Abstract
Bacteria inhabiting the gut of insects provide many benefits to their hosts, such as aiding in food digestion, reproduction, and immunity, tissue homeostasis, adaptation to environment and resistance to pathogen and pesticides. The cowpea beetle, Callosobruchus maculatus, is a serious cosmopolitan pest of pulses. This beetle has lent itself as a guinea pig for several ecological studies. It harbors a consortium of bacterial communities in its gut, but the evidence for their role in its physiology is fragmentary. In this work, we hypothesized that gut microbiota mediates C. maculatus resistance to dichlorvos (DDVP or O,O-dimethyl O-2,2-dichlorovinylphosphate) and represent the target of Lippia adoensis (Gambian Tea Bush) essential oil (EO). Symbiotic and aposymbiotic beetles were exposed to artificial cowpea seeds earlier treated with DDVP or EO. Adult mortality and changes in gut bacterial community composition and abundance were examined at F1 and F5 generations. The susceptibility of experimental beetles to DDVP was significantly affected by their symbiotic status. The adult mortality decreased across generations in DDVP treatments, and remained significantly higher in aposymbiotic groups. In EO treatments, the mortality was consistent irrespective of symbiotic status and experimental generations. When compared to DDVP and the Control, EO treatments had significantly lower bacterial richness and diversity, as well as lower abundance of Proteobacteria, Firmicutes, and Bacteroidetes. These results support our hypothesis and describe the responses of gut microbial communities to pesticide treatments. This could be of interest for developing new management strategies of this pest.
Subject terms: Symbiosis, Microbiome
Introduction
Insects are associated with bacteria which colonize their body, cells and guts1 and contribute to their fitness in a variety of ways2–4. Bacteria play vital roles in host fitness and their interaction has greatly impacted the adaptation of host insects in their ecological niches5,6. For example, recent studies showed that gut endosymbionts contribute to host nutrition7,8, modulate host foraging behavior9 and enhance host resistance to entomophages10, and entomopathogens11.
Insect pest resistance is one of the major problems that characterize the use of synthetic insecticides. One of the many ways insects develop resistance to pesticides is through metabolic means such as detoxification. Generally, insects acquire resistance to pesticides through the expression (up-regulation or down-regulation) of detoxification enzymes12–14. The most commonly described are cytochrome P450 mono-oxygenase, glutathione-S-transferases and carboxylases15,16, which degrade xenobiotics into water soluble components easily excreted by the insects17.
Over the past decades, intestinal bacteria prompted interests for their prominent role in the process of detoxification which confer resistance in insects7,18. For example, Kikuchi et al.19 reported that bacteria of the genus Burkholderia impart protection against organophosphorus pesticides in stinkbugs. Intestinal bacteria from the fifth instars larvae of Spodoptera frugiperda were shown to degrade synthetic insecticides lambda-cyhalothrin, deltamethrin, chlorpyrifos ethyl, spinosad and lufenuron7.
The cowpea beetle, Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae), is the most destructive pest of stored cowpea worldwide20. This pest infests cowpea from the field before harvest and causes substantial damages to the stored seeds, owing to its short biological cycle (25–28 days) and higher fecundity rates21. The adults are not harmful as they do not require any food or water along their lifespan (~2 weeks)22,23, but mate multiple times to produce eggs and sustain their progeny24,25. The adults are ready for mating within 24–36 hours post emergence, and search for oviposition substrate (seeds)22. The larvae bore into the seeds’ endosperm, undergo a series of molts26 and cause considerable quantitative and qualitative losses in the storage. Moreover, this pest is reported to harbor rich and diverse bacterial communities in their guts27,28 which can play a role in host resistance and adaptation to pesticides29. However, studies on how gut bacterial communities structure and composition are affected when C. maculatus is exposed to pesticides across multiple generations are still fragmentary.
Dichlorvos (O,O-dimethyl O-2,2-dichlorovinylphosphate or DDVP) (Fig. 1) is one of the most popular organophosphate (OP) insecticides widely used to control C. maculatus in developing nations, such as Cameroon and Nigeria20,30. However, the repeated use of this OP has led to an increase in its level in several ecosystems and development of resistance in C. maculatus populations31.
Figure 1.
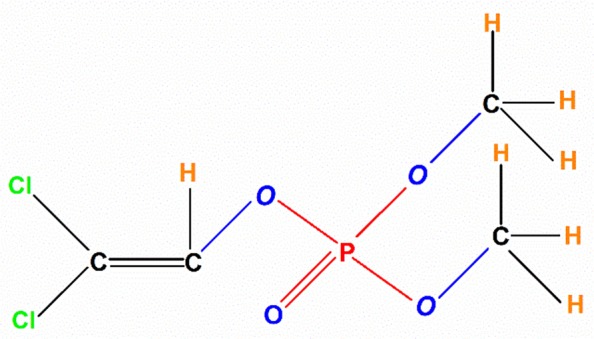
Chemical structure of dichlorvos (O,O-dimethyl,O-2,2-dichlorovinylhosphate).
Essential oils (EOs) are secondary metabolites produced by aromatic plants to protect themselves from herbivorous insects32,33. One of such aromatic plant is Lippia adoensis Hochst, commonly known as “Gambian Tea Bush”, “Bush Tea”, “Healer Herb” or “Butter Herb”34,35. It is known locally as “Ligi or Gossolderi” (in Fulfulde language) or “Fever Tea” in northern Cameroon. Lippia adoensis EO is reported previously to possess a variety of chemical constituents, mostly monoterpenes (supplementary), which confer on them a broad spectrum of insecticidal activities36. EOs are less toxic to the environment (biodegradable), humans and non-target organisms and their chemical constituents have multiple modes of actions on insects37. They are also reported to target the gut microbiome and suppress their contribution in the establishment of resistance in insects38,39.
In this study, we examine the hypothesis that gut microbial communities could mediate and/or sustain C. maculatus resistance to DDVP. In contrast, L. adoensis EO could target gut bacterial communities (including those that are resistant to DDVP) by disrupting their activities in order to enhance the susceptibility of the beetle to the toxin. We produced aposymbiotic beetles from the larval stage (as adult does not feed) by inoculating two antibiotics (Ciprofloxacin and Gentamycin) in artificial cowpea seeds on which normal eggs were allowed to develop. Likewise, the experimental seeds were produced by applying DDVP or EO on artificial cowpea seeds, to which, symbiotic and aposymbiotic beetles were exposed across multiple generations. The effects of this long term exposure to both pesticides on adult mortality and on the structure, composition and diversity of gut microbiota were assessed at first and fifth generation.
Results
Adult mortality
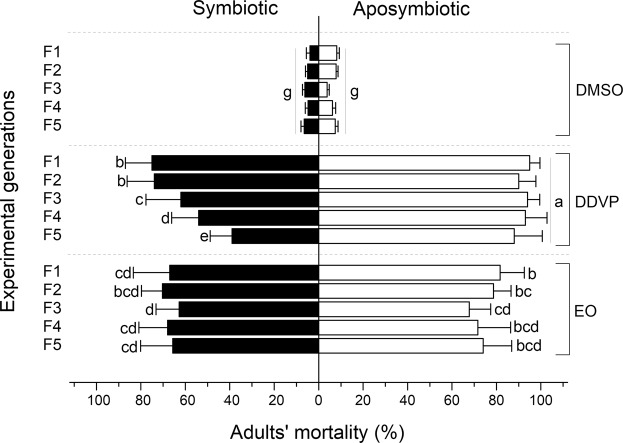
The susceptibility of experimental beetles was significantly affected by symbiotic status (Ordinary Least Squares Regression Model, F = 95.952; df = 1 r2 = 0.988; t = 3.936; P < 0.0001), pesticides used (F = 191.036; df = 2; r2 = 0.969; t = 4.598; P < 0.0001) and by experimental generations (F = 8.531; df = 4; r2 = 0.895; t = 2.751; P < 0.0001). However, the susceptibility of aposymbiotic beetles treated with DDVP did not vary significantly across generations (F = 94.95; df = 2; r2 = 0.673; t = 3.481; P = 0.148) (Fig. 2). The symbiotic status and experimental generations did not affect the susceptibility of beetles treated with DMSO only (control) (F = 28.894; df = 1, 4; r2 = 0.776; t = 2.027; P = 0.282) as very low and similar mortality was recorded between the symbiotic and aposymbiotic beetles across generations (Fig. 2).
