SUMMARY
Immune checkpoints normally stop the body from mounting an immune response against healthy cells. Some cancers can acquire these checkpoints so that the tumour cells are not recognised by the immune system
Inhibiting the checkpoints therefore enables the tumour cells to be recognised and allows an immune response to be activated against them
Immune checkpoint inhibitors can improve the survival of some patients with advanced malignancies. These include malignant melanoma, renal cell carcinoma, urothelial bladder cancer and non-small cell lung cancer
Trials have shown that immune checkpoint inhibitors have significant benefits over conventional therapies so they are increasingly being used in routine clinical practice
However, a significant proportion of patients will not respond to immune checkpoint inhibitors and retain a poor prognosis. The optimal use of these drugs requires further study
Immune-related adverse events commonly include pneumonitis, hepatitis, nephritis, colitis and endocrinopathies. However, nearly any organ system can be affected. These toxicities present clinicians with a new challenge of recognising them early and acting promptly
Keywords: avelumab, durvalumab, ipilimumab, melanoma, nivolumab, non-small cell lung cancer, pembrolizumab, bladder cancer
Introduction
In the last 40 years, our understanding of the relationship between immune surveillance and tumour proliferation has advanced at a rapid pace. This has resulted in the development of immunotherapies such as the immune checkpoint inhibitors. Examples include ipilimumab, nivolumab, pembrolizumab, durvalumab and avelumab. These monoclonal antibodies are given by infusion.
Immune checkpoint inhibitors have already become the first-line treatment for patients with advanced melanoma and non-small cell lung cancer. Efficacy has also been shown in the second-line setting and there are ongoing phase III trials looking into their effectiveness in other cancer subtypes, such as lymphoma.1
Mechanisms of action
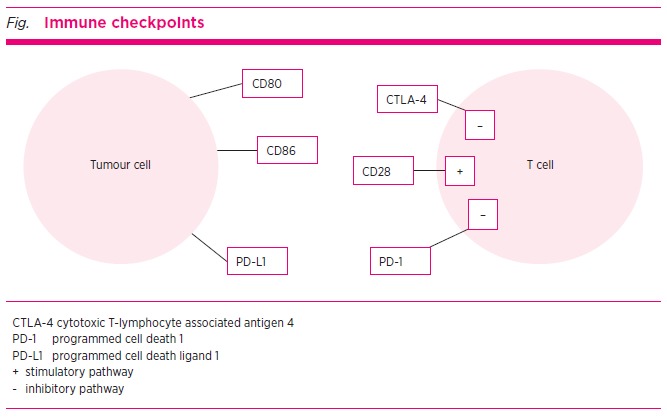
One of the most fundamental characteristics of the human immune system is its ability to differentiate between self and non-self cells, such as tumour cells. This process is regulated by a balance between co-stimulatory and inhibitory signals, collectively known as immune checkpoints. Maintaining this balance is crucial for preventing an autoimmune reaction against normal cells.
T cells are lymphocytes involved in cell-mediated immune responses. T-cell activation begins when an antigen binds to a T-cell receptor.2 This component of the immune response is highly precise, as the receptor on the T cell is specific for one particular antigen. In addition to this antigen-specific binding, a ‘second signal’ is needed for T-cell activation. This involves co-stimulatory receptors such as CD28.
The two-step process acts as a fail-safe, to prevent an inappropriate immune response causing damage to healthy tissues. If a second signal is not received, the T cells become anergic.
Two pathways are central to the immune process:
cytotoxic T-lymphocyte associated antigen 4 (CTLA-4)
programmed cell death 1 (PD-1) molecule.
The CTLA-4 pathway is the best studied and its predominant role is as an immune dampener to prevent the initial activation of T cells in lymph nodes. PD-1 regulates the interaction of already activated T cells in extra-lymphatic tissues (see Fig.).2
Fig.
Immune checkpoints
Highly mutant tumours are commonly able to acquire, or ‘hijack’ the immune checkpoints. This allows tumour cells to be inappropriately recognised as self tissues and so they restrain the T cell’s ability to mount an effective antitumour response.
The immune checkpoint inhibitors stop the inhibitory effects of tumour cells on T cells. By inhibiting the immune checkpoints, immune-mediated antitumour activity is restored.
