Carbapenem-resistant Klebsiella pneumoniae sequence type 258 (CRKP-ST258) can cause chronic infections in lungs and airways, with repeated episodes of bacteremia. In this report we addressed whether the recruitment of myeloid cells producing the anti-inflammatory cytokine interleukin-10 (IL-10) modulates the clearance of CKRP-ST258 in the lungs and establishes bacterial persistence.
KEYWORDS: interleukin-10, Klebsiella pneumoniae ST258, monocytic-myeloid-derived suppressor cells, neutrophils
ABSTRACT
Carbapenem-resistant Klebsiella pneumoniae sequence type 258 (CRKP-ST258) can cause chronic infections in lungs and airways, with repeated episodes of bacteremia. In this report we addressed whether the recruitment of myeloid cells producing the anti-inflammatory cytokine interleukin-10 (IL-10) modulates the clearance of CKRP-ST258 in the lungs and establishes bacterial persistence. Our data demonstrate that during pneumonia caused by a clinical isolate of CRKP-ST258 (KP35) there is an early recruitment of monocyte-myeloid-derived suppressor cells (M-MDSCs) and neutrophils that actively produce IL-10. However, M-MDSCs were the cells that sustained the production of IL-10 over the time of infection evaluated. Using mice unable to produce IL-10 (IL-10−/−), we observed that the production of this cytokine during the infection caused by KP35 is important to control bacterial burden, to prevent lung damage, to modulate cytokine production, and to improve host survival. Importantly, intranasal transfer of bone marrow-derived M-MDSCs from mice able to produce IL-10 at 1 day prior to infection improved the ability of IL-10−/− mice to clear KP35 in the lungs, decreasing their mortality. Altogether, our data demonstrate that IL-10 produced by M-MDSCs is required for bacterial clearance, reduction of lung tissue damage, and host survival during KP35 pneumonia.
INTRODUCTION
One major cause of pneumonia in health care facilities worldwide is carbapenem-resistant Klebsiella pneumoniae sequence type 258 (CRKP-ST258). CRKP-ST258 strains encode KPC β-lactamases, which makes these bacteria resistant to different β-lactams, including carbapenems (1, 2). CRKP-ST258 isolates are the etiologic agent of different diseases, including pneumonia, sepsis, and urinary tract infections (3). CRKP-ST258 pneumonia in health care facilities is associated with high mortality rates (50%) although in some cases these bacteria can also establish chronic infections with frequent episodes of sepsis (4).
Similar to the well-studied laboratory reference strain K. pneumoniae ATCC 43816 (KPPR1), CRKP-ST258 expresses the typical pathogen-associated molecular patterns (PAMPs) of Gram-negative bacteria (3) that potently activate proinflammatory signaling on epithelial and immune cells through Toll-like receptor 4 (TLR4)/MyD88/NF-κB (4). Therefore, we believe that the ability of CRKP-ST258 to cause chronic infections is not because of impaired bacterial recognition by the immune system. In contrast to KPPR1, CRKP-ST258 isolates have acquired resistance to killing by neutrophils (4, 5). During CRKP-ST258 infection, the initial neutrophil response is rapidly supplanted by the recruitment of suppressive monocytic myeloid-derived suppressor cells (M-MDSCs) (4), which have been described as major suppressors of the inflammatory response during cancer and infection (6).
During KPPR1 pneumonia, MDSCs are recruited at later time points, and their function is associated with lung recovery and remodeling after infection (7). MDSCs are a heterogeneous group of anti-inflammatory cells best described in cancer (8). This group of myeloid cells is composed of at least three different subsets: monocytic (M-MDSCs), granulocytic (G-MDSCs) (9), and eosinophilic (Eo-MDSCs) cells (10). Each subset shares the same surface markers of their proinflammatory counterparts (inflammatory monocytes, neutrophils, and eosinophils, respectively) but also expresses anti-inflammatory mediators able to inhibit T cell function and proliferation, such as arginase-1, inducible nitric oxide synthase (iNOS), and interleukin-10 (IL-10) (9).
IL-10 is a major anti-inflammatory cytokine produced by nearly all leukocytes, with widely suppressive effects on several immune cell types (11). During bacterial infections, IL-10 modulates the proinflammatory immune response, allowing clearance of the pathogen with reduced tissue damage (12). In the case of IL-10 overproduction, the immune response is not robust enough to clear the infective pathogen, leading to chronic infections or elevated host mortality (13, 14). In this report we evaluated whether the early recruitment of IL-10-producing M-MDSCs observed during pneumonia caused by a clinical isolate of CRKP-ST258 (KP35) (4, 15) plays a major role in the establishment of a persistent infection and delayed bacterial clearance. Using transgenic mice expressing IL-10 and enhanced green fluorescent protein (eGFP) (IL-10/GFP mice), we demonstrate that M-MDSCs recruited early to the lungs of KP35-infected mice are the main myeloid source of IL-10 in the lungs from 48 to 240 h postinfection. The central role of this myeloid population was shown by the attenuation of pulmonary damage by the adoptive transfer into IL-10−/− mice of MDSCs able to produce IL-10. Altogether, IL-10 production in response to CRKP-ST258 infection has an important function in enhancing bacterial clearance from the airways.
RESULTS
Neutrophils and M-MDSCs account for the early production of IL-10 in lungs of KP35-infected mice.
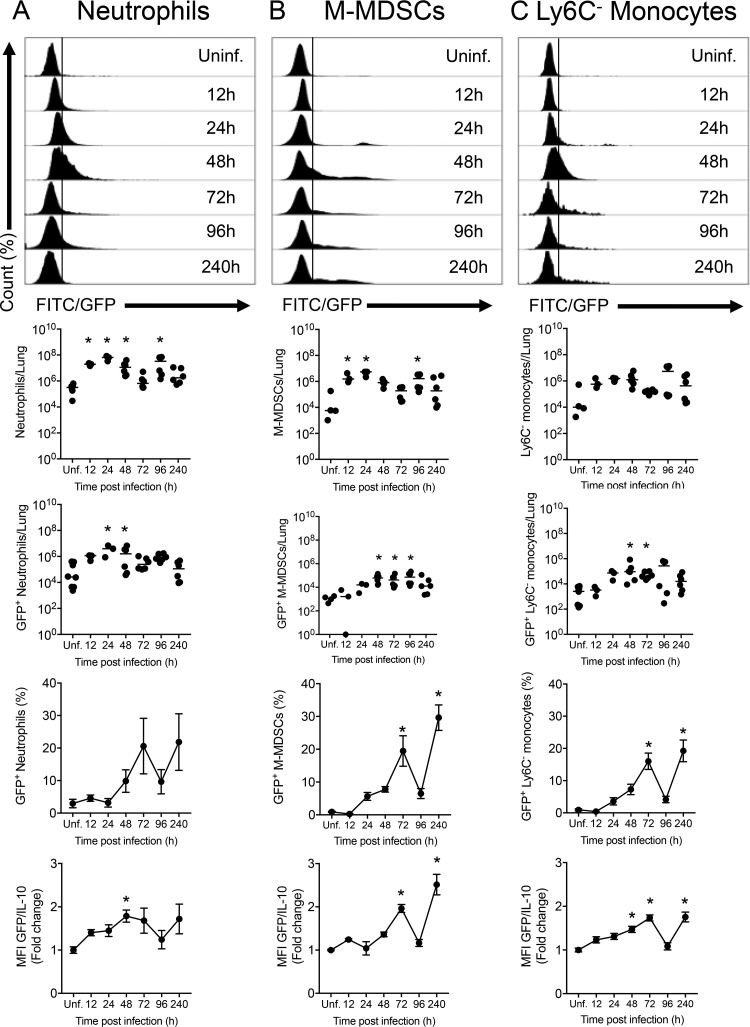
To evaluate the ability of M-MDSCs to produce IL-10 at different times post-KP35 infection, reporter IL-10/GFP VertX mice were intranasally inoculated with KP35. IL-10-producing cells were then quantified by flow cytometry. Similar to wild-type (WT) mice (4), IL-10/GFP mice displayed severe symptomatology after KP35 infection, with a peak symptom score at 48 h postinfection (hpi), a survival rate of 100%, a reduction in the epithelial/endothelial barrier function, and elevated bacterial burden in the lung tissue and bronchoalveolar lavage fluid (BALF) at 10 days postinfection (dpi) (see Fig. S1 in the supplemental material). The infection was characterized by a rapid recruitment of neutrophils and M-MDSCs, a decrease in the number of alveolar macrophages, and a mild recruitment of Ly6C− monocytes, eosinophils, and interstitial macrophages (Fig. 1 and Fig. S2). As expected, several innate immune cells produced IL-10 in response to KP35. IL-10 production started at 24 hpi, with the arrival of neutrophils, identified as the most abundant IL-10-producing cells during the first 48 hpi (Fig. 1A). From 48 hpi onward, the population of IL-10-producing neutrophils returned to baseline levels, and recruited M-MDSCs actively produced IL-10 until 240 hpi (Fig. 1B). Ly6C− monocytes were also an important source of IL-10 at 48 and 72 hpi (Fig. 1C). Moreover, eosinophils were identified as IL-10-producing cells at 72 and 96 hpi (Fig. S2A). Alveolar macrophages and interstitial macrophages did not show a significant increase in IL-10 production (Fig. S2B and C).
