Polyploidy has evolved many times across the kingdom of life. The relationship between cell growth and chromosome replication in bacteria has been studied extensively in monoploid model organisms such as Escherichia coli but not in polyploid organisms. Our study of the polyploid cyanobacterium Synechococcus elongatus demonstrates that replicating chromosome number is restricted and regulated by DnaA to maintain a relatively stable gene copy number/cell volume ratio during cell growth. In addition, our results suggest that polyploidy confers resistance to UV, which damages DNA. This compensatory polyploidy is likely necessitated by photosynthesis, which requires sunlight and generates damaging reactive oxygen species, and may also explain how polyploid bacteria can adapt to extreme environments with high risk of DNA damage.
KEYWORDS: DNA replication, DnaA, polyploidy, RpoC, cyanobacteria
ABSTRACT
Homologous chromosome number (ploidy) has diversified among bacteria, archaea, and eukaryotes over evolution. In bacteria, model organisms such as Escherichia coli possess a single chromosome encoding the entire genome during slow growth. In contrast, other bacteria, including cyanobacteria, maintain multiple copies of individual chromosomes (polyploid). Although a correlation between ploidy level and cell size has been observed in bacteria and eukaryotes, it is poorly understood how replication of multicopy chromosomes is regulated and how ploidy level is adjusted to cell size. In addition, the advantages conferred by polyploidy are largely unknown. Here we show that only one or a few multicopy chromosomes are replicated at once in the cyanobacterium Synechococcus elongatus and that this restriction depends on regulation of DnaA activity. Inhibiting the DnaA intrinsic ATPase activity in S. elongatus increased the number of replicating chromosomes and chromosome number per cell but did not affect cell growth. In contrast, when cell growth rate was increased or decreased, DnaA level, DnaA activity, and the number of replicating chromosomes also increased or decreased in parallel, resulting in nearly constant chromosome copy number per unit of cell volume at constant temperature. When chromosome copy number was increased by inhibition of DnaA ATPase activity or reduced culture temperature, cells exhibited greater resistance to UV light. Thus, it is suggested that the stepwise replication of the genome enables cyanobacteria to maintain nearly constant gene copy number per unit of cell volume and that multicopy chromosomes function as backup genetic information to compensate for genomic damage.
INTRODUCTION
Chromosomal ploidy has diversified during evolution in all three domains of life, bacteria, archaea, and eukaryotes. Most eukaryotic organisms are diploid, but some plants are triploid or polyploid. Likewise, ploidy level varies in bacteria. Many bacteria adopted as models for cell biological studies, such as Escherichia coli and Bacillus subtilis, possess a single circular chromosome (genome) per cell (monoploid) during slow growth and become mero-origoploid during fast growth. In the case of Caulobacter crescentus, cells are monoploid irrespective of growth rate (1, 2). Alternatively, some bacteria maintain multiple chromosome copies per cell (polyploid) irrespective of growth rate as observed in cyanobacteria (3, 4), Deinococcus (5, 6), Thermus thermophilus (7), and symbiotic or parasitic bacteria such as Buchnera (8), Neisseria (9), Borrelia (10), and Epulopiscium (11). Chloroplasts and mitochondria, which evolved in eukaryotic cells from bacterial endosymbionts, are also polyploid. In addition, polyploidy has been reported in several lineages of archaea (12–16).
Although mechanisms that regulate polyploidy are not known, a positive association between ploidy level and cell size has been observed in a certain species of polyploid prokaryotes and eukaryotes. In polyploid prokaryotes such as the Gram-positive bacteria Epulopiscium spp. (17), the cyanobacterium Synechococcus elongatus (18), and the halophilic archaeon Halobacterium salinarum (13), larger cells possess more copies of individual chromosomes than smaller cells. Similarly, artificial polyploidy in the yeast Saccharomyces cerevisiae was associated with greater cell size (19). In plant epidermal cells as well, a positive association of ploidy with cell size has been observed (20). It has been assumed that cytoplasmic cell volume changes in a manner depending on ploidy level which regulates rate of ribosome biogenesis, gene expression level, or other factors in yeasts and plants (21, 22). However, the studies did not rule out the opposite possibility that cell volume regulates the ploidy level. Thus, it is unclear whether there is any causal relationship between ploidy level and cell size in polyploid organisms and, if so, whether cell growth increases ploidy level or increased ploidy level leads to increased cell size (21, 22). Further, the biological significance of this relationship remains obscure. Monoploidy is apparently advantageous for proliferation compared to polyploidy due to the lower energetic costs and reduced risks associated with DNA replication during cellular proliferation. Nevertheless, polyploidy has evolved many times in both prokaryotes and eukaryotes, implying that polyploidy confers certain survival advantages in specific environments.
Precise chromosomal DNA replication is essential for inheritance of advantageous genetic traits during proliferation. Molecular studies in model bacteria such as E. coli have shown that chromosome replication is tightly controlled mainly at the initiation stage of DNA replication. The initiator protein DnaA first binds to the replication origin (oriC) and then recruits components of the replisome (23, 24). DnaA AAA+ ATPase is highly conserved among bacteria. It binds both ATP and ADP, but only ATP-bound DnaA is capable of forming an oligomeric structure at the oriC region and initiating DNA replication. In E. coli, DnaA level is constant throughout cell cycle progression, whereas the ratio of ATP-DnaA to the total DnaA pool peaks just before chromosome replication in the cell cycle (25, 26). A similar regulatory mechanism for DnaA activity during the cell cycle has been observed in other bacteria (23, 27, 28). In contrast, DnaA activity in C. crescentus is regulated by a change in the total DnaA level (29, 30). It is believed that regulation of DnaA activity ensures chromosome replication once per cell cycle in monoploid bacteria, although the specific mode of regulation (i.e., DnaA level or ratio of ATP-DnaA to total DnaA) has diverged among species (31). In contrast to these monoploid bacteria, regulatory mechanisms for DNA replication are still poorly understood in polyploid bacteria.
Cyanobacteria are photosynthetic, and it has been reported that many species are polyploid (32–36). Recent studies in Synechococcus elongatus PCC 7942 have begun to reveal the regulatory mechanism of polyploid DNA replication in cyanobacteria. In S. elongatus, DNA replication is initiated at the oriC region and replication requires DnaA as in monoploid bacteria (37, 38). However, all multicopy chromosomes (four to six) are not replicated simultaneously; rather, only one or two chromosomes are replicated at any stage of the cell cycle (37–40). In addition, chromosomal copy number per cell changes and exhibits a positive and linear relation with cell size (18, 39, 40). Furthermore, despite the increase in cell volume, protein concentration remains constant (18); thus, it has been suggested that the increase in chromosomal copy number with growth allows the cell to maintain individual mRNA and protein concentrations (18). However, it is still unclear how replication of multiple chromosome copies is regulated so that only a few are replicated at once and how cell size and copy number are coordinated. In addition, it is not known whether all chromosomal copies contribute mRNA and protein production to maintain a constant protein concentration per cell.
Here we show that initiation of DNA replication and the number of replicating chromosomes are regulated by DnaA and that the rate of cell growth determines the number of replicating chromosomes by modulating DnaA level and activity in S. elongatus. In addition, it is shown that increasing the ploidy level also increases cellular resistance to UV irradiation, suggesting that possessing multicopy chromosomes allows the organism to cope with DNA damage.
RESULTS
Only a few chromosomal copies are replicated at once while genes are transcribed from all chromosomal copies in cyanobacteria.
Previous reports suggested that the number of replicating chromosomes is restricted to one (or in some case two) in S. elongatus (37–40). Zheng and O’Shea recently reported that chromosomal copy number and protein concentration per cell volume are maintained constant independently of cell size and growth rate (18) in S. elongatus. These observations imply that genes are transcribed from all chromosome copies to maintain a constant concentration of transcripts and proteins; however, the transcriptional activity at chromosomes was not examined.
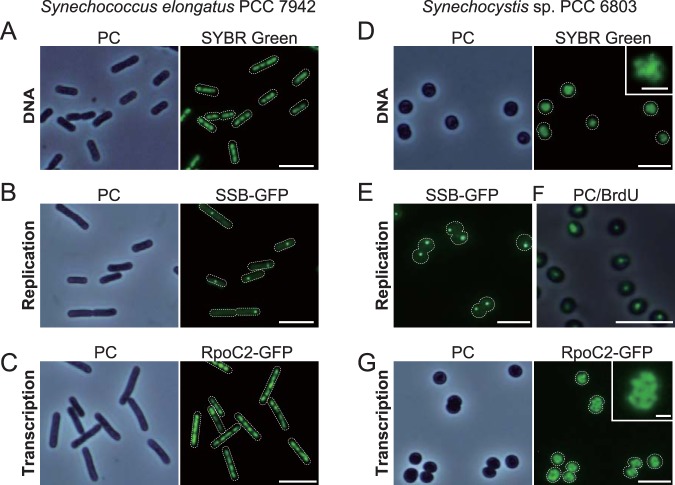
When S. elongatus cells were fixed and stained with a DNA-specific fluorescent dye, SYBR Green I, multiple (3 to 6) copies of chromosomes were observed aligned along the long axis of the cell (Fig. 1A). To examine transcriptional activities of multicopy chromosomes, we visualized replicating chromosomes and transcribing chromosomes in live S. elongatus cells. In addition, to assess the generality of the results, the same assays were applied to Synechocystis sp. strain PCC 6803 (here called Synechocystis), a model cyanobacterium phylogenetically distant from S. elongatus (41, 42). To this end, we expressed GFP-tagged single-strand binding protein (SSB; see Fig. S1 and S2 in the supplemental material), which is known to localize at replication forks (40, 43), and RNA polymerase beta subunit (RpoC2; Fig. S3 and S4). Consistent with previous reports (37, 39, 40), the majority of S. elongatus cells exhibited only one SSB focus (replication fork) per cell during growth under illumination (Fig. 1B). In contrast, RNA polymerase was observed on all chromosome copies (Fig. 1C).
FIG 1.
Replication and transcription of multicopy chromosomes in S. elongatus and Synechocystis. Mid-log-phase cultures were inoculated into fresh inorganic medium and cultured for 6 h under illumination at 70 µE m−2 s−1. (A and D) Microscopic images of SYBR Green-stained nucleoids in S. elongatus (A) and Synechocystis (D) cells. A magnified and high-contrast view of SYBR Green-stained Synechocystis cells is shown in the inset (D). The phase-contrast (PC) and SYBR Green-stained images of the same fields are also shown. (B and E) Fluorescence microscopic images of SSB-GFP expressers showing localization of SSB in S. elongatus (B) and Synechocystis (E) cells. PC and SSB-GFP images of the same field are shown for S. elongatus. (C and G) Fluorescence microscopic images of RpoC2-GFP expressers showing localization of RNA polymerase in S. elongatus (C) and Synechocystis (G) cells. A magnified view of RpoC2-GFP Synechocystis cells is shown in the inset (G). PC and RpoC2-GFP images of the same fields are also shown. (F) Synechocystis cells were labeled with BrdU for 1 h (from hour 5 to 6) and then fixed for immunofluorescence staining with the anti-BrdU antibody. The PC and immunofluorescence images are merged. Bars, 5 µm (1 µm for insets of panels D and G).
