Abstract
In this study, full-length (1282–1330 bp) α-expansin 1 (EXPA1) gene from three different accessions belonging to Saccharum complex (Saccharum officinarum—SoEXPA1, Erianthus arundinaceus—EaEXPA1, and Saccharum spp. hybrid—ShEXPA1) was isolated using RAGE technique and characterized. The intronic and coding regions of isolated expansin genes ranged between 526–568 and 756–762 bp, respectively. An open reading frame encoding a polypeptide of 252 amino acids was obtained from S. officinarum and commercial sugarcane hybrid, whereas 254 amino acids were obtained in E. arundinaceus, a wild relative of Saccharum. Bioinformatics analysis of deduced protein revealed the presence of specific signature sequences and conserved amino acid residues crucial for the functioning of the protein. The predicted physicochemical characterization showed that the protein is stable in nature with instability index (II) value less than 40 and also clearly shown the dominance of random coil in the protein structure. Phylogenetic analysis revealed high conservation of EXPA1 among Saccharum complex and related crop species, Sorghum bicolor and Zea mays. The docking study of EXPA1 protein showed the interaction with xylose, which is present in xyloglucan of plant cell wall, elucidated the role of the expansin proteins in plant cell wall modification. This was further supported by the subcellular localization experiment in which it is clearly seen that the expansin protein localizes in the cell wall. Relative expression analysis of EXPA1 gene in Saccharum complex during drought stress showed high expression of the EaEXPA1 in comparison with SoEXPA1 and ShEXPA1 indicating possible role of EaEXPA1 in increased water-deficit stress tolerance in E. arundinaceus. These results suggest the potential use of EXPA1 for increasing the water-deficient stress tolerance levels in crop plants.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1719-3) contains supplementary material, which is available to authorized users.
Keywords: Expansin, Sugarcane, Saccharum complex, Subcellular localization, Docking
Introduction
Cell wall is a distinctive feature of plant cell, and its major functions are to determine cell structure, provide tensile strength and protect against mechanical and osmotic stress. Expansin protein loosens and softens the cell wall through a non-enzymatic mechanism which is set off by inducing the reversible slippage of cellulose microfibrils in the plant cell wall (Cosgrove 2000a). Expansin proteins are relatively conserved, and typical mature protein has a molecular weight of ~ 25–30 kDa, which corresponds to 250–300 amino acids. They are classified into four families, namely α-expansin (EXPA), β-expansin (EXPB), expansin-like A (EXLA) and expansin-like B (EXLB), and reported to be involved in cell enlargement and in a variety of other developmental processes in which cell wall modification occurs (Sampedro et al. 2005a, b). Expansins not only help in cell growth but also play a part during biotic and abiotic stress conditions in plants (Sun et al. 2011). However, the precise mechanism by which this occurs remains unclear.
Since its first isolation in 1992 from cucumber, there is continuous isolation of expansin genes from different plants such as tobacco, maize and rice. There are limited studies showing the involvement of expansin genes in growth of the plant and its involvement in different abiotic stresses. In Arabidopsis thaliana, it was studied and proved that the EXPA7 was involved and required in root hair growth (Lin et al. 2011). Expression of EXPA1 gene is high in young growing potato tubers (Jung et al. 2010) and high in stamen filaments, gynoecium styles and expanding leaves in Gladiolus grandiflorus (Azeez et al. 2010) that shows its involvement in organ development of the plant. EXPA1 gene is highly expressed during drought stress condition compared to two other α-expansins (EXPA2 and EXPA3) in Craterostigma plantagineum (Jones and McQueen-Mason 2004). During abiotic stresses such as salinity, drought, cold and heat, the transcript level of EXPA1, EXPA4 and EXPA5 genes in Nicotiana tabacum increases (Kuluev et al. 2016). There was a significant increase in expression level of EXPA1 gene during heat stress in Agrostis grass species (Xu et al. 2007). Few reports have shown that expansins are involved in plant adaptation during environmental stress condition by regulating the cell expansion and the cell growth (Wu and Cosgrove 2000; Wu et al. 2001; Yang et al. 2004). More recently, overexpression of OsEXPA10 in rice resulted in increased plant height and enhanced tolerance to diseases (Tan et al. 2018).
Sugarcane, belonging to genus Saccharum, comes under Poaceae family along with Zea mays and Sorghum bicolor. The Saccharum genus and other closely related genera such as Erianthus, Miscanthus, Narenga and Sclerostachya constitute an interbreeding group which is assumed to be involved in the origin of sugarcane and thus referred to as the “Saccharum complex” (Mukherjee 1957). In one of the earlier reports, different accessions from Saccharum complex were analyzed for better drought tolerance and it was observed that the Erianthus arundinaceus IK 76-81 possessed many of the physiological and molecular parameters of drought-tolerant characteristics (Augustine et al. 2015). Also in that study, real-time PCR expression analysis using the primers designed by previously isolated EXPA1 gene from Zea mays and Oryza sativa was carried out between Erianthus arundinaceus IK 76-81 and commercial Co 86032. The expression of EXPA1 was higher in Erianthus arundinaceus IK 76-81 when compared to Co 86032 during drought stress. It was suggested that IK 76-81 is a drought tolerance accession which can be used in sugarcane crop improvement and the expansin gene can be a good candidate gene for drought tolerance development (Augustine et al. 2015). Sugarcane, an important commercial crop, faces a severe threat because of water-deficit stress. Owing to increasing importance of drought tolerance required in sugarcane, in this study for the first time α-expansin 1 (EXPA1) gene from three different accessions of Saccharum complex, viz. S. officinarum—SoEXPA1, E. arundinaceus—EaEXPA1, and Saccharum spp commercial hybrid—ShEXPA1, was isolated and characterized using bioinformatics analysis, homology modeling and expression profile study during different time intervals of drought stress. The results may be helpful to discover drought resistance mechanism and for further improvement in commercial sugarcane varieties.
Materials and methods
Saccharum complex plant materials and growth conditions
Saccharum complex plant materials maintained in the gene bank at ICAR-Sugarcane Breeding Institute, Coimbatore, India, Erianthus arundinaceus IK 76-81, S. officinarum NG 77-18 and Saccharum spp. hybrid Co 86032 were used in this study. Co 86032 is a widely cultivated commercial sugarcane variety in tropical regions of India. E. arundinaceus IK 76-81 is a known drought-tolerant accession of wild sugarcane germplasm (Augustine et al. 2015). S. officinarum NG 77-18 is source for sucrose genes, and this had been successfully exploited in sugarcane improvement programmes, and most of the present day commercial varieties are derivatives of interspecific hybrids involving S. officinarum. Single bud sett cuttings of these three accessions were planted in pots (45 cm × 40 cm) in 1:1:1 mixture (sand, red soil and farm yard manure) and were grown under green house conditions (16-/8-h light and dark photoperiod). Young leaf tissues (third leaf from top) were harvested from 90-day-old plants and stored at − 80 °C until future use.
