Abstract
Werner syndrome (WS) is an autosomal recessive progeroid disorder caused by mutations in the WRN gene (WRN). Most Japanese WS patients are born from a consanguineous marriage with homozygous WRN mutations. We herein report a rare WS patient born from non-consanguineous parents with compound heterozygous WRN mutations with a novel heterogeneous c.1720+1G>A substitution plus the most frequent heterogeneous c.3139-1G>C substitution among Japanese. Although the present case showed clinical characteristics common to previous Japanese WS patients, he had not developed any malignant tumors as of 43 years of age, suggesting that WS patients with this particular genetic mutation have a different phenotype than others.
Keywords: werner syndrome, compound heterozygous, Japanese
Introduction
Werner syndrome (WS) is an autosomal recessive progeroid disorder. The majority of WS patients are born from a consanguineous marriage with homozygous WRN gene (WRN) mutations and usually show similar clinical features, such as a senile appearance, subcutaneous calcification, painful ulcers, age-related disorders and malignant tumors (1). We herein report a rare WS patient with compound heterozygous WRN mutations, including a novel heterogeneous c.1720+1G>A substitution plus the most frequent heterogeneous c.3139-1G>C substitution among Japanese (2). The patient, who is now 43 years old, has not yet developed any malignant tumors.
Case Report
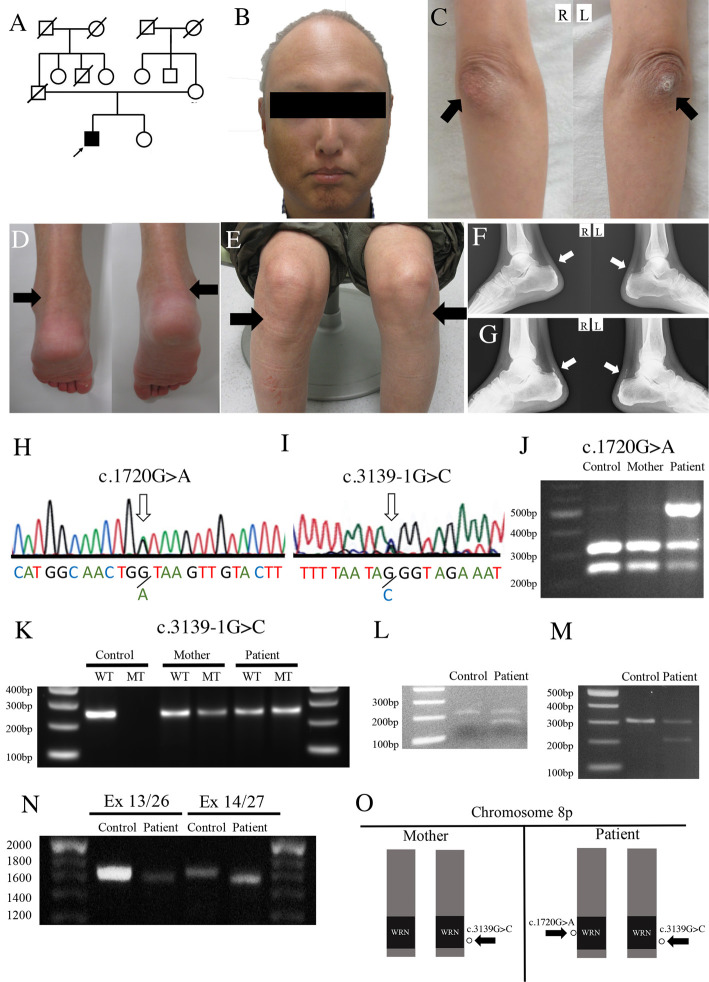
An eight-year-old Japanese boy noticed pain in his posterior neck and lumbar region, and the pain had spread throughout his body by the time he was 13. He subsequently developed bilateral cataracts at the age of 19 and gray hair and baldness by the time he turned 25. At 30 years of age, his pain became much more acute, especially in the bilateral Achilles tendons. At 38 years of age, he was admitted to our hospital for examinations. There was no family history of progeroid syndrome, age-related disorders or consanguineous marriage (Figure A).
