Abstract
Excessive intake of fructose increases lipogenesis in the liver, leading to hepatic lipid accumulation and development of fatty liver disease. Metabolic alterations in the liver due to fructose intake have been reported in many studies, but the effect of fructose administration on hepatic gluconeogenesis is not fully understood. The aim of this study was to evaluate the acute effects of fructose administration on fasting-induced hepatic gluconeogenesis. C57BL/6J mice were administered fructose solution after 14 h of fasting and plasma insulin, glucose, free fatty acids, and ketone bodies were analysed. We also measured phosphorylated AKT and forkhead box O (FoxO) 1 protein levels and gene expression related to gluconeogenesis in the liver. Furthermore, we measured glucose production from pyruvate after fructose administration. Glucose-administered mice were used as controls. Fructose administration enhanced phosphorylation of AKT in the liver, without increase of blood insulin levels. Blood free fatty acids and ketone bodies concentrations were as high as those in the fasting group after fructose administration, suggesting that insulin-induced inhibition of lipolysis did not occur in mice administered with fructose. Fructose also enhanced phosphorylation of FoxO1 and suppressed gluconeogenic gene expression, glucose-6-phosphatase activity, and glucose production from pyruvate. The present study suggests that acute fructose administration suppresses fasting-induced hepatic gluconeogenesis in an insulin-independent manner.
Keywords: Fructose, Gluconeogenesis, Insulin, AKT, FoxO1, G6Pase
Abbreviations: ChREBP, carbohydrate response element binding protein; CREB, cAMP response element binding protein; EDTA, ethylenediaminetetraacetic acid; FFA, free fatty acid; FoxO, forkhead box O; G6Pase, glucose-6-phosphatase; PEPCK, phosphoenolpyruvate carboxykinase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; PIP 3, phosphatidylinositol-(3,4,5)-trisphosphate; PI3K, phosphoinositide-3-kinase; SREBP, sterol-regulatory element binding protein
Highlights
-
•
Fructose administration does not increase blood glucose and insulin levels.
-
•
Fructose administration suppressed fasting-induced hepatic gluconeogenic gene expression and G6Pase activity.
-
•
Fructose accelerates FoxO1 phosphorylation through the AKT-FoxO1 pathway.
-
•
We propose that fructose intake suppresses fasting-induced hepatic gluconeogenesis in an insulin-independent manner.
1. Introduction
The liver plays an essential role in the maintenance of plasma glucose homeostasis by adjusting the balance between hepatic glucose utilization and production via the glycolytic and gluconeogenic pathways. Under fasting conditions, liver gluconeogenesis is accelerated by several hormones such as glucagon and catecholamines, to produce glucose in amounts that are necessary to meet the metabolic demands of the body [[1], [2], [3], [4]]. In the postprandial state, dietary carbohydrates are generally supplied to the bloodstream as a form of glucose [5]. An increase in blood glucose levels induces insulin secretion from the pancreatic β-cells, and metabolism in peripheral organs is switched from catabolism to anabolism [6].
Insulin is a key hormone that inhibits hepatic gluconeogenesis in the postprandial state. Phosphoinositide-3-kinase (PI3K) -AKT signaling is the most important downstream pathway of insulin action [7,8]. Activated AKT phosphorylates forkhead box O (FoxO) 1, a member of the FoxO family of forkhead transcription factors [9]. Under fasting conditions, FoxO1 positively regulates the expression of genes related to gluconeogenesis such as, glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK) [10,11]. Transcriptional activity of FoxO1 is reduced by phosphorylation through AKT, in the presence of insulin, resulting in the inhibition of G6Pase and PEPCK gene expression [12].
Insulin is deeply involved in metabolic changes in peripheral organs after carbohydrate intake. On the other hand, some of the carbohydrates alter liver metabolism in an insulin-independent manner. Fructose, a simple sugar, is a major component of sucrose and high-fructose corn syrup, two of the most commonly used sweeteners. Fructose intake has increased markedly over the last centuries, in parallel with the rise in intake of sucrose and high-fructose corn syrup. The increase in fructose consumption causes several metabolic diseases such as obesity, steatosis, insulin resistance, and non-alcoholic fatty liver disease [[12], [13], [14], [15]]. The relationship between fructose and lipid metabolism has been investigated in numerous studies [[16], [17], [18], [19], [20]]. Fructose is taken up into the liver in an insulin-independent manner, and fructose-derived precursors activate sterol-regulatory element binding protein (SREBP)-1c and carbohydrate response element binding protein (ChREBP) [19]. Insulin is considered less involved in the alteration of lipid metabolism caused by fructose, since it is widely accepted that blood insulin levels are marginally or not at all increased after fructose intake [21,22].
The mechanism of lipid metabolism regulated by fructose is becoming clearer; however, it is still not clear whether fructose intake suppresses gluconeogenesis induced by fasting. In this study, we focused on the effects of acute fructose consumption on hepatic gluconeogenesis. We found that fructose administration suppressed gluconeogenic gene expressions concomitantly with the phosphorylation of FoxO1, without increase in blood insulin levels.
2. Experimental methods
2.1. Animals
Five-week-old C57BL/6J male mice were obtained from Japan SLC Inc. (Shizuoka, Japan) and fed with a normal chow diet (MF; CLEA Japan, Tokyo, Japan) ad libitum for two weeks in order to establish their baseline metabolic status. Mice were maintained in a 12 h light–dark cycle at 22 °C and cared for according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (https://www/ncbi.nlm.nih.gov/books/NBK54050/) and institutional guidelines. All the animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Shizuoka (no. 165126).
2.2. Fructose administration
In experiment-I, mice were administered fructose (Nacalai Tesque, Kyoto, Japan) solution (2 g/kg body weight) intragastrically after 14 h fasting. The mice were sacrificed at 5 and 30 min after fructose administration. Control mice received glucose (Wako Pure Chemical Industries, Osaka, Japan) solution (2 g/kg body weight), instead of fructose.
In experiment-II, mice were administered fructose solution in the same manner as in experiment-I. The mice were sacrificed at 1–5 h after fructose administration. Blood samples were collected from the orbital sinus under anaesthesia (isoflurane) [23]. Ethylenediaminetetraacetic acid (EDTA) (Wako Pure Chemical Industries, Osaka, Japan) was used as an anticoagulant. After blood sampling, mice were sacrificed by cervical dislocation, and tissue samples were collected. Plasma and tissue samples were stored at −80 °C until analysis.
