Influenza kills 30,000 to 40,000 people each year in the United States and causes 10 times as many hospitalizations. A common complication of influenza is bacterial superinfection, which exacerbates morbidity and mortality from the viral illness.
KEYWORDS: influenza, interferons, MRSA, pneumococcus, pneumonia
ABSTRACT
Influenza kills 30,000 to 40,000 people each year in the United States and causes 10 times as many hospitalizations. A common complication of influenza is bacterial superinfection, which exacerbates morbidity and mortality from the viral illness. Recently, methicillin-resistant Staphylococcus aureus (MRSA) has emerged as the dominant pathogen found in bacterial superinfection, with Streptococcus pneumoniae a close second. However, clinicians have few tools to treat bacterial superinfection. Current therapy for influenza/bacterial superinfection consists of treating the underlying influenza infection and adding various antibiotics, which are increasingly rendered ineffective by rising bacterial multidrug resistance. Several groups have recently proposed the use of the antiviral cytokine interferon lambda (IFN-λ) as a therapeutic for influenza, as administration of pegylated IFN-λ improves lung function and survival during influenza by reducing the overabundance of neutrophils in the lung. However, our data suggest that therapeutic IFN-λ impairs bacterial clearance during influenza superinfection. Specifically, mice treated with an adenoviral vector to overexpress IFN-λ during influenza infection exhibited increased bacterial burdens upon superinfection with either MRSA or S. pneumoniae. Surprisingly, adhesion molecule expression, antimicrobial peptide production, and reactive oxygen species activity were not altered by IFN-λ treatment. However, neutrophil uptake of MRSA and S. pneumoniae was significantly reduced upon IFN-λ treatment during influenza superinfection in vivo. Together, these data support the theory that IFN-λ decreases neutrophil motility and function in the influenza-infected lung, which increases the bacterial burden during superinfection. Thus, we believe that caution should be exercised in the possible future use of IFN-λ as therapy for influenza.
INTRODUCTION
In the United States, influenza results in 300,000 hospitalizations and 30,000 to 40,000 deaths each year (1). While many people are infected with influenza virus annually, most recover without interventional therapy. However, in severe cases, bacterial superinfection is a common complication, causing increased morbidity and mortality. Tissue specimens from the 1918 influenza pandemic, which occurred before the advent of antibiotics, reveal a staggering 95% rate of bacterial superinfection (2). That pandemic claimed an estimated 675,000 lives in the United States and millions of lives worldwide (1). To this day, bacterial superinfection is responsible for over one-third of pediatric deaths from influenza each year in the United States (3).
Streptococcus pneumoniae is considered the “classical” pathogen found during influenza superinfection; in postmortem cultures of lung tissue from victims of the 1918–1919 pandemic, 23.5% were found to have S. pneumoniae, and another 8.1% were found to have Staphylococcus aureus (2). The earliest murine models of influenza superinfection focused on the use of S. pneumoniae, as it readily replicates in the mouse lung, and even a small inoculum will eventually kill mice (4). However, many groups have been moving toward modeling superinfection with influenza and methicillin-resistant S. aureus (MRSA), due to the recent rise in MRSA superinfections relative to S. pneumoniae superinfections. In fact, from 2004 to 2012, including the 2009 H1N1 pandemic, 48% of bacterial isolates from pediatric patients who died from influenza were identified to be methicillin-resistant S. aureus, with another 23% identified as methicillin-sensitive S. aureus as well as 22% identified as S. pneumoniae (5). Data for the current influenza season show that these trends continue. In the 2017–2018 season, one-half of pediatric patients who died from influenza tested positive for bacterial superinfection. A total of 54.6% of bacterial isolates from these patients were typed to be S. aureus, with 50% of those isolates testing positive for methicillin resistance (6). While mice clear MRSA more easily than S. pneumoniae from the lung, immune responses to each bacterium differ in both humans and mice (7–9).
Six to seven days after influenza infection, both mice and humans exhibit a “window of susceptibility” to bacterial infection (10, 11). The influenza virus infects respiratory epithelial cells, which in response produce high levels of type I interferon (IFN) (IFN-α and IFN-β) to induce an antiviral gene program in the lung (12). Type I IFN has been shown to mediate susceptibility during influenza to superinfection with myriad bacteria, including the most common superinfecting pathogens, S. aureus and S. pneumoniae, as well as Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa (13–15). While type I IFN plays a significant role in the pathogenesis of bacterial superinfection, type III IFN (IFN-λ, interleukin-28 [IL-28], and IL-29) is produced at even higher levels than type I IFN in response to influenza (16).
IFN-λ is thought to exert its antiviral effects through the same JAK/STAT pathway as type I IFN (17, 18) and similarly induces antiviral gene expression in the respiratory epithelium (19). Researchers have recently proposed IFN-λ as a therapeutic for influenza (20, 21), demonstrating that mice treated early during influenza infection with pegylated IFN-λ2 (PEG-IFN-λ2) display reduced immunopathology and mortality during influenza infection. However, it was recently shown that mice lacking the receptor for IFN-λ display reduced bacterial burdens during influenza/S. aureus superinfection (22). This observation, combined with the copious similarities between type I and III IFNs, suggests that IFN-λ therapy during influenza may exacerbate bacterial superinfection. Thus, we investigated the consequences of therapeutic IFN-λ on susceptibility to bacterial superinfection during influenza.
RESULTS
IFN-λ treatment increases bacterial burden during influenza superinfection.
