Abstract
Clinical trials and real-world data have proven that hepatitis C virus (HCV) in most infected patients can be eradicated by direct-acting antivirals (DAAs). However, the proper retreatment regimen for hemodialysis patients with HCV infection who have previously failed to respond to DAAs has not been clarified. We herein report, for the first time, the successful retreatment with glecaprevir and pibrentasvir, of three hemodialysis patients with genotype 1 or 2 HCV infection, who had previously failed to respond to combination therapy with an HCV-NA5A inhibitor (daclatasvir) and an HCV protease inhibitor (asunaprevir).
Keywords: HCV, retreatment, glecaprevir, pibrentasvir, hemodialysis
Introduction
The prevalence of hepatitis C virus (HCV) infection in hemodialysis patients is high and is associated with a poor prognosis (1,2). Thus, the effective and safe treatment of hemodialysis patients with HCV infection is crucial. In addition, because HCV infection is associated with a poor renal allograft prognosis (3), renal transplantation candidates should consider anti-HCV therapy. Until recently, interferon-based therapy was a standard therapy for hemodialysis patients (similarly to non-hemodialysis patients). However, the outcome of interferon (IFN)-based therapy for hemodialysis patients was not sufficiently effective, partly due to the high rate of adverse events (4).
The development of direct acting antivirals (DAAs) has changed the landscape of anti-HCV therapy for hemodialysis patients. Combination therapy with DAAs has been proven to show high efficacy and safety in hemodialysis patients. We previously reported that the first IFN-free DAA combination therapy regimen in Japan with an NA5A inhibitor (daclatasvir) and an HCV protease inhibitor (asunaprevir) was highly effective and safe in the treatment of genotype 1 HCV-infected dialysis patients, and that it was associated with a sustained virological response (SVR) rate of more than 95% (5,6). Subsequently, several clinical trials and real-world data have clearly shown that IFN-free DAA therapy was highly effective and well-tolerated in hemodialysis patients (7,8). However, caution must be exercised when treating dialysis patients with IFN-free DAAs. Some potent DAAs, such as the NS5B inhibitor sofosbuvir and the anti-viral drug ribavirin are not recommended for patients with severe renal dysfunction, including those on hemodialysis as they are metabolized via the kidney (9). Additionally, hemodialysis patients are generally treated with various drugs; thus, attention should be paid to the possible development of drug-drug interactions among DAAs and concomitantly administered drugs.
Despite the high efficacy of IFN-free DAA therapy, around 5-10% of patients treated with DAAs fail to respond to the treatment (6,10-14). However, an effective retreatment regimen for HCV-infected hemodialysis patients who have previously failed to respond to DAAs, especially NS3 inhibitor and NS5A inhibitor combination therapy, has not been clarified.
Combination therapy with an HCV NS5A inhibitor (glecaprevir) and an HCV protease inhibitor (pibrentasvir) is a novel recently approved anti-HCV therapy. Clinical trials have shown that this regimen is highly effective and safe for HCV-infected patients, including patients with renal dysfunction and those who have previously failed to respond to DAA therapy (15-17). Thus, this regimen might be suitable for the retreatment of hemodialysis patients who have previously failed to respond to DAA therapy. However, the outcomes of glecaprevir and pibrentasvir treatment in these patients have not been reported thus far.
In this case report, for the first time, we show the outcomes of retreatment with glecaprevir and pibrentasvir, in three hemodialysis patients with genotype 1 or 2 HCV infection, who had previously failed to respond to HCV NS5A inhibitor and HCV protease inhibitor combination therapy.
Three hemodialysis patients agreed to participate in a clinical study of the safety and efficacy of glecaprevir and pibrentasvir in patients with hepatitis C infection. The study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the institutional ethics review committee of Hokkaido University Hospital. Written informed consent to participate in the study was obtained from each patient.
