Summary
Background
Epidermolysis bullosa (EB) is a group of rare and currently incurable genetic blistering disorders. As more pathogenic driven therapies are being developed, the need for EB-specific validated outcomes measures designed for use in clinical trials is becoming important.
Objectives
We previously reported on development of an instrument for scoring clinical outcomes of research for Epidermolysis Bullosa (iscorEB), a new combined clinician and patient reported outcomes tool. We proceeded to test the reliability and construct validity of iscorEB in this study.
Methods
Observational study consisting of independent one-day assessments (6 assessors) at 2 academic hospitals. The assessments consisted of iscorEB clinician (iscorEB-c), Birmingham Epidermolysis Bullosa Severity (BEBS), and global severity assessment for physicians; and iscorEB patient (iscorEB-p), Quality of Life in Epidermolysis Bullosa (QOLEB) and Children’s Dermatology Life Quality Index (CDLQI) for patients. Construct validity and intra-class correlation coefficients (ICC) for inter-observer, intra-observer, and test-retest reliability were calculated.
Results
Thirty one patients with a mean age of 19.5 years (1.8–45.2) were included. Disease severity was mild in 42%, moderate in 29% and severe in 29% of cases. The inter-observer ICC was 0.96 for both the clinician-reported section of iscorEB-c and BEBS. The ICC for intra-observer reliability was 0.91 and 0.70 for the skin and mucosal domains of iscorEB-c, respectively. Cronbach’s alpha for iscorEB-c was 0.89. iscorEB-p’s test-retest reliability was 0.97, and Cronbach’s alpha was 0.84. The clinical score differentiated between subjects with mild, moderate and severe disease, and both clinical and patient subscores discriminated between recessive dystrophic EB and other EB subtypes.
Conclusion
iscorEB has robust reliability and construct validity, including strong ability to distinguish EB types and severities. Further studies are planned to test its responsiveness to change.
Introduction
The inherited forms of epidermolysis bullosa (EB) are a group of rare, devastating, and currently incurable genetic blistering disorders characterized by fragility of skin and mucosa. The estimated incidence and prevalence of EB in the United States are 19 per million live births and 8 per million, respectively. (1) The most recent classification system for EB includes 4 major types (simplex, junctional, dystrophic, and Kindler syndrome) determined by the location of cleavage in relation to the epidermal-dermal basement membrane zone and further refined by phenotype, inheritance pattern, the affected protein, and genetics.(2) The severity of EB depends on the subtype and ranges from limited skin involvement to a devastating, multisystem disorder with secondary complications in many organ systems and reduced life expectancy.
There is no cure or well-accepted disease modifying treatment for EB at this time. Treatment is palliative with the aims of promoting patient well-being, optimizing wound healing (3), and monitoring for and treating secondary complications (4). New therapies targeting the pathogenesis of EB are currently being studied in pre-clinical or early phase trials. These therapies have primarily focused on recessive dystrophic EB (RDEB) in which collagen VII, the main component of anchoring fibrils in the superficial dermis, is absent or significantly reduced. Correcting collagen VII deficiency may be achieved through hematopoietic progenitor cell transplantation (5,6), grafting of autologous, genetically-corrected keratinocytes and fibroblasts (7,8), or replacement of collagen VII. (9,10) While restoration of collagen VII expression can be used as a biologic endpoint for these therapies, validated and accepted measures of meaningful improvement from the clinical and patient perspectives are also needed.
We lack a robust and reliable EB-specific outcome measurement tool that could be used in clinical research. Existing instruments have significant limitations, such as design flaws and insufficient validation that restrict their widespread use in clinical trials. The Birmingham Epidermolysis Bullosa Severity score (BEBS) was created to assess EB severity and emphasizes many of the chronic and irreversible changes of EB, such as scarring. (11) BEBS is potentially less sensitive to capturing improvement due to a therapeutic intervention, and its responsiveness has not been tested. The recently published Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) separates disease activity from scarring, but its responsiveness has not been tested either.(12) In addition, both BEBS and EBDASI do not capture complications in non-skin or non-mucosal organ systems, nor do they capture patient-reported perceptions of severity. Quality of Life in Epidermolysis Bullosa (QOLEB) is an EB-specific, patient-reported quality of life instrument, but it has not been widely studied in children, who are disproportionally affected by severe forms of EB.(13) Generic dermatologic quality of life measures such as the Dermatology Life Quality Index (DLQI) (14) and Children’s Dermatology Life Quality Index (CLDQI) (15,16) do not capture dimensions specific to EB, making them less sensitive for this patient population. The DLQI and the CLDQI were used in a descriptive study of Scottish EB patients (17), but have not been used in clinical trials of therapies for EB.
