Highlights
-
•
Evaluation of organ-motion based robust optimisation for breast VMAT.
-
•
Organ-motion based robust optimisation generates clinically acceptable plans.
-
•
Technique validated by recalculating robust plans on real patient CBCT data.
-
•
The technique generated plans that are robust to changes to the patient shape.
Keywords: Robust optimisation, VMAT, Breast cancer, Organ-motion, IMC
Abstract
Aims
In patients undergoing locoregional radiotherapy (RT) for breast cancer including the internal mammary chain (IMC), VMAT has been shown to be superior to tangential-field radiotherapy in terms of target coverage and minimising dose to heart and lungs. In this study we describe and validate organ motion-based robust optimisation for generating breast and locoregional lymph node VMAT plans that are robust to inter-fractional changes.
Materials and methods
In this retrospective study of five patients with left-sided breast cancer requiring locoregional breast radiotherapy including the IMC, non-robust plans were generated in the nominal scenario (planning-CT) and corresponding robust plans were created by optimising over a range of simulated CTs representing worst-case scenario shape changes to the breast. Both plans were re-calculated on CBCT images (n = 67) acquired prior to RT to generate estimates of delivered fractional dose. Plan robustness to inter-fractional changes was assessed in terms of the estimated target coverage and OAR dose.
Results
Organ motion-based robust optimisation was able to generate clinically acceptable treatment plans in the nominal scenario on the planning CT with no significant differences to OAR dose between the robust and non-robust planning techniques. All plans (robust and non-robust) achieved the mandatory target coverage requirements. Estimates of delivered dose demonstrated a significant improvement in breast target coverage for the robust plans compared to non-robust plans. For the breast CTV, 92% of the robust plans achieved the optimal D98% > 95% clinical goal as compared to 71% of the non-robust plans (p < 0.01). 94% of robust plans achieved acceptable superficial breast coverage, as compared to 55% for the non-robust technique.
Conclusions
Organ motion-based robust optimisation VMAT is able to produce clinically acceptable organ-at-risk sparing plans for locoregional breast radiotherapy (including the IMC) that are robust to inter-fractional changes, therefore reducing the likelihood of reactive adaptive re-planning.
1. Introduction
Inclusion of the internal mammary chain (IMC) in the target volume for locoregional breast radiotherapy has been proven to improve disease free and overall survival in recently published clinical trials [1], [2], [3].The Royal College of Radiologists, UK has published guidance stating that IMC radiotherapy (IMC-RT) should be considered for women with higher risk breast cancer [4]. However, inclusion of the IMC for locoregional breast radiotherapy increases dose to the heart [5], [6], [7]. A linear no-threshold relationship between mean heart dose and major coronary events has previously been demonstrated [8]. In order to maximise the survival benefits of IMC-RT, cardiac-sparing techniques are recommended [4].
The HeartSpare-Plus dosimetry study demonstrated that the use of voluntary deep inspiratory breath hold (vDIBH) is capable of reducing mean heart dose to below 4 Gy in the context of IMC-RT. However, in order to deliver higher doses to locoregional lymph nodes (especially the IMC) volumetric modulated arc therapy (VMAT) is superior when compared to tangential treatments [9].
VMAT approaches for breast cancer have previously been developed and used in dosimetry studies and clinical trials [9], [10], [11], [12], [13]. A challenging aspect of delivering VMAT for breast cancer is the lack of robustness of the treatment plans in terms of inter- and intra-fraction motion, particularly when significant breast swelling occurs during the treatment course [14]. Tangent-based RT plans mitigate this issue by incorporating skin flash in the field apertures to ensure irradiation of all breast tissue should the target increase in size from the time of the planning CT. However, for inverse-planned approaches such as VMAT, the incorporation of adequate skin flash is difficult to achieve.
Attempts have been made to address the skin flash problem for inverse planning for breast RT. The most common approach is to add virtual bolus (VB) in the region of the PTV outside of, or close to, the external contour during optimisation [15] with final dose calculation performed with the VB removed [14]. This approach has been shown to generate VMAT plans with skin flash adequate to achieve acceptable superficial dose coverage [14] with dosimetric advantages over VMAT plans generated without VB in terms of estimated delivered dose to the target [16]. However, plan degradation is inevitable upon removal of the VB for final dose calculation. The further the target is expanded outside of the body in the virtual plan, the larger the plan degradation, meaning it is difficult to achieve acceptable VMAT plans with equivalent skin flash compared to a tangent plan. Therefore, VMAT plans generated using VB may not be sufficient for patients who demonstrate significant swelling during their treatment course and reactive re-planning may still be necessary.
