Abstract
Inflammatory myofibroblastic tumor (IMT), a rare sarcoma, is primarily treated via resection of the mass. However, in cases of recurrence or unresectable tumors, no standard care exists. While crizotinib, an anaplastic lymphoma kinase (ALK) inhibitor, is only approved for non-small-cell lung cancer with ALK mutation, it is reportedly effective for other malignant tumors with ALK mutation. We herein report a case involving a 37-year-old woman with retroperitoneal IMT with ALK mutation, who experienced recurrence after complete resection, in whom crizotinib treatment resulted in complete response. ALK-inhibitor efficacy against malignancies with ALK mutations should be investigated in future.
Keywords: ALK mutation, crizotinib, inflammatory myofibroblastic tumor
Introduction
Precision medicine has become one of the most concerning issues among oncologists. Pembrolizumab, for all patients with unresectable or metastatic and microsatellite instability-high or mismatch repair-deficient solid tumors, was the first approved cancer treatment (US Food and Drug Administration) based on a biomarker analysis. In the future, cancer treatments may be determined based on the biological findings of the tumor rather than its location. We herein report a case of recurrent inflammatory myofibroblastic tumor (IMT) with anaplastic lymphoma kinase (ALK) mutation, in which a complete response was achieved using crizotinib, a tyrosine kinase inhibitor targeting ALK. The treatments for recurrent IMTs remain controversial, partly because of its rarity; thus, the accumulation of research on this condition can be meaningful. In addition, we would like to introduce the first reported phenomenon where the rearrangement of the ALK gene with multiple partner fusion genes was identified in the IMT of a single patient.
Case Report
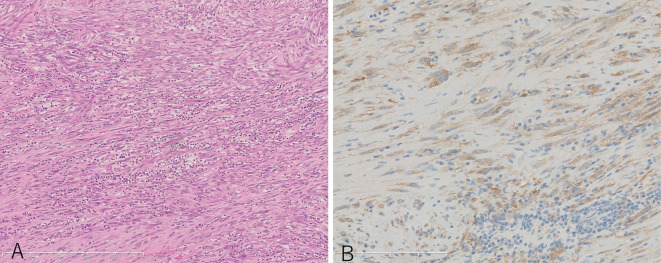
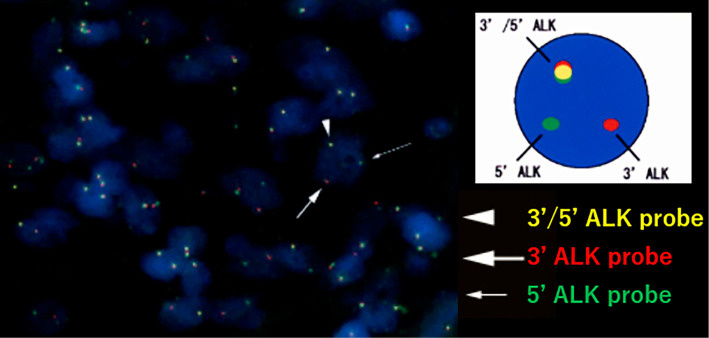
A 37-year-old woman presented to our hospital after a right adrenal gland mass was incidentally detected by ultrasound sonography in an annual medical checkup. She did not have any symptoms. Computed tomography (CT) and magnetic resonance imaging (MRI) revealed a hypervascular mass of 6 cm in diameter adjacent to the right adrenal gland and inferior vena cava (IVC) (Fig. 1). The mass showed the same signal as muscle on T1-weighted imaging and a high signal intensity on T2-weighted imaging. Contrast enhancement was observed gradually from the margin to the center. Positron emission tomography-CT revealed no area of uptake other than the retroperitoneal mass. The patient’s laboratory data, including her adrenocorticotropic hormone, cortisol, dehydroepiandrosterone sulfate, aldosterone, renin, adrenaline, noradrenaline, and dopamine levels were normal, indicating that the function of the adrenal gland was normal. Laparoscopic surgery was conducted in November 2015 to confirm the diagnosis, and the right adrenal gland mass (70×60×50 mm) was removed without any residual lesion. The tumor was composed of spindle cells in a storiform growth pattern with infiltrating inflammatory cells that were mainly composed of plasma cells (Fig. 2A). An immunohistochemical analysis revealed that the tumor cells were positive for ALK (in the cytoplasm) (Fig. 2B) and negative for S-100, desmin, c-kit, and CD34. ALK gene rearrangement was detected by fluorescent in situ hybridization (Fig. 3); thus, the tumor was diagnosed as IMT with ALK rearrangement. We performed RNA sequencing to identify the partner gene and observed that the ALK gene had multiple fusion partner genes: TPM3 (tropomyosin 3), MPRIP (myosin phosphatase Rho interacting protein), KLC1 (kinesin light chain 1), KIF5B (kinesin family member 5B), EML4 (echinoderm microtubule associated protein like 4), and HIP1 (huntingtin interacting protein 1), in descending order from the point of the read counts. Thoracoabdominal examinations using CT were conducted every 3 months, and the first recurrence was observed 7 months after the operation (Fig. 4A). The largest mass was located between the IVC and the right kidney. Small nodal paravertebral