Abstract
Objective
To test the hypothesis that fusidic acid would not increase the treatment effect of disinfecting with povidone-iodine alone in children with impetigo.
Design
Randomised placebo controlled trial.
Setting
General practices in Greater Rotterdam.
Participants
184 children aged 0-12 years with impetigo.
Main outcome measures
Clinical cure and bacterial cure after one week.
Results
After one week of treatment 55% of the patients in the fusidic acid group were clinically cured compared with 13% in the placebo group (odds ratio 12.6, 95% confidence interval 5.0 to 31.5, number needed to treat 2.3). After two weeks and four weeks the differences in cure rates between the two groups had become smaller. More children in the placebo group were non-compliant (12 v 5) and received extra antibiotic treatment (11 v 3), and more children in the placebo group reported adverse effects (19 v 7). Staphylococcus aureus was found in 96% of the positive cultures; no strains were resistant to fusidic acid.
Conclusions
Fusidic acid is much more effective than placebo (when both are given in combination with povidone-iodine shampoo) in the treatment of impetigo. Because of the low rate of cure and high rate of adverse events in the placebo group, the value of povidone-iodine in impetigo can be questioned.
What is already known on this topic
Impetigo is the most common skin infection in children
Fusidic acid, which is advocated as topical treatment in several countries, has never been investigated in a placebo controlled study
What this study adds
In combination with povidone-iodine, treatment with fusidic acid is much more effective than placebo
None of the strains of Staphylococcus aureus isolated at baseline showed resistance to fusidic acid
The value of treatment with povidone-iodine alone can be questioned
Introduction
Impetigo is the most common skin infection in children; it is caused mainly by Staphylococcus aureus and sometimes by Streptococcus pyogenes (group A).1,2 Because of the supposedly benign spontaneous course of impetigo, some authors suggest that an expectant attitude with disinfection but no antibiotic treatment would suffice in mild cases.3–5 Antiseptic treatment with chlorhexidine or povidone-iodine is often advocated as a useful adjunct to antibiotic treatment.3,5
Immediate antibiotic treatment is advised for most cases of impetigo, to achieve a quick cure and prevent spread of the infection to other children.4–6 Oral antibiotic treatment has long been the first choice, because of better treatment results and because topical antibiotic treatment is more likely to induce sensitisation and bacterial resistance.7 In recent years, however, the resistance of staphylococci to oral antibiotics such as erythromycin has increased dramatically.1,8–11 At the same time, topical antibiotic treatment with mupirocin has been shown to give results equal to or even better than oral treatment.1,9,12,13 In general, children comply better with topically administered treatment than with oral treatment,13 and fewer systemic side effects occur.8
Fusidic acid is an antibiotic that has been available for a long time and is mainly used topically.14 It is recommended as the first choice topical antibiotic in the Dutch College of General Practitioners' guidelines on the treatment of impetigo.3 Some authors discourage the topical use of fusidic acid because of its value in systemic treatment,5,15 although other authors recommend that mupirocin should be reserved for treatment of nasal carriage of S aureus in specific groups of patients.3,16 A recent meta-analysis of three randomised trials found the overall clinical effect of fusidic acid cream in patients with impetigo to be equal to that of mupirocin,17 but the effectiveness of fusidic acid has never been assessed in comparison with placebo. The cost of fusidic acid compares favourably with that of mupirocin.
We compared the effectiveness of fusidic acid cream and placebo cream, both added to a disinfecting treatment with povidone-iodine, in the treatment of impetigo in children. Our hypothesis was that fusidic acid cream would not improve the treatment effect of povidone-iodine.
Methods
Participants
We asked general practitioners in the Greater Rotterdam area to report patients aged 0-12 years with non-bullous impetigo presenting at their surgery. We excluded patients who were immunocompromised; patients with extensive lesions (estimated area more than 5% of the total skin surface), infections of deeper skin structure, temperature >38.5°C, hypersensitivity to povidone-iodine, or hyperthyroidism; patients who had used topical or systemic antibiotics in the previous 48 hours; and patients for whom informed consent was not obtained. The medical ethics committee of Erasmus University and University Hospital Rotterdam approved the trial protocol.