Figure 2.
Variation in the susceptibility of symbiotic and aposymbiotic beetles over five generations. Means with different letters between generations of each pesticide treatment are significantly different after comparison with parametric New Duncan’s Multiple Range Test at p = 0.05.
Overall, aposymbiotic beetles were the most susceptible populations to DDVP and L. adoensis EO, compared to symbiotic ones (F = 350.245; df = 1; P < 0.0001 and F = 19.245; df = 1; P < 0.0001) (Fig. 2). The mortality of symbiotic beetles significantly decreased from the third generation in DDVP treatments (ANOVA, F = 20.382; df = 4; P < 0.0001), while it remained higher but consistent across generations in aposymbiotic beetles (F = 65.34; df = 4; P = 0.667) (Fig. 2). The successive generations did not significantly affect the susceptibility of C. maculatus to L. adoensis EO (F = 13.302; df = 4; P = 0.5058 and F = 13.302; df = 4; P = 0.274 for symbiotic and aposymbiotic beetles, respectively) (Fig. 2).
Diversity of operational taxonomic units (OTUs)
A total of 63,709 raw reads were generated from all treatments (Control, DDVP and EO) at F1 and F5 generations (35,069 and 28,640 reads, respectively). After quality trimming, filtering and downstream analyses, 55,620 high quality sequences were obtained (31,328 and 24,292 from F1 and F5, respectively) and generated on average 194 ± 69 OTUs (205 ± 37 and 183 ± 100 OTUs from F1 and F5, respectively). Experimental generations did not significantly affect the OTUs composition of the Control and DDVP treated samples (Ordinary Least Squares Regression Model, F = 0.137; r2 = 0.86; t = 0.688; df = 1; P = 0.73 and F = 54.16; r2 = 0.86; t = 0.688; df = 1; P = 0.26, respectively) (Table 1). However, the number of OTUs from EO treated samples varied significantly across generations (Ordinary Least Squares Regression Model, F = 27.021; r2 = 0.939; t = 1.73; df = 1; P = 0.007), and was significantly reduced compared to the Control and DDVP treatments (ANOVA, F = 7.078; df = 2; P < 0.001 and F = 7.078; df = 2; P < 0.0001, respectively) (Table 1). There was a non-significant increase of OTUs number at F5 in DDVP treatments compared to their amount at F1 (ANOVA, F = 7.82; df = 1; P = 0.056) (Table 1).
Table 1.
Richness and diversity parameters of gut microbiome from C. maculatus as affected by DDVP and EO.
| Generations | F1 | F5 | ||||
|---|---|---|---|---|---|---|
| Parameters | Control | DDVP | EO | Control | DDVP | EO |
| Coverage (%) | 77.08 ± 2.9b | 71.96 ± 1.56bc | 87.38 ± 2.19a | 68.4 ± 1.32c | 72.08 ± 2.24bc | 89.42 ± 1.54a |
| OTUs1 | 74 ± 4a | 77 ± 5a | 54 ± 6b | 76 ± 4a | 84 ± 5a | 22 ± 3c |
| Chao1 | 7.39 ± 1ab | 7.47 ± 0.04a | 5.93 ± 0.15c | 6.87 ± 0.18b | 7.18 ± 0.1ab | 4.47 ± 0.3d |
| Shannon2 | 4.78 ± 0.13a | 4.98 ± 0.1a | 3.58 ± 0.3b | 4.61 ± 0.14a | 4.56 ± 0.15a | 2.47 ± 0.11c |
| Simpson2 | 0.92 ± 0.02a | 0.94 ± 0.01a | 0.67 ± 0.03c | 0.82 ± 0.03b | 0.76 ± 0.04d | 0.57 ± 0.03d |
| IL (kb)3 | 1.3–1.5 | 1.3–1.5 | 1.3–1.5 | 1.3–1.5 | 1.3–1.5 | 1.3–1.5 |
| Cutoffs | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
Different letters (a, b, c, d) within rows are statistically different after parametric New Duncan’s Multiple Range Test at P = 0.05. 1The operational taxonomic units (OTUs) were defined with 3% dissimilarity level; 2The richness estimator (Chao1) and diversity indices (Shannon and Simpson) were calculated according to Good’s method72 and the mothur program, respectively; 3Isoform length (kb). Each datum represents the Mean ± SE of 3 replicates.
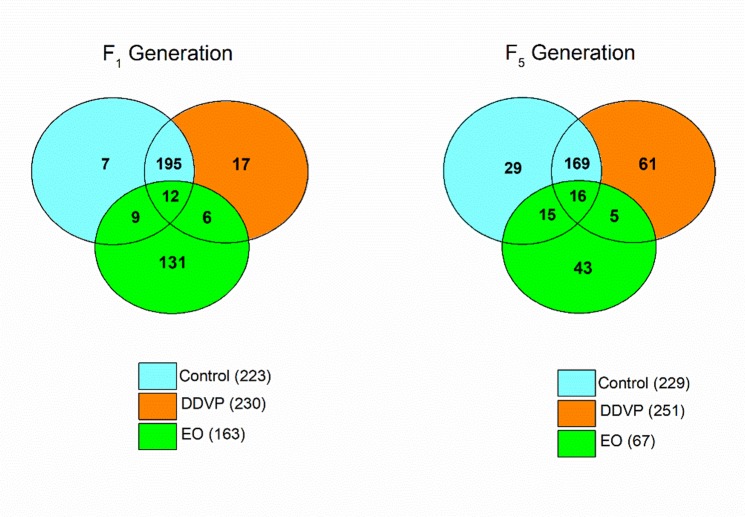
Overall, of the 615 OTUs and 547 OTUs detected from all samples at F1 and F5, respectively, only 12 and 16 core OTUs were shared among the three samples at F1 and F5, respectively (Fig. 3). The Control and DDVP treated samples shared the majority of OTUs detected at F1 and F5 generations (with 195 OTUs and 169 OTUs, respectively), but the EO treated samples recorded the highest number of unique OTUs at F1 generation (131 OTUs) (Fig. 3).
Figure 3.
Venn diagram showing the distribution of unique and shared OTUs within and between samples.
Bacterial richness and diversity
The non-parametric richness indexes (Shannon and Simpson) evaluated at 97% similarity, showed similar comparative trends in the prediction of the number of OTUs from DDVP and Control samples. The Shannon diversity index provides not only species richness (i.e. the number of species present) but how the abundance of each species is distributed (the evenness of the species) among all the species in the community. Here, the bacterial diversity were similarly higher in the Control and DDVP samples at both tested generations (Control: Shannon = 4.78 ± 0.13 and Shannon = 4.98 ± 0.1; DDVP: Shannon = 4.61 ± 0.14 and Shannon = 4.56 ± 0.15, at F1 and F5, respectively), but significantly lower in EO samples (Shannon = 3.58 ± 0.3 and Shannon = 2.47 ± 0.11, at F1 and F5, respectively) (F = 1699.178; df = 1; P = 0.032) (Table 1).
The Multi Response Permutation Procedure (MRPP) analysis based on Bray-Curtis distances revealed no significant difference between the gut microbiotas of Control and DDVP treated beetles (A = 0.09208; P = 0.558). However, the microbiome of EO treated beetles was found to be significantly different from the gut microbiomes of the Control and DDVP treated beetles (Bray-Curtis distance statistics, A = 0.009871; P = 0.0089 & A = 0.0002764; P < 0.0201). Similar trends were observed after the analysis of similarity (ANOSIM) performed on the various treatment groups. There was no significant difference in the structure, composition and abundance of the Control and DDVP treated beetles (R = 0.7518, P = 0.201), which were both different from the EO treated beetles (EO- Control: R = 0.798; P = 0.0014 & EO-DDVP: R = 0.9602; P < 0.001).
