Abstract
Introduction:
New persistent opioid use following surgery is a common iatrogenic complication, developing in roughly 6% of patients after elective surgery. Despite increased awareness of misuse and associated morbidity, opioids remain the cornerstone of pain management in bariatric surgery. The potential impact of new persistent opioid use on long-term postoperative outcomes is unknown. We sought to determine the relationship between new persistent opioid use and 1-year postoperative outcomes for patients undergoing bariatric surgery.
Methods:
Using data from the MBSC registry, we identified patients undergoing primary bariatric surgery between 2006–2016. Using previously validated patient-reported survey methodology, we evaluated patient opioid use preoperatively and at 1 year following surgery. New persistent use was defined as a previously opioid naïve patient who self-reported opioid use 1 year after surgery. We used multivariable logistic regression models to evaluate the association between new persistent opioid use, risk-adjusted weight loss, and psychologic outcomes (psychological wellbeing, body image, and depression).
Results:
27,799 patients underwent primary bariatric surgery between 2006 and 2016. Among opioid-naïve patients, the rate of new persistent opioid use was 6.3%. At 1-year after surgery, patients with new persistent opioid user lost significantly less excess body weight compared to those without new persistent use (57.6% vs 60.3%; p <0.0001). Patients with new persistent opioid use had significantly worse psychological wellbeing (35.0 vs 33.1; p <0.0001), body image (19.9 vs 18.0; p <0.0001), and depression scores (2.4 vs 5.0; p <0.0001). New persistent opioid users also reported less overall satisfaction with their bariatric surgery (75.1% vs 85.7%; p <0.0001)
Conclusions:
New PERSISTENT opioid use is common following bariatric surgery and associated with significantly worse physiologic and psychologic outcomes. More effective screening and postoperative surveillance tools are needed to identify these patients, who likely require more aggressive counseling and treatment to maximize the benefits of bariatric surgery.
Keywords: Bariatric Surgery, Opioid, New Persistent Opioid Use, Outcomes, Patient Reported
INTRODUCTION
Iatrogenic opioid dependence is an increasingly common postoperative complication with approximately 6–10% of opioid naïve patients developing chronic opioid use after major or minor surgical procedures.(1–5) While bariatric surgery is a safe and effective treatment for obesity, with less than 3% of patients experiencing a serious complication, 4–9% of patients develop new persistent opioid use following surgery.(6–12) Bariatric surgery patients may be particularly vulnerable to opioid misuse and dependence due to high prevalence of risk factors in this population, such as metal health disorders, and the risk of “addiction-transfer”.(13, 14) Despite increased awareness of national prescription opioid abuse and surgeon overprescribing habits, opioids remain the cornerstone of postoperative pain management following bariatric surgery.(15–17)
The effects of opioid use on surgical outcomes are unclear. Preoperative opioid use has been linked to decreased physical function in orthopedic patients and increased risk of morbidity and healthcare utilization following non-emergent abdominopelvic procedures.(18–21) However, for previously opioid-naïve patients, the impact of new persistent opioid use postoperative outcomes is unknown. In bariatric surgery specifically, the association between new persistent opioid use and postoperative outcomes such as weight loss, psychological wellbeing, and satisfaction has not been evaluated. It is possible that opioid use does not affect bariatric postoperative outcomes. On the other hand, new persistent opioid use may be associated with worse perioperative outcomes, prompting bariatric surgeons to establish prescribing guidelines and opioid surveillance strategies. Additionally, patients may be further motivated to seek alternative pain management strategies in an effort to maximize their desired surgical results. Given the paucity of data on the possible implications of new persistent opioid use, it remains difficult to comprehensively counsel patients on risks and anticipated outcomes following surgery.
In this context, we used clinical registry data from a statewide quality improvement collaborative to evaluate the association between new persistent opioid use and postoperative physiologic and psychologic outcomes following bariatric surgery.
MATERIALS AND METHODS
Data Source and Population
This study utilizes data from the Michigan Bariatric Surgery Collaborative (MBSC). The MSBC is a statewide clinical registry that includes information from >95% of patients undergoing bariatric surgery in the state of Michigan. The MBSC has previously been described in great detail (6, 22). This collaborative includes 45 hospitals and employs trained data abstractors that perform extensive chart review regarding patients’ demographics, comorbidities, perioperative care and process details, and postoperative outcomes for multiple bariatric operations. Institutional review boards at each participating hospital have approved data collection and participation in the MBSC.