Recently, robust-optimisation tools have become available within commercial RT treatment planning systems (TPSs) enabling plans to be optimised concurrently in different geometrical scenarios by including range and setup uncertainties [17], [18], [19]. Although these techniques were originally developed for proton beam therapy [19], [20], they are now being applied for photon-based RT. Jensen showed that such methods can be applied to breast VMAT plans and that these plans were more robust than tangent 3DCRT plans to setup errors [17]. However, such methods do not account for changes in patient shape during their radiotherapy course.
Alongside range and setup uncertainties, additional robust-optimisation strategies have recently become available within commercial TPSs including organ-motion (OM) robust-optimisation which optimises the plan in the nominal (planning CT) scenario and over a number of simulated CTs that have been generated by a Deformable Image Registration (DIR) based on user-defined organ motion.
In this study we aimed to validate, for the first time, OM-based robust-optimisation available within a commercial TPS for generating breast VMAT plans that include the internal mammary chain and axillary nodes. Unlike previous studies that simulated robust evaluation using perturbed dose recalculations [17], [21], we validated the OM robust optimisation method by recalculating the treatment plans on cone beam CT (CBCT) data for a selection of patients previously treated for breast cancer.
2. Methods
2.1. Patient selection
Five consecutive patients with left-sided breast cancer requiring locoregional breast radiotherapy including the IMC (40.05 Gy in 15 fractions) who had previously been treated within a single-centre study and provided written informed consent were selected for this investigation. These patients were treated prior to the development of the OM robust optimisation described in this manuscript. All patients had three CTVs (whole breast, IMC, and axillary levels 2–4) and OARs (heart, left lung, right lung, and right breast) delineated by experienced clinical oncologists based on ESTRO guidelines [22]. Planning target volumes were generated from corresponding CTVs using 5 mm isotropic margins. All target volumes were clipped 5 mm from the surface. All patients were treated using vDIBH and had daily-online CBCT positional matching. Table 1 details information about the patients used in this study.
Table 1.
Patient characteristics. Case 5 only had 7 CBCTs as they had to be re-planned during their treatment course due to the observed changes in external contour.
| Case number | Axillary levels treated | Number of CBCTs for analysis | Patient specific comments |
|---|---|---|---|
| 1 | 2–4 | 15 | Large breast target volume and exhibited shape changes throughout RT course |
| 2 | 2–4 | 15 | Exhibited changes in shape (external contour increases) especially towards the end of RT course |
| 3 | 2–4 | 15 | Small changes to external shape observed towards the end of RT course |
| 4 | 2–4 | 15 | Good set up with no change to external shape throughout RT course |
| 5 | 2–4 | 7 | Exhibited large changes in shape (Fig. 1) during RT course and was re-planned due to the differences. |
CBCT, Cone beam CT.
2.2. Organ Motion-based robust VMAT planning technique
The organ-motion based robust optimisation VMAT technique consisted of three steps:
-
1.
Non-robust plans were generated in the nominal scenario on the planning CT.
-
2.
Simulated CTs were generated in order to represent worst-case swelling scenarios using OM features within a commercial TPS.
-
3.
Robust plans were generated.
All work was carried out using the RayStation v7 TPS (RayStation v7, RaySearch laboratories, Stockholm, Sweden).
2.2.1. Non-robust optimisation
The non-robust VMAT treatment plans were generated on the planning CT. Class-solutions for the beams and optimisation objectives aimed to achieve the clinical goals taken from a previously published dosimetric study [9] and listed in Table 2. Two partial 6MV arcs were used with start/stop angles of 179°/310°, collimator angles of 30°/330°, and maximum delivery times of 45 s per beam to enable treatment to be delivered in an acceptable number of breath-holds. Plans were optimised to PTVs that were clipped 5 mm from the external contour to avoid complex modulations that can arise due to lack of build-up and lateral scatter in the superficial region. The beam parameters and optimisation objectives were then modified to ensure clinical goal compliance for each patient, for example beams were angled to avoid entering through the contralateral breast.
Table 2.
Clinical goals for target structures and OARs alongside average results from all five cases for the non-robust and robust plans in the nominal (planning CT) geometry. For the PTVs, the D95% and D98% were mandatory and optimal, respectively. All OAR constraints were mandatory. All plans were normalised such that the Breast PTV achieved a D50% of prescription dose meaning a statistical test for this metric is not recorded.