2.3. Plasma analysis
Circulating insulin in the mice plasma was measured using a commercially available ultra-sensitive ELISA (Morinaga Institute of Biological Science Incorporated, Kanagawa, Japan), according to the manufacturer's instructions. Concentrations of plasma glucose, free fatty acids (FFA), and ketone bodies were analysed using the Glucose CII test (Wako Pure Chemical Industries, Osaka, Japan), NEFA C-test (Wako Pure Chemical Industries, Osaka, Japan) and the Wako auto kit for total ketone bodies (Wako Pure Chemical Industries, Osaka, Japan), respectively.
2.4. Western blot analysis
AKT and phosphorylated AKT protein levels in the liver whole cell lysate were measured using the western blotting technique. Samples were homogenised in RIPA Lysis Buffer (Merck Millipore, Temecula, CA), containing a phosphatase inhibitor cocktail (Nacalai Tesque, Kyoto, Japan) and a protease inhibitor cocktail (Active Motif, Carlsbad, CA). After three freeze/thaw cycles, the supernatant was separated by centrifugation (15,000 g, 15 min, at 4 °C).
The amount of the phosphorylated FoxO1 levels in the cytosol were also measured using the western blotting technique. The liver was homogenised in buffer A, consisting of: 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Dojindo laboratories, Kumamoto, Japan) pH 7.8, 25 mM potassium chloride (Wako Pure Chemical Industries, Osaka, Japan), 1 mM EDTA, 0.2% Triton X100 (Bio-Rad Laboratories Incorporated, Hercules, CA) with a protease inhibitor cocktail and a phosphatase inhibitor cocktail. The supernatant was recovered after centrifugation (800 g, 10 min, at 4 °C).
These supernatant samples (20–40 μg protein) were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Each band was detected using the following antibodies: anti-AKT (#9272; Cell Signaling Technology, Beverly, MA), anti-phospho-AKT (Ser 473; #4058; Cell Signaling Technology, Beverly, MA), anti-phospho-FoxO1 (Ser 256; #84192; Cell Signaling Technology, Beverly, MA), and anti-Actin (#4970; Cell Signaling Technology, Beverly, MA). HRP-linked anti-rabbit IgG antibody (#NA934, GE Healthcare, Buckinghamshire, UK) and HRP-linked anti-mouse IgG antibody (#7076; Cell Signaling Technology, Beverly, MA) were used as secondary antibodies. Bands were detected using a chemiluminescent ECL Prime Western Blotting Reagent (GE Healthcare, Buckinghamshire, UK) and their intensity was quantified using a C-DiGit luminescent image scanner (LI-COR, Lincoln, NE).
2.5. Liver glycogen determination
A small piece of frozen liver was homogenised to determine liver glycogen concentration. Homogenised liver was treated with 0.3 M perchloric acid (Wako Pure Chemical Industries, Osaka, Japan) and 2 M hydrochloric acid (Wako Pure Chemical Industries, Osaka, Japan), incubated for 2 h at 100 °C and then neutralised with 0.67 M sodium hydroxide (Wako Pure Chemical Industries, Osaka, Japan) for subsequent determination of glucose concentration via enzymatic analysis using the Glucose CII-test.
2.6. RNA extraction and quantitative real-time PCR analysis
Total RNA was extracted from liver using RNA iso plus (Takara Bio Incorporated, Shiga, Japan). Reverse transcription (RT) was performed with PrimeScript RT reagent Kit with gDNA Eraser (Takara Bio Incorporated, Shiga, Japan) using 1 μg of RNA. Real-time PCR was performed with SYBR Premix TaqII (Takara Bio Incorporated, Shiga, Japan). Primer sequences used in this study are listed in Table 1. Target gene expression was normalised to the housekeeping 18S rRNA gene expression, using the standard curve method.
Table 1.
Nucleotide sequences of quantitative real-time RT-PCR.
| Target | Sense sequence | Antisense sequence |
|---|---|---|
| G6Pase | 5′-CCAGAGTGCTCTACCCCAAT-3′ | 5′-CCACCACAAAGTACTCCTGTTTC-3′ |
| PEPCK | 5′-CCACAGCTGCTGCAGAAC-3′ | 5′-GAAGGGTCGCATGGCAAA-3′ |
| PGC-1α | 5′-CCTGCACCACCAACTGCTTA-3′ | 5′-TCATGAGCCCTTCCACAATG-3′ |
| FoxO1 | 5′-TACCTGCCAGGGCACAAG-3′ | 5′-GGGTACCACAAAAACCAGGA-3′ |
| 18S rRNA | 5′-GGGAGCCTGAGAAACGGC-3′ | 5′-GGGTCGGGAGTGGGTAATTTT-3′ |
G6Pase, glucose-6-phosphatase; PEPCK, phosphoenolpyruvate carboxykinase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; FoxO, forkhead box O.
2.7. G6Pase activity
For the analysis of G6Pase activity, the harvested livers were homogenised in ice-cold saline and these homogenates were centrifuged (600 g, 10 min, at 4 °C). The supernatants were collected, aliquoted, and stored at −80 °C until analysis. The liver lysates were incubated for 15 min at 37 °C in 65 mM sodium cacodylate (Wako Pure Chemical Industries, Osaka, Japan) buffer (pH 6.5) containing 10 mM EDTA and 10 mM glucose-6-phosphate (Wako Pure Chemical Industries, Osaka, Japan). The reaction was stopped by adding ice-cold 10% trichloroacetic acid (Wako Pure Chemical Industries, Osaka, Japan) and the samples were centrifuged (600 g, 10 min, at 4 °C). The supernatants were incubated for 10 min at 37 °C with ammonium molybdate (Wako Pure Chemical Industries, Osaka, Japan) and 1-amino-2-naphthol-4-sulfonic acid (Wako Pure Chemical Industries, Osaka, Japan). After adding triethanolamine (Wako Pure Chemical Industries, Osaka, Japan) solution, the absorbance was measured at 780 nm.
2.8. Intraperitoneal pyruvate tolerance test after fructose administration
Glucose or fructose solutions were administered into the mice after 14 h of fasting. Two h after the administration of glucose or fructose, pyruvate (Wako Pure Chemical Industries, Osaka, Japan) (2 g/kg body weight) was injected intraperitoneally. Blood samples were collected from the tail vein of the mice, before and after 30, 60, 90, and 120 min of the pyruvate injection. The blood glucose levels were measured using Breeze2 (BAYER, Leverkusen, Germany). The control mice received a saline solution instead of the glucose or fructose solutions, before the pyruvate tolerance test.