Case Reports
Case 1
The patient was a 57-year-old woman with genotype 2b HCV infection who had been undergoing hemodialysis for 23 years due to diabetic nephropathy-induced chronic renal failure. At 55 years of age, she was treated with NS5A inhibitor (daclatasvir) and HCV protease inhibitor (asunaprevir) combination therapy, as an HCV serotype examination indicated HCV serotype 1 infection. After the initiation of daclatasvir and asunaprevir therapy, the HCV RNA level remained detectable for 3 months; thus, the treatment was terminated.
Twelve weeks of glecaprevir and pibrentasvir combination therapy was initiated when she was 57 years of age. An HCV genotyping test, conducted before the initiation of the therapy, indicated that the HCV genotype was 2b. At the initiation of combination therapy, the patient’s platelet count was 12.7×104/μL, her alanine transaminase (ALT) level was 15 IU/L, her HCV RNA titer was 5.2 log IU/mL, and IL28B (rs8099917) was TG (Table 1). As shown in Figure A, the HCV RNA titer quickly decreased and reached an undetectable level at 4 weeks after the initiation of retreatment; it remained undetectable at 12 weeks after the completion of treatment (SVR12). This patient completed 12 weeks of glecaprevir and pibrentasvir treatment without severe adverse events. At two weeks after the initiation of treatment, this patient felt nausea; however, it resolved without any medications.
Table 1.
Baseline Characteristics of the Three HCV Infected Hemodialysis Patients who Previously Failed to Respond to Daclatasvir and Asunaprevir.
| Case 1 | Case 2 | Case3 | ||||
|---|---|---|---|---|---|---|
| Age (years) | 57 | 69 | 68 | |||
| Sex | Female | Female | Female | |||
| Baseline white blood cell count (/μL) | 5,730 | 2,500 | 2,500 | |||
| Baseline hemoglobin level (g/dL) | 17.5 | 13.6 | 14.2 | |||
| Baseline platelet count (×103/μL) | 127 | 111 | 87 | |||
| Baseline ALT level (IU/L) | 15 | 7 | 10 | |||
| Baseline AST level (IU/L) | 14 | 14 | 12 | |||
| Baseline HCV RNA level (log10 IU/mL) | 5.2 | 6.9 | 4.2 | |||
| HCV genotype | 2b | 1b | 1b | |||
| FIB-4 index | 1.6 | 3.3 | 3.0 | |||
| IL28 B gene (rs8099917) | TG | TT | TT | |||
| Previous treatment | DCV/ASV | DCV/ASV | DCV/ASV | |||
| Response to previous treatment | Non-response | Viral breakthrough | Relapse | |||
| Etiology of renal dysfunction | DM | DM | Nephrosclerosis | |||
| HD duration (years) | 23 | 13 | 14 | |||
| NS5A RAVs | NA | R30Q, L31M, Y93H | L31M, Y93H | |||
| NS3 RAVs | NA | none | D168E |
HCV: hepatitis C virus, IFN: interferon, ALT: alanine transaminase, DCV: daclatasvir, ASV: asunaprevir, RAVs: resistance associated variants, ART: antiretroviral therapy, Tx: treatment, ND: not detected, NA: not analyzed
Figure.