| ROI | Statistic | Clinical goal | Nominal (planning CT) scenario for all five cases |
p-values | |||
|---|---|---|---|---|---|---|---|
| robust |
Non-robust |
||||||
| median | IQ range | median | IQ range | ||||
| Breast PTV | D98 (Gy) | >38.05 | 37.9 | 37.7–38.0 | 38.1 | 37.8–38.2 | 0.13 |
| D95 (Gy) | >38.05 | 38.6 | 38.5–38.6 | 38.7 | 38.4–38.8 | 0.47 | |
| D50 (Gy) | 40.05 ± 0.80 | 40.1 | 40.1–40.1 | 40.1 | 40.1–40.1 | n/a | |
| D2 (Gy) | <42.85 | 41.6 | 41.3–41.6 | 41.2 | 41.2–41.2 | 0.04 | |
| Axillary PTV | D98 (Gy) | >38.05 | 38.4 | 38.4–38.5 | 38.6 | 38.5–38.8 | 0.04 |
| D95 (Gy) | >38.05 | 38.7 | 38.7–38.9 | 38.9 | 38.8–39.1 | 0.04 | |
| D50 (Gy) | 40.05 ± 0.80 | 39.6 | 39.6–39.7 | 39.8 | 39.8–39.8 | 0.08 | |
| D2 (Gy) | <42.85 | 40.6 | 40.4–40.7 | 40.8 | 40.7–40.8 | 0.22 | |
| IMC PTV | D98 (Gy) | >38.05 | 37.6 | 37.2–38.0 | 38.5 | 37.9–38.8 | 0.04 |
| D95 (Gy) | >38.05 | 38.3 | 38.1–38.6 | 38.9 | 38.7–39.1 | 0.04 | |
| D50 (Gy) | 40.05 ± 0.80 | 39.8 | 39.8–40.0 | 40.1 | 40.0–40.2 | 0.04 | |
| D2 (Gy) | <42.85 | 40.8 | 40.8–40.9 | 41.0 | 41.0–41.0 | 0.04 | |
| Heart | mean (Gy) | <6.00 | 3.8 | 3.6–5.5 | 3.9 | 3.7–5.5 | 0.68 |
| Left Lung | V17Gy (%) | <35 | 33.1 | 32.4–34.2 | 33.4 | 32.5–33.8 | 0.71 |
| mean (Gy) | <15 | 13.6 | 13.6–13.8 | 13.6 | 13.5–13.9 | 0.72 | |
| Right Lung | mean (Gy) | <3.5 | 3.3 | 3.2–3.3 | 3.3 | 3.2–3.3 | 0.32 |
| Right Breast | mean (Gy) | <3.5 | 3.3 | 3.3–3.3 | 3.3 | 3.3–3.4 | 0.65 |
IQ = inter-quartile. PTV = Planning Target Volume.
2.2.2. Generation of simulated CTs
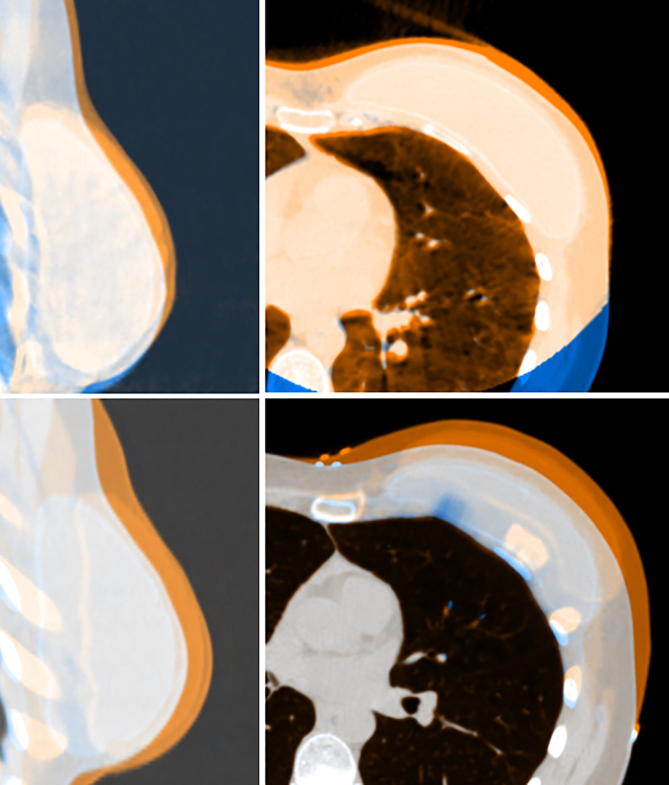
The organ-motion tools within the TPS were used to generate deformed CTs. The planning CT was chosen as the source image and the breast CTV as the motion ROI with 1.5 cm anterior and left organ-motion uncertainty. The left lung was chosen as a fixed ROI as the CBCT match was to the chest wall. The TPS generated three simulated CTs: one with 1.5 cm left motion of the breast CTV; one with 1.5 cm anterior motion of the breast CTV; and one with a combination of anterior and left motion of the breast CTV. The ROIs associated with the planning CT were mapped to each simulated CT according to the simulated motion. These parameters were chosen to represent worst-case scenario breast swelling that we have observed in our clinic (Fig. 1).