RNA isolation and cDNA preparation
Young leaves stored at − 80 °C were used for isolation of total RNA using TRIzol method. Genomic DNA contamination was removed using RNA-free DNaseI (Invitrogen, USA). Quality of RNA was checked on 1.2% agarose gel and quantified using NanoDrop (Thermo Fisher Scientific Company, USA). About 1 μg of RNA samples was taken for cDNA synthesis using RevertAid First strand cDNA synthesis kit by following manufacturer’s instructions (Thermo Fisher Scientific Company, USA) (Chomczynski and Mackey 1995).
Isolation and sequencing of EXPA1 from Saccharum complex
As this is the first study to isolate EXPA1 gene from Saccharum complex, the gene was isolated through RAGE technique by walking using primers developed from the conserved region of the α-expansin 1 from six different plants: Oryza sativa (AF394543.1), Brassica juncea (GU222694.1), Zea mays (AF332169.1), Sorghum bicolor (XM_002456505.1), Triticum aestivum (AY485121.3) and Lycopersicon esculentum (U82123.1). EXPA1 gene sequence of these plant species was retrieved from NCBI GenBank. The gene was isolated from E. arundinaceus IK 76-81, S. officinarum NG 77-18 and Saccharum spp. hybrid Co 86032 belonging to Saccharum complex. Genomic DNA was used for isolation of full-length EXPA1 gene, and cDNA was used as template to obtain the coding region of genes. Primer pairs used for PCR amplification are given in Supplementary Table S1. PCR amplification was carried out as follows: initial denaturation of 4 min at 94 °C, 35 cycles (94 °C for 30 s, 61 °C for 30 s, 72 °C for 1 min) and final extension of 10 min at 72 °C. PCR product was analyzed on 1.2% agarose gel, and desired DNA fragment was purified using the MEGA quick-spin™ PCR and Agarose Gel DNA Extraction System (Intron Biotechnology INC., Korea). Eluted fragment was ligated into pTZ57R/T vector, and the ligated product was mobilized into Escherichia coli DH5α cells using InsTA Clone™ PCR cloning kit (Fermentas). Recombinant plasmid was isolated using QIAprep Spin Miniprep Plasmid isolation kit (Qiagen, Germany) and was sequenced by Sanger method. Primary analysis of obtained sequence was carried out by comparing the isolated genes with its closely related crops such as Z. mays and S. bicolor. To further confirm, obtained gene sequence was translated into protein using Expasy translate tool (https://web.expasy.org/translate/).
Bioinformatics and phylogenetic analysis
Expasy’s ProtParam server was used to compute physicochemical characterization (Gasteiger et al. 2005) such as theoretical molecular weight (MW), isoelectric point (pI), instability index (II) (Guruprasad et al. 1990), aliphatic index (AI) (Ikai 1980) and grand average hydropathy (GRAVY) (Kyte and Doolottle 1982). SignalP 4.1 server was used to detect the presence of signal peptide (Petersen et al. 2011) within the predicted protein encoded by the newly isolated gene from Saccharum complex. The predicted protein was also subjected to domain prediction using SMART server (http://smart.embl-heidelberg.de/). The multiple sequence alignments of isolated EXPA1 from Saccharum complex and their closely related species Z. mays and S. bicolor were generated by CLC workbench, and conserved motifs were analyzed. Secondary structure of expansins was predicted using different algorithms, viz. GOR IV (Garnier et al. 1996) (http://npsa-prabi.ibcp.fr/cgi-87bin/npsa_automat.pl?page=/NPSA/npsa_gor4.html), HNN (Guermeur 1997) (https://npsa-prabi.ibcp.fr/cgi-88bin/npsa_automat.pl?page=/NPSA/npsa _hnn.html) and PSIPRED Protein Sequence Analysis Workbench (McGuffin et al. 2000) (http://bioinf.cs.ucl.ac.uk/psipred/) programs. RaptorX server was used for tertiary structure prediction (Kallberg et al. 2012). Obtained tertiary structure was refined by energy minimization using 3Drefine server. Quality of the refined model was evaluated by the inspection of the Psi/Phi Ramachandran plot generated using RAMPAGE server (Lovell et al. 2003) and by Verify 3D of SAVES server (Bowie et al. 1991; Luthy et al. 1992). Verify 3D was used to determine or to test the compatibility of the 3D structure with its own amino acid sequence (1D). It determines the percentage of amino acid residues scored ≥ 0.2 in the 3D/1D profile.
Swissdock server, dedicated to docking of small molecules on the target proteins, was used for docking study (Grosdidier et al. 2011). Swissdock uses EADock algorithm to identify the binding sites of the ligands on the respective protein. Xylose, present in xyloglucan of the plant cell wall, was chosen as the ligand to study protein–ligand docking. Expansin protein plays a major role in plant cell wall modification, and xylose is present in xyloglucan of plant cell wall which makes xylose as an ideal ligand for protein–ligand docking study. The predicted and refined expansin tertiary structure was subjected to docking using xylose to study ligand–protein interaction (Basu and Sarkar 2014). Blind docking of the selected ligand (xylose) with expansin protein was carried out. The parameters selected for docking were “accurate” with no flexibility of side chain. The result was viewed and studied with the software UCSF Chimera (Pettersen et al. 2004).
Phylogenetic analyses of newly isolated EXPA1 sequences along with EXPA1 gene sequences from different plants species were performed. From NCBI database, EXPA1 gene from different plants species was retrieved, and along with the newly isolated EXPA1 sequences the phylogenetic tree was constructed using molecular evolutionary genetic analysis version 6 (MEGA6) software (Tamura et al. 2013).
Subcellular localization
A construct for subcellular localization of EaEXPA1 was prepared using pCAMBIA1302 as a vector. The vector pCAMBIA1302 contains mGFP5 (modified GFP5), a variant of green fluorescent protein (GFP). For better expression of GFP in transformed cells, researchers have altered cryptic intron in the wild GFP to produce different variants of GFP such as enhanced GFP (EGFP), cycle 3 GFP (GFPC3) and modified GFP5 (mGFP5). The mGFP5 is made suitable for expression in plant cell with the following changes such as removal of cryptic plant intron; nucleotides were mutated to improve folding of the apoprotein during posttranslational maturation (V163A, S175G) and coupled with I167T mutation provide equalized UV and blue light excitation (Haseloff and Siemering 1998; Stewart 2001). The codon region of the gene EaEXPA1 was amplified without termination codon using specific primers (Supplementary Table S2). The amplified fragment was digested and fused in frame with mGFP5 under control of CaMV35S promoter using NcoI and SpeI restriction sites. The vector without the expansin gene was used as the control. Onion epidermal cell layers were peeled and placed inside up on the MS plates. The subcellular localization construct plasmid along with the control plasmid was bombarded after coating it on gold particle of size 1.5–3 μm using particle gun (PDS-1000/He; Bio-Rad) into onion epidermal cells. After 24 h of incubation in MS media at 25 °C in dark, the onion epidermal cells were observed under fluorescent microscope (EVOS FL, Life Technologies, USA) for GFP fluorescence (Chujo et al. 2007).
