Abstract
Matrix metalloproteinase-7 (MMP-7) is a secreted endopeptidase that degrades a broad range of substrates. Recent studies have identified MMP-7 as an early biomarker to predict severe acute kidney injury (AKI) and poor outcomes after cardiac surgery; however, the role of MMP-7 in the pathogenesis of AKI is unknown. In this study, we investigated the expression of MMP-7 and the impact of MMP-7 deficiency in several models of AKI. MMP-7 was induced in renal tubules following ischemia/reperfusion injury or cisplatin administration, and in folic acid-induced AKI. MMP-7 knockout mice experienced higher mortality, elevated serum creatinine, and more severe histologic lesions after ischemic or toxic insults. Tubular apoptosis and interstitial inflammation were more prominent in MMP-7 knockout kidneys. These histologic changes were accompanied by increased expression of FasL and other components of the extrinsic apoptotic pathway, as well as increased expression of pro-inflammatory chemokines. In a rescue experiment, exogenous MMP-7 ameliorated kidney injury in MMP-7 knockout mice after ischemia/reperfusion. In vitro, MMP-7 protected tubular epithelial cells against apoptosis by directly degrading FasL. In isolated tubules ex vivo, MMP-7 promoted cell proliferation by degrading E-cadherin and thereby liberating β-catenin, priming renal tubules for regeneration. Taken together, these results suggest that induction of MMP-7 is protective in AKI by degrading FasL and mobilizing β-catenin, thereby priming kidney tubules for survival and regeneration.
Keywords: MMP-7, acute kidney injury, apoptosis, FasL, proliferation, β-catenin
Graphical Abstract

INTRODUCTION
AKI is a clinical condition characterized by an abrupt decrease in kidney function, and it is associated with high morbidity and mortality.1 AKI is highly prevalent, particularly among hospitalized patients and those undergoing major surgery.2–4 Current treatment for AKI is mainly supportive in nature, and there is no therapeutic modality with proven efficacy thus far.5 In this context, an early detection for AKI is paramount for eventually developing an effective intervention, as current diagnostic criteria including serum creatinine and urine output are the late manifestations of the condition.6
Over the past decades, nephrology community has made great efforts to discover and validate biomarkers for monitoring kidney injury in the early phase of AKI. Several biomarkers such as kidney injury molecule-1 (Kim-1), neutrophil gelatinase-associated lipocalin (NGAL), and tissue inhibitor of metalloproteinase-2/IGF-binding protein-7 (TIMP-2/IGFBP7) have been identified, and some of them are successfully applied in the clinic.6–11 Recently, we reported that urinary matrix metalloproteinase-7 (uMMP-7) is an early and noninvasive biomarker that predicts severe AKI in patients after cardiac surgery.12 In two cohorts of 721 patients, uMMP-7 outperforms all other biomarkers tested, suggesting that it is a potentially better predictor for AKI.12
MMP-7 is a secreted zinc- and calcium-dependent endopeptidase.13 Structurally, MMP-7 is one of the smallest MMPs, and it is synthesized as an inactive zymogen.14 Upon activation, the N-terminal proregion is removed through proteolysis, which results in the final active enzyme.15 In general, the primary function of MMP-7 is to breakdown extracellular matrix by digesting casein, gelatins, fibronectin, and proteoglycan. MMP-7 is also capable of cleaving other substrates and plays a role in E-cadherin ectodomain shedding,15 tumor necrosis factor-α (TNF-α) release, and activation of other proteinases.16, 17 Such an ability of MMP-7 acting on a broad spectrum of substrates renders it a potential player in controlling wide-ranging biologic processes such as tissue remodeling, apoptosis and inflammation.13, 18–20 Several MMPs such as MMP-2, MMP-8 and MMP-9 have been implicated in regulating kidney injury and recovery after AKI.21–24 However, the role of MMP-7 in the pathogenesis of AKI is completely unknown.
In normal adult kidneys, MMP-7 expression is barely detectable. However, it is markedly induced in renal tubular epithelium after injury.12, 15 MMP-7 is transcriptionally regulated by β-catenin, the intracellular effector of canonic Wnt signaling.17, 18 Previous studies show that MMP-7 is induced in CKD and functions as a biomarker and pathogenic mediator of kidney fibrosis.15, 25, 26 Genetic ablation of MMP-7 in vivo mitigates fibrotic lesions by preserving epithelial cell integrity.15
In this study, we examined MMP-7 expression in multiple models of AKI. Using MMP-7−/− null mice, we demonstrate that endogenous MMP-7 protects against AKI by cleaving Fas ligand (FasL) and mobilizing β-catenin. Our studies illustrate that the induction of MMP-7 in AKI is renal protective by priming tubular cells for survival and regeneration.
RESULTS
Induction of MMP-7 is a common finding in various models of AKI
To investigate the regulation of MMP-7 in AKI, we used both ischemic and nephrotoxic models induced by ischemia/reperfusion (IR) and cisplatin, which represent distinct etiologies leading to AKI in clinical setting.27, 28 As shown in Figure 1a, quantitative real-time RT-PCR (qRT-PCR) revealed that MMP-7 mRNA was markedly induced in the kidneys at different time points after IR. Comparable MMP-7 induction was found at 1 day after IR and 3 days after cisplatin (Figure 1b). Renal MMP-7 protein was also induced after IR or cisplatin, as shown by Western blot analyses (Figure 1, c and d). We further examined MMP-7 protein in the kidneys by immunohistochemical staining. As illustrated in Figure 1e, MMP-7 protein was barely detectable in control kidney, but induced in the injured kidneys after IR or cisplatin. Similar induction of MMP-7 was observed in AKI induced by folic acid (Supplementary Figure S1).
Figure 1.
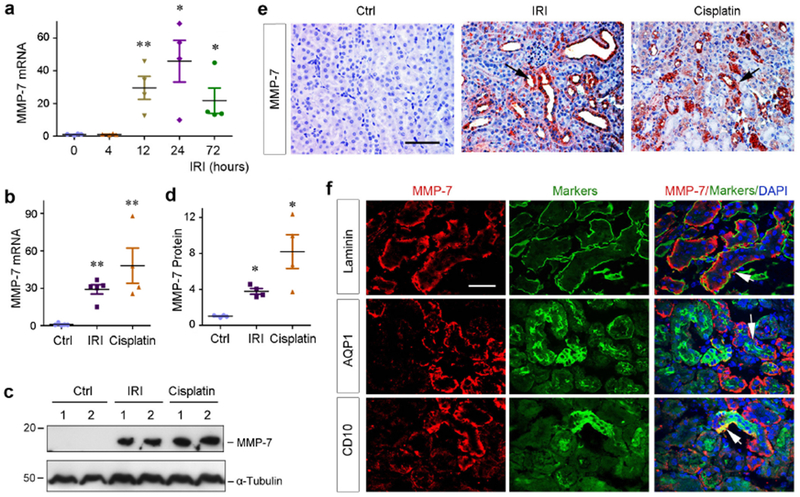
Induction of MMP-7 is a common finding in various AKI. (a) Time-dependent induction of MMP-7 in mouse kidneys after IRI. Total RNA was isolated from control kidney and injured kidneys at different time points after IRI as indicated. Relative abundance of renal MMP-7 mRNA was assessed by qRT-PCR, and fold induction over the controls was reported. **P<0.01, *P<0.05 versus sham controls (n=4). (b) Both ischemic and toxic AKI induces renal MMP-7 mRNA expression. Relative level of renal MMP-7 mRNA was assessed by qRT-PCR at different time points after IRI (1 day) and cisplatin (3 days), respectively. **P< 0.01 vs sham controls (n=4-5). (c, d) Western blot analyses of renal expression of MMP-7 protein in injured kidneys after AKI induced by IRI and cisplatin. Representative western blot (c) and quantitative data (d) were presented. Numbers (1-2) indicate each individual animal in a given group. *P< 0.05 vs sham controls. (e) Representative micrographs show MMP-7 protein expression and localization in control and injured kidneys at 1 day after IRI or 3 days after cisplatin. Arrows indicate positive staining. Scale bar, 60 μm. (f) Co-immunostaining shows tubular localization of MMP-7 after IRI. Cryosections from the kidney at 1 day after IRI were double-stained with antibodies against MMP-7 (red) or different markers (green) including laminin, AQP1 and CD10, respectively. Arrows indicate positive staining. Scale bar, 50 μm.