The general practitioner notified the research nurse of suitable patients, and the nurse visited the children at home, usually the same day. The nurse recorded the duration of impetigo, nature of the lesions (redness, crusts, pustules, and painfulness), number of lesions, localisation and estimated area of lesions, body temperature, presence of regional lymphadenopathy, recent use of antibiotics, demographic data, and pre-existence of eczema. After obtaining written informed consent, the nurse took a bacterial swab of the lesions and started the treatment.
Interventions
The lesions were washed gently with povidone-iodine shampoo (Betadine shampoo; ASTA, Diemen, Netherlands), 75 mg/ml twice daily, in the morning and the evening. The study cream was applied three times a day (once after each shampoo and once at midday). The study cream was either 2% fusidic acid cream (Fucidin; Leo Pharmaceutical Products BV, Weesp, Netherlands) or placebo cream containing 13.5 g vaseline-cetomacrogol cream, 8.25 g glycerol 85%, 0.03 g sorbic acid, and distilled water up to 30 g. Patients were advised to use the study cream for a maximum of 14 days or until the lesions had disappeared and to use common hygienic measures (cutting nails short, use of personal towels).
The research nurse visited participants at home for evaluation at 7, 14, and 28 days after the start of treatment and recorded data on clinical cure, compliance, use of other drugs, violation of the protocol, and side effects. The nurse took a swab for bacterial culture of the lesions if they were still present. General practitioners were free to prescribe other treatment such as oral antibiotics if the impetigo worsened or did not improve, but they were encouraged to comply with the study protocol for at least the first week. When patients withdrew from the protocol regimen, the evaluation visits were carried out as planned.
Assignment and masking
The placebo cream, prepared by the pharmacist, did not differ in colour, smell, or consistency from the fusidic acid cream. An independent statistician provided a computer generated list of random set numbers in permuted blocks of six. The hospital pharmacist packed the study medication in identical blank tubes with a code number according to the randomisation list. We randomised the patients in blocks and stratified them for presence of pre-existing eczema at the site of the impetigo. The research nurse, who was unaware of the treatment allocation, allocated patients to the first available number of study cream in the corresponding block. The pharmacist kept the list of code numbers in a sealed envelope and was not involved in analysis of data or assessment of outcome. Unblinding took place after the primary statistical analysis had been done.
Outcomes
Primary outcome measures were clinical cure, improvement, and size of affected area, as assessed by the research nurse, and bacterial cure after 7, 14, and 28 days. We defined clinical cure as the complete absence of lesions or the lesions having become dry and without crusts; remaining local redness of intact skin was acceptable. In cases of incomplete cure the nurse recorded the number, localisation, and size of the affected area of the persisting lesions. The size of the affected area was estimated by comparing the size of the actual lesions with a range of examples with different exactly measured areas on paper. We defined improvement as a decline in affected area, number of lesions, or both. We defined bacterial cure as the elimination of the causative pathogen(s) in persisting lesions or the unavailability of a swab if lesions were cured. Adverse effects were secondary outcome measures. We considered baseline characteristics, causative pathogen, and resistance of the pathogen to fusidic acid at baseline as possible confounding factors.