EXPA1 gene differential expression analysis during drought stress
Different accessions belonging to Saccharum complex, i.e., Saccharum spp. hybrid Co 86032 and E. arundinaceus IK 76-81, were vegetatively propagated using single bud setts in pots. Three-month-old plants were subjected to drought stress, and leaf samples from the middle part of fully expanded third leaf were collected for RNA isolation during 5th day, 10th day and 15th day of stress along with the sample of normal irrigated plant which acts as a control. Total RNA isolation and cDNA preparation were done as mentioned above. Gene (EXPA1)-specific primers, forward primer: ACGGCCACCAACTTCTG, and reverse primer: CGGTACTGCGCGATCTG, for real-time polymerase chain reaction were designed using Integrated DNA Technology (IDT) Server. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as the internal control (Park et al. 2015; Selvarajan et al. 2018). All PCRs were performed for 40 cycles (1 cycle at 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min and subsequently 1 cycle of melt curve, 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s was followed) (Dharshini et al. 2016). qRT-PCR was performed using MESAGREEN master mix and StepOne real-time PCR system (Applied Biosystems, Burlington, Canada) as per the manufacturer’s instruction. The CT values for both the target and internal control genes were used for the quantification of transcripts by comparative CT method normalization. Later, the products were analyzed through melt curve analysis to check the specificity of PCR amplification. To minimize the error, each reaction was performed with three biological and three technical replicates. The expression of target gene was calculated using the formula: 2−∆∆CT, where ∆∆CT = ∆CT sample − ∆CT GAPDH (Livak and Schmittgen 2001). ∆∆CT values reflect the relative expression of the target gene upon exposure to drought stress.
Statistical analysis
Statistical analysis of the expression analysis data was carried out in three biological and three technical replicates. Student’s t test was performed, and the probability (P) value ≤ 0.05 was considered as statistically significant.
Results
Isolation and sequencing of EXPA1 from Saccahrum complex
Expansin (EXPA1) gene belonging to α-expansin family was isolated from Saccharum complex such as E. arundinaceus IK 76-81 (EaEXPA1), S. officinarum NG 77-18 (SoEXPA1) and Saccharum spp. hybrid Co 86032 (ShEXPA1) through RAGE technique. Full-length EXPA1 gene sequences of 1282–1330 bp were obtained. The intron length of these three EXPA1 varied from 526 to 568 nucleotides while the complete coding region (exon region) transcript of 756–762 bp was obtained with start codon (ATG) to stop codon (TGA). The EXPA1 coding region of NG 77-18 (S. officinarum) and Co 86032 (Saccharum spp. hybrid) is 756 bp while coding region of EXPA1 isolated from IK 76-81 (E. arundinaceus) is 762 bp. Isolated EXPA1 gene has two intron and three exon regions forming protein with amino acids ranging between 252 and 254. Details of EXPA1 genes isolated from Saccharum complex are given in Table 1.
Table 1.
α-Expansin family genes isolated from Saccharum complex
| Expansin gene | Species clones | Accession number | Full length (bp) | Intron (no) | Intron position | Intron size (bp) | CDS (bp) | Protein (amino acid) | |
|---|---|---|---|---|---|---|---|---|---|
| Intron 1 | Intron 2 | ||||||||
| EaEXPA1 | E. arundinaceus IK 76-81 | KY550230 | 1330 | 2 | 146–249 | 563–1026 | 568 | 762 | 254 |
| SoEXPA1 | S. officinarum NG 77-18 | KY596114 | 1282 | 2 | 140–248 | 562–978 | 526 | 756 | 252 |
| ShEXPA1 | Saccharum sp hybrid Co 86032 | MG736917 | 1285 | 2 | 140–248 | 562–981 | 529 | 756 | 252 |
Isolated EXPA1 gene showed high sequence homology between the three accessions of Saccharum complex and with its closely related crops Z. mays and S. bicolor. EXPA1 showed more than 96% homology similarity between the Saccharum complex and its close counter parts in both CDS region and amino acid sequence. The highest homology (99.2%) in CDS region is between ShEXPA1 and EaEXPA1, whereas the lowest (95.9%) is between ShEXPA1 and Z. mays. EXPA1 isolated from sugarcane variety Co 86032 showed higher nucleotide sequence similarity with NG 77-18 than IK 76-81. In the translated amino acid sequence, the highest (99.6%) homology was recorded between Co 86032 and NG 77-18 and the lowest (95.3%) homology was recorded between Z. mays and IK 76-81. Details of CDS and protein similarity percentage are given in Table 2. Full-length nucleotide sequence of EXPA1 was submitted to NCBI GenBank database under the accession numbers KY550230, KY596114 and MG736917.
Table 2.
Percentage of similarity in CDS region and protein among the Saccharum complex and to its closely related Zea mays and Sorghum bicolor
| Similarity (%) | CDS | Protein | ||||||
|---|---|---|---|---|---|---|---|---|
| Zea mays | Sorghum bicolor | IK 76-81 | NG 77-18 | Zea mays | Sorghum bicolor | IK 76-81 | NG 77-18 | |
| IK 76-81 | 96.2 | 96.6 | – | 95.3 | 97.2 | – | ||
| NG 77-18 | 96.2 | 97.6 | 97.9 | – | 96.0 | 98.0 | 99.2 | – |
| Co 86032 | 95.9 | 97.4 | 97.6 | 99.2 | 95.7 | 97.6 | 98.8 | 99.6 |
Bioinformatics and phylogentic analysis
Analysis of the sequences using SignalP 4.1 server revealed a cleavage site in between the residue numbers 24 and 25 in all the expansins that have been reported in this study. Domain analysis using SMART server showed the presence of two domains. The first domain is the N-terminal domain which consists of six-stranded Double-psi beta-barrel (DPBB) which is similar to that of family 45 of glycosyl hydrolases (GH45). Hence, this N-terminal domain is often described as GH45-like. After the N-terminal domain is the second domain, namely C-terminal domain, which is in homology with grass pollen allergens, having β-sandwich fold. In EaEXPA1, the N-terminal domain starts from 66th amino acid and ends at 151th amino acid, whereas in SoEXPA1 and ShEXPA1 it is from 64th to 149th amino acids. The C-terminal domain in EaEXPA1 is from 162th amino acid till 239th amino acids, and in other two accessions it is from 160th to 237th amino acid.