MMP-7 was predominantly localized in renal tubular epithelium (Figure 1e, arrows), whereas interstitial cells were mostly negative. To further confirm this, we performed double-immunostaining for MMP-7 and laminin. As shown in Figure 1f, MMP-7 was predominantly localized in renal tubular compartment at 1 day after IR. Co-staining for MMP-7 and aquaporin 1 (AQP1) or CD10 revealed that MMP-7 was mainly induced in the proximal tubules, particularly in the S3 segment, of the kidneys in this model (Figure 1f).
Deletion of endogenous MMP-7 aggravates ischemic AKI
We next investigated the role of endogenous MMP-7 in the pathogenesis of AKI. To this end, we utilized MMP-7−/− null mice in which MMP-7 gene is globally knocked out. MMP-7−/− mice were phenotypically normal, without overt physical or morphological abnormalities.15 Age- and sex-matched wild-type (WT) and MMP-7 knockout (KO) mice in the same genetic background were subjected to IR injury (IRI) for 1 day. As shown in Figure 2a, serum creatinine level in MMP-7 KO mice at 1 day after IRI was higher than that in WT controls. MMP-7−/− kidneys exhibited more severe morphological injury, particularly in the outer stripe of out medulla region, characterized by loss of brush border, tubular cell death and cast formation (Figure 2b, asterisks). Quantitative assessment of kidney injury between WT and KO mice was presented in Figure 2c. Meanwhile, ablation of MMP-7 caused marked upregulation of renal NGAL, a biomarker of tubular injury,29 as illustrated in Figure 2 (d through f).
Figure 2.
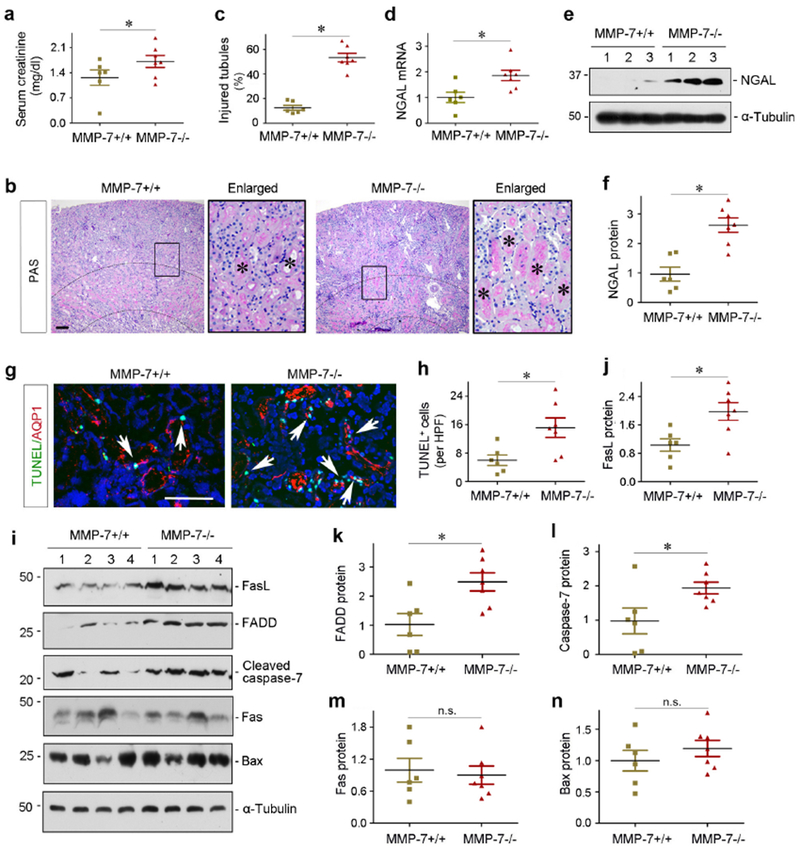
Deletion of endogenous MMP-7 aggravates ischemic AKI in mice. (a) Serum creatinine level in MMP-7+/+ wild-type and MMP-7−/− null mice at 1 day after IRI. *P< 0.05 (n=6-7). (b) Representative micrographs show kidney morphology in MMP-7+/+ and MMP-7−/− mice at 1 day after IRI. Asterisks in the enlarged boxed areas indicate injured tubules. Dashed lines show renal corticomedullary junction area. Scale bar, 50 μm. (c) Semi-quantitative assessment of kidney injury (percentage of injured tubules) at 1 day after IRI. *P< 0.05 (n=6-7). (d) qRT-PCR analyses show an increased NGAL mRNA expression in the injured kidneys of MMP-7−/− mice at 1 day after IRI, compared with MMP-7+/+ controls. *P< 0.05 (n=6-7). (e, f) Western blot analyses of renal NGAL protein in MMP-7+/+ and MMP-7−/− mice at 1 day after IRI. Representative western blot (e) and quantitative data (f) are presented. *P < 0.05 (n=6-7). Numbers (1-3) indicate each individual animal in a given group. (g) Representative micrographs show apoptotic proximal tubular cells detected by co-staining of TUNEL (green) and AQP1 (red) in MMP-7+/+ and MMP-7−/− mice. Arrows indicate apoptotic cells. Scale bar, 100 pm. (h) Quantitative determination of apoptotic cells at 1 day after IRI. Data are presented as numbers of apoptotic cells per high power field (HPF). *P< 0.05 (n=6-7). (i) Representative western blotting show the protein levels of renal FasL, FADD, cleaved caspase-7, Fas and Bax. Numbers (1-4) indicate each individual animal in a given group. (j-n) Quantitative determination of renal protein levels of FasL (j), FADD (k), cleaved caspase-7 (l), Fas (m) and Bax (n) in MMP-7+/+ and MMP-7−/− mice at 1 days after IRI. *P< 0.05 (n=6-7).
Ablation of MMP-7 promotes tubular cell apoptosis after AKI
To explore the mechanism underlying renal protection of MMP-7 in AKI, we examined cell apoptosis in WT and KO kidneys. As shown in Figure 2g, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining revealed more apoptotic cells in KO kidneys at 1 day after IRI, compared to WT controls. Most of these apoptotic cells appeared to be at proximal tubule, as shown by co-immunostaining with AQP1 (Figure 2g). Quantitative data on apoptotic cells were presented in Figure 2h.
We further investigated the mechanism of apoptosis by dissecting both intrinsic and extrinsic pathways. As shown in Figure 2, i and j, FasL, a type-II transmembrane protein that belongs to the TNF family,30 was induced in KO mice at 1 day after IRI, compared with WT controls. The Fas-associated protein with death domain (FADD) was also induced (Figure 2, i and k), although Fas itself exhibited little change (Figure 2, i and m). Activation of the extrinsic pathway consequentially caused an increased cleaved caspase 7 in MMP-7−/− kidneys (Figure 2, i and l). However, renal expression of Bax, a proapoptotic member of Bcl-2 family playing a central role in mediating intrinsic pathway,31 was not significantly different between WT and KO mice ( Figure 2, i and n). Therefore, deletion of MMP-7 promotes tubular cell apoptosis mainly via activation of the FasL-mediated extrinsic pathway.
Ablation of MMP-7 augments renal inflammation after AKI
We also studied the role of MMP-7 in renal inflammation after AKI. As shown in Figure 3, a through d, major proinflammatory cytokines such as monocyte chemoattractant protein-1 (MCP-1), regulated on activation normal T cell expressed and secreted (RANTES) and TNF-α were induced in MMP-7−/− kidneys at 1 day after IRI, compared with WT controls. Immunostaining also revealed a marked induction of MCP-1 and TNF-α in MMP-7−/− kidneys, which predominantly localized in renal tubules (Figure 3e). Of note, MCP-1 was undetectable in the kidneys of WT and KO mice under normal conditions (Supplementary Figure S2).
Figure 3.