Preparation of an S. elongatus SSB-GFP expresser. (A) The gfp ORF was fused with the ssb gene just before the stop codon. The construct was integrated into the chromosomal ssb locus by homologous recombination, and the gentamicin resistance gene (Gmr) was used as the selection marker for the transformant. (B) Insertion of the gfp and Gmr genes into the chromosomal ssb locus of DnaAWT and DnaAR328H cells was confirmed by PCR using the primers indicated in panel A by arrows. The wild-type cell was used as a negative control. (C) Immunoblot analyses showing the expression of GFP-SSB in HA-DnaAWT and HA-DnaAR328H cells. HA-DnaAWT and HA-DnaAR328H cells without GFP-SSB were used as negative controls. Total proteins extracted from respective strains were subjected to analyses. GFP-SSB was detected with the anti-GFP antibody, and HA-DnaA was detected with the anti-HA antibody as a loading control. Download FIG S1, EPS file, 0.6 MB (667.6KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Preparation of a Synechocystis SSB-GFP expresser. (A) The gfp ORF was fused with the ssb gene just before the stop codon. The construct was integrated into the Synechocystis chromosomal ssb locus by homologous recombination. The gentamicin resistance gene (Gmr) was used as the selection marker for the transformant. (B) Insertion of the gfp and Gmr genes into the chromosomal ssb locus was confirmed by PCR using the primers indicated in panel A by arrows. The wild-type cell was used as a negative control. (C) Immunoblot analyses showing the expression of GFP-SSB in the transformant. The wild-type cell was used as a negative control. Total proteins extracted from the wild type and the transformant were subjected to analysis. GFP-SSB was detected with the anti-GFP antibody. As a loading control, Coomassie brilliant blue (CBB) staining of the protein samples resolved by SDS-PAGE is also shown. Download FIG S2, EPS file, 1.3 MB (1.3MB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Preparation of an S. elongatus RNA polymerase-GFP expresser. (A) The gfp ORF was fused with the rpoC2 gene just before the stop codon. The construct was integrated into the chromosomal rpoC2 locus by homologous recombination. The gentamicin resistance gene (Gmr) was used as a selection marker for the transformant. (B) Insertion of the gfp and Gmr genes into the chromosomal rpoC2 locus was confirmed by PCR using the primers indicated in panel A by arrows. The wild-type cell was used as a negative control. (C) Immunoblot analyses showing the expression of RpoC2-GFP in the transformant. The wild-type cell was used as a negative control. Total proteins extracted from the wild-type and the RpoC2-GFP cells were subjected to analysis. RpoC2-GFP was detected with the anti-GFP antibody. As a loading control, Coomassie brilliant blue (CBB) staining of the protein samples resolved by SDS-PAGE is also shown. Download FIG S3, EPS file, 2.3 MB (2.3MB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Preparation of a Synechocystis RNA polymerase-GFP expresser. (A) The gfp ORF was fused with the rpoC2 gene just before the stop codon. The construct was integrated into the chromosomal rpoC2 locus by homologous recombination. The spectinomycin resistance gene (Specr) was used as the selection marker for the transformant. (B) Insertion of the gfp and Specr genes into the chromosomal rpoC2 locus was confirmed by PCR using the primers indicated in panel A by arrows. The wild-type cell was used as a negative control. (C) Immunoblot analyses showing the expression of RpoC2-GFP in the transformant. The wild-type cell was used as a negative control. Total proteins extracted from the wild-type and RpoC2-GFP cells were subjected to analysis. RpoC2-GFP was detected with the anti-GFP antibody. As a loading control, Coomassie brilliant blue (CBB) staining of the protein samples resolved by SDS-PAGE is also shown. Download FIG S4, EPS file, 0.5 MB (548.1KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Synechocystis possesses more chromosome copies (10 to 20) than S. elongatus (3 to 6) (3, 36). When genomic DNA of Synechocystis was stained with SYBR Green I, many small foci were observed in each cell (Fig. 1D). In contrast, SSB-GFP was detected at only one focus per cell (Fig. 1E). In a similar manner, only one chromosome per cell (or two in the case of dividing cells) incorporated 5-bromo-2′-deoxyuridine (BrdU), an analog of thymidine incorporated into newly synthesized DNA, during a 1-h observation period (Fig. 1F). In contrast, RNA polymerase localized on all chromosome copies (Fig. 1G). These results indicate that the number of replicating chromosomes is restricted to one or two while all chromosome copies are used as the templates for transcription both in S. elongatus and in Synechocystis.
DnaA binding to the oriC region depends on photosynthesis.
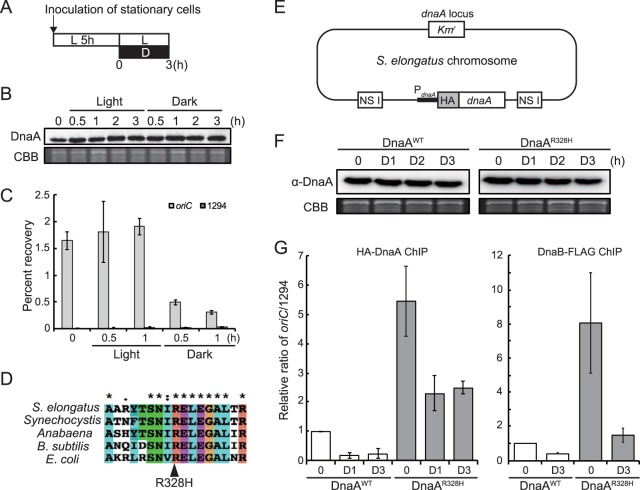
To address how replicating chromosome number is regulated, we examined the relationships of DnaA protein level and activity with chromosomal replication and chromosomal copy number in S. elongatus. It was previously shown that genomic DNA replication absolutely depends on photosynthetic activity in S. elongatus, so replication ceases under darkness (44). To examine whether DNA replication correlates with DnaA activity, we first compared DnaA protein level and oriC-binding activity between light and dark conditions. Cells were grown in an inorganic medium for 5 h under illumination and then kept under light or transferred to dark conditions (Fig. 2A). Immunoblot analysis using anti-DnaA antibody showed that DnaA protein level was constant for 3 h under both light and dark conditions (Fig. 2B and Fig. S5). In contrast, chromatin immunoprecipitation using the anti-DnaA antibody and subsequent quantitative PCR (ChIP-qPCR) analysis of the oriC region showed that the affinity of DnaA to oriC decreased by approximately two-thirds after 30 min under darkness (Fig. 2C). When an inhibitor of photosynthetic electron flow, 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) or 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB), was added to the culture under light, oriC binding of DnaA was almost completely abolished although DnaA level remained constant (Fig. S5 and Fig. S6A, B, and C). These results suggest that DNA replication activity is associated with the oriC-binding activity of DnaA rather than DnaA protein level. Further, oriC binding and DNA replication appear to depend on photosynthesis, which was confirmed in subsequent studies.
FIG 2.
Effects of light and inhibition of DnaA intrinsic ATPase activity on affinity for the genomic oriC region. (A) Schematic diagram of the culture conditions for panels B, C, F, and G. Mid-log-phase cultures of the S. elongatus wild-type, DnaAWT, or DnaAR328H strains were inoculated into fresh inorganic medium and cultured for 5 h under 70-µE m−2 s−1 illumination. At hour 0, one culture was kept under illumination and another culture was transferred to darkness. Both were cultivated for a further 3 h. (B) Immunoblot analysis showing the DnaA protein level under the light or dark condition. Proteins extracted from wild-type cells at the indicated time points were reacted with the anti-DnaA antibody (upper panel). Coomassie brilliant blue (CBB) staining of protein samples resolved by SDS-PAGE is shown in the lower panel as a gel loading control. (C) ChIP-qPCR analysis showing the affinity of DnaA for the oriC region under light and dark conditions. The DnaA-chromatin complex was immunoprecipitated with the anti-DnaA antibody. The samples were quantified by qPCR using primers specific to the oriC region (oriC, gray bar) and Syf1294 gene (1294, dark gray bar). The percent recovery against the total amount of input DNA is indicated. (D) A partial amino acid sequence alignment of the DnaA proteins from S. elongatus, Synechocystis, Anabaena sp. strain PCC 7120 (Anabaena), Bacillus subtilis 168, and Escherichia col. The conserved arginine residue is indicated by the arrowhead. (E) Schematic diagram of the S. elongatus chromosome expressing HA-DnaAWT (DnaAWT) or HA-DnaAR328H (DnaAR328H). DNA encoding HA-DnaAWT or HA-DnaAR328H under the control of the dnaA promoter was introduced into the chromosomal neutral site I (NS I). The native dnaA gene was then deleted by insertion of the kanamycin resistance gene (Kmr). (F) Immunoblot analysis showing DnaAWT and DnaAR328H protein levels in culture under light and dark conditions. Proteins extracted from respective cells at the indicated time points were reacted with the anti-HA antibody (upper panel). CBB staining of the protein samples resolved by SDS-PAGE is shown in the lower panel as a gel loading control. (G) ChIP-qPCR analysis showing the affinity of DnaA (left) and DnaB (right) protein for the oriC region in DnaAWT and DnaAR328H cells under light and dark conditions. The DnaA (or DnaB)-chromatin complex was immunoprecipitated with anti-HA (or FLAG) antibody. For the DnaB assay, DnaB-FLAG was expressed under the control of the dnaB promoter (44). The samples were quantified by qPCR using primers specific to the oriC region and the Syf1294 gene (1294), which is farthest from oriC in the circular chromosome. The value indicated is the ratio of percent recovery (oriC/1294) normalized to the value of DnaAWT at hour 0 (defined as 1.0).
Comparison of DnaA expression levels in S. elongatus that was cultured under different conditions. Immunoblot analyses showing temporal changes in DnaA and SSB-GFP protein levels in S. elongatus. Mid-log-phase cultures of SSB-GFP cells were inoculated into fresh inorganic medium (hour 0) and then cultured for 9 h under the indicated illumination intensities and temperatures. Total proteins extracted from the cells at the indicated time points were reacted with the anti-DnaA antibody (upper panel) and anti-GFP antibody (lower panel). Samples at hour 0 are identical in the all blots. In order to clearly visualize the temporal change in protein levels for the 30°C and 250-µE m−2 s−1 treatment group, an image acquired with a shorter exposure time than the others is shown. Download FIG S5, EPS file, 0.5 MB (560.9KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effect of photosynthetic inhibitors on affinity of DnaA and DnaB proteins to genomic oriC region. (A) Schematic diagram of the culturing condition for panels B to E. A saturated cell culture of the S. elongatus wild type or HA-DnaAR328H was inoculated into fresh inorganic medium and cultured with illumination (70 µE m−2 s−1) for 5 h. Then DCMU or DBMIB was added to the culture (hour 0), and cells were further cultured with illumination for 3 h. (B) Immunoblot analysis showing the DnaA protein level in a culture treated with DCMU or DBMIB under illumination. Total proteins extracted from cells at the indicated time points were reacted with the anti-DnaA antibody (upper panel). Coomassie brilliant blue (CBB) staining of the protein samples resolved by SDS-PAGE is shown in the lower panel as a loading control. (C and D) ChIP-qPCR analysis showing the effect of DCMU or DBMIB on the affinity of DnaA (C) and DnaB (D) for the oriC region. The DnaA- (or DnaB)-chromatin complex was immunoprecipitated with the anti-DnaA (or anti-FLAG) antibody. For the DnaB assay, a strain expressing DnaB-FLAG under the control of the dnaB promoter (44) was used. The samples were quantified by qPCR using the primers specific to the oriC region (oriC; gray bar) and the Syf1294 gene (1294; white bar), which is furthest from oriC in the circular chromosome. The percent recovery against the amount of total input DNA is indicated. (E) ChIP-qPCR analysis showing the effect of DCMU on the affinity of HA-DnaAR328H for the oriC region. The samples were subjected to ChIP-qPCR analysis with the anti-HA antibody. The value indicated is the ratio of percent recovery (oriC/1294). Download FIG S6, EPS file, 0.7 MB (748.5KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ATP-dependent binding of DnaA to oriC in S. elongatus.
