Abstract
Purpose:
The complex and varied presentation of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) has made it difficult to diagnose, study, and treat. Its symptoms and likely etiology involve multiple components of endocrine and immune regulation including the hypothalamic-pituitary-adrenal, the hypothalamic-pituitary-gonadal axis, and their interactive oversight of immune function. We propose that the persistence of ME/CFS may involve changes in the regulatory interactions across these physiological axes. We also propose that the robustness of this new pathogenic equilibrium may at least in part explain the limited success of conventional single-target therapies.
Methods:
We constructed a comprehensive model of female endocrine-immune signaling consisting 28 markers linked by 214 documented regulatory interactions. This detailed model was then constrained to adhere to experimental measurements in a subset of 17 candidate immune markers measured in peripheral blood of ME/CFS subjects and healthy controls before, during, and after a maximal exercise challenge. A set of 26 competing numerical models satisfied this data to within 5% error.
Findings:
Mechanistically informed predictions of endocrine immune markers that were either unmeasured or exhibited high subject-to-subject variability pointed to possible context-specific overexpression in ME/CFS at rest of CRH, CXCL8, estrogen, FSH, GNRH1, IL-23, and luteinizing hormone, and under-expression of ACTH, cortisol, IFNγ, IL-10, IL-17, and IL-1α. Simulations of rintatolimod and rituximab treatment predicted a shift in the repertoire of available endocrine-immune regulatory regimes. Rintatolimod was predicted to make available substantial remission in a significant subset of subjects, in particular those with low IL-1α, IL-17, and cortisol, intermediate progesterone and FSH, and high estrogen levels. Rituximab treatment was predicted to support partial remission in a smaller subset of ME/CFS subjects specifically those with low norepinephrine, IL-1α, CXCL8, and cortisol, intermediate, intermediate FSH and GNRH1, and elevated expression of TNFa, LH, IL-12, and B cell activation.
Implications:
Applying a rigorous filter of known signaling mechanisms to experimentally measured immune marker expression in ME/CFS has highlighted potentially new context-specific markers of illness. These novel endocrine and immune markers may offer useful candidates in delineating new subtypes of ME/CFS and may inform on refinements to the inclusion criteria, and instrumentation of new and ongoing trials involving rintatolimod and rituximab treatment protocols.
Keywords: chronic fatigue syndrome, endocrine regulation, immune regulation, numerical modelling, rintatolimod, rituximab
INTRODUCTION
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex disorder where a key presenting feature consists of a state of persistent debilitating fatigue lasting more than six months. Though diagnostic criteria have evolved from early case definitions1 characteristic features commonly include pathological fatigue and malaise that are worsened after exertion, cognitive dysfunction, immune dysfunction, unrefreshing sleep, pain, autonomic dysfunction, neuroendocrine and immune symptoms2. Although this would point to a multifactorial etiology involving hormonal, neurological, and immunological factors, initial efforts at biomarker identification were focused on immune dysregulation3,4, perhaps owing to the significant occurrence of infectious illness as correlate of onset3,5.
In general, stress has a dysregulating influence on hormonal systems with consequences for metabolism and reproductive function in women. In addition to the systems described above, the immune system has also been implicated in the etiology of ME/CFS6,7. In healthy subjects, close coordination between the hypothalamic-pituitary-gonadal (HPG) and immune systems has been described, giving rise to regular fluctuations over the course of the menstrual cycle as well as acute variations in response to HPG activation8,9. The primary stress response axis, the hypothalamic-pituitary-adrenal (HPA) axis, can be stimulated by peripheral inflammatory responses to infection or trauma resulting in the sympathetic activation of a positive feedback loop whereby hypothalamic inflammatory signaling drives and is sustained by local production of IL-1β, IL-6, and TNFα10. Indeed, ME/CFS has frequently been observed to follow infection by Epstein-Barr virus, in which context it tends to be associated with chronic dysregulation of inflammatory cytokine production3. Rintatolimod (ampligen), a specific activator of inflammatory mediator toll-like receptor 3 (TLR3) has been deployed in ME/CFS treatment for decades, with varying efficacy11,12. With immune B cells serving as a reservoir for latent EBV infection, B cell depletion by Rituximab has also been deployed in clinical trials and has seen some success in treating ME/CFS13. Combined with observations of deficient Epstein-Barr virus-specific B cell responses in ME/CFS patients14, this suggests that dysregulated B cell function and persistent latent viral infection may be significant contributing factors to ME/CFS. However, efforts to identify specific markers of B cell dysfunction have been inconsistent, with various reports of altered maturation15, serum B cell activating factor13, and conflicting results of gene expression studies7,16. Nonetheless, a general neuro-immune model of ME/CFS has been proposed, where an initial infection may lead to chronic peripheral immune activation and sustained neuroinflammation, with cyclic fluctuations in T cell balance contributing to observed patterns of relapse and remission17.
Since women are at higher risk of ME/CFS than men5,18, it is thought that female sex hormones play an important role in the onset, persistence, and symptom burden of this illness19. Despite evidence of sex-specific differences in susceptibility and response to infection by pathogens20 and autoimmunity21, the potential role of cross-talk between sex hormone regulation and oversight of immune function in ME/CFS has not been well explored. The same may be said of immune crosstalk with metabolic hormones22 in ME/CFS with the possible exception of recent work implicating dysregulation of leptin23,24 in illness severity. Indeed, stress hormone regulation has arguably been the main focus of endocrine involvement in ME/CFS and has been relatively well documented25. Unfortunately, these components have been studied largely in isolation with limited consideration of their regulatory interactions with adjacent and overlapping systems.
To explore the potential for altered co-regulation of endocrine and immune axes as a mediator of ME/CFS and its persistence, our group had assembled a basic computational model of regulatory logic coordinating the principal feedforward and feedback actions of the hypothalamus-pituitary-adrenal (HPA) axis, the hypothalamus-pituitary-gonadal (HPG) axis, and the innate and adaptive branches of the peripheral immune system. We found that even this coarse-grained model could support multiple steady states, two of which were proximal to immune marker profiles exhibited by ME/CFS subjects26,27. Though useful in demonstrating the potential involvement of endocrine-immune co-regulatory control in supporting the persistence of this condition, these first models remained coarse in resolution. This work extends our initial proof of concept study with the assembly of a more detailed endocrine-immune circuitry where regulatory dynamics are supported by a more sophisticated logic that captures the actions of low and high affinity receptor signaling as well as competing influences of weak versus strong mediators. These effects are adjusted to directly align model predictions with experimental measurements of immune markers sampled in peripheral blood at 8 points in time before, during, and after a maximal exercise challenge. Instead of broad immune functional sets, individual cytokines are now networked with a more detailed model of sex, stress and metabolic hormones through regulatory interactions extracted from the published scientific literature using automated natural language processing (NLP). This literature-informed model of endocrine-immune regulatory logic aligned supported experimentally observed immune responses to maximal exercise within 5% error in 26 competing candidate models. The regulatory constraints imposed by these competing model circuits predicted widespread dysregulation of endocrine function in ME/CFS patients at rest, characterized by blunted HPA regulation, HPG overactivation, and an immune profile dominated by IL-8 and IL-23. Together, these patterns may contribute to the pathologies experienced by ME/CFS patients. Simulations based on this family of models mimicking the effects of TLR3 activation and B cell depletion suggest that these interventions may in fact alter the repertoire of stable regulatory behaviors in favor of a more robust normal regulation or in the case of the latter even render ME/CFS dynamically unstable outright.
PATIENTS AND METHODS
Participants
A total of 88 female subjects (43 with ME/CFS and 45 healthy controls (HC)) were selected without exclusion for ethnicity from the patient population of the Institute for Neuroimmune Medicine at Nova Southeastern University (NSU), directed by Nancy Klimas, M.D. All subjects signed an informed consent approved by the Institutional Review Board of Nova Southeastern University, Fort Lauderdale, FL. Included subjects presented with acute onset and with an illness duration of at least 4 years. ME/CFS was diagnosed according to current research case definitions1,28: fatigue of greater than 6 months duration and at least 4 of 8 symptoms including exercise-induced relapse, myalgia, arthralgia, headache of a new and different type, nonrestorative sleep, cognitive complaints, sore throat, and tender lymph nodes. All ME/CFS study subjects presented with a SF-36 summary physical score (PCS) below the 50th percentile, based on population norms. Heathy controls (HC) were self-defined as sedentary (no regular exercise program, sedentary employment), and matched to ME/CFS cases by age (+/−5 years), race/ethnicity, and BMI (+/−5).
