Abstract
Purpose
Spectral-domain optical coherence tomography (SD-OCT) commonly reveals lamellar-hole-associated epiretinal proliferation (LHEP) as an avascular homogenous layer of premacular material with medium reflectivity, as recently described in various traction maculopathies, mostly in lamellar macular holes (LMH). We have used multimodal imaging to examine a patient suffering from unilateral advanced atrophic LMH presenting LHEP with perifoveal exudative vascular anomalous complex (PEVAC) and intra-LHEP edema fluctuating under anti-vascular endothelial growth factor (anti-VEGF) therapy.
Observation
A 77-year-old male presented with decreased vision in the left eye attributable to longstanding LMH. He complained of worsening symptoms for six months. Whereas SD-OCT showed classic tractional epiretinal gliosis in the right eye, the left eye exhibited atrophic LMH and a significant amount of LHEP containing hyperreflective round lesions and hyporeflective cystoid spaces. Fluorescein/indocyanine green angiography demonstrated PEVAC with large anomalous vessels and exudation. OCT angiography revealed abnormal vessels originating from the deep retinal plexus. After anti-vascular endothelial growth factor (anti-VEGF) therapy, the intraretinal edema seemed to decrease.
Conclusions and importance
Perifoveal exudative vascular anomalous complex can occur in eyes with advanced LMHs causing edema inside LHEP. Pathologic vessels appear to originate from the deep retinal plexus. Given that LHEP formation is proposed to be a glial-cell-driven process, Müller cells may play a decisive role in the pathogenesis of the presented vascular malformation. Because of spontaneous fluctuation of the associated edema, the role of anti-VEGF remains questionable, while a functional response to therapy might be limited according to the progressive atrophic lamellar defect with intraretinal tissue loss.
Keywords: Lamellar macular hole, Lamellar-hole-associated epiretinal proliferation, Perifoveal exudative vascular abnormality, Premacular proliferation
1. Introduction
Premacular proliferation in lamellar macular holes (LMHs) was first described as a dense epiretinal membrane by spectral-domain optical coherence tomography (SD-OCT).1,2 More recently, this premacular tissue has been termed ‘lamellar hole-associated epiretinal proliferation’ (LHEP) because of its predominance in lamellar macular defects.3, 4, 5 By means of SD-OCT, Itoh and colleagues demonstrated that LHEP can also be seen in other traction maculopathies, and therefore that it does not represent an exclusive feature of LMHs.6
LHEP is characterized as an avascular homogenous layer of medium reflectivity covering the premacular surface and surrounding the foveal defect without common features of retinal traction. The amount of LHEP in eyes with LMHs enlarges during long-time follow-up and correlates with photoreceptor layer defects associated with poor visual acuity.7,8 Histopathology suggests a crucial role for glial cells and vitreous cortex remnants in the development of premacular proliferation in eyes with traction maculopathies.
Chronic neurodegenerative processes in macular telangiectasia (MacTel) are widely known also to cause loss of retinal tissue resembling LMH.9, 10, 11 However, reports on the connection of LHEP and retinal vascular dysfunction are scarce, with only one short report by Semoun et al., 2016 describing an intraretinal neovascular network presenting with LHEP, but without any exudation or communication between the two entities.12 Another report by Doshi et al. has described intra-LHEP edema but did not notice causative intraretinal vascular abnormalities in the simultaneous presence of the possible confounders age-related macular degeneration (AMD) and post-operative macular edema.13
We describe below a case presenting with intraretinal vascular abnormalities seemingly communicating with edematous LHEP found above a lamellar macular hole, which we interpret as a perifoveal vascular anomalous complex (PEVAC), defined by Querques14 and Sacconi15 as a unilateral, idiopathic, isolated, perifoveal aneurysm.
2. Case report
A 77-year-old male presented with decreased vision in the left eye attributable to atrophic large LMH as confirmed by SD-OCT. He complained of worsening symptoms for six months without significant metamorphopsia. The best-corrected visual acuity of the patient was 20/25 and 20/200 in the right and left eyes, respectively. Whereas SD-OCT showed no retinal pathology except for a classic epiretinal membrane in the right eye, the left eye featured a significant amount of LHEP containing numerous small hyperreflective round structures, single lesions with a hyporeflective lumen and a hyperreflective wall, together with large hyporeflective cystoid spaces. In both eyes, anterior segment examination was unremarkable except for moderate corticonuclear cataract formation. Intraocular pressure was normal. With regard to retinal pathology, family history was positive for age-related macular degeneration. Medical history included cardiac arrhythmia and arterial hypertension sufficiently controlled, but was negative for diabetes.