Rarefactions
The rarefaction is a computational analysis of species accumulation based on the repeated re-sampling of all clusters. The curve is therefore the representation of statistical expectation for the observed accumulation curves, enabling the comparison of the statistically expected species richness of each community at the same sampling effort or abundance. The flattening of the rarefaction curves recorded here indicates that the sampling was of adequate depth and that additional sampling would produce few additional operational taxonomic units (OTUs) (Fig. 4).
Figure 4.
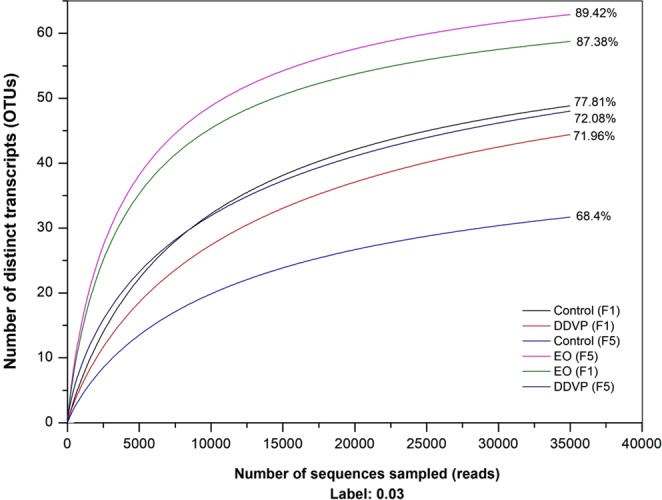
Multiple samples rarefaction curves based on 16S rRNA genes pyrosequencing. This was used to evaluate the completeness of the sequencing effort in describing the diversity of guts associated bacteria of C. maculatus. The OTUs were defined by 3% distances. Percentage values represent coverage indices which are given from a Chao1 analysis.
Bacterial diversity analysis
At Phylum level
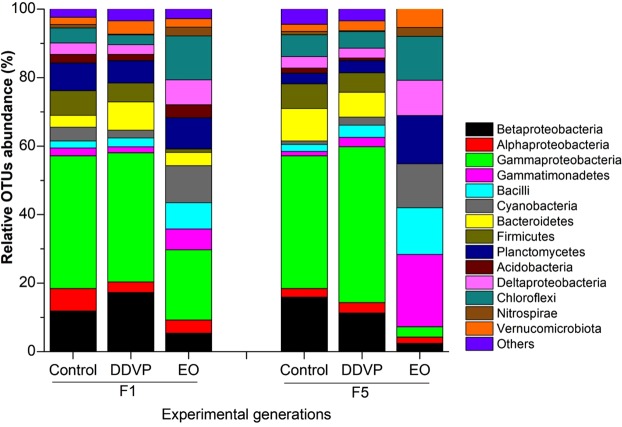
Proteobacteria (gamma: 35.60% and beta: 13.26%), Bacteroidetes (7.89%) and Firmicutes (6.09%) were the most dominant bacterial phyla equally represented at F1 and F5 generations in DDVP and Control samples, although their proportions were slightly different (Fig. 5). The proportions of gammaproteobacteria, betaproteobacteria and Firmicutes were significantly reduced at F1 and F5 in EO samples in comparison with the Control and DDVP. Bacteroidetes, Firmicutes and Acidobacteria present at F1 in EO samples were completely missing at F5. Gammatimonadetes, Bacilli, Cyanobacteria, Chloroflexi and Deltaproteobacteria detected in low abundance (≤2%) in the Control and DDVP (F1 and F5) samples, proliferated significantly in EO samples at F1 and F5(Fig. 5). Overall, the gut bacterial communities in C. maculatus were dominated by Proteobacteria (γ, α & β) (53.67 ± 11.36%), Bacteroidetes (7.87 ± 3.61%) and Firmicutes (6.09 ± 2.04%) (Fig. 5). Other bacterial phyla consisted of Planctomycetes, Chloroflexi, Verrucomicrobiota and unclassified taxa (others) (Fig. 5).
Figure 5.
Relative abundance of the dominant bacterial phyla in all samples revealed by 454-pyrosequencing. The relative number of OTUs was defined as the percentage of the species sequences in total successfully classifiable sequences in the samples using SILVA databank. Data was obtained at an average threshold of 97%. Phylogenetic groups accounting for less than 1% of all classified sequences are summarized as “Others”.
At Family and genus levels
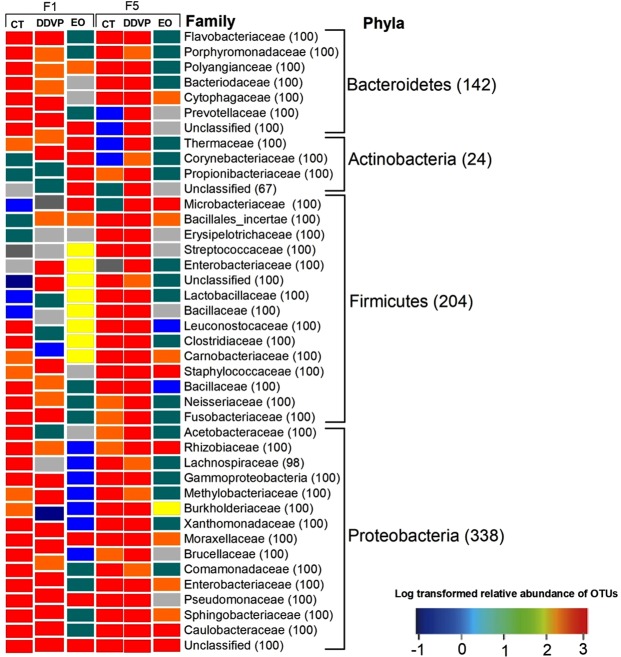
The OTUs assigned to Proteobacteria matched to the classes alpha, beta, delta and gammaproteobacteria and to eleven bacterial families (Comamonadaceae, Enterococcaceae, Enterobacteriaceae, Methylobacteriaceae, Moraxellaceae, Acetobacteraceae, Morganellaceae, Pseudomonadaceae, Sphingomonadaceae, Rhizobiaceae, and Polyangiaceae); four families from Firmicutes (Staphylococcaceae, Carnobacteriaceae, Lactobacillaceae, and Streptococcaceae); four families from Bacteroidetes (Bacteroidaceae, Porphyromonadaceae, Sphingobacteriaceae, and Flavobacteriaceae) and finally three families from Actinobacteria (Micrococcaceae, Brevibacteriaceae and Propionibacteriaceae) (Fig. 6). Other bacterial families were represented by only a few bacterial sequences. The percentage of unclassified sequences was not taken into consideration during the calculation of their abundance. Overall, 51.69% of bacterial genera were identified from the different samples, representing a total of 42 genera. Most of the dominant genera were from Proteobacteria: these are Acinetobacter (4.73%), Pseudomonas (2.03%), Citrobacter (2.03%), Klebsiella (1.69%), Orbus (1.69%) and Comamonas (1.03%). The Firmicutes were predominantly represented by Lactobacillus (3.04%), Lactococcus (3.04%), Staphylococcus (2.03%) and Exiguobacterim (1.35%). Dysgonomonas (9.80%) is the most dominant genus from Bacteroidetes phyla. Burkholderiales represent 10.93% of the total bacterial order detected although no genus was identified.
Figure 6.
Gut bacterial community composition of Callosobruchus maculatus as affected by DDVP and EO. The heat map represents the log-transformed relative abundance of each bacterial family from each sample and the blue colors represent the absence of OTUs. Only high quality trimmed sequences were used to generate the heat map. The non-abundant OTUs (<1%) are not represented in this chart. The distance of log-transformed clusters from the three samples and bacterial families are indicated in the right side of the figure. The numbers between parentheses in front of each taxon represent their relative abundance. DDVP: O,O-dimethyl,O-2,2-dichlorovinylphosphate; EO: essential oil; CT: control.
