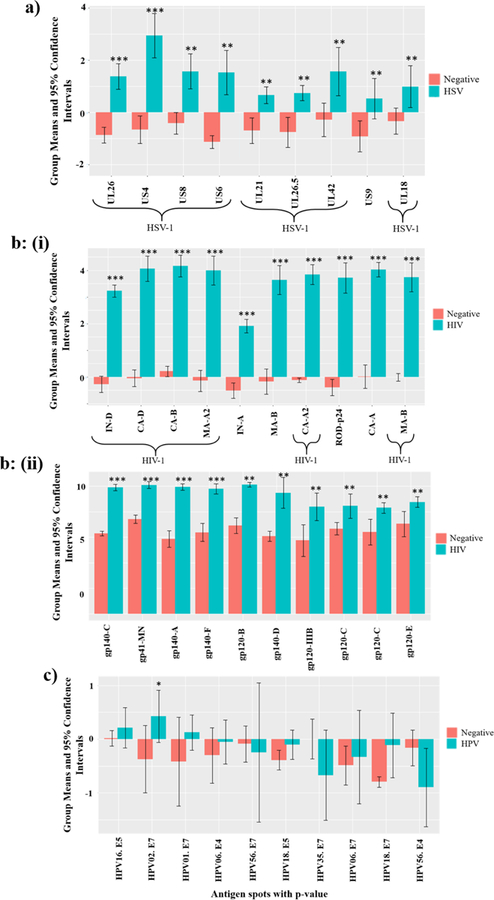
a) Reactivity of HSV+ serum samples with HSV proteins on the microarray. b) (i) Reactivity of HIV+ serum samples with HIV proteins produced by IVTT, (ii) Reactivity of HIV+ serum samples with HIV+ proteins produced in eukaryotic cells, c) Reactivity of HIV and HPV double positive serum samples with HPV IVTT proteins. *** denotes significance with p<e−4, ** indicates significance for p<0.05, * shows significance for p<0.1. Y-axis displays the average intensity of the spots with log base 2.
Similarly, Fig. 3b (i) shows the top ten HIV antigens produced by IVTT that were found to be most significantly reactive with HIV+ sera compared to the reactivity of negative sera (p<e−4). These antigens represent HIV-1 group M viruses, clades B, D and A2 which are responsible for a large part of the global AIDS pandemic. In addition to HIV-1 subtypes, the HIV+ patient samples also showed positive reactivity with HIV-2 antigens from both major groups, A and B. Furthermore, we compared the reactivity of the HIV+ and negative sera with purified HIV glycoproteins produced in eukaryotic cells. We found ten HIV glycoproteins that had ≥5x intensity differences and were statistically significant with p <<0.05 (Fig. 3b (ii)). These antigens were derived from HIV-1 clades A-F, which together comprise more than 90% of the worldwide AIDS pandemic. The HIV+ sera also showed significant reactivity with HSV-1 glycoprotein G (US4) with ~2x intensity and p <0.05 which might be due to HSV-1 infection.
HIV-infected individuals have higher levels of HPV seropositivity, with an increased frequency of infection with multiple HPV types simultaneously compared to HIV negative people. Therefore, we analyzed HIV+ sera which were reactive with HPV antigens and compared their reactivity with that of triple-virus negative samples. To increase the signal intensity, we added AP conjugated goat anti-human IgA antibodies along with goat anti-human IgG secondary antibodies at 1:25 dilution for this assay only. HPV reactivity was weaker than HIV or HSV reactivity, but we observed near-significant reactivity with three HPV antigens: HPV-1 E7, HPV-2 E7 (p <0.1), and HPV-16 E5, with ~2x intensity in comparison to the negative spots as shown in Fig. 3c. In addition, we observed significant reactivity (~2x intensity, p<0.05) of these patient samples with HSV-1 antigens glycoprotein G (US4), and capsid protein (UL26). HSV-2 antigens such as DNA polymerase subunit (UL42) and trans-activator tegument protein of early genes (UL48) also demonstrated near significant positive reactivity with p <0.1. This is likely due to HSV-1 and HSV-2 infection in one or more of these HIV-infected subjects.