The absence of a widely accepted, comprehensive, EB-specific outcome instrument is a significant unmet need in EB clinical research. The instrument for scoring clinical outcomes of research for Epidermolysis Bullosa (iscorEB) was developed with this gap in mind using a rigourous methology and international collaboration involving EB clinical experts and patients with EB that derived and refined the content of iscorEB with an emphasis on items that may change over time, particularly as a result of intervention. (18) iscorEB is unique in that it evaluates cutaneous, mucosal and other organ impact of EB and includes clinician- and patient-reported outcomes in a single instrument. Our central hypothesis for this study is that iscorEB is a reliable and valid outcome instrument that measures clinically relevant and patient-important outcomes specific to EB. Thus, the objective of this study was to evaluate the reliability and construct validity of iscorEB among care providers and patients with EB.
Methods
We conducted this study at 2 academic, tertiary institutions with specialized EB Clinics: Children’s Hospital Colorado (affiliated with the University of Colorado School of Medicine) in Aurora, CO and The Hospital for Sick Children in Toronto, Ontario (affiliated with the University of Toronto). The institutional review boards at each site reviewed and approved the study.
Study population
We recruited a convenience sample of participants from the EB clinics at each hospital and through referrals from two EB advocacy groups: the Dystrophic Epidermolysis Bullosa Research Association (www.debra.org) and the Jackson Gabriel Silver Foundation (now called Epidermolysis Bullosa Research Partnership, www.ebrp.org). Eligible subjects had a physician-verified diagnosis of EB simplex, junctional EB, or dystrophic EB based on clinical findings, biopsy, and/or genetic testing; were between 1 and 50 years old; and were physically capable of travelling to and from the study site and participating in the study activities. Subjects and families unable to read and understand English with at least moderate fluency were excluded. We recruited an equal number of pediatric (1–17 years of age) and adult (≥ 18 years of age) subjects. In addition, we planned to distribute enrolment across three levels of severity determined by the principal investigator at each site using the following definitions: mild was localized skin involvement (typically less than 5% body surface area) and no associated systemic complications, moderate was potential for generalized skin involvement and ≤ 2 non-skin manifestations or complications, and severe was generalized skin involvement and 3 or more non-skin manifestations or complications. Written informed consent was obtained from all subjects or their parents/guardians.
Study instrument
The clinician-reported section of iscorEB (iscorEB-c) has 5 domains: skin involvement, mucosal involvement, internal organ involvement, laboratory abnormalities, and complications / procedures, totalling 114 points. Items are completed based on a complete examination of the skin and mucosa and review of the medical history. The patient-reported section (iscorEB-p) contains 15 straightforward questions and covers seven domains: pain, itch, essential functions, sleep, daily activities, mood and impact. Each question ranges from 0–8 points, yielding a maximum of 120 points.
Study Procedures
Physician assessments
Six physicians with a range of perspectives in the care of EB participated in this study: ALB, IL-C, AWL, and EP are pediatric dermatologists, JAF is a pediatrician specializing in children with special medical needs, and JT is a pediatric bone marrow transplant physician using this procedure to treat EB. Four of the six physicians participated in each evaluation day. Training meetings to review the format of the sessions and the use of the outcomes instruments were held prior to each evaluation.
Before the first clinical assessment, each subject was placed in a private room, removed his or her clothing and bandages, and standardized photographs were taken. The first assessment included review of the subject’s medical history and recent laboratory studies and interventions, as well as a complete examination of the skin and mucosa. Following a break, the second assessment consisted of an examination of the skin and mucosa only. In the case of 6 subjects with RDEB, photographs were used for the second skin assessment, as these subjects could not tolerate remaining unbandaged for an extended period.
Physicians examined subjects independently or in pairs but were not allowed to discuss the examination findings or historical data. Each physician completed iscorEB-c and BEBS independently during their first assessment. In addition, each physician rated the subject’s global disease severity (Physician Global Assessment) as mild, moderate, or severe based on his or her overall clinical impression. For the second assessment, subjects were evaluated in a different order than the first round. Only the skin and mucosal domains of iscorEB-c (which rely on clinical judgment) were completed after the second assessment.