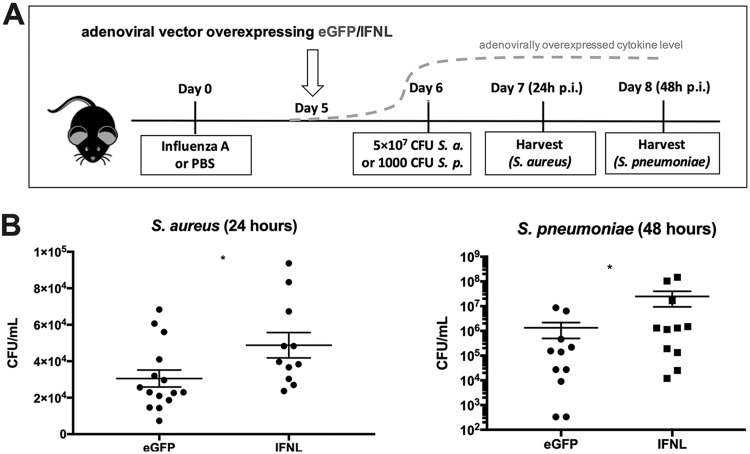
To test the effect of IFN-λ therapy on bacterial superinfection during influenza, we treated mice with an adenoviral vector to overexpress IFN-λ (Ad-IFN-λ) or control green fluorescent protein (GFP) (Ad-GFP) in the lung. Ad-IFN-λ treatment 5 days following influenza A/PR/8/34 H1N1 infection resulted in a 4-fold increase in the level of IFN-λ protein in bronchoalveolar lavage fluid (BALF), from a mean of 740.9 ± 56.81 to 3,114 ± 583.8 pg/ml (see Fig. S1 in the supplemental material). Surprisingly, levels of type I and II IFNs were unchanged by IFN-λ treatment (Fig. S1). Twenty-four hours following adenoviral treatment, mice were challenged with 5 × 107 CFU MRSA USA300 and harvested 24 h later or challenged with 1 × 103 CFU Streptococcus pneumoniae and harvested 48 h later (Fig. 1A). While mice quickly clear S. aureus from the lung and will survive this inoculum of MRSA, S. pneumoniae will replicate in the murine lung, eventually causing death. Moreover, both bacteria have been important causes of secondary bacterial infection during influenza throughout history and are still relevant today. Thus, we assessed the effect of Ad-IFN-λ treatment on both influenza/MRSA and influenza/S. pneumoniae superinfection. In mice given either bacterial challenge, Ad-IFN-λ treatment significantly increased the bacterial burden in the lung 24 h after bacterial infection (Fig. 1B). The influenza viral burden was unchanged (Fig. S2). Together, these data suggest that IFN-λ therapy has negative consequences for bacterial superinfection due to modulation of the immune response to influenza.
FIG 1.
IFN-λ treatment increases bacterial burden during influenza superinfection. Mice were infected with 25 PFU influenza A/PR/8/34 H1N1 or PBS as a control and 5 days later given 1 × 1010 VP of an adenoviral vector to overexpress IFN-λ (Ad-IFN-λ) or GFP (Ad-GFP). Twenty-four hours following adenoviral treatment, mice were challenged with 5 × 107 CFU methicillin-resistant Staphylococcus aureus (S. a.) USA300 (n = 4; four independent experiments) and harvested 1 day later or challenged with 1 × 103 CFU Streptococcus pneumoniae (S. p.). *, P < 0.05. (A) Diagram showing the experimental design. (B) Lung bacterial burden of S. aureus 24 h after S. aureus challenge and lung bacterial burden of S. pneumoniae 48 h after S. pneumoniae challenge.
IFN-λ treatment does not affect antimicrobial peptide expression in the lung.
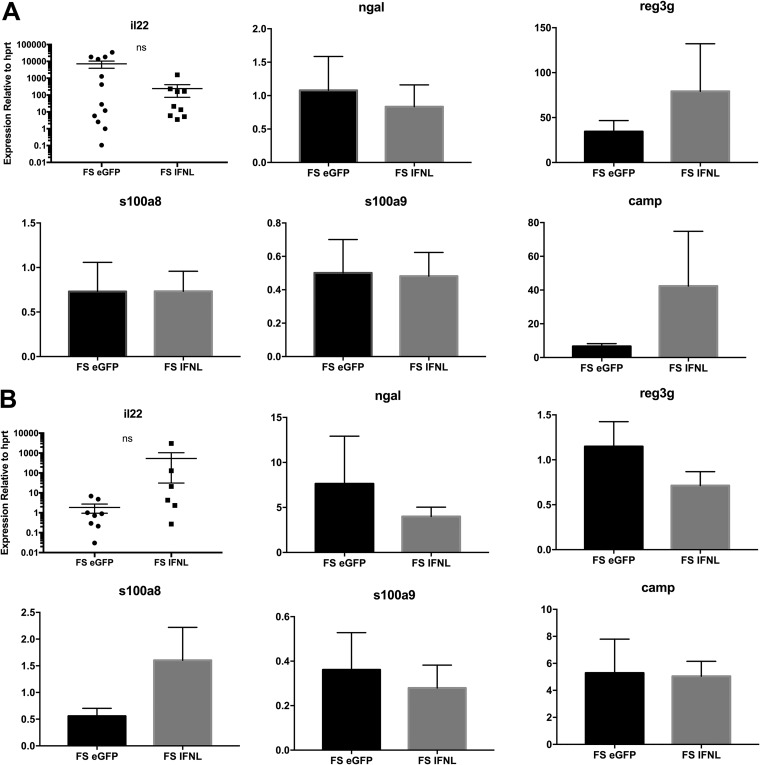
Little is known about the role of IFN-λ in superinfection. It was previously reported that mice lacking the receptor for IFN-λ have decreased bacterial burdens during both pulmonary MRSA infection and influenza/MRSA superinfection. This decrease in bacteria correlated with increased expression of IL-22 and its associated antimicrobial peptide NGAL (neutrophil gelatinase-associated lipocalin) (22). The release of antimicrobial peptides is a key bacterial defense mechanism for both myeloid cells and the lung epithelium during S. aureus infection (23, 24). Moreover, we have previously shown that administration of exogenous NGAL decreases the bacterial burden in influenza/MRSA superinfection (23). Thus, we tested the effect of IFN-λ treatment on antimicrobial peptide expression during influenza/MRSA superinfection. IFN-λ treatment did not alter the expression of NGAL in BALF cells or whole lung (Fig. 2) or the associated type 17 cytokine IL-17 (Fig. S3) or IL-22 (Fig. 2). Expression levels of other lung antimicrobial peptides (regenerating islet-derived protein 3 gamma and cathelicidin antimicrobial peptide) and calprotectin (s100a8:s100a9 dimer), a neutrophil antimicrobial peptide that suppresses S. aureus growth (25), were also unchanged in both BALF cells and whole lung (Fig. 2). These results suggest that IFN-λ does not increase the bacterial burden by inhibiting antimicrobial peptide expression.
FIG 2.
IFN-λ treatment does not affect antimicrobial peptide expression in the lung. Mice were infected with 25 PFU influenza virus A/PR/8/34 H1N1 or PBS as a control and 5 days later given 1 × 1010 VP of Ad-IFN-λ or Ad-GFP. Twenty-four hours following adenoviral treatment, mice were challenged with 5 × 107 CFU MRSA and harvested 1 day later. (A) Whole lung lobes were snap-frozen in liquid nitrogen, and RNA was extracted and assayed by real-time PCR for expression of IL-22 and the antimicrobial peptides ngal, reg3g, s100a8, s100a9, and camp (n = 4; two to three independent experiments). (B) Bronchoalveolar lavage fluid was centrifuged to pellet cells. The supernatant was removed, and the cell pellet was frozen at −80°C for RNA extraction and subsequent real-time PCR. FS, MRSA infection preceded by influenza virus infection for 6 days. ns, not significant.