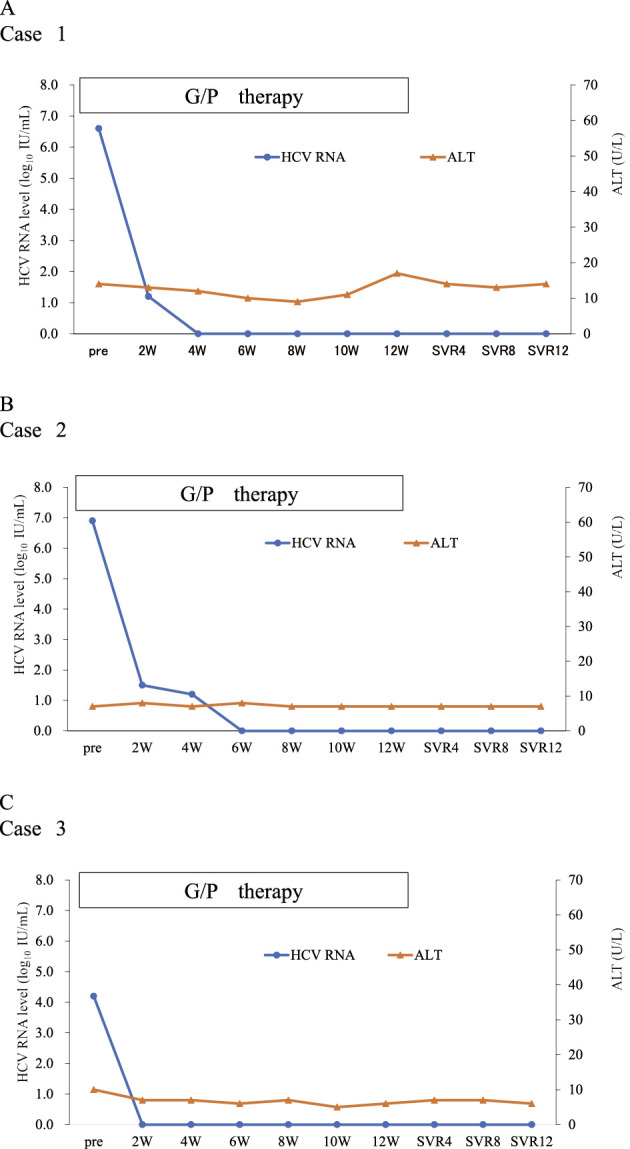
The virologic response and clinical course to glecaprevir and pibrentasvir retreatment in three hemodialysis patients who previously failed to respond to daclatasvir and asunaprevir. Changes in the serum hepatitis C virus (HCV) titer and alanine aminotransferase (ALT) level are shown. A: The clinical course of Case 1. B: The clinical course of Case 2. C: The clinical course of Case 3. HCV: hepatitis C virus, ALT: alanine transaminase
Case 2
The patient was a 69-year-old woman with genotype 1b HCV infection who had been undergoing hemodialysis for 13 years because of diabetic nephropathy-induced chronic renal failure and who had previously failed to respond to NS5A inhibitor (daclatasvir) and HCV protease inhibitor (asunaprevir) combination therapy, which was initiated when she was 67 years of age. Her HCV-RNA titer became undetectable at 4 weeks after the initiation of treatment; however, at the end of treatment, HCV-RNA became detectable. Regarding resistance associated variants (RAVs), at the time of the initiation of daclatasvir and asunaprevir treatment, this patient had NS5A RAVs of L31M and A92T, and after failure to respond to daclatasvir and asunaprevir treatment, the emergence of NS5A RAVs of R30H/Q, L31M, and Y93H, and NS3 RAVs of D168T was detected by direct sequencing (18). At the initiation of glecaprevir and pibrentasvir treatment, NS5A RAVs of R30Q, L31M, and Y93H were observed, but no RAVs were detected in the NS3 region (Table 2). At the initiation of treatment, the patient’s platelet count was 11.1×104/μL, her ALT level was 7 IU/L, her HCV RNA titer was 6.9 log IU/mL, and IL28B (rs8099917) was TT (Table 1). As shown in the Figure B, the HCV RNA titer decreased and reached an undetectable level at 6 weeks after the initiation retreatment and remained undetectable at 12 weeks after the completion of treatment (SVR12). This patient completed 12 weeks of glecaprevir and pibrentasvir treatment without adverse events.
Table 2.