Inhibiting the immune checkpoints reduces the body’s ability to dampen the immune response. This causes a marked increase in immune-mediated toxicity and attacks on healthy tissues. It is this mechanism that accounts for the broad range of immune-related adverse events associated with immune checkpoint inhibitors.3
CTLA-4 pathway
The CTLA-4 receptor is part of an inhibitory pathway. This downregulates T-cell function and acts in conjunction with the co-stimulatory receptor CD28. The CTLA-4 molecule and CD28 are expressed on T cells. Both bind the ligands CD80 and CD86 which are located on the surface of antigen-presenting cells.
CTLA-4 binds CD80/CD86 with a significantly greater affinity and avidity than CD28. This binding results in a CTLA-4 and CD80/CD86 complex which has an immune dampening effect and leads to T-cell anergy. Conversely, if a complex of CD28 with CD80/CD86 is formed, then a co-stimulatory signal is produced and T-cell activation occurs. The relative ratio of CD80/CD86 binding with CD28 versus CTLA-4 will determine whether a T cell will undergo activation or anergy.4
Tumour cells can generate inappropriate CTLA-4 signalling, enabling them to evade normal immune surveillance. Ipilimumab4 and tremelimumab are both fully human monoclonal antibodies that bind CTLA-4 and antagonise the binding of CTLA-4 with CD80/CD86. This then allows for increased binding of CD28 with CD80/CD86 leading to immune recognition of the tumour cells and T-cell activation.
PD-1 pathway
The PD-1 pathway has an inhibitory effect on the immune system. It downregulates T-cell function. The PD-1 molecule is expressed on T cells and binds to programmed cell death ligand 1 (PD-L1), which is found on antigen-presenting cells. This interaction between PD-1 and PD-L1 produces a signal that inhibits T-cell proliferation, resulting in immune dampening and T-cell anergy.5
Tumour cells can develop the ability to harness this mechanism resulting in inappropriate PD-L1 expression and activation of the inhibitory signalling pathway. This enables tumours to evade antigen-specific T-cell immune responses.
Nivolumab and pembrolizumab are both fully human monoclonal antibodies that inhibit the interaction between PD-1 and PD-L1. This prevents the downregulation of T cells and tumour cell evasion of normal immune surveillance.2,5 Recognition of the tumour cells enhances antitumour immune-mediated activity.
CTLA-4 inhibitors
Ipilimumab, a fully human monoclonal antibody, is a CTLA-4 inhibitor. By blocking the inhibitory effect of CTLA-4 on T cells, ipilimumab can stimulate a T-cell-mediated immune response against the cancer cells.6
Ipilimumab was approved after it was shown to prolong median overall survival from six months to 10 months in patients with advanced melanoma in a phase III randomised controlled trial.7,8 Before this, the median overall survival had ranged from 6–7 months with dacarbazine chemotherapy alone. However, when ipilimumab was later compared to more recently developed PD-1 immune checkpoint inhibitors, it was found to be inferior as a monotherapy.
Tremelimumab is another CTLA-4 inhibitor. In April 2008, a phase III trial in advanced melanoma was discontinued after a review of interim data indicated that tremelimumab was not superior to standard chemotherapy. The clinical role of tremelimumab has since remained limited.
PD-1 inhibitors
Programmed cell death ligand 1 (PD-L1) is expressed on the surface of multiple tissue types, including kidneys and lungs, and is important in normal immune function. PD-1 inhibitors prevent the ligand from binding to its receptor, thus allowing the immune system to recognise cancer cells.
Nivolumab has efficacy in the treatment of advanced melanoma, non-small cell lung cancer and renal cell carcinoma.9 Pembrolizumab has been used in the treatment of advanced melanoma, non-small cell lung cancer and advanced urothelial bladder cancer.
Durvalumab has efficacy in advanced urothelial bladder cancer and stage III non-small cell lung cancer. Avelumab has been approved for a rare skin cancer called Merkel cell carcinoma.