We identified all patients age ≥ 18 years in the MBSC registry who underwent primary Roux-en-Y gastric bypass, sleeve gastrectomy, adjustable gastric band placement, or duodenal switch/biliopancreatic diversion from June 26, 2006 and December 31, 2016. Patients undergoing revisional surgery were excluded from this analysis. Patients with incomplete or missing one year postoperative survey data were also excluded.
Outcomes Measures
The primary outcome for this study was percent excess body weight loss (EBWL) at 1 year after the primary operation, as determined from the patient medical record. Additional postoperative 1-year patient outcomes were assessed using MBSC patient self-reported surveys. Information gathered in the 1-year outcomes survey included physiologic and psychologic measures. Physiologic outcomes included overall physical satisfaction as well as total body weight loss. Psychologic outcomes were assessed via the Pyschological Wellbeing survey, Body-Q survey and depression questionnaire PHQ-8, which were included in the MBSC 1-year postoperative survey. The Psychological Wellbeing survey provides information on patients’ self-perception within the past two weeks, with scores ranging from 10–40. The Body-Q survey provides information on a patient’s self-perceived body image, with scores ranging from 7–28. The depression questionnaire PHQ-8 is a self-reported tool that provides information on the diagnosis and degree of a patient’s reported depression, with scores ranging from 0–24. Postoperative psychological outcomes were compared to preoperative baseline scores. An additional secondary outcome was self-reported alcohol use disorder, which was also obtained from the MBSC 1-year postoperative survey.
Definitions of Opioid Use
Opioid use was assessed based on patient self-reported use on the preoperative and 1-year postoperative MBSC survey. Patients were asked on each survey whether they currently used “prescription pain killers” with a list of commonly prescribed opioid medications including Vicodin, Percocet, Darvocet, Lorcet, Roxicet, Tylenol #3, Oxycodone, Morphine, Methadone, Fentanyl patch, Duragesic patch, and Dilaudid. All patients were assigned to one of three categories based on their self-reported opioid use status. “Chronic use” status was assigned to patients who reported use of a narcotic pain medication on the preoperative MBSC survey. “New persistent use” status was defined as patients who denied use of opioid medications preoperatively, but reported use of an opioid on the one-year postoperative survey. “Non-use” status was assigned to patients who denied use of opioid pain medication at the preoperative and 1-year postoperative survey.
Study Variables
Data regarding demographic information (such as age, gender, race, and BMI), mental health comorbid conditions (such as anxiety disorder, depression, and bipolar disorder), and 30-day postoperative complications were collected by data extractors for all patients via direct, clinical chart abstraction. Musculoskeletal (MSK) disorder was defined as obesity-related pain, injuries or disorders or the muscles, nerves, tendons, ligaments, joints, cartilage and spinal disc, e.g. arthritis, osteoarthritis (hands, feet, hip, back, knee), polyarthritis, back pain, sciatica or carpel tunnel syndrome. Rheumatoid arthritis was excluded from MSK disorder. Serious complications include intraabdominal abscess formation (requiring drainage/reoperation), bowel obstruction/hernia (requiring operation), anastomotic leak, band-related problems (requiring reoperation), bleeding (transfusion >4 units), respiratory failure (requiring intubation for 2–7 day), renal failure (requiring in-hospital dialysis) wound infection/dehiscence, VTE, myocardial infarction/cardiac arrest, renal failure (requiring long-term dialysis), respiratory failure (requiring intubation for >7 days or tracheostomy), and death. Data regarding preoperative tobacco use and alcohol use were collected from the MBSC preoperative survey.
Statistical Analysis
We utilized Pearson χ2 test for categorical variables and independent sample t-tests for continuous variables to assess whether these baseline patient characteristics were independent of the assigned opioid use categorical variable (non-user vs new persistent opioid user at 1-year following surgery). We then performed Pearson χ2 tests to assess whether there was an association between unadjusted 1-year surgical outcomes and opioid use. For risk adjustment, we performed a stepwise regression to evaluate the covariate-adjusted association between new persistent opioid use and the primary study outcomes. Adjustment covariates included gender, white race, private insurance, hypertension, hyperlipidemia, diabetes, GERD, CAD, sleep apnea, mobility limitations, asthma, mental health disorder, urinary incontinence, musculoskeletal disorder, kidney disease, liver disorder, and surgical procedure performed (RYGB, lap-band, sleeve gastrectomy, or BPD/DS).
Factors that were statistically significant in univariate analysis were included as risk-adjusted variables. We then compared new persistent opioid users to non-users using risk-adjusted outcomes at baseline and 1-year after surgery. We also compared these groups for magnitude of change between baseline and 1-year after surgery.