lesions showed the uptake of fluorodeoxyglucose and a small mass between the IVC and the pancreas was detected by MRI; these were also likely to be recurrent lesions. Since the suspected recurrent lesions were multiple and unresectable, we assessed that they were inoperable. The patient, whose Eastern Cooperative Oncology Group Performance Status was 0, started crizotinib therapy at a dose of 500 mg per day. Before starting crizotinib, we obtained written informed consent from the patient for off-label use and the approval of crizotinib use in our study by the Ethics Committee of Kobe City Medical Center General Hospital. We discontinued crizotinib one month later, because the patient’s aspartate transaminase (AST) and alanine transaminase (ALT) levels increased to 47 U/L and 112 U/L, respectively. Two weeks later, her liver enzymes recovered to within normal limits, and crizotinib therapy was restarted at a dose of 250 mg/day, which was increased to 400 mg/day three weeks later. ALT transiently increased to 73 U/L but finally reached within normal limits. The patient experienced grade 1 visual disturbance (afterimage) and grade 1 diarrhea; however, they were tolerable. Positron emission tomography-CT revealed that she had achieved a complete response (Fig. 4B); thus, we discontinued crizotinib. The total administration period was five months. Local recurrence was observed again at nine months after treatment, at the same location as the first recurrence (Fig. 4C). This time the mass was small, and she underwent surgical resection. ALK staining of the resected tumor was positive. The patient is now free from any treatment and is receiving regular imaging surveillance.
Figure 1.
The magnetic resonance imaging (MRI) findings at the time of the diagnosis. A: Transverse plane (arrow). B: Coronal plane (arrow).
Figure 2.
The histopathological examination of the first resected mass. A: Hematoxylin and Eosin staining ×10. Spindle cells and inflammatory cells were observed. B: ALK staining ×20. Tumor cells were positive for ALK.
Figure 3.
The ALK gene rearrangement that was detected by fluorescent in situ hybridization.
Figure 4.
The Positron Emission Tomography-Computed Tomography (PET-CT) findings during the course of treatment. A: First recurrence. B: Complete response after five months of crizotinib treatment. C: Second local recurrence nine months after the discontinuation of crizotinib.
Discussion
IMT is a rare mesenchymal tumor with an unclear etiology. It can arise in various locations, and is locally aggressive. The first choice of treatment for IMT is surgical resection. Local recurrence may occur once or more than once, but distant metastasis is rare (1). Approximately 50% of IMTs harbor ALK gene rearrangement with various fusion partner genes (2). It is known that the pathological features of IMTs, such as the mitotic rate, presence or absence of necrosis, or cellular atypia, do not correspond with the clinical outcome (3,4). On the other hand, ALK rearrangement has been associated with local recurrence but not with distant metastasis, according to a previously reported immunohistochemical study of IMTs (4). ALK immunostaining is categorized into three patterns, which appear to be determined by fusion partners (3). The three staining patterns are as follows: smooth cytoplasmic staining, granular cytoplasmic staining, and distinctive nuclear membrane staining; our case showed smooth cytoplasmic staining, which is reasonable for TPM3-ALK fusion. There is no consensus on the optimal treatment of recurrence, as was observed in our patient. In previous case reports, crizotinib has been considered to be effective for ALK-positive IMT. Table presents the six cases in which crizotinib was used for ALK-positive IMT in adults. The patients were young and their tumor locations varied. In most cases crizotinib first resulted in a partial or better response; however, the final outcomes differed. Our case is the first report of a Japanese patient and is an additional example of that suggests that crizotinib is effective for ALK-positive IMT, regardless of where the tumor is.
