Abstract
Pulmonary fibrosis refers to a heterogeneous group of disorders that scar the lung, most often irreversibly. To date, there are limited effective treatments for these conditions, despite decades of research in this area of investigation. In pulmonary fibrosis, the principle cell responsible for producing the vast majority of scar tissue is the fibroblast, making these cells ideally suited for drug targeting. For decades, the major experimental approach to blocking the activity of lung fibroblasts has been either to inhibit the interaction of fibroblast growth factors with their receptors or interfere with downstream effector molecules regulating extracellular matrix production. However, emerging evidence now indicates that lung fibroblasts also undergo dramatic metabolic reprogramming in the setting of growth factor stimulation. These discoveries, along with preclinical investigations showing marked reductions in lung fibrosis after targeting specific metabolic pathways, has led to a total rethinking of drug development in the pulmonary fibrosis field. Here, we review the major metabolic pathways and highlight some of the key metabolic events that occur in the transition of fibroblasts from quiescent to activated states. Moreover, we discuss the emerging evidence linking changes in fibroblast metabolism to pulmonary fibrosis and propose how targeting specific metabolic pathways could be employed in the treatment of fibrotic lung diseases.
Keywords: Fibroblast, Myofibroblast, Lung, Metabolism, Glycolysis, Pulmonary fibrosis
INTRODUCTION
Pulmonary fibrosis refers to a diverse group of conditions that impair respiratory gas exchange by scarring and thickening the distal airspaces of lung.1, 2 As scar tissue progresses, the lung becomes more rigid, making it more difficult to inflate and deflate the distal pulmonary air sacs and transport oxygen and other respiratory gases across the lung’s epithelial surfaces.3 When these events unfold, delivery of oxygen to the bloodstream becomes significantly impaired, leading to a host of other complications, including severe functional limitations, and at times, organ failure and death.
Currently, there are over 200 different types of fibrotic respiratory conditions that have been described.3 In some of these cases, the factors contributing to development of pulmonary fibrosis are easily identifiable, such as after a viral infection or drug ingestion, or in individuals with certain predisposing conditions (e.g. scleroderma) or environmental exposures (e.g. asbestos). However, in many other situations the cause of pulmonary fibrosis cannot be uncovered, even after an exhaustive search. In these situations, one of several different idiopathic interstitial lung diseases is diagnosed, based on the specifics of radiographic and histopathological features.2 Despite the fact that pulmonary fibrosis represents a heterogeneous group of disorders, and each condition can have different inciting events, the primary cells responsible for producing the vast majority of scar tissue in all cases are activated lung fibroblasts.
Lung fibroblasts are highly versatile cells that possess a remarkable capacity to respond and adapt to changes in their microenvironment.4 Under healthy conditions, lung fibroblasts reside mostly in a quiescent state, exhibiting little to no proliferative or synthetic (e.g. collagen production) activities. However, lung fibroblasts undergo dramatic transformation in response to pulmonary insults, leading to massive growth and proliferation of the population and to the production of large amounts of extracellular matrix materials.4, 5 Although this transformation serves many useful purposes in injured lung tissues, like helping to restore barrier protection and coordinate tissue repair, sustained activation of lung fibroblasts is also recognized to play a central role in the pathobiology of pulmonary fibrosis, and its many complications, including architectural distortion of the airways and impairments in gas exchange.3, 5
Because activated lung fibroblasts, also called myofibroblasts, are known to contribute to the pathobiology of pulmonary fibrosis, researchers and clinicians have long sought to identify novel ways to block their activities.6–9 Indeed, for decades one of the major approaches to reducing pulmonary fibrosis in experimental models has been to block the interaction of fibroblast growth factors with their receptors (transforming growth factor beta 1/transforming growth factor receptor) or to inhibit key effector molecules downstream of these interactions.6, 8 Although these approaches have proven to be effective in reducing pulmonary fibrosis in rodent models, similar success has not been achieved in humans, emphasizing the pressing need for alternative therapeutic approaches.
In the cancer field, it has long been recognized that alterations in cellular metabolism play an essential role in helping tumor cells acquire and maintain their malignant properties. Although it has taken much too long to apply this knowledge to other fields, it is now appreciated that cellular metabolism is altered in all forms of disease. Here, our focus is on pulmonary fibrosis and the metabolic changes that occur as lung fibroblasts transition from quiescent to activated states.10, 11 In addition to reviewing the major metabolic pathways, we will highlight recent discoveries that have significantly advanced our understanding of the metabolic reprogramming of lung fibroblasts in pulmonary fibrosis. Moreover, we discuss some of the emerging evidence supporting the concept of targeting cellular metabolism in the treatment of pulmonary fibrosis.