Study Design
Subjects were challenged with a supervised symptom-limited maximum graded exercise test (GXT) performed under the McArdle protocol on a fully-automated Life Fitness cycle Model 95Ri and the Oxycon Mobile ergospirometry testing device. Subjects pedaled at an initial output of 60 W for 2 minutes, followed by an increase of 30 W every 2 minutes. This was continued until one of the following endpoints: 1) maximal oxygen consumption (VO2max) was reached; 2) respiratory exchange ratio > 1.15; 3) the subject discontinued the challenge. Blood samples (8 mL) were collected before the test after a 30-minute rest period, at maximal effort, and at 10, 20, 30, and 60 minutes post-stress, with additional blood draw at approximately 12 hours and 24 hours post-stress. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque extraction and stored in liquid nitrogen; plasma was stored at −80C.
Assessments
PBMC samples from each timepoint were analyzed by flow cytometry on a Beckman/Coulter FC500 using commercially available antibodies to record frequencies of B cell (CD19+) and NK cell (CD3-CD56+) populations. Plasma concentrations of IFNγ, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-13, IL-15 IL-17, IL-23, and TNFα were measured by Q-Plex multiplex ELISA (Quansys Biosciences, Logan, Utah). Details of the protocol and assay variability have been reported previously by our group3,29. Finally, serum samples were analyzed for concentrations of the predominant estrogen estradiol (E2) and progesterone by immunoelectro-chemiluminescence (IECL) assays on a Roche Cobas 6000 analyzer (Roche Diagnostics, Basel, Switzerland), following all manufacturer’s instructions for instrument maintenance and assay calibration and test procedures with inter-assay CV’s that are consistently <4%.
Statistical Analysis
Differences in marker expression were tested for significant effects of condition, timepoint, and condition-time interactions by 2-way ANOVA; raw F test null probability p values were adjusted for multiple comparisons by the Benjamini-Hochberg procedure with a false discovery rate of 0.05 in R version 3.4.230. Continuous measurements were converted to discrete values using a variational Bayesian update scheme for expectation maximization of Gaussian mixture models31,32. The full set of measurements for each marker was used to define the most representative number of discrete activation levels (e.g. whether the cytokine measured behaved according to binary or multi-valued logic). Each variable was then summarized by taking the median of inverse hyperbolic sine (arcsinh)-transformed values for HC and ME/CFS patients at each timepoint. These summarized values were discretized by k-means clustering using the previously-defined maximum activation levels to determine the number of clusters: the discretized values for entities which were found to vary significantly by ANOVA were used as input trajectories for model parameterization. Predicted behaviors for network entities were analyzed after parameterization. ANOVA was performed on predicted trajectories excluding the start state values; the start states were compared separately by Wilcoxon rank-sum test. Figures were prepared using the ggplot2 package in R33. Graph topological metrics (e.g. betweenness centrality) were calculated using MATLAB.
Mechanistic Modeling of Endocrine-immune Signaling
The model assembled and reported previously by our group27 has been extended in this work to include additional regulators (nodes) of HPG and HPA axis function, as well as regulators of the HPT axis and a much more detailed description of the immune signaling. Regulatory interactions (edges) between these entities were drawn from the Pathway Studio (Elsevier, Amsterdam NL) knowledge database, a repository extracted from the published scientific literature using the MedScan34 natural language processing engine. Edges were verified independently using our implementation of a Bayesian sentiment analysis classifier35. Disagreements between MedScan and this platform were reviewed and adjudicated by the authors.
The scope of the validated regulatory network was further constrained to focus on regulators directly involved in feedforward and feedback control. Nodes with no outgoing edges (sink nodes) were removed with the exception of B cells and NK cells since experimental measurements of these immune cell populations were available. Direct regulatory associations which duplicated the actions of a sequence of indirect associations were also removed to promote parsimony. For example, while the Pathway Studio database contained many associations directly linking physiological stress to a range of immune mediators, these actions were more accurately accounted for by representing these as downstream effects of HPA axis regulation. Redundant regulatory actions such as these were removed after careful consideration of the references supporting them.
Endocrine Regulatory Logic
As mentioned above dysregulation of the HPA stress response axis has long been associated with ME/CFS. Indeed, a simple computational model of HPA function previously reported by our group readily supported an alternate stable resting state characterized by persistently low levels of circulating cortisol36. This basic model was extended in subsequent work and remains the central representation of stress response circuitry used here. The three main elements of the HPA (CRH, ACTH, and CORT) are represented in the network model, with stress included as an input signal. Norepinephrine and dopamine are also included as highly-connected elements of the stress response. Stress also acts on the HPG axis through norepinephrine release into the ovaries, and also inhibits the release of GnRH, luteinizing hormone, estrogen, and progesterone37. Findings such as these support the existence of complex co-regulatory interactions between the stress response axis and reproductive hormone regulation. Estrogen for example has been shown to increase corticotropin secretion in both female monkeys and rats37, supporting a feedback loop between the HPG and HPA axes. In this work we retain key components of the female HPG axis (estrogen, FSH, GNRH1, LH, and progesterone) used in the basic model reported previously27 and have augmented this with more detailed representation of cross-axis regulatory interactions with adjacent systems such as the HPA axis. We have shown in related work that this representation of the HPG axis is capable of recapitulating the regular oscillations characteristic of the menstrual cycle when simulated with ternary logic38.
Immune Regulatory Logic
Highly-connected immune markers implicated in ME/CFS were selected for inclusion in the current model in addition to peripheral blood markers for which experimental measurements were available in this study population. Specifically, these immune markers included the cytokines IFNγ, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-13, IL-15, IL-17, IL-23, and TNFα. Since evidence of dysregulated B cell14 and NK cell function39 has been reported in ME/CFS, these cell populations were also included in the model.
Model Parameterization and Simulation of Regulatory Dynamics
The biological signaling model is represented as a directed and weighted graph, where an edge represents the regulatory action of one node onto another and where a positive or negative edge polarity indicates a stimulatory or inhibitory mode of action respectively. Network parameters describing signal activation thresholds and the weighted contextual response of each marker node to all combinations of its input mediators were derived according to a discrete logical formalism40–43. These parameters were derived by constraining the model to satisfy a set of qualitative (e.g. steady states) and quantitative observations (experimental data). As is the case in this work, the complexity of the regulatory network models often exceeds that of the available data both in terms of the amount of data and the breadth of the markers surveyed. Here we address the issue of insufficient and incomplete data by translating the parameterization problem into a constraint satisfaction problem38 which we solved using OR-tools (Google), an open-source library of algorithms for operations research44. Constraint satisfaction has proven to be an efficient problem solving technology employed by the Artificial Intelligence community to efficiently solve large combinatorial problems45. In our identification of parameter sets we enforced adherence of model predictions to experimental data, and also constrained the model to support specific clinically observed behaviors. Specifically, we assumed that measurements taken at rest (T0) represented a stable steady state in both ME/CFS patients and healthy controls, and that the trajectory of response to exercise should accommodate a return to this stable resting state in control subjects after 24 hours; the ME/CFS patient trajectory was not similarly constrained. Additionally, the ME/CFS steady state was constrained to align with an under-expression of cortisol and overexpression of estrogen as previously reported27.