Table.
Summary of the Past IMT Cases Using Crizotinib.
| Reference | Age, Sex | Tumor site | Duration of crizotinib use | Result | Major side effects |
|---|---|---|---|---|---|
| 6 | 43 y, F | Uterus | Not mentioned | DLT→ceritinib | Not mentioned, but intolerable |
| 7 | 26 y, M | Cerebrum | 16 months | PR→PD→ceritinib | QT prolongation |
| 8 | 32 y, M | Lung, chest wall, muscle, omentum | 8 months | PR→PD→ceritinib | Not mentioned |
| 9 | 50’s, F | Uterine | 6 months | PR | tolerable |
| 10 | 45 y, F | Liver, adrenal gland, lumber spine | 27 months | CR | Not mentioned |
| 11 | 44 y, M | Abdomen, pelvis | 22 months | CR | tolerable |
IMT: inflammatory myofibroblatic tumor, F: female, M: male, DLT: dose limiting toxicities, PR: partial response, PD: progressive disease, CR: complete response
Crizotinib was first developed as a c-Met (mesenchymal-epithelial transition factor) inhibitor, which is now widely known as an ALK inhibitor. Grade ≥3 adverse events included ALT elevation in 11% of cases, AST elevation in 4% of cases, neutropenia in 9% of cases, QT prolongation in 2% of cases, and interstitial lung disease in 0.6% of cases. Grade 1 eye disorder, which was observed in this case, was reported in 62% of patients in a clinical trial of crizotinib against non-small-cell lung cancer (5). A few cases of intestinal lung disease and liver dysfunction resulted in death in this phase 3 trial; however, most side effects are tolerable. This suggests that crizotinib is a safely manageable drug, and our case also supports the safety of the drug. Similar to crizotinib, off-target effects may lead to the development of new drugs. In conclusion, IMTs occur in various locations; however, regardless of the tumor location, the use of ALK inhibitors, such as crizotinib, may be effective for tumors harboring ALK mutations.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent for inclusion in the study, or its equivalent, was obtained from the patient.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 19: 859-872, 1995. [DOI] [PubMed] [Google Scholar]
- 2. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol 61: 428-437, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Cook JR, Dehner LP, Collins MH, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol 25: 1364-1371, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 31: 509-520, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 368: 2385-2394, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Pickett JL, Chou A, Andrici JA, et al. Inflammatory myofibroblastic tumors of the female genital tract are under-recognized: a low threshold for ALK immunohistochemistry is required. Am J Surg Pathol 41: 1433-1442, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chennouf A, Arslanian E, Roberge D, et al. Efficiency of crizotinib on an ALK-positive inflammatory myofibroblastic tumor of the central nervous system: a case report. Cureus 9: e1068, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mansfield AS, Murphy SJ, Harris FR, et al. Chromoplectic TPM3-ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib. Ann Oncol 27: 2111-2117, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Subbiah V, McMahon C, Patel S, et al. STUMP un“stumped”: anti-tumor response to anaplastic lymphoma kinase (ALK) inhibitor based targeted therapy in uterine inflammatory myofibroblastic tumor with myxoid features harboring DCTN1-ALK fusion. J Hematol Oncol 8: 66, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacob SV, Reith JD, Kojima AY, Williams WD, Liu C, Vila Duckworth L. An unusual case of systemic inflammatory myofibroblastic tumor with successful treatment with ALK-inhibitor. case Rep Pathol 2014: 470340, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 363: 1727-1733, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]