Analysis of HSV, HIV and HPV positive saliva samples
After demonstrating the device’s potential using serum samples, we wanted to develop a diagnostic test which can utilize oral samples to minimize invasive sample collection. We tested saliva samples from four HIV+, four HIV and HSV double+, four HIV and HPV double+ and 4 triple negative individuals. Since, saliva has lower antibody titers, we reduced the dilution from 1:25 to 1:1 in blocking buffer (anti-IgG secondary antibody: 1:25) and used the same 17.5 minute assay. Images of the post-assay protein microarrays and a heat map of the reactivity (Fig. 4a and 4b) show reactivity of the positive saliva samples in comparison to triple negative samples.
Figure 4: Saliva antibody reactivity with HSV, HIV and HPV proteins on microarray.
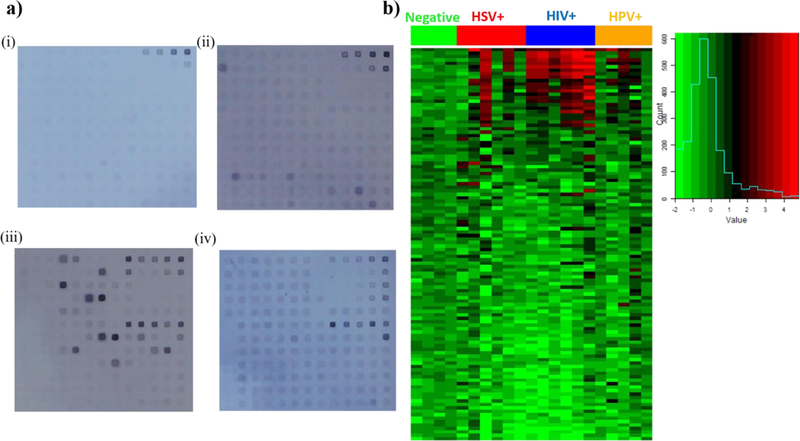
a) Microarrays following 17.5 minute assay with (i) triple negative donor saliva (n=4 patient samples), (ii) HSV+ donor saliva (n=6 patient samples), (iii) HIV+ donor saliva (n=4 patient samples, two repeats), and (iv) HIV+, HPV+ saliva (n=5 patient samples). b) Heat map of saliva antibody reactivity with the color histogram on right indicating intensity on a log base 2 scale
HIV, HSV double+ saliva showed slightly greater reactivity in comparison to the triple negative saliva with HSV-1 glycoprotein G (US4) and (UL42) DNA polymerase subunit from HSV-1 and HSV-2 (Fig. 5a). The large error bars are due to the relatively weak signals and the small number of samples used.
Figure 5: Most significantly reactive proteins with positive sample saliva antibodies in comparison to triple negative serum samples.
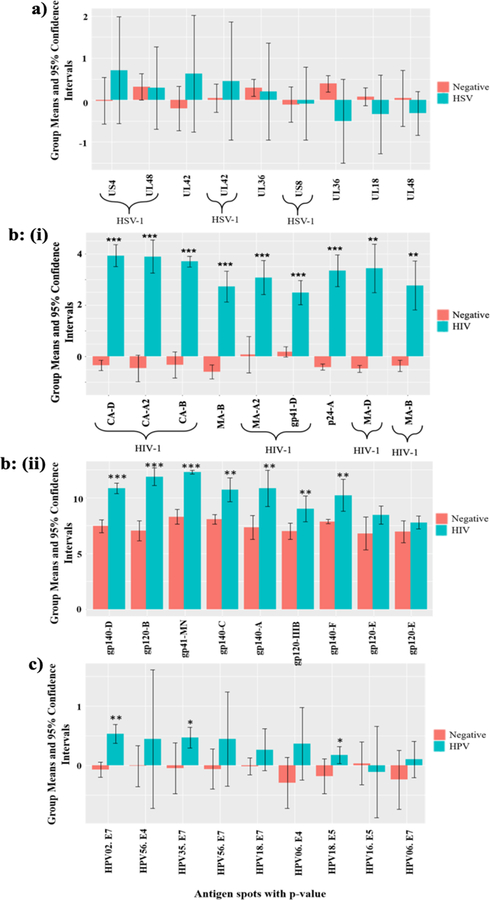
a) Reactivity of HSV+ saliva samples with HSV proteins on the microarray b) (i) Reactivity of HIV+ saliva samples with HIV proteins produced by IVTT, (ii) Reactivity of HIV+ saliva samples with HIV+ proteins produced in eukaryotic cells, c) Reactivity of HIV and HPV double positive saliva samples with HPV IVTT proteins. *** denotes significance with p<e−4, ** indicates significance for p<0.05, * shows significance for p<0.1. Y-axis displays the average intensity of the spots with log base 2.