Melanoma
Ipilimumab was the first immune checkpoint inhibitor to show benefit in advanced melanoma. However, more recently other double-blind randomised controlled trials have shown the PD-1 inhibitors nivolumab and pembrolizumab to be more efficacious (see Table).9-11
Table. Phase III trials of immune checkpoint inhibitors in advanced melanoma.
| Trials (number of patients) | Treatment | Efficacy | Treatment-related adverse effects | |
|---|---|---|---|---|
| CheckMate 2389 (906) | Ipilimumab (10 mg/kg every 3 weeks) | 12-month progression-free survival | 60.8% | 95.8% |
| Nivolumab (3 mg/kg every 2 weeks) | 70.5% | 85.2% | ||
| CheckMate 06710 (945) | Ipilimumab (3 mg/kg every 3 weeks) | 24-month overall survival | 45% | 86% |
| Nivolumab (3 mg/kg every 2 weeks) | 59% | 86% | ||
| Nivolumab (1 mg/kg) + ipilimumab (3 mg/kg every 3 weeks), then nivolumab (3 mg/kg every 2 weeks) | 64% | 96% | ||
| KEYNOTE-00611 (834) | Ipilimumab (3 mg/kg every 3 weeks) | 24-month overall survival | 43% | 73% |
| Pembrolizumab (10 mg/kg every 3 weeks) | 55% | 72.9% | ||
| Pembrolizumab (10 mg/kg every 2 weeks) | 55% | 79.5% | ||
CheckMate 238
The CheckMate 238 study was a double-blind, phase III randomised controlled trial which directly compared ipilimumab with nivolumab as first-line therapy for advanced melanoma. This trial included patients aged over 15 years who had undergone complete surgical resection of either a stage IIIb, IIIc or IV melanoma.
The 12-month rate of recurrence-free survival was 70.5% in the nivolumab group and 60.8% in the ipilimumab group. Treatment-related adverse events were high in both groups and reported in 85.2% of the patients given nivolumab and 95.8% of patients given ipilimumab. Treatment was discontinued due to toxicities in 7.7% of the nivolumab group and 41.7% of the ipilimumab group.9 As a result of the significant toxicity with ipilimumab, the dose was reduced in subsequent studies, with the aim of reducing morbidity and treatment discontinuation rates.
CheckMate 067
The CheckMate 067 study was another double-blind, phase III trial which randomised patients in a 1:1:1 ratio to receive either ipilimumab, nivolumab or ipilimumab in combination with nivolumab, for untreated, unresectable advanced melanoma.
After a minimum follow-up of 36 months, the median overall survival had not been reached in the nivolumab plus ipilimumab group, but was 37.6 months with nivolumab and 19.9 months with ipilimumab monotherapy. The overall survival rate at three years was 58% with the combination therapy group compared with 52% in the nivolumab group and 34% in the ipilimumab group. This trial was not designed to detect a difference between the two nivolumab-containing groups, but did show significantly improved overall survival and progression-free survival with nivolumab, compared to ipilimumab monotherapy. Treatment-related adverse events occurred in 96% of the patients in the nivolumab plus ipilimumab group, 86% of the nivolumab group, and 86% of the ipilimumab group. Respectively, these adverse events led to the withdrawal of 39%, 12% and 16% of the patients.10
KEYNOTE-006
The KEYNOTE-006 study was a double-blind, phase III randomised controlled trial in patients with advanced melanoma. Patients were assigned in a 1:1:1 ratio to pembrolizumab every two weeks, pembrolizumab every three weeks or ipilimumab every three weeks.
The two pembrolizumab-containing groups showed higher six-month progression-free survival rates compared with the ipilimumab group (46.4% and 47.3% vs 26.5%). The respective 12-month overall survival rates were 74.1% and 68.4% versus 58.2%. There was no statistically significant difference detected between the two pembrolizumab-containing groups. The rates of immune-related adverse events of grade 3 to 5 (death) were lower in the pembrolizumab groups (13.3% and 10.1%) than in the ipilimumab group (19.9%).11
Interpretation
In these trials the PD-1 inhibitors nivolumab and pembrolizumab significantly outperformed the CTLA-4 inhibitor ipilimumab as monotherapy for patients with advanced melanoma. Combining these therapies has yielded further positive results, but trials to date lack the statistical power to detect a significant difference between combination therapy and nivolumab or pembrolizumab monotherapy.
There are two key groups that benefit from combination therapy. These are firstly patients with BRAF mutation positive melanoma and, secondly, patients with brain metastases. Unfortunately, this benefit is often coupled with increased toxicity. Patients must be well informed regarding the toxicities of combination immunotherapy, balanced against any potential benefit. Patients with poor functional status or significant comorbidities may not be eligible for combination therapy.
