Abstract
Giardia duodenalis is an ubiquitous parasitic pathogen that causes significant morbidity and mortality worldwide. Failures in drug therapy are commonly due to poor patient compliance as a result of the need for repeated administration, off target drug effects and increasing parasite drug resistance. In this study the in vitro efficacy and selectivity of the aminoguanidine compound robenidine and 2 structural analogues against Giardia were determined. After 5 h exposure to each compound the IC50 was as low as 0.2 μM with corresponding MLCs as low as 2.8 μM. This is in contrast to metronidazole which required 24 h to exhibit inhibitory activity. A modified adherence assay, developed for this study, demonstrated that three of the compounds inhibited in vitro adherence of the parasite. The lead compound exhibited rapid giardicidal activity (<5hr). In addition, microscopy studies demonstrated damage to the plasma membrane of trophozoites. In conclusion, a class of aminoguanidines, represented by robenidine, has shown antigiardial activity warranting further investigation.
Keywords: Giardia duodenalis, Giardiasis, Drug development, Antigiardial, Giardicidal, Adherence
Graphical abstract
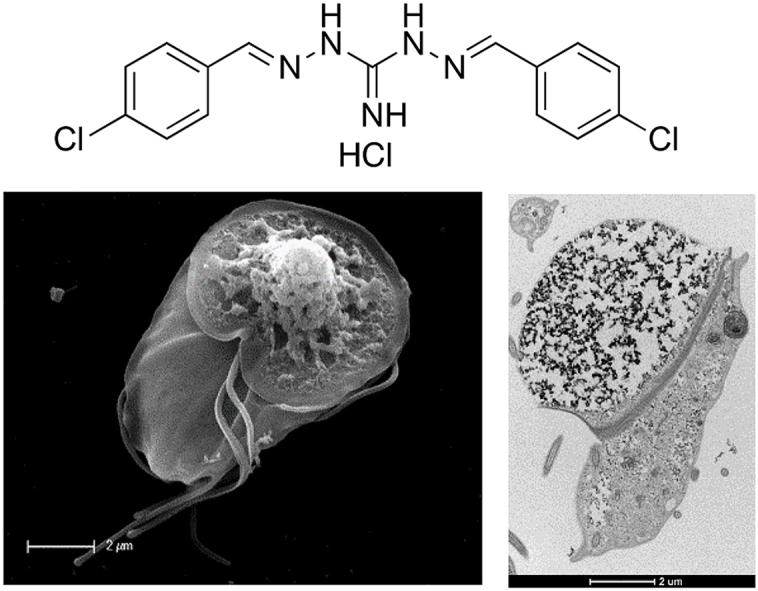
Highlights
-
•
Robenidine has faster antigiardial activity in vitro compared to metronidazole.
-
•
Novel structures developed with improved antigiardial activity compared to robenidine.
-
•
Giardia is unable to recover after short exposure times to novel antigiardials
-
•
Electron microscopy images show detailed structural damage after robenidine exposure.
1. Introduction
Giardia duodenalis (syn. Giardia lamblia, Giardia intestinalis) is a bi-nucleate protozoan pathogen estimated to cause between 130 million and 262 million human infections annually (Kirk et al., 2015), making it the most common protozoal pathogen worldwide (Halliez and Buret, 2013; Upcroft and Upcroft, 1998, 2001). Although infections are common in developed countries they are more prevalent in developing nations. Giardiasis has been recognised by the World Health Organisation as a neglected disease causing widespread morbidity worldwide (Savioli et al., 2006). Giardia infection is acquired via ingestion of cysts, either directly through a faecal-oral route or by contaminated food or water (Savioli et al., 2006; Thompson, 2000). Giardia results in a malabsorptive gastrointestinal disease with symptoms including diarrhoea, bloating and abdominal cramping (Buret, 2008). Symptoms may be acute or chronic and re-occurring. Persistent infection, especially in children and immunocompromised hosts, results in long term effects including malnutrition, developmental delay and failure to thrive syndrome (Wright et al., 2003).
Current antigiardial drugs used to treat giardiasis are drawn from the nitroimidazole, nitrothiazole, nitrofuran, acridine, benzimidazole, quinolone and aminoglycoside structural classes (Wright et al., 2003). The most frequently used nitroimidazoles, metronidazole and tinidazole, have a treatment success rate of 80–90%; while albendazole, a benzimidazole, has a reported efficacy of 62–95% (Wright et al., 2003). Treatment failures with these drugs are frequently reported and many exhibit unwanted side effects including but not limited to, nausea, fatigue and malaise (Wright et al., 2003). Metronidazole, is known to cause vomiting, weakness and headaches and is potentially carcinogenic (Nagel and Aronoff, 2015; Bendesky et al., 2002; Jokipii and Jokipii, 1979). Furthermore, treatment failure due to the development of resistant organisms has been reported for all commonly used antigiardial drugs (Nagel and Aronoff, 2015; Jokipii and Jokipii, 1979).
