Abstract
Objective:
Multiplexed metabolic phenotyping systems are available from multiple commercial vendors, and each system includes unique design features. Although expert opinion supports strengths and weaknesses of each design, empirical data from carefully controlled studies to test the biological impact of design differences is lacking.
Methods:
Wildtype C57BL/6J mice of both sexes underwent phenotyping in OxyMax (Columbus Instruments International) and Promethion (Sable Systems International) systems located within the same room of a newly constructed animal research facility, in a crossover design study. Phenotypes were examined under chow (2920x)-fed conditions, and again after four weeks of 60% high fat diet (D12492) feeding.
Results:
Food intake, physical activity, and respiratory gas exchange data significantly diverged between systems, depending upon sex of animals and diet supplied. Estimates of energy expenditure based on gas exchange in both systems accounted for a fraction of consumed calories that was greater in males than females.
Conclusions:
Design differences quantitatively impact the assessment of metabolic endpoints and therefore the qualitative interpretation of various interventions. Importantly, current multiplexed systems remain blind to multiple additional endpoints including digestive efficiency and selected forms of energy flux (nitrogenous, anaerobic, etc.) which account for a physiologically / pathophysiologically-significant fraction of total energy flux.
Keywords: Energy, Metabolism, Calorimetry, OxyMax, Promethion
Introduction
Increased interest in comprehensive assessments of energy homeostasis for obesity research has driven field-wide adoption of multiplexed phenotyping systems, available from multiple vendors. Critically, few institutions host multiple systems from different vendors, and therefore few studies have been enabled to carefully and empirically test commonly held (but rarely tested) assumptions about the impact of different system designs. It remains unclear how studies of the same biological manipulations, when performed in distinct multiplexed phenotyping systems, may lead to differential conclusions due to the nuanced differences in phenotyping equipment.
Thus, the current project was carried out to address the following fundamental questions: (i) Do multiplexed phenotyping systems from different vendors yield similar results and precipitate similar conclusions about the biological mechanisms manipulated, despite substantially different equipment design? (ii) Does interpretation of results from studies using different systems require equipment-specific interpretation? (iii) If differences in results are observed from multiple systems, how can we know whether either of the systems is correct? (iv) Finally, what pitfalls and limitations should be considered when using any multiplexed system? Importantly, this study was not designed to identify mechanisms that underlie any observed differences between systems.
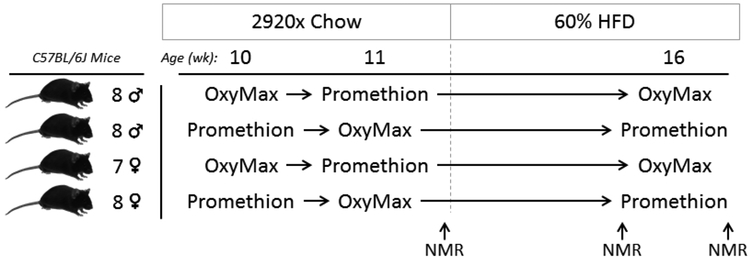
To address these questions, we examined metabolic phenotypes of wildtype C57BL/6J mice under chow- and 60% HFD-fed conditions using both OxyMax (Columbus Instruments International) and Promethion (Sable Systems International) multiplexed phenotyping systems, in a longitudinal crossover-design study (Figure 1).
Figure 1. Illustration of experimental design.
Male and female C57BL/6J mice fed 2920x chow diet were subjected to a cross-over design experiment, evaluating metabolic phenotypes exhibited in OxyMax (Columbus Instruments International) and Promethion (Sable Systems International) multiplexed systems. Mice were examined either in OxyMax or Promethion systems at 10 weeks of age, then examined in the opposite system at 11 weeks of age. Mice were then switched to a high fat diet, providing 60% of calories from fat (60% HFD). At 16 weeks of age, mice were again examined in the same system as employed at 10 weeks of age. Body composition was assessed using NMR at 12 weeks of age before initiating 60% HFD, and immediately before and after multiplexed system phenotyping in week 16.
Methods
Animals and Design
Nine-week old C57BL/6J mice (Jackson Laboratories) arrived at the University of Iowa and were acclimated for one week. Mice were maintained at 25°C on a 12 h light cycle, and fed 2920x chow (3.1 kcal/g, 16% of kcal from fat) unless otherwise noted. At ten weeks of age, mice were randomly assigned to multiplexed phenotyping systems for four days (Figure 1), then returned to standard housing. At eleven weeks of age, mice were moved to the opposite system for four days in a crossover design. At twelve weeks of age, mice underwent body composition analyses. All mice were then fed a 60% high fat diet (HFD; D12492, 5.24 kcal/g, 60% of kcal from fat) for the remainder of the study. Animals were singly-housed throughout the study to minimize effects of repeatedly re-grouping males. At sixteen weeks of age, mice underwent body composition analyses before and after multiplexed phenotyping. Both multiplexed phenotyping systems, home caging, and NMR equipment are all located within the University of Iowa Metabolic Phenotyping Core Facility, within a radius of approximately 20 meters. Such proximity is expected to minimize differences among treatment groups from noise, vibration, HVAC, temperature, light, odor, and personnel. Both systems were calibrated weekly using standardized gas mixtures immediately before animals entered chambers. Ambient temperatures inside chambers were indistinguishable between systems (range observed: 29.7 – 30.8 °C).
Body composition:
Body composition was assessed using time-domain nuclear magnetic resonance (NMR), as previously described (8).