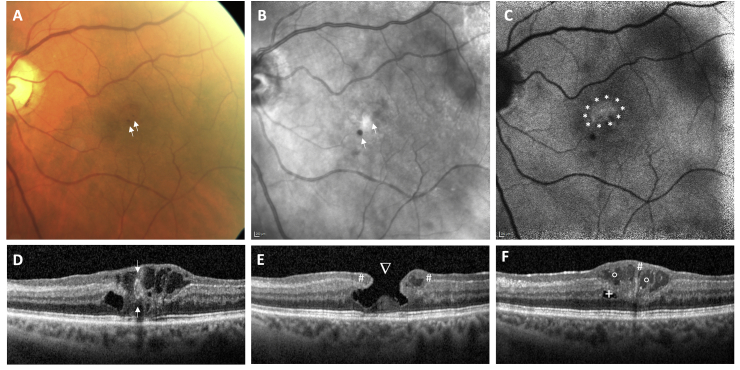
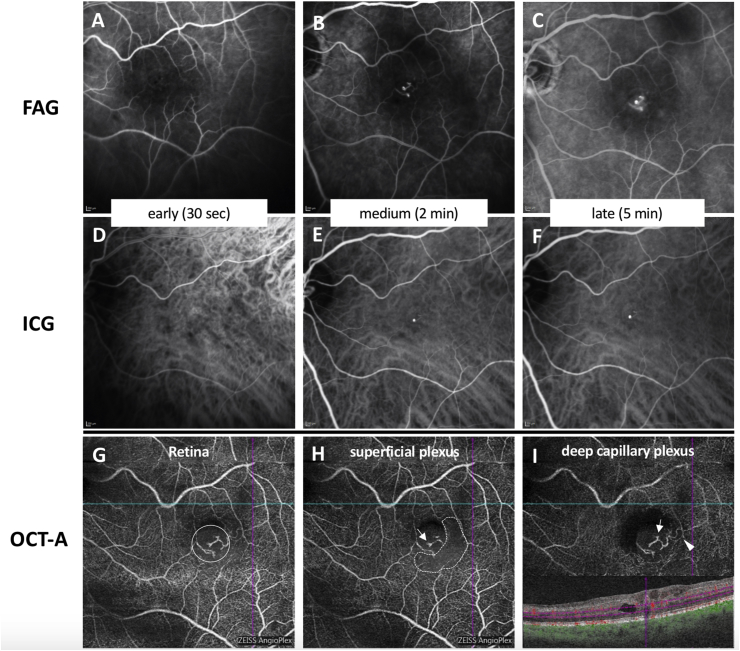
Multimodal imaging included fundus photography, SD-OCT, near-infrared (NIR) imaging and blue autofluorescence (BAF) confocal laser scanning ophthalmoscopy, and fluorescein (FAG) and indocyanine green (ICG) angiography, all performed by using Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany) and OCT angiography (OCT-A; Angioplex, Cirrus 5000 OCT, Carl Zeiss Meditec AG, Jena, Germany). Fundus photography demonstrated the temporal parafoveal area of the left eye with two red dot-like lesions but without gliosis or retinal folds (Fig. 1A). Infrared imaging and BAF displayed the funduscopic red lesions as hyporeflective dots (Fig. 1B and C). Additionally, a hyperreflective area was seen on BAF correlating to the lamellar macular defect with retinal tissue loss. Spectral-domain OCT confirmed the diagnosis of LMH with intraretinal cavitation and tissue loss, especially affecting the inner nuclear layer and outer plexiform layer (Fig. 1D–F). A thinning of the outer plexiform layer was seen with a central foveal bump. The central foveal thickness was significantly reduced, and the ellipsoid zone (EZ) was disrupted. Lamellar hole-associated epiretinal proliferation covered wide areas of the macula, extending even beyond the 49 OCT B-scans centered on the fovea. Of note, multiple large and irregularly formed hyporeflective lesions within the LHEP could be detected. These hyporeflective lesions were accompanied by hyperreflective foci. Fluorescein and indocyanine green angiography (Fig. 2 A-C and D-F) demonstrated an anomalous vascular network that clearly presented with intraretinal and intra-LHEP exudation (see also the late phase angiogram in Fig. 2C). The hyperfluorescent structures correlated with a dilated anomalous vascular network. OCT angiography revealed that the abnormal vessels originated from the deep retinal plexus extending to the superficial vascular plexus (Fig. 2G–H), suggesting the diagnosis of a perifoveal exudative vascular anomalous complex (PEVAC). The PEVAC additionally presented with an area of capillary rarefication that could be detected in the superficial capillary plexus slab (Fig. 2H), accompanied by a microvascular loop that could be seen temporal of the fovea in the deep plexus (Fig. 2I). No flow was detected inside the LHEP on OCT-A.