Fig. 1.
(top) example of CBCT image match for patient 5 and (bottom) corresponding fusion between the planning CT (blue) and one of the OM simulated CTs orange. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2.3. Robust optimisation
Within a copy of the non-robust plan with its patient-specific refined beam parameters and optimisation objectives, all minimum and maximum optimisation objectives for PTVs were set to optimise both in the nominal scenario (planning CT) and the simulated scenarios based on OM. The TPS used a minmax optimisation approach where the plan was optimised simultaneously in all four geometrical scenarios (nominal planning CT and three simulated CTs) and the worst objective value from these geometries was used in the objective function [23]. Robust optimisation consisted of three optimisation runs each of 60 iterations.
2.3. Technique validation
In order to validate the proposed methodology, the non-robust and robust plans were re-calculated on the patient CBCTs (Elekta XVI, Elekta, Stockholm, Sweden) which represented real-world setup uncertainty, changes in external shape, and examples of significant breast swelling during the RT course (Table 1). CBCT images were obtained in two breath holds of 20 s each. Half-scans were performed with a gantry angle of 295–120° (100 kV, 200 mAs, 9 mGy). 67 CBCT images across five cases were used for analysis.
CBCT images were imported into the TPS in treated geometry including all daily setup corrections applied prior to treatment. CBCT dose calculation was facilitated using previously validated [24] tools available within the TPS and a density of 0.95 g/cm3 was applied to missing tissue outside the field-of-view (FOV) of the CBCT. DIR (ANACONDA hybrid DIR, RayStation v7) was performed between every CBCT and corresponding planning CT for each case using the CBCT FOV and the external ROI limited by the FOV used as controlling ROIs. The DIRs were used to map CTVs and OARs from the planning CT to every CBCT. For each CBCT, a superficial breast CTV ROI was generated which consisted of the breast CTV within 1 cm of the external contour. Each fractional dose recalculation was scaled to 15 fractions in order to assess clinical goal compliance. Dose-volume metrics for the mapped ROIs were compared between the non-robust and robust plans for all 67 CBCT dose recalculations which represented estimates of delivered fractional dose for both planning techniques.
2.4. Statistical analysis
The Wilcoxon matched-pairs rank sum test was used to test for statistical significance between robust and non-robust plans for all dose metrics with a significance level of 0.05. Comparisons of PTV (clipped 5 mm from the surface) metrics were analysed for the nominal plan (planning CT) whereas CTV metrics were assessed for the CBCT tests. All statistical analysis was performed using Python v2.7.6.
3. Results
3.1. Organ motion-based robust VMAT planning technique
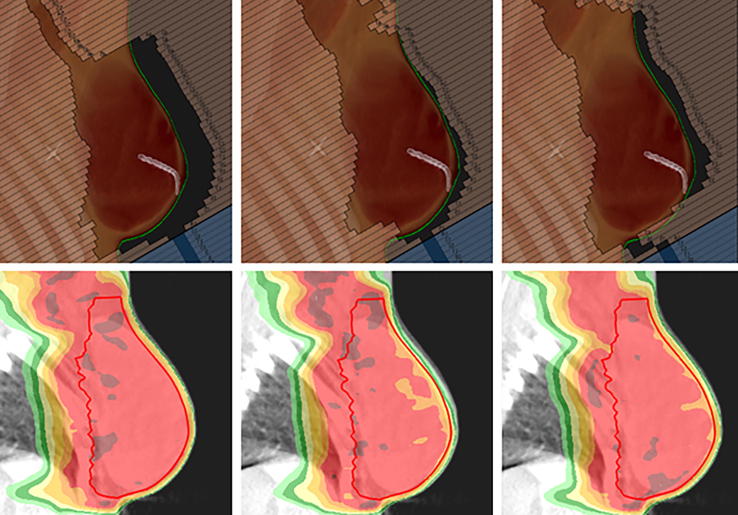
The proposed method was able to generate clinically acceptable treatment plans with all robust and non-robust plans satisfying all mandatory clinical goals (Table 2) apart from case 1 where a mean heart dose of 7.1 Gy was accepted for both the non-robust and robust plans due to the target size and location adjacent to the heart. Fig. 2 illustrates the skin flash that was achieved using the robust optimisation method.
Fig. 2.