RESULTS
Regulatory Network Structure and Parameterization
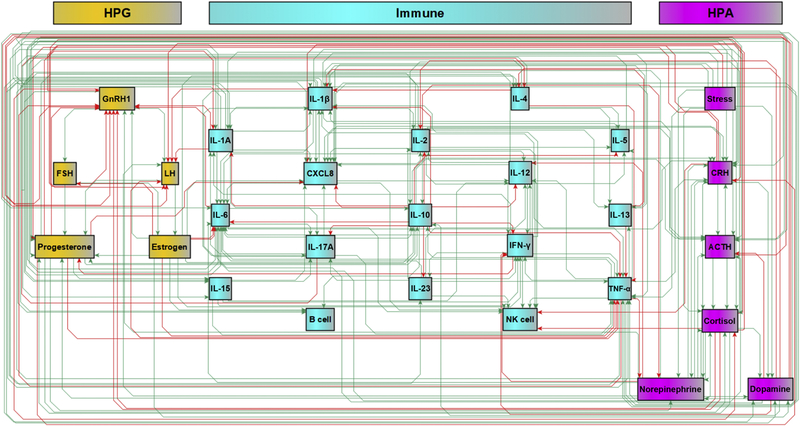
A survey of published literature on elements of the HPA, HPG, and immune systems implicated in ME/CFS identified 28 biological markers, including hormones, neurotransmitters, cytokines, and cell populations, with physiological stress as an input stimulus. Automated text mining of the Pathway Studio literature database and validation of statements about regulatory interactions between these entities identified 214 interactions (Figure 1). The structure of this regulatory circuit model was supported by a total of 21,146 references, with a median of 16.0 references and a mean of 58.9 references supporting each interaction (Appendix A, Figure A1). Based on expert adjudication of divergence in interpretation with a competing Bayesian text mining engine, approximately 4% of edge polarities assigned by Pathway Studio’s MedScan were judged to be an incorrect interpretation of the supporting text. The connection density of our network model (27.3%) is in line with reported estimates of connectivity in protein signaling networks46. Betweenness centrality for individual mediator nodes in the network was highly variable, with TNFα, IL-1β, IL-10, and progesterone each occurring in >50% of shortest paths, highlighting the latter as highly influential regulators in this model (Appendix A, Figure A2).
Figure 1.
Proposed regulatory model of endocrine signaling pathways and molecules implicated in ME/CFS, incorporating elements of the HPA, HPG, and immune systems. The model comprises 28 entities and 214 regulatory edges.
Marker Differential Expression and Discretization
As described in the previous section ME/CFS (n=43) and healthy control (n=45) subjects were challenged with a graded maximal exercise test with serial blood samples collected at 8 time points before during and after challenge. Plasma levels of inflammatory cytokines and the abundance of immune cell subpopulations are depicted in Appendix A, Figure A3. A 2-way ANOVA supported significant effects for condition (ME/CFS vs HC) and/or timepoint in IL-1β, IL-2, IL-4, IL-5, IL-6, IL-13, IL-5, and NK cells (Table 1). Continuous expression values for these 8 exercise responsive and/or condition sensitive markers were converted into discrete activation levels using a variational Bayesian gaussian estimation31,32. This number of discrete states was then used to support a k-means clustering of the median expression at each time point for both conditions, producing discretized exercise response trajectories for each group (Appendix A, Figure A4). Thus, the response trajectories transformed to discrete values offer qualitative representations of the continuous measurements shown in Figure A3, and serve as constraints in a logical modeling formalism. Markers in the numerical model for which experimental measurements were not found to vary significantly were left unconstrained or “free” during parameterization.
Table 1.
A 2-way ANOVA of each measured variable as a function of condition (ME/CFS vs healthy control), timepoint, and interactions. Variables with at least one significant effect were constrained; others were left free.
| Variable | Condition | Time | Condition × Time | Constrained (Y/N) |
|---|---|---|---|---|
| IL-lα | 0.413 | 0.171 | 0.643 | N |
| IL-1β | 0.017 | 0.173 | 0.722 | Y |
| IL-2 | <0.001 | 0.523 | 0.899 | Y |
| IL-4 | <0.001 | 0.585 | 0.550 | Y |
| IL-5 | 0.002 | 0.088 | 0.293 | Y |
| IL-6 | <0.001 | 0.433 | 0.035 | Y |
| IL-8 | 0.481 | 0.760 | 0.869 | N |
| IL-10 | 0.673 | 0.984 | 0.231 | N |
| IL-12 | 0.825 | 0.588 | 0.580 | N |
| IL-13 | <0.001 | 0.403 | 0.009 | Y |
| IL-15 | 0.041 | 0.337 | 0.343 | Y |
| IL-17 | 0.257 | 0.802 | 0.627 | N |
| IL-23 | 0.462 | 0.963 | 0.991 | N |
| IFNγ | 0.298 | 0.918 | 0.967 | N |
| TNFα | 0.584 | 0.244 | 0.894 | N |
| B cell | 0.672 | 0.369 | 0.991 | N |
| NK cell | <0.001 | <0.001 | 0.285 | Y |
Model Parameterization and Alignment with Exercise Response Trajectories
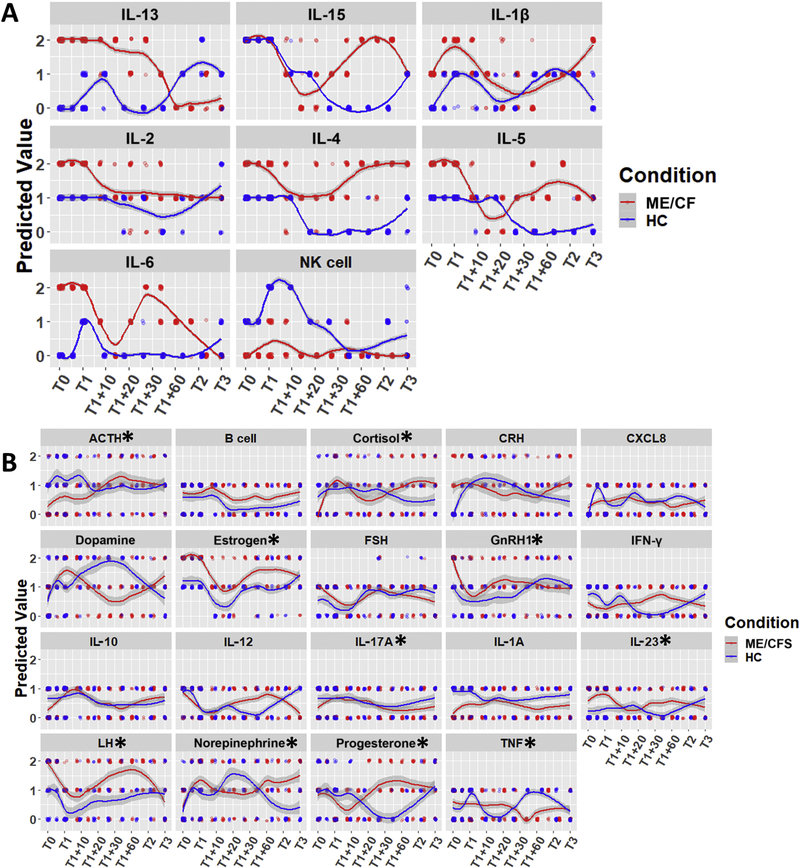
These discrete state response trajectories, in conjunction with the regulatory network structure (Figure 1), served to establish a set of constraints from which regulatory logic parameters were derived in accordance with methods described in our previous work38. As mentioned above, the resolution offered by this group size supported the detection of statistically significant variations in 8 of the 17 measured immune markers. Measurements for this subset of 8 immune markers served to define constraints for the parameter estimation problem. Specifically, allowable parameter sets supported model predictions of the expression of these 8 markers which exactly matched their expression at rest in both ME/CFS and healthy control while also deviating as little as possible from values measured longitudinally during the course of the exercise challenge. In addition to this and independently from the data, qualitative interpretations from the literature of high estrogen and low cortisol levels were applied to constrain the ME/CFS condition at rest only. We found 26 parameter sets which accommodated the available exercise response data to within 5% error in addition to exactly matching the resting steady state discrete expression profiles for ME/CFS and healthy control subjects. The values predicted by these top 26 models for the measured immune markers are shown in Figure 2A, demonstrating close adherence to the discretized experimental data. This suggests that the set of candidate mechanisms embodied in the endocrine-immune circuity model offer a framework for accurately reproducing the immune response to exercise in this cohort of subjects.
Figure 2.
Predicted trajectories for all network entities across 26 top-performing solutions; subjects underwent maximum stress at T1. A) Predicted trajectories for measured and constrained immune entities showing adherence to data. B) Putative trajectories for unconstrained entities. Lines depict LOESS curves with 95% confidence intervals (points are jittered to show relative frequency; * indicates p<0.05 for both ME/CFS condition and its interaction with time, BH-corrected ANOVA).