Fig. 1.
Multimodal imaging of perifoveal exudative anomalous vascular complex (PEVAC) and lamellar hole-associated epiretinal proliferation (LHEP). (A) Fundus photography, (B) near-infrared imaging, and (C) blue-autofluorescence revealed two small red (hyporeflective) dots in the temporal part of the fovea (arrows →). Blue-autofluorescence revealed alterations of the RPE (area surrounded by stars *). OCT (D–F) showed a lamellar macular hole (arrowhead ∇) with a hollow structure of medium reflectivity and a hyperreflective wall, highly suggestive of PEVAC; and intraretinal cavities (plus sign +) and LHEP (hash #) with intra-LHEP fluid (hollow circle ◦). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Comparison of vessel imaging. By FAG (A–C), microvascular abnormalities with dilations and mild exudation can be detected on medium and late frames. One of the microvascular dilations also corresponds to hyperreflectivity on ICG (D–F). Microvascular abnormalities can be detected most precisely by OCT-A (G–I),. On the retina slab (G), the abnormal vessels (surrounded by a large circle) can easily be seen originating from the deep vascular plexus (I) and presenting with prominent microvascular dilations (arrows) located in the deep (I) and superficial vascular plexus (H). An area of capillary rarefication can be detected (dotted line) in the superficial capillary plexus slab (H). In the deep plexus (I), a microvascular loop can be seen temporal of the fovea (arrowhead).
The total period of follow-up was six months. Because of the advanced stage of LMH and its atrophic appearance with EZ defects, the patient was initially closely monitored. Given the lack of a tractional epiretinal membrane, we did not recommend macular surgery with internal limiting membrane peeling.
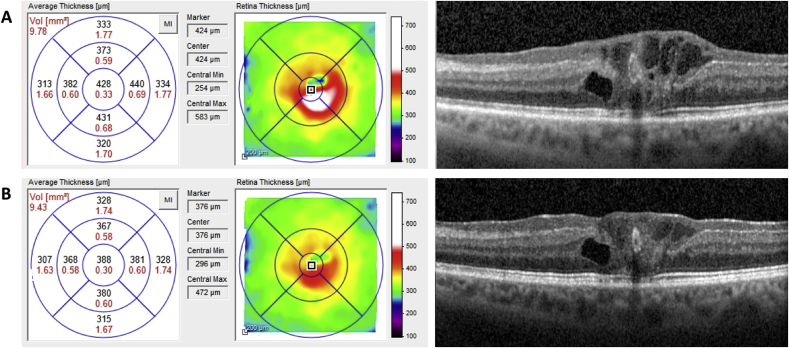
After three months, visual function remained unchanged. However, a significant increase of intra-LHEP edema with increased macular thickness was detected (Fig. 3A), and therefore, anti-vascular endothelial growth factor (VEGF) intravitreal therapy was recommended. Following three intravitreal ranibizumab injections every four weeks (Lucentis®; Novartis Pharma AG, Basel, Switzerland), SD-OCT revealed a significant decrease of foveal thickness (3B) and reduction of LHEP cystoid spaces. Visual acuity improved to 20/125 during that period of time.
Fig. 3.
Time course of retinal thickness on OCT. After three Ranibizumab injections, the thickness decreased from a maximum of 583 μm (A) to 472 μm (B), and the PEVAC lesion seemed partially to shrink. After a cessation of Ranibizumab treatment for three months, the thickness increased again to a maximum of 614 μm (not shown), driven by intra-LHEP cysts.