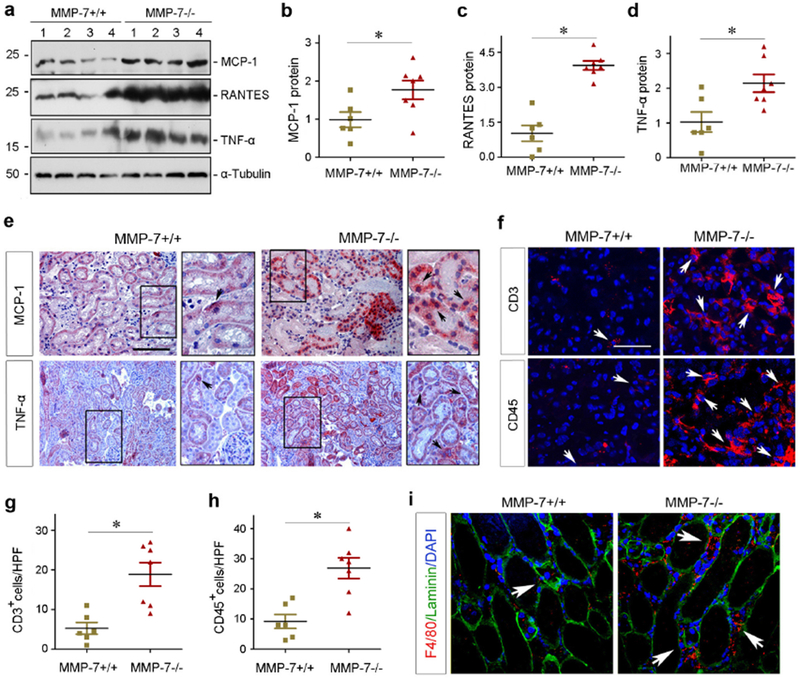
Ablation of endogenous MMP-7 induces pro-inflammatory cytokine expression and promotes renal inflammation after ischemic AKI. (a-d) Western blot analyses of MCP-1, RANTES, and TNF-α protein abundance in MMP-7+/+ and MMP-7−/− kidneys at 1 day after IRI. Representative western blot (a) and quantitative data (b-d) were presented. Numbers (1-4) indicated each individual animal in a given group. *P < 0.05 (n=6-7). (e) Representative micrographs show MCP-1 and TNF-α protein in MMP-7+/+ and MMP-7−/− kidneys at 1 day after IRI. Arrows in the enlarged boxes indicate positive staining. Scale bar, 100 μm. (f) Immunofluorescence staining reveals an increased infiltration of CD3+ T cells and CD45+ leukocytes in MMP-7−/− kidneys at 1 day after IRI. Arrows indicate positive staining. Scale bar, 50 μm. (g, h) Quantitative data show the numbers of CD3+ (g) or CD45+ cells (h) per high-power field (HPF). *P< 0.05 (n=6-7). (i) Co-immunofluorescence staining for F4/80 (red), basement membrane protein laminin (green) and nuclei (blue) shows an increased macrophages infiltration at 1 day after IRI in MMP-7−/− mice, compared with MMP-7+/+ controls. Arrows indicate interstitial macrophages. Scale bar, 50 μm.
Consistent with the induction of various cytokines, increased numbers of CD3+ T cells and CD45+ leukocytes were observed in the interstitium of MMP-7−/− kidneys, comparing to WT controls (Figure 3, f through h). Similarly, more prominent infiltration of the F4/80+ macrophages was found in the interstitium of MMP-7−/− kidneys (Figure 3i). We further investigated the potential effects of MMP-7 on macrophage polarization in vitro. As presented in Supplementary Figure S3, MMP-7 clearly promoted macrophage M2 polarization, while it had no effect on Ml activation and polarization. Furthermore, ablation of MMP-7 also enhanced renal infiltration of the Ly6G+ cells which include neutrophils, monocytes and granulocytes at 1 day after IRI (Supplementary Figure S3).
Loss of MMP-7 worsens AKI induced by cisplatin
To assess a broad implication of MMP-7 in AKI, we next tested whether MMP-7 also plays a role in ameliorating nephrotoxic AKI induced by cisplatin. As shown in Figure 4a, a dramatic difference in mortality rate was observed between WT and KO mice after cisplatin. Within 3 days after cisplatin, 21 out of 29 MMP-7 KO mice died (72.4% mortality rate), whereas only 4 out of 20 WT mice deceased (20%) in the same period. Among the survived mice, serum creatinine level at 3 days after cisplatin was elevated in MMP-7−/− mice, compared to WT controls (Figure 4b). Both renal NGAL mRNA and protein levels were also induced in the survived MMP-7−/− mice after cisplatin, compared to WT controls (Figure 4, c, h and i). Consistently, MMP-7−/− kidneys also displayed more severe morphological injury, characterized by tubular cell dropout and loss of brush borders (Figure 4, d and e).
Figure 4.
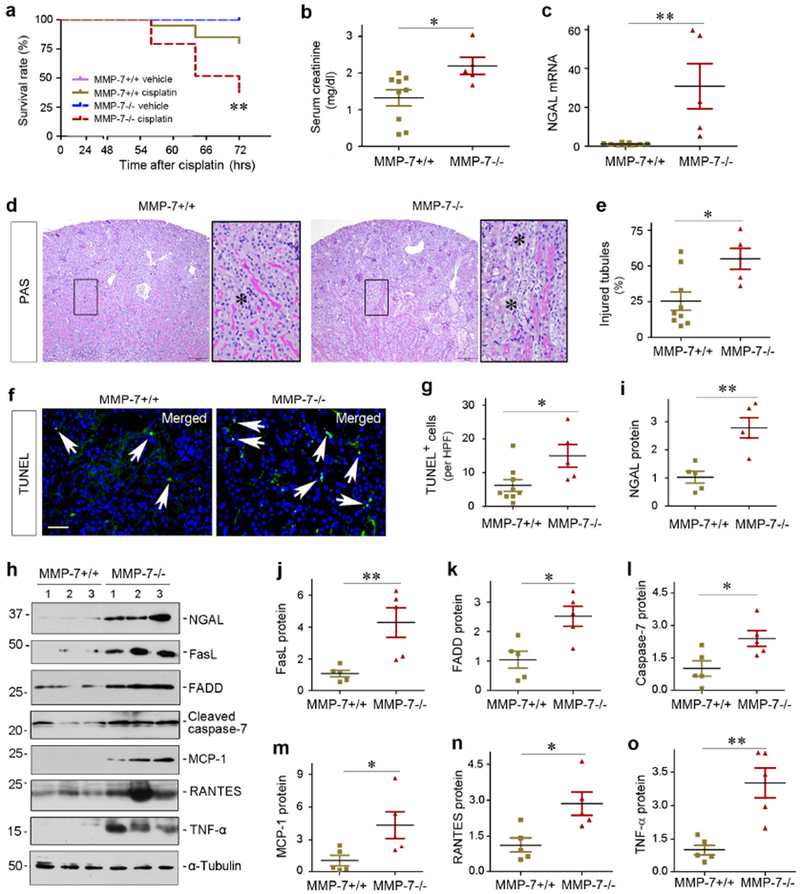
Loss of endogenous MMP-7 aggravates toxic AKI induced by cisplatin. (a) Survival curve shows higher mortality rate in MMP-7−/− mice at 3 days after cisplatin, compared with MMP-7+/+ controls. **P< 0.01 (n=20-29). (b) Serum creatinine level in MMP-7+/+ and MMP-7−/− mice at 3 days after cisplatin injection. *P< 0.05 (n=5-9). (c) qRT-PCR analysis reveals an increased NGAL mRNA in injured kidneys of MMP-7−/− mice at 3 days after cisplatin, compared with MMP-7+/+ controls. **P< 0.01 (n=5-9). **P< 0.01 (n=5-9). (d) Representative micrographs of the kidneys in MMP-7+/+ and MMP-7−/− mice at 3 days after cisplatin. Asterisks in the enlarged boxed areas indicate injured tubules. Scale bar, 150 μm. (e) Injured tubules in MMP-7+/+ and MMP-7−/− mice at 3 days after cisplatin. The percentages of injured tubules (%) are presented. *P< 0.05 (n=5-9). (f) Representative micrographs show apoptotic cells detected by staining in MMP-7+/+ and MMP-7−/− mice at 3 days after cisplatin. Arrows indicate apoptotic cells. Scale bar, 50 μm. (g) Quantitative determination of apoptotic cells at 3 days after cisplatin. Data are presented as numbers of apoptotic cells per high power field (HPF). *P< 0.05 (n=5-9). (h) Representative western blot analyses of various proteins in the injured kidneys of MMP-7+/+ and MMP-7−/− mice at 3 days after cisplatin. Numbers (1-3) indicate each individual animal in a given group. (i-o) Quantitative determination of the relative protein levels in MMP-7+/+ and MMP-7−/− kidneys at 3 days after cisplatin. Proteins of NGAL (i), FasL (j), FADD (k), caspase-7 (l), MCP-1 (m), RANTES (n) and TNF-α (o) are presented. **P< 0.01, *P< 0.05 (n=5).