CELLULAR METABOLISM: MORE THAN JUST ENERGY PRODUCTION
Cellular metabolism refers to the complex set of chemical reactions that permit cells to perform their essential duties. While cellular metabolism is often discussed as a linear sequence of events, there is actually a complex, multidirectional, interplay among all metabolic pathways.10 That said, for most cells, cellular metabolism begins with the uptake of glucose from the environment. From there, glucose typically undergoes immediate phosphorylation by one of several kinases (e.g. hexokinase) to retain the sugar in the cytoplasm and ready it for subsequent metabolism.10 In most cases, this involves a series of 9 additional enzymatic reactions, which, along with the first phosphorylation step, are collectively called glycolysis (Figure 1). Glycolysis literally means glucose splitting and the end-product of this pathway is the cleavage of one glucose molecule into two individual 3-carbon compounds called pyruvate.10 Additionally, glycolysis also yields two molecules of adenosine triphosphate (ATP) and two molecules of nicotinamide adenine dinucleotide (NADH), which are utilized as metabolic fuel for numerous other biochemical reactions.
Figure 1. An overview of major metabolic pathways in resting and activated fibroblasts.
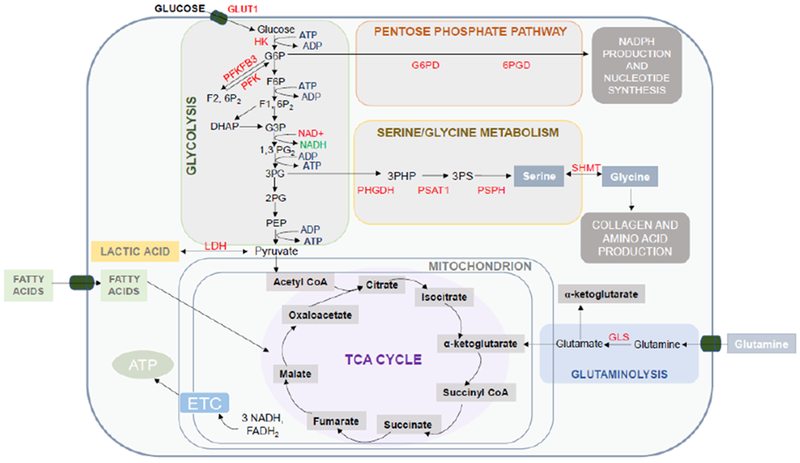
Cellular metabolism begins with the entry of glucose and subsequent metabolism in glycolysis (10-step series of reactions in the cytoplasm). Glycolysis yields two pyruvate molecules; this gets converted to lactate by the enzyme LDH or is shuttled into mitochondria for the tricarboxylic acid cycle (TCA). The TCA cycle yields 3 NADH molecules and 1 FADH2 molecule which serve as electron donors for the electron transport chain and production of ATP. Other major metabolic pathways employed by fibroblasts include the pentose phosphate pathway and de novo serine/glycine metabolism. These pathways rely on metabolic intermediates from glycolysis. Additionally, activated lung utilize glutaminolysis for promoting pro-fibrotic activities. Glutaminolysis refers to the process by which glutamine is converted to alpha-ketoglutarate. Abbreviations: GLUT1, Glucose transporter-1; HK, Hexokinase; G6PD- glucose-6-phosphate dehydrogenase; 6GPD, 6-phosphogluconate dehydrogenase; NADPH- nicotinamide adenine dinucleotide phosphate; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; PFK, phosphofructokinase-1; PHGDH, phosphoglycerate dehydrogenase; PSAT1, phosphoserine aminotransferase; PSPH, phosphoserine phosphatase; SHMT, serine hydroxymethyltransferase 2; LDH, lactate dehydrogenase; GLS, glutaminase; NADH, nicotinamide adenine dinucleotide; FADH2, flavin adenine dinucleotide; ETC, electron transport chain; ATP, adenosine triphosphate.