Bacterial structure distribution
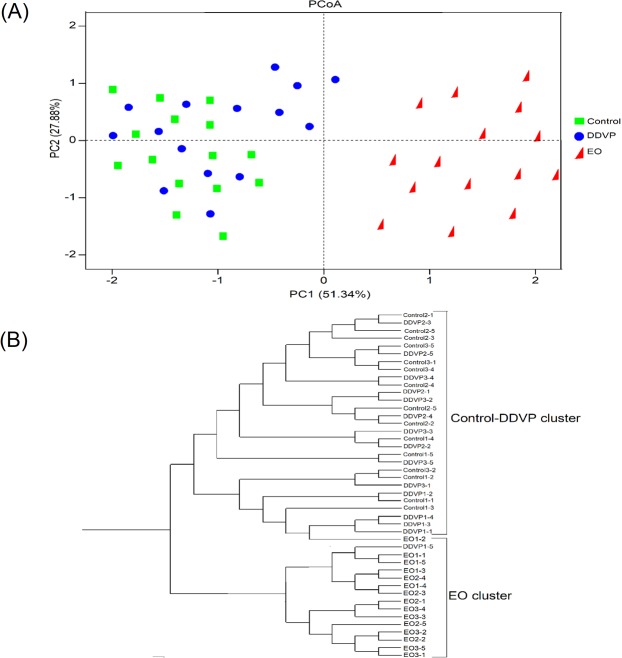
Bacterial community structure did not vary significantly across generations in Control and DDVP treated samples (F = 581.677; df = 1; P = 0.158 and F = 581.677; df = 1; P = 0.058, respectively) (Fig. 7A), but vary significantly in EO treated samples at F1 and F5 (F = 1699.178; df = 4; P < 0.001 and F = 1699.178; df = 4; P < 0.0001, respectively) (Fig. 7A). A cladogram representing the clustering of the different samples based on the composition of their microbiotas show that the Control and DDVP groups are closely related but both are distantly related with the EO treated beetles (Fig. 7B).
Figure 7.
Multivariate Principal Coordinates Analysis (PCoA) plots showing the distance between C. maculatus gut samples (A). Each color point represents individuals from each treatment group. The cladogram represents the clustering of the different samples based on the composition of their microbiotas (B). The analysis was created by using an average linkage from the weighted UniFrac distance matrices. Each node supported by a Jackknife analysis with greater than 100% accuracy. This analysis was based on the relative abundance of bacterial phyla and proteobacterial classes. Figures before treatment groups represents the various biological replicates and sample ID (for example, Control1-1 means first biological replicate and the first individual from the control group).
Discussion
Several insects have developed extraordinary capacity to survive exposure to xenobiotics normally designed to manage them40. Furthermore, insects harbor a wide variety of gut symbionts2,41,42, which play pivotal roles in their adaptation to the environment following exposure to pesticides19,43. In an attempt to reduce the incidence of insecticide resistance in C. maculatus, coupled with the necessity of developing an alternative control strategy, we tested the hypothesis that gut microbiota of C. maculatus are involved in its resistance to dichlorvos and L. adoensis EO could break this resistance and enhance the susceptibility of the beetle across generations.
On one hand, the results showed that the adult mortality in both pesticides was significantly higher in aposymbiotic beetles compared to the symbiotic ones and the control groups. On the other hand, the susceptibility of symbiotic C. maculatus declined from the third generation in DDVP treatments whereas in EO treatments, it remained consistently higher across all tested generations (Fig. 2). These findings suggest that symbiotic beetles have developed the ability to tolerate DDVP molecules, presumably sustained by their gut bacteria, as their population crash in aposymbiotic beetles resulted in significant increase of their susceptibility compared to the symbiotic ones and remained consistent across generations.
The mortality of EO treated beetles (symbiotic and aposymbiotic) was not significantly affected across generations, contrary to DDVP where the susceptibility of symbiotic beetles dropped irrevocably from F3. This suggests that, the symbiotic beetles started developing resistance to DDVP from F3 generations and the sustainability or heritability of the resistance trait in the following generations depended on the symbiotic status and pesticides used. After F3, L. adoensis EO succeeded to reduce resistance in the following generations, thereby causing failures in the beetle’s adaptation attempts or defense mechanism mediated bacterially. Previously, gut microbial symbionts were shown to be implicated in insect-plant interactions by increasing the adaptations of insects to plant toxins17,44. The results from this study showed an opposite trend whereby allelochemicals of L. adoensis EO sustained the susceptibility of C. maculatus potentially by reducing the most dominant populations of gut bacteria.
The 454-pyrosequencing analysis of gut microbiome provided empirical evidence on bacterial phyla potentially involved in the interaction with DDVP and EO. Proteobacteria, Bacteroidetes and Firmicutes were abundantly detected in the Control and DDVP treated samples at F1 and F5 generations (Fig. 5). However, their proportions in EO treated beetles were significantly reduced across generations and these correlated with the increase in adult mortality. Possibly, these bacterial phyla could be associated to DDVP-biodegradation in C. maculatus, allowing the host to survive exposure to DDVP across generations by increasing the host tolerance to it. Moreover, the presence of the genera Acinetobacter, Citrobacter, Pseudomonas and Burkholderiales (although unclassified) genera in C. maculatus gut could be associated to their ability to develop resistance to DDVP components. Similarly, recent studies have shown that bacterial species from Proteobacteria phyla of the genera Citrobacter, Burkholderia and Pseudomonas were implicated in biodegradations of trichlorphon and fenitrothion (both OP pesticides) by B. dorsalis and Bemissia tabacci as well as in the environment29,45,46.
Conversely, when C. maculatus was treated with L. adoensis EO, the populations of Proteobacteria, Bacteroidetes and Firmicutes (the most dominant bacterial phyla in untreated beetles) were significantly reduced and the vulnerability of treated beetles increased and remained consistent across generations. This could be an indication that Proteobacteria, Bacteroidetes and Firmicutes may represent to some extent, the primary targets of chemical constituents of L. adoensis EO. Recent studies on insect gut microbiology have shown that Proteobacteria and Firmicutes were the most dominant bacterial phyla in the midgut of Lepidopterans, including Lymantria dispar, Helicoverpa armigera, and Bombyx mori47–49. At the family level, Enterococcaceae was the most abundantly represented in the gut of C. maculatus, followed by Enterobacteriaceae (Fig. 6) as earlier discovered in three life stages of Bactrocera dorsalis50. This could be an indication that Enterococcaceae and Enterobacteriaceae might play important roles in ecological adaptations and survival of C. maculatus.
The use of antibiotics to suppress the gut microbial communities did not affect the survival of aposymbiotic beetles as the very little mortality recorded was similar to that of the symbiotic ones. This suggests that the mortality recorded was not caused by the use of antibiotics (in aposymbiotic beetles), but by the pesticide treatments (EO and DDVP). However, the suppression of gut bacteria by antibiotics resulted in higher adult mortality rates compared to the symbiotic ones. This suggests that, without its intestinal bacterial, C. maculatus could not successfully survive exposure to DDVP. The high number of chemical constituents detected from L. adoensis EO26 may come into play toward maintaining the consistent susceptibility of the beetles across generations by targeting the most abundant bacterial communities (Fig. 5). Many previous works demonstrated the interactions between gut bacteria and phytochemicals51–54, but the specific conserved functions of gut bacteria in the cowpea beetles need further investigations of the whole transcriptomes of the insect. At the very least, our results give evidence that gut bacterial communities in C. maculatus might mediate DDVP degradation making them resistant to it. These bacteria might also be the target of plant toxins from EO making C. maculatus more susceptible to phytochemicals. We therefore believe that plant allelochemicals may have evolved antibiotic functions to disrupt gut bacterial communities in C. maculatus.