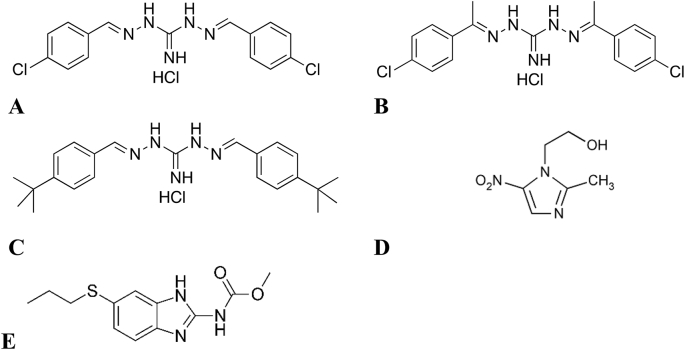
The combination of ineffective treatments resulting from adverse side effects and emerging resistance to all classes of antigiardial drugs provides an imperative to identify and develop low side effect, low toxicity antigiardial compounds. In this study we explored the potential of robenidine, a symmetrical chloroaromatic compound linked via a guanidinal core, as a lead compound for the development of novel antigiardial drugs (Fig. 1). Robenidine has been in use in the commercial poultry and rabbit industries as an anticoccidial agent since the early 1970s (Kantor et al., 1970). As part of an on-going antigiardial drug development program, in-house screening identified robenidine as possessing antigiardial effects, but with undesired off target actions (Abraham et al., 2016). We thus sought to more closely examine exemplar guanidinal linked aromatic compounds as potential antigiardial agents with an improved safety profile. Herein, we evaluated the antigiardial activity of robenidine and the activity of two structural analogues, (E)-N'-((E)-1-(4-chlorophenyl)ethylidene)-2-(1-(4-chlorophenyl)ethylidene)hydrazine-1-carboximidhydrazide hydrochloride (NCL 062) and N′,2-bis((E)-4-(tert-butyl)benzylidene)hydrazine-1-carboximidhydrazide hydrochloride (NCL 099) (Fig. 1).
Fig. 1.
Chemical structures of the four compounds investigated in this study. A. robenidine; B. NCL 062; C. NCL 099; D. Metronidazole; and E. Albendazole.
2. Materials and methods
2.1. Chemicals
Robenidine was provided by Neoculi Pty Ltd (Burwood, Vic, Australia). NCL 062 and NCL 099 were synthesised at the University of Newcastle (Abraham et al., 2016). The remaining drugs used in this study were sourced from Sigma Chemical Company (St Louis, Missouri).
Chemicals used for the culture of Giardia in vitro were sourced as follows: glucose and L-cysteine (ACROS organics, Thermo Fisher Scientific, Scoresby, Vic), ammonium iron (III) citrate, ascorbic acid (Sigma-Aldrich, Castle Hill, NSW), potassium dihydrogen orthophosphate (UNIVAR, Ingleburn, NSW), bovine bile (Fluka analytical (BD)), di-potassium hydrogen orthophosphate (Fronine Laboratories and Supplies, Riverstone, NSW).
2.2. Cell culture
G. duodenalis (BAH2c2 and BAH12 strain) was cultivated according to the method of Clark and Diamond in Keister's modified TYI-S-33 medium, supplemented with heat inactivated foetal bovine serum (Hyclone™, Thermo Fisher Scientific, Scoresby, Victoria, Australia) at 37 °C in plastic 9 mL screw-capped test tubes (nunc, Thermo Fisher Scientific, Scoresby, Victoria, Australia). Subcultures were performed once a confluent monolayer was observed, approximately 2–3 times per week (Clark and Diamond, 2002).
2.3. In vitro drug efficacy assays
2.3.1. Resazurin reduction assay
The in-vitro compound efficacy was determined using a resazurin reduction assay as previously described (Benere et al., 2007). The media of confluent cultures was replaced with fresh media and the cultures were placed on ice for 40 min to detach trophozoites. Trophozoites were enumerated using a haemocytometer and 50 000 trophozoites were added to each test well of a 96 well plate. Doubling or tripling dilutions of the test compounds were added to wells beginning at 25 or 50 μM compound concentration (previously prepared in DMSO). Metronidazole and DMSO (vehicle only) were used as controls. Plates were incubated under anaerobic conditions, using anaerobic gas generating sachets, (AnaeroGen, Thermo Fisher Scientific, Scoresby, Victoria, Australia) for 24 h at 37 °C. After incubation the media was removed and replaced with an equal volume of warm PBS. Resazurin (Alamarblue, Thermo Fisher Scientific, Scoresby, Victoria, Australia) was then added at 10% of the total volume of the wells. Plates were further incubated (1.5 h) for colour development and absorbance read at 570 nm and 630 nm. The percentage resazurin reduction was then calculated using the following formula:
| ((εoxi630 x A570) – (εoxi570 x A630))/((εred570 x C630)-(εred x C570)) x 100 |
Where: εoxi630 = 34798, εoxi570 = 80586, A570 = absorbance at 570 nm, A630 = absorbance at 630 nm, εred570 = 155677, εred630 = 5494, C630 = absorbance of negative control well at 630 nm and C570 = absorbance of negative control well at 570 nm.
The effect of the compounds on the proliferation of Giardia trophozoites was determined following the protocol outlined above, with the following modifications. The number of trophozoites was decreased to 1000 per well and the cells were incubated in the presence of each compound for 96 h at 37 °C.
2.3.2. Modified adherence assay
A second screening method, based on counting adherent trophozoites, was developed and used to validate the primary screen method, modified from earlier adherence assay protocols (Crouch et al., 1986). The assay was prepared in 24 well plates with plastic coverslips placed in the bottom. Trophozoites were prepared as above by placing on ice and ∼5 × 105 cells/mL were added to each well. Drugs prepared in DMSO were added at the required concentration, with the DMSO concentration never exceeding 1%. Assay plates were incubated for 5 h at 37 °C under anaerobic conditions, the media removed and cells adhering to the coverslips were fixed with glutaraldehyde or methanol. Once the coverslips were dry, cells were stained with a Romanowsky stain variant (Rapid stain), fixed to a glass slide and imaged at 10x or 100x magnification. Images (10x magnification) were processed with DotCount v1.2 software (http://reuter.mit.edu/, 2008–2012 © Martin Reuter) to count the number of cells present and data analysed using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. Images (100x magnification) were assessed for obvious morphological changes.