Clinical Features and Change in RAVs after Failure to Respond to the Therapies.
| Case 1: genotype 2b | Case 2: genotype 1b | Case 3: genotype 1b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS5A RAVs |
NS3 RAVs |
NS5A RAVs |
NS3 RAVs |
NS5A RAVs |
NS3 RAVs |
|||||||
| Pre-DCV /ASV | NA | NA | L31M, A92T | - | - | - | ||||||
| Post-DCV/ASV | NA | NA | R30H/Q, L31M, Y93H | D168T | L31M, Y93H | D168E | ||||||
| Pre-G/P | NA | NA | 30Q, L31M, Y93H | - | L31M, Y93H | D168E | ||||||
HCV: hepatitis C virus, IFN: interferon, ALT: alanine transaminase, DCV: daclatasvir, ASV: asunaprevir, G/P: glecaprevir and pibrentasvir, RAVs: resistance associated variants, NA: not analyzed
Case 3
The patient was a 68-year-old woman with genotype 1b HCV infection who had been undergoing hemodialysis due to nephrosclerosis since she was 54 years of age. When she was 66 years of age, she was treated with daclatasvir and asunaprevir combination therapy for 24 weeks. A response was achieved at the end of treatment; however, at 12 weeks after the completion of treatment, HCV-RNA became detectable. The analysis of HCV RAVs at the initiation of daclatasvir and asunaprevir therapy, showed that this patient had no NS5A RAVs or NS3 RAVs. After the response failure, the emergence of NS5A RAVs of L31M and Y93H, and an NS3 RAV of D168E was detected by direct sequencing (18) (Table 2). At the initiation of glecaprevir and pibrentasvir treatment, NS5A RAVs of L31M and Y93H, and NS3 RAVs of D168E were observed. At the initiation of retreatment, the patient’s platelet count was 8.7×104/μL, her ALT level was 12 IU/L, her HCV RNA titer was 4.2 log IU/mL, and IL28 B (rs8099917) was TT (Table 1). As shown in Figure C, HCV RNA was undetectable at 2 weeks after the initiation of retreatment and remained undetectable at 12 weeks after the completion of treatment (SVR12). This patient completed 12 weeks of glecaprevir and pibrentasvir treatment. At 2 weeks after the initiation of treatment, this patient experienced an itching sensation, but it resolved without medication. At 61 days after the completion of treatment, this patient suffered from hematencephalon, which was resolved by conservative management. The attending physician reported that the incidence of hematencephalon might have been unrelated to glecaprevir and pibrentasvir treatment because it occurred at long time after the completion of treatment.
Discussion
The optimal retreatment regimen for hemodialysis patients who have previously failed DAA therapy has not been fully clarified. In this case report, for the first time, we report that HCV eradication was achieved after 12 weeks of glecaprevir and pibrentasvir treatment in three hemodialysis patients with genotype 1 or 2 HCV infections, who had previously failed to respond to NS5A and HCV protease inhibitor combination therapy. This result may provide a new treatment approach for patients with severe renal dysfunction who have previously failed to respond to DAA treatment.
Recently developed DAAs have dramatically improved efficacy and safety of HCV treatment. This revolutionary change prevailed in HCV infected patients who were historically difficult to treat, such as patients with HIV co-infection, renal dysfunction, or liver transplantation (5,6,10,11,19-22). Treatment with these DAAs achieved an SVR rate of more than 90% in these patients, with successful HCV eradication in most patients. However, a certain number of these patients fail to respond to DAA treatment. Typically, it is recommended that retreatment regimens for such patients include classes of DAAs that were not included during the initial treatment, because of the emergence of RAVs after failure. These strategies are recommended by the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) guidelines. We previously showed the possibility that the addition of ribavirin to sofosbuvir and ledipasvir might improve the treatment outcomes of patients who fail to respond to daclatasvir and asunaprevir treatment (23). However, ribavirin and sofosbuvir cannot be administered to hemodialysis patients because these drugs are metabolized through the kidney. Thus, the retreatment regimen for hemodialysis patients is limited to NA5A and HCV protease inhibitors, which are usually administrated during the initial DAA treatment. Toyoda et al. and our group, reported that the SVR rates of patients who previously failed treatment with an NS5A inhibitor (daclatasvir), a protease inhibitor (asunaprevir) who were treated with an NS5A inhibitor (elbasvir) and a protease inhibitor (grazoprevir) were insufficient [55.6% (10/18) and 0% (0/1), respectively] (10,14). Thus, there is a dire need for an effective retreatment regimen for such patients.