IFN-λ treatment decreases neutrophils in bronchoalveolar lavage fluid during superinfection.
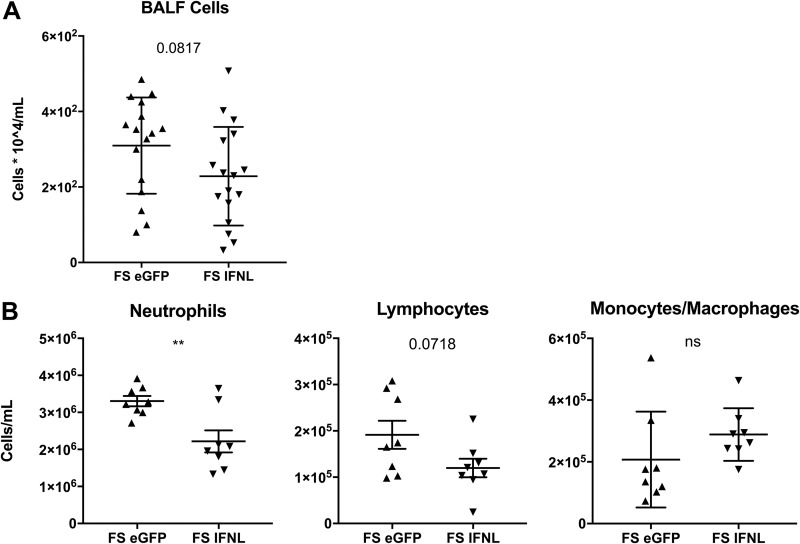
No other reported data were available for how IFN-λ affects infection with bacteria that commonly complicate influenza. Thus, we examined the known role of IFN-λ in influenza infection alone as well as in other immunopathological diseases to hypothesize how it may affect influenza/bacterial superinfection. IFN-λ has been shown to be a potent suppressor of neutrophil trafficking and function in murine models of collagen-induced arthritis, colitis, and, most importantly, influenza infection (21, 26, 27). To our knowledge, the role of IFN-λ in neutrophil recruitment toward sites of bacterial infection is unknown. To assess the effect of IFN-λ on neutrophils responding to pulmonary MRSA infection (with and without preceding influenza infection), we characterized infiltrating cells in the BALF by differential counting. Although the total number of cells in BALF was not significantly changed by IFN-λ treatment, a trend of reduced cell numbers was apparent (Fig. 3A). Specifically, neutrophil recruitment to the airway was significantly reduced (Fig. 3B). During MRSA pneumonia alone, IFN-λ treatment did not alter the bacterial burden or BALF cell numbers (Fig. S4). These data suggest that IFN-λ increases the bacterial burden during influenza/MRSA superinfection by suppressing neutrophil recruitment to the airway.
FIG 3.
IFN-λ treatment decreases neutrophils in bronchoalveolar lavage fluid during influenza/MRSA superinfection. Mice were infected with influenza, given Ad-IFN-λ or Ad-GFP 5 days later, and challenged with 5 × 107 CFU MRSA 24 h later, as described in the legend of Fig. 1. (A) Total cell counts from bronchoalveolar lavage fluid (n = 4; four independent experiments). (B) Differential counting of BALF cells (n = 4; two independent experiments). **, P < 0.01; ns, not significant.
IFN-λ treatment increases granulocyte chemokines in the lung but does not affect neutrophil production or trafficking.
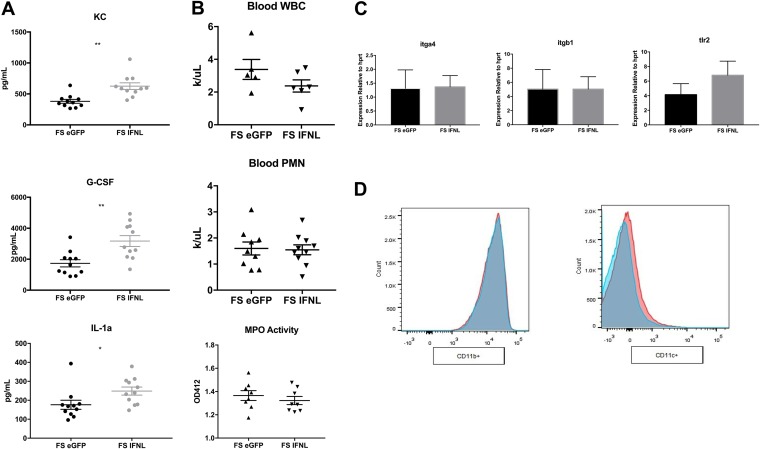
We sought to determine the mechanism by which IFN-λ treatment suppresses airway neutrophil recruitment. First, we measured levels of inflammatory cytokines in the lung. IFN-λ treatment significantly increased the levels of only 3 of the 23 assayed cytokines, CXCL1/keratinocyte chemoattractant (KC), granulocyte colony-stimulating factor (G-CSF), and IL-1α (Fig. 4A and Fig. S3). All three are neutrophil chemokines, suggesting a “frustrated” granulocyte chemokine release by the lung in response to a defect in neutrophil production or trafficking. Neutropenia is a common side effect of type I IFN administration (28, 29), which led us to think that IFN-λ might decrease numbers of circulating neutrophils as well. To test for a defect in neutrophil production, we assayed blood from mice with an automated hematology instrument. IFN-λ treatment did not result in decreases in total leukocyte or neutrophil counts in whole blood (Fig. 4B), confirming that the reduction of neutrophils in BALF upon IFN-λ treatment is not due to leukopenia or neutropenia.
FIG 4.