Top row displays beams-eve-view (BEV) for one of the VMAT segments (case 5) for (left) a OM robustly optimised plan; (centre) a non-robust plan; and (right) a plan that was optimised using a VB technique where the VB extended 1 cm outside of the patient external ROI. The external ROI is shown as a green contour. Bottom row displays corresponding dose recalculations on the CBCT acquired for fraction 4 of the patient’s treatment. The breast CTV is shown as a red contour and the red colourwash represents 95% of the prescription dose (38.05 Gy). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2 shows that there were no significant differences to OAR dose between the two planning techniques. Statistical differences between the planning techniques were observed for all three PTVs but all mandatory clinical goals were achieved for all plans and only 3% (2 out of 60) PTV clinical goals differed by more than 2% between the robust and non-robust plans.
3.2. Technique validation
3.2.1. CBCT dose calculation
A previously validated method was used to assign density to the CBCT images [24]. In addition, the same method of bulk-density assignment was applied to the planning CT for all five cases and the mean dose to the heart and left lung and the median dose (D50%) to the three CTVs for the bulk-density calculation were compared to the look-up-table (LUT) calculation. For all dose statistics across all five cases the median difference between the bulk density and LUT calculations was −0.2 Gy (−0.6%) with a range of −0.7 Gy (−2.2%) to 0.1 Gy (0.4%). Due to the global assignment of 0.95 g/cm3 outside of the CBCT FOV, delivered dose statistics to the right lung were not considered for analysis.
3.2.2. Deformable image registration approval
All ROIs that were mapped from the planning CT to each CBCT according to the corresponding DIR were approved by a trained clinical oncologist. No manual edits to the mapped ROIs were made. The ROIs that were mapped and used for delivered dose comparison were the heart, left lung, right breast, and the three CTVs (breast, axillary, and IMC).
3.2.3. CBCT dose comparison
Table 3 details the results for the CBCT dose calculations of the non-robust and robust plans. No statistical difference was observed between the non-robust and robust plans for the OARs, apart from the right breast dose where only one fifth of right breast doses differed by more than 2% between the non-robust and robust calculations and none of the observed differences were clinically significant. If case 1, who exhibited a mean heart dose of 7.1 Gy on the nominal plans is excluded from analysis, the proportion of CBCT calculations achieving mean heart dose <6 Gy increased to 87% and 81% for the robust and non-robust plans, respectively.
Table 3.
Clinical goals for target structures and OARs alongside average results from all 67 CBCT dose calculations for the non-robust and robust plans. 93% of prescription (37.25 Gy) was used for the CBCT target coverage constraints. The number of calculation pairs (robust vs non-robust CBCT calculations) that differ by 2% is reported along with the proportion of robust and non-robust calculations achieving the clinical goal.
| ROI | Statistic | Clinical goal | Robust |
Non-robust |
p-values | % of pairs above 2% difference | % of plans CBCT dose calculations meeting clinical goal |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| median | IQ range | median | IQ range | Robust | Non-robust | |||||
| Breast CTV | D98 (Gy) | >37.25 | 38.5 | 38.0–38.6 | 37.9 | 37.3–38.4 | <0.01 | 24 | 97 | 76 |
| D95 (Gy) | >37.25 | 38.8 | 38.4–39.1 | 38.6 | 38.0–38.9 | <0.01 | 17 | 100 | 91 | |
| D50 (Gy) | 40 ± 0.8 | 40.2 | 39.8–40.3 | 40.1 | 39.8–40.2 | <0.01 | 0 | 100 | 100 | |
| D2 (Gy) | <42.85 | 41.7 | 41.5–41.9 | 41.4 | 41.2–41.6 | <0.01 | 0 | 100 | 100 | |
| Axillary CTV | D95 (Gy) | >37.25 | 39.7 | 39.0–39.9 | 39.8 | 39.2–40.0 | <0.01 | 0 | 100 | 100 |
| D50 (Gy) | 40 ± 0.8 | 40.4 | 40.0–40.7 | 40.6 | 40.3–40.9 | <0.01 | 0 | 74 | 65 | |
| D2 (Gy) | <42.85 | 41.4 | 41.1–41.8 | 41.9 | 41.2–42.1 | <0.01 | 3 | 98 | 100 | |
| IMC CTV | D95 (Gy) | >37.25 | 38.3 | 37.8–39.2 | 39.4 | 38.1–39.7 | <0.01 | 26 | 80 | 82 |
| D50 (Gy) | 40 ± 0.8 | 40.1 | 39.6–40.6 | 40.7 | 40.0–41.0 | <0.01 | 17 | 76 | 45 | |
| D2 (Gy) | <42.85 | 41.5 | 41.1–42.2 | 41.9 | 41.2–42.3 | 0.30 | 15 | 85 | 92 | |
| Superficial Breast CTV | D95 (Gy) | >37.25 | 38.5 | 37.9–38.8 | 37.3 | 36.2–38.0 | <0.01 | 70 | 94 | 55 |
| Heart | mean (Gy) | <6 | 3.9 | 3.4–6.0 | 4.0 | 3.5–5.8 | 0.23 | 30 | 74 | 82 |
| Left Lung | V17Gy (%) | <35 | 30.5 | 29.5–32.2 | 30.8 | 29.8–32.0 | 0.72 | 48 | 97 | 97 |
| mean (Gy) | <15 | 13.1 | 12.8–13.8 | 13.2 | 12.8–13.5 | 0.32 | 55 | 100 | 100 | |
| Right Breast | mean (Gy) | <3.5 | 3.3 | 3.2–3.3 | 3.3 | 3.2–3.4 | <0.01 | 21 | 91 | 86 |
CBCT = Cone beam CT. IQ = inter-quartile. CTV = Clinical Target Volume.