Two-sided p values less than 0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
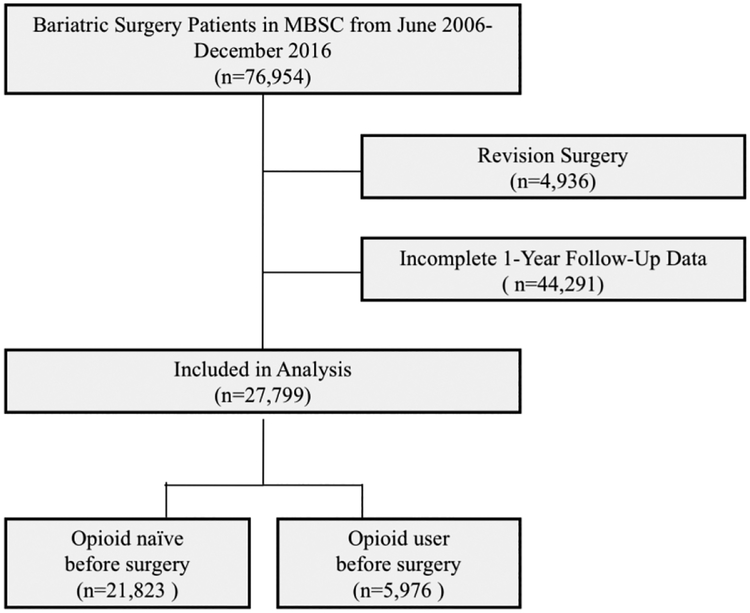
Figure 1 shows details of the study cohort. During the study period, 27,799 patients underwent primary bariatric surgery and were included in this analysis. Of these, 21,823 (79%) were opioid-naïve preoperatively and 5,976 (21%) reported opioid use prior to surgery. Among opioid-naïve patients, 1,370 or 6.3% developed new persistent opioid use after bariatric surgery.
Figure 1.
Study cohort and sample criteria. Patients who underwent primary bariatric surgery from June 26, 2006 to December 31, 2016 were included if they met the following criteria: age ≥ 18 years, non-revisional bariatric surgery, complete baseline and 1-year postoperative follow-up data.
Table 1 presents characteristics of opioid-naïve patients stratified by whether or not they developed new persistent opioid use. Compared to patients who did not develop persistent use, new persistent opioid users were older (mean age 48.3 vs. 46.6), less likely to be White (78.4% vs. 83.7%), more likely to currently use tobacco (9.6% vs. 6.3%), more likely to have various comorbidities, more likely to have undergone Roux-en-Y gastric bypass (41.3% vs. 34.9%), and more likely to have suffered a postoperative complication (9.3% vs 6.0%). New persistent users were also more likely to carry a diagnosis of depression (54.3% vs. 40.2%) and bipolar disorder (54.3% vs. 40.2%). However, new persistent users were less likely have diagnosed anxiety (48.4% vs. 61.8%) or be married / living with a significant other (62.1% vs. 68.4%).
Table 1.
Baseline characteristics of preoperative opioid-naïve patients
| Naïve Patients with No Persistent Opioid Use | Naïve Patients with New Persistent Use | P value | |
|---|---|---|---|
| Patients (n) | 20,453 | 1,370 | |
| Age, mean (± SD) | 46.6 (11.7) | 48.3 (12.0) | <0.0001 |
| Male (%) | 20.3 | 19.1 | 0.2782 |
| White (%) | 83.7 | 78.4 | <0.0001 |
| Comorbidities (%) | |||
| Diabetes | 31.9 | 40.0 | <0.0001 |
| CVDa | 52.9 | 63.4 | <0.0001 |
| Hypertension | 51.0 | 61.7 | <0.0001 |
| CADb | 5.0 | 7.8 | <0.0001 |
| Serious lung disease | 6.3 | 10.4 | <0.0001 |
| Starting BMIc (%) | |||
| >40 | 16.3 | 14.3 | 0.0545 |
| 40–49 | 52.5 | 49.9 | 0.0608 |
| 50–59 | 24.6 | 25.8 | 0.3060 |
| ≥60 | 6.6 | 9.9 | <0.0001 |
| Mean (± SD) | 47.1 (7.89) | 48.3 (8.8) | <0.0001 |
| Mental Health Disorder (%) | |||
| Anxiety | 61.8 | 48.4 | <0.0001 |
| Depression | 22.1 | 29.1 | <0.0001 |
| Bipolar | 40.2 | 54.3 | <0.0001 |
| Musculoskeletal Disorders (%) | 70.6 | 82.2 | <0.0001 |
| Married or living with significant other (%) | 68.4 | 62.1 | <0.0001 |
| Insurance type (%) | |||
| Private | 78.4 | 63.4 | <0.0001 |
| Medicare or Medicaid | 12.2 | 29.1 | <0.0001 |
| Current Tobacco Use (%) | 6.3 | 9.6 | <0.0001 |
| Substance Abuse (%) | 1.0 | 1.2 | 0.4795 |
| Procedure Type (%) | |||
| Roux-en-Y Gastric Bypass | 34.5 | 41.3 | <0.0001 |
| Sleeve Gastrectomy | 54.0 | 46.6 | <0.0001 |
| Adjustable Band Placement | 10.6 | 10.8 | 0.8401 |
| BPD/DSd | 0.9 | 1.2 | 0.2082 |
| Complications (%) | 6.0 | 9.3 | <0.0001 |
| Serious Complications® (%) | 1.6 | 2.9 | 0.0003 |
CVD cardiovascular disease,
CAD coronary artery disease,
BMI body mass index (calculated as kg/m2),
BPD/DS biliopancreatic diversion with duodenal switch.
