Abstract
In nature, an oxo-bridged Mn4CaO5 cluster embedded in Photosystem II (PSII), a membrane-bound multi-subunit pigment protein complex, catalyzes the water oxidation reaction that is driven by light-induced charge separations in the reaction center of PSII. The Mn4CaO5 cluster accumulates four oxidizing equivalents to enable the four-electron four-proton catalysis of two water molecules to one dioxygen molecule and cycles through five intermediate S-states, S0 – S4 in the Kok cycle. One important question related to the catalytic mechanism of the oxygen-evolving complex (OEC) that remains is, whether structural isomers are present in some of the intermediate S-states and if such equilibria are essential for the mechanism of the O-O bond formation. Here we compare results from electron paramagnetic resonance (EPR) and X-ray absorption spectroscopy (XAS) obtained at cryogenic temperatures for the S2 state of PSII with structural data collected of the S1, S2 and S3 states by serial crystallography at neutral pH (~6.5) using an X-ray free electron laser at room temperature. While the cryogenic data demonstrate the presence of at least two structural forms of the S2 state, the room temperature crystallography data can be well-described by just one S2 structure. We discuss the deviating results and outline experimental strategies for clarifying this mechanistically important question.
Introduction
In oxygenic photosynthesis, light-driven water oxidation to molecular oxygen is carried out by the oxygen-evolving complex (OEC) in Photosystem II (PSII), which is a multisubunit protein complex in the thylakoid membrane of plants, algae, and cyanobacteria (Wydrzynski and Satoh 2005, Renger 2008). The OEC consists of four oxo-bridged Mn atoms and one Ca atom (Mn4CaO5) ligated to the D1 protein and chlorophyll-protein CP43 subunits by carboxylate and histidine ligands (Umena et al. 2011). During water oxidation, the Mn4CaO5 complex cycles through five intermediate states, collectively called the S-states, labelled S0 – S4 in the Kok cycle (Kok et al. 1970). S0 is the most reduced state, while S1, S2 and S3 represent sequentially higher oxidation states in the OEC. O2 is released during the S3→[S4]→S0 transition, where S4 is a transient state. Thus, the Mn4CaO5 cluster accumulates four oxidizing equivalents before the release of O2. The formal oxidation state of each S-state has been assigned as Mn4III,III,III,IV for S0, Mn4III,III,IV,IV for S1 Mn4III, IV,IV,IV for S2, and Mn4IV,IV,IV,IV for S3 (Haumann et al. 2005, Dau and Haumann 2008, Yano and Yachandra 2014). Increased delocalization of the charge in the Mn complex has also been proposed, especially, in the higher S-states, using data from resonant inelastic X-ray spectroscopy (RIXS) experiments (Glatzel et al. 2004, Glatzel et al. 2013).
Recently, we reported the structures of the catalytic intermediate states of PSII in the dark (0F; S1-rich), 1F (S2-rich), 2F (S3-rich), and 3F (S0-rich)) states with resolutions of 2.04 – 2.08 Å (Kern et al. 2018). Data were collected at an X-ray Free Electron Laser (XFEL) with femtosecond X-ray pulses at room temperature (RT), by in situ visible light excitation to advance the S-states. Improved resolution approaching 2 Å unambiguously reveals oxygen and metal atomic positions in the catalytic center in each state. Kβ1,3 X-ray emission spectra (XES) were simultaneously collected from crystals to provide confirmation of catalytic advancement by probing the changes in the oxidation state of the metal cluster (Kern et al. 2013, Kern et al. 2014, Fuller et al. 2017, Fransson et al. 2018, Kern et al. 2018), together with the oxidation state changes of the mobile quinone QB site, evidenced by its electron density changes. Availability of the structural data for each stable intermediate state now enables us to reexamine previous X-ray spectroscopy data based on the new structural information.
One of the important questions regarding the OEC is the presence or absence of structural isomers in each S-state and its catalytic role (Pantazis et al. 2012, Renger 2012, Cox et al. 2014, Isobe et al. 2014). Spin isomers indeed exist, as shown by the various EPR studies in the S2 state (reviewed in (Haddy 2007, Pokhrel and Brudvig 2014)). Yet, whether such isomers play an important role in the catalytic mechanism has not been demonstrated experimentally. For example, the importance of the spin isomorphism observed in the S2 state has been suggested in recent studies (Pantazis et al. 2012, Cox et al. 2014, Isobe et al. 2014), in relation to the formation of the S3 state, where the chemical environment is prepared for the O-O bond formation to occur in the following steps. Similarly, there have been suggestions of isomorphism in the S1 (Stotal = 0 and Stotal = 1) and S3 states (Stotal = 3 and an undetermined higher spin state that is not detected by EPR), all of which are detected under cryogenic temperatures (Boussac et al. 2009, Cox et al. 2014, Lubitz et al. 2014, Isobe et al. 2014, Isobe et al. 2016, Shoji et al. 2018). The presence and population of the isomers in each S-state under physiological conditions remains to be established. The difficulty to prove the role of isomers, also stems from the fact that each set of experimental data contains its own uncertainty and they are often collected under different experimental conditions. Thus, understanding if the structural and electronic heterogeneity is functionally important or caused due to variations in experimental conditions becomes important.
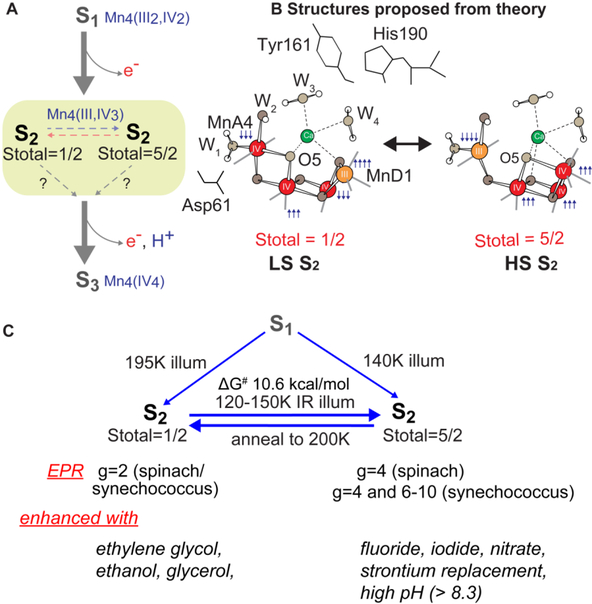
In this review, we focus on the S2 state by examining the room temperature S2 state crystal structure and comparing it with the previous results from EPR and synchrotron X-ray spectroscopy at cryogenic temperature. In the S2 state, EPR studies by several groups (Krewald et al. 2016, reviewed in (Haddy 2007, Pokhrel and Brudvig 2014)) suggest the presence of isomorphous structures within the same redox/intermediate S-state, i.e. S2 with a high-spin (HS, Stotal=5/2) and a low spin (LS, Stotal=1/2) form (Fig. 1A, B). As discussed above, it has been proposed that such geometric and electronic structural flexibility in S2 may play a role in the formation of the S3 state through water binding (Ugur et al. 2016, Pantazis et al. 2012, Cox et al. 2013, Cox and Messinger 2013, Isobe et al. 2014, Boussac et al. 2018). Our XAS studies using synchrotron radiation (SR) at cryogenic temperature also shows differences in the geometric and electronic structure of the cryo-trapped HS and LS S2 states (Chatterjee et al. 2016). Such structural and magnetic redox-isomers, if present at RT, will likely play a role during the catalysis for determining the directionality and the kinetics of the reaction. The room temperature crystallography study provides a tool to visualize the role of such structural heterogeneity. We show that, under our experimental conditions of crystallography at neutral pH (~6.5), the dominant form in the S2 state is the right-open structure with a low spin configuration that geometrically resembles the dark stable S1 state structure. However, differences are observed in the atomic distances and positions of surrounding waters between the S1 and the S2 states.