Validation of Predicted Sex Hormone Expression
As a separate segment of this same dataset, the HPG hormones estrogen (estradiol) and progesterone were measured in the same 43 ME/CFS patients and 45 healthy controls at 4 of the 8 time points (T0, T1, T2 and T3) but were not used to constrain parameter identification for the model (Appendix A, Figure A5). While a requirement for elevated estrogen levels was applied to describe ME/CFS at rest, this constraint was informed by a qualitative interpretation of the literature and not from the data. Moreover, the remainder of the estrogen response trajectory was unconstrained in ME/CFS, as was the entirety of the estrogen response trajectory in the healthy control group. Parameter selection was completely uninformed by any prior knowledge or experimental measurement of progesterone levels in either subject group. As such, these hormone measurements may be tested against the immunologically-informed predictions from the network model as a validation step. In a 2-way ANOVA of estrogen and progesterone measurements over time, we found significant variation in estrogen according to health condition with elevated levels in ME/CFS patients throughout the exercise response (p=0.002), with t tests at each independent timepoint consistently showing a marginally significant increase in ME/CFS patients (p<0.1) for this hormone. A 2-way ANOVA of progesterone measurements indicated a marginally significant difference in progesterone levels across groups (p=0.070), however individual t tests at each independent timepoint did not support these differences at this level of resolution. Nonetheless, the mechanistically predicted response trajectories in Figure 2 are not inconsistent with the hormone measurements shown in Figure A5. The model predicted constitutively upregulated estrogen levels in ME/CFS subjects throughout the course of exercise challenge and recovery, while progesterone was predicted to be elevated only transiently during recovery. Progesterone is of special interest, because our simulations predicted the greatest differences between ME/CFS and measurements in healthy subjects at timepoints immediately following peak exercise stress (T1+10, T1+20, T1+30, T1+60). Though predictions of progesterone expression show good alignment with experimental measurements made at time points T0, T1, T2 and T3, no experimental data was available for further validation of this significant transient response.
Predicting Variations in Exercise Response
These putative mechanisms were then used to mechanistically filter the measured responses serving to constrain model parameters. This same mechanistic framework was used to predict expected values for unmeasured markers and markers with high within-group variability that were not used to constrain parameter optimization (Figure 2B). Such predictions may highlight new potentially significant and mechanistically consistent differences in endocrine and immune response to exercise in ME/CFS subjects. Based on results from a 2-way ANOVA, a number of predicted trajectories for these unmeasured or high heterogeneity markers diverged significantly across time according to illness condition (time × condition interaction) suggesting an alternate regulation of these markers in response to physiological stress in ME/CFS. Specifically, these markers were ACTH, cortisol, estrogen, GNRH1, IL-17, IL-23, LH, and TNFα. Additionally, IL-1α, B cell activation, CRH, and dopamine levels were predicted to vary across condition but independently of time.
Predicting Novel Endocrine-immune Markers of ME/CFS at Rest
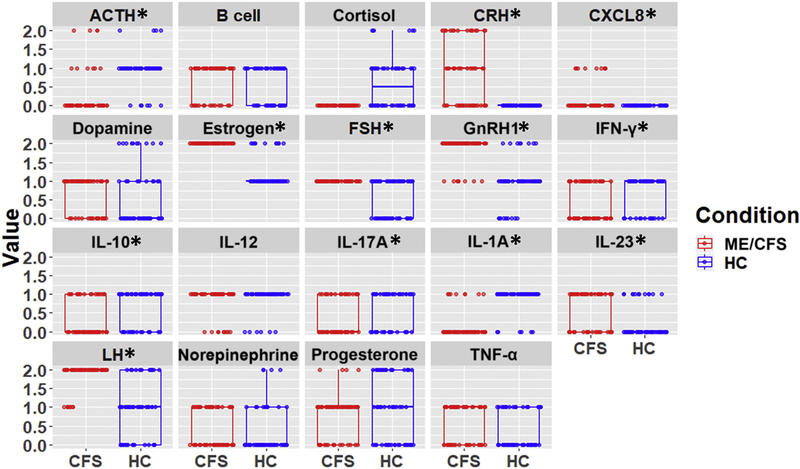
A basic hypothesis in his work has been that ME/CFS presents as a new regulatory setpoint for an alternative homeostatic state. To further understand what these setpoints might be for novel endocrine and immune markers non-parametric Wilcoxon rank-sum tests were applied to the differences in the predicted steady-state expression of all unmeasured markers as well as high heterogeneity markers using all 26 competing numerical models. Because of the number of comparisons, the Benjamini-Hochberg correction was applied to these Wilcoxon tests. Endocrine mediators predicted to be constitutively overexpressed in ME/CFS at rest were CRH, estrogen, FSH, GNRH1, and LH as well as immune mediators CXCL8 and IL-23. Constitutively downregulated entities were the stress hormones ACTH and cortisol as well as immune cytokines IFNγ, IL-10, IL-17, and IL-1α (Figure 3).
Figure 3.
Frequencies of predicted values for unmeasured entities in ME/CFS and HC at rest over 26 solutions with minimal error (* p<0.05, BH-corrected Wilcoxon test).
Although a group-wise comparison of average expression at rest in ME/CFS did not achieve statistical significance in the experimental data (Table 1) for IL-8, IFNγ, IL-10, IL-17, IL-1α, and IL-23, it is important to note that conventional univariate statistical tests do not account for the broader co-regulatory context. Indeed, the model-predicted values for these markers are necessarily in compliance with documented immune regulatory mechanisms. The different models may thus represent subtle differences in regulatory logic that may be characteristic of different patient subpopulations and this divergence from group-wise univariate test results may indicate that these markers are especially sensitive to the within-group heterogeneity of this illness.
Simulating the Therapeutic Disruption of ME/CFS
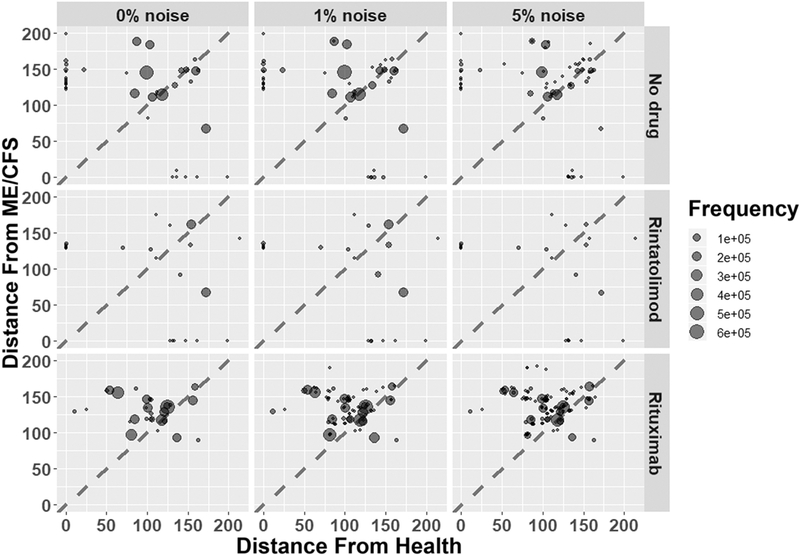
Several pharmaceutical agents have been recently assessed in clinical trials for the treatment of ME/CFS. Among the most prominent are the B cell depleting CD20 antibody rituximab15,47 and the specific TLR3 agonist rintatolimod, which promotes innate inflammatory cytokine production and NK cell activation11,12,48,49. We simulated these courses of treatment in ME/CFS subjects across the family of 26 data-compliant models. Interventions were modeled as fixing the biological targeting the network models to lower or higher values depending on the particular mode of action of the drug. The endocrine-immune network response was then simulated over a horizon of up to 100 transition events to observe whether immune and endocrine profiles evolve towards a new stable steady state, preferably one that more closely resembles normal healthy equilibrium. The similarity of the new predicted steady state to the both the healthy and ME/CFS resting states was expressed as the Euclidean distance, normalized by betweenness centrality of each endocrine and immune marker (Appendix A, Figurer A2) such that matching key mediators (e.g. TNFα, IL-1β, or progesterone) was favored over alignment with less influential markers (e.g. IL-13 or IL-23). In an attempt to canvas a broad range of conditions, 200,000 simulations were conducted by selecting a random initial state from the complete set of states supported by the regulatory circuitry (on the order of 1011 states). We conducted these simulations under conditions of 0%, 1%, and 5% random noise to estimate the robustness of these solutions to biological variability.