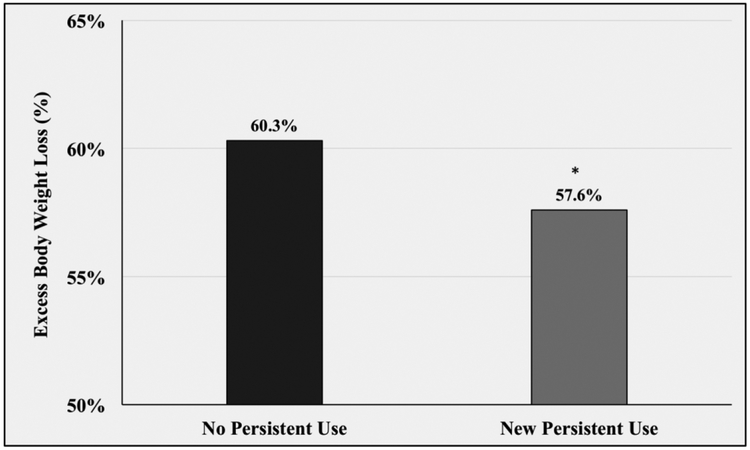
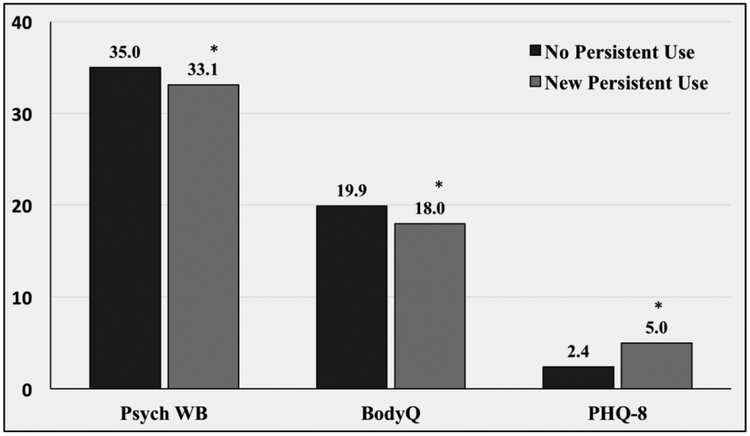
Table 2 shows the results of the postoperative risk-adjusted analyses. New persistent opioid users had significantly less excess body weight loss one year following surgery compared to non-use patients (57.6% vs. 60.3%, p<0.0001) (Figure 2). New persistent opioid users also had worse psychologic outcomes compared to their non-opioid use counterparts. Specifically, baseline psychological wellbeing was similar between the two groups prior to surgery (31.6 vs. 32.7; p=0.0798). One year after surgery, however, new persistently users had less improvement in their scores with significantly worse psychological wellbeing (33.1 vs 35.0; p<0.0001). A similar trend was seen in BodyQ Scores. Despite similar baseline reports (11.9 vs 12.0; p=0.9674), new persistent opioid users had less improvement and a significantly worse postoperatively BodyQ scores (18.0 vs 19.9, p<0.0001). Preoperatively, new persistent opioid users reported more severe depression symptoms (5.5 vs. 4.0; p=0.0007). Following surgery both non-users and new persistent users had improvement in their symptoms. However, new persistent users had significantly less improvement and continued to have statistically greater PHQ-8 scores (5.0 vs 2.4; p=<0.0001). (Figure 3)
Table 2:
1-Year Risk Adjusted Outcomes by Opioid Use Status
| Naïve Patients with No Persistent Opioid Use (mean) | Naïve Patients with New Persistent Use (mean) | P value | |
|---|---|---|---|
| 1-Year Post-Operative (± SD) | |||
| Weight (lbs.) | 200.4 (45.6) | 207.2 (51.1) | <0.0001 |
| Weight loss (lbs.) | 90.0 (38.0) | 88.8 (41.5) | 0.3974 |
| Excess Body Weight Loss (%) | 60.3 (20.3) | 57.6 (23.3) | <0.0001 |
| BMIa | 32.5 (6.5) | 33.8 (7.7) | <0.0001 |
| Change in BMI | 14.6 (5.9) | 14.44 (6.5) | 0.1615 |
| Psychological well-being (± SD) | |||
| Baseline | 32.7 (6.6) | 31.6 (7.5) | 0.0798 |
| 1-year | 35.0 (6.2) | 33.1 (7.0) | <0.0001 |
| BodyQ Score (± SD) | |||
| Baseline | 12.0 (4.7) | 11.9 (4.8) | 0.9674 |
| 1-year | 19.9 (5.3) | 18.0 (5.7) | <0.0001 |
| PHQ-8 Score (± SD) | |||
| Baseline | 4.0 (4.5) | 5.5 (5.4) | 0.0007 |
| 1-year | 2.4 (3.6) | 5.0 (5.2) | <0.0001 |
| Satisfaction with Bariatric Surgery (%) | 85.7 | 75.1 | <0.0001 |
| AUD (%)b | 2.6 | 1.9 | 0.2089 |
BMI body mass index (calculated as kg/m2),
AUD alcohol use disorder
Figure 2.