Patient-reported outcomes
Prior to the first clinical assessment, each subject completed iscorEB-p. In addition, subjects 18 years and older completed QOLEB, while subjects 4–17 years old completed the CDLQI. Subjects 3–7 years of age were allowed assistance from an adult to complete the CDLQI. At the end of the day, subjects completed iscorEB-p again.
Statistical analysis
Based on feasibility, we initially proposed a sample size of 40 subjects and 5 physicians. Assuming an ICC of 0.7, this size allowed 95% confidence intervals of ± 0.12 for inter-observer reliability and ± 0.16 or smaller for test-retest reliability. Our final size of 31 subjects and 6 physicians yielded 95% confidence intervals of ± 0.15 for inter-observer reliability and ± 0.20 for test-retest reliability, which we felt were acceptable levels of precision.
To assess inter-observer, intra-observer, and test-retest reliability, we used a repeated-measures, random effects ANOVA model to calculate the ICC. The models contained an overall mean, random variation between subjects, and residual errors; the latter two were used to calculate the correlations. The domains and total scores of iscorEB-c were compared between physicians to determine inter-observer reliability. Correlation was estimated as the ratio of the variance between subject scores to the total variance in the sample. The skin and mucosal domains of iscorEB-c from repeat clinical assessments by the same physician were used to determine intra-observer reliability. Repeat iscorEB-p assessments were used to calculate test-retest reliability.
To assess internal consistency of iscorEB-c and iscorEB-p, we calculated the Cronbach’s alpha. To test the convergent validity of iscorEB, domains from iscorEB-c were compared to the BEBS and iscorEB-p was compared to QOLEB or CDLQI using Spearman’s correlation.
Finally, to test if iscorEB scores could discriminate between different subtypes and severities of EB, we hypothesized that subjects with RDEB would have higher iscorEB scores than those with other EB subtypes and that subjects with more severe EB would have higher iscorEB scores. A repeated-measures, mixed model using linear contrasts with corresponding F-statistics was used to compare iscorEB-c scores, domain subscores, and iscorEB-p scores across groups defined by the median Physician Global Assessment score and across subjects with RDEB versus other disease subtypes.
All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 31 subjects participated in the study, 17 at the Hospital for Sick Children and 14 at the University of Colorado. Subject characteristics are shown in Table 1. ALB, EP and JT each assessed all 31 subjects, while IL-C and AWL assessed 17 subjects, and JF assessed 14 subjects. The mean time between the first and second set of physician assessments was 3.2±1.0 hours (range 2.1–5.1 hours), and the mean time between completions of the patient questionnaires was 3.4±0.5 hours (range 2.5–4 hours).
Table 1.
Demographic and Clinical Characteristics
| N (%) | |
|---|---|
| Age – yr (range) | 19.5±13.4 (1.8–45.2) |
| Children (< 18 years old) | 16 (51.6) |
| Male sex | 17 (54.8) |
| Race /ethnicity | |
| White / Non-Hispanic | 19 (61.3) |
| White / Hispanic | 8 (25.8) |
| Black | 1 (3.2) |
| Asian | 3 (9.7) |
| EB Type, Subtype | |
| Simplex, Generalized | 2 (6.5) |
| Simplex, Localized | 8 (25.8) |
| Dominant Dystrophic | 5 (16.1) |
| Recessive Dystrophic, generalized | 7 (22.6) |
| intermediate | 9 (29.0) |
| Recessive Dystrophic,generalized severe | |
| Disease severity, based on screening | |
| Mild | 11 (35.5) |
| Moderate | 10 (32.3) |
| Severe | 10 (32.3) |
| Physician Global Assessment | |
| Mild | 13 (41.9) |
| Moderate | 9 (29.0) |
| Severe | 9 (29.0) |
The iscorEB scores, intra-class correlation coefficients (ICC) for inter-observer, intra-observer, and test-retest reliability, and Cronbach’s alpha for this sample are reported in Table 2. The inter-observer ICC was 0.96 for both the clinician-reported section of iscorEB (iscorEB-c) and BEBS, showing comparable reliability between investigators on the two instruments. The ICC for intra-observer reliability was 0.91 and 0.70 for the skin and mucosal domains of iscorEB-c, respectively. Cronbach’s alpha for the clinical portion of iscorEB was 0.89, again comparable to BEBS. In addition, domain scores ranged from 0.85–0.88, suggesting each clinical domain is relevant to the instrument. For the patient-reported section of iscorEB (iscorEB-p), the ICC for test-retest reliability was 0.97, and Cronbach’s alpha was acceptable at 0.84, which was slightly lower than that for QOLEB but higher than that for CDLQI.