Fig. 1.
S2-g2 and S2-g4 transition.(A) Transition scheme from S1 to the S2-g2 and S2-g4 states in PSII from plants and the oxidation states of the Mn and the total spin of S-states. (B) Proposed HS and LS S2 state structures by Pantazis et al. 2012 and Isobe et al. 2014 C, Formation and the conversion between the S2-g2 and S2-g4 state and the difference in their physical properties. A detailed discussion can be found in several reviews and papers (Haddy 2007, Pokhrel and Brudvig 2014, Boussac et al. 2015, Boussac et al. 2018).
Background
Among the S-states, the S2 state is the most studied state due to the presence of rich EPR signals and nearly 100% conversion by illumination starting from the dark stable S1 state. The subsequent S2 to S3 state transition is accompanied by noticeable Mn-Mn distance changes (Glöckner et al. 2013, Guiles et al. 1990, Liang et al. 1994, Pushkar et al. 2008), and several factors such as Ca-depletion, site-specific mutations, and chemical treatments (e.g. with fluoride) are known to block this advance (Debus 1992, Haddy 2007, Pokhrel and Brudvig 2014, Boussac et al. 2015). The requirement for a structural change, and its susceptibility to many chemical and biochemical treatments, makes the S2 to S3 transition one of the critical steps for the water oxidation reaction during the S-state cycle.
In the S2 state, two types of EPR signals have been assigned to the Mn cluster (Fig. 1, 2A). The multiline signal (MLS) centered at g = 2, exhibiting at least 18 partially resolved hyperfine lines at X-band (~9 GHz), is assigned to a low spin (Stotal = 1/2, i.e. MnIII/MnIV and MnIV/MnIV are anti-ferromagnetically-coupled, respectively) ground state, LS S2 state (Dismukes and Siderer 1981, Hansson and Andréasson 1982, Brudvig et al. 1983, de Paula and Brudvig 1985, Randall et al. 1995, Peloquin et al. 2000, Charlot et al. 2005, Haddy 2007, Kulik et al. 2007, Cox et al. 2011). Another broad featureless EPR signal at g ≥ 4.1, attributed to a higher spin multiplicity (Stotal = 5/2, i.e. ferromagnetically-coupled three MnIV with anti-ferromagnetically-coupled one MnIII) ground state, HS S2 state, is also observed under different experimental conditions (Casey and Sauer 1984, Zimmermann and Rutherford 1984, Boussac et al. 1996, Boussac et al. 1998b, Boussac et al. 1998a, Peloquin and Britt 2001, Haddy et al. 2004).
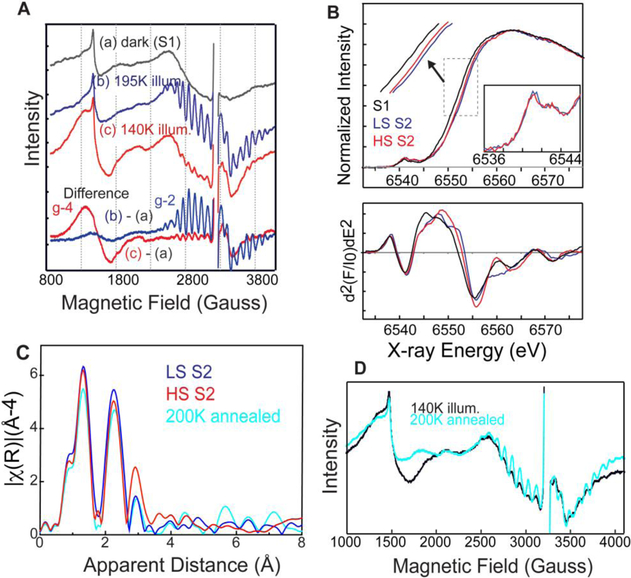
Fig. 2.
EPR and XAS spectra of spinach PSII. (A) EPR spectra of spinach PSII samples illuminated at (b) 195 K (blue) and (c) 140K with NIR (red) along with corresponding (a) dark (black) EPR spectra. The difference spectra are between the spectra after illumination and the spectra of the same dark-adapted sample. (B) Mn XANES spectra (top) and their second derivative spectra (bottom) of HS (red) and LS (blue) S2 states, in comparison with S1 (black) states. (C) Mn EXAFS spectra of HS (red) and LS (blue) S2 states. The spectrum of the 140K illuminated sample (HS-rich) after 200K annealing is also shown (cyan) (D) EPR spectrum of the sample annealed at 200K (cyan) after 140K with illumination compared to the 140K with NIR (black) spectrum. The figure is adapted from Chatterjee et al. 2016.
The high (Stotal = 5/2) and low spin (Stotal = ½) forms in the S2 state are interrelated, on the basis of the observation of the amplitude conversion of the S2-g4 EPR signal to the S2-g2 EPR signal (Casey and Sauer 1984, Beck and Brudvig 1986, Zimmermann and Rutherford 1986, Hansson et al. 1987). The distribution of high spin and low spin species and the g-values and hyperfine coupling values of these spin states are sensitive to several parameters, such as (a) species (higher-plant, thermophile or nonthermophile cyanobacterial PSII) in different pHs, (b) the presence of chemical additives like alcohol (methanol or ethanol) or sucrose and glycerol (often used as cryo-protectant) in the sample, (c) substitution of the native Ca2+ in the OEC (Ca2+-PSII) by Sr (Sr2+-PSII), and (d) halide substitution in PSII with Br− or I− replacing the Cl− of the native state (Fig. 1C). A detailed discussion can be found in several reviews (Haddy 2007, Pokhrel and Brudvig 2014, Boussac et al. 2015, Boussac et al. 2018). Briefly, in spinach PSII samples illuminated at 200 K, both S2-g2 and S2-g4 signals are observed in the presence of sucrose, while with glycerol, ethylene glycerol, or ethanol, the MLS is enhanced and the S2-g4 EPR signal is suppressed (Zimmermann and Rutherford 1986). Some treatments such as (c) and (d) instead stabilize the Stotal = 5/2 state. Illumination by near-infrared (NIR) light at low temperature (~150 K) has been shown to convert the Stotal = 1/2 form to the Stotal = 5/2 form without further advancement of the S-state of the OEC (Zimmermann and Rutherford 1986, Boussac et al. 1998b). Subsequent annealing in the dark converts the Stotal = 5/2 form back to the Stotal = 1/2 form (Casey and Sauer 1984), showing that these two forms are interconvertible. PSII samples treated with F−, NO3-, or I− or when Ca2+ is replaced by Sr2+ have been reported to show an enhanced S2-g4 signal with the line widths and g values being slightly different (Boussac and Rutherford 1988). In cyanobacterial PSII wild type, along with the S2-g4 signal additional EPR signals at g = 6 to 10 are also observed when samples are illuminated with IR light. The pure g = 4 signal is only observed when the native Ca2+ or Cl− is substituted (Boussac et al. 2015). In this species, the intensity of these low EPR field signals are weak, suggesting that the S2-g2 state is the primary feature in the wild type. The reason for such species dependence is not known, as the crystal structure is only available for PSII from the thermophilic cyanobacteria Synechococcus elongatus or Synechococcus vulcanus. However, it is likely due to small differences in the hydrogen-bonding network in the water channels surrounding the OEC that could lead to subtle differences in the electronic structure.