We further examined apoptosis in WT and KO kidneys at 3 days after cisplatin. As shown in Figure 4, f and g, TUNEL staining revealed more apoptotic cells in MMP-7 KO kidneys after cisplatin, compared to WT controls. Furthermore, expression of FasL, FADD and cleaved caspase-7 were upregulated in MMP-7−/− kidneys (Figure 4, h through l). In addition, multiple pro-inflammatory cytokines including MCP-1, RANTES and TNF-α were induced in KO kidneys at 3 days after cisplatin, compared to WT controls (Figure 4, h, m, n and o). In short, loss of MMP-7 exacerbates cisplatin-induced AKI as well.
MMP-7 protects tubular cells against apoptosis in vitro
We next investigated the role of exogenous MMP-7 in regulating tubular cell survival after injury by using in vitro system. As shown in Figure 5, a and b, substantial apoptosis was detected in human proximal tubular epithelial cells (HKC-8) after incubation with staurosporine (STS), a potent apoptosis inducer.32, 33 However, incubation with human recombinant MMP-7 largely protected HKC-8 cells from STS-induced apoptosis (Figure 5, a and b). Western blot analyses demonstrated that STS induced FasL and FADD, which was prevented by MMP-7 (Figure 5c). Immunostaining also confirmed that MMP-7 largely prevented STS-induced caspase-3 activation in vitro, which was abolished by MMP inhibitor II, a MMP-7 selective inhibitor (Figure 5, d and e). To model IRI in vivo, we treated HKC-8 cells with hypoxia/reoxygenation (H/R) injury in the absence or presence of MMP-7. As shown in Figure 5f, H/R caused FasL and FADD upregulation and MMP-7 abolished their induction. Similar results were obtained when HKC-8 cells were treated with cisplatin (Figure 5g).
Figure 5.
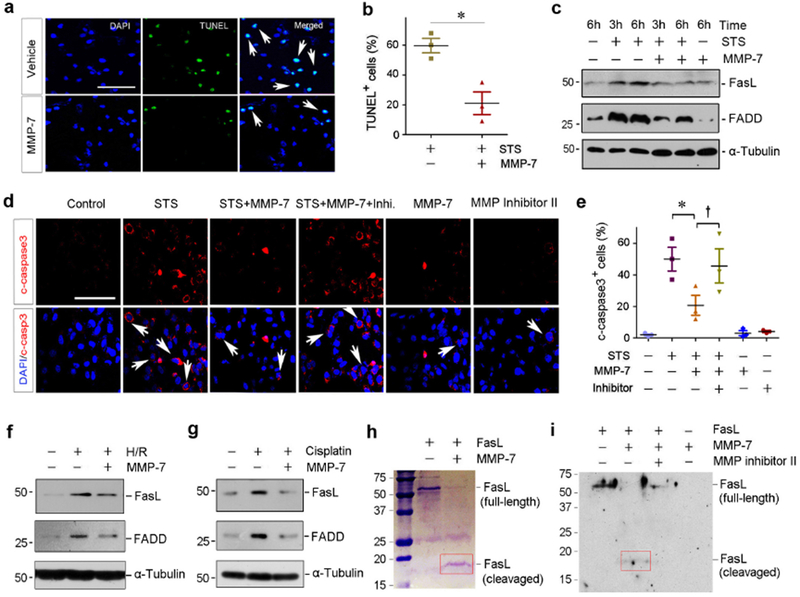
MMP-7 protects kidney tubular cells against apoptosis by cleaving FasL in vitro. (a) Human recombinant MMP-7 protein protects tubular cells against apoptosis induced by staurosporine (STS). HKC-8 cells were incubated with recombinant MMP-7 or vehicle, followed by treatment with STS (0.1 nM) as indicated. Apoptosis was assessed by TUNEL staining. Representative micrographs of TUNEL staining (a) and quantitative data of apoptotic cells are presented (b). Arrows indicate apoptotic cells. *P< 0.05 vs vehicle (n=3). (c) Representative western blotting shows that MMP-7 decreased FasL and FADD protein in HKC-8 cells following STS treatment. (d, e) MMP-7 modulates caspase-3 activation in HKC-8 cells after STS, which is dependent on its enzymatic activity. HKC-8 cells were treated with STS in the absence or presence of MMP-7 and MMP inhibitor II as indicated. Representative micrographs of immunostaining for cleaved caspase-3 (d) and quantitative data (e) are presented. *P< 0.05 vs vehicle (n=3). †P< 0.05 vs MMP-7 without MMP inhibitor II (n=3). Scale bar, 100 μm. Arrows indicate positive staining. (f) Representative western blotting shows that MMP-7 abolished FasL and FADD expression induced by hypoxia/reoxygenation (H/R). HKC-8 cells were incubated in the absence or presence of MMP-7 protein (25 nM) in hypoxic condition for 6 hours, followed by reoxygenation for 2 hours. (g) Representative western blotting shows that MMP-7 abolished FasL and FADD protein expression induced by cisplatin. HKC-8 cells were incubated with cisplatin (25 μg/ml) for 24 hours in the absence or presence of MMP-7 protein (25 nM). (h) Representative SDS-PAGE shows that MMP-7 directly degrades FasL. Recombinant mouse FasL (2 pg) was incubated with 50 nM MMP-7 for 60 min. Full-length FasL migrates at the location of ~50 kDa. Red box indicates the degraded fragments of FasL. (i) Western blot analyses show FasL and its degraded fragments after incubation with MMP-7 in the absence or presence of MMP inhibitor II. FasL degraded fragment is indicated by Red box.
We then explored whether MMP-7 can directly digest FasL by its proteolytic activity. To this end, full-length FasL protein was incubated with MMP-7 in an acellular test tube. As shown in Figure 5h, MMP-7 was clearly able to cleave FasL, resulting in a smaller fragment with molecular weight of 18 kDa. Western blot analyses gave similar results (Figure 5i). In addition, MMP inhibitor II prevented MMP-7-mediated FasL degradation (Figure 5i).
Exogenous MMP-7 restores renal protection after AKI
To further confirm the role of MMP-7 in AKI, we carried out a rescue experiment by injecting exogenous MMP-7 to KO mice. As depicted in Figure 6a, WT and KO mice were subjected to IRI. At 4 hours after IRI, MMP-7 KO mice were injected with MMP-7 at 0.2 or 0.4 mg/kg body weight, respectively. At 1 day after IRI, serum creatinine level elevated in MMP-7−/− mice, compared to WT controls (Figure 6b). Interestingly, renal function was largely preserved in MMP-7−/− mice after injection of MMP-7 (Figure 6b), suggesting that exogenous MMP-7 is able to restore renal protection in vivo. The kidneys from MMP-7−/− mice received exogenous MMP-7 exhibited less morphological injury, compared to vehicle controls (Figure 6c, asterisks). Quantitative assessment of kidney histologic injury is presented in Figure 6d. Meanwhile, qRT-PCR revealed that exogenous MMP-7 at 0.4 mg/kg suppressed renal NGAL expression in MMP-7−/− mice (Figure 6, e through g). Of note, injection of MMP-7 to WT mice at 0.4 mg/kg did not exhibit any appreciable effect on kidney morphology and function (Supplementary Figure S4).
Figure 6.
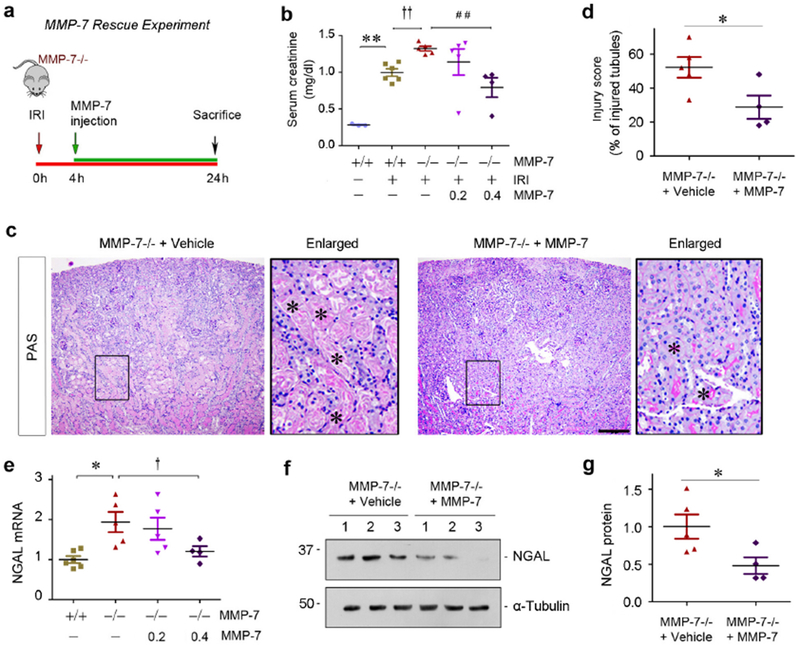
Exogenous MMP-7 rescues its renoprotection after AKI in MMP-7−/− null mice. (a) Rescue experiment design. At 4 h after IRI, a single dose of MMP-7 was injected intravenously, and mice were sacrificed at 24 h. (b) Serum creatinine level in MMP-7+/+ and MMP-7−/− mice injected with either vehicle or recombinant MMP-7 at different dosage after IRI. **P< 0.01 vs sham control (n=3-6); ††P< 0.01 vs MMP-7+/+ mice (n=5-6); ##P< 0.01 vs MMP-7−/− mice (n=4-5) with exogenous MMP-7. (c) Representative micrographs show morphological injury of the kidneys in MMP-7−/− mice injected with vehicle or MMP-7. Asterisks in the enlarged boxed areas indicate injured tubules. Scale bar, 200 μm. (d) Quantitative assessment of morphological injury is presented. *P< 0.05 (n=4-5). (e) qRT-PCR analyses show renal NGAL mRNA expression in various groups as indicated. *P< 0.05 vs sham control; †P<0.05 vs MMP-7−/− mice with exogenous MMP-7 (n=4-6). (f, g) Western blot analyses of renal NGAL protein in MMP-7−/− mice injected with vehicle or MMP-7. Representative western blot (f) and quantitative data (g) were presented. Numbers (1-3) indicate each individual animal in a given group. *P< 0.05 (n=4-5).