One year postoperative excess body weight loss stratified by opioid use status. * p <0.05 compared to risk-adjusted weight loss of non-opioid users.
Figure 3.
One year psychologic outcomes stratified by opioid use status. Psych WB; Psychological Wellbeing. BodyQ; Body Image. PHQ-8; Depression. * p <0.05 compared to scores of non-opioid users.
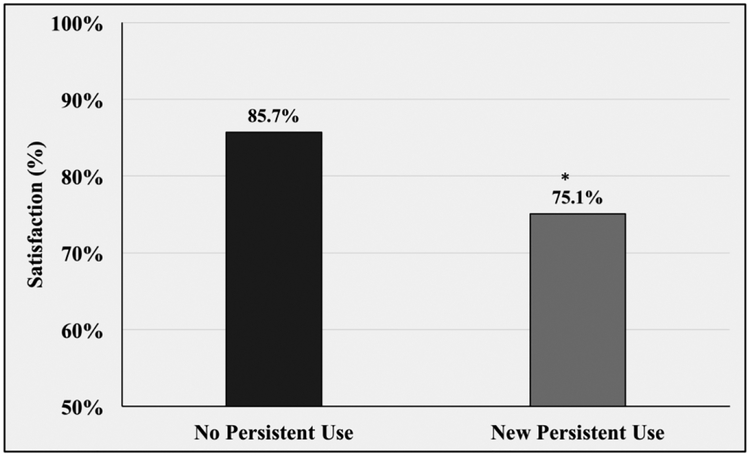
Patients who developed new persistent opioid use were also significantly less satisfied with their surgery. 75.1% of new persistent users reported overall satisfaction with their bariatric surgery compared to 85.7% satisfaction among patients without new persistent use (p<0.0001). (Figure 4)
Figure 4.
Satisfaction of bariatric surgery stratified by opioid use status. * p <0.05 compared to satisfaction of non-opioid users.
DISCUSSION
This study has two key findings. First, we found that in this large sample from a single state, 79% of patients undergoing bariatric surgery were opioid-naïve prior to surgery, and 6.3% of these patients developed new persistent opioid use. New persistent users were more likely to have various comorbidities and experience a postoperative complication, as well as more likely to be a minority, have public insurance, and not live with a spouse or significant other. While the higher rate of postoperative complications in new persistent opioid users may account for some of the increased opioid use, the majority of new persistent users did not suffer a complication. Additionally, many short-term and late postoperative complications following bariatric surgery do not cause significant pain (e.g. bleeding, renal failure, stricture) and therefore complications are unlikely to account for the development of new persistent opioid use in the majority of patients. This suggests that potential factors contributing to developing new persistent opioid use include medical factors as well as social constructs. Second, we found that new persistent use was associated with worse physiologic and psychologic outcomes. New persistent users achieved less excess body weight loss (57.6% vs 60.3%) and had less improvement in psychological wellbeing, BodyQ scores, and depression scores compared to their non-use counterparts. The overall worse outcomes in these patients highlights new persistent opioid use as a potential risk factor and marker of decreased effectiveness of bariatric surgery.