Figure.
The patient’s (A) family tree, (B) characteristic “bird-like face” and senile appearance, (C, arrows) cutaneous hyperkeratosis in bilateral elbows, (D, arrows) knees and (E, arrows) ankles. (F and G, arrows) X-ray showing the segmented calcification of the Achilles tendons at the diagnosis and five years later. The patient’s (H and I, arrows) direct DNA sequence of the WRN gene revealed a c.1720+1G>A substitution in intron 14 and c.3139-1G>C substitution in intron 25 (2), which was (J) confirmed by PCR-RFLP and (K) AS-PCR for the patient and his mother. RT-PCR spanning (L) exons 13 to 15, (M) exons 25 to 27, (N) exons 13 to 26 and exons 14 to 27, confirming that (O) skips in exons 14 and 26 take place in different alleles.
He was 162 cm tall and weighed 50 kg. A physical examination revealed that he had a “bird-like face”, senile appearance (Figure B), a high-pitched voice and hyperkeratosis in the bilateral elbows (Figure C, arrows), knees (Figure D, arrows) and ankles (Figure E, arrows). X-ray showed segmented calcification of his Achilles tendons (Figure F, arrows).
A laboratory examination revealed liver dysfunction (aspartate aminotransferase 90 IU/L; normal 13-30 U/L, alanine aminotransferase 121 IU/L; normal 10-42 U/L), hypertriglyceridemia (triglyceride 411 mg/dL; normal 40-150 U/L) and diabetes mellitus (fasting blood glucose 179 mg/dL; normal 70-110 mg/dL, hemoglobin A1c of national glycohemoglobin standardization 6.9%; normal 4.6-6.2%). Dual-energy X-ray absorptiometry revealed a low bone density (cancellous bone mineral content of the 2nd, 3rd and 4th lumbar vertebrae: 0.822 g/cm2; normal 0.895-1.137 g/cm2, T-score -1.6; normal > -1.0).
Based on the diagnostic criteria (3), he was suspected of having WS. After obtaining his written informed consent, a direct DNA sequence analysis was performed for WRN using a blood sample obtained during admission, revealing a c.1720+1G>A substitution (2) in intron 14 and c.3139-1G>C substitution in intron 25 of human WRN (Figure H and I). Polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) using BsrI performed on DNA samples of the patient and his mother (his father was already deceased) confirmed that the c.1720+1G>A substitution (2) was in the patient only (Figure J). Allele-specific PCR (AS-PCR) performed for c.3139-1G>C using a common forward primer and two sets of reverse primers confirmed that the mutation was in both the patient and his mother (Figure K, Supplementary material). Reverse transcription (RT)-PCR was performed on RNA samples from the patient's leukocytes using flanking primers for exons 13 and 15 and exons 25 and 27, confirming skips in exons 14 and 26 due to c.1720+1G>A (2) and c.3139-1G>C, respectively (Figure L and M, Supplementary material). We subsequently conducted PCR using primers with specific base sequences for exons 13 and 26 and exons 14 and 27, which confirmed skips in exons 14 and 26 in different alleles, indicating that c.1720+1G>A (2) and c.3139-1G>C exist on different alleles (Figure N and O, Supplementary material).
He was treated with cimetidine and minodoronic acid for the calcification of his Achilles tendons, atorvastatin for dyslipidemia, metformin for diabetic mellitus and salicylic acid and pregabalin for his whole-body pain at our outpatient clinic for five years after discharge. During this time, the calcification of his bilateral Achilles tendons progressed slightly (Figure G). However, cancer screening has shown no malignancy thus far (currently 43 years old).