We further examined the effects of exogenous MMP-7 on apoptosis and inflammation in MMP-7 KO mice. As illustrated in Figure 7, a and b, apoptotic cells were reduced in the kidneys of KO mice injected with MMP-7, compared vehicle controls. Meanwhile, a decreased mRNA expression of MCP-1 and RANTES was evident in KO kidneys received exogenous MMP-7, compared to vehicle controls (Figure 7, c and d). There was a tendency of reduced renal TNF-α and IL-6 mRNA in KO mice received MMP-7 as well, although not statistically significant (Figure 7, e and f). Consistently, significant inhibition of several key apoptosis- and inflammation-regulatory proteins, including FasL, FADD, cleaved caspase-7, MCP-1, RANTES and TNF-α, was detected in KO kidneys after MMP-7 injection (Figure 7, g and h).
Figure 7.
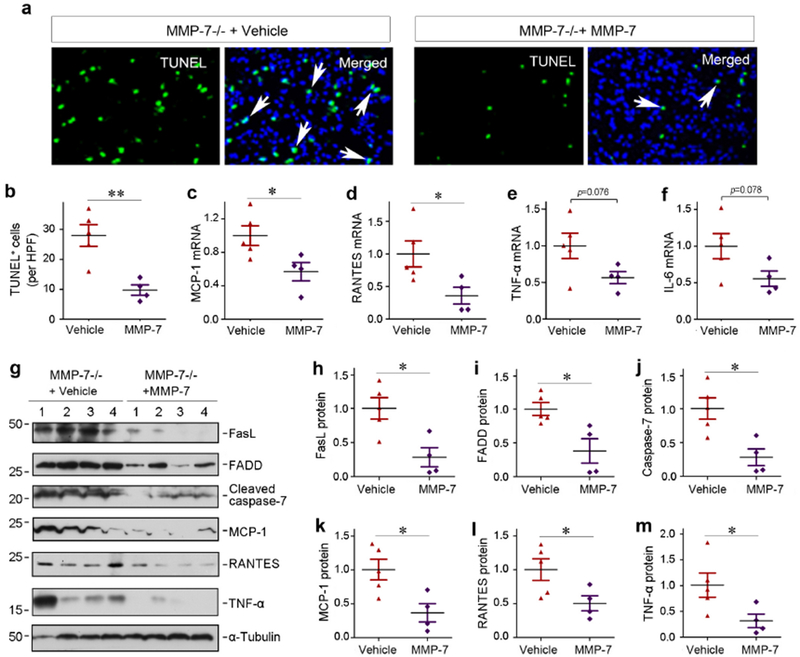
Exogenous MMP-7 ameliorates apoptosis and renal inflammation in MMP-7−/− null mice in vivo. (a, b) TUNEL staining shows a reduced apoptosis in the injured kidneys of MMP-7−/− mice injected exogenous MMP-7 after IRI. Representative micrographs (a) and quantitative data (b) are presented. Arrows in the TUNEL/DAPI merged images indicate apoptotic tubular cells. **P< 0.01 (n=4-5). (c-f) qRT-PCR analyses show the relative mRNA levels of renal MCP-1, RANTES, TNF-α, and IL-6 in MMP-7−/− mice injected with vehicle or exogenous MMP-7 after IRI. *P< 0.05 (n=4-5). (g) Representative western blotting show a reduced expression of both apoptosis-regulatory proteins and pro-inflammatory chemokines in MMP-7−/− mice injected with vehicle or exogenous MMP-7. (h-m) Quantitative determination of the relative protein levels of renal FasL, FADD, cleaved caspase-7, MCP-1, RANTES and TNF-α. Numbers (1-4) indicated each individual animal in a given group. *P < 0.05 (n=4-5).
MMP-7 primes renal tubules to regeneration in vivo and ex vivo
We finally investigated the effect of MMP-7 on tubular cell proliferation, a crucial event leading to kidney regeneration after AKI.34 As shown in Figure 8, a and b, the proliferating cell nuclear antigen (PCNA)+ cells were reduced in renal tubules of MMP-7 KO kidneys at 1 day after IRI, compared to WT controls. However, the numbers of tubular PCNA+ cells were largely restored in MMP-7−/− kidneys received exogenous MMP-7 (Figure 8, a and b), suggesting that MMP-7 promotes tubular cell proliferation.
Figure 8.
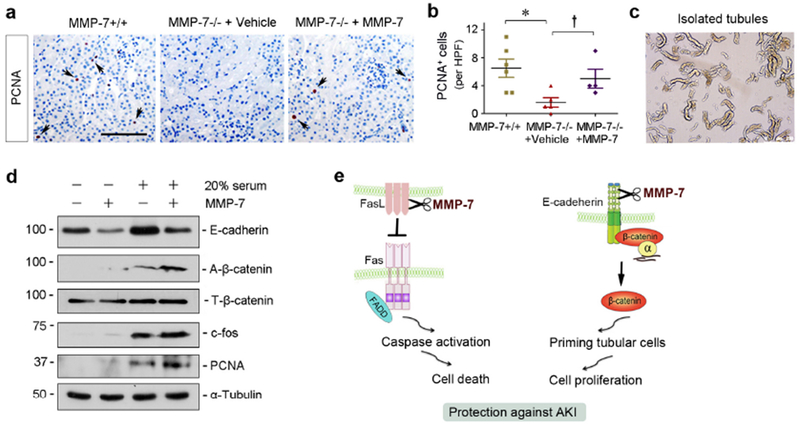
MMP-7 promotes tubular cell proliferation after AKI in vivo and ex vivo. (a) Representative micrographs show PCNA+ tubular cells in MMP-7−/− mice injected with vehicle or exogenous MMP-7 after IRI. (b) Quantitative determination of PCNA+ tubular cells per high power field (HPF) at 1 day after IRI. *P < 0.05 vs MMP-7+/+ mice (n=5-6); †P < 0.05 vs MMP-7−/− mice injected with vehicle (n=4-5). (c) Isolated tubules from corticomedullary region of healthy adult mice and ex vivo culture. (d) Western blot analyses show that MMP-7 cleaved E-cadherin, activated β-catenin, augmented c-fos and PCNA induction in the isolated tubules ex vivo. (e) Schematic diagram shows that MMP-7 cleaves FasL to prevent cell death, and meanwhile it primes tubular cells to proliferation by activating β-catenin via E-cadherin degradation. The combined actions will work in concert and lead to renal protection after AKI by mitigating cell injury and promoting regeneration.
To elucidate how MMP-7 promotes tubular cell proliferation, we isolated renal tubules from mouse kidneys. Isolated tubules were incubated with MMP-7 in the medium with or without 20% serum (Figure 8c). As shown in Figure 8d, MMP-7 degraded E-cadherin in isolated tubules in the absence or presence of serum. Accordingly, MMP-7 promoted β-catenin activation in renal tubules ex vivo (Figure 8d). This is largely due to the release and activation of E-cadherin-bound β-catenin, because total β-catenin level was unaltered (Figure 8d). MMP-7 itself did not significantly induce cell proliferation in isolated tubules, as little expression of proliferation-related proteins such as c-fos and PCNA was observed (Figure 8d). However, MMP-7 augmented c-fos and PCNA expression induced by mitogens-rich serum in renal tubules ex vivo (Figure 8d). These results suggest that MMP-7, via promoting the liberation of E-cadherin-bound β-catenin, primes renal tubules to proliferation by providing a permissive setting for mitogens to function.