In many model bacterial species, binding of DnaA to the oriC region is controlled by the ATP/ADP ratio, as only the ATP-bound form is capable of forming the oligomeric structure at the oriC region required for initiation of DNA replication (45). To assess the mechanism of light/photosynthesis-dependent activation of DnaA activity in S. elongatus, we examined the effect of changing the proportion of ATP-bound DnaA on oriC-binding activity under constant illumination.
The arginine residue at position 334 of E. coli DnaA is essential for ATP hydrolysis, and the amino acid substitution R334H inactivates this intrinsic ATPase activity, which leads to accumulation of DnaA-ATP (46). This arginine is conserved in DnaA of many organisms (47), including cyanobacteria (Fig. 2D). In order to examine the effect of ATP binding to DnaA on oriC affinity in S. elongatus, we expressed HA-DnaA (DnaAWT) or HA-DnaA R328H (DnaAR328H) (which corresponds to R334H of E. coli DnaA) under the control of the dnaA promoter from the genomic neutral site (NS I) on an endogenous dnaA-knockout background (Fig. 2E and Fig. S7). Binding to oriC was then examined by chromatin immunoprecipitation (ChIP). For these studies, HA tag was fused to the N terminus of DnaAWT or DnaAR328H so that the protein could be precipitated with an anti-HA antibody instead of anti-DnaA because the affinity of the polyclonal anti-DnaA antibody would likely differ between DnaAWT and DnaAR328H.
Revision of the start codon of S. elongatus dnaA gene. (A) Amino acid sequence (deduced from the annotated ORF sequences) alignment of the DnaA N-terminal regions of S. elongatus (syf), Synechococcus sp. strain UTEX2973 (syu), Cyanothece sp. strain PCC 7425 (cyn), Anabaena sp. (ana), Synechocystis (syn), and Bacillus subtilis 168 (bus). (B) DNA sequence of S. elongatus dnaA gene around the annotated start codon (CyanoBase, http://genome.kazusa.or.jp/cyanobase). Based on the protein expression analyses in panel C, the GTG codon 60 bp downstream of the annotated translational start codon (ATG) turned out to be the actual start codon of the dnaA gene in S. elongatus. (C) Three constructs (a to c) for expression from the chromosomal neutral site I (NS I). In construct a, the HA-tag-encoding sequence was fused with the annotated start codon (ATG) of dnaA and expressed by the IPTG-inducible trc promoter. In construct b, the HA-tag-encoding sequence was fused with the revised start codon (GTG) of dnaA and expressed by the 60-bp 5′ upstream sequence flanking the revised start codon (GTG). In construct c, the HA-tag-encoding sequence was fused with the revised start codon (GTG) of dnaA and expressed by the 300-bp 5′ upstream sequence flanking the revised start codon (GTG). After introduction of each HA-tagged dnaA, the endogenous dnaA gene was deleted in all strains. (D) Immunoblot analysis showing the size of HA-DnaA expressed in the respective strains. Mid-log-phase cultures were inoculated into fresh medium with (+) or without (−) 1 mM IPTG. Total proteins extracted from respective transformants (for construct a, with or without IPTG induction) were subjected to immunoblot analysis with the anti-DnaA antibody (upper panel) and the anti-HA antibody (lower panel). In construct a, HA-DnaA protein was expressed only by IPTG induction, while the smaller endogenous DnaA protein was expressed both with and without IPTG, suggesting that this construct possesses a promoter other than the trc promoter. In addition, in constructs b and c, HA-DnaA was expressed by the predicted dnaA promoter (upstream of GTG; US60 or US300). The HA-DnaA expressed by constructs b and c was smaller than that expressed by construct a. These results indicate that the actual start codon of the dnaA gene is a “GTG” 60 bp upstream of the “GTG” functioning as a promoter of the dnaA gene. Download FIG S7, EPS file, 0.7 MB (766.6KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Both DnaAWT and DnaAR328H proteins were expressed in respective transformants at similar levels, and the expression levels remained constant for 3 h after cells were transferred to darkness (Fig. 2F). Similarly to endogenous DnaA (Fig. 2C), DnaAWT bound to the oriC region in cells cultured under illumination and the affinity was reduced when the cells were transferred to darkness (Fig. 2G). The R328H mutation elevated DnaA activity due to an approximately 6-fold increase in oriC affinity compared to DnaAWT under illumination (Fig. 2G). When the DnaAR328H cells were transferred to darkness, the affinity of DnaAR328H to oriC decreased but the level was still higher than DnaAWT (Fig. 2G). These results suggest that the ATP-bound form of DnaA possesses higher affinity for the oriC site than the ADP-bound form as shown in other model bacteria. In addition, the light/photosynthesis-dependent activation of DnaA is, at least partly, independent of DnaA intrinsic ATPase activity because the affinity of constitutive DnaAR328H for oriC decreased when the cells were transferred to darkness or treated with DCMU (Fig. 2G and Fig. S6E). This assumption is based on the following observations in E. coli. The R334H mutation completely abolished the intrinsic ATPase activity of DnaA in vitro (46). However, ATP bound to the mutated DnaA was gradually hydrolyzed by the addition of crude extract of E. coli in vitro (46). In addition, ATP bound to DnaA was slowly hydrolyzed by ATPases other than DnaA in vivo (23).
To examine the effect of DnaAR328H expression and light/photosynthesis on replication fork components other than DnaA, we compared affinity of DnaB to oriC between DnaAWT and DnaAR328H transformants by ChIP-qPCR analysis. DnaB is a DNA helicase recruited to the oriC region in a DnaA- and DnaC-dependent manner in bacteria. Similar to DnaA, the affinity of DnaB to oriC was higher in DnaAR328H cells than in DnaAWT cells under both light and dark conditions (Fig. 2G). In addition, the affinity of DnaB to oriC decreased in both DnaAR328H and DnaAWT cells after transfer to darkness. These results suggest that the increased affinity of DnaA to oriC by light/photosynthesis or by an increase in ATP-DnaA by the R328H mutation promoted the formation of replication forks.
The oriC-binding activity of DnaAR328H was 6-hold higher than that of DnaAWT under illumination, although the expression level of DnaAR328H protein was comparable to that of DnaAWT (Fig. 2G). Thus, it is suggested that DnaA activity is limited, probably by moderation of ATP hydrolysis, even under illumination to restrict the number of replicating chromosomes as shown below.
Simultaneous replication of multiple chromosome copies by increasing ATP-DnaA.
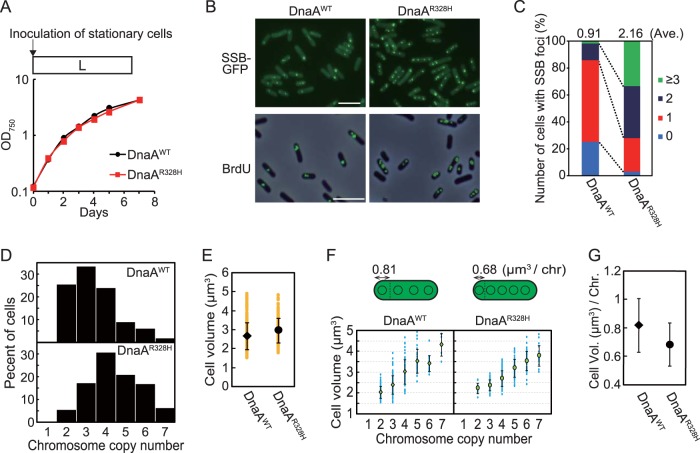
As mentioned above, only one copy of multiple chromosomes is replicated in wild-type S. elongatus (37, 39, 40). This finding led us to examine the effect of artificially elevating DnaA activity by expressing DnaAR328H on the number of replicating chromosome copies. To this end, we expressed GFP-tagged SSB to visualize replicating chromosomes in DnaAWT and DnaAR328H cells. Consistent with previous reports (37, 39, 40) and the results shown in Fig. 1, the majority of DnaAWT cells exhibited only one SSB focus per cell during growth under illumination (Fig. 3A to C). In contrast, the majority of DnaAR328H cells exhibited two or more SSB foci under the same growth conditions (Fig. 3B and C). When DnaAWT and DnaAR328H cells were labeled with BrdU, one or two BrdU foci were detected in DnaAWT cells (Fig. 3B) whereas two or more foci were detected in DnaAR328H cells 6 h after inoculation under illumination (Fig. 3B). These results indicate that elevation of DnaA activity by enhancing the ratio of ATP-DnaA to total DnaA results in simultaneous replication of multiple chromosome copies.
FIG 3.
Chromosome replication in HA-DnaAWT and HA-DnaAR328H cells under illumination. (A) To visualize replicating chromosomes by fluorescence microscopy, GFP-tagged SSB protein was expressed in DnaAWT and DnaAR328H cells. Mid-log-phase cultures were inoculated into fresh inorganic medium (hour 0) and cultured under illumination. Growth curves of DnaAWT (black) and DnaAR328H (red) cells are shown. (B) Fluorescence microscopic images showing replicating chromosomes in DnaAWT and DnaAR328H cells. In the upper panel, SSB-GFP was observed in living DnaAWT and DnaAR328H cells at hour 6. In the lower panel, newly synthesized DNA was visualized by immunofluorescence microscopy with the anti-BrdU antibody. Cells were labeled with BrdU for 1 h (hour 5 to 6) and then fixed for immunofluorescence staining. The phase-contrast and immunofluorescence images are merged. Bar, 5 µm. (C) Frequencies of cells exhibiting zero (blue), one (red), two (deep blue), or three or more (green) SSB-GFP foci in DnaAWT and DnaAR328H cultures at hour 6 (n ≥ 300 cells for each strain). The average number of SSB foci is indicated above the bars. (D) Histograms showing number of chromosomes per cell in DnaAWT and DnaAR328H cultures at hour 6. The number of chromosomes was determined based on micrographs of SYBR Green-stained cells. (E) Distribution of DnaAWT and DnaAR328H cell volumes at hour 6. Orange points represent volumes of single cells (n ≥ 300 cells for each strain). The black diamond (DnaAWT) and circle (DnaAR328H) represent the means, and the error bars represent the standard deviations (n ≥ 300 cells for each strain). (F) Distribution of DnaAWT and DnaAR328H cell volumes and chromosomal numbers at hour 6. Blue points represent single cells. The green diamond (DnaAWT) and circle (DnaAR328H) represent the means, and the error bars represent the standard deviations (n ≥ 300 cells for each strain). (G) Mean cell volume occupied by one chromosome in DnaAWT and DnaAR328H cells at hour 6. The black diamond (DnaAWT) and circle (DnaAR328H) represent the means, and error bars represent the standard deviations (n ≥ 300 cells for each strain).