3. Discussion
Although, in this case, a diagnosis of a lamellar macular hole (LMH) with lamellar hole-associated epiretinal proliferation (LHEP) can be clearly made, the vascular alterations detected on OCT angiography require thorough differential diagnosis. Aside from pathologic findings secondary to conditions such as diabetes, arterial hypertension, or retinal vascular occlusion, the perifoveal location and intraretinal origin of the vascular lesion described in our case are highly suggestible of (i) retinal angiomatous proliferation (RAP) belonging to the spectrum of age-related macular degeneration (AMD), (ii) perifoveal exudative vascular anomalous complex (PEVAC) as described for the first time by Querques in 201114, or (iii) the spectrum of idiopathic macular telangiectasia.15 Because of the complete absence of Drusen, RAP can be excluded in our case. As the right eye showed no vascular alterations, the diagnosis of MacTel Type 2 also does not apply, leaving PEVAC and MacTel 1 for further consideration.
Given the clear delineation of vascular alterations with a large lumen and hyperreflective wall, multimodal imaging strongly suggests the diagnosis of PEVAC. Interestingly, our case shows two new features until now commonly associated with MacTel 1, but not described for PEVAC, i.e., (i) the development of LMH,9, 10, 11,15 and (ii) the possible anatomical response to intravitreal anti-VEGF therapy. The finding that the central macular thickness of our patient significantly decreased from 583 to 472 μm after three injections of Ranibizumab and re-increased to 614 μm without further treatment might hints at some involvement of VEGF in the PEVAC exudation pathway. Nevertheless, this proposal needs further confirmation, as PEVAC-associated edema can spontaneously increase and decrease during follow-up without any therapy, as shown by Sacconi et al.15 However, while still persistent after anti-VEGF, the PEVAC lesion also seemed to respond in a limited manner by partial shrinking (Fig. 3A and B).
Interestingly, long-term follow-up of LHEP has found not only that the size of LHEP increases on average, but that LHEP can also migrate from the retinal surface into the macular hole towards the outer retinal layers.17 Therefore, the first possible etiologic scenario in the patient described here might be the coincident presence of a lamellar hole with associated epiretinal proliferation and PEVAC, which gained connection to LHEP tissue because of long-standing disease and LHEP migration towards the vascular lesion. On the other hand, LHEP immunohistochemical staining has found the presence of anti–glial fibrillary acidic protein and anti–glutamine synthetase clone 6, highly suggestive of Müller cells.3 Additionally, lamellar holes associated with macular telangiectasia frequently present the ‘ILM-drape sign’, which contributes to the purported Müller-cell-linked pathogenesis of MacTel Type 29. Müller cell loss is a pathognomonic finding in MacTel Type 2 and has been linked to vascular destabilization.18 Thus, the second possible etiologic scenario might be that Müller cell dropout in deep retinal layers has led to vascular destabilization, generating the telangiectatic vessels demonstrated on OCT angiography. Secondarily, the migration of Müller cells towards the retinal surface might have contributed to LHEP formation. Despite being highly speculative, this second scenario is supported by the fact that we describe here the first case of intra-LHEP edema hinting at direct communication between the vascular dysfunction in the deep retinal layers and the LHEP filling the macular hole and extending onto the surface of the retina. Additional clinical and histopathological studies are needed to obtain further evidence for this proposal.
4. Conclusions
PEVAC can occur in eyes with advanced LMHs causing edema inside LHEP and intraretinal layers. Macular telangiectasia might serve as an alternative differential diagnosis. PEVAC appears to originate from the deep retinal plexus in the present case. Given that LHEP formation is proposed to be a glial-cell-driven process, Müller cells may play a decisive role in the pathogenesis of the presented vascular abnormality. Functional response to therapy might be limited according to the progressive atrophic lamellar defect with intraretinal tissue loss.
Patient consent
Consent to publish this case report has been obtained in writing from the patient.
Funding
No funding or grant support.