Prior studies have evaluated the prevalence of perioperative opioid use in the bariatric surgery population and reported that new persistent opioid use occurred in 4.0 – 8.8% of patients.(10–12) We report a 6.3% risk of developing new persistent opioid in previously opioid naïve patients, which is similar to these previously reported rates. While the rate of new persistent opioid use in bariatric patients is similar to that of the broader surgical population, bariatric patients may be more vulnerable to misuse and dependence given the risk of “addiction transfer”.(2) Previous work has found that bariatric surgery patients develop alcohol use disorders at disproportionately high rates compared to the general population.(23–25) “Addiction-transfer”, or exchange of one compulsive behavior for another, is recognized as a possible contributor to this maladaptive behavior, and the risk of “addiction-transfer” may confer a similar risk of misuse to patients with new persistent opioid use.(14, 26)
Perioperative outcomes in patients with preoperative opioid use have recently been evaluated. In orthopedic surgery, chronic opioid use is associated with postoperative hyperalgesia, decreased quality of life, and decreased physical function.(19, 21) In abdominopelvic surgery preoperative use is linked to increased healthcare utilization and increased odds of morbidity (1.36; 95% CI 1.04 – 1.78).(18, 20) Regarding postoperative use, studies have demonstrated that early adoption of nonmedical use of prescription opioids is associated with increased risk of abuse.(27) However, the impact of new persistent use and perioperative outcomes has not been explored. Our findings contribute to the literature by evaluating the independent association of new persistent opioid use and surgical outcomes in opioid naïve patients. Specifically, this study demonstrates that new persistent users achieve less improvement in physiologic and psychologic measures after bariatric surgery, which has not been reported in previous studies.(27) Other patient behaviors and modifiable characteristics, such as tobacco use and morbid obesity, have been linked to an increased risk of postoperative complications with interventions aimed at modifying these behaviors in the bariatric population. (28–30) As opposed to tobacco use and BMI, it is unknown if new persistent opioid use has a direct causative effect on bariatric surgical outcomes. Therefore, in addition to viewing new persistent use as a potential modifiable risk factor, it should also be considered a marker of patients at increased risk for suboptimal results following surgery.
This study has several limitations. First, we included patients from a single state and therefore the results may not be fully generalizable. However, patients in Michigan are unlikely to systematically vary from bariatric patients across the country. Second, we utilized patient-reported opioid use which may result in some inaccuracies. Specifically, we queried patients for active opioid prescriptions and may not have captured those with illicit use, therefore underestimating the risk of developing persistent use, Nevertheless, patient-reported medication use is similar to clinical practice, where we rely on patients to disclose their medications. Furthermore, recent work has demonstrated substantial agreement between medical record abstraction and patient reporting of comorbidities in urology patients, providing further support for the use of patient-reported data as a means of data collection.(31) Finally, our study does not evaluate the indication for opioid prescription or evaluate patients’ opioid consumption. It is conceivable that patients’ prescriptions are for a new medical indication and not related to their bariatric surgery. However, previous work suggests that the rate of new persistent opioid use in non-surgical patients is only 0.4%, so it is unlikely that rate of new persistent opioid use in this population is fully attributable to new pathology.(2)
Despite these limitations, our findings demonstrate that 6.3% of opioid naïve bariatric surgery patients develop iatrogenic new persistent opioid use. Notably, these patients experience less weight loss and less improvement in psychological measures compared to their non-opioid use counterparts. The full clinical significance of these differences is unknown. However, given the broad patients’ goals and motivations for pursuing bariatric surgery, these differences may serve to motivate both providers and patients to decrease opioid use and minimize exposure to the serious risks associated with opioids. As a result of this work surgeons should address postoperative opioid use in several ways. First, while understanding that pain should not be disregarded or undertreated, surgeons should follow current prescribing guidelines and actively reduce excess opioid prescribing.(32, 33) In addition, providers should develop surveillance programs that monitor postoperative medications in order to intervene promptly on patients with persistent opioid use and recognize those at increased risk of poor outcomes. Further study is needed to develop effective screening tools to identify patients at risk for new persistent opioid use. Tools specific to bariatric surgery patients may be most effective as many psychosocial factors and behaviors associated with postoperative opioid use are highly prevalent in this population.(34, 35) Finally, the findings of this study suggest surgeons must appropriately counsel patients on risks and associated outcomes of new persistent opioid use in order to emphasize the importance of appropriate pain management strategies and motivate patients to minimize opioid use.