FIG 1.
Neutrophils, M-MDSCs, and Ly6C− monocytes are the main IL-10 producers during KP35 infection. (A to C) IL-10/GFP VertX mice were intranasally infected with 1 × 108 CFU of KP35, and IL-10 production by myeloid cells was evaluated by flow cytometry at 12, 24, 48, 72, 96, and 240 hpi on neutrophils, M-MDSCs, and Ly6C− monocytes, as indicated. For each cell population, data are presented as the number of total lung cells, the numbers of lung GFP+ (IL-10+) cells, the percentage of GFP+ (IL-10+) cells relative to the total number of cells, and the fold change in mean fluorescence intensity (MFI) of GFP in the total cell population. WT uninfected mice were included to establish the autofluorescence level (data not shown). *, P < 0.05, for comparison of each group with the uninfected control (by one-way ANOVA with a Tukey posttest). FITC, fluorescein isothiocyanate.
IL-10 production during KP35 infection is critical for host survival and bacterial clearance.
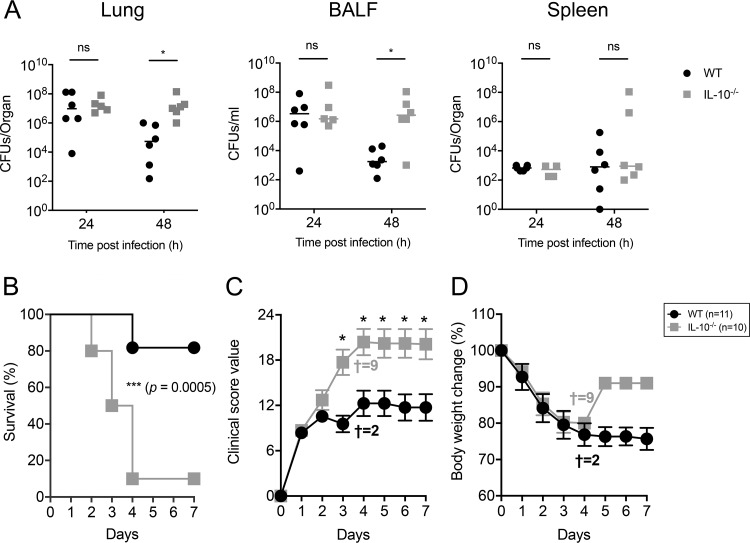
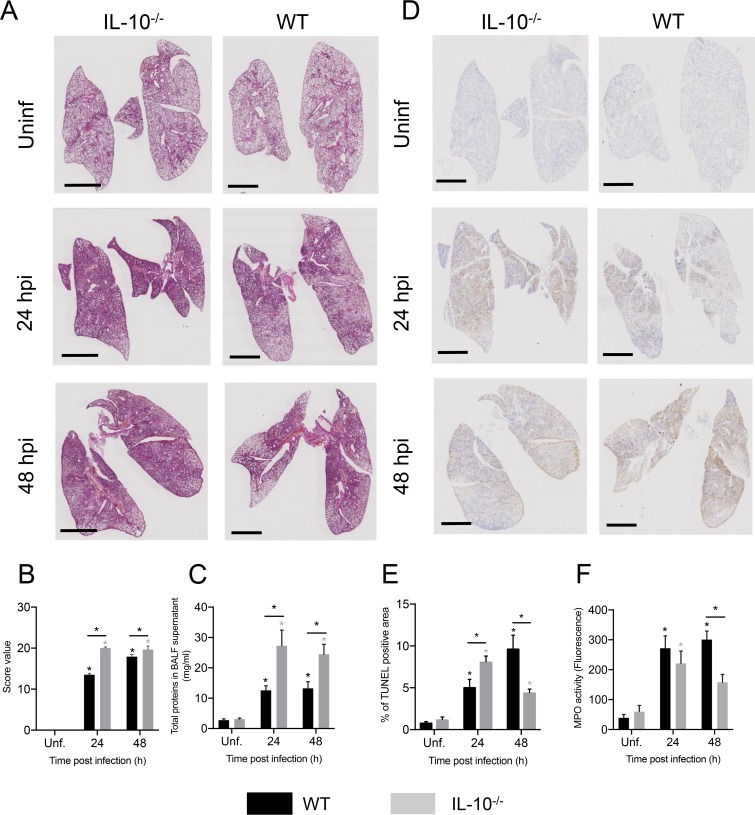
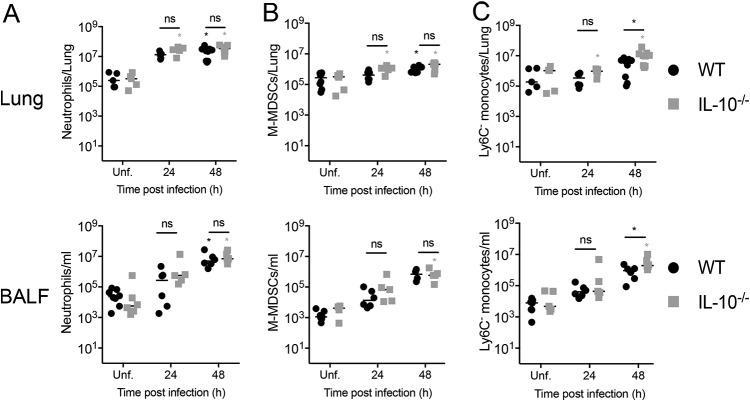
Next, we evaluated whether the production of IL-10 is required for the clearance of KP35 from the lungs. To address this aim, WT and IL-10 knockout (IL-10−/−) mice were intranasally infected with KP35, and bacterial burden was evaluated at 24 and 48 hpi. At 48 hpi, IL-10−/− mice had elevated bacterial counts in the lung tissue and BALF but not in the spleen (Fig. 2A). IL-10−/− mice also had increased mortality (Fig. 2B), with 81% (9/11) of WT mice surviving after KP35 infection up to 7 days whereas only 10% (1/10) of IL-10−/− mice survived. Consistently, IL-10−/− mice also had more severe symptomatology than WT mice (Fig. 2B and C). At 24 hpi, IL-10−/− mice presented increased lung inflammation (Fig. 3A to C) and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) (Fig. 3D and E). At 48 hpi, lung inflammation remained higher in IL-10−/− mice (Fig. 3A to C), but these mice presented a decrease in TUNEL-positive area compared to that of WT mice, which presented an increasing trend over time (Fig. 3D and E). Consistently, IL-10−/− mice had reduced myeloperoxidase (MPO) activity in BALF (Fig. 3F). Despite this, IL-10−/− mice presented an elevated lung injury, evidenced in a higher histopathology score and total BALF protein amount. Interestingly, the increased lung injury observed in IL-10−/− mice did not correlate with increased infiltration to the airways of inflammatory myeloid cells, such as neutrophils, M-MDSCs, eosinophils, and macrophages, which remained at levels between those of IL-10−/− and WT mice at 24 and 48 h after KP35 infection (Fig. 4 and Fig. S3).