DISCUSSION
In this study, using MMP-7−/− null mice in vivo, tubular cells in vitro and isolated tubules ex vivo, we demonstrate that MMP-7 is renal protective by preventing tubular cell apoptosis and promoting tubular regeneration. As summarized in Figure 8e, MMP-7 directly degrades FasL, thereby protecting tubular cells from death induced by extrinsic apoptotic pathway. Meanwhile, MMP-7 can degrade E-cadherin via its ectodomain shedding, which leads to the liberation and release of E-cadherin-bound β-catenin,15 thereby priming renal tubules for responding to mitogenic signals. These studies illustrate that the induction of MMP-7 in renal tubules after AKI is an early attempt for the kidney to alleviate injury and promote regeneration.
Consistent with an elevation of MMP-7 in the urine of AKI patients,12 renal MMP-7 expression is markedly induced in three models of AKI induced by IRI, cisplatin and folic acid (Figures 1 and S1). MMP-7 protein is predominantly localized in the injured proximal tubules, particularly in the S3 segment after IRI (Figure 1). These results suggest that urinary MMP-7 in AKI patients is most likely to be released by the injured tubules. Although the mechanism of MMP-7 induction is not interrogated in the present study, it is known that MMP-7 is transcriptionally controlled by Wnt/β-catenin.17 In this context, it is conceivable to speculate that Wnt/β-catenin activation after AKI triggers MMP-7 expression, which is then secreted into tubular lumen and urine. In line with this view, Wnt/β-catenin is activated in both ischemic and nephrotoxic AKI and tubule-specific deletion of β-catenin aggravates kidney injury,32 whereas Wnt agonist reduces tissue damage and improves renal function after IRI.35
AKI is characterized by tubular cell apoptosis and renal inflammation, followed by the proliferation of the survived tubular cells, leading to repair and recovery.34, 36, 37 Cell apoptosis is regulated by two distinct pathways: the extrinsic, death receptor-dependent and intrinsic, mitochondria-dependent routes.38, 39 In both IRI- and cisplatin-induced AKI, ablation of MMP-7 induces FasL, suggesting that MMP-7 suppresses the death receptor-mediated, extrinsic apoptotic pathway. This conclusion is supported by several lines of evidence. First, loss of MMP-7 also increases FADD and promotes caspase 7 activation, thereby activating the downstream cascade of the extrinsic apoptotic pathway. Second, MMP-7 can directly cleave purified FasL protein in an acellular system (Figure 5), confirming its ability to destroy FasL via its proteolytic degradation. Third, rescue experiments validate that MMP-7 mitigates apoptosis after IRI by repressing FasL/FADD/caspase-7 activation in MMP-7 null mice (Figure 7). It remains unclear, however, whether additional MMP-7 can ameliorate ischemic or nephrotoxic AKI in wild-type mice. Nevertheless, our studies suggest that intrinsic MMP-7 plays an important role in maintaining resistance to AKI. Notably, some studies suggest that MMP-7 may also degrade Fas receptor itself, which contributes to an apoptosis-resistant phenotype in tumor cells.40 However, we found no difference in renal Fas expression in WT and KO mice after IRI (Figure 2), suggesting that Fas is not a primary target of MMP-7 in AKI. Furthermore, Bax expression is not changed in MMP-7 KO kidney after IRI (Figure 2), arguing for that MMP-7 has little effect on the activity of intrinsic, mitochondria-dependent apoptotic pathway.
It is worthwhile to stress that the impact of MMP-7 on apoptosis could be context-dependent. While MMP-7-mediated cleavage of FasL may protect tumor cells from chemotherapeutic drug cytotoxicity,41, 42 at least one study suggests that MMP-7 generates active soluble FasL that potentiates epithelial cell apoptosis.43 In the setting of AKI, MMP-7-mediated cleavage of FasL clearly leads to its inactivation, thereby protecting tubular cells from apoptotic death. Notably, MMP-7 has been shown to promote renal interstitial fibroblast apoptosis by inducing FasL expression.44 Therefore, induction of MMP-7 in AKI may have dual functions in different tissue compartments. On one hand, it protects tubular cells from apoptosis by inactivating FasL during the initial injury phase. On the other hand, it could play a role in the resolution of fibroblasts by promoting their apoptosis via FasL induction in the repair phase.44
One novel finding in the present study is that MMP-7 primes renal tubules to regenerate by preparing a permissive environment for cell proliferation (Figure 8e). Tubular epithelium usually harbors tight and cohesive cell-cell adhesion, and displays ‘contact inhibition’ that curtails cell proliferation in normal conditions. By degrading E-cadherin (Figure 8e), MMP-7 would enable renal tubules to loosen the cell-cell contact, and get ready for proliferation in response to mitogens. In agreement with this notion, loss of MMP-7 in vivo blunts renal PCNA expression after IRI (Figure 8). Conversely, injections of exogenous MMP-7 in KO mice restore PCNA expression in tubular epithelium (Figure 8), which is accompanied by an improved renal function and histology (Figure 6). Using an ex vivo approach, we show that MMP-7 effectively degrades E-cadherin in cultured renal tubules, which leads to the liberation of β-catenin and potentiates mitogen-mediated cell proliferation in cultured renal tubules (Figure 8). Based on these observations, it is plausible to propose that MMP-7-mediated cleavage of E-cadherin creates a permissive setting, which enables tubular cells to dissociate with their neighbors and proliferate in response to mitogens.
Loss of MMP-7 is also associated with an increased expression of several pro-inflammatory cytokines such as MCP-1, TNF-α, and RANTES in AKI (Figure 3), which regulate various aspects of inflammation and immunity.45, 46 Not surprisingly, induction of inflammatory cytokines is associated with an increased renal infiltration of CD3+ T cells, CD45+ leukocytes and F4/80+ macrophages (Figure 3). The mechanism by which MMP-7 inhibits inflammation remains to be elucidated, but it could be related to the degradation of FasL as well, because FasL also participates in the regulation of inflammation by an apoptosis-independent mechanism.47, 48 Furthermore, MMP-7 appears to promote anti-inflammatory M2 macrophage polarization, while it has no effect on pro-inflammatory Ml macrophage activation (Supplementary Figure S3). This could be another mechanism by which MMP-7 elicits its anti-inflammatory action. Given that inflammation is a major component that incites renal injury in AKI,1, 37 these results suggest that MMP-7 may also ameliorate AKI by mitigating renal inflammation.
The present study has some limitations and pitfalls. For examples, the activity of MMP-7 in AKI in vivo is not explicitly examined, despite the clear upregulation of its mRNA and protein. Another pitfall is that serum creatinine levels are determined by using colorimetric Jaffe method, but not a HPLC-based assay. However, a direct comparison has confirmed a good agreement between HPLC and colorimetric methods in mice.49 Finally, while PCNA is used as a marker of cell cycle progression (Figure 8), recent studies suggest that such markers including PCNA and Ki-67 do not reliably indicate cell proliferation in AKI,50 and therefore more studies are needed to clarify these issues. It should be noted that the renal protective effects of MMP-7 are probably limited to the early phase of AKI. Similar to Wnt/β-catenin,51 exaggerated or sustained MMP-7 upregulation after AKI could elicit detrimental actions in the long run, leading to CKD progression. In this regard, we previously show that MMP-7 is a pathogenic mediator of renal fibrosis.15
In summary, we herein report that MMP-7, a secreted endopeptidase recently identified as a valuable biomarker for AKI,12 is induced in kidney tubular epithelium after IRI, cisplatin or folic acid. We show that MMP-7 is renal protective by alleviating apoptosis via degrading FasL and promoting tubular regeneration via digesting E-cadherin. Therefore, the early induction of MMP-7 after AKI is necessary for the kidneys to prime renal tubules for survival and regeneration. These studies provide novel insights into the role and mechanism of MMP-7 in protecting renal tubules after AKI.