In order to compare frequency of DNA replication initiation between DnaAWT and DnaAR328H cells on a genome-wide basis, relative copy numbers of chromosomal regions in these strains were analyzed by next-generation sequencing. When model bacteria possessing a single chromosome (genome) per cell, such as E. coli, are rapidly grown in nutrient-rich medium, DNA is replicated in a multifork mode. In this case, the oriC/terC ratio is more than 2, thus yielding a V-shaped profile in the depth of sequencing reads (lowest at terC and highest at oriC) (37). In the case of cyanobacteria possessing multiple chromosome copies, of which only one copy is being replicated, the ratio of terC and oriC to total DNA is almost 1.0, thus yielding an almost linear profile in the depth of sequencing reads (37) (Fig. S8A, DnaAWT) throughout the genome. The sequencing profile of DnaAR328H cells 6 h after inoculation under illumination exhibited a slight V-shape, in contrast to the sequencing profile of DnaAWT cells (highest at oriC region; Fig. S8A). However, the ratio of oriC to terC was less than 2 (Fig. S8A). These results suggest that some chromosomes are replicated simultaneously in DnaAR328H cells under illumination but that the number is still restricted, most likely by ATPase proteins other than DnaA. Nonetheless, this restriction was compromised to a certain extent when the intrinsic ATPase activity was abolished by the R328H mutation.
High-throughput sequencing of genomic DNA and chromosomal copy number in mid-log-phase cultures of DnaAWT and DnaAR328H. (A) Depth of genomic DNA read through the genome 6 h after inoculation in DnaAWT and DnaAR328H strains was analyzed by Illumina MiSeq. Relative sequencing coverage ratios of Illumina MiSeq reads (1-kb window for the left graphs and 100-kb window for the right graphs) at respective genomic regions in HA-DnaAWT and HA-DnaAR328H cells are shown. The ratio of read depth is shown in the graphs. The sequencing coverage of each region was normalized to the total read depth. (B) Flow cytometry analysis showing the DNA level and relative cell volume of DnaAWT (upper panel) and DnaAR328H (lower panel) cells 6 h after inoculation. Dots represent single cells. DNA level was determined by intensity of SYTOX Green fluorescence. Download FIG S8, EPS file, 0.7 MB (782.7KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relationship between cell growth and DNA replication in S. elongatus.
Chromosome copy number exhibits a positive, linear correlation with cell volume in S. elongatus (18, 39, 40). However, it is not known whether increased chromosomal copy number leads to increased cell volume or if cell growth leads to an increased number of chromosomal copies. To address whether there is such a causal relationship between chromosomal copy number and cell size, we examined the relationships among growth rate, cell volume, and frequency of chromosomal replication.
First, we investigated the effect of changing the chromosome copy number on cell growth and cell volume by comparing DnaAR328H and DnaAWT cells. When DnaAR328H and DnaAWT were cultured under illumination, DnaAR328H cells possessed more chromosome copies than DnaAWT cells (Fig. 3D). Thus, an increase in the number of replicating chromosome by expression of DnaAR328H led to an increase in chromosomal copy number per cell. However, DnaAR328H and DnaAWT cells exhibited similar growth rates (Fig. 3A) and cell volumes (Fig. 3E). Thus, the cell volume occupied by one chromosome in DnaAR328H cells was smaller than that in DnaAWT cells (Fig. 3F and G). Similar results were also observed by measuring cell volume and DNA level by flow cytometry (Fig. S8B). These results suggest that initiation frequency and number of chromosome copies have little impact on cellular growth rate and volume.
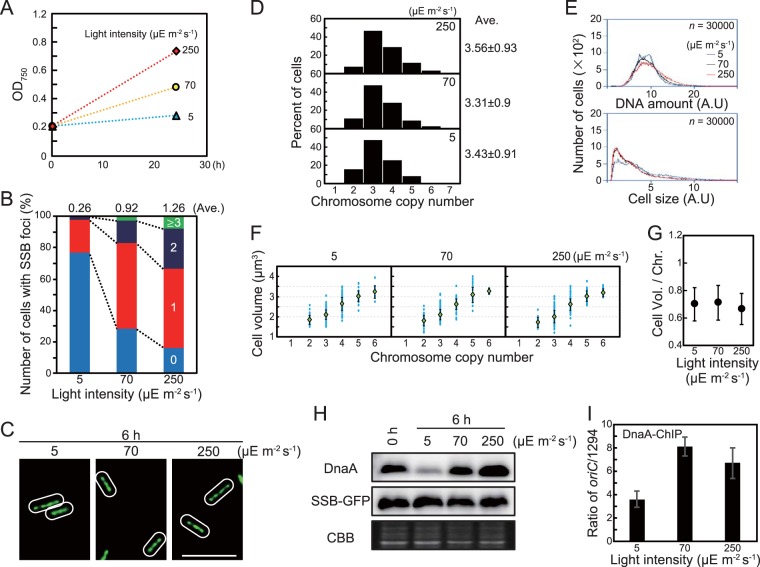
Next, to examine the effect of cell growth on chromosomal replication and chromosome copy number, we cultured S. elongatus under different light intensities (Fig. 4A). S. elongatus is an obligate photoautotroph, so growth rate depends on light intensity. Cultures expressing SSB-GFP exhibited faster growth at higher light intensity [0.01 h−1 at 5 µE (µmol photons) m−2 s−1, 0.03 h−1 at 70 µE m−2 s−1, and 0.05 h−1 at 250 µE m−2 s−1] (Fig. 4A). As light intensity and growth rate increased, the number of SSB foci per cell also increased (average of 0.26 foci per cell at 5 µE m−2 s−1, 0.92 at 70 µE m−2 s−1, and 1.26 at 250 µE m−2 s−1) (Fig. 4B). On the other hand, cells growing under different light intensities exhibited similar chromosome copy numbers and cell volumes and thus similar positive correlations between cell volume and chromosomal copy number (Fig. 4C to G). Comparable results were also observed by measuring cell volume and DNA level by flow cytometry (Fig. S9A). Changing the light intensity also influenced DnaA level. Specifically, DnaA level decreased when light intensity was reduced to 5 µE m−2 s−1 (hour 6) from 70 µE m−2 s−1 (hour 0) (Fig. 4H and Fig. S5) and increased when the light intensity was raised to 250 µE m−2 s−1 (hour 6) from 70 µE m−2 s−1 (hour 0) (Fig. 4H and Fig. S5). In addition, the oriC-binding activity of DnaA was lower at 5 µE m−2 s−1 than at 70 or 250 µE m−2 s−1 (Fig. 4I; note that in the ChIP-qPCR analysis, sample input DnaA depends on the cellular DnaA level). These results suggest that an increase or decrease in cellular growth rate leads to a corresponding increase or decrease in chromosomal replication activity to maintain a constant chromosomal copy number per unit cell volume at a constant temperature.
FIG 4.
Effect of growth rate on number of replicating chromosomes, chromosome copy number, and cell size. To prepare cultures with different growth rates, S. elongatus was cultured under different intensities of illumination at 30°C. (A) Growth rate at 5, 70, and 250 µE m−2 s−1. A mid-log-phase SSB-GFP culture was inoculated into fresh inorganic medium (hour 0) and then cultured under the indicated illumination intensity. (B) Frequency of cells exhibiting zero (blue), one (red), two (deep blue), or three or more (green) SSB-GFP foci at hour 6 (n ≥ 300 cells per condition). The average number of SSB foci is indicated above the bars. (C) Fluorescence microscopic images showing SYBR Green staining of nucleoids at hour 6. Phase-contrast and SYBR Green images are shown. Bar, 5 µm. (D) Histograms showing number of chromosomes per cell at hour 6. Number of chromosomes was determined based on micrographs of SYBR Green-stained cells (n ≥ 300 cells per condition). (E) Flow cytometry analysis showing the DNA level (upper panel) and relative cell volume (lower panel) at hour 6. DNA level was calculated by intensity of SYTOX Green fluorescence. Blue line, 5 µE m−2 s−1; black line, 70 µE m−2 s−1; red line, 250 µE m−2 s−1; A.U., arbitrary unit. (F) Distribution of cell volume and chromosomal copy number. The blue points represent single cells. The green diamond represents the mean, and the error bar represents the standard deviation (n ≥ 300 cells per condition). (G) Mean cell volume occupied by one chromosome at hour 6. Error bar represents the standard deviation (n ≥ 300 cells per condition). (H) Immunoblot analysis showing DnaA and SSB-GFP protein levels. Proteins extracted from cells at hours 0 and 6 were reacted with anti-HA antibody (top) and anti-GFP antibody (middle). Coomassie brilliant blue (CBB) staining of the protein samples resolved by SDS-PAGE is shown in the bottom panel as a gel loading control. (I) ChIP-qPCR analysis showing the affinity of DnaA for the oriC region at hour 6. The value indicated is the ratio of percent recovery (oriC/1294). Error bars represent the standard deviation (n = 3). Three independent experiments yielded similar results, and results from one experiment are shown (for panels A to H).
Flow cytometry analysis of cell volume and DNA level of cells grown under different illumination intensities or temperatures. Flow cytometry analysis showing the DNA level and cell volume as in Fig. 4A (A) and Fig. 5E (B). Dots represent single cells. DNA level was determined by intensity of SYTOX Green fluorescence. Download FIG S9, EPS file, 0.5 MB (551KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
When the cells were transferred from light to dark or photosynthesis was inhibited with DCMU or DBMIB, cellular growth and chromosome replication ceased whereas DnaA level remained constant (Fig. 2 and Fig. S5 and S6). In contrast, when the light intensity was reduced and cellular growth slowed down, DnaA level decreased (Fig. 4 and Fig. S5). Thus, DnaA level changes only when cells are growing and the growth rate changes to match chromosome replication rate, but does not change when cellular growth and chromosome replication cease.