FIG 2.
IL-10 production is required for KP35 clearance and host survival. WT and IL-10−/− mice were intranasally infected with 1 × 108 CFU of KP35. (A) Bacterial burden was evaluated in lung, BALF, and spleen at 24 and 48 hpi. (B to D) Survival rate, severity of the disease, and body weight change were evaluated over 7 days († represents the number of dead mice at 4 dpi). For bacterial burden, * P < 0.05 (Mann-Whitney U test comparing results between both groups at each time point); for the survival curve, ***, P = 0.0005 (log rank test); for disease severity (clinical score), *, P < 0.05 (two-way ANOVA with a Tukey posttest comparing results for both groups at each time point). ns, not significant.
FIG 3.
IL-10 production modulates lung damage during KP35 infection. WT and IL-10−/− mice were intranasally infected with 1 × 108 CFU of KP35, and different parameters of lung damage were evaluated, including lung histopathology by H&E staining (A), lung histopathological score (B), total BALF proteins (C) and total lung cell apoptosis by TUNEL assay (D) and TUNEL-positive area (E). (F) Neutrophil activation was measured by the quantification of MPO activity in the BALF. Scale bar, 2 mm. *, P < 0.05, for a comparison of results between groups at each time point or between the infected group and the respective uninfected (Unf) group (two-way ANOVA with a Tukey posttest).
FIG 4.
IL-10 production does not influence immune cell recruitment to the airways of KP35-infected mice. Recruitment of neutrophils (A), M-MDSCs (B), and Ly6C− (C) monocytes was measured in the lung and BALF of uninfected and infected WT and IL-10−/− mice at 24 and 48 hpi. *, P < 0.05, for a comparison of results between groups at each time point or between the infected group and the respective uninfected (Unf) group (two-way ANOVA with a Tukey posttest).
IL-10 production has a major effect on proinflammatory cytokine production.
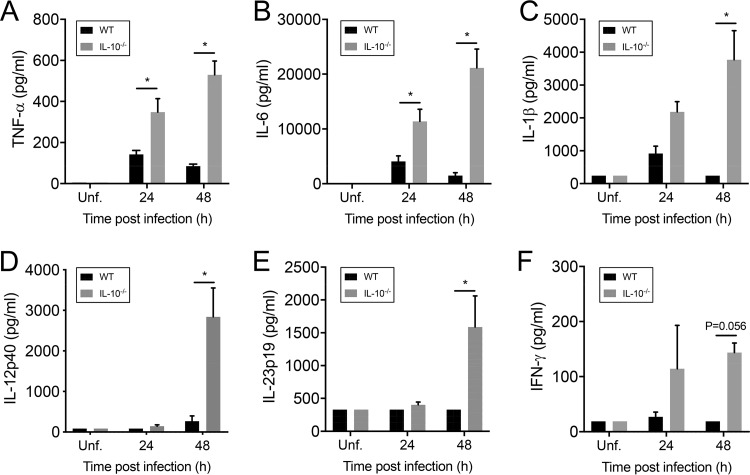
Proinflammatory cytokine production was measured in BALF of infected mice. WT mice produced reduced amounts of tumor necrosis factor alpha (TNF-α), IL-1β, and IL-6 at 24 and 48 hpi (Fig. 5A to C). Furthermore, negligible amounts of IL-12p40, IL-23p19, and gamma interferon (IFN-γ) were detected in these mice (Fig. 5D to F). In contrast, elevated levels of TNF-α and IL-6 were detected in BALF samples of IL-10−/− mice at 24 hpi, and levels were even higher at 48 hpi (Fig. 5A and B). Moreover, increased amounts of IL-1β, IL-12p40, IL-23p19, and IFN-γ were detected at 48 hpi (Fig. 5C to F).
FIG 5.
IL-10 modulates proinflammatory cytokine production during KP35 pneumonia. (A to F) The production of TNF-α, IL-6, IL-1β, IL-12p40, IL-23p19, and IFN-γ in BALF, as indicated, from WT and IL-10−/− mice was analyzed by Luminex technology at 24 and 48 hpi. *, P < 0.05, for a comparison of results of both groups at each time point (two-way ANOVA with a Tukey posttest).
IL-10 provided by MDSCs is important for KP35 clearance, prevention of lung injury, and host survival.
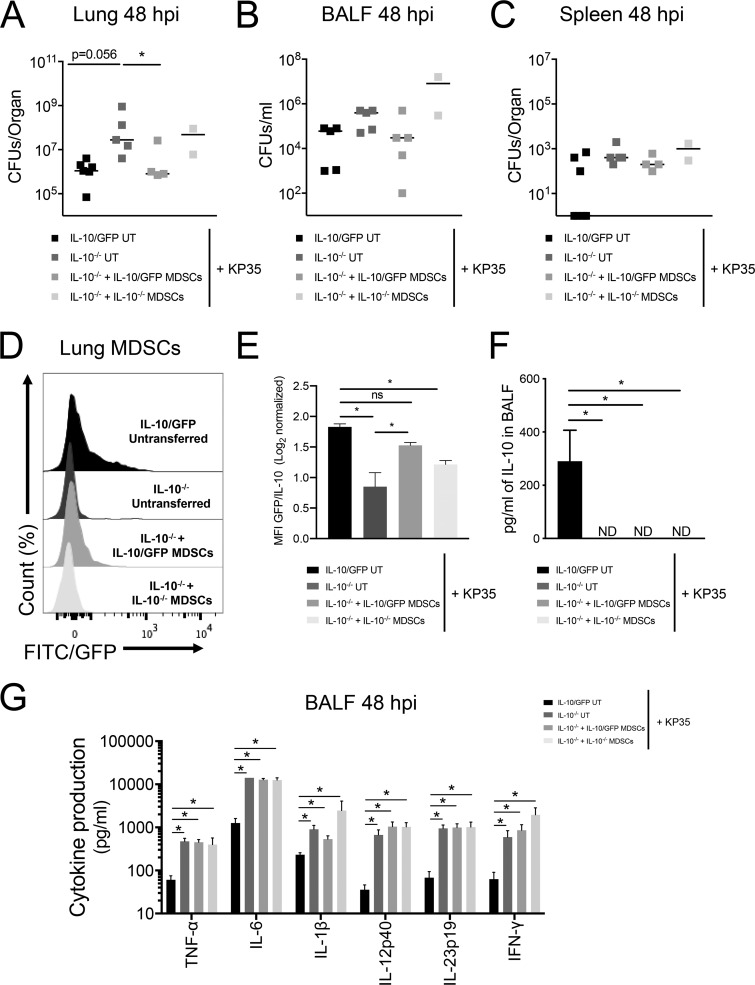
MDSCs from bone marrow of IL-10/GFP mice (BM-MDSCs) were treated and differentiated with granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) over 5 days and then intranasally transferred into IL-10−/− mice. Published data demonstrate that these cells express the typical MDSC markers, are not able to clear bacteria, and suppress T cell and neutrophil function (4, 16, 17). Consistently in this report, differentiated cells expressed the characteristic surface markers of MDSCs (CD11b, Ly6C, Ly6G, F4/80, CD115, major histocompatibility complex class II [MHC-II], and CD11c) and were not able to kill KP35 in vitro (Fig. S4). Intranasal transfer of BM-MDSCs able to produce IL-10 in IL-10−/− mice at 1 day prior to infection improved KP35 clearance capacity in the lung tissue (Fig. 6A) but not in BALF (Fig. 6B) or spleen (Fig. 6C). Even though total IL-10 production (in picograms/milliliter) was not detected in BALF of IL-10−/− mice with or without BM-MDSC transfer, we specifically detected the production of IL-10 by transferred IL-10/GFP-expressing BM-MDSCs (IL-10/GFP BM-MDSCs) in the lungs of IL-10−/− mice by flow cytometry (Fig. 6D to F). Production of IL-10, however, was not enough to modulate the production of proinflammatory cytokines, which remained higher in all IL-10−/− groups than in control mice without transferred IL-10/GFP (Fig. 6G).