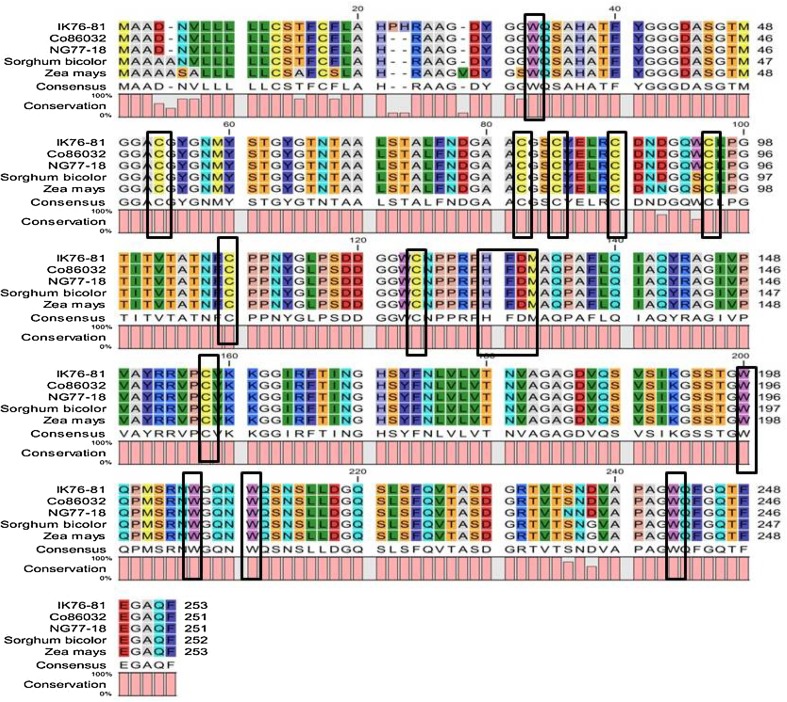
Multiple sequence alignment analysis using CLC workbench was able to identify conserved motif and residues in EXPA1 protein. There is conserved His-Phe-Asp (HFD) motif in the center region and one conserved tryptophan (W) residue at N-terminal; meanwhile, there are four tryptophans (W) residue at the C-terminal. A total of eight conserved cysteine (C) residues were recognized, of which seven are in N-terminal and one in C-terminal region (Fig. 1). Expasy’s ProtParam analysis revealed that the molecular weight of all the three isolated expansin proteins exceeds 26500 daltons, theoretical pI of EaEXPA1 was 5.87, while ShEXPA1 and SoEXPA1 recorded 5.69. EXPA1 protein from these three accessions is stable with score < 40 in instability index (II) (Guruprasad et al. 1990). EXPA1 protein shows low GRAVY index which infers that the protein could interact better with water as it will be less hydrophobic in nature. Lower GRAVY index of EXPA1 from E. arundinaceus suggests that this could interact better to maintain water balance in plant. The details of physicochemical characterization are shown in Table 3. Secondary structure of EXPA1 predicted using GORIV, HNN and Psipred revealed the dominance of random coils (62% to 66%) followed by extended strands (18% to 27%) and α-helices (6% to 18%) (Supplementary Table S3).
Fig. 1.
Multiple sequence alignment using CLC workbench. The conserved regions, namely HFD, tryptophan (W) and cysteine (C) residues, are enclosed in boxes
Table 3.
Physicochemical characterization of EXPA1 protein from Saccharum complex
| Expansin gene | Molecular weight | Theoretical pI | Instability index (II) | Aliphatic index | GRAVY |
|---|---|---|---|---|---|
| IK 76-81 EXPA1 | 26804.83 | 5.87 | 33.33 (stable) | 61.78 | − 0.141 |
| NG 77-18 EXPA1 | 26597.60 | 5.69 | 33.03 (stable) | 62.27 | − 0.133 |
| Co 86032 EXPA1 | 26570.57 | 5.69 | 33.63 (stable) | 62.27 | − 0.123 |
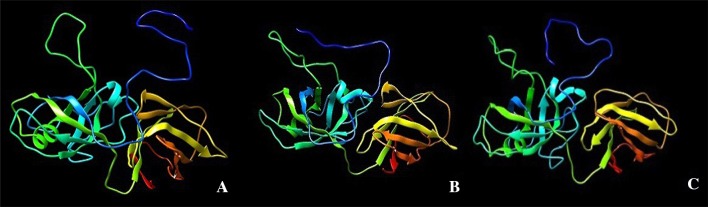
Tertiary structure of the protein was predicted using RaptorX server (Fig. 2). The 3Drefine server refines the tertiary structure by optimization of hydrogen bonding network combined with atomic-level energy minimization on the optimized model using a composite physics and knowledge-based force fields (Bhattacharya et al. 2016). These structures were further evaluated using Ramachandran plot by RAMPAGE server (Supplementary Fig. 1) and Verify 3D (SAVES server). In all the three accessions, after the model was energy minimized, it was seen in Ramachandran plot that the favored region is more than 90%. Better results were seen both in Ramachandran plot and in Verify 3D before and after energy minimization. The purpose of Verify 3D is to determine or to test the compatibility of the atomic model (3D) with its own residue (1D) by assigning a structural class based on its location, environment and comparing the results to good structure (Eisenberg et al. 1997). With more than 80% of the amino acid having 3D/1D score ≥ 0.2 in Verify 3D, all the isolated expansins are reliable and compatible with its own sequence. Detailed results of Ramachandran plot and Verify 3D after energy minimization are given in Supplementary Table S4.
Fig. 2.
Tertiary structure model of IK 76-81 (a), Co 86032 (b) and NG 77-18 (c). Tertiary structure model was determined by RaptorX server
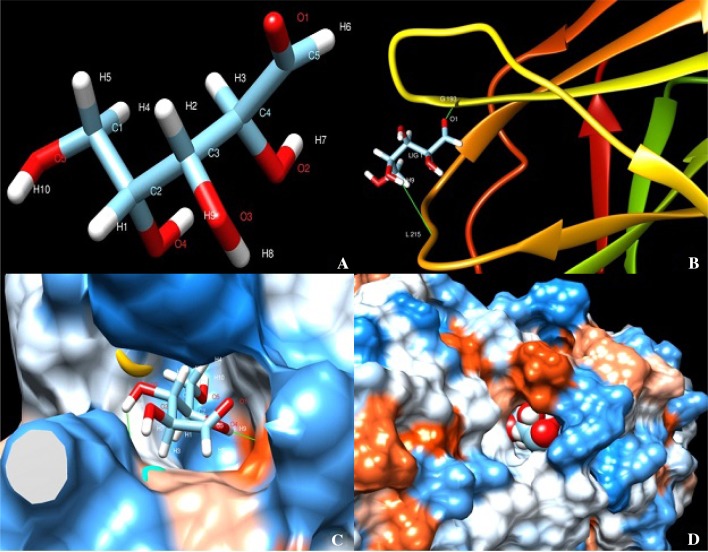
Protein–ligand of expansin protein (EXPA1) from all the accessions were successfully docked using xylose as ligand in swissdock server. Swissdock gave results in terms of full fitness and ΔG free energy of binding (Kcal/mol). Docking generated around 80 binding clusters with different binding cavities; based on the best full fitness and best ΔG score the clusters were selected. The best full fitness for IK 76-81, NG 77-18 and Co 86032 is − 952.43, − 920.39 and − 845.13, respectively. The best ΔG Kcal/mol is − 6.55, − 6.78 and − 6.66 for IK 76-81, NG 77-18 and Co 86032, respectively. In all the three accessions, xylose forms two hydrogen bonds with EXPA1; in IK 76-81 it forms H bonding between O1 atom of xylose and H atom of GLY193, and a second H bonding is formed between H9 atom of xylose and O atom of LEU215. In NG 77-18, H bonding is formed between H8 atom of xylose and O atom of ASP74 and another is formed between H9 atom of xylose and O atom of VAL184. In Co 86032, H7 and H9 atom of Xylose forms H bond with VAL155 and GLY79, respectively. Visualization of the docking was carried out in UCSF Chimera (Pettersen et al. 2004) as shown in Fig. 3.