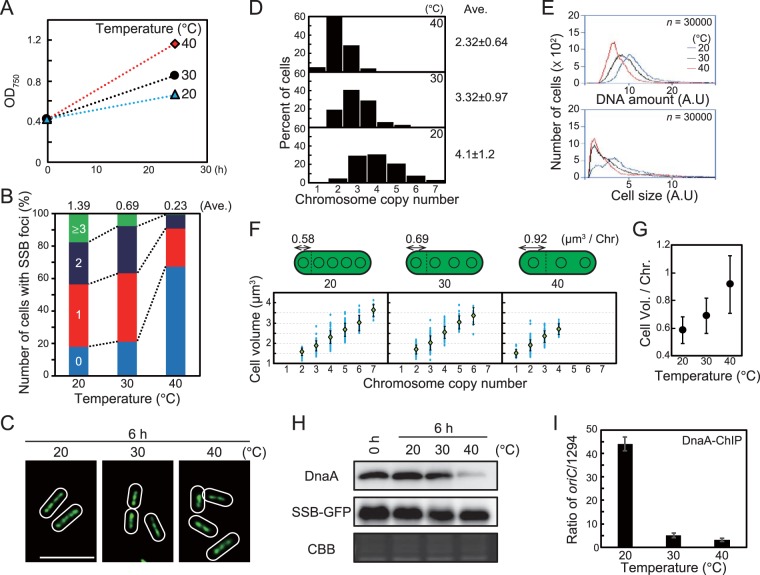
We also examined the relationship between cell growth and chromosomal replication and copy number at different temperatures. As temperature increased, cell growth accelerated (0.01 h−1 at 20°C, 0.03 h−1 at 30°C, and 0.05 h−1 at 40°C; Fig. 5A). In contrast to results at constant temperature (30°C) and variable light intensity (Fig. 4), the number of SSB foci per cell increased at lower temperature (Fig. 5B) even though cell growth was slower (Fig. 5A). Consistent with these observations, microscopic analysis and flow cytometry showed that chromosome copy number per cell was higher at lower temperature 6 h after temperature reduction (Fig. 5C, D, and E) while cell volume per chromosome was reduced at lower temperature (Fig. 5F and G). Similar results were also observed by measuring cell volume and DNA level by flow cytometry (Fig. S9B). The level and oriC-binding activity of DnaA were higher at lower temperature (Fig. 5H and I; note that in the ChIP-qPCR assay, sample input DnaA level depends on cellular DnaA level). Although cell volume per chromosome depended on temperature, cell volume and chromosomal copy number exhibited a linear correlation at all temperatures examined. These results suggest that the number of replicating chromosomes (initiation frequency) and chromosomal copy number are regulated by a certain temperature-affected factor.
FIG 5.
Effect of temperature on growth rate, number of replicating chromosomes, chromosome copy number, and cell size. (A) Growth rate of S. elongatus at 20, 30, and 40°C. A mid-log-phase SSB-GFP culture at 30°C was inoculated into fresh inorganic medium (hour 0) and then cultured at the indicated temperature under 70-µE m−2 s−1 illumination. (B) Frequency of cells exhibiting zero (blue), one (red), two (deep blue), or three or more (green) SSB-GFP foci at hour 6 (n ≥ 300 cells per condition). The average number of SSB foci is indicated above the bars. (C) Fluorescence microscopic images showing SYBR Green staining of nucleoids at hour 6. SYBR Green images are shown. Bar, 5 µm. (D) Histograms showing number of chromosomes per cell at hour 6. Number of chromosomes was determined based on micrographs of SYBR Green-stained cells (n ≥ 300 cells per condition). (E) Flow cytometry analysis showing the DNA level (upper) and relative cell volume (lower) at hour 6. DNA level was calculated by intensity of SYTOX Green fluorescence. Blue line, 20°C; black line, 30°C; red line, 40°C; A.U., arbitrary unit. (F) Distribution of cell volume and chromosomal copy number. The blue points represent single cells. The green diamond represents the mean, and the error bar represents the standard deviation (n ≥ 300 cells per condition). The schematic illustrations above the graphs show the average cell volume occupied by one chromosome. (G) Mean cell volume occupied by one chromosome at hour 6. Error bar represents the standard deviation (n ≥ 300 cells per condition). (H) Immunoblot analysis showing DnaA and SSB-GFP protein levels. Proteins extracted from cells at hour 0 and 6 were reacted with the anti-HA antibody (top) and anti-GFP antibody (middle). Coomassie brilliant blue (CBB) staining of the protein samples resolved by SDS-PAGE is shown in the bottom panel as a gel loading control. (I) ChIP-qPCR analysis showing the affinity of DnaA for the oriC region at hour 6. The value indicated is the ratio of percent recovery (oriC/1294). Error bars represent the standard deviation (n = 3). Three independent experiments yielded similar results. Results from one experiment are shown (for panels A to H).
Multicopy chromosomes confer greater UV resistance to S. elongatus.
Photoautotrophic organisms require light for growth. However, UV in sunlight damages DNA directly and the photosystems generate reactive oxygen species (ROS) that also damage DNA (48). Thus, photosynthetic organisms are exposed to higher risk of DNA damage during cell growth than many other heterotrophic organisms. Polyploid bacteria often inhabit extreme environments such those with high temperatures (e.g., Thermus thermophilus) and possess resistance to high UV (e.g., Deinococcus radiodurans). In a similar manner, polyploid chloroplasts and mitochondrial DNA are also exposed to oxidative stresses that result from photosynthesis and respiration (49, 50). Thus, one plausible advantage of multiple chromosome copies is to compensate for a damaged copy using the information from the undamaged replicate.
In order to assess this possibility, we investigated the relationship between ploidy level and cellular resistance to UV in S. elongatus. Populations with different ploidy levels were generated by cultivation of DnaAWT cells and DnaAR328H cells at 20, 30, or 40°C (Fig. 3 and 5). After cultivation in liquid culture under light for 6 h, cells were spotted onto agar medium. After UV irradiation, cells were cultured on agar medium at 30°C to allow surviving single cells to produce colonies (Fig. 6A and B). Cell viability just after UV irradiation was quantified based on the number of single colonies (Fig. 6C and E). As temperature increased and ploidy level decreased (Fig. 5), susceptibility to UV increased in the wild type (Fig. 6E). Ploidy was higher in DnaAR328H than DnaAWT cells at respective temperatures, and resistance to UV was also greater in DnaAR328H cells than in DnaAWT cells at respective temperatures (Fig. 6C and D). Thus, cells of higher ploidy were more resistant to UV irradiation, supporting the hypothesis that polyploidy benefits organisms under higher risk of DNA damage.
FIG 6.
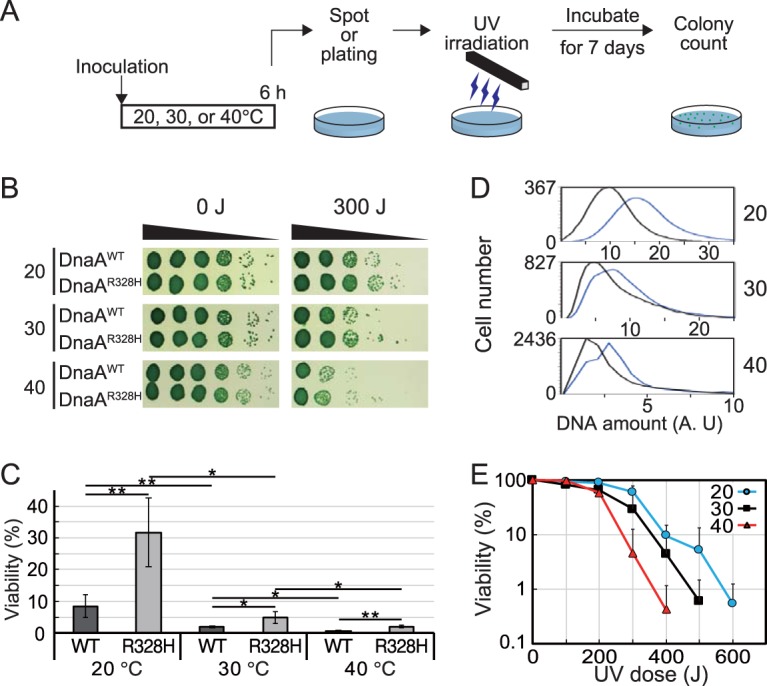
Effect of polyploidy level on UV resistance of the cells. (A) Schematic diagram of the assay. A mid-log-phase DnaAWT or DnaAR328H culture was inoculated into fresh inorganic medium and then cultured for 6 h at the indicated temperature under 70-µE m−2 s−1 illumination. Cell density was adjusted to an OD750 of 0.2, and a serial dilution series (10−1 to 10−5) was prepared using fresh medium. The diluted cells were spotted (for panel B) and plated (for panels C and D) on agar medium and then irradiated under 0- to 600-J m−2 UV-C (254 nm), respectively. The plates were further incubated at 30°C under illumination (70 µE m−2 s−1) for 7 days to allow surviving cells to form colonies. (B and C) The cell survival rate was calculated by counting the colony formation units. Results of DnaAWT and DnaAR328H cells cultured at 20°C, 30°C, or 40°C under 300-J m−2 UV-C irradiation are shown. Error bars represent the standard deviation (n = 3 biological replicates). Two-tailed t tests between the indicated strains or conditions were performed. *, P < 0.05; **, P < 0.01. (D) Flow cytometry analysis showing the DNA level at hour 6 in DnaAWT and DnaAR328H cells cultured at 20°C, 30°C, or 40°C. Black line, DnaAWT; blue line, DnaAR328H; A.U., arbitrary unit. (E) The survival rate of the wild-type cells was determined after irradiation with 0- to 600-J m−2 UV-C at 20, 30, or 40°C. Error bars represent the standard deviation (n = 3 biological replicates).
DISCUSSION
DnaA-dependent regulation of multicopy chromosomes in cyanobacteria.
Our results suggest that DNA replication in S. elongatus is absolutely dependent on photosynthesis through regulation of DnaA activity (Fig. 2; see also Fig. S6 in the supplemental material) and that the number of replicating chromosomes is restricted so that chromosome replication frequency is matched to cellular growth rate. Through this coordination, chromosome copy number increases and decreases according to cell size (Fig. 4 and 5), thereby maintaining protein and metabolite concentrations during cell growth.
Supporting this conclusion, inhibition of DnaA intrinsic ATPase activity by the R328H mutation increased the number of replicating chromosomes during cell growth (Fig. 3). However, DNA replication was still limited to a certain chromosome copy number in DnaAR328H cells (Fig. 3; Fig. S8). In addition, the oriC-binding activity of DnaAR328H decreased when photosynthesis and cell growth were blocked by transfer to darkness or chemical inhibition of photosynthesis, although DnaAR328H cells still exhibited higher oriC-binding activity than DnaAWT cells (Fig. 2; Fig. S6). These results suggest that in addition to the intrinsic ATPase activity of DnaA, other, as-yet-unknown factors also regulate DnaA activity in S. elongatus. There are several steps during initiation in which regulatory systems have been found to control bacterial DNA replication (for a review, see reference 23). For example, oriC binding of ATP-DnaA is inhibited by SeqA in E. coli, Spo0A in B. subtilis, and CtrA in C. crescentus, all of which occupy the oriC region.
In addition, there are also likely DnaA-independent mechanisms that regulate the number of replicating chromosomes in cyanobacteria. In this study, we showed that the number of replicating chromosome is also restricted to one in Synechocystis cells during growth (Fig. 1). As in S. elongatus, chromosome replication in Synechocystis depends on photosynthesis (44). However, even when DnaA protein is depleted, Synechocystis grows in a manner similar to the wild type, and it is likely that initiation and regulation of chromosome replication in Synechocystis are governed mainly by unknown DnaA-independent mechanisms (38) that also function in S. elongatus.
Relationship between cell growth and initiation of chromosome replication.