FIG 6.
IL-10 produced by BM-MDSCs is critical for restoring bactericidal capacity in IL-10−/− mice. BM-MDSCs from IL-10−/− and IL-10/GFP VertX mice were generated in vitro. One day before the infection (day −1), 1 × 106 BM-MDSCs were transferred intranasally into IL-10−/− mice. At day 0 each group was intranasally infected with 1 × 108 CFU of KP35, and at 48 h postinfection, mice were euthanized. (A to C) Bacterial burden in lung tissue, spleen, and BALF was determined. (D and E) IL-10 production by MDSCs was identified by flow cytometry in the lung tissue. (F and G) Cytokine production in BALF was quantified by Luminex assay. *, P < 0.05, for a comparison of results for each parameter (by one-way ANOVA with a Tukey posttest). UT, untransferred.
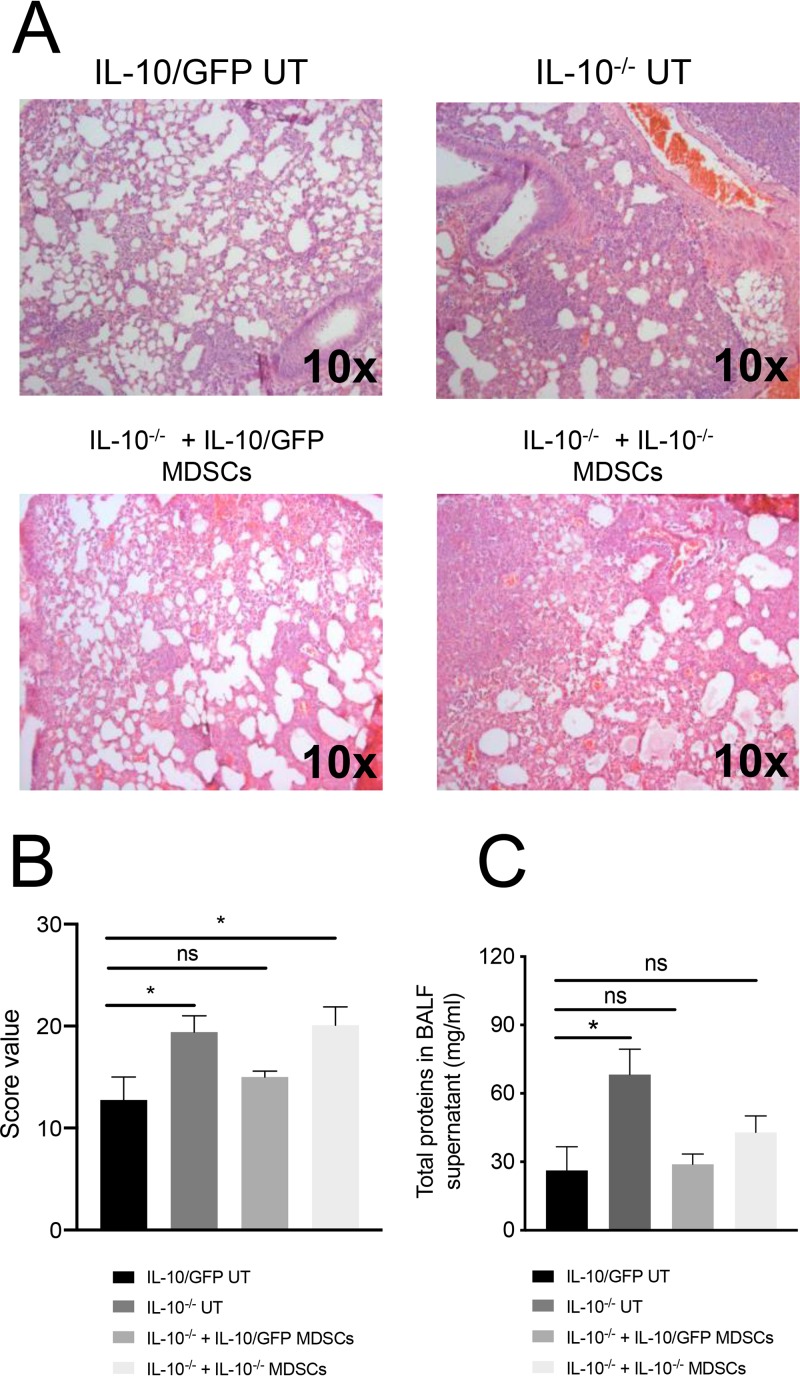
IL-10 production by transferred IL-10/GFP BM-MDSCs not only improved KP35 clearance but also slightly reduced lung injury. In fact, IL-10−/− mice that received IL-10/GFP BM-MDSCs presented a lung histopathological score equivalent to that of mice that did not receive IL-10/GFP as well as equivalent amounts of total BALF proteins (Fig. 7).
FIG 7.
IL-10 produced by BM-MDSCs modulates lung injury during KP35 pneumonia. BM-MDSCs from IL-10−/− and IL-10/GFP VertX mice were generated in vitro. One day before the infection (day −1), 1 × 106 BM-MDSCs were transferred intranasally into IL-10−/− mice. At day 0 each group was intranasally infected with 1 × 108 CFU of KP35; at 48 h postinfection, mice were euthanized and lung histopathology was assessed through H&E staining (A). Lung injury was quantified through a histopathological score (B) and through the quantification of total proteins in the BALF (C). *, P < 0.05; ns, not significant, for a comparison of results for each parameter (by one-way ANOVA with a Tukey posttest).
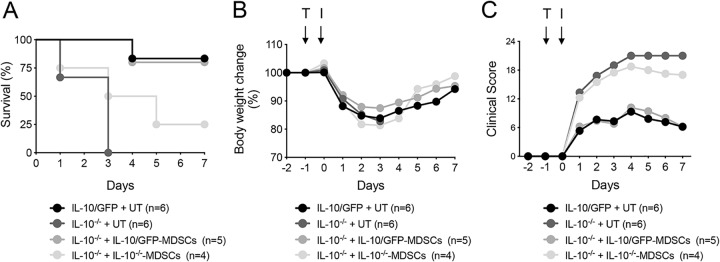
When IL-10−/− mice received IL-10/GFP BM-MDSCs, they showed increased survival (P < 0.05) (Fig. 8A) and reduced symptomatology (Fig. 8B and C) (P < 0.05), which was equivalent to levels in untransferred IL-10/GFP mice (Fig. 8). Interestingly, adoptive transfer of IL-10−/− mouse BM-MDSCs slightly increased host survival from 0% to 25% although these mice still presented an elevated clinical score (Fig. 8). Adoptive transfer of BM-MDSCs from IL-10/GFP or IL-10−/− mice to IL-10/GFP-recipient mice did not influence the resistance observed in these mice (data not shown). Cellular infiltration was not affected with the exception of reduced neutrophils in the total lung homogenate (Fig. S5A) but not in the BALF (Fig. S5B).
FIG 8.
IL-10 produced by BM-MDSCs is critical for host survival during KP35 pneumonia. (A to C) BM-MDSCs from IL-10−/− and IL-10/GFP VertX mice were generated in vitro. One day before the infection (day −1), 1 × 106 BM-MDSCs were transferred (T) intranasally into receptor IL-10−/− mice. At day 0 each group was intranasally infected (I) with 1 × 108 CFU of KP35, and survival rate, body weight, and disease severity, as indicated, were followed over 7 days. Survival rate was evaluated with a log rank test. Disease severity levels between IL-10−/− mice (untransferred [UT]) and IL-10−/− mice that received IL-10/GFP BM-MDSCs by transfer were compared by two-way ANOVA with a Tukey posttest.