2.4. Mechanism of action
2.4.1. Transmission electron microscopy
To determine any effects robenidine has on the ultrastructure of Giardia trophozoites, transmission electron microscopy (TEM) was performed. Giardia BAH2c2 trophozoites were exposed to 3 X IC50 of robenidine or metronidazole for 1 h and placed on ice for 40 min to detach trophozoites then washed twice with PBS (900×g, 10 min 4 °C) and fixed with a combination of glutaraldehyde and formaldehyde overnight. Cells were washed with PBS +4% sucrose before fixation with osmium tetroxide for 1 h. Samples were dehydrated through a graded ethanol series (70–100%) followed by suspension in propylene oxide for ten minutes. Samples were centrifuged and suspended in 1:1 mixture of propylene oxide and epoxy resin for an hour before centrifugation. Following overnight suspension in 100% epoxy resin samples were resuspended in fresh resin and polymerized at 70 °C for 24 h. After sectioning samples were stained with uranyl acetate and lead citrate. Sections were imaged with a FEI tecnai G2 Spirit Transmission Electron microscope (Adelaide Microscopy, University of Adelaide).
2.4.2. Scanning electron microscopy
To determine the effect that robenidine and metronidazole has on the cell surface of the Giardia trophozoites, scanning electron microscopy (SEM) was performed as follows. Giardia BAH2c2 trophozoites were prepared as for TEM, however they were exposed to compounds for 2 or 4 h. After fixation with glutaraldehyde cells were attached to glass coverslips (pre-treated with poly-L-Lysine for 10 min) and washed with PBS + 4% sucrose before dehydration through a graded ethanol series (70–100%). Following ethanol dehydration coverslips were dried using a critical point drier, coated with platinum and observed using a ZIESS SEM (CMCA, University of Western Australia).
2.5. Giardia recovery assay
G. duodenalis trophozoites were harvested on ice as outlined above and 5 × 105 cells/mL were added to 1.5 mL centrifuge tubes. Cells were exposed to 5x the IC50 of robenidine, metronidazole or DMSO (1%) only for 5 h under anaerobic conditions at 37 °C. After exposure cells were collected by placing tubes on ice for 40 min followed by centrifugation at 900×g for 5 min. The supernatant was removed and cells were resuspended in 8 mL of fresh media (in a 9 mL culture tube). The number of trophozoites was counted using a haemocytometer and cells were incubated for 48 h with cell numbers being determined at 24 and 48 h.
2.6. In vitro cytotoxicity
Human cells lines, Hep G2 (ATCC HB-8065) and HEL 299 (ATCC CCL-137), were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco cat no. 12430) supplemented with 10% foetal bovine serum and 1% PenStrep (100U/ml penicillin and 100 μg/mL streptomycin). Cells were passaged every 3 days. Assays were performed in 96 well plates in duplicate. Wells were seeded with 50 000 cells. Twenty four hours post seeding cells were washed and fresh media added. Two hours post wash, compounds diluted in DMSO were added in doubling dilution at a concentration of 1% DMSO. After 24 h exposure, WST-1 reagent at a final concentration of 10% was added to each well and absorbance at 450 nm was recorded after 1 h. The GI50 values were determined using the nonlinear regression function of GraphPad Prism v6 software.
2.7. Statistical analysis
The results for the in vitro drug efficacy studies were analysed using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. For the resazurin reduction assay the mean and standard error of the mean were determined with each assay completed in triplicate. The IC50 was calculated using the log (inhibitor) vs. normalised response – variable slope function in Graphpad Prism. For the adherence assay mean and standard error of the mean were calculated and data analysed using an unpaired t-test, relative to untreated control.
3. Results and discussion
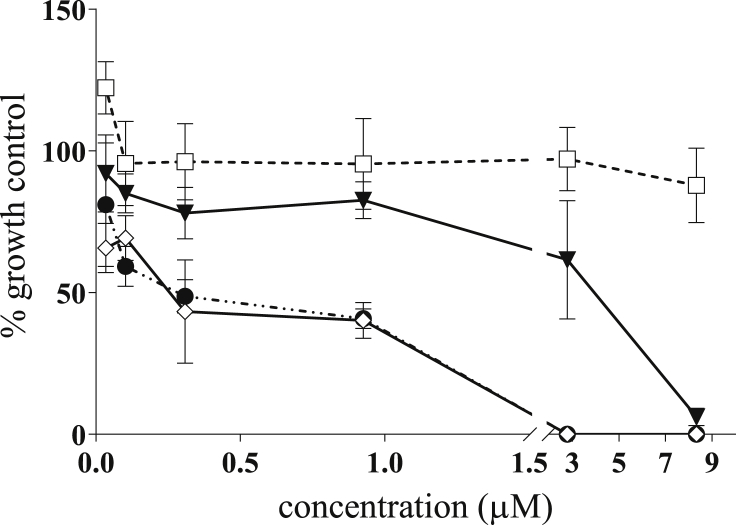
In this study, we demonstrated that robenidine, NCL 062 and NCL 099 have potent rapid in vitro activity against G. duodenalis (Fig. 2). The IC50 of each compound was determined using a resazurin reduction assay, which measures the metabolic activity of cells, using the log (inhibitor) vs. normalised response – variable slope function in Graphpad Prism. The compounds were tested against an assemblage A (BAH2c2, 24 h) and an assemblage B (BAH12, 24 h) isolate (Table 1). The parent molecule, robenidine, had antigiardial activity with an IC50 of 2.9 ± 2.9 μM and a minimum lethal concentration (MLC), the concentration at which no metabolism of resazurin was observed, of 2.8–8.3 μM after 24 h. This is similar to the current gold-standard treatment, metronidazole, which returned an IC50 of 2.0 ± 1.0 μM and an MLC of 2.8 μM. Both of the robenidine analogues tested displayed potent antigiardial efficacy with IC50 values of 3.0 ± 0.3 and 0.8 ± 0.6 μM respectively for NCL 099 and NCL 062. Variation in the MLCs was observed with NCL 062 having an MLC of 0.9 μM and NCL 099 with an MLC of 8.3 μM. Activity against the assemblage B isolate was similar to the results for the assemblage A isolate (Table 1). The effect of the compounds on the proliferation of Giardia trophozoites (BAH2c2) was also determined. The IC50 determined above and the IC50 of proliferation were similar for all compounds except NCL 099 where the IC50 of proliferation was 4-fold higher than the IC50. (Table 1).
