Abstract
Introduction
Pseudoaneurysms of the renal artery are fairly uncommon and mostly asymptomatic. They develop mostly in the right renal artery and in female patients.
Report
In this report, a female patient with an intraparenchymal renal artery pseudoaneurysm with one year follow up is described. She presented with non-specific abdominal pain. A computed tomography scan revealed hydronephrosis of the right kidney and a giant, intracapsular, contained rupture of a pseudoaneurysm of the right renal artery. The patient was admitted to hospital and underwent a successful selective embolisation of the pseudoaneurysm. Follow up at one year showed normal renal function and an excluded aneurysm.
Discussion
Although relatively uncommon, renal artery pseudoaneurysms should be considered in the work up of patients with colicky flank pain. As a treatment option, endovascular approaches are appealing because they are less invasive. Successful treatment can prevent resection of the affected kidney.
Keywords: Endovascular procedures, False aneurysm, Rare disease, Renal artery, Spontaneous rupture, Hydronephrosis
Highlights
-
•
This report describes the rare case of a young female patient with contained rupture of a right renal artery pseudoaneurysm.
-
•
The pseudoaneurysm was treated by endovascular coiling, preserving the kidney and its function.
-
•
A spontaneous pseudoaneurysm is a rare cause of colicky flank pain.
-
•
Endovascular treatment is a good treatment option.
-
•
Most pseudoaneurysms present after direct penetrating injury and not spontaneously.
Introduction
Renal artery aneurysms can be divided into true aneurysms and pseudoaneurysms, based on the composition of the vascular wall. They are mostly asymptomatic and occur predominantly in female patients. In addition, they develop mostly extraparenchymally and in the right kidney.1, 2 The incidence of true renal artery aneurysms varies between 0.01 and 1% in the general population.1, 2 The aetiology of pseudoaneurysms is thought to be secondary to trauma.3 The incidence of spontaneous rupture is unknown; however, this can lead to a life threatening haemorrhage that requires direct treatment.
In this report, a young female patient with a giant intraparenchymal renal artery pseudoaneurysm, treated by selective endovascular embolisation (coiling) is described.
Informed consent was obtained from the patient.
Case report
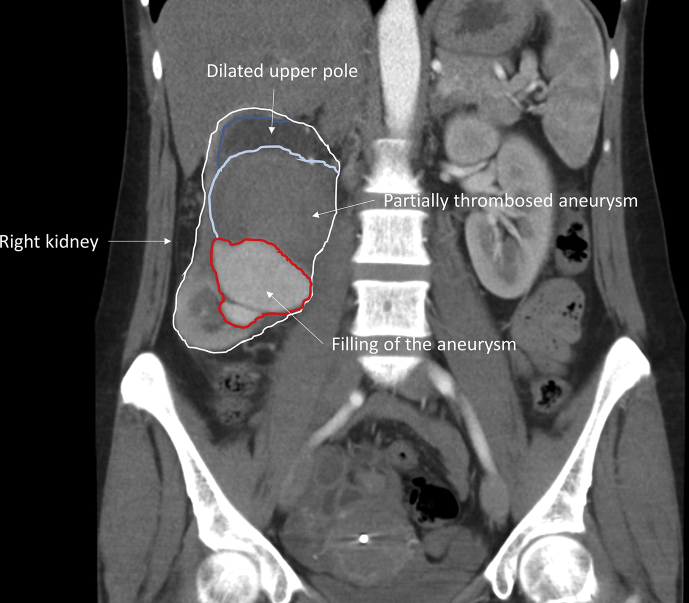
A 42 year old female patient presented at the Emergency Department with complaints of colicky right flank pain, nausea, and vomiting for three days. There was no history of fever, haematuria, dysuria or changes in defaecation pattern. Her past medical and family history revealed no relevant abnormalities. On physical examination, the patient was haemodynamically stable. She complained of tenderness on palpation of the right hemi-abdomen, without clinical signs of peritonitis. Laboratory results showed microcytic anaemia (haemoglobin concentration 4.6 mmol/L), leukocytosis (total white blood cell count 15.4 × 109/L) and C reactive protein of 51 mg/L. Renal function was normal with a creatinine 54 μmol/L and an estimated glomerular filtration rate of 112 mL/min/1.73 m2. A urine sample was taken and revealed traces of protein and erythrocytes. Abdominal ultrasound and contrast enhanced computed tomography (CT) scan showed hydronephrosis of the right kidney and a giant intracapsular, partially thrombosed pseudoaneurysm of the inferior branch of the right renal artery (Fig. 1). A trace of fluid surrounding the lower part of the aneurysm suggested a contained rupture.