Microbiological procedures
The nurse used sterile cotton wool swabs (Transwab; Medical Wire and Equipment, Corsham, UK) to obtain swab specimens of the wound. The largest five lesions were swabbed with one swab by gently rubbing the wound surface. The swabs were immediately placed in Stuart's transport medium and kept at 4°C until inoculation (within 24 hours). We cultured the swabs semiquantitatively on Columbia blood agar plates (Becton-Dickinson, Etten-Leur, Netherlands), which were incubated anaerobically; phenol red mannitol salt agar; and phenol red mannitol salt broth.18 We identified bacteria according to accepted international standards, on the basis of morphology of colonies on the Columbia blood agar and phenol red mannitol salt agar.19 Suspected colonies were cultured overnight on Columbia blood agar. We then did a catalase test and a latex agglutination test (Staphaurex Plus; Murex, Dartford, UK). We used antisera to the Lancefield groups A, B, C, F, and G cell wall carbohydrates (PathoDx, Strep Grouping latex agglutination kit; DPC, Los Angeles, CA) to identify β haemolytic streptococci. All isolates were stored at −70°C in liquid media containing glycerol. We used Vitek II equipment (bioMerieux Vitek, Hazelwood, MO) for susceptibility testing of isolates of S aureus. We used disk diffusion to test for susceptibility to mupirocin.20The panel of antibiotics for staphylococci consisted of erythromycin, fusidic acid, and mupirocin. We used the guidelines of the National Committee for Clinical Laboratory Standards to categorise strains as resistant, intermediately sensitive, or sensitive to the antibiotic used.21,22
Statistical analysis
We analysed the results both by intention to treat and per protocol. We used logistic regression analysis to calculated crude and adjusted odds ratios for dichotomous outcome measures. Baseline characteristics that had at least a weak relation (P<0.25) with the outcome variable in univariate logistic regression analyses were considered to be potentially adjusting variables. If continuous baseline characteristics showed a non-linear relation with the outcome variable, we transformed these into categorical variables with two or three levels. We used a forward selection procedure (Wald) to determine which adjusting variables entered the final multivariate model. For the change in affected area—a continuous outcome—we used linear regression analysis, with the logarithm of the ratio of the area of the lesions at three visits and the area at baseline as dependent variables. With a postulated spontaneous cure of 50% at one week and a detectable absolute difference of 25% in the treatment group, with α=0.05 and β=0.10, the planned study population was 85 children in each group.
Results
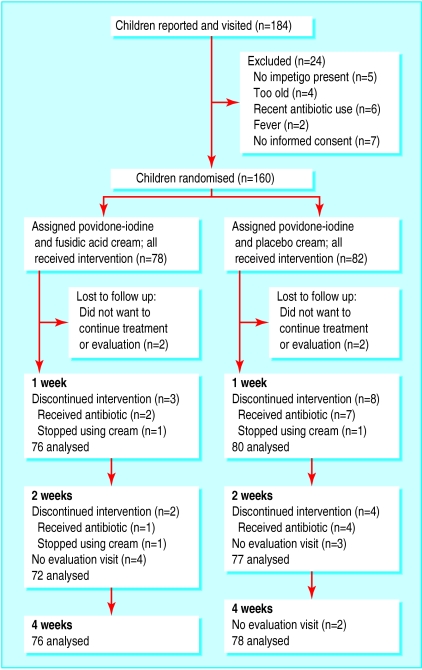
From February 1999 to November 2000, 58 general practitioners reported 184 children with impetigo. After the inclusion visit of the research nurse, 160 children were included and randomised (fig). The groups were comparable with respect to baseline characteristics (table 1). Non-Dutch patients were mainly of Turkish or Moroccan origin, reflecting the distribution of the population in the catchment area.
Table 1.
Baseline characteristics of study population (n=160). Values are numbers (percentages) unless stated otherwise
| Fusidic acid cream (n=78) | Placebo cream (n=82) | |
|---|---|---|
| Mean (SD) age (years) | 4.8 (2.9) | 5.1 (2.7) |
| Boys | 47 (60) | 51 (62) |
| Dutch origin | 47 (60) | 48 (59) |
| Attending day care or school | 59 (76) | 66 (80) |
| Mean (SD) No of lesions | 11.4 (13.3) | 13.2 (14.5) |
| Mean (SD) affected area (cm2) | 5.6 (7.3) | 7.3 (11.4) |
| Mean (SD) body temperature (°C) | 36.5 (0.7) | 36.6 (0.7) |
| Lymphadenopathy | 32 (41) | 25 (30) |
| Mean (SD) duration of impetigo (days) | 9.6 (8.0) | 9.2 (10.2) |
| Localisation: | ||
| Head | 61 (78) | 61 (74) |
| Trunk | 22 (28) | 29 (35) |
| Limbs | 36 (46) | 33 (40) |
| Pre-existing eczema | 10 (13) | 12 (15) |
| Pathogen isolated: | ||
| Staphylococcus aureus | 66 (85) | 61 (74) |
| Streptococcus pyogenes (A) | 1 (1) | 4 (5) |
| Both | 1 (1) | 7 (9) |
| None | 10 (13) | 10 (12) |
| Fusidic acid resistance (S aureus): | ||
| Resistant | 0/67 (0) | 0/68 (0) |
| Intermediately sensitive | 3/67 (4) | 7/68 (10) |
| Sensitive | 64/67 (96) | 61/68 (90) |
| Mupirocin resistance (S aureus) | 1/67 (1) | 0/68 (0) |
| Erythromycin resistance (S aureus) | 6/67 (9) | 8/68 (12) |
As the number of missing evaluation visits was very small and was similar in the two groups (fig), we decided not to impute data but to exclude these children from the analysis for that particular evaluation moment.