After comparing the reactivity of HIV+ saliva samples to the reactivity of negative saliva samples, we ranked the most significantly reactive antigens based on the p-value. These donors showed 6–30 fold intensity differences for reactivity with HIV-1 proteins produced by IVTT such as the transmembrane glycoprotein, gp41 from clade D, capsid protein, p24 from clades A2, B and D and matrix protein, p17 from clades A2 and B. The top nine most significantly reactive antigens from HIV-1 and HIV-2 are shown in Fig. 5b (i). Similar to HIV+ sera, the positive reactivity among HIV-1 antigens were from clades B, D and A2. The saliva samples also showed reactivity with HIV-2 matrix antigens from groups A and B. The HIV+ saliva samples demonstrated significant reactivity with seven glycoproteins produced in eukaryotic cells (p<<0.05); 6–30 fold more intense than in triple negative samples as shown in Fig. 5b (ii). HIV single positive saliva samples didn’t present any significant HSV+ or HPV+ reactivity.
We then compared the HIV and HPV double positive saliva samples with triple negative saliva samples and similar to serum samples, we added AP conjugated anti-IgA antibodies to increase the signal intensity. As shown in Fig. 5c, HIV, HPV double + saliva showed significant and near significant reactivity (~2x intensity) with HPV-2 E7 (p<0.05), HPV-35 E7 (p<0.1), HPV-18 E5 (p<0.1), HPV-56 E4, HPV-56 E7, HPV-18 E7, and HPV-6 E4. In addition, we observed significant reactivity (~2x intensity) of these samples with HSV-1 tegument protein (UL21, p<0.05) and near significant reactivity with HSV-2 large tegument protein (UL36, p<0.1), HSV-1 glycoprotein G (US4), capsid protein (UL18), glycoprotein C (UL44), and virion protein VP16 (UL48). As for the HIV+, HPV+ serum samples, this likely resulted from HSV-1 and HSV-2 infection of one or more of the subjects.
Discussion
Currently, the diagnosis and treatment of viral infections with HIV, HPV and HSV is based on a handful of highly expressed biomarkers and each infection is diagnosed with different assays which causes delayed diagnoses and added expense (42). Many co-infections by HPV and HSV in HIV infected people go undetected which then results in full blown infections and encompass huge epidemiological burden (43). While a multiplexed HIV, HBV, HCV and syphilis screening is in development; the combined and simultaneous infections by HIV, HPV and HSV are at high-risk and needs continuous monitoring (43). Although metagenomic shotgun sequencing is powerful in screening a broad range of viruses and detected both HPV and HSV (combined) in a dermal swab sample, it is limited due to longer assay times (72 hours) with high costs (44) and needs nucleic acid extraction and amplification. Bonito et al. utilized Luminex-based multiplexed platform and found the presence of both HPV-SD2 and HSV-1 in raw sewage samples (45). Cao et al. utilized DNA microarray to simultaneously characterize pathogens including HSV and HPV with high sensitivity (102 – 103 copies), but the target genes were specific for single biomarker (glycoprotein B gene for HSV and L1 gene for HPV) and the hybridization process took ~1.5 hours (46). In this work, we demonstrate for the first time a novel microfluidic platform capable of detecting multiple biomarkers as a result of single viral infection or co-infection with HIV (both HIV-1 and HIV-2), HSV (both HSV-1 and HSV-2) and HPV (L1, E4, E7 for HPV-1, 2, 6, 11, 16, 18, 35, 56) within 17.5 minutes. This may be particularly relevant for HIV infected populations who often have other infections that interact with HIV and vice versa. For example, genital HSV infection increases the acquisition risk of HIV infection which leads to worsened clinical presentation of HSV infection due to the HIV induced immune-suppression. In addition, HIV-infected persons encompass a heavy burden of HPV-associated disease linked mostly to progressive immune suppression (47). With the presented microfluidic device, we observed that HIV+ serum and saliva samples showed significant reactivity with both HSV (US4, UL26, UL21) and HPV (HPV-2 E7 and HPV-35 E7) antigens. Therefore, in order to avoid false negatives and achieve precise diagnosis, we demonstrate, for the first time, diagnosis of all three pathogens within 17.5 minutes, necessitating the importance of a multiplexed