IFN-λ treatment increases levels of granulocyte chemokines in the lung but does not affect neutrophil production, trafficking, or ROS generation. Mice were infected with influenza, given Ad-IFN-λ or Ad-GFP 5 days later, and challenged with 5 × 107 CFU MRSA 24 h later, as described in the legend of Fig. 1. (A) Cytokine production in lung homogenates (n = 4; three independent experiments). (B) Total white blood cell (WBC) counts and differential counting of blood neutrophils (n = 4; two independent experiments). The left lobe of the lung was perfused with PBS, homogenized, and assayed for myeloperoxidase (MPO) activity as a measure of reactive oxygen species generation (n = 4; three independent experiments). PMN, polymorphonuclear leukocytes. (C) Real-time PCR of the bronchoalveolar lavage cell pellet for neutrophil adhesion molecules and bacterial receptors (n = 4; three independent experiments). (D) Expression of CD11b on live CD45+ Ly6G+ lung neutrophils (n = 4; two independent experiments). Ad-IFN-λ-treated samples are displayed in pink, and Ad-GFP-treated samples are in blue. *, P < 0.05; **, P < 0.01.
To test if IFN-λ treatment alters neutrophil trafficking to the lung, we assessed the expression of adhesion molecules in the BALF cell pellet, which is mainly composed of neutrophils (80 to 90%) (Fig. 3B). For successful emigration to the lung, neutrophils must first tether to the lung endothelium by binding integrin α4β1 to endothelial vascular cell adhesion molecule 1 (VCAM-1) (30). Firm binding and adhesion are established by CD11/CD18 binding to endothelial intracellular adhesion molecule 1 (ICAM-1). Importantly, CD18 blockade reduces neutrophil migration to pulmonary S. aureus infection, as the dependence on CD18 for neutrophil emigration to the lung is highly stimulus specific (31). IFN-λ treatment did not alter the expression of the genes encoding the neutrophil trafficking receptor VLA-4 (igta4:itgb1) (Fig. 4C) or the expression of CD11b/c on the surface of Ly6G+ lung neutrophils (Fig. 4D), suggesting that IFN-λ likely does not alter integrin/CD11-mediated neutrophil adhesion to the lung endothelium.
These data suggest that IFN-λ therapy does not suppress neutrophil production in the bone marrow and does not inhibit neutrophil recruitment to the airway by altering adhesion molecule expression. Interestingly, neutrophils from IFN-λ-treated mice exhibit reduced chemotaxis in both TAXIscan and transwell assays toward leukotriene B4 (LTB4) (26, 27), suggesting a general defect in neutrophil movement. Patients with reduced neutrophil chemotaxis or oxidative burst exhibit increased susceptibility to S. aureus infection (32). Moreover, to evade the immune system, S. aureus produces factors to inhibit both neutrophil chemotaxis and phagocytosis (33–35). As IFN-λ has been shown to inhibit neutrophil chemotaxis both ex vivo and in vitro, we asked if neutrophil phagocytosis of S. aureus was also reduced by IFN-λ treatment.
To kill MRSA, neutrophils must first sense the bacteria through Toll-like receptor 2 (TLR2) recognition of peptidoglycan. Treatment with a TLR2 agonist reduces bacterial burden and mortality during MRSA pneumonia in mice (36), and mice lacking TLR2 have altered neutrophil recruitment and bacterial phagocytosis in various infection models (37). Additionally, neutrophil killing of S. aureus relies upon the intracellular generation of reactive oxygen species (ROS), and patients defective in ROS generation are more susceptible to S. aureus infection (32). Neither TLR2 expression (Fig. 4C) nor antimicrobial peroxidase activity (Fig. 4B) was affected by IFN-λ overexpression. These data suggest that IFN treatment may not alter killing of S. aureus by neutrophils. However, data from other models suggest that IFN-λ treatment specifically impairs neutrophil movement (27), demonstrating that IFN-λ impairs neutrophil chemotaxis both in vitro and ex vivo.
IFN-λ does not affect bacterial TLR expression or ROS production in neutrophils but strongly inhibits bacterial phagocytosis during superinfection.
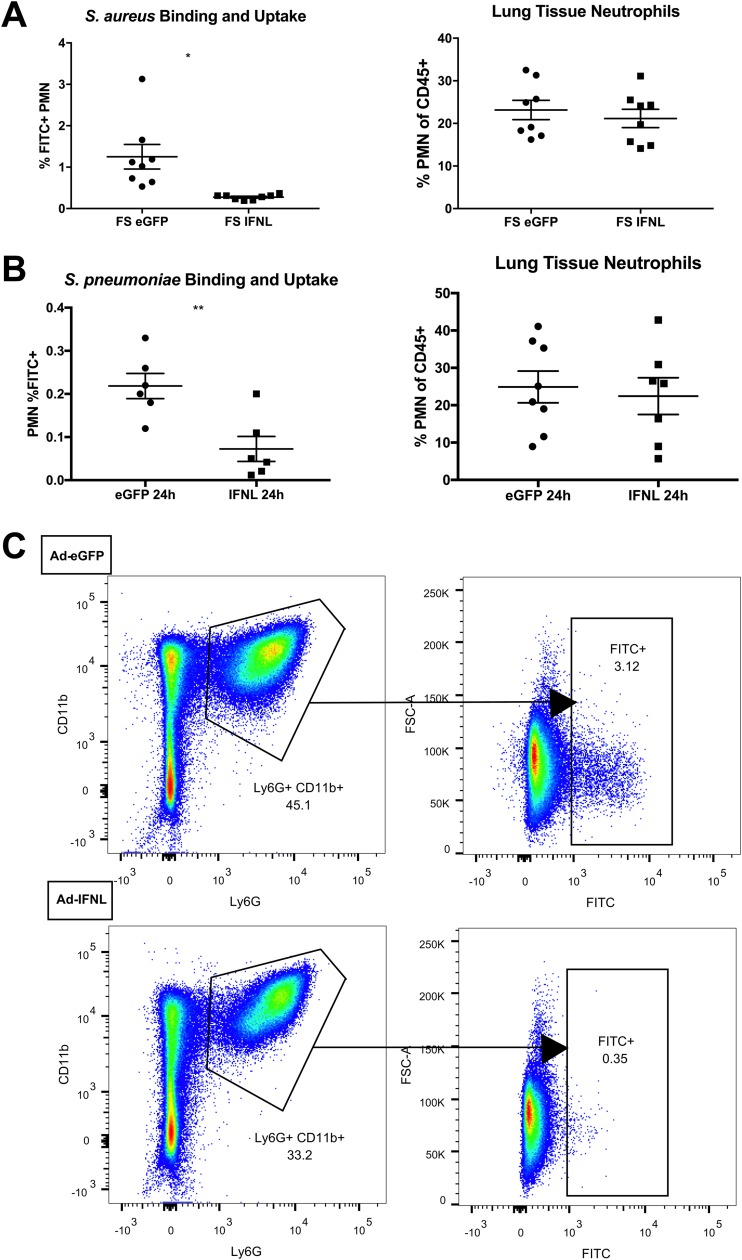
To assess the ability of neutrophils to phagocytose bacteria in vivo, we challenged influenza-infected mice with fluorescein isothiocyanate (FITC)-labeled MRSA or S. pneumoniae and analyzed lungs by flow cytometry 24 or 48 h later. The percentage of FITC-positive (FITC+) neutrophils (defined as FITC+ live CD45+ CD11b+ Ly6G+ cells) in the lung decreased over 4-fold from 1.3% to 0.2% (Fig. 5A), implying that IFN-λ decreases neutrophil phagocytosis of MRSA during superinfection. Moreover, when mice were challenged with FITC-labeled S. pneumoniae, neutrophil phagocytosis was also markedly decreased (Fig. 5B). While IFN-λ overexpression impaired neutrophil infiltration into BALF (Fig. 3B), the percentage of neutrophils in lung tissue was not altered (Fig. 5A and B). However, flow cytometry analysis showed a striking decrease in neutrophil phagocytosis in vivo in IFN-λ-treated mice (Fig. 5C). Together with data reported for models of sterile inflammation and influenza infection alone (21, 26), these data suggest that IFN-λ generally reduces neutrophil movement, preventing chemotaxis to and phagocytosis of bacteria during influenza/bacterial superinfection.