METHODS
Animals and AKI models
MMP-7 knockout mice with C57BL/6J genetic background were obtained from the Jackson Laboratory. Age- and sex-matched C57BL/6J mice were used as WT controls. IRI was performed by using an established protocol, as described elsewhere.32 Cisplatin was administered by the single intraperitoneal injection at a dose of 30 mg/kg as described elsewhere.52 Folic acid was injected intraperitoneally at a dose of 250 mg/kg, as described previously.32
In MMP-7 rescue experiment, five groups of mice were used: 1) sham control in WT mice (n=3); 2) IRI and injected with vehicle in WT mice (n=6); 3) IRI and injected with vehicle in MMP-7 KO mice (n=5); 4) IRI and injected with MMP-7 recombinant protein in KO mice (low dose, n=5); 5) IRI and injected with MMP-7 recombinant protein in KO mice (high dose, n=4). Mice were injected through tail vein with vehicle or MMP-7 recombinant protein (#444270; EMD Chemicals) at 0.2 and 0.4 mg/kg body weight, respectively. Additional groups of WT mice were injected with exogenous MMP-7 or vehicle as well. All animal studies were performed by use of the procedures approved by the Animal Ethic Committee at Nanfang Hospital and the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Determination of serum creatinine
Serum creatinine level was determined by use of the QuantiChrom creatinine assay kit, protocols specified by the manufacturer (BioAssay Systems, Hayward, CA). The level creatinine was expressed as milligrams per 100 ml (dl).
Real-time RT-PCR
Total RNA isolation and qRT-PCR was performed on an ABI PRISM 7000 sequence detection system as described previously.53 The sequences of primer pairs for different genes were shown in Supplementary Table 1.
Western blot analysis
Protein expression was analyzed by Western blot analysis. The primary antibodies used were described in the Detail Methods published online.
Histology and immunohistochemical staining
Paraffin-embedded mouse kidney sections were prepared by a routine procedure. The sections were stained with Periodic acid-Schiff (PAS) staining reagents by standard protocol. Immunohistochemical staining was performed according to the established protocol as described previously.53
Immunofluorescence staining
Kidney cryosections were co-immunostained with antibodies described in the Detail Methods published online.
Detection of apoptotic cells
Apoptotic cell death was determined by using TUNEL staining with Dead End Fluorometric Apoptosis Detection System, as described previously.32 For some kidney sections, co-staining after TUNEL with anti-AQP-1 antibody was performed to characterize the identity of apoptotic cells.
Cell culture
Human proximal tubular epithelial cell line (HKC-8) was maintained as described previously.15 HKC-8 cells were incubated with human MMP-7 recombinant protein at 25 nM in the absence or presence of MMP inhibitor II, followed by treating with 0.1 μM staurosporine for various periods of time as indicated to induce apoptosis, and then subjected to TUNEL staining and western blot analyses, respectively. HKC-8 cells were also treated with cisplatin at 25 μg/ml in the absence or presence of human MMP-7 at the concentration of 25 nM. For imitating IRI, we used cellular model of hypoxia/reoxygenation (H/R) injury. Briefly, HKC-8 cells were cultured in glucose-free medium in a tri-gas incubator (94% N2, 5% CO2 and 1.0% O2) for 6 hours in the absence or presence of MMP-7 (25 nM), followed by incubating in complete medium under normal conditions for 2 hours reoxygenation. Cell lysates were then analyzed by western blotting.
Mouse macrophage cell line (RAW264.7) was obtained from American Type Culture Collection (ATCC, Manassas, VA). RAW264.7 cells were incubated with LPS (5 ng/ml) and IFN-γ (20 ng/ml) or IL-4 (20 ng/ml) in the absence or presence of MMP-7 (25 nM) for 24 hours to induce M1 or M2 polarization, respectively. Cell lysates were then analyzed by western blotting for M1 and M2 macrophage markers.
Ex vivo tubule culture
Mouse kidney was minced and digested with the pre-warmed solution of collagenase IV in the Hank’s balanced salt solution. Kidney tubules were separated on a density gradient by ultracentrifugation and the band containing predominantly proximal tubules was collected. Suspended tubules were cultured in mediums in the absence or presence of MMP-7, respectively.
Statistical analyses
All data were expressed as mean ± SEM. Statistical analysis of the data was performed using SigmaStat software (Jandel Scientific Software, San Rafael, CA). Comparison between groups was made using one-way ANOVA, followed by the Student-Newman-Keuls test. Animal survival rate was analyzed by Log-rank (Mantel-Cox) Test. P< 0.05 was considered significant.
Supplementary Material
Detailed Methods.
Figure S1. MMP-7 is induced in AKI triggered by folic acid. (a) qRT-PCR shows an increased MMP-7 mRNA expression in the kidney at 2 days after folic acid injection. **P<0.01 versus controls. (b) Representative micrographs show MMP-7 protein expression and localization in control and injured kidneys at 2 days after folic acid injection. Arrows indicate positive staining. Scale bar, 50 μm.
Figure S2. Deletion of MMP-7 does not cause renal abnormality under physiological conditions. (a) Genotyping of MMP-7+/+ and MMP-7−/− mice. (b) Kidney morphology and renal expression of MCP-1 protein in MMP-7+/+ and MMP-7−/− mice in normal conditions. Scale bar, 60 μm.
Figure S3. MMP-7 promotes M2 macrophage polarization in vitro and reduces neutrophil infiltration in vivo. (a) MMP-7 does not affect Ml macrophage polarization. Mouse macrophages (RAW264.7) were incubated with LPS (5 ng/ml) and IFN-γ (20 ng/ml) in the absence or presence of MMP-7 protein (25 nM) for 24 hours. Cell lysates were immunoblotted for Ml macrophage markers iNOS and TNF-α. (b) MMP-7 promotes M2 macrophage polarization. RAW264.7 cells were incubated with IL-4 (20 ng/ml) in the absence or presence of MMP-7 protein (25 nM) for 24 hours. Cell lysates were immunoblotted for M2 macrophage marker Arg-1. (c, d) Ablation of MMP-7 promoted renal infiltration of neutrophils at 1 day after IRI. Representative micrographs (c) and quantitative data (d) are presented. Arrows indicate Ly6G+ staining. Scale bar, 50 μm. *P < 0.05 (n =3).
Figure S4. Administration of exogenous MMP-7 in wild-type MMP-7+/+ mice does not affect kidney morphology and function. (a, b) Exogenous MMP-7 did not affect kidney function in MMP-7+/+ mice. At 24 hours after MMP-7 injection (0.4 mg/kg), serum creatinine and BUN were assessed. (c) Representative micrographs show that exogenous MMP-7 did not affect kidney morphology. Kidney sections of MMP-7+/+ mice after injection without or with MMP-7 (0.4 mg/kg) were subjected to PAS staining. Scale bar, 100 μm.
Table S1. Nucleotide sequences of the primers used for RT-PCR.
Translational Statement.
Matrix metalloproteinase-7 (MMP-7) is an early and valuable biomarker for predicting severe AKI and poor outcomes in patients after cardiac surgery. The present study indicates that induction of endogenous MMP-7 is renal protective in AKI by priming kidney tubules to survival and regeneration. These results suggest that some urinary biomarkers in patents manifest an initial attempt of the kidney to protect against injury and to promote repair after AKI. Whether infusion of exogenous MMP-7 can protect kidneys against AKI in patients warrants further investigation.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health grants DK064005 and DK106049, and the National Science Foundation of China grants 81521003 and 81770715. F. H was supported by the National Science Foundation of China grant 81770737.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have declared that no conflict of interest exists.
SUPPLEMENTARY MATERIAL
Supplementary information is available at Kidney International’s website.