Figure 1.
Computed imaging of the giant haematoma in the right kidney (coronal view).
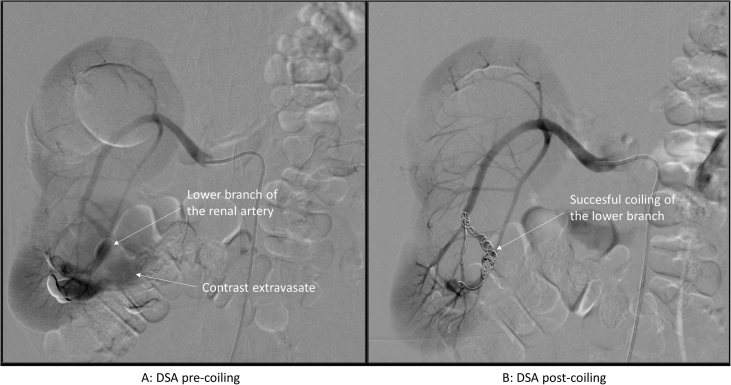
The patient was admitted to hospital to undergo a selective embolisation (coiling) of the inferior branch of the right renal artery leading to the pseudoaneurysm. This endovascular procedure was performed under local anaesthesia. A 6F sheath was inserted in the right common femoral artery. Selective catheterisation of the right renal artery was followed by catheterisation of its inferior branch with a 2.7 F Progreat® microcatheter (Terumo Interventional Systems, Tokyo, Japan). The microcatheter was passed into the pseudoaneurysm (Fig. 2A), and coil embolisation was performed of the renal artery branch leading to the pseudoaneurysm using five 10 mm Tornado coils (Cook Medical LLC, Bloomington, USA). A completion angiogram showed complete aneurysm obliteration, preserving blood flow to the main renal artery branches (Fig. 2B).
Figure 2.
Angiography of endovascular treatment of the aneurysm (A, catheterisation; B selective coiling of the lower renal artery).
Over the following days, the haemoglobin level improved after transfusion of one unit of erythrocytes to a haemoglobin concentration of 5.8 mmol/L. Renal function remained stable with creatinine levels of 50–54 μmol/L and an estimated glomerular filtration rate (GFR) of 110–114 mL/min/1.73 m2. An abdominal ultrasound showed no active bleeding in the right kidney. The patient was discharged five days after the procedure and was seen in the outpatient clinic after four weeks for repeat laboratory tests. At that time, her complaint of flank pain had diminished, leaving only a minor swelling of the right flank. Furthermore, the laboratory results showed no abnormalities in renal function, with creatinine levels remaining stable at 56 μmol/L and estimated glomerular filtration rate of 110 mL/min/1.73 m2 (previous values were 54 μmol/L and 112 mL/min/1.73 m2, respectively).
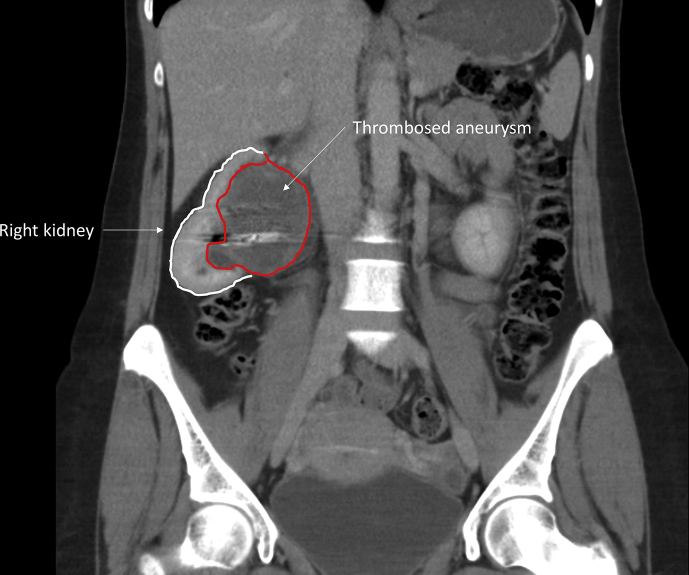
Follow up at 12 weeks with a renal scintigraphy, with intravenous 103 MBq 99mTc-succimer, showed similar renal function in the left (60%) and right (40%) kidneys. A contrast enhanced CT scan after nine months showed a reduction in aneurysm size from eight to five cm and no active filling of the aneurysm sac (Fig. 3). At 12 months of follow up renal function remained stable (creatinine 57 μmol/L), and estimated glomerular filtration rate 109 mL/min/1.73 m2. The patient was discharged from surgical follow up after one year and was referred to the nephrologist for further follow up.