OxyMax:
A sixteen-channel OxyMax system (Columbus Instruments International) was used. Individual cages (interior dimensions 20.2 × 10.5 (floor) × 12.1 cm tall, with a polycarbonate mesh floor 2.7 cm above the bottom of the chamber) include a floor-mounted food hopper (10 mg resolution) and a ceiling-mounted water bottle. X- and Y-axis (horizontal plane) photoelectric beam motion detectors are positioned around the cage. Air flow was positive (500 mL/min) and gas analyses were recorded once every ~17 minutes per cage. Eight cages each are mounted inside of two thermally-controlled cabinets, maintained to 30°C (to account for the lack of accessibility to bedding, and therefore inability to thermoregulate via burrowing behaviors).
Promethion:
A sixteen-channel Promethion system (Sable Systems International) was used. Individual cages (interior dimensions of 31.5 × 15.5 (floor), up to 34.5 × 19.0 (ceiling) × 13.0 cm tall) include a ceiling-mounted food hopper (3 mg resolution) and water spigot. Cages include a ceiling-mounted small “hut” into which mice can climb, which permits body mass measurements. X- and Y-axis (horizontal plane) photoelectric beam motion detectors are positioned around the cage. A running wheel accessory was available but excluded from the current study. Air flow through the chamber was negative (2,000 mL/min) and gas analyses were recorded once per minute. Cages are mounted inside of thermally-controlled cabinets that are maintained at 30°C, to match parameters utilized in the OxyMax system.
Statistics:
Although data were recorded for four days (Monday to Friday), only data from 6 AM Wednesday to 6 AM Friday were used for statistical analyses to ensure sufficient acclimation (please see Supplemental Figures 1–4 for complete, annotated gas analysis, X+Y physical activity, and cumulative food intake tracings). Metabolic rate was estimated from oxygen consumption (VO2) and carbon dioxide production (VCO2) rates by both systems first using the modified Weir formula (19), where [Heat production = 3.941(VO2) + 1.106(VCO2)], or the formulae derived from Zuntz and Schumburg (20), refined by Lusk (12), where [Heat production = 3.815(VO2) + 1.232(VCO2)]. Respiratory exchange ratio (RER) was calculated by both systems as the ratio of VCO2 to VO2. To compare generalized linear models incorporating main effects of instrument and sex with other covariates such as food intake, physical activity, total or lean body mass, the Akaike Information Criterion, corrected for small sample sizes (AICc) was calculated to identify the most parsimonious model (7). All analytical comparisons were performed by generalized linear modeling (SAS), ANOVA or t-test (GraphPad Prism) as indicated. Differences were considered significant at p<0.05.
Results
Part 1: Assessment of metabolic endpoints during chow feeding
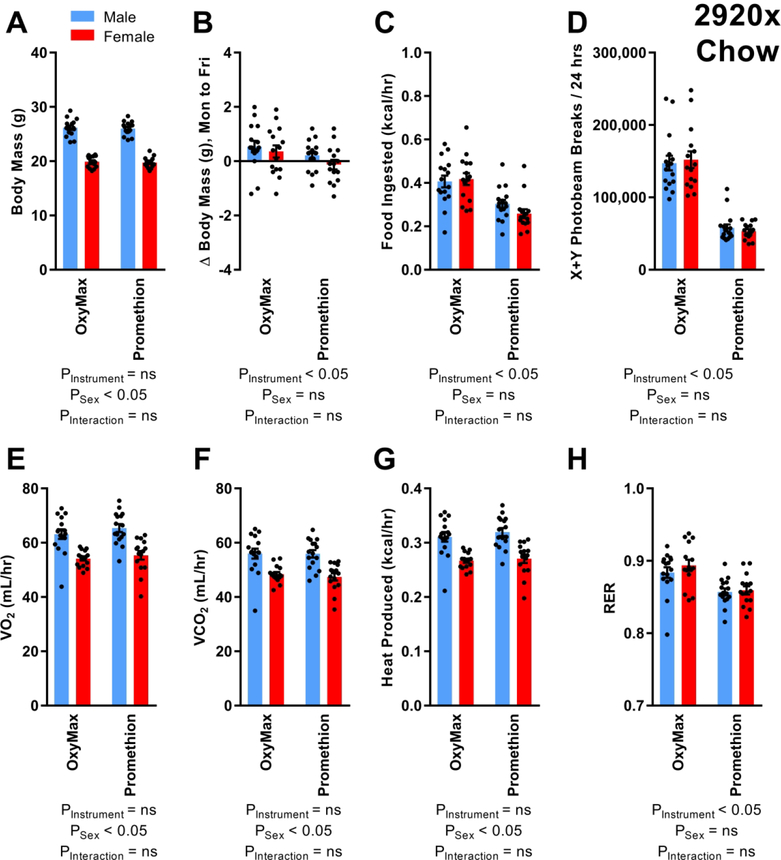
Our first objective was to compare metabolic phenotypes of wildtype mice maintained on a standard chow diet, when assessed using two distinct multiplexed instruments. A crossover-design approach was used, in which mice of each sex were sequentially tested in both instruments (Figure 1). The crossover design ensured that mice entering each system were matched for body mass, though as expected, males were significantly larger than females (Figure 2A). Body mass stability was assessed across the week of testing, to evaluate whether either system differentially affected energy balance. A small but statistically significant difference in body mass gain was observed, with animals in OxyMax exhibiting greater weight gain (Figure 2B). Food intake was greater in the OxyMax system (Figure 2C). Major differences in cage design confound the direct comparison of total physical activity in the X+Y planes. Thus, a difference in photobeam interruptions between systems (Figure 2D) should not necessarily be interpreted as evidence of differences in activity. No differences in activity counts were observed between males versus females, and no interaction between machine and sex was observed. Both oxygen consumption (VO2) and carbon dioxide production (VCO2) rates were significantly increased in the males regardless of system (Figures 2E, 2F). Heat production rates, estimated using either the modified Weir equation (Figure 2G) or the equation from Lusk (Supplemental Figure 5A), were similar between systems, though males exhibited greater heat production than females. Respiratory exchange ratio (RER) was lower in the Promethion system (Figure 2H). Within each sex, no difference was observed in lean body masses at week 11 between animals placed into each system (Supplemental Figure 6).