Recently, density functional theory (DFT) calculations suggested theoretical structural models corresponding to the two spin states (Pantazis et al. 2012, Isobe et al. 2014, Bovi et al. 2013, Narzi et al. 2014) and concluded that the two spin states, LS S2 and HS S2, are almost isoenergetic. Ab initio molecular dynamics simulations by Bovi et al. (2013) showed that they can interconvert over a low barrier (ΔG# of 10.6 kcal mol−1). In the proposed models by Pantazis et al. (2012), the two spin states arise from a different location of MnIII; for g = 2, MnIII is located in the corner of the cubane motif (MnD1), while for g>4, it is located at the tail MnA4 (Fig. 1B). In this study, the two spin isomers differ structurally, where LS S2 is “open” (also called “right open structure”) with all 4 Mn connected in di-μ-oxo Mn-Mn interactions while HS S2 is a “closed” structure with three Mn connected via di-μ-oxo interactions and one Mn having a mono-μ-oxo interaction, this structure is also referred to as a closed “cubane” with a “dangling Mn” or as the “left open structure”. It has been suggested that such isomorphism makes O5 unique, and that O5 could become a likely candidate for the slow-exchanging water in the S2 state (Pantazis et al. 2012, Cox et al. 2013, Cox and Messinger 2013). It was further proposed that the structural change from LS S2 to HS S2 is a required (Narzi et al. 2014, Retegan et al. 2014) or favored (Ugur et al. 2016) intermediate step in the S2 to S3 transition.
EPR (Cox et al. 2013, Cox et al. 2014, Lubitz et al. 2014) and theoretical studies (Bovi et al. 2013, Siegbahn 2013, Narzi et al. 2014, Isobe et al. 2015, Shoji et al. 2015, Retegan et al. 2016, Ugur et al. 2016) proposed that there is an insertion of a water molecule in the Mn4CaO5 cluster during the S2 to S3 transition. As the quality of the room temperature crystallography data of the S3 state has been improved compared to previous studies (Young et al. 2016, Suga et al. 2017), the exact position of the inserted water molecule is becoming clear, although its protonation state is still uncertain (Kern et al. 2018). It has been suggested from theory that this water insertion into the Mn4CaO5 cluster is coupled with the interconversion of the two spin isomers, ‘open’ LS S2 and ‘closed’ HS S2 cubane, of the S2 state (Fig. 1B) as part of the S2 to S3 transition. These suggested models, largely based on theoretical calculations, can be summarized as follows: (1) For a terminal water-derived ligand to fill the open coordination site on MnD1, a chemical change, such as deprotonation of a water molecule, occurs during the interconversion of the two spin isomers, ‘open’ LS S2 and ‘closed’ HS S2. It is suggested that the oxidation and formation of the TyrZ radical in the LS S2 state reorients the dipole moment from the cationic imidazolium of His190 to Asp61 making this region of the OEC the locus of the negative charge, regardless of the formal oxidation state of MnA4 (Retegan et al. 2014). This is proposed to trigger proton transfer from one of the waters (W1) on MnA4 to Asp61, shifting the LS S2 ↔ HS S2 equilibrium in favor of the high spin configuration. In this model, only the HS S2 state would be able to progress to the S3 state, and thus LS S2 needs to convert to HS S2 in order to advance (Narzi et al. 2014, Retegan et al. 2014, Yamaguchi et al. 2017). (2) Another suggestion is that the formation of the TyrZ radical affects the pKa of W3 triggering its movement toward either MnA4 or MnD1 depending on whether the cubane is in the closed (HS) or open (LS) configuration (Ugur et al. 2016). The authors propose a spontaneous movement of W3 (OH−) in both spin states for closed cubane structures but only in the high spin state in the open cubane form (Ugur et al. 2016). The interconversion between LS S2 and HS S2 state is proposed to be kinetically feasible due to the calculated small energy gap of 0.3–1.2 kcal mol−1 between the spin/conformational states. This suggests that the OEC might switch between the two conformations (open → closed) and/or spin states (low spin → high spin) in order to proceed to the S3 state. However, there is no experimental evidence confirming these predicted pathways so far. Therefore, taking snapshots during each S-state transition under physiological temperatures becomes a valuable approach, as described in the later section.
The S2 to S3 transition involves proton and electron transfers (Dau and Haumann 2008, Cox and Messinger 2013, Klauss et al. 2012, Klauss et al. 2015). The proton release is proposed to take place before electron transfer to remove the excess positive charge in the S2 state, thereby reducing the redox potential of the OEC (Klauss et al. 2012, Klauss et al. 2015, Noguchi 2015). It is also important to understand the role of Ca, as Ca2+ depletion blocks the S2 to S3 transition (Debus 1992). This indicates that the hydrogen-bond network of water molecules near Ca may play an important role in the release of a proton and water insertion (Isobe et al. 2015, Shoji et al. 2015, Nakamura et al. 2016, Kim et al. 2018). Therefore, to understand the changes taking place in the S2 to S3 transition that involves electron, proton, and water transfers, it is important to probe the structural changes of the water network occurring during the S2 to S3 transition. This is key towards understanding the mechanism of water oxidation.
Cryo-trapped HS and LS S2 states from Spinach studied by Mn X-ray Absorption Spectroscopy
We have investigated the isomorphism in the S2 state using Mn K-edge X-ray absorption spectroscopy combined with supporting EPR characterization (Chatterjee et al. 2016). Fig. 2 shows the EPR and XAS spectra of the HS (g = 4) and LS (g = 2) S2 state species from spinach PSII preparations. The two spin isomers of the S2 state in the spinach PSII samples were prepared by illumination at 140 or 195 K. The 195 K illumination for the LS (g=2) S2 state was performed in a dry ice/ethanol bath and samples were illuminated for 10 min using a 400 W tungsten lamp. For the HS (g=4) S2 state, the 140 K illumination, samples were continuously illuminated for 10 min using a 400 W tungsten lamp with filters to allow only near-infrared (NIR) light to reach the sample while the temperature was maintained with a continuous stream of liquid nitrogen-cooled nitrogen gas. We confirmed the presence of the HS (g=4) and LS (g=2) S2 state by measuring the EPR spectra (Fig. 2A). From the XANES and XES spectra of the LS S2 and HS S2 states, we observe that HS S2 appears slightly reduced compared to the LS form, suggesting that the effective charge density of the HS form may be lower than that of LS (Fig. 2B). As formal oxidation state and number of unpaired spins should be the same between the HS and LS S2 state (although the total spin number differs, 1/2 or 5/2, due to exchange coupling of the four Mn), we speculated that different protonation states of the oxygen ligands or changes in geometry of the cluster in these two states shifts the effective charge density on Mn. The extended X-ray absorption fine structure (EXAFS) of the LS S2 and HS S2 states suggests that their structures are different from each other. The EXAFS data for the LS S2 state fit well with the proposed right open structure with three short Mn-Mn interactions around 2.7 – 2.8 Å and one long Mn-Mn interaction around 3.3 Å. Meanwhile, the HS S2 state fit well with two short Mn-Mn interactions around 2.7 – 2.8 Å and two long Mn-Mn interactions around 3.1 – 3.3 Å. These Mn-Mn interactions of the HS S2 state do not match well with the closed cubane structure (left open) proposed by theoretical studies, where the numbers of short and long Mn-Mn interactions and Mn-Ca interactions remain the same in the HS S2 and the LS S2 states. We further showed that when we anneal the HS (g = 4) S2 state to 200K in the dark, the EPR spectrum of the S2-g4 form converts back to the S2-g2 form (Fig. 2D). The EXAFS of the annealed S2-g4 form also goes back to the S2-g2 form, confirming the observation made by EPR spectroscopy. This implies a certain flexibility of the OEC in its geometric and electronic structure, although one state may be more preferable than the other under a given condition. Whether the HS S2 state serves as an intermediate state between the LS S2 and the S3 state as proposed from theoretical modeling remains a question that we wish to answer from high-resolution crystal structures of these intermediate states at room temperature. Although the S2 to S3 transition is accompanied by structural changes of the cluster, the current data indicates that the closed-cubane-like structure may not be an intermediate that appears during this transition (see next section; Kern et al. 2018).