For the intention to treat analysis the proportion of children cured clinically at one week was 55% in the fusidic acid cream group and 13% in the placebo group, resulting in a number needed to treat of 2.3. At two weeks and four weeks the results were still in favour of fusidic acid, but the differences were smaller and the results were no longer significant (tables 2 and 3). The final multivariate model contained, apart from treatment, the size of the affected area (adjusted odds ratio 12.6, 95% confidence interval 5.0 to 31.5). Clinical improvement and cure combined yielded a crude odds ratio of 5.2 (fusidic acid v placebo); this hardly changed after adjustment. The mean affected area declined steadily in the fusidic acid group, whereas in the placebo group the mean affected area had increased after one week's treatment (table 4). No difference was found between children with and without eczema (results not shown).
Table 2.
Clinical effect and bacterial cure (intention to treat analysis). Values are numbers (percentages)
| Fusidic acid cream (n=76) | Placebo cream (n=80) | |
|---|---|---|
| One week | ||
| Clinical effect: | ||
| Cure | 42/76 (55) | 10/80 (13) |
| Improvement | 25/76 (32) | 37/80 (46) |
| Failure | 9/76 (11) | 33/80 (41) |
| Bacterial cure | 63/69 (91) | 23/72 (32) |
| Two weeks | ||
| Clinical effect: | ||
| Cure | 53/72 (73) | 46/77 (60) |
| Improvement | 17/72 (23) | 20/77 (26) |
| Failure | 2/72 (3) | 11/77 (14) |
| Bacterial cure | 62/70 (89) | 52/70 (74) |
| Four weeks | ||
| Clinical effect: | ||
| Cure | 70/76 (92) | 69/78 (88) |
| Improvement | 5/76 (7) | 7/78 (9) |
| Failure | 1/76 (1) | 2/78 (3) |
| Bacterial cure | 71/75 (95) | 70/75 (93) |
Table 3.
Clinical cure: results of logistic regression analysis. Odds ratios (95% CI) fusidic acid cream (F) versus placebo (P)
| Intention to treat
analysis
|
Per protocol
analysis
|
||||
|---|---|---|---|---|---|
| Cured | Cured or improved | Cured | Cured or improved | ||
| One week: | (F, n=76; P, n=80) | (F, n=73; P, n=72) | |||
| Crude | 8.7 (3.9 to 19.3) | 5.2 (2.3 to 11.9) | 8.5 (3.6 to 19.1) | 5.2 (2.2 to 12.4) | |
| Adjusted | 12.6 (5.0 to 31.5) | 5.5 (2.3 to 13.4) | 3.0 (5.0 to 33.8) | 5.2 (2.2 to 12.4) | |
| Two weeks: | (F, n=72; P, n=77) | (F, n=68; P, n=65) | |||
| Crude | 1.9 (0.9 to 3.8) | 5.8 (1.2 to 27.3) | 1.8 (0.8 to 3.6) | 4.6 (0.9 to 22.7) | |
| Adjusted | 1.9 (0.8 to 4.1) | 5.2 (1.1 to 24.9) | 1.9 (0.8 to 4.7) | 4.1 (0.8 to 21.9) | |
| Four weeks: | (F, n=76; P, n=78) | (F, n=71; P, n=66) | |||
| Crude | 1.5 (0.5 to 4.5) | 2.0 (0.2 to 22.2) | 1.1 (0.3 to 3.5) | 1.1 (0.1 to 17.6) | |
| Adjusted | 1.8 (0.5 to 5.9) | 2.3 (0.1 to 50.5) | 1.1 (0.3 to 3.7) | 1.7 (0.0 to 26.6) | |
Table 4.