Figure 2. C57BL/6J mice fed 2920x chow, at 10–11 weeks of age.
(A) Body masses of mice entering OxyMax and Promethion systems. (B) Change in body mass over four 24-hour cycles. (C) Food ingested, corrected for digestible calories per gram. (D) Photoelectric beam breaks in X+Y planes. (E) Rate of O2 consumption. (F) Rate of CO2 production. (G) Heat production as estimated using the Weir equation. (H) Respiratory exchange ratio. For all endpoints, n=16 males and 15 females. Comparisons performed by two-way repeated-measures ANOVA. Summary data are presented as mean ± SEM.
Heat production may be influenced by factors including body size and composition, food intake and physical activity, and therefore we used generalized linear modeling to compare heat production in the two systems after adjusting for these covariates. Heat production, estimated using the Weir equation, remained unchanged between systems after adjustment for total body mass, lean body mass, food intake, or combinations of total body mass plus food intake, or lean body mass plus food intake (Supplemental Table 1). The Akaike Information Criterion, corrected (AICc) method identified the adjustment for “sex, instrument, and lean body mass” as the best-fit model to account for observed variability in heat production by animals on chow diet. Importantly, because total and lean body masses between sexes differed considerably (Supplemental Figure 6), interpretation of heat production data after normalization to total or lean body masses when including both sexes in the model is confounded (2); thus, such corrections are included in the current manuscript primarily for completeness. Qualitatively and quantitatively similar results were observed when the Lusk equation was used to estimate heat production (Supplemental Table 2).
Part 2: Detection of the metabolic consequences of 60% HFD feeding
Our second objective was to examine the effects of 60% HFD feeding upon energy homeostasis, as assessed by the two multiplexed systems. Convergence of conclusions drawn from both systems provides confidence in conclusions regarding the effect of HFD upon individual endpoints, whereas divergence in conclusions drawn between systems highlights context-dependence of the effect of diet upon such endpoints.
Before and after four weeks of 60% HFD feeding, body composition was assessed by NMR (Supplemental Figure 6). While all animals gained significant total, lean, fat and fluid masses, a few minor but statistically significant differences were detected in the total and fat mass gains of individual mice randomly assigned to OxyMax versus Promethion. Males gained more mass than females, as expected.
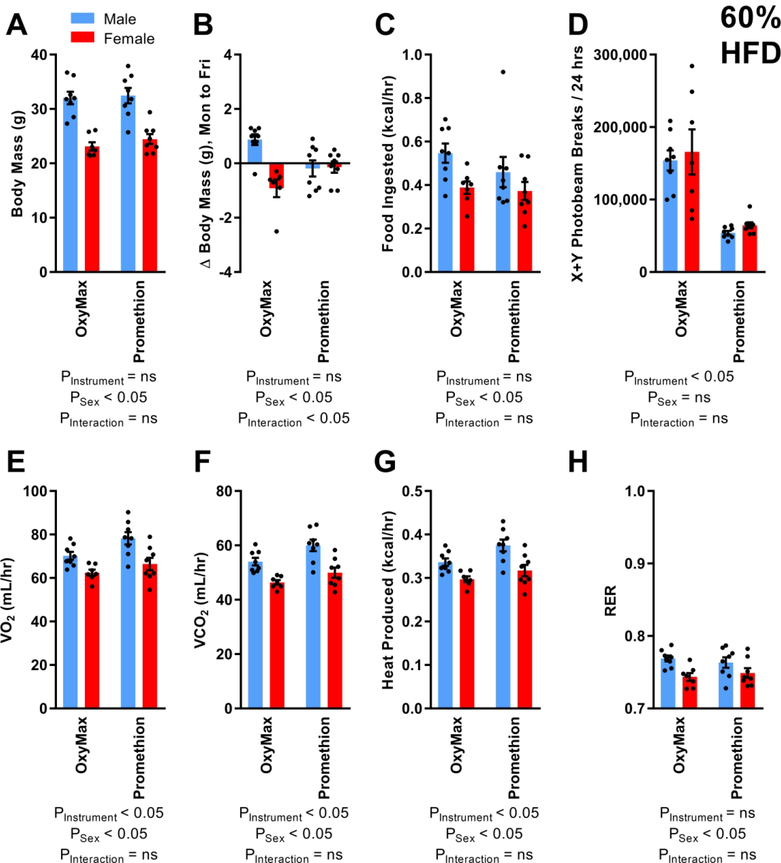
Body masses of animals entering the phenotyping systems in week 16 were, on average within sexes, indistinguishable (Figure 3A). Over the week of testing in week 16, animals in Promethion exhibited no average change in body mass, however a sex-dependent effect of housing in OxyMax was observed in which males gained weight while females lost weight (Figure 3B). Food intake was similar between systems but females consumed less than males (Figure 3C). Photoelectric beam break activity was lower in Promethion (Figure 3D). In contrast to observations during the chow-feeding phase, VO2 (Figure 3E), VCO2 (Figure 3F), and heat production as estimated by the Weir equation (Figure 3G) or Lusk equation (Supplemental Figure 5B) were all significantly increased in Promethion during HFD feeding. RER was similar between systems, but lower in females (Figure 3H).