Funding:
Margaret E. Smith receives funding from the National Institutes of Health Obesity Surgery Scientist Training Grant DK108740–02. Jay S. Lee receives funding from the National Institutes of Health National Research Service Award postdoctoral fellowship CA009672–23.
Amir A. Ghaferi receives funding from the Agency for Healthcare Research and Quality (Grant #: 5K08HS02362 and P30HS024403) and a Patient Centered Outcomes Research Institute Award (CE-1304–6596).
Footnotes
Presented in part at the Society of American Gastrointestinal and Endoscopic Surgeons 2018 Annual Meeting, Seattle, WA, April 14, 2018.
Conflicts of Interest: None
DISCLOSURES
Dr. Margaret Smith has no conflicts of interest or financial ties to disclose.
Dr. Jay Lee has no conflicts of interest or financial ties to disclose.
Aaron Bonham has no conflicts of interest or financial ties to disclose.
Dr. Oliver Varban and Dr. Jonathan Finks receive salary support from Blue Cross Blue Shield of Michigan as the Associate Directors of the Michigan Bariatric Surgery Collaborative.
Dr. Arthur Carlin receives an honorarium from Blue Cross Blue Shield of Michigan as chair of the Executive Committee of the Michigan Bariatric Surgery Collaborative.
Dr. Ghaferi receives salary support from Blue Cross Blue Shield of Michigan as the Director of the Michigan Bariatric Surgery Collaborative
REFERENCES
- 1.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Archives of internal medicine. 2012;172(5):425–30. [DOI] [PubMed] [Google Scholar]
- 2.Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA surgery. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ (Clinical research ed). 2014;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic Opioid Dependence in the United States: Are Surgeons the Gatekeepers? Annals of surgery. 2017;265(4):728–30. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Hu HM, Edelman AL, Brummett CM, Englesbe MJ, Waljee JF, et al. New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(36):4042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkmeyer NJ, Dimick JB, Share D, Hawasli A, English WJ, Genaw J, et al. Hospital complication rates with bariatric surgery in Michigan. Jama. 2010;304(4):435–42. [DOI] [PubMed] [Google Scholar]
- 7.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA surgery. 2014;149(3):275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courcoulas AP, Yanovski SZ, Bonds D, Eggerman TL, Horlick M, Staten MA, et al. Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA surgery. 2014;149(12):1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. The New England journal of medicine. 2009;361(5):445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King WC, Chen JY, Belle SH, Courcoulas AP, Dakin GF, Flum DR, et al. Use of prescribed opioids before and after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2017;13(8):1337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohanty S, Lee JS, Ross RA, Stricklen A, Carlin AM, Ghaferi AA. New Persistent Opioid Use after Bariatric Surgery. Journal of the American College of Surgeons.225(4):S123. [Google Scholar]
- 12.Raebel MA, Newcomer SR, Bayliss EA, Boudreau D, DeBar L, Elliott TE, et al. Chronic opioid use emerging after bariatric surgery. Pharmacoepidemiology and drug safety. 2014;23(12):1247–57. [DOI] [PubMed] [Google Scholar]
- 13.Ivezaj V, Saules KK, Wiedemann AA. “I didn’t see this coming.”: why are postbariatric patients in substance abuse treatment? Patients’ perceptions of etiology and future recommendations. Obesity surgery. 2012;22(8):1308–14. [DOI] [PubMed] [Google Scholar]
- 14.Yoder R, MacNeela P, Conway R, Heary C. How Do Individuals Develop Alcohol Use Disorder After Bariatric Surgery? A Grounded Theory Exploration. Obesity surgery. 2017. [DOI] [PubMed] [Google Scholar]
- 15.Bartels K, Mayes LM, Dingmann C, Bullard KJ, Hopfer CJ, Binswanger IA. Opioid Use and Storage Patterns by Patients after Hospital Discharge following Surgery. PloS one. 2016;11(1):e0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription Opioid Analgesics Commonly Unused After Surgery: A Systematic Review. JAMA surgery. 2017;152(11):1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prevention CfDCa. DrugoverdosedeathsintheUnited States hit record numbers in 2014 [updated August 20, 2017; cited 2017 Novebmer 28]. Available from: http://www.cdc.gov/drugoverdose/epidemic/.