In clinical practice, PD-1 inhibitor monotherapy is now well established as the first-line treatment in Australia for patients with the BRAF wild-type form of advanced melanoma. The use of ipilimumab and nivolumab in combination has now been approved by the Therapeutic Goods Administration (TGA) for patients with unresectable stage III or IV melanoma.
Non-small cell lung cancer
The KEYNOTE-024 study was an open label, phase III trial in 305 patients with previously untreated advanced non-small cell lung cancer, with more than 50% PD-L1 expression on biopsy. They were randomised to either pembrolizumab or the investigator’s choice of platinum-based chemotherapy.
After a median follow-up of 11.2 months, the median progression-free survival was 10.3 months in the pembrolizumab group compared with six months in the chemotherapy group. The median overall survival at six months was 80.2% with pembrolizumab and 72.4% with chemotherapy. The response rate was also higher in the pembrolizumab group with the median duration of that response being significantly longer and associated with less immune-related adverse events than chemotherapy.12
The trial showed that pembrolizumab resulted in significantly longer progression-free survival, overall survival and fewer adverse events than with platinum-based chemotherapy. Pembrolizumab is now the first-line treatment for advanced non-small cell lung cancer in patients with PD-L1 expression higher than 50%. It can also be used in the second-line setting for patients with advanced non-small cell lung cancer who have been unable to tolerate or have progressed despite platinum-based chemotherapy.
Most patients with locally advanced or unresectable non-small cell lung cancer will experience disease progression despite combination chemo-radiotherapy. The PACIFIC trial randomised 713 patients with locally advanced or unresectable stage III non-small cell lung cancer whose disease had not progressed on combination platinum-based chemo-radiotherapy. It compared sequential treatment with the PD-L1 inhibitor durvalumab versus placebo.13
The median progression-free survival was 16.8 months with durvalumab versus 5.6 months with placebo. This effect was consistent across all patient subgroups analysed. There was slightly higher treatment-related toxicity seen in the durvalumab group (29% vs 26%), most commonly pneumonia, but severe toxicity was similar between groups.13 Durvalumab is now TGA-approved for use in Australia, but is not yet PBS-listed and is only available through a drug company access scheme.
Urothelial bladder carcinoma
The KEYNOTE-045 study was a phase III trial that studied 542 patients with advanced urothelial cancer that had recurred or progressed after platinum-based chemotherapy. They were randomised to receive either second-line pembrolizumab or the investigator’s choice of chemotherapy with paclitaxel, docetaxel or vinflunine.
After a median follow-up of 14.1 months, the median overall survival was 10.3 months in the pembrolizumab group and 7.4 months in the chemotherapy group. The degree of PD-L1 expression did not appear to affect the outcome. Additionally, there were fewer treatment-related adverse events in the pembrolizumab group than in the chemotherapy group (60.9% vs 90.2%).14 Pembrolizumab is now TGA-approved for this indication, although it is not PBS-listed and is only available through a drug company access scheme.
Lymphoma
Studies have shown the PD-1 inhibitors nivolumab and pembrolizumab to be effective in the treatment of refractory or relapsed lymphoma. Their use has resulted in improved partial and complete responses and is evolving to become central in the treatment of lymphoma. Current studies are now assessing PD-1 inhibitors in combination with immunomodulatory therapies for lymphoma, as well as searching for predictive biomarkers.1
Renal cell carcinoma
In advanced renal cell carcinoma, treatment options have until recently been limited to anti-angiogenic therapies. A randomised controlled trial compared nivolumab to everolimus, an inhibitor of the mTOR pathway and the current standard of care at the time. The median overall survival was 25 months with nivolumab compared with 19.6 months with everolimus. The progression-free survival was only marginally improved by 0.2 months with nivolumab (4.6 months vs 4.4 months). The treatment-related adverse events were lower in the nivolumab group at 19% compared with 37% in patients receiving everolimus.15 Nivolumab is now approved in Australia for the treatment of patients with advanced, clear cell renal cell carcinoma who have undergone previous treatment with an anti-angiogenic therapy.