Room temperature crystal structure of the S2 state from T. elongatus
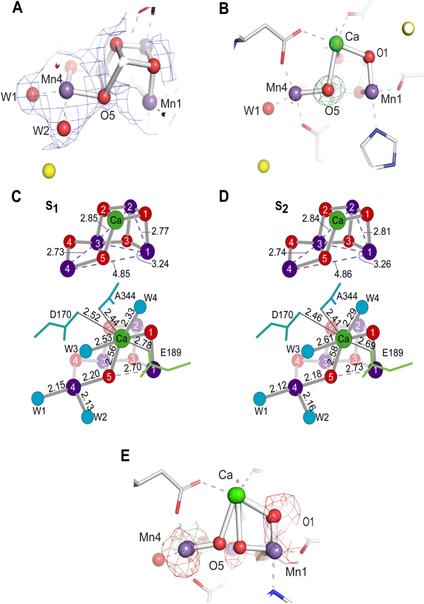
Our improved crystallography datasets (Kern et al. 2018) allow us to examine the chemical structures of the catalytic center in each S-state, and in time-intervals between the S-state transitions. During the crystallography data acquisition, the S-state advancement was also confirmed by Kβ1,3 XES collected simultaneously with the diffraction data, which serves as a diagnostic tool for the oxidation state of the OEC. This combined XES/XRD approach thus allows connecting the oxidation state changes of Mn to the light-induced structural changes of the OEC and the protein. Based on the estimation from XES (in situ) as well as MIMS (ex situ, room temp.) and EPR (ex situ, cryo) we can conclude that the S1 and S2 states are the predominant species (>90 %) in our dark (0F) and 1F data, respectively. Fig. 3A shows the 2mFo-DFc electron density and the model of the S2 (1F) state. Bridging oxygens and terminal water positions can be clearly located in the Mn4CaO5 cluster of the OEC with the current resolution of 2.08 Å. The geometry of three 2.7–2.8 Å and one 3.2–3.3 Å Mn-Mn distances is fundamentally the same as that in the dark S1 state, which is also expected from the similarity of the S1 and S2 state EXAFS studies (Glöckner et al. 2013), with a right-open cubane Mn3CaO4 moiety plus a di-μ-oxo bridged 4th Mn.
Fig. 3.
Comparison of the MnCaO5 cluster of the OEC between the S1 and S2 states. (A) 2mFo-DFc density (blue mesh) of the OEC in the S2 state shown at 0.7σ contour level. In addition, the mFo-DFc difference density between the data and the model was plotted at 3.0σ contour level but no peaks are visible as the differences have low intensity, indicating a good fit of the model to the experimental data (B) O5 omit map density shown of the OEC in the S2 state, contoured at 3.0σ (green mesh). (C, D) Atomic distances in the OEC averaged across both monomers in S1 and S2 states, respectively. Ca remains 8-coordinate upon insertion of Ox by way of movement of D1-Glu189 away from the OEC. E, mFo-DFc difference density between the data and the model shown at +3.0σ (green) and −3.0σ (red) contour. This density was calculated using the 1:1 mixture of the open cubane (right open) and closed cubane (left open) models (Fig. 1B). The figure is adapted from Kern et al. (2018).
Upon S1 to S2 transition, a charge separation occurs at P680 and an electron is transferred to the QB site and one Mn is oxidized from +3 to +4. It has been widely accepted that the formal oxidation state of Mn in the S1 and the S2 state is Mn4 (III2,IV2) and Mn4 (III,IV3), respectively, based on the EPR exchange coupling scheme and supported by theory (Kulik et al. 2007, Krewald et al. 2015). The Mn4CaO5 cluster in the S2 state shows a clear right-open structure, in which there is no bond between Mn1 and O5. The Mn4-O5 distance is ~2.2 Å, while Mn1-O5 is ~2.70 Å in S1 and 2.73 Å in S2 (Fig. 3C, D). The trend in distance changes (albeit within the coordinate errors), suggests an increased asymmetry of the Mn4-O5-Mn1 positions and a shortening of Mn4-W1 when going from the S1 to the S2 state. This is consistent with Mn4 being oxidized at this stage, matching well with the formation of the S2 state with the total spin (Stotal) of 1/2. However, this needs to await further confirmation by higher resolution data. The possibility that a small fraction of the HS S2 state with a closed-cubane structure is also present is discussed in the next section.
Implication of the isomorphism and heterogeneity in the S2 state
As discussed above, there are many indications for the presence of two forms of the S2 state (high-spin Stotal = 5/2 and low spin Stotal = 1/2 states, Fig. 1A) at least at cryogenic temperatures (Boussac et al. 1998a, Pantazis et al. 2012, Bovi et al. 2013, Cox et al. 2013, Isobe et al. 2014, Chatterjee et al. 2016, Boussac et al. 2018). Nevertheless, the right-open S2 state seems to be the one that best explains the diffraction data obtained at room temperature (Fig. 3A, C). To investigate if our data are also compatible with the presence of a different structural model for the S2 state, we focused on the position of the O5 atom, which would undergo the largest structural change between the two proposed S2 state models. In addition, an elongation of the Mn3-Mn4 or the shortening of the Mn1-Mn3 distances are expected according to the theoretical models for the two S2 states. Fig. 3B shows the Fobs-Fcalc omit map in the region of the OEC obtained from the S2 (1F) data and a model in which the O5 was omitted. A clear single positive density feature is visible at the position that was modeled for the O5 atom, indicating that the data allow to locate only one oxygen position and that the modeled position matches well with the experimental data. As a control, we considered the 1:1 mixture of the open cubane (right-open) and closed cubane (left-open) models, in which the coordinates for the closed cubane model were taken from the reference, Pantazis et al. 2012, and Fobs-Fcalc electron density maps (the difference between the experimental data and the model) were calculated (Fig. 3E). Clear difference density features at several positions, including a negative feature at the position of oxygen 5 in the closed cubane model, indicated that the 1:1 mixture model is not a good fit to the data. This implies that the right-open structure is a suitable model for the S2 (1F) state data under our experimental condition. Although a slight increase of B-factors for Mn and bridging oxygens between the 0F and 1F data in both monomers can be seen, this can be explained mostly by the difference in resolution/data quality between the two data sets (2.05 vs 2.08 Å), and no clear evidence is observed under our experimental conditions for increased disorder in the S2 (1F) state data (Kern et al. 2018).
Although we do not see any evidence for the presence of more than one S2 structure in the 1F data under our conditions, we cannot exclude the presence of minor concentrations (< 20%) and further studies will be required to elucidate if such forms exist as transient states between the S2 and S3 states as proposed by some theoretical studies, in relation to the water-insertion pathway (Pantazis et al. 2012, Bovi et al. 2013, Siegbahn 2013, Isobe et al. 2014, Narzi et al. 2014, Isobe et al. 2015, Shoji et al. 2015, Retegan et al. 2016, Ugur et al. 2016). We have taken the first step into this direction, by collecting X-ray diffraction data for two time points during the S2-S3 transition (150 μs and 400 μs after the 2nd flash; Kern et al. 2018). The electron densities obtained and the isomorphous difference maps indicated some motion of Mn1 and Mn4 at the earlier stage (150 μs after the 2nd flash) with O5 still staying close to Mn4 (~2.2 Å). A clear insertion of an additional oxygen next to Mn1 was observed in the later time point (400 μs after the 2nd flash). At this point, there is no evidence supporting the proposed role of a left-open HS S2 state during this transition.