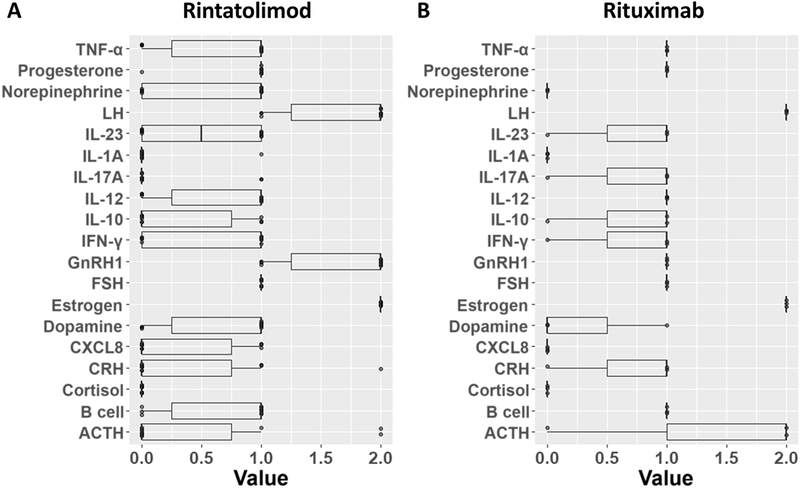
Rituximab was modeled as inhibition of B cells, IFNγ, and IL-4, since B cells have been reported to produce these cytokines in the context of autoimmunity50; rintatolimod was modeled as induction of IL-12 and TNFα48,51. Results of these simulations are depicted in Figure 4. In the absence of any drug, healthy and pathological steady states were reached with roughly equivalent frequencies; increasing noise tended to make all attractors less available. Rintatolimod was predicted to sharply upset the attractor landscape, destabilizing most of the available attractors but retaining both healthy and ME/CFS states. Simulated rituximab treatment destabilized both healthy and ME/CFS attractors. However, the remaining attractors tended to be closer to normal health than to ME/CFS, indicating a potential reduced pathology relative to the untreated ME/CFS state. The outcome of these simulations is summarized in Table 2. Models predicting the most favorable response to treatment were surveyed to assess the degree of agreement between their respective predictions of endocrine-immune profile for ME/CFS at rest (Figure 5) to highlight profiles likely to be characteristic of good candidates for rituximab or rintatolimod treatment. 10 of the 26 models supported a return to the healthy homeostasis reference state under rintatolimod treatment, and 3 models supported return to an attractor with a Euclidean distance from health of less than 50 under rituximab treatment. These subsets of candidate models predicted that rintatolimod is most likely to benefit patients with low IL-1α, IL-17, and cortisol, intermediate progesterone and FSH, and high estrogen. The 3 out of 26 models supporting a favorable response to rituximab described an ME/CFS state characterized by low norepinephrine, IL-1α, CXCL8, and cortisol, intermediate, intermediate FSH and GNRH1, and high TNF, LH, IL-12, and B cell activation.
Figure 4.
Results of simulated drug treatments. Each attractor is represented by a point, sized according to the number of simulations reaching it. Axes represent the Euclidean distance of each attractor from the Health and ME/CFS states multiplied by the betweenness centrality of each entity. Points above the diagonal are more similar to Health than to ME/CFS (less pathological); points below the diagonal are more similar to ME/CFS than to Health (more pathological).
Table 2.
Results of surveys on the basins of attraction. The number of simulations ending in a Healthy or ME/CFS attractor (Euclidean distance of 0) is indicated, along with the simulations ending in the Healthy attractor as a fraction of simulations ending in Health or ME/CFS. *Since simulated rituximab treatment rendered the Healthy and ME/CFS attractors unavailable, a distance cutoff of 50 was used.
| Noise | Drug | Healthy | ME/CFS | Fraction |
|---|---|---|---|---|
| 0% noise | No drug | 33031 | 509 | 0.985 |
| 0% noise | Rintatolimod | 2584 | 385 | 0.867 |
| 0% noise | Rituximab* | 4065 | 0 | NA |
| 1% noise | No drug | 47291 | 13128 | 0.783 |
| 1% noise | Rintatolimod | 11152 | 7723 | 0.591 |
| 1% noise | Rituximab* | 28963 | 0 | NA |
| 5% noise | No drug | 9604 | 2641 | 0.784 |
| 5% noise | Rintatolimod | 4462 | 1397 | 0.762 |
| 5% noise | Rituximab* | 8695 | 0 | NA |
Figure 5.
Predicted ME/CFS states associated with favorable response to treatment by A) rintatolimod or B) rituximab. The subset of models found to reach states with a distance-from-health of 0 (rintatolimod) or less than 50 (rituximab) under drug treatment were canvassed for their predictions of resting ME/CFS values for unconstrained network entities.
DISCUSSION
In this work we assemble a network model of 28 endocrine and immune markers linked by 214 regulatory signaling mechanisms documented in the literature including those elements reported to be involved in ME/CFS pathology. We found 26 competing parameter sets which allowed this regulatory circuit to exactly reproduce the expression profile measured at rest in 8 characteristic immune markers for both the ME/CFS and healthy control conditions as well as align with the exercise response dynamics of both groups to with 5% error. It is important to note that the complexity of the regulatory model exceeds the coverage supported by the available data. In this case, measurements for important hormones were unavailable and the range of conditions was limited to exercise challenge of a specific type. This results in a parameter identification problem that is highly under-constrained or where many model solutions exist that satisfy the data equally well. As such, the 26 candidate models examined here represent only a small fraction of all possible solutions. However, they are all equally consistent with the available data, and if they are assumed to comprise a representative fraction of all the valid models, certain qualitative conclusions may still be drawn. The finding that a single circuit model of endocrine-immune regulation can support both the healthy and ME/CFS phenotypes is in itself significant. This suggests that ME/CFS may consist of altered regulatory function without permanent damage to the underlying regulatory circuitry, such that substantial remission may be achievable. This finding, which is here supported by longitudinal exercise-response data applied to a much more detailed model of endocrine-immune function, remains consistent with earlier work by our group using a much coarser grained representation and resting state data only27. Another important contribution is the explicit application of signaling network based on known documented mechanisms directly in our analysis of experimental data to reinforce the coordinated and context-specific interdependency of immune and endocrine markers. For example, enforcing documented co-regulatory structure to the 8 exercise responsive markers identified in this data and described above confirmed elevated expression in ME/CFS at rest of IL-1β, IL-4, IL-5 and IL-6 as reported previously by our group4. However, the model and data in this work also suggest elevated IL-2 and IL-13, previously reported as unchanged and reduced in expression respectively in resting ME/CFS subjects. Interestingly, closer examination of this earlier data revealed that although increased group size (n=40 ME/CFS, 59 HC) supported a statistically significant difference, the fold change in median expression of these markers was very weak (~1.1, 1.2 respectively). In this same previous work, relatively high intragroup variability in cytokine expression was observed (Median absolute deviation / median > 0.50), especially in the ME/CFS group. Conventional univariate statistical tests are ill-suited to this situation, and more complex variants that formally account for the interdependencies between markers and control for context are required. Here, rather than attempt to extract this interdependency structure from the data using regression-based approaches, we applied known documented signaling mechanisms to this end. Indeed, controlling in the context of documented regulatory mechanisms suggests here that IL-10, IL-17 and IFN-γ expression, previously reported as unchanged on average between groups4, may in fact be lower in ME/CFS subjects at rest in the context of joint marker expression. Controlling for co-regulated expression also predicts depressed levels of IL-1α and elevated levels of IL-2, IL-8, IL-23 and TNF-α in ME/CFS at rest, differences that were either undetected or contrary to reported median expression changes using conventional group-wise statistics.
Of note in this work this co-regulatory context is expanded beyond the immune system to include endocrine mediators. This is of special significance for an illness which disproportionately affects one sex over the other as regular variations over the course of the menstrual cycle profoundly influence immune function8. Not surprisingly, dysregulation of the HPA and HPG axes, specifically overexpression of estrogen, FSH, GNRH1, and LH in ME/CFS subjects, is predicted based on the regulatory circuit model presented here. These hormones are important regulators of the menstrual cycle, which is known to be dysregulated in women with ME/CFS52. These results highlight the importance of considering cyclic fluctuations in hormonal regulation when considering complex metabolic disorders such as ME/CFS, especially in women. Interestingly, one case of ME/CFS associated with membranous dysmenorrhea spontaneously resolved after the discontinuation of hormonal contraceptive treatment53. Despite this observation, other studies have failed to find significant group-wise changes in sex hormones in ME/CFS54,55. Once again however, these results are based on group-wise average expression and are not controlled in the context of co-expression in other markers.