DISCUSSION
CRKP-ST258 is a major health concern worldwide (2). Available data describe major differences between CRKP-ST258 and KPPR1 strains in terms of pathogenesis, virulence, and clinical manifestations (Fig. 9A and B) (4, 18, 19). In terms of virulence, the 50% lethal dose (LD50) of KPPR1 in mice is between 3 × 103 and 1.8 × 104 CFU in wild-type mice (19, 20), in contrast to levels for CRKP-ST258 isolates, for which the LD50 is much higher, typically around 1 × 108 CFU (4, 18). Moreover, CRKP-ST258 can establish chronic and persistent infections (4). Under these conditions, CRKP-ST258 colonization allows adaptation to environmental conditions, such as antibiotic treatment, as well as to the host defenses (4, 5).
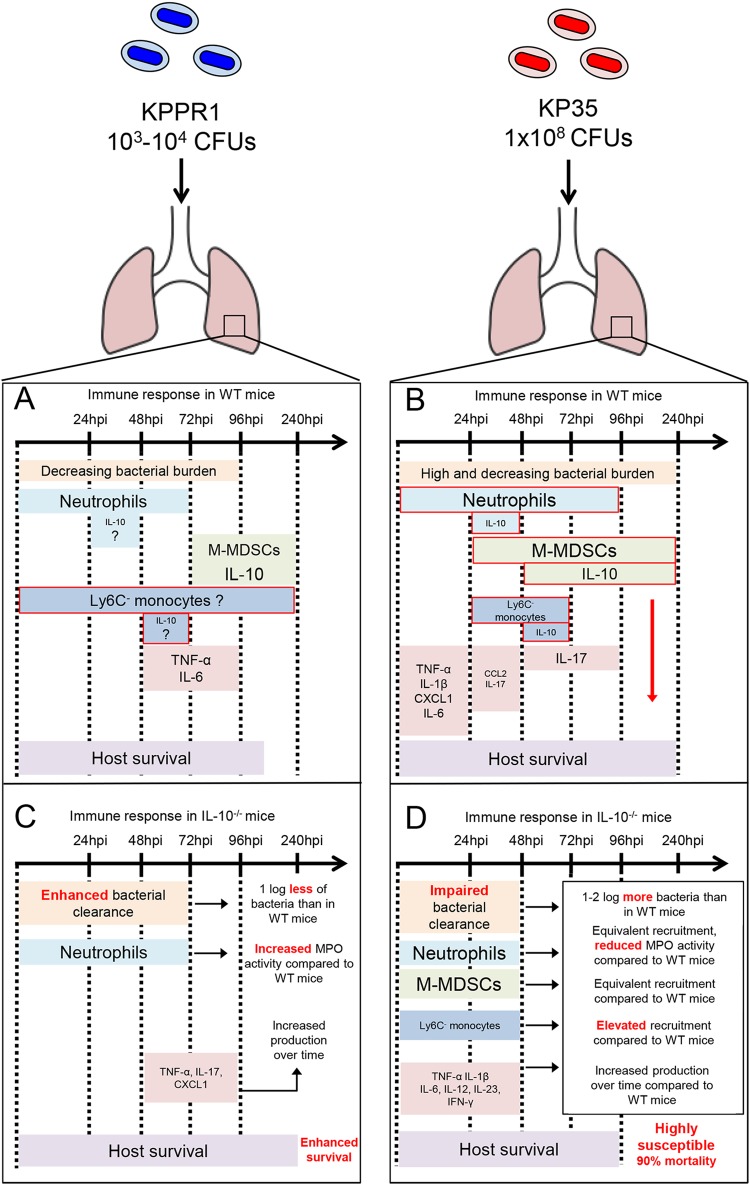
FIG 9.
Graphical summary of major differences of the immune responses and the role of IL-10 during KP35 and KPPR1 pneumonia. Major differences in the immune responses against sublethal doses of KPPR1 and KP35 have been described in WT and IL-10−/− mice. (A) The immune response against KPPR1 is characterized by a rapid bacterial clearance and a rapid infiltration of neutrophils but a much slower infiltration of M-MDSCs at 72 hpi. WT mice infected with KPPR1 produce TNF-α and IL-6 from 48 hpi to at least 96 hpi. (B) During KP35 pneumonia the immune response is characterized by early recruitment of neutrophils and M-MDSCs able to produce IL-10 and other suppressive effectors. As a consequence, these mice present weaker production of proinflammatory cytokines and a high but decreasing bacterial burden in the lung tissue. Incredibly, these mice are very resistant to KP35 and CRKP-ST258. (C) In the absence of IL-10 the immune response against KPPR1 is more efficient, presenting enhanced bacterial clearance in the first 72 hpi, increased neutrophil activation and proinflammatory cytokine production, and enhanced survival compared to levels in mice able to produce IL-10. (D) On the other hand, in the absence of IL-10, KP35 infection leads to a diminished bacterial clearance capacity in the first 48 hpi and equivalent numbers of neutrophils and M-MDSCs but impaired neutrophil activation and increased proinflammatory cytokine production during the first 48 hpi, with around 90% mortality at 4 dpi.
One of the most remarkable characteristics of CRKP-ST258 murine pneumonia is the nature of the immune response that is evoked, characterized by reduced production of proinflammatory cytokines despite robust recruitment of neutrophils during the first 24 hpi (4). This attenuated inflammatory response correlates with elevated levels of IL-10 in the BALF (4). In the present report, we have demonstrated that during KP35 infection, the production of IL-10 by the early recruited M-MDSCs directs the immune response and is critical for host survival. Specifically, the production of IL-10 prevents uncontrolled cytokine production, reduces lung damage, and improves bacterial clearance although this comes at the expense of a delayed bacterial clearance in the airways.
IL-10 is a major anti-inflammatory cytokine that modulates excessive lung inflammation during infection (21). Lack of IL-10 during acute pulmonary infections typically leads to more severe disease, characterized by increased proinflammatory cytokine production, increased recruitment/activity of neutrophils and, in some cases, increased mortality (7, 21). Whether lack of IL-10 increases or decreases host susceptibility depends on the specific pathogen. For example, during Streptococcus pneumoniae pneumonia, a high infective dose unmasks a critical role of IL-10 that favors host survival (21). However, during KPPR1 infection, IL-10 neutralization led to improved host survival (Fig. 9C) (22). The data provided in this report are consistent with previous reports on the role of IL-10 in lung immunity but also highlight major differences from what has been described in classical KPPR1 pneumonia (7, 22). During a sublethal infection of KPPR1, the lack of IL-10 enhances neutrophil activity and bacterial clearance although it also leads to elevated cytokine production, lung damage, and more severe pathology (Fig. 9C) (7). Similarly, lack of IL-10 during KP35 pneumonia also led to increased production of proinflammatory cytokines and greater lung damage; however, we observed elevated bacterial burden in the airways, impaired neutrophil activation, and increased host mortality during KP35 infection (Fig. 9D). This highlights important differences between CRKP-ST258 and KPPR1 in the nature of the innate immune responses that are activated in response to these common pathogens (Fig. 9A and B) and also unmasks important differences in the roles of IL-10 between CRKP-ST258 and KPPR1 pneumonia (Fig. 9C and D).
The kinetics of IL-10 production and the cellular source of IL-10 are also major differences between pneumonia caused by CRKP-ST258 and that caused by other pathogens. IL-10 production is induced in myeloid cells after PAMP recognition by pattern recognition receptors (PRRs) (11). In this scenario, any leukocyte may potentially produce IL-10. During S. pneumoniae pneumonia, macrophages and T cells have been identified as the main IL-10-producing cells (23). IL-10 induction is rapid, and high levels can be detected at 48 hpi when Ly6C+ monocytes are absent from the lung tissue (21). In contrast to pulmonary infection with Mycobacterium bovis in which the early production of IL-10 is driven mostly by neutrophils at 24 hpi (24), during KPPR1 infection recruited MDSCs were identified as an important IL-10 source but not until 72 hpi when the infection was being resolved (7). The immune response to CRKP-ST258 is distinct from responses to these other infections; once M-MDSCs are recruited to the lung tissue at 48 hpi, they immediately start the production of IL-10 and continue for at least 10 dpi. This kinetic of IL-10-producing MDSCs is consistent with the reduced production of proinflammatory cytokines at 48 hpi observed in WT but not IL-10−/− mice. Our data are consistent with previous reports that describe the ability of IL-10 to inhibit TNF-α and IL-12 production (25, 26) and IL-1β through the suppression of the NLRP3-inflammasome (27).