Fig. 3.
Protein–ligand docking using Swissdock viewed in USCF Chimera. a Xylose ligand. b Representation of docking in IK 76-81 using molecular back bone structure. c, d Protein–ligand interaction in NG 77-18 and Co 86032 represented using sphere surface model representation of protein–ligand interaction
A total of 38 EXPA1 sequences of which 35 EXPA1 sequences from different plant species retrieved from NCBI database and 3 EXPA1 isolated from Saccharum complex were used to construct a phylogenetic tree using MEGA6 (Fig. 4). Phylogenic tree clearly showed that the newly isolated EXPA1 genes from Saccharum complex make a separate group with its close relatives Z. mays and S. bicolor.
Fig. 4.
Phylogenetic tree of 38 EXPA1 sequence from different plants was constructed by Neighbor-Joining method with 1000 bootstrap replicates implemented in MEGA6. It is clearly seen that EXPA1 from Saccharum complex falls in a same cluster with its close relatives Z. mays and S. bicolor. This shows that EXPA1 is conserved in nature signifying lower evolution rate
Subcellular localization
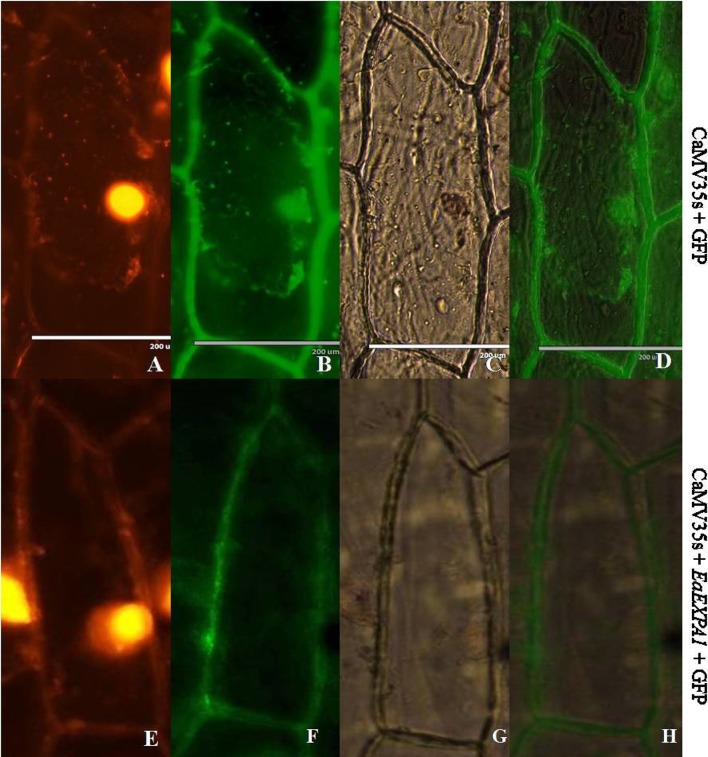
Vector for subcellular localization was constructed using pCAMBIA1302 as backbone, in which EaEXPA1 gene was fused in frame with mGFP5 under the control of CaMV35S promoter using the restriction sites NcoI and SpeI (CaMV35S + EaEXPA1 + mGFP5). Fused construct and control (CaMV35S + mGFP5) plasmids (Supplementary Fig. 2) were bombarded into onion epidermal cells. In control vector (CaMV35S + mGFP5), bombarded onion epidermal cells, the green fluorescent protein (GFP) fluorescence was visualized throughout cells, whereas cells transformed with CaMV35S + EaEXPA1 + mGFP5 vector displayed a strong green fluorescence in cell walls and no fluorescence was detected in the other regions of the cell. The result shows that signal peptide in the gene EaEXPA1 directs it to localize in the cell wall (Fig. 5).
Fig. 5.
Subcellular localization of EaEXPA1 in epidermal onion cells. Both control plasmid (35 s + mGFP5) and EaEXPA1 fused with mGFP5 (35 s + EaEXPA1 + mGFP5) were bombarded in epidermal onion cells. Above figure shows propidium iodide staining (a, e), GFP fluorescence (b, f), bright field (c, g), merged picture with GFP fluorescence and bright field (d, h) in which the expression of the expansin gene in the cell wall can be clearly observed
EXPA1 is responsive to water-deficit stress conditions
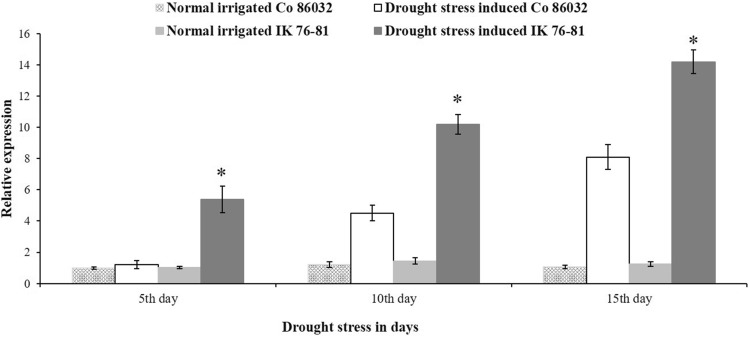
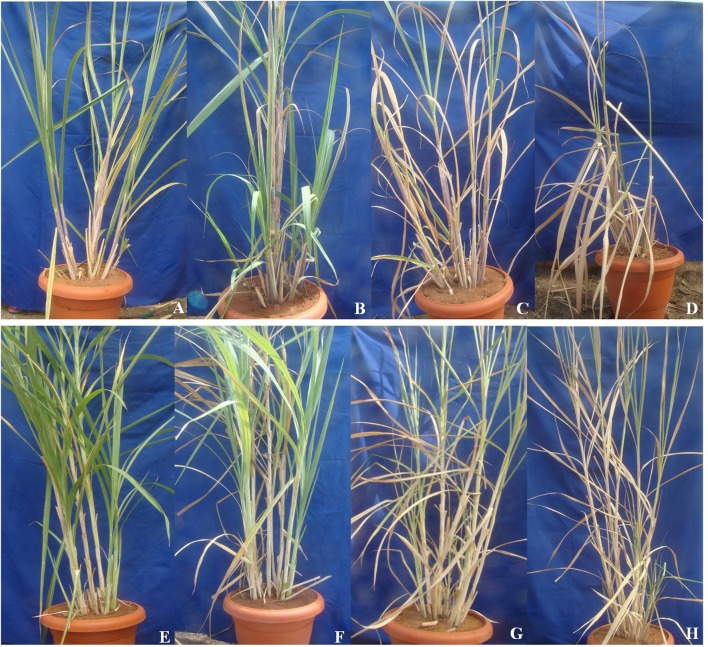
Expression of EXPA1 gene was studied in Saccharum complex under water-deficit stress conditions. An increase in accumulation of EXPA1 transcripts when exposed to limited water conditions was observed. During drought stress, the relative expression of EXPA1 is higher in E. arundinaceus IK 76-81, a wild relative of sugarcane, than that of commercial sugarcane variety Co 86032. Expression level of EXPA1 gene increases gradually from 5th to 15th day of stress when compared to the control plants (0th day of stress). Expression of EXPA1 gene in Erianthus is two- to threefold higher when compared to commercial variety during stress (Fig. 6). Individual relative expression readings of all the three biological and three technical replicates are given in supplementary Fig. 3. Morphological variation in both Saccharum spp. hybrid Co 86032 and E. arundinaceus IK 76-81 is well seen at different stages of the drought stress (Fig. 7). Among these accessions, the wild relative of sugarcane E. arundinaceus was able to withstand the drought better than that of commercial sugarcane Co 86032.