Figure 3.
Follow up computed tomography imaging at 9 months (coronal view).
Discussion
This report discusses the case of a young female patient with an uneventful medical history who presented at the Emergency Department with colicky pain (complaints mimicking kidney stones) in the right flank. After routine laboratory results (showing anaemia and elevated infection parameters), an ultrasound was carried out to exclude inflammatory or traumatic causes for the abdominal pain. The ultrasound showed a partially thrombosed pseudoaneurysm of the right renal artery, which was confirmed by the (immediately following) contrast enhanced CT scan: a giant intraparenchymal pseudoaneurysm in the right kidney with hydronephrosis, explaining the patient's clinical symptoms.
True aneurysms and pseudoaneurysms are two different conditions each with their own aetiology. Both can be diagnosed by ultrasound, contrast enhanced CT, or magnetic resonance imaging. An intra- or extraparenchymal location, saccular or fusiform shape, and the size of the aneurysm determine its classification. The clinical presentation differs from asymptomatic, anaemia, flank pain, haematuria, abdominal mass, hypertension, or even haemorrhagic shock.3 The aetiology of true aneurysms of the renal artery include atherosclerosis, fibromuscular dysplasia, or intrinsic collagen deficiencies (e.g. Ehlers–Danlos syndrome, Marfan syndrome, and neurofibromatosis). Studies have shown that pseudoaneurysms arise from direct arterial injuries that cause disruption of arterial wall continuity. These injuries can be categorised as either traumatic, after a percutaneous procedure, post surgery, or due to inflammation (vasculitis), drugs (amphetamine), or neoplasia. However, most pseudoaneurysms present after direct penetrating injury of the renal artery, for example abdominal trauma or surgical/percutaneous procedures.3, 4
Interestingly, the patient did not present with any of these causes, which would indicate that this was a spontaneously developed pseudoaneurysm. However, it is unclear if she suffered trauma at a younger age. She was not haemodynamically unstable, but was anaemic (haemoglobin 4.6 mmol/L). The thin layer of cortex in the upper part of the kidney that was caused by compression of the pseudoaneurysm indicated that the aneurysm must have been present for a longer period of time. The acute onset of pain and the small amount of fluid surrounding the pseudoaneurysm suggested a contained rupture. Fortunately, the stable condition allowed diagnostics to be performed properly and multiple treatment options to be considered, eventually leading to the endovascular coiling. Nevertheless, it should be noted that in most patients with suspicion of a serious illness nowadays, a CT scan is performed with a relatively low threshold, which enables these rare conditions to be discovered.
In small true aneurysms (<2 cm) observation with follow up can be appropriate. However, this is not the case in pseudoaneurysms, because haemorrhage from such a lesion can be acute, rapid, and potentially lethal.4 An appealing treatment of intraparenchymal renal artery pseudoaneurysms is by endovascular catheter angio-embolisation, using thrombogenic agents or occluding coils. This results in minimal loss of renal parenchyma3 and has a high technical success rate for this condition.5 The endovascular treatment options clearly depend on the anatomy and might be established by coiling of the afferent artery or stenting of the affected vessel. A less invasive treatment option is an ultrasound guided percutaneous injection of thrombogenic material into the aneurysmal sac.4 This can be used in selected, haemodynamically stable patients, but provides less control during the procedure. Surgery should be reserved for those patients in whom less invasive treatment fails, and nephrectomy should be avoided whenever possible. In this case, successful treatment with endovascular coiling was achieved and the patient remained haemodynamically stable in the days after the procedure. Follow up demonstrated no adverse events. After an extensive literature search, only one similar case of a spontaneously developed pseudoaneurysm has been reported6 (PubMed search described in Appendix I).