assay. On the other hand, we observed varied reactivity of US4 in both HSV+ serum and saliva samples which can lead to test ambiguities and incomplete diagnosis. In order to minimize these risks and provide improved diagnosis of HSV infection, we identified multiple antigens such as capsid proteins (UL26, UL26.5, UL18), glycoprotein E (US8), tegument protein (UL21, US9), and DNA polymerase processivity factor (UL42), that are characteristic of antibodies produced during HSV (type 1 and type 2) infection. Therefore, contrary to the tests based on single antigens for diagnosing HIV and HSV (48), this technology will be able to provide clear, distinct antigens or antigen sets for serodiagnostic biomarker, vaccine, and therapeutic product development.
Although significant reactivity was obtained with serum samples, its sample preparation is time consuming and needs phlebotomists to draw blood. Therefore, to develop a diagnostic test for large scale detection at community health centers and dental clinics, we validated our platform with saliva samples. This is particularly important while handling of pediatric and geriatric patients in order to reduce the number of blood draws or when access to healthcare is limited in remote locations where phlebotomists are unavailable (49). Although, antibody detection for HIV from oral fluids has been developed, its dependence on single antigen (p24) might lead to false negatives. In addition, both HPV and HSV from saliva samples were detected with antibodies specific to secretory IgA by nephelometry and ELISA, respectively, which takes time and lacks specificity. In this work, with saliva samples, we obtained significant reactivity of multiple proteins from all three viruses, HIV, HSV and HPV similar to serum samples. Although antibody titers in saliva are generally low, we obtained six common antigens from serum and saliva samples which belongs to both HIV-1 (CA-B, CA-D, MA-A2, CA-A2, MA-B) and HIV-2 (MA-B, p24) and can be utilized as antigen sets for HIV diagnosis. While saliva reactivity was not found to be significant for HSV, US4 and UL42 from HSV-1 showed positive reactivity in both serum and saliva samples. HPV-2 E7 was common between HPV+ serum and saliva samples but due to asymptomatic nature of both HSV and HPV infections and sample preparations, it seems more appropriate to utilize serum samples for HSV and HPV diagnosis. Therefore, this device can also be used at the point-of-care in doctor’s or dentist’s office to screen for infections that affect care, using saliva samples. For example, HPV has been implicated in oropharyngeal cancer and identifying its symptoms via oral or dental examinations (saliva) may be the key to early detection and diagnosis. In addition, an oral test for detection of antibodies will allow large-scale population testing at community health centers, STD clinics and dental settings, thereby reducing the number of deaths worldwide (49).
Overall, this work demonstrates a highly promising approach for identification, analysis, and monitoring of antibodies to specific antigens or antigen sets as a result of single or multiple infections. This technology, based on acoustic microstreaming, assesses the reactivity of serum and saliva samples with the spotted antigens in less than twenty minutes. We observed diverse antibody responses and significant positive reactivity with multiple antigens from each virus. Hence this platform enables simultaneous screening of multiple antigens from various infections in different samples at reduced cost and time which are especially relevant for use in resource limited settings.
Conclusions
An acoustic microstreaming based LCAT-VCAT device that can assess the reactivity of antibodies from both serum and saliva samples to detect multiple infections is reported. Within 17.5 minutes of operation, diverse antibody responses and significant positive reactivity with multiple antigens from HSV, HIV and HPV to prevent false negatives is demonstrated. This study validates the use of integrated high-throughput microarray to show significant cross-reactivity between HSV and HIV infected serum samples, HSV and HIV-HPV dual positive serum and saliva samples. Finally, colorimetry-based analysis applied in the present work enables easy on-site testing which is suitable for point-of-care diagnosis.