Fig. 6.
Relative expression of EXPA1 gene during drought stress in Saccharum complex. The error bars indicate SD. *Indicates significant difference in expression between E. arundinaceus IK 76-81 and Co 86032 during drought stress (P ≤ 0.05 by student T test)
Fig. 7.
Morphological changes of the commercial Saccharum hybrid Co 86032 and Erianthus arundinaceus IK 76-81 during different days of drought stress. Morphological changes of Co 86032 under normal irrigation (a), 5th (b), 10th (c) and 15th (d) day of drought stress and Erianthus arundinaceous under normal irrigation (e), 5th (f), 10th (g) and 15th (h) day of drought, respectively
Discussion
In this study, α-expansin 1 (EXPA1) was isolated and characterized in Saccharum complex. Multiple sequence analysis showed high homology, ranging from 95 to 99% CDS and protein similarity, both within the Saccharum complex and with its closely related crops, Z. mays and S. bicolor. The number of introns and exons is same as that of previously reported EXPA1 gene from different plant species (Harmer et al. 2002). High level of gene sequence similarities was observed between related species and genera in other crops (Lakhanpal et al. 2018). This showed the conserved nature of expansin genes in the closely related species or genera. It also revealed the presence of conserved HDF motif, five tryptophan and eight cysteine residues in α-expansin protein, the same as present in other plant species EXPA1 protein. High conservation of EXPA1 indicates the importance of structure–function relationship and low rate of evolution across species.
Physicochemical characterization was carried out in Expasy’s ProtParam server. Instability index (II) showed that EXPA1 protein of all the three accessions is stable as the instability index is < 40 and has a low GRAVY index which infers that the protein could interact better with water as it will be less hydrophobic in nature. Random coils play an important function in protein for flexibility and conformation changes such as enzymatic turnover (Buxbaum 2007). The presence of large random coils in EXPA1 protein indicates that it might exhibit high flexibility and conformation changes under stress conditions. 3-D structure of putative EXPA1 was in line with already known protein in other crops. Preservation of amino acids residues concerned substantiates that EXPA1 function is conserved through its structural features across species. Phylogenetic analyses revealed that expansin genes from Saccharum complex were in the same cluster with its close relatives Z. mays and S. bicolor. This showed that EXPA1 is conserved in nature signifying lower evolution rate. In a very recent study, a total of 92 expansin genes: 51 EXPA, 38 EXPB and three EXPLA were reported from sugarcane cultivar SP80-3280 (Santiago et al. 2018). These expansins also contain HFD conserved motif, two domains (N-terminal domain and pollen allergen C-terminal domain) and number of introns which coincide with the present study and findings. These conserved residues and motif such as HFD, cysteine (C) and tryptophan (W) residues were seen in expansin gene across the species (Choi et al. 2006).
The presence of signal peptide in the protein of the three isolated sugarcane expansins (EXPA1) was detected using SignalP4.1 server. The presence of the signal peptide in the sugarcane EXPA1 protein in the N- terminal domain region is in agreement with the earlier reported expansin proteins from other plant species (Cosgrove 2000a, b). The N-terminal domain which is similar to family-45 glycosyl hydrolase and pollen allergen C-terminal domain was also present in previously reported expansin proteins (Cosgrove 2015). Thus, most of the regions of expansin protein are conserved and may be due to their important role in plant growth and development as indicated by Tan et al. (2018).
Xyloglucan is abundant hemicellulose in plant cell wall which is made up of glucose and xylose. Docking study of expansin protein by using xylose as a ligand suggests the interaction between the protein and cell wall. This explains the possible interaction and its involvement in cell wall loosening. Subcellular localization also proves that the EXPA1 protein is localized in cell wall of plant. Expansin localized to cell wall is also observed in other expansins isolated from different species (Vannerum et al. 2011; Zenoni et al. 2011). The presence of a signal peptide, the docking study and the subcellular localization study evidence the possible interaction of EaEXPA1 with plant cell wall.
The gene’s expression level varies during different levels of water-deficit stress conditions. The wild species, E. arundinaceus, had higher EXPA1 gene expression under water-deficit stress conditions than the commercial variety which had the lower expression of the gene. The higher expression of expansin gene in E. arundinaceus might have helped the cell to maintain its flexibility under water-deficit condition which in turn helps the plant survive under abiotic stress condition as noticed in other crops (Lu et al. 2013). This analysis revealed that EXPA1 could play an important role in regulation during drought stress. Moreover, E. arundinaceus has a lowest GRAVY index, suggesting that this EXPA1 in E. arundinaceus might interact better with water and helps to maintain the water balance in plant. It was earlier seen in different reports that expansins help in the resurrection of the plant during abiotic stresses such as drought, cold and salinity stresses (Marowa et al. 2016). In an earlier report, it was observed that the overexpression of Poa pratensis EXPA1 in transgenic tobacco plants were able to withstand the heat stress much better than the untransformed tobacco plants (Xu et al. 2014). It was seen under drought stress conditions expansin gene overexpression helps the plants to perform better than the normal plants (Lu et al. 2013). One of the mechanisms in increased tolerance level of the plant toward drought stress is the increase in degree of cell wall flexibility/cell membrane permeability, and perhaps, expansins are expected to play an important role in cell wall modifications during stress conditions. However, further studies need to be carried out to understand the role of expansins in cell wall flexibility under stress conditions.