IL-10 has a major role in protecting the host from damaging inflammation. In response to bacterial infection, MDSC recruitment and activity are typically associated with resolution and/or chronicity (28). This has been described during pulmonary infections caused by Pseudomonas aeruginosa (28) and nonpulmonary infections with major pathogens such as Staphylococcus aureus, Salmonella enterica, and Porphyromonas gingivalis (10, 13, 14, 29–34). In each of these cases, recruited MDSCs inhibit the innate and adaptive immune responses, causing chronic infection. This immunosuppressive role of IL-10-producing MDSCs in the lungs is important during lipopolysaccharide (LPS) endotoxic shock and post-influenza pneumococcal pneumonia (35). IL-10 also affects other types of immune cells. IL-10 production protects B cells from death, improving their antigen presentation and antibody isotype change (36–39). The relevance of B cell function in K. pneumoniae infections was demonstrated in a recent study that showed that capsule antibodies enhance the serum-mediated clearance of CRKP-ST258 and promote neutrophil-mediated phagocytic clearance of these organisms (40).
In our model, we provide strong evidence that the production of IL-10 by M-MDSCs and also by other myeloid cells is a major mechanism of lung protection during CRKP-ST258 pneumonia. The data presented give important insights into how IL-10 modulates lung injury; first, lung hematoxylin and eosin (H&E) staining and further analyses led us to identify major differences in the area compromised, the luminal exudate and parenchyma pneumonia in lungs from WT and IL-10−/− mice during the first 48 h. Total BALF proteins, another approximation of lung injury, were found at higher concentration in BALF of IL-10−/− mice during the first 48 h. When we looked at proinflammatory cytokine production, IL-10−/− mice presented elevated levels of proinflammatory cytokines involved in lung permeabilization and injury such as TNF-α, IL-1β, IL-6, and IL-12p40. Finally, the fact that transfer of BM-MDSCs able to produce IL-10 reduced lung inflammation in IL-10−/− mice in response to KP35 pneumonia gave important information regarding the key role of these cells and IL-10 in the modulation of lung injury.
It is quite remarkable that other cells such as Ly6C− monocytes and eosinophils are also important sources of IL-10 during KP35 pneumonia. Ly6C− monocytes, also known as alternative or nonclassical monocytes (41), have been identified as important cells involved in wound repair (41, 42). Specifically, nonclassical monocytes can differentiate into anti-inflammatory macrophages (42) and promote the repair of the endothelium, disrupted either by neutrophil extravasation or by excessive production of TNF-α or IL-1β (43). The increased amount of nonclassical monocytes observed in the lung tissue and the BALF of IL-10−/− mice at 48 hpi could explain why these mice show reduced TUNEL signal and MPO activity compared to levels in WT mice at 48 hpi. Whether these cells are modulating cell apoptosis or neutrophil activity, their suppressive activities appear to be IL-10 independent. More experiments need to be done to establish the real contribution of nonclassical monocytes during CRKP-ST258 pneumonia. On the other hand, suppressive eosinophils, called Eo-MDSCs, have been recently identified during S. aureus infection (10). These cells suppress T cell function through the production of nitric oxide (10). It is not clear whether the eosinophils identified in our model can be defined as Eo-MDSCs, and more studies are needed to establish the real contribution of these cells in the modulation of the inflammatory response during CRKP-ST258 pneumonia.
The increased survival and bacterial clearance observed in the lung tissue of IL-10−/− mice receiving a transfer of IL-10-producing BM-MDSCs confirm the relevant role of these cells during KP35 pneumonia. However, the fact that the transfer of these cells did not reduce the production of proinflammatory cytokines also indicates that the transient IL-10 production by other cells such as neutrophils, Ly6C− monocytes, and eosinophils is required to achieve an optimal and efficient immune response in terms of host survival, lung damage, and bacterial clearance.
Finally, even though the transfer of non-IL-10-producing BM-MDSCs did not have an effect on bacterial clearance and lung inflammation, it had a positive impact on host survival by either delaying host mortality or improving host survival from 0% to 25%. It has been reported that BM-MDSCs can efficiently suppress T cell activation through the activity of arginase-1, iNOS, Cox2, vascular endothelial growth factor (VEGF), Ido1, and other factors (28). It is highly possible that these molecules also have an important role in the modulation of the inflammatory response during KP35 infection; however, more studies are needed to establish the real contribution and the mechanisms involved.
CRKP-ST258 infections have become a common and challenging clinical problem. As illustrated by the accumulating data from murine models that appear to reflect what is observed in patients, these organisms are highly resistant to normal immune clearance and often cause persistent infections. Our data demonstrate that during KP35 pneumonia, the production of IL-10 by the recruited MDSC population is critical in the modulation of the proinflammatory immune response and in the prevention of host mortality although persistent infection is promoted.
MATERIALS AND METHODS
Mice.
C57BL/6 wild-type (WT) mice and B6.129P2-Il10tm1Cgn (IL-10−/−) and B6(Cg)-Il10tm1.1Karp (IL-10-GFP) mice were originally obtained from The Jackson Laboratories (Bar Harbor, ME) and maintained in the animal facility of Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile. All experimental protocols were reviewed and approved by the Scientific Ethical Committee for Animal and Environment Care (Comité Etico Científico en Cuidado Animal y Ambiente) of the Pontificia Universidad Católica de Chile (protocols number 150721004 and 150721005). All animal work was performed according to the Guide for Care and Use of Laboratory Animals (44). Experiments using animals were overseen by trained personnel daily, and a clinical score was evaluated as previously described (21, 45).
Klebsiella pneumoniae infection.
KP35 was grown on Luria-Bertani (LB) medium until an optical density of 0.5 was reached. Then, 1 ml was centrifuged and resuspended at a final concentration of 1 × 108 CFU on 50 μl of phosphate-buffered saline (PBS). Six to 8-week-old WT, IL-10−/−, and IL-10/GFP VertX mice were anesthetized with a ketamine (80 mg/kg)-xylazine (4 mg/kg) solution and intranasally infected. Body weight was recorded daily; animals that lost more than 20% of their original weight were euthanized.
BALF collection.
One milliliter of bronchoalveolar lavage fluid (BALF) was obtained from uninfected and infected mice at 24 and 48 h postinfection (hpi) by instilling 2 ml of sterile 1× PBS into a cannulated mouse trachea. Then the cellular fraction was separated from the supernatant by centrifugation at 1,800 rpm for 20 min. The cellular fraction was used to measure the infiltrating immune cells, and supernatant was used to quantify cytokine production, MPO activity, and total protein.
Flow cytometry.
Whole lungs were recovered at different times postinfection, minced with sterile scissors, and incubated in PBS-collagenase (1 mg/ml) for 1 h at 37°C, with agitation. Homogenized lungs were filtered using a 70-μm-pore-size filter, and cells were recovered by centrifugation in sterile 1× PBS and washed once with ammonium chloride-potassium (ACK) lysis buffer and once with PBS. In parallel, cells from bronchoalveolar lavage fluid (BALF) were recovered by centrifugation and washed with ACK lysis buffer. Then, cells from lungs and BALF were stained with Live/Dead fixable viability stain 510 (BD Biosciences) in PBS. Cells were washed twice, resuspended in PBS–2% fetal bovine serum (FBS), and stained with the following mix of antibodies: CD45-BV786 (BD Biosciences), CD11b-phycoerythrin (PE) (BD Biosciences), Siglec-F-PE-CF594 (BD Biosciences), Ly6G-Alexa Fluor 700 (Biolegend), CD11c-PE-Cy7 (BD Biosciences), MHC-II–BV650 (BD Biosciences), CD64-Alexa Fluor 647 (Biolegend), Ly6C-BV605 (BD Biosciences), and CD24-BV421 (BD Biosciences). Before analysis, CountBright absolute counting beads (Life Technologies) were added to quantify each cell population. The gating strategy used to identify each cell type is described in Fig. S6 in the supplemental material.