Conclusion
This young female patient who presented with a giant pseudoaneurysm of the renal artery was successfully treated by endovascular coiling and to date, does not have any complications from the procedure. In addition, her renal function remained stable. In patients with flank pain that cannot be attributed to the more common causes, a renal artery pseudoaneurysm should be considered and treated promptly to prevent a life threatening haemorrhage. In this patient, the affected kidney could be preserved with adequate diagnostics and endovascular treatment. At the one year follow up the aneurysm remains excluded and her renal function is normal.
Funding
None.
Conflict of interest
None.
Acknowledgments
The authors acknowledge the helpful contribution of Thomas Vissers, librarian at the Haaglanden Medical Centre.
Appendix I.
PubMed search
(“Aneurysm, False”[Mesh] OR (fals*[ti] AND aneurysm*[ti]) OR false aneurysm*[tiab] OR pseudoaneurysm*[tiab] OR pseudo-aneurysm*[tiab] OR pseudaneurysm*[tiab]) AND (“Renal Artery”[Mesh] OR renal arter*[tiab] OR intrarenal arter*[tiab] OR renal*[ti] OR intrarenal*[ti] OR kidney*[ti] OR nephr*[ti]) AND (“Embolization, Therapeutic”[Mesh] OR emboliza*[tiab] OR embolize*[tiab] OR embolisa*[tiab] OR embolise*[tiab] OR angioembolis*[tiab] OR angioemboliz*[tiab] OR embolotherap*[tiab] OR embolo-therap*[tiab])
((“Aneurysm, False”[Mesh] OR (fals*[ti] AND aneurysm*[ti]) OR false aneurysm*[tiab] OR pseudoaneurysm*[tiab] OR pseudo-aneurysm*[tiab] OR pseudaneurysm*[tiab]) AND (“Renal Artery”[Mesh] OR renal arter*[tiab] OR intrarenal arter*[tiab] OR renal*[ti] OR intrarenal*[ti] OR kidney*[ti] OR nephr*[ti])) NOT (“Embolization, Therapeutic”[Mesh] OR emboliza*[tiab] OR embolize*[tiab] OR embolisa*[tiab] OR embolise*[tiab] OR angioembolis*[tiab] OR angioemboliz*[tiab] OR embolotherap*[tiab] OR embolo-therap*[tiab])
Search performed in September 2018.
References
- 1.Eskandari M.K., Resnick S.A. Aneurysms of the renal artery. Semin Vasc Surg. 2005;18:202–208. doi: 10.1053/j.semvascsurg.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Coleman D.M., Stanley J.C. Renal artery aneurysms. J Vasc Surg. 2015;62:779–785. doi: 10.1016/j.jvs.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Cura M., Elmerhi F., Bugnogne A., Palacios R., Suri R., Dalsaso T. Renal aneurysms and pseudoaneurysms. J Clin Imag. 2011;35:29–41. doi: 10.1016/j.clinimag.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Ngo T.C., Lee J.J., Gonzalgo M.L. Renal pseudoaneurysm: an overview. Nat Publishing Group. 2010;7:619–625. doi: 10.1038/nrurol.2010.163. [DOI] [PubMed] [Google Scholar]
- 5.Etezadi V., Gandhi R.T., Benenati J.F., Rochon P., Gordon M., Benenati M.J. Endovascular treatment of visceral and renal artery aneurysms. J Virol. 2011;22:1246–1253. doi: 10.1016/j.jvir.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Hadjipavlou M., Abbaraju J., Serafimov J., Madaan S. Spontaneous rupture of a renal artery pseudoaneurysm with no precipitating risk factor. J Royal Soc Med (JRSM) open. 2018;9:1–5. doi: 10.1177/2054270418758568. [DOI] [PMC free article] [PubMed] [Google Scholar]