Discussion
We encountered a Japanese WS patient with compound heterozygous mutations of the WRN: one is a novel heterogeneous c.1720+1G>A substitution, while the other is the most frequent heterogeneous c.3139-1G>C substitution among Japanese (Figure H-K) (2). Most of the WRN mutations were related to exon skipping and the loss of function, showing similar clinical characteristics (4). In the present case, we confirmed that the novel c.1720+1G>A (2) also resulted in exon 14 skipping (Figure L). A previous report showed that the heterozygous c.3139-1G>C mutation, accompanied by exon 26 skipping, reduces the expression of WRN protein and the activity of DNA helicase (5), suggesting that the heterozygous c.1720+1G>A mutation (2), accompanied by exon 14 skipping, might also be related to a reduction in the WRN protein expression and DNA helicase activity. Although many Japanese WS patients are born from a consanguineous marriage and are homozygous for c.3139-1G>C (6), the present patient was not born from consanguineous parents and was compound heterozygous (Figure A, N and O). Of note, there have been several reports indicating that the number of WS patients from non-consanguineous marriages has been gradually increasing (3,7).
WS patients often suffer from skin atrophy and subcutaneous calcification, resulting in painful ulcers (8); age-related disorders, such as type 2 diabetes mellitus; hypertension, dyslipidemia, osteoporosis, cardiovascular disease and malignant tumor (3). Malignant tumors are a leading cause of death in WS patients (4), including thyroid neoplasms (16.1%), malignant melanoma (13.3%), soft tissue sarcomas (10.1%), hematologic/lymphoid neoplasms (9.3%) and osteosarcoma (7.7%) (9). Although the present case with a novel mutation (c.1720+1G>A) showed similar clinical characteristics to previous Japanese WS patients, such as age-related disorders and symptoms related to ectopic calcification (Table), he has not developed any malignant tumors thus far (currently 43 years old), suggesting that WS patients with this a compound heterozygous mutation have a different phenotype than others (2).
Table.
Clinical Characteristics of Japanese Werner Syndrome Patients.
| Ref. 8 | Ref. 3 | Present case (2018) | ||
|---|---|---|---|---|
| Genetically confirmed (n=7) | Genetically confirmed (n=47) | Clinically diagnosed (n=146) | ||
| Age | 42.9% in 50s, 42.9% in 40s, 14.3% in 30s | 62.7% in 60s, 22.7% in 50s, 10.8% in 40s, 1.1% in 30s, and 0.5% in 20s | 38 | |
| Gender | Male 3, Female 4 | Male 82, Female 93, unknown 18 | Male | |
| Height (cm) | n.m. | Male 158.3±8.6, Female 148.5±8.6 | 162.0 | |
| Body weight (kg) | n.m. | Male 45.3±8.3, Female 37.7±8.3 | 50.0 | |
| Gene mutations | c.3139-1G>C/ c.3139-1G>C |
n.m. | n.m. | c.1720+1G>A/ c.3139-1G>C |
| Clinical symptoms | ||||
| Gray hair, loss of hair | 100% | 97.9% | 98.2% | + |
| Bird-like face | n.m. | 93.3% | 97.2% | + |
| Bilateral cataract | 100% | 86.0% | 75.5% | + |
| Abnormality of the voice | 85.70% | 82.2% | 91.3% | + |
| Skin atrophy | 100% | 93.2% | 99.0% | + |
| Skin ulcer | 71.40% | 86.3% | 88.5% | + |
| Clavus or callus | n.m. | 90.4% | 92.4% | + |
| Flat foot | n.m. | 94.6% | 84.3% | + |
| Family history | ||||
| Consanguinity | n.m. | 35.3% | 40.6% | - |
| Parenthood | n.m. | 34.4% | 34.4% | - |
| Complications | ||||
| Diabetes mellitus | n.m. | 55.8% | 70.5% | + |
| Hypertension | n.m. | 30.9% | 37.0% | + |
| Dyslipidemia | n.m. | 85.4% | 60.7% | + |
| LDL-C<140 | n.m. | 50.0% | 43.6% | - |