On the basis of previous transcriptomic and microarray analyses carried out on C. maculatus gut55,56, the future works will focus on evaluating the expression profiles of detoxification genes to understand the extent of gut microbiome involvement in molecular resistance or susceptibility in this cowpea pest.
Conclusion
The development of resistance in pest insects is a major challenge to sustainable agriculture and pest management. The present study demonstrated that the Control and DDVP treated C. maculatus shares similar core bacteria, dominated by Proteobacteria, Bacteroidetes and Firmicutes. These bacterial phyla may allow the adults C. maculatus to survive on DDVP treated grains, thereby making it inappropriate to control the beetle populations in the field. The results also revealed that L. adoensis EO could represent an alternative pesticide to DDVP through its capacity to uphold the vulnerability of the beetles across generations, hence maintaining their populations below economic thresholds. From an ecological perspective, the findings herein broaden our understanding of the implications of gut bacteria in the adaptation mechanisms of C. maculatus to pesticides. This could help to develop new tools to aid the implementation of eco-friendly control strategies and reduce the environmental impact of synthetic chemicals.
Methods
Insects rearing and maintenance
Twenty kilograms of dried cowpea seeds (Vigna unguiculata) (black eye beans) were collected from Lara locality in Northern Cameroon (latitude 10°10′00.0″N and longitude 14°31′00.0″E). The seeds were cleaned and disinfested by storing them at −20 °C for 10 days and thereafter left undisturbed under laboratory conditions (27 ± 3 °C, 55 ± 5% relative humidity and 10: 14 Light: Dark photoperiod) for 5 days for acclimatization and to avoid moldiness20. To minimize variations in the insect populations, adult C. maculatus that emerged from infested field collected seeds were reared on disinfested cowpea seeds for about eight generations until required for bioassays.
Lippia adoensis essential oil (EO) and dichlorvos (DDVP)
Fifty grams of fresh L. adoensis leaves were harvested at the vicinity of the University of Ngaoundere, Cameroon (latitude 7°19′39″N and longitude 13°35′04″E), shade-dried for one week and ground. The essential oil was extracted by hydro-distillation using a modified Clevenger-type apparatus (XWD-C-1000, Shanghai XinWangDe Laboratory Equipment Co., China) for 6 h and the oil was extracted with n-hexane. The recovered crude essential oil (EO) was stored in an airtight glass container and kept at 4 °C20. The chemical constituents of L. adoensis EO were determined and quantified by Gas chromatography/mass spectrometry as previously described26. Dichlorvos or DDVP (O,O-dimethyl,O-2,2-dichlorovinylphosphate) 100 EC 103 g/L (PESTANAL®, Analytical Standard, C4H7Cl2O4P) was obtained from Sigma-Aldrich (China) (ID: 45441, molecular weight: 220.98; CAS number: 62-73-7) at 100% purity.
The EO and DDVP were separately dissolved at equal proportions (1:1) in dimethylsulfoxide or DMSO (solvent) prior to treatments.
Artificial cowpea grains
Five kilograms of disinfested cowpea seeds were soaked in distilled water for 30 minutes to allow the removal of testae. Decorticated seeds were air-dried overnight under room temperature and ground into fine flour. The resulting flour was used to prepare two types of artificial seeds resulting flour (see details in Supplementary 1(3)). The normal seeds (flour without any antibiotics), and the antibiotic groups (flour mixed with 10 µg of Ciprofloxacin per gram of flour and 4 µg of Gentamycin per gram of flour at the preparation)55. Mineral elements (methyl-p-hydroxybenzoate, choline chlorite, L-ascorbic acid and sodium benzoate) were added among the ingredients to compensate those removed during seed decortication and to boost the females’ fecundity.
The concentrations of antibiotics represent their minimum inhibitory concentration (MIC) and the two antibiotics were selected for their potencies to clear the gut microbiota of C. maculatus based on the colony forming units (CFU) of gut homogenates (Supplementary 1). In each treatment group, the artificial cowpea seeds were left undisturbed for 24 h and equilibrated with atmospheric moisture, prior to coating with 10% gelatin55. The preparation procedures were carried out under aseptic conditions. The artificial cowpea seeds were air-dried under a laminar flow hood and packaged in plastic bag until use for bioassays.
Production of symbiotic and aposymbiotic beetles
10 mated C. maculatus couples were introduced in two separate glass jars (500 mL size), respectively, each containing 50 g of normal artificial seeds and antibiotic treated seeds, respectively. The females were allowed to lay eggs on the seeds in which the larvae were allowed to develop till adult emergence. The beetles emerging from the normal seeds are symbiotic (containing intact gut microbiome) and those emerging from antibiotics treated seeds are experimentally deprived of gut bacteria but their aposymbiotic state was confirmed by culture dependent and molecular techniques as described below.
Confirmation of aposymbiotic status of experimental beetles
The aposymbiotic status of the newly emerged beetles was checked using the culture dependent technique and the qPCR analyses targeting the 16S rRNA genes, respectively. Ten normal and ten antibiotics treated beetles, were randomly selected and individually dissected. Individual guts were removed and homogenized and the homogenate was serially diluted, spread on standard Luria-Bertani (LB) agar plates (10 g tryptone, 5 g yeast extract, and 10 g NaCl in 950 mL deionized water at pH 7.0) and incubated at 30 °C overnight. The Colony Forming Units (CFU) resulting from the bacterial colonies were calculated in each group. The estimation of cultivable bacteria in symbiotic beetles (normal) gave 6.514 × 105 ± 13.72 × 102 CFUs g−1.gut−1 while the antibiotic treated beetles gave 254 ± 9.32 CFUs g−1.gut−1 representing just 0.039% of the total bacterial communities of the normal beetles (Independent T-Test, F = 19.65; t = 11.502; df = 1, 28; P < 0.0001).
The bacterial genomic DNA was extracted from ten individual gut suspensions (earlier prepared) using the CTAB/SDS method57 and the recovered DNA samples were checked for quality by spectrophotometry and purified. Five nanograms of purified DNA was used per qPCR reaction targeting the bacterial 16S rRNA gene using universal primers 27 F/533R. Each sample was prepared by pooling 10 individual guts representing 10 replicates from symbiotic and aposymbiotic beetles each, using SYBRGreen in 384-well plates on an ABI 7300 Real-Time PCR detection system (Applied Biosystems Inc, Foster City, CA, USA). The 18 S rRNA was used as internal control gene for the determination of gut bacterial content from each sample. The gut microbial communities from the symbiotic beetles gave 10.01 × 106 ± 2.4 × 106 CFUs per gut while in aposymbiotic ones, an average gut bacterial content of 2.5 × 106 ± 6.08 × 104 CFUs (Independent T-Test, F = 89.692; t = 1.835; df = 1, 18; P < 0.0001). After this estimation, the antibiotic treated beetles were confirmed to be aposymbiotic (which is a conventional relative term, used widely in similar experimental settings, see refs2,3,4,38) and were used in subsequent experiment.
Bioassays
Symbiotic and aposymbiotc beetles were assayed separately. The test was carried out in Petri dishes (Ø = 90 mm), each containing 10 g of artificial cowpea seeds divided into three treatment groups (EO, DDVP & Control) of 15 replicates each. Each treatment group comprised three biological replicates and each biological replicate contained 5 individual beetles. The EO groups (seeds treated with 10 µL of EO per gram of overall seed weight), the DDVP groups (seeds treated with 15 µL of DDVP per gram of overall seed weight), and finally the control groups (seeds treated with DMSO only). The working concentrations of EO and DDVP represent their effective concentration (EC50) determined after a dose-response assessment among several dosages (Fig. S2, Supplementary 1).
Five C. maculatus couples were introduced into each petri dish. The insects remained in contact with the grains for 6 days and adult mortality was recorded daily for 6 days until no mortality was seen20. The dead insects were removed and the surviving ones were left in respective experimental Petri dishes and selected for breeding of the next generation58. Emerging adults from different treatments (including controls) were used to set up separate lines. The resistance levels of experimental beetles were based on adult mortality rates at each generation and insects were considered dead if they were unable to turn over after gentle prod on their abdomen26. All treatments were maintained at 27 ± 3 °C temperature, 55 ± 5% relative humidity (RH) and 14:10 (Light: Dark) photoperiod. Only females were chosen for microbial analyses due to their high capacity to tolerate pesticides, shown by lower mortality rates recorded across generations compared to males (Fig. S3, Supplementary 1).