Mean change in size of affected area. Affected area at baseline=100%
| Fusidic acid cream | Placebo cream | Adjusted P value (intention to treat analysis) | |
|---|---|---|---|
| One week | −66% | 27% | ⩽0.001 |
| Two weeks | −90% | −38% | 0.24 |
| Four weeks | −99% | −95% | 0.67 |
Non-compliance
In the first week, three patients in the fusidic acid cream group and eight patients in the placebo group did not comply with the treatment protocol. Nine patients, seven of whom were in the placebo group, had received an antibiotic treatment from their general practitioner and stopped using the study cream, in most cases because the impetigo had worsened or had not improved. Two patients, one in each group, had stopped using the study cream before the impetigo was cured but had not used any other treatment. Minor violations of the protocol, such as omission of a single application of povidone-iodine shampoo or study cream, were recorded in 14 cases (not shown in table). Another six patients were non-compliant in the second week (four in the placebo group and two in the fusidic acid group) and another five patients received extra antibiotic treatment—four patients in the placebo group and one in the fusidic acid group.
Side effects
Side effects were experienced in 26 cases—seven occurred in the fusidic acid group and 19 in the placebo group. The most frequent side effects were pain (two in the fusidic acid group v six in the placebo group) and burning (one v four) during administration of the povidone-iodine shampoo. Other side effects were redness (two in each group), burning from the study cream (one in placebo group), itching from both applications (two in placebo group), and pain and irritation from both applications (one in each group).
Bacteriological results
For all the baseline visits and 431 (94%) of the 459 follow up visits, swabs were available or lesions were cured. S aureus was found in 135 (96%) of the 140 positive cultures at baseline (table 1); S pyogenes and mixed infections were found in a minority of cases. No strain of staphylococcus was found to be resistant to fusidic acid. Ten of the 140 strains were intermediately sensitive to fusidic acid. At baseline, 20 (12.5%) of the 160 swab cultures showed no bacterial growth. In the intention to treat analysis, the proportion of children with bacterial cure at one week was 91% in the fusidic acid group compared with 32% in the placebo group (tables 2 and 5).
Table 5.
Bacterial cure: results of logistic regression analysis. Odds ratios (95% CI) fusidic acid cream (F) versus placebo (P)
| Intention to treat analysis | Per protocol analysis | |
|---|---|---|
| One week: | (F, n=69; P, n=72) | (F, n=70; P, n=63P) |
| Crude | 22.4 (8.5 to 59.2) | 23.9 (8.4 to 68.0) |
| Adjusted | 28.3 (9.5 to 84.7) | 43.9 (13.1 to 147.1) |
| Two weeks: | (F, n=70; P, n=70) | (F, n=66; P, n=58) |
| Crude | 2.7 (1.1 to 6.7) | 2.4 (0.9 to 6.6) |
| Adjusted | 2.9 (1.1 to 7.8) | 2.8 (0.9 to 8.1) |
| Four weeks: | (F, n=75; P, n=75) | (F, n=70; P, n=63) |
| Crude | 1.3 (0.3 to 4.9) | 0.5 (0.1 to 3.1) |
| Adjusted | 1.1 (0.3 to 4.4) | 0.4 (0.1 to 2.7) |
Discussion
This is the first study to compare fusidic acid cream with placebo in treating impetigo. It shows that application of fusidic acid cream in combination with povidone-iodine shampoo is much more effective than placebo cream combined with povidone-iodine shampoo.
The treatment effect of fusidic acid cream compared with placebo after one week was even larger after adjustment for potential confounders. After two and four weeks the differences between the two treatments were smaller and no longer significant. If it was not due to the possibly benign spontaneous course of impetigo, this finding was probably caused by the fact that more patients in the placebo group had “crossover treatment” with antibiotics. Fourteen per cent of the patients in the placebo group compared with 4% in the fusidic acid group returned to their general practitioner because of failure of treatment and received an extra, usually oral, antibiotic treatment.