| HDL-C<40 | n.m. | 17.2% | 25.9% | + |
| TG<150 | n.m. | 59.3% | 55.9% | + |
| Fatty liver | n.m. | 50.0% | 42.1% | + |
| Cerebral hemorrhage | n.m. | 2.5% | 1.1% | - |
| Cerebral infarction | n.m. | 2.4% | 4.4% | - |
| Coronary heart disease | n.m. | 11.1% | 16.1% | - |
| Arteriosclerosis obliterans | n.m. | 21.6% | 25.8% | - |
| Malignant tumors | n.m. | 44.4% | 40.2% | - |
| Osteoporosis | n.m. | 60.7% | 66.4% | + |
| Markers for ectopic calcification (normal range) | ||||
| Serum calcium (mg/dL) (8.5-10.0) | 9.3±0.28 | n.m. | n.m. | 9.9 |
| Serum inorganic phosphate (mg/dL) (2.5-4.5) | 3.4±0.40 | n.m. | n.m. | 3.1 |
| Serum intact-parathyroid hormone (PTH)(pg/mL) (10.0-65.0) | 45±13.7 | n.m. | n.m. | 15 |
| Serum 1,25-(OH)2 Vitamin D (mg/mL) (20-60) | 53±10.8 | n.m. | n.m. | n.e. |
| Serum bone-specific alkaline phosphatase (BAP) (U/L) (7.9-29.0) | 28.3±27.8 | n.m. | n.m. | 9.4 |
| Ectopic calcification | ||||
| Elbow | 85.70% | n.m | n.m. | + |
| Knee | 85.70% | n.m. | n.m. | + |
| Ankle | 85.70% | 76.5% | 83.6% | + |
| Pain | ||||
| Elbow | 71.40% | n.m. | n.m. | + |
| Knee | 57.10% | n.m. | n.m. | + |
| Ankle | 71.40% | n.m. | n.m. | + |
LDL-C: low density lipoprotein cholesterol, HDL-C: high density lipoprotein cholesterol, TG: triglyceride, n.m.: not mentioned, n.e.: not evaluated
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was partly supported by a Grant-in-Aid for Scientific Research (B) 17H0419619, (C) 15K0931607, 17H0419619 and 17K1082709, and by Grants-in-Aid from the Research Committees (Kaji R, Toda K, and Tsuji S) from Japan Agency for Medical Research and Development (AMED).
Supplementary Material
List of primers used for PCR analysis.
References
- 1. Yu CE, Oshima J, Fu YH, et al. . Positional cloning of the Werner's syndrome gene. Science 272: 258-262, 1996. [DOI] [PubMed] [Google Scholar]
- 2. Yokote K, Chanprasert S, Lee L, et al. . WRN mutation update: mutation spectrum, patient registries, and translational prospects. Hum Mutat 38: 7-15, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takemoto M, Mori S, Kuzuya M, et al. . Diagnostic criteria for Werner syndrome based on Japanese nationwide epidemiological survey. Gerontol Int 13: 475-481, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Huang S, Lee L, Hanson NB, et al. . The spectrum of WRN mutations in Werner syndrome patients. Hum Mutat 27: 558-567, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moser MJ, Kamath-Loeb, AS, Jacob JE, Bennett SE, Oshima J, Monnat RJ Jr. WRN helicase expression in Werner syndrome cell lines. Nucleic Acids Res 28: 648-654, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsumoto T, Imamura O, Yamabe Y, et al. . Mutation and haplotype analyses of the Werner's syndrome gene based on its genomic structure: genetic epidemiology in the Japanese population. Hum Genet 100: 123-130, 1997. [DOI] [PubMed] [Google Scholar]
- 7. Yamaga M, Takemoto M, Takada-Watanabe A, et al. . Recent trends in WRN gene mutation patterns in individuals with Werner syndrome. J Am Geriatr Soc 65: 1853-1856, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Honjo S, Yokote K, Fujimoto M, et al. . Clinical outcome and mechanism of soft tissue calcification in Werner syndrome. Rejuvenation Research 11: 809-819, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Lauper JM, Krause A, Vaughan TL, Monnat RJ Jr. Spectrum and risk of neoplasia in Werner syndrome: a systematic review. PLOS ONE 8: e59709, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of primers used for PCR analysis.