Lung histopathological analysis.
Complete lungs were removed from mice at 24 and 48 hpi, fixed in 4% paraformaldehyde, dehydrated through the exposure to different concentrations of ethanol, and infiltrated with paraffin wax. Then, lung tissue was embedded in paraffin wax blocks. Paraffin blocks were sliced with a conventional microtome in 5-μm sections with an angle blade of 10°. Then, cut slices were placed at 60°C for 1 h, mounted in glass slides, and stained with hematoxylin and eosin (H&E) and TUNEL. Once stained, samples were analyzed in a Leica SCN400 slide scanner for digital imaging (magnification, ×40) or in an Olympus BX51 light microscope at a magnification of ×10; total lung digital images were collected and analyzed with the ImageScope, version 11, software and ImageJ software, respectively. TUNEL staining was quantified as the percentage of TUNEL-positive area in each group using ImageJ software (https://imagej.nih.gov/ij/docs/examples/stained-sections/index.html). Lung damage was quantified in H&E-stained sections by a pathologist according to parameters listed in Table S1.
Protein and cytokine quantification in BALF.
BALF from IL-10−/− and WT mice was collected and centrifuged at 1,800 rpm for 15 min. BALF supernatant was collected and stored at −80°C until used. Total BALF protein content was measured by a conventional Bradford protein assay. Levels of IL-10, TNF-α, IL-6, IL-1β, IL-12p40, and IFN-γ (in picograms/milliliter) were measured on a Luminex 200 instrument (Merck Millipore), using a mouse magnetic Luminex screening assay (R&D Systems), according to the manufacturer’s instructions.
MPO activity in BALF.
Myeloperoxidase (MPO) chlorination activity was evaluated in BALF using an Enzchek MPO activity assay kit according to the manufacturer’s instructions. Briefly, in a 96-well plate, BALF obtained from uninfected and infected WT and IL-10−/− mice was exposed to a mixture of 5 mM H2O2 and the probe 3′-(p-aminophenyl) fluorescein (APF) at room temperature in the dark for 10 min. Fluorescence (emission wavelength, 530 nm; excitation wavelength, 485 nm) was quantified in a Cytation3 plate reader.
Bone marrow-derived MDSC generation, adoptive transfer, and bactericidal capacity.
Bone marrow MDSCs were generated as previously described (16, 17) with some modifications. Briefly, total bone marrow cells were purified from donor IL-10−/− and IL-10/GFP VertX mice. Cells were counted, and 2.5 × 106 bone marrow cells were cultured for 5 days in 100-mm culture dishes with 10 ml of RPMI 1640 medium supplemented with 2 mM l-glutamine, 10 mM HEPES, 20 μM 2-mercaptoethanol (2-ME), 1% streptomycin-penicillin, 10% heat-inactivated FBS, and 40 ng/ml of G-CSF and GM-CSF. After 5 days, cells were washed with sterile 1× PBS and counted. A total of 1 × 106 cells were intranasally transferred to receptor IL-10−/− and IL-10/GFP VertX mice. At 24 h posttransfer, mice were intranasally infected with 1 × 108 CFU of KP35. Survival rate and severity of disease were followed over 7 days. Before the adoptive transfer, BM-MDSCs were stained and phenotypically characterized by flow cytometry using the following antibodies: CD11b-peridinin chlorophyll protein (PerCP)-Cy5 (BD), Ly6C-BV605 (BD), Ly6G-AF700 (BioLegend), F4/80-allophycocyanin (APC) (BD), CD115-PE (BD), MHC-II-BV650 (BD), and CD11c-PE-Cy7 (BD). To evaluate the bactericidal capacity of BM-MDSCs, 105 BM-MDSCs were seeded in 24-well plates for 1 h at 37°C to allow cell adherence. After 1 h, BM-MDSCs were infected with KP35 (multiplicity of infection [MOI] of 1), and at 4 h postinfection the cells were placed on ice and lysed with distilled H2O. Serial dilutions were seeded on LB agar, and plates were incubated overnight.
Statistical analyses.
A one-way analysis of variance (ANOVA) test followed by a Tukey multiple-comparison test was performed to analyze IL-10-producing cells, total BALF protein concentration, and bacterial burden in BALF, lung, and spleen over time in IL-10/GFP-expressing infected mice and for all the comparisons for BM-MDSC transfer experiments. Survival curves were compared using a log rank test. In all cases, a P value of <0.05 was considered statistically significant. Two-way ANOVA, followed by Tukey multiple-comparison tests, was performed to analyze cellular infiltration, lung histopathology, cytokine production, and MPO activity in WT and IL-10−/− mice at 24 and 48 hpi. Bacterial burdens in lung, BALF, and spleen between WT and IL-10−/− mice were compared with a Student t test (P value of <0.05). All comparisons were performed using the GraphPad Prism software, version 7.0a, for Macintosh (GraphPad software).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from Fondo Nacional de Ciencia y Tecnología de Chile (grant no. 1170964), the Millennium Institute on Immunology and Immunotherapy (P09/016-F), the Comisión Nacional de Investigación Científica y Tecnológica (grant no. 21140214), and NIH (R35 HL135800 and NIH K08 HL138289).
We thank Luis Larrondo and Consuelo Olivares, from the Faculty of Biological Sciences, Pontificia Universidad Católica de Chile, for providing access to the plate reader Cytation 3 and Alexis Kalergis from the Faculty of Biological Sciences, Pontificia Universidad Católica de Chile, for providing access to the flow cytometer BDFortessa.
We declare that we have no financial conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00665-18.