2.9. Statistical analysis
Results are presented as mean ± SEM. The data were analysed using the one-way ANOVA test. Significant differences between each group was compared using the Student's t-test (JMP 5.1.2; SAS, Cary, NC). Statistical significance was defined as P < 0.05.
3. Results
3.1. Blood insulin and hepatic phosphorylated AKT levels after fructose administration
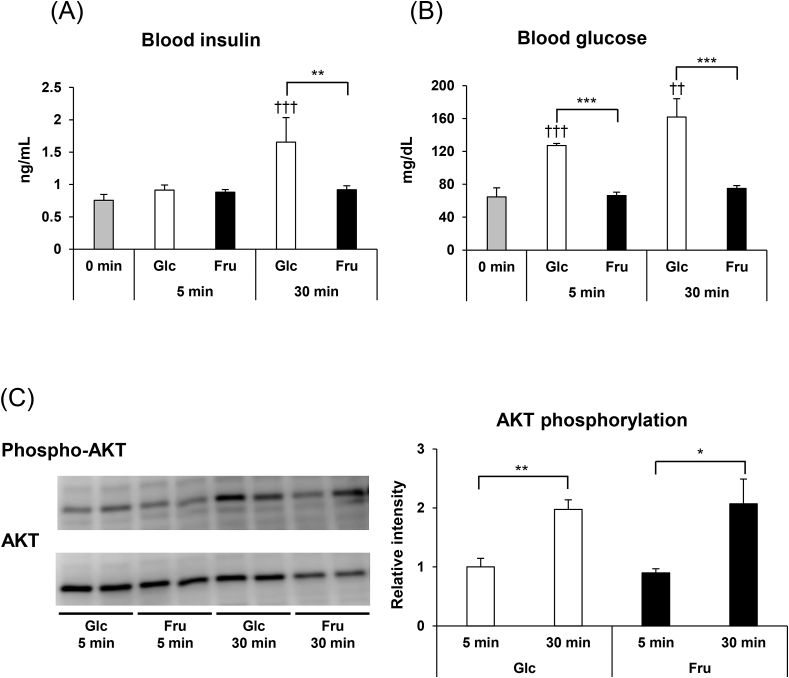
In experiment-I, changes in the blood insulin levels and phosphorylated AKT levels in the liver were investigated, after acute fructose administration in mice. In the control group, the same amount of glucose was administered instead of fructose. Five min after glucose administration, blood insulin levels remained the same as before the glucose administration (Fig. 1A), but were 2.2-fold higher 30 min after glucose administration. On the other hand, 30 min after the administration of fructose, the blood insulin levels increased only by 1.2-fold. We also observed that the blood glucose levels increased immediately after glucose administration (Fig. 1B), but no increase in the blood glucose levels were seen after fructose administration. These results suggest that fructose intake does not increase blood insulin and glucose levels, in accordance with previous studies [21,22].
Fig. 1.
(A) Blood insulin levels and (B) glucose levels, before glucose (Glc) or fructose (Fru) administration, and 5 and 30 min after administration. Mice were given glucose or fructose (2.0 g/kg body weight) after 14 h of fasting. Values are presented as means ± SEM (n = 3–7). **P < 0.01; ***P < 0.001 vs. the glucose administered mice at the same time-points. ††P < 0.01; †††P < 0.001 vs. the mice under fasting condition. (C) Phosphorylation of AKT in the liver, 5 and 30 min after glucose (Glc) or fructose (Fru) administration. Phospho-AKT/total AKT in the liver was measured by western blotting. Representative blots are shown. Values in the graph are expressed as a relative intensity compared to the values at 5 and 30 min after glucose administration (n = 4–5). *P < 0.05; **P < 0.01.
Next, we measured the phosphorylation level of AKT, which is downstream of the insulin signal pathway. AKT has several phosphorylation sites and its activation is induced by the phosphorylation of Thr473 [24]. To investigate the changes mediated by fructose on AKT phosphorylation, we measured the levels of phosphorylated AKT at 5 and 30 min after fructose administration in mice. Five min after administration, the level of AKT phosphorylation was not significantly different among the fructose and glucose administered mice (Fig. 1C). In contrast, phosphorylated AKT levels increased significantly in both groups of mice 30 min after administration, but there were no significant differences between the glucose- and fructose-administered mice. Although blood insulin levels were not increased in response to fructose administration, AKT phosphorylation in the liver was enhanced, suggesting that fructose administration induced AKT activation in an insulin-independent manner.
3.2. Blood FFA, ketone bodies, and glucose levels, and changes in the liver glycogen contents after fructose administration
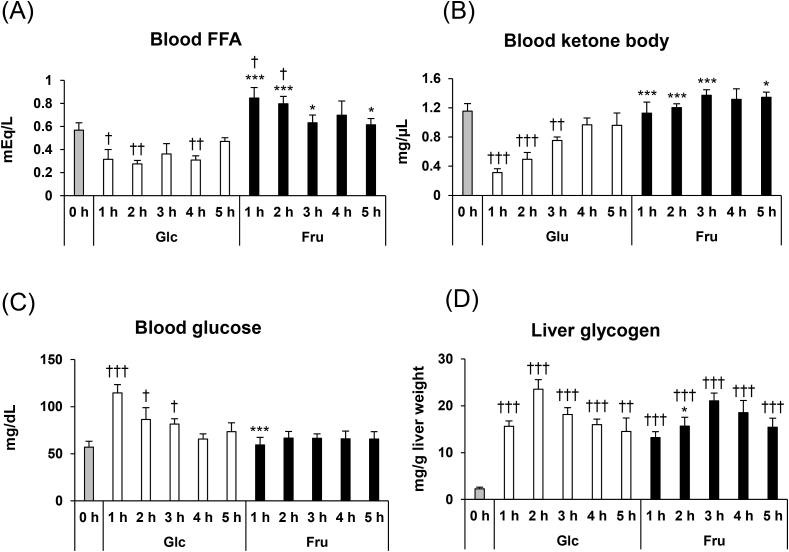
In experiment-I, we confirmed that the blood insulin levels were increased only in the glucose-administered mice and not in fructose-administered mice. Postprandial insulin release promotes anabolic reactions in the peripheral tissues such as adipocyte, and decreases blood FFA and ketone bodies levels [25,26]. Therefore, we hypothesised that the administration of glucose, not fructose, suppressed the FFA and ketone bodies levels. In experiment-II, to confirm this hypothesis, we measured the blood FFA and ketone bodies levels after glucose and fructose administration. The blood levels of FFA and ketone bodies rapidly decreased after glucose administration (Fig. 2A and B). In contrast, blood FFA levels increased in the mice after fructose administration. Blood ketone bodies levels did not change after fructose administration.