Acknowledgements
This work was supported by the Schlumberger Faculty for the Future Award (award number SF-202940), and the National Institutes of Health under award number R43AI102288-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interests
The authors declare no competing financial interests with the work described in this manuscript.
References
- 1.UNAIDS DATA 2017. [Internet] Available from: http://www.unaids.org/en/resources/documents/2017/2017_data_book
- 2.WHO | Herpes simplex virus [Internet] WHO. Available from: http://www.who.int/mediacentre/factsheets/fs400/en/
- 3.WHO | 24 October 2014, vol. 89, 43 (pp. 465–492) [Internet] WHO Available from: http://www.who.int/wer/2014/wer8943/en/ [Google Scholar]
- 4.Seitz R Human Immunodeficiency Virus (HIV). Transfus Med Hemotherapy 2016;43(3):203–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasoglu S, Tekin HC, Inci F, Knowlton S, Wang S, Wang-Johanning F, et al. Advances in Nanotechnology and Microfluidics for Human Papillomavirus Diagnostics. Proc IEEE 2015. February;103(2):161–78. [Google Scholar]
- 6.Cowan FM, Copas A, Johnson AM, Ashley R, Corey L, Mindel A. Herpes simplex virus type 1 infection: a sexually transmitted infection of adolescence? Sex Transm Infect 2002. October 1;78(5):346–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald A, Link K. Risk of Human Immunodeficiency Virus Infection in Herpes Simplex Virus Type 2–Seropositive Persons: A Meta-analysis. J Infect Dis 2002. January 1;185(1):45–52. [DOI] [PubMed] [Google Scholar]
- 8.Kalantari-Dehaghi M, Chun S, Chentoufi A, Pablo J, Liang L, Dasgupta G, et al. Discovery of potential diagnostic and vaccine antigens in herpes simplex virus-1 and −2 by proteome-wide antibody profiling. J Virol 2012. February 8;JVI05194–11. [DOI] [PMC free article] [PubMed]
- 9.Ahdieh L, Klein RS, Burk R, Cu-Uvin S, Schuman P, Duerr A, et al. Prevalence, Incidence, and Type-Specific Persistence of Human Papillomavirus in Human Immunodeficiency Virus (HIV)-Positive and HIV-Negative Women. J Infect Dis 2001. September 15;184(6):682–90. [DOI] [PubMed] [Google Scholar]
- 10.Hysterectomy Among Women With HIV: Indications and Incidence [Internet] Available from: http://www.natap.org/2007/HIV/052207_11.htm [DOI] [PubMed]
- 11.Cornett JK, Kirn TJ. Laboratory Diagnosis of HIV in Adults: A Review of Current Methods. Clin Infect Dis 2013. September 1;57(5):712–8. [DOI] [PubMed] [Google Scholar]
- 12.Singh A, Preiksaitis J, Ferenczy A, Romanowski B. The laboratory diagnosis of herpes simplex virus infections. Can J Infect Dis Med Microbiol 2005;16(2):92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devegowda D, Doddamani P, Vishwanath P. Human papillomavirus screening: Time to add molecular methods with cytology. Int J Health Allied Sci 2014. July 1;3(3):145. [Google Scholar]
- 14.Inan H, Wang S, Inci F, Baday M, Zangar R, Kesiraju S, et al. Isolation, Detection, and Quantification of Cancer Biomarkers in HPV-Associated Malignancies. Sci Rep 2017. June 12;7(1):3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costantini F, Sberna C, Petrucci G, Manetti C, de Cesare G, Nascetti A, et al. Lab-on-chip system combining a microfluidic-ELISA with an array of amorphous silicon photosensors for the detection of celiac disease epitopes. Sens Bio-Sens Res 2015. December 1;6:51–8. [Google Scholar]
- 16.Shafiee H, Wang S, Inci F, Toy M, Henrich TJ, Kuritzkes DR, et al. Emerging Technologies for Point-of-Care Management of HIV Infection. Annu Rev Med 2015;66(1):387–405. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Guo S, Carvalho WSP, Jiang Y, Serpe MJ. Portable point-of-care diagnostic devices. Anal Methods 2016. November 10;8(44):7847–67. [Google Scholar]