p < 0.05 shown in bold.
Despite the statistical differences observed, both planning techniques achieved similar plan quality in terms of mandatory daily coverage (D95 > 93% of prescription dose) for all three CTVs. For the breast CTV, 92% of the robust plans achieved the optimal D98% > 38.05 Gy clinical goal, this lowered to 71% of the non-robust plans indicating improved breast CTV coverage for the robust plans. 100% and 91% of CBCT dose calculations achieved D95% > 38.05 Gy for the breast CTV for robust and non-robust plans, respectively.
There was a statistically significant difference between the non-robust and robust plans in terms of coverage dose metrics to the superficial breast CTV with only 55% of CBCT dose calculations for the non-robust plans achieving the mandatory D95% > 37.25 Gy, this increased to 94% of the robust plans and in 70% of CBCT calculations there was at least 2% difference between the D95% coverage metric between the two techniques.
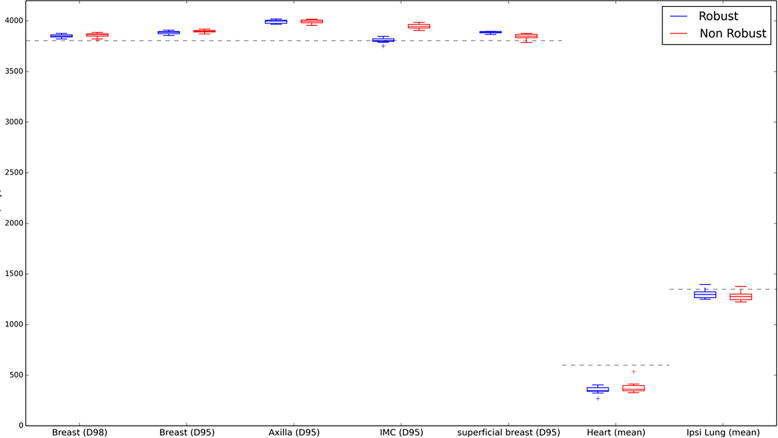
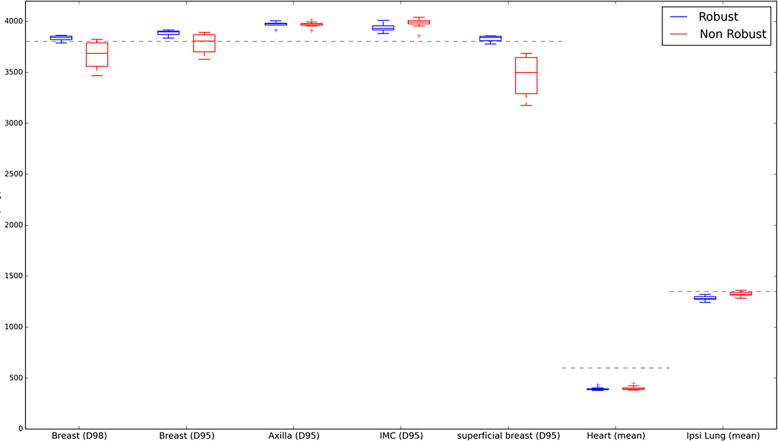
Fig. 3, Fig. 4 compare box-plots of robust and non-robust plan dose recalculations for all CBCT images for cases 4 and 2, respectively. Case 4 exhibited good set up and a stable external contour shape throughout the RT course whereas case 2 had a variable shape with large increases in external contour especially evident towards the end of the RT course. As displayed in Fig. 3, Fig. 4, the robust plans remained clinically acceptable for both cases across all fractions. Fig. 4 shows that for case 2 the increase in external contour resulted in compromised target coverage to the breast CTV for the non-robust plan and this was especially evident in the superficial breast region. This can also be observed in Fig. 2 (bottom row) where for case 5 the robust plan provided adequate target coverage but the non-robust plan did not.