Changes in the water network
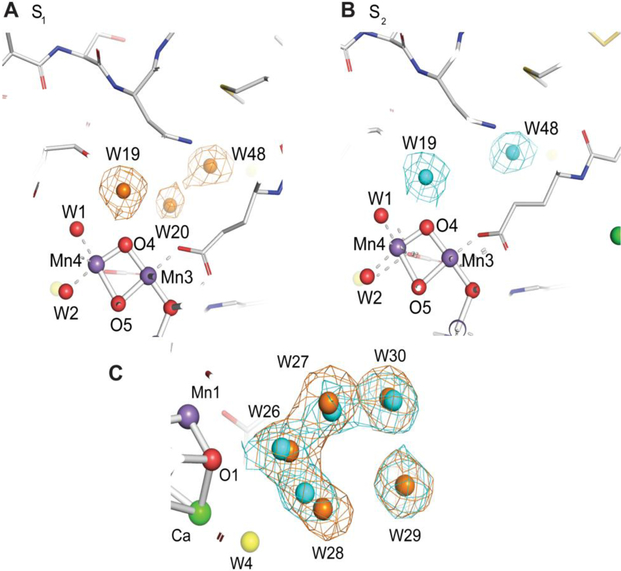
Upon the S1 to S2 transition, it is known that one electron is extracted from the OEC, but without the release of the proton into the bulk, thus building up a positive charge in the OEC (Dau and Haumann 2008, Cox and Messinger 2013, Klauss et al. 2015). The response to such changes may appear in the hydrogen-bonding network around the OEC, because a proton may be parked near the OEC without being released to the bulk, or the proton still stays at the Mn4CaO5 cluster in the S2 state, but the extra charge at the cluster is compensated by a rearrangement of the surroundings. We have observed changes in two sites around the OEC, one is next to oxygen O1 at a cluster of 5 waters (W26 - W30) and the other next to oxygen O4 at the W20 region (Fig. 4; Kern et al. 2018). Waters of the former five membered water ring change positions upon every S-state transition. In contrast, the change of W20 is observed only in the S1 to S2 step. In the dark-adapted S1 state the electron density for W20 is visible with about 70% occupancy (Fig. 4A), but this electron density disappears between 0F (S1) and 1F (S2) (Fig. 4B). This could mean that either water W20 in the S2 state disappears or is not observed due to high mobility. ‘Disappearance’ means that the water molecule (W20) does not show up in the original position but may have moved to a new position. Higher mobility, on the other hand, means that the water molecule is still present in the same location, but due to, for example, flexibility in its orientation, the position is not well-defined. As a consequence, its occupancy becomes very low and the water cannot be clearly observed. While these two phenomena are difficult to differentiate from the crystallographic data, we speculate that W20 is not observed due to the higher mobility. This is based on the observation that no additional water density shows up in the proximal region upon the disappearance of the W20 density. In the recent work by Suga et al. (2017), the disappearance of the same water (W665 in their nomenclature) was reported in the 2F-dark difference map. Our result shows that this change in the W20 density actually occurs during the S1 to S2 transition, and not during the S2 to S3 transition. The removal of a weakly bound W20 is accompanied by small changes of the water network around Mn4 and O4, including W19 (bound to O4) and W1 (bound to Mn4). The W20 density is already weak in the dark state in comparison to other waters, indicating its higher mobility or lower occupancy. It is likely that the overall changes observed above are a consequence of the oxidation of Mn4 from +3 to +4 in the S1 to S2 transition and the connected increase in charge of the cluster (Klauss et al. 2012, Sakamoto et al. 2017). Thus, we speculate that the structural changes observed in the W20 region are a consequence of charge compensation around the OEC. Interestingly, the W20 water density becomes visible again in the 3F (S0) data, implying that its hydrogen-bonding network comes back upon the S3 to S0 transition.
Fig. 4.
Comparison of the water environment of the OEC between the S1 and S2 states. (A, B) Water environment of the OEC at the O4 water chain (A, B) and next to O1(C) in the S1 (0F, brown) and S2 (1F, blue) states. We note that Water 20 is highly unstable in position and there is not sufficient density in the S2 state data to model the water 20 position. (C) the overlaid 2mFo-DFc density maps at 1.5σ contour level of the S1 (0F, brown) and S2 (1F, blue) states show the changes in water positions. The O1-W26 distance changes from 3.09 to 3.01 Å upon transition from S1 to S2. The figures are adapted from Kern et al. (2018).
Summary
In this review, we examined the room temperature crystal structure to investigate the presence/absence of the structural isomers in the S2 state, in comparison to other spectroscopic studies that include X-ray absorption spectroscopy and EPR, and theoretical calculations. In our crystallography experiment under catalytically functional conditions (at room temperature, pH 6.5), the dominant form of the Mn4CaO5 cluster in the S2 state is the right-open structure that is similar to the low spin configuration (Stotal=1/2) form from the EXAFS result. We did not observe the closed-cubane form of the cluster (left-open) of the S2 state at this pH. The X-ray diffraction data collected at the intermediate time points (150 and 400 μs) during the S2-S3 transition, albeit at limited resolution, also did not provide any indication for the presence of a left-open (closed cubane) form of the OEC. Thus, the results suggest that the presence/appearance of the high-spin, closed cubane form may not be necessary for the S2 to S3 transition under catalytic conditions. On the other hand, the low-spin and the high-spin form of the S2 state do appear under other experimental conditions as evidenced by the series of EPR studies, and our EXAFS studies under cryogenic temperature have shown that the OEC structures of these two forms differ in its Mn-Mn distances. However, the structure of this high-spin form may not be exactly the same as the closed cubane form proposed so far, as the current EXAFS result does not match with the metal-metal distances expected in the closed cubane motif. Thus, high-resolution crystal structures for several time points during the S2-S3 transition together with structures under different conditions (e.g. higher pH) will be necessary to understand the actual structure of the high-spin form, and to bridge between the catalytic mechanism and the structural parameters.
Acknowledgements –
This work was supported by the NIH Grants GM110501 (J.Y.), GM126289 (J.K.) and GM055302 (V.K.Y.), the Director, Office of Science, Office of Basic Energy Sciences (OBES), Division of Chemical Sciences, Geosciences, and Biosciences of the Department of Energy (DOE) (J.Y., V.K.Y.), the Artificial Leaf Project (K&A Wallenberg Foundation 2011.0055) and Vetenskapsrådet (2016–05183) (J.M.), and the Ruth L. Kirschstein National Research Service Award (GM116423–02, F.D.F.). Use of the LCLS, SLAC National Accelerator Laboratory, is supported by the U.S. DOE, Office of Science, OBES under Contract No. DE-AC02–76SF00515. We thank the Deutsche Forschungsgemeinschaft (DFG) for financial support to the collaborative research center on Protonation Dynamics in Protein Function (SFB 1078, project A5 Zouni/Dobbek (M.I., A.Z.)).