In this study, we reported the successful retreatment with 12 weeks of glecaprevir and pibrentasvir of three dialysis patients who had previously failed to respond to NS5A inhibitor and HCV protease inhibitor combination therapy. Although a phase 3 trial in Japan reported that glecaprevir and pibrentasvir were highly effective for patients who failed to respond to initial DAA treatment, hemodialysis patients were not included in the retreatment group (15). In addition, our report is the first to show the successful retreatment of genotype 2 HCV-infected patient in whom NS5A inhibitor and HCV protease inhibitor combination therapy had previously failed. Thus, this case report might shed light on the retreatment approaches for such patients.
In Case 1, the HCV serotype examination before the initiation of treatment indicated that the HCV serotype was 1; thus, daclatasvir and asunaprevir were prescribed. However, after the initiation of treatment, HCV-RNA was still detectable. An HCV genotyping examination using serum conserved from before the initiation of daclatasvir and asunaprevir treatment showed that the HCV genotype was 2b. Inoue et al. reported that at least 1.8% (3/168) patients who were treated with daclatasvir and asunaprevir, showed discrepancies in their HCV serotype and genotype examination results. Importantly, all of the three patients failed to respond to daclatasvir and asunaprevir (24). Thus, it is possible that a certain number of patients, for whom only an HCV serotype examination has been conducted (because this is the only examination approved in Japan) and who have failed to respond to daclatasvir and asunaprevir treatment, might have HCV genotype 2 infection. Further studies are needed to investigate the outcomes of glecaprevir and pibrentasvir retreatment in such cases.
In a Japanese phase 3 trial, glecaprevir and pibrentasvir was highly effective for patients who had previously failed to respond to DAAs, except for those with NS5A P32deletion (15). In this case report, this mutation was not observed before the initiation of glecaprevir and pibrentasvir treatment. Thus, the appropriate retreatment regimen for hemodialysis patients with NS5A P32deletion remains to be clarified.
The proper retreatment of hemodialysis patients who fail to respond to glecaprevir and pibrentasvir remains to be clarified. The addition of non-nucleoside inhibitors of HCV NS5B and/or interferon-which can be administered to hemodialysis patients-to NS5A inhibitor and HCV protease inhibitor combination therapy, might be therapeutic options for the retreatment of such patients; however, further studies are required.
In conclusion, this case report indicated that glecaprevir and pibrentasvir combination therapy is safe and effective for hemodialysis patients with genotype 1 or 2 HCV infection who previously have failed to respond to HCV NS5A inhibitor and HCV protease inhibitor combination therapy. Large-scale studies are required to further validate the results of the present study.
Author's disclosure of potential Conflicts of Interest (COI).
Goki Suda: Research funding, Bristol Myers Squibb and MSD. Naoya Sakamoto: Research funding, Gilead Sciences and AbbVie.
Financial Support
This study was supported by the Japan Agency for Medical Research and Development (AMED) Grant JP18fk0210018h0002 and JSPS KAKENHI (Grant Number 16K09334).