- 18.Yeh E-C, Fu C-C, Hu L, Thakur R, Feng J, Lee LP. Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip. Sci Adv 2017. March 1;3(3):e1501645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branavan M, Mackay RE, Craw P, Naveenathayalan A, Ahern JC, Sivanesan T, et al. Modular development of a prototype point of care molecular diagnostic platform for sexually transmitted infections. Med Eng Phys 2016. August 1;38(8):741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Workman S, Wells SK, Pau C-P, Owen SM, Dong XF, LaBorde R, et al. Rapid detection of HIV-1 p24 antigen using magnetic immuno-chromatography (MICT). J Virol Methods 2009. September 1;160(1):14–21. [DOI] [PubMed] [Google Scholar]
- 21.Grant BD, Smith CA, Castle PE, Scheurer ME, Richards-Kortum R. A paper-based immunoassay to determine HPV vaccination status at the point-of-care. Vaccine 2016. November 4;34(46):5656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin CD, Linder V, Sia SK. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012. May 22;12(12):2118–34. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y-G, Moon S, Kuritzkes DR, Demirci U. Quantum dot-based HIV capture and imaging in a microfluidic channel. Biosens Bioelectron 2009. September 15;25(1):253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shafiee H, Jahangir M, Inci F, Wang S, Willenbrecht RBM, Giguel FF, et al. Acute On-Chip HIV Detection Through Label-Free Electrical Sensing of Viral Nano-Lysate. Small 2013. August 12;9(15):2553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inci F, Tokel O, Wang S, Gurkan UA, Tasoglu S, Kuritzkes DR, et al. Nanoplasmonic Quantitative Detection of Intact Viruses from Unprocessed Whole Blood. ACS Nano 2013. June 25;7(6):4733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Sierra N, Martró E, Castellà E, Llatjós M, Tarrats A, Bascuñana E, et al. Evaluation of an Array-Based Method for Human Papillomavirus Detection and Genotyping in Comparison with Conventional Methods Used in Cervical Cancer Screening. J Clin Microbiol 2009. July;47(7):2165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso R, Roa PL, Suárez M, Bouza E. New Automated Chemiluminescence Immunoassay for Simultaneous but Separate Detection of Human Immunodeficiency Virus Antigens and Antibodies. J Clin Microbiol 2014. May 1;52(5):1467–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaculovicova M, Michalek P, Krizkova S, Macka M, Adam V. Nanotechnology-based analytical approaches for detection of viruses. Anal Methods 2017;9(16):2375–91. [Google Scholar]
- 29.de la Rica R, Stevens MM. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nanotechnol 2012. December;7(12):821–4. [DOI] [PubMed] [Google Scholar]
- 30.Ng AHC, Uddayasankar U, Wheeler AR. Immunoassays in microfluidic systems. Anal Bioanal Chem 2010. June 1;397(3):991–1007. [DOI] [PubMed] [Google Scholar]
- 31.Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat Med 2011. August;17(8):1015–9. [DOI] [PubMed] [Google Scholar]
- 32.Guo T, Patnaik R, Kuhlmann K, Rai AJ, Sia SK. Smartphone dongle for simultaneous measurement of hemoglobin concentration and detection of HIV antibodies. Lab Chip 2015. August 11;15(17):3514–20. [DOI] [PubMed] [Google Scholar]
- 33.Laksanasopin T, Guo TW, Nayak S, Sridhara AA, Xie S, Olowookere OO, et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci Transl Med 2015. February 4;7(273):273re1–273re1. [DOI] [PubMed] [Google Scholar]