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/ppl.12947
References
- Beck WF, Brudvig GW (1986) Binding of amines to the O2-evolving center of photosystem-II. Biochemistry 25: 6479–6486 [DOI] [PubMed] [Google Scholar]
- Boussac A, Rutherford AW (1988) Nature of the inhibition of the oxygen-evolving enzyme of photosystem II Induced by NaCl washing and reversed by the addition of Ca2+ or Sr2+. Biochemistry 27: 3476–3483 [Google Scholar]
- Boussac A, Girerd J-J, Rutherford AW (1996) Conversion of the spin state of the manganese complex in photosystem II induced by near-infrared light. Biochemistry 35: 6984–6989 [DOI] [PubMed] [Google Scholar]
- Boussac A, Rutherford AW, Sugiura M (2015) Electron transfer pathways from the S-2-states to the S-3-states either after a Ca2+/Sr2+ or a Cl-/I- exchange in photosystem II from. Biochim Et Biophys Acta-Bioenergetics 1847: 576–586 [DOI] [PubMed] [Google Scholar]
- Boussac A, Un S, Horner O, Rutherford AW (1998a) High-spin states (S >= 5/2) of the photosystem II manganese complex. Biochemistry 37: 4001–4007 [DOI] [PubMed] [Google Scholar]
- Boussac A, Sugiura M, Rutherford AW, Dorlet P (2009) Complete EPR spectrum of the S-3-state of the oxygen-evolving photosystem II. J Am Chem Soc 131: 5050–5051. [DOI] [PubMed] [Google Scholar]
- Boussac A, Kuhl H, Un S, Rögner M, Rutherford AW (1998b) Effect of near-infrared light on the S2-state of the manganese complex of photosystem II from Synechococcus elongatus. Biochemistry 37: 8995–9000 [DOI] [PubMed] [Google Scholar]
- Boussac A, Ugur I, Marion A, Sugiura M, Kaila VRI, Rutherford AW (2018) The low spin - high spin equilibrium in the S-2-state of the water oxidizing enzyme. Biochim Biophys Acta-Bioenerg 1859: 342–356 [DOI] [PubMed] [Google Scholar]
- Bovi D, Narzi D, Guidoni L (2013) The S2 state of the oxygen-evolving complex of photosystem II explored by QM/MM dynamics: spin surfaces and metastable states suggest a reaction path towards the S3 state. Angew Chem Int Edit 52: 11744–11749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudvig GW, Casey JL, Sauer K (1983) The effect of temperature on the formation and decay of the multiline EPR signal species associated with photosynthetic oxygen evolution. Biochim Biophys Acta 723: 366–371 [Google Scholar]
- Casey JL, Sauer K (1984) EPR detection of a cryogenically photogenerated intermediate in photosynthetic oxygen evolution. Biochim Biophys Acta 767: 21–28 [Google Scholar]
- Charlot M-F, Boussac A, Blondin G (2005) Towards a spin coupling model for the Mn4 cluster in photosystem II. Biochim Biophys Acta-Bioenerg 1708: 120–132 [DOI] [PubMed] [Google Scholar]
- Chatterjee R, Han G, Kern J, Gul S, Fuller FD, Garachtchenko A, Young ID, Weng TC, Nordlund D, Alonso-Mori R, Bergmann U, Sokaras D, Hatakeyama M, Yachandra VK, Yano J (2016) Structural changes correlated with magnetic spin state isomorphism in the S2 state of the Mn4CaO5 cluster in the oxygen-evolving complex of photosystem II. Chem Sci 7: 5236–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N, Messinger J (2013) Reflections on substrate water and dioxygen formation. Biochim Biophys Acta-Bioenerg 1827: 1020–1030 [DOI] [PubMed] [Google Scholar]
- Cox N, Pantazis DA, Neese F, Lubitz W (2013) Biological water oxidation. Acc Chem Res 46: 1588–1596 [DOI] [PubMed] [Google Scholar]
- Cox N, Retegan M, Neese F, Pantazis DA, Boussac A, Lubitz W (2014) Electronic structure of the oxygenevolving complex in photosystem II prior to O-O bond formation. Science 345: 804–808 [DOI] [PubMed] [Google Scholar]
- Cox N, Rapatskiy L, Su JH, Pantazis DA, Sugiura M, Kulik L, Dorlet P, Rutherford AW, Neese F, Boussac A, Lubitz W, Messinger J (2011) Effect of Ca2+/Sr2+ substitution on the electronic structure of the oxygen-evolving complex of photosystem II: a combined multifrequency EPR, 55Mn-ENDOR, and DFT study of the S2 state. J Am Chem Soc 133: 3635–3648 [DOI] [PubMed] [Google Scholar]
- Dau H, Haumann M (2007) Time-resolved X-ray spectroscopy leads to an extension of the classical S-state cycle model of photosynthetic oxygen evolution. Photosynth Res 92: 327–343 [DOI] [PubMed] [Google Scholar]
- Dau H, Haumann M (2008) The manganese complex of photosystem II in its reaction cycle - Basic framework and possible realization at the atomic level. Coord Chem Rev 252: 273–295 [Google Scholar]
- de Paula JC, Brudvig GW (1985) Magnetic properties of manganese in the photosynthetic oxygen-evolving complex. J Am Chem Soc 107: 2643–2648 [Google Scholar]
- Debus RJ (1992) The manganese and calcium-ions of photosynthetic oxygen evolution. Biochim Biophys Acta 1102: 269–352 [DOI] [PubMed] [Google Scholar]
- Debus RJ (2008) Protein ligation of the photosynthetic oxygen-evolving center Coord Chem Rev 252: 244–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes GC, Siderer Y (1981) Intermediates of a polynuclear manganese cluster involved in photosynthetic oxidation of water. Proc Natl Acad Sci USA 78: 274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson T, Chatterjee R, Fuller FD, Gul S, Weninger C, Sokaras D, Kroll T, Alonso-Mori R, Bergmann U, Kern J, Yachandra VK, Yano J (2018) X-ray emission spectroscopy as an in situ diagnostic tool for X-ray crystallography of metalloproteins using an X-ray free-electron laser. Biochemistry 57: 4629–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller FD, Gul S, Chatterjee R, Burgie ES, Young ID, Lebrette H, Srinivas V, Brewster AS, Michels-Clark T, Clinger JA, Andi B, Ibrahim M, Pastor E, de Lichtenberg C, Hussein R, Pollock CJ, Zhang M, Stan CA, Kroll T, Fransson T, Weninger C, Kubin M, Aller P, Lassalle L, Brauer P, Miller MD, Amin M, Koroidov S, Roessler CG, Allaire M, Sierra RG, Docker PT, Glownia JM, Nelson S, Koglin JE, Zhu DL, Chollet M, Song S, Lemke H, Liang MN, Sokaras D, Alonso-Mori R, Zouni A, Messinger J, Bergmann U, Boal AK, Bollinger JM, Krebs C, Hogbom M, Phillips GN, Vierstra RD, Sauter NK, Orville AM, Kern J, Yachandra VK, Yano J (2017) Drop-on-demand sample delivery for studying biocatalysts in action at X-ray free-electron lasers. Nat Methods 14: 443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glöckner C, Kern J, Broser M, Zouni A, Yachandra V, Yano J (2013) Structural changes of the oxygen-evolving complex in photosystem II during the catalytic cycle. J Biol Chem 288: 22607–22620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzel P, Bergmann U, Yano J, Visser H, Robblee JH, Gu W, de Groot FMF, Christou G, Pecoraro VL, Cramer SP, Yachandra VK (2004) The electronic structure of Mn in oxides, coordination complexes, and the oxygen-evolving complex of photosystem II studied by resonant inelastic X-ray scattering. J Am Chem Soc 126: 9946–9959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzel P, Schroeder H, Pushkar Y, Boron T III, Mukherjee S, Christou G, Pecoraro VL, Messinger J, Yachandra VK, Bergmann U, Yano J (2013) Electronic structural changes of Mn in the oxygen-evolving complex of photosystem II during the catalytic cycle. Inorg Chem 52: 5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiles RD, Zimmermann JL, McDermott AE, Yachandra VK, Cole JL, Dexheimer SL, Britt RD, Wieghardt K, Bossek U, Sauer K, Klein MP (1990) The S3 state of photosystem II: Differences between the sructure of the manganese complex in the S2 and S3 states determined by X-ray absorption spectroscopy. Biochemistry 29: 471–485 [DOI] [PubMed] [Google Scholar]