Fig. 2.
(A) Blood FFA, (B), ketone bodies (C) glucose levels, and (D) liver glycogen contents before and after 1–5 h of glucose (Glc) or fructose (Fru) administration. Mice were administered glucose or fructose (2.0 g/kg body weight) after 14 h of fasting. Values are presented as means ± SEM (n = 5–6). *P < 0.05; ***P < 0.001 vs. the glucose-administered mice at the same time-point. †P < 0.05; ††P < 0.01; †††P < 0.001 vs. the mice under fasting condition. FFA, free fatty acids.
Blood glucose levels peaked at 1 h after glucose administration (Fig. 2C). After reaching this peak, blood glucose levels decreased gradually. Fructose administration did not change blood glucose levels. The liver glycogen contents increased immediately after both fructose and glucose administration (Fig. 2D). These results suggest that both glucose and fructose are taken up into the liver and utilised for glycogen synthesis.
3.3. Hepatic phosphorylated FoxO1 levels after fructose administration
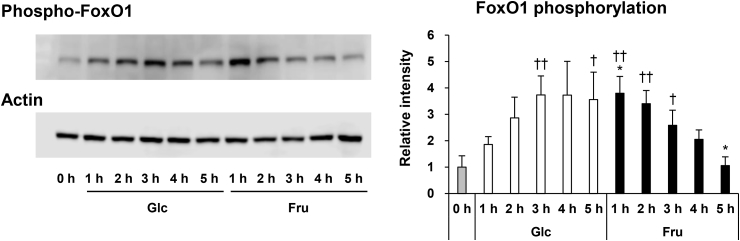
In experiment-I, we observed that fructose administration enhanced AKT phosphorylation in the liver without increasing blood insulin levels. Since activated AKT phosphorylates FoxO1, and phosphorylated FoxO1 is translocated from the nucleus to the cytosol [27], we confirmed the phosphorylation of FoxO1 after the administration of fructose. An increase in phosphorylated FoxO1 levels was seen at 1 h after fructose administration, and then these levels gradually decreased (Fig. 3). Phosphorylated FoxO1 levels gradually increased after glucose administration, compared to fructose administration, and reached a peak at 3 h after administration. Different patterns of phosphorylation were observed in mice with glucose and fructose administration.
Fig. 3.
Phosphorylated FoxO1 protein levels in the liver before and after 1–5 h of glucose (Glc) or fructose (Fru) administration. Phosphorylated FoxO1 protein in the liver was measured by western blotting. Actin was used as an internal control. Representative blots are shown. Values in the graph are expressed as a relative intensity compared to the values before administration (n = 4). *P < 0.05 vs. the glucose administered mice at the same time-point. †P < 0.05; ††P < 0.01 vs. the mice before administration. FoxO, forkhead box O.
3.4. Gene expression related to gluconeogenesis in the liver after fructose administration
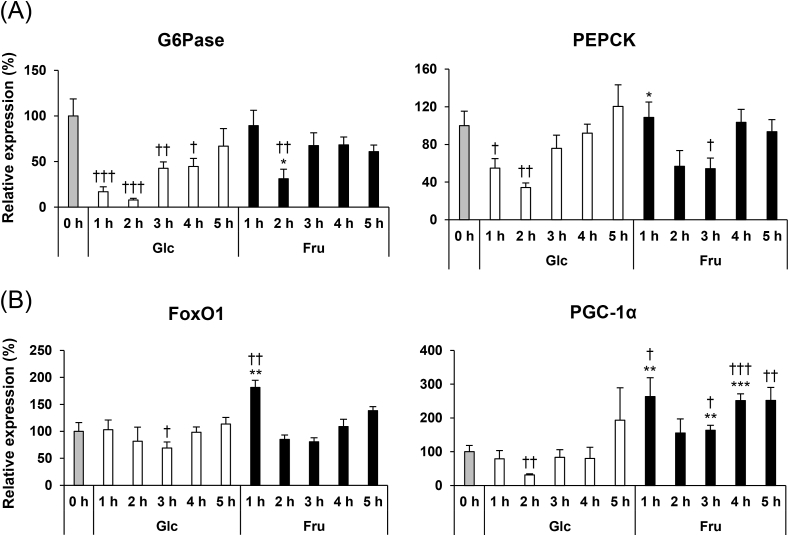
As shown in Fig. 3, fructose administration enhances the phosphorylation of FoxO1 in the liver. Under fasting conditions, FoxO1 is located in nucleus and accelerates the transcription of gluconeogenesis related genes such as G6Pase and PEPCK, in the liver. The phosphorylated FoxO1 is translocated out of the nucleus, which leads to a decrease in the levels of hepatic gluconeogenic gene expression [12,27]. Therefore, we hypothesised that fructose administration decreases the gluconeogenic gene expression through AKT and FoxO1 phosphorylation. To investigate this hypothesis, we measured the gene expression levels of G6Pase and PEPCK by quantitative real-time RT-PCR (Fig. 4A). The expression of G6Pase and PEPCK in the glucose-administrated mice decreased drastically at 1 h after administration. Expression levels of both these genes reached a minimum value at 2 h after administration, and a time-dependent increase was observed thereafter. In contrast, in the fructose-administrated mice, the expression levels of G6Pase and PEPCK genes did not change at 1 h after administration. However, G6Pase and PEPCK gene expression levels significantly decreased at 2 and 3 h after fructose administration. The decrease in G6Pase gene expression levels at 2 h after fructose administration was moderate compared to the glucose-administered mice, and this difference was statistically significant.
Fig. 4.
mRNA expression levels of genes related to gluconeogenesis in the liver of the mice before and after glucose (Glc) or fructose (Fru) administration. The results are displayed as percent increase/decrease, from the level of mRNA expressed in the mice before administration considered as 100%. Values are presented as means ± SEM (n = 5–6). *P < 0.05; **P < 0.01; ***P < 0.001 vs. the glucose administered mice at the same time-point. †P < 0.05; ††P < 0.01; †††P < 0.001 vs. the mice before administration. G6Pase, glucose-6-phosphatase; PEPCK, phosphoenolpyruvate carboxykinase; FoxO, forkhead box O; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha.