Microbiological bioassay
Five surviving females from each of the three biological replicates of different treatment groups (Control, EO, and DDVP) at F1 and F5 generations, symbiotic and aposymbiotic were dissected and their individual guts were considered for gut microbial analysis based on high-throughput 454 pyrosequencing of V2-V3 regions of the 16S rRNA gene59,60. The three sequencing samples were randomly selected among replicates which show higher tolerance to each pesticide. To evaluate the effects of long term exposure to DDVP and EO on gut microbiota, gut samples from all treatments at F1 and F5 generations were considered for the analysis.
Insect dissection
Five insects from each of the three biological replicates (making 15 individuals per treatment group) were collected, anesthetized by placing them at −20 °C for 30 minutes. Soon after, they were washed in 70% ethanol for 2 min and rinsed 3 times in sterile distilled water before dissection. To prevent contamination of the samples, the dissection was carried out aseptically with two pairs of sterilized forceps on a sterilized glass slide spread with 50 µL of sterile distilled water under a stereomicroscope50. The whole process was done in a laminar flow hood to avoid aerial contaminations. The intact guts were individually and separately transferred into Eppendorf tubes containing 750 μL TE buffer (10 mM Tris-Cl, pH 8; 1 mM EDTA, pH 8), disaggregated and manually homogenized with an Eppendorf adapted pestle and directly inoculated onto LB agar plates61. The homogenate was used for bacterial colony screening and total DNA extraction.
DNA Extraction, molecular cloning and 454-Pyrosequencing
Genomic DNA samples were extracted using the CTAB method57 on five individual gut homogenates from each biological replicate and treatment. The quantity and quality of extracted gDNA were determined using a NanoDrop Spectrophotometer (Eppendorf AG, Germany) and stored at −20 °C prior to the analysis. The pyrosequencing of V2-V3 hyper-variable regions of the 16S rRNA gene amplicons was carried out on a 454 GS FLX Titanium system (Roche, Penzberg, Germany)60,62. Universal barcoded primers Ba27F (5′-AGA GTT TGA TCM TGG CTC AG-3′) and Ba519R (5′-TAT TAC CGC GGC KGC TG-3′) containing A and B sequencing adaptors (454 Life Sciences), key sequences and multiplex identifiers were used to prepare the pyrosequencing amplicons63. Before pyrosequencing, amplicons were purified from gel using AxyPrep DNA gel extraction kit (Axygen Scientific, Inc.), quantified using Quant-iT Pico Green dsDNA assay (Invitrogen, Germany) and pooled in an equimolar ratio before being subjected to emulsion PCR and breaking. GS FLX Titanium chemistry was used to perform pyrosequencing from A-end according to the supplier protocol.
Statistical analysis
All mortality data from each treatment group were corrected using Abbott’s formula64 and tested for homogeneity of variances using Levene’s tests. To check important factors that influence the susceptibility of the experimental beetles, variables of overall mortality were analyzed (each one separately) using the ordinary least squares regression model with symbiotic status, pesticides and generations as effects. Multivariate tests (using DDVP, EO and DMSO data as dependent variables) and multifactorial analyses (using the symbiotic status and experimental generations as fixed factors) were performed to analyze the variations of mortality within and between all the parameters (symbiotic status, experimental generations and pesticide treatments). The one-way analysis of variance (ANOVA) was used to analyze differences in mortality and sequencing data (Table 1). Colony Forming Units of antibiotics treated and untreated samples were analyzed using an independent T-Test. Tukey’s HSD test at P = 0.05 significance, was used for mean separations within and between treatments. Colony Forming Units (CFU) data were log-transformed to narrow down data variations. Statistical analyses were carried out using SPSS 20.0 software (Statsoft Inc, Carey, J, USA). Bray–Curtis and unweighted UniFrac distance matrices were used to calculate the beta diversity and the analysis of similarity (ANOSIM) was performed to evaluate the similarity of C. maculatus gut bacterial community among treatments using the software PRIMER 7.0. All results (alpha and beta diversity, survival, & sequence data) are presented as mean ± standard deviations (SD) of the three biological replicates. OriginPro 8.5.1 software was used to construct graphs.
Bioinformatics analysis
Bioinformatics analysis was done separately for each of the three samples (control, DDVP and EO) using Quantitative Insights Into Microbial Ecology (QIIME) 1.9.0 pipeline65. Raw pyrosequencing reads (54,885) were denoised using the “pre.cluster” command (http://www.mothur.org/wiki/Pre.cluster) in mothur platform (version 1.25.0; http://www.mothur.org)66. Any mismatch in the barcode, more than two primer mismatches, homopolymers of more than 8 bases and sequences less than 160 bp were discarded for the analysis. Chimeric sequences were identified and removed using UCHIME, and the remaining unique sequences were clustered into Operational Taxonomic Units (OTUs) using the Greengenes database filtered at 97% similarity, which is the consensus threshold for species boundaries67. A total of 47,766 high-quality pyrosequencing reads were produced according to barcode- and primer-sequence filtering, and were used for further analysis.
To obtain additional information regarding species diversity composition, sequence reads were subjected to redundancy treatment with Mothur software to count the number of identical tags. Taxonomic classification of OTUs was done via the ribosomal database project (RDP), with naïve Bayesian settings at 0.5 confidence threshold68. Rarefaction analysis was used to compare sequencing depth and bacterial species richness within and between F1 and F5 generations. Only OTUs that reached 97% identity level were used for alpha diversity (Shannon and Simpson) and richness (Chao1) analysis. Dataset was normalized at 300 sequences per sample, and this rarified threshold was used to identify the OTUs which were highly affected by the pesticides69. Mothur program was used to construct Venn diagram to show OTUs that are unique or shared between treatments (Control, DDVP and EOs)70. Principal components analyses were generated using Bayesian algorithms71 and dendrograms showing the similarity of bacterial communities in different treatments were constructed through a jackknife-beta-diversity script from QIIME65.
Ethical Statement
Callosobruchus maculatus is indigenous in Cameroon. Lippia adoensis fresh leaves were collected from the experimental field of the Department of Biological Sciences, Faculty of Science, University of Ngaoundere (Cameroon) and cowpea seeds were bought from local farmers. Therefore, no permit was required for their collection and manipulation. The study was approved by the scientific committee of the Faculty of Science and validated by the Scientific Committee of the University of Ngaoundere (Decision no. 2015/093) in a full PhD Program assigned to the author M.A.
Supplementary information
Acknowledgements
We thank the Chinese Government for the scholarship (No. 2015120T22) granted to the first author (MA). This work was financially supported by the National Natural Science Foundation of China (31661143045) and the International Atomic Energy Agency (CRP No. 17153 and No. 18269). Authors are grateful to Professor Boaz Yuval (Department of Entomology, The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot 76100, Israel) and two anonymous reviewers for their valuable comments on this manuscript.
Author Contributions
M.A., E.N.N., C.Y.N. and N.Y.N., conceived and designed the experiments; M.A. performed the laboratory works and wrote the first draft of the manuscript; M.A., M.A.R., O.A.G. and A.A.A. analyzed the data, edited and corrected the manuscript. All authors gave final approval for publication.
Data Availability
Metagenomic data generated from this study has been submitted to the Sequence Read Archive (SRA) of NCBI under the BioProject ID: PRJNA530839. Other additional data are available from corresponding authors upon request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mazarin Akami, Email: makami1987@gmail.com.
Chang-Ying Niu, Email: niuchangying88@163.com.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42843-1.