Figure 3. C57BL/6J mice fed 60% HFD for 5 weeks, at 16 weeks of age.
(A) Body masses of mice entering OxyMax and Promethion systems. (B) Change in body mass over four 24-hour cycles. (C) Food ingested, corrected for digestible calories per gram. (D) Photoelectric beam breaks in X+Y planes. (E) Rate of O2 consumption. (F) Rate of CO2 production. (G) Heat production as estimated using the Weir equation. (H) Respiratory exchange ratio. For all endpoints, n=8 males in OxyMax and 8 in Promethion, plus 7 females in OxyMax and 8 in Promethion. Comparisons performed by two-way ANOVA. Summary data are presented as mean ± SEM.
Heat production estimates by the Weir (Supplemental Table 3) and Lusk (Supplemental Table 4) equations were again adjusted for combinations of covariates using generalized linear modeling. With the exception of “sex, instrument, and physical activity” (where instrument p=0.08), the estimated heat production was significantly different between OxyMax and Promethion systems in all other statistical models, and the AICc method identified “sex, instrument, and total body mass” as the best-fit model to explain observed data.
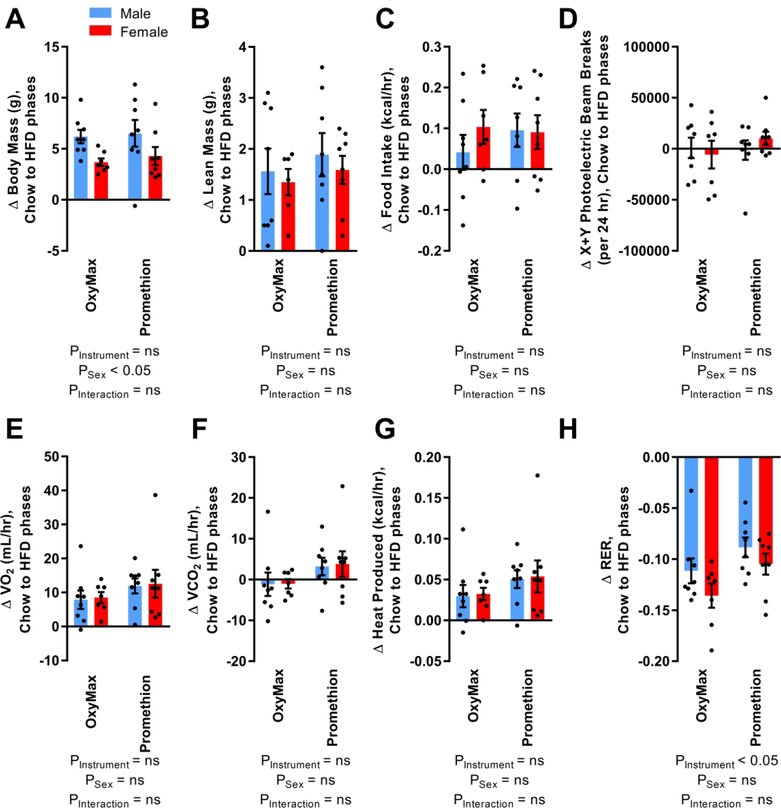
The modified Weir equation is now more commonly employed than the equation derived from Lusk, so we took advantage of the dataset to examine how the heat production estimates from each equation correlate, and how co-variates may impact this relationship. Reassuringly, we determined that the relationship between the results of the two equations for heat production is independent of the magnitude of heat production, diet, or the physical activity of the animals, and thus the use of either equation to analyze the current datasets had no qualitative effect on system comparison (not shown). Finally, we examined whether HFD feeding differentially impacted major phenotypes when assessed by the two systems. Comparing phenotypes in week 10 versus week 16 within animal demonstrated that HFD caused increased weight gain in males versus females, regardless of instrument (Figure 4A). Animals in both systems gained significant total (5.2±0.5 g, p<0.05 vs zero) and lean body masses (1.6±0.2 g, p<0.05 vs zero), consumed more calories (0.08±0.02 kcal/hr, p<0.05 vs zero), consumed more O2 (10.28±1.41 mL/hr, p<0.05 vs zero), produced more heat (0.04±0.01 kcal/hr, p<0.05 vs zero) and exhibited reduced RER (−0.109±0.006, p<0.05 vs zero), while no changes in CO2 production rate (1.28±1.27 mL/hr) or physical activity (1277±4837 X+Y photoelectric beam breaks/day) were detected. Neither instrument nor sex influenced changes in lean body mass, caloric ingestion, physical activity, VO2, VCO2, or heat production as estimated by the modified Weir equation (Figures 4B–G). While RER was significantly reduced by HFD feeding in both systems, the magnitude of reduction as assessed by OxyMax was greater (Figure 4H), because RER during chow feeding was higher in OxyMax (Figure 2H).
Figure 4. Changes in metabolic endpoints within individual mice serially examined in OxyMax or Promethion, during 2920x chow (week 10) versus 60% HFD feeding (week 16).