Once pyruvate is produced, this molecule tends to have one of two fates: it is either converted to lactate by the enzyme lactate dehydrogenase (LDH) or transferred to mitochondria for further breakdown by the tricarboxylic acid (TCA) cycle.10, 12 For many decades, biochemistry textbooks have taught that the conversion of pyruvate to lactate occurs only under low oxygen tension conditions. However, it is now appreciated that there are many clinical scenarios in which cells preferentially choose to metabolize pyruvate to lactic acid under aerobic conditions.13, 14 The best example of this is in cancer cells, in which the phenomenon is often dubbed the Warburg effect after the German physician who first described these events.14 As will be discussed later, activated fibroblasts, like cancer cells, engage in aerobic glycolysis for their own specific purposes.15
In contrast to lactate production, which occurs in the cytoplasm, breakdown of pyruvate by the TCA cycle occurs in the matrix of mitochondrion. Once there, pyruvate is immediately converted to acetyl-CoA by the enzyme pyruvate dehydrogenase, which combines with oxaloacetate to trigger the first step of the TCA cycle. In total, one complete TCA cycle involves eight chemical reactions driven by eight different enzymes (Figure 1). Consistent with all cyclical events, the final end-product of the TCA cycle is oxaloacetate, which combines with a new molecule of acetyl-CoA to further propagate the cycle.10 In addition to generating oxaloacetate, the TCA cycle also yields three molecules of NADH and one molecule of flavin adenine dinucleotide, both of which are utilized by the electron transport chain (ETC) for ATP production. Notably, at times, flow through the TCA cycle is interrupted due to controlled inactivation of TCA enzymes. Interestingly, emerging evidence indicates that enzymatic inactivation of TCA enzymes is important not only to prevent metabolic intermediates from accumulating in the mitochondrial matrix but also to permit the export of metabolites to other parts of the cell. Of note, activated lung fibroblasts appear to engage in this export process, also called cataplerosis, to augment collagen gene transcription.16 At times, metabolic intermediates other than pyruvate can also feed the TCA cycle, particularly amino acids like alanine and glutamine.17 Import of these alternative substrates to the TCA cycle is often referred to as anaplerosis.
METABOLIC ADAPTATIONS OF ACTIVATED FIBROBLASTS (MYOFIBROBLAST)
It is now appreciated that lung fibroblasts undergo dramatic metabolic reprogramming in response to activation, thereby helping to facilitate growth, proliferation and their synthetic activities.4, 5, 18 One hallmark feature of these metabolic reprogramming events is the upregulation in aerobic glycolysis. For example, Xie and others have shown that several rate-limiting enzymes involved in glycolysis are dramatically upregulated in activated lung fibroblasts and that this associates with a marked increase in glycolytic flux15, 18 Although these studies did not determine why glycolysis is upregulated, several theories have recently been proposed. First, increased glycolysis is thought to help activated lung fibroblasts meet their energy demands. While glycolysis is generally considered an inefficient means for generating ATP (two ATP molecules by glycolysis vs 36 ATP by oxidative phosphorylation), aerobic glycolysis can actually produce ATP at faster rate than oxidative phosphorylation, providing fibroblast with speed over quantity in terms of energy production. Additionally, aerobic glycolysis also generates byproducts that could be important for fibroblasts, namely lactate. Lactate production is believed to be essential for several reasons including its effects in- and outside of cells. For example, the production of lactate restores cellular NAD+ levels, through the activity of LDH, which not only permits glycolysis to continue but also serves to maintain redox balance in cells. Additionally, lactate production also has important effects outside of cells, including helping in the activation of TGFβ1 through lowering extracellular pH (after conversion to lactic acid) and serving as an energy source for neighboring cells.13, 19
In addition to these benefits, aerobic glycolysis is also believed to be important for the construction of new cells. This is achieved by diverting glycolytic intermediates to other metabolic pathways involved in the synthesis of nucleotides, lipids or amino acid. For example, glucose-6-phosphate is an essential substrate for the pentose phosphate pathway (PPP), producing ribose-5-phosphate for the synthesis of nucleotides (ATP and GTP)10, 20 and NADPH for the de novo synthesis of lipids. Glycolytic intermediates are also diverted to various other metabolic pathways depending on the specific needs of a cell. For example, Nigdelioglu et al showed that activated lung fibroblasts divert high levels of glycolytic intermediates to the de novo serine synthesis pathway.21 This produces nonessential amino acids like glycine that are highly abundant in collagen and needed for the formation of pulmonary scars.