FIG 5.
IFN-λ inhibits phagocytosis of MRSA during superinfection. (A and B) Mice were infected with influenza and 5 days later given Ad-IFN-λ or Ad-GFP as described in the legend of Fig. 1. Twenty-four hours later, mice were challenged with 5 × 107 CFU of FITC-labeled MRSA (A) or FITC-labeled S. pneumoniae (B) and sacrificed 1 day later. The left lobe of the lung was digested to a single-cell suspension and stained for flow cytometry. We report the percentage of FITC+ neutrophils as analyzed by flow cytometry (percentage of live CD45+ Ly6G+ CD11b+ cells that were FITC+) as well as the percentage of total immune cells in the lung that are neutrophils (percentage of live CD45+ cells that were Ly6G+ CD11b+) (n = 4; two independent experiments). (C) Representative flow cytometry plot showing the gating strategy for determining FITC positivity in neutrophils from mice treated with Ad-GFP or Ad-IFN-λ during influenza/MRSA superinfection. *, P < 0.05; **, P < 0.01. FSC, forward scatter.
DISCUSSION
IFN-λ has recently been proposed as a potentially attractive therapy for influenza (20, 21). There is a dire need for more influenza therapeutics, as a broadly effective vaccine has not yet been developed, and current therapies are both time restricted and limited in effect (38). With the continual mismatch of influenza vaccines to circulating viral strains (39) and the ever-present threat of another influenza pandemic, more broadly effective treatments for influenza are certainly necessary. Galani et al. have shown that pegylated IFN-λ (PEG-IFN-λ) therapy during influenza reduces immunopathology and mortality by reducing the cytokine storm and neutrophil infiltration (21). However, bacterial superinfection commonly complicates influenza, increasing morbidity and mortality. While reducing neutrophil recruitment to the lung ameliorates disease during influenza alone, these neutrophils are crucial for survival during Staphylococcus aureus lung infection (40). Moreover, mice lacking the IFN-λ receptor exhibit decreased bacterial burden during pulmonary MRSA infection as well as influenza/MRSA superinfection, suggesting that IFN-λ contributes to disease during bacterial infection of the lung (22). Especially with the rise of drug-resistant pathogens such as MRSA as secondary bacterial pathogens, considering the risk for superinfection when evaluating a therapeutic for influenza is critical.
We report that overexpression of IFN-λ during influenza results in an increased lung bacterial burden upon superinfection with either MRSA or Streptococcus pneumoniae. These data support findings by Planet et al. showing that mice lacking the IFN-λ receptor have lower bacterial burdens during pulmonary MRSA infection as well as influenza/MRSA superinfection (22). Specifically, Planet et al. found increases in the expression of IL-22 and its associated antimicrobial peptide lipocalin 2 in IFN-λ receptor knockout mice. We have previously shown that exogenous lipocalin 2 decreases the bacterial burden during influenza/MRSA superinfection (23). However, we found no change in IL-22 or lipocalin 2 expression upon IFN-λ overexpression. However, IFN-λ overexpression does not exactly recapitulate the phenotype of the total receptor knockout, as we see no difference in bacterial burdens during single S. aureus infection (see Fig. S4 in the supplemental material), while in the IFNLR1 knockouts, the S. aureus burden is decreased. It is also likely that there is a differential requirement for IL-22 and the associated antimicrobial peptide expression during transient IFN-λ overexpression versus constitutive IFN-λ receptor knockout. Thus, we investigated what else might be responsible for the acute IFN-λ-induced increase in the bacterial burden.
We demonstrate that IFN-λ decreases BALF neutrophil accumulation during influenza/MRSA superinfection. Galani et al. also showed a decrease in BALF neutrophils as well as total BALF cells, along with reduced peribronchial and parenchymal cell infiltration, upon PEG-IFN-λ administration during influenza infection alone. Notably, Galani et al. administered PEG-IFN-λ 2 days after viral infection, while we treated mice 5 days after viral infection, leading to a significant overexpression of IFN-λ at harvest 2 days later. While weight loss from our influenza virus infection mimicked the weight loss reported by Galani et al. with similar inocula (21, 41), treatment with IFN-λ 5 days after viral infection did not reproduce their reported reduction in viral burden. As current therapies for influenza decrease in effectiveness the later they are given during infection (38), our delay in treatment until 5 days after viral infection is likely responsible for this discrepancy. We did not observe a decrease in BALF neutrophil accumulation either 24 or 48 h following influenza/S. pneumoniae superinfection (Fig. S5), which suggests that the decrease in BALF neutrophils that we see in influenza/MRSA superinfection is not the cause of the increased bacterial burden.
Aside from differences in the timing of therapeutic intervention, bacterial superinfection drastically changes the immunological landscape of the lung. Interestingly, we saw no increase in bacterial burden upon IFN-λ treatment during MRSA pneumonia without preceding influenza (Fig. S4). This suggests that influenza-induced cytokines work in concert with IFN-λ during bacterial superinfection to reduce antibacterial immunity. Type I IFN is also broadly produced in response to influenza and strongly contributes to superinfection susceptibility (14). We expect that the high levels of type I IFN in the influenza-infected mouse may synergize with our administered IFN-λ to produce this increase in bacterial burden that we see during superinfection but not bacterial infection alone. Many other cytokines are induced by influenza and play significant roles in its pathogenesis, including type 17 cytokines (23) and IL-1 family members (42). There is likely an interactive role for these players as well in the complex cytokine environment of the influenza-infected lung.