(A) Change in total body mass. (B) Change in lean body mass. (C) Change in rate of calories ingested. (D) Change in activity estimated by photoelectric beam interruptions in the X+Y planes. (E) Change in rate of O2 consumption. (F) Change in rate of CO2 production. (G) Change in heat production rate as estimated by the modified Weir equation. (H) Change in respiratory exchange ratio. For all panels, n=8 males in OxyMax and 8 in Promethion + 7 females in OxyMax and 8 in Promethion. Summary data are presented as mean ± SEM.
Part 3: Estimating unmeasured endpoints contributing to energy homeostasis
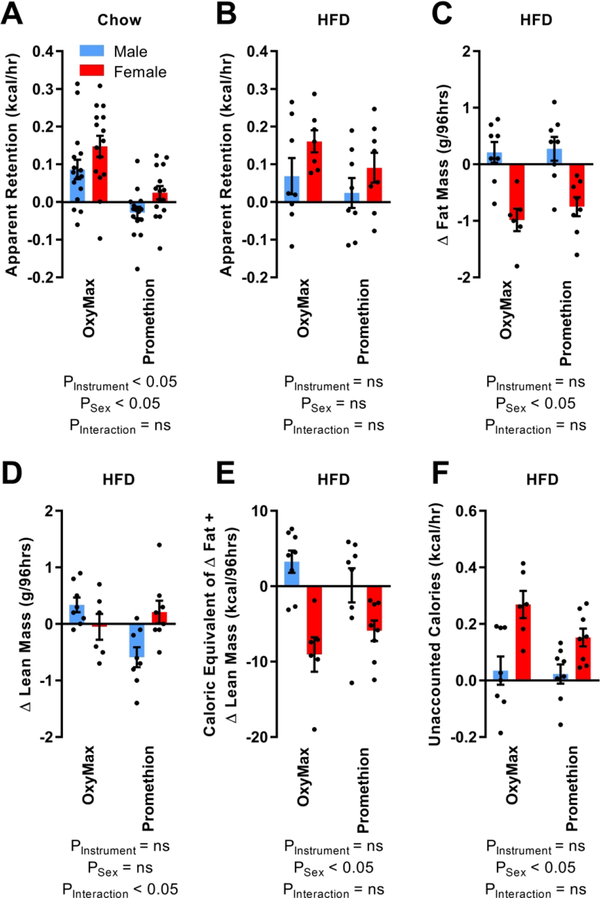
Direct comparison of daily energy intake by mice in each system to estimated heat production provides an approach to estimating the magnitude of other physiological processes which are known to contribute to energy homeostasis, but are not assessed in multiplexed phenotyping systems such as those employed in the current study. Subtraction of calories expended as heat from the calories ingested during chow (Figure 5A) or HFD feeding (Figure 5B) reveals a large magnitude of energy that is apparently retained by the animals. As NMR was performed immediately before and after animals were studied in week 16, changes in body composition over this week were then used to estimate the calorific equivalent of changes in fat (Figure 5C) and lean (Figure 5D) masses, using the rough estimates of 9 kcal/g for fat tissue and 4 kcal/g for lean tissue (Figure 5E). Subtracting this energy equivalent from the calories that were otherwise unaccounted for (Figure 5B) yields an estimate of the magnitude of energy that is unaccounted for by either system through heat production plus tissue growth (Figure 5F). Calories unaccounted for in the OxyMax system averaged approximately 0.14±0.05 kcal/hr (21±12% of total intake), vs 0.09±0.03 kcal/hr (17±7% of total intake) in the Promethion system (p=ns between systems, but p<0.05 for either system vs zero). This effect was due almost completely to losses observed specifically in female mice (versus zero: p=ns for males in OxyMax or Promethion, while p<0.05 for females in either system). Ultimately, the Weir equation plus the caloric equivalent of growth accounted for 95±5% of calories consumed during HFD feeding in males (94±8% in OxyMax / 96±7% in Promethion), but only 47±8% for females (33±12% in OxyMax / 57±10% in Promethion). Future studies to dissect the interactions of sex, ambient temperature, diet, and instrumentation-specific environmental influences are warranted to understand this observation.
Figure 5. During 60% HFD feeding, not all ingested calories are accounted for by heat production as estimated by the modified Weir equation.
(A) Calories apparently retained by mice maintained on 2920x chow, at 10–11 weeks of age, calculated as digestible calories consumed minus heat produced as estimated by the modified Weir equation. (B) Calories apparently retained by mice fed 60% HFD for 5 weeks, at 16 weeks of age. (C) Change in fat mass during week 16. (D) Change in lean mass during week 16. (E) Caloric equivalent of changes in fat mass and lean mass during week 16, as estimated by multiplying fat mass change by 9 kcal/g, and lean mass change by 4 kcal/g. (F) Digestible calories consumed that are not accounted for by O2/CO2 exchange using the Weir equation. In panel F, all animals combined regardless of instrument and sex (mean 0.11 ± SEM of 0.03, n=30) are significantly different from zero (1-sample t-test, t=4.1765, df=29, p=0.0002). Similar results are observed within OxyMax alone (0.14±0.05, t=2.8933, df=13, p=0.01) or Promethion alone (0.09±0.03, t=3.1383, df=15, p=0.007). This effect is driven by females, as unaccounted calories in males in both instruments are not statistically different from zero (0.03±0.03, t=0.9866, df=15, p=0.34) but unaccounted calories in females are different from zero regardless of instrument (0.20±0.03, t=6.5890, df=13, p<0.0001). For panels B-F, n=8 males in OxyMax and 8 in Promethion + 7 females in OxyMax and 8 in Promethion. Summary data are presented as mean ± SEM.