Along with an increase in glycolysis, glutaminolysis is also increased in activated lung fibroblasts.16, 17 Glutaminolysis is the metabolic process by which the amino acid glutamine is converted to glutamate and α-ketoglutarate by the enzymes glutaminase and glutamine dehydrogenase, respectively. Ge et.al. showed that glutaminase levels were significantly increased in both TGFβ1-stimulated mouse lung fibroblasts and fibroblasts from idiopathic pulmonary fibrosis patients.16 Moreover, intracellular glutamine levels were found to be reduced in these cells, presumably from increased glutaminase activity (See Figure1). Recently, enhanced glutaminolysis has been shown to be important for several reasons, including to drive collagen gene transcription through α-ketoglutarate-mediated mammalian target of rapamycin (mTOR) activation and to feed de novo proline synthesis through the action of pyrroline-5-carboxylate synthestase. Additionally, Xie et al showed that byproducts of glutaminolysis, namely succinate, can also help to stabilize HIF1α expression, thereby augmenting levels of glycolytic enzymes and enhancing metabolic reprogramming.15
PRECLINICAL STUDIES TARGETING CELLULAR METABOLISM IN PULMONARY FIBROSIS
Therapeutic approaches to targeting metabolic pathways are being tested in the treatment of a wide variety of diseases, including cancer, heart disease and neurologic disorders.22–24 Although such approaches have yet to be employed in the treatment of pulmonary fibrosis a growing body of evidence has demonstrated the safety and efficacy of these approaches in experimental models and in in vitro systems (Table 1).
Table 1.
Metabolic targets for inhibiting myofibroblast activation.
| Metabolic Pathway | Target | Anticipated Outcome |
|---|---|---|
| Glycolysis | PFKFB3 | ↓ Collagen production ↓ Proliferation |
| Pentose Phosphate Pathway | G6PD | ↓ NADPH production ↓ Nucleotide synthesis ↓ Proliferation |
| Serine/Glycine Pathway | PHGDH PSAT1 PSPH SHMT |
↓ Collagen production |
| Lactic Acid Fermentation | LDH | ↓ TGF-β activation |
| Glutaminoylsis | GLS | ↓ HIF-1α activation ↓ TGF-β activation ↓ Collage production |
| Energy conservation | AMPK | ↓ Proliferation ↓ Lipid synthesis ↓ Collage production |
| Pro-growth pathway | mTOR | ↓ Collage production |
Abbreviations: PFKFB3- phosphofructo-2-kinase; G6PD- glucose-6-phosphate dehydrogenase; 6-phosphogluconate dehydrogenase; PHGDH, phosphoglycerate dehydrogenase; PSAT1, phosphoserine aminotransferase; PSPH, phosphoserine phosphatase; SHMT, serine hydroxymethyltransferase 2; LDH, lactate dehydrogenase; GLS, glutaminase; AMPK, 5’ AMP- activated protein kinase; mTOR, mammalian target of rapamycin.
One strategy recently employed to treat pulmonary fibrosis in mice was to inhibit glycolysis. For example, Xie et al showed that pharmacological or genetic approaches to inhibiting the glycolytic enzyme 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) not only reduced fibroblast activation in vitro but also dramatically ameliorated the severity of pulmonary fibrosis in mice.15 Importantly, this therapeutic approach demonstrated efficacy in both bleomycin- and the TGFβ1–induced experimental models, suggesting the broad applicability of this treatment strategy. That said, it remains to be determined whether chronic use of glycolytic inhibitors will be safe in animals or, more importantly, in humans.
Another novel metabolic approach that has yet to be tested in vivo but exhibits dramatic effects on fibroblasts in culture is to inhibit key enzymes involved in glutaminolysis. For example, two different research groups have shown that pharmacological (CB-839 or BPTES) and genetic approaches to inhibiting glutaminolysis can significantly suppress collagen gene transcription in lung fibroblasts.16, 17
Because collagen protein contains high concentrations of the amino acids glycine and proline, several groups have tested whether inhibiting amino acid biosynthesis might be effective in reducing fibrotic responses. In support of this approach, Hamanaka et al found that inhibiting the enzyme phosphoglycerate dehydrogenase (PHGDH) with a novel compound called NCT-503 dramatically reduced the severity of bleomycin-induced pulmonary fibrosis in mice.21, 25 To date, it has yet to be determined whether inhibiting other enzymes involved the biosynthesis of amino acids would be equally effective and whether such approaches will be safe over the long-term due to the importance of these amino acids in many other proteins.
Finally, another approach that has shown promise in mice is to inhibit the enzyme 5’ AMP-activated protein kinase (AMPK). AMPK is a serine-threonine kinase whose enzymatic activity increases in cells when ATP stores are depleted, such as in clinical situations associated with hypoxia, ischemia, and inflammation.10, 26 In these situations, the main function of AMPK is to restore energy balance by inhibiting processes that consume ATP and by stimulating processes the lead to the production of new ATP. Relevant to this, Rangarajan et al recently showed that AMPK activation is reduced in lungs’ of mice during pulmonary fibrosis, despite the presence of factors that should activate the enzyme.27 Armed with this information, Rangarajan et al. hypothesized that augmenting AMPK activation might reduce fibrotic remodeling in the bleomycin-injured mouse lung. Consistent with this line of reasoning, treatment of mice with the AMPK activator metformin was found to markedly reduce the severity of pulmonary fibrosis to bleomycin.27 Interestingly, these investigators found that AMPK activation was also reduced in fibroblasts from patients with IPF, suggesting that AMPK activation might also be effective in the treatment of pulmonary fibrosis in humans.