During bacterial superinfection, IFN-λ therapy produced no significant decrease in type I IFN expression or protein levels. We also saw no decrease in tumor necrosis factor alpha (TNF-α), IFN-γ, CCL3, or CCL4 levels, which were reported by Galani et al. to be reduced by PEG-IFN-λ administration. Instead, IFN-λ treatment during bacterial superinfection specifically increased neutrophil chemokines in the lung, while BALF neutrophils were decreased. This is consistent with previous findings that neutrophil depletion during S. aureus pulmonary infection increases lung KC and G-CSF levels (23), suggesting a “frustrated” chemokine production by the lung in response to a lack of neutrophils.
The reduction in BALF neutrophils implies that IFN-λ induces a defect in neutrophil production or migration. However, upon assessment of circulating blood cells, we saw no difference in neutrophils or total leukocytes. IFN-λ has been shown to suppress neutrophil migration in vitro by transwell and EZTAXIscan assays (26, 27), suggesting a defect in the migration of neutrophils into airspaces where MRSA aggregates reside (43). Surprisingly, while IFN-λ treatment decreased BALF neutrophils, lung neutrophils were not altered, as measured by flow cytometry. It must be noted that this flow cytometry was performed on lavaged lungs, which may explain the disparity between these two measurements. Importantly, bronchoalveolar lavage samples only the epithelial surface of the respiratory tract (44), whereas flow cytometry was performed on a single-cell suspension digested from whole lung. Together, these data suggest that IFN-λ may specifically impair neutrophil migration across the lung epithelium.
Although IFN-λ treatment does not alter the number of neutrophils in the lung, it results in a marked decrease in neutrophil phagocytosis of MRSA in vivo. This effect of IFN-λ appears to be specific, as it alters phagocytosis but not myeloperoxidase activity or the expression of adhesion molecules. As the cytoskeletal changes of a cell responsible for in vivo migration mimic those necessary for phagocytosis, including actin remodeling and microtubule assembly, these data suggest that IFN-λ may be exerting a broader effect on neutrophil cellular motility and cytoskeletal rearrangement.
Together, these data strongly suggest that the use of IFN-λ as a therapeutic for influenza may result in adverse outcomes for patients who contract a secondary bacterial infection. We show that neutrophils, which are essential for the control of superinfecting pathogens (13), are reduced in BALF following IFN-λ treatment. Moreover, neutrophil binding and uptake of MRSA are reduced with IFN-λ treatment, which correlates with an increase in the MRSA burden in the lung. Importantly, IFN-λ administration also decreases binding and uptake of S. pneumoniae during influenza superinfection, which also correlates with an increase in the lung bacterial burden. Although IFN-λ may reduce influenza severity, both our data and the findings of other groups show that it worsens bacterial superinfection (22). While new therapeutics targeting influenza are sorely needed, it is crucial that we take the risk of bacterial superinfection into account when evaluating new treatments.
MATERIALS AND METHODS
Murine infections.
Six- to eight-week-old male C57BL/6 mice were purchased from Taconic Biosciences (Hudson, NY) and maintained under pathogen-free conditions. All studies were performed on age- and sex-matched mice. All animal studies were conducted with approval from the University of Pittsburgh Institutional Animal Care and Use Committee. All murine treatments (influenza virus, adenovirus, S. aureus, and S. pneumoniae) were administered by oropharyngeal aspiration.
Mice were infected with 25 PFU of influenza H1N1 A/PR/8/34 (41) or phosphate-buffered saline (PBS). Five days later, mice were treated with 1 × 1010 viral particles (VP) of an adenoviral vector overexpressing mIFN-λ3/IL-28B (Vector BioLabs, Malvern, PA) or the enhanced GFP (eGFP) control in 50 μl of PBS (Genome Editing, Transgenic, and Virus Core, University of Pittsburgh). One day later, mice were challenged with 5 × 107 CFU methicillin-resistant Staphylococcus aureus USA300 in 50 μl of PBS and harvested an additional 24 h later, or mice were challenged with 1 × 103 CFU Streptococcus pneumoniae serotype 3 (ATCC 6303) in 50 μl of PBS and harvested 48 later.
Bacterial labeling and quantification.
FITC labeling of S. aureus was performed as follows. Bacteria were grown overnight to stationary phase with shaking at 37°C in casein hydrolysate yeast extract-containing modified medium (1806 CCY modified medium; ATCC). After measurement of the optical density at 660 nm (OD660), 10 μl of 10 mg/ml FITC in dimethylformamide (DMF) was added to 1 ml of bacteria and incubated with shaking at room temperature for 1 h. Following incubation, bacteria were spun at 10,000 × g for 5 min and washed with PBS twice. FITC labeling of S. pneumoniae was performed as follows. Bacteria were grown for 6 h with shaking at 37°C in Todd-Hewitt broth (BD Biosciences, Franklin Lakes, NJ). Next, 100 μl of this culture was used to inoculate a 100-ml flask of Todd-Hewitt broth and grown for an additional 12 h with continued shaking at 37°C. After measurement of the OD600, 10 μl of 10 mg/ml FITC in DMFO was added to 1 ml of bacteria, and the mixture was incubated with shaking at room temperature for 1 h. Following incubation, bacteria were spun at 10,000 × g for 5 min and washed with PBS twice. FITC-labeled bacteria were resuspended in PBS to bring the concentration to 5 × 107 bacteria per 50 μl. The bacterial burden was determined by plating serial 10-fold dilutions of the lung homogenate (right upper lobe of the lung homogenized in 1 ml sterile PBS).
Analysis of lung inflammation.
At harvest, mouse lungs were lavaged with 1 ml sterile PBS. This lavage fluid was centrifuged at 10,000 × g for 5 min to pellet cells, and the supernatant was frozen for cytokine measurement by an enzyme-linked immunosorbent assay (ELISA) for IFN-β and IFN-λ (mouse IFN-β or IFN-λ2/3 DuoSet; R&D Systems, Minneapolis, MN). Cell pellets from lavage fluid were resuspended in 500 ml sterile PBS and counted on a hemocytometer to enumerate infiltrating cells. These cells were then either processed by cytospin and stained for differential counting or centrifuged again at 10,000 × g for 5 min and then immediately frozen at −80°C for RNA extraction.