REFERENCES
- 1.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 2011;7:189–200. [DOI] [PubMed] [Google Scholar]
- 2.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 2014;10:193–207. [DOI] [PubMed] [Google Scholar]
- 3.Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med 2017;376:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int 2012;81:819–825. [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw SM, Wald R. Acute kidney injury: Timing of renal replacement therapy in AKI. Nat Rev Nephrol 2016;12:445–446. [DOI] [PubMed] [Google Scholar]
- 6.Moledina DG, Parikh CR. Phenotyping of acute kidney injury: beyond serum creatinine. Semin Nephrol 2018;38:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulluck H, Maiti R, Chakraborty B, et al. Neutrophil gelatinase-associated lipocalin prior to cardiac surgery predicts acute kidney injury and mortality. Heart 2018;104:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medic B, Rovcanin B, Vujovic KS, et al. Evaluation of novel biomarkers of acute kidney injury: the possibilities and limitations. Curr Med Chem 2016;23:1981–1997. [DOI] [PubMed] [Google Scholar]
- 9.Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: A meta-analysis. Am J Kidney Dis 2015;66:993–1005. [DOI] [PubMed] [Google Scholar]
- 10.Adler C, Heller T, Schregel F, et al. TIMP-2/IGFBP7 predicts acute kidney injury in out-of-hospital cardiac arrest survivors. Crit Care 2018;22:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Chen C, Teng S, et al. Urinary matrix metalloproteinase-7 predicts severe AKI and poor outcomes after cardiac surgery. J Am Soc Nephrol 2017;28:3373–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol 2012;302:F1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke B, Fan C, Yang L, et al. Matrix Metalloproteinases-7 and Kidney Fibrosis. Front Physiol 2017;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou D, Tian Y, Sun L, et al. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol 2017;28:598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grindel BJ, Martinez JR, Pennington CL, et al. Matrilysin/matrix metalloproteinase-7(MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol 2014;36:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He W, Tan RJ, Li Y, et al. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/beta-catenin activity in CKD. J Am Soc Nephrol 2012;23:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rims CR, McGuire JK. Matrilysin (MMP-7) catalytic activity regulates beta-catenin localization and signaling activation in lung epithelial cells. Exp Lung Res 2014;40:126–136. [DOI] [PubMed] [Google Scholar]
- 19.Surendran K, Simon TC, Liapis H, et al. Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int 2004;65:2212–2222. [DOI] [PubMed] [Google Scholar]
- 20.Duarte S, Baber J, Fujii T, et al. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol 2015;44–46: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu RK, Donaworth E, Siroky B, et al. Loss of matrix metalloproteinase-8 is associated with worsened recovery after ischemic kidney injury. Ren Fail 2015;37:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko T, Shimizu A, Mii A, et al. Role of matrix metalloproteinase-2 in recovery after tubular damage in acute kidney injury in mice. Nephron Exp Nephrol 2012;122:23–35. [DOI] [PubMed] [Google Scholar]
- 23.Lee SY, Horbelt M, Mang HE, et al. MMP-9 gene deletion mitigates microvascular loss in a model of ischemic acute kidney injury. Am J Physiol Renal Physiol 2011;301:F101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunugi S, Shimizu A, Kuwahara N, et al. Inhibition of matrix metalloproteinases reduces ischemia-reperfusion acute kidney injury. Lab Invest 2011;91:170–180. [DOI] [PubMed] [Google Scholar]
- 25.Ho J, Rush DN, Krokhin O, et al. Elevated Urinary Matrix Metalloproteinase-7 Detects Underlying Renal Allograft Inflammation and Injury. Transplantation 2016;100:648–654. [DOI] [PubMed] [Google Scholar]
- 26.Afkarian M, Zelnick LR, Ruzinski J, et al. Urine matrix metalloproteinase-7 and risk of kidney disease progression and mortality in type 2 diabetes. J Diabetes Complications 2015;29:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramesh G, Ranganathan P. Mouse models and methods for studying human disease, acute kidney injury (AKI). Methods Mol Biol 2014;1194:421–436. [DOI] [PubMed] [Google Scholar]
- 28.Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol 2012;303:F1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005;365:1231–1238. [DOI] [PubMed] [Google Scholar]
- 30.Waring P, Mullbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol 1999;77:312–317. [DOI] [PubMed] [Google Scholar]
- 31.Elmore S Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou D, Li Y, Lin L, et al. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int 2012;82:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simenc J, Lipnik-Stangelj M. Staurosporine induces apoptosis and necroptosis in cultured rat astrocytes. Drug Chem Toxicol 2012;35:399–405. [DOI] [PubMed] [Google Scholar]
- 34.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 2011;121:4210–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuncewitch M, Yang WL, Corbo L, et al. WNT agonist decreases tissue damage and improves renal function after ischemia-reperfusion. Shock 2015;43:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang-Panesso M, Humphreys BD. Cellular plasticity in kidney injury and repair. Nat Rev Nephrol 2017;13:39–46. [DOI] [PubMed] [Google Scholar]
- 37.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2012;2:1303–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004;305:626–629. [DOI] [PubMed] [Google Scholar]
- 39.Wajant H The Fas signaling pathway: more than a paradigm. Science 2002;296:1635–1636. [DOI] [PubMed] [Google Scholar]
- 40.Strand S, Vollmer P, van den Abeelen L, et al. Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumour cells. Oncogene 2004;23:3732–3736. [DOI] [PubMed] [Google Scholar]
- 41.Wang WS, Chen PM, Wang HS, et al. Matrix metalloproteinase-7 increases resistance to Fas-mediated apoptosis and is a poor prognostic factor of patients with colorectal carcinoma. Carcinogenesis 2006;27:1113–1120. [DOI] [PubMed] [Google Scholar]
- 42.Mitsiades N, Yu WH, Poulaki V, et al. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res 2001;61:577–581. [PubMed] [Google Scholar]
- 43.Powell WC, Fingleton B, Wilson CL, et al. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol 1999;9:1441–1447. [DOI] [PubMed] [Google Scholar]
- 44.Zhou D, Tan RJ, Zhou L, et al. Kidney tubular beta-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci Rep 2013;3:1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandenbroucke RE, Vanlaere I, Van Hauwermeiren F, et al. Pro-inflammatory effects of matrix metalloproteinase 7 in acute inflammation. Mucosal Immunol 2014;7:579–588. [DOI] [PubMed] [Google Scholar]
- 46.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4:617–629. [DOI] [PubMed] [Google Scholar]
- 47.Mande P, Zirak B, Ko WC, et al. Fas ligand promotes an inducible TLR-dependent model of cutaneous lupus-like inflammation. J Clin Invest 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bien K, Sokolowska J, Baska P, et al. Fas/FasL pathway participates in regulation of antiviral and inflammatory response during mousepox infection of lungs. Mediators Inflamm 2015;2015:281613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keppler A, Gretz N, Schmidt R, et al. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int 2007;71:74–78. [DOI] [PubMed] [Google Scholar]
- 50.Lazzeri E, Angelotti ML, Peired A, et al. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat Commun 2018;9:1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou D, Tan RJ, Fu H, et al. Wnt/beta-catenin signaling in kidney injury and repair: a double-edged sword. Lab Invest 2016;96:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou D, Tan RJ, Lin L, et al. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int 2013;84:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu H, Tian Y, Zhou L, et al. Tenascin-C is a major component of the fibrogenic niche in kidney fibrosis. J Am Soc Nephrol 2017;28:785–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed Methods.
Figure S1. MMP-7 is induced in AKI triggered by folic acid. (a) qRT-PCR shows an increased MMP-7 mRNA expression in the kidney at 2 days after folic acid injection. **P<0.01 versus controls. (b) Representative micrographs show MMP-7 protein expression and localization in control and injured kidneys at 2 days after folic acid injection. Arrows indicate positive staining. Scale bar, 50 μm.
Figure S2. Deletion of MMP-7 does not cause renal abnormality under physiological conditions. (a) Genotyping of MMP-7+/+ and MMP-7−/− mice. (b) Kidney morphology and renal expression of MCP-1 protein in MMP-7+/+ and MMP-7−/− mice in normal conditions. Scale bar, 60 μm.
Figure S3. MMP-7 promotes M2 macrophage polarization in vitro and reduces neutrophil infiltration in vivo. (a) MMP-7 does not affect Ml macrophage polarization. Mouse macrophages (RAW264.7) were incubated with LPS (5 ng/ml) and IFN-γ (20 ng/ml) in the absence or presence of MMP-7 protein (25 nM) for 24 hours. Cell lysates were immunoblotted for Ml macrophage markers iNOS and TNF-α. (b) MMP-7 promotes M2 macrophage polarization. RAW264.7 cells were incubated with IL-4 (20 ng/ml) in the absence or presence of MMP-7 protein (25 nM) for 24 hours. Cell lysates were immunoblotted for M2 macrophage marker Arg-1. (c, d) Ablation of MMP-7 promoted renal infiltration of neutrophils at 1 day after IRI. Representative micrographs (c) and quantitative data (d) are presented. Arrows indicate Ly6G+ staining. Scale bar, 50 μm. *P < 0.05 (n =3).
Figure S4. Administration of exogenous MMP-7 in wild-type MMP-7+/+ mice does not affect kidney morphology and function. (a, b) Exogenous MMP-7 did not affect kidney function in MMP-7+/+ mice. At 24 hours after MMP-7 injection (0.4 mg/kg), serum creatinine and BUN were assessed. (c) Representative micrographs show that exogenous MMP-7 did not affect kidney morphology. Kidney sections of MMP-7+/+ mice after injection without or with MMP-7 (0.4 mg/kg) were subjected to PAS staining. Scale bar, 100 μm.
Table S1. Nucleotide sequences of the primers used for RT-PCR.