CONCLUSIONS
It is now firmly established that lung fibroblasts are metabolically reprogramed in the mouse and human lung in pulmonary fibrosis. Moreover, it has been shown that these metabolic reprogramming events are critical to driving pro-fibrotic behaviors and contributing to the development and progression of lung fibrosis. Finally, there is emerging preclinical evidence to suggest that targeting specific metabolic pathways could be effective in the treatment of disease. Although similar approaches have yet to be tested in humans, the possibility of employing such approaches has led to enormous excitement and optimism in the field.
Acknowledgments
This work was funded by NIH Grant No: NIH R01 HL131784 (RS) and R01HL136833 (F.R.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter discussed in this manuscript.
REFERENCES
- 1.Chambers RC, Mercer PF. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann Am Thorac Soc. 2015; 12 Suppl 1:S16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001; 345:517–25. [DOI] [PubMed] [Google Scholar]
- 3.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012; 122:2756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008; 5:334–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast: one function, multiple origins. Am J Pathol. 2007; 170:1807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fertala J, Romero F, Summer R, et al. Target-Specific Delivery of an Antibody That Blocks the Formation of Collagen Deposits in Skin and Lung. Monoclon Antib Immunodiagn Immunother. 2017; 36:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014; 370:2071–82. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G Pharmacotherapy for idiopathic pulmonary fibrosis: current landscape and future potential. Eur Respir Rev. 2017; 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King TE Jr., Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014; 370:2083–92. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Summer R. Cellular Metabolism in Lung Health and Disease. Annu Rev Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero F, Shah D, Duong M, et al. A pneumocyte-macrophage paracrine lipid axis drives the lung toward fibrosis. Am J Respir Cell Mol Biol. 2015; 53:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher AB. Intermediary metabolism of the lung. Environ Health Perspect. 1984; 55:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faubert B, Li KY, Cai L, et al. Lactate Metabolism in Human Lung Tumors. Cell. 2017; 171:358–71 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009; 324:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie N, Tan Z, Banerjee S, et al. Glycolytic Reprogramming in Myofibroblast Differentiation and Lung Fibrosis. Am J Respir Crit Care Med. 2015; 192:1462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge J, Cui H, Xie N, et al. Glutaminolysis Promotes Collagen Translation and Stability via alpha-Ketoglutarate-mediated mTOR Activation and Proline Hydroxylation. Am J Respir Cell Mol Biol. 2018; 58:378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard K, Logsdon NJ, Benavides GA, et al. Glutaminolysis is required for transforming growth factor-beta1-induced myofibroblast differentiation and activation. J Biol Chem. 2018; 293:1218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernard K, Logsdon NJ, Ravi S, et al. Metabolic Reprogramming Is Required for Myofibroblast Contractility and Differentiation. J Biol Chem. 2015; 290:25427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kottmann RM, Kulkarni AA, Smolnycki KA, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am J Respir Crit Care Med. 2012; 186:740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassett DJ, Fisher AB. Pentose cycle activity of the isolated perfused rat lung. Am J Physiol. 1976; 231:1527–32. [DOI] [PubMed] [Google Scholar]
- 21.Nigdelioglu R, Hamanaka RB, Meliton AY, et al. Transforming Growth Factor (TGF)-beta Promotes de Novo Serine Synthesis for Collagen Production. J Biol Chem. 2016; 291:27239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ardehali H, Sabbah HN, Burke MA, et al. Targeting myocardial substrate metabolism in heart failure: potential for new therapies. Eur J Heart Fail. 2012; 14:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas B, Beal MF. Mitochondrial therapies for Parkinson’s disease. Mov Disord. 2010; 25 Suppl 1:S155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luengo A, Gui DY, Vander Heiden MG. Targeting Metabolism for Cancer Therapy. Cell Chem Biol. 2017; 24:1161–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamanaka RB, Nigdelioglu R, Meliton AY, et al. Inhibition of Phosphoglycerate Dehydrogenase Attenuates Bleomycin-induced Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2018; 58:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao B, Sanders MJ, Underwood E, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011; 472:230–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangarajan S, Bone NB, Zmijewska AA, et al. Metformin reverses established lung fibrosis inableomycin model. Nat Med. 2018; 24:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