In this study, we showed that DnaA level, DnaA activity, and number of replicating chromosomes changed depending on cellular growth rate (Fig. 4 and 5). In addition, chromosome copy number per unit cell volume was nearly constant during cell growth at a constant temperature (Fig. 4 and 5). On the other hand, increasing the number of replicating chromosomes and chromosome number per cell by inhibition of DnaA ATPase activity did not affect cell growth (Fig. 3). These results suggest that the number of replicating chromosomes is regulated by cell growth through regulation of DnaA level and activity in S. elongatus.
Regulation of chromosome replication depending on cellular growth has also been demonstrated in monoploid bacteria such as E. coli, in which chromosome replication is initiated when the cell grows to a certain fixed volume (51, 52). However, in both monoploid and polyploid bacteria, it is still unclear how a cell senses its volume to regulate chromosome replication. When cell volume increases, concentrations of RNAs transcribed from chromosomes and proteins translated from these mRNAs are predicted to decrease until additional chromosome copies are generated by replication (Fig. 7). Therefore, chromosomal replication is likely triggered by a reduction in the concentration of an as-yet-unknown factor or factors by cell growth. For example, chromosomal replication may be initiated when the concentration of a replication repressor decreases below a certain threshold. This notion is supported by the observation that DnaA level and activity, number of replicating chromosomes, and chromosome number per cell volume were lower at higher temperature in S. elongatus (Fig. 5). Increased temperature accelerates chemical reactions, including enzymatic reactions; thus, transcriptional and translational activities increase with temperature. At higher temperature, cells require lower levels of template chromosomal DNA and thus a smaller number of chromosomal copies to sustain protein and metabolite levels than at lower temperature.
FIG 7.
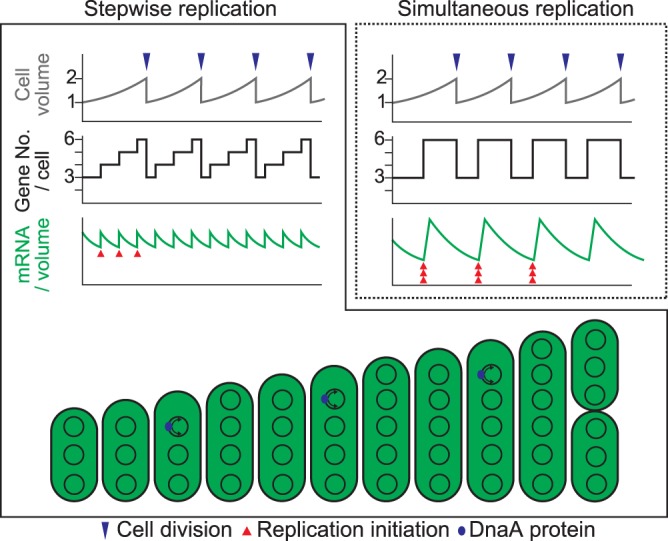
Correlation between cell growth and chromosomal replication in S. elongatus. Schematic diagrams showing changes in cell volume (top), gene copy number per cell (middle), and mRNA level per unit volume (bottom) during cell growth in the case of stepwise (as observed in this study) or simultaneous replication of multicopy chromosomes. Blue and red arrowheads indicate cell division and replication of a chromosomal copy, respectively.
Biological significance of polyploidy in bacteria.
Polyploidy is widespread in bacteria, archaea, and eukaryotes, but the advantages conferred by multicopy genomes are unclear. Results of this study suggest that polyploid bacteria are able to change chromosomal copy number per cell during cell growth to maintain nearly constant gene copy number per unit cell volume (Fig. 7) and that multiple copies of chromosomes in bacteria act as backup genetic information to compensate for damage to the other chromosomal copies. In accord with the first notion, compared to monoploid organisms or polyploid organisms exhibiting simultaneous replication of chromosomes, in which gene number per unit cell volume changes up to 2-fold (53), stepwise replication of chromosomal copies in polyploids such as S. elongatus reduces changes in the gene number/cell volume ratio during cell growth and division. Thus, the stepwise replication of multiple chromosomal copies enables a cell to keep nearly constant chromosome number per unit cell volume, which coordinates chromosomal number with cell size (Fig. 7) and probably contributes to maintaining a constant mRNA and protein concentration during cell growth as observed in S. elongatus (18). Consistent with chromosome copies as backup genetic information, some known polyploid eukaryotes inhabit extreme environments (7, 12–16) that can damage DNA (48). In addition, cyanobacterial growth depends on sunlight, which contains UV, and photosynthesis generates ROS, and both UV radiation and ROS damage DNA (48, 54). In this study, S. elongatus cells of higher ploidy exhibited greater UV resistance. Thus, despite the higher metabolic cost of polyploidy than of monoploidy, possessing multiple copies of a chromosome that replicates asynchronously probably benefits the organism by compensating for a damaged copy.
MATERIALS AND METHODS
Strains and culture conditions.
Strains used in this study were Synechococcus elongatus PCC 7942 (WT) and several transformants (described below) as well as Synechocystis sp. PCC 6803 (GT-I strain) (55) and several transformants (described below) including a thymidine kinase (TK) strain (38) expressing thymidine kinase for the BrdU assay and a strain expressing DnaB-FLAG under the control of the dnaB promoter (44). All cyanobacterial strains were cultured at 30°C unless otherwise indicated in BG-11 liquid medium with air bubbling and illumination of 70 µE m−2 s−1 unless otherwise indicated. DCMU or DBMIB was added to the culture at a final concentration of 5 µM to inhibit photosynthetic electron flow.
ChIP-qPCR analyses.
Combined chromatin immunoprecipitation and qualitative PCR (ChIP-qPCR) analyses were performed according to the method of Hanaoka and Tanaka (56) with minor modifications. Cells were fixed with 1% formaldehyde for 15 min at room temperature. The cross-link reaction was stopped by the addition of glycine (final concentration of 125 mM) and incubation at room temperature for 5 min. Cells were harvested by centrifugation, washed twice with ice-cold Tris-buffered saline (TBS) (20 mM Tris-HCl, pH 7.4, 150 mM NaCl), and stored at −80°C. Fixed cells were broken using Beads Crusher (Taitec) with glass beads (<106 μm; Sigma Aldrich) in TBS at 4°C, and the genome was fragmented to approximately 500 bp using a Covaris sonication system (MS Instrument Inc.). After centrifugation for 15 min to precipitate the insoluble fraction, the supernatant containing genomic DNA was subjected to immunoprecipitation using an anti-HA antibody (clone 16B12; BioLegend), anti-SyfDnaA antibody (38), or anti-FLAG M2 antibody (F1804; Sigma) at a dilution of 1:250. Precipitated DNA was quantified by qPCR using primer sets oriC-F and oriC-R for the oriC region and 1294-F and 1294-R for the genomic region farthest from oriC in the circular genome. Primer sequences are listed in Text S1 in the supplemental material.
Supplemental methods. Contains Table S1 (primers used in this study). Download Text S1, DOCX file, 0.7 MB (767.8KB, docx) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Immunofluorescence microscopy.
Cells were fixed with 1% paraformaldehyde and 10% dimethyl sulfoxide dissolved in methanol at −30°C for 5 min. After washing twice with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.4), fixed cells were treated with 0.05% Triton X-100 in PBS for 15 min at room temperature. After washing twice with PBS, the fixed cells were further permeabilized with 0.2 mg ml−1 lysozyme (dissolved in 25 mM Tris-HCl, pH 7.5, 10 mM EDTA) at 37°C for 30 min. After two additional washes with PBS, genomic DNA was digested in situ with 4 M HCl at 37°C for 1 h. After washing twice with PBS, cells were blocked with Blocking One (Toyobo) at room temperature for 30 min and then were reacted with the anti-BrdU antibody (clone BMC9318; Roche) diluted in Blocking One (1:20) at 37°C overnight. After washing twice with PBS, cells were reacted with Alexa Fluor 488 goat anti-mouse antibody (Invitrogen) diluted in Blocking One (1:200). After two final washes with PBS, cells were observed by fluorescence microscopy.
DNA staining for flow cytometry and observation of nucleoids by fluorescence microscopy.
Cells were harvested by centrifugation, fixed with 1% glutaraldehyde for 10 min, and washed with PBS. Fixed cells were stained with SYBR Green I (Invitrogen) (final concentration was 1:1,000) to count chromosomal copy number by fluorescence microscopy or with 10 µM Sytox Green for flow cytometry analysis. After staining, cells were subjected to flow cytometry (BD Accuri C6) as previously described (37).
UV irradiation and evaluation of cell viability by spotting assay.
Mid-log-phase cultures of S. elongatus DnaAWT or DnaAR328H at 30°C were inoculated into fresh inorganic medium (BG-11) and cultured under 70-µE m−2 s−1 illumination at 20, 30, or 40°C. A portion of the culture was harvested 6 or 24 h after the inoculation and then adjusted to an OD750 of 0.2 for preparation of a serial dilution series (10−1 to 10−5) using fresh medium. The diluted cells were spotted on BG-11 agar plates and then irradiated with 0 to 600 J m−2 UV-C (254 nm) under a UV lamp (UVP; Upland). The plates were further incubated at 30°C under 70-µE m−2 s−1 illumination for 7 days, and cell survival rate was calculated by counting the colonies.
ACKNOWLEDGMENTS
We thank Masato Kanemaki, Toyoaki Natsume, Masayuki Su’etsugu, and Hiraku Takada for technical advice and members of the Miyagishima laboratory for their technical support.
This work was supported by grants-in-aid for Scientific Research from the Japan Society for the Promotion of Science (no. 16H07418 to R.O., no. 18H04827 to R.O., and no. 17H01446 to S.-Y.M.) and by NIG postdoctoral fellowship (to R.O.).
R.O. and S.-Y.M. designed the study; R.O. performed the experiments; A.N., T.S.H., S.W., T.C., and H.Y. contributed new reagents/analytic tools; R.O. and Y.K. performed sequencing analyses; R.O. and S.-Y.M. wrote the paper.
The authors declare no competing financial interests.
Footnotes
Citation Ohbayashi R, Nakamachi A, Hatakeyama TS, Watanabe S, Kanesaki Y, Chibazakura T, Yoshikawa H, Miyagishima S-Y. 2019. Coordination of polyploid chromosome replication with cell size and growth in a cyanobacterium. mBio 10:e00510-19. https://doi.org/10.1128/mBio.00510-19.
Contributor Information
Jorg Soppa, Goethe University.
Richard Losick, Harvard University.