Our hypothesis that fusidic acid cream would not increase the treatment effect of disinfecting with povidone-iodine is firmly rejected. The results do not support conservative treatment with disinfecting measures alone. Treatment with povidone-iodine combined with placebo cream had a very disappointing cure rate of 13% at one week. The mean affected area after one week of treatment was even larger than at the start of treatment. Also, administration of the povidone-iodine more often caused pain and burning in the placebo group, which may be explained by prolonged healing of the lesions in this group. The low cure rates in this study and the reported side effects raise questions about the value of povidone-iodine as an adjunctive treatment for impetigo. Doubts have already been raised about the value of other disinfecting measures in the treatment of impetigo.6,23,24 Future studies comparing povidone-iodine with placebo may provide more evidence.
A small number of swab results (12.5%) were negative at baseline—because we were studying patients with a clinical diagnosis of impetigo we had no reason to exclude these patients. S aureus was the pathogen most often found, which accords with other investigations showing that the predominant organism in impetigo has changed from S pyogenes to S aureus in recent years.1,11 We did not find resistance to fusidic acid in our study population, indicating that many years of use of topical fusidic acid has not resulted in appreciable resistance in staphylococci in the general population. The resistance to erythromycin in staphylococci was higher, in accordance with the international trend. Although oral antibiotics are used for impetigo in many countries, this finding provides an extra argument for topical treatment with fusidic acid cream.
Our recent meta-analysis showed that fusidic acid and mupirocin were equally effective in treating impetigo,17 but fusidic acid costs less. Regional antibiotic policies may differ in restricting the use of one of these two antibiotics.3,5,16
We conclude that topical fusidic acid cream is an effective treatment for impetigo, with very few side effects, and can be considered a first choice in the treatment of impetigo in general practice. The value of sole or adjunctive treatment with povidone-iodine can be questioned.
Figure.
Flow and follow up of participants
Acknowledgments
We thank all the participating general practitioners; Mariet Op ‘t Veld, research nurse for the field work, and her assistants Jannie Blom, Carine van Egten, Cocky van der Ent, Wil Hartendorp, Marjon de Jong, and Thea Zweers; Roel Verkooyen for helping with data entry; Susan Snijders for the laboratory work; Frederike Engels for preparing the study medication; Paul Mulder for preparing the list of random numbers; and Arianne Verhagen and Joost Zaat for their critical comments on a draft of the paper.
Footnotes
Funding: Grant from the Fonds Alledaagse Ziekten (common illnesses fund) of the Dutch College of General Practitioners.
Competing interests: APO was reimbursed four years ago by Leo Pharmaceutical Products for attending symposiums and has received fees for speaking at satellite symposiums.
References
- 1.Dagan R, Bar-David Y. Double-blind study comparing erythromycin and mupirocin for treatment of impetigo in children: implications of a high prevalence of erythromycin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 1992;36:287–290. doi: 10.1128/aac.36.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruijnzeels MA, van Suijlekom-Smit LWA, van der Velden J, van der Wouden JC. The child in general practice. Rotterdam/Utrecht: Erasmus Universiteit/NIVEL; 1993. [Google Scholar]
- 3.Boukes FS, van der Burgh JJ, Nijman FC, Sampers GM, Simon B, Romeijnders AC, et al. NHG-Standaard bacteriële huidinfecties [Dutch College of General Practitioners’ guideline on bacterial skin infections] Huisarts Wet. 1999;41:427–437. [Google Scholar]
- 4.Resnick DS. Staphylococcal and streptococcal skin infections: pyodermas and toxin-mediated syndromes. In: Harper J, Oranje A, Prose N, editors. Textbook of pediatric dermatology. 1st ed. Oxford: Blackwell; 2000. pp. 369–372. [Google Scholar]