Table 2.
Summary of assessments, their reliability and internal consistency
| Instrument or subscore (reference range) | Mean±SD | Range | Inter-observer reliability (ICC) | Intra-observer or test-retest reliability (ICC) | Cronbach’s alpha |
|---|---|---|---|---|---|
| iscorEB clinician (0–114) | 11.3±10.9 | 0–47.1 | 0.96 | 0.89 | |
| Skin (0–60) | 4.1±5.4 | 0–26.1 | 0.88 | 0.91 | 0.85 |
| Mucosal (0–20) | 3.2±2.0 | 0–8 | 0.82 | 0.70 | 0.88 |
| Internal organ (0–12) | 1.6±1.7 | 0–7 | 0.97 | 0.88 | |
| Laboratory abnormalities (0–11) | 1.5±2.1 | 0–7 | 0.98 | 0.86 | |
| Complications / procedures (0–11) | 0.9±1.4 | 0–5 | 0.91 | 0.88 | |
| BEBS (0–100) | 13.4±14.3 | 0–59 | 0.96 | 0.89 | |
| iscorEB patient (0–120) |
32.4±16.2 | 0–70 | - | 0.97 | 0.84 |
| QOLEB (0–75), N=15 | 16.3±8.8 | 2–33 | - | 0.91 | |
| CDLQI (0–30), N=15 | 11.4±4.9 | 2–20 | - | 0.67 |
SD-standard deviation
BEBS- Epidermolysis Bullosa Severity score
QOLEB- Quality of Life Epidermolysis Bullosa
CDLQI- Childhood Dermatology Life Quality Index
The correlation of iscorEB with similar instruments is shown in Table 3. Overall, iscorEB-c correlated well with BEBS. The internal organ, laboratory abnormalities and complications domains correlated less well than skin and mucosal involvement with the BEBS total score. iscorEB-p had a strong correlation with QOLEB, while the correlation with CDLQI was reasonable but less robust. The overall correlation of iscorEB-c and iscorEB-p was reasonable (0.67).
Table 3.
Convergent validity of iscorEB. Comparison with similar instruments and between the clinical and patient portions of the instrument.
| iscorEB portion or domain | Comparison (N=31 unless specified otherwise) Spearman’s rho (p-value) |
|---|---|
| iscorEB-c versus BEBS (total score) | |
| iscorEB-c total score | 0.94 (<.0001) |
| Skin | 0.90 (<.0001) |
| Mucosal | 0.86 (<.0001) |
| Internal organ | 0.73 (<.0001) |
| Laboratory abnormalities | 0.83 (<.0001) |
| Complications / procedures | 0.77 (<.0001) |
| iscorEB-p versus QOLEB (N=15) | 0.91 (<.0001) |
| iscorEB-p versus CDLQI (N=15) | 0.76 (0.0009) |
| iscorEB-c total score versus iscorEB-p | 0.67 (<.0001) |
| Skin | 0.61 (.0003) |
| Mucosal | 0.77 (<.0001) |
| Internal organ | 0.33 (.067) |
| Laboratory abnormalities | 0.63 (.0002) |
| Complications / procedures | 0.67 (<.0001) |
BEBS- Epidermolysis Bullosa Severity score
QOLEB- Quality of Life Epidermolysis Bullosa
CDLQI- Childhood Dermatology Life Quality Index
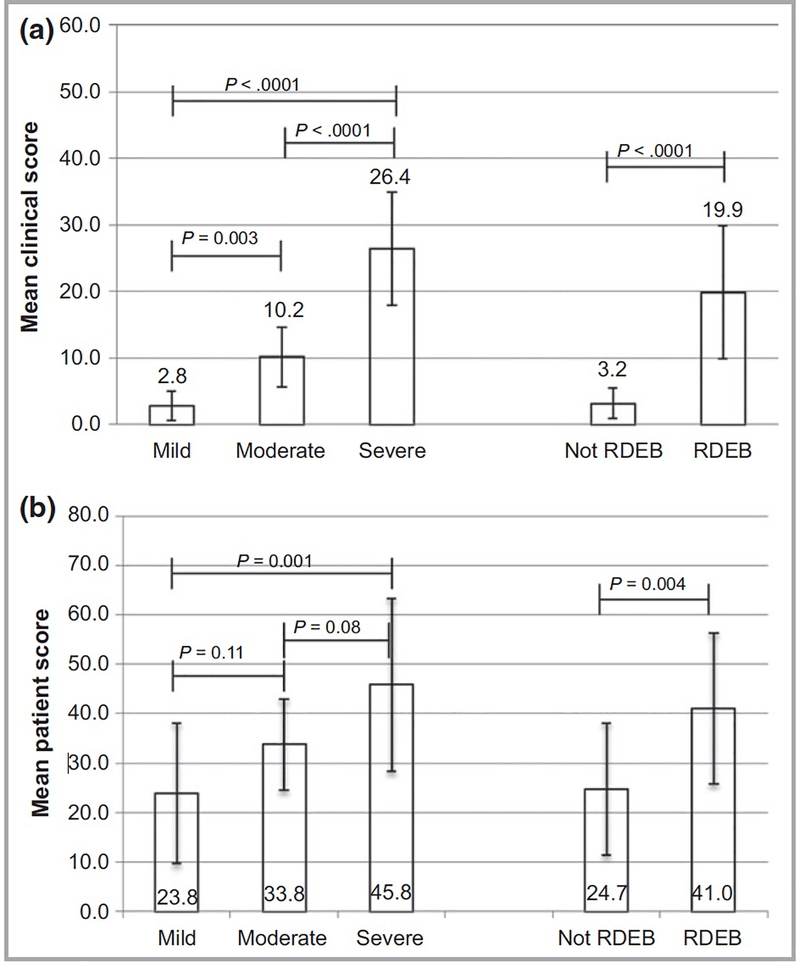