Fig. 2.
Metabolic inhibitory activity of robenidine, NCL 062 and NCL 099 against Giardia duoenalis BAH2c2 strain, assemblage A. Trophozoites were exposed to the compounds for 5 h before removal of the compounds and addition of resazurin. Absorbance was measured (630 and 570 nm), percent reduction of resazurin calculated and data presented as a percentage of the growth control. Treatment groups are: square - metronidazole; triangle –NCL 099; diamond - robenidine; and circle - NCL 062. Error ± SEM, n = 3. Data represents a typical experiment. Note the break in the x-axis indicates a change in the scale of drug concentration.
Table 1.
Antigiardial IC50 values and MLC for robenidine, NCL 099, NCL 062, metronidazole and albendazole against Giardia duodenalis assemblage A and B after 24 h. Data were obtained using a resazurin metabolic assay. IC50prol refers to the concentration to cause 50% inhibition in the proliferation assay.
| Compound | Assemblage A (μM) |
Assemblage B (μM) |
RAW264.7 (μM) |
|||
|---|---|---|---|---|---|---|
| IC50 | IC50prol | MLC | IC50 | MLC | GI50 | |
| Robenidine | 2.9 ± 2.9 | 1.2 ± 0.7 | 2.8–8.3 | 4.7 ± 0.6 | 16.6 | 17.1 |
| NCL099 | 3.0 ± 0.3 | 11.1 ± 0.8 | 8.3 | 3.3 ± 0.2 | 5.5 | 7.3 |
| NCL062 | 0.8 ± 0.6 | 2.0 ± 1.3 | 0.9 | 1.8 ± 0.0008 | 3.1 | 4.0 |
| Metronidazole | 2.0 ± 1 | 1.7 ± 2.0 | 2.8 | 3.8 ± 0.5 | 16.6 | NT |
| Albendazole | <0.02 | 0.8 ± 0.7 | 0.02–0.07 | NT | NT | NT |
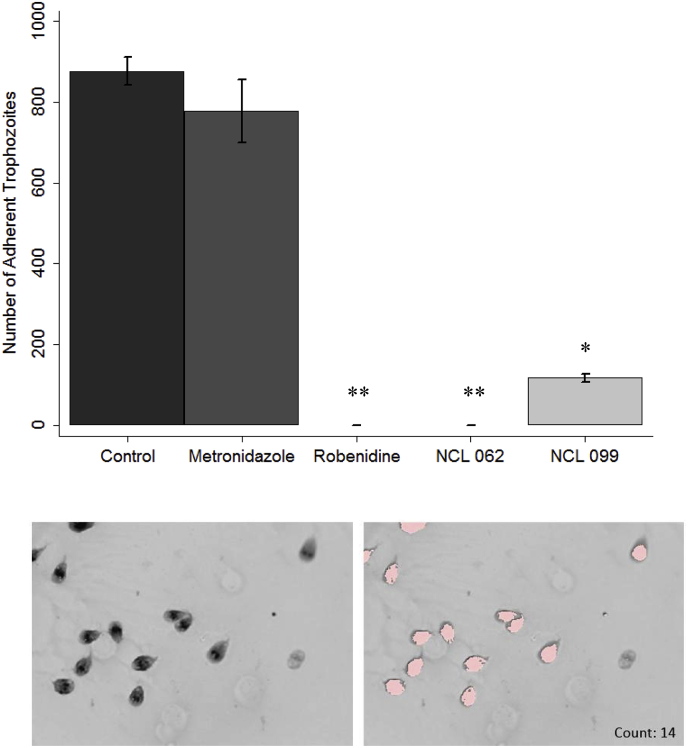
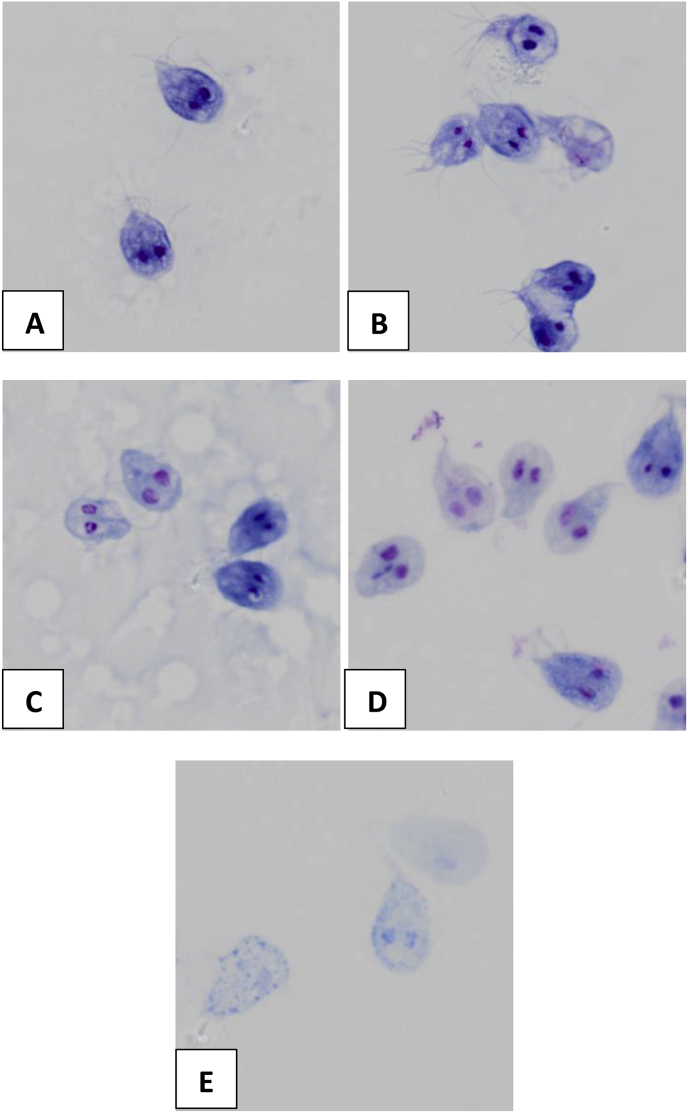
As trophozoite adherence is also a key characteristic in the establishment and development of Giardia infection, the effect of compounds on trophozoite adherence was examined further using a modified adherence assay. In contrast to metronidazole, robenidine, NCL 062 and NCL 099 significantly (p ≤ 0.05) affected the adherence of trophozoites at concentrations of 4 μM after 5 h exposure. With robenidine and NCL 062a complete inhibition of adherence was observed at 4 μM (Fig. 3). NCL 099 significantly affected adherence of trophozoites at 4 μM, but did not completely inhibit adherence. The effect of NCL 099 on cell membrane integrity was also observed in stained images of the trophozoites taken at 100 x magnification (Fig. 4). At the IC50 of NCL 099 (3.0 μM) a decrease in the membrane stain intensity was observed. At 2 x the IC50 of NCL 099 a disintegration of the cell membrane was observed (Fig. 4E). A decrease in the intensity of the membrane stain after treatment with robenidine at the IC50 (2.9 μM) was also observed