REFERENCES
- 1.Marczynski GT, Shapiro L. 2002. Control of chromosome replication in Caulobacter crescentus. Annu Rev Microbiol 56:625–656. doi: 10.1146/annurev.micro.56.012302.161103. [DOI] [PubMed] [Google Scholar]
- 2.Quardokus EM, Brun YV. 2003. Cell cycle timing and developmental checkpoints in Caulobacter crescentus. Curr Opin Microbiol 6:541–549. doi: 10.1016/j.mib.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Griese M, Lange C, Soppa J. 2011. Ploidy in cyanobacteria. FEMS Microbiol Lett 323:124–131. doi: 10.1111/j.1574-6968.2011.02368.x. [DOI] [PubMed] [Google Scholar]
- 4.Sargent EC, Hitchcock A, Johansson SA, Langlois R, Moore CM, LaRoche J, Poulton AJ, Bibby TS. 2016. Evidence for polyploidy in the globally important diazotroph Trichodesmium. FEMS Microbiol Lett 363:fnw244. doi: 10.1093/femsle/fnw244. [DOI] [PubMed] [Google Scholar]
- 5.Hansen MT. 1978. Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. J Bacteriol 134:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minton KW. 1994. DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans. Mol Microbiol 13:9–15. doi: 10.1111/j.1365-2958.1994.tb00397.x. [DOI] [PubMed] [Google Scholar]
- 7.Ohtani N, Tomita M, Itaya M. 2010. An extreme thermophile, Thermus thermophilus, is a polyploid bacterium. J Bacteriol 192:5499–5505. doi: 10.1128/JB.00662-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komaki K, Ishikawa H. 1999. Intracellular bacterial symbionts of aphids possess many genomic copies per bacterium. J Mol Evol 48:717–722. doi: 10.1007/PL00006516. [DOI] [PubMed] [Google Scholar]
- 9.Tobiason DM, Seifert HS. 2006. The obligate human pathogen, Neisseria gonorrhoeae, is polyploid. PLoS Biol 4:e185. doi: 10.1371/journal.pbio.0040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitten T, Barbour AG. 1992. The relapsing fever agent Borrelia hermsii has multiple copies of its chromosome and linear plasmids. Genetics 132:311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bresler V, Montgomery WL, Fishelson L, Pollak PE. 1998. Gigantism in a bacterium, Epulopiscium fishelsoni, correlates with complex patterns in arrangement, quantity, and segregation of DNA. J Bacteriol 180:5601–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malandrin L, Huber H, Bernander R. 1999. Nucleoid structure and partition in Methanococcus jannaschii: an archaeon with multiple copies of the chromosome. Genetics 152:1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breuert S, Allers T, Spohn G, Soppa J. 2006. Regulated polyploidy in halophilic archaea. PLoS One 1:e92. doi: 10.1371/journal.pone.0000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildenbrand C, Stock T, Lange C, Rother M, Soppa J. 2011. Genome copy numbers and gene conversion in methanogenic archaea. J Bacteriol 193:734–743. doi: 10.1128/JB.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Miao D, Zhang F, Wu Z, Liu J, Xiang H. 2013. Characterization of the minimal replicon of pHM300 and independent copy number control of major and minor chromosomes of Haloferax mediterranei. FEMS Microbiol Lett 339:66–74. doi: 10.1111/1574-6968.12052. [DOI] [PubMed] [Google Scholar]
- 16.Spaans SK, van der Oost J, Kengen SW. 2015. The chromosome copy number of the hyperthermophilic archaeon Thermococcus kodakarensis KOD1. Extremophiles 19:741–750. doi: 10.1007/s00792-015-0750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendell JE, Clements KD, Choat JH, Angert ER. 2008. Extreme polyploidy in a large bacterium. Proc Natl Acad Sci U S A 105:6730–6734. doi: 10.1073/pnas.0707522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X-Y, O’Shea EK. 2017. Cyanobacteria maintain constant protein concentration despite genome copy-number variation. Cell Rep 19:497–504. doi: 10.1016/j.celrep.2017.03.067. [DOI] [PubMed] [Google Scholar]
- 19.Mundkur BD. 1953. Interphase nuclei and cell sizes in a polyploid series of Saccharomyces. Cell Mol Life Sci 9:373–374. doi: 10.1007/BF02167638. [DOI] [PubMed] [Google Scholar]
- 20.Melaragno JE, Mehrotra B, Coleman AW. 1993. Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5:1661–1668. doi: 10.1105/tpc.5.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugimoto-Shirasu K, Roberts K. 2003. “Big it up”: endoreduplication and cell-size control in plants. Curr Opin Plant Biol 6:544–553. doi: 10.1016/j.pbi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Wood E, Nurse P. 2015. Sizing up to divide: mitotic cell-size control in fission yeast. Annu Rev Cell Dev Biol 31:11–29. doi: 10.1146/annurev-cellbio-100814-125601. [DOI] [PubMed] [Google Scholar]
- 23.Katayama T, Ozaki S, Keyamura K, Fujimitsu K. 2010. Regulation of the replication cycle: conserved and diverse regulatory systems for DnaA and oriC. Nat Rev Microbiol 8:163–170. doi: 10.1038/nrmicro2314. [DOI] [PubMed] [Google Scholar]
- 24.Skarstad K, Katayama T. 2013. Regulating DNA replication in bacteria. Cold Spring Harb Perspect Biol 5:a012922. doi: 10.1101/cshperspect.a012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurokawa K, Nishida S, Emoto A, Sekimizu K, Katayama T. 1999. Replication cycle-coordinated change of the adenine nucleotide-bound forms of DnaA protein in Escherichia coli. EMBO J 18:6642–6652. doi: 10.1093/emboj/18.23.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donachie WD, Blakely GW. 2003. Coupling the initiation of chromosome replication to cell size in Escherichia coli. Curr Opin Microbiol 6:146–150. doi: 10.1016/S1369-5274(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 27.Jonas K, Chen YE, Laub MT. 2011. Modularity of the bacterial cell cycle enables independent spatial and temporal control of DNA replication. Curr Biol 21:1092–1101. doi: 10.1016/j.cub.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Fernandez C, Gonzalez D, Collier J. 2011. Regulation of the activity of the dual-function DnaA protein in Caulobacter crescentus. PLoS One 6:e26028. doi: 10.1371/journal.pone.0026028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonas K, Liu J, Chien P, Laub MT. 2013. Proteotoxic stress induces a cell-cycle arrest by stimulating Lon to degrade the replication initiator DnaA. Cell 154:623–636. doi: 10.1016/j.cell.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leslie DJ, Heinen C, Schramm FD, Thüring M, Aakre CD, Murray SM, Laub MT, Jonas K. 2015. Nutritional control of DNA replication initiation through the proteolysis and regulated translation of DnaA. PLoS Genet 11:e1005342. doi: 10.1371/journal.pgen.1005342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonas K. 2014. To divide or not to divide: control of the bacterial cell cycle by environmental cues. Curr Opin Microbiol 18:54–60. doi: 10.1016/j.mib.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Simon RD. 1977. Macromolecular composition of spores from the filamentous cyanobacterium Anabaena cylindrica. J Bacteriol 129:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binder BJ, Chisholm SW. 1990. Relationship between DNA cycle and growth rate in Synechococcus sp. strain PCC 6301. J Bacteriol 172:2313–2319. doi: 10.1128/jb.172.5.2313-2319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Binder BJ, Chisholm SW. 1995. Cell cycle regulation in marine Synechococcus sp. strains. Appl Environ Microbiol 61:708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu B, Yang G, Zhao W, Zhang Y, Zhao J. 2007. MreB is important for cell shape but not for chromosome segregation of the filamentous cyanobacterium Anabaena sp. PCC 7120. Mol Microbiol 63:1640–1652. doi: 10.1111/j.1365-2958.2007.05618.x. [DOI] [PubMed] [Google Scholar]
- 36.Zerulla K, Ludt K, Soppa J. 2016. The ploidy level of Synechocystis sp. PCC 6803 is highly variable and is influenced by growth phase and by chemical and physical external parameters. Microbiology 162:730–739. doi: 10.1099/mic.0.000264. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe S, Ohbayashi R, Shiwa Y, Noda A, Kanesaki Y, Chibazakura T, Yoshikawa H. 2012. Light-dependent and asynchronous replication of cyanobacterial multi-copy chromosomes. Mol Microbiol 83:856–865. doi: 10.1111/j.1365-2958.2012.07971.x. [DOI] [PubMed] [Google Scholar]
- 38.Ohbayashi R, Watanabe S, Ehira S, Kanesaki Y, Chibazakura T, Yoshikawa H. 2016. Diversification of DnaA dependency for DNA replication in cyanobacterial evolution. ISME J 10:1113–1121. doi: 10.1038/ismej.2015.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain IH, Vijayan V, O’Shea EK. 2012. Spatial ordering of chromosomes enhances the fidelity of chromosome partitioning in cyanobacteria. Proc Natl Acad Sci U S A 109:13638–13643. doi: 10.1073/pnas.1211144109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen AH, Afonso B, Silver PA, Savage DF. 2012. Spatial and temporal organization of chromosome duplication and segregation in the cyanobacterium Synechococcus elongatus PCC 7942. PLoS One 7:e47837. doi: 10.1371/journal.pone.0047837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shih PM, Wu D, Latifi A, Axen SD, Fewer DP, Talla E, Calteau A, Cai F, Tandeau de Marsac N, Rippka R, Herdman M, Sivonen K, Coursin T, Laurent T, Goodwin L, Nolan M, Davenport KW, Han CS, Rubin EM, Eisen JA, Woyke T, Gugger M, Kerfeld CA. 2013. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc Natl Acad Sci U S A 110:1053–1058. doi: 10.1073/pnas.1217107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Alda JAO, Esteban R, Diago ML, Houmard J. 2014. The plastid ancestor originated among one of the major cyanobacterial lineages. Nat Commun 5:4937. doi: 10.1038/ncomms5937. [DOI] [PubMed] [Google Scholar]
- 43.Mangiameli SM, Veit BT, Merrikh H, Wiggins PA. 2017. The replisomes remain spatially proximal throughout the cell cycle in bacteria. PLoS Genet 13:e1006582. doi: 10.1371/journal.pgen.1006582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohbayashi R, Watanabe S, Kanesaki Y, Narikawa R, Chibazakura T, Ikeuchi M, Yoshikawa H. 2013. DNA replication depends on photosynthetic electron transport in cyanobacteria. FEMS Microbiol Lett 344:138–144. doi: 10.1111/1574-6968.12166. [DOI] [PubMed] [Google Scholar]
- 45.Scholefield G, Errington J, Murray H. 2012. Soj/ParA stalls DNA replication by inhibiting helix formation of the initiator protein DnaA. EMBO J 31:1542–1555. doi: 10.1038/emboj.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su'etsugu M, Kawakami H, Kurokawa K, Kubota T, Takata M, Katayama T. 2001. DNA replication-coupled inactivation of DnaA protein in vitro: a role for DnaA arginine-334 of the AAA+ Box VIII motif in ATP hydrolysis. Mol Microbiol 40:376–386. doi: 10.1046/j.1365-2958.2001.02378.x. [DOI] [PubMed] [Google Scholar]
- 47.Roth A, Messer W. 1995. The DNA binding domain of the initiator protein DnaA. EMBO J 14:2106. doi: 10.1002/j.1460-2075.1995.tb07202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiseman H, Halliwell B. 1996. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 313:17. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF. 2009. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res 37:2539–2548. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu P, Demple B. 2010. DNA repair in mammalian mitochondria: much more than we thought? Environ Mol Mutagen 51:417–426. doi: 10.1002/em.20576. [DOI] [PubMed] [Google Scholar]
- 51.Wallden M, Fange D, Lundius EG, Baltekin Ö, Elf J. 2016. The synchronization of replication and division cycles in individual E. coli cells. Cell 166:729–739. doi: 10.1016/j.cell.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 52.Si F, Li D, Cox SE, Sauls JT, Azizi O, Sou C, Schwartz AB, Erickstad MJ, Jun Y, Li X, Jun S. 2017. Invariance of initiation mass and predictability of cell size in Escherichia coli. Curr Biol 27:1278–1287. doi: 10.1016/j.cub.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paijmans J, Bosman M, Ten Wolde PR, Lubensky DK. 2016. Discrete gene replication events drive coupling between the cell cycle and circadian clocks. Proc Natl Acad Sci U S A 113:4063–4068. doi: 10.1073/pnas.1507291113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castenholz RW, Garcia-Pichel F. 2012. Cyanobacterial responses to UV radiation, p 481–499. In Whitton BA. (ed), Ecology of cyanobacteria II. Springer, Dordrecht. [Google Scholar]