Discussion
With increasingly sophisticated genetic and pharmacological approaches available to manipulate biological systems controlling energy balance, there is increased need for validation and comparison of the phenotyping tools that are employed by biomedical researchers. The current study sought to clarify the nuanced differences in assessments of metabolic endpoints in mice studied in two commonly used multiplexed metabolic phenotyping systems. We took advantage of the fact that we recently installed two widely utilized, commercially-available systems (OxyMax from Columbus Instruments International, and Promethion from Sable Systems International) within meters of each other, in the same large room inside a newly constructed academic research core facility. This provided an outstanding opportunity to tightly control for environmental variables. When combined with a crossover design and relatively large number of inbred C57BL/6J mice from a major and internationally-respected commercial vendor, confidence that any observed effects could indeed be attributed to differences between the phenotyping systems is high.
Based on the data presented here, we conclude that the OxyMax and Promethion systems differ in their assessments of multiple aspects of energy flux in mice, and that the differences observed are a result of the different system designs. In brief, we observed significant differences in estimates of aerobic heat production, food intake and RER in mice studied with the Promethion system compared to the OxyMax system, depending upon diet supplied and sex of animals studied. We can only hypothesize potential causes of the observed differences, and additional nuanced studies to dissect causes for the differences observed in the current study are warranted. Differences may result directly from differential sensitivities of the systems (e.g. - resolution of endpoint detection, endpoint detection limits, or time-resolution between measurements). Differences may also result indirectly, due to effects of system design upon the cage microenvironment (e.g. - the presence or absence of bedding, ambient noise from a cage fan, etc.) and study design (e.g. - diets supplied and ambient temperature), which could influence subject behavior and physiology. Clearly, future studies are warranted to understand the biological mechanisms by which the array of differences in system designs (floor type, air flow rate, noise levels and types, food hopper designs, environmental enrichment, etc.) influence outcomes. Such information is required to direct future system designs, though thorough investigations of such details are beyond the purpose of the current study.
It is worth noting that despite the obvious utility of multiplexed phenotyping systems, these systems do not quantify all aspects of energy homeostasis in animals (despite marketing claims to “comprehensively” assess metabolic phenotypes). In the current study, it was estimated that while fed a HFD, a large fraction of calories that were consumed by the animals in either system were unaccounted for by aerobic heat production, and that this difference was most pronounced in females. Multiple possible contributors to this large discrepancy exist. First, spillage of food may contribute to this discrepancy, though over a 96-hour testing period, the average 0.11 kcal/hr loss of this 5.24 kcal/g HFD would equate to approximately 2.0 g of the food (D12491, which is dyed a bright blue color), which should be easily visualized in either system by technicians. As very little spillage was noted and therefore unaccounted for in either system in the current study, spillage is unlikely to account for the bulk of these ‘missing’ calories. Additional mechanisms which may account for these calories – and are not assessed by any current multiplexed system – may include altered digestive efficiency, protein / nitrogen metabolism, and anaerobic metabolism.
For example, no current system is designed to collect urine and feces efficiently, which is required to assess digestive efficiency. Digestive efficiency reflects the fraction of calories consumed that are absorbed by an animal (and their associated microbiota), versus lost to stool. Digestive efficiency is a major contributor to energy homeostasis, and it represents the target of the single longest-running FDA-approved anti-obesity therapeutic compound, Orlistat (Alli / Xenical) (11). Under chow-fed conditions, mice typically absorb approximately 80% of consumed calories, and under HFD-fed conditions this fraction jumps to 90–95%. Dietary, genetic, surgical and pharmacological interventions which modify energy homeostasis via changes in digestive efficiency will be completely missed when researchers only employ multiplexed phenotyping equipment such as described herein (18). Complementary, highly quantitative methods adopted from the fields of analytical and physical chemistry, such as bomb calorimetry, are required to evaluate such endpoints (8).
Current multiplexed phenotyping systems are also limited by design to the detection of “aerobic” forms of energy expenditure because they all utilize the same respirometric method of measuring metabolic rate. By definition, these methods rely upon a series of assumptions, many of which are untested or untestable (10). One such assumption is that nitrogenous and anaerobic metabolic processes are constant and indistinguishable from zero. Studies published by our group and others over the last 40 years examining these assumptions in endotherms including mice, various birds and desert species, and humans (5, 6, 14, 15, 17) via the use of combined (direct calorimetry + respirometry) calorimetry systems have documented that the processes to which respirometry remains blind will typically account for between 5–40% of total energy expenditure. Further, this contribution is highly modifiable by body composition, diet, pharmacological agents, and genetics. Recently we have determined that the gut microbiota are major contributors to this “non-aerobic” form of energy expenditure, providing up to 10% of total daily energy expenditure through mechanisms that respirometry cannot detect (3, 16). Our previous studies examining the effect of a switch from chow (Teklad 7013) to a 45% HFD (D12451) in male C57BL/6J mice demonstrated that while the “aerobic” fraction (by respirometry) accounted for only 93% of the “total” resting metabolic rate (by direct calorimetry) during chow feeding, this fraction jumped to 100% within one week when mice were switched to HFD (5). These findings are consistent with the current observation that after 4 weeks of 60% HFD, “aerobic” heat production accounts for essentially all calories consumed in male mice, and prompt further investigation into the contribution of “aerobic” versus “non-aerobic” processes in female mice fed various diets. Further supporting this concept and highlighting its translational significance, Pittet demonstrated in the mid-1970’s that human obesity is associated with a major suppression of the “non-aerobic” fraction of energy expenditure – a discovery largely ignored for >40 years (14, 15). Unfortunately, no commercial sources for direct calorimetry-based systems currently exist, and we are unaware of any reports of multiplexed direct calorimetry-based systems. This highlights a major unmet technical need for the obesity research community, and an opportunity for enterprising biotechnologists.