Taken together these mechanistically adherent differences suggest a general overactivation of the HPG axis and inactivation of the HPA axis in ME/CFS with a heightened sensitivity to inflammatory stimuli. IL-1β, IL-6 and TNF-α (all predicted in this work to be elevated in ME/CFS) are drivers of sterile inflammation, especially in the brain56,57. The predicted upregulation of CXCL8 and IL-23 could also be taken as an indication of increased inflammation in the brain, as both of these cytokines are associated with pathological neuroinflammation58–60. GNRH1 agonists and recombinant FSH administered as a fertility treatment have been found to exacerbate multiple sclerosis (MS), increasing blood-brain barrier permeability to peripheral blood mononuclear cells and a greater abundance of cells producing IL-8. Progesterone and estrogen levels were also increased in these patients61. Thus, HPG dysregulation may increase blood-brain barrier permeability, allowing infiltration of immune cells into the brain to establish or sustain a low-grade neuroinflammatory profile as put forward by Morris and Maes17. Indeed, white-matter lesions have long been reported in connection with ME/CFS62,63. More recent studies have found abnormalities in cerebrospinal fluid independent of psychiatric diagnoses64–66, consistent with neuroinflammation.
The origin of this neuroinflammatory dysregulation is unclear, with many competing hypotheses being proposed. Epstein-Barr and hepatitis viral infections have been implicated as potential causes14,67, with evidence that these viruses can persistently inhibit innate and adaptive immunity. Mitochondrial dysfunction has also been identified in ME/CFS patients68, and the extracellular release of mitochondrial DNA in the hypothalamus has been proposed as an initiator of neuroinflammation via mast cell activation69. These activated mast cells may then go on to establish a self-sustaining positive feedback of neuroinflammation and autoimmunity70–72, with further complex interactions with other endocrine systems73,74. Our approach is independent of any assumptions about the ultimate underlying cause of ME/CFS. Observed patterns of immune dysregulation in the study cohort are sufficient for us to project concomitant differences in the HPA and HPG axes informed by known regulatory relationships between these systems. In future work we hope to extend the network model to include more-explicit representation of other cell types and systems implicated in ME/CFS pathology such as mitochondrial metabolism and mast cells.
While efforts to treat ME/CFS have often focused on therapeutic modulation of immune mediators, these trials have met with mixed results. Rituximab has been effective in only a subset of patients, with no clear explanation for its mechanism of action47. Likewise, rintatolimod (Ampligen) is sometimes effective11,12, but a means of identifying good candidates for this course of treatment remains similarly elusive. The mechanistic effects of rintatolimod in vivo have not been very well defined. While the latter has been found to delay T cell depletion in the context of HIV infection75, data regarding its influence on systemic cytokine production are sparse, especially as divergent responses have been described in primates and rodents76. Our simulations did not predict a complete rescue in most cases as a result of either rintatolimod or rituximab therapy. While rintatolimod treatment was predicted to reach a target healthy remission state in some cases, these represented only a small fraction of all simulations. Rituximab treatment was not predicted to deliver remission to a target healthy state, but instead to reduce the availability of highly pathological steady states. These simulations offer a possible explanation for the wide variability in the reported efficacy of these drugs in clinical trials. In general, rintatolimod is predicted to destabilize most attractors, but does not necessarily disrupt the ME/CFS state, indicating that rintatolimod may either induce a more-or-less complete remission or have no appreciable effect. Rituximab, on the other hand, is more likely to support alternate stable resting states which are more similar to health than the initial ME/CFS pathological state, resulting in a partial remission. These outcomes are highly dependent on both the initial endocrine-immune profile and regulatory tone represented here by different candidate models. Indeed, predicted treatment-responsive endocrine-immune expression profiles supported by these different models agree unanimously in only a select few markers. This result is not surprising given that ME/CFS is diagnosed based on adherence to a broad set of physiological symptoms and the occurrence of multiple disease phenotypes in experimental studies of ME/CFS has been highlighted as a significant challenge77. Variability in the efficacy of different treatments is likely to be a consequence of this heterogeneity within study populations. Indeed, our analysis of the family of competing models which equally satisfied the experimental data suggested that inclusion criteria in further studies of rintatolimod and rituximab could be informed by subject stratification based on differences in the simultaneous co-expression patterns of cortisol, FSH, progesterone and estrogen.
5. Conclusions
Computer simulations based on a literature-informed mechanistic model of endocrine-immune co-regulatory dynamics tuned to experimental exercise data support a potential etiology for ME/CFS where sustained HPG overactivation may permits the initiation and maintenance of peripheral inflammation potentially leading to low-grade neuroinflammation. The partial efficacy of rituximab and rintatolimod treatment are both predicted to alter the landscape of available steady states such that pathological states are less available. Results suggest that future studies of ME/CFS and related clinical trials in women should consider the impact of the menstrual cycle on other endocrine and immune regulatory processes. Measurements of HPA and HPG hormones, especially cortisol, ACTH, estrogen, GNRH, and LH, may be of considerable value in developing rigorous biomarkers for ME/CFS diagnosis and delineating treatment-responsive subgroups.
Supplementary Material
Highlights.
Variations in immune function and dysregulation of sex hormones in ME/CFS are mutually informative
Existing drugs including e.g. rintatolimod and rituximab may serve to destabilize pathological attractors such as ME/CFS
Different endocrine and immune profiles may support distinct patient subtypes and inform on therapeutic response in rintatolimod and rituximab treatment
Acknowledgments
This work was supported by NIH awards R01 NS090200–01 (Fletcher PI); R56 AI065723–06A1 (Fletcher PI); R01AR057853–01 (Klimas PI) as well as by the US Department of Defense Congressionally Directed Medical Research Program (CDMRP) (http://cdmrp.army.mil/) under award GW140142 (Broderick/Craddock - PI; Whitley Partnering PI), as well as GW093042 (Broderick - PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Mandatory Disclaimer
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Defense.
Appendix A. Supplementary material
Figures A1–A5
REFERENCES
- 1.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–959. http://www.ncbi.nlm.nih.gov/pubmed/7978722. Accessed August 25, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223–249. doi: 10.1515/reveh-2015-0026 [DOI] [PubMed] [Google Scholar]
- 3.Broderick G, Katz BZ, Fernandes H, et al. Cytokine expression profiles of immune imbalance in post-mononucleosis chronic fatigue. J Transl Med. 2012;10:191. doi: 10.1186/1479-5876-10-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broderick G, Fuite J, Kreitz A, Vernon SD, Klimas N, Fletcher MA. A formal analysis of cytokine networks in Chronic Fatigue Syndrome. Brain Behav Immun. 2010;24(7):1209–1217. doi: 10.1016/j.bbi.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz BZ, Shiraishi Y, Mears CJ, Binns HJ, Taylor R. Chronic Fatigue Syndrome After Infectious Mononucleosis in Adolescents. Pediatrics. 2009;124(1):189–193. doi: 10.1542/peds.2008-1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broderick G, Craddock RC, Whistler T, Taylor R, Klimas N, Unger ER. Identifying illness parameters in fatiguing syndromes using classical projection methods. Pharmacogenomics. 2006;7(3):407–419. doi: 10.2217/14622416.7.3.407 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen CB, Alsøe L, Lindvall JM, et al. Whole blood gene expression in adolescent chronic fatigue syndrome : an exploratory cross-sectional study suggesting altered B cell differentiation and survival. J Transl Med. 2017. doi: 10.1186/s12967-017-1201-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S, Kim J, Jang B, et al. Fluctuation of peripheral blood T, B, and NK cells during a menstrual cycle of normal healthy women. J Immunol. 2010;185(1):756–762. doi: 10.4049/jimmunol.0904192 [DOI] [PubMed] [Google Scholar]