References
- 1. Iwasa Y, Otsubo S, Sugi O, et al. Patterns in the prevalence of hepatitis C virus infection at the start of hemodialysis in Japan. Clin Exp Nephrol 12: 53-57, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol 18: 1584-1593, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Mathurin P, Mouquet C, Poynard T, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology 29: 257-263, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Fabrizi F, Dixit V, Messa P, Martin P. Pegylated interferon mono-therapy of chronic hepatitis C in the dialysis population: systematic review and meta-analysis. Ther Apher Dial 19: 611-621, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Suda G, Kudo M, Nagasaka A, et al. Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. J Gastroenterol 14: 733-740, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Suda G, Furusyo N, Toyoda H, et al. Daclatasvir and asunaprevir in hemodialysis patients with hepatitis C virus infection: a nationwide retrospective study in Japan. J Gastroenterol 53: 119-128, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Atsukawa M, Tsubota A, Koushima Y, et al. Efficacy and safety of ombitasvir/paritaprevir/ritonavir in dialysis patients with genotype 1b chronic hepatitis C. Hepatol Res 47: 1429-1437, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 386: 1537-1545, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Suda G, Ogawa K, Kimura M, et al. Novel treatment of hepatitis C virus infection for patients with renal impairment. J Clin Transl Hepatol 4: 320-327, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suda G, Kurosaki M, Itakura J, et al. Safety and efficacy of elbasvir and grazoprevir in Japanese hemodialysis patients with genotype 1b hepatitis C virus infection. J Gastroenterol 2018 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 11. Suda G, Ogawa K, Morikawa K, Sakamoto N. Treatment of hepatitis C in special populations. J Gastroenterol 53: 591-605, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology 59: 2083-2091, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumada H, Suzuki Y, Karino Y, et al. The combination of elbasvir and grazoprevir for the treatment of chronic HCV infection in Japanese patients: a randomized phase II/III study. J Gastroenterol 52: 520-533, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toyoda H, Atsukawa M, Takaguchi K, et al. Real-world virological efficacy and safety of elbasvir and grazoprevir in patients with chronic hepatitis C virus genotype 1 infection in Japan. J Gastroenterol 2018 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 15. Kumada H, Watanabe T, Suzuki F, et al. Efficacy and safety of glecaprevir/pibrentasvir in HCV-infected Japanese patients with prior DAA experience, severe renal impairment, or genotype 3 infection. J Gastroenterol 53: 566-575, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chayama K, Suzuki F, Karino Y, et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 1 hepatitis C virus infection with and without cirrhosis. J Gastroenterol 53: 557-565, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toyoda H, Chayama K, Suzuki F, et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection. Hepatology 2017 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ito J, Suda G, Yamamoto Y, et al. Prevalence and characteristics of naturally occurring sofosbuvir resistance-associated variants in patients with hepatitis C virus genotype 1b infection. Hepatol Res 46: 1294-1303, 2016. [DOI] [PubMed] [Google Scholar]
- 19. Sho T, Suda G, Nagasaka A, et al. Safety and efficacy of sofosbuvir and ribavirin for genotype 2 hepatitis C Japanese patients with renal dysfunction. Hepatol Res 48: 529-538, 2018. [DOI] [PubMed] [Google Scholar]
- 20. Kawaoka T, Imamura M, Morio K, et al. Three patients treated with daclatasvir and asunaprevir for recurrent hepatitis C after liver transplantation: case report. Hepatol Res 46: 707-712, 2016. [DOI] [PubMed] [Google Scholar]
- 21. Sogni P, Gilbert C, Lacombe K, et al. All-oral direct-acting antiviral regimens in HIV/hepatitis C virus-coinfected patients with cirrhosis are efficient and safe: real-life results from the prospective ANRS CO13-HEPAVIH cohort. Clin Infect Dis 63: 763-770, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Sulkowski M, Hezode C, Gerstoft J, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet 385: 1087-1097, 2015. [DOI] [PubMed] [Google Scholar]
- 23. Suda G, Ogawa K, Yamamoto Y, et al. Retreatment with sofosbuvir, ledipasvir, and add-on ribavirin for patients who failed daclatasvir and asunaprevir combination therapy. J Gastroenterol 52: 1122-1129, 2017. [DOI] [PubMed] [Google Scholar]
- 24. Inoue J, Kanno A, Wakui Y, et al. Identification of genotype 2 HCV in serotype-1 hepatitis C patients unresponsive to daclatasvir plus asunaprevir treatment. Tohoku J Exp Med 241: 21-28, 2017. [DOI] [PubMed] [Google Scholar]