The right upper lobe of each lung was mechanically homogenized and plated for bacterial CFU counting, and cytokines in lung homogenates were analyzed with the Bio-Plex Pro mouse cytokine 23-plex array (Bio-Rad, Hercules, CA). The right middle and lower lobes of each lung were snap-frozen in liquid nitrogen for RNA extraction.
For assessment of myeloperoxidase (MPO) activity, the left lobe of each lung was perfused with PBS until white, snap-frozen in liquid nitrogen, and then mechanically homogenized for MPO activity assessment (MPO activity assay kit [colorimetric]; Abcam, Cambridge, UK).
Flow cytometry.
To obtain a single-cell suspension, lungs were mechanically dissected and then incubated with shaking at 37°C for 30 min in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1 mg/ml collagenase. After collagenase treatment, tissue was forced through a 70-μm filter and treated with ACK buffer to lyse erythrocytes. The resulting single-cell suspension was pretreated with anti-CD16/32 for 5 min to block Fc receptor binding before incubation with fixable viability dye and fluorochrome-conjugated anti-surface marker monoclonal antibodies (mAbs) for 30 min at 4°C. The following antibodies were used: anti-CD45, anti-CD11b, anti-CD11c, anti-Ly6G, anti-SiglecF, anti-F4/80, anti-CD24, anti-CD64, and anti-major histocompatibility complex class II (MHC-II). Live/Dead fixable aqua stain (Life Technologies, Carlsbad, CA) was used to determine cell viability. Samples were collected using an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (vX.0.7; TreeStar, Ashland, OR). Flow gating began with doublet exclusion by comparing forward light-scatter area versus height and then debris exclusion by comparing forward versus side light scatter. Dead cells were excluded based on viability dye staining. Neutrophils were identified as CD45+ Ly6G+ cells.
Blood cell quantification.
Blood was taken from the heart via cardiac puncture upon tissue collection, placed into K2-EDTA tubes, and assayed within 30 min of recovery using a Hemavet 950FS hematology system (Drew Scientific, Miami Lakes, FL).
Real-time PCR.
RNA was isolated from whole lung lobes snap-frozen in liquid nitrogen using the Absolutely RNA miniprep kit (Agilent Technologies, Santa Clara, CA), and its concentration was analyzed by spectrophotometry (NanoDrop ND-1000; Thermo Fisher Scientific) or isolated from −80°C frozen bronchoalveolar lavage cell pellets using the MagMax-96 total RNA isolation kit (Invitrogen, Carlsbad, CA). RNA was reverse transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), which was assayed by real-time PCR for gene expression with Assay on Demand TaqMan primer and probe sets (Life Technologies, Grand Island, NY).
Statistical analysis.
Data were analyzed using GraphPad Prism 7 (GraphPad, La Jolla, CA). Analyses comparing two groups were performed by an unpaired t test with Welch’s correction, unless data were not parametrically distributed, in which case Mann-Whitney analysis was used. S. pneumoniae CFU data were log transformed before statistical analysis due to their distribution. Two-way analysis of variance (ANOVA) was used to compare repeated measures over time. Mortality data were analyzed by a log rank (Mantel-Cox) test. All figures show combined data from multiple replicate studies as means ± standard errors of the means (SEM). The indicated n values are numbers of animals per independent experiment. Statistical significance is indicated in the figure legends. P values of between 0.05 and 0.1 are displayed numerically.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by NHLBI grant R01HL107380 (J.F.A.) and NIAID grant T32AI089443 (H.E.R.).
Experiments were designed by H.E.R. and J.F.A. and carried out by H.E.R., C.C.M., W.Q.Z., and K.J.M. K.M.R. provided consultation on this project, and J.W. provided the influenza virus used in these experiments.
We have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00114-19.
REFERENCES
- 1.CDC. 2 November 2017. Past pandemics. CDC, Atlanta, GA: https://www.cdc.gov/flu/pandemic-resources/basics/past-pandemics.html. Accessed 16 February 2018. [Google Scholar]
- 2.Morens DM, Taubenberger JK, Fauci AS. 2008. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. 2018. Influenza-associated pediatric deaths in the United States, 2010-2016. Pediatrics 141:e20172918. doi: 10.1542/peds.2017-2918. [DOI] [PubMed] [Google Scholar]
- 4.McCullers JA, Webster RG. 2001. A mouse model of dual infection with influenza virus and Streptococcus pneumoniae. Int Congr Ser 1219:601–607. doi: 10.1016/S0531-5131(01)00631-8. [DOI] [Google Scholar]
- 5.Wong KK, Jain S, Blanton L, Dhara R, Brammer L, Fry AM, Finelli L. 2013. Influenza-associated pediatric deaths in the United States, 2004-2012. Pediatrics 132:796–804. doi: 10.1542/peds.2013-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. 2018. Influenza-associated pediatric mortality. CDC, Atlanta, GA: https://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed 16 February 2018. [Google Scholar]
- 7.Slupsky CM, Cheypesh A, Chao DV, Fu H, Rankin KN, Marrie TJ, Lacy P. 2009. Streptococcus pneumoniae and Staphylococcus aureus pneumonia induce distinct metabolic responses. J Proteome Res 8:3029–3036. doi: 10.1021/pr900103y. [DOI] [PubMed] [Google Scholar]
- 8.Preu L, Bischoff M, Veith NT, Rosenbruch M, Theegarten D, Laschke MW, Meier C, Tschernig T. 2016. Initial host response to bacteria in the murine lung differs between Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pneumoniae. Inflammation 39:513–517. doi: 10.1007/s10753-015-0274-2. [DOI] [PubMed] [Google Scholar]