Next, we measured the mRNA levels of the transcriptional factor and the transcriptional coactivator that regulates the gluconeogenic gene expression. Gene expression of FoxO1 and PGC-1α decreased significantly at 3 and 2 h after glucose administration, respectively (Fig. 4B). On the other hand, the expression levels of these two gene increased significantly at 1 h after fructose administration. There was no significant difference in the FoxO1 gene expression levels between the glucose- and fructose-administered mice, at 2 h after administration. PGC-1α gene expression levels were significantly higher in the mice at 3 and 4 h after fructose administration when compared to that after glucose administration.
3.5. Hepatic G6Pase activity after fructose administration
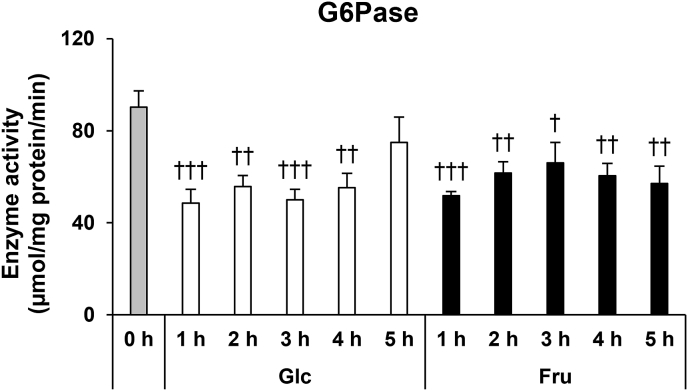
As shown in Fig. 4A, G6Pase gene expression levels decreased after administration of fructose. To investigate whether there is a change in enzyme activity that accompanies the changes in G6Pase expression levels after fructose administration, we prepared an enzyme solution from the excised liver and measured its G6Pase activity. The activity of G6Pase decreased by 43% and 46%, immediately after the administration of fructose and glucose, respectively (Fig. 5). Until 4 h after administration, the G6Pase activity in both fructose and glucose administered mice was significantly lower than the G6Pase activity measured before administration. In fructose-administered mice, G6Pase activity was also significantly lower at 5 h after administration. There were no significant differences in the G6Pase activity between the glucose and fructose administered mice at all time points after administration.
Fig. 5.
G6Pase activity in the liver of the mice before and after glucose (Glc) or fructose (Fru) administration. Values are presented as means ± SEM (n = 5–6). †P < 0.05; ††P < 0.01; †††P < 0.001 vs. the mice before administration. G6Pase, glucose-6-phosphatase.
3.6. Glucose production from pyruvate after fructose administration
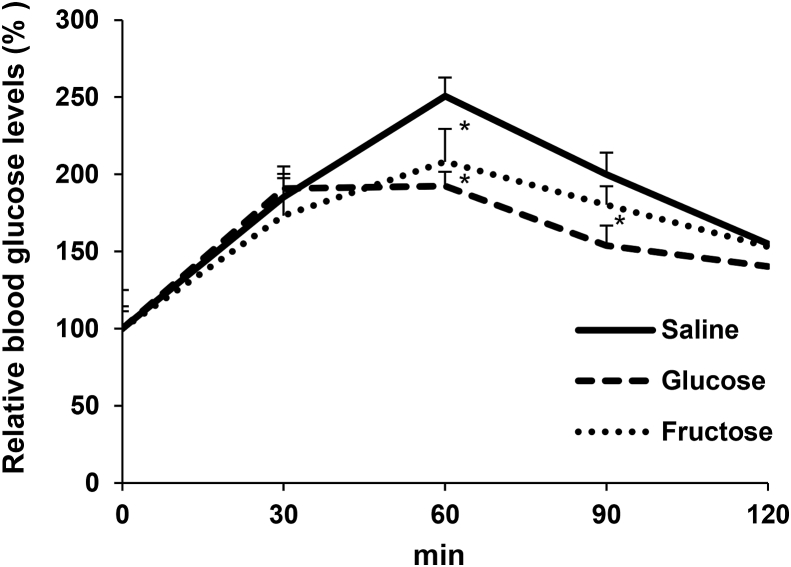
As shown in Figs. 4 and 5, we observed that fructose suppressed gluconeogenic gene expression and G6Pase activity in the liver. Based on these results, we hypothesised that fructose administration suppresses fasting-induced gluconeogenesis in the liver. To investigate this hypothesis, we measured the glucose production from pyruvate after fructose administration. After a 14 h fasting period, we administered saline, glucose, or fructose solution to the mice intragastrically. Two h after administration, we injected pyruvate intraperitoneally into these mice. The blood glucose levels increased at 30 min after injection of pyruvate, among all groups (Fig. 6). Sixty min after pyruvate administration, the maximum blood glucose levels in the saline group increased 2.5-folds, when compared to 2.1 and 1.9-folds in the fructose- and glucose-administered mice, respectively, which is significantly lower than that in the saline group. These results indicate that fructose administration actually diminishes glucose production, probably due to the reduction of gluconeogenic gene expression and G6Pase activity.
Fig. 6.
Blood glucose levels after administration of pyruvate. Mice were administered glucose or fructose after 14 h fasting. After 2 h glucose or fructose administration, mice were injected with pyruvate (2.0 g/kg body weight). Blood glucose levels in the mice injected saline, glucose, or fructose administration, at 0 min were 104 ± 3, 131 ± 11 and 101 ± 5 mg/dL, respectively. Values are presented as means ± SEM (n = 4–6). *P < 0.05 vs. the saline administered mice at the same time-point.
4. Discussion
Numerous studies have indicated that insulin is the major postprandial factor in regulating gluconeogenesis [12,[26], [27], [28], [29], [30]]. Glucose administration does not suppress gluconeogenesis in the insulin-depleted mice model [31], suggesting that insulin plays an important role in postprandial glucose metabolism. It is widely accepted that the fructose intake does not enhance pancreatic insulin secretion [21,22]. Blood insulin levels did not increase after fructose administration in our study, which is consistent with the previous reports [21,22]. Postprandial increase in insulin levels is known to inhibit adipose tissue lipolysis promoted by fasting [26]. Suppression of adipose tissue lipolysis by insulin reduces the release of FFA into the blood stream, and as a consequence, the blood FFA concentration immediately decreases. Insulin also suppresses β-oxidation and the concentration of blood ketone bodies in the liver [25]. In our study, blood FFA and ketone bodies levels decreased with the elevation of insulin levels after glucose administration, but not after fructose administration in mice. These results also suggest that insulin is not released by fructose administration.