Overall, iscorEB-c demonstrated good discriminant ability between subjects in this cohort with mild, moderate and severe EB and between subjects with RDEB versus other forms of EB (Figure 1A). Each clinical domain could distinguish between subjects with mild versus severe disease and RDEB versus other forms of EB, but differentiation for the mucosal, internal organ, and complications/procedures domains was not statistically significant between subjects with moderate to severe severity for the mucosal domain or between mild to moderate severity for the internal organ and complications/procedures domains. Likewise, iscorEB-p could distinguish between subjects with mild versus severe disease and RDEB versus other forms of EB (Figure 1B), but differentiation between mild to moderate and moderate to severe disease was not statistically significant. For subjects with RDEB, iscorEB-c correlated with age (r=0.51, p<.001). Age did not correlate with iscorEB-c in subjects who did not have RDEB nor with iscorEB-p in either disease subgroup.
Figure 1. Discriminant validity of iscorEB-c and iscorEB-p across disease severity and subtype.
Mean iscorEB-c (A) and iscorEB-p (B) score are shown for disease severity and RDEB versus other disease subtypes. For disease severity overall, F2,28=56.3 (p<.0001) for iscorEB-c, and F2,28=6.51 (p=0.005) for iscorEB-p. Error bars represent standard deviations.
DISCUSSION
Ideal outcome instruments for use in clinical trials of treatments for EB should be EB-specific, able to assess clinically-relevant and patient-important signs and symptoms that are likely to change due to intervention, non-invasive, easy-to-use, and complementary to biologic markers (e.g. collagen VII expression in the case of RDEB).
In this study, we have demonstrated that iscorEB, which combines clinical and patient-reported outcomes, is a reliable and valid outcomes instrument that satisfies many of these characteristics. The novelty of our tool is that combines the clinician- and patient-reported outcomes into a composite tool that could be used as a comprehensive score or as subscores. The combined score decreases the need for using different scoring systems in a clinical trial. Furthermore, we documented that patients with mild forms of EB have very low clinician scores (mean-2.84, 2.5% of iscorEB-c) but moderate range patient scores (mean-23.85, 20% of iscorEB-p), emphasizing that a clinician-only perspective minimizes the disease severity in the milder spectrum of disease.
In terms of inter-observer reliability, the clinical portion of iscorEB compared favorably with BEBS. Our team of assessors came from both dermatologic and non-dermatologic backgrounds, showing that a range of physicians who care for a patient with EB can utilize these outcomes instruments to assess EB patients. This derives from our prior work on the development of the score which emphasized that items being part of the clinician portion should be specific, descriptive and easy to measure by a non-specialist (e.g not necessary to have an ophthalmologist score the eye changes).(18) In this study, only the skin and mucosal portions of iscorEB-c were repeated to test intra-observer reliability, as these are the only two parts of the assessments that rely on clinical judgment. The ICCs for these domains were 0.91 and 0.70, respectively. While acceptable, the lower ICC for mucosal involvement suggests this domain could be further refined. Our investigators remarked that measuring mouth opening was more difficult than expected, and the scale was more suited to an adult’s mouth than a child’s.
Internal reliability (Cronbach’s alpha) for iscorEB-c, its domains, and iscorEB-p was high, showing that each section of the instrument contributes to the score. Within the mucosal, internal organ, and laboratory domains of iscorEB-c, certain items performed less well, either due to low rates of endorsement or low rates of response, and will be re-evaluated further as we refine the instrument. For example, signs and symptoms of airway involvement were assessed in each subject, but it was extremely rare to have a score higher than zero due to lack of findings. However, the presence of these findings, although uncommon, would be clinically significant and likely to contribute to disease severity. Thus, we plan to keep these items in the instrument. Similarly, although many subjects did not have laboratory studies that could be evaluated, we feel these items are relevant to disease severity and plan to keep them in the instrument. In the context of a clinical trial these items are likely to be done consistently, therefore it is important to keep them as part of the tool.
We compared iscorEB-c with BEBS and found a good correlation of both the total score and domain scores with the BEBS total score, supporting that iscorEB-c is assessing relevant disease manifestations. iscorEB-p also compared favorably with QOLEB and reasonably well with the CDLQI. Except for internal organ involvement, iscorEB-c and iscorEB-p showed a reasonable correlation.
In our study population, iscorEB-c demonstrated good ability to discriminate between patients with mild, moderate and severe EB. These results are similar to those found during pilot testing of iscorEB as previously reported.(18) In addition, iscorEB-c is able to distinguish between RDEB and other disease subtypes as a whole. We chose not to evaluate these disease subtypes separately in our analyses due to the small number of patients in each group. A similar trend was seen for the iscorEB-p, although the differences between mild to moderate and moderate to severe EB were not statistically significant. To our knowledge, a formal definition of clinically meaningful disease change for EB does not exist. Our data suggest that a change of 16 points in the iscorEB-c score (going from moderate to severe or vice-versa) is likely to be clinically significant. Additional studies in a larger patient population are needed to test this hypothesis.
iscorEB was designed with the intent to be used in clinical trials. Thus, it emphasizes clinical characteristics that will likely change because of intervention. The skin involvement domain assesses active blisters, erosions and associated crusts / scabs in addition to chronic wounds and infection. The score reflects the type of lesions, as well as the percentage of involvement for an affected region; each region of the body is weighted as in the Psoriasis Activity Severity Index (PASI).(19) As a child’s proportions are different from that of an adult, we plan to include a pediatric scale in the next version of iscorEB. The skin component of BEBS also evaluates the percentage of the body affected but includes “healing skin, erythema, and atrophic scarring” in the assessment. Scarring is unlikely to change, making this assessment less useful for clinical trials. The newly described EBDASI distinguishes disease activity (blisters and erosions) from chronic changes. Active lesions are assessed based on the number and size of lesions in a body region. We did not compare iscorEB to EBDASI as EBDASI had not been published when our study was planned. Both BEBS and EBDASI do not capture complications in non-skin or non-mucosal organ systems. Our prior work with item generation and expert consensus showed that including these areas was important. The internal organ, laboratory abnormalities, and complications / procedures domains of iscorEB comprise about 30% of the total clinical score, and our Cronbach’s alpha values support their contributions to the instrument.
Both the CDLQI and EBQOL are measures of quality of life, while iscorEB-p was designed to assess patient perceptions of aspects of their disease that may change due to intervention. There are notable differences in the content of these instruments that reflect this intent. For instance, EBQOL includes one question about pain, while iscorEB-p assesses overall pain as well as skin, mouth, eye, and bone/joint pain. iscorEB-p also assesses itch, which has been found to be a significant concern of patients with EB.(20) iscorEB-p does not emphasize independence, relationships, finances, and emotional complexities (other than mood) like EBQOL. We do not view these differences as a deficiency of iscorEB-p. Rather, they simply highlight our emphasis on assessing outcomes that may change due to intervention and that are perceived to be important by patients affected by EB.
This study must be considered in the context of several limitations. The sample size was smaller than initially calculated; however, the p-values suggest that data is reliable. Many of the subjects, particularly those with mild or moderate disease, did not have data for certain items due to lack of severity or information, thus creating a floor effect. Some of these items (e.g. scoring of the mouth opening) were modified in the new version of the instrument. Others, such as missing laboratory information, are less likely to be absent in the context of a trial, thus minimizing this limitation. Certain domains, particularly, the internal organ, complications / procedures need to be further assessed in a larger group of patients. Despite our efforts, participants with certain EB subtypes such as Kindler syndrome and junctional EB could not be recruited due to rarity of their condition. However, we felt that the spread of disease severity will allow generalizing our findings to other suptypes that are not included but may be easily classified into one of the severity categories. There was no ceiling effect observed; the mean cohort iscorEB was a small portion of the maximum values.
In conclusion, measuring the activity and severity of EB is a challenge due to its rarity, clinical heterogeneity, and wide range of affected ages. We feel iscorEB has several advantages over BEBS and EBDASI. In this study, we have built upon our prior work that supported the content validity of iscorEB. We have demonstrated that iscorEB has good psychometric properties such as reliability and construct validity, including strong ability to differentiate between varying severities of EB. Based on this data, the instrument has been updated to include a different multiplier for skin scoring in children < 8 years, validated scoring for mouth opening, adding different weight to inflammation and squamous cell carcinoma. Our next steps further testing of the modified instrument in a broader range of EB subtypes and measuring responsiveness in longitudinal cohorts and intervention trials (stem cell transplant).
Bulleted Statements.
What is known?
Outcome measure testing EB severity are limited.
There is a high need for valid tools to assess EB severity in a context of a clinical trials.
What does the study add?
instrument for scoring clinical outcomes of research for Epidermolysis Bullosa (iscorEB), is a new combined clinician and patient reported outcomes tool.
iscorEB has robust reliability and construct validity, including strong ability to distinguish EB types and severities.
ACKNOWLEDGEMENTS
The authors acknowledge Hanna Fadzeyeva, Heather M. Blondin and Jennifer L. Sternberg, research assistants, for help with the study procedures and Drs. Lori Crane and Allan Prochazka for thoughtful review of this manuscript.
Funding: Epidermolysis Bullosa Research Partnership and the Epidermolysis Bullosa Medical Research Fund. The study was also supported by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082–04. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Abbreviations used
- BEBS
Birmingham Epidermolysis Bullosa Severity
- CDLQI
Children’s Dermatology Life Quality Index
- EB
epidermolysis bullosa
- EBDASI
Epidermolysis Bullosa Disease Activity and Scarring Index
- ICC
intra-class correlation coefficient
- iscorEB
instrument for scoring outcomes of research for epidermolysis bullosa
- iscorEB-c
instrument for scoring outcomes of research for epidermolysis bullosa – clinician portion
- iscorEB-p
instrument for scoring outcomes of research for epidermolysis bullosa – patient portion
- QOLEB
Quality of Life in Epidermolysis Bullosa
- RDEB
recessive dystrophic epidermolysis bullosa
Footnotes
Conflicts of interest: None Declared
References
- 1.Fine J-D. Inherited epidermolysis bullosa. Orphanet J Rare Dis. BioMed Central; 2010. May 28;5(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fine J-D, Bruckenr-Tuderman L, Eady RA et al. Inherited epidermolysis bullosa: Updated recommendations on diagnosis and classification. Journal of American Dermatology. Elsevier Inc; 2014. 70:1103–26. Elena, Bruckner is not spelled correctly here. [DOI] [PubMed] [Google Scholar]
- 3.Pope E, Lara-Corrales I, Mellerio J, et al. A consensus approach to wound care in epidermolysis bullosa. Journal of American Dermatology. Elsevier Inc; 2012. March 1;67(5):904–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellerio JE, Weiner M, Denyer JE, Pillay EI, LUCKY AW, Bruckner A, et al. Medical management of epidermolysis bullosa: Proceedings of the IInd International Symposium on Epidermolysis Bullosa, Santiago, Chile, 2005. International Journal of Dermatology. Blackwell Publishing Ltd; 2007. August 1;46(8):795–800. [DOI] [PubMed] [Google Scholar]
- 5.Tolar J, Wagner JE. Allogeneic blood and bone marrow cells for the treatment of severe epidermolysis bullosa: repair of the extracellular matrix. The Lancet. 2013. October;382(9899):1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, Keene DR, Woodley DT, et al. Bone Marrow Transplantation for Recessive Dystrophic Epidermolysis Bullosa. N Engl J Med. 2010. August 12;363(7):629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siprashvili Z, Nguyen NT, Bezchinsky MY, Marinkovich MP, Lane AT, Khavari PA. Long-Term Type VII Collagen Restoration to Human Epidermolysis Bullosa Skin Tissue. Human Gene Therapy. 2010. October;21(10):1299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siprashvili Z, Nguyen NT, Gorell ES, Loutit K, Khuu P, Furukawa LK, et al. Safety and Wound Outcomes Following Genetically Corrected Autologous Epidermal Grafts in Patients With Recessive Dystrophic Epidermolysis Bullosa. JAMA. 2016. November 1;316(17):1808–14. [DOI] [PubMed] [Google Scholar]
- 9.Woodley DT, Keene DR, Atha T, Huang Y, Lipman K, Li W, et al. Injection of recombinant human type VII collagen restores collagen function in dystrophic epidermolysis bullosa. Nat Med. 2004. July;10(7):693–5. [DOI] [PubMed] [Google Scholar]
- 10.Woodley DT, Wang X, Amir M, Hwang B, Remington J, Hou Y, et al. Intravenously injected recombinant human type VII collagen homes to skin wounds and restores skin integrity of dystrophic epidermolysis bullosa. Journal of Investigative Dermatology. 2013. July;133(7):1910–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss C, Wong A, Davies P. The Birmingham Epidermolysis Bullosa Severity score: development and validation. Br J Dermatol. 2009. May;160(5):1057–65. [DOI] [PubMed] [Google Scholar]
- 12.Hons I et al. Development, reliability, and validity of a novel Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI). Journal of American Dermatology. Elsevier Inc; 2014. January 1;70(1):89–97.e13. [DOI] [PubMed] [Google Scholar]
- 13.Frew JW, Martin LK, Nijsten T, Murrell DF. Quality of life evaluation in epidermolysis bullosa (EB) through the development of the QOLEB questionnaire: an EB-specific quality of life instrument. Br J Dermatol. Blackwell Publishing Ltd; 2009. December;161(6):1323–30. [DOI] [PubMed] [Google Scholar]
- 14.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clinical and Experimental Dermatology. 1994. May;19(3):210–6. [DOI] [PubMed] [Google Scholar]
- 15.Holme SA, Man I, Sharpe JL, Dykes PJ, Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003. February;148(2):285–90. [DOI] [PubMed] [Google Scholar]
- 16.Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995. June;132(6):942–9. [DOI] [PubMed] [Google Scholar]
- 17.Horn HM, Tidman MJ. Quality of life in epidermolysis bullosa. Clinical and Experimental Dermatology. 2002. November;27(8):707–10. [DOI] [PubMed] [Google Scholar]
- 18.Schwieger-Briel A, Chakkittakandiyil A, Lara-Corrales I, Aujla N, Lane AT, LUCKY AW, et al. Instrument for scoring clinical outcome of research for epidermolysis bullosa: a consensus-generated clinical research tool. Pediatric Dermatology. 3rd ed. 2015. January;32(1):41–52. [DOI] [PubMed] [Google Scholar]
- 19.Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–44. [DOI] [PubMed] [Google Scholar]
- 20.Danial C, Adeduntan R, Gorell ES, Lucky AW, Paller AS, Bruckner AL, et al. Evaluation of Treatments for Pruritus in Epidermolysis Bullosa. Pediatric Dermatology. 2015. September;32(5):628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]