Nitrogen-based metabolism is an important contributor to total energy flux, and measures of nitrogen intake (in food) or urine nitrogen content are appropriately used to estimate this contributor to energy flux. Indeed, the un-modified Weir equation for estimating energy flux includes a term for nitrogen metabolism (19). Unfortunately, these methods also rely on assumptions, including that all relevant nitrogen species which are consumed are completely absorbed, and that urine-based recovery is complete. Losses to the environment (e.g. - sweat or saliva) are likely minor, but are almost uniformly ignored. Perhaps most importantly, assessments of nitrogen-based metabolic processes are also necessarily performed over long time periods, and therefore the ability to assess minute-to-minute changes in rates of nitrogen metabolism are impossible using these techniques. Further, the caloric oxygen equivalent of all substrates are not the same. For example, Mansell & Macdonald demonstrated that when various fuel types (including those associated with gut microbiota such as acetoacetate, hydroxybutyrate, and ethanol, in addition to dietary protein) are oxidized, the energy equivalents of consumed oxygen deviate significantly from the relationship defined by dietary fats and carbohydrates (13). These issues further underscore the need and potential utility for direct calorimetry-based systems that do not rely solely upon gas respirometry-based estimates of energy expenditure.
The discovery that both OxyMax and Promethion systems fail to account for any large fraction of total energy turnover is itself of importance to the field. In addition, the determination that there is a different detection of the magnitude of this ‘missing’ fraction in males versus females is also of interest, and may contribute to sex differences in weight gain (Figure 5F). It is possible, given the different design of food supply mechanisms in each system, that differences in spillage recovery may contribute to observed differences. It is possible that this difference may represent sex-specific alterations in digestive efficiency. It is also conceivable that because animals in the Promethion system live in a standard bedding floor and therefore have access to feces, whereas animals in the OxyMax are housed on a suspended floor which restricts access to feces, that sex-dependent differences in coprophagic behaviors and tendency to consume bedding materials may impact measurable food intake behaviors and digestive efficiency and therefore differentially impact the fraction of calories which are accounted for by the two systems.
Study Limitations
The primary purpose of the current study was to evaluate the impact of employing two different commercially-available multiplexed phenotyping systems on assessments of energy homeostasis. As a result, the experimental paradigm was optimized to detect differences between systems, rather than to optimize the external validity of either system’s approach. Thus, there are an array of important study limitations that must be noted. For example, because mice thermoregulate in part through burrowing behaviors, and because the Promethion system provides access to bedding material but the OxyMax system does not, it was decided that energy homeostasis assessments would be performed in both systems while maintained with a thermoneutral 30°C chamber ambient temperature. This elevated ambient temperature choice may conceivably minimize differences that could be detected between systems, and future comparisons of the two systems at standard housing temperatures (22–25°C) may yield additional useful information with regard to the use of these multiplexed systems. Further, the use of a 30°C ambient temperature may conceivably contribute to the observed “unaccounted for” calories in females fed a HFD (Figure 5F), through effects upon body composition (Figure 5B–E). Future studies need to be performed to carefully quantify such missing calories in both sexes at various temperatures and in the context of various diets.
Additionally, it should be noted that no separate “acclimation” period was provided for animals entering either system, though both manufacturers suggest that users include acclimation phases. Our study design was purposeful, as we intended to empirically document the time-course of acclimation in either system, both to test the effects of acclimation and to compare acclimation time-courses between systems. Indeed, from the current study it appears that 48 hours of acclimation is sufficient to establish normal rhythmicity in most or all endpoints in either system (Supplemental Figures 1–4). Nonetheless, the external validity of this acclimation time course requires further study in the context of various covariates.
Many additional comparisons between systems are possible to further dissect the influence of system design upon endpoint evaluation. For example, it is apparent from close examination of full time-course tracings (Supplemental Figures 1–2) that larger differences in gas exchange between light and dark phases are observed from subjects examined in the Promethion system, despite no differences in 24-hour averages (Figures 2–3), though no formal statistical comparison of these possible amplitude changes were performed herein. Ambient temperature and psychological stress have both been associated with alterations in such rhythmicity (1, 4), which underscores yet another area that requires future establishment of the external validity of either multiplexed system.
Finally, for the purpose of the current study, the modified Weir and Lusk equations were utilized to estimate aerobic heat production. Kevin Hall’s group has recently described an updated equation that may provide even more accurate estimates, in which the proportion of total energy expenditure accounted for by protein metabolism can be estimated from the proportion of protein in the diet (9). It is logical that use of this newer equation will likely modify the quantitative results comparing each phase of the current study (i.e. - 2920x chow diet provides 24% kcal from protein, versus D12492 provides 20% kcal from protein), however, the use of any other equation would not be expected to have substantial effects upon the comparison of OxyMax versus Promethion systems within either phase. Again, continued refinement of technologies and equations used to estimate heat production from simple-to-measure endpoints such as oxygen and carbon dioxide exchange from animals of each sex in the context of varied diets, ambient temperatures, etc. and in various species is warranted.