- 9.Lorenz TK, Heiman JR, Demas GE. Sexual activity modulates shifts in TH1/TH2 cytokine profile across the menstrual cycle: an observational study. Fertil Steril. 2015;104(6):1513–21.e1–4. doi: 10.1016/j.fertnstert.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burfeind KG, Michaelis KA, Marks DL. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin Cell Dev Biol. 2016;54(4):42–52. doi: 10.1016/j.semcdb.2015.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strayer DR, Carter WA, Stouch BC, et al. A double-blind, placebo-controlled, randomized, clinical trial of the TLR-3 agonist rintatolimod in severe cases of chronic fatigue syndrome. PLoS One. 2012;7(3):1–9. doi: 10.1371/journal.pone.0031334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell WM. Efficacy of rintatolimod in the treatment of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). Expert Rev Clin Pharmacol. 2016;9(6):755–770. doi: 10.1586/17512433.2016.1172960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lunde S, Kristoffersen EK, Sapkota D, et al. Serum BAFF and APRIL Levels, T-Lymphocyte Subsets, and Immunoglobulins after B-Cell Depletion Using the Monoclonal Anti-CD20 Antibody Rituximab in Myalgic Encephalopathy/Chronic Fatigue Syndrome. PLoS One. 2016;11(8):e0161226. doi: 10.1371/journal.pone.0161226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loebel M, Strohschein K, Giannini C, et al. Deficient EBV-specific B- and T-cell response in patients with Chronic Fatigue Syndrome. PLoS One. 2014;9(1). doi: 10.1371/journal.pone.0085387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley AS, Ford B, Bansal AS. Altered functional B cell subset populations in patients with chronic fatigue syndrome compared to healthy controls. Clin Exp Immunol. 2013;172(1):73–80. doi: 10.1111/cei.12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouquet J, Gardy JL, Brown S, et al. RNA-Seq Analysis of Gene Expression, Viral Pathogen, and B-Cell/T-Cell Receptor Signatures in Complex Chronic Disease. Clin Infect Dis. 2017;64(4):476–481. doi: 10.1093/cid/ciw767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris G, Maes M. A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome. Metab Brain Dis. 2013;28(4):523–540. doi: 10.1007/s11011-012-9324-8 [DOI] [PubMed] [Google Scholar]
- 18.Cleare AJ, Reid S, Chalder T, Hotopf M, Wessely S. Chronic fatigue syndrome. BMJ Clin Evid. 2015;2015 http://www.ncbi.nlm.nih.gov/pubmed/26415100. Accessed August 25, 2017. [PMC free article] [PubMed] [Google Scholar]
- 19.Smylie AL, Broderick G, Fernandes H, et al. A comparison of sex-specific immune signatures in Gulf War illness and chronic fatigue syndrome. BMC Immunol. 2013;14(1):1–14. doi: 10.1186/1471-2172-14-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClelland EE, Smith JM. Gender Specific Differences in the Immune Response to Infection. Arch Immunol Ther Exp (Warsz). 2011;59(3):203–213. doi: 10.1007/s00005-011-0124-3 [DOI] [PubMed] [Google Scholar]
- 21.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. 2012;38(2–3):J282–J291. doi: 10.1016/j.jaut.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. doi: 10.1038/nature21363 [DOI] [PubMed] [Google Scholar]
- 23.Stringer EA, Baker KS, Carroll IR, et al. Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: Evidence of inflammatory pathology. J Transl Med. 2013;11(1). doi: 10.1186/1479-5876-11-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montoya JG, Holmes TH, Anderson JN, et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci. 2017:201710519. doi: 10.1073/pnas.1710519114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aschbacher K, Adam EK, Crofford LJ, Kemeny ME, Demitrack MA, Ben-Zvi A. Linking disease symptoms and subtypes with personalized systems-based phenotypes: A proof of concept study. Brain Behav Immun. 2012;26(7):1047–1056. doi: 10.1016/j.bbi.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritsch P, Craddock TJ, del Rosario RM, et al. Succumbing to the laws of attraction: Exploring the sometimes pathogenic versatility of discrete immune logic. Syst Biomed. 2013;1(3):179–191. doi: 10.4161/sysb.28948 [DOI] [Google Scholar]
- 27.Craddock TJA, Fritsch P, Rice MA, et al. A role for homeostatic drive in the perpetuation of complex chronic illness: Gulf war illness and chronic fatigue syndrome. PLoS One. 2014;9(1). doi: 10.1371/journal.pone.0084839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J Chronic Fatigue Syndr. 2003;11(1):7–115. doi: 10.1300/J092v11n01_02 [DOI] [Google Scholar]
- 29.Fletcher M, Zeng X, Barnes Z, Levis S, Klimas NG. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. 2009;7(1):96. doi: 10.1186/1479-5876-7-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. 2017. http://www.r-project.org/.
- 31.Subedi S, McNicholas PD. Variational Bayes approximations for clustering via mixtures of normal inverse Gaussian distributions. Adv Data Anal Classif. 2014;8(2):167–193. doi: 10.1007/s11634-014-0165-7 [DOI] [Google Scholar]
- 32.Chen M-S, Wang H-F, Hwang C-P, Ho T-Y, Hung C-H. A Variational Bayesian Approach for Unsupervised Clustering. In: Springer, Singapore; 2016:651–660. doi: 10.1007/978-981-10-0539-8_63 [DOI] [Google Scholar]
- 33.Wickham H ggplot2: Elegant Graphics for Data Analysis. 2009. http://ggplot2.org.
- 34.Novichkova S, Egorov S, Daraselia N. MedScan, a natural language processing engine for MEDLINE abstracts. Bioinformatics. 2003;19(13):1699–1706. http://www.ncbi.nlm.nih.gov/pubmed/12967967. Accessed November 25, 2017. [DOI] [PubMed] [Google Scholar]
- 35.Dey L, Chakraborty S, Biswas A, Bose B, Tiwari S. Sentiment Analysis of Review Datasets Using Naïve Bayes’ and K-NN Classifier. Int J Inf Eng Electron Bus. 2016;8(4):54–62. doi: 10.5815/ijieeb.2016.04.07 [DOI] [Google Scholar]
- 36.Ben-Zvi A, Vernon SD, Broderick G. Model-based therapeutic correction of hypothalamic-pituitary-adrenal axis dysfunction. PLoS Comput Biol. 2009;5(1). doi: 10.1371/journal.pcbi.1000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toufexis D, Rivarola M, Lara H. Stress and the Reproductive Axis. Neuroendocrinology. 2015;26(9):573–586. doi: 10.1111/jne.12179.Stress [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedghamiz H, Chen W, Rice MJ, Whitley D, Broderick G. Selecting Optimal Models Based on Efficiency and Robustness in Multi-valued Biological Networks In: IEEE International Conference on Bioinformatics and Bioengineering. Washington DC; 2017. doi: 10.1109/BIBE.2017.00040 [DOI] [Google Scholar]
- 39.Brenu EW, van Driel ML, Staines DR, et al. Longitudinal investigation of natural killer cells and cytokines in chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 2012;10(1):88. doi: 10.1186/1479-5876-10-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas R, Kaufman M. Multistationarity, the basis of cell differentiation and memory. II. Logical analysis of regulatory networks in terms of feedback circuits. Chaos. 2001;11(1):180–195. doi: 10.1063/1.1349893 [DOI] [PubMed] [Google Scholar]
- 41.Thomas R, Kaufman M. Multistationarity, the basis of cell differentiation and memory. I. Structural conditions of multistationarity and other nontrivial behavior. Chaos. 2001;11(1):170–179. doi: 10.1063/1.1350439 [DOI] [PubMed] [Google Scholar]
- 42.Thomas R, Thieffry D, Kaufman M. Dynamical Behavior Of Biological Regulatory Networks .1. Biological Role Of Feedback Loops And Practical Use Of The Concept Of The Loop-Characteristic State. Bull Math Biol. 1995;57(2):247–276. doi: 10.1016/0092-8240(94)00036-C [DOI] [PubMed] [Google Scholar]
- 43.De Jong H Modeling and Simulation of Genetic Regulatory Systems: A Literature Review. Inst Natl Rech en Inform en Autom. 2002;9(September):67–103. doi: 10.1089/10665270252833208 [DOI] [PubMed] [Google Scholar]