and affected cells appeared to have enlarged nuclei (Fig. 4C). Previously published work has also demonstrated the mechanism of action of robenidine in bacteria is linked to disruption of the cell membrane in susceptible species (Ogunniyi et al., 2017). No cells could be imaged at higher concentrations of robenidine and NCL 062. Metronidazole had no significant effect on cell adherence at the time or concentration tested in this experiment, which is to be expected based on its known mechanism of action (Fig. 3) (Nagel and Aronoff, 2015).
Fig. 3.
Inhibition of adherence of Giardia duodenalis by metronidazole (Mz), robenidine, NCL 062 and NCL 099. Cells were exposed to the compounds for 5 h before staining. Stained cells were imaged at 10x magnification and counted using DotCount™ software. Each assay was completed in triplicate and 5 images taken per sample. Top. Number of adherent trophozoites after exposure to 4 μM of each compound for 5 h; Bottom. An example of the Dotcount software identifying trophozoites at higher magnification. Left: stained trophozoites, Right: highlighted trophozoites identified by the Dotcount software. Each highlighted cell is counted individually. Error ± SEM. * p-value <0.05, ** p-value <0.0001.
Fig. 4.
Morphological effect of Robenidine and NCL 099 on Giardia trophozoites. Giardia trophozoites were exposed to each compound for 24 h before fixation with glutaraldehyde and staining following the Diff quik protocol. A-control cells, B – cells treated with metronidazole (IC50, 2 μM), C- robendine treated cells (IC50, 2 μM), D – NCL 099 treated cells (IC50, 3 μM), E − NCL 099 treated cells (2 x IC50).
Despite robenidine being used for the past 40 years as an anticoccidial agent its mechanism of action against protozoa is not known. Previous studies of the mechanism of action of robenidine have been inconclusive with some suggesting that ATPase is the primary target while others have identified no obvious morphological effect in E. tenella on mitochondria or the nucleus, with chiefly the Golgi body and endoplasmic reticulum being affected (Lee and Millard, 1972; Wong et al., 1972). In the same study, swelling of the perinuclear space was noted, it was suggested this could be due to the overproduction of proteins potentially indicating an increase in metabolism causing the cells to self-destruct (Lee and Millard, 1972). In the present study, we undertook electron microscopy studies as a first step to elucidate a possible mechanism of action of robenidine against Giardia.