Conclusion
It is essential to increase the sugarcane yield to meet the increasing demand for sugar and its byproducts. Sugarcane productivity is challenged by numerous abiotic stresses. Among these, drought stress causes 50% yield loss. Expansin genes reported to play a vital role in both cell growth, and biotic and abiotic stress tolerance. Comparative expression experiment of EXPA1 on wild type E. arundinaceous and commercial Saccharum hybrid showed significantly higher levels of EXPA1 in Erianthus when compared to Saccharum hybrid during drought treatment. Localization study revealed that EXPA1 expression is confined to cell wall. This study provides an initial step in gaining knowledge about the role of EXPA1 in Saccharum complex under drought stress. This newly isolated and characterized expansin (EXPA1) gene from Saccharum complex could be a good candidate gene for improving drought tolerance in sugarcane and other agricultural crops.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Director, ICAR-Sugarcane Breeding Institute, Coimbatore, Tamil Nadu, India, for providing the facilities. This work was supported by the Department of Biotechnology (DBT) (Grant no. 102/IFD/SAN/1151/2017-2018), Government of India. One of the authors, Ashwin Narayan J, thanks Council of Scientific and Industrial Research (CSIR), New Delhi, India, for the award of Senior Research Fellowship (SRF) (09/706/0003/2018-EMR-I).
Author contributions
JAN, NS, MNP and CA conceived and designed the research. JAN, SD, VMN, TSSP, KK and GSS performed the experiments. JAN, MNP and CA analyzed the results and drafted the manuscript. BR revised the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
M. N. Premachandran, Email: premsbi@rediffmail.com
C. Appunu, Phone: +91 9345061233, Email: cappunu@gmail.com
References
- Augustine SM, Syamaladevi DP, Premachandran MN, Ravichandran V, Subramonian N. Physiological and molecular insights to drought responsiveness in Erianthus sp. Sugar Tech. 2015;17(2):121–129. doi: 10.1007/s12355-014-0312-7. [DOI] [Google Scholar]
- Azeez A, Sane AP, Tripathi SK, Bhatnagar D, Nath P. The gladiolus GgEXPA1 is a GA-responsive α-expansin gene expressed ubiquitously during expansion of all floral tissues and leaves but repressed during organ senescence. Postharvest Biol Technol. 2010;58:48–56. doi: 10.1016/j.postharvbio.2010.05.006. [DOI] [Google Scholar]
- Basu A, Sarkar A (2014) Molecular docking and ligand-protein interaction study of the expansin protein AtEXPA23 and EXLX1, In: Proceedings of 1st International Science and Technology Congress, pp 1–8
- Bhattacharya D, Nowotny J, Cao R, Cheng J. 3Drefine: an interactive web server for efficient protein structure refinement. Nucleic Acids Res. 2016;44(W1):W406–W409. doi: 10.1093/nar/gkw336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie JU, Luthy R, Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991;253(5016):164–170. doi: 10.1126/science.1853201. [DOI] [PubMed] [Google Scholar]
- Buxbaum E. Fundamentals of protein structure and function. New York: Springer; 2007. [Google Scholar]
- Choi D, Cho HT, Lee Y. Expansins: expanding importance in plant growth and development. Physiol Plant. 2006;126(4):511–518. doi: 10.1111/j.1399-3054.2006.00612.x. [DOI] [Google Scholar]
- Chomczynski P, Mackey K. Modification of the TRI reagent DNA/protein isolation procedure for isolation of RNA from polysaccharide and proteoglycan rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- Chujo T, Takai R, Akimoto-Tomiyama C, Ando S, Minami E, Nagamura Y, Kaku H, Shibuya N, Yasuda M, Nakashita H, Umemura K. Involvement of the elicitor-induced gene OsWRKY53 in the expression of defense-related genes in rice. Biochim Biophys Acta Gene Struct Expr. 2007;1769(7–8):497–505. doi: 10.1016/j.bbaexp.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. New genes and new biological roles for expansins. Curr Opin Plant Biol. 2000;3(1):73–78. doi: 10.1016/S1369-5266(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Plant expansins: diversity and interactions with plant cell walls. Curr Opin Plant Biol. 2015;25:162–172. doi: 10.1016/j.pbi.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharshini S, Chakravarthi M, Ashwin Narayan J, Manoj VM, Naveenarani M, Kumar R, Meena M, Ram B, Appunu C. De novo sequencing and transcriptome analysis of a low temperature tolerant Saccharum spontaneum clone IND 00-1037. J Biotechnol. 2016;231:280–294. doi: 10.1016/j.jbiotec.2016.05.036. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Luthy R, Bowie JU. VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol. 1997;277:396–404. doi: 10.1038/356083a0. [DOI] [PubMed] [Google Scholar]
- Garnier J, Gibrat JF, Robson B. GOR secondary structure prediction method version IV. Methods Enzymol. 1996;266:540–553. doi: 10.1016/S0076-6879(96)66034-0. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins, Appel RD, Bairoch A. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The proteomics protocols handbook. New York: Human Press; 2005. pp. 571–607. [Google Scholar]
- Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39(2):270–277. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermeur Y (1997) Combinaison de classifieurs statistiques, Application a la prediction de structure secondaire des proteins. Ph. D. Thesis, Universite Paris
- Guruprasad K, Reddy BVP, Pandit MW. Correlation between stability of a protein and its dipeptide composition: a novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng Des Sel. 1990;4:155–164. doi: 10.1093/protein/4.2.155. [DOI] [PubMed] [Google Scholar]
- Harmer S, Orford S, Timmis J. Characterisation of six α-expansin genes in Gossypium hirsutum (upland cotton) Mol Genet Genom. 2002;268(1):1–9. doi: 10.1007/s00438-002-0721-2. [DOI] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR. The uses of green fluorescent protein in plants. In: Chalfie M, Kain SR, editors. Green fluorescent protein: properties, applications, and protocols. Chichester: Wiley; 1998. pp. 191–220. [Google Scholar]
- Ikai AJ. Thermo stability and aliphatic index of globular proteins. J Biochem. 1980;88:1895–1898. doi: 10.1093/oxfordjournals.jbchem.a133168. [DOI] [PubMed] [Google Scholar]
- Jones L, McQueen-Mason S. A role for expansins in dehydration and rehydration of the resurrection plant Craterostigma plantagineum. FEBS Lett. 2004;559(1–3):61–65. doi: 10.1016/S0014-5793(04)00023-7. [DOI] [PubMed] [Google Scholar]