Fig. 3.
Box-plot for all robust and non-robust CBCT recalculations for case 4. The grey dotted line represents optimal target coverage and mandatory OAR clinical goals.
Fig. 4.
Box-plot for all robust and non-robust CBCT recalculations for case 2. The grey dotted line represents optimal target coverage and mandatory OAR clinical goals.
4. Discussion
A methodology to generate VMAT plans using OM-based robust optimisation available within a commercial TPS was developed and validated by recalculating the treatment plans on CBCT data for a selection of patients. The OM-based technique was able to generate clinically acceptable plans in the nominal scenario and was validated to be robust to changes to the patient external ROI during their treatment course.
The results demonstrate that it is important to review the superficial breast target coverage as well as the whole breast when assessing the recalculated dose on the CBCTs. This is because the large volume of the breast target can mask poor superficial coverage. Although the non-robust plan generated clinically acceptable delivered doses for cases where the external ROI remained stable throughout the treatment course (Fig. 3), a significant improvement in whole breast coverage, especially in superficial regions, was observed for cases who exhibited an increase in the external ROI (Fig. 2, Fig. 4). Case 5 (Fig. 2) had to be re-planned during their treatment course due to the large increases in external contour. However, if the OM-based robust optimisation technique had been used for this patient a re-plan would not have been necessary as the OM-based robust plan would have satisfied the target coverage requirements.
As well as generating robust and non-robust plans, an additional robust plan was also created for case 5 using the VB method; the VB ROI was defined as an isotropic 1 cm expansion of all PTVs limited to outside of the external ROI. A density of 1 g/cm3 was applied to the VB ROI during optimisation and was removed for final dose calculation. Fig. 2 displays that although this method is able to generate some skin flash, it was not sufficient to adequately cover the breast target in situations of large increases to the external ROI (Fig. 2, bottom right). Furthermore, the implementation of a VB approach can lead to reduced dose to the axillary target. As the VB is grown only in regions where the target is close to the surface and are subject to external contour changes, the VB ROI does not typically extend superiorly to the axillary levels. Therefore, upon removal of the VB, re-prescribing the plan to achieve a D50% breast target dose of 40.05 Gy will result in the lowering of the median dose to the axillary PTV below the prescribed value.
The proposed methodology for generating robust breast VMAT plans using OM relies on a non-robust plan being generated first. This step is important as it enables the planner to quickly generate a patient-specific optimisation problem. After the non-robust plan has been generated, due to the multiple optimisation scenarios, the robust optimisation takes much longer to complete. The length of time needed to perform OM-based robust optimisation is a clear limitation of this technique. Table 2 shows that by copying the non-robust plans and optimising robustly without any further interventions we were able to generate plans of similar quality, in terms of mandatory clinical goal compliance, to the non-robust distributions in the nominal scenario (planning CT) meaning the robust-optimisation step could be automated via scripting within the TPS. Furthermore, the robust optimisation results in plans that were more robust to increases in the external ROI, meaning that undertaking reactive adaptive re-planning (including re-scanning, contouring, planning, and checking) is less likely.
In order to validate the OM-based robust optimisation technique, CBCT dose calculations have relied on bulk-density assignment and the ROIs used for dosimetric comparison have been mapped across to each CBCT using a DIR. Despite inherent uncertainties associated with both of these operations, their validity has been assessed quantitatively (bulk-density dose calculation) and qualitatively (clinical oncologist review and approval of the DIR-mapped ROIs). Furthermore, the CBCT analysis was used to compare the robustness of the two planning techniques, meaning any errors in dose calculation or ROI propagation would have affected both techniques in the same way. Due to the bulk density assignment of 0.95 g/cm3 outside the CBCT FOV, CBCT dosimetric comparison of the right lung was not possible. However, due to the beam geometries, it is not expected that the robust and non-robust plans would deliver significantly different doses to the contralateral structure.
It is recommended that patients planned with VMAT for breast cancer are treated in breath-hold and are set up according to appropriate locally-defined imaging protocols. For patients demonstrating large setup variations, a daily online imaging protocol may be adopted. Finally, the techniques used to generate OM-based robust plans require that the TPS has functionality to enable such optimisations.
5. Conclusion
VMAT plans generated using organ motion-based robust optimisation available within a commercial TPS are clinically acceptable in the nominal scenario and are robust to both typical and extreme changes to the patient external ROI during their treatment course.