Immunotherapy-related adverse events
The use of immune checkpoint inhibitors in clinical practice brings with it a spectrum of new toxicities. In addition to infusion reactions, there are immune-related adverse events that can affect almost any organ site. These include pneumonitis, hepatitis, nephritis, colitis and endocrinopathies. The new challenge for health professionals is recognising these toxicities early and acting promptly. In general, immune-related adverse events occur within two weeks to three months after the immune checkpoint inhibitor is given. However, immune-related adverse events have been reported as long as one year after discontinuation of treatment.
The most common toxicities are lethargy, rash with pruritus, liver toxicity, diarrhoea with colitis and hypophysitis. However, due to the broad range of possible toxicities, there should always be a low threshold for investigating any symptoms with radiological and biochemical tests. Although there are no clear guidelines on routine monitoring during treatment with an immune checkpoint inhibitor, in an otherwise asymptomatic patient it would be reasonable to check a full blood count, with kidney, liver and thyroid function every 2–4 weeks.
The mainstay of treatment for immune-related adverse reactions involves either dose reduction or cessation of the drug, and consideration of immunosuppression. Often with moderate toxicity, the immunotherapy drug can be temporarily withheld and resumed when symptoms have resolved. Additionally, a short course of oral prednisone (0.5 mg/kg) can be given if symptoms have not resolved within one week. For severe toxicities, the immunotherapy should be permanently discontinued and intravenous methylprednisolone (1–2 mg/kg/day) given. Once symptoms have improved, prednisone can be gradually weaned over the course of 1–2 months. In rare cases where prednisone is ineffective, infliximab, intravenous immunoglobulin and plasmapheresis may be required. The management for all immune-related toxicities is discussed in significantly greater detail in the ESMO clinical practice guidelines16 and eviQ.17
Duration of therapy
There is a very limited evidence base detailing the total duration of immune checkpoint inhibitor therapy. It is likely that the majority of patients are treated longer than necessary and a recent analysis18 has shown this, identifying that patients who discontinued treatment earlier due to toxicities achieved the same benefit as those who completed their planned treatment course. However, there is a paucity of high-quality trial data and the duration of treatment is left to the best judgement of the treating oncologists or departmental policies.
Conclusion
Immune checkpoint inhibitors can improve progression-free survival and overall survival in some patients with advanced malignancies. However, a significant proportion of patients do not respond and still have a poor prognosis. There are ongoing trials with novel immunotherapy combinations which aim to treat refractory disease and identify predictive biomarkers to select likely responders from non-responders. There are also ongoing trials looking at the use of immunotherapy in the adjuvant setting, especially in melanoma.
It is likely that these drugs will become increasingly used in clinical practice, with many novel immunotherapies currently being developed and trialled. The benefits and sequelae of these drugs are being more frequently seen so health professionals will need to be alert for the emerging burden of chronic cancer-related disease and the identification and management of treatment-related adverse effects.
Footnotes
Conflict of interest: none declared
REFERENCES
- 1.Galanina N, Kline J, Bishop MR. Emerging role of checkpoint blockade therapy in lymphoma. Ther Adv Hematol 2017;8:81-90. 10.1177/2040620716673787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti- CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 2018;8:86. 10.3389/fonc.2018.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158-68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 4.Camacho LH. CTLA-4 blockade with ipilimumab: biology, safety, efficacy, and future considerations. Cancer Med 2015;4:661-72. 10.1002/cam4.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 2017;8:561. 10.3389/fphar.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 2011;11:852-63. 10.1038/nri3108 [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 9.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. CheckMate 238 Collaborators CheckMate 238 Collaborators. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824-35. 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 10.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345-56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. KEYNOTE-006 investigators Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521-32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 12.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. KEYNOTE-024 Investigators KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 13.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. PACIFIC Investigators PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 2017;377:1919-29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 14.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. KEYNOTE-045 Investigators KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015-26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. CheckMate 025 Investigators CheckMate 025 Investigators. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803-13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haanen JB, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al.; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28 suppl_4:iv119-42. https://doi.org/ 10.1093/annonc/mdx225 [DOI] [PubMed]
- 17.Evi Q. Management of immune-related adverse events (irAEs) [Internet]. Sydney: Cancer Institute NSW; 2018. www.eviq.org.au/clinical-resources/side-effect-and-toxicity-management/immunological/1993-management-of-immune-related-adverse-events [cited 2019 Mar 1] [Google Scholar]
- 18.Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol 2017;35:3807-14. 10.1200/JCO.2017.73.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]