- Jung J, O’Donoghue EM, Dijkwel PP, Brummell DA. Expression of multiple expansin genes is associated with cell expansion in potato organs. Plant Sci. 2010;179:77–85. doi: 10.1016/j.plantsci.2010.04.007. [DOI] [Google Scholar]
- Kallberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J. Template-based protein structure modeling using the RaptorX web server. Nat Protoc. 2012;7(8):1511–1522. doi: 10.1038/nprot.2012.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuluev B, Avalbaev A, Mikhaylova E, Nikonorov Y, Berezhneva Z, Chemeris A. Expression profiles and hormonal regulation of tobacco expansin genes and their involvement in abiotic stress response. J Plant Physiol. 2016;206:1–12. doi: 10.1016/j.jplph.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolottle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lakhanpal N, Verma D, Kaur R, Singh K. Characterization of cold responsive uncoupling protein1 (UCP1) gene from Brassica juncea L. (Czern. and Coss.) J Plant Biochem Biotechnol. 2018;27(1):108–117. doi: 10.1007/s13562-017-0421-y. [DOI] [Google Scholar]
- Lin C, Choi HS, Cho HT. Root hair-specific EXPANSIN A7 is required for root hair elongation in Arabidopsis. Mol Cells. 2011;31(4):393–397. doi: 10.1007/s10059-011-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lovell SC, Davis IW, Arendall WB, III, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Calpha geometry: phi, psi and Cbeta deviation. Proteins: structure. Funct Genet. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- Lu P, Kang M, Jiang X, Dai F, Gao J, Zhang C. RhEXPA4, a rose expansin gene, modulates leaf growth and confers drought and salt tolerance to Arabidopsis. Planta. 2013;237(6):1547–1559. doi: 10.1007/s00425-013-1867-3. [DOI] [PubMed] [Google Scholar]
- Luthy R, Bowie JU, Eisenberg D. Assessment of protein models with three-dimensional profiles. Nature. 1992;356(6364):83–85. doi: 10.1038/356083a0. [DOI] [PubMed] [Google Scholar]
- Marowa P, Ding A, Kong Y. Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016;35(5):949–965. doi: 10.1007/s00299-016-1948-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16(4):404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- Mukherjee SK. Origin and distribution of Saccharum. Bot Gaz. 1957;119:55–61. doi: 10.1086/335962. [DOI] [Google Scholar]
- Park JW, Benatti TR, Marconi T, Yu Q, Solis-Gracia N, Mora V, da Silva JA. Cold responsive gene expression profiling of sugarcane and Saccharum spontaneum with functional analysis of a cold inducible saccharum homolog of NOD26-like intrinsic protein to salt and water stress. PLoS One. 2015;10(5):e0125810. doi: 10.1371/journal.pone.0125810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from trans-membrane regions. Nat Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Sampedro J, Cosgrove DJ, et al. The expansin superfamily. Genome Biol. 2005;6(12):242. doi: 10.1186/gb-2005-6-12-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro J, Lee Y, Carey RE, dePamphilis C, Cosgrove DJ. Use of genomic history to improve phylogeny and understanding of births and deaths in a gene family. Plant J. 2005;44(3):409–419. doi: 10.1111/j.1365-313X.2005.02540.x. [DOI] [PubMed] [Google Scholar]
- Santiago TR, Pereira VM, de Souza WR, Steindorff AS, Cunha BA, Gaspar M, Fávaro LC, Formighieri EF, Kobayashi AK, Molinari HB. Genome-wide identification, characterization and expression profile analysis of expansins gene family in sugarcane (Saccharum spp.) PloS One. 2018;13(1):e0191081. doi: 10.1371/journal.pone.0191081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajan D, Mohan C, Dhandapani V, Nerkar G, Jayanarayanan AN, Mohanan MV, Murugan N, Kaur L, Chennappa M, Kumar R, Meena M. Differential gene expression profiling through transcriptome approach of Saccharum spontaneum L. under low temperature stress reveals genes potentially involved in cold acclimation. 3 Biotech. 2018;8(4):195. doi: 10.1007/s13205-018-1194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. The utility of green fluorescent protein in transgenic plants. Plant Cell Rep. 2001;20(5):376–382. doi: 10.1007/s002990100346. [DOI] [PubMed] [Google Scholar]
- Sun T, Zhang Y, Chai T. Cloning, characterization, and expression of the BjEXPA1 gene and its promoter region from Brassica juncea L. Plant Growth Regul. 2011;64(1):39–51. doi: 10.1007/s10725-010-9533-2. [DOI] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Wang M, Shi Z, Miao X. OsEXPA10 mediates the balance between growth and resistance to biotic stress in rice. Plant Cell Rep. 2018;37:993–1002. doi: 10.1007/s00299-018-2284-7. [DOI] [PubMed] [Google Scholar]
- Vannerum K, Huysman MJ, De Rycke R, Vuylsteke M, Leliaert F, Pollier J, Lutz-Meindl U, Gillard J, De Veylder L, Goossens A, Inze D. Transcriptional analysis of cell growth and morphogenesis in the unicellular green alga Micrasterias (Streptophyta), with emphasis on the role of expansin. BMC Plant Biol. 2011;11(1):128. doi: 10.1186/1471-2229-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YJ, Cosgrove DJ. Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J Exp Bot. 2000;51(350):1543–1553. doi: 10.1093/jexbot/51.350.1543. [DOI] [PubMed] [Google Scholar]
- Wu Y, Thorne ET, Sharp RE, Cosgrove DJ. Modification of expansin transcript levels in the maize primary root at low water potentials. Plant Physiol. 2001;126(4):1471–1479. doi: 10.1104/pp.126.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Tian J, Belanger FC, Huang B. Identification and characterization of an expansin gene AsEXP1 associated with heat tolerance in C3 Agrostis grass species. J Exp Bot. 2007;58(13):3789–3796. doi: 10.1093/jxb/erm229. [DOI] [PubMed] [Google Scholar]
- Xu Q, Xu X, Shi Y, Xu J, Huang B. Transgenic tobacco plants overexpressing a grass PpEXP1 gene exhibit enhanced tolerance to heat stress. PLoS One. 2014;9(7):e100792. doi: 10.1371/journal.pone.0100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zheng B, Mao C, Qi X, Liu F, Wu P. Analysis of transcripts that are differentially expressed in three sectors of the rice root system under water deficit. Mol Genet Genom. 2004;272(4):433–442. doi: 10.1007/s00438-004-1066-9. [DOI] [PubMed] [Google Scholar]
- Zenoni S, Fasoli M, Tornielli GB, Dal Santo S, Sanson A, de Groot P, Sordo S, Citterio S, Monti F, Pezzotti M. Overexpression of PhEXPA1 increases cell size, modifies cell wall polymer composition and affects the timing of axillary meristem development in Petunia hybrida. New Phytol. 2011;191(3):662–677. doi: 10.1111/j.1469-8137.2011.03726.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.