- 34.Luevano M, Bernard H-U, Barrera-Saldaña HA, Trevino V, Garcia-Carranca A, Villa LL, et al. High-throughput profiling of the humoral immune responses against thirteen human papillomavirus types by proteome microarrays. Virology 2010. September 15;405(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasgupta G, Chentoufi AA, Kalantari M, Falatoonzadeh P, Chun S, Lim CH, et al. Immunodominant “Asymptomatic” Herpes Simplex Virus 1 and 2 Protein Antigens Identified by Probing Whole-ORFome Microarrays with Serum Antibodies from Seropositive Asymptomatic versus Symptomatic Individuals. J Virol 2012. April;86(8):4358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tovar AR, Patel MV, Lee AP. Lateral air cavities for microfluidic pumping with the use of acoustic energy. Microfluid Nanofluidics 2011. June 1;10(6):1269–78. [Google Scholar]
- 37.Okabe Y, Chen Y, Purohit R, Corn RM, Lee AP. Piezoelectrically driven vertical cavity acoustic transducers for the convective transport and rapid detection of DNA and protein binding to DNA microarrays with SPR imaging—A parametric study. Biosens Bioelectron 2012. May 15;35(1):37–43. [DOI] [PubMed] [Google Scholar]
- 38.Liao U, Tovar A, Felgner P, Lee AP. A Microfluidic Approach and Enhancement Towards a Colorimetric Enzyme-Linked-Immunosorbant-Assay for Diagnostic Detection of Infectious Diseases 2007. January 1;71–2.
- 39.Garg N, Westerhof TM, Liu V, Liu R, Nelson EL, Lee AP. Whole-blood sorting, enrichment and in situ immunolabeling of cellular subsets using acoustic microstreaming. Microsyst Nanoeng 2018. February 26;4:17085. [Google Scholar]
- 40.Nivedita N, Garg N,P. Lee A, Papautsky I. A high throughput microfluidic platform for size-selective enrichment of cell populations in tissue and blood samples. Analyst 2017;142(14):2558–69. [DOI] [PubMed] [Google Scholar]
- 41.Garg N, Vallejo D, Boyle D, Nanayakkara I, Teng A, Pablo J, et al. Integrated On-Chip Microfluidic Immunoassay for Rapid Biomarker Detection. Procedia Eng 2016. January 1;159:53–7. [Google Scholar]
- 42.Mok J, Mindrinos MN, Davis RW, Javanmard M. Digital microfluidic assay for protein detection. Proc Natl Acad Sci 2014. February 11;111(6):2110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pant Pai N, Daher J. Multiplexed testing for HIV and related bacterial and viral co-infections at the point-of-care: quo vadis? Expert Rev Mol Diagn 2015. April;15(4):463–9. [DOI] [PubMed] [Google Scholar]
- 44.Wylie KM, Wylie TN, Buller R, Herter B, Cannella MT, Storch GA. Detection of Viruses in Clinical Samples Using Metagenomic Sequencing and Targeted Sequence Capture. J Clin Microbiol 2018. September 19;JCM01123–18. [DOI] [PMC free article] [PubMed]
- 45.Di Bonito P, Iaconelli M, Gheit T, Tommasino M, Della Libera S, Bonadonna L, et al. Detection of oncogenic viruses in water environments by a Luminex-based multiplex platform for high throughput screening of infectious agents. Water Res 2017. October 15;123:549–55. [DOI] [PubMed] [Google Scholar]
- 46.Cao B, Wang S, Tian Z, Hu P, Feng L, Wang L. DNA Microarray Characterization of Pathogens Associated with Sexually Transmitted Diseases. PLOS ONE 2015. July 24;10(7):e0133927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sexually Transmitted Viral Infections in Women: HIV, HSV, and HPV | Patient Care [Internet] Available from: http://www.patientcareonline.com/infections-medicine-journal/sexually-transmitted-viral-infections-women-hiv-hsv-and-hpv
- 48.Methods And Compositions of Protein Antigens For The Diagnosis And Treatment of Herpes Simplex Viruses Type 1 And 2 [Internet] 2016. Available from: https://patents.google.com/patent/US20160158344A1/en
- 49.Malamud D, Rodriguez-Chavez IR. Saliva as a Diagnostic Fluid. Dent Clin North Am 2011. January;55(1):159–78. [DOI] [PMC free article] [PubMed] [Google Scholar]