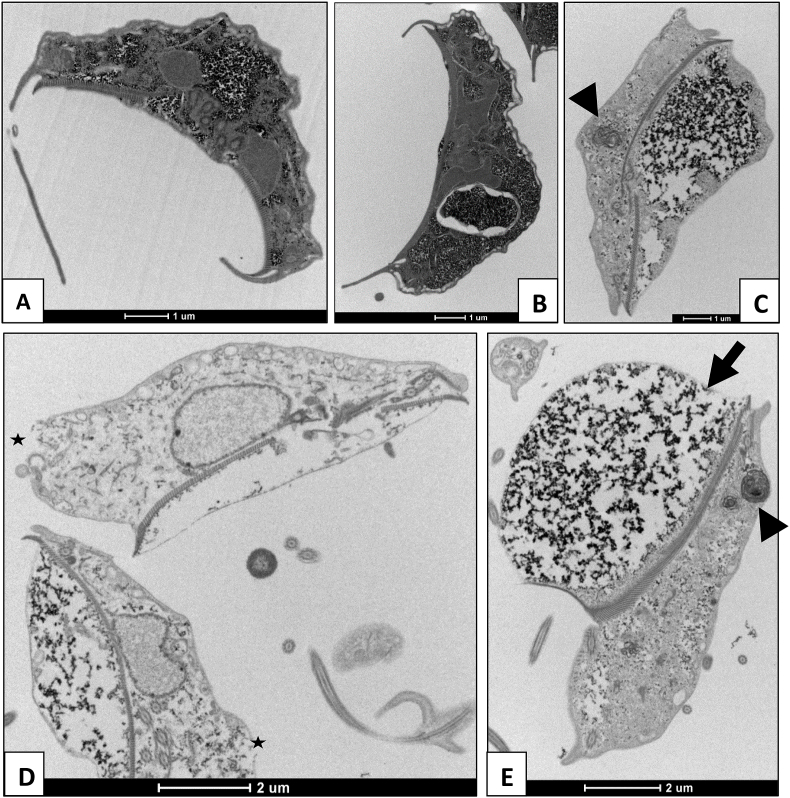
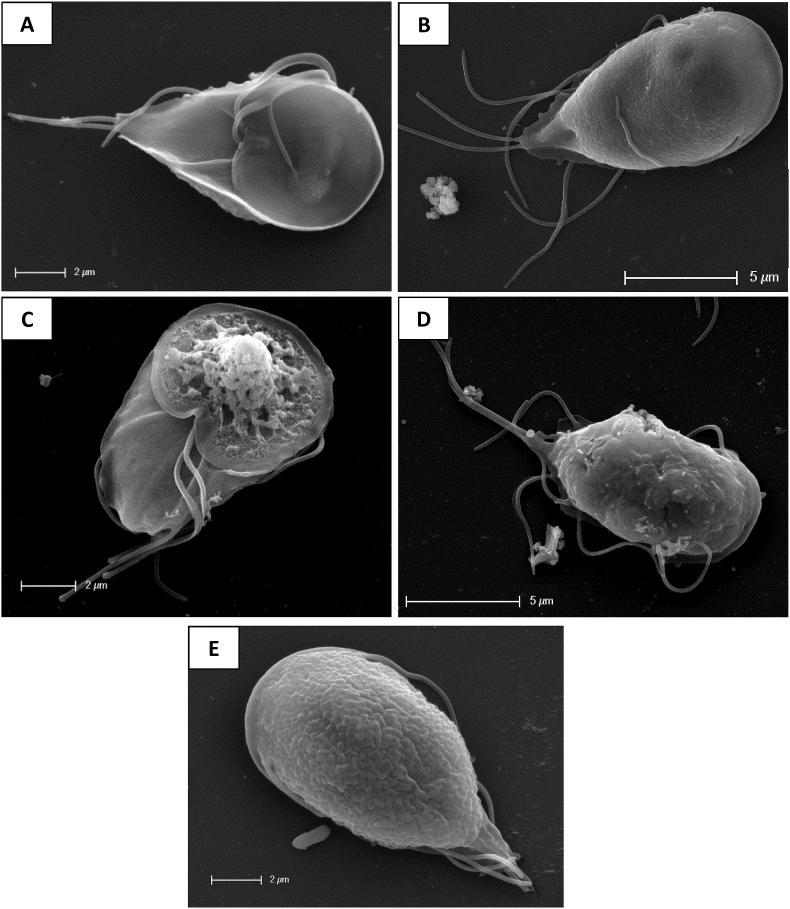
Electron microscopy showed gross morphological changes in the trophozoites after 1 h of exposure to robenidine (Fig. 5). TEM studies revealed that the treated trophozoites developed extreme membrane blebbing, most significantly affecting the adhesive disc, scattered with electron dense material. In addition, unusual vacuolar membranous structures appeared within the cytoplasm. Several trophozoites also exhibited rupturing of the dorsal cytoplasmic membrane and all observed trophozoites had various degrees of disintegration of the cytoplasmic space. SEM studies also showed extreme membrane blebbing, as seen in TEM, after 2 h of exposure. Furthermore, these images showed that there was severe damage to the cell wall of the engorged adhesive disk with distinct lesions observed on the surface. Based on the electron microscopy results of this study it appears that robenidine exposure results in a general swelling of the cells leading to rupture of the cell membrane and ultimately cell death (Fig. 6).
Fig. 5.