Summary
In conclusion, our studies support the ongoing and major utility of both OxyMax and Promethion phenotyping systems, but they also highlight critical quantitative differences that result from the use of each system, and complex diet- and sex-dependent effects upon measures of energy flux in each system. Caution is therefore advised for researchers directly comparing results (quantitative and qualitative) of studies examining animals in each distinct type of multiplexed system. Use of either single system in isolation may lead to results that contradict results obtained from a different system type, due primarily to system design, rather than due to biological mechanisms. Such differences may help explain contradictory results reported by users at independent research institutions. It is important to appreciate that work to establish the external validity of either system is ongoing by various groups, and required to clarify the impacts of sex, diet, ambient temperature, noise, and many other variables upon energy flux. The current study provides preliminary data that may prompt hypotheses to explain the observed differences in results obtained between systems, however, it is critical to recognize that the current study was not designed to test causal relationships, and thus cannot be used to directly justify the superiority or validity of either design. Finally, researchers must be cognizant of the inability of any current multiplexed system to detect or measure multiple additional physiologically and pathophysiologically-significant mechanisms that contribute to energy homeostasis, such as digestive efficiency and non-aerobic metabolic processes. Additional work is needed to understand the validity, strengths, weaknesses, and interactive effects of multiplexed system designs in studies of metabolic physiology.
Supplementary Material
Study Importance Questions.
What is already known about this topic?
Multiplexed phenotyping systems are available from several commercial vendors
Expert opinion dominates consideration of the advantages, disadvantages, and shortcomings of these types of systems
Well-controlled, empirical, direct comparisons of competitor systems are rarely performed
What does your study add?
The University of Iowa’s Diabetes Research Center uniquely maintains both OxyMax (Columbus Instruments International) and Promethion (Sable Systems International) multiplexed phenotyping systems in a single room of a new core facility, permitting a tightly controlled crossover-design study
The two phenotyping systems yielded results that differed in many ways, which are likely secondary to differences in system design
Both systems remain blind to important contributors to total energy balance including digestive efficiency and nitrogenous or anaerobic forms of energy flux
Acknowledgments
This work was made possible by staff of the University of Iowa Metabolic Phenotyping Core.
Funding: The investigators were supported by grants from the NIH (HL134850, HL084207, HL127764, HL112413, UL1TR002537), and the American Heart Association (15SFRN23730000, 16SFRN31810000, 18EIA33890055). The investigators and University of Iowa Metabolic Phenotyping Core also gratefully acknowledge the generous support of the Fraternal Order of Eagles.
Footnotes
Disclosures: The authors declare no conflict of interest.
Literature Cited
- 1.Abreu-Vieira G, Xiao C, Gavrilova O, and Reitman ML. Integration of body temperature into the analysis of energy expenditure in the mouse. Molecular metabolism 4: 461–470, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arch JR, Hislop D, Wang SJ, and Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. International journal of obesity (2005) 30: 1322–1331, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bahr SM, Weidemann BJ, Castro AN, Walsh JW, deLeon O, Burnett CM, Pearson NA, Murry DJ, Grobe JL, and Kirby JR. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine 2: 1725–1734, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatnagar S and Dallman MF. The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain research 851: 66–75, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Burnett CM and Grobe JL. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Molecular metabolism 3: 460–464, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnett CM and Grobe JL. Direct calorimetry identifies deficiencies in respirometry for the determination of resting metabolic rate in C57Bl/6 and FVB mice. American journal of physiology Endocrinology and metabolism 305: E916–924, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnham KP and Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach: Springer; New York, 1998. [Google Scholar]
- 8.Grobe JL. Comprehensive Assessments of Energy Balance in Mice. Methods in molecular biology (Clifton, NJ) 1614: 123–146, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT, and Ravussin E. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. The American journal of clinical nutrition 104: 324–333, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaiyala KJ and Ramsay DS. Direct animal calorimetry, the underused gold standard for quantifying the fire of life. Comparative biochemistry and physiology Part A, Molecular & integrative physiology 158: 252–264, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, and Singh S. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 315: 2424–2434, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lusk G The elements of the science of nutrition. Philadelphia, PA: W.B. Saunders Company, 1928. [Google Scholar]
- 13.Mansell PI and Macdonald IA. Reappraisal of the Weir equation for calculation of metabolic rate. The American journal of physiology 258: R1347–1354, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Pittet P, Chappuis P, Acheson K, De Techtermann F, and Jequier E. Thermic effect of glucose in obese subjects studied by direct and indirect calorimetry. The British journal of nutrition 35: 281–292, 1976. [DOI] [PubMed] [Google Scholar]
- 15.Pittet P, Gygax PH, and Jequier E. Thermic effect of glucose and amino acids in man studied by direct and indirect calorimetry. The British journal of nutrition 31: 343–349, 1974. [DOI] [PubMed] [Google Scholar]
- 16.Riedl RA, Atkinson SN, Burnett CML, Grobe JL, and Kirby JR. The Gut Microbiome, Energy Homeostasis, and Implications for Hypertension. Current hypertension reports 19: 27, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsberg GE and Hoffman TC. Direct calorimetry reveals large errors in respirometric estimates of energy expenditure. The Journal of experimental biology 208: 1035–1043, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Weidemann BJ, Voong S, Morales-Santiago FI, Kahn MZ, Ni J, Littlejohn NK, Claflin KE, Burnett CM, Pearson NA, Lutter ML, and Grobe JL. Dietary Sodium Suppresses Digestive Efficiency via the Renin-Angiotensin System. Sci Rep 5: 11123, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of physiology 109: 1–9, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuntz N and Schumburg WAEF. Studien zu einer Physiologie des Marsches. Berlin: Hirschwald, 1901. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.