- 44.Perron L Operations research and constraint programming at google. In: Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Vol 6876 LNCS.; 2011:2. doi: 10.1007/978-3-642-23786-7_2 [DOI] [Google Scholar]
- 45.Nethercote N, Stuckey PJ, Becket R, Brand S, Duck GJ, Tack G. MiniZinc: Towards a Standard CP Modelling Language In: Principles and Practice of Constraint Programming – CP 2007. Berlin, Heidelberg: Springer Berlin Heidelberg; 2007:529–543. doi: 10.1007/978-3-540-74970-7_38 [DOI] [Google Scholar]
- 46.Dong J, Horvath S. Understanding network concepts in modules. BMC Syst Biol. 2007;1:1–20. doi: 10.1186/1752-0509-1-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mensah F, Bansal A, Berkovitz S, et al. Extended B cell phenotype in patients with myalgic encephalomyelitis/chronic fatigue syndrome: a cross-sectional study. Clin Exp Immunol. 2016;184(2):237–247. doi: 10.1111/cei.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engel AL, Holt GE, Lu H. The pharmacokinetics of toll-like receptor agonists and the impact on the immune system. Expert Rev Clin Pharmacol. 2011;4(2):275–289. doi: 10.1586/ecp.11.5.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsumoto M, Tatematsu M, Nishikawa F, et al. Defined TLR3-specific adjuvant that induces NK and CTL activation without significant cytokine production in vivo. Nat Commun. 2015;6(1):6280. doi: 10.1038/ncomms7280 [DOI] [PubMed] [Google Scholar]
- 50.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20(3):332–338. doi: 10.1016/j.coi.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicodemus CF, Berek JS. TLR3 agonists as immunotherapeutic agents. Immunotherapy. 2010;2(2):137–140. doi: 10.2217/imt.10.8 [DOI] [PubMed] [Google Scholar]
- 52.Boneva RS, Lin J-MS, Unger ER. Early menopause and other gynecologic risk indicators for chronic fatigue syndrome in women. Menopause. 2015;22(8):826–834. doi: 10.1097/GME.0000000000000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veldman J, Van Houdenhove B, Verguts J. Chronic fatigue syndrome: A hormonal origin? A rare case of dysmenorrhea membranacea. Arch Gynecol Obstet. 2009;279(5):717–720. doi: 10.1007/s00404-008-0795-0 [DOI] [PubMed] [Google Scholar]
- 54.Cevik R, Gur A, Acar S, Nas K, Sarac AJ. Hypothalamic-pituitary-gonadal axis hormones and cortisol in both menstrual phases of women with chronic fatigue syndrome and effect of depressive mood on these hormones. BMC Musculoskelet Disord. 2004;5(1):47. doi: 10.1186/1471-2474-5-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gur A, Cevik R, Nas K, Colpan L, Sarac S. Cortisol and hypothalamic-pituitary-gonadal axis hormones in follicular-phase women with fibromyalgia and chronic fatigue syndrome and effect of depressive symptoms on these hormones. Arthritis Res Ther. 2004;6(3):R232. doi: 10.1186/ar1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brough D, Denes A. Interleukin-1α and brain inflammation. IUBMB Life. 2015;67(5):323–330. doi: 10.1002/iub.1377 [DOI] [PubMed] [Google Scholar]
- 57.Dantzer R Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500(1–3):399–411. doi: 10.1016/j.ejphar.2004.07.040 [DOI] [PubMed] [Google Scholar]
- 58.vom Berg J, Prokop S, Miller KR, et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease–like pathology and cognitive decline. Nat Med. 2012;18(12):1812–1819. doi: 10.1038/nm.2965 [DOI] [PubMed] [Google Scholar]
- 59.He Q, Shi X, Zhou B, et al. Interleukin 8 (CXCL8)-CXC chemokine receptor 2 (CXCR2) axis contributes to MiR-4437-associated recruitment of granulocytes and natural killer cells in ischemic stroke. Mol Immunol. 2018;101:440–449. doi: 10.1016/j.molimm.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 60.Shichita T, Ago T, Kamouchi M, Kitazono T, Yoshimura A, Ooboshi H. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J Neurochem. 2012;123:29–38. doi: 10.1111/j.1471-4159.2012.07941.x [DOI] [PubMed] [Google Scholar]
- 61.Correale J, Farez MF, Ysrraelit MC. Increase in multiple sclerosis activity after assisted reproduction technology. Ann Neurol. 2012;72(5):682–694. doi: 10.1002/ana.23745 [DOI] [PubMed] [Google Scholar]
- 62.Lange G, DeLuca J, Maldjian J a, Lee H, Tiersky L a, Natelson BH. Brain MRI abnormalities exist in a subset of patients with chronic fatigue syndrome. J Neurol Sci. 1999;171(1):3–7. doi:S0022510X99002439[pii] [DOI] [PubMed] [Google Scholar]
- 63.Keenan PA. Brain MRI abnormalities exist in chronic fatigue syndrome. J Neurol Sci. 1999;171(1):1–2. doi: 10.1016/S0022-510X(99)00242-7 [DOI] [PubMed] [Google Scholar]
- 64.Natelson BH, Mao X, Stegner AJ, et al. Multimodal and simultaneous assessments of brain and spinal fluid abnormalities in chronic fatigue syndrome and the effects of psychiatric comorbidity. J Neurol Sci. 2017;375:411–416. doi: 10.1016/j.jns.2017.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wyller VB, Reme SE, Mollnes TE. Chronic fatigue syndrome/myalgic encephalo-myelitis--pathophysiology, diagnosis and treatment. Tidsskr Nor Laegeforen. 2015;135(23–24):2172–2175. doi: 10.4045/tidsskr.15.1180 [DOI] [PubMed] [Google Scholar]
- 66.Wostyn P, De Deyn PP. The putative glymphatic signature of chronic fatigue syndrome: A new view on the disease pathogenesis and therapy. Med Hypotheses. 2018;118(May):142–145. doi: 10.1016/j.mehy.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 67.Russell A, Hepgul N, Nikkheslat N, et al. Persistent fatigue induced by interferon-alpha: a novel, inflammation-based, proxy model of chronic fatigue syndrome. Psychoneuroendocrinology. 2018;(November):1–10. doi: 10.1016/j.psyneuen.2018.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagy-Szakal D, Barupal DK, Lee B, et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci Rep. 2018;8(1):10056. doi: 10.1038/s41598-018-28477-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hatziagelaki E, Adamaki M, Tsilioni I, Dimitriadis G, Theoharides TC. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome-Metabolic Disease or Disturbed Homeostasis due to Focal Inflammation in the Hypothalamus? J Pharmacol Exp Ther. 2018;367(1):155–167. doi: 10.1124/jpet.118.250845 [DOI] [PubMed] [Google Scholar]
- 70.Tettamanti L, Kritas SK, Gallenga CE, et al. IL-33 mediates allergy through mast cell activation: Potential inhibitory effect of certain cytokines. J Biol Regul Homeost Agents. 32(5):1061–1065. http://www.ncbi.nlm.nih.gov/pubmed/30334399. Accessed February 4, 2019. [PubMed] [Google Scholar]
- 71.Varvara G, Tettamanti L, Gallenga CE, et al. Stimulated mast cells release inflammatory cytokines: potential suppression and therapeutical aspects. J Biol Regul Homeost Agents. 32(6):1355–1360. http://www.ncbi.nlm.nih.gov/pubmed/30574739. Accessed February 4, 2019. [PubMed] [Google Scholar]
- 72.Gallenga CE, Pandolfi F, Caraffa A, et al. Interleukin-1 family cytokines and mast cells: activation and inhibition. J Biol Regul Homeost Agents. 2019;33(1). http://www.ncbi.nlm.nih.gov/pubmed/30656901. Accessed February 4, 2019. [PubMed] [Google Scholar]
- 73.Zouikr I, Karshikoff B. Lifetime modulation of the pain system via neuroimmune and neuroendocrine interactions. Front Immunol. 2017;8(MAR). doi: 10.3389/fimmu.2017.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Theoharides TC, Weinkauf C, Conti P. Brain cytokines and neuropsychiatric disorders. J Clin Psychopharmacol. 2004;24(6):577–581. doi: 10.1097/01.jcp.0000148026.86483.4f [DOI] [PubMed] [Google Scholar]
- 75.Thompson KA, Strayer DR, Salvato PD, et al. Results of a double-blind placebo-controlled study of the double-stranded RNA drug polyI:polyC12U in the treatment of HIV infection. Eur J Clin Microbiol Infect Dis. 1996;15(7):580–587. http://www.ncbi.nlm.nih.gov/pubmed/8874076. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell WM, Nicodemus CF, Carter WA, Horvath JC, Strayer DR. Discordant biological and toxicological species responses to TLR3 activation. Am J Pathol. 2014;184(4):1062–1072. doi: 10.1016/j.ajpath.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maclachlan L, Watson S, Gallagher P, et al. Are current chronic fatigue syndrome criteria diagnosing different disease phenotypes? PLoS One. 2017;12(10):1–16. doi: 10.1371/journal.pone.0186885 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.