Transmission Electron Microscopy of Giardia duodenalis trophozoites: A control, and after exposure to B metronidazole for 4 h (3 x IC50) or C-E robenidine for 1 h (3 x IC50). Membrane blebbing at the adhesive disc can be seen in robenidine treated cells (arrows) as well as membranous structures within the cytoplasm (arrow head), disintegration of the cytoplasmic space and rupturing of the cell membrane (star). Images taken with a FEI tecnai G2 Spirit Transmission Electron microscope.
Fig. 6.
Scanning electron microscopy of Giardia duodenalis trophozoites: A control ventral, B control dorsal, C exposed to robenidine 2 h (3 x IC50) ventral, D exposed to robenidine 4 h (3 x IC50) dorsal. Multiple ruptures as well as blebbing at the adhesive disk can be seen on the ventral surface (C) while tiny blebbing and rupturing of the dorsal surface can be seen in D. E metronidazole exposed cells (3 x IC50) 4 h, dorsal.
It is possible that robenidine causes the plasma membrane of the protozoan to become destabilized, altering membrane properties leading to cell swelling and modifications of the cytoplasmic space. Another possible theory is that robenidine has a similar mechanism of action as the thiazolides which demonstrate a disintegration of the cytoplasmic space as observed by TEM and development of membrane ruptures on the adhesive disc resulting in a loss of osmotic potential (Muller et al., 2006). In addition, research into the mechanism of action of CGP 40215A, effectively a structural analogue of robenidine where the chlorides have been substituted with amine groups, identified a strong bond with the AT region of DNA (Nguyen et al., 2002). The strong bond was facilitated by the guanidine backbone which is conserved in robenidine, potentially providing another alternative mechanism of action for this series of compounds. The binding of robenidine analogues with appropriate structural, spatial and hydrogen bond characteristics to the AT region of DNA could result in a cascade of events resulting in a disruption of normal cellular process and eventually, cell death.
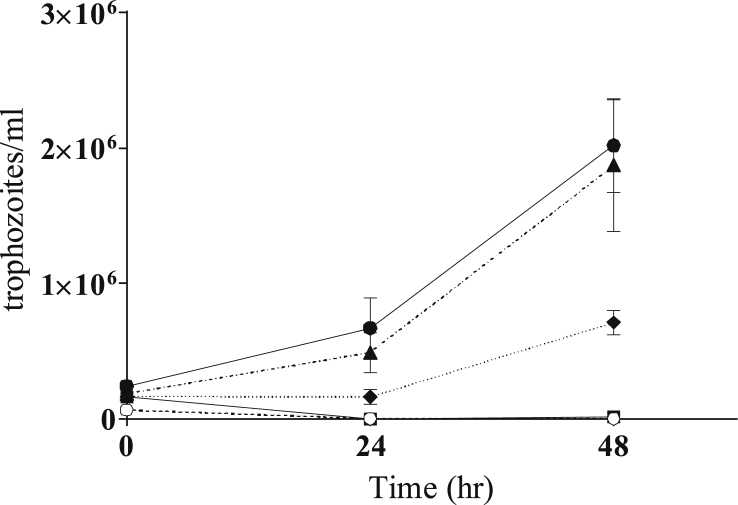
Trophozoites exposed to robenidine and NCL 062 were unable to recover after short-term exposure (5 h) at 5x the IC50, again, in contrast to metronidazole which did not have any permanent effects after short exposure times, most likely due to the mechanism of metronidazole which requires metabolism to form nitro radicals (Upcroft and Upcroft, 2001) (Fig. 7). NCL 099 treated trophozoites were able to recover after short-term exposure but growth was slower when compared to untreated and metronidazole treated cells.
Fig. 7.
Recovery assay of Giardia duodenalis exposed to robenidine and metronidazole. Giardia trophozoites were exposed to robenidine or metronidazole for 5 h at 5x the IC50. Key: closed circle – growth control, triangle – metronidazole, square – robenidine, open circle – NCL 062, diamond – NCL 099. Cell numbers were determined at 24 and 48 h post exposure. Error ± SD.
In addition to the potent in vitro and giardicidal activity, all three of the aminoguanidines studied here have the advantage of being highly insoluble in aqueous solution thereby potentially increasing the concentration at the site of infection by limiting systemic absorption from the gastrointestinal tract. While robenidine has been used extensively in the poultry and rabbit industries, we observed off-target antibacterial effects and cytotoxicity towards human lung fibroblast (HEL 299; GI50 = 17.7 μM) and human hepatocellular carcinoma (Hep G2; GI50 = 25.5 μM) cell lines. Cytotoxicity against murine macrophage cells for robenidine and both analogues was also observed (RAW 264.7; GI50 robenidine = 17.1 ± 4.4, NCL 062 = 4.0 ± 1.3, NCL 099 7.3 ± 2.8).
In conclusion, the results presented in this study demonstrated that the class of aminoguanidines related to robenidine, have potential to be developed as antigiardial agents. The three analogues presented here were potent, quick acting and have the potential to concentrate at the target site due to aqueous insolubility. These compounds provide a necessary starting point in the search for analogues with greater selectivity for Giardia and less off target effects against both host cells and the autochthonous bacteria of the gastrointestinal tract.
Acknowledgements
We would like to thank Prof. R. C. A. Thompson for the provision of the Giardia strains used in these experiments. The authors also acknowledge the facilities, and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy, Characterisation & Analysis, The University of Western Australia (A/Prof Peta Clode, Lyn Kirilak), and Adelaide Microscopy, The University of Adelaide (Lyn Waterhouse), facilities funded by the University, State and Commonwealth Governments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2019.04.003.