- 18.Cron DC, Englesbe MJ, Bolton CJ, Joseph MT, Carrier KL, Moser SE, et al. Preoperative Opioid Use is Independently Associated With Increased Costs and Worse Outcomes After Major Abdominal Surgery. Annals of surgery. 2017;265(4):695–701. [DOI] [PubMed] [Google Scholar]
- 19.Pivec R, Issa K, Naziri Q, Kapadia BH, Bonutti PM, Mont MA. Opioid use prior to total hip arthroplasty leads to worse clinical outcomes. International orthopaedics. 2014;38(6):1159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waljee JF, Cron DC, Steiger RM, Zhong L, Englesbe MJ, Brummett CM. Effect of Preoperative Opioid Exposure on Healthcare Utilization and Expenditures Following Elective Abdominal Surgery. Annals of surgery. 2017;265(4):715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. The Journal of bone and joint surgery American volume. 2011;93(21):1988–93. [DOI] [PubMed] [Google Scholar]
- 22.Scally CP, Varban OA, Carlin AM, Birkmeyer JD, Dimick JB. Video Ratings of Surgical Skill and Late Outcomes of Bariatric Surgery. JAMA surgery. 2016;151(6):e160428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King WC, Chen JY, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, et al. Prevalence of alcohol use disorders before and after bariatric surgery. Jama. 2012;307(23):2516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reslan S, Saules KK, Greenwald MK, Schuh LM. Substance misuse following Roux-en-Y gastric bypass surgery. Substance use & misuse. 2014;49(4):405–17. [DOI] [PubMed] [Google Scholar]
- 25.Svensson PA, Anveden A, Romeo S, Peltonen M, Ahlin S, Burza MA, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity (Silver Spring, Md). 2013;21(12):2444–51. [DOI] [PubMed] [Google Scholar]
- 26.Bak M, Seibold-Simpson SM, Darling R. The potential for cross-addiction in post-bariatric surgery patients: Considerations for primary care nurse practitioners. Journal of the American Association of Nurse Practitioners. 2016;28(12):675–82. [DOI] [PubMed] [Google Scholar]
- 27.McCabe SE, West BT, Morales M, Cranford JA, Boyd CJ. Does early onset of non-medical use of prescription drugs predict subsequent prescription drug abuse and dependence? Results from a national study. Addiction (Abingdon, England). 2007;102(12):1920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inadomi M, Iyengar R, Fischer I, Chen X, Flagler E, Ghaferi AA. Effect of patient-reported smoking status on short-term bariatric surgery outcomes. Surgical endoscopy. 2018;32(2):720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livingston EH, Arterburn D, Schifftner TL, Henderson WG, DePalma RG. National Surgical Quality Improvement Program analysis of bariatric operations: modifiable risk factors contribute to bariatric surgical adverse outcomes. Journal of the American College of Surgeons. 2006;203(5):625–33. [DOI] [PubMed] [Google Scholar]
- 30.Moller AM, Villebro N, Pedersen T, Tonnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet (London, England). 2002;359(9301):114–7. [DOI] [PubMed] [Google Scholar]
- 31.Ye F, Moon DH, Carpenter WR, Reeve BB, Usinger DS, Green RL, et al. Comparison of Patient Report and Medical Records of Comorbidities: Results From a Population-Based Cohort of Patients With Prostate Cancer. JAMA oncology. 2017;3(8):1035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blay E Jr., Nooromid MJ, Bilimoria KY, Holl JL, Lambert B, Johnson JK, et al. Variation in post-discharge opioid prescriptions among members of a surgical team. American journal of surgery. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill MV, Stucke RS, Billmeier SE, Kelly JL, Barth RJ, Jr. Guideline for Discharge Opioid Prescriptions after Inpatient General Surgical Procedures. Journal of the American College of Surgeons. 2017. [DOI] [PubMed] [Google Scholar]
- 34.Dev R, Parsons HA, Palla S, Palmer JL, Del Fabbro E, Bruera E. Undocumented alcoholism and its correlation with tobacco and illegal drug use in advanced cancer patients. Cancer. 2011;117(19):4551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janda AM, As-Sanie S, Rajala B, Tsodikov A, Moser SE, Clauw DJ, et al. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology. 2015;122(5):1103–11. [DOI] [PubMed] [Google Scholar]