The elevation of blood insulin levels promptly enhances the phosphorylation of AKT and FoxO1 in the peripheral organs [7,8]. We observed that glucose administration induces the phosphorylation of AKT and FoxO1 due to an increase in blood insulin levels. Unexpectedly, fructose administration also induced the phosphorylation of AKT and FoxO1, despite low blood insulin levels. Previous studies have indicated that fructose administration directly regulates the AKT-FoxO1 pathway, independent of insulin [32,33]. PI3K activity in the muscle was shown to be higher after a long-term administration of fructose in mice, when compared to glucose administration [32]. Another study, utilizing a rat intestine, reported that the perfusion of the small intestinal lumen with fructose solution enhances GLUT5 activity and protein expression by activating AKT [33]. AMPK is one of the candidate molecules that could activate the AKT pathway. Previous studies have shown that AMPK can activate the hepatic PI3K/AKT signaling pathway [34,35]. Moreover, fructose injection immediately enhances AMPK activity in the hypothalamus due to the decline in the ATP/AMP ratio [36]. Altogether, these results indicate that fructose may activate the AKT-FoxO1 pathway in the liver without increasing the blood insulin levels. Gluconeogenesis in the liver is regulated by hormones and transcription factors. Glucagon, an essential regulator of glucose homeostasis under fasting condition, activates transcriptional factors PGC-1α and FoxO1 to enhance the expression of gluconeogenic genes [37,38]. We have previously reported that fasting increases the hepatic gluconeogenic gene expression in a time-dependent manner [39]. In this study, we observed that fructose administration suppressed the gene expression of G6Pase and PEPCK induced by fasting. However, there was the time lag for the decrease in expression of these gluconeogenic genes in response to glucose and fructose. As shown in Fig. 3, FoxO1 phosphorylation levels in the fructose-administered mice increased earlier than in the glucose-administered mice. However, the decrease in G6Pase and PEPCK gene expression occurred later in the fructose-administered mice, suggesting a discrepancy between the results of FoxO1 phosphorylation and gluconeogenic gene expression. Considering this discrepancy, it can be assumed that another mechanism, other than the AKT-FoxO1 pathway, is involved in regulating gluconeogenic gene expression after fructose administration.
cAMP response element binding protein (CREB) is one of the transcriptional factors involved in the regulation of gluconeogenic genes [38]. Glucagon activates the cAMP-PKA signaling pathway and phosphorylates CREB at its Ser133 site [40]. Phosphorylated CREB binds to the cAMP-response element in the promoter region of the G6Pase and PEPCK gene and increases their gene expression levels [38,41]. Furthermore, the CREB binding site is also present in the promoter region of PGC-1α and FoxO1 gene [42,43]. In this study, the gene expression levels of PGC-1α and FoxO1 immediately increased after fructose, but not glucose, administration. It has been previously reported that fructose activates the cAMP-PKA signaling pathway through reduction of hepatic intracellular ATP concentration, which further activates CREB [44]. The CREB pathway might be involved in the increase of FoxO1 and PGC-1α expression after fructose administration, suggesting that fructose intake regulates the gene expression levels of the gluconeogenic genes through both AKT-FoxO1 and CREB pathway. On the other hand, it has also been reported that CREB pathway is suppressed by insulin [41]. We assumed that the suppression of the CREB pathway, mediated by insulin, is involved in the prompt decrease of gluconeogenic gene expression levels after glucose administration.
G6Pase is an enzyme that catalyses the last enzymatic reaction of glycogenolysis and gluconeogenesis. It is a multi-subunit, integral membrane protein of the endoplasmic reticulum that is composed of a catalytic subunit and transporters, and transports glucose-6-phosphate from the cytosol to the endoplasmic reticulum lumen [45]. G6Pase activity is attenuated during the postprandial period through mechanisms involving insulin and/or intrahepatic nutrient signals [[46], [47], [48]]. In our study, G6Pase activity was immediately decreased by glucose and fructose administration after fasting. In a previous study that administered glucose to mice, suppression of G6Pase activity was reported, along with an increase in blood insulin level [46]. Based on this report, the decline of G6Pase activity by glucose administration in our study can be explained by the increase in blood insulin levels. G6Pase activity is inhibited by several amphiphilic compounds, such as fatty acids and phosphoinositide [48,49]. Particularly, phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) exhibits a potent inhibitory effect on G6Pase activity [48]. PIP3 is the product of the PI3K-dependent phosphorylation of phosphatidylinositol-(4,5)-diphosphate. Since insulin activates PI3K, suppression of G6Pase activity by insulin seems to be involved in the increase in PIP3. As mentioned earlier, a previous study has reported that fructose intake activates PI3K activity [32]. Based on previous findings about the effect of fructose on PI3K activity, we suggest that the insulin-independent activation of PI3K leads to a decrease in G6Pase activity after acute fructose administration.
Glycogen is also reported to be an inhibitor of G6Pase activity [49]. Glycogen particles exist close to the endoplasmic reticulum lumen in the liver [49]. Glycogen particles contains saturated and unsaturated FFA such as palmitic acid and oleic acid. Most of these fatty acids are efficient inhibitors of G6Pase activity [50]. As shown in Fig. 2D, the glycogen content of the liver was depleted under fasting conditions. The liver glycogen contents were recovered by the administration of fructose as well as glucose. Recovery of liver glycogen contents after fructose administration may be involved in the suppression of G6Pase activity.
In conclusion, we found that the intake of fructose enhances the phosphorylation of AKT and FoxO1 in the liver, but the blood insulin levels remain unaltered. These results indicate that the intake of fructose enhances AKT-FoxO1 phosphorylation in an insulin-independent manner. At the same time, fructose administration suppressed gluconeogenic gene expression and G6Pase activity in the liver. In the pyruvate tolerance test, fructose administration suppressed the production of glucose from pyruvate. These results suggest that, acute fructose administration suppresses hepatic gluconeogenesis, probably due to the decrease in the expression of gluconeogenic genes and G6Pase activity. The present study provides evidence for the acute effect of fructose in the hepatic gluconeogenesis. Inhibition of fasting-induced gluconeogenesis by fructose administration would contribute to an increase in the supply of substrates necessary for lipid synthesis, exacerbating fat accumulation and steatosis. However, the mechanisms behind the changes induced by the intake of fructose are not yet fully understood. More investigations are needed to fully elucidate the mechanisms of fructose-induced metabolic changes in the liver.