- Haddy A (2007) EPR spectroscopy of the manganese cluster of photosystem II. Photosynth Res 92: 357–368 [DOI] [PubMed] [Google Scholar]
- Haddy A, Lakshmi KV, Brudvig GW, Frank HA (2004) Q-band EPR of the S2 state of photosystem II confirms an S = 5/2 origin of the X-band g = 4.1 signal. Biophys J 87: 2885–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson Ö, Aasa R, Vänngard T (1987) The origin of the multiline and g = 4.1 electron-paramagnetic resonance signals from the oxygen-evolving system of photosystem II. Biophys J 51: 825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson Ö, Andréasson L-E (1982) EPR-detectable magnetically interacting manganese ions in the photosynthetic oxygen-evolving system after continuous illumination. Biochim Biophys Acta 679: 261–268 [Google Scholar]
- Haumann M, Müller C, Liebisch P, Iuzzolino L, Dittmer J, Grabolle M, Neisius T, Meyer-Klaucke W, Dau H (2005) Structural and oxidation state changes of the photosystem II manganese complex in four transitions of the water oxidation cycle (S0 -> S1, S1 -> S2, S2 -> S3, and S3,S4 -> S0) characterized by X-ray absorption spectroscopy at 20 K and room temperature. Biochemistry 44: 1894–1908 [DOI] [PubMed] [Google Scholar]
- Hillier W, Wydrzynski T (2001) Oxygen ligand exchange at metal sites – implications for the O2 evolving mechanism of photosystem II. Biochim Et Biophys Acta-Bioenergetics 1503:197–209 [DOI] [PubMed] [Google Scholar]
- Hillier W, Wydrzynski T (2008) 18O-Water exchange in photosystem II: Substrate binding and intermediates of the water splitting cycle. Coord Chem Rev 252: 306–317 [Google Scholar]
- Isobe H, Shoji M, Shen JR, Yamaguchi K (2015) Strong coupling between the hydrogen bonding environment and redox chemistry during the S2 to S3 transition in the oxygen-evolving complex of photosystem II. J Phys Chem B 119: 13922–13933 [DOI] [PubMed] [Google Scholar]
- Isobe H, Shoji M, Shen JR, Yamaguchi K (2016) Chemical equilibrium models for the S-3 state of the oxygen-evolving complex of photosystem II. Inorg Chem 55: 502–511 [DOI] [PubMed] [Google Scholar]
- Isobe H, Shoji M, Yamanaka S, Mino H, Umena Y, Kawakami K, Kamiya N, Shen JR, Yamaguchi K (2014) Generalized approximate spin projection calculations of effective exchange integrals of the CaMn4O5 cluster in the S-1 and S-3 states of the oxygen evolving complex of photosystem II. Phys Chem Chem Phys 16: 11911–11923 [DOI] [PubMed] [Google Scholar]
- Kern J, Chatterjee R, Young ID, Fuller FD, Lassalle L, Ibrahim M, Gul S, Fransson T, Brewster AS, Alonso-Mori R, Hussein R, Zhang M, Douthit L, de Lichtenberg C, Mun Hon Cheah, Shevela D, Wersig J, Seufert I, Sokaras D, Pastor E, Weninger C, Kroll T, Sierra RG, Aller P, Butryn A, Orville AM, Liang M, Batyuk A, Koglin JE, Carbajo S, Boutet S, Moriarty NW, Holton JM, Dobbek H, Adams PD, Bergmann U, Sauter NK, Zouni A, Messinger J, Yano J, Yachandra VK (2018) Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 563: 421–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J, Alonso-Mori R, Tran R, Hattne J, Gildea RJ, Echols N, Glöckner C, Hellmich J, Laksmono H, Sierra RG, Lassalle-Kaiser B, Koroidov S, Lampe A, Han GY, Gul S, DiFiore D, Milathianaki D, Fry AR, Miahnahri A, Schafer DW, Messerschmidt M, Seibert MM, Koglin JE, Sokaras D, Weng TC, Sellberg J, Latimer MJ, Grosse-Kunstleve RW, Zwart PH, White WE, Glatzel P, Adams PD, Bogan MJ, Williams GJ, Boutet S, Messinger J, Zouni A, Sauter NK, Yachandra VK, Bergmann U, Yano J (2013) Simultaneous femtosecond X-ray spectroscopy and diffraction of photosystem II at room temperature. Science 340: 491–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J, Tran R, Alonso-Mori R, Koroidov S, Echols N, Hattne J, Ibrahim M, Gul S, Laksmono H, Sierra RG, Gildea RJ, Han G, Hellmich J, Lassalle-Kaiser B, Chatterjee R, Brewster AS, Stan CA, Glöckner C, Lampe A, DiFiore D, Milathianaki D, Fry AR, Seibert MM, Koglin JE, Gallo E, Uhlig J, Sokaras D, Weng TC, Zwart PH, Skinner DE, Bogan MJ, Messerschmidt M, Glatzel P, Williams GJ, Boutet S, Adams PD, Zouni A, Messinger J, Sauter NK, Bergmann U, Yano J, Yachandra VK (2014) Taking snapshots of photosynthetic water oxidation using femtosecond X-ray diffraction and spectroscopy. Nature Comm 5:4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Bao H, Burnap RL, Debus RJ (2018) Impact of D1-V185 on the water molecules that facilitate O-2 formation by the catalytic Mn4CaO5 cluster in photosystem II. Biochemistry 57: 4299–4311 [DOI] [PubMed] [Google Scholar]
- Klauss A, Haumann M, Dau H (2012) Alternating electron and proton transfer steps in photosynthetic water oxidation. Proc Natl Acad Sci USA 109: 16035–16040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauss A, Haumann M, Dau H (2015) Seven steps of alternating electron and proton transfer in photosystem II water oxidation traced by time-resolved photothermal beam deflection at improved sensitivity. J Phys Chem B 119: 2677–2689 [DOI] [PubMed] [Google Scholar]
- Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic oxygen evolution. A linear four step mechanism. Photochem Photobiol 11: 457–475 [DOI] [PubMed] [Google Scholar]
- Krewald V, Retegan M, Neese F, Lubitz W, Pantazis DA, Cox N (2016) Spin state as a marker for the structural evolution of nature’s water splitting catalyst. Inorg Chem 55: 488–501 [DOI] [PubMed] [Google Scholar]
- Krewald V, Retegan M, Cox N, Messinger J, Lubitz W, DeBeer S, Neese F, Pantazis DA (2015) Metal oxidation states in biological water splitting. Chem Sci 6: 1676–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik LV, Epel B, Lubitz W, Messinger J (2007) Electronic structure of the Mn4OxCa cluster in the S-0 and S-2 states of the oxygen-evolving complex of photosystem II based on pulse Mn-55-ENDOR and EPR Spectroscopy. J Am Chem Soc 129: 13421–13435 [DOI] [PubMed] [Google Scholar]
- Liang WC, Latimer MJ, Dau H, Roelofs TA, Yachandra VK, Sauer K, Klein MP (1994) Correlation between structure and magnetic spin state of the manganese cluster in the oxygen-evolving complex of photosystem II in the S2 state: determination by X-ray absorption spectroscopy. Biochemistry 33: 4923–4932 [DOI] [PubMed] [Google Scholar]
- Lubitz W, Cox N, Rapatskiy L, Lohmiller T, Navarro MP, Ames W, Pantazis D, Neese F, Boussac N, Messinger J (2014) Light-induced water oxidation in photosynthesis. J Biol Inorg Chem 19: S350–S350 [Google Scholar]
- Messinger J (2004) Evaluation of different mechanistic proposals for water oxidation in photosynthesis on the basis of Mn4OxCa structures for the catalytic site and spectroscopic data. Phys Chem Chem Phys 6: 4764–4771 [Google Scholar]