- 55.Kanesaki Y, Shiwa Y, Tajima N, Suzuki M, Watanabe S, Sato N, Ikeuchi M, Yoshikawa H. 2012. Identification of substrain-specific mutations by massively parallel whole-genome resequencing of Synechocystis sp. PCC 6803. DNA Res 19:67–79. doi: 10.1093/dnares/dsr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanaoka M, Tanaka K. 2008. Dynamics of RpaB–promoter interaction during high light stress, revealed by chromatin immunoprecipitation (ChIP) analysis in Synechococcus elongatus PCC 7942. Plant J 56:327–335. doi: 10.1111/j.1365-313X.2008.03600.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preparation of an S. elongatus SSB-GFP expresser. (A) The gfp ORF was fused with the ssb gene just before the stop codon. The construct was integrated into the chromosomal ssb locus by homologous recombination, and the gentamicin resistance gene (Gmr) was used as the selection marker for the transformant. (B) Insertion of the gfp and Gmr genes into the chromosomal ssb locus of DnaAWT and DnaAR328H cells was confirmed by PCR using the primers indicated in panel A by arrows. The wild-type cell was used as a negative control. (C) Immunoblot analyses showing the expression of GFP-SSB in HA-DnaAWT and HA-DnaAR328H cells. HA-DnaAWT and HA-DnaAR328H cells without GFP-SSB were used as negative controls. Total proteins extracted from respective strains were subjected to analyses. GFP-SSB was detected with the anti-GFP antibody, and HA-DnaA was detected with the anti-HA antibody as a loading control. Download FIG S1, EPS file, 0.6 MB (667.6KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Preparation of a Synechocystis SSB-GFP expresser. (A) The gfp ORF was fused with the ssb gene just before the stop codon. The construct was integrated into the Synechocystis chromosomal ssb locus by homologous recombination. The gentamicin resistance gene (Gmr) was used as the selection marker for the transformant. (B) Insertion of the gfp and Gmr genes into the chromosomal ssb locus was confirmed by PCR using the primers indicated in panel A by arrows. The wild-type cell was used as a negative control. (C) Immunoblot analyses showing the expression of GFP-SSB in the transformant. The wild-type cell was used as a negative control. Total proteins extracted from the wild type and the transformant were subjected to analysis. GFP-SSB was detected with the anti-GFP antibody. As a loading control, Coomassie brilliant blue (CBB) staining of the protein samples resolved by SDS-PAGE is also shown. Download FIG S2, EPS file, 1.3 MB (1.3MB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Preparation of an S. elongatus RNA polymerase-GFP expresser. (A) The gfp ORF was fused with the rpoC2 gene just before the stop codon. The construct was integrated into the chromosomal rpoC2 locus by homologous recombination. The gentamicin resistance gene (Gmr) was used as a selection marker for the transformant. (B) Insertion of the gfp and Gmr genes into the chromosomal rpoC2 locus was confirmed by PCR using the primers indicated in panel A by arrows. The wild-type cell was used as a negative control. (C) Immunoblot analyses showing the expression of RpoC2-GFP in the transformant. The wild-type cell was used as a negative control. Total proteins extracted from the wild-type and the RpoC2-GFP cells were subjected to analysis. RpoC2-GFP was detected with the anti-GFP antibody. As a loading control, Coomassie brilliant blue (CBB) staining of the protein samples resolved by SDS-PAGE is also shown. Download FIG S3, EPS file, 2.3 MB (2.3MB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Preparation of a Synechocystis RNA polymerase-GFP expresser. (A) The gfp ORF was fused with the rpoC2 gene just before the stop codon. The construct was integrated into the chromosomal rpoC2 locus by homologous recombination. The spectinomycin resistance gene (Specr) was used as the selection marker for the transformant. (B) Insertion of the gfp and Specr genes into the chromosomal rpoC2 locus was confirmed by PCR using the primers indicated in panel A by arrows. The wild-type cell was used as a negative control. (C) Immunoblot analyses showing the expression of RpoC2-GFP in the transformant. The wild-type cell was used as a negative control. Total proteins extracted from the wild-type and RpoC2-GFP cells were subjected to analysis. RpoC2-GFP was detected with the anti-GFP antibody. As a loading control, Coomassie brilliant blue (CBB) staining of the protein samples resolved by SDS-PAGE is also shown. Download FIG S4, EPS file, 0.5 MB (548.1KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of DnaA expression levels in S. elongatus that was cultured under different conditions. Immunoblot analyses showing temporal changes in DnaA and SSB-GFP protein levels in S. elongatus. Mid-log-phase cultures of SSB-GFP cells were inoculated into fresh inorganic medium (hour 0) and then cultured for 9 h under the indicated illumination intensities and temperatures. Total proteins extracted from the cells at the indicated time points were reacted with the anti-DnaA antibody (upper panel) and anti-GFP antibody (lower panel). Samples at hour 0 are identical in the all blots. In order to clearly visualize the temporal change in protein levels for the 30°C and 250-µE m−2 s−1 treatment group, an image acquired with a shorter exposure time than the others is shown. Download FIG S5, EPS file, 0.5 MB (560.9KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effect of photosynthetic inhibitors on affinity of DnaA and DnaB proteins to genomic oriC region. (A) Schematic diagram of the culturing condition for panels B to E. A saturated cell culture of the S. elongatus wild type or HA-DnaAR328H was inoculated into fresh inorganic medium and cultured with illumination (70 µE m−2 s−1) for 5 h. Then DCMU or DBMIB was added to the culture (hour 0), and cells were further cultured with illumination for 3 h. (B) Immunoblot analysis showing the DnaA protein level in a culture treated with DCMU or DBMIB under illumination. Total proteins extracted from cells at the indicated time points were reacted with the anti-DnaA antibody (upper panel). Coomassie brilliant blue (CBB) staining of the protein samples resolved by SDS-PAGE is shown in the lower panel as a loading control. (C and D) ChIP-qPCR analysis showing the effect of DCMU or DBMIB on the affinity of DnaA (C) and DnaB (D) for the oriC region. The DnaA- (or DnaB)-chromatin complex was immunoprecipitated with the anti-DnaA (or anti-FLAG) antibody. For the DnaB assay, a strain expressing DnaB-FLAG under the control of the dnaB promoter (44) was used. The samples were quantified by qPCR using the primers specific to the oriC region (oriC; gray bar) and the Syf1294 gene (1294; white bar), which is furthest from oriC in the circular chromosome. The percent recovery against the amount of total input DNA is indicated. (E) ChIP-qPCR analysis showing the effect of DCMU on the affinity of HA-DnaAR328H for the oriC region. The samples were subjected to ChIP-qPCR analysis with the anti-HA antibody. The value indicated is the ratio of percent recovery (oriC/1294). Download FIG S6, EPS file, 0.7 MB (748.5KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Revision of the start codon of S. elongatus dnaA gene. (A) Amino acid sequence (deduced from the annotated ORF sequences) alignment of the DnaA N-terminal regions of S. elongatus (syf), Synechococcus sp. strain UTEX2973 (syu), Cyanothece sp. strain PCC 7425 (cyn), Anabaena sp. (ana), Synechocystis (syn), and Bacillus subtilis 168 (bus). (B) DNA sequence of S. elongatus dnaA gene around the annotated start codon (CyanoBase, http://genome.kazusa.or.jp/cyanobase). Based on the protein expression analyses in panel C, the GTG codon 60 bp downstream of the annotated translational start codon (ATG) turned out to be the actual start codon of the dnaA gene in S. elongatus. (C) Three constructs (a to c) for expression from the chromosomal neutral site I (NS I). In construct a, the HA-tag-encoding sequence was fused with the annotated start codon (ATG) of dnaA and expressed by the IPTG-inducible trc promoter. In construct b, the HA-tag-encoding sequence was fused with the revised start codon (GTG) of dnaA and expressed by the 60-bp 5′ upstream sequence flanking the revised start codon (GTG). In construct c, the HA-tag-encoding sequence was fused with the revised start codon (GTG) of dnaA and expressed by the 300-bp 5′ upstream sequence flanking the revised start codon (GTG). After introduction of each HA-tagged dnaA, the endogenous dnaA gene was deleted in all strains. (D) Immunoblot analysis showing the size of HA-DnaA expressed in the respective strains. Mid-log-phase cultures were inoculated into fresh medium with (+) or without (−) 1 mM IPTG. Total proteins extracted from respective transformants (for construct a, with or without IPTG induction) were subjected to immunoblot analysis with the anti-DnaA antibody (upper panel) and the anti-HA antibody (lower panel). In construct a, HA-DnaA protein was expressed only by IPTG induction, while the smaller endogenous DnaA protein was expressed both with and without IPTG, suggesting that this construct possesses a promoter other than the trc promoter. In addition, in constructs b and c, HA-DnaA was expressed by the predicted dnaA promoter (upstream of GTG; US60 or US300). The HA-DnaA expressed by constructs b and c was smaller than that expressed by construct a. These results indicate that the actual start codon of the dnaA gene is a “GTG” 60 bp upstream of the “GTG” functioning as a promoter of the dnaA gene. Download FIG S7, EPS file, 0.7 MB (766.6KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
High-throughput sequencing of genomic DNA and chromosomal copy number in mid-log-phase cultures of DnaAWT and DnaAR328H. (A) Depth of genomic DNA read through the genome 6 h after inoculation in DnaAWT and DnaAR328H strains was analyzed by Illumina MiSeq. Relative sequencing coverage ratios of Illumina MiSeq reads (1-kb window for the left graphs and 100-kb window for the right graphs) at respective genomic regions in HA-DnaAWT and HA-DnaAR328H cells are shown. The ratio of read depth is shown in the graphs. The sequencing coverage of each region was normalized to the total read depth. (B) Flow cytometry analysis showing the DNA level and relative cell volume of DnaAWT (upper panel) and DnaAR328H (lower panel) cells 6 h after inoculation. Dots represent single cells. DNA level was determined by intensity of SYTOX Green fluorescence. Download FIG S8, EPS file, 0.7 MB (782.7KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Flow cytometry analysis of cell volume and DNA level of cells grown under different illumination intensities or temperatures. Flow cytometry analysis showing the DNA level and cell volume as in Fig. 4A (A) and Fig. 5E (B). Dots represent single cells. DNA level was determined by intensity of SYTOX Green fluorescence. Download FIG S9, EPS file, 0.5 MB (551KB, eps) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental methods. Contains Table S1 (primers used in this study). Download Text S1, DOCX file, 0.7 MB (767.8KB, docx) .
Copyright © 2019 Ohbayashi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.