Funding
This study was supported by the Council for Science, Technology, and Innovation (CSTI), Cross-ministerial Strategic Innovation Promotion program (SIP, number 15J10165); “Technologies for creating next-generation agriculture, forestry, and fisheries” (funding agency: Bio-oriented Technology Research Advancement Institution, National Agriculture and Food Research Organization, NARO); and the Tojuro Iijima Foundation for Food Science and Technology (Chiba, Japan). This study was also supported by Grants-in-Aid for Scientific Research (KAKENHI, numbers 15K00827) and Grant-in-Aid for JSPS Research Fellow (KAKENHI, numbers 15J10165) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT, Tokyo), the Mishima Kaiun Memorial Foundation (Tokyo, Japan), and a University of Shizuoka Grant for Scientific and Educational Research.
Authorship
Conceived and designed the experiments: T. S., S. M. Performed experiments: T. S., Y. W., Y. N., M. I. Analysed data: T. S., A. M., S. M. Wrote the paper: T. S., S. M.
Acknowledgments
We thank the members of the Laboratory of Nutritional Biochemistry (Graduate School of Nutritional and Environmental Sciences, University of Shizuoka) for their technical assistance.
Footnotes
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100638
Contributor Information
Tomoki Sato, Email: tsato1@uci.edu.
Yui Watanabe, Email: s17218@u-shizuoka-ken.ac.jp.
Akihito Morita, Email: moritaa@u-shizuoka-ken.ac.jp.
Shinji Miura, Email: miura@u-shizuoka-ken.ac.jp.
Transparency document
References
- 1.Cederblad F., Ewald U., Gustafsson J. Effect of glucagon on glucose production, lipolysis, and gluconeogenesis in familial hyperinsulinism. Horm. Res. 1998;50:94–98. doi: 10.1159/000023242. [DOI] [PubMed] [Google Scholar]
- 2.Chhibber V.L., Soriano C., Tayek J.A. Effects of low-dose and high-dose glucagon on glucose production and gluconeogenesis in humans. Metabolism. 2000;49:39–46. doi: 10.1016/s0026-0495(00)90638-3. [DOI] [PubMed] [Google Scholar]
- 3.Mine T., Kojima I., Ogata E. Role of calcium fluxes in the action of glucagon on glucose metabolism in rat hepatocytes. Am. J. Physiol. 1993;265:G35–G42. doi: 10.1152/ajpgi.1993.265.1.G35. [DOI] [PubMed] [Google Scholar]
- 4.Stumpo D.J., Kletzien R.F. Gluconeogenesis in rat liver parenchymal cells in primary culture: permissive effect of the glucocorticoids on glucagon stimulation of gluconeogenesis. J. Cell. Physiol. 1981;107:11–19. doi: 10.1002/jcp.1041070103. [DOI] [PubMed] [Google Scholar]
- 5.Huntington G.B. Starch utilization by ruminants: from basics to the bunk. Am Soc Anim Sci. 1997;75:852–867. doi: 10.2527/1997.753852x. [DOI] [PubMed] [Google Scholar]
- 6.Hardie D.G. Organismal carbohydrate and lipid homeostasis. Cold Spring Harb Perspect Biol. 2012;4:a006031. doi: 10.1101/cshperspect.a006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohn A.D., Takeuchi F., Roth R.A. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J. Biol. Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 8.Brozinick J.T., Birnbaum M.J. Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J. Biol. Chem. 1998;273:14679–14682. doi: 10.1074/jbc.273.24.14679. [DOI] [PubMed] [Google Scholar]
- 9.Tikhanovich I., Cox J., Weinman S. FOXO transcription factors in liver function and disease. J. Gastroenterol. Hepatol. 2013;28:125–131. doi: 10.1111/jgh.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renga B., Mencarelli A., D'Amore C., Cipriani S., Baldelli F., Zampella A. Glucocorticoid receptor mediates the gluconeogenic activity of the farnesoid X receptor in the fasting condition. FASEB J. 2012;26:3021–3031. doi: 10.1096/fj.11-195701. [DOI] [PubMed] [Google Scholar]
- 11.Hirota K., Sakamaki J., Shimamoto Y., Shimamoto Y., Nishihara S., Kodama N. A combination of HNF-4 and Foxo1 is required for reciprocal transcriptional regulation of glucokinase and glucose-6-phosphatase genes in response to fasting and feeding. J. Biol. Chem. 2008;283:32432–32441. doi: 10.1074/jbc.M806179200. [DOI] [PubMed] [Google Scholar]
- 12.Barthel A., Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2003;285:E685–E692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer E.J., Gleason J.A., Dansinger M.L. Dietary fructose and glucose differentially affect lipid and glucose homeostasis. J. Nutr. 2009;139:1257S–1262S. doi: 10.3945/jn.108.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tappy L., Le K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 15.Lanaspa M.A., Cicerchi C., Garcia1 G., Li N., Roncal-Jimenez C.A., Rivard C.J. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki M., Dobrzyn A., Man W.C., Chu K., Sampath H., Kim H.J. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and independent mechanisms. J. Biol. Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 17.Katsurada A., Iritani N., Fukuda H., Matsumura Y., Nishimoto N., Noguchi T. Effects of nutrients and hormones on transcriptional and post-transcriptional regulation of fatty acid synthase in rat liver. Eur. J. Biochem. 1990;190:427–433. doi: 10.1111/j.1432-1033.1990.tb15592.x. [DOI] [PubMed] [Google Scholar]
- 18.Towle H.C., Kaytor E.N., Shih H.M. Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annu. Rev. Nutr. 1997;17:405–433. doi: 10.1146/annurev.nutr.17.1.405. [DOI] [PubMed] [Google Scholar]
- 19.Lee A.H., Scapa E.F., Cohen D.E., Glimcher L.H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park O.J., Cwsar D., Faix D., Wu K., Shackleton C.H., Hellerstein M.K. Mechanisms of fructose-induced hypertriglyceridaemia in the rat. Activation of hepatic pyruvate dehydrogenase through inhibition of pyruvate dehydrogenase kinase. Biochem. J. 1992;282:753–757. doi: 10.1042/bj2820753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H.S., Paik H.Y., Lee K.U., Lee H.K., Min H.K. Effects of several simple sugars on serum glucose and serum fructose levels in normal and diabetic subjects. Diabetes Res. Clin. Pract. 1988;4:281–287. doi: 10.1016/s0168-8227(88)80030-5. [DOI] [PubMed] [Google Scholar]