- Nakamura S, Ota K, Shibuya Y, Noguchi T (2016) Role of a Water Network around the Mn4CaO5 Cluster in Photosynthetic Water Oxidation: A fourier transform infrared spectroscopy and quantum mechanics/molecular mechanics calculation study. Biochemistry 55: 597–607 [DOI] [PubMed] [Google Scholar]
- Narzi D, Bovi D, Guidoni L (2014) Pathway for Mn-cluster oxidation by tyrosine-Z in the S-2 state of photosystem II. Proc Natl Acad Sci USA 111: 8723–8728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T (2008) FTIR detection of water reactions in the oxygen-evolving center of photosystem II, Philos Trans R Soc Lond Ser B Biol Sci 363: 1189–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T (2015) Fourier transform infrared difference and time-resolved infrared detection of the electron and proton transfer dynamics in photosynthetic water oxidation. Biochim Biophys Acta-Bioenerg 1847: 35–45 [DOI] [PubMed] [Google Scholar]
- Pantazis DA, Ames W, Cox N, Lubitz W, Neese F (2012) Two interconvertible structures that explain the spectroscopic properties of the oxygen-evolving complex of photosystem II in the S2 State. Angew Chem Int Edit 51: 9935–9940 [DOI] [PubMed] [Google Scholar]
- Peloquin JM, Britt RD (2001) EPR/ENDOR characterization of the physical and electronic structure of the OEC Mn cluster. Biochim Biophys Acta 1503: 96–111 [DOI] [PubMed] [Google Scholar]
- Peloquin JM, Campbell KA, Randall DW, Evanchik MA, Pecoraro VL, Armstrong WH, Britt RD (2000) 55Mn ENDOR of the S2-state multiline EPR signal of photosystem II: Implications on the structure of the tetranuclear cluster. J Am Chem Soc 122: 10926–10942 [Google Scholar]
- Pokhrel R, Brudvig GW (2014) Oxygen-evolving complex of photosystem II: correlating structure with spectroscopy. Phys Chem Chem Phys 16: 11812–11821 [DOI] [PubMed] [Google Scholar]
- Pushkar Y, Yano J, Sauer K, Boussac A, Yachandra VK (2008) Structural changes in the Mn4Ca cluster and the mechanism of photosynthetic water splitting. Proc Natl Acad Sci USA 105:1879–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall DW, Sturgeon BE, Ball JA, Lorigan GA, Chan MK, Klein MP, Armstrong WH, Britt RD (1995) 55Mn ESE-ENDOR of a mixed valence Mn(III)Mn(IV) complex: Comparison with the Mn cluster of the photosynthetic oxygen-evolving complex. J Am Chem Soc 117: 11780–11789 [Google Scholar]
- Renger G (2008) Functional Pattern of Photosystem II. In: Primary processes of photosynthesis. RSC Publishing, 237–290 [Google Scholar]
- Renger G (2012) Mechanism of light induced water splitting in Photosystem II of oxygen evolving photosynthetic organisms. Biochim Biophys Acta-Bioenerg 1817: 1164–1176 [DOI] [PubMed] [Google Scholar]
- Retegan M, Cox N, Lubitz W, Neese F, Pantazis DA (2014) The first tyrosyl radical intermediate formed in the S-2-S-3 transition of photosystem II. Phys Chem Chem Phys 16: 11901–11910 [DOI] [PubMed] [Google Scholar]
- Retegan M, Krewald V, Mamedov F, Neese F, Lubitz W, Cox N, Pantazis DA (2016) A five-coordinate Mn(IV) intermediate in biological water oxidation: spectroscopic signature and a pivot mechanism for water binding. Chem Sci 7: 72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Shimizu T, Nagao R, Noguchi T (2017) Monitoring the Reaction Process During the S-2 -> S-3 Transition in photosynthetic water oxidation using time-resolved infrared spectroscopy. J Am Chem Soc 139: 2022–2029 [DOI] [PubMed] [Google Scholar]
- Shoji M, Isobe H, Yamaguchi K (2015) QM/MM study of the S-2 to S-3 transition reaction in the oxygen-evolving complex of photosystem II. Chem Phys Lett 636: 172–179 [Google Scholar]
- Shoji M, Isobe H, Tanaka A, Fukushima Y, Kawakami K, Umena Y, Kamiya N, Nakajima T, Yamaguchi K (2018) Understanding two different structures in the dark stable state of the oxygen-evolving complex of photosystem II: Applicability of the jahn–teller deformation formula. Chem Photo Chem 2: 257–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegbahn PEM (2009) Structures and energetics for O2 formation in photosystem II. Acc Chem Res 42: 1871–1880 [DOI] [PubMed] [Google Scholar]
- Siegbahn PEM (2013) Water oxidation mechanism in photosystem II, including oxidations, proton release pathways, O-O bond formation and O-2 release. Biochim Biophys Acta-Bioenerg 1827: 1003–1019 [DOI] [PubMed] [Google Scholar]
- Suga M, Akita F, Sugahara M, Kubo M, Nakajima Y, Nakane T, Yamashita K, Umena Y, Nakabayashi M, Yamane T, Nakano T, Suzuki M, Masuda T, Inoue S, Kimura T, Nomura T, Yonekura S, Yu LJ, Sakamoto T, Motomura T, Chen JH, Kato Y, Noguchi T, Tono K, Joti Y, Kameshima T, Hatsui T, Nango E, Tanaka R, Naitow H, Matsuura Y, Yamashita A, Yamamoto M, Nureki O, Yabashi M, Ishikawa T, Iwata S, Shen JR (2017) Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 543: 131–135 [DOI] [PubMed] [Google Scholar]
- Ugur I, Rutherford AW, Kaila VRI (2016) Redox-coupled substrate water reorganization in the active site of photosystem II-The role of calcium in substrate water delivery. Biochim Biophys Acta-Bioenerg 1857: 740–748 [DOI] [PubMed] [Google Scholar]
- Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 angstrom. Nature 473: 55–60 [DOI] [PubMed] [Google Scholar]
- Wydrzynski T, Satoh S (eds) (2005) Photosystem II: The light-driven water:plastoquinone oxidoreductase. Springer, Dordrecht [Google Scholar]
- Yamaguchi K, Shoji M, Isobe H, Yamanaka S, Umena Y, Kawakami K, Kamiya N (2017) On the guiding principles for understanding of geometrical structures of the CaMn4O5 cluster in oxygen-evolving complex of photosystem II. Proposal of estimation formula of structural deformations via the Jahn-Teller effects. Mol Phys 115: 636–666 [Google Scholar]
- Yano J, Yachandra VK (2014) Mn4Ca Cluster in Photosynthesis: Where and how water is oxidized to dioxygen. Chem Rev 114: 4175–4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ID, Ibrahim M, Chatterjee R, Gul S, Fuller FD, Koroidov S, Brewster AS, Tran R, Alonso-Mori R, Kroll T, Michels-Clark T, Laksmono H, Sierra RG, Stan CA, Hussein R, Zhang M, Douthit L, Kubin M, de Lichtenberg C, Pham LV, Nilsson H, Cheah MH, Shevela D, Saracini C, Bean MA, Seuffert I, Sokaras D, Weng TC, Pastor E, Weninger C, Fransson T, Lassalle L, Brauer P, Aller P, Docker PT, Andi B, Orville AM, Glownia JM, Nelson S, Sikorski M, Zhu DL, Hunter MS, Lane TJ, Aquila A, Koglin JE, Robinson J, Liang MN, Boutet S, Lyubimov AY, Uervirojnangkoorn M, Moriarty NW, Liebschner D, Afonine PV, Waterman DG, Evans G, Wernet P, Dobbek H, Weis WI, Brunger AT, Zwart PH, Adams PD, Zouni A, Messinger J, Bergmann U, Sauter NK, Kern J, Yachandra VK, Yano J (2016) Structure of photosystem II and substrate binding at room temperature. Nature 540: 453–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann JL, Rutherford AW (1984) Electron-Paramagnetic-Res Studies of the oxygen-evolving enzyme of photosystem-II. Biochimica Et Biophysica Acta 767: 160–167 [Google Scholar]
- Zimmermann JL, Rutherford AW (1986) Electron-Paramagnetic resonance properties of the S-2 state of the oxygen-evolving complex of photosystem-II. Biochemistry 25: 4609–4615 [Google Scholar]