- 5.Hay RJ, Adriaans BM. Bacterial infections. In: Champion RH, Burton JL, Ebling FJG, editors. Rook/Wilkinson/Ebling, Textbook of dermatology. 6th ed. Oxford: Blackwell; 1998. pp. 1109–1111. [Google Scholar]
- 6.Carruthers R. Prescribing antibiotics for impetigo. Drugs. 1988;36:364–369. doi: 10.2165/00003495-198836030-00006. [DOI] [PubMed] [Google Scholar]
- 7.Baltimore RS. Treatment of impetigo: a review. Pediatr Infect Dis. 1985;4:597–601. doi: 10.1097/00006454-198509000-00061. [DOI] [PubMed] [Google Scholar]
- 8.Espersen F. Resistance to antibiotics used in dermatological practice. Br J Dermatol. 1998;139:4–8. doi: 10.1046/j.1365-2133.1998.1390s3004.x. [DOI] [PubMed] [Google Scholar]
- 9.Mertz PM, Marshall DA, Eaglstein WH, Piovanetti Y, Montalvo J. Topical mupirocin treatment of impetigo is equal to oral erythromycin therapy. Arch Dermatol. 1989;125:1069–1073. [PubMed] [Google Scholar]
- 10.De Neeling AJ, van Leeuwen WJ, Schouls LM, Schot CS, van Veen-Rutgers A, Beunders AJ, et al. Resistance of staphylococci in the Netherlands: surveillance by an electronic network during 1989-1995. J Antimicrob Chemother. 1998;41:93–101. doi: 10.1093/jac/41.1.93. [DOI] [PubMed] [Google Scholar]
- 11.Rogers M, Dorman DC, Gapes M, Ly J. A three-year study of impetigo in Sydney. Med J Aust. 1987;147:63–65. doi: 10.5694/j.1326-5377.1987.tb133260.x. [DOI] [PubMed] [Google Scholar]
- 12.Bass JW, Chan DS, Creamer KM, Thompson MW, Malone FJ, Becker TM, et al. Comparison of oral cephalexin, topical mupirocin, and topical bacitracin for treatment of impetigo. Pediatr Inf Dis J. 1997;16:708–709. doi: 10.1097/00006454-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Britton JW, Fajardo JE, Krafte-Jacobs B. Comparison of mupirocin and erythromycin in the treatment of impetigo. J Pediatr. 1990;117:827–829. doi: 10.1016/s0022-3476(05)83352-9. [DOI] [PubMed] [Google Scholar]
- 14.Godtfredsen WO, Roholt K, Tybring L. Fucidin: a new orally active antibiotic. Lancet. 1962;i:928–931. doi: 10.1016/s0140-6736(62)91968-2. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert M. Topical 2% mupirocin versus 2% fusidic acid ointment in the treatment of primary and secondary skin infections. J Am Acad Dermatol. 1989;20:1083–1087. doi: 10.1016/s0190-9622(89)70137-7. [DOI] [PubMed] [Google Scholar]
- 16.Morley PAR, Munot LD. A comparison of sodium fusidate ointment and mupirocin ointment in superficial skin sepsis. Curr Med Res Opin. 1988;11:142–148. doi: 10.1185/03007998809110457. [DOI] [PubMed] [Google Scholar]
- 17.Van Amstel L, Koning S, van Suijlekom-Smit LWA, Oranje A, van der Wouden JC. De behandeling van impetigo contagiosa, een systematisch overzicht [Treatment of impetigo contagiosa, a systematic review] Huisarts Wet. 2000;43:247–252. [Google Scholar]
- 18.VandenBergh MF, Yzerman EP, van Belkum A, Boelens HA, Sijmons M, Verbrugh HA. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J Clin Microbiol. 1999;37:3133–3140. doi: 10.1128/jcm.37.10.3133-3140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical microbiology procedures handbook. Washington, DC: American Society for Microbiology; 1992. [Google Scholar]
- 20.Finlay JE, Miller LA, Poupard JA. Interpretive criteria for testing susceptibility of staphylococci to mupirocin. Antimicrob Agents Chemother. 1997;41:1137–1139. doi: 10.1128/aac.41.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. 7th ed. Wayne, PA: NCCLS; 2000. . (Approved standard M2-A7.) [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility testing, 11th informational supplement. Wayne, PA: NCCLS; 2001. . (M100–S11.) [Google Scholar]
- 23.Ruby RJ, Nelson JD. The influence of hexachlorophene scrubs on the response to placebo or penicillin therapy in impetigo. Pediatrics. 1973;52:854–859. [PubMed] [Google Scholar]
- 24.Dajani AS, Hill PL, Wannamaker LW. Experimental infection of the skin in the hamster simulating human impetigo: II. Assessment of various therapeutic regimens. Pediatrics. 1971;48:83–90. [PubMed] [Google Scholar]