References
- 1.Kikuchi Y, Hosokawa T, Fukatsu T. Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl. Environ. Microbiol. 2007;73:4308–4316. doi: 10.1128/AEM.00067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Yosef M, Behar A, Jurkevitch E, Yuval B. Bacteria-diet interactions affect longevity in the medfly - Ceratitis capitata. J. Appl. Entomol. 2008;132:690–694. doi: 10.1111/j.1439-0418.2008.01330.x. [DOI] [Google Scholar]
- 3.Ben-Yosef M, Pasternak Z, Jurkevitch E, Yuval B. Symbiotic bacteria enable olive flies (Bactrocera oleae) to exploit intractable sources of nitrogen. J. Evol. Biol. 2014;27:2695–2705. doi: 10.1111/jeb.12527. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Yosef M, Pasternak Z, Jurkevitch E, Yuval B. Symbiotic bacteria enable olive fly larvae to overcome host defences. Royal. Soc. Open. Sci. 2015;2:150170. doi: 10.1098/rsos.150170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu C-C, Spencer JL, Curzi MJ, Zavala JA, Seufferheld MJ. Gut bacteria facilitate adaptation to crop rotation in the western corn rootworm. Proc. Natl. Acad. Sci. 2013;110:11917–11922. doi: 10.1073/pnas.1301886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soen, Y. Environmental disruption of host–microbe co-adaptation as a potential driving force in evolution. Front. Genet. 5, 10.3389/fgene.2014.00168 (2014). [DOI] [PMC free article] [PubMed]
- 7.de Almeida LG, de Moraes LAB, Trigo JR, Omoto C, Cônsoli FL. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation. PloS One. 2017;12:e0174754. doi: 10.1371/journal.pone.0174754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen AK, Moran NA. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol. Ecol. 2014;23:1473–1496. doi: 10.1111/mec.12421. [DOI] [PubMed] [Google Scholar]
- 9.Akami M, et al. Intestinal bacteria modulate the foraging behavior of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae) PloS One. 2019;14:e0210109. doi: 10.1371/journal.pone.0210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver KM, Moran NA, Hunter MS. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12795–12800. doi: 10.1073/pnas.0506131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownlie JC, Johnson KN. Symbiont-mediated protection in insect hosts. Trends Microbiol. 2009;17:348–354. doi: 10.1016/j.tim.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Montella IR, Schama R, Valle D. The classification of esterases: an important gene family involved in insecticide resistance-A review. Mem Inst Oswaldo Cruz. 2012;107:437–449. doi: 10.1590/S0074-02762012000400001. [DOI] [PubMed] [Google Scholar]
- 13.Whalon, M. E., Mota-Sanchez, D. & Hollingworth, R. M. Global pesticide resistance in arthropods. 169 pp. (Cabi, 2008).
- 14.Akami Mazarin, Njintang Nicolas Y., Gbaye Olajire, Niu Chang-Ying, Nukenine E-N. Comparative expression of two detoxification genes by Callosobruchus maculatus in response to dichlorvos and Lippia adoensis essential oil treatments. Journal of Pest Science. 2019;92(2):665–676. doi: 10.1007/s10340-018-01075-4. [DOI] [Google Scholar]
- 15.Crava CM, Brütting C, Baldwin IT. Transcriptome profiling reveals differential gene expression of detoxification enzymes in a hemimetabolous tobacco pest after feeding on jasmonate-silenced Nicotiana attenuata plants. BMC Genomics. 2016;17:1005. doi: 10.1186/s12864-016-3348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feyereisen R. Insect CYP genes and P450 enzymes. Insect Biochem Mol Biol. 2012;8:236–316. doi: 10.1016/B978-0-12-384747-8.10008-X. [DOI] [Google Scholar]
- 17.Hammer TJ, Bowers MD. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia. 2015;179:1. doi: 10.1007/s00442-015-3327-1. [DOI] [PubMed] [Google Scholar]
- 18.Dowd, P. Symbiont-mediated detoxification in insect herbivores. Microbial Mediation of Plant-Herbivore Interactions, 411–440 (1991).
- 19.Kikuchi Y, et al. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. 2012;109:8618–8622. doi: 10.1073/pnas.1200231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akami M, et al. Essential oil optimizes the susceptibility of Callosobruchus maculatus and enhances the nutritional qualities of stored cowpea Vigna unguiculata. Royal. Soc. Open. Sci. 2017;4:170692. doi: 10.1098/rsos.170692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiroesele B, Thomas K, Seketeme S. Control of cowpea weevil, Callosobruchus maculatus (F.)(Coleoptera: Bruchidae), using natural plant products. Insects. 2014;6:77–84. doi: 10.3390/insects6010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck, C. W. & Blumer, L. S. A handbook on bean beetles, Callosobruchus maculatus. National Science Foundation (2011).
- 23.Fox CW. Multiple mating, lifetime fecundity and female mortality of the bruchid beetle, Callosobruchus maculatus (Coleoptera: Bruchidae) Funct. Ecol. 1993;7:203–208. doi: 10.2307/2389888. [DOI] [Google Scholar]
- 24.Wilson CJ, Tomkins JL. Female Callosobruchus maculatus can maximize long-term fitness through polyandry. Behav. Ecol. 2014;26:502–509. doi: 10.1093/beheco/aru218. [DOI] [Google Scholar]
- 25.Ursprung C, Den Hollander M, Gwynne DT. Female seed beetles, Callosobruchus maculatus, remate for male-supplied water rather than ejaculate nutrition. Behav. Ecol. Sociobiol. 2009;63:781–788. doi: 10.1007/s00265-009-0711-z. [DOI] [Google Scholar]
- 26.Akami M, et al. Persistence and comparative pesticidal potentials of some constituents of Lippia adoensis (Hochst. ex Walp.)(Lamiales: Verbenaceae) essential oil against three life stages of Callosobruchus maculatus (Fab.)(Coleoptera: Bruchidae) Br Biotechnol J. 2016;13:1–16. doi: 10.9734/BBJ/2016/26087. [DOI] [Google Scholar]
- 27.Goodacre SL, Fricke C, Martin OY. A screen for bacterial endosymbionts in the model organisms Tribolium castaneum, T. confusum, Callosobruchus maculatus, and related species. Insect science. 2015;22:165–177. doi: 10.1111/1744-7917.12096. [DOI] [PubMed] [Google Scholar]
- 28.Prakash R. Isolation of bacteria from Callosobruchus maculatus, coleopteran pest of stored products. J. Ecotoxicol. Environ. Monit. 2011;21:541. doi: 10.18466/cbujos.71031. [DOI] [Google Scholar]
- 29.Itoh H, et al. Bacterial population succession and adaptation affected by insecticide application and soil spraying history. Frontiers in Microbiology. 2014;5:457. doi: 10.3389/fmicb.2014.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gbaye OA, Oyeniyi EA, Ojo OB. Resistance of Callosobruchus maculatus (Fabricius)(Coleoptera: Bruchidae) Populations in Nigeria to Dichlorvos. Jordan J. Biol. Sci. 2016;9:41–46. doi: 10.12816/0027007. [DOI] [Google Scholar]
- 31.Faraone N, Hillier NK, Cutler GC. Plant essential oils synergize and antagonize toxicity of different conventional insecticides against Myzus persicae (Hemiptera: Aphididae) PloS One. 2015;10:e0127774. doi: 10.1371/journal.pone.0127774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food and Chemical Toxicology. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 33.Regnault-Roger Catherine, Vincent Charles, Arnason John Thor. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annual Review of Entomology. 2012;57(1):405–424. doi: 10.1146/annurev-ento-120710-100554. [DOI] [PubMed] [Google Scholar]
- 34.Folashade KO, Omoregie EH. Essential oil of Lippia multiflora Moldenke: A review. J. Appli. Pharm. Sci. 2012;2:15–23. doi: 10.7324/JAPS.2012.2524. [DOI] [Google Scholar]
- 35.Ravindran, P. N. The Encyclopedia of herbs and spices. 505 pp (CABI, 2017).