- 22.Crapo P.A., Scarlett J.A., Kolterman O.G., Sanders L.R., Hofeldt F.D., Olefsky J.M. The effects of oral fructose, sucrose, and glucose in subjects with reactive hypoglycemia. Diabetes Care. 1982;5:512–517. doi: 10.2337/diacare.5.5.512. [DOI] [PubMed] [Google Scholar]
- 23.Parasuraman S., Raveendran R., Kesavan R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010;1:87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alessi D.R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 25.McGarry J.D., Foster D.W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 26.Girard J. Insulin's effect on the liver: “Direct or indirect?” continues to be the question. J. Clin. Investig. 2006;116:8–10. doi: 10.1172/JCI27743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuzaki H., Daitoku H., Hatta M., Tanaka K., Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl. Acad. Sci. Unit. States Am. 2003;100:11285–11290. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M.A. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB Binding Protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O-Sullivan I., Zhang W., Wasserman D.H., Liew C.W., Liu J., Paik J. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat. Commun. 2015;6:1–12. doi: 10.1038/ncomms8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gastaldelli A., Toschi E., Pettiti M., Frascerra S., Quiñones-Galvan A., Sironi A.M. Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes. 2001;50:1807–1812. doi: 10.2337/diabetes.50.8.1807. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzaka T., Shimano H., Yahagi N., Amemiya-Kudo M., Okazaki H., Tamura Y. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 2004;53:560–569. doi: 10.2337/diabetes.53.3.560. [DOI] [PubMed] [Google Scholar]
- 32.Bhanot S., Salh B.S., Verma S., McNeill J.H., Pelech S.L. In vivo regulation of protein-serine kinases by insulin in skeletal muscle of fructose-hypertensive rats. Am. J. Physiol. 1999;277:E299–E307. doi: 10.1152/ajpendo.1999.277.2.E299. [DOI] [PubMed] [Google Scholar]
- 33.Cui X.L., Schlesier A.M., Fisher E.L., Cerqueira C., Ferraris R.P. Fructose-induced increases in neonatal rat intestinal fructose transport involve the PI3-kinase/Akt signaling pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G1310–G1320. doi: 10.1152/ajpgi.00550.2004. [DOI] [PubMed] [Google Scholar]
- 34.Xiao F., Huang Z., Li H., Yu J., Wang C., Chen S. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng T., Yang X., Wu D., Xing S., Bian F., Li W. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3β pathway. Br. J. Pharmacol. 2015;172:3284–3301. doi: 10.1111/bph.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cha S.H., Wolfgang M., Tokutake Y., Chohnan S., Lane M.D. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16871–16875. doi: 10.1073/pnas.0809255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haeusler R.A., Kaestner K.H., Accili D. FoxOs function synergistically to promote glucose production. J. Biol. Chem. 2010;285:35245–35248. doi: 10.1074/jbc.C110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herzig S., Long F., Jhala U.S., Hedrick S., Quinn R., Bauer A. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 39.Sato T., Yoshida Y., Morita A., Mori N., Miura S. Glycerol-3-phosphate dehydrogenase 1 deficiency induces compensatory amino acid metabolism during fasting in mice. Metabolism. 2016;65:1646–1656. doi: 10.1016/j.metabol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Altarejos J.Y., Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puigserver P., Rhee J., Donovan J., Walkey C.J., Yoon J.C., Oriente F. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 42.Oh K.J., Han H.S., Kim M.J., Koo S.H. CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep. 2013;46:567–574. doi: 10.5483/BMBRep.2013.46.12.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wondisford A.R., Xiong L., Chang E., Meng S., Meyers D.J., Li M. Control of foxo1 gene expression by co-activator p300. J. Biol. Chem. 2014;289:4326–4333. doi: 10.1074/jbc.M113.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Speicher T., Köhler U.K., Chouke`r A., Werner S., Weiland T., Wendel A. Fructose protects murine hepatocytes from tumor necrosis factor-induced apoptosis by modulating JNK signaling. J. Biol. Chem. 2012;287:1837–1846. doi: 10.1074/jbc.M111.266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soty M., Chilloux J., Delalande F., Zitoun C., Bertile F., Mithieux G. Post-translational regulation of the glucose-6-phosphatase complex by cyclic adenosine monophosphate is a crucial determinant of endogenous glucose production and is controlled by the glucose-6-phosphate transporter. J. Proteome Res. 2016;15:1342–1349. doi: 10.1021/acs.jproteome.6b00110. [DOI] [PubMed] [Google Scholar]
- 46.Ashmore J., Baird A., Nesbett F.B. Studies on carbohydrate metabolism in rat liver slices. J. Biol. Chem. 1954;218:77–88. [PubMed] [Google Scholar]
- 47.Daniele N., Rajas F., Payrastre B., Mauco G., Zitoun C., Mithieux G. Phosphatidylinositol 3-kinase translocates onto liver endoplasmic reticulum and May account for the inhibition of glucose-6-phosphatase during refeeding. J. Biol. Chem. 1999;274:3597–3601. doi: 10.1074/jbc.274.6.3597. [DOI] [PubMed] [Google Scholar]
- 48.Mithieux G., Daniele N., Payrastre B., Zitoun C. Liver microsomal glucose-6- phosphatase is competitively of phosphatidylinositol 3-kinase. J. Biol. Chem. 1998;273:17–19. doi: 10.1074/jbc.273.1.17. [DOI] [PubMed] [Google Scholar]
- 49.Danie`le N., Bordet J.C., Mithieux G. Unsaturated fatty acids associated with glycogen may inhibit glucose-6 phosphatase in rat liver. J. Nutr. 1997;127:2289–2292. doi: 10.1093/jn/127.12.2289. [DOI] [PubMed] [Google Scholar]
- 50.Mithieux G., Bordet J.C., Minassian C., Ajzannay A., Mercier I., Riou J.P. Characteristics and specificity of the inhibition of liver glucose-6-phosphatase by arachidonic acid. Eur. J. Biochem. 1993;213:461–466. doi: 10.1111/j.1432-1033.1993.tb17782.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.