Conflicts of interest
The following authors have no financial disclosures to make: JS, EV, DV, AW, SGP, RGS.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgment
The authors wish to thank Prof. Dr. Faik Gelisken, Department of Ophthalmology, Eberhard Karls University Tuebingen, for his help in the characterization of this case.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajoc.2019.03.008.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Witkin A.J., Ko T.H., Fujimoto J.G. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113(3):388–397. doi: 10.1016/j.ophtha.2005.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parolini B., Schumann R.G., Cereda M.G., Haritoglou C., Pertile G. Lamellar macular hole: a clinicopathologic correlation of surgically excised epiretinal membranes. Investig Ophthalmol Vis Sci. 2011;52(12):9074–9083. doi: 10.1167/iovs.11-8227. [DOI] [PubMed] [Google Scholar]
- 3.Pang C.E., Maberley D.A., Freund K.B. LAMELLAR HOLE-ASSOCIATED epiretinal proliferation: a clinicopathologic correlation. Retina. 2016;36(7):1408–1412. doi: 10.1097/IAE.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 4.Pang C.E., Spaide R.F., Freund K.B. Comparing functional and morphologic characteristics of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Retina. 2015;35(4):720–726. doi: 10.1097/IAE.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 5.Pang C.E., Spaide R.F., Freund K.B. Epiretinal proliferation seen in association with lamellar macular holes: a distinct clinical entity. Retina. 2014;34(8):1513–1523. doi: 10.1097/IAE.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 6.Itoh Y., Levison A.L., Kaiser P.K., Srivastava S.K., Singh R.P., Ehlers J.P. Prevalence and characteristics of hyporeflective preretinal tissue in vitreomacular interface disorders. Br J Ophthalmol. 2016;100(3):399–404. doi: 10.1136/bjophthalmol-2015-306986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schumann R.G., Compera D., Schaumberger M.M. Epiretinal membrane characteristics correlate with photoreceptor layer defects in lamellar macular holes and macular pseudoholes. Retina. 2015;35(4):727–735. doi: 10.1097/IAE.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 8.Compera D., Schumann R.G., Cereda M.G. Progression of lamellar hole-associated epiretinal proliferation and retinal changes during long-term follow-up. Br J Ophthalmol. 2018;102(1):84–90. doi: 10.1136/bjophthalmol-2016-310128. [DOI] [PubMed] [Google Scholar]
- 9.Charbel Issa P., Scholl H.P., Gaudric A. Macular full-thickness and lamellar holes in association with type 2 idiopathic macular telangiectasia. Eye. 2009;23(2):435–441. doi: 10.1038/sj.eye.6703003. [DOI] [PubMed] [Google Scholar]
- 10.Karth P.A., Raja S.C., Brown D.M., Kim J.E. Outcomes of macular hole surgeries for macular telangiectasia type 2. Retina. 2014;34(5):907–915. doi: 10.1097/IAE.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 11.Gregori N., Flynn H.W., Jr. Surgery for full-thickness macular hole in patients with idiopathic macular telangiectasia type 2. Ophthalmic Surg Laser Imag: Off. J. Int. Soc. Imag. Eye. 2010;41 Online:1–4. doi: 10.3928/15428877-20100726-10. [DOI] [PubMed] [Google Scholar]
- 12.Semoun O., Miere A., Srour M. Lamellar hole associated with prominent intraretinal vessels. Retina. 2016;36(6):e43–44. doi: 10.1097/IAE.0000000000000930. [DOI] [PubMed] [Google Scholar]
- 13.Doshi R.R., Lowrance M.D., Kim B.T., Davis J.L., Rosenfeld P.J. Epiretinal macular edema associated with thick epiretinal membranes. Ophthalmic Surg. Laser. Imag. Retina. 2013;44(5):508–512. doi: 10.3928/23258160-20130909-19. [DOI] [PubMed] [Google Scholar]
- 14.Querques G., Kuhn D., Massamba N., Leveziel N., Querques L., Souied E.H. Perifoveal exudative vascular anomalous complex. J Fr Ophtalmol. 2011;34(8):e551–554. doi: 10.1016/j.jfo.2011.03.002. 559. [DOI] [PubMed] [Google Scholar]
- 15.Sacconi R., Freund K.B., Yannuzzi L.A. The expanded spectrum of perifoveal exudative vascular anomalous complex. Am J Ophthalmol. 2017;184:137–146. doi: 10.1016/j.ajo.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Compera D., Cereda M.G., Schumann R.G., Bottoni F. Development and progression of a lamellar macular hole with lamellar hole-associated epiretinal proliferation. Retin Cases Brief Rep. 2017 doi: 10.1097/ICB.0000000000000605. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Powner M.B., Gillies M.C., Tretiach M. Perifoveal müller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010;117(12):2407–2416. doi: 10.1016/j.ophtha.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.