REFERENCES
- 1.Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 2.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paczosa MK, Mecsas J. 2016. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn D, Peñaloza H, Wang Z, Wickersham M, Parker D, Patel P, Koller A, Chen EI, Bueno SM, Uhlemann AC, Prince A. 2016. Acquired resistance to innate immune clearance promotes Klebsiella pneumoniae ST258 pulmonary infection. JCI Insight 1:e89704. doi: 10.1172/jci.insight.89704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi SD, Porter AR, Dorward DW, Brinkworth AJ, Chen L, Kreiswirth BN, DeLeo FR. 2016. Phagocytosis and killing of carbapenem-resistant ST258 Klebsiella pneumoniae by human neutrophils. J Infect Dis 213:1615–1622. doi: 10.1093/infdis/jiw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI. 2017. Myeloid-derived suppressor cells. Cancer Immunol Res 5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, Khare A, Levy DE, Lee JS, Mallampalli RK, Chan YR, Ray A, Ray P. 2013. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol 6:189–199. doi: 10.1038/mi.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. 2016. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldmann O, Beineke A, Medina E. 2017. Identification of a novel subset of myeloid-derived suppressor cells during chronic staphylococcal infection that resembles immature eosinophils. J Infect Dis 216:1444–1451. doi: 10.1093/infdis/jix494. [DOI] [PubMed] [Google Scholar]
- 11.Penaloza HF, Schultz BM, Nieto PA, Salazar GA, Suazo I, Gonzalez PA, Riedel CA, Alvarez-Lobos MM, Kalergis AM, Bueno SM. 2016. Opposing roles of IL-10 in acute bacterial infection. Cytokine Growth Factor Rev 32:17–30. doi: 10.1016/j.cytogfr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Cyktor JC, Turner J. 2011. Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect Immun 79:2964–2973. doi: 10.1128/IAI.00047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam JW, Kullas AL, Mena P, Bliska JB, van der Velden AW. 2014. CD11b+ Ly6Chi Ly6G- immature myeloid cells recruited in response to Salmonella enterica serovar Typhimurium infection exhibit protective and immunosuppressive properties. Infect Immun 82:2606–2614. doi: 10.1128/IAI.01590-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tebartz C, Horst SA, Sparwasser T, Huehn J, Beineke A, Peters G, Medina E. 2015. A major role for myeloid-derived suppressor cells and a minor role for regulatory T cells in immunosuppression during Staphylococcus aureus infection. J Immunol 194:1100–1111. doi: 10.4049/jimmunol.1400196. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Simmonds A, Greenman M, Sullivan SB, Tanner JP, Sowash MG, Whittier S, Uhlemann AC. 2015. Population structure of Klebsiella pneumoniae causing bloodstream infections at a New York City tertiary care hospital: diversification of multidrug-resistant isolates. J Clin Microbiol 53:2060–2067. doi: 10.1128/JCM.03455-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, Tolar J, Ochoa AC, Blazar BR. 2010. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. 2010. Tumor-induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity 32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Tzouvelekis LS, Miriagou V, Kotsakis SD, Spyridopoulou K, Athanasiou E, Karagouni E, Tzelepi E, Daikos GL. 2013. KPC-producing, multidrug-resistant Klebsiella pneumoniae sequence type 258 as a typical opportunistic pathogen. Antimicrob Agents Chemother 57:5144–5146. doi: 10.1128/AAC.01052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong H, Carter RA, Leiner IM, Tang YW, Chen L, Kreiswirth BN, Pamer EG. 2015. Distinct contributions of neutrophils and CCR2+ monocytes to pulmonary clearance of different Klebsiella pneumoniae strains. Infect Immun 83:3418–3427. doi: 10.1128/IAI.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawlor MS, Hsu J, Rick PD, Miller VL. 2005. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol Microbiol 58:1054–1073. doi: 10.1111/j.1365-2958.2005.04918.x. [DOI] [PubMed] [Google Scholar]
- 21.Peñaloza HF, Nieto PA, Muñoz-Durango N, Salazar-Echegarai FJ, Torres J, Parga MJ, Alvarez-Lobos M, Riedel CA, Kalergis AM, Bueno SM. 2015. Interleukin-10 plays a key role in the modulation of neutrophils recruitment and lung inflammation during infection by Streptococcus pneumoniae. Immunology 146:100–112. doi: 10.1111/imm.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. 1995. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol 155:722–729. [PubMed] [Google Scholar]
- 23.Duell BL, Tan CK, Carey AJ, Wu F, Cripps AW, Ulett GC. 2012. Recent insights into microbial triggers of interleukin-10 production in the host and the impact on infectious disease pathogenesis. FEMS Immunol Med Microbiol 64:295–313. doi: 10.1111/j.1574-695X.2012.00931.x. [DOI] [PubMed] [Google Scholar]
- 24.Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. 2007. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity 27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong L, Jordan N, Millar A. 1996. Interleukin 10 (IL-10) regulation of tumour necrosis factor alpha (TNF-alpha) from human alveolar macrophages and peripheral blood monocytes. Thorax 51:143–149. doi: 10.1136/thx.51.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahim SS, Khan N, Boddupalli CS, Hasnain SE, Mukhopadhyay S. 2005. Interleukin-10 (IL-10) mediated suppression of IL-12 production in RAW 264.7 cells also involves c-rel transcription factor. Immunology 114:313–321. doi: 10.1111/j.1365-2567.2005.02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y, Vent-Schmidt J, McGeough MD, Wong M, Hoffman HM, Steiner TS, Levings MK. 2015. Tr1 cells, but not Foxp3+ regulatory t cells, suppress NLRP3 inflammasome activation via an IL-10-dependent mechanism. J Immunol 195:488–497. doi: 10.4049/jimmunol.1403225. [DOI] [PubMed] [Google Scholar]
- 28.Peñaloza HF, Álvarez D, Muñoz-Durango N, Schultz BM, González PA, Kalergis AM, Bueno SM. 2018. The role of myeloid-derived suppressor cells in chronic infectious diseases and the current methodology available for their study. J Leukoc Biol doi: 10.1002/JLB.MR0618-233R. [DOI] [PubMed] [Google Scholar]
- 29.Rieber N, Brand A, Hector A, Graepler-Mainka U, Ost M, Schafer I, Wecker I, Neri D, Wirth A, Mays L, Zundel S, Fuchs J, Handgretinger R, Stern M, Hogardt M, Doring G, Riethmuller J, Kormann M, Hartl D. 2013. Flagellin induces myeloid-derived suppressor cells: implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease. J Immunol 190:1276–1284. doi: 10.4049/jimmunol.1202144. [DOI] [PubMed] [Google Scholar]
- 30.Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, Muirhead DE, Kielian T. 2014. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J Immunol 192:3778–3792. doi: 10.4049/jimmunol.1303408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heim CE, Vidlak D, Kielian T. 2015. Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. J Leukoc Biol 98:1003–1013. doi: 10.1189/jlb.4VMA0315-125RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oz HH, Zhou B, Voss P, Carevic M, Schroth C, Frey N, Rieber N, Hector A, Hartl D. 2016. Pseudomonas aeruginosa airway infection recruits and modulates neutrophilic myeloid-derived suppressor cells. Front Cell Infect Microbiol 6:167. doi: 10.3389/fcimb.2016.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su L, Xu Q, Zhang P, Michalek SM, Katz J. 2017. Phenotype and function of myeloid-derived suppressor cells induced by Porphyromonas gingivalis infection. Infect Immun 85:e00213-17. doi: 10.1128/IAI.00213-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada KJ, Heim CE, Aldrich AL, Gries CM, Staudacher AG, Kielian T. 2018. Arginase-1 expression in myeloid cells regulates S. aureus planktonic but not biofilm infection. Infect Immun 86:e00206-18. doi: 10.1128/IAI.00206-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Namkoong H, Ishii M, Fujii H, Yagi K, Asami T, Asakura T, Suzuki S, Hegab AE, Kamata H, Tasaka S, Atarashi K, Nakamoto N, Iwata S, Honda K, Kanai T, Hasegawa N, Koyasu S, Betsuyaku T. 2018. Clarithromycin expands CD11b+ Gr-1+ cells via the STAT3/Bv8 axis to ameliorate lethal endotoxic shock and post-influenza bacterial pneumonia. PLoS Pathog 14:e1006955. doi: 10.1371/journal.ppat.1006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malisan F, Briere F, Bridon JM, Harindranath N, Mills FC, Max EE, Banchereau J, Martinez-Valdez H. 1996. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J Exp Med 183:937–947. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shparago N, Zelazowski P, Jin L, McIntyre TM, Stuber E, Pecanha LM, Kehry MR, Mond JJ, Max EE, Snapper CM. 1996. IL-10 selectively regulates murine Ig isotype switching. Int Immunol 8:781–790. doi: 10.1093/intimm/8.5.781. [DOI] [PubMed] [Google Scholar]
- 38.Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. 2002. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood 100:4537–4543. doi: 10.1182/blood-2002-05-1525. [DOI] [PubMed] [Google Scholar]
- 39.Tangye SG, Ferguson A, Avery DT, Ma CS, Hodgkin PD. 2002. Isotype switching by human B cells is division-associated and regulated by cytokines. J Immunol 169:4298–4306. doi: 10.4049/jimmunol.169.8.4298. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi SD, Porter AR, Freedman B, Pandey R, Chen L, Kreiswirth BN, DeLeo FR. 2018. Antibody-mediated killing of carbapenem-resistant ST258 Klebsiella pneumoniae by human neutrophils. mBio 9:e00297-18. doi: 10.1128/mBio.00297-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CH, Petri B, Ransohoff RM, Charo IF, Jenne CN, Kubes P. 2015. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med 212:447–456. doi: 10.1084/jem.20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, Jordan BT, Peirce SM, Botchwey EA. 2017. Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci Rep 7:447. doi: 10.1038/s41598-017-00477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ginhoux F, Jung S. 2014. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 44.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC. [Google Scholar]
- 45.Mook-Kanamori B, Geldhoff M, Troost D, van der Poll T, van de Beek D. 2012. Characterization of a pneumococcal meningitis mouse model. BMC Infect Dis 12:71. doi: 10.1186/1471-2334-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.