Funding
This research was supported by a Linkage Project grant from the Australian Research Council in collaboration with Neoculi Pty. Ltd (LP110200770) and Neoculi (Australia) Research Grants (#16632 and #17159).
Conflicts of interest
SWP is director of Neoculi Pty Ltd who are seeking to develop these analogues for use in humans and animals. There are no other conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abraham R.J., Stevens A.J., Young K.A., Russell C., Qvist A., Khazandi M., Wong H.S., Abraham S., Ogunniyi A.D., Page S.W., O'Handley R., McCluskey A., Trott D.J. Robenidine analogues as gram-positive antibacterial agents. J. Med. Chem. 2016;59:2126–2138. doi: 10.1021/acs.jmedchem.5b01797. [DOI] [PubMed] [Google Scholar]
- Bendesky A., Menendez D., Ostrosky-Wegman P. Is metronidazole carcinogenic? Mutat. Res. 2002;511:133–144. doi: 10.1016/s1383-5742(02)00007-8. [DOI] [PubMed] [Google Scholar]
- Benere E., Inocencio da Luz R.A., Vermeersch M., Cos P., Maes L. A new quantitative in vitro microculture method for Giardia duodenalis trophozoites. J. Microbiol. Methods. 2007;71:101–106. doi: 10.1016/j.mimet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Buret A.G. Pathophysiology of enteric infections with Giardia duodenalius. Parasite. 2008;15:261–265. doi: 10.1051/parasite/2008153261. [DOI] [PubMed] [Google Scholar]
- Clark C.G., Diamond L.S. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 2002;15:329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch A.A., Seow W.K., Thong Y.H. Effect of twenty-three chemotherapeutic agents on the adherence and growth inhibition of Giardia lamblia in vitro. Trans. R. Soc. Trop. Med. Hyg. 1986;80:893–896. doi: 10.1016/0035-9203(86)90249-x. [DOI] [PubMed] [Google Scholar]
- Halliez M.C., Buret A.G. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J. Gastroenterol. 2013;19:8974–8985. doi: 10.3748/wjg.v19.i47.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokipii L., Jokipii A.M. Single-dose metronidazole and tinidazole as therapy for giardiasis: success rates, side effects, and drug absorption and elimination. J. Infect. Dis. 1979;140:984–988. doi: 10.1093/infdis/140.6.984. [DOI] [PubMed] [Google Scholar]
- Kantor S., Kennett R.L., Jr., Waletzky E., Tomcufcik A.S. 1,3-Bis(p-chlorobenzylideneamino)guanidine hydrochloride (robenzidene): new poultry anticoccidial agent. Science. 1970;168:373–374. doi: 10.1126/science.168.3929.373. [DOI] [PubMed] [Google Scholar]
- Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C. l., Hald T., Hall A.J., Keddy K.H., Lake R.J., Lanata C.F., Torgerson P.R., Hayelaar A.H., Angulo F.J. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, Protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12:1–21. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.L., Millard B.J. Fine-structural changes in eimeria-tenella, from infections in chick-embryos and chickens, after exposure to anticoccidial drug robenidene. Parasitology. 1972;65:309. doi: 10.1017/s0031182000045091. [DOI] [PubMed] [Google Scholar]
- Muller J., Ruhle G., Muller N., Rossignol J.F., Hemphill A. In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob. Agents Chemother. 2006;50:162–170. doi: 10.1128/AAC.50.1.162-170.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J.L., Aronoff D.M. Updated Edition. 2015. Metronidazole. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. J.E. Bennett, R. Dolin and M.J. Blaser: 350-357.e352. [Google Scholar]
- Nguyen B., Lee M.P.H., Hamelberg D., Joubert A., Bailly C., Brun R., Neidle S., Wilson W.D. Strong binding in the DNA minor groove by an aromatic diamidine with a shape that does not match the curvature of the groove. J. Am. Chem. Soc. 2002;124:13680–13681. doi: 10.1021/ja027953c. [DOI] [PubMed] [Google Scholar]
- Ogunniyi A.D., Khazandi M., Stevens A.J., Sims S.K., Page S.W., Garg S., Venter H., Powell A., White K., Petrovski K.R., Laven-Law G., Totoli E.G., salgado H.R., Pi H., Coombs G.W., Shinabarger D.L., Turnidge J.D., Paton J.C., McCluskey A., Trott D.J. Evaluation of robenidine analog NCL195 as a novel broad-spectrum antibacterial agent. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0183457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savioli L., Smith H., Thompson A. Giardia and cryptosporidium join the ‘neglected diseases initiative’. Trends Parasitol. 2006;22:203–208. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. 2000;30:1259–1267. doi: 10.1016/s0020-7519(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Upcroft J., Upcroft P. My favorite cell: Giardia. Bioessays : News Rev. Mol. Cell. Dev. Biol. 1998;20:256–263. doi: 10.1002/(SICI)1521-1878(199803)20:3<256::AID-BIES9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Upcroft P., Upcroft J.A. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 2001;14:150–164. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D.T., Horng J.S., Wilkinson J.R. Robenzidene, an inhibitor of oxidative phosphorylation. Biochem. Biophys. Res. Commun. 1972;46:621–627. doi: 10.1016/s0006-291x(72)80185-2. [DOI] [PubMed] [Google Scholar]
- Wright J.M., Dunn L.A., Upcroft P., Upcroft J.A. Efficacy of antigiardial drugs. Expert Opin. Drug Saf. 2003;2:529. doi: 10.1517/14740338.2.6.529. 5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.