Declarations of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2019.04.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Thorsen L.B.J., Offersen B.V., Danø H., Berg M., Jensen I., Pedersen A.N. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. 2016;34:314–320. doi: 10.1200/JCO.2015.63.6456. [DOI] [PubMed] [Google Scholar]
- 2.Whelan T.J., Olivotto I.A., Parulekar W.R., Ackerman I., Chua B.H., Nabid A. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poortmans P.M., Collette S., Kirkove C., Van Limbergen E., Budach V., Struikmans H. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 4.www.rcr.ac.uk, P. r.; 2016. www.rcr.ac.uk. Retrieved 2018.
- 5.Taylor C.W., Povall J.M., McGale P., Nisbet A., Dodwell D., Smith J.T. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys. 2008;72:501–507. doi: 10.1016/j.ijrobp.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 6.Taylor C.W., Kirby A.M. Cardiac side-effects from breast cancer radiotherapy. Clin Oncol. 2015;27:621–622. doi: 10.1016/j.clon.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Hjelstuen M.H.B., Mjaaland I., Vikström J., Dybvik K.I. Radiation during deep inspiration allows loco-regional treatment of left breast and axillary-, supraclavicular-and internal mammary lymph nodes without compromising target coverage or dose restrictions to organs at risk. Acta Oncol. 2012;51:333–344. doi: 10.3109/0284186X.2011.618510. [DOI] [PubMed] [Google Scholar]
- 8.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Brønnum D. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 9.Ranger A., Dunlop A., Hutchinson K., Convery H., Maclennan M.K., Chantler H. A dosimetric comparison of breast radiotherapy techniques to treat locoregional lymph nodes including the internal mammary chain. Clin Oncol. 2018;30(6):346–353. doi: 10.1016/j.clon.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Popescu C.C., Olivotto I.A., Beckham W.A., Ansbacher W., Zavgorodni S., Shaffer R. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010;76:287–295. doi: 10.1016/j.ijrobp.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 11.Sakumi A., Shiraishi K., Onoe T., Yamamoto K., Haga A., Yoda K. Single-arc volumetric modulated arc therapy planning for left breast cancer and regional nodes. J Radiat Res. 2012;53:151–153. doi: 10.1269/jrr.11159. [DOI] [PubMed] [Google Scholar]
- 12.Osman S.O., Hol S., Poortmans P.M., Essers M. Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation. Radiother Oncol. 2014;112:17–22. doi: 10.1016/j.radonc.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Viren T., Heikkilä J., Myllyoja K., Koskela K., Lahtinen T., Seppälä J. Tangential volumetric modulated arc therapy technique for left-sided breast cancer radiotherapy. Radiat Oncol. 2015;10:79. doi: 10.1186/s13014-015-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sankar A., Velmurugan J. Different intensity extension methods and their impact on entrance dose in breast radiotherapy: a study. J Med Phys. 2009;34:200–205. doi: 10.4103/0971-6203.56079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bethesda M. ICRU Report 62: prescribing, recording and reporting photon beam therapy (supplement to ICRU report 50). International Commission on Radiation Units and Measurements; 1999.
- 16.Tyran M. Safety and benefit of using a virtual bolus during treatment planning for breast cancer treated with arc therapy. J Appl Clin Med Phys. 2018;19:463–472. doi: 10.1002/acm2.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen C., Acosta Roa A. Robustness of VMAT and 3DCRT plans toward setup errors in radiation therapy of locally advanced left-sided breast cancer with DIBH. Physica Med. 2018;45:12–18. doi: 10.1016/j.ejmp.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Byrne M., Hu Y., Archibald-Heeren B. Evaluation of RayStation robust optimisation for superficial target coverage with setup variation in breast IMRT. Australas Phys Eng Sci Med. 2016;39:705–716. doi: 10.1007/s13246-016-0466-6. [DOI] [PubMed] [Google Scholar]
- 19.Liu W., Zhang X., Li Y., Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39:1079–1091. doi: 10.1118/1.3679340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredriksson A., Forsgren A., Hardemark B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med Phys. 2011:1672–1684. doi: 10.1118/1.3556559. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen D., Corbet C., Largeron G. Is robust optimization better than virtual. Radiother Oncol. 2018 EP-1896. [Google Scholar]
- 22.Offersen B., Boersma L., Kirkove C. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 23.Fredriksson A., Bokrantz R. A critical evaluation of worst case optimization methods for robust intensity-modulated proton therapy planning. Med Phys. 2014;41(8) doi: 10.1118/1.4883837. [DOI] [PubMed] [Google Scholar]
- 24.Dunlop A., McQuaid D., Nill S., Murray J. Comparison of CT number calibration techniques for CBCT-based dose calculation. Strahlenther Onkol. 2015:970–978. doi: 10.1007/s00066-015-0890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.