- 9.Strehlitz A, Goldmann O, Pils MC, Pessler F, Medina E. 2018. An interferon signature discriminates pneumococcal from staphylococcal pneumonia. Front Immunol 9:1424. doi: 10.3389/fimmu.2018.01424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCullers JA, Rehg JE. 2002. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis 186:341–350. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 11.McNamee LA, Harmsen AG. 2006. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun 74:6707–6721. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenmann J. 1982. From interference to interferon: a brief historical introduction. Philos Trans R Soc Lond B Biol Sci 299:3–6. doi: 10.1098/rstb.1982.0101. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Moltedo B, Moran TM. 2012. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J Virol 86:12304–12312. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. 2011. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 186:1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee B, Robinson KM, McHugh KJ, Scheller EV, Mandalapu S, Chen C, Di YP, Clay ME, Enelow RI, Dubin PJ, Alcorn JF. 2015. Influenza-induced type I interferon enhances susceptibility to Gram-negative and Gram-positive bacterial pneumonia in mice. Am J Physiol Lung Cell Mol Physiol 309:L158–L167. doi: 10.1152/ajplung.00338.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jewell NA, Cline T, Mertz SE, Smirnov SV, Flano E, Schindler C, Grieves JL, Durbin RK, Kotenko SV, Durbin JE. 2010. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J Virol 84:11515–11522. doi: 10.1128/JVI.01703-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 18.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 19.Crotta S, Davidson S, Mahlakoiv T, Desmet CJ, Buckwalter MR, Albert ML, Staeheli P, Wack A. 2013. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog 9:e1003773. doi: 10.1371/journal.ppat.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson S, McCabe TM, Crotta S, Gad HH, Hessel EM, Beinke S, Hartmann R, Wack A. 2016. IFNlambda is a potent anti-influenza therapeutic without the inflammatory side effects of IFNalpha treatment. EMBO Mol Med 8:1099–1112. doi: 10.15252/emmm.201606413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galani IE, Triantafyllia V, Eleminiadou EE, Koltsida O, Stavropoulos A, Manioudaki M, Thanos D, Doyle SE, Kotenko SV, Thanopoulou K, Andreakos E. 2017. Interferon-lambda mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity 46:875.e6–890.e6. doi: 10.1016/j.immuni.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Planet PJ, Parker D, Cohen TS, Smith H, Leon JD, Ryan C, Hammer TJ, Fierer N, Chen EI, Prince AS. 2016. Lambda interferon restructures the nasal microbiome and increases susceptibility to Staphylococcus aureus superinfection. mBio 7:e01939-15. doi: 10.1128/mBio.01939-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson KM, McHugh KJ, Mandalapu S, Clay ME, Lee B, Scheller EV, Enelow RI, Chan YR, Kolls JK, Alcorn JF. 2014. Influenza A virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production. J Infect Dis 209:865–875. doi: 10.1093/infdis/jit527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rigby KM, DeLeo FR. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 26.Blazek K, Eames HL, Weiss M, Byrne AJ, Perocheau D, Pease JE, Doyle S, McCann F, Williams RO, Udalova IA. 2015. IFN-lambda resolves inflammation via suppression of neutrophil infiltration and IL-1beta production. J Exp Med 212:845–853. doi: 10.1084/jem.20140995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broggi A, Tan Y, Granucci F, Zanoni I. 2017. IFN-lambda suppresses intestinal inflammation by non-translational regulation of neutrophil function. Nat Immunol 18:1084–1093. doi: 10.1038/ni.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 29.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958–965. doi: 10.1016/S0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 30.Tasaka S, Richer SE, Mizgerd JP, Doerschuk CM. 2002. Very late antigen-4 in CD18-independent neutrophil emigration during acute bacterial pneumonia in mice. Am J Respir Crit Care Med 166:53–60. doi: 10.1164/rccm.2105034. [DOI] [PubMed] [Google Scholar]
- 31.Ramamoorthy C, Sasaki SS, Su DL, Sharar SR, Harlan JM, Winn RK. 1997. CD18 adhesion blockade decreases bacterial clearance and neutrophil recruitment after intrapulmonary E. coli, but not after S. aureus. J Leukoc Biol 61:167–172. doi: 10.1002/jlb.61.2.167. [DOI] [PubMed] [Google Scholar]
- 32.Miller LS, Cho JS. 2011. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ, Heezius EC, Poppelier MJ, Van Kessel KP, van Strijp JA. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith EJ, Visai L, Kerrigan SW, Speziale P, Foster TJ. 2011. The Sbi protein is a multifunctional immune evasion factor of Staphylococcus aureus. Infect Immun 79:3801–3809. doi: 10.1128/IAI.05075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dossett JH, Kronvall G, Williams RC Jr, Quie PG. 1969. Antiphagocytic effects of staphylococcal protein A. J Immunol 103:1405–1410. [PubMed] [Google Scholar]
- 36.Chen YG, Zhang Y, Deng LQ, Chen H, Zhang YJ, Zhou NJ, Yuan K, Yu LZ, Xiong ZH, Gui XM, Yu YR, Wu XM, Min WP. 2016. Control of methicillin-resistant Staphylococcus aureus pneumonia utilizing TLR2 agonist Pam3CSK4. PLoS One 11:e0149233. doi: 10.1371/journal.pone.0149233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fournier B. 2012. The function of TLR2 during staphylococcal diseases. Front Cell Infect Microbiol 2:167. doi: 10.3389/fcimb.2012.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canini L, Conway JM, Perelson AS, Carrat F. 2014. Impact of different oseltamivir regimens on treating influenza A virus infection and resistance emergence: insights from a modelling study. PLoS Comput Biol 10:e1003568. doi: 10.1371/journal.pcbi.1003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sautto GA, Kirchenbaum GA, Ross TM. 2018. Towards a universal influenza vaccine: different approaches for one goal. Virol J 15:17. doi: 10.1186/s12985-017-0918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robertson CM, Perrone EE, McConnell KW, Dunne WM, Boody B, Brahmbhatt T, Diacovo MJ, Van Rooijen N, Hogue LA, Cannon CL, Buchman TG, Hotchkiss RS, Coopersmith CM. 2008. Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance. J Surg Res 150:278–285. doi: 10.1016/j.jss.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Luo J, Alcorn JF, Chen K, Fan S, Pilewski J, Liu A, Chen W, Kolls JK, Wang J. 2017. AIM2 inflammasome is critical for influenza-induced lung injury and mortality. J Immunol 198:4383–4393. doi: 10.4049/jimmunol.1600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson KM, Ramanan K, Clay ME, McHugh KJ, Rich HE, Alcorn JF. 2018. Novel protective mechanism for interleukin-33 at the mucosal barrier during influenza-associated bacterial superinfection. Mucosal Immunol 11:199–208. doi: 10.1038/mi.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hook JL, Islam MN, Parker D, Prince AS, Bhattacharya S, Bhattacharya J. 2018. Disruption of staphylococcal aggregation protects against lethal lung injury. J Clin Invest 128:1074–1086. doi: 10.1172/JCI95823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunninghake GW, Gadek JE, Kawanami O, Ferrans VJ, Crystal RG. 1979. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol 97:149–206. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.