- 36.Izakmehri Khadijeh, Saber Moosa, Mehrvar Ali, Hassanpouraghdam Mohammad Bagher, Vojoudi Samad. Lethal and Sublethal Effects of Essential Oils fromEucalyptus camaldulensisandHeracleum persicumAgainst the Adults of Callosobruchus Maculatus. Journal of Insect Science. 2013;13(152):1–10. doi: 10.1673/031.013.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebadollahi A. Chemical constituents and toxicity of Agastache foeniculum (Pursh) Kuntze essential oil against two stored-product insect pests. Chil. J. Agric. Res. 2011;71:212. doi: 10.4067/s0718-58392011000200005. [DOI] [Google Scholar]
- 38.Berasategui A, et al. Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol. Ecol. 2017;26:4099–4110. doi: 10.1111/mec.14186. [DOI] [PubMed] [Google Scholar]
- 39.Ignasiak, K. & Maxwell, A. Antibiotic-resistant bacteria in the guts of insects feeding on plants: prospects for discovering plant-derived antibiotics. BMC Microbiol. 17, 10.1186/s12866-017-1133-0 (2017). [DOI] [PMC free article] [PubMed]
- 40.Karunamoorthi K, Sabesan S. Insecticide resistance in insect vectors of disease with special reference to mosquitoes: a potential threat to global public health. Health Scope. 2013;2:4–18. doi: 10.17795/jhealthscope-9840. [DOI] [Google Scholar]
- 41.Ben-Yosef M, Jurkevitch E, Yuval B. Effect of bacteria on nutritional status and reproductive success of the Mediterranean fruit fly Ceratitis capitata. Physiol. Entomol. 2008;33:145–154. doi: 10.1111/j.1365-3032.2008.00617.x. [DOI] [Google Scholar]
- 42.Sharon G, et al. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. 2010;107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Z, et al. Transmission modes of a pesticide-degrading symbiont of the oriental fruit fly Bactrocera dorsalis (Hendel) Appl. Microbiol. Biotechnol. 2017;101:8543–8556. doi: 10.1007/s00253-017-8551-7. [DOI] [PubMed] [Google Scholar]
- 44.Pizzolante, G. et al. Cultivable gut bacteria provide a pathway for adaptation of Chrysolina herbacea to Mentha aquatica volatiles. BMC Plant Biol. 17, 10.1186/s12870-017-0986-6 (2017). [DOI] [PMC free article] [PubMed]
- 45.Cheng D, et al. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel) Microbiome. 2017;5:13. doi: 10.1186/s40168-017-0236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie Wen, Meng Qing-shu, Wu Qing-jun, Wang Shao-li, Yang Xin, Yang Ni-na, Li Ru-mei, Jiao Xiao-guo, Pan Hui-peng, Liu Bai-ming, Su Qi, Xu Bao-yun, Hu Song-nian, Zhou Xu-guo, Zhang You-jun. Pyrosequencing the Bemisia tabaci Transcriptome Reveals a Highly Diverse Bacterial Community and a Robust System for Insecticide Resistance. PLoS ONE. 2012;7(4):e35181. doi: 10.1371/journal.pone.0035181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broderick NA, Raffa KF, Goodman RM, Handelsman J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl. Environ. Microbiol. 2004;70:293–300. doi: 10.1128/AEM.70.1.293-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Werren JH. Symbionts provide pesticide detoxification. Proc. Natl. Acad. Sci. 2012;109:8364–8365. doi: 10.1073/pnas.1206194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiang H, et al. Microbial communities in the larval midgut of laboratory and field populations of cotton bollworm (Helicoverpa armigera) Can. J. Microbiol. 2006;52:1085–1092. doi: 10.1139/W06-064. [DOI] [PubMed] [Google Scholar]
- 50.Andongma AA, et al. Pyrosequencing reveals a shift in symbiotic bacteria populations across life stages of Bactrocera dorsalis. Sci. Rep. 2015;5:9470. doi: 10.1038/srep09470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ceja-Navarro JA, et al. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015;6:7618. doi: 10.1038/ncomms8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung SH, et al. Host plant species determines symbiotic bacterial community mediating suppression of plant defenses. Sci. Rep. 2017;7:39690. doi: 10.1038/srep39690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammerbacher A, et al. A common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce. Plant. Physiol. 2013;162:1324–1336. doi: 10.1104/pp.113.218610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohl KD, Weiss RB, Cox J, Dale C, Dearing MD. Gut microbes of mammalian herbivores facilitate intake of plant toxins. Ecol. Lett. 2014;17:1238–1246. doi: 10.1111/ele.12329. [DOI] [PubMed] [Google Scholar]
- 55.Guo F, et al. Antagonistic regulation, yet synergistic defense: effect of bergapten and protease inhibitor on development of cowpea bruchid Callosobruchus maculatus. PloS One. 2012;7:e41877. doi: 10.1371/journal.pone.0041877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sayadi A, Immonen E, Bayram H, Arnqvist G. The de novo transcriptome and its functional annotation in the seed beetle Callosobruchus maculatus. PloS One. 2016;11:e0158565. doi: 10.1371/journal.pone.0158565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.William S, Feil H, Copeland A. Bacterial genomic DNA isolation using CTAB. Sigma. 2012;50:6876. [Google Scholar]
- 58.Jin T, Zeng L, Lin Y, Lu Y, Liang G. Insecticide resistance of the oriental fruit fly, Bactrocera dorsalis (Hendel)(Diptera: Tephritidae), in mainland China. Pest Manag. Sci. 2011;67:370–376. doi: 10.1002/ps.2076. [DOI] [PubMed] [Google Scholar]
- 59.Chakravorty S, Helb D, Burday M, Connell N, Alland D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J Microbiol Meth. 2007;69:330–339. doi: 10.1128/jcm.01228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nacke H, et al. Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PloS One. 2011;6:e17000. doi: 10.1371/journal.pone.0017000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong CNA, Ng P, Douglas AE. Low‐diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ. Microbiol. 2011;13:1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cardinale, M., Kaiser, D., Lueders, T., Schnell, S. & Egert, M. Microbiome analysis and confocal microscopy of used kitchen sponges reveal massive colonization by Acinetobacter, Moraxella and Chryseobacterium species. Sci. Rep. 7, 10.1038/s41598-017-06055-9 (2017). [DOI] [PMC free article] [PubMed]
- 63.Pilloni G, Granitsiotis MS, Engel M, Lueders T. Testing the limits of 454 pyrotag sequencing: reproducibility, quantitative assessment and comparison to T-RFLP fingerprinting of aquifer microbes. PloS One. 2012;7:e40467. doi: 10.1371/journal.pone.0040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abbott W. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 65.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schloss PD, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mirón Leticia, Mira Alex, Rocha-Ramírez Víctor, Belda-Ferre Pedro, Cabrera-Rubio Raúl, Folch-Mallol Jorge, Cardénas-Vázquez René, DeLuna Alexander, Hernández Ana Lilia, Maya-Elizarrarás Elisa, Schondube Jorge E. Gut Bacterial Diversity of the House Sparrow (Passer domesticus) Inferred by 16S rRNA Sequence Analysis. Metagenomics. 2014;3:1–11. doi: 10.4303/mg/235853. [DOI] [Google Scholar]
- 68.Nguyen N-P, Warnow T, Pop M, White B. A perspective on 16S rRNA operational taxonomic unit clustering using sequence similarity. npj Biofil. Microbiom. 2016;2:16004. doi: 10.1038/npjbiofilms.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cole JR, et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2013;42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ling Z, et al. Pyrosequencing analysis of the human microbiota of healthy Chinese undergraduates. BMC Genomics. 2013;14:390. doi: 10.1186/1471-2164-14-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Good IJ. The population frequencies of species and the estimation of population parameters. Biometrika. 1953;40:237–264. doi: 10.1093/biomet/40.3-4.237. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Metagenomic data generated from this study has been submitted to the Sequence Read Archive (SRA) of NCBI under the BioProject ID: PRJNA530